Post-turbinectomy nasal packing with Merocel versus glove finger Merocel: A prospective, randomized, controlled trial
Comparison of anterior palatoplasty and uvulopalatal flap placement for treating mild and moderate obstructive sleep apnea
Psoriasis, chronic tonsillitis, and biofilms: Tonsillar pathologic findings supporting a microbial hypothesis
Quality of life in patients with larynx cancer in Latin America: Comparison between laryngectomy and organ preservation protocols
www.entjournal.com A Vendome Publication MARCH 2018 • VOL. 97, NO. 3


EDITORIAL BOARD
EDITORIAL BOARD MEMBERS
Editor-in-Chief
Robert T. Sataloff, MD, DMA, FACS
Professor and Chairman, Department of Otolaryngology–Head and Neck Surgery, and Senior Associate Dean for Clinical Academic Specialties, Drexel University College of Medicine Philadelphia, PA
Jean Abitbol, MD
Jason L. Acevedo, MD, MAJ, MC, USA
Jack B. Anon, MD
Gregorio Babighian, MD
Peter C. Belafsky, MD, PhD
Bruce Benjamin, MD
Gerald S. Berke, MD
Michael J. Brenner, MD
Kenneth H. Brookler, MD
Karen H. Calhoun, MD
Steven B. Cannady, MD
Ricardo Carrau, MD
Swapna Chandran, MD
Chien Chen, MD
Dewey A. Christmas, MD
Nicolle T. Clements, MS
Daniel H. Coelho, MD, FACS
David M. Cognetti, MD
Maura Cosetti, MD
James V. Crawford, MD
David H. Darrow, MD, DDS
Rima Abraham DeFatta, MD
Robert J. DeFatta, MD, PhD
Hamilton Dixon, MD
Paul J. Donald, MD, FRCS
Mainak Dutta, MS, FACS
Russell A. Faust, PhD, MD
Ramón E. Figueroa, MD, FACR
Charles N. Ford, MD
Paul Frake, MD
Marvin P. Fried, MD
Richard R. Gacek, MD
Andrea Gallo, MD
Frank Gannon, MD
Emilio Garcia-Ibanez, MD
Soha Ghossani, MD
William P. R. Gibson, MD
David Goldenberg, MD
Jerome C. Goldstein, MD
Richard L. Goode, MD
Samuel Gubbels, MD
Reena Gupta, MD
Joseph Haddad Jr., MD
Missak Haigentz, MD
Christopher J. Hartnick, MD
Mary Hawkshaw, RN, BSN, CORLN
Garett D. Herzon, MD
Thomas Higgins, MD, MSPH
Jun Steve Hou, MD
John W. House, MD
Glenn Isaacson, MD
Steven F. Isenberg, MD
Stephanie A. Joe, MD
Shruti S. Joglekar, MBBS
Raleigh O. Jones, Jr., MD
Petros D. Karkos, MD, AFRCS, PhD, MPhil
David Kennedy, MD
Seungwon Kim, MD
Robert Koenigsberg, DO
Karen M. Kost, MD, FRCSC
Jamie A. Koufman, MD
Stilianos E. Kountakis, MD, PhD
John Krouse, MD
Ronald B. Kuppersmith, MD, MBA, FACS
Rande H. Lazar, MD
Robert S. Lebovics, MD, FACS
Keat-Jin Lee, MD
Donald A. Leopold, MD
Steve K. Lewis, BSc, MBBS, MRCS
Daqing Li, MD
Robert R. Lorenz, MD
John M. Luckhurst, MS, CCC-A
Valerie Lund, FRCS
Karen Lyons, MD
A.A.S. Rifat Mannan, MD
Richard Mattes, PhD
Brian McGovern, ScD
William A. McIntosh, MD
Brian J. McKinnon, MD
Oleg A. Melnikov, MD
Albert L. Merati, MD, FACS
Joseph P. Mirante, MD, MBA, FACS
Ron B. Mitchell, MD
Steven Ross Mobley, MD
Jaime Eaglin Moore, MD
Thomas Murry, PhD
Ashli K. O’Rourke, MD
Ryan F. Osborne, MD, FACS
J. David Osguthorpe, MD
Robert H. Ossoff, DMD, MD
Enrique Palacios, MD, FACR
Michael M. Paparella, MD
Kourosh Parham, MD, PhD
Arthur S. Patchefsky, MD
Meghan Pavlick, AuD
Spencer C. Payne, MD
Kevin D. Pereira, MD, MS (ORL)
Nicolay Popnikolov, MD, PhD
Didier Portmann, MD
Gregory N. Postma, MD
Matthew J. Provenzano, MD
Hassan H. Ramadan, MD, FACS
Richard T. Ramsden, FRCS
Gabor Repassy, MD, PhD
Dale H. Rice, MD
Ernesto Ried, MD
Alessandra Rinaldo, MD, FRSM
Joshua D. Rosenberg, MD
Allan Maier Rubin, MD, PhD, FACS
John S. Rubin, MD, FACS, FRCS
Amy L. Rutt, DO
Anthony Sclafani, MD, FACS
Raja R. Seethala, MD
Jamie Segel, MD
Moncef Sellami, MD
Michael Setzen, MD, FACS, FAAP
Stanley Shapshay, MD
Douglas M. Sidle, MD
Herbert Silverstein, MD
Jeffrey P. Simons, MD
Raj Sindwani, MD, FACS, FRCS
Aristides Sismanis, MD, FACS
William H. Slattery III, MD
Libby Smith, DO
Jessica Somerville, MD
Thomas C. Spalla, MD
Matthew Spector, MD
Paul M. Spring, MD
Brendan C. Stack, Jr., MD, FACS
James A. Stankiewicz, MD
Jun-Ichi Suzuki, MD
David Thompson, MD
Lester D.R. Thompson, MD, FASCP
Helga Toriello, PhD, FACMG
Ozlem E. Tulunay-Ugur, MD
Galdino Valvassori, MD
Emre Vural, MD
Donald T. Weed, MD, FACS
Neil Weir, FRCS
Kenneth R. Whittemore, MD
David F. Wilson, MD
Ian M. Windmill, PhD
Ian J. Witterick, MD,MSc, FRCSC
Richard J. Wong, MD
Naoaki Yanagihara, MD
Eiji Yanagisawa, MD, FACS
Ken Yanagisawa, MD, FACS
Anthony Yonkers, MD
Mark Zacharek, MD
Joseph Zenga, MD
Liang Zhou, MD
CLINIC EDITORS
Dysphagia
Jamie A. Koufman, MD
Peter C. Belafsky, MD, PhD
Gregory N. Postma, MD
Facial Plastic Surgery
Anthony P. Sclafani, MD, FACS
Geriatric Otolaryngology
Kourosh Parham, MD, PhD, FACS
Karen M. Kost, MD, FRCSC
Head and Neck
Ryan F. Osborne, MD, FACS
Paul J. Donald, MD, FRCS
Reena Gupta, MD
Imaging
Enrique Palacios, MD, FACR
Ramón E. Figueroa, MD, FACR
Laryngoscopic
Robert T. Sataloff, MD, DMA, FACS
Otoscopic
John W. House, MD
Brian J. McKinnon, MD
Pathology
Lester D.R. Thompson, MD, FASCP
Pediatric Otolaryngology
Rande H. Lazar, MD
Rhinoscopic
Eiji Yanagisawa, MD, FACS
Dewey A. Christmas, MD
Joseph P. Mirante, MD, MBA, FACS
Ken Yanagisawa, MD, FACS
Special Topics
Robert T. Sataloff, MD, DMA, FACS
Thyroid and Parathyroid
David Goldenberg, MD
42 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018

Editor-in-Chief Robert T. Sataloff, MD, DMA, FACS 219 N. Broad St., 10th Fl., Philadelphia, PA 19107 entjournal@phillyent.com Ph: 215-732-6100
Managing Editor Linda Zinn
Manuscript Editors Martin Stevenson and Wayne Kuznar
Associate Editor, Reader Engagement Megan Combs
Creative Director Eric Collander
National Sales Manager Mark C. Horn mhorn@vendomegrp.com Ph: 480-895-3663
Traffic Manager Judi Zeng
Please send IOs to adtraffic@vendomegrp.com
All ad materials should be sent electronically to: https://vendome.sendmyad.com
Customer Service/Subscriptions
www.entjournal.com/subscribe Ph: 888-244-5310 email: VendomeHM@emailpsa.com
Reuse Permissions Copyright Clearance Center info@copyright.com Ph: 978-750-8400 Fax: 978-646-8600
Chief Executive Officer Jane Butler
Chief Marketing Officer Dan Melore
Vice President, Finance Bill Newberry
Vice President, Custom Media Jennifer Turney Director, Circulation Rachel Beneventi
ENT-Ear, Nose & Throat Journal (ISSN: Print 0145-5613, Online 1942-7522) is published 9 times per year in Jan/Feb, Mar, Apr/May, June, July, Aug, Sept, Oct/ Nov and Dec, by Vendome Group, LLC, 237 West 35th Street, 16th Floor, New York, NY 10001-1905.

©2018 by Vendome Group, LLC. All rights reserved. No part of ENT-Ear, Nose & Throat Journal may be reproduced, distributed, transmitted, displayed, published, or broadcast in any form or in any media without prior written permission of the publisher. To request permission to reuse this content in any form, including distribution in education, professional, or promotional contexts or to reproduce material in new works, please contact the Copyright Clearance Center at info@ copyright.com or 978.750.8400.
EDITORIAL: The opinions expressed in the editorial and advertising material in this issue of ENT-Ear, Nose & Throat Journal are those of the authors and advertisers and do not necessarily reflect the opinions or recommendations of the publisher, editors, or the staff of Vendome Group, LLC. ENT-Ear, Nose & Throat Journal is indexed in MEDLINE/PubMed and Current Contents/Clinical Medicine and Science Citation Index Expanded. Editorial offices are located at 812 Huron Rd., Suite 450, Cleveland, OH 44115. Manuscripts should be submitted online at www.editorialmanager. com/entjournal. Instructions for Authors are available at www.entjournal. com.
SUBSCRIPTIONS: For questions about a subscription or to subscribe, please contact us by phone: 888-244-5310; or email: VendomeHM@emailpsa.com. Individual subscriptions, U.S. and possessions: 1 year $225, 2 years $394; International: 1 year $279, 2 years $488; Single copies $28; outside the U.S., $40.
POSTMASTER: send address changes to Ear, Nose & Throat Journal, PO Box 11404 Newark, NJ 07101-4014.
44 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
ADVERTISER INDEX Pages American Rhinologic Society 45 Arbor Pharmaceuticals ...................... 85, 86 Beutlich Pharmaceuticals, LLC ............... 95 CANT Corporation 49 Compulink Business Systems ................. 81 Cook Medical 59 Eagle Surgical Products LLC 53 Eosera, Inc......................................... CVR 3 Fyzical Therapy and Balance 41 Haag-Streit .............................................. 67 InHealth Technologies ............................. 47 Lannett Company 71, 72 Lumenis Ltd. ............................................ 89 McKeon Products, Inc. ...................... CVR 4 Optim LLC 43 Optinose ............................................ 75, 77 Reliance Medical 61 Spectrum Audiology ................................ 56 SurgiTel .............................................. CVR 2 Xlear, Inc. 51 Xoran Technologies ................................. 93

ORIGINAL ARTICLES
64 Post-turbinectomy nasal packing with Merocel versus glove finger Merocel: A prospective, randomized, controlled trial
Rani Abu Eta, MD; Ephraim Eviatar, MD; Jacob Pitaro, MD; Haim Gavriel, MD
69 Comparison of anterior palatoplasty and uvulopalatal flap placement for treating mild and moderate obstructive sleep apnea
Süheyl Hayto lu, MD; Osman Kür at Arikan, MD; Nuray Bayar Muluk, MD; Birgül Tuhanio lu, MD; Mustafa Çörtük, MD
79 Psoriasis, chronic tonsillitis, and biofilms: Tonsillar pathologic findings supporting a microbial hypothesis
Herbert B. Allen, MD; Saagar Jadeja, BS; Rina M. Allawh, MD; Kavita Goyal, MD
83 Quality of life in patients with larynx cancer in Latin America: Comparison between laryngectomy and organ preservation protocols
Alvaro Sanabria, MD, PhD, FACS; Daniel Sánchez, MD; Andrés Chala, MD; Andres Alvarez, MD
91 “Split to save”: Accessing mandibular lesions using sagittal split osteotomy with virtual surgical planning
Stanley Yung-Chuan Liu, MD, DDS; Douglas Sidell, MD; Leh-Kiong Huon, MD; Carlos Torre, MD
ONLINE EXCLUSIVES
E1 Relationship between dysarthria and oraloropharyngeal dysphagia: The current evidence
Brandon J. Wang, BA, BBA; Felicia L. Carter, MA, CCC-SLP; Kenneth W. Altman, MD, PhD
E10 Prospective evaluation of the early effects of radiation on the auditory system frequencies of patients with head and neck cancers and brain tumors after radiotherapy
Akram Hajisafari, Msc; Mohsen Bakhshandeh, PhD;
Seyed Mahmoud Reza Aghamiri, PhD;
Mohammad Houshyari, MD; Afshin Rakhsha, MD;
Eftekhar Rajab Bolokat, Msc; Abbas Rezazadeh, PhD
E18 Pharyngeal pack placement in minor oral surgery: A prospective, randomized, controlled study
Badr A. Al-Jandan, FRCD(C);
Faiyaz Ahmed Syed, MDS; Ahed Zeidan, MD; Hesham Fathi Marei, MSc, MFDS (RCS Eng), PhD; Imran Farooq, MSc
E22 Aerophagia and subcutaneous emphysema in a patient with Rett syndrome
Christine M. Clark, MD; Shivani Shah-Becker, MD; Abraham Mathew, MD; Neerav Goyal, MD, MPH
E25 Alternative therapies for chronic rhinosinusitis: A review
Aaron S. Griffin, MBBS, BSci; Peter Cabot, PhD, BSci; Ben Wallwork, PhD, MBBS, FRACS; Ben Panizza, MBA, MBBS, FRACS
E34 CO2-laser–assisted diverticulotomy remains an effective and safe method for treating Zenker diverticulum
Mazin Merdad, MD, MPH; Nitin Bhatia, MD; Elie E. Rebeiz, MD, FACS
E38 Bilateral pyriform sinus parathyroid adenomas
Thomas Muelleman, MD; Sreeya Yalamanchali, MD; Yelizaveta Shnayder, MD
E41 The utility of enlarging symptomatic nasal septal perforations
Philip G. Chen, MD; Stephen Floreani, MBBS; Peter-John Wormald, MD
E44 Incidence of epistaxis after endoscopic pituitary surgery: Proposed treatment algorithm
Lee A. Zimmer, MD, PhD; Norberto Andaluz, MD
46 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 EDITORIAL OFFICE Robert T. Sataloff, MD, DMA, FACS, Editor-in-Chief • 219 N. Broad St., 10th Fl. • Philadelphia, PA 19107 CONTENTS MARCH 2018 • VOL. 97, NO. 3
DEPARTMENTS 44 Advertiser Index 48 ENT Journal Online 50 Editorial 54 Otoscopic Clinic 55 Imaging Clinic 57 Pathology Clinic 58 Thyroid and Parathyroid Clinic 62 Special Topics Clinic E49 Laryngoscopic Clinic E51 Rhinoscopic Clinic

JOURNAL ONLINE
Ear, Nose & Throat Journal's website is easy to navigate and provides readers with more editorial content each month than ever before. Access to everything on the site is free of charge to physicians and allied ENT professionals. To take advantage of all our site has to offer, go to www.entjournal. com and click on the "Registration" link. Once you have filled out the brief registration form, you will have full access. Explore and enjoy!
ONLINE EXCLUSIVES
Relationship between dysarthria and oraloropharyngeal dysphagia: The current evidence
Brandon J. Wang, BA, BBA; Felicia L. Carter, MA, CCC-SLP; Kenneth W. Altman, MD, PhD
There is a high prevalence of dysphagia among patients with neuromuscular diseases and cerebrovascular diseases, and its consequences can be profound. However, the correlation between dysarthria and oral-oropharyngeal dysphagia remains unclear. We conducted a literature review to define the clinical presentation of both dysarthria and dysphagia in patients with neuromuscular and cerebrovascular diseases. We performed a systematic PubMed search of the English-language literature since 1995. Objective and subjective outcomes instruments were identified for both dysarthria and dysphagia. Studies that included....
Prospective evaluation of the early effects of radiation on the auditory system frequencies of patients with head and neck cancers and brain tumors after radiotherapy
Akram Hajisafari, Msc; Mohsen Bakhshandeh, PhD; Seyed Mahmoud Reza Aghamiri, PhD; Mohammad Houshyari, MD; Afshin Rakhsha, MD; Eftekhar Rajab Bolokat, Msc; Abbas Rezazadeh, PhD
Patients with head and neck cancer after radiotherapy often suffer disability such as hearing disorders. In this study, the effect of radiotherapy (RT) on hearing function of patients with head and neck cancer after RT was determined according to the total dose delivered to specific parts of the auditory system. A total of 66 patients treated with primary or postoperative radiation therapy for various cancers....
Pharyngeal pack placement in minor oral surgery: A prospective, randomized, controlled study
Badr A. Al-Jandan, FRCD(C);
Faiyaz Ahmed Syed, MDS; Ahed Zeidan, MD; Hesham Fathi Marei, MSc, MFDS (RCS Eng), PhD;
Imran Farooq, MSc
We conducted a prospective, randomized, controlled study to investigate the influence of pharyngeal pack placement on postoperative nausea, vomiting, and throat pain after minor oral surgery. Our study group was made up of 80 patients—45 men and 35 women, aged 19 to 52 years (mean: 27.3)—who underwent a minor oral surgical procedure under general anesthesia. Patients were randomly assigned to one of three groups: 20 patients who received a pharyngeal pack under videolaryngoscopic guidance....
Aerophagia and subcutaneous emphysema in a patient with Rett syndrome
Christine M. Clark, MD; Shivani Shah-Becker, MD; Abraham Mathew, MD; Neerav Goyal, MD, MPH
A patient with Rett syndrome presented to our Emergency Department with extensive subcutaneous emphysema in the cervical region, chest wall, upper extremities, and back. Diagnostic evaluation revealed a mucosal tear in the posterior pharyngeal wall and an abscessed retropharyngeal lymph node, but she had no known history of trauma to account for these findings. This report discusses the occurrence of subcutaneous emphysema in the context of a rare neurodevelopmental disorder and proposes accentuated aerophagia, a sequela of Rett syndrome, as the most likely underlying mechanism.
Alternative therapies for chronic rhinosinusitis: A review
Aaron S. Griffin, MBBS, BSci; Peter Cabot, PhD, BSci; Ben Wallwork, PhD, MBBS, FRACS; Ben Panizza, MBA, MBBS, FRACS
The use of alternative medicine in chronic rhinosinusitis (CRS) continues to increase in popularity, for the most part without meeting the burden of being based on sound clinical evidence. New and emerging treatments, both natural and developed, are numerous, and it remains a challenge for otolaryngologists as well as general practitioners to keep up to date with these therapies and their efficacy. In this systematic review, we discuss a number of alternative therapies for CRS, their proposed physiologic mechanisms, and evidence supporting their use. This analysis is based on....
CO2-laser–assisted diverticulotomy remains an effective and safe method for treating Zenker diverticulum
Mazin Merdad, MD, MPH; Nitin Bhatia, MD; Elie E. Rebeiz, MD, FACS
Our objectives were to review our experience with laserassisted diverticulotomy (LAD) in the treatment of Zenker diverticulum (ZD) and compare our results with those in published literature on other endoscopic and surgical techniques. We conducted a retrospective chart review of 57 patients who underwent LAD treatment of ZD in a single tertiary care institution. Data on surgical complications, length of stay, and follow-up were collected. Age ranged from 56 to 89 years. Endoscopic exposure of the diverticulum was not possible in 2 patients. All 55....
48 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 www.entjournal.com
Bilateral pyriform sinus parathyroid adenomas
Thomas Muelleman, MD; Sreeya Yalamanchali, MD; Yelizaveta Shnayder, MD
Parathyroid glands undergo a variable descent during embryologic development and can be found anywhere in the neck from the level of the mandible to the mediastinum. To the best of our knowledge, we present the first report of a patient who was found to have bilateral parathyroid adenomas in her pyriform sinuses. A middle-aged woman with renal failure and secondary hyperparathyroidism presented with dysphagia and was found to have bilateral....
The utility of enlarging symptomatic nasal septal perforations
Philip G. Chen, MD; Stephen Floreani, MBBS; Peter-John Wormald, MD
Nasal septal perforations cause a subset of patients to suffer with significant impairments in quality of life. While smaller perforations can often be surgically repaired, perforations exceeding 2 cm are challenging to close. These repairs are highly technical, and there is a lack of consensus regarding the most effective means to do so. The authors performed a retrospective chart review of patients with....

Incidence of epistaxis after endoscopic pituitary surgery: Proposed treatment algorithm
Lee A. Zimmer, MD, PhD; Norberto Andaluz, MD
Endoscopic transsphenoidal surgery for pituitary tumors is an increasingly common practice. Little has been reported on the incidence and treatment of postoperative epistaxis in this population. The aim of this study was to analyze the incidence of postoperative epistaxis and formulate a treatment algorithm based on our experience. We performed a case series with chart review. A total of 434 consecutive patients who had endoscopic transsphenoidal pituitary surgery were identified between April 2006 and November 2013. The incidence, clinical management, and outcomes were recorded. Based on the data, a treatment algorithm....
ONLINE DEPARTMENTS
Laryngoscopic Clinic: Reinke edema
Jessie C. Everaert, BS; Jonathan J. Romak, MD; Robert T. Sataloff, MD, DMA, FACS
Rhinoscopic Clinic: Endoscopic findings of bilateral fungal balls
Jong Seung Kim, MD; Sam Hyun Kwon, MD, PhD
Volume 97, Number 3 www.entjournal.com 49 ENT JOURNAL ONLINE
Geriatric surgery in otolaryngology

More than one-third of all inpatient surgical procedures are performed on patients age 65 and over.1 This is not surprising. In 2015, people ≥65 years constituted 15% of the U.S. population, and this percentage is expected to grow to 24% by 2060.2 In 2010, nearly 40% of hospital discharges (including short-stay hospitals) involved patients ≥65 years of age.3 This means that approximately 200 million operations a year are performed on elderly (≥65 years) patients. While these data are not specific to otolaryngology, it is likely that the age profile of our patients is similar; and, with changing population demographics, it is certain that the percentage of otolaryngology patients who are elderly will increase.
We have been trained to understand that pediatric patients are not just “small adults.” Similarly, geriatric patients are not just “old adults.” They have special problems and require knowledgeable diagnostic and therapeutic intervention. The American Academy of Otolaryngology–Head and Neck Surgery acknowledged the importance of geriatric otolaryngology by publishing with Thieme a multidisciplinary textbook of geriatric otolaryngology–head and neck surgery in 2015.4 That text makes it clear that special knowledge of geriatric otolaryngology is important in all subspecialties except pediatric otolaryngology, and such knowledge is especially important in surgical decision making.
On one hand, surgery should not be denied to patients simply because they are “old.” Moreover, “old” is hard to define. More and more people are living beyond 100 years, so denying surgery to an 80-year-old and condemning him/her to suffer from a potentially correctable problem for another 15 to 20 years is not right. We need to be concerned about quality of life. On the other hand, surgery that is unlikely to improve quality of life, or that presents a high risk of ending life without a concomitant benefit (such as some surgery for advanced head and neck cancer), might not be appropriate in this population.
Numerous articles on surgery in the elderly have been published—far too many to reference in an editorial. Discussions of this topic appear regularly in publications of the American College of Surgeons, for example. The underlying theme of most of these articles is the need to improve preoperative assessment. Surgical decision making must focus on more than surgical mortality and morbidity. We must consider maintenance of independence, quality of life, return to at least preoperative functional activity levels, the likely consequences of each person’s physiologic reserve, the cognitive effects associated with general anesthesia in the elderly, and the patient’s desires regarding quality of life and longevity.
Ideally, with the help of a healthcare team, the surgeon needs to consciously assess cognitive function, nutrition, risk of falls, geriatric syndromes, and other special healthcare issues in all elderly patients for whom surgery is contemplated. Such preoperative assessments help not only in surgical decision making, but also in perioperative care of elderly patients.
50 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
EDITORIAL

Elderly patients have increased risk of postoperative morbidity, sometimes after even relatively short general anesthesia. Problems may include long-term cognitive impairment, delirium, deep vein thrombosis, myocardial ischemia, infection, and others. It is essential for the physician, patient, and family to review these risks and make sure everyone agrees that they are justified and weighed against potential benefits. Physician and patient education are required, but much of the necessary knowledge is still being developed.
While there is no specific measure that will guide us in decisions about whether to perform surgery, assessments of frailty can be helpful and enlightening. This topic, as well as other tools for clinical assessment that predict adverse outcomes in geriatric patients, is covered in other literature.4-10 Interesting studies in geriatric trauma patients have shown the value of frailty assessment. For example, Joseph et al looked at elderly trauma patients (a particularly vulnerable population) and found that a 50-variable frailty index predicted unfavorable discharge disposition in geriatric patients.11 In a follow-up paper published during the same year, they validated a 15-variable trauma-specific frailty index (TSFI).12 The TSFI proved to be an effective instrument for predicting discharge disposition in geriatric trauma patients. Similar studies in otolaryngology patients have not been performed. Other frailty instruments have been used to predict surgical survival and outcomes, although most otolaryngologists are not using frailty assessment routinely and knowledgably. There are exceptions, such as the University of Pittsburgh Medical Center, where targeted assessments of elderly patients have shown great value.
Nearly all otolaryngologists care for elderly patients, and the percentage of elderly patients in our practices will continue to increase. As a field, it is past time for us to study otolaryngology-specific implications of advanced age, to apply and study assessment tools that have proven useful in other specialties, and to develop assessments and guidelines of our own to assist our trainees and our patients in providing optimal care for patients 65 and older.
References
1. Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. National Health Statistics Reports. U.S. Department of Health and Human Services. October 26, 2010. www.cdc.gov/nchs/data/nhsr/nhsr029.pdf. Accessed Jan. 26, 2018.
2. U.S. Census Bureau. 2014 national population projections summary tables. Table 6: Percent distribution of the projected population by sex and selected age groups for the U.S.: 2015 to 2060. www. census.gov/data/tables/2014/demo/popproj/2014-summarytables.html. Accessed Jan. 26, 2018.
3. Centers for Disease Control and Prevention. Number of discharges from short-stay hospitals, by first-listed diagnosis and age: United States, 2010. www.cdc.gov/nchs/data/nhds/3firstlisted/2010first3_ numberage.pdf. Accessed Jan. 26, 2018.
4. Sataloff RT, Johns MM, Kost KM (eds.) Geriatric Otolaryngology. New York: Thieme Medical Publishers and the American Academy of Otolaryngology–Head and Neck Surgery; 2015.
5. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA 2005;294(6):716-24.
6. Woods NF, LaCroix AZ, Gray SL, et al. Frailty: Emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc 2005;53(8): 1321-30.
7. Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA 1998;279(8):585-92.
8. Zafonte RD, Hammond FM, Mann NR, et al. Relationship between Glasgow Coma scale and functional outcome. Am J Phys Med Rehabil 1996;75(5):364-9.
9. Foreman BP, Caesar RR, Parks J, et al. Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. J Trauma 2007;62(4):946-50.
10. Shah MK, Al-Adawi S, Burke DT. Age as predictor of functional outcome in anoxic brain injury. J Appl Res 2004;4(3):380-4.
11. Joseph B, Pandit V, Rhee P, et al. Predicting hospital discharge disposition in geriatric trauma patients: Is frailty the answer? J Trauma Acute Care Surgery 2014;76(1):196-200.
12. Joseph B, Pandit V, Zangbar B, et al. Validating trauma-specific frailty index for geriatric trauma patients: A prospective analysis. J Am Coll Surg 2014;219(1):10-17.
Robert T. Sataloff, MD, DMA, FACS Editor-in-Chief
Ear, Nose & Throat Journal
52 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 EdITORIAL
OTOSCOPIC CLINIC
Schwartze sign
Kevin A. Peng, MD; John W. House, MD
A 34-year-old man presented to the otology clinic complaining of right progressive hearing loss over the previous 4 years. Otoscopic examination revealed a reddish blush visible on the cochlear promontory beyond an intact right tympanic membrane (figure 1). Both 512- and 1024-Hz tuning forks lateralized to the right ear, and bone conduction was greater than air conduction on the right using both tuning forks. Audiometry revealed a moderate conductive hearing loss on the right with absent reflexes.
Computed tomography (CT) was performed, revealing demineralization at the fissula ante fenestram bilaterally (figure 2). At the time of surgery, a fixed stapes was noted, confirming the clinical diagnosis of otosclerosis.

Hermann Schwartze (1837-1910) was a German otologist at the University of Halle. In 1878, he published The Pathological Anatomy of the Ear, a comprehensive
From House Clinic, Los Angeles, Calif.
text cataloging diseases of the outer, middle, and inner ear.1 Although ankylosis of the stapes was discussed in his book, several years would pass before Politzer coined the term otosclerosis in 1893.2
It remains unclear when Schwartze first noted the red discoloration of the cochlear promontory now associated with his name, but by 1960, the Schwartze sign had become common parlance in otolaryngology publications. It is estimated to affect fewer than 10% of patients with otosclerosis, and it reflects the increased vascularity of otospongiotic bone in the otic capsule.
References
1. Schwartze H, Green JO. The Pathological Anatomy of the Ear. Boston: Houghton, Osgood and Co.; 1878.
2. Mudry A. Adam Politzer (1835-1920) and the description of otosclerosis. Otol Neurotol 2006;27(2):276-81.
54 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
Figure 1. Otoscopy of the right tympanic membrane demonstrates a reddish blush on the cochlear promontory.
Figure 2. CT demonstrates demineralization at the fissula ante fenestram on the right, which is present bilaterally.
IMAGING CLINIC
An atypical presentation of hemifacial spasm secondary to neurovascular compression
Hemifacial spasm, also called tic convulsive, is a painless, involuntary contraction of the muscles innervated by the ipsilateral facial nerve, although less commonly it can present with weakness.1,2 As is the case in its trigeminal counterpart, tic douloureux, hemifacial spasm has a diverse array of potential etiologies. The most widely accepted of these is neurovascular compression, first proposed by Schultze.3 Other documented causes include space-occupying lesions, traumatic injury, and Bell palsy.1
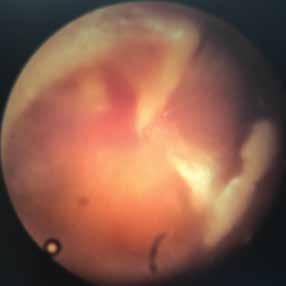
Our case involved a 57-year-old man presenting with weakness of the left face for the previous 18 months with acute worsening over the course of that day. Transient ischemic attack was ruled out, although a vascular loop of the ipsilateral vertebral artery was observed on magnetic resonance angiogram (MRA) of the brain to be impinging on the facial nerve (figure). The patient was treated with muscle relaxants with some relief of the symptoms, and he was discharged to be followed for further evaluation of the symptoms.



Hemifacial spasm affects 0.8/100,000 people.1 It presents most often in the fifth to sixth decades of life and is nearly twice as common in females.4,5 Symptoms typically manifest initially in the orbicularis oculi and progress downward, although 4% progress in the opposite direction.1 With its nonspecific symptomatology, the differential diagnosis for hemifacial spasm is wide and includes facial tic, myokymia, blepharospasm, and tardive dyskinesia.6
Diagnostic workup for hemifacial spasm consists of a complete neurologic exam, electromyography, mag-
netic resonance (MR) imaging, and MRA.3 Botulinum toxin injection and microvascular decompression are the mainstays of treatment.4 Symptoms are controlled
From the Department of Radiology, Head and Neck Section, Tulane University Medical Center, New Orleans.
Volume 97, Number 3 www.entjournal.com 55
Enrique Palacios, MD, FACR; Radia Ksayer, MD; Jeremy Nguyen, MD
A B C D
Figure. Noncontrast MR examination at the level of the cerebellopontine angle reveals a vascular loop corresponding to the distal portion of the left vertebral artery, compressing the area at the origin of the left facial nerve at the pontomedullary junction (A and B). In the axial (C) and coronal (D) T2W images, the arrows point to the flow void of the vessel. In the axial image of the source angiogram (C) and in the coronal view of the time-of-flight image (D), the arrows identify the vessel to better advantage than in A and B. The thin arrow in panel C identifies the left anterior inferior cerebellar artery.
acutely with anticonvulsants, muscle relaxants, and benzodiazepines.7
Visualization of the vascular structure and the nerve is best achieved in oblique sagittal gradient MR imaging, which is reported to have a 75.9% sensitivity for identification of facial nerve compression by the anterior inferior cerebellar artery or the posterior inferior cerebellar artery, the first and second most common offending vessels, respectively.8
Neurovascular compression involving the vertebral artery, which accounts for a minority of cases (17%), can be evaluated with 100% sensitivity due to the vessel’s larger caliber.8,9 MRA and computed tomography angiography have demonstrated similar utility in identification of pathologic vessels.10
References
1. Samii M, Günther T, Iaconetta G, et.al. Microvascular decompression to treat hemifacial spasm: Long-term results for a consecutive series of 143 patients. Neurosurgery 2002;50(4):71218; discussion 718-19.
2. Mauriello JA Jr., Leone T, Dhillon S, et al. Treatment choices of 119 patients with hemifacial spasm over 11 years. Clin Neurol Neurosur 1996;98(3):213-16.
3. Zappia JJ, Wiet RJ, Chouhan A, Zhao JC. Pitfalls in the diagnosis of hemifacial spasm. Laryngoscope 1997;107(4):461-5.
4. Lorentz I. Treatment of hemifacial spasm with botulinum toxin. J Clin Neurosci 1995;2(2):132-5.
5. Auger RG, Whisnant JP. Hemifacial spasm in Rochester and Olmsted County, Minnesota, 1960 to 1984. Arch Neurol 1990; 47(11):1233-4.
6. Palacios E, Breaux J, Alvernia JE. Hemifacial spasm. Ear Nose Throat J 2008;87(7):368-70.
7. Wang A, Jankovic J. Hemifacial spasm: Clinical findings and treatment. Muscle Nerve 1998;21(12):1740-7.
8. Nagaseki Y, Omata T, Ueno T, et al. Prediction of vertebral artery compression in patients with hemifacial spasm using oblique sagittal MR imaging. Acta Neurochir (Wien) 1998;140(6):565-71.
9. Payner TD, Tew JM Jr. Recurrence of hemifacial spasm after microvascular decompression. Neurosurgery 1996;38(4):686–90; discussion 690-1.
10. El Refaee E, Langner S, Baldauf J, et al. Value of 3-dimensional highresolution magnetic resonance imaging in detecting the offending vessel in hemifacial spasm: Comparison with intraoperative high definition endoscopic visualization. Neurosurgery 2013;73(1):58-67.

56 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
ImAgINg CLINIC
PATHOLOGY CLINIC
Dentigerous cyst
Lester D.R. Thompson, MD
A dentigerous cyst is a development cyst that surrounds and envelops the crown of an unerupted tooth, attached at the crown-root (cemento-enamel or cervical) junction. Dentigerous cysts account for about 20% of all odontogenic cysts, developing during a peak age of 10 to 30 years, with a male predilection (3:2). The lesion presents in the mandible (3rd molar region) about twice as often as the maxilla (near maxillary canines).
Patients are usually asymptomatic, so these cysts are incidentally discovered during routine dental imaging. However, pain may be experienced with bone expansion or resorption of adjacent teeth. Imaging studies (orthopantomographs) usually show a unilocular radiolucency surrounding the crown of the affected tooth (figure 1), with a well-defined sclerotic border. Cyst enucleation or extraction is generally employed, although marsupialization is sometimes used for larger lesions.
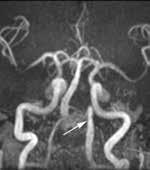
Histologically, there is a difference in findings depending on whether the lesion is inflamed. The noninflamed cyst shows 2 to 3 cell layers of cuboidal to squamoid
cells adjacent to fibrous connective tissue (figure 2), rarely showing ciliated, mucous, or sebaceous cells. The inflamed cyst shows a much thicker, proliferative epithelium, with hyperplastic rete, chronic inflammation (figure 2), and sometimes hyalinized keratin (Rushton bodies). Cholesterol clefts are common.
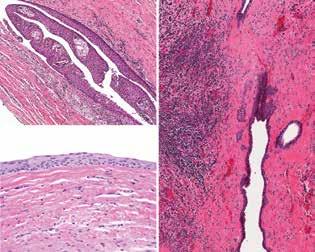
The differential diagnosis includes a dental follicle, an eruption cyst (a soft-tissue cyst overlying the erupting tooth), a glandular odontogenic cyst, and a unicystic ameloblastoma, while an odontogenic keratocyst may also be considered.

Suggested reading
Johnson NR, Gannon OM, Savage NW, Batstone MD. Frequency of odontogenic cysts and tumors: A systematic review. J Investig Clin Dent 2014;5(1):9-14.
Lin HP, Wang YP, Chen HM, et al. A clinicopathological study of 338 dentigerous cysts. J Oral Pathol Med 2013;42(6):462-7.
From the Department of Pathology, Southern California Permanente Medical Group, Woodland Hills Medical Center, Woodland Hills, Calif.
Volume 97, Number 3 www.entjournal.com 57
Figure 1. A unilocular radiolucency around tooth #32 (arrow) shows a crown-root junction of the cyst in this orthopantomograph.
Figure 2. Left: A few layers of epithelium are noted, lacking a palisading of the nuclei and showing an abrupt junction with the surrounding fibrous tissue. Right: Inflammation is noted in the adjacent stroma, with several projections of the epithelium into the stroma.
THYROID AND PARATHYROID CLINIC
A midline mediastinal parathyroid cyst
Guy
A 49-year-old woman presented with progressive dysphagia of several months’ duration accompanied by a pressure sensation at the lower anterior base of her neck. Her medical history was significant for type II diabetes, hypertension, hypertriglyceridemia, obesity, anxiety, and depression. Lab values were unremarkable. Physical examination revealed no neck mass. The remainder of her head and neck examination was within normal limits. On ultrasound, a cystic lesion measuring 4.0 × 2.7 × 3.6 cm was seen, abutting and immediately caudal to the thyroid isthmus. There were no pathologic findings within the thyroid gland and no neck lymphadenopathy.
Computed tomography (CT) of the neck with intravenous contrast revealed a cystic mass of similar dimensions adhering to the anterior tracheal surface

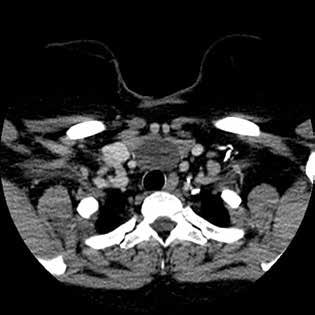
and occupying the space between the thyroid isthmus and the aortic arch. Because of mass effect, there was splaying of the inferior poles of the thyroid lobes and the left and right innominate veins (figure 1).
The cytologic findings of an ultrasound-guided fine-needle aspiration biopsy were of rare macrophages, inflammatory cells, and proteinaceous debris, consistent with cystic contents; there was no evidence of malignant cells.
The patient underwent exploration of the lower neck and upper anterior mediastinum by a cervical approach. A cystic lesion at the superior anterior mediastinum was identified. It was meticulously dissected and entirely removed. Histopathology revealed a unilocular cystic cavity containing parathyroid cells in rare residual groups on an otherwise flat lining and in focal nests
58 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
From the Department of Surgery, Division of Otolaryngology–Head and Neck Surgery (Dr. Slonimsky, Dr. Baker, and Dr. Goldenberg) and the Department of Pathology (Dr. Crist), The Pennsylvania State University, College of Medicine, Hershey.
Slonimsky, MD; Aaron Baker, MD; Henry Crist, MD; David Goldenberg, MD
A B
Figure 1. A: Axial CT with contrast of the neck and chest demonstrates a homogeneous cystic lesion overlying the inferior border of the thyroid gland, anterior border of the trachea, and the great mediastinal vessels. B: Coronal CT of the cyst demonstrates its cervical and mediastinal components with splaying of the innominate veins.

within its fibrous wall, indicative of a parathyroid cyst (figure 2).
Parathyroid cysts are rare lesions of the neck and mediastinum with a peak incidence in the fourth and fifth decades of life.1-3 These cysts are generally divided into nonfunctional or functional, based on their hormonal activity, and can rarely lead to hypercalcemic crisis. 3-5 There are several theories for the pathogenesis of these cysts, including remnants of branchial clefts, retention cysts, coalescence of numerous minute cystic lesions, or degeneration of an adenoma.3
The clinical presentation is varied and ranges from an incidental finding on imaging or physical examination in the asymptomatic patient to a neck mass with compressive symptoms, recurrent laryngeal nerve palsy, and sometimes hyperparathyroidism.2-4,6 The mainstay of diagnosis includes neck ultrasonography, CT, or magnetic resonance imaging, with nonspecific findings of a benign-appearing homogeneous cystic lesion.7 Detection of parathyroid hormone in fine-needle aspiration can facilitate the diagnosis, as parathyroid cysts can be easily misdiagnosed as thyroid cysts or other cystic lesions of the neck.8 Histopathology will reveal a smooth cystic lesion lined with cuboidal epithelium and nests of parathyroid cells within its wall.9
Nonfunctioning asymptomatic cysts can be observed with routine follow-up. Symptomatic nonfunctioning cysts can be treated with fluid aspiration2 or sclerotherapy in recurrent cases with the risk of fibrosis.10,11 Although needle aspiration may lead to definitive resolution in some cases,12 surgical resection is warranted in many cases as a diagnostic procedure, for the treatment of hyperparathyroidism (in cases of functional cysts), and because of the rare possibility of
parathyroid carcinoma presenting as a cystic neck or mediastinal mass.13,14

The location and size of the parathyroid cyst will dictate the appropriate surgical approach. The cervical approach will enable the resection of lesions in the neck and upper mediastinum, whereas large mediastinal lesions and lower or posterior mediastinal lesions will require thoracotomy, median sternotomy, or thoracoscopy.15
References
1. Cappelli C, Rotondi M, Pirola I, et al. Prevalence of parathyroid cysts by neck ultrasound scan in unselected patients. J Endocrinol Invest 2009;32(4):357–9.
2. Ippolito G, Palazzo FF, Sebag F, et al. A single-institution 25-year review of true parathyroid cysts. Langenbecks Arch Surg 2006; 391(1):13–18.
3. Shields TW, Immerman SC. Mediastinal parathyroid cysts revisited. Ann Thorac Surg 1999;67(2):581–90.
4. Suzuki K, Sakuta A, Aoki C, Aso Y. Hyperparathyroidism caused by a functional parathyroid cyst. BMJ Case Rep 2013 June 21;2013. pii: bcr2012008290
5. Khan A, Khan Y, Raza S, et al. Functional parathyroid cyst: A rare cause of malignant hypercalcemia with primary hyperparathyroidism—a case report and review of the literature. Case Rep Med 2012;2012:851941.
6. Jarnagin WR, Clark OH. Mediastinal parathyroid cyst causing persistent hyperparathyroidism: Case report and review of the literature. Surgery 1998;123(6):709–11.
7. Kato H, Kanematsu M, Kiryu T, et al. Nonfunctional mediastinal parathyroid cyst: Imaging findings in two cases. Clin Imaging 2008;32(4):310–13.
8. Ujiki MB, Nayar R, Sturgeon C, Angelos P. Parathyroid cyst: Often mistaken for a thyroid cyst. World J Surg 2007;31(1):60–4.
9. Fortson JK, Patel VG, Henderson VJ. Parathyroid cysts: A case report and review of the literature. Laryngoscope 2001;111(10): 1726–8.
10. Kaplanoglu V, Kaplanoglu H, Ciliz DS, Duran S. A rare cystic lesion of the neck: Parathyroid cyst. BMJ Case Rep 2013 Oct 11;2013. pii: bcr2013200813
11. Kim JH. Ultrasound-guided sclerotherapy for benign non-thyroid cystic mass in the neck. Ultrasonography 2014;33(2):83–90.
12. Prinz RA, Peters JR, Kane JM, Wood J. Needle aspiration of nonfunctioning parathyroid cysts. Am Surg 1990;56(7):420–22.
13. Vazquez FJ, Aparicio LS, Gallo CG, Diehl M. Parathyroid carcinoma presenting as a giant mediastinal retrotracheal functioning cyst. Singapore Med J 2007;48(11):e304-7.
14. Pirundini P, Zarif A, Wihbey JG. A rare manifestation of parathyroid carcinoma presenting as a cystic neck mass. Conn Med 1998;62(4):195–7.
15. Dell’Amore A, Asadi N, Bartalena T, et al. Thoracoscopic resection of a giant mediastinal parathyroid cyst. Gen Thorac Cardiovasc Surg 2014;62(7):444–50.
60 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 ThyROId ANd PARAThyROId CLINIC
Figure 2. Nests of parathyroid cells are seen within the wall of the cyst with residual foci lining the cyst wall (arrow) (hematoxylin and eosin stain, original magnification ×100).

SPECIAL TOPICS CLINIC
Complete resolution of guttate psoriasis after tonsillectomy
Psoriasis is a chronic, inflammatory, immunemediated disease that affects 3.2% of adults in the United States.1 Streptococcal infections have been associated with several systemic disease processes including glomerulonephritis and rheumatic heart disease. A well-established link between psoriasis and streptococcal infection also has been reported in the literature.1,2 Specifically, this association appears to be with both guttate and plaque psoriasis.
Although the exact etiology is unclear, it is believed that a streptococcal trigger residing in the palatine tonsils might activate T cells in the skin through molecular mimicry.1 HLA-Cw*0602 homozygosity has been linked to streptococcal-associated psoriasis.3 Several studies have reported improvement or complete resolution of psoriasis after tonsillectomy.1,2,4-8
A 26-year-old man presented to our office with a history of recurrent streptococcal tonsillitis. One episode of tonsillitis was treated with oral levofloxacin by his primary care physician. However, because the patient
developed joint pain after several days of treatment, it was discontinued.
Two weeks later, the patient began to develop a rash over his hands (figure 1), trunk, back, and extremities. The patient was evaluated by a dermatologist and was subsequently diagnosed with guttate psoriasis. It was further described as a salmon-pink, teardrop-shaped papular rash with a fine scale. This rash was treated with topical steroids.


Several months later the rash resolved, but it returned with another episode of tonsillitis. Given the recurrent episodes of tonsillitis, the patient underwent tonsillectomy 2 months later. After surgery, the rash completely resolved (figure 2). On his back, some hypopigmented lesions from scarring remained, but these are expected to improve over time. The patient currently does not require treatment for psoriasis; his rash has not returned to date.
Several studies have demonstrated the efficacy of tonsillectomy for the treatment of streptococcal-associated
62 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
From the Department of Otolaryngology–Head and Neck Surgery, Philadelphia College of Osteopathic Medicine (Dr. Cohn and Dr. Pfeiffer); and the Department of Otolaryngology–Head and Neck Surgery, Jefferson Health–Methodist Hospital (Dr. Vernose), Philadelphia.
Jason E. Cohn, DO; Michael Pfeiffer, DO; Gerard Vernose, MD
Figure 1. Photo shows the guttate psoriasis on the patient’s hands before tonsillectomy.
Figure 2. This photo shows the patient’s hands 2 months after tonsillectomy. The psoriasis has resolved completely.
psoriasis. One meta-analysis revealed that 290 of 410 (70.7%) patients with psoriasis who underwent tonsillectomy experienced improvement of their psoriasis.1 The authors concluded that evidence is insufficient to support tonsillectomy in most patients with psoriasis. However, a tonsillectomy might be beneficial when psoriasis exacerbations are closely associated with recurrent tonsillitis.1
A randomized, controlled trial demonstrated that patients with plaque psoriasis undergoing tonsillectomy had significantly improved health-related quality of life and psoriasis-related stress.5 A Cochrane database protocol states that adult tonsillectomy for psoriasis is beneficial, but the evidence for this is not robust.6

It has been shown, especially with guttate psoriasis, that tonsillectomy has a potential benefit in psoriasis disease burden.1,5,7,8 Our patient demonstrated complete recovery after tonsillectomy. The decision to perform a tonsillectomy in this case was appropriate because the patient’s psoriasis flares corresponded with episodes of tonsillitis. It is important to highlight this case because it involved an adult, although tonsillitis-associated guttate psoriasis is typically reported in the pediatric population.4,7,8 Additionally, we demonstrated a significant improvement in this disease process with photographic evidence. Future prospective studies looking at the efficacy of tonsillectomy on psoriasis are encouraged.
References
1. Rachakonda TD, Dillon JS, Florek AG, Armstrong AW. Effect of tonsillectomy on psoriasis: A systematic review. J Am Acad Dermatol 2015;72(2):261-75.
2. Sigurdardottir SL, Thorleifsdottir RH, Valdimarsson H, Johnston A. The role of the palatine tonsils in the pathogenesis and treatment of psoriasis. Br J Dermatol 2013;168(2):237-42.
3. Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, et al. HLA-Cw6 homozygosity in plaque psoriasis is associated with streptococcal throat infections and pronounced improvement after tonsillectomy: A prospective case series. J Am Acad Dermatol 2016;75(5):889-96.
4. Saita B, Ishii Y, Ogata K, et al. Two sisters with guttate psoriasis responsive to tonsillectomy: Case reports with HLA studies. J Dermatol 1979;6(3):185-9.
5. Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, et al. Patient-reported outcomes and clinical response in patients with moderate-to-severe plaque psoriasis treated with tonsillectomy: A randomized controlled trial. Acta Derm Venereol 2017;97(3): 340-5.
6. Owen CM, Chalmers RJ, O’Sullivan T, Griffiths CE. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev 2000(2):CD001976.
7. Hone SW, Donnelly MJ, Powell F, Blayney AW. Clearance of recalcitrant psoriasis after tonsillectomy. Clin Otolaryngol Allied Sci 1996;21(6):546-7.
8. McMillin BD, Maddern BR, Graham WR. A role for tonsillectomy in the treatment of psoriasis? Ear Nose Throat J 1999;78(3):155-8.
Volume 97, Number 3 www.entjournal.com 63 SPECIAL TOPICS CLINIC
Post-turbinectomy nasal packing with Merocel versus glove finger
Merocel: A prospective, randomized, controlled trial
Rani Abu Eta, MD; Ephraim Eviatar, MD; Jacob Pitaro, MD; Haim Gavriel, MD
Abstract
Nasal packs are widely used after septoplasty and turbinectomy. We conducted a prospective, randomized, controlled clinical trial including 100 patients who underwent septoplasty with/or without turbinectomy randomized into two groups. In the first group (the Merocel group), a standard tampon was inserted at the end of surgery. In the second group (the glove finger group), the tampon was first placed inside a glove finger. The main outcomes measured were pain and bleeding during the postoperative period and during tampon removal. Consumption of pain killers and tranexamic acid were also recorded. The mean visual analog scale score 12 hours after surgery and during tampon removal in the Merocel group were 6.78 and 8.92, respectively, compared to 4.06 and 5.27, respectively, in the glove finger group (p < 0.001). A statistically significant difference in the bleeding rate and tranexamic acid consumption during tampon removal in favor of the Merocel group was shown (p < 0.001). The use of Merocel in a glove finger is significantly less painful, although a higher chance of bleeding is reported. The influence of the surgeon’s experience in using this technique needs further investigation.
Introduction
Septoplasty and reduction of the inferior turbinates are the most commonly used surgical interventions to enhance nasal airways compromised secondary to nasal septal deviations and turbinate hypertrophy.
From the Department of Otolaryngology Head and Neck Surgery, Assaf Harofeh Medical Center, Zerifin, Israel, affiliated to the Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv, Israel.
Corresponding author: Dr. Haim Gavriel, Department of Otolaryngology Head and Neck Surgery, Assaf Harofeh Medical Center, Zerifin 70300, Israel. Email: haim.ga@012.net.il
Nasal packs are widely used after these operations to prevent nasal bleeding, to support the mucoperichondrial flaps, and to minimize the risk of forming septal hematomas and prevent adhesions.1
Since Hippocrates first defined nasal tampon packing in the fifth century BC, 2 various materials have been proposed for nasal packing, such as gauzes with or without medication (e.g., paraffin), Telfa cellulose and foam, Merocel (a polyvinyl acetate sponge; Medtronic Xomed; Jacksonville, Fla.), expanding sponges, ready-made or inflatable tampons, and Foley catheters. Ideally, nasal packs should be easy to insert and remove with minimal discomfort, and they should also effectively prevent postoperative bleeding. 3,4
The use of nasal packing may result in several side effects that include pain, dysphagia, eustachian tube blockage, sinusitis, synechia and toxic shock syndrome.5-7 Aspiration of nasal packing and airway obstruction have also been reported.8 However, the most frequent patient complaints appear to be pain and bleeding during removal of nasal tampons.
Merocel dressings are widely used after endonasal surgery.9,10 To reduce pain during their removal, several studies have proposed using Merocel within a glove finger after septoplasty and endoscopic sinus surgery.7,11 However, a higher risk of bleeding post-turbinectomy is expected and, to the best of our knowledge, no study on the use of Merocel in a glove finger has been reported in the English literature.
In this study, we prospectively compared the effects of two different types of nasal packs on pain and bleeding after turbinectomy with or without septoplasty: Merocel standard 8-cm nasal dressing and Merocel in a glove finger.
64 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 ORIGINAL ARTICLE
Patients and methods
A prospective, randomized, controlled clinical trial comparing Merocel nasal pack and Merocel within a glove finger regarding pain and bleeding before and during tampon removal was conducted. The study was approved by the Institute Ethical Committee.
The study group included 100 consecutive patients with an indication for septoplasty with turbinectomy or turbinectomy alone. Twelve operations were performed under general anesthesia and 88 under local anesthesia. Local homeostasis was achieved with lidocaine HCl 1% and epinephrine 1:100,000.
Excluded were patients younger than 18 years, patients with a history of previous endoscopic sinus surgery (ESS) or underlying immunologic diseases, patients with Samter’s triad, those allergic to latex, and those who reported using anticoagulants or antiaggregants.
Surgical technique. Surgery was carried out under general or local anesthesia. After proper premedication, the nasal cavities were packed with 2% lidocaine with epinephrine (1: 100,000) and then removed after several minutes. Angled turbinate scissors were used for this procedure. One blade was inserted beneath the inferior turbinate and the other on top of it after the turbinate was fractured medially. Resection included the turbinate mucosa and, partially, the bone. The extent of the resection depended on the degree of turbinate hypertrophy.
The patients were randomized into two groups according to the last digit of their identity number. Patients in the first group were packed at the end of surgery with a standard 8-cm Merocel tampon (Merocel group). Patients in the second group were packed with a Merocel tampon of the same size that was placed in a glove finger of a regular sterile glove (glove finger group).
All tampons in both groups were secured to the nose with tape and a 2-0 silk stich—through the anterior edge of the tampon in the Merocel group and through the anterior edge of the glove finger in the glove finger group. The same nasal packing materials were used for both sides of the nose for each patient. The nasal tampons were removed 48 hours postoperatively.
Surgical outcomes. The main outcomes measured were pain and bleeding during the postoperative period. Pain was evaluated by a nurse using a visual analog scale (VAS) numbered from 0 to 10 (0 representing the least pain and 10 the maximum pain) at two times: 12 hours after surgery and during the removal of the tampons. Patient painkiller intake was divided into three categories: category A, per os: dipyrone 1 g
(metamizole in the United States), acetaminophen 1 g, or oxycodone 5 mg and naloxone 2.5mg); category B, per os: oxycodone 5mg and acetaminophen 325 mg or tramadol 50 mg; and category C, intravenous: tramadol 100 mg.
Bleeding after the removal of the nasal tampon was evaluated according to the following scale created by the authors of this study: 0 = no bleeding; 1 = blood seeping from the nose; 2 = continuous bleeding from the nose; 3 = bleeding stopped in the operating room.
The need for intravenous tranexamic acid was recorded in both groups.
Statistical analyses. Categorical variables were reported as numbers (percentages), and continuous variables as means (standard deviations [SD]) or medians (interquartile ranges [IQR]). Continuous variables were tested for normal distribution using the Shapiro–Wilk test and quantile-quantile (q-q) plots. The chi-square test or Fisher exact test was used to compare categorical variables; continuous variables were compared by the independent-samples t test or the Mann-Whitney U test. A two-tailed p value of <0.05 was considered statistically significant. Analyses were performed with R (R Project for Statistical Computing) version 3.1.2.
Results
A total of 100 patients (87 men and 13 women; mean age: 22 years, range 18 to 69 years) were enrolled prospectively during the study period. The Merocel group consisted of 41 patients with a median age of 29 years, and the glove finger group consisted of 59 patients with a median age of 22 years (p = 0.012) (table 1).
Comorbidities were identified in 5 (5%) patients: 3 patients had hypertension, 1 patient had ischemic heart disease, and 1 patient had hypercholesterolemia.
The median VAS scores 12 hours after surgery and during tampon removal in the Merocel group were 7 and 9, respectively, compared to VAS scores of 4 and 5, respectively, in the glove finger group (p < 0.001) (table 1).
All patients in the Merocel group consumed an analgesic; 11 (18.6%) patients in the glove finger group did not (p = 0.002). Six (14.6%) patients in the Merocel group needed category A painkillers, 25 (61.0%) needed category B painkillers, and 10 (24.4%) needed category C painkillers. In the glove finger group, 11 (18.6%) patients needed no painkillers at all, 40 (67.8%) patients were treated with category A painkillers, and 8 (13.6%) with category B painkillers.
Thirty-five patients (85.4%) in the Merocel group showed no signs of bleeding during tampon removal
Volume 97, Number 3 www.entjournal.com 65
POST-TURbINECTOmy NASAL PACkINg wITh mEROCEL vERSUS gLOvE fINgER mEROCEL: A PROSPECTIvE, RANdOmIzEd, CONTROLLEd TRIAL
Key: IQR = interquartile range; SMR = submucous resection;VAS = visual analog scale.
compared to only 2 (3.4%) patients in the glove finger group ( p < 0.001). Mild bleeding was observed in the other 6 (14.6%) patients in the Merocel group. However, in the glove finger group, mild bleeding was detected in 27 (45.8%) patients, continuous bleeding in another 27 (45.8%) patients, and in 3 (5.1%) patients the bleeding was stopped only in the operating room ( p < 0.001) (table 2).
In the Merocel group, 2 (4.9%) patients needed intravenous administration of tranexamic acid (1 g) after tampon removal due to continuous bleeding compared to 19 (32.2%) patients in the glove finger group (p = 0.001) (table 2).
Discussion
Septoplasty and reduction of the inferior turbinates are among the most common surgical interventions in otorhinolaryngology. Since these operations may lead to significant bleeding postoperatively, nasal packing is widely used to reduce bleeding. The use of nasal packing may, by itself, lead to severe complications. One of the most significant concerns of patients is pain during removal of the tampon while that of the surgeon is postoperative bleeding.12
Merocel packing is the most commonly used material after nasal surgery, with well-known advantages and reported complications and disadvantages such as pain and bleeding. Endeavors to alleviate the disadvantages of Merocel tampons by using glove fingers during ESS and post-septoplasty have been previously reported.1,3-5 In these studies, the major benefit of using a Merocel tampon in a glove finger was the reduced pain reported by the patients during tampon removal. No significant difference was observed in these studies regarding bleeding after the removal of the tampons. However, none of these studies investigated the outcomes after inferior turbinectomy.
Major bleeding during or after septoplasty is seldom observed due to the good visualization of the surgical field during the operation.7 In cases of significant
66 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 AbU ETA, EvIATAR, PITARO,
gAvRIEL
Merocel group (n = 41) Glove finger group (n = 59) p Value Sex, n (%) male 35 (85.4) 52 (88.1) 0.685 female 6 (14.6) 7 (11.9) median age, yr (IQR) 29 (21-41.5) 22 (20-25) 0.012 Surgery, n (%) SmR conchotomy 37 (90.2) 54 (91.5) >0.999 Conchotomy 4 (9.8) 5 (8.5) Anesthesia, n (%) Local 33 (80.5) 55 (93.2) 0.066 general 8 (19.5) 4 (6.8) Analgesics, n (%) 41 (100) 48 (81.4) 0.002 Pain killers, n (%) None 0 (0) 11 (18.6) <0.001 Category A 6 (14.6) 40 (67.8) Category b 25 (61.0) 8 (13.6) Category C 10 (24.4) 0 (0) vAS during hospitalization, median (IQR) 7 (6-7) 4 (3-5) <0.001 vAS during removal, median (IQR) 9 (9-9.5) 5 (4-6) <0.001
Table 1. Differences in pain and pain control in the Merocel group vs. the glove finger group
Merocel group (n = 41) Glove finger group (n = 59) p Value Tranexamic acid, n (%) 2 (4.9) 19 (32.2) 0.001 bleeding, n (%) 6 (14.6) 57 (96.6) <0.001 bleeding grade, n (%) No bleeding 35 (85.4) 2 (3.4) <0.001 blood seeping from nose 6 (14.6) 27 (45.8) Continuous bleeding from nose 0 (0) 27 (45.8) bleeding stopped in OR 0 (0) 3 (5.1)
Table 2. Differences in bleeding between the Merocel and glove finger groups

bleeding, cauterization is feasible and successful in most cases. ESS harbors great risk to major vessels with possible significant bleeding. However, the magnified surgical field and the ability of the surgeon to work with two hands enables better bleeding control. ESS should be finalized with no significant bleeding and, therefore, the use of a tampon in a glove finger is mostly effective.
Inferior turbinectomy poses a unique challenge regarding nasal packing. In many cases, it will not be performed with endoscopic surgery, and the surgeon relies on his or her ability to view the turbinates throughout their entire length. Moreover, major bleeding from major branches of the sphenopalatine artery is usually encountered from the posterior half of the injured turbinate. Therefore, optimal packing should be used post-turbinectomy, especially when it is performed by a lesser-trained surgeon.
The results of the current study indicate that nasal packing with Merocel carries significantly higher pain scores when compared to the use of Merocel in a glove finger, either after septoplasty with inferior turbinectomy or turbinectomy alone. We attributed the lower degree of pain reported in patients in the glove finger group to less adherence of the Merocel glove finger to the structures inside the nose, thereby lowering the amount of superficial tissue injured by the exiting tampon and resulting in significantly less consumption of painkillers.13,14
Our study also demonstrates statistically significant greater postoperative bleeding when using a Merocel tampon in a glove finger, with a significantly greater need for intravenous tranexamic acid. Unfortunately, the advantage of less pain due to less adherence of the packing to the tissue and a smaller amount of tissue trauma during the pack removal brings with it the disadvantage of bleeding. As the amount of tissue in close contact with a Merocel tampon in a glove finger is reduced compared to Merocel, a higher chance of blood vessels not directly affected by the influence of the tampon is anticipated, leading to a higher chance of postoperative bleeding.
It is important to mention that all patients reported in this series were operated on by our skilled residents, experienced with these types of surgeries. However, we believe that an experienced consultant surgeon would have targeted the placement of the Merocel tampon in a glove finger to the higher bleeding risk areas of the nose, thereby possibly lowering the chances of postoperative bleeding.
Conclusion
The use of Merocel in a glove finger is significantly less painful for the patient than Merocel directly inserted into the nostril, although a higher risk of bleeding is demonstrated. We are preparing for a comparative study to investigate the effect of the surgeon’s experience on the bleeding rate post-turbinectomy when using Merocel in a glove finger.
References
1. Acio lu E, Edizer DT, Yi it Ö, Alkan Z. Nasal septal packing: Which one? Eur Arch Otorhinolaryngol 2012;269(7):1777–81.
2. Singer AJ, Blanda M, Cronin K, et al. Comparison of nasal tampons for the treatment of epistaxis in the emergency department: A randomized controlled trial. Ann Emerg Med 2005; 45(2):134–9.
3. Ozcan C, Vayisoglu Y, Kiliç S, Görür K. Comparison of rapid rhino and merocel nasal packs in endonasal septal surgery. J Otolaryngol Head Neck Surg 2008;37(6):826–31.
4. Bresnihan M, Mehigan B, Curran A. An evaluation of Merocel and Series 5000 nasal packs in patients following nasal surgery: A prospective randomised trial. Clin Otolaryngol 2007;32(5):352–5.
5. Ardehali MM, Bastaninejad S. Use of nasal packs and intranasal septal splints following septoplasty. Int J Oral Maxillofac Surg 2009;38(10):1022-4.
6. Taasan V, Wynne JW, Cassisi N, Block AJ. The effect of nasal packing on sleep-disordered breathing and nocturnal oxygen desaturation. Laryngoscope 1981;91(7):1163-72.
7. Celebi S, Caglar E, Develioglu ON, et al. The effect of the duration of merocel in a glove finger on postoperative morbidity. J Craniofac Surg 2013;24(4):1232-4.
8. Koudounarakis E, Chatzakis N, Papadakis I, et al. Nasal packing aspiration in a patient with Alzheimer’s disease: A rare complication. Int J Gen Med 2012;5:643-5.
9. Badran K, Malik TH, Belloso A, Timms MS. Randomized controlled trial comparing Merocel and RapidRhino packing in the management of anterior epistaxis. Clin Otolaryngol 2005;30(4):333–7.
10. Corbridge RJ, Djazaeri B, Hellier WP, Hadley J. A prospective randomized controlled trial comparing the use of merocel nasal tampons and BIPP in the control of acute epistaxis. Clin Otolaryngol Allied Sci 1995;20(4):305–7.
11. Garth RJ, Brightwell AP. A comparison of packing materials used in nasal surgery. J Laryngol Otol 1994;108(7):564–6.
12. Eliashar R, Gross M, Wohlgelernter J, Sichel JY. Packing in endoscopic sinus surgery: Is it really required? Otolaryngol Head Neck Surg 2006;134(2):276-9.
13. Lemmens W, Lemkens P. Septal suturing following nasal septoplasty, a valid alternative for nasal packing? Acta Otorhinolaryngol Belg 2001;55(3):215-21.
14. Weber R, Keerl R, Hochapfel F, et al. Packing in endonasal surgery. Am J Otolaryngol 2001;22(5):306–20.
68 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
AbU ETA, EvIATAR, PITARO, gAvRIEL
Comparison of anterior palatoplasty and uvulopalatal flap placement for treating mild and moderate obstructive sleep apnea
Süheyl Haytoğlu, MD; Osman Kürşat Arikan, MD; Nuray Bayar Muluk, MD; Birgül Tuhanioğlu, MD; Mustafa Çörtük, MD
Abstract
We prospectively compared the efficacy of anterior palatoplasty and the uvulopalatal flap procedure for the treatment of patients with mild and moderate obstructive sleep apnea syndrome (OSAS). Our study group was made up of 45 patients who had been randomly assigned to undergo one of the two procedures. Palatoplasty was performed on 22 patients—12 men and 10 women, aged 28 to 49 years (mean: 39.2)—and the flap procedure was performed on 23 patients—14 men and 9 women, aged 28 to 56 years (mean: 41.3). Our primary outcomes measure was the difference in pre- and postoperative apnea-hypopnea index (AHI) as determined by polysomnography at 6 months after surgery. Surgical success was observed in 18 of the 22 palatoplasty patients (81.8%) and in 19 of the 23 flap patients (82.6%). Compared with the preoperative values, mean AHIs declined from 17.5 to 8.1 in the former group and from 18.5 to 8.6 in the latter; the improvement in both groups was statistically significant (p < 0.001). In addition, significant postoperative improvements in both groups were seen in mean visual analog scale (VAS) scores for snoring, in Pittsburgh Sleep Quality Index values, and in Epworth Sleepiness Scale scores (p < 0.001 for all). VAS scores for pain at rest were significantly lower in the palatoplasty group than in the flap group at 2, 4, and 8 hours postoperatively and on postoperative days 4 through 7 (p <
From the ENT Clinic, Adana Numune Training and Research Hospital, Adana, Turkey (Dr. Haytoğlu, Prof. Arikan, and Dr. Tuhanioğlu); the ENT Department, Kırıkkale University Faculty of Medicine, Kırıkkale, Turkey (Prof. Muluk); and the Pulmonary Diseases Department, Karabük University Faculty of Medicine, Karabük, Turkey (Dr. Çörtük). The study described in this article was conducted at Adana Numune Training and Research Hospital. Corresponding author: Prof. Nuray Bayar Muluk, ENT Department, Kırıkkale University Faculty of Medicine, Birlik Mahallesi, Zirvekent 2. Etap Sitesi, C-3 blok, No: 62/43 06610 Çankaya, Kırıkkale, Turkey. Email: nurayb@hotmail.com
0.002). Likewise, VAS scores for pain during swallowing were significantly lower in the palatoplasty group at 2, 4, 8, and 16 hours and on days 4 through 7 (p < 0.009). We conclude that both anterior palatoplasty and uvulopalatal flap procedures are effective for the treatment of mild and moderate OSAS in patients with retropalatal obstruction. However, our comparison of postoperative pain scores revealed that anterior palatoplasty was associated with significantly less morbidity.
Introduction
Obstructive sleep apnea syndrome (OSAS) is characterized by episodes of partial or complete obstruction of the upper airway during sleep, which results in interruptions (apnea) or reductions (hypopnea) of the flow of air; the transient awakening that follows leads to the restoration of upper airway permeability.1 In the upper airway, the retropalatal area is the most common site of the obstructive process.2,3
Since Fujita et al first described uvulopalatopharyngoplasty (UPPP) in 1981,4 many studies have been published about its efficacy and complication rates. Studies have shown that while short-term success rates ranged from 76 to 95%, rates after 1 year fell to only 46 to 50%.5-9
Since UPPP has been associated with high recurrence and complication rates and patient discomfort, other procedures have been introduced to restore the space at the retropalatal area.9,10 One of them is the uvulopalatal flap procedure, which was first described by Powell et al in 1996.11 Studies showed that flap placement yielded success rates similar to those of UPPP but with less postoperative pain.11-13 Nevertheless, postoperative pain is still a common complication of the uvulopalatal flap procedure, as is temporary velopharyngeal insufficiency and a permanent foreign-body sensation in the pharynx.12,13
Volume 97, Number 3 www.entjournal.com 69 ORIGINAL ARTICLE
A modified cautery-assisted palatal stiffening procedure was introduced by Pang and Terris in 2007 as an alternative to the flap procedure.14 They subsequently called this procedure anterior palatoplasty.15 In their first study, which looked at a small series of patients with mild OSAS, they observed surgical success in 6 of 8 patients (75.0%) as determined by polysomnography, as well as a reduction in daytime sleepiness scores in 11 of 13 patients (84.6%).14 In a subsequent study of 77 patients published in 2009, Pang et al found a success rate of 71.8% for anterior palatoplasty with or without tonsillectomy at a mean of 33.5 months of follow-up.15 With anterior palatoplasty, temporary velopharyngeal insufficiency and postoperative pain are also common, but a foreign-body sensation is not.
In a 2013 study by Marzetti et al, success rates for anterior palatoplasty and the uvulopalatal flap procedure were 86 and 84%, respectively.16 The authors reported lower pain scores in the palatoplasty group. In both groups, they performed a tonsillectomy first if the tonsil size was greater than grade 2. Previous studies of palatal surgeries in OSAS patients had not considered the possible effects of tonsillectomy on success rates and postoperative discomfort, especially pain.
In this article, we describe our comparison of the efficacy of anterior palatoplasty and the uvulopalatal flap procedure for the treatment of mild and moderate OSAS in patients with retropalatal obstruction who did not require a tonsillectomy.
Patients and methods
For this prospective study, we recruited 50 patients who had been diagnosed with mild or moderate OSAS in the ENT Clinic at Adana Numune Training and Research Hospital from June 2012 through January 2014. All diagnoses had been based on the results of polysomnography. All patients had undergone a complete head and neck examination, including flexible fiberoptic nasopharyngoscopy to evaluate the posterior airway space. All were found to have retropalatal collapse, based on the Müller maneuver.
Our inclusion criteria were an apnea-hypopnea index (AHI) of greater than 5 and less than 30, a body mass index (BMI) of less than 30 kg/m 2 , a tonsillar hypertrophy grade of 0 or 1,17 and the presence of retropalatal obstruction. Exclusion criteria included previous palatal surgery, the presence of a retrolingual obstruction, and the presence of a chronic disease that prevented surgery.
Of the 50 patients, 25 were randomly assigned to undergo anterior palatoplasty and 25 were randomized to uvulopalatal flap surgery. Tonsillectomy was not performed in either group. During surgery, sutures became unraveled in 3 palatoplasty patients and 2 flap
patients, and thus these cases were not included in our final analysis. As a result, our final study population was made up of 45 patients. The palatoplasty group included 22 patients—12 men and 10 women, aged 28 to 49 years (mean: 39.2)—and the flap group included 23 patients—14 men and 9 women, aged 28 to 56 years (mean: 41.3).
Surgical procedures. Anterior palatoplasty. All palatoplasties were performed with general anesthesia. First, the mucosa and submucosal tissue (including fat) were removed as a horizontal rectangular strip (70 to 100 mm long and 40 to 50 mm wide) from the lingual surface of the soft palate down to the muscle layer. Bipolar cautery was used for hemostasis. The remaining tissue was sutured with 4-0 Vicryl and a round-body curved needle.
Uvulopalatal flap procedure. All flap procedures were likewise performed with general anesthesia. After insertion of a Dingman mouth gag, the projection formed by folding the uvula toward the soft palate was determined and the mucosa, submucosa with glands, and the fat on the lingual surface of the uvula and soft palate according to the determined area were removed with a scalpel. Then the uvular tip was amputated. Once bleeding was controlled, horizontal incisions were made to the upper part of the posterior plicas of the tonsils. The uvula was folded into its new position and was fixed with 3-0 Vicryl sutures.
During the postoperative period, patients were administered analgesia in the form of acetaminophen at 250 mg/5 ml in doses of 10 to 15 mg/kg per dose 4 times a day.18 Patients were permitted to take additional acetaminophen as long as the total daily dose did not exceed 4 g. All acetaminophen intake was recorded.
Data collection. In addition to demographic information, we compiled pre- and postoperative data on patients’ AHI, snoring, sleep quality, daytime sleepiness, and postoperative pain. We also recorded the duration of surgery and the amount of intraoperative blood loss.
AHI. The primary outcomes measure was the difference between pre- and postoperative AHI at 6 months after surgery as determined by polysomnography. The other findings were secondary outcomes.
Snoring. Snoring was assessed by bed partners preoperatively and 6 months postoperatively with the use of a visual analog scale (VAS) of 0 (none) to 10 (extremely loud).
Quality of sleep. We assessed the quality of sleep with the Pittsburgh Sleep Quality Index (PSQI), which is one of the most commonly used scales in sleep research.19 It was originally designed for use in clinical populations as a simple and valid assessment of both sleep quality and sleep disturbance that might affect sleep quality.
70 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
hAyTO LU, ARIkAN, mULUk, TUhANIO LU, ÇÖRTÜk


The advantages of the PSQI include its ability to (1) discern patterns of sleep dysfunction over a stipulated period of 1 month by assessing qualitative as well as quantitative data and (2) allow for the calculation of a simple global score that can convey both the number and severity of sleep problems for use in both clinical practice and research.
The PSQI consists of 19 individual items that produce seven component scores and a global sleep quality score. The seven components include sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleeping medications, and daytime dysfunction. These components were chosen on the basis of the standard areas that are assessed by clinicians during routine examinations of sleep problems. The PSQI is based on variable response categories pertaining to a patient’s usual bedtime, usual wake time, the number of hours actually slept, and the number of minutes that passed before falling asleep, as well as forced-choice Likert-type responses.
The total PSQI score can range from 0 to 21. A score of more of than 5 indicates poor sleep quality.
Daytime sleepiness. The Epworth Sleepiness Scale (ESS) is an 8-item questionnaire designed to capture an individual’s propensity to fall asleep during commonly encountered situations.13 Each item score ranges from 0 to 3, and the total score can range from 0 to 24. In adults, an ESS score of 10 or more is taken to indicate increased daytime sleepiness.20
Pain. Patients’ postoperative pain was evaluated at 2, 4, 8, 16, and 24 hours and once each on days 2, 3, 4, 5, 6, and 7. We assessed pain at rest and pain during swallowing on a VAS of 0 (none) to 10 (worst pain imaginable).
Statistical analysis. Statistical analysis was performed with the Statistical Package for the Social Sciences software (v. 16.0). Analyses were performed with the chi-square test, Mann-Whitney U test, and Wilcoxon
signed rank test. A p value <0.05 was considered statistically significant.
Ethical considerations. This study was conducted in accordance with the principles of the Declaration of Helsinki.21 Approval of the study protocol was granted by the Ethics Committee at Adana Numune Training and Research Hospital. All patients provided written informed consent.
Results
Sex and age. There were no statistically significant differences between the two study groups in terms of sex (p = 0.668) or age (p = 0.290).
BMI, duration of surgery, and blood loss. There were no statistically significant differences in BMI between the two groups and no significant differences within groups pre- and postoperatively. The mean duration of surgery was 29.1 minutes in the palatoplasty group and 32.9 minutes in the flap group (p = 0.057). The mean amount of intraoperative blood loss in the two groups was 6.8 and 7.4 ml, respectively (p = 0.991) (table 1).
AHI. The mean AHI in the palatoplasty group improved significantly from 17.5 before surgery to 8.1 at 6 months postoperatively (p < 0.001). In the flap group, the AHI fell from 18.5 to 8.6 (p < 0.001). There were no significant differences between the two groups either pre- or postoperatively (table 2).
Based on the AHI, we determined that the surgical success rates were 81.8% in the palatoplasty group (18 of 22 patients) and 82.6% in the flap group (19 of 23 patients). We defined success as described by Sher et al 22 as a postoperative reduction of at least 50% in the AHI combined with a postoperative AHI of less than 10.
Snoring. The VAS score for snoring was 8.1 preoperatively and 3.0 postoperatively (p < 0.001) in the palatoplasty group and 8.6 and 2.8, respectively, in the
* Indicates p value according to the Mann-Whitney U test, reflecting differences between the two groups. † Indicates p value according to the Wilcoxon signed rank test, reflecting differences between pre- and postoperative values.
Volume 97, Number 3 www.entjournal.com 73
COMPARISON OF ANTERIOR PALATOPLASTY AND UVULOPALATAL FLAP PLACEMENT FOR TREATING MILD AND MODERATE OBSTRUCTIVE SLEEP APNEA
Palatoplasty group Flap group Statistical difference* Variable Mean ± SD Range Mean ± SD Range bmI Preoperative 28.0 ± 1.6 24.8 to 29.9 27.3 ± 1.8 23.4 to 29.9 p = 0.266 Postoperative 27.9 ± 1.4 25.1 to 29.7 27.3 ± 1.7 23.0 to 29.6 p = 0.195 Statistical difference† p = 0.797, z = –0.257 p = 0.722, z = –0.356 duration of surgery, min 29.1 ± 6.4 20.0 to 40.0 32.9 ± 5.9 23.0 to 42.0 p = 0.057 blood loss, ml 6.8 ± 2.9 5.0 to 15.0 7.4 ± 4.2 5.0 to 20.0 p = 0.991
Table 1. Comparison of BMI values, duration of surgery, and amount of intraoperative blood loss between the two groups
Key: VAS = visual analog score (0 to 10); PSQI = Pittsburgh Sleep Quality Index (0 to 21); ESS = Epworth Sleepiness Scale (0 to 24).
* Indicates p value according to the Mann-Whitney U test, reflecting differences between the two groups.
† Indicates p value according to the Wilcoxon signed rank test, reflecting differences between pre- and postoperative values.
flap group (p < 0.001). Again, the differences between groups were not significant (table 2).
Sleep quality. The PSQI score in the palatoplasty group was 5.9 preoperatively and 4.3 postoperatively (p < 0.001); in the flap group, the corresponding scores were 6.7 and 4.7 (p < 0.001). The differences between the two groups at both assessments were not significant (table 2).
Daytime sleepiness. ESS values in the palatoplasty group improved from 13.6 to 6.4 (p < 0.001), and in the flap group they improved from 10.8 to 5.4 (p < 0.001). The differences between the two groups were not significant (table 2).
Postoperative pain. VAS scores for postoperative pain at rest were significantly lower in the palatoplasty group than in the flap group at postoperative hours 2, 4, and 8 and on days 4 through 7 (p < 0.002). Scores in the palatoplasty group peaked throughout the first 24 hours after surgery. In the flap group, scores were likewise highest on the day of surgery, and they generally declined afterward (figure 1).
Likewise, scores for pain during swallowing were significantly lower in the palatoplasty group at 2, 4, 8, and 16th hours and on days 4 through 7 (p < 0.009). Scores in both groups generally declined after day 1 (figure 2).
Other complications. No primary or secondary hemorrhage or velopharyngeal insufficiency was observed in any patient. In the flap group, 8 patients reported a continuing foreign-body sensation in the pharynx at 6 months postoperatively; only 1 patient in the palatoplasty group reported the same.
Discussion
The goal of surgery in OSAS patients is to create greater airway dimensions while causing minimal postoperative complications after the operation.23 One of the complications that surgeons wish to avoid is postoperative discomfort.
In our study, we found that anterior palatoplasty and the uvulopalatal flap procedure were equally efficacious in treating OSAS. There were no significant differences in outcomes between the two groups in terms of the duration of surgery, intraoperative blood loss, and postoperative AHI, snoring, sleep quality, and daytime sleepiness. Moreover, in both groups, the AHI, snoring, PSQI, and ESS values were all significantly lower at 6 months postoperatively than they were preoperatively. The only significant difference we observed between the two groups was that the degree of postoperative
74 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 hAyTO LU, ARIkAN,
TUhANIO LU, ÇÖRTÜk
mULUk,
Palatoplasty group Flap group Statistical difference* Variable Mean ± SD Range Mean ± SD Range AhI Preoperative 17.5 ± 8.2 5.8 to 29.9 18.5 ± 7.9 6.1 to 29.7 p = 0.555 Postoperative 8.1 ± 7.3 1.4 to 32.5 8.6 ± 6.9 2.1 to 32.1 p = 0.593 Statistical difference† p < 0.001, z = –4.043 p < 0.001, z = –4.000 Snoring (vAS score) Preoperative 8.1 ± 1.7 5 to 10 8.6 ± 1.4 5 to 10 p = 0.376 Postoperative 3.0 ± 2.7 0 to 10 2.8 ± 2.9 0 to 10 p = 0.736 Statistical difference† p < 0.001, z = –3.835 p < 0.001, z = –3.911 Sleep quality (total PSQI) Preoperative 5.9 ± 1.9 4 to 9 6.7 ± 1.5 4 to 10 p = 0.090 Postoperative 4.3 ± 1.2 3 to 8 4.7 ± 1.2 3 to 9 p = 0.142 Statistical difference† p < 0.001, z = –3.588 p < 0.001, z = –3.498 daytime sleepiness (total ESS) Preoperative 13.6 ± 3.3 9 to 20 10.8 ± 3.3 6 to 18 p = 0.010 Postoperative 6.4 ± 3.3 3 to 18 5.4 ± 4.8 1 to 20 p = 0.010 Statistical difference† p < 0.001, z = –4.023 p < 0.001, z = –3.599
Table 2. Comparison of AHI, snoring, sleep quality, and daytime sleepiness values between the two groups pre- and postoperatively

pain at rest and during swallowing was less in the palatoplasty group.

The success rates in our study—81.8% in the palatoplasty group and 82.6% in the flap group—were similar to those observed in previous studies.14-16 As mentioned, Pang and Terris reported a success rates of 75.0%,14 and Pang et al reported a success rate of 71.8%15 in their studies of anterior palatoplasty.
In the previously mentioned comparison of anterior palatoplasty and uvulopalatal flap placement by Marzetti et al, the success rates with both procedures—86 and 84%, respectively—were very similar to ours.16 In that study, mean ESS values fell from 8.5 ± 3.7 preoperatively to 4.9 ± 3.2 postoperatively (p < 0.001) after anterior palatoplasty and from 8.1 ± 3.5 to 5.2 ± 3.2 after the flap procedure (p < 0.001). A satisfactory reduction in the volume of snoring on polysomnography data was achieved in both groups. In addition, responses to the Müller maneuver improved from 2.7 ± 1.0 to 1.1 ± 0.9 (p < 0.001) after anterior palatoplasty and, to a lesser extent, from 2.8 ± 1.1 to 1.8 ± 1.1; p < 0.05) after the flap procedure. The mean duration of pain was 7.1 and 10.8 days, respectively; mean pain scores during the first 3 days were 5.1 and 6.8. The authors suggested that anterior palatoplasty was more practical and resulted in less discomfort than the flap procedure.
In a study published in 2013, Ugur et al compared the long-term efficacy of anterior palatoplasty and modified uvulopalatopharyngoplasty (mUPPP) in 50 patients.24 The authors evaluated snoring
and daytime sleepiness before and 24 months after surgery, along with postoperative pain. They found that mean snoring VAS scores and ESS scores were significantly lower after surgery in both groups (p < 0.025 for both). Pain scores were significantly lower in the palatoplasty group (p < 0.001). Patient satisfaction scores were 85% in the palatoplasty group and 70% in the mUPPP group.
Cekin et al compared UPPP and uvulopalatal flap placement in OSAS patients and found that snoring was relieved in 85% of the UPPP patients and 83.3% of the flap patients 90 days after surgery.13 The degree of postoperative pain was greater in the UPPP group, and the mean duration of pain was significantly longer. The authors concluded that the uvulopalatal flap procedure was preferable to UPPP.
Akcam et al compared the severity of pain occurring after different surgical procedures and sought to determine appropriate analgesic requirements for the first 24 hours postoperatively.25 In their study, patients underwent either anterior palatoplasty, lateral pharyngoplasty, or tongue base suspension suturing. Tramadol delivered by a patient-controlled analgesia device and, when necessary, rescue pethidine (meperidine in the United States) were used for pain relief.
Postoperative pain scores in the tongue base suspension group of the Akcam study were higher than those in the palatoplasty group at all time points except at hour 12, and they were higher than those in the lateral pharyngoplasty group except at hour 10; pain scores were significantly higher in the lateral pharyn-

76 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
hAyTO LU, ARIkAN, mULUk, TUhANIO LU, ÇÖRTÜk
Figure 1. Graph shows the VAS scores for postoperative pain at rest in the two groups. Scores were significantly lower in the palatoplasty group at postoperative hours 2, 4, and 8 and on days 4 through 7 (p < 0.002).
Figure 2. Graph shows the VAS scores for postoperative pain during swallowing in the two groups. Scores were significantly lower in the palatoplasty group at 2, 4, 8, and 16 hours and on days 4 through 7 (p < 0.009).
goplasty group than in the palatoplasty group at hour 1. 25 Mean total tramadol consumption was highest in the tongue base suspension group and lowest in the palatoplasty group.
Akcam et al concluded that patient-controlled analgesia with tramadol effectively treated the pain caused by anterior palatoplasty and lateral pharyngoplasty, but alleviation of the pain caused by tongue base suspension suturing usually required a rescue opioid analgesic. In our study, pain scores were lower in the palatoplasty group than in the flap group, which might reflect the fact that anterior palatoplasty is a less invasive procedure.
In our study, the degree of postoperative pain at rest was significantly less in the palatoplasty group than in the flap group at 2, 4, and 8 hours and on days 4 through 7. Pain scores in the palatoplasty group peaked at 24 hours. In the flap group, pain at rest values peaked between 8 and 16 hours.
Postoperative pain scores during swallowing were significantly lower in the palatoplasty group than in the flap group at 2, 4, 8, and 16 hours and on days 4 through 7. Scores peaked at 24 hours in the palatoplasty group and at 16 hours in the flap group.
In conclusion, our study found that anterior palatoplasty and uvulopalatal flap placement were equally effective in the treatment of patients with mild and moderate OSAS secondary to retropalatal obstruction. However, we recommend anterior palatoplasty because it is associated with less postoperative pain.
References
1. Azagra-Calero E, Espinar-Escalona E, Barrera-Mora JM, et al. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med Oral Patol Oral Cir Bucal 2012;17(6):e925-9.
2. Rosen CL. Obstructive sleep apnea syndrome (OSAS) in children: Diagnostic challenges. Sleep 1996;19(10 Suppl):S274-7.
3. Yagi H, Nakata S, Tsuge H, et al. Morphological examination of upper airway in obstructive sleep apnea. Auris Nasus Larynx 2009;36(4):444-9.
4. Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: Uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981; 89(6):923-34.
5. Pelausa EO, Tarshis LM. Surgery for snoring. Laryngoscope 1989; 99(10 Pt 1):1006-10.
6. Simmons FB, Guilleminault C, Miles LE. The palatopharyngoplasty operation for snoring and sleep apnea: An interim report. Otolaryngol Head Neck Surg 1984;92(4):375-80.
7. Katsantonis GP, Friedman WH, Rosenblum BN, Walsh JK. The surgical treatment of snoring: A patient’s perspective. Laryngoscope 1990;100(2 Pt 1):138-40.
8. Levin BC, Becker GD. Uvulopalatopharyngoplasty for snoring: Long-term results. Laryngoscope 1994;104(9):1150-2.
9. Larsson LH, Carlsson-Nordlander B, Svanborg E. Four-year follow-up after uvulopalatopharyngoplasty in 50 unselected patients with obstructive sleep apnea syndrome. Laryngoscope 1994;104(11 Pt 1):1362-8.
10. Han D, Ye J, Lin Z, et al. Revised uvulopalatopharyngoplasty with uvula preservation and its clinical study. ORL J Otorhinolaryngol Relat Spec 2005;67(4):213-19.
11. Powell N, Riley R, Guilleminault C, Troell R. A reversible uvulopalatal flap for snoring and sleep apnea syndrome. Sleep 1996;19(7):593-9.
12. Neruntarat C. Uvulopalatal flap for snoring on an outpatient basis. Otolaryngol Head Neck Surg 2003;129(4):353-9.
13. Cekin E, Cincik H, Ulubil SA, et al. Comparison of uvulopalatopharyngoplasty and uvulopalatal flap in Turkish military personnel with primary snoring. Mil Med 2009;174(4):432-6.
14. Pang KP, Terris DJ. Modified cautery-assisted palatal stiffening operation: New method for treating snoring and mild obstructive sleep apnea. Otolaryngol Head Neck Surg 2007;136(5):823-6.
15. Pang KP, Tan R, Puraviappan P, Terris DJ. Anterior palatoplasty for the treatment of OSA: Three-year results. Otolaryngol Head Neck Surg 2009;141(2):253-6.
16. Marzetti A, Tedaldi M, Passali FM. Preliminary findings from our experience in anterior palatoplasty for the treatment of obstructive sleep apnea. Clin Exp Otorhinolaryngol 2013;6(1):18-22.
17. Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am 1989;36(6):1551-69.
18. Goldman RD, Scolnik D. Underdosing of acetaminophen by parents and emergency department utilization. Pediatr Emerg Care 2004;20(2):89-93.
19. Buysse DJ, Reynolds CF III, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989;28(2):193-213.
20. Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991;14(6):540-5.
21. No authors listed. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2000;284(23):3043-5.
22. Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996;19(2):156-77.
23. Lye KW, Waite PD, Meara D, Wang D. Quality of life evaluation of maxillomandibular advancement surgery for treatment of obstructive sleep apnea. J Oral Maxillofac Surg 2008;66(5):968-72.
24. Ugur KS, Kurtaran H, Ark N, et al. Comparing anterior palatoplasty and modified uvulopalatopharyngoplasty for primary snoring patients: Preliminary results. B-ENT 2013;9(4):285-91.
25. Akcam T, Arslan HH, Deniz S, et al. Comparison of early postoperative pain among surgical techniques for obstructive sleep apnea. Eur Arch Otorhinolaryngol 2012;269(11):2433-40.
78 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
hAyTO LU, ARIkAN, mULUk, TUhANIO LU, ÇÖRTÜk
Psoriasis, chronic tonsillitis, and biofilms: Tonsillar pathologic findings supporting a microbial hypothesis
Herbert B. Allen, MD; Saagar Jadeja, BS; Rina M. Allawh, MD; Kavita Goyal, MD
Abstract
Group A Streptococcus has been identified as a possible etiologic agent in psoriasis in epidemiologic, immunologic, immunopathologic, medical, and surgical studies. Tonsillectomy has been shown to provide considerable relief to 75% of patients with plaque psoriasis. Even with the substantial evidence supporting group A Streptococcus as a causative pathogen in psoriasis, it is an elusive pathogen because it is not culturable, nor does it exhibit any positive serologic evidence of its presence. One possible reason for the negative cultures and negative serology findings with group A Streptococcus is the development of biofilms. We conducted a pathologic study to determine whether biofilms were present in the tonsillar tissues of 10 patients with psoriasis—6 men and 4 women, aged 25 to 64 years (mean: 48)—and in 10 age- and sex-matched controls with chronic tonsillitis who did not have psoriasis. We found that biofilms were present in every tonsillectomy specimen we examined, including those of the controls. Whereas psoriasis has been considered a “double hit” phenomenon, we believe that the development of skin lesions is likely attributable to the presence of the gene PSORS together with the biofilm in psoriasis patients rather than to the biofilm itself. Biofilms have been identified in both extra- and intracellular locations. We believe our findings add further evidence supporting a microbial pathogenesis of this disease.
Introduction
Psoriasis is a chronic immunomediated inflammatory disease that affects 2 to 3% of adults.1 Patients with psoriasis develop erythematous, scaly plaques on their skin along with disfiguring nail and joint involvement. Affected patients are more likely to experience major
From the Department of Dermatology, Drexel University College of Medicine, Philadelphia.
Corresponding author: Herbert B. Allen, MD, Department of Dermatology, Drexel University College of Medicine, 219 N. Broad St., 4th Floor, Philadelphia, PA 19107. Email: hba25@drexel.edu
depression and have a higher risk of developing certain cardiovascular comorbidities, further contributing to the debilitating nature of the disease.1,2
The pathogenesis of psoriasis is multifactorial. It involves an intricate interplay between the immune system, individual genetics, and the environment. One environmental factor of particular interest is the connection between tonsillar infection with group A Streptococcus and the development and maintenance of the psoriatic phenotype. It has been well reported that group A streptococcal pharyngitis often precedes the development of guttate psoriasis.3
Epidemiologic studies have shown that the prevalence of environmental group A Streptococcus correlates with the incidence of psoriasis.4 Moreover, psoriasis patients who have been treated with long-term penicillin injections have reported a very significant improvement in their Psoriasis Area and Severity Index (PASI) score.5 This connection notwithstanding, there is insufficient evidence demonstrating that persistent group A streptococcal infection is present in plaque psoriasis because (1) the group A pathogen Streptococcus pyogenes is not easily cultured and (2) elevated antistreptolysin titers are not present in all affected individuals.6
One possible explanation for the lack of culturability and the lack of serum markers is evasion of the host’s immune response via the formation of intricate biofilms within the tonsils. Evasion of the immune system promotes bacterial growth and survival despite the use of antistreptococcal agents, which are unable to penetrate the biofilms.
Biofilms are clusters of microorganisms that adhere to biologic or nonbiologic surfaces. They exist in a matrix of extracellular polysaccharides, amyloid, adhesive fibers, and DNA. The term extracellular polysaccharides refers to the biomass outside the bacteria, which makes up the bulk of the biofilm; the term intracellular biofilms refers to the biofilms within the host cells. The bacteria manufacture both the extracellular polysaccharides and the amyloid, which form the infrastructure of the biofilm.
Volume 97, Number 3 www.entjournal.com 79 ORIGINAL ARTICLE
In a meta-analysis of studies of tonsillectomy and psoriasis, Thorleifsdottir et al concluded that tonsillectomy was a worthwhile treatment in 70% of psoriasis patients.7 In a recent surgical study, Rachakonda et al found that tonsillectomy reversed the development of plaque psoriasis in 13 of 15 patients.8
In this article, we describe our investigation aimed at evaluating the presence of biofilms in tonsillectomy specimens obtained from patients with psoriasis.

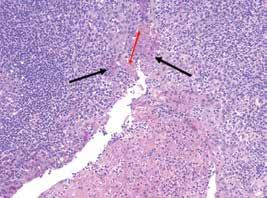
Materials and methods
We performed a histopathologic analysis of tonsillectomy specimens obtained from 10 patients—6 men and 4 women, aged 25 to 64 years (mean: 48)—who had been given a clinical diagnosis of plaque psoriasis. We compared the findings in these specimens with those of an age- and sex-matched control group of patients with chronic tonsillitis who did not have psoriasis. All tonsillar specimens were stained with routine hematoxylin and eosin, periodic acid–Schiff (PAS), and Congo red. Routine light microscopy was employed for evaluation.
All protocols were approved by the Drexel University College of Medicine Institutional Review Board.
Results
On gross examination, we found no appreciable differences in the tonsillectomy specimens between the psoriasis patients and the controls. On histopathologic evaluation, all tonsillectomy specimens from the psoriasis patients demonstrated positive extra- and intracellular staining with both PAS and Congo red. The positive PAS staining indicated the presence of polysaccharides (figure 1, A), while the positive Congo red staining indicated the presence of amyloid (figure 1, B). The tonsillectomy specimens from the controls exhibited similar features.
Uninvolved crypts that showed no PAS or Congo red staining were present in both cohorts (figure 2). Using these crypts as internal controls could possibly make for a better evaluation.
Discussion
To the best of our knowledge, this is the first study to look for the presence of tonsillar extra- and intracellular biofilms in patients with psoriasis. As evidenced by the positive
PAS and Congo red staining within the tonsillar epithelial cells, our results showed that group A streptococcal organisms persist within intracellular biofilms in psoriasis patients. Extracellular biofilms are evident, as well. These findings raise important considerations regarding the role of group A streptococcal infection in the pathogenesis of psoriasis.
One of these considerations is that the psoriatic phenotype may be propagated by chronic group A streptococcal tonsillitis. This is supported by the work of El-Rachkidy et al, who found that a significantly greater number of patients with chronic plaque psoriasis had elevated titers of IgG-reactive proteins to S pyogenes compared with healthy age- and sex-matched controls.9 Support for this consideration was reported by Zhang et al, who found Toll-like receptor 2 in the upper dermal capillaries in psoriatic plaques.10 It appears that both the innate and adaptive immune systems may be involved.
The concept of group A Streptococcus as a pathogen in psoriasis was further corroborated in a study by Saxena and Dogra, who found that patients exhibited a significant improvement in PASI scores after receiving long-term penicillin or azithromycin therapy for chronic plaque psoriasis.11 However, they noted that 20% of their patients had developed a recurrence of their symptoms at 1 year of follow-up.5,11 We hypothesize that these recurrences were attributable to the antibiotics addressing the transient bacteremia that occurs in chronic group A streptococcal tonsillitis because antibiotics would kill any cells released (exported) from the biofilm and not allow those microbes to generate an immune response. The organisms still exist latently within both extra- and intracellular biofilms and thus they are protected from the effects of the antibiotics.
A word about the controls in our study: We believe psoriasis is a “double hit” phenomenon in which the
B: Congo red staining is positive for amyloid in the cell’s cytoplasm, which forms the infrastructure of biofilms (black arrows). The nuclei of these cells are clearly present. Large areas of staining (without nuclei) represent extracellular biofilms (white arrows) (original magnification ×40).
80 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
ALLEN, JAdEJA, ALLAwh, gOyAL
Figure 1. Images show stains of tonsillar specimens obtained from a patient with psoriasis. A: PAS staining shows an extracellular biomass (red arrow) that consists mostly of polysaccharides. An intracellular biofilm with retained nuclei (black arrows) is also seen (original magnification ×40).
A B

environmental component is group A Streptococcus and the genetic component is one of the PSORS genes or another gene.12 Thus, in patients with chronic tonsillitis, psoriasis would become evident only in those who have a gene for the disease. Therefore, the tonsillar microbiologic/pathologic findings would be the same in chronic tonsillitis in patients with and without psoriasis. The high levels of Streptococcus-specific IgG in some controls, as noted by El-Rachkidy et al,9 help support this hypothesis.
We, among others, have previously described the presence of biofilms and their role in the pathogenesis of a variety of skin diseases, including atopic dermatitis, Lyme disease, and tinea versicolor.13,14 In these disease processes, the biofilms were extracellular rather than intracellular. More recently, we reported that intracellular biofilms were found in hippocampal specimens in patients with Alzheimer disease.15
The distinction between extra- and intracellular biofilms is particularly important because intracellular biofilms provide another level of protection against both the immune system and antibiotics, and thus they are increasingly difficult to eradicate.16 In the context of psoriasis, tonsillar intracellular biofilms may serve as a nidus of infection and can provide antigens that stimulate skin-homing T cells through molecular mimicry.17 This chronic state may help explain the relapsing and remitting nature of the psoriatic phenotype.
The current treatment paradigm for psoriasis is based on using topical and systemic agents to modify the immune pathways that contribute to keratinocyte proliferation and the development of psoriatic lesions. Despite the availability of multiple treatment modalities, including newer systemic immunomodulatory agents, patients often require life-long treatment and are subject to a host of side effects. Given our findings, it may be that immunomodulatory therapies work too far downstream and do not directly address the causative entity. Additional weight should likely be given to tonsillectomy in psoriasis because it has the capability of being curative with a single procedure. Also, studies investi-
gating the relationship between chronic group A streptococcal tonsillitis and the psoriatic phenotype, as well as the utility of antistreptococcal and biofilm-dispersing agents in the management of psoriasis, should be encouraged.
References
1. Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch Dermatol 2010;146(8):891-5.
2. Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296(14):1735-41.
3. Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol 1992;128(1):39-42.
4. McFadden JP, Baker BS, Powles AV, Fry L. Psoriasis and streptococci: The natural selection of psoriasis revisited. Br J Dermatol 2009;160(5):929-37.
5. Saxena VN, Dogra J. Long-term use of penicillin for the treatment of chronic plaque psoriasis. Eur J Dermatol 2005;15(5):359-62.
6. Kim SK, Kang HY, Kim YC, Lee ES. Clinical comparison of psoriasis in Korean adults and children: Correlation with serum anti-streptolysin O titers. Arch Dermatol Res 2010;302(4):295-9.
7. Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, et al. Improvement of psoriasis after tonsillectomy is associated with a decrease in the frequency of circulating T cells that recognize streptococcal determinants and homologous skin determinants. J Immunol 2012;188(10):5160-5.

8. Rachakonda TD, Dhillon JS, Florek AG, Armstrong AW. Effect of tonsillectomy on psoriasis: A systematic review. J Am Acad Dermatol 2015;72(2):261-75.
9. El-Rachkidy RG, Hales JM, Freestone PP, et al. Increased blood levels of IgG reactive with secreted Streptococcus pyogenes proteins in chronic plaque psoriasis. J Invest Dermatol 2007; 127(6):1337-42.
10. Zhang J, Shaver C, Neidig L, et al. Toll-like receptor 2 and its relationship with Streptococcus in psoriasis. Skinmed 2017; 15(1):27-30.
11. Saxena VN, Dogra J. Long-term oral azithromycin in chronic plaque psoriasis: A controlled trial. Eur J Dermatol 2010;20(3): 329-33.
12. Chang YT, Chou CT, Shiao YM, et al. Psoriasis vulgaris in Chinese individuals is associated with PSORS1C3 and CDSN genes. Br J Dermatol 2006;155(4):663-9.
13. Gantz M, Allen HB. Psoriasis, atopic dermatitis, Lyme disease and tinea versicolor: All caused by microbes but none a classic infection. J Clin Exp Dermatol Res 2016;4:362.
14. Allen HB, Vaze ND, Choi C, et al. The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol 2014;150(3):260-5.
15. Allen HB, Allawh R, Touati A, et al. Alzheimer’s [sic] disease: The novel finding of intracellular biofilms. J Neuroinfect Dis 2017;8(2):247.
16. Scott VC, Haake DA, Churchill BM, et al. Intracellular bacterial communities: A potential etiology for chronic lower urinary tract symptoms. Urology 2015;86(3):425-31.
17. Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol 2009;30(10):494-501.
82 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 ALLEN,
JAdEJA, ALLAwh, gOyAL
Figure 2. Tonsillar crypt in a patient with psoriasis shows no staining with Congo red. Tonsillar epithelial cells line the crypt (original magnification ×10).
Quality of life in patients with larynx cancer in Latin America: Comparison between laryngectomy and organ preservation protocols
Alvaro Sanabria, MD, PhD, FACS; Daniel Sánchez, MD; Andrés Chala, MD; Andres Alvarez, MD
Abstract
The effect on quality of life (QOL) of laryngectomy and organ preservation protocols is important in decision making. The aim of this cross-sectional study was to evaluate QOL outcomes of patients with advanced laryngeal tumors who were treated with laryngectomy or organ preservation protocols in Latin America. A total of 35 patients from three oncology units were enrolled. Patients with stage III/IV laryngeal cancer who were treated using organ preservation protocols or laryngectomy were assessed with the University of Washington QOL Questionnaire. The most important domains that affected QOL for both groups were speech and activity. In the laryngectomy group, the next most important domains were appearance, taste, pain, and recreation, whereas in the organ preservation group, they were saliva, recreation, mood, and swallowing. There were no statistically or clinically significant differences in the global score or the 7 days of QOL assessments before patients were interviewed. Global QOL assessments were similar when comparing laryngectomy and organ preservation protocols.
Introduction
Laryngeal cancer is one of the most common tumors of the respiratory tract. Chemoradiotherapy and surgery are believed to offer similar final outcomes; however, laryngectomy impairs quality of life (QOL) as a result of definitive tracheostomy and the loss of vocal speech.1
From the Department of Surgery, Universidad de Antioquia, Medellin, Colombia (Dr. Sanabria and Dr. Sánchez); Fundación Colombiana de Cancerología–Clínica Vida, Medellin, Colombia (Dr. Sanabria); the Department of Surgery, Universidad de Caldas, Manizales, Colombia (Dr. Chala); and the Department of Surgery, Universidad del Rosario, Bogota, Colombia (Dr. Alvarez).
Corresponding author: Alvaro Sanabria, MD, PhD, FACS, Department of Surgery, Universidad de Antioquia, Calle 67 No. 53 - 108, Medellin, Colombia. Email: alvarosanabria@gmail.com
Organ preservation protocols focusing on larynx preservation are widely offered to patients with T3 tumors without massive invasion of cartilage.2 For patients with more advanced tumors, the effectiveness of chemoradiotherapy compared with total laryngectomy is controversial, with an increasing acceptance of laryngectomy as a more effective strategy.3 In arriving at a therapeutic modality, most physicians and patients consider QOL concerns to be as important as long-term survival.4
One factor favoring organ preservation treatments is the possibility of breathing and talking with natural organs but with a higher rate of adverse effects related to the long-term sequelae of chemotherapy or radiotherapy, such as xerostomia and swallowing disorders. Laryngectomy, therefore, is not usually considered to be the first alternative due to attitudes related to poorer QOL.
Many studies have evaluated QOL outcomes in cohorts of patients who underwent organ preservation or laryngectomy; few randomized controlled trials, however, have compared the results between both strategies. The few randomized comparisons that have been conducted show similar or better results in organ preservation treatments.5,6 Most of this information comes from QOL assessments in English literature and primarily in patients from developed countries with specific social and economic characteristics.
Data from patients in developing countries and in different cultural settings are lacking. Despite QOL findings coming from Brazilian investigators,7,8 data are scarce for Spanish-speaking countries.9,10 Because QOL assessments change according to socioeconomic factors, results are difficult to extrapolate to different populations.
The aim of this study was to evaluate the QOL outcomes of patients with advanced laryngeal tumors who were treated with laryngectomy or organ preservation protocols in Latin America.
Volume 97, Number 3 www.entjournal.com 83 ORIGINAL ARTICLE
Patients and methods
This study was approved by the Ethics in Research Committee of the Hospital Pablo Tobón Uribe, and informed consent was obtained for inclusion. Patients with laryngeal cancer classified as stage III or IV who were treated under organ preservation protocols (including chemoradiotherapy or exclusive radiotherapy) or laryngectomy were included. The choice of treatment was at the discretion of the treating physicians, who were following the guideline established by the National Comprehensive Cancer Network.11
All patients must have completed their definitive treatment and were being followed at the Head and Neck Section of the Hospital Pablo Tobón Uribe Oncology Unit, Medellin; Hospital Universitario de Caldas, Manizales; and the Hospital Mederi, Bogotá, Colombia.
Patients with physical impairments that prevented reading, hearing, or understanding the QOL scale, those for whom the last treatment date was more than 5 years ago, and those who did not consent to participate were excluded.
Patient data were collected prospectively during routine clinical visits from February 2013 to January 2015.
To evaluate QOL outcomes, we used the Spanish validated version of the University of Washington Quality of Life (UW-QOL) questionnaire for patients with head and neck cancer, assisting those patients who could not read the instrument to obtain the relevant information. This instrument has been evaluated in many clinical settings and has been translated to and validated in many languages.12-17
The UW-QOL has three domains (symptom, priority of symptoms, and global health-related QOL) with separate items (pain, appearance, activity level, recreation, swallowing, chewing, speech, shoulder function, taste, and saliva production) scored on a scale from 0 to 100.
The burden of symptoms was defined following the coding instruction of the UW-QOL instrument, according to the rank that patients gave to each symptom. We also obtained patient demographics and information on stage and European Cooperative Oncology Group functional status from the clinical charts.
The categorical variables are presented as percentages and ranges, and the continuous variables are shown as the means and standard deviation. For the analysis, some continuous variables were categorized. The significance level was set at p < 0.05. Between-group differences were assessed with the nonparametric Mann-Whitney and Kruskal-Wallis tests due to the non-normal distribution of variables and the small sample size. We used the Stata statistical software v9.1 (StataCorp; College Station, Texas).
Results
We included 35 patients, 14 of whom were treated with an organ preservation protocol; 21 patients underwent laryngectomy. The demographic data and clinical and treatment characteristics are shown in table 1. The mean age was 61.4 ± 9.5 years (median: 62; range: 41 to 87). A total of 94.3% of the 35 patients were men. Patients were interviewed for symptoms 17.7 ± 21.4 months after diagnosis (median: 10.9).
Ten patients (71.4%) who were treated with an organ preservation protocol needed definitive tracheostomy, and half of the patients in each group had a gastrostomy tube. A total of 22.9% had recurrence at the time of the interview. The only baseline difference between the two groups was that the group that underwent laryngectomy had a lower proportion of adjuvant chemoradiotherapy.
The burden of symptoms on the UW-QOL instrument is shown in table 2. The most important factors on QOL for both groups were speech and activity, respectively. In the laryngectomy group, the next most important factors were appearance, taste, pain, and recreation; for the organ preservation group, the next most important factors were saliva, recreation, mood, and swallowing. The descriptive distribution of domains of the UW-QOL is shown in table 3.
Statistically significant differences were found in activity and speech, with worse scores for the laryngectomy group. Although not statistically significant, more than a 10-point decrement (clinically relevant) was found in the shoulder, anxiety, swallowing, taste, and appearance domains among the patients who underwent laryngectomy, and in the saliva domain among the patients who received an organ preservation protocol. Both social and physical subscales had a dif-
Table 1. Clinical characteristics in patients with advanced laryngeal cancer treated with or without laryngectomy
84 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
SANAbRIA, SáNChEz, ChALA, ALvAREz
Without laryngectomy (n = 14) (n, %) Laryngectomy (n = 21) (n, %) p Value Sex (male) 12 (85.7) 21 (100) 0.07 Age (years)* 60.5 ± 10.2 (61) 62.0 ± 9.2 (62) 0.67 T3/4 11 (78.6) 21 (100) 0.16 N2-3 5 (35.7) 11 (52.4) 0.68 Radiotherapy 14 (100) 15 (71.4) 0.02 Chemotherapy 12 (85.7) 5 (23.8) <0.01 gastrostomy 7 (50) 11 (52.4) 0.89 Recurrent disease 5 (35.7) 3 (14.3) 0.14 ECOg 2-3 3 (21.4) 5 (23.8) 0.46 *Kruskal-Wallis test Key: ECOG = Eastern Cooperative Oncology Group.


Table 2. Significant problems in patients treated with or without laryngectomy
trials and meta-analyses.19,20
ference of >10 points between groups, with lower scores observed for the laryngectomy group. No statistically or clinically significant differences in the global score or QOL assessments were observed during the 7 days before the interview.
Discussion
QOL assessment has become important in decision making for patients with head and neck cancer. The impact of a disease and its treatment is correlated with survival, ability to return to work, and social functioning.18 Many instruments with a general or specific focus have been designed to evaluate QOL in patients with head and neck cancer. Although generic QOL instruments can be used in a wide range of patients with different types of tumors, they lack responsiveness for specific ailments of each disease. The UW-QOL for head and neck cancer is a widely used instrument with high reliability that has been validated in the Spanish language, which enables its use in populations of developing countries in Latin America.16
Due to its location in the body and its close relationship with feeding, communication, and interpersonal relationships, head and neck cancer imposes an important burden on QOL. Moreover, its related treatments (chemotherapy, radiotherapy, and surgery) adversely affect functions such as swallowing, speech, breathing, and mastication, which can lead to even poorer QOL.
Since the 1990s, organ preservation protocols for patients with advanced tumors have been considered to offer outcomes similar to those of laryngectomy followed by radiotherapy, with the enormous advantage of larynx preservation and the consequent gain in QOL, as demonstrated in randomized, controlled
The introduction of these protocols relegated laryngectomy to a secondary method in the treatment of advanced tumors due to the negative impact of a definitive tracheostomy with the loss of vocal ability and the related consequences of this procedure on appearance. 21 However, for transglottic tumors with massive cartilage invasion, laryngectomy has recently been demonstrated to offer advantages in terms of disease-free and overall survival compared with chemoradiotherapy, producing a renaissance of indications for and the use of total laryngectomy.22 Nonetheless, patients and physicians are aware of the negative effects of total laryngectomy on QOL, which impedes its wide use.
QOL assessments have been made in patients who underwent laryngectomy or organ preservation protocols and have shown similar or better results with organ preservation in the most recent studies.5,6 However, the perception of laryngectomy as a disastrous event has not been proven by data. List et al 23 and Eadie and Bowker24 suggested that the ability to cope with impairments produced by laryngectomy shortens early differences compared with organ preservation in QOL scores.
In periodic assessments, Deleyiannis et al25 found that QOL scores return to values similar to the presurgical stage after 2 years postlaryngectomy, whereas Hammerlid et al 26 found that a 1-year period is sufficient to compensate for the acute effects of treatment on QOL score. Therefore, although functional limitations persist over time, the overall QOL scores reach those that are similar to those of other diseases with an intact larynx.27
In our study, we found that the UW-QOL instrument could define the specific ailments that decrease QOL in patients with head and neck cancer who had undergone (or had not undergone) laryngectomy. For patients who have undergone laryngectomy, the importance of appearance due to the scar, neck deformity produced by the surgery, and the presence of a definitive tracheostomy in the neck were ranked as important domains that affect QOL.
The taste factor also ranked high due to the loss of smell and the difficulty in rehabilitating this sense after surgery. In contrast, patients in the organ preservation group revealed that saliva and swallowing were important factors in QOL. The recreation factor ranked high in both groups and represents a new finding. No data exist that shed
Volume 97, Number 3 www.entjournal.com 87 QUALITY OF LIFE IN PATIENTS WITH LARYNX CANCER IN LATIN AMERICA: COMPARISON BETWEEN LARYNGECTOMY AND ORGAN PRESERVATION PROTOCOLS
Without laryngectomy (n =
Laryngectomy (n = 21) Problem domain n % Problem domain n % Speech 6 42.9 Speech 16 76.2 Activity 6 42.9 Activity 15 71.4 Saliva 6 42.9 Appearance 10 47.6 Recreation 5 35.7 Taste 9 42.9 mood 4 28.6 Pain 9 42.9 Swallowing 4 28.6 Recreation 9 42.9 Pain 3 21.4 mood 8 38.1 Appearance 3 21.4 Anxiety 7 33.3 Anxiety 3 21.4 Swallowing 7 33.3 Chewing 3 21.4 Shoulder 5 23.8 Taste 3 21.4 Chewing 5 23.8 Shoulder 1 7.1 Saliva 3 14.3
14)
light on the meaning of this finding for patients with head and neck cancer, although it may be a proxy for depression or life enjoyment.
The most important finding of this study was that QOL global scores were similar in both groups, even though individual domains such as activity and speech ranked lower in the laryngectomy group. These results are similar to those reported in other studies. In 42 patients, Hanna et al could not find global differences in QOL between patients who underwent laryngectomy or organ preservation protocols, as measured with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, Head and Neck Module.28
The same results were obtained by other authors.25,29-31 The consistency of these findings confirms that after an initial period of adaptation (as long as 2 years), patients who undergo laryngectomy reach QOL scores that are similar to those of patients treated with chemoradiotherapy and weakens the notion that laryngectomy is a disastrous event in a patient’s life. This lack of difference also may be explained by the high frequency of definitive tracheostomy in the organ preservation group, which reduces the potential advantages offered by organ preservation treatments in the voice and breath domains.
In other studies, the rate of tracheostomy was approximately 30%, whereas in our study, nearly three-fourths of patients had a tracheostomy. It is impossible to discard any of these hypotheses due to the design of this study.
Most studies on QOL in laryngectomy or organ preservation protocols were performed in developed countries with specific socioeconomic and cultural characteristics. Until now, little information was available on outcomes in patients in developing countries. A study by Vilaseca et al in 49 laryngectomized patients found mean scores on the UW-QOL that ranged from 72 for taste to 89 for chewing.32 In our study patients, domain scores varied from 26 for speech to 80 for saliva.
Most of our scores were comparatively lower than those reported elsewhere, which indicates that the relative importance of domains is different in our population and that these differences should be considered when treatments are offered. In the Scotland Laryngectomy Audit, Robertson et al found a global score of 72.9 with high importance for speech, swallowing, activity, mood, and appearance. 27 In our study, taste, pain, and recreation were important domains. They are not currently reported in other studies and could represent a different weight for items in the QOL questionnaire as rated by Latin American patients.
Some weaknesses of this study should be noted. The first is related to the small sample size, which can explain clinically meaningful but not statistically significant differences in some domain scores. The second limitation is related to the design of this study. It was not a randomized trial, and treatment decisions were made by surgeons; thus, comparisons can introduce bias. Third, although a clear treatment protocol has been established, not all patients received the same treatments, doses, and procedures. All of these factors can affect the measurement of
88 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
SANAbRIA, SáNChEz, ChALA, ALvAREz
QOL.
Without laryngectomy (n = 14) mean ± SD (median) Laryngectomy (n = 21) mean ± SD (median) p Value* global 69.8 ± 18.7 (74.7) 61.4 ± 18.4 (66.3) 0.13 Previous QOL 71.4 ± 32.3 (75) 66.3 ± 37.4 (75) 0.71 QOL last 7 days 60.0 ± 27.2 (50) 67.4 ± 26.8 (60) 0.35 Social subscale 75.1 ± 21.1 (74.6) 65.9 ± 18.9 (70) 0.27 Physical subscale 65.6 ± 21.1 (65.4) 57.0 ± 20.1 (62.5) 0.29 Speech 57.1 ± 31.9 (70) 26.8 ± 28.1 (30) <0.01 Activity 73.2 ± 26.8 (75) 52.4 ± 26.1(50) 0.03 Appearance 78.6 ± 19.2 (75) 65.5 ± 23.0 (75) 0.09 Saliva 59.3 ± 38.3 (70) 80.5 ± 25.6 (100) 0.10 Taste 70.0 ± 35.9 (70) 52.9 ± 38.2 (70) 0.17 Shoulder 88.6 ± 27.7 (100) 73.3 ± 37.7 (100) 0.18 Anxiety 76.4 ± 33.7 (100) 65.2 ± 31.9 (70) 0.23 Swallowing 62.3 ± 24.9 (70) 51.5 ± 31.2 (70) 0.36 Chewing 61.5 ± 41.6 (50) 66.6 ± 42.8 (100) 0.66 Recreation 67.8 ± 28.5 (75) 65.5 ± 24.3 (75) 0.71 mood 67.8 ± 31.7 (75) 64.3 ± 33.1 (75) 0.76 Pain 76.8 ± 22.9 (75) 75 ± 28.5 (100) 0.98
Table 3. Comparison of UW-QOL scale between patients treated with or without laryngectomy
UW-QOL
QOL = quality of life.
*Kruskal-Wallis test Key:
= University of Washington quality of Life questionnaire, SD = standard deviation,

In conclusion, in a cohort of Latin American patients with laryngeal cancer, global QOL assessments were similar when comparing laryngectomy with organ preservation protocols. Important differences in specific domains should be identified to better explain the consequences of treatment. The relevance of domains and the scores of the UW-QOL instrument differ from those reported in developed countries; thus, local evaluations of QOL should be integrated in decision making.
References
1. Department of Veterans Affairs Laryngeal Cancer Study Group, Wolf GT, Fisher SG, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med 1991;324(24):1685-90.
2. Forastiere AA, Weber RS, Trotti A. Organ preservation for advanced larynx cancer: Issues and outcomes. J Clin Oncol 2015; 33(29):3262-8.
3. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: Changes in demographics, patterns of care, and survival. Laryngoscope 2006;116(9 Pt 2 Suppl 111):1-13.
4. McNeil BJ, Weichselbaum R, Pauker SG. Speech and survival: Tradeoffs between quality and quantity of life in laryngeal cancer. N Engl J Med 1981;305(17):982-7.
5. Hillman RE, Walsh MJ, Wolf GT, et al. Functional outcomes following treatment for advanced laryngeal cancer. Part I—Voice preservation in advanced laryngeal cancer. Part II—Laryngectomy rehabilitation: The state of the art in the VA System. Research Speech-Language Pathologists. Department of Veterans Affairs Laryngeal Cancer Study Group. Ann Otol Rhinol Laryngol Suppl 1998;172:1-27.
6. Terrell JE, Fisher SG, Wolf GT. Long-term quality of life after treatment of laryngeal cancer. The Veterans Affairs Laryngeal Cancer Study Group. Arch Otolaryngol Head Neck Surg 1998; 124(9):964-71.
7. Vartanian JG, Carvalho AL, Yueh B, et al. Long-term qualityof-life evaluation after head and neck cancer treatment in a developing country. Arch Otolaryngol Head Neck Surg 2004; 130(10):1209-13.
8. Vartanian JG, Kowalski LP. Acceptance of major surgical procedures and quality of life among long-term survivors of advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 2009;135(4):376-9.
9. Alvarez-Buylla Blanco M, Herranz González-Botas J. Evolución de la calidad de vida en pacientes intervenidos de carcinomas de orofaringe, laringe o hipofaringe [in Spanish]. Acta Otorrinolaringol Esp 2011;62(2):103-12.
10. Nazar GP, Gonzalez I, Messina A. Evaluación de la calidad de vida en pacientes tratados por cáncer de laringe [in Spanish]. Rev Otorrinolaringol Cir Cabeza Cuello 2004;64:9.
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines). Head and neck cancers. Version 2.2017. https://www.nccn.org/professionals/ physician_gls/pdf/head-and-neck.pdf. Accessed January 12, 2018.
12. Rogers SN, Lowe D, Brown JS, Vaughan ED. A comparison between the University of Washington Head and Neck DiseaseSpecific measure and the Medical Short Form 36, EORTC QOQ-C33 and EORTC Head and Neck 35. Oral Oncol 1998; 34(5):361-72.
13. Omoro SA, Fann JR, Weymuller EA, et al. Swahili translation and validation of the Patient Health Questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med 2006;36(3):367-81.
14. Vartanian JG, Carvalho AL, Yueh B, et al. Brazilian-Portuguese validation of the University of Washington Quality of Life Questionnaire for patients with head and neck cancer. Head Neck 2006;28(12):1115-21.
15. D’cruz AK, Yueh B, Das AK, et al. Validation of the University of Washington quality of life questionnaires for head and neck cancer patients in India. Indian J Cancer 2007;44(4):147-54.
16. Nazar G, Garmendia ML, Royer M, et al. Spanish validation of the University of Washington Quality of Life questionnaire for head and neck cancer patients. Otolaryngol Head Neck Surg 2010;143(6):801-7.
17. Chang MY, Rogers SN, Lowe D, et al. The Korean version of the University of Washington Quality of Life Questionnaire for Patients with head and neck cancer, and its use in an initial validation study of 56 patients. Int J Oral Maxillofac Surg 2012;41(10):1201-5.
18. Østhus AA, Aarstad AK, Olofsson J, Aarstad HJ. Prediction of survival by pretreatment health-related quality-of-life scores in a prospective cohort of patients with head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg 2013;139(1):14-20.
19. Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother Oncol 2011; 100(1):33-40.
20. Pignon JP, le Maître A, Bourhis J, MACH-NC Collaborative Group. Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): An update. Int J Radiat Oncol Biol Phys 2007;69(2 Suppl):S112-4.
21. Harwood AR, Rawlinson E. The quality of life of patients following treatment for laryngeal cancer. Int J Radiat Oncol Biol Phys 1983;9(3):335-8.
22. Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg 2014; 140(9):855-60.
23. List MA, Ritter-Sterr CA, Baker TM, et al. Longitudinal assessment of quality of life in laryngeal cancer patients. Head Neck 1996;18(1):1-10.
24. Eadie TL, Bowker BC. Coping and quality of life after total laryngectomy. Otolaryngol Head Neck Surg 2012;146(6):959-65.
25. Deleyiannis FW, Weymuller EA Jr, Coltrera MD, Futran N. Quality of life after laryngectomy: Are functional disabilities important? Head Neck 1999;21(4):319-24.
26. Hammerlid E, Silander E, Hörnestam L, Sullivan M. Healthrelated quality of life three years after diagnosis of head and neck cancer—a longitudinal study. Head Neck 2001;23(2):113-25.
27. Robertson SM, Yeo JC, Dunnet C, et al. Voice, swallowing, and quality of life after total laryngectomy: Results of the west of Scotland laryngectomy audit. Head Neck 2012;34(1):59-65.
28. Hanna E, Sherman A, Cash D, et al. Quality of life for patients following total laryngectomy vs chemoradiation for laryngeal preservation. Arch Otolaryngol Head Neck Surg 2004;130(7):875-9.
29. Williamson JS, Ingrams D, Jones H. Quality of life after treatment of laryngeal carcinoma: A single centre cross-sectional study. Ann R Coll Surg Engl 2011;93(8):591-5.
30. Finizia C, Hammerlid E, Westin T, Lindström J. Quality of life and voice in patients with laryngeal carcinoma: A posttreatment comparison of laryngectomy (salvage surgery) versus radiotherapy. Laryngoscope 1998;108(10):1566-73.
31. LoTempio MM, Wang KH, Sadeghi A, et al. Comparison of quality of life outcomes in laryngeal cancer patients following chemoradiation vs. total laryngectomy. Otolaryngol Head Neck Surg 2005;132(6):948-53.
32. Vilaseca I, Chen AY, Backscheider AG. Long-term quality of life after total laryngectomy. Head Neck 2006;28(4):313-20.
90 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
SANAbRIA, SáNChEz, ChALA, ALvAREz
“Split to save”: Accessing mandibular lesions using sagittal split osteotomy with virtual surgical planning
Stanley Yung-Chuan Liu, MD, DDS; Douglas Sidell, MD; Leh-Kiong Huon, MD; Carlos Torre, MD
Abstract
Large, benign intramandibular lesions are frequently removed by resection followed by extensive free tissue transfer or delayed bone grafting. We outline a protocol to remove benign mandibular lesions using sagittal split osteotomy (SSO) with virtual surgical planning (VSP) to mitigate risks involved with this effective, tissue-saving approach. Patients with benign mandibular lesions accessed by SSO with VSP during 2014 were included in this study. Computed tomographic data were imported into VSP software. Using VSP, the exact locations of mandibular lesions and the inferior alveolar nerve canal were delineated. SSO was designed virtually and provided surgeons exact measurements to gain access to lesions and to avoid vital structures intraoperatively. SSO with VSP preserved the cortical mandibular bone and the inferior alveolar neurovascular bundle in 3 patients with benign mandibular lesions. Twelve months after surgery, no patient had pathologic fracture, prolonged paresthesia (except for the patient who required inferior alveolar nerve resection), or malocclusion. No patient required bone grafting. There were no functional or aesthetic jaw deficits. SSO is an effective approach to access intramandibular lesions. The technique does not result in loss of mandibular bone, and patients return to full masticatory function compared with those who require resection
From the Department of Otolaryngology–Head and Neck Surgery, Stanford University School of Medicine, Stanford, Calif. (Dr. Liu); the Division of Pediatric Otolaryngology, Department of Otolaryngology–Head and Neck Surgery, Stanford University Medical Center, Stanford, Calif. (Dr. Sidell); the Department of Otolaryngology–Head and Neck Surgery, Cathay General Hospital, Taipei, Taiwan (Dr. Huon); and the Department of Otolaryngology–Head and Neck Surgery, University of Miami, Miller School of Medicine, Miami, Fla. (Dr. Torre).
Corresponding author: Stanley Yung-Chuan Liu, MD, DDS, Department of Otolaryngology, Stanford University, 801 Welch Rd., Stanford, CA 94304. Email: ycliu@stanford.edu
and reconstruction. VSP may mitigate technical challenges associated with SSO.
Introduction
Sagittal split osteotomy (SSO) was conceived more than 60 years ago to correct maxillofacial deformities. It involves sagittal osteotomies of the mandible that allow repositioning of the dentate segment to address congenital or acquired dentofacial deformity.1 SSO has occasionally been used as an alternative to expose and remove benign intramandibular lesions.2-5 Advantages include avoidance of large mandibular resections for benign but extensive lesions, preservation of cortical bone and associated dentition (particularly important for growing individuals), rapid return to premorbid jaw function, and avoidance of secondary bone grafting or free tissue transfer.
Despite these advantages, SSO is underused for access to mandibular lesions because of the potential for complications, such as unintended fractures or injury to the inferior alveolar nerve (IAN). Recent advances in virtual surgical planning (VSP) may greatly reduce these risks.3,6
We describe how VSP can be used to plan SSO that removes mandibular lesions while preserving the entirety of mandibular cortical bone and minimizing the risk of injury to adjacent vital structures.
Patients and methods
Patients with biopsy-proven benign intramandibular lesions accessed by SSO with VSP during 2014 were included. All patients were managed by the Stanford University otolaryngology service.
Preoperative planning. Preoperative computed tomography (CT) scans in DICOM (Digital Imaging and Communications in Medicine) format are imported
Volume 97, Number 3 www.entjournal.com 91 ORIGINAL ARTICLE
into VSP software (Materialise N.V.; Leuven, Belgium). Planning includes (1) localizing and demarcating the lesion of interest, (2) outlining ideal positions of osteotomies, and (3) delineating the intrabony course of the IAN to avoid severing or tearing the IAN during sagittal split.
The facial-lingual position of the IAN in the mandibular body is best seen in the coronal view, and the nerve can be traced through its entire length (figure 1). Two planned osteotomies are a ramus osteotomy extending just past the lingula and an anterior mandibular vertical osteotomy. The position of the anterior osteotomy is often dictated by the location of the lesion.
Optimal methods of fixation also can be planned before the operation with the customary combination of bicortical positional screws at the ramus and fracture plates using nonlocking screws across the vertical osteotomy at the body.
Intraoperative considerations. SSO begins with a subperiosteal exposure of the lateral mandibular body and anterior ramus including the lingula, behind which the IAN inserts and begins its intramandibular course.
The ideal incision is a linear one starting about 1 cm inferior to the mucogingival junction of the second molar. This incision is carried down to bone with a blade; this region is almost always avascular with no muscle attachments. A periosteal elevator can then be inserted through this pocket, and the lateral masseter muscle can be lifted subperiosteally with ease.
Electrocautery divides the mucosal junction between the lateral and medial pterygoid muscles along the anterior ramus. The medial pterygoid muscle is reflected to allow identification of the lingula, which is identified preoperatively using VSP. Its precise distance from the anterior border of the ramus is known, and a nerve hook can be used with a marker at the predetermined length.
A mark can be made on the saw blade so that the medial ramus osteotomy does not extend past the lingula (resulting in full-length ramus fractures) or underextend (incarcerating the IAN after the mandible is split).
With SSO, only three osteotomies are made with the saw (medial ramus osteotomy, anterior vertical mandibular body osteotomy, and an osteotomy connecting the two). The osteotomies are followed by a controlled fracture with osteotomes (figure 2, A and B). An ideal SSO allows the mandible to open like a book (figure 2, C).
With VSP, the position of the IAN is known, providing two advantages. First, the depth of the anterior osteot-

omy can be planned. During the split, the osteotomes can also be guided appropriately prior to visualizing the IAN (figure 1).
After the removal of the lesion, fixation of the split mandibular cortices involves positional bicortical screws at the ramus and a fracture plate across the anterior vertical mandibular osteotomy (figure 2, D). Before fixation, maintaining occlusion with intermaxillary fixation (IMF) is important. The condyle is seated by pushing the lateral cortex of the SSO (the condylar segment) posteriorly. Jaw movement is assessed after fixation by releasing the patient from IMF. Occlusion should be as it was before surgery.
Results
Three patients treated with this approach are presented in detail. All underwent successful SSO exposure and complete removal of mandibular lesions. They achieved full recovery of mandibular function including institution of a regular diet within 6 weeks of surgery. Patient 2 required IAN resection, but the other 2 patients had full return of IAN sensation postoperatively.
Clinical scenario 1: Extensive cyst in the mandibular body and ramus. A 55-year-old man presented with
92 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018
LIU, SIdELL, hUON, TORRE
Figure 1. Using virtual surgical planning, the inferior alveolar nerve (IAN) can be traced through its entire intramandibular course. Illustrations courtesy of Dr. Huon.

intermittent left lower facial swelling. Magnetic resonance imaging of the head and neck showed infiltration of an intraosseous lesion in the left mandibular body with erosion near the mental foramen, suspicious for a peripheral nerve sheath tumor.
Figure
depict
process of a sagittal split osteotomy. A: Sagittal split osteotomies are initiated with a reciprocating saw. Three osteotomies are made (medial/posterior horizontal, anterior vertical, and superior connecting). B: The inferior osteotomy requires a controlled fracture using osteotomes. With virtual surgical planning, the position of the inferior alveolar nerve (IAN) is known. This guides the depth of the anterior osteotomy and the directionality of osteotomes. C: This is the point in the procedure when the ideal sagittal split osteotomy opens like a book. After splitting the distal, or dentate, segment and condylar segment of the mandibular ramus, the lesion and inferior alveolar nerve are clearly identified, and the lesion is removed without unnecessary bone loss. D: Bone cortices are reset after positioning the condylar segment upward and backward to the articular fossa. Rigid fixation is done using two bicortical screws at the ramus and a rigid plate across the anterior lower border. (In panels B to D, A = dentate segment; B = condylor segment.)
slowly enlarging right lower facial swelling. The CT scan showed a 6 × 3 × 3-cm radiolucent lesion associated with a third molar dislocated to the posterior ramus (figure 3). The lesion had eroded through the buccal cortex near the mental nerve, where an incisional biopsy had been performed, and a diagnosis of dentigerous cyst was confirmed (figure 3).
SSO using VSP was performed. After the mandibular split, the cystic lining along the inner buccal and lingual cortices was removed easily in one piece along with the third molar. Bony cortices were reapproximated and fixated. The patient’s jaw was banded closed for 1 week, and the patient was gradually released to full diet in 6 weeks. There were no limitations to jaw function at the 1-year follow-up. Lower facial and mandibular bony contours were preserved.


Clinical scenario 2: Inferior alveolar nerve sheath tumor. A 15-year-old boy presented with acute onset of left mental nerve paresthesia associated with
SSO using VSP was performed. The IAN was exposed and traced along its entire length from the ramus to the mental foramen (figure 4) after sagittal split and separation of the facial and lingual cortices of the mandible. The IAN was excised in its entirety within this region. Despite erosion of the medullary bone, bicortical positional screws at the ramus and an anterior fracture plate with nonlocking screws were adequate to maintain jaw stability. No masticatory or aesthetic deficits were apparent at the 1-year follow-up. The patient’s facial growth had not been impeded by the operation.
Clinical scenario 3: Multiple cysts in the mandibular body and ramus. A 19-year-old man presented with numerous asymptomatic intramandibular radiolucencies in the ramus and body of the mandible found during routine panoramic radiography. Incisional biopsy had been performed previously in the anterior mandibular body, with removal of overlying cortical bone. Pathologic diagnosis was inconclusive. To obtain more tissue, biopsy of the lesions in the posterior ramus was necessary.
SSO using VSP was performed, and the exposure allowed for extensive visualization between the split
and radiograph, a 6 × 3 × 3-cm cystic lesion is seen within the mandibular body and ramus with a cortical defect noted near the mental foramen. The lesion is associated with a third molar that is displaced toward the posterior ramus.
Figure 3.
94 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 LIU, SIdELL,
hUON, TORRE
A B C D
In this illustration
2. Illustrations
the

ramus and body cortices. Multiple bony cavities with scant cystic lining were found in the ramus. With the exposure obtained, lesions within the posterior ramus were removed with endoscopic assistance. Preservation of the IAN in the ramus was also confirmed with endoscopic visualization. Biopsy was consistent with a history of giant cell granulomas that had regressed. The patient returned to a regular diet in 2 weeks. He had no functional or aesthetic deficits at the 1-year follow-up.
Discussion
We describe three clinical scenarios in which SSO allowed optimal access to the lesion of interest in the mandible while preserving bony integrity. Classic segmental resection followed by secondary bone grafting or primary free tissue transfer are more usual approaches in these scenarios. SSO was effective in saving healthy bony tissue, preserving bony integrity, allowing patients an immediate return to premorbid occlusion and mastication, and obviating the need for reconstructive surgery. In these circumstances, VSP provided surgeons with important landmarks and measurements that assisted in carrying out SSO safely.
We propose the use of SSO with VSP for the management of (1) benign and extensive intramandibular lesions that otherwise would have required mandibular resection, (2) IAN lesions, and (3) multiple lesions within the mandibular ramus and body. This approach is particularly ideal for growing individuals because jaw development is not disrupted, in contrast with resection.
The SSO approach has been reported in the literature only sporadically. Complications related to SSO include injury to the IAN, postoperative malocclusion, and unanticipated fractures and unfavorable splits.7,8
Although reports demonstrate the efficacy of SSO with VSP, discussion about ways to make the SSO more predictable and reproducible for all surgeons has been inadequate. VSP helps surgeons appreciate the location of the lesion relative to other structures of interest, to design optimal osteotomies to ensure good mandibular splits, and to avoid injury to the IAN. It is also cost-effective when one considers the alternative approach, which requires resection and reconstruction. We hope that VSP encourages surgeons to consider “split to save.”
Conclusion
For access to extensive or multiple benign intramandibular lesions, SSO prevents the need for extensive resection and reconstruction, avoids injury to the IAN, and allows rapid return to full jaw function. These benefits are particularly pertinent for growing individuals and medically compromised patients who are not ideal candidates for resection and reconstruction. VSP mitigates risks involved with SSO.
References
1. Trauner R, Obwegeser H. The surgical correction of mandibular prognathism and retrognathia with consideration of genioplasty. II. Operating methods for microgenia and distoclusion. Oral Surg Oral Med Oral Pathol 1957;10(9):899-909.

2. Lee HG, Rhee SH, Noh CA, Shin SH. Enucleation of large keratocystic odontogenic tumor at mandible via unilateral sagittal split osteotomy: A report of three cases. J Korean Assoc Oral Maxillofac Surg 2015;41(4):208-12.
3. Casap N, Zeltser R, Abu-Tair J, Shteyer A. Removal of a large odontoma by sagittal split osteotomy. J Oral Maxillofac Surg 2006; 64(12):1833-6.
4. Mahmood L, Demian N, Weinstock YE, Weissferdt A. Mandibular nerve schwannoma resection using sagittal split ramus osteotomy. J Oral Maxillofac Surg 2013;71(11):1861-72.
5. Wong GB. Surgical management of a large, complex mandibular odontoma by unilateral sagittal split osteotomy. J Oral Maxillofac Surg 1989;47(2):179-84.
6. Rittersma J, van Gool AV. Surgical access to multicystic lesions, by sagittal splitting of the lower jaw. J Maxillofac Surg 1979; 7(3):246-50.
7. Shawky M, Mosleh M, Jan AM, Jadu FM. Meta-analysis of the incidence of lingual nerve deficits after mandibular bilateral sagittal split osteotomy. J Craniofac Surg 2016;27(3):561-4.
8. Steenen SA, van Wijk AJ, Becking AG. Bad splits in bilateral sagittal split osteotomy: Systematic review of fracture patterns. Int J Oral Maxillofac Surg 2016;45(8):971-9.
96 www.entjournal.com ENT-Ear, Nose & Throat Journal March 2018 LIU, SIdELL,
hUON, TORRE
Figure 4. Illustration and photograph show the sagittal split osteotomy of the left mandible and the preoperative virtual surgical plan model in this inferior alveolar nerve sheath tumor (A = dentate segment; B = conydlar segment; IAN = inferior alveolar nerve).



















































