





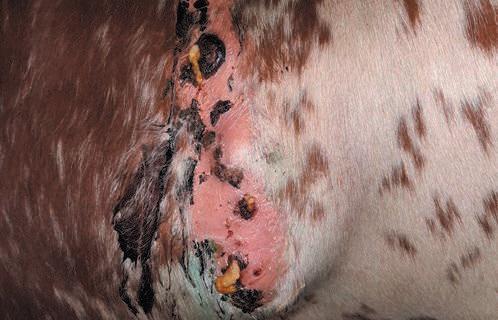
A case of a cow that had undergone a caesarean section in the summer of 2023 was reported to the GD Veekijker hotline in the first quarter of 2024. After an apparent normal recovery, the cow started showing signs of a purulent infection at the old caesarean section incision site, some six months after the procedure. On the advice of the Veekijker veterinarian, further tests were carried out, including both histological and bacteriological testing. The histological tissue examination indicated botryomycosis.
Botryomycosis is an inflammatory reaction following bacterial infection of the skin (cutaneous form) and/or the underlying tissues or intestines (visceral form) (see Photo 1). Chronic, granulomatous and purulent masses appear, which consist of an accumulation of inflammatory tissue and cells and which may contain various bacterial species. In cattle, botryomycosis can be caused by Pseudomonas aeruginosa, but other bacteria such as Staphylococcus aureus and Trueperella pyogenes can also cause this type of inflammation. The consequences for the affected animals are significant: antibacterial therapy has little effect and there is a risk of infertility and abortion. In terms of prevention, it is important to take strict hygiene precautions during a surgical procedure (such as a caesarean section), and to take account of the risk of P. aeruginosa being resistant to chlorhexidine.
In the case reported during the first quarter of 2024, bacteriological testing revealed a mix of species that contained a large proportion of Trueperella pyogenes. Given the outcome of the testing and the severity of the inflammation, the cow in question was euthanised. Similar cases had occurred earlier in 2023, involving several cows from various farms. Nevertheless, botryomycosis is not a common finding in the Netherlands. In consultation with the veterinarians and livestock farmers of those cases, the Veekijker veterinarians and pathologist at GD who were involved wrote an article for ‘Trends from monitoring’ in the Dutch Journal of Veterinary Medicine.

In 2023, the NWVA (Food and Consumer Products Safety Authority) has received 1,563 reports of clinical suspicions of BTV-3 infections and 4,438 positive PCR test results since the start of the BTV serotype 3 outbreak in the Netherlands in September 2023 (reference date 15-Apr-2024). No clinical cases of suspected new infections with BTV-3 were reported by the NVWA in the first quarter of 2024. Among the positive PCR test results, Wageningen Bioveterinary Research (WBVR) makes a distinction between results with high and low Ct values when interpreting the results, thereby distinguishing between animals that were infected long ago (the PCR result can remain positive for a long time) and those infected more recently. There is a relationship between the Ct value and the viral load (the
number of viral particles present in the sample): the lower the Ct-value, the more viral particles are present. A low Ct value can therefore be assumed to be a recent infection, whereas a high value is assumed to have been caused by detection of residues of the viral genome from a previous bluetongue infection.
During the previous bluetongue outbreak of BTV serotype 8, vertical transmission had been established and congenital abnormalities (of the brain in particular) were observed in calves. In the fourth quarter of 2023, pathological findings provided evidence for vertical transmission of BTV-3 as well: BTV-3 was detected in the spleens of three stillborn/full-term aborted calves. In the first quarter of 2024, an
abnormal premature calf was born at a farm where clinical signs of a bluetongue infection had been observed in October 2023. The calf, which was aborted at 7 months’ gestation and sent to Royal GD for necropsy, was diagnosed to have had hydrocephalus (see Photo 2). The skull, spinal column and legs were normal and no abnormalities were seen in the other internal organs. BTV-03 was detected by PCR in spleen tissue at a low Ct value (i.e. a large amount of the virus). Blood was not available for testing for BTV antibodies. Other significant infectious causes of abortion (and congenital malformations of the brain) were excluded and the bacteriological examination was negative.
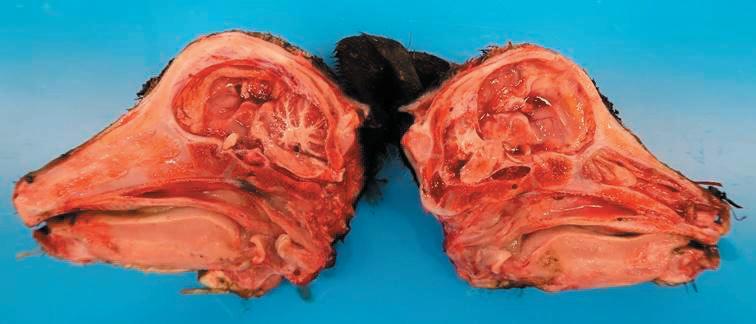
Hydrocephalus has been reported to occur following infection with BTV serotype 8. It is thought that this congenital malformation is a possible consequence of transplacental infection with BTV-3 early in gestation (at between 70 and 130 days).
On instructions from LNV (the Ministry of Agriculture, Nature and Food Quality), GD
launched a project at the end of 2023 to investigate the clinical image of BTV-3 and its impact on cattle and sheep farms further. This project has now been completed. An average of 24.5 per cent of the cattle at the five cattle farms studiedshowed clinical symptoms of bluetongue during the study period. The variation in the percentage of cattle with signs of disease was however
large (ranging from 8.1 to 50.4 per cent). The clinical signs most commonly observed in this project were swollen, red and/or damaged coronary bands of the claws, lameness, stiffness, painful joints, red eyes and nasal discharge. Of the affected animals, 91.6 per cent were reported to have been cured after 13 weeks, and were sick on average for 22 days.
An initial descriptive analysis of the impact of BTV-3 on animal mortality and production in cattle farms was carried out at the end of 2023. A more in-depth analysis followed and determined what the effect of the BTV-3 outbreak has been for Dutch dairy farms over the entire timespan of the 2023 outbreak, corrected for a multitude of other possible factors and compared to previous years. Various other metrics were analysed in addition to mortality and milk production: transport for slaughter, udder health, fertility, metabolism and use of antibiotics. The data covered the period from 1 January 2020 to 31 December 2023. Farms were categorised into three groups: those who
Positive PCR results for BTV-3 have been reported from Belgium, Germany and England during the first quarter. It is unclear whether these are referring to clinically sick animals or to those that were tested for other reasons and may therefore have been infected quite some time ago. The new strain of BTV-8 that was detected in 2023 is deemed to be endemic in France, which means that the number of infections is no longer structurally reported and shown. Any
self-reported bluetongue symptoms, those in an area containing farms that reported bluetongue symptoms but did not report any themselves, and those in areas with no reports of bluetongue. The last of these categories also covers all Dutch farms in the years before the BTV-3 outbreak and has been used as the reference category.
Mortality among cattle aged over one year was about twice as high as normal at farms of all types (dairy, beef, stock rearing and suckler cow farms). For calves aged up to one year, general mortality was not elevated at any of the types of farm, although mortality among calves without ear notches (aged 0 to
14 days) at dairy farms was somewhat above normal (by a factor of 1.2). Increased mortality was not seen at veal farms. Milk production decreased by over a kilogram per cow per day for several weeks and udder health was also poorer on farms with BTV-3 infections. The metabolic metrics did not differ significantly between the farm categories. Whether BTV-3 infections have affected cattle fertility remains to be seen during 2024. Finally, it is noteworthy that antibiotics were used two to three times more often than normal at non-dairy farms (except veal farms) with BTV-3 infections.
changes in the epidemiological situation will be reported by France. The impact of the new BTV-8 strain on cattle mortality was investigated in the two French départements where the strain was detected. Increased mortality was observed among cattle aged older than 2 years in both areas, by factors of between 1.29 and 1.59 with respect to the three preceding years. In the département of Aveyron, mortality among cattle aged 6 to 24 months was also 1.21 to 1.34 times higher
than in the three preceding years. BTV-8 is also present on Sardinia (Italy), in addition to BTV-4. The BTV-8 strain that is present in Sardinia is very similar to the one present in France and does not seem to be the same as the BTV-8 strain that is present on the Italian mainland. Sardinia has also reported infections in the first quarter of 2024, without specifying the serotype.
Implementing Regulation (EU)
Lumpy Skin Disease (LSD) Viral infection. The Netherlands is officially disease-free.
Foot and Mouth Disease (FMD) Viral infection. The Netherlands has been officially disease-free since 2001.
D, E No infections ever detected.
D, E No infections detected.
Implementing Regulation (EU) 2018/1882 of Animal Health Regulation (AHR) 2016/429 (categories B to E)
Bluetongue (BT) Bluetongue serotype 3 outbreak in the Netherlands since September 2023. C, D, E New infections are not suspected. Congenital abnormality through vertical transmission of BTV-3 has been observed.
Bovine genital campylobacteriosis
Bacterium. The Netherlands has been disease-free since 2009. Monitoring of AI and embryo stations and of animals for export.
D, E Campylobacter fetus spp. Veneralis not detected.
VETERINARY
Implementing Regulation (EU) 2018/1882 of Animal Health Regulation (AHR) 2016/429 (categories B to E)
Bovine Viral Diarrhoea (BVD) Viral infection.
Control measures compulsory for dairy farms but voluntary on beef cattle farms.
Brucellosis (zoonosis, infection through animal contact or inadequately prepared food)
Bacterium.
The Netherlands has been officially diseasefree since 1999. Monitoring via antibody testing of blood samples from aborting cows.
Enzootic bovine leukosis Viral infection.
The Netherlands has been officially diseasefree since 1999. Monitoring via antibody testing of bulk milk and blood samples from slaughtered cattle.
Epizootic Haemorrhagic Disease (EHD) Viral infection. Detected since 2022 in cattle in Europe (Spain, Italy, Portugal and France).
Infectious Bovine Rhinotracheitis (IBR) Viral infection.
Control measures compulsory for dairy farms but voluntary on beef cattle farms.
C, D, E The status* of 90 per cent of dairy farms is ‘BVD-free’ or ‘BVD not suspected’. *
The status of 21 per cent of all non-dairy farms is favourable (BVD-free or BVD not suspected).
* The BVD status as determined according to the GD programme.
B, D, E No infections detected.
C, D, E No infections detected.
Anthrax (zoonosis, infection through contact with animals)
Paratuberculosis
Rabies (zoonosis, infection through bites or scratches)
Bovine tuberculosis (TB) (zoonosis, infection through animal contact or inadequately prepared food)
Trichomonas
Q fever
(zoonosis, infection through dust or inadequately prepared food)
Bacterium.
Not detected in the Netherlands since 1994. Monitoring through blood smears from fallen stock.
Bacterium.
In the Netherlands, the control programme is compulsory for dairy farms. 99 per cent take part.
Viral infection.
The Netherlands has been officially diseasefree since 2012 (an illegally imported dog).
Bacterium.
The Netherlands has been officially diseasefree since 1999. Monitoring of slaughtered cattle.
Bacterium.
The Netherlands has been disease-free since 2009. Monitoring of AI and embryo stations and of animals for export.
Bacterium. In the Netherlands, this is a different strain to the one on goat farms and a relationship with cases of disease in humans has not been established.
From the first quarter of 2023 onwards, this is once again part of the standard post-mortem protocol when cows calve prematurely.
D, E No infections detected.
C, D, E The status of 81 per cent of dairy farms is ‘IBR-free’ or ‘IBR not suspected’.
The status of 21 per cent of all non-dairy farms was favourable (IBR-free or IBR not suspected).
D, E No infections detected.
E The PPN (Paratuberculosis Programme Netherlands) status of 82 per cent of dairy farms is ‘A’ (‘not suspected’).
B, D, E No infections detected.
B, D, E No infections detected.
C, D, E Tritrichomonas foetus not detected.
E No infections detected in aborted cattle foetuses.
P.O. Box 9, 7400 AA Deventer
Netherlands
T. +31 (0)88 20 25 575
info@gdanimalhealth.com www.gdanimalhealth.com
VETERINARY DISEASES SITUATION IN THE NETHERLANDS Category (AHR)



Table continuation
Surveillance – Highlights Third Quarter 2023
Article 3a.1 Notification of zoonoses and disease symptoms, ‘Rules for Animal Husbandry’ from the Dutch Animals Act
Leptospirosis
(zoonosis, infection through animal contact or inadequately prepared food)
Bacterium. Control measures compulsory for dairy farms but voluntary on beef cattle farms.
Listeriosis (zoonosis, infection through inadequately prepared food)
Salmonellosis (zoonosis, infection through animal contact or inadequately prepared food)
Yersiniosis (zoonosis, infection through animal contact or inadequately prepared food)
Regulation (EC) no. 999/2001
Bacterium. Infections have occasionally been detected in cattle.
Bacterium. Control measures compulsory for dairy farms but voluntary on beef cattle farms.
Bacterium. Infections detected occasionally in cattle, mostly in aborted foetuses.
Bovine Spongiform Encephalopathy (BSE) Prion infection. The OIE status for the Netherlands is ‘negligible risk’. Monitoring has not revealed any further cases since 2010 (total from 1997 to 2009: 88 cases).
Other infectious diseases in cattle
Malignant Catarrhal Fever (MCF) Viral infection. There are occasional cases in the Netherlands of infection with type 2 ovine herpes virus.
Liver fluke Parasite. Liver flukes are endemic in the Netherlands, particularly in wetland areas.
Neosporosis Parasite. In the Netherlands, this is an important infectious cause of abortion in cows.
Tick-borne diseases
External parasite that can transmit infections. Ticks infected with Babesia divergens, Anaplasma phagocytophilum and Mycoplasma wenyonii can be found in the Netherlands.
- The status of 98.2 per cent of dairy farms is ‘leptospirosis-free’.
The status of 30.9 per cent of non-dairy farms is ‘leptospirosis-free’.
More animals are arriving with a leptospirosis status poorer than ‘free’ than in the previous quarter.
No new leptospirosis infections.
- Infections detected in four cows and one aborted foetus presented for necropsy.
The bulk milk results of 98.5 per cent of dairy farms are favourable (nationwide programme).
- Yersinia enterocolitica was detected in one case, an aborted foetus.
- No infections detected.
- Two infections confirmed upon necropsy.
- Infections were detected at sixteen farms and in a single cow presented for necropsy.
- No infections detected in aborted foetuses submitted for necropsy.
- No samples submitted for examination.



Since 2002, Royal GD has been responsible for animal health monitoring in the Netherlands, in close collaboration with the veterinary sectors, the business community, the Ministry of Agriculture, Nature and Food Quality, vets and farmers. The information used for the surveillance programme is gathered in various ways, whereby the initiative comes in part from vets and farmers, and partly from Royal GD. This information is fully interpreted to achieve the objectives of the surveillance programme – the rapid identification of health problems on the one hand and the following of more general trends and developments on the other. Together, we team up for animal health, in the interests of animals, their owners and society at large.