LIVE FACULTY
Eric S. Daar, MD

Chief, Division of HIV Medicine
Harbor-UCLA(UniversityofCalifornia,LosAngeles)MedicalCenter
Investigator,LundquistInstitute
Professor of Medicine
David Geffen School of Medicine at UCLA Torrance, California
Raymund R. Razonable, MD, FIDSA, FAST
Professor of Medicine
ProgramDirector,InfectiousDiseasesFellowshipProgram
Vice Chair, Division of Infectious Diseases
MayoClinicCollegeofMedicineandScience Rochester, Minnesota
Phyllis Tien, MD

Professor of Medicine, Division of Infectious Diseases
UniversityofCalifornia(UC),SanFrancisco Staff Physician
San Francisco Veterans Affairs Health Care System
San Francisco, California
“CALL-A-COLLEAGUE” FACULTY
Lucy Horton, MD, MPH

Associate Professor of Medicine
InfectiousDiseaseSpecialist

UCSanDiegoHealth
SanDiego,California
Lewis Teperman, MD, FACS


ViceChair,OrganTransplantSurgery
Director,OrganTransplantation
ProfessorofSurgeryandMedicine
Zucker School of Medicine at Hofstra
Northwell Health
New York, New York
Charles P. Vega, MD, FAAFP

Clinical Professor, Family Medicine
Director,UCIrvinePrograminMedicalEducationforthe Latino Community
Associate Dean, School of Medicine
University of California, Irvine Irvine, California
TARGET AUDIENCE
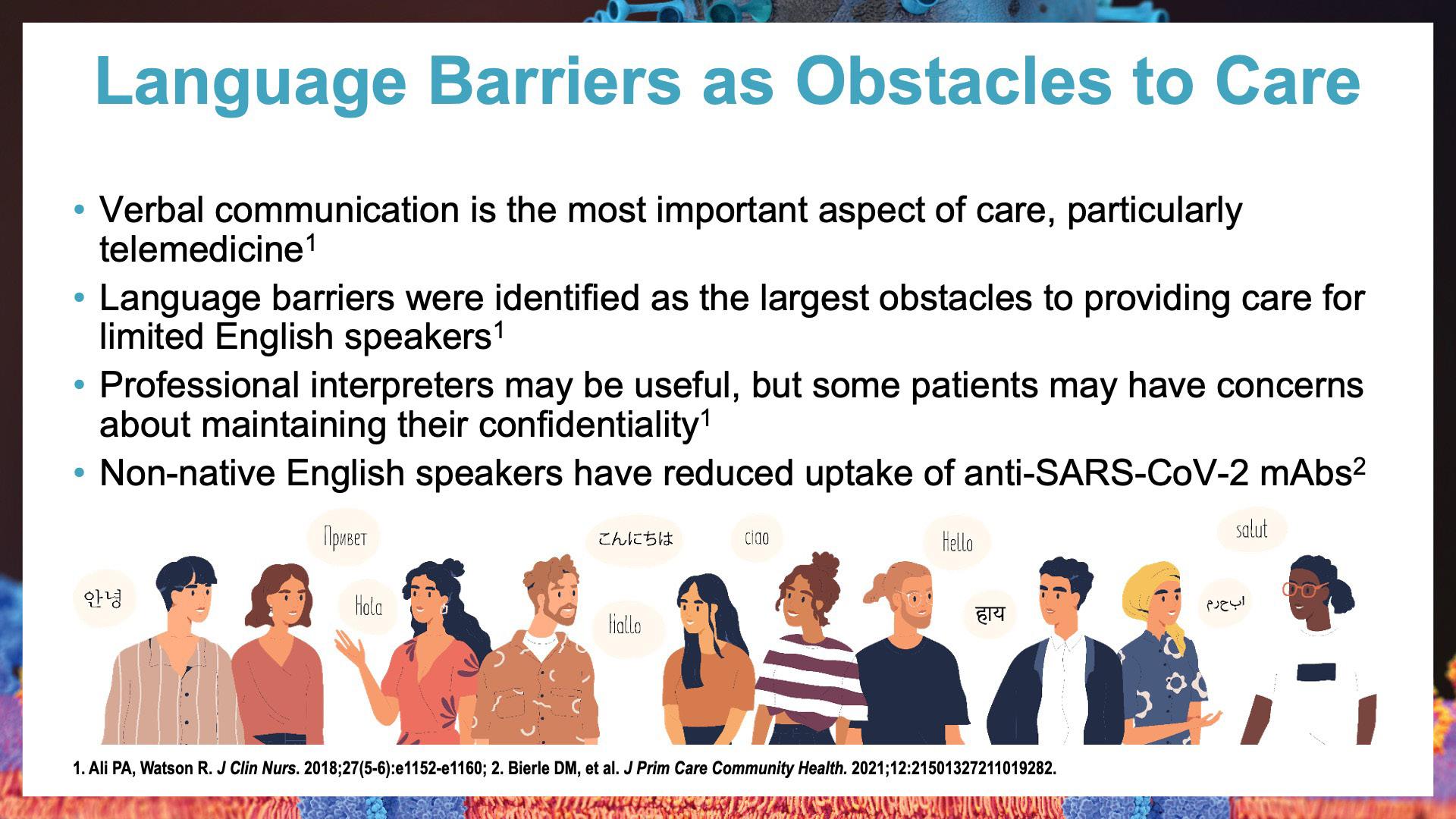
This program is intended to educate infectious disease, internal medicine, and other clinicians involved in the management of patients who are at heightened risk for poor outcomes for coronavirus disease 2019 (COVID-19).

PROGRAM DESCRIPTION
Multiple anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) monoclonal antibodies (mAbs) have gained Emergency Use Authorization (EUA) from the US Food and Drug Administration for both treatment and prevention of COVID-19, though the presence of multiple new variants and subvariants has rapidly shifted mAb efficacy and EUA status. Clinicians struggle to remain up to date with a rapidly changing field that has remained a public health concern. A panel of experts will present data on and discuss current evidence and regulatory status for these anti-SARS-CoV-2 mAbs and available antivirals, updates to National Institutes of Health guidelines on patient identification and stratification for both treatment and prevention, implementation through COVID-19 clinic models, and strategies to reduce health care disparities. The panel will utilize additional experts to augment discussion and provide expertise on multiple topics.
EDUCATIONAL OBJECTIVES
After completing this activity, participants will be better prepared to:
• Describe the clinical profiles, trial data, and
preexposure prophylaxis
status
anti-SARS-CoV-2 mAbs
Physician Accreditation Statement
This activity has been planned and implemented in accordance with the accreditation
of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Global Education Group (Global) and Integritas Communications. Global is accredited by the ACCME to provide

medical education for physicians.
Physician Credit Designation
Global Education Group designates this live activity for a maximum of 2.0 AMA PRA Category1Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Pharmacist Accreditation Statement
Global Education Group is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education with Commendation.
Credit Designation
Global Education Group designates this continuing education activity for 2.0 contact hour(s) (0.20 CEUs) of the Accreditation Council for Pharmacy Education. (Universal Activity Number - 0530-9999-22-108-L01-P)
This is a knowledge- based activity.
•
candidates for preexposure prophylaxis
treatment with anti-SARS-CoV-2 mAbs, including immunocompromised
Nurse Practitioner Continuing Education
This activity has been planned and implemented in accordance with the Accreditation Standards of the American Association of Nurse Practitioners (AANP) through the joint providership of Global Education Group and (name of other entity). Global Education Group is accredited by the American Association of Nurse Practitioners as an approved provider of nurse practitioner continuing education. Provider number: 110121. This activity is approved for 2.0 contact hour(s) (which includes 0.1 hour(s) of pharmacology).
Global Contact Information
For information about the approval of this program, please contact Global at 303-395-1782 or cme@globaleducationgroup.com.
Instructions to Receive Credit
Participants must 1) read the learning objectives and faculty disclosures; 2) view the educational activity; and 3) complete the posttest with a 70% or better and submit the evaluation form directly after the activity. Pharmacist learners must check the CPE monitor within 30 days of the activity for their credit.
Fee Information& Refund/Cancellation Policy
There is no fee for this educational activity.
Disclosures of Conflicts of Interest
Global Education Group (Global) adheres to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the Accreditation Council for Continuing Medical Education (ACCME) and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous.
All persons in a position to control the content of an accredited continuing education program provided by Global are required to disclose all financial relationships with any ineligible company within the past 24 months to Global. All financial relationships reported are identified as relevant and mitigated by Global in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Global to assure objectivity and that the activity is free of commercial bias.
All relevant financial relationships have been mitigated.
The faculty have the following relevant financial relationships with ineligible companies:
Eric S. Daar, MD
Consultant Fees: Gilead, Merck; Contracted Research: Gilead, ViiV
Lucy Horton, MD ConsultingFees: Gilead, GSK
Raymund R. Razonable, MD Contracted Research: Gilead (funds to institution), Roche, Regeneron; Honoraria: Member of DSMB for Novartis
Lewis Teperman, MD Nothing to disclose
Phyllis Tien, MD
Contracted Research: Gilead, Merck

Charles P. Vega, MD Consultingfees: GlaxoSmithKline plc., Johnson & Johnson
The plannersandmanagers at Global Education Group and Integritas Communications have no relevant financial relationships to disclose.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. Global Education Group (Global) and Integritas Communications do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications on dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.