

Sound science reinforced by sound evidence
Stefano Fazzini (Tor Vergata Hospital, Rome, Italy) goes into the details and the data behind Shockwave Intravascular Lithotripsy (IVL; Shockwave Medical)— a technology he states is “changing the boundaries” in peripheral arterial disease (PAD).

ARE WE STILL CONFINED BY THE complexity of calcium when treating vascular disease? Endovascular treatment is a frontline therapy for patients with PAD and the presence of heavy calcification represents the most hostile anatomical challenge we face as vascular specialists.
A poor vessel preparation can lead to dissections and high residual stenosis and consequently increased utilisation of stents. Stenting outcomes can also be significantly affected by calcium with poor stent expansion leading to acute/early failure through stent thrombosis and/or crush. Drug uptake with a drug-coated balloon (DCB) has also been proven to be reduced by the barrier of calcium.1
As disease becomes more complex, the need for effective and consistent vessel preparation is important and this can facilitate improved outcomes and a leave nothing behind strategy. This approach has become a reality, and specialty percutaneous transluminal angioplasty (PTA) or debulking devices can provide a solution.2
One of the most innovative technologies available today is Shockwave IVL. IVL can significantly improve effectiveness at the vessel preparation phase.
Peripheral arteries differ from coronary arteries, which have a high level of elastic fibres; they are predominantly muscular and affected with medial calcification and consequent loss of vessel compliance. Together with increasing luminal gain and minimising complications, a better vessel compliance is the key to a superior vessel prep with IVL and the novelty that differentiates it from other techniques.
Calcific plaques impair vascular distensibility and need high pressures to be modified, leading to complications such as flow-limiting dissections, embolisation, or life-threatening complications such as a rupture (e.g. in the iliac arteries).
The changing paradigm of IVL uses shockwaves delivered on an intuitive lowpressure (4 ATM) balloon platform to avoid vessel barotrauma. This unique mechanism of action derives from a miniaturisation of shockwave treatment used in the treatment
of kidney stones for over 30 years with adaptations made for safety and effectiveness, and is easy to use to modify cardiovascular calcium. The IVL source, in contrast to that for extracorporeal lithotripsy, has close proximity to the calcium and the goal is only to fracture, not pulverise.
These shockwaves are a specific form of sonic pressure waves produced by emitters arrayed within a balloon angioplasty catheter. A small electrical discharge at the emitters vaporises the fluid and creates a rapidly expanding bubble within the balloon.
Shockwaves fracture superficial and deep calcium with an effective pressure of ~50 ATM, changing vessel compliance and allowing an improved luminal gain, while safely passing through the soft tissue; fractures occur when shockwaves encounter tissue with higher acoustic impedance, like calcium, while they have no effect on soft tissue due to having similar impedance to water.
One of the most appealing safety features is the lower risks of embolisation because calcium remains in situ, it is disrupted and not pulverised, with the internal elastic lamina acting as a membrane.3
Compared to the use of atherectomy, IVL is not a complex and time-consuming procedure, but requires a few simple indications for use to improve effectiveness, such as a proper sizing. It should be 10% oversized compared to the reference vessel diameter.4
Despite being a relatively new tool, IVL has a growing data pool to back up the science with a randomised controlled trial (RCT) that demonstrated superiority in procedural success vs. conventional PTA in femoropopliteal lesions, with a reduction in complications and 75% reduction in the need for provisional stenting.4
The PAD III RCT demonstrated superior vessel preparation in an IVL group compared to a PTA group, with no complications (embolisations, perforations, thrombus, no flow), atraumatic treatment (max ballooning
pressure: 6.3 ATM vs. 11.3 ATM, p<0.0001), reduced dissections (type ≥C dissections: 3.5% vs. 15.1%, p=0.03) and bailout stenting (4.6% vs. 18.3%, p=0.0002).4
William A Gray and Gunnar Tepe, coprincipal investigators of DISRUPT PAD III, recently published two-year outcomes that confirm previous successful data: primary patency favoured IVL over PTA at one year (80.5% vs. 68%; p=0.017) and remained favourable through two years (74.4% vs. 57.7%; p=0.005).5
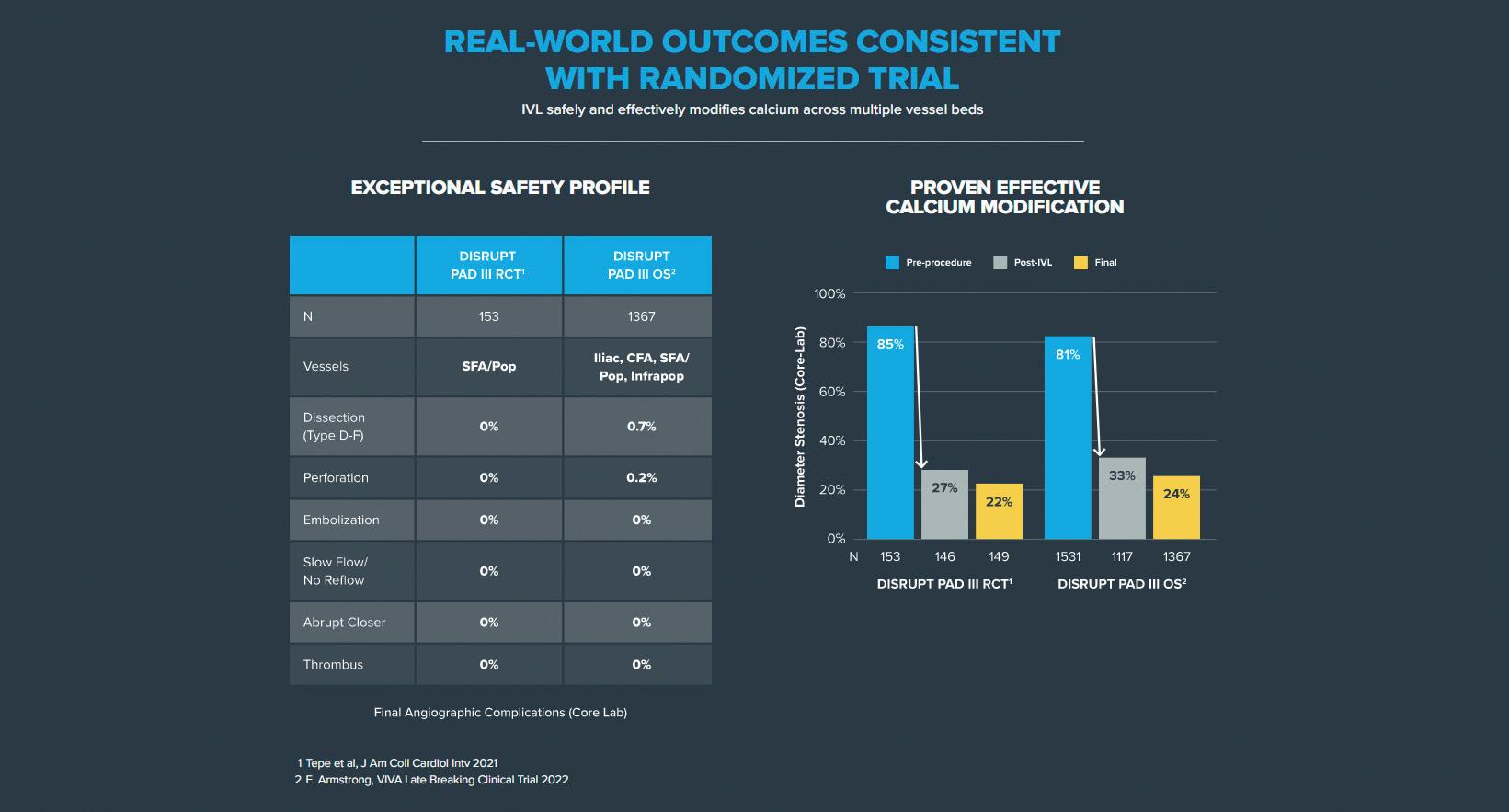
The PAD III RCT was reinforced by the PAD III observational study (OS)—a large all-comers registry that showed IVL to be an effective tool across all peripheral vessel beds, achieving consistently low residual stenoses and extremely low complications.6
The Disrupt PAD III OS with 1,373 patients and 1,677 lesions represents the largest prospective ‘real-world’ evidence for the treatment of heavily calcified PAD, and showed consistent outcomes with the randomised trial: an exceptional safety profile without final angiographic complications and proven effective calcium modification (residual final stenosis of 22% vs. 25%, PAD III RCT vs. OS, respectively).6,7
These optimal outcomes from the PAD III OS reinforce the predictability of IVL and
its ability to consistently modify calcium across multiple peripheral vessel beds (iliac, femoral, popliteal and infrapopliteal arteries), challenging lesions (severe calcification, chronic total occlusions, long [>15cm] and eccentric lesions) and complex patients (chronic limb-threatening ischaemia, dialysis, and female patients).
The safety characteristics of IVL mean it is an effective tool in territories that have previously been difficult to treat with debulking technologies. Due to the low rates of dissections and distal emboli and no need for embolic protection devices, bifurcation disease can be simplified. The reduction in bailout stenting that has been observed also favours the use of IVL as vessel prep in ‘high-flexion zones’ and territory considered
Compliance is key and opens the door to successful treatments, changing previous procedure plans and preserving many future options.”Stefano Fazzini
Shockwave IVL M5+ delivers up to 10 cycles of 30 pulses (two per second) through five emitters arrayed in 60mmlength balloon platform.
tool to assess the effects of endovascular revascularisation in patients with peripheral calcified lesions. A better vessel compliance is well detected by a better Doppler waveform (monophasic changing to multiphasic) and duplex can confirm results from angiograms.
IVL is emerging as a powerful tool for the treatment of heavily calcified peripheral arteries and enabling new vascular pathways. It is changing the boundaries in PAD and transfemoral access for large-bore devices.
References
1. Fanelli F, Cannavale A, Gazzetti M, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol. 2014;37(4):898–907.
‘no-stent zones’; in these districts, stents historically have not performed well when calcium is a contributing factor.

In the iliac district, IVL can open new pathways to become an adjunctive treatment for both occlusive (reducing kissing stent, stent length, covered stent, stent recoil) and aortic disease to facilitate hostile calcified access for large-bore devices (reducing iliac ruptures and use of open/endoconduits).
Recently, we presented at the 2022 European Society for Vascular Surgery (ESVS) annual meeting (20–23 September, Rome, Italy) our IVL experience to facilitate aortic endograft delivery in heavily calcified iliac access.8 There are two main indications, access only and associated iliac occlusive disease, in which an increased vessel compliance and luminal gain play a key role.
A third indication for using IVL prior to aortic endografts is allowing proper iliac limbs expansion. Heavily calcified iliac arteries could pose an obstacle to the correct expansion of iliac extension, resulting in an increased risk for limb occlusion. Even in this case, an accurate vessel preparation with IVL could prevent this risk, avoiding adjunctive stenting inside the iliac limbs.
The goal of changing vessel compliance aims to restore natural mechanics to the vessel, adding elasticity and increasing pulsatility. This is a paradigm shift in how we should approach calcium. Compliance is key and opens the door to successful treatments, changing previous procedure plans and preserving many future options.
Duplex ultrasonography could be a potential intraoperative adjunctive imaging
2. Ormiston W, Dyer-Hartnett S, Fernando R, et al. An update on vessel preparation in lower limb arterial intervention. CVIR Endovascular. 2020 Nov 27;3(1):86.
3. Kereiakes DJ, Virmani R, Hokama JY, et al. Principles of intravascular lithotripsy for calcific plaque modification. JACC: Cardiovascular Interventions. 2021;14(12):1275–92.
4. Tepe G, Brodmann M, Werner M, et al. Intravascular lithotripsy from the randomised Disrupt PAD III trial. JACC: Cardiovascular Interventions. 2021;14(12)1352–61.
5. Tepe G, Brodmann M, Bachinsky W, et al. Intravascular lithotripsy for peripheral artery calcification: mid-term outcomes from the randomized Disrupt PAD III trial. Journal of the Society for Cardiovascular Angiography & Interventions 2021 Jun 28;14(12):1352–1361.
6. Adams G, Soukas PA, Mehrle A, et al. Intravascular lithotripsy for treatment of calcified infrapopliteal lesions: results from the Disrupt PAD III observational study. J Endovasc Ther 2022 Feb;29(1):76–83.
7. Armstrong EJ, Soukas PA, Sammas N, et al. Intravascular lithotripsy for treatment of calcified, stenotic iliac arteries: a cohort analysis from the Disrupt PAD III study. Cardiovasc Revasc Med. 2020 Oct;21(10):1262–1268.
8. Fazzini S, Bosiers M, et al. A paradigm shift in tackling hostile calcified access. ESVS annual meeting, Rome, 2022 Sep.
Fazzini
Case report
IVL: A paradigm shift in the treatment of common femoral artery lesions

Raphaël Coscas (Ambroise Paré Hospital, AP-HP and Paris-Saclay University, Paris, France) illustrates the use and benefits of Shockwave Intravascular Lithotripsy (IVL; Shockwave Medical) in a common femoral artery (CFA) case. Owing in large part to the therapy’s ability to modify vessel compliance, Coscas believes endovascular repair of the CFA using IVL as the main preparation tool for calcified lesions “should be discussed routinely”.

ENDOVASCULAR REPAIR OF THE CFA is subject to controversy. Open surgery remains considered the ‘gold standard’ due to its excellent long-term patency,1 but early local complications (wound dehiscence, infection, crural nerve injuries) are frequent drawbacks that can impair the comfort and the walking capability of the patient for several weeks.2 Endovascular repair is a compelling approach in CFA lesions, especially when they do not involve the bifurcation. The French randomised TECCO trial demonstrated that endovascular repair was associated with fewer early complications and similar patency rates at 24 months when compared to open surgery.3 However, many physicians are still reluctant to use endovascular repair for CFA lesions since these lesions are
generally highly calcified and endovascular repair is fraught with technical failures. Implanting a stent in the CFA is possible but it could create challenges for future femoral access. These points highlight the need for specific preparation tools for CFA lesions.
Over several years, endovascular repair of the CFA in an outpatient setting has become a first-line approach in our centre. IVL totally changed our perception of endovascular repair of the CFA as it allows better preparation of heavily calcified lesions. IVL improves the luminal gain and decreases the rates of dissections and stent placement as demonstrated by the Disrupt PAD III trial.4
Here we present the case of an 84-year-
old man with severe disabling claudication of the right lower limb due to a highly calcified stenosis of the CFA. He has no major comorbidities and lives alone at home. Duplex scan evaluation revealed a tight stenosis of the common femoral artery with a heavily calcified circumferential lesion and a peak systolic velocity of 380cm/s. The duplex waveform was monophasic below the lesion. A non-significant iliac stenosis and severe lesions of the distal arteries were also evident. A computed tomography angiography (CTA) scan confirmed a highly calcified stenosis of the CFA sparing the bifurcation (Figure 1). The CFA diameter was measured as 7.4mm. The contralateral CFA presented as calcified but not stenosed and thus suitable for puncture. An endovascular repair of the CFA using an IVL preparation was decided as our preferred strategy.
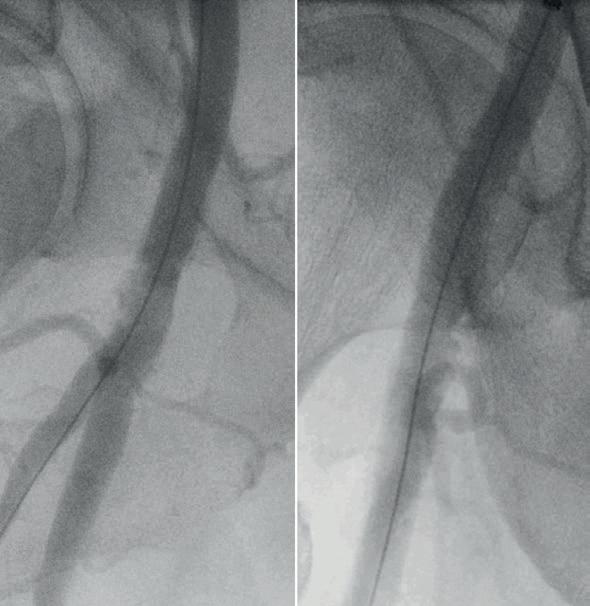
The procedure was scheduled as a sameday discharge intervention and performed under local anaesthesia with sedation in an operating room using standard fluoroscopic guidance with a mobile C-arm. A 7Fr 45cm sheath was brought from the contralateral femoral artery with a crossover technique. The initial angiography showed the lesion, which appeared to be a near occlusion of the CFA (Figure 2), with a low flow in the profunda femoris artery (PFA). A 0.014” wire was positioned antegrade in the PFA due to lesion morphology to preserve the bifurcation. An 8x60mm M5+ IVL catheter

(Shockwave Medical) was selected to prepare the lesion. The use of a slightly oversized balloon allows a perfect apposition to the lesion to deliver the sonic pressure waves. Care was given to deliver the pulses using an overlap strategy to bring the sonic waves at different levels of the lesion to fracture the plaque (Figure 3). The fact that the balloon is inflated at a low pressure (four atmospheres) allowed for inflation in the distal portion of the PFA without having the fear of damaging it. A total of 300 pulses was delivered on the lesion to optimise the effect of IVL. The postpreparation angiography was performed with two different incidences (anterior and oblique view) showing a successful preparation. The luminal gain was satisfactory without
significant residual stenosis or dissection. An 8x60mm drug-coated balloon was inflated at eight atmospheres for three minutes as the anti-restenotic therapy. Completion angiography showed a successful procedure with good flow in the CFA, the PFA and the superficial femoral artery (SFA; Figure 4). The patient was discharged home the same day with dual antiplatelet therapy. At six-month follow-up, the patient presented as healthy and could walk without any limitation. Duplex scan evaluation confirmed a satisfactory mid-term result of the procedure without any significant restenosis and a triphasic flow at the level of and below the lesion.

This case illustrates the use and benefits of IVL in CFA lesions. The availability of an 8mm M5+ facilitates optimal sizing for most CFA lesions. IVL therapy expands the endovascular treatment opportunities for CFA lesions. It modifies the compliance of the vessel by creating microfractures in the plaque, allowing for a controlled expansion with limited complications. In cases where a stent may finally be needed, this ‘leave nothing behind’ approach is usually viable, and maintains options for future procedures. Sufficient preparation using IVL allows for better stent deployment and apposition. Recent data from various centres and registries support the use of IVL in CFA lesions.5–7 In today’s practice, endovascular repair of the CFA using IVL as the main
preparation tool for calcified lesions should be discussed routinely.
References
1. Aboyans V, Ricco JB, Bartelink MEL, et al. Editor's choice - 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(3):305–68.
2. Halpin D, Erben Y, Jayasuriya S, et al. Management of isolated atherosclerotic stenosis of the common femoral artery: a review of the literature. Vasc Endovascular Surg 2017;51(4):220–7.
3. Goueffic Y, Della Schiava N, Thaveau F, et al. Stenting or surgery for de novo common femoral artery stenosis. JACC Cardiovasc Interv. 2017;10(13):1344–54.
4. Tepe G, Brodmann M, Werner M, et al. Intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized Disrupt PAD III trial. JACC Cardiovasc Interv. 2021;14(12):1352–61.
5. Colacchio EC, Salcuni M, Gasparre A, et al. Midterm results of intravascular lithotripsy for severely calcified common femoral artery occlusive disease: a single-center experience. J Endovasc Ther. 2022:15266028221105188.
6. Baig M, Kwok M, Aldairi A, et al. Endovascular intravascular lithotripsy in the treatment of calcific common femoral artery disease: a case series with an 18-month follow-up. Cardiovasc Revasc Med. 2022;43:80–4.
7. Brodmann M, Schwindt A, Argyriou A, Gammon R. Safety and feasibility of intravascular lithotripsy for treatment of common femoral artery stenoses. J Endovasc Ther. 2019;26(3):283–7.
Raphaël Coscas is a professor of vascular surgery at Ambroise Paré Hospital, AP-HP and Paris-Saclay University in Paris, France.
[IVL therapy] modifies the compliance of the vessel by creating microfractures in the plaque, allowing for a controlled expansion with limited complications.”3 4 Figure 3. Care was given to deliver the pulses using an overlap strategy to bring the sonic waves at different levels of the lesion to fracture the plaque
Case report
Improving compliance is the key to “best endovascular” CFA treatment
In this case report, Narayanan Thulasidasan (Guy’s & St Thomas’ NHS Foundation Trust, London, UK) demonstrates how the M5+ Intravascular Lithotripsy (IVL) catheter (Shockwave Medical) has transformed endovascular treatment options for the common femoral artery (CFA), “opening up minimally invasive therapy to many more patients considered high risk for open surgery without compromising on effectiveness”.



ENDOVASCULAR TREATMENT OF the CFA was first indirectly reported in 19941 and then specifically in 2004.2–3 However, two decades later—despite multiple singlearm studies, a small RCT,4 and a recent retrospective propensity-matched comparison of over a thousand cases5—it remains at best infrequently utilised outside a small number of centres, and at worst regarded with outright hostility. At our institution we take a pragmatic approach; whilst the majority of patients with symptomatic CFA atherosclerotic disease continue to be treated with common femoral endarterectomy and patch plasty, we do consider endovascular treatment of the CFA in patients with elevated perioperative risk (of wound infection or from an anaesthetic perspective), and those with high frailty score/poor mobility and/or shorter life expectancy.
In chronic limb-threatening ischaemia (CLTI) patients for whom endovascular treatment of the CFA is deemed most appropriate, careful consideration must be given to the choice of technique. Distribution of disease (including involvement of the profunda femoris artery [PFA] origin), CFA diameter, and, importantly, the presence of nodular calcification—which frequently occurs in CFA lesions and predicted poorer outcomes in the CAULIFLOWER study5 — must all be taken into account. Due to the suboptimal response of such heavily calcified lesions to simple balloon angioplasty without prior vessel preparation, these patients were often previously excluded from endovascular treatment of the CFA if they had arteries that were too large for Supera (Abbott) stenting, or patency of both the PFA and superficial femoral artery (SFA), conferring increased risk of distal embolisation from atherectomy. IVL, however, with its exceptional safety profile (0% distal embolisation and 0.7% type D–F dissection)6 and our own experience of its strong performance in this lesion type, has shifted the paradigm for risk and efficacy of

endovascular treatment of the CFA in this setting. An 89-year-old female patient was referred to our institution with bilateral painful legs and a large, deep, infected pre-tibial ulcer on the right. Her medical history included hypertension, hyperlipidaemia, chronic obstructive pulmonary disease, chronic kidney disease (stage 3a), osteoarthritis and restless leg syndrome, and she was noted to have irondeficiency anaemia and new atrial fibrillation. Computed tomography angiography (CTA) at the referring hospital revealed diffuse heavy vascular calcification, patent right iliac arteries, multifocal stenotic disease in the CFA and SFA and then a heavily calcified chronic total occlusion (CTO) of the popliteal artery reconstituting in the P3 segment with three-vessel tibial run-off.
The possibility of primary below-knee amputation was discussed, but the patient was understandably adamant that she did not want this, as her mobility was already restricted and she feared losing what independence she had left. Therefore, with the primary objectives of limb salvage and pain control, a multidisciplinary team decision was made to attempt total endovascular revascularisation. Due to continuous lower limb movement secondary to restless legs syndrome, the procedure was performed under general anaesthesia (using a totally intravenous technique to reduce the risk of postoperative delirium). The planned strategy was to treat the right CFA with IVL from a contralateral groin approach, thus facilitating antegrade right CFA access, which would expedite crossing and primary stenting of the popliteal CTO in order to minimise the anaesthetic time in this multi-morbid patient.
Left CFA retrograde access was obtained, heparinisation commenced with target activated clotting time >200 seconds, and a
7Fr crossover sheath (Flexor Balkin, Cook Medical) advanced to the right external iliac artery (EIA). Intravascular ultrasound (IVUS; EagleEye, Philips) was employed in order to minimise use of iodinated contrast, revealing 60% right CFA stenosis with reference vessel diameter between 8 and 9mm. An 8mm M5+ catheter was easily advanced to the 5.5mm-diameter right SFA, and five cycles of IVL were delivered to its stenotic proximal/mid segments. With experience, we are comfortable oversizing the Shockwave catheter well beyond the minimum recommended 1.1:1 ratio, as the semi-compliant balloon is never inflated above the lowest required pressure to achieve wall apposition so the risk of dissection is extremely low. This flexibility in sizing allows significant cost saving by enabling use of a single Shockwave catheter at multiple levels. The M5+ catheter was then withdrawn to the right CFA and a further five cycles of IVL delivered, with slight adjustments in the position of the catheter between cycles to vary the incident angle of the lithotripsy pulse on the calcium. After IVL vessel preparation, plain balloon angioplasty (Sterling, Boston Scientific) was performed to 5mm in the SFA and 8mm in the CFA, followed by application
of a 6mm paclitaxel-coated balloon (Ranger, Boston Scientific) to the SFA. Repeat IVUS and angiography confirmed significant luminal gain in the treated segments, and, importantly, no dissections in the right CFA.


Using a micropuncture technique, ultrasound-guided antegrade access to the right CFA was then obtained in the segment treated with IVL, and a 6Fr sheath easily inserted. For speed, subintimal crossing of the popliteal CTO with a Hi-Torque Command 18 ST guidewire (Abbott) was accepted, with targeted re-entry to the proximal P3 segment using an Outback catheter (Cordis). The CTO was pre-dilated to high pressure with a 6mm Athletis

balloon (Boston Scientific), facilitating nominal deployment of a 5.5x200mm Supera stent from P3 to the distal SFA. IVUS demonstrated the proximal end of the stent had landed in a residual stenosis, so proximal extension with a 6mm BioMimics3D stent (Veryan Medical) was undertaken. Completion IVUS and angiography confirmed excellent stent position, expansion and flow with preservation of the threevessel run-off. A ProStyle (Abbott) was used for closure of the 7Fr left CFA access, and manual compression for the 6Fr right CFA access. Check ultrasonography confirmed the right CFA remained widely patent.
Ultrasound follow-up at five months confirmed patency of all treated segments, and the patient has experienced a significant reduction in pain (which is now confined to the location of the slowly healing ulcer) and retains her mobility. This case demonstrates how IVL applied to the CFA not only effectively treats calcified plaque, but can also enable immediate percutaneous access to optimally treat more distal disease by changing the vessel compliance sufficiently to facilitate antegrade puncture. We consider IVL to be a significant addition to our armamentarium for the endovascular
treatment of the CFA, opening minimally invasive therapy to many more patients considered high risk for open surgery without compromising on effectiveness.
References

1. Mandalam KR, Rao VR, Sandhyamani S, et al. Focal occlusive disease of the common femoral artery: a report of 20 cases. Cardiovasc Surg. 1994 Aug;2(4):498–502.
2. Silva JA, White CJ, Quintana H, et al. Percutaneous revascularization of the common femoral artery for limb ischemia. Catheter Cardiovasc Interv. 2004 Jun;62(2):230–3.


3. Stricker H, Jacomella V. Stent-assisted angioplasty at the level of the common femoral artery bifurcation: midterm outcomes. J Endovasc Ther. 2004 Jun;11(3):281–6.
4. Gouëffic Y, Della Schiava N, Thaveau F, et al. Stenting or surgery for de novo common femoral artery stenosis. J Am Coll Cardiol Intv. 2017 Jul;10 (13):1344–1354.
5. Nakama T, Takahara M, Iwata Y, et al. One-year outcomes of thromboendarterectomy vs endovascular therapy for common femoral artery lesions: CAULIFLOWER study results. JACC Cardiovasc Interv. 2022 Jul 25;15(14):1453–1463.
6. Armstrong EJ. Intravascular Lithotripsy for the treatment of peripheral artery calcification: results from the Disrupt PAD III observational study. Oral presentation at VIVA 2022, latebreaking clinical trials session.
Narayanan Thulasidasan is a consultant interventional radiologist at Guy’s & St Thomas’ NHS Foundation Trust in London, UK.
We consider IVL to be a significant addition to our armamentarium for the endovascular treatment of the CFA.”
Preserving options in no-stent zones
Bella Huasen (Lancashire Teaching Hospitals NHS Foundation Trust, Preston, UK) outlines a recent femoropopliteal occlusion case, during which Shockwave Intravascular Lithotripsy (IVL; Shockwave Medical) proved to be an effective vessel preparation strategy in this ‘no-stent zone’.

THERE ARE NO SIGNS OF atherosclerotic disease regression internationally, with an increasing number of chronic limb-threatening ischaemia (CLTI) patients presenting with heavily calcified disease and ulcerations. Whilst healthcare authorities work on prevention strategies, we as endovascular clinicians must work hard on ‘cures’ to avoid amputation. In our trust, cases are discussed at the CLTI

multidisciplinary team meeting every week, and those with calcific disease are listed for endovascular intervention with Shockwave IVL. IVL is helping fight against calcium by increasing vessel wall compliance, thus minimising significant dissection and procedural complications and

resulting in acute procedural success with a significantly reduced need for stenting. In our CLTI algorithm, IVL is not classed as an alternative but as an adjunctive therapy to our current treatment pathway by providing consistent vessel preparation for a definitive treatment strategy. This is a simple and effective tool, often used as part of day-case work, and so the learning curve is short.
We present an 85-year-old female with multiple comorbidities including hypertension, diabetes mellitus type 2, and cardiac disease, who came to our care with Rutherford 5 CLTI involving painful ulcers affecting the second and third toe, limiting mobility and self-confidence. Computed tomography angiography (CTA) imaging confirmed an occlusive lesion of the distal superficial femoral artery (SFA) into the proximal popliteal artery (POPA) with little flow into the foot. We were keen to avoid

treatment that would require stent insertion alone due to the associated risk of stent failure in this region, where high pressure and mechanical dynamics in limb flexion create deformations and the literature suggests many existing stent designs are not yet successfully

able to withstand the repetitive movement that impacts artery-stent combination.
The procedure was performed as a day case. On-table Doppler ultrasound confirmed the CTA findings. Subcutaneous 1% lidocaine local anaesthetic was infiltrated under ultrasound guidance followed by puncture of the common femoral artery in antegrade access using the Seldinger technique. Initially, a 4Fr sheath was inserted. Angiogram confirmed occlusive disease of the distal SFA-POPA. A long, 7Fr Advantage sheath (Terumo) and 0.014” Advantage track wire (Terumo) were used to cross the occlusion. This was followed by a 7x60mm Shockwave M5+ IVL catheter (Shockwave Medical), which was used across the calcified length of the lesion using all the 300 pulses available on the catheter. It is important to up size, to obtain maximum benefits. Focus was made to cover the entire lesion area and


cross cover the entry-exit site of subintimal spaces. Successful vessel preparation was achieved with low residual stenosis and without dissection or distal embolisation. Treatment of the target lesion was concluded using a 7mm sirolimus balloon (Selution, MedAlliance) which was inflated for three minutes. Finally, the crural arteries were treated using 1.5–2.0–2.5mm tapered balloons. A 6Fr Angioseal (Terumo) was used for haemostasis. There were no immediate or late complications. The patient was on dual antiplatelet therapy for three months. There was no further intervention in an 18-month period with almost complete resolution of the ulcers.
Bella Huasen is an endovascular and interventional radiology consultant at Lancashire Teaching Hospitals NHS Foundation Trust in Preston, UK.
IVL allows me to treat CLTI patients safely and effectively with minimal complications due to increased vascular compliance of the vessel.”
Case report
THE PERUGIA VASCULAR AND endovascular team has been a strong believer in the endovascular revolution since the early nineties, exploring the possibilities provided by new devices to treat aortic and thoracic disease.
Nowadays, iliofemoral accesses represent
one of the most important concerns when dealing with complex aortic repair. Many custom-made or off-the-shelf thoracoabdominal endografts require largebore sheaths to facilitate delivery, and for patients with diseased, calcified vascular accesses this can lead to complications with
both perioperative and long-term morbidity and mortality,1 which forces physicians to modify their approach and prevents perfect tailoring to the vascular anatomy.
Shockwave IVL (Shockwave Medical) expands feasibility and safety during these challenging procedures, allowing a complete endovascular management by delivering sonic pressure waves into the vessel wall to fracture superficial and deep calcium. This changes vessel compliance, creating more elasticity in the vessel and thus granting the possibility to take into account only visceral vessels and aortic features, overcoming most of the vascular accesses’ limitations.
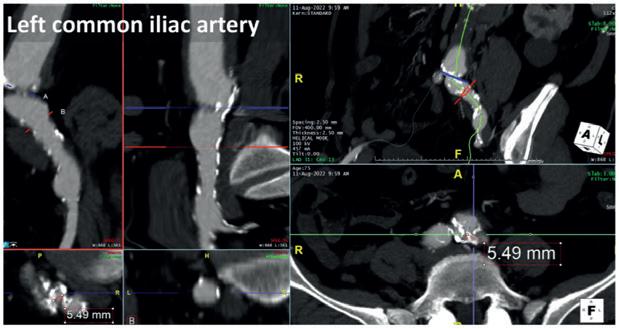
Herein we report the case of a 75-year-old male patient treated in 2009 for infrarenal ruptured abdominal aortic aneurysm (AAA) who developed a proximal pararenal anastomotic pseudoaneurysm of 55mm in maximum axial diameter (Figure 1)



As the patient was a poor candidate for open repair, we opted for an E-nside fourinner-branched, pre-cannulated endograft (Artivion). The major concern about the proposed approach was that the vascular
accesses as a tight circumferential calcified stenosis affected the bilateral origins of the common iliac arteries (Figure 2). Because of this obstructive disease, the femoral pulses were weak, with biphasic wave form bilaterally detected by duplex ultrasound during preoperative evaluation.
Furthermore, two large lumbar arteries arose from the aortic bifurcation and therefore the plan consisted of a distal landing inside the previous aorto-aortic surgical graft, avoiding sacrificing these vessels in order to reduce the risk for spinal cord ischaemia (Figure 3). Moreover, the aortic arch and descending thoracic aorta, as shown in Figure 4, presented irregular floating thrombotic apposition, imposing the need to avoid an upper extremity access and thus the need for both iliac arteries to accomplish endovascular reconstruction.
After preparing both femoral accesses for percutaneous manipulation with two Proglide vascular closure devices (Abbott) using the pre-close technique, an M5+ 8x60mm catheter was advanced over a 0.014” just at the level

IVL: The last piece of the puzzle to overcome the challenge of hostile calcified access in complex endovascular aortic managementGiacomo Isernia and Gioele Simonte (Azienda Ospedaliera di Perugia, Perugia, Italy) outline a branched endovascular aneurysm repair (BEVAR) case in which the M5+ Intravascular Lithotripsy (IVL) catheter (Shockwave Medical) was “effective in modifying arterial compliance to provide luminal gain and reduce procedural complications”.
The new M5+ catheter halves the time needed for treatment, doubling the pulse delivery to two pulses/second.”
of the right common iliac artery stenosis, delivering five cycles of energy (150 pulses overall; Figure 5). The new M5+ catheter halves the time needed for treatment, doubling the pulse delivery to two pulses per second. The exact same procedure was repeated using the remaining five cycles of energy with the same catheter on the contralateral side, to further modify the vessel compliance at the origin of the left common iliac artery.
After vessels preparation, a 24Fr Dryseal introducer sheath (W L Gore) was easily advanced from the right percutaneous common femoral access until reaching the surgical infrarenal aortic graft lumen. The E-nside off-the-shelf endograft was finally deployed through the Dryseal sheath at the planned position.
Taking advantage of the left common femoral access, a 16Fr introducer sheath (Cook Medical) was smoothly inserted until
the distal edge of the just-released innerbranched endograft. An additional steerable 12Fr Reach introducer sheath (Oscor) was coaxially pushed and bent at the cranial edge of the endograft.
In this favourable configuration, exploiting both femoral accesses and the inner branched pre-cannulated technology, visceral artery bridging was effortlessly and promptly completed.
Control angiography was performed using a CO2 automatic injector (Angiodroid) because of a moderate renal impairment. The resulting angiography showed effective pseudoaneurysm exclusion with patent target visceral vessels without signs related to possible iliac complications (i.e. dissection, rupture, thrombus).



In summary, Shockwave IVL was effective in modifying arterial compliance to provide luminal gain and reduce procedural
complications. Postoperative duplex assessment revealed a polyphasic wave in both common femoral arteries as well as a clinically noticeable pulse improvement.
A control computed tomography angiography (CTA) was performed on postoperative day two as per institutional protocol, confirming the intraoperative findings and revealing a significant luminal gain at the treated segments that was especially evident on the right (Figure 6).


References
1. O Donnell TFX, Deery SE, Boitano LT, et al. The long-term implications of access complications during endovascular aneurysm repair. J Vasc. Surg. 2021 April;73(4):1253–1260.



