© 2024 Integritas Communications.
All rights reserved. No part of this eHealth Source may be used or reproduced in any manner whatsoever without written permission except in the case of brief quotations embedded in articles or reviews.
Integritas Communications
55 Barr Lane, Monroe, NY 10950
www.integritasgrp.com www.exchangecme.com
FACULTY Candace T. Westgate,
 DO, MPH, FACOG
DO, MPH, FACOG
Medical Director
AHEAD Hereditary Cancer Risk Assessment Programs
Adventist Health – St. Helena Women's Center
St. Helena, California
Dr. Candace Westgate is board certified in obstetrics and gynecology (OB-GYN) and has completed additional certifications in Advanced Laparoscopy, Menopausal Management, and Cancer Genetics, as well as the American College of Obstetrics and Gynecology’s program in Women’s Genomics Counseling and the 2018 City of Hope intensive course in hereditary cancer risk assessment. She is the founder and director of the Adventist Health Early All-Around Detection (AHEAD) program, a population health cancer risk assessment and prevention program that features a multidisciplinary team focused on the management of familial and hereditary cancer risk for patients and their families.
Dr. Westgate received a master’s degree in public health from Tulane University in New Orleans, Louisiana, with a focus on program management and maternal and child health. She graduated from A.T. Still University College of Osteopathic Medicine in Kirksville, Missouri, and completed her OB-GYN internship and residency at Georgetown University Hospital in Washington, DC.
Cancer risk assessment and prevention strongly align with her philosophy of practice: “Preventive care is important to me. I believe in partnering with and empowering patients on their journey to find health, treating the whole patient and not focusing on only the disease.” For this reason, Dr. Westgate has stepped up as a leader in her community and within her organization.
PREAMBLE Target Audience
The educational design of this activity addresses the needs of primary care and other frontline clinicians related to early cancer detection.
Program Overview
Cancer presents enormous medical, economic, and social burdens, with ≥2 million new cases diagnosed and an estimated 611,000 deaths projected to occur in 2024. Despite significant advances in therapy, cancer is still the second leading cause of death in the United States and the leading cause of mortality in people younger than 80 years. The costs of treating cancer, including drugs, hospitalization, and ambulatory care, as well as lost productivity and absenteeism, exceed $257 billion annually. Early detection is crucial to reducing these cancer burdens, improving outcomes and quality of life by making it possible for therapy to begin sooner, thereby decreasing treatment costs and complexity and reducing morbidity and mortality. Blood-based multi-cancer early detection (MCED) tests have been developed to support population-based screening of asymptomatic individuals for dozens of cancer types. This multimedia educational activity has been designed to help primary care clinicians—the most important facilitators of preventive health care and cancer screening—understand the science behind MCED tests, interpret trial data, and explore the potential role for MCED testing in the routine cancer screening paradigm. The activity will also address the incorporation of MCED testing into primary care practice, including the use of shared decision-making to determine patient
eligibility and appropriate diagnostic follow-up in response to a positive test.
Educational Objectives
After completing this activity, the participant should be better able to:
• Discuss benefits and limitations of current guidelinerecommended screening modalities and the potential role of blood-based tests to improve early cancer detection
• Describe the use of cell-free DNA (cfDNA) for the early detection of multiple cancer types
• Evaluate the recent clinical data on available and emerging blood-based multi-cancer early detection (MCED) screening tests and their utility in early cancer detection
• Implement MCED screening into primary care practice, including patient eligibility evaluation, counseling, shared decisionmaking, and clinical care pathways following a positive result
Physician Accreditation Statement
Integritas Communications is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physician Credit Designation
Integritas designates this enduring activity for a maximum of 1.0
AMA PRA Category 1 Credit™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Nurse Practitioner Continuing Education
This activity has been planned and implemented in accordance with the Accreditation Standards of the American Association of Nurse Practitioners (AANP) through the joint providership of Global and Integritas Communications. Global is accredited by the American Association of Nurse Practitioners as an approved provider of nurse practitioner continuing education. Provider number: 110121. This activity is approved for 1.0 contact hour (which includes 0.0 hour of pharmacology).
Integritas Contact Information
For more information about the approval (ACCME credit) of this program, please contact Integritas at info@exchangecme.com
Global Contact Information
For information about the approval (AANP credit) of this program, please contact Global at 303-395-1782 or cme@globaleducationgroup.com
Instructions to Receive Credit
In order to receive credit for this activity, the participant must score at least 75% on the posttest and complete the program evaluation.
Fee Information & Refund/Cancellation
Policy
There is no fee for this educational activity.
Disclosures of Conflicts of Interest
Integritas and Global Education Group adhere to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the Accreditation Council for Continuing Medical Education (ACCME) and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous. All persons in a position to control the content of an accredited continuing education program provided by Integritas are required to disclose to Integritas all financial relationships with any ineligible company within the past 24 months. All financial relationships reported are identified as relevant and mitigated by Integritas in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Integritas to assure objectivity and that the activity is free of commercial bias. All relevant financial relationships have been mitigated.
The faculty has the following relevant financial relationships with ineligible companies:
Candace T. Westgate, DO, MPH, FACOG Consulting Fees: Myriad Genetics, Inc.; Speakers Bureau: AstraZeneca plc, GRAIL, LLC, Myriad Genetics, Inc.
The planners and managers at Integritas Communications and Global Education Group have no relevant financial relationships to disclose.
Disclosure
of Unlabeled Use
This educational activity may contain discussion of published and/ or investigational uses of agents that are not indicated by the US Food and Drug Administration. Integritas and Global do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
INTRODUCTION

VIDEO 1: Introduction to the Multi-Cancer Early Detection eHealth Source
Cancer presents enormous medical, economic, and social burdens, with an estimated ≥2 million new cases diagnosed and 611,000 deaths in the United States this year.1 With 5-year relative survival rates 3 to 8 times higher for cancers diagnosed at local stages as compared to distant stages, increasing the detection of cancer at early stages is an important public health initiative to reduce cancer-associated morbidity and mortality.2,3 However, population-level screening recommendations for asymptomatic individuals are only available for breast, prostate, cervical, colorectal, and lung cancers.4
New blood-based assays represent a potential paradigm shift for cancer screening as they can identify biomarkers (eg, methylated
cell-free DNA [cfDNA]) for many types of cancers, including those without a currently recommended screening modality.5-7 With one assay currently available in the United States and others on the horizon, primary care providers should be familiar with the data surrounding blood-based multi-cancer early detection (MCED) tests. To be prepared to incorporate this new screening modality into primary care practice, clinicians should know how to identify the correct patients to screen, understand the efficacy of the various tests, identify appropriate follow-up care pathways, and utilize shared decision-making to help patients understand the benefits and drawbacks of MCED testing.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49.
2. Hubbell E, Clarke CA, Smedby KE, Adami H, Chang ET. Potential for cure by stage across the cancer spectrum. Cancer Epidemiol Biomarkers Prev. 2024;33(2):206-214.
3. Centers for Disease Control and Prevention (CDC). Incidence and relative survival by stage at diagnosis for common cancers. USCS Data Brief, no. 25. United States Cancer Statistics (USCS) 2021; https://www.cdc.gov/cancer/uscs/about/data-briefs/ no25-incidence-relative-survival-stage-diagnosis.htm. Accessed March 12, 2024.
4. US Preventive Services Task Force (USPSTF). Published Recommendations for Cancer. www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics/ uspstf-and-b-recommendations. Accessed March 12, 2024.
5. Ahlquist DA. Universal cancer screening: revolutionary, rational, and realizable. NPJ Precis Oncol. 2018;2:23.
6. Hackshaw A, Cohen SS, Reichert H, Kansal AR, Chung KC, Ofman JJ. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br J Cancer. 2021;125(10):1432-1442.
7. Guerra CE, Sharma PV, Castillo BS. Multi-cancer early detection: the new frontier in cancer early detection. Annu Rev Med. 2024;75:67-81.
CHAPTER 1. CURRENT STATUS OF PREVENTIVE CANCER SCREENING AND THE NEED FOR IMPROVEMENT
Cancer Epidemiology and Mortality
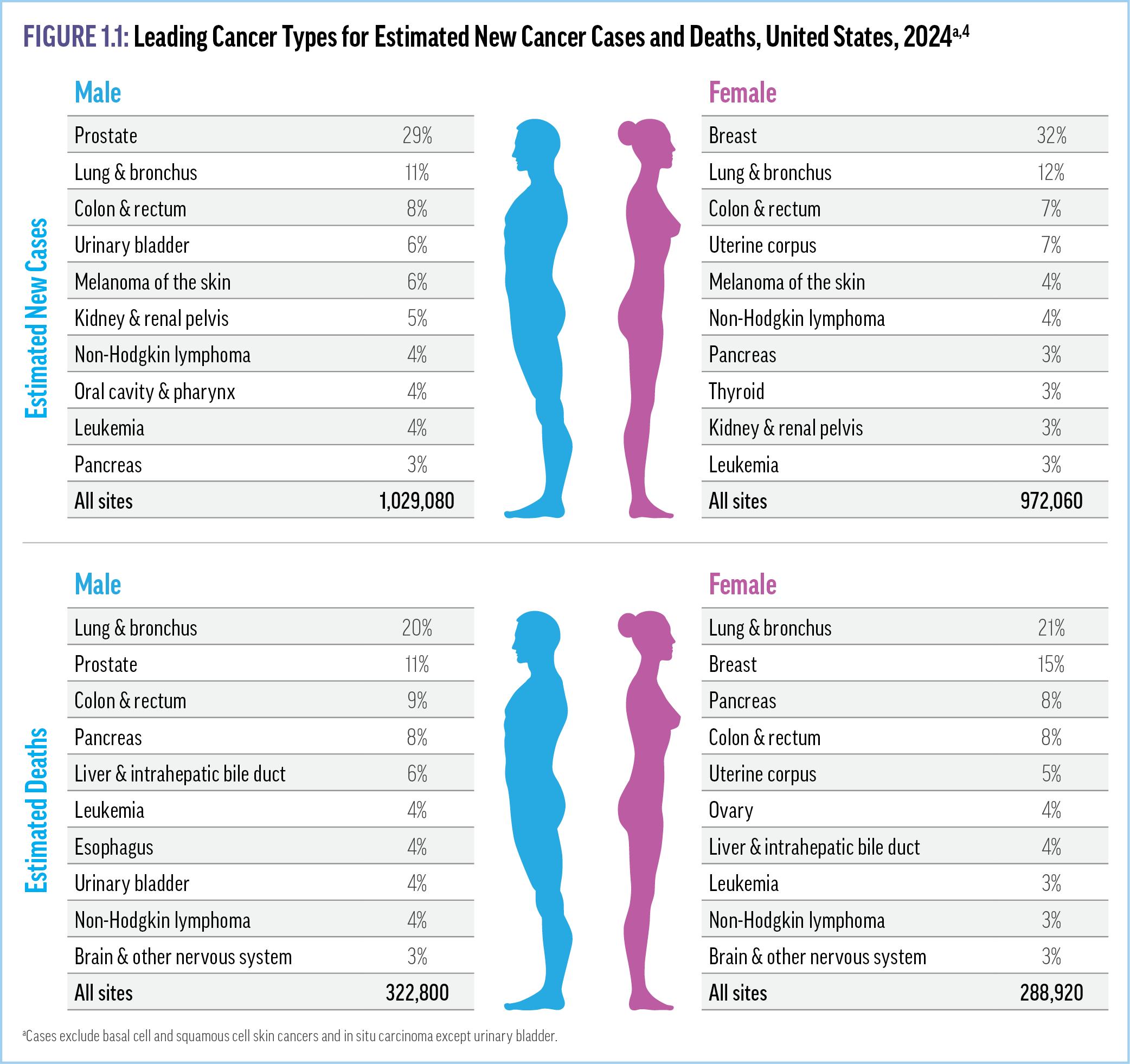
Despite significant advances in therapy, cancer is still the second leading cause of death in the United States and the leading cause of mortality in people younger than 80 years.1 Although nearly 40% of Americans will receive a cancer diagnosis at some point during their lifetimes, it is most frequently diagnosed among individuals aged 65 to 74 years old (mean age at diagnosis, 66 years).2,3 Figure 1.1 details the most common cancer types In the United States.4 Although the overall incidence of cancer is higher among men than women, the most commonly afflicted are African American men and White women, with Asian/Pacific Islanders (A/ PI) of both sexes the least commonly diagnosed.2
Factors Influencing Cancer Survival
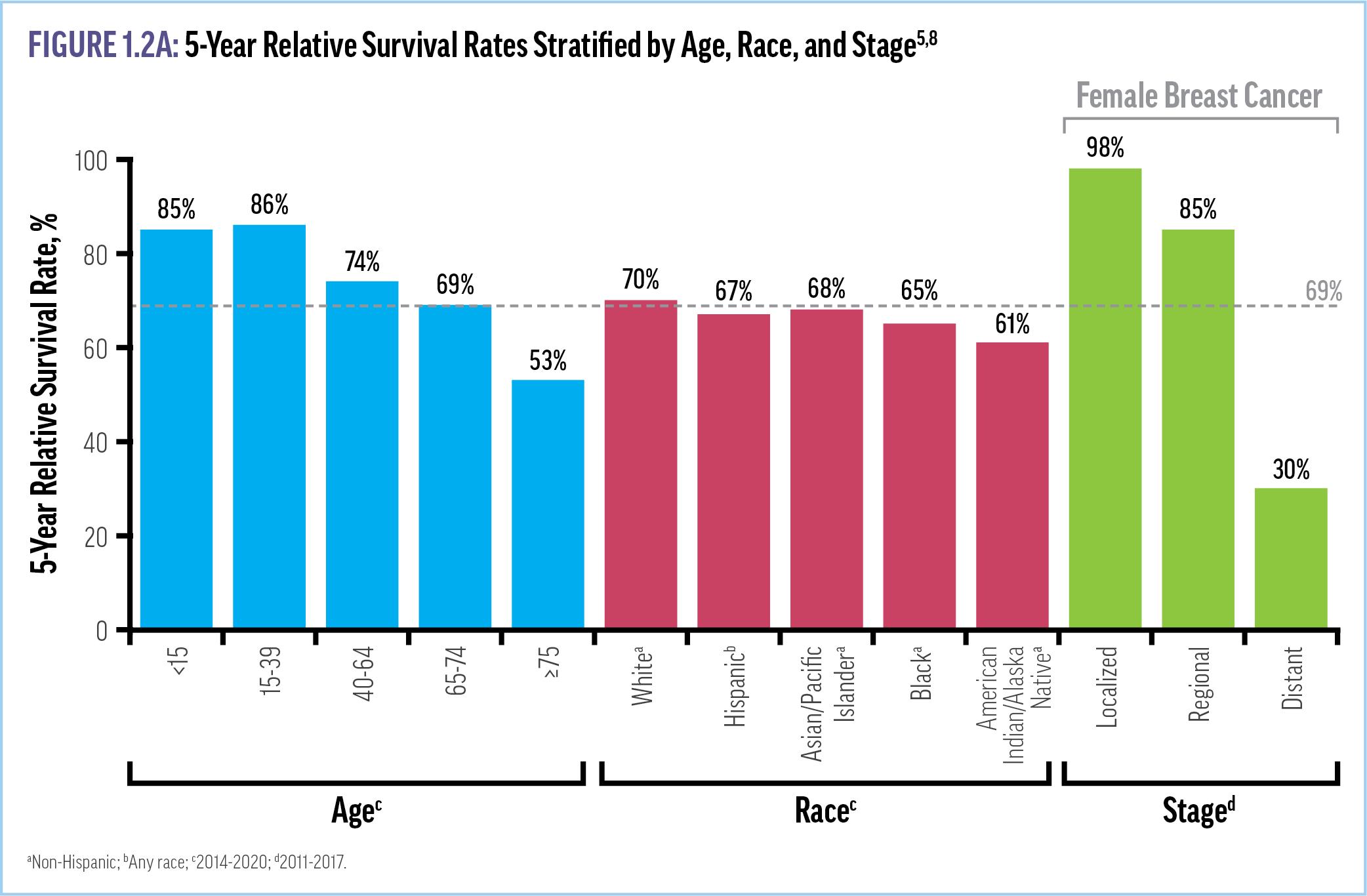
The average 5-year relative survival rate for patients with any malignancy is 69.2%, although a number of factors influence the chances of survival in a given individual, including age, race, tumor location, and stage at diagnosis (Figure 1.2A).2,5,8 In fact, the 5year relative survival rate is at least 3 times higher for tumors detected at a localized stage, when they can be surgically excised or treated with simpler drug regimens, than for those detected at a distant stage.5,6 For example, the 5-year survival rate for female breast cancer is 98% when diagnosed at a localized stage as
compared to 30% when diagnosed at a metastatic stage.5 Further, between 2006 and 2015, cancers with distant metastases (stage 4) accounted for only 18% of new cases but 48% of cancer-related deaths within 5 years.7
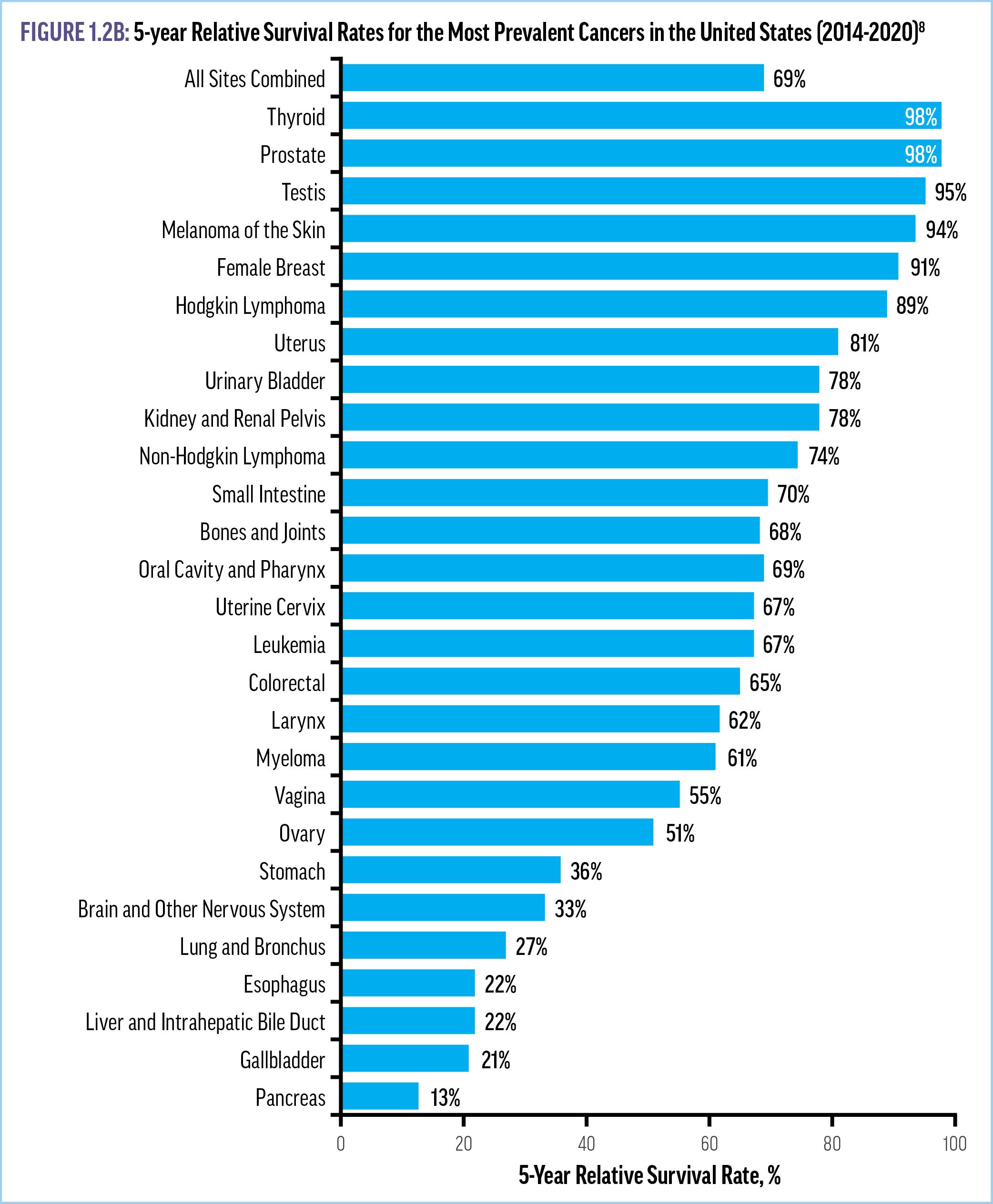
The 5-year relative survival rate is highest for cancers of the thyroid (98%), prostate (98%), testis (95%), and melanoma of the skin (94%), and lowest for cancers of the pancreas (13%), gallbladder (21%), liver/bile duct and esophagus (22%), and lung (27%) (Figure 1.2B).8 Additionally, after adjusting for sex, age, and disease stage at diagnosis, the risk of death is 33% higher for Black patients and 51% higher for Indigenous American patients compared with White patients.9 While the underlying causes are not fully understood, these disparities by race have persisted over time and can be at least partially explained by differences in the timely receipt of recommended treatments.6-8 Importantly, reduced smoking, advances in treatment, and better screening resulted in
a 33% decline in the overall mortality rate for all cancer sites combined from 1991 to 2001.4 Further, a recent modeling analysis of cure fraction rates across all stages for 21 cancer types and additional subtypes found significant benefits from early detection: the cure fractions were much higher at early stages of diagnosis and substantially reduced for metastatic cancers, demonstrating that survival differences by stage are not due to lead-time bias, but in fact a result of earlier diagnosis and treatment.6 Given the ongoing trend of an aging US population and projections for significant increases in cancer incidence in the coming years, strategies to ensure early diagnosis across diverse patient populations are vital to lessen the burdens of cancer.10
Benefits and Limitations of Current
Modalities
Preventive screening is crucial to early cancer detection. However, current guidelines from the US Preventive Services Task Force (USPSTF) recommend screening for only a few types of cancer that account for less than half of all new cancer cases: breast, cervical, and colorectal cancer in people without risk factors; lung cancer in people at risk (eg, smokers); and prostate cancer in men.11 The current screening recommendations from the USPSTF are summarized in Table 1.1
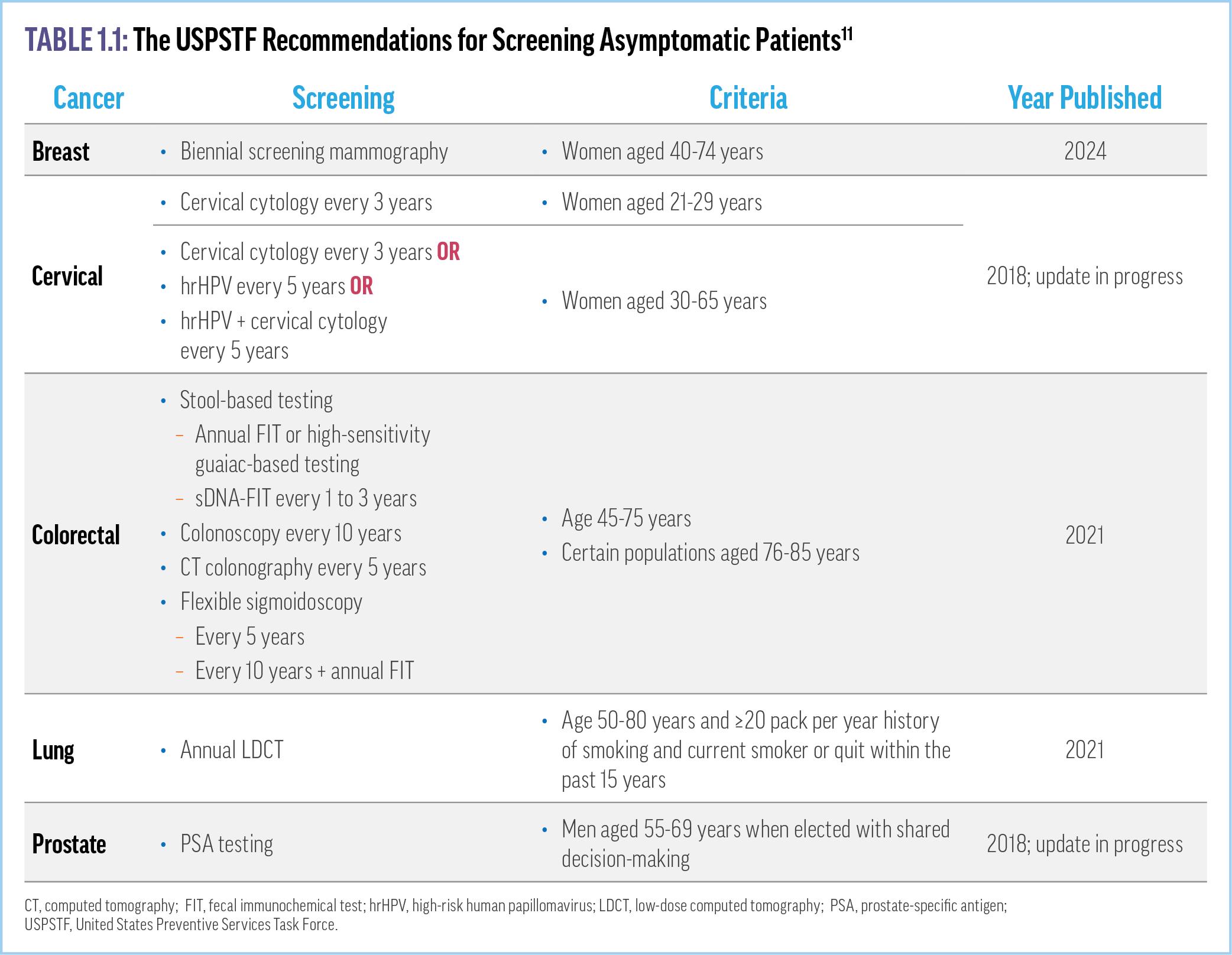
The benefits seen with preventive cancer screening are significant. For example, the percentage of lung cancers diagnosed at a localized stage increased by 3% in the 5 years
following the USPSTF recommendation to perform annual low-dose computed tomography (LDCT) for high-risk individuals.8 Moreover, another study showed that LDCT-based screening reduced the risk of lung cancer mortality over 10 years by 39% compared with no intervention.12 The overall mortality rate for colorectal cancer has decreased by approximately 2% per year since 2010; however, the mortality rate among adults less than 50 years of age has increased by about 1.2% annually, as this population was not originally included in colorectal cancer screening recommendations.1,2 The mortality rates for breast and prostate cancers continue to decline after initial rapid reductions when mammography screening and PSA testing were introduced, respectively.2
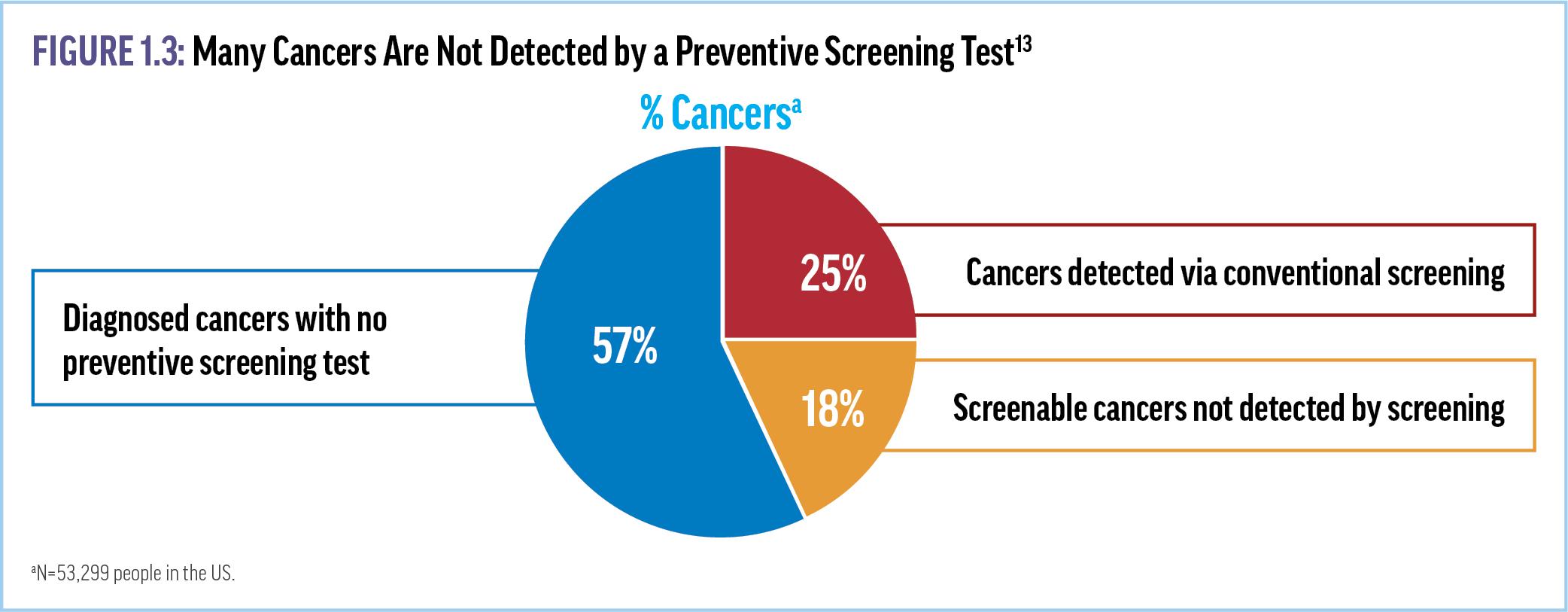
Despite the documented progress, USPSTF recommendations have significant limitations. For instance, only a quarter of newly identified cancers are discovered via these screening guidelines (Figure 1.3).13
Other limitations of current screening methods include the “one organ at a time” approach, which requires the use of different (and often expensive) testing modalities for each type of cancer as well as larger “numbers needed to screen (NNS).”14 In fact, a recent analysis of participants with ≥30 pack-years of smoking history
from the National Lung Screening Trial showed that 68% of the primary cancer incidence after randomization were non-lung cancers, which were only identified based on patient self-report or death certificates.15 Thus, even in this high-risk population, focused screening with a single-organ approach missed most cancers. Furthermore, there are no population-wide screening protocols for 54% of the observed primary malignancies, including esophageal, hepatic, ovarian, pancreatic, and stomach cancers.15 These data highlight the potential utility of multi-cancer screening strategies that can efficiently detect a wider range of cancer types with a single test.
A number of other barriers can interfere with cancer screening and early disease detection in primary care. Patient barriers include absence of symptoms to trigger an evaluation; misunderstanding or discounting symptoms; lack of access to screening or diagnostic tests; financial challenges, including lack of insurance coverage; fear of cancer diagnosis; lack of confidence in the health care system; and patient reluctance to undergo screening.16 For example, only about half of eligible women have had a recent mammogram and approximately 70% of adults ≥21 years old are not up to date on at least 1 routine cancer screening.17 Race, gender, and socioeconomic status are other factors that can affect access to and use of appropriate screening.18 Disparities in cancer screening are well documented, with the lowest rates observed among racial and ethnic minorities, uninsured people, patients who do not have a usual source of care, individuals without a college education, and people who live below the national poverty line.19-22 Efforts to improve the broad and equitable use of early cancer detection strategies are necessary to further reduce the morbidity and mortality associated with cancer.
Modeling Studies Demonstrate the Benefits of Multi-cancer Screening

VIDEO 2: Preventive Cancer Screening Gaps That Could Be Filled by MCED Tests
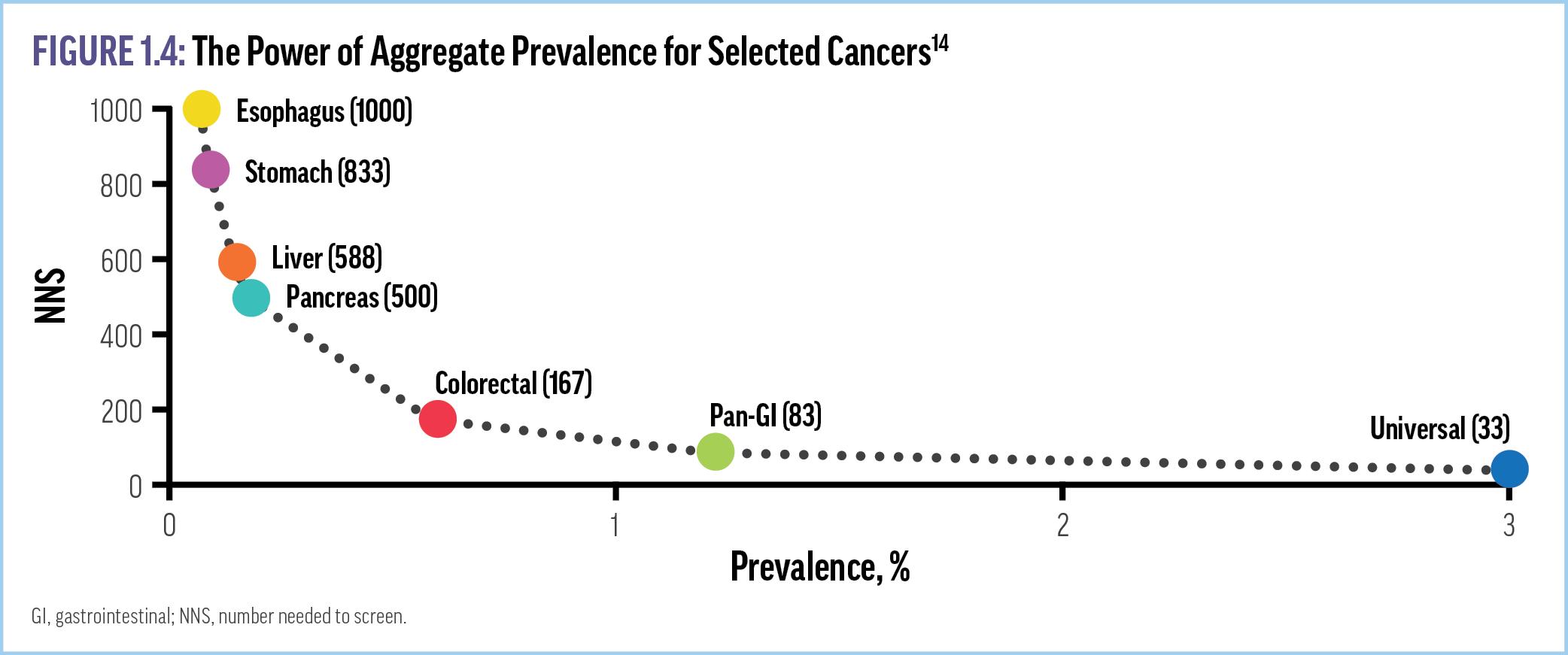
One strategy to address the limitations of current guidelinerecommended screening is the use of multi-cancer early detection (MCED) tests (Supplemental Video 2). These tests have the ability to screen for many cancers at once. One statistical modeling analysis found that adding MCED to usual screening could identify (intercept) nearly 3 times as many cancers compared with routine screening alone, with at least 17% fewer cancer deaths per year.23 Another analysis predicted that adding MCED to routine screening could potentially reduce 5-year cancer mortality by 39% in those patients whose cancer is intercepted, for an absolute reduction of 26% of all cancer-related deaths.24 Further, MCED testing is likely to significantly reduce the NNS. For instance, the NNS to detect pancreatic cancer with an organ-specific approach is currently
approximately 500, whereas a universal MCED test reduces the NNS for all cancers to 33 (Figure 1.4).14 Studies have also modeled economic benefits from adding MCED tests to screening paradigms. For example, it is estimated that 2 to 4 patients who receive a diagnosis of stage 1 cancer can be treated for the same cost as just 1 patient whose disease is first identified at stage 4.25 Another analysis found that adding MCED testing to the guidelinerecommended screening decreased the cost of confirmatory diagnostic testing for positive screens per cancer detected from $89,042 to $7,060.26 Notably, the costs of MCED tests and potential follow-up surveillance testing should be considered when evaluating the results of these modeling studies.
Early detection may be particularly valuable in historically underserved populations. One recent modeling analysis predicted that screening to intercept cancer at an earlier stage (eg, stage III vs stage IV) will produce similar reductions in mortality rates across various racial/ethnic populations.18 Thus, improved screening strategies may help reduce disparities in cancer care. Improving broad and equitable access to MCED testing in addition to usual care will likely facilitate cancer detection at earlier stages, thereby accelerating cancer diagnoses, simplifying treatment for
many patients, reducing overall health care costs, and saving lives.27
Key Takeaways
• While guideline-recommended screening protocols have produced some benefits (eg, reduced mortality rates for some cancers), cancer continues to impose high public health and economic burdens.
• Racial, ethnic, and socioeconomic disparities in cancer burdens, mortality rates, and access to screening persist.
• MCED tests have the potential to reduce cancer mortality rates, costs of diagnosis and treatment, and screening disparities.
References
1. Centers for Disease Control and Prevention(CDC) : National Center for Health Statistics. National Vital Statistics System, Provisional Mortality on CDC WONDER Online Database. http://wonder.cdc.gov/mcd-icd10-provisional.html. Accessed March 7, 2024.
2. National Cancer Institute (NCI): Surveillance Epidemiology, and End Results Program. Cancer Stat Facts. https://seer.cancer.gov/statfacts/. Accessed March 6, 2024.
3. NCI. Cancer statistics. https://www.cancer.gov/about-cancer/understanding/ statistics. Accessed March 8, 2024.
4. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49.
5. CDC. Incidence and relative survival by stage at diagnosis for common cancers. USCS Data Brief, no. 25. United States Cancer Statistics (USCS) 2021; https:// www.cdc.gov/cancer/uscs/about/data-briefs/no25-incidence-relative-survivalstage-diagnosis.htm. Accessed May 7, 2024.
6. Hubbell E, Clarke CA, Smedby KE, Adami H, Chang ET. Potential for cure by stage across the cancer spectrum in the United States. Cancer Epidemiol Biomarkers Prev. 2024;33(2):206-214.
7. Clarke CA, Hubbell E, Kurian AW, Colditz GA, Hartman AR, Gomez SL. Projected reductions in absolute cancer-related deaths from diagnosing cancers before metastasis, 2006-2015. Cancer Epidemiol Biomarkers Prev. 2020;29(5):895-902.
8. NCI. Surveillance, Epidemiology, and End Results Program (SEER). SEER*Explorer: An interactive website for SEER cancer statistics. https://seer.cancer.gov/explorer/. Accessed March 11, 2024.
9. Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109(9):djx030.
10. Weir HK, Thompson TD, Stewart SL, White MC. Cancer incidence projections in the United States between 2015 and 2050. Prev Chron Dis. 2021;18:E59.
11. US Preventive Services Task Force (USPSTF). Published Recommendations for Cancer. www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics/ uspstf-and-b-recommendations. Accessed March 12, 2024.
12. Pastorino U, Silva M, Sestini S, et al. Prolonged lung cancer screening reduced 10year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol. 2019;30(7):1162-1169.
13. NORC at the University of Chicago. Only 14% of cancers are detected through a preventive screening test. 2022; https://www.norc.org/PDFs/GRAIL/StateSpecific%20PCDSs%20chart%201213.pdf. Accessed May 20, 2024.
14. Ahlquist DA. Universal cancer screening: revolutionary, rational, and realizable. NPJ Precis Oncol. 2018;2:23.
15. Chang ET, Janes SM, Hackshaw A, Clarke Dur CA, Buist DSM, Hubbell E. Overall and non-lung cancer incidence in the National Lung Screening Trial (NLST) as indicators of potential for multi-cancer screening. J Clin Oncol. 2023;41(suppl 16):abstr 10633.
16. Brill JV. Screening for cancer: the economic, medical, and psychosocial issues. Am J Manag Care. 2020;26(suppl 14):S300-S306.
17. Prevent Cancer Foundation. 2024 Early Detection Survey. 2024; https:// www.preventcancer.org/early-cancer-detection-better-outcomes/2024-earlydetection-survey-prevent-cancer-foundation/#results. Accessed May 20, 2024.
18. Clarke CA, Kurian A, Hubbell E, Gomez S. Potential for earlier cancer diagnosis to reduce cancer deaths: differences by sex and race/ethnicity. Value Health. 2021;24(suppl 1):S42.
19. NCI. Prostate Cancer Screening. Online Summary of Trends in US Cancer Control Measures. 2024; https://progressreport.cancer.gov/detection/prostate_cancer. Accessed May 9, 2024.
20. American Cancer Society. Breast Cancer Facts & Figures 2019-2020. Atlanta, GA; 2019.
21. Joseph D, King, JB, et al. Vital Signs: Colorectal Cancer Screening Test Use—United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(10):253-259.
22. Liu D, Schuchard H, Burston B, Yamashita T, Albert S. Interventions to reduce healthcare disparities in cancer screening among minority adults: a systematic review. J Racial Ethn Health Disparities. 2021;8(1):107-126.
23. Sasieni P, Smittenaar R, Hubbell E, Broggio J, Neal RD, Swanton C. Modelled mortality benefits of multi-cancer early detection screening in England. Br J Cancer. 2023;129(1):72-80.
24. Hubbell E, Clarke CA, Aravanis AM, Berg CD. Modeled reductions in late-stage cancer with a multi-cancer early detection test. Cancer Epidemiol Biomarkers Prev. 2021;30(3):460-468.
25. Ofman JJ, Fendrick AM, Raza A. Novel multicancer early detection technology— potential value to employers and the workforce. Am J Manag Care. 2020;26(10 Spec No.):SP363.
26. Hackshaw A, Cohen SS, Reichert H, Kansal AR, Chung KC, Ofman JJ. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br J Cancer. 2021;125(10):1432-1442.
27. Guerra CE, Sharma PV, Castillo BS. Multi-cancer early detection: the new frontier in cancer early detection. Annu Rev Med. 2024;75:67-81.
CHAPTER 2. BLOOD-BASED CANCER DETECTION: AN EVOLVING SCIENCE
Blood-Based Biomarkers as Identifiers of Cancer
To improve outcomes across cancer types, it is essential to identify and effectively treat malignancies as early as possible. Historically, tissue biopsy—the “gold standard” for tumor diagnosis and classification—has been used to identify if cancer is present, characterize the type of tumor and its stage, and analyze biomarker expression for insights into patient prognosis and potential treatment options. However, tissue biopsies can be invasive, costly, risky, and potentially painful.1,2 These procedures require that the tumor is accessible and can only provide detailed information about cancer at the site and the time of biopsy, potentially failing to capture a complete and accurate picture of the extent and aggressiveness of the tumor.1,3 Newer minimally invasive blood-based tests, also known as “liquid biopsy,” have shown the potential to accelerate cancer diagnoses and improve patient outcomes. Based on a routine blood draw, testing results can reveal real-time information about the presence of tumors and their stages, patient prognoses, potential treatment responses and resistance, recurrence/metastases, and biologic risk stratification.4,5
Even at the earliest stages of tumor development, cancerous cells undergo characteristic genetic changes that reflect and reveal the specific underlying pathophysiologic processes driving the
disease. These changes can be identified in tumor cells via tissue biopsies or liquid biopsies that analyze biomarkers released from the primary tumor, metastases, or tumor microenvironment.6 Potential biomarkers include cell-free circulating nucleic acids and circulating tumor cells.7-9 Cell-free nucleic acids, including cell-free DNA (cfDNA), circulating tumor DNA (ctDNA), and cell-free RNA (cfRNA), have been extensively studied for detection of new or residual cancer, minimally invasive molecular profiling, and identification of resistance mutations.7,10,11 cfDNA in particular has become the basis for new cancer screening modalities as cfDNA from tumor cells (ie, ctDNA) can be distinguished from noncancerrelated cfDNA.3
The Role of Methylated cfDNA in Cancer Detection
cfDNA fragments are found in plasma of healthy individuals at an average concentration of 30 ng/mL (range: 0-100 ng/mL), with the level depending on patient age, overall health status, and degree of physical activity.12 In patients with cancer, tumor cell apoptosis and necrosis increase average cfDNA concentrations approximately sixfold to 180 ng/mL (range: up to 1000 ng/mL), with levels varying based on tumor type and location.12 The sizes of these DNA fragments also differ; fragments from those with cancer are typically smaller.13 Advances in molecular technologies have been used to evaluate cfDNA for cancer-related genetic alterations, cfDNA length profiles, and epigenetic modifications that are specific for various malignancies.14
In addition to numerous genetic changes—including copy number variation, chromosome rearrangements, and point mutations— cancer is associated with epigenetic changes that regulate genomic function and activity by altering the 3-dimensional
conformation of the genome and/or protein-DNA interactions, or through chemical modifications on DNA strands (Figure 2.1). 11,17
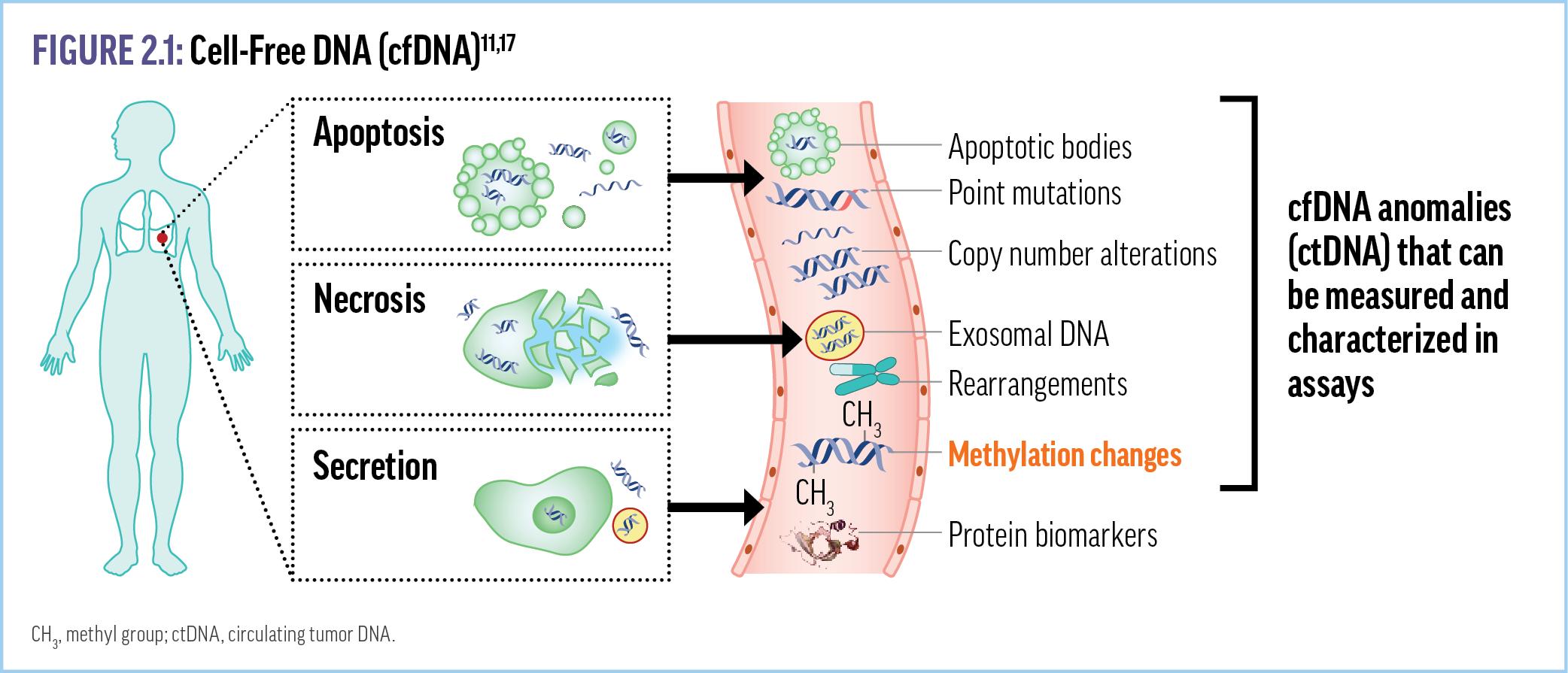
One well-characterized epigenetic change that has been investigated as a tumor biomarker is DNA methylation, in which methyl groups are attached to nucleotides to silence or express certain genes.18 Hypo- or hyper-methylation of DNA can contribute to tumorigenesis or progression via further tumor-promoting genetic damage, including silencing the expression and/or eliminating the function of tumor suppressor proteins.18-21
Evaluating DNA methylation patterns was originally proposed as a cancer screening modality because these epigenetic changes occur early in tumorigenesis and are highly pervasive across tumor types.22 Moreover, many different malignancies exhibit a high degree of concordance in DNA methylation patterns across tissues or within the tissue of origin.22-24 Therefore, DNA methylation patterns are indicative of individual cell types, providing a fingerprint or tissue-specific marker that indicates the origin of the cfDNA fragment.25 Each cancer type generally has a distinct methylation pattern that is unique and identifiable in the cfDNA released by those cancers. For example, methylation patterns in cfDNA derived from patients with pancreatic cancer
are distinct from the patterns observed in cfDNA from patients with lung cancer.25 Next-generation sequencing (NGS) tools are now available that can quickly assess the thousands of potential methylation patterns in cfDNA.
With a single blood draw, multi-cancer early detection (MCED) tests and NGS can be used to identify many different cancers via the circulating tumor-derived materials.3 These tests have been shown to identify many cancers (2 to >50 types) efficiently at earlier stages than traditional screening modalities.26,27 These tests can also identify the specific organ in which the tumor is located. Once a potential cancer is identified, the patient should then undergo a more targeted workup to confirm a cancer diagnosis. Thus, the advent of blood-based MCED screening has the potential to provide effective, presymptomatic, populationwide screening for cancer, with the potential for significant reductions in cancer morbidity and mortality.7
Evaluating the Efficacy of MCED Tests
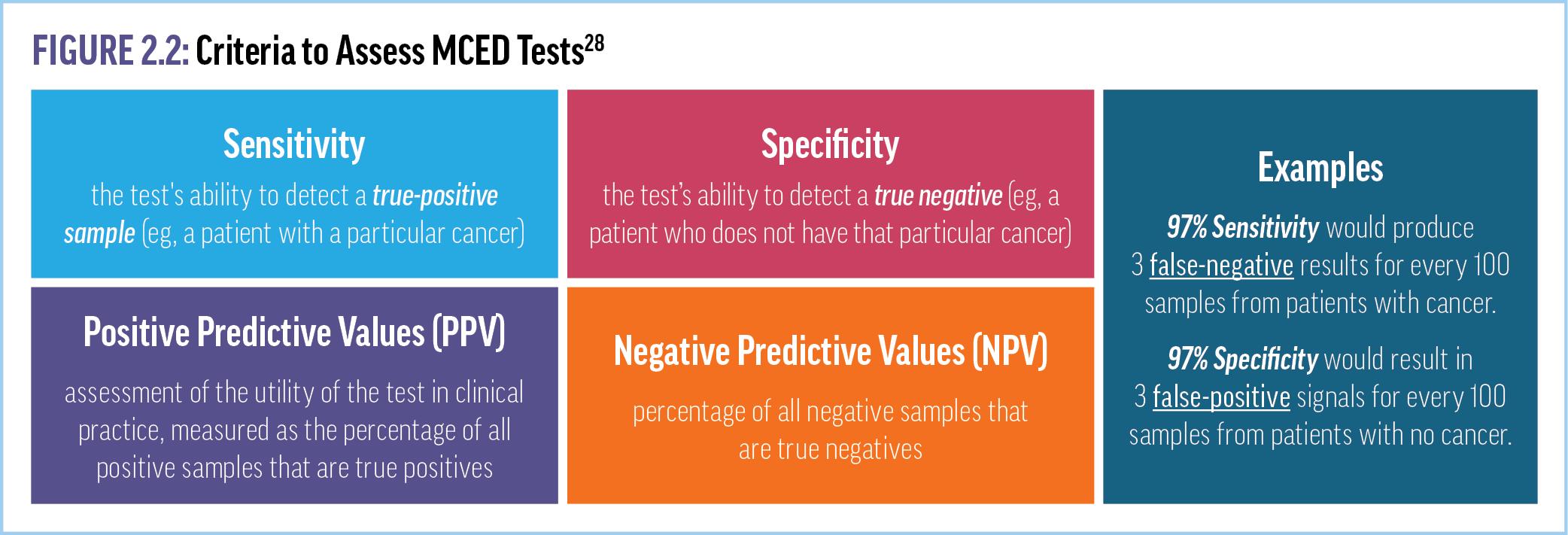
Over the last decade, significant scientific and clinical research has focused on developing different MCED assays, especially those based on analyzing methylated cfDNA. To appropriately integrate MCED tests into practice, clinicians must be aware of the sensitivities, specificities, and positive and negative predictive values associated with various MCED tests. The sensitivity of a test describes its ability to detect a true positive sample (eg, a patient with a particular cancer), whereas specificity is the ability of the test to detect a true negative (eg, a patient who does not have that particular cancer) (Figure 2.2).28 As examples, a test with a sensitivity of 97% would produce 3 false-negative results for every 100 samples from patients with cancer, whereas a specificity of 97% would result in 3 false-positive signals for every
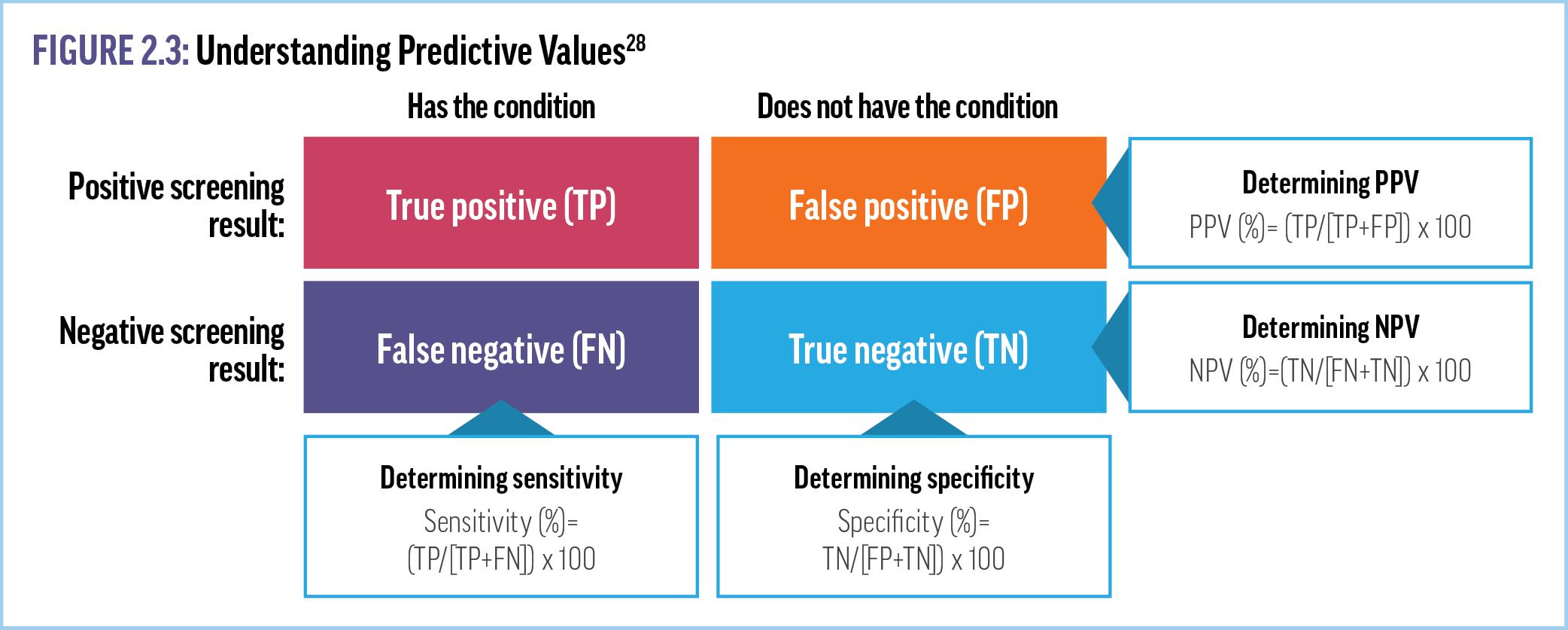
100 samples from patients with no cancer. Positive predictive values (PPVs), which can be thought of as an assessment of the utility of the test in clinical practice, are measured as the percentage of all positive samples that are true positives (Figure 2.3).28 Negative predictive values (NPVs) are the opposite—the percentage of all negative samples that are true negatives.28
Supplementary Video 3 includes explanations of these numbers in practical terms for use when interpreting test results and communicating them to patients.

VIDEO 3: MCED Test Performance Measures in Practical Terms
Key Takeaways
• Genetic and epigenetic changes drive cancer tumorigenesis, growth, and metastasis.
• The analysis of cfDNA methylation profiles in blood can identify multiple cancer types and their tissue of origin, demonstrating potential for use in population-level cancer screening.
• The sensitivity, specificity, and predictive values of multi-cancer screening reflect the accuracy of the test’s cancer detection and localization strategies and clinical utility in practice.
References
1. Rodríguez J, Avila J, Rolfo C, et al. When tissue is an issue the liquid biopsy is nonissue: a review. Oncol Ther. 2021;9(1):89-110.
2. Sharma S, George P, Waddell N. Precision diagnostics: integration of tissue pathology and genomics in cancer. Pathology. 2021;53(7):809-817.
3. Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379(18):1754-1765.
4. Aarthy R, Mani S, Velusami S, Sundarsingh S, Rajkumar T. Role of circulating cell-free DNA in cancers. Mol Diagn Ther. 2015;19(6):339-350.
5. Mathai RA, Vidya RVS, Reddy BS, et al. Potential utility of liquid biopsy as a diagnostic and prognostic tool for the assessment of solid tumors: implications in the precision oncology. J Clin Med. 2019;8(3):373.
6. Palanca-Ballester C, Rodriguez-Casanova A, Torres S, et al. Cancer epigenetic biomarkers in liquid biopsy for high incidence malignancies. Cancers (Basel). 2021;13(12):3016.
7. Ahlquist DA. Universal cancer screening: revolutionary, rational, and realizable. NPJ Precis Oncol. 2018;2:23.
8. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977
9. Salvianti F, Gelmini S, Costanza F, et al. The pre-analytical phase of the liquid biopsy. N Biotechnol. 2020;55:19-29.
10. Galbiati S, Damin F, Ferraro L, et al. Microarray approach combined with ddPCR: an useful pipeline for the detection and quantification of circulating tumour dna mutations. Cells. 2019;8(8):769.
11. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
12. Fernández-Lázaro D, García Hernández JL, García AC, Córdova Martínez A, MielgoAyuso J, Cruz-Hernández JJ. Liquid biopsy as novel tool in precision medicine: origins, properties, identification and clinical perspective of cancer's biomarkers. Diagnostics (Basel, Switzerland). 2020;10(4):215.
13. Aucamp J, Bronkhorst AJ, Badenhorst CPS, Pretorius PJ. The diverse origins of circulating cell-free DNA in the human body: a critical re-evaluation of the literature. Biol Rev Camb Philos Soc. 2018;93(3):1649-1683.
14. Grabuschnig S, Bronkhorst AJ, Holdenrieder S, et al. Putative origins of cell-free DNA in humans: a review of active and passive nucleic acid release mechanisms. Int J Mol Sci. 2020;21(21):8062.
15. Kondo Y. Epigenetic cross-talk between DNA methylation and histone modifications in human cancers. Yonsei MedJ. 2009;50(4):455-463.
16. Nakagawa H, Fujita M. Whole genome sequencing analysis for cancer genomics and precision medicine. Cancer Sci. 2018;109(3):513-522.
17. Cisneros-Villanueva M, Hidalgo-Pérez L, Rios-Romero M, et al. Cell-free DNA analysis in current cancer clinical trials: a review. Br J Cancer. 2022;126(3):391-400.
18. Locke WJ, Guanzon D, Ma C, et al. DNA methylation cancer biomarkers: translation to the clinic. Front Genet. 2019;10:1150.
19. Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21(35):5427-5440.
20. Daskalos A, Nikolaidis G, Xinarianos G, et al. Hypomethylation of retrotransposable elements correlates with genomic instability in non-small cell lung cancer. Int J Cancer. 2009;124(1):81-87.
21. Prada D, González R, Sánchez L, Castro C, Fabián E, Herrera LA. Satellite 2 demethylation induced by 5-azacytidine is associated with missegregation of chromosomes 1 and 16 in human somatic cells. Mutat Res. 2012;729(1-2):100-105.
22. Zhang J, Huang K. Pan-cancer analysis of frequent DNA co-methylation patterns reveals consistent epigenetic landscape changes in multiple cancers. BMC Genomics. 2017;18(suppl 1):1045.
23. Hoadley KA, Yau C, Hinoue T, et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173(2):291-304.e6.
24. Yang X, Gao L, Zhang S. Comparative pan-cancer DNA methylation analysis reveals cancer common and specific patterns. Briefin Bioinform. 2017;18(5):761-773.
25. Moss J, Magenheim J, Neiman D, et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat Commun. 2018;9(1):5068.
26. Beer TM. Novel blood-based early cancer detection: diagnostics in development. Am J Manag Care. 2020;26(14 suppl):S292-S299.
27. Schrag D, Beer TM, McDonnell CH III, et al. Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study. Lancet. 2023;402(10409):1251-1260.
28. Trevethan R. Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice. Front Pub Health. 2017;5:307.
CHAPTER 3. THE DATA SUPPORTING THE USE OF MCEDs
Methylated cfDNA–based MCED Tests in Development
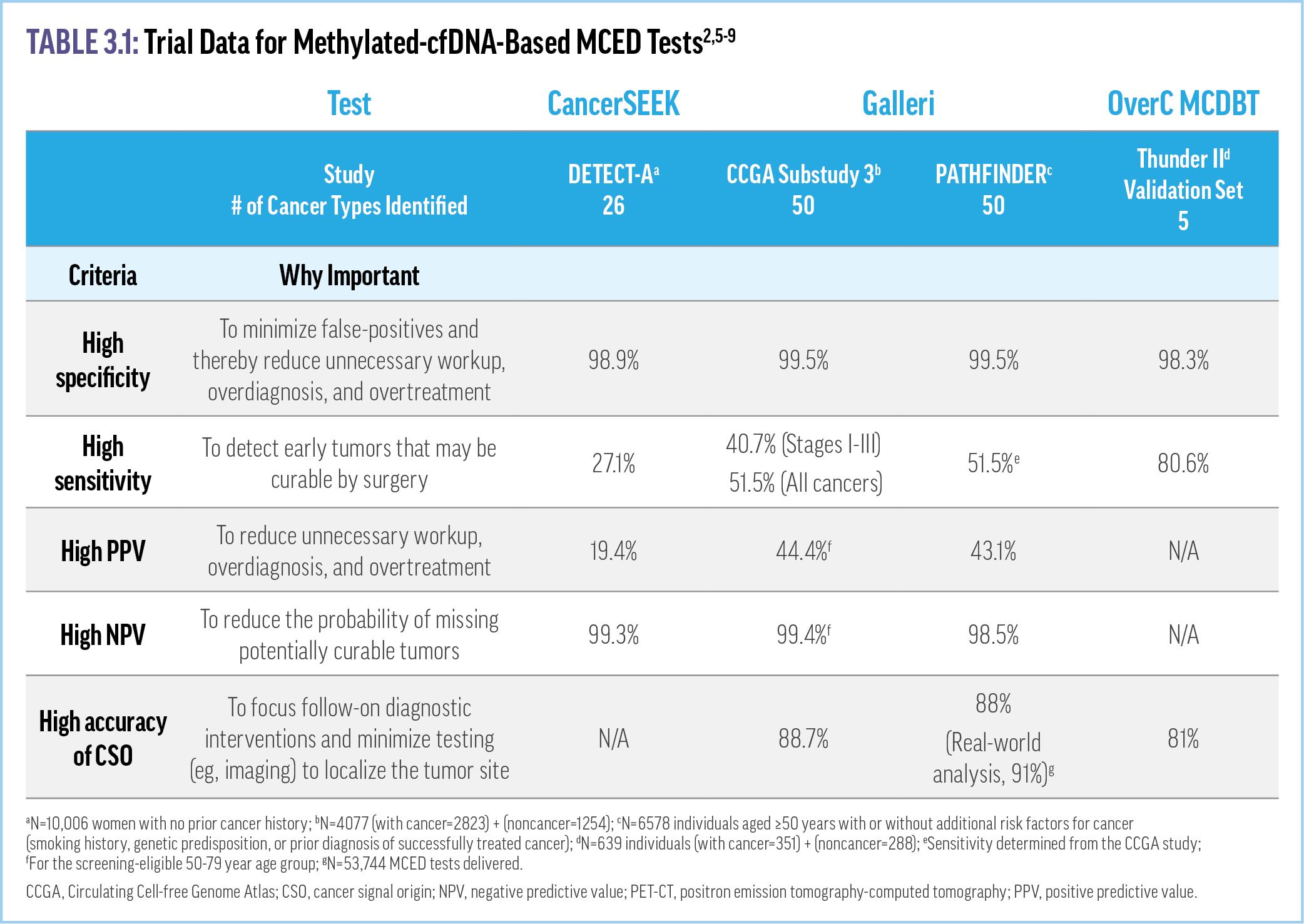
Blood-based biopsy holds the potential to radically change cancer screening and management. Increasing evidence supports the use of methylated cfDNA-based MCEDs for cancer screening.1 For an MCED test to be effective, it should demonstrate high specificity, high positive predictive value (PPV), and an ability to successfully predict the cancer signal tissue of origin (CSO) to minimize false-positives and unnecessary diagnostic workups.2 The test should also have high sensitivity and high negative predictive value (NPV) to detect tumors earlier in development and reduce the likelihood of missing potentially curable tumors.2 A number of cfDNA-based MCED assays (eg, CancerSEEK, OverC™ Multi-Cancer Detection Blood Test [MCDBT]) are in various stages of development, with only the Galleri® test currently commercially available.3,a Current evidence supporting the utility of cfDNA-based MCED tests is detailed below and in Table 3.1
aThe OneTest™ MCED test is also commercially available, but it is used to predict “an individual’s risk of being identified as having cancer in the coming 12-month period,” not whether cancer is present at the time of the test.4
CancerSEEK
The CancerSEEK test uses machine learning to analyze ctDNA mutations and protein biomarkers to identify 26 different cancer types. An early version of the test was used in the DETECT-A study, the first clinical trial of an MCED test.3 This study of 10,006 women aged 65-75 years with no prior cancer history used positron emission tomography-computed tomography (PET-CT) or other forms of imaging to confirm cancer signals and the CSO identified by CancerSEEK.7 This study demonstrated the feasibility of cfDNAbased MCED tests, with a specificity of 99.6%, sensitivity of 27.1%, PPV of 40.6%, and NPV of 99.3% when the blood test was combined with any form of imaging (Table 3.1).7 The CancerSEEK blood test alone, without any imaging, demonstrated 98.9% specificity and 19.4% PPV.7 CancerSEEK received US Food and Drug Administration (FDA) Breakthrough Device Designation for the
detection of ovarian and pancreatic cancer in 2019, and a refined version of the test is currently being tested in the ASCEND trial.2,10
Galleri
The Galleri MCED test utilizes next-generation sequencing of cfDNA methylation patterns in conjunction with machine learning to identify a cancer signal and its tissue of origin.8 The first clinical trial of the Galleri test was the Circulating Cell-free Genome Atlas (CCGA) Study. This study was a 3-part prospective, casecontrolled, observational study of more than 15,000 participants to determine whether the Galleri test could detect and localize a large number of cancer types with a specificity high enough to support general population–based screening.2 The initial proof-of-concept portion of the study showed that examining cfDNA methylation patterns outperformed sequencing approaches focused on genetic mutations or chromosome changes for detecting multiple cancer types.11,12 Using two different early versions of Galleri for a training and validation substudy, more than 50 different cancers were identified.13 In the clinical validation substudy, the sensitivity rose significantly depending on the specific cancer and stage at detection, with an average of 51.5% for all cancers (16.8% for stage I, 40.4% for stage II, 77.0% for stage III, 40.7% for stage I-III cancers, and 90.1% for stage IV.6 Galleri also demonstrated a 99.5% specificity, 44.4% PPV, 99.4% NPV, and an overall CSO accuracy of 88.7% (Table 3.1).6,13 With a low false positive rate (0.5%), the CCGA studies showed that this test has a high degree of specificity and CSO accuracy across many different cancer types, including many that currently lack national screening recommendations and are associated with lower mortality risks when they are diagnosed at earlier stages.6,14
More evidence of Galleri’s utility is provided by the prospective, interventional PATHFINDER study, which was designed to evaluate the implementation of Galleri in practice.8,15 Recently published PATHFINDER results, involving more than 6600 patients aged ≥50 years without a prior diagnosis of cancer, reported a positive cancer signal in 92 patients (1.4%).8 Of these positive tests, a refined version of Galleri (two versions were utilized in the study) correctly identified a cancer signal in 35 patients, correlating to a positive predictive value (PPV) of 43.1% and a specificity of 99.5%.8 The CSO accuracy was 88% for the first- or secondpredicted origin.8 (Table 3.1) The median time to diagnostic resolution was 79 days: 57 days for participants who received a true positive result and 162 days for a false-positive result.8 A realworld analysis of >53,000 Galleri tests presented at the American Society of Clinical Oncology 2023 annual conference demonstrated the Galleri test’s performance was consistent with previous clinical studies across age, sex, and geographic location. For example, the real-world CSO accuracy was greater than 90%.9 The cancer signal detection rate of 0.95% (lower in females than males) increased with age and was consistent with that demonstrated in PATHFINDER. Further, 67.4% and 61.9% of the malignancies detected in males and females, respectively, represented cancers without population screening recommendations. The Galleri test is currently being investigated in large clinical trials for a number of different populations, including women attending mammography screening (STRIVE), smokers and former smokers at high risk of lung cancer attending low-dose CT screening (SUMMIT), and patients eligible for other guideline-recommended cancer screenings (PATHFINDER 2).16 The Galleri test was granted FDA Breakthrough Device Designation and has been available since June 2021 under a waiver for high-
complexity testing by the Clinical Laboratory Improvement Amendments of 1988.12
OverC MCDBT
The THUNDER I and THUNDER II substudies are prospective, observational trials for the development and validation of the OverC MCDBT, an MCED test that specifically targets cancers of the lung, colon/rectum, liver, ovary, pancreas, and esophagus.13,17 Similar to the CCGA study, these trials included training and validation sets with two different versions of OverC.5,17 The original THUNDER I study showed a specificity of 95.1-98.9% and sensitivity of 69.1-75.1% depending on the test version used.5 Reported results of the Thunder II substudy include a specificity of 99.5% for the training set and 98.3% for the validation set, whereas the sensitivity of the test was 79.9% and 80.6% in the training and validation sets, respectively (Table 3.1).13,17 Further, the CSO accuracy was reported as 81%.17 Based on these results, the OverC MCDBT test was granted Breakthrough Device Designation by the FDA in early 2023.18 This assay is being examined in 2 ongoing prospective trials: the PREDICT study of persons aged 40-75 years with cancer or benign diseases in tumor sites and healthy participants, and the PREVENT study of asymptomatic individuals aged 40-75 years who have not received a colonoscopy, abdominal MRI/CT, low-dose CT, or chest CT within 5 years.16,19
Meta-analyses Confirm the Efficacy of MCED Tests
Many other tests are currently in development and validation phases (eg, CanScan, CancerRadar, Adela, PanSeer).3,10,20 It can
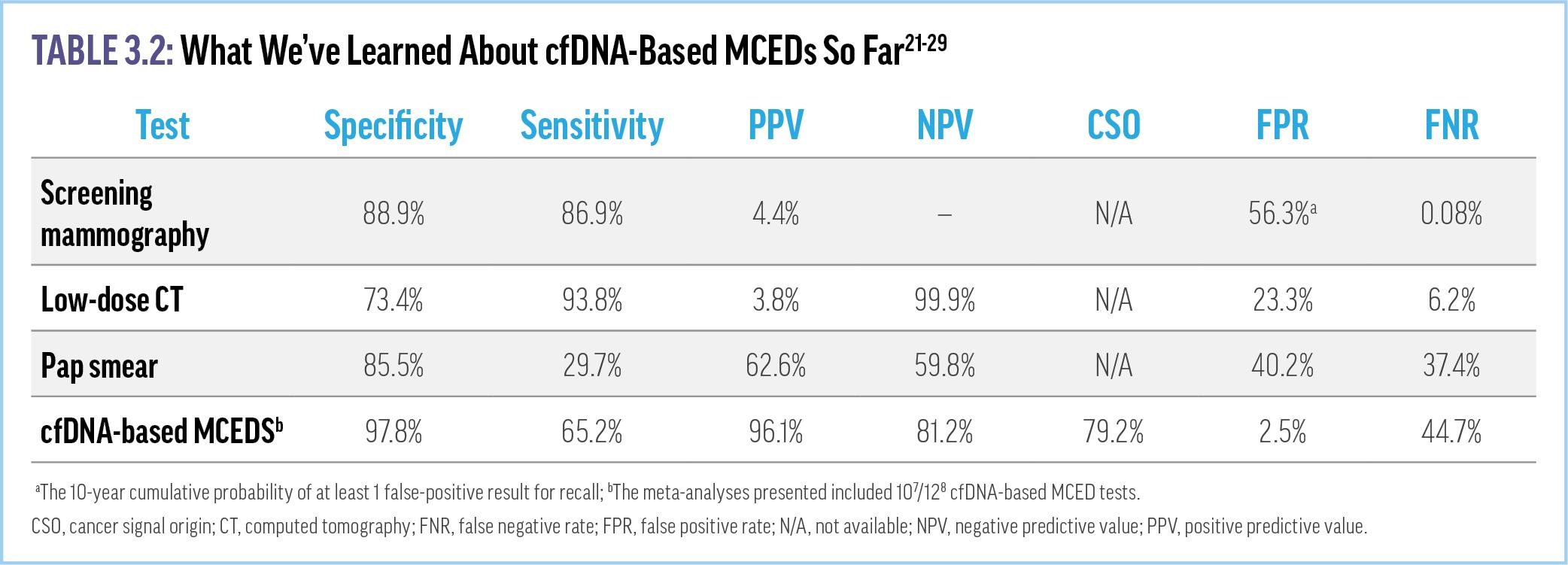
be difficult for clinicians to distinguish among the tests and their abilities to detect true cancer signals with a minimum of false positives. Recent meta-analyses of multiple (≥10) cfDNA-based MCED tests presented at the Spring 2023 ASCO and American Association for Cancer Research annual conferences support their use in early cancer detection.21,22 The results showed an overall MCED test specificity of 97.5%, a PPV of 96%, and falsepositive rate of 2.5% (Table 3.2). These rates are significantly better than such single-organ screening modalities as diagnostic mammography and low-dose CT for lung cancer (diagnostic mammography PPV, 4.4%) (Supplementary Video 4) 23-25 As with other cancer screening strategies, none of the versions of MCED testing is 100% accurate and they have been developed to maximize specificity. Prioritizing specificity over sensitivity will help minimize false positives. However, the lower sensitivities of the tests increase the risk for false negatives and the potential for missed cancers

VIDEO 4: MCED Test Efficacy Compared to Current Screening Modalities
With at least 10 MCED tests in development, clinicians may struggle with which test to choose for various patients when more become commercially available.3,10,30 Thus, the National Cancer Institute has planned the Vanguard Study on Multi-Cancer Detection. Slated to begin in 2024, this randomized controlled trial will compare the emerging and available MCED tests.31 The Vanguard study is a part of the larger Cancer Moonshot Initiative introduced in early 2022, which is designed to expedite evaluations of MCED tests so that they can be easily integrated into practice upon their approval.32
Key Takeaways
• Individual clinical trial results and meta-analyses have demonstrated the utility of methylated cfDNA-based multi-
cancer screening tests, including high specificity, sensitivity, PPV, and CSO accuracy and low false-positive rates.
• Currently, the only commercially available methylated cfDNA–based MCED assay is the Galleri test, which detects more than 50 cancer types with high PPV, specificity, and CSO accuracy.
• Many MCED tests are in various stages of development and clinical trials; the Vanguard study will provide important information to consider when more than one test is commercially available.
References
1. Liu MC. Transforming the landscape of early cancer detection using blood tests— Commentary on current methodologies and future prospects. Br J Cancer. 2021;124(9):1475-1477.
2. Duffy MJ, Diamandis EP, Crown J. Circulating tumor DNA (ctDNA) as a pan-cancer screening test: is it finally on the horizon? Clin Chem Lab Med. 2021;59(8):1353-1361.
3. Guerra CE, Sharma PV, Castillo BS. Multi-cancer early detection: the new frontier in cancer early detection. Annu Rev Med. 2024;75:67-81.
4. OneTest. The science behind OneTest for cancer. https://onetestforcancer.com/ onetest-science/. Accessed April 26, 2024.
5. Gao Q, Lin YP, Li BS, et al. Unintrusive multi-cancer detection by circulating cell-free DNA methylation sequencing (THUNDER): development and independent validation studies. Ann Oncol. 2023;34(5):486-495.
6. Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-1177.
7. Lennon AM, Buchanan AH, Kinde I, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020;369(6499):eabb9601.
8. Schrag D, Beer TM, McDonnell CH, 3rd, et al. Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study. Lancet. 2023;402(10409):1251-1260.
9. Westgate C, Kingsbury D, Poliak M, et al. Early real-world experience with a multicancer early detection (MCED) test. J Clin Oncol. 2023;41(suppl 16):abstr 10519.
10. Farooq M, Leevan E, Ahmed J, et al. Blood-based multi-cancer detection: a state-ofthe-art update. Curr Probl Cancer. 2024;48:101059.
11. Liu MC, Jamshidi A, Venn O, et al. Genome-wide cell-free DNA (cfDNA) methylation signatures and effect on tissue of origin (TOO) performance. J Clin Oncol. 2019;37(suppl 15):3049.
12. Oxnard GR, Klein EA, Seiden M, et al. Simultaneous multi-cancer detection and tissue of origin (TOO) localization using targeted bisulfite sequencing of plasma cell-free DNA (cfDNA). Ann Oncol. 2019;30:v912.
13. Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cellfree DNA. Ann Oncol. 2020;31(6):745-759.
14. National Cancer Institute (NCI): Surveillance Epidemiology, and End Results Program Cancer Stat Facts. https://seer.cancer.gov/statfacts/. Accessed March 6, 2024.
15. Beer TM, McDonnell III CH, Nadauld L, et al. Interim results of PATHFINDER, a clinical use study using a methylation-based multi-cancer early detection test. J Clin Oncol. 2021;39(suppl 15):Abstract 3070.
16. ClinicalTrials.gov. Accessed March 19, 2024.
17. Gao Q, B. L, Xu J, Wang C. Early detection and localization of multiple cancers using a blood-based methylation assay (ELSA-seq). J Clin Oncol. 2021;39(3_suppl):459.
18. Seymour C. FDA grants breakthrough device designation to OverC multi-cancer detection blood test. 2023; https://www.onclive.com/view/fda-grants-breakthroughdevice-designation-to-overc-multi-cancer-detection-blood-test. Accessed April 26, 2024.
19. Carr DJ, Welch HG. Assessing the clinical utility of liquid biopsies across 5 potential indications from therapy selection to population screening: a review. JAMA Intern Med. 2023;183(10):1144-1151.
20. FDA grants breakthrough device designation for Geneseeq's multi-cancer early detection solution. 2024. https://www.prnewswire.com/news-releases/fda-grantsbreakthrough-device-designation-for-geneseeqs-multi-cancer-early-detectionsolution-302024692.html. Accessed March 18, 2024.
21. Park JH, Oh Y, Chung LI-Y, et al. Systematic review and meta-analysis of the accuracy and applicability of blood-based multi-cancer early detection (MCED) in the general population. Cancer Res. 2023;83(suppl 7):Abstr 783.
22. Park J, Oh Y, Chung L, et al. Systematic review and meta-analysis of the accuracy and applicability of blood-based multi-cancer early detection (MCED) in the general population. J Clin Oncol. 2023;41(suppl 16):abstr 3069.
23. Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49-58.
24. Church TR, Black WC, Aberle DR, et al for the National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991.
25. Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
26. Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5(3):e222440-e222440.
27. Myers DA, Wood B. A case of stage IIA anal squamous cell carcinoma (SCC) diagnosed using a multi-cancer early detection (MCED) test. Cancer Res. 2023;83(suppl 7):6503.
28. Vahedpoor Z, Behrashi M, Khamehchian T, Abedzadeh-Kalahroudi M, Moravveji A, Mohmadi-Kartalayi M. Comparison of the diagnostic value of the visual inspection with acetic acid (VIA) and Pap smear in cervical cancer screening. TaiwanJ Obstet Gynecol. 2019;58(3):345-348.
29. Xiang J, Liu Q, Shaknovich R, Venn O. Clonal B-cell expansion and the potential challenges to blood-based early cancer detection. Cancer Res. 2023;83(suppl 7):779.
30. Cisneros-Villanueva M, Hidalgo-Pérez L, Rios-Romero M, et al. Cell-free DNA analysis in current cancer clinical trials: a review. Br J Cancer. 2022;126(3):391-400.
31. Minasian L, Castle PE. Cancer screening research network/multi-cancer early detection evaluation. 2022; https://prevention.cancer.gov/sites/default/files/ 2023-02/Cancer-Screening-Research-Network-MCED-20220615.pdf. Accessed March 18, 2024.
32. American Association for Cancer Research (AACR). Moonshot redux to focus on prevention, screening. Cancer Discov. 2022;12(4):876.
CHAPTER 4. IMPLEMENTING MCED SCREENING INTO PRIMARY CARE PRACTICE
Introduction
Adoption of blood–based cancer screening requires strategies to optimize benefits and minimize potential harms. Notably, MCED tests are not intended to replace current cancer screening methods, but rather should be used in conjunction with those strategies. In the United States, implementing appropriate cancer screening regimens—and therefore the use of novel screening technologies—is predominantly the responsibility of primary care clinicians.1 Thus, it is essential that primary care providers are current on available cancer screening options, resources, and recommendations, while also being prepared to work with patients to interpret and act on test results.2
Eligibility Criteria
Currently, no MCED test has been included in national cancer screening guidelines or other consensus recommendations. The MCED Consortium—a nonprofit US/UK public-private consortium that includes clinicians, advocacy groups, payers, and industry experts—has been working toward developing consensus guidelines and information about MCED tests.3 In 2023, the MCED Consortium published several White Papers to aid clinicians seeking to incorporate MCED testing into practice (available in the CRC ExchangeCME.com/MCEDeHealthResources). Patient eligibility
criteria for MCED testing suggested by the MCED Consortium and other publications (including the test manufacturer’s recommendations) include age ≥50 years, family or personal history of cancer, known genetic mutations, and/or risk factors including smoking history, environmental exposure, obesity, and immunosuppression (Figure 4.1).3,4 Criteria that designate an individual ineligible for MCED testing include age <21 years, having an active cancer diagnosis or treated for cancer in last 3 years, or pregnancy.4,5 Some sample case scenarios for determining eligibility for MCED testing are discussed in Supplementary video 5
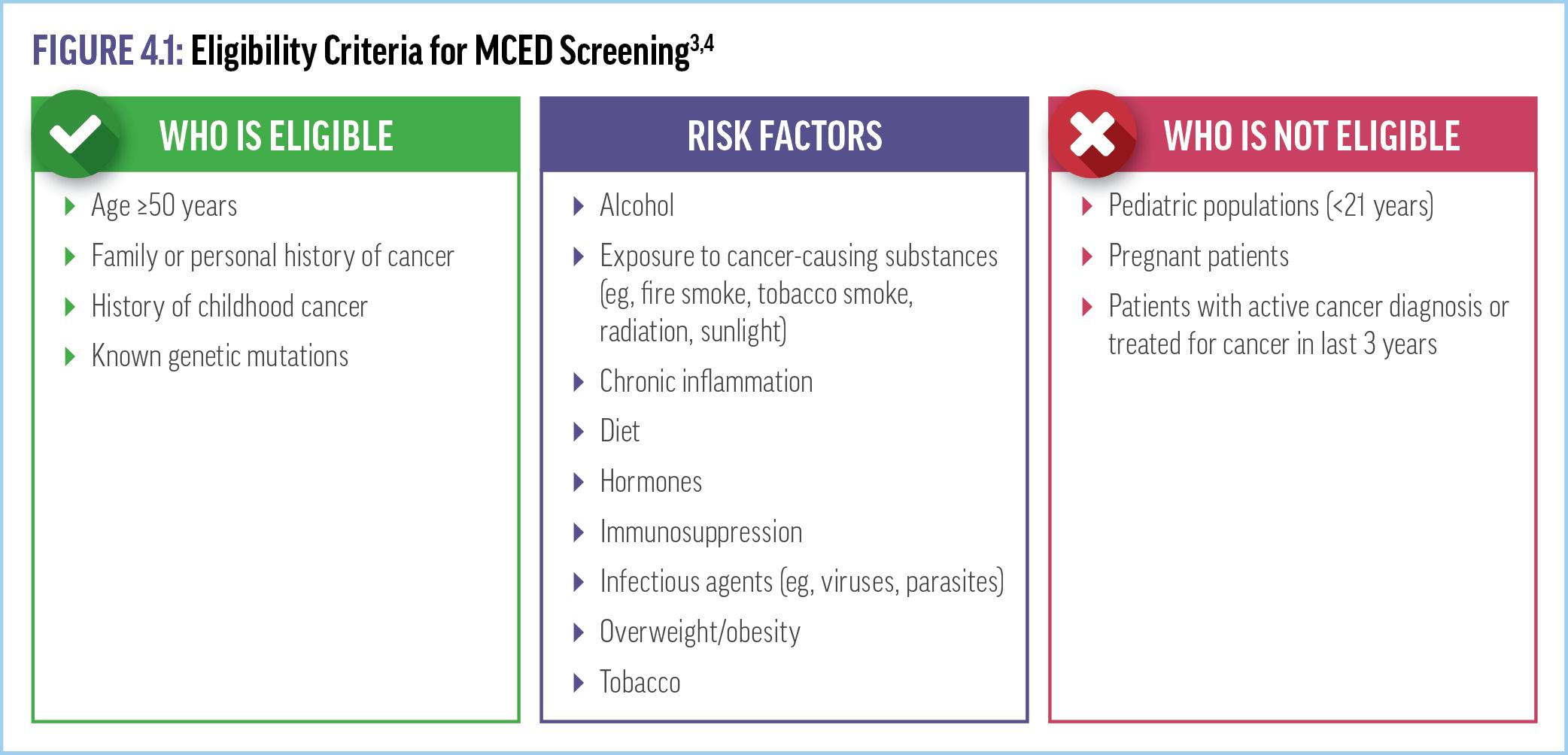

VIDEO 5: Eligibility Criteria for MCED Screening
Patient Counseling
Successful clinical adoption of tests that proactively screen for multiple cancers simultaneously requires some dedication to patient counseling about potential advantages and disadvantages (Table 4.1). Potential benefits of MCED screening clearly include reduced morbidity and mortality associated with identifying tumors at earlier stages, especially when patients are asymptomatic.3,6 Other advantages include screening for multiple cancers with 1 test as well as intercepting cancers that do not have a practical or recommended screening modality, especially those that are usually caught at later stages and are harder to treat (eg, pancreatic, ovarian).3,6 In contrast, potential harms include the consequences of false positives (eg, unnecessary diagnostic workups, increased anxiety and stress, overtreatment of indolent cancers) or false negatives, which may delay the identification and treatment of a malignancy.3,4,6-8
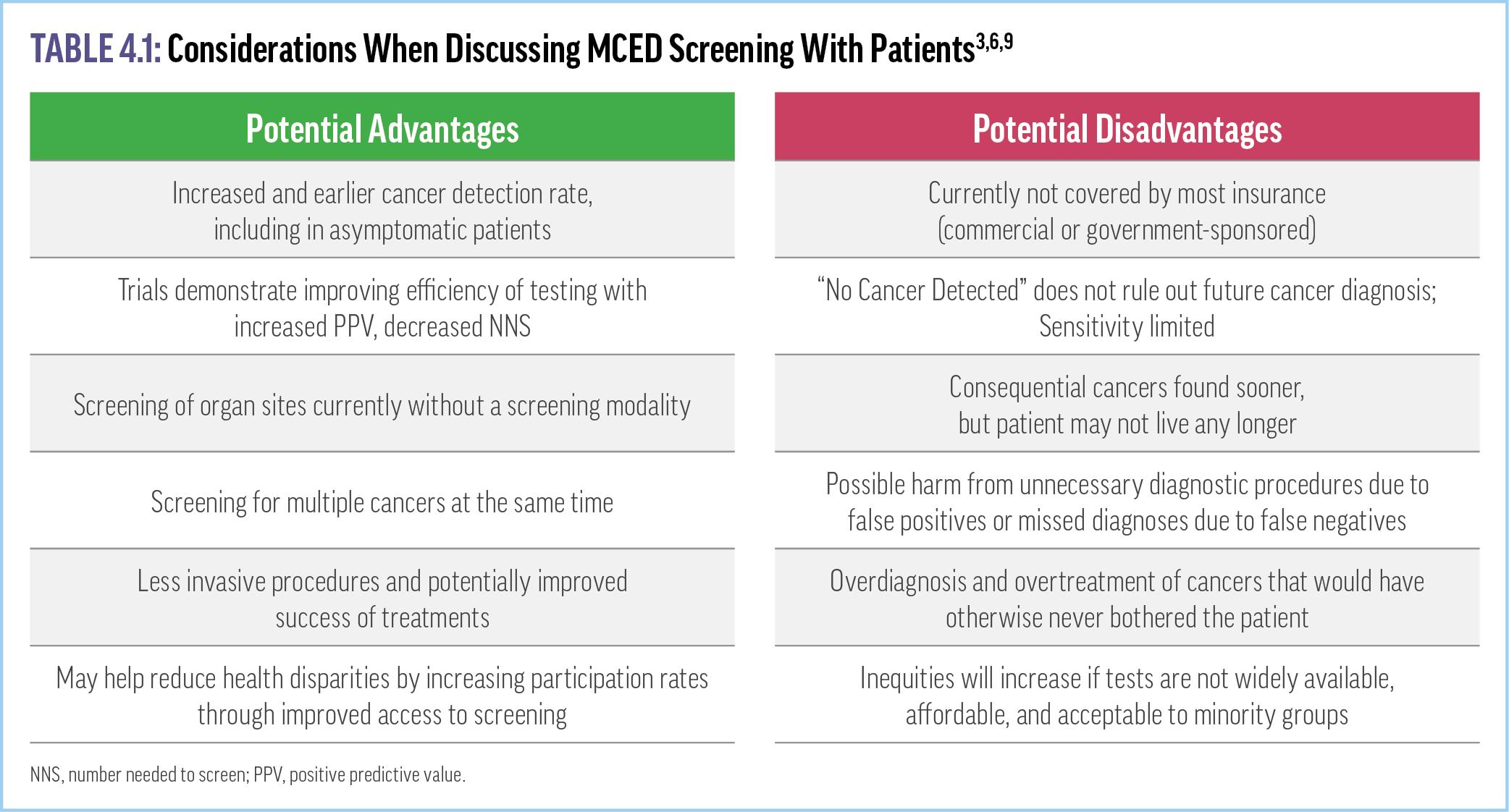
Thus, patient counseling prior to screening should include specific conversations about the possibility of false positives and false negatives, with the decision to move forward based on shared decision-making with each patient. A recent review noted that education balancing the current benefits and risks is lacking.10 Yet surveys of primary care patients reveal that as many as 4 in 5 have a high level of interest in undergoing MCED testing, primarily driven by expectations of recommendations by their health care provider, convenience, and the ability to identify early-stage disease.11 An interim analysis of the PATHFINDER study supports the notion that patients are accepting of MCED testing. Among the more than 6000 patients surveyed, 43.7% reported being “extremely satisfied,” and another 45% were “satisfied” or “very satisfied” with MCED testing.12 For now, it is up to the clinician and patient to determine whether the potential benefits of screening outweigh the costs and potential harms.
MCED Testing Workflow (Figure 4.2)
Once the decision has been made for an individual to move forward, the MCED test requires a simple blood draw and processing by the appropriate laboratory. Described below is the workflow for the Galleri test, the only currently commercially available MCED test to identify the presence of cancer. Notably, individual testing processes may differ and evolve as more tests become available and real-world data continue to accrue. Galleri is ordered by a health care provider via a test requisition form through the mail or electronically.5 Specimen collection kits, including two 10-mL Streck cfDNA BCT® blood collection tubes, are shipped upon request to health care providers or directly to patients. Following the collection of whole blood, specimens must be stored between 1°C and 40°C for no more than 7 days before the start of processing by the lab.5 Results are delivered through a provider portal within 2 weeks. Clinicians should be aware that Galleri’s manufacturer offers telehealth services to consult with patients and order the test for them if they do not have access to it through their regular clinician.

Insurance Coverage
One of the significant challenges to the widespread incorporation of MCED testing into the cancer screening paradigm is cost. The currently available MCED test is covered by only a few insurance plans and has an out-of-pocket cost of $949. Pricing for tests that are still in development is not yet known. However, several initiatives have moved forward to improve coverage of both MCED testing and any diagnostic follow-up. For example, the bipartisan Medicare Multi-Cancer Early Detection Screening Coverage Act of 2023—a bill to authorize Medicare to evaluate and cover MCED tests and future test methods approved by the FDA—was introduced in Congress and is awaiting review by the Committee on Finance.14,15 In addition, in late 2023, the Galleri-Medicare REACH study (Real-world Evidence to Advance Multi-Cancer Early Detection Health Equity) was initiated with Medicare coverage for testing and follow-up diagnostic services for study participants.16 The REACH initiative will provide MCED testing to 50,000 Medicare beneficiaries with the goal of evaluating the clinical impact of multicancer screening in this population, which includes racial and ethnic minorities as well as seniors from historically underserved communities.16
Patient-Centered Care After a Positive Test and Follow-up Care Pathways
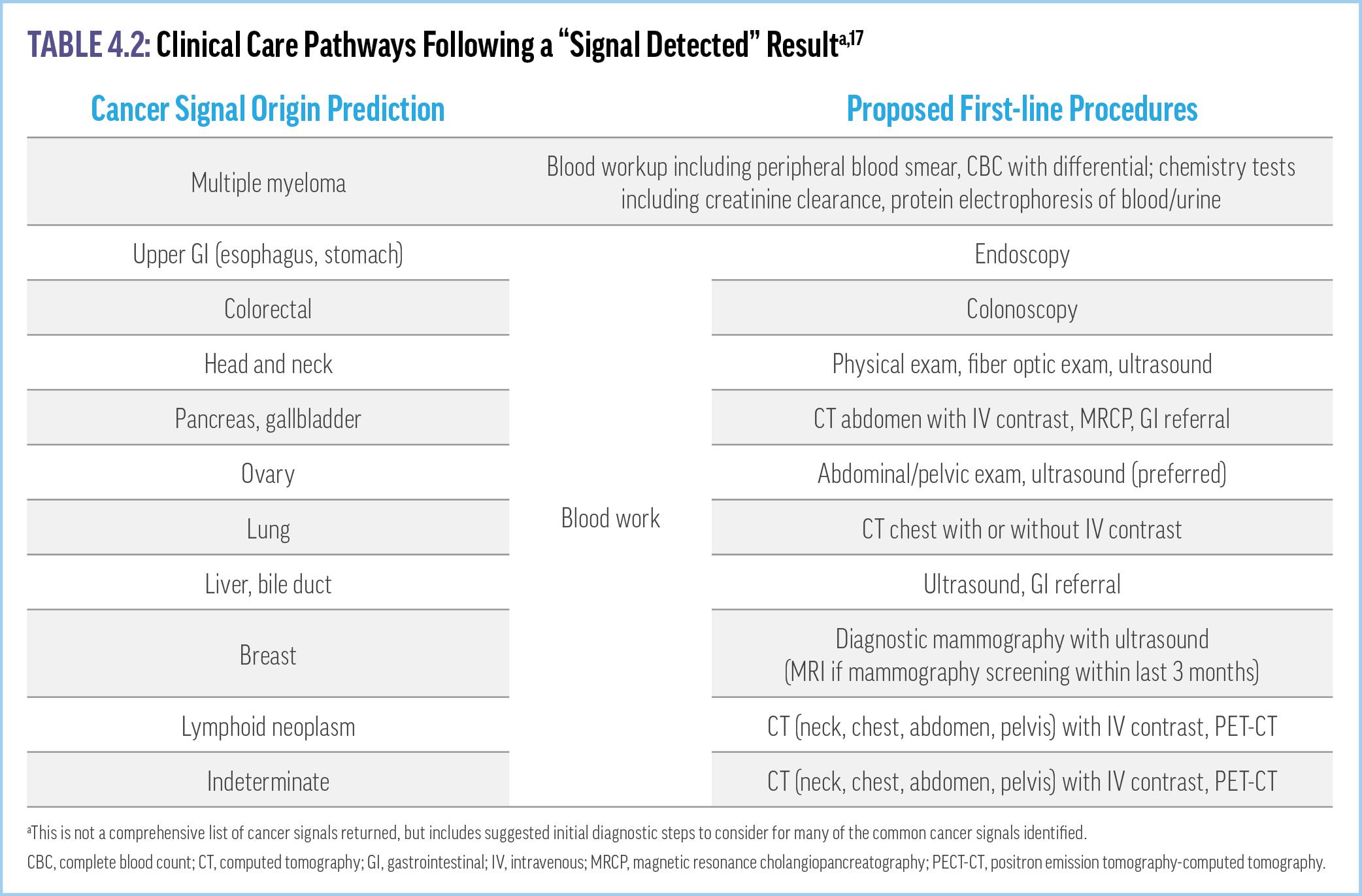
Clinicians should be prepared to counsel patients with evidencebased guidance on what MCED test results do, and do not, mean. Follow-up for a test that produces a positive signal for cancer should be individualized based on the specificity and sensitivity of the specific MCED test, the invasiveness of additional diagnostic evaluations, the potential efficacy of available treatments, and other patient-specific factors. Recommended first-line procedures
for diagnostic confirmation following a positive cancer signal may include laboratory tests, bone scans, magnetic resonance imaging, positron emission tomography, ultrasound, X-ray, or tissue biopsy (Table 4.2).17 Monitoring pathways should also be developed for people with a false positive MCED test (eg, no radiologic or clinical evidence of cancer after a positive MCED finding), including repeat MCED testing. Of note, Galleri’s manufacturer will provide free retesting 3 to 6 months following a false positive signal.18 Clinicians must also be prepared when they refer patients with positive MCED results to specialists who may not be familiar with testing specificity, PPV, or CSO accuracy. Further, Galleri’s manufacturer offers a panel of third-party experts who can consult on challenging cases.18 Answers to a number of common clinician questions regarding MCED testing can be found in Supplementary Video 6

VIDEO 6: Answers to Common Implementation Questions About MCED Tests
For negative MCED test results, clinicians should ensure that patients understand that these findings do not eliminate the risk of developing cancer at some point, but rather indicate that they likely did not have cancer when the blood was drawn. There are also no consensus recommendations on when to repeat testing after a negative result. Thus, it is a decision to be made with the patient, taking into account any risk factors and adherence to other cancer screening guidelines.
Key Takeaways
• There are no national guidelines regarding eligibility criteria for MCED testing, but the current recommendations include age ≥50 years and/or an increased cancer risk.
• MCED testing is intended to complement, not replace, current screening strategies.
• The choice to undergo MCED testing should result from shared decision-making after counseling the patient on potential benefits and consequences of testing.
• Effective integration of MCED screening into primary care practices includes the establishment of workflows surrounding testing logistics, follow-up evaluations, and transitions of care.
References
1. Wender R, Wolf AMD. Increasing cancer screening rates in primary care. Med Clin North Am. 2020;104(6):971-987.
2. Smith RA, Oeffinger KC. The importance of cancer screening. Med Clin North Am. 2020;104(6):919-938.
3. Multi-cancer Early Detection (MCED) Consortium: Care Delivery Workgroup. Multicancer Early Detection (MCED) Screening Guidance: A Recommended Care Pathway for Clinical Use of MCED Tests. 2023.
4. Putcha G, Gutierrez A, Skates S. Multicancer screening: one size does not fit all. JCO Precision Oncol. 2021(5):574-576.
5. Galleri. Provider overview. https://www.galleri.com/hcp. Accessed March 25, 2024.
6. Klein EA, A D, Cohn A, et al. Clinical validation of a targeted methylation-based multicancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-1177.
7. Guerra CE, Sharma PV, Castillo BS. Multi-cancer early detection: the new frontier in cancer early detection. Annu Rev Med. 2024;75:67-81.
8. Selby K, Elwyn G, Volk RJ. Multi-cancer early detection tests, primary care, and shared decision making. Ann Intern Med. 2023;176(5):718-720.
9. Welch HG, Kramer B. The crazy confluence of Congress, liquid biopsies, Medicare, and health inequities. STAT. 2022. https://www.statnews.com/2022/01/12/medicareshouldnt-cover-liquid-biopsies-early-cancer-detection/. Accessed May 20, 2024.
10. Greene MP, Vassy JL. Helping patients understand multi-cancer early detection tests: a scoping review. Per Med. 2024:1–7. https://doi.org/10.2217/pme-2023-0090.
11. Myers RE, Hallman MH, Shimada A, et al. Primary care patient interest in multi-cancer early detection for cancer screening. J Pers Med. 2023;13(11):1613.
12. Beer TM, McDonnell III CH, Nadauld L, et al. Interim results of PATHFINDER, a clinical use study using a methylation-based multi-cancer early detection test. J Clin Oncol. 2021;39(suppl 15):Abstract 3070.
13. Agarwal G, Carlson JW, Broyles DR. Implementation of a multi-cancer early detection (MCED) test using a centralized model within (w/i) a multi-state health system. J Clin Oncol. 2023;41 (suppl 16):1526.
14. Prevent Cancer Foundation. Multi-cancer early detection. https:// www.preventcancer.org/multi-cancer-early-detection/coverage-and-legislation/ #coverage-act. Accessed April 26, 2024.
15. Congress.gov. S.2085 - A bill to amend title XVIII of the Social Security Act to provide for Medicare coverage of multi-cancer early detection screening tests. https:// www.congress.gov/bill/118th-congress/senate-bill/2085/all-actions? overview=closed#tabs. Accessed April 16, 2024.
16. GRAIL to initiate REACH study to evaluate clinical impact of Galleri multi-cancer early detection (MCED) test among the medicare population [press release]. 2023. https:// grail.com/press-releases/grail-to-initiate-reach-study-to-evaluate-clinical-impact-ofgalleri-multi-cancer-early-detection-mced-test-among-the-medicare-population/. Accessed April 26, 2024.
17. Nadauld LD, McDonnell CH III, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13(14).
18. Rubin R. Questions swirl around screening for multiple cancers with a single blood test. JAMA. 2024;331(13):1077-1080.
CLINICAL RESOURCE CENTER™
Guidelines
US Preventive Services Task Force. Published A and B Recommendations for Cancer.
www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics/ uspstf-and-b-recommendations
US Preventive Services Task Force. Breast Cancer: Screening. Final Recommendation Statement. April 30, 2024
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/ breast-cancer-screening#bcei-recommendation-title-area
US Preventive Services Task Force. Prostate Cancer: Screening. Final Recommendation Statement. May 8, 2018
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/ prostate-cancer-screening
Multicancer Early Detection Consortium. White paper on Multicancer Early Detection (MCED) Screening Guidance: A Recommended Care Pathway for Clinical Use of MCED Tests. June 27, 2023
https://static1.squarespace.com/static/615c87aaea640d19cc98840b/t/ 6499fcaddf755715cc844f39/1687813293969/ MCED+Consortium+Care+Delivery+Paper_Final.pdf
Clinician Resources
Prevent Cancer Foundation
Multi-cancer early detection coverage and legislation. https://www.preventcancer.org/multi-cancer-early-detection/coverage-andlegislation/
Surveillance, Epidemiology,
and End Results (SEER)
*Explorer
An interactive website for SEER cancer statistics. Surveillance Research Program, National Cancer Institute, Division of Cancer Control and Population Sciences. https://seer.cancer.gov/explorer/
Galleri Test Website
Menlo Park, CA: Grail LLC. https://www.galleri.com/hcp/the-galleri-test
Suggested Readings
Cell-free DNA analysis in current cancer clinical trials: a review.
Cisneros-Villanueva M, et al. Br J Cancer. 2022;126(3):391-400.
https://www.nature.com/articles/s41416-021-01696-0.pdf
Projected reductions in absolute cancer–related deaths from diagnosing cancers before metastasis, 2006–2015.
Clarke CA, et al. Cancer Epidemiol Biomarkers Prev. 2020;29(5):895-902.
https://aacrjournals.org/cebp/article/29/5/895/72197/Projected-Reductions-inAbsolute-Cancer-Related
Circulating tumor DNA (ctDNA) as a pan-cancer screening test: is it finally on the horizon?
Duffy MJ, et al. Clin Chem Lab Med. 2021;59(8):1353-1361. https://www.degruyter.com/document/doi/10.1515/cclm-2021-0171/html
Multi-cancer early detection: the new frontier in cancer early detection.
Guerra CE, et al. Annu Rev Med. 2024;75:67-81. https://www.annualreviews.org/content/journals/10.1146/annurevmed-050522-033624
New genomic technologies for multi-cancer early detection: rethinking the scope of cancer screening.
Hackshaw A, et al. Cancer Cell. 2022;40(2):109-113. https://www.cell.com/cancer-cell/fulltext/S1535-6108(22)00014-9
Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA.
Liu MC, et al. Ann Oncol. 2020;31(6):745-759.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8274402/pdf/ nihms-1712146.pdf
Multicancer screening: one size does not fit all.
Putcha G, et al. JCO Precis Oncol. 2021:574-576. https://ascopubs.org/doi/pdf/10.1200/PO.20.00488?role=tab
Modelled mortality benefits of multi-cancer early detection screening in England.
Sasieni P, et al. Br J Cancer 2023;129(1):72-80. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10307803/pdf/ 41416_2023_Article_2243.pdf
Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study.
Schrag D, et al. Lancet. 2023;402(10409):1251-1260.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01700-2/ fulltext
Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice.
Trevethan R. Front Public Health. 2017;5:307. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5701930/pdf/ fpubh-05-00307.pdf
Related Activities
Can You Compete in Early Cancer Detection?
https://exchangecme.com/MCEDCanYouCompete
Hot Topics in Blood-Based Cancer Screening
Key Information on Multi-Cancer Early Detection From 2023 Spring Oncology Meetings
https://exchangecme.com/MCED2023
TOP 10 Early Cancer Detection Queries
What You Need to Know About Blood-Based Screening in Primary Care
https://exchangecme.com/MCEDTop10
Multi-Cancer Early Detection in Primary Care
What You Need to Know About New Blood-Based Screening Tools
https://exchangecme.com/MCEDNACEOnline




 DO, MPH, FACOG
DO, MPH, FACOG






















