
MIDDLE EAST & AFRICA

COUNTRY FOCUS: Morocco
DISEASE FOCUS: Malaria
TOPICAL FOCUS: Complementary and Alternative Medicine
ASSOCIATION FOCUS: Biochemistry and Biotechnology Professionals Society of Kenya

AUGUST 6-8












COUNTRY FOCUS: Morocco
DISEASE FOCUS: Malaria
TOPICAL FOCUS: Complementary and Alternative Medicine
ASSOCIATION FOCUS: Biochemistry and Biotechnology Professionals Society of Kenya

AUGUST 6-8









3 | Issue No.11 | Apr-Jun 2024
FOUNDER & PUBLISHER
Francis Juma
SENIOR EDITOR
Alphonse Okoth
ASSISTANT LEAD EDITOR
Jackie Muinde
EDITOR
Vincent Moranga
BUSINESS DEVELOPMENT DIRECTOR
Virginia Nyoro
BUSINESS DEVELOPMENT ASSOCIATE
Elly Okutoyi
HEAD OF DESIGN
Clare Ngode
DESIGN
Yvonne Njambi
ACCOUNTS
PUBLISHED BY: FW Africa
P.O. Box 1874-00621, Nairobi Kenya
Tel: +254 725 34 39 32
Email: info@fwafrica.net
Company Website: www.fwafrica.net
Welcome to issue 11 of Healthcare Middle East & Africa Magazine!
Sub-Saharan Africa faces a complex landscape of health challenges characterized by high rates of infectious diseases, emerging non-communicable diseases, and limited healthcare infrastructure. The region remains the epicenter of the HIV/ AIDS epidemic, accounting for the majority of global cases. Management strategies include widespread antiretroviral therapy (ART) programs, prevention of mother-tochild transmission initiatives, and public health campaigns promoting safe practices and testing. These efforts have significantly reduced the transmission rates and improved the quality of life for many individuals living with HIV/AIDS.
Malaria, a major public health concern, has seen significant progress in Sub-Saharan Africa over the last two years. In this issue, we highlight the region’s efforts to manage the disease, focusing on the strides made and the areas that still require attention. Initiatives such as insecticide-treated bed nets, indoor residual spraying, and the introduction of new antimalarial drugs have played crucial roles in reducing malaria morbidity and mortality rates. However, challenges such as drug resistance, funding constraints, and healthcare access continue to impede further progress.
patients that they have a range of choices to suit their individual needs and preferences.
Beyond disease management, we spotlight Medland Hospital, the first private facility to introduce a full-time operational cardiovascular surgery department in Zambia. Opened in June 2019, this 72-bed facility provides access to comprehensive and individualized patient care in a friendly, safe, and comfortable environment of international standards based on principles of collaboration, compassion, and innovation. The hospital’s state-of-the-art technology and highly skilled medical professionals are transforming cardiovascular care in the region, offering procedures and treatments that were previously unavailable locally.
HealthCare Africa (ISSN 2307-3535) is published 4 times a year by FW Africa. Reproduction of the whole or any part of the contents without written permission from the editor is prohibited. All information is published in good faith. While care is taken to prevent inaccuracies, the publishers accept no liability for any errors or omissions or for the consequences of any action taken on the basis of information published.
Jonah Sambai MIDDLE
Our exploration extends beyond disease management to the significant rise in the adoption of alternative and interactive medicine globally. This trend, which emphasizes treating the whole person—mind, body, and spirit, is gaining traction worldwide, including in regions like Sub-Saharan Africa. Practices such as traditional herbal medicine, acupuncture, and homeopathy are being integrated with conventional treatments, offering more personalized and effective healthcare solutions. This diversity of options reassures


Additionally, learn about Morocco’s efforts to achieve universal health coverage, a program that aims to provide health coverage to vulnerable populations, including the poor and those with chronic diseases. Morocco’s ambitious plan includes expanding access to healthcare services, improving the quality of care, and reducing out-of-pocket expenses for patients.
Accompanying these features and articles is the latest news from Africa and globally, along with the newest devices and innovations driving the healthcare sector. From breakthroughs in medical technology to policy changes and healthcare reforms, we bring you comprehensive coverage of the developments shaping the future of healthcare in the region and beyond.
Enjoy the read!
Alphonse Okoth Senior Editor HCMEA





34
ASSOCIATION FOCUS: BBPSK
Empowering Scientific Collaboration for Researchers in Biochemistry


38
TOPICAL FOCUS: COMPLEMENTARY AND ALTERNATIVE MEDICINES
Navigating the Shift: Exploring the Rise of Complementary and Alternative Medicines

COUNTRY FOCUS: MOROCCO
44 Morocco : Chasing the UHC dream
50
DISEASE FOCUS: MALARIA
Combatting Malaria in Africa: Innovations, Challenges, and Hope for the Future
Mirador secures
US$400M to revolutionize precision medicine for immune diseases
USA - Mirador Therapeutics Inc., a biotechnology startup founded by Mark C. McKenna and led by several former executives of Prometheus Biosciences, has been launched with the goal of revolutionizing precision medicine for immune-mediated inflammatory and fibrotic diseases.
Following this launch, Mirador has raised more than US$400 million in financing led by ARCH Venture Partners, with early investments from OrbiMed and Fairmount.
Other investors include Fidelity Management & Research Company, Point72, Farallon Capital Management, Boxer Capital, TCGX, Invus, Logos Capital, Moore Strategic Ventures, Blue Owl Healthcare Opportunities, Sanofi Ventures, Woodline Partners LP, Venrock Healthcare Capital Partners, RTW Investments, and Alexandria Venture Investments.
This is the largest private financing achieved by a biotech firm in 2024, as well as one of the sector’s most significant Series A rounds in recent years according to BioPharma Dive.
Mirador will focus on generating first-in-class or best-in-class precision medicines by expediting development with Mirador360, its patented precision development engine that takes use of recent advances in human genetics and data science.

HOSPITALS
FDA approves Duvyza for Duchenne muscular dystrophy

USA - The Food and Drug Administration has approved a groundbreaking drug to treat Duchenne muscular dystrophy, marking a significant milestone in the medical landscape.
Duvyza, developed by Italian pharmaceutical company Italfarmaco, has will now be available for use in all Duchenne patients aged six and above, irrespective of their disease’s genetic profile.
INVESTMENT
The drug will be distributed in the U.S. by Italfarmaco’s subsidiary, ITF Therapeutics.
Duvyza represents a revolutionary advancement as the first non-steroidal treatment for Duchenne muscular dystrophy.
Administered in pill form, it is designed to mitigate inflammation and muscle degeneration. Clinical trials have demonstrated statistically significant improvements in motor function when compared to a placebo.
Operating as an HDAC inhibitor, Duvyza targets enzyme activity associated with muscle damage, thereby slowing the deterioration of muscle fibers characteristic of Duchenne muscular dystrophy.
– The African Union Development Agency-NEPAD (AUDANEPAD) has received €10 million (US$10.67m).
The investment was from the European Commission and €4 million (US$4.27m) from Belgium for the African Medicines Regulatory Harmonisation (AMRH) initiative to continue advancing the establishment of African Medicines Agency (AMA).
The European Commission, Belgian Presidency of the Council of the European Union, and European Medicines Agency (EMA) came together to fortify their support to the African Medicines Agency (AMA) during the high-level event on the European Union (EU) – African Union (AU) partnership on Global Health for equitable access.
This support for regulatory strengthening in Africa is part of the Team Europe Initiative on
manufacturing and access to vaccines, medicines and health technologies in Africa (MAV+).
The contributions to AMRH complement the recently announced cooperation with the European Medicines Agency to support the establishment of AMA.

NETHERLANDS - Philips Holding USA and Philips Respironics, subsidiaries of Royal Philips, have reached a definitive agreement with the US Department of Justice (DOJ) and US Food and Drug Administration (FDA).
This deal, announced on January 29, 2024, follows the FDA’s inspection of Philips Respironics’ Murrysville facility in 2021, which prompted conversations
between the DOJ, representing the FDA, and Royal Philips in July 2022.
The agreement focuses on Philips Respironics’ US businesses, which include manufacturing sites in Murrysville and New Kensington, a service facility in Mount Pleasant, and the Respironics headquarters in Pittsburgh, Pennsylvania.
The deal addresses Philips Respironics’ business operations in the United States, including its production plants in Murrysville and New Kensington, service center in Mount Pleasant, and Respironics headquarters in Pittsburgh, Pennsylvania.
The consent decree outlines a systematic plan of action, milestones, and deliverables designed to ensure regulatory compliance.
SOUTH AFRICA - The South African Medical Research Council (SAMRC) and Thermo Fisher Scientific Inc., an American supplier of life sciences solutions and biotechnology services, have inked a Memorandum of Agreement (MOA).
The MOA is to collaborate on establishing a Centre of Excellence and training program in the field of molecular biology and life sciences in South Africa.
The primary objective of this collaboration is to tackle the challenges faced by the African continent in enhancing the skills of graduates and laboratory personnel, particularly in practical applications and exposure to cutting-edge technologies in molecular biology and life sciences.
The Centre of Excellence, slated for launch in September 2024, will be

headquartered in Pretoria.
It will focus on delivering accredited learning modules and training programs covering general laboratory practice, theoretical science, bioinformatics, laboratory management, finance processes, and soft skills development.
Both entities will collaborate on developing a certified training program curriculum, leveraging Thermo Fisher’s technical expertise in the sector.

TANZANIA - The Radiological Society of North America (RSNA) and GE Healthcare have teamed up to enhance breast cancer detection and treatment in Tanzania.
The partnership aims to equip radiologists at Muhimbili National Hospital (MNH), part of the Muhimbili University of Health and Sciences (MUHAS), with cutting-edge mammography technology and comprehensive training.
This announcement was made coinciding with the unveiling of the breast center at Muhimbili National Hospital, where GE Healthcare and RSNA will celebrate this collaborative effort.
Muhimbili National Hospital, the largest public hospital in Tanzania, will be the recipient of the latest mammography technology as part of this initiative.
The objective is to empower clinicians to enhance the detection, diagnosis, and treatment of breast cancer across the country, ultimately aiming to lower Tanzania’s alarming 50% breast cancer mortality rate through early detection and expanded clinical education.
As part of the collaboration, GE Healthcare will install the Pristina Mammography Suite at MUHAS, incorporating state-of-the-art technology such as 2D and 3D digital breast tomosynthesis and Contrast Enhanced Mammography (CEM).










EGYPT -The Cleopatra Hospitals Group has announced that it intends to invest
US$41 million over the next 18 months as part of its profit expectations strategy before interest, taxes, and depreciation over the next two years.
The 40-bed first phase will include a radiology centre, an emergency department, operating rooms, and a pharmacy.
The second phase will expand the capacity to 254 beds, he said on the sidelines of a press conference to announce the Group’s partnership with Novartis Egypt to support patients with arteriosclerosis.
The 4-storey hospital is being developed over an area of 40,000 square metres and Phase 1 is scheduled to open in August 2025.
KENYA – Ilara Health, the healthtech company digitizing and consolidating highly fragmented primary care in Kenya, has announced the close of a US$4.2 million pre-series A round of equity and debt.
The funding round was led by DOB Equity, and saw follow-on equity investment from AAIC INVESTMENT, Angaza Capital, Black Pearl Investments, Perivoli Innovations, as well as debt investment from Alphamundi and Kiva Capital.
New to the round are Philips Foundation and Boehringer Ingelheim.
The latest capital injection will be used to scale the firm’s tech-enabled primary care model across Kenya before expanding to other regions.
The investment will facilitate Ilara Health’s focus for the next 12 months which is to achieve significant topline growth, keep growing the already large partner clinics network, and incorporate the next phase of growth into its model, which includes the launch of the
employee health services through B2B health & occupational services, initially in select locations and later throughout the country.
This is to further strengthen the Ilara Health branded clinics and achieve revenue growth, proving the path to profitability as the operating base remains broadly consistent.
THE LATEST CAPITAL INJECTION WILL BE USED TO SCALE THE FIRM’S TECH-ENABLED
The company will also be taking up the expansion of Cleopatra October, located in Western Cairo and adding more outpatient clinics soon.
The primary areas of concentration for this investment include the completion of the initial and secondary phases of the Sky Hospital project situated in East Cairo, the expansion of Cleopatra October facilities located in West Cairo, and the enlargement of outpatient clinic centers.
The investment figure excludes any potential expansions beyond Cairo.
The company has confirmed that the capital expenditures will be entirely funded through internal sources and existing bank facilities.
INDIA - Metropolis Healthcare, an established and well-known Indian diagnostics company, has opened a diagnostic center in Malegaon, Maharashtra.
The 1,400-square-foot facility can process about 3,000 samples each month while providing quick turnaround times and high-quality reports.
Dadaji Bhuse Saheb, Guardian Minister of Palghar district, and Dr. Sachin Thakare, President of the Indian Medical Association in Malegaon, attended the ceremony.
The new lab is located on Camp Road, near SBI Bank and the New Jain Mandir in Matrusmruti, Malegaon. Metropolis provides complete tests and reports at moderate costs.
This facility will provide an important addition to Malegaon’s healthcare system, giving nearby people access to high-quality diagnostic services, cutting-edge technology, and experienced medical experts.

KENYA -The Pharmacy and Poisons Board (PPB), Kenya’s drug regulator, has announced the recall of Benylin Pediatric 100ml cough syrup manufactured by Johnson & Johnson {Pty}, South Africa.
This action follows information received by PPB regarding the recall of Benylin Pediatric cough syrup batch No. 329304 by the National Agency for Food and Drug Administration and
Control (NAFDAC) of Nigeria.
The affected batch of Benylin Pediatric 100ml syrup, manufactured in May 2021 with an expiry date of April 2024, is intended to relieve cough, hay fever, and allergies in pediatric patients.
NAFDAC’s decision for this recall was based on quality concerns arising from an unacceptable high level of diethylene glycol detected through laboratory analysis conducted by NAFDAC.
Upon receipt of this information, PPB has immediately commenced investigations, initiating a rapid response that includes sampling batches of Benylin Pediatric 100ml syrup within shelf life for screening levels of ethylene glycol and diethylene glycol.
PPB has advised all pharmaceutical outlets, healthcare facilities, healthcare workers, and the public to immediately quarantine the product and cease distribution, sale, issuing, or use.
INDIA - Sun Pharmaceutical, based in India, has announced that the Australian Therapeutic Goods Administration (TGA) has approved its Winlevi (clascoterone) cream 1% for treating acne vulgaris in patients aged 12 and older.
The cream’s active ingredient, clascoterone, operates through a mechanism that competes with androgens, particularly dihydrotestosterone (DHT), for binding to the androgen receptors within the sebaceous gland and hair follicles.
The skin of patients with acne vulgaris typically produces higher levels of androgens like testosterone and DHT, leading to increased sebum production and inflammation.
Winlevi’s ability to bind to androgen receptors, competing with DHT, inhibits
this process, thereby reducing sebum production and inflammation.
Sun Pharma, the world’s fourthlargest specialty generics company, has a portfolio spanning specialty, generics, and consumer healthcare products.
MDaaS
led

NIGERIA – MDaaS Global, a health tech company and operator of BeaconHealth Diagnostics, has raised a US$3 million Pre-Series A round of financing to further develop the company’s proprietary technology platform BeaconOS.
The round was co-led by Aruwa Capital Management and follow-on investor Newtown Partners. It also landed additional follow-on investment from Ventures Platform, one of the company’s earliest institutional investors. This new round of financing brings MDaaS Global’s total investment to date to US$6.8 million.
The additional capital will also be used to expand its healthcare network to all 36 states in Nigeria through a mix of company-owned and affiliate clinics. This strategic move propels MDaaS Global closer to its mission of democratizing access to high-quality diagnostics and healthcare services across the continent.
With this injection of capital, the company will develop new applications on the BeaconOS platform that solve critical issues in the healthcare system: An affiliate clinic portal will facilitate collaboration with partner clinics across the country, allowing for realtime patient referrals, seamless results management, and standardized quality control.

USA Royalty Pharma, a major life sciences innovation financier, has announced that it will pay US$525 million to biotechnology startup ImmuNext for the right to receive royalties and milestone payments from an autoimmune disease medicine that the privately held business licensed to Sanofi.
Sanofi purchased the drug from ImmuNext in 2017 and promised milestone payments of up to US$500 million plus tiered royalties on potential sales.
This new development follows the promising results of a mid-stage study of patients with relapsed multiple sclerosis published last year.
According to BioPharma, the French manufacturer Sanofi advanced the medicine, known as frexalimab, to a Phase 3 trial.
This deal will ensure that Royalty receives “substantial” milestone payments from Sanofi and full royalties on up to US$2 billion in global sales of the medicine.
A portion of royalties on sales above that threshold will be split with ImmuNext shareholders. Royalty announced that tiered royalties vary from high single-digit to low doubledigits.
Royalty said that with frexalimab in its portfolio, it will have 15 investigational medicines with the potential to produce combined peak annual royalties “significantly greater” than US$1 billion.
Approved products will supplement the cash flow from existing marketed pharmaceuticals, such as GSK’s asthma treatment Trelegy and Roche’s spinal muscular atrophy medication Evyrsdi.
USA – Fulcrum Therapeutics, a clinicalstage biopharmaceutical company, has inked a partnership and licensing agreement valued at up to US$1.05 billion with Sanofi to develop and commercialize losmapimod, an oral small molecule.
Losmapimod is currently undergoing evaluation as a potential treatment for facioscapulohumeral muscular dystrophy (FSHD), a rare and debilitating genetic disorder characterized by progressive muscle weakness.
Under the agreement, Fulcrum Therapeutics will receive an initial payment of US$80 million and potentially earn an additional US$975 million through specified regulatory and
AbbVie,
sales-based milestones.
The collaboration also entails tiered escalating royalties, commencing in the low teens, on Losmapimod’s annual net sales outside the United States.
Additionally, Fulcrum Therapeutics and Sanofi will evenly split future global development expenses for the drug.
Losmapimod is being evaluated in a global Phase 3 clinical trial for treating FSHD. Topline data are anticipated in the fourth quarter of 2024.
Preliminary findings from the Phase 2 clinical trial, ReDUX4, demonstrated encouraging results, including a deceleration in disease progression and improvements in muscle health.
USA – AbbVie, a global pharmaceutical giant, has embarked on a significant venture into psychiatric treatments by collaborating with Gilgamesh Pharmaceuticals, a US-based biotech company.
This strategic partnership, bolstered by an option-to-license agreement, aims to develop innovative therapies for psychiatric disorders.
The collaboration entails AbbVie providing Gilgamesh with an upfront payment of US$65 million, alongside potential milestone payments and royalties totaling up to US$1.95 billion.
The core focus of this collaboration lies in the development of neuroplastogens, compounds that induce rapid and durable neuroplasticity without the psychoactive side effects often associated with traditional psychedelic compounds.
Neuroplastogens have emerged as a promising avenue for addressing psychiatric disorders, offering the potential for substantial clinical benefits

while minimizing adverse effects.
In addition to ongoing clinical endeavors, Gilgamesh is pursuing an investigational new drug (IND) application for GM-5022, an oral nonhallucinogenic neuroplastogen.
These innovative compounds hold promise for addressing the significant unmet need in psychiatric care, offering potential solutions for mood and anxiety disorders.
The collaboration between AbbVie and Gilgamesh comes at a time of growing interest and investment in psychedelic medicine for psychiatric disorders.



















CHINA – WestGene, a leading biotech company dedicated to mRNA technology, announces the historic FDA IND approval of its mRNA therapeutic cancer vaccine, WGc-043.
This milestone achievement signifies a breakthrough in cancer treatment and marks the world’s first approval of an EB virus-related mRNA therapeutic cancer vaccine.
Founded by distinguished scientists
Dr. Yuquan Wei, Academician of the Chinese Academy of Sciences, and Dr. Xiangrong Song, Westgate has emerged as a pioneering force in mRNA technology research and innovative drug development.
This FDA approval underscores WestGene’s unwavering commitment to advancing the boundaries of biomedicine, driven by a relentless pursuit of scientific excellence.
The approval of WGc-043 represents a beacon of hope for patients grappling with advanced EB virus-related cancers.
The EB virus, intricately linked with a spectrum of malignancies, including nasopharyngeal carcinoma (NPC), natural killer T-cell lymphoma (NKTL), and various other cancers, presents a formidable challenge in oncology.
WGc-043 emerges as a potential game-changer in this battle, offering promising efficacy, low toxicity, and broad applicability.
The vaccine has already demonstrated its superiority in investigator-initiated trials (IIT) conducted in NPC and NKTL, showcasing enhanced safety and efficacy compared to existing mRNA therapeutic cancer vaccines.
Upon successful launch, WGc043 is poised to offer a much-needed treatment option for patients with advanced EB virus-positive solid tumors and hematologic malignancies.
USA – Johnson & Johnson (J&J) has announced a definitive agreement to acquire Proteologix, a private biotech company, for US$850 million in cash, with additional milestone payments possible.
This move, expected to close in mid2024, underlines J&J’s commitment to strengthening its dermatology portfolio and addressing unmet needs in conditions like atopic dermatitis (AD).
Proteologix specializes in developing bispecific antibodies for immunemediated diseases.
Its pipeline includes PX128, a phase I-ready candidate targeting interleukin (IL)-13 and TSLP, designed for severe AD and moderate-to-severe asthma.
Additionally, Proteologix is developing PX130, a bispecific antibody
targeting IL-13 and IL-22 for moderateto-severe AD.
This acquisition aligns with J&J’s strategic focus on expanding its dermatology portfolio.
With Proteologix’s pipeline, J&J aims to offer differentiated and complementary bispecific antibodies while addressing immune-mediated diseases.
Adding PX128 and PX130 strengthens J&J’s position in dermatology and reinforces its commitment to innovation in healthcare.
Elsewhere, J&J made a significant strategic move by purchasing Ambrx Biopharma for US$2 billion, solidifying its footprint in the antibody-drug conjugate (ADC) sector.
Through this acquisition, J&J secures
access to notable drugs such as ARX517, targeted for metastatic castrationresistant prostate cancer, and ARX788, designed for metastatic HER2+ breast cancer.

SOUTH AFRICA South Africa’s President, Cyril Ramaphosa, has signed the National Health Insurance (NHI) Bill into law. This bill guides the change of South Africa’s health care system to attain universal health coverage. This new law aims to address fundamental socioeconomic imbalances and inequities from the past. Access to excellent care will be defined by need rather than ability to pay, resulting in better health outcomes and preventing
APPROVALS
preventable deaths.
The signing event was held in the Union Buildings in Pretoria. It was attended by the Minister of Health, Dr Joe Phaahla, who, among other guests, conducted a question-and-answer session with the media immediately following the signing.
The signing is a significant step toward a more equal society, coming only two weeks before a highly competitive election.
The National Health Insurance (NHI) Act aims to eliminate a two-tier healthcare system in which a publicly funded sector serving 84% of the population is overwhelmed and inefficient, while others have access to superior treatment through private insurance.
The legislation will gradually reduce the role of private insurance, establish a new public fund to give South African people free access and determine the rates and prices that private doctors and healthcare providers may charge for NHI-funded benefits.
USA - Azurity Pharmaceuticals has announced the FDA’s approval of Myhibbin™, the first ready-to-use mycophenolate mofetil oral suspension. Mycophenolate mofetil is a crucial antimetabolite immunosuppressant, shielding transplanted organs from rejection by the recipient’s immune system.
With over 46,000 transplant procedures performed in the United States in 2023 alone, the necessity for daily medication to avert rejection reinforces the significance of Myhibbin’s approval. It’s specifically indicated for organ rejection prophylaxis in adult and pediatric recipients three months and older who have undergone allogeneic
kidney, heart, or liver transplants, typically in conjunction with other immunosuppressive agents.
Commercial availability of Myhibbin in pharmacies across the United States is anticipated in the second quarter of 2024.
Myhibbin’s approval represents a breakthrough in transplant medicine, providing a vital therapeutic option for individuals navigating the intricate post-transplant landscape. The approval of Myhibbin comes on the heels of Azurity Pharmaceuticals’ proactive response to quality assurance concerns, exemplifying the company’s steadfast commitment to product integrity and patient safety.
New
The World Health Organization (WHO) has prequalified Euvichol-S, a novel oral cholera vaccine (OCV), for cholera treatment.
This substantial development was revealed on April 12 and has received overwhelming support from Gavi, the Vaccine Alliance, and UNICEF.
Euvichol-S, manufactured by EuBiologicals Co., Ltd. in the Republic of Korea, provides a simpler formulation with comparable efficacy to existing vaccines.
Its simplified formulation allows for rapid production scale-up, contributing to increased global vaccination supply.
Notably, this clearance broadens the WHO prequalification list, which already contains Euvichol and Euvichol Plus.
This approval is expected to increase the global availability of oral cholera vaccines in 2024. An estimated 50 million doses will be available for the stockpile, up from 38 million in 2023.
This discovery responds to the growing need for cholera vaccinations, which has been accompanied by a global increase in cholera incidence since 2021.
UNICEF, the main agency for vaccine procurement and delivery, has obtained access to Euvichol-S doses and is dedicated to speeding up their distribution to affected countries.

USA The FDA recently granted accelerated approval for Bristol Myers Squibb’s Breyanzi to treat patients with relapsed or refractory follicular lymphoma (FL).
This approval offers a promising option for patients undergoing at least two prior systemic therapies.
Breyanzi, a CD19-directed chimeric antigen receptor (CAR) T cell therapy, significantly advances FL treatment.
Administered as a one-time infusion, it delivers a precise dose of 90 to 110 × 10⁶ CAR-positive viable T cells, targeting malignant cells with enhanced precision and efficacy.
The FDA’s decision is based on compelling data from the Phase II TRANSCEND FL clinical trial, which was conducted globally across multiple centers.
This open-label, single-arm study evaluated Breyanzi’s efficacy and safety, with impressive results shaping the approval process.
Key outcomes from the trial included an overall response rate of 95.7%, with a notable complete response rate of 73.4%.
Response to treatment was rapid, typically observed within one month, and durable, with the median duration of response not yet reached. Even at 12 and 18 months post-treatment, most patients remained in response.
Safety remains paramount, and Breyanzi has demonstrated a consistent safety profile throughout clinical trials.
Cytokine release syndrome (CRS), a common concern with CAR T cell therapies, was manageable. Any grade was reported in 53% of subjects, and Grade >3 CRS was observed in 4% of patients.
UAE – The Department of Health (DoH) in Abu Dhabi has forged a strategic partnership with the British pharmaceutical giant GSK to support healthcare infrastructure and advance vaccine distribution capabilities. This collaboration, announced during Abu Dhabi Global Healthcare Week (ADGHW), aims to establish a regional vaccine distribution hub in the Emirate, signifying a milestone in healthcare innovation.
Under the Memorandum of Understanding (MoU) signed between DoH and GSK, the two entities will collaborate closely to ensure the successful establishment and operation of the vaccine distribution hub. The partnership will also maximize this initiative’s strategic and healthcare impacts while fostering knowledge exchange and capability building.
Establishing a regional vaccine distribution hub in Abu Dhabi aligns
HOSPITALS

with the UAE’s proactive approach to addressing public health challenges.
As the country navigates demographic shifts, prevention emerges as a core principle driving healthcare strategies, with robust immunization programs playing a pivotal role.
Abu Dhabi Global Healthcare Week is a platform for driving collaboration, innovation, and investment in the healthcare sector.

INDIA - Agilus Diagnostics, an Indiabased diagnostic laboratory network, has partnered with SEAS International LLC of Oman to open a cutting-edge referral laboratory in Oman.
The company stated that this strategic alliance seeks to provide high-
quality diagnostic testing services to Oman’s 5 million people.
The collaboration aims to strengthen the diagnostic service provider’s international offering.
Its international network includes various centres and pick-up points (PUPs) throughout the South Asian Association for Regional Cooperation (SAARC), Sub-Saharan Africa, the Commonwealth of Independent States, and the Middle East.
Agilus Diagnostics has the largest network of NABL-accredited labs as of March 31, 2023, according to the CRISIL Report, with an extensive diagnostics network in India spread across 25 states and five union territories, covering over 1,000 towns and cities and over 532 districts.
UAE – Burjeel Holdings, a prominent healthcare services provider in the MENA region, has recently inaugurated an advanced Day Surgery Center in Al Ain’s Al Dhahir area in the UAE.
The region’s cutting-edge facility, the first of its kind, strengthens Burjeel’s commitment to delivering toptier patient care and fostering holistic community well-being.
Sheikh Nahyan bin Zayed Al Nahyan, Chairman of the Zayed Charitable and Humanitarian Foundation, inaugurated it.
This strengthens Burjeel’s commitment to delivering top-tier patient care and fostering holistic community well-being.
Equipped with state-of-the-art diagnostic and treatment technologies, the Day Surgery Center aims to revolutionize patient care by offering efficient surgical interventions for
various medical conditions while concurrently reducing the need for prolonged hospital stays.
A highly qualified and boardcertified team of doctors spearheads the Center, strategically positioning it to enhance patient experience and well-being through community-led healthcare initiatives.
The Center specializes in various medical fields, including Orthopedics, Gastroenterology, ENT, Urology, Dermatology, Ophthalmology, and General Surgery.
It ensures a rapid and comprehensive treatment experience for patients across various healthcare needs.
With a strong emphasis on community health, the Center boasts a robust physiotherapy and rehabilitation department to support overall wellbeing.

USA - Elevance Health, Inc. has completed the purchase of Paragon Healthcare, a Texas-based provider of infusion services, strengthening Elevance Health’s commitment to providing access to specialty pharmaceuticals for those with chronic
and severe diseases.
This acquisition is part of Elevance Health’s larger aim to deliver integrated healthcare services, and it is consistent with a trend of consolidation in the healthcare business as corporations strive to provide more complete care.
Elevance Health intends to broaden Paragon Healthcare’s geographic reach and increase its therapeutic solutions to ensure that its members have prompt and accessible access to drugs.
The goal is to improve the health of humanity by assisting consumers, families, and communities throughout their care journeys.
Elevance Health serves about 117 million people through its medical, digital, pharmaceutical, behavioral, clinical, and complex care solutions portfolio.
UK - AstraZeneca, the BritishSwedish pharmaceutical company, has withdrawn its COVID-19 vaccine worldwide amid rare blood clot concerns.
The decision comes amidst declining demand for the vaccine and the emergence of newer alternatives that are better equipped to tackle evolving virus variants.
Since its approval in December 2020, AstraZeneca’s vaccine, developed in collaboration with Oxford University, has been widely distributed, with over three billion doses administered globally. However, demand has waned significantly in recent years, prompting the company to cease manufacturing and supplying the vaccine.
The withdrawal is unrelated to concerns over the vaccine’s safety or efficacy, clarified the pharmaceutical giant.
Instead, it reflects a strategic response to changing market dynamics and the availability of updated vaccines tailored to combat newer virus variants.
According to AstraZeneca, the decision to withdraw the vaccine from the market was made months ago, and the process of withdrawing licenses to market the vaccine was already underway.
Despite these challenges, AstraZeneca emphasized its pride in the vaccine’s contribution to the global fight against the pandemic.
UAE— M42, a technology-enabled global health powerhouse, has joined forces with AstraZeneca, a leader in science-led biopharmaceuticals, to enhance preventative healthcare and next-generation precision medicine.
M42, AstraZeneca’s chosen clinical genomics partner in the Middle East, will screen hereditary breast cancer genes to develop tailored medicines and prevention methods for each patient. Breast cancer is the most frequent disease in women globally and the top cause of death in the UAE, accounting for 11.6% of all cancer deaths each year, according to the Ministry of Health and Prevention (MoHAP).
Approximately 15% of breast cancers are caused by genetic mutations in the BRCA1 and BRCA2 genes, which can be inherited from either parent.
This innovative endeavor highlights M42’s strong multi-omics sequencing capabilities in oncological diagnostics, a fast-evolving field with enormous potential to enhance patient outcomes as the global cancer burden rises. M42 will assist in determining each patient’s inherited risk for cancer by simplifying cancer gene sequencing, allowing for early or even preventative interventions.
M42 is also establishing one of the Gulf’s first companion diagnostics projects in partnership with AstraZeneca. This project will provide patients with data to optimize personalized drug-based therapies.

SAUDI ARABIA - amplifAI Health, a health technology company headquartered in Saudi Arabia, and Healthspan Digital Inc., a Toronto-based data-driven healthtech company, have announced a strategic partnership aimed at revolutionizing the understanding and management of vascular aging.
This collaboration integrates amplifAI Health’s AI-powered, rapid, and cost-effective thermal hyperspectral technology with Healthspan Digital’s advanced longevity clinical protocols.
The innovative fusion of AI with thermal hyperspectral imaging holds immense potential for unlocking groundbreaking therapeutic and diagnostic advancements in this field.
The newfound partnership seeks to enhance healthspan and improve the
quality of life not only in the Kingdom of Saudi Arabia but also across the rest of the GCC and beyond, especially as global life expectancy continues to rise.
THE NEWFOUND PARTNERSHIP SEEKS TO ENHANCE HEALTHSPAN AND IMPROVE THE QUALITY OF LIFE ACROSS THE GCC AND BEYOND
KENYA The Kenya Medical Research Institute (KEMRI) has renewed its fiveyear Memorandum of Understanding with the American Centres for Disease Control and Prevention (CDC), enhancing Kenya’s cutting-edge research operations.
The historic signing occurred at the CDC Headquarters in Atlanta, Georgia, in front of His Excellency President William Ruto and other top government officials.
Prof Elijah Songok, Acting Director General of KEMRI, signed on behalf of KEMRI, while Dr. Kayla Laserson, Director of Global Health Centre, CDC, signed on behalf of the CDC.
This MoU aims to improve mutually beneficial collaboration between KEMRI and the CDC.
The collaboration will include human health research and program implementation at KEMRI facilities
and Kenyan communities, as well as research and capacity development for public health risks and emergencies. The partnership will also involve distributing and using research findings to inform policy formation, train public health professionals, enhance research leadership, and strengthen laboratory capacities.
Furthermore, the MOU covers staff exchanges, sharing research information and resources in compliance with Kenyan laws, and other mutually beneficial health-related research activities.
It includes 45 years of CDC partnership with Kenya’s public health and laboratory systems, 21 years of collaboration through the President’s Emergency Programme for AIDS Relief (PEPFAR), and the implementation the Global Health Security Agenda.

UGANDA— The Aga Khan University Hospital has inaugurated the Nakawa Specialty Centre on Old Port Bell Road, offering Ugandans a wide range of specialized outpatient health care services.
This development was announced through a statement by Aga Khan University (AKU) on their LinkedIn
page, where they expressed gratitude to Mr. and Mrs. Aziz Manji and their family for their generous donation towards the establishment of the Aziz & Zubeda Manji Building, which houses the facility.
This is the first facility on the upcoming Aga Khan University’s Kampala campus and will provide chemotherapy, dialysis, and diagnostic imaging services, including CT scans, mammography, neurophysiology, and ultrasound.
The facility is part of a larger development project that includes a seven-story University Centre and a nine-story student housing building currently under construction. The Aga Khan University Hospital, Kampala, is slated to begin construction in 2025.
The center will offer access to various specialists in gynecology, pediatrics,
cardiology, neurophysiology, oncology, and endocrinology.
Additionally, physiotherapy, mammography, dentistry, laboratory, and pharmacy services will be available.
The upcoming campus will also feature academic facilities, student accommodation, and a university hospital.
This investment is one of AKU’s most significant in East Africa. Through its education programs, it aims to develop much-needed leaders and healthcare professionals and provide high-quality healthcare to Uganda and the wider East African region.
With the inauguration of this facility, Nakawa Specialty Centre joins the hospital’s three other medical centers in Kampala, located at Acacia Mall, Metroplex Mall Naalya, and the DTB building.
CONGO —The World Health Organization (WHO) has revealed that vaccines have saved an estimated 51.2 million lives in Africa over the last five decades, with each infant life saved adding nearly 60 years to life, a significant achievement in public health, according to a recent report. These achievements have been made possible by the Expanded Programme on Immunization (EPI), a WHO-entered effort in 1974 to ensure that every child has equitable access to lifesaving immunizations, regardless of geographical or socioeconomic status. The research assessing vaccines’ lifesaving impact was released on April 24 as part of this year’s African Vaccination Week and World Immunization Week, entitled “Safeguarding Our Future: Humanly Possible,” which runs from

April 24 to April 30.
With ongoing support from WHO, UNICEF, Gavi, the Vaccine Alliance, and other partners, most countries in the region now provide antigens for 13 vaccine-preventable diseases, up from the initial six introduced with the EPI. Remarkable progress has been made, including reducing measles deaths, with an estimated 19.5 million deaths
prevented in the last 22 years.
Furthermore, the number of meningitis deaths in the region decreased by 39% between 2000 and 2019.
Maternal and neonatal tetanus is practically eradicated, and after years of concerted effort, the African continent will be certified free of indigenous wild poliovirus in 2022.

SOUTH AFRICA The South African Health Products Regulatory Authority (SAHPRA) and other health professional regulators have taken proactive measures to curb codeine misuse, particularly among young people, through the Codeine Care Initiative.
This initiative aims to meticulously track the supply chain of the drug, from manufacturers to end-users, enabling health workers to intervene effectively.
It will force pharmacies and other medical institutions to digitally record the identity numbers of people who have been given medications such as codeine, used for pain relief, and other cough mixtures.
The concept for the Codeine Care Initiative originated in the early 2010s, initiated by the Pharmaceutical Society of South Africa, highlighting the collaborative effort among stakeholders to address this pressing issue.
Codeine, an opioid present in certain pain relief medications and cough mixtures, is frequently abused, especially among youth.
With this new development, SAHPRA is developing new guidelines to regulate codeine usage, slated for public review in June.
The Codeine Care Initiative represents a crucial step towards addressing codeine abuse comprehensively, safeguarding public health, and curbing the illicit use of this potent drug.
USA – Blackstone Life Sciences has unveiled Uniquity Bio, a new player in immunology and inflammation, backed by a hefty US$300 million investment.
Uniquity Bio is set to embark on Phase II trials for its flagship asset, solrikitug, a monoclonal antibody licensed from MSD targeting thymic stromal lymphopoietin (TSLP).
Uniquity aims to commence Phase II trials targeting chronic obstructive pulmonary disease (COPD) and asthma, two prevalent respiratory conditions with substantial market opportunities.
These trials align with market estimates of US$18.6 billion and US$7.2 billion for COPD and asthma, respectively, in the US, as reported by GlobalData’s Pharma Intelligence Centre.
Solrikitug, designed to inhibit TSLP, a pivotal cytokine in the inflammatory cascade, has received FDA clearance for
clinical trials.
This demonstrates its potential in a range of immunology and inflammation applications.
Uniquity Bio’s strategic approach combines scientific rigor with operational flexibility, enabling rapid progress without compromising quality.
With Blackstone’s unwavering support, the company aims to accelerate the development of solrikitug and expand its portfolio, leveraging extensive expertise and capital resources.
The global immunology market’s exponential growth underscores the urgency and potential for innovative therapies.
Uniquity Bio’s entry into this dynamic landscape signals a commitment to advancing therapeutics and forging strategic collaborations to maximize impact.
Cadila Pharmaceuticals introduces Redshot FCM to address Iron deficiency
INDIA - Cadila Pharmaceuticals has introduced an innovative iron injection named Redshot FCM to combat Iron Deficiency Anemia, a condition that is both preventable and treatable.
Redshot FCM, fortified with an advanced ferric carboxymallose formulation, is tailored for administration to both adults and pediatric patients over one year of age, particularly those with oral iron intolerance.
In a recent announcement, Cadila emphasized that Redshot Injection, an intravenous iron preparation, not only delivers effective iron doses but also boasts an exemplary safety profile.
With superior tolerability and minimal risk of anaphylaxis, Redshot accelerates the improvement of hemoglobin levels and efficiently
replenishes depleted iron stores.
Redshot offers heightened tolerance, facilitating the utilization of high doses in a single administration through intravenous delivery of iron.

NIGERIA—The African Export-Import Bank (Afreximbank) and MobiHealthCare Limited (MobiHealth) have signed a project preparatory facility agreement to increase MobiHealth’s telemedicine services in Nigeria.
This will be followed by a feasibility assessment of similar programmes in Egypt, GhA feasibility assessment of similar programs in Egypt, Ghana, Kenya, and Côte d’Ivoireprograms will follow this to expand MobiHealth’s integrated telemedicine platform into four other nations, which was initially piloted in Nigeria.
This plan is expected to quickly build a network of telemedicine clinics throughout Africa, complete with outpatient facilities.
The project preparation facility, worth up to US$1.5 million, will make the project bankable, potentially unleashing additional investments worth US$65 million.
This financing will hasten the implementation of digital healthcare solutions, improving access, efficiency, and quality of care.
The MobiHealth platform allows local and diaspora medical experts to diagnose and prescribe treatments to patients remotely, minimizing waiting and travel times, helping hardto-reach people, and removing barriers for underserved communities.
This outcome is remarkable for a female-led firm and emphasizes the difficulties that such startups frequently encounter, particularly in obtaining financing.
Afrexk will also be the project’s designated lead arranger, supervising senior debt syndication.




WHO appoints Dr. Mike Ryan as Deputy Director-General
SWITZERLAND - Dr. Mike Ryan has been appointed as the new Deputy Director-General of the World Health Organization (WHO).

Ryan will assume the position alongside his current role as the Executive Director of Health Emergencies, Preparedness, and Response.
With nearly 25 years of experience in managing acute risks to global health, Dr. Ryan first joined WHO in 1996, within a newly established unit responding to emerging and epidemic disease threats.

Before becoming Executive Director of the Health Emergencies Programme during the COVID-19 pandemic in 2019, Ryan served as Assistant DirectorGeneral for Emergency Preparedness and Response in WHO’s Health Emergencies Programme from 2017 to 2019.
In this capacity, he has led WHO’s operational responses to high-impact events such as the COVID-19 pandemic and the SARS epidemic. Additionally, he served as a Senior Advisor on Polio Eradication for the Global Polio Eradication Initiative from 2013 to 2017, deploying to countries in the Middle East.
Dr. Ryan is a founding member of the Global Outbreak Alert and Response Network (GOARN), which has aided the response to hundreds of disease outbreaks around the world.
SWITZERLAND - Medicines for Malaria Venture (MMV) has announced the appointment of Martin Fitchet as its new Chief Executive Officer (CEO).
In his new capacity, Martin will oversee the development and delivery of new antimalarial medicines to advance towards a malaria-free future.
With over 25 years of experience spanning pharmaceuticals, MedTech, and Global Public Health, Martin joins

from Johnson & Johnson, where he most recently held the position of Global Head of Global Public Health.
During his tenure, he played pivotal roles in spearheading the development and global access to innovative pharmaceuticals for various diseases, including cardiovascular and metabolic diseases, HIV, tuberculosis, Ebola, and COVID-19.
Martin assumes the role of CEO as MMV celebrates its 25th anniversary, succeeding David Reddy, who led the organization for 13 years.
Under Reddy’s stewardship, MMV brought forth 15 new medicines, estimated to have saved the lives of 15.4 million people.

Vishal
INDIA - Apollo Hospitals has appointed Vishal Lathwal as Chief Executive Officer (CEO) for its homecare business.
In his new role, Vishal will spearhead the company’s trajectory towards the next growth phase, leveraging his visionary approach to business and profound understanding of the home healthcare space.
With over 15 years of diverse professional experience spanning the consulting, automobile, and healthcare sectors, Vishal brings a wealth of expertise in building and expanding direct-to-consumer (D2C) offerings.
Having served as a founding member of Max@Home, the homecare division of Max Healthcare, Vishal played a pivotal role in establishing and elevating the brand to become one of the nation’s largest providers.
Leveraging this extensive experience, he is poised to play a vital role in formulating and executing core business strategies at Apollo Home Healthcare.
As the youngest executive to assume this role within the company and boasting a proven track record of strengthening market position and driving growth, Lathwal will prioritize cultivating relationships with key stakeholders, identifying growth opportunities, and managing financial performance.
Moreover, he will ensure the delivery of high-quality home healthcare services, which is in line with Apollo’s esteemed ethos.

INDIA - Madhu Sasidhar has been appointed the President and CEO of Apollo Hospitals Group- hospital subsidiary.
Dr Madhu’s appointment is part of a planned transition that has been in place since he joined Apollo as Chief Strategy Officer in October 2023.
As part of this transition, Dr. K Hari Prasad, the outgoing President of Apollo Hospitals, will retire from his position after a remarkable 27-year career at Apollo where he has worked diligently.
In this role, Dr. Madhu will lead Apollo’s hospital business, with a focus on continuing to provide the world’s finest clinical outcomes while also improving Apollo’s signature patient care and experience.
Dr. Madhu previously worked at Cleveland Clinic in a variety of roles, including President of Cleveland Clinic Tradition Hospital.
Dr. Madhu is a practicing physician who has received US board certifications in internal medicine, lung medicine, and critical care.
He is a published author, having written multiple book chapters, journal articles, and peer-reviewed publications.
Dr. Madhu is also a self-taught programmer with skills in large data systems, and he holds a patent through Cleveland Clinic Innovations for a technology he designed.
USA - Ardigen, a company that harnesses advanced AI methods for novel precision medicine, has appointed Livia Legg to its Chief Commercial Officer position.
Livia will also become a member of the Board of Management for the firm.
With her extensive background in business development and commercial operations, Livia is expected to greatly support Ardigen’s sales efforts and help the company reach its ambitious growth

goals.
Livia has a history of creating largescale ventures, and her leadership and knowledge will be crucial to Ardigen’s strategic goals of tripling its operations and increasing its market share.
Her appointment demonstrates Ardigen’s dedication to strengthening its management group and reaffirming its goal of raising the likelihood of a successful medication development process.
She most recently held the positions of General Manager of ChemPartner Corporation US & EU in 2015 and Chief Commercial Officer at Shanghai ChemPartner.
Throughout her career, Livia has held significant positions at MDS Pharma Services and Merck Pharmaceutical Company since 1997, in addition to her international leadership roles as Vice President, Executive Director, and Head of Global Business Development at QPS, LLC from 2002 to 2015.

USA - Dr. Terry Gilliland has been appointed CEO of Geisinger, a health and wellness organization.

Gilliland will be in charge of the 10-hospital system, a health plan with over 500,000 members, a research institute, and the Geisinger College of Health Sciences.
Gilliland takes on this role as Geisinger forecasts a US$37 million operating deficit for 2023, up from a US$239 million loss in 2022.
Dr. Gilliland has vast expertise and has held leadership positions in various major healthcare delivery and payer organizations.

Dr. Gilliland was previously the chief medical officer and chief science officer at Cogitativo, an artificial intelligence and machine learning firm focusing on healthcare.
He previously held the positions of executive vice president of healthcare quality and affordability at Blue Shield of California and senior vice president and chief medical officer at Sentara Healthcare.
Dr. Gilliland earned a Bachelor of Science in Biology from Stanford University, a Doctorate of Medicine from the University of California, Los Angeles, and a Master of Science in Management from the Stanford Graduate School of Business.


Celebrating 5 years of putting Zambia’s healthcare sector on the map
By JACKIE MUINDE


DR. MOHAMED
EL SAHILI, CEO, MEDLAND HOSPITAL
Medland Hospital offers a unique perspective as one of Zambia’s finest comprehensive healthcare providers. In an interview with Francis Juma, Founder & CEO, HealthCare Middle East & Africa Magazine, Dr. Mohamed El Sahili, CEO of Medland elaborate more on the healthcare provider as it celebrates its fifth anniversary of impactful operation.
Medical Doctor. Entrepreneur/Businessman. CEO. Chief Vision Officer. International Speaker. Award winner. Board member. These are some of the many ways that Dr. Mohamed El Sahili (or Moe Sahili, by his close friends) is referred to, in addition to being a strategic visionary and innovative thinker.
Extremely passionate about any initiative he takes on, Dr. El Sahili has spent the past five years involved in the project that is bound to make the most impact on his entrepreneurial journey: as the CEO and Chief Visionary Officer (CVO) of Medland Hospital, Zambia’s leading privatesector healthcare operator.
Boasting as the first private facility to introduce a full-time operational cardiovascular surgery department in Zambia, Medland Hospital has been a game changer in the country’s
healthcare space, providing highly specialized medical services for Zambians and citizens within the region.
Located in the county’s capital city, Lusaka, Medland Hospital is dedicated to providing access to comprehensive and individualized patient care in a friendly, safe, and comfortable environment of international standards based on collaboration, compassion and innovation principles.
Opened in June 2019, the US$35 million facility is a 72-bed space facility that offers services such as Cardiac Surgeries and Interventional Cardiology, Surgical Oncology, Orthopedics & Trauma, Urology, Ear, Nose and Throat (ENT), General Surgery including Minimally invasive procedures, Nephrology, Obstetrics & Gynecology, Ophthalmology, Pathology, Radiology, Pediatrics & Neonatology, General Medicine, IVF & Fertility, and AntiAgeing Medicine.
Zambia and other neighboring countries, except South Africa, do not have hospital centers of excellence to treat cases requiring specialized treatment and lack specialist diagnostic and treatment centers to treat cardio-vascular, liver, renal, and cancer diseases. Medland Hospital came to fill the gap. It provides medical tourism and receives patients from Zambia, Zimbabwe, Malawi, Botswana, Angola, and as far north as Kenya.
“What we offer is what I call the Medland experience,” says Dr. El Sahili, adding, “We do provide all kinds of services available worldwide. We have regular medical visits and advanced services like cardio-vascular surgeries, surgical oncology, and advanced orthopedic surgery.”
The hospital was created based on a comprehensive specialized facility that provides discreet and individual quality care for day and overnight stays. By providing a sustainable infrastructure that delivers international standards across all healthcare system levels, Medland Hospital ensures that the patient experience can match any facility outside the continent. The facility has created almost 300 full-time jobs, 80 percent locals, and 20 percent expatriate staff.
Dr. Mohamed El Sahili was born in Saida, Lebanon. After completing his high school education, he moved to Toulouse, France, for two years before returning to Beirut to finalize his studies in medicine at the Lebanese University. His medical
journey didn’t stop there; he obtained a further specialty in Anesthesia and Reanimation at Beirut Arab University, after which he relocated to Geneva to kickstart his career and delve into the studies of Anti-Aging Medicine.
From a young age, he wanted to be a doctor. “I envisioned making a difference in people’s lives by providing access to affordable, timely, and quality health care. Today, Medland Hospital has made this vision a reality,” he says.
After graduating, he joined the family business in Zambia and managed them, including Fairy Bottling Zambia. The firm’s primary products were natural mineral waters and carbonated drinks. After implementing a company-wide business strategy, the firm rose to the ranks of the nation’s top five. Dr. El Sahili managed the merger and acquisition negotiations that led to The Coca-Cola Company’s complete acquisition of the company.
Concurrently, he worked on acquiring bottling businesses in Zambia and 13 other countries. His impactful work was recognized in 2018 when he was awarded the Africa Food Industry Champion at the Africa Food Awards in Nairobi, Kenya.
In his commitment to public service, Dr El Sahili chaired the Lusaka Water Security Initiative for three years up to 2018. This initiative, which combined forces across the public and private
I ENVISIONED MAKING A DIFFERENCE IN PEOPLE’S LIVES BY PROVIDING ACCESS TO AFFORDABLE, TIMELY, AND QUALITY HEALTH CARE. TODAY, MEDLAND HOSPITAL HAS MADE THIS VISION A REALITY
sectors and international organizations, sought to enable people’s right to access clean and clear water.
The meticulous planning, designing, and implementation of the Medland Hospital setup marked a significant milestone in his entrepreneurial journey.
Besides being CEO and CVO of Medland Hospital, Dr. El Sahili also serves on the boards of critical economic international organizations, such as the African Business Roundtable, the Baobab College, the Flying Doctors, and the Corporate Council on Africam.
Dimitra Papalexiou, a Greek-American, is the
TEAM OF DOCTORS PERFORMING SURGICAL LIGATION OF CONGENITAL CORONARY FISTULA
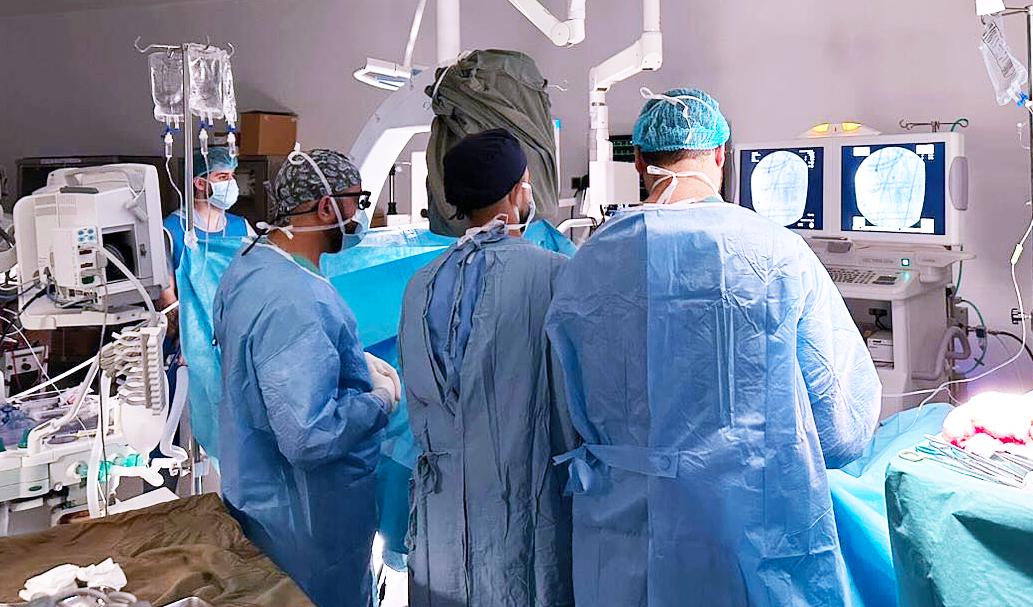

Hospital Administrator at Medland Hospital and a member of the American College of Healthcare Executives (ACHE).
Dr Collin West is the Medical Director. He is also the secretary general of Zambia Orthopedic and Trauma Association of Zambia.
Patience Shavuna, a Zambian, is the Human Resource Manager, and Marie Claire Makuza, an American, is the Universal Health Coverage (UHC) Coordinator. Marie has been named African Global Health Ambassador in Zambia and is the hospital’s national and international spokesman.
COVID-19 was an unseen pandemic that caused a global crisis, and it happened barely eight months after Medland Hospital launched.
As the outbreak spread across the World and into Zambia, the hospital had to act fast, in some cases changing some of its policies and procedures as the pandemic hit. In one groundbreaking move, the hospital set up a COVID-19 response committee few weeks before the World Health Organization (WHO) declared the outbreak a pandemic.
“For a newly established hospital, the policies were still fresh. An example is the emergency response plan drafted before opening the facility. We never knew that there would be a pandemic.
Still, we had it in place because it’s part of our state-of-the-art facility to be perfect on all levels,” states Dr. El Sahili, adding that they immediately moved into putting together an anticipated list of challenges, looking closely at the areas of supply chain, human resources and other details which would affect the overall day-to-day running of the hospital.
With the guidance of public authorities, Medland became one of the first accredited hospitals in Zambia to conduct PCR screening tests through its existing PCR laboratory.
Dr. El Sahili expressed his positivity to the pandemic by saying it accelerated the trust between the hospital and its patients. With the international travel bans, Medland Hospital became a hospital of choice for patients seeking treatments abroad, fulfilling one of their chief objectives. The facility, therefore, benefitted from the increased contact with locals, cultivating a solid bond built on trust and accountability with patients and the community.
“Before COVID, no one could believe that cardiac surgery could be done in Zambia, but because of the lockdown, they had to try our services, and when they had access to that care of excellence, they changed their minds, and it became more of a real relationship that is being built between a patient and the facility,” he says.
Dr. El Sahili led the fight against the pandemic,
and he was honored with the Waterfalls Global Award and the UAE Community Service Medal for his role in enacting a novel emergency plan that contributed to expanding the nation’s capacity for screening, immunization and treatment.
Dr. El Sahili launched a successful international initiative called Q-Medland during the Covid-19 pandemic. Q-HUB (an acronym for Quality Healthcare Unit and Beyond, pronounced as “Cube”) was created after he realized that the units were more than just places to receive medical care; they were also thriving community centers that provided locals with different forms of empowerment.
In May 2021, the facility strengthened the communal bond by sending its Q-Medland Units to decentralize access to COVID-19-related services. Dr. El Sahili says that these units serve communities, specifically those in rural areas, by addressing health issues and raising awareness among the general population.
Q-Medland mobile clinics were located in Livingstone, Lilayi, and Chongwe, among other areas. They provided COVID-19 screening, testing, vaccination services, and primary health care services such as general GP consultations, health checks and screenings, minor surgical
treatments, medication administration, nebulization and oxygen therapy, and routine lab testing.
“It’s the perception that you create within the general population, and the trust people have in you and the care they would receive,” says Dr. El Sahili.
Medland Hospital has also developed relationships with oncology research institutions to provide updated chemotherapy protocols. It is also a member of the International Hospital Federation and one of the first 100 signatories to Ethical Principles in Healthcare (EPIHC) worldwide. Dr. El Sahili sits on the Africa Healthcare Federation and the Corporate Council on Africa boards, lending further credibility to Medland’s prestige.
These partnerships have been possible through what Dr. El Sahili describes as a direct dialogue between the private and public sectors. The channels of communication between the private sector and the government are open. “Sometimes they do face challenges like resistance and over-excitement, but the channels of communication are open,” he retaliates.
Dr. El Sahili insists that Zambia must be promoted as a regional medical hub, and he
DOCTORS PERFORMING FIRST SUCCESSFULL VARICOCELE EMBOLIZATION AT MEDLAND HOSPITAL
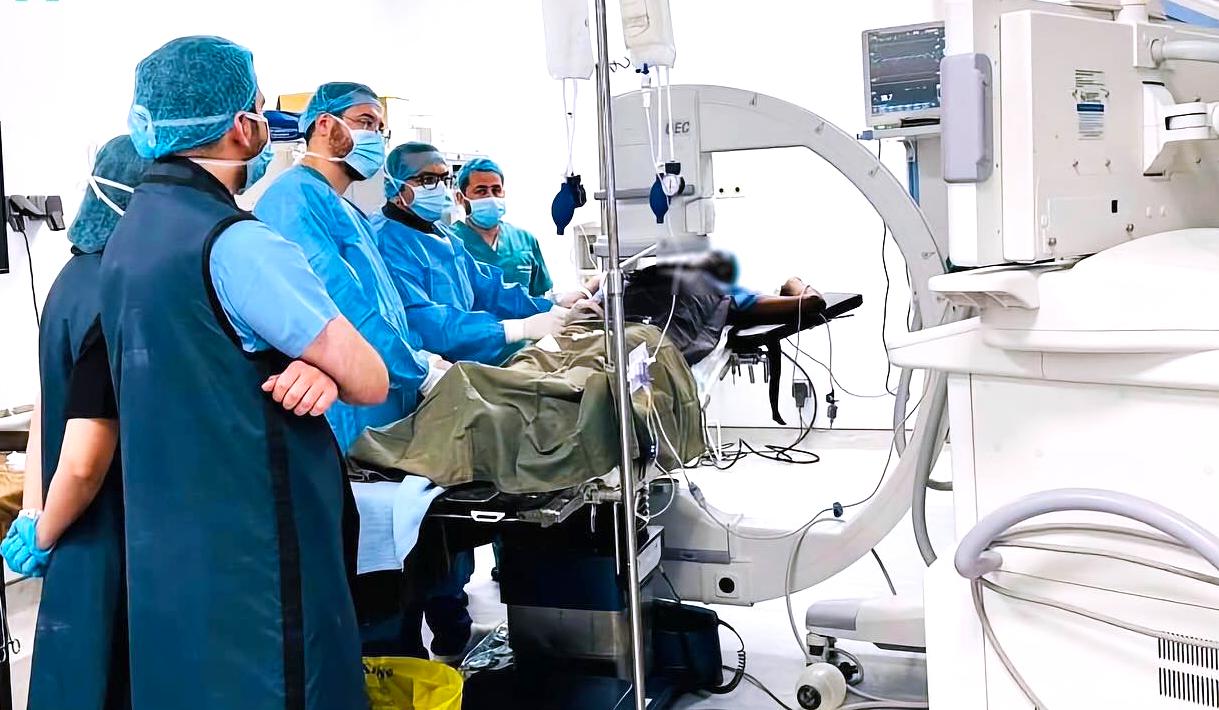
calls for investors, specifically health investors, to consider investing in the country. Zambian patients are increasingly seeking high-quality healthcare services, which has increased demand
MEDLAND WILL BE PARTNERING MORE OR VENTURING JOINTLY WITH INTERNATIONAL ENTITIES TO IMPLEMENT
A BIGGER VISION THAT ADDRESSES THE FACT THAT ZAMBIA CAN BECOME A MEDICAL REGIONAL HUB.
for private hospitals. The middle class is willing to pay for better healthcare services and is driving the growth of private hospitals in the country.
Providing further insights into the environment in the country and the role the medical sector can play in enhancing Zambia’s role as a regional medical hub, and the general lack of awareness about the country’s many advantages, considering it borders seven countries such as Angola, DRC, Mozambique, Malawi, Tanzania, Botswana and Zimbabwe, he reveals that the messaging has not been direct and forthright from all involved in the sector.
“For a long time, Zambia has marketed itself by advocating for investments in agriculture, mining, and other areas, while the healthcare sector has not been on government and private sector investors’ map. However, when you look at Medland Hospital today, we are receiving patients from Zambia, Zimbabwe, Malawi, Botswana, Angola, and even Kenya, believe it or not. It is the perception you create within the general population, the trust people have in you, and the care they receive,” Dr. El Sahili remarked.
He added that the Medland Hospital project has created a call to other investors looking at Zambia as a regional medical hub. “We are, therefore, playing an important role in enabling other stakeholders to come to Zambia. Medland is playing an important role in that direction,” he said adding that in the coming future, Medland will be partnering more or venturing jointly with international entities to implement a bigger vision that addresses the fact that Zambia can become a
medical regional hub.
However, Dr El Sahili highlights that the goal to make Zambia a regional hub can only happen if the players in the private sector move from a competition mindset to a leadership and coalition mindset. “If we don’t do that, we won’t be able to push for a smoother adoption of regulation updates or upgrades and implementation. The public sector can only change, amend or adopt new regulations when sitting with one private sector, not with a distracted private sector or a dismantled one, where everyone is thinking about their own needs, their benefits,” he pointed out.
Medland Hospital, as a member of Healthcare Without Harm’s Healthcare Climate Challenge, was the first private African facility to join the United Nations-backed race to net zero campaign, committing to achieving net zero emissions by 2050.
The facility’s Vision 853 is based on a Triple-W structure: Women and Youth Empowerment, Wellness and Health, and Water and Waste Management. In line with this, the facility recently appointed a Chief Sustainability officer. “The CSO has to make sure that sustainability is achieved in-house in terms of our way of dealing with our procurement, our services, our hospitality, and our side of doing business,” Dr. El Sahili explained.
He highlights that the facility is paying attention to primary care. Recently, they have invested in developing its home care departments. “Medland is working on enhancing its telemedicine services to respond to the needs of people living in rural areas or underprivileged communities who may not be able to travel to you every time they increase in a health care challenge,” he says.
As Medland Hospital celebrates the huge progress and impact it has had on the healthcare industry in Zambia over the last five years, the organization’s team is gearing up for a brighter future and even more impact on its patients, the communities it serves, and the broader economy of the country.
“There will be a huge change in the coming years at Medland Hospital. First, we are now very committed to our sustainability agenda, and the appointment of our Chief Sustainability Officer attests to that,” Dr. El Sahili says.
Secondly, the team is considering adopting various new technologies into its operations, including artificial intelligence (AI). It recently installed an advanced 4D ultrasound scanning machine in the diagnostic and imaging department. He believes that new technologies are set to transform how healthcare is delivered and even how diseases will be diagnosed and treated.
“I may be wrong, but I believe that within the coming decade, which is near for us as a
generation, we will not even see the medical faculties or schools as they exist today. I think it’s very important for us to know that change is coming, and we must be ready for it,” he added.
The third priority area is to help develop human resources within its facility and the broader healthcare sector in the country. The hospital recently launched its education program, which will improve capacity building and allow better technical skills transfer across its team to augment the government’s efforts.
The public health sector in Zambia has been experiencing a human resource crisis for several decades. Public health facilities reportedly function below the required capacity of qualified health personnel, which is attributed to the best workers leaving for more advanced economies such as the UK, Canada, Australia, and the USA.
“Medland is an internship site accredited by authorities, and we are here to offer proper education programs that can transfer technological skills to our local talents,” Dr. El Sahili points out, adding that they are using technology to support that academic side.
The hospital will also boost its partnerships with like-minded companies from the UAE and other countries in the future, believing that such partnerships will help build its capacity to meet its ambitious objectives.
As the hospital turns five, Dr. El Sahili applauds the great work the facility’s team has done to get it to where it is today. He hopes to expand Medland Hospital’s dream of community impact, transparency, and sustainability in the coming years.
HCMEA
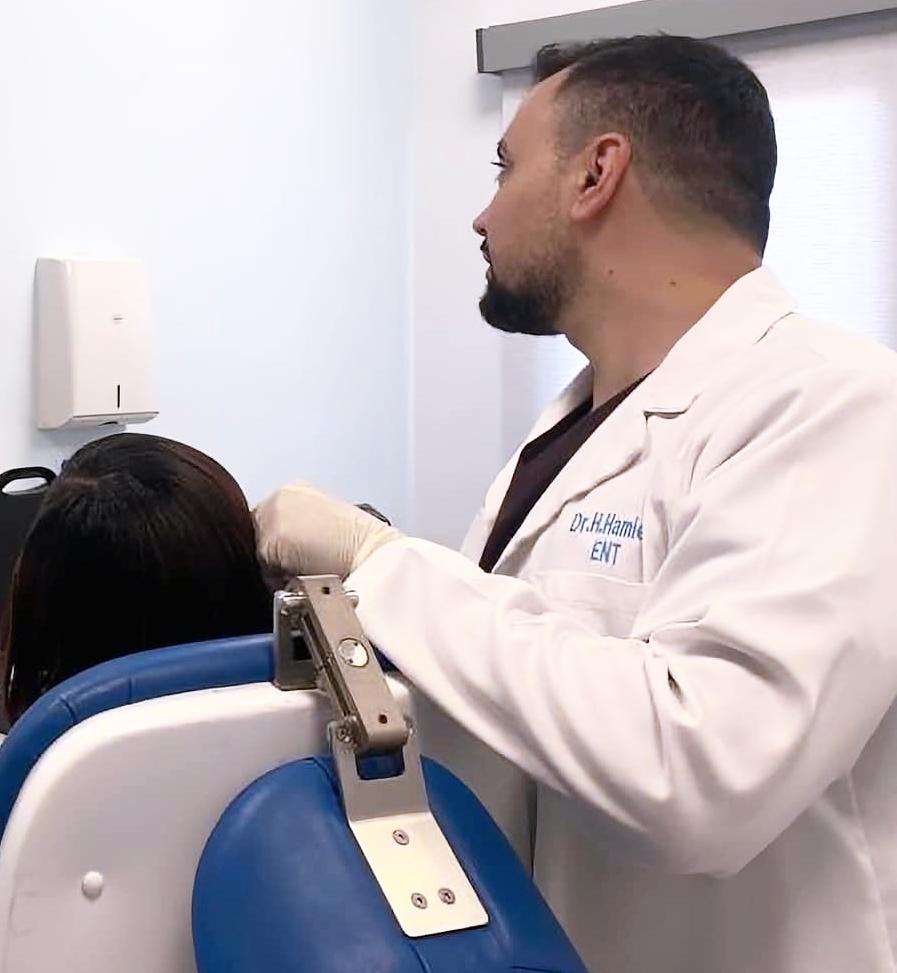
A DOCTOR PERFORMING EAR ENDOSCOPY AT MEDLAND HOSPITAL


Established in 1948, Sheba operates 120 departments and clinics. It has 1,430 beds, about 1,250 physicians, 2,300 nurses and 1,500 other healthcare workers. It employs 1,700 other staff, and nearly 1,000 longterm volunteers, researchers and foreign interns. It handles over



1,000,000 patient visits a year, including 200,000 emergency visits annually, and conducts more than two million medical tests of all types each year. Sheba is supported by donations from a network of philanthropists and friends from around the world.









Located in Riyadh city, capital of Saudi Arabia, started operations in 1995. KFMC complex consists of 4 hospitals: The Main Hospital, The Women’s Specialized Hospital, The Children’s Specialized Hospital and The Rehabilitation Hospital. It has a total capacity
of 1200 beds, 30 fully equipped operating rooms and contains the largest number of intensive care beds in the region. It currently treats 50,000 inpatients and more than 600,000 outpatients annually.

Ichilov, as it’s commonly known, is Israel’s largest acute care facility, a world-class 1500-bed public academic medical center that began with one building in 1963. It treats 400,000 patients and 1.8 million patients visit the hospital every year. The center
has 6000 specialists, of which 1300 doctors and 2055 nurses. It encompasses three hospitals: Ichilov General Hospital and Ida Sourasky Rehabilitation Center, Lis Maternity Hospital, and Dana Children’s Hospital.

The Hadassah Medical Center opened its doors in 1939. It is the oldest and most prestigious medical institution in Israel. Across its two campuses, Hadassah Medical Center has over 1,300 beds, 31 operating theaters, and
nine special intensive care units, and runs five schools of medical professions. The facility operates two university hospitals as well as schools of medicine, dentistry, nursing, and pharmacology

Jordan University Hospital (JUH) prides itself to be the first academic teaching hospital in the Hashemite Kingdom of Jordan and one of the first university teaching hospitals at the level of the Arab World. Established January 1971 the Hospital currently has a bed capacity of 550, 8 specialized intensive care
units and 24 surgical operation theaters. It annually serves more than 500,000 patients in the outpatients clinics, carries out 25,000 surgical operations, and receives 94,000 Emergency cases in the ER. Jordan university hospital’s team consists of 2,600 employees

Johns Hopkins Aramco Healthcare is a joint project that started in 2014 between Saudi Aramco, energy and integrated global petrochemicals company, and Johns Hopkins School of Medicine, the academic medical teaching and research arm of Johns Hopkins University. It
was created as a brand-new health service for Saudi Aramco employees and their dependents. The 483 bed capacity facility has about 255,000 eligible medical recipients, including Aramco employees from Saudi Arabia’s Eastern Province, as well as their dependents and retirees.

Founded in 1938, Rambam Hospital is the largest medical center in northern Israel and fifth largest in Israel. Some 80,000 people are hospitalized there every year, and another 600,000 are treated in its outpatient clinics and
medical institutes. The medical center has 36 departments with 1000 beds, 45 medical units, 9 institutes, 6 laboratories and 30 administrative and maintenance departments. The facility employs Four thousand employees.


Established in 1975, this facility provides the highest level of specialized medical treatment within educational, research, and integrated environments. The hospital has a large 2000+ bed capacity. It has further helped develop and enhance
the examination and treatment procedure within the entire hospital of 1549 bed capacity along with a substantial staff strength of 14,650, which includes medical, research, nursing, administrative and technical staff.

Ichilov, as it’s commonly known, is Israel’s largest acute care facility, a world-class 1500-bed public academic medical center that began with one building in 1963. It treats 400,000 patients and 1.8 million patients visit the hospital every year. The center
has 6000 specialists, of which 1300 doctors and 2055 nurses. It encompasses three hospitals: Ichilov General Hospital and Ida Sourasky Rehabilitation Center, Lis Maternity Hospital, and Dana Children’s Hospital.

Marmara University Hospital was founded in 1991, however, by 2010 the hospital was completely reconstructed to be one of the most equipped and advanced centers of Turkey. It has a 658 bed capacity with 62 emergency
department short stay unit, 29 surgery rooms, 60 adult intensive care beds with third level ventilators, 9 intensive care isolation rooms and 195 polyclinic rooms. Over 250,000 patients are treated there every year.
ByJACKIE MUINDE
Biochemistry and Biotechnology Professionals Society of Kenya (BBPSK), formerly known as the Biochemical Society of Kenya (BSK), is the leading association for scientists specializing in biochemistry, biotechnology, synthetic biology, molecular biology, bioinformatics, and genetics. Since its relaunch in 2019, BBPSK has remained dedicated to advancing these broader fields for the benefit of humanity and the planet.
In an interview with Healthcare Middle East and Africa Magazine, Dr. Patrick Okanya, the chairperson of BBPSK, provided further insights into the association.
HCMEA: We’re delighted to have you here for this interview. Before we dive into the association, could you please take a moment to introduce yourself?
DR. OKANYA: I’m a biochemist by profession. I completed my undergraduate studies in biochemistry and chemistry and pursued my master’s degree in biochemistry at the University of Nairobi. Later, I obtained my Ph.D. from the University of Saarland in Germany, focusing on research in microbial drugs. Upon returning to Kenya, I joined the Technical University of Kenya, where I currently serve as a senior lecturer in biochemistry.
HCMEA: What kind of person are you, and what personal values have enabled you to take on leadership roles in the association?
DR. OKANYA: I consider myself a people person who values transparency and honesty. When it

DR. PATRICK OKANYA, CHAIRPERSON BBPSK
comes to leadership, I think I am gifted because I started being a leader in primary school where I was a class prefect and later a headboy. In my first year in high school, I was a class prefect and later the hall prefect. I was also a head boy in high school.
As an undergraduate, I was a member of the Student Organization of Nairobi University (SONU) and the Chiromo campus congressperson. Leadership qualities have been following me somehow. Most of the time I don’t even put much effort in campaigning but get nominated, maybe it is because of how I carry myself out.
HCMEA: That sounds like a compelling mission. Can you provide an overview of the association, including its origins and key management roles and their responsibilities?
DR. OKANYA: The Biochemical Society of Kenya (BSK), which evolved into BBPSK, was established in 1986 at the University of Nairobi for biochemists engaged in research and teaching, reflecting the limited scope of the field at the time, as only the University of Nairobi offered biochemistry courses.

As time progressed, related disciplines like Biotechnology, Synthetic Biology, Molecular Biology, Bioinformatics, and Genetics emerged as independent fields. This evolution led to a pivotal meeting where the decision to expand beyond biochemistry alone was made, resulting in the birth of BBPSK. The society was officially registered in 2019 under the Societies Act of the Laws of Kenya, with the late Ms. Lucy Kariuki serving as our founding chair.
BBPSK is affiliated with the Federation of African Societies of Biochemistry and Molecular Biology (FASBMB), which in turn is under the International Union of Biochemistry and Molecular Biology (IUBMB).
Our organization comprises several key positions: chairperson (myself), vice chair, secretary-general, organizing secretary, treasurer, assistant treasurer, legal advisor, and digital and communication director.
The chairperson’s primary role is to preside over all meetings, with the vice chair assuming this responsibility when needed. The secretary-general manages meeting minutes and issues memos for meeting notifications. The organizing secretary represents us at workshops and conferences, while the treasurer oversees financial matters.
To enhance our outreach efforts, we recently appointed a digital communication director with an IT background. This director maintains our website and ensures that our social media platforms (including Twitter, Facebook, and WhatsApp)
remain active and engaging.
HCMEA: How does one become a member of BBPSK, and what are the membership requirements?
DR. OKANYA: We draw most of our members from academic institutions that offer biochemistry, biotechnology, and biomedical sciences. We have chatter representatives in public
BBPSK IS AFFILIATED WITH THE FEDERATION OF AFRICAN SOCIETIES OF BIOCHEMISTRY AND MOLECULAR BIOLOGY (FASBMB), WHICH IN TURN IS UNDER THE INTERNATIONAL UNION OF BIOCHEMISTRY AND MOLECULAR BIOLOGY (IUBMB).
universities but in private universities, we have members only.
We also have members from research organizations like Kenya Medical Research Institute (KEMRI), The International Centre of Insect Physiology and Ecology (ICIPE), the International Livestock Research Institute (ILRI), and Kenya Forestry Research Institute (KEFRI) since they have recruited biotechnologies and biochemists.
Others are from related industries, like the pharmaceutical industries and the chemical industries, or those that supply reagents and equipment that have done Biotechnology, Biochemistry, or Chemistry.
HCMEA: How did you personally join the association and eventually rise to its leadership?
DR. OKANYA: Having a background in Biochemistry, I wanted to fit where my peers are. So, I joined BSK while doing my undergraduate at the University of Nairobi. We used to have opportunities to present our work through poster presentations, have networking opportunities, and be in the company of our peers and it was a good anchor for the job market since we knew someone.
It was also through the society that I did my master’s and Ph.D. I was an active member when it was being registered and that’s how I got elected as a vice chairperson and after the passing on of the founding chairperson, Madam Lucy Kariuki, the constitution allowed me to proceed as the chairperson of the association.
HCMEA: What pivotal role does the association play in advancing the healthcare sector?

DR. OKANYA: Biochemists and biotechnologists contribute significantly to healthcare by conducting research that informs disease management strategies. For instance, we research about a disease and how it can be managed. What is the causative agent, is it a virus or a bacterium? How does this bacteria or virus behave? What is the life cycle?
Once we know that, we come up with mitigation measures of what point in the lifecycle to kill it and if we are going to use a drug or produce a vaccine. It’s not just about the theory, we want to get solutions for the theory that is learned in class by providing either products in the form of medicine or vaccines or information that can guide policy.
HCMEA: What core values and strategies have driven the association’s growth?
DR. OKANYA: There are several things that we are doing that have product strategy one being the IT. We actively engage with our members through social media platforms, where we promote opportunities such as scholarships and workshops for undergraduates. Additionally, we run a robust mentorship program pairing students with industry professionals who guide them in areas like writing motivational letters, crafting CVs, and applying for scholarships.
Transparency and honesty are foundational values in all our activities. We prioritize inclusivity, diversity, and gender sensitivity, ensuring active participation from undergraduates
to professors. Our online membership recruitment drive has also been instrumental in expanding our reach and engagement. This combination of innovative strategies and values has been pivotal in propelling our association’s growth and impact.
HCMEA: What services does BBPSK provide to its members?
DR. OKANYA: We provide diverse platforms for sharing scientific findings, including webinars and conferences. When our members conduct research on topical issues, they have opportunities to present their work through webinars on platforms like Zoom or Google Meet. Additionally, we organize physical workshops and conferences where members can network, collaborate, and write joint grant proposals to secure funding from specific donors.
Furthermore, we actively promote research and training programs in biochemistry and related fields through collaborations with national and international scientific bodies. We prioritize maintaining research links with professionals in biochemistry and related disciplines across Africa and beyond.
For our members seeking employment opportunities, we offer support such as recommendation letters and participation in our mentorship program. Through this program, students are paired with professional mentors who provide guidance and support in various aspects of career development.
HCMEA: In time where adoption of technology is rampant in all and every sector, how can technology promote innovation in your profession?
DR. OKANYA: Technology supports curriculum development and provides access to emerging scientific trends. We leverage online platforms to disseminate information about new technologies and organize educational events for our members.
HCMEA: Shifting to another area that has garnered global attention, what sustainability concerns does the association address?
DR. OKANYA: In Kenya, financial sustainability is crucial, given challenges like rising taxes. For instance, a member could have a very brilliant idea that would help us but when it comes to implementing, finances become a challenge. To make sure we don’t lose our members, we’ve come up with easy solutions. We implement cost-effective measures to maintain membership and offer subsidized rates for workshops and conferences.
HCMEA: What key challenges has the association faced, and how have you overcome them?
DR. OKANYA: One challenge we faced was a leadership gap after losing our founding chair in 2022. Additionally, our association is fully registered within the Attorney General’s (AG) chambers but has not yet been passed and enacted by an act of Parliament of Kenya.
The delay in parliamentary approval poses several
challenges. It impedes individuals who have studied biochemistry or biotechnology from enrolling as members, and it may become a requirement for practitioners to obtain a license from us before commencing their practice.
Passing the act of Parliament is crucial for our association to receive government backing and support. It would provide the necessary regulatory framework for us to champion initiatives related to our field, such as research funding or establishing new facilities. Having governmental support ensures that we have the resources and authority needed to carry out our activities effectively.
HCMEA: So, you mean you may need a regulatory body established by the government to oversee this profession?
DR. OKANYA: Yes, that is the direction we aim to take over time. It’s not an easy path; the founders of BSK attempted it, but it didn’t work out. However, that doesn’t mean we should give up. It’s crucial, especially now, to unite all professionals within our society under one umbrella to speak with one voice and gain strength in numbers.
When the government supports an association, it can effectively regulate it. This support would enable us to implement initiatives that benefit our country. It would also provide funding and licenses to establish pharmaceutical industries, start drug or vaccine production, and develop other products based on our profession. Achieving this would significantly contribute to the growth of our association.
HCMEA: In what other ways can the government support your industry?
DR. OKANYA: The government can support small entrepreneurs and innovators with incentives, capital, and licensing to scale up production. We also encourage government support for biotechnology startup industries, particularly in drug or vaccine manufacturing, where biochemists and biotechnologists can actively contribute to innovation and development.
HCMEA: Apart from government support, have you explored other types of partnerships?
DR. OKANYA: Absolutely. We are actively seeking partners and collaborators to sponsor our workshops and facilitate our planned activities through exhibits. While having good ideas is important, implementation often requires financial support. To overcome this challenge and sustain our activities, we recognize the need to form multiple partnerships.
Furthermore, we aim to expand our reach regionally through peer-to-peer collaborations. For instance, we supervise students working on research topics aligned with our interests in neighboring countries like Tanzania and Uganda.
HCMEA: You mentioned expanding regionally; are there similar associations in neighboring countries?
DR. OKANYA: BBPSK is currently the only active society of

biochemists in East Africa. However, we are actively engaging with professionals from Uganda, Tanzania, Rwanda, and Burundi to establish similar associations. Although many African societies in the past have become inactive, we aim to revive and strengthen regional collaboration.
This year, we are organizing a congress in Nairobi, thanks to the Federation of African Societies of Biochemistry and Molecular Biology (FASBNB), to engage professionals from these countries and emphasize the importance of active professional societies in biomedical sciences.
Additionally, we have partnered with the Pan-African University of Science and Technology Innovation (PAUSTI) based at JKUAT. Their students from across Africa are registering with our association, and we aim for them to champion similar associations in their home countries.
HCMEA: What are the association’s plans for the next five years?
DR. OKANYA: Over the next five years, BBPSK plans to introduce certificates and tokens of appreciation for professors who volunteer as mentors and participants in our workshops and conferences. We are also exploring ways to recognize and reward our most active members contributing significantly to our association’s growth.
Moreover, we are advocating for improvements in university curricula to meet current needs. Additionally, we plan to launch more webinars on topical and emerging technologies and host an annual conference to foster knowledge exchange and collaboration within the biochemistry and biotechnology community.

Anoticeable shift in healthcare and wellness approaches has emerged in recent years, with conventional medicine no longer monopolizing treatment options. Patients increasingly explore CAM as a supplementary or alternative approach to conventional treatments. The National Center for Complementary and Integrative Health (NCCIH) defines alternative medicine as seeking non-mainstream approaches to replace conventional medicine. In response, healthcare centres are innovating to meet this demand.
CAM encompasses diverse therapies, practices, and systems that are not typically part of conventional medical care. From herbal remedies to acupuncture and yoga to aromatherapy, CAM offers a multifaceted approach to health that resonates with individuals seeking alternatives to traditional treatments.
These practices offer a different perspective on healing, focusing on the mind-body connection and treating the root cause of ailments rather than just the symptoms. While CAM therapies are often used in conjunction with conventional medicine, they can also be effective on their own for a variety of health conditions.
According to Global Research Market, the CAM market size reached US$162.04 billion in 2022 and is projected to grow at a CAGR of 21.82% to reach US$529.59 billion by 2028. This surge reflects the growing demand for alternative healthcare options. This article explores the factors driving CAM adoption, including growing awareness, increasing disposable income, and government initiatives.
As consumers’ demand for natural remedies for various health concerns increases, many companies are expanding their product lines to include more natural remedies and botanicals. For instance, the wellness company GNC now offers
a wide range of herbal supplements, including ashwagandha, echinacea, and turmeric.
The Ayurveda subsegment is expected to dominate the traditional alternative medicine market owing to technological advancements and increased government support. The Ayurveda market is leveraging technology to improve product quality, increase efficiency, and enhance the consumer experience. For instance, the Indian government launched a digital platform called the Ayurveda Health and Wellness Centre to provide telemedicine and e-consultation services to patients.
The mind-healing segment is anticipated to witness the fastest growth rate between 2024 and 2030. The market presents several opportunities for growth, including the development of new mental health treatments and therapies, expansion of mental health services to
By SHEHREBANU KHUZAIMA


ONE OF THE KEY BENEFITS OF CAM IS ITS HOLISTIC APPROACH TO HEALTH, WHICH CONSIDERS THE WHOLE PERSON – BODY, MIND, AND SPIRIT.
underserved populations, and integration of mental healthcare into primary care. Integration of mental healthcare into primary care has been an area of growth in recent years.
Many primary care clinics now offer mental health services alongside physical health services, which has helped increase the accessibility of mental healthcare and reduce the stigma surrounding mental health treatment. The Transcendental Meditation (TM) subsegment is expected to dominate the mind-healing market driven by programs and institutes such as the Maharishi University of Management in Iowa, U.S., which offers a comprehensive education in transcendental meditation and its related practices.
Technological innovations such as virtual reality (VR) and holographic presence are revolutionizing treatment modalities, making them more accessible. Coworth Park’s introduction of Hebridean sound treatment in October 2023 exemplifies this trend. This therapy utilizes calming sound frequencies for a deeply relaxing and revitalizing experience, contributing to the evolving landscape of holistic wellness offerings.
Holistic approach to health
One of the key benefits of CAM is its holistic approach to health, which considers the whole person – body, mind, and spirit. This approach recognizes that all aspects of a person’s life are interconnected and can impact their overall wellbeing. By addressing the underlying causes of illness and promoting balance in the body, CAM therapies aim to support the body’s natural healing abilities.
Personalized treatment plans
CAM practitioners often take a personalized approach to treatment, considering each individual’s unique needs and preferences. This
can lead to more tailored and effective treatment plans that address the specific health concerns of the individual. By focusing on the individual as a whole, CAM therapies can help promote overall health and well-being.
Many CAM therapies are natural and noninvasive, which can result in fewer side effects compared to conventional medications. For example, acupuncture, a popular CAM therapy, involves the insertion of thin needles into specific points on the body to promote healing and relieve pain. Acupuncture is generally well-tolerated and has minimal side effects, making it a safe and effective treatment option for many people.
Stress reduction and mental health benefits
CAM therapies such as yoga, meditation, and mindfulness practices can help reduce stress, anxiety, and improve mental health. These practices focus on calming the mind, promoting relaxation, and improving emotional well-being. By incorporating these practices into their daily routine, individuals can experience improved mental clarity, reduced stress levels, and a greater sense of overall well-being.
CAM therapies have been shown to be effective in managing chronic pain conditions such as arthritis, fibromyalgia, and back pain. Techniques such as massage therapy, chiropractic care, and herbal remedies can help reduce pain, improve mobility, and enhance quality of life for individuals living with chronic pain.
Despite its growing popularity, CAM is not without challenges and controversies. One of the main challenges is the lack of standardized regulations and accreditation for CAM practitioners and therapies. This can lead to variability in the quality and safety of CAM treatments, raising concerns about patient safety and efficacy. Additionally, some CAM modalities lack robust scientific evidence to support their effectiveness, prompting scepticism and criticism from the medical community. As a result, there is ongoing debate surrounding the integration of CAM into mainstream healthcare and the need for rigorous research to validate its efficacy.
Complementary and Alternative Medicines (CAM) offer diverse therapeutic options that
appeal to individuals seeking holistic and personalized approaches to health and wellness. CAM continues to evolve and expand its influence in the healthcare landscape, from ancient healing traditions to modern integrative therapies. As more research is conducted and integrative models of care are developed, CAM has the potential to play an increasingly prominent role in promoting health and healing for individuals and communities worldwide.
In conclusion, the definition of alternative and interactive medicine remains dynamic and complex. With a steady rising of adoption of complementary and alternative medicine, many health care practices are striving to become innovative providers. They are changing the outlook of the health care system, by leaning towards a more comprehensive style of healing that incorporates all aspects of wellness.
As studies show an evolution in the health care system in the last three decades, it is essential to stay informed and remain open minded about alternative treatment, to be able to provide a comprehensive and personalized plan to patients. As alternative and interactive medicine continues to gain momentum, it is important for the medical community to adapt and evolve to better meet the diverse needs of patients and contribute to improved health outcomes for all.
PROJECTED GROWTH OF CAM MARKET SIZE BY 2028 21.82%
NUMBERS NEEDLE THERAPY, A FORM OF CAM
By Dr Lucas Nyabero, CEO Elara Health Innovations Inc
Among the well-thought-out facets of the Global Fund is the Resilient and Sustainable Systems for Health (RSSH) initiative. RSSH is central to many of the Global Fund’s primary goals, including sustainability, transition, and repositioning, ultimatelyleading to a stronger healthcare system that serves all citizens effectively. This hidden gem within the Global Fund initiative plays a crucial role in building robust healthcare systems while the larger portion of the Global Fund focuses on combating the three major diseases: HIV/AIDS, tuberculosis, and malaria.
The strategic implementation and utilization of RSSH makes it the “little engine that could,” in steadily fortifying healthcare infrastructures. The recognition of this potential by the Ministry of Health (MoH) could accelerate the transformation of a country’s healthcare systems. RSSH should be seen as an investment rather than merely a means of subsistence for the healthcare sector. Successful countries invest RSSH funds in developing their healthcare systems, whereas others may use

these funds primarily for salaries and operational expenses. Understanding RSSH’s potential can lead to substantial improvements in healthcare provision and positive patient outcomes, ultimately achieving Universal Health Coverage (UHC) and the establishment of a well-run healthcare system.
A crucial component of developing a resilient healthcare system is the establishment of data-driven decisionmaking enabled by data generation, collection, and analysis capabilities. This data-driven approach informs decisions, policies, and guidelines, allowing for continuous improvement and innovation at various touch points within the healthcare system.
Emphasizing Return on Investment (ROI) within healthcare programs promotes sustainability thinking. Contrary to popular belief, focusing on ROI does not compromise quality. Instead, it fosters profitability, growth, and sustainability, ultimately resulting in affordable healthcare services. A well-compensated workforce benefits the economy from the multiplier effect of competitive compensation, expanding the tax base and by extension strengthening the healthcare system.
Well-designed RSSH projects should include resilience to pandemics as well as considerations for climate change effects and how to mitigate them. Climate changes have led to unforeseen health issues, like Malaria in surprising and unexpected places like Florida. Climate change repercussions have led to atypical pathogen which pose a great challenge to the healthcare system. The harsh season temperatures were sufficient to control pathogens are now milder. Scientific forcasting suggest that more unpredictable health threats will arise. RSSH can help inoculate healthcare systems against complete breakdowns by leveraging lessons from Malaria and COVID-19 response data analysis to inform the development of new surveillance systems.
Geo political issues stemming from epidemics and or pandemics can lead toregional political unrest. Early detection is key to avoid such conflicts. RSSH can facilitate the establishment of robust epidemiological surveillance systems, utilizing data to identify threats, devise viable solutions, and marshal resources to contain them. This proactive approach coupled with robust coordination can curtail the spread of diseases and pandemics like Ebola and COVID-19,, data, coordination, and international collaboration which are key to a successful response are aspects that RSSH investment can support.
The hallmark of a high-quality healthcare system is
consistently positive patient outcomes. Achieving sustainable outcomes indicates a well-designed system with established standards of care and responsiveness to citizens’ health challenges. Consistency in healthcare results leads to predictable costs and facilitates better budgeting. Quality also involves learning from failures to inform system improvements. All these steps are supported by a strong healthcare system, a goal envisioned by the Global Fund through RSSH.
The genius of RSSH lies in recognizing its potential to invest in the future and reap the rewards in a timely fashion. Conversely, using RSSH funds primarily for operational costs and overheads, with minimal infrastructure support, undermines this potential. While the Global Fund focuses on TB, HIV, and malaria, the RSSH framework is designed to integrate with and enhance these efforts, boosting overall healthcare system performance.
It is not too late for countries to reconsider their RSSH utilization strategies. Focusing on investing these funds in infrastructure can provide the necessary support for transitioning from the Global Fund. Building the various aspects of the healthcare system as outlined above will lead to self-sustaining and effective healthcare systems.
Post-Global Fund planning should be a priority. Honest analysis of healthcare system shortfalls, innovative approaches to healthcare challenges, collaboration with all communities, inclusion of traditional healthcare techniques, responsiveness to healthcare challenges, and a deliberate focus on ROI are essential for effective healthcare functions.
Governments are motivated to provide for their citizens. An
effective and efficient healthcare system will lead to healthy communities which in turn will increase productivity, leading to high GDP, a larger tax base therefore more tax collection and resources to tackle other priorities, A healthy people is a healthy productive nation. RSSH investments will ensure the building of an appropriate, efficient, effective, predictable, and affordable healthcare system. The result of which will support the prosperity of the great Nation.
Food security seems like a misplaced RSSH discussion. Consider this, Nutrition is a key part of wellness and health. Good nutrition reduces healthcare spending in a roundabout way. RSSH funding can be used to set up a system that will ascertain what nutritional elements would best protect the public from common illnesses. Nutritional elements that will promote healing, Nutritional elements that build healthy resilient bodies from birth. As the saying goes, “…let your kitchen be your pharmacy…” RSSH investment will go a long way to identify how to reduce healthcare system utilization by prevention and healing time.
The walk from here to a Transition to a strong sustainable healthcare system is not a pipe dream. It is a realistic goal that can be achieved. Below is an example of how to achieve it. For governments that have unutilized RSSH fund, there are viable options. To facilitate an orderly transition Global Fund needs to reinvigorate RSSH as a key strategy for a transition from Global Fund. The repositioning of CCM will consolidate the gains and share their learnings from the administration of Global Funds with MoH. It is time to plan and invest for the post-Global Fund MoH. HCMEA

By ELLY OKUTOYI
As the global momentum toward achieving Universal Health Coverage (UHC) grows, African and other developing nations are increasingly at the forefront of this movement. Despite historically lagging behind in healthcare services compared to the developed world, many nations in these regions are now making significant progress towards providing affordable high- quality healthcare for all in line with World Health Organization standards. One such Country is Morocco.
Officially known as the Kingdom of Morocco, the African-Arabian country is found in the Maghreb region of North Africa, right in the heart of the larger Sahara Desert. Spreading over a surface area of 446.55 square kilometers, the Kingdom boasts of a general population of 38.2 million people as at March 30th 2024, indicating a growth of 1.02%, an increase from 2023. The North African Country is headquartered at the city of Rabat.
With a rich history spanning about over 300,000 years back, the Kingdom came into
existence in 788 after the establishment of the Idrisdid dynasty by Idris. The Alawy dynasty, which rules the country to this day, seized power in 1631, and over the next two centuries expanded diplomatic and commercial relations with the Western World. Morocco’s strategic location near the mouth of the Mediterranean drew renewed European interest; in 1912, France and Spain divided the country into respective protectorates, reserving an International Zone Tangier. Following intermittent riots and revolts against colonial rule, in 1956, Morocco regained its independence and reunified.
Generally, the Moroccan healthcare system is in two tiers-the public and the private sectors. The public healthcare system is known as National Health Insurance Fund (CNAM) and is funded by public coffers. This provides access to basic medical care, including preventive care, hospitalization and emergency services. The public sector runs 2,689 primary health care facilities and 144 hospitals at different levels, with
a total bed capacity of 22,146. Although the Country has both public and private health facilities, private hospitals are preferred owing to long ques and lower quality medical care experienced at the public healthcare facilities. The private sector healthcare market on the other hand is growing rapidly with more than 356 private clinics and 7,518 physicians. The private health sector holds a bed capacity of over 27,000. This includes ultra-modern facilities such as Clinique Internationale in Marrakech, Clinic Averroes founded in 1975 by Dr. Ahmed Laaabi also in Marrakech and Anfa Fertility Centre in Casabalanca.
Despite the challenges facing the Moroccan healthcare system, there have been several notable achievements in recent years that offer hope for improvement. One significant achievement is the implementation of universal health coverage through the Ramed program in 2011. This program aims to provide health coverage to vulnerable populations, including those who are poor or have chronic diseases.
The Ramed was replaced in 2023 by the generalization of social coverage for the benefit of all Moroccans, which was launched on July 29, 2020, by King Mohammed VI. This project requires a rigorous reform of social systems and programs following the crisis linked to the COVID pandemic, which has highlighted several shortcomings, particularly the weakness of social protection networks.
Over the years, there have been significant improvements in key health indicators. Life expectancy at birth has seen a remarkable rise, increasing from 47 years in 1967 to 74.8 years in 2013. Infant mortality rates have seen a substantial decline, with numbers falling from 113.6 per thousand live births in 1967 to 28.8 in 2013. Similarly, maternal mortality has witnessed a significant decrease, dropping from 359 per hundred thousand live births in 1981 to 112 in 2013.
The healthcare system has also made

great strides in vaccination coverage, with a commendable 94.5% coverage rate and equitable distribution across different regions. This has resulted in the elimination of targeted diseases like poliomyelitis and diphtheria, as well as a notable decrease in the incidence of diseases such as measles.
The expansion of health coverage has been a priority, evident through a substantial increase in the number of basic healthcare establishments and hospitals throughout the country. Moreover, there has been a notable rise in the number of doctors, with a ratio of 1 doctor per 1775 inhabitants in 2009, which improved to 1.5 doctors per 1000 inhabitants in 2014.
Another significant achievement is the development of the pharmaceutical industry in Morocco. The industry has been successful in meeting 70% of the national demand for drugs, ensuring a more self-sufficient and accessible healthcare system for the population. According to the Country’s Minister of Health and Social Protection Dr. Khalid Ait Taleb, Morocco plans to promote local production through collaboration between the Ministries of Commerce and Industry and that of Health and
Social Protection- “In collaboration with the Ministry of Industry and Commerce, we are actively working on promoting the ‘Made in Morocco’ brand and local production. This is because Morocco has significant potential; we can easily become an African hub, and not only that but also a part of the European hub. Other countries are now looking to reshore their production, which will considerably impact their healthcare costs. Hence, Morocco can benefit from this situation and become a key player in both African and European pharmaceutical distribution” Said Dr. Taleb.
These accomplishments in Moroccan healthcare reflect the country’s commitment to improving the wellbeing of its citizens and provide a solid foundation for the continued growth and advancement of the healthcare sector.
The Moroccan authorities have also invested in expanding healthcare infrastructure, including building new hospitals and clinics and upgrading existing facilities. Additionally, several e-health services, such as telemedicine and electronic health records, have been developed to improve healthcare access and efficiency. For instance, the
ORGANIZE THE CARE PATHWAY TERRITORIALLY, TO LIGHTEN THE LOAD
REDUCE
Government has begun construction of Ibn Sina University Hospital in Rabat. This will be largest Public Hospital in Morocco with a bed capacity of over 1000 beds, at a cost of Dh. 1.55 billion.
UNIVERSITY HOSPITAL CENTER IN TANGIER , ONE OF THE HEALTHCARE FACILITIES IN MORROCCO
Meanwhile, in spite of the significant progress made in recent years, the Moroccan healthcare system still grapples with several challenges that limit its ability to deliver high-quality healthcare to all citizens. One significant challenge is the unequal distribution of healthcare resources, with urban areas having better healthcare access and resources as compared to rural areas. This disparity is particularly pronounced in the areas of healthcare infrastructure, medical equipment, and specialist care.
One other significant challenge choking Morocco just like any other developing nation is inadequate funding for healthcare, which limits the ability to provide essential medicines and technologies, improve healthcare infrastructure, and increase healthcare access. Additionally, there is a shortage of healthcare workers, particularly doctors and nurses, especially in rural areas. This shortage results in longer wait times for patients and limited access to specialist care.
Furthermore, non-communicable diseases (NCDs) are increasingly prevalent in Morocco, posing a significant health burden. NCDs account for over 80% of deaths in Morocco, and the healthcare system is not adequately equipped to manage these diseases. The prevalence of risk factors for NCDs, such as tobacco use, unhealthy diets, and physical inactivity, is also high in Morocco.
Moreover, insufficient health insurance coverage: 38% of the population does not have medical coverage, and those who do have coverage may still face high out-of-pocket expenses for healthcare services.
The Moroccan healthcare system struggles with the management of health data and information, which limits its ability to make informed decisions, monitor healthcare quality, and plan healthcare strategies.
Finally, it is evident that the main source

of these difficulties is generally the lack of optimal, rational, and effective governance; good governance has become an essential factor in efforts to strengthen health systems and its impact on health is now more decisive. The overall governance of the system does not empower the actors, neither does it encourage quality.
The Moroccan Ministry of Health’s budget is expected to see a 9.1% increase in 2024, reaching around MAD 30.7 billion (USD 3.48 billion), according to the new 2024 budget. The budgetary increase is primarily focused on enhancing the healthcare infrastructure across the country, as detailed in the official report released by the Ministry of Economy and Finance.
In recent years, the Ministry of Health and Social Protection, along with University Hospital Centers (CHU), have received substantial financial support. Notably, the 2024 budget is poised to introduce 5,500 new budgetary positions, contributing to a cumulative total of nearly 42,700 positions created between 2017 and 2024, with 35,500 allocated to the Department of Health.
In 2024, the country is slated to implement vital initiatives integral to the ongoing transformation of the national healthcare system. These initiatives encompass projects initiated by King Mohammed VI. Key initiatives for 2024 include addressing investment expenses related to the construction and equipping of new CHUs, along with comprehensive enhancements to Primary Healthcare Facilities (ESSP) throughout the country.
Additionally, these projects entail the deployment of an integrated information system, as well as the continued modernization of hospital infrastructure and equipment, aligning with the nationwide implementation of Mandatory Health Insurance (AMO). The Ministry of Health is also set to initiate new hospital infrastructure projects and bring existing hospital construction projects to completion. The measures aim to improve healthcare and provide citizens with quality medical services. The budget increase for 2024 is expected to have a positive impact on healthcare accessibility and quality throughout Morocco.
While the recent achievements in the Moroccan healthcare system are promising, several perspectives can guide future efforts to improve the country’s healthcare system.

The health map project which is currently being carried out aims to organize the care pathway territorially, to lighten the load on health structures, reduce waiting times and properly distribute care between regions.
In this scheme, the university hospital center plays a crucial role as a leading specialty, research, and training institution and as a structure to which the other hospitals in the region are linked within the framework of the Regional Health Groups.
The Public-Private Partnership is another means that aims to improve health services for the population, which will also make it possible to pool the resources of the public and private sectors and allow, for example, the health professionals of the latter to offer health care at the level of public health structures by taking advantage of their technology platform.
Addressing the unequal distribution of healthcare resources between urban and rural areas is essential. To achieve this, there must be a concerted effort to invest in rural healthcare infrastructure, medical equipment, and specialist care, as well as increase the number of healthcare workers in these areas.
Notably, adequate funding for healthcare is necessary to provide essential medicines, medical equipment, and technologies, and improve healthcare infrastructure. Increased funding will also facilitate the recruitment and retention of
KHALID AIT TALEB , MORROCCO’S HEALTH MINISTER
NUMBER OF PRIMARY HEALTH CARE FACILITIES IN MORROCCO

CLINIQUE INTERNATIONALE DE MARRAKECHONE OF THE BEST HOSPITALS IN MOROCCO
healthcare workers, particularly in rural areas. Managing and preventing NCDs should be a priority. This requires a comprehensive approach that includes promoting healthy behaviors, improving healthcare access and quality, and addressing social determinants of health that contribute to the development of NCDs.
There are several propositions to enhance the Moroccan health system and pave the way for further improvements. First, the digitization of the health system can bring significant benefits. By embracing digital technologies, such as electronic health records and telemedicine, healthcare delivery can be streamlined, ensuring efficient management of patient data and enabling remote consultations. Additionally, reorganizing the care pathway from the community level to the regional level can improve access and coordination of healthcare services, allowing for better integration and collaboration among healthcare providers.
To strengthen the public healthcare sector, efforts should be made to make public hospitals more attractive. This can be well achieved by empowering them to generate their resources, promoting financial sustainability, and implementing measures that enhance the quality and efficiency of services. Encouraging public-private cooperation can also play a vital role in leveraging the strengths of both sectors, fostering innovation, and expanding access to healthcare for all.
Another perspective of great importance is the
implementation of an integrated and intersectoral policy focused on prevention and health education. By addressing health promotion and prevention strategies, such as disease prevention campaigns and education programs, the burden of illness can be reduced, and individuals can be empowered to make informed choices about their health. This requires collaboration across different sectors, including education, public health, and community organizations, to create a comprehensive and effective approach to improving population health.
By embracing these propositions and perspectives, Morocco can lay the foundation for a more efficient, inclusive, and patient-centered health system that prioritizes prevention, embraces innovation, and ensures equitable access to quality healthcare services for all its citizens.
Challenges such as resource disparities, inadequate funding and rampant noncommunicable diseases not withstanding achievements like Universal Health Coverage and improvements in Health Indicators show progress.
To enhance the system, addressing resource disparities, increasing funding and workforce, managing non-communicable diseases, embracing technologies and focusing on prevention are quite key. These steps would pave way for an efficient, inclusive and patientcentered healthcare system in the Northern Africa Country.

ADVERTISE IN HEALTHCARE AFRICA MAGAZINE & WEBSITE?
• Reach the healthcare, pharmaceutical and biotech industry in Africa & Middle East using one publication and website
• The first magazine of its kind in the region that is also available in a digital format, providing our advertisers with a regional and worldwide audience

• The magazine and website are read by key decision makers with direct responsibilities for purchase of new technologies, products and services across Africa, Middle East and globally.
• HealthCareMEA.com is the best read and visited healthcare, pharmaceuticals and biotech news website in Africa and the Middle East.







By VINCENT MORANGA
Just like any struggle for independence, malaria eradication journey has not been easy especially in the Africa region where declining efficacy of existing, lower-cost interventions such as insecticides, anti-malarial medications, and rapid diagnostic tests exist. Although, malaria is widespread throughout tropical and subtropical regions of the world, Africa carries a disproportionately high share of the global malaria burden, both in terms of total malaria cases and malaria deaths.
According to data from the World Health Organization (WHO), in 2022, the African Region bore the heaviest malaria burden, with 94% of cases and 95% of deaths globally. This represented 233 million malaria cases and 580,000 deaths, a slight reduction from 2021.
Despite this increase, progress has been made in recent years with increased coverage of preventive measures and improved access to treatment. This article explores the steps made in the African region to curb the spread of this disease.
Africa bears the brunt of the world’s malaria burden, with the
Centers for Disease Control and Prevention (CDC) estimating that the continent alone loses US$12 billion in economic productivity each year due to loss of work time. In Uganda alone, Dr Jimmy Opigo, the Health Ministry’s Manager for the malaria control program, estimates that malaria kills approximately 16 Ugandans per day and causes an estimated annual economic loss of US$500 million (UGX 1.76 trillion) due to treatment and lost work time.
Moreover, children under the age of five are the most vulnerable, accounting for over 80% of all malaria deaths in 2022, according to the latest data from WHO. Of these fatalities, about 96% occurred in twenty-nine countries worldwide, with four African countries accounting for more than half of all cases: Nigeria (27%), the Democratic Republic of the Congo (12%), Uganda (5%), and Mozambique (4%).
While malaria case incidence in the WHO African Region decreased from 373 to 225 per 1000 people at risk between 2000 and 2019, it rose to 234 in 2020, primarily due to service disruptions during the COVID-19 pandemic. Lockdowns
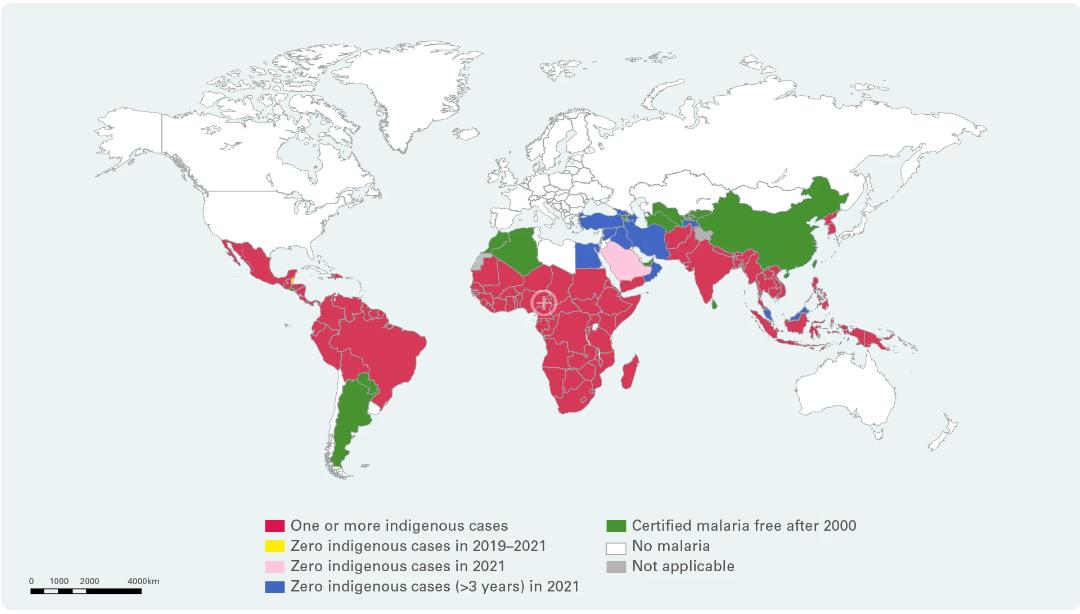
Source: WHO
and supply chain issues disrupted routine immunization and cases of infectious diseases such as malaria, HIV/AIDS, and TB, leading to a surge in both incidence and mortality rates, exacerbating already stalled progress against the disease.
According to the 2022 World Malaria Report, malaria cases grew to 241 million in 2020, with 627,000 deaths, representing 14 million more cases and 69,000 more deaths than in 2019. About two-thirds of these additional deaths (47,000) were due to disruptions in malaria prevention, diagnosis, and treatment during the pandemic.
Given the significant impact of COVID-19, Dr. Tedros Adhanom Ghebreyesus, WHO Director-General, was keen to highlight during the launch of the 2022 Malaria report that “Even before the COVID-19 pandemic struck, global gains against malaria had leveled off.” He added, “Now, we need to harness that same energy and commitment to reverse the setbacks caused by the pandemic and step up the pace of progress against this disease.”
Despite the challenges imposed by COVID-19, by the end of 2020, about three-quarters (72%) of insecticide-treated mosquito nets (ITNs) had been distributed in malaria-endemic countries as planned. Thirteen nations in Africa’s Sahel subregion provided preventive antimalarial drugs to 11.8 million more children during the high-transmission rainy season in 2020 than they did in 2019 when the COVID-19 epidemic began.
Climate change has worsened malaria transmission dynamics by changing the disease’s geographic distribution and lengthening transmission seasons. Warmer temperatures,
changes in rainfall, human activity, increased vector survival and bite rates, pathogen replication inside vectors, and shorter reproduction rates have all contributed to their spread. As a result, mosquito-borne diseases are expanding and reemerging in locations where they have remained dormant for years.
The invasion of Anopheles stephensi in Sub-Saharan Africa, where malaria is most prevalent and more than 40 percent of the population lives in cities, has sparked a concern since it is suspected to have fueled the comeback of malaria in Djibouti. Furthermore, based on new mathematical modeling and observations in Djibouti, experts believe the species can potentially raise Plasmodium falciparum incidence by 50 percent.
In Kenya, the Kenya Medical Research Institute (KEMRI) has also detected Anopheles stephensi in the Laisamis and Saku sub-counties of Marsabit County in northern Kenya. Studies indicate that this unique vector can proliferate in various environments, including jerry cans, tires, open tanks, sewers, overhead tanks, and underground tanks. This sets it apart from Anopheles gambiae and Anopheles funestus, which typically thrive in water pools, rice paddies, surface run-offs, hoof prints, or streams.
Its proliferation in urban and peri-urban areas poses a significant threat to malaria transmission in formerly Anopheles-free areas since it transmits both Plasmodium falciparum and Plasmodium vivax malaria parasites.
Besides climate change, resistance to current control methods, such as insecticides and antimalarial medicines, is becoming
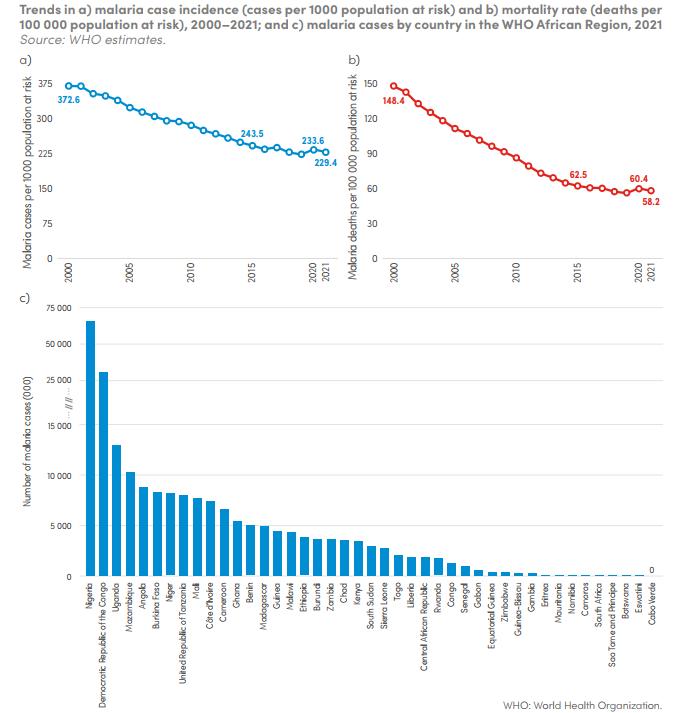
Source: World Health Organization
increasingly concerning and threatens the malaria control initiative. Experts such as Dr. Dunia Munyakanage, a vector control supervisor at the Rwanda Biomedical Centre, point out that charter, a fixed-dose combination of artemether-lumefantrine, a significant malaria treatment medicine, has also developed resistance. Scientists are compelled to design novel treatments for the Anopheles stephensi mosquito, finding a way to resist artemisinin, a vital component of coartems.
Amidst recent advancements, WHO has intensified its focus on recommending antimalarial drug replacement should efficacy levels drop below 90%. Consequently, a combination of artemisinin-based combination therapies (ACTs) is now
regarded as highly efficacious. Universal Corporation Limited (UCL) based in Kenya made history in November 2023 by becoming the first African company to secure WHO prequalification for the manufacture of sulfadoxinepyrimethamine with amodiaquine (SPAQ), a pivotal antimalarial medication.
While malaria eradication has long been the ultimate goal, numerous initiatives have predominantly focused on precise malaria diagnosis, vector control, and the advancement of new antimalarial drugs. However, recent strides in malaria vaccines have brought us closer to realizing this elusive objective, instilling optimism for a future where
vaccinations play a pivotal role in disease prevention.
WHO has recommended two vaccines, RTS, S/AS01 (Mosquirix), and R21/Matrix-M, for children living in areas with moderate to high transmission of Plasmodium falciparum malaria, as a breakthrough for child health and malaria control.
Since 2019, over 2 million children in Ghana, Kenya, and Malawi have been vaccinated with the RTS,S/AS01 malaria vaccine as part of a pilot program. Furthermore, over 30 additional African countries have expressed interest in receiving the vaccine. Additionally, 18 million doses of RTS,S have been sent to 12 African nations for use between 2023 and 2025.
The ongoing vaccination campaign has yielded promising results, with a notable decrease in malaria illness, a 13% reduction in overall child mortality, and significant declines in hospitalizations. Under the guidance of WHO, the Malaria Vaccine Implementation Programme (MVIP) has been instrumental in coordinating these efforts. Additionally, the financing provided by Gavi, the Vaccine Alliance, amounting to US$160 million for the 2022–2025 period, ensures the expanded deployment of malaria vaccines in Gavi-eligible countries.
Studies demonstrating the safety and efficacy of these vaccines have resulted in a notable decrease in cases of severe malaria and a reduction in child fatalities in Africa. This has led Thabani Maphosa, Managing Director of Country Programmes Delivery at Gavi, to highlight renewed hope in the fight against malaria, “This vaccine has the potential to be very impactful in the fight against malaria, and when broadly deployed alongside other interventions, it can prevent tens of thousands of future deaths every year.”
WHO anticipates that following these rollouts, yearly global demand for malaria vaccines will increase to 40–60 million doses by 2026 alone, with a goal of 80–100 million doses annually by 2030. In addition to the RTS,S vaccine, developed and produced by GSK, and
in the future supplied by Bharat Biotech, a second vaccine, R21, developed by Oxford University and manufactured by the Serum Institute of India (SII), is expected to play a significant role in future vaccination efforts. This vaccine was WHO prequalified in December 2023, paving the way for its inclusion in the ongoing vaccination drive.
Effective malaria management relies heavily on accurate and prompt diagnosis, so advancements in diagnostic technologies are becoming crucial. These technologies include rapid diagnostic tests (RDTs) and molecular diagnostics, which facilitate swift identification of malaria infections, particularly in regions with limited resources.
However, the emergence of Plasmodium falciparum parasites carrying deletions in the Pfhrp2/3 genes poses a significant challenge to the reliability of conventional RDTs. These genes encode antigens targeted by many RDTs, and their absence can lead to false-negative results. This necessitates the diversification of diagnostic tools to address this vulnerability.
Molecular diagnostics, such as PCR assays, are, therefore, becoming common as they offer higher sensitivity and specificity than RDTs and are invaluable for detecting genetic variations impacting diagnostic accuracy. They are particularly proficient at identifying non-falciparum species, which are often missed by traditional RDTs. However, making molecular techniques more accessible and affordable in resource-limited settings remains a hurdle.
The eradication of malaria in Africa is within reach, provided that key obstacles are addressed through focused research and development initiatives. Improving the sensitivity of diagnostic instruments for non-falciparum species and enhancing their applicability across various diagnostic settings are critical steps in this process. This could include identifying novel biomarkers, refining assay procedures, innovating point-of-care diagnostic equipment, and accelerating vaccine development efforts.
In Malawi, the microfluidic point-of-care (mPOC) immunoassay is now being used as it has shown comparable accuracy to a commercial HRP2 enzyme-linked immunosorbent assay (ELISA) test while being 12 times quicker and easier to use. According to Peter Lillehoj, associate professor of mechanical engineering at Rice’s Brown School of Engineering, mPOC can rapidly and effectively diagnose malaria, particularly cerebral malaria, at the point of care, which might lead to early detection and treatment of severe cases.
Meanwhile, in Kenya, the Ministry of Health (MOH) has taken steps to install an automated malaria testing system (XN-31 analyzer) at the Ahero County Hospital Laboratory in Kisumu County. This state-of-the-art analyzer, with exceptional sensitivity and specificity, is designed to detect and count malaria-infected red blood cells within a minute. The implementation of the XN-31 analyzer holds promise for addressing malaria diagnostic challenges and enhancing the accuracy and efficiency of treatment for this endemic disease.
WITH STUDIES DEMONSTRATING THE SAFETY AND EFFICACY OF THIS VACCINE, THERE HAS BEEN A NOTABLE DECREASE IN CASES OF SEVERE MALARIA AS WELL AS A DECREASE IN CHILD FATALITIES IN AFRICA
Malaria’s devastating toll on Africa has been undeniable, claiming millions of lives annually and inflicting significant economic repercussions. Although climate change, insecticide resistance, and evolving vector species still pose new challenges to malaria control efforts, risking hard-won gains, there is reason for optimism. Africa must sustain its momentum and foster collaboration to address the multifaceted challenges posed by malaria.
Innovative approaches, more robust healthcare systems, and increased international cooperation are essential to realizing a malaria-free world. Encouragingly, the number of malaria-endemic countries has decreased from 108 in 2000 to 84 in 2021, signifying substantial advancements in eradication efforts. Cabo Verde stands out as the most recent success story, having reported no malaria deaths since 2018. It joins the ranks of Mauritius and Algeria as the third country in the WHO African region to achieve malaria-free certification.

SPAIN – Bruker Corporation, a leader in the post-genomic era, has showcased its latest innovations at the ESCMID Global 2024 conference in a bid to revolutionize microbial identification, antimicrobial susceptibility testing, and infectious disease diagnostics.
With a focus on streamlining clinical workflows and enhancing patient care, Bruker introduced novel diagnostic tools aimed at expediting treatment decisions and improving outcomes.
Bruker introduced the MBT Pathfinder® IVD robot, designed to automate target preparation for the MALDI Biotyper® system.
This advancement promises to eliminate time-consuming manual tasks, enhancing efficiency and accuracy in microbial identification.
The IVD-CE MALDI Biotyper®, coupled with the MBT Sepsityper® IVD Kit, enables the identification of pathogens within 20 minutes from positive blood cultures.
Additionally, Bruker provides clinical workflows for rapid antibiotic resistance testing, facilitating timely and targeted treatment strategies.
Antimicrobial susceptibility testing (AST) is crucial for guiding antibiotic therapy, and Bruker’s UMIC® tests deliver precise results for single antibiotics and combinations.
The MICRONAUT range offers AST of multiple antibiotics in a single test, streamlining laboratory workflows while ensuring compliance with CLSI/ EUCAST recommendations.
These advancements empower healthcare professionals to make informed treatment decisions tailored to individual patient needs


USA — Siemens Healthineers, a German company that provides healthcare solutions and services, has released an app for Apple Vision Pro.
The Cinematic Reality app allows surgeons, medical students, and patients to view immersive, interactive holograms of the human body captured through medical scans in their real-
world environment.
This new tool is vital for visualizing renderings, which can aid in surgery planning and medical education, as well as helping patients visualize treatments.
This app is intended to use the power of Apple Silicon and Metal, laying a solid foundation for future development.
The software serves to provide a more realistic method of viewing organs or bodily components, allowing doctors to better explain clinical issues to patients, discuss clinical questions about referrals, and teach medical students.
In the future, this could help surgeons with pre-operative planning, enable multidisciplinary communication among doctors in many professions, or help non-radiologists and patients better understand scans and situations.
USA - Mayo Clinic, a nonprofit American academic medical center dedicated to integrated healthcare, education, and research, has introduced a new program aimed at expediting the development and implementation of digital health solutions within hospitals.
This initiative, known as Solutions Studio, was unveiled on March 12th at the annual HIMSS conference in Orlando, Florida.
As part of the Mayo Clinic Platform, Solutions Studio focuses on leveraging data insights and technology adoption across its product and service portfolio.
The primary objective of the program is to accelerate the development, validation, and deployment of digital health solutions while seamlessly integrating them into healthcare workflows.
By doing so, it aims to mitigate the high costs and complexities often faced by digital health startups during the commercialization process, thus fostering greater adoption of solutions within hospitals.
Mayo Clinic’s overarching mission

is to simplify and hasten digital health innovation and adoption by granting access to global, federated, de-identified data and facilitating quick, scalable integration into clinical workflows.
Solutions Studio will benefit digital health enterprises by providing clean, curated, de-identified data from various worldwide regions for model creation and refinement.
Additionally, it will offer a library of tools with analytics and federated training capabilities, along with the ability to conduct objective assessments of model performance using the Mayo Clinic Platform Validate.
USA - Siemens Healthineers has introduced a groundbreaking solution to streamline intraoperative imaging with the Ciartic Move3, a self-driving C-arm system.
With the increasing demand for minimally invasive procedures and the shortage of medical staff, there’s a growing need for automation to enhance efficiency and reduce the burden on surgical teams.
The Ciartic Move3’s holonomic, omnidirectional wheels will enable precise movements even in tight spaces, while its remote-control functionality allows for effortless positioning and retrieval of stored positions.
This automation not only saves time
but also provides accurate imaging, potentially reducing overall treatment times by up to 50%, especially in spine, pelvic, and distal radius surgeries.
By relieving surgical staff from the manual task of repositioning the C-arm, the Ciartic Move3 not only saves time but also reduces physical strain and the risk of error.
Its ability to store multiple positions and image parameters further enhances workflow efficiency.
Moreover, the system’s active sensing technology provides collision protection, ensuring safety within the operating room.
The inclusion of touch-sense handles facilitates motor-assisted movements, making it easier to steer the system and operate it remotely, even from a sterile area.
This advancement is particularly significant amidst the global shortage of medical staff, as it alleviates the burden on surgical teams and minimizes the need for postponements due to staff shortages.
IRAQ – The Health Resources and Services Availability Monitoring System (HeRAMS) initiative has transformed Iraq’s healthcare management by making vital information easily accessible for planning and decisionmaking.
The project, which began in January 2022, seeks to offer comprehensive data on critical health resources and services to decision-makers at all levels by midJune 2024.
HeRAMS has considerably strengthened Iraq’s health information systems by standardizing the collection, analysis, and dissemination of information linked to key health services across the country.
The effort promotes health equity and equal access to vital health services
in Iraq by creating, maintaining, routinely updating, and distributing a master list of health facilities, resources, and services.
The project implementation strategy was guided by WHO’s “Digital Implementation Investment Guide,” which ensured compatibility with the aims of Iraq’s national health system.
Active participation from both ministries was required to assure the project’s ownership and sustainability.
The first phase of HeRAMS implementation in 2022 and 2023 produced an online dynamic dashboard and approximately 500 pages of descriptive analysis, including an advanced model for obtaining health services.

KENYA - Kenyatta University Teaching, Research, and Referral Hospital (KUTRRH) has reached a historic milestone by successfully completing a rare operation to repair the main artery without the need for open surgery.
The operation, termed Thoracic Endovascular Aortic Repair (TEVAR), represents a significant development in medical care within Kenya’s public healthcare system.
This first-of-its-kind non-invasive strategy for treating thoracic aortic aneurysms and dissections in a public hospital in Kenya gives patients quicker recovery times than typical open-heart surgery.
Previously, surgeons depended on open surgery techniques for such diseases, but TEVAR is increasingly recommended due to its faster recovery time and lower risk of significant consequences, particularly in qualified individuals.
The first operation, done on a female patient, demonstrates KUTRRH’s dedication to pioneering revolutionary medical techniques.
In addition to the TEVAR milestone, KUTRRH recently introduced ultracyberknife technology aimed at enhancing cancer treatment capabilities.
With approximately 35 patients benefiting from this advanced technology each week, the hospital continues to lead the fight against cancer in the country.
EGYPT—The Ministry of Economic Planning, in collaboration with the Ministry of Health and Population, has launched a new service that sends congratulatory text messages to parents of newborns through the integrated
health office services system, effective April 1, 2024.
The initiative aims to educate parents about the critical importance of timely vaccinations for their children, emphasizing the administration of routine vaccinations within 48 hours of the scheduled date and guiding them to the nearest health facility for necessary doses.
The initiative targets all newborns in Egypt, as well as non-Egyptians residing within Egyptian territories.
It aims to raise awareness about the importance of vaccinations against diseases such as polio, tuberculosis, Hepatitis B, neonatal tetanus, diphtheria, pertussis, bacterial influenza B, measles, German measles, and mumps.
UAE — XCath, an early-stage medical device company committed to advancing endovascular treatment robotic systems, showcased cutting-edge medical technology during the Abu Dhabi Global Healthcare Week (ADGHW).
Dr. Vitor Mendes Pereira, a seasoned neurosurgeon under the auspices of the Abu Dhabi Department of Health (DoH), conducted a live telerobotic surgery trial for emergency stroke treatment.
From Abu Dhabi, Dr. Pereira remotely treated a patient located 7,000km away in South Korea.
Using advanced technology, he performed a mechanical thrombectomy procedure, swiftly removing blood clots from the patient’s brain to mitigate the effects of the stroke.
Dr. Pereira, renowned for conducting the world’s inaugural neurovascular robotic procedure in 2019, seamlessly controlled and executed the clot removal procedure using telerobotic means, accentuating the precision and efficacy of remote surgical interventions.
The procedure, executed with cutting-edge robotic technology, entailed Dr. Pereira operating via a robotic controller while the silicone model and bedside unit were stationed in Seoul.
The surgery utilized neurovascular devices such as Stryker AXS Infinity LS, Trevo Trakb21, and Trevo NXT, which showcased the potential for advanced medical interventions conducted remotely.
FROM ABU DHABI, DR. PEREIRA REMOTELY TREATED A PATIENT LOCATED 7,000KM AWAY IN SOUTH KOREA.

AUSTRIA - Siemens Healthineers has introduced the Magnetom Flow1, marking its debut in the realm of magnetic resonance imaging (MRI) technology with a closed helium circuit and innovative features aimed at revolutionizing MRI systems.
The unveiling took place in Vienna, showcasing a platform designed to redefine MRI capabilities while addressing critical issues such as resource conservation and operational efficiency.
The Magnetom Flow1 boasts a closed helium circuit and eliminates the need for a quench pipe, significantly reducing the amount of liquid helium required for cooling.
Owing to its Dry Cool technology, helium consumption has been dramatically reduced from as much as 1,500 litres to a mere 0.7 litres, leading to cost savings and resource preservation.
The system’s bore size of 60 cm ensures comprehensive coverage of MRI applications while leveraging artificial intelligence for image reconstruction enables shorter measurement times and improved image quality.
In addition to its reduced helium usage, the Magnetom Flow boasts lower energy consumption, owing to features such as Eco Gradient Mode and Eco Power Mode.
Siemens Healthineers is also committed to sustainability, and the Magnetom Flow1 exemplifies this dedication.
•
•
•
•



The Africa Business Summit is the ground-breaking executive level conference and expo on the future of strategic leadership, entrepreneurship, sustainability & african transformation
The program at the 3-day Summit comprises of a number of premium sessions such as Leadership Dialogues, CEO Roundtables, Plenary and Panel Discussions and a B2B Deal Connect Service as well as presentations by influential people and change makers who are making waves – and inspiring the next generation of leaders in Africa




