Paclitaxel-coated devices: US FDA removes red flag after review finds data do not support mortality risk


In a letter to healthcare providers dated 11 July 2023, the US Food and Drug Administration (FDA) communicates that the risk of mortality associated with paclitaxel-coated devices to treat peripheral arterial disease (PAD) is no longer supported based on the totality of the available data and analyses.
This update signals a lowering of the red flag raised in a 2019 letter from the Administration—published in response to a meta-analysis that indicated a late mortality signal—warning that treatment of PAD with paclitaxelcoated balloons and paclitaxel-eluting stents was “potentially associated with increased mortality”.
Alongside the letter, the US FDA has updated its recommendations for healthcare providers regarding the use of paclitaxel-coated balloons and stents for PAD. As well as removing reference to the possibility of increased mortality with these devices, the amended guidance softens the language around the monitoring of patients who have been treated with paclitaxelcoated stents and balloons, stating that healthcare providers should continue ‘routine’ rather than ‘close’ monitoring of these patients, as had previously been stated.
The safety of paclitaxel—used in peripheral interventions to prevent restenosis—was called into question by data put forward in 2018 by Konstantinos Katsanos (University of Patras, Patras, Greece) et al that pointed to an increased risk of death at two and five years following the use of paclitaxel-coated balloons and paclitaxel-eluting stents in the femoropopliteal artery.

The FDA responded, notifying healthcare providers in early 2019 about a late mortality signal in patients treated for PAD in the femoropopliteal artery with paclitaxel-coated balloons and paclitaxeleluting stents. Their most recent update on the topic, prior to that shared on 11 July 2023, was posted in August 2019.
In its new update, the FDA notes that “additional data from the pivotal randomised controlled trials (RCTs) has become available,” and that the Administration has worked with device manufacturers and external stakeholders to develop the protocol and analysis plan for new data generation.
The FDA references the fact that device manufacturers collaborated in an updated meta-analysis, which included “additional studies, more complete vital status information, and longer-term follow-up compared to prior studies”. Patient
follow-up in these studies ranged from two to five years, the Administration notes, and led it to conclude that the updated RCT meta-analysis “does not indicate that the use of paclitaxelcoated devices is associated with a late mortality signal”. Furthermore, the FDA states that it also reviewed additional analyses of the risk for late mortality, including the SWEDEPAD trial interim analysis, the VOYAGER PAD study, the German BARMER Health Insurance study, the US Veterans Health Administration study and the Medicare SAFE-PAD study. “None of these studies, with mean or median follow-up ranging from 1.7 to 3.5 years, found a risk for late mortality associated with paclitaxel-coated devices,” the FDA communicates, adding that longer-term follow-up in several of these studies is ongoing.
Societies and industry express support for use of paclitaxel-coated devices
The day following the release of the FDA update, the Cardiovascular Interventional Radiological Society of Europe (CIRSE) published an editorial in CardioVascular Interventional Radiology (CVIR) expressing its support for the use of paclitaxel-coated devices in femoropopliteal disease treatment. The editorial, written and accepted for publication in CVIR before the publication of the FDA letter, refutes a mortality signal for paclitaxel.
Written on behalf of CIRSE’s Endovascular Subcommittee, the authors—including corresponding author Robert Morgan (St George’s University Hospitals NHS Foundation Trust, London, UK)—“advocate that the benefits of paclitaxelcoated devices used in the femoropopliteal segment in terms of increased primary patency and reduced TLR [target lesion revascularisation] warrant their use in the routine treatment of patients with femoropopliteal disease”.
The following day, the Society for Cardiovascular Angiography & Interventions (SCAI) issued a statement from

Continued on page 2
“With effective use of apron and ceiling and table shields, operator [radiation] doses can be reduced to the equivalent of one to two days of natural background radiation,” investigators write in the conclusion of a recently published study.

RICHARD W HARBRON (Royal Victoria Infirmary, Newcastle upon Tyne, UK) and colleagues— including senior author Bijan Modarai (King’s College London and Guy’s and St Thomas’ NHS Foundation Trust, London, UK)— recently shared this finding in a European Journal of Vascular and Endovascular Surgery (EJVES) Editor’s Choice paper.
“The radiation doses received by staff members performing fluoroscopically guided interventional procedures are potentially high, raising concerns of increased risk of cancer and noncancer effects such as cataracts,” Harbron et al outline in their introduction. They continue by noting that endovascular aneurysm repair (EVAR) procedures “are associated with especially high doses to both staff and patients, due to the procedural complexity and the thickness and density of the abdominal region”.
It was the investigators’ objective in the present study to estimate operator organ radiation doses from fluoroscopically guided infrarenal EVAR procedures, using
Continued on page 3
September 2023 / Issue 99 www.vascularnews.com
this
Featured in
issue: 9 Artificial intelligence Emerging technology Profile Jürgen Falkensammer
Additional data from the pivotal randomised controlled trials has become available.”
20 Deep venous valve technologies Addressing a “large unmet need worldwide”
13 Rohini J Patel Smoking cessation
Computer model indicates optimal shielding can reduce operator radiation doses close to “background” levels
Paclitaxel-coated devices: US FDA removes red flag after review finds data do not support mortality risk
Continued from page 1
its president, George Dangas (Icahn School of Medicine and Mount Sinai, New York, USA), who welcomed the Administration’s decision to remove restrictions on the use of paclitaxel-coated devices to treat PAD.

Dangas stated: “SCAI applauds the FDA for its decision to reverse restrictions on using paclitaxel-coated devices to treat [PAD], based on continued efforts to collect and analyse data related to the mortality risk for these devices.”
There was also a response from industry, with Boston Scientific—which manufactures the Eluvia drug-eluting vascular stent system and the Ranger drug-coated balloon—publishing a statement on 12 July. Michael R Jaff, chief medical officer and vice president, Peripheral Interventions, Boston Scientific, commented: “We are pleased that, after continued analysis of data and collaboration among a broad set of stakeholders, the FDA has determined the large body of long-term clinical data do not support an excess mortality risk for paclitaxel-coated devices used to treat patients with PAD.”
Andrew Holden (Auckland City Hospital, Auckland, New Zealand) shared with Vascular News that he had “mixed emotions” to reading the news of the FDA’s announcement. He first detailed his excitement that “globally, now, patients can benefit from these drug-coated devices”. He elaborated: “One of the things we often debated over the last three years or so was the validity of the original meta-analysis, but prior to that we had a huge body of evidence that clearly showed that paclitaxel-coated devices were associated with much better durability of endovascular treatment, lower incidence of restenosis, and therefore lower incidence of reintervention. And the sad fact is that hundreds of thousands of patients since 2019 have had unnecessary reinterventions and associated morbidity and even mortality with that. Now, we can look forward to an era where that no longer occurs.” He also spoke of feeling pride on reading the update. “I feel very proud to have been part of a group of physicians and industry leaders who have worked tirelessly and collectively to assemble and present the data that were needed to convince the FDA to make this recent update,” he said.


Finally, Holden expressed that he felt challenged by the FDA’s decision to remove the red flag on paclitaxel. “We often think of the cost to patients of this meta-analysis finding, but also the cost to industry and research. There is no doubt that our ability to advance these technologies has been halted for a number of years. This has cost
companies and physicians a huge amount of time and resources, and I am glad that we are now approaching an era where we can see ongoing development. But there are also other challenges—we have learnt that trial design can be improved, and we have also learnt to reassess the value of meta-analyses, particularly when they attempt to assess endpoints that are not the primary endpoints of the trials studied. These poorly performed meta-analyses potentially can do real harm,” he shared.
Holden summarised his reaction by stating that, overall, he is “very excited” to see this announcement. He said in closing: “I would like to thank the FDA for having the courage to make such a clear announcement, and the future for patients is looking a whole lot brighter.”
Updated meta-analysis “forthcoming”
Peter Schneider (University of California San Francisco, San Francisco, USA) also shared his thoughts on the FDA statement with Vascular News, noting the “unambiguous” nature of its message “in the sense that they do not see a mortality signal, they urge us to take good care of our patients […] and emphasise the need for concomitant medical therapy and [optimisation of] the treatment of atherosclerotic risk factors”.
“I think we can use paclitaxel now without hesitation, without the concern that there is some damage being done that contributes toward longterm mortality,” he added.
Schneider made specific reference to the FDA’s mention that they had reviewed “the totality of the data” in order to make their decision. He stressed that the amount of available data on the topic has grown “substantially” since the initial statements of concern that the FDA expressed and, in addition to published data, pointed out that an updated patient-level meta-analysis “will be forthcoming”. This will include additional five-year follow-up data and patients that had previously been lost to follow-up, he detailed. Concluding, Schneider shared his overarching opinion that “we should strongly consider incorporating drug-delivering therapies into our peripheral vascular procedures” following the FDA’s latest announcement. “I think this means a tremendous benefit to our patients who now can receive the most efficacious therapies we have.”


News in brief
The latest stories from the vascular world

n ARTIFICIAL INTELLIGENCE: Can we trust artificial intelligence (AI)? Will AI replace physician judgement? Tom Carrell (Barrington, UK), Richard Linder (Sandy, USA) and Randy Moore (Calgary, Canada) recently addressed these and other key questions during a CX Vascular Live roundtable discussion.

For more on this story go to page 4.
n PAD: There has been major progress in the treatment of peripheral arterial disease (PAD) globally in the last few years, “but there are still issues that need to be addressed”, Marianne Brodmann (Graz, Austria) told Vascular News for an advertorial, reviewing her experience as co-director of the recent BD PAD Summit. The summit was led by course chair Bruno Migliara (Pescheria del Garda, Italy), alongside co-directors Brodmann, Koen Deloose (Dendermonde, Belgium), and Marco Manzi (Abano Terme, Italy).
For more on this story go to page 15.
n CAROTID STENOSIS: Interim results from the ECST-2 randomised controlled trial (RCT) have shown no evidence that carotid stenosis patients with a low-to-intermediate stroke risk, treated with optimised medical therapy, will benefit from additional carotid revascularisation via carotid endarterectomy (CEA) or carotid artery stenting (CAS). Paul Nederkoorn (Amsterdam, The Netherlands) presented these late-breaking findings at the 2023 European Stroke Organisation Conference. For more on this story go to page 25.
Editor-in-chief: Roger Greenhalgh | Publisher: Roger Greenhalgh | Content Director: Urmila Kerslake | Head of Global News: Sean Langer
Editor: Jocelyn Hudson Jocelyn@bibamedical.com | Editorial contribution: Jamie Bell, Will Date, Bryan Kay, Éva Malpass, Benjamin Roche and Clare Tierney
Design: Terry Hawes, Wes Mitchell and David Reekie
Advertising: Rav Pankhania Rav@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
September 2023 | Issue99 2 VascularNews linkedin.com/company/Vascular-news @VascularNews Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd | BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323 Printed by: Buxton Press Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2023. All rights reserved.
you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
If
Cover Story
“The future is looking a whole lot brighter”
I think we can use paclitaxel now without hesitation, without the concern that there is some damage being done that contributes toward longterm mortality.”
Peter Schneider
Peter Schneider
Continued from page 1
detailed exposure information contained in radiation dose structured reports.
Harbron and colleagues share in their methods section that they calculated conversion factors relating to kerma area product (PKA) to primary operator organ doses using Monte Carlo methods for 91 beam angles and seven X-ray spectra typical of clinical practice. The investigators wrote a computer programme that was designed to select the appropriate conversion factor for each exposure listed in a structured report and multiplied it by the respective PKA, and detail that this system was used to estimate operator doses for 81 EVAR procedures for which structured reports were available. Harbron and colleagues state that they also investigated the impact of shielding scenarios and variations in operator positions.
Writing in EJVES, the authors report
that the median estimated effective dose without shielding was 113μSv (interquartile range [IQR] 71–252μSv) and specify that the highest median organ doses were for the colon (154μSv, IQR 81–343μSv) and stomach (133μSv, IQR 76–307μSv). They note that these dose estimates represent all exposures, including fluoroscopy and nonfluoroscopic digital acquisitions.
The authors continue that, with minimal shielding provided by 0.25mm of lead (Pb) covering the torso and upper legs, the effective dose was reduced by a factor of around six, and that—with additional shielding from ceiling and table shields—a 25- to 50-fold reduction in dose is achievable. Finally, they share that estimated doses were highest where the primary beam was pointed directly away from the operator.
In this discussion of their findings, Harbron et al stress that their results “need to be interpreted in terms of typical yearly caseloads”. To this point, they reference surgeon-specific clinical outcome data collected by the National Vascular Registry for 533 vascular surgeons in the UK. They specify that the latest available data, covering the period 2017–2019, “suggest that the median number of infrarenal aortic aneurysm repairs performed by a surgeon as primary operator was 18”. However, they also note that the range
in number of procedures performed by a single surgeon was 1–95 “due to subspecialisation and centralisation”. They add that operators who perform “more complex, longer” fenestrated and branched EVAR procedures are exposed to even greater levels of radiation. “High-volume specialists may perform over 100 of these procedures on top of their annual workload of 20–30 infrarenal EVARs,” they write. Harbron and colleagues also highlight that it is also possible some operators may be genetically more susceptible to the effects of radiation exposure.
In the discussion of their findings, the investigators mention that the procedures described in their study were all performed in a hybrid vascular
suite with a fixed imaging system. They note that previous studies have shown these fixed systems may be associated with higher patient and occupational radiation exposures than a traditional C arm. However, they stress that this effect “may be offset by reducing operator time and advanced radiation reducing technologies such as fusion imaging”. The authors address a number of errors and uncertainties in their research. For example, they write: “If beam angle and X-ray energy can be accounted for in dose estimation, the largest source of uncertainty is operator position, which could easily range from -50% to +100%. The relationship between operator position and dose cannot be modelled by a simple application of the inverse square law because of the extended and non-isotropic nature of the scattering source.” As a result of this, they stress that their results “should be regarded as applicable only to the primary operator standing at the femoral access site”.
Considering what their paper adds to the literature, the authors write that it “improves estimates of the radiation doses to primary operators during fluoroscopically guided aneurysm repair by taking into account beam angle and X-ray-specific information for each individual exposure during the procedure”. They anticipate that their study “will help operators ensure their exposure is kept as low as reasonably achievable and will raise awareness of dose reduction strategies”.
3 Issue99 | September 2023
If beam angle and X-ray energy can be accounted for in dose estimation, the largest source of uncertainty is operator position, which could easily range from -50% to +100%.”
Computer model indicates optimal shielding can reduce operator radiation doses close to “background” levels
Journal Highlight
With additional shielding from ceiling and table shields, a 25- to 50-fold reduction in dose is achievable
25-
50-fold
“A hugely exciting time”: Experts focus in on artificial intelligence in the vascular field
Can we trust artificial intelligence (AI)? Will AI replace physician judgement? Experts recently addressed these and other key questions amidst an expansion of AI technologies in the vascular space.

AI is “here to stay”. This is according to Randy Moore (Calgary, Canada), vascular surgeon and chief medical officer (CMO) of ViTAA Medical Solutions, during a recent CX Vascular Live roundtable discussion. Moore was joined by Richard Linder (Sandy, USA), chair and chief executive officer of Xenter, and Tom Carrell (Barrington, UK), founder and CMO of Cydar Medical and formerly a vascular surgeon, to examine the topic of AI in the medical field.
“Can we trust AI?” was Moore’s opening question to Linder and Carrell, having stated that the discussion would focus on some of the “more controversial” aspects of the topic.
Linder’s response was twofold: “not yet” and “it depends”. He elaborated: “I think […] the quality of data, the size of the data, the transparency […] and the sourcing of the data, and how these algorithms are drafted are very important pieces of that question.”
Carrell concurred that “it depends”. He views AI as an “enabling” technology, or a “complementary additional ability,” adding an extra layer of assurance on top of clinical judgement. However, Carrell warned, “you cannot trust anything—you
cannot trust your own clinical judgement 100%, you cannot trust the AI 100%”.
Moore summarised that perhaps AI should be categorised as a “trusted advisor”. On this note, however, he acknowledged that one of the main challenges here has to do with a “natural assumption” that, due to the “huge bodies of data” being entered, the outputs are truthful. “We have to make sure that the data that are being entered and being used to drive the algorithms are representative of the general population and free of bias,” he stressed.
Linder agreed with Moore’s point, sharing that he and the team at Xenter have looked at the concept of physical intelligence for gathering the real-time data that are needed to develop a clinical decision. These data are put into a healthcare cloud, he explained, offering a standardised, global alternative to datasets such as specific healthcare records or healthcare systems.
At Cydar Medical, Carrell shared that the team is using data generated through real-world product use to address the issue of bias. Moore added that at ViTAA Medical Solutions, while the team is also focused on developing representative AI technology, “more importantly,” they
have built in “explainable” as opposed to “black box” AI. Moore explained that this allows the team to conduct an auditing process and ensures that physicians are aware of the outputs that are being generated so they can make a more informed clinical decision.
The conversation then moved on to whether AI will ever replace physician judgement. “No,” was Carrell’s short answer, reiterating his belief that the two are “complementary”. He detailed: “I think there are things that humans can do and will be able to do better than AI, things that are kind of outliers where you are relying on other bits of information, other bits of experience.”
Carrell does believe AI has a place, however. In contrast to what he summarised as the “plasticity” inherent in human decision-making, AI is “very good at doing some things that humans find time consuming and laborious”.
Linder continued that the diagnostic capabilities of a physician—honed by often decades-long experience of seeing patients—is “not going to be replaced by AI”. He used the example that the tone of how a patient might say ‘I feel great’ could vary from laboured to upbeat, which are “two totally different responses”. He stressed that AI in the form of voice recognition would simply not pick up such nuanced details.
While Moore concurred that physician judgement will never be replaced by AI, he warned that physicians who do not incorporate AI into their practice will be replaced by those who do.
“Completely,” Carrell said in agreement, adding that AI “is here today, it is coming, and we are seeing it around in all sorts of elements in our daily life”. He equated ignoring AI with ignoring the advent of multidisciplinary teams, or the advent of good clinical practice.
According to Linder, AI could be put to beneficial use in the development of clinical decision support tools. In this capacity, it might be able to answer some key questions, Linder communicated, including, for instance, the accuracy and size of a dataset. “There will be a value placed on those decision support tools,” Linder posited, “and I think that is kind of where we all have to focus for each of our companies and products too.”
The discussion then turned to accuracy, with Moore posing the question of how to deal with AI that “hallucinates”. Carrell responded by noting that, at Cydar, the team makes sure the information that is being presented is “visually inspectable”. He elaborated that, as Cydar technology deals primarily with imaging data, it is visually presented to the user so they can fact check it, leading the user to ask the pertinent questions: “Does that make sense? Is that matching up to what I am expecting to see?”
Linder continued that he and his team use “multiple modalities” to validate the accuracy of any AI-generated data. The combination of intravascular ultrasound (IVUS), optical coherence tomography (OCT) and angiography can be used to “tri-register,” for example— where multiple imaging modalities are partnered with physiological data and presented in a simple way to enable fast decision-making. “That is the type of platform technology that we are trying to develop,” Linder noted.
Finally, the trio considered some of the challenges ahead for AI. Linder described data security as “probably one of the most significantly looming issues” in the field. “Patients ultimately own their data, and they want to make sure that they receive their data, that it is secure, and that they can understand and comprehend the clinical decisions [made],” he stressed.
In addition to this, Moore pointed out the fact that, while there are clinicians who are interested in bringing technology forward, AI “adds an extra layer of complexity to the processes that already require complex [quality management system] processes”.
Taking a step back to look at the broader picture, Carrell opined that this is “a hugely exciting time”. He expressed his hope that “we have finally got the tools” to address some challenges that have been around for a quarter of a century or so.
Linder, while agreeing with Carrell, also cautioned that the power of predictive data and the regulatory side of things “need to catch up,” but postulated that when it does, AI will have a big impact. “We just need to keep an eye on […] clinical outcomes and make sure that we are improving those,” he stressed.
4 September 2023 | Issue99 Feature
L-R: Tom Carrell, Richard Linder and Randy Moore
We just need to keep an eye on […] clinical outcomes and make sure that we are improving those.”
Richard Linder
Our decades of expertise in providing cardiac and vascular surgeons with solutions to treat patients with aortic diseases—coupled with our recent acquisitions and partnerships— have collectively empowered our intentional focus on offering life-changing aortic-centric technologies.
Advancing
Learn more at artivion.com Artivion, CryoLife, and Jotec are trademarks owned by Artivion, Inc. or its subsidiaries. © 2022 Artivion, Inc. All rights reserved. Note: All products and indications are not available/approved in all markets. MLENG1528.000 (2022-01)
Aortic Technologies with Purpose™
Novel drug candidate for slowing AAA growth demonstrates safety in humans
The local delivery of a glucose-derived compound in small- and medium-sized abdominal aortic aneurysms (AAAs) has been deemed safe, with “promising” early efficacy data indicating its potential in stabilising or slowing AAA sac growth. According to Stephen Cheng (University of Hong Kong), these first-in-human study findings— which he presented at this year’s Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM 2023; 14–17 June, National Harbor, USA)—merit further evaluations within randomised controlled trials.
“The main message is that this is a group of patients where, currently, there are no effective treatments to slow the growth of [abdominal aortic] aneurysms,” Cheng tells Vascular News. “And, therefore, the idea of using a drug that is delivered only once inside the aneurysm sac sounds attractive—especially if it leaves nothing behind and all the future treatment options are left open.”
The multicentre study in question saw patients with an AAA (diameter <5.5cm) recruited to receive a one-time, local administration of 25ml of 1,2,3,4,6-pentagalloyl glucose (PGG) solution via transfemoral access. The study’s primary endpoints were technical success and safety—determined by the occurrence of major adverse events at 30 days. Cheng et al have reported a 100% rate of technical success, and found that the only safety-related concern was that four of the 21 enrolled patients showed a transient elevation of liver
enzyme levels. However, these levels returned to normal within 30 days and triggered no clinical symptoms.
“When we talked to the pharma scientists, who are really looking at the molecular aspects of why these drugs work, the answer was that PGG is largely metabolised in the liver,” Cheng adds. “This is a way that the liver responds to any [raised level] of glucose in the metabolism pathway. That is the explanation that has been given to us, but we have seen no adverse effects in the patients. It will certainly be an area we will be closely monitoring as to how patients behave afterwards but, so far, they are all fine and the one patient who did have a very high enzyme level returned to normal in about a month’s time.” With this being a first-in-human study, Cheng is quick to point out that any conclusions drawn from the results regarding efficacy of the PGG solution in slowing AAA growth are preliminary.
Prediction model offers new option for forecasting AAA growth
A predictive model incorporating 3D shape features such as aneurysmal flow lumen and outer wall structure extracted from computed tomography (CT) imaging can improve the ability to predict the growth of abdominal aortic aneurysms (AAAs).
THIS IS ACCORDING TO research presented at VAM 2023 by Anirudh Chandrashekar (Stony Brook Medicine, Stony Brook, USA), who tells Vascular News that the research has the potential to add to existing metrics for predicting aneurysmal growth, used to inform surveillance intervals and timing for surgical intervention.

Alongside senior author Regent Lee (University of Oxford, Oxford, UK), Chandrashekar and colleagues set out to develop a means of predicting aneurysm growth beyond the current gold standard—namely assessment of the diameter of the aneurysmal sac. “What we wanted to do was to devel¬op a prediction paradigm to individualise the follow-up and risk factor management for these patients,” Chandrashekar comments. “This may better inform the timing of surgery down the line.”
The first arm of the project saw the researchers develop a deep learning process to extract and categorise geometric features of the aneurysmal sac that may be associated with AAA growth, including curvature of the sac and surface irregularity.
The latest step of their research, which was presented here at VAM, looks at both the lumen and outer aneurys¬mal wall, and how these correlate with AAA growth. “The methodology is extremely novel, and this is the first time we are incorporating the lumen in this whole decision model,” says Chandrashekar.
To test their model, the researchers have conducted a retrospective analysis of serial CT images taken during surveillance in 192 patients with infrarenal AAAs, categorising
Nevertheless, as per their secondary endpoint of freedom from aneurysm sac enlargement, the researchers report average AAA diameter changes from baseline of 0.2mm, 1.1mm, 2mm and 0.8mm at six, 12, 24 and 36 months, respectively. Follow-up computed tomography angiography (CTA) data further indicated average volume changes of 2.5%, 9.6%, 24.3%, and 11.6%, respectively—and, at 12 months, none of the aneurysms had grown by more than 5mm in diameter, while only three had a volume growth >10%.
Prior studies have indicated that the average rate of AAA sac growth is around 3–3.5mm per year, according to Cheng. He states that this figure was used as a target in the present study, adding that the treatment threshold in Caucasians is an aneurysm diameter of 5.5cm and, “if we can slow the growth by 50% [to about 1.7mm], then we can push back the threshold for intervention from five years to 10 years, and that would bring expected benefits”.
“But, I must reiterate that this is a first-in-human study of a relatively small number of patients,” he adds. “The main focus was on patient safety rather than long-term aneurysm sac [growth].”
patients into either “slow” or “fast” cohorts based upon the progression of their aneurysm.
Chandrashekar reported that integrating the lumen and outer wall structure as unique components within the 3D statistical shape model captured the lumen-thrombus interface, marking this as superior to max diameter, undulation index and radius of curvature in the prediction of AAA growth phenotype, with a p-value of <0.001. “We can take a CT image, isolate the aneurysm section, extract out the aneurysm shape and extract measurements from the defined shape to a level that is clinically acceptable, and we can do this automatically,” says Chandrashekar of the top line messages of the research.
“Secondly, we are able to improve on already published metrics to predict
aneursymal growth, by incorporating not only the aneurysmal sac, but also how the flow lumen interacts with the surrounding thrombus and the surrounding aneurysmal wall,” he adds, commenting that this is “extremely novel in itself.”
Further work has been conducted to validate the model in an independent cohort of patients, which “excitingly,” according to Chandrashekar, shows that the growth model still holds to be predictive. “Going on from there the next step is to try to establish a prospective study, a longitudinal study following aneurysm patients over time, extracting out additional clinical metrics, for example blood pressure, medication regimen and all those metrics that you would obtain in a randomised controlled study, to see whether this model truly is effective.”
Senior author Regent Lee told Vascular News: “I congratulate Dr Chandrashekar in spearheading this international collaborative project and for delivering refinement of our AAA growth prediction model as an independent postdoctoral researcher.”
“The results presented here further highlight the concept of CT imagederived indices as ‘standalone’ biomarkers to predict AAA growth. With appropriate regulatory approvals, this can be further field validated at scale by utilising existing data already stored in the clinical picture archiving and communication systems (PACS) archives.”
6 September 2023 | Issue99 Conference Coverage
AORTIC
If we can slow the growth by 50% [to about 1.7mm], then we can push back the threshold for intervention from five years to 10 years.”
What we wanted to do was to develop a prediction paradigm to individualise the follow-up and risk factor management for these patients.”
Anirudh Chandrashekar
surveillance

low threshold for further interventions “crucial” following rescue of prior EVAR with PMEG



The findings of a recent study on reinterventions and sac dynamics after fenestrated endovascular aneurysm repair (FEVAR) with a physician-modified endograft (PMEG) for index aneurysm repair and following prior EVAR led researchers to conclude that “vigilant” surveillance and a low threshold for further interventions are “crucial” following PMEG for rescue of prior EVAR with loss of proximal seal.
NICHOLAS J SWERDLOW (Beth Israel Deaconess Medical Center, Boston, USA) shared these findings at VAM 2023 on behalf of senior author Marc L Schermerhorn (Beth Israel Deaconess Medical Center) and colleagues.
Swerdlow et al note in their study abstract that, while the high frequency of reinterventions after FEVAR with a PMEG has been well-studied, the impact of prior EVAR on reinterventions and sac behaviour following these procedures remains unknown. In the present study, therefore, the researchers analysed three-year rates of reinterventions and sac dynamics
following PMEG for index aneurysm repair compared with PMEG for prior EVAR with loss of proximal seal.
The investigators analysed 122 consecutive FEVARs with PMEGs at a tertiary care centre that was submitted to the US Food and Drug Administration (FDA) in support of an investigational device exemption (IDE) trial. They excluded patients with aortic dissection, type I–III thoracoabdominal aneurysms, non-elective procedures and prior aortic surgery other than EVAR, for a final cohort of 92 patients.
Patients were divided into those who underwent PMEG for index aneurysm
repair (index-PMEG) and those who underwent PMEG for rescue of prior EVAR with loss of proximal seal (rescue-PMEG).

Results
Swerdlow shared with the audience that, of the 92 patients included in the analysis, 55 (60%) underwent index-PMEG and 37 (40%) underwent rescue-PMEG. He added that rescuePMEG patients were older—78 years (interquartile range [IQR] 75–83) vs. 73 years (69–78), p<0.001. Otherwise, there were no statistically significant differences in baseline demographics and procedural characteristics.



The presenter reported that perioperative mortality was 1.8% for index-PMEG and 2.7% for rescuePMEG (p=0.8) and that, at three years, overall survival was 83% for indexPMEG and 72% for rescue-PMEG (p=0.08).
In addition, he noted that freedom from reintervention was significantly
higher for index-PMEG than rescuePMEG, specifically 79% vs. 45% at three years (p<0.001).
Swerdlow then shared sac dynamic findings. He revealed that, at three years following index-PMEG, aneurysm diameter was stable in 58% of patients and decreased in 42% of patients, with no cases of sac expansion.
At three years following rescuePMEG, however, he noted that aneurysm diameter was stable in 31% of patients, decreased in 31% of patients and increased in 38% of patients (p=0.05).
Conclusions
The presenter stated in his conclusion that FEVAR with PMEGs for index aortic repair and rescue of prior EVAR with loss of proximal seal are “two distinctly different entities.”
He summarised that, following FEVAR with a PMEG for index aneurysm repair, less than a quarter of patients had undergone reintervention at three years and sac expansion was “rare”.


At three years following PMEG rescue of prior EVAR with loss of proximal seal, however, it was observed that over half of patients had undergone reintervention and over a third had ongoing sac expansion, which led Swerdlow to underscore the importance of “vigilant” surveillance and a low threshold for further interventions in this group of patients.



“Vigilant”
and
Freedom from reintervention was significantly higher for index- than rescue-PMEG.”
*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the cardiovascular field Editorially independent Available in print and digital formats and through our social channels Visit cardiovascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today 7 Issue99 | September 2023 Device Updates
Marc Schermerhorn




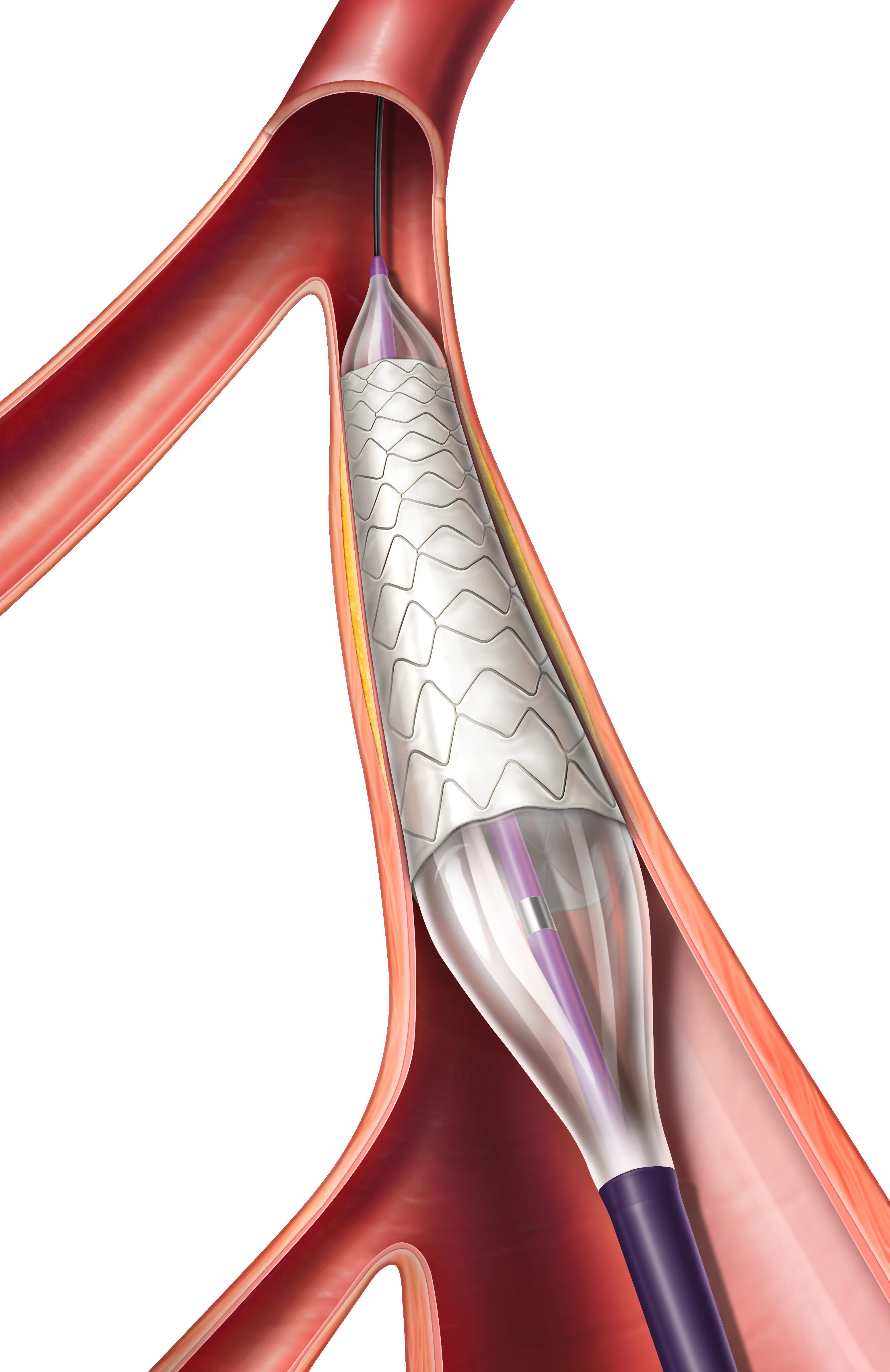
* Evidence from pre-clinical studies. ACS Biomater Sci Eng 2020 6 2588–2599. INDICATION: The IMPEDE Embolization Plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature. INDICATION: The IMPEDE-FX Embolization Plug is indicated for use with the IMPEDE Embolization Plug to obstruct or reduce the rate of blood flow in the peripheral vasculature. CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Shape Memory Medical and IMPEDE are registered trademarks of Shape Memory Medical. Generates new healing possibilities www.shapemem.com Returns clarity Conforms to the anatomy Delivers unmatched volume LIT1142 Rev A Regenerative smart polymer stimulates the immune response and healing process to promote healthy new cellular growth as the material slowly bioabsorbs without chronic inflammation* Generates new healing possibilities Beyond embolization IMPEDE® Embolization Plug IMPEDE-FX Embolization Plug
Postcode matters: Residing in areas of economic distress related to poor EVAR outcomes
Ahsan Zil-E-Ali
A new study in the Journal of Vascular Surgery (JVS) finds that a patient’s postcode is a crucial indicator for advanced stages of an abdominal aortic aneurysm (AAA) at the time of endovascular aneurysm repair (EVAR), with a higher risk of mortality and an effect on long-term follow-up. Ahsan Zil-E-Ali and co-authors (Penn State University, Hershey, USA) have analysed the largest vascular surgery quality improvement dataset in the USA and present the importance of a patient’s postcode in the outcomes of EVAR for AAA.

According to the US Census Bureau, 21.1% of the US population lives in impoverished areas with wide ranges across different states. For understanding this areabased poverty, the Economic Innovation Group (EIG) provided a more comprehensive understanding of the US population through the distressed communities index (DCI) that this study in JVS utilised to analyse the EVAR outcomes. This index incorporates education, housing, employment, poverty, income, change in jobs, and business establishments to understand a
community—and gives a score to each postcode ranging from 0–100, of which the higher the score, the more distressed the community is.
The present study is the first in the USA that analyses almost 60,000 EVAR patients to investigate the outcomes based on postcode DCI and presents alarming findings requiring attention. These findings demonstrate that patients living in areas with economic distress have relatively higher number of comorbidities, including hypertension and coronary artery disease, and receive care for symptomatic AAA,
which indicates an advanced disease with a higher mortality risk. It was also perturbing that patients in these distressed communities may also be lost to long-term follow-up after EVAR, limiting our ability to ensure optimal care in case of sac expansion or any other complication. Another observation was that these patients also have a higher probability of declining functional status at long-term follow-up, implying a much higher disease burden in these populations.
These observations provide important insights into understanding the prevailing socioeconomic disparities in the USA in the context of vascular surgery and a patient’s residence and inform us about the care gaps that may be intervened upon to mitigate the burden. These gaps can stem from limited insurance options, lack of an established primary care provider, access to early screening, geographic proximity to medical centres, or general health knowledge. A detailed needbased analysis of the communities and their available resources by the locals collaborating with experts can not only assist in guiding the inhabitants of economically distressed areas but can
facilitate the preparedness of healthcare services.
The inferior outcomes reported in this study are just the tip of the iceberg, as these inequities result from systemic biases, health illiteracy, and even access to preventive care. The solution to these disparities may not be straightforward and could require tailored approaches for each disease and specialty; however, vascular surgery patients, particularly
with AAA may require early screening, health awareness, and enhanced communication with patients, which could be essential first steps. In the surveillance period for EVAR, special arrangements should be discussed for patients with limited resources to ensure equity can be upheld along with the delivery of standard care. Connecting with other healthcare stakeholders, including policymakers and institutions, can help vascular surgeons advocate for their patients at various stages of care and address restricting factors found in low socioeconomic communities. This study unwraps many avenues of investigation in understanding the US population based on postcodes, the needs of poverty-stricken patients requiring vascular surgeries, and most importantly, the care gaps leading to disparities that require the noticing of everyone involved in healthcare delivery.
New
study reports positive performance of AI-powered assessment of biomarkers for growth prediction of AAAs
The characterisation of aortic tissue by means of three key biomechanics-based biomarkers bundled into a compound Regional Areas of Weakness (RAW) Map showed “very good performance” in an artificial intelligence (AI)-based prediction of faster than average growth for a population of abdominal aortic aneurysm (AAA) patients under serial monitoring, according to a new study published in the Journal of Vascular Surgery-Vascular Science (JVS-VS).
THE RETROSPECTIVE STUDY was conducted into 36 AAA patients undergoing surveillance with electrocardiographically (ECG)-gated computed tomography angiography
(CTA) at the University of Calgary in Alberta, Canada, by a team of researchers led by vascular surgeon Randy Moore and Elena Di Martino, a professor of biomedical engineering,
using the emerging ViTAA Medical Solutions technology which aims to provide an algorithm-driven route to precision care.
The RAW Mapping assessment of aortic weakness incorporates time-averaged wall-shear stress, in vivo principal strain and the intraluminal thrombus thickness, with the research team concluding from the published analysis that “the use of features based on the functional and local characterisation of the aortic tissue resulted in a superior performance in terms of faster than average growth prediction when compared to models mostly based on geometrical assessments”.
The technology deploys an AI model to predict accelerated aneurysmal
growth, with the current study looking at RAW Mapping’s ability to predict growth and AAA evolution within a year. Future work will focus on expanding the investigation and growth prediction over a longer surveillance period, the investigators reported. Further research will also aim to broaden the applicability of the methodology to different imaging modalities and protocols used to monitor AAAs.
“The ability to access functional information related to tissue weakening and disease progression at baseline for individual aortas has the potential to benefit patient monitoring, risk stratification and treatment selection, and optimise precision-based aortic care,” the researchers added.
9 Issue99 | September 2023 Opinion
Ahsan Zil-E-Ali is a postdoctoral research fellow at Penn State University (Hershey, USA).
Point of View
The inferior outcomes reported in this study are just the tip of the iceberg, as these inequities result from systemic biases, health illiteracy, and even access to preventive care.”
Patient voice central to new trial addressing nerve catheter PLACEMENT in major amputation care

David Bosanquet (South East Wales Vascular Network, Cardiff, UK) is chief investigator of a new randomised trial—PLACEMENT—which addresses the topic of pain control around the time of a major amputation. Here, he speaks to Vascular News about the importance of such a trial, the challenges associated with conducting a randomised study, and the central involvement of the patient voice in this new research.
What is the PLACEMENT trial about?
DB: The PLACEMENT trial will assess whether placing a nerve catheter—through which it is possible to administer five days’ worth of local anaesthetic— impacts pain and other outcomes compared to no nerve catheter in patients who have undergone a major amputation. In terms of available data on the topic, we previously conducted a meta-analysis, and there are observational and some relatively poor-quality randomised data out there. However, there are no good, solid, robust data to conclusively evaluate if a nerve catheter improves outcomes, which is why we have developed this study: to answer that key question.
Why is this trial important?
DB: PLACEMENT is a good opportunity to educate readers about the importance of good pain control around the time of amputation. Good pain control was recently picked up as a key priority in the James Lind Alliance priority setting. We asked patients, carers and healthcare professionals, ‘What are the important research priorities in amputation and how do you order them?’ Priority number four was highlighted as ‘What are the best ways to prevent or treat pain, including phantom pain, after amputation?’ So, pain control has been identified as an important priority when it comes to research around amputation. In addition, although I do not have conclusive evidence for this, anecdotally amputation surgery has received significantly less interest from the vascular research community compared to aortic interventions, stents for peripheral vascular disease or carotid surgery. Moreover, looking at something like money spent in big randomised controlled trials (RCTs) or recruitment to RCTs, you find that a number of patients go into RCTs for carotid surgery and lower limb intervention, but very few are recruited for amputation surgery trials.
Could you outline how the study links with the Limbless Association Volunteer Visitor Scheme?
DB: During study setup for PLACEMENT, we put together focus groups of patients and presented the research to them. We asked about things like primary outcomes and how we should collect data, and the groups provided us with some really important feedback. A few individuals spoke about their time during amputation surgery, and they said that while the clinical team can inform them about risks of surgery, when it comes to quality of life, that information is often not given to patients, as we do not have lived experience as clinicians. That prompted us to set up a smaller working group to
find out if this was something that we could address. We then found out that the Limbless Association Volunteer Visitor Scheme already fills this gap, and so we linked with them. The Limbless Association now formally supports PLACEMENT. As a direct result of the patient input from these focus groups, every recruiting participant to PLACEMENT will be given information about the Limbless Association Volunteer Visitor Scheme, which is free. Within the scheme, every patient gets buddied up with somebody who has already had an amputation, and they get time to talk through the process with them either before or after surgery, about how it will affect their life. These are people who are experts in their own right, and they can provide invaluable information about what life is really like after an amputation, how to engage services which support people after amputation, and direct patients towards other avenues of the scheme’s support network. I think that is really exciting. We speak to patients before every study, and they have an integral part in shaping and designing them, but for them to play such a major role is really quite interesting. We are hoping to do a bit of extra research around this point, asking patients to engage with the programme and tell us whether or not they find it helpful and why.
Across the UK there may be centres where this is
done already and done very well, but certainly, there will be lots of centres where this is done either on an ad hoc basis or hardly at all.
In June, the Limbless Association celebrated three years of their National Lottery funding, and PLACEMENT was presented at that meeting. This is a randomised control trial— could you outline some of the challenges associated with conducting this type of research?
DB: I think the biggest challenge is going to be the equipoise of treating clinicians. There is a widely held view that catheters are of benefit in patients who have undergone an amputation, and so it will be challenging trying to get that balance between doing what you think is best for the patient based on the limited data that are available compared to the longer-term view and broader question about providing robust evidence to base practice on. Equipoise is a challenging issue, and, unsurprisingly, PLACEMENT is not exempt from that challenge.

It is important to remember that there are lots of examples of RCTs done in vascular surgery and general surgery where it was thought ‘the answer’ to the study was a foregone conclusion, and yet when the study was conducted it was found that the opposite was true. Therefore, I do appreciate that there is that question mark over whether we should we just put catheters in every single patient, but we do not have good clinical evidence about their effectiveness, we do not really understand what has the impact on long-term follow up, and we do not know about cost-effectiveness.
In addition, there is significant variability in practice on an international level. We have had fellows come from countries such as Egypt and Bahrain and they have never seen a nerve catheter, for example. Even in the UK, we conducted a survey and found that approximately 60% of centres use a catheter most of the time. While that is not exactly a 50:50 split, there is clearly still variation within UK practice as to what people do.
When do you hope to start the trial?
DB: We recently received ethical and UK Medicines and Healthcare products Regulatory Agency (MHRA) approval and hope to commence the trial in September.
10 September 2023 | Issue99 Q&A
PERIPHERAL
We speak to patients before every study, and they have an integral part in shaping and designing them, but for them to play such a major role is really quite interesting.”
David Bosanquet

DELIVER PLACEMENT ACCURACY SEE OUR APPROACH When you reach for a balloon expandable covered stent, you require placement accuracy. The LifeStream™ Expandable Vascular Covered Stent was developed for the challenging anatomy of iliac arteries and engineered to facilitate accurate placement. Not for use in patients with uncorrected bleeding disorders or in patients who cannot receive recommended antiplatelet and/or anticoagulation. The LifeStream Balloon Expandable Vascular Covered Stent is indicated for the treatment of atheroslerotic lesions in common and external iliac arteries with reference vessel diameters between 4.5 mm and 12.0 mm, and lesion lengths up to 100 mm. Please consult product labels and instructions for use for indications, contraindications, hazards, warnings and precautions. BD, the BD Logo, and LifeStream are trademarks of Becton, Dickinson and Company or its affiliates. © 2023 BD. All rights reserved. © 2023 Illustration by Mike Austin. BD-86429
DESIGNED TO







*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the interventional field Editorially independent Visit interventionalnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
Significant life and limb gains for claudicants who stop smoking before lowerextremity bypass
LONG-TERM OVERALL survival (OS) and amputation-free survival (AFS) are outcomes that rebound in claudicants who quit smoking prior to elective surgery—and they mirror those of never smokers. But patients who do not kick the habit have significantly worse outcomes, lighting up the question: should stubbing out for good be “a requirement” before intervention?
That was the message from Rohini J Patel (University of California San Diego, San Diego, USA), who presented data at this year’s Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM 2023; 14–17 June, National Harbor, USA) on long-term outcomes in the smoking claudicant after elective lower extremity bypass. The findings were published simultaneously in the Journal of Vascular Surgery (JVS).

Patel noted that patients and vascular specialists must grasp that structured smoking cessation should be a more
prominent part of vascular office visits before and after lower extremity bypass and can “even be considered a requirement prior to elective procedures in claudicants”, a group that represents a unique non-emergent vascular patient population that can require lower extremity bypass.
Smoking is known to increase complications, including poor wound healing and coagulation abnormalities, and have cardiac and pulmonary ramifications, said Patel. “Across specialties, elective surgical procedures are commonly denied to active smokers. Given the base population of active smokers with vascular disease, smoking cessation is encouraged but is not required the way it is in general surgery,” she explained.
The research team queried the Vascular Quality Initiative Vascular
Implant Surveillance and Interventional Outcomes Network (VISION) database
over a 16-year period to tackle the question of how actively smoking claudicants fare after elective lower extremity bypass. They then carried out two separate propensity score matches on patient records that included 609 (10%) never smokers, 3,388 (55.3%) former smokers, and 2,123 (34.7%) current smokers who underwent bypass for claudication. One analysis examined the outcomes of former smokers and compared these to outcomes achieved in patients who had never smoked. The second analysis contrasted outcomes between current smokers and former smokers. The primary outcome measures included five-year OS, limb salvage (LS), freedom from target lesion reintervention (FTR) and AFS.
Health gains do not shift unless claudicants quit smoking
There were no differences recorded with respect to any of these measures between 497 well-matched pairs of former smokers and those who had never smoked. The second analysis, which focused on ascertaining outcome differences between 1,451 wellmatched pairs of current and former smokers, found that there was no
They first got together to develop the idea for a trial to compare open versus endo treatment for CLTI in 2007. “It really was the blind leading the just as blind,” quipped Menard. As Farber described, he personally had very little experience in clinical trials at the time. “There was a lot of insecurity,” he said. “Were we really the right people to do this?” he recalled thinking back then.
Upon receiving positive feedback from within the vascular surgery ranks, Farber related, they began the National Institutes of Health (NIH) application portion of their journey.
difference in LS or FTR, but revealed “a significant increase in OS and AFS” in former smokers compared with current smokers, suggesting that giving up smoking reaps rewards in terms of both limb and life preservation.
“Our study found that former smokers have better OS and AFS when compared to current smokers, while former smokers mimic never smokers at five-year outcomes for OS, LS, FTR and AFS,” Patel averred.
Patel states: “This study emphasises that we as providers must spend more time and effort working with patients to quit smoking prior to elective lower extremity bypass in claudicants.”
Limitations of the study include that the database contained no information on the duration or intensity of smoking among the study population.
According to senior author Mahmoud Malas (University of California San Diego): “Previous studies have shown that smoking cessation might not necessarily affect an immediate outcome in patients. We have found through this VISION database that even quitting one month prior to surgical intervention can change longterm outcomes.
“Former smokers do better than current smokers and former smokers mimic the results of patients who have never smoked. As vascular surgeons, we need to play a more active role in these discussions with patients in the clinic.”
THE PRINCIPAL INVESTIGATORS BEHIND the BEST-CLI trial struck a conciliatory tone during the inaugural Frank J Veith Distinguished Lecture at VAM 2023 in which they laid bare the blood, sweat and tears shed on their journey to complete the landmark study.
The headline findings that emerged out of the trial—that both open and endovascular procedures were equally safe, and that chronic limb-threatening ischaemia (CLTI) patients deemed suitable for either approach who had an adequate great saphenous vein experienced better overall clinical outcomes after open bypass surgery—sparked rancour in the interventional surgical communities.

Yet, Alik Farber (Boston Medical Center, Boston, USA) and Matthew Menard (Brigham and Women’s Hospital, Boston, USA) used the maiden Veith Lecture to shine a light down a path toward further advances in end-stage peripheral arterial disease (PAD) care. The pair used the platform to tell the story of their journey in order that others might take the leap of faith they did to tackle “impactful questions in science,” Farber told VAM 2023 attendees.
At first blush, as they prepared to go down the NIH route, they thought vascular surgeons should do the trial owing to the fact they carry out both procedural modalities.
Despite their arguments, the application failed. Yet, they persevered. They responded to the criticisms. Other specialties were included. More funding was secured. Some of that rancour seen in the aftermath of the release of their findings late last year emerged back then over trial design too. But eventually, the specialties united, and they got NIH grant approval.
They had many difficulties along the way. “This trial was extremely difficult to enrol,” said Farber. “There were multiple curveballs.” The Katsanos meta-analysis. COVID-19. Running out of money.
They got there, presenting at the American Heart Association and publishing the results in the New England Journal of Medicine. “We hope our story encourages others to pursue their research ambitions, even in the face of obstacles, self-doubt and judgment of others,” said Farber.
Menard paid tribute to the man for whom the new VAM lecture is named: Frank Veith (New York University Medical Center, New York, USA).
“It is impossible to [over]estimate how much of a maverick Frank has been, and the enormity of what he has contributed to the field,” he said. Menard sees a kindred spirit in Veith in the sense of how the limb-salvage pioneer looks at the foot. Menard, too, likes to try the less conventional. “But one thing I have not done, and Frank did it a long time ago: he did a prosthetic bypass to the foot,” said Menard. “It worked. Here is another thing that Frank did: 13 prior failed procedures—he did a bypass from the common iliac artery to the peroneal artery. And it worked.” Veith was also an endovascular believer before most had cottoned on to its potential, he said. Menard’s point was centred on progress in CLTI made over the decades. That extends to turf battles— endo versus open. “Hopefully, never more,” he said. “Turf battles in the vascular community have been and still are distracting, destructive, highly counterproductive and of very little service to our patients. I charge the audience to sincerely bring their part to fight an entirely new battle: that is to move beyond this 25-year-old paradigm of endo versus open.”
13 Issue99 | September 2023 Conference Coverage
BEST-CLI investigators implore a move beyond endo versus open “battle” in the name of scientific advance
I charge the audience to sincerely bring their part to fight an entirely new battle: that is to move beyond this 25-yearold paradigm of endo versus open.”
PERIPHERAL
Matthew Menard
Rohini J Patel
The pulmonary arterial tree—it is time we branch out

Nicolas J Mouawad (McLaren Health System, Bay City, USA) urges vascular surgeons to “get out of their comfort zone” and become more involved in pulmonary embolism (PE) care.
WITH OVER ONE MILLION cases of deep vein thrombosis (DVT) and/or PE diagnosed each year in the USA alone,1 the management of patients with venous thromboembolic disease (VTE) is a critical public health concern.2 So much so that in 2008, the US Surgeon General declared a formal call to action against VTE. Despite an initial modest increase in awareness, it has been the recent COVID-19 pandemic that truly erupted a flurry of VTE therapies and catapulted this pathology from the sidelines to center-stage. Although DVT and PE are a continuum of the same disease state, untreated acute PE has a mortality of 30%.3 The severity of impact is primarily based on the embolic burden and the resultant effect on the right ventricle (RV), in addition to underlying comorbid conditions. The vicious cycle commences with acute increases in pulmonary arterial pressure secondary to the embolic obstruction which increases right ventricular afterload, increasing RV myocardial oxygen consumption and impairing RV contractility. This in turn subsequently affects the left side manifest by decreased cardiac output that can eventually lead to cardiogenic shock and death.
Pulmonary embolism response teams (PERTs) have emerged as an effort to battle the crisis of pulmonary emboli. Akin to “doctor-heart” for ST-elevation myocardial infarctions (STEMIs) and code stroke activations, the PERT is a multidisciplinary team focused on early triage, assessment, risk stratification and rapid coordination of an organised response to mobilise resources as necessary for PE care. These traditionally have been composed of interventional medical specialties with surgical counterparts as backup. The role of the vascular surgeon in the management of PEs varies widely and is based on their interest, their comfort (particularly navigating the heart), geography and the institutional politics.
The main issue in my opinion is that it feels outside of our “comfort zone” to be in the thorax. Whether surgically or by endovascular means, the thorax has historically been a black box—a void—for the vascular surgeon. From an interventional perspective, it is a domain of cardiothoracic surgery, interventional cardiology and interventional radiology, among some others. Furthermore, dedicated training paradigms have not
been established for formal education in navigating the heart and the pulmonary vasculature for vascular surgeons. Most of us that are involved in PE care learned it from our interventional colleagues, training courses, or “on the job”. But why do we not take a more active role in this disease process? After all, we are vascular specialists very comfortable in diseases of the arteries, veins and lymphatics, whether medical, minimally invasive or maximally open.
And who gets an intervention?
Unfortunately, risk stratification of patients with PE remains in development. The most common system separates them into low risk, intermediate risk, and high risk. An in-depth evaluation of cost, resource utilisation, risk and safety profiles as well as clinical efficacy, such as HiPeitho, Peerless 2, STORMPE, PE-TRACTS, among others, are currently underway to help answer many of these questions.
For those that qualify for intervention based on currently used criteria, vascular access is obtained in the standard fashion with ultrasound guidance. Caval venography is performed to ensure no anatomic abnormality, thrombosis or clot in transit. The right heart is then catheterised—I am a fan of the angled pigtail more so than a balloon-tipped catheter such as the Swan-Ganz as I feel its shape mirrors the anticipated trajectory. For each one of my PE interventions, a full right heart catheterisation is performed. A comfort with waveform analysis traversing right atrium, right ventricle and into the main pulmonary artery is paramount. These are standard displays in a cardiac catheterisation laboratory although not usual in the operating suite, so depending on your site of care, it is
important to equip your lab with the ability to transmit and display these data. Clearly it will help monitor critical patient vitals and also assist in evaluating the effectiveness of some interventions. The procedure is then completed in the standard fashion. Just as we have adopted many new disruptive technologies for the management of our patients, the pulmonary vasculature is an extension of the vascular tree we are trained to treat. I submit that it is time we branch out in the pulmonary arterial tree and become comfortable navigating the heart. We are trained for quick decision making in high-stakes situations. It’s time to dust off our old physiology textbooks and revisit right heart pressures, pulmonary vascular resistance and dynes/sec! A multidisciplinary group is imperative for the management of patients with PE—and for a successful PERT—and vascular surgeons should get out of their comfort zone and play an active role in this patient population and pathology. We have a duty to
surgery
our trainees to develop training paradigms to tackle all components of vascular disease and offer a familiarity and applicability of endovascular concepts while addressing barriers to implementation. Through continued awareness, education, and support, we can help cement vascular surgery as an integral component of comprehensive PE care and focus on improving PE patient outcomes.
Nicolas J Mouawad is chief and medical director of vascular and endovascular surgery at McLaren Health System in Bay City, USA.

References
1. Lutsey PL, Zakai NA. Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol. 2023 Apr;20(4):248–262. doi: 10.1038/ s41569-022-00787-6. Epub 2022 Oct 18. PMID: 36258120; PMCID: PMC9579604.
2. US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism 2008. http://www.surgeongeneral.gov/topics/deepvein.
3. Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and non-thrombotic pulmonary embolism. Exp Clin Cardiol. 2013 Spring;18(2):129–38. PMID: 23940438; PMCID: PMC3718593.
14 Opinion
hematology vascular
cardiac
pulmonary/ critical care vascular medicine radiology
surgery
cardiology emergency
Nicolas J Mouawad
medicine
Whether surgically or by endovascular means, the thorax has historically been a black box— a void—for the vascular surgeon.”
Promising future previewed for PAD treatment at Riga summit
There has been major progress in the treatment of peripheral arterial disease (PAD) globally in the last few years, “but there are still issues that need to be addressed”, Marianne Brodmann (Medical University of Graz, Graz, Austria) told Vascular News, reviewing her experience as co-director of the recent BD PAD Summit (20–21 June, Riga, Latvia). She said the event highlighted the “consensus and strong will” among physicians to “improve” the picture when it comes to fighting the disease.
The summit was led by course chair Bruno Migliara (Pederzoli Hospital, Pescheria del Garda, Italy), alongside co-directors Brodmann, Koen Deloose (AZ Sint-Blasius Hospital, Dendermonde, Belgium), and Marco Manzi (Policlinico Abano Terme, Abano Terme, Italy).
Aimed at “shaping the future of PAD patient care”, its programme was divided into five sections. Sessions on diagnostic tools and algorithms were complemented by presentations on access and crossing lesions, treating lesions and optimising patency, and finally, on the future of PAD treatment. Across a day and a half, over 170 attendees were offered 24 hours of hands-on sessions, as well as the opportunity to hear 31 presentations by 20 key opinion leaders in the field. Rapid-fire debates followed most presentations, while a research and development session offered education on further new advances.
Brodmann opened the conference by addressing what she called the historical underappreciation of the problem of PAD—236 million adults worldwide have the condition—when compared with other conditions such as coronary artery disease and stroke.
More recently, she said, there has been “increasing recognition that PAD is an important cause of cardiovascular morbidity and mortality”, with particular attention given to its status as a “leading cause of physical disability”, especially when it progresses to critical limb ischaemia (CLI).
The problem is growing, she suggested, noting a study which found that the prevalence of the disease jumped by 45% globally between 2000 and 2015. “It can only be changed by awareness campaigns […] from healthcare providers as well as the general population,” she told Interventional News
Part of the issue, Brodmann suggested, lies with difficulties in diagnosing PAD. This set the stage for the beginning of the first session on diagnostic tools, where Christos Rammos (University of DuisburgEssen, Essen, Germany) highlighted a selection of new options in angiography, among them carbon dioxide angiography. Following him came Daniel van den Heuvel (St Antonius Hospital, Nieuwegein, The Netherlands), who assessed duplex ultrasound as a method for developing an “objective endpoint” in
the definition of optimal results from revascularisation procedures.
Algorithms came to the forefront next, with Brodmann stepping in to deliver the absent Maria Ruffino’s (Institute of Imaging of Southern Switzerland, Lugano, Switzerland) presentation on the need for clarity on the best aortoiliac lesion treatment. A compelling discussion followed, where it was suggested by one audience member that lack of evidence-based treatment in this area could be due to physicians’ preference. In conversation with Interventional News, Brodmann said: “This is indeed the case. Therefore, there is a need for creating strong treatment algorithms in different areas.” This, she suggested, was a need that could be met by physicians.

Deloose, meanwhile, also contributed to the session by telling the audience to base their treatment algorithms on their prepping algorithms—which should in turn be based on how you pass the lesion. PAD below the knee (BTK) was addressed during this session by Marc Sirvent (General University Hospital of Granollers, Granollers, Spain), who, in an assessment of different strategies, highlighted the “essential” nature of good angioplasty technique.
Next up came a set of talks on access and crossing lesions. Ralf Langhoff (Sankt Gertrauden Hospital, Berlin, Germany) set the tone by alerting listeners to the fact that up to 11% of peripheral vascular interventions result in access complications, underscoring the importance of addressing them. Also speaking in this session was Giacomo Isernia (Azienda Ospedaliera di Perugia, Perugia, Italy), who outlined why the guidewire is “essential” in complex occlusion cases. Yann Gouëffic (University Hospital of Nantes, Nantes, France) addressed the audience too, stating on the subject of vessel preparation
Bruno Migliara (pictured right) leads one of the 31 presentations that made up the summit. A total of 20 key opinion leaders were among those who took the stage

devices that “no studies have shown” that they offer a decisive advantage in cutting intimal hyperplasia.
Following one packed day of presentations came another, with the session on lesion treatment opening with further discussion from Rammos, who presented on a rotational atherothrombectomy device. In the subsequent talks on lesions, Mercedes Guerra (University Guadalajara Hospital, Guadalajara, Spain) gave a strong presentation on the need for better classification of extremely calcified lesions, which she said require their own treatment approach.
Migliara spoke to Interventional News following the summit to praise Guerra’s talk, which he said “analysed very well and deeply one of the most challenging and discussed questions: how to treat calcium”. The presentation and discussion which followed illuminated the need to “better define the best treatment choice for every different type of lesion”, he added.
Migliara himself presented during this session, returning to the rotational atherothrombectomy device explored by Rammos, which he said held particular promise for the treatment of in-stent restenosis. Its advantage, he averred to Interventional News, was that it “allow[s] retreatments without increasing their complexity”. In the debate following his talk, he suggested its repeated use for debulking poses fewer risks than bypass.
One highlight of the conference was what Migliara described as its “best debate”, between Deloose and Sherif El Kerdawy (Wadi El Neel Hospital, Cairo, Egypt) on the benefits of covered and uncovered stents in iliac artery treatment. Through this “scientific but also friendly debate”, Migliara said, “we understood better where the best choice is uncovered and where [it is] covered.” The audience swayed to the side of El Kerdawy: a post-debate poll found 42.9% on the side of the covered stent and 57.1% on the side of the uncovered.
“I learned many new ideas not only about techniques, but also about new materials and devices,” Migliara said of the concluding session on the future of PAD. Drawing special attention to vessel preparation and resorbable stents for BTK, he also praised a talk by Marta Lobato (Hospital de Cruces, Barakaldo, Spain) on new arterialisation techniques.
Migliara thanked those who spoke at the summit. Their “interesting presentations” and “active discussions” provided opportunities for learning, he said in review, and allowed him and his co-directors “to achieve the aim of this meeting”.
Disclaimer: The opinions and clinical experiences presented herein are for informational purposes only. The results from this Advertorial may not be predictive for all patients. Individual results may vary depending on a variety of patient specific attributes. The clinicians have been compensated by Becton, Dickinson and Company to participate in this advertorial. BD Switzerland Sarl, Terre Bonne Park – A4, Route De Crassier, 17, 1262 Eysins, Vaud. Switzerland, Tel: +41 21 556 30 00. Fax: +41 44 722 5370. bd.com. BD, the BD logo are the property of Becton, Dickinson and Company or its affiliates. © 2023 BD. All rights reserved. BD-94905
15 Advertorial Issue99 | September 2023 THIS ADVERTORIAL IS SPONSORED BY BD
Jürgen Falkensammer
Jürgen Falkensammer (Wilhelminenhospital Vienna, Vienna, Austria) speaks to Vascular News about his career so far. The current president of the Austrian Society of Vascular Surgery, Falkensammer recalls in particular a fellowship in the USA that opened his eyes to the possibilities of endovascular surgery and “shaped [his] whole career”. He also shares his thoughts on some major challenges in the field, stressing that younger surgeons must be sufficiently trained in both endovascular and open techniques in the interest of optimal patient care.
Why did you decide to pursue a career in medicine and why, specifically, did you choose to specialise in vascular surgery?
As a school pupil, I was very interested in science and within this broad subject area I had to make a choice between studying two very different options: one was archaeology and the other was medicine. I considered medicine as the more future-proof option. Also, there are several physicians in my family and to me, it felt like everybody liked his or her job. Early on, I learned that medicine could be dedication, not just a job. Why did I choose vascular surgery? As a medical student, I was part of a research group around Bernd Puschendorf, whose main scientific focus was the identification of a reliable marker of myocardial infarction. This awoke my interest in cardiovascular medicine. A considerable amount of research was going on in this field. However, I also learned that I would not spend my entire life in a lab. One of our collaborators was Johannes Bonatti, a cardiac surgeon. He was the cool man! I performed several clinical electives in cardiac surgery departments at home and abroad. It was frustrating! These guys were always performing the same procedures, bypass surgery and valves! That was, at least, my impression as a medical student. Only, some of them went beyond the heart, down the aorta. And, some of them experimented with less invasive techniques. The decision eventually came down to either cardiac or vascular surgery. Finally, a vascular surgery training post was the first option that came up, and so my decision was made!
Who were your career mentors and what was the best advice that they gave you?
My most significant mentor was certainly Afshin Assadian. We first met at a conference in Berlin. He was inspiring, talking about endovascular procedures being performed in the OR rather than an angio suite. We discussed the importance of surgical simulation and training, open and endovascular. In fact, he offered me a job right there, on the spot. Anyway, I had only a couple of months to finish my formal training. I joined Afshin’s team right afterwards. What followed was a 10-year race on the fast track with arguably one of the best teams of surgeons one can wish for.
Afshin’s best advice? This man is very careful giving you specific advice. If anything, he may challenge you to reflect. It is more the way he works that has been an inspiration to me. It is the way he does the job. First, there is a vision. Second, a strategy. And then, focus! Getting things done is definitely something I have learned from him.
What has been the most important development in vascular surgery over the course of your career so far?
I bet everyone will give you the same answer on this one! Certainly, that was the advent of endovascular
surgery. When I started my vascular surgery career, right after medical school, our senior vascular surgeons did not take this ‘new stuff’ very seriously. They were teasing our much more innovative radiologists, pretending we would have to convert all these endovascular aneurysm repair (EVAR) patients to open surgery, because this ‘wire game’ was not going to work out. I think many otherwise experienced, highly skilled vascular surgeons in Austria were thinking like that, and we have certainly lost a lot of time in adopting these now commonplace practices.
What are the biggest challenges currently facing vascular surgery?
One of the biggest challenges that all surgical specialties are facing is human resource development. In times of strict working time regulations and an increasing awareness of work-life balance, how can we provide for the high-quality treatment of future patients? How can we recruit—in fact, I should rather say, how can we attract young and able graduates? Vascular surgery is characterised by the rapid advance of new techniques and new endovascular devices. How can we provide a sufficient caseload for open as well as endovascular surgery to ensure that our team of tomorrow is able to perform either at the highest level? Simulator training is one tool that will help improve education. Centralisation may also play a role.
You embarked upon a research fellowship at the Mayo Clinic Jacksonville in 2004. How did this experience shape your career in vascular surgery back in Europe?
In Jacksonville, there was a small team, and they were proficient in both open and endovascular surgery. Both senior surgeons, Andrew Oldenburg and Albert Hakaim, had gained their endovascular expertise when they were already highly experienced open vascular surgeons. That left an enormous impression on me! Essentially, my experience in Jacksonville shaped my whole career. I knew I had to adopt endovascular techniques in order to truly become a vascular specialist.
What are some of your current research interests?
My current research is focused on the treatment of patients with complex aortic pathologies. Custommade fenestrated branched endografts have enabled us to treat older and sicker patients. Do they profit in the intermediate- and long-term? How can we improve patient selection? How can we optimise durability?
What are your goals over the next year as president of the Austrian Society of Vascular Surgery?
My primary goals all have to do with training and education. We should provide our trainees with formal courses to cover theoretical as well as hands-on training. Also, compulsory simulator training should become integrated in the vascular trainees’ logbook.
16 September 2023 | Issue99 Interview
PROFILE
How can we provide a sufficient caseload for open as well as endovascular surgery to ensure that our team of tomorrow is able to perform either at the highest level?”
alisonlang.com
As an executive board member for CX Aortic Vienna, could you comment on the importance of multidisciplinary meetings such as this?
I think there is a lot of competition between disciplines, especially between vascular surgeons and interventional radiologists. Multidisciplinary meetings like this enable us to communicate on neutral grounds. We must understand we have a common goal, which is the best possible treatment of our patients. And it is in the best interest of our patients to be able to choose the best technique—open, endovascular or hybrid. It is important we share this message at meetings.
Could you outline one of your most memorable cases?
A couple of days after I had started working at the Wilhelminenhospital in Vienna, a patient with a ruptured AAA was transferred from the ER to our operating suite. A fit octogenarian in stable condition. I expected the usual stressful situation from intubation until the placement of the aortic clamp. Far from it! Slight sedation, local anaesthesia in the groin, small oblique groin incisions, blocking balloon, etc. Ninety minutes later, the guy was awake in intensive care. I knew I was in the right place!
What advice would you give to someone looking to start a career in medicine?
Do you mean to someone who wants to study medicine to be a doctor? Go for it! There is a wide spectrum of opportunities. Medicine can truly be a mission rather than a job, if you let it be. Or do you rather mean starting a career in the sense of ‘climbing the ladder’ to be able to create, to manage? There is probably an inherent discrepancy between a healthy worklife balance and carving out a career, independent of the profession. Anyway, there is a great need for physicians with good management abilities, at all levels.
What are your hobbies and interests outside of medicine?
My hobbies and interests include hiking, mountain biking, running and reading. Also, I consider myself a family man, with two kids, a girl (16) and a boy (12). My wife is a successful in-hospital urologist.
Education
2000: Doctor of Medicine, Medical University of Innsbruck (Innsbruck, Austria)
June 2001–September 2010: Residency (General Surgery and Vascular Surgery), Medical University of Innsbruck
Postgraduate positions
July 2005–June 2006: Research fellowship, Mayo Clinic Jacksonville (Jacksonville, USA)
October 2010–December 2010: Consultant of vascular surgery, Medical University of Innsbruck
January 2011–present: Consultant of vascular surgery, Wilhelminenhospital Vienna (Vienna, Austria)
June 2016–November 2018: Professor of Vascular Surgery, Sigmund Freud University (Vienna, Austria)
2019: Associate professor, Medical University of Vienna (Vienna, Austria)
17 Issue99 | September 2023 Interview
Adding 8–12mm diameter devices to the Shockwave Peripheral Intravascular Lithotripsy toolkit
Mazin Foteh (Aortic Clinic at Baylor Scott & White Health, Plano, USA) contrasts the benefits of Shockwave Medical’s new Shockwave L6 Peripheral Intravascular Lithotripsy Catheter alongside those of its sister device, the Shockwave M5+ Peripheral Intravascular Lithotripsy Catheter.
The Shockwave L6 device is a purposebuilt IVL catheter, perfectly designed for complex iliac occlusive disease. Calcified plaque blockages in this region are distinctly different from lesions elsewhere in the arterial system. These are much larger blood vessels, which are often thick and heterogeneously calcified. Although the Shockwave M5+ IVL catheter was instrumental in introducing this technology to an underserved space, in some scenarios it lacked the size and energy profile to compete with this degree of calcium. The most important feature of the Shockwave L6 catheter is its larger diameter offerings (8, 9, 10 and 12mm).
Today, we can appropriately match the Shockwave balloon catheter to the vessel and optimise outcomes. Secondly, the compact emitter placement is instrumental in providing a uniform energy profile across the length of the balloon. There are a total of three paired emitters, which fire in groupings of two. This translates to consistent sonic output without diminishment at the ends.
The Shockwave M5+, on the other hand, delivers peak pressure at the centre electrode. In practice this equates to improved case efficiency, as we are no longer trying to centre the electrode at the most dense area of plaque. Finally, all of the Shockwave L6 catheters can be delivered over an 0.018” wire and in 7/8F sizes. Tracking over a larger wire removes resistance and sets a platform for additional therapies such as stents.

To date, the endovascular treatments for calcified iliac plaques have centred on the “crack and pave” technique, which harnesses barotrauma as the mechanism of action. The Shockwave L6 catheter allows a rethinking of this method, now relying purely on low-pressure inflations and the delivery of sonic pressure waves to the hardened calcium.
This results in a much gentler, worry-free technique, causing calcified lesions to yield in a manner that is without the risk of rupture. Additionally, if stenting is necessary, we can rest assured that optimal luminal gain can be achieved. This particular arterial bed is very frequently laden with calcium deposits, therefore the need for new techniques is present.
The Shockwave L6 catheter is the first technology to address this need and may one day challenge the notion that all iliac blockages require a stent. My practice is centred around large-bore devices for endovascular aneurysm repair (EVAR), thoracic EVAR (TEVAR), and fenestrated EVAR (FEVAR). Iliac stenosis and poor access are quite common in this population.
Prior to the Shockwave L6, we were forced to create a runway for delivery. This meant advanced and expensive techniques such as endovascular and
open surgical conduits. These procedures can add cost, but also morbidity. I can now successfully treat most iliac lesions in this setting with the Shockwave L6 alone. The Shockwave M5+ was an excellent precursor to the Shockwave L6 catheter. Its longer length certainly provided a nice platform to treat multi-segment disease, especially in the external iliac as well as the fem-pop distribution. However, its energy profile differs greatly from the Shockwave L6, with the maximal energy coming from the centre electrode. This feature affects the way the balloon is used, generally requiring significant overlap of the inflations and sometimes even trying to centre the middle emitter on the peak plaque.
however, the healthy reference vessel diameter (RVD) was 9.5mm. He would require a fenestrated repair of the AAA along with complex iliac stenosis treatment, and extensive bilateral common femoral endarterectomies.
Further, we were concerned we would not be able to advance the 19F fenestrated graft. The endograft was prepared, and then both femoral arteries were exposed. We attempted delivery of the endograft via the right common iliac access. This failed. We then attempted delivery on the contralateral side. Despite multiple attempts, we made little progress. A 10x30mm Shockwave L6 catheter was selected, and a total of 150 pulses were delivered to each iliac artery at just 4atm. This not only allowed for the delivery of our FEVAR graft but also the full unrestricted expansion of the endograft limbs in the common iliac arteries.
At the completion of the AAA, we took extra time to perform endarterectomies of the deep femoral arteries. His completion CT scan revealed a good seal of the AAA, patency of the renal fenestrations and unrestricted flow to the bilateral femoral arteries.
Case 2: The Shockwave M5+ tackles 90% stenosis of the distal SFA
MLD = 4.5mm
RVD = 9.5mm
Furthermore, it is somewhat limited in its applicability in larger vessels, simply based on the balloon size. I find that for medium-sized vessels with moderate calcium burden that this balloon is favoured. Whereas for focal, dense plaque in larger vessels, the Shockwave L6 is the catheter of choice. My experience with this system has taught me that appropriate balloon sizing is the single-most important predictor of a successful outcome.
Case 1: The Shockwave L6 device in the setting of FEVAR and complex iliac stenosis
An 82-year-old man with a history of coronary artery disease (CAD), hypertension, hyperlipidaemia, and peripheral arterial disease (PAD) presents with rest pain as well as a large pararenal abdominal aortic aneurysm (AAA).
His computed tomography angiography (CTA) revealed very dense and highly diseased iliac vessels with calcium deposition along most of the bilateral common iliac distribution.
The minimum luminal diameter (MLD) of the common iliac arteries measured roughly 4.5mm;
The patient is a 75-year-old man with a history of hypertension, hyperlipidaemia, CAD, chronic kidney disease and diabetes mellitus, who presents with a non-healing wound of the right lower extremity for three months. He had undergone diagnostic work—his ankle-brachial index (ABI) was found to be 0.73 with a toe pressure of 50mmhg, and a duplex ultrasound revealed a densely calcified superficial femoral artery (SFA) stenosis of 90%. He was taken to the cath lab and underwent a right lower-extremity arteriogram. This confirmed a 90% stenosis of the distal SFA, with normal threevessel run off.
Due to the heavy calcium, we opted for a Shockwave M5+ 6x60mm and delivered 200 pulses to the lesion. There was no appreciable residual stenosis at the completion of the procedure, and his postprocedure ABI improved to 1.
This is a great example of how IVL can be used as a standalone therapy without the need for adjunctive treatment.
Mazin Foteh is director of aortic therapy and innovation and co-director of the Aortic Clinic at Baylor Scott & White Health in Plano, USA. He is a paid consultant to Shockwave Medical. The views expressed are those of the author and not necessarily those of Shockwave Medical.
Shockwave L6 Device is Available in the US Only.

18 Advertorial September 2023 | Issue99
THIS ADVERTORIAL IS SPONSORED BY SHOCKWAVE MEDICAL
My experience with this system has taught me that appropriate balloon sizing is the single-most important predictor of a successful outcome.”
IVL Device Choice = Shockwave L6 10mm
Mazin Foteh
Shockwave L6
Peripheral IVL Catheter
Shockwave M5+, Shockwave M5, Shockwave S4 and Shockwave L6 Safety Information In the United States: Rx only.
Indications for Use—The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary or cerebral vasculature.

Contraindications—Do not use if unable to pass 0.014” (M5, M5+, S4) or 0.018” (L6) guidewire across the lesion-Not intended for treatment of instent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings—Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device—Use the generator in accordance with recommended settings as stated in the Operator’s Manual.
Precautions—use only the recommended balloon inflation medium— Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology.
Adverse effects—Possible adverse effects consistent with standard angioplasty include–Access site complications –Allergy to contrast
or blood thinner– Arterial bypass surgery—Bleeding complications— Death—Fracture of guidewire or device—Hypertension/Hypotension— Infection/sepsis—Placement of a stent—renal failure—Shock/pulmonary edema—target vessel stenosis or occlusion—Vascular complications. Risks unique to the device and its use—Allergy to catheter material(s)— Device malfunction or failure—Excess heat at target site. Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. www.shockwavemedical.com SPL-68917 Rev. A.
19 Issue99 | September 2023 Advertorial
Case 1
Case 2
Figure 1. Pre-procdural CTA
Figure 4. Graft delivery
Figure 1. Pre-procedural angiogram
Figure 2. Pre-procedural angiogram
Figure 5. Final angiogram
Figure 2. IVL treatment angiogram
Figure 3. Post-IVL angiogram
Figure 6. Post-procedural CTA
Figure 3. Final angiogram
Deep venous valve technologies set to address “large unmet need worldwide”
The pursuit of a cure for deep venous valvular reflux—long considered to be the “holy grail” of deep venous disease—is underway, with new technologies set to address a longstanding unmet clinical need across the globe.
Chronic venous disease affects nearly 25 million in the USA alone, and among those patients, the deep venous reflux population is close to two million people. Erin Murphy (Charlotte, USA) and Marc Glickman (Irvine, USA) put forward these figures to set the scene for a deep dive into deep venous valvular reflux during a recent CX Vascular Live discussion. They were joined by Ramon Varcoe (Sydney, Australia) to outline the scale of the issue, as well as potential solutions in the pipeline.
“The population is large, it is being treated most of the time with conservative therapy, so I think there is a large demand—untreated, unmet and underdiagnosed,” said Glickman in summary of the overarching issue. “This is a large unmet need worldwide.” Moving beyond the US statistics, he stressed that India, China and Brazil have even larger patient populations with deep venous reflux.
Varcoe concurred that deep venous reflux represents an “enormous global burden,” not only in terms of healthcare costs, but also in patients’ livelihoods. To this point he described the difficulties associated with living with venous ulcers, and the fact that vascular surgeons “really struggle” with these patients in terms of applying compression therapy and using other conservative measures.
Murphy, director of the venous and lymphatic programme at Atrium Health’s Sanger Heart & Vascular Institute, noted that there has been “considerable progress” over the last 10 years in treatment options for patients with reflux in the
superficial system, and also in the deep venous obstruction space, with various new stents now available. “Why do you think it is taking so long to establish new technology in the deep venous valve realm?” she posed to the panel.

“For the last 50 to 60 years, everyone has considered finding a cure for deep venous valvular reflux as the holy grail of deep venous disease,” remarked Glickman, highlighting the importance of, and difficulty associated with finding a solution to, the problem. He put forward two issues at play here—one is the challenging nature of correctly diagnosing deep venous valvular insufficiency and the other, a lack of standardisation in making that diagnosis, resulting in the severity of the disease being “missed throughout the medical community”.
Varcoe also stressed the “huge variability” in how this disease is diagnosed, and mentioned another issue, that surgery in these patients is “next to impossible” due to challenging anatomical conditions.
Glickman also noted another challenge in the fact that vascular surgery training tends to focus on arterial disease and less on the venous world, resulting in only “very minor” innovation in the latter.
New technologies “on the horizon”
Glickman expressed his belief that the potential for growth in the deep venous valve market is “enormous”, moving the focus of the conversation towards two new technologies that could improve patient outcomes in this challenging space. On this
note, Varcoe expressed his excitement that there are some new technologies “on the horizon” that “might make a huge difference to patients’ lives”.
Glickman is the senior vice president and chief medical officer at Envveno Medical, the company behind the VenoValve, and shared that his training as a vascular surgeon informed him that “most of our endovascular procedures have started out as open procedures that have worked and that have migrated to endovascular,” citing the examples of carotid stenting, ablation and stripping, and the treatment of abdominal aortic aneurysms. In the deep venous reflux space, however, he noted that an open surgical technique is missing.
About seven years ago, Glickman recalled, the VenoValve was developed. He detailed that it is made from a porcine aortic monocusp on a stainless steel frame that is surgically implanted into the femoral vein in the mid-thigh region. The procedure takes about an hour and 15 minutes.
Glickman noted that three-year data from a firstin-human trial conducted in Colombia, which were recently shared at the 2023 American Venous Forum, showed improvement in reflux time, improvement and consistency of the Venous Clinical Severity Score (VCSS), and a reduction in pain. The team are presently conducting another trial—SAVVE—at 24 sites in the USA.
He highlighted that the company is now working on a transcatheter version of its VenoValve device, with the plan being to commence a first-in-human study. Varcoe, who has been involved in clinical studies on the BlueLeaf (InterVene), shared that this novel device is “completely different” to the VenoValve. “What we do with this device is we make a valve leaflet from the blood vessel wall itself—so we leave nothing behind, we are not transplanting anything, we are literally creating a flap-like valve, which can be either monocuspid or bicuspid and it can be done multiple times in the same vein segment,” he explained. The device is percutaneous, Varcoe added, and is therefore inserted over a guidewire, with the procedure guided by both intravascular ultrasound and fluoroscopy. “It takes around an hour and a half to make a series of valves and we hope that over time those valve cusps endothelialise and become part of the native structure of the vein wall,” he said.
In terms of data, Varcoe noted that 41 patients have now been enrolled in a global clinical trial of the technology. The data demonstrate the safety and efficacy of the device in its early stages, Varcoe
20 September 2023 | Issue99 Feature
VENOUS
L-R: Marc Glickman, Ramon Varcoe and Erin Murphy
reported, and that the procedure can be done without creating deep vein thromboses, which was an “initial fear”. He added that the data have shown some “dramatic, remarkable improvement” in VCSS scores. “Patients I have seen who have had ulcers for 20 years have had them healed within six weeks, and that is extremely satisfying,” he shared.
In addition to VenoValve and BlueLeaf, Cook Medical announced earlier this summer that the first patient has been treated in a clinical study to evaluate a new venous valve designed for treating chronic venous insufficiency (CVI). The patient was treated by principal investigator Mauricio Alviar (Barranquilla, Colombia). The valve’s safety and efficacy are now being tested in a global, multisite clinical trial. The global principal investigator of the study is Paul Gagne (Darien, USA). “When leg veins function poorly, patients suffer with leg swelling, leg pain, leg ulcers, disability, and possible amputation. Therefore, it is important to restore blood flow out of the leg veins, back to the heart. Part of doing this successfully required restoring the function of the venous valves,” Gagne said in a press release at the time.
What is next?

While these technologies are in their infancy, both Glickman and Varcoe are cautiously optimistic about the future. Glickman noted that the focus at Envveno Medical will be finishing up the trial for US Food and Drug Administration (FDA) approval by the end of this year, after which it will take six months to get the approval. The plan is to conduct a large trial in the USA. He summarised that the percutaneous device is probably five years out, and VenoValve “probably two years out from being in people’s hands”.
Jorge Ulloa (Bogota, Colombia), one of the key

figures behind research of the surgical VenoValve, told Vascular News the future looks bright in the venous valve space following 65 years of development.



“So far, we are at the pivotal phase of the study [of the surgical version of the valve],” he said, relaying how more than 50 cases have now been performed in the USA to date. This follows the first-in-human trial carried out at Ulloa’s institution in Bogota. The surgical valve “seems to have a promising future, due to the length and results from the follow-up, that we have presented extensively in the last three years,” the Fundacion Santa Fe-Universidad de los Andes vascular surgeon continued. “There is another player
everything looks like it will continue this way. Of course, only time and follow-up of these cases will show us the path to continue.”
Ulloa believes more new technology will emerge in the venous valve space in the coming years. “I envision a myriad of new devices that will emulate our experience,” he said. “Some will work, some [will not]. So far, there are more than four major companies designing their own concepts, and they will have to run through all the necessary stages in order to prove that they are safe and functional. All of these efforts will only benefit PTS [post-thrombotic syndrome] patients—and that is a good thing.”
On BlueLeaf, Varcoe stated that the team involved is “in a process of innovating and iterating the device”. It is about to launch the fourth generation of the device, which allows for the treatment of larger veins and the creation of more circumferential valve cusps. He added that the company is going through the pre-submission process with the FDA about what an investigational device exemption trial might look like, which it hopes to be able to start within months. “I see a future where this technology is successful,” Varcoe averred, “whichever deep valve technologies do end up being available to us.”

from Cook Medical, which has already implanted a first device via endovascular deployment, and we are on the way to migrating to [an endovascular device] also.” Ulloa is similarly involved in the first-inhuman trial of the endovascularly delivered version of the valve. In the meantime, continuing study of the surgical valve is showing “a plateau in terms of stabilisation in pain, VCSS and reflux time,” he said. “It means that there is no tendency to return to baseline at 36 months of follow-up. That is something that has never happened in previous series, and
There will undoubtedly be challenges along the way, however. Glickman acknowledged that paradigm shifts in medicine “take time”, emphasising the complexity of the regulatory landscape. He also pointed out the importance of continued education in this space to address a lack of understanding of deep venous insufficiency within the medical community.
“I have just really enjoyed seeing the focus be put back on venous disease,” Varcoe said in closing. “I am seeing huge interest in venous disease now and it is a really great time to be involved in the field.”
21 Issue99 | September 2023 Feature
I am seeing huge interest in venous disease now and it is a really great time to be involved in the field.”
A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the venous arena Editorially independent Available in print and digital formats and through our social channels Subscribe today Efforts to move towards routine patch closure or eversion endarterectomy among all carotid surgeons with an increased risk ipsilateral equivalent, and that bovine routine patch closure, rather than they add, “many surgeons prefer University Hospitals NHS Foundation Trust, (CX) audience should take back to their time presentation of the BASIL-2 trial led chief stark message: patient who needs belowfemoropopliteal revascularisation is likely endovascular-first strategy rather than vein Ithreatening ischaemia (CLTI), best endovascular treatmentamputation-free survival than vein bypass-first strategy all seems pointing towards attempting an bypass-first strategy treatment underwent major amputation Median survival for the whole cohort was 3.8 years—3.3 continued. “There is no significant difference 30-day groups quite different.” It is very, very crucial that whatever you do first, it does not have to be Roger Greenhalgh First results of BASIL-2 randomised controlled trial revealed at CX 2023 venousnews 10 EACTS 2021 Antithrombotics this years event 18 Osman Ahmed *Available for US and EU readers only **Available worldwide Visit venousnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription**
Ramon Varcoe
Data in the pipeline for “toolbox” of saphenous vein ablation options
In an interview at this year’s Leipzig Interventional Course (LINC 2023; 6–9 June, Leipzig, Germany), Raghu Kolluri (OhioHealth, Columbus, USA) outlined the “vast list” of options that are now available for the treatment of patients who require saphenous vein ablation.

From radiofrequency, which first gained approval over a quarter of a century ago, to surgical, laser and non-thermal ablation options, the treatment landscape for these patients has evolved over time, Kolluri began. “All these are tools in the toolbox to take care of our patients’ pathology depending on their anatomy and presentation,” he explained.
Turning his attention to data, Kolluri referenced SPECTRUM as the most important trial in the superficial venous space in the near future. “I cannot think of a larger trial or more important trial in this space because (a) it is the largest post-market study in the space of superficial venous intervention and (b) it is going to assess the role of closure of the great saphenous vein in the treatment of venous ulcers,” he opined. In terms of what is missing from the literature, Kolluri did stress that there
is no head-to-head comparison of the available treatments to assess which has the fewest complications.
The system medical director of Vascular Medicine at OhioHealth, Kolluri also touched on the importance of multispecialty treatment, stressing that “holistic care” is crucial in the venous space. “If [you are] unable to offer the full spectrum of venous treatments, [you have] got to collaborate,” he said, highlighting the importance of his vascular surgery, interventional cardiology and interventional radiology colleagues in his day-to-day practice.
“There are large venous aneurysms that I send routinely to my vascular surgery colleagues who are my partners in my practice,” Kolluri continued.
“And on the other hand, the vascular surgeons will send their complicated thrombotic patients with reflux to me
because I deal with hypercoagulable states and venous interventions.”
Kolluri also made note that he advises colleagues to “jump in with both feet” rather than making venous intervention a side operation to the main procedure the patient is undergoing.
Finally, Kolluri listed some unmet needs in the saphenous vein ablation space, including which procedure to tackle first in post-thrombotic patients, and whether to treat reflux or obstruction first in venous ulcer patients. He remarked that while there have been great advancements, there is still room for growth within the field.
“You have data from the EVRA trial, which tell us that for small wounds, you probably do not need to treat the deep system. But for the larger wounds, we all believe that we need to treat the deep system first before treating the superficial system,” Kolluri noted,
LAMA trial: MOCA “may have a role in a small number of selected patients”
FIVE-YEAR RESULTS OF THE LAMA randomised controlled trial (RCT) show that both mechanochemical ablation (MOCA) and modern endovenous laser ablation (EVLA) technology are associated with low procedural and post-procedural pain, while clinical outcomes in the short and medium term are worse for MOCA. This led senior author Daniel Carradice (Hull York Medical School, Hull, UK) to conclude that MOCA “may have a role in a small number of selected patients such as those with a fear of needles”.
First author Arthur Lim (Hull York Medical School, Hull, UK) presented the five-year results at the recent European Venous Forum (EVF 2023; 22–24 June, Berlin, Germany). He began by stating that endovenous thermal ablation (EVTA) is the current first-line treatment for superficial venous incompetence (SVI). Despite this, he noted that it requires tumescent anaesthesia, which has been reported to cause perioperative discomfort.
MOCA offers a non-thermal, non-tumescent alternative to EVTA, and Lim detailed that it combines mechanical and chemical damage to achieve vein occlusion. In addition, he stressed that the longer-term outcomes of MOCA are little known.
The LAMA trial is a single-centre RCT that included adult patients with single-axis unilateral symptomatic SVI who were treated with either MOCA, using the ClariVein device (Merit Medical) with or without phlebectomy, or EVLA—a type of thermal ablation—using the VenaCure laser (AngioDynamics) with or without phlebectomy.
Lim informed EVF attendees that the investigators recruited a total of 150 patients in the LAMA trial, noting that 69 patients in the laser group and 69 in the MOCA group had completed follow-up at one year. At the one-year follow-up point, Lim recapped that both groups had similar procedural pain scores. However, he added, MOCA had “significantly lower” anatomical occlusion compared to laser. In addition, both groups had similar Venous clinical severity scores (VCSS). “Our results are similar to other RCTs of MOCA,” he said.
After sharing these early results, Lim then underlined the goal for longer-term follow-up in the LAMA trial, which was to determine how reduced anatomical occlusion rates impacted venous health and quality of life.
Lim revealed that 52 patients in the laser group and 57 in the MOCA group completed follow-up out to five years. He reported that, for anatomical occlusion, initial success was achieved in all but two patients in the laser group and all bar one in the MOCA group. Five years after treatment, however, it was observed that EVLA had a “significantly higher” anatomical occlusion rate compared to MOCA. Regarding total reinterventions, the presenter continued, MOCA had a “significantly higher” number compared to laser—specifically, 16 for MOCA and six for laser.
Lim also communicated clinical outcome scores, first sharing that VCSS scores for both groups were similar at one year and also at five years after treatment. “The improvement in VCSS is maintained
while acknowledging that there is currently no science to support this approach.
Additionally, Kolluri highlighted that the venous field is still lacking data comparing thermal ablation for saphenous reflux to physiciancompound foam, which he noted was used in approximately 50% of patients in the EVRA trial.
When it comes to venous research over the next five to 10 years, Kolluri’s stance is that the need to assess whether “the deep is responsible for the ulcers or the superficial is” should be at the forefront of future study. Secondly, he expressed the importance of understanding what a “true haemodynamically significant lesion” should be defined as. He also touched on the need for a research trial to understand the success rates of physician-compounded foam in the treatment of axial reflux and how the intervention compares to thermal and non-thermal treatments.
from baseline after one year and five years for both groups,” the presenter stated.
For disease-specific quality of life, Lim added that both groups had similar scores at one year and five years after treatment, with the improvement maintained at both one and five years for both groups. Results were similar for generic quality of life, with both groups showing similar scores at one and five years.
“To conclude, MOCA has significantly lower anatomical occlusion at five years,” Lim said, adding that this treatment option saw a higher rate of reintervention for symptomatic clinical recurrence. The presenter also reiterated that the investigators were unable to detect any difference in clinical outcomes between laser and MOCA at five years.
Speaking to Vascular News, Carradice summarises that LAMA supports the case that the likely disadvantage of MOCA is reduced anatomical occlusion over time, and that this reduced anatomical occlusion is clinically relevant as it is linked to much higher reintervention. He continues that while there is no significant difference at five years in clinical severity or quality of life between the two treatment methods, this should be interpreted with caution, as it is only after a high rate of reintervention and the smaller numbers in the subgroup analysis are susceptible to type 2 statistical error.
22 September 2023 | Issue99 Conference Coverage
LAMA supports the case that the likely disadvantage of MOCA is reduced anatomical occlusion over time.”
VENOUS
If [you are] unable to offer the full spectrum of venous treatments, [you have] got to collaborate.”
Raghu Kolluri


Now back in print YOUR VENOUS NEWSPAPER OF CHOICE RETURNS IN SEPTEMBER... Avoid the rush to subscribe by either scanning this QR code or by visiting https://bibapublishing.com/ subscriptions/

New data “confirm” Roadsaver carotid stent safety and efficacy
Presenting first-time data from large multicentre European studies at this year’s Leipzig Interventional Course (LINC 2023; 6–9 June, Leipzig, Germany), Arne Schwindt (St Franziskus Hospital Münster, Münster, Germany) and Ralf Langhoff (Sankt Gertrauden Hospital, Berlin, Germany) delivered results that “confirm” the safety and efficacy of the Roadsaver (Terumo) dual-layer micromesh carotid stent (DLMS).
First to the podium, Schwindt identified 30-day outcomes in male versus female patients treated using the Roadsaver device. He explained that the majority of patients were treated using a femoral approach, adding that radial access was more common in the female group. The researchers found no difference in the use of embolic protection in their cohort; however, Schwindt noted that in the cases where protection was employed, female patients more frequently received distal protection. Their primary endpoint concerned any major adverse event (MAE) between the female and male groups. Additionally, Schwindt explained that of their 2,000-patient cohort,
participants were evenly split between symptomatic and asymptomatic.
Schwindt and his team found that the MAE rate in their patient population was 2.2%—recorded as 2.0% for males and 2.6% for females—which he reported to the LINC 2023 audience— was not statistically significant. Schwindt did pinpoint a trend toward

higher stroke risk in their study’s female group, though this was also not statistically significant. Among the entire cohort, stroke risk was seen in 1.9%—which expanded to 1.6% in male and 2.6% in female patients.
“We saw that the majority of these events still take place within 48 hours to three days after the procedure,” Schwindt noted, “but we see these curves flatten out and nothing much happens towards the end of the 30-day period.” Concluding his presentation, Schwindt emphasised the “very low” MAE rate in both male and female patients and “[similarly]” low 30-day rates of minor or major stroke, despite a “small trend” toward lower incidence in males. Schwindt stated: “Given that both groups undercut the stroke rates defined by the S2 guidelines from Germany, currently the strictest guidelines concerning clinical outcome, CAS with dual layer micromesh stent can be concerned as a valid treatment option for males and females alike.”
Following Schwindt, Langhoff subsequently presented firsttime data on the 30-day safety results in patients treated using the Roadsaver device—their investigation assessed the influence of
predilatation on outcomes. “A major difference,” Langhoff posited, became apparent when observing transient ischaemic attack (TIA), which in patients who had received predilatation, was seen to be “significantly” more prevalent. His team identified a 1.6% to 2.0% split, which emphasised the effect of predilatation that Langhoff himself “did not expect”.
Summarising their findings, Langhoff stated that there was low overall need for predilatation in real-world elective carotid artery stenting (CAS) patients treated with the Roadsaver DLMS. Equally, he noted that there were low and comparable 30-day MAE rates between the groups with and without predilatation, “[confirming]” the safety of the Roadsaver device when performing CAS.
Interim results from the ECST-2 randomised controlled trial (RCT) have shown no evidence that carotid stenosis patients with a low-to-intermediate stroke risk, treated with optimised medical therapy, will benefit from additional carotid revascularisation via carotid endarterectomy (CEA) or carotid artery stenting (CAS).
NEVERTHELESS, PAUL NEDERKOORN (Amsterdam UMC, Amsterdam, The Netherlands)— who presented these late-breaking findings at the European Stroke Organisation Conference (ESOC; 24–26 May, Munich, Germany)—noted that a longer clinical follow-up is now required. The aforementioned interim results were observed at two years, with ECST-2 set to continue following patients for a total of five years.


In an attempt to provide an update to the original European carotid surgery trial (ECST), which was published in the late 1990s and found CEA to be effective in symptomatic patients with carotid stenosis >80%, ECST-2 hypothesised that there would be no benefit from additional carotid revascularisation alongside optimised medical therapy in patients who have carotid stenosis ≥50% and a low-to-intermediate risk of stroke. Nederkoorn stated during his presentation that, in the decades following the ECST and NASCET RCTs, medical management—the comparator in both trials—has improved “significantly”.
ECST-2 was designed as a multicentre, prospective RCT with blinded-outcome adjudication, and saw patients randomised to either immediate carotid revascularisation plus optimised medical therapy
or optimised medical therapy alone. Patients were considered eligible if they had an asymptomatic or symptomatic carotid stenosis ≥50% and an estimated five-year stroke risk <20%, calculated using the Carotid Artery Risk (CAR) score. Recruitment was halted after the inclusion of 428 patients, Nederkoorn reported.
For the composite endpoint defined for these interim results—the twoyear rate of any stroke, myocardial infarction (MI) or periprocedural death—Nederkoorn and colleagues found a hazard ratio of 0.96 with optimised medical therapy alone in comparison to optimised medical therapy plus revascularisation (95% confidence interval [CI] 0.53–1.76).
The speaker noted that there was “a sign of more harm” initially with revascularisation, signified by a higher rate of stroke, MI or periprocedural death in the first few months following randomisation, but that these rates converged later on and Kaplan-Meier analyses ultimately showed no significant difference between the two groups at 24 months.
While the numbers involved were “very small,
precluding valid conclusions”, Nederkoorn reported no differences across any of the predefined subgroups in ECST-2—including between symptomatic and asymptomatic stenoses. There was, however, a slight trend towards optimised medical therapy being associated with a greater treatment effect in patients with an increased risk of ipsilateral stroke (CAR score >10%).
From this interim analysis of the ECST-2 intention-to-treat population, the investigators concluded that there is no evidence patients with stenosis ≥50% and a low-to-intermediate stroke risk will benefit from carotid revascularisation in addition to optimised medical therapy.
As well as emphasising that, despite this finding, further follow-up is both warranted and planned in ECST-2, Nederkoorn stated that a complete, two-year analysis including evaluations of silent infarcts on magnetic resonance imaging (MRI) is now scheduled too. Also, the results of an MR plaque-imaging substudy from ECST-2 will soon be available, which Nederkoorn told NeuroNews is a “crucial tool” within a novel prediction rule that is still to be designed but may elucidate future stroke risks and treatment decisions in this domain of patients.
A subsequent discussion at ESOC 2023 saw Seemant Chaturvedi (University of Maryland School of Medicine, Baltimore, USA) posit that the medical therapy given in the trial’s comparator arm could have been optimised “even further” in accordance with some current guideline recommendations, potentially improving that group’s outcomes to a still-greater extent—a point that Nederkoorn agreed with.
25 Issue99 | September 2023 Conference Coverage
Interim data find “no evidence of benefit” for carotid revascularisation additional to optimised medical therapy
CAROTID
We saw that the majority of these [major adverse] events still take place within 48 hours to three days after the procedure.”
Arne Schwindt
Paul Nederkoorn presenting at ESOC 2023
Arne Schwindt
Clinical News
Nectero Medical receives US FDA clearance of IND application to initiate Phase II/III clinical trial of Nectero EAST system
Nectero Medical recently announced that the US Food and Drug Administration (FDA) has granted investigational new drug (IND) clearance for the company to initiate a prospective, multicentre, randomised clinical trial (the stAAAble study) to evaluate the safety and efficacy of the Nectero endovascular aneurysm stabilisation treatment (EAST) system in patients with infrarenal abdominal aortic aneurysms (AAAs), maximum diameter 3.5–5cm.
“We believe the Nectero EAST system has the potential to address a significant unmet clinical need and improve outcomes in AAA patients who currently have no proven treatment options. Nectero EAST aims to be the first therapy to stabilise growth of small- to mid-sized AAAs,” commented Jack Springer, president and chief executive officer of Nectero Medical. “The IND clearance and initiation of a landmark pivotal study reflect our continuing commitment to bringing transformative therapies to improve the lives of patients with aneurysmal disease.”
The Nectero EAST system is a single-use, endovascular system for the treatment of infrarenal AAAs and is comprised of a dual-balloon delivery catheter and stabiliser mixture containing pentagalloyl glucose (PGG). The system delivers PGG locally into the aneurysmal wall where it binds to elastin and collagen to strengthen the aortic vessel wall and potentially reduce the risk of further degradation. A press release details that the procedure does not require any specialised tools or training, takes less than an hour to complete, leaves no permanent implant behind and does not preclude any future interventions.
“I am really encouraged by the initial data of the Nectero EAST system to potentially slow the growth of AAA and thereby minimise the likelihood of rupture and/or need for major intervention,” commented Dan Clair (Vanderbilt University Medical Center, Nashville, USA), co-principal investigator for the stAAAble study. “If proven safe and efficacious at stabilising AAA growth, the Nectero EAST system has the potential to transform the lives of thousands of patients with aneurysmal

disease. We are honoured to be one of the centres participating in this groundbreaking study that is likely to have a pronounced impact on the future management of AAA patients.”
The IND submission was supported by a prospective, first-in-human study of 21 patients treated outside the USA. Early Phase I clinical results were recently published in the Journal of Vascular Surgery and showed that a single, localised PGG administration to patients with small- to mediumsized infrarenal AAAs was safe and demonstrated the potential to slow the growth of AAA.
The stAAAble study will be conducted largely in the USA and is expected to initiate enrolment in the next few months.

post angioplasty and then again at 15 minutes post inflation. The degree of vessel recoil was assessed angiographically and then core lab adjudicated. These data showed a mean percent recoil of 6% in the Serranator group vs. 55% in the plain balloon angioplasty group representing a 49% reduction in recoil using serration angioplasty. Notably, there were no flow limiting dissections in either lesion treatment group, suggesting that optimal inflation technique impacts dissection rates, but not recoil rates.
“The recoil phenomenon is well understood as one of the greatest headwinds to successful BTK treatment and ultimately wound healing,” stated Michael Lichtenberg (Klinikum Hochsauerland, Arnsberg, Germany) who participated in the study.
system (BeGraft) for the endovascular treatment of patients with aortic aneurysms involving one or more of the major visceral arteries.
The ZFEN+ is predicated on the commercially available Zenith fenestrated abdominal aortic aneurysm (AAA) endovascular graft, but extends the proximal margin of aneurysmal disease that can be treated endovascularly to include patients with more complex aortic disease. For patients with complex aortic disease, the only currently established treatment option is open surgical repair.
RECOIL
study: Serranator demonstrates 49% less recoil than plain balloon angioplasty in BTK arteries
Cagent Vascular has announced the results of its below-the-knee (BTK) RECOIL study. This core labadjudicated Recoil analysis— the first of its kind, according to the company— evaluated vessel recoil in lesions treated with Serranator versus plain balloon angioplasty.
The study results demonstrated that lesions treated using serration angioplasty exhibited 49% less average recoil than plain balloon angioplasty. Stefan Stahlhoff (Arnsberg Clinic, Arnsberg, Germany) and Venita Chandra (Stanford University, Stanford, USA) led the RECOIL study as co-principal investigators.
The Serranator percutaneous transluminal angioplasty (PTA) serration balloon catheter is the first and only angioplasty balloon that is US Food and Drug Administration (FDA) cleared and CE marked that embeds serration technology into a semi-compliant balloon for treating peripheral arterial disease (PAD), a press release reads. The device is designed to create multiple longitudinal lines of interrupted micro-serrations to aid arterial expansion.
Thirty-six patients with 39 lesions in up to 22cm in length were matched 1:1 using either the Serranator or plain balloon angioplasty. The study objectives were to assess the ability to define and measure post treatment recoil in infrapopliteal arteries and to show preliminary evidence as to the differences between serration angioplasty and standard balloon angioplasty as defined by posttreatment recoil, lumen gain, and dissection. In each procedure the lumen diameter was measured immediately
“The 49% difference demonstrated in the RECOIL study between Serranator and [plain balloon angioplasty] is statistically significant and in my view also clinically relevant. Wound healing requires a durable lumen to support brisk blood flow, and 55% recoil for [plain balloon angioplasty] treated lesions is a signal that our current 40-year [plain balloon angioplasty] standard therapy is inadequate. I believe this study is a further step in demonstrating serration angioplasty as a definitive treatment for BTK lesions.”
Chandra added: “We know that BTK interventions are highly susceptible to vessel recoil, however the impact of recoil on residual stenosis at 15 minutes post procedure makes the true severity of this challenge clear. Our [chronic limb-threatening ischaemia] patient population is particularly vulnerable and at greater risk of limb loss and the associated increase in mortality that accompanies amputation. While this initial RECOIL study did not follow patient outcomes, in the future we intend to evaluate how impactful serration angioplasty’s recoil improvement is to healing BTK wounds and savings limbs. The results from this comparative RECOIL study are indicative that serration angioplasty may be the tool that finally addresses this longstanding challenge with [plain balloon angioplasty].”
US FDA approves study of ZFEN+ for the treatment of aortic aneurysms
The US Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an investigational device exemption (IDE) study on the Zenith fenestrated+ endovascular graft (ZFEN+).

The clinical study will assess the safety and effectiveness of the ZFEN+ in combination with the Bentley InnoMed BeGraft balloon-expandable fenestrated endovascular aortic repair (FEVAR) bridging stent graft

“The clinical community has been asking for a durable, low-profile commercial device that allows them to easily treat more patients,” Johnny LeBlanc, director of Aortic Product Management, Cook Medical, was quoted as saying in a press release. “We pioneered this technology with key thought leaders and continue to listen and innovate to the market needs. The ZFEN+ has received breakthrough device designation from the FDA, so we are excited to see this innovation progress into a clinical study. The ZFEN+ is the next generation and future of fenestrated technologies.”
“Outside the USA, Bentley has supplied 300,000 BeGraft stents to patients in 80 countries around the world. We are pleased that this study approval of the FDA brings us one step closer to making our leading technology also available to American patients suffering from vascular diseases,” said Sebastian Büchert, CEO, Bentley.
The ZFEN+ clinical study is a prospective, multicentre, single-arm study that will include sites in the USA and Europe. At this time, the FDA has approved enrolment of the first 30 patients. Ultimately, Cook intends to enrol 102 patients in the pivotal study. The primary safety endpoint is a composite measure of device technical success and procedural safety within 30 days. Likewise, the primary effectiveness endpoint is a composite measure of freedom from aneurysmrelated mortality and freedom from clinically significant reintervention through 12 months post procedure. A composite measure is a group of variables collected during the clinical study and analysed.
“The ZFEN+ endovascular graft will meet a significant need for our patients,” said Gustavo Oderich (University of Texas Health Science Center at Houston, Houston, USA), who is the global principal investigator of the study.
“There are not many options to treat complex aortic aneurysms other than open repair or off-label use of devices. Even long after off-the-shelf devices become available, the ZFEN+ will offer a tailored approach specific to the patient anatomy. The ZFEN+ clinical study will provide a benchmark for safety and effectiveness of a less invasive option to treat complex abdominal aortic aneurysms.”
26 Market Watch September 2023 | Issue99
Cagent Vascular’s Serranator




CONTROVERSIES CHALLENGES CONSENSUS INNOVATION EDUCATION EVIDENCE Controversies Update Vascular & Endovascular REGISTER NOW CXSYMPOSIUM.COM 23–25 APRIL 2024 TUESDAY-THURSDAY NEW VENUE, E xCeL LONDON, UNITED KINGDOM Peripheral Arterial Controversies Aortic Controversies Acute Stroke Controversies Vascular Trauma Controversies Venous & Lymphatic Controversies Vascular Access Controversies The Hurting Leg Controversies
compatibility and 2mm to 4mm balloon diameters. All Advance Serenity products are available in both the USA and Canada and will be available in Europe in the coming months as of July 2023.
“After looking at the needs of patients with this condition, we built
Boston, USA), co-principal investigator of the TRANSCEND clinical trial.
“The SurVeil DCB is the next generation DCB as established by results from the TRANSCEND trial, which is the only head-to-head pivotal study that has been conducted versus the market-leading DCB.”

options. According to Endospan, the Nexus and Nexus Duo systems provide a minimally invasive alternative, reducing procedure and hospitalisation times. The company advises that the Nexus aortic arch stent graft system is an investigational device, limited by US law to investigational use.
 Terumo Aortic’s Thoraflex Hybrid
Terumo Aortic’s Thoraflex Hybrid
Shape Memory Medical announces sublicense of intellectual property
Shape Memory Medical has announced that it has entered into a sublicense agreement with “a global medtech market leader” in a press release. Under the agreement, Shape Memory Medical will sublicense its proprietary shape memory polymer technology for a narrow indication in a therapeutic area outside of Shape Memory Medical’s cardiovascular, endovascular and neurovascular focus, in return for an upfront licence payment, and future milestones and royalties.

While pursuing this and other potential sublicense opportunities, Shape Memory Medical remains committed, the press release states, to the clinical and commercial development of its proprietary technology within its core business— aortic, peripheral vascular, and neurovascular embolisation.
Commenting on the agreement, Shape Memory Medical’s president and CEO, Ted Ruppel, said: “We are excited to have executed this first sublicense agreement with a highly
Conference calendar
9–13 September 2023
Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Annual Congress 2023 Copenhagen, Denmark cirsecongress.cirse.org/about/ theannualcongress
17–21 September 2023
UIP 2023 World Congress Miami Beach, USA myavls.org/annual-congress-2023. html
respected medical device manufacturer. Given the unique properties of our shape memory polymers in clinical applications, we believe this will be the first of several arrangements in which our materials will help improve and further differentiate market-leading medical products, to benefit patients and grow market share for our partners.”


The Shape Memory Medical team, the press release adds, “combined expertise in polymer chemistry and formulation, medical device development, manufacturing, and clinical research to develop a medical technology platform and a pipeline of medical devices for a variety of clinical indications”. The company’s core shape memory polymer technology offers “unique” properties including vascular space filling, radiopacity, low radial force, porosity to support thrombus formation, and stimulation of the immune response and healing system. Shape Memory Medical’s intellectual property portfolio covers the development, manufacture, and clinical application of its novel shape memory polymer technology.
26–29 September 2023
European Society for Vascular Surgery (ESVS) Annual Meeting 2023 Belfast, UK esvs.org/events/annual-meeting/ annual-meeting-2023


28–29 September 2023
London Aorta 2023 London, UK londonaorta.org
US FDA seeks to “modernise” clinical trials with new draft guidance
The US Food and Drug Administration (FDA) has released draft guidance with updated recommendations for good clinical practices (GCPs) aimed at modernising the design and conduct of clinical trials.
In a statement, the regulator said that the updates are intended to help pave the way for more efficient clinical trials to facilitate the development of medical products, with an aim to be “more agile without compromising data integrity or participant protections”.
The draft guidance is adopted from the International Council for Harmonisation’s (ICH) recently updated E6(R3) draft guideline that was developed to enable the incorporation of rapidly developing technological and methodological innovations into the clinical trial enterprise.
“A more robust clinical trial ecosystem that is capable of producing reliable evidence more efficiently may support more informed decisionmaking in developing medical products to help patients,” said FDA commissioner Robert M Califf. “These draft recommendations propose a major step forward in this work. Building quality into the design and conduct of trials and encouraging the use of innovative trial designs and health
29 October–2 November 2023
The VEINS (Venous Endovascular Interventional Strategies) and VIVA (Vascular Interventional Advances) 2023 Las Vegas, USA viva-foundation.org/future-meetings
8–10 November 2023
Paris Vascular Insights (PVI) Paris, France paris-vascular-insights.com
14–18 November 2023
VEITHsymposium 2023 New York, USA veithsymposium.org/index.php
technologies are essential to truly advance clinical trials and generate meaningful results.”

GCPs ensure the safety of trial participants, as well as the integrity of the data generated from trials. Over the years, the clinical trial enterprise has been viewed as costly, inefficient and constrained by inadequate collaboration and insufficient utilisation of technology, data sources and innovations in design and conduct. The COVID-19 pandemic highlighted many of these challenges, while also spurring the development of new approaches, FDA’s statement adds.
“These draft recommendations were developed with the aim to streamline trials, making them more efficient and flexible as the trial enterprise continues to evolve,” said M Khair ElZarrad, director of the FDA’s Center for Drug Evaluation and Research’s Office of Medical Policy. “We hope these recommendations, once finalised, will encourage thoughtful approaches to conducting clinical trials with a focus on participant safety and data integrity.”
ElZarrad led the ICH Expert Working Group in developing the ICH E6(R3) draft guideline. Academic clinical trial experts from various ICH member countries also played an important role in informing the work of the expert group.
22–24 November 2023
The Vascular Societies’ Annual Scientific Meeting Dublin, Ireland vascularsociety.org.uk

1–3 December 2023
20th European Angiology Days Online europeanangiologydays.net
3–5 March 2024
27th European Vascular Course


Maastricht, The Netherlands vascular-course.com
14–16 March 2024
IM Endo Forum - International Multidisciplinary Endovascular Forum Edition 2024 Florence, Italy imendoforum.com
23–25 April 2024 Charing Cross (CX) Symposium 2024 London, UK cxsymposium.com
News 30 Market Watch September 2023 | Issue99 Vascular News is a trusted, independent source of news and opinion in the vascular and endovascular world. Sign up for a free print subscription* and e-newsletter subscription** www.vascularnews.com *Available for US and EU readers only ** Available worldwide
Industry



*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the vascular arena Editorially independent Visit vascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels

Visit our website for more information on use, indications, contraindications, warnings/precautions and availability within your market. Manufactured by: Bolton Medical Inc, 799 International Parkway, Sunrise, Florida 33325, USA Product availability subject to local regulatory approval. Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. USA_0266-A Apr23 • PM-06885 terumoaortic.com Discover solutions for every segment of the aorta Low Profile Without Compromise Relay®Pro Expanded Versatility ٷ Greatest applicability to broader patient population ٷ Most aggressive tapers New dissection & transection indications Now indicated to treat all thoracic pathologies Indicated for all Thoracic Pathologies ٷ Indicated for use in all disease types (aneurysm, dissection, transection) ٷ Multiple configurations for a more personalized approach ٷ Relay®Pro NBS - approved for distal extension of Thoraflex™ Hybrid Expanded Treatment Options ٷ Bare stent and non-bare stent options
Wide range of diameters and lengths
Upon request configurations to fit specific patient needs
ٷ
ٷ
































































































 Terumo Aortic’s Thoraflex Hybrid
Terumo Aortic’s Thoraflex Hybrid


















