10 minute read
Pioneers in healthcare solutions
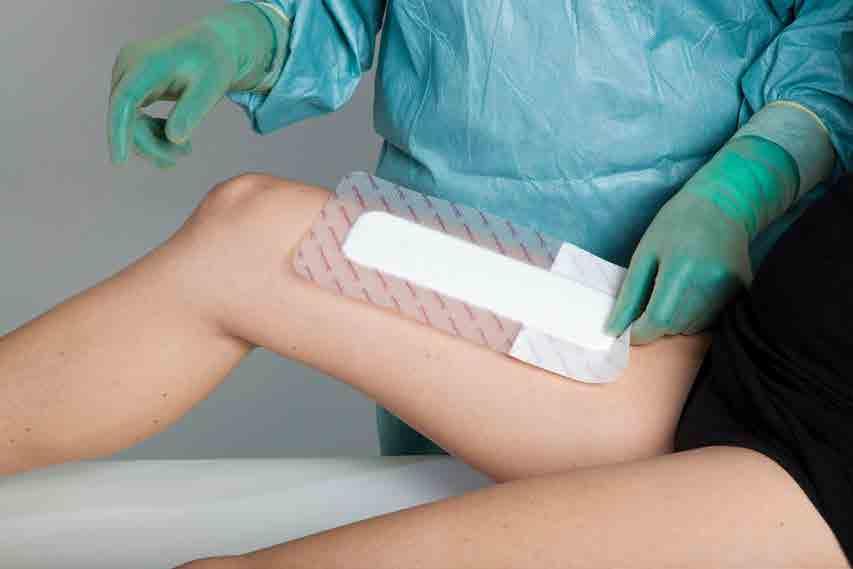
Mepilex® Border Post-Op is an all-inone dressing that absorbs and retains blood and surgical exudates


Swedish-based Mölnlycke Health Care is a world leader in the supply of single-use surgical and wound care products. As Victoria Hattersley finds out from Eric De Kesel, executive vice-president, Operations, the company continues to grow and to enhance its globally-renowned product offering for customers, healthcare institutions and patients alike.


The roots of Mölnlycke Health Care go back to 1849, when it was established in Gothenburg, Sweden as a textile producer. Today it is present in 33 countries all over the world and active in more than 100. Its operations cover every continent, with 14 production plants – two in the US, 6 in Asia (across Malaysia and Thailand) and the remainder throughout Europe (Belgium, the Czech Republic, Poland, Finland, France and the UK).
The company’s history has been one of steady growth, boosted by regular investments and acquisitions. A significant milestone came about in 2005 when it was acquired by Apax Partners and merged with Regent Medical and Medlock Medical (also specialists in single-use surgical products). This followed Mölnlycke’s own acquisition of Johnson & Johnson’s single-use surgical product line. Investor AB, founded by the Wallenberg family, took over Apax in 2007 and has since owned Mölnlycke Health Care. Both of these developments have enabled the company to establish a firm presence in this highly competitive segment of the healthcare industry.
Investment in organic expansion is also key. For example, the company has been steadily increasing its influence in the US market over the past few years. In 2011 it began the construction of a new manufacturing facility at Brunswick Landing (Maine), on the site of the former Naval Air Station Brunswick. In 2014 Mölnlycke announced its plans to expand its other US production site in Wiscasset, Maine.
But the company has not been neglecting its European roots in this drive for global expansion – after all, this remains its core market. Indeed, as Mr De Kesel tells us: “Recently we announced that we will be opening a new site in the Czech Republic in 2017 for our ProcedurePak® product as this is going to play a key role in our activities moving forward. Most of the business for this product is in the European market.”
Aside from investment and acquisition, the company has recently issued bonds for 500 million euros and made its first capital distribution to Investor – amounting to EUR 130 million.

The new Mepilex® XT reduces the risk of maceration and pain.


FLEXTRUS – THE NATURAL CHOICE FOR DEMANDING PACKAGING CUSTOMERS
Flextrus is a leader in barrier flexible packaging with base in Northern Europe. We supply a comprehensive range of innovative and environmentally responsible materials intended for the healthcare industry, including thermoformable base webs, printed and coated medical papers, tailor-made film laminates and high barrier materials. We hold quality, flexibility and delivery as cornerstones of our solutions.
Flextrus is a proud supplier to Mölnlycke Health Care.
www.flextrus.com
Scan Global Logistics
With an extensive and reliable network, dedicated people, top management commitment and an agile business philosophy, Scan Global Logistics has played a significant role in shaping and developing the Mölnlycke Healthcare´s supply chain.
As a proof of this, the partnership has grown extensively where Scan Global Logistics is now the sole provider of all sea and airfreight services globally.
To deliver excellent service along the process has been key for Scan Global Logistics since this partnership was formed in 2007.


Mölnlycke Health Care’s surgical drapes are manufactured to the highest standards

A healthcare products pioneer
It was in the 1940s that Mölnlycke first entered the field for which it is now so highly regarded throughout the world – that of wound care products. In fact, since it entered this field the company has been a pioneer, introducing a number of revolutionary products. In 1949 it introduced Mesorb, a new wound dressing principle; this was followed in the 1960s by OP-Plast – a surgical drape material that increased hospital efficiency and protected against surgical infections.
In 1985 Mölnlycke began work on Safetac® , a soft silicone based wound dressing adhesive. This was a particularly important product for the company and the industry as a whole, since it led the way in what Mr De Kesel refers to as ‘gentle’ dressing. “Since that time,” he tells us, “the patent has run out and many other companies are trying to make their own versions, which is testament to the effectiveness of our solution.”
The year 1999 saw the introduction of ProcedurePak. This is a hospital efficiency concept, comprising of customised procedure trays which are assembled to contain all the single-use components required for a particular surgicalintervention. Developed in order to replace the large number of individually packed items, ProcedurePak trays can generate significant savings for hospitals – both in terms of time and money. It has been estimated that, using this solution, preparation time for surgical procedures can be cut by more than half compared to traditional operation room preparation times.
ProcedurePak remains a key focus for the company’s growth plans, but its portfolio also encompasses a wide range of other highly regarded products. The range falls broadly into ‘wound dressings’ and ‘surgical solutions’, but within these are several subcategories such as advanced wound care products, surgical gloves, patient warming products, scar treatment products, surgical staff clothing and drapes.
To name just one other highlight from Mölnlycke’s product portfolio: Exufiber® is a sterile, nonwoven fibre dressing designed to be used on a wide range of highly exuding wounds, including leg and foot ulcers, pressure ulcers, surgical wounds and so on. When this product comes into contact with the wound it transforms into a gel which in turns helps to create a moist healing environment and facilitates ease of removal during dressing changes. It is also worth noting that the company’s products can often be used in combination with each other, and this is no exception. If required, Exufiber can be employed alongside products such as Mepilex Border® and Safetac, to further improve the healing process.
Continuous innovation
Mölnlycke Health Care has never rested on its laurels: its R&D team is continuously working on new solutions to ensure the company maintains its position at the forefront of surgical product development. A particular focus for the future will be on the ‘gentle’ end of the wound-dressing spectrum.
To this end, Mr De Kesel is keen to mention some newer additions to the product portfolio, such as Mepilex XT – a soft, comfortable foam dressing design to treat a wide range of exuding acute and chronic wounds in all healing stages, including leg and foot ulcers, pressure ulcers and traumatic wounds. With its use of unique, integrated exudate channels, Mepilex is re-defining the possibilities for foams. These integrated channels can absorb both low and high viscosity exudate, drawing it away from the wound bed and spreading it away from the absorbent foam pad whilst the above-mentioned Mölnlycke Safetac technology seals the wound edge, preventing leakage and maceration.
He adds: “In the future we will also be focusing increasingly on the growing area of negative pressure wound therapy (NPWT). Here, for example, we produce systems under our Avance® brand name.” This system includes a gentle adhesive film which incorporates Safetac and is designed to minimise stress and pain for the patient. It is lightweight, easy to understand and operate, and can be used for grafts, burns and wounds of varying severity.
“When it comes to surgical solutions we are still focused primarily on ways to increase efficiency. For example, in the US the ObamaCare act means there is greater pressure on hospitals to carry out surgical procedures in
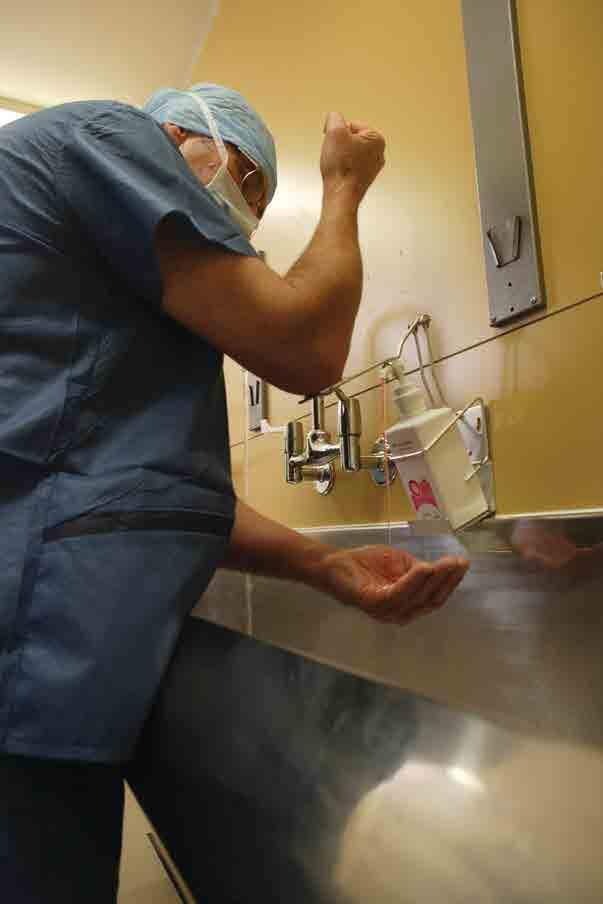
HibiScrub being used to provide protection against surgical site infections Mepilex® Heel is a shaped foam dressing, that minimises pain and wound or skin damage at dressing change

Sontara®
For over 35 years, Mölnlycke AB and Sontara® have collaborated in a strong partnership to support better and safer surgical procedures through conversion to single-use surgical gowns. Our Sontara® spunlace is a unique nonwoven blend made from pulp and polyester fibers. Combining with Mölnlycke’ s customer-oriented product development led to a Sontara® leadership position for “The Comfort in Single Use.” Sontara® offers the ideal balance between comfort and protection for surgeons, bringing a superior soft and breathable spunlace fabric suitable for all surgical procedures.
BARRIER® Classic models produced with Sontara® fabric meet the EN 13795 and AAMI PB 70 Level 3 norms, allowing for surgical interventions with superior softness and increased comfort.
a way that optimises the healing process and reduces the need for further treatments. Many hospitals simply cannot afford re-admissions, so products such as ours are essential when it comes to their bottom line.”
For Mölnlycke, nurturing long-term supplier relationships is essential if it is to maintain its reputation for industry-leading innovation and quality. “We like to develop close relationships with our key suppliers over a long period of time. We don’t cherry pick as some companies do – playing the game of switching suppliers each year according to which offer the best prices. We have to work together with them when it comes to product development, and we can only do this if we know and trust them thoroughly. After all, we are essentially a converter so we rely, for one thing, on the raw material producers.”
A company that cares
For Mölnlycke, it is not enough simply to be successful in an economic sense: the company also takes great pride in the recognition it has achieved for the clear stance it adopts on environmental and social issues.
In terms of its environmental obligations, it is certified according to ISO 14001. Its sustainability strategy, according to Mr De Kesel, is focused on three key areas: CO2 reduction (which it has cut by an impressive 17 per cent since 2012); material recycling (currently at 95 per cent); and finally the cutting of overall water consumption.
Mr De Kesel adds: “sustainable business is not created overnight. That’s why we adopt a long-term approach; combining our successful business practices with responsible behaviour. Our code of conduct provides a good foundation for our business ethics – ethics that we also expect our suppliers to adhere to.”
“We are proud of our strong Swedish heritage. But we are equally proud of our organisation’s diversity and ability to invest in and develop local employees when we enter new markets or regions.”
When it comes to it social issues, Mölnlycke is fully aware of its obligations as a leading global player. To give just one example of its work in this field: for more than 10 years the company has been involved with Operation Smile, the charity which provides free surgery to repair cleft lip, cleft palate and other facial deformities for children around the world. Just last year, it donated 50 per cent of the funding to support a mission in Fortaleza, Brazil, during which 84 patients received life-changing cleft palate and cleft lip surgery.
But there is something else that Mr De Kesel is keen to emphasise: “We are very proud of the way we treat our employees and how they respond to this, and carry out regular surveys on workplace satisfaction. Last year we found that: 91 per cent of our employees said that they were proud to work with us; 80 per cent said they felt motivated at work; and 87 per cent said they felt respected by their manager. We intend to maintain, and indeed improve upon, these impressive figures in the years to come.”
Future strategy
Looking ahead, Mr De Kesel says there are no major investments planned following the expansions already implemented in the US and the Czech Republic. Instead, growth will be more organic with a series of smaller investments. “For example, we are opening offices in Brazil and have also expanded our regional presence in the US and Singapore.
He concludes: “In terms of acquisitions, there is nothing planned but we certainly won’t rule it out if a suitable opportunity arises. What I can say is that we have ambitious growth targets and anything that can help to fuel this will be considered carefully.” n










