ESVS publishes “pioneering” clinical practice guidelines on radiation safety


The European Society for Vascular Surgery (ESVS) has released clinical practice guidelines on radiation safety, which the writing committee notes are the first guidelines on the topic to be published under the auspices of a vascular surgical society. The guidelines were published online ahead of print in the European Journal of Vascular and Endovascular Surgery (EJVES) and presented for the first time at the 2022 ESVS annual meeting (20–23 September, Rome, Italy).
Bijan Modarai (Guy’s and St Thomas’ NHS Foundation Trust and King’s College, London, UK) and Stéphan Haulon (Hôpital Marie Lannelongue, GHPSJ, Paris, France) co-chaired the guideline writing committee that included 13 members in total which, as well as vascular surgeons and interventional radiologists, included a radiation protection scientist and a medical physicist.

In EJVES, the authors outline the aim of the guidelines as being “to inform the reader about radiation physics and radiation dosimetry, raising awareness of the risks of ionising radiation, and describing the methods available to protect against radiation exposure”.
The need for guidelines on radiation safety has grown in line with the rise of endovascular procedures over the last two decades, Modarai, Haulon et al state in the introductory chapter. They note that the risks of radiation exposure, however, are “not universally recognised” due to a “poor


understanding of key concepts and paucity of educational material directly relevant to vascular surgery”.
The authors cover measuring radiation exposure and the associated risks of exposure, legislation regarding exposure limits for radiation-exposed workers, and radiation safety practices and protection equipment in the operating room, among other topics.
At ESVS 2022, Modarai stated that the “pioneering” new document, as described by writing committee member Anders Wanhainen (Uppsala University, Uppsala, Sweden), is long-awaited—the culmination of two years of work. He expressed that while the guidelines will help to raise awareness amongst stakeholders and set the standard for individuals and institutions, these also underline the fact that this is an evolving area of practice and highlight areas where more research is needed.





The future is radiation free
In a chapter on future technologies and gaps in evidence, Modarai et al underline the fact that several recommendations are supported by level C evidence and are relying on expert opinion of the committee,
Cardiac and vascular surgeons collaborate at the vanguard
CX AORTIC VIENNA RETURNS THIS year for its third edition (24–26 October, Digital), bringing together world-leading specialists from the cardiac and vascular fields to discuss all facets of aortic care from selection to investigation, diagnosis, techniques and technologies. The meeting will showcase the latest approaches—open and endovascular—for the treatment of complex aortic problems spanning the aortic valve to the iliac arteries.
Having attracted a global, online audience during its first two editions, CX Aortic Vienna will continue to reach out to the worldwide aortic surgery community. The 2022 edition features three days of high-quality digital programming, to be broadcast live from 24–26 October for the wider global audience, delivering a total of 15 hours of aortic education, which will also be available on-demand to registered attendees after the event. CX Aortic Vienna Digital Edition registrants can claim up to 14 EU/US reciprocal CME credits for both livestreamed and on-demand content.
The format for the digital edition will follow the CX style of short talks, debates, and audience interaction, presented live via broadcast. The programme includes open and endovascular aortic techniques and technologies, including edited aortic cases.

For the first time, the meeting will also



Featured in this issue: November 2022 | Issue 96 www.vascularnews.com SPACE-2: New data on carotid interventions page 31 12
Gustavo Oderich Endovascular thoracoabdominal aortic repair
Profile: Markus Steinbauer page 20 See clearly. Treat optimally. Fiber Optic RealShape (FORS) technology paves the way to radiation free 3D device guidance Learn more about our clinical journey www.philips.com/FORS-technology 220911 _CX Aortic Vienna_225x54-5mm.indd 1 10/10/2022 09:52
Continued
on page 4 Continued on page 6
There is a need for the vascular community and allied disciplines to instigate studies that will strengthen the evidence base for radiation protection matters.”
The global leader in aortic













More than




patients treated worldwide
medtronic.com/aortic UC202306914IE ©2022 Medtronic. All rights reserved. Medtronic and the Medtronic logo are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. 10/2022
800,000
You’re invested. So are we.

Dedication
Aortic support where and when it matters.






Durability


Strength in products. Strength in evidence.

Credibility Technical expertise.

ESVS publishes “pioneering” clinical practice guidelines on radiation safety





















Continued from page 1
which they say “highlights the need for the vascular community and allied disciplines to instigate studies that will strengthen the evidence base for radiation protection matters”. New technologies—including 3D navigation, robotic tracking, and artificial intelligence—that require a reduced need for X-ray “should be embraced and evaluated carefully,” for example.
At ESVS 2022, Joost van Herwaarden (Utrecht University Medical Center, Utrecht, The Netherlands), who disclosed a research collaboration with Philips, spoke on the benefits and drawbacks of some of these technologies, speaking first on robotic navigation. He highlighted its ability to reduce radiation exposure for the operator and increase accuracy of catheter positioning, while acknowledging that the technology still carries the limitations associated with fluoroscopy use, due to its requirement for navigation.
Another technology is intravascular ultrasound (IVUS). “You get circular information about the vessel wall, the lumen, and also about aortic side branches, as well as improved arterial wall and stenosis characterisation, and improved sizing for your percutaneous transluminal angioplasty,” he said, noting however that visualisation length is limited to the ultrasound transducer, which is “why you still need fluoroscopy for navigation of your endovascular devices” with the use of IVUS.
According to Van Herwaarden, electromagnetic tracking addresses the limiting factors of greyscale images, offering instead brightly coloured images, as well as 3D navigation of devices, unlimited viewing angles, and no radiation, with the limited numbers of wires and catheters being a drawback.
Fiber Optic RealShape (FORS) technology from Philips also shows potential in this space, offering the benefits of 3D navigation, unlimited viewing angles, and the radiation-reducing benefits associated with the use of light instead of fluoroscopy, Van Herwaarden highlighted. He noted that one drawback is the fact there are only a limited number of devices available.
Finally, the presenter gave an overview of the pros and cons of image fusion. He stated that there is one system in this space—the Cydar Medical system—that offers automated registration. This technology compares the anatomy visible on live fluoroscopy with anatomy of a preoperative computed tomography angiography
(CTA) and automatically produces 3D overlay, he explained. According to the presenter, this technology offers “a significant reduction of contrast agent and also reduction of procedure time,” despite having a somewhat “complex setup”. Image fusion is “widely available” and, in the presenter’s opinion, “should be used in all complex procedures”.
Van Herwaarden summarised that these technologies show radiation-reducing potential, the small number of available publications suggest that these techniques are “still minimally applied”. According to the presenter, the future of these technologies lies in their combined use. For example, he said that, while electromagnetic tracking and FORS are “still under development,” they do offer “an even greater promise for simplifying complex procedures and realising dose reduction for patients and for staff,” when used in combination with IVUS or a robotic navigation system.
“Performing endovascular interventions without any form of radiation is certainly the future,” he concluded.




















Education and training are paramount
Another chapter of the guidelines is dedicated to education and training. Speaking to Vascular News on the topic, Isabelle Van Herzeele (Ghent University Hospital, Ghent, Belgium) highlighted the necessity of starting early with education and training in radiation safety. “Ideally we should start training about radiation protection in medical school or in nursing school, so that everybody is aware of the dangers and knows the theory about justification (do benefits outweigh the risks), optimisation (obtain image of sufficient quality to guide treatment while respecting the ALARA principle in real life) and dose limits (be aware of possible side effects)”. She noted also that several technologies exist to remove radiation as a factor at the practical training stage.
“Nowadays, you should have the opportunity to train in a radiation-free environment, so using simulation to learn steps, basic endovascular skills, and how to manoeuvre your C-arm without being exposed,” she said.
Furthermore, Van Herzeele underlined the benefits of continued training in radiation safety, especially regarding the changing technological landscape outlined by Van Herwaarden. “Techniques and the materials that we use are evolving, and whenever big changes are being made in your radiation safety environment, additional training should take place, with the overarching goal of ensuring we can treat our patients safely and protect everybody within the team.”
Van Herzeele also expressed her hope that these new guidelines will not only provide awareness on the risks of radiation, as well as practical steps for reducing exposure, but also provide vascular team members with some “backup” if ever faced with addressing a manager about the need to improve radiation safety protection within the endovascular operating room.
News in brief
n EARLY EVAR IN WOMEN:





A multinational collaboration of researchers has received endorsement from the Global Cardiovascular Research Funders Forum (GCRFF) Multinational Clinical Trials Initiative for the WARRIORS
(Women’s abdominal aortic aneurysm research: repair immediately or routine surveillance) trial. Vascular News spoke with chief investigator Janet Powell (Imperial College London, London, UK) about what is next for the trial in light of the GCRFF endorsement, and why research in this space is important.

For more on this story go to page 11.
n ATHERECTOMY:
In the USA, atherectomy use in peripheral vascular interventions “more than doubled” from 2010 to 2019, with office-based procedures a “major driver” of this increase.
This is according to published US data from the Vascular Quality Initiative, which also revealed wide regional variability in the use of atherectomy.
For more on this story go to page 17.
n PULMONARY EMBOLISM:
Results of the FLASH registry demonstrate the “excellent safety profile” of the FlowTriever system (Inari Medical) in 800 “real-world” patients. This is according to Catalin Toma (University of Pittsburgh Medical Center, Pittsburgh, USA), who presented outcomes for the full US cohort of FLASH at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT; 16–19 September, Boston, USA). The results were simultaneously published in EuroIntervention
For more on this story go to page 22.
Published by:
of
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788



































Reprint requests and







regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2022. All rights reserved.




Write to us!
BIBA Medical,
America, 155 North Wacker Drive – Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
Printed by:
write to jocelyn@bibamedical.com
4 facebook.com/VascularNews www.vascularnews.com
Editor-in-chief: Roger Greenhalgh Publisher: Roger Greenhalgh Content Director: Urmila Kerslake Editor: Jocelyn Hudson Jocelyn@bibamedical.com Editorial contribution: Jamie Bell, Will Date, Bryan Kay and Clare Tierney Design: Terry Hawes, Wes Mitchell and David Reekie Advertising: Shilpa Suthar shilpa@bibamedical.com Subscriptions: subscriptions@bibamedical.com Please contact the Vascular News team with news or advertising queries Tel: +44 (0) 20 7736 8788
BIBA Publishing, which is a subsidiary
BIBA Medical Ltd
North
Buxton Press
all correspondence
If you have comments on this issue or suggestions for upcoming editions
Make sure you get your copy of Next issue February 2023 Cover Story 19.7% in 2019 to In the USA, atherectomy use for peripheral vascular interventions increased from 8.5% in 2010




























































E-vita™ Thoracic 3G Stent Graft System E-nside™ TAAA Multibranch Stent Graft System
Cardiac and vascular surgeons collaborate at the vanguard of aortic care
offer an in-person component from its home city, Vienna, Austria, where CX Aortic Vienna has been invited to provide two 90-minute primetime, cuttingedge English-language sessions for attendees of the Dreiländertagung—the joint meeting of the Austrian Society of Vascular Surgery (ÖGG), the Swiss Society for Vascular Surgery (SGG) and the German Society of Vascular Surgery (DGG)—on Thursday 20 October.
World-leading faculty
The full programme has been curated by a Vascular, Endovascular and Cardiothoracic executive board comprised of leaders in the field of aortic care including CX Aortic Vienna founding chair Roger Greenhalgh (London, UK), alongside Tilo Kölbel (Hamburg, Germany) who will moderate the inperson session at Dreiländertagung, Afshin Assadian (Vienna, Austria), Roberto Chiesa (Milan, Italy), Martin Grabenwöger (Vienna, Austria), Stéphan Haulon (Paris, France), Gustavo Oderich (Houston, USA), Markus Steinbauer (Regensburg, Germany) and Alexander Zimmermann (Zürich, Switzerland).
“This is, as always, a focus upon the aorta as managed by cardiac aortic surgeons, open vascular surgeons and endovascular surgeons; from the aortic
valve at one end, to the iliacs at the other end,” says Greenhalgh of the event’s 2022 edition. “CX Aortic Vienna, for those of you who have been to it before, is for all of those who manage the aorta,” he adds. “This is not just the surgeons, but the physicians—we have radiologists, imaging experts, vascular scientists and vascular nurses. All who manage aortic patients are welcome to come and join us for the digital edition on 24, 25 and 26 October this year.”
“October is the time for aortic disease and to discuss newest trends, technologies and features in aortic therapy,” comments Kölbel. “We are focusing on both open and endovascular techniques, and we are inviting the cardiovascular and vascular communities to come to CX Aortic Vienna, with a presence during the Dreiländertagung, and very much to the virtual event that happens a few days later.”
Multidisciplinary approach
The importance of multidisciplinary aortic approaches will be a key strand running through CX Aortic Vienna’s content and discussion, and the 2022 event will continue its mission to bring together specialists of all skills—be they cardiac, vascular or endovascular—to provide a comprehensive overview of cutting-edge aortic treatment.
“CX Aortic Vienna is the unique chance to get together with cardiac surgeons and all kinds of vascular specialists that are treating the aorta,” says Zimmermann, discussing the importance of the multidisciplinary focus at the heart of CX Aortic Vienna.
“The problem is that we have a transition zone in the arch and we—as vascular surgeons—move further and further into the ascending aorta with our endovascular techniques. This can only be done with the support of the cardiac surgeons, and this is

Mortality drops for acute type A aortic dissection, still high for patients not receiving surgery
The chance of a patient surviving after an acute type A aortic dissection has improved significantly, but mortality remains high if not recognised early and repaired surgically. This is according to new research from a team at Michigan Medicine at the University of Michigan (Ann Arbor, USA).
A TEAM OF RESEARCHERS examined early mortality rates for over 5,600 patients admitted to the hospital and examined hourly with acute type A aortic dissection between 1996 and 2018 from the International Registry for Acute Aortic Dissection (IRAD).
Findings published in JAMA Cardiology reveal that 5.8% of patients with acute type A aortic dissection died within the first two days after hospital arrival, a mortality rate of 0.12% per hour. The rate is significantly lower than that reported in the 1950s, which estimated that 37% of patients died within the first 48 hours, with an increasing mortality rate of 1–2% per hour.
“We believe that advances in diagnosis and management, especially a focus on early surgical repair, may have contributed in part to these improvements in mortality for acute
the reason why we really look forward to CX Aortic Vienna because everyone comes together addressing this complex field.”
Talking points
The programme encompasses discussion and debate spanning key talking points in the aortic space including aortic arch interventions, thoracic dissection, thoracic imaging, thoracoabdominal techniques, juxtarenal, abdominal aortic and iliac artery therapies.
“I have had the honour and the privilege during my career to treat the aorta from the aortic valve all the way to the femoral arteries,” notes Joseph Coselli (Houston, USA), who has been among the expert speakers to have participated in previous editions of CX Aortic Vienna and is returning in 2022. “The entire history of aortic surgery, and vascular surgery to the same extent, has been one of technical and clinical evolution.”
Coselli adds: “The Charing Cross meetings are among the best with regard to the broad spectrum of technology and clinical information and sharing among experts. I would invite everyone to take the opportunity to learn a lot about vascular and aortic surgery at CX Aortic Vienna.”
From 2023 onwards, CX Aortic Vienna will be held annually in October as a hybrid meeting in-person in Vienna whilst simultaneously livestreaming globally.
Key points:
CX Aortic Vienna Digital Edition registrants can claim up to 14 EU/US reciprocal CME credits for both livestreamed and on-demand content
From 2023 onwards, CX Aortic Vienna will be held annually in October as a hybrid meeting in-person in Vienna whilst simultaneously livestreaming globally
aortic dissection,” said Kim Eagle, senior author of the paper and director of the University of Michigan Health Frankel Cardiovascular Center.
Of all the patients, 91% either received surgery or were intended for surgery, with the others managed medically due to advanced age and complications, such as stroke and kidney failure. Nearly 24% of those receiving medical treatment alone died within two days, compared to 4.4% of patients treated with surgical repair—a death rate more than five times higher.
“Patients who were managed medically were likely not surgical candidates due to their comorbidities,” said Bo Yang, a professor of cardiothoracic surgery at University of Michigan Medical School, in a press release announcing the results of the study. Yang was not involved in the study, the release notes. “The medically
managed patients could die from aortic dissection-associated complications— such as malperfusion, cardiac tamponade, aortic rupture and acute aortic insufficiency, which can be treated with surgery—or from their existing medical conditions, which could be worsened by the aortic dissection.”
Only 1% of patients deemed suitable for surgery died before the procedure. These patients died after an average of nearly nine hours from being admitted to the hospital, exceeding the six-hour median time to surgery for all patients.
Interhospital transfer is needed in more than 70% of aortic dissection cases, causing inherent delays. Before this study, Eagle says, it was thought that early death from this condition was so prohibitive that operating urgently, even in hospitals with limited volume of aortic dissection surgery and resources, was the preferred strategy.
However, there is evidence that surgery at a low-volume hospital can double the risk of dying while undergoing repair compared to the highest volume providers. Additionally, mortality rates for open repair of acute type A aortic dissection are nearly three times higher when the operation is not performed by a dedicated aortic surgeon.
“Hospital mortality at a highvolume centre like U-M [University of Michigan], where aortic dissection
patients are taken care only by highly experienced aortic surgeons, can be as low as 5%, while the same patient operated on at a low-volume centre may be 20% or higher,” Eagle said. “With this new information, it is clear that the ‘cost’, or risk, of a four-to-six-hour delay caused by transfers is more than offset by the lower risk of surgery at experienced hospitals.”
Cases are rare. Approximately three in 100,000 people suffer aortic dissection each year. The condition most commonly affects older men, and a person experiencing the tear may feel a “knifelike, tearing pain through the back,” according to IRAD.
It is estimated that up to 50% of patients will die before ever reaching the hospital, making the overall mortality for aortic dissection substantially higher, the press release from Michigan Medicine notes.
“There is a need to identify the highrisk population of aortic dissection, such as those with a family history of aortic aneurysm and dissection, especially at a younger age, or known pathogenic genetic variants, so that we can replace the proximal aorta electively to prevent acute type A aortic dissection,” Yang said. “For young people under 55 years old with severe chest pain, we have to prove if patients have aortic dissection or otherwise.”
November 2022 | Issue966
Top Stories
Continued from page 1
CX Aortic Vienna Digital Edition registrants can claim up to 14 EU/ US reciprocal CME credits for both livestreamed and on-demand content.
14
stands

Patency that
the test of time Mwipatayi BP et al. A systematic review of covered balloon expandable stents for treating aorto-iliac occlusive disease. Journal of Vascular Surgery, 2020. Visit getinge.com to learn more. PN011632 Rev AA Advanta V12 Balloon expandable covered stent As the only balloon expandable covered stent with clinical evidence demonstrating long-term patency up to 8 years, Advanta V12 treats patients with even the most challenging TASC C&D lesions with durable results.
Fully automated software using artificial intelligence could become a “crucial adjunct” for EVAR follow-up

A software named PRAEVAorta (Nurea), using artificial intelligence (AI), has the potential to enable a fast, reproducible, and fully automated analysis of abdominal aortic aneurysm (AAA) sac pre- and post-endovascular aneurysm repair (EVAR). This is according to Caroline Caradu (Bordeaux University Hospital, Bordeaux, France) and colleagues, who recently reported the results of a single-centre, retrospective analysis of fully automatic volume segmentation using deep learning approaches in the Journal of Vascular Surgery (JVS).
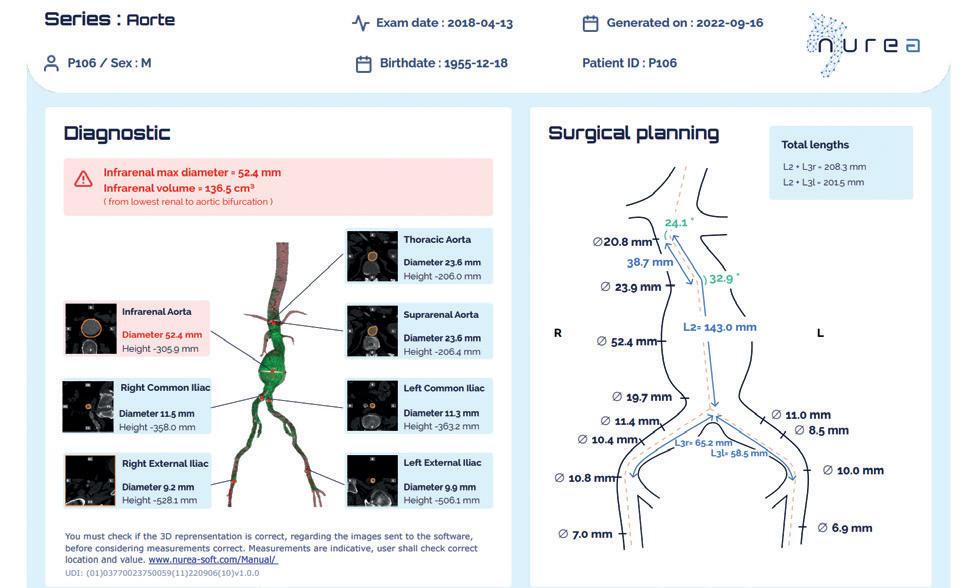
Precise follow-up remains the “Achilles’ heel” of EVAR, the researchers note in the introduction to their report, detailing that the procedure’s longterm success has been “hindered” by postoperative complications such as endoleaks, endotension, stent graft migration, and iliac limb occlusion. They highlight that these complications cannot always be detected by visual inspection of computed tomography (CT) scans, and state that “an urgent need exists for more accurate and reliable postprocedure surveillance parameters by which to assess the behaviours of post-EVAR aneurysm sacs”. New technologies able to detect endoleaks and analyse their effects on the aneurysm sac are “highly relevant”, they specify.
The research group—which includes investigators from Bordeaux University Hospital and Imperial College London (London, UK)—note that EVAR surveillance relies on serial measurements of the maximal sac diameter despite significant inter- and intraobserver variability. However, Caradu et al write that volumetric measurements are more sensitive and that their implementation has been hampered by the time required for their implementation.
PRAEVAorta, which the authors describe as an


volumes and surfaces and the proximal neck and maximum aneurysm diameters measured using the fully automatic and manually corrected segmentation methods (Pearson’s coefficient correlation >0.99; p<0.0001).
In addition, they note that a comparison between the fully automatic and manually corrected segmentation methods revealed a mean Dice similarity coefficient of 0.95±0.015, Jaccard index of 0.906±0.028, sensitivity of 0.929±0.028, specificity of 0.965±0.016, volumetric similarity of 0.973±0.018, and mean Hausdorff distance/slice of 8.7±10.8mm.
Finally, Caradu et al reveal that the segmentation time was nine times faster with the fully automatic method (2.5 minutes vs. 22 minutes per patient with the manually corrected method; p<0.0001), and that a preliminary analysis also demonstrated that a diameter increase of 2mm can actually represent a >5% volume increase.
The authors conclude that PRAEVAorta showed “excellent reproducibility” and “required less time and reduced the risk of human error”. They suggest that the software could become a “crucial adjunct” to endovascular aneurysm repair (EVAR) follow-up through early detection of sac evolution, which might reduce the risk of secondary rupture.
Caradu and colleagues acknowledged some limitations of their analysis in the discussion of their findings. The study was restricted to infrarenal AAAs, for example, due to the fact that the precise characterisation of patients with complex aneurysms, including the pararenal and visceral segments, “remains to be validated”. They write that further software development and validation are needed to enable automated reporting on the presence and type of endoleaks, adding that the development of volumetric analysis of the sealing zone “is also in progress”.
of JVS, which opened with a special communication on the application of AI in vascular surgery. In the article, Konstantinos Spanos (Larissa University Hospital, Larissa, Greece) and colleagues opine that AI and machine learning technologies are “rapidly revolutionising the healthcare system and patient management” and outline why vascular surgeons “should pay attention” to developments in the space.
“It is of paramount importance for healthcare providers and insurance companies to understand the state of AI technologies and how these can be used to improve the efficacy and safety of, and access to, healthcare services to achieve cost-effectiveness in healthcare status,” they write.
Spanos et al suggest that AI and its applications could benefit vascular physicians at both the clinical and the administrative levels. In regard to AAA disease, for example, they reference current international guidelines that highlight the importance of follow-up for these patients, something that the work of Caradu and colleagues addresses. “It is a clinical issue of paramount importance to predict which patients will require closer follow-up and potentially reintervention to prevent postoperative complications,” the authors stress.
Beyond assisting in the prediction of AAA growth and rupture risk, Spanos and colleagues hypothesise that the use of AI could also represent a method for determining the indications and optimal surgical treatment. They suggest that it might be able to assist in planning postoperative monitoring, and also in expediting the development of personalised decision-making and treatment for AAA patients, for instance.
Spanos et al also touch upon the applications of AI in cerebrovascular disease and peripheral arterial disease (PAD). For the former, the authors mention among various potential uses that AI could play a role in computer-aided prediction of stroke. They note that while the application of AI technologies to PAD is “in its infancy,” the potential is “tremendous”.
“innovative, fully automated software,” had previously demonstrated “fast and robust detection of the characteristics of infrarenal [AAAs] on preoperative imaging studies”. In the present study, they note, the team assessed the robustness of these data on post-EVAR CT scans. Caradu and colleagues explain that they compared fully automatic and semiautomatic segmentation manually corrected by a senior surgeon using a dataset of 48 patients—48 early post-EVAR CT scans with 6,466 slices and 101 follow-up CT scans with 13,704 slices.
In JVS, the authors report that their analyses confirmed the excellent correlation of the post-EVAR
In closing, the investigators state that their results, in addition to current literature, “bring interesting perspectives to the use of AI for clinical practice”. In time, they believe, “these tools will be used for optimising preoperative planning and sizing, to better assess aneurysmal evolution, and to anticipate post-EVAR complications, with the potential to individually tailor postoperative surveillance protocols”.
In the spotlight: Application of AI in vascular diseases
Caradu et al’s work appeared in the September edition
The authors also consider an economic perspective on the application of AI in healthcare. “Reported evidence is lacking regarding the economic effects of AI solutions for the healthcare system,” they write. They reference one meta-analysis on the topic, however they stress that “more comprehensive economic analyses are required before deciding for or against implementing AI technology in healthcare”.


Speaking to Vascular News, Caradu and senior author Eric Ducasse (Bordeaux University Hospital) remark on the the future direction of the PRAEVAorta software: “In a research-use-only version, it integrates a prediction tool giving hints on how the aneurysm will evolve based on data classification of geometry and blood flow characteristics.
“Moreover, this AI is on the verge of proposing a consolidated solution for an advanced and complete 3D visualisation of the entire vascular network dedicated to vascular disease quantification (for instance in peripheral arterial disease) to enable early vascular disease diagnostic, follow-up, and prevention.”
November 2022 | Issue968
C M Y CM MY CY CMY K
Artificial Intelligence
These tools will be used for optimising preoperative planning and sizing, to better assess aneurysmal evolution, and to anticipate postEVAR complications.”
Nurea AI solution for fully automated aortic analysis and preoperative planning measurements
Konstantinos Spanos
AORTIC VIENNA











CLASSICAL OPEN AND


CX Aortic Vienna was a revolution in aortic approaches and updated our knowledge in the speciality
All the speakers were excellent. It was a perfect overview, and I was able to look back at the sessions on-demand
It was a great use of my time. Intensive learning!
cxaortic.com
ENDOVASCULAR SOLUTIONS CARDIAC, VASCULAR AND ENDOVASCULAR AORTIC ADVANCES DIGITAL EDITION, LONDON 24–26 OCTOBER 2022 REGISTER NOW
choice.





www.shapemem.com LIT1096 Rev A 0297 TrelliX® Embolic Coil IMPEDE® Embolization Plug IMPEDE-FX Embolization Plug IMPEDE-FX RapidFill® Device 5X © 2022 Shape Memory Medical Inc. All rights reserved. 807 Aldo Avenue, Suite 109, Santa Clara, CA 95054 USA +1.408.649.5175 | info@shapemem.com | www.shapemem.com Indications: The IMPEDE Embolization Plug, the IMPEDE-FX Embolization Plug, and IMPEDE-FX RapidFill are indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature. The TrelliX Embolic Coil System is intended to obstruct or occlude blood flow in vascular abnormalities of the neurovascular and peripheral vessels. Indications include intracranial aneurysms, other neurovascular abnormalities such as arteriovenous malformations and arteriovenous fistulae, and arterial and venous embolizations in the peripheral vasculature. Refer to the IFU – supplied with each device – for a complete statement of the indications, contraindications, warnings, and instructions for use. Inferior Mesenteric (IMA) and Renal Accessory Artery angiographic image courtesy of Alexander Maβmann, MD, Saarland University Medical Center, Homburg/Saar, Germany. The images are illustrative and do not represent the actual size of any products. Patent: www.shapemem.com/patents Shape Memory Medical products and associated components are not available in all countries or regions. Please contact your Shape Memory Medical representative for details regarding product availability. Shape Memory Medical, IMPEDE, IMPEDE-FX RapidFill, and TrelliX are all registered trademarks of Shape Memory Medical. IMPEDE® Embolization Plug GENERATES NEW HEALING POSSIBILITIES CONFORMS TO THE ANATOMY UNPARALLELED VOLUME RETURNS CLARITY Supports thrombus formation throughout porous scaffold Reshaping your view of clinical success through the science of smart polymer Smart
Radiolucent smart polymer IMPEDE-FX Embolization Plug
WARRIORS randomised trial aims to examine early EVAR in women
A multinational collaboration of researchers has received endorsement from the Global Cardiovascular Research Funders Forum (GCRFF) Multinational Clinical Trials Initiative for the WARRIORS (Women’s abdominal aortic aneurysm research: repair immediately or routine surveillance) trial, allowing the investigators to seek priority funding from their partner organisations.

THE TRIAL AIMS TO ANSWER the question, should women have their aneurysms repaired electively using endovascular aneurysm repair (EVAR) at smaller diameters than men to improve their survival and quality of life? Imperial College London (London, UK) is co-ordinating the study that will include collaboration with vascular surgeons from Canada, Denmark, Germany, The Netherlands, Sweden and the USA. The researchers mention that new partners such as Australia may also be joining.
The investigators note in a press release that the need for this trial, which seeks to recruit nearly 1,200 women, stems from the poor outcomes suffered by women with abdominal aortic aneurysm (AAA). Although women contribute 15–20% of total AAA burden, and one-third of ruptures, they have been significantly underrepresented in trials which guide current AAA repair, the investigators detail, adding that women have smaller arteries, a four-fold higher rupture risk and lose eligibility for EVAR at smaller AAA diameters. Treated at the current threshold, the researchers note that a greater proportion receive either higherrisk open surgery or no repair at all. Those that do receive elective repair, do worse, with nearly double the rate of operative mortality (open surgery 6%, EVAR 2.3%), higher postoperative
complication rates and longer hospital stays.
“We have learnt that women worry a lot about their AAA and modelling has suggested that repair of AAA at 4cm for women might result in improved quality of life and reduced overall cost,” the investigators write. “These potential benefits as well as reduction in aneurysm-related mortality, would need to be balanced against the operative risk of early repair.” They state that these areas of uncertainty, regarding the optimal strategy for AAA repair in women, are what the trial seeks to answer.
According to the investigators, WARRIORS would be the first randomised trial of AAA management with multinational, wide-ranging, expertise and to have received endorsement from the GCRFF. However, they stress that this is the just the first step. They elaborate: “To gain funding within each participating country and to implement the trial successfully we will need considerable support from the vascular and multidisciplinary community. The disadvantage of women with AAA can no longer be ignored, and we hope that you
Large study of thoracic aortic aneurysm backs guidelines
A large Kaiser Permanente (Oakland, USA) study provides high-quality evidence that most of the 33,000 patients diagnosed each year in the USA with a thoracic aortic aneurysm are not likely to experience an aortic dissection and may not need open-heart surgery.
“WE BUILT THE LARGEST-EVER COHORT of patients with thoracic aortic aneurysm to study their natural history,” said lead author Matthew D Solomon, a physician researcher at the Kaiser Permanente Division of Research and a Kaiser
will support us, in what will hopefully be a major step towards readdressing the imbalance in AAA outcomes for women and men. We also hope that this initiative will pave the way to obtain evidence about the management of other underserved patient groups, minorities and rarer diseases managed by vascular surgeons.” The researchers note that the trial will be nested in a wider registry of women with small AAA, the registry initiative being led by researchers from Germany and the USA.
Vascular News spoke with Janet Powell (Imperial College London, London, UK), chief investigator of WARRIORS, about what is next for the trial.

What does the GCRFF endorsement mean?
JP: The GCRFF facilitates trials that require international collaboration. You cannot move forward unless you get your trial endorsed by this body, and once it has been endorsed, you get privileged access to funding by their member organisations.
What are the next steps?
We have got about a year of hard graft and paperwork ahead of us before we can hopefully start recruiting patients in early 2024. Having said that, since the press release announcing the GCRFF endorsement went out in early September, there has been a lot of movement, with both Italy and New Zealand showing interest in joining the trial. The interest from New Zealand is of particular significance because, firstly, their healthcare system is quite similar to ours, and secondly, their heart foundation is a member of the GCRFF. That is one of the main reasons we decided to issue a press release—in today’s world, where so much happens beyond the scientific journals and through other avenues instead, such as social media,
Permanente cardiologist. “This research was critical because of the lack of evidence to guide clinicians and the ongoing debate in the field as to how large an aneurysm should be before recommending a patient undergo a very high-risk surgery.”
The study, published in JAMA Cardiology, is the largest to date to support the current consensus guidelines that recommend surgery for most patients with a thoracic aneurysm that is 5.5cm or larger.

These guidelines are specific to patients who do not have certain genetic conditions that increase their risk of experiencing an aortic aneurysm or dissection.
The study included 6,372 Kaiser Permanente patients in Northern California who were identified as having a thoracic aortic aneurysm between 2000 and 2016. Of these, 6,092 (96%) were diagnosed with an aneurysm that was initially less than 5.5cm, and 280 (4%) were initially diagnosed with an aneurysm 5.5cm or larger. All the patients were enrolled in a computerised population management system to ensure they received appropriate, ongoing imaging to assess the size and growth of their
we thought that it would be a great way to get some support behind this project. We are also hoping to garner industry support, and I think that as well as the endograft industry we should think also about involving artificial intelligence (AI) companies.
Why is this trial important?
I think multinational trials such as this are the only way that we can assess the benefits of certain treatments in underserved patient groups. Research questions such as ours are very difficult to answer based on data from a single country in a short space of time, except perhaps if you were to carry out the study in the USA. We simply do not have the populations available. There have long been registries for uncommon vascular diseases, but they tell us only about what happens, rather than provide evidence of how patients should be treated.
To find out more, or be put in touch with your country team, please contact the WARRIORS investigators at Imperial College London—Colin Bicknell, Anna Pouncey and Janet Powell— at warriors@imperial.ac.uk.
aneurysm. None had a genetic syndrome known to increase risk for an aortic aneurysm or dissection.
For patients with an aneurysm less than 5cm, the five-year risk of experiencing an aortic dissection was less than 1%, and for patients with 5–5.4cm aneurysms it was 1.5%.
But for patients with an aneurysm 5.5cm or larger, the story changed: the five-year predicted risk of a dissection for an aneurysm 5.5–5.9cm was 3.6%, and for patients with an aneurysm 6cm or larger, the five-year risk of experiencing a dissection jumped to more than 10%.
“Our study shows that regular monitoring, coupled with aggressive blood pressure control and lifestyle changes, is a safe strategy for most patients until the aneurysm reaches the 5.5cm mark, when surgery becomes necessary,” said Solomon, who is the founder and director of the Kaiser Permanente Center for Thoracic Aortic Disease. “The fact that we found a clear inflection point in risk at 6cm supports the current guidelines and will help inform the debate of when to do surgery.”
11 AORTIC Issue96 | November 2022 Research Updates
Although women contribute 15–20% of total AAA burden, and onethird of ruptures, they have been significantly underrepresented in trials which guide current AAA repair.”
Janet Powell
International collaboration key to proliferation of endovascular thoracoabdominal repair
“We have to work together to pool data that demonstrate equivalence or superiority to open surgical repair,” Gustavo Oderich (University of Texas Health Science Center at Houston, Houston, USA) said of endovascular thoracoabdominal aortic aneurysm (TAAA) repair at this year’s European Society for Vascular Surgery (ESVS) annual meeting (20–23 September, Rome, Italy). He stressed that while the technique is “here to stay,” it is one that will require international, multicentre collaboration in order to be seen as on a par with, or even superior to, the current “gold standard” of open repair.
Oderich delivered these key messages as part of his Nicolai Volodos honorary lecture at ESVS 2022, and Volodos’s pioneering work in the endovascular space formed the backdrop to his presentation. “I got quite philosophical when preparing this talk and wondering about the difficulties that Dr Volodos encountered while doing research under the Soviet Union,” Oderich said. “Volodos has described that he was unable to borrow from other surgeons’ experiences, or receive critical evaluation or comments from others. International conferences, discussions with other physicians, input from engineers—all of these factors that are so important to the creation of the devices that we rely on in the endovascular era—he was not privy to any of this.”
Despite the confines within which Volodos worked, Oderich remarked that he was a “true pioneer” in the endovascular field, carrying out many “firsts”. He noted, for example, that Volodos performed the first thoracic endovascular repair, and also the first hybrid arch repair.
Now, three decades later, Oderich remarked that the vascular community has “embraced” endovascular therapy in many areas of the aorta and for a variety of pathologies, ranging from aneurysms to dissections. “However,” he noted, “there are areas of the world where endovascular repair of TAAAs is still not considered the first line of treatment”.
What do the data say?
Society clinical practice guidelines on aortic disease, which Oderich noted are being updated and will be published later this year, state that open thoracoabdominal aortic repair “remains the gold standard” for low- and intermediate-risk patients, at least in the USA. The reason for this, the presenter believes, comes down to the data that are currently available. The data on endovascular repair mostly come from single centres, or series with relatively short follow-up that are “often plagued with high reintervention rates,” he said.
With this in mind, Oderich put forward the key question: “What is the benchmark that we have to compare to, to be able to say—at some point—that endovascular is truly the first line of therapy?”
The speaker first considered the question of mortality. He noted that, despite many advances in critical care and surgical techniques, the mortality rate “in the best hands” for open surgical thoracoabdominal repair averages about 10% for an extent II thoracoabdominal aneurysm. However, the speaker highlighted that only one third of these patients are actually treated in high-volume centres. The majority are treated in mid- or low-volume centres where the mortality is “substantially higher” than it is in high-volume centres, averaging 20%.
He also pointed out other factors in addition to mortality that must be considered. “These patients
are subjected to a very high rate of life-changing complications, notably respiratory failure, dialysis, still spinal cord injury, and mortality,” he informed ESVS attendees. Since the era of Stanley Crawford, however Oderich did say that there is one area in which there have been “substantial improvements”: paraplegia and spinal cord injury. “Now, with distal perfusion, the rates of paraplegia have gone down from 30% to 5% and 4% in contemporary series,” he detailed.
Durability is one of the frequently cited reasons why many people still believe open surgery should be the first line of therapy, Oderich stated, citing freedom from redo aortic operation rates of 90–95% at five to 10 years in open surgical series. However, he stressed that redo aortic surgery is not the same as secondary intervention. “We should hold open surgery to the same standard as we do for endovascular,” he remarked.
“Secondary intervention implies any secondary procedure that is associated or triggered with the index operation and that includes not only redo aortic surgery but target vessel problems and other non-aortic problems. If we look carefully at open surgical series, the weight of these problems is not low, and in fact many of them carry substantially higher morbidity and risk.”
Oderich also referenced his own, ongoing work in this area. In a series of 500 thoracoabdominal aneurysms, he and his team demonstrated the benefits of endovascular versus open approaches for known aortic procedures. “In the area of aortic interventions,” he summarised, “endovascular does worse, but when we look at freedom from any secondary intervention, in fact, endovascular has done slightly better than open”.
The presenter also mentioned the US Aortic Research Consortium, which he described as “one of the neatest” collaborative efforts in this field of research. “[The project] really allows us to accrue patients in a rapid manner and at some point soon we hope to start doing prospective, randomised studies,” he said, mentioning that enrolment is now up to over 2,700 patients in the USA.
Oderich’s message at this point was hopeful: “We can achieve very low mortality rates [with endovascular repair], in the single digits, despite the fact that we still have a lot to learn from this technique.”
New technologies show “a lot of promise”
The presenter also addressed technological developments in the endovascular field, noting for example the potential of artificial intelligence to detect which patients are going to be at high risk of failure. He referenced some collaborative work that he and his team at the University of Texas are doing with researchers from the University of Stanford (Stanford, USA)—specifically a casecontrolled study involving analysis of cases that failed versus cases with similar anatomy that did not fail by carrying out computational fluid analysis that allows the researchers to scale the haemodynamics, pressure, and flow rates.
New technologies also show potential in addressing the issue of radiation, which Oderich describes as the “bane” of endovascular operators’ existence. He showed a chart demonstrating that, since 2011, there has been a plateau in terms of fluoroscopy time in his cases. Technologies such as Centerline Biomedical’s IntraOperative Positioning System (IOPS) and others show “a lot of promise” in radiation reduction, among other benefits, he informed ESVS attendees.
Collaboration is key
At the end of his lecture, Oderich stressed the importance of creating an environment in which researchers can work together. The International Aortic Research Consortium is an example of such a collaborative effort. “There is a lot of work to do in terms of funding, organisation, and structure, however we have already 2,600 or so cases in multiple centres and a number of projects that were delivered from this collaboration, and I can tell you a lot of these projects have led to change in my practice,” he said.
“Endovascular thoracoabdominal repair is here to stay,” Oderich concluded, noting, however, that there is work to be done. “We have to work together to pool data that demonstrate equivalence or superiority to open surgical repair, and we are not done yet. There is a lot of resistance to that in many parts of the world.”
Beyond collaborative data collection, Oderich stressed that the ultimate goal is to offer both open and endovascular options to all patients—as is the case at the University of Texas Health Science Center at Houston—with the patient involved in all decisionmaking related to their treatment.

12 November 2022 | Issue96ESVS 2022
AORTIC
We have to work together to pool data that demonstrate equivalence or superiority to open surgical repair, and we are not done yet.”
Enrolment in the US Aortic Research Consortium has now reached over patients in the USA
2, 700
Gustavo Oderich
Emergency EVAR protocol should be “first-line intervention” for the right patients
In a recently published study, the introduction of endovascular aneurysm repair (EVAR) into the management of ruptured abdominal aortic aneurysm (AAA) decreased the 30-day mortality in unstable patients. Authors Melissa Jones (Peter Lougheed Centre, Calgary, Canada) and colleagues write that such an emergency EVAR protocol “should be considered the first-line intervention for the appropriate patient”.
IN THE JOURNAL OF VASCULAR Surgery, Jones et al give an overview of the background to their study, underlining a “paucity of literature” to support the mortality benefit of EVAR on ruptured AAA. They also note that an EVAR-first strategy is recommended by the Society of Vascular Surgery (SVS), however this is rated a lowquality recommendation. To address these issues, Jones and colleagues write that an emergency EVAR protocol was introduced at the Peter Lougheed Centre, a tertiary care centre in Calgary, Canada, in 2004.
Furthermore, the investigators claim that there is no published literature on the longstanding outcomes of emergency EVAR protocols on 30-day
mortality after EVAR. The present report, they write, represented 17 years of experience with the management of ruptured AAA incorporating EVAR. In their analysis, the investigators evaluated all adult patients with a ruptured AAA who underwent a surgical or endovascular intervention at the Peter Lougheed Centre between March 2001 and December 2018.
The researchers identified 376 patients with ruptured AAA between 2001 and 2018—75 preprotocol and 301 postprotocol—with a decreasing incidence of ruptured AAA during the study period. They report that the introduction of the protocol in 2004 was associated with increased EVAR use (63.6% vs. 6.7%; p<0.001).
Jones and colleagues reveal that patients managed according to the protocol were more frequently unstable (systolic blood pressure of ≤80mmHg, 46.5% postprotocol vs. 22.7% preprotocol; p<0.001), with a lower average systolic blood pressure (87.4mmHg postprotocol vs. 83.2mL/ min preprotocol; p<0.001). In addition, they state that the risk-adjusted 30-day mortality was 23.2% with the emergency EVAR protocol, versus 35.8% preprotocol (p=0.0727).
Finally, the researchers outline the results of a subgroup analysis, which demonstrated improved 30day mortality for unstable patients (systolic blood pressure of ≤80mmHg) at 38% (vs. 62.4% preprotocol introduction; p=0.019). A cumulative sum, they add, demonstrated worse-than-expected mortality outcomes in the preprotocol period, and stability of surgical performance over 15 years after protocol introduction.
of an emergency EVAR protocol demonstrated stable surgical performance for all patients with a ruptured AAA and evidence of improved 30-day mortality for unstable patients with a ruptured AAA,” Jones et al conclude in JVS. EVAR has become a mainstay intervention since the protocol introduction, they write, adding that the overall incidence of ruptured AAA is declining despite an increase in comorbid patients.
Thirty-day mortality for unstable patients:
Preprotocol:
In the discussion of their findings, the authors consider areas of future research.
RECOMMENDATION
Detailed pre-operative procedural planning is recommended to reduce exposure in endovascular procedures.1
Postprotocol:
“On reflection of a 17year experience with EVAR for ruptured AAA, the implementation
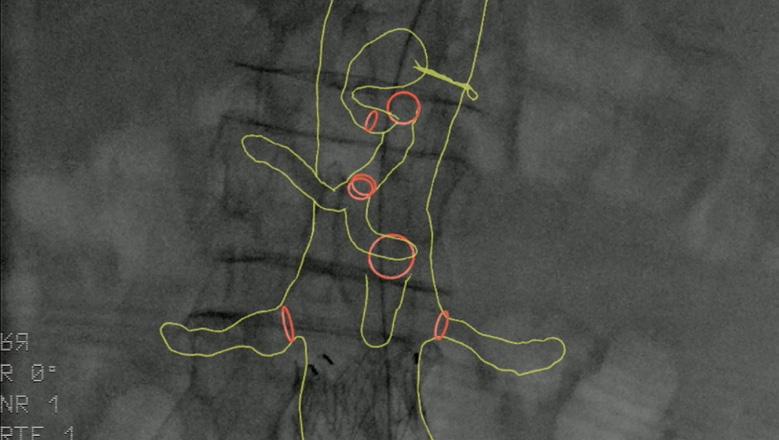
SAFETY GUIDELINES
RECOMMENDATION 18**
Image fusion should be considered during aortic endovascular procedures to reduce radiation exposure.1
“Although this study focused on the impact of the emergency EVAR protocol on preprotocol and postprotocol 30-day ruptured AAA mortality, further investigation into operative details (time to the operating room, procedure length, blood loss, use of intraaortic occlusion balloon), anatomic details (aneurysm size, neck characteristics), and comorbidities (heart disease, pulmonary disease) would help to characterise additional mortality risk factors for patients with a ruptured AAA,” Jones and colleagues suggest.
During pre-operative planning, Cydar EV Maps processes the CT scan (even if poor quality) to accurately segment the anatomy. This, along with our detailed planning and measurement tools, provides you with the right insights and information at the right time.
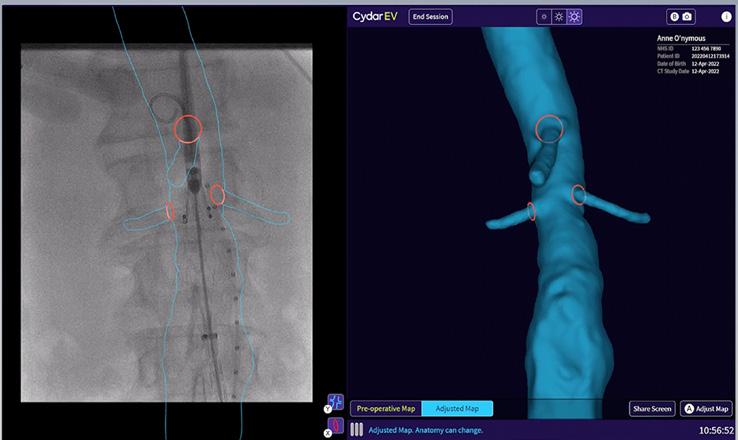
Cydar EV Maps automatically adjusts the 3D overlay when changes occur due to C-arm position, patient movement or posture change, minimizing the need for additional registrations.
RECOMMENDATION 26†
Prolonged use of steep gantry angulation is not recommended during endovascular procedures.1
With Cydar EV Maps, you are able to visualize and navigate vessels, minimizing the need for steep lateral angulation and reducing radiation exposure to clinical teams and patients.
Issue96 | November 2022 13Journal Highlight
Image courtesy of Gabriel C. Inaraja
Perez 2 NEW RADIATION
PUBLISHED BY EUROPEAN SOCIETY OF VASCULAR SURGERY 1
17*
† Class III Level B* Class I Level C ** Class IIa Level B 2797 CLEARED FDAHIPAA & GDPR Compliant. ISO 27001 & ISO 13485 Certification. Cydar EV is a class IIB medical device under EU regulation MDR 2017/745. CYD-AD 4.5_2 SIMPLY POWERING PROGRESS 1. Modarai B et al. ESVS 2022. https://doi.org/10.1016/j.ejvs.2022.09.005. 2. Full case presented September 15, 2022, Vascupedia live webinar.
62.4%
38%


*6F up to 8x17 mm and 7F from 8x27mm up to 10 mm. www.ivascular.global iCover BX ePTFE covered stent The visibility you deserve Smallest profiles 6F introducer compatibility up to 8mm* High flexibility Enhanced visibility Unique stent with radiopaque markers to facilitate the implantation To arrive and treat the most angled arteries Proprietary technology to encapsulate the stent into an inner and outer ePTFE layer TechCover
Six-month data from the Surmodics SWING BTK first-in-human trial presented at AMP Europe
Six-month data from the Surmodics SWING first-in-human (FIH) study of the company’s Sundance sirolimus drug-coated balloon (DCB) were shared at the 2022 Amputation Prevention Symposium Europe (AMP Europe; 9–11 October, Lugano, Switzerland).
THE SWING STUDY IS A PROSPECTIVE, multicentre, single-arm feasibility study to evaluate the safety and performance of the Sundance sirolimus DCB when used to treat occlusive disease of the infrapopliteal arteries.
The study’s primary safety endpoint data showed no perioperative deaths or major amputations at 30 days and just one major reintervention was reported among the 35 trial subjects, a Surmodics press release reports. Data for the primary efficacy endpoint show a late lumen loss (LLL) of 1mm (±0.79mm) across 35 lesions at six months, indicating that the large luminal gain achieved immediately after the procedure was sustained six months post-treatment.
“At six months we observed a consistent improvement in Rutherford category and functional measures, as well as an excellent primary patency of 88.5%, which compares favourably to other DCBs used in the infrapopliteal circulation,” said SWING trial co-lead investigator Ramon Varcoe, vascular surgeon at the Prince of Wales Hospital and associate professor of vascular surgery at the University of New South Wales (Sydney, Australia).
The SWING trial enrolled patients with stenotic or occluded lesions of the infrapopliteal arteries, a reference vessel diameter (RVD) of 2–4mm, and a total lesion length of ≤230mm for treatment with the Sundance sirolimus DCB at eight sites in Australia,
New Zealand, and multiple locations in Europe. They will be followed for 36 months following the index procedure.
“The novel coating on the Sundance sirolimus DCB was evaluated in a challenging, predominantly CLTI [chronic limb-threatening ischaemia] population with a high proportion of diabetes and moderate-severe calcification,” said trial co-lead investigator Andrew Holden, director of interventional radiology at the



University of Auckland (Auckland, New Zealand).
“This first-in-human study demonstrates that the Sundance sirolimus DCB could be a safe and promising treatment for occlusive disease of the infrapopliteal arteries.”
The Sundance sirolimus DCB utilises a “nextgeneration” coating technology consisting of microcrystalline sirolimus and a proprietary excipient to maximise drug transfer, which, according to Surmodics, enhances sirolimus delivery and sustains therapeutic levels in the artery.
In a press release, Surmodics notes that sirolimus is a potent anti-inflammatory and antiproliferative compound, and has been used successfully in coronary drug-eluting stents.

“The delivery of sirolimus to the vessel wall during mechanical dilatation provides an ancillary action of inhibiting the proliferation of cells, with the intended purpose of reducing restenosis,” the company adds. Surmodics advises that the Sundance sirolimus DCB is not available for sale anywhere in the world, and is currently for investigational use only.
Key points:
The SWING trial enrolled patients with stenotic or occluded lesions of the infrapopliteal arteries, a reference vessel diameter of 2–4mm, and a total lesion length of ≤230mm for treatment with the Sundance sirolimus DCB at eight sites in Australia, New Zealand, and multiple locations in Europe
At six months, the investigators observed a consistent improvement in Rutherford category and functional measures, as well as an “excellent” primary patency of 88.5%
New way to track peripheral arterial disease aids quest for better treatments
Cardiovascular experts at UVA Health (Charlottesville, USA) have found a new way to track peripheral arterial disease (PAD). The researchers say the approach will greatly benefit efforts to better understand the condition and to improve treatment options for patients.
THE UVA RESEARCHERS WERE able to use a new magnetic resonance imaging (MRI) technique at the end of exercise to understand the effects of PAD in the calves of patients with the disease and distinguish them from normal volunteers. The approach they used, called chemical exchange saturation transfer, or CEST, produced results comparable to the current gold standard, which does not produce an image. CEST, they found, offered added benefits without requiring highly specialised equipment unavailable to many hospitals and researchers.
“The beauty of CEST is that it creates an image of energy stores in the muscle, which we can match to images of blood flow,” said researcher and imaging expert Christopher Kramer, the chief of UVA Health’s Division
of Cardiovascular Medicine and a professor of cardiology and radiology at the University of Virginia School of Medicine. “This gives us a new understanding of how atherosclerosis in the leg arteries causes problems in the muscles downstream.”
The new diagnostic approach identified at UVA will advance efforts to better understand and treat PAD.
To see if CEST would work for this purpose, the research team conducted a clinical trial comparing CEST with the current gold-standard approach, phosphorus
magnetic resonance spectroscopy. The researchers used CEST to image 35 volunteers with PAD and compared the results with imaging obtained from 29 control subjects after they performed calf exercise in the MRI scanner. They found that CEST was effective at identifying PAD in the lower legs, differentiating patients from normal subjects, and the results compared favourably to phosphorus magnetic resonance spectroscopy.
CEST, they concluded, could offer many advantages for researchers. CEST has better special resolution, creates an image and does not require costly equipment needed for phosphorus magnetic resonance spectroscopy. That means more centres could take advantage of
CEST can also be combined with other magnetic resonance imaging methods that measure blood flow in the calf to better understand the effects of PAD, the researchers note.
CEST with the techniques for measuring muscle blood flow with MRI enables an exciting new approach to studying potential benefits of established and novel therapies in this disease,” Kramer said. Kramer and his collaborators have published their findings in the medical journal Circulation: Cardiovascular Imaging
15Peripheral Arterial Disease
“Combining
Combining CEST with the techniques for measuring muscle blood flow with MRI enables an exciting new approach to studying potential benefits of established and novel therapies in this disease.”
Christopher Kramer
This first-inhuman study demonstrates that the Sundance sirolimus DCB could be a safe and promising treatment for occlusive disease of the infrapopliteal arteries.”
Andrew Holden
Andrew Holden Ramon Varcoe
Dan Addison University of Virginia Communications
SAVAL trial finds no gains with custom drug-eluting stents in PAD below the knee
At the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2022 annual meeting (10–14 September, Barcelona, Spain), Hans van Overhagen (Haga Teaching Hospital, Den Haag, The Netherlands), as European principal investigator, presented the primary results of the SAVAL trial.
COMPARING THE SAVAL DRUG-ELUTING stent (DES) below-the-knee (BTK) vascular stent system (Boston Scientific) and percutaneous transluminal angioplasty (PTA) in the infrapopliteal lesions of chronic limb-threatening ischaemia (CLTI) patients, the trial failed to meet its primary effectiveness and safety endpoints.
The presenter explained that the SAVAL trial is a prospective, multicentre, randomised superiority trial of patients with symptomatic infrapopliteal artery lesions and Rutherford category 4–5 CLTI. The patients were assigned 2:1 to treatment with Saval DES or PTA.
Providing further details of the study, Van Overhagen stated that a total of 201 patients were enrolled and randomly assigned to treatment (n=130 DES, n=71 PTA) at 39 study centres in Belgium, France, The Netherlands, the USA, and Japan.
In terms of primary results, Van Overhagen reported a high technical success rate of 100% in the DES group and 98.7% in the PTA group.
However, “the primary effectiveness endpoint of superior 12-month primary patency was not met,” the
presenter relayed—the figure was 68% in the DES group and 71% in the PTA group. “The primary safety endpoint of non-inferior 12-month major adverse event (MAE)-free rate was [also] not met,” Van Overhagen supplemented, citing the results of 91.6% and 95.3% respectively for the DES and PTA groups, while freedom from all-cause mortality was 92.2% versus 89.9%.
Van Overhagen shared the rationale behind conducting the study: CLTI is a condition with “few effective endovascular options for infrapopliteal artery revascularisation” and DESs have yielded positive results in previous studies. This reflects what Van Overhagen highlighted in an earlier presentation he gave on DESs— both an endovascular approach and revascularisation are key to optimal treatment of CLTI.
In view of the primary results, Van Overhagen told delegates that “patient
Preliminary MOTIV BTK outcomes positive for bioresorbable scaffold use in below-the-knee lesions
Thomas Rand (Klinik Florisdorf, Vienna, Austria) recently presented on the preliminary results to 12 months of the Motiv bioresorbable scaffold (Reva Medical) postmarket trial at the recent Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2022 annual meeting (10–14 September, Barcelona, Spain). With the objective of evaluating safety and efficacy for the treatment of patients with rest pain or minor tissue loss due to the presence of lesions of a maximum length of 100mm at the level of the below-the-knee (BTK) arteries, Rand shared in a FIRST@CIRSE session that at 12 months, primary patency—the efficacy endpoint—was 88%, and the technical success rate, 99%.
THE MOTIV BTK STUDY IS A prospective, single-arm, multicentre trial, in which 58 patients with BTK artery lesions of a maximum length of 100mm have been enrolled. The study is being carried out on 60 limbs with 76 Motiv scaffolds, Rand specified. The Motiv scaffold has been approved for use in Europe since 2018 when, according to Reva Medical, the device became the first bioresorbable scaffold to receive CE mark for use below the knee.
“The special thing with the Motiv scaffold,” Rand opined, “is that it is sirolimus-eluting and bioresorbable,
so you have two good things.” The presenter noted that this is in keeping with the ‘leave nothing behind’ concept. “The scaffold itself is made from TyroCore, which is derived from tyrosine amino acid,” Rand then detailed, and this design is responsible for “low inflammation and irritation during degradation and [no calcification].” The thin struts “have been evaluated as best-in-class,” Rand supplemented.
Moving on to address the preliminary study outcomes, Rand described them as “really very good, [with] a 99% technical success in all patients.”
follow-up will continue through three years in-office with vital status assessment through five years, as specified in the study protocol […] We believe that given the impact of CLTI on limb and life, continued innovation to provide minimally invasive treatments is needed”.
In another session, Van Overhagen presented on the devices currently available and evidence to support the use of DESs specifically.
He began one presentation by underlining that the “cornerstone” of CLTI is revascularisation—”we try to get unobstructed blood flow all the way into the foot and avoid amputation”. The presenter went on to acknowledge that “we all know that endovascular treatment is less invasive. This is why, where possible, we use an endovascular approach”.
The presenter then proceeded to explain the origin of drug-eluting balloons (DEB) and DES: “[they] have been introduced in order to try to reduce restenosis […] we try to diminish the amount of intimal hyperplasia”. The Dutch PADI trial, for which Van Overhagen was principal investigator, compared PTA with bare metal stents (BMS) to DES and, at two-year follow-up, there were almost twice as many major amputations in the former group, a trend which was also observed at five years.
“Our initial conclusions were that DES BTK in CLTI achieved improved patency, a trend towards fewer major amputations [and indicated] a need for dedicated self-expandable and longer stents,” Van Overhagen reported. Further analysis out to ten years has also shown DES BTK to be safe and cost-effective, the presenter concluded.
Primary patency at six months was 90%, and the 12-month preliminary result 88%. The limb salvage rate until now at 12 months, as conveyed by Rand was 97%, and the 14% (n=8) death rate was entirely unrelated to the device or procedure. The freedom from clinically driven target lesion revascularisation (CD-TLR) was “a very nice” 98.3% at 12 months, Rand also underlined, with a “decline in Rutherford stages observed during this time.”
“As a conclusion, already, we can say with the Motiv scaffold we had excellent tracking and visibility and a problem-free delivery.” Rand proceeded to emphasise the scaffold’s ease of use and the 90% vessel patency rate, before sharing that the CD-TLR and limb salvage rates remained stable from six to 12 months.
“The major concept behind this study, which I really like, is the ‘nothing left behind’ concept, and this is really fulfilled in this study,” Rand finished by informing delegates.
Enrolment in MOTIV IDE clinical trial begins
Following this presentation, Reva Medical announced that enrolment in the MOTIV pivotal trial has been initiated at clinical centres in both the USA and Europe. The study, which is being conducted under a US Food and Drug Administration (FDA) investigational device exemption (IDE), will evaluate the use of the Motiv sirolimus-eluting bioresorbable vascular scaffold for treatment in patients suffering from chronic limbthreatening ischaemia (CLTI).
The MOTIV study is a global, randomised controlled trial (RCT) that was designed to evaluate the safety and efficacy of the Motiv scaffold for the treatment of infrapopliteal lesions in patients with CLTI by randomised comparison with standard balloon angioplasty.
The study, led by coprincipal investigators Ehrin Armstrong (Adventist Health, St Helena, USA) and Andrej Schmidt (Universitätsklinikum Leipzig, Leipzig, Germany) will follow up to 292 patients at approximately 35 clinical centres in the USA and Europe.
16 PERIPHERAL November 2022 | Issue96Below-The-Knee Interventions
PERIPHERAL
The primary effectiveness endpoint of superior 12-month primary patency was not met.
68
%
71
%
in the DES group and
The figure was in the PTA group.
The special thing with the Motiv scaffold is that it is sirolimuseluting and bioresorbable, so you have two good things.”
In the USA, atherectomy use in peripheral vascular interventions (PVIs) “more than doubled” from 2010 to 2019, with officebased procedures a “major driver” of this increase. This is according to published US data from the Vascular Quality Initiative (VQI), which also revealed wide regional variability in the use of atherectomy.
According to authors Tonga Nfor (Aurora St Luke’s Medical Center, Milwaukee, USA) and colleagues, small, older studies have indicated that the use of atherectomy devices has become common in PVIs despite what they describe as the “paucity of strong clinical guidelines”.
The study, published in the Journal of Vascular Surgery (JVS), was a retrospective analysis of prospectively collected data from the VQI registry, which the investigators say provides a “unique opportunity” to fill knowledge gaps in this field of research using “real-world” data originating from across the USA. The team identified all patients who had undergone endovascular PVIs for occlusive lower-extremity arterial disease from 2010 to 2019. Procedures in which an atherectomy device had been used as the primary or secondary device were classified as the atherectomy group, Nfor et al write.
In the study period, the authors note that 205,377 PVIs were performed for 152,693 unique patients. They report in JVS is that, during the 10-year study period, 16.6% of the PVIs had used atherectomy, with the data showing that use “significantly increased” from 8.5% in 2010 to 19.7% in 2019.
Geographic variation was evident in the study results, with Nfor and colleagues stating that they found a significant difference in the prevalence of atherectomy use across 17 geographic regions, ranging from 8.2% to 29%. These regions were further split into North, East, South, and West, the authors write, revealing that the observed frequency of atherectomy use in PVIs during the 10-year period was highest in the South at 22.5%, followed by the West (19.9%), North (17.3%), and East (12.6%; p<0.0001).
The 10-year VQI data also clarified predictors of atherectomy use. According to the study authors, the strongest predictor of use was performance of PVI in an office setting (odds ratio [OR], 10.08; 95% confidence interval [CI], 9.17–11.09) or ambulatory centre (OR, 4; 95% CI, 3.65–4.39) versus
a hospital setting.
Furthermore, the geographic trend in atherectomy use followed the distribution of the proportion of office-based PVI procedures—South (6.1%), followed by the West (4%), North (3.9%), and East (0.5%).
In the USA, atherectomy use for peripheral vascular interventions increased from 8.5% in 2010
“To provide patients with the highest quality of care, physicians must allow their decision-making to be guided only by the clinical factors and the best interest of the patient, and should be mindful of any biases that might influence their practice, including the reimbursement structure and financial incentives,” the researchers stress.

Despite the financial incentives that exist, the authors acknowledge that clinical considerations “play an important role in interventional decisions that guide patient care”. For example, they note that patients in the hospital setting will tend to have more acute disease with greater urgency and a greater risk of complications. “It follows naturally that operators might want to avoid long, complicated procedures that will subject patients to more risk, which might partly explain the lower use of atherectomy,” the investigators remark.
There are a number of limitations to the present study which the authors address in their discussion. For example, while the VQI database offers researchers a “large sample of patients from multiple regions,” they acknowledge that a selection bias could still occur because the nature of the database is “dynamic” and different centres were added cumulatively over time.
Despite this and other drawbacks, Nfor and colleagues claim that, to the best of their knowledge, the present study was “the largest to investigate specific clinical and nonclinical predictors of atherectomy use and also to examine the trend and geographic distribution”.
19.7% in 2019 to
They note that the presence of severe (OR, 2.6; 95% CI, 2.4–2.85) or moderate (OR, 1.5; 95% CI, 1.4–1.69) lesion calcification was also predictive of atherectomy use, as well as some other factors including elective status, insurance provider, lesion length, prior PVI, claudication symptoms, and diabetes mellitus.
The researchers also examined the reimbursement aspect of the atherectomy discussion, highlighting differences in compensation levels for hospitalbased versus office-based procedures, and for angioplasty versus angioplasty with atherectomy. For angioplasty performed in a hospital, the physician total relative value units (RVU) was 12.94,
Routine use of atherectomy for infrainguinal PVI called into question
In a commentary on Nfor and colleagues’ report, Caitlin Hicks (Johns Hopkins University School of Medicine, Baltimore, USA) underscores the “overwhelming message” of the analysis: that atherectomy is being overused in the USA.
“This is not an attack on physicians who work in OBLs,” Hicks stresses at the outset of her commentary—titled ‘Atherectomy overuse is a real problem’—but rather a “statement of the facts,” of which she assembles four from this latest addition to the available research:
Atherectomy use for infrainguinal PVI has been “increasing rapidly” in the USA
No high-quality evidence that atherectomy improves outcomes compared with alternative endovascular therapies is available
Atherectomy is much more frequently used in OBL settings than in hospital-based settings
averaging about US$466, they report. For the same procedure performed in an office-based laboratory [OBL], the total RVU was 100.68 (procedure total), translating to US$3,628. Nfor et al further reveal that, when angioplasty was performed with atherectomy in a hospital, the physician RVU was 17.61, averaging US$635. However, when atherectomy with angioplasty was performed in an OBL, the reimbursement increased to 345 RVU— approximately US$12,444. The authors, however, point out that these total RVU reimbursement figures for office and ambulatory centre procedures includes the technical and facility fees.
Reimbursement for atherectomy procedures is substantially higher than that for stenting and balloon angioplasty in the outpatient setting
“Based on these facts, the routine use of atherectomy for infrainguinal PVI is suspicious at best,” Hicks remarks. She acknowledges that cases exist for which atherectomy has proven beneficial—calcific disease, popliteal lesions that cannot or should not be stented, and recalcitrant lesions, for example. However, Hicks also notes that the data have shown the use of atherectomy in up to 100% of cases by some physicians—a fact that is “not right” in her view.
Hicks concludes: “As a field, vascular surgeons need to come together and unite on this issue. If we do not police ourselves regarding the appropriate use of high-cost technologies, the Centers for Medicare and Medicaid will most certainly do it for us.”
17Issue96 | November 2022
Atherectomy
“Significant” increase in atherectomy use in the USA largely driven by officebased procedures, VQI data show
The routine use of atherectomy for infrainguinal PVI is suspicious at best.”
Caitlin Hicks
Three-year data bolster use of the BeGraft as a bridging stent in FEVAR
Stéphan Haulon (Hôpital Marie Lannelongue, GHPSJ, Paris, France) has extensive clinical experience using the BeGraft (Bentley, Hechingen, Germany) as a bridging stent in fenestrated endovascular aneurysm repair (FEVAR) procedures. He has conducted a pioneering study on the topic, with the latest published data pointing to excellent patency out to three years. In this interview, Haulon speaks to Vascular News about how the BeGraft has changed his approach to certain fenestrated procedures, outlines the key features of a good bridging stent and underscores how his dataset and others pave the way for an on-label indication for the BeGraft as a bridging stent in FEVAR procedures.

In what ways has the BeGraft as a bridging stent changed how you implant a fenestrated graft?
I think the BeGraft has changed our workflow and the ancillary tools that we are using. We have almost exclusively changed to using 6Fr catheters to get into the target vessel, whereas previously we were using 7Fr sheaths routinely. The access you need from the iliacs is smaller with a 6Fr catheter, especially when using a pre-loaded system. If you use a pre-loaded renal fenestrated endograft you can access both renal arteries from the side of the endograft delivery system, and then on the other side you only need to access the two visceral vessels. In cases such as this we can use a 14Fr sheath where we might once have used a 20Fr or larger sheath. It is risky to use large sheaths in the iliacs because they can cause rupture, and so if we can avoid using them that makes the procedure safer.
What are the main features of a good bridging stent?
I think you need to combine a good endograft design with a good bridging stent in order to achieve positive outcomes. In my experience, using endografts with the fenestration positioned at the origin of the target vessel—meaning that there is no gap between the origin of target vessel and the fenestration—provides a very stable platform for BeGraft as a bridging stent. In addition, the bridging stent needs to be 6Fr compatible so that you can easily and safely position it in the target vessel. It also needs to be flexible so that it can adapt to the various forces that are exerted on the target vessel. In the renal artery, for example, the first 20mm moves with the aorta and then the distal portion moves with respiratory motion, and so a flexible stent is really important here if you want to avoid issues during follow-up. Furthermore, it is necessary to have various diameters and lengths available so that you can adapt your approach to the patient anatomy and safely perform the procedure. With the BeGraft you have a lot of options available in this regard.
How does the BeGraft compare to other bridging stents on the market?
I think the BeGraft is very flexible, and the 200 microns thickness of the ePTFE provides a secure coverage of the cobalt-chromium stent. It is always difficult to compare stents, however, in my experience, the BeGraft is perfectly suited for use as a bridging stent in FEVAR and we now have strong data to support this.
You conducted a study with the BeGraft as a bridging stent in FEVAR. What were the endpoints?

We were looking primarily at the patency of fenestrated endografts for short necks or juxtarenal aneurysms. In addition, we were looking for things like type III endoleaks and stent fractures.
Three-year data from this study have been published. Could you briefly summarise the main outcomes at this time point?
The patency was excellent and there were only two secondary procedures. I think these outcomes are a result of good patient selection, sizing and graft design. If you design the appropriate fenestrated endograft, then the BeGraft will provide a perfect platform.
Furthermore, I think we had such a low number of technical issues because we perform a cone-beam computed tomography (CT) scan at the end of each procedure, and I think this should be routine. If you capture an issue at the end of the procedure with your
vessels during respiratory motion. All patients enrolled in that study underwent a pre-operative CT scan in inspiratory and expiratory motion, and then we did the same scan postoperatively with the endograft and the BeGraft in all target vessels and performed again inspiratory and expiratory acquisition of the CT scan. We looked at how the bridging stent modified the way those visceral vessels behaved and it was very interesting to see that there was only a minimal impact. We were actually not changing the way those target vessels behave.
Bentley is working on an on-label indication for the BeGraft in FEVAR. Why now?
While it takes a lot of effort and a lot of financial commitment to get on-label approval, I think Bentley understood that it was time to undertake such a project. Moreover, physicians have been pushing for a dedicated bridging stent for a long time and working hard on compiling the data to support an on-label indication. In Europe, I think that very soon the BeGraft Peripheral will have an indication for FEVAR and the BeGraft Plus will soon have an indication for branched endovascular repair (BEVAR). To date we have all had to use the bridging stents in an off-label setting for FEVAR procedures, and so I and other physicians are very pleased that an on-label indication is on its way.
cone-beam CT, you can fix it right away, and then you do not need a secondary procedure.
Will there be further follow-up of these patients?
All our patients are in a prospective cohort, and so we continue to collect data on these patients. We have been using the BeGraft as our primary bridging stent for around seven years now, and we have more than 500 fenestrated cases in our database. There is also a dedicated prospective study currently running in Germany to evaluate the BeGraft as a bridging stent for fenestrated endografts. It is great that data are coming from different centres to consolidate our findings.
You also performed a study on the movements of stents once they have been implanted. Could you outline the key findings from this study?
We know, and as mentioned in your previous question, that there is some movement in all the target
18 Advertorial November 2022 | Issue96 THIS ADVERTORIAL IS SPONSORED BY BENTLEY
We have been using the BeGraft as our primary bridging stent for around seven years now, and we have more than 500 fenestrated cases in our database.”
Case images: Selective angiograms confirming patency and sealing after implantation of a BeGraft into the left renal artery during FEVAR and into the coeliac trunk during BEVAR
Stéphan Haulon
BeGraft Plus
LIKE
www.bentley.global SIMPLY RELIABLE JUST
OUR DELIVERY
Markus Steinbauer
Despite his initial intention to become a transplant surgeon, Markus Steinbauer (Krankenhaus Barmherzige Brüder, Regensburg, Germany) recalls how the rise of endovascular techniques, and the possibilities for procedural advancement they posed in vascular surgery, led him to change career course. Steinbauer, now head of the Department of Surgery at the Krankenhaus Barmherzige Brüder and president of the German Society of Vascular Surgery (DGG), considers how the field of vascular surgery has changed over the course of his career, anticipates future challenges vascular surgeons might face—including those associated with an ageing population—and expresses his interest in seeing further developments in artificial intelligence and imaging to improve endovascular treatment modalities and reduce radiation.

Why did you decide to pursue a career in medicine and why, in particular, did you choose to specialise in vascular surgery?
I was attracted to medicine as a career by its diversity as a scientific discipline and the opportunities to interact and communicate with patients and colleagues in a trustworthy and special relationship.
Originally, I aimed to become a transplant surgeon. However, during my training in general/transplant surgery at the end of the last millennium, I was deeply impressed by and attracted to the rapid and unbelievable development of endovascular surgery and the tremendous potential advances it posed in the future of vascular surgery. For this reason, I decided to specialise in vascular surgery and I have never regretted this decision, not even during complex and long-lasting nocturnal procedures.
Who were your career mentors and what was the best advice that they gave you?
My first scientific mentor was Konrad Messmer, who is one of the leading scientists in microcirulatory research. He taught me that science is pivotal for the further development of medical disciplines, and also that precise and structured planning, in combination with intense collaboration, are essential elements for the successful realisation of a project.
Piotr Kasprzak was another mentor of mine. He shared his devotion and fascination for vascular and endovascular surgery and commitment to constant technical development according to the motto ‘standstill is regression’.
What has been the most important development in vascular surgery during your career so far?
The most important development has been the endovascular ‘revolution’, combining new concepts of imaging with new endovascular techniques and devices. This has allowed us to treat complex lesions in vulnerable patients. To learn and perform the first aortic interventions in abdominal aortic aneurysm (AAA) patients with basic C-arms and to apply the development of these techniques to complex procedures from the aortic arch to the iliacs has been one of the most impressive evolutions for me in my career in vascular surgery.
Another important development has been the implementation of various techniques and devices in the endovascular treatment of peripheral occlusive disease (POD). These changes and developments were only made possible by the implementation of the Hybrid operating room (OR) concept and the dramatic improvement of imaging in our specialty.
What has been the biggest disappointment?
As a young scientist working on microcirculation and angiogenesis, I placed great hope in the use of angiogenetic factors in no-option POD patients. Inducing a relevant collateralisation by using angiogenetic factors by injection or gene therapy would have been a striking therapeutic concept. Unfortunately, the promising preclinical results were not proven in relevant clinical trials.
How do you anticipate the field might change in the next decade, and what development would you most like to see realised?
As is the case in oncology, I hope that an individual diagnosis of specific molecular markers and targets in the vessel wall may help to personalise a specific prophylaxis and treatment of atherosclerosis.
Furthermore, I would like to see further development in artificial intelligence and imaging, for example by mixed virtual reality to improve endovascular treatment modalities and to reduce radiation. The technical prerequisites are already available; we now have to adapt these techniques and use them for the benefit of our patients.
What are the biggest challenges currently facing vascular surgery?
The biggest challenges facing vascular surgery are population ageing and, perhaps more importantly, the dramatic negative changes in nutrition and exercise/ sports behaviour in western societies. To recruit and educate/train young vascular surgeons to handle the expected tremendous workload of vascular patients will be a demanding but valuable challenge.
What are your current areas of research?
At present I am focused on four main areas of research. These are vascular graft infections, open surgery in chronic limb-threatening ischaemia (CLTI) and POD, imaging, and endovascular aortic treatment.
What do you think has been the most important paper published this year?
The paper with highest relevance for our clinical work in the future might be ‘Long-term cardiovascular outcomes of COVID-19’ by Yan Xie et al. This was published in February in Nature Medicine. In the paper, the authors describe an increase in the long-term risk and burden of cardiovascular diseases in COVID-19 patients. We have to elucidate the underlying mechanisms for these late vascular complications and find new solutions for prevention.
However, I think the paper that interested me the most this year was was ‘Neuroimmune cardiovascular interfaces control atherosclerosis,’ by Sarajo Mohanta et al, published this April in Nature. The authors describe a new mechanism of the development and progression of atherosclerosis—a direct interaction of the peripheral nervous system with diseased arteries by neuroimmune cardiovascular interfaces (NICIs). This might be highly relevant for the POD and diabetic foot patients in the future and at might give us the
20 PROFILE November 2022 | Issue96
Interview
alisonlang.com
I would like to see further development in artificial intelligence and imaging, for example by ‘mixed virtual reality’ to improve endovascular treatment modalities and to reduce radiation.”
opportunity to develop therapeutic concepts including new surgical approaches.
What have been the highlights of your German Society of Vascular Surgery presidency?

My personal highlight was the organisation of the very successful and happy annual meeting (Jahrestagung) of the German Society of Vascular Surgery in October 2021 under COVID-19 conditions—the first hybrid Jahrestagung of the DGG. The revival of an onsite congress with cordial meetings and discussions with friends was the best event of my presidency. Furthermore, I am looking forward to the Dreiländertagung 2022 with our Austrian and Swiss friends in Vienna, Austria. Finally, I deeply enjoyed the multifaceted and trustworthy conversations/meetings with our members and colleagues and the tremendous input and devotion they gave to the development of our society.
What have been your most memorable cases?
A very memorable case was my first standalone surgical handling of an aortoenteric fistula in a haemodynamically unstable patient with graft infection as a young vascular surgeon. I learned that an uncompromising surgery and therapeutic endurance are the key factors for the control of these disastrous complications in vascular surgery.
Later on, as a newly elected head of the Department of Vascular Surgery at the Krankenhaus Barmherzige Brüder, the successful treatment of a ruptured thoracoabdominal aortic aneurysm (TAAA) in a young female patient with Marfan syndrome with open surgery gave my colleagues and me the confidence that our team and infrastructure were ready for further challenges.
What advice would you give to someone looking to start a career in medicine?
I have four pieces of advice for anyone looking to pursue a medical career. First of all, medicine/surgery is a dedication and not a job. Secondly, it is imperative that you serve the patient and not the hospital manager. I also advise the younger generation to choose vascular surgery as their specialty, as I strongly believe it is the best of all specialties. And, finally, keep calm and carry on!
What are your hobbies and interests outside of medicine?
One of my main hobbies is golf as, like vascular surgery, it teaches you humility and modesty. Another is skiing—both downhill and cross country skating— and I am the former coach for ski instructors of the German Ski Association. My interests are history, wine, and travelling with my family and friends.
Clinical experience
2007–present: Head of the Department of Vascular Surgery and head of the Vascular Centre, Krankenhaus Barmherzige Brüder, Regensburg, Germany
2010–2014: Chairman, Krankenhaus Barmherzige Brüder
2003–2007: Senior physician at the Department of Vascular and Endovascular Surgery, University of Regensburg, Germany
1995–2003: Research fellow
at the Department of Surgery, University of Regensburg
1994–1995: Research fellow at the Institute for Surgical Research, University of Munich, Munich, Germany
Education
1992–1994: Ludwig Maximilian University of Munich, Munich, Germany
1991: University of Vienna, Vienna, Austria
1987–1990: Ludwig Maximilian University of Munich
Society memberships (selected)
German Society of Vascular Surgery
European Society for Vascular Surgery
Germany Society of Phlebology
German Society of Surgery
Section Surgical Research of the German Society of Surgery
Society presidencies
2021–2022: German Society of Vascular Surgery
2020: Society of Bavarian Surgeons
21Issue96 | November 2022
Interview
RESCUE trial of the Bashir endovascular catheter demonstrates reduction in pulmonary artery obstruction in patients with acute PE
Thrombolex presented the final results of its National Institutes of Health (NIH)sponsored RESCUE trial at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT; 16–19 September, Boston, USA).
This investigational device exemption (IDE) trial demonstrated that pharmacomechanical catheter-directed thrombolysis therapy using the Bashir endovascular catheter (Thrombolex) led to a significant improvement in right ventricular function with an excellent safety profile. The magnitude of reduction in pulmonary artery obstruction in the RESCUE trial was markedly greater than what has been reported in other contemporary pulmonary embolism (PE) trials, a press release reports.
The RESCUE trial is a prospective, multicentre trial evaluating the Bashir catheter in 109 patients with intermediate risk acute PE at 18 sites in the USA. The Bashir catheter was used to deliver 7mg of recombinant tissue plasminogen activator (r-tPA) into each pulmonary artery over a five-hour infusion period. The primary efficacy endpoint was the
core lab-assessed change in the computed tomography angiography (CTA)-derived mean right ventricular to left ventricular (RV/LV) diameter ratio at 48 hours, and the primary safety endpoint was serious adverse events, including major bleeding at 72 hours.
The median device placement time was 15 minutes and length of hospital stay was 2.8 days. At 48 hours after delivering r-tPA, the RV/LV ratio decreased from baseline by 0.56, a reduction of
33.3% (p<0.0001). A key secondary efficacy endpoint was the core lab assessed reduction in pulmonary artery obstruction as measured by the refined Modified Miller Index (MMI), which demonstrated a reduction of 35.9% (p<0.0001). Compared to other contemporary core lab-assessed CDT trials, this reduction in pulmonary artery obstruction was more than two-fold greater with the Bashir catheter, per mg of r-tPA administered.
“The RESCUE trial demonstrated extremely rapid resolution of thrombus and a remarkable reduction in PA obstruction, with a less than a 1% rate of major bleeding. This represents a major advance in the treatment of acute PE,” said Kenneth Rosenfield (Massachusetts General Hospital, Boston, USA), co-principal investigator of the RESCUE study.


“We are absolutely delighted with the results of the RESCUE trial and thank all those who made this possible, especially our physician investigators and their patients,” said Marvin Woodall, executive chairman of Thrombolex.
FLASH results demonstrate “excellent safety profile” of the FlowTriever system in full US cohort
Results of the FLASH registry demonstrate the “excellent safety profile” of the FlowTriever system (Inari Medical) in 800 “real-world” patients. This is according to Catalin Toma (University of Pittsburgh Medical Center, Pittsburgh, USA), who presented outcomes for the full US cohort of FLASH at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT; 16–19 September, Boston, USA). The results were simultaneously published in EuroIntervention
TOMA REPORTED A 1.4% MAJOR bleeding rate and 0.4% rate of other major adverse events (MAEs) in what the presenter described as the largest prospective interventional study in pulmonary embolism (PE). All-cause mortality was <1% at the 30-day visit in this “real-world” population with prevalent baseline predictors of mortality, the presenter added, noting also that haemodynamics improved ontable, acute vitals and right ventricular (RV) strain normalised, and clinical recovery continued through six months.
The presenter noted that acute all-cause mortality following PE has remained high (>10%) over two decades, adding that, following PE, the 30-day readmission rate is nearly 25%, and up to half of patients report persistent dyspnoea,
exercise intolerance, and/or functional limitations at three to six months.
According to Toma, guidelines suggest considering advanced therapies, including mechanical thrombectomy, when thrombolytics fail or are contraindicated, or haemodynamic deterioration occurs. However, he said, major bleeding risks with thrombolyticbased treatment strategies remain a concern, and their use is limited by corresponding contraindications and consequent intensive care unit (ICU) monitoring.
Mechanical thrombectomy can rapidly extract thrombus and relieve RV strain without thrombolytics, the presenter said, stating that “additional data on the risk-to-
benefit ratio could shift the standard of care”. One example of a mechanical thrombectomy device is FlowTriever, he highlighted, noting that this system is 510(k)-cleared by the US Food and Drug Administration (FDA) and CE mark-approved for the treatment of PE.
FLASH is a prospective, multicentre, single-arm registry designed to evaluate the safety and effectiveness of the FlowTriever system for treatment of PE through six months, with the decision to intervene being at the discretion of treating physicians or local PE response team (PERT). The primary endpoint is an MAE composite endpoint of devicerelated mortality within 48 hours, major bleeding within 48 hours, and intraprocedural device- or procedurerelated adverse events.
At TCT, Toma reported a primary MAE rate of 1.8% (14/788), which combined a 0% rate of device-related deaths (0/788), 1.4% rate of major bleeding (11/788), and 0.4% rate of intraprocedural adverse events (3/788). Regarding all-cuase mortality, he noted rates of 0.3% at the 48-hour visit (2/794), 0.8% at the 30day visit (6/734), and 5% rate at the six-month visit (28/588). Importantly, the procedure led to significant improvement in invasive haemodynamics with decreased pulmonary
pressures and improved cardiac output. Finally, Toma revealed that the rate of all-cause readmissions at the 30-day visit was 6.2%, with 1.4% related to PE treatment (10/711) and 4.8% unrelated to PE treatment (34/714).
Looking ahead, Toma highlighted that “data from the ongoing PEERLESS randomised controlled trial will provide important evidence regarding the safety and effectiveness of FlowTriever mechanical thrombectomy compared with catheter-directed thrombolysis”.
22 VENOUS November 2022 | Issue96Pulmonary Embolism
Bashir endovascular catheter
Kenneth Rosenfield
Data from the ongoing PEERLESS randomised controlled trial will provide important evidence regarding the safety and effectiveness of FlowTriever mechanical thrombectomy compared with catheter-directed thrombolysis.”
This represents a major advance in acute PE treatment.”
Catalin Toma
New mechanical thrombectomy systems unlock possibilities for the treatment of venous thromboembolism

Mechanical thrombectomy with the ClotTriever and FlowTriever systems (Inari Medical) opens up new possibilities for the management of deep vein thrombosis (DVT) and pulmonary embolism (PE), widening the treatment window and eliminating the side-effects associated with lysis. This is according to Bernhard Gebauer (Charité – University Medicine Berlin, Berlin, Germany) and Gerd Grözinger (University of Tübingen, Tübingen, Germany), who discussed developments in the diagnosis and treatment of venous thromboembolism at this year’s Cardiovascular and Interventional Radiological Society of European (CIRSE) annual meeting (10–14 September, Barcelona, Spain). In the discussion, both interventionalists focused on the benefits of mechanical thrombectomy, in particular with the ClotTriever and FlowTriever systems, and how these technologies have opened up new possibilities in their treatment algorithms.
AT THE OUTSET OF THE DISCUSSION, both physicians gave an overview of general trends in the treatment of DVT and PE. Grözinger was keen to get Gebauer’s take on why the prognosis for PE “has not significantly improved” in recent years. Gebauer, who is head of the Interventional Radiology Department at Charité, stressed that this is a “severe” disease, with most patients experiencing impaired heart function. The main reason why prognosis has not improved, he believes, is because the treatment options on anticoagulation or lysis are “very limited”. Gebauer explained that often thrombus is old and organised, and when this is the case anticoagulation or lysis are not effective. “I think that removing the thrombus and getting away from the underlying disease is probably helping us to improve treatment for these patients in the future,” he remarked, pointing to the benefits of mechanical thrombectomy in this patient population.
Gebauer also commented on the option of systemic thrombolysis. He noted that, while this method is now recommended in the guidelines for some patient groups, the high dose of medicine needed for the technique to work could result in complications, including risk of bleeding. He also pointed out that the technique is not a suitable option for many patients. “A lot of patients we treat have had trauma
or recent surgery, and so giving them thrombolysis is contraindicated,” he noted. In addition, he mentioned that physicians will sometimes experience the “very serious event” of severe cerebral bleedings from thrombolysis in these patients. Grözinger, who is deputy medical director at the University of Tübingen, added that treatment with thrombolysis might also be suboptimal for old and organised thrombus. Mechanical thrombectomy might be a solution here, Gebauer suggested.
This technology also shows potential in DVT, the experts pointed out when discussing the perfect time window for treating this condition. The ClotTriever has the potential to overcome the “strict timeframe” of two weeks for treatment that used to exist, Grözinger
said, referencing its ability to deal with thrombus of various ages. “The time window has extended tremendously.”
The experts considered which patients benefit the most from interventional treatment for either a DVT or PE, especially with regard to long-term outcomes. Gebauer expressed his opinion that patients with older thrombus in whom guideline-recommended therapies give suboptimal results are the patients who benefit the most from these new therapies. “The direct removal of thrombus leads to improvement in these patients,” he noted.
For pulmonary embolism, which Gebauer stressed is “more of an emergency”, he believes that intermediate high-risk (IHR) patients benefit the most from intervention. “High-risk patients have a very high mortality rate, and in these patients, you can also improve the mortality, however they would still have a high mortality rate because of their underlying disease and impaired cardiac function,” he explained.
Grözinger added that there is another set of patients in whom he would always recommend intervention: “If we see the pelvic veins are involved, we would rather go straight to interventional treatment,” he said, “especially with these new devices where you can more or less eliminate bleeding risks and other side-effects without lysis”. He summarised that the approach at his institution centres on a “strong belief” in lysis-free thrombectomy.
Despite it having been established that patients with older thrombus benefit more from mechanical thrombectomy than from conservative treatments, Gebauer underlined the fact that patient selection is not simple. “It is not easy to identify the correct patients because very often you do not know the thrombus age itself,” he said. He stated that imaging can provide some “hints” as to the age of the thrombus, and that sometimes onset of patient symptoms can also help in identifying the right patients for treatment.
According to Grözinger, magnetic resonance (MR) venography is more reliable than onset of symptoms and patient history in determining thrombus age in DVT. He noted the example of an MR venography showing organised thrombus and vessel wall scars despite the patient saying that symptoms began three to five days earlier. “In cases such as this, there is a fresh part of the thrombus, but the underlying disease is often much older,” he explained. This is where these new treatment options unlock the possibilities for the treatment of venous thromboembolism.
Indications for use: The ClotTriever® Thrombectomy System is indicated for (1) the non-surgical removal of thrombi and emboli from blood vessels, and (2) injection, infusion and/or aspiration of contrast media and other fluids into or from a blood vessel. The ClotTriever thrombectomy system is intended for use in the peripheral vasculature including deep vein thrombosis (DVT).
Indications, Contraindications, warnings and instructions for use can be found in the product labeling supplied with each device.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
All trademarks are property of their respective owners.
The FlowTriever® Retrieval/Aspiration System is indicated for (1) the non-surgical removal of emboli and thrombi from blood vessels; and (2) injection, infusion and/or aspiration of contrast media and other fluids into or from a blood vessel. The FlowTriever system is intended for use in the peripheral vasculature and for the treatment of pulmonary embolism.
Indications, Contraindications, warnings and instructions for use can be found in the product labeling supplied with each device.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
All trademarks are property of their respective owners.
Inari Medical, Inc. headquarters: 6001 Oak Canyon, Suite 100 | Irvine CA 92618
Inari Medical Europe GmbH, a subsidiary of Inari Medical, Inc. | Messeplatz 10 | 4058 Basel, Switzerland
Issue96 | November 2022
I think that removing the thrombus and getting away from the underlying disease is probably helping us to improve treatment for these patients in the future.” Bernhard Gebauer
The time window [for deep vein thrombosis treatment] has extended tremendously.”
— Gerd Grözinger
ClotTriever device
THIS
ADVERTORIAL IS SPONSORED BY INARI MEDICAL
VENOUS
Two recently published studies reveal new data about oedema reduction, patient satisfaction, and quality of life with the Dayspring active compression therapy system (Koya Medical).
THE FIRST STUDY, PUBLISHED IN THE Journal of Vascular Surgery: Venous and Lymphatic Disorders (JVS-VL), is the multicentre, randomised, crossover head-to-head trial (NILE). In their JVSVL report, Stanley Rockson (Stanford University School of Medicine, Stanford, USA) and colleagues evaluated the safety and efficacy of the Dayspring device for treating lymphoedema versus an advanced pneumatic compression device (APCD) in 50 women with unilateral breast cancer-related lymphoedema.
The investigators found that the Dayspring device was more effective than an APCD, reporting significantly greater adherence (95.6% vs. 49.8%) and greater satisfaction (90% vs. 14%) with the Dayspring device versus an ACPD.
Furthermore, Rockson et al found that participants experienced mean improvements on the lymphoedema quality of life questionnaire (LYMQOL) and significantly greater mean reduction in oedema volume (64.6% vs. 27.7%; 0<0.05) with the Dayspring device versus an ACPD.
“Lymphoedema is an incurable condition that requires lifelong maintenance, often forcing people to be tied to their compression devices for hours at a time. However, with the mobility-enabled Dayspring system, I am seeing not only a meaningful reduction in patients’ oedema, but also a significant improvement in their satisfaction and overall quality

ABRE clinical study 36-month data show sustained effectiveness of Abre venous self-expanding stent system
Medtronic has announced the 36-month final results from the ABRE clinical study. The purpose of the ABRE clinical study was to evaluate the safety and effectiveness of the company’s Abre venous self-expanding stent system, intended for the treatment of symptomatic iliofemoral venous outflow obstruction.
of life,” said Rockson, who is a cardiologist and professor in the Falk Cardiovascular Research Center at Stanford University School of Medicine and principal investigator of the NILE clinical study. “These results highlight the significant benefits the Dayspring system provides in the way we can manage and treat lymphoedema and venous disease.”
The second study is an open-label investigation of the Dayspring system for the treatment of lymphoedema and venous diseases in the lower extremities, which was recently published in Scientific Reports, Nature. More specifically, this is a multicentre, open-label, 12-week clinical study of 24 patients with primary or secondary unilateral lower extremity lymphoedema that evaluated changes in limb oedema and quality of life following treatment with the Dayspring device.
In this paper, also authored by Rockson and colleagues, the investigators report that patients experienced mean improvements in LYMQOL compared with baseline (p<0.05) after three months of use. Furthermore, they reveal that limb volume improved (up to 50% reduction in oedema) with Dayspring use, with an average reduction in affected limb oedema of 39.4%.
The results of these studies were also recently presented at the American Vein and Lymphatic Society (AVLS) 2022 annual congress (13–16
THE STUDY RESULTS WERE presented in a late-breaking clinical trial session at the American Vein and Lymphatic Society (AVLS) 2022 annual meeting (13–16 October, New Orleans, USA). Stephen Black (Guy’s and St Thomas’ Hospital, London, UK), co-principal investigator for the ABRE study, presented the data.
“The 36-month ABRE data have continued to demonstrate the longterm durability of interventions in patients suffering from deep venous disease,” Black said. “The results show a sustained result in both technical aspects, but more importantly, in patient outcomes.”
The ABRE study included a complex set of patients. Within this patient group, 47.5% of patients were categorised as having post-thrombotic syndrome (PTS) and 35.8% of PTS patients presented with a complete venous occlusion confirmed by the core lab. The mean lesion length was 112.4mm and 44% of patients had stents that extended below the inguinal ligament.
Of note, the study results showed:
October, New Orleans, USA).
“These key data publications further expand our body of clinical evidence supporting the use of the Dayspring active compression system for people living with lymphoedema and chronic venous conditions,” said Thomas Maldonado, medical director of the Venous Thromboembolic Center at New York University Langone Health (New York, USA). “We look forward to continuing to advance evidence supporting Dayspring and helping even more people with lymphoedema and venous diseases maintain mobility while continuing to experience effective compression treatment.”
In addition to these two publications, Koya Medical recently announced that the Centers for Medicare & Medicaid Services (CMS) has confirmed the durable medical equipment (DME) benefit category determination, payment schedule and five final Level II Healthcare Common Procedure Coding System (HCPCS) codes for the Dayspring active compress system in its final biannual Medicare benefit decisions update.
According to Koya Medical, the Dayspring system is the first non-pneumatic active dynamic compression treatment designed for patient mobility and cleared by the US Food and Drug Administration (FDA) to treat lymphoedema and chronic venous conditions.
Overall, effectiveness following treatment with the Abre venous stent was sustained through 36 months as evidenced by a KaplanMeier estimated primary patency rate of 81.6% and a Kaplan-Meier estimated freedom from clinically driven target lesion revascularisation (CD-TLR) rate of 89.3%.

No stent fractures or delayed stent migrations were reported through 36 months.
Sustained and clinically meaningful improvements were observed through 36 months compared to baseline as measured by EQ-5D and VEINES-QoL quality of life.
Sustained and clinically meaningful
improvements through 36 months as measured by Villalta and VCSS venous functional assessments indicates less severity of PTS disease and venous disease overall.
“Medtronic is strongly committed to the deep venous market. As the 36-month results show, the Abre venous stent system safely and effectively treats venous disease,” said Dave Moeller, president of the Peripheral Vascular Health Operating Unit at Medtronic. “This study included many patients with highly challenging cases, showing that Abre is meaningfully able to improve patients’ quality of life—including those with severe disease—for a long period.”
24 November 2022 | Issue96
New Data
Data point to benefits of non-pneumatic active compression for the treatment of lymphoedema and venous disease
Credit: Business Wire
Dayspring active compression therapy system
Abre venous stent
Experts establish international standard set of outcome measures for patients with VTE
The International Consortium for Health Outcomes Measurement (ICHOM) venous thromboembolism (VTE) working group has developed a standard set of outcome measures for patients with VTE. The consensus recommendation—published in the September edition of The Lancet Haematology—is designed to “facilitate the implementation of the use of patient-centred outcomes in daily practice”.
AUTHORS ADAM GWOZDZ (King’s College London, London, UK), Cindy de Jong (Leiden University, Leiden, The Netherlands) and colleagues explain in their report that the ICHOM assembled an international working group of VTE experts and patient representatives “to develop a standardised minimum set of outcomes and outcome measurements for integration into clinical practice and potentially research to support clinical decision making and benchmarking of quality of care”.
The group selected a total of 15 core outcomes important to patients and healthcare professionals, and then
categorised these outcomes into four domains: patient-reported outcomes, long-term consequences of the disease, disease-specific complications, and treatment-related complications. They write that the outcomes and outcome measures were designed to apply to all patients with VTE aged 16 years or older.
Gwozdz, de Jong et al selected a measurement tool package for inclusion in the core standard set. “Additional measures can be introduced to the user by a cascade opt-in system that allows for further assessment if required,” the authors note.
In a section on implementation, the
researchers advise that the final set of outcome measures is now available online for use within clinical practice and potentially research. They elaborate: “After signing up for free through ICHOM Connect, all materials related to the set (i.e. a flyer, reference guide, and data dictionary) can be downloaded.”

The investigators state that the overarching aim of their work is to achieve a globally adopted standard set. However, they acknowledge that there are “different resources, digital infrastructures, and healthcare contexts in low-income, middle-income, and highincome countries that can affect the speed and success of implementation”. They write that “training and education, commitment, and enabling attitudes of healthcare professionals are believed to facilitate implementation” and “offset more structural challenges within the healthcare system”.

“We anticipate that the introduction of this set will contribute substantially towards increasing value in VTE care,” Gwozdz, de Jong and colleagues state in their concluding remarks. They also recognise the benefit of the document for patients, asserting that implementation of this set will “empower patients with VTE to actively participate in their care and, together with involved professionals, make better informed decisions about healthcare options”.
Speaking to Venous News, senior author
Frederikus Klok (Leiden University Medical Center, Leiden, The Netherlands) notes that the ICHOM will provide support to any healthcare professional or institution that sets out to implement the outcome measures. In addition, he advises that the set will be regularly reassessed and updated if relevant.

25Issue96 | November 2022 Venous Thromboembolism
We anticipate that the introduction of this set will contribute substantially towards increasing value in VTE care.”
Adam Gwozdz
Cindy de Jong
*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the venous arena Editorially independent Available in print and digital formats and through our social channels Visit venousnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today
Renal denervation back on track with studies adding “important data to the field”
More than eight years after results of the SYMPLICITY HTN-3 trial led the authors of a New England Journal of Medicine (NEJM) editorial to observe that the “renal denervation train” had reached a “grinding halt”, investigators have expressed renewed optimism that the procedure is back on track as a promising treatment option for hypertension.
This comes on the back of a release of data from two sham-controlled renal denervation trials at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT; 16–19 September, Boston, USA), including long-term results from the SYMPLICITY HTN-3 trial, and primary results from the RADIANCE II trial—described as “important data for the field” by discussants in a late-breaking clinical science session at which the data were presented.
Deepak Bhatt (Brigham and Women’s Hospital Heart & Vascular Center and Harvard Medical School, Boston, USA), who presented the long-term findings from SYMPLICITY HTN-3, acknowledged in his presentation that at six months, though renal denervation using the Symplicity (Medtronic) radiofrequency renal denervation system had been shown to be safe, the trial’s efficacy endpoint was not met.
At TCT 2022, Bhatt shared new data from the trial, which enrolled a total of 535 patients with treatmentresistant hypertension from 88 centres across the USA, all of whom were randomised 2:1 to undergo renal denervation (364) with an early generation of the Symplicity system or a sham procedure (171). All patients received stable, maximum tolerated doses of three or more antihypertensive drugs. The latest analysis included 101 patients from the sham control arm who crossed over to receive renal denervation after six months.


Despite the trial’s primary endpoint failure at six months, the long-term data presented by Bhatt point towards significantly greater reductions in office systolic blood pressure in patients who had undergone renal denervation versus those receiving a sham procedure at one (-18.9mmHg vs. -6.3mmHg, p<0.0001), two (-24.1mmHg vs. -4.3mmHg, p<0.0001) and three (-26.4mmHg vs. -5.7mmHg, p<0.0001) years, while the number of antihypertensive drugs were shown to be similar between patients in each arm of the trial.
Noting that the earlier data from the trial should confer a degree of caution to the interpretation of the long-term findings, Bhatt concluded that the latest data support the notion that durable blood pressure reductions with radiofrequency renal denervation in the presence of maximal medical therapy are “safely achievable”.
“I think the results that I have presented here and which were published in The Lancet are very encouraging, and I think it helps unify the field and more recent findings with renal denervation,” Bhatt told Vascular News, adding that the analysis complements other studies in renal denervation to have met their primary endpoints. “Viewed in that context, [and] examining the totality of data [indicating] that there is really something there,” he added, “renal denervation is, in fact, reducing blood pressure. It does not mean it should replace medicines or lifestyle modifications, but in the right patient, I think it could be a useful adjunct.”
In what is seen as a further positive development for proponents of renal denervation, the same TCT 2022 late-breaking clinical science session saw Ajay Kirtane (Columbia University Irving Medical Center New York-Presbyterian Hospital, New York, USA) present
the primary results from RADIANCE II, looking at the blood pressure-lowering efficacy of ultrasound renal denervation using the Paradise (Recor Medical) system at two months.
Through the trial, investigators from 60 study centres in eight countries screened 1,038 individuals for trial eligibility, with 224 patients with uncontrolled hypertension ultimately randomised 2:1 to undergo renal denervation with the Paradise system (150) or a sham procedure (74).
Patients were required to remain off antihypertensive medications throughout the two months of follow-up unless specified blood pressure criteria were exceeded. At the two-month primary efficacy endpoint, Kirtane reported, patients treated with renal denervation had a mean reduction in daytime ambulatory systolic blood pressure of -7.9mmHg, compared to a reduction of -1.8mmHg in the sham control group, corresponding to a statistically significant between-group difference of -6.3mmHg (p<0.0001).
Similar reductions in blood pressure were observed in nighttime and 24-hour measures, as well as measurements taken at home and in the physician office. There were also no major adverse events in either group through 30 days, Kirtane reported. The trial’s primary safety endpoint will be measured at six months, and patients will be followed for a total of five years.
“The reason that we did the RADIANCE II trial is to try and build upon prior trials, such as RADIANCE-HTN SOLO and TRIO, which had demonstrated that renal denervation could lower blood pressure in comparison with a sham,” Kirtane commented to Vascular News, referring to earlier studies to have assessed the use of the Paradise system. “I think it pretty clearly showed that renal denervation lowered blood pressure at two months in comparison with the sham, especially on a backdrop of patients with no medication.
“We have also shown that, in a backdrop of a combination therapy with three separate agents, renal denervation can lower blood pressure, and in many ways this is a confirmatory trial, but a larger trial that will hopefully lead to regulatory approval in the USA.”
In discussions that followed Kirtane’s presentation at TCT, speakers welcomed the findings of RADIANCE
II, and their wider importance to the understanding of renal denervation as a treatment for hypertension. “We should not forget that this is the sixth sham-controlled trial that showed and confirmed the efficacy of this approach using different modalities, but also using different patient populations,” said Felix Mahfoud (Saarland University Hospital, Hamburg, Germany), who has himself been heavily involved in renal denervation research including as an investigator in the RADIANCE-HTN SOLO and TRIO trials.
Mahfoud asked Kirtane to offer his view on what is required for the treatment to find its way into clinical practice, to which Kirtane responded that additional safety and, importantly, clinical outcomes data will be vital. “That is important, because while blood pressure is recognised as a surrogate of adverse events, and we know that for patients that are uncontrolled that lowering blood pressure is a good thing, I think we have seen enough examples in our field of potential questions to clinical efficacy that the onus is on us to do those kinds of trials,” Kirtane said.
Naomi Fisher (Brigham and Women’s Hospital and Harvard Medical School, Boston, USA) offered her perspective on the trial as a specialist in hypertension, describing the condition as the “number one global burden of disease risk factor in the world”.
RADIANCE II
The change in ambulatory systolic blood pressure was -7.9 mmHg for the ultrasound renal denervation group compared to -1.8mmHg for the sham group.
“We need innovation,” she commented. “Our control rates are poor and they are only falling, if you look at large statistical datasets showing that we are doing a worse and worse job as time goes on. We have had pharmacology around since the 1950s for high blood pressure, our last new drug class was introduced in 2007, that is how long ago that we had an innovation.
SYMPLICITY HTN-3
“Finally, we have evidence for an innovative therapy that is really desperately needed to help control blood pressure.”
At 12 months, renal denervation patients had significantly greater office systolic blood pressure reductions compared with sham control after crossover imputation (-18.9 mmHg vs -6.3 mmHg, p<0.0001, respectively).
November 2022 | Issue9626 Renal Denervation
Ajay
Kirtane
Deepak
Bhatt
The results of these two key trials were presented at TCT 2022
Aperto® drugcoated balloon technology shows promise in dialysis access patients
The Aperto drug-coated balloon (DCB) technology (Cardionovum) is “one of the very promising new technologies” for treating central vein stenosis and restenosis in dialysis access patients, Michael Lichtenberg (Arnsberg Vascular Center, Arnsberg, Germany) tells Vascular News.

SHUNT STENOSIS AND restenosis represent common threats to the function of arteriovenous fistulas (AVFs) and shunt grafts in patients on haemodialysis. Patients often develop a consecutive neointimal hyperplasia in haemodialysis access vessels as well as along the needle puncture site.
The new Aperto paclitaxel-releasing, high-pressure DCB dilatation catheter provides a dual shunt treatment quality

for the prevention and dilatation of intimal hyperplasia. The device can treat AVF or polytetrafluoroethylene (PTFE) shunt graft venous outflow lesions successfully, leading to a substantial reduction of haemodialysis shunt stenosis and restenosis, for a prolonged dialysis access survival. The benefits of Aperto have also been demonstrated in the literature.1
Lichtenberg, who is director of the Angiology Department at the Arnsberg Vascular Center, has some clinical experience with the Aperto DCB technology, and shared his thoughts on the technology with Vascular News at this year’s Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual meeting (10–14 September, Barcelona, Spain). He spoke about some of the major difficulties associated with treating patients with central vein stenosis and restenosis, the latest treatments available, and some key insights from the APERTO CVS trial assessing the Aperto DCB technology.
What are some of the major difficulties associated with treating central vein stenosis and restenosis?
“Patients who are on chronic dialysis really depend on arteriovenous access for their dialysis treatment.
Unfortunately, we know that arteriovenous access could have a lot of issues with stenosis and restenosis, which also counts for central veins, and if the central veins are blocked—i.e. they are stenotic or restenotic— it could mean that these patients lose their arteriovenous access for dialysis.”
What are the latest state-ofthe-art treatments for this patient population?
“Unfortunately, standard balloon angioplasty is the most commonly used therapy option at the moment for restenosis or stenotic AVFs and central vein stenosis. There are new technologies like scoring devices and drug-eluting balloon angioplasties, which are now coming into focus, because we have learned that these new technologies have much better patency rates and freedom from loss of patency than standard balloon angioplasty. It has become clear that we need these new concepts to maintain patency.”
As a physician, what do you look for in a DCB in terms of features?
“You cannot use every DCB in stenotic
and restenotic lesions, and the reason for this relates to device diameters. For central vein stenosis, you need DCBs with large diameters, for example 10mm or 12mm. That means you need dedicated products, dedicated DCBs for these indications. Therefore, I am really looking forward to having these new devices available, because they will expand treatment options.”
Can you tell us about the APERTO CVS trial?
“The Aperto DCB is one of the very promising new technologies for stenotic and restenotic lesions, and especially for central vein stenosis. The Aperto is available with large diameters and so with this device it is possible to treat central vein stenosis. The APERTO CVS trial prospectively analyses the efficacy and safety of this DCB technology for central vein stenosis. We have already recruited a couple of patients and the interim data are very promising.”
References 1. Y Yin, Y Shi, T Cui, et al. Efficacy and safety of paclitaxel-coated balloon angioplasty for dysfunctional arteriovenous fistulas: a multicentre randomised controlled trial. American Journal of Kidney Diseases 2021; 71(1): 19–27.
27AdvertorialIssue96 | November 2022
C M Y CM MY CY CMY K 221006_ApertoADVnew_Trim.pdf 14 06/10/2022 15:42:06 THIS ADVERTORIAL IS SPONSORED BY CARDIONOVUM®
Michael Lichtenberg
SYMPOSIUM ON ENDOVASCULAR THERAPY
2023, I’D LIKE TO
Discover best techniques and devices to treat cardiovascular disease.
Utilize multidisciplinary teams to improve patient outcomes of endovascular therapy.


Compare simple and complex arterial access during diverse endovascular procedures performed by worldrenowned specialists.
Access the most relevant and pertinent data that I can utilize in challenging situations in my practice.

CHECKED YES TO ANY/ALL OF THE ABOVE? JOIN US.
16 –19 JANUARY 2023 LOEWS MIAMI BEACH FLORIDA INTERNATIONAL
iset.org
IN
VASCULAR
Arteriovenous graft use linked to venous thromboembolism risk in haemodialysis patients
In an observational cohort study of Medicare beneficiaries receiving haemodialysis, the use of an arteriovenous graft (AVG) compared with an arteriovenous fistula (AVF) was associated with an increased risk of deep vein thrombosis (DVT) and/or pulmonary embolism (PE). Conversely, researchers also found that there was little to no difference according to the access type in the rates of major bleeding or other cardiovascular outcomes. These results were recently published in Kidney Medicine
In their report, authors Nicholas S Roetker (Hennepin Healthcare Research Institute, Minneapolis, USA) and colleagues write that the risks of major bleeding, thrombosis, and cardiovascular events are elevated in patients receiving maintenance haemodialysis. The team’s objective was to compare the risk of these outcomes in haemodialysis patients according to the permanent vascular access type.
Using data from the United States Renal Data System (2010–2015), the researchers included in their study patients with kidney failure who were
greater than 18 years, had Medicare as the primary payer, were not using an oral anticoagulant, and were newly using an arteriovenous (AV) access for haemodialysis.
Roetker et al compared 17,763 AVG and 60,329 AVF users, estimating the three-year incidence rates and incidence rate ratios (IRRs) of various outcomes, specifically major bleeding, venous thromboembolism (VTE), ischaemic stroke, myocardial infarction, cardiovascular death, and chronic limbthreatening ischaemia.
In Kidney Medicine, the authors report that the use of an AVG,
Registry data highlight longterm trends in haemodialysis access profile of failed kidney transplant patients
New findings, recently published in the Journal of Vascular Access (JVA), provide insight into the haemodialysis access profile of failed kidney transplant patients treated in the Catalonia region of Spain over an 18-year period.
RESEARCHERS RAMON ROCA-TEY (Hospital Universitari Mollet, Barcelona, Spain) and colleagues note that data on vascular access use in failed kidney transplant patients returning to haemodialysis are limited. In the present study, therefore, the investigators sought to analyse the vascular access profile of this patient group, the factors associated with the likelihood of haemodialysis reinitiation through arteriovenous fistula (AVF), and the effect of vascular access in use at the time of kidney transplant on kidney graft outcome.
Roca-Tey et al write that they examined data from the Catalan Renal Registry on failed kidney transplant patients restarting haemodialysis and incidence haemodialysis patients with native kidney
compared with that of an AVF, was associated with an increased risk of VTE (10.8 vs. 5.3 events per 100 person-years; adjusted IRR, 1.74; 95% confidence interval [CI], 1.63–1.85) but not with the risk of major bleeding (IRR, 1.04; 95% CI, 0.93–1.17). They add that the use of an AVG was also potentially associated with a slightly increased risk of cardiovascular death (IRR, 1.09; 95% CI, 1.01–1.16).
The investigators acknowledge some limitations of their study, noting for example that the analysis included only patients with a functioning AV access, and that the scope of outcomes considered provides an “incomplete picture” of the risks facing AV access users. “While this analysis was primarily concerned with assessing bleeding, thrombotic, and cardiovascular events, the risk
Haemodialysis
of a variety of other serious clinical outcomes should be considered when choosing a vascular access,” they write.
Furthermore, the authors note that their study included Medicare fee-for-service beneficiaries with Part D coverage who were not receiving oral anticoagulation; as such, they comments that “the results may not be generalisable to other kidney failure populations”.
In their conclusion, Roetker and colleagues summarise that the use of an AVG, relative to an AVF, in haemodialysis is associated with an increased risk of VTE. They remark on the implications of these findings:
“Given recent guidelines emphasising selection of the ‘right access’ for the ‘right patient,’ the results of this study should potentially be considered as one additional factor when selecting the optimal access for haemodialysis”.
failure over the period from 1998–2016.
The authors report in JVA that the vascular access profile of 675 failed kidney transplant patients at haemodialysis reinitiation compared with that before kidney transplant and with 16,731 incident patients starting haemodialysis was: AVF 79.3% vs. 88.6% and 46.2% (p=0.001 and p<0.001), arteriovenous graft (AVG) 4.4% vs. 2.6% and 1.1% (p=0.08 and p<0.001), tunnelled central catheter 12.4% vs. 5.5% and 18% (p=0.001 and p<0.001) and nontunnelled catheter 3.9% vs. 3.3% and 34.7% (p=0.56 and p<0.001).
In addition, they reveal that the likelihood of haemodialysis reinitiation by AVF was significantly lower in patients with cardiovascular disease, kidney transplant duration of over five years, those dialysed through AVG or tunnelled central catheter before kidney transplant, and those of the female sex.
The authors add that analysis of Kaplan-Meier curves showed a greater kidney graft survival in patients dialysed through arteriovenous access than in patients using catheters just before kidney transplant. Finally, Cox regression analysis showed that patients on haemodialysis through arteriovenous access at the time of kidney transplant had lower probability of kidney graft loss compared to those with catheter, Roca-Tey and colleagues write in JVA
The authors conclude that the vascular access profile of failed kidney transplant patients returning to haemodialysis and incident patients starting haemodialysis was different. They also note that, compared to before kidney transplant, the proportion of failed kidney transplant patients restarting haemodialysis with an AVF decreased significantly at the expense of a tunnelled central
catheter, and that patients on haemodialysis through arteriovenous access at the time of kidney transplant showed greater kidney graft survival compared with those using a catheter.
According to the researchers, there are two clinical implications that can be derived from their findings. “To reduce the proportion of failed kidney transplant patients returning to haemodialysis with a central line, better vascular access management is needed,” they state, outlining the first implication. The second implication, Roca-Tey et al believe, arises from the impact of arteriovenous access on kidney graft outcomes. They elaborate: “If our results are confirmed in further studies, including a larger number of kidney transplant patients and not simply patients returning to haemodialysis, routine arteriovenous access closure after a successful kidney transplantation should not be performed.”
In the discussion of their findings, the authors state that a number of factors limit the strength of their findings. The use of a population registry database for this study they highlight as a key weakness, for example. “The variables used are restricted in number and can have low clinical specificity,” they note, giving the example that the number of litigated accesses, as well as those with spontaneous thrombosis and not salvaged, were not recorded.
Furthermore, the researchers acknowledge a weakness pertaining to the effect of vascular access on kidney graft survival—a secondary aim of the study. They elaborate: “Kidney transplant patients who returned to haemodialysis were considered instead of all kidney transplant patients, and this may be a bias in our study.”
Issue96 | November 2022 29Haemodialysis
ACCESS
The results of this study should potentially be considered as one additional factor when selecting the optimal access for haemodialysis.”
Data from the Catalan Renal Registry were examined over
the
period
1998–2016

Consensus Update Vascular & Endovascular SEE YOU IN 2023 CXSYMPOSIUM.COM Peripheral Arterial Consensus Aortic Consensus Acute Stroke Consensus Venous & Lymphatic Consensus Vascular Access Consensus The Hurting Leg Consensus INNOVATIONEDUCATION EVIDENCE 25–27 APRIL 2023 TUESDAY-THURSDAY HILTON LONDON METROPOLE, UNITED KINGDOM CONTROVERSIES CHALLENGES CONSENSUS
CAROTID
Randomised trial finds carotid endarterectomy or stenting not superior to best medical therapy in asymptomatic patients
Five-year outcomes of a multicentre, randomised controlled trial (RCT) indicate that carotid endarterectomy (CEA) or carotid artery stenting (CAS) are not superior to best medical therapy (BMT) alone for moderate-to-severe asymptomatic carotid artery stenosis.
TILMAN REIFF (UNIVERSITY Hospital of Heidelberg, Heidelberg, Germany) and colleagues recently reported data from the SPACE-2 trial in The Lancet Neurology. The authors claim that—to the best of their knowledge—this is the first randomised trial to directly compare CEA plus BMT, CAS plus BMT, and BMT alone over a five-year period in patients with asymptomatic carotid artery stenosis.
“The optimal treatment for patients with asymptomatic CAS is under debate,” the investigators write, noting that, since BMT has improved over time, the benefit of CEA or CAS is “unclear”. They add that randomised data comparing the effect of CEA and CAS versus BMT alone are lacking, and therefore aimed to directly compare CEA plus BMT with CAS plus BMT and both with BMT only in SPACE-2.
SPACE-2 was a multicentre RCT carried out at 36 study centres in Austria, Germany, and Switzerland, Reiff et al detail in their report. The researchers enrolled participants aged 50–85 years with asymptomatic carotid artery stenosis at the distal common carotid artery or the extracranial internal carotid artery of at least 70% according to European Carotid Surgery Trial criteria (equivalent to at least 50–99% according to North American
Symptomatic Carotid Endarterectomy Trial [NASCET] criteria).
Reiff and colleagues note that SPACE-2 was initially designed as a three-arm trial including one group for BMT alone. However, the design was amended due to slow recruitment to become two substudies with two arms each comparing CEA plus BMT with BMT alone (SPACE-2a) and CAS plus BMT with BMT alone (SPACE-2b).
“Originally, we planned to recruit 3,640 patients; however, the study had to be stopped prematurely due to insufficient recruitment,” the authors write. They report that a total of 513 patients across SPACE-2, SPACE-2a, and SPACE-2b were recruited and surveyed between 9 July 2009 and 12 December 2019, of whom 203 (40%) were allocated to CEA plus BMT, 197 (38%) to CAS plus BMT, and 113 (22%) to BMT alone. The median follow-up was 59.6 months.
In their Lancet paper, Reiff and colleagues report that the cumulative incidence of any stroke or death from any cause within 30 days or an ipsilateral stroke within five years— the primary efficacy endpoint of the study—was 2.5% (95% confidence interval [CI], 1–5.8) with CEA plus BMT, 4.4% (95% CI, 2.2–8.6) with CAS plus BMT, and 3.1% (95% CI,
1–9.4) with BMT alone.
The investigators also relay that, in both the CEA and CAS group, five strokes and no deaths occurred in the 30-day period after the procedure. During the five-year followup period, they note, three ipsilateral strokes occurred in both the CAS plus BMT and BMT alone groups, with none in the CEA plus BMT group.
Reiff et al conclude that CEA plus BMT or CAS plus BMT were not found to be superior to BMT alone regarding risk of stroke or death within 30 days or ipsilateral stroke during the five-year observation period. However, they stress that their results “should be interpreted with caution” due to the small sample size.
Indeed, they note that the small sample size of the trial was its main limitation. “With the low number of observed outcome events, only a few further events would be needed to change the results significantly.” Despite this, they highlight that the present analysis “reveals important indications for possibly relevant existing associations, can be used for further pooled analyses, and provides data for future meta-analyses in a
topic area in which currently only few randomised trials are available”.
Randomisation is key
In a commentary on the study, also published in The Lancet Neurology, Alison Halliday (University of Oxford, Oxford, UK) underscores the importance of randomised trials in this field of study. Halliday, who is principal investigator of the Asymptomatic Carotid Surgery Trials, remarks:
“Despite its relatively small size, SPACE-2 provides important longterm follow-up data because of the randomised trial methodology. Large procedural registries usually cease follow-up within days or a few months, and patients on medical treatment alone cannot be meaningfully compared without randomisation.
“The future of interventions for carotid artery stenosis depends on randomised comparisons of different treatment approaches to ensure that bias is minimised and, at the same time, that enough patients are included. Alternatively, individual patient meta-analysis of similar trials can be used to support future interventions. SPACE-2 is of value to future individual patient meta-analyses of these three treatment options.”
Silk Road Medical announces first patient enrolled in ROADSTER 3 post-approval study
Silk Road Medical has announced enrolment of the first patient in ROADSTER 3, which the company claims is the the first prospective, multicentre, single-arm study to assess real-world treatment of standard surgical risk patients with carotid artery disease using transcarotid artery revascularisation (TCAR). The procedure was performed by Animesh Rathore at Sentara Norfolk General Hospital in Norfolk, USA.
ROADSTER 3 is a post-approval study designed to prospectively evaluate real-world use of TCAR among standard surgical risk patients and will enrol a maximum of 400 per protocol patients across up to 60 sites. The primary endpoints include a hierarchical composite of major adverse events (death, stroke, or myocardial infarction) through 30 days post-procedure, plus ipsilateral stroke from day 31 to 365 post-procedure. The incidence of cranial nerve injury within 30 days post-procedure is a
key secondary endpoint. With the enrolment of the first patient, the launch of the ROADSTER 3 study achieves the first of a series of milestones set forth by the US Food and Drug Administration (FDA)’s post-approval requirements.
“The successful enrolment of the first patient in ROADSTER 3 by Dr Rathore and the team at Sentara Norfolk General Hospital represents a significant milestone in our efforts to extend the body of clinical evidence supporting TCAR in this
expanded patient population,” said Erica Rogers, president and chief executive officer of Silk Road Medical. “Our goal with the study is to confirm the safety and efficacy of TCAR in a prospective realworld standard surgical risk patient population to firmly establish this less invasive treatment option for all carotid stenosis patients.”
“Positive outcomes in high surgical risk patients treated with TCAR have been well documented over the years,” said Rasesh M Shah, site principal investigator at Sentara Norfolk General Hospital for the ROADSTER 3 study. “A study to confirm the efficacy of TCAR in the treatment of standard surgical risk patients is incredibly valuable as the physician community looks to improve its overall approach to treating carotid patients, independent of their surgical risk. I am honoured to be participating in this first ever study to collect prospective data on the treatment of a recently approved patient population using TCAR.”

Issue96 | November 2022 31Trial Updates
Despite its relatively small size, SPACE-2 provides important long-term follow-up data because of the randomised trial methodology.”
Alison Halliday
Skull model showing carotid arteries
Launch
The psychology of surgery
Vascular trainee Claire Dawkins addresses the “heavy burden” of dealing with surgical complications, and considers how trainees can navigate the difficulties of surgical training and a subsequent consultant career without neglecting their mental health.

A PATIENT I OPERATED ON DIED. IT WAS not anything I had done wrong and it was not my fault. But knowing that does not prevent the feelings of guilt I feel because of it. It made me ask ‘what is there in our training that can prepare us for dealing with these often devastating complications and how do we develop a support network to help us to do so?’
I have seen senior colleagues deal with complications in a variety of ways. I have seen complete persona change, with previously calm, relaxed individuals withdrawing and secondguessing themselves, ruminating over whether they did the right thing for their patient. Some overcompensate, becoming anxiously meticulous to the detriment of their wider responsibilities. Or the total opposite, a ‘bury head in sand’ approach, with consultants avoiding the patient and never discussing the case. Neither of these approaches are beneficial to
the patient or the surgeon. The term ‘second victim’, which has been used to describe surgeons in these situations, is particularly fitting.
Surgeons are expected to be able to cope with complications, with those who do not being considered weak. But this stereotype goes beyond resilience and is actually very concerning. As doctors, our primary aim is first do no harm. But as a surgeon you are continuously causing harm, albeit with the intention of doing good. Gaining the trust of a patient sufficiently for them to give their consent for you to operate on them is both a huge honour and a great responsibility. However, this makes dealing with complications even more difficult and a heavy burden to bear.
So what is available to help surgeons face this burden? I feel very fortunate to be working as part of a good team. I have leant on registrar and consultant colleagues in the past to help me cope with complications and I have had the privilege to have been someone to confide in when other surgeons have found themselves in the same situation. Sadly not everyone is as fortunate as to have such a team to rally around them.
The multidisciplinary team (MDT) is a key starting point when considering the management of many patients, with shared decision-making spreading the responsibility between all MDT members. Often in patients experiencing significant complications I find myself asking whether the decision-making was correct; was it the right operation for the right patient at the right time? It is the preoperative management that is usually more important than intraoperative decision. Knowing the patient has been through a robust
Ukrainian surgeons present clinical update on Human Acellular Vessel in vascular trauma at ESVS 2022
Humacyte recently announced the presentation of a clinical update on the Human Acellular Vessel (HAV) for the treatment of vascular trauma. The update was presented by Ukrainian surgeon collaborators, Oleksandr Sokolov (Dnipro State Medical University, Dnipro, Ukraine) and Vasyl Shaprynskyi (State Institution of Science “Research and Practical Center for Preventive and Clinical Medicine” State Administrative Department, Kyiv, Ukraine) at this year’s ESVS Annual Meeting (ESVS 2022; 20–23 September, Rome, Italy).
HUMACYTE NOTES IN A press release that the company’s investigational HAV is designed to offer off-the-shelf availability and resistance to infection and to address long-standing limitations in vascular tissue repair and replacement.
Shaprynskyi presented a talk entitled, “The first experience of using the Human Acellular Vessels in Ukraine for the treatment of patients with vascular trauma,” while Sokolov spoke virtually from Ukraine and presented, “Vascular trauma due to blast injury. Experience of Dnipro in Russian-Ukrainian war 2022.”
“Shaprynskyi and Sokolov have been instrumental in establishing their hospitals as medical strongholds during the Russian-Ukrainian war and reported that blast trauma, causing massive tissue
damage and infected wounds, accounts for approximately 82% of incoming vascular trauma cases to their medical centres,” the press release reads. Trauma to the extremities makes up the majority of injuries, the statement adds, noting that these are primarily vascular injuries to the lower extremities and shoulders.
“Access to the HAV, a biologic conduit, has improved our ability to perform vascular reconstructions by eliminating the need to harvest a venous conduit and saving time required to look for a useable vein, assisting greatly in limb salvage. While we continue to face this crisis in our country, partnerships with groups like Humacyte allow us to overcome many limitations in wartime medical care that we previously experienced such as lack of readily
MDT process does not totally negate the feelings of guilt following patients’ complications but it can alleviate it to some degree.
A quick Google search reveals a number of resources available to surgeons to help them in exactly this sort of crisis: helplines, apps and courses. I, personally, have never used them, and it is only when thinking of writing about this topic that I thought to look them up. It is not something that is talked about when all is well, so when complications occur then accessing these aids does not necessarily spring to mind.
How can we navigate the difficulties of surgical training and a subsequent consultant career without neglecting our mental health? Talking through these situations gives others an insight into the issues and starts to normalise these feelings to colleagues and junior surgeons. We need to get to know ourselves and our team, learn what triggers you and others and the best way to recognise that you or your colleagues are struggling. Failing that immediate support structure, remembering to turn to professional bodies and their websites can offer a number of resources to support surgeons in precisely these predicaments can offer help. However, it is key for the speciality as a whole to discuss these issues, normalising a human reaction to complications. This enables the surgeon— the ‘second victim’—to process their feelings as a normal and healthy part of managing complications amidst a strong and supportive team.
CLAIRE DAWKINS is a vascular trainee at the Newcastle Upon Tyne Hospitals NHS Foundation Trust in Newcastle, UK and a committee member for the Rouleaux Club, the UK national vascular trainee society.
available conduits that are resistant to infection, particularly important in the contaminated battlefield setting,” said Shaprynskyi.
Shaprynskyi and Sokolov reported that surgeons in Ukraine have utilised the HAV to treat patients with a multitude of wartime injuries. Sokolov provided a clinical update on a patient with a blast injury to the shoulder who received a repair using the HAV. The patient is now beyond threemonth follow-up without complication. Another patient who suffered a blast injury to the lower leg underwent successful HAV implantation and is now one-month past surgery without complication. Shaprynskyi reported on a patient with a gunshot wound to the right thigh that was initially treated
with a synthetic graft, but ultimately the graft failed due to infection, putting the patient at risk of limb loss. The HAV was used to replace the infected graft, and three months later the HAV is supplying blood flow to the limb and is infection-free.
Humacyte states that it worked closely with the Office of International Programs of the US Food and Drug Administration (FDA) and the Ukrainian Ministry of Health to provide the HAV as an additional treatment option to those affected with vascular injury in Ukraine. Humacyte is currently evaluating the HAV in a Phase 2/3 clinical trial in vascular trauma for use as a vascular replacement to restore blood flow to a limb, when saphenous veins or synthetic grafts are not feasible.
November 2022 | Issue9632 Launch Pad
Pad
Access to the HAV, a biologic conduit, has improved our ability to perform vascular reconstructions by eliminating the need to harvest a venous conduit and saving time required to look for a useable vein, assisting greatly in limb salvage.”
Vasyl Shaprynskyi
Human Acellular Vessel
Subscribe today













*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the cardiovascular field Editorially independent Visit cardiovascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription**
Available in print and digital formats and through our social channels
Clinical News
PEERLESS commenced earlier this year and compares FlowTriever to catheter-directed thrombolytics in pulmonary embolism.
Xeltis has announced what it describes as “very promising” preliminary efficacy and safety results from one of the centres participating in the AXESS study—a first-in-human (FIH) clinical trial of its restorative haemodialysis access graft, Axess.
The company has also reported that enrolment in the trial is now complete, with 20 patients having been successfully implanted with the Axess graft.
During haemodialysis, the Axess device demonstrated 100% cumulative primary and secondary patency, with an average follow-up time of 5.8 months and no device-related complications, in patients implanted by Matteo Tozzi and his team at the University of Insubria (Varese, Italy)—one of six European sites participating in the trial.
These data were presented at the Porto Vascular Conference (PVC; 7–8 October 2022, Porto, Portugal), organised by the European Society of Vascular Surgery (ESVS). To date, Axess has enabled more than 1,200 haemodialysis sessions in just over a year as part of the AXESS FIH trial. Sixty of these sessions have taken place in Varese, a Xeltis press release states.
“Our initial clinical experience with Axess is very promising, from implanting to puncturing, and the data on functionality observed so far are very encouraging,” Tozzi commented.
Xeltis’ Axess device is a synthetic, bio-restorative vessel graft that enables early puncturing needed to connect to the haemodialysis machine. Unlike any currently available options—according to Xeltis—it is also designed to turn into a patient’s own living blood vessel, like an arteriovenous fistula, as its porous structure gets gradually colonised by patient’s own tissue cells.
According to the experience in Varese, Axess improved compliance over time, which may result from new tissue forming in the device.
Inari Medical announces randomised controlled trial evaluating clinical outcomes of the ClotTriever system in DVT
Inari Medical has announced planned enrolment of the DEFIANCE randomised controlled trial (RCT), which is designed to compare the clinical outcomes of patients with iliofemoral deep vein thrombosis (DVT) treated with the ClotTriever system versus anticoagulation only.
The trial will enrol 300 patients at up to 60 centres worldwide. DEFIANCE is Inari’s second RCT and it will run in parallel to the PEERLESS trial.
“Anticoagulation is still the predominant therapy used in the management of DVT and in some studies has been associated with post-thrombotic syndrome (PTS) in up to 50% of patients,” said Steven Abramowitz (MedStar Health, Washington, DC, USA), co-principal investigator. “PTS is a debilitating condition with symptoms including swelling, difficulty walking, skin changes and poorly healing open wounds. Research has shown PTS quality of life scores can be as low as those of patients suffering from heart failure or cancer. Mechanical thrombectomy procedures, using technology like ClotTriever, rapidly remove large volumes of DVT and may thereby reduce the risk of PTS.”
“DEFIANCE is the first RCT to compare mechanical thrombectomy to anticoagulation for the treatment of DVT,” said Xhorlina Marko (Beaumont Health, Dearborn, USA), co-principal investigator. “The primary endpoint for
(ACCESS) study that showed that Sirogen use in end-stage renal disease (ESRD) patients 65 years and older resulted in clinically meaningful improvement in arteriovenous fistula (AVF) maturation and durability.
Kansal commented: “We are excited to participate in this randomised clinical trial that addresses an important unmet clinical need. The elderly population is the fastest growing segment of the haemodialysis population and these patients urgently need a solution to improve AV fistula maturation.”
Sriram Iyer, chief scientific officer of Vascular Therapies commented: “Initiating enrolment of the ACCESS 2 clinical study is an important milestone for Vascular Therapies. We would like to thank our investigators and their research teams for their interest in Sirogen and desire to evaluate a new potential therapy for haemodialysis patients.”
Vascular Therapies advises that the Sirogen drug development programme has received Fast Track status from the US Food and Drug Administration (FDA).
Shape Memory Medical completes enrolment in the AAA-SHAPE early feasibility study
Shape Memory Medical has announced the completion of patient enrolment in AAA-SHAPE, the company’s prospective, multicentre early feasibility safety study of the Impede-FX RapidFill device when used for abdominal aortic aneurysm (AAA) sac management during elective endovascular aneurysm repair (EVAR).
principal investigator for AAASHAPE New Zealand. “Impede-FX RapidFill could be a significant tool for addressing this need, so we could not be more pleased to participate in AAA-SHAPE.”
Michel Reijnen (Rijnstate Hospital, Arnhem, The Netherlands), principal investigator for AAA-SHAPE Netherlands added: “Although we are still early in the course of patient follow-up, we are seeing signals of impressive sac shrinkage at six months and one year. We look forward to the ongoing evaluation of these patients and how their aneurysms respond longer-term.”
the trial is a hierarchical composite of treatment failure and PTS severity at six months. The trial has the potential to influence guidelines and change the standard of care.”
“Physicians need to know in which patients to use our devices and what outcomes to expect. This is our basic responsibility,” said Thomas Tu, Inari’s chief medical officer. “We have studied over 2,000 patients, published 250 peer reviewed publications, and DEFIANCE marks our sixth major clinical study. These high-quality studies will establish a new standard of care for patients and further distance us from competition.”
Vascular Therapies initiates enrolment in ACCESS 2 clinical trial
Vascular Therapies has announced that the first patient in the ACCESS 2 clinical trial was enrolled by Nikhil Kansal at Harbor-UCLA Medical Center in Torrance, USA.
The Phase 3 prospective randomised ACCESS 2 study will enrol 120 patients from centres in the USA and UK. The study is being conducted to validate an encouraging subgroup analysis from the first Phase 3
“We are pleased to reach this critical milestone in our AAA-SHAPE clinical programme,” said Ted Ruppel, president and chief executive officer of Shape Memory Medical. “We would like to thank the patients, investigators, and clinical study teams for their important contributions, and we look forward to evaluating the follow-up data to come.” The AAA-SHAPE study, which enrolled a combined 35 patients across two centres in New Zealand and in three centres in The Netherlands, will follow patients for two years.
Shape Memory Medical notes that Impede-FX RapidFill, the study device, incorporates a proprietary shape memory polymer, which is a high volume, porous embolic material that self-expands upon contact with blood.
According to the company, AAASHAPE is the first prospective clinical trial to evaluate the application of this novel material in AAA patients and its potential to improve aneurysm sac regression rates following EVAR.
“We have been talking for years about the need to directly manage the AAA sac due to the link between aneurysm failure to regress post EVAR and increased mortality risk,” said Andrew Holden (Auckland City Hospital, Auckland, New Zealand),
Impede-FX RapidFill device
First US patient enrolled in Selution SLR IDE peripheral study
The first US patient has been enrolled in the US Food and Drug Administration (FDA) SELUTION4BTK (below-the-knee) clinical trial evaluating Selution SLR, MedAlliance‘s novel sirolimuseluting balloon. This follows investigational device exemption (IDE) approval in the USA in May 2022, with enrolment of the first patient occurring in Germany one week after approval.
“We are very excited to finally have drug-eluting technology in the USA to treat this difficult patient population,” commented the trial’s principal investigator (PI) Ehrin Armstrong (University of Colorado, Denver, USA). “We are encouraged by the positive early outcomes with this novel SELUTION SLR sirolimus drugeluting balloon (DEB) in Europe and Asia. We hope this FDA IDE trial will demonstrate significant benefit for patients who currently have limited treatment options.”
The aim of the SELUTION4BTK clinical trial is to demonstrate the superior efficacy and equivalent safety of Selution SLR compared to plain (uncoated) balloon angioplasty in the treatment of BTK arteries in chronic limb-threatening ischaemia (CLTI) patients. The trial is a prospective, multicentre, single blinded, randomised study (ClinicalTrials.gov Identifier: NCT05055297).
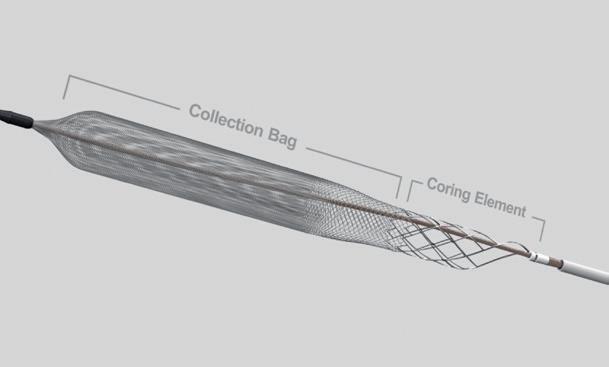
A total of 377 patients are being randomised 1:1 to either SELUTION SLR or control treatment. This is the first study of its kind where ‘real-world’ patients with CLTI can be included, according to MedAlliance. Patients are being enrolled at approximately 40 sites across the USA, Europe and Asia. This first US patient was enrolled at Vascular Solutions (Cary, USA) by Siddhartha Rao.
“We are very pleased to be
34 Market Watch November 2022 | Issue96
Xeltis presents “promising” first-in-human data on restorative haemodialysis access graft
ClotTriever catheter
Clinical News
participating in this ground-breaking study,” said Rao. “We hope that this promising technology will fulfil a huge need for our patients with CLTI, saving life and limb. We look forward to the rapid enrolment of this trial.”
“We were the first company to be granted Breakthrough Device designation for a DEB by the FDA and are proud to enrol the very first US patient in a sirolimus DEB study,” added Jeffrey B Jump, MedAlliance chairman and CEO. “Many companies have been trying for years to bring sirolimus to the USA and we are very pleased with our team for achieving this significant milestone. US patients will now have an alternative to paclitaxel DEB, addressing the concerns expressed by the FDA.”
MedAlliance advises that Selution SLR is available in Europe and all other countries where the CE mark is recognised.

BD launches first-in-human trial of a peripheral sirolimus drug-coated balloon BD has announced the start of enrolment in a first-in-human trial of a peripheral sirolimus drug-coated balloon (DCB).

The PREVISION trial is a
prospective, multicentre, single arm, non-randomised study designed to evaluate the safety of the BD sirolimus DCB in the treatment of peripheral arterial disease (PAD) in the femoropopliteal arteries.
A company press release notes that BD initiated this trial to determine the viability of sirolimus as a future treatment option for patients with PAD. The first patient was successfully treated by principal investigator Andrew Holden at Auckland City Hospital (Auckland, New Zealand).
“It is an honour to enrol the first patient in the PREVISION study,”
across multiple sites in Australia, New Zealand, and Singapore. The trial will enrol and follow-up on approximately 50 patients over the coming months.
Terumo announces study results underscoring safety and efficacy of radial to peripheral interventions in treatment of complex lower extremity PAD Terumo Medical Corporation has announced late-breaking data showing the safety and efficacy of radial to peripheral (R2P) interventions with the R2P Misago self-expanding peripheral stent in lower extremity endovascular interventions via a radial approach. The results were presented at this year’s Transcatheter Cardiovascular Therapeutics (TCT) conference (16–19 September, Boston, USA).
access has been shown to minimise bleeding and vascular complications, lessen mortality rates, reduce costs, and shorten length of stay compared to femoral access in patients undergoing percutaneous coronary intervention (PCI), and we are now seeing growing evidence for similar benefits in peripheral arterial intervention. Our study contributes significantly to this expanding body of evidence as it clearly demonstrates the safety and efficacy of radial access in PAD intervention.”
said Holden. “The burden of PAD continues to impact patients and challenge physicians around the globe. A continued focus on developing nextgeneration technology is important for the patients suffering from PAD.”
PREVISION is being conducted
Presented by Yulanka CastroDominguez (University Hospitals’ Harrington Heart & Vascular Institute, Cleveland, USA), the results showed that using a radial approach in treatment of complex lower extremity peripheral arterial disease (PAD) allowed 92.3% of patients to be discharged the same day. In addition, there were no serious access site complications for any of the patients enrolled in the study.
“In the invasive management of patients with PAD, the ‘radial first’ approach is beginning to gain momentum,” said Michael Martinelli, chief medical officer of Terumo Medical Corporation. “Radial artery
The study was a prospective, multicentre, core-lab reviewed, clinical endpoint committee (CEC) adjudicated, single-arm study aimed at evaluating the safety and feasibility of radial access when used in the treatment of complex lower extremity PAD. A total of 120 PAD patients with Rutherford classification 2–5, from eight medical centres from across the USA were enrolled from 29 June, 2020 to 24 June, 2021 and followed up to one year.
In the final analysis, the primary safety endpoint included evaluation of radial access related complications, to include: access site bleeding, hand ischaemia, haematoma, nerve damage, perforation, pseudoaneurysm, radial artery occlusion, embolic stroke or transient ischemic attack. Based on CEC adjudication, primary safety events categorised as serious access site-related adverse events did not occur in any patient. Radial access was successfully achieved in 100% of patients.
Subscribe today Available in print and digital formats and through our social channels
Visit neuronewsinternational.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription**



Editorially independent

35Market Watch xxxx Issue96 | November 2022 *Available for US and EU readers only **Available worldwide
A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders
A specialised news source in the neuro interventional arena
Product News
Medtronic receives CE mark for the first balloonexpandable covered stent indicated for ChEVAR

“To support this business expansion, Getinge has invested in doubling our stent production capacity in order to provide all of our global customers with the devices needed to treat patients,” the company’s senior director of Global Marketing and Product Management, Vascular Systems, Patty Burns, said of the partnership.
Bentley acquires GoBack catheter from Upstream Peripheral Medical Technologies
Bentley today announced that it has acquired the rights of the GoBack catheter from Upstream Peripheral Technologies.
Penumbra and Asahi Intecc partner to introduce Indigo system to Japan Penumbra and Asahi Intecc, a Japanese medical device manufacturer, announced that they will collaborate to introduce Penumbra’s Indigo Aspiration System into the Japanese market upon regulatory approval.
“By bringing together our newest innovations with Asahi’s leadership and expertise in the Japanese medical device market, we can provide physicians in Japan with technology that has been proven highly effective, helping patients to return home quickly and in good health,” said Adam Elsesser, president and chief executive officer of Penumbra.
Penumbra’s Indigo Aspiration System can be used to remove emboli and thrombi from vessels of the peripheral arterial and venous systems, and for treatment of pulmonary embolism. A minimally invasive device, Indigo enables the restoration of blood flow in such cases as acute limb ischaemia and venous thrombus.

Penumbra’s newest generation offering combines hypotube-based Indigo Aspiration Catheters with Lightning Intelligent Aspiration, a unique computer-aided clot detection technology that can differentiate between clot and blood, designed to reduce blood loss and the need for clotdissolving drugs, which may lower the risk of bleeding complications.
“Our companies share the mission of helping expand the available treatment options for serious conditions that impact an increasing number of people in Japan,” said Masahiko Miyata, president and chief executive officer of Asahi Intecc. “We believe that, together, we are able to provide physicians with the latest innovations that best meet their patients’ needs and help advance patient care.”
Penumbra’s neurovascular thrombectomy and neurovascular and peripheral embolization devices will continue to be distributed by its longtime partner, Medico’s Hirata. This includes the latest RED reperfusion catheters, which are designed with optimised trackability and aspiration efficiency to help navigate the complex anatomy of the brain and deliver efficient aspiration for the removal of blood clots in a broad range of stroke patients.
Medtronic announced that it has recently received CE mark for its Radiant balloon-expandable covered stent, the first and currently, only, covered stent indicated for use in chimney endovascular aneurysm repair (ChEVAR) with the Endurant II/IIs stent graft system.
Used together, the Endurant II/IIs stent graft and Radiant covered stent offer a standardised, fully on-label, off-the-shelf solution for short-neck, juxtarenal abdominal aortic aneurysms (AAA). This enables safe and effective endovascular repair in both urgent and elective juxtarenal AAA cases.
The Radiant product’s design offers predictable, accurate delivery and deployment, while providing the flexibility and radial strength necessary for a chimney covered stent.
The covered stent is intended to maintain perfusion to the renal arteries when used in combination with the Endurant II/IIs stent graft system for AAA patients with inadequate sealing zones.
Vascade vascular and venous closure devices gain CE mark
Haemonetics Corporation has received CE mark certification for its Vascade vascular closure and Vascade MVP venous vascular closure systems.
The CE marking will allow Haemonetics to engage in the next steps of country-specific entrance of both products into the European Union (EU) and forms the basis for entry into other geographies that recognise CE marking, the company said in a press release.
“The acquisition of the GoBack catheter marks the start of inorganic growth for Bentley,” said Sebastian Büchert, Bentley’s CEO. “We launched our first of six existing product families to the market in 2012 and have experienced significant growth since then. Now, only 10 years later, we are able to acquire this very innovative product. This strategic move further completes our product offering to hospitals and physicians to the benefit of our joint patients.”

The GoBack catheter will simplify crossing and re-entry in occluded arteries. A press release notes that the product’s unique feature is a curved nitinol needle that can be manipulated by the physician using the device handle. Thanks to its small profile, the device can even be used in the smaller arteries in the lower leg.
With this CE mark, Medtronic will roll out a comprehensive training programme and continue to invest in clinical data to optimise ChEVAR outcomes for physicians and the patients they treat.
“Having a stent graft specifically approved for ChEVAR reflects a milestone towards standardisation of the technique and the materials used. In combination with the strict preoperative protocol regarding planning and sizing, ChEVAR will stabilise its role and approach in the existing endovascular alternatives to treat aneurysms with inadequate infrarenal sealing zones,”

Konstantinos Donas, head of the Department of Vascular Surgery and director of the Research Vascular Centre, Asklepios Clinic Langen, University of Frankfurt (Frankfurt, Germany) said of the Radiant stent graft.
In partnership with Getinge
The Radiant product comes from the long-term collaboration between Medtronic and Getinge, leveraging Getinge’s proven Advanta V12 design and eight sizes of the Getinge (Atrium Medical) stent portfolio. It will be produced by Getinge and distributed by Medtronic.
The Vascade system is designed for “small-bore” femoral arterial and venous closure, generally used in interventional cardiology and peripheral vascular procedures. The Vascade MVP system is designed for “mid-bore” multi-access femoral venous closure—generally used in electrophysiology procedures— and is the only US Food and Drug Administration (FDA)-approved closure device for use following cardiac ablation procedures requiring two or more access sites within the same limb. Both devices include proprietary collapsible disc technology and a resorbable collagen patch to achieve hemostasis. Vascade and Vascade MVP are designed to save time for hospital staff, while helping patients reach haemostasis faster with fewer complications on average.
In 2021, Vascade MVP received an FDA indication for same-day discharge following atrial fibrillation (AF) ablation. While not all countries in the EU are able to offer same-day discharge to patients, all patients can benefit from the quicker time to ambulation and reduced discomfort that this product provides.
“We see a significant opportunity to bring the advantages of our unique vascular closure products to hospitals within the EU, and look forward to taking steps towards commercialising in the market,” said Stew Strong, president, Global Hospital at Haemonetics. “With the VASCADE portfolio earning CE mark certification, we can meaningfully improve hospital operations throughout the region and further our goal of raising the standard of care for patients around the globe.”
“Bentley acquiring the GoBack makes me feel proud. I am sure that with Bentley’s global footprint more physicians will have access to the catheter,” said Dan Rottenberg, CEO of Upstream Peripheral Technologies. “Our vision is to save limbs and reduce the number of amputations. With Bentley’s strong network more patients will have access to such treatment.”
The GoBack catheter is CE marked and US Food and Drug Administration (FDA) cleared. Launched in 2019, the device is now available in 23 countries. It is Bentley’s intention to extend the global availability to all of the 80 markets in which the company is already active, the press release details.
Martijn Nugteren, director Sales & Marketing, Bentley, says, “We will be really busy in the months ahead. Not only because we want to make the product commercially available in additional markets, but also due to the fact that we are going to rebrand the GoBack to BeBack. This is to make sure that our new product will be recognised as another leading product underneath Bentley’s brand umbrella.”
Currently produced in Israel and initially shipped from there, a production transfer to the Bentley production facility in Hechingen, Germany, will be completed by 2025.
36 Market Watch November 2022 | Issue96
Indigo system with Lightning 7
Bentley celebrates the acquisition of the GoBack catheter
Vascade system
Radiant balloonexpandable covered stent
Product
Merit Medical launches the Prelude Roadster guide sheath
Merit Medical Systems has announced the US commercial release of the Prelude Roadster guide sheath. The Prelude Roadster is the newest addition to the Merit Vascular-Peripheral Access portfolio, which includes introducers and other products, including access kits, vessel dilators, and accessories.
The device is designed for deliverability, visibility, and resilience in tortuous peripheral vasculature anatomies. Indicated for use in a variety of procedures, the device will also help deliver devices used to diagnose and treat the large patient population who suffer from peripheral artery disease (PAD).
“Patients with peripheral vascular disease often have complex vessel anatomies that make treatment challenging,” said Sandeep Nathan, interventional cardiologist and director of the Coronary Care Unit at the University of Chicago Medicine (Chicago, USA) and a consultant of Merit Medical. “The Roadster sheath is designed to help physicians deliver the diagnostic and therapeutic devices needed to achieve successful outcomes.”
“The Prelude Roadster is an example of Merit’s commitment to understanding, innovating, and delivering products to meet the needs of physicians. After decades of offering vascular access products, we are always searching for ways to help physicians diagnose and treat successfully, improving the lives of patients worldwide,” said Fred P Lampropoulos, Merit Medical’s chairman and CEO.
Viz.ai receives FDA 510(k) clearance for automated RV/ LV analysis algorithm Viz.ai recently announced it has received US Food and Drug Administration (FDA) 510(k) clearance for an automated right ventricle (RV)/ left ventricle (LV) ratio algorithm, a new component of the Viz pulmonary embolism (PE) solution.
According to a company press release, the algorithm is designed to quickly and accurately measure the diameter of the ventricles of the heart to provide the ratio of the maximum RV diameter versus the maximum LV diameter.

Launched in November 2021, Viz PE uses deep learning to identify suspected central and segmental pulmonary emboli. With the integration of the RV/LV algorithm, the solution now includes an automated assessment of potential right ventricle dilation. Viz. ai claims that the information further enables care teams to ensure that the right clinical decision is made at the right time—no matter where the patient resides in the healthcare system—to ensure quick and appropriate care.




“The Viz.ai automated CT [computed tomography] scan clot detection system improves diagnostic acumen and expedites care for patients with acute pulmonary embolism,” said Kenneth Rosenfield (Massachusetts General Hospital, Boston, USA), co-founder of the PERT Consortium. “But the true killer in patients with PE is failure of the right heart. With this clearance, the Viz PE Solution now includes both detection of clot in the lungs and degree of strain on the right heart. This will enable clinicians to quickly triage patients and treat them appropriately, by providing a powerful tool for early detection and risk stratification. This expedited critical decision-making will undoubtedly save lives.”
Terumo Aortic announces new technology add-on payment For Thoraflex Hybrid device in the USA Terumo Aortic recently announced that the US Centers for Medicare and Medicaid Services (CMS) has granted approval of a new technology add-on payment (NTAP) for Terumo Aortic’s Thoraflex Hybrid device under the inpatient prospective payment system (IPPS).
Thoraflex Hybrid is the first of its kind device used in frozen elephant trunk (FET) repair in the USA, according to Terumo Aortic. It was granted Breakthrough Device designation by the US Food and Drug Administration (FDA) in 2020 followed by FDA approval for commercial sale in the USA earlier this year.

The hybrid device allows patients with suitably limited disease to be treated in a single stage procedure rather than two procedures which has previously been the conventional pathway in the USA for this group of patients, Terumo Aortic details in a press release.
The add-on payment will allow hospitals to be reimbursed for the incremental costs relating to the implantation of the Thoraflex Hybrid FET device to support the treatment of patients with complex aortic arch disease—this is in addition to the diagnosis related group (DRG) reimbursement.
37Market WatchIssue96 | November 2022
*Available for US and EU readers only **Available worldwide Subscribe today Available in print and digital formats and through our social channels Visit vascularspecialistonline.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** An official publication of the Society for Vascular Surgery (SVS) A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders Editorially independent Viz.ai mobile viewer for the Viz PE module
News
Industry News
Interventions, Boston Scientific. “The first analysis will focus on gaining a deeper understanding of the long-term patient outcomes relating to the use of our products indicated for treatment of PAD.”
Truveta announces collaboration with Boston Scientific to advance postprocedure patient insights and help address healthcare disparities

Truveta announced a strategic collaborative agreement with Boston Scientific to improve long-term patient care and gain insights into healthcare disparities. Through this collaboration, Boston Scientific researchers will be able to access data from Truveta, which contains de-identified medical records from more than 65 million US patients.
“Truveta will enable us to gather insights on a breadth of devices and disease states, including peripheral artery disease (PAD), venous thromboembolic disease, and segments of interventional oncology,” said Michael Jaff, vascular medicine specialist and chief medical officer and vice president, Medical Affairs, Innovation and Technology, Peripheral
Calendar of events
Calendar of events
24–26 October

CX Aortic Vienna 2022 Digital Edition Digital cxaortic.com
30–31 October
The VEINS Las Vegas, USA viva-foundation.org/veins-programming
31 October–3 November

Vascular InterVentional Advances (VIVA) Las Vegas, USA viva-foundation.org/
According to the US Centers for Disease Control, PAD affects approximately 6.5 million people aged 40 and older in the USA. This month is PAD Awareness Month, and Truveta recently shared new insights from its de-identified data, including findings that of the more than 11,000 PAD patients who received revascularisation procedures, Black or African American patients with PAD (1.0%) were less likely to undergo revascularisation than white patients diagnosed with PAD (2.6%). Of those who are treated with an interventional procedure, Black or African American patients were also less likely to receive drug-eluting stents than their white counterparts. Additionally, insights into major adverse outcomes, like lower-limb amputation, were discovered. For example, patients treated with drugeluting stents had lower rates (4.8%) of amputation compared to all patients who received revascularisation (8.5%).
“Device manufacturers realise it is critical to have data on the clinical outcomes for patients treated with their devices following regulatory approvals, yet those data can be messy, fragmented, and difficult to obtain and analyse,” said Terry Myerson, chief executive officer of Truveta. “Truveta solves this problem, empowering researchers with real-world data on how any drug or device is currently being used every day across the USA.”
Gore acquires InnAVasc Medical Gore has announced the acquisition of InnAVasc Medical, a privately held medical technology company focused on advancing care for patients with end-stage renal disease who utilise graft circuits for dialysis treatment.


Jeffrey Lawson and Shawn Gage from Duke University School of Medicine (Durham, USA) developed the InnAVasc device, which is specifically designed to allow for safe, easy, reproduceable and durable access for dialysis treatment of patients with graft circuits.
The investigational InnAVasc device is designed to protect the graft from backwall punctures and reduce the damage associated with frequent needle sticks which occur over the lifespan of a dialysis graft. This can lead to circuit failure and shortened circuit life. “To be stuck with two needles three times a
week for haemodialysis for 52 weeks, that is 312 times a needle goes into a patient’s graft each year,” said Stephen Hohmann, vascular surgeon at Texas Vascular Associates (Dallas, USA). “So having a graft that has the ability to decrease risk potential and longterm injury is definitely something that would be a game-changer.”
“Backwall punctures and damage due to excessive needling are painful and can cause unwanted bleeding, delay or stoppage of treatment, and reduced graft durability,” said Prabir Roy-Chaudhury, professor of medicine at the Division of Nephrology and Hypertension at the University of North Carolina (Chapel Hill, USA). “I greatly appreciate how this technology is intended to be so patient-centric, addressing this important interface need, for both clinicians and home caregivers.”
“We see an array of synergies working with Gore. The company is well recognised for its advanced material capabilities. [It has a] long history of designing graft solutions […] used in dialysis access procedures today, and we are excited to collaborate on future innovations,” said InnAVasc CEO Joseph Knight.
15–19 November
VEITHsymposium New York, USA veithsymposium.org/index.php
23–25 November
Paris Vascular Insights (PVI) Paris, France parisvascularinsights.com/
23–25 November
The Vascular Societies’ Annual Scientific Meeting Brighton, UK vascularsociety.org.uk
23–25 March
IM Endo Forum – International Multidisciplinary Endovascular Forum Florence, Italy imendoforum.com
25–27 April
Charing Cross (CX) Symposium 2023 London, UK cxsymposium.com
6–9 June
Leipzig Interventional Course (LINC) 2023
Leipzig, Germany leipzig-interventional-course.com
14–17 June
Society for Vascular Surgery (SVS) Vascular Annual Meeting 2023 National Harbor, USA Vascular.org/vam-2023
Vascular News is a trusted, independent source of news and opinion in the vascular and endovascular world.


Sign up for a free print subscription* and e-newsletter subscription** www.vascularnews.com
for US and EU readers only ** Available worldwide

*Available
November 2022 | Issue9638 Market Watch Please be advised that the events listed below are subject to change. Please check the relevant website for further details as the event may be cancelled, postponed, or become a virtual event.
2023
Please be advised that the events listed below are subject to change. Please check the relevant website for further details as the event may be cancelled, postponed, or become a virtual event.
Through
long-term
with
have created an innovative aortic


and provide a long-lasting
With it, build your
that works for them.
aortic

















our
collaboration
you, we
portfolio.
patients’ trust
treatment
We’re your
ally. Learn more, visit gmd.cm/aortic-ally
YOUR EXPERTISE AND OUR INNOVATIONS KEEP PATIENTS LIVING THEIR EVERYDAY LIFE
Products listed may not be available in all markets. GORE, Together, improving life and designs are trademarks of W. L. Gore & Associates. © 2022 W. L. Gore & Associates GmbH 22566791-EN MAY 2022
Certainty
Every aspect of the TREO® Abdominal Stent-Graft System is engineered for performance.


As an EVAR device with dual proximal fixation and active leg fixation, TREO® offers the next advancement in abdominal stent-graft solutions.
Low Profile without compromise
Relay®Pro offers a low profile system without compromising performance. The platform utilizes the same stent design, materials and foundational Dual Sheath Technology of the proven Relay®Plus.
The Relay®Pro system has a wide range of sizes and tapers allowing each patient access to the right solution, every time.
Simplified TREO® Relay®Pro Now FDA Approved
Visit our website for more information on use, indications, contraindications, warnings/precautions and availability within your market. Manufactured by: Bolton Medical Inc, 799 International Parkway, Sunrise, Florida 33325, USA Product availability subject to local regulatory approval. ALL_0172-A Sep 21 • PM-05118 terumoaortic.com Discover solutions for every segment of the aorta
















































































































































































































































































































