By Bryan Kay, Urmila Kerslake and Aaron Kudhail


One of the earliest multispecialty public gatherings of the vascular community following the release of the first results from the BESTCLI randomized controlled trial (RCT) raised some of the early

fault lines developing between different interpretations of the study’s key data.
The co-principal investigators (PIs)—Alik Farber, MD, Matthew Menard, MD, and Kenneth Rosenfield, MD—have hailed the study as the largest RCT comparing revascularization treatment strategies in patients with CLTI, and have outlined how it will provide important information regarding their management.
The trio stand at the vanguard of a study that set out to be a multidisciplinary endeavor. Yet, early disagreements exist even among the PIs themselves, a facet touched on before a packed standalone BEST-CLI session at the 2022 VEITHsymposium in New York City (Nov. 15–19) by Rosenfield himself. Results from BEST-CLI showed that surgical
www.vascularspecialistonline.com THE OFFICIAL NEWSPAPER OF THE DECEMBER 2022 Volume 18 Number 12 18
23
02
12
Presorted Standard U.S. Postage PAID Permit No. 384 Lebanon Jct. KY
JVS-VL Journal dedicated to venous disease set to go online-only
VenoValve New firstin-human data emerge demonstrating benefits of prosthetic device In this issue:
Guest editorial Arthur E. Palamara, MD, on Envision, private equity and patient care
Comment & Analysis Bhagwan Satiani, MD, on learning culture, leaders and Melinda French Gates
See page 6 Read the full story on page 4 VASCULAR COMMUNITY STARTS PROCESS OF WRESTLING WITH FINDINGS FROM BEST-CLI ascularV pecialists CHANGE SERVICE REQUESTED 9400 W. Higgins Road, Suite 315 Rosemont, IL 60018
in vascular: Annual meeting dedicated to tackling key issues related to women’s vascular health and female workforce seeks to ‘enable and empower’
Women
Linda Harris
GUEST EDITORIAL
Associate Medical Editors
Bernadette Aulivola, MD | O. William Brown, MD | Elliot L. Chaikof, MD, PhD
| Carlo Dall’Olmo, MD | Alan M. Dietzek MD, RPVI, FACS | Professor HansHenning Eckstein, MD | John F. Eidt, MD
| Robert Fitridge, MD | Dennis R. Gable, MD | Linda Harris, MD | Krishna Jain, MD | Larry Kraiss, MD | Joann Lohr, MD
| James McKinsey, MD | Joseph Mills, MD | Erica L. Mitchell, MD, MEd, FACS
| Leila Mureebe, MD | Frank Pomposelli, MD | David Rigberg, MD | Clifford Sales, MD | Bhagwan Satiani, MD | Larry Scher, MD | Marc Schermerhorn, MD | Murray L. Shames, MD | Niten Singh, MD | Frank J. Veith, MD | Robert Eugene Zierler, MD
Resident/Fellow Editor
Christopher Audu, MD
Executive Director SVS Kenneth M. Slaw,
PhD Director of Marketing & Communications Bill Maloney Managing Editor SVS Beth Bales Marketing & Social Media Manager Kristin Crowe
Envision, private equity and patient care: Substituted values 2.0
 By Arthur E. Palamara, MD
By Arthur E. Palamara, MD
He was only 41, already burdened with multiple health problems. Diabetes, hypertension and renal failure had put him on dialy sis, caused the amputation of his left leg, and now left him with poor circulation in his right leg. Signs of gangrene were already present in his toes. Only bringing more blood to his leg would pre vent him from being a bilateral amputee, a condition that would place him entirely dependent on his family, who were already stressed by his debilitating illness. His two teenage sons visited him daily to give him courage. Only his mind remained mostly intact. And even then, his mental functions waxed and waned.
Published by BIBA Publishing, which is a subsidiary of BIBA Medical Ltd.
Publisher Roger Greenhalgh
Content Director Urmila Kerslake
Managing Editor Bryan Kay bryan@bibamedical.com
Editorial contribution
Jocelyn Hudson, Will Date, Jamie Bell, Clare Tierney, Anthony Strzalek, Benjamin Roche and Aaron Kudhail
Design Terry Hawes
Advertising Nicole Schmitz nicole@bibamedical.com
Letters to the editor
vascularspecialist@vascularsociety.org BIBA Medical, Europe 526 Fulham Road, London SW6 5NR, United Kingdom
BIBA Medical, North America 155 North Wacker Drive – Suite 4250, Chicago, IL 60606, USA
Scheduled for an angioplasty in the early afternoon, his pro cedure was delayed by staffing issues: there were insufficient “anesthesia providers” to cover the case. The days of having a fully-trained anesthesiologist to provide anesthetic care were long gone. Previously, high-risk individuals like him were seen the night before, recommendations made, then deliv ered anesthesia the following day. Now, care was provided by anesthesiologists employed by a private equity firm, Envision Healthcare/KKR, an entity with different goals. Here, profit ability was valued over patient care. Staffing was a problem, and high turnover resulted in a condition where the patient became a “case.” Whoever was available carried out the case, be it a doctor, a certified nurse practitioner or an anesthesia assistant. The shortage was made worse by the pandemic. Since large Wall Street firms cared little for their employees, the employees felt no loyalty to their employer, and moved from one hospital to another. The days of institutional loyalty were long over.
Vascular Specialist is the official newspaper of the Society for Vascular Surgery and provides the vascular specialist with timely and relevant news and commentary about clinical developments and about the impact of healthcare policy. Content for Vascular Specialist is provided by BIBA Publishing.
Content for the News From SVS is provided by the Society for Vascular Surgery. The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or the Publisher. The Society for Vascular Surgery and BIBA Publishing will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services, or the quality or endorsement of advertised products or services, mentioned herein. | The Society for Vascular Surgery headquarters is located at 9400 W. Higgins Road, Suite 315, Rosemont, IL 60018.
| POSTMASTER: Send changes of address (with old mailing label) to Vascular Specialist, Subscription Services, 9400 W. Higgins Road, Suite 315, Rosemont, IL 60018. |
RECIPIENT: To change your address, e-mail subscriptions@bibamedical.com | For missing issue claims, e-mail subscriptions@bibamedical. com. | Vascular Specialist (ISSN 1558-0148) is published monthly for the Society for Vascular Surgery by BIBA Publishing. | Printed by Vomela Commercial Group | ©Copyright 2022 by the Society for Vascular Surgery
The afternoon dragged on and the patient had not eaten since the day before. He received insulin that morning, but the insulin had little food to work on. The patient was sleepy but arousable. He smiled weakly, opening his eyes, and muttered a few incomprehensible words. His vital signs remained stable. One o’clock became two o’clock, then three then four. Finally, an anesthesia provider was found. He was placed on the radiol ogy table and the case was set to begin.
The nurse anesthetist, just assigned to the case, was very competent, but knew little about the “case” except an outline of his major problems. The procedure, minimally invasive, and done with local anesthesia and sedation, carried little risk of blood loss or serious complications. She gave the usual medications, the patient was prepped, and the procedure was about to begin. As the surgeon began, he felt no pulse in the femoral artery into which he was to place his needle. Wires and balloons would subsequently be inserted to open the blocked arteries. That was not to be.
“Are we okay?”
“Let me check,” the nurse anesthetist responded.
He could hear the whirring of the blood pressure machine attempting, but failing, to find a blood pressure. The heart monitor recorded a stable heart rhythm, but the oxygen monitor bleeped a lower pitch with each heartbeat, consistent with falling oxygenation.
2
Medical Editor Malachi Sheahan III, MD
The ‘great resignation,’ traveling nurses, and professional ennui have reduced the number of trained, experienced personnel. New hires are often unacquainted with complex procedures, which increases the chances for unfavorable outcomes
Vascular Specialist | December 2022
Arthur E. Palamara




Processed With Patents and patents pending see: ww w.mimedx.com/patents. AMNIOCORD, AMNIOEFFECT, AMNIOFIX, AXIOFILL, PURION and MIMEDX are trademarks of MIMEDX Group, Inc. ©2022 MIMEDX Group, Inc All Rights Reser ved ww w.mimedx.com US-AC-2200009 v1.0 Thin Sheets | Thick Sheets | Particulate & Paste Scan Here bit.ly/3XOBiVi Learn more: mimedx.com NEW! NEW!
FROM THE COVER: SUMMIT DEDICATED TO TACKLING KEY ISSUES RELATED TO WOMEN’S VASCULAR HEALTH AND FEMALE WORKFORCE SEEKS TO ‘ENABLE AND EMPOWER’
LINDA HARRIS, MD, IS A PROLIFIC ATTENDEE ON the national and regional vascular meeting circuit, a noteworthy academic vascular surgeon with trainees and papers on the scientific session plate, and a curious questioner of new research offered up from the podium. A few years back, she started to take serious note of a recurring theme. Harris, the vascular surgery program director at University at Buffalo in Buffalo, New York, had spotted a problem with papers delving into issues and outcomes related to disparities.
“I was going to all the major national and regional meetings, and every meeting I would hear multiple papers saying women do worse, African Americans do worse, next paper, repeat, so we would hear the same mantra repeated over again that we acknowledge, ‘We have a problem with our patients,’ and yet we weren’t going anywhere,” she tells Vascular Specialist
The time had come, Harris figured, for a dedicated forum, “a venue [where] we can dive deeper into what the problem is, and, better yet, what can we do to change the outcome so that the answer is not women do worse, Blacks do worse, etc.,” she says.
So was born the Women’s Vascular Summit, set to enter its 5th edition into the books. Harris, a course director and meeting founder, describes the meeting as having scored huge successes over the course, tackling both sides of its dual purpose: drilling down deeper on issues around women’s vascular health, and helping elevate and empower women vascular surgeons.
This year’s iteration sees the summit take another leap forward: the creation of three research grants for mid-level women vascular surgeons who are interested in aortic dis-
ease. Scheduled to take place at Harris’ own Jacobs School of Medicine in Buffalo from April 28–29, the 2023 edition of the meeting also coincides with special issues of both Seminars in Vascular Surgery and Annals of Vascular Surgery that are dedicated to women’s vascular health and set to be released during the year.
To be sure, Harris points out she is no critic of those regional or national vascular meetings. Rather, she feels they are not equipped to scale the enormity of the task of establishing the why of the disparate outcomes. “One of the problems is that when the books were written, the patients were considered to be White males, because we assumed women didn’t have cardiovascular disease,” Harris explains. “Women didn’t get chest pain; women didn’t get peripheral arterial disease [PAD] or aneurysms. We know that’s not true. But the symptomatology, the way it’s written, is for White males. So, when women present, they often have atypical symptoms. They’re really not atypical symptoms. As one of my friends said, they’re really women’s symptoms.
“I believe in all of the meetings and organizations,” Harris continues. “The problem is they’re all very overburdened with multiple things they’re trying to accomplish. There is not the time during those meetings to really delve deeper into what the issues are. We used to have time periods for women’s get-togethers. Even when we have them, we maybe have two hours if we’re lucky. That’s not enough time. If we continue doing that, we won’t make progress.”
Likewise, the summit aims to facilitate progress for the generations of women vascular surgeons following the trail of people like Harris and fellow course directors such as
Amy Reed, MD, chief of vascular surgery at the University of Minnesota; Mariel Rivero, MD, an assistant professor of surgery also from the University at Buffalo; and Elizabeth Genovese, MD, director of the limb salvage program at the Medical University of South Carolina.
“When I started,” Harris recalls of the early years of her career, “I’d walk into a room at major meetings, and there would be two or three other women and 100s of men. The opportunity for advancement was more difficult.”
Rather than foster a scenario where women leapfrog men, Harris says, the spirit of the summit is about creating “ equal opportunity.”
“Part of the problem is women haven’t always been given opportunities—99% of the time it is unconscious bias,” she says. “It’s not that our male colleagues are trying to put us down. But they just don’t always think of giving the opportunity of, ‘You might be good for this position,’ or, ‘Would you like to be a PI [principal investigator]?’”
The meeting also seeks to provide a platform to tackle what Harris describes as an intrinsic conditioning often present among women in the workplace—scenarios where “women don’t feel as if they are prepared for a position even when they are more qualified than their male colleagues who will say they are prepared for a position.”
Fundamentally, this strand of the summit—which consists of a day-and-a-half of programming—is about fostering diversity and equity, she says, and providing options for those women vascular surgeons who aspire to make natural career progression. “We’re trying to have a venue where women are comfortable communicating with each other, helping to promote [each other] and work together.”
cents on the dollar. How did this happen to the largest U.S. physician staffing firm, owned by KKR, one of the most financially successful private equity firms in the world? How can KKR extricate itself and protect its investment? And what happens to its doctors and patients?”
Private equity firms like to boast about their closely guarded “secret sauce” recipe for how they buy a company, load it with debt, introduce new high-tech practices that increase efficiency and revenue, and then exit at a profit. But KKR and Envision demonstrate these assertions are empty. KKR acquired Envision in 2018 in a leveraged buyout that burdened the company with billions in debt. But KKR’s plan for paying off the debt, and garnering a high return for its investors, was low-tech.
An article in Bloomberg by Eliza Ronalds-Hannon and Davide Scigliuzzo, from Oct. 5, 2022, details its immense challenge: Envision is in dire straits since it lacks the ability to support the roughly $7 billion debt that KKR structured into its 2018 buyout. Angelo Gordon & Co. and Centerbridge Partners, fund managers, were offering more than $1 billion of new capital. Even more unpalatable is the requirement that Envision must divest itself of its most profitable asset: AmSurg. Envision is in
deep trouble because of high labor costs, patient-protection (surprise billing) legislation, as well as a drop in hospital visits. Further compounding Envision’s prospects are rising interest rates. Its position is additionally threatened owing to complex loans and bonds that make it difficult to determine Envision’s actual worth. Potential creditors have little protection and face steep challenges if faced with a default. A looming recession further dampens Envision’s chances of a successful outcome.
What does this mean for anesthesiologists, surgeons and patients? Anesthesiologists are dedicated doctors who deal with complex patients in critical situations. Since they have little faith in their employers’ ability to pay them, and anticipate little future security, many have left for greener pastures. Locally here, three anesthesiologists resigned in the past month, while others are retiring. As such, hospitals have trouble recruiting new anesthesia providers. This lack of an essential service causes delays and postponement of cases. Complex cases are performed later in the day when adequately trained operating room personnel are not available. (Complications and mortality increase by 10% when cases are done “off-hours” or on weekends.)
The “great resignation,” traveling nurses, and professional ennui have reduced the
number of trained, experienced personnel. New hires are often unacquainted with complex procedures, which increases the chances of unfavorable outcomes.
While most operations are straightforward, vascular cases are especially complex since many are hybrid. “Team” experience is the best safeguard to reduce errors.
The anesthesia turnover is so great that it leads to loss of confidence in the person at the head of the table. With enthusiastic but inexperienced personnel providing anesthesia care, many anesthesia providers are unfamiliar with the surgeon and the procedure, and cannot anticipate what could potentially go wrong. Since most cases are being covered by nurse anesthetists or anesthesia assistants, albeit supervised by an anesthesiologist, newly minted providers lack the expertise to avoid trouble.
The margin between success and failure is thin. Medicine is very personal. The surgeon looks the patient in the eye and assures them that they can expect a favorable outcome. It is incumbent that all of us as providers see patients as a person with a husband or wife, children and a life story. To the patient, the fact that the surgeon is unfamiliar with the anesthesia provider elevates their level of tension. Concern rises when this crucial element in the surgery is
found to be untried and untested.
The concerns cited above are real but correctable. Entities such as Envision/ KKR—interested as they are in their own profitability—provide no demonstrable benefit to patients, surgeons or hospitals. They provide even less benefit to the likes of anesthesiologists and anesthesia assistants themselves who are placed in an undesirable position through no fault of their own. These dedicated caregivers are additionally exposed to litigation, financial damages and professional sanctions.
Envision/KKR’s position will become more precarious in 2023 and can only lead to under-performance of services. This will, by necessity, negatively impact on the quality of care. It is apparent that the solution lies in anesthesiologists (and mid-level providers) being hired by hospitals and treated as the dedicated professionals that they are.
Hospitals may consider this an unnecessary administrative headache and financial burden, but their obligation to patients as well as protection of their reputation, make this an inevitable strategy. Private equity in healthcare consumes much, but yields little.
ARTHUR E. PALAMARA is a vascular surgeon in Hollywood, Florida.
4 Vascular Specialist | December 2022
➽ GUEST EDITORIAL ENVISION, PRIVATE EQUITY
PATIENT CARE: SUBSTITUTED VALUES
AND
2.0 continued from page 2
Impressive

The REALITY Study, sponsored by the VIVA physicians,1 demonstrates how the use of the HawkOne™ directional atherectomy system, followed by the IN.PACT™ Admiral™ drug-coated balloon (DCB), can help achieve positive patient outcomes in treating peripheral artery disease. Learn more by visiting medtronic.com/realitystudy
HawkOne Directional Atherectomy System



















IN.PACT Admiral Drug-Coated Balloon
































results. Your new REALITY.
92.6% 8.8% 12-month freedom from CD-TLR† Outcomes in complex, long, heavily calcified lesions2: Bailout stent rate
FROM THE COVER:
VASCULAR COMMUNITY STARTS PROCESS OF WRESTLING WITH FINDINGS
bypass with adequate single-segment great saphenous vein (GSV) is a more effective revascularization strategy for patients with chronic limb-threatening ischemia (CLTI) who are deemed to be suitable for either an open or endovascular approach, the investigators reported. In patients without a suitable single-segment saphenous vein, both surgical and endovascular strategies were found to be effective in treating patients with CLTI, leading the investigators to conclude that there is “a complementary role for both revascularization strategies in these patients.”
The session saw commentary being led by both Eric Secemsky, MD, representing a perspective from the endovascular community, and Michael Conte, MD, providing a view from the vascular surgery sphere.



Secemsky, section head of interventional cardiology at Beth Israel Deaconess Medical Center in Boston, said BEST-CLI provided robust critical evidence but bore limitations in terms of the inclusion of major reintervention in the primary endpoint, the representativeness of non-surgical specialties in the trial, as well as the “generalizability” of the patients enrolled. “Patient preference, surgical candidacy, prognosis, suitable anatomy, technical proficiency, costs and timely access to care remain important considerations when deciding whether to pursue a surgical versus endovascular strategy, “ he pointed out.

Conte, chief of vascular and endovascular surgery at the University of California, San Francisco, said the trial showed that open surgery and endovascular intervention “are both safe and have complementary roles in the treatment of CLTI patients.” He said that open bypass with GSV provides more effective revascularization in suitable candidates, and “is likely under-utilized in current practice,” adding that “an endo-first or endo-only approach to all patients with CLTI is not evidence-based care.” Centers
FROM BEST-CLI
continued from page 1
carrying out less than 20% bypass in CLTI “should probably take stock.”
During the session, Farber, for his part, delved into potential trial weaknesses. “The study is not perfect; it had many limitations,” he said. “I think we need to be honest about it and lay those out.” It was a pragmatically designed trial, with the possibility for selection and operator bias in enrollment and intervention, he noted. “And equipoise and eligibility: Although everybody understands it exists, they were different across sites. There was also procedural heterogeneity.”

Farber acknowledged the trial’s cohort 2 was likely underpowered. “The anatomical complexity is yet to be evaluated,” he added. “The percentage of female patients was lower than targeted. There is no question that the Katsanos meta-analysis and its effects probably has an effect on the use of drug-coated technologies in the trial.”

Rosenfield noted the “controversy” the trial will generate: “[Amongst the BEST-CLI investigators] we have differences in the way we think it should be interpreted. My perspective is a little bit more muted than sort of the ‘Okay, this just tells the whole story about how you have to treat CLTI patients.’” His “top line,” he said, is that bypass fundamentally bears an important role in the treatment of CLTI, underscoring how the trial also showed that both procedures are safe. The lesser-discussed cohort 2, Rosenfield said, gets to one of his points of focus—that the study “raises a lot of questions that still need to be answered.”
The trial answers questions “about those patients who were randomized in the trial,” he said, continuing, “We need to unpack better what were the characteristics of those patients who were entered into the trial.” That will help specialists determine the degree to which “we can generalize the findings: to which patients does this apply?”—Bryan Kay
†12-month data reported includes patients beyond the follow-up window.
References
1 The REALITY Study was independently sponsored and conducted by the VIVA Physicians. The study prospectively enrolled 102 participants whose treatment outcomes were independently adjudicated by angiographic and duplex ultrasound core labs and a clinical events committee. The research was funded by Medtronic through the manufacturer’s ERP program.
2 Rocha-Singh KJ, Sachar R, DeRubertis BG, et al. Directional atherectomy before paclitaxel coated balloon angioplasty in complex femoropopliteal disease: The VIVA REALITY Study. Catheter Cardiovasc Interv. September 2021;98(3):549-558.
Brief Statements
HawkOne™ Directional Atherectomy System
Important Information: Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device.
Indications for Use: The HawkOne™ directional atherectomy system is intended for use in atherectomy of the peripheral vasculature. The HawkOne catheter is indicated for use in conjunction with the SpiderFX™ embolic protection device in the treatment of severely calcified lesions. The HawkOne catheter is NOT intended for use in the coronary, carotid, iliac, or renal vasculature.
Caution: Federal (USA) law restricts this product for sale by or on the order of a physician.
IN.PACT Admiral Paclitaxel-coated PTA Balloon Catheter
Indications for Use: The IN.PACT Admiral Paclitaxel-coated PTA Balloon Catheter is indicated for percutaneous transluminal angioplasty, after appropriate vessel preparation, of de novo, restenotic, or in-stent restenotic lesions with lengths up to 360 mm in superficial femoral or popliteal arteries with reference vessel diameters of 4–7 mm. Contraindications: The IN.PACT Admiral DCB is contraindicated for use in: • Coronary arteries, renal arteries, and supra-aortic/cerebrovascular arteries • Patients who cannot receive recommended antiplatelet and/or anticoagulant therapy • Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system • Patients with known allergies or sensitivities to paclitaxel • Women who are breastfeeding, pregnant, or are intending to become pregnant or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and whether there is a potential for adverse reaction in nursing infants from paclitaxel exposure. Warnings: • A signal for increased risk of late mortality has been identified following the use of paclitaxel-coated balloons and paclitaxel-eluting stents for femoropopliteal arterial disease beginning approximately 2–3 years post-treatment compared with the use of non-drug coated devices. There is uncertainty regarding the magnitude and mechanism for the increased late mortality risk, including the impact of repeat paclitaxel-coated device exposure. Physicians should discuss this late mortality signal and the benefits and risks of available treatment options with their patients. • Use the product prior to the Use-by Date specified on the package. • Contents are supplied sterile. Do not use the product if the inner packaging is damaged or opened. • Do not use air or any gaseous medium to inflate the balloon. Use only the recommended inflation medium (equal parts contrast medium and saline solution). • Do not move the guidewire during inflation of the IN.PACT Admiral DCB. • Do not exceed the rated burst pressure (RBP). The RBP is 14 atm (1419 kPa) for all balloons except the 200 and 250 mm balloons. For the 200 and 250 mm balloons the RBP is 11 atm (1115 kPa). The RBP is based on the results of in vitro testing. Use of pressures higher than RBP may result in a ruptured balloon with possible intimal damage and dissection. • The safety and effectiveness of using multiple IN.PACT Admiral DCBs with a total drug dosage exceeding 34,854 μg of paclitaxel in a patient has not been clinically evaluated.
Precautions: • This product should only be used by physicians trained in percutaneous transluminal angioplasty (PTA). • This product is designed for single patient use only. Do not reuse, reprocess, or resterilize this product. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or create a risk of contamination of the device, which could result in patient injury, illness, or death.
• Assess risks and benefits before treating patients with a history of severe reaction to contrast agents.
• The safety and effectiveness of the IN.PACT Admiral DCB used in conjunction with other drugeluting stents or drug-coated balloons in the same procedure or following treatment failure has not been evaluated.
• The extent of the patient’s exposure to the drug coating is directly related to the number of balloons used. Refer to the Instructions for Use (IFU) for details regarding the use of multiple balloons and paclitaxel content. • The use of this product carries the risks associated with percutaneous transluminal angioplasty, including thrombosis, vascular complications, and/or bleeding events. • Vessel preparation using only pre-dilatation was studied in the clinical study. Other methods of vessel preparation, such as atherectomy, have not been studied clinically with IN.PACT Admiral DCB. • This product is not intended for the expansion or delivery of a stent. Potential adverse effects: The potential adverse effects (e.g., complications) associated with the use of the device are: abrupt vessel closure; access site pain; allergic reaction to contrast medium, antiplatelet therapy, or catheter system components (materials, drugs, and excipients); amputation/loss of limb; arrhythmias; arterial aneurysm; arterial thrombosis; arteriovenous (AV) fistula; death; dissection; embolization; fever; hematoma; hemorrhage; hypotension/hypertension; inflammation; ischemia or infarction of tissue/organ; local infection at access site; local or distal embolic events; perforation or rupture of the artery; pseudoaneurysm; renal insufficiency or failure; restenosis of the dilated artery; sepsis or systemic infection; shock; stroke; systemic embolization; vessel spasms or recoil; vessel trauma which requires surgical repair. Potential complications of peripheral balloon catheterization include, but are not limited to the following: balloon rupture; detachment of a component of the balloon and/or catheter system; failure of the balloon to perform as intended; failure to cross the lesion. Although systemic effects are not anticipated, potential adverse events that may be unique to the paclitaxel drug coating include, but are not limited to: allergic/immunologic reaction; alopecia; anemia; gastrointestinal symptoms; hematologic dyscrasia (including leucopenia, neutropenia, thrombocytopenia); hepatic enzyme changes; histologic changes in vessel wall, including inflammation, cellular damage, or necrosis; myalgia/ arthralgia; myelosuppression; peripheral neuropathy. Refer to the Physicians’ Desk Reference for more information on the potential adverse effects observed with paclitaxel. There may be other potential adverse effects that are unforeseen at this time.Please reference appropriate product Instructions for Use for a detailed list of indications, warnings, precautions, and potential adverse effects. This content is available electronically at manuals.medtronic.com. Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
medtronic.com/realitystudy
UC202107921a EN ©2022 Medtronic. All rights reserved. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. For distribution in the USA only. 10/2022
6 Vascular Specialist
➽
Co-principal investigator Matthew Menard (top, and bottom left) introduces a panel of experts who participated in a packed BEST-CLI discussion session at the 2022 VEITHsymposium in New York. Speakers included Michael Conte (middle right) and Eric Secemsky (bottom right), representing both the vascular surgical and endovascular communities


Heparin coated The only graft of its kind combing the benefits of ePTFE, knitted polyester and a heparin coating. Compared to standard ePTFE grafts, Fusion Bioline delivers: • Significantly shorter time to hemostasis1 • Reduced hemostatic agent usage1 • Superior handling 2,3 When peripheral bypass is on your schedule... Fusion Bioline vascular graft saves you time Solutions. Selection. Support. www.getinge.com Fusion Bioline products are manufactured by Maquet Cardiovascular, LLC / 45 Barbour Pond Drive, Wayne, NJ 07470 All products protected by the following international and U.S. patent(s): http://patents.maquet.com. · Getinge and Maquet are trademarks or registered trademarks of Getinge AB, its subsidiaries or affiliates in the United States or other countries Getinge and Maquet are registered with the U.S. Patent and Trademark Office. Copyright 2021 Getinge AB or its subsidiaries or affiliates · All rights reserved CAUTION: Federal (US) law restricts this device to sale by or on the order of a physician. Refer to Instructions for Use for current indications, warnings, contraindications, and precautions. MCV00107527 REVA 1. Lumsden AB, Morrissey NJ. Comparison of Safety and Primary Patency Between the FUSION BIOLINE Heparin-Coated Vascular Graft and EXXCEL Soft ePTFE (FINEST) Trial Co-investigators. Randomized controlled trial comparing the safety and efficacy between the FUSION BIOLINE heparin-coated vascular graft and the standard expanded polytetrafluoroethylene graft for femoropopliteal bypass. J Vasc Surg. 2015 Mar;61(3):703-12.e1. 2. Data on file at Maquet. 3. Cronwett & Johnston, et al. Rutherford’s Vascular Surgery 8th ed., Vol 2, Elsevier, 2014.
After success in 2022, co-chairs planning 2023 Gala
Co-chairs of next year’s SVS Foundation Gala are looking to build on the tremendous success of 2022’s “Cheers to 75 Years” Gala, which netted the SVS Foundation more than $200,000. “We are all so proud of what we were able to raise for such an important cause,” said Venita Chandra, MD. “We’re looking forward to continuing that success, hopefully on an even grander scale this coming year.”
Chandra and Matthew Eagleton, MD, co-chaired the 2022 Gala, under the chairmanship of Ronald Dalman, MD. With Eagleton’s election to SVS vice president, Leigh Ann O’Banion, MD, has joined the team as Chandra’s co-chair.

The 2023 gala, with a “Roaring Twenties” theme, will be held the evening of Friday, June 16, on the grounds of the Gaylord National Resort and Convention Center in National Harbor, Maryland, just outside Washington, D.C. The Vascular Annual Meeting (VAM), June 14–17, will take place at the Gaylord.

While details are not yet set in stone, both Chandra and O’Banion assured members it is going to be a “roaring good time.”
They reminded potential participants that it’s never too early to start thinking about donations for the live and silent auction that are such a big part of the evening.
Past contributions have included stays in vacation destinations across the world; fine wines and liquors; expeditions in fishing, hiking, even whiskey drinking; artwork; tickets to entertainment venues, museums and other attractions; books and much more.
Anyone with an internet connection may sign up to
participate in the silent auction, which will open for bids approximately 10 days before the Gala and end during it. As was the case last year, organizers will earmark items near the VAM location for early bidding and awarding.
Entertainment for the evening is also still in the planning stages. Of last year, O’Banion said, “The music was fantastic. I have never seen that many people on the dance floor.”
“Get ready to put on your dancing shoes!” said Chandra.
So many positives flow from the Gala, they said. It permits vascular surgeons and their guests to spend more time together in a social atmosphere. Most importantly, the money goes to the SVS Foundation.
“Last year we earned $200,000 which enabled funding for grants, for projects in underserved communities, and for research into treatments for vascular disease,” said Chandra.
“We want to remind people that at the end of the day, this is what we’re doing all this for. We can have fun and raise money for a good cause at the same time.”
The SVS Foundation funds the future of vascular health through its four pillars of research and innovation; community vascular care and patient education; disease prevention; and diversity, equity and inclusion.
Learn more about the Foundation at vascular.org/SVS-Foundation; read the 2022 Annual Report at vascular.org/Foundation22Report
TOPICS SELECTED FOR VAM 2023 SESSIONS
SOCIETY FOR VASCULAR SURGERY (SVS) committee members involved in programming for the 2023 Vascular Annual Meeting (VAM) have selected more than 20 topics for further development into educational sessions. These were chosen from among 55 program proposals submitted in an open call for content suggestions.
“In preparing the postgraduate educational program for VAM 2023, we wanted to continue to utilize those formats which the attendees found the most engaging. We also wanted to make some tweaks to respond to feedback that was received from both the educational needs assessment as well as the VAM22 evaluation,” said William Robinson, MD, chair of the Postgraduate Education Committee (PGEC).
VAM 2023 will be June 14–17 at the Gaylord National Resort and Convention Center in National Harbor, Maryland, just outside Washington, D.C. Educational programming runs across all four days. Exhibits will be open June 15 and 16.
Three small group sessions will provide information on retirement, embolization tips and tricks and, in collaboration with the American Venous Forum (AVF), deep vein thrombosis.

To avoid early-morning programming following the SVS Foundation Gala on Friday evening, SVS breakfast sessions will take place only on Friday. (Industry-sponsored breakfast sessions remain on the agenda for Thursday.) Breakfast session topics are: clinical outcomes, pulmonary embolism, and policy/ advocacy and government relations, to take advantage of VAM’s location near the seat of the country’s government.
“One change for VAM 2023 is that we will have a larger number of 90-minute sessions, in lieu of
STUDENTS AND RESIDENTS: ATTEND VAM 2023 ON A SCHOLARSHIP
MEDICAL STUDENTS AND GENERAL SURGERY residents have the chance to change their lives.
Perhaps they are still seeking some direction for their medical careers. Perhaps they’re considering vascular surgery but aren’t sure that’s right for them.
A trip to the Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM) could be just what they need to point them to their future. Applications opened Dec. 5 for the SVS Diversity Medical Student Vascular Annual Meeting Travel Scholarship and the SVS General Surgery Resident/Medical Student Vascular Annual Meeting Travel Scholarship.
Nathalie Baroum attended VAM 2021 on a travel scholarship after finishing her first year of medical school. She was already interested in vascular surgery but wasn’t entirely sure about heading that direction because of her lack of exposure to the specialty. “Overall, receiving the scholarship and experiencing both the conference and the specialized meetings for the recipients really solidified for me that I wanted to go into the field,” she said.
Applications will close at 3 p.m. CST on Jan. 9. Visit vascular.org/VAMDiversityTravel23 and vascular.org/ VAMtravel23
a smaller number of threehour courses, so we can better address the wide range of clinical and non-clinical content areas that are important to the membership,” Robinson said.
Topics for 15 concurrent sessions are:
Clinical learning and practice environments
Results of the BEST-CLI and BASIL-2 (Bypass vs. angioplasty in severe ischemia of the leg— a United Kingdom investigation) trials
The role of the oncologyvascular surgeon
Deep venous stenting tips and tricks, in collaboration with the AVF
Acute stroke management
Vascular wounds
Intermittent claudication
Vascular trauma
Complex aortic disorders
Evolution of the modern Journal of Vascular Surgery
Worst cases
Hemodialysis
Acute limb ischemia
Aortic dissection
Peripheral arterial disease
Robinson said when choosing topics, committee members attempt to include and develop strong proposals which members have taken the time to submit; ensure that VAM leaders are addressing timely and controversial topics; ensure that over multi-year cycles, SVS is adequately addressing all the disease states members cover; and leverage the expertise of a broad, diverse group of SVS members in delivering the meeting content.
Registration is expected to open in March. Learn more about VAM at vascular.org/VAM
SUBMIT RESEARCH
TO VRIC AND VAM
THE SOCIETY FOR VASCULAR SURGERY (SVS) is looking for research abstracts on vascular disease and treatment for the 2023 Vascular Research Initiatives Conference (VRIC) and the Vascular Annual Meeting (VAM).
Submissions will close at 6 p.m. CST on Jan. 11, 2023, for VRIC, which will be May 10, 2023, in Boston. VRIC focuses on emerging vascular science and is considered the SVS’ annual meeting for basic and translational research. Learn more at vascular.org/VRIC23. VRIC is held in conjunction and in the same location as the American Heart Association’s “Vascular Discovery: From Genes to Medicine” meeting May 10–13, 2023.
VAM abstract submissions will close at 3 p.m. CST Jan. 11, 2023. Recent meetings have presented research across the vascular spectrum, including aortic disease, innovations, hemodialysis access, revascularization options, healthcare disparities and telemedicine. This year, abstract authors must include one self-assessment question for post-presentation evaluation with their submissions. View the submission guidelines at vascular.org/VAM23AbstractProcess
8 Vascular Specialist | December 2022
◆
◆
◆
◆
◆
◆
◆
◆
◆
◆
◆
◆
◆
◆
◆
VAM DIGEST
William Robinson
The SVS Foundation hopes to emulate the success of the 2022 Gala in 2023
Compiled by Beth Bales



MEDICAL ® silkroadmed.com/standard-surgical-risk
By Jocelyn Hudson
The Sundance sirolimus drug-coated balloon (DCB) has an “excellent” safety profile in a “challenging, real-world, predominantly CLTI [chronic limb-threatening ischemia] population,” and has a primary patency rate of 80% at 12 months in a per-protocol analysis population. Ramon Varcoe, MD, from Prince of Wales Hospital in Sydney, Australia, presented these findings from the SWING first-in-human study at the VEITHsymposium 2022 (Nov. 15–19) in New York City.
Varcoe, who is co-lead investigator of the trial, added that the research team observed no major amputations, “very low” rates of major adverse events, and “impressive” luminal gain, which was sustained out to six-month angiogram. In addition, Varcoe reported that Rutherford category and the functional outcome measures were improved. “The Sundance [Surmodics] sirolimus-coated balloon is a novel device,” Varcoe informed VEITH attendees. He elaborated: “It has a microcrystalline surface, but also a very sophisticated proprietary excipient, which, rather than using nanoparticles or

microreservoirs, uses chemistry to deliver the drug into the blood vessel wall and have it retain that much more so than the other devices on the market.”

The presenter noted that SWING is a prospective, multicenter, single-arm feasibility study that looked at patients with stenotic or occluded lesions. The researchers enrolled 35 patients over eight sites in Australia, New Zealand and Europe.
Noting some key inclusion criteria, Varcoe detailed that patients had to be either Rutherford 4 or 5. He added the caveat that Rutherford 3 patients were included, but numbers were capped at 20% of the total cohort. Furthermore, patients had to have de novo or restenotic lesions, and at least 50% stenosis by visual estimate of the investigator. They could have up to two distinct lesions in the same or different below-the-knee (BTK) artery, and had to have successfully treated inflow, as well as an unimpaired outflow artery in continuity to the ankle or foot. The investigators performed both an intention-to-treat and a per-protocol analysis, Varcoe informed the audience.
“The reason for that was because [the trial] was conducted over the COVID-19 pandemic period, so we lost seven patients to the primary endpoint of angiography,” he noted, adding that there were also three post-protocol deviations. For these reasons, the research team focused on the 25 patients who had the per-protocol analysis.
The presenter stressed that the patients included represent a “real-world” population, including a high proportion of patients who are smokers and have diabetes, as well as a majority of patients with Rutherford 4 or 5 disease. “They also had high proportions of moderate-to-severe calcification in excess of 18%, and around a third of these patients had total occlusions,” he added.
Ramon Varcoe
death, “so it was a safe device.”
The team also saw a rate of allcause death that was 0%, target lesion amputations were 0%, and a “very low” rate of clinically driven target lesion revascularization at 8%.
Varcoe added that the minimal luminal diameter at the end of the procedure was “very high,” which he thinks “represents modern-day angioplasty techniques,” and reported a late lumen loss of 1mm at six months—results that compare “very favorably” to other equivalent DCB trials below the knee, he remarked.
The study had two primary endpoints. The first was a primary safety endpoint— freedom from major adverse limb event (MALE) and perioperative death at 30 days following the index procedure. The primary efficacy endpoint was the rate of late lumen loss at six months, as assessed by quantitative vascular angiography. Both primary endpoints of the SWING trial were achieved, Varcoe revealed. Varcoe reported that, in the per-protocol population, there were no major amputations, no major reinterventions, and complete freedom from perioperative
As six months, the researchers observed a primary patency of 88.5%. Varcoe noted that primary patency was retained and consistently retained out to 12 months at 80%—a figure that he said is “very good for this part of the vasculature.” Varcoe added that, “pleasingly,” the team also saw improvement in Rutherford Becker classification, which was sustained and improved out to 12 months. Regarding quality of life, he noted that patient-reported outcome measures were again consistently improved out to that 12-month endpoint. In closing, Varcoe shared his belief that the Sundance device “warrants evaluation in a large-scale pivotal trial” based on these latest findings.
 By Jamie Bell
By Jamie Bell
THE SWISS FEDERAL ASSEMBLY HAS VOTED IN favor of accepting medical devices with the Food and Drug Administration (FDA) marketing authorization in Switzerland.
A motion for “more freedom of action in the procurement of medical products for supply of the Swiss population” was discussed and put to a vote in the country’s parliament on Nov. 28, with many industry groups, including Swiss Medtech, supporting the acceptance of FDA-approved devices.
In a press release, Swiss Medtech described this as a “necessary and urgently needed decision,” and stated that it is “essential” for this order to now be implemented swiftly and pragmatically. It further draws on Australia and Israel as examples of countries in which “efficient procedures” to recognize FDA approvals in parallel with the CE mark have “proven successful,” and “can be achieved in an uncomplicated manner.”
“Swiss Medtech very much welcomes the policymakers’ important and forward-thinking decision,” added Peter
Biedermann, managing director of Swiss Medtech. “It is a response to circumstances that could no longer be ignored. Specifically, problems with the implementation of the new European Medical Device Regulation [MDR], and the negative consequences concerning availability, product range and quality of medical devices throughout Europe. As innovations are increasingly being introduced first to the market in the U.S., new products reach Europe with a delay, at best.”
The Swiss Federal Council—the executive body of the country’s federal government—opposed this proposition, citing the administrative burden that would be brought about by this regulatory shift, and patient safety concerns caused by risk classification discrepancies between the U.S. and Europe.
On May 30, 2022, the potential acceptance of FDA products was voted on by the 46 members of the Swiss Council of States (the upper house of the country’s Federal Assembly), with 23 voting to accept and 12 voting to reject.

Shortly following this decision, the Federal Council asserted in a press release that there was no need to ac cept non-CE-marked medical devic es, such as FDA-approved devices, at that time—describing the extension of simplified market access to other countries outside
of the European Union as “disproportionate”—before noting that it would re-evaluate this situation at the end of 2024. It added that the supply of safe medical devices in Switzerland was “currently guaranteed,” referencing various backup measures it took back in 2021 to ensure this “even without an updated MRA [Mutual Recognition Agreement].”
The parliamentary vote that took place earlier this week was borne out of the introduction of the European MDR on May 26, 2021, with the MRA and all related trade-facilitating effects for medical devices between the European Union and Switzerland also ceasing to apply from the same date. And, while devices “in conformity” with the MDR can be certified and placed on the market until May 25, 2024, marketed devices must be certified directly under the MDR from the following day (May 26) onwards. With this deadline drawing ever closer, concerns have been raised regarding regulatory challenges, supply chain gaps and device shortages, and the impact this could have on patient care and industry alike.
The motion to accept FDA approval of devices in Switzerland was brought up to the 200-seat Swiss National Council (the lower house of the country’s Federal Assembly), with 100 votes in favor and 79 against being cast. As such, both chambers of Switzerland’s national parliament have now voted to adopt the initiative, and will instruct the Swiss Federal Council to adapt legislation to allow devices with FDA clearance onto the market.
10 Vascular Specialist | December 2022
TRIAL 12-MONTH DATA: NOVEL
‘GREAT
PAD MEDTECH
SWING
SIROLIMUS DCB SHOWS
PROMISE’ IN ‘CHALLENGING’ CLTI POPULATION Swiss parliament votes to accept FDA-approved medical devices
FDA
6 MONTHS MONTHS 12 PRIMARY PATENCY 88.5% 80%
Campus


References: 1. Rundback J, Chandra P, Brodmann M, et al. Novel laser-based catheter for peripheral atherectomy: 6-month results from the Eximo Medical B-LaserTM IDE study. Catheter Cardiovasc Interv. 2019;94(7):1010-1017. 2. Shammas NW, Chandra P, Brodmann M, et al. Acute and 30-day safety and effectiveness evaluation of Eximo Medical’s B-LaserTM a novel atherectomy device, in subjects affected with infrainguinal peripheral arterial disease: results of the EX-PAD-03 trial. Cardiovas Revasc Med. 2020;21(1):86-92. 3. Auryon. Instructions for use. AngioDynamics; 2020. RISK INFORMATION Caution: Federal (USA) law restricts the use of the system by or on the order of a physician. Refer to Directions for Use and/or User Manual provided with the product for complete Instructions, Warnings, Precautions, Possible Adverse Effects and Contraindications prior to use of the product. INDICATIONS FOR USE The AURYON Atherectomy System is indicated for use in the treatment, including atherectomy, of infrainguinal stenoses and occlusions, including in-stent restenosis (ISR). AngioDynamics, the AngioDynamics logo, Auryon, and the Auryon logo are trademarks and/or registered trademarks of AngioDynamics, Inc., an affiliate or a subsidiary. © 2020 AngioDynamics, Inc. US/PA/AD/405 Rev 01 9/2020 Auryon-PAD.com The future has arrived. Deliver it to your patients. To secure early access for your practice, visit: Conquer every lesion you encounter with the most advanced peripheral atherectomy technology ever: the Auryon system1-3 Clear all lesion types, including severe calcifications, with a single device Revolutionize how you treat, above and below the knee Practice with confidence by minimizing the risk of embolization Say goodbye to tough.
Learning culture, errors and Melinda French Gates
Workplace culture is fascinating to observe because it is nebulous and hard to measure. There is also often a perceived gap between the existing and the intended culture in terms of how the organization deals with human errors or unintended mistakes. In Mayo Clinical Proceedings, Shanafelt and colleagues point to the incongruence between artifactual and espoused values in medicine. In other words, how we embrace the fact that to err is human in the professional culture domain.1 But our contradictory behavior reflects the profession’s aspiration to perfectionism and low self-compassion. This behavior is then transmitted to our students and trainees.
I started thinking about this in the context of the existing culture in many if not most academic healthcare educational programs. One salient feature in these training programs is that besides being learners ourselves, there is a requirement that we commit to teaching learners (students and trainees). Most of us commit to it in academic surgery to varying degrees. I knew very early on in residency and private practice that I loved to teach and learn as well. I learned from many academic and private practice surgeons in my own training. The commonality was that there was a “learning” culture.
Is academic culture synonymous with learning culture? It depends on how one defines “academic,” and what the existing culture is like. Academic is defined as “relating to education and scholarship,” so clearly either full-time academic faculty or voluntary faculty enthusiastic about teaching and learning are both included. What,
then, is learning culture? Adam Grant, who carried out work for relates his work for Melinda French Gates, says psychologic safety is its foundation, allowing people the freedom to admit their lack of knowledge, have the humility to re-learn, take risks, and make mistakes. It then comes down to whether there is an existing culture of learning, which, broadly speaking, includes teaching, education, and scholarship. Therefore, it is the learning part that is the essential ingredient, which is critical in any academic environment whether at a university or a private teaching hospital.
Grant relates his work for Gates, co-director of the Gates Foundation, as a perfect example of a learning culture when she agreed to provide psychologic safety to employees at the foundation by allowing everyone to see her reactions to her own negative reviews, which were shared by live video conferencing.2 He points out that allowing vulnerability by leaders is important and shows that they are also seeking to learn and improve themselves. In a non-clinical sense, learning culture is quite different from the performance culture that my generation experienced, where any decision was purely about results, with little room to fail. Arthur Kaplan, PhD, founder of the division of medical ethics at NYU Grossman School of Medicine, goes even further and has argued that even in the clinical area, other than “culpable” mistakes such as drugs or alcohol, etc., we need to learn from them. “We tend to
pay attention to errors and want to know how to punish them,” he said. “The correct moral position, I think, is [to] prevent error.”3
In a recent Search and Physician Wellness Services Organizational Culture Survey, a supportive management— non-clinical in this case—approach to mistakes was seen as one of three ranked as most important to physician satisfaction. Others have also advocated to minimize the culture of fear and blame in the clinical context.

In 2013, the National Academy of Medicine proposed a framework for healthcare systems called the Learning Health System (LHS) to transform care delivery.4 The LHS was intended to facilitate “continuous knowledge development, improvement, and application,” while also recognizing the importance of vision, leadership, investment, and culture change.”5
This requires a top-down push by senior leadership, but, more importantly, all stakeholders must be brought into the learning framework and supported by their immediate leader. While I am not aware of conclusive results associated with this approach, the impact on morale, work satisfaction and employee retention make sense. My point is that there is an authenticity gap related to learning in academic surgery. Consistency of people at the top and those under them has been described as “cultural coherence.” Leaders stand to benefit from cultural cohesion with high levels of connectivity with colleagues, employees and indeed the entire organization.6 Our leaders may think they are in alignment
with the values and culture of learning in their faculty, trainees and employees—but many are not. Leaders should strive for this coherence and function as teachers and mentors, provide resources including continuing education time, and be seen as learners themselves. In return, they should require and expect up-to-date knowledge. To be clear, my observations relate to taking risks and making mistakes in the management and patient care context— they are not about jeopardizing patient safety. If non-culpable errors are made, a leader should counsel and protect trainees and employees, and use incidents as
an opportunity to gain experience rather than to punish. This applies particularly to medical students and residents. They need to be taught self-compassion and be protected, but not coddled by us to lessen the learning anxiety at most training programs. This involves a conscious culture change in order to lower physician burnout and attrition rates. Leaders want to hold people accountable for their decisions and actions.
on page 14
STITCH
A REFLECTION ON 2022… CORNER
By Christopher Audu, MD
THIS MONTH’S COLUMN CHRONICLES THE exciting year we’ve had here at Corner Stitch. As the section editor, I’ve been most proud of the various authors who have contributed, and the range of topics discussed in the different monthly write-ups this year.
To take a brief trip down the archive lane, we welcomed a new set of interns (who are now six months in!),
learned how to write winning abstracts for meeting presentations (May 2022—Dr. DeCarlo), welcomed new fellows (April 2022—Dr. Aru), discussed the role of mentorship (VAM edition—Shane Dong, M3), and even profiled the founders of the Audible Bleeding podcast (September 2022) and the new Journal of Vascular Surgery internship program (November 2022).
counsel patients? Moreover, what are key clinical trials that we all should know as trainees? Also, in 2023, we will welcome a new set of trainees at the resident and fellowship level. And there will be numerous conferences to attend— in particular the Vascular Annual Meeting (VAM).
Christopher Audu
It’s been a banner year, and the diversity of voices has helped shape the topics so that they are relevant to the trainee navigating the training environment. Thank you to all the contributors this year.
The year 2023 will bring a new set of challenges and topics. For instance, we now have the results of the BESTCLI trial to help guide treatment options in our peripheral arterial disease patients. How will this affect trainees as we

Looking forward to the new year, we at Corner Stitch intend to continue the trend of having trainee voices share their experiences on a variety of topics. I hope you look forward to these exciting monthly installments. We have an exciting lineup, and are always open to suggestions.
As the 2022 calendar year draws to an close, have a safe holiday season… and if you’re on call, may the vascular surgery force be with you!
CHRISTOPHER AUDU is a vascular surgery resident at the University of Michigan in Ann Arbor, Michigan, and the Vascular Specialist resident/fellow editor.

12 Vascular Specialist | December 2022
COMMENT&
ANALYSIS
ACADEMIC CULTURE
Leaders should strive for this coherence and function as teachers and mentors, provide resources including continuing education time, and be seen as learners themselves. In return, they should require and expect upto-date knowledge
Bhagwan Satiani
continued



Driving Change. TOGETHER. REFERENCE: - Technical and Clinical Outcome of Topical Wound Oxygen in Comparison to Conventional Compression Dressings in the Management of Refractory Nonhealing Venous Ulcers. Wael A. Tawfick WA, Sultan S. s. Vascular Endovascular Surgery 2013; 47:30–37 Delivering Exceptional Outcomes Multi-modality Therapy for VLUs. Combining non-contact cyclical compression, oxygen and humidification, this seamless addition to your care plan can be used in any clinical setting or self-administered by the patient at home, improving compliance and access to care for all. Faster, Sustained Healing for Venous Leg Ulcers Visit www.AOTInc.net for more information BE A PART OF THE CHANGE HIGHER HEALING RATE at 12 weeks 46% 76% | vs. SOC* LOWER RECURRENCE RATE at 36 months 47% 6% FASTER TIME TO HEALING 107 DAYS 57 DAYS MRSA ELIMINATION 0% CCD 46% | vs. SOC | vs. SOC | vs. SOC *Standard of Care (SOC) was Conventional Compression Dressings (CCD)
COMMENT& ANALYSIS ADVOCACY
Building the payment reform drumbeat: Looking ahead to the 118th Congress
By Matthew J. Sideman, MD
WITH YET ANOTHER ELECTION SEASON YIELDING somewhat unexpected results (e.g., a “red wave” that didn’t materialize), we find ourselves looking ahead to a 118th Congress that will include razor-thin majorities in both chambers. The political dynamics of a new, but small, Republican majority in the House, paired with the continuation of a slim Democratic majority in the Senate, means that a key driver of successful advocacy in the 118th Congress will be developing and maintaining coalitions—among both stakeholder organizations and bipartisan lawmakers.
Luckily, the Society for Vascular Surgery (SVS) has been an early adopter of this approach and is well-positioned to maintain its leadership role to resume work on several of our legislative priorities when the new Congress convenes in January.

Medicare payment reform
As we are all unfortunately aware, flaws associated with the current Medicare Physician Fee Schedule (MPFS) continue to generate annual payment cuts. While there are many variables driving these cuts, a primary factor relates to the budget neutrality requirements within the fee schedule, which have (recently) resulted in significant annual cuts to the Medicare conversion factor. Congress stepped in to mitigate the impact of these cuts for calendar years 2021 and 2022, with deliberations ongoing for additional relief in 2023. That being said, these year-end Band-Aid efforts are simply not enough and vascular surgeons, and others across the House of Medicine, are in desperate need of systemic payment reform that will provide financial stability.
The SVS is among the organizations pressuring lawmakers to convene Congressional proceedings (hearings and/or roundtables) to further identify what is and is not working in the current system and, more importantly, discuss what reforms are necessary to immediately stabilize the Medicare payment system and establish future payment models that are inclusive, and meaningful, for all specialties and practice settings. As part of this effort, the SVS submitted a response to a congressional “Request for Information” (RFI) at the end
of October that outlined potential reforms to the MACRA (Medicare Access and CHIP Reauthorization Act of 2015) law, and noted other structural issues with the MPFS (e.g., budget neutrality, etc.) that are significant drivers of instability within the system.
The SVS also is continuing its work to advance policy to provide additional targeted relief for those most impacted by the clinical labor update finalized in the 2022 rule. As the leader of the Clinical Labor Coalition (CLC), the SVS has done extensive work to raise awareness of these specific cuts. As of the writing of this article, inclusion of the clinical labor relief policy in year-end legislation remains a possibility. If that provision is excluded at year-end, the CLC will seek introduction of stand-alone legislation in the 118th Congress.
Prior authorization relief
A record number of bipartisan lawmakers and stakeholder organizations supported legislation relating to prior authorization relief in the 117th Congress. Despite the House passing this critical legislation in September, a flawed “score” from the Congressional Budget Office (CBO) derailed efforts to advance the bill through the Senate. The more than 500 supporting organizations will continue to work on this issue in the next Congress and will work with the CBO (and other relevant governmental bodies) to ensure the costs associated with the bill are accurately characterized.
Public health, physician training, wellness and other issues
Beyond the urgent efforts relating to payment, the SVS continues its forward-looking approach regarding other
LEARNING CULTURE, ERRORS AND MELINDA FRENCH GATES
The message may translate into “no mistakes are tolerated,” which makes people pull back and make few decisions at all. To become healthy, the academic culture must be a learning one. A single leader with vision can have an enormous impact on establishing a healthy culture—or changing an ill one.7 And what allows us to feel safe so we can all thrive? Self-aware leaders.
References
1. Shanafelt TD et al. Healing the Professional Culture of Medicine. Mayo Clin Proc. August 2019;94(8):1556-1566
https://doi.org/10.1016/j.mayocp.2019.03.026.
2. https://www.strategy-business.com/article/Building-aculture-of-learning-at-work.
3. Bean M, Carbajal E. ‘We can’t punish our way to safer medical practices’: 2 experts on criminalization of medical errors. https://www.beckershospitalreview.com/patientsafety-outcomes/we-can-t-punish-our-way-to-safer-medicalpractices-2-experts-on-criminalization-of-medical-errors.

4. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. National Academies Press (US);
health-related policy issues. With nearly 30% of vascular surgeons over the age of 60, we must continue to advocate for the expansion of funding for graduate medical education. Without a robust pipeline of in-training physicians and surgeons, we simply will not be able to meet the healthcare demands of a rapidly aging population. In addition, we continue to raise awareness regarding the importance of continued advances in population health and the need for lawmakers on Capitol Hill to further embrace screening and prevention efforts as a means to thwart costs associated with management of chronic illnesses.
What is the role of SVS members?
Despite changing majorities and leadership transitions on Capitol Hill, one thing remains constant: lawmakers need to hear from their constituents—and that’s you. We are all battle-worn from fighting payment cuts over the last several years, but we must continue to beat the drum regarding the importance of broad reforms to our current payment systems. While the SVS advocacy team is engaged in these efforts on all levels, your personal stories—about how payment reductions impact your practice and your patients—can be invaluable in term of cutting through political “noise” to help lawmakers really understand what is at stake in their home states and districts. To that end, here are a couple actions you can take: Become a “key contact” in your state/district by joining that SVS’ REACH 535 program. This collaborative advocacy effort is designed to ensure we have SVS members available to engage all lawmakers. Respond
to legislative “calls to action.” Send pre-written messages to your lawmakers to share the vascular perspective when key policies are introduced and/or advancing in Congress. Contribute to SVS PAC. Engaging in political advocacy helps ensure there are lawmakers on Capitol Hill who are ready and willing to champion the issues important to vascular surgery.
On behalf of the SVS Advocacy Council, thank you to everyone who has supported our engagement efforts throughout the year. December will undoubtedly be a busy month with several “unknowns” remaining. However, I am 100% certain that we increase our chances of success when we work together to amplify our messages on Capitol Hill and within the healthcare community.
2013.http://www.ncbi.nlm.nih.gov/books/NBK207225/.
5. Realizing a learning health system through process, rigor, and culture change. Healthcare Volume 8, Supplement 1, June 2021. https://doi.org/10.1016/j.hjdsi.2020.100478.
6. Global Culture 2021. PWC Report.
7. Runge MS. Medicine needs a culture change to retain talented physicians https://www.statnews.com/2019/01/28/ medicine-culture-change-retain-talented-physicians/.
BHAGWAN SATIANI is a Vascular Specialist associate medical editor.
14 Vascular Specialist | December 2022
➽
continued from page 12 C M Y CM MY CY CMY K
MATTHEW J. SIDEMAN is chair of the SVS Advocacy Council.
“With nearly 30% of vascular surgeons over the age of 60, we must continue to advocate for the expansion of funding for graduate medical education”
Matthew J. Sideman (inset) discusses the new Congress

Consensus Update Vascular & Endovascular SEE YOU IN 2023 CXSYMPOSIUM.COM Peripheral Arterial Consensus Aortic Consensus Acute Stroke Consensus Venous & Lymphatic Consensus Vascular Access Consensus The Hurting Leg Consensus INNOVATION EDUCATION EVIDENCE 25–27 APRIL 2023 TUESDAY-THURSDAY HILTON LONDON METROPOLE, UNITED KINGDOM CONTROVERSIES CHALLENGES CONSENSUS
EUROPE
VSGBI spotlights data-driven financial incentives for PAD revascularization
By Clare Tierney
The President’s Symposium at the Vascular Society of Great Britain and Ireland (VSGBI) annual scientific meeting (Nov. 23–25) in Brighton, England, saw Rob Sayers, MD, from the University of Leicester, presenting, “CLTI CQUIN [Commissioning for Quality and Innovation] has raised the profile of PAD and will lead to fewer amputations.” Sayers’ take-home message for delegates was that the National Health Service (NHS) Commissioning for Quality and Innovation (CQUIN) for chronic limb-threatening ischemia (CLTI) will prove a valuable package of measures when it comes to raising awareness of peripheral arterial disease (PAD) and improving outcomes for patients, but that the question now lies in “how best we measure [these improvements].”

The CQUIN, Sayers began by outlining, was started in April 2022, and the premise of it is for the NHS to give “financial rewards for excellence [in the hope] that that translates into better patient outcomes.” The presenter noted, with relevance to the VSGBI audience, that their society had made sure to inform all its members of the CQUIN at its launch, and that the Society’s own PAD quality improvement framework (QIF) is complementary in its patient outcome focus.
Then providing background to the CQUIN process, Sayers shared that “you are more likely to be successful if your bid is pa-
tient-focused [and it is easy to implement],” adding that it is “competitive.” The resulting financial incentive that a successful bid bestows “depends on the size of your unit,” Sayers proceeded, and in the case of the CLTI CQUIN, “most [units] will receive between £500,000 and £1.5 million.”
Speaking to the merits of the CLTI CQUIN, Sayers described it as a “well written and clear proposal,” adding that there are benefits for patients.”
Moreover, the successful application could perhaps be set against the backdrop of “a lot of support from the NHS for various vascular services […which were] particularly hard-hit by the pandemic.” Sayers went on to stipulate that “[this] was noticed, and we became a protected specialty.”
Regarding the conditions of the CLTI CQUIN funding, “it requires you to revascularize patients within five days of referral,” Sayers detailed for the symposium audience.
“If you revascularize 60% of your patients within five days, you get the full payment, and if you revascularize less than 40%, you do not get any money at all.” The amount awarded for achieving results in between these benchmark figures is graduated, Sayers expanded.
Data monitoring is key to the CQUIN’s operation, delegates then heard. This is carried out “by local commissioners who
compare data such as HES [Hospital Episode Statistics], and NVR [National Vascular Registry…] so if you were tempted to underre port, that would be spotted,” Sayers cautioned.
Moving on to address how the CQUIN can help patients, Sayers underlined that the goal is to “raise awareness of PAD and the PAD QIF, and CLTI, among clinicians and managers.” This then increases the chance of reinvestment to improve services, such as limb salvage clinics.
As of April 2023, the CQUIN will be up for renewal, Sayers conveyed, and the decision will be based on a number of factors, including whether participating units themselves “found [it] worthwhile.” However, there are limitations when it comes to how successful the CQUIN may be at achieving improved outcomes: “It requires very reliable data capture,” Sayers admitted. It “may [also be argued that] the targets are challenging” and “not particularly evidence-based,” he furthered.
Sayers rounded off by taking a long and wide view of VSGBI’s role in putting patients at the heart of members’ work and calling successive presidents to prioritize data gathering to facilitate this.
Maintaining relationships with exam boards so that the specialty adheres to “very good” professional standards was also among Sayers’ pieces of advice.
“There is no doubt that the Society needs to continue to foster its good relationship with NHS England,” he asserted, before opining that “work with commissioners” can “no doubt” serve as a solution to the problem of the post-COVID vascular services backlog.
Questions following the symposium centered on data monitoring and the quality of datasets, which reflected Sayers’ message that this is pivotal in the success of measures like those comprising the CLTI CQUIN.
In addition, Sayers was posed the question of whether “in order to ensure the development and appropriate investment in CLTI management, do you think some units will need to take one for the team and fail?”
The presenter responded by referring to the “current data” from the CQUIN—“there are 55 units in England that are eligible for the CQUIN scheme [of whom] about 10–12 are currently below the threshold of 40% and [there are] probably another 5% [whose data] we do not [have].”
He reiterated that the CQUIN is “about improving your service to improve outcomes for patients,” and concluded, therefore, that those units that are currently falling short have the choice “whether they want to try to improve things for patients,” rather than it being about “taking one for the team.”
ACCOLADES
Deadlines coming for scholarships, grants, awards
Deadlines arrive by March 1, with some as early as this month, for a number of Society for Vascular Surgery (SVS) and SVS Foundation awards, grants and scholarships, writes Beth Bales.
SVS awards
DEC. 31: The SVS Section on Outpatient and Office Vascular Care (SOOVC) Presentation Award, recognizing vascular surgeons who have completed clinical research projects in an outpatient-based setting; and the SOOVC Research Seed Grant ($5,000 each) to provide three vascular surgeons with funds to pay a data analyst.
DEC. 31: International Scholars Program, funding four $5,000 scholarships to qualified young vascular surgeons from outside North America. Recipients not only attend the Vascular Annual Meeting (VAM) but also can visit clinical, teaching and research facilities in the United States and Canada.
JAN. 9, 2023: Both the SVS General Surgery Resident/Medical Student Vascular Annual Meeting Travel Scholarship and the SVS
Diversity Medical Student Vascular Annual Meeting Travel Scholarship, both of which help defray costs for aspiring vascular surgeons with the opportunity to attend VAM in June.
FEB. 1, 2023: Excellence in Community Practice Award, bestowed on members who have exhibited outstanding leadership within their communities as practicing vascular surgeons.
MARCH 1, 2023: The Lifetime Achievement Award and the Medal for Innovation in Vascular Surgery. The first is SVS’ highest honor, recognizing an individual’s outstanding and sustained contributions to the profession and the SVS, alongside his or her exemplary professional practice and leadership. Jonathan Towne , MD, received the award in 2022. The Medal for Innovation—awarded intermit-
tently—honors individuals whose contributions have had a transformative impact on the science or practice of vascular surgery. The most recent honoree is Robert Kistner, MD, who received the accolade in 2019. Visit vascular.org/Awards for information on SVS awards.
SVS Foundation Awards
JAN. 11, 2023: The Resident Research Award and the Vascular Research Initiatives Conference (VRIC) Trainee Award. The Resident Research Award recipient will present his or her research at a plenary during VAM 2023 in June. The SVS Foundation hopes to motivate physicians early in their training to pursue research that explores the biology of vascular disease and potential translational therapies. Applications for the VRIC Trainee Award is automatic; recipients are those with top-scoring abstracts submitted for presenta-
tion at the conference, set for May 10, 2023, in Boston.
FEB. 1, 2023: Student Research Fellowship, intended to stimulate laboratory and clinical vascular research by undergraduate and medical school students attending universities in the United States and Canada.
MARCH 1, 2023: Clinical Research Seed Grant, to encourage interest in the development of clinical investigators among SVS members, particularly junior ones; the grant provides direct support for pilot clinical projects that can develop into larger studies funded by industry or government sources. The investigators are expected to conduct patient-oriented research, interacting directly with human subjects.
Visit vascular.org/ FoundationGrantsAwards
16 Vascular Specialist | December 2022
“If you revascularize 60% of your patients within five days, you get the full payment, and if you revascularize less than 40%, you do not get any money at all”
ROB SAYERS
VSGBI President Rob Sayers
SIMULATION
BY VASCULAR SURGEONS FOR VASCULAR SURGEONS: PAD SKILLS TAKE CENTER STAGE





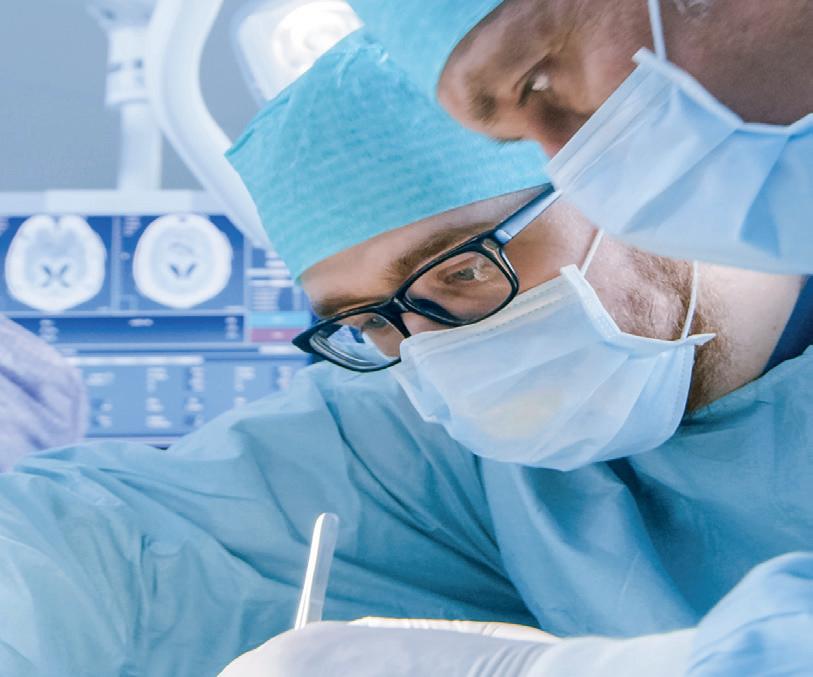
THE RECENT SOCIETY FOR VASCULAR Surgery (SVS) Complex Peripheral Vascular Intervention (CPVI) Skills Course delved into both case-based and hands-on learning, allowing participants the opportunity to practice the latest procedures on cadavers and benchtop models during small-group simulations.

The much-anticipated gathering, held in October, was staged by course directors Patrick Geraghty, MD, Vikram Kashyap, MD, and Daniel McDevitt, MD.
The trio assembled what they had billed as “an all-star faculty” of 19 renowned surgeons well-versed in interventions for peripheral arterial disease (PAD). Kashyap said the course was designed “by vascular surgeons for vascular surgeons.” It included a demonstration of a percutaneous deep vein arterialization system, which is currently under evaluation.— Beth Bales and Bryan Kay
•
•
www.vascularspecialistonline.com 17
New Single-Use Surgical Doppler System Simple to Use, Lightweight and Easy to Hold
8 MHz single-use, sterile probes ready for immediate use
Attendees of the October CPVI course spent a full day rotating between 15 hands-on stations where they were able to learn, practice and even perfect new procedure techniques on benchtop models and cadavers. The 2023 class will be Oct. 1-2, 2023.
•
Easily confirm blood flow with the high resoluiton, color display of the waveforms on the DMX Doppler
Reduce the risk of infection during surgery caused by non-sterile products being used within the sterile field Scan Here to Learn More www.huntleigh-healthcare.us Vascular Specialist Print Ad - DIOP.indd 1 09/09/2022 15:32:31
More than 10 copies of Rutherford’s Vascular Surgery and Endovascular Therapy, a seminal two-book reference set for treating vascular disease, are now—or soon will be— helping doctors come to the aid of patients in Nepal.


The Society for Vascular Surgery (SVS) and the SVS Foundation facilitated the donation to an under-funded area of Nepal with few resources for vascular surgeons after receiving a request from a representative of an industry partner, W.L. Gore. SVS staff boxed up some of the Society’s limited copies of the latest version of the reference work, and they are now in the hands of Robin Man Karmacharya, MD, and his team at Dhulikhel Hospital,
A NEW REVIEW TEXTBOOK
developed by a vascular surgery resident with the aim of helping fellow trainees prepare for milestones like the Vascular Surgery In-Training Exam (VSITE) has accumulated sales of around 500 copies across some 14 countries.
Such has been the success of The Vascular Surgery Review Book, author Thomas Creeden, DO, from the University of Massachusetts in Worcester, Massachusetts, who birthed the title just before the 2022 Vascular Annual Meeting (VAM) in June, already has outline plans

outside Kathmandu, Nepal. Rutherford publisher Elsevier sent more copies, which were en route as of late November.
The industry representative said he had been approached by a United Statesbased physician. This region in Nepal has only eight vascular surgeons and vascular trainees serving an area containing 36 million people. The providers make an average of just $15–20 (U.S.) a day and so would greatly appreciate the donation, the physician said.
“We would like to thank you so much for all the help,” said one of the medical team members. “The books will be of great use for training programs here and will be of great resource for teaching learning activities in vascular surgery in Nepal.”
Learn more about the SVS Foundation and consider a year-end donation at vascular. org/SVSFoundation.
JVS:VL IS SET TO GO ONLINE ONLY AT BEGINNING OF 2023
THOUGH ITS COVER COLOR IS BLUE, the Journal of Vascular Surgery: Venous and Lymphatic Disorders (JVS:VL) is going green. As of January 2023, the publication will be published exclusively online.

“Society for Vascular Surgery [SVS] members, and millions of people throughout the United States and the world, are concerned about the environmental impact of the choices we make,” said JVS:VL editor-in-chief Ruth L. Bush, MD.
“Eliminating the need for a print publication provides positive environmental benefits and also will reduce production costs,” she said. “Readers have sought this change for some time now, and we’re happy it’s occurring. The digital future is now!”
SVS members can remain up to date on all the latest research by signing up for regular content alerts at www.jvsvenous.org and click on “Alerts” in the “Connect” section at right.
This will provide the opportunity to sign up for alerts for all four JVS publications. There is a great deal of content easily accessible through the website, much more so than in any single print copy.
Subscribers also will be able to continue to enjoy all their current JVS:VL benefits in addition to these exclusive online features:
◆ The Visual Abstract Gallery: Scroll through the abstracts in JVS:VL to gain a better understanding of selected published articles and quickly identify which are most relevant to specific research interests
JVS-VL is set to go online only from January 2023, the SVS announced
To access JVS-VL online, SVS members should visit the JVS site at www.jvascsurg.org, select “SVS Member Login” and use their SVS username and password. Those who need a reminder of their login can visit vascular. org/forgot. For any further questions, reach out to membership@vascularsociety.org.
FREE-ACCESS ARTICLES FROM DECEMBER ISSUE OF JVS
The Journal of Vascular Surgery (JVS) has four free-access articles in its December issue that will be available online through March 1.
They are:
1. “Factors associated with lower preoperative quality of life in patients with chronic limb-threatening ischemia in the BEST-CLI trial” (Editor’s Choice). Visit vascular.org/ BESTQofL
for a second edition to be released next fall.
The book has registered as one of top-selling titles on Amazon within the vascular and thoracic surgery category, Creeden tells Vascular Specialist. “Copies have sold in 14 countries, and have made their way into many of the current vascular surgery training programs,” he says.
With the next annual VSITE just around the corner, Creeden hopes other trainees and programs might make use of a nearly 300-page book designed to be high-yield— both easier and quicker to work through than weighty modern-day textbooks.
The planned second edition is set to contain clinical updates, new study results, procedural steps for common vascular surgeries, and additional illustrations.
◆ The JVS Online Journal Club: Through active presentation and participation in this completely online journal club, JVS leaders educate vascular surgical trainees in the interpretation of high-level clinical and basic science articles and how to appropriately apply the information to clinical practice and future research
◆
Audible Bleeding podcast: This audible resource for trainees and practicing vascular surgeons focuses on interviews with leaders in the field, board preparation and dissemination of best clinical practices and high-impact innovations in vascular surgery
“We look forward to your continued support of JVS:VL as we move in this exciting new direction,” Bush added.
2. “Rates of progression of carotid instent stenosis and clinical outcome” (Offers Continuing Medical Education credit). Visit vascular.org/JVSStenosisFollowup1222
3. “Decreasing trends in reintervention and re-admission after endovascular aneurysm repair in a multiregional implant registry.” Visit vascular.org/ JVS-EVARTimeTrends1222
4. “Use of an app-based exercise therapy program including cognitive behavioral techniques for the management of intermittent claudication” (Audible Bleeding). Visit vascular.org/JVSAppExerciseTherapy1222
18 Vascular Specialist | December 2022
LITERATURE
SENT TO NEPAL TO HELP FURTHER VASCULAR CARE
RUTHERFORD
Trainee-developed VSITE aid records soaring sales
Rutherford textbooks arrives in Nepal
Compiled by Beth Bales and Bryan Kay
VENOUS DISEASE
By Peter Henke, MD
THE SOCIETY FOR VASCULAR SURGERY (SVS) HAS published an update to the SVS/American Venous Forum (AVF) 2011 clinical practice guidelines on the care of patients with varicose veins.


This update was developed in collaboration with the AVF and the American Vein and Lymphatic Society (AVLS), and has also been endorsed by the Society for Vascular Medicine and the International Union of Phlebology.

The “2022 Society for Vascular Surgery, American Venous Forum and American Vein and Lymphatic Society Clinical Practice Guidelines for the Management of Varicose Veins of the Lower Extremities” is the first of two parts, and presents evidence-based recommendations on the diagnosis of superficial venous disease and treatment of superficial truncal venous reflex.
Superficial venous disease of the lower extremities is due to refluxing valves, which creates a situation of venous hyper-
tension. This is manifested as leg heaviness, pain, varicosities and occasionally skin changes and ulceration. This is an extremely common disease and primarily affects people as they age, although the disease can be seen in younger patients.
The first step is accurate and consistent diagnosis of the reflux in the superficial veins. Duplex ultrasound scanning is recommended as the diagnostic test of choice. Duplex ultrasound scanning—which delivers no radiation—is considered best due to standardized protocols for sonographers and standardized definitions for abnormal reflux. The guideline also recommends using trained vascular ultrasonographers in an accredited vascular laboratory.
The guideline recommends that patients with symptomatic varicose veins and reflux are best treated with intervention rather than prolonged long-term compression stockings. Use of compression stock-
ings, while an important mode of therapy, does not relieve the underlying cause of the venous reflux and insufficiency. The techniques to treat systematic axial vein reflux are primarily endoluminal with thermal or non-thermal ablation techniques rather than the older surgical vein stripping.
Treatment of tributary varicosities can be by direct phlebectomy or ultrasound-guided foam sclerotherapy.
The guideline also addresses the role of treatment of incompetent perforator veins in patients with varicose veins, as well as the limitations of perforator vein treatment in patients with mild to moderate venous insufficiency. Lastly, the guideline recommends performing truncal ablation in the superficial veins combined with varicose vein treatment during the same session, if possible.
“The new SVS/AVF/AVLS guideline on varicose veins is the first high-quality multi-society guideline where each recommendation is based on an independent systematic review and meta-analysis,” said Peter Gloviczki, MD, co-chair of the guideline writing group. “The primary goal was to provide recommendations supported by the latest scientific data, with a secondary goal that adopting these guidelines will markedly decrease the number of inappropriate procedures performed in patients with chronic venous disease.”

Read the new guideline at vascular.org/JVSVL-VaricoseVeinUpdate. It will also be in an issue of Journal of Vascular Surgery: Venous and Lymphatic Disorders in early 2023.
In tandem, the SVS held a virtual roundtable discussion in early December aimed at helping expand physicians’ knowledge base and clinical effectiveness in treating patients with varicose veins.
www.vascularspecialistonline.com 19
SOOVC
VAM
Excellence
Distinguished
International Scholars Program International Scholars Program
Presentation Award/Research SOOVC Presentation Award/Research Seed Grants Seed Grants VAM Travel Scholarships
Travel Scholarships
in Community Practice Excellence in Community Practice Award Award Lifetime Achievement Award Lifetime Achievement Award Medal for Innovation in Vascular Medal for Innovation in Vascular Surgery Surgery
Fellow Designation Distinguished Fellow Designation Dec. 31 Dec. 31 JJan. an. 9, 2023 9, 2023 Feb. 1, 2023 Feb. 1, 2023 March 1, 2023 March 1, 2023 Mentored Research Career Development Mentored Research Career Development Awards (K, NHBLI, AHRQ programs) Awards (K, NHBLI, AHRQ programs) Resident Research Award Resident Research Award Vascular Research Initiatives Conference Vascular Research Initiatives Conference Trainee Award Trainee Award Student Research Fellowship Student Research Fellowship Clinical Research Seed Grant Clinical Research Seed Grant (Multiple Deadlines) (Multiple Deadlines)
Jan. 11, 2023 Jan. 11, 2023 Feb. 1, 2023 Feb. 1, 2023 March 1, 2023 March 1, 2023 SVS Foundation Awards: vascular.org/FoundationAwards SVS Awards: vascular.org/SVSAwards
PETER HENKE is a member of the SVS Document Oversight Committee (DOC).
“The primary goal was to provide recommendations supported by the latest scientific data, with a secondary goal that adopting these guidelines will markedly decrease the number of inappropriate procedures performed in patients with chronic venous disease”
PETER GLOVICZKI
Updated multisociety guidelines for treatment of varicose veins see every recommendation based on independent systematic review and meta-analysis
Peter Henke
IN.PACT™ AV Drug-Coated Balloon (DCB)
First & only
The
first and
Separate trials evaluating target lesion primary patency for IN.PACT AV DCB at 36 months and Lutonix™* DCB at 24 months.†
Probability of target lesion primary patency
Probability of target lesion primary patency
2.5% vs.
PTA at 24 months
PTA at 24
Probability of target lesion primary patency
Probability of target lesion primary patency
100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0%
100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0%
Log-rank p < 0.001 at 6, 7, 12, 24, and 36 months
Log-rank p < 0.001 at 6, 7, 12, 24, and 36 months
IN.PACT AV DCB 43.1% Standard PTA 28.6%
IN.PACT AV DCB 43.1% Standard PTA 28.6%
0 1 3 6 7 9 12 18 24 30 36
0 1 3 6 7 9 12 18 24 30 36
Months
Months
Log-rank p = 0.087
Log-rank p = 0.087
Standard PTA 24.4%
Standard PTA 24.4%
0 1 3 6 7 9 12 18 24 30 36
0 1 3 6 7 9 12 18 24 30 36
Months
Months
*Third-party brands are trademarks of their respective owners.
†Primary patency endpoints are defined differently; results are from different studies and may vary in a head-to-head comparison; charts are for illustration purposes only.
‡IN.PACT AV Access Trial: Target Lesion Primary Patency Rate was defined as freedom from clinically driven target lesion revascularization (CD-TLR) or access circuit thrombosis measured through 36 months (1,080 days) post-procedure.
Lutonix DCB 26.9%
Lutonix DCB 26.9%
§Lutonix AV Clinical Trial: Target Lesion Primary Patency was defined as freedom from clinically driven reintervention of the target lesion or access thrombosis measured through 24 months. only DCB with superior, sustained results at
36 months for AV fistula lesions versus PTA.1,2
36-month results
months 14.5% vs.
IN.PACT AV DCB‡1
PTA at 36 months
2.5% vs.
14.5%
Lutonix DCB§2
vs. PTA at 36 months
SVS FOUNDATION UNVEILS TRIO OF NEW MEMBERS TO BOARD OF DIRECTORS
By Beth Bales
THE SOCIETY FOR VASCULAR Surgery (SVS) Foundation Board has three new directors. They are Anil Hingorani, MD, Geetha Jeyabalan, MD, and Peter Nelson, MD, who formerly was on the Foundation’s Development Committee. Each will serve a three-year term.
The SVS Foundation funds research into vascular disease and treatments, sponsors programs and carries out patient education to prevent and treat circulatory disease.
Hingorani is a professor at New York University Langone Brooklyn. His interests include research and patient education, as well as diversity, equity and inclusion (DEI) issues.

Jeyabalan is a vascular surgeon with MedStar Heart & Vascular Institute in Annapolis, Maryland. She graduated from University of Michigan Medical School, where she was a member of Alpha Omega Alpha Honor So-

14.5% vs. PTA at 36 months
14.5% vs. PTA at 36 months
ciety, then received her general surgery and vas cular surgery training at the University of Pittsburgh Medical Center (UPMC). She started her career as faculty at UPMC and now serves as the chief of vascular surgery at Anne Arundel Medical Center. She also is the vice-chair of the Community Practice Section of the SVS.
“I’m enthusiastic to see more SVS Foundation focus on decreasing healthcare disparities in vascular care,” she said.
Nelson is the Mary Louise Todd Chair for Cardiovascular Research at the University of Oklahoma College of Medicine. He also is professor, vice chair for research, and vascular surgery section chief in the Department of Surgery.

He has served as chair of the SVS Foundation Development Committee and is co-investigator for an SVS Foundation pilot program exploring solutions to access to vascular care in underserved Native American, underrepresented minority and rural communities in Oklahoma.
Nelson became an enthusiastic foundation donor after, as he put it, “I realized that the foundation invests in both community and academic surgeons to develop impactful clinical programs. It supports important clinical and translational research; it provides educational materials for all providers and patients fight-
ing vascular disease; and it supports young, bright vascular surgeons early in their careers to get involved.” The Foundation’s funding of groundbreaking work from SVS members has “enormous impact on the lives of our patients.”
Recent foundation accomplishments included the successful inaugural Vascular Health Step Challenge, held throughout September, which is national Peripheral Arterial
Disease (PAD) Awareness Month. Participants aimed to walk 60 miles each during the month, raising funds from family members, friends and co-workers. The 60mile goal represented the 60,000 miles of blood vessels in the human body, and the fund-raising goal was $60,000. The challenge helped bring awareness of the benefits of walking on vascular health. Proceeds will help purchase fitness trackers for patients in need.
In keeping with its mission, the foundation also recently added a fourth pillar of DEI to its existing three pillars, which are research and innovation, community vascular care and patient education, and disease prevention.
Initiatives may include targeted scholarships, awards and research grants for diversity candidates, research projects focused on healthcare disparities or bringing programs to underserved areas.
The core mission of the foundation is to optimize the vascular health and well-being of patients and the public through support of research that leads to discovery of knowledge and innovative strategies, as well as education and programs, to prevent and treat circulatory disease.
GEETHA JEYABALAN
Learn more about the SVS Foundation at vascular.org/SVS-Foundation.
Precautions
References
1 Holden A. The IN.PACT AV Access Study: Results through 36 Months. Presented at Charing Cross 2022.
2 Trerotola SO, Saad TF, Roy-Chaudhury P; Lutonix AV Clinical Trial Investigators. The Lutonix AV Randomized Trial of Paclitaxel-Coated Balloons in Arteriovenous Fistula Stenosis: 2-Year Results and Subgroup Analysis. J Vasc Interv Radiol. January 2020;31(1):1-14.e5.
IN.PACT™
Brief Statement
AV Drug-coated PTA Balloon Catheter Indications for Use
The IN.PACT AV Paclitaxel-coated PTA Balloon Catheter is indicated for percutaneous transluminal angioplasty, after appropriate vessel preparation, for the treatment of obstructive lesions up to 100 mm in length in the native arteriovenous dialysis fistulae with reference vessel diameters of 4 to 12 mm.
Contraindications
The IN.PACT AV DCB is contraindicated for use in the following anatomy and patient types:
• Coronary arteries, renal arteries, and supra-aortic/cerebrovascular arteries
Patients who cannot receive recommended antiplatelet and/or anticoagulant therapy
• Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system
Patients with known allergies or sensitivities to paclitaxel
• This product should only be used by physicians trained in percutaneous transluminal angioplasty (PTA). Assess risks and benefits before treating patients with a history of severe reaction to contrast agents. Identify allergic reactions to contrast media and antiplatelet therapy before treatment and consider alternatives for appropriate management prior to the procedure.
• This product is not intended for the expansion or delivery of a stent.
Do not use the IN.PACT AV DCB for pre-dilatation or for post-dilatation.
• This product is designed for single patient use only. Do not reuse, reprocess, or resterilizethis product. Reuse, reprocessing, or resterilizationmay compromise the structural integrity of the device and/or create a risk of contamination of the device, which could result in patient injury, illness, or death.
• The use of this product carries the risks associated with percutaneous transluminal angioplasty, including thrombosis, vascular complications, and/or bleeding events.
The safety and effectiveness of the IN.PACT AV DCB used in conjunction with other drug-eluting stents or drug-coated balloons in the same procedure has not been evaluated.
• The extent of the patient’s exposure to the drug coating is directly related to the number of balloons used. Refer to the Instructions for Use (IFU) for details regarding the use of multiple balloons and paclitaxel content.
• Appropriate vessel preparation, as determined by the physician to achieve residual stenosis of ≤ 30%, is required prior to use of the IN.PACT AV DCB. Vessel preparation of the target lesion using high-pressure PTA for pre-dilatation was studied in the IN.PACT AV Access clinical study. Other methods of vessel preparation, such as atherectomy, have not been studied clinically with IN.PACT AV DCB.
• Women who are breastfeeding, pregnant, or are intending to become pregnant, or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and whether there is a potential for adverse reaction in nursing infants from paclitaxel exposure.
Warnings
2.5% vs. PTA at 24 months
2.5% vs. PTA at 24 months
• A signal for increased risk of late mortality has been identified following the use of paclitaxel-coated balloons and paclitaxel-eluting stents for femoropopliteal arterial disease beginning approximately 2-3 years post-treatment compared with the use of non-drug coated devices. There is uncertainty regarding the magnitude and mechanism for the increased late mortality risk, including the impact of repeat paclitaxel-coated device exposure. Inadequate information is available to evaluate the potential mortality risk associated with the use of paclitaxel-coated devices for the treatment of other diseases/conditions, including this device indicated for use in arteriovenous dialysis fistulae.
Physicians should discuss this late mortality signal and the benefits and risks of available treatment options for their specific disease/ condition with their patients.
• Use the product prior to the Use-by date specified on the package.
• Contents are supplied sterile. Do not use the product if the inner packaging is damaged or opened.
Do not use air or any gaseous medium to inflate the balloon. Use only the recommended inflation medium (equal parts contrast medium and saline solution).
Do not move the guidewire during inflation of the IN.PACT AV DCB.
Do not exceed the rated burst pressure (RBP). The RBP is based on the results of in vitro testing. Use of pressures higher than RBP may result in a ruptured balloon with possible intimal damage and dissection.
The safety of using multiple IN.PACT AV DCBs with a total drug dosage exceeding 15,105 μgpaclitaxel has not been evaluated clinically.
Potential Adverse Effects
Potential adverse effects which may be associated with balloon catheterization may include, but are not limited to, the following: abrupt vessel closure, allergic reaction, arrhythmias, arterial or venous aneurysm, arterial or venous thrombosis,death, dissection, embolization, hematoma, hemorrhage, hypotension/hypertension, infection, ischemia or infarction of tissue/organ, loss of permanent access, pain, perforation or rupture of the artery or vein, pseudoaneurysm, restenosis of the dilated vessel, shock, stroke, vessel spasms, or recoil.
Potential complications of peripheral balloon catheterization include, but are not limited to, the following: balloon rupture, detachment of a component of the balloon and/or catheter system, failure of the balloon to perform as intended, failure to cross the lesion. These complications may result in adverse effects.
Although systemic effects are not anticipated, potential adverse effects not captured above that may be unique to the paclitaxel drug coating include, but are not limited to, the following: allergic/immunologic reaction, alopecia, anemia, gastrointestinal symptoms, hematologic dyscrasia (including leucopenia, neutropenia, thrombocytopenia), hepatic enzyme changes, histologic changes in vessel wall, including inflammation, cellular damage, or necrosis, myalgia/ arthralgia, myelosuppression, peripheral neuropathy.
Refer to the Physicians’ Desk Reference for more information on the potential adverse effects observed with paclitaxel. There may be other potential adverse effects that are unforeseen at this time.
Please reference appropriate product Instructions for Usefor a detailed list of indications, warnings, precautions, and potential adverse effects. This content is available electronically at www.manuals.medtronic.com.
CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician.
medtronic.com/AVdata
UC202216301 EN ©2022 Medtronic. All rights reserved. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. For distribution in the USA only. 05/2022
www.vascularspecialistonline.com 21
P
OUTREACH
“I’m enthusiastic to see more SVS Foundation focus on decreasing healthcare disparities in vascular care”
From left to right: Anil Hingorani, Geetha Jeyabalan and Peter Nelson
SOCIETY BRIEFS
Raise a smile to benefit SVS Foundation
 Compiled by Beth Bales and Bryan Kay
Compiled by Beth Bales and Bryan Kay
PEOPLE THE WORLD OVER ARE ticking down to the holidays, end-of-2022 gatherings and shopping according to their traditions and wishes. For those who do some of their shopping online at Amazon, please remember the SVS Foundation.
Shopping that starts at www.smile.amazon. com turns that gift for the newspaper delivery person into a donation for the SVS Foundation. It’s simple: designate the SVS Foundation as the designated “smile” organization and Amazon will donate 0.5% of every eligible purchase to the Foundation.
The practice is good year-round. Every purchase that starts with a “smile” means contributions to the Foundation.
It pays to smile! Please do so at www. smile.amazon.com and help fund the future of vascular health.
SVS launches latest ‘Week of Action’ amid recurring threat of Medicare payment cuts
THE SOCIETY FOR VASCULAR SURGERY (SVS) staged its latest “Week of Action” in early December aimed at targeting lawmakers before the end of the current Congressional session.
The advocacy effort took place Dec. 5–9 and involved a series of engagement activities providing SVS members with an opportunity to collaboratively amplify messages on Capitol Hill.
Vascular surgeons again face significant Medicare payment cuts as 2022 draws to a close, with the changes set to take effect on Jan. 1, 2023 unless Congress takes action.
“We are urging congressional support for the inclusion of new funds in yearend legislation for the explicit purpose of increasing the non-facility/office-based practice expense relative value units negatively impacted by CMS’ [Centers for Medicare and Medicaid Services] clinical labor update policy,” according to the SVS.
“This effort, coupled with parallel initiatives to provide relief from the underlying fee schedule conversion factor adjustment, as provided in legislation introduced by Reps. Ami Bera, MD and Larry Bucshon, MD (H.R. 8800), will help provide critical stability for vascular surgeons and ensure a positive foundation for ongoing deliberations regarding systemic reforms to Medicare physician reimbursement.”
For questions related to SVS advocacy activities, contact SVSAdvocacy@vascularsociety.org.
VOTING ENDS DEC. 18 ON BYLAWS REVISIONS
SOCIETY
FOR VASCULAR SURGERY (SVS) MEMBERS
in good standing have until Dec. 18 to cast their ballots to change the composition of the Society’s Nominating Committee.
Online voting began Dec. 1 on consideration of three revisions to Article X of the SVS bylaws regarding the committee’s composition. The committee’s size would remain at seven members but would expand the diversity of perspective.
The questions address: 1) Reducing the number of past presidents on the Nominating Committee from three to two; the most current past president, who serves on the Executive Board, will join the Nominating Committee one year removed from this service. 2) Adding the SVS Diversity, Equity and Inclusion (DEI) Committee chair, or vice chair if the chair is unavailable, as a voting member of the Nominating Committee, replacing the chair of the Leadership Committee. The Leadership Committee chair was added when the committee’s name was the Leadership and Diversity Committee, but diversity has since moved to the DEI Committee. 3) Adding the chair of the Young Surgeons Section, or the vice chair if the chair is unavailable, as a voting member of the Nominating Committee. The Young Surgeons Section chair has been serving as a non-voting ex-officio member.
The process for nominating SVS officers for 2022–23 begins in January 2023. The special referendum ensures that any changes enacted will be in time to affect the 2023 nominating process and elections.

Read the proposed changes at vascular.org/2023NomCommBylawsChange
SAVS extends travel scholarship application cut-off for 2023 meeting
THE SOUTHERN ASSOCIATION for Vascular Surgery (SAVS) announced a deadline extension to Dec. 5 for 20 travel scholarships that were made available to medical students and general surgery resi dents to attend the 2023 iteration of the SAVS annual meeting (Jan. 18–21).

Half of the 20 $1,000 grants for the gathering in Rio Grande, Puerto Rico— partially funded by W.L. Gore through the Association of Program Directors in Vascular
Surgery (APDVS)—are reserved for women and underrepresented minorities.
To qualify, applicants must be either a medical student (years 1–3) or a general surgery resident (postgraduate years 1–3) interested in vascular surgery and able to attend the meeting from Wednesday, Jan. 18, through Saturday, Jan. 21, according to SAVS.
“As the purpose of these scholarships is to increase interest in vascular surgery, students and residents already applying for training positions are not eligible,” the association noted.
The travel scholarships include complimentary registration for the offshore meeting alongside the $1,000 award to be used for travel reimbursement.
Webinar examines 3D printing for vascular imaging
Members learned some of the ins and outs of three-dimensional (3D) printing for vascular imaging at a Society for Vascular Surgery (SVS) webinar on Dec. 12.
The SVS’ Health Information Technology (HIT) Committee sponsored the webinar that delved into an emerging field in the vascular sphere.
With 3D printing, vascular surgeons get a replica of a patient’s unique vascular anatomy—from heart valves to blood vessels—before performing open surgery on endovascular procedures. “This offers the vascular surgeon increased detail to optimize the procedure and patient outcomes,” said Judith Lin, MD, chair of the HIT Committee.
She and Daniel Kassavin, MD, moderated the webinar.
Topics included, among other topics, a 3D printing overview and presurgical planning using 3D modeling. There will be time for questions and discussion.
SVS dues reminder
TO AVOID A LAPSE IN THEIR benefits, Society for Vascular Surgery (SVS) members are encouraged to pay their 2023 member dues by Dec. 31.
These benefits of SVS membership include access to the Journal of Vascular Surgery publications at reduced or no cost; the customizable SVS Branding Toolkit, discounts on education programs— including the Vascular Annual Meeting (VAM) and Coding and Reimbursement Workshop—as well as networking, practice management resources and more.
For 2023, the SVS has created the Early Active member category specifically for those vascular surgeons completing their training. Dues are lower, just $275 for the year, instead of the $695 fee for Active members, to help these new members transition to working life.
SVS also has simplified the application process for Early Active membership, dropping a formal application and any required letters of recommendation.
The 575 members who have finished training within the past three years have been notified that they have automatically become Early Active members.
Members will automatically transition to full Active status after achieving their Vascular Surgery Board (VSB) certification and a minimum of two years of Early Active membership.
22
Vascular Specialist | December 2022
ACTIVE AND SENIOR
Positive long-term, three-year observational data from a cohort of patients that participated in the previously concluded VenoValve (Envveno Medical) first-inhuman clinical trial were recently presented at the VEITHsymposium 2022 (Nov. 15–19) in New York City.
Principal investigator Jorge Hernando Ulloa, MD, from University of the Andes in Bogota, Colombia, reported that the VenoValve patients, who are now an average of 36 months post VenoValve implantation, continue to benefit from the VenoValve and have experienced no relapses of severe chronic venous insufficiency (CVI) and no recurrences of venous ulcers. Safety events were limited to one thrombosis after discontinuation of anticoagulation medication.
The presenter added that average improvements in reflux, CVI disease manifestations (revised Venous Clinical Severity Score [rVCSS]), and pain (Visual Analog Scale [VAS]) remained stable at 63%, 52%, and 84% respectively, when compared to pre-surgery levels, for the cohort of eight patients that agreed to be followed at conclusion of the one-year first-in-human trial. One patient experienced an increase in rVCSS due to dermatitis, which Ulloa noted was unrelated to the VenoValve or vascular disease.
A company press release details that the VenoValve is a first-in-class, surgically implanted replacement venous valve that is currently being evaluated in the SAVVE (Surgical Anti-reflux Venous Valve Endoprosthesis) U.S. pivotal trial—a prospective, non-blinded, single-arm, multicenter study of 75 CVI patients.

VenoValve is intended to restore proper directional blood flow for patients with CVI of the deep veins of the leg. Envveno Medical estimates that approximately 2.5 million people in the U.S. that suffer from the debilitating impacts of severe deep venous CVI would be candidates for implantation of the VenoValve device.
VIZ.AI RECENTLY ANNOUNCED NEW data from a large aortic dissection artificial intelligence (AI) real-world study that supports the use of its AI technology for the detection of suspected aortic dissection.
Data from the new study, which were presented this week at VEITHsymposium 2022 (Nov. 15–19) in New York City, validate the Viz.ai’s dissection detection algorithm, according to the company.
An abstract from Viz.ai, in collaboration with Avicenna.ai, entitled “Real-world validation of a deep learning AI-based detection algorithm for suspected aortic dissection,” reported the performance of the Viz Aortic Dissection Algorithm on 1,303 computed tomography (CT) angiography scans collected from over 200 U.S. cities.
The algorithm demonstrated a sensitivity of 94.2%, specificity of 97.3%, as well as a positive predictive value of 80.1% and negative predictive value of 99.3%.
The authors concluded: “These findings
provide significant real-world validation of a deep-learning AI-based detection algorithm for suspected aortic dissection. Automated detection may have a positive downstream effect on patient triage leading to accelerated care coordination, earlier diagnosis, timely initiation of life-saving interventions, and better patient outcomes.”
According to a company press release, Viz Aortic accelerates time-to-notification to specialists giving them access to clinically-relevant imaging and patient information for appropriate patient treatment plans.
The solution includes AI-powered alerts, high-fidelity mobile image viewing, relevant clinical information, and HIPAA-compliant communication to facilitate workflow and improve patient care for all aortic conditions.
Meanwhile, Viz.ai recently announced a partnership with Illuminate, a developer of proprietary natural language processing (NLP) and AI software that discovers at-risk patients from electronic medical records (EMR), assesses disease severity, and facilitates follow-up surveillance for a variety of diseases.

The partnership will enable healthcare providers to make timely, critical decisions for aortic aneurysm patients while improving compliance with pre- and post-surgical surveillance programs, the company said in a press release.
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm – 13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation. CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available.
GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts. CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available.
* As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
Products listed may not be available in all markets.
CBAS is a trademark of Carmeda AB, a wholly owned subsidiary of W. L. Gore & Associates, Inc. GORE, Together, improving life, VBX, VIABAHN and designs are trademarks of W. L. Gore & Associates. © 2022 W. L. Gore & Associates, Inc. 22689847-EN NOVEMBER 2022
www.vascularspecialistonline.com 23 CLINICAL
DEVICE
&
NEWS
Compiled by Jocelyn Hudson and Bryan Kay
Viz.ai announces positive new data from large aortic dissection AI realworld study
First-in-human patients continue to benefit from VenoValve at average of three years post-surgery, new data show
VenoValve
* The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis indication includes de novo or restenotic lesions in iliac arteries, including those at the aortic bifurcation. The GORE® VIABAHN® Endoprosthesis indication includes lesions in the iliac arteries only.

1. Piazza M, Squizzato F, Dall’Antonia A, et al. Outcomes of self expanding PTFE covered stent versus bare metal stent for chronic iliac artery occlusion in matched cohorts using propensity score modelling. European Journal of Vascular & Endovascular Surgery 2017;54(2):177-185.
2. Panneton JM, Bismuth J, Gray BH, Holden A. Three-year follow-up of patients with iliac occlusive disease treated with the Viabahn Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy 2020;27(5):728-736.
3. Sabri SS, Choudhri A, Orgera G, et al. Outcomes of covered kissing stent placement compared with bare metal stent placement in the treatment of atherosclerotic occlusive disease at the aortic bifurcation. Journal of Vascular & Interventional Radiology 2010;21(7):995-1003.
see accompanying prescribing information in this journal. Products listed may not be available in all markets.
Please
GORE,
JULY
COVERED STENTS OFFER PROMISING ADVANTAGES for treating complex iliac occlusive disease The GORE® VIABAHN® Device family of covered stent grafts gives you the flexibility and conformability to safely and confidently address even the most complex cases.1,2,* Balloon expandable covered iliac kissing stents versus bare metal stents at the aortic bifurcation Patency rates One-year Covered stents Bare metal stents 92% 78% Two-year Covered stents Bare metal stents 92% 62% See all the data HIGHER PATENCY RATES AT THE AORTIC BIFURCATION3 P = .023
Together, improving life, VBX, VIABAHN and designs are trademarks of W. L. Gore & Associates. ©2022 W. L. Gore & Associates, Inc. 22470700-EN
2022




 By Arthur E. Palamara, MD
By Arthur E. Palamara, MD































































 By Jamie Bell
By Jamie Bell



































 Compiled by Beth Bales and Bryan Kay
Compiled by Beth Bales and Bryan Kay




