Salivary α-amylase levels in vertigo: Can it be an autonomic dysfunction?
Mucocele development after endoscopic sinus surgery for nasal polyposis: A long-term analysis
Positivity rates of in vitro inhalant/ respiratory and food allergy tests in the northern midwestern United States
Acute infectious laryngitis: A case series
No difference in disease-free survival after oral cancer resection with close tumor margins in patients with and without postoperative radiotherapy
www.entjournal.com A Vendome Publication SEPTEMBER 2018 • VOL. 97, NO. 9
s
ss
ss





































The new two-piece magnetically coupled solution for non-surgical closure Round Oval www.inhealth.com We speak ENT ©2017 InHealth Technologies Manufactured by Freudenberg Medical, LLC (161010.06) The voice of experience since 1978 Magnetic4
Closure
Nasal Septal Perforation Prosthesis
3
ü We'reseeing40 -4 5 patientsperdayforbalance therapyonly.

ü Ourhearingaidsaleshaveincreased59%.
ü Ourtotalpatientvisitsareup15%fornewpatientsand5%forreturnpatients.
ü Ourallergy / immunotherapypopulationhasgrown20%since joining.

The
MostProfitable Ancillary ServiceforPrivatePracticeENTs our peers wanted to share an update since adding a FYZICAL Balance Center to t eir ENT practice:
is the single best thing we’ve ever done in our practice. e can’t comprehend why an otolaryngologist would not do this. It’s a no-brainer and it’s taking our profession by storm. e an t stress enough how ama ing o an opportunity this really is. ou will grow every part o your pra ti e through this vehi le.
#1
“This
e couldn’t be more excited about our future. Get in touch with FYZICAL. You absolutely, positively, 100% need to see for your own eyes what this can do for your practice before it’s too late.” Tuscaloosa ENT Group lore Balance at ame an er is over the systems an pro e ures behin this opportunity. Call 941-227-4314 to register now or learn more about this event. * * Discover more at www.BusinessofBalance.com
EDITORIAL BOARD
EDITORIAL BOARD MEMBERS
Editor-in-Chief
Robert T. Sataloff, MD, DMA, FACS
Professor and Chairman, Department of Otolaryngology–Head and Neck Surgery, and Senior Associate Dean for Clinical Academic Specialties, Drexel University College of Medicine Philadelphia, PA
Jean Abitbol, MD
Jason L. Acevedo, MD, MAJ, MC, USA
Jack B. Anon, MD
Gregorio Babighian, MD
Peter C. Belafsky, MD, PhD
Bruce Benjamin, MD
Gerald S. Berke, MD
Michael J. Brenner, MD
Kenneth H. Brookler, MD
Karen H. Calhoun, MD
Steven B. Cannady, MD
Ricardo Carrau, MD
Swapna Chandran, MD
Chien Chen, MD
Dewey A. Christmas, MD
Nicolle T. Clements, MS
Daniel H. Coelho, MD, FACS
David M. Cognetti, MD
James V. Crawford, MD
David H. Darrow, MD, DDS
Rima Abraham DeFatta, MD
Robert J. DeFatta, MD, PhD
Hamilton Dixon, MD
Paul J. Donald, MD, FRCS
Mainak Dutta, MS, FACS
Russell A. Faust, PhD, MD
Ramón E. Figueroa, MD, FACR
Charles N. Ford, MD
Paul Frake, MD
Marvin P. Fried, MD
Richard R. Gacek, MD
Andrea Gallo, MD
Frank Gannon, MD
Emilio Garcia-Ibanez, MD
Soha Ghossani, MD
William P. R. Gibson, MD
David Goldenberg, MD
Jerome C. Goldstein, MD
Richard L. Goode, MD
Samuel Gubbels, MD
Reena Gupta, MD
Joseph Haddad Jr., MD
Missak Haigentz, MD
Christopher J. Hartnick, MD
Mary Hawkshaw, RN, BSN, CORLN
Garett D. Herzon, MD
omas Higgins, MD, MSPH
Jun Steve Hou, MD
John W. House, MD
Glenn Isaacson, MD
Steven F. Isenberg, MD
Stephanie A. Joe, MD
Shruti S. Joglekar, MBBS
Raleigh O. Jones, Jr., MD
Petros D. Karkos, MD, AFRCS, PhD, MPhil
David Kennedy, MD
Seungwon Kim, MD
Robert Koenigsberg, DO
Karen M. Kost, MD, FRCSC
Jamie A. Koufman, MD
Stilianos E. Kountakis, MD, PhD
John Krouse, MD
Ronald B. Kuppersmith, MD, MBA, FACS
Rande H. Lazar, MD
Robert S. Lebovics, MD, FACS
Keat-Jin Lee, MD
Donald A. Leopold, MD
Steve K. Lewis, BSc, MBBS, MRCS
Daqing Li, MD
Robert R. Lorenz, MD
John M. Luckhurst, MS, CCC-A
Valerie Lund, FRCS
Karen Lyons, MD
A.A.S. Rifat Mannan, MD
Alexander Manteghi, DO
Richard Mattes, PhD
Brian McGovern, ScD
William A. McIntosh, MD
Brian J. McKinnon, MD
Oleg A. Melnikov, MD
Albert L. Merati, MD, FACS
Joseph P. Mirante, MD, MBA, FACS
Ron B. Mitchell, MD
Steven Ross Mobley, MD
Jaime Eaglin Moore, MD omas Murry, PhD
Ashli K. O’Rourke, MD
Ryan F. Osborne, MD, FACS
J. David Osguthorpe, MD
Robert H. Osso , DMD, MD
Enrique Palacios, MD, FACR
Michael M. Paparella, MD
Kourosh Parham, MD, PhD
Arthur S. Patchefsky, MD
Meghan Pavlick, AuD
Spencer C. Payne, MD
Kevin D. Pereira, MD, MS (ORL)
Nicolay Popnikolov, MD, PhD
Didier Portmann, MD
Gregory N. Postma, MD
Matthew J. Provenzano, MD
Hassan H. Ramadan, MD, FACS
Richard T. Ramsden, FRCS
Gabor Repassy, MD, PhD
Dale H. Rice, MD
Ernesto Ried, MD
Alessandra Rinaldo, MD, FRSM
Joshua D. Rosenberg, MD
Allan Maier Rubin, MD, PhD, FACS
John S. Rubin, MD, FACS, FRCS
Amy L. Rutt, DO
Anthony Sclafani, MD, FACS
Raja R. Seethala, MD
Jamie Segel, MD
Moncef Sellami, MD
Michael Setzen, MD, FACS, FAAP
Stanley Shapshay, MD
Douglas M. Sidle, MD
Herbert Silverstein, MD
Je rey P. Simons, MD
Raj Sindwani, MD, FACS, FRCS
Aristides Sismanis, MD, FACS
William H. Slattery III, MD
Libby Smith, DO
Jessica Somerville, MD
omas C. Spalla, MD
Matthew Spector, MD
Paul M. Spring, MD
Brendan C. Stack, Jr., MD, FACS
James A. Stankiewicz, MD
Jun-Ichi Suzuki, MD
David ompson, MD
Lester D.R. ompson, MD, FASCP
Helga Toriello, PhD, FACMG
Ozlem E. Tulunay-Ugur, MD
Galdino Valvassori, MD
Emre Vural, MD
Donald T. Weed, MD, FACS
Neil Weir, FRCS
Kenneth R. Whittemore, MD
David F. Wilson, MD
Ian M. Windmill, PhD
Ian J. Witterick, MD,MSc, FRCSC
Richard J. Wong, MD
Naoaki Yanagihara, MD
Eiji Yanagisawa, MD, FACS
Ken Yanagisawa, MD, FACS
Anthony Yonkers, MD
Mark Zacharek, MD
Joseph Zenga, MD
Liang Zhou, MD
CLINIC EDITORS
Dysphagia
Peter C. Belafsky, MD, PhD
Gregory N. Postma, MD
Facial Plastic Surgery
Anthony P. Sclafani, MD, FACS
Geriatric Otolaryngology
Kourosh Parham, MD, PhD, FACS
Karen M. Kost, MD, FRCSC
Head and Neck
Ryan F. Osborne, MD, FACS
Paul J. Donald, MD, FRCS
Reena Gupta, MD
Imaging
Enrique Palacios, MD, FACR
Ramón E. Figueroa, MD, FACR
Laryngoscopic
Robert T. Sataloff, MD, DMA, FACS
Otoscopic
John W. House, MD
Brian J. McKinnon, MD
Pathology
Lester D.R. Thompson, MD, FASCP
Pediatric Otolaryngology
Rande H. Lazar, MD
Rhinoscopic
Eiji Yanagisawa, MD, FACS
Dewey A. Christmas, MD
Joseph P. Mirante, MD, MBA, FACS
Ken Yanagisawa, MD, FACS
Special Topics
Robert T. Sataloff, MD, DMA, FACS
Thyroid and Parathyroid
David Goldenberg, MD
258 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
The ENT Ergonomic Solution
To help you get the best vision and ergonomics, SurgiTel offers a loupe and LED light combo for ear, nose, and throat physicians.

•Adjustable beam direction to see directly into small channels and narrow surgical sites






•Clear and color-corrected light beam


•Adjustable working distance for ease of use

Are You?
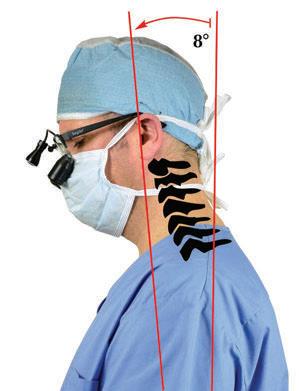
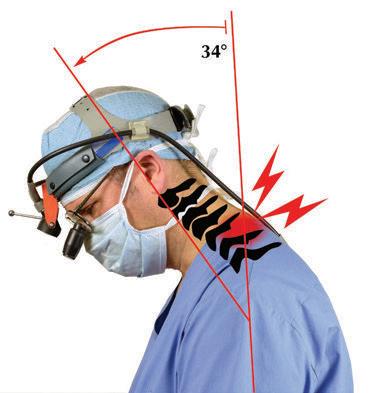
Traditionally designed loupes may force you to tilt your head and neck forward more than 25 degrees (left), leading to neck pain and eventual injury.1
SugiTel loupes feature patented designs which reduce head tilt (right), so you can work with a safe and comfortable posture.

If you look like the clinican on the left and not on the right, you may end your career early and in pain. Contact your local SurgiTel representative to experience the ergonomic difference!
1. Pulat, BM, Fundamentals of Industrial Ergonomics, Chapter 7: 177. Prospect Heights, Illinois: Waveland Press, Inc., 1992.

SurgiTel www.SurgiTel.com 1-800-959-0153 Vision & Ergonomics at Work follow us: Contact your Local Representative NOW for an In-Office Demonstration! www.SurgiTel.com/MyRep 1-800-959-0153
Clinician
Traditional Designs SurgiTel Loupes
Which
DEPARTMENT OF OTOLARYNGOLOGY
HEAD AND NECK SURGERY
UNIVERSITY OF OKLAHOMA COLLEGE OF MEDICINE
POSITION AVAILABLE: LARYNGOLOGIST
DATE AVAILABLE: IMMEDIATELY
The Department of Otolaryngology Head and Neck Surgery of the University of Oklahoma College of Medicine has a position available for a full-time otolaryngologist at the Assistant or Associate Professor level. Specific expertise is required in laryngology. Minimum requirements include: Doctoral degree (M.D. or equivalent), Board certification/ eligibility, a demonstrable commitment to teaching and an interest in collaborative research. Responsibilities will include program development and patient care, resident and medical student education, and research.
Letters of interest with accompanying CV should be directed to: Greg A. Krempl, M.D., F.A.C.S., Attn: Nancy Geiger, Department of Otolaryngology Head and Neck Surgery, 800 Stanton L. Young Blvd, Suite AAT 1400 , Oklahoma City, OK 73104or via e-mail to nancygeiger@ouhsc.edu. The University of Oklahoma is an Affirmative Action and Equal Opportunity Employer. Individuals with disabilities and protected veterans are encouraged to apply.
Editor-in-Chief Robert T. Satalo , MD, DMA, FACS 219 N. Broad St., 10th Fl., Philadelphia, PA 19107 entjournal@phillyent.com Ph: 215-732-6100
Managing Editor Linda Zinn
Manuscript Editors Martin Stevenson and Wayne Kuznar
Associate Editor, Reader Engagement Megan Combs
Creative Director Eric Collander
National Sales Manager Mark C. Horn mhorn@vendomegrp.com Ph: 480-895-3663
Tra c Manager Eric Collander
Please send IOs to adtra c@vendomegrp.com
All ad materials should be sent electronically to: https://vendome.sendmyad.com
Customer Service/Subscriptions
www.entjournal.com/subscribe Ph: 888-244-5310 email: VendomeHM@emailpsa.com
Reuse Permissions Copyright Clearance Center info@copyright.com Ph: 978-750-8400 Fax: 978-646-8600
Chief Executive O cer Jane Butler
Chief Marketing O cer Dan Melore
Vice President, Finance Bill Newberry
Vice President, Custom Media Jennifer Turney Director, Circulation Rachel Beneventi
ENT-Ear, Nose & roat Journal (ISSN: Print 0145-5613, Online 1942-7522) is published 9 times per year in Jan/Feb, Mar, Apr/May, June, July, Aug, Sept, Oct/ Nov and Dec, by Vendome Group, LLC, 237 West 35th Street, 16th Floor, New York, NY 10001-1905.
©2018 by Vendome Group, LLC. All rights reserved. No part of ENT-Ear, Nose & roat Journal may be reproduced, distributed, transmitted, displayed, published, or broadcast in any form or in any media without prior written permission of the publisher. To request permission to reuse this content in any form, including distribution in education, professional, or promotional contexts or to reproduce material in new works, please contact the Copyright Clearance Center at info@ copyright.com or 978.750.8400.
EDITORIAL: e opinions expressed in the editorial and advertising material in this issue of ENT-Ear, Nose & roat Journal are those of the authors and advertisers and do not necessarily re ect the opinions or recommendations of the publisher, editors, or the sta of Vendome Group, LLC. ENT-Ear, Nose & roat Journal is indexed in MEDLINE/PubMed and Current Contents/Clinical Medicine and Science Citation Index Expanded. Editorial o ces are located at 812 Huron Rd., Suite 450, Cleveland, OH 44115. Manuscripts should be submitted online at www.editorialmanager.com/entjournal. Instructions for Authors are available at www.entjournal.com.

SUBSCRIPTIONS: For questions about a subscription or to subscribe, please contact us by phone: 888-244-5310; or email: VendomeHM@emailpsa.com. Individual subscriptions, U.S. and possessions: 1 year $225, 2 years $394; International: 1 year $279, 2 years $488; Single copies $28; outside the U.S., $40.
POSTMASTER: send address changes to Ear, Nose & roat Journal, PO Box 11404 Newark, NJ 07101-4014.
260 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
Balloon dilation for Eustachian tube dysfunction



The superior, durable results your patients deserve. With the safety you can count on.




A randomized, controlled trial comparing balloon dilation with ongoing medical therapy as treatment for persistent Eustachian tube dysfunction reported zero complications and significant symptom improvement through 12 months for patients treated with the XprESS™ ENT Dilation System.1
dilation with XprESS is SAFE 0
ET balloon dilation SUPERIOR to medical management
DURABLE symptom improvement through 12 months
www.url_loremipsumdolores


1 Meyer TA, O’Malley E, Schlosser RJ, et al. A randomized controlled trial of balloon dilation as a treatment for persistent Eustachian tube dysfunction with 1-year follow-up. Otol Neurotol. 2018. DOI: 10.1097/ MAO.0000000000001853
INDICATIONS FOR USE: To access and treat the maxillary ostia/ethmoid infundibula in patients 2 years and older, and frontal ostia/recesses and sphenoid sinus ostia in patients 12 years and older using a transnasal approach. The bony sinus outflow tracts are remodeled by balloon displacement of adjacent bone and paranasal sinus structures. To dilate the cartilaginous portion of the Eustachian tube for treating persistent Eustachian tube dysfunction in patients 18 years and older using a transnasal approach. Please see Instructions for Use (IFU) for a complete listing of warnings, precautions, and adverse events as well as cleaning, sterilizing and care for surgical instruments.
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician.


ENTELLUS MEDICAL and XPRESS are trademarks of Entellus Medical, Inc.

Entellus Medical, Inc. 1738-762 rA 07/2018

























NEW CLINICAL DATA Read more at: go.ent.stryker.com/ETDRCT1YearData
©2018
Balloon Dilation (N=28) Control (N=27) Baseline(N=54) 6 Weeks (N=51) 3 Months (N=52) 6 Months (N=51) 12 Months (N=49) 1 2 3 4 5 6 7 0 1 2 3 4 5 6 7 4.6 2.12.12.12.1 MILD (NO PROBLEM) MODERAT E SEVERE M EAN OVERALL ETDQ-7 SCORE MEAN OVERALL ETDQ-7 SCORE ∆=-2.9±1.4 ∆= -0.6±1.0 p<0.0001 p<0.0001 for
from baseline to all follow-up periods BaselineFollow-up BaselineFollow-up
Balloon Dilation (N=28) Control (N=27) Baseline(N=54) 6 Weeks (N=51) 3 Months (N=52) 6 Months (N=51) 12 Months (N=49) 1 2 3 4 5 6 7 0 1 2 3 4 5 6 7 4.6 2.12.12.12.1 MILD (NO PROBLEM) MODERAT E SEVERE M EAN OVERALL ETDQ-7 SCORE MEAN OVERALL ETDQ-7 SCORE ∆=-2.9±1.4 ∆= -0.6±1.0 p<0.0001 p<0.0001 for change from baseline to all follow-up periods BaselineFollow-up BaselineFollow-up ET balloon
% COMPLICATION RATE
change
ORIGINAL ARTICLES
278 Salivary α-amylase levels in vertigo: Can it be an autonomic dysfunction?
Tanzer Korkmaz, MD; Yusuf Ozgur Bicer, MD; Erdinc Serin, MD; Sinan Seyhan, MD; Serap Koybasi Sanal. MD
284 Mucocele development a er endoscopic sinus surgery for nasal polyposis: A long-term analysis
Hakim Benkhatar, MD; Idir Khettab, MD; Philippe Sultanik, MD, PhD;
Ollivier Laccourreye, MD; Pierre Bon ls, MD, PhD
296 Positivity rates of in vitro inhalant/ respiratory and food allergy tests in the northern midwestern United States
Michael S. Benninger, MD; Thomas Daly, MD; Kevin Graffmiller, MD
306 Acute infectious laryngitis: A case series
Aaron J. Jaworek, MD; Kranthi Earasi, MD; Karen M. Lyons, MD; Srihari Daggumati, BS; Amanda Hu, MD, FRCSC; Robert T. Sataloff, MD, DMA, FACS
314 No di erence in disease-free survival a er oral cancer resection with close tumor margins in patients with and without postoperative radiotherapy
Britta Kaltoft Welinder, MD; Mads Lawaetz, MD; Laura M. Dines, MD, PhD; Preben Homøe, MD, PhD, DMSc
324 Outcomes of reapplication to otolaryngology residency: A prospective cohort study
Colin Fuller, MD, MS; J. Kenneth Byrd, MD; Michael Groves, MD
ONLINE EXCLUSIVES
E1 e role of meteorologic factors and air pollution on the frequency of pediatric epistaxis
M. Volkan Akdoğan, MD; Evren Hızal, MD; Mustafa Semiz, PhD; Özgül Topal, MD; Hakan Akkaş, MD; Aydın Kabataş, MSc; Selim S. Erbek, MD
E6 Renal cell carcinoma metastatic to the sinonasal cavity: A review and report of 8 cases
Pierre-Louis Bastier, MD; Dorothée Dunion, MD; Guillaume de Bonnecaze, MD; Elie Serrano, MD, PhD; Ludovic de Gabory, MD, PhD
E13 Upper aerodigestive tract frostbite from inhalation of automotive nitrous oxide
Stephen A. Chan, BS; Kristan P. Alfonso, MD; Brett T. Comer, MD
E15 Nondisseminated rhinosporidiosis with multisite involvement in the head and neck
K Devaraja, MS; Prem Sagar, MS; Chirom Amit Singh, MS; Rajeev Kumar, MS
E18 Audiologic pro le in patients with ankylosing spondylitis: A controlled study of 30 patients
Lumy Yagueshita, MD; Lucas Resende Lucinda, MD; Valderilio Azevedo, PhD; Gislaine Richter Minhoto Wiemes, PhD; Nicole Richter Minhoto Wiemes, MD; José Fernando Polanski, PhD
E23 Bilateral spontaneous temporomandibular joint herniation: A case report and literature review
Daniel C. O’Brien, MD, MAS; Kaylee R. Purpura, MD; Adam M. Cassis, MD
E28 Histoplasmosis of the head and neck in the immunocompetent patient: Report of 2 cases
Ashley P. O’Connell Ferster, MD; Aaron Jaworek, MD; Amanda Hu, MD, FRCSC
262 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 EDITORIAL OFFICE Robert T. Sataloff, MD, DMA, FACS, Editor-in-Chief • 219 N. Broad St., 10th Fl. • Philadelphia, PA 19107 CONTENTS SEPTEMBER 2018 • VOL. 97, NO. 9
DEPARTMENTS 264 ENT Journal Online 265 Advertiser Index 266 Guest Editorial 270 Rhinoscopic Clinic 272 Laryngoscopic Clinic 274 Pediatric Otolaryngology Clinic 276 Facial Plastic Surgery Clinic E32 Otoscopic Clinic E34 Rhinoscopic Clinic
MEETING HIGHLIGHTS:
The 14th Annual Kennedy
Lecture:
Guest Speaker: Noam Cohen, MD, FARS

Associate Professor of Otorhinolaryngology –Head and Neck Surgery and Director of Rhinology Research at The Perelman School of Medicine at The University of Pennsylvania; Adjunct Associate Member of The Monell Chemical Senses Center and a Staff Surgeon at the Philadelphia VA Medical Center
Topic: “Patholophysiology of Refractory CRS: Translating Basic Science into Clinical Outcomes
• Film FESStival - A contest for the most interesting video case of sinus or skull base surgery


• Resident’s Cadaveric Lab (Limited Space)



• Poster Hall
• Exhibit Hall
• Welcome, Poster and DWK Lecturer Cocktail Reception
• Guest Countries: Colombia, Japan, Portugal, South Africa, Turkey
• Panels:
- Combined AAOA/ARS Panel: Biologics –The demise of the sinus surgeon?
- Biologic Basics of Nasal Polyps
- Pain in Rhinlogy
- Frontal Sinus Surgery
- Applications of Topical Therapies
- Big Data Reviews




- Empty Nose Syndrome

- Economics of Endoscopic Skull Base
- Inverting Pappiloma





October 5-6, 2018


Westin Peachtree Plaza Hotel

Atlanta, GA

Women in Rhinology
Mentorship Program and Resident’s & Fellows
Combined Educational Session: “All I Really Need to Know I’ve learned in Otorhinolaryngology” Saturday, October 6, 2018; 1-2pm
Presented by Cherie-Ann O. Nathan, MD, Professor and Chair, Louisiana State University Health Science Center

HOUSING: https://www.wynjade.com/aaohnsf18/ars (Rooms are filling up quickly)


REGISTRATION: http://www.american-rhinologic.org/annual_registration
Details at http://www.american-rhinologic.org/annual_meeting
www.american-rhinologic.org
ARS 64th ANNUALMEETING
Contact: Wendi Perez, Executive Administrator, ARS, PO Box 269, Oak Ridge, NJ 07438 | Tel: 973-545-2735 | Fax: 973-545-2736 | wendi@amrhso.com
JOURNAL ONLINE
Ear, Nose & Throat Journal's website is easy to navigate and provides readers with more editorial content each month than ever before. Access to everything on the site is free of charge to physicians and allied ENT professionals. To take advantage of all our site has to offer, go to www.entjournal. com and click on the “Registration” link. Once you have lled out the brief registration form, you will have full access. Explore and enjoy!
ONLINE EXCLUSIVES
The role of meteorologic factors and air pollution on the frequency of pediatric epistaxis
M. Volkan Akdoğan, MD; Evren Hızal, MD; Mustafa Semiz, PhD; Özgül Topal, MD; Hakan Akkaş, MD; Aydın Kabataş, MSc; Selim S. Erbek, MD
Fluctuations in atmospheric temperature, humidity, and air pollution are associated with the incidence of epistaxis. To date, no study in the literature has evaluated the e ect of air pollution and meteorologic conditions on the pediatric population. We aimed to evaluate the e ect of meteorologic factors and air pollution on the frequency of epistaxis in children. Children presenting to an outpatient clinical setting at a tertiary care hospital during a 5-year period....
Renal cell carcinoma metastatic to the sinonasal cavity: A review and report of 8 cases
Pierre-Louis Bastier, MD; Dorothée Dunion, MD; Guillaume de Bonnecaze, MD; Elie Serrano, MD, PhD; Ludovic de Gabory, MD, PhD
Renal cell carcinoma (RCC) metastatic in the sinonasal cavity is rare. In many cases, it represents the initial presentation of RCC. We conducted a retrospective chart review to report the clinical presentation, imaging, and treatment of RCC metastases in the sinonasal cavity at two tertiary care referral centers. Our population was made up of 8 patients—6 men and 2 women, aged 55 to 86 years (mean: 66.9; median: 63.5)—who had been diagnosed with cancer in the sinonasal cavity. e most common complaints were epistaxis, nasal obstruction, and diplopia. Cancers were located in the ethmoid sinus (n = 3), nasal cavity (n = 2), sphenoid sinus (n = 2), and maxillary sinus (n = 1); in our series....
Upper aerodigestive tract frostbite from inhalation of automotive nitrous oxide
Stephen A. Chan, BS; Kristan P. Alfonso, MD; Brett T. Comer, MD
Nitrous oxide, a cryogenic gas, may be abused as an inhalant for its euphoric properties. If inhaled, nitrous oxide may cause frostbite to the oral cavity and upper aerodigestive tract, with possible airway compromise due to edema. In this article we describe what is, to the best of our knowledge, the rst case of intentional inhalation of nitrous oxide from an automotive nitrous oxide canister and discuss the management and mechanism of the patient’s injury....
Nondisseminated rhinosporidiosis with multisite involvement in the head and neck
K Devaraja, MS; Prem Sagar, MS;
Chirom Amit Singh, MS; Rajeev Kumar, MS
Rhinosporidiosis is a communicable disease prevalent in tropical countries that a ects one or more mucocutaneous sites such as the nasal cavity, pharynx, skin, bronchus, genitals, and bone, in isolation or together. We report a case of multicentric rhinosporidiosis involving the nasal cavity, oropharynx, larynx, and cheek skin without disseminated disease outside the head and neck. Although the appearance of mucocutaneous lesions in our patient was similar to that of papilloma or neoplasm, the distinct clinicopathologic characteristics of the rhinosporidiosis guided us in managing the case successfully. In our own experience with 11 patients with rhinosporidiosis on whom we operated over the past 5 years, the nasal cavity and pharynx were the most commonly involved sites in the head and neck. Surgical excision of all lesions along with cauterization of the base and long-term dapsone therapy is the current standard of care for multicentric rhinosporidiosis....
Audiologic pro le in patients with ankylosing spondylitis: A controlled study of 30 patients
Lumy Yagueshita, MD; Lucas Resende Lucinda, MD;
Valderilio Azevedo, PhD;
Gislaine Richter Minhoto Wiemes, PhD; Nicole Richter Minhoto Wiemes, MD
José Fernando Polanski, PhD
Recent studies have identi ed sensorineural hearing loss as a possible manifestation of ankylosing spondylitis. We conducted a study of 30 patients with ankylosing spondylitis to characterize their audiologic pro le and to correlate their disease activity and functional indices with their hearing thresholds. e study group was made up of 18 men and 12 women, aged 25 to 58 years (mean: 46.5), who were diagnosed with ankylosing spondylitis. We compared their ndings with a socially and demographically matched group of 30 healthy controls. All 60 participants underwent an audiologic assessment, consisting of pure-tone audiometry, speech audiometry, and tympanometry. We used validated indices to assess disease activity and functional status, and we compiled information on the time of diagnosis and the types of medications used to treat the ankylosing spondylitis. We found that the average of the mean....
264 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
www.entjournal.com
Bilateral spontaneous temporomandibular joint herniation: A case report and literature review
Daniel C. O’Brien, MD, MAS; Kaylee R. Purpura, MD; Adam M. Cassis, MD
In this article we report the case of a 41-year-old man with bilateral aural fullness and hearing loss. On examination he was found to have bilateral, dehiscent anterior canal walls with herniation of the mandibular condyle. is herniation partially obstructed the canals and contributed to his symptoms. To the best of our knowledge, this is only the third reported case of bilateral spontaneous temporomandibular joint herniation, and only 28 cases of unilateral....
Histoplasmosis of the head and neck in the immunocompetent patient: Report of 2 cases
Ashley P. O’Connell Ferster, MD; Aaron Jaworek, MD; Amanda Hu, MD, FRCSC
Histoplasmosis of the head and neck is rarely seen in immunocompetent patients. We report 2 new cases of histoplasmosis of the head and neck in immunocompetent patients, one an 80-year-old man and the other a 57-year-old man. e older man presented with oral cavity histoplasmosis; his symptoms included pain, dysphagia, and ulcerative lesions. e younger man had laryngeal histoplasmosis,
which resulted in hoarseness and dyspnea. We discuss the methods of diagnosis and the classic ndings in histoplasmosis, including the microscopic appearance of caseating granulomas, the results of periodic acid–Schi staining and Gomori staining, and antibody detection of histoplasmosis. We also review the treatment options with antifungals, including amphotericin B and the oral conazole drugs. With an accurate diagnosis and proper treatment, both of our patients recovered well and their symptoms resolved. Because their symptoms overlapped with those of other, more common disease processes, an accurate diagnosis of these patients was essential to treating their infection.....
ONLINE DEPARTMENTS
Otoscopic Clinic: Primary pleomorphic adenoma of the middle ear and mastoid
Asnake Bitew, MD; Tsion Sahle, MD; Miriam Redleaf, MD, FACS
Rhinoscopic Clinic: Endoscopic view of antrochoanal polyp with a dental implant
Hwang Chul Shin, MD; Jong Seung Kim, MD
ADVERTISER INDEX
Volume 97, Number 9 www.entjournal.com 265 ENT JOURNAL ONLINE Pages Pages Acclarent, Inc. .......................................................301 American Rhinologic Society................................263 Arbor Pharmaceuticals..................................311, 312 Audigy Medical......................................................279 Beutlich Pharmaceuticals......................................305 Carl Zeiss..............................................................323 Compulink Business Systems...........................CVR3 Cook Medical........................................................303 Entellus Medical....................................................261 Eosera, Inc.............................................................291 Feather Safety Razor Co., Ltd...............................275 Fyzical Therapy and Balance................................257 Grason-Stadler, Inc...............................................271 Haag-Streit USA....................................................295 Haag-Streit Reliance.............................................307 InHealth Technologies.......................................CVR2 Interacoustics USA........................................298, 299 Lumenis.................................................................309 MAICO Diagnostics...............................................315 McKeon Products, Inc...............................327, CVR4 Medtronic..............................................................289 Meeting Achievements..........................................269 MTI, Inc..................................................................285 OmniGuide Surgical..............................................287 Optim LLC.............................................................293 Shire......................................................................317 Spectrum Audiology..............................................283 Stryker Medical.....................................................319 SurgiTel..................................................................259 WRS Health...........................................................273
GUEST EDITORIAL
Building highly reliable of ce-based surgery
[Excerpted from the Keynote Address presented at the Annual Meeting of the American Society of Geriatric Otolaryngology, Scottsdale, Arizona; January 19, 2018.]
With a shock I watched the mouth of the 1-week-old infant ll with blood. It had seemed so simple when my senior partner said, “You don’t need to go to the OR. Just snip it,” in response to my query as to whether the infant with tongue-tie should be booked for formal division and closure under general anesthesia. Although I had performed frenuloplasty in the OR on numerous occasions, I had never “just snipped it,” nor had I ever considered performing the procedure on a 1-week-old in the clinic. But I recognized that I had made a serious error when I made the second snip to make it perfect. Until that moment I had never considered the implications of performing the supposedly simple procedure in the clinic, several hundred yards through a rabbit’s warren of hallways from the well-sta ed OR suite.
Reviewing options quickly, I picked up the infant in the crook of my arm, grabbed a 4 × 4 gauze sponge, and applied pressure with my index nger. Telling my technician to call the OR and tell them I was on my way, I stepped through the door to face several dozen pairs of curious eyes, and two frightened faces. In as calm a voice as I could muster, I said “I got a little bit of bleeding so am going to take him to the OR for a stitch. Come along with me and we will do the paperwork when we get there.”
e rest of the story was uneventful, but when re ecting on the event over the intervening three and a half decades, I realize that I had failed to fully consider the implications of what my partner had proposed before I was doing it.
I suspect events such as the one related above are not rare. Moreover, I suspect many readers of this editorial will have similar stories from their own practices or those of their colleagues. is commentary was driven by the assumption that the recent increase in the numbers and complexity of o ce-based procedures has likely led to an increase in both the frequency and severity of unanticipated, and occasionally devastating, events.
e death of Joan Rivers in an outpatient endoscopy suite focused public attention on the risks of performing common procedures in uncommon settings.1
Interestingly, in its infancy otolaryngology was a leader in o ce-based (and even kitchen-table) procedures. Over the century or so of the specialty’s existence,
otolaryngologists have performed many procedures in their o ces. Patients who underwent tonsillectomy at home “on the kitchen table” are still encountered occasionally, and many practicing today recall rigid bronchoscopy and esophagoscopy performed in the clinic “back room” during their residencies.
Changes in technology, desire for patient comfort, and recognition of the danger of some procedures eventually led to the migration of many procedures into the hospital. Many of these were performed initially as inpatient procedures, but over the past 4 decades, they were increasingly done as outpatient procedures. A plethora of “surgicenters” has dramatically changed surgery.
Driven by forces a ecting all of medicine and facilitated by new technology, procedures once done in the operating room are moving into the o ce setting. erefore, we need to reexamine systems of care, to ensure that these procedures can be performed in the new setting as safely as possible. One strategy to achieve this may be to study and apply the principles of high-reliability organizations.
High-reliability organizations (HROs) operate in high-risk, high-tempo, and high-stakes environments but have an accident rate far lower than would be expected. ese organizations can be considered “positive deviants” and are worthy of investigation to determine the “secret sauce” that enables them to function as they do. In fact, they have been studied extensively by organizational psychologists, most notably in the investigation of ight operations on U.S. Navy aircra carriers conducted by a research team at UC Berkeley in the 1980s.2
266 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
HROs are painfully aware that many situations are more complex than they initially appear. This should not be surprising to those of us in the medical profession.
Combining the knowledge gleaned from frontline operational leadership and frontline deck personnel with direct observations led to the ndings of the UC Berkeley team, which are now accepted as valid and form the basis of ongoing investigation and scholarship. ey concluded that the following characteristics are essential: sensitivity to operations, reluctance to simplify, preoccupation with failure (asking what could happen), commitment to resilience, deference to expertise (not always apparent who has it), and commitment to perpetual training. ese observations represent the foundational science of HROs.
Some authorities have proposed that leaders of healthcare organizations study HROs and attempt to integrate their characteristics into their own operations, with the goal of improving patient outcomes and avoiding untoward events and accompanying bad outcomes.
A recent “how to” text by Sculli and Paull at the Veterans Administration National Center for Patient Safety provides insight into how this might take place.3
As a guide for leadership, the text provides speci c recommendations, o en in the form of checklists, to assist in integrating HRO principles into one’s organization.
Sculli and Paull provide several key recommendations that could improve patient safety in o ce-based surgery. ey note that sensitivity to operations, also referred to as situational awareness (SA), is harder to achieve than it would appear. ey emphasize that frontline workers o en possess extensive knowledge that is unknown to leadership. Andriessen and Fahlbruch emphasize the importance of establishing formal networks to ensure transmission of informal information “up the chain.”4 More recently, Marx 5 and Dekker6 have pointed out that organizations must “buy” this information from frontline sta by establishing a “just culture” in which employees feel comfortable in sharing what is o en unwelcome information.
Sculli and Paull point out that checklists are invaluable in maintaining e ective SA, to augment short- and long-term memory. Just as checklists are now standard in the OR environment, so should they be used in o ce-based surgery. In fact, multiple checklists are necessary to meet the needs of patients and their families, the o ce’s business and technical sta , and those responsible for instrument setup, teardown, and reprocessing. ese checklists should be dra ed and reviewed by appropriate stakeholders before beginning to perform the procedure(s).
HROs have a reluctance to simplify. ey do not fall prey to the natural tendency to simplify what is complex, thus mistakenly downplaying the risks. HROs are painfully aware that many situations are more complex than they initially appear. is should not be surprising
to those of us in the medical profession, as nearly every disease process or treatment algorithm is found to be more complex than it initially appears. An HRO constantly seeks to dissect and understand complexity in hopes of improving the organization’s ability to predict failure points in processes.
e peril of oversimpli cation applies when one is setting up an o ce procedure. For example, one might simplify the post-procedure period to “we will watch them for 30 minutes.” In actuality, “watching for 30 minutes” involves determining where the observation will occur, who will do the observing, what they are observing for, what instrumentation might be needed, what is the family’s role, will patients be escorted to their vehicles, etc. It is useful to enumerate these steps and requirements before the fact.
e HRO characteristic of deference to expertise mandates that preplanning to identify complexities should include all likely stakeholders.
Regarding preoccupation with failure, an HRO continuously is alert for “things that might go wrong.” HROs are concerned about failures they can anticipate, but they are even more concerned about the possibility of failures they have not considered. In other words, they anticipate and expect that unpleasant surprises are inevitable. erefore, an HRO is constantly looking for an early variation that might indicate an approaching failure. e term “failure to rescue” in our healthcare environment o en implies that what really happened was “failure to recognize early signs of approaching disaster.”
A useful exercise as one sets up o ce-based surgery is to consider and list the multiple possibilities for failure associated with a procedure. Not only can this enhance an o ce sta ’s ability to predict, recognize, and respond to events, but it also serves as a reminder that an unpleasant surprise might lurk just ahead.
Volume 97, Number 9 www.entjournal.com 267 GUEST EDITORIAL
Cultures that criticize those who speak up when events seem to be degenerating effectively guarantee that no one will speak up until they are completely sure an adverse event is about to happen.
Closely linked to preoccupation with failure is the HRO’s commitment to resilience, its willingness to identify “Plan B.” Resilience is a ected by many factors. An HRO is constantly asking the “what if” question, to better prepare. e recent emphasis on in situ crisis management simulation training in hospitals is an example of a strategy to enhance organizational resilience. Considering actions that might be required can be easily incorporated into the process of listing possible untoward events while setting up o ce-based surgery. Being resilient includes asking what resources will be needed if a “what if” occurs. For example, is a cardiac monitor needed? What drugs are necessary for resuscitation if needed? Who should be involved, and what are their roles? What resources are nearby, such as a cardiology office in building? Who will notify the family in the waiting room, and how? If emergency medical services must be contacted, where are they, where is the phone number kept, and what is their typical ETA? Who is responsible for contacting the emergency department (ED) of the receiving facility, and where is that phone number? Considering (and listing) what might be needed before the fact is essential to establishing safe and reliable office-based surgery.
As mentioned earlier, deference to expertise—the expectation that anyone, even the lowest ranking sailor, can immediately halt operations if he or she suspects an untoward event might be developing—is a critical component responsible for the low accident rate during active ight operations on an aircra carrier. Not only is there an expectation that anyone will speak up quickly, but also that the individual will not be criticized if he or she is found to have been incorrect. In fact, they are praised for speaking up, which encourages them to speak up again.
Cultures that criticize those who speak up when events seem to be degenerating e ectively guarantee that no one will speak up until they are completely sure an adverse event is about to happen, which may be too late. Unfortunately, this is common in medical establishments. However, it is possible to encourage employees to speak up by continuously reminding and reinforcing the behavior. is behavior is susceptible to extinction if senior team members criticize other team members. In the author’s institution, all personnel are encouraged to call for help even if they are unsure whether an adverse event is developing. Maintaining this culture requires constant re-education, especially of those who arrive from institutions that do not have the same expectation.
An HRO recognizes that human performance will decline over time unless constantly reinforced by commitment to perpetual training. is decline is encountered particularly in skills required to address rare events. Integrated into the establishment of an o ce-based surgical practice should be a consideration for continuous training to enable sta to manage untoward events optimally. Setting up periodic refresher training to include low- delity simulation is an ideal strategy. If the o ce uses cardiac monitoring, low-cost apps are available that can be used to simulate common events such as bradycardia, etc.
In conclusion, the increasing numbers and complexity of o ce-based surgical procedures inevitably will lead to more challenges for otolaryngologists seeking to institute these procedures in their own o ces. Even though the procedure itself may be identical to that done in a hospital or well-sta ed surgicenter, there are di erent challenges inherent in the move from OR to o ce that may escape detection until some unforeseen event occurs. An awareness of the characteristics of HROs, and adopting some of the strategies they use when establishing o ce-based surgery, has the potential to improve outcomes, reduce the likelihood of unexpected adverse events, and increase the likelihood of rescue should such events occur.
References
1. Yahr E. What went wrong with Joan Rivers’s last medical procedure: Lawsuit. Washington Post. January 28, 2015. https:// www.washingtonpost.com/news/arts-and-entertainment/ wp/2015/01/28/what-went-wrong-with-joan-riverss-lastmedical-procedure-lawsuit/?utm_term=.2afa1dcaf447/. Accessed Aug. 14, 2018.
2. Rochlin GI, La Porte TR, Roberts KH. e self-designing highreliability organization: Aircra carrier ight operations at sea. Navy War College Review; 1987. http://www.refresher.com/ Archives/!sdhro.html/. Accessed Aug. 14, 2018.
3. Sculli GI, Paull DE. Building a High-Reliability Organization: A Toolkit for Success. Brentwood, Tenn.: HCPro; 2015.
4. Erik Andriessen JH, Fahlbruch B (eds). How to Manage Experience Sharing: From Organisational Surprises to Organisational Knowledge. Amsterdam: Elsevier Science Ltd.; 2004.
5. Marx D. Whack-a-Mole: e Price We Pay for Expecting Perfection. Plano, Texas: By Your Side Studios; 2009.
6. Dekker S. Just Culture: Balancing safety and Accountability. Boca Raton, Fla.: CRC Press; 2012.
David E. Eibling, MD, FACS Professor and Vice Chairman of Education Department of OtolaryngologyHead and Neck Surgery University of Pittsburgh School of Medicine
268 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 GUEST EDITORIAL











































































































































RHINOWORLD CHICAGO June 6-9, 2019 Sheraton Grand Chicago THE PREMIER CONGRESS FOR RHINOLOGISTS Combined International Rhinology Meeting Robert Kern MD - 2019 ISIAN President Brent Senior MD - 2019 IRS President James Palmer MD - 2019 ARS President David Kennedy MD - ISIAN General Secretary Metin Onerci MD - IRS General Secretary Joseph Jacobs MD - ARS EVP For questions or more information visit RhinoWorld2019.com Contact: Wendi Perez, Executive Administrator, ARS +1-973-545-2735 ext. 6 | wendi@amrhso.com Polly Rossi, CMP-HC, CMM, Meeting Logistics +1-219-465-1115 | polly@meetingachievements.com Kevin Welch MD, Rakesh Chandra MD & David Conley MD - Program Chairs Featuring Pre-Course Dissections on June 5, 2019 Registration opens October 1, 2018 THE DATE
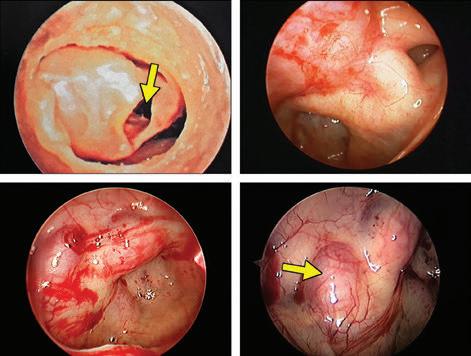
Endoscopic view of the carotid artery in the sphenoid sinus
 Eiji Yanagisawa, MD, FACS; Dewey A. Christmas, MD; Joseph P. Mirante, MD, MBA, FACS
Eiji Yanagisawa, MD, FACS; Dewey A. Christmas, MD; Joseph P. Mirante, MD, MBA, FACS
Because of its posterior location and intimate proximity to the internal carotid artery, optic nerve, and skull base, the sphenoid sinus was the last paranasal sinus frontier to be frequently approached surgically. Surgical approaches to the sphenoid sinus became more accepted in the early 1900s as intranasal techniques improved. e use of the endoscope and then powered instrument techniques introduced in the 1990s1 have made sphenoid sinus surgery widely accepted.
e sphenoid sinus has been described as the most variable, in shape and size, of any bilateral cavity or organ in the human body.2 In early anatomic studies, the internal carotid artery was found to indent the posterolateral wall of the sphenoid sinus in approximately 65% of specimens.3 Also, bony wall dehiscence over the internal carotid artery was not an uncommon nding in large studies4 ( gure, A).
Because of the important anatomic structures indenting the lateral wall of the sphenoid sinus, entry into it should always be done through an inferior and medial approach when dissection is carried out through the ethmoid sinus. Visualization of the lateral sphenoid wall can then be carried out with an angled endoscope ( gure, B and C) to be sure that the internal carotid is not dangerously bulging into or dehiscent into the sinus cavity. When the sphenoid dissection is carried out transnasally through the natural sphenoid ostium or through the anterior wall of the sphenoid sinus, similar precautions should be carried out before any lateral dissection is performed. Any so -tissue biopsy or tissue removal in the lateral portion of the sphenoid sinus should be approached with extreme caution and good visualization because of possible dehiscence over the internal carotid artery ( gure, D).
Acknowledgment
e authors thank Grayson Bertaina for his assistance in preparing this article.
References
1. Christmas DA Jr., Krouse JH. Powered instrumentation in functional endoscopic sinus surgery. I: Surgical technique. Ear Nose roat J 1996;75(1):33-6, 39-40.
2. Congdon ED. e distribution and mode of origin of septa and walls of the sphenoid sinus. Anat Rec 1920;18(2):97-123.
3. Van Alyea OE. Sphenoid Sinus: Anatomic study, with considerations of the clinical signi cance of the structural characteristics of the sphenoid sinus. Arch Otolaryng 1941;34(2):225-53.
4. Dixon FW. A comparative study of the sphenoid sinus: A study of 1600 skulls. Ann Otol Rhinol Laryngol 1937;46(3):687-8.
270 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
From the Section of Otolaryngology, Yale New Haven Hospital–St. Raphael Campus and the Yale University School of Medicine, New Haven, Ct. (Dr. Yanagisawa); the Department of Otolaryngology, the Halifax Medical Center, Daytona Beach, Fla. (Dr. Christmas and Dr. Mirante); and Florida State University School of Medicine, Daytona Beach (Dr. Mirante).
RHINOSCOPIC CLINIC
C B D
Figure. A: is cadaver dissection of the sphenoid sinus shows a very large dehiscent carotid artery almost lling the sphenoid sinus. e artery has been opened (arrow). B and C: Each of these cases shows the carotid artery bulging into the lateral wall of the sphenoid sinus. D: A partially dehiscent carotid artery is seen (arrow).
A


Nonarytenoid laryngeal granulomas
Marissa Evarts, DO; Jonathan Romak, MD, Robert T. Satalo , MD, DMA, FACS
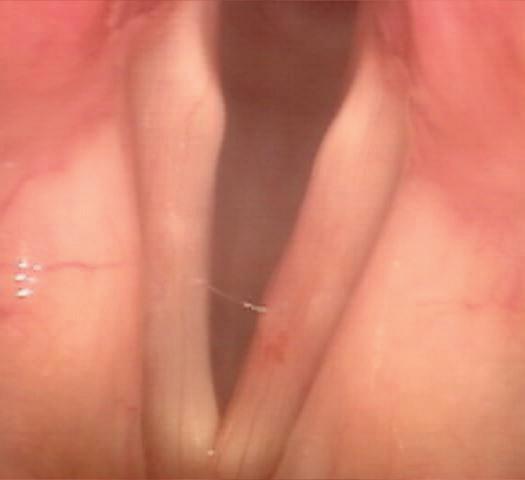
A 69-year-old woman with dysphonia was taken to the operating room for excision of a le true vocal fold cyst refractory to voice therapy and re ux control. She had previously undergone Nissen fundoplication for severe re ux and inability to tolerate anti-re ux medications. Examination 1 week postoperatively revealed swelling at the excision site on the le vocal fold. She was started on a prednisone taper and a strict low-acid diet. Upon her re-examination 2 weeks later, a large, le true vocal fold granuloma was observed at the operative site, along the middle third of the true vocal fold ( gure 1).

e patient subsequently underwent microdirect laryngoscopy, excision of a le true vocal fold granuloma, angiolytic KTP laser treatment of the attachment site, and dexamethasone injection. Two weeks postoperatively, she was doing well with no evidence of recurrent granuloma ( gure 2).
Contact granulomas are benign lesions of the larynx that characteristically develop posteriorly at the vocal process of the arytenoid. e most common presenting symptom is hoarseness, with sore throat, dyspnea on
exertion, globus, stridor, and “cut-o voice” occurring less commonly.1
Contact granulomas result from trauma, o en due to laryngopharyngeal re ux, chronic cough, and throat clearing, as well as vocal abuse. ey are perpetuated by repeated contact between vocal processes during phonation.2 is prevents adequate wound healing and leads to ulcer formation with subsequent reactive tissue overgrowth, o en presenting as a nodular, reddish lesion.3 ese granulomas, however, lack typical features of granulomatous lesions on light microscopy. Instead, histopathology of contact granulomas includes hyperplastic epithelium, granulation tissue, and chronic in ammatory in ltrate, with no reported cases of malignant transformation.4,5
Very rarely, contact and postintubation granulomas are located on the middle third or anterior portion of the vocal folds, with few reported in the literature. e senior author (R.T.S.) has described a case of bilateral granuloma and varicosity in the midportion of the vocal folds, as well as a laryngeal granuloma of the false vocal fold.6,7
Continued on page 277
From the Department of Otolaryngology and Facial Plastic Surgery, Philadelphia College of Osteopathic Medicine (Dr. Evarts); and the Department of Otolaryngology–Head and Neck Surgery, Drexel University College of Medicine, Philadelphia (Dr. Evarts, Dr. Romak, and Dr. Satalo ).
272 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
LARYNGOSCOPIC CLINIC
Figure 1. Videostroboscopy reveals the le true vocal fold granuloma.
Whether it’smanaging the ongoing changes of Meritbased Incentive Payments, attracting new patients to your practice, or documenting new standards of care, ENT-Cloud has you covered.


We provide support in billing, marketing, clinical services and so much more.You just want to focus on your patients - ENT-Cloud can juggle everything else at your practice so that you don’t have to.
much more.
With more than 19 years of experience with the Otolaryngology community, ENT-Cloud has a proven track record of helping practices streamline the revenue cycle, build a brand in their community, and speed up patient charting. We don’t just provide software, we provide solutions for the busy physician.


















Come chat with us at the AAO-HNSF in Atlanta, Georgia BOOTH 3141
1-866-495-4002 ENT-Cloud.com LearnMore@ENT-Cloud.com
ENT-Cloud
by WRS Health EDITORS’ CHOICE #1 EMR MAGAZINE
in Your Practice?
Powered
PEDIATRIC OTOLARYNGOLOGY CLINIC
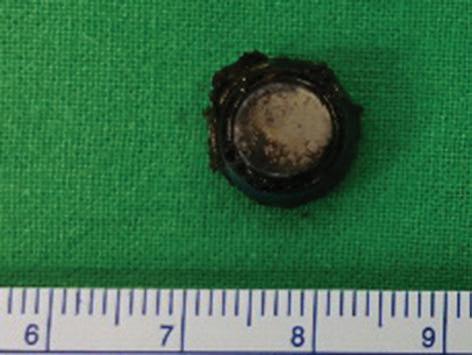
Button battery insertion in nose manifested
as infraorbital cellulitis
Sheng-Yao Cheng, MD; Cheng-Ping Shih, MD
A 3-year-old boy presented to our emergency department with a 3-day history of fever and progressively painful swelling over the le infraorbital and medial canthal region. Crusts and discharge from his le nostril were noted on physical examination. Blood tests showed leukocytosis and an increased level of C-reactive protein. Computed tomography (CT) revealed a hyperdense material in the le nasal cavity with prominent swelling in the so tissue overlying the le maxilla ( gure 1).

In the operating room, a button battery and necrotic tissue surrounding it were removed from the le nasal cavity ( gure 2), and septal perforation also was found. A er 4 days of intravenous antibiotics, the child’s fever and facial symptoms resolved.
Ingestion or insertion of a button battery is a threat to children. Once a battery is lodged in one location, it
rapidly can lead to signi cant mucosal damage through the external electrolytic current that hydrolyzes tissue uid.1 erefore, early identi cation and removal are important.
Infraorbital cellulitis developing from a nasal foreign body is relatively rare and should be considered when diagnosing a child with a presentation similar to the one described in this article.
Funding/support
is work was supported by grants from Tri-Service General Hospital, National Defense Medical Center, Taiwan.
Reference
1. Litovitz T, Whitaker N, Clark L, et al. Emerging battery-ingestion hazard: Clinical implications. Pediatrics 2010;125(6):1168–77.
From the Department of Otolaryngology–Head and Neck Surgery, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
274 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
Figure 2. Photo shows the button battery and necrotic tissue that have been removed from the le nasal cavity.
R L
Figure 1. CT reveals a hyperdense material (asterisk) in the le nasal cavity with prominent swelling in the so tissue overlying the le maxilla (arrow).
Feather Safety Razor




































Ef cient closure of earlobe cleft with biopsy punch


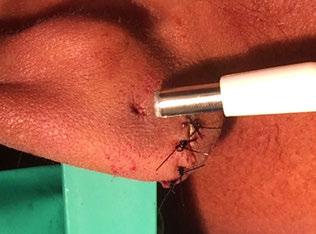 Chetan Y. Sa , MD; Anthony P. Sclafani, MD
Chetan Y. Sa , MD; Anthony P. Sclafani, MD
Several techniques for ear lobule reconstruction after ear piercing or ear gauging have been described in the literature. Modified Z-plasty maneuvers, linear reconstruction, tissue rearrangement, and other procedures have been used to repair a complete or partial cleft of the ear lobule.1-6 Furthermore, use of a biopsy punch with a guidewire or a modified needle base to excise the epithelial tract have been described.7,8 However, previously described punch biopsy techniques can be imprecise, leading to improper or incomplete excision of the tract or conversely
leading to tangential excision of more epithelium than is required.
We describe using a precisely sized biopsy punch with the base of a scalpel to more uniformly excise the earlobe cle in a quick, e cient manner ( gure). e rm scalpel handle provides a broad base with tactile feedback in determining how much pressure needs to be applied with the punch biopsy to uniformly excise the epithelial tract, while simultaneously stabilizing the earlobe so tissue. A er excision, the circular wound can be closed primarily with a good cosmetic result.
From the Department of Otolaryngology–Head and Neck Surgery, New York Presbyterian Hospital–Weill Cornell Medical Center, New York City (Dr. Sa and Dr. Sclafani); the Department of Otolaryngology–Head and Neck Surgery, New York Presbyterian Hospital–Columbia University Medical Center, New York City (Dr. Sa ).
276 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
FACIAL PLASTIC SURGERY CLINIC B C D
A
Figure 1. A: Incomplete earlobe cle is seen laterally. Full laceration of the earlobe has been closed in a traditional manner with interrupted nylon sutures a er de-epithelializing both edges of the laceration. B and C: Biopsy punch seen above earlobe cle is sized to just span the cle , with the scalpel handle providing support medially to the ear. e punch excises the epithelial tract with a circular motion while the scalpel handle provides tactile feedback medially. D: e so -tissue defect visible a er excision of the epithelial tract is minimized via use of the biopsy punch and scalpel handle.
Continued from page 272
Based on the diameter of the ear cle , the appropriately sized biopsy punch can be chosen (3 to 6 mm diameter).
e lobule is then anesthetized and hydroin ated with lidocaine with 1:100,000 epinephrine. Next, with the scalpel placed on the medial surface of the earlobe to provide support, the biopsy punch is pressed rmly with a circular motion into the lobule, centered closely over the earlobe cle . e entire epithelial tract and a small cu of epithelium from the medial and lateral surface of the earlobe is excised, leaving fresh edges of epithelium and dermis for primary closure with dermal 4-0 Vicryl and epidermal 5-0 nylon sutures.
e patient is advised to apply bacitracin to the incision twice daily for 1 week. e nylon sutures are removed in 5 to 7 days; the earlobe can be repierced, if desired, in 6 to 8 weeks.
Many techniques can be used to de-epithelialize and close an ear cle that remains a er long-term earring use or ear gauging. Procedures range from use of a #11 scalpel blade to a biopsy punch with a guidewire to excise the epithelial tract.1-8 We have highlighted an e cient and accurate method for reconstructing the ear lobule. A biopsy punch with the support of the at and even surface of a scalpel handle provides the appropriate balance to excise a smaller tract, including all necessary epithelium, leaving a defect that easily can be closed primarily with an excellent cosmetic outcome.
References
1. Abenavoli FM. Split earlobe: Repair using a half Z-plasty technique. Plast Reconstruct Surg 1996;98(2):372-3.
2. Arasaratnam RB, Patel SK, Pramechander D, et al. Repair of large holes in stretched earlobes. Clin Otolaryngol 2011;36(6):597-8.
3. de la Sotta P, Paredes N, Lasalle MA. Repair of dilated earlobe due to plug piercing. Dermatol Surg 2010;36(10):1621-3.
4. Snell BJ, Caplash Y. A novel way to repair the earlobe a er eargauging. J Plast Reconstructr Aesthet Surg 2013;66(1):140-1.
5. Vujevich J, Goldberg LH, Obagi S. Repair of partial and complete earlobe cle s: A review of 21 methods. J Drugs Dermatol 2007;6(7):695-9.
6. Vujevich J, Obagi S. Repair of partial earlobe cle using a “purse‐string” repair. Dermatol Surg 2006;32(7):969-71.
7. Dessy LA, Buccheri EM, Anniboletti T. Modi ed punch technique for incomplete earlobe cle repair. Aesthet Plast Surg 2006;30(6):731-2.
8. Taher M, Metelitsa A, Salopek TG. Surgical pearl: Earlobe repair assisted by guidewire punch technique: A useful method to remove unwanted epithelial tracts caused by body piercing. J Am Acad Dermatol 2004;51(1):93-4.
Figure 2. No evidence of the granuloma is seen 2 weeks a er excision and angiolytic KTP laser treatment.
e natural course and treatment of contact versus postintubation granulomas di er, as contact granulomas have a high likelihood of recurrence (92%) when removed surgically.8 e mainstay of treatment of contact granulomas is conservative, consisting initially of anti-re ux medication and voice therapy. Surgical removal is reserved primarily for cases refractory to medical treatment or when the diagnosis is in doubt, and “bloodless” in-o ce techniques such as KTP laser treatment can o er increased accessibility, decreased morbidity, and lower recurrence rates than traditional cold steel microlaryngoscopy techniques, but at the expense of complete histologic evaluation.9,10
References
1. Bradley PJ. Arytenoid granuloma. J Laryngol Otol 1997;111(9):801-3.
2. Leonard R, Kendall K. E ects of voice therapy on vocal process granuloma: A phonoscopic approach. Am J Otolaryngol 2005;26(2):101-7.
3. Bohlender J. Diagnostic and therapeutic pitfalls in benign vocal fold diseases. GMS Curr Top Otorhinolaryngol Head Neck Surg 2013;12 Doc01.doi3205/cto000093.
4. Devaney KO, Rinaldo A, Ferlito A. Vocal process granuloma of the larynx—recognition, di erential diagnosis and treatment. Oral Oncol 2005;41(7):666-9.
5. Heller AJ, Wohl DL. Vocal fold granuloma induced by rigid bronchoscopy. Ear Nose roat J 1999;78(3):176-8, 180.
6. Anderson T, Hawkshaw M, Satalo RT. Bilateral granuloma and varicosity in the midportion of the vocal folds. Ear Nose roat J 2002;81(6):374.
7. Satalo RT, Spiegel JR, Hawkshaw MJ. Laryngeal granulomas of the false vocal fold. Ear Nose roat J 1995;74(10):687.
8. Ylitalo R, Lindestad PA. A retrospective study of contact granuloma. Laryngoscope 1999;109(3):433-6.
9. Karkos PD, George M, Van Der Veen J, et al. Vocal process granulomas: A systematic review of treatment. Ann Otol Rhinol Laryngol 2014;123(5):314-20.
10. Mascarella MA, Young J. In-o ce excision en masse of a vocal process granuloma using the potassium-titanyl-phosphate laser. J Voice 2016;30(1);93-5.
Volume 97, Number 9 www.entjournal.com 277
FACIAL PLASTIC SURGERY CLINIC LARYNGOSCOPIC CLINIC
Salivary α-amylase levels in vertigo: Can it be an autonomic dysfunction?
Tanzer Korkmaz, MD; Yusuf Ozgur Bicer, MD; Erdinc Serin, MD; Sinan Seyhan, MD; Serap Koybasi Sanal, MD
Abstract
We aim to demonstrate possible autonomic dysfunction based on salivary α-amylase measurements during and a er the vertigo attacks associated with Ménière disease (MD) and benign paroxysmal positional vertigo (BPPV). Patients admitted to the emergency room with a diagnosis of vertigo attacks caused by either MD (n = 15) or BPPV (n = 9) constituted the study groups. e control group (n = 10) consisted of volunteer patients admitted to the emergency department with minor so -tissue trauma. e rst saliva samples were obtained immediately during the attacks and the second and third samples were obtained on the third and eenth days of the attack, respectively. In the controls, the rst sample was obtained a er admission to the hospital and the second sample was obtained on the third day. Salivary α-amylase levels were evaluated. e di erence between salivary α-amylase levels in patients with MD and BPPV was not signi cant. e amylase value measured early a er the BPPV attack was signi cantly lower than that of the controls (p = 0.008). Although not signi cant, an undulating pattern of salivary α-amylase levels was observed with both diseases. An autonomic imbalance could be partly demonstrated by salivary α-amylase measurement early a er the attack in patients with BPPV. erefore, amylase may be a promising marker that is worth further investigation.
Introduction
Vertigo is a common symptom encountered in the emergency department (ED). It adversely a ects many quality-of-life aspects. Benign paroxysmal positional vertigo (BPPV) and, to a lesser extent, Ménière disease
From the Department of Emergency, Izmir Medicalpark Hospital, Izmir, Turkey (Dr. Korkmaz); Department of Otolaryngology, Abant İzzet Baysal University, Faculty of Medicine, Bolu, Turkey (Dr. Bicer, Dr. Seyhan, and Dr. Sanal); and Department of Biochemistry, Ministry of Health, İstanbul Training and Research Hospital, İstanbul, Turkey (Dr. Serin). e study was conducted at Abant İzzet Baysal University, Faculty of Medicine, Bolu.
Corresponding author: Tanzer Korkmaz, MD, Department of Emergency, Izmir Medicalpark Hospital, Izmir, Turkey. Email: tanzerkorkmaz@gmail.com
(MD) account for a considerable number of patients seeking medical help in EDs.
BPPV is characterized by brief, recurrent attacks of vertigo triggered by changes in head position. e attacks may be followed by residual dizziness in some patients. With the use of canalith repositioning maneuvers, management of BPPV is usually easy for most patients.1 MD is a disease of the peripheral vestibular system characterized by repetitive attacks of vertigo, tinnitus, and low-frequency hearing loss. Although many factors such as genetics, allergy, autoimmunity, and stress are presumed to have a role in the etiology of MD, the exact pathophysiologic changes have not been elucidated.2,3 e hypothalamic pituitary adrenal axis, autonomic nervous system, and immune system are interrelated components of the stress response. e role of stress has been documented in inner ear diseases in many studies.4,5 Studies showed the vestibular system and the autonomic system to be closely related since vestibular activity influences the cardiovascular system. Central anatomic connections between vestibular nuclei and the autonomic pathways have been identified.6 In addition, stress hormones have been reported to modify inner ear functions such as threshold shi s.7
Horner reported prolactin to have a role in MD by its e ect on osmoregulatory mechanisms.6 Together with growth hormone, prolactin is now considered a major stress-induced hormone.8 Yildiz et al showed a marked asymmetric sympathetic hypofunction in the area of the postauricular region of the involved ear in patients with MD.9 e hypofunction was demonstrated with the use of sympathetic skin responses in the postauricular area. Yamada et al reported autonomic nervous dysfunction to be a predisposing factor in MD using heart rate variability.10 However, the question of whether it occurs as a triggering factor or a consequence of vertigo in MD was not determined in that study.
MD and BPPV are di erent entities. Although idiopathic in most cases, BPPV can be observed a er MD attacks. BPPV that occurs a er inner ear diseases, including MD, has been shown to have a higher recurrence rate and a longer recovery period.11,12 Kim and Lee showed
278 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 ORIGINAL ARTICLE
Connect Patients to More of Your Services
Tap into the potential of your ancillary o erings. From sleep medicine to immunotherapy to audiology, we help you steer patients to your nonsurgical services.

An Allergy Upgrade
A prominent Texas-based ENT practice needed to optimize their allergy work ow across multiple clinics. Implementation of our allergy business solutions resulted in:
120 hours saved per year with auto-generation of prescriptions
Greater antigen quality through antigen-expiration alerts


Greater e ciency through consistent, scalable processes across locations




An Audiology Increase


A well-established Maryland-based ENT practice wanted to increase audiology utilization. Within six months, our cultural, operational, marketing, and nancial process changes resulted in:
30% increase in hearing aid revenue
130% increase in department pro tability
Learn more about Audigy Medical’s consulting and practice-support services at AudigyMedical.com/member-stories.
an association between residual dizziness in BPPV and sympathoneural autonomic dysfunction.13 Pezzoli et al also hypothesized autonomic dysfunction to have a role in orthostatic dizziness a er recovery of BPPV attacks.14 However, they failed to show any signi cant relation between BPPV and orthostatic dizziness through the use of the head-up tilt test.
Salivary α-amylase is a peptide with sympathetic activity and so a ects the autonomic nervous system. Many studies in the literature use salivary α-amylase as a marker of sympathetic activity.15-17 Instead of employing venous puncture, obtaining saliva is a noninvasive and cost-e ective method to measure α-amylase.
Our objective in this study was to demonstrate possible autonomic dysfunction with the use of salivary α-amylase measurements during and a er the vertigo attacks of MD and BPPV.
Patients and methods
is study was approved by the Ministry of Health, İstanbul Training and Research Hospital Ethical Committee for Clinical Research with approval number of 2014/459. Informed consent was obtained from each participant.
Patients. Patients with a diagnosis of MD attacks or BPPV were recruited from EDs. Patients 18 to 65 years old who agreed to participate were included in the study. A bedside con rmation of the attacks was performed by the otolaryngologists according to the criteria recommended by the Committee on Hearing and Equilibrium of the American Academy of Otolaryngology–Head and Neck Surgery.18 e control group consisted of patients presenting to the ED with acute, minor so -tissue injuries (i.e., bruises) who were willing to participate in the study. e routine workup was performed for these patients together with the saliva sampling.
Exclusion criteria included use of medications with an e ect on the central nervous system, drinking alcohol or acidic beverages, or doing physical exercise within the preceding 1 hour.
Saliva collection and amylase analysis. Saliva was collected three times from all patients with MD and BPPV and two times from participants in the control group. e rst sample was obtained shortly a er the patients arrived. For patients with MD, the rst sample was obtained during the attack (before any medication use). e second sample was obtained on the third day, and the third sample was obtained on the eenth day.
Saliva collection was performed as follows: the patients were asked to collect their saliva for 5 minutes. A er its collection, the sample was frozen at -20°C. All samples were unfrozen at 4 to 8°C on the same day. A supernatant was prepared by centrifuging the samples at 1,500 rpm for 10 minutes. Salivary α-amylase analysis of these
supernatants was performed a er adequate dilution using Siemens Advia 2400 kits (Erlangen, Germany).
Statistical analysis. SPSS for Windows (version 17.0, IBM; Armonk, New York) was used for statistical analysis. All data are presented as the mean ± standard deviation. Associations between salivary α-amylase measures were evaluated using the Wilcoxon signed rank test. Comparison between group means was carried out using the Kruskal-Wallis test at the signi cance level of p < 0.05. Multiple comparisons were performed using the post hoc Mann-Whitney U test with Bonferroni correction. Bonferroni correction was conducted manually, and values at the level of p < 0.016 were considered signi cant for post hoc analysis.
Results
Patients who did not come for the repeated saliva measurements were excluded from the study. Also, if it was determined that patients did not have MD or there was a residual vertiginous symptom at the time of the second or third measurement, they were excluded from the study.
Overall, 15 patients in the MD group, 9 patients in the BPPV, and 10 participants in the control group were included in the study. e MD group consisted of 10 men and 5 women aged 18 to 62 years (mean: 39 years). e BPPV group consisted of 2 men and 7 women aged 21 to 65 years (mean: 38 years), and the control group included 6 men and 4 women aged 24 to 62 years (mean: 46 years).
Patients in the MD group had experienced at least two attacks of vertigo previously. Five of the 15 patients had experienced at least 10 attacks. All patients with BPPV were diagnosed with posterior canal BPPV.
In patients with MD, the mean level of salivary α-amylase on rst, second, and third measurements were 2,676 ± 4,110 U/l, 1,106 ± 1,411 U/l, and 1,811 ± 2,338 U/l, respectively. Values were 2,087 ± 1,357 U/l, 236 ± 237 U/l, and 813 ± 1,169 U/l in sequential order in patients with BPPV, and 1,020 ± 2,089 U/l and 2,210 ± 8,736 U/l on the rst and second measurements of the control group.
e statistical analysis revealed no signi cant di erence among the three groups with respect to the rst salivary α-amylase measurements (p > 0.05). e second salivary α-amylase measurement on the third day of the vertigo attack (de ned as early a er the attack) was signi cantly di erent in the BPPV group compared with controls (p = 0.024).
No signi cant di erence was detected in salivary α-amylase levels between the patients with MD and those with BPPV (p = 0.055); however, a signi cant di erence between the BPPV and control groups was found (p = 0.008). No signi cant di erence was found in salivary α-amylase measurements between the MD and control groups. In the BPPV group, however, a signi cant di erence was observed between the rst and second salivary α-amylase
280 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
KORKMAZ, BICER, SERIN, SEYHAN, SANAL
measurements (p = 0.038) and between the second and third salivary α-amylase measurements (p = 0.008). e second measurements in the control group could be considered normal values since those patients had only minor so -tissue injuries. A comparison of those values to the rst measurements taken in the MD and BPPV groups revealed no signi cant di erence between groups.
Discussion
Salivary α-amylase is a member of glucosyl hydrolases and is produced mainly in the parotid glands. Its main role is degradation of carbohydrates. Numerous investigators have proposed salivary α-amylase to be a marker of the adrenergic system. It was shown to be secreted from the salivary glands mainly in response to beta adrenergic and partly to alpha adrenergic stimuli.
Salivary α-amylase has been advocated as a biologic marker of physiologic and psychological stress. e interaction between the autonomic nervous system and stress is well known. Many studies have demonstrated a marked increase in salivary α-amylase in anxiety-related diseases, post-traumatic stress, and mental disorders.15,17-19
Sympathetic innervation and the presence of stress hormone receptors in the inner ear tissue are evident.20 Steroid hormones are shown to slowly modify the inner ear physiology via changing gene expressions or nongenomic pathways. Possible multiple interactions between the sympathetic and the complex feedback neuroendocrine systems have been proposed. Via interacting with the immune system, these interactions and the cytokines contribute to inner ear pathologies such as tinnitus, hearing loss, and vertigo.21 In noise trauma, for example, even though glucocorticoids are not the sole actors, a more rapid recovery has been observed in adrenalectomized animals.22
Vasopressin, also known as antidiuretic hormone, is secreted in the hypothalamus and is associated with corticotrophin-releasing hormone. It stimulates adenylate cyclase activity both in the stria vascularis and semicircular canal epithelium and has been reported to be elevated under psychological stress conditions.23-25 Vasopressin levels are increased with endolymphatic hydropic states and are associated with vertigo attacks.26,27 Takeda et al demonstrated that the administration of vasopressin, which increases the activity of aquaporin 2, to guinea pigs over 1 week resulted in the development of hydrops.26
Because evidence supports stress-related inner ear pathologies, we aimed to demonstrate possible autonomic dysfunction in MD and BPPV with salivary α-amylase measurements. e rst salivary α-amylase measurements (during the vertigo episode) in all the groups were highest compared with subsequent measurements. is nding may be attributed to sympathetic over-reactivity
caused by the stress of the attack itself in addition to the stress provoked by the atmosphere of the ED.
Patients with MD demonstrated the highest levels of salivary α-amylase during their attacks. Our statistical analysis supports our hypothesis of increased sympathetic activity during the vertigo attacks, although the di erence was not signi cant. However, a noteworthy nding was the undulating pattern of the salivary α-amylase measurements; salivary α-amylase levels decreased initially at the second measurement and increased again in the following days. We therefore believe that repeating such types of studies with a bigger sample size may reveal more striking results.
e results of the second amylase measurements provide a clue about the role of the autonomic nervous system in these diseases. e di erence between the second measurements of salivary α-amylase was signi cantly lower in BPPV patients than in the MD and control patients. erefore, a depression in sympathetic tone or a parasympathetic overactivation might be responsible.
e autonomic nervous system with its sympathetic and parasympathetic subdivisions should be in balance to avoid disease states. Disruption of this balance in either hyperadrenergic or hypervagal mixed states can result in a variety of symptoms such as dizziness, palpitations, anxiety, fatigue, syncope, and gastrointestinal symptoms.28
Although autonomic dizziness is generally considered to be lightheadedness, vertigo in association with autonomic dysfunction is being reported more frequently.28-30 Low et al reported vertigo as a symptom of orthostatic hypotension in 37% of cases.29 Although the lightheadedness is believed to occur as a result of a transient acute decrease in cerebral blood ow, the mechanism of vertigo is still poorly understood.28
e underlying mechanisms of BPPV have been better elucidated than those of MD or other hydropic states. Residual dizziness is a commonly encountered condition a er BPPV.14 Kim and Lee reported a 43% rate of dizziness a er successful canalith repositioning maneuvers.13 is by itself points to a connection of some type between BPPV and the autonomic nervous system. e mentioned reports demonstrated autonomic dysfunction by means of the head-up tilt test. Our aim in this study was to determine whether this autonomic dysfunction was also possible during vertigo attacks by using a relatively simple method—measurement of salivary α-amylase levels during the attacks.
In BPPV, salivary α-amylase levels were lowest on the third day and then rose again on the 15th day. is result points to a possible imbalance in the autonomic nervous system in BPPV. is possibility is important during follow-up; the clinician should keep in mind that an autonomic dysfunction is possible and should evaluate patients by repeating the maneuvers.
Volume 97, Number 9 www.entjournal.com 281 SALIVARY α-AMYLASE LEVELS IN VERTIGO: CAN IT BE AN AUTONOMIC DYSFUNCTION?
Salivary α-amylase is accepted to re ect sympathetic tone. A possible autonomic imbalance in peripheral vertigo might be due to a change in parasympathetic tone. is possibility needs further studies.
e timing of the second and third measurements of salivary α-amylase in our study can be criticized. As no globally accepted normal range of salivary α-amylase currently exists, we compared its levels in patients with di erent diseases and in patients presenting to the ED with minor so -tissue trauma and no history of vertigo. e second measurements in the control group can be considered normal values.
Cochleovestibular physiology is a ected by the autonomic nervous system. Many mechanisms in the etiology of MD and BPPV have been proposed. e results of our study related to salivary α-amylase levels suggest an autonomic imbalance early a er the canalith repositioning maneuvers in BPPV but do not support autonomic dysfunction in MD.
To the best of our knowledge, this study is the rst to measure salivary α-amylase in patients with inner ear diseases. Further studies with a larger sample size are required to identify salivary α-amylase as a possible biomarker of autonomic imbalance in patients with vertigo. Diagnosis of vertigo in the ED can be challenging, and there is a continued need for a reliable method to di erentiate causes of vertigo.
Acknowledgment
e authors thank Burcin Balaban, MD, for consulting with the patients in the ED.
References
1. Bhattacharyya N, Baugh RF, Orvidas L, et al. Clinical practice guideline: Benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 2008;139(5 Suppl 4):S47-81.
2. Sajjadi H, Paparella MM. Ménière's disease. Lancet 2008;372(9636):406-14.
3. Semaan MT, Alagramam KN, Megerian CA. e basic science of Ménière’s disease and endolymphatic hydrops. Curr Opin Otolaryngol Head Neck Surg 2005;13(5):301-7.
4. Fowler EP Jr, Zeckel A. Psychosomatic aspects of Ménière's disease. J Am Med Assoc 1952;148(15):1265-8.
5. Andersson G, Hägnebo C, Yardley L. Stress and symptoms of Ménière's disease: A time-series analysis. J Psychosom Res 1997;43(6):595-603.
6. Horner KC. e emotional ear in stress. Neurosci Biobehav Rev 2003;27(5):437-46.
7. Giraudet F, Horner KC, Cazals Y. Similar half-octave TTS protection of the cochlea by xylazine/ketamine or sympathectomy. Hear Res 2002;174(1-2):239-48.
8. Horner KC, Guieu R, Magnan J, et al. Prolactinoma in some Ménière's patients—is stress involved? Neuropsychopharmacology 2002;26(1):135-8.
9. Yildiz SK, Koybasi S, Turkoglu SA, et al. Sympathetic skin responses from postauricular region in Ménière’s disease. Clin Neurophysiol 2007;118(9):1991-8.
10.Yamada M, Mizuta K, Ito Y, et al. Autonomic nervous function in patients with Ménière’s disease evaluated by power spectral analysis of heart rate variability. Auris Nasus Larynx 1999;26(4):419-26.
11.Su P, Liu YC, Lin HC. Risk factors for the recurrence of postsemicircular canal benign paroxysmal positional vertigo a er canalith repositioning. J Neurol 2016;263(1):45-51.
12.Lee NH, Ban JH, Lee KC, Kim SM. Benign paroxysmal positional vertigo secondary to inner ear disease. Otolaryngol Head Neck Surg 2010;143(3):413-7.
13.Kim HA, Lee H. Autonomic dysfunction as a possible cause of residual dizziness a er successful treatment in benign paroxysmal positional vertigo. Clin Neurophysiol 2014;125(3):608-14.
14.Pezzoli M, Garzaro M, Pecorari G, et al. Benign paroxysmal positional vertigo and orthostatic hypotension. Clin Auton Res 2010;20(1):27-31.
15.Schumacher S, Kirschbaum C, Fydrich T, Ströhle A. Is salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders? A review of preliminary ndings and the interactions with cortisol. Psychoneuroendocrinology 2013;38(6):729-43.
16.Rohleder N, Nater UM, Wolf JM, et al. Psychosocial stressinduced activation of salivary alpha‐amylase: An indicator of sympathetic activity? Ann N Y Acad Sci 2004;1032:258-63.
17.Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009;34(4):486-96
18.[No authors listed]. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Ménière's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg 1995;113(3):181-5.
19.Chatterton RT Jr, Vogelsong KM, Lu YC, et al. Salivary α‐amylase as a measure of endogenous adrenergic activity. Clin Physiol 1996;16(4):433-48.
20.Rarey KE, Curtis LM, ten Cate WJ. Tissue speci c levels of glucocorticoid receptor within the rat inner ear. Hear Res 1993;64(2):205-10.
21.Ryan AF, Pak K, Low W, et al. Immunological damage to the inner ear: Current and future therapeutic strategies. Adv Otorhinolaryngol 2002;59:66-74.
22.Ma YL, Gerhardt KJ, Curtis LM, et al. Combined e ects of adrenalectomy and noise exposure on compound action potentials, endocochlear potentials and endolymphatic potassium concentrations. Hear Res 1995;91(1-2):79-86.
23.Zenner HP, Zenner B. Vasopressin and isoproterenol activate adenylate cyclase in the guinea pig inner ear. Arch Otorhinolaryngol 1979;222(4):275-83.
24.Schacht J. Hormonal regulation of adenylate cyclase in the stria vascularis of the mouse. Hear Res 1985;20(1):9-13.
25.Dugué B, Leppänen EA, Teppo AM, et al. E ects of psychological stress on plasma interleukins-1 beta and 6, C-reactive protein, tumour necrosis factor alpha, anti-diuretic hormone and serum cortisol. Scand J Clin Lab Invest 1993;53(6):555-61.
26.Takeda T, Kakigi A, Saito H. Antidiuretic hormone (ADH) and endolymphatic hydrops. Acta Otolaryngol Suppl 1995;519:219-22.
27.Sawada S, Takeda T, Saito H. Antidiuretic hormone and psychosomatic aspects in Ménière's disease. Acta Otolaryngol Suppl 1996;528:109-12.
28.Pappas DG Jr. Autonomic related vertigo. Laryngoscope 2003;13(10):1658-71.
29.Low PA, Opfer-Gehrking TL, McPhee BR, et al. Prospective evaluation of clinical characteristics of orthostatic hypotension. Mayo Clin Proc 1995;70(7):617-22.
30.Grubb BP, Kosinski DJ, Boehm K, Kip K. e postural orthostatic tachycardia syndrome: A neurocardiogenic variant identi ed during head-up tilt table testing. Pacing Clin Electrophysiol 1997;20(9 Pt 1):2205-12.
282 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
KORKMAZ, BICER, SERIN, SEYHAN, SANAL
Mucocele development after endoscopic sinus surgery for nasal polyposis: A long-term analysis
Hakim Benkhatar, MD; Idir Khettab, MD; Philippe Sultanik, MD, PhD; Ollivier Laccourreye, MD; Pierre Bon ls, MD, PhD
Abstract
e aim of the present study was to determine the prevalence of long-term mucocele development a er functional endoscopic sinus surgery (FESS) for nasal polyposis, to search for a statistical relationship with preoperative variables and to analyze the management of this complication. A retrospective analysis of 153 patients who underwent FESS for nasal polyposis, with a minimum of 7 years of follow-up, was performed. Mucocele diagnosis was based on regular clinical and radiologic evaluation. Univariate and multivariate statistical analysis was performed. e postoperative mucocele rate was 13.1% (20 patients). e mean delay between surgery and mucocele diagnosis was 6.25 years. A high preoperative Lund-Mackay score (>19) was a risk factor for postoperative mucocele (p = 0.04). Asthma and aspirin intolerance did not increase the risk of this complication. Endoscopic marsupialization of mucoceles was successful in 19 patients, with only one recurrent frontal mucocele. One patient required external approaches for two frontal mucoceles. In conclusion, mucocele risk a er FESS for nasal polyposis is signi cant, especially in case of a high preoperative Lund-Mackay score (>19). Long-term clinical follow-up is recommended, imaging being prescribed based on symptoms or abnormal ndings on clinical examination. Endoscopic marsupialization is very effective, but frontal mucoceles are more likely to recur.
Introduction
Functional endoscopic sinus surgery (FESS) is now widely accepted in the setting of medical treatment failure in patients with nasal polyposis.1,2 Nevertheless, the extent of surgery is still debated, ranging from polypectomy to radical ethmoidectomy.3 In our experience, FESS has shown to be e ective to control nasal polyp recurrence with good functional results.4 Although the incidence of major and minor immediate complications of FESS have been well documented,5,6 only a few studies have documented long-term complications.
One of the most threatening scenarios a er FESS is mucocele development because of the associated visual and neurologic risks.7-9 is long-term complication can occur many years a er surgery.10,11 Sinus or ethmoid cell obstruction a er mucosal trauma and mucosal in ammation have an important pathophysiologic role in mucocele formation;12-14 therefore, patients who undergo FESS for nasal polyposis might be at higher risk of postoperative mucocele.
Based on a retrospective analysis of 153 patients who underwent FESS for nasal polyposis with a minimum follow-up of 7 years, we aimed to: (1) determine the prevalence of long-term mucocele development, (2) search for potential statistical relationships with various variables, and (3) analyze the management and ultimate outcome of this long-term complication.
Patients and methods
From the ENT–Head and Neck Surgery Department (Dr. Benkhatar, Prof. Laccourreye, and Prof. Bon ls) and the Department of Radiology (Dr. Khettab), European Hospital Georges Pompidou, AP-HP, Paris; Faculty of Medicine Paris Descartes, University Paris V, Paris (all authors); the Department of Hepatology, Cochin Hospital, AP-HP, Paris (Dr. Sultanik); and Inserm UMS20, Pasteur Institute, Paris (Dr. Sultanik).
Corresponding author: Benkhatar Hakim, ORL, Hôpital Européen Georges Pompidou, 20 rue Leblanc, 75015 Paris, France. Email: hakim.benkhatar@egp.aphp.fr
Diagnostic criteria and patient selection. Our retrospective study included 153 patients with nasal polyposis who had failed medical treatment (twice-daily washing of the nasal cavities with sterile saline solution, twice daily intranasal beclomethasone spray in each nasal cavity, and oral steroid administration [i.e., prednisone, 1 mg/kg body weight per day for a 6-day period]).
ese patients were consecutively managed with FESS by the same physician (P.B.) from 1991 to 2008. FESS
284 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 ORIGINAL ARTICLE





was suggested to the patient if more than three systemic courses of prednisone per year had proved to be necessary to achieve a signi cant quality of life.
e initial diagnosis of nasal polyposis was based on two criteria: (1) the visualization of bilateral polyps in the nasal cavities on endoscopic examination, and (2) the existence of bilateral ethmoidal opaque areas on multislice computed tomography (CT) with a 1.25-mm slice thickness (120 Kv, 20 mA), located either in the anterior or the posterior ethmoid sinuses. e CT scan was performed at clinical baseline to obtain a reliable Lund-Mackay score.
Table 1 presents the patients’ preoperative variables.
e severity of nasal polyposis was evaluated with a 4-point clinical scale (0 = no polyp; 1 = small polyps not reaching the lower edge of the middle turbinate; 2 = medium-sized polyps extending between the upper and lower edges of the inferior turbinate; and 3 = large polyps extending below the lower edge of the inferior turbinate).
Severity also was evaluated by calculating the Lund-Mackay score and searching for associated morbidities such as asthma, bronchial hyperreactivity a er a challenge test with methacholine (performed for nonasthmatic patients according to the guidelines of the European Respiratory Society),15 and aspirin or another nonsteroidal anti-in ammatory drug intolerance.
Patients with primary ciliary dyskinesia, ChurgStrauss syndrome, or cystic brosis were excluded from this study.
Initial surgical procedure and follow-up a er initial FESS. e FESS procedure4 was performed bilaterally
with the assistance of computerized sinus navigation. It began with the inferior resection of the middle turbinate and fenestration of the maxillary antrum. en, the posterior ethmoid cells were totally removed and the fovea ethmoidalis identi ed. e anterior ethmoid cells were also widely opened, and the frontal ostium was identi ed a er Draf I sinusotomy. Mucosa of the frontal recess area was le untouched. A sphenoidotomy ended the procedure.
Minimum length of follow-up was 7 years, with a mean of approximately 11 years (range: 7 to 21 years).
Postoperative clinical evaluation was conducted two to four times a year a er FESS. At each visit, mean symptom severity, polyp size, and steroid consumption were evaluated. Regular sinus imaging (CT scan initially and cone-beam CT subsequently) was performed (with coronal, axial, and sagittal views) every 3 to 4 years a er surgery and/or if clinical evaluation suggested potential development of a mucocele.
When a mucocele was suspected on sinus imaging (showing a well-delineated, cyst-like, homogeneous opacity with rounded bony outline and smooth bone erosion), magnetic resonance imaging (MRI) was implemented to con rm the diagnosis or to study the surrounding orbit and brain. MRI showed di erent patterns of T1-weighted signals (from low T1 in young mucoceles to high T1 in older mucoceles) and a high T2 signal, associated with a thin mucosal lining.
Frontal sinus mucoceles were classi ed according to Sama’s classi cation: medial, intermediate, and lateral.16 Endoscopic marsupialization of the mucocele was performed in patients with symptoms or, in asymptomatic patients, if erosion of the orbital walls or skull base was found on imaging.
Statistical analysis. Statistical analysis searched for a potentially signi cant relation between this long-term complication and the variables depicted in table 1. e prognostic value of each variable was analyzed using a Cox model, adjusted with time between surgery and complication (in cases of multiple surgeries for this complication in the same patient, only the rst event was included). Univariate and multivariate analyses were performed.
Survival curves were determined using the median of continuous variables as a distinction threshold between groups. A log-rank test was performed to analyze the di erence between groups. Statistical signi cance was set at the bilateral p value = 0.05. Statistical analysis used the Statistical Package for the Social Sciences so ware ver. 20.0 for Macintosh.
Results
Prevalence of long-term mucocele development. Mucocele development was observed in 20 patients (13.1%). Before surgery, 15 of the 20 (75%) patients
286 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
BENKHATAR, KHETTAB, SULTANIK, LACCOURREYE, BONFILS
Variable Value Sex ratio (M:F) 1.6 Mean age 48 ± 1 yr Nasal polyposis grade 1 25.5% 2 33.3% 3 41.2% Prior surgeries Polypectomy 9.2% Partial ethmoidectomy 15.0% Lund-Mackay score Mean 19 Median 19 Asthma 50.9% Bronchial hyperactivity 66.6% NSAID/aspirin intoloerance 22.2%
Table 1. Preoperative variables
MAXIMUM VERSATILITY FOR PRECISE CO2 ENERGY DELIVERY.

The BEACON Advanced Energy Laser combines our industry-leading Enhanced Safety Fibers with free beam energy delivery, giving you the versatility to choose.


System Features:
Versatile: Industry-leading enhanced safety fibers + Line of Sight (LOS) capabilities.




Easy-to-Use: OR workflow inspired user interface with smartphone like navigation.

Built for Performance: Innovative design ensures mobility with integrity. Multiple laser modes enable desired tissue interaction.

OmniGuide’s polymer-based Enhanced Safety Fibers feature multiple layers of highly reflective mirrors, which efficiently transmit laser energy the length of the fiber. Visit www.omni-guide.com/beacon
call 888.666.4484 for
or
additional information. © 2018 OmniGuide, Inc. All rights reserved. 1-0168-074-00-00 rev.0
were asymptomatic, with mucoceles being discovered on sinus imaging during follow-up (table 2). Five patients (25%) were symptomatic, with mucoceles causing facial pain in 8 cases, proptosis in 3 cases, and diplopia in 2 cases (each of the 5 patients had multiple mucoceles in di erent locations).
e mean delay between the initial procedure and this complication was 75 months (6.25 years), ranging from 11 months to 16.5 years. Among the 39 diagnosed
mucoceles, frontal and anterior ethmoid mucoceles were predominant (61.5%). Strictly frontal mucoceles were medial in 3 cases, intermediate in 1 case, and lateral in 1 case. Other mucoceles developed in the posterior ethmoid (28.2%), the sphenoid (7.7%) and, rarely, in the maxillary sinus (2.6%).
Potential statistical relationship with various variables. In univariate and multivariate analysis, the preoperative Lund-Mackay score was the only variable that signi cantly
Key: EE = endonasal endoscopic; K = Kuhn cell; T = trephination through eyebrow incision; SS = stenting with Silastic sheet (1 week); FSO = frontal sinus obliteration with abdominal fat; FRS = frontal recess stenosis.
288 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
BENKHATAR, KHETTAB, SULTANIK, LACCOURREYE, BONFILS
PatientDelay (mo)Diagnosis Location Approach 1 81Follow-up CT Posterior ethmoid EE 2 25Follow-up CT Anterior ethmoid EE 3 58Follow-up CT Posterior ethmoid ×2 EE 4 26Follow-up CT Anterior ethmoid ×2 EE 5 11Follow-up CT Frontoethmoid EE 6 44Follow-up CT Anterior ethmoid + maxillary EE 98Follow-up CT Posterior ethmoid EE 7 156Facial pain Frontoethmoid EE 8 112Follow-up CT Posterior ethmoid ×2 EE 9 13Diplopia Fronto-orbito-ethmoid EE 10 30Facial pain Sphenoid + posterior ethmoid EE 11 80Follow-up CT Anterior ethmoid EE 12 76Follow-up CT Frontoethmoid EE 13 26Proptosis Frontoethmoid EE 14 101Follow-up CT Posterior ethmoid + sphenoid EE 15 22Follow-up CT Medial frontal + anterior ethmoid EE 16 73Facial pain Sphenoid EE 17 15Facial pain Anterior ethmoid ×2 EE 116Follow-up CT Frontoethmoid EE 18 181Follow-up CT Posterior ethmoid EE 198Facial pain Anterior ethmoid EE 19 50Follow-up CT Posterior ethmoid EE 65Proptosis Medial frontal (right side) + K4 EE + T + SS 93Proptosis Lateral frontal (right side) + anterior ethmoid EE + FSO 20 19Diplopia Anterior ethmoido-orbital (right side) EE 62Facial pain Anterior ethmoid (left side) EE 105Facial pain Intermediate and medial frontal (right side) + contralateral FRS EE (Draf III) 151Facial pain Recurrent intermediate frontal (right side) + medial frontal (left side) + posterior ethmoid EE (Draf III) + SS
Table 2. Patients presenting with mucocele
IRRIGATION. SUCTION. SYNCHRONIZATION.
Now there’s a better way to provide saline washes. The HydroCleanse™ Sinus Wash Delivery System from Medtronic combines pressurized sinus irrigation with builtin suction capability for a more efficient procedure. Disposable and easy to use, it allows you to irrigate the sinuses while limiting pooling and effluent discharge.
§ Disposable and easy to use
§ 360° fan spray





§ Pressurized saline irrigation







§ Built-in suction capability
§ Choice of angled catheter tips




HydroCleanse™ Sinus Wash Delivery System
IMPROVE THE SALINE WASH EXPERIENCE. CHOOSE HYDROCLEANSE.


Rx only. Refer to product instruction manual/package insert for instructions, warnings, precautions and contraindications.
For further information, please call Medtronic ENT at 800.874.5797 or consult Medtronic’s website at www.medtronicent.com
© 2018 Medtronic. All rights reserved. Medtronic, Medtronic logo and Further, Together are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. UC201902765 EN 07.2018
indicated an increased risk for mucocele development (p = 0.04). Asthma and aspirin intolerance were not associated with an increased risk of mucocele development.
Management of mucoceles and outcomes. As mentioned above, 28 procedures for 39 mucoceles were performed in 20 patients (table 2). Endoscopic marsupialization of the mucocele into the nasal cavity (made as large as the anatomy allowed and helped by the navigation system) was performed for 19 patients (26 procedures).
One of the 39 operated mucoceles recurred (for a recurrence rate of 2.7%); the mucocele was in the frontal sinus. e patient with this recurrence had rst presented with an intermediate frontal mucocele and contralateral frontal recess stenosis; at that time, a Draf III procedure was undertaken. Almost 4 years later, he presented with a recurrence of that mucocele with an associated medial frontal mucocele on the opposite side. e frontal sinusotomy was closed by scar tissue and was reopened to gain access to both mucoceles. One-week stenting with a Silastic sheet was implemented. To date, this patient has not required another revision surgery.
One patient presenting with frontal mucoceles required surgery via external approaches. First, he presented with a medial frontal mucocele and Kuhn type 4 cell, which required additional sinus trephination through an eyebrow incision. One-week stenting with a Silastic sheet was implemented. Two years later, he presented with a lateral frontal mucocele (with complete closure of the frontal ostium by neo-osteogenesis) on the same side, and therefore a frontal sinus obliteration procedure (with abdominal fat) was performed. To date, this patient has not required another revision surgery.
Facial pain and ophthalmic symptoms disappeared in all symptomatic cases.
Discussion
Prevalence of mucocele development. During the decades since the introduction of FESS, post-FESS mucoceles have been increasingly reported.17,18 Most cases of chronic rhinosinusitis have non-evolving pathologies, with no persisting mucosal in ammation a er FESS (e.g., sinus fungus ball). In nasal polyposis, the mucosa remains in ammatory with subsequent recurrent polyps. e persistent in ammation modi es the postoperative follow-up and may increase the risk of mucocele development.
e strength of the present study is the large number of patients (153 consecutive patients) with a similar disease and long-term follow-up (mean >11 years).
Mucocele development was noted in 20 patients (13.1%). is rate is higher than those reported in previous studies: 0%,19,20 2.5%, 21 and 2.6%22 a er FESS procedures for patients with nasal polyposis (table 3). However, these publications reported a shorter follow-up, with a mean of 3 years.19-22 In our study, the minimum follow-up was 7 years and the mean delay between the initial procedure and the mucocele diagnosis was 75 months (more than 6 years).
Previous publications only reported rates of symptomatic mucoceles.17-22 Since berendoscopy is unlikely to detect early intrasinus mucoceles, and asymptomatic mucoceles can only be detected a er surgery by regular sinus imaging, previous studies might have underestimated the real proportion of mucoceles.
Given the prevalence of asymptomatic mucoceles, regular sinus imaging should be considered a er nasal polyposis surgery in selected patients. However, radiologic screening can be associated with a signi cant cost and a risk of radiation exposure. Our ndings led us to prefer the use of cone-beam CT to signi cantly reduce
290 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
BENKHATAR, KHETTAB, SULTANIK, LACCOURREYE, BONFILS
Author/yr Number of patientsType of surgeryFollow-up (mo)Mucoceles (%) Klossek et al, 199719 50 rET + MTR + SPH +FI36 0 Jankowski et al, 199720 34 rET + MTR + SPH32-36 0 29 fET ± FRD ± MTR ± SPH18-31 3.4 Chobillon and Jankowski, 200421 501 rET + MTR + SPH72 (max) 2.5 Devars du Mayne et al, 201122 77 fET ± MTR ± SPH36 (min) 2.6 50 PO 36 (min) 0 Guerrero et al, 200734 110 fET ± FRD ± SPH24 (min) 0 Present study, 2018 153 fET ± FRD ± partial MTR ± SPH 137 13.1
Table 3. Mucocele formation after nasal polyp surgeries
Key: rET = radical ethmoidectomy; MTR = middle turbinate resection; SPH = sphenoidectomy; FI = postoperative frontal irrigation; fET =functional ethmoidectomy; FRD = frontal recess dissection; po = polypectomy.


WWW.EARCAREMD.COM Available at CVS, Amazon and Target. EOSERA EVERYTHING EAR CARE Ear Itch MD™ coming soon in 2019
the radiation exposure, and to limit its use as a mucocele screening tool only for (1) symptomatic patients presenting with headache, facial pressure, or orbital symptoms and (2) asymptomatic patients with abnormal ndings on clinical examination (frontal swelling, endonasal bulge on endoscopic examination, recurrent polyps).
Additionally, increased watchfulness may be necessary during the follow-up in patients with a high preoperative Lund-Mackay score (>19) or a previous history of mucoceles.
Risk factors for mucocele development. Our study demonstrated that a high preoperative Lund-Mackay score was an independent risk factor for mucocele development. To our knowledge, there is no other study reporting a signi cant risk factor. A higher Lund-Mackay score is related to lower sinonasal mucociliary clearance, leading to a higher grade of sinus opaci cation.
In cases of nasal polyposis, mucociliary dysfunction is mainly caused by in ammation, which induces excessive mucin production (leading to highly viscoelastic mucus and mucostasis), rather than ciliary cell impairment.23-25 is fact agrees with prevailing theories of mucocele development that include obstruction and in ammation.12-14 In addition, cytokines such as IL-1β and TNF-α can induce both mucin production and bone resorption.14,25,26 erefore, this suggests that in cases of surgically induced mucosal entrapment, the higher the patient’s in ammatory responsiveness, the higher the level of mucin production and bone resorption. is mechanism could explain the higher risk of mucocele development in severe nasal polyposis.
As previously suggested, the preponderance of frontoethmoid mucocele may be related to the fact that mucosal contacts are more likely to happen in narrow areas.14According to these ndings, preoperative oral steroid therapy, which reduces polyp size before surgery and increases visibility during FESS, 27 could theoretically help to achieve a better sinus opening and reduce the risk of short-term ostial stenosis (particularly in the frontal recess area) and long-term mucocele development. Consequently, we prescribe a 5-day course of prednisone (1 mg/kg) before surgery.
In our study, asthma and aspirin intolerance were not associated with this complication. Some studies showed that the number of eosinophils in patients with the Samter triad was four times higher than in patients with nasal polyposis and asthma alone, and 15 times higher than in patients with nasal polyposis alone.28,29 Consequently, we suggest that eosinophils may not have an important role in mucocele formation, whereas lymphocytes and monocytes appeared to be involved in that process.13 Moreover, recent studies suggest that eosinophils in ltrating the periosteum are more likely
to promote osteoblastic activity and neo-ostegenesis in patients with nasal polyposis.30
Management of mucoceles. In our study, 39 mucoceles were managed in 20 patients. Endoscopic marsupialization was achieved for 37 mucoceles (19 patients) with only 1 case of recurrent medial frontal mucocele (recurrence rate of 2.7%). is result agrees with other series that described low recurrence rates a er endoscopic mucocele marsupialization, ranging from 0% with a mean follow-up of 17.6 months31 to 0.9% with a mean follow-up of 4.6 years.17 A large series also showed a recurrence rate of 1.6% a er microendoscopic mucocele marsupialization, with a median follow-up of 12 years.18
According to the literature, it is not necessary to attempt to repair bone defects caused by mucoceles.11,16,18 In fact, a recent study demonstrated a possible reversal of the bone resorption process over years, i.e., complete self-regeneration of the eroded bone.32 Interestingly, it is possible that pro-osteoblastic medical treatments such as vitamin D supplementation might ease bone regeneration, as vitamin D de ciency is more frequently encountered in patients with nasal polyposis than in the general population.33
In our study, one patient required external surgical approaches for two frontal mucoceles. As suggested by a recent series, external approaches are still a part of the surgeon’s armamentarium in the treatment of frontal mucoceles, especially in cases of lateral frontal mucoceles or extensive frontal ostium neo-osteogenesis.16
Extended endoscopic frontal sinusotomies (Draf IIa to III) are e ective in cases of medial or intermediate frontal mucocele, limited frontal drainage pathway, and/or associated type III/IV frontoethmoid cells.16 ese di cult cases highlight the need to properly inform patients about the long-term risk of mucocele development a er nasal polyposis surgery.
Conclusion
is article presents a study of mucocele development a er FESS for nasal polyposis in a large cohort of 153 patients with a mean follow-up of approximately 11 years. is complication occurred in a signi cant number of patients (13.1%), and the mean delay between the initial procedure and the mucocele diagnosis was about 6 years, with almost 40% of the mucoceles being asymptomatic and diagnosed on sinus imaging. A high preoperative Lund-Mackay score (>19) was correlated with a higher risk of mucocele development (p = 0.04). Endoscopic marsupialization is the standard of treatment and is associated with a low recurrence rate, but frontal mucocele treatment remains challenging and may require advanced techniques, such as extended endoscopic frontal sinusotomy or an external approach.
292 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
BENKHATAR, KHETTAB, SULTANIK, LACCOURREYE, BONFILS

Everywhere ENT Clinicians Need to Be Outpatient Clinic | ICU | Bedside ER | Radiation Oncology | OR TM Designed to empower ENT clinicians with the visibility & mobility they need Contact Us Today : 800.225.7486 / sales@optim-llc.com Flexible Laryngoscopes www.optim-llc.com/aao2018 Please visit us in Atlanta at the AAO-HNSF Annual Meeting Booth 1312 Learn more about our diagnostic visualization products and meeting presence at Experience the ENTity™ Difference No cables. No additional equipment. No Limits.
ese ndings stress the importance of a prolonged clinical evaluation of any patient undergoing FESS for nasal polyposis. In our opinion, systematic radiologic screening is not to be recommended to detect mucoceles, given the cost of unnecessary exams and the radiation exposure risk (although it is signi cantly reduced by using cone-beam CT). Nevertheless, increased watchfulness may be necessary during the follow-up in patients with a high preoperative Lund-Mackay score (>19) or a history of mucoceles.
Any patient undergoing FESS for nasal polyposis must be informed of the long-term risk of mucocele development that can lead to intraorbital or intracranial complications, and occasionally di cult revision surgeries.
References
1. Lund VJ. Surgical outcomes in chronic rhinosinusitis and nasal polyposis. Rhinology 2006;44(2):97.
2. Georgalas C, Cornet M, Adriaensen G, et al. Evidence-based surgery for chronic rhinosinusitis with and without nasal polyps. Curr Allergy Asthma Rep 2014;14(4):427.
3. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012;50(1):1-12.
4. Bon ls P. Evaluation of the combined medical and surgical treatment in nasal polyposis. I: Functional results. Acta Otolaryngol 2007;127(4):436-46.
5. Dalziel K, Stein K, Round A, et al. Endoscopic sinus surgery for the excision of nasal polyps: A systematic review of safety and e ectiveness. Am J Rhinol 2006;20(5):506-19.
6. Hopkins C, Browne JP, Slack R, et al. Complications of surgery for nasal polyposis and chronic rhinosinusitis: e results of a national audit in England and Wales. Laryngoscope 2006;116(8):1494-9.
7. Kim YS, Kim K, Lee JG, et al. Paranasal sinus mucoceles with ophthalmologic manifestations: A 17-year review of 96 cases. Am J Rhinol Allergy 2011;25(4):272-5.
8. Saylam G, Bayır O, Girgin D, et al. Permanent central diabetes insipidus as a complication of sphenoid sinus mucocele. Am J Otolaryngol 2014;35(5):658-60.
9. Weidmayer S. Frontal mucocele with intracranial extension causing frontal lobe syndrome. Optom Vis Sci 2015;92(6):e138-42.
10. Devars du Mayne M, Moya-Plana A, Malinvaud D, et al. Sinus mucocele: Natural history and long-term recurrence rate. Eur Ann Otorhinolaryngol Head Neck Dis 2012;129(3):125-30.
11. Scangas GA, Gudis DA, Kennedy DW. e natural history and clinical characteristics of paranasal sinus mucoceles: A clinical review. Int Forum Allergy Rhinol 2013;3(9):712-17.
12. Lund VJ. Anatomical considerations in the aetiology of frontoethmoidal mucoceles. Rhinology 1987;25(2):83-8.
13. Lund VJ, Harvey W, Meghji S, Harris M. Prostaglandin synthesis in the pathogenesis of fronto-ethmoidal mucoceles. Acta Otolaryngol 1988;106(1-2):145-51.
14. Lund VJ, Henderson B, Song Y. Involvement of cytokines and vascular adhesion receptors in the pathology of fronto-ethmoidal mucocoeles. Acta Otolaryngol 1993;113(4):540-6.
15. Sterk PJ, Fabbri LM, Quanjer PH, et al. Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. O cial Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:53–83.
16. Sama A, McClelland L, Constable J. Frontal sinus mucocoeles: New algorithm for surgical management. Rhinology 2014;52(3):267-75.
17. Har-El G. Endoscopic management of 108 sinus mucoceles. Laryngoscope 2001;111(12):2131-4.
18. Bockmühl U, Kratzsch B, Benda K, Draf W. Surgery for paranasal sinus mucocoeles: E cacy of endonasal micro-endoscopic management and long-term results of 185 patients. Rhinology 2006;44(1):62-7.
19. Klossek JM, Peloquin L, Friedman WH, et al. Di use nasal polyposis: Postoperative long-term results a er endoscopic sinus surgery and frontal irrigation. Otolaryngol Head Neck Surg 1997;117(4):355-61.
20. Jankowski R, Pigret D, Decroocq F. Comparison of functional results a er ethmoidectomy and nasalization for di use and severe nasal polyposis. Acta Otolaryngol 1997;117(4):601-8.
21. Chobillon MA, Jankowski R. Relationship between mucoceles, nasal polyposis and nasalisation. Rhinology 2004;42(4):219-24.
22. Devars du Mayne M, Prulière-Escabasse V, Zerah-Lancner F, et al. Polypectomy compared with ethmoidectomy in the treatment of nasal polyposis. Arch Otolaryngol Head Neck Surg 2011;137(2):111-17.
23. Slater A, Smallman LA, Logan AC, Drake-Lee AB. Mucociliary function in patients with nasal polyps. Clin Otolaryngol Allied Sci 1996;21(4):343-7.
24. Rogers DF. Airway mucus hypersecretion in asthma: An undervalued pathology? Curr Opin Pharmacol 2004;4(3):241-50.
25. Ali MS, Pearson JP. Upper airway mucin gene expression: A review. Laryngoscope 2007;117(5):932-8.
26. Song KS, Lee WJ, Chung KC, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem 2003;278(26):23243-50.
27. Ecevit MC, Erdag TK, Dogan E, Sutay S. E ect of steroids for nasal polyposis surgery: A placebo-controlled, randomized, double-blind study. Laryngoscope 2015;125(9):2041-5.
28. Kaldenbach T, Schäfer D, Gosepath J, et al. Signi cance of eosinophilic granulocytes in relation to allergy and aspirin intolerance in patients with sinusitis polyposa [in German]. Laryngorhinootologie 1999;78(8):429-34.
29. Sampson AP, Cowburn AS, Sladek K, et al. Profound overexpression of leukotriene C4 synthase in bronchial biopsies from aspirin-intolerant asthmatic patients. Int Arch Allergy Immunol 1997;113(1-3):355-7.
30. Wang M, Ye T, Liang N, et al. Di ering roles for TGF-β/Smad signaling in osteitis in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol Allergy 2015;29(5):152-9.
31. Kennedy DW, Josephson JS, Zinreich SJ, et al. Endoscopic sinus surgery for mucoceles: A viable alternative. Laryngoscope 1989;99(9):885-95.
32. Terranova P, Karligkiotis A, Digilio E, et al. Bone regeneration a er sinonasal mucocele marsupialization: What really happens over time? Laryngoscope 2015;125(7):1568-72.
33. Stokes PJ, Rimmer J. e relationship between serum vitamin D and chronic rhinosinusitis: A systematic review. Am J Rhinol Allergy 2016;30(1):23-8.
34. Guerrero J, Molina B, Echeverría L, et al. Endoscopic sinonasal surgery: Study of 110 patients with nasal polyposis and chronic rhinosinusitis [in Spanish]. Acta Otorrinolaringol Esp 2007;58(6):252-6.
294 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
BENKHATAR, KHETTAB, SULTANIK, LACCOURREYE, BONFILS
TAKE YOUR NEXT STEP.

Every move you make is under the microscope, so why not choose the one specifically designed to meet the needs of an ENT surgeon. The Haag-Streit Hi-R 700 ENT microscope offers mobility, precise positioning, superior depth of field and a continuous illumination diaphragm.
Get the ENT microscope you’ve waited your whole career to see. Contact us to arrange an evaluation.



AN ENT MICROSCOPE WORTHY OF THE SURGEON’S ART. PRECISION. BALANCE. PERFORMANCE. 800.787.5426 HSmicroscopes.com/ENT © 2018 Haag-Streit USA. All Rights Reserved.
Seeitat AAO-HNSF Booth#1732
Positivity rates of in vitro inhalant/ respiratory and food allergy tests in the northern midwestern United States
Michael S. Benninger, MD; omas Daly, MD; Kevin Gra miller, MD
Abstract
Rates of allergy-test positivity vary by country and by regions within countries. Several studies have looked at allergy test results to determine the most common allergens. Many of these studies have been based on surveys or on studies of small numbers of tests. Positivity rates for allergy tests are poorly de ned in the northern midwestern region of the United States. We conducted a study to identify the rates of positive allergy tests for both inhalant/respiratory allergens and food allergens in the upper Midwest. We extracted from our laboratory database the results of all test samples sent for one of eight allergen panels that had been analyzed between Sept. 1, 2014, and Sept. 1, 2015. All testing was performed at e Cleveland Clinic with the Phadia ImmunoCAP system. e percentage of positive tests, the distribution of the most frequently positive tests, and the class of in vitro responses were identi ed. A total of 148,628 test results for 63 di erent allergens were identi ed. Of the 125,190 tests for inhalant/respiratory allergens, the most frequently positive were dog dander (24% of tests), cat dander (23%), dust mites (23% for both Dermatophagoides pteronyssinus and Dermatophagoides farinae), and June grass (21%). Of the 23,438 food tests, the most frequently positive test results were for milk (18%), peanut (17%), wheat (16%), and egg white (15%). Most of the results fell into classes 1 through 3, although there was still a notable number of very high responses (class 5 and 6). ese ndings suggest that there is wide variability in the positivity of
From the Head and Neck Institute (Dr. Benninger and Dr. Gra miller) and the Pathology and Laboratory Medicine Institute (Dr. Daly), e Cleveland Clinic, Cleveland, Ohio.
Corresponding author: Michael S. Benninger, MD, Head and Neck Institute, e Cleveland Clinic, 9500 Euclid Ave., A-71, Cleveland, OH 44139. Email: benninm@ccf.org
Previous presentation: e information in this article has been edited for publication and updated as necessary from its original form as a podium presentation at the 26th Congress of the European Rhinologic Society in conjunction with the 35th Congress of the International Society of Infection and Allergy in the Nose and the 17th Congress of the International Rhinologic Society; July 3-7, 2016; Stockholm.
in vitro allergy tests and that the likelihood of a positive result in screening panels can be estimated. Evaluating such rates will help identify the most and least common allergens and will help to cost-e ectively re ne allergy screening panels.
Introduction
Allergic disease is a highly prevalent condition with several clinical manifestations, including allergic rhinitis and sinusitis, allergic dermatologic disorders, allergic conjunctivitis, asthma, and food sensitivities. It is di cult to identify its true prevalence because diagnoses may not be con rmed, studies may be looking at only one of the allergic diseases, and many studies rely on survey responses. When looking at allergic rhinitis, for example, its reported prevalence ranges from 10 to 30% in adults and is as high as 40% in children.1 ere is also evidence that the prevalence of allergies may be increasing, particularly in Western countries.2
e diagnosis of allergic disease is based on a classic history with con rmation by allergy testing or response to treatment. Allergy testing can be performed either through skin testing or in vitro (blood) testing for speci c IgE directed against potential allergens.3 Recent clinical guidelines for the management of allergic rhinitis developed by the American Academy of Otolaryngology–Head and Neck Surgery state, “Clinicians should perform and interpret, or refer to a clinician who can perform and interpret, speci c IgE (skin or blood) allergy testing for patients with a clinical diagnosis of AR who do not respond to empiric treatment, or when the diagnosis is uncertain, or when knowledge of the speci c causative allergen is needed to target therapy.”3
Allergy testing, be it skin or blood testing, can be expensive and is associated with some minor morbidity. Test positivity rates may vary depending on the level of suspicion of the ordering provider, the prevalence of a speci c allergy in a given location, and the type of clinical practice. Individual allergens can be pursued on the basis of speci c symptoms, or broad panels can be performed to screen for any allergen positivity.
296 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 ORIGINAL ARTICLE
In an attempt to re ne a cost-e ective allergy test panel, Bousquet et al looked at large numbers of skin allergy tests to identify which were highly positive and to determine the size of the panel that might be necessary to identify most of the positive allergens.4 ey tested approximately 3,000 patients and found that roughly two-thirds were sensitized to at least one allergen, and they identi ed eight allergens (grass pollen, Dermatophagoides pteronyssinus, birch pollen, cat dander, Artemisia, olive pollen, Blatella, and Alternaria) that allowed them to detect more than 95% of sensitized patients.
eir study overlapped several European countries, which suggested that there were some di erences among them.4 Despite the di erences, only 13 allergens were needed to identify all sensitized patients. Although there have been studies that evaluated the prevalence of food allergies, 5 not many have looked at
positivity rates of allergy testing for foods. Moreover, there has been a lack of good studies looking at large numbers of in vitro allergy tests to identify the frequency of positive tests or di erences among speci c allergens. We conducted this study to identify positive rates of in vitro allergy testing (both inhalant/respiratory and food) in a reference laboratory in the northern Midwest region of the United States.
Patients and methods
We searched our automated database to identify the results of specimen testing for any of eight large allergen panels o ered at e Cleveland Clinic. ese included a mixture of results from patients seen at Cleveland Clinic sites throughout northeast Ohio, as well as results sent to us by reference laboratory clients who were primarily located in the upper Midwest (northern Ohio, northern Illinois, and southern Wisconsin).
Volume 97, Number 9 www.entjournal.com 297 POSITIVITYRATESOFINVITROINHALANT/RESPIRATORYANDFOODALLERGYTESTSINTHENORTHERN MIDWESTERN UNITED STATES
Allergen Tests, nPos, n (%)Allergen Tests, nPos, n (%) Alternaria tenuis 5,508884 (16)Johnson grass1,188155 (13) Australian pine 1474 (3)June grass 2,580543 (21) Bahia grass 14721 (14)Lamb’s quarters2,570240 (9) Bermuda grass 4,124582 (14)Marsh elder 2,916338 (12) Birch tree 1,632195 (12)Mountain juniper3,072293 (10) Box elder 4,369747 (17)Mouse epithelium2,936172 (6) Cat dander 5,5161,285 (23) Mucor racemosus 3,081124 (4) Cladosporium herbarum 5,511387 (7)Oak tree 5,493710 (13) Cocklebur 14913 (9)Orchard grass 14725 (17) Cockroach 4,123461 (11) Penicillium notatum 4,372244 (6) Common ragweed 5,488944 (17)Pigweed 3,328307 (9) Cottonwood tree 3,077418 (14)Rabbit epithelium1475 (3) Dermatophagoides farinae 5,5181,286 (23)Redtop grass 14725 (17) Dermatophagoides pteronyssinus 4,126932 (23)Russian thistle2,918344 (12) Dog dander 5,5161,325 (24)Sheep sorrel 41126 (6) Elm tree 4,103539 (13)Sweet vernal grass14724 (16) English plantain 1,02197 (10)Sycamore tree3,343368 (11) Epicoccum purpurascens 1478 (5)Timothy grass4,738828 (17) Feather mix 1472 (1)Walnut tree 4,117581 (14) Giant ragweed 14816 (11)White ash tree3,076510 (17) Goldenrod 1497 (5)White mulberry tree2,921112 (4) Helminthosporium halodes 1467 (5)White pine 1472 (1) Hickory/pecan tree 4,116449 (11)Willow tree 1479 (6) Horse dander 14710 (7) House dust 1,026299 (29) (Hollister-Stier)
Table 1. Positivity rates for 48 inhalant/respiratory allergens
New Orion Rotary Chair for Micromedical VisualEyes™

Rotational testing is gold standard for bilateral weaknesses identified in caloric
Product demonstrations daily AAO-HNSF booth #2833 h f t


T R to p tw V



bala nce lit d
The future of balance is here! Rotary Chair functionality and easyto-use USB goggles with the most precise eye tracking available are two of the great features of the VisualEyes™software.

The comprehensive rotary chair test battery with customizable protocols includes Sinusoidal Harmonic Acceleration (SHA), Step Test, VOR Suppression and Visual VOR.
Micromedical VisualEyes™ 515/525 is an investment that will grow with your clinic, using a platform that can meet your changing needs into the future.
















Find out more at micromedical.com interacoustics.com


Micromedical by Interacoustics




Touch activated sof tware








Mic o I ntera
Eclipse Vestibular Solution

ib l k d

•Vestibular Evoked Myogenic Potentials (cVEMP and oVEMP)
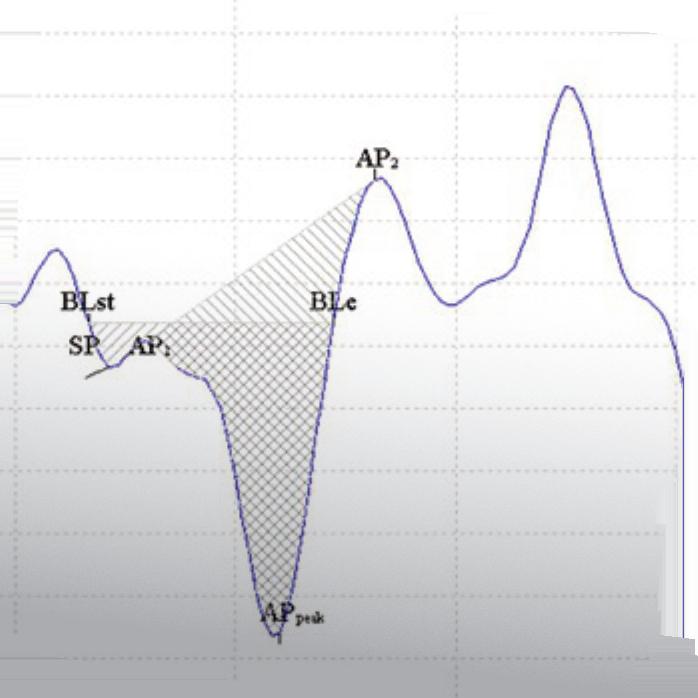

•Electrocochleography (ECochG)



•Neuro Rate Study
Ideal for:






•Neurological balance centers

•Rehab balance centers


•ENT practices

See us at AAO-HNSF booth #2833
The VEMP monitor assists the patient in maintain maintaining adequate muscle contraction action during testing t
Interacoustics USA 10393 West 70th Street Eden Prairie, MN 55344 T +1-800 947 6334 info@interacoustics-us.com
ABR/OAE Balance Assessm Assessment Fitting Systems Middle
Analyzers Audiometers
Interacoustics is a world leading diagnostic solutions provider in the fields of hearing and balance assessment. Since 1967 we have designed and manufactured our innovative diagnostic solutions for the world of audiology with a constant focus on providing our customers with quality, dependable products.
Ear
to muscle tonus during recording
VEMP recording with responses scaled according
Th VEMP monito assist th patien i
ese samples had been analyzed between Sept. 1, 2014, and Sept. 1, 2015.
All speci c IgE testing was performed at e Cleveland Clinic with the Phadia ImmunoCAP system ( ermo Fisher Scienti c; Uppsala, Sweden) in accordance with manufacturer-speci ed protocols. e prevalence of each allergen was calculated as the number of positive results of any class (using a lower cuto of 0.35 kU/L) divided by the total number of times an allergen was assayed across all panels.
Institutional Review Board approval for the study protocol was obtained, and no patient identi ers were included in the study.
Results
A total of 148,628 individual results for 63 di erent allergens were generated during the study period. A large majority were from respiratory/inhalant panels.
Respiratory/inhalant allergies. e frequency of positive reactions to the inhalant allergens ranged as high as 29%; 30 antigens had positivity rates of at least 10% (table 1). e most commonly found inhalant antigens were dog dander (24%), cat dander (23%), dust mites (23%), and June grass (21%) (table 2).
Food allergies. Two food panels yielded 23,438 individual tests for 14 allergens. e positivity rate for these allergens ranged as high as 18%, with 6 allergens that exceeded 10%. e most common positive allergen tests were for milk (18%), peanut (17%), wheat (16%), and egg white (15%) (table 3).
Allergy class. e distribution of class results was similar across most antigens, with most falling into the class 1 to class 3 range ( gure 1). However, a notable number of very high responses (class 5 and 6) were seen for most allergens. Dog dander, cat dander, dust mites, and peanut had the greatest class 4 and 5 responses ( gure 2).
Discussion
Identifying the prevalence of allergies is di cult because (1) allergic disorders are fairly common, (2) many patients self-treat and are therefore not identied in the healthcare system or literature, and (3) patients are o en diagnosed with an allergy without conrmation by objective testing. In addition, particularly with allergic rhinitis, there is a notable overlap with allergic rhinitis and nonallergic rhinitis, and there is a growing number of reports indicating that there are patients who may have a local allergic response in the nose (local allergic rhinitis) who do not have positive results on either skin or in vitro testing.6 Many of the
prevalence studies have relied on surveys, again without objective con rmation.
Allergy testing is routinely performed to con rm a diagnosis, to detect speci c allergies, to identify areas for prevention and avoidance, to direct pharmacotherapy, and to identify antigens for immunotherapy. Although there is debate about the e ectiveness of skin testing vis-à-vis blood testing for identifying allergies, both are good screening methods, and they have been used interchangeably in guidelines.3
A few well-done prevalence studies have been performed. e National Health and Nutrition Examination Survey (NHANES) prospectively evaluated patients in the United States by skin testing to identify the prevalence of allergic disease and speci c allergens. NHANES II7 evaluated patients aged 6 to 74 years from 1976 through 1980, and NHANES III8 looked at patients aged 6 to 59 from 1988 through 1994.
NHANES III investigators looked at 10 inhalant allergies and the rate of skin-test positivity. ey found that
300 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 BENNINGER,
GRAFFMILLER
DALY,
Table
Allergen Tests, nPositive, n (%) Dog dander 5,516 1,325 (24) Cat dander 5,516 1,285 (23) Dust mites (Dermatophagoides farinae) 5,518 1,286 (23) June grass 2,580 543 (21) Common ragweed 5,488 944 (17) Timothy grass 4,738 828 (17) Alternaria tenuis 5,508 884 (16) Oak tree 5,493 710 (13) Cockroach 4,123 461 (11)
2. Most common positive inhalant/respiratory allergens
Food Tests,
n (%) Milk 2,217 396 (18) Peanut 2,220 388 (17) Wheat 2,213 348 (16) Egg white 2,218 330 (15) Soybean 2,219 242 (11) Corn 2,215 242 (11) Shrimp 2,218 210 (9) Clam 1,273 56 (4) Cod sh 1,270 43 (3)
Table 3. Most common food allergen positivity rates
nPositive,


ACCLARE NT AER A® EustachianTubeBalloon Dilation System GO TO THE SOURCE. The only balloon dilation system*speci cally designed to address persistent Eustachian tube dysfunction at its source. OpenMyEars.com | Acclarent.com *In the U.S. only. ACCLARENT AERA® is intended for use by physicians who are trained on Acclarent technology. Eustachian tube balloon dilation has associated risks, including tissue and mucosal trauma, infection, or possible carotid artery injury. Prior to use, it is important to read the Instructions for Use and to understand the contraindications, warnings, and precautions associated with these devices. The safety of the device as used under local anesthesia has not been evaluated. Caution: Federal (US) law restricts the sale, distribution or use of these devices to, by or on the order of a physician. Third party trademarks used herein are trademarks of their respective owners. This site is intended for visitors from the United States and published by Acclarent, Inc., which is solely responsible for its contents. ©2017 Acclarent, Inc. All rights reserved. 061003-170803 Visit us at AAO-HNSF Booth #2223
dust mites were the most frequently positive allergen (27.5% of tests), followed by perennial rye grass (26.9%), short ragweed (26.2%), cockroach (26.1%), Bermuda grass (18.1%), and cat dander (17%). Study participants were positive for at least one indoor allergen 43% of the time and to one outdoor allergen 40% of the time. Some 15% of patients were test-positive for only one allergen, with 4.3% being cockroach and 4.2% being dust mites.8
ese gures represented a dramatic increase in positivity over the rates found during NHANES II, which had been conducted only a few years earlier. In NHANES II, only 20% of participants were positive for one allergen—7.6% for an indoor allergen, and 17.7% for an outdoor allergen. Positivity rates were 6.2% for house dust mites and 2.3% for both cat and dog dander, while rates were 10.2% for rye grass, 10.1% for ragweed, and 4.4% for Bermuda grass.7
It is di cult at times to correlate the true prevalence of allergic disease with positive test results. In most cases, patients are sent for testing with only a suspicion of allergic disease, and a positive test result does not necessarily mean that positivity rates are consistent with the presence of true allergic disease. However, surveys of positive allergen rates do serve to help identify the most common allergens, and they may correlate with the severity of associated diseases such as allergic rhinitis and asthma.
Quest Diagnostics Health Trends investigators analyzed test results from more than 2 million patient encounters over a 4-year period, and they found that the overall allergen-sensitization rate increased by 5.8% during the course of the 4-year study period.9 e greatest increases were seen in positive test results for ragweed (15%) and molds (12%).9
eir study also found wide variability in testing results throughout the United States.9 For example, ragweed positivity was only 11% in Miami and San Francisco but 29% in Phoenix. Mold positivity was 7% in Seattle and 20% in both Riverside, Calif., and San
Bernardino, Calif. e ranking of the ve cities with the highest rates by categories of allergen showed that Baltimore was worst for foods, Phoenix for ragweed, Dallas for molds, Miami for dust mites, and Denver for animals.
In addition, there was very little overlap among the top ve cities in relationship to the ve major allergies, with Boston appearing three times and Las Vegas and Dallas twice among the 25 worst cities for allergies.9 A broad distribution of positive rates was also seen with foods. ese data illustrate the importance of identifying possible allergens that are relevant to the local environment and determining which screening tests and panels are sensitive to the allergies that are common within that geographic area.
Our study represents one of the largest evaluations of screening allergy tests ever reported within a speci c region, with 125,190 potential inhalant/respiratory allergens and 23,438 individual food tests. Although there are di erences between our study and others regarding both the frequency of positive tests and the most common positive tests, there are some similarities, as well. Dog dander (24%), cat dander (23%), dust mites (23% for both D farinae and D pteronyssinus), and June grass (21%) were the most common inhalant/respiratory allergens in our study, while milk (18%), peanut (17%), wheat (16%), and egg white (15%) were the most commonly positive food allergens. ese results are for the most part similar to those reported in other studies, including in the NHANES III study and the Quest Diagnostics Health Trends report, although there are some di erences with regard to speci c allergens.7,8,10 e in vitro class responses we found are also similar to those in the Quest study.9
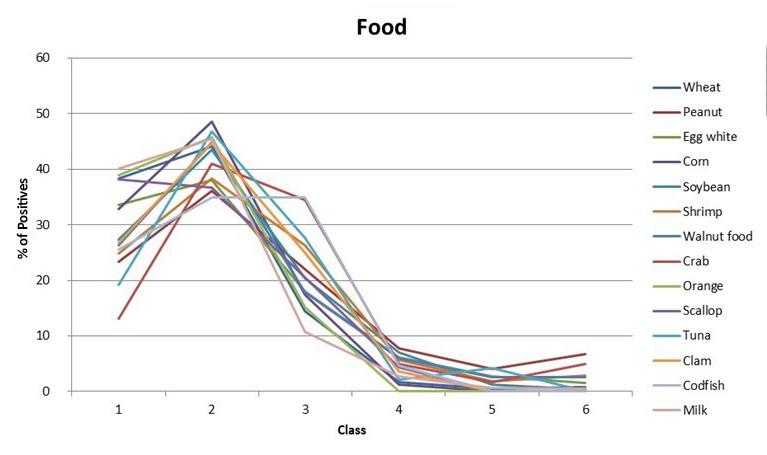
On the other hand, our positivity rates were notably lower than those identi ed in the GA 2LEN III study.4 Of note, cockroach positivity was relatively low (11%) in our study; indeed, it was less than half of that seen in other studies.8 is might be a bit surprising given
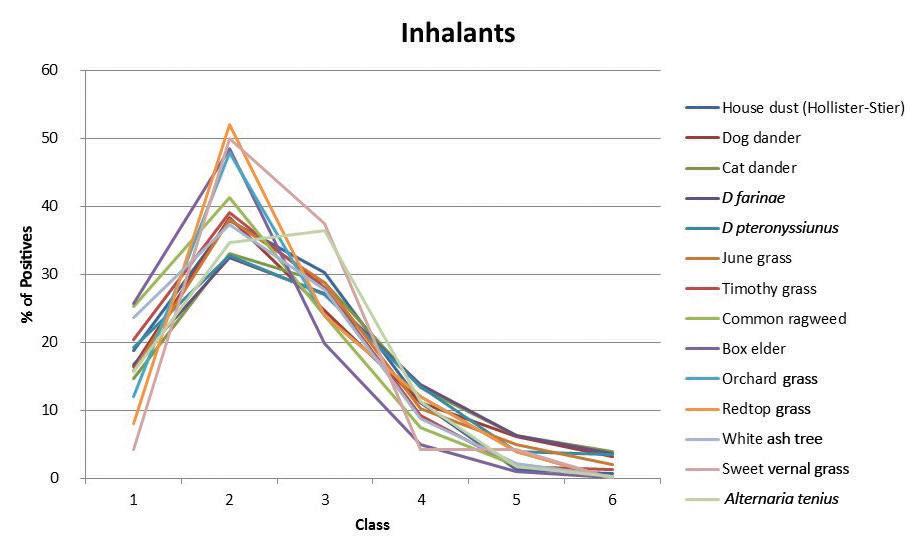
302 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 BENNINGER, DALY, GRAFFMILLER
Figure 1. Graphs show the in vitro class response for the most common inhalant/respiratory allergens (A) and food allergens (B).
Promote remucosalization. 2
In a multicenter, prospective, randomized controlled study remucosalization at the nasal septum donor site significantly improved early and overall by 10.03% at 2 weeks and 8.13% across 12 weeks, respectively. 2


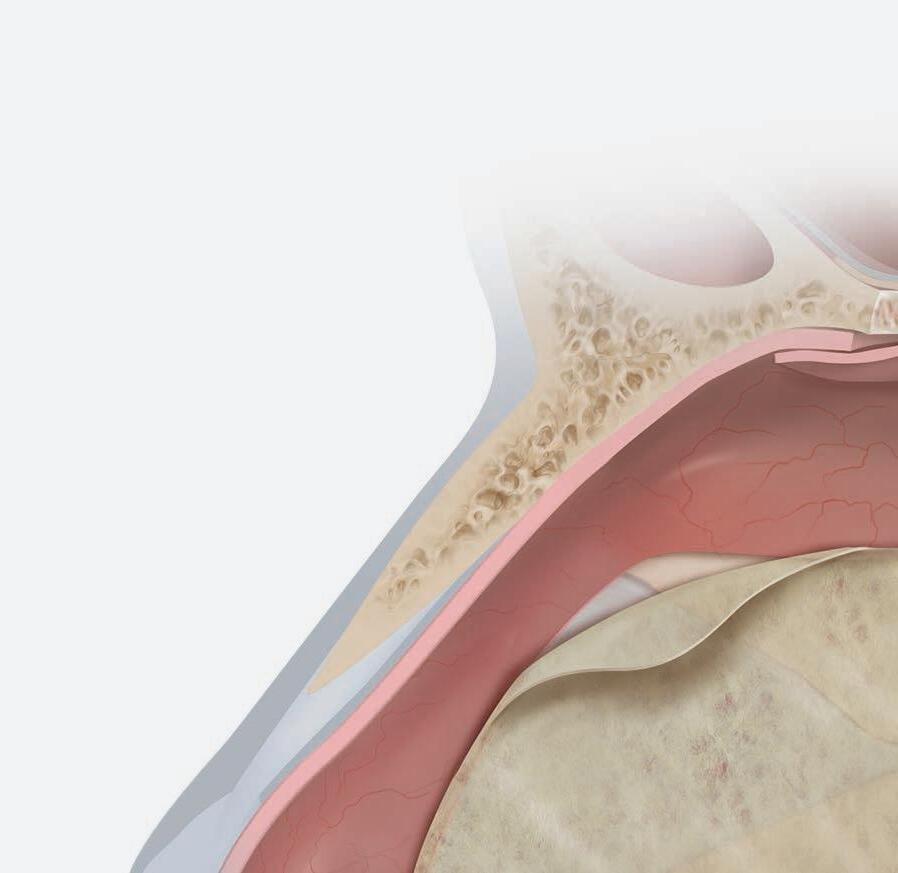
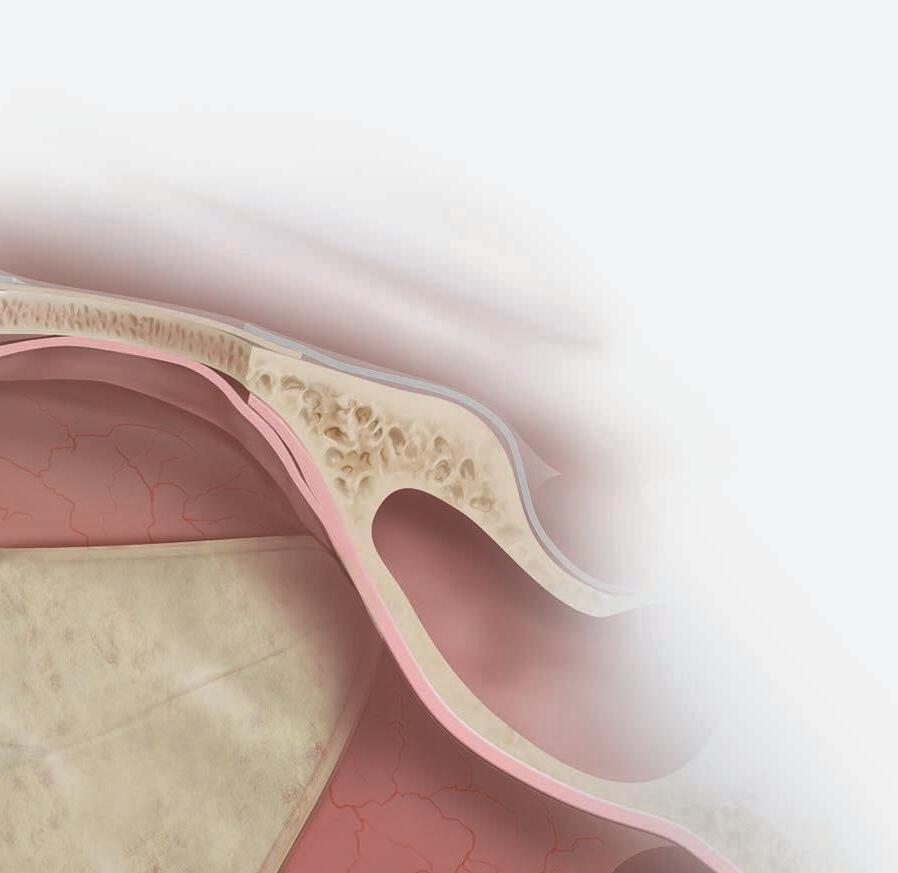
2003;5(6):528-529.
A,
intestine submucosal grafts improve remucosalization and progenitor cell recruitment to sites of upper airway tissue remodeling. Int Forum Allergy Rhinol. 2018;00:1–7.

cookmedical.com
RESTORE PATIENT TISSUE 1
1. Ambro BT, Zimmerman J, Rosenthal M, Pribitkin EA, Nasal septal perforation repair with porcine small intestinal submucosa. Arch Facial Plast Surg.
© COOK 06/2018 OHNS-D42808-EN-F Contact us at cookohns@cookmedical.com.
2. Nayak JV, Rathor
Grayson JW, et al. Porcine small
the many large industrial cities located in the upper Midwest and the large underprivileged populations in the inner cities.
One of the conclusions of the GA 2LEN III study was that, depending on the speci c country, as few as 8 to 13 allergens were all that were needed to identify all sensitized subjects.4 is might allow for re nement of screening allergy test batteries in a way that would be more cost-e cient than current methods.
Although treatment paradigms di er between Europe and the United States, and multiantigen immunotherapy in the United States may result in a desire to identify more positive allergens, there is some cost bene t in trying to reduce the panel size for allergy tests. Our study did not identify a huge opportunity for reducing the size of testing panels since 30 of the inhalant/respiratory allergy tests were positive in at least 10% of tests, although known cross-reactivity among certain allergens, such as grasses, might allow for some reduction in the size of the panels. However, some of the allergens tested yielded very low positivity rates; for example, rates for feather mix and white pine were 1%, rabbit epithelium was 3%, and 18 allergens yielded rates of less than 10%. is suggests that inclusion of these antigens in large panels may not be helpful unless there are speci c suggested triggers that might justify them.
Evaluating the prevalence of food allergies is a bit more challenging than doing so for inhalant allergies. Some persons experience reactions to foods that may not be allergic. According to Sampson, “Food intolerances (nonallergic food hypersensitivities) are adverse responses caused by some unique physiologic characteristic of the host, such as metabolic disorders
(e.g., lactase de ciency). Food hypersensitivities/allergies are adverse immunologic reactions that might be due to IgE- or non-IgE–mediated immune mechanisms.”5
Food allergies also vary according to age. While the prevalence of inhalant allergies increases with age, the prevalence of food allergies decreases, so positivity rates of food allergy screening tests would be expected to be much higher in children than in adults.11 While food allergy positivity rates may be well above 25 to 30% in small children, these rates drop to less than that by the later teenage years.11 IgE sensitization drops over time for milk and egg in particular. 5 Screening panels for foods, particularly those panels that are performed with in vitro testing, tend to be relatively small, and therefore the opportunity to reduce their size would seem to be limited, other than panels for sh perhaps. e limitations of our study are related to the inability to correlate our ndings with speci c symptoms or diagnoses. is study was not a random survey of the population but rather an analysis of ordered tests. We assume that the tests were ordered because patients exhibited symptoms, physical ndings, or diagnoses suggestive of allergic disease and therefore some unidenti ed clinical correlate was involved. It would be likely then that in most patients, positive allergy test results were associated with a clinical allergy.
A potential controversy concerns whether skin tests or in vitro tests should be used for screening. It seems that blood testing is easier to perform in many patient populations, particularly in primary care and emergency department settings. e authors of the recent “International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis” suggest that “Perhaps the strongest statement that can be made on behalf of tIgE [total IgE] is its ability to generally identify patients or populations with atopic or allergic disease.”6 ey also wrote, “Based on the reviewed literature, sIgE [serum IgE] testing is an acceptable alternative to skin testing....”6
Other potential limitations of our study are related to the clinical usefulness of our data in patient care, even though the large number of tests we studied would be useful in identifying the most common potential allergens in patients with allergy-mediated diseases.
Continued on page 322

304 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 BENNINGER, DALY, GRAFFMILLER
Figure 2. Scatter plot illustrates the likelihood of a strong positive class response by allergen. e greatest class 4 and 5 responses are seen with dog dander, cat dander, dust mites (D farinae), and peanut.

Acute infectious laryngitis: A case series
Aaron J. Jaworek, MD; Kranthi Earasi, MD; Karen M. Lyons, MD; Srihari Daggumati, BS; Amanda Hu, MD, FRCSC; Robert T. Satalo , MD, DMA, FACS
Abstract
Although acute laryngitis is common, it is o en managed by primary physicians. erefore, video images documenting its signs are scarce. is series includes 7 professional voice users who previously had undergone baseline strobovideolaryngscopy (SVL) during routine examinations or during evaluations for other complaints and who returned with acute laryngitis. Sequential SVL showed not only the expected erythema, edema, cough, and dysphonia, but also new masses in 5 of the 7 subjects. All the signs returned to baseline. is series is reported to highlight the reversible structural changes that can be expected in patients with acute laryngitis and the value of conservative management.
Introduction
Acute infectious laryngitis is one of the most common disorders of the larynx and is o en associated with upper respiratory tract infections (URIs), as reported more than a decade ago in a Cochrane review.1 Laryngeal in ammation can cause hoarseness, sore throat, and di culty swallowing. ese symptoms usually subside within 3 weeks but may persist much longer (months).
It can be di cult to distinguish between bacterial and viral origins.1 Viral laryngitis can be caused by many organisms, including but not limited to, in uenza virus, adenovirus, and even Varicella zoster virus. Acute bacterial laryngitis presents similarly; however, purulent secretions are observed more commonly in patients with a bacterial infection.2 Treatment for viral
From the Department of Otolaryngology–Head and Neck Surgery, Drexel University College of Medicine, Philadelphia (Dr. Jaworek, Dr. Lyons, and Dr. Sataloff); Specialty Physician Associates, Bethlehem, Pa. (Dr. Jaworek); Drexel University College of Medicine, Philadelphia (Dr. Earasi and Mr. Daggumati); and the Department of Otolaryngology–Head and Neck Surgery, the University of British Columbia, Vancouver (Dr. Hu). e cases described in this article occurred at Drexel University College of Medicine.
Corresponding author: Robert T. Satalo , MD, DMA, FACS, 219 N. Broad St., 10th Floor, Philadelphia, PA 19107. Email: RTSatalo @ PhillyENT.com
causes remains supportive, with adequate hydration and at least relative voice rest, while the bacterial form may bene t from the addition of an antibiotic.
Most patients with URI symptoms are evaluated and treated by a primary care physician. It is much less common for the otolaryngologist to treat an acute episode unless there is a complicating feature (e.g., protracted course, persistent symptoms a er the URI, recurrent episodes, etc.). For this reason, high-quality images of the larynx during an acute infection o en are not available. We present a series of patients with acute infectious laryngitis and their coinciding laryngoscopic images to highlight salient features.
Each patient presented here was established within the practice and had had strobovideolaryngoscopy performed before, during, and a er an acute episode of infectious laryngitis.
e examinations performed before the current visits o en were performed routinely at the patient’s request, to establish the normal baseline appearance of their vocal folds when they were healthy. In some cases, they were performed incidentally during evaluation for other complaints, but none was performed for laryngitis or any other acute laryngeal problem. ese examinations established the “normal” basline laryngoscopic appearance for each of these patients.
Case reports
Patient 1. A 37-year-old woman presented with a history of cough for 1 week and a gradual onset of hoarseness that began 2 days before the visit. ese symptoms progressed to aphonia for 3 days and a sensation of throat swelling. Strobovideolaryngoscopy (SVL) revealed a new right mid-membranous vocal fold mass, moderate erythema and edema of the true vocal folds and arytenoids, and thick mucopurulent secretions ( gure 1, A). e proximal trachea also appeared inamed. Acute laryngotracheitis was diagnosed, and amoxicillin-clavulanate and prednisone were prescribed for 7 days.
306 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 ORIGINAL ARTICLE

















BUILT TO LAST. AND LAST. AND LAST. 800.735.0357 | Builttosupport.com © 2018 Haag-Streit USA. All Rights Reserved.
Reliance 710 Premiere Chair
Since 1898, Reliance Medical Products has had an unmatched reputation for reliability and craftsmanship, building everything from exam chairs to ENT cabinets and accessories that stand up to anything. Including the test of time. And we’d like to thank all the doctors along the way who’ve helped us reach this proud milestone.
how our history of excellence can help build a stronger future for you. Visit us at AAO-HNSF booth #1732. A REPUTATION 120 YEARS IN THE MAKING.
1926 Reliance Barber Chair
Learn
Visit us at AAO-HNSF Booth #1732
Reliance Model 680 Chair
Follow-up SVL 8 days later demonstrated resolution of the patient's right vocal fold mass, and the true vocal folds were no longer erythematous. e appearance of her arytenoids and consistency of her secretions had returned to baseline ( gure 1, B).


Patient 2. A 29-year-old man experienced hoarseness that progressed to aphonia 4 days before his o ce visit. Cough, increased mucus production, and a sensation of swelling of the throat were reported, as well. SVL revealed moderate edema and severe erythema of the arytenoids, and erythema and edema of the true vocal folds with increased vascularity ( gure 2, A). ick mucus was visualized in the larynx, as well as bilateral mid-membranous in ammatory masses with an exudative appearance. A diagnosis of acute infectious laryngitis was made, and amoxicillin-clavulanate was prescribed for 7 days.
e patient did not return until 15 weeks later, at which time SVL con rmed resolution of the infection ( gure 3, B).

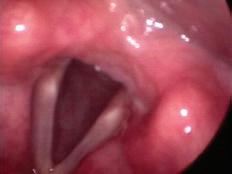
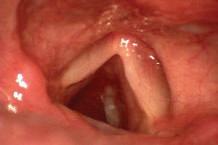
Patient 4. A 35-year-old woman presented with acute hoarseness and cough of 1 to 2 weeks’ duration. SVL revealed moderate erythema and edema of the arytenoids and Reinke edema. Severe in ammation was observed involving the true vocal folds, subglottis, trachea, and the interarytenoid region ( gure 4, A). Purulent secretions were evident, and amoxicillin-clavulanate was prescribed for 7 days.
SVL 3 weeks later demonstrated resolution of the acute changes, and only the baseline pathology remained, which included laryngopharyngeal re ux (LPR) and vocal fold scar ( gure 2, B).
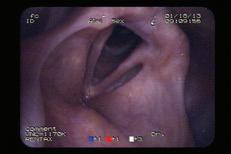
Patient 3. A 17-year-old female singer had a history of vocal fold nodules and re ux laryngitis. She reported a productive cough for the previous 24 hours. SVL revealed edema of the true vocal folds and erythema of the larynx. ick yellow mucus was observed from within the trachea ( gure 3, A). A diagnosis of acute laryngotracheitis was made, and the patient was started on amoxicillin-clavulanate for 10 days.
Follow-up 3 weeks later with SVL revealed improvement in Reinke edema, erythema of the larynx and trachea, and the consistency of secretions ( gure 4, B).
Patient 5. A 34-year-old man presented with hoarseness and cough for 2.5 weeks. His voice had deteriorated gradually over the previous few days to a whisper. SVL revealed new lesions on the posterior third of each musculomembranous vocal fold that appeared in ammatory ( gure 5, A). A diagnosis of acute laryngitis was made, and the patient was treated with amoxicillin for 14 days. Fluconazole and nystatin were prescribed empirically to treat suspected superimposed. fungal laryngitis, especially since prednisone also was prescribed for 2 weeks to help with the in ammation.


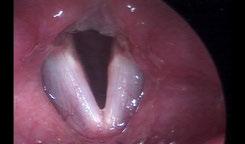
e patient returned 1 week later for follow-up. SVL revealed resolution of the true vocal fold lesions and accompanying erythema and edema ( gure 5, B). e patient’s hoarseness also had improved markedly.
Patient 6. A 31-year-old woman presented with sore throat, odynophagia, hoarseness, and a cough productive of yellow sputum for 1 week. She reported vocal fatigue, reduced projection, and loss of range when
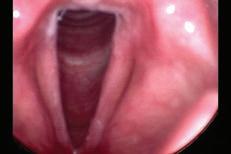


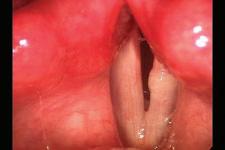

308 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
JAWOREK, EARASI, LYONS, DAGGUMATI, HU, SATALOFF
Figure 1. Patient 1 during acute infectious laryngitis episode (A) and a er resolution of the laryngitis (B).
Figure 2. Patient 2 during acute infectious laryngitis episode (A) and a er resolution of the laryngitis (B).
Figure 3. Patient 3 during acute infectious laryngitis episode (A) and a er resolution of the laryngitis (B).
Figure 4. Patient 4 during acute infectious laryngitis episode (A) and a er resolution of the laryngitis (B).
Figure 5. Patient 5 during acute infectious laryngitis episode (A) and a er resolution of the laryngitis (B).
singing. She had been staying hydrated and taking a decongestant, expectorant, and cough suppressant. SVL revealed three areas of swelling along the vibratory margin of the le vocal fold with a “tongue and groove” e ect along the contralateral vocal fold. ere were prominent vessels along this patient’s vocal folds, and her larynx appeared erythematous ( gure 6, A). Decreased mucosal wave and vibration of the true vocal folds were observed. e patient was treated with amoxicillin-clavulanate for 10 days along with guaifenesin for congestion.
At follow-up 1 week later, the patient’s voice had improved markedly. She reported that she had been able to speak “normally” a er 5 days of treatment. SVL revealed resolution of the in ammatory vocal fold masses, return to baseline of laryngeal erythema and edema, and the vibratory characteristics of the vocal folds had improved. ( gure 7, B).
Summary of symptoms
On presentation, all 7 patients reported hoarseness, 6 had cough, 3 had increased mucus/phlegm, and 3 had aphonia. SVL revealed laryngeal edema and erythema in all 7 patients, a new vocal mass in 5 patients compared with their baseline examinations, increased mucus in 4 patients, and tracheal involvement in 3 patients. e duration of symptoms before and a er the patients’ initial visits is shown in gure 8.

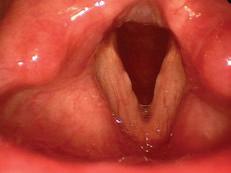
Discussion
e patient returned 7 weeks later for follow-up. At that time, she reported that she had had a sore throat for the previous week and that she had begun coughing up yellow sputum. She was treated with levo oxacin for 7 days and oseltamivir for 5 days. When she returned 3 weeks later, follow-up SVL showed resolution of her in ammatory masses, and the mucosal wave and vibratory characteristics of the vocal folds had returned to baseline ( gure 6, B).
Patient 7. A 29-year-old woman presented with an acute episode of hoarseness progressing to aphonia, which she had experienced 3 days before her appointment. She also reported a sore throat, odynophagia, and cough for 5 days. She had been taking a cough suppressant, antihistamine, decongestant, and acetaminophen to relieve her symptoms, and she had increased oral hydration. SVL revealed increased Reinke edema and new bilateral mid-membranous vocal fold masses ( gure 7, A). e amplitude and wave form of the vocal folds were decreased. She was diagnosed with acute laryngitis and treated with amoxicillin-clavulanate for 10 days and a methylprednisolone taper.
ese 7 patients provided novel insights into the presentation of acute infectious laryngitis. Of particular interest was the development of acute in ammatory vocal fold masses along the vibratory margin and increased laryngeal edema and erythema compared to baseline. Voice change and laryngeal edema and erythema were present in all 7 patients. Cough was a presenting symptom in 6, and new vocal fold masses along the vibratory margin were present in 5 patients. An increased amount of thick mucus involving the larynx was observed in 4 patients, with additional patients reporting a productive cough. e duration of symptoms ranged from 15 days to 35 days. e mean time to symptom resolution a er initiation of medical therapy was 16 days. Follow up SVL con rmed resolution of the acute ndings in all 7 patients.
Acute infectious laryngitis is characterized by inammation of the larynx that usually resolves within 3 weeks. e condition o en accompanies upper respiratory tract infections with symptoms that may include hoarseness, sore throat, odynophonia, odynophagia, dysphagia, dyspnea, cough, congestion, postnasal drip, and mucus. e hoarseness o en is accompanied by lowering of pitch that persists for 3 to 8 days in most cases.1 e voice may sound breathy and/or raspy, and compensatory muscle tension dysphonia o en is present.
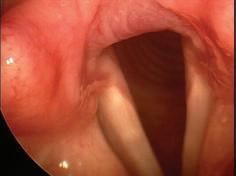
SVL frequently reveals erythema and edema of the larynx involving the true vocal folds, resulting in alteration of the mucosal wave and vibratory characteristics. In ammatory masses also can develop on the true vocal folds, further exacerbating the dysphonia and altered pitch.
Acute infectious laryngitis is caused by numerous pathogens. Among these, viral pathogens are believed to be most prevalent, following patterns similar to
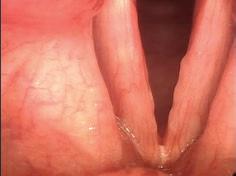
310 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
JAWOREK, EARASI, LYONS, DAGGUMATI, HU, SATALOFF
Figure 6. Patient 6 during acute infectious laryngitis episode (A) and a er resolution of the laryngitis (B).
Figure 7. Patient 7 during acute infectious laryngitis episode (A) and a er resolution of the laryngitis (B).
For the treatment of AOMT in pediatric patients due to S. aureus, S. pneumoniae, H. influenzae, M. catarrhalis, and P. aeruginosa.
The difference is in the delivery.
T E (ciprofloxacin and fluocinolone acetonide) is a sterile solution—not a suspension1
• Blow-fill-seal (BFS) technology provides sterile manufacturing and packaging2













• Containers are formed, filled, and sealed in a continuous, automated operation3
• BFS technology reduces human intervention in the fill/finish process3
• Every dose is sterile, precise, and preservative free1
• No drop counting. No mixing or shaking required1
• Demonstrated efficacy and safety in 2 clinical trials1,5
INDICATIONS
OTOVEL (ciprofloxacin and fluocinolone acetonide) is indicated for the treatment of acute otitis media with tympanostomy tubes (AOMT) in pediatric patients (aged 6 months and older) due to S. aureus, S. pneumoniae, H. influenzae, M. catarrhalis, and P. aeruginosa.
IMPORTANT SAFETY INFORMATION
Contraindications
OTOVEL is contraindicated in:




• Patients with known hypersensitivity to fluocinolone acetonide or other corticosteroids, ciprofloxacin or other quinolones, or to any other component of OTOVEL.
• Viral infections of the external ear canal, including varicella and herpes simplex infections and fungal otic infections.
The following Warnings and Precautions have been associated with OTOVEL: hypersensitivity reactions, potential for microbial overgrowth with prolonged use, and continued or recurrent otorrhea.
The most common adverse reactions are otorrhea, excessive granulation tissue, ear infection, ear pruritis, tympanic membrane disorder, auricular swelling, and balance disorder. For additional Important Safety Information, please see Brief Summary of Prescribing Information on adjacent page, and full Prescribing Information available at www.otovel.com.
References: 1. Otovel [package insert]. Atlanta, GA: Arbor Pharmaceuticals, LLC. 2016. 2. Data on file. Arbor Pharmaceuticals, LLC. 3. Guidance for industry: sterile drug products produced by aseptic processing current good manufacturing practice. Food and Drug Administration. https://www.fda.gov/downloads/Drugs/Guidances/ucm070342.pdf. Published September 2004. Accessed March 15, 2018. 4. Orange Book: Approved drug products with therapeutic equivalence evaluations. US Food and Drug Administration. https://www.accessdata.fda.gov/scripts/ cder/ob/default.cfm. Updated May 17, 2013. Accessed uly 15, 2016. 5. Spektor , Pumarola P, Ismail , et al. Efficacy and safety of ciprofloxacin plus fluocinolone in otitis media with tympanostomy tubes in pediatric patients: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg 2017 143(4):341-349.
Otovel is a registered trademark of Laboratorios Salvat, S.A. with the US Patent and Trademark Office and under license by Arbor Pharmaceuticals, LLC. Trademarks are the property of their respective owners. 2018
Arbor Pharmaceuticals, LLC. All rights reserved.
AOMT acute otitis media with tympanostomy tubes BID twice daily.
Printed in USA. PP-OTO-US-0268
We’d like to deliver Starter Packs to your practice. Order yours at otovel.com/hcp/resources
combination ear drop in single-dose vials4
Single-use vials contain 1 premeasured dose each dose BID/7 days1
• The first and only antibiotic/steroid
•
Delivered in simple, single-dose vials1
OTOVEL® (ciprofloxacin and fluocinolone acetonide) otic solution
Brief Summary of Prescribing Information
1 INDICATIONS AND USAGE
OTOVEL is indicated for the treatment of acute otitis media with tympanostomy tubes (AOMT) in pediatric patients (aged 6 months and older) due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa
2 DOSAGE AND ADMINISTRATION
• OTOVEL is for otic use only. It is not for ophthalmic use, or for injection.
The recommended dosage regimen is as follows:
• Instill the contents of one single-dose vial 0.25 mL into the affected ear canal twice daily (approximately every 12 hours) for 7 days. Use this dosing for patients aged 6 months of age and older.
• Warm the solution by holding the vial in the hand for 1 to 2 minutes. This is to avoid dizziness, which may result from the instillation of a cold solution into the ear canal.
• The patient should lie with the affected ear upward, and then instill the medication.
• Pump the tragus 4 times by pushing inward to facilitate penetration of the medication into the middle ear.
• Maintain this position for 1 minute. Repeat, if necessary, for the opposite ear [see Instructions for Use]
3 DOSAGE FORMS AND STRENGTHS
Otic Solution: Each single-dose vial of OTOVEL (ciprofloxacin 0.3 % and fluocinolone acetonide 0.025 %) delivers 0.25 mL of solution equivalent to ciprofloxacin 0.75 mg and fluocinolone acetonide 0.0625 mg.
4 CONTRAINDICATIONS
OTOVEL is contraindicated in:
• Patients with known hypersensitivity to fluocinolone acetonide or other corticosteroids, ciprofloxacin or other quinolones, or to any other components of OTOVEL.
• Viral infections of the external ear canal, including varicella and herpes simplex infections and fungal otic infections.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
OTOVEL should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria and itching. Serious acute hypersensitivity reactions may require immediate emergency treatment.
5.2 Potential for Microbial Overgrowth with Prolonged Use
Prolonged use of OTOVEL may result in overgrowth of non-susceptible bacteria and fungi. If the infection is not improved after one week of treatment, cultures should be obtained to guide further treatment. If such infections occur, discontinue use and institute alternative therapy.
5.3 Continued or Recurrent Otorrhea
If otorrhea persists after a full course of therapy, or if two or more episodes of otorrhea occur within 6 months, further evaluation is recommended to exclude an underlying condition such as cholesteatoma, foreign body, or a tumor.
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, 224 patients with AOMT were treated with OTOVEL for a median duration of 7 days. All the patients received at least one dose of OTOVEL. There were 220 patients who received at least one dose of ciprofloxacin (CIPRO) and 213 patients received at least one dose of fluocinolone acetonide (FLUO). The most common adverse reactions that occurred in 1 or more patients are as follows:
Group
1Selected adverse reactions that occurred in ≥ 1 patient in the OTOVEL group derived from all reported adverse events that could be related to the study drug or the drug class.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ciprofloxacin and fluocinolone acetonide otic solution, 0.3% / 0.025% outside the US. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
• Immune system disorders: allergic reaction.
• Infections and infestations: candidiasis.
• Nervous system disorders: dysgeusia, paresthesia (tingling in ears), dizziness, headache.
• Ear and labyrinth disorders: ear discomfort, hypoacusis, tinnitus, ear congestion.
• Vascular disorders: flushing.
• Skin and subcutaneous tissue disorders: skin exfoliation.
• Injury, poisoning and procedural complications: device occlusion (tympanostomy tube obstruction).
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy Risk Summary
OTOVEL is negligibly absorbed following otic administration and maternal use is not expected to result in fetal exposure to ciprofloxacin and fluocinolone acetonide (12.3)].
8.2 Lactation Risk Summary
OTOVEL is negligibly absorbed by the mother following otic administration and breastfeeding is not expected to result in exposure of the infant to ciprofloxacin and fluocinolone acetonide.
8.4 Pediatric Use
OTOVEL has been studied in patients as young as 6 months in adequate and wellcontrolled clinical trials. No major differences in safety and effectiveness have been observed between adult and pediatric patients.
8.5 Geriatric Use
Clinical studies of OTOVEL did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
10 OVERDOSAGE
Due to the characteristics of this preparation, no toxic effects are to be expected with an otic overdose of OTOVEL.
Distributed by: Arbor Pharmaceuticals, LLC Atlanta, GA 30328
Under license of Laboratorios SALVAT, S.A.
OTOVEL is a registered trademark of Laboratorios SALVAT, S.A.
U.S. Patent No: 8,932,610
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
For more detailed information, see the full prescribing information for Otovel at www.otovel.com or contact Arbor Pharmaceuticals, LLC at 1-866-516-4950.
Adverse Reactions1 Number (%) of Patients OTOVEL N=224 CIPRO N=220 FLUO N=213 Otorrhea 12 (5.4%)9 (4.1%)12 (5.6%) Excessive granulation tissue 3 (1.3%)0 (0.0%)2 (0.9%) Ear infection 2 (0.9%)3 (1.4%)1 (0.5%) Ear pruritus 2 (0.9%)1 (0.5%)1 (0.5%) Tympanic membrane disorder 2 (0.9%)0 (0.0%)0 (0.0%) Auricular swelling 1 (0.4%)1 (0.5%)0 (0.0%) Balance disorder 1 (0.4%)0 (0.0%)0 (0.0%)
Table 1: Selected Adverse Reactions that Occurred in 1 or more Patients in the OTOVEL
those for URI. ey include parain uenza, rhinovirus, in uenza virus, and adenovirus. Bacterial pathogens that have been associated with acute laryngitis include Moraxella catarrhalis, Haemophilus in uenzae, and Streptococcus pneumoniae, with M catarrhalis being the most common.2 Extension to the larynx of an infection involving the upper aerodigestive tract can occur. In some cases, a sputum culture may be helpful to direct therapy.
e treatment for viral and bacterial laryngitis includes supportive measures. At least relative voice rest, humidi cation, analgesics, and hydration should be considered along with mucolytics, decongestants, and glucocorticoid steroids. In addition, antibiotics may be prescribed in cases in which a bacterial infection is suspected (e.g., tracheitis, purulent secretions, immunocompromised state, protracted course, positive culture, etc.). ey also may be used when the etiology is uncertain but pressing voice commitments are imminent.
A Cochrane Review completed in 2015 investigated the bene ts of antibiotic usage for acute laryngitis.3 A er reviewing the only two placebo-controlled, randomized trials that met their inclusion criteria, the authors concluded that antibiotics appeared to have no bene t; however, they acknowledged that one of the studies demonstrated a signi cant reduction in severity of reported vocal symptoms a er 1 week on erythromycin (vs. placebo) and a signi cant reduction in cough a er 2 weeks with antibiotics.1
Since the patients in our series were professional voice users, expeditious return to baseline vocal function was a primary concern. erefore, prescribing an antibiotic was justi ed based on the conclusions of the study included in the Cochrane Review, as well as on the uncertainty of the etiology and the need for rapid return to safe phonation.
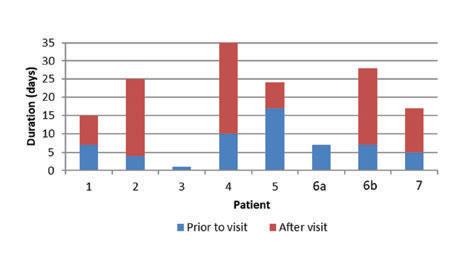
LPR, a chronic form of laryngitis, can have signs and symptoms that overlap with those of acute infectious laryngitis. e distinguishing features of re ux laryngitis include a longer duration of symptoms (usually over many weeks, months, or even years), exacerbating factors (symptoms with meals or certain foods and during speci c activities), response to proton pump inhibitor/H2 blocker, and erythema/edema commonly most prominent on or isolated to the region of the arytenoids.4 When the two pathologies coexist, it is prudent to allow the acute laryngitis episode to resolve before making a nal assessment of the need for long-term treatment for the re ux, but acute treatment of LPR is advisable to decrease in ammation caused by both LPR and infection. When any uncertainty in the diagnosis remains, a 24-hour pH impedance test can be helpful.
Another form of chronic laryngitis, prolonged ulcerative laryngitis, also has been and reported by the senior author (RTS), among others.5 Symptoms and abnormal SVL may persist for many weeks (up to 5 months or longer in some cases). Antibiotics and corticosteroids are ine ective, and the etiology remains unknown, although the senior author suspects that bio lms may play a role.
Conclusion
Acute infectious laryngitis presents commonly during URI. rough SVL images, the changes along the musculomembranous vocal folds have been de ned clearly and documented in this case series. Further investigation with prospective, randomized, controlled trials is needed to improve our understanding of ways to di erentiate viral from bacterial causes, to determine whether the structural changes seen in this series of voice professionals also occur routinely in other patients, to help guide appropriate use or nonuse of antibiotics without inappropriate delays awaiting culture results (especially for voice professionals), and to identify the most e ective treatment strategies in various patient populations.
References
1. Reveiz L, Cardona AF, Ospina EG. Antibiotics for acute laryngitis in adults. Cochrane Database Syst Rev 2007;2:CD004783
2. Dworkin JP. Laryngitis: Types, causes, and treatments. Otolaryngol Clin North Am 2008;41(2):419-36.
3. Reveiz L, Cardona AF. Antibiotics for acute laryngitis in adults. Cochrane Database Syst Rev 2015;5:CD004783
4. Joniau S, Bradshaw A, Esterman A, Carney AS. Re ux and laryngitis: A systematic review. Otolaryngol Head Neck Surg 2007;136(5):686-92.
5. Satalo RT. Common infections, in ammations and other conditions: Management in singers and actors. In: Satalo RT. Professional Voice: e Science and Art of Clinical Care. 3rd ed. San Diego: Plural Publications; 2005:809.
Volume 97, Number 9 www.entjournal.com 313 ACUTEINFECTIOUSLARYNGITIS: A CASESERIES
Figure 8. Graph shows the duration of symptoms in the 7 patients before and a er their visits. (Note: 6a and 6b represent two separate episodes in the same patient. e total duration of symptoms was not known for patients 3 and 6a.)
No difference in disease-free survival after oral cancer resection with close tumor margins in patients with and without postoperative radiotherapy
Britta Kalto Welinder, MD; Mads Lawaetz, MD; Laura M. Dines, MD, PhD; Preben Homøe, MD, PhD, DMSc
Abstract
We conducted a retrospective follow-up study to determine if adjunctive radiotherapy (RT) a ected disease-free survival in patients with oral squamous cell carcinoma (SCC) who were found to have close surgical margins a er tumor resection. Our study population was made up of 110 patients—72 men and 38 women, aged 30 to 94 years (median: 66) at the time of diagnosis. eir follow-up ranged from 12 days to 5.2 years (median: 3.6 yr). Of this group, 40 patients had free margins, 55 patients had close margins, and 15 had involved margins a er surgery. Only 31 of these patients received postoperative RT, including 17 who had close margins. We would expect to nd better postoperative local tumor control with combined surgery and RT, but we found no statistically signi cant di erence in disease-free survival between the surgery-plus-RT group and the surgery-only group (p = 0.72). We also found no signi cant di erence in disease-free survival between patients with a tumor of the oor of mouth and those with a tumor of the tongue (p = 0.34). In the study population as a whole, the disease-free survival rate was 81.0% and the overall survival rate was 78.2%. Our ndings support the trend toward a watchand-wait approach before initiating postoperative RT for patients with close surgical margins. e decision should be carefully discussed between the surgeon, the oncologic radiotherapist, and the patient.
From the Department of Otorhinolaryngology, Head and Neck Surgery and Audiology, Rigshospitalet, University Hospital of Copenhagen, Copenhagen East, Denmark (all authors); and the Department of Otorhinolaryngology and Maxillofacial Surgery, Zealand University Hospital, University of Copenhagen, Køge, Denmark (Dr. Homøe). e study described in this article was conducted at Rigshospitalet, University Hospital of Copenhagen.
Corresponding author: Britta Kalto Welinder, MD, Department of Otorhinolaryngology, Head and Neck Surgery and Audiology, Rigshospitalet, University Hospital of Copenhagen, Blegdamsvej 9, 2100 Copenhagen East, Denmark. Email: brittakalto @hotmail.com
Introduction
e choice of treatment for oral squamous cell carcinoma (SCC) according to the Danish Head and Neck Cancer Group (DAHANCA) guidelines depends on tumor stage and histopathologic factors. Surgery is the preferred monotherapy for patients with a low risk of recurrent disease (T1/2N0M0), while a combination of surgery and either radiotherapy (RT) or chemoradiotherapy is recommended for high-risk patients.
Successful treatment with radical surgery demands a minimum macroscopic resection margin of 10 mm and a minimum microscopic margin of 5 mm to achieve free margins.1 e width of the margin cited on the pathology report will o en di er from the 10- and 5-mm margins that the surgeon intended. is can be attributable to several factors, such as the anatomy of the involved oral cavity, the shrinking of the tumor and surrounding tissue a er removal, xation of the tissue, and microscopic changes.2-5 Involved margins demand additional treatment, but o en there is a group of patients at intermediate risk for recurrent disease whose resection margins are 1 to 5 mm.
While there is general agreement on the type of treatment for patients at low and high risk for recurrence, there is controversy regarding the primary treatment for patients with close surgical margins, who are at intermediate risk, as studies have found di erent locoregional control and long-term survival rates a er combined surgery and RT versus surgery alone.6-8
In 2012, a review by Brown et al found that both overall survival rates and disease-free survival rates were better in patients who were treated with combined therapy than in those treated only surgically (94 vs. 84%, no p value reported, and 68 vs. 38%, p <0.005, respectively)8 Conversely, a similar study by Wong et al6 published that same year found that locoregional control rates were better in the surgery-only group, although not signicantly so (15 vs. 8%, p = 0.29). Another study by Brown
314 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 ORIGINAL ARTICLE

et al,9 published in 2007, found that long-term survival rates were signi cantly better in the surgery-only group (74 vs. 54%, p = 0.002), although locoregional control rates were slightly worse in patients who did not receive postoperative RT (15 vs. 23%, p = 0.19).
When planning treatment, the bene t of combined therapy should be considered along with the potential long-term side e ects of RT, such as xerostomia and osteoradionecrosis.9,10
Some authors have advocated a watch-and-wait approach a er surgery before deciding whether to administer RT.9,11 ey contend that repeat surgery and subsequent curative RT can be performed in the case of a recurrence. On the other hand, the DAHANCA guidelines recommend immediate additional RT to reduce the risk of a recurrence.
In this article, we describe our study to determine if adjunctive RT had an e ect on disease-free survival in patients with oral SCC who were found to have close surgical margins a er tumor resection.
Patients and methods
For this retrospective follow-up study, we reviewed the records of 178 consecutively presenting patients with oral SCC who had been treated at Rigshospitalet, University Hospital of Copenhagen, from January 2010 through December 2011 and followed until May 31, 2015 at the latest.
Patients were excluded if they had presented with a recurrence of oral SCC (n = 38), if their tumor had been su ciently removed during a biopsy procedure (n = 12), if they had been previously treated with RT in the head and neck area (n = 11), if histology revealed only carcinoma in situ (n = 5), or if their tumor was a metastasis (n = 2). A er these exclusions, our study population consisted of 110 patients—72 men and 38 women, aged 30 to 94 years (median: 66) at the time of diagnosis.
Data collection. Data were retrieved from our hospital’s electronic medical records and pathology reports. In addition to demographic data, we compiled information on each patient’s date of diagnosis, date of primary surgery, recurrence at the original tumor site or at a nodal site, any distant metastasis, tobacco history, and the date and cause of death, if applicable. ese data were compared with previously collected data on the same cohort described in a study published by two of the present study’s authors, Lawaetz and Homøe.11 Resection margins were recorded in millimeters on all edges (anterior, posterior, lateral, medial, and deep). e smallest margin was then categorized according to the e Royal College of Pathologists as either clear, close, or involved for margins that were located more than 5 mm, 1 to 5 mm, or less than 1 mm from the primary
tumor, respectively.1 e margin closest to the middle of the oral cavity was categorized as the medial margin; the opposite margin, the lateral; the margin closest to the front of the oral cavity, the anterior; and the margin closest to the back, the posterior. e margin closest to the dysplasia or carcinoma in situ did not qualify as a tumor margin and was not used in this study.
e decision as to whether postoperative RT should be administered was discussed at a multidisciplinary team (MDT) conference with an attending head and neck surgeon and an oncologic radiotherapist a er the pathology report had been completed. Based on the decision reached at the MDT conference, patients gave their informed consent regarding either postoperative RT or a postoperative watch-and-wait strategy.
Recurrence was evaluated by the head and neck surgeon on the basis of a clinical examination and ndings on computed tomography, magnetic resonance imaging, and/or positron-emission tomography; ultrasonography was performed when relevant. Imaging ndings were con rmed by ne-needle aspiration cytology or histologic examination.
Overall survival was de ned as the time from the initial diagnosis to the date of death from any cause. Disease-free survival was de ned as the time from surgery to the initial development of a recurrence or a local, regional, or distant metastasis.
Statistical analysis. Univariate analyses were performed with the Statistical Package for the Social Sciences so ware (v. 19.0). e chi-square test and Fisher exact test were used to explore relationships between categorical variables. Rates of overall and disease-free survival were estimated with the use of the Kaplan-Meier model. Univariate comparison of survival was performed using the log-rank test.
Ethical considerations. According to the Danish Ethical Committee, ethical approval was not necessary at the time of data collection because the patients were not contacted. e study was therefore granted an exemption.
Results
For this study cohort, data regarding tumor site, tumor size, TNM classi cation, margin status, tumor thickness, perineural invasion, character of the invasive front, vascular permeation, resection margins on all surfaces, postoperative RT, and re-resection a er the primary surgery were described by Lawaetz and Homøe.11 As they reported then, the 110 patients in the present study were comparable to patient samples in similar retrospective studies with regard to age, sex, and tumor distribution.
Follow-up. e duration of follow-up ranged from 12 days to 5.2 years (median: 3.6 yr). One patient died 12
316 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
WELINDER, LAWAETZ, DINES, HOMØE
A
Number: 1642
ACT EARLY*
Age 1-2 years Age 3-4 years

78% of HOS patients developed an abdominal hernia1
72% of HOS patients had otitis media2
A rare combination of common childhood complaints could indicate Hunter syndrome1,2 REFER TO A SPECIALIST
more information visit:
1.5
TODAY Silas,
5 © 2018 Shire S42756 08/18 Shire AAO-HNSF
Silas,
Exhibit Booth
68% of HOS patients were diagnosed with enlarged tonsils or adenoids1 Median age of onset and prevalence data from HOS (Hunter Outcome Survey). 1. Wraith JE et al. Genet Med 2008; 10(7): 508–516. 2. Keilmann A et al. J Inherit Metab Dis 2012; 35(2): 343–353.
RE
OU M
G
UNTER SYNDROME?
HUNTER SYNDROME IS A PROGRESSIVE GENETIC DISEASE
Y
ISSIN
H
hunterpatients.com
* Consider the importance of early assessment, diagnosis, and follow-up by a specialist For
days postoperatively from pneumonia and sepsis; he had not been evaluated at the MDT conference regarding postoperative RT before his death. No patients were lost to follow-up.
Tumor site. Tumors were located in the tongue in 52 patients (47.3%), the oor of mouth in 47 (42.7%), the gums in 6 (5.5%), and the retromolar space in 5 (4.5%).
Type of surgical margins. In total, 40 patients (36.4%) had microscopically free resection margins, 55 (50.0%) had close resection margins (including the patient who died 12 days postoperatively, whose data are hereinafter not included in our analysis unless noted), and 15 (13.6%) had involved margins. Postoperative RT had been administered to 2, 17, and 12 patients, respectively (table 1).
Re-resection. ree patients underwent surgical re-resection. Two of these 3 patients were among the 37 with closed margins who had not received postoperative RT. Of the remaining 35 patients, 16 refused a second operation; the reason for the other 19 patients was not recorded. e other patient who underwent re-resection had involved margins.
Recurrence. In all, 23 of the 109 evaluable patients (21.1%) experienced a recurrence. e duration from treatment to recurrence ranged from 55 days to 3.6 years (median: 234 days).
Margin status. With respect to margin status, recurrence was seen in 4 of the 40 patients with free margins (10.0%), 15 of the 54 evaluable patients with close margins (27.8%), and in 4 of the 15 patients with involved margins (26.7%). e distribution of recurrences between the RT and non-RT subgroups by margin status is shown in table 1.
Tumor site. With respect to the primary tumor site, recurrence was seen in 14 of the 52 patients with a tongue tumor (26.9%), in 8 of 47 with a oor of mouth tumor (17.0%), in 1 of 6 patients with a tumor of the gums (16.7%), and in none of the 5 patients with a retromolar space tumor. We found no signi cant association between tumor recurrence and speci c tumor location (p = 0.34). In all, 12 patients had a local recurrence, 8 had a nodal recurrence, and 3 had a distant metastasis. e distribution of recurrences
between the RT and non-RT subgroups by tumor site and by TNM category is shown in table 2.
Risk factors for patients with close margins. Among the patients with close surgical margins, the RT group had signi cantly more nodal involvement (p = 0.009), more advanced overall cancer stage (p = 0.033), and a greater incidence of perineural invasion (p = 0.028) than the non-RT group. No di erence was seen regarding T and M categories, perivascular invasion, bone invasion, and invasive front (table 3).
Survival. A total of 35 patients (31.8%) died during the study period. e interval between diagnosis and death ranged from 29 days to 5.1 years (median: 2.1 yr).
Disease-free survival. e 3-year disease-free survival rate among the 110 patients was 81.0% ( gure, A). Among the patients with close margins, we found no statistically signi cant di erence in disease-free survival between those who did and did not receive postoperative RT (p = 0.72). We also found no signi cant di erence between patients with oor of mouth tumors and tongue tumors (p = 0.34).
Overall survival. e overall survival rate at 3 years was 78.2% ( gure, B).
Smoking. Of the 110 patients, 69 (62.7%) reported active smoking at the time of the diagnosis, 29 (26.4%) had previously smoked, and 12 (10.9%) had never smoked. We were able to obtain information on pack-years from 77 of the 98 current and previous smokers (78.6%); the duration of their consumption ranged from 6 to 180 pack-years (median: 40).
Among the 54 evaluable patients with close margins, 17 of 17 RT patients were active or previous smokers, as were 32 of 37 non-RT patients. We found no statistically signi cant di erence in smoking in the group with close margins between the RT and non-RT patients (p = 0.16). We also found no signi cant di erence in disease-free
318 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 WELINDER,
LAWAETZ, DINES, HOMØE
Type of surgical margin Free, n = 40Close, n = 54* Involved, n = 15 No. pts. 2381737123 Recurrences, n (%)04 (10.5)4 (23.5) 11 (29.7)4 (33.3)0
Table 1. Distribution of surgical margins and recurrences according to postoperative radiotherapy status in 109 evaluable patients
* One patient with close margins died 12 days postoperatively before any other treatment could be planned, and his data are not included in this table.
Total (T, N, M) Tumor site RT Non-RT Tongue (n = 52) 5 (4, 1, 0)9 (4, 4, 1) Floor of mouth (n = 47)3 (1, 1, 1)5 (2, 2, 1) Gums (n = 6) 01 (1, 0, 0) Retromolar space (n = 5) 0 0
Table 2. Number of recurrences and TNM classi cation according to postoperative radiotherapy status in the entire study group (N = 110)

survival between the active and previous smokers compared with the nonsmokers (p = 0.086).
Discussion
In our 3-year follow-up study, we found no signi cant association between tumor recurrence and speci c tumor location in the oral cavity (p = 0.34), although the patients with oor of mouth tumors had a signi cantly higher incidence of inadequate resection margins, as reported previously by Lawaetz and Homøe.11 Close
or involved margins were signi cantly more common in the deep edges than in the other four edges.
Floor of mouth anatomy is challenging for surgeons because of natural boundaries, so -tissue extension, and poor overview. McMahon et al have shown that a reduction in the risk of inadequate surgical margins is possible with the use of improved imaging and by rigorous surgical approaches.5
In patients with close margins, our nding that there were no signi cant di erences in T and M categories, perivascular invasion, bone invasion, and invasive front between the RT and non-RT patients corresponds with ndings reported in other studies.6,9,12,13
DAHANCA guidelines recommend monotherapy—primarily surgery, but in rare cases RT—for patients with stage I (T1N0), stage II (T2N0), and some stage III (T1N1, T2N1) oral SCCs. Surgical removal of the primary tumor is preferred if radical resection can be achieved with a functionally and aesthetically acceptable result.
If a tumor is not radically removed (i.e., if a micro- or macroscopic tumor is too close to the resection margin), postoperative RT is recommended. All stage III and IV cancers are treated with combination therapy. In our study, we did not adhere entirely to these guidelines because not all patients with close or even involved surgical margins received RT (quite a few because they refused it).
Even when frozen sections are obtained regularly during surgery, the exact surgical margin cannot be con rmed until the tissue has been evaluated histopathologically. Our study found that perioperative evaluations of the adequacy of surgical margins were used in the postoperative evaluation to decide whether postoperative RT should be administered. However, any di erence between the width of a surgeon’s resection margins and a pathologist’s resection margins should be viewed in light of the fact that tissue shrinks by 21
320 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 WELINDER, LAWAETZ, DINES, HOMØE
Variable RT (n = 17) Non-RT (n = 37) p value T category 0.125 T1 6 24 T2 9 11 T3 or T4 2 2 N category 0.009† N0 8 31 N+ 9 6 M category 1.0 Mx 0 1 M0 17 36 Cancer stage 0.033† I or II 8 28 III or IV 9 8 No information 0 1 Perineural invasion 0.028† Yes 10 9 No 5 25 No information 2 3 Perivascular invasion 0.265 Yes 15 34 No 2 1 No information 0 2 Bone invasion 1.0 Yes 0 1 No 17 36 Invasive front 0.273 Noncohesive 13 21 Cohesive 4 13 No information 0 3
Table 3. Findings in the 54 evaluable patients* with close surgical margins according to postoperative radiotherapy status
* One patient with close margins died 12 days postoperatively before any other treatment could be planned, and his data are not included in this table.
† Statistically signi cant di erence.
to 59% during the interval between surgery and histopathologic analysis.2-4,13
It is well known that combined therapy with surgery and postoperative RT can increase the incidence of severe and irreversible side e ects such as xerostomia and osteoradionecrosis of the mandible, both of which have an in uence on quality of life.9,10
e trend toward a watch-and-wait approach regarding postoperative RT in patients with close margins makes it possible for patients to receive potentially curative RT later if there is tumor recurrence.6,7,14 In our study, patients with close margins who received postoperative RT had signi cantly more nodal involvement, signi cantly more advanced cancer stage, and a signi cantly greater frequency of perineural invasion than did patients with close surgical margins who did not receive RT. However, this nding might have been the result of a selection bias, since cancer stage and histopathologic characteristics were used at the MDT conference as a basis on which to advise patients as to whether they should undergo postoperative RT.9 Nevertheless, our results still indicate that a watch-andwait strategy should at least be considered for selected patients with close surgical margins.

We found that smoking had no e ect on outcomes. Data on other potential confounding variables, such as alcohol use and immunosuppression therapy, were not available for all patients.
In our study, the 3-year disease-free survival rate was 81.0%. No other studies have reported a 3-year disease-free survival rate or a 3-year recurrence rate. However, because of our small sample size, these data should be interpreted with caution. Wong et al did report a 2-year local recurrence rate of 12.5%.6
e overall survival rate at 3 years in our study was 78.2%. is rate was higher than the previously reported rates of 28 to 69% in other European studies.11,15 e di erence is most likely attributable to the fact that our study had a shorter follow-up period.
e main nding in our study is that although some patients experienced recurrence, it was seen in both the RT and non-RT groups. Another signi cant nding was that 26 of 37 patients who would have been treated with RT according to the DAHANCA guidelines did not receive it and did not experience a recurrence. Although those without RT had less advanced disease, they still avoided RT and its complications. is is consistent with the ndings of other studies and should be taken into careful consideration when advising patients as to whether RT or a watch-and-wait approach should be o ered.6,9
In conclusion, our ndings support the trend toward a watch-and-wait approach instead of postoperative RT for patients with close surgical margins and perhaps even for patients with involved margins. Although our retrospective study has some limitations, its results support the need for large, high-quality, prospective, randomized, controlled trials if adherence to current guidelines regarding postoperative RT for inadequate close surgical margins should prove to have only a minor bene t in terms of tumor recurrence and overall survival. However, until such trials are conducted, we nd it morally acceptable to pursue a watch-and-wait policy regarding postoperative RT, depending on the TNM status and histopathologic results in patients with inadequate surgical margins.
e decision should be carefully discussed among the surgeon, the oncologic radiotherapist, and the patient.
Volume 97, Number 9 www.entjournal.com 321 NO DIFFERENCE IN DISEASE-FREE SURVIVAL AFTER ORAL CANCER RESECTION WITH CLOSE TUMOR MARGINS IN PATIENTS WITH AND WITHOUT POSTOPERATIVE RADIOTHERAPY
Figure. Graphs show 3-year disease-free survival (A) and overall survival (B) in the 110 patients with oral SCC.
e patient also should be informed about symptoms of tumor recurrence and the need for close follow-up, especially during the rst 2 years.
References
1. Helliwell TR, Woolgar JA. Datasets for Histopathology Reports on Head and Neck Carcinomas and Salivary Neoplasms. 2nd ed. London: e Royal College of Pathologists; 2005.
2. Spiro RH, Guillamondegui O Jr., Paulino AF, Huvos AG. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck 1999;21(5):408-13.
3. Mistry RC, Qureshi SS, Kumaran C. Post-resection mucosal margin shrinkage in oral cancer: Quanti cation and signi cance. J Surg Oncol 2005;91(2):131-3.
4. Johnson RE, Sigman JD, Funk GF, et al. Quanti cation of surgical margin shrinkage in the oral cavity. Head Neck 1997;19(4):281-6.
5. McMahon JD, Devine JC, Hetherington J, et al. Involved surgical margins in oral and oropharyngeal carcinoma—an anatomical problem? Br J Oral Maxillofac Surg 2011;49(3):172-5.
6. Wong LS, McMahon J, Devine J, et al. In uence of close resection margins on local recurrence and disease-speci c survival in oral and oropharyngeal carcinoma. Br J Oral Maxillofac Surg 2012;50(2):102-8.
7. Sutton DN, Brown JS, Rogers SN, et al. e prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2003;32(1):30-4.
8. Brown JS, Shaw RJ, Bekiroglu F, Rogers SN. Systematic review of the current evidence in the use of postoperative radiotherapy for oral squamous cell carcinoma. Br J Maxillofac Surg 2012;50(6):481-9.
9. Brown JS, Blackburn TK, Woolgar JA, et al. A comparison of outcomes for patients with oral squamous cell carcinoma at intermediate risk of recurrence treated by surgery alone or with post-operative radiotherapy. Oral Oncol 2007;43(8):764-73.

10. Bekiroglu F, Ghazali N, Laycock R, et al. Adjuvant radiotherapy and health-related quality of life of patients at intermediate risk of recurrence following primary surgery for oral squamous cell carcinoma. Oral Oncol 2011;47(10):967-73.
11. Lawaetz M, Homøe P. Risk factors for and consequences of inadequate surgical margins in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;188(6):642-6.
12. Binahmed A, Nason RW, Abdoh AA. e clinical signi cance of the positive surgical margin in oral cancer. Oral Oncol 2007;43(8):780-4.
13. Ch’ng S, Corbutt-Burns S, Stanton N, et al. Close margin alone does not warrant postoperative adjuvant radiotherapy in oral squamous cell carcinoma. Cancer 2013;119(13):2427-37.
14. Blackburn TK, Bakhtawar S, Brown JS, et al. A questionnaire survey of current UK practice for adjuvant radiotherapy following surgery for oral and oropharyngeal squamous cell carcinoma. Oral Oncol 2007;43(2):143-9.
15. Rogers SN, Brown JS, Woolgar JA, et al. Survival following primary surgery for oral cancer. Oral Oncol 2009;45(3):201-11.
POSITIVITY RATES OF IN VITRO INHALANT/RESPIRATORY AND FOOD ALLERGY TESTS IN THE NORTHERN MIDWESTERN UNITED STATES
Continued from page 304
Although the reference laboratory we used is in the upper Midwest region of the United States, the large number of allergens and the signi cant overlap among regions might make seasonal data applicable to most regions throughout the United States and the perennial allergens applicable more broadly.
In conclusion, wide variability in positive in vitro allergy tests exists, and the likelihood of a positive result in screening panels can be estimated. e most common positive antigens in our study were dog dander (24%), cat dander (23%), dust mites (23% each for D farinae and D pteronyssinus), and June grass (21%).
e most common positive food allergen tests were for milk (18%), peanut (17%), wheat (16%), and egg white (15%). e positivity rates for shell sh ranged from 4 to 9%, and the rates for sh were low (3 to 5%). Evaluating these rates will help further identify the most and least common allergens, and there is potential to use the results to identify opportunities to cost-e ectively re ne allergy screening panels.
References
1. McCrory DC, Williams JW, Dolor RJ, et al. Management of allergic rhinitis in the working-age population. Evid Rep Technol Assess (Summ) 2003;(67):1-4.
2. Andersson M. Emerging treatments for allergic rhinitis. Expert Opin Emerg Drugs 2003;8(1):63-9.
3. Seidman MD, Gurgel RK, Lin SY, et al; Guideline Otolaryngology Development Group, AAO–HNSF. Clinical Practice Guideline: Allergic Rhinitis. Otolaryngol Head Neck Surg 2015;152:(1 Suppl):S1-43.
4. Bousquet PJ, Burbach G, Heinzerling LM, et al. GA2LEN skin test study III: Minimum battery of test inhalant allergens needed in epidemiological studies in patients. Allergy 2009;64(11):1656-62.


5. Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004;113(5):805-19; quiz 820.
6. Wise SK, Lin SY, Toskala E, et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int Forum Allergy Rhinol 2018;8(2):108-352.
7. Gergen PJ, Turkeltaub PC, Kovar MG. e prevalence of allergic skin test reactivity to eight common aeroallergens in the U.S. population: Results from the second National Health and Nutrition Examination Survey. J Allergy Clin Immunol 1987;80(5):669-79.
8. Arbes SJ Jr., Gergen PJ, Elliott L, Zeldin DC. Prevalence of positive skin test responses to 10 common allergens in the US population: Results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol 2005;116(2):377-83.
9. Kaufman HW, Odeh MA, Bost WH, Ragothaman P. Allergies across America. Health Trends Allergy Report 2010. www. QuestDiagnostics.com/HealthTrends, 1-40.
10. Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med 2001;344(1):30-7.
11. Osborne NJ, Koplin JJ, Martin PE, et al; HealthNuts Investigators. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol 2011;127(3):668-76.e1-2.
322 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018
WELINDER, LAWAETZ, DINES, HOMØE
Continuing to lead the future.
Advanced visualization at your fi ngertips.

In 1953, ZEISS invented OPMI® 1, the progenitor of modern surgical microscopes. Since then ZEISS has continued to develop and innovate visualization solutions. Your visualization system needs to be fully integrated within today’s high tech landscape. It should be able to record and connect, so others can see what you see, and experience what you experience. In an increasingly digital world, data should be intuitive, seamlessly accessible, transferrable and secure, so you can provide even better patient care. We understand.
Find out more about how ZEISS invented the beginnings and is continuing to lead the future at: www.zeiss.com/we-understand/ENT







SUR.10361 EN_30_030_0091I
Outcomes of reapplication to otolaryngology residency: A prospective cohort study
Colin Fuller, MD, MS; J. Kenneth Byrd, MD; Michael Groves, MD
Abstract
Although the eld of otolaryngology has experienced a decline in the number of applicants to our residency programs, otolaryngology remains a highly competitive eld with an extremely strong applicant pool. Many highly quali ed candidates cannot obtain a position in our eld each year, and many of these candidates choose to reapply the next year. Data are lacking regarding reapplicants' success rate and the best gap year employment and training options for these reapplicants. Reapplicants were studied prospectively via a two-stage survey during the 2014-2015 and 2015-2016 application cycles. Success rates for the overall group were compared to those from published data, and success rates between subgroups were also compared. First-time reapplicants in the study performed extremely well. eir match rate (19/22) was not signi cantly di erent from that of traditional otolaryngology applicants (551/619, p = 0.73) and was signi cantly higher than that of nontraditional applicants not in our cohort (23/62, p < 0.001). No signi cant di erence was found between applicants by employment/training activities, with both researchers (11/12) and surgical interns (8/10, p = 0.57) performing well. Predictors of reapplicant success could not be assessed because only 3 reapplicants in the cohort were unsuccessful. First-time otolaryngology reapplicants remain a highly competitive group of applicants to our eld, regardless of employment/training activities undertaken a er graduating medical school.
Introduction
By any measure, the eld of otolaryngology–head and neck surgery is one of the most competitive specialties in the United States. For example, in 2016, the average United States Medical Licensing Exam (USMLE) Step 1
From the Department of Otolaryngology–Head and Neck Surgery, Augusta University, Augusta, Ga.
Corresponding author: Colin Fuller, MD, MS, Department of Otolaryngology–Head and Neck Surgery, 1120 Fi eenth St., Augusta, GA 30909. Email: cfuller@augusta.edu
Funding/support: is study was funded by the authors’ institution through the senior author’s (M.G.) research fund.
score for matched applicants to otolaryngology programs was 248, higher than every eld except dermatology (249) and integrated plastic surgery (250).1 In years past, high levels of competition have led to many highly quali ed applicants going “unmatched” but still desiring to pursue a career in the specialty.
e rst di cult decision these applicants face is whether it is worthwhile to reapply to the specialty. Unfortunately, data are lacking regarding their odds of a successful match as a “reapplicant.” In addition to their yearly report of applicant totals and match rates, the National Resident Matching Program (NRMP) publishes additional competitiveness data for matched and unmatched applicants in most specialties, including USMLE scores, Alpha Omega Alpha Honor Medical Society (AOA) standing, number of publications, and other measures, in their “Charting Outcomes in the Match” publication every 2 to 3 years.
Before 2016, the data were reported for both United States allopathic senior medical students (traditional applicants) and the heterogeneous group “independent applicants,” which includes reapplicants, foreign medical graduates, osteopathic medical students, those applying to specialties for a second residency, and others. For 2016, even the independent applicant data were not available. e otolaryngology match rate for reapplicant U.S. allopathic graduates is unknown and/or unreported but may be higher or lower than for the entire independent group.
During the “gap year” between application cycles, motivated reapplicants will have several months to improve their applications, and they are o ered several options that may help them to do so. ey may enter a training program in a closely related eld (usually general surgery), or they may engage in clinical or basic science research within a research-oriented otolaryngology department. Each option has advantages, making it attractive for di erent reasons.
A research fellowship allows candidates to improve their research credentials and obtain letters of reference from well-published faculty members, while a year of residency training in a related eld will allow the candidate to exhibit clinical decision-making skills similar
324 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 ORIGINAL ARTICLE
to what is demanded of an otolaryngology resident. Research fellowships may or may not o er the candidate a competitive salary. A surgery resident may have the option to continue in general surgical training at a postgraduate year-2 (PGY-2) level if the reapplication to otolaryngology is unsuccessful. e challenge of deciding whether and how to reapply is o en compounded by the mere hours to make this profoundly impactful decision during the NRMP’s Supplemental O er and Acceptance Program (SOAP) (previously known as “the scramble”). ere are few data regarding which option, if any, o ers the best chance for reapplicants to obtain a position in an otolaryngology residency, and no prospective data on the topic exist. erefore, we undertook this prospective study to help answer this question and to obtain prospective data on the match rate for otolaryngology reapplicants.
Participants and methods
is prospective cohort study was conducted with IRB approval from August 2015 through April 2017, with data collection performed by survey in two stages. e rst stage of the survey obtained demographic data and information regarding the reapplicants’ competitiveness within the applicant pool, including under-represented minority status, USMLE score, AOA status, number of publications authored, and the number of interviews obtained during the previous year’s application cycle, as well as whether they were conducting a clinical internship or research during their gap year (herea er referred to as interns or researchers).
Reapplicants also provided an institutional email address that allowed the study team to verify their association with a teaching hospital. is email address was used in late March 2017 to provide subjects with a linked second-stage survey that asked them whether their reapplication was successful and whether they matched to the institution at which they were performing their gap year activity.
Subjects were recruited initially from two sources. Applicants to our institution’s residency program for the 2014-2015 cycle were reviewed on the NRMP database to determine whether they were successful in obtaining an otolaryngology position. ose who were unsuccessful were sent an email invitation to join the survey study.
e second source of subjects was a popular online message board frequented by otolaryngology applicants to discuss the application process (www.otomatch.com). With permission from the website administrator, a message was posted describing the study and inviting reapplicants to participate. For the second year of the study, we used the message board only and reserved the email for additional recruitment, but it was not needed. e study enrollment period each year lasted 1 month, beginning in the early fall before the start of the residency interview season for most programs.
U.S. allopathic graduates who had applied unsuccessfully once to otolaryngology residencies were included in the study. Exclusion criteria included osteopathic graduates, international graduates, military applicants, applicants for whom otolaryngology was not their rst choice specialty, and those planning to couples match.
Data obtained from the study were held in strict con dence from faculty, who could use it to identify applicants in the active applicant pool. e data were collected by the corresponding author, a resident, who did not participate in applicant interviews or the ranking of applicants. Although baseline demographic data including sex and under-represented minority status were obtained, these data were intentionally limited to reassure applicants that anonymity would be preserved.
Instead of asking for an exact USMLE score, applicants were asked to specify into which ve-point range their USMLE scores fell to further de-identify the data. To incentivize participation, a $200 gi certi cate to a popular online store (Amazon.com) was awarded to the winner of a random drawing at the end of each year of the study. e winner was noti ed via email.
A er the second year of the study, a post hoc analysis was made to compare reapplicants in our study with the match rate of all traditional and independent applicants during the years of the study. When the match rate for our cohort was compared to nontraditional applicants, we subtracted our cohort from the nontraditional totals to avoid duplication of these applicants into both pools.
Comparisons between groups were made with the Student t test for continuous variables, the chi-square test for dichotomous variables, and the Mann-Whitney U test for the ordinal variable of USMLE score ranges. ese computations were performed using free-to-use online so ware from graphpad.com and socscistatistics.com.
Results
Twenty-three reapplicants satisfying all inclusion criteria agreed to be surveyed over both years of the study. A response rate was not calculable from data published by the NRMP. However, 100% of applicants who completed our rst survey also completed the second survey.
No statistically signi cant di erence was found between interns and researchers regarding underrepresented minority status, USMLE scores, or number of interviews in the prior application cycle. A trend toward higher numbers of research projects for researchers was observed, although no comparison demonstrated statistical signi cance. (table, gure)
e number of researchers and interns in our cohort was nearly evenly split, with 12 researchers and 11 interns participating. Nineteen applicants successfully obtained an otolaryngology residency position: 11 of 12 (91.7%) researchers and 8 of 11 (72.7%) interns. All successful
Volume 97, Number 9 www.entjournal.com 325 OUTCOMESOFREAPPLICATIONTOOTOLARYNGOLOGYRESIDENCY: A PROSPECTIVECOHORTSTUDY
*Numbers are expressed as totals or means.
†p
Key:
reapplicants obtained their positions in the main residency match; none obtained positions outside the match or for PGY-2 positions. One of the interns who did not match into otolaryngology changed course and decided to apply to a di erent specialty, bringing the match rate to 8 of 10 (80%) for interns and 19 of 22 (86.4%) overall. e di erence in match rate between researchers and interns was not statistically signi cant (p = 0.57). With only three reapplicants failing to match, we did not perform analyses of predictors of match success. Both unmatched interns were able to continue in their training at a PGY-2 level; the unmatched researcher began at a PGY-1 level. ree of 11 (27.3%) matched researchers successfully matched to the program where they were completing their research gap year activities and 8 did not. Two of 8 (25.0%) matched interns matched to the institution where they had been working the past year and 6 did not. Researchers and interns matched with near equal rates to their gap year institution (p = 1.00).


In the 2015-16 application cycle, 272 of 314 (86.6%) traditional applicants successfully matched into otolaryngology. In the 2016-2017 cycle, 279 of 305 (91.5%) tra-
ditional applicants successfully matched, yielding a total of 551 of 619 (89.0%). ere was no signi cant di erence between this match rate and that of our reapplicant cohort (p = 0.73).
In the 2015-16 application cycle, 56 nontraditional applicants applied to otolaryngology in the main residency match, and 30 of them matched. In the 2016-17 cycle, 28 nontraditional applicants applied to otolaryngology in the main residency match, and 12 matched, yielding a 2-year nontraditional match rate of 42 of 84 (50.0%). Subtracting our surveyed applicant pool (19/22) from this group results in a match rate of 37.1% (23/62). e di erence between the two match rates was signi cant (p < 0.001).
Unfortunately, since the NRMP does not publish the number of U.S. allopathic graduates that apply to each specialty each year, determining the overall response rate to our survey invitation is not possible. However, the number of U.S. allopathic graduates that are successful is published yearly. Our study featured 19 of the 29 U.S. allopathic graduates that successfully matched during the 2 years of the study.2
Discussion
is study is the rst prospective study of otolaryngology reapplicants that examines two di cult questions they face:
(1) What are the odds of matching as a reapplicant, and (2) is an reapplicant in better position to match a er completing a year of research versus a year of surgical training.
Our study indicates that highly motivated reapplicants likely have an excellent chance to obtain a residency position in our specialty, regardless of whether they pursued research or internships. e match rate for reapplicants in our cohort was even slightly higher than the match rate for traditional applicants during the years we conducted our study.
Before this study, the only available data reported the success rate of nontraditional applicants, which is a heterogeneous group that also includes international medical graduates and osteopathic medical students.
326 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 FULLER, BYRD, GROVES
IR p Value† Total no. 1112 No. males 680.68 Under-represented minorities310.32 AOA members 561.00 First-author papers accepted0.551.420.12 Non– rst-author papers accepted0.733.670.09 First-author presentations1.272.420.19 Previous applications 67.9164.250.58 Previous interviews 9.7310.330.84
Table. Baseline characteristics of interns and researchers*
Value of chi-square or Student t test, where appropriate.
I = interns; R = residents; AOA = Alpha Omega Alpha Honor Society.
Figure. A: Analysis of United States Medical Licensing Exam (USMLE) Step 1 scores for interns and researchers indicated no di erence in scores between the two groups. B: Analysis of USMLE Step 2 clinical knowledge scores for interns and researchers indicated no di erence in scores between the two groups. (p = p value of Mann-Whitney U test comparison between groups.)
Henry Ford Health System, one of the nation’s largest group practices, is actively recruiting Board certified/ Board eligible academic Otoloaryngologists in the following areas:

· Head and Neck Surgical Oncology
· Sleep Medicine
· Rhinology
· General Otolaryngology
The department has fellowship trained specialists in the areas of laryngology, neuro-otology, head and neck cancer, head and neck endocrine, rhinology, pediatric otolaryngology, facial plastic and reconstructive surgery and sleep medicine. The department supports a very competitive otolaryngology residency program with an outstanding national reputation for clinical educational and research programs, and has earned a spot in US News & World Report’s Top Hospital in ENT for the 2nd year in a row.
We offer a competitive salary with an incentive opportunity and a full benefit package including health, dental, moving costs, licensure reimbursement, four retirement savings plans, paid vacation and CME time and allowance. Clinical faculty academic appointments are available through Wayne State University School of Medicine.
Contact: Current Curriculum Vitae (CV) should be sent to:
Kathleen Yaremchuk, MD, MSA, Chair, Department of
Otolaryngology
Head and Neck Surgery
c/o
Amber Arnold
Aarnold2@hfhs.org or fax (313) 874-7989
H ENRY F ORD M EDICAL G ROUP
Our data show that rst-time allopathic reapplicants who responded to the survey had a signi cantly better match rate than the pool of other nontraditional applicants. Although the comparison likely still included several rst-time allopathic reapplicants that did not participate in our study, the di erence in success between all rst-time allopathic reapplicants and all remaining nontraditional applicants may be even higher than suggested by this study.
A 2013 study surveyed orthopedics residency program directors and chairpersons regarding reapplicants, and the results indicate that university-a liated programs favored research years for reapplicants, whereas community-based programs did not.3 However, no data on the actual performance of reapplicants were compiled.
A 2011 cross-sectional study of dermatology reapplicants demonstrated several factors that were associated with improved match outcomes.4 Unsurprisingly, these included higher USMLE scores (including Steps 1, 2, and 3), AOA standing, more listed honors, a letter of recommendation from an attending dermatologist, and more research experience. General medicine internships were better for gap year activities than other internships. Overall reapplicant success was low (20.8%).
A retrospective study from 2016 evaluated the success rate of orthopedics reapplicants over the previous 20 years by tracking NRMP data and data from LinkedIn and professional websites for all applicants to the authors’ institution.5 Most (>90%) reapplicants pursued an internship, and there was no signi cant di erence in reapplicant success based on gap year activities. Overall success was 40 to 50%.
Given these published statistics, we were surprised to nd that our data showed such success for our reapplicant cohort. Respondents to our study might have self-selected as highly motivated candidates who felt so con dent in their chances that they did not feel intimidated by sharing their data with an academic institution, which could have arti cially in ated our apparent success rate via survey sampling bias. Of the 2 years, our survey reached 22 of 84 nontraditional applicants (26.2%); however, it is not possible to determine the survey response rate among rst-time allopathic applicants based on available data.
This low-powered study showed no significant di erence between the success rate of researchers and interns. Researchers had an increased number of research activities as of early autumn, when study enrollment occurred, but this trend was not signi cant. Ascertaining whether this activity re ected old research activities undertaken before the failed match cycle or if the researchers were able to publish additional projects within the 6- or 7-month time frame between their failed match and study enrollment is not possible.
Application dynamics continue to shi year to year. e 2016-17 application cycle was one of the kindest in many years to otolaryngology applicants. e number of applicants was the lowest in years; the match rate for traditional applicants was 92.1% and the overall match rate was 87.9%.2 In 2013, the match rate for traditional applicants was just 71.3% and the overall match rate was 65.6%,6 both all-time lows. e recent, higher match rate for traditional applicants probably results in fewer and lower-quality reapplicants the next year. However, the average USMLE Step 1 score for otolaryngology applicants has risen each year, and this trend suggests that the quality of reapplicants should continually improve as the overall applicant pool becomes stronger by this measure.
Given both these trends, it is reasonable to consider that the applicant pool is shrinking because the “bottom” of the applicant pool has been eliminated from the specialty, as was suggested in a pair of publications in 2017.7,8 e data we present should prove encouraging to those candidates who may be considered less competitive: Even if an applicant fails to match on the rst attempt, reapplication remains viable for motivated applicants who are dedicated to the eld of otolaryngology.
Acknowledgments
We wish to acknowledge the otomatch.com community for participating in this study, and we acknowledge administrator Sam Reyes for his diligence in vetting applicant survey studies.
References
1. Charting outcomes in the match for U.S. allopathic seniors, 2016, in National Resident Matching Program. 2016. Washington, D.C. https://www.nrmp.org/wp-content/uploads/2016/09/ChartingOutcomes-US-Allopathic-Seniors-2016.pdf.
2. National resident matching program. Results and data: 2017 Main Residency Match. National Resident Matching Program. 2017. Washington, D.C. https://www.nrmp.org/wp-content/ uploads/2017/06/Main-Match-Results-and-Data-2017.pdf.
3. Amin NH, Jakoi AM, Cerynik DL, et al. How should unmatched orthopaedic surgery applicants proceed? Clin Orthop Relat Res 2013;471(2):672-9.
4. Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol 2011;147(2):196-202.
5. Rivero S, Ippolito J, Martinez M, et al. Analysis of unmatched orthopaedic residency applicants: Options a er the match. J Grad Med Educ 2016;8(1):91-5.
6. National resident matching program, results and data: 2013 Main Residency Match. National Resident Matching Program. 2013. Washington, D.C. http://www.nrmp.org/wp-content/ uploads/2013/08/resultsanddata2013.pdf.
7. Bowe SN, Schmalbach CE, Laury AM. e state of the otolaryngology match: A review of applicant trends, “impossible” quali cations, and implications. Otolaryngol Head Neck Surg 2017;156(6):985-90.
8. Schmalbach CE. 2017: e year otolaryngology had to “scramble.” Otolaryngol Head Neck Surg 2017;156(6):975.
328 www.entjournal.com ENT-Ear, Nose & Throat Journal September 2018 FULLER, BYRD, GROVES










































SMART is Better TRANSFORM YOUR PRACTICE WITH ARTIFICIAL INTELLIGENCE Our Advantage SMART Practice™ suite of All-in-One Solutions use real-time data from your clinic to optimize efficiency, improve outcomes, and increase revenue. EHR | PM | RCM | ASC ANALYTICS | PATIENT ENGAGEMENT TELEHEALTH | 2015 ONC CERTIFIED ENT SPECIFIC EHR & PM Leader for 33 Years www.compulinkadvantage.com/ENTEHR | 800.456.4522 AAO-HNSF 2018 Annual meeting & oto experience. Booth #3025
HELPING PEOPLE GET MORE RESTFUL SLEEP.


HELPING PEOPLE PREVENT SWIMMER’S EAR.

HELPING PEOPLE PROTECT THEIR HEARING.
HELPING PEOPLE TRAVEL IN COMFORT.

Versatility is the key to our success.

Mack’s® Pillow Soft® Silicone Putty Earplugs

The #1 selling earplugs, year after year, from the #1 doctor recommended earplug brand.
available at
VisitusatAAO-HNSF Booth#2224


































































































































































































 Eiji Yanagisawa, MD, FACS; Dewey A. Christmas, MD; Joseph P. Mirante, MD, MBA, FACS
Eiji Yanagisawa, MD, FACS; Dewey A. Christmas, MD; Joseph P. Mirante, MD, MBA, FACS




















































 Chetan Y. Sa , MD; Anthony P. Sclafani, MD
Chetan Y. Sa , MD; Anthony P. Sclafani, MD







































































































































































































