Vol. 14 1technation.com ADVANCING THE BIOMEDICAL / HTM PROFESSIONAL FEBRUARY 2023
LEGISLATION
CYBERSECURITY AND REMANUFACTURING
46
Professional of the Month Larry Sanlin, BMET III
Next Gen HTM Michael Heusser, CCE, CHTM
Roundtable Ultrasound Systems
Right to Repair Are patients safer?
MDUFA V & HTM
ADDRESSES 510(K),
PAGE
14
16
42
56














1.855.888.8762 USOCMEDICAL.COM • GREAT CUSTOMER SERVICE • EXPANDED SERVICE CAPABILITIES TO THE GULF AND EAST COASTS • EXPANDED MODALITY COVERAGE • WORLD-CLASS R&D • PROFESSIONAL ENGINEERS WITH DECADES OF INDUSTRY EXPERIENCE ISO 9001:2015 & 13485:2016 Certified LOCATION! CALL US TODAY! USOC has announced they will be adding Endoscopes to their capabilities: New State-of-the-Art Facility in Texas Additional features of the multi-milliondollar Texas facility will include: NEW
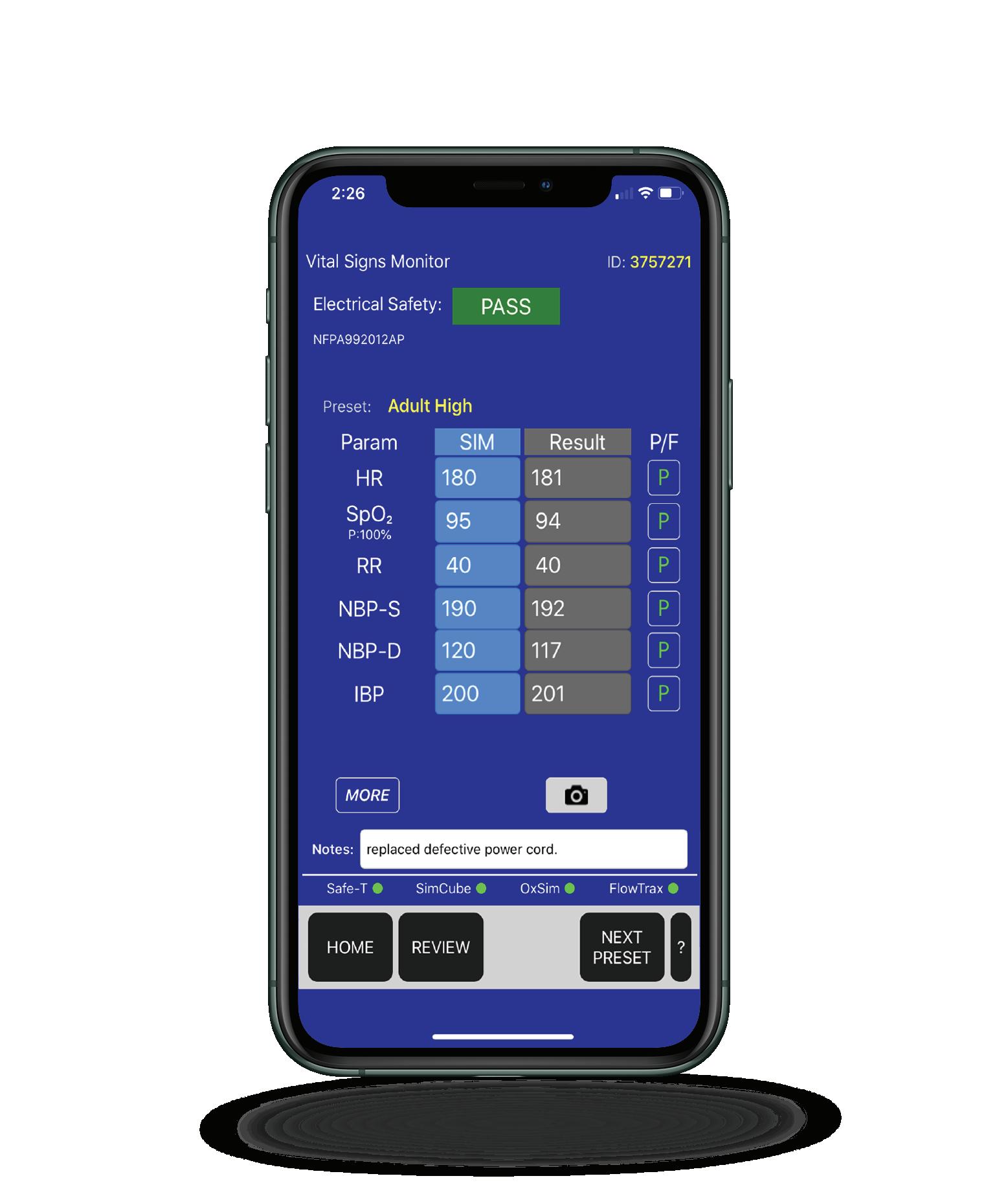
CMMS
MediMizer
D A T R END’ S VI S I ON-P A D TEC H NOLOG Y ™ INTEGRATES YOUR CMMS WITH YOUR MEDICAL TESTING EQUIPMENT
Completed TestResults
API

On-Device CMMS Integration

Assets, Procedures,
Work Orders
vPad-Rugged 2 Automated and manual 120-240V testing with wireless interface to corporate CMMS.








vPad-Mini™
TRUSTED AROUND THE WORLD FOR OVER 30 YEARS

Once an OEM for Dynatech, Datrend is one of the oldest biomedical test equipment manufacturers.

From our Legacy products, the Oxitest and Infutest, Datrend’s focus has been on testing accuracy and assisting biomedical professionals in getting their jobs done with integration that maximizes the technician’s time.
Datrend’s Vision-Pad Technology™ has over twelve integrations with CMMS systems, including EQ2, Nuvolo, and MediMizer.
COUNT ON DATREND’S INTEGRATED PRODUCTS
vPad-IV
• Improve Efficiency
• Increase Accuracy
• Automate Testing
• Cloud Integration

• Intuitive Technology
• Innovative Platform
1.877.254.7086 Sales@QRS-Solutions.com ISO 17025:2017 Calibrations ISO 13485:2016 Medical Devices www.QRS-Solutions.com Mention Promo Code TN23 (Expires April 1, 2023)
Phase 3
venTest
vPad-CO2
vPad-RF ESU Analyzer
ROI REALIZATION THROUGH TEST EQUIPMENT AUTOMATION Disclaimer: All trademarks and logos are the property of their respective owners.
vPad-A1 All-in-one Vital Signs Simulator
Our Future is Your Future.
We understand that your future depends on quality education in a rapidly growing technological field. Our program offerings are designed to provide you with the skills and expertise needed for servicing today’s imaging modalities.
Parts, Equipment & Training


With one of the largest inventories in the industry, if we don’t have the part or device you’re looking for, we can help you source it from a variety of trusted partners around the globe.
Extended Services
We have engineers and installers on-board to make your purchase a priority. Whether it’s a full installation or de-installation, or technical troubleshooting, RSTI eXchange is Engineered for Life™



Technical Support
Our certified techs know our catalog and equipment like the backs of their hands. You’ll always get expert advice from the friendly and capable RSTI eXchange team.
(833) 229-7784 | rsti-training.com | rsti@rsti-training.com
TRAINING - PARTS - EQUIPMENT OPTIONS – ISO CERTIFIED OHIO STATE BOARD OF CAREER SCHOOLS AND COLLEGES REG. NO. 93-09-1377T Visit rsti-exchange.com for more information. INDUSTRY ORIGINAL SINCE 1985 TRAINING & PARTS LEADER VISIT US AT ICE! CHECK OUT OUR TWO EDUCATION SESSIONS! SATURDAY, FEBRUARY 18 10:30 AM & 1 PM BOOTH #212 SEE US AT
PROBO MEDICAL
Your Ultrasound Partner
Your Ultrasound Partner
FREE TECH SUPPORT
FREE TECH SUPPORT
QUALITY, TESTED PARTS
QUALITY, TESTED PARTS
ULTRASOUND SERVICE TRAINING
ULTRASOUND SERVICE TRAINING
PROBE REPAIR WITH FREE LOANERS
PROBE REPAIR WITH FREE LOANERS


NEXT DAY RENTALS
NEXT DAY RENTALS
SERVICE ACROSS THE US
SERVICE ACROSS THE US
Mention this ad for 10% OFF your next part or repair.
Mention this ad for 10% OFF your next part or repair.
HAVING PROBLEMS WITH YOUR ULTRASOUND PROBE?
HAVING PROBLEMS WITH YOUR ULTRASOUND PROBE?


Scan the QR code and get an instant quote by using our free ultrasound probe evaluation tool.
Scan the QR code and get an instant quote by using our free ultrasound probe evaluation tool.
WWW.PROBOMEDICAL.COM
WWW.PROBOMEDICAL.COM
PROBO MEDICAL
WHAT’S INSIDE MATTERS
WHAT’S INSIDE MATTERS
We deliver an inside advantage you can’t get elsewhere:
We deliver an inside advantage you can’t get elsewhere:
• Int er nal su ppl y c hai n su ppo rti n g hund re ds of ul tras ound pro b es a nd MRI c oil s
• Int er nal su ppl y c hai n su ppo rti n g hund re ds of ul tras ound pro b es a nd MRI c oil s
• Engi n eer in g ex pe r tise in cl ud es tra nsd u cer
ar r ay s, pl as ti c m oldin g, 3 D pri n ti n g, ca bl e harne ss fabrica ti on , a nd pr ec ision len s es
• Repair proc e sses info rme d by ou r le gacy in FD A reg is tere d ma nuf act uri n g
X-ray of GE C3-10-D
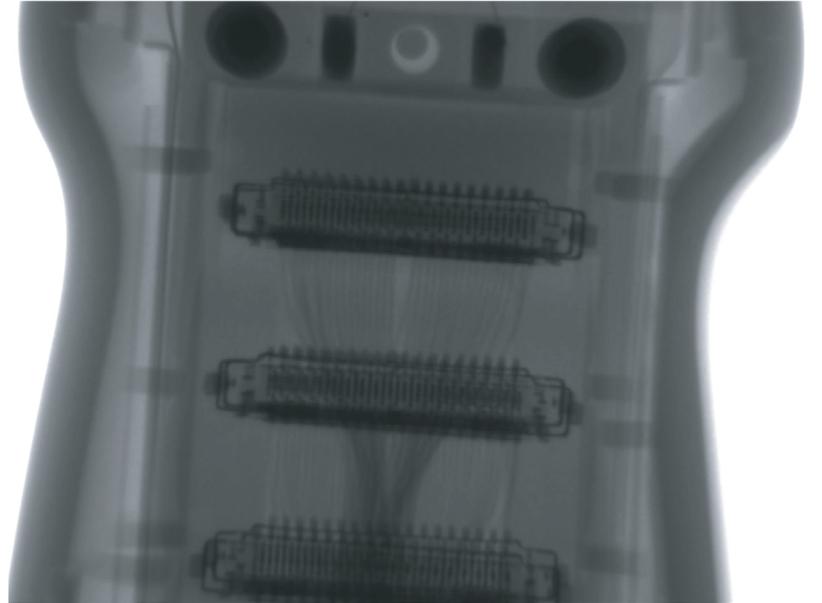
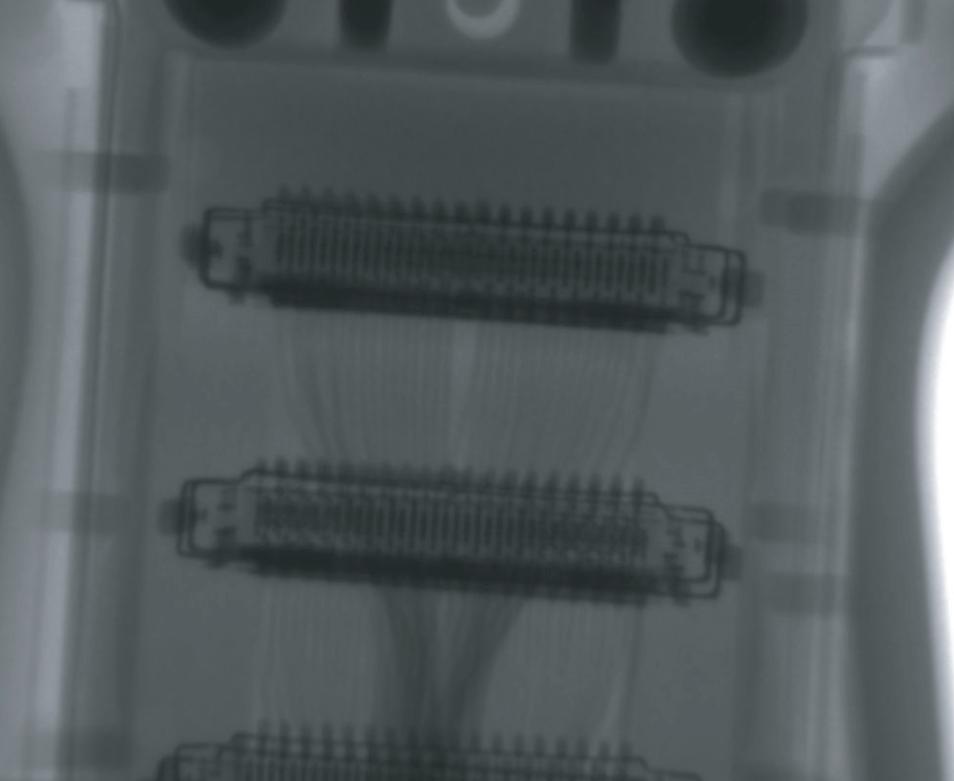
MORE R EAS ONS THAN E VER T O EVER T O INSIST ON INNOVATUS IMAGING IN SIDE! Visit us at the HTMA-OH and ICE this winter and get the Inside Advantage
@
customercare@innovatusimaging.com
844.687.5100
innovatusimaging.com
PI TTS BU R GH • TU LSA • DE NV ER
MORE
EAS ONS THAN
INSIST ON INNOVATUS IMAGING IN SIDE! Visit us at the HTMA-OH and ICE this winter and get the Inside Advantage PI TTS BU R GH • TU LSA • DE NV ER X-ray of GE C3-10-D customercare@innovatusimaging.com 844.687.5100 innovatusimaging.com @
• Engi n eer in g ex pe r tise in cl ud es tra nsd u cer ar r ay s, pl as ti c m oldin g, 3 D pri n ti n g, ca bl e harne ss fabrica ti on , a nd pr ec ision len s es • Repair proc e sses info rme d by ou r le gacy in FD A reg is tere d ma nuf act uri n g
R
E VER T O EVER T O
CONTENTS
P.12 SPOTLIGHT
p.12 Department of the Month: The Houston Methodist Biomedical Engineering Department
p.14 Professional of the Month: Larry Sanlin, BMET III
p.16 Next Gen HTM: Michael Heusser, CCE, CHTM
p.18 Shifting Gears: 200 MPH on a Motorcycle is a Drag
P.23 INDUSTRY UPDATES
p.23 News & Notes
p.26 Welcome to TechNation
p.27 Sponsored Content: Medical Equipment Doctor
p.28 Ribbon Cutting: 1904 HTM
p.30 AAMI Update
p.32 ECRI Update
42
46
P.36 THE BENCH
p.36 Biomed 101
p.39 Tools of the Trade
p.41 Webinar Wednesday
P.42 ROUNDTABLE
p.42 Ultrasound Systems
P.46 COVER STORY
p.46 MDUFA V & HTM: Legislation
Addresses 510(k), Cybersecurity and Remanufacturing
P.53 EXPERT ADVICE
p.53 Career Center
p.54 20/20 Imaging Insights: Test Your Knowledge of Ultrasound Probe Failures

p.56 Right to Repair
p.58 The Future
p.61 Cybersecurity
herein are those of the writer and/or advertiser, and not necessarily those of
TechNation (Vol. 14, Issue #2) February 2023 is published monthly by MD Publishing, 1015 Tyrone Rd., Ste. 120, Tyrone, GA 30290. POSTMASTER: Send address changes to TechNation at 1015 Tyrone Rd., Ste. 120, Tyrone, GA 30290. TechNation magazine is dedicated to providing medical equipment service professionals with comprehensive, reliable, information concerning medical equipment,
service and supplies. It is published monthly by MD Publishing, Inc. Subscriptions are
free of charge
within the United States. Publisher reserves the right to determine qualification for a free subscriptions. Every
and
in the articles and
the
©2023
parts,
available
to qualified individuals
precaution is taken to ensure accuracy of content; however, the information, opinions,
statements expressed
advertisements
publisher.
February 2023 | TechNation 9
PUBLISHER John M. Krieg
VICE PRESIDENT Kristin Leavoy
VICE PRESIDENT Jayme McKelvey OF SALES
ACCOUNT Megan Cabot
EXECUTIVES
ART DEPARTMENT
Emily Hise
Karlee Gower
Taylor Hayes
Kameryn Johnson
EDITORIAL
CONTRIBUTORS
John Wallace
Roger Bowles
K. Richard Douglas
Jim Fedele
Joie Marhefka
Manny Roman
Connor Walsh
David Witt
Steven J. Yelton
DIGITAL SERVICES Cindy Galindo
Kennedy Krieg
EVENTS Kristin Leavoy
WEBINARS
HTMJOBS.COM

ACCOUNTING
CIRCULATION
Linda Hasluem
Kristen Register
Sydney Krieg
Diane Costea
Joanna Manjarrez
EDITORIAL BOARD
Rob Bundick, Director HTM & Biomedical Engineering, ProHealth Care
Carol Davis-Smith, CCE, FACCE, AAMIF, Owner/ President of Carol Davis-Smith & Associates, LLC
Jim Fedele, CBET, Senior Director of Clinical Engineering, UPMC
Bryant Hawkins Sr., Site Manager, Children’s Hospital of New Orleans
Benjamin Scoggin, MBA, MMCi, Director, Clinical Engineering | Biomedical Operations, Equipment Distribution, Clinical IT, DHTS, Duke Health Technology Solutions
Allison Woollford, Biomedical Equipment Specialist at Duke University Health System

MD Publishing / TechNation Magazine 1015 Tyrone Rd., Ste. 120, Tyrone, GA 30290 800.906.3373 • Fax: 770.632.9090 Email: info@mdpublishing.com www.mdpublishing.com Proud supporters of
P.65 BREAKROOM p.65 The Vault p.67 Where in the World is Ben C.? p.74 #IAmTechNation p.70 Service Index p.73 Alphabetical Index Like us on Facebook, www.facebook.com/TechNationMag Follow us on LinkedIn, www.linkedin.com/company/technation-magazine 74 10 TechNation | February 2023

DEPARTMENT OF THE MONTH
The Houston Methodist Biomedical Engineering Department
BY K. RICHARD DOUGLAS
Houston, Texas is one of the bestknown cities in America. The city and surrounding communities form a metropolis that extends to the Galveston Bay. With a population exceeding 2.3 million, it is the fourth largest city in the U.S., covering 637 square miles. Houston was founded in 1836 and offers attractions like Space Center Houston, the largest fine arts museum in the Southwest and the downtown aquarium.
A city of this size depends on health care systems with the capacity to serve such a large population. That capability is embraced by Houston Methodist Hospital, affiliated hospitals and other facilities scattered across the Houston metro area. We recently profiled a small two-technician department, but it takes a biomed department at the other end of the spectrum to manage medical devices for such a large-scale health network.
With eight hospitals, an academic institute and 2,509 operating beds, Houston Methodist includes 27,947 employees, with many working in biomed. The 85-member biomedical engineering department is led by System Director Anthony Maroulis. Other members of leadership include Campus Director of Clinical Engineering Marc Bateman, Managers of Clinical Engineering Terresa Everhart, Javier Ruiz, Miguel Meza and Coreaun Jackson and Program Coordinators Brandon Hight and Nathan Stathos.
Leadership is part of GE Healthcare.
The team’s clinical engineers include Zach Smith, Cristian Delgado, Bailyn Piecewicz and Hani Khalil. The department also consists of two administration assistants, five biomed equipment tech leads, 23 biomed equipment tech IIs, 23 biomed equipment tech IIIs, 15 biomed equipment tech Is, 4 biomed radiology service engineers and 1 data entry specialist.
Bateman says that the biomed team services more than
91,000 medical devices in-house thanks to the exceptional training options available to the department’s technicians.
In addition to managing equipment at the eight hospitals, the large biomed team also serves more than 20 orthopedic and sports medicine locations offering physical and occupational therapy across the Greater Houston area.
They are also responsible for equipment at the Specialty Physician Group with 848 physicians at 191 locations and the Primary Care Group with 169 physicians at 42 locations.
The hospital is affiliated with Weill Cornell Medicine, NewYork-Presbyterian Hospital and the Texas Annual Conference of the United Methodist Church. The Houston Methodist Academic Institute, which is a research and education institute, has a faculty of 742, with 2,110 credentialed researchers.
BIG TEAM, BIG PROJECTS
The challenges to address COVID-19 surges taxed the resources of health care facilities across the nation, but it required innovation on a grand scale for the biomed team at Houston Methodist.
“At the onset of the COVID-19 pandemic, there was a glaring need to reduce exposure to patients within the hospital for our RT department. Thankfully, our Hamilton G5 ventilators have a detachable interaction panel. RT asked to have vents inside the COVID room and place the interaction panel outside to minimize exposure for the RT therapist to go inside the room. The interaction panel was attached to a table outside the patient room and we had to do this for 90-100 G5 patient rooms,” Smith says.
He says that together with the IT medical device integration team, they successfully opened a virtual ICU (vICU). “We had a key role to play in ensuring the patient physiological monitors were capable of being used in this new, innovative setting,” Smith says.
The team tackled many other projects related to the vICU to meet the needs of patients and visitors during the pandemic. Another project was centered around Alaris pump interoperability and tying them to patient records.
SPOTLIGHT
12 TechNation | February 2023
“Pharmacy, nursing, biomed, multiple IT groups, and the manufacturer have worked together to automate the delivery of IV drugs across the system,” Smith says.
He says that the result of the project is that a nurse at the bedside can now scan barcodes on the drug, patient, pump channel which will program the pump with the physician order. It also automates the charting of drug delivery in the patient record in the EHR.
“Another benefit is that there is now a standardized drug library used at all hospitals, so a standard of care is met as well as contributing to the system ISO certification,” Smith adds. The team has also worked to improve asset equipment disposition.
“We have taken the manual process of gathering signatures from department leaders to retire no longer needed equipment from our inventory to a system that leverages the platform Docusign. Using Docusign, we have saved time and resources by going digital, allowing time for other tasks,” Smith says.
The biomed team has also handled its share of problem solving, including automated notifications when defibrillators have been put into service.
“In order to adequately service our automated external defibrillators (AEDs) located throughout the hospital, we have worked with our RTLS vendor, GE, to send an email notification each time it is moved from its glass-protected case and put in use. This way, we can make sure it is inspected by our technicians after use,” Smith says. Realtime location systems (RTLS) have not only been used by the team with the defibs, but has also been a valuable tool for locating ER pumps.
“Leveraging RTLS, we created par level of IV pumps in our ER and were able to locate them to bring them back. This helped solve the issue of idle pumps and the shortages that incurred. The RTLS beacons on the pumps could help pinpoint where the IV pumps were so they could be gathered and returned in the ER,” Smith says. Team members also solved a problem that resulted from power outages.
“Our Da Vinci robots used in our ORs took a significant time to reboot when there was a power blip. In ORs that do not have a built-in UPS, we added an external UPS and it solved this issue – the Da Vinci now stays up and running when there is a sudden outage,” Smith says. Another project related to defibs provided valuable data to clinicians.
“Our hospital code blue committee wanted to find a way to transmit their code files from all our defibs in the hospital after code blue (cardiac arrest) events. We worked with our defib vendor, Zoll, and added WiFi cards to each of our defibs, allowing us to transmit data for our clinicians to review after each code. This was done for over 250 defibs. This has helped them further educate the code blue team,” Smith says.
Away from the workplace, team members remain involved in the HTM community.
“There is an assortment of professional associations that our biomed technicians are involved in. AAMI and ACCE for example. Some are also active in the local HTMA Texas and we also have a biomed instructor that serves at a local community college,” Maroulis says.
“Partnering with GE HealthCare we have implemented an apprentice program. The apprentice program has been a big success at Houston Methodist. We have used the talent pool to shape future careers, and at the same time, create a workforce that we can hire. Students from local colleges spend 12 months working with senior BMETS, where they gain a lot of experience,” Maroulis says.
With big responsibilities and big challenges, this talented team of technicians and engineers has solved problems and addressed pandemic-related trials to the benefit of patients and clinical staff. They are also partnering to train the next generation of biomeds learning in an ideal environment.
 The Houston Methodist Biomedical Engineering Department manages close to 100,000 devices.
The Houston Methodist Biomedical Engineering Department manages close to 100,000 devices.
February 2023 | TechNation 13
“Leveraging RTLS, we created par level of IV pumps in our ER and were able to locate them to bring them back. This helped solve the issue of idle pumps and the shortages that incurred.”
PROFESSIONAL OF THE MONTH Larry Sanlin, BMET III
From the Ocean Floor to Tech III
BY K. RICHARD DOUGLAS
U.S. Navy submarine can load up with 15,000 pounds of food at the beginning of a deployment. Aerosol cans, such as spray deodorant or hair spray, are not authorized to be brought onboard. The life of a submariners is unique in many ways. Submariners, when underway, operate in eight-hour shifts. It is a tight community.
Submariners also take on a lot of knowledge as part of life on a submarine. This ability to learn and comprehend translates well to other professions; including biomed.
“I served the U.S. Navy until 1996. After getting out of the U.S. Navy, I looked for a job working as a biomed technician in Chicago, however I ended up taking another job as a field service technician in 2002,” says Larry Sanlin, BMET III, Banner Baywood and Heart Hospital, Banner Health in Mesa, Arizona.
Sanlin says that in 2009, he was laid off from the field service technician job and he applied for a job as a facility maintenance technician. The next day, he received a call from the biomed director asking him to come in to interview for a position as a maintenance technician.
“I was hired and 11 years later, I am a Tech III and managing biomeds at four facilities,” he says.
Sanlin has combined biomed knowledge from his military days with on-the-job training and study to get to where he is today.
“I was hired to repair beds and stretchers. When I wasn’t working on beds, I started doing PMs on equipment and reading tech manuals, expanding my knowledge of biomed equipment and its operations. Also, I gained biomed knowledge during my time in the U.S. Navy on the submarine,” he says.
His military experience provided a firm foundation for the work he does today.
“I did have my 19 years of military training in electronics, which was very helpful, as well as my experience as a submariner, gave me the ability to absorb the required information to succeed as a biomed tech,” Sanlin says.
PROJECTS – ON THE JOB AND OFF
The accumulated experience that led to his current position resulted in the responsibility for overseeing biomeds at four campuses at his health system. That has included several projects.
“I managed two major upgrades [to] Philips telemonitor systems. My knowledge of the Philips system during the last upgrade at Banner Baywood helped complete the upgrade in record time and ahead of schedule. I identified issues that needed to be resolved to finish the upgrade. Also, at this time, I am managing a total replacement of the nurse call systems at three hospitals (Baywood, Heart and Goldfield) facilities. Changing from West-Com to ASCOM nurse call system, which is a million-dollar project. I just completed a BD Alaris major firmware upgrade to 2,600 pieces of Alaris LVP and PCU on time with medium staff,” Sanlin says.
A change in personnel also required Sanlin to step up to the challenge.
“After losing my director and shop manager in a 30-day period, I had to step up and manage the shop
SPOTLIGHT A 14 TechNation | February 2023
and take on the responsibility of four hospitals, while being short three biomed technicians. I had to manage a $2 million dollar nurse call replacement at one facility, replacement and upgrade of telemonitoring at two facilities,” Sanlin says.
There was also the challenge of training and maintaining a 95 percent preventative maintenance completion rate and completing firmware upgrades, while getting the Alaris project completed in 35 days. Sanlin says that he enjoys teaching electronics to fellow biomeds.

Off the job, Sanlin’s interests are varied. He builds computers and servers and has been involved in the martial arts and helps out at his church.
“My interest in computers started when I was attending Tuskegee University at the age of 17. I wanted to become a mechanical engineer. In addition to being accepted at Tuskegee’s mechanical engineering program, I was also accepted into the mechanical engineering program at Texas A&M. I have spent a lot of time in computer labs learning code and writing programs. As a result of what I have learned, I presently have two personal video servers with the capacity of 60 TB movies and TV shows, a home security server, which allows me to monitor my home from anywhere in the world. It’s a hobby I thoroughly enjoy,” he says.
Sanlin says that when he left the U.S. Navy, he relocated to Arizona to start his civilian job with a company called Instron.
“I also started attending church at Living by the Word Family Church. The pastor of the church found out I had training in self-defense, so he asked me to join his martial arts school,” he says.
“I started as a white belt and advanced up to 2nd dan (second degree black belt in Tang Soo Do Karate) and I taught basic and advanced self-defense to kids and adults mixing military and Tang Soo Do self-defense skills,” Sanlin adds.
Along with the martial arts and computer projects, Sanlin manages his church’s audio system and streaming channel.
“I accepted the church’s offer and have been managing their audio system from 1996 and continue to manage their audio system presently. Another thing is the church wanted a website. I knew nothing at the time about creating a website, but I love a challenge, so I told the church to give me 30 days to learn how to create a website. I did learn how to create a website and I have created four different websites for the church,” he says.
Sanlin has been married for 25 years and enjoys his job. Certainly, they appreciate him as well. There is much to be learned under the sea.
BIOMETRICS
FAVORITE BOOK: Technical manuals, cookbooks and martial arts manuals
FAVORITE MOVIE:
I enjoy westerns and martial arts.
HIDDEN TALENT:
I have the ability to comprehend technical information and use it to repair equipment. Also, hold two black belts in two different styles of karate.
FAVORITE FOOD: Popcorn, deep dish pizza and seafood
WHAT’S ON MY BENCH?
I take a 15-minute break and enjoy bacon with a biscuit. This helps me get through the day.
FAVORITE PART OF BEING A BIOMED?
The ability to work on many pieces of equipment and to know that the equipment I repair helps patients.
February 2023 | TechNation 15
NEXT GEN POWERED BY YP
AT MD
Michael Heusser, CCE, CHTM
Middlesex Health Manager of Clinical Engineering Michael Heusser, CCE, CHTM, holds a Master of Science degree in Biomedical Engineering with a focus in Clinical Engineering.

TechNation recently learned more about this up-and-coming HTM professional.
Q: WHERE DID YOU GROW UP?
A: Oxford, Connecticut
Q: WHERE DID YOU RECEIVE YOUR HTM TRAINING/ EDUCATION?

A: University of Connecticut
Q: HOW DID YOU FIRST DISCOVER HTM?
A: I learned about clinical engineering in my introduction to biomedical engineering class taught by John Enderle.
Q: HOW DID YOU CHOOSE TO GET INTO THIS FIELD?
A: During college I considered going to medical school, but enjoyed engineering. When I learned about this field, I felt it would be the perfect blend. I confirmed this when I volunteered at St. Raphael’s Hospital, now Yale New Haven Health. My time there was a great experience. It introduced me to the field and allowed me to prove to myself that my talents would be a great fit in HTM as opposed to industry.
Q: WHAT DO YOU LIKE MOST ABOUT YOUR POSITION?
A: I enjoy the variety of challenges that I deal with in my position. It always makes for an interesting and different day.
Q: WHAT INTERESTS YOU THE MOST ABOUT HTM?
A: The part of HTM that interests me the most would be strategic capital planning and data analytics.
Q: WHAT HAS BEEN YOUR GREATEST ACCOMPLISHMENT IN YOUR FIELD THUS FAR?
A: My greatest accomplishment was the work done with my team to develop a sticker program to ensure the proper cleaning of medical equipment. This program allows users to know at the point of cleaning a device which chemical is appropriate for use on the device to help prevent damage caused by cleaning with an incompatible chemical.
Q: WHAT GOALS DO YOU HAVE FOR YOURSELF IN THE NEXT 5 YEARS?
A: Over the next 5 years I would like to continue to innovate and develop solutions to challenges that my institution and the industry as a whole are facing to be able to share them with others in the field.
FUN FACTS
FAVORITE HOBBY: Playing video games
FAVORITE SHOW OR MOVIE: “Supernatural”
FAVORITE MEAL:
2 eggs over easy with a side of bacon, home fries and toast
WHAT WOULD YOUR SUPERPOWER BE? Time travel
1 THING ON YOUR BUCKET LIST: Visit Switzerland
SOMETHING YOUR CO-WORKERS DON’T KNOW ABOUT YOU: My high school nickname
SPOTLIGHT
16 TechNation | February 2023
The Total Package
2
TECHNICAL TRAINING
1
REPLACEMENT PARTS
Quality Imaging parts for CT, Mammography, MRI, Cath labs, and general X-Ray
Hands-on technical training for all imaging modalities in Nashville, TN. Now offering an Apprentice program! 4 PRE-OWNED
Inspected, tested, and certified by our team in our 60k square foot facility in Nashville, TN.


3
Service support for all major imaging manufacturers including GE, Philips, Siemens, Hologic and Toshiba/Canon.



FOR EMPOWERING THE ENGINEER
EQUIPMENT
TECH SUPPORT & SERVICE
SOLUTIONS triimaging.com | 855.401.4888 Located in Nashville, TN • ISO 13485:2016 certified
SHIFTING GEARS
200 MPH on a Motorcycle is a Drag
BY K. RICHARD DOUGLAS
iding a motorcycle in traffic can be unnerving when drivers don’t see the biker. Imagine going over 200 miles per hour on a motorcycle. That requires nerves of steel and quick reaction times.
A helmet, but no seat belt; just the wind whipping by. That is just part of the experience that has become old hat for David Barron, owner of Universal Medical Systems in Waxahachie, Texas.
Barron’s specialization is MRI/CT/X-ray service and sales, but he also has substantial experience riding a drag bike, finding ways to get every last bit of horsepower out of an engine and carrying on a family tradition.
“I made the transition to drag bikes in the early ’90s when a high school friend, Jeff Redmon, was racing at the Motorplex on the weekends. I was helping him crew and I got some seat time here and there on some faster bikes and rode for some teams in Division 4, then started racing some ET brackets and grudge races in the late ’90s,” Barron says.
In 2010, he was introduced to the pro stock motorcycle class by a mutual acquaintance of the Underdahl Racing family.
“When I approached them at the Fall Nationals in Texas, and asked what it would take to transition to a pro stock motorcycle, Greg Underdahl told me he needed to see if I could handle a bike of that magnitude by just holding the throttle wide open and releasing the clutch; then progressed to 330 feet, 660 feet and progressing into a quarter-mile (1,320 feet) pass in a couple of test sessions,” Barron says.
Underdahl then told Barron that he felt comfort -
able selling him one of his bikes and helped Barron get licensed shortly after on a Suzuki he bought in 2011.
“I always had a family member riding anything from Honda CR125s, Suzuki RM250, Yamaha YZ490 two stroke dirt bikes growing up, but always stayed interested in the dragstrip when watching my dad, uncles, grandpa, cousins race many late nights and playing or riding around in the pits,” Barron remembers.
He says that in 1985, the family relocated from a suburb of Fort Worth to Waxahachie, Texas. Waxahachie just happened to be 10 minutes from a national dragstrip, built by former NHRA funny car driver Billy Meyer, which was completed in 1986.
“It seemed like it was my destiny to continue the tradition of our family’s drag racing history after going to the first national event held there and never missed an opportunity since. All my family, including my kids, raced down that strip in competition. I am immensely proud of the four generations of drag racers that started before I was born with my grandpa in 1970,” Barron says.
His grandpa and dad had matching 1939 Chevy coupes they raced under the moniker “The Red Barron,” a play on their last name taken from the famous World War I pilot.
“I remember seeing the painted coupes with Snoopy flying his doghouse on the sides. I remember as a kid watching the Charlie Brown and Peanuts series where Snoopy often dreamed of fighting the World War I ace pilot, Baron Manfred Von Richthofen, aka, The Red Baron. I still have many of my grandma’s trophies from drag racing when she competed in the Powder Puff Women’s Drag Racing Series in Ft. Worth in the late 1960s and early 1970s before I was born,” Barron says.
Barron says that he tries to attend events close to
SPOTLIGHT
18 TechNation | February 2023
R
home, but he also races in the NHRA Camping World Drag Racing Series that starts in Gainesville, Florida, in March and consists of 16 to 18 races, ending in Pomona, California, in November.

BEHIND THE SCENES
Barron’s drag racing weekends look much different than his duties during the work week. There is a lot of work that goes into preparing a drag bike for competition.
“Crew arrives on Thursday and starts setting up and getting the bike ready to run checks and balances, qualify on Friday and Saturday and tune to the best you can,” he says.
Barron says that returning emails and calls, making sure you have your bases covered, weighing and inspections are always a prerequisite prior to qualifying, hoping you can get it together and tuned in three attempts to make the top 16.
“If you can qualify, you have a chance at more seat time and possible round wins on Sunday in eliminations. It has to become routine, so it takes a while to create and become smooth in that aspect. Hopefully, we are racing on Sunday, and going home, or to the next event late; that means we had a good day,” Barron says.
He says that it is a competitive field, but you must be wary about who you want to allow in your inner circle.
“In the end, we are all out there to win, but I have seen some shady dealings and personalities in the process just to gain an edge. It is like a poker game; hold your cards close and only show them when it is necessary,” Barron adds.
Barron’s work on-the-job also requires turning a wrench, but doesn’t include hurling himself down a quarter mile at high speed.
“I was working for an RF shielding enclosure company
and a gentleman with a third-party service company approached me and asked me if I would like to learn to work on the machines that I was building the rooms for. I then participated in a long and tenuous shadow program with a veteran engineer that showed me many good things,” he says.
He says that he has also worked for, and with, the OEM and taken continuing education courses over the past 20 years that have allowed him to train on multi-OEM modalities.
“I am a senior field engineer for North America for Aspect Imaging. I advise other field engineers with technical support, and just started an open MRI center in my hometown, that I am immensely proud to be a part of as well,” Barron says.
What has Barron learned after a decade of drag racing motorcycles?

“It is like life; you gain good friendships. You have bad experiences, but if you walk away at the end of the day knowing you did your best, it’s satisfying just to be able to perform. It is my way of releasing tension and stress because I trust what I am doing and have done it off and on for 10 years,” he says.
He says that he has seen guys race every event, make every qualifying field, and seen guys show up that do not race much, come out and win everything.
“So, I believe anyone can win on any given day. It is just a machine in the end that you must optimize to the best of your abilities, with regards to riding it. Do not let anyone distract you or discourage you, stay in your zone and be confident,” Barron says.
The Red Barron team is seeking full and partial sponsorships for the 2023 racing season. Barron can be reached with inquiries at redbarronracing14@gmail.com.
February 2023 | TechNation 19
Left photo: David Barron enjoys going fast when not working on medical imaging devices. Racing is a family tradition that included his grandmother and grandfather. Right photo: Universal Medical Systems Owner David Barron knows his way around medical imaging equipment and high-powered engines.
VERIFY THE INTEGRITY OF EQUIPMENT
The Insulation tester, Leak Tester Tester and Cable Continuity Tester are easy-to-use devices for verifying the functionality of equipment for safety

McGan Insulation Tester


Detect & locate defects such as pinholes, cracks and bare spots in the jacket or coating of laparoscopic and bi-polar electrosurgical instruments
Cable Continuity Tester
Leak Tester Tester
Test the functionality of automated & handheld endoscope leakage testers with healthmark’s new Leak Tester Tester.
Test the quality of monopolar and bipolar cords with this user-friendly, durable device. A green light notifies the user that the cable passed testing.
For more of Healthmark’s intelligent solutions for instrument care and infection control, visit HMARK.COM
INTELLIGENT SOLUTIONS FOR INSTRUMENT CARE & INFECTION CONTROL
HMARK.COM | 800.521.6224


















3 Centers of Excellence 24/7 technical support Up to savings compared to OEMs 40% workspace 6S 35+ years of experience 200k shelf-to-ship parts 40 customer service representatives ISO certification 13 4 8 5: 2016 field service engineers & technicians 14 0














You asked for an end-to-end imaging equipment solution at a value. 626 delivers it. Acquisitions to expand our services to our customers The world moves fast. We move faster. - The right choice as a partner ISO 13485:2016 Certified 90% We are dedicated to quality management and we ensure best practice in everything we do of our customers would recommend our services to a colleague Consistent YoY Growth Strategic company acquisitions to expand our services to our customers include Walsh Imaging in 2017, ISS in 2019 and Innovatus, CR-DR business in 2020. Recognized by Broward Health Medical Center for corporate community giving Florida Biomedical Society recognizes as an industry leader in 2019 Walsh Imaging recognized as tier Del Medical dealer We are your multi-modality, multi-manufacturer contrast injector service and support solution. We want
your first
call WeAre626.com We are your multi-modality, multi-manufacturer service and support solution. Our number of expert, full-time engineers on staff ready for service +130 Remote diagnostic solution CMMC-1 Cyber certified (800) 516-0990 | weare626.com ISO 13485:2016 CERTIFIED
to be
— and last —
NEWS & NOTES
Updates from the HTM Industry
REMEMBERING AN HTM PIONEER, GBIS CO-FOUNDER

Clarence Michael “Mike” O’Rear Sr., a United States Army Veteran, passed away Thursday, December 15, 2022.
Georgia Biomedical Instrumentation Society GBIS Executive Director Horace Hunter shared the news. “Dr. Mike O’Rear passed Thursday, December 15, 2022. He performed the duties of GBIS Trustee for many years and served several other positions to include president, secretary and treasurer. He was one of the original GBIS pioneers to help develop GBIS into a state of Georgia biomed association,” Hunter said. “Dr. Mike” was known by many and had a giving spirit. He always volunteered to be in charge of the education and training classes for the GBIS annual conferences.
“Dr. Mike would light up a room when he entered. He enjoyed having his picture taken with everyone he would meet. He cherished family, friends and community above himself,” Hunter said. “He was a backbone to the GBIS family and an inspiration to his fans. To say he left a legacy would be an understatement and his positive impact will continue to be felt forever.”
GBIS will be developing a memorial to honor Dr. Michael C. O’Rear.
MD Publishing Founder and President John Krieg remembers how important O’Rear was to the healthcare technology management (HTM) field. “He was a true pillar in our industry,” Krieg said. MD Publishing Vice President Kristin Leavoy recalled how wonderful it was to see O’Rear at the recent MD Expo in Atlanta. It was perfect for O’Rear to be in attendance as MD Expo celebrated its 20th anniversary.
“Dr. Mike” is survived by his wife of 60 years Dianne, of Jasper, Georgia; son Mike O’Rear, of Jasper; daughter Sharon Farist, of Chattanooga, Tennessee; brother Dennis O’Rear, of Chattanooga; sister Debbie Holder and her husband Gary, of Whitwell, Tennessee; grandchildren Dylan Farist, of Jasper; Matthew Farist, of Chattanooga; and Danielle Farist, of Tuscaloosa, Alabama.
Mike and Dianne moved to Memphis in 1969, after he received his associate degree in electrical engineering, where he first started his career as a professor of electrical engineering at State Technical Institute at Memphis. While teaching, Mike also attended college part time and achieved his B.S. in electronic engineering technology in 1973 and his M.S. electronic engineering technology in 1975. He
did his master thesis on biomedical engineering technology. O’Rear left the teaching field in 1974 to work for Sharp Manufacturing Company of America, as the manager of the microwave oven engineering plant. In 1984, he left Sharp and began working for Sanyo Manufacturing Corporation as the department manager of the Color Television Engineering Department. Then, in 1986 Mike moved his family to Marietta, Georgia, where he started at Chattahoochee Technical College as the lead instructor of electronics and computer engineering technology and biomedical engineering technology.
In January 2012, he became the adjunct professor of biomedical engineering technology. He decided to go back to college, and he attended Georgia State University, where he earned his Ph.D. in human resource development (training) in 1995, after that he became known as “Dr. Mike.” He was not just a professor to his students, “Dr. Mike” truly loved each and every one of his students. He was always there for them and willing to help them with anything they needed, even if it was for help with assignments from different professors or if they had already graduated and were working and just needed some assistance with anything. The family would like to let all of his previous students know that he truly loved and cared for them.
He was a member of the Institute of Electrical and Electronic Engineers, the Atlanta Area of Biomedical Association, Georgia Biomedical Instrumentation Association. During his free time he enjoyed spending time with his family, especially his grandchildren. They would watch college football, go to the gun range, play games and tell jokes. Mike was in The U.S. Army where he served for 6 years. He joined the George State Defense Force, was an active member of the DAV. “The family would like to let everyone know that they were proud to share him with you!” according to an obituary.
The HTM community will forever be thankful.
INDUSTRY UPDATES
February 2023 | TechNation 23
INDUSTRY UPDATES
MITA REACTS TO YEAR-END CONGRESSIONAL FUNDING PACKAGE
The Medical Imaging & Technology Alliance (MITA) remains discouraged that Congress has not acted to address the need for financial stability and predictability in the Medicare physician payment system, though applauds the inclusion of certain provisions from the year’s earlier Food & Drug Administration (FDA) user fee agreement.
“Economic headwinds are affecting physician providers, including radiologists, across the care continuum. A partial increase to the Medicare conversion factor still has the potential to create access to care challenges and negative real-world impacts for patients,” said Patrick Hope, executive director of MITA. “Congress should eliminate these cuts fully and work with stakeholders to find a long-term, predictable, and sustainable solution.”
MITA is encouraged to see one element of the user fee agreement package, a provision which instructs FDA to regulate contrast agents and radiopharmaceuticals as drugs, has been included in the end-of-year funding legislation package. Earlier this year, MITA commented that the current regulation of all medical imaging agents as drugs is correct, and that to reclassify these products as devices would violate the clear definitions of drugs and devices under the Federal Food, Drug, and Cosmetic Act (FDCA) and the intent of the law.
FDA ISSUES DRAFT GUIDANCE, REQUEST COMMENTS
The U.S. Food and Drug Administration (FDA) has issued this draft guidance: Voluntary Malfunction Summary Reporting (VMSR) Program for Manufacturers.
This draft guidance proposes recommendations to help manufacturers understand and use the VMSR program. The guidance, once finalized, is intended to further explain, but not change, the conditions of the VMSR program. The VMSR program reduces the number of individually reported device malfunction medical device reports (MDRs) that manufacturers must submit and allows the FDA to get a more efficient understanding of certain malfunction issues via a summary report.
The VMSR Program enables the FDA to efficiently detect potential safety signals and better use agency resources to focus on addressing the highest risks, such as deaths and serious injuries, associated with medical devices. For eligible device types, this voluntary program allows manufacturers to report certain device malfunctions in summary form on a quarterly basis, rather than on an individual basis. Reports from this voluntary program are publicly available in the MAUDE database. Importantly, reports of a death or serious injury are not allowed to be submitted via the VMSR Program and the FDA may still require individual malfunction reports for devices that are eligible for the program, such as when individual reports are necessary to address a public health issue.
Submit comments under docket number FDA-2022-D-2873 at www.regulations.gov by February 7, 2023 to ensure the FDA considers comments on this draft guidance before it begins work on the final version of the guidance.
MD EXPO HOUSTON OFFERS VARIETY OF CE TOPICS
The spring 2023 MD Expo set for Houston, Texas offers a wide variety of continuing education topics for healthcare technology management professionals.
MD Expo Houston is set for April 11-13. HTM professionals can’t afford to miss MD Expo and its top-notch ACI-approved education, packed exhibit hall and unique networking events. Some of the sessions being offered include:
• Back to the Basics of Measurement
• Effect of Equipment Age on Reliability
• Using Your AAMI Certification to Help Advance Your Career
• Women in the Healthcare Technology Field
• Imaging Service – Keys to Developing and Sustaining Your Imaging Service Program
• Adding to the Toolbox – Tips to Grow into a Better Leader
HTM leaders presenting sessions at MD Expo include:
• Binseng Wang, VP of Program Management
• Torgeir Rui, Lead Data Analyst, Program Management,
Sodexo Healthcare Technology Management
• Ty Greenhalgh, Industry Principal, Healthcare, Medigate by Claroty
• Brian Wilson, ISE 3, Trimedx
• David Scott Sr. BMET, UCHealth
• Justin Barbour, Host, Better Biomed YouTube Channel/ FOBI Medical
• Andrea Brainard, CBET, CHTM, Director HTM, Children’s Health
• Ryan Harris, Director of HTM, Texoma Medical Center (UHS of Delaware)
• Greg Czajka, CHTM, Support Services Operations Director, Advocate Aurora Health
• Bryant Hawkins, Unit Director II, Crothall Healthcare
• Carol Davis-Smith
Register today and sign up for the newsletter to stay up to date on all MD Expo news at MDExpoShow.com.

24 TechNation | February 2023
626 ACQUIRES MOUNTAIN STATES BIOMEDICAL SERVICES
626 Opco has announced the acquisition of Mountain States Biomedical Services (MSBS).
“MSBS could not be a better fit. Since acquiring ISS back in 2019, our injector business has grown exponentially, and it only makes sense to further invest to support that growth. We know MSBS will bring tremendous value to 626 as they share our core values of quality and urgency while having a customer centric approach. We are very excited about our future with MSBS,” stated Phil Revien, CEO of 626.
Mountain States Biomedical Services, based in Aurora, Colorado, has become a premier source of contrast injector





service and support as well as biomedical services in North America, a news release states.
“My team and I are beyond excited to start working with Phil, Michael and the 626 family. We have always prided ourselves on the quality of our service, our growth and our customer retention. These qualities align very well with 626. Knowing that we are teaming up with a company of similar values and culture and we join a list of brands that have grown aggressively once partnering with 626, provides confidence as we begin this next chapter,” stated MSBS Founder and Owner Robert Monette.

A&G Biomedical is your one stop shop for all your Biomedical and Surgical equipment needs. We are a trusted and reliable source with certified Biomed Technicians who specialize in equipment sales, repairs, PM’s, and much more. We at A&G Biomedical, are committed to offering the best service and great savings without sacrificing quality.

888-890-0192 | sales@agbiomedical.com | www.agbiomedical.com Clinical Engineering • OR • Purchasing Materials Manager February 2023 | TechNation 25
QRS SOLUTIONS
Q: WHAT PRODUCT, SERVICE, OR SOLUTIONS DOES YOUR COMPANY PROVIDE TO THE INDUSTRY?


QRS Solutions represents Datrend Systems line of Biomedical Test Instruments in the U.S. as well as providing training and is an authorized service and calibration center. We also provide calibration services for most Biomedical Test Instruments as well as general Test Equipment and are an ISO17025:2017 accredited laboratory as well as ANSI/NCSL Z544-1-1994(R2002).


Q: TELL US WHAT DIFFERENTIATES YOUR COMPANY FROM THE COMPETITION?
QRS Solutions has been providing sales, training and services for more than 20 years to the biomed community. We are the leader in test automation and assist our customers with the transition to automated testing. We also offer Metrology training to the HTM community, emphasizing the importance of good Metrology practices when performing Preventive Maintenance (Calibrations) and Corrective Maintenance (Calibration Adjustments).
For more information, visit qrs-solutions.com.
INDUSTRY UPDATES Proudly Manufactured in the U.S.A Visit Our Website www.elitebiomedicalsolutions.com Or Call 1.855.291.6701 WELCOME TO THE TECHNATION COMMUNITY!
SEE OUR AD ON PAGE 5
26 TechNation | February 2023
Karie Heiselt, Managing Member
The Doctor Will See You Now.
Southern California’s Medical Equipment Doctor is on track to receive its ISO 13485 certification
ocated in Orange County, California, Medical Equipment Doctor (MED) specializes in the sale, purchase, rental, and repair of refurbished medical devices and instruments. In 2017, industry veteran and the Doctor himself, Albert Negron, set out on his own to start a company in his garage with nothing but one plastic folding table, two Home Depot orange buckets, and 10 years of medical device sales experience. He made a logo, applied his expertise and with that, Operation: MED was underway.
Today, gone are the times wherein going to work involves pressing a clicker to reveal orange buckets hiding behind a garage door. In fact, the company recently relocated to a new facility.
MED upgraded its facility with the addition of space from next door in November of 2022. The acquistion added 6,000-square-feet of space for the growing company. The sales and customer service team are located upstairs, and all biomedical service are downstairs in the new unit. MED is working to complete a full remodel on the space to compliment the influx of service it is receiving. As part of the expansion, MED will add in-house endoscope and ultrasound probes service.
MED’s current space houses administration, human resources, accounting and marketing offices.
Albert has since carefully and successfully handpicked a group of industry-seasoned professionals like himself to create an office team that feels more like family. And not just that, one that continues to expand with impeccable force through each passing quarter.
In the five short years since setting up shop, Albert has instituted an eminent company founded on a basis that centralizes culture, growth and community. MED’s holistic scope of service, from rental to repair, is dedicated to helping hospitals and surgery centers sustain and fulfill their working needs. With the Doctor’s help, clinicians are able to stay fully equipped so they can continue to provide the best patient care possible.
“Our focus at Medical Equipment Doctor is to partner with you to keep your budget and costs in check through

providing top-level pre-owned equipment sales, service and rental, for all your medical equipment needs. This has our company being recognized nationally as the affordable solution to purchasing new. Over the years we have had the good fortune to get to know many of you in the health care and medical equipment industry,” Albert said. “It has been a true pleasure. “

“The name of our company came from people referring to us at trade shows and gatherings as their; ‘Medical Equipment Doctor’ so we carried it forward into our name and brand,” he added. “We are passionate about what we do and always keep the safety and comfort of the patients that use our equipment at the forefront of every step we take and the care with which we approach it. Let’s connect and discuss your needs and how we can partner up with you on all your medical equipment needs.”
Included in the different kinds of equipment MED services are:
• Patient monitoring
• Infusion pumps
• Surgical equipment
• Respiratory equipment
• Cables
• Imaging equipment
As the year is beginning to wind down, Medical Equipment Doctor is excited to announce that the company is currently on track to have its ISO 13485 certification by mid-2023. Each month, the MED team members continue to surpass their goals and reach new heights. The aim remains to serve the community to the highest of their ability. One thing that sets them apart from all of the others is their dedication to customer service. All salesmen, technicians, and everything in between are all under the same roof, working tirelessly to ensure the best quality and the quickest turnaround times possible for biomedical equipment.
Above all, Medical Equipment Doctor cares. Though not working directly with the patients themselves, the main driver in being motivated for success is the role they get to play in helping people. Healthcare takes a village, and from the mouth of the Doctor and former Home-Depot-bucketin-garage owner himself, “You take care of the patient, we’ll take care of the equipment!”
For more information, visit medicalequipdoc.com.
L SPONSORED CONTENT Respiratory equipment Imaging equipment As the year is beginning to wind down, Medical Equipment Doctor is excited to announce that the company is currently on track to have its ISO 13485 certification by mid-2023. Each month, the MED team members continues to surpass their goals and reach new heights. The aim remains to serve the community to the highest of their ability. One thing that sets them apart from all of the others is their dedication to everything in between are all under the same roof, working tirelessly to ensure the best quality and the quickest turnaround times possible for biomedical equipment. Above all, Medical Equipment Doctor cares. Though not working directly with the patients themselves, the main driver in being motivated for success is the role they get to play in helping people. Healthcare takes a village, and from the mouth of the Doctor and former Home-Depot-bucket-in-garage owner himself, “You take care of the patient, we’ll take care of the equipment!” For more information, visit medicalequipdoc.com. SPONSORED CONTENT THE DOCTOR WILL SEE YOU NOW. Southern California’s Medical Equipment Doctor is on track to receive its ISO 13485 certification February 2023 | TechNation 27
RIBBON CUTTING 1904
HTM
This month’s Ribbon Cutting article features 1904 HTM. 1904 HTM is a diagnostic imaging service and support company with ACI-certified field service engineers (FSEs) and OEM-training on the most common health care brands and modalities, including C-arms, R/F, ultrasound and women’s health. The broad experience that our FSEs have gives us the ability to fill gaps in our client’s workforces, allowing them to focus on other issues.


Our computerized maintenance management system (CMMS) partnership allows our users to instantly initiate service requests as easily as taking a picture and sending a text. The same interface can provide access to existing work orders, scheduling preferences, and maintenance history for every piece of equipment, across multiple sites and locations.
Clients don’t have to be huge healthcare delivery organizations (HDOs) in order to leverage data analytics. By analyzing maintenance and usage, we assist our clients in designing a medical equipment management plan (MEMP) that allows them to prioritize critical compliance and operational issues through preventive maintenance before they become problems that disrupt patient care.
TechNation recently found out more from 1904 HTM Company Founder Scott Fishman, MA, CRES, 1st Lieutenant (Ret)
Q: HOW DOES YOUR COMPANY STAND OUT IN THE MEDICAL EQUIPMENT FIELD?
A: Our company culture prioritizes balance, purpose and service. Our organization is composed of experienced professionals in complementary fields and disciplines to address the needs of our clients (and their patients) in an ever-evolving environment. Our diverse backgrounds – both military and civilian – in service, business, management, finance, HR and logistics all become force multipliers. This allows us to do the same work, in less time, with greater accuracy.
Our purpose is to help our clients find balance by relieving them of equipment service in an otherwise overwhelming workload. Our holistic approach to client and vendor collaboration then creates a mutually beneficial relationship based on open communication and respect, to resolve issues before they become problems. The collaborative process between clients, vendors and partners creates effective solutions that exceed expectations.
Q: DO YOU HAVE ANY GOALS YOU WOULD LIKE TO ACHIEVE IN THE NEAR FUTURE?
A: Our goal is to foster sustainability in the diagnostic imaging service segment. The shortage of experienced field service engineers came as no surprise to anyone in the industry. That shortage, accelerated by COVID, compounded the problem by overstressing senior talent resulting in early retirements. Our efforts are focused on educating and mentoring new talent while providing existing FSEs with much needed backup and support resources.
Our education efforts include working with local colleges and career centers to provide opportunities for hands-on internships and/or apprenticeships. We customize the educational curriculum and program for each student.
We also offer franchise opportunities and à la carte options for small repair shops to receive much needed support services. What began as a network of independent engineers is becoming a formal coalition of highly trained and experienced professionals. By building a formal coalition, we’re able to support each other when workloads increase or expertise on specific equipment is required. This also allows us to support multi-location clients with consistent service standards.
Q: IS THERE ANYTHING ELSE YOU WOULD LIKE OUR READERS TO KNOW?
A: We provide value to our clients by anticipating and resolving issues before they can become costly problems. Whether a client is a multi-location hospital system or a single out-patient clinic, our diverse backgrounds and experience allow us to customize solutions for each client’s specific needs.
For more information, visit 1904htm.com.
INDUSTRY UPDATES
28 TechNation | February 2023
World class biomedical testing solutions for healthcare environments
Whether you’re a manufacturer of electrical medical equipment, a biomedical engineer or a healthcare technician, Rigel Medical have a test solution for you.


Electrical Safety Testing
Patient Simulation
Defibrillator Testing
Electrosurgery Testing
Infusion Pump Testing
Ventilator Testing
Calibration
Scan the QR code to find out more or visit rigelmedical.com/TN1
AAMI UPDATE
What’s Changing for International Standards? Conversations from ISC 2022
In late 2022, the global medical device standards community came together in-person for a practical review of recent changes to the regulatory landscape and valuable lessons learned in the global medical device marketplace. Popularly called “THE international standards conference,” or ISC, the AAMI/FDA/BSI International Conference on Medical Device Standards and Regulation was hosted at AAMI headquarters in Arlington, Virginia. What follows are some highlights from the international event:
ENABLING INNOVATION THROUGH REGULATION?
It was a tale of two devices. One was an AI-enabled medical device with premarket approval (PMA). Another was a consumer hardware product – a smartwatch – with a software application that’s received 510(k) premarket clearance. But at the end of the day, they shared an identical primary function – detecting atrial fibrillation.
“At what point in our industry do these two devices converge? At what point does that blurred line become confusing or challenging for companies to get products to market?” asked Jennifer McCaney, Ph.D., executive director of UCLA Biodesign, in her keynote address for ISC 2022. “One of the things that I examine is how change in technology [is] impacting the industry.”
McCaney’s work focuses on early-stage medical innovation training and development programs for aspiring innovators. She is also an associate professor of medicine and management at UCLA Health.
“How do we accelerate approval of new technology? How do we advance standards and regulatory policies so they can keep up with exponential
change?” she asked.
In many ways there has been significant – even breakthrough – progress. McCaney cited a decade-old Stanford study that painted an unfavorable view of the regulatory landscape in the United States 10 years ago, one that increased cost and slowed innovation. But that’s not the story of U.S. standards and regulations today.
We’ve seen “an increase in regulatory policy that enables innovation” thanks to FDA initiatives like the Digital Health Center of Excellence, McCaney said. According to a 2022 report by McCaney and others at UCLA Biodesign, a “sea change” is underway when it comes to the preferred path to bring new products to market – investment from the FDA appears to be paying off when it comes to launching medical devices globally.

GLOBAL UDIS ARE CLOSER THAN YOU THINK
Unique device identifiers (UDIs) are “a first step” towards creating an identification scheme just like what you’d see on products in a grocery store. However, instead of identifying a box of cereal, UDIs help keep track a life-saving device.
Jay Crowley, vice president of Medical Devices for USDM Life Science and Lena Cordie-Bancroft, lead of the Medical Devices for BSI spoke about expectations the medical device industry will need to meet to implement UDIs during the ISC 2022.
They mentioned how, on July 22, 2022, the FDA published its final guidance on UDIs, describing submission requirements for certain Class I devices considered consumer health products.
“When fully implemented, the label of most devices will include a UDI in human- and machine-readable form, which will ultimately improve
INDUSTRY UPDATES
30 TechNation | February 2023
patient safety, modernize device postmarket surveillance, and facilitate medical device innovation,” the agency reported at the time.
“It was really beneficial, I think, for the entire industry when the Final Rule was published … and as manufacturers started to face the hurdles and address the challenges [of adopting a UDI system] they realized it wasn’t as difficult as it had been perceived,” said Cordie-Bancroft. “So, now the challenge is getting it adopted in the health care facilities. Getting it used and applied in health care records and closing that loop between regulation, the manufacturers and the users.”
And then there’s global adoption as well …
The pair noted that different countries and jurisdictions are creating their own unique UDIs, which could prove problematic.

Fortunately, according to Crowley, the International Medical Device Regulators Forum has provided internationally applicable guidance for the concepts that regulators should take. “And at a high level, regulators are following that. So, we’re seeing concepts that are the same,” he said.
“A UDI assigned to a medical device in the U.S. can be used in other countries,” Cordie-Bancroft added. “The application of that UDI in various databases could be different … but generally, once you assign one, it can be used globally. That’s the ultimate goal. And we’re a lot closer than people think we are.”
THE REGULATORY ‘TSUNAMI WAVE’ APPROACHES
Regulatory standards are changing rapidly around the world. The problem is that innovators often are unaware or unprepared for the full extent of these changes, even years after they’re initially an -
nounced. A prime example is new European regulation for in vitro diagnostics, the EU’s IVDR.
That’s according to Kimberly Oleson, founder and principal consultant for Lucent Clinical Consulting, who surveyed IVD manufacturers about the new EU regulations during a special preconference symposium to ISC 2022.
“We did live audience polling so we could understand current trends for these key issues, and about one-third of our audience members claimed that their companies were ready for the change and comfortable,” said Oleson, “and yet, zero attendees said that they had read the IVD regulation and actually understood it.”
According to Oleson, IVDs are a hot commodity in booming markets, such as cancer diagnostics, a $50-billion market that’s expected to grow threefold by 2028. “If the market grows that fast, you’ve got to have enough of these diagnostics products available” to meet exponentially growing demand, she said.
And yet, Oleson has already witnessed that EU countries are not all following the new processes in the same way. “Because countries are learning and everyone is experimenting … the cycle time to get your IVD protocol approved could take up to 11 months,” she said. In some cases, “there are not enough notified bodies out there for this expected tsunami wave of certificate requests ... get in line right now!”
Worse still are stories of companies backing out of Europe entirely.
“It just hurts me to know that a company thinks it has to back away. That gets in the way of innovation, and that’s not we want,” she said. “We want products available and helping patients as soon as possible … and so, we want to help manufacturers to stay on top of this as much as possible.”
February 2023 | TechNation 31
Lena Cordie-Bancroft and Jay Crowley, vice president of Medical Devices for USDM Life Science, discuss expectations for ISC 2022.
ECRI UPDATE

How Predictive Replacement Planning Can Help
BY MARC SCHLESSINGER, MBA, FACHE
To make more effective and efficient decisions regarding budgeting and managing medical equipment, it’s essential that health care organizations focus on capital budgeting, medical equipment selection and recall management.
Capital budgeting is the process of allocating funds for the purchase of long-term assets, such as medical equipment. The goal of capital budgeting is to ensure that a health care organization has the funds necessary to purchase needed equipment and can do so while maintaining a balanced budget.
Equipment selection is an area of critical focus during the process. To select the right equipment, health care organizations should understand the benefits and drawbacks of the various different types of medical equipment.
Product recall management also should be a high priority. If a piece of medical equipment is recalled, a health care organization must have a plan for how to handle the recall to ensure patient safety.
By focusing on these key areas, health care organizations can make sound decisions that will benefit patients and the bottom line.
But how can organizations improve these areas? The answer is the use of predictive replacement planning (PRP). Let’s explore PRP and how it can help your health care enterprise improve:
• Capital budgeting
• Medical equipment selection
• Recall management
UNDERSTANDING PRP
PRP is a process that health care organizations use to identify and plan the replacement process for medical devices and information systems. PRP helps organizations manage the lifecycle of technology assets and plan for the replacement of systems prior to the end of their usable life.
The PRP process begins with identification of devices and systems approaching the end of usable life. Organizations then develop an unbiased five- or 10-year replacement schedule for these devices and systems. This plan may include the selection of new devices and systems, the development of budgets and timelines, and the assignment of responsibilities for the replacement process.
The plan is not based solely on the age of the equipment or subjective desires or influences of department directors or physicians. Instead, a predictive replacement plan considers multiple objective factors including:
• Organizational goals
• Patient needs
• Data from device recalls
• Support from the original equipment manufacturer (OEM)
• Availability of parts from OEM and aftermarket sources
• Changing technologies
• Device usage frequency
• Clinical needs of health care providers
Replacing medical devices and information systems before they reach the end of usable life helps organizations manage technology assets and keep pace with the latest and most efficient devices and systems.
PRP is a valuable tool that can help health care
INDUSTRY UPDATES
32 TechNation | February 2023
institutions reduce capital spending, decrease equipment downtime and maintenance costs, and prevent clinical obsolescence.
ACCURATE PREDICTIONS OF EQUIPMENT REPLACEMENTS
PRP can help improve capital budgeting processes by accurately predicting when medical equipment will need to be replaced. PRP uses data analytics to identify patterns and trends in equipment use and failure rates to forecast when a particular piece of medical equipment will need to be replaced. This information can help health care institutions budget for replacement equipment in a more timely and accurate manner, improving their overall capital budgeting process and patient safety.
PRP also can help improve the overall quality of care provided by health care institutions. By accurately predicting when equipment will fail, PRP can help health care institutions plan for and schedule repairs and replacements in a timelier manner, and help prevent equipment failures from impacting patient care.
PRP can be an invaluable tool for health care institutions looking to improve their capital budgeting process.
IDENTIFYING THE MOST CRITICAL PIECES OF EQUIPMENT
PRP can help health care institutions improve equipment selection by identifying the most critical pieces of equipment and providing estimated replacement timelines. This information can help institutions make better decisions about which equipment to invest in and which ones to replace sooner rather than later.
The most critical pieces of equipment are those that have the most significant impact on patient care. Numerous factors can make equipment critical. One of the most important factors is the number of patients the equipment impacts. Equipment used to treat many patients is more critical than equipment that treats a small number of patients.
Age is another critical factor to consider in the process. Old and/or outdated equipment can have a negative impact on patient care, making timely replacement essential to excellent patient care. Finally, reliable equipment is obviously more vital
than unreliable equipment. Institutions should consider replacing equipment that isn’t reliable
It’s worth noting that the most critical pieces of equipment are not always the most expensive. In fact, they may be relatively low-cost items that are essential for day-to-day operations of the hospital, such as beds, infusion pumps and wheelchairs. By identifying these items and planning for their replacement, health care organizations can confirm that they have the necessary equipment to provide quality care to patients.
ENSURING REPLACEMENT EQUIPMENT AVAILABILITY
When a piece of medical equipment is recalled, a health care facility must be able to replace it quickly. The process of managing recalls is essential for protecting the safety of patients, but it can be challenging to achieve with limited resources.
With the help of PRP, a health care enterprise can be prepared for recalls by planning for the availability of replacement equipment. This is critical to safeguarding patients not affected by a product recall. If a hospital doesn’t have a replacement available, it cannot use the recalled equipment and may have to send patients to other facilities. This can be a significant inconvenience for patients and can lead to revenue loss for the hospital.
IMPROVEMENTS
PRP is a proven method for improving a health care facility’s budgeting, equipment selection and product recall management process. A quality PRP system will provide accurate predictions of equipment replacements, identify the most critical pieces of equipment and identify available replacement equipment in the event of a recall.
Learn how health systems, aging services facilities, and ambulatory care centers can benefit from ECRI Predictive Replacement Planning Services by visiting www.ecri.org/ solutions/predictive-replacement-planning.
Marc Schlessinger, MBA, FACHE, is a senior associate, accident and forensic investigation at ECRI .

February 2023 | TechNation 33












772B Special Purpose Relocatable Power Taps The PowerMATE Family Permanently Mount to IV Pole *With available Locking Bold with Nut Current Monitoring Display on CM Model 15 ft Medical Grade Power Cord Power Cord Management Includes a 1-Year Warranty Get the Power Support You Need with BIOMEDICAL When It Comes To CENTRIFUGES, One Name Stands Out • Free Tech Support • Depot Repair • Rental Units • Re-manufactured Parts • New Parts • Exchanges Your Centrifuge Solutions Center www.ozarkbiomedical.com 800-457-7576 34 TechNation | February 2023



Reachable. Reliable. There when you need us. United Infusion is a leader in infusion pump sales, rental and repair for medical professionals. CONTACT US TODAY! (919) 609-9975 info@unitedinfusion.com
BIOMED 101 Hospitals Urgently Need Circular Economy Solutions, Right to Repair Medical Devices
 BY DANIEL J. VUKELICH, ESQ
BY DANIEL J. VUKELICH, ESQ
limate change is a public health threat impacting everyone. As water and air pollution increase, so do deadly chronic respiratory diseases.1 Increased temperatures due to climate change lead to increased ground-level ozone, which cause airway inflammation and damages lung tissue. 2 That 5% of all greenhouse gas emissions are caused by the health sector – the institutions charged with returning us to good health – should be a call to action to all health care workers to reduce waste and lower emissions. 3
By reprocessing single use medical devices, hospitals reduce costs, waste and emissions. They also build a more resilient supply chain by keeping devices in circulation longer, reducing the demands of the global supply chain. At a time when U.S. hospitals are expected to lose $54 billion, circular economy solutions that eliminate waste and invest in sustainability are desperately needed.4 Urvashi Bhatnager and Paul Anastas, authors of “The Sustainability Scorecard: How to Implement and Profit from Unexpected Solutions,”
use reprocessing of medical devices as a textbook example for how to simultaneously lower costs and improve an organization’s carbon footprint.
Similarly, the “Right to Repair” movement is enabling consumers to extend the life of other products, including mobile phones or tractors, demonstrating that circular economy solutions cross industries. Single-use device (SUD) reprocessing is highly regulated by FDA and other medical device oversight agencies across the globe, providing a safe, regulated, circular economy solution for health care. However, like with other products, some manufacturers seek to undermine consumers (or hospitals) rights to do with their products what they will, including repair and reprocessing.
THE RIGHT TO REPROCESS
Hospitals and surgical centers should view all medical equipment as assets that health care facilities – the consumers of these items – have a right to repair or reprocess.
The larger right to repair movement gaining attention in Congress, state legislatures and in regulatory agencies, underscores the rights consumers have to repair the products they buy, including the rights to choose service or repair providers not affiliated with the original manufacturer. When it comes to medical equipment, hospitals are just like
THE BENCH
C
36 TechNation | February 2023
Commercial reprocessing companies clean, inspect and repair SUDs before they test, disinfect/sterilize, package, and ship the product for another safe use. Photo: AMDR
any other consumers of products.
Electronics consumers are well aware of planned obsolescence measures some manufacturers build into mobile phones, MP3 players and earbuds. The same is true for hospitals and surgical centers who often have little option but to accept the latest model equipment from device manufacturers, which may mean accepting devices with wasteful, built-in planned obsolescence.
By viewing SUDs as assets and reminding original equipment manufacturer (OEM) partners that they must work together to overcome cost, waste and supply chain disruptions, reprocessing programs must not be hindered. However, even though use of reprocessed SUDs requires FDA or other agency clearance or approval and is a well-established practice at over 10,300 hospitals worldwide, some manufacturers are routinely seeking to infringe on this right.
EXAMPLES OF EFFORTS THAT HINDER A HOSPITAL’S RIGHT TO REPROCESS SUDS
AMDR urges hospitals and surgical centers to partner with medical device makers and reprocessors that seek to maximize the value of device assets (extend their life) and therefore reduce costs, waste and emissions. Efforts that inhibit a hospital’s right to reprocessing and undermine reprocessing programs include:
1. OEM Staff Influence: When some OEMs lend staff to the operating room, hospitals may become overly reliant on that staff and it may amount to greater costs and consumption by the hospital. AMDR is aware of some OEMs, once entrenched, who threaten to pull the staff unless the hospital agrees to stop reprocessing some SUDs. AMDR urges hospitals and surgical centers to only work with vendors supportive of reprocessing programs.
2. Software ‘upgrades:’ Another threat to the rights of hospitals to reprocess SUDs is some OEM use of software “updates” or “upgrades” that prevent reprocessed versions of these devices from working with consoles or generators. By manufacturing the devices with microchips and updating the software on the generators, some OEMs are causing more waste, cutting out competition, and increasing a hospital’s reliance on the OEM by preventing reprocessed versions of medical devices from working. AMDR urges hospitals and surgical centers to work with vendors that do NOT restrict the facility’s ability to reprocess and strongly counter such measures (such as refusing software “updates”) when they encounter them as antithetical to the interests of the health institution.
3. Forced obsolescence: The use of “erasable programma-
ble read-only memory” (EPROM) microchips in the handles of surgical and diagnostic medical devices are being used to intentionally make it difficult to use the device more than once. Employed based on unsupported “patient safety” claims, these chips are inserted to shut the device off after a single use. AMDR has evidence that an OEM with its own reprocessing division (not an AMDR member), includes such chips, not just to discourage reprocessing, but to discourage reprocessing with competing vendors.
Further, billed as “innovation,” some medical device manufacturers introduce new models of existing technology that are designed to make it impossible to reprocesses and reuse them. When such generational innovation disallows reprocessing, the hospital loses the savings and environmental benefits due to the OEM’s locking out reprocessing. While many technology innovations come with clinical and technological advantages to prior models, the launch of devices with reprocessing “kill switches” or “locks” – without scientifically backed clinical advantages – is becoming more and more common. AMDR encourages hospitals and surgical centers to inform these companies they wish such activity to stop, to resist new makes and new models with such “kill chips,” and to reward with your business companies that support reprocessing.
More information, including a resource page that further addresses each of these topics, is available at www.AMDR.org.
REFERENCES
1. Amato G, Cecchi L, Amato M, et al., “Climate change and respiratory diseases,” European Respiratory Review, 2014; 23(132):161-69.
2. Amato G, Cagani Ca, Cecchi L, et al., “Climate change, air pollution, and extreme events leading to increasing prevalence of allergic respiratory diseases,” Multidisciplinary Respiratory Medicine, 2013:12.
3. Health Care Climate Footprint Report, Health Care Without Harm, September 2019
4. “Financial Effects of COVID-19: Hospital Outlook for the Remainder of 2021,” Kaufman, Hall and Associates, p.3, 2021
Daniel J. Vukelich, Esq, is president and CEO of the Association of Medical Device Reprocessors.

February 2023 | TechNation 37
Commercial reprocessing companies clean, inspect and repair SUDs before they test, disinfect/ sterilize, package, and ship the product for another safe use.





















































































































































































































































































































































































































































































































































































































































































SERVICING DEFIBRILLATORS, VITAL SIGN MONITORS, EKG/ECG’S, VENTILATORS, PUMPS, AED’S, & MANY MORE! MEDICAL EQUIPMENT SALES AND SERVICE ON-SITE TEST EQUIPMENT CALIBRATION Call or email us today to learn how we can work for you. Toll-free: 888.310.7322 • info@sebiomedical.com • www.sebiomedical.com BIOMEDS HELPING BIOMEDSTM MEDICAL EQUIPMENT SALES AND SERVICE www.sebiomedical.com On-Site Test CalibrationEquipment Eliminate shipping costs Reduce down time Increase productivity
TOOLS OF THE TRADE
Radcal
QA X-Ray Testing Equipment
Radcal Corporation’s Touch Professional series, Accu-Gold+ Touch Pro, includes a new software-based measuring system capable of performing multiple operations with just one exposure. The Accu-Gold+ Touch is the most versatile stand-alone system in Radcal’s product line, supporting Radcal’s unparalleled line of gold standard ion chambers, solid-state dose sensors, solid-state multi-sensors and current probes. These systems have a Customizable Data capture profile page and are an excellent solution for the full range of applications including
radiography, fluoroscopy, mammography, CT, dental and survey. The Pro Series adds wired and wireless computer interfaces to the Touch stand-alone system. With this interface, the Accu-Gold 2.0 Windows software provides a feature-rich and user-friendly environment in which to perform your measurements. Stream-lined Excel reporting and advanced waveform analysis are among many useful capabilities. Android and IOS applications available.
For more information, visit radcal.com.

THE BENCH
February 2023 | TechNation 39
Does pump month make you feel weak? We are here to
PUMP YOU UP!
We handle it all from finding the pumps, cleaning, PMs, minor repairs, batteries and returning to the floor! We offer daily and end of project reporting and hourly infusion pump specialist available to assist during pump month. If you mention you saw in TechNation, we will provide a team bonus when you contract us for these services!


Contract with us in the month of February for anytime in 2023 and earn your team lunch when we are on-site.

1 (888)-492-3400 • multimedicalsystems.com
Attacks
Healthcare:
IN CASE YOU MISSED IT
Watch these webinars on-demand
“Busting Myths of Contrast Power Injectors”
althea-group.com

cynerio.com
All too often health care environments are hit by cyberattacks that are generalized with terms like “ransomware” and “network incident,” but rarely provide details that can aid others in combating future attacks. The unfortunate reality is that the mechanics and types of attacks vary greatly and must be better understood to properly protect patients, facilities and finances. The Cynerio team discussed the most common types of cyberattack that lead to health care breaches. From ransomware to RATs and everything in between, the team provided simple descriptions, publicly available incidents, technical details and preventative steps that can be taken by every facility regardless of size, resources or funding. The presenters provided additional information via a question-and-answer session with attendees. Watching it on-demand is eligible for 1 credit from the ACI.
• “This webinar helps me be aware about “Active Attacks on Healthcare,” which TechNation magazine has been a forward-thinking publication, and done a great job bringing important issues forward to the HTM community and its professionals. Also, by sharing lessons learned and best practices that we can implement into our daily operation.”
– Salim Kai, MS, CBET, enterprise director clinical engineering at University of Cincinnati Medical Center.
Althea Modality Manager Michael Gossman dispelled false pretenses, myths and anxiety associated with contrast power injectors. He provided a quick introduction on what a contrast power injector is, including modalities and purpose. Then, he addressed three main myths associated with this equipment. The three myths are:
• Pressure – Why is high pressure used?/Safety to patients
• High liability/Testing tolerances
• This equipment should only be serviced by the OEM
• More than 100 healthcare technology management (HTM) professionals registered for this modality-specific webinar. Watching it on-demand is eligible for 1 credit from the ACI.
• “It was good to understand the differences in pressure and how it’s applied.”
– Donna Parker, imaging business manager, Hattiesburg Clinic
“Key Considerations for RTLS Technology Imple-
cognosos.com

Presenter Paula Dyciaco, director, healthcare product, at Cognosos, examined what to expect when deploying RTLS, and how the right pre-implementation, implementation and post-implementation approach enables teams to deploy with confidence. The webinar also featured an informative question-and-answer session. Some of the questions asked were: Why is full coverage in a hospital important?; What about multiple technology techs? Isn’t flexibility better?; and What are some of the other data points needed to complete and accurate ROI – outside of number of beds, assets and square footage? Watching it on-demand is eligible for 1 credit from the ACI.
Attendees provided feedback in a survey that included the question, “Why did you attend today’s webinar?”
• “Our hospital is implementing RTLS tagging. I wanted to gain more knowledge on the topic.”
– Raul Garcia, BMET Lead, Cedars Sinai Medical Center.
“Active
on
Examples, Protections and Lessons Learned”
February 2023 | TechNation 41
mentation Confidence”
ROUNDTABLE Ultrasound Systems
TechNation invited several companies to share their expert insights regarding ultrasound systems with readers in this roundtable article. Participants include Avante Health Solutions Repair Operations Manager Chris Parker, Probo Medical Director of Training and Field Service Hobie Sears and Innovatus Imaging Vice President of Sales and Marketing Matt Tomory.
Q: WHAT ARE SOME MUST-HAVE FEATURES FACILITIES SHOULD CONSIDER WHEN PURCHASING AN ULTRASOUND SYSTEM?

PARKER: Some common features facilities should look for are: application software that is easy to navigate; user interface that is organized and has a simple layout; ease of maintenance and serviceability; and standard options such as networking.
SEARS: This will depend on your facility, but one of the most noted must-have features in recent years is that the ultrasound sound system is built on Windows 10. There is more availability on units on Windows 10 than a few short years ago, so that does make it a bit easier. Wireless networking is another feature that has become common place, when getting a new unit be sure to verify it is coming with wireless networking. One last thing that everyone should consider are the cybersecurity suites being built into many systems available today, not something that you use when scanning, but certainly a powerful feature of many units.
TOMORY: The ultrasound system technology curve has somewhat flattened over recent years so there are many systems that produce image quality far exceeding what was available 5 years ago. Workflow and efficiency enhancements are high on the list –this decreases exam time and increases patient throughput. Ergonomics are also a critical consideration. I have many sonographer friends (and am also married to one) and debilitating repetitive
ROUNDTABLE
42 TechNation | February 2023
motion injuries to arms, wrists and shoulders are on a dangerous rise. System layout, workflow efficiencies, probe/cable design, system adjustability should all be optimized for sonographer comfort and safety.
Q: WHAT ARE SOME OF THE LATEST FEATURES FACILITIES SHOULD CONSIDER WHEN PURCHASING ULTRASOUND DEVICES?
PARKER: Some new technology features to consider are:
• Artificial Intelligence (AI) since there are tasks on the system that can be automated. In today’s age this technology is becoming commonplace. As new software is released, there will be more options relating to AI in the very near future.
• Shear wave elastography, which is used to measure tissue stiffness. This is commonly used to measure the liver for fibrosis. This option is replacing the FibroScan method, which was very common but less reliable.
• 3D/4D technology. There are clinical options, hardware, and applications which have drastically improved the 3D/4D image quality. Original equipment manufacturers (OEMs) are starting to include a separate GPU for image acquisition. This really gives 3D/4D rendering a boost, prevents the PC from being overtasked, and helps provide a clear and precise image without lag.
SEARS: What is key about new features is the actual plan to utilize them, it doesn’t make sense to pay extra money for high-end features unless you intend to use them. So, part of the consideration process should look into the actual usage of those new features. With that said, features in cardiac ultrasound such as strain, heart modeling and AI enhanced measurement packages are certainly in high demand. In the OB market, more advanced 3D/4D tools and more options, tools around 4D imaging are always in demand. Radiology or general imaging departments should be looking toward the updated versions on some technologies, such as contract enhanced ultrasound, updated elastography technology as well as newer measurement tools and AI assisted tools. MicroFlow color imaging is also a feature that deserves a look. Newer features are sometimes necessary or become useful, but sometimes those new features don’t pan out in the ultrasound market, so choose wisely.
TOMORY: Artificial intelligence is more common today for measurements and diagnostics which decreases scan times and may enhance diagnostic outcomes. 3D/4D ultrasound for cardiac exams is
common today whereas it was used infrequently only 5-10 years ago. Ultrasound fusion imaging is another breakthrough where the ultrasound image is overlayed or displayed next to an established CT, MRI or PET exam to guide or correlate anatomy.
Q: WHAT ARE THE ADVANTAGES AND CHALLENGES OF A HANDHELD POCUS SYSTEM COMPARED TO A CART-BASED ULTRASOUND SYSTEM?
PARKER: Advantage: mobility. For example, in an emergency department where time is critical, the sonographer can quickly perform a scan and have the data ready for a physician in a matter of minutes.
Advantage: affordability.
Disadvantage: display size. On a cart-based system, the display is in the 22- to 24-inch range with OLED technology. The average size of the display on a POCUS is approximately 15 inches.
Disadvantage: processing power. When comparing a POCUS system and cart-based system, size is the major factor – not just the physical size but the hardware package size inside the machine. While the technology has come a very long way and image quality is impressive on some, the hardware is still limited due to the size of the package in POCUS systems. In a cart-based system, you have a much larger footprint for larger and more powerful FPGAs and other ICs that you cannot fit into the POCUS system. This affects the image quality and overall processing power of the machine.
SEARS: The obvious advantages of POCUS systems are their ability to be stationed where they are needed in your facility. Today’s POCUS systems have come far from those that started out in this niche market. These systems have better image quality, have more options, and can perform some higher functions, all at a much better price than a full console system. On the disadvantage side, they will just not compete in the long run with the imaging and available tools/higher features on a console system.
TOMORY: Point-of-care ultrasound systems (POCUS) continue to improve. I recently scanned with a $2,000 unit from a major manufacturer and was amazed at the image quality and features. While the portability and convenience cannot be argued, console-based systems still provide the most features and highest image quality.
Q: WITH TECHNOLOGY ADVANCING SO QUICKLY, DO YOU THINK THAT IT’S POSSIBLE FOR A SINGLE SERVICE PROVIDER TO ADDRESS SYSTEMS AND PROBES?
PARKER: Yes, it is very common in today’s market. Outside of the OEM, there are now multiple third-
February 2023 | TechNation 43
party vendors that sell, service and repair systems, probes and parts. Third-party service providers like Avante Health Solutions also offer the opportunity to purchase quality equipment at a fraction of the cost.

SEARS: In the end, even newer units are still ultrasound units at their core. While new advanced features give systems more abilities, generally it doesn’t change the concept of how the ultrasound data is captured, processed and displayed. Service providers that understand ultrasound service have little trouble adapting to the newer technologies and understanding how to repair these units and support the ultrasound transducers.
TOMORY: Just like we go to specialists when we or loved ones have health problems, the advancement in technology is best addressed by specialists; an organization dedicated to one specialty day in and day out. Dedicating all engineering, resources, technology, training and mission is the best way to support your ultrasound systems and transducers.
Q: WHAT ELSE DO YOU THINK TECHNATION READERS NEED TO KNOW ABOUT PURCHASING AND SERVICING ULTRASOUND DEVICES?
PARKER: Over the last 10 years, I have seen ultrasound technology change rapidly. The systems are getting far more advanced, and the number of parts is being condensed down for serviceability. From a purchasing standpoint, I think customers should get the right people involved to ensure they are getting the most for their dollar. From an


imaging, applications and user ability standpoint, the sonographers should be very involved in the purchase since they will be using the equipment regularly. The healthcare technology management team should also be involved from a serviceability standpoint to ensure they are comfortable handling the maintenance and care in-house.
SEARS: When it comes to any significant purchase, having a solid relationship with those vendors you work with is crucial. This goes for the system purchase and the service provider. Your service provider should also have experience with the product line/manufacturer for your ultrasound unit. One last item is, does your service provider offer training programs for your biomed staff should you decide to train your staff to undertake the ultrasound service in part or completely?
TOMORY: Ultrasound systems are highly optioned and it is always more cost effective to add options with the system sale as opposed to later on. So, consider the needs of the department both now and in the future when placing an order. Long-term support is also a strong consideration – the system will likely be in service 5-7 years or longer, so service support post warranty needs to be considered. Clinical engineering should always be involved so questions around training, service keys and manuals, parts, etc. are addressed.
ROUNDTABLE
Hobie Sears Probo Medical, Director of Training and Field Service
Matt Tomory Innovatus Imaging, Vice President of Sales and Marketing
44 TechNation | February 2023
Chris Parker Avante Health Solutions, Repair Operations Manager
OEM NE W

MDUFA V & HTM
LEGISLATION ADDRESSES 510(K), CYBERSECURITY AND REMANUFACTURING
BY K. RICHARD DOUGLAS
COVER STORY
46 TechNation | February 2023
he FDA regulates more than 190,000 medical devices manufactured by more than 18,000 companies. Those devices can be found in more than 21,000 health care facilities around the world.
Every business day, on average, the FDA approves marketing authorization to 12 new or modified devices.
The process of reviewing and approving new medical devices is crucial to global health care. As new technological advances are discovered and developed into cutting-edge medical devices, they must be brought to market quickly and efficiently, maintaining a high safety profile and hardened against cyberattacks.
In early spring 2022, the FDA submitted its $8.4 billion budget request to Congress for fiscal year 2023. All government agencies rely on the Congress to allocate and approve funding for those agencies to continue operations.
The agency specifically mentioned an effort to “advance medical product safety” as part of the justification for the $356 million that exceeds the fiscal year 2022 budget.
The FDA has fees to review submissions. These fees are negotiated between the medical technology industry and the FDA. Once negotiated, Congress must pass into law, and the president must sign, an agreement that lasts five years.
While the FDA commits to certain metrics in the submission and approval process, the increasing standards come with a price – higher fees.
In its MDUFA performance goals and procedures, beginning in 2023, the FDA has stated: “Total Time to Decision for premarket approval applications (PMAs) and premarket notification (510(k)) submissions, provided that the total funding of the device review program adheres to the assumptions underlying this agreement.”
Also, in its Fiscal Year 2023 Budget Summary Fact Sheet, the FDA states in part: “The budget also proposes new authorities which would require medical device manufacturers to address cybersecurity issues.”
The agency needs to maintain a set of regulatory standards to maintain the safety of medical devices and support a process that is familiar to the manufacturers of medical devices.
The FDA needs funding in order to review and approve medical devices and drugs. In the Office of Device Evaluations, there are a substantial number of employees required to take on this task. This funding
comes by way of the fees charged.
The fifth Medical Device User Fee Amendments (MDUFA) recently passed on September 30, 2022. This made it MDUFA V. The series of agreements date back to 2002.
Some of the metrics that were made a part of MDUFA V require the FDA to add staff to meet the demands of both greater levels of submissions as well as the challenges of reviewing devices for cybersecurity.
Surprisingly, the approval by Congress was “clean” without riders or rider bills; a rarity in Washington. It was also a bi-partisan effort.
Industry trade groups were pleased with the FDAs commitment to bring new devices to market in a more expedited manner and further prioritizing patient safety.
“MITA looks forward to working with the FDA to implement MDUFA V, including the Agency’s targeted Total Product Lifecycle Advisor Program (TAP) pilot project designed to facilitate early interaction between FDA and industry on novel innovative products,” says Peter Weems, director of Policy and Strategy at the Medical Imaging & Technology Alliance (MITA).
He says that “with increased User Fee funds, new performance goals and heightened oversight, we believe the agreement encourages the timely authorization and delivery of safe and effective medical innovations that improve patient care, especially as the Agency continues to confront the effects of the pandemic.”
“As signed into law, the final agreement excluded certain policies that have been at the center of longstanding discussions between industry, FDA and Congress. MITA looks forward to working with Congress and the Agency to continue to advance policies that enhance patient safety, including policies that clarify the regulation of remanufacturing activities and promote shared cybersecurity responsibility among all health care stakeholders,” Weems says.
The FDA’s Center for Devices and Radiological Health (CDRH) launched the TAP pilot effective January 1, 2023. The program was one of the agreements made between medical device sponsors and the agency. For the initial soft launch, FDA intends to enroll up to 15 devices in the Office of Health Technology 2 (OHT2): Office of Cardiovascular Devices, according to the FDA. The goals of the program include a more fluid and involved process for both manufacturers and the agency, identifying risks early in the process and improving the efficiency of the premarket review process.
CHANGES AND COMMITMENTS
Every five years, the FDA has a new set of requirements in order to receive the funding needed to accomplish its mission. In the passage of MDUFA V, not only does the agency need to add staff, but it also has committed to
T February 2023 | TechNation 47
speed up the 510 (k) and premarket process.
The faster turn-around time will help device manufacturers, although some fees will jump substantially. The hope that there would be language as a part of MDUFA V to hold device manufacturers’ feet to the fire as it relates to cybersecurity fell short. While the FDA remains committed to a focus on cybersecurity, there is other legislation pending that also addresses this critical component.
The submission fees usually go up under MDUFA V. Prices are adjusted for inflation and with inflation at historic highs, it was a certainty that there would be a more substantial increase. The increase was 55.90 percent. De Novo Classification Requests increased by 17.79 percent.
The standard fee for 510(k) submissions for fiscal year 2023 is $19,870. If the firm applying has small-business status, the small business fee is $4,967. The De Novo classification standard request fee is $132,464. The small business De Novo fee is $33,116. All establishments pay the annual establishment registration fee of $6,493. The FDA requires cybersecurity documentation for medical devices.
“MDUFA V represents a substantial investment in the future of the agency’s medical device program and would provide for important improvements, including new hiring targets, greater engagement with developers of innovative technologies based on lessons learned from the pandemic, broadened international harmonization efforts and expanded opportunities to ensure patient perspectives are an integral part of medical device development,” says an FDA spokesperson.
Another industry trade group with an interest in MDUFA V expressed its support for passage of funding.
“The law preserves everything that works well in the current system, adding support of specific needs to fulfill the critical mission of device review, and reflects lessons learned about shifting workloads and priorities in device development and review during a global pandemic,” said Scott Whitaker, the president and CEO of the Advanced Medical Technology Association (AdvaMed) in an op-ed article.
Whitaker went on to say, “Industry user fees will help fund more FDA employees to evaluate new medical devices for patient use, with first-ever additional funding if the agency meets clearly defined process targets. Adding to the FDA’s workforce will help the agency manage a constantly increasing device review workload, as innovations soar from the smallest start-ups to the largest companies.”
STRENGTHENING CYBERSECURITY IN NEW DEVICES
When a health care user purchases a piece of medical equipment, it often arrives with “off-the-shelf” vulnerabilities. The device isn’t always hardened against cyberthreats by the medical device manufacturers. Not only does this pose a real danger to patients, but there is no recourse for holding the device maker responsible.
Rep. Michael Burgess (M.D.) (R-TX-26) is the sponsor of H.R.7084, known as the “Protecting and Transforming Cyber Health Care” (PATCH) Act of 2022. According to Congress’ bill-tracking website: “This bill requires premarket applications for cyber devices (i.e., medical devices that include software or connect to the Internet) to include information relating to cybersecurity, including plans to monitor for cybersecurity risks and address vulnerabilities through regular product updates.”
The bill was introduced in the House in March of 2022. Its purpose is to ensure cybersecurity throughout the life cycle of a device. The Act compels the manufacturer to take several specific steps to monitor postmarket vulnerabilities and exploits.
Although the bill has remained in the House Energy and Commerce Subcommittee on Health since March, it lays out requirements that address cybersecurity by going on to state:
“Any person who submits a premarket submission for the cyber device shall include such information as the Secretary may require to ensure that the cyber device meets such cybersecurity requirements as the Secretary determines to be appropriate to demonstrate a reasonable assurance of safety and effectiveness, including at a minimum the cybersecurity requirements under subsection (b). The Secretary may establish exemptions to the requirements under this subsection.”
The improvement that the PATCH Act would create is that it would put teeth in the requirement that manufacturers meet certain base requirements and that there are penalties for not complying. Under current rules, the requirement for certain cybersecurity measures is implied, but are often viewed as simply “guidance” and not necessity.
“Even if there is not a ‘patch’ or ‘update’ yet available for a known vulnerability or critical update – medical device manufacturers are not required to alert providers in a timely manner, which is essential to patient safety, as well as building and maintaining a strong, robust medical device safety net. The FDA is seeking greater authority to regulate medical devices
COVER STORY 48 TechNation | February 2023
from Congress – which the PATCH Act would do – and is something CHIME strongly supports,” says Mari Savickis, vice president of public policy at the College of Healthcare Information Management Executives (CHIME).
She says that even if there is not a “patch” or “update” yet available for a known vulnerability or critical update – medical device manufacturers are not required to alert providers in a timely manner, which is essential to patient safety, as well as building and maintaining a strong, robust medical device safety net. The FDA is seeking greater authority to regulate medical devices from Congress – which the PATCH Act would do – and is something CHIME strongly supports.
FDA specifically requested additional authority from Congress in the budget submission for FY23 which mimics some of what is in the PATCH Act, according to Savickis.
The agency is onboard with fortifying the ability of new medical devices, brought to market, to be resistant to cyberattack.
“FDA is requesting additional funding from Congress to develop a more comprehensive cybersecurity program for medical devices which will help to identify and mitigate vulnerabilities that could compromise medical devices. This may be accomplished by developing policies, engaging with stakeholders to inform FDA policies across the total product life cycle of the device, and monitoring for emerging technologies,” says an FDA spokesperson.
The agency’s spokesperson also said that “even without additional funding, FDA remains committed to addressing potential cybersecurity risks. For example, to aid industry, FDA continues to: 1) train reviewers across the Offices of Health Technology on cybersecurity to improve review consistency and 2) move quickly to finalize the medical device cybersecurity premarket guidance (FY23 A-list) so it can be imple -
mented across all premarket review staff. For its health care organization stakeholders, FDA is providing educational resources so they can learn more about medical device cybersecurity and how to prepare for and respond to medical device cybersecurity incidents (including ransomware).”
CONGRESS TRYING TO DEFINE REMANUFACTURING
A related bill that will be watched by many in the ISO and biomed communities is the “Clarifying Remanufacturing to Protect Patient Safety Act of 2022.”
According to the House of Representative’s website, the summary of H.R. 7253, which was introduced in the House on March 28, 2022, is:
“This bill specifies that entities that remanufacture medical devices in a manner that could change the performance or safety specifications or the intended use of the device must register with the Food and Drug Administration as producers of medical devices and comply with related requirements.”
Different groups within the medical device community take different positions on the bill. The Medical Imaging & Technology Alliance (MITA) strongly supports it. The Alliance for Quality Medical Device Servicing opposes the bill.
The bill was referred to the House Energy and Commerce Subcommittee on Health where it has sat since March 2022. For now, further legislation that impacts medical devices from the planning stage through the maintenance and repair stages will return to center stage.
The head of the FDAs Center for Devices and Radiological Health (CDRH), Jeff Shuren, has stated that 2023 will be a “big transition year” post-pandemic, allowing the agency to return to more normal conditions.
This will be a time period for the pace of the most sophisticated medical devices to come to market in the most expeditious and efficient manner.
“The law preserves everything that works well in the current system, adding support of specific needs to fulfill the critical mission of device review, and reflects lessons learned about shifting workloads and priorities in device development and review during a global pandemic.”
February 2023 | TechNation 49
–Scott Whitaker, the president and CEO of the Advanced Medical Technology Association (AdvaMed)




Find Your Next Job Here .com The biggest difference is that there is actually someone there, an actual live human, not just some algorithm chat bot. – E. Messenger, now BMET with Renovo Solutions “ ” LOOKING TO FILL A POSITION? Visit htmjobs.com/start-posting/ to post a job. Companies that post with us: Agiliti, TRIMEDX, OSF HealthCare, InfuSystem Inc., Canon Medical Systems, Renovo Solutions, InterMed, Associated Imaging Services, and Piedmont Healthcare. April 11–13, 2023 • Houston, TX SAVE the DATE The Woodlands Waterway Marriott Hotel & Convention Center | Houston, TX REGISTER FOR FREE AT HTMJOBS.COM
Please take a look at these opportunities to join RENOVO SOLUTIONS, the industry’s largest privately held HTM provider. We value knowledge, reliability, and integrity in our employees. If you are interested in being a part of a team that is committed to making a difference in the field of Healthcare Technology Management, we invite you to apply for one of our open positions. We are always looking for talented, passionate, hard-working people to join our team.


Radiology Equipment Technician I

The Radiology Equipment Technician I (RADT I) performs scheduled maintenance and builds a knowledge base of a variety of common medical imaging devices & systems at multiple locations. Examples of these imaging devices & systems are; Portable radiographic, portable fluoroscopic, radiographic unit digital and conventional, radiographic/fluoroscopic digital & conventional Ultrasound, etc. The RADT I also provides the following services; incoming equipment inspections, installation and calibration and repair of medical imaging devices & systems.
Biomedical Technician III
As the largest independent, technology-enabled clinical asset management company in the United States, TRIMEDX provides strategic planning and management of clinical assets to drive reduction in operational expenses, free up capital for new strategic initiatives and deliver improved safety and cyber protection. TRIMEDX was built by providers, for providers and leverages a history of expert clinical engineers to manage over $30 billion in clinical assets across thousands of locations.

Canon Medical Systems USA, Inc., a world leader in diagnostic imaging, is in search of qualified candidates to fill our open positions. Canon Medical Systems offers a competitive salary and benefits package, we support a diverse workplace and are an equal opportunity employer. We invite you to join and become part of our Canon family.

The InterMed Group is a technolog management company fulfilling the needs of our customers for over 20 years. InterMed sells and services biomedical equipment for clients across the country and is growing quickly. As a result, InterMed is on the search for high-quality candidates to serve our many customers and contracts.

Agiliti is a nationwide company of passionate medical equipment management experts who believe every interaction has the power to change a life. We proudly serve within hospitals, healthcare facilities, and our 90+ local service centers to ensure quality medical equipment is in the right place at the right time for effective patient care. Make an impact in healthcare and grow your career with Team Agiliti!


InfuSystem is a growing healthcare service provider, specializing in medical devices and related products and services for patients in hospitals, clinics, ambulatory surgery centers, and other major service centers. We provide direct payer rentals, pump and consumable sales, and biomedical services and repair, serving all 50 states and Canada. Headquartered in Rochester Hills, Michigan, we have Centers of Excellence in Kansas, California, Massachusetts, and Ontario, Canada.
For more than a century, Piedmont Healthcare has been a recognized leader in delivering expert care. Last year, Piedmont served over 2.7 million patients - performing over 88,368 surgeries, delivering 16,746 babies, providing nearly one million outpatient encounters, completing 382 organ transplants and handling more than 627,230 emergency room visits. For most, that would be a great track record. For us, it’s a good start.
Associated Imaging Services has been offering nuclear medicine and ultrasound solutions to our customers since 1990. We specialize in the sales and service of new and refurbished nuclear medicine cameras and ultrasound systems throughout Kansas, Oklahoma, Texas, and the surrounding areas.

Healthcare Technology Manager Customer Engineer III Traveling Technical Service Specialist Biomedical Equipment Technician (BMET) Clinical Engineer II Field Service Technician I Field Service Engineer (Nuclear Medicine) VIEW FULL DETAILS www.htmjobs.com VIEW FULL DETAILS www.htmjobs.com VIEW FULL DETAILS www.htmjobs.com VIEW FULL DETAILS www.htmjobs.com VIEW FULL DETAILS www.htmjobs.com VIEW FULL DETAILS www.htmjobs.com VIEW FULL DETAILS www.htmjobs.com VIEW FULL DETAILS www.htmjobs.com VIEW FULL DETAILS www.htmjobs.com
LIVE:
FEBRUARY 1 | Sodexo

Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
FEBRUARY 8 | Pronk
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
FEBRUARY 15 | Ordr
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
All webinars and podcast are eligible for 1 CE credit from the ACI.

CELEBRATING9 Y !SRAE
ON-DEMAND:
sponsored by Medigate “Left To Our Own Devices: Will We Implement Best Practices?”

sponsored by Cynerio “Real-Time Medical Device Visibility and Security in Action”

PODCASTS:
sponsored by MMS “The Next Generation of Biomeds: Mayra Becerra, The BioMed Girl”
LEARN, GROW AND BE INSPIRED.
webinarwednesday.live
CAREER CENTER When Excitement Turns to Worry
BY KATHLEEN FURORE
ou’ve accepted an invitation to interview for a position that sounds great. But now you’re reading negative comments in online reviews about what working for the company is really like. How should you handle the interview? Are there any questions that might help you determine if there’s any merit to those reviews?
Anyone likely would be worried to read negative comments about a company that only minutes before they were hoping to work at, says Adrienne Couch, a human resources analyst at LLCservices, a company that provides insights on successful LLC formation.
“However, what you read should not affect your view of the company. It is important to remember you don’t understand the full context of the reviews and the backstory,” Couch says, noting that people more often share negative experiences than positive ones. “So, do not panic, and continue getting ready for the interview.”
What should you do to prepare?
Be ready with questions based on the negative reviews. That advice comes from Fran Haasch, founding attorney at Fran Haasch Law Group in Palm Harbor, Florida. “For instance, if the online reviews cite that the company has a poor work-life balance, your question should be, ‘What does the company do for helping employees maintain a healthy work-life balance?’ ” Haasch says. If the interviewer offers only vague responses instead of definitive answers, it could be a red flag that those negative comments might have some merit, she notes.
Couch agrees and goes a step farther by suggesting mentioning the negative reviews during the
questioning. Just don’t do it in a negative or biased way, she stresses.
“Since the reviews will leave you with some doubts, you can ask questions about these reviews in a smart way,” Couch says. “For example, if there was a bad negative review of the company culture, you can politely ask, ‘Could you tell me more about your company culture. I came across a review on the company website claiming the company culture does not include X factors, and I would like to know more.’ “
Robert Donnelly, the CFO of Marketplace Fairness (a website designed to help readers make better decisions with their investments), says it is important to go into the interview with a realistic perspective – hence it is OK to mention concerns about negative reviews. “This will show that you’re interested in the company and that you’ve done your research, but it will also give you a chance to get more information about the concerns that you have,” Donnelly says.
Respect the company’s standpoint and try to consider their point of view. Always remember that: online reviews can be one-sided and filled with past employees who ended on a sour note with the company, Haasch says. “Your perception of the work culture of an entire company shouldn’t change based on three reviews by ex-employees,” she stresses.
As Donnelly concludes, “Keep in mind that not every review is accurate, and that you may have a different experience if you were to work for the company.”
Kathleen Furore is a Chicago-based writer and editor who has covered personal finance and other business-related topics for a variety of trade and consumer publications. You can email her your career questions at kfurore@yahoo.com.

EXPERT ADVICE February 2023 | TechNation 53
Y
Test Your Knowledge of Ultrasound Probe Failures
BY TED LUCIDI, CBET
t aying ahead in healthcare technology management is all about staying informed and up to date on new developments, trends, procedures and processes associated with current technology. From academia to OEMs to ISOs, organizations which continuously engage in research and development, design, testing, verification and validation have a proven impact on efficiencies, costs and outcomes. For example, organizations involved with design, manufacturing and engineering are constantly testing new ideas for products that increase performance, simplify operations, increase workflow efficiencies and reduce costs.
As a premium service provider, Innovatus Imaging is continuously improving upon existing and qualifying new processes. The goal is to not just repair a device and get it working again, but to restore performance and reset the device’s life cycle. Continuous learning is as important as continuous quality improvement in all aspects of health care. Having teams dedicated to testing operational and performance thresholds for ultrasound probes and MRI coils enables the teams at Innovatus Imaging’s Centers of Excellence to develop better methodologies for device repair and inform end users of best practices and processes for care and handling that result in more-favorable, cost-saving outcomes.

Test your ultrasound knowledge by seeing how well you can answer the following three questions.
WHAT IS THE MOST FREQUENTLY REPORTED FAILURE ON AN ULTRASOUND PROBE?
Most probes sent-in for repair to our Ultrasound Center of Excellence have a reported problem related to performance (image dropout, image artifacts, etc.). Based on data from over 175,000 probe repairs, the majority of costly performance-related failures are actually preventable.
A strong percentage of performance problems are a result of not addressing one or more minor problems. Now, more than ever, probes are not only cleaned after every use, but exposed to harsh disinfectants. Today, normal wear includes slow and gradual degradation of the seals surrounding the acoustic lens, and separation of the seams that join the various parts of the probe housing. The results are corrosion to the underlying acoustic array and associated electronics, as well as shorted circuits leading to catastrophic electronic failures. It’s vital for sonographers to perform frequent visual inspections to every probe in their department, and alert HTM teams of any concerns.
WHAT IS THE MOST FREQUENTLY ENCOUNTERED ROOT CAUSE OF AN ARRAY FAILURE ON AN ULTRASOUND PROBE?
Historically, most professionals believed that the main failure of the acoustic array was performance degradation or just internal failures. Although these are valid root causes, the most common root cause is related to physical damage. Adding to the confusion is the fact that, most times, there is no outward sign of trauma or physical damage to the probe housing. The thickness of the acoustic elements or “crys -
20/20 IMAGING INSIGHTS
EXPERT ADVICE
S 54 TechNation | February 2023
tals” within the array can be less than 0.5 mm. The majority of acoustic arrays in ultrasound probes utilize PZT (lead zirconate titanate). To the layperson, PZT is a ceramic-like compound. What happens when your ceramic coffee mug falls to the floor? With an ultrasound probe, the force of an accidental fall to the floor is more than enough to induce significant damage to the fragile elements. The result is often dark vertical (hypoechoic) lines in the image.


After evaluating over a quarter-million probes, with a very high degree of confidence, our teams are able to distinguish between an acute failure of the array due to trauma, and array/electronic failures related to normal wear.
WHAT TYPE OF DISINFECTANTS ARE BEST FOR MY ULTRASOUND PROBE?


Today, for ultrasound probes alone, health care facilities have the ability to choose from over 100 different OEM-approved chemical disinfectants and even more which are not OEM-approved. Each disinfectant has very specific instructions for use (IFUs). Key points include exposure time, application methods and rinsing practices.

You may not know that disinfectants containing alcohols and ammonium chloride solutions can induce significant damage to plastics and rubber materials. And most importantly, you may not know that most of the disinfectants used on ultrasound probes contain these chemicals. The use of alcohols and ammonium chlorides, over time, can lead to excessive stiffness, brittleness, shrinking, and staining of the lens, strain relief, sealants, housings, cable sheathings, etc.
Typically, the choices that end-users have, for disinfectants are limited by supply chain in combination with the infection control department. These departments may be more focused on efficacy and cost than on the effects to the medical devices on which the disinfectants are used. Even OEM-approved chemicals (and the methods in which they are used) have the potential to affect materials over time. It’s important to work with supply chain and infection control to source OEM-approved disinfectants with little to no alcohol and ammonium chloride content and be sure that end-users are following the recommended practices.
Innovatus Imaging is an FDA-registered manufacturer of ultrasound probes for several prominent OEMs. Whether through device manufacturing or depot repair, the above insights and practices add up to data-driven repair methodologies as a result of years of research, design, testing and successful outcomes. The results are proven repair solutions designed to restore the performance of your ultrasound probes and MRI coils at a cost much more budget-friendly than an exchange or replacement.
SPONSORED CONTENT February 2023 | TechNation 55
Ted Lucidi, CBET, is the director of commercial operations and business analytics at Innovatus Imaging.
RIGHT TO REPAIR
Are patients SAFER with or without medical Right to Repair?
BY KEVIN O’REILLY
The most compelling arguments for medical Right to Repair I hear are the ones that show its impact on patients.
Nader Hammoud, the biomedical engineering manager at John Muir Health, puts it in particularly stark terms. “If the iPhone isn’t fixed, you’re not going to have a phone,” Hammoud told WIRED. “If you don’t fix a vent[ilator], the patient is dead.”
Hammoud has had to rush into the hospital in the middle of the night to fix a broken device – often using parts harvested from other devices – multiple times over the course of his career. The urgency is all too real; if “you don’t get that device up and running in an hour or two hours, that patient will die,” he said.
While patient safety is a critical consideration related to medical Right to Repair, it’s not the only important factor. In the BMJ, a peer-reviewed medical journal, authors Shuhan He, Debbie Lai, Grace Jin and Jarone Lee argue that we should adopt a SAFER framework – one that accounts for issues surrounding safety and security, adaptability, fiscal responsibility, environmental impact and regulatory compliance – for advancing such legislation.
Each of these aspects are critical to make sure that the laws we pass are ones that improve our health care system. How does medical Right to Repair legislation measure up to these categories?
SAFETY
In addition to stories such as Hammoud’s, that show how repair limits limit hospitals’ ability to deliver effective care, it’s important to look at empirical evidence. Both highlight the need for medical Right to Repair.
Based on analysis of millions of complaints, a 2018 FDA report concluded that, “the objective evidence indicates that many OEMs and third-party entities provide high quality, safe and effective servicing of medical devices,” and that, “the continued availability of third-party entities to service and repair medical devices is critical to the functioning of the U.S. health care system.”
SECURITY
Cyber attacks on health care organizations are both costly – the average total cost for the most expensive cyber attack experienced was $4.4 million – and dangerous for patients, with 57% of healthcare IT and security practitioners surveyed reporting that the attacks caused poor patient outcomes.
These risks, however, should not be used as an excuse to lock out independent repair. Medical device cybersecurity expert Billy Rios told U.S. PIRG that accepted best practices for addressing cyber issues do not impact independent maintenance or repair. Rather, equipment makers misrepresent repair as a cybersecurity concern to limit competition in the repair market.
“The manufacturers’ concerns are not the most important,” Rios said. “This is about safety and saving lives. The doctors and the patients need to have a say in that calculation as well.”
The BMJ article authors also note that, “Key security features of any Medical Internet of Things include authentication, encryption and secure storage which should not be manipulable. Functionality that ensures security includes data logging and monitoring of any repair that is made.” None of these measures are at odds with medical Right to Repair.
ADAPTABILITY
The paper states Right to Repair, “should not impede product improvements and innovation,” and I couldn’t agree more. Ensuring that we continue to invent and
EXPERT ADVICE
56 TechNation | February 2023
improve upon medical device technology is critical to two of Right to Repair’s key goals – promoting patient safety and reducing health care costs.

The authors note that “There may at times be trade-offs between product designs that render medical devices easier to use, rather easier to repair.”
Repairability is an important design consideration, and I’d argue OEMs can do better. But model Right to Repair legislation does not impose any design requirements on manufacturers. Again, these goals can be balanced.
FISCAL RESPONSIBILITY
Hospitals have faced financial challenges in the wake of the pandemic, and the cost of health care in the United States is too damn high: it’s nearly twice the average of other wealthy countries. Roughly $1.1 trillion of hospital costs are related to medical equipment.
Repair and maintenance is a significant portion of those costs – and repair restrictions make it more expensive. Without medical Right to Repair, hospitals can be forced into pricey OEM service contracts. Biomeds estimate that OEM repair services run roughly 10-15 percent of the original cost of the device. That’s roughly double the cost of independent service, and as much as three times the cost of in-house repair.

For example, a single $1 million dollar MRI machine, ensuring repair options could save a hospital as much as $100,000 a year. Imagine the possible savings across other modalities – not to mention our whole health care system.
ENVIRONMENTAL IMPACT
When OEMs restrict repair materials, hospitals don’t have complete control of the repair of medical devices, and refurbishers aren’t able to restore them for future use. That means too many are destined to end up in the
landfill, when they could be used by other hospitals in the U.S. or around the world.
In addition to disposal problems, the production and transportation of medical devices contributes to the overall greenhouse gas emissions of the health care industry, which are estimated to be as much as 10% of total U.S. emissions.
Repair materials enable refurbishment, which together can reduce e-waste, emissions and the squandering of medical equipment that could be saving lives. That sounds like a win-win-win to me.
REGULATORY COMPLIANCE
The final section of the BMJ paper hones in on the importance of balancing intellectual property rights with repair access.
The Federal Trade Commission’s “Nixing the Fix” report states that it is possible: “the assertion of IP rights does not appear to be a significant impediment to independent repair.” Another significant governmental body, the U.S. Copyright Office, recently ruled that the repair and maintenance of medical devices does not amount to a copyright crime.
RIGHT TO REPAIR IS SAFER THAN REPAIR RESTRICTIONS
This doctor-developed framework is an important way to evaluate the potential impact of medical Right to Repair legislation. Based on our evaluation, the results seem clear – the SAFER option is to give hospital and independent biomeds access to the repair materials they need.
Kevin O’Reilly is PIRG’s Right to Repair campaign director. Follow him on Twitter and LinkedIn.

An industry leader for all your endoscopy needs. • All Inclusive Service Agreement
Colonoscopes
Gastroscopes
Bronchoscopes
Duodenoscopes
Video Processors
Light Sources
Replacement Filters
Preventative Maintenance CALL US TODAY 615.220.8728 spencerj@endoti.com February 2023 | TechNation 57
•
•
•
•
•
•
•
•
THE FUTURE Collaboration to Enhance HTM Education
BY STEVE YELTON
Iwould like to outline a program that we have been using for Cincinnati State Technical and Community College’s HTM Program for many years. I feel this can be a rewarding aspect of any HTM educational program.
Since the early 1980s the HTM program at Cincinnati State has taught the HTM courses (formerly known as the biomed courses) using a collaborative method. This method included a faculty member at the college working with an adjunct instructor from a local hospital.
Currently, we employ a hybrid approach to delivering our HTM courses. Some of the lecture and laboratory material is presented online via the Blackboard platform and some is presented face-toface. The collaboration occurs mostly with the face-to-face portion of the class. Our face-to-face time is comprised of approximately 50% of the time on campus and 50% of the time at the hospital. Currently, the hospital partner teaches the face-toface piece on campus and at the hospital with help from a college faculty member.
The college has biomed as well as electronics laboratories on campus. We also have a nursing program, so we have access to numerous medical devices that the students may troubleshoot and repair. Our biomedical courses focus on repair of medical devices with some design work. Where the collaboration is extremely helpful is in the areas of the hospital that we are not able to replicate in the college laboratories. There are many medical systems that we do not have on campus.
I admit there are some challenges as we continue to navigate a future that includes COVID-19 and its variants. However, these challenges have been
overcome as we proceed with this important part (in my opinion) of our HTM program. Cincinnati State is continuing to place cooperative education students and is working to enhance our placements in local hospitals. This is an integral part of this collaboration.
As I meet with my educational colleagues at AAMI and others, most of them have similar collaborations with their local hospitals. The college sees many benefits to this. Our HTM program was built on collaborations like this. Our hospital and corporate partners have ownership in the HTM program. By this, I mean they have a strong interest in supporting and growing a vibrant HTM educational program. Our partners have always supported us with equipment donations, guest speakers, advisory board members and more. This support takes the collaboration to another level.
An added bonus is, as we go through our accreditation processes, this collaboration proves to be a very positive aspect of our program. We are able to provide actual hands-on education on medical systems that we do not have on campus. Most notably are imaging modalities, intensive care areas and operating suites. We are able to instruct students on X-ray technology of all kinds. They learn about X-ray rooms as well as mobile X-ray. They are able to study CT systems as well as MRI. Students have classes and laboratories in all of these areas as well as cath lab. To have actual laboratory experiences in these areas is extremely beneficial to us and would not be possible without the collaboration.
Our students are required to work in a related cooperative education (co-op) placement. They normally complete their co-op assignments at one hospital. Most students complete more than one semester as a co-op employee, but they generally return to the same hospital so that they are able to
EXPERT ADVICE
58 TechNation | February 2023
avoid the “new employee” learning curve and move into more sophisticated experiences. Since this is normally the case, having the opportunity to attend classes in a different hospital is a nice perk for the students.













In our particular collaboration, numerous members of the hospital HTM department take part in the educational process. The director is the actual adjunct instructor from the hospital, but numerous technicians take part in classes and laboratories that are part of their specialty. Since our students participate in a cooperative education program, this gives the partici pating hospital a chance to interact with prospective employees. The students also get an opportunity to see the hospital operation. This is a win-win for everyone.
Through this collaboration as well as our coopera tive education program, students graduate from the program with significant practical experience in the HTM field. I feel that this collaboration teaches beyond the classroom. The student learns the importance of timeliness, work ethic, teamwork, as well as equipment repair and the hospital environment. Many times, the collaborating hospital hires a student from the class upon graduation. This is an advantage to both. This collaboration has been extremely rewarding for me and hopefully for our collaborators also.

WHERE KNOWLEDGE, EXPERTISE, AND INTEGRITY MEAN NO WORRIES 512 477 1500 KEIMEDICALIMAGING COM February 2023 | TechNation 59
Steven J. Yelton, P.E.; is a senior HTM engineer for a large health network in Cincinnati, Ohio and is a professor emeritus at Cincinnati State Technical and Communi ty College where he teaches biomedical instrumentation (HTM) courses.
Diag nos t ic Solut ion s i s a c u s tome r se r v ice ba se d pa r t s pr ov ide r t hat s pe c ia l i ze s i n a l l i mag i ng mod a l it ie s a nd manufacturers. Created to offer hos pita l s a nd I SO’s a cos t ef fe c t i ve a nd t i me sav i ng solut ion for or de r i ng i mag i ng r e place me nt pa r t s , e qu ipme nt move s , u lt r a sou nd pr obe r e pa i r a nd on-site se r v ice.









Contac t u s today, we a re conf ident you w i l l see u s a s T H E Pa r t s Solut ion!

diagnostic-solutions.com
LE N DING A H E LP ING HAN D AT E V E RY S T E P. Join our newsletter! 1technation.com/yp/ SCAN BELOW PUTTING CUSTOMERS FIRST SINCE 1987 TOSHIBA • GE • PHILIPS • SIEMENS AND MORE! Call: 508.730.9544 or 508.559.9441 www.InternationalXrayBrokers.com admin@intxray.com INTERNATIONAL X-RAY BROKERS IS NOW AN AUTHORIZED DISTRIBUTOR FOR CUSTOM BUILT SURGICAL TABLES! ALL MANUFACTURES & MODALITIES WE ALSO BUY AND SELL PRE-OWNED MEDICAL IMAGING EQUIPMENT. 60 TechNation | February 2023
330.296.9729
CYBERSECURITY Obtaining Zero Trust with IOMT Visibility Solutions
 BY CONNOR WALSH, CISSP
BY CONNOR WALSH, CISSP
In many modern health care facilities, a “perimeter-based security strategy” is used to segment and/or isolate medical devices from the Internet. Although this provides a basic level of network security, the non-existence of east-west traffic security and visibility, can lead to several possible vulnerabilities that can be exploited by a threat actor. Zero-trust networking is a giant step up from the traditional perimeter-based security and is something that every HTM professional should consider implementing using an IOMT visibility solution.
Zero trust networking is the principal of “never trust, always verify.” In other words, no device will be allowed connectivity in or out of your network until you can confirm, with confidence, that the device is what it is supposed to be and doing what it is supposed to be doing. And any time a device configuration changes, that trust must be verified again. The biggest first step into deploying a zero-trust network at your facility is obtaining visibility into your attack surface. Identifying not only where these devices are, but also what they consist of, such as OS, make, application, TCP/UDP port usage, vulnerabilities, and in the case of HTM, even FDA alerts. However, doing this manually would be very time consuming and require constant attention. This is where the IOMT visibility solution comes into play.
IOMT is a subset of IOT and stands for “Internet of Medical Things.” There are several dedicated IOMT visibility solutions that look at providing protection and zero-trust for a medical facility. These tools work in similar ways, first by building a robust medical
device inventory, as mentioned above, by passively or actively “discovering” devices on your network and aggregating each type of device into groups. Once a device has been discovered, the IOMT solution will look at “classifying” each device by attempting to characterize each discovered device and assigning it to an established policy. These policies can be developed based on almost anything, such as, “Is this device domain joined?,” “What type of traffic does this device generate?” and “What ports are open?” After classification, the next stage is to “assess,” where the IOMT solution will determine whether the discovered/classified devices are following facility policy. This is very important because it leads into the final “control” stage, where the IOMT solution can take active measures to enforce policy or remove devices that do not conform.
An IOMT visibility solution can help a medical facility obtain zero-trust networking and provide additional risk mitigation that perimeter-based security cannot provide. Investing, deploying and learning one of these devices at your facility will show leadership that you are prioritizing the protection of your facility’s sensitive data. In today’s ever-changing cyber landscape, it is important to know what devices you have, and be able to act quickly when new threats arise with real-time data at your disposal.
Connor Walsh, CISSP, works in the VHA Healthcare Technology Management Program Office.
EXPERT ADVICE February 2023 | TechNation 61












Accidents happen without warning. How do you respond? Conduct accident investigations more efficiently and effectively with ECRIʼs new online Healthcare Incident Management and Investigation training course. Developed by ECRIʼs team of experienced, unbiased investigators, this is the only course of its kind. 7-point Incident Management and Investigation (IMI) Plan 6 Interactive Modules with Real Case Studies 2 Educational Credit Hours Prevent recurrence and limit risk to the organization. Learn more at www.ecri.org/himi or call us today at 610-825-6000, x5891, to inquire about group discount pricing.













Hassle-free equipment security with ACCESSIBLE cable tethers PD1 Medical provides healthcare facilities with a simple, yet versatile approach to equipment security. The Med Tether works with equipment in all shapes and sizes and uses a security key making installation easy! Secure your equipment today and avoid the hassle and large expense of purchasing replacements. pd1medical.com NOW AVAILABLE ON PARTSSOURCE.COM! We’re on LinkedIn! FOLLOW US: linkedin.com/company/technation-magazine 64 TechNation | February 2023
THE VAULT
Do you consider yourself a history buff? Are you widely regarded among coworkers as an equipment aficionado? Here is your chance to prove it! Check out “The Vault” photo. Tell us what this medical device is and earn bragging rights. Each person who submits a correct answer will be entered to win a $25 Amazon gift card. To submit your answer, visit 1TechNation.com/vault-february-2023. Good luck!

SUBMIT A PHOTO
Send a photo of an old medical device to editor@mdpublishing.com and you could win a $25 Amazon gift card courtesy of TechNation !
 JANUARY PHOTO GIBBS Generator & X-Ray Tube (early 1930s)
FEBRUARY PHOTO
JANUARY PHOTO GIBBS Generator & X-Ray Tube (early 1930s)
FEBRUARY PHOTO
BREAKROOM February 2023 | TechNation 65


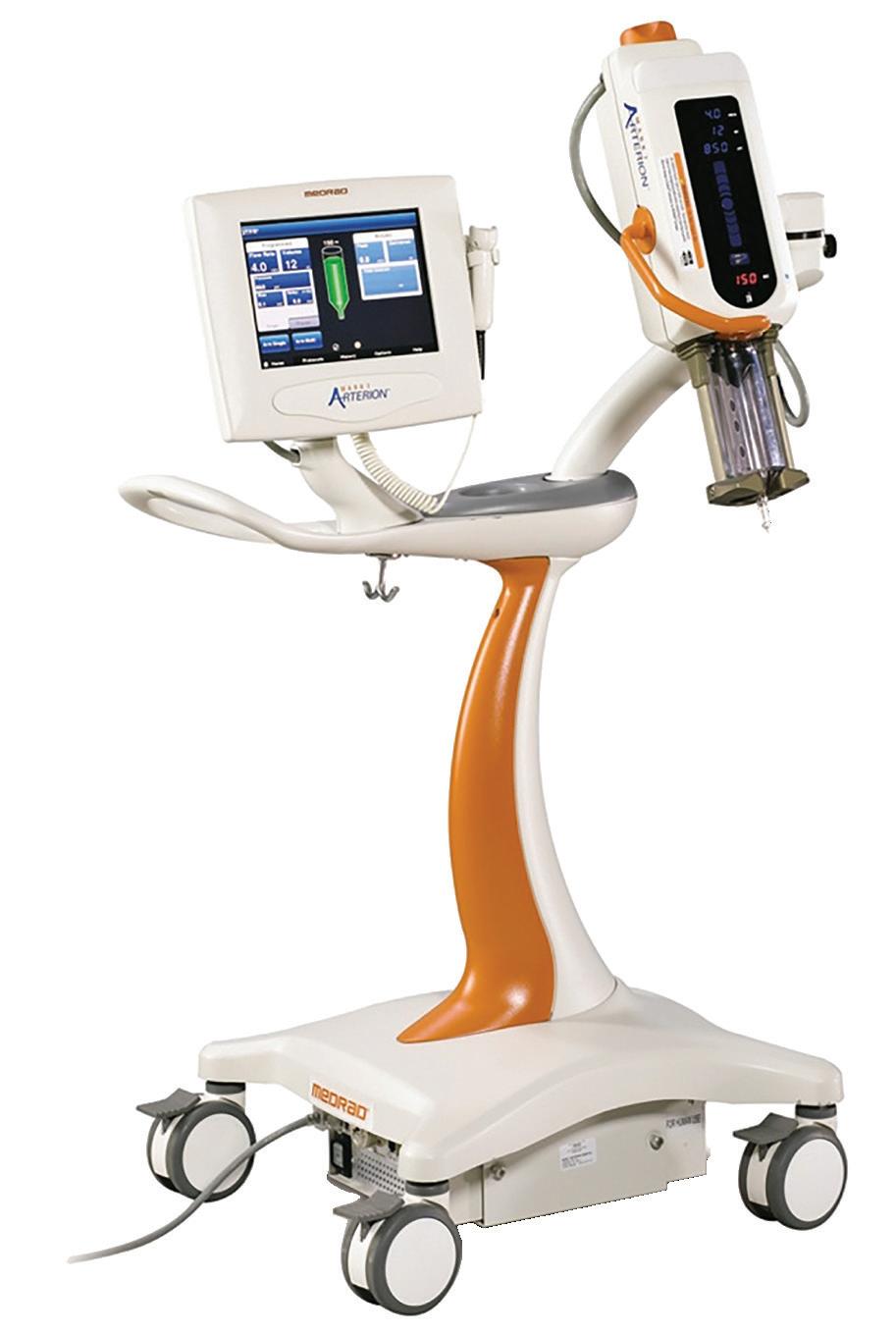


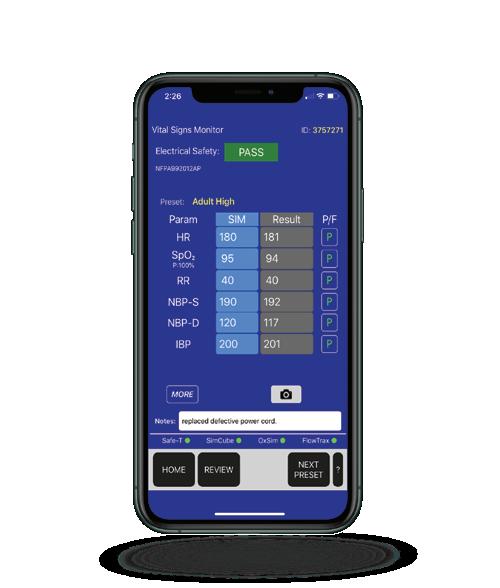

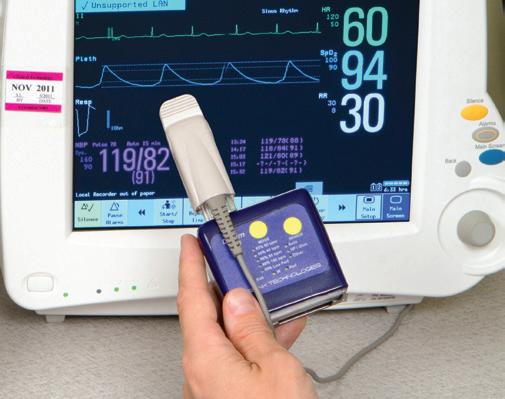



Contrast Injector Training • 100s of Error Codes not found in OEM Lit • Online and Onsite Training Available • Training BMETs since 2008 • Up to $5,500 in FREE Parts, PM Kits and Service • Massive Troubleshooting Library WWW.MAULLBIOMEDICAL.COM | 440-724-7511 | STEVE@MAULLBIOMEDICAL.COM ON-SITE AVAILABLE 66 TechNation | February 2023
WHERE IN THE WORLD IS BEN C?









us what Ben Calibrating has been up to! Follow MedWrench on Facebook at facebook.com/medwrench & on LinkedIn at linkedin.com/company/medwrench
1: Like the MedWrench Facebook or LinkedIn company page. Step 2: Post your picture of Ben C. to Facebook or LinkedIn & tag MedWrench in your photo. Step 3: Post a funny caption with your picture. Step 4: Use #BenC Atlanta, GA Airport Atlanta, GA Milwaukee, WI Lake Tahoe, CA Tri-ImagingMadison,Solutions, TN
BY: BREAKROOM February 2023 | TechNation 67
Show
Step
SPONSORED





Operate Efficiently, Perform Real Time, Simplify Compliance www.truasset.com 214-276-1280 sales@truasset.com • Security, RTLS, and Test Equipment Integrations • Customizable Service Request System • Pre-Order Parts/Service Reminder for PM’s One Month Ahead • Define Dashboard KPI’s • Manage Technician Productivity Your Comprehensive CMMS Solution! Call us to learn more about our newest features! Continue your free subscription of TechNation magazine! SUBSCRIBE TODAY! Advancing the Biomedical /HTM Professional ADVANCINGTHEBIOMEDICAL HTMPROFESSIONAL JUNE 2022 14 Company Showcase Elite Biomedical Solutions 24 Company ShowcaseTheInterMedGroup 60 Roundtable Training/Education ACCREDITATION SURVEYTIPS L PREPARATIONRELIEVESANXIETY 1technation.com ADVANCINGTHEBIOMEDICAL HTMPROFESSIONAL PAGE52 APRIL2022 1technation.com ADVANCINGTHEBIOMEDICAL/HTMPROFESSIONAL 12 Professional of the Month StephanieDrake 16 DepartmentoftheMonth JohnMuirHealthWalnutCreek MedicalCenterHTMDepartment 46 Roundtable CMMS 66 Cybersecurity MedicalDeviceProcurement: WhyIt’sOKtoBetheBadGuy JULY2022 SECURE NETWORK HTM, IT COLLABORATION VITAL FOR HOSPITALS PAGE 52 1technation.com ADVANCINGTHEBIOMEDICAL HTMPROFESSIONAL 12 DepartmentoftheMonth ClinicalThePeaceHealthOregonNetwork EngineeringDepartment 18 ProfessionaloftheMonth Hosameldin‘Sam’Elsemany 48 Roundtable Radiography 68 40Under40 HTM’sYoungProfessionals AUGUST2022 BACK TO THE FUTURETRENDS IN TRAINING PAGE 52 1technation.com/subscribe 68 TechNation | February 2023
Renaissance Nashville Hotel Nashville, TN

The Imaging Conference and Expo (ICE) is the only conference dedicated to Imaging Directors, Radiology Administrators, and Imaging Engineers from hospital imaging departments, freestanding imaging centers and group practices. ICE offers valuable CE credits from the ASRT and ACI (pending approval) and, keeping in line with our successful conferences in the past, offers comprehensive educational opportunities for attendees.

The Woodlands Waterway Marriot Houston, TX
MD Expo strives to provide healthcare technology management professionals with a unique, intimate and rewarding conference second to none. Clinical engineers, biomedical technicians, directors and managers, procurement/asset managers and others responsible for medical technology will gather in a one-of-a-kind warm and welcoming environment to network with peers, learn the latest technologies and advances in HTM. Find out what everyone has been talking about; this is one event you can’t afford to miss!
April 11–13, 2023 •
Houston, TX

Turf Valley Resort Baltimore, MD

May 11-12, 2023
Caribe Royale Orlando Orlando, FL
MD Expo strives to provide healthcare technology management professionals with a unique, intimate and rewarding conference second to none. Clinical engineers, biomedical technicians, directors and managers, procurement/asset managers and others responsible for medical technology will gather in a one-of-a-kind warm and welcoming environment to network with peers, learn the latest technologies and advances in HTM. Find out what everyone has been talking about; this is one event you can’t afford to miss!
What are HTM Mixers? Think of them as MD Expo 2.0 – a slightly modified, smaller, shorter-duration and less-crowded event that still provides valuable continuing education, networking and vendor engagement opportunities. HTM Mixers were created during the novel coronavirus (COVID-19) pandemic when larger events were not possible. The mixers were a hit and served as a regional conference for HTM professionals eager to earn continuing education credits, explore solutions in an exhibit hall and network with peers.
Orlando, FL • October 29-31, 2023


Registration open attendice.com htmmixer.com Registration open mdexposhow.com call for presentations is open mdexposhow.com SUPPORTED BY February 2023 | TechNation 69
Company Info AD PAGE PARTS SERVICE TRAINING Company Info AD PAGE PARTS SERVICE TRAINING SERVICE INDEX Anesthesia USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P Asset Management Capital i capitali.us • 417-708-2924 59 Cables PD1 Medical pd1medical.com • 64 Calibration Rigel Medical, Seaward Group www.seaward-groupusa.com • 813-886-2775 29 Cardiology Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010 38 P P CMMS Capital i capitali.us • 417-708-2924 59 TruAsset, LLC www.truasset.com • 214-276-1280 68 Computed Tomography AllParts Medical www.allpartsmedical.com • 866-507-4793 45 P P Diagnostic Solutions diagnostic-solutions.com • 330-296-9729 60 P P International X-Ray Brokers internationalxraybrokers.com/ • 508-559-9441 60 RSTI www.rsti-training.com • 800-229-7784 6 P P P Tri-Imaging Solutions www.triimaging.com • 855-401-4888 17 P P P Contrast Media Injectors Maull Biomedical Training www.maullbiomedicaltraining.com • 440-724-7511 66 P Defibrillator SakoMED sakomed.com • (844) 433-7256 75 P P Diagnostic Imaging 626 Holdings weare626.com • 800-516-0990 22 P Avante Health Solutions avantehs.com • 21 P P Diagnostic Solutions diagnostic-solutions.com • 330-296-9729 60 P P International X-Ray Brokers internationalxraybrokers.com/ • 508-559-9441 60 Probo Medical www.probomedical.com • 3174947872 7 P P Tri-Imaging Solutions www.triimaging.com • 855-401-4889 17 Education/Training Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6703 26 P Probo Medical www.probomedical.com • 3174947872 7 Tri-Imaging Solutions www.triimaging.com • 855-401-4888 17 P Employment/Recruiting HTM Jobs www.htmjobs.com • 50 Endoscopy Endo Technologies, Inc. endoti.com • 866-813-0480 57 Healthmark Industries hmark.com • 800-521-6224 20 Multimedical Systems www.multimedicalsystems.com • 888-532-8056 40 P Fetal Monitoring Multimedical Systems www.multimedicalsystems.com • 888-532-8056 40 P General ALCO Sales & Service Co. www.alcosales.com • 800-323-4282 73 PD1 Medical pd1medical.com • 64 Infection Control Healthmark Industries hmark.com • 800-521-6224 20 Infusion Pumps A&G Biomedical www.agbiomedical.com • 888-890-0192 25 P AIV aiv-inc.com • 888-656-0755 34 P P Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6701 26 P P 70 TechNation | February 2023
Company Info AD PAGE PARTS SERVICE TRAINING Company Info AD PAGE PARTS SERVICE TRAINING Infusion Pump Repair www.infusionpumprepair.com • 855-477-8866 63 Multimedical Systems www.multimedicalsystems.com • 888-532-8056 40 P United Infusion unitedinfusion.com • 919-609-9975 35 P P USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P Installs/Deinstalls Tri-Imaging Solutions www.triimaging.com • 855-401-4889 17 Labratory Ozark Biomedical www.ozarkbiomedical.com • 800-457-7576 34 P P Mammography International X-Ray Brokers internationalxraybrokers.com/ • 508-559-9441 60 RSTI www.rsti-training.com • 800-229-7784 6 P P P Monitors/CRTs USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P MRI Diagnostic Solutions diagnostic-solutions.com • 330-296-9729 60 P P Innovatus Imaging www.innovatusimaging.com • 844-687-5100 8 Online Resource HTM Jobs www.htmjobs.com • 50 Webinar Wednesday www.webinarwednesday.live • 800-906-3373 52 P PACS RSTI www.rsti-training.com • 800-229-7784 6 P Patient Monitors AIV aiv-inc.com • 888-656-0755 34 P P Avante Health Solutions avantehs.com • 21 P P Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6701 26 P P SakoMED sakomed.com • (844) 433-7256 75 P P USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P Refurbish AIV aiv-inc.com • 888-656-0755 34 Rental/Leasing Avante Health Solutions avantehs.com • 21 Repair ALCO Sales & Service Co. www.alcosales.com • 800-323-4282 73 Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6702 26 United Infusion unitedinfusion.com • 919-609-9975 35 Replacement Parts Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6701 26 P P Software TruAsset, LLC www.truasset.com • 214-276-1280 68 Surgical ALCO Sales & Service Co. www.alcosales.com • 800-323-4282 73 P Healthmark Industries hmark.com • 800-521-6224 20 Telemetry AIV aiv-inc.com • 888-656-0755 34 P P Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6701 26 P P Multimedical Systems www.multimedicalsystems.com • 888-532-8056 40 P USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P Test Equipment BC Group International, Inc www.BCGroupStore.com • 314-638-3800 BC P P Pronk Technologies, Inc. www.pronktech.com • 800-609-9802 2, 66 SERVICE INDEX February 2023 | TechNation 71
the
calengineeringdepartmentisledbySystemDirector AnthonyMaroulis.Othermembersofleadershipinclude CampusDirectorofClinicalEngineeringMarcBateman, Managers ClinicalEngineeringTerresaEverhart,Javier Ruiz,MiguelMezaandCoreaunJacksonandProgram CoordinatorsBrandonHightandNathanStathos.Theteam’s clinicalengineersincludeZachSmith,CristianDelgado, BailynPiecewiczandHaniKhalil.Thedepartmentalso consistsoftwoadministrationassistants,fivebiomed equipmenttechleads, biomedequipmenttechIIs,23 biomedequipmenttechIIIs,15biomedequipmenttechIs, biomedradiologyserviceengineersand dataentry specialist.Batemansaysthatthebiomedteamservicesmorethan 91,000medicaldevicesin-housethankstotheexceptional
trainingoptionsavailabletothedepartment’stechnicians. Inaddition managingequipmentattheeighthospitals, thelargebiomedteamalsoservesmorethan20orthopedic andsportsmedicinelocationsofferingphysicalandoccupationaltherapyacrosstheGreaterHoustonarea. PhysicianTheyarealsoresponsibleforequipmentattheSpecialty Groupwith848physiciansat191locationsandthe PrimaryCareGroupwith169physiciansat42locations. ThehospitalisaffiliatedwithWeillCornellMedicine, NewYork-PresbyterianHospitalandtheTexasAnnual Conference UnitedMethodistChurch.TheHoustonMethodistAcademicInstitute,whichis researchand educationinstitute,has facultyof742,with2,110credentialedresearchers.BIGTEAM,BIGPROJECTS ThechallengestoaddressCOVID-19surgestaxedthe resourcesofhealthcarefacilitiesacrossthenation,but requiredinnovationonagrandscaleforthebiomedteamat HoustonMethodist.“AttheonsetoftheCOVID-19pandemic,therewas glaringneedtoreduceexposuretopatientswithinthe hospitalforourRTdepartment.Thankfully,ourHamiltonG5 ventilatorshaveadetachableinteractionpanel.RTasked haveventsinsidetheCOVIDroomandplacetheinteraction paneloutsidetominimizeexposure theRTtherapisttogoinsidetheroom.Theinteractionpanelwasattachedtoa tableoutsidethepatientroomandwehad dothisfor 90-100G5patientrooms,”Smithsays. Hesaysthattogetherwiththe medicaldeviceintegrationteam,theysuccessfullyopened virtualICU(vICU).“We had keyroletoplayinensuringthepatientphysiologicalmonitorswerecapable beingusedinthisnew,innovative setting,”Smithsays.TheteamtackledmanyotherprojectsrelatedtothevICU tomeettheneedsofpatientsandvisitorsduringthe pandemic.AnotherprojectwascenteredaroundAlarispump interoperabilityandtyingthemtopatientrecords. “Pharmacy,nursing,biomed,multipleITgroups,andthe
ablesellinghimone hisbikesandhelpedBarrongetlicensedshortlyafter Suzukiheboughtin 2011. “Ialwayshad familymemberridinganything twofromHondaCR125s,SuzukiRM250,YamahaYZ490 stroke bikesgrowingup,butalwaysstayed uncles,interestedinthedragstripwhenwatchingmydad, grandpa,cousinsracemanylatenightsand playing ridingaround thepits,”Barronremembers. suburbsaysthatin1985,thefamilyrelocatedfrom ofFortWorthtoWaxahachie,Texas.Waxa-hachiejusthappenedto 10minutesfrom drivernationaldragstrip,builtbyformerNHRAfunnycar BillyMeyer,whichwascompletedin1986.“Itseemedlikeitwasmydestiny continue the goingtraditionofourfamily’sdragracinghistoryafter thefirstnationaleventheldthereandnevermissedanopportunitysince.All family,includingmykids,raceddownthatstripincompetition. am
In2010,hewasintroduced theprostock motorcycleclassby mutualacquaintanceofUnderdahlRacingfamily. “When approachedthematthe Nationalsin Texas,andaskedwhat wouldtake transitiontoprostockmotorcycle,GregUnderdahltoldmehe neededtoseeif couldhandleabike thatmagni-tudebyjustholdingthethrottlewideopenand releasingtheclutch;thenprogressed 330feet, 660feetandprogressinginto quarter-mile(1,320 feet)passinacoupleoftestsessions,”Barronsays. Underdahl then Barron that felt comfort-beras kidwatching CharlieBrownandPeanutsWorldserieswhereSnoopyoftendreamedoffighting War pilot,BaronManfredVonRichthofen,aka,TheRedBaron. stillhavemanyofmygrandinma’strophiesfromdragracingwhenshecompeted Ft.thePowderPuffWomen’sDragRacingSeriesin Worth thelate1960sandearly1970sbeforewasborn,”Barronsays. Barronsaysthat triestoattendeventsclose
TechNation February2023 2302-TN.indd Navy submarine load up with of food beginning of aAerosol deodorant spray, are authorized to be brought onboard. submariners is ways. Submariners, when underway, operate eight-hour tight community. Submariners take on knowledge on submarine. This ability learn and comprehend translates to other professions; including biomed. “I served Navy until After getting the U.S. looked for working as technician Chicago, however ended up taking another job service technician 2002,” Larry Sanlin, Banner Baywood and Heart Hospital, Banner Health Mesa, Arizona. Sanlin says 2009, laid off from service technician job and applied for facility maintenance technician. next day, received the biomed director asking come in to for a position maintenance technician. was hired years Tech managing four facilities,” says. Sanlin has combined biomed knowledge from military days on-the-job and study where he was hired beds stretchers. When working started PMs on equipment and tech manuals, expanding knowledge of equipment operations. gained knowledge my time Navy on submarine,” he military experience provided foundation work he today. did have years military training electronics, which very helpful, well as my experience as submariner, gave ability the required information succeed as tech,” says. PROJECTS JOB accumulated experience that his current resulted responsibility overseeing biomeds four campuses at his system. That included several projects. managed upgrades Philips telemonsystems. My knowledge of system the last Banner Baywood helped complete the record ahead schedule. issues to the upgrade. Also, this am managing replacement nurse systems at three hospitals (Baywood, Heart and facilities. Changing from to ASCOM call system, million-dollar project. completed major firmware upgrade to pieces PCU on with medium Sanlin says. change in also required Sanlin to challenge. “After losing director and manager period, step up manage the shop PROFESSIONAL OF THE MONTH Larry Sanlin, BMET III From the Ocean Floor to Tech III K. DOUGLAS SPOTLIGHT A February SHIFTING GEARS200MPHonaMotorcycleisaDrag ding motorcycleintraffic erscanbeunnervingwhendrivgoingdon’tseethebiker.Imagover200milesper houron motorcycle.That quickrequiresnervesofsteeland reactiontimes. Ahelmet,but seatbelt;justthewindwhipping That partoftheexperiencethathasbecome old SystemsforDavidBarron,ownerofUniversalMedical Waxahachie,Texas.Barron’sspecialization MRI/CT/X-rayservice andsales,but alsohassubstantialexperience riding dragbike,findingways geteverylastbit horsepower of engineandcarryingon familytradition. “Imade transitiontodragbikesintheearly ’90swhen highschoolfriend, Redmon,was racing theMotorplexontheweekends. was helpinghimcrewand gotsomeseattimehereand there on some faster bikes and rode for some teams inDivision4,thenstartedracingsome bracketsandgrudgeracesinthelate’90s,”Barronsays.
Company Info AD PAGE PARTS SERVICE TRAINING SERVICE INDEX CONTINUED QRS Solutions www.qrs-solutions.com/ • 877-254-7086 5 P P Rigel Medical, Seaward Group www.seaward-groupusa.com • 813-886-2775 29 Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010 38 P P Tubes/Bulbs Tri-Imaging Solutions www.triimaging.com • 855-401-4888 17 P P Ultrasound AllParts Medical www.allpartsmedical.com • 866-507-4793 45 P P Avante Health Solutions avantehs.com • 21 P P Innovatus Imaging www.innovatusimaging.com • 844-687-5100 8 MW Imaging www.mwimaging.com • 877-889-8223 4 P P Probo Medical www.probomedical.com • 3174947872 7 P P Ventilators SakoMED sakomed.com • (844) 433-7256 75 P P X-Ray AllParts Medical www.allpartsmedical.com • 866-507-4793 45 P P Innovatus Imaging www.innovatusimaging.com • 844-687-5100 8 International X-Ray Brokers internationalxraybrokers.com/ • 508-559-9441 60 RSTI www.rsti-training.com • 800-229-7784 6 P P P Tri-Imaging Solutions www.triimaging.com • 855-401-4888 17 P P P SUBMIT YOUR NOMINATIONS FOR Advancing the Biomedical /HTM Professional
love to feature you, your colleague or the entire department in one of TechNation’s monthly features! 1
We’d
Biomed/CE
QR code
email
Think of a
Department deserving of recognition. Scan the
or
editor@mdpublishing.com.
3 2 Professional of
Month,
DEPARTMENT OFTHEMONTH TheHoustonMethodistBiomedicalEngineeringDepartment uston,Texas oneofthebestknowncitiesinAmerica.Thecity andsurroundingcommunitiesforma metropolisthatextends theGalvestonBay.With populationexceeding 2.3million, isthefourthlargest cityintheU.S.,covering637square miles.Houstonwasfounded 1836 andoffersattractionslikeSpace CenterHouston,thelargestfinearts museumintheSouthwestandthe downtownaquarium. cityofthissizedependsonhealthcaresystemswiththe capacitytoservesuch largepopulation.Thatcapabilityis embraced HoustonMethodistHospital,affiliatedhospitals andotherfacilitiesscatteredacrosstheHoustonmetroarea. Werecentlyprofiledasmalltwo-techniciandepartment,but takes biomeddepartmentattheotherend thespectrum managemedicaldevices such large-scalehealth network.Witheighthospitals,anacademicinstituteand2,509 operatingbeds,HoustonMethodistincludes27,947employees,withmanyworkinginbiomed.The85-memberbiomedi-
We will contact you for additional information if your nomination is accepted! SPOTLIGHT BY RICHARDDOUGLAS
Shifting
Gears and Department of the Month!
H
immenselyproud thefourgenerationsofdragracersthatstartedbefore wasbornwithmygrandpa 1970,”Barronsays. coupesgrandpaanddadhadmatching1939Chevy Barron,”theyracedunderthemoniker“TheRed play theirlastnametakenfromthe famousWorldWar pilot. Snoopy“Irememberseeingthepaintedcoupeswith flyinghisdoghouseon sides. rememSPOTLIGHT BY RICHARD RDOUGLAS TechNation February2023 2302-TN.indd 1/6/23 9:08 72 TechNation | February 2023







ALPHABETICAL INDEX Bed & Stretcher Parts, Wheelchair Parts & Casters GET 10% OFF! ORDER ONLINE AND GET 10% OFF YOUR 1ST WEB ORDER! coupon code: FIRSTORDER alcosales.com 800.323.4282 • www.alcosales.com SPICE UP YOUR INBOX Sign up for the monthly TechNation newsletter and get a first look at magazines, promos, webinars, MD Expos, and more! 1technation.com/technation-newsletter-subscription/ 626 Holdings 22 A&G Biomedical 25 AIV 34 ALCO Sales & Service Co. 73 AllParts Medical 45 Avante Health Solutions 21 BC Group International, Inc BC Capital i 59 College of Biomedical Equipment Technology 11 Diagnostic Solutions 60 ECRI Institute ……………………… 62 Elite Biomedical Solutions 26 Endo Technologies, Inc. 57 Healthmark Industries 20 HTM Jobs 50 Infusion Pump Repair 63 Innovatus Imaging 8 International X-Ray Brokers 60 KEI Medical Imaging 59 Maull Biomedical Training 66 Multimedical Systems 40 MW Imaging 4 Ozark Biomedical 34 PD1 Medical 64 Probo Medical 7 Pronk Technologies, Inc. 2, 66 QRS Solutions 5 Rigel Medical, Seaward Group 29 RSTI 6 SakoMED 75 Southeastern Biomedical, Inc 38 Tri-Imaging Solutions 17 TruAsset, LLC 68 United Infusion 35 USOC Bio-Medical Services 3 Webinar Wednesday 52 February 2023 | TechNation 73
#IamTechNation


J oin us as we celebrate the TechNation community. You - each and every reader, Webinar Wednesday attendee, HTM Jobs user and MD Expo attendee - are the most important part of the TechNation community. Share a photo of yourself, a colleague or the entire biomed team on social media and tag it with #IamTechNation. Then, check each issue of the magazine to see yourself and all of the men and women that are TechNation.
 Photo by Reagan Jordan
Photo by Reagan Jordan
BREAKROOM
Introducing Renown Healths newest Clinical Engineering Tech 2. Congratulations Rylie on your promotion. You earned it the old fashioned way, you worked for it.
Photo by Salih Siddeg
Replaced UPS in Philips EasyDiagnost Eleva
Photo by Anthony J. Coronado, MBA
74 TechNation | February 2023
Another successful Joint Commission inspection for Kaiser ClinTech at Baldwin Park Medical Center.. Zero deficiencies and not one PM inspection found out of date!! “Best of the Best” style!!
Photo by Vaida Tomasetti
Working on a massive deployment project. Burning that midnight oil every day. So grateful for our wonderful team and their dedication.













Looking to lower repair cost? Need a FREE loaner and FREE evaluation? SakoMed is here to help, offering 2-3 days turn around time on all repairs. REFURBISHED MEDICAL EQUIPMENT SALES AND SERVICES 27751 LA PAZ RD. STE A LAGUNA NIGUEL, CA • 92677 (844) 433-SAKO • INFO@SAKOMED.COM • Anesthesia Machines • Respiratory Ventilators • Electro Surgical Units www.sakomed.com • Patient Monitors & Telemetries • Defibrillators and AEDs • Parts and Accessories
The DA-2006P is a microprocessor-based analyzer that is used in the testing of defibrillators. It measures energy output and provides information about the pulse.





The DA-2006P is used on manual, semi-automatic and automatic defibrillators with monophasic,biphasic and pulsed biphasic outputs.

DA-2006P Product Page
5000 V, 1000 J CAPACITY $5634 DA-2006P DEFIBRILLATOR ANALYZER with PACER SIMPLE TO OPERATE. myBC MOBILE INTEGRATION via BLUETOOTH* TESTS AEDs INTERNAL PADDLE ADAPTERS PACER TESTING CAPABILTY MONOPHASIC, BIPHASIC & PULSED BIPHASIC *Purchase adapter for legacy models. Internal Bluetooth® Coming Soon. (800) 242.8428 ISO 9001:2015 Registered and Certified ISO/IEC 17025:2017 Accredited ISO 13485:2016 Certified BCGroupStore.COM Android
iOS-iPhone-iPad myBC mobile
OS











































 The Houston Methodist Biomedical Engineering Department manages close to 100,000 devices.
The Houston Methodist Biomedical Engineering Department manages close to 100,000 devices.

























































































 BY DANIEL J. VUKELICH, ESQ
BY DANIEL J. VUKELICH, ESQ





































































































 BY CONNOR WALSH, CISSP
BY CONNOR WALSH, CISSP































 JANUARY PHOTO GIBBS Generator & X-Ray Tube (early 1930s)
FEBRUARY PHOTO
JANUARY PHOTO GIBBS Generator & X-Ray Tube (early 1930s)
FEBRUARY PHOTO











































 Photo by Reagan Jordan
Photo by Reagan Jordan





















