Laser atherectomy redefined Breakthrough 355nm technology opens next frontier in treatment
www.vascularspecialistonline.com


Laser atherectomy redefined Breakthrough 355nm technology opens next frontier in treatment
www.vascularspecialistonline.com

Luis R. Leon Jr., MD, reviews the historical trajectory of laser in medicine and the development of the Auryon laser system.
Laser technology was not the invention of a single person, but rather the culmination of more than a century of ideas and observations. Laser— which stands for light amplification by stimulated emission of radiation—and its technological roots can be traced back to the start of the 20th century. Since then, laser technology has exponentially spread and is now widely encountered in our dayto-day lives.
The first time the technology was applied in human medicine was in New York in 1961, when it was used to treat an eye tumor. The tumor was destroyed with the use of a single pulse that lasted a thousandth of a second. Since then, laser has been used in several areas of human health, including in the treatment of cardiovascular disease. Among these disease states, laser is now commonly used in minimally invasive treatment of peripheral artery disease (PAD).
the original Excimer laser, including bulky capital equipment with long warm-up times, highly energetic and chaotic interaction in contrast media, and arterial wall damage caused by the laser photon energy. Indeed, the initial pursuit of laser atherectomy led to high dissection, perforation and bailout stenting rates. For these reasons, the Auryon Atherectomy System (AngioDynamics) was a welcome addition to the laser atherectomy space.

Every year, new devices are being added to our existing armamentarium in our fight against PAD. Although balloon and stent technologies have rapidly evolved over the last few years, some lesions remain difficult to treat with traditional percutaneous transluminal revascularization techniques.
Atherectomy is a common technique for the revascularization of PAD patients. Several devices have progressed the field of atherectomy since the initial excitement of early atherectomy devices in the mid1980s, including laser technology.
The CVX-300 Excimer laser system (Philips) was the first on the market for vascular applications. Using XeCl gas excitation to generate light energy at 308nm wavelength, the Excimer laser produces high-pulse energy to abalate tissue in front of the catheter, ultimately breaking down plaque into harmless microscopic particles. However, multiple logistical and clinical issues remain with
The Auryon system produces high-energy ultraviolent light at 355nm wavelength through an array of optical fibers, transmitting energy at pre-set fluency levels of 50 and 60mJ/ mm2 to the diseased artery. The system works over 0.014” guidewires and has several available catheter sizes. For the smaller catheters (0.9, 1.5 and 1.7mm), there is a centralized guidewire lumen. The larger catheters (2.0 and 2.35mm) have an eccentric guidewire lumen and include aspiration and off-center features (2.35mm only). While the aspiration feature helps mitigate embolic risk, the off-center feature allows for debulking larger arteries beyond the catheter diameter. While most run times last only a few minutes, it is not recommended to exceed 10 minutes of lasing.
In preclinical studies, the Auryon system showed a higher affinity for ablation of fibrotic material over endothelium, thus reducing the risk of vessel injury.1 We began using the Auryon system shortly after its U.S. market release to treat patients with highly complex, calcific lesions in both above- and below-the-knee segments. This included both antegrade and retrograde pedal approaches.
Our group was one of 10 sites that reported results from the PATHFINDER postmarket, multicenter registry. This was a real-world, prospective, single-arm evaluation of the safety and efficacy of the Auryon system for de novo, restenotic and in-stent restenotic (ISR) lesions in the infrainguinal arteries of PAD patients. The mean age of the 102

Auryon
The Auryon system confers multiple logistic, practical, theoretical and real advantages over other available atherectomy systems
subjects included was 68.4±10.21 years; 61.8% of subjects were male; 44.6% had chronic limb-threatening ischemia (CLTI); and 47.3% had tibial lesions. The mean residual stenosis at the end of the procedure was 24.4±15.5%, with a 69% rate of technical procedural success (<30% stenosis). Residual stenoses were similar among subjects with ISR (25.5±14.9%), chronic total occlusion (CTO; 28.1±17%),
and severe calcification (36.5±21.6%) lesions. Mean ankle-brachial index (ABI), Rutherford and Walking Impairment Questionairre (WIQ) scores were improved at both six and 12 months. Ninety-seven of the subjects (95.1%) met the primary safety endpoint of not experiencing a periprocedural major adverse event before discharge.2
In short, the Auryon system confers multiple logistic, practical, theoretical and real advantages over other available atherectomy systems. They can be summarized in seven important aspects.
First, the Auryon system is highly efficient. It includes an intuitive, Windows-based touchscreen in a small console that favorably compares with existing laser competitors.
Second, the system is also highly adaptable to all levels of calcification and lesion composition, and at all location levels.
Third, the Auryon system interacts with specific tissues in a very particular manner that minimizes the chances of arterial perforation.1
Fourth, its very short, <25ns pulse width allows delivery of greater peak power to treat severely calcified lesions.
Fifth, the Auryon technology is nonreactive to iodinated contrast, allowing simultaneous ablation and observation of fluoroscopy images.3
Sixth, the system includes built-in aspiration to minimize distal embolization, avoiding the routine use of embolic protection devices with other available systems.
Last, an increasing number of our patients require pedal access. The very low profile needed for the smaller Auryon devices allows us to treat patients using distal retrograde access and, if desired, the entire intervention can be performed from a distal approach.
Like any other device, the Auryon system has been substantially modified since its inception in 2018. Indeed, in just six years, it has become our main tool for vessel prep because of its minimal preparation and set-up time, ease of use and safety profile— with minimal risks of embolization, vessel perforation, dissections or any other complication.
References
1. Herzog A, et al. Selective tissue ablation using laser radiation at 355nm in lead extraction by a hybrid catheter; a preliminary report. Lasers Surg Med. 2016 Mar;48(3): 281–7
2. Das TS, et al. Solid state, pulsed-wave 355nm UV laser atherectomy debulking in the treatment of infrainguinal peripheral arterial disease: The PATHFINDER Registry. Catheter Cardiovasc Interv. 2024 May;103(6):949–962
3. Herzog A, Steinberg I, Gaisenberg E, Nomberg R, and Ishaaya AA. A route to laser angioplasty in the presence of fluoroscopy contrast media, using a nanosecond-pulsed 355-nm laser. IEEE Journal of Selected Topics in Quantum Electronics 22, no. 3 (2015): 342–347
LUIS R. LEON JR. is a vascular and endovascular surgeon at Sonoran Vein and Endovascular in Tucson, Arizona. He is a paid consultant for AngioDynamics.
Several improvements have been implemented over the last six years, including:
1. Crossing tight up-and-over aortic bifurcations
The Auryon device tip has been modified to make crossing the aortic bifurcation easier.
2. Optimization of catheter tip design
The device has a circumferential blade design to help with pushability, which was optimized for improved performance and reduced friction with guidewires, and markers were added to improve visibility under X-ray.
3. Laser power and number of ablation laser fibers
The 1.7mm catheter contains the most ablating fibers yet in the Auryon catheter lineup. Unlike the larger catheters which have a lumen dedicated to suction, the 1.7mm catheter was able to occupy this space with more fibers, conferring the largest ablation power yet. The addition of the 1.7mm device is a welcome modification that also enables using 0.018” guidewires, which have better support and less chance of kinking, as compared to the 0.014” wires.

The Auryon laser catheters (left to right): the 0.9mm, 1.5mm, 1.7mm, 2.0mm and 2.35mm repectively. The latter two both include aspiration
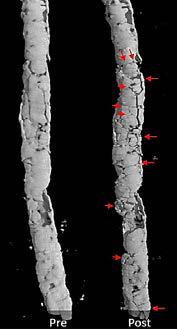
Jason N. MacTaggart, MD, and Robert Tahara, MD, discuss the impact made by the Auryon Atherectomy System on the disruption of medial arterial calcification, drilling into results from a cadavaric study that showed its effects under micro-computed tomography (CT) scanning.
MacTaggart, a professor of surgery in the Division of Vascular Surgery at the University of Nebraska Medical Center and Omaha VA, and adjunct professor of biomechanics at the University of Nebraska Omaha, delves into how micro-CT imaging has placed the outcomes from Auryon laser atherectomy treatment under a far higher-resolution microscope, while Tahara, medical director at Allegheny Vein & Vascular in Bradford, Pennsylvania, provides insight into what those effects look like on the frontlines of clinical practice.
JM: Micro-CT is kind of like regular old CAT [computed axial tomography] scanning, except it is ultra-high resolution. With regular CAT scans, we speak in millimeters; with microCT, it’s micrometers. What we see is a substantially increased resolution for things we want to visualize.
RT: I think that is a really important point because, when compared with the ultrasound that I use routinely, there are a couple of orders of magnitude difference. With clinical ultrasound, it is measured in millimeters or fractions of a millimeter, and what Dr. MacTaggart is talking about is really tens of thousands of that.
JM: It’s stunning when you see these images. When you CAT scan a human, and you see a calcified popliteal artery, you zoom in and you have, maybe, seven or eight pixels that represent the calcified artery in this clinical image. But when you have a micro-CT, you have many more pixels, which allows you to see smaller imperfections. When you’re looking at calcified vessels, you can see what looks like a fingerprint of the calcification pattern versus a blob of white on the clinical CAT scan. So, when you’re thinking about


micro-CT as a research tool in the lab, you can see so much more, particularly when you are looking at calcium. This is important because different calcification patterns might respond differently to different therapies.
What did the micro-CT study of the Auryon laser system’s effects on medial arterial calcification show?
JM: We were originally taught that lasers don’t really do much for calcification. But, when we started this research with the Auryon laser, it became apparent that it does affect calcium. I think we’re just getting started in figuring this out. There are probably going to be different protocols and settings on the machine that you might use to attack calcium versus a clot or neointimal tissue. It’s a really exciting time to be doing this sort of research with tools like micro-CT and lasers. You can’t put a patient in a microCT scanner, but you can put an artery in a micro-CT scanner, then scan that same artery with the clinical CAT scanner, and potentially develop some correlations between what clinical CAT scans show you and what the micro-CTs would look like if you could put that patient in there.
RT: In today’s vascular world, we’re not seeing most things being driven by hard, basic science up front. What we are really seeing is people, like myself and others, tinkering. What we saw empirically with Auryon under ultrasound guidance is that
we were getting these much bigger flow channels. We were seeing a real change in functional compliance, where we were able to use much lower pressure for angioplasties. At that time, it didn’t make any sense, because everybody “knew” that laser “doesn’t work” in calcium. With what we were seeing, the theory then evolved that the effect had to be photoacoustic, like soundwaves. When Dr. MacTaggart’s research was brought to my attention, it showed how well this basic science actually dovetails with the effect that we are seeing [clinically], particularly with the AngioDynamics device.
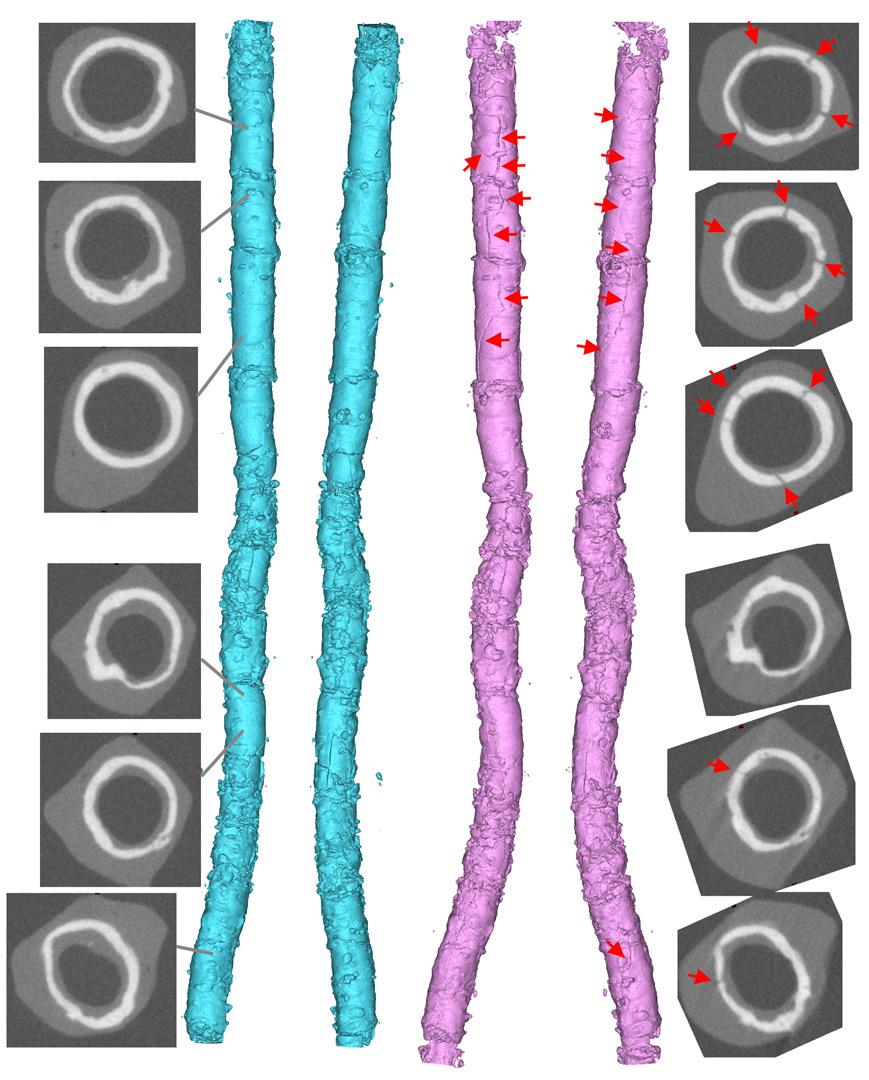
JM: For this study, we used a bank of donated arteries, characterized them mechanically, and imaged them with micro-CT. There were a variety of lesions, from total occlusions to no occlusions with circumferential calcium. We created conditions as close as we possibly could [to a living vessel] on the bench, fully mounting the artery into a module, and then putting it into the micro-CT without disturbing how the artery is stretched or twisted. We treated a number of popliteal and tibial vessels, and found that what people like Dr. Tahara were seeing in the hybrid operating rooms and cath labs—that there is some sort of action occurring on the calcium—was, in fact, occurring. It didn’t happen in all of them, but it was very interesting to compare these pretreatment and post-treatment vessels and see new cracks in calcium from the laser.
“When you have a microCT, you have many, many more pixels, which allows you to see smaller imperfections”
JASON N. MACTAGGART
conclusions can you draw about the device performance and how does it compare to other devices on the market?
RT: Focusing on the calcium issue and why I switched over to the Auryon laser as my workhorse device, we had a notable case that I could not bridge with the Spectranetics laser [now owned by Philips]. What I will sometimes do with these “rock in the road” lesions is take the catheter up, pull the wire back because I can’t cross it, and just sit there and pulverize it. But, in this case, I couldn’t bridge it. So, I brought the patient back a week later and used the Auryon device, and was able to grease through it in about five

minutes. The basic science is that the Auryon laser—because of the physics, characteristics and how it transmits the energy, the wavelength, and so on—actually has more energy delivery at the tip. When you look at it one-to-one against the other clinically widespread-use lasers in the peripheral world, that seems to be the big difference. JM: In our study, we saw that the laser seemed to be dependent upon real blood, and the hematocrit had to be physiological in order for it to work. That was critical for the laser. For instance, if we had a benchtop model that compared the Shockwave [intravascular lithotripsy (IVL)] device to the laser, running saline through the vessel, we’d see that the Shockwave device would crack up calcium but the laser wouldn't [because there was no blood present]. This goes to show how sensitive some of these models are to certain variables and is why it always comes back around to what people see clinically, and the question of does the model fit what we see in patients.
What clinical differences have you noticed?
RT: Until Dr. MacTaggart started the microCT [study], this was completely theoretical on my part: I think the photoacoustic effect of the laser is arguably far more important than any direct effect at the tip of the catheter. That’s why we prefer laser over the other types of modalities because all of the other modalities’ effects are contingent upon them directly excising, sanding or mechanically hitting the disease or the artery itself, whereas the laser has a dual function—photochemical, which is basically ablative, and [photomechanical or] photoacoustic. The secondary gain I have had with the Auryon system is because I [now have the option to] approach everything from a pedal access—unless I have gone the transtibial route. Because the blood flow is pushing everything to the tip of the laser, if we knock something off and embolize things, which is a known
(1) Zilver (Cook Medical) stent under CTA (top) versus micro-CT (bottom). Images courtesy of Alexey Kamenskiy, UNO Biomchanics; (2) Pre- and post-Auryon micro-CT of calcification in a posterior
Proven success in below-the-knee arteries, where





a diverse array of plaque morphology
Venkatesh Ramaiah, MD, chief of vascular and endovascular surgery and complex vascular services at HonorHealth in Scottsdale, Arizona, details how he started using the Auryon Atherectomy System and why it became a mainstay in his treatment of peripheral arterial disease (PAD).
Ramaiah’s experience using atherectomy devices goes back two decades, to a time, he says, when the technology first started to gain traction in PAD practice. Likewise, he first used laser technology at his institute alongside the late internationally recognized cardiovascular surgeon Edward (Ted) B. Diethrich, MD. Over the years, the successor to Diethrich at Arizona Heart Hospital has used a slew of atherectomy devices, from the SilverHawk (Medtronic), to the Jetstream (Boston Scientific), Diamondback (Abbott), Phoenix (Philips) and Rotarex (BD) systems.
As Ramaiah relates, he first started using the treatment modality in the superficial femoral artery (SFA) and popliteal artery segments in the face of stent fractures and high recurrence rates of intimal hyperplasia. “These patients would keep coming back every three to six months,” he explains, with atherectomy combined with drug-coated balloons (DCBs) becoming “the principal modality of treatment for the SFA and popliteal artery over the years.”
It was within this evolving landscape that the Auryon laser system came to the fore, quickly emerging, for Ramaiah’s part, as the standout modality. Ramaiah pinpoints the technology’s simplicity and minimal ancillary equipment among its virtues, explaining how it has become the mainstay treatment in his PAD armamentarium, principally above the knee, but also increasingly for below-the-knee disease. With the Auryon system, he says, the minimal learning curve and, crucially, the chances of embolization evidenced with other atherectomy devices have, thus far, not proven to be an issue.
“We had used lasers in the past, but this was different: it has a much longer
wavelength, 355nm, and a shorter pulse, 10–25ns. It tracks beautifully. It’s very simple to use over a 0.014” guidewire. It reacts and treats all kinds of plaque morphology. Apparently, it has a very high affinity to lesion tissue compared to the endothelium of the vessel. And, of course, the mechanism of action is vaporization of the lesion without causing a large amount of heat generation, and I think that is the fundamental principle of the Auryon laser catheter.”
Ramaiah has been using the Auryon laser system for about five years now, describing how he first introduced the technology to his practice in the outpatient setting and highlighting its reliability, portability and lack of a need for calibration. He started out on short SFA and popliteal lesions, then progressing to chronic total occlusions
“The mechanism of action is vaporization of the lesion without causing a large amount of heat generation, and I think that is the fundamental principle of the Auryon laser catheter”
VENKATESH RAMAIAH
(CTOs) and calcific disease.
His go-to device—the 1.5mm version of the Auryon laser catheter, which goes through a 5F sheath—has become an
important facet of his outpatient practice, he explains, treating lesions in the SFA, common femoral, popliteal and larger tibial vessels. “I’ve noticed that even if you shoot an angiogram after the use of the laser and the lesion may look like you haven’t done much, the laser atherectomy allows much more balloon angioplasty and much more plaque modification, plaque shift, and it creates much larger luminal gain,” Ramaiah explains. “I have not seen any embolization with use of the Auryon 1.5mm laser catheter, nor have I seen any ruptures, dissections or perforations.”
At this juncture, following use of the Auryon technology he tends to stent very little in the SFA-popliteal region, he adds. Turning to the 2.0mm Auryon laser catheter, Ramaiah points to some important highlights. “It has suction attached to it, so, over a 6F sheath, it has additional aspiration along with the atherectomy effect of the laser,” he says. “Again, this is for larger vessels, and also for more complex lesions, especially those with calcification and the possibility of thrombus. In those situations, I use the 2.0 with the suction features so you don’t embolize.”
Over the years, Ramaiah says he and his team’s results with the Auryon device shore up. “They are very good in terms of the laser use without any major complications,” he explains, noting how, below the knee, he now tends to deploy the Auryon 0.9mm laser catheter before balloon angioplasty.
“For below-the-knee vessels, especially in single vessels, especially in calcific vessels, the 0.9 laser tracks really well and gives you complete change in the morphology of the plaque so that after balloon angioplasties— whether you use a cutting balloon, a scoring balloon or just regular balloons—your outcomes are much better. Because the lumen is so small in the below-knee vessels, balloon angioplasty alone can sometimes lead to dissection.”
Ramaiah considers that some of his most notable results have come in cases of instent restenosis (ISR)—the 2.0 and 2.35mm catheters are indicated for ISR.
“When patients have stents in the SFApopliteal [segment], this laser works really well, and if you combine that with DCB, we’ve noticed that these patients do not come back every three months, and the duration of patency of these in-stent treatments has been much better, exceeding almost a year,” he says.
Venkatesh Ramaiah is a paid consultant of AngioDynamics.

Peripheral arterial disease (PAD) is thought to affect an estimated 12–15 million Americans.1 A “gold standard” for management of chronic limb-threatening ischemia (CLTI) is still lacking. For endovascular therapies, current consensus appears to be moving toward avoiding permanent implants, with a focus on debulking followed by drug-coated balloon (DCB) angioplasty. Atherectomy has been shown to have numerous advantages, which include debulking of plaque burden resulting in lower subsequent dissection during angioplasty and need for stenting. 2 Additionally, it may allow for deeper penetration of drugs when using DCBs. This case report from Karthikeshwar Kasirajan, MD, describes the use of a new generation of atherectomy devices that combines atherectomy with simultaneous thrombectomy to minimize risk of distal embolization.
A 55-year-old male with prior history of aortic valve replacement with a bioprosthetic valve was admitted for sepsis and echocardiogram evidence of vegetations in his aortic valve. He subsequently had his aortic valve replaced. Since discharge, the patient has experienced significant lifestylelimiting right calf claudication. The patient did not feel exercising was improving his claudication distance and felt very debilitated by his inability to walk. Arterial duplex imaging demonstrated a right popliteal occlusion, with an ankle brachial index (ABI) of 0.73. The left side was normal with an ABI of 1.09.
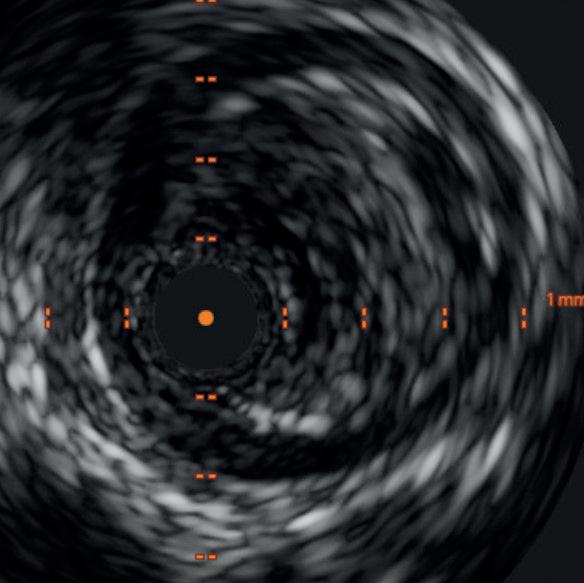
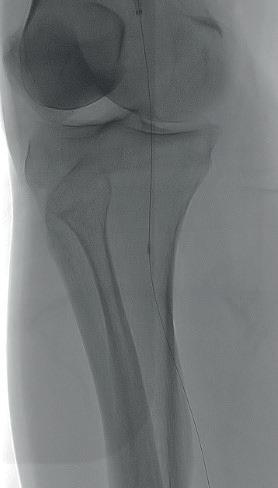
Diagnostic angiogram demonstrated a right popliteal occlusion extending into his proximal tibial vessels (Figure 1). An 0.014” wire was able to easily cross the lesion and pass into the posterior tibial vessel. Intravascular ultrasound (IVUS) demonstrated chronic intraluminal thrombus
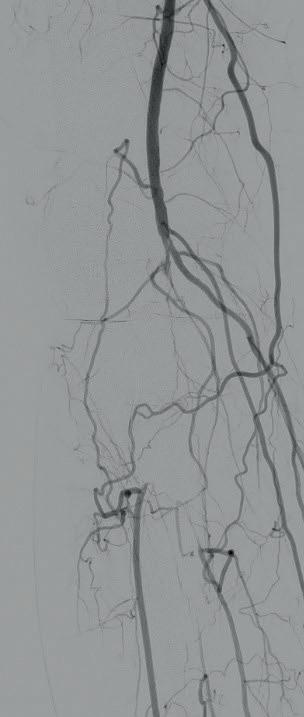
with limited evidence of atherosclerosis (Figure 2). A 2.0mm Auryon laser catheter was used to perform a simultaneous athrectomy-thrombectomy (Figure 3). After a single pass, repeat angiogram demonstrated >70% luminal gain (Figure 4). Low pressure (2atm) balloon angioplasty was then performed (Figure 5). Completion angiogram (Figure 6) demonstrated no residual stenosis, with brisk flow into the posterior tibial vessel. The patient developed a palpable posterior tibial pulse, and, at six months, has no claudication symptoms.
In a study evaluating the pathology of lower extremity atherosclerotic lesions, Narula et al evaluated blood vessels from 239 lower extremity lesions performed for chronic arterial occlusion. Seventy-three percent of the stenotic lesions demonstrated acute or chronic luminal thrombus.3 Zeller et al

Karthikeshwar Kasirajan
investigated the type of embolic debris captured using distal protection during interventions in 123 patients.4 All interventions resulted in debris in the protection device on histological exam. The majority of the lesions on angiogram were not pre-identified as thrombotic. However, 52% of the analyzed material was thrombus. Hence, the ability to address luminal thrombus during peripheral interventions may decrease distal embolization. In the REALITY study, despite the use of distal filters in 97% of patients, 12.8% of patients had clinically significant distal embolization when using a directional atherectomy device.5 In the same study, debris was captured in 93.9% of patients.
The Auryon laser works by vaporizing luminal debris, minimizing the risk of distal embolization. Additionally, the 2.0 and 2.35mm catheters also have a built-in aspiration capability that helps decrease the risk. This represents the new generation of devices that combine aspiration with atherectomy to improve outcomes.
References
1. Cirqui MH, Aboyans V. Epidemiology of peripheral artery disease. Cir Res. 2015;11(9):1509–26.
2. Mittleider D, Russell E. Peripheral atherectomy: applications and techniques. Tech Vasc Interv Radiol. 2016;19(2):123–35.
3. Narula N, et al. Pathology of peripheral artery disease in patients with critical limb ischemia. J Am Coll Cardiol 2018;72(18):2152–63.
4. ZellerT, Schmidt A, Rastan A, et al. New approach to protected percutaneous transluminal angioplasty in lower limbs. J Endovascular Ther. 2013;20(3):409–419.
5. Rocha-Singh KJ, Sachar R, DeRubertis BG, et al. Directional atherectomy before paclitaxel coated balloon angioplasty in complex femoropopliteal disease: The VIVA REALITY study. Cath and Cardovasc Interventions. 2021;98(3):549–558.
KARTHIKESHWAR KASIRAJAN is a clinical professor of surgery at Stanford TriValley in Pleasanton, California. He is a paid consultant of AngioDynamics.






