
5 minute read
Upper Sixth Talks
Figure 1. Electron microscopic image of C. difficile
Bacteria enter the host via the faecal-oral route, yet unless the microbiome is imbalanced, it remains within the gut. CD is acid resistant, so it can survive the low pH environment in the stomach, and it has resistance to some common broad-spectrum antibiotics. CD is present in 2-5% of healthy individuals. Alterations in the gut microbiome can cause CD infection. When the normal gut flora is disturbed, CD faces less competition so they can multiply rapidly and release toxins. The disturbance in the gut can be caused by antibiotics (especially fluoroquinolone), laxatives and recent food poisoning (a lot of the microbiota is lost).
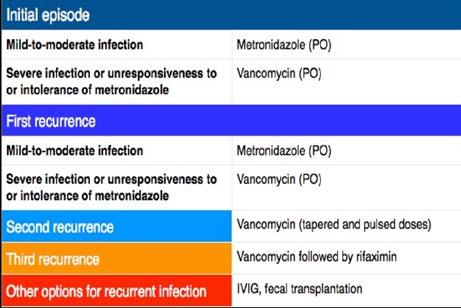
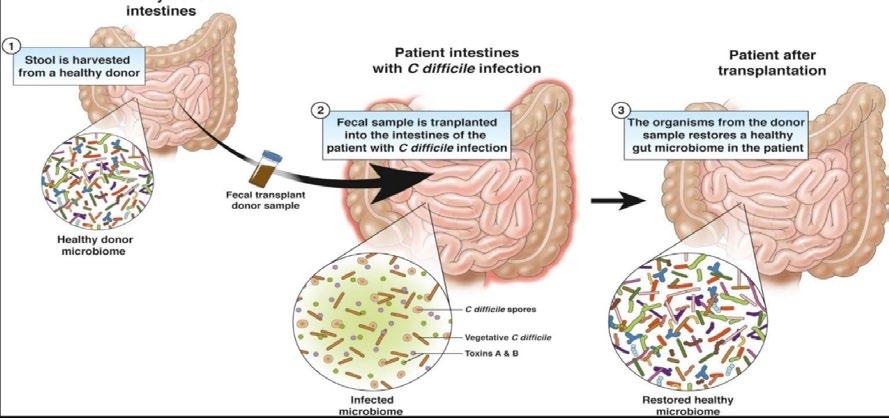
Table 1. The possible course of treatment.
Symptoms are fever, abdominal cramps, nausea, appetite loss and diarrhoea. This is primarily treated with oral antibiotics such as vancomycin. In many cases, patients experience recurring episodes of this infection. The bacteria can form spores that are spread in diarrhoea, which are alcohol resistant. Spores can be spread if a doctor goes from patient to patient without washing their hands, or if they use alcohol-based hand sanitiser. The CDC recommends killing the spores using diluted bleach. Unless the spores are killed, they can result in re-infection or colonisation of another host. Reoccurrences are treated with the same antibiotic that treated the first infection, however, on subsequent infection, the doses are higher.
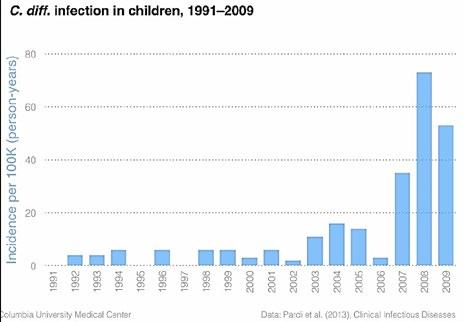
Figure 2. the massive increased rates of CDI in children in the US over time.
TREATMENT
Although antibiotics are effective in most CD cases, the addition of more antibiotics increases the chance of antibiotic resistance due to horizontal gene transfer. Treatments alternative to antibiotics may be considered. Faecal microbiota transplant (FMT) has shown promising results in clinical trials with an 80-95% cure rate. It is when stool, from healthy donors, is transplanted into patients with C. difficile infection. The introduction of other bacteria can compete with CD and increases the resistance to future colonisation.
This treatment has shown much promise, however, the gut microbiome is linked to increased risk of metabolic disease and cardiovascular disease. If a transplant could help a patient to recover from CDI, it could also give the recipient a type of microbe that alters their risk of having other diseases. By transplanting faeces, the bacteria could unwittingly pass on alterations in their microbiome, leading to disease susceptibility later in life. Before the transplant, the patients should be aware of the possible consequences and should only be used to treat CDI after antibiotic approaches have failed. Donors have to complete many questionaries, be screened for recent antibiotic use, intestinal infection, irritable bowel disease and other infectious diseases before consideration. In addition, related stool donors are preferred as they have a greater chance of success. The stool is collected from the donor and sent to a lab as soon as possible, it is then mixed with saline water and screened for pathogens, the sample is then stored at -80 degrees Celsius. There are two delivery methods, to the upper and lower GI tract, these methods were compared and results showed that lower GI tract delivery by colonoscope was the most efficacious. Some side effects include diarrhoea, abdominal cramps and nausea. However not all FMTs are successful, this may be due to not making any lifestyle changes after FMT, or rejection similar to when an organ transplant occurs.
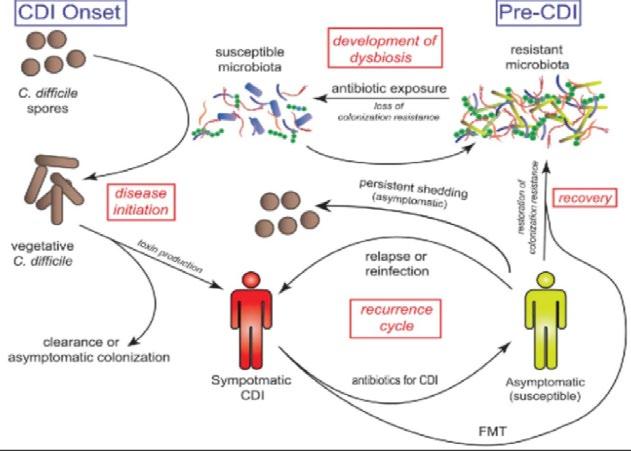
Figure 3. The process of infection and possible consequences
Figure 4. The principals of faecal microbiota transplant
Genetics of Asthma
Michelle Wong
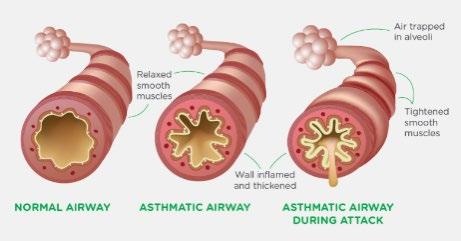
Asthma is a common lung condition that causes occasional breathing difficulties. Asthma causes chronic inflammation in the airways making them narrow and more difficult to breathe through. People with asthma can have asthma exacerbation, or asthma attacks, which are triggered by something in the environment and causes immune cells to generate inflammation in the lungs, thus can make them even narrower and potentially be life-threatening.
WHAT CAUSES IT?
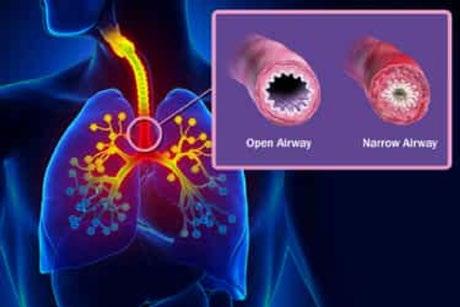
In the lungs, there are typically lots of eosinophils below the epithelium in the lamina propria. The eosinophils are white blood cells that carry a cargo of granules, soluble chemical mediators like histamines, leukotriene, prostaglandin, and platelet-activating factor. When eosinophils sense an environmental trigger, such as cigarette smoke in the airway, they release their granules. The chemical mediators are spilled out and start degrading lipids, proteins, and nucleic acids, destroying all major cell components. This creates a strong inflammatory reaction in the bronchial wall. Smooth muscles around the bronchioles spasm, which narrows the airway. This action is exacerbated by the increased mucus secretion in the narrow airways.
WHY IS IT CAUSED?
Although the specific causes of asthma are ultimately unknown, it is thought to be caused by a combination of genetic and environmental factors. Certain genes have been identified to increase the risk of redeveloping asthma, and having a family history of asthma seems to increase risk as well. For environmental factors, there is the hygiene hypothesis which suggests that reduced early immune system exposure to bacteria and viruses might increase the risk of later developing asthma, possibly by altering the overall proportion of immune cell subtype. Research has shown that asthma has an important genetic component, but no clear pattern of inheritance has been observed. The heritability estimates of asthma vary between 36% to 79% as there are parts that are still unknown.
WHAT IS KNOWN?
There is a Familial Association of Asthma and Allergy that has a Genetic Component
Familial concordance of a disorder can be because of shared environment and genes. Twin studies can help to determine the relative contribution of a shared environment and genes to a phenotype. Concordance of asthma in monozygotic twins and dizygotic twins can be compared and when possible, the concordance is also compared in twins who were reared together or apart. Another type of experimental design that can identify a genetic component to the trait of interest is segregation analysis where the transmission of a trait is examined in families to see if it conforms to a genetic or environmental pattern. All these studies have shown that familial concordance is at least partly due to shared genes and most authors concluded that genetic contribution is more important than environmental influences.
Asthma and Allergy Show Polygenic Inheritance and Genetic Heterogeneity
Segregation analysis of phenotypes has not identified a consistent Mendelian pattern such as dominant recessive or sex-linked. A nonMendelian pattern of inheritance is characteristic of complex genetic disorders such as asthma and allergy. Genetic heterogeneity such as different combinations of gene variants contributes to the phenotype in different families.









