






Marian Aalberts*, Marjolein E. Sanders and Maarten F. Weber Royal GD, Arnsbergstraat 7, 7418 EZ Deventer, The Netherlands; *presenting author
Introduction
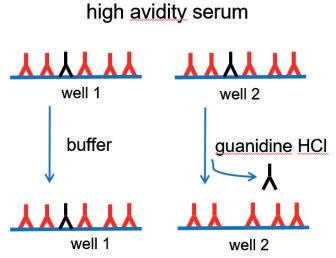
During the course of an immune response, antibody avidity is increased due to the selective expansion of B lymphocytes that produce antibodies with higher antigen affinity. Low and high avidity antibodies can be distinguished by an adapted antibody ELISA, which includes a washing step with a chaotropic agent (e.g. guanidine HCl) to detach the bonds between low avidity antibodies and antigen (Fig. 1).
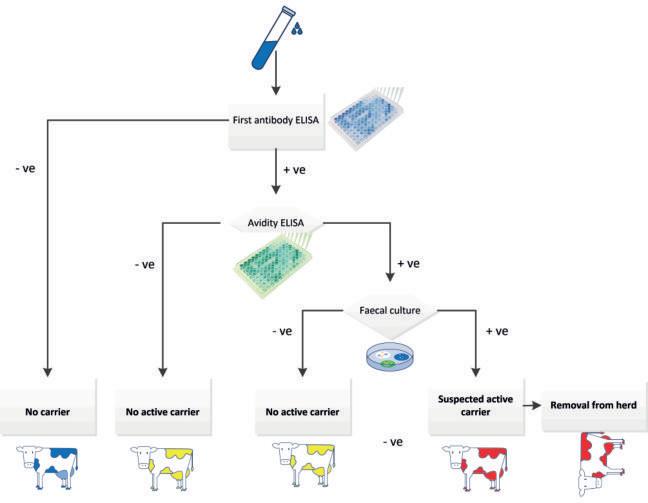
We developed an avidity antibody ELISA that can distinguish between acute Salmonella infections and Salmonella carriers in chronically infected cattle herds. To identify carriers, biennial serial testing by antibody-ELISA and faecal culture of cattle with a first positive ELISA result is recommended (Santman-Berends et al. 2021). Cattle that are both antibody and culture positive are presumed to be active carriers (persistent shedders), whereas cattle that remain antibody positive at subsequent tests without a positive faecal culture are suspected of latent carriership (intermittent shedding of Salmonella).
Aim of the study
Adaptation of an in-house ELISA detecting antibodies against Salmonella to identify potential active and latent carriers. This poster focuses on the identification of potential active carriers among antibody-positive cattle, to decrease the number of faecal cultures required in herd tests (Fig. 2).
Materials and methods
• Sera from 350 ELISA positive cattle for which subsequent faecal culture results were available (40 culture positive, 310 culture negative).
• Sera were tested using an indirect ELISA for IgG antibodies against LPS of Salmonella enterica subsp. enterica serogroups B and D, with an adapted test protocol: Between serum incubation (and washing) and conjugate incubation, plates were incubated with varying concentrations of guanidine HCL.
• Antibody avidity was calculated as the OD (at 450 nm) after 2M guanidine HCl incubation divided by OD at 0M (ratio 2M/0M).
• Logistic regression analysis was performed with positive faecal culture as a dependent variable and ratio 2M/0M and PP ratio (OD of sample/OD of positive control) at 0M as potential predictors.
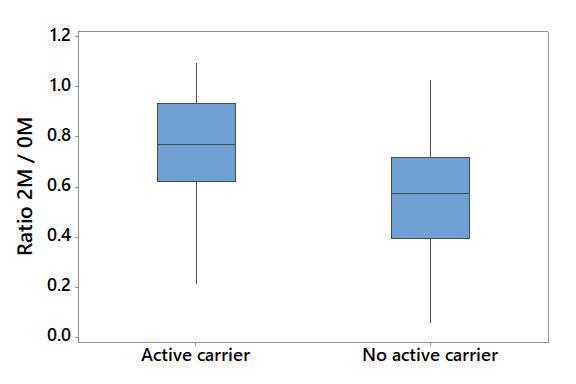
• The ratio 2M/0M was significantly higher in sera from active carriers (faecal culture positive) in comparison to cattle that were no active carriers (faecal culture negative; Fig. 3).
• A logistic regression model based on the ratio 2M/0M improved the prediction of active carriership (Table 1).
• An ROC analysis revealed that the model could effectively detect active carriers with high sensitivity (AUC = 0.726; 95% CI: 0.651 - 0.801).
• Similar results were obtained using individual milk samples instead of sera (data not shown).
Conclusion
• An antibody avidity ELISA for Salmonella serogroups B and D antibodies can be used to preselect cattle for faecal culture in the identification of active Salmonella carriers.
• The range of avidity cutoffs that permit cost reduction whilst preserving sufficient diagnostic sensitivity (>85%), is limited (data not shown).
Reference Santman-Berends IMGA, Mars MH, Weber MF, van Duijn L, Waldeck HWF, Biesheuvel MM, van den Brink KMJA, Dijkstra T, Hodnik JJ, Strain SAJ, de Roo A, Veldhuis AMB and van Schaik G (2021) Control and Eradication Programs for Six Cattle Diseases in the Netherlands. Front. Vet. Sci. 8:670419.


3
2
4


m.aalberts@gdanimalhealth.com www.gdanimalhealth.com