STUDY: COMBINED ANTIPLATELET, NOAC USE LINKED TO WORSE LIMB OUTCOMES AFTER SUPRAINGUINAL BYPASS
 By Bryan Kay
By Bryan Kay
A NEW ANALYSIS THAT found combined antiplatelet and novel oral anticoagulant (NOAC) therapy after suprainguinal bypass was associated with worse limb outcomes and equivalent survival compared to antiplatelet use alone has “weaknesses” but is “hypothesis-generating,” the senior author insisted during a scientific session at the 2022 annual meeting of the Midwestern Vascular Surgical Society (MVSS) in Grand Rapids, Michigan (Sept. 15–17).
William Robinson, MD, the chief of vascular surgery at Southern Illinois University School of Medicine in Springfield, Illinois, was responding to a question from the floor of the gathering that called into question aspects of the study’s patient population.


The research comes as recent trials report that NOACs, or direct oral anticoagulants (DOACs), alongside antiplatelet use, reduce limb and cardiovascular events when compared to antiplatelet therapy alone after infrainguinal surgical revascularization. Robinson, Syed Zaidi, MD— who delivered the findings at MVSS 2022—et al retrospectively analyzed patients in the Vascular Quality Initiative who had undergone the bypass from 2014 until last year.
this issue:
VINCENT ROWE
VINCENT ROWE HAS BEEN ON the regional vascular meeting circuit this fall delivering invited lectures that drill down on the need to focus on the social determinants of health in order to tackle enduring disparities of vascular outcomes, not least of which in the area of peripheral arterial disease (PAD) and amputation, a personal area of profes sional focus. He spoke before both the MVSS and the New England Society for Vascular Surgery (NESVS) on the topic during September and October, and it also formed a central portion of his WVS Presidential Address in Victoria, Canada, as he brought his 2021–22 term as president to a close at the 2022 WVS annual meeting (Sept. 17–20). In all three lectures, Rowe drew on an upclose example of how the artificial lines that bound the sorts of communities impacted by disparities can play out.
Vincent Rowe, MD, and Raghu Motaganahalli,
“This is the zip code we live in, and this is the zip code just below us,” he said of his home in Los Angeles, gesturing toward a slide in which he showed maps of the two zones and how the pair of areas neatly fit together. The street on which he lives, Rowe pointed out, marks the boundary between the two zip codes. “All of sudden,” he said, referring to a recent inquiry he made into home values in the area, he realized a dishevelled-looking property across the street was worth about $100–150 per square foot more than his own.
“I’m like, ‘Come on, this guy’s house is a dump compared to ours.’ But it doesn’t matter. He’s in the zip code that
By Bryan Kay
PAC T
018
PresortedStandard U.S.Postage PAID PermitNo.384 LebanonJct.KY pecialistsascularVCHANGESERVICEREQUESTED 9400W.HigginsRoad, Suite315 Rosemont,IL60018 www.vascularspecialistonline.comTHE OFFICIAL NEWSPAPER OF THE OCTOBER 2022 Volume 18 Number 10 In
10 Inaugural award Midwestern Vascular initiates new prize for disparities research 26 NESVS Stéphan Haulon discusses future of aortic arch technology in New England lecture 02 From the editor Malachi Sheahan III, MD, takes aim at the NRA 08 SVS Executive Board A demographic breakdown of voting in the SVS officer elections IN .
™
Drug-Coated Balloon (DCB) A new option for PAD treatment. For indications, safety, and warnings, see the IN.PACT 018 DCB advertisement in this publication. See page 4
See page 20
MD, delivered Presidential Addresses at the Western Vascular Society (WVS) and the Midwestern Vascular Surgical Society (MVSS) that spelled out ways in which vascular surgery can advance—and thrive.
REGIONAL VASCULAR PRESIDENTS MEASURE STATE OF SPECIALTY “Our profession doesn’t have much of a flexibility to lose individuals” RAGHU MOTAGANAHALLI “Why don’t we do something? Why don’t we get this big truck? Say it’s our pipeline to surgery”
Voting eligible: Geographic region breakdown
THE EDITOR
Associate Medical Editors
Bernadette Aulivola, MD | O. William Brown, MD | Elliot L. Chaikof, MD, PhD
| Carlo Dall’Olmo, MD | Alan M. Dietzek MD, RPVI, FACS | Professor HansHenning Eckstein, MD | John F. Eidt, MD
| Robert Fitridge, MD | Dennis R. Gable, MD | Linda Harris, MD | Krishna Jain, MD | Larry Kraiss, MD | Joann Lohr, MD
| James McKinsey, MD | Joseph Mills, MD | Erica L. Mitchell, MD, MEd, FACS
| Leila Mureebe, MD | Frank Pomposelli, MD | David Rigberg, MD | Clifford Sales, MD | Bhagwan Satiani, MD | Larry Scher, MD | Marc Schermerhorn, MD | Murray L. Shames, MD | Niten Singh, MD | Frank J. Veith, MD | Robert Eugene Zierler, MD Resident/Fellow Editor
Christopher Audu, MD Executive Director SVS Kenneth M. Slaw, PhD Director of Marketing & Communications Bill Maloney Managing Editor SVS Beth Bales Marketing & Social Media Manager Kristin Crowe
An open letter to the National Rifle Association
 By Malachi Sheahan III, MD
By Malachi Sheahan III, MD
Published by BIBA Publishing, which is a subsidiary of BIBA Medical Ltd.
Publisher Roger Greenhalgh

Content Director Urmila Kerslake
Managing Editor Bryan Kay bryan@bibamedical.com
Editorial contribution
Jocelyn Hudson, Will Date, Jamie Bell, Clare Tierney, Anthony Strzalek, Aaron Kudhail and Adam Pearce
Design Terry Hawes
Advertising Nicole Schmitz nicole@bibamedical.com
Letters to the editor vascularspecialist@vascularsociety.org
BIBA Medical, Europe 526 Fulham Road, London SW6 5NR, United Kingdom
BIBA Medical, North America 155 North Wacker Drive – Suite 4250, Chicago, IL 60606, USA
I think it is time we talked about guns.
I know you don’t like to discuss these things after a school shooting, so I waited. It has been four months since Uvalde. It seems there have been three more since, but I had to look that up. Some school shootings don’t even make the news these days.
I have spent a lot of time trying to figure out what happened to you, and if there is a way forward. Look, you were never really easy to talk to, but at least there was debate. A chance for compromise. When you were formed in 1872 by Civil War General Ambrose Burnside, the goal was to promote rifle practice and improve marksmanship on a scientific basis. Burnside was pretty annoyed by the horrendous aim of his Union soldiers. It
authored the “No compromise. No gun legislation” ethos that the NRA carries to this day.
Behind its new activist leadership, NRA membership soared. Your goal became the rollback of the 1968 restrictions and the abolition of the Bureau of Alcohol Tobacco and Firearms (ATF). By increasing your lobbying pressure, the NRA compelled Congress to pass the Firearm Owners Protection Act of 1986. This law eased restrictions on interstate firearms sales and prohibited the creation of a national database of gun ownership. Complaining about the NRA’s new tactics, Republican Senator Bob Dole stated, “You have to have a litmus test every five minutes, or you’re considered wavering.”
The spirit of the NRA became increasingly antigovernment. A mailing to members declared a proposed assault rifle ban “gives jackbooted government thugs more power to take away our constitutional rights, break in our doors, seize our guns, destroy our property and even injure and kill us.”
After anti-government sentiments drove the April 1995 federal building bombing in Oklahoma City, many NRA members became uncomfortable with this rhetoric. Former U.S. President George H.W. Bush was one. He resigned in protest.
The April 1999 Columbine High School massacre proved to be your litmus test. I am sure it would have been great to lay low and offer empty platitudes to the dead. Unfortunately for you, the NRA annual meeting was scheduled to occur 10 days later in Denver, a few miles away. Just one day after the shooting, the NRA held a conference call to discuss the options. Respectfully cancel or delay the convention? Or defiantly press on? Luckily we don’t have to guess about the substance of
Vascular Specialist is the official newspaper of the Society for Vascular Surgery and provides the vascular specialist with timely and relevant news and commentary about clinical developments and about the impact of healthcare policy. Content for Vascular Specialist is provided by BIBA Publishing. Content for the News From SVS is provided by the Society for Vascular Surgery. | The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or the Publisher. The Society for Vascular Surgery and BIBA Publishing will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services, or the quality or endorsement of advertised products or services, mentioned herein. | The Society for Vascular Surgery headquarters is located at 9400 W. Higgins Road, Suite 315, Rosemont, IL 60018. | POSTMASTER: Send changes of address (with old mailing label) to Vascular Specialist, Subscription Services, 9400 W. Higgins Road, Suite 315, Rosemont, IL 60018. |
RECIPIENT: To change your address, e-mail subscriptions@bibamedical.com | For missing issue claims, e-mail subscriptions@bibamedical. com. Vascular Specialist (ISSN 1558-0148) is published monthly for the Society for Vascular Surgery by BIBA Publishing. | Printed by Vomela Commercial Group | ©Copyright 2022 by the Society for Vascular Surgery
Vascular Specialist | October 20222
Medical Editor Malachi Sheahan III, MD
FROM
Carter was a former U.S. Border Patrol director who led an operation along the southern border with a title too racist to print in my family-friendly publication. Let’s just say Carter was not a fan of Mexicans
Malachi Sheahan III, MD, is the chief medical editor of Vascular Specialist His opinions do not reflect SVS policy or positions.

References: 1. Rundback J, Chandra P, Brodmann M, et al. Novel laser-based catheter for peripheral atherectomy: 6-month results from the Eximo Medical B-LaserTM IDE study. Catheter Cardiovasc Interv 2019;94(7):1010-1017. 2. Shammas NW, Chandra P, Brodmann M, et al. Acute and 30-day safety and effectiveness evaluation of Eximo Medical’s B-LaserTM , a novel atherectomy device, in subjects affected with infrainguinal peripheral arterial disease: results of the EX-PAD-03 trial. Cardiovas Revasc Med. 2020;21(1):86-92. 3. Auryon. Instructions for use. AngioDynamics; 2020. RISK INFORMATION Caution: Federal (USA) law restricts the use of the system by or on the order of a physician. Refer to Directions for Use and/or User Manual provided with the product for complete Instructions, Warnings, Precautions, Possible Adverse Effects and Contraindications prior to use of the product. INDICATIONS FOR USE The AURYON Atherectomy System is indicated for use in the treatment, including atherectomy, of infrainguinal stenoses and occlusions, including in-stent restenosis (ISR). AngioDynamics, the AngioDynamics logo, Auryon, and the Auryon logo are trademarks and/or registered trademarks of AngioDynamics, Inc., an affiliate or a subsidiary. © 2020 AngioDynamics, Inc. US/PA/AD/405 Rev 01 9/2020 Auryon-PAD.comThe future has arrived. Deliver it to your patients. To secure early access for your practice, visit: Conquer every lesion you encounter with the most advanced peripheral atherectomy technology ever: the Auryon system1-3 Clear all lesion types, including severe calcifications, with a single device Revolutionize how you treat, above and below the knee Practice with confidence by minimizing the risk of embolization Say goodbye to tough.
VASCULAR PRESIDENTS MEASURE STATE OF SPECIALTY
matters. The school district is different, income level is different, because that zip code goes in a different direction. So I think we have to be careful, because we can’t just categorize it just on zip code. Hopefully we can start to get more data from our patients, and we’ll have those self-reported determinants to be able to understand. They’ll tell us their educational level, they’ll tell us their mean income, and then we can start to understand better how that all impacts our health.” Pushing the envelope, Rowe raised targeted screening for PAD as a way to get ahead of even tual surgery like amputation in order catch communities vulnerable to disparate outcomes at the prevention stage.
Over in Grand Rapids, Michigan, at the MVSS annual meeting (Sept. 15–17), meanwhile, the day after Rowe gave a visiting lecture on disparities in vascular disease management at the Midwestern meeting, MVSS Presi dent Raghu Motaganahalli focused his own Presidential Address on the rich fabric of vascular surgery and the contributions made by international medical graduates (IMGs)—a population from which he hails—to vascular surgery practice. The vascular chief from Indiana Univer sity School of Medicine compared the vascular profession to an intricate silk rug from Persia, saying that, like these carpets, “we are knitted” by the many individuals who make up the specialty, each coming in different shapes and forms, and with different beliefs, “but, at the end of the day, our profession is far more rich by having those individuals.” Motaganahalli cast the position of IMGs and the hurdles they must negotiate in the U.S. healthcare system against the specter of the coming physician—and vascular surgeon—shortages facing the country. “We are
only about 4,000 individuals in a population of about 4oo million, and our profession doesn’t have much of a leeway, much of a flexibility, to lose individuals,” he told MVSS 2022. “Our profession is clearly at a crossroads when we either accept that portions of our care are relegated to the other professions or specialists, or improve upon our workforce.” According to American College of Surgeons (ACS) data, observed Motaganahalli, there are about 2,800 IMG surgeons operating in the U.S. Vascular surgery’s share of that number equates to 7%, he said. Of the 4,000 vascular surgeons in the U.S., just 15% are women—“are we happy about this?” Motaganahalli continued. “Each vascular surgeon caters to about 80,000 patients, which underscores the value of each one of you. Compared to several other specialties, this is a profession that caters to a very large volume of patients.” While placing a lens over the contributions made by IMGs, Motaganahalli also zeroed in on the position of women vascular surgeons. “I’d like to use this forum to recognize the need to recruit more women into our specialty. As I showed you, 15% of our current workforce are women, but the current trends are increasing, telling us one third of our future workforce will be women. While that is a promising sign, our work is not done yet.” In the same vein, he raised the importance of recognizing IMGs and “their value to enhance the diversity of our profession,” explaining, “Currently, 17% of our vascular surgery workforce is comprised of interna tional medical graduates.” The IMG application pipeline is stagnant, with about 14% of current trainees hailing from that demographic, he said. “Clearly, in a timeline where we have a shortage of manpower, I think we need to come up with a better system, or better existing mechanism
for us to create some novel pathways to help address the physician shortage.”
From an entirely different angle, Rowe suggested a novel approach to tackle the vascular workforce pipeline problem—and potentially diversify the field. Invoking the example of a kid who parlayed a passion for Gran Turismo on the PlayStation into a career as a racing car driver, he raised the potential of a mobile surgical simulation suite to raise the stakes in sparking interest in vascular surgery. “Why don’t we do something? Why don’t we get this big truck? Say it’s our pipeline to surgery,” Rowe said, showing a slide of a refrigerated semi-trailer. “Why don’t we outfit it—put in a laparoscopic station, put a suturing station, put a robotics station back there? Industry can afford it. They’ll put an endovascular station back there. Then we’ll take this big truck, and we park it wherever we want to.” The tackling of both the social determinants of vascular health—the focus of Rowe’s address—and the workforce pipeline issues, to which both Rowe and Mota ganahalli referred, find common ground in what the out going MVSS president called “the diversity bonus,” from a eponymous book by Scott E. Page. “If we truly want to be viable as a professional organization, as a profession itself, we have the duty to protect and strengthen the fabric of our profession,” Motaganahlli implored. “Different kinds of thinkers outperform homogeneous groups on complex tasks,” he said. “You need to have a diverse group of people addressing these tasks so as to have what we call diversity bonuses.” At the close of his WVS 2022 address, Rowe asked, “Can we improve our vascular team?,” before answering, “I think we can, we have to do it; the time is now, and I think together we can celebrate as a group.”
Brief Statement
IN.PACT 018 Paclitaxel-coated PTA Balloon Catheter
Indications for Use The IN.PACT 018 paclitaxel-coated PTA Balloon Catheter is indicated for percutaneous transluminal angioplasty, after appropriate vessel preparation, of de novo, restenotic, or in-stent restenotic lesions with lengths up to 360 mm in superficial femoral or popliteal arteries with reference vessel diameters of 4–7 mm. Contraindications: The IN.PACT 018 DCB is contraindicated for use in: • Coronary arteries, renal arteries, and supra-aortic/cerebrovascular arteries • Patients who cannot receive recommended antiplatelet and/or anticoagulant therapy Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system Patients with known allergies or sensitivities to paclitaxel Women who are breastfeeding, pregnant, or are intending to become pregnant or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and whether there is a potential for adverse reaction in nursing infants from paclitaxel exposure. Warnings A signal for increased risk of late mortality has been identified following the use of paclitaxelcoated balloons and paclitaxel- eluting stents for femoropopliteal arterial disease beginning approximately 2–3 years post-treatment compared with the use of non-drug coated devices. There is uncertainty regarding the magnitude and mechanism for the increased late mortality risk, including the impact of repeat paclitaxel-coated device exposure. Physicians should discuss this late mortality signal and the benefits and risks of available treatment options with their patients. • Use the product prior to the Use-by Date specified on the package. • Contents are supplied sterile. Do not use the product if the inner packaging is damaged or opened. • Do not use air or any gaseous medium to inflate the balloon. Use only the recommended inflation medium (equal parts contrast medium and saline solution). • Do not move the guidewire during inflation of the IN.PACT 018 DCB. • Do not exceed the rated burst pressure (RBP). The RBP is 10 atm (1013 kPa). The RBP is based on the results of in vitro testing. Use of pressures higher than RBP may result in a ruptured balloon with possible intimal damage and dissection. • The safety and effectiveness of using multiple IN.PACT 018 DCBs with a total drug dosage exceeding 34,854 μg of paclitaxel in a patient has not been clinically evaluated. Precautions • The safety and effectiveness of the IN.PACT Admiral DCB (0.035 in guidewire compatible), as established in the clinical studies that were performed primarily via femoral access, can be considered supportive for the IN.PACT 018 DCB. Vessel preparation using only pre-dilatation was studied in the IN.PACT Admiral DCB clinical studies. Other methods of vessel preparation, such as atherectomy, have not been studied clinically.
The IN.PACT 018 DCB has not been evaluated in a clinical study. • This product should only be used by physicians trained in percutaneous transluminal angioplasty (PTA). • This product is designed for single patient use only. Do not reuse, reprocess, or resterilize this product. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or create a risk of contamination of the device, which could result in patient injury, illness, or death. • Assess risks and benefits before treating patients with a history of severe reaction to contrast agents. The safety and effectiveness of the IN.PACT 018 DCB used in conjunction with other drug-eluting stents or drug-coated balloons in the same procedure or following treatment failure has not been evaluated. The extent of the patient’s exposure to the drug coating is directly related to the number of balloons used. Refer to the Instructions for Use (IFU) for details regarding the use of multiple balloons and paclitaxel content. The use of this product carries the risks associated with percutaneous transluminal angioplasty, including thrombosis, vascular complications, and/ or bleeding events. • This product is not intended for the expansion or delivery of a stent. Potential Adverse Effects The potential adverse effects (e.g., complications) associated with the use of the device are: abrupt vessel closure; access site pain; allergic reaction to contrast medium, antiplatelet therapy, or catheter system components (materials, drugs, and excipients); amputation/loss of limb; arrhythmias; arterial aneurysm; arterial thrombosis; arteriovenous (AV) fistula; death; dissection; embolization; fever; hematoma; hemorrhage; hypotension/hypertension; inflammation; ischemia or infarction of tissue/ organ; local infection at access site; local or distal embolic events; perforation or rupture of the artery; pseudoaneurysm; renal insufficiency or failure; restenosis of the dilated artery; sepsis or systemic infection; shock; stroke; systemic embolization; vessel spasms or recoil; vessel trauma which requires surgical repair. Potential complications of peripheral balloon catheterization include, but are not limited to: balloon rupture; detachment of a component of the balloon and/ or catheter system; failure of the balloon to perform as intended; failure to cross the lesion. Although systemic effects are not anticipated, potential adverse events that may be unique to the paclitaxel drug coating include, but are not limited to: allergic/immunologic reaction; alopecia; anemia; gastrointestinal symptoms; hematologic dyscrasia (including leucopenia, neutropenia, thrombocytopenia); hepatic enzyme changes; histologic changes in vessel wall, including inflammation, cellular damage, or necrosis; myalgia/arthralgia; myelosuppression; peripheral neuropathy. Refer to the Physician’s Desk Reference for more information on the potential adverse effects observed with paclitaxel. There may be other potential adverse effects that are unforeseen at this time.
reference appropriate product Instructions for Use for a detailed list of indications,
CAUTION: Federal (USA) law
This
is
electronically at manuals.medtronic.com.
this device to sale by or on the order of a physician.
4 Vascular Specialist | October 2022
Please
warnings, precautions, and potential adverse effects.
content
available
restricts
medtronic.com/INPACT018 UC202305734 EN ©2022 Medtronic. All rights reserved. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. 09/2022 †Data on file with Medtronic. The safety and effectiveness of the IN.PACT Admiral DCB (.035 in guidewire compatible), as established in the clinical studies that were performed primarily via femoral access, can be considered supportive for the IN.PACT 018 DCB. The IN.PACT 018 DCB has not been evaluated in a clinical study. FROM THE COVER: REGIONAL
continued from page 1

Available in 130 cm and 200 cm catheter lengths Crosses tight lesions IN.PACT ™ 018 Drug-Coated Balloon (DCB) Low profile. High performance. Expand your treatment options with the low-profile DCB designed for better deliverability.† New 0.018" DCB platform Discover more at medtronic.com/INPACT018 5 Fr compatible (4–6 mm diameter) 5F Proven drug formulation of IN.PACT™ Admiral™ DCB
AN OPEN
➽frequently in the ensuing years. So instead of meaningful discussion after a school shooting, we got thoughts and prayers.
If Columbine was your turning point as an organization, Sandy Hook was ours as a nation. A moral reckoning, and one that we failed. Could we accept the vision of 20 first graders crumpled dead in their classrooms? Tiny bodies shredded by an AR-15 that had been modified by the manufacturer for close combat in confined spaces, such as a grammar school. A gun that had been advertised by the slogan “Consider your Man Card reissued”. The horror of Sandy Hook was so great that most gun advocates knew there was no logical way to debate. Out of desperation, some even resorted to calling it a hoax. Could our nation stomach all of this and do nothing? Apparently so.
People often think the NRA does the bidding of the firearms industry, but I am certain the opposite is true. After Sandy Hook, the only significant legislation that came close to passing was the Manchin-Toomey Proposal, which would have strengthened and expanded background checks for firearms sales. The NRA was even involved in the negotiations to prepare the bill. The National Shooting Sports Foundation (NSSF), the firearms industry’s trade association, signaled their endorsement of the proposal. But, unbelievably, the NRA withdrew support and declared that the vote would be “scored.” Meaning if a senator did not vote the way the you wanted, their precious NRA grade would be affected. Senator Joe Manchin saw his A grade plummet to a D, just for voting for the legislation that the NRA helped to draft. The NSSF, predictably, fell in line and financed commercials opposing the bill. Manchin-Toomey failed to get the required 60% vote and was defeated on April 17, 2013. The threat of an NRA score even scared five democrats into voting no.
Why do you have such a hold over politicians? Most of your members support common-sense measures. That must be inconvenient for you. To be truly valuable you need a unified voting base. Politicians love single-issue voters. No need to worry about fixing things or making anyone’s life better. Just vote a certain way on a certain issue. I bet if asked, “Should a guy who beats the crap out of his wife and kids be able to buy a gun?”, most of your members would vote no. Inconvenient. Ask any member of a high school debate team the key to a good faith debate, and they will tell you—defining the terms. So, to obscure a good faith debate, you appeal to emotion. Everything is a slippery slope. You label these “red flag laws” and convince your members that if some lunatic can’t buy a gun, they will be next.
Politicians are also afraid of the fruitcakes and wackos in your organization. These are the folks most likely to show up at a town hall and scream at their representatives. Those pejorative terms aren’t mine, though. They’re yours. In the recorded call leading up to the Denver convention after Columbine,
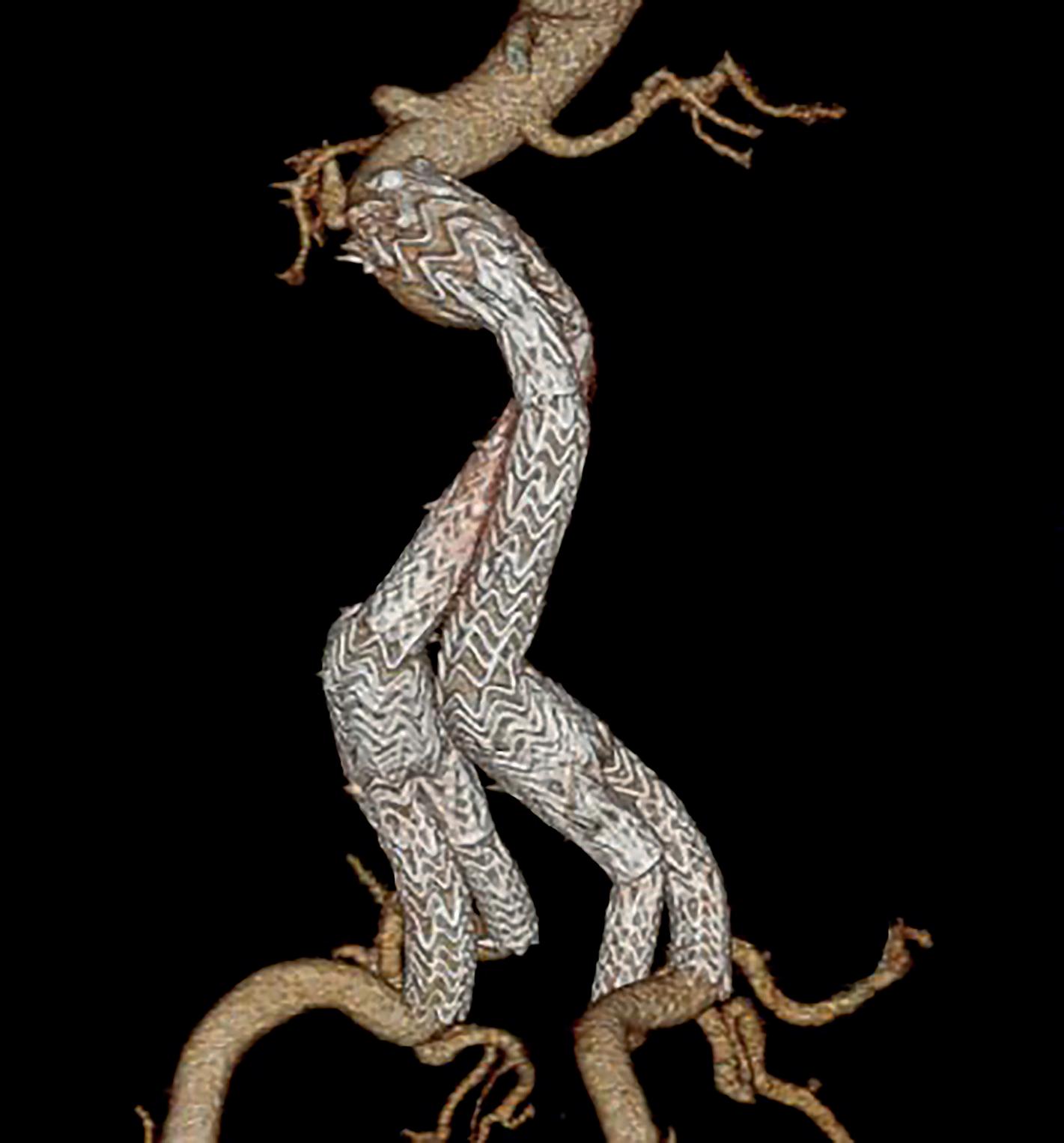
A RECENT SNAPSHOT OF PEDIATRIC VASCULAR TRAUMA INJURIES
your leadership expressed fear that “The fruitcakes are gonna show up,” as PR consultant Tony Makris put it. Marion Hammer, your first female president, seemed to agree, “…you’re gonna have the wackos… dressing like a bunch of hillbillies and idiots…” Maybe it is ironic that your leadership has disdain for some of its most politically valuable constituents. Although I think irony may be lost on an association that opposes all efforts to curb gun violence while garnering tax-exempt status as a social welfare organization.
A study of major pediatric vascular injuries delivered in the Vascular and Endovascular Surgery Society (VESS) Paper Session during the Vascular Annual Meeting (VAM) in June found that 70.2% of the cohort analyzed presented with penetrating trauma. More specifically, noted authors Amanda Tullos, MD, a research fellow at Lousiana State University in New Orleans, and Malachi Sheahan III, the institution’s vascular chief, 60.8%, or 45 of 74, were secondary to gunshots. Arterial injuries were the most common (67 of 74, 90%). In 55.2% (32 of 58) of the patients who underwent surgery, the vascular repair was performed by a vascular surgeon; the remaining (44.8%, 26 of 58) were conducted by trauma surgeons.
Tullos and Sheahan charted the demographics, injuries, and outcomes of the pediatric vascular trauma population, identifying any risk factors associated with complications or death. “Although pediatric vascular trauma is rare, it can result in severe functional deficits or death,” they noted, concluding: “Pediatric trauma is a leading cause of death in patients under 18 years of age. Although vascular trauma is uncommon in pediatric patients, it is associated with high morbidity and mortality.”—Bryan Kay
The NRA’s tactics have caused a sea change in conservative opinion over the past 40 years. Before becoming an NRA darling, Ronald Reagan actually passed strict gun control measures as governor of California. Conservative Chief Justice Warren Burger said, “The Gun Lobby’s interpretation of the Second Amendment is one of the greatest pieces of fraud, I repeat the word fraud, on the American People by special interest groups that I have ever seen in my lifetime.” Even Justice Antonin Scalia said, “The Second Amendment is not a right to keep and carry any weapon whatsoever in any manner whatsoever and for whatever purpose.”
Today the U.S. is awash in guns. More than one for every person. Despite your best efforts to curb research on gun violence through stripping the Centers for Disease Control and Prevention (CDC) of research funding and the Tiahrt Amendment, the facts are pretty damning. Guns are involved in 51% of suicides and 73% of homicides. Since 2015, toddlers have killed more Americans than terrorists. Worldwide, 97% of children four and under killed by guns happen in the U.S. More children
die in a year by gunfire than on-duty police officers and active military members. The explosion of AR-15 purchases happened after the Assault Rifle Ban expired in 2004. A weapon formerly used mainly by law enforcement and the military was now marketed to the public by over 500 manufacturers. To compete, they would make improvements, which, in this context, means making the rifle more lethal.
Many people from urban areas underestimate the emotional connection individuals from more rural regions can have to guns. Some of their fondest memories can be hunting and going to shooting ranges with their family. Heck, I live in Louisiana and work with a surgeon who unironically owns a cannon! Interestingly though, while the number of guns is increasing, the number of homes with a gun has actually decreased. So who is buying all of these AR-15s? Somehow I don’t think these purchases are driven by a love of the Great Outdoors.
A 2020 study by the National Shooting Sports Foundation (NSSF) labeled the fastest-growing segment of customers Urban Defenders, or those likely to buy guns because they “don’t trust others around them.” So while gun advocates often bristle at the suggestion that the AR15 is a weapon of war, the industry clearly promotes it as such. Consider this advertisement from gun manufacturer Daniel Defense: “whether you’re on the battlefield … or protecting your family in the middle of the night.”
It’s time to put to rest all of the myths about protecting kids from guns. Uvalde ended the notion that “good guys with guns” can save us. And please Google “Triangle Shirtwaist Factory” before suggesting schools should have a single entry and exit. But I don’t really expect a good faith argument here. The Protection of Lawful Commerce in Arms Act of 2005 protects gun manufacturers from civil lawsuits resulting from the “misuse” of their products by others. NRA president Wayne LaPierre called it the most significant piece of pro-gun legislation in 20 years. I agree. Let’s roll it back. Holding gun manufacturers once again responsible under the 1972 Consumer Product Safety Act may be the best recourse the public has.
Finally, gun advocates often make the bad faith argument that “cars are dangerous too, maybe we should ban them.” Well, if Honda decides to start equipping Civics with Mad Max style flamethrowers, then, yes, we must take action. But maybe we can start addressing gun violence in the same manner as we did car fatalities. The formation of the 1970 National Highway Traffic Safety Administration led to a number of improvements and hazard-mitigating regulations in the automotive industry. Motor vehicle casualties have steadily fallen among children and teens. In fact, firearms now surpass vehicular accidents as the number one cause of death in children. It is time to establish a national, safety-driven organization with complete access to all gun-related data. We need to know where to put our resources. Smarter policing? Gun regulations? Improved mental and social services? It is far past time to launch a data-driven campaign to end gun violence. Oh, I almost forgot to mention that I saw that you are having a myriad of financial problems. Sending my thoughts and prayers.
References:
1. Nicholas Kristof. Preventing Mass Shootings Like the Vegas Strip Attack. New York Times. Oct 2, 2017.
2. Melinda Wenner Moyer. More Guns Do Not Stop More Crimes, Evidence Shows. Scientific American. Oct 1, 2017. home.NRA.org
3. David Frum. Mass Shootings Don’t Lead to Inaction—They Lead to Loosening Gun Restrictions. The Atlantic. Oct 3, 2017.
4. Antonio Cediel, Amber Goodwin, Michael McBride and Ciera Walker. Why Do We Ignore Initiatives That Reduce Gun Violence? New York Times. Oct 6, 2017.
5. Ron Elving. The NRA Wasn’t Always Against Gun Restrictions. NPR Oct 10, 2017. https://www.npr.org/2021/11/09/1049054141/a-secrettape-made-after-columbine-shows-the-nras-evolution-on-schoolshootings
6 Vascular Specialist | October 2022
LETTER TO THE NATIONAL RIFLE ASSOCIATION continued from page 2FROM THE EDITOR
I think irony may be lost on an association that opposes all efforts to curb gun violence while garnering tax-exempt status as a social welfare organization
Why 25 years of patient-centered innovation really matters
Twenty-five years of patient im pact and durability is worth celebrating in the life of any medical device. And when a device reaches this milestone, it is celebrating more than just 25 years in the treatment landscape. It is a legacy of making a difference for physicians and their patients.
The GORE ® EXCLUDER ® AAA Endoprosthesis for abdominal aor tic aneurysm (AAA) is commem orating 25 years of commercial availability since earning its CE mark in September 1997. It has been used to treat more than 440,000 pa tients worldwide* and has become the most-studied EVAR device according to company-sponsored trials and registries shown on ClinicalTrials.gov for currently available stent grafts.

“The EXCLUDER® AAA Endoprosthesis has been on the market helping patients for well over two decades: A truly remarkable ac complishment and the longest stretch in the industry,” said Makaroun, chief of the Division of Vascular Surgery at the University of Pitts burgh Medical Center and an investigator in each EXCLUDER® device clinical study.
“The close collaboration between medical community and manufacturer has allowed for numerous innovations and improvements along the way, providing for more accurate deployment and better outcomes.”
“As EVAR became more prevalent and ad ditional patient needs were uncovered, it was important that W. L. Gore & Associates—the global materials science company behind the device—continue exploring and improving EVAR solutions,” said Davison, Abdominal Aortic Global Business leader at Gore.

“We recognized the broader potential of the device but knew that there was still a need to keep improving, keep innovating, to help address even more patients’ unique needs and anatomies.”
Over the next two decades, Gore developed and studied additional solutions within its EVAR portfo lio, receiving approvals for larger trunk and contralateral limb diam eters, an iliac branch device, and a next generation of the EXCLUDER® device that is conformable and offers optional angulation control—allowing physicians more treatment options to consider for their patient’s anatomy.
Davison said that at Gore, collab oration with the medical commu nity is key to developing solutions that continue to advance patient care. “We are immensely grateful to the physicians who have put their trust in our devices to help their patients maintain their quality of life and those who have partnered with us as we continue ex ploring future solutions for AAA patients,” he said.
For more information on the GORE® EXCLUDER® AAA Endoprosthesis and the GORE® EXCLUDER® device family, scan the above QR code.

*Based on the number of

trunk-ipsilateral legs distributed. ADVERTORIAL | SPONSORED BY GORE
One mainstay endovascular aneurysm repair (EVAR) device is celebrating not just a quarter century of being commercially available, but the innovation it has inspired along the way. Michel Makaroun, MD, and Willy Davison, PhD, discuss the evolution and innovation of the GORE® EXCLUDER® device family over time.
Michel Makaroun
Willy Davison
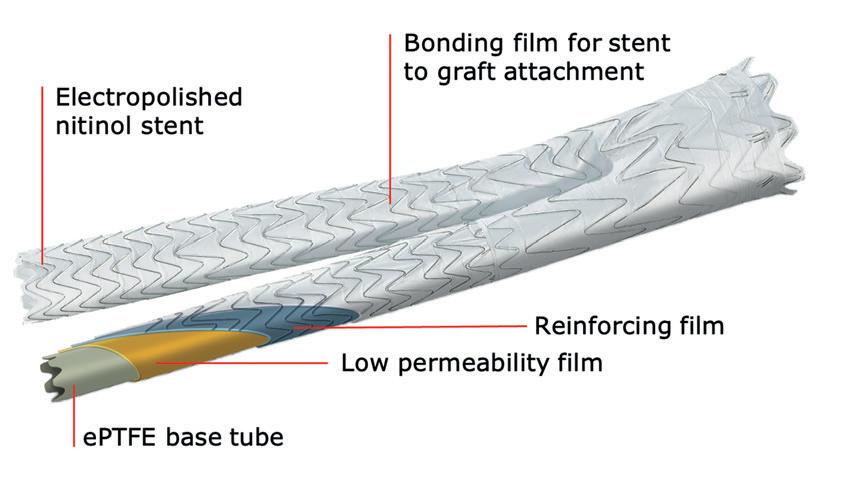
Pre-implant Four-year follow-up showing aortoiliac aneurysms treated with bilateral iliac branch devices EXCLUDER® device design features a nitinol stent, sutureless stent-to-graft attachment and proprietary ePTFE film layers Introduced in July 2004 www.vascularspecialistonline.com 7 Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. Please see accompanying prescribing information in this journal. Products listed may not be available in all markets. GORE, Together, improving life, EXCLUDER and designs are trademarks of W. L. Gore & Associates. ©2022 W. L. Gore & Associates, Inc.
“As EVAR became more prevalent and additional patient needs were uncovered, it was important that
W.
L. Gore
&
Associates—the global materials science company behind the device—continue exploring and improving EVAR solutions”
WILLY
DAVISON
SOCIETY OFFICER ELECTIONS 2022: A GLIMPSE INTO THE ELECTORATE
IN 2020, SOCIETY FOR VASCULAR Surgery (SVS) leadership proposed, and SVS members resoundingly approved with a 97.7% majority (170-4), a bylaws change affecting officer elections. The change man dated that the SVS Nominating Committee propose two candidates for each open offi cer position and that voting be open to all qualified SVS members, regardless of their ability to be present at the Annual Business Meeting (ABM) at the Vascular Annual Meet ing (VAM).

While the existing bylaws had permitted the committee to advance a slate of multi ple candidates, historically this had been un common, with the Nominating Committee more typically advancing just one candidate
for each open position and the membership present at the ABM endorsing that selection. SVS leadership proposed the bylaws change to ensure that members would always have a choice in the election of their officers and, therefore, empower them to truly influence the direction of the SVS.
The second facet of the bylaws change— opening up the election to all qualified SVS members—required a transition to electron ic voting. This change permitted 10 times more SVS members to vote than usually attend the ABM, further empowering the membership to shape and direct the SVS. The results of this change were immediate ly evident as the inaugural internet election in 2020 engaged 482 voters, substantially
GORE® EXCLUDER® AAA Endoprosthesis
GORE® EXCLUDER® AAA Endoprosthesis: INDICATIONS FOR USE: Trunk-Ipsilateral Leg and Contralateral Leg Endoprosthesis. The GORE® EXCLUDER® AAA Endoprosthesis is intended to exclude the aneurysm from the blood circulation in patients diagnosed with infrarenal abdominal aortic aneurysm (AAA) disease and who have appropriate anatomy as described below: Adequate iliac/ femoral access; Infrarenal aortic neck treatment diameter range of 19–32 mm and a minimum aortic neck length of 15 mm; Proximal aortic neck angulation ≤ 60°; Iliac artery treatment diameter range of 8–25 mm and iliac distal vessel seal zone length of at least 10 mm. Aortic Extender and Iliac Extender Endoprosthesis. The Aortic and Iliac Extender Endoprostheses are intended to be used after deployment of the GORE® EXCLUDER® AAA Endoprosthesis. These extensions are intended to be used when additional length and/or sealing for aneurysmal exclusion is desired.
CONTRAINDICATIONS: The GORE® EXCLUDER® AAA Endoprosthesis is contraindicated in: patients with known sensitivities or allergies to the device materials; patients with a systemic infection who may be at increased risk of endovascular graft infection. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. GORE® EXCLUDER® Iliac Branch Endoprosthesis (IBE): INDICATIONS FOR USE IN THE U.S.: Iliac Branch and Internal Iliac Components. The GORE® EXCLUDER® Iliac Branch Endoprosthesis (IBE) is intended to be used with the GORE® EXCLUDER® AAA Endoprosthesis or the GORE®EXCLUDER® Conformable Endoprosthesis to isolate the common iliac artery from systemic blood flow and preserve blood flow in the external iliac and internal iliac arteries in patients with a common iliac or aortoiliac aneurysm, who have appropriate anatomy, including: Adequate iliac/femoral access; Minimum common iliac diameter of 17 mm at the proximal implantation zone of the IBE; External Iliac artery treatment diameter range of 6.5–25 mm and seal zone length of at least 10 mm; Internal iliac artery treatment diameter range of 6.5–13.5 mm and seal zone length of at least 10 mm; Adequate length from the lowest major renal artery to the internal iliac artery to accommodate the total endoprosthesis length, calculated by adding the minimum lengths of required components, taking into account appropriate overlaps between components. GORE® EXCLUDER® Components used in conjunction with GORE® EXCLUDER® Iliac Branch Endoprosthesis: Trunk-Ipsilateral Leg Component. The Trunk-Ipsilateral Leg is intended to provide proximal seal and fixation for the endovascular repair of the aneurysm. For more information on the Trunk-Ipsilateral Leg Component indications for use and deployment, see the GORE® EXCLUDER® AAA Endoprosthesis or the GORE® EXCLUDER® Conformable Endoprosthesis Instructions for Use. Contralateral Leg Endoprosthesis Component. The Contralateral Leg Endoprosthesis is intended to bridge the GORE® EXCLUDER® Device Trunk-Ipsilateral Component to the GORE® EXCLUDER® Iliac Branch Endoprosthesis following deployment of the GORE® EXCLUDER® Iliac Branch Endoprosthesis. Additionally, the Contralateral Leg Endoprosthesis is intended to be used for distal extension of the Iliac Branch Component in the external iliac artery. The Iliac Branch Component can treat external iliac artery diameters up to 13.5 mm.
more than the 250–300 typically present at the ABM. In 2021 and 2022 the number of members that voted grew to 611 and 602 respectively.
leadership success, strategic vision, and a growing awareness and mindfulness of SVS diversity, equity, and inclusion principles.
Michael Dalsing
Since internet vot ing was established in 2020 for that year’s election, the goal has been first to double the number of votes that used to be cast at the in-person ABM at VAM, which averaged 300. This goal has been met with the last two elections engaging 611 and 602 votes, respec tively. But aspirations of the SVS Nominating Committee and Executive Board are much higher, with sights set on achieving 1,000 votes amongst eligible voters in the election. Currently, only Active and Senior Members in good standing at the time of the election are eligible to vote.
The Nominating Committee continues to carry out its crucial charge in identifying, vetting, and advancing highly qualified nom inees based on commitment and service to the SVS, time availability, track record of
The 2022 election for SVS officers was held May 23–June 3, during which time 602 members cast their votes for vice president, secretary, and several bylaw referendum changes. The election was well-publicized, with SVS members receiving 44 reminders and prompts to vote via various media and social media channels, in addition to 39 re minders and posts regarding the candidate Town Hall, during which candidates and is sues were announced and introduced. In all, members were sent more than 70 notices and reminders during the week prior and during the 10-day election period.
The transition to internet voting was initi ated to engage every voting eligible member across the country to participate. There are approximately 2,900 eligible voting members in the SVS. Compared to other medical soci eties that hold internet elections, a 20% voter turnout is considered healthy and above aver age. The goal of the Nominating Committee and the Executive Board is to achieve at least 1,000 votes, or 33% of eligible voters
In looking at the pool of 602 voters in the 2022 election, the demographics of the voter pool largely parallel the demographics of the overall voter-eligible SVS membership, although there are some differences.
With regard to gender, 80% of those who voted identified as male, 18 % female, and 2% undisclosed. This compares to 84%, 11%, and 5% in the overall voter-eligible member
This ability to extend the Iliac Branch Component distally with any Contralateral Leg Endoprosthesis expands the external iliac artery treatment range up to 25 mm. For more information on the Trunk-Ipsilateral Leg and Contralateral Leg Endoprosthesis Component indications for use and deployment, see the GORE® EXCLUDER® AAA Endoprosthesis Instructions for Use. Aortic Extender and Iliac Extender Components. The Aortic and Iliac Extender Components can be used after deployment of the GORE® EXCLUDER® Iliac Branch and GORE® EXCLUDER® AAA Endoprostheses or the GORE® EXCLUDER® Conformable Endoprosthesis. These extensions are used when additional length and/or sealing for aneurysmal exclusion is desired. For more information on Aortic Extender and Iliac Extender indications for use and deployment, see the GORE® EXCLUDER® AAA Endoprosthesis or the GORE® EXCLUDER® Conformable Endoprosthesis Instructions for Use CONTRAINDICATIONS: The GORE® EXCLUDER® Iliac Branch Endoprosthesis (IBE) is contraindicated in: patients with known sensitivities or allergies to the device materials. All components of the GORE® EXCLUDER® Iliac Branch Endoprosthesis (IBE), the GORE® EXCLUDER® AAA Endoprosthesis and GORE® EXCLUDER Conformable Endoprosthesis contain ePTFE, FEP, nitinol (nickel-titanium alloy) and gold. Patients with a systemic infection who may be at increased risk of endovascular graft infection. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. GORE® EXCLUDER® Conformable AAA Endoprosthesis: INDICATIONS FOR USE: The GORE® EXCLUDER® Conformable AAA Endoprosthesis is intended to exclude the aneurysm from the blood circulation in patients diagnosed with infrarenal abdominal aortic aneurysm (AAA) disease and who have appropriate anatomy as described below: Adequate iliac/femoral access; Infrarenal aortic neck treatment diameter range of 16–32 mm and a minimum aortic neck length of 15 mm; Proximal aortic neck angulation ≤ 60°; Iliac artery treatment diameter range of 8–25 mm and iliac distal vessel seal zone length of at least 10 mm. The GORE® EXCLUDER® Conformable Aortic Extender Endoprosthesis (Aortic Extender) is intended to be used after deployment of the GORE® EXCLUDER® Conformable Trunk-Ipsilateral Leg Component. The Aortic Extender is to be used when additional length and/or sealing for aneurysmal exclusion is desired.
CONTRAINDICATIONS: The GORE® EXCLUDER® Conformable AAA Endoprosthesis is contraindicated in: Patients with known sensitivities or allergies to the device materials. Patients with a systemic infection who may be at increased risk of endovascular graft infection. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available.
W. L. Gore & Associates, Inc. Flagstaff, Arizona 86004 goremedical.com Products listed may not be available in all markets. GORE, Together, improving life, EXCLUDER and designs are trademarks of W. L. Gore & Associates, Inc. © 2022 W. L. Gore & Associates, Inc. 22682633-EN SEPTEMBER 2022
8 Vascular Specialist | October 2022
The SVS Executive Board highlights details of the most recent Society for Vascular Surgery (SVS) elections to inform members and further strengthen the process.
“Aspirations of the SVS Nominating Committee and Executive Board are set on achieving 1,000 votes amongst eligible voters in the election”
YOUR SVS
ship. The percentage of female voters was slightly larger than the voter-eligible SVS membership, 18% vs. 11%; and the percent age of males slightly lower, 80% vs. 84%.
With regard to age, 28% of voters were 60+ years of age, followed by 27% each for those 51–60 and 41–50, 9% were under 40 years of age. This compares to 42% over 60 in the overall voter-eligible membership, 17% 51–60; 19% 41–50; and 4% 31–40. In general, those who voted were slightly younger than represented in the voter-eligible membership with 36% of the vote from members under age 40, compared to 22% in the voter-eligible membership.
With regard to geographic location, the population of voters was very close in par allel to the voter-eligible SVS membership, with the South Atlantic at 21% for both vot ers and voter-eligible membership; Mid-At lantic nearly identical with 17% and 16%, respectively; East North Central slightly over-sampled with 16% and 13%; New En gland slightly over-sampled with 10% vs. 7%; the Pacific slightly under-sampled with 13% vs. 14%; and West South Central identical with 8%.
Lastly, with regard to ethnicity, it is dif ficult to make comparisons as the percent ages of members who identify as minorities underrepresented in medicine (URiM) are small. Overall, the percentage of voters who represent as non-Caucasian ethnicities ap pears slightly higher than in the voter-eligible membership, 29% vs. 25%, but this may be due to more non-Caucasian members identi fying as a different ethnicity compared to the voter-eligible membership profile.
The transition to open internet elections in the SVS is going into its third full-year. The work of the Nominating Committee is challenging because the SVS is blessed with an abundance of qualified nominees, and it is likely that due to the high quality of can didates, elections will be predictably close, so every vote matters. “It takes your vote to shape the Society you want to see into the fu ture,” noted SVS President Michael Dalsing, MD. The SVS Executive Board and Nominat ing Committee are currently deliberating further improvements to broaden diversity and choice in candidates, and increase mem ber engagement. If you have comments, email president@vascularsociety.org.
SVS PREPARES FOR IMPORTANT MEMBER SURVEY TO BE HELD IN NOVEMBER CENSUS
THE SOCIETY FOR VASCULAR Surgery (SVS) will survey members in November to continue to track demographic changes, how members would like the SVS to strengthen its value of membership, and what they consider the most important strategic trends to address in the next three to five years.
This biennial census survey is focused on key demographic and strategic information, asking respondents about their needs, interests and member satisfaction. Participation is vital to the Society’s commitment to ensure diversity, equity and inclusion, and to provide an opportunity for all to participate. The survey will be held Nov. 2–22. Participation is essential as the Strategic Board prepares for its January 2023 retreat.
The data collected will help leaders understand how members perceive current member benefits and what potential gaps remain; how they access educational information and information about the Society; what professional issues and trends are most impacting their work; and political advocacy engagement and their
perceptions of the Society’s effectiveness in tackling these advocacy issues.
Other questions will provide information on critical strategic priorities members want the SVS to focus on; what drives members to join and stay with the SVS; how members prefer to get information on critical issues; establish their involvement in other vascular societies and where SVS fits within the overall picture from their perspective; education, travel and quality improvement initiatives; as well as programming and how to fund it. Participants also can enter a draw for a chance to win prizes.
“We want to better understand your professional needs and challenges and your experiences with SVS,” said SVS President Michael Dalsing, MD. “Your feedback is critical to ensure that SVS can best support you and other allied professionals in the vascular field. SVS members helped us to chart our current course and it is time to look to the next three- to five-year horizon.“
Participation invitations will be emailed Nov. 2 from Avenue M. Group LLC, an independent market research firm and SVS’ partner for this survey.—Beth Bales
www.vascularspecialistonline.com 9
Voting eligible: Geographic region breakdown Voting eligible: Gender Breakdown ● Male - 84% ● Female - 11% ● Prefer not to disclose - 5% Voters: Age breakdown ● 60+ - 28% ● 51-60 27% ● 41-50 27% ● Not given - 9% ● 31-40 9% ● 30 and under - 0% Voting eligible: Age breakdown ● 60+ - 42% ● 51-60 17% ● Not given 18% ● 41-50 19% ● 31-40 4% ● 30 and under - 0% Voting eligible: Ethnicity breakdown ● Caucasian 56% ● Hispanic 19% ● Prefer to self-describe - 12% ● American Indian or Alaska Native - 4% ● Not given 3% ● Prefer not to respond - 2% ● Middle Eastern or North African 2% ● Asian or Asian Indian 2% ● Black or African American - 0% ● Native Hawaiian or Pacific Islander 0% Voters: Geographic region breakdown Voters: Gender breakdown ● Male - 80% ● Female - 18% ● Prefer not to disclose - 2% Voters: Ethnicity breakdown ● Caucasian -64% ● Asian or Asian Indian 13% ● Hispanic 7% ● Middle Eastern or North African - 6% ● Not given 4% ● Black or African American - 3% ● Prefer not to respond - 3% ● South Atlantic - 21% ● Mid-Atlantic - 17% ● East North Central 16% ● Pacific 13% ● New England - 10% ● West South Central - 8% ● West North Central - 5% ● Mountain 4% ● East South Central 4% ● International Tier 1 2% ● Unknown - 0% ● South Atlantic 21% ● Mid-Atlantic 16% ● Pacific 14% ● East North Central - 13% ● West South Central - 8% ● New England - 7% ● Mountain 5% ● East South Central 5% ● West North Central - 5% ● International Tier 1 5% ● Not given 1% ● International Tier 3 0% ● International Tier 2 0% Region and state breakdown 2022 officer election: By the numbers South SVS subscribers: 1779 35%= SVS subscribers: 1302 26%= SVS subscribers: 993 20%= SVS subscribers: 952 19%= Northeast Midwest West
RACIAL DISPARITIES
RESEARCH ON DIALYSIS ACCESS SURGERY ATTRACTS MVSS AWARD

Black patients were found at significantly higher risk of having additional surgical procedures, including both maintenance and new creations.
By Bryan Kay
THE AUTHORS BEHIND A NEW STUDY THAT found “significant differences” in hemodialysis vascular access outcomes after first-time arteriovenous (AV) fistula or graft creation “directly related” to a patient’s race said the findings speak “to an injustice within the field of vascular surgery.” The research won the Midwestern Vascular Sur gical Society’s inaugural MVSS Disparities Research Award at the organization’s 2022 annual meeting in Grand Rapids, Michigan (Sept. 15–17).
The data were presented by Shannon McDonnell, a medical student from Loyola University’s Stritch School of Medicine in Maywood, Illinois, who was mentored by Pegge Halandras, MD, from Loyola University Medical Center. The research team carried out a single-institution, retrospective cohort study of 926 patients un dergoing AV procedures from 2007 to 2021, capturing subsequent AV creations and main tenance procedures.
Of the total pool, 45% identified as White and 37% as Black, McDonnell reported. A total of 494 (53%) patients had no addition al procedures, 258 (28%) had one additional
procedure, 95 (10%) had two additional procedures, and 78 (9%) had three or more procedures.
“Black patients have higher frequency of hypertension, COPD [chronic obstructive pulmonary disease], diabetes and anemia,” McDonnell told MVSS 2022. “Just over half of our patients required only the initial fistula or graft place ment. Black patients were at a significantly higher risk of having additional AV access surgical procedures, including both maintenance and new creations as compared to their counterparts of other races.
“Further exploration of these disparities is necessary in order to discover the root of these inequities in care.”
In acknowledging limitations in the study, McDonnell pointed to race’s frequent association with lower socioeco nomic status. “Lower socioeconomic status influences a patient’s utilization of healthcare and can lead to poorer out comes, and worse managed chronic conditions,” she said.
McDonnell also pointed to some reasons why patients may only have one procedure at the study team’s institution, such as a patient receiving a kidney transplant or choosing to continue to dialyze through a catheter instead of under going another procedure.
ANNALS OF VASCULAR SURGERY ANNOUNCES SPECIAL ISSUE DEDICATED TO ORIGINAL WORK BY WOMEN

The Annals of Vascular Surgery peer-review journal has announced an upcoming special issue dedicated to original work by women that is set to be guest edited by Caitlin W. Hicks, MD, the associate fellowship program director for vascular surgery and endovascular therapy and associate professor of surgery at Johns Hopkins Medicine in Baltimore, Maryland.
The issue, which will be published in September 2023 to coincide with Women in Medicine Month, will feature original scientific work whose first and last authors identify as women, with men welcomed as co-authors. Hicks will be supported by an all-female editorial team.
“We encourage all women to submit your best original scientific work related to vascular surgery for peer review and possible publication in our special issue,” Hicks said. “Please note that we will not accept review articles, case reports, or commentaries given the high volume of competitive original scientific works that we anticipate.”
Interested researchers are being encouraged to submit an abstract for review by the editorial team, with those selected then offered the opportunity to submit a full manuscript. Issue editors note that abstracts may have been presented at, or submitted to, a meeting, but should be free from embargo by September 2023.
They should be uploaded by Monday, Dec. 5, with all authors notified of their abstract’s selection or rejection by Monday, Dec. 19. Selected manuscript submissions will be due by Feb. 1, 2023. “Annals of Vascular Surgery is excited about the opportunity to highlight the amazing research being performed by women in our specialty,” added Hicks.
Questions about the process should be directed to Hicks directly at chicks11@jhmi.edu, or Annals of Vascular Surgery managing editor Camilla Davies at cdavies.avs@ gmail.com. The full abstract link is: www.surveymonkey. com/r/AVSWomen Bryan Kay
WARRIORS randomized trial aims to examine early EVAR in women

The trial aims to answer the question of whether women should have their aneu rysms repaired electively using endovas cular aneurysm repair (EVAR) at smaller di ameters than men to improve their survival and quality of life. Imperial College London in London, England, is coordinating the study, which will include collaboration with vascular surgeons from Canada, Denmark, The Netherlands, Sweden and the U.S. The researchers note that new partners from lo cations such as Australia may also be joining.
The investigators noted in a press release announcing the endorsement last month that the rationale and need for this trial, which seeks to recruit nearly 1,200 women, stems from the poor outcomes suffered by women with abdominal aortic aneurysms (AAAs). Although women contribute 15–20% of total AAA burden, and one-third of ruptures, they have been significantly un der-represented in trials which guide current AAA repair, the investigators detail, adding that women have smaller arteries, a four-fold
higher rupture risk and lose eligibility for EVAR at smaller AAA diameters. Treated at the current threshold, the researchers note that a greater proportion receive either high er-risk open surgery or no repair at all. Those that do receive elective repair, do worse, with nearly double the rate of operative mortal ity (open surgery 6%, EVAR 2.3%), higher postoperative complication rates and longer hospital stays.
“We have learnt that women worry a lot about their AAA and modeling has suggested that repair of AAA at 4cm for women might result in improved quality of life and reduced overall cost,” the investigators write. “These potential benefits, as well as reduction in aneurysm-related mortality, would need to be balanced against the operative risk of early repair.” They state that these areas of uncertainty, regarding the optimal strategy for AAA repair in women, are what the trial seeks to answer.
The endorsement provided by the GCRFF allows the investigators to move forward
and seek priority funding from their partner organizations. The team is also hoping for some support from industry for specific as
pects of the trial and/or associated registry.
According to the investigators, WAR RIORS would be the first randomized trial of AAA management with multinational, wide-ranging, expertise and to have received endorsement from the GCRFF. However, they stress that this is the just the first step.
They elaborate: “To gain funding within each participating country, and to implement the trial successfully, we will need consider able support from the vascular and multidis ciplinary community. The disadvantage of women with AAA can no longer be ignored, and we hope that you will support us in what will hopefully be a major step towards read dressing the imbalance in AAA outcomes for women and men. We also hope that this initiative will pave the way to obtain evidence about the management of other underserved patient groups, minorities and rarer diseases managed by vascular surgeons.”
To find out more, contact warriors@ imperial.ac.uk.
10 Vascular Specialist | October 2022
A
multinational collaboration of researchers has received endorsement
from the Global
Cardiovascular Research
Funders
Forum (GCRFF) Multinational Clinical
Trials Initiative for the WARRIORS (Women’s abdominal aortic aneurysm research: Repair immediately or routine surveillance) trial, writes Jocelyn Hudson.
WOMEN’S
RCT “ The disadvantage of women with AAA can no longer be ignored, and we hope that you will support us in what will hopefully be a major step towards readdressing the imbalance in AAA outcomes for women and men”
WARRIORS TRIAL INVESTIGATORS
Shannon Mc Donnell wins the MVSS’ in augural Dispar ities Research Award at the 2022 annual meeting
patients with CAD really stable?
XARELTO®.


INDICATION







WARNING:
DISCONTINUATION OF XARELTO® INCREASES THE RISK OF THROMBOTIC EVENTS,














SPINAL/EPIDURAL HEMATOMA
A. Premature discontinuation of XARELTO® increases the risk of thrombotic events

Premature discontinuation of any oral anticoagulant, including XARELTO®, increases the risk of thrombotic events. If anticoagulation with XARELTO® is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant.
B. Spinal/epidural hematoma
Epidural or spinal hematomas have occurred in patients treated with XARELTO® who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling

patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
• Use of indwelling epidural catheters
• Concomitant use of other drugs that affect hemostasis, such as non-steroidal antiinflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants, see Drug Interactions
• A history of traumatic or repeated epidural or spinal punctures
• A history of spinal deformity or spinal surgery
• Optimal timing between the administration of XARELTO® and neuraxial procedures is not known




Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary.
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis.
events. An increased rate of stroke was observed during the transition from XARELTO® to warfarin in clinical trials in atrial fibrillation patients. If XARELTO® is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant.

• Risk of Bleeding: XARELTO® increases the risk of bleeding and can cause serious or fatal bleeding. Promptly evaluate any signs or symptoms of blood loss and consider the need for blood replacement. Discontinue in patients with active pathological hemorrhage.
An agent to reverse the anti-factor Xa activity of rivaroxaban is available. Because of high plasma protein binding, rivaroxaban is not dialyzable.
Concomitant use of other drugs that impair hemostasis increases risk of bleeding. These include aspirin, P2Y12 platelet inhibitors, dual antiplatelet therapy, other antithrombotic agents, fibrinolytic therapy, NSAIDs, selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs).
Risk of Hemorrhage in Acutely Ill Medical Patients at High Risk of Bleeding: Acutely ill medical patients with the following conditions are at increased risk of bleeding with the use of XARELTO® for primary VTE prophylaxis: history of bronchiectasis, pulmonary cavitation, or pulmonary hemorrhage; active cancer (ie, undergoing acute, in-hospital cancer treatment); active gastroduodenal ulcer or history of bleeding in the three months prior to treatment; or dual antiplatelet therapy. XARELTO® is not for use for primary VTE prophylaxis in these hospitalized, acutely ill medical patients at high risk of bleeding.
• Spinal/Epidural Anesthesia or Puncture: When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma, which can result in long-term or permanent paralysis. To reduce the potential risk of bleeding associated with concurrent use of XARELTO® and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of XARELTO®. Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of XARELTO® is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known. An indwelling epidural or intrathecal catheter should not be removed before at least 2 half-lives have elapsed (ie, 18 hours in young patients aged 20 to 45 years and 26 hours in elderly patients aged 60 to 76 years), after the last administration of XARELTO®. The next dose should not be administered earlier than 6 hours after the removal of the catheter. If traumatic puncture occurs, delay the administration of XARELTO® for 24 hours. Monitor frequently to detect signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), or bowel and/or bladder dysfunction. Instruct patients to immediately report any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
• Use in Patients with Renal Impairment: Nonvalvular Atrial Fibrillation: Periodically assess renal function as clinically indicated (ie, more frequently in situations in which renal function may decline) and adjust therapy accordingly. Consider dose adjustment or discontinuation in patients who develop acute renal failure while on XARELTO®. Clinical efficacy and safety studies with XARELTO® did not enroll patients with CrCl <30 mL/min or end-stage renal disease (ESRD)
dialysis.
Are your
Consider
XARELTO® is the ONLY DOAC indicated in combination with aspirin* to significantly reduce the risk of major CV events† in patients with CAD1-8 *XARELTO® 2.5 mg twice daily + aspirin 75 mg to 100 mg once daily; †Major cardiovascular events include CV death, MI, and stroke. BID = twice daily; DOAC = direct oral anticoagulant; CAD = coronary artery disease; CV = cardiovascular; MI = myocardial infarction; QD = once per day. Tablets not shown to actual size.
XARELTO®, in combination with aspirin, is indicated to reduce the risk of major cardiovascular events (cardiovascular death, myocardial infarction, and stroke) in adult patients with coronary artery disease (CAD). Please read additional Important Safety Information on the following pages and read accompanying Brief Summary of full Prescribing Information, including Boxed WARNINGS for XARELTO® Scan QR code for more data: IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS • Active pathological bleeding • Severe hypersensitivity reaction to XARELTO® (eg, anaphylactic reactions) WARNINGS AND PRECAUTIONS • Increased Risk of Thrombotic Events after Premature Discontinuation: Premature discontinuation of any oral anticoagulant, including XARELTO®, in the absence of adequate alternative anticoagulation increases the risk of thrombotic
on
XARELTO® (2.5 mg BID) ASPIRIN (75 mg-100 mg QD) Arterial Thrombus
(A) PREMATURE
(B)
WARNINGS AND PRECAUTIONS (cont’d)
• Use in Patients with Renal Impairment (cont’d):
Treatment of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), and Reduction in the Risk of Recurrence of DVT and of PE: In patients with CrCl <30 mL/min, rivaroxaban exposure and pharmacodynamic effects are increased compared to patients with normal renal function. There are limited clinical data in patients with CrCl 15 to <30 mL/min; therefore, observe closely and promptly evaluate any signs or symptoms of blood loss in these patients. There are no clinical data in patients with CrCl <15 mL/min (including patients on dialysis); therefore, avoid the use of XARELTO® in these patients. Discontinue XARELTO® in patients who develop acute renal failure while on treatment.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery: In patients with CrCl <30 mL/min, rivaroxaban exposure and pharmacodynamic effects are increased compared to patients with normal renal function. There are limited clinical data in patients with CrCl 15 to <30 mL/min; therefore, observe closely and promptly evaluate signs or symptoms of blood loss in these patients. There are no clinical data in patients with CrCl <15 mL/min (including patients on dialysis); therefore, avoid the use of XARELTO® in these patients. Discontinue XARELTO® in patients who develop acute renal failure while on treatment.
Prophylaxis of Venous Thromboembolism in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding: In patients with CrCl <30 mL/min, rivaroxaban exposure and pharmacodynamic effects are increased compared to patients with normal renal function. There are limited clinical data in patients with CrCl 15 to <30 mL/min; therefore, observe closely and promptly evaluate any signs or symptoms of blood loss in these patients. There are no clinical data in patients with CrCl <15 mL/min (including patients on dialysis); therefore, avoid the use of XARELTO® in these patients. Discontinue XARELTO® in patients who develop acute renal failure while on treatment.
Reduction of Risk of Major Cardiovascular Events in Patients with CAD and Reduction of Risk of Major Thrombotic Vascular Events in Patients with PAD, Including Patients after Recent Lower Extremity Revascularization Due to Symptomatic PAD: For patients with CrCl <15 mL/min, no data are available, and limited data are available for patients with a CrCl of 15 to 30 mL/min. In patients with CrCl <30 mL/min, a dose of 2.5 mg XARELTO® twice daily is expected to give an exposure similar to that in patients with moderate renal impairment (CrCl 30 to <50 mL/min), whose efficacy and safety outcomes were similar to those with preserved renal function. Clinical efficacy and safety studies with XARELTO® did not enroll patients with end-stage renal disease (ESRD) on dialysis.
Pediatric Patients: There are limited clinical data in pediatric patients 1 year or older with moderate or severe renal impairment (eGFR <50 mL/min/1.73 m2); therefore, avoid use of XARELTO® in these patients. There are no clinical data in pediatric patients younger than 1 year with serum creatinine results above 97.5th percentile; therefore, avoid the use of XARELTO® in these patients.
• Use in Patients with Hepatic Impairment: No clinical data are available for adult patients with severe hepatic impairment. Avoid use in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy, since drug exposure and bleeding risk may be increased. No clinical data are available in pediatric patients with hepatic impairment.
• Use with P-gp and Strong CYP3A Inhibitors or Inducers: Avoid concomitant use of XARELTO® with known combined P-gp and strong CYP3A inhibitors or inducers.
• Risk of Pregnancy-Related Hemorrhage: In pregnant women, XARELTO® should be used only if the potential benefit justifies the potential risk to the mother and fetus. XARELTO® dosing in pregnancy has not been studied. The anticoagulant effect of XARELTO® cannot be monitored with standard laboratory testing. Promptly evaluate signs or symptoms suggesting blood loss (eg, a drop in hemoglobin and/or hematocrit, hypotension, or fetal distress).
• Patients with Prosthetic Heart Valves: Use of XARELTO® is not recommended in patients who have had transcatheter aortic valve replacement (TAVR), based on the results of the GALILEO study, which reported higher rates of death and bleeding in patients randomized to XARELTO® compared to those randomized to an antiplatelet regimen. Safety and efficacy of XARELTO® have not been studied in patients with other prosthetic heart valves or other valve procedures. Use of XARELTO® is not recommended in patients with prosthetic heart valves.
• Acute PE in Hemodynamically Unstable Patients/Patients Who Require Thrombolysis or Pulmonary Embolectomy: Initiation of XARELTO® is not recommended acutely as an alternative to unfractionated heparin in patients with pulmonary embolism who present with hemodynamic instability or who may receive thrombolysis or pulmonary embolectomy.
• Increased Risk of Thrombosis in Patients with Antiphospholipid Syndrome: Direct-acting oral anticoagulants (DOACs), including XARELTO®, are not recommended for use in patients with triple-positive antiphospholipid syndrome (APS). For patients with APS (especially those who are triple positive [positive for lupus anticoagulant, anticardiolipin, and anti-beta 2-glycoprotein I antibodies]), treatment with DOACs has been associated with increased rates of recurrent thrombotic events compared with vitamin K antagonist therapy.
DRUG INTERACTIONS
• Combined P-gp and strong CYP3A inhibitors increase exposure to rivaroxaban and may increase risk of bleeding.
• Combined P-gp and strong CYP3A inducers decrease exposure to rivaroxaban and may increase risk of thromboembolic events.
• XARELTO® should not be used in patients with CrCl 15 to <80 mL/min who are receiving concomitant combined P-gp and moderate CYP3A inhibitors (eg, erythromycin) unless the potential benefit justifies the potential risk.

• Coadministration of enoxaparin, warfarin, aspirin, clopidogrel, and chronic NSAID use may increase risk of bleeding.
• Avoid concurrent use of XARELTO® with other anticoagulants due to increased bleeding risk, unless benefit outweighs risk. Promptly evaluate signs or symptoms of blood loss if patients are treated concomitantly with aspirin, other platelet aggregation inhibitors, or NSAIDs.
USE IN SPECIFIC POPULATIONS
• Pregnancy: The limited available data on XARELTO® in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. Use XARELTO® with caution in pregnant patients because of the potential for pregnancy-related hemorrhage and/or emergent delivery. The anticoagulant effect of XARELTO® cannot be reliably monitored with standard laboratory testing. Consider the benefits and risks of XARELTO® for the mother and possible risks to the fetus when prescribing to a pregnant woman.
Fetal/Neonatal adverse reactions: Based on the pharmacologic activity of Factor Xa inhibitors and the potential to cross the placenta, bleeding may occur at any site in the fetus and/or neonate.
Labor or delivery: The risk of bleeding should be balanced with the risk of thrombotic events when considering use in this setting.
There are no adequate or well-controlled studies of XARELTO® in pregnant women, and dosing for pregnant women has not been established. Post-marketing experience is currently insufficient to determine a rivaroxaban-associated risk for major birth defects or miscarriage.
• Lactation: Rivaroxaban has been detected in human milk. There are insufficient data to determine the effects of rivaroxaban on the breastfed child or on milk production. Consider the developmental and health benefits of breastfeeding along with the mother’s clinical need for XARELTO® and any potential adverse effects on the breastfed infant from XARELTO® or from the underlying maternal condition.
• Females and Males of Reproductive Potential: Females of reproductive potential requiring anticoagulation should discuss pregnancy planning with their physician. The risk of clinically significant uterine bleeding, potentially requiring gynecological surgical interventions, identified with oral anticoagulants, including XARELTO®, should be assessed in females of reproductive potential and those with abnormal uterine bleeding.
• Pediatric Use: XARELTO® was not studied and therefore dosing cannot be reliably determined or recommended in children less than 6 months who were less than 37 weeks of gestation at birth, had less than 10 days of oral feeding, or had a body weight of less than 2.6 kg.
Clinical studies that evaluated safety, efficacy, and pharmacokinetic/pharmacodynamic data support the use of XARELTO® 10-mg, 15-mg, and 20-mg tablets in pediatric patients.
For the XARELTO® 2.5-mg tablets, there are no safety, efficacy, and pharmacokinetic/ pharmacodynamic data to support the use in pediatric patients. Therefore, XARELTO® 2.5-mg tablets are not recommended for use in pediatric patients.
Although not all adverse reactions identified in the adult population have been observed in clinical trials of children and adolescent patients, the same warnings and precautions for adults should be considered for children and adolescents.
• Geriatric Use: In clinical trials the efficacy of XARELTO® in the elderly (65 years or older) was similar to that seen in patients younger than 65 years. Both thrombotic and bleeding event rates were higher in these older patients.
OVERDOSAGE
• Overdose of XARELTO®
Discontinue XARELTO® and initiate appropriate therapy if bleeding complications associated with overdosage occur. An agent to reverse the anti-factor Xa activity of rivaroxaban is available.
lead to
ADVERSE REACTIONS
• Most common adverse reactions in adult patients with XARELTO® were bleeding complications.

• Most common adverse reactions in pediatric patients were bleeding, cough, vomiting, and gastroenteritis.
Please read accompanying Brief Summary of full Prescribing Information, including Boxed WARNINGS for XARELTO®

References: 1. XARELTO® [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc. 2. Bevyxxa® [prescribing information]. South San Francisco, CA: Portola Pharmaceuticals. Inc; 2020. 3. Eliquis® [prescribing information]. Princeton, NJ and New York, NY: Bristol-Myers Squibb Company and Pfizer Inc.; 2021. 4. Bevyxxa® (betrixaban)—Notice of Permanent Discontinuance of Manufacturing in April 2020: Portola Pharmaceuticals, Inc. 5. Pradaxa® [prescribing information]. Ridgefield, CT: Boehringer lngelheim Pharmaceuticals, Inc.; 2021. 6. Savaysa® [prescribing information]. Basking Ridge, NJ: Daiichi Sankyo, Inc.; 2021. 7. Eikelboom JW, Connolly SJ, Bosch J, et al; COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377[14):1319-1330. 8. Online supplement to: Eikelboom JW, Connolly SJ, Bosch J. et al; COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl Med. 2017 ;377[14]: 1319-1330.
XARELTO® is licensed from Bayer HealthCare AG, 51368 Leverkusen, Germany. © Janssen Pharmaceuticals, Inc. 2022 09/22 cp-330633v1



Janssen Pharmaceuticals, Inc.
















IMPORTANT SAFETY INFORMATION (cont’d)
may
hemorrhage.
cp-62551v9
Brief Summary of Prescribing Information for XARELTO® (rivaroxaban)
XARELTO (rivaroxaban) tablets, for oral use
XARELTO (rivaroxaban) for oral suspension
See package insert for Full Prescribing Information
WARNING: (A) PREMATURE DISCONTINUATION OF XARELTO INCREASES THE RISK OF THROMBOTIC EVENTS, (B) SPINAL/EPIDURAL HEMATOMA
A. Premature discontinuation of XARELTO increases the risk of thrombotic events
Premature discontinuation of any oral anticoagulant, including XARELTO, increases the risk of thrombotic events. If anticoagulation with XARELTO is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant [see Dosage and Administration (2.3, 2.4) in Full Prescribing Information, Warnings and Precautions, and Clinical Studies (14.1) in Full Prescribing Information].
B. Spinal/epidural hematoma
Epidural or spinal hematomas have occurred in patients treated with XARELTO who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
• use of indwelling epidural catheters
• concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants
• a history of traumatic or repeated epidural or spinal punctures
• a history of spinal deformity or spinal surgery
• optimal timing between the administration of XARELTO and neuraxial procedures is not known [see Warnings and Precautions and Adverse Reactions].
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary [see Warnings and Precautions]
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis [see Warnings and Precautions]
INDICATIONS AND USAGE
Reduction of Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
XARELTO is indicated to reduce the risk of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation.
There are limited data on the relative effectiveness of XARELTO and warfarin in reducing the risk of stroke and systemic embolism when warfarin therapy is well-controlled [see Clinical Studies (14.1) in Full Prescribing Information].
Treatment of Deep Vein Thrombosis
XARELTO is indicated for the treatment of deep vein thrombosis (DVT).
Treatment of Pulmonary Embolism
XARELTO is indicated for the treatment of pulmonary embolism (PE).
Reduction in the Risk of Recurrence of Deep Vein Thrombosis and/or Pulmonary Embolism
XARELTO is indicated for the reduction in the risk of recurrence of DVT and/or PE in adult patients at continued risk for recurrent DVT and/or PE after completion of initial treatment lasting at least 6 months.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
XARELTO is indicated for the prophylaxis of DVT, which may lead to PE in adult patients undergoing knee or hip replacement surgery.
Prophylaxis of Venous Thromboembolism in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding
XARELTO is indicated for the prophylaxis of venous thromboembolism (VTE) and VTE related death during hospitalization and post hospital discharge in adult patients admitted for an acute medical illness who are at risk for thromboembolic complications due to moderate or severe restricted mobility and other risk factors for VTE and not at high risk of bleeding [see Warnings and Precautions and Clinical Studies (14.5) in Full Prescribing Information].
Reduction of Risk of Major Cardiovascular Events in Patients with Coronary Artery Disease (CAD)
XARELTO, in combination with aspirin, is indicated to reduce the risk of major cardiovascular events (cardiovascular death, myocardial infarction, and stroke) in adult patients with coronary artery disease.
Reduction of Risk of Major Thrombotic Vascular Events in Patients with Peripheral Artery Disease (PAD), Including Patients after Lower Extremity Revascularization due to Symptomatic PAD
XARELTO, in combination with aspirin, is indicated to reduce the risk of major thrombotic vascular events (myocardial infarction, ischemic stroke, acute limb ischemia, and major amputation of a vascular etiology) in adult patients with PAD, including patients who have recently undergone a lower extremity revascularization procedure due to symptomatic PAD.
Treatment of Venous Thromboembolism and Reduction in Risk of Recurrent Venous Thromboembolism in Pediatric Patients
XARELTO is indicated for the treatment of venous thromboembolism (VTE) and the reduction in the risk of recurrent VTE in pediatric patients from birth to less than 18 years after at least 5 days of initial parenteral anticoagulant treatment.
Thromboprophylaxis in Pediatric Patients with Congenital Heart Disease after the Fontan Procedure
XARELTO is indicated for thromboprophylaxis in pediatric patients aged 2 years and older with congenital heart disease who have undergone the Fontan procedure.
CONTRAINDICATIONS
XARELTO is contraindicated in patients with:
• active pathological bleeding [see Warnings and Precautions]
• severe hypersensitivity reaction to XARELTO (e.g., anaphylactic reactions) [see Adverse Reactions]
WARNINGS AND PRECAUTIONS
Increased Risk of Thrombotic Events after Premature Discontinuation
Premature discontinuation of any oral anticoagulant, including XARELTO, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. An increased rate of stroke was observed during the transition from XARELTO to warfarin in clinical trials in atrial fibrillation patients. If XARELTO is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant [see Dosage and Administration (2.3, 2.4) and Clinical Studies (14.1) in Full Prescribing Information].
Risk of Bleeding
XARELTO increases the risk of bleeding and can cause serious or fatal bleeding. In deciding whether to prescribe XARELTO to patients at increased risk of bleeding, the risk of thrombotic events should be weighed against the risk of bleeding.
Promptly evaluate any signs or symptoms of blood loss and consider the need for blood replacement. Discontinue XARELTO in patients with active pathological hemorrhage. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
Concomitant use of other drugs that impair hemostasis increases the risk of bleeding. These include aspirin, P2Y12 platelet inhibitors, dual antiplatelet therapy, other antithrombotic agents, fibrinolytic therapy, non-steroidal anti-inflammatory drugs (NSAIDs) [see Drug Interactions], selective serotonin reuptake inhibitors, and serotonin norepinephrine reuptake inhibitors.
Concomitant use of drugs that are known combined P-gp and strong CYP3A inhibitors increases rivaroxaban exposure and may increase bleeding risk [see Drug Interactions].
Risk of Hemorrhage in Acutely Ill Medical Patients at High Risk of Bleeding
Acutely ill medical patients with the following conditions are at increased risk of bleeding with the use of XARELTO for primary VTE prophylaxis: history of bronchiectasis, pulmonary cavitation, or pulmonary hemorrhage, active cancer (i.e., undergoing acute, in-hospital cancer treatment), active gastroduodenal ulcer in the three months prior to treatment, history of bleeding in the three months prior to treatment, or dual antiplatelet therapy. XARELTO is not for use for primary VTE prophylaxis in these hospitalized, acutely ill medical patients at high risk of bleeding.
Reversal of Anticoagulant Effect
An agent to reverse the anti-factor Xa activity of rivaroxaban is available. Because of high plasma protein binding, rivaroxaban is not dialyzable [see Clinical Pharmacology (12.3) in Full Prescribing Information] Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of rivaroxaban. Use of procoagulant reversal agents, such as prothrombin complex concentrate (PCC), activated prothrombin complex concentrate or recombinant factor VIIa, may be considered but has not been evaluated in clinical efficacy and safety studies. Monitoring for the anticoagulation effect of rivaroxaban using a clotting test (PT, INR or aPTT) or anti-factor Xa (FXa) activity is not recommended.
Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis [see Boxed Warning]
To reduce the potential risk of bleeding associated with the concurrent use of XARELTO and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of XARELTO [see Clinical Pharmacology (12.3) in Full Prescribing Information]. Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of XARELTO is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known.
An indwelling epidural or intrathecal catheter should not be removed before at least 2 half-lives have elapsed (i.e., 18 hours in young patients aged 20 to 45 years and 26 hours in elderly patients aged 60 to 76 years), after the last administration of XARELTO [see Clinical Pharmacology (12.3) in Full Prescribing Information]. The next XARELTO dose should not be administered earlier than 6 hours after the removal of the catheter. If traumatic puncture occurs, delay the administration of XARELTO for 24 hours.
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/ analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
Use in Patients with Renal Impairment
Nonvalvular Atrial Fibrillation
Periodically assess renal function as clinically indicated (i.e., more frequently in situations in which renal function may decline) and adjust therapy accordingly [see Dosage and Administration (2.1) in Full Prescribing Information]. Consider dose adjustment or discontinuation of XARELTO in patients who develop acute renal failure while on XARELTO [see Use in Specific Populations]
Treatment of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), and Reduction in the Risk of Recurrence of DVT and of PE
In patients with CrCl <30 mL/min, rivaroxaban exposure and pharmacodynamic effects are increased compared to patients with normal renal function. There are limited clinical data in patients with CrCl 15 to <30 mL/min; therefore, observe closely and promptly evaluate any signs or symptoms of blood loss in these patients. There are no clinical data in patients with CrCl <15 mL/min (including patients on dialysis); therefore, avoid the use of XARELTO in these patients.
Discontinue XARELTO in patients who develop acute renal failure while on treatment [see Use in Specific Populations]
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
In patients with CrCl <30 mL/min, rivaroxaban exposure and pharmacodynamic effects are increased compared to patients with normal renal function. There are limited clinical data in patients with CrCl 15 to <30 mL/min; therefore, observe closely and promptly evaluate any signs or symptoms of blood loss in these patients. There are no clinical data in patients with CrCl <15 mL/min (including patients on dialysis); therefore, avoid the use of XARELTO in these patients.
Discontinue XARELTO in patients who develop acute renal failure while on treatment [see Use in Specific Populations]
Prophylaxis of Venous Thromboembolism in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding
In patients with CrCl <30 mL/min, rivaroxaban exposure and pharmacodynamic effects are increased compared to patients with normal renal function. There are limited clinical data in patients with CrCl 15 to <30 mL/min; therefore, observe closely and promptly evaluate any signs or symptoms of blood loss in these patients. There are no clinical data in patients with CrCl <15 mL/min (including patients on dialysis); therefore, avoid the use of XARELTO in these patients.
Discontinue XARELTO in patients who develop acute renal failure while on treatment [see Use in Specific Populations]
Pediatric Patients
There are limited clinical data in pediatric patients 1 year or older with moderate or severe renal impairment (eGFR <50 mL/min/1.73 m2); therefore, avoid the use of XARELTO in these patients.
There are no clinical data in pediatric patients younger than 1 year with serum creatinine results above 97.5th percentile; therefore, avoid the use of XARELTO in these patients [see Dosage and Administration (2.2) in Full Prescribing Information and Use in Specific Populations].
Use in Patients with Hepatic Impairment
No clinical data are available for adult patients with severe hepatic impairment.
Avoid use of XARELTO in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy since drug exposure and bleeding risk may be increased [see Use in Specific Populations]
No clinical data are available in pediatric patients with hepatic impairment.
Use with P-gp and Strong CYP3A Inhibitors or Inducers
Avoid concomitant use of XARELTO with known combined P-gp and strong CYP3A inhibitors [see Drug Interactions].
Avoid concomitant use of XARELTO with drugs that are known combined P-gp and strong CYP3A inducers [see Drug Interactions].
Risk of Pregnancy-Related Hemorrhage
In pregnant women, XARELTO should be used only if the potential benefit justifies the potential risk to the mother and fetus. XARELTO dosing in pregnancy has not been studied. The anticoagulant effect of XARELTO cannot be monitored with standard laboratory testing. Promptly evaluate any signs or symptoms suggesting blood loss (e.g., a drop in hemoglobin and/or hematocrit, hypotension, or fetal distress) [see Warnings and Precautions and Use in Specific Populations].
Patients with Prosthetic Heart Valves
On the basis of the GALILEO study, use of XARELTO is not recommended in patients who have had transcatheter aortic valve replacement (TAVR) because patients randomized to XARELTO experienced higher rates of death and bleeding compared to those randomized to an anti-platelet regimen. The safety and efficacy of XARELTO have not been studied in patients with other prosthetic heart valves or other valve procedures. Use of XARELTO is not recommended in patients with prosthetic heart valves.
XARELTO® (rivaroxaban)
Acute PE in Hemodynamically Unstable Patients or Patients Who Require Thrombolysis or Pulmonary Embolectomy
Initiation of XARELTO is not recommended acutely as an alternative to unfractionated heparin in patients with pulmonary embolism who present with hemodynamic instability or who may receive thrombolysis or pulmonary embolectomy.
Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome Direct-acting oral anticoagulants (DOACs), including XARELTO, are not recommended for use in patients with triple-positive antiphospholipid syndrome (APS). For patients with APS (especially those who are triple positive [positive for lupus anticoagulant, anticardiolipin, and anti-beta 2-glycoprotein I antibodies]), treatment with DOACs has been associated with increased rates of recurrent thrombotic events compared with vitamin K antagonist therapy.
ADVERSE REACTIONS
The following clinically significant adverse reactions are also discussed in other sections of the labeling:
• Increased Risk of Stroke After Discontinuation in Nonvalvular Atrial Fibrillation [see Boxed Warning and Warnings and Precautions]
• Bleeding Risk [see Warnings and Precautions]
• Spinal/Epidural Hematoma [see Boxed Warning and Warnings and Precautions]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. During clinical development for the approved indications, 34,947 adult patients were exposed to XARELTO.
Hemorrhage
The most common adverse reactions with XARELTO were bleeding complications [see Warnings and Precautions]
Nonvalvular Atrial Fibrillation
In the ROCKET AF trial, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 4.3% for XARELTO vs. 3.1% for warfarin. The incidence of discontinuations for non-bleeding adverse events was similar in both treatment groups. Table 1 shows the number of patients experiencing various types of bleeding events in the ROCKET AF trial.
Table 1: Bleeding Events in ROCKET AF*- On Treatment Plus 2 Days
Parameter
XARELTO N=7111 n (%/year)
Warfarin N=7125 n (%/year)
XARELTO vs. Warfarin HR (95% CI)
Major Bleeding† 395 (3.6) 386 (3.5) 1.04 (0.90, 1.20)
Intracranial Hemorrhage (ICH)‡ 55 (0.5) 84 (0.7) 0.67 (0.47, 0.93) Hemorrhagic Stroke§ 36 (0.3) 58 (0.5) 0.63 (0.42, 0.96) Other ICH 19 (0.2) 26 (0.2) 0.74 (0.41, 1.34)
Gastrointestinal (GI)¶ 221 (2.0) 140 (1.2) 1.61 (1.30, 1.99)
Fatal Bleeding# 27 (0.2) 55 (0.5) 0.50 (0.31, 0.79)
ICH 24 (0.2) 42 (0.4) 0.58 (0.35, 0.96)
Non-intracranial 3 (0.0) 13 (0.1) 0.23 (0.07, 0.82)
Abbreviations: HR = Hazard Ratio, CI = Confidence interval, CRNM = Clinically Relevant Non-Major.
* Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories. These events occurred during treatment or within 2 days of stopping treatment.
† Defined as clinically overt bleeding associated with a decrease in hemoglobin of ≥2 g/dL, a transfusion of ≥2 units of packed red blood cells or whole blood, bleeding at a critical site, or with a fatal outcome.
‡ Intracranial bleeding events included intraparenchymal, intraventricular, subdural, subarachnoid and/or epidural hematoma.
§ Hemorrhagic stroke in this table specifically refers to non-traumatic intraparenchymal and/or intraventricular hematoma in patients on treatment plus 2 days.
¶ Gastrointestinal bleeding events included upper GI, lower GI, and rectal bleeding.
# Fatal bleeding is adjudicated death with the primary cause of death from bleeding.
Figure 1 shows the risk of major bleeding events across major subgroups.
Figure 1: Risk of Major Bleeding Events by Baseline Characteristics in ROCKET AF – On Treatment Plus 2 Days
Treatment of Deep Vein Thrombosis (DVT) and/or Pulmonary Embolism (PE)
EINSTEIN DVT and EINSTEIN PE Studies
In the pooled analysis of the EINSTEIN DVT and EINSTEIN PE clinical studies, the most frequent adverse reactions leading to permanent drug discontinuation were bleeding events, with XARELTO vs. enoxaparin/ Vitamin K antagonist (VKA) incidence rates of 1.7% vs. 1.5%, respectively. The mean duration of treatment was 208 days for XARELTO-treated patients and 204 days for enoxaparin/VKA-treated patients.
Table 2 shows the number of patients experiencing major bleeding events in the pooled analysis of the EINSTEIN DVT and EINSTEIN PE studies.
Table 2: Bleeding Events* in the Pooled Analysis of EINSTEIN DVT and EINSTEIN PE Studies
Parameter
XARELTO† N=4130 n (%)
Enoxaparin/ VKA† N=4116 n (%)
Major bleeding event 40 (1.0) 72 (1.7)
Fatal bleeding 3 (<0.1) 8 (0.2)
Intracranial 2 (<0.1) 4 (<0.1)
Non-fatal critical organ bleeding 10 (0.2) 29 (0.7)
Intracranial‡ 3 (<0.1) 10 (0.2)
Retroperitoneal‡ 1 (<0.1) 8 (0.2)
Intraocular‡ 3 (<0.1) 2 (<0.1)
Intra-articular‡ 0 4 (<0.1)
Non-fatal non-critical organ bleeding§ 27 (0.7) 37 (0.9)
Decrease in Hb ≥ 2 g/dL 28 (0.7) 42 (1.0)
Transfusion of ≥2 units of whole blood or packed red blood cells 18 (0.4) 25 (0.6)
Clinically relevant non-major bleeding 357 (8.6) 357 (8.7)
Any bleeding 1169 (28.3) 1153 (28.0)
* Bleeding event occurred after randomization and up to 2 days after the last dose of study drug. Although a patient may have had 2 or more events, the patient is counted only once in a category.
† Treatment schedule in EINSTEIN DVT and EINSTEIN PE studies: XARELTO 15 mg twice daily for 3 weeks followed by 20 mg once daily; enoxaparin/VKA [enoxaparin: 1 mg/kg twice daily, VKA: individually titrated doses to achieve a target INR of 2.5 (range: 2.0-3.0)]
‡ Treatment-emergent major bleeding events with at least >2 subjects in any pooled treatment group
§ Major bleeding which is not fatal or in a critical organ, but resulting in a decrease in Hb ≥ 2 g/dL and/or transfusion of ≥2 units of whole blood or packed red blood cells
Reduction in the Risk of Recurrence of DVT and/or PE EINSTEIN CHOICE Study

In the EINSTEIN CHOICE clinical study, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 1% for XARELTO 10 mg, 2% for XARELTO 20 mg, and 1% for acetylsalicylic acid (aspirin) 100 mg. The mean duration of treatment was 293 days for XARELTO 10 mg-treated patients and 286 days for aspirin 100 mg-treated patients.
Table 3 shows the number of patients experiencing bleeding events in the EINSTEIN CHOICE study.
Table 3: Bleeding Events* in EINSTEIN CHOICE
Parameter
XARELTO† 10 mg N=1127 n (%)
Acetylsalicylic Acid (aspirin)† 100 mg N=1131 n (%)
Major bleeding event 5 (0.4) 3 (0.3)
Fatal bleeding 0 1 (<0.1)
Non-fatal critical organ bleeding 2 (0.2) 1 (<0.1)
Non-fatal non-critical organ bleeding‡ 3 (0.3) 1 (<0.1)
Clinically relevant non-major (CRNM) bleeding§ 22 (2.0) 20 (1.8)
Any bleeding 151 (13.4) 138 (12.2)
* Bleeding event occurred after the first dose and up to 2 days after the last dose of study drug. Although a patient may have had 2 or more events, the patient is counted only once in a category.
† Treatment schedule: XARELTO 10 mg once daily or aspirin 100 mg once daily.
‡ Major bleeding which is not fatal or in a critical organ, but resulting in a decrease in Hb ≥ 2 g/dL and/or transfusion of ≥ 2 units of whole blood or packed red blood cells.
§ Bleeding which was clinically overt, did not meet the criteria for major bleeding, but was associated with medical intervention, unscheduled contact with a physician, temporary cessation of treatment, discomfort for the patient, or impairment of activities of daily life.
In the EINSTEIN CHOICE study, there was an increased incidence of bleeding, including major and CRNM bleeding in the XARELTO 20 mg group compared to the XARELTO 10 mg or aspirin 100 mg groups.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
In the RECORD clinical trials, the overall incidence rate of adverse reactions leading to permanent treatment discontinuation was 3.7% with XARELTO.
The rates of major bleeding events and any bleeding events observed in patients in the RECORD clinical trials are shown in Table 4.
Table 4: Bleeding Events* in Patients Undergoing Hip or Knee Replacement Surgeries (RECORD 1 3)
XARELTO 10 mg Enoxaparin†
Total treated patients N=4487 n (%) N=4524 n (%)
Major bleeding event 14 (0.3) 9 (0.2)
Fatal bleeding 1 (<0.1) 0
Bleeding into a critical organ 2 (<0.1) 3 (0.1)
Bleeding that required re-operation 7 (0.2) 5 (0.1)
Note: The figure above presents effects in various subgroups all of which are baseline characteristics and all of which were pre-specified (diabetic status was not pre-specified in the subgroup but was a criterion for the CHADS2 score). The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
Extra-surgical site bleeding requiring transfusion of >2 units of whole blood or packed cells 4 (0.1) 1 (<0.1)
Any bleeding event‡ 261 (5.8) 251 (5.6)
XARELTO® (rivaroxaban) XARELTO® (rivaroxaban)
XARELTO 10 mg Enoxaparin†
Surgery Studies N=3281 n (%) N=3298 n (%)
Major bleeding event 7 (0.2) 3 (0.1)
Fatal bleeding 1 (<0.1) 0
Bleeding into a critical organ 1 (<0.1) 1 (<0.1)
Bleeding that required re-operation 2 (0.1) 1 (<0.1)
Extra-surgical site bleeding requiring transfusion of >2 units of whole blood or packed cells 3 (0.1) 1 (<0.1)
Any bleeding event‡ 201 (6.1) 191 (5.8)
Knee Surgery Study
N=1206 n (%) N=1226 n (%)
Major bleeding event 7 (0.6) 6 (0.5)
Fatal bleeding 0 0
Bleeding into a critical organ 1 (0.1) 2 (0.2)
Bleeding that required re-operation 5 (0.4) 4 (0.3)
Extra-surgical site bleeding requiring transfusion of >2 units of whole blood or packed cells 1 (0.1) 0
Any bleeding event‡ 60 (5.0) 60 (4.9)
* Bleeding events occurring any time following the first dose of double-blind study medication (which may have been prior to administration of active drug) until two days after the last dose of double-blind study medication. Patients may have more than one event.
† Includes the placebo-controlled period for RECORD 2, enoxaparin dosing was 40 mg once daily (RECORD 1-3)
‡ Includes major bleeding events
Following XARELTO treatment, the majority of major bleeding complications (≥60%) occurred during the first week after surgery.
Prophylaxis of Venous Thromboembolism in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding
In the MAGELLAN study, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events. Cases of pulmonary hemorrhage and pulmonary hemorrhage with bronchiectasis were observed. Patients with bronchiectasis/pulmonary cavitation, active cancer (i.e., undergoing acute, in-hospital cancer treatment), dual antiplatelet therapy or active gastroduodenal ulcer or any bleeding in the previous three months all had an excess of bleeding with XARELTO compared with enoxaparin/placebo and are excluded from all MAGELLAN data presented in Table 5. The incidence of bleeding leading to drug discontinuation was 2.5% for XARELTO vs. 1.4% for enoxaparin/placebo. Table 5 shows the number of patients experiencing various types of bleeding events in the MAGELLAN study.
Table 5: Bleeding Events in MAGELLAN* Study–Safety Analysis Set - On Treatment Plus 2 Days
MAGELLAN Study¶
XARELTO 10 mg N=3218 n (%) Enoxaparin 40 mg / placebo N=3229 n (%)
Major bleeding‡† 22 (0.7) 15 (0.5)
Critical site bleeding 7 (0.2) 4 (0.1)
Fatal bleeding§ 3 (<0.1) 1 (<0.1) Clinically relevant non-major bleeding events (CRNM) 93 (2.9) 34 (1.1)
* Patients at high risk of bleeding (i.e. bronchiectasis/pulmonary cavitation, active cancer, dual antiplatelet therapy or active gastroduodenal ulcer or any bleeding in the previous three months) were excluded.
† Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories. These events occurred during treatment or within 2 days of stopping treatment.
‡ Defined as clinically overt bleeding associated with a drop in hemoglobin of ≥2 g/dL, a transfusion of ≥2 units of packed red blood cells or whole blood, bleeding at a critical site, or with a fatal outcome.
§ Fatal bleeding is adjudicated death with the primary cause of death from bleeding.
¶ Patients received either XARELTO or placebo once daily for 35 ±4 days starting in hospital and continuing post hospital discharge or received enoxaparin or placebo once daily for 10 ±4 days in the hospital.
Reduction of Risk of Major Cardiovascular Events in Patients with CAD
the COMPASS trial overall, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 2.7% for XARELTO 2.5 mg twice daily vs. 1.2% for placebo on background therapy for all patients with aspirin 100 mg once daily. The incidences of important bleeding events in the CAD and PAD populations in COMPASS were similar.
Table 6 shows the number of patients experiencing various types of major bleeding events in the
Table
Parameter
trial.
- Bleeding into the surgical site requiring reoperation (non-fatal, not in critical organ)
‡ Defined as i) fatal bleeding, or ii) symptomatic bleeding in a critical area or organ, such as intraarticular, intramuscular with compartment syndrome, intraspinal, intracranial, intraocular, respiratory, pericardial, liver, pancreas, retroperitoneal, adrenal gland or kidney; or iii) bleeding into the surgical site requiring reoperation, or iv) bleeding leading to hospitalization.
CI: confidence interval; HR: hazard ratio; ISTH: International Society on Thrombosis and Hemostasis
Reduction of Risk of Major Thrombotic Vascular Events in Patients with Peripheral Artery Disease (PAD), Including Patients after Lower Extremity Revascularization due to Symptomatic PAD
The incidence of premature permanent discontinuation due to bleeding events for XARELTO 2.5 mg twice daily vs. placebo on background therapy with aspirin 100 mg once daily in VOYAGER was 4.1% vs. 1.6% and in COMPASS PAD was 2.7% vs. 1.3%, respectively.
Table 7 shows the number of patients experiencing various types of TIMI (Thrombolysis in Myocardial Infarction) major bleeding events in the VOYAGER trial. The most common site of bleeding was gastrointestinal.
Table 7: Major Bleeding Events* in VOYAGER- On Treatment Plus 2 Days
XARELTO† N=3256 Placebo† N=3248 XARELTO vs. Placebo HR (95 % CI)Parameter n (%) Event rate %/year n (%) Event rate %/year
TIMI Major Bleeding (CABG/non-CABG) 62 (1.9) 0.96 44 (1.4) 0.67 1.4 (1.0, 2.1)
Fatal bleeding 6 (0.2) 0.09 6 (0.2) 0.09 1.0 (0.3, 3.2)
Intracranial bleeding 13 (0.4) 0.20 17 (0.5) 0.26 0.8 (0.4, 1.6)
Clinically overt signs of hemorrhage associated with a drop in hemoglobin of ≥5 g/dL or drop in hematocrit of ≥15%
46 (1.4) 0.71 24 (0.7) 0.36 1.9 (1.2, 3.2)
* Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories.
† Treatment schedule: XARELTO 2.5 mg twice daily or placebo. All patients received background therapy with aspirin 100 mg once daily.
CABG: Coronary artery bypass graft; CI: confidence interval; HR: hazard ratio; TIMI: Thrombolysis in Myocardial Infarction Bleeding Criteria
Other Adverse Reactions
Non-hemorrhagic adverse reactions reported in ≥1% of XARELTO-treated patients in the EINSTEIN DVT and EINSTEIN PE studies are shown in Table 8.
Table 8: Other Adverse Reactions* Reported by ≥1% of XARELTO-Treated Patients in EINSTEIN DVT and EINSTEIN PE Studies
Body System Adverse Reaction
EINSTEIN DVT Study
XARELTO 20 mg N=1718 n (%) Enoxaparin/VKA N=1711 n (%)
Gastrointestinal disorders Abdominal pain 46 (2.7) 25 (1.5)
General disorders and administration site conditions Fatigue 24 (1.4) 15 (0.9)
Musculoskeletal and connective tissue disorders Back pain 50 (2.9) 31 (1.8) Muscle spasm 23 (1.3) 13 (0.8)
Nervous system disorders Dizziness 38 (2.2) 22 (1.3)
Psychiatric disorders Anxiety 24 (1.4) 11 (0.6) Depression 20 (1.2) 10 (0.6) Insomnia 28 (1.6) 18 (1.1)
EINSTEIN PE Study
(0.9, 2.0) 1.1 (0.6, 2.0) 1.4 (0.7, 2.8) 0.7 (0.2, 1.9)
7 (<0.1) 6 (<0.1) 1.2 (0.4, 3.5)
- Bleeding leading to hospitalization (non-fatal, not in critical organ, not requiring reoperation) 188 (1.1) 91 (0.5) 2.1 (1.6, 2.7)
Major GI bleeding 117 (0.7) 49 (0.3) 2.4 (1.7, 3.4)
* Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories. These events occurred during treatment or within 2 days of stopping treatment in the safety analysis set in COMPASS patients.
† Treatment schedule: XARELTO 2.5 mg twice daily or placebo. All patients received background therapy with aspirin 100 mg once daily.
XARELTO 20 mg N=2412 n (%) Enoxaparin/VKA N=2405 n (%)
Skin and subcutaneous tissue disorders Pruritus 53 (2.2) 27 (1.1)
* Adverse reaction with Relative Risk >1.5 for XARELTO versus comparator Non-hemorrhagic adverse reactions reported in ≥1% of XARELTO-treated patients in RECORD 1-3 studies are shown in Table 9.
Table 9: Other Adverse Drug Reactions* Reported by ≥1% of XARELTO-Treated Patients in RECORD 1-3 Studies
Body System Adverse Reaction
XARELTO 10 mg N=4487 n (%) Enoxaparin† N=4524 n (%)
Injury, poisoning and procedural complications Wound secretion 125 (2.8) 89 (2.0)
Musculoskeletal and connective tissue disorders Pain in extremity 74 (1.7) 55 (1.2) Muscle spasm 52 (1.2) 32 (0.7)
Nervous system disorders
Syncope 55 (1.2) 32 (0.7)
Skin and subcutaneous tissue disorders Pruritus 96 (2.1) 79 (1.8) Blister 63 (1.4) 40 (0.9)
* Adverse reaction occurring any time following the first dose of double-blind medication, which may have been prior to administration of active drug, until two days after the last dose of double-blind study medication
Includes the placebo-controlled period of RECORD 2, enoxaparin dosing was 40 mg once daily (RECORD 1-3)
Table 4: Bleeding
Events* in Patients Undergoing
Hip
or Knee Replacement Surgeries
(RECORD 1 3) (continued)
Hip
In
COMPASS
6: Major Bleeding Events in COMPASS - On Treatment Plus 2 Days*
XARELTO† N=9134 n (%/year) Placebo† N=9107 n (%/year) XARELTO vs. Placebo HR (95 % CI) Modified ISTH Major Bleeding‡ 263 (1.6) 144 (0.9) 1.8 (1.5, 2.3) - Fatal bleeding event Intracranial hemorrhage (ICH) Non-intracranial 12 (<0.1) 6 (<0.1) 6 (<0.1) 8 (<0.1) 3 (<0.1) 5 (<0.1) 1.5 (0.6, 3.7) 2.0 (0.5, 8.0) 1.2 (0.4, 4.0) - Symptomatic bleeding in critical organ (non-fatal) - ICH (fatal and non-fatal) Hemorrhagic Stroke Other ICH 58 (0.3) 23 (0.1) 18 (0.1) 6 (<0.1) 43 (0.3) 21 (0.1) 13 (<0.1) 9 (<0.1) 1.4
†
XARELTO® (rivaroxaban) XARELTO® (rivaroxaban)
XARELTO®
Pediatric Patients
Treatment of Venous Thromboembolism and Reduction in Risk of Recurrent Venous Thromboembolism in Pediatric Patients
The safety assessment is based on data from the EINSTEIN Junior Phase 3 study in 491 patients from birth to less than 18 years of age. Patients were randomized 2:1 to receive body weight-adjusted doses of XARELTO or comparator (unfractionated heparin, low molecular weight heparin, fondaparinux or VKA).
Discontinuation due to bleeding events occurred in 6 (1.8%) patients in the XARELTO group and 3 (1.9%) patients in the comparator group.
Table 10 shows the number of patients experiencing bleeding events in the EINSTEIN Junior study.
In female patients who had experienced menarche, ages 12 to <18 years of age, menorrhagia occurred in 23 (27%) female patients in the XARELTO group and 5 (10%) female patients in the comparator group.
Table 10: Bleeding Events in EINSTEIN Junior Study – Safety Analysis Set - Main Treatment Period*
Parameter
XARELTO† N=329 n (%)
Comparator Group‡ N=162 n (%)
Major bleeding§ 0 2 (1.2)
Clinically relevant non-major bleeding¶ 10 (3.0) 1 (0.6)
Trivial bleeding 113 (34.3) 44 (27.2) Any bleeding 119 (36.2) 45 (27.8)
* These events occurred after randomization until 3 months of treatment (1 month for patients <2 years with central venous catheter-related VTE (CVC-VTE). Patients may have more than one event.
† Treatment schedule: body weight-adjusted doses of XARELTO; randomized 2:1 (XARELTO: Comparator).
‡ Unfractionated heparin (UFH), low molecular weight heparin (LMWH), fondaparinux or VKA.
§ Defined as clinically overt bleeding associated with a decrease in hemoglobin of ≥2 g/dL, a transfusion of ≥2 units of packed red blood cells or whole blood, bleeding at a critical site, or with a fatal outcome.
¶ Defined as clinically overt bleeding, which did not meet the criteria for major bleeding, but was associated with medical intervention, unscheduled contact with a physician, temporary cessation of treatment, discomfort for the patient, or impairment of activities of daily life.
Non-bleeding adverse reactions reported in ≥5% of XARELTO-treated patients are shown in Table 11.
Table 11: Other Adverse Reactions* Reported in XARELTO-Treated Patients by ≥5% in EINSTEIN Junior Study
Adverse Reaction
XARELTO N=329 n (%)
Comparator Group N=162 n (%)
Pain in extremity 23 (7) 7 (4.3)
Fatigue† 23 (7) 7 (4.3)
* Adverse reaction with Relative Risk >1.5 for XARELTO versus comparator.
† The following terms were combined: fatigue, asthenia.
A clinically relevant adverse reaction in XARELTO-treated patients was vomiting (10.6% in the XARELTO group vs 8% in the comparator group).
Thromboprophylaxis in Pediatric Patients with Congenital Heart Disease (CHD) after the Fontan Procedure The data below are based on Part B of the UNIVERSE study which was designed to evaluate the safety and efficacy of XARELTO for thromboprophylaxis in 98 children with CHD after the Fontan procedure who took at least one dose of study drug. Patients in Part B were randomized 2:1 to receive either body weightadjusted doses of XARELTO or aspirin (approximately 5 mg/kg).
Discontinuation due to bleeding events occurred in 1 (1.6%) patient in the XARELTO group and no patients in the aspirin group.
Table 12 shows the number of patients experiencing bleeding events in the UNIVERSE study.
Table 12: Bleeding Events in UNIVERSE Study - Safety Analysis Set - On Treatment Plus 2 Days
Parameter
XARELTO* N=64 n (%)
XARELTO®
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of XARELTO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: agranulocytosis, thrombocytopenia
Hepatobiliary disorders: jaundice, cholestasis, hepatitis (including hepatocellular injury)
Immune system disorders: hypersensitivity, anaphylactic reaction, anaphylactic shock, angioedema
Nervous system disorders: hemiparesis
Skin and subcutaneous tissue disorders: Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms (DRESS)
DRUG INTERACTIONS
General Inhibition and Induction Properties
Rivaroxaban is a substrate of CYP3A4/5, CYP2J2, and the P-gp and ATP-binding cassette G2 (ABCG2) transporters. Combined P-gp and strong CYP3A inhibitors increase exposure to rivaroxaban and may increase the risk of bleeding. Combined P-gp and strong CYP3A inducers decrease exposure to rivaroxaban and may increase the risk of thromboembolic events.
Drugs that Inhibit Cytochrome P450 3A Enzymes and Drug Transport Systems
Interaction with Combined P-gp and Strong CYP3A Inhibitors
Avoid concomitant administration of XARELTO with known combined P-gp and strong CYP3A inhibitors (e.g., ketoconazole and ritonavir) [see Warnings and Precautions and Clinical Pharmacology (12.3) in Full Prescribing Information]
Although clarithromycin is a combined P-gp and strong CYP3A inhibitor, pharmacokinetic data suggests that no precautions are necessary with concomitant administration with XARELTO as the change in exposure is unlikely to affect the bleeding risk [see Clinical Pharmacology (12.3) in Full Prescribing Information]
Interaction with Combined P-gp and Moderate CYP3A Inhibitors in Patients with Renal Impairment
XARELTO should not be used in patients with CrCl 15 to <80 mL/min who are receiving concomitant combined P-gp and moderate CYP3A inhibitors (e.g., erythromycin) unless the potential benefit justifies the potential risk [see Warnings and Precautions and Clinical Pharmacology (12.3) in Full Prescribing Information]
Drugs that Induce Cytochrome P450 3A Enzymes and Drug Transport Systems
Avoid concomitant use of XARELTO with drugs that are combined P-gp and strong CYP3A inducers (e.g., carbamazepine, phenytoin, rifampin, St. John’s wort) [see Warnings and Precautions and Clinical Pharmacology (12.3) in Full Prescribing Information]
Anticoagulants and NSAIDs/Aspirin
Coadministration of enoxaparin, warfarin, aspirin, clopidogrel and chronic NSAID use may increase the risk of bleeding [see Clinical Pharmacology (12.3) in Full Prescribing Information]
Avoid concurrent use of XARELTO with other anticoagulants due to increased bleeding risk unless benefit outweighs risk. Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with aspirin, other platelet aggregation inhibitors, or NSAIDs [see Warnings and Precautions]
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
The limited available data on XARELTO in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. Use XARELTO with caution in pregnant patients because of the potential for pregnancy related hemorrhage and/or emergent delivery. The anticoagulant effect of XARELTO cannot be reliably monitored with standard laboratory testing. Consider the benefits and risks of XARELTO for the mother and possible risks to the fetus when prescribing XARELTO to a pregnant woman [see Warnings and Precautions].
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Aspirin* N=34 n (%)
Major Bleeding† 1 (1.6) 0 Epistaxis leading to transfusion 1 (1.6) 0
Clinically relevant non-major (CRNM) bleeding§ 4 (6.3) 3 (8.8)
Trivial bleeding 21 (32.8) 12 (35.3) Any bleeding 23 (35.9) 14 (41.2)
* Treatment schedule: body weight-adjusted doses of XARELTO or aspirin (approximately 5 mg/kg); randomized 2:1 (XARELTO: Aspirin).
† Defined as clinically overt bleeding associated with a decrease in hemoglobin of ≥2 g/dL, a transfusion of the equivalent of ≥2 units of packed red blood cells or whole blood, bleeding at a critical site, or with a fatal outcome.
§ Defined as clinically overt bleeding, which did not meet the criteria for major bleeding, but was associated with medical intervention, unscheduled contact with a physician, temporary cessation of treatment, discomfort for the patient, or impairment of activities of daily life.
Non-bleeding adverse reactions reported in ≥5% of XARELTO-treated patients are shown in Table 13.
Table 13: Other Adverse Reactions* Reported by ≥5% of XARELTO-Treated Patients in UNIVERSE Study (Part B)
Adverse Reaction
XARELTO N=64 n (%)
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnancy is a risk factor for venous thromboembolism and that risk is increased in women with inherited or acquired thrombophilias. Pregnant women with thromboembolic disease have an increased risk of maternal complications including pre-eclampsia. Maternal thromboembolic disease increases the risk for intrauterine growth restriction, placental abruption and early and late pregnancy loss.
Fetal/Neonatal Adverse Reactions
Based on the pharmacologic activity of Factor Xa inhibitors and the potential to cross the placenta, bleeding may occur at any site in the fetus and/or neonate.
Labor or Delivery
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding and this risk may be increased during labor or delivery [see Warnings and Precautions]. The risk of bleeding should be balanced with the risk of thrombotic events when considering the use of XARELTO in this setting.
Data
Human Data
There are no adequate or well-controlled studies of XARELTO in pregnant women, and dosing for pregnant women has not been established. Post-marketing experience is currently insufficient to determine a rivaroxaban-associated risk for major birth defects or miscarriage. In an in vitro placenta perfusion model, unbound rivaroxaban was rapidly transferred across the human placenta.
Animal Data
Aspirin N=34 n (%)
Cough 10 (15.6) 3 (8.8)
Vomiting 9 (14.1) 3 (8.8)
Gastroenteritis†
* Adverse reaction with Relative Risk >1.5 for XARELTO versus aspirin.
† The following terms were combined: Gastroenteritis: gastroenteritis, gastroenteritis viral Rash: rash, rash maculo-papular, viral rash
8 (12.5) 1 (2.9)
6 (9.4) 2 (5.9)
Rivaroxaban crosses the placenta in animals. Rivaroxaban increased fetal toxicity (increased resorptions, decreased number of live fetuses, and decreased fetal body weight) when pregnant rabbits were given oral doses of ≥10 mg/kg rivaroxaban during the period of organogenesis. This dose corresponds to about 4 times the human exposure of unbound drug, based on AUC comparisons at the highest recommended human dose of 20 mg/day. Fetal body weights decreased when pregnant rats were given oral doses of 120 mg/kg during the period of organogenesis. This dose corresponds to about 14 times the human exposure of unbound drug. In rats, peripartal maternal bleeding and maternal and fetal death occurred at the rivaroxaban dose of 40 mg/kg (about 6 times maximum human exposure of the unbound drug at the human dose of 20 mg/day).
Lactation
Risk Summary
Rivaroxaban has been detected in human milk. There are insufficient data to determine the effects of rivaroxaban on the breastfed child or on milk production. Rivaroxaban and/or its metabolites were present in the milk of rats. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for XARELTO and any potential adverse effects on the breastfed infant from XARELTO or from the underlying maternal condition (see Data)
Rash†
(rivaroxaban)
(rivaroxaban)
Animal Data
Following a single oral administration of 3 mg/kg of radioactive [14C]-rivaroxaban to lactating rats between Day 8 to 10 postpartum, the concentration of total radioactivity was determined in milk samples collected up to 32 hours post-dose. The estimated amount of radioactivity excreted with milk within 32 hours after administration was 2.1% of the maternal dose.
Females and Males of Reproductive Potential
Females of reproductive potential requiring anticoagulation should discuss pregnancy planning with their physician.
The risk of clinically significant uterine bleeding, potentially requiring gynecological surgical interventions, identified with oral anticoagulants including XARELTO should be assessed in females of reproductive potential and those with abnormal uterine bleeding.
Pediatric Use
The safety and effectiveness of XARELTO have been established in pediatric patients from birth to less than 18 years for the treatment of VTE and the reduction in risk of recurrent VTE. Use of XARELTO is supported in these age groups by evidence from adequate and well-controlled studies of XARELTO in adults with additional pharmacokinetic, safety and efficacy data from a multicenter, prospective, openlabel, active-controlled randomized study in 500 pediatric patients from birth to less than 18 years of age. XARELTO was not studied and therefore dosing cannot be reliably determined or recommended in children less than 6 months who were less than 37 weeks of gestation at birth; had less than 10 days of oral feeding, or had a body weight of less than 2.6 kg [see Dosage and Administration (2.2) in Full Prescribing Information, Adverse Reactions, Clinical Pharmacology (12.3) and Clinical Studies (14.8) in Full Prescribing Information].
The safety and effectiveness of XARELTO have been established for use in pediatric patients aged 2 years and older with congenital heart disease who have undergone the Fontan procedure. Use of XARELTO is supported in these age groups by evidence from adequate and well-controlled studies of XARELTO in adults with additional data from a multicenter, prospective, open-label, active controlled study in 112 pediatric patients to evaluate the single- and multiple-dose pharmacokinetic properties of XARELTO and the safety and efficacy of XARELTO when used for thromboprophylaxis for 12 months in children with single ventricle physiology who had the Fontan procedure [see Dosage and Administration (2.2) in Full Prescribing Information, Adverse Reactions, Clinical Pharmacology (12.3) and Clinical Studies (14.9) in Full Prescribing Information].
Clinical studies that evaluated safety, efficacy, pharmacokinetic and pharmacodynamic data support the use of XARELTO 10 mg, 15 mg, and 20 mg tablets in pediatric patients. For the XARELTO 2.5 mg tablets, there are no safety, efficacy, pharmacokinetic and pharmacodynamic data to support the use in pediatric patients. Therefore, XARELTO 2.5 mg tablets are not recommended for use in pediatric patients.
Although not all adverse reactions identified in the adult population have been observed in clinical trials of children and adolescent patients, the same warnings and precautions for adults should be considered for children and adolescents.
Geriatric Use
Of the total number of adult patients in clinical trials for the approved indications of XARELTO (N=64,943 patients), 64 percent were 65 years and over, with 27 percent 75 years and over. In clinical trials the efficacy of XARELTO in the elderly (65 years or older) was similar to that seen in patients younger than 65 years. Both thrombotic and bleeding event rates were higher in these older patients [see Clinical Pharmacology (12.3) and Clinical Studies (14) in Full Prescribing Information]
Renal Impairment
In pharmacokinetic studies, compared to healthy adult subjects with normal creatinine clearance, rivaroxaban exposure increased by approximately 44 to 64% in adult subjects with renal impairment. Increases in pharmacodynamic effects were also observed [see Clinical Pharmacology (12.3) in Full Prescribing Information]
Nonvalvular Atrial Fibrillation
Patients with Chronic Kidney Disease not on Dialysis
In the ROCKET AF trial, patients with CrCl 30 to 50 mL/min were administered XARELTO 15 mg once daily resulting in serum concentrations of rivaroxaban and clinical outcomes similar to those in patients with better renal function administered XARELTO 20 mg once daily. Patients with CrCl <30 mL/min were not studied, but administration of XARELTO 15 mg once daily is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment [see Clinical Pharmacology (12.3) in Full Prescribing Information].
Patients with End-Stage Renal Disease on Dialysis
Clinical efficacy and safety studies with XARELTO did not enroll patients with end-stage renal disease (ESRD) on dialysis. In patients with ESRD maintained on intermittent hemodialysis, administration of XARELTO 15 mg once daily will result in concentrations of rivaroxaban and pharmacodynamic activity similar to those observed in the ROCKET AF study [see Clinical Pharmacology (12.2, 12.3) in Full Prescribing Information]. It is not known whether these concentrations will lead to similar stroke reduction and bleeding risk in patients with ESRD on dialysis as was seen in ROCKET AF.
Treatment of DVT and/or PE and Reduction in the Risk of Recurrence of DVT and/or PE
In the EINSTEIN trials, patients with CrCl values <30 mL/min at screening were excluded from the studies, but administration of XARELTO is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment (CrCl 30 to <50 mL/min) [see Clinical Pharmacology (12.3) in Full Prescribing Information]. Observe closely and promptly evaluate any signs or symptoms of blood loss in patients with CrCl 15 to <30 mL/min. Avoid the use of XARELTO in patients with CrCl <15 mL/min.
Prophylaxis of DVT Following Hip or Knee Replacement Surgery
The combined analysis of the RECORD 1-3 clinical efficacy studies did not show an increase in bleeding risk for patients with CrCl 30 to 50 mL/min and reported a possible increase in total venous thromboemboli in this population. In the RECORD 1-3 trials, patients with CrCl values <30 mL/min at screening were excluded from the studies, but administration of XARELTO 10 mg once daily is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment (CrCl 30 to <50 mL/min) [see Clinical Pharmacology (12.3) in Full Prescribing Information]. Observe closely and promptly evaluate any signs or symptoms of blood loss in patients with CrCl 15 to <30 mL/min. Avoid the use of XARELTO in patients with CrCl <15 mL/min.
Prophylaxis of Venous Thromboembolism in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding
Patients with CrCl values <30 mL/min at screening were excluded from the MAGELLAN study. In patients with CrCl <30 mL/min a dose of XARELTO 10 mg once daily is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment (CrCl 30 to <50 mL/min) [see Clinical Pharmacology (12.3) in Full Prescribing Information]. Observe closely and promptly evaluate any signs or symptoms of blood loss in patients with CrCl 15 to <30 mL/min. Avoid use of XARELTO in patients with CrCl <15 mL/min.
Reduction of Risk of Major Cardiovascular Events in Patients with CAD and Reduction of Risk of Major Thrombotic Vascular Events in Patients with PAD, Including Patients After Recent Lower Extremity Revascularization due to Symptomatic PAD
Patients with Chronic Kidney Disease not on Dialysis
Patients with a CrCl <15 mL/min at screening were excluded from COMPASS and VOYAGER, and limited data are available for patients with a CrCl of 15 to 30 mL/min. In patients with CrCl <30 mL/min, a dose of 2.5 mg XARELTO twice daily is expected to give an exposure similar to that in patients with moderate renal impairment (CrCl 30 to <50 mL/min) [see Clinical Pharmacology (12.3) in Full Prescribing Information], whose efficacy and safety outcomes were similar to those with preserved renal function.
Patients with End-Stage Renal Disease on Dialysis No clinical outcome data is available for the use of XARELTO with aspirin in patients with ESRD on dialysis since these patients were not enrolled in COMPASS or VOYAGER. In patients with ESRD maintained on intermittent hemodialysis, administration of XARELTO 2.5 mg twice daily will result in concentrations of rivaroxaban and pharmacodynamic activity similar to those observed in moderate renal impaired patients in the COMPASS study [see Clinical Pharmacology (12.2, 12.3) in Full Prescribing Information]. It is not known whether these concentrations will lead to similar CV risk reduction and bleeding risk in patients with ESRD on dialysis as was seen in COMPASS.
Pediatric Use
No dosage adjustment is required in patients 1 year of age or older with mild renal impairment (eGFR 50 to ≤ 80 mL/min/1.73 m2). There are limited clinical data in pediatric patients 1 year or older with moderate or severe renal impairment (eGFR <50 mL/min/1.73 m2); therefore, avoid the use of XARELTO in these patients.
There are no clinical data in pediatric patients younger than 1 year with serum creatinine results above 97.5th percentile; therefore, avoid the use of XARELTO in these patients [see Dosage and Administration (2.2) in Full Prescribing Information]
Hepatic Impairment
In a pharmacokinetic study, compared to healthy adult subjects with normal liver function, AUC increases of 127% were observed in adult subjects with moderate hepatic impairment (Child-Pugh B).
The safety or PK of XARELTO in patients with severe hepatic impairment (Child-Pugh C) has not been evaluated [see Clinical Pharmacology (12.3) in Full Prescribing Information]
Avoid the use of XARELTO in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy. No clinical data are available in pediatric patients with hepatic impairment.
OVERDOSAGE
Overdose of XARELTO may lead to hemorrhage. Discontinue XARELTO and initiate appropriate therapy if bleeding complications associated with overdosage occur. Rivaroxaban systemic exposure is not further increased at single doses >50 mg due to limited absorption. The use of activated charcoal to reduce absorption in case of XARELTO overdose may be considered. Due to the high plasma protein binding, rivaroxaban is not dialyzable [see Warnings and Precautions and Clinical Pharmacology (12.3) in Full Prescribing Information]. Partial reversal of laboratory anticoagulation parameters may be achieved with use of plasma products. An agent to reverse the anti-factor Xa activity of rivaroxaban is available.
Active Ingredient Made in Germany
Manufactured for: Janssen Pharmaceuticals, Inc. Titusville, NJ 08560
Licensed from: Bayer HealthCare AG 51368 Leverkusen, Germany © 2011-2021 Janssen Pharmaceutical Companies cp-62545v11
Data
XARELTO® (rivaroxaban) XARELTO® (rivaroxaban)
SVS weighs in on 2023 Medicare payment rules for physicians and hospital outpatient services
By Trisha Crishock, Megan Marcinko and Jill Rathbun
The Society for Vascular Surgery (SVS) has submitted com ment letters in response to the proposed rules issued by the Centers for Medicare and Medicaid Services (CMS) on the Medicare Physician Fee Schedule (MPFS) and the Medicare Hospital Outpatient Perspective Payment System (OPPS) and Ambulatory Surgical Center (ASC) Payment System.
Both proposed rules are for calendar year (CY) 2023.
Each year CMS announces proposed rules for the MPFS and the OPPS, usually in July, and provides a 60-day comment period when the public can submit input on the proposed rules. After reviewing the comments, CMS issues final rules on the MPFS and OPPS, typically on or around Nov. 1; rules taken effect on Jan. 1 of the following calendar year.
Members of the SVS Coding and Performance Measures committees review these rules each year and assist in devel oping the SVS comment letters submitted to CMS.
This year’s MPFS and OPPS proposed rules contained several important issues that impact vascular surgeons.
MPFS rule comment letter
In the comments on the CY 2023 MPFS proposed rule, SVS members stressed the need for stability in Medicare physician payment. CMS proposes to reduce the MPFS conversion fac tor by nearly 4.5%, rebase and revise the Medicare Economic Index (MEI) and move forward with year two of the four-year implementation of the clinical labor-pricing update finalized in the CY 2022 MPFS rule. This will result in payment cuts that are unsustainable for vascular surgeons.
This recurring scenario with the annual MPFS payment rule that necessitates congressional action to mitigate Medi care cuts demonstrates that the Medicare physician payment system is broken. Factors that adversely impact the physician payment system are the lack of an annual inflationary update and the statutory budget-neutrality requirements that trig ger across-the-board decreases in payment for all physicians when increases are provided for any physician fee schedule service. Updates to the conversion factor have consistently failed to keep up with inflation, in large part due to the fee schedule budget-neutrality requirements. We strongly stated to CMS that “our policy-makers, both within the adminis tration and in Congress, have a duty to ensure a Medicare system that provides financial stability through a baseline positive annual update reflecting inflation in practice costs, and eliminate, replace or revise budget-neutrality require ments to allow for appropriate changes in spending growth.”

Percutaneous arteriovenous fistula creation
We requested that CMS reconsider its rejection of the RVS Update Committee (RUC)-recommended work relative val ue units (wRVUs) of 7.50 for Current Procedural Terminol ogy (CPT) code 368X1 and 9.60 for 368X2. CMS proposed
work RVUs of 7.20 for CPT code 368X1 and 9.30 for CPT code 368X2 do not accurately reflect the intensity of the physician work associated with these services. We believe the RUC-recommended values for these codes account for the complexity and intensity of the procedures and are sup ported through the survey data collected. We urged CMS to accept the RUC-recommended work RVUs of 7.50 for CPT code 368X1 and 9.60 for CPT code 368X2.
SVS commended CMS for accepting the RUC-recom mended increased work values for the hospital inpatient and observation codes. However, we reiterated our strong objection to CMS’ continued refusal to incorporate the 2021 office evaluation and management (E/M) visit increases to the visits bundled into global surgery payment.
CMS requested public comment on strategies to improve the accuracy of payment for the global surgical packages. We expressed strong opposition to the CMS position that physicians are not performing the follow-up care with their patients that is includ ed in the global surgi cal package. Noting the many flaws of the RAND study, which CMS uses to defend its position that physicians are not seeing patients for follow-up care, we urged CMS to follow the established process of identifying individ ual codes as potential ly misvalued if there is concern with the postoperative visits as signed to a particular service. A blanket approach to address all 010-day and 090-day global surgical packages inappropri ately impacts physicians performing surgeries.
Quality Payment Program
In response to several CMS proposed changes to the Quality Payment Program (QPP) Merit-Based Incentive Payment System (MIPS) benchmark and reporting requirements, we raised our continued concerns about increased reporting requirements and how they conflict with CMS’ goals of re ducing administrative burden within the MIPS program. Annual program changes increase the administrative burden and complexity of the MIPS program and divert physician time and resources from providing patient care. Physicians who comply with MIPS reporting requirements should be offered a bonus potential for having increased reporting re quirements as an incentive to do that work instead of being penalized. We urged CMS to hold harmless all eligible cli nicians and groups from a MIPS penalty during the 2023 performance year due to the ongoing circumstances of the COVID-19 pandemic. These flexibilities should be continued until COVID cases are markedly reduced.
SVS responded to CMS’ request for information (RFI) on the potential development of a quality measure which would assess the percentage of patients with diabetes who receive neurologic and vascular assessment of their lower extrem ities to determine ulcer risk and if they receive a follow-up plan of care. We expressed general support for a new mea sure related to diabetes control that includes a neurologic and vascular assessment and suggested that including a referral to a specialist would enhance the measure’s effectiveness in reducing diabetes-related lower-extremity amputation. However, we noted such a measure would not be appropriate for specialists who are seeing patients after an ulcer has been identified. This measure would be appropriate for primary care providers at the group level within the same specialty and those treating the patients’ underlying conditions who have frequent contact with the patients.
OPPS rule comment letter
We appreciate that CMS has acknowledged the impact of
the recent Supreme Court decision on American Hospital Association v Becerra, which requires CMS to apply a rate of ASP (average sales price) +6% to drugs and biologicals. We urged CMS to provide as much detail as possible in the final rule related to the conversion factor modifications that will impact HOPPS payments as a result of the revised 340B drug payment policy, and provide opportunities for public input regarding how it will conduct retrospective policy changes if such changes are necessary.
In CMS’ comprehensive Ambulatory Payment Classifi cation (APC) methodology, if two J1 services are reported together and meet specific frequency and cost criteria, a com plexity adjustment can be applied, thus moving the payment rate to the next highest APC. We requested that CMS allow a complexity adjustment for CPT code pair 37187 (venous thrombectomy) and 37248 (venous balloon angioplasty) based on frequency and cost data. CMS currently recognizes CPT code pair 37187 (venous thrombectomy) with 37238 (venous stent) for com plexity adjustment con sideration.
In the 2020 OPPS/ ASC final rule, CMS established a prior au thorization process for certain hospital outpatient department (HOPD) services, which is now being ap plied to vein ablation.
We are concerned that CMS continues to mis interpret an increase in the volume of utili zation for certain procedures in the HOPD setting as “un necessary,” when such increases may reflect an appropriate site-of-service shift, a change in practice guidelines, decreases in corresponding/related services, or changes in coverage determinations.
We urged CMS to holistically review data (i.e., site of service, guidelines, coverage determinations, etc.) prior to expanding prior authorization requirements.
Temporarily office-based
CMS reviewed CY 2021 volume and utilization data for sever al procedures, including two vascular duplex scan procedures (CPT codes 93985 and 93986).
Based on low volume, CMS is proposing to continue to designate these two vascular duplex scan procedures as tem porarily office-based for CY 2023. We expressed appreciation for CMS’ proposal to extend the temporary designation for CY 2023 to allow for further analysis before determining if it is appropriate to permanently designate them as office-based.
Additionally, SVS opposes CMS’ proposal to change the terminology currently applied to “skin substitutes” to “wound care management products” and encouraged CMS to work with specialty societies and the American Medical Association CPT Editorial Panel to address termi nology confusion.
CMS will review the SVS comment letters along with those submitted by other stakeholders and members of the public and will issue final CY 2023 rules for both the MPFS and the OPPS later this fall.
Information on the finalized rules will be provided in SVS publications. Resources will be available to assist SVS mem bers in understanding and preparing for the implementation of the new Medicare MPFS and OPPS payment policies in the new year.
Read the full letters at vascular.org/ FeeSchedule23Comment and vascular.org/ HOPPS23Comment.
TRISHA CRISHOCK, MEGAN MARCINKO AND JILL RATHBUN are members of the SVS Washington, D.C., office
18 Vascular Specialist | October 2022
ADVOCACY
NEW FINDINGS, RECENTLY published in the Journal of Vascular Access (JVA), provide insight into the hemodialy sis access profile of failed kidney transplant patients treated in the Catalonia region of Spain over an 18-year period.

Researchers Ramon Roca-Tey, MD, a se nior consultant in nephrology from Hospital Universitari Mollet in Barcelona, Spain, and colleagues note that data on vascular access use in failed kidney transplant patients re turning to hemodialysis are limited. In the present study, therefore, the investigators sought to analyze the vascular access profile of this patient group, the factors associated with the likelihood of hemodialysis reinitia tion through an arteriovenous fistula (AVF), and the effect of vascular access in use at the time of kidney transplant on kidney graft outcomes.
In their report, Roca-Tey et al write that they examined data from the Catalan Renal Registry on failed kidney transplant patients restarting hemodialysis and incidence hemo dialysis patients with native kidney failure over the period from 1998–2016.

The authors report in JVA that the vascular access profile of 675 failed kidney transplant
pared with that before kidney transplant, and with 16,731 incident patients starting hemodialysis was 79.3% vs. 88.6% and 46.2% (p=0.001 and p<0.001) for AVF; 4.4% vs. 2.6% and 1.1% (p=0.08 and p<0.001) for arteriovenous graft (AVG); 12.4% vs. 5.5% and 18% (p=0.001 and p<0.001) for tun neled central catheter; and 3.9% vs. 3.3% and 34.7% (p=0.56 and p<0.001) for non-tun neled catheter. In addition, they reveal that the likelihood of hemodialysis reinitiation by AVF was significantly lower in patients with cardiovascular disease, a kidney trans plant duration of more than five years, those dialyzed through AVG or tunneled central catheter before kidney transplant, and those of the female sex.
The authors add that analysis of Ka plan-Meier curves showed a greater kidney graft survival in patients dialyzed through arteriovenous access than in patients using catheters just before kidney transplantation. Finally, Cox regression analysis showed that patients on hemodialysis through arterio venous access at the time of kidney trans plantation had lower probability of kidney graft loss compared to those with catheters, Roca-Tey and colleagues write.
access profile of failed kidney transplant pa tients returning to hemodialysis and incident patients starting hemodialysis was different. They also note that, compared to before a kidney transplant, the proportion of failed kidney transplant patients restarting hemo dialysis with an AVF decreased significantly at the expense of a tunneled central catheter, and that patients on hemodialysis through arteriovenous access at the time of a kidney transplant showed greater kidney graft sur vival compared with those using a catheter.
According to the researchers, there are two clinical implications that can be derived from their findings. “To reduce the propor tion of failed kidney transplant patients returning to hemodialysis with a central line, better vascular access management is needed,” they state, outlining the first im plication. The second implication, Roca-Tey et al believe, arises from the impact of arte riovenous access on kidney graft outcomes. They elaborate: “If our results are confirmed in further studies, including a larger num ber of kidney transplant patients and not simply patients returning to hemodialysis, routine arteriovenous access closure after a successful kidney transplantation should not be performed.”


In the discussion of their data, the au thors state that a number of factors limit the strength of their findings. They highlight the use of a population registry database for this study as a key weakness, for example. “The variables used are restricted in number and can have low clinical specificity,” they note, giving the example that the number of liti gated accesses, as well as those with spon taneous thrombosis and not salvaged, were not recorded. Furthermore, the researchers acknowledge a weakness pertaining to the effect of vascular access on kidney graft sur vival—a secondary aim of the study. They write: “Kidney transplant patients who returned to hemodialysis were consid ered instead of all kidney transplant patients, and this may be a bias in

www.vascularspecialistonline.com 19
“If our results are confirmed in further studies, including a larger number of kidney transplant patients and not simply patients returning to hemodialysis, routine arteriovenous access closure after a successful kidney transplantation should not be performed”
RAMON ROCA-TEY
REGISTRY DATA HIGHLIGHT LONG-TERM TRENDS IN HEMODIALYSIS ACCESS PROFILE OF FAILED KIDNEY TRANSPLANT PATIENTS VASCULAR ACCESS O C T O B E R 2 3 - 2 4 , 2 0 2 2 R O S E M O N T , I L L I N O I S V A S C U L A R . O R G / C P V I
MIDWESTERN VASCULAR
CEA, TCAR ‘CONTINUE TO SHOW SUPERIOR OUTCOMES’ TO TRANSFEMORAL STENTING
Researchers led by a vascular team in Indianapolis found that carotid endarterectomy (CEA) remains the most-used strategy in carotid revascularization, with CEA and TCAR showing decreased odds of stroke or death compared to TF-CAS.
By Bryan Kay
THOSE WERE AMONG THE MAIN findings delivered by Hanaa Dakour Aridi, MD, an integrated vascular surgery resident at Indiana University in Indianapolis, during the 2022 annual meeting of the Midwestern Vascular Surgical Society (MVSS) in Grand Rapids, Michigan (Sept. 15–17), after a analy sis of regional variation in patient selection,

practice patterns and outcomes based on techniques used in carotid revascularization logged in the Vascular Quality Initiative (VQI) from 2016–2021.
The research team divided 19 geographic regions into three quantiles based on the aver age annual volume of carotid procedures per formed: low (956 cases), medium (1,533) and high (1,845) centers. They looked at a total of 126,768 carotid revascularization cases, with most patients asymptomatic. CEA was the most common procedure (>60%) across all regional groups, with 13% performed using transfemoral carotid artery stenting (TF-CAS) and 17% with transcarotid artery revascular ization (TCAR).
“Overall, there was a trend towards in creased utilization of TCAR since its intro duction in 2016 up to 2021,” Aridi told MVSS 2022. “This seems to have been mirrored by an increase in the percentage of CEA cases.”
Aridi reported the research team found no significant differences in stroke and death between CEA and TCAR across the three re gional volume groups. “However, TF-CAS was associated with a higher odds of stroke and death compared to CEA, and TCAR was associated with a decreased odds of stroke or death compared to TF-CAS,” she said. “These variations were also seen when we analyzed the regions individually.”
Despite significant variation in clinical prac tice, Aridi concluded, “no significant regional variation was observed in the outcomes of carotid revascularization. TCAR and CEA continue to show superior outcomes to TF-
CAS across all regional groups. Our study identifies some gaps in adherence to societal guidelines, mainly in terms of intraoperative procedural variability that can potentially lead to worse outcomes. It also highlights the need for uniformity in management of patients with carotid artery disease.”
Raghu Motaganahalli, MD, the study’s se nior author, told Vascular Specialist: “What we are seeing is TCAR has grown at the cost of CEA, not at the cost of TF-CAS,” further not ing, “We would love to see growth happening at the cost of TF-CAS because it is probably at a higher risk of stroke and complications.”
FROM THE COVER: STUDY: COMBINED ANTIPLATELET, NOAC USE LINKED TO WORSE LIMB OUTCOMES AFTER SUPRAINGUINAL BYPASS
continued from page 1
Zaidi noted that rates of antiplatelet usage were “pretty steady” over the eightyear study period, whereas warfarin use “steadily decreased,” with NOAC administration “steadily increasing.”
Univariate analysis showed that at one year, “the NOACplus-antiplatelet group had a statistically significantly reduced primary patency rate compared to antiplatelet alone.”Multivariate analysis demonstrated that NOAC use, prior bypass, diabetes and acute ischemic limb presentation “all were independently significant predictors of a reduced primary [and] primary assisted patency at one year.” Likewise, secondary patency rates showed a similar finding, Zaidi stated.
Further, at 12 months, univariate analysis showed NOAC use associated with a lower major adverse limb event-free survival rate compared to the antiplatelet group. “We found several independent predictors on multivariate analysis— NOAC use, prior bypass, patient being nonambulatory, rest pain or tissue loss and acute ischemic limb presentation—of increased major adverse limb events,” said Zaidi.
NOAC use was also associated with reduced major amputation-free survival at one year, he continued, with multivariate analysis showing that non-ambulatory status, having an axillary bypass graft origin, tissue loss or acute ischemic limb presentation were associated with increased rates of major amputation.
Univariate analysis demonstrated that overall survival in the NOAC group was 89% at one year compared to 91.3%
in the antiplatelet group, Zaidi added. “We did find that warfarin ultimately had similar outcomes as NOAC therapy but, ultimately, did not include it in the final analysis due to its decreased usage over time,” he noted.

“Combined antiplatelet and NOAC therapy after suprainguinal bypass was associated with worse limb outcomes and patency but equivalent statistically significant survival compared to antiplatelet therapy alone,” Zaidi concluded. “NOAC use in suprainguinal bypass has typically been extrapolated from infrainguinal data and can’t be recommended for routine use based on our experience. Additional prospective investigations are required to determine the role of NOACs and optimal antithrombotic therapy after suprainguinal bypass.”
The questioner from the floor who noted some concern over the findings asked whether the study was dealing with equivalent patients. “I’m a little concerned that, if you publish this, you’re probably not comparing apples to apples; you’re going to give the recommendation that patients shouldn’t go on [rivaroxaban], or whatever,” he said.
In response, Zaidi explained that the message was more “to be discriminating” about “who should get NOACs, [and] who shouldn’t.”
Rising from the audience to explain further, senior author Robinson said he wanted to add some caveats. “We do know NOACs are being used a lot, and we don’t have a lot of data for their use. You bring up a lot of good points: Certainly, we can’t identify hypercoagulant states. Do we know every anatomic aspect of these patients in the VQI? No ...
“There is a fair bit of data you can control for, and I think using both propensity score matching and pretty aggressive multivariate analysis within that—you can’t do much better shy of a randomized trial is the bottom line. If you look at the randomized trials, we actually don’t have any of that information either.”
Expanding on where the study might lead, Robinson added, “I do think [this study] adds a little bit, mostly being that it’s hypothesis-generating.”
20 Vascular Specialist | October 2022
“NOAC use in suprainguinal bypass has typically been extrapolated from infrainguinal data”
SYED ZAIDI
“TF-CAS was associated with a higher odds of stroke and death compared to CEA, and TCAR was associated with a decreased odds of stroke or death compared to TF-CAS” HANAA DAKOUR ARIDI
Hanaa Dakour Aridi takes to the MVSS 2022 podium
Study probes use of NOACs such as rivaroxaban




MEDICAL ®
AORTIC
Mortality drops for acute type A aortic dissection, still high for patients not receiving surgery
By Jocelyn Hudson
THE CHANCE OF A PATIENT SURVIVING AFTER AN acute type A aortic dissection has improved significantly, but mortality remains high if not recognized early and repaired surgically. This is according to new research from a team at Michigan Medicine at the University of Michigan in Ann Arbor, Michigan.
A team of researchers examined early mortality rates for more than 5,600 patients admitted to hospital and examined hourly with acute type A aortic dissection between 1996 and 2018 from the International Registry for Acute Aortic Dissection (IRAD).
Findings published in JAMA Cardiology reveal that 5.8% of patients with acute type A aortic dissection died within the first two days after hospital arrival, a mortality rate of 0.12% per hour. The rate is significantly lower than that reported in the 1950s, which estimated that 37% of patients died within the first 48 hours, with an increasing mortality rate of 1–2% per hour.
“We believe that advances in diagnosis and management, especially a focus on early surgical repair, may have contribut ed in part to these improvements in mortality for acute aortic dissection,” said Kim Eagle, MD, senior author of the paper and director of the University of Michigan Health Frankel Cardiovascular Center.
Of all the patients, 91% either received surgery or were intended for surgery, with the others managed medically due to advanced age and complications such as stroke and kidney failure. Nearly 24% of those receiving medical treatment alone died within two days, compared to 4.4% of patients treated with surgical repair—a death rate more than five times higher.
“Patients who were managed medically were likely not surgical candidates due to their comorbidities,” said Bo Yang, MD, a professor of cardiothoracic surgery at University of Michigan Medical School, in a press release announcing the results of the study. Yang was not involved in the study, the release notes. “The medically managed patients could die from aortic dissection-associated complications—such as malperfusion, cardiac tamponade, aortic rupture and acute aortic insufficiency, which can be treated with surgery—or from their existing medical conditions, which could be wors ened by the aortic dissection.”
Only 1% of patients deemed suitable for surgery died be fore the procedure. These patients died after an average of
nearly nine hours from being admitted to the hospital, ex ceeding the six-hour median time to surgery for all patients.
Interhospital transfer is needed in more than 70% of aortic dissection cases, causing inherent delays. Before this study, Eagle says, it was thought that early death from this condition was so prohibitive that operating urgently, even in hospitals with limited volume of aortic dissection surgery and resourc es, was the preferred strategy.
However, there is evidence that surgery at a low-volume hospital can double the risk of dying while undergoing repair compared to the highest volume providers. Additionally, mortality rates for open repair of acute type A aortic dissec tion are nearly three times higher when the operation is not performed by a dedicated aortic surgeon.
“Hospital mortality at a high-volume center like U-M [University of Michigan], where aortic dissection patients are taken care only by highly experienced aortic surgeons, can be as low as 5%, while the same patient operated on at a low-volume center may be 20% or higher,” Eagle said. “With this new information, it is clear that the ‘cost,’ or risk, of a four-to-six-hour delay caused by transfers is more than offset by the lower risk of surgery at experienced hospitals.”
Cases are rare. Approximately three in 100,000 people suffer aortic dissection each year. The condition most com monly affects older men, and a person experiencing the tear may feel a “knife-like, tearing pain through the back,” ac cording to IRAD.
It is estimated that up to 50% of patients will die before ever reaching the hospital, making the overall mortality for aortic dissection substantially higher, the press release from Michigan Medicine points out.
“There is a need to identify the high-risk population of aortic dissection, such as those with a family history of aor tic aneurysm and dissection, especially at a younger age, or known pathogenic genetic variants, so that we can replace the proximal aorta electively to prevent acute type A aortic dissection,” Yang said. “For young people under 55 years old with severe chest pain, we have to prove if patients have aortic dissection or otherwise.”
EASTERN VASCULAR STUDY SUGGESTS LOWER RATES OF AORTIC RUPTURE, MORTALITY AFTER TEVAR FOR UNCOMPLICATED TBAD IN SUBACUTE PHASE COMPARED TO MEDICAL MANAGEMENT
By Bryan Kay
THORACIC ENDOVASCULAR AORTIC
repair (TEVAR) carried out for uncompli cated type B aortic dissection (TBAD) saw worse mortality and aortic-related outcomes when performed in the acute phase com pared to those treated in the subacute phase and those managed medically.
That was the chief conclusion of an in vestigation of TEVAR timing presented at the 2022 Eastern Vascular Society (EVS) an nual meeting in Philadelphia (Sept. 29–Oct. 1) by Matthew Muller and colleagues from Albert Einstein College of Medicine in New York City. Patients with acute uncomplicated TBAD between 2007 and 2019 were identi fied using the TriNetX Analytics Network, a
federated network of nationwide healthcare organizations with de-identified data.
The study looked at 17,018 patients with TBAD, with 918 (5.4%) undergoing acute TEVAR, 328 (1.9%) subacute, and 15,772 (92.7%) being treated nonoperatively.
After 1:1 propensity matching, the research team found that the acute TEVAR group had higher rates of endovascular reintervention (7.76% vs. 1.09%; p<0.001) and aortic rup ture at 30 days (3.93% vs. 1.09%; p<0.001) and three years (9.51% vs. 3.93%; p<0.001), when compared to medical management. The subacute group had significantly higher rates of endovascular intervention at three years when compared to medical manage
ment (9.17% vs. 3.06%; p=0.001). The rate of aortic rupture at three years was significantly higher in the acute TEVAR group compared to the subacute TEVAR group (10.2% vs. 3.51%; p=0.001). There were no significant differences in 30-day and three-year mortality rates, or three-year rates of open reinterven tion, between the three groups.
Muller told the EVS gathering that the acute TEVAR group was found to have high er rates of mortality compared to both the medical management and subacute TEVAR groups but this finding was not found to be
statistically significant. Additionally, the sub acute TEVAR group had lower three-year mortality rates compared to the medical management group, but this, too, was not found to be statistically significant. “Our data shows that when TEVAR is performed in the subacute phase, there are lower rates of aor tic rupture and mortality compared to medi cal management,” Muller concluded. “These are similar findings to previously discussed studies looking at the timing of TEVAR with regards to both complicated and uncompli cated type B aortic dissections.”

22 Vascular Specialist | October 2022
DISSECTION
“With this new information, it is clear that the ‘cost,’ or risk, of a four-to-six-hour delay caused by transfers is more than offset by the lower risk of surgery at experienced hospitals”
KIM EAGLE
Matthew Muller presents at EVS 2022


PROVEN SUSTAINED HEALING Visit www.AOTInc.net for research articles, patient and physician testimonials – and more. A Unique At-Home Therapy: Delivers the ability to close chronic, non-healing wounds using OXYGEN, CYCLICAL COMPRESSION and HUMIDIFICATION. A Game Changer for Patients, Clinicians & Payors: Addresses access to care with a self-administered in-home therapy that overcomes traditional healthcare barriers. A Seamless Addition to Your Care Plan: TWO2 is an adjunct treatment that can be used with gas permeable dressings, CCD, UNNA Boot, or TCC left in place. DELIVERING EXCEPTIONAL OUTCOMES O2 for VLU HIGHER HEALING RATE at 12 weeks 46%76% | vs. SOC* LOWER RECURRENCE RATE at 36 months 47%6% FASTER TIME TO HEALING 107 DAYS 57 DAYS MRSA ELIMINATION 0% CCD 46% | vs. SOC | vs. SOC | vs. SOC *Standard of Care (SOC) was Conventional Compression Dressings (CCD)
ENGLAND
NESVS meeting features paper showing relationship between increased distance from medical center and higher treatment costs among complex aortic patients
Research detailing that patients traveling farther for complex aortic surgery have higher procedural costs, postoperative imaging costs, and comprehensive one-year costs was among the new science being presented at the 2022 annual meeting of the New England Society for Vascular Surgery (NESVS) in Newport, Rhode Island (Oct. 14–16).
By Bryan Kay
INVESTIGATORS, INCLUDING FIRSTnamed author Zach M. Feldman, MD, a vas cular surgery resident at Massachusetts Gen eral Hospital in Boston, and senior author Mark F. Conrad, MD, drew the conclusion after conducting a retrospective review of
all patients in the Vascular Quality Initiative (VQI) and Vascular Implant Surveillance and Interventional Outcomes Network (VISION)
databases undergoing complex endovascular aortic repair (EVAR).
Between 2011 and 2018, they looked at
8,782 patients, including 4,822 with complex EVARs, 2,672 complex thoracic EVARs (TE VARs), and 1,288 complex open abdominal aortic aneurysm (AAA) repairs. Median trav el distance was 22.8 miles, with the research team establishing that patients traveling far ther were more likely female and to have had prior aortic surgery. “These patients should be targeted for increased care coordination for improved outcomes and healthcare sys tem burden,” the researchers concluded.
Elsewhere, a team from the University of Massachusetts Medical School in Worcester led by Andres Schanzer, MD, delivered data on their initial experience with the emerg ing Fiber Optic RealShape (FORS) imaging technology.
They presented findings from 21 proce dures carried out with FORS guidance that were matched to 63 non-FORS procedures. Ninety-five FORS cannulation tasks were attempted (87 visceral target artery, eight contralateral bifurcated gate), they report ed, with technical success achieved in 81 cannulations (85%). Furthermore, 15 (16%) were completed without any fluoroscopy, they added. “Use of FORS guidance was as
sociated with lower median procedural and fluoroscopy times, dose area product, and air kerma.”
Meanwhile, a team from Beth Israel Dea coness Medical Center in Boston, led by Marc Schermerhorn, MD, demonstrated use of FORS in internal iliac artery navigation in a video presentation. “Much of the attention garnered by FORS is for use in complex aor tic surgery … however, we have found that the FORS system is also particularly useful for navigation of the internal iliac artery,” they reported. The video showed two exam ples of internal iliac artery navigation with the FORS system. “Specifically, they demon strate the ability to employ extreme angles that would either not be possible with con ventional fluoroscopy or subject personnel to high radiation doses,” the team add.
‘SIGNIFICANT’ INCREASE IN ATHERECTOMY USE IN US LARGELY DRIVEN BY OFFICE-BASED PROCEDURES, VQI DATA SHOW
In the United States, atherectomy use in peripheral vascular interventions (PVIs) “more than doubled” from 2010 to 2019, with officebased procedures a “major driver” of this increase. This is according to published U.S. data from the Vascular Quality Initiative (VQI), which also revealed wide regional variability in the use of atherectomy. By Jocelyn Hudson
According to authors Tonga Nfor, MD, a cardiologist at Au rora St Luke’s Medical Center, in Milwaukee, Wisconsin, and colleagues, small, older studies have indicated that the use of atherectomy devices has become common in PVIs despite what they describe as the “paucity of strong clinical guidelines.” The study, published in the Journal of Vascular Surgery (JVS), was a retrospective analysis of prospective ly-collected data from the VQI registry, which the investiga tors say provides a “unique opportunity” to fill knowledge gaps in this field of research using real-world data originating across the U.S. The team identified all patients who had un dergone endovascular PVIs for occlusive lower-extremity arterial disease from 2010 to 2019. Procedures in which an atherectomy device had been used as the primary or sec ondary device were classified as the atherectomy group, Nfor et al write.
In the study period, the authors note that a total of 205,377 PVIs were performed for 152,693 unique pa tients. The key finding, they report in JVS, is that, during the 10-year study period, 16.6% of the PVIs had used atherectomy, with the data showing that use “significantly increased” from 8.5% in 2010 to 19.7% in 2019.
Geographic variation was evident in the study results, with Nfor and colleagues stating that they found a signifi cant difference in the prevalence of atherectomy use across 17 geographic regions, ranging from 8.2% to 29%.
These regions were further split into North, East, South, and West, the authors write, revealing that the observed frequency of atherectomy use in PVIs during the 10-year period was highest in the South at 22.5%, followed by the West (19.9%), North (17.3%), and East (12.6%; p<0.0001).
According to the study authors, the strongest predictor
of use was performance of PVI in an office setting (odds ratio [OR], 10.08; 95% confidence interval [CI], 9.17–11.09) or ambulatory center (OR, 4; 95% CI, 3.65–4.39) versus a hospital setting. Furthermore, the geographic trend in atherectomy use followed the distribution of the pro portion of office-based PVI procedures—South (6.1%), followed by the West (4%), North (3.9%), and East (0.5%).
They note that the presence of severe (OR, 2.6; 95% CI, 2.4–2.85) or moderate (OR, 1.5; 95% CI, 1.4–1.69) lesion calcification was also predictive of atherectomy use.
The researchers also examined the reimbursement aspect of the atherectomy discussion. For angioplasty performed in a hospital, the physician total relative value units (RVUs) was 12.94, averaging about $466, they report. For the same procedure performed in an office-based labo ratory [OBL], the total RVU was 100.68 (procedure total), translating to $3,628.
Nfor et al further reveal that, when angioplasty was per formed with atherectomy in a hospital, the physician RVU was 17.61, averaging $635. However, when atherectomy with angioplasty was performed in an OBL, the reimburse ment increased to 345 RVU—approximately $12,444.
The authors, however, point out that these total RVU reimbursement figures for office and ambulatory center procedures includes the technical and facility fees.
“To provide patients with the highest quality of care, physicians must allow their decision-making to be guided only by the clinical factors and the best interest of the pa tient, and should be mindful of any biases that might influ ence their practice, including the reimbursement structure and financial incentives,” the researchers stress.
Despite the financial incentives that exist, the authors acknowledge that clinical considerations “play an import ant role in interventional decisions that guide patient care.”
For example, the investigators note that patients in the hospital setting will tend to have more acute disease with greater urgency and a greater risk of complications.
Routine use of atherectomy for infrainguinal PVI called into question
In a commentary on Nfor and colleagues’ report, Caitlin Hicks, MD, a vascular surgeon from Johns Hopkins Univer sity School of Medicine, Baltimore, Maryland, underscores the “overwhelming message” of the analysis: that atherec tomy is being overused in the U.S.

“This is not an attack on physicians who work in OBLs,” Hicks stresses at the outset of her commentary, but rather a “statement of the facts,” of which she assembles four from this latest addition to the available research:
◆ Atherectomy use for infrainguinal PVI has been “in creasing rapidly” in the U.S.
◆ No high-quality evidence is available that atherectomy improves outcomes compared with alternative endo vascular therapies
◆ Atherectomy is much more frequently used in OBL settings than in hospital-based settings
◆ And reimbursement for atherectomy procedures is substantially higher than that for stenting and balloon angioplasty in the outpatient setting
24 Vascular Specialist | October 2022
PAD
NEW
VASCULAR
“The FORS system is also particularly useful for navigation of the internal iliac artery”
Caitlin Hicks
CANADIAN VASCULAR
Virtual visits versus in-person: Telemedicine study detects ‘substantial’ cost savings for patients
By Bryan Kay
VIRTUAL CONSULTATIONS ARE

al,
in-person
physical exam or not seeing anything in person, as opposed to a video camera, that may include additional costs later.” Javidan, from the division of vascular surgery at the Univer sity of Toronto in Toronto, was part of a research team led by Faysal Naji, MD, from the department of vascular surgery at McMaster University in Hamilton.


Some 22 studies were included in the review and involved around 29,000 patients. The study group’s limited meta-anal ysis of three randomized-controlled trials (RCTs) assessing diabetic foot ulcers did not detect any statistically significant differences in terms of ulcer healing, mortality or amputa tion rates, they noted. “In terms of other clinical outcomes, we grouped together what we were able to find based off the data available,” Javidan said. “This had to do with re admission rates, with surveys evaluating patient-reported outcome measures. And, largely, across clinical outcomes measured, there were no appreciable differences—meaning that telemedicine and virtual consultations were really no better or no worse across the board between virtual care and in-patient care.”
and 2.2 to 12 gallons of fuel saved.” Among seven studies evaluating patient satisfaction, patients generally felt tele medicine was either comparable or better than in-person visits, Javidan and colleagues found.
The researchers had felt there was a need to assess the merits of telemedicine—particularly through the lens of vas cular surgery practice—from a higher level after analyzing a plethora of level three and four evidence, mostly in the form of cohort studies and case series. “There was no real review out there summarizing things, putting things together and aggregating results,” Javidan said.
Naji added, “As COVID calmed down a little bit, we no ticed that telemedicine was still being used, kind of begging the question that maybe there were some hidden benefits to these shifts in practice patterns that we weren’t identifying.”
it comes to
and meta-analysis of all primary studies
comes, a systematic
surgery has demonstrated.
in
“In considering, telemedicine’s effectiveness moving for ward, one thing we have to keep in the back of our minds is: Are there other healthcare costs to the system with the use of virtual consultations?” first-named investigator, Arshia Javidan, MD, observed in an interview with Vascular Special ist, after delivering data from the study at the 2022 annual meeting of the Canadian Society for Vascular Surgery (CSVS) in Vancouver (Sept. 9–10). “So, for example, are additional investigations, or potentially unnecessary investigations, be ing ordered when we’re seeing a patient virtually, not doing a
From a cost savings point of view, continued Javidan, the study found that patients were saving time, money and fuel through the use of virtual consultations. In terms of travel distance savings, the number “was relatively substantial at between 31 and 150 miles, 39 to 115 minutes of travel time,
Summing up their findings at CSVS 2022, the research team noted: “Telemedicine has the potential to augment vascular surgery practice while reducing resource use for patients and providers alike. Additional high-quality evidence comparing telemedicine to in-person clinical encounters is required to further elucidate the effect that telemedicine and virtual consultations have on clinical outcomes.”
New Single-Use Surgical Doppler System







www.vascularspecialistonline.com 25
Simple to Use, Lightweight and Easy to Hold • 8 MHz single-use, sterile probes ready for immediate use • Easily confirm blood flow with the high resoluiton, color display of the waveforms on the DMX Doppler • Reduce the risk of infection during surgery caused by non-sterile products being used within the sterile field Scan Here to Learn More www.huntleigh-healthcare.us Vascular Specialist Print Ad - DIOP.indd 1 09/09/2022 15:32:31
COMPARABLE TO care received
when
gener
clinical, process and patient satisfaction out
review
evaluating telemedicine
vascular
“[In terms of savings, the numbers] were relatively substantial at between 31 and 150 miles, 39 to 115 minutes of travel time, and 2.2 to 12 gallons of fuel saved” ARSHIA JAVIDAN
Arshia Javidan (left) and Faysal Naji
CORNER
SIGNAL-TO-NOISE RATIO: THOUGHTS ON FINE-TUNING FEEDBACK DURING VASCULAR SURGERY TRAINING
 By Christopher Audu, MD
By Christopher Audu, MD
I RECENTLY TOOK THE REGISTERED PHYSICIAN Vascular Imaging (RPVI) exam, and while studying for it, one of the concepts that stood out was the signal-to-noise ratio (SNR) in vascular ultrasound, and how it accounted for the quality of images. Don’t worry. I have no intention of putting up the Doppler equation in this month’s writeup. It’s just that, for those of us in the trenches of surgical training, this concept of SNR is very relatable.
No one doubts that the long road to becoming com petent, confident and compassionate surgeons requires constant evaluation and re-evaluation during training. At any given time as trainees, however, there are multiple voices in our ear and in our heads. We’ve all gotten that feedback or evaluations that leaves us overwhelmed and/ or is contradictory.

So, how do we begin to decipher what’s important?
The patient comes first
Perhaps we should start where any great surgeon starts— with the patient in front of us. There is no greater litmus test about how we’re doing in training than to know that our clinical decisions are providing the right care for the right patient at the right time. Do I have a knowledge or skillset deficit that puts patients at risk of harm? If so, that needs to be remedied.
blindspot that is potentially harmful to patient care? Then that evaluation needs to be taken seriously, and that blind spot fixed. Providing appropriate care for our patients is the most important signal—even if it means that doing so, sometimes, can generate a lot of noise.
The transducer
The RPVI exam impressed upon me the importance of the transducer properties in improving SNR. In training, this is akin to having the right mentors whose opinion you genuinely respect. We all have surgeons that we’d like to emulate our practice after followong training. Seek out these people after working with them on various rotations and ask for honest feedback. For me, these surgeons help me determine what’s working and what’s not.
But if I’m being really honest, sometimes the best feedback comes from the non-surgeons in our profession al lives—from the operating room nurses who see our efforts and have direct comparison to how everyone else they have trained has done, to the administrative staff who have access to my CV. I have personally found these people to be some of our strongest allies in helping cross the finish line in training. For the most part, they all want us to genuinely succeed.
And of course, one cannot forget a most important set of people—the loved ones in your personal life. From parents and friends to spouses and kids, these people provide grounding to reality. While they may not always have medical advice to proffer, their evaluation and advice
can help us become a more compassionate person—and they are often adept at recog nizing burnout before you do.
Internal reflection
Last, but most importantly, is you and your goals. Introspection is key to goal align ment in training. This gets at the concept of meaningful work. What are your personal and pro fessional goals for training? Sure we all want to become excellent surgeons, but are there nuances unique to you and your situation? Perhaps seeking opportunities that get you to that goal while letting go of ones that are a waste of time, will allow for a more meaningful and fulfilling experience that will translate into more competence and confidence in training. There is no surefire answer here, but learning to define appropriate boundaries that align with your ambitions and goals can be a good place to start.
Christopher Audu
In training, there are a lot of voices—including your own. This month, as we barrel toward the end of the cal endar year, I hope that your SNR remains high so that the image of who you’re becoming as a surgeon remains crisp and aligned with the image of whom you want to become.
CHRISTOPHER AUDU is a vascular surgery resident at the University of Michigan in Ann Arbor, Michigan, and the Vascular Specialist resident/fellow editor.

NESVS Robert R. Linton Lecture features complex aortic masterclass from Europe
World-leading aortic surgeon Stéphan Haulon, MD, from the Aortic Centre at Hôpital Marie Lannelongue in Paris, France, took attendees of the 2022 New England Society for Vascular Surgery (NESVS) annual meeting on a whirlwind, 15-year journey through the evolution of aortic arch endografting from his personal vantage point— the most advanced view in the world, 2021–22 NESVS President Andres Schanzer, MD, said as he introduced the 2019–20 president of the European Society for Vascular Surgery (ESVS).
Haulon started the 2022 Robert R. Linton Lecture by introducing his first case in this arena, from 2007, which involved undertaking “a very simple endovascular approach.” From there, he explained, with more confidence, he proceeded to perform various combinations of fenestrations and debranching, choosing patients selectively. “But we started moving to more and more challenging cases, such as chronic dissections,” Haulon explained, showing slides of his first such case in which the dissected innominate artery necessitated a crossover bypass, and involved two fenestrations covering the left common carotid and left subclavian arteries. Eventually, Haulon continued, he and colleagues began “to move away from this early experience with the fenestrated endograft,” showing the current device they are using—a graft with inner branches. Then came a “game-changer,” he said, with the advent of hybrid treatment, which he said “we had to fight for” in order to get access to the requisite devices.
“Then we started doing those procedures under fusion guidance, and that was a huge difference.” Now, he continued, “we can locate the origin of the coronary arteries, you can see the endograft tracking, we could have a large field where we could see both the left
ventricle and the top of the arch ... the origin of the target vessel, the innominate bifurcation—all those markers that we need during the procedures.”
From this initial experience, he and colleagues learned much, seeing much better outcomes in a later cohort of patients. “In [our] second paper, [we showed] no mortality, [and] the stroke rate went down to less than 10%, and mostly minor stroke,” Haulon noted.
He further referenced the latest emerging option, a total percuntaneous approach to aortic arch repair recently described in a type-A dissected patient.
Looking even further ahead into the future of aortic techology, he told NESVS: “I don’t think open surgery is still the golden standard for arch repair; [but] it’s not the old standard either. We need expertise in both fields, and to discuss cases.” What’s next? “The acute type A dissections,” Haulon said. One device in particular bears scrutiny, “a very simple device with only three fenestrations.” Work is ongoing with the graft but it could be another “game-changer,” Haulon concluded. Bryan Kay
COMMENT& ANALYSIS
STITCH 26 Vascular Specialist | October 2022
Stéphan Haulon at NESVS 2022
SOOVC subsection introduces two awards
By Beth Bales
THE SOCIETY FOR VASCULAR SURGERY’S SUBsection for those who provide service in outpatient settings has created two professional development opportunities for its members, a research grant and a presentation award to present already completed research.
Applications for the Sub-Section on Outpatient and Office Vascular Care (SOOVC) Presentation Award and the SOOVC Research Seed Grant are due Dec. 31. Up to three recipients for each award may be selected.
The SOOVC Research Seed Grant will provide vascular surgeons $5,000 for a data analyst to analyze research data for insights, quality improvement and patient care. Research must have been completed in an office-based lab (OBL) or ambulatory surgery center (ASC). Criteria for evaluation will include:
◆ How the research will positively impact care and/or procedures provided in the outpatient setting
◆ Why this research is necessary
◆ Whether the research will benefit patients, physicians, payors and the healthcare system at large
Three recipients will be selected, and the grant is renewable for a second year, based on first-year out comes. Applicants must be an SVS Active or Can didate member—including trainees, fellows and residents—practicing in an OBL or ASC setting.
Research presentations will take place at the SOOVC session during the Vascular Annual Meet ing (VAM). Learn more and apply at vascular.org/ OBLResearchGrant
The SOOVC Presentation Award will rec ognize vascular surgeons who have complet ed clinical research projects in an OBL or ASC practice. Three recipients will be selected to present their research at the SOOVC ses sion during VAM 2023 in National Harbor,
Maryland. Priority will be given to projects that will serve communities with the greatest health disparities and those projects that include commu nity partnerships.
The significance and innovative nature of the pro posed project, as determined by the potential to impact vascular health, awareness and prevention, also will factor into the selection process.
Applicants must be an SVS Active or Candidate Member practicing in an OBL or ASC setting. Vascu lar interventionalists, trainees, fellows and residents are eligible. Visit vascular.org/OBLPresentation Award for information and to apply.
“There is an identified need for research that spe cifically targets the OBL or ASC settings,” said Anil Hingorani, MD, chair of the SOOVC sub-section.
“We want to encourage this research, which we expect to lead to improved care within the settings or improvements in the way we perform procedures in the OBL.”
The presentation awards, particularly for 2023, will highlight existing research, he said.
“We’re excited about these new awards, which came from suggestions from our members. As a whole, vascular surgeons love research and the
VENOUS DISEASE
EXPERTS ESTABLISH INTERNATIONAL STANDARD SET OF OUTCOME MEASURES FOR PATIENTS WITH VTE
THE INTERNATIONAL CONSORTIUM FOR HEALTH OUTCOMES Measurement (ICHOM) venous thromboembolism (VTE) working group has developed a standard set of outcome measures for patients with VTE. The consensus recommendation—published in the September edition of The Lancet Haematology—is designed to “facilitate the implementation of the use of patient-centered outcomes in daily practice”.
Authors Adam Gwozdz, MBBS, from King’s College London, England, Cindy de Jong, MD, from the Leiden University, Leiden, The Netherlands, and colleagues explain in their report that the ICHOM assembled an interna tional working group of VTE experts and patient representatives “to develop a standardized minimum set of outcomes and outcome measurements for integration into clinical practice and potentially research to support clinical decision-making and benchmarking of quality of care”.
The group selected a total of 15 core outcomes important to patients and healthcare professionals, and then categorized these outcomes into four do mains: patient-reported outcomes, long-term consequences of the disease, disease-specific complications, and treatment-related complications. They write that the outcomes and outcome measures were designed to apply to all patients with VTE aged 16 years or older. Gwozdz, de Jong et al selected a measurement tool package for inclusion in the core standard set. “Additional measures can be introduced to the user by a cascade opt-in system that allows for further assessment if required,” the authors note.
In a section on implementation, the researchers advise that the final set of outcome measures is now available online for use within clinical practice and potentially research. They elaborate: “After signing up for free through ICHOM Connect, all materials related to the set (i.e. a flyer, reference guide, and data dictionary) can be downloaded.”
chance to use science to improve our procedures and our care. We expect these grants and awards to be pretty popular.”
The SOOVC sub-section is part of the SVS Com munity Practice Section, for those members who practice in the community setting. Learn more at vascular.org/SOOVC
Applicants can apply for both the research grant and, subsequently, the Presentation Award after the research is complete.
The investigators state that the overarching aim of their work is to achieve a globally adopted standard set. However, they acknowledge that there are “different resources, digital infrastructures, and healthcare contexts in low-in come, middle-income, and high-income countries that can affect the speed and success of implementation.” They write that “training and education, commitment, and enabling attitudes of healthcare professionals are believed to facilitate implementation” and “offset more structural challenges within the healthcare system.”
“We anticipate that the introduction of this set will contribute substantially towards increasing value in VTE care,” Gwozdz, de Jong and colleagues state in their concluding remarks. They also recognize the benefit of the docu ment for patients, asserting that implementation of this set will “empower patients with VTE to actively participate in their care and, together with involved professionals, make better informed decisions about healthcare options.” Jocelyn Hudson
By Beth Bales
esponding to members’ requests for more information on financial education, a na tionally known estate and tax attorney will provide information and guidance on financial wellness at a Society for Vascular Surgery (SVS) webinar on Nov. 2.
The free webinar, “MONIES for the Vas cular Surgeon: Managing, Organizing and Navigating Investment choices for Econom ic Success,” will be held from 7 to 8:30 p.m., CDT. Registrants will receive materials help ful for financial planning.
Alan Gassman, a board-certified trust and estates lawyer, regularly lectures to medical, legal and accounting groups about financial, tax and estate planning, and cred itor protection.
His presentation will include financial in formation for vascular surgeons at all career stages—including residents and fellows—
spending many years in training. Yet, vas cular surgeons are involved in some of the most sophisticated business models, espe cially those running their own practices with or without OBL [office-based labs] or ASC [ambulatory surgery center] participation,” said Robert Molnar, MD, chair of the SVS Community Practice Section.
“No matter what practice type a surgeon finds himself or herself practicing, this we binar will provide information and tools to help ensure financial wellness.”
A recent survey of all SVS members showed great interest in learning more about financial wellness, he said.
Gassman has a master’s degree in taxation and an undergraduate degree in business with a concentration in accounting, plus a law degree. He speaks annually at the Mote Vascular Foundation Conference and nation al symposia.
In-person attendees will receive Gassman’s presentation slides plus the books 8 Steps to a Successful Estate Plan, by Gassman, and Grow Your Medical Practice, and Get Your Life Back, which he co-authored with David Finkel and Parikisth Singh, MD.
is clear that physicians receive very lit tle
in business/finances,
The webinar addresses this need and will cover issues not only for every career stage but also in all practice settings: private, sin gle-specialty group, employed, and academ ic surgeons.
Learn more and register at: vascular. org/MONIES. Join the SVS Community Practice Section (vascular.org/ CommunityPracticeSection) to receive information on future educational opportunities being planned by the section. Email svscps@ vascularsociety.org for more information.
www.vascularspecialistonline.com 27
“We want to encourage this research, which we expect to lead to improved care within the settings or improvements in the way we perform procedures in the OBL”
ANIL HINGORANI
OBL R
and will cover: ◆ Investment options ◆ Unnecessary costs that reduce retirement resources ◆ Creditor protection ◆ Estate planning ◆ Tax consequences ◆ Insurance needs ◆ Asset protection “It
education
despite
FINANCIAL WELLNESS IS FOCUS OF NOV. 2 WEBINAR VASCULAR PRACTICE
AN UPDATE ON QUALITY MEASURES FOR VASCULAR SURGERY
By Lily E. Johnston, MD, Jeniann Yi, MD, and Evan C. Lipsitz, MD
AN UPCOMING NATIONAL reporting change/addition from the Centers for Medicare and Medicaid Services (CMS) includes three important elements of which Society for Vascular Surgery (SVS) members should be aware.

The SVS Performance Measures Commit tee (PMC) represents SVS member interests regarding national quality reporting required by CMS for participation in the Quality Pay ment Program (QPP).
Physicians can report through the Mer it-based Incentive Payment System (MIPS) or Alternative Payment Models (APMs). Be ginning in 2023, CMS is adding MIPS Value Pathways (MVPs), designed to “align and connect measures and activities across the quality, cost and improvement activities per formance categories of MIPS.”
There are three important, related devel
opments to highlight for SVS members.
The first concerns changes to the quality measure roster in the 2023 CMS Proposed Rule. Currently, there are fewer than 20 qual ity measures available for vascular surgeons’ use in MIPS reporting. The SVS is responsi ble for maintaining and updating only four of these measures at present. CMS is planning a net reduction to 194 quality measures, in cluding the elimination of two SVS-spon sored measures:
◆ #258 (percent of patients undergoing open, non-ruptured, infrarenal AAA re pair without major complications and discharge to home no later than post operative day #7), and
◆ #260 (percent of asymptomatic patients undergoing carotid endarterectomy dis charged to home no later than postop erative day #2)
The rationale cited for removal is “insuf ficient volume of data submitted for per formance year 2020 to establish a historic benchmark.”
In other words, the measure cannot be benchmarked and validated. Two related measures, for endovascular aneurysm re pair (EVAR) and carotid stenting, may be similarly removed. As the reporting year cited was almost entirely encompassed by the COVID-19 public health emergency, the lack of reportable data is unsurprising. If fi nalized, elimination of these measures will reduce the reporting options for vascular surgeons, thus impacting the development and utilization of relevant MVPs.
The second development is that the SVS has rejoined the National Quality Forum (NQF), a decision carefully considered by the SVS Quality Council and Executive Board. Membership will allow the SVS to leverage the NQF’s considerable resources and experience in measure development and maintenance.
This occurs at a time when the vascular community needs to develop new measures to address the changing QPP landscape. In addition, historically CMS is more likely to accept NQF-endorsed measures, given the extensive testing and vetting process required to receive this endorsement.
Finally, members of the PMC can serve as individual NQF members, who are in turn eligible to serve on expert panels and work groups. This is another avenue for SVS and its members to influence national healthcare policy.
The third development is the plan to de velop MVP(s) designed specifically for vas cular surgeons. While several new MVPs are forthcoming, none specifically address the vascular space. The PMC has discussed options, including the development of sepa rate disease-focused (e.g., aneurysm, venous disease, lower-extremity occlusive disease) pathways versus a global MVP that all vascu lar surgeons could participate in, regardless of practice focus.
We expect the MVP development process to take at least a year or more, and in either iteration, several new vascular quality mea sures must be developed. Given the work required to develop and maintain MVPs, the
PMC felt that developing a single, more glob al option would serve vascular surgeons and the SVS most effectively. The quality mea sure area is dynamic: new measure devel opment must respond to CMS’ evolution in priorities. These priorities include increased use of claims-based measures (designed to reduce reporting burden by relying on ex isting administrative data), patient-reported outcomes and consideration of health equi ty, while balancing the need to remain clin ically relevant, measure what matters, and, where possible, incorporate existing quality programs such as the Vascular Quality Ini tiative (VQI) to reduce additional adminis trative burden and maximize participation, efficiency and validity.
References
1 Quality Payment Program: MIPS Value Pathways. Available at: https://qpp.cms. gov/mips/mips-value-pathways. Accessed 9/17/22
2. Federal Register: CMS CY 2023 Proposed Rule, Available at: https://www.federalregister.gov/ documents/2022/07/29/2022-14562/ medicare-and-medicaid-programs-cy-2023payment-policies-under-the-physician-feeschedule-and-other. Accessed 9/17/22
3. Quality Payment Program: Quality Performance Category: Traditional MIPS Requirements. Available at: https://qpp. cms.gov/mips/quality-requirements. Accessed 9/17/22
LILY E. JOHNSTON and JENIANN YI are SVS PMC members. EVAN C. LIPSITZ is the committee chair.
28 Vascular Specialist | October 2022 Prep for Exams, Stay Current with the Specialty • is perfect for: u Qualifying, certification and recertification exam prep u ABSITE and VSITE exam prep u Satisfying CME/MOC requirements u RPVI certification u Staying current with the specialty u 13 topic areas u VESAP5 Vascular Lab (available as separate purchase) O 4 modules for diagnostics and imaging (When successfully passed, this satisfies the three-year CME requirement of ARDMS to maintain RPVI certification) u Updated content and questions based on current practice, trends and guidelines u Topics that align with VSCORE u Learning and exam modes u Companion app for off-line use VISIT VSWEB.ORG/VESAP
CMS
Vascular surgeons step up for PAD awareness campaign during September
By Kristin Crowe
WALKING IS INSTRUMENTAL TO KEEPING THE 60,000 miles of blood vessels in the human body healthy— especially for patients with peripheral arterial disease (PAD). That is why throughout the month of September, also known as PAD Awareness Month, the Society for Vascular Surgery (SVS) Public and Professional Outreach (PPO) Subcommittee and the SVS Foundation brought life to a new campaign—the Vascular Health Step Challenge.
Throughout the campaign, SVS members, patients and mem bers of the general public were challenged to walk 60 miles while working toward a collective fundraising goal of $60,000.
The funds raised throughout the campaign will support the SVS Foundation in amplifying the importance of vascular health while also promoting healthy vascular health habits in communities across the country.
Over 400 walkers rose to the occasion, logging over 35,000 miles throughout September; a total of 880 individuals donated to the campaign, and together they raised more than $80,000.
Individuals were welcome to create or join teams, or to par ticipate individually. Many participants joined teams with their professional colleagues, families and peers; and some institutions hosted a walking event, all of which contributed to the comradery of the fundraiser.
“Walking is such an integral part of a healthy life for each of us, patient and healthcare professional alike,” said Benjamin Pearce, MD, who chairs the SVS PPO subcommittee. “I think it is incred ible the range of disease processes impacted positively by walk
ing—heart disease, stroke, abdominal aortic aneurysm and venous reflux to name several. But even more amazing is that walking can be a therapeutic agent for our patients with PAD, and may be the only intervention they need to improve their life.
“Each of the participants—donors and walkers—in the first annual Vas cular Health Step Challenge has rallied behind our patients to get the word out about walking and PAD, and provide much-needed assistance to our patients. We can’t wait to walk and raise even more next year!”
The SVS Foundation plans to host the Vascular Health Step Challenge again next September and encourages all SVS members to get involved. For more information, contact svsfoundation@ vascularsociety.org

The SVS and the SVS Foundation also extended “the sincerest message of thanks” to the campaign’s presenting sponsor, Ad vanced Oxygen Therapy Inc. (AOTI), Globetrotter-level sponsor Abbott and Explorer-level sponsor Peripheral Vascular Associates.
INTERACTING WITH EMR BEST PRACTICE ADVISORY ‘SIGNIFICANTLY’ IMPROVES AAA SCREENING RATE
By Bryan Kay
POSITIVELY INTERACTING WITH best practice advisory notifications is cor related with an increased rate of abdominal aortic aneurysm (AAA) screening, according to an analysis of all patients who triggered these communications alerts in an electron ic medical record system (EMR) at a single tertiary medical center.
Nearly 1,300 patients triggered a notifica tion over the three-year study period carried out by a team of researchers from St. Louis University in St. Louis, Missouri, led by firstnamed author Amin Mohamed Ahmed, MD, a vascular surgery fellow at the institution, and senior author Matthew Smeds, MD, a professor of surgery. Presenting the data at the Midwestern Vascular Surgical Society annual meeting in Grand Rapids, Michigan (Sept 15–17), the investigators found that providers interacted with—rather than dis missed—the alerts in 20.4% of patients.
BENJAMIN PEARCE
SIROLIMUS


The overall screening rate in the cohort was 28.2%. “Interacting with the best prac tice advisory significantly increased the odds of being appropriately screened for a AAA [odds ratio (OR) 2.48)],” they reported
SIX-MONTH DATA FROM BELOW-THEKNEE, FIRST-IN-HUMAN TRIAL PRESENTED IN EUROPE
SIX-MONTH DATA FROM THE SURMODICS SWING FIRST-IN-HUMAN (FIH) study of the company’s Sundance sirolimus drug-coated balloon (DCB) were shared at the 2022 Amputation Prevention Symposium (AMP) held Oct. 9–11 in Lugano, Switzerland.
The SWING study is a prospective, multicenter, single-arm feasibility study to evaluate the safety and performance of the Sundance sirolimus DCB when used to treat occlusive disease of the infrapopliteal arteries.
The study’s primary safety endpoint data showed no perioperative deaths or major amputations at 30 days and just one major reintervention was reported among the 35 trial subjects, a Surmodics press release reports. Data for the primary efficacy endpoint show a late lumen loss (LLL) of 1mm (±0.79mm) across 35 lesions at six months, indicating that the large luminal gain achieved immediately after the procedure was sustained six months post-treatment. “At six months we observed a consistent improvement in Rutherford cat egory and functional measures, as well as an excellent primary patency of 88.5%, which compares favorably to other DCBs used in the infrapopliteal circulation,” said SWING trial co-lead investigator Ramon Varcoe, MD, a vascular surgeon at the Prince of Wales Hospital and associate professor of vascular surgery at the University of New South Wales in Sydney, Australia.
The SWING trial enrolled subjects with stenotic or occluded lesions of the infrapop liteal arteries, a reference vessel diameter of 2–4mm, and a total lesion length of ≤230mm for treatment with the Sundance sirolimus DCB at eight sites in Australia, New Zealand, and multiple locations in Europe. They will be followed for 36 months following the index procedure. “The novel coating on the Sundance sirolimus DCB was evaluated in a chal lenging, predominantly CLTI [chronic limb-threatening ischemia] population with a high proportion of diabetes and moderate-severe calcification,” said trial co-lead investigator Andrew Holden, MD, director of interventional radiology at the University of Auckland in Auckland, New Zealand. “This first-in-human study demonstrates that the Sundance sirolimus DCB could be a safe and promising treatment for occlusive disease of the infrap opliteal arteries.”—Clare Tierney
www.vascularspecialistonline.com 29
“Each of the participants—donors and walkers—in the first annual Vascular Health Step Challenge has rallied behind our patients to get the word out about walking and PAD and provide much needed assistance to our patients”
EXERCISE THERAPY AORTIC ANEURYSM
SAVE THE SDATE! AVE THE DATE! 400 walkers 35,000 miles 80,000880 donors dollars
SOCIETY BRIEFS
SVS adds ‘service’ criterion to Distinguished Fellows process
Compiled by Beth Bales, Kristin Crowe and Bryan Kay
THE SOCIETY FOR VASCULAR SURGERY (SVS) has added service to the Society as an important component of its Distinguished Fellows process, an honor annually bestowed upon a handful of members.
This accolade goes to Active, International or Senior vascular-surgeon members who have distinguished themselves in a sustained matter by making substantial contributions in two of three categories: research, service or education.
Examples of service to the SVS include but are not limited to being a member or chair of an SVS committee, council, task force, writing group or section; the SVS Executive Board or Strategic Board of Directors; the Vascular Surgery Board (VSB); the Association of Program Directors of Vascular Surgery (APDVS); and/or of a Journal of Vascular Surgery (JVS) editorial board.
In addition, members of the SVS Awards and Distinctions Committee will consider presentations at the SVS Vascular Annual Meeting (VAM) or other SVS educational program, authorship of an SVS publication, or a member of the SVS Foundation or the Patient Safety Organization (PSO).
“The SVS Awards and Distinctions Committee felt strongly that for people to be distinguished members of our Society, they should have exhibited service to our Society and that service should be part of the selection process,” said SVS Treasurer Keith Calligaro, MD.
As treasurer, he chairs the SVS Awards and Distinctions Committee, which selects those members who will be put forward as candidates for the designation for voting by all SVS Distinguished Fellows.
“The Society for Vascular Surgery offers innumerable opportunities to become involved, including writing groups for guidelines, innumerable committees and task forces, JVS, VSB and more,” Calligaro said.
“Nor does service include only committee work. Members can offer to review articles for the Journal of Vascular Surgery publications, regardless of whether or not they are academic vascular surgeons or community practice vascular surgeons.
“Other members may want to mentor younger surgeons or offer to create and lead webinars on an important topic.”
The SVS Executive Board enthusiastically endorsed the addition, Calligaro added. The nomination/application deadline for 2023 is March 1, 2023.
Distinguished Fellows are recognized during the Opening Ceremony at VAM and receive their certificates at the Presidents Dinner at the annual meeting.
For the full and current list of SVS Distinguished Fellows, visit vascular.org/ DistinguishedFellows
WEEK OF ACTION’ RAISES AWARENESS OF SVS ADVOCACY ACTIVITIES
FROM SENDING EMAILS TO LAWMAKERS, POSTING ON SOCIAL MEDIA to amplify legislative issues, and donating to the Society for Vascular Surgery Political Action Committee (SVS PAC), SVS members embraced a variety of activities to support the Society’s first-ever advocacy “Week of Action,” Sept. 26–30.
“These seemingly simple advocacy activities, when done collaboratively across the SVS membership, help to significantly raise the profile of our issues on Capitol Hill and help to secure legislative victories that will benefit our practices and the patients we serve,” said SVS President Michael C. Dalsing, MD.
Activities included sending pre-written messages to lawmakers urging action to prevent pending Medicare payment reductions and signing up to become an SVS REACH 535 key contact to strengthen the SVS’ grassroots foundation.
In writing about her own experiences, Megan Tracci, MD, vice-chair of the SVS Advocacy Council, said: “Remember that vascular surgeons are not as isolated or powerless as it can sometimes feel—the SVS advocacy effort is stronger than it has ever been. Together we can—and will—make a difference.” Mark Mattos, MD, co-chair of SVS PAC, added the additional sentiment that he had participated in all aspects of the “Week of Action.” “I signed up because I want to make a difference for our patients, for my practice, for my partners, and for you, my SVS colleagues. I want to make a difference for all of us!”
Learn more about the Society’s advocacy efforts at vascular.org/advocacy.
Prepare to submit research for VAM, VRIC
The Society for Vascular Surgery (SVS) wants members’ research on vascular disease for the 2023 Vascular Research Initiatives Conference (VRIC) and the 2023 Vascular Annual Meeting (VAM).
VRIC will be held Tuesday, May 10, 2023, at the Boston Marriott Copley Place in Boston. VAM is set for June 14–17, 2023, at the Gaylord National Resort and Convention Center in National Harbor, Maryland, outside Washington, D.C.
VRIC submissions open Oct. 26. These submissions are coordinated with the American Heart Association’s submission site for the “Vascular Discovery 2023: From Genes to Medicine” conference. That conference will be May 10–13, 2023, in the same location as VRIC.
The VAM submission site will be open from Wednesday, Nov. 16, through 3 p.m. Central Standard Time Wednesday, Jan. 11, 2023.
Learn more about VRIC at vascular. org/VRIC23. VAM submission categories and guidelines are expected to be available by mid-October at vascular.org/ VAMGuidelines23. Learn more about VAM at vascular.org/VAM
Slew of regional vascular societies welcome new leaders for 2022–23
THE MANY “BENEFITS OF BELONG ing” to the Society for Vascular Surgery (SVS) include discounts on two impor tant reference titles in the diagnosis and treatment of vascular injury: Rich’s Vascular Trauma and Rutherford’s Vascular Surgery and Endovascular Therapy
Both works are published in associa tion with the SVS. Members are entitled to receive 30% off when using the SVS discount code, SVS30.
The fourth edition of Rich’s Vascular Trauma has now been fully updated, and reflects recent changes in vascular injury patterns, wounds and trauma care.
It draws from current research and a wide variety of peer-reviewed publica tions to keep surgeons up to date with the latest evidence-based management strategies and techniques, according to book publisher Elsevier.
Released in November 2021, the book was written and edited by vascular surgeons who are also trauma specialists with proficiency in both open surgical and endovascular techniques. The editors are Todd E. Rasmussen, MD, and Nigel R.M. Tai (in England).
The SVS discount reduces the price of Rich’s to $160.99. Visit vascular.org/ Rich’s for more information and to buy the book.
The two-volume 10th edition of Ru therford’s Vascular Surgery and Endovascu lar Therapy is considered an “outstanding reference for vascular surgeons, vascular medicine specialists, interventional radiologists and cardiologists, and their trainees who depend upon Rutherford’s in their practice,” according to Elsevier.
Former SVS Presidents Anton Sidawy, MD, and Bruce Perler, MD, are once again editors.
With the SVS code, the price of Ru therford’s drops to $297.49.
Visit vascular.org/Rutherford10 to purchase the set and get more information.
A number of regional vascular societies across the U.S. and Canada introduced their 2022–23 slew of officers during their annual meetings during the early fall months.
Among the leaders taking office in the U.S. were Jeffrey Jim, MD, who took over as the Midwestern Vascular Surgical Society (MVSS) president; Peter Faries, MD, who assumed the same role at the head of the Eastern Vascular Society (EVS); Wei Zhou, MD, the new president of the Western Vascular Society (WVS); and Sean Roddy, MD, now the New England Society for Vascular Surgery (NESVS) top office holder.
In Canada, John Harlock, MD, assumed the reins of the Canadian Society for Vascular Surgery (CSVS) for the next 12 months.
Taking their leave from the presidential stage after the 2021–22 term were Raghu Motaganahalli, MD, of the MVSS; Robert Rhee, MD, of the EVS; Vincent Rowe, MD, at the WVS; Andres Schanzer, MD, atop NESVS; and Luc Dubois, MD, from the CSVS.
30 Vascular Specialist | October 2022
SVS members: Don’t forget 30% discount on Rich’s, Rutherford’s
Ukrainian surgeons present clinical update on Human Acellular Vessel in vascular trauma at ESVS 2022
HUMACYTE RECENTLY ANNOUNCED THE presentation of a clinical update on the Human Acellular Vessel (HAV) for the treatment of vascular trauma. The
was presented by Ukrainian surgeon collaborators, Oleksandr Sokolov, MD, from Dnipro State Medical University in Dnipro, Ukraine, and Vasyl Shaprynskyi, MD, from the State Institution of Science Research and Practical Center for Preventive and Clinical Medicine State Administrative Department in Kyiv, Ukraine, at this year’s European Society for Vascular Surgery (ESVS) annual meeting (Sept. 20–23) in Rome, Italy.
Humacyte notes in a press release that the company’s investigational HAV is designed to offer off-the-shelf availability and resistance to infection and to address long-standing limitations in vascular tissue repair and replacement.
Shaprynskyi attended the conference in person and presented a live talk entitled, “The first experience of using the Human Acellular Vessel in Ukraine for the treatment of patients with vascular trauma,” while Sokolov spoke
Aortic
device in US
THE CENTERS FOR MEDICARE AND Medicaid Services (CMS) has granted approval of a new technology add-on payment (NTAP) for Terumo Aortic’s Thoraflex Hybrid device—used to treat aortic arch disease—under the inpatient prospective payment system (IPPS).
The add-on payment is effective in CMS’ FY 2023 fiscal year, starting on Oct. 1, and has been assigned the maximum new technology add-on payment—65% of the average cost of the technology for a case involving the use of the Thoraflex Hybrid device.
The Thoraflex Hybrid received CE mark approval in 2012, with more than 13,000 devices sold commercially around the world over the past 10 years.
Thoraflex Hybrid is the first of its kind device used in frozen elephant trunk (FET) repair in the U.S., according to Terumo Aortic. It was granted Breakthrough Device designation by the Food and Drug Administration (FDA) in 2020, followed by FDA approval for commercial sale in the U.S. earlier this year.
This hybrid device allows patients with suitably limited disease to be treated in a single-stage procedure rather than two, which has previously been the conventional pathway in the U.S. for this group of patients.
The add-on payment will allow hospitals to be reimbursed for the incremental costs relating to the implantation of the Thoraflex Hybrid device to support the treatment of patients with complex aortic arch disease—in addition to the diagnosis related group (DRG) reimbursement.
virtually from the Ukraine and presented, “Vascular trauma due to blast injury. Experience of Dnipro in RussianUkrainian war 2022.”
“Shaprynskyi and Sokolov have been instrumental in establishing their hospitals as medical strongholds during the Russian-Ukrainian war and reported that blast trauma, causing massive tissue damage and infected wounds, accounts for approximately 82% of incoming vascular trauma cases to their medical centers,” the press release reads. Trauma to the extremities makes up the majority of injuries, the statement adds, noting that these are primarily vascular injuries to the lower extremities and shoulders.
“Access to the HAV, a biologic conduit, has improved our ability to perform vascular reconstructions by eliminating the need to harvest a venous conduit and saving time required to look for useable vein, assisting greatly in limb salvage.
While we continue to face this crisis in our country, partnerships with groups like Humacyte allow us to overcome many limitations in wartime medical care that we previously experienced, such as lack of readily available conduits that are resistant to infection, particularly important in the contaminated battlefield setting,” said Shaprynskyi.

Shaprynskyi and Sokolov reported that surgeons in Ukraine have utilized the HAV to treat patients with a multitude of wartime injuries. Sokolov provided a clinical
results demonstrate ‘excellent safety profile’ of the FlowTriever system in full US cohort
RESULTS OF THE FLASH REGISTRY demonstrate the “excellent safety profile” of the FlowTriever system (Inari Medical) in 800 “real-world” patients. This is according to Catalin Toma, MD, from the University of Pittsburgh Medical Center, Pittsburgh, who presented outcomes for the full U.S. cohort of FLASH at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT; Sept. 16–19) in Boston.
The results were simultaneously published in EuroIntervention. Toma reported a 1.4% major bleeding rate and 0.4% rate of other major adverse events (MAEs) in what the presenter described as the largest prospective interventional study in pulmonary embolism (PE).
All-cause mortality was <1% at the 30-day visit in this real-world population with prevalent baseline predictors of mortality, the presenter added, noting also that hemodynamics improved on-table, acute vitals and right ventricular (RV) strain normalized, and clinical recovery continued through six months. The presenter noted that acute all-cause mortality following PE has remained high (>10%) over two decades.
update on a patient with a blast injury to the shoulder who received a repair using the HAV. The patient is now beyond three-month follow-up without complication. Another patient who suffered a blast injury to the lower leg underwent successful HAV implantation and is now one month past surgery without complication. Shaprynskyi reported on a patient with a gunshot wound to the right thigh that was initially treated with a synthetic graft, but ultimately the graft failed due to infection, putting the patient at risk of limb loss. The HAV was used to replace the infected graft, and three months later the HAV is supplying blood flow to the limb and is infection-free.
Humacyte worked closely with the Office of International Programs of the Food and Drug Administration (FDA) and the Ukrainian Ministry of Health to provide the HAV as an additional treatment option to those affected with vascular injury in Ukraine. Humacyte is currently evaluating the HAV in a phase 2/3 clinical trial in vascular trauma for use as a vascular replacement to restore blood flow to a limb, when saphenous veins or synthetic grafts are not feasible.
The HAV has received priority designation for the treatment of vascular trauma by the U.S. secretary of defense. The HAV is an investigational product and has not been approved for sale by the FDA or any international regulatory agency.
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts. CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II. Refer to Instructions for Use at eifu.goremedical.com for a
of
precautions and contraindications for
available.
www.vascularspecialistonline.com 31
complete description
all applicable indications, warnings,
the markets where this product is
GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface* Consult Instructions for Use eifu.goremedical.com *As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS Heparin Surface. Products listed may not be available in all markets. GORE, Together, improving life, VIABAHN and designs are trademarks of W. L. Gore & Associates. © 2021 W. L. Gore & Associates, Inc. 21373436-EN DECEMBER 2021 01: 4.5” x 5.625” 21373436-EN-VSX-Indications-Ad.indd 1 12/8/21 11:10 AM Terumo
announces new technology add-on payment for Thoraflex Hybrid
FLASH
CLINICAL&DEVICENEWS Compiled by Jocelyn Hudson, Will Date and Bryan Kay
update
Humacyte device
Catalin Toma
MORE POSSIBILITIES


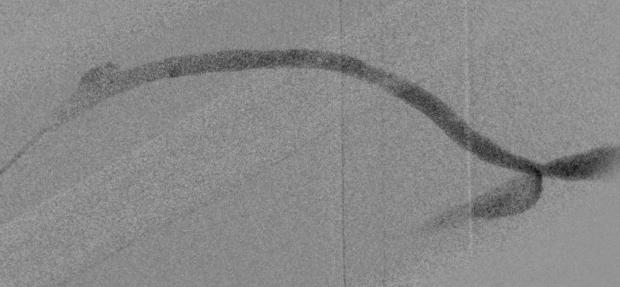















GORE® VIABAHN® Endoprosthesis OPEN
Restoring durable AV graft flow upon diagnosis of recoil post-PTA at the AV graft venous anastomosis Recoil post-PTA Post VIABAHN® Device placement See the NEW case study W. L. Gore & Associates, Inc.—Flagstaff, Arizona 86004—goremedical.com Images courtesy of Daniel V. Patel, M.D. Used with permission. Please see accompanying prescribing information in this journal. Products listed may not be available in all markets. GORE, Together, improving life, VIABAHN and designs are trademarks of W. L. Gore & Associates. © 2022 W. L. Gore & Associates, Inc. 21372352-EN JANUARY 2022
 By Bryan Kay
By Bryan Kay




 By Malachi Sheahan III, MD
By Malachi Sheahan III, MD











































































 By Christopher Audu, MD
By Christopher Audu, MD



























