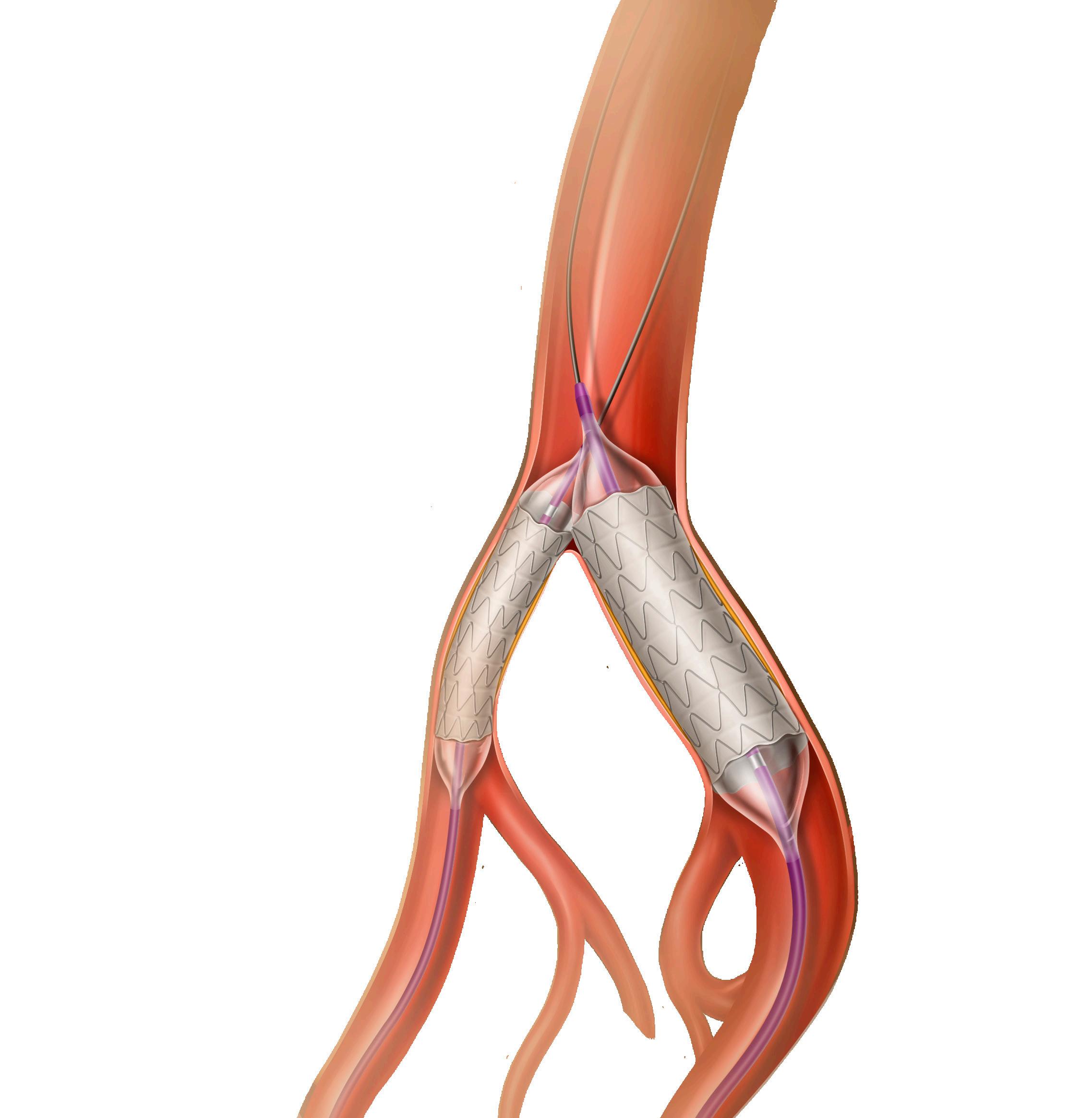
BASIL-2 AND BEST-CLI: A TALE OF TWO LIMB TRIALS
By Michael S. Conte, MD
OPTIMAL TREATMENT OF patients with chronic limbthreatening ischemia (CLTI) has been an ongoing debate within the vascular community, fueled by growing numbers of patients, evolving technologies, provider bias, and a lack of highquality evidence.

Few randomized controlled trials (RCTs) have focused on this patient population. Now, within a sixmonth period, the primary results of the long-awaited BEST-CLI and BASIL-2 RCTs have been reported. At the top level, their results seem wholly discordant with respect to limb-based versus survival outcomes. A deeper dive into the designs of these two trials, their
See page 4
VASCULAR SURGERY MOVES TO ASSESS TRAINEES’ COMPETENCE WITH EPAs

 By Beth Bales
By Beth Bales
FOR SURGEONS, “EPA” MEANS not the “Environmental Protection Agency” but “Entrustable
Professional Activity”—and they’re going to change surgery training.
A pilot rollout for vascular surgeons will begin in select centers in the fall with Vascular Surgery Board
See page 8
VAM 2023 BECKONS
Our annual gathering is almost here. After months of planning, our premiere educational event in vascular surgery, the Vascular Annual Meeting (VAM), opens June 14. Every president before me has said nothing can top this year’s event—and yet, somehow, year after year, we do. This year is no exception, writes SVS President Michael C. Dalsing, MD.




2 From the Editor
BEST-CLI vs. BASIL-2: Different questions yield different answers
5 Comment & Analysis
This month’s Corner Stitch tackles the trainee return to the OR
11 The Big Interview Frank J. Veith, MD, goes in-depth about his book ahead of inaugural lecture



12 AI Bias and the silver bullet: Gender and machine learning in AAAs
www.vascularspecialistonline.com
VAM 2023 IS FINALLY HERE. MONTHS OF PLANNING AND HARD work are almost complete. The Program Committee, headed by Andres Schanzer, MD, and the Postgraduate Education Committee, with Will Robinson, MD, at the helm, have put in countless hours selecting top-notch abstracts and developing clinical and non-clinical educational sessions for your knowledge and inspiration. Diversity, equity and inclusion (DEI) topics are incorporated into each session to highlight discussion within the very fabric of the meeting. From talking with both Schanzer and Robinson, I know that each and every attendee will be able to leave VAM with information they can use immediately in their practices and/or careers. It could take the form of research results, how-tos on performing particular procedures, thoughts on the road to retirement or practice management tips and tricks. We once again have sessions for a variety of career stages and interests. Each SVS membership section and subsection—physician assistants, women, young surgeons, those in community practice and those operating in an office-based lab—is hosting sessions of particular interest to the section members. Plus:
◆ Special sessions aimed at international members
◆ Our always-interesting E. Stanley Crawford Critical Issues Forum, this year on the vascular surgery workforce pipeline
◆ A session on transitioning to retirement

◆ Maximizing various facets of vascular care in attendees’ practices

◆ Events for medical students and residents
◆ The inaugural Frank J. Veith Distinguished Lecture, named for our former president, and to focus on limb salvage
◆ A special session on the results of two landmark clinical trials: BESTCLI (Best endovascular versus best surgical therapy in patients with critical limb ischemia) and BASIL-2 (Bypass versus angioplasty in severe ischemia of the leg)
See page 14
THE OFFICIAL NEWSPAPER OF THE Presorted Standard U.S. Postage PAID Permit No. 384 Lebanon Jct. KY ascularV pecialists CHANGE SERVICE REQUESTED 9400 W. Higgins Road, Suite 315 Rosemont, IL 60018
this issue: JUNE 2023 Volume 19 Number 06
In
VAM 2023 PREVIEW SPECIAL
Medical Editor Malachi Sheahan III, MD
Associate Medical Editors
Bernadette Aulivola, MD | O. William
Brown, MD | Elliot L. Chaikof, MD, PhD
| Carlo Dall’Olmo, MD | Alan M. Dietzek
MD, RPVI, FACS | Professor HansHenning Eckstein, MD | John F. Eidt, MD
| Robert Fitridge, MD | Dennis R. Gable, MD | Linda Harris, MD | Krishna Jain, MD | Larry Kraiss, MD | Joann Lohr, MD
| James McKinsey, MD | Joseph Mills, MD | Erica L. Mitchell, MD, MEd, FACS
| Leila Mureebe, MD | Frank Pomposelli, MD | David Rigberg, MD | Clifford Sales, MD | Bhagwan Satiani, MD | Larry Scher, MD | Marc Schermerhorn, MD | Murray
L. Shames, MD | Niten Singh, MD | Frank
J. Veith, MD | Robert Eugene Zierler, MD
Resident/Fellow Editor
Christopher Audu, MD
Executive Director SVS
Kenneth M. Slaw, PhD
Director of Marketing & Communications Bill Maloney
Managing Editor SVS Beth Bales
Marketing & Social Media Manager
Kristin Crowe
Communications Specialist
Marlén Gomez
Published by BIBA News, which is a subsidiary of BIBA Medical Ltd.
Publisher Roger Greenhalgh

Content Director Urmila Kerslake
Managing Editor Bryan Kay bryan@bibamedical.com
Editorial contribution
Jocelyn Hudson, Will Date, Jamie Bell, Clare Tierney, Eva Malpass and Benjamin Roche
Design Terry Hawes
Advertising Nicole Schmitz nicole@bibamedical.com
Letters to the editor vascularspecialist@vascularsociety.org
BIBA Medical, Europe
526 Fulham Road, London SW6 5NR, United Kingdom
BIBA Medical, North America
155 North Wacker Drive – Suite 4250, Chicago, IL 60606, USA
Vascular Specialist is the official newspaper of the Society for Vascular Surgery and provides the vascular specialist with timely and relevant news and commentary about clinical developments and about the impact of healthcare policy. Content for Vascular Specialist is provided by BIBA News. Content for the news from SVS is provided by the Society for Vascular Surgery. | The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or the Publisher. The Society for Vascular Surgery and BIBA News will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services, or the quality or endorsement of advertised products or services, mentioned herein. | The Society for Vascular Surgery headquarters is located at 9400 W. Higgins Road, Suite 315, Rosemont, IL 60018. POSTMASTER: Send changes of address (with old mailing label) to Vascular Specialist, Subscription Services, 9400 W. Higgins Road, Suite 315, Rosemont, IL 60018. | RECIPIENT: To change your address, e-mail subscriptions@bibamedical.com | For missing issue claims, e-mail subscriptions@bibamedical. com. | Vascular Specialist (ISSN 1558-0148) is published monthly for the Society for Vascular Surgery by BIBA News. Printed by Vomela
Commercial Group | ©Copyright 2023 by the Society for Vascular Surgery
FROM THE EDITOR
BEST-CLI vs. BASIL-2: Different questions yield different answers
Medical editor Malachi Sheahan III, MD, takes a look at seemingly conflicting results from the landmark BEST-CLI and BASIL-2 trials, and asks what they really reveal.
This past April, BASIL-2 chief investigator Andrew Bradbury, MD, presented trial results at the Charing Cross (CX) International Symposium in London. The authors concluded that an endovascular-first approach to patients with critical limb-threatening ischemia (CLTI) who required an infrapopliteal intervention was associated with better amputation-free survival. These findings seem to be a direct contradiction to the bypass-first approach supported by the BEST-CLI study. So what do we do when two major trials report conflicting data? That’s right, start a Flame War on Twitter. But before we bury the interventionalists in memes, let’s look at what these studies may actually be telling us. BASIL-2 was an open-label, pragmatic, multicenter, randomized trial performed mainly in the United Kingdom. The primary outcome was amputation-free survival, which was higher in the endovascular treatment group than the bypass cohort (hazard ratio [HR] 1.35, p=0.037). But what drove this disparity? There was no difference in major amputation rate between the endo and open groups (18% vs. 20%) over the study period. Therefore, the difference in outcomes was mainly derived from the lower mortality in the endo patients (45% vs 53%).
Intuitively, one would think that the higher mortality in the open group would be associated with the risk of general anesthesia and the increased morbidity of surgery. The 30-day morbidity and mortality, however, were not statistically different between the treatment modality groups. What then drove this difference in survival? First, it should be noted that overall survival was fairly dismal, as one would expect from an aging population with severe peripheral vascular disease. It is often easy to get lost in the canyon between statistical significance and clinical significance. My back of the napkin math tells me that as few as five more deaths in the endo group over the study period might have robbed the endpoint of its statistical “significance.” Looking at the two groups, they seem pretty well matched, but the raw prevalence of previous myocardial infarction (24% vs. 13%) and previous dialysis (6% vs. 3%)
The tale of the tape
were higher in the bypass cohort. Could this have been enough to generate a few more late deaths?
It is also important to note that in this intent-to-treat analysis, only 145 of the 172 patients in the bypass group underwent surgery. Since the difference in outcomes was mainly driven by late mortality, a look at the survival in each group of those actually receiving the treatment would be helpful.
Interestingly, the BASIL-2 results also seem to contradict a previously published subgroup analysis from the BASIL-1 trial, which suggested better outcomes with vein bypass than angioplasty in infrapopliteal revascularization.
Cohort 1 of the BEST-CLI trial was comprised of CLTI patients with an adequate saphenous vein who were randomized to either an endovascular- or surgery-first strategy. The study concluded that an initial surgical approach led to a lower risk of major adverse limb event or death. Cohort 1 enrolled more than four times as many patients as BASIL-2 and had many crucial differences in design. Participants were required to be good operative risks. The patients were younger and demonstrated more gender and ethnic diversity. Infrapopliteal revascularization was a require-
ment for BASIL-2 but only performed in about 60% of the BEST-CLI cohort 1. Perhaps most importantly, the primary measured outcome of BEST-CLI was a combined freedom from death, major amputation, and major reintervention, while BASIL-2 focused on amputation-free survival. Both BASIL-2 and BEST-CLI found significantly higher reintervention rates in the endo groups. So where to go from here? Luckily, the BEST-CLI and BASIL-2 investigators have a data-sharing agreement in place that should elucidate many of these questions in the future. Patient-level data and more detailed anatomic information are needed to provide any true guidance to the ideal therapy for CLTI. Also, a disclaimer. These trials employed complex statistical modeling. This passage from the BASIL-2 manuscript made my eyes glaze over: “Binary secondary outcomes measured at a single timepoint were analysed using a mixed effects log-binomial model to generate an adjusted risk ratio and risk difference (with an identity link function).” So, to paraphrase Michael Scott, I know exactly what I’m doing, but in a much more real sense, I have no idea what I’m doing. To this end, I have asked Michael Conte to provide a “smart guy” commentary to complement my own in this issue of Vascular Specialist
While we await further analysis, should we employ an endo- or open-first strategy for CLTI? The answer is likely neither. The best approach is one suited to the individual patient. Of course, one could then argue that the best physician for this problem is one who can expertly offer either treatment paradigm. But I’ll leave that for the Twitterati. Don’t @ me.
Vascular Specialist | June 2023 2
BEST-CLI (Cohort 1) BASIL 2 Total Enrollment 1434 345 Median Follow-Up 2.7 years 3.3 years Primary Outcome Amputation/Major Reintervention/Death Amputation/Death Age 66.9 72.5 White 72.2% 91% Female 28.5% 19% Adequate GCV Conduit Required? Yes No Good Surgical Risk Requirement? Yes No Infrapopliteal Revascularization Required? No Yes
Malachi Sheahan III
“ While we await further analysis, should we employ an endo- or open-first strategy for CLTI? The answer is likely neither”
AOTI KEPT MY FOOT


Diabetic limb amputation is devastating… and preventable.



David was on the road to amputation. His diabetes led to a wound that would not heal despite three surgeries. He was told he may lose his foot.
Within five months of using TWO2 multi-modality therapy, his wound closed. He kept his foot and is back on his feet doing the things he loves. TWO2 delivers faster, sustained healing for diabetic foot ulcers and is proven to reduce amputations by 71%. 1
MB1091-A REFERENCE: 1. Yellin J, et al. Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes Advances in Wound Care, 2021. Disclaimer: Testimonials are based on actual patient experiences. Patients have given their consent. Any person depicted is an actor portrayal and not an actual patient. LET’S MAKE AMPUTATION A THING OF THE PAST. Visit us at Booth #611 VAM, June 14-17
FROM THE COVER: BASIL-2 AND BEST-CLI: A TALE OF TWO LIMB TRIALS
populations and outcomes reported to date is needed to interpret their meaning.
The BEST-CLI trial was funded by the U.S. National Heart, Lung and Blood Institute and involved 133 centers in the U.S., 12 in Canada, and five in other countries. It was designed to compare the effectiveness of endovascular intervention versus open bypass surgery among CLTI patients with infrainguinal occlusive disease who were deemed acceptable candidates for either treatment.1 The study was conducted as two parallel RCTs based on preoperative assessment of an adequate great saphenous vein (GSV) for bypass. The primary endpoint was major adverse limb event (MALE)-free survival, defined in accordance with the published SVS Objective Performance Goals (MALE: major amputation or major reintervention [new open bypass, major open graft revision, thrombectomy or thrombolysis]).2 A total of 1,434 subjects were randomized in cohort 1 (adequate GSV) and 396 in cohort 2.

Analyzed by intention-to-treat (ITT), patients assigned to open surgery in cohort 1 (median follow-up time 2.7 years) experienced significantly lower rates of MALE or death (32% relative risk [RR]), major amputation (27% RR), any major reintervention (65% RR), and less than half the total number of major reinterventions in the treated limb over time.3 These findings were robust across virtually all pre-defined patient subgroups. In cohort 2, there was no significant difference in the primary endpoint after a median of 1.6 years of follow-up. Importantly, perioperative mortality, long-term survival, and overall major adverse cardiovascular event (MACE) rates were not different by assigned treatment in either cohort. The BEST-CLI investigators concluded that both procedures were equally safe, and that CLTI patients deemed suitable for either approach who had an adequate GSV experienced better overall clinical outcomes after open bypass surgery.
BASIL-2 was funded by the UK National Institute of Health Research and involved 39 centers in the UK, one in Sweden and one in Denmark. It was designed to compare the effectiveness of endovascular intervention versus open bypass with vein among CLTI patients requiring treatment of infrapopliteal (IP) disease, with or without a more proximal infrainguinal intervention.4 Patients had to be deemed suitable for either procedure, with an anticipated life expectancy of at least six months. The primary endpoint was major amputation-free survival (AFS). Among 345 patients enrolled and followed for a median of 40 months, those assigned to bypass surgery experienced a 35% greater incidence of major amputation or death.5 This result was driven entirely by excess long-term mortality in the surgical group.
The BASIL-2 investigators concluded that while limbbased outcomes were similar, patients treated by an endovascular-first strategy were more likely to be alive with an intact limb compared to those who underwent surgery first.
What gives with these seemingly opposite results?
Let’s start with similarities between the two RCTs. Both trials were designed to compare revascularization strategies among patients deemed suitable for either, with equipoise determined locally by site investigators. Immediate technical success rates reported for endovascular interventions were strikingly similar (85% in BEST-CLI, 87% in BASIL-2). Approximately 70% of subjects in both trials had diabetes. More than 70% in both trials were treated with an antiplatelet agent, and similar proportions were treated with a statin. Both trials had to deal with the major impacts of a COVID pandemic on healthcare delivery and clinical research. And once again these trials highlight that, despite concerted efforts at guideline-based medical therapy, mortality rates in CLTI approximate 10% or more per year. However, differences in the designs,
continued from page 1
enrolled populations, treatments received and trial execution are all likely related to the disparate outcomes reported. Key characteristics of the two RCT cohorts, most importantly the anatomic complexity of disease treated, remain incompletely described at this time. Comparison of the populations is important for understanding both the contrasting results and the generalizability of each trial. BEST- CLI was more than five times the size of BASIL-2 and had broader inclusion criteria.
BEST-CLI enrolled more women (28% vs 18%) and more non-white (28% vs. 9%) subjects. The BASIL-2 study population was defined as requiring an IP intervention. In BEST-CLI an IP procedure was performed in just over half of all subjects, and only a minority had treatment of IP disease alone (16% in cohort 1). The severity and management of femoropopliteal disease in BASIL-2 is unclear and likely different from BEST-CLI. Patients in BASIL-2 were older (72 vs. 67 yrs), and, notably, those randomized to open surgery had a higher prevalence of prior MI (24% vs. 13% for the endo arm). Together with the six-month life expectancy entry criterion for BASIL-2, these factors may explain worse long-term survival among surgical patients in BASIL-2. Perioperative mortality after bypass surgery was twice as high in BASIL-2 (6% vs 1.7% in BEST-CLI cohort 1).
Both RCTs were “pragmatic” and, thus, an array of endovascular and open interventions were applied. Befitting the infrapopliteal design of BASIL-2, there were twice as many bypasses originating from the popliteal artery in BASIL-2 (41%) versus cohort 1 of BEST-CLI
(16%). Data reported to date on endovascular devices and techniques are hard to compare but plain balloon angioplasty was the most common procedure below the knee in both trials. In BEST-CLI, vascular surgeons performed 73% of endovascular interventions, compared to BASIL-2 where interventional radiologists performed 84%. These differences reflect the healthcare systems of the United Kingdom and U.S. in relation to vascular care. AFS is an important outcome in CLTI but is dominated by mortality rather than limb events, and does not reflect the burden of reinterventions and unresolved or recurrent symptoms that are experienced. As such, it is an insensitive measure of the quality of limb revascularization. Just as in coronary disease RCTs, need for repeat revascularizations and other major limb events are highly relevant to compare treatment strategies. BASIL-2 was likely quite underpowered for limb-specific outcomes, a problem amplified by extending follow-up time rather than enrolling more new subjects to reach a targeted number of events, since most MALEs occur within the first year. Reinterventions in CLTI are largely driven by ongoing symptoms during follow-up surveillance.
The impacts of the COVID-19 pandemic on healthcare delivery and trial execution in these trials were likely disparate. BASIL-2 investigators noted that face-to-face follow-up assessments were substantially affected after March 2020. In looking at some of the published top-line results, major amputation rates were higher in both arms of BASIL-2 (20% open and 18% endo vs. 10.4% and 14.9%, respectively, in BEST-CLI cohort 1); reintervention rates were lower in the endovascular arm of BASIL-2 (32% vs. 43% in BEST-CLI cohort 1); and mortality after open bypass was significantly worse in BASIL-2. It is presently unclear if these differences between study outcomes reflect the patients enrolled, the care delivered, or both.
All RCTs have limitations of which generalizability is paramount. CLTI patients are heterogeneous in systemic risk, severity of limb threat, and anatomic complexity of occlusive disease. The “endo-first vs. open-first” debate is and has always been an oversimplification, and a disservice to this clinical complexity. The results of BESTCLI clearly demonstrate advantages of surgical bypass in a subset of CLTI patients. To better understand the relative effectiveness of these complementary strategies, trials and registries need to incorporate staging such as WIfI (Wound, ischemia and foot infection) and GLASS (Global Limb Anatomic Staging System)6 in their design and reporting. We anticipate much further and more granular data in regard to important patient subsets in these trials, as well as secondary endpoints and economic analyses. It remains for the trial investigators to further unravel the puzzle by comparing like-to-like patients and identifying predictive factors for outcomes. The good news is that we now have a large trove of high-quality data in CLTI from these two RCTs, thanks to the great efforts of the investigators, the sponsors, and most importantly the patients who volunteered to participate. These data will undoubtedly advance evidence-based practice in CLTI and improve future study designs in this arena.
References
1. Menard MT, Farber A, Assmann SF, et al J Am Heart Assoc 2016. PMID: 27402237
2. Conte MS, Geraghty PJ, Bradbury AW, et al J Vasc Surg 2009. PMID:19897335
3. Farber A, Menard MT, Conte MS, et al New Engl J Med 2022. PMID:36342173
4. Popplewell MA, Davies H, Jarrett H, et al. Trials 2016. PMID: 26739146
5. Bradbury AW, Mookes CA, Popplewell M, et al Lancet 2023. PMID: 37116524
6. Conte MS, Bradbury AW, Kolh P, et al J Vasc Surg 2019. PMID:31159978
Vascular Specialist | June 2023 4
MICHAEL S. CONTE is Edwin J. Wylie, MD, Chair in Vascular Surgery at the University of California San Francisco (UCSF).
“
To better understand the relative effectiveness of these complementary strategies, trials and registries need to incorporate staging such as WIFI and GLASS in their design and reporting ”
Michael S. Conte
COMMENT& ANALYSIS CORNER STITCH
CHANGING GEARS: A GUIDE TO CRUISING BACK TO THE OPERATING ROOM
By Kenneth Tran, MD

FOR SURGEONS-IN-TRAINING, professional development time (colloquially known as “the research years”) is a welcome break from the rigors of surgical residency. A time to rest, reflect, and embark on new growth opportunities. A reprieve from the 6:00 a.m. (or earlier) morning rounds, middle of the night calls from the emergency department, and discerning gaze from our attendings. However, inevitably, the time comes to get back into clinical gear to finish the job that you started (residency). Cue the nervous jitters and sweaty palms. This is a universal feeling nearly all of us have felt before returning to patient care after a long hiatus. Rest assured, the first three years of your surgical training have prepared you well for your return—but there are a few things you can do to make the transition back as smooth as possible.
Trip down memory lane
Like all things in surgery, the devil is in the detail. While the basics of clinical care, such as determining operative candidacy or perioperative management, are likely old hat, little things will certainly have escaped your memory. This is especially true in the operating room (OR). Which side of the anastomosis is easier to sew first for a below-knee bypass? What sheath size does this device need again?
How does attending X like their cases draped and positioned? Revisiting old case logs and surgical notes from years past may be helpful to jog your memory of these minute details. This is also a good time to dust off the old anatomy and operative technique textbooks. Like riding a bicycle, technically performing both open and endovascular cases “comes back fast.” However, a few afternoons of deliberate practice with a set of Castroviejos, 6-0 Prolene, some expired PTFE grafts and your favorite Spotify playlist is a nice way to get back into the swing. Likewise, for endovascular cases in particular, a proactive visit to the endovascular device storage room to get re-acquainted with the names of commonly used wires, catheters and sheaths is beneficial.
Showing your age
One of the joys of vascular surgery is the sheer pace of innovation in our field, with a never-ending stream of new devices and techniques to master. Coming back from research, I found myself encountering new acronyms that I had never heard of before. What is a DVA exactly? Is the TBE the same as TAMBE? Seemingly overnight, TCAR became a widely adopted procedure that I was now expected to be facile with. A quick meeting with your friendly neighborhood device
GOVERNMENT GRAND ROUNDS
representatives is helpful in this regard. They often can share with you the newest updated endograft configuration booklets, which are invaluable for case planning. A few months prior to starting back, you may also find it useful to listen in on your division’s weekly case conferences to get a grasp on current clinical algorithms. For example, our vascular lab and limb salvage program had adopted pedal acceleration time as a new surrogate marker for foot perfusion, which may affect which patients are offered intervention.
It’s all about the team
Returning from research years often coincides with transitioning from being a junior to senior resident. In your new role, managing a cadre of interns, junior residents and advanced practice providers becomes supremely important. Cases need to be appropriately and fairly assigned, consults evaluated and staffed efficiently, and patient concerns and dispositions thoroughly addressed. These tasks cannot be performed without a well-functioning team. I personally found team management was one of the
most difficult aspects of my final years of residency. This aspect of training was seldom discussed, nor do we often have formal education or coaching to improve our “soft skills”—which are arguably right up there with gaining operative skill in order to be an effective surgeon. If they can spare the time, grabbing coffee to chat with the current chief residents on service is immensely helpful in this regard. Your colleagues can give you a primer on any new workflow changes, service logistics or other aspects of patient care that have changed since you were last on service. Leaning into your new role as chief resident is a daunting task, but is without a doubt a rewarding and beneficial experience as you embark on your journey to independent practice.
The week before you start
One of my friends told me that he channeled his nerves into preparation. For him, that meant rounding with the team a few days before he was to start running the service. This helped get him back into the wake-up schedule, and he got to know the patient on service and their plan. He was also able to pick the mind of the chief resident he was taking over from. He got to meet the support staff. He also cites the benefit of knowing the operative schedule so that he could prep for the cases. Finally, he says he went down to the hybrid room on a quiet afternoon and reminded himself of “what the buttons did.”
The first month back is going to be stressful. There’s just no way around it, but I hope this column helps someone make that transition back a little smoother.
Advocacy exists to serve: Surgeons, patients and our mission
 By Margaret C. Tracci, MD
By Margaret C. Tracci, MD
One of the most gratifying aspects of working to advocate for surgeons is that, at its heart, this is completely transparent and is done simply to serve. Serve what? To serve surgeons, by supporting their ability to flourish in practice, pushing back against the seemingly endless accumulation of regulatory and other encumbrances, and focusing a light on surgeon well-being. To serve our patients, by advocating for the highest quality care and tackling impediments to access. To serve our mission, by raising public and patient awareness of the role of vascular surgery and highlighting the importance of research, care innovation and the healthy growth of the vascular surgeon workforce.
Over the years, the Society for Vascular Surgery (SVS) has built a robust infrastructure to serve its members through advocacy, supported by both surgeon volunteers on its committees and councils and by an expert professional staff, based both in Rosemont, Illinois, and Washington, D.C. The Policy and Advocacy Council works closely with policy and advocacy staff to facilitate communication and coordination
among its member committees, across councils, with the Strategic Board.
The Council’s committees include: Government Relations, which takes the lead on federal legislative and regulatory issues; Coding, which plays a critical role through the American Medical Association’s Relative Value Scale (RVS) Update Committee (RUC) and beyond in the valuation and reimbursement of vascular services; the PAC Steering Committee, which raises and disburses funds to support all advocacy efforts; the VA Vascular Surgeons Committee, representing the unique position and perspective of the U.S. Department of Veterans Affairs (VA) surgeons and veterans; and the Performance Measures Committee, doing duty across councils to ensure alignment.
What does coordination and collaboration of advocacy across the SVS mean? This means that the policy and priorities of the entire society are drawn from the expertise of the councils and committees and the values of our vascular surgeon members. The Clinical Practice Council, for ex-
ample, has helped define our advocacy agenda through the recommendations developed by the Wellness Committee, the insight into the impact of current payment policies offered by the Section on Outpatient and Office Vascular Care (SOOVC), and the initial work of the Population Health Task Force. Once policy and priorities are set, the SVS continues to serve through its three advocacy pillars: grassroots, political and legislative advocacy.
Our professional staff is continually working to move our priorities forward in D.C. And as Andrew Kenney wrote a few months ago, this operation is infinitely stronger with the support of surgeons engaging our legislators through in-person meetings, letters, calls, and social media in a grassroots campaign. So when you think of advocacy, know that SVS advocacy truly exists to serve. To get involved, reach out at advocacy@vascularsociety.org
MARGARET C. TRACCI is the vice chair of the SVS Advocacy Council.
www.vascularspecialistonline.com 5
KENNETH TRAN is a vascular surgery resident at Stanford University, California.
“A few months prior to starting back, you may also find it useful to listen in on your division’s weekly case conferences to get a grasp on current clinical algorithms”
Adding 8–12mm diameter devices to the Shockwave Peripheral Intravascular Lithotripsy toolkit
Mazin Foteh, MD, contrasts the benefits of Shockwave Medical’s new Shockwave L6 Peripheral Intravascular Lithotripsy Catheter alongside those of its sister device, the Shockwave M5+ Peripheral Intravascular Lithotripsy Catheter.
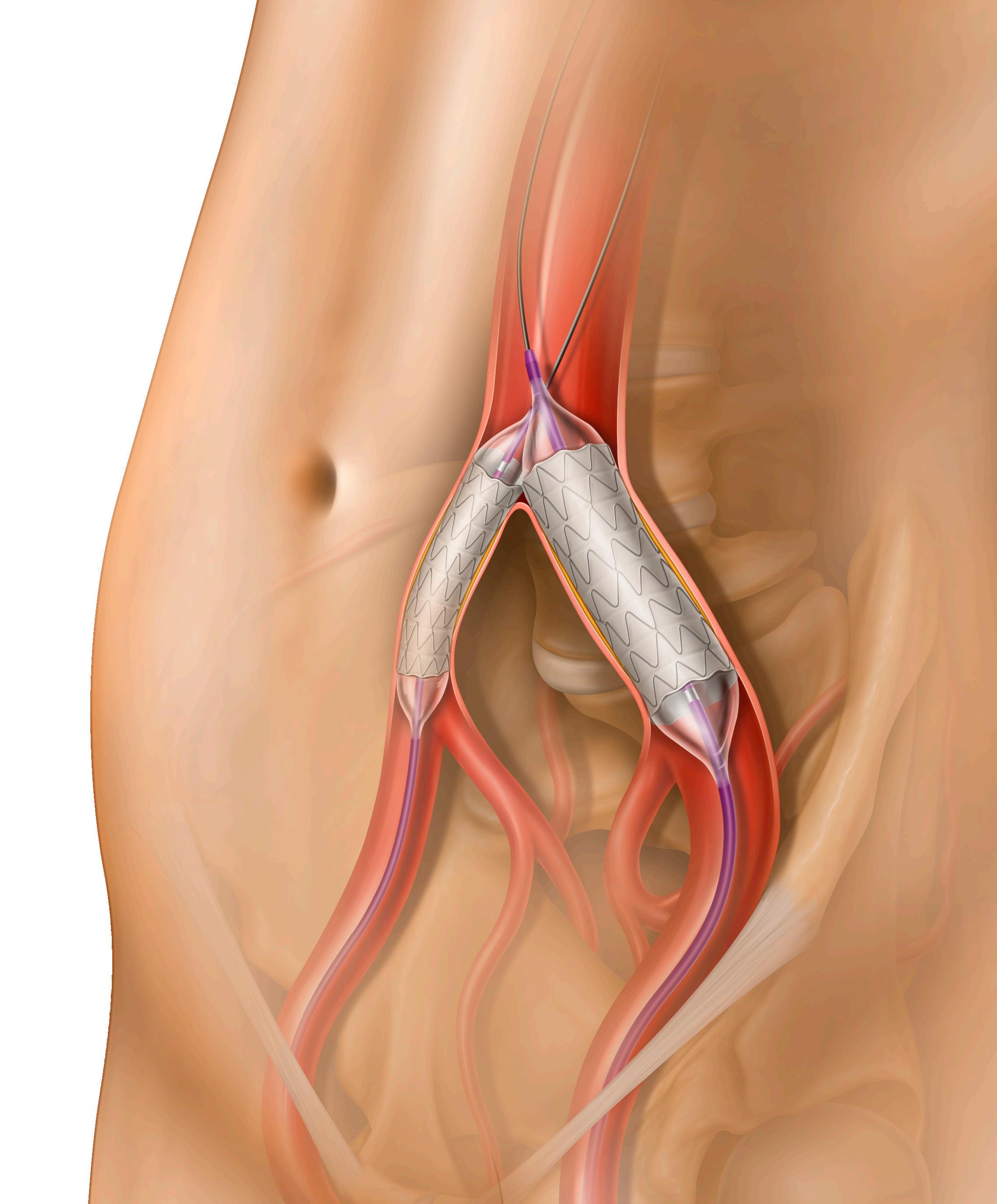
The Shockwave L6 device is a purpose-built IVL catheter, perfectly designed for complex iliac occlusive disease. Calcified plaque blockages in this region are distinctly different from lesions elsewhere in the arterial system. These are much larger blood vessels, which are often thick and heterogeneously calcified. Although the Shockwave M5+ IVL catheter was instrumental in introducing this technology to an underserved space, in some scenarios it lacked the size and energy profile to compete with this degree of calcium. The most important feature of the Shockwave L6 catheter is its larger diameter offerings (8, 9, 10 and 12mm).
Today, we can appropriately match the Shockwave balloon catheter to the vessel and optimize outcomes. Secondly, the compact emitter placement is instrumental in providing a uniform energy profile across the length of the balloon. There are a total of three paired emitters, which fire in groupings of two. This translates to consistent sonic output without diminishment at the ends.
The Shockwave M5+, on the other hand, delivers peak pressure at the center electrode. In practice this equates to improved case efficiency, as we are no longer trying to center the electrode at the most dense area of plaque. Finally, all of the Shockwave L6 catheters can be delivered over an 0.018” wire and in 7/8F sizes. Tracking over a larger wire removes resistance and sets a platform for additional therapies such as stents.

To date, the endovascular treatments for calcified iliac plaques have centered on the “crack and pave” technique, which harnesses barotrauma as the mechanism of action. The Shockwave L6 catheter allows a rethinking of this method, now relying purely on low-pressure inflations and the delivery of sonic pressure waves to the hardened calcium.
This results in a much gentler, worry-free technique, causing calcified lesions to yield in a manner that is without the risk of rupture. Additionally, if stenting is necessary, we can rest assured that optimal luminal gain can be achieved. This particular arterial bed is very frequently laden with calcium deposits, therefore the need for new techniques is present.
The Shockwave L6 catheter is the first
technology to address this need and may one day challenge the notion that all iliac blockages require a stent. My practice is centered around large-bore devices for endovascular aneurysm repair (EVAR), thoracic EVAR (TEVAR), and fenestrated EVAR (FEVAR). Iliac stenosis and poor access are quite common in this population.
Prior to the Shockwave L6, we were forced to create a runway for delivery. This meant advanced and expensive techniques such as endovascular and open surgical conduits. These procedures can add cost, but also morbidity. I can now successfully treat most iliac lesions in this setting with the Shockwave L6 alone.
The Shockwave M5+ was an excellent precursor to the Shockwave L6 catheter. Its longer length certainly provided a nice platform to treat multi-segment disease, especially in the external iliac as well as the fem-pop distribution. However, its energy profile differs greatly from the Shockwave L6, with the maximal energy coming from the center electrode. This feature affects the way the balloon is used, generally requiring significant overlap of the inflations and sometimes even trying to center the middle emitter on the peak plaque.
Furthermore, it is somewhat limited in its applicability in larger vessels, simply based on the balloon size. I find that for medium-sized vessels with moderate calcium burden that this balloon is favored. Whereas for focal, dense plaque in larger vessels, the Shockwave L6 is the catheter of choice.
My experience with this system has taught me that appropriate balloon sizing is the single-most important predictor of a successful outcome.
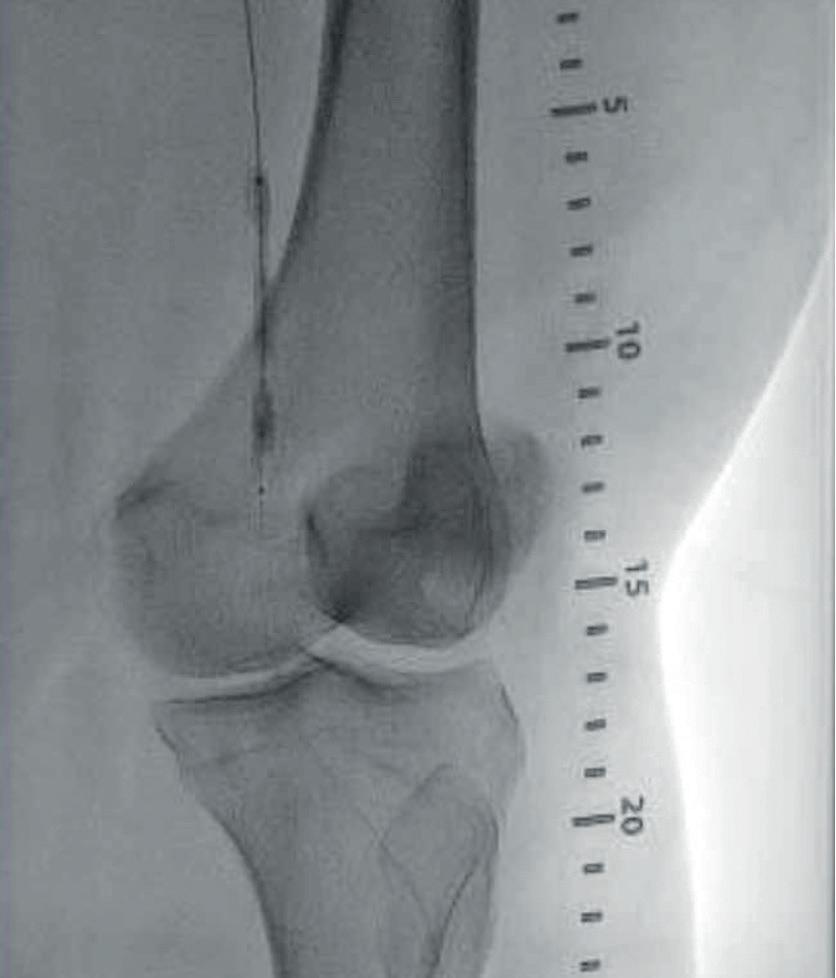
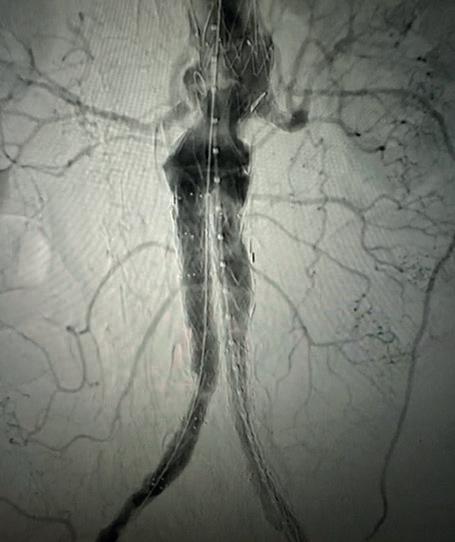
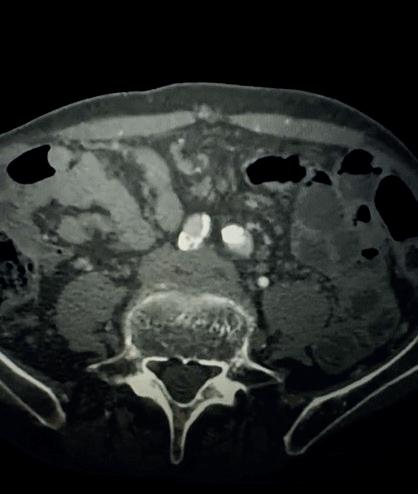
Case 1: The Shockwave L6 device in the setting of FEVAR and complex iliac stenosis
An 82-year-old man with a history of coronary artery disease (CAD), hypertension, hyperlipidemia, and peripheral arterial disease (PAD) presents with rest pain as well as
a large pararenal abdominal aortic aneurysm (AAA). His computed tomography angiography (CTA) revealed very dense and highly diseased iliac vessels with calcium deposition along most of the bilateral common iliac distribution.
The minimum luminal diameter (MLD) of the common iliac arteries measured roughly 4.5mm; however, the
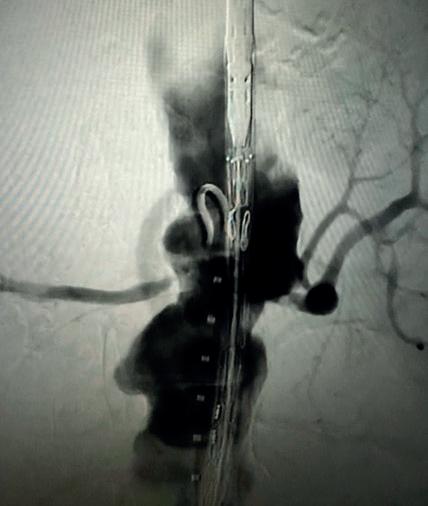
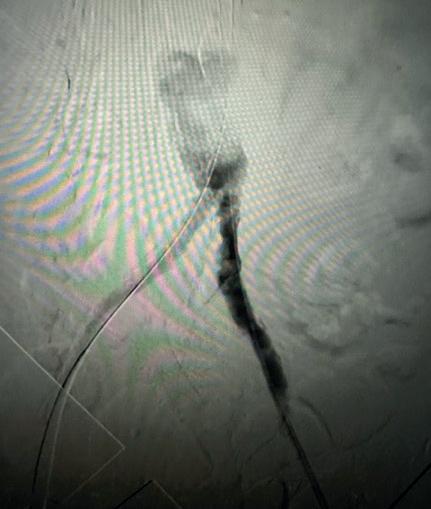
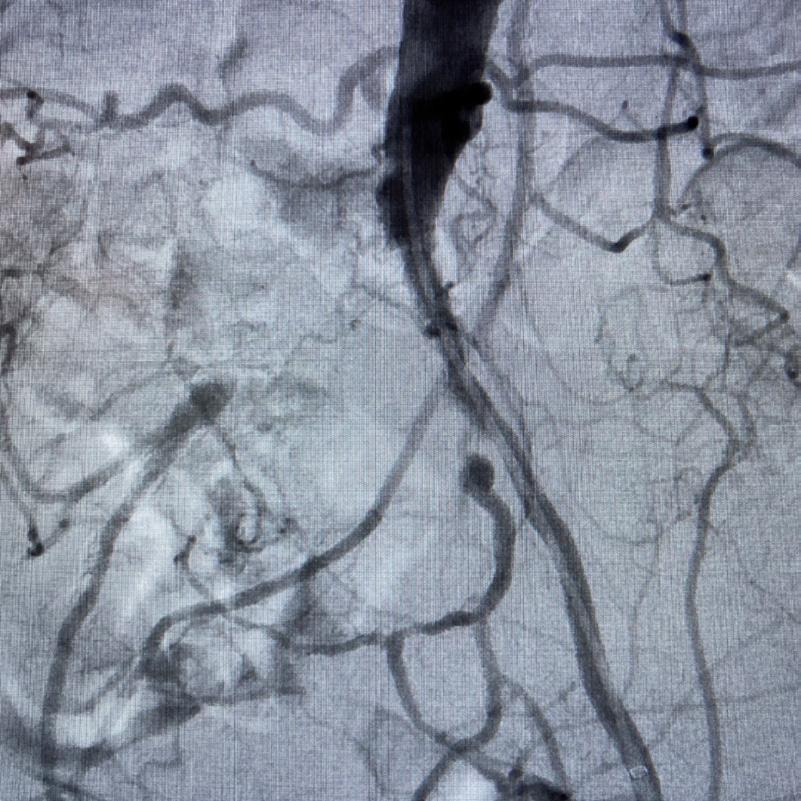
Case 2: The Shockwave M5+ tackles 90% stenosis of the distal SFA
The patient is a 75-year-old man with a history of hypertension, hyperlipidemia, CAD, chronic kidney disease and diabetes mellitus, who presents with a non-healing wound of the right lower extremity for three months.
He had undergone diagnostic work—his ankle-brachial index (ABI) was found to be 0.73 with a toe pressure of 50mmhg, and a duplex ultrasound revealed a densely calcified superficial femoral artery (SFA) stenosis of 90%.
He was taken to the cath lab and underwent a right lower-extremity arteriogram. This confirmed a 90% stenosis of the distal SFA, with normal three-vessel run off.
Due to the heavy calcium, we opted for a Shockwave M5+ 6x60mm and delivered 200 pulses to the lesion. There was no appreciable residual stenosis at the completion of the procedure, and his post-procedure ABI improved to 1.
healthy reference vessel diameter (RVD) was 9.5mm. He would require a fenestrated repair of the AAA along with complex iliac stenosis treatment, and extensive bilateral common femoral endarterectomies.
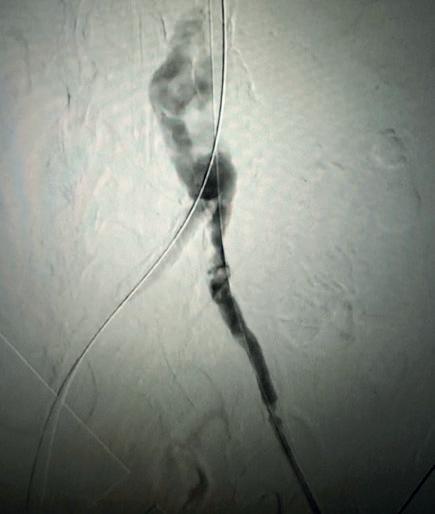
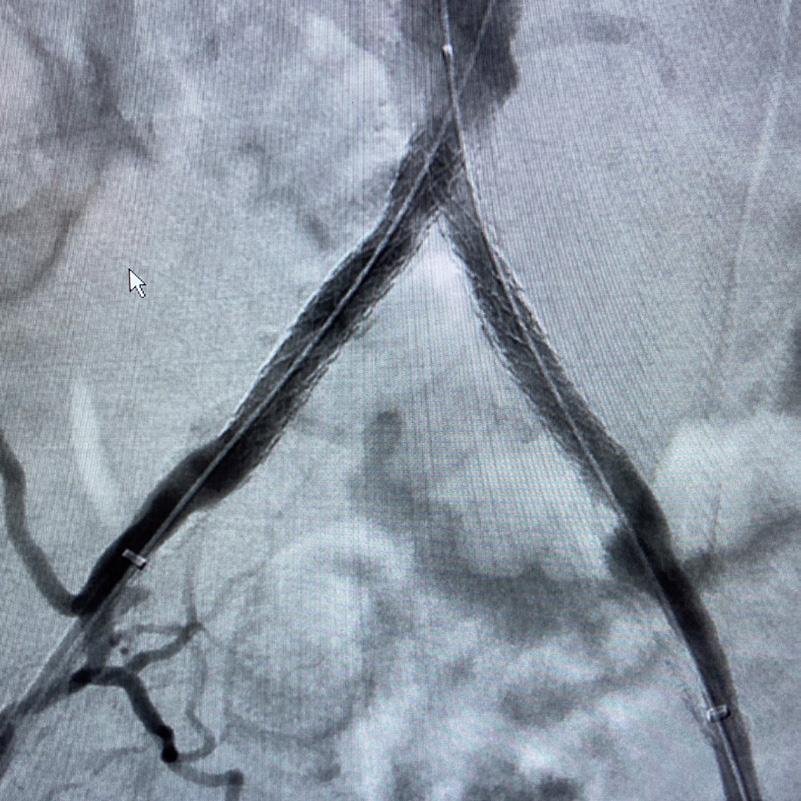
Further, we were concerned we would not be able to advance the 19F fenestrated graft. The endograft was prepared, and then both femoral arteries were exposed. We attempted delivery of the endograft via the right common iliac access. This failed. We then attempted delivery on the contralateral side. Despite multiple attempts, we made little progress. A 10x30mm Shockwave L 6 catheter was selected, and a total of 150 pulses were delivered to each iliac artery at just 4atm. This not only allowed for the delivery of our FEVAR graft but also the full unrestricted expansion of the endograft limbs in the common iliac arteries.
At the completion of the AAA, we took extra time to perform endarterectomies of the deep femoral arteries. His completion CT scan revealed a good seal of the AAA, patency of the renal fenestrations and unrestricted flow to the bilateral femoral arteries.
This is a great example of how IVL can be used as a standalone therapy without the need for adjunctive treatment.
MAZIN FOTEH is director of aortic therapy and innovation and co-director of the Aortic Clinic at Baylor Scott & White Health in Plano, Texas. He is a paid consultant to Shockwave Medical. The views expressed are those of the author and not necessarily those of Shockwave Medical.

Vascular Specialist | June 2023 6
“My experience with this system has taught me that appropriate balloon sizing is the single-most important predictor of a successful outcome”
THIS ADVERTORIAL IS SPONSORED BY SHOCKWAVE MEDICAL
MLD = 4.5mm RVD = 9.5mm IVL Device Choice = Shockwave L6 10mm
Mazin Foteh
Shockwave L6 Peripheral IVL Catheter
CASE 1
CASE 2
Shockwave M5+, Shockwave M5, Shockwave S4 and Shockwave L6 Safety Information
In the United States: Rx only. Indications for Use—The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary or cerebral vasculature.

Contraindications—Do not use if unable to pass 0.014” (M5, M5+, S4) or 0.018” (L6) guidewire across the lesion-Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.

Warnings—Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device—Use the generator in accordance with recommended settings as stated in the Operator’s Manual.
Precautions—use only the recommended balloon inflation medium—Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology.

Adverse effects–Possible adverse effects consistent with standard angioplasty include–Access site complications –Allergy to contrast or blood thinner– Arterial bypass surgery—Bleeding complications— Death—Fracture of guidewire or device—Hypertension/Hypotension—Infection/sepsis—Placement of a stent—renal failure—Shock/pulmonary edema—target vessel stenosis or occlusion—Vascular complications. Risks unique to the device and its use—Allergy to catheter material(s)— Device malfunction or failure—Excess heat at target site.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. www.shockwavemedical.com. SPL -68917 Rev. A.
www.vascularspecialistonline.com 7
1 1 4 2 2 5 3 3 6
Figure 1. Pre-procdural CTA
Figure 4. Graft delivery
Figure 1. Pre-procedural angiogram
Figure 2. Pre-procedural angiogram
Figure 5. Final angiogram
Figure 2. IVL treatment angiogram
Figure 3. Post-IVL angiogram
Figure 6. Post-procedural CTA
Figure 3. Final angiogram
FROM THE COVER: VASCULAR SURGERY MOVES TO ASSESS TRAINEES’ COMPETENCE WITH EPA
(VSB) implementation expected to begin in July 2024.
The American Board of Surgery (ABS) announced the move to competency-based assessment of surgical trainees in February 2022, introducing the ABS EPA Project. The project for general surgery residency programs, for instance, is set to launch in July.
It’s a slightly different version of the Norwegian concept of “entrustment” as a “core way of thinking about when a healthcare professional is ready to be unsupervised,” Brigitte Smith, MD, tells Vascular Specialist

Smith, associate professor of vascular surgery and program director for the vascular surgery fellowship at the University of Utah in Salt Lake City, is leading work to develop the concept of an EPA program for the Association of Program Directors in Vascular Surgery (APDVS). APDVS directors reviewed the program and discussed pilot site recruitment at the APDVS Spring Meeting in March.
Smith said supervisors generally assess performance in terms of medical knowledge, test scores, technical skills and a numerical threshold of procedures and/or years of training.
However, more than 20 years ago, from Europe came the idea of assessing trainees thusly, said Smith: “Do I trust you to do this task independently or do you still need supervision?” It provides a more holistic and intuitive workplace-based assessment to assure graduates are ready to treat patients.
Physicians frequently do this with trainees, she said. “Without really thinking about it, they make a judgment about whether trainees can do a consult by themselves, whether they can be left in the operating room (OR). You can think of it as, ‘Are they ready to do things by themselves?’”
EPA development for vascular surgery
VASCULAR SURGERY EPAs
The 15 areas in which vascular surgery trainees must show competence are:
● Cerebrovascular disease
● Dialysis access
● Traumatic/latrogenic vascular injury
● Peripheral artery aneurysm
● Claudication
● Chronic limb-threatening ischemia
● Acute limb ischemia
● Amputation
● Chronic venous disease
● Acute venous thromboembolism
● Asymptomatic aortoiliac aneurysm
● Symptomatic or ruptured aortoiliac aneurysm
● Chronic mesenteric ischemia
● Acute mesenteric ischemia
● Type B aortic dissection (TBAD)
The SVS EPA Writing Group is currently recruiting pilot programs; interested members should contact Brigitte Smith at apdvs@vascularsociety.org
began nearly two years ago, in June 2021. The Vascular Surgery Board (VSB) of the ABS and the APDVS hope to launch a pilot of approximately 20 programs this fall, which will give developers a chance to identify hiccups and refine the program as it evolves.
Vascular surgery’s EPAs will include proficiency in 15 observable areas of work as the minimum bar for trainees before they would be entrusted to work on their own before graduating from residency or fellowship, Smith said.
“It’s the absolute floor, or minimum bar to complete training and practice vascular surgery” she said. General surgery, meanwhile, includes 18 EPAs. According to the ABS, each EPA includes a description, essential functions, scope and expected behaviors. The Board has created entrustment scales to assess EPAs as follows:
● Limited participation
● Direct supervision
● Indirect supervision
● Practice ready
Smith said she foresees hesitance to change a system many perceive is working, and that the “I walked uphill in the snow both ways” adage applies to medicine. Some physicians may think, “I was fine; I was ready to operate; ergo our system is fine, it’s not broken, don’t fix it.”

However, medicine has changed dramatically in even the past decade, with an older population that is living longer and experiencing more complicated health problems, and hospital systems focused on length of stay.
s continued from page 1
“The learner 20, 30 years ago wasn’t being pushed for a quick discharge; he or she could treat the same patient for a longer period of time, could order blood work and other procedures to figure things out,” she said. “The attention on cost, efficiency, and safety that’s prominent now, and important, has nonetheless changed the experience for trainees. They don’t operate by themselves as much.”
Assessments should change to meet changing circumstances, she said. “We need to be accountable to the public that trainees can meet this minimum bar of competence. At the same time, trainees will have a definitive set of expectations to work toward.”
EPAs attempt to address this need. “Holistically, can we trust this person to care for patients with aortic aneurysm disease? Can we trust them to see the patient, work them up, consider the whole range of care, deliver care and operate?” notes Smith.
The ABS is partnering with the Society for Improving Medical Professional Learning (SIMPL) to create the technology platform for the EPA project, including the mobile platform, data storage and reporting structures. “We’re trying to make it really user-friendly so there’s no reason not to do these,” Smith said.
Currently, assessment typically takes place potentially quite a bit after interactions with trainees.
“The idea with the EPA is that the supervisor is on the app and completing the assessment within 72 hours of the interaction,” Smith explained. “As soon as the resident is done with the consult, you do the EPA. It’s less prone to recall bias and more accurate.”
Increased frequency of assessment will mean a larger dataset—data assessment points that happened in real time—that will help with judgments on a trainee’s competencies and also help program directors refine the system, she said.
An unknown is what happens if a trainee isn’t ready. Smith estimated that maybe just a small percentage of trainees would not be ready “on time” and perhaps need a few more months of focused training in a particular area. That, in turn, brings on such questions as who will pay the trainee’s salary in the event of extended training.
“Overall, I’d say the vast majority of people will finish on time. Some will be ready early,” she said. “If they’re not, it will be focused remediation and support to ensure competence.”
But making sure trainees are patient-ready is the important point, she added. To be sure, patient safety is the fundamental focus.
Nobody likes change, she acknowledged. And most people perceive this new system as being more work and want to know what existing evaluations can be jettisoned.
“Hopefully this will take the place of a few things over time, such as end-of-rotation evaluations” Smith said.
“But ultimately, we owe it to our patients to assure them that before trainees leave the supervised environment to treat patients independently, they’ve demonstrated and we’ve documented competence.”
THE MECHANICS: HOW AN EPA WILL WORK
Brigitte Smith outlined how Entrustable Professional Activities (EPAs) might work in assessing a trainee who is overseeing the evaluation and management of a patient.
The rating scale strives to let the supervising surgeon assess “How much do I trust this learner?” And the possible answers are that the trainee needs direct supervision (also known as “limited participation”), indirect supervision, can work unsupervised and, finally, is “practice-ready.”
The EPA project writing group recently met to craft the “behavioral anchors” for each activity, including what does direct supervision look like? Can the trainee make an accurate diagnosis from an image?
Each level includes the three categories of care: preoperative/ assessment; intraoperative/procedural; and postoperative/disposition.
At level 1, for example, in terms of preoperative care for cerebrovascular disease, a trainee:
● Knows basic cerebrovascular anatomy, takes generic history, performs basic neurologic exam, and knows basic imaging needs
● Develops limited differential diagnosis, requires oversight for workup, knows there are available guidelines for treatment reference
● Communicates basic information
But a practice-ready trainee can manage more complex operations and take care of most cases. The trainee:
● Oversees care of complex patients and problems
● Conducts informed consent for complex procedures (endovascular and open) and critical situations, interprets imaging studies, has a thorough understanding of all relevant trials and applies evidence-based medicine to treatment selection, understands benefits/limitations of medical vs. endo vs. open treatment, and selects appropriately
The goal is standardization across the specialty, said Smith. When a surgeon in Montana says a trainee needs direct supervision “it means the same as when it’s applied to a surgeon in Florida,” she said.
“We need to standardize the language and establish a shared mental model of what competence looks like.”
8 Vascular Specialist | June 2023
“Holistically, can we trust this person to care for patients with aortic aneurysm disease? Can we trust them to see the patient, work them up, consider the whole range of care, deliver care and operate?”
BRIGITTE SMITH
Brigitte Smith


SEE OUR APPROACH When you reach for a balloon expandable covered stent, you require placement accuracy. The LifeStream™ Expandable Vascular Covered Stent was developed for the challenging anatomy and engineered to facilitate accurate placement Dr. Ray Norby shows a kissing stent procedure featuring the advantage of the 6F LifeStream™ Balloon Expandable Vascular Covered Stent in a patient who could not tolerate a 7F sheath. Initial Angio Kissing Stents Placed Result The LifeStream™ Balloon Expandable Vascular Covered Stent is indicated for the treatment of atheroslerotic lesions in common and external iliac arteries with reference vessel diameters between 4.5 mm and 12.0 mm, and lesion lengths up to 100 mm. Not for use in patients with uncorrected bleeding disorders, who receive recommended antiplatelet and/or anticoagulation therapy, or who are judged to have a lesion that prevents full expansion of the implant. Please consult product labels and instructions for use for indications, contraindications, hazards, warnings and precautions. BD, the BD Logo, and LifeStream are trademarks of Becton, Dickinson and Company or its affiliates. © 2023 BD. All rights reserved. © 2023 Illustration by Mike Austin. BD-86589 bd.com BD, Tempe, AZ, USA, 1 800 321 4254
ACCURACY Images courtesy of Dr. Norby.
DESIGNED TO DELIVER PLACEMENT
‘Nobody believed us’: Vascular giant discusses overcoming skeptics to make specialty-defining breakthroughs
Malachi Sheahan III, MD, Vascular Specialist’s medical editor, speaks to living legend and limb-salvage pioneer Frank J. Veith, MD, on the contents of his hard-hitting new book, The Medical Jungle: A Pioneering Surgeon’s Battle to Revolutionize Vascular Care and Challenge the Medical Mafia, ahead of the inaugural named lecture established in his honor taking place at this month’s Vascular Annual Meeting (VAM).

Frank Veith needs little introduction to the world of vascular surgery, either in the United States or anywhere around the globe. He is one of the profession’s pioneers. A vascular working life that started more than a half century ago, Veith’s book—memoir-cum-manifesto—covers the highlights from the start, middle and later parts of his career. VAM will see the birth of its fulcrum: The Frank J. Veith Distinguished Lecture was announced during VAM 2022 in honor of the former SVS president’s near-career-long dedication to the avoidance of amputation, and will focus on the recently published BEST-CLI trial. The talk is to be delivered by trial principal investigators, Alik Farber, MD, and Matthew Menard, MD, and will look at the journey that resulted in BEST-CLI’s evidence, and what lies beyond it for the treatment of CLTI..
Veith devotes a significant portion of The Medical Jungle to limb salvage, as well as topics ranging from his early work in lung transplantation, his involvement in North America’s first-ever endovascular aneurysm repair (EVAR), to the question of vascular surgery as an independent specialty and his philosophy on the mentorship of trainees.

Here, he tackles these themes and more in a frank exchange, which is an edited version of a video interview that features as a VAM 2023 special on the Vascular Specialist website.
MS: Dr. Veith, in your book, you describe yourself as extremely shy as a student and early trainee. How do you account for the evolution in your personality?
FV: That’s a great question. I don’t know how to answer. I still consider myself pretty shy. You know, I don’t jump up and ask questions at meetings, and stuff like that. I had to be a little more forceful and outgoing. With practice, you get better at public speaking, but I would always get nervous when I would give a talk, particularly at a big meeting. But somehow, you get through it. And if you prepare, things usually go pretty well.
MS: For vascular surgeons of my generation, it’s really fascinating to see how much we were involved with transplant surgery in the early days. Can you describe how you got interested in transplant surgery—and as a profession, did we drop the boat on this?
FV: Well, it was very simple. I trained at the Brigham with Franny Moore and Joe Murray, who were pioneers in kidney transplantation and, to some extent, in liver transplantation … But I also wanted to be in an area that was somewhat unconquered and that got me into lung transplantation, which really had not been done with any degree of success. So we started out by trying to figure out why [lung transplantation] hadn’t been successful. Because it was just another organ that involved some vascular surgical techniques [in order] to do the transplant.
MS: You describe very well in the book how you looked to differentiate yourself—and you really took to limb salvage early on. Can you describe how that came about?

FV: It was, of course, when I went to Montefiore, which was, in New York City, really a second-rate, or somewhat second-rate, institution in the Bronx. It was not glamorous, like Cornell, Columbia, Mount Sinai, NYU [New York University]. I wanted to do the normal stuff, aneurysms, carotid, etc., and we didn’t have
many of those cases for a variety of reasons. We did have an abundance of poor patients with threatened limbs—with gangrene or ulceration—who were faced with amputation, and the thinking of the day was, well, “If they have such a limb-threatening lesion, they need a below-knee amputation and ‘rapid rehabilitation.’” We found that that didn’t work. So we started with great care and concern, obviously, to challenge the thinking of the day, which was not to do reconstructive vascular surgery for limb salvage. We found that if we were very careful with our techniques, using some of the methods that I’d learned to do—AV [arteriovenous] fistulas, micro and semi-micro anastomoses—that many of these limbs could be saved. We were able to save more than 90% of the patients who presented to us with a threatened limb.
MS: You were also one of the early advocates—maybe the first—for EVAR for ruptured aneurysms. Can you tell us a bit more about that?
FV: We actually did the first-ever EVAR outside of Argentina. That was a stroke of good fortune. As I say, I had always been interested in endovascular techniques because I’d become very friendly
Vascular Specialist | June 2023 10
THE BIG INTERVIEW
2022
Top: Frank J. Veith takes to the rostrum at VAM 2022 in Boston as his named lectureship is announced. Middle and bottom: His pull-nopunches memoir tackles the central pivot points and landmarks of his career, including the pioneering limb salvage work that is the feature of the new VAM lecture
with Barry Katzen, a well-known interventional radiologist. Because of our friendship and my early support for him, he used to invite me to his interventional meetings. So I was exposed to the benefits of those endovascular techniques. And I was a very early believer, despite many of my colleagues not being believers in angioplasty, stenting, and so forth. I was always interested in the idea of doing EVAR because we had a lot of very sick aneurysm patients who didn’t do well with an open aneurysm repair. When that became available, we embraced it. Remarkably, we were very lucky that the procedure proved to be a big success. The patient— who was totally inoperable and with very bad heart and lung disease—got better. He was up and sitting in a chair reading Playboy the next day. For me, that was an epiphany. I said, “God, if we don’t—as vascular surgeons—learn how to do these techniques, we’re going to be out of a job and become extinct.” … Of course, nobody believed us again. It was very discouraging. But then, with time, after about four or five years, and persistence in presenting our work, and because of our unbelievable successes in these very difficult patients, other vascular surgeons started to embrace the technology.
MS: You were an early proponent of collaboration with interventional radiology. And during your SVS Presidential Address, collaboration was one of your three missions for our specialty. You say later that we failed. Why do you think things happened that way?
FV: Human nature. The book highlights a lot of the frailties, or bad traits, of human nature that you don’t think are going to apply in medicine. I mean: lust for power, control, self interest, greed and, sometimes, jealousy. Those traits which you’d like to think aren’t very popular in medicine, because we’re supposedly a humanistic occupation. Those traits are just as common, maybe even more common, in medicine and surgery, in hospitals, other institutions, and medical schools, as they are in other walks of life. That really is the theme of the book. Young people in particular have to be aware that human nature is not always benign. In fact, it can be very malignant. You have to deal with that and be persistent, and recognize that sometimes you cannot always overcome it. And that certainly applied to limb salvage and lung transplantation, and absolutely applied to the endovascular [arena].
MS: Your ideas were in many ways the father of the Heart and Vascular Center concept.

FV: Yes. We tried to do that. [But] either a chairman of radiology, or perhaps the chairman of surgery, and other places, didn’t want to let go of their patient loads, etc. One of the recommendations that I made, way back in 1996, was that we have vascular centers that were independent of medicine and surgery, and radiology, and could function as isolated centers. That just didn’t work in many cases. In a few places it worked, but, in most cases, either a surgeon or medical specialist, or interventionalists, wanted to be dominant. We felt that that is not going to work—that you have to share leadership, you have to share financial rewards, and you have to teach each other without being threatened. And that, of course, is not easy.
MS: Turning toward the creation of the VEITHsymposium and your ideas in developing it—what was lacking in terms of vascular gatherings at that time? What was the niche you were trying to fill with the symposium?

FV: I had great good fortune because the meeting was started as a very small meeting by Dr. Henri Haimovici, who was a prominent vascular surgeon, very academically oriented, and very smart in writing and speaking. It was a tiny meeting. It had maybe 10 or 12 faculty and less than 100 attendees. It was in a fleabag hotel in New York. So when I became chief [at Montefiore], I inherited that meeting. Again, we were lucky. It was in New York, which was easy to get to. We embraced industry from the beginning, which no one else was doing at that time. Coming up with the idea of the short talk—again, good luck, more than good thinking—was so I could get more faculty on the program. And, gradually, we grew to the point where it became a major international vascular meeting. We also embraced—from the very beginning—inter-
A career in surgery through the decades
ventional radiologists and cardiologists because they had a lot to contribute.
MS: One of your other goals for the specialty outlined in your presidential address—which I think you have falsely characterized as having failed—was independence for vascular surgery. I think that what I hear from you is you don’t understand how much you actually won, and how different things are than when you started. It is difficult for people of a certain generation to know how subservient we were to general surgery, especially in the 1970s, 80s and 90s ,when we were basically not even a subspecialty of general surgery at times.
FV: We did make some inroads. I mean, we got residencies that were zero and five—vascular surgery residency. But the problem now is in many institutions—and I’ve been in two of them, or maybe three actually, because you can include the Cleveland Clinic—vascular surgery doesn’t [have independence] because it’s not a separate specialty. It doesn’t have a seat at the table where resources are doled out. You have to go through your general surgery chairman, or your cardiac surgery chairman, in order to get a sign off on what you want to do—and we really are a separate
1982:


1983:
1984:
1991:
1992:
specialty. We’re certainly as separate as neurosurgery or orthopedic surgery, or gynecology, and, yet, that barrier has not yet been breached. I’m not fighting it anymore, but I think, sooner or later, we have to come into accordance with other countries. Vascular surgery should be a totally separate specialty. Why? Because we sort of are: we don’t do general surgery anymore. We may cover for them, but we really are separate because we’re different. Our techniques are different. And we certainly need the separation from interventional cardiology and interventional radiology that will allow us to compete with them on a level playing field. Because interventional cardiology, who are very talented and very much supported by institutions, outnumber us by about five to one.
MS: This brings us to the topic of energy. With all of the things you’ve achieved, I think what we all want to know is: where do you get your energy?
FV: It’s luck and genes. I mean, the two things that you obviously have, and I was greatly fortunate to have, was pretty good health. And if you don’t have your health, you don’t have your energy. If I get tired now, everything sort of stops. So you have to keep at it. That’s your genes and your parents, and you can’t do anything to pick them. And then commitment to want to do stuff that matters.
MS: The last section of the book is on mentorship. I think I’m probably one of the last American vascular surgeons who you did not train. What do you think is the key to mentoring our trainees? FV: Some of it is luck. I always picked people who wanted to go into academics, and then I would beat them unmercifully to publish stuff, and they used to joke about it and belittle me for it. But the other thing is to treat people at all levels well and as equals. You’ve got to pay people well. You split the income pretty much—as bad as that can be in vascular surgery. Sometimes, you split the good cases and you put your trainees as first authors on papers, even though you may end up writing the paper. And, they sort of get the bug. Nobody is going to remember what I did in 10 or 20 years. But hopefully the people that I train know that I helped them get to where they are—that’s a good legacy, because they’ll be around for a long time. It goes against human nature, but you’ve got to be forcefully unselfish. Because, in the end, it comes back to benefit your interest a lot more if you support people than if you exploit them.

www.vascularspecialistonline.com 11
1963: Frank. J Veith (middle) pictured alongside Francis D. Moore, MD, (left) and Robert Zollinger, MD
Veith is shown in a newspaper feature that documents difficulties encountered in obtaining donor lungs
A year later, an article from the New York Daily News focuses on angioplasty and bypass as alternatives in limb salvage
Veith (front, second from right) is photographed with pioneers in clinical organ transplantation
An article from Newsday details a Soviet World War Two veteran’s limb-saving bypass performed by Veith and colleagues
1984 1992
An operating room photo of the first-ever EVAR in North America
1963 1982 1983 1991
We started with great care and concern, obviously, to challenge the thinking of the day, which was not to do reconstructive vascular surgery for limb salvage
BIAS AND THE SILVER BULLET: GENDER PARTIALITY SEEN WITHIN AI ALGORITHMS FOR AAA IDENTIFICATION
Artificial intelligence (AI) has become a central focus within various spheres, from commerce to medicine—including applications in patient care. Sharon Kiang, MD, talks to Éva Malpass about the specter of bias and how a “hybrid” approach between AI and human specialty may be the best solution going forward.
SHARON KIANG, MD, CONSIDERS IT the “silver bullet” clinical perception of AI. In the Loma Linda University School of Medicine associate professor’s reckoning, it’s no mere nebulous metaphor but the underpinnings of her latest research into disease prediction models, and the gendered biases they found within their foundational algorithms.
“Originally, we weren’t looking at bias. We were just looking to see if the aortic model, using machine learning and AI algorithms, could predict and understand the progression of disease and, if possible, therefore predict outcomes,” Kiang tells Vascular Specialist Operating on the assertion that human error and a “human-bound limitation of resources” confound patient care today, Kiang’s team believe that AI could alleviate a portion of manual “yearly factors,” which can take up significant time.
Kiang has not long since finished presenting some of her latest findings at the 2023 Women’s Vascular Summit (April 28–29) in Buffalo, New York, where she extrapolated

on the advent of AI’s more frequent deployment, and the greater ease with which its limitations can be seen.“We started reading about how bias is unintentionally put into algorithms, and so went to look at our own data to see if there are biases there,” she says. Kiang selected gender as their specified bias variable, in part because disparities within post-intervention clinical outcomes in female aneurysm patients have already been reported. “We wanted to see if our machine was able to fix that and equalize its ability to learn,” Kiang explains.
Kiang told the Women’s Vascular Summit about how she and col leagues investigated gendered bias in convolutional neural networks, or deep learning, for abdominal aor tic aneurysm (AAA). Their findings revealed that the machine was un able to overcome this bias and could not identify aneurysms in females as it could in males.
“This may be that female aortas
are smaller,” Kiang notes, so limiting the machine’s accuracy. Offering further explanation, she stated that their data may have also been “flawed,” creating bias in the algorithm, or that it may not accurately represent “the question being asked of the machine.”
Although she hypothesized that a perfect algorithm could theoretically be designed— blinded to race, gender, financial, and geographical disparities, so in essence a “blinded unicorn algorithm”—Kiang states that questions arise about whether this would make it a “useful” algorithm at all. Where the “balance” lies, she continues, is in a realistic AI architecture that can support these variables in a “representative” and “fair” way.
But, time is of the essence. “AI moves fast” Kiang asserts, observing that her growing body of research is developing closely alongside that of organizations with predominantly financial motives, who are keen to “keep pushing.” Apart from a brief diversion to comment on the novel and rapid development of ChatGPT—“Dude, we haven’t even discussed the ethical implications of [ChatGPT], right?”—Kiang’s viewpoint remains focused on the positive change AI can effect in the care of humans globally. “It sounds Ivory Tower-ish, but my mind is centered on the benefit to populations—not just at a specialty level, or even at a hospital level, but at a national, global level,” Kiang says.
AI does not come without limitations, Kiang makes clear; however, apprehension has most commonly been “driven by fear.”
“On a superficial level people suggest that they will be out of a job, and I believe that’s unfounded,” she says, highlighting the only legitimate fear might be for support staff whose job could be performed more efficiently by AI.
“I’m not here to say we should not advance technology so that people can be less efficient in the care that they provide pa-
VENOUS DISEASE CLTI
Arecent study concludes that non-thrombotic iliac vein lesion (NIVL) patients have better primary patency after venous stenting than patients with venous thrombotic disorders. Olivier Espitia, MD, from CHU de Nantes in Nantes, France, and colleagues report this main finding from a multicenter cohort study in the European Journal of Vascular and Endovascular Surgery (EJVES).
The authors detail that it was their aim to assess primary stent patency predictive factors in three groups of patients with history of lower limb vein thrombosis: NIVL, acute deep vein thrombosis (aDVT) and post-thrombotic syndrome (PTS).
The investigators included consecutive patients from January 2014 to December 2020 from seven hospitals. The team reported the anticoagulant and antiplatelet therapy strategies employed after venous stenting and compared these between the three patient groups. In their results, Espitia et al state that the study included 377 patients in total,
comprising 134 in the NIVL group, 55 in the aDVT group and 188 PTS patients, and that median follow-up was 28.2 months.
The authors report in EJVES that primary patency was statistically significantly higher in the NIVL group (99.3%) compared with the PTS group (68.6%; p<0.001) and the aDVT group (83.6%; p=0.002). In addition, the researchers note that PTS patients received a statistically significantly greater number of stents and had more stents below the inguinal ligament.
Finally, Espitia et al report that discontinuation of antiplatelet therapy at the last assessment was 83.6% for NIVL, 100% for aDVT, and 95.7% for the PTS group (p<0.001). Discontinuation of anticoagulation therapy was 93.2% for NIVL, 25% for aDVT, and 70.3% for the PTS group (p<0.001).
“The only predictor of worse primary patency in the aDVT group was long-term anticoagulation before stenting,” they write.—Jocelyn Hudson
tients. But neither am I saying we should build Skynet and drive the human race out of need,” she adds.
A “hybrid” approach between AI and human specialty may be the best solution, Kiang proposes, a “human understanding of relationships” filling the gaps where AI is most lacking. But a “critical mass” of people who believe in the positive function of AI is needed, she continues. “If you don’t have that buy-in, you will have a lopsided relationship that isn’t truly a hybrid model. In this case, you will have executive bean-counters who don’t know anything about bootsto-the-ground patient care, leaning on AI predictions. I can see that as a problem moving forward.”
Kiang describes her experience of the complex contemporary reception to AI, detailing how audiences are becoming more “receptive” to her ongoing AI research compared with previous years.
“We are hitting while the iron is hot right now, and it’s very hot,” she says. Kiang believes people are now more “open-minded, or are being forced to be.” Emphasizing the importance of “clean” messaging when presenting research on this technology, Kiang says she aims to continue demonstrating the realistic clinical applications of AI that have the potential to better patient care in the future.
NEW ANALYSIS PROBES BEST-CLI TRIAL AGAINST REAL-WORLD SETTING DATA
A NEW ANALYSIS OF CHRONIC LIMB-THREATENING ischemia (CLTI) treatment outcomes was presented at the Society for Cardiovascular Angiography & Interventions (SCAI) 2023 Scientific Sessions (May 18–20) in Phoenix. Since the initial results of the BEST-CLI trial found surgery superior to endovascular revascularization, questions have lingered. The new analysis, led by Eric Secemsky, MD, sought to analyze a broader clinical population via the Medicare population. The endpoint used for the patient group was a composite of major adverse limb events (MALE) and death.
A total of 66,153 patients were included—10,125 autologous grafts, 7,867 non-autologous and 48,161 endovascular. Compared to BEST-CLI cohort 1, patients were older, more often female and had a greater burden of comorbidities. Endovascular operators were less likely to be surgeons (55.9% vs. 73% in BEST-CLI) and more likely to be interventional cardiologists (25.5% vs. 13.0%). The risk of death or MALE was higher with surgery (56.6% autologous vs. 42.6% BEST-CLI cohort 1; 51.6% non-autologous vs. 42.8% BEST-CLI cohort 2) but similar with endovascular. Of those receiving endovascular treatment, major interventions occurred less frequently compared to the trial (10% real-world vs. 23.5% cohort 1; 8.6% real-world vs. 25.6% cohort 2).—Jocelyn Hudson
12 Vascular Specialist | June 2023
Primary patency after venous stenting best in NIVL patients, study finds
MACHINE LEARNING
“You will have executive bean-counters who don’t know anything about boots-to-the-ground patient care, leaning on AI predictions”
SHARON KIANG
Sharon Kiang
Less invasive, long lasting
Ellipsys™ Vascular Access System


Only the Ellipsys vascular access system uses a single point of venous access,1 offering the simplest, most minimally invasive option for arteriovenous fistula (AVF) creation.†1,2 It provides an immediate, suture-free, permanently fused anastomosis1 that is clinically proven to improve longevity3-5 and reduce the risk of complications.3,5
medtronic.com/ellipsys
 Distal Tip
† Compared to surgical arteriovenous fistulas.
Distal Tip
† Compared to surgical arteriovenous fistulas.
FROM THE COVER: VAM 2023
BECKONS continued from page 1
◆ Sessions covering the full range of vascular disease
◆ A career fair for anyone who is contemplating a change of direction
◆ Collaboration with both the Society for Vascular Nursing (SVN) and the Vascular Quality Initiative (VQI), also both holding their annual meetings during VAM 2023
In addition, we’ve added a family-friendly event for Wednesday evening, SVS Connect: Building Community, for people to get together, renew old acquaintances and meet new colleagues. We have also arranged a Celebration of Diversity Reception on Thursday.
And, in a change from past years, the last presentation in every plenary session will feature a video on a particular procedure or repair.
There’s so much more on the schedule than I can mention here. For information on the full VAM 2023 lineup, visit vascular.org/OnlinePlanner23
And I hope to see many of you in National Harbor, Maryland, next month.
Yours,
MICHAEL C. DALSING, MD SVS President
BEST-CLI-foused sessions, papers dot VAM program
THE GROUND-BREAKING BEST-CLI TRIAL FEATURES across several sessions during the Thursday (June 15) of VAM 2023, from the inaugural Frank J. Veith Distinguished Lecture to an industry-sponsored slot.
The study’s first appearance occurs during Industry Breakfast Session 2 (sponsored by Boston Scientific) from 6:45–8 a.m. under the title, “BEST-CLI in light of modern peripheral drug-eluting technologies: What conclusions can we draw?” Moderated by Michael Jaff, DO, and Levester Kirksey, MD, speakers include Elina Quiroga, MD, Anahita Dua, MD,and Kristian Hochberg, MD.
Plenary Session 2, which kicks off at 8 a.m., includes two BEST-CLIfocused papers. The first, “The impact of revascularization strategy on clinical failure, hemodynamic failure and chronic limb-threatening ischemia [CLTI] symptoms in the BEST-CLI trial,” is being presented by one of its principal investigators, Matthew Menard, MD. Working to dive into the issue of sustained clinical and hemodynamic benefit following revascularization for CLTI and its need in resolving symptoms and preventing limb loss, Menard and colleagues sought to compare rates of clinical and hemodynamic failure, as well as resolution of initial and prevention of recurrent CLTI following endovascular vs. bypass revascularization in the BEST-CLI trial. It takes place from 8:11–8:22 a.m.
That is immediately followed by “Secondary interventions following open vs. endovascular
References
1Hull JE, Jennings WC, Cooper RI, Waheed U, Schaefer ME, Narayan R. The pivotal multicenter trial of ultrasound-guided percutaneous arteriovenous fistula creation for hemodialysis access. J Vasc Interv Radiol. February 2018;29(2):149-158.e5.
2Shahverdyan R, Beathard G, Mushtaq N, et al. Comparison of Ellipsys percutaneous and proximal forearm gracz-type surgical arteriovenous fistulas. Am J Kidney Dis. October 2021;78(4):520-529.e1.
3Hull JE, Jennings WC, Cooper RI, Narayan R, Mawla N, Decker MD. Long-term results from the pivotal multicenter trial of ultrasound-guided percutaneous arteriovenous fistula creation for hemodialysis access. J Vasc Interv Radiol. Published online June 2, 2022.
4Franco G, Mallios A, Bourquelot P, Jennings W, Boura B. Ultrasound evaluation of percutaneously created arteriovenous fistulae between radial artery and perforating vein at the elbow. J Vasc Access. September 2020;21(5):694-700.
5Mallios A, Bourquelot P, Franco G, et al. Midterm results of percutaneous arteriovenous fistula creation with the Ellipsys vascular access system, technical recommendations, and an algorithm for maintenance. J Vasc Surg. December 2020;72(6):2097-2106.
Brief Statement
Indications
The Ellipsys system is indicated for the creation of a proximal radial artery to perforating vein anastomosis via a retrograde venous access approach in patients with a minimum vessel diameter of 2.0 mm and less than 1.5 mm of separation between the artery and vein at the fistula creation site who have chronic kidney disease requiring dialysis.
Contraindications
The Ellipsys system is contraindicated for use in patients with target vessels that are < 2 mm in diameter. The Ellipsys System is contraindicated for use in patients who have a distance between the target artery and vein > 1.5 mm.
Warnings
• The Ellipsys system has only been studied for the creation of an AV fistula using the proximal radial artery and the adjacent perforating vein. It has not been studied in subjects who are candidates for surgical fistula creation at other locations, including sites distal to this location.
• The Ellipsys™ system is not intended to treat patients with significant vascular disease or calcification in the target vessels.
• The Ellipsys system has only been studied in subjects who had a patent palmar arch and no evidence of ulnar artery insufficiency.
• Use only with the Ellipsys Power Controller, Model No. AMI-1001.
• The Ellipsys Catheter has been designed to be used with the 6 F Terumo Glidesheath Slender *. If using a different sheath, verify the catheter can be advanced through the sheath without resistance prior to use.
• Use ultrasound imaging to ensure proper placement of the catheter tip in the artery before retracting the sheath, since once the distal tip of the catheter has been advanced into the artery, it cannot be easily removed without creation of the anastomosis. If the distal tip is advanced into the artery at an improper location, complete the procedure and remove the catheter as indicated in the directions for use. It is recommended that a follow-up evaluation of the patient is performed using appropriate clinical standards of care for surgical fistulae to determine if any clinically significant flow develops that require further clinical action.
medtronic.com/ellipsys
are trademarks of a Medtronic company. 09/2022

revascularization for CLTI in the BEST-CLI trial” from 8:23–8:34 a.m. Being presented by Michael S. Conte, MD, the secondary analysis of the trial examines the rates of major reintervention, any reintervention, and the composite of any reintervention, amputation or death by intention-to-treat assignment in both trial cohorts (cohort 1 with suitable single segment great saphenous vein, and cohort 2 lacking suitable vein).
At 9.30 a.m. the first-ever Veith Lecture takes place under the title “BEST-CLI: The journey to evidence and beyond,” delivered by Menard and his fellow principal investigator, Alik Farber.
Then, in the afternoon, a dedicated BEST-CLI and BASIL-2 session takes place at 3:30 p.m, headed up by Joseph Mills, MD, alongside Menard and Farber as moderators. The slot compares and contrasts the two landmark CLTI trials under the title, “BEST-CLI and BASIL 2: Debate, analysis and implementation.” It includes a summary of the BEST-CLI and BASIL-2 findings and trial limitations, followed by a debate.

“Patients with CLTI who have adequate saphenous vein should be treated with bypass,” will be the cause taken up Conte. He will be pitted against Brian G. DeRubertis, MD, who will propose that “Almost all patients with CLTI should be first treated with endovascular therapy.”
Meanwhile, Vincent L. Rowe, MD, will look at how best to capture meaning from the endpoints in both studies; Misty D. Humphries, MD, will ask, “What is BEST for CLTI?” Administrative database or randomized controlled trial?; and Kristina A. Giles, MD, looks at what will be “easy and not so easy” for specialists to implement from BESTCLI and BASIL-2.—Bryan Kay
Precautions
• This product is sterilized by ethylene oxide gas.
• Additional procedures are expected to be required to increase and direct blood flow into the AVF target outflow vein and to maintain patency of the AVF. Care should be taken to proactively plan for any fistula maturation procedures when using the device.
• In the Ellipsys study, 99% of subjects required balloon dilatation (PTA) to increase flow to the optimal access vessel and 62% of subjects required embolization coil placement in competing veins to direct blood flow to the optimal access vessel. Prior to the procedure, care should be taken to assess the optimal access vessel for maturation, the additional procedures that may be required to successfully achieve maturation, and appropriate patient follow-up. Please refer to the “Arteriovenous Fistula (AVF) Maturation” section of the labeling for guidance about fistula flow, embolization coil placement, and other procedures to assist fistula maturation and maintenance.
• The Ellipsys System is intended to only be used by physicians trained in ultrasound guided percutaneous endovascular interventional techniques using appropriate clinical standards for care for fistula maintenance and maturation including balloon dilatation and coil embolization.
• Precautions to prevent or reduce acute or longer-term clotting potential should be considered. Physician experience and discretion will determine the appropriate anticoagulant/antiplatelet therapy for each patient using appropriate clinical standards of care.
Potential Adverse Events
• Potential complications that may be associated with creation and maintenance of an arteriovenous fistula include, but may not be limited to, the following:
• Total occlusion, partial occlusion or stenosis of the anastomosis or adjacent outflow vein
• Stenosis of the central AVF outflow requiring treatment per the treatment center’s standard of care
• Failure to achieve fistula maturation
• Incomplete vessel ligation when using embolization coil to direct flow
• Steal Syndrome
• Hematoma
• Infection or other complications
• Need for vessel superficialization or other maturation assistance procedures.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Important Information: Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device.
14 Vascular Specialist | June 2023
UC202301636 EN ©2022 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands
Welcome to your 2023 Vascular Annual
special
section
Meeting
preview
QUALITY TAKES CENTER STAGE AT VQI MEETING, INITIATIVE MAKING MUCH OUT OF ZERO
By Beth Bales
OVER THE PAST SEVERAL MONTHS, the Society for Vascular Surgery’s Vascular Quality Initiative (VQI) hit some important milestones, including registering procedure number 1,000,000 in its database, enrolling center number 1,000, as well as 100-plus quality charters initiated in 2022. There are also the 10,000-plus new procedures added to the clinical registry every month.
In short, VQI has collected a lot of zeros. But it’s about more than collecting: it’s about how the members of the VQI use these zeros to become quality improvement heroes.
This year’s VQI@VAM—the VQI’s annual meeting—will celebrate those zeros. But the meeting is about so much more than nothing, said VQI Medical Director Jens Eldrup-Jorgensen, MD.
The meeting will be held from 12 to 5 p.m. Tuesday June 13, followed immediately by the VQI Poster Networking Reception
until 6:30 p.m., and from 8 a.m. to 5 p.m. Wednesday (June 14).
Highlights of this year’s VQI@VAM include the launch of a new national initiative, the announcement of scholarships for vascular fellows pursuing quality improvement projects, an update on that program, announcement of VQI participation awards, and the popular reception on Tuesday evening.
VQI launched the Fellows in Training (FIT) initiative in 2022 and will graduate its first set of graduates during the meeting. FIT is a 12- to 18-month program for individuals completing their medical residencies or fellowships in any vascular disease-focused specialty, such as vascular surgery, cardiology, radiology or vascular medicine.
It fosters an understanding of quality processes and metrics through mentorship in the VQI. The program is a collaboration with the Association of Program Directors in Vascular Surgery (APDVS), American College of Cardiology (ACC) and the Society for Vascular Medicine (SVM).
Gary Lemmon, MD, an integral part of FIT, will announce the winners of the Jack Cronenwett Scholarship, permitting these recipients to continue for a second year.
The update and awards will be from 11:15 to 11:40 a.m. Wednesday (June 14).
The scholarships are named for Cronenwett not just because of everything he means to vascular surgery and quality initiatives, but also because he has already mentored so many physicians, said Eldrup-Jorgensen. “It’s a part of his career that should be publicized and applauded. It’s not just his work with quality, but because of the excellent mentor he was to generations of current excellent vascular surgeons.”
Cronenwett, in fact, will meet with the first group of FIT Scholarship Award winners Thursday morning (June 15). He founded the New England Vascular Study group which led to the creation of VQI, and received the Lifetime Achievement Award at VAM 2016.

Eldrup-Jorgensen is saving FIT program highlights for Adam Johnson, MD, chair of the FIT Committee, to discuss at the meeting, but said assessments of the program from participants and mentors indicated the experience was beneficial. “The true indication that it really did work was when so many people applied for the Year Two scholarship,” he said.
On the Wednesday morning of the meeting, Lemmon will announce a new major initiative on smoking cessation.
Prior to that presentation, Cassius I. Ochoa Chaar, MD, will discuss “A Comprehensive multidisciplinary inpatient-based approach to smoking cessation for patients with vascular disease” at 10 a.m. Wednesday.
Priscilla Callahan Leone of the Food and Drug Administration (FDA) follows as a guest speaker from 10:08 to 10:28 a.m.” Lemmon will present more on the initiative until 10:45 a.m.
“Smoking cessation is of primary importance to patients, particularly vascular patients, who are particularly affected by smoking,” said Eldrup-Jorgensen.
He and SVS Patient Safety Organization Director of Quality Betsy Wymer will introduce a patient toolkit physicians can use to help their patients quit smoking. Underscoring the topic’s importance, VAM 2023 includes smoking cessation in two abstract presentations.
VQI@VAM’s Tuesday sessions include panel discussions on a variety of topics as well as drilling into semi-annual regional reports. Wednesday sessions are designed for physicians, nurses, data managers, quality improvement professionals and administrators. View the full meeting agenda at vascular.org/OnlinePlanner23. Organizers point out that VQI@VAM requires a separate ticket from VAM registration.
Thank
3M, Abbott, Boston Scientific Corporation, CompHealth, CVRx, Janssen Pharmaceuticals, Medtronic, Philips, Penumbra, Shockwave, Surmodics, Teleflex,
Abbott, Boston Scientific Corporation, Cook Medical, Cordis®, Medtronic and W L Gore
15 www.vascularspecialistonline.com
VQI@VAM
Exhibitor Booth No 3M Health Care 902 3M Health Care Meeting Suite 1333 Abbott 517 Advanced Oxygen Therapy Inc 611 Aidoc 1011 ALPINION USA 1028 Amputee Associates 1006 AngioAdvancements 502 AngioDynamics 516 Aroa 1126 Artivion 1102 BD 827 BIBA Medical 1128 Bipore Medical Devices Inc 1027 BLOXR Solutions 631 Boston Scientific 527 Cardiovascular Systems, Inc 606 Cedaron Medical 533 Centerline Biomedical, Inc 513 CompHealth 931 Cook Medical 711 Cordis® 806 Exhibitor Booth No Teleflex 534 Terumo Aortic 817 The Permanente Medical Group 511 Thompson Surgical Instruments Inc 834 TRUVIC Medical 632 UltraLight Optics Inc 1116 Vascular Technology Inc 602 Viz ai 910 VQI (Vascular Quality Initiative) 512 Wexler Surgical, Inc 707 Ziehm Imaging 903 Exhibitor Booth No Core Sound Imaging 1010 CutisCare 911 CVRx 503 CVSUSTAIN 1026 Designs for Vision Inc 627 Elsevier Inc 706 Endologix Meeting Suite 629 Endovascular Today 807 Fivos (formerly Medstreaming) 803 Forme Financial 804 Getinge 1127 Gore & Associates 717 Hackensack Meridian Health 1131 Hayes Locums 1106 Humacyte Inc 610 Inari Medical 703 iThera Medical 1012 Janssen Pharmaceuticals Inc 506 Janssen Scientific Affairs, LLC 1115 Koya Medical 1103 LeMaitre 811 LifeNet Health 1002 Exhibitor Booth No Medistim 1118 Medtronic 917 Mercy Clinic 528 MiMedx Group Inc 1107 Mindray 1112 Nectero Medical 1105 Penumbra Inc 727 Philips 802 ProgenaCare 1121 Remington Medical, Inc 927 Ronin Surgical Corp 514 Rooke Products by Osborn Medical 906 Scanlan International Inc 907 Shape Memory Medical 526 Shape Memory Medical Meeting Suite 1337 Shockwave Medical 702 Silk Road Medical 603 Society for Vascular Ultrasound 1117 Softek Illuminate 1003 SurgiTel 1007 Surmodics, Inc 507 Tactile Medical 505 A-Z EXHIBITOR LIST
INDUSTRY@VAM
You
Vascular
Terumo
&
Educational
to Our Sponsors!
Annual Meeting Sponsorships:
Aortic and W L Gore
Associates, Inc
Grants:
& Associates, Inc
Jens EldrupJorgensen
PEDIATRICS

Assessing the needs of young vascular patients
By Beth Bales
IN THE PAST 18 MONTHS, A JOINT TASK FORCE studying the needs of pediatric vascular patients has made great strides, conducting a needs assessment, identifying top priorities and planning resources for surgeons of these young patients. Next up: a meeting in June for interested members from both the Society for Vascular Surgery (SVS) and the American Pediatric Surgical Association (APSA). SVS members Dawn Coleman, MD, and John White, MD, co-lead for the SVS; Regan Williams, MD, and David Rothstein, MD, co-lead for APSA.
The June meeting of what will be known as the Pediatric Vascular Surgery Development Group will be held from 12 to 1:30 p.m. Thursday at the Gaylord National Resort and Convention Center, with interested SVS members able to attend live during the SVS Vascular Annual Meeting and APSA members attending virtually. All four co-leaders anticipate the group then beginning to meet quarterly.

It’s the first-of-its-kind get-together at an annual meeting to gather both vascular and pediatric surgeons. “We’re looking for input from everyone who has interest or experience in taking care of children with vascular disease,” said White.
Since its formation in late 2021, the task force has conducted a thorough needs assessment and prioritized four pillars of critical care: blunt and penetrating vascular trauma, iatrogenic trauma, hemodialysis access and ECMO (extracorporeal membrane oxygenation) life support.

Trauma isn’t the No. 1 issue in pediatric vascular patients, but it is one of the most common and, importantly, it’s often a time-sensitive challenge, according to Coleman. “Delays in care can compromise the long-term and functional outcomes of children, in addition to immediate life and limb threat,” she said. “Thus, it’s a high-impact focus for our team.”



Many more invasive procedures on the heart and kidneys occur now than 10 and 20 years ago, said White. “Patients will have complications from these procedures, and we need to be prepared to address them.” ECMO has been used more frequently with children recently, especially during COVID-19, but also for the wide variety of respiratory disorders children acquire. “Its usefulness in children has been proven and it requires vascular cannulation. So we, again, need to explore best techniques,” White added.
Williams and Rothstein said the task force grew out of several themes. For one, general surgery residents (and consequently graduating pediatric surgery fellows) have progressively less and less exposure to vascular surgery, particularly open surgery. As a result, practicing pediatric surgeons, especially early-career ones, have little experience in vascular

surgery operations. Additionally, vascular surgery diseases are rare in pediatric surgery but opportunities for collaboration with vascular surgeons abound. “The best care for our pediatric patients is often provided in a collaborative fashion and we can improve this collaboration by defining areas of common ground, breaking down administrative barriers and measuring our outcomes,” they said.
In the works are supplements from each organization’s journals that will include a set of descriptive disease-specific articles and access to a physician toolkit. Task force members want to accelerate developing best practices for these critical domains already identified and eventually expand into other areas and engage stakeholders from other disciplines.
“It’s clear that there’s opportunity for this multidisciplinary collaborative to engage stakeholders from other disciplines that care for pediatric patients with vascular disease,” said Coleman. “The landscape of care provision is variable based on local resources—for example, a free-standing children’s hospital where there may be no vascular surgical support ... We could be setting the foundation for a truly powerful, multidisciplinary team as this group moves forward.”
All four co-leaders are excited about the inaugural interest group meeting at VAM and the collaboration between APSA and SVS. “We acknowledge that most collaborative care is local, and rather than defining how this process should work in a uniform manner for every hospital, we have focused on defining the problem (where might a vascular surgeon provide expertise?), stimulating conversations in our respective societies, providing some care guidelines for specific disease processes, and facilitating collaboration by exploring logistical/administrative/dogmatic barriers to sharing care of relevant patients,” according to Rothstein and Williams.
“We are so excited to collaborate with APSA,” said Coleman. “The joint task force truly prioritizes these special patients, acknowledging that vascular challenges and needs will look different at different centers.”
Vascular Specialist | June 2023
“Delays in care can compromise the longterm and functional outcomes of children, in addition to immediate life and limb threat”
16 Platinum Gold Silver Bronze Booth No 527 Booth No 717 Booth No. 503 The Society for Vascular Surgery would like to thank the following companies for their support of the 2023 Vascular Annual Meeting and participation in the SVS Industry Alliance Program. Thank
Partners! Booth No. 917 Booth No. 517 Booth No. 711 Booth No. 611 Abbott Shockwave Medical Philips Janssen Pharmaceuticals, Inc. 3M Health Care Cook Medical Advanced Oxygen Therapy Inc.
DAWN COLEMAN
You SVS Industry Alliance
VASCULAR NURSING
SVN CONFERENCE TO FOCUS ON MEDICINE, WELLNESS
By Beth Bales
The 41st annual Society for Vascular Nursing (SVN) Conference will stress not only the care of vascular patients, but also the care of nurses themselves, as well as building the entire community of vascular nursing.
“I’m a firm believer that we really have to talk about the whole person, the essence of our being,” said SVN President Nancy Crowell, RN. “We often forget that topic in healthcare.”
Thus, mental health takes the spotlight at the conference, set for 8 a.m. to 5 p.m. the Wednesday and Thursday (June 14 and 15) of the meeting, in conjunction with the Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM). The SVN conference requires a separate registration.
Both the keynote and closing addresses focus on mental health, and Crowell will help lead guided meditation, which includes slow, flowing movements, and deep rhythmic breathing.
Apoorva Katikaneni, manager of grants and partnership at Hope for the Day, an
organization which focuses on suicide prevention and mental health, will present the opening address.
Other highlights of the two-day conference include:
◆ Sessions with the SVS Physician Assistants Section, including the new—and highly-anticipated—“Jeopardy Cases Over Cocktails,” with teams vying for highest score on vascular-related topics. The two groups also will enjoy a “grab-and-go” lunch Thursday, and a question-and-answer discussion on topics of joint interest
◆ The SVN reception, which follows “Jeopardy”
◆ A compression station, highlighting wound care
◆ An overview on imaging by the Society for Vascular Ultrasound, plus a station on Doppler ultrasounds
◆ Several breakout sessions on research and practice management, and professional development. Topics
RELAX, RECHARGE AT OPENING DAY SOCIAL EVENT
OPENING DAY AT THE SOCIETY FOR Vascular Surgery’s Vascular Annual Meeting (VAM) starts with an educational session for the World Federation of Vascular Societies (WFVS), moves on to the Opening Ceremony, followed by a very full day of abstract and education sessions, meetings, industry symposiums and more.
With VAM Wednesday (June 14) packed full of important vascular topics, what better way to unwind and to celebrate the kick-off of this epic four-day gathering of vascular professionals than with fun?
New this year is the first SVS Connect@ VAM: Building Community, a free, blockparty-style and family-friendly networking event that will include food and beverages, games, entertainment and activities. The event is sponsored by Terumo Aortic and Philips.
“People will have spent the day moving from place to place to place, all to catch as many of the sessions we’re offering as they can,” said Andres Schanzer, MD, chair of the SVS Program Committee, which plans much of VAM’s educational content.
“We’re introducing this event—and we want it to become a new tradition—to encourage everyone to take a breath, or seven, and relax and recharge. Bring the family if they’re along for the trip. Bring yourself!”
“It’s strictly casual,” he emphasized.
“Save the fancy clothing for Friday’s ‘Great Gatsby’ Gala on Friday night,” he said. “We want people to focus on kicking back and visiting with some of the people they haven’t seen since last year’s VAM. The emphasis is on family and fun.”
Though the event is free, tickets are required and may be obtained when registering for VAM. Those already registered can include “Connect” tickets at vascular.org/VAM23reg
VISIT ‘SVS CENTRAL’ FOR HEADSHOTS
THE SOCIETY FOR VASCULAR SURGERY (SVS) WILL ONCE AGAIN OFFER VAM attendees the opportunity to receive free headshots. These professional headshots are a valuable addition to any professional’s career portfolio. The headshot booth will be open from 10 a.m. to 2 p.m. on Thursday and Friday of VAM 2023, close to “SVS Central,” the Society’s own member resource. Both will be just outside the entrance to the Exhibit Hall.
“We urge all of our members who don’t have an up-to-date professional photo to stop by and have their photos taken,” said SVS Communications Committee Chair William Shutze, MD. “A photo helps demonstrate your professionalism, either of you alone, or as part of your practice or division.”
include a review of the characteristics of individuals admitted for major amputations; an update on the “Systemic review of the prevalence of undiagnosed peripheral artery disease (PAD) among patients undergoing orthopedic procedures”; heterogeneity in response to supervised exercise therapy in PAD; for professional development, developing a vein practice; demonstrating the value as a vascular advanced practice provider (APP); and developing a journal submission
◆ Hands-on workshops focusing on venous and arterial issues
◆ Presentations by nurses, nurse providers and surgeons on vascular disease and treatment
◆ The Presidential Luncheon from 12:30 to 1:30 p.m. on Wednesday June 14 and the closing ceremony and awards from 4:45 to 5 p.m. Thursday June 15. Leslie Green, a patient, will present the closing keynote address, “Reflecting Love”
Vascular nurses practice in a variety of settings and
VASCULAR LIVE@VAM
BE SURE TO SAVE TIME FOR THE Vascular Live theater-in-the-round presentations, with exhibitors presenting new ideas, new technologies and discussing the latest trends in vascular surgery. All Vascular Live events take place in Exhibit Hall C. These are not CME eligible.
Thursday, June 15
10 to 10:25 a.m.
Sponsored by W.L. Gore & Associates “Optimizing therapy solutions with Gore branched technology”
Speaker: Kendal M. Endicott, MD
12:15 to 12:40 p.m.
Sponsored by CVRx
“Baroreflex Activation Therapy: A novel extravascular procedure for heart failure”
1 to 1:25 p.m.
Sponsored by Shockwave
“Going big with IVL: The new Shockwave L6 in the treatment of calcified iliac and common femoral arteries”
Speakers: Karan Garg, MD, and Michael Siah, MD
3 to 3:25 p.m.
Sponsored by 3M
“Exploring the current state of amputation site management: Strategies to prevent surgical site complications”
Speaker: Ellen Dillavou, MD

the conference aims to meet all attendees’ needs, Crowell said. “I think we’ve done that. We have other associations collaborating with us, and we’ve created a robust agenda, with a great lineup of speakers providing updates on advancements in vascular healthcare and state-of-theart technology.”
SVN also strives to build the SVN community by enhancing relationships among the members.
“We solidify our knowledge and the skills we’ve gained, but we also talk about some of the things we don’t talk about. We’re not only addressing aortic, carotid and revascularization topics, we’re talking about holism in healthcare and providing practical applications to create and sustain inner peace. I think it’s going to be fantastic.”
Learn more about the meetvascular.org/SVN23
All registrants will have access to recordings of sessions held in the main ballroom at a later date.
5:15 to 5:40 p.m.
Sponsored by Penumbra
“Lightning Bolt 7 and 30-day results of the STRIDE study on thrombectomy for acute limb ischemia”
5:50 to 6:15 p.m.
Sponsored by Teleflex “MANTA® Vascular Closure Device”
Friday, June 16
9:30 to 9:55 a.m.
Sponsored by Abbott
“JETi Hydrodynamic Thrombectomy: A dual-action design for efficient clot removal”
Speaker: Kevin Onofrey, MD
12:15 to 12:40 p.m.
Sponsored by W.L Gore & Associates
“Treating complex PAD: Evolving evidence informing my approach”
Speakers: Steve Abramowitz, MD, and Caitlin Hicks, MD
1 to 1:25 p.m.
Sponsored by Shockwave
“The new Shockwave L6 for large-bore access and the treatment of calcified iliac arteries”
Speakers: Nicolas Mouawad, MD, and Leigh Ann O’Banion, MD
3 to 3:25 p.m.
Sponsored by Abbott
“Unlocking potential: Maximizing the benefits of the interwoven Supera stent”
www.vascularspecialistonline.com
17
Compiled by Beth Bales and Marlén Gomez
Nancy Crowell
SOCIETY BRIEFS
Leadership in challenging times topic of July 12
‘SVS Presents’
THE SOCIETY FOR VASCULAR Surgery’s next “SVS Presents” webinar will focus on the concept of leadership during challenging times.
The first of two webinars will be from 7 to 8 p.m. CDT July 12 and will feature two disaster preparedness experts who will discuss their experiences during the mass-casualty incidents of the Boston Marathon bombing and the Las Vegas mass shooting.
This interview-style webinar will include topics on how leaders can prepare their institution, lessons learned, post-traumatic stress disorder (PTSD) and how to involve trainees in preparedness.
April Boyd, MD, chair of the SVS Education Committee will moderate. Speakers are:
◆ Deborah Kuhls, MD, chief of trauma at University Medical Center’s Adult and Pediatric Trauma Centers in Las Vegas— Nevada’s only level 1 adult and the state’s only pediatric trauma center. She was in-house during the 2017 Las Vegas shooting and acted as senior surgeon. She teaches multiple disaster preparedness courses

◆ Susan Briggs, MD, a senior surgeon at Massachusetts General Hospital and team commander of the United States Trauma and Critical Care Team for the U.S. Department of Health and Human Services National Disaster Medical System. Briggs has participated in numerous domestic and international disasters ,such as the World Trade Center terrorist attack, earthquakes in Haiti and Iran and Hurricane Katrina
Each will speak about their particular incidents and provide a variety of resources related to disaster preparedness. Moderator questions will be based on the methodology of the SVS Leadership Development Program.
The SVS will also provide a variety of ongoing resources related to disaster preparedness.
The second webinar is set for 7 to 8 p.m. CDT Oct. 4 and will focus on leading during natural disasters like hurricanes, earthquakes and pandemics.
Learn more about the SVS Presents series and these particular webinars at vascular.org/SVSPresents
 Compiled by Beth Bales and Marlén Gomez
Compiled by Beth Bales and Marlén Gomez
DEVELOPMENT OF MVPs FOR VASCULAR SURGEONS
By Evan Lipsitz, MD, Caitlin W. Hicks, MD
WITH THE INTRODUCTION OF THE MEDICARE ACCESS AND CHIP Reauthorization Act of 2015 (MACRA), the Centers for Medicare & Medicaid Services (CMS) required physicians to participate in the Quality Payment Program (QPP) to be eligible for a bonus and avoid penalties. Physicians were required to report various measures through either the Merit-based Incentive Payment System (MIPS) or an Alternative Payment Model (APM). The vast majority of vascular surgeons report through MIPS.

Beginning in 2023, CMS added MIPS Value Pathways (MVPs) as a new way to report that is designed to “align and connect measures and activities across the quality, cost and improvement activities performance categories of MIPS.” The SVS Quality and Performance Measures Committee (QPMC) represents member interests regarding national quality reporting and has taken a lead role in developing these pathways for the vascular community.
The MIPS reporting measures consist of four performance categories, each of which comprises a percentage of the total score. These categories are quality (30%), cost (30%), improvement activities (15%) and promoting interoperability (25%). The concept of the MVP framework is to align these four categories of measures and activities for a given specialty or condition.
Specialty-specific measures will be chosen to provide fewer and more targeted measures with enhanced and more granular feedback. Some activities, such as promoting interoperability, are common to all specialties and conditions, and these are viewed as foundational across all MVPs. Reducing administrative burden on physicians with an increased use of claims-based data, especially those relating to population-based health, is another tenet of MVPs.
MVP development instructions and criteria are available on the CMS QPP website. There are several options for creating one or more MVPs within a given specialty. One option is to create a broad-based MVP in which all specialists could participate. The other option is to create several more condition-focused MVPs, at least one of which would be available and relevant to all physicians within a specialty. The SVS has chosen the latter approach, as it aligns more directly with CMS aims.
Specific information to be included in an MVP includes general concepts such as a clearly defining measurement intent, linking of measures and activities, incorporating the patient voice, and supporting digital measure reporting. Quality measures (four in total) must include at least one outcome measure, and outcome measures that can be captured from a Qualified Clinical Data Registry (QDCR) and/or be electronically reported are preferred. Cost measures should be specific to the condition of focus or, if not, be applicable to all physicians in the specialty. Improvement activities (two to four) should improve quality of care and be complementary to the other measures within the MVP. Interoperability includes standard MIPS interoperability measures, as well as population health measures.
There are currently 12 active MVPs available for the 2023 performance year, but none for vascular surgery as of yet. The SVS QPMC is actively supporting the development of a few MVPs that are specific to disease conditions relevant to vascular surgery. MVP submission and review is a multi-step process with ongoing CMS feedback. The MVP registration window for the 2024 performance period runs from April 3 through Nov. 30, 2023. For those planning to report Consumer Assessment of Healthcare Providers & Systems (CAHPS) for the MIPS survey as a measure, registration is due by June 30. More information is available at qpp. cms.gov, qpp.cms.gov/mips/mips-value-pathways and qpp.cms.gov/mips/ mips-value-pathways/submit-candidate
EVAN LIPSITZ is the chair and CAITLIN HICKS the vice chair of the SVS QPMC.
Officer voting open, ends during VAM
VOTING IS UNDER WAY FOR ELECTION of Society for Vascular Surgery officers for 2023–24 and will continue through 2 p.m. CDT Thursday, June 15.
This is the first year that voting not only takes place for such an extended period of time, but also during the SVS Vascular Annual Meeting (VAM). Up for election this year are the offices of SVS vice president and treasurer. Candidates are, for vice president, succeeding to president-elect and then president, are Kellie Brown, MD, and Keith Calligaro, MD. Candidates for treasurer are Thomas Forbes, MD, and Palma Shaw, MD.
Voting is a privilege restricted to Active and Senior members in good standing, who may vote only once. SVS members had a chance to “Meet the Candidates” at a town hall May 17. A recording of that session is available at vascular.org/CandidateWebinar Candidates’ biographies and answers to specific questions are available at vascular.org/23_24Candidates
“We hope that by extending the voting period and offering it during VAM, with hundreds of members in attendance, more people will take advantage of the chance to let their voices be heard,” said Nominating Committee Chair Kim Hodgson, MD. “It’s our members’ opportunity to impact the future direction of our Society.”
Leaders hope that more than 1,000 members cast votes in the elections this year. Election results will be announced during the Annual Business Meeting Saturday, June 17, at VAM.
UCLA-SVS vascular review course set for Aug. 23–26
THE ANNUAL “COMPREHENSIVE REVIEW and Update of What’s New in Vascular and Endovascular Surgery” is set to take place Aug. 23 to 26 at the Beverly Hilton, in Beverly Hills, California.
The 3.5-day review course is a joint effort of the Division of Vascular and Endovascular Surgery at the University of California, Los Angeles (UCLA) and the SVS.
It provides an in-depth review of the vascular specialty for those preparing for Vascular Surgery Board examinations and also provides basic didactic education for vascular residents and fellows in training.
The course recognizes the four major pillars of vascular surgery practice: open surgery, endovascular interventions, medical management, and diagnostic imaging and noninvasive testing.
For more information on the meeting— including the schedule and faculty—visit vascular.org/UCLAReviewCourse23
18
Vascular Specialist | June 2023
Evan Lipsitz Caitlin Hicks
FOR THE TREATMENT OF JUXTARENAL AORTIC aneurysms, reinterventions after physician-modified endovascular grafts (PMEGs) are non-detrimental to long-term survival. This is one of the main findings of a recently published study by Ayumi Tachida, MS, a medical student from the University of Washington School of Medicine in Seattle, senior author Sara Zettervall, MD, an assistant professor of vascular surgery at the institution, and colleagues.
The authors note in their Journal of Vascular Surgery (JVS) paper that the frequency and impact of reintervention on mortality after PMEGs is unknown. It was the aim of the present study, therefore, to describe reinterventions after PMEG for the treatment of juxtarenal aneurysms and their effect on survival.

Tachida et al used 11 years’ worth of data (2011–2022) from a prospective investigational device exemption clinical trial in their study. The trial in question—officially titled “Physician-modified endovascular grafts for the treatment of elective, symptomatic or ruptured juxtarenal aortic aneurysm: An investigator-initiated study”—is being led
by Benjamin Starnes, MD, chief of vascular surgery at the University of Washington, who is also a co-author of the JVS paper. The trial aims to enroll 300 participants with an estimated completion date of January 2035.
On the Clinical Trials webpage for the study, it is noted that not all patients presenting with symptomatic or ruptured aortic aneurysms are candidates for endovascular repair, with the reasons for exclusion noted to predominantly involve a lack of a suitable proximal aortic neck. “Solutions to this problem involve multi-branched or ‘fenestrated’ endografts, which are being assessed in other clinical trials,” the study description continues. However, it is highlighted that grafts in these trials require between six and 12 weeks to manufacture and deliver to the investigational site.
“We seek to evaluate the safety and efficacy of PMEG using a US Food and Drug Administration [FDA]-approved, off-the-shelf device in order to increase the applicability of these technologies to more patients and, thus, save more lives,” the description reads.
In the study reported in JVS, reinterventions after PMEG were categorized as open or percutaneous and major or minor by Society for Vascular Surgery reporting standards ,according to high or low magnitude based on physiological impact. The investigators also categorized reinterventions by timing, as well as those interventions which occurred within one week of PMEG. They compared survival between patients who did and did not undergo reintervention and between reintervention subcategories.
Writing in JVS, Tachida and colleagues relay that a total of 170 patients underwent PMEG in the 11-year study time period, 50 (29%) of whom underwent a total of 91 reinterventions (mean reinterventions/patient, 1.8). They
report that freedom from reintervention was 84% at one year and 60% at five years. The authors specify that reinterventions were most often percutaneous (80%), minor (55%) and low magnitude (77%), with the most common reintervention being renal stenting (26%). They also note that there were 10 early reinterventions within one week of PMEG and that two aortic-related mortalities occurred after reintervention.
A RECENT PROPOSAL HAS CALLED FOR “whole-person, multidisciplinary interventions” after an interrogation of the interplay between lower-extremity peripheral arterial disease (PAD) and mental health impacts. Published in the Journal of The American College of Cardiology, researchers have put forward a “biopsychosocial” PAD management roadmap, offering solutions to current care “obstacles” to better attend to both behavioral and social health needs throughout vascular treatment.
Kim G. Smolderen, PhD, clinical psychologist, and Carlos Mena-Hurtado, MD, interventional cardiologist, from Yale University in New Haven, Connecticut, et al note that with PAD, multimorbidity is “common,” which includes both mental and physical health conditions that the authors assert disproportionately affect underrepresented groups and younger people.
Rooted in “maladaptive” health behaviors, the authors believe these conditions are directly undermining cardiovascular health and disease management. This link can be seen between depression and anxiety in the context of endovascular and surgical revascularization.

The “PAD experience,” including the clinical and financial burden of the comorbid conditions linked with PAD, have received “little consideration,” Smolderen, Mena and colleagues opine. This lack,
they believe, is driving the need to “expand the PAD care paradigm” to a biopsychosocial one, which acknowledges the contribution of both behavior and psychosocial factors on disease management. Their roadmap identifies four domains that, once addressed, can redesign vascular specialty care services to attend to behavioral and social health needs. Identifying first a “fundamental problem” in the way physical and mental health are divided when providing care, the authors pinpoint the “major advocacy efforts” that are needed across professional organizations serving populations of PAD.
Second, they outline the evolution of highly technological and procedural care which has been “valued disproportionately” over preventative and psychosocial care in the context of reimbursement and code availability. To rectify this, they believe payment reform is needed.
Third, the authors emphasize that despite several evidencebased interventions stemming from high-level randomized controlled trial evidence, there is a prevalent “lack of awareness” among health administrators regarding the “scope of practice” of allied mental and behavioral health physicians in the context of chronic disease. They believe adopting a formal approach to assessing behavioral health could be a solution.
Their fourth area of proposed reform concerns integrated behavioral care practice guidelines for PAD management, and the formation of better evidence-based workflows derived from comparative effectiveness and implementation research.
There were no differences in survival between patients who underwent reintervention and those who did not, Tachida et al communicate. However, they continue, survival differed based on the timing of reintervention.
Tachida and colleagues further share that, after adjusted analysis, reintervention within one week of PMEG was associated with an increased risk of mortality, both compared with late reintervention (hazard ratio [HR], 11.1; 95% confidence interval [CI], 2.7–46.5) and no reintervention (HR, 5.2; 95% CI, 1.6–16.8).
Study finds no correlation between TEVAR timing and uncomplicated type B aortic dissection outcomes
WITH PROPER PATIENT selection, short-term outcomes of thoracic endovascular aortic repair (TEVAR) in acute uncomplicated acute type B aortic dissection (uTBAD) are similar irrespective of the timing of treatment after dissection. This is according to a study recently published in the Journal of Vascular Surgery (JVS).
Authors Adam Beck, MD, from the University of Alabama at Birmingham in Birmingham, Alabama, and colleagues write in their introduction that the timing of TEVAR after the onset of uTBAD “remains controversial.” The objective of their study was to evaluate the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) post-approval study data for the impact of TEVAR timing for uTBAD on early and late outcomes.
Patients with uTBAD (n=206), were divided into groups defined by the SVS/Society of Thoracic Surgeons reporting guidelines based on the timing of treatment after the onset of dissection. The three groups were: treatment within 24 hours (n=8), between one to 14 days (n=121) and between 15 to 90 days (n=77).
The authors report that postoperative stroke, congestive heart failure and renal ischemia were more common in the <24-hour group
without an increase in mortality. Unadjusted 30-day mortality across the groups, they continue, was lowest in the early TEVAR group (0%, 3.3% and 5.2%; p=0.68), as was the one-year mortality (0%, 8.3% and 18.2%; p=0.06). However, they add the caveat that these results were not statistically different at any time point. Furthermore, Beck and colleagues reveal that reintervention out to three years was not different between the groups, and that multivariable analysis demonstrated the need for a postoperative therapeutic lumbar drain to be the only predictive risk factor for mortality (hazard ratio=7.595; 95% confidence interval 1.73–33.337; p=0.007).
In the conclusion of their study, Beck et al acknowledge that patients with uTBAD treated within 24 hours were unusual (n=8) and the group was “too small for valid statistical comparison.” They add that these patients “likely represent a high-risk subgroup, which is manifested in a higher risk of complications.”
Although there was a “trend” toward improved survival in the acute phase, the researchers summarize that outcomes did not differ compared with the subacute phase with relation to early mortality, postoperative complications, or one-year survival.
www.vascularspecialistonline.com 19
CLINICAL&DEVICENEWS Compiled by Jocelyn Hudson and Éva Malpass
Reinterventions after physician-modified endovascular grafts ‘non-detrimental’ to long-term survival
Sara Zettervall
New ‘biopsychosocial’ roadmap set out to address PAD treatment and mental health impacts

DEEPLY COMMITTED TO INNOVATION SEEING OPPORTUNITY WHERE OTHERS DON’T. W. L. Gore & Associates, Inc. Flagstaff, Arizona 86004 goremedical.com Products listed may not be available in all markets. GORE, Together, improving life and designs are trademarks of W. L. Gore & Associates. © 2023 W. L. Gore & Associates, Inc. 231001708-EN MAY 2023 See the latest innovation in aortic branch technology We are continuously innovating to overcome yesterday’s limitations, creating new possibilities in care for your patients.


 By Beth Bales
By Beth Bales


















 By Margaret C. Tracci, MD
By Margaret C. Tracci, MD

































 Distal Tip
† Compared to surgical arteriovenous fistulas.
Distal Tip
† Compared to surgical arteriovenous fistulas.











 Compiled by Beth Bales and Marlén Gomez
Compiled by Beth Bales and Marlén Gomez



