




































































2
1
REPLACEMENT PARTS
Quality Imaging parts for CT, Mammography, MRI, Cath labs, and general X-Ray
Hands-on technical training for all imaging modalities in Nashville, TN. Now offering an Apprentice program! 4 PRE-OWNED
Inspected, tested, and certified by our team in our 60k square foot facility in Nashville, TN.


3
Service support for all major imaging manufacturers including GE, Philips, Siemens, Hologic and Toshiba/Canon.




can’t
Supply chain issues and low-quality parts from offshore vendors can wreak havoc on your timeline and bottom line. You need a support team who understands the pressures you face and provides solutions — from new and T.Certified pre-owned products to top-quality repair parts — that fit your budget.

We’re in YOUR corner. Call today to schedule repairs or to purchase parts and equipment.



“We appreciate the opportunity to support the training needs of the imaging service industry,” RSTI CEO Todd Boyland, CRES, CPSM, said. “It was equally as fun getting to share this with the RSTI team and the group of students in classes that week too.”
SINCE 1985, RSTI HAS PROVIDED DIAGNOSTIC IMAGING TRAINING. OFFERING A WIDE SELECTION OF OVER 60 COURSES EACH YEAR, RSTI HAS TRAINED MORE THAN 15,000 SERVICE PROFESSIONALS FROM OVER 50 COUNTRIES IN RADIOLOGY, MAMMOGRAPHY, MR, CT, ULTRASOUND, NETWORKING, PACS AND DICOM.
CONTACT US: 23/24 CALENDAR:
ISO 9001:2015 CERTIFIED (IQC CERTIFICATE NO. Q-1158) STATE OF OHIO REG. NO. 93-09-1377T


P.12 SPOTLIGHT
p.12 Department of the Month: The St. Elizabeth Healthcare Clinical Engineering Department
p.14 Company Showcase: Collaborative Medical Solutions
p.18 Professional of the Month: Broderick Richard
p.20 Shifting Gears: The Big Reveal
p.22 Next Gen: Bryant Hawkins Jr.
P.24 INDUSTRY UPDATES
p.24 News & Notes
p.30 [Sponsored Content] Medical Equipment Doctor Is In
p.32 Welcome to TechNation
p.34 AAMI Update
p.36 Ribbon Cutting: NVRT Labs
p.38 ECRI Update
52 46
P.41 THE BENCH
p.41 Tools of the Trade
p.42 Biomed 101
p.45 Webinar Wednesday
P.46 FEATURE ARTICLES
p.46 Roundtable: Endoscopes

p.52 Cover Story: Women in the Industry
P.58 EXPERT ADVICE
p.58 Career Center
p.60 [Sponsored Content] Innovatus Imaging
p.63 The Future
P.64 CONNECTED
p.64 Cybersecurity
p.68 HIMSS
p.71 Networking Notes
p.73 Get Connected Company Directory

PUBLISHER John M. Krieg
VICE PRESIDENT Kristin Leavoy
VICE PRESIDENT Jayme McKelvey OF SALES
EDITORIAL John Wallace
CONTRIBUTORS
Roger Bowles
K. Richard Douglas
Jim Fedele
Joie Marhefka
Manny Roman
Connor Walsh
David Witt
Steven J. Yelton
ACCOUNT
EXECUTIVES
ART DEPARTMENT
Megan Cabot
Emily Hise
Karlee Gower
Taylor Hayes
Kameryn Johnson
DIGITAL SERVICES
Cindy Galindo
Kennedy Krieg
Haley Wells
EVENTS Kristin Leavoy
WEBINARS
HTMJOBS.COM

ACCOUNTING
CIRCULATION
Linda Hasluem
Kristen Register
Sydney Krieg
Diane Costea
Joanna Manjarrez
Rob Bundick, Director HTM & Biomedical Engineering, ProHealth Care
Carol Davis-Smith, CCE, FACCE, AAMIF, Owner/ President of Carol Davis-Smith & Associates, LLC
Jim Fedele, CBET, Senior Director of Clinical Engineering, UPMC
Bryant Hawkins Sr., Site Manager, Children’s Hospital of New Orleans

Benjamin Scoggin, MBA, MMCi, Director, Clinical Engineering | Biomedical Operations, Equipment Distribution, Clinical IT, DHTS, Duke Health Technology Solutions
Allison Woollford, Biomedical Equipment Specialist at Duke University Health System
Register now for the next DNV CHOP-B Certification Course
- Starts September 18 - 3-Weeks Online
- Live Webinars
Fall 1 Cohort begins September 18
Fall 2 Cohort begins October 20

At the beginning of the Civil War, in Covington, Kentucky, Henrietta Cleveland and Sarah Peter, with the support of the Catholic church, turned a grocery store in the city into a hospital. It was the first St. Elizabeth Hospital. The new hospital was staffed by nuns and named after St. Elizabeth of Hungary.
A second location was established in Covington after the end of the war. Those two locations grew into a health care system that today serves Northern Kentucky and Southeastern Indiana.
The St. Elizabeth Healthcare Clinical Engineering Department manages the medical equipment inventory for the health system including more than 30,000 medical devices, including radiology, biomedical equipment, laboratory and CE/IT medical device integration.
The CE team includes Director D’Juan James, MBA; Imaging Specialist Aaron Fischer; CE Supervisor Penny Frederick, CBET, MBA; CE Supervisor Brad Swecker; CE/IT Specialist Nathan Fischer; Service Logistics Coordinator Gina Loudermilk and 20 technical professionals.
The department includes six imaging specialists and two OR equipment specialists.
“St. Elizabeth Healthcare operates five hospital facilities throughout Northern Kentucky and Southeast Indiana: St. Elizabeth Dearborn, St. Elizabeth Edgewood, St. Elizabeth Florence, St. Elizabeth Ft. Thomas, and St. Elizabeth Grant, for a combined total of 1,200 patient beds,” James says.
He says the system also operates an ambulatory care center, hospice center, three freestanding
imaging centers and is in partnership with St. Elizabeth Physicians (SEP), which includes a network of over 185 physician offices located in Kentucky, Indiana and Ohio.
The CE team manages and negotiates service contracts to determine if a service agreement is necessary based on financial and service history data, proprietary equipment, department request, number of devices within the organization and equipment to technician sustainability ratio (ETSR), which is used to determine if the department has the full-time employee capacity to support the device in-house or outsource service contract.
James says that the vast majority of the department’s data collection is from its CMMS database (Medimizer). He says that this includes financial and service history data, service contract management and preventative maintenance compliance.
“In addition, we are now using Internet of Things (IOT) among other resources to track IP addresses, MAC addresses, operating systems, medical device vulnerabilities, remediations, alerts and recalls, and equipment utilization management,” he says.
James adds that there is a high level of integration between CE and IT.
“All medical device integration projects typically go through a technical/security discovery meeting with either our ancillary care or acute care subgroups within IT depending on the device type, network configurations, infrastructure type (on premises or cloud based), licensing requirements and security parameters,” he says.
As is the case with all biomed departments, there are projects and challenges that the St. Elizabeth’s team has tackled.
“St. Elizabeth outsources their telemetry monitoring and Tele-ICU services (virtual health). Technical
challenges come along with that. The vendor uses their own computer hardware to integrate with our Philips patient monitoring system, gaining remote access to view/surveillance our patients,” James says.
He says that CE manages the installation, integration and troubleshooting of the remote hardware system.
“We experience intermittent or hard done network dropouts which causes the vendor to lose visibility to patient vitals momentarily. Our team works diligently troubleshooting by immediately enlisting the help of a nurse to assist us in gaining remote access to allow us to reboot the PC or reseating hardware connections, and sometimes, reconfiguring the display settings on the remote screen resolves the issue,” James says.
He says that the CE department is involved in many projects such as capital planning for a new cancer center, slated to open in late 2024, business plan proposals; providing ROI contributions and maintenance strategies, standardizing infusion pump management at the most recently acquired hospital, as well as upgrading the Philips patient monitoring system software to the latest software revision.
“Philips offers a software application called patient information center (PIC iX) that provides visibility into patient data and enhancing clinical workflow. Our central station monitoring across the system are on two different software revisions; Rev B and C. Revision B has been end of life for several years and C will soon follow. We are
currently upgrading our monitoring system in stages to Philips newest 4.1 revision platform,” James says.
He says that the project required effective communication to nursing leaders without impacting patient care as it resulted in equipment downtime.
“CE assisted Philips with the deinstallation of the old MP series monitors (non-compatible) as part of the upgrade as well as staging, incoming inspection and inventory of the new MX series monitors into CMMS database,” James says.
The team also put its resources into a telemetry department remodel project that included a complete remodel of the telemetry unit and reduction from 32 to 26 beds.
“Our department worked with an outside vendor and IT department to run all new cabling and secure network drops. Installed and programmed the central monitoring PCs. Installed wireless access points and configuration of telemetry transmitters to communicate with the wireless network. In addition, installed patient overview hallway monitors for better visibility for the nursing staff to view patient vitals,” James says.
As a result of its efforts, the CE team carries on Henrietta Cleveland’s vision of helping ensure quality health care services to the residents of Covington and the Northern Kentucky/Southeastern Indiana region the health care system serves.

ollaborative Medical Solutions Principal Trey McIntyre explains that the company was formed after nearly 20 years in the pre-owned medical imaging equipment space.
“Actually, about four years ago, it was an international client from those early days in that business who came to us with an endoscopy opportunity. We had become friends over the years, and it was great to connect again and work together,” McIntyre says.
“While endoscopy equipment was somewhat of a new category, many of the same principles apply,” he adds. “People want someone reliable when buying pre-owned equipment; someone who will provide quality solutions and follow through. Relationships matter to us, and good relationships require product quality and availability, and ongoing support. We work hard to build long-lasting partnerships around the world by growing our inventory, improving our quality processes and being consistent in communication.”
TechNation found out more about Collaborative Medical Solutions during a recent Q&A with McIntyre.
Q: WHAT ARE SOME ADVANTAGES THAT YOUR COMPANY HAS OVER THE COMPETITION?
MCINTYRE: Our global reach and experience in international sales and support has been a key to our success and will be the biggest part of our growth in future. Our team has served more than 30 countries around the world, and we’re proud that nearly all our clients are repeat customers.
Q: WHAT ARE SOME CHALLENGES THAT YOUR COMPANY FACED LAST YEAR?
MCINTYRE: It may sound absurd to say, but the slowdowns of the pandemic still influence our industry. Project
timelines for delivering new equipment to hospitals and outpatient facilities have been pushed out or otherwise interrupted, and that has effects everywhere in the endoscopy market, from service and repair lead-times to pre-owned equipment availability, and so on. By offering many different brands of endoscopy equipment – from Olympus, Fuji, Pentax, and many others – we’ve managed to maintain inventory levels and still offer great opportunities and solutions.
Everyone is still challenged to find staff to meet demand, and this industry is no different. We’re happy to have grown our team and be able to help find creative ways to work together.
Q: CAN YOU EXPLAIN YOUR COMPANY’S CORE COMPETENCIES AND UNIQUE SELLING POINTS?
MCINTYRE: We take a consultative approach with our clients, and really try to understand everyone’s unique needs and challenges. Our focus is on the best brands and technology that can help our global client base with the equipment they need for their practice. Previously owned scopes and towers can add a lot of value as part of an overall strategy to manage and upgrade endoscopy equipment.
The great partnerships and other relationships we’ve formed with organizations and people who share our values have been an incredible resource we can bring together to offer unique and powerful solutions.
Q: CAN YOU SHARE SOME COMPANY SUCCESS — ONE TIME THAT YOU “SAVED THE DAY” FOR A CUSTOMER?
MCINTYRE: Endoscopy asset managers deal with tight budgets and compressed timelines quite a lot in today’s health care industry. There was a facility that was struggling to find a solution to update their technology. We were able to lay out a strategy that showed exactly how they could get the technology they needed, including customizable warranty options, which was a significant concern. What made it all work was that we were able to give value for the much older scopes and equipment that they’ve been using – they were surprised to be
able to reduce their purchase price with what they considered “outdated” assets. Beyond that, it was important to them to keep their patient schedule intact while finalizing financing options. We were able to provide loaner scopes and processors so there were no interruptions at all.



It’s a common case, but another example we’re proud of. We received a request for older model scopes from a very small country that seems to struggle to purchase the endoscopy equipment they need from reputable providers. The commerce and logistics challenges often make people shy away, which can be understandable, but that’s where our international experience shows best. To be able to deliver quality scopes to them, at a price that makes sense for their budget, means a lot. Those are the customers who need support, and we know we’ll work together on the next opportunity when it comes, as well.

MCINTYRE: As part of our international growth, we’ve brought on Barney Greig to oversee sales, marketing, and sourcing in the European and other markets. Barney comes with a wealth of experience in used medical devices, crucial in such a specialized niche market,
where product and market knowledge is vital.
To understand its challenges, its nuances, its players and its often-underestimated value within the health care landscape has been incredibly helpful.
More importantly, he’s immediately shown that he shares our key values: always focused on long-term relationships, creating an excellent customer experience, and offering technical and customer support long after the point of sale. He is based in England, but loves to travel and meet with clients, vendors and partners in person from Germany to New Zealand where he was born and raised.
Q: CAN YOU DESCRIBE YOUR COMPANY’S FACILITY?
MCINTYRE: Our big news is that we’re opening a new U.S. headquarters this fall. We are in the later stages of the construction project, and the whole team is excited about what’s coming. It’s larger than our current space, and will better fit the growth we’ve seen, as well as our expansion plans for the future. We’ll almost double the QA/QC capability, carry more endoscopy inventory that’s ready to ship, plus give the new faces on our customer support and operations teams some elbow room.
For more infomation, visit cmsscopes.com.
LIVE:
SEPT 13 | Kontakt.io

Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
SEPT 20 | Chronos Imaging

Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
SEPT 27 | Phoenix Data
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
SEPT 28 | Tools of the Trade LIVE Demo with BC Group


Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
ON-DEMAND:
sponsored by HTMJobs
“HTM Jobs: The Future of HTM Careers”

sponsored by BC Group “Infusion Pump Testing with the IPA-3100”
sponsored by Cynerio
“Generative AI: The Future of Healthcare Cybersecurity”
sponsored by Cynch!

“Solid Productivity Gains with Best of Breed CMMS and Asset Management Platform”

PODCASTS:
sponsored by Healthmark

“Fire in the OR! Why Checking Cords for Integrity & Continuity is Important”
sponsored by MMS

“Learning Marketable Skills as a Biomed”
All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.


The greater Houston area represents one of the largest population centers in the U.S. and the largest economic and cultural center in the South. The city’s port is the second largest in the U.S. Working in this vibrant area offers residents many avenues for employment and many bedroom communities to choose from.
One of the region’s health care providers is CHI St. Luke’s The Woodlands Hospital in Conroe, north of Houston. The hospital’s CE department has previously been spotlighted in TechNation
Broderick Richard is a Senior BMET in the clinical engineering department at The Woodlands.
Richard was already making a contribution to health care in another hospital system when he discovered biomed.
“Actually, I started off in EMS at Harris Health Systems LBJ Hospital and while transporting patients to and from Ben Taub I ran across a gentleman name Joe Boone. I asked him, ‘I always see you in scrubs but never see you with a patient. What do you do?’ He replied, ‘biomed.’ I was like, ‘what is that?’ So, the next morning while at LBJ before I started my shift, I started asking around about biomed and a co-worker offered to take me to the manager of biomed at LBJ; Coreaun
Jackson. Coreaun showed me the shop and we talked about biomed for almost an hour, and from that moment, I knew I wanted to change professions. I didn’t realize they would pay me to play with electronics all day,” Richard says.
To facilitate his new career, Richard obtained an associate degree in electronic engineering technology with a specialization in biomedical electronics from Houston Community College.
Since entering the field, Richard has been a Clinical Engineer I and Clinical Engineer II, before his 2023 promotion to Senior BMET.
His specialty area is anesthesia. He is manufacturer-trained by GE for the GE Carestation 600 Series, GE Avance CS2, GE Avance as well as Draeger Fabius GS Premium, GS/OS, Fabius Plus, Fabius Plus XL, Fabius Tiro and Fabius MRI. He has also received OEM training on Philips PIC IX System and Philips MX Monitors.
Richard says that any challenges he has experienced are simply the ones to be expected.
“Challenges in biomed, I feel, are pretty much the same for most biomeds; getting to the equipment once it is down. There are times you get called to repair a machine and you have no clue what it does. My go to question in this case is ‘Show me what you did?’ About 75 percent of the time, the machine works just fine and the error is fixed and then they say it’s only working because you are here,” Richard says.
Away from work, Richard spends time with his family or involved in high school football; a Texas tradition.
“On Friday nights during football season, you can catch me on the sidelines refereeing high school football,” he says.
He says that “Friday Night Lights” refers to high school football.
“I normally don’t attend games outside of the ones I’m working. Trying to get to college football. It’s a sport I love and played growing up. Once I had the opportunity to get back on the field, I jumped at it. Also, standing on the sidelines, I get a pretty good view of the game,” Richard says.
He says that he also likes to spend time “cheering my kiddos” as they participate in their prospective sports.
This biomed keeps the Texas tradition alive by not only cheering on his local high school football team but taking an active part in the sport as well.
FAVORITE FOOD: Snow crabs
HIDDEN TALENT: Refereeing
WHAT’S ON MY BENCH?
My trusty certifier and manometer. Most of my calls can be resolved with these items and my screwdriver.
My favorite part about being a biomed is the fact I can be the hero in the background without having to be in the limelight. The satisfaction of knowing just about every person that walks through my hospital door for treatment plays a role in getting them back to their family safe and ready to enjoy life. I always look at it like a music producer, everybody always knows the singer but the producer behind the song plays just as big of a part as the singer.
or fans of college sports, the team’s mascot is as well-known as any star player on the team. Real college sports fans can reel off a list of popular mascots. What is less known, is the amount of work and commitment these mascots make. The person, hidden below the costume, is as committed to the team and its success as any other team member. The person in the costume is also an athlete like the players in uniform.
During the 2023 graduation season, when most colleges and high schools were immersed in milestone days for thousands of students, one event may have stood out as extra unique and exciting for participants and sports fans.
At the 2023 University of South Carolina (USC) graduation ceremony participants were in for a big reveal as the student beneath the school’s beloved mascot “Cocky” costume would be unveiled for the first time. Sports is a big part of college life at the University of South Carolina. The Gamecocks compete in 21 varsity sports.
The student who was revealed at this year’s commencement ceremony just happened to be receiving her degree in biomedical engineering. But, before continuing her studies in the school’s master’s degree in biomedical engineering program, Sarah Sylvester surprised everyone at the graduation ceremony and created a viral video in the process.
Yet, after two years of donning the costume and thrilling college sports fans, what originally drew her to become a famous mascot?
“Believe it or not, I was never a mascot in the past, nor did I ever want to become a mascot. As cliche as it sounds, I just wanted to be ‘Cocky.’ I always knew I wanted to go to the University of South Carolina. My mother and sister are alumni and so I grew up visiting, exploring the campus, and going to football games. It was the only place I applied for college. My freshman year I went to every single event and sporting game possible. Oftentimes, I would find myself watching Cocky, in
love with his character, the way he interacted with others, and the joy and laughter he always brought to the environment,” Sylvester says.
She says that one day she told some close friends, “I am going to figure out how and I am going to do that one day.” The rest was history.
“I made the proper connections, auditioned and here we are. My pure passion and love for the character and university led me to the position,” she adds.
From ringing the opening bell on the New York Stock Exchange on College Colors Day to doing a nationwide televised interview with David Muir on ABC World News, Sylvester as Cocky or herself, has had some incredible experiences.
“Holding the position as Cocky has given me invaluable opportunities and the memories of a lifetime. Even the smallest experiences such as high-fiving or hugging a student when they might be having a tough day hold the biggest places in my heart. There were also many larger experiences that were proven to be once-in-a-lifetime opportunities. One of the most memorable experiences for me has been ‘coming out of the box’ in the middle of the football field at home football games. At USC, the pregame tradition for years has been for the song ‘2001: A Space Odyssey’ to play as ‘Cocky’s magic box’ is in the center of the football field, where at the big crescendo, the curtains drop to reveal Cocky jumping around, to then jump out of the box and down to the end zone to lead the entrance of the football team onto the field,” Sylvester says.
She says that the first time she got to lead this experience was absolutely magical and she wouldn’t trade it for the world.
“In addition to partaking in countless memorable experiences at home in Columbia, South Carolina, I also got to travel abroad to represent the university in countless ways as well. One of the most unique experiences was being invited to ring the opening bell on the floor of the New York Stock Exchange. In September of 2022, Cocky as well as a few other notable SEC mascots were invited to come to New York City. There, they wanted us to lead the ceremonious ringing of the opening bell to spark the beginning of the day in the New York Stock
Exchange to represent national College Colors Day. We got to stand up on the platform and ring that large famous bell. Afterwards, we got to sign the wall of celebrities that have gotten to do the same over the years,” she remembers.

Sylvester describes that experience as “absolutely surreal.” “Another experience that will always be one of my most treasured is competing and placing in my own national’s competition. Every year in Daytona, Florida, there is the NCA and NDA national’s competition where cheer and dance teams, and their mascots, compete for a national title. For mascots to compete, they have to receive a bid to go, either at camp or via submitting a video to audition. I did the latter and beat out several mascots for a chance to compete at nationals,” Sylvester says.
She says that mascots compete by creating a skit with a soundtrack and props, the theme of which is entirely up to the performer’s choice and perform it in front of judges and a large audience.
“They then only announce the ones who ‘place,’ which are the top five. Being that Cocky hadn’t placed in several years, and that I was creating the skit and props entirely by myself with the help of one close friend and my father, my goal that I had set for myself was to place, and that I did. Hearing my (or Cocky’s, rather) name called as a placer on that big stage fulfilled a goal of mine and re-established my knowledge that I can do anything I set my mind to. All of these experiences have proven nothing short of that,” Sylvester says.
Running parallel to all of the functions she fulfilled as Cocky, there was still the primary reason that Sylvester enrolled at USC. It was the reputation of the degree program


she participated in.
What led Sylvester to pursue a biomedical engineering degree and an interest in medical devices?
“Growing up I always knew I wanted to be in engineering. I always loved STEM, and math in particular, and excelled at it; although, I wasn’t always sure of which pathway I wanted to take with it. In high school, I became part of a volunteer program where I shadowed departments all over the local hospital, most notably the biomedical engineering department, the emergency room and the operating room,” she says.
Sylvester says that it was there that she learned of her passion for the medical field and how it operates as well.
“After some research, I discovered the field of biomedical engineering and specifically that I could take that pathway to focus on developing medical devices to further enhance the medical field. I knew that the University of South Carolina offered that program, and it was history from there,” she adds Because she was part of an accelerated master’s program during her senior year, her pursuit of a Master of Engineering degree in Biomedical Engineering at USC will only take her one year.
Future patients may never realize that they are benefiting from a medical device that in whole, or in part, was developed by an engineer who was once also a college mascot known to fans far and wide. That may be fitting, since for two years, USC fans had no idea who was underneath the costume of their beloved mascot Cocky. For Sylvester, either way, she has a lifetime of stories to tell.



For more photos, view this article online at 1TechNation.com.

ryant Hawkins Jr. was introduced to the world of healthcare technology management by his father and namesake Bryant Hawkins. His father is a successful HTM professional with 30 years of experience. Bryant Hawkins Jr. holds an associate degree in biomedical equipment technology and is currently a biomedical technician with AdventHealth Fish Memorial Hospital in Florida.

TechNation found out more about this Next Gen HTM pro via a recent question-and-answer session.
Q: WHERE DID YOU GROW UP?
A: I grew up in Slidell, Louisiana.
Q: WHERE DID YOU RECEIVE YOUR HTM TRAINING/ EDUCATION?
A: I received my college education at the College of Biomedical Equipment Technology and before starting in the field I also did an internship and AdventHealth Palm Coast.

Q: WHY DID YOU CHOOSE TO GET INTO THIS FIELD?
A: My father was the one who introduced me into this field. I was unsure what I wanted to pursue when I was finishing my tenure in high school. He told me that I should go into this field as he felt like it would be something I would enjoy and that even if I did not enjoy it I could use it to fund my education. Fortunately for me, I greatly enjoy this field.
Q: WHAT DO YOU LIKE MOST ABOUT YOUR POSITION?
A: I enjoy the fact that I help people by doing my job correctly. It is a very fulfilling feeling.
Q: WHAT INTERESTS YOU THE MOST ABOUT HTM?
A: All the different kinds of medical equipment there are. Before starting, I never realized how ignorant I
was to just how many kinds of machines there are to help save people’s lives.
Q: WHAT HAS BEEN YOUR GREATEST ACCOMPLISHMENT IN YOUR FIELD THUS FAR?
A: My greatest accomplishment has to be seeing how far I’ve grown intellectually in the field from when I started to now. In the beginning, my knowledge was minimum on the workings on the equipment and hospital environment, but now I can say I feel quite comfortable navigating myself through issues. Whether it’s a call from the OR or having to troubleshoot with the telemetry system, I always find myself being able to find a solution to the problems.
Q: WHAT GOALS DO YOU HAVE FOR YOURSELF IN THE NEXT 5 YEARS?
A: One goal I have for myself in the next 5 years is to advance to Tech 2 status and go to some training sessions.
FAVORITE HOBBY: Piano and photography
FAVORITE SHOW OR MOVIE: “That ‘70s Show”
FAVORITE MEAL: Pizza
WHAT WOULD YOUR SUPERPOWER BE? Flight
1 THING ON YOUR BUCKET LIST: Travel to a different continent.
SOMETHING YOUR CO-WORKERS DON’T KNOW ABOUT YOU: I can play 5 musical instruments.
The Insulation tester, Leak Tester Tester and Cable Continuity Tester are easy-to-use devices for verifying the functionality of equipment for safety



Detect & locate defects such as pinholes, cracks and bare spots in the jacket or coating of laparoscopic and bi-polar electrosurgical instruments
Test the functionality of automated & handheld endoscope leakage testers with healthmark’s new Leak Tester Tester.
Test the quality of monopolar and bipolar cords with this user-friendly, durable device. A green light notifies the user that the cable passed testing.
For more of Healthmark’s intelligent solutions for instrument care and infection control, visit HMARK.COM
TechNation delivers a new video series that empowers healthcare technology management (HTM) professionals. The series highlights the tools that biomeds can use to do their jobs more efficiently.
The video series builds on the longstanding and popular “Tools of the Trade” magazine feature. The series will have live broadcast and on-demand recordings, similar to the current Webinar Wednesday series. Viewing live broadcast will be eligible for 1 CE credit from the ACI.
The Philips MX40 IntelliVue was featured as the Tool of the Trade in the May issue of TechNation. In the inaugural broadcast, Glenn Schneider, MHSA, chief service officer of Elite Biomedical Solutions, provided a live demo on the Philips MX40 IntelliVue.
The next installment of the Tools of the Trade video series brought over 40 registrations and featured Voytek Medical’s Bear Trap and URSA product lines.
“This will save so much money on equipment leads and keep the inventory up,” attendee Douglas Booth, BMET III, Southern NH Medical Center, shared after the live presentation. July brought another broadcast for this series, featuring
J.C. Newell of NRC LLC. Newell discussed candidate services provided by her company, also featured as the Tool of the Trade in the July issue of TechNation.
Details for upcoming Tools of the Trade broadcasts can be found on WebinarWednesday.live. Each month a new tool will be featured in print and with a live demo.
For more information, scan the QR code below:

In its continued effort to promote and applaud the men and women of healthcare technology management (HTM), TechNation magazine introduces the Tech Choice Awards also known as the Wrenchies!
The award categories are:
• Professional of the Year
• Department of the Year
• YP of the Year
• ISO Employee of the Year
• Director/Manager of the Year
• Lifetime Achievement Award
• Military BMET of the Year
• Humanitarian Award
• Women in Leadership Award
• Ingenuity Award
• Outstanding Vendor of the Year
• Association of the Year
• Industry Influencer of the Year
The inaugural awards will be announced in the March 2024 issue of TechNation. Nominations are being accepted in a variety of categories through November 3, 2023. Up to 13 Wrenchies will be awarded with the winners selected based 100% on votes from those who make up the HTM industry.
The top nominations for each category will be highlighted in the January 2024 issue of TechNation with voting allowed until January 12, 2024. Winners will be announced in the March issue of TechNation and highlighted in a special digital supplement.
Nominations are open online at 1technation.com/ tech-choice-awards.
MD Expo, the signature healthcare technology management (HTM) conference hosted by MD Publishing, returns to the Sunshine State this fall. Registration is now open for the October 29-31 conference and is FREE for hospital employees, students and active military members.

MD Expo strives to provide HTM professionals with a unique, intimate and rewarding conference second to none. Clinical engineers, biomedical technicians, directors and managers, procurement/asset managers and others responsible for medical technology will gather in a one-of-a-kind warm and welcoming environment to network with peers, learn the latest technologies and advances in HTM. Find out what everyone has been talking about; this is one event you can’t afford to miss!
MD Expo features:
• Industry-leading speakers covering the hottest topics in HTM, compliance, IT, cybersecurity, management and equipment service
• Certified education approved for 36 CEU credits
• The industry’s most unique networking events to connect and share best practices with other leading HTM professionals
• World-class exhibit hall with the latest technology, products and services
Several of the top-flight presenters recently took some time to share more about their presentations and MD Expo.
University of Chicago Medical Center System Director of Clinical Engineering Shawn Molloy will present the session “Developing a Family Out of a Team – Know your role and your place” at the MD Expo. He described it as “a playful and inspirational dive into developing a strong family-like environment in your CE department through understanding your role and everyone else around you.”
He described MD Expo as an out-of-this-world event.
“MD Expo is an essential experience to reinforce the support structure and backbone of our career field. It gives you the true visibility of the scope of our career field. I compare it to when astronauts see the Earth for the first time and their feeling of one humanity. That is the same feeling I get when I attend the MD Expo and it revitalizes my engagement in the field,” Molloy said.
“I am always humbled by seeing and conversing with my HTM heroes and also speaking with the new blood to the organization and seeing the ideas and new life they bring to the HTM community,” he added. “I love to see our leaders and communicators sharing their love, experience and knowledge with the HTM community.”
Children’s Health (Dallas) Healthcare Technology Management Senior Director Andrea Brainard, CBET, CHTM, is slated to host a panel discussion on women in HTM.
“This will be a questions-and-answers forum with questions garnered from LinkedIn, past presentations and audience questions,” she explained. “My panelists include women that are in various disciplines within HTM. We get down to the nitty gritty of being a woman in HTM in hopes to not only get more women into HTM, but to allow everyone to share in our experiences and knowledge.”
When asked what attendees can expect at MD Expo, Brainard spoke from experience having attended several in recent years.
“Attendees can expect a vast array of educational opportunities, seminars, panel discussion and product demos in the exhibit hall. There are also great networking opportunities to meet both vendors and HTM colleagues in the industry,” she shared. “MD Expo is great way to collaborate with other HTM colleagues, make new connections, learn a lot of new stuff and have some fun doing all it.”
Intermountain Health’s Director of Field Service for the HTM Department Mike Powers, MBA, AAMIF, CHTM, CDP, will present on the Health Sector Coordinating Council’s (an advisory group to HHS for Cybersecurity) “Health Industry Cybersecurity – Managing Legacy Technology Security (HIC-MaLTS)” document. After three years of efforts this 117-page document helps organizations have concrete guidance to deal with technologies that have aged to the point that the risk posed by their software cannot be reasonably mitigated.
He said, “An MD Expo is a trade show/educational seminar series opportunity to learn from industry experts, meet new vendors for unique solutions to operational challenges and catch up with friends and colleagues in the field.”
Milwaukee School of Engineering Adjunct Professor in Electrical Engineering and Computer Science
Larry Fennigkoh, Ph.D., PE, is another veteran MD Expo presenter who will share his knowledge in Orlando.
He shared that his presentation will benefit anyone involved in the support and maintenance of physiological monitors, electrocardiographs, heart-lung machines or intra-aortic balloon pumps.
For anyone thinking about attending MD Expo Orlando, Fennigkoh said to find a way.
“Stop just considering attending but start planning to attend now. Beg, plead, or negotiate with your employer to let and help you attend – even paying some or all the costs needed to do so. These conferences remain some of the absolute best value-added and most needed PM that you can give yourself,” he said. “One of the reasons so many others start to lose the passion, excitement, and fun for a job that they once loved, is because they didn’t nurture, regularly feed, and take care of their career-related bodies. Attending the upcoming MD Expo may be just the professional and personal health spa experience that you need.”
The elite members of the HTM community will be on hand for the education, exhibit hall and networking events including the Boos & Brews Halloween Party.
Attendees won’t want to miss the finale event with the MD Expo Halloween Costume Contest. Ghosts and goblins beware, the staff boasts some scary good prizes that will be awarded for the best costumes. With great music, food and a signature cocktail it would be ghoulish to miss this party!
Register today at MDExpoShow.com.
FSI Services has partnered with health care management professional, Theodore Pappas, to help health care systems re-evaluate their space management approach from a cost-savings and software perspective.
“Space is the largest, most valuable asset within a health care organization,” said Pappas, healthcare facility information specialist, of FSI. “All departments are impacted, from design and construction, finance, compliance and safety, real estate, and property management. Within those teams, I see space managed at a very basic level within hospitals. What they need is the right tools to make educated, cost-effective decisions before jumping to – let’s build, expand, buy.”
In the past, hospitals relied on a physical pen and paper process – large scale paper plots in tubes, binders, disks with files. The industry has since evolved, with several cloud-based solutions that allow technicians to access information on their phone, tablet or computer – regardless of their location.
“Ted’s experience was exactly what we needed to elevate our space management product – CMS Space Manager – in the market,” explained Chris Lang, director of strategic business development at FSI. “We’re hearing more from customers and at industry events that hospital staff are expected to do more with less. Taking advantage of a comprehensive tool that reduces manual tasks and saves money is how teams solve for this expectation.”
With Space Manager, benefits include:
• Reduce overspend on materials: Users can import polylined CAD drawings and instantly calculate square
footage to help accurately estimate remodeling costs.
• 100% regulatory reimbursement: Easily maintain accurate rooms, floors, and utilization square footage calculations to ensure 100% Medicare and Medicaid reimbursement.
• Eliminate manual square footage calculations: With a few mouse clicks, calculate square feet.
• Maintain a centralized database with every building’s CAD drawings in one place.
Vizzia Technologies has announced new technology partnerships to expand its portfolio of digital solutions for smart, connected hospitals.
“Vizzia is proud to collaborate with these world-class technology leaders,” said Dave Wiedman, chief commercial officer of Vizzia Technologies. “We are committed to delivering innovative solutions that improve healthcare efficiency and patient care.”

The new technology partners are:
• Zebra Technologies offers health care-grade hardware solutions that are used by 14 of the top 20 hospitals on the U.S. News & World Report 2022-2023 “Best Hospitals” honor roll. Vizzia has joined the Zebra PartnerConnect program and added its Bluetooth Low Energy (BLE) devices.
• ElectrifAi is a global leader in machine learning (ML) and artificial intelligence (AI) and its Inventory Optimization module generates predictive PAR levels of critical
hospital equipment. Vizzia has piloted ElectrifAi at a 500-bed hospital, which yielded a 52% improvement in key metrics.
• Sonicu is an IoT provider of critical environmental monitoring solutions, trusted by more than 500 health care customers in all 50 states to meet CDC and Joint Commission compliance. Vizzia has verified the Sonicu technology at the Vizzia IoT Lab onsite at the University of New Mexico.
• HCI provides digital patient engagement solutions to transform the patient experience and streamline caregiver workflows for more than 900 hospitals and 1,500 clinics in all 50 states. Vizzia clients have requested integration with patient communication systems to include Digital Whiteboards.
Meet Vizzia executives and partners at HIMSS Georgia annual conference in Atlanta (Oct. 3) and MD Expo Fall conference in Orlando (Oct. 29-30).
Radiological Service Training Institute (RSTI) recently celebrated a record-breaking enrollment milestone! It is said that a picture is worth a thousand words and the video crew, photographers and drone footage captured that and more.

Surpassing enrollment numbers since their doors opened almost 40 years ago, was a special moment to capture
“We appreciate the opportunity to support the training needs of the imaging service industry,” RSTI CEO Todd

Boyland, CRES, CPSM, said. “It was equally as fun getting to share this with the RSTI team and the group of students in classes that week too.”
Since 1985, RSTI has provided diagnostic imaging training. Offering a wide selection of over 60 courses each year, RSTI has trained more than 15,000 service professionals from over 50 countries in radiology, mammography, MR, CT, ultrasound, networking, PACS and DICOM.
626 recently acquired Custom X-Ray Services in Arizona, according to a press release.
“We are ecstatic to welcome Custom X-Ray to the 626 family,” shared Philip Revien, chief executive officer of 626. “Our longstanding strategy of acquiring respected, founder-run brands that are family-oriented, profitable, growth-oriented companies in markets where 626 needs deeper FSE coverage and deeper-rooted customer relationships has always served us well.”
“We endeavored to strengthen our presence in Arizona; and see Custom X-Ray as the perfect fit to immediately improve our service delivery to 626’s and Custom X-Ray’s current and future customers,” he added.
Established in 1968, Custom X-Ray is a veteran- and woman-owned legacy business that provides depend -
able, knowledgeable and dedicated customer service. Custom X-Ray offers a variety of analog and digital medical imaging technologies to its customers and represents the leading manufacturers of medical imaging systems.
“My parents founded this company to provide the highest quality products and services,” shared Shawna Wiertzema, chief operating officer for Custom X-Ray.
“We firmly believe that service, not the product, should be considered the most important factor when selecting an imaging equipment sales and service company to handle critical applications and project planning. Both the team behind the service and our focus on the customer have enabled Custom X-Ray’s sustained growth and lasting customer relationships,” she added.
Rigel Medical has invested further in the expansion and growth of its U.S. operations with a senior appointment.
Lewis Lennard has joined the U.S. operation in Tampa, Florida, as a product manager. Bringing eight years’ experience to the role, he will be responsible for managing the medical product portfolio and supporting brand sales across North America.

He will also be involved in analyzing market trends and identifying opportunities to support new business development as well as monitoring and reporting on market behaviors and trends to inform strategic planning and marketing.
The move by Lennard, who was previously an application engineer with Rigel Medical based in the United Kingdom, comes at a time when the market for electrical safety test products in North America continues to show huge potential for growth, according to a news release.
“Rigel Medical is fast building a reputation and presence in the industry at a time when the market is rapidly growing,” Lennard said. “I look forward to working alongside a team focused on providing high-quality products, unsurpassed customer service and innovation that adapts and expands to meet market standards.”
Seaward is part of Metrawatt’s GMC Instrumentation Group, a collaboration of separate specialist test equipment manufacturing businesses based in Germany, Switzerland and the USA.
“I very much look forward to working with Lewis as we continue to strengthen our U.S. team. He has excelled throughout his time in Seaward, going above and beyond in many areas,” Sean Daley, vice president and chief operating officer at Seaward USA, said. “The growth opportunities in the North American marketplace align with his ambition and will add further success to Rigel Medical’s growth in the territory.”
Getinge has announced clearance from the U.S. FDA for Servo-air Lite, a wall gas independent non-invasive mechanical ventilator.
“We are happy to broaden our ventilator product offering for the U.S. market,” says Elin Frostehav, president acute care therapies at Getinge. “This significantly increases our addressable ventilation market in the U.S., by now being able to target the non-invasive hospital segment with our ventilation offering.”
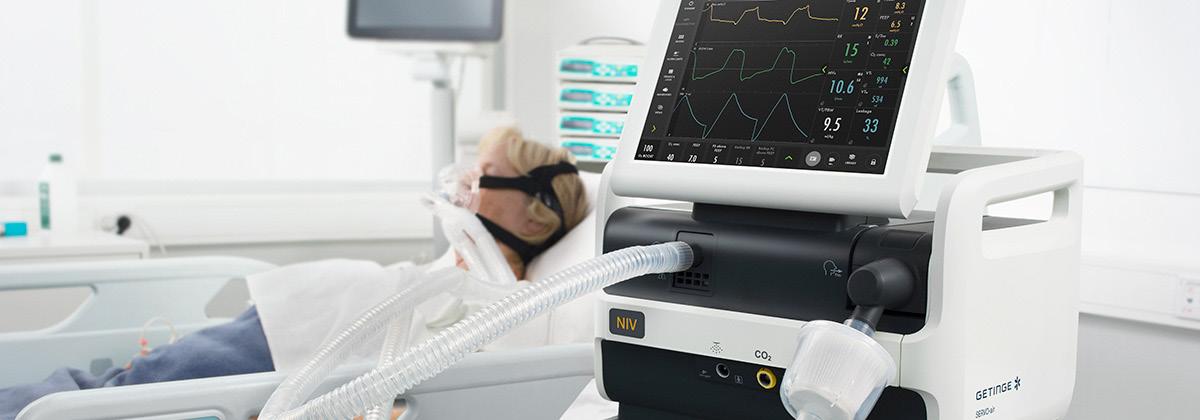
Servo-air Lite is Getinge’s turbine-driven ventilator for

non-invasive ventilation. Like all Servo ventilators, it offers ICU-quality ventilation but is more geared towards spontaneously breathing patients in need of extra breathing support. With its powerful turbine and long-lasting battery backup power, it can also be operated independent of wall gas and is suitable for intrahospital transports. It features embedded workflows, support for high flow therapy, CO2 monitoring option and tools to support escalation of therapy if needed.
The product is expected to be available for customers in the U.S. from this month.
• REPAIR AND PM SERVICE
• HIGHLY TRAINED AND CERTIFIED TECHNICIANS
• OEM NEW AND RECERTIFIED PARTS

• QUICK TURNAROUND TIMES
• COMPREHENSIVE PARTS AND LABOR WARRANTY
MAKE CARDIOTRONIX YOUR NEXT SERVICE CALL (855)-4DEFIBS IF IT’S MEDICAL EQUIPMENT RELATED, WE HAVE YOUR SOLUTION!




CARDIOTRONIXHEALTH.COM

Located in Orange County, California, Medical Equipment Doctor (MED) specializes in the sale, purchase, rental, and repair of refurbished medical devices and instruments. In 2017, industry veteran and the Doctor himself, Albert Negron, set out on his own to start a company in his garage with nothing but one plastic folding table, two Home Depot orange buckets, and 10 years of medical device sales experience. He made a logo, applied his expertise and with that, Operation: MED was underway.
Today, gone are the times wherein going to work involves pressing a clicker to reveal orange buckets hiding behind a garage door. In fact, the company augmented in a second unit within its HQ building in Tustin, California, earlier this year.
The most exciting facet of MED’s expansion is an in-house endoscope and ultrasound probes repair depot, which celebrated its launch in April 2023. At the hands of industry-renowned technicians, several with notably over 20 years of repair experience, Medical Equipment Doctor will sell and repair both large and small scopes, not to forget your rigid models as well.
In all domestic hospitals, GI labs, and surgery centers alike, MED knows how vital functioning endoscopes are to not only facilities’ revenue, but to their patients moreover. Because of this, they are dedicated to making sure when equipment, you don’t have to miss a step. When you send in for a repair, Medical Equipment Doctor provides you with an interim loaner device, offering same-day delivery or next-day shipping for centers outside of the local region.
In conjunction with its competitive pricing, MED’s endoscope repair depot offers free evaluations and some of the quickest return times in the industry. Above all, they do everything they can to ensure you don’t have to halt any procedures at the hands of broken equipment.
In the five short years since setting up shop, Albert has instituted an eminent company founded on a basis that centralizes culture, growth and community. MED’s holistic scope of service, from rental to repair, is dedicated to helping hospitals and surgery centers sustain and fulfill their working needs. With the Doctor’s help, clinicians are able to stay fully equipped so they can continue to provide the best patient care possible.
“Our focus at Medical Equipment Doctor is to partner with you to keep your budget and costs in check through providing top-level pre-owned equipment sales, service and rental, for all your medical equipment needs. This has our company being recognized nationally as the affordable solution to purchasing new. Over the years we have had the good fortune to get to know many of you in the health care and medical equipment industry,” Albert said. “It has been a true pleasure. “
“The name of our company came from people referring to us at trade shows and gatherings as their; ‘Medical Equipment Doctor’ so we carried it forward into our name and brand,” he added. “We are passionate about what we do and always keep the safety and comfort of the patients that use our equipment at the forefront of every step we take and the care with which we approach it. Let’s connect and discuss your needs and how we can partner up with you on all your medical equipment needs.”
Included in the different kinds of equipment MED services are:
• Patient monitoring
• Infusion pumps
• Surgical equipment
• Respiratory equipment
• Cables
• Imaging equipment
As we approach the back half of the year, Medical Equipment Doctor is excited to announce that the company is currently on track to have its ISO 13485 certification by the end of 2023. Each month, the MED team members continue to surpass their goals and reach new heights. The aim remains to serve the community to the highest of their ability. One thing that sets them apart from all of the others is their dedication to customer service. All salesmen, technicians, and everything in between are all under the same roof, working tirelessly to ensure the best quality and the quickest turnaround times possible for biomedical equipment.
Above all, Medical Equipment Doctor cares. Though not working directly with the patients themselves, the main driver in being motivated for success is the role they get to play in helping people. Healthcare takes a village, and from the mouth of the Doctor and former Home-Depot-bucket-in-garage owner himself, “You take care of the patient, we’ll take care of the equipment!”






















 CRAIG HARMON PRINCIPAL/FOUNDER
CRAIG HARMON PRINCIPAL/FOUNDER
Q: WHAT PRODUCT, SERVICE, OR SOLUTIONS DOES YOUR COMPANY PROVIDE TO THE INDUSTRY?
Products: New and ReCertified Defibrillators and AED’s, Ventilators, CPR assist devices, and IV Therapy devices
Services: Single-Source bundled services, On-Site Maintenance, Equipment “refresh” programs, service all brands, legacy equipment supported
Q: TELL US WHAT DIFFERENTIATES YOUR COMPANY FROM THE COMPETITION?
Without a doubt, it is our commitment to customer service deliverables. We are not a break/fix organization, we are a relationship driven company. We create cost effective solutions that deliver long term financial results.
For more information, visit cardiotronixhealth.com.
Q: WHAT PRODUCT, SERVICE, OR SOLUTIONS DOES YOUR COMPANY PROVIDE TO THE INDUSTRY?
We are a global provider of pre-owned endoscopy equipment focused on Olympus, Fuji, and Pentax scopes and towers, as well as Stryker and Karl Storz. By working together, we can help find value in your excess equipment or older technology as part of an overall upgrade strategy.
Q: TELL US WHAT DIFFERENTIATES YOUR COMPANY FROM THE COMPETITION?
We take a collaborative approach with the goal of long-term relationships by offering quality equipment and creative solutions, beyond the usual buy-sell model.
For more information, visit cmsscopes.com.














Any builder can attest to the fact that a strong foundation is needed for a sturdy house. Neglect the groundwork, and the walls and roof will never hold. Everything is bound to come tumbling down. For the members of the AAMI Medical Device Security Working Group, some form of this maxim must have been top of mind. The group recently developed a new standard on security risk management for device manufacturers – ANSI/AAMI SW96 – building it on the cement and steel already laid down by two technical information reports (TIRs) on the same subject.
ANSI/AAMI SW96:2023, Standard for medical device security—Security risk management for device manufacturers, is the first consensus standard to provide specific requirements for managing security across a product’s entire life cycle. The document, released earlier this year, is based on guidance previously published in AAMI’s TIR57:2016 and TIR97:2019.
• AAMI TIR57:2016/(R)2023; Principles for medical device security—Risk management, is primarily focused on addressing security risk during product design.
• AAMI TIR97:2019/(R)2023; Principles for medical device security—Postmarket risk management for device manufacturers, offers guidance related to managing security risk during and after product production.
The two TIRs are meant to be used together and within the risk management framework defined by the
International Organization for Standardization’s global standard, ISO 14971:2019.
The publication of ANSI/AAMI SW96:2023 comes at a time when managing medical device security risks is becoming more difficult by the day. A study from 2022 in The Journal of the American Medical Association found that ransomware attacks against health care organizations more than doubled between 2016 and 2021, for example. And a 2023 cybersecurity report by the U.S. Department of Health and Human Service’s Office of Information Security noted that health care data breaches “have consistently trended upward” over the past decade. Cybercriminals, the HHS reports, “are continuously seeking to make their attacks more resilient, more disruptive and harder to counter.”
The issue of cybersecurity has become so critical that the 2023 “omnibus” bill signed into law by President Joe Biden includes a section on “Ensuring Cybersecurity of Medical Devices” that amends product submission requirements spelled out in the Federal Food, Drug and Cosmetic Act. Responding to the amendment this March, the FDA published new guidance on medical device security that advises manufacturers to submit “a plan to monitor, identify and address … postmarket cybersecurity vulnerabilities and exploits” and to “design, develop and maintain processes and procedures to provide a reasonable assurance that the device and related systems are cybersecure.”
While SW96 was developed before the FDA guidance came out, the standard provides device manufacturers with a roadmap to comply with its recommendations. Postmarket monitoring of device vulnerabilities and exploits, for example, is among the topics addressed in SW96, as are important cybersecurity measures like patching and creating a software bill of materials (SBOM).
In a summary of the standard and its implications
written by four industry leaders who played major roles in its development (Charles S. Farlow, et al., Biomedical Instrumentation & Technology), the authors say SW96 strengthens security risk management by focusing on several “elements” that are key to the risk management process. Summarizing the group’s research, Charles Farlow stated, “SW96 advances the state of the art and provides a set of well-documented requirements for security risk management.” One section of the standard, for example, is all about security risk analysis, while another covers the evaluation of “overall security residual risk acceptability.” Under “General Requirements for Security Risk Management,” it explains what manufacturers need to include in their security risk management plans. And within clause 10, “Production and Post-production Activities,” it specifies that device makers must establish “a process for identifying and managing security incidents.”
The authors of the BI&T analysis also point to the

standard’s supporting annexes and explain how they were developed to expand on topics “of particular interest to practitioners in the security risk management field.” The annexes include detailed information on everything from working with third-party service organizations to best practices in threat modeling.
The BI&T authors note that TIR57 and TIR97 “have been widely successful” as resources for industry stakeholders, but that TIRs are limited by the simple fact of their status as TIRs rather than standards. These guidance documents are reviews of technical issues and statements of expert opinion, but because they are TIRs, they do not include any requirements that medical device manufacturers must follow.
Now, with ANSI/AAMI SW96:2023, device makers have a playbook they can use to stay ahead of existing and emerging cyberthreats while adhering to federal guidance on the subject. For years, manufacturers had their foundation, but now they finally have a house built to last.

Founder and CEO Matthew Bassuk explains that his company’s name “NVRT Labs” is pronounced “Invert Labs.” It is a company on the cutting-edge of virtual reality training for healthcare technology management (HTM) professionals.
TechNation found out more about the company in a recent question-and-answer session with Bassuk.
Q: WHAT ARE SOME OF THE SERVICES AND PRODUCTS YOU OFFER? IS THERE A SPECIFIC OR NEW ONE YOU ARE EXCITED ABOUT RIGHT NOW?
BASSUK : We just launched our VR training platform for HTM professionals as of July. We couldn’t be more excited to be the first and only extended reality company to bring virtual reality training to our industry. At a time when technicians everywhere are being required to have more and more skill sets yet training budgets are constantly being tightened, we are thrilled to be offering a truly innovative solution to close the skill gap and save organizations training dollars. Our platform launched with an already amazing library of procedural modules, and we are continuing to add more and more BMET content monthly on everything from defibrillators and fluid warmers to imaging and ultrasound, etc.
Q: HOW DOES YOUR COMPANY STAND OUT?
BASSUK : I think NVRT Labs is starting to be noticed in the industry for the focus we have on future technologies in the HTM space. Truthfully, we are the ones who are doing the really cool and cutting-edge
development that this industry has been craving for years. Whether it’s our VR training content, our AR design services, or the AI projects we are working on, we think about technology from an HTM industry perspective first and that should stand out. I think applying emerging technologies specifically to the medical device repair space is filling a void that’s been open for too long. Hopefully that continues to turn some heads.
BASSUK : In the short-term we are continuing to ramp up our platform adoption and add content as fast as physically possible. Our internal goal is to get a VR headset to every BMET student and into every biomed shop in the country. Thinking about the future, I envision that we leave no stone unturned with regards to technology driven products. We want NVRT Labs to be synonymous not only with extended realities and VR training but with anything related to paradigm-shifting technology in the HTM space.
BASSUK : I am beyond excited for us to be launching our journey in the TechNation community. We have been part of this audience for a long time, attending all the MD Expos and events, and working with amazing organizations and professionals who are also a part of this community. I primarily want the readers to know that we are a resource that is cut from the same cloth. Should anyone in this space have a technology idea or need, our lines are always open for collaborations and partnerships.
For more information, visit nvrtlabs.com and check out the content library.


Amid increasing electricity prices and increased emphasis on sustainability, health care organizations are looking for ways to reduce their energy consumption. Medical imaging departments are among the larger energy consumers in health care, so substantially reducing power consumption in these areas could result in considerable cost savings.
Health care organizations are large consumers of energy, making them particularly vulnerable to energy supply shortages and price increases, as experienced in recent years. In addition to affecting the financial health of the institution, high energy usage also has a societal impact, making it harder for organizations or communities to meet carbon neutrality goals. Facilities therefore have clear incentives to scrutinize their electricity usage.
“Historically, hospitals have focused on patient care and high-quality service regardless of the energy toll,” notes Francisco Rodriguez-Campos, MSc, Ph.D., MRSO (MRSC), a principal project officer in ECRI’s Device Evaluation group. “But today, we’re seeing interest in finding ways to reduce energy use without compromising care. The imaging suite is a good place to start.”
A common target for energy-saving improvements is one of the bigger consumers of electricity within a health care facility: the imaging suite. CT and MR imaging scanners in particular require a great deal of electricity.
In addition to the energy demand during scans, a large amount of energy is required to keep these systems cool, even when they are not in active use.
“The climatization requirements of the devices in the imaging suite are a key driver for high energy use,” explains Rodriguez-Campos.
Most imaging systems have dedicated water cooling systems that run independently of the institution’s climate control, while some now use air cooling systems that use the air in the imaging suite for cooling, placing demand on the facility’s heating, ventilating and air conditioning (HVAC) systems.
To help organizations save money and work toward sustainability goals, ECRI summarizes below research showing the amount of energy used by different imaging systems, then offers tips for reducing energy consumption.
In a 2015 study, researchers directly monitored the energy usage of three CT scanners, four MR scanners, and their cooling systems at a health care facility in Switzerland for one year (Heye et al. 2020). The total energy consumption of the imaging and cooling systems represented 4% of the facility’s total yearly consumption. Of the energy used for these systems, 44% was to power cooling systems and 56% was for patient imaging exams.
The three CT scanners used 78,679 kWh over the course of the year. Energy usage was broken down into categories based on periods of use: (1) Active periods (includes patient preparation, planning, net scan and reconstruction time): 23,797 kWh. The net scan portion (actual patient scan time during which energy consumption increases) was 10,741 kWh. And (2) Idle periods (time between patients): 42,867 kWh (about four times the amount of power used for scanning patients). During the remaining time, the scanners were off,
though ancillary systems continued to consume power.
The four MR scanners – one 3.0-tesla (T) system and three 1.5 T systems – used 467,348 kWh in one year, including 265,334 kWh for active periods and 36,268 kWh for idle periods. In addition, more than a third of the power consumption of the MR scanners was during the off state. Unlike CT scanners, which aren’t associated with significant amounts of energy use when shut down, MR scanners require constant magnet cooling even when the system is off. For CT scanners, nearly two-thirds of electricity was used during idle time, which includes the demand of adjunct cooling systems, and only one-third of power consumption was used during actual imaging exams.
Note that the scanners in this 2015 study did not have certain power-saving technology that is more widely available today. For example, today’s users can take advantage of newer off/standby states that reduce the use of electricity. Also, new technology is downsizing the magnet cooling systems in MR scanners to help reduce electricity usage. MR scanners’ use of electricity is largely driven by the helium cooling heads. Plus, there’s room for new developments. Rodriguez-Campos notes that “novel device designs could be devised to, for example, generate
electricity from the moving parts within an imaging device, like the internal tube and detector that keep spinning after a CT scanner exposure.”
Heye T, Knoerl R, Wehrle T, et al. The energy consumption of radiology: energy- and cost-saving opportunities for CT and MRI operation. Radiology. 2020;295(3):593-605. doi:10.1148/radiol.2020192084
Furlan L, Di Francesco P, Tobaldini E, et al. The environmental cost of unwarranted variation in the use of magnetic resonance imaging and computed tomography scans. Eur J Intern Med. 2023;111:47-53. doi:10.1016/j.ejim.2023.01.016
COCIR. COCIR Self-Regulatory Initiative for the Ecodesign of Medical Equipment. Status Report 2018. COCIR European Coordination Committee of Radiological, Electromedical and Healthcare IT Industry. COCIR Self-Regulatory Initiative for the Ecodesign of Medical Equipment Steering Committee; June 2018. Accessed May 23, 2023. https://www.cocir.org/ fileadmin/6_Initiatives_SRI/SRI_Status_Report/ COCIR_SRI_Status_Report_2018_-_June_2019.pdf
1. For CT scanners, use optimized schedulers (orchestrators) to maximize time spent performing exams and minimize idle periods.
2. For MR scanners, explore the possibility of using waste heat recovery methods that recycle the heat produced by the system rather than spending additional energy to neutralize the excess heat.
3. Explore the implementation of a smart cooling system that uses a scanner’s status to adjust the operation of adjunct cooling capabilities.
4. Use a modular configuration for cooling systems that allows scalability of ancillary systems instead of each newly installed scanner requiring its own system.
5. During the procurement process, request information from the vendor regarding the energy consumed during scanning as well as during the idle and system-off states.
6. Replace outdated HVAC systems in the imaging suite with more efficient equipment.
7. Monitor energy usage with energy management solutions that can provide granular, real-time information about energy usage. Some applications can give real-time consumption by specific areas of the hospital.
8. Ask vendors to modify the scanner’s defaults and other parameters to minimize energy use in idle and system-off states, which is something only manufacturers can do.
9. Ask vendors if they take any measures to reduce greenhouse gas (GHG) emissions and if they are implementing features in their equipment to help facilities achieve their GHG goals. A study by Furlan et al. published in February 2023 found that the use of CT and MR is a major contributor to GHG emissions.
10. Avoid refurbished devices, which are older and therefore may not have the highest energy efficiency; about 5% of medical imaging devices sold in 2016 were refurbished. There has been a reduction of about 20% in energy usage from 2005 to 2012 in medical devices (COCIR 2018), which indicates that manufacturers are making their devices more energy efficient, and refurbished equipment may not be able to match these improvements.



SEPT. 28, 2023 @ 2PM ET Register at webinarwednesday.live
The IPA-3100 is the most compact, feature dense single channel analyzer on the market. It is a high accuracy and easy-to-use system incorporating full touch screen control with an intuitive interface. It has built-in Bluetooth wireless technology and is myBC mobile compatible. The patented design uses a dual syringe stepper motor driven system that provides continuous monitoring of the fluid flow without the need to stop and perform intermittent drains. This provides a more realistic flow path for the infusion device under test and therefore more accurate readings.

The IPA-3100 has configurable test parameters which allows for specific test routines specified by
various manufacturers. Tests can be stored manually or automatically with up to 250 different records. These test records can be downloaded to a computer to then create reports that can be printed.
The IPA-3100’s Bluetooth wireless technology syncs to myBC mobile and allows for wireless testing and control. With the use of myBC mobile or BC Flow, multiple test configurations can be stored for quick testing. Records of each test can be created and then saved for later viewing and analysis. Reports can also be generated from test records for in-depth analysis and/or traceability.
For more information, visit bcgroupintl.com.
Medical equipment cybersecurity is the hot topic in the healthcare technology management (HTM) industry these days and with good reason. Cyber threats against health care providers have increased significantly in recent years forcing medical equipment manufacturers and those who maintain the equipment to develop products, tools and processes to mitigate the risks.
Based on discussions occurring within the government and accreditation organizations like The Joint Commission, many leaders in the HTM industry speculate that it is only a matter of time before regulatory agencies will begin to move more of their cybersecurity recommendations for hospitals from “nonbinding” to regulated requirements.
“The HTM community has known for years that it’s necessary for hospitals to protect their clinical equipment from hackers and malware, but there is general agreement among those that follow the topic that we will soon see more suggestions and standards become regulations,” says Barbara Maguire, vice president of quality and healthcare technology management at ISS Solutions Inc.
While many in the HTM industry feel overwhelmed by this prospect, ignoring the issue will not make it go away. Fortunately, developing an effective medical equipment cybersecurity program can be accomplished with a tool you should already have – your computerized maintenance management software (CMMS).
“The first and maybe most important step in setting up an effective medical equipment cybersecurity program is to make sure all the medical equipment your hospital owns or leases long-term is inventoried in your CMMS, especially if it is networked,” Maguire says. “That includes often forgotten devices in your
medical offices and ambulatory care centers.”
Along with a complete and accurate inventory, hospitals should capture pertinent security information for each device so that when vulnerabilities or alerts are found, affected devices can quickly be identified. This includes whether the device is networked, its IP and MAC address, its ePHI capabilities, and its operating system.
Emphasizing this point, the U.S. Federal Bureau of Investigation (FBI) conducted its own research into how hospitals should protect their medical equipment and released a report last year that listed a number of cybersecurity recommendations including:
• Maintain an electronic inventory management system for all medical devices and associated software, including vendor-developed software components, operating systems, version and model numbers.
• Use inventory results to identify critical medical devices, operational properties and maintenance timeframes.
• Consider replacement options for affected medical devices as part of purchasing process; if replacing the medical device is not feasible, take other mitigation precautions, such as isolating the device from network or auditing the device’s network activities.
Those in HTM know that it’s much easier said than done to capture all this data, and even more difficult to keep it up to date. There are different “checkpoints” in a device’s life cycle that are particularly important in collecting the pertinent data to help protect it.
• Onboarding – working with supply chain, the receiving dock, and other departments involved in equipment purchasing, all HTM departments should have a process in place to be notified when new equipment arrives and is being installed so that it can be inventoried, tagged and added to the CMMS with all required information.
• During Maintenance – anytime a device receives
maintenance – whether it’s planned maintenance (PM) or corrective – the technician should verify that the CMMS contains up-to-date information about the device, including its IT components. This data check should be part of maintenance checklists to ensure this task is not forgotten. Remember that it’s always easier to get the information when the device is in front of you versus having to go find it later when missing data is identified.
• Regular Scheduled Inventory Audits – it’s now considered a best practice to do a manual sweep of the hospital’s medical equipment at least every few years to verify the inventory and associated data is accurate. If your validation process during checkpoints one and two are being followed closely, you can limit this manual audit just to devices that have not been seen since the last audit and perform visual audits in departments to look for un-inventoried devices that slipped through the cracks.
• Disposal – an often-forgotten but critical step in a device’s life cycle is to document its proper disposal in the CMMS. This includes documenting that ePHI stored in the equipment was properly removed. No matter how important accurate data is, finding and entering the data is not always a technician’s favorite part of his/her job. Therefore, you want to make sure certain fields are required in your CMMS so that pertinent data is captured and/or verified during each of these checkpoints. Depending on which CMMS you use, you also may be able to build in workflows and checklists that automatically instruct your technicians what data they need to collect and/or validate during each checkpoint, as well as automatically fill in certain data points associated with the make/model.
“We spent a lot of time configuring our CMMS – Nuvolo – to streamline collection of this data and to develop risk criteria so we can focus our resources on the riskiest devices,” Maguire says. “This has helped us tremendously in keeping the inventory accurate.”
Once your inventory and its data are accurate, you can then begin the process of assessing the equipment’s cybersecurity risk and documenting it in your CMMS. Don’t forget about those non-networked mobile devices that store ePHI. If they go missing, you still need to report it to the Office of Inspector General (OIG) if enough patient records go missing with them.
If your CMMS is configurable, you’ll also want to build in workflows that allow you to quickly identify all devices impacted by a cybersecurity threat and
generate work orders (if mitigation steps are needed) that are assigned to the proper team(s) on each affected device.
Many hospitals also have purchased security monitoring software that not only monitors the network for cyber threats but also identifies new devices added to the network and changes in their IT components, such as an operating system upgrade. Taking that one step further, hospitals can also integrate some CMMS products with their network monitoring tool so that the information feeds directly to their CMMS, enabling them to monitor cyber threats from their CMMS and automatically issue work orders to threats with pertinent information like the type/make/model, location of the device, who supports it, and whether it’s in use. This integration also will feed information on newly discovered networked devices and changes to IT components to the CMMS, effectively keeping the data updated.
“Having visibility of a device and its details is only the first step in cyber risk reduction,” states Ty Greenhalgh, HCIPP, healthcare industry principal at Medigate by Claroty. “With adversaries increasing their attack speed, efficient operationalization to mitigate vulnerabilities at scale will only come from an integrated workflow tool like a CMMS.”
With that in mind, Greenhalgh stresses that HTM organizations wanting mature medical equipment cybersecurity programs should also be able to assess each device’s cybersecurity risk and document it in their CMMS, apply appropriate protections based on those risks and document them in their CMMS, and enable workflows in their CMMS that help orchestrate fast responses to threats.
Whether you’re a large health system or a small stand-alone hospital, all health care providers have a responsibility to protect the privacy and safety of their patients and should implement a medical equipment cybersecurity program.
Maguire agrees, “The robustness of your medical equipment cybersecurity program is dependent on the resources and risk tolerance of the organization, but doing nothing is no longer an option.”
Heidi Horn, MA, AAMIF is president of Heidi Horn HTM Consulting LLC. Horn currently serves on the AAMI Board of Directors Executive Committee as treasurer.






goaims.com
Implementing a CMMS solution for multiple departments can be a complex process that requires careful planning and coordination. Matthew Binko, training manager at Phoenix Data Systems, shared how setting clear objectives, following best practices, and anticipating potential challenges can enable organizations to maximize the benefits of a CMMS solution and improve the efficiency and reliability of its maintenance activities. Additional insights were shared during an informative question-andanswer portion of the webinar. A recording of the webinar includes the Q&A session.
Attendees provided feedback via a survey that included the question, “What was your single biggest takeaway from today’s webinar?”
“The various solutions that can be used throughout the departments,” - Sal Cruz from Cook Children’s Medical Center Director of Clinical Engineering
Watch on-demand on WebinarWednesday.live.
us.sodexo.com

Attendees were able to join Binseng Wang, ScD, CCE, vice president, program management at Sodexo HTM as he presented a series of questions to challenge their knowledge. The task of maintaining and managing medical equipment to ensure patients can receive timely and safe care has evolved in the past 40 years. During this time, many theories and practices have been created but not all of them have been validated. Some have been proven wrong, while others are not effective. The combination of the ever-increasing amount of equipment in hospitals and dwindling availability of HTM professionals is forcing everyone to re-examine their jobs to determine what is truly necessary and beneficial for patients and care providers, i.e., how can we work smarter and not harder. Wang also provided additional insights during an insightful question-and-answer session with attendees.
Attendees provided feedback via a survey that asked, “Why did you attend today’s webinar?”
“First time attending Webinar Wednesday and it was thoroughly enjoyable and a learning experience. The presenter was excellent and very knowledgeable. Will certainly attend future Webinar Wednesdays if time permits,” - Wendell Pascual from Kaiser Permanente Zion Medical Center Biomed Tech
Watch on-demand on WebinarWednesday.live.
Know Your Job: Test Your Knowledge”
“Implementing a CMMS Solution for Multiple Departments: Best Practices and Challenges”
Editor’s Note: The following article contains edited answers to accommodate space constraints. For the unedited version visit 1TechNation.com.
En doscopes are important medical devices that come in a variety of models for many different procedures. TechNation invited experts to share their knowledge regarding these devices in this month’s Roundtable article. Sharing their insights are: F UJIFILM Healthcare Americas Corporation Executive Director of Endoscopy Product & Marketing Anthony Borrelli; Olympus Corporation of the Americas Executive Director of Marketing for Endoscopy Philip Doyle; Endo Technologies CEO Spencer Johnston; Medical Equipment Doctor’s Damon Kelley; Agiliti Director of Customer Training and Education Michael Matthews, MBA, CLSSGB; and Healthmark Industries Clinical Education Specialist John Whelan, BSN, RN.
BORRELLI: Important and recent advancements in the fields of endoscopy and endosurgery surround products that serve dual purposes and allow clinicians to perform a greater scope of procedures with the same technology. Two examples of Fujifilm empowering clinicians to achieve more with less include dual-channel endoscopes and the LUXEO Surgical System with ELUXEO Vision.
DOYLE: This year has brought exciting innovations to the U.S. market for GI endoscopy. Offering improved visualization of gastrointestinal bleeding and anatomical structure, Olympus’ new EVIS X1 Endoscopy System received regulatory clearance and is being prepared for launch this year, along with two compatible endoscopes: the GIF-1100, a videoscope for use in the upper digestive tract, and the CF-HQ1100DL/I, a colonovideoscope for use in the lower digestive tract.
JOHNSTON: With the upcoming launch of the new Olympus system, AI has taken the forefront of advancements in the industry. This new development will assist doctors in locating polyps and will lead to better care for the patients. Along with AI, we are seeing a decrease in physical logs and an uptick in the amount of computer screens in the reprocessing space. These screens are giving step-by-step instructions throughout the entire process, which helps less trained techs perform at a higher level.
KELLEY: Continued advancement in video endoscopy CCD chips and white light cancer detection.
MATTHEWS: Many industries are evaluating AI to determine if there are new opportunities or efficiencies that can be gained through automation – and flexible endoscopy is no exception. Several companies in the sterile processing field are already developing AI that can assist reprocessing staff in identifying early warning signs of damage as part of a borescope inspection of the internal channels of the endoscope. This will enable technicians to identify when scopes are damaged or inadequately cleaned – without requiring extensive time or training.
WHELAN: Ongoing concerns related to ineffective processing and cross contamination with flexible endoscopes have led to increased development of completely disposable endoscopes as well as duodenoscopes with disposable endcaps. Clinical research, FDA MAUDE database reports, and national standards/guidelines have increased focus on especially the need to carefully inspect reusable flexible endoscopes (best practice is with lighted magnification); and the need to actively dry endoscope channels –even after AER processing. Because of that, there has been an increased use of portable channel drying units as well as dedicated channel drying cabinets.
BORRELLI: Before getting endoscopy equipment serviced or repaired, users should first contact their scope manufacturer or service provider and inform them of the specific damage and/or reason for return. After that, and before sending the equipment for service or repair, the endoscope must be reprocessed per the manufacturer’s recommendations to prevent subsequent handling of a contaminated instrument. Standard manufacturer’s reprocessing instructions should be applied to an endoscope which is fully intact and has no leaks. However, if a breach has been detected in the watertight integrity of the endoscope, depending upon
the characterizations of the leak (size, location, etc.), special reprocessing recommendations may be provided by the endoscope manufacturer, which may include scope pressurization during fluid immersion. In certain cases, a leak may be so large that standard attempts to pressurize an endoscope during reprocessing to eliminate fluid invasion may not be possible. It’s also important to check with each manufacturer of an automated endoscope reprocessor (AER), if one is utilized, to confirm their specific instructions and product claims for the automated reprocessing of an endoscope with a leak.
DOYLE: First and foremost, prior to handling an endoscope, make sure to wear personal protective equipment (PPE): Put on gloves, a lab coat and any other necessary protective gear to prevent direct contact with potentially infectious material. Request documentation from the parties responsible for the point of use/ precleaning, leakage testing, manual cleaning and high-level disinfection or sterilization processes to ensure that they have been performed. This documentation should include the date, time, personnel involved, cleaning and disinfection agents used, and any observed issues or abnormalities. I encourage our customers to take full advantage of the training we, as the original equipment manufacturer (OEM), offer. We have dedicated staff that offer onsite education and equipment uptime support focused on repair reduction and cleaning, disinfection and sterilization to assist with infection prevention.
JOHNSTON: The precleaning and leak test stages are crucially important to increase the life span of the scopes. Having an expert reprocessing tech is oftentimes the difference in a facility that can maintain their scopes versus a facility that wears their scopes out quickly. Lastly, communication is one of the biggest and easiest fixes we can offer. By offering specifics on the repairs and what the staff is having trouble with can point our techs in the right direction so that we can tailor the repair to the need of the doctor or staff.
KELLEY: Much depends on the damage the scope has, however, whenever possible the scope should be fully sterilized. Except when you have a confirmed leak in the scope, doing so would only further damage it. In this case carefully wiping down the scope and cleaning the best you can is necessary while using a red bag when shipping to signify that it is not completely clean.
MATTHEWS: First, clinical engineers should make sure they are following Department of Transportation regulations related to shipping contaminated endoscopes. Second, they should also be proactive in working and communicating with reprocessing staff to
understand the stages that an endoscope must go through prior to patient use. This will enable clinical engineers to understand where there are opportunities for cross-contamination risks when an endoscope is damaged.
WHELAN: Standards/guidelines have directed for cleaning verification testing (after manual cleaning and before the AER) – for all “high risk endoscopes.” It would be prudent to make sure any endoscope going out for or returning from repair undergoes such testing. Additionally, borescopic examination of exterior surfaces and accessible channels allows identification of damage as well as residual bioburden.
Q: HOW CAN BIOMEDS HELP LOWER THE RISK OF PATIENT INFECTION WHEN IT COMES TO ENDOSCOPES?
BORRELLI: Biomedical engineering can help reduce potential patient infection risks associated with endoscopes by ensuring the proper maintenance of all equipment involved with endoscope reprocessing including but not limited to endoscopes, automated endoscope reprocessors (AERs) and their accessories/ channel adapters, channel flushing aids, cleaning brushes, etc. In addition, biomeds can help to ensure that all related scope-specific reprocessing instructions and validated reprocessing equipment instructions are readily available for staff. Staff should be trained not only on the intricacies of endoscope reprocessing and infection control practices, but also on identifying scope irregularities, signs of damage and problems with scope functionality as such abnormalities can impact reprocessing outcomes. When available, quick reference guides or reprocessing wall charts should be posted to support staff adherence to manufacturers’ instructions.
DOYLE: Biomeds should conduct regular maintenance and inspection of endoscope reprocessing equipment, such as automated endoscope reprocessors (AERs) and drying cabinets. This equipment is intended to properly and effectively eliminate residual contamination. Biomeds should also perform routine checks, calibration and repairs of equipment to maintain optimal
performance. They can also monitor AERs’ performance, water quality and documentation compliance to identify any deviations or issues.
JOHNSTON: Moving from reusable to disposable buttons and tubing will go a long way in minimizing the risk of bioburdens. Aside from that, using proper procedures when cleaning/brushing the scopes will ensure that bioburdens never gain a foothold in the first place. We see scopes come in with crud and crust built up for months on the distal end, and the staff never noticed at the facility. We work to get the scopes back to a safe level by replacing these parts and providing training to the staff on what to look for and how to prevent bioburdens.
KELLEY: Always follow OEM instructions for the cleaning and sterilizing of all endoscopes.
MATTHEWS: A proactive and deliberate preventive maintenance inspection process is important to finding endoscopes that are potential sources of infection risks to patients. In fact, there have been several studies related to flexible endoscope infections that have demonstrated that a common root cause of infections is endoscope damage. Endoscopes can also be damaged in ways that allow them to function during a procedure – but they can no longer be cleaned effectively. A consistent inspection process can help capture some of these “unseen” factors where infection risk can unknowingly be passed along to patients.
WHELAN: Delivering consistent, ongoing quality control for any/all automated processing equipment – i.e., inspections, PMs, repairs – and complete documentation of same. Additionally, following manufacturers’ IFUs for filter changes (e.g., pre-AER, AER, storage cabinets), help ensure that processes are safe and effective. One of the newer standards recommendations is focused on making sure leak testers (handheld or automated) are delivering the correct PSI. Note that one of the most common endoscope leak testers still in use does not provide such QC on its own – supplemental testing is required.






BORRELLI: Assuming healthcare delivery organizations have evaluated the clinical impact of new technology and have confirmed it will 1) provide better care, 2) improve efficiency, 3) support financial objectives, 4) lower maintenance costs, and 5) potentially attract new patients, then it’s time to evaluate the following factors to determine the TCO and ROI of the equipment. Deciding whether to lease or purchase your endoscopy equipment can have an impact on your TCO. When it comes to leasing equipment, one might face lower up-front costs, more frequent opportunities to upgrade technology, and lower maintenance costs, but they will also likely pay more overtime as they won’t gain equity or have any ROI down the road. When purchasing equipment, you tend to face lower long-term costs. Equipment ownership can increase the value of your practice, particularly when you eventually sell the equipment.
Health care organizations need to evaluate what kind of service program the technology provider offers; is it all-encompassing, capitated all-encompassing, or is it “cost-per-service?” Be sure to fully understand the possibilities and limitations of your service program to have greater confidence in your future service budget and support.
TCO and ROI will be highly impacted by the quality, durability and longevity of your equipment. To maximize the life of your equipment, it’s important to partner with and purchase from technology manufacturers who prioritize the importance of training and scope handling, and who offer this training on a routine and ongoing basis.
DOYLE: Olympus’ first priority is patient care and safety; however, we recognize that finances do play a role in building your endoscopy portfolio. Total cost of ownership (TCO) is an analysis that places a single value on the complete life cycle of a capital purchase. Factors that are considered in this analysis include: capital cost; labor cost; reprocessing cost; supplies and accessories; and service cost.
JOHNSTON: Olympus recommends that each scope only get around 300 uses per scope per year. We like to think that’s like an oil change number in the sense that you could likely push them a little more, but it’s a great equation for a facility to determine how many scopes they should have by dividing total amount of colon cases versus amount of colon scopes. I highly recommend checking in frequently with us to determine when the best time to buy is or what model to purchase. If we
have some notice, we can create a plan with the facility to upgrade on their timeline, while allowing them to budget over the long term.
MATTHEWS: Flexible endoscopes are increasingly complex and delicate, which can lead to increasingly expensive repairs. One of the best ways to decrease your total cost of ownership and increase ROI is by reducing unnecessary repairs over the life of the scope. In general, catching damage early tends to result in less expensive repairs versus waiting until the scope no longer functions. Repair vendors should provide tools to biomedical and reprocessing technicians to help them to identify early warning signs of damage and minimize unnecessary repair costs. These vendors should also provide reporting to monitor repair consumption and offer education to minimize common gaps in the care and handling of endoscopes that can contribute to additional costly damage to scopes.
WHELAN: This is multifactorial; and includes considerations for number and type of endoscopic procedures, inventory required and related costs, methods for processing (HLD versus sterilization) and their related costs, and estimated repair frequency and dollar amounts. Consideration should also be given to owning versus leasing scopes. The older a scope is, the more likely it is to have damage; and this is a patient risk.
Q: WHAT ELSE DO YOU THINK TECHNATION READERS NEED TO KNOW ABOUT PURCHASING AND SERVICING ENDOSCOPES?
BORRELLI: I believe the most important thing TechNation readers should keep in mind is that quality of “purchasing” and “servicing” is highly driven by their vendor selection. When selecting their technology provider(s), they should seek a true partnership versus a transactional relationship. They should seek a responsive partner that drives continuous technological innovation, creates training opportunities, and provides clinical support on an ongoing basis. When it comes to investing in technology, they should ensure that the product portfolio is a complete package, combining visualization/image clarity, ergonomics/control, access, in addition to quality service packages.
DOYLE: Health care facilities must consider a wide range of factors when purchasing and servicing endoscopes. How many scopes do we need to maintain the timely flow of patients through our department?
What mix of different types and sizes of upper and lower scopes, plus specialty scopes, will we need to handle the variety of anatomical and clinical needs that we may see? Which scopes have a reputation for durability and
also offer the technical performance and post-sales support our facility needs to be successful? How can OEM service help us keep our lab up and running through loaners, quick turnaround times, and original manufacturer parts and expert knowledge, and how can the OEM support our staff to reduce service costs through education about proper care and handling of these valuable assets?
JOHNSTON: Some facilities celebrate not having to send their scopes in for repair for a year or more. I would argue that periodic service to your scopes is paramount to lowering cross contamination risks. The glue bands oxidize over time, creating pockets where bodily fluids get in, but don’t necessarily come out during reprocessing. We keep track of your repairs and offer pattern recognition behind the scenes to offer training to the staff. The service contract for your equipment should be a partnership between your staff and mine, and we should communicate accordingly. Quotes are free to gauge the market pricing for a wide variety of scopes, so please don’t hesitate to reach out.
KELLEY: To reduce any loss of revenue during an inevitable equipment repair downtime, consider



purchasing devices for backup procedures. Often you can obtain a slightly older model at tremendous cost savings that operates the same as your newer model and is compatible with your current system.

MATTHEWS: Three big challenges for reprocessing staff are high turnover rates, demand for regular training and education and internal pressures to expedite their cleaning procedures to meet surgical case demands. Given these challenges, it’s no surprise that our internal research has demonstrated that over 80% of damage is related to poor care and handling practices. Clinical engineers, reprocessing staff, clinical staff and repair vendors should collaborate on strategies to improve processes that are customized to fit the individual challenges at their facilities.
WHELAN: The best protection for our patients is a proactive versus reactive approach. This can best be realized through a multidisciplinary system approach – where clinical engineering is at the table regularly with processing professionals, infection preventionists, clinicians and administration. Every discipline has “a piece of the pie” and a unique and deserved perspective.


During the 1960s, a commercial jingle directed at women announced; “You’ve come a long way baby to get to where you’ve got to today.” While the company promoting the commercial was a cigarette maker, the sentiment was right. Much had transpired since the 1920s. The Women’s Suffrage Movement had labored for 100 years to win women the right to vote, and in August of 1920, the 19th Amendment was passed and women finally won that fundamental right of citizenship.
During World War II, when men were off to war, it was the women who kept the country going and who labored to build the weaponry the U.S. troops needed to push the effort forward.
Artist Norman Rockwell immortalized the allegorical icon “Rosie the Riveter” in 1942, an image that represented all of the important women who worked in America’s defense industry; including factories and shipyards. (This writer’s mother was one of them.)
While many women were also engaged in the important work of acting as a single parent during the war effort, the image of Rosie was one of women using tools, working in construction and in steel mills and doing jobs that had traditionally been held my men.
The image of women using power tools and shop machines showed conclusively that women could do these jobs right alongside the men. While many women have worked in jobs that are often thought of as traditionally female, like teachers or nurses, there are also men who work in those roles.
The Rosie the Riveters of the war era had to fill those jobs to continue the production of goods needed by their country. They didn’t plan, from their school days, to go into those industries, but instead answered the call when they were most needed.
Today, many women find that they enjoy electronics or working with tools from the time they are in school. Many decide that a less-traditional path is more fulfilling and they enter professions that had previously been dominated by males. Even while still representing a minority in those professions, they have carved out a niche that has helped break stereotypes about who can perform that job.
This has included an evolution in the HTM profession, as women have gained in numbers as both technicians and managers. There are many women making a difference in the HTM field and moving the profession and processes forward. While the numbers are not 50/50, the image of the profession as a viable career alternative for women continues to gain momentum.
Several women who have had an impact on HTM have provided perspectives on how the field can attract more women and what the prospects are in the future for women in HTM. Each of these women also illustrate the

At a time when the HTM field suffers from an exodus of experienced biomeds, any effort to broaden the pool of qualified applicants would make sense. This would include methods and efforts to introduce more women to the profession. Women offer special insights, ideas and opinions about how successful campaigns might be conducted.
“Women already working in the HTM field have a unique opportunity to make a significant impact in attracting more women to join this field. By actively engaging in educational outreach, we can visit schools and introduce students to the exciting world of HTM,” says Mayra Becerra, a senior biomed with Memorial Healthcare Systems and the creator of the BioMedGirl YouTube channel.
She says that additionally, serving as role models and mentors, “we can provide guidance and support to women aspiring to enter engineering or health care fields, as well as those seeking meaningful career paths.”
“Personally, I find social media to be an effective platform for showcasing the capabilities of women in HTM. By sharing our experiences and accomplishments, we can inspire others and demonstrate that women can excel as HTM professionals,” Becerra says.
“We face many challenges attracting qualified techs,” says Carol L. Wyatt, MPA, CBET, CHTM, director, SCS at Baylor Scott and White Health in Dallas, Texas.
“We need to grab future teammates’ interest early by talking to students at middle school, high school and college career fairs. They will not know what HTM has to offer unless we get out there and share the knowledge,” she says.
Allison Woolford, CBET, biomedical equipment specialist, clinical engineering-periop team lead at the Duke University Health System says that those in the biomed field need to go into the community and advocate for the field.
“Community colleges, clinical engineering departments and medical device companies could put together one-day summer camps or Saturday camps that are geared towards female middle and high school students,” Woolford suggests.
“Clinical engineering departments and private industries can work with their local schools to have a job shadowing program, where once a quarter, students can come and see what the field is like. Let them fix a vital signs machine or do a PM on a syringe pump. Teach them why accuracy is important for the care of patients,” she adds.
Kayla L. Heppler, MHA, clinical engineering director with Intermountain Health in Utah says that women need to know there are health care career opportunities outside of clinician roles.
“Speaking as someone who stumbled into this field, I think that more women will discover the field as the HTM industry attempts to develop how we find talent. When I was in high school, I knew that I wanted to do something to help people. I wanted to work in health care, but the problem was that I am
way too squeamish to be a nurse or anything patient facing,” she says.
She ended up pursuing a bachelor’s in finance because she didn’t know what else to pursue besides a generic business degree.
“After finishing my degree, I fell into a financial analyst role in Intermountain Healthcare. The way I stumbled into clinical engineering was through my friendships and connections, and I’ve been in HTM ever since. Had I known more about this field prior to attending college, I might have changed my course. I am happy that I still stumbled into this industry,” Heppler says.
She says that she believes that there are plenty of young women out there who want to work in health care, but do not have the ability to work in a patient facing area, like her.
“While HTM certainly can have some off and on interface with patients directly, it is mostly behind the scenes work. I know that my peers in my organization have been making efforts to go to job fairs and high schools to educate people on what this field is all about. We also are establishing an apprentice program and connections with the state department of labor and the U.S. Department of Defense. I think that the more our women HTM techs and leaders participate in the education and recruitment efforts, the more they can attract other women to the field,” Heppler says.
Vanessa Goldsmith, site manager at Ascension Sacred Heart Bay/Emerald Coast/Gulf for TRIMEDX Healthcare Equipment Services, says that to help more women discover HTM, it is important to have female representation at recruitment events such as college and job fairs.
“With the HTM field being known as predominately male, utilizing female faces and voices in various media platforms helps increase awareness around the field being a viable and rewarding career for women looking for careers in tech,” she says.
An emphasis on STEM and its application in the real world could help direct more women into HTM.
“All of the areas of STEM, including HTM, still need more exposure earlier in children’s school curriculum. Classes in middle and high school that show the application of the STEM fields to the real world would help students identify how skills such as problem solving, can be applied to everyday situations,” says Samantha Jacques, Ph.D., FACHE, AAMIF, vice president of McLaren Clinical Engineering Services (MCES) at McLaren Health Care in Grand Blanc Township, Michigan.
She says that although students need good foundations in the fundamentals, nothing sparks imagination in students more than seeing how it applies to real life and how that application can be a career path for them to follow.
“I agree that we need more women in HTM, but we also need more men in the field. This profession still lacks the visibility to the general public, even though it’s been around for decades. We need to get in front of kids in middle school and high school during career fairs; we need to use social media to spread the word to the younger generation; and we need to support local colleges that have biomed programs – essentially, we need to use any means possible to get the word out,”
says Andrea Brainard, CBET, CHTM, senior director of healthcare technology management at Children’s Health in Dallas, Texas.
Where do these HTM professionals see women in the field in five years?
“In the past five years, there has been an increase of women not only in the technician roles, but also more visible in leadership roles. I believe we will continue to see more women enter the field and be promoted to management roles. The one area I haven’t see a lot of improvement is in the imaging service area. There is still a lack of women in those roles and I would love to see some more expansion into those aspects of device repair,” Jacques says.
Goldsmith says that the stereotype of tech roles being for males is slowly going away, and more women are venturing out into roles like HTM.
“This will continue to allow for steady growth and career development in the coming years,” she says.
Becerra says that she envisions a promising future for women in the HTM field.
“While the current industry average stands at 22 percent female representation, it is encouraging to note the gradual increase observed in recent years. With the growing emphasis on inclusion and diversity within many organizations, I am optimistic about the potential to achieve a significant milestone of 40 percent women in the HTM industry, with a substantial number occupying management roles,” she says.
“If we can show the benefits of joining and remaining in the HTM field and we can offer competitive salaries, I see women flourishing in five years, 10 years and even 20 years,” Wyatt says.
Heppler says that it’s hard to imagine and predict the future because of how fast health care and the HTM industry is constantly evolving.
“I would imagine and hope that we would see a steady increase of women in this industry. I think women bring a unique perspective to the field and I have been seeing a slow increase of women entering the field, which is exciting. I just hope the trend continues, with all the efforts being made by not only my organization, but the HTM industry as a whole,” she says.
Woolford says that in five years, she sees more women in team leader and management roles.
“By helping to remove gender specific stigmas and increasing exposure to this field, more women will see that this career field has a place for them,” she says.
“Hopefully in five years, we see an exponential increase in the number of women in HTM as we work to bring HTM to the forefront as a career choice,” Brainard says.
She says that HTM, as a career, provides stability, a sense of accomplishment and a rewarding feeling at the end of the day knowing your job saves patient lives.
“With the rapid growth of technology in general, more women are working in STEM fields as compared to the 1970s,
but there’s still a gap to our male counterparts and I’m hoping that as more and more women get into HTM, they will recruit more women to also get into this field,” Brainard adds.

Regardless of role or responsibilities, the women in HTM find the field rewarding and fulfilling.
“I really like knowing that I have the skills necessary to be a member of a team that is critical to a person’s physical health and well-being,” Woolford says.
She says that she enjoys working in the operating room.
“It’s a different atmosphere when you put on the scrubs designated for the OR. It is as if we are our own little community in the hospital. When a call comes from the OR, you know that it is a matter of life and death. When the problem is resolved, there is a sigh of relief that fills the room. The staff know that now, they are able to provide the best care to that person on the table,” Woolford adds.
Another thing she enjoys about the profession is the relationships you build within the HTM and hospital community.
“We are like a big family. We cheer each other on and celebrate accomplishments; professionally and personally. We want to see each other succeed because we understand that our success in turn helps the patient in the long run,” Woolford says.
“In HTM, I like the diversity of the field. There is always a new challenge that allows me to grow and learn something new. I enjoy being able to play a vital role in the care of those in need,” Goldsmith says.
Jacques says that one thing she likes about HTM is that it offers a different challenge each day.
“Two days are never the same and the changing hospital landscape, the advancements in technology, and the collaborations to be successful make each day something new. I can always continue to learn, adapt and grow my skills,” she says.
Wyatt says that the best thing about the HTM profession is that it offers varied opportunities to highlight one’s skills.
“Whether your strength is troubleshooting medical devices, managing operations, database administration, cybersecurity, or any of the other dozen fields, HTM has a place for you. And I have taken advantage of a few different areas as my skills and knowledge evolved. But for me, the best thing about working in HTM is the opportunity to make a difference and have a positive impact to make someone’s life better, whether they know it or not,” she says.
One aspect that Becerra enjoys the most about the field is having a significant impact on patient care.
“It brings me great satisfaction to know that my contributions play a part in saving lives and improving health care outcomes. As HTM professionals, we play a vital role in the health care system by meticulously maintaining and ensuring the proper operation of medical devices. Our primary focus is on patient safety, diligently working to ensure that all medical equipment is safe and reliable for use in patient care,” she says.
Brainard says that what she likes about HTM requires a two-fold answer.
“First, I love that HTM is still a small, tightly knit community. We all have the same basic goal: ensuring medical devices are safe and ready for patient care. Since becoming an HTM leader, my personal goals have grown and evolved somewhat, but the drive and purpose of our HTM team remains the same. The second part of the answer is that I love being a part of cultivating new HTM talent, recruiting new technicians into the field, and providing them opportunities to learn and grow and be the best techs they can be,” she says.
With women represented across the HTM profession at every level, and an obvious passion among them for the jobs they do every day, it can only behoove the field to grow their numbers and introduce the profession to a new generation of females as a career option.
NOTE: The upcoming MD Expo in Orlando, Florida will feature a panel discussion on women in HTM. For more information, visit tinyurl.com/mr23vcyc
• Completely free and confidential registration and application process
• 500+ open positions across the United States (part + full-time, temp, internships and externships)
Personally connect candidates with


• A talent network of 2,500 qualified, actively looking HTM and Imaging professionals
• A variety of posting options ranging from single-job listings to unlimited-membership packages
• Social media, print, and eNews promotion
“My HR department advertised on various Government sites and our web site but we did not get a single applicant in over 120 days. Fairbanks Alaska is hard to recruit for but I took out an Ad on HTM Jobs and got two good applicants in less than 30 days . I am hiring them both. Thanks HTM Jobs.”
- D. Anderson, Tanana Chiefs Conference
Featured Employers: Agiliti, InterMed, TRIMEDX, Renovo Solutions, Texas Health Resources, SPBS, Inc., and Universal Medical Resources.
The headline “LinkedIn Bets on Skills Over Degrees as Future Labor Market Currency” is from a June 9 Bloomberg story that predicts employers are going to be focusing on an applicant’s proven skills instead of college degrees and prior job titles. And data shows that already is happening.
According to a recent study from American Student Assistance (ASA), completed in collaboration with Jobs for the Future, 81% of employers believe in prioritizing skills over degrees, and 72% of employers believe that a degree is not a reliable way to assess the quality of a candidate. So, what exactly is skills-first hiring? And how can applicants make sure they provide the kind of information that will appeal to a company using a skill-first approach?
“Skills-first hiring is the concept that employers should consider the skills a prospective employee has gained through education, career training, or work experience, rather than simply relying on a degree as a threshold for employment,” explains Julie Lammers, senior vice president, advocacy and corporate social responsibility for ASA.
Matt Diggity, founder and CEO of Diggity Marketing, describes it as “an alternative to the traditional model, which tends to prioritize formal education and previous job titles. Now, it’s more about what you can do right now, not where you studied or where you’ve worked in the past.”
Diggity gives SEO and digital marketing as examples of fields “where this shift is particularly poignant.”
“The digital landscape evolves so quickly that static degrees often can’t keep pace. It’s a realm
where the keen ability to adapt and learn – and most importantly, to apply learned skills effectively – is key,” he explains.
Skills-first-focused employers are looking for “durable skills” (also known as “soft skills”) that include teamwork, problem solving, critical thinking, collaboration and flexibility, as well as character skills like fortitude and leadership — “things that are essential in almost every job,” Lammers explains. According to a study by America Succeeds, 70% of the most requested skills in more than 82 million job postings fall into the durable skills category. Why the transition to a skills-based model?
“Technical skills are often easy to train someone on through a certification process or on the job, but it is these durable skills that are desperately desired by employers and harder to train on later in life,” Lammers says.
To appeal to employers using a skills-based hiring model, Lammers and Diggity offer this advice: Showcase relevant skills and accomplishments. This will make your resume and cover letter shine. Remember that “skills” don’t mean technical skills alone.
“In today’s world, soft skills like communication, teamwork and adaptability are highly valued,” Diggity stresses. “Showcase instances where you have used these skills to create a significant impact.” Highlight your continuous learning initiatives. Recent courses and certifications can set you apart.
“It’s always a plus in the dynamic world of digital marketing, for example, to show you’re proactive about staying on top of the latest trends and techniques,” Diggity says.
Take advantage of career readiness learning opportunities as early as possible. Internships, project-based learning and entrepreneurship experiences offer the opportunity to build the durable skills
employers desire, Lammers notes.
“For example, the CAPS Network works to match students with the project needs of organizations through a consulting model. In this way, young people can build skills like problem solving, teamwork, adaptability, and creativity by co-working with employers to solve a real-world business challenge,” Lammers says.
Another organization Lammers suggests is the Network for Teaching Entrepreneurship, which helps young people learn how to take risks, how to fail safely and quickly, and how to problem solve.
“These lifelong, transferable skills are all skills that every young person and prospective employee needs to be career ready,” Lammers says.
There also is the U.S. Chamber of Commerce Foundation’s pilot phase of the Employer Provided Innovation Challenges initiative, an online platform that connects high school and postsecondary learners with employers to create solutions to real-world, employer-led, work-based learning challenges.

“Through this program, employers will articulate a specific business challenge they are looking to address, and students will be invited to work as a team to find a solution and build durable skills along the way,” Lammers says.
Kathleen Furore is a Chicago-based writer and editor who has covered personal finance and other business-related topics for a variety of trade and consumer publications. You can email her your career questions at kfurore@yahoo.com.

 BY TED LUCIDI, CBET
BY TED LUCIDI, CBET
ealth care clinicians using evidence-based medicine for diagnosis and treatment have been improving quality outcomes and reducing costs for years. Data-driven protocols enable clinicians to eliminate guess work and provide treatment at higher confidence levels.
The same applies to medical device service and depot-based repair. When providers base repair processes on scientifically valid research and methodology, they are able to increase consistency, reliability, operational efficiencies and, ultimately, device longevity, all while decreasing costs. Yet few repair providers in today’s price-driven market have the resources to continually test and develop these methodologies. Doing so requires high levels of:
• Clinical and technical expertise and judgement
• Valid evidence
• Ability to execute efficiently and affordably
• Sophisticated instruments and test devices
Having the above requires more than what a typical repair shop with skilled technicians can provide. It requires engineering depth, research and testing capabilities, and leadership that understands what to test, and how to identify valid evidence around which to build best practices. Relying on scientifically valid evidence and data-driven analytics to guide repair procedures is critical for results that enable devices to perform as originally intended while positively impacting operational efficiencies and financial goals. At Innovatus Imaging, we call this Data-Driven Repair (DDR). Following are three benefits of Data-Driven Repair that can help you maximize device life cycle while providing perfor-
mance consistent with OEM intent and clinician expectations.
Repair providers face increasing pressure to resolve problems quickly and cheaply, yet doing so without valid data for best practices can have consequences. Those that engage in multiple types and levels of testing are more prepared to solve problems right the first time and achieve optimal outcomes.
It’s important to ask providers what data they use to back up their practices. Is the data backed by high-level evidence from very well controlled trials ... or low-level evidence such as personal experience and opinion. While low-level evidence can be impactful, the strongest evidence for building best practices and optimal outcomes comes from controlled testing and trials.
With more than 180,000 successful probe repairs and 40,000 MRI coil repairs, and the longest warranty periods in the industry, Innovatus Imaging continues to collect and apply proprietary evidence for repair methodologies used in its operations. Ongoing testing programs help gather evidence and identify thresholds at which efficacy diminishes further impacting quality outcomes.
While advanced testing capabilities are not always necessary for immediate favorable outcomes, providers that engage in high level testing programs can build proprietary DDR methods and provide in-depth information about your devices. For ultrasound probe repairs, acoustic testing (hydrophone measurements and radiation force balance) is a good example. OEMs design their probes, in conjunction with their systems, to deliver a specific level of acoustic energy to the patient. Acoustic testing measures the amount
of energy delivered to the patient. Innovatus utilizes hydrophone testing of OEM new probes to enable engineers to benchmark and document acoustic performance and uses the data to build an acoustic profile for a given probe model. As a result, DDR can provide clients with confidence that Innovatus’ repaired probes perform as originally intended.
Accountability and documentation are another benefit of DDR. Now, more than ever, guidance from an ISO 13485:2016 certified Quality Management System (QMS) should be the minimal criteria when seeking potential repair providers for a medical device. Organizations that adhere to the globally accepted 13485:2016 standard and understand medical device manufacturing will, by design, produce outcomes containing many key elements necessary for safe, effective and reliable repairs. It’s important for providers to supply documentation that supports the work performed on their clients’ devices as well as the testing criteria used to release the devices back into clinical use. Not only does Innovatus provide a comprehensive pre-repair assessment, but also supplies a post-repair Certificate of Conformance which is consistent with OEM production. In this manner, Innovatus clients know what deficiencies were identified and what processes were used to restore safety, functionality, efficacy and OEM intended design.

In an industry with so much at stake, quick fixes and lowest-cost options are no longer valid qualifiers for choosing a repair provider. The choice needs to be backed up by evidence, not just personal experience as to what will work fast, keep a budget in-line and get a device working again. DDR represents methodologies developed over 30 years of proprietary research, testing and experience which guide our manufacturing and repair of ultrasound probes and MRI coils. It also integrates insights and methodologies from scientific studies, quality management systems, globally recognized performance standards and exclusive analytics from more than 180,000 successful probe and 40,000 coil repairs.
For more information on data driven repair and how it can help re-define your ultrasound probe and MRI coil service strategy, reach out to TedL@innovatusimaging.com.



• US based support
• Well organized implementations delivered on time

• Fast system performance


• Numerous integrations, including with the top cybersecurity applications
• 30 years of innovation focused completely on healthcare
“HEMS One” empowers HTM / Biomed teams to do their best work! Schedule your demo of HEMS today: 888-312-4367 or EQ2LLC.com
At iServe Biomedical, we strive to provide only the best quality replacement parts and accessories on the market. We offer an extensive selection of OEM Compatible replacements for top brands such as Philips, GE Healthcare, Nellcor, Masimo, Nihon Kohden, Spacelabs, Draeger, B.D/Carefusion/ Alaris, GE/Corometrics, Baxter, and more. Our products are designed to meet the highest standards of quality and reliability, ensuring that healthcare professionals can provide the best possible care for their patients. Whether you need replacement parts for your medical equipment or accessories to enhance their functionality, iServe Biomedical is your one-stop-shop for all your needs. Contact us today for a quote!







 BY JOIE N. MARHEFKA
BY JOIE N. MARHEFKA
While technical skills are certainly necessary in healthcare technology management (HTM), soft skills cannot be overlooked. Communication, teamwork, leadership, creative problem solving and time management are essential for a successful career in HTM. I encourage my students to take certain electives and participate in activities that strengthen these soft skills. Doing so will help them develop a strong resume and succeed in their HTM career. While some of these suggestions are specific to college students, many are not and can be used to enhance soft skills at any point in one’s career.
Communication is one of the most essential skills for an HTM career. Time and time again, I hear this from the people that supervise our interns and hire our graduates. In particular, the ability to communicate with nurses and others who have different technical backgrounds is stressed. Our students are required to take both a writing class and a public speaking class, and I try to help them understand the importance of these courses and skills. For students at other schools who may not require these courses, I’d highly recommend taking them anyway. For professionals, I suggest joining Toastmasters International, a nonprofit organization that teaches public speaking as a way to practice and develop communication skills. Toastmasters has clubs throughout the world, as well as some that meet exclusively online, and offers opportunities to practice public speaking and to receive feedback and mentoring. Any of these activities will likely improve confidence as well as public speaking ability.
Teamwork is another valuable skill, which can be built outside of the classroom. In any position, it is important to be able to work with a variety of people from different backgrounds and with different expertise. Joining an intramural or recreational sports team is one good way to practice working with others. Participating in a club or other organization, where
members work together toward a common goal, would also be helpful. Volunteering with a group, such as Habitat for Humanity or a local food bank, is an opportunity to work with others to accomplish a task and thus strengthen teamwork skills.
Leadership skills can be beneficial as well, especially for advancing beyond an entry-level position. Classes in leadership are available, both as for-credit college courses and as continuing education, which is one way to develop these skills. Outside the classroom, joining a leadership organization, which is available in many cities, is a path to develop as a leader. For example, I have had friends and family members complete the Leadership Harrisburg and Leadership Pittsburgh programs. In addition, volunteering as a leader for a club or professional organization can be a good way to practice and refine leadership skills. I encourage my students to seek leadership positions in student government or with other clubs on campus. For professionals, opportunities may be available with a local HTM organization or with professional societies.
Creative problem-solving skills are also useful in a field like HTM. Students can enroll in a design class to work on their problem solving. Participating in a design challenge or competition is another way to develop and practice creative problem solving. Problem-solving skills can also be improved through projects in other classes as well as on the job.
Finally, time management is a skill that is beneficial in any career. Courses and seminars, many of which are available online, can be used to learn new approaches to time management. A good way to practice time management, as well as to demonstrate it on a resume or interview, is to participate in activities, such as those discussed above. This will likely require planning and prioritization, which are essential for good time management.
The soft skills discussed above, communication, teamwork, leadership, creative problem-solving, and time management, are essential for any career, especially one in HTM. Finding ways to improve these skills while in school and through lifelong learning will help to build a resume and to succeed and grow in a career.
ne of the core foundational documents of any HTM cybersecurity program is the MDS2 (Manufacturer Disclosure Statement for Medical Device Security) which provides information on your medical device or Software as a Medical Device (SaMD) mapped directly to security controls established by National Institute of Science and technology (NIST), International Electrotechnical Commission (IEC) and International Standards Organization (ISO). Medical Device Manufacturers (MDMs) supply an MDS2 on their product at the time of sale or upon request from health delivery organizations (HDOs). The MDS2 allows the MDM the opportunity to communicate critical security related information through a standardized form that assists HDOs in the evaluation of a medical device’s security capabilities, how to best handle any issues when they occur as well as compare devices during the product selection process.
The MDS2 standard was developed by Healthcare Information and Management Systems Society (HIMSS) and the National Electrical Manufacturers Association (NEMA), and most recently revised by NEMA and the Medical Imaging & Technology Alliance (MITA) back in 2018 with the latest version of its standard, ANSI/NEMA HN 1-2019 MDS2 published in 2019. The new version provides medical device manufacturers with an expanded MDS2 form to collect and report information related to their products’ security capabilities to their customers. MDMs fill in responses to a series of 240 questions that cover 23 different security capabilities about the device.
The questions asked in an MDS2 provide the HDO with information needed to properly evaluate, purchase, implement, sustain and secure the device in their organization while ensuring it meets their contracting and security requirements at the time of purchase such as:
• Does the device perform automatic installation of software updates?
• Does the manufacturer notify the customer when updates are approved for installation?
• Does the device require vendor or vendor-authorized service to install patches or software updates?
• Does the medical device manufacturer allow security updates from any third-party manufacturers (e.g., Microsoft) to be installed without approval from the manufacturer?
• Does the device have the capability to receive remote installation of patches or software updates?
• Does the device contain anti-malware software?
• Does the manufacturer have a process in place to assess device vulnerabilities and updates?
• Does the manufacturer have an approved list of third-party software that can be installed on the device?
• What types of private data are stored on the device, and how are they transmitted?
• Does the device support and enforce unique IDs and passwords for all users and roles (including service accounts)?
• Is the SBOM for this product available?
Note: Some of the questions may need follow up with the MDM to determine if service and support contracts are needed and should be included in the planned purchase and sustainment costs of the medical device.
These recommendations are adapted from material found on the ECRI (www.ECRI.org) and AAMI (www.aami.org) websites:
• Make sure a MDS2 form is requested for each medical device during the prepurchasing/procurement phase or for systems in your current inventory lacking an MDS2.
• Verify with the MDM that they are using the latest version of the MDS2. If an outdated form is received, request that the manufacturer provide you with a completed MDS2 based on the latest version. The manufacturer should update the MDS2 form for each of its devices as updates/ new software versions are released.
• Verify model, OS, firmware and software version of the device matches the version on the MDS2 or matches what will “ship.”
• The MDS2 form is meant to provide a way of reporting the technical, model-specific elements of information needed by an HDO to begin medical device security risk assessments. Note: There may be variations based on SKUs, operating system and software version installed. If you have more than one variation, be sure you have the proper MDS2 for each variation in your inventory and its risk score rated accordingly.
• If the MDM hasn’t yet completed one for your device, you can send a copy or link to the MDS2 at NEMA (www.nema.org), see QR code for direct link. NEMA also recommends referencing the instructions document (HN 1-2019) and example worksheet.

• The MDS2 assists in determining whether the device meets your security requirements “as is,” can be compliant with compensating controls, or cannot meet your requirements even with compensating controls. HTM, IT and risk management should review the MDS2 to assist in product comparisons and final purchasing decisions. NOTE: All answers on an MDS2 may not be
applicable to all devices and should be evaluated accordingly.
• MDS2 questions are formatted with a response of “Yes,” “No,” “N/A,” (Not Applicable) or “See Note” with little to no room for misinterpretation or confusion. NOTE: Some questions require technical details to be provided in the “Notes” field, review these fields closely. You may also request any supplemental documentation including network diagrams, security documentation (listing of ports, protocols, and services used), software dependencies, device and software inventory, a Software Bills of Materials (SBOM) which is asked in the MDS2, etc.
• Verify that the MDM has filled the form out properly and completely. If insufficient information is provided in the MDS2 form, request that the MDM expound to meet your standards and/or request a conference call with the manufacturer’s technical team for clarification with other members of your risk assessment team. NOTE: Not all questions can be answered, but more questions answered helps create a better security profile.
• Use the MDS2 to determine if the device generates, stores and/or transmits Protected Health Information (PHI), how much data is stored on the device, how it is
protected, the type of data, and if the data is stored permanently or temporarily. If data is permanent, the manufacturer should be able to provide instructions how it can be removed. This will also help determine what breach notification action must be taken per the HIPAA Breach Notification Rule with Health and Human Services (HHS).
• Upload or link to your MDS2 forms to your device entry in your asset management database, Computerized Maintenance Management System (CMMS), Governance, Risk Management, Compliance (GRC) system, Internet of Medical Things (IoMT) solution or store in a simple networked repository with shared access to all parties involved (HTM, IT, Security, etc.). Some software solution vendors even allow the data from the MDS2 to be imported and ingested. This may facilitate and automate future risk management and vulnerability remediation efforts.
• Check with the MDM periodically (some MDMs have websites or portals) for updated MDS2 forms or when product changes occur – like changes to the operating system and/or major version changes of installed software. Additional software tools are also available from several CMMS vendors and third-party security vendors that can use MDS2 information in the creation of a risk profile or a Common Vulnerability Scoring System (CVSS) risk score for a particular device which rely heavily on current and updated information.
• Detailed information collected on a MDS2 allows for an accurate risk assessment as well as the best configuration for proper operation, patching, updates, security and guidance used in the care and feeding of the medical device as deemed by the MDM, while it resides in your hospital organization.
Very rarely are these documents updated and unfortunately remain a static document. As mentioned previously, HDOs should request a new MDS2 when there is a significant or major change/update to the operating system or software version so an accurate risk analysis and proper patching can occur.
Several MDS2 forms received from MDMs are created using outdated or old forms from 2008 and 2013. If you are using an IoMT solution and this data is being used, make sure your MDS2 documents are current as this can affect your risk analysis and accuracy of properly identifying your medical devices in your cybersecurity program.
Several third-party security systems can store and even process the information on an MDS2 sheet to assist and provide insight in notifying HDOs of potential vulnerabilities and some even provide pre-procurement and post-procurement risk analysis. Some also ingest the number of PHI on a device which is needed in to properly report breaches to HHS per HIPPA when a vulnerability is identified.
Many third-party IoMT solutions also have a large collection of MDS2s. This is great for organizations without a way of
archiving MDS2 documents or lack a cache of their own, but unfortunately most are old, outdated or based on an older version of the MDS2 form and this may affect a proper risk assessment. If not current, this will ultimately leave gaps in your security posture if you are relying on this for an accurate assessment of your medical device inventory cybersecurity health. Another thing to consider is how deeply the data from the MDS2 is integrated into the rest of the solution and how it is used. If the data is outdated, it can cause false positives for vulnerabilities that may not exist potentially wasting valuable time and resources verifying devices that may have already been patched. Work with your IoMT solution provider to verify the data is accurate and up to date as well as how it is used. If you also manage non-medical devices or research devices, using the MDS2 as a template for documenting those devices can also be used to help with preprocurement as well as address patching strategy, risk assessment, and security for systems like security door systems, temperature control systems, elevators, security cameras, IoT devices, nurse call systems, etc. These systems generally can’t receive enterprise baselines and don’t currently have a standardized method of proper documentation and risk assessment. Inherently they also have a higher potential attack surface than medical devices because of lower security standards, unpatched and unknown vulnerabilities and poor documentation. They would benefit from using similar best practices and documentation outlined in the MDS2 form.
With innovation and maintaining updated MDS2, HDOs will be able to easily ingest the information into their CMSS or IoMT security program along with information given on SBOMs to better assess what they have, what can be patched and what needs to be patched/updated. SBOMs are still in their nascent stage with the potential to tap into a regularly automated updated SBOM feeds directly from the manufacturer, which could automate the updating of MDS2 documents stored on MDMs websites or an HDOs CMMS system providing updated information in near real time.
Zack Hornberger from MITA states that the “MDS2 aims to makes sure that no one is left behind on cybersecurity – which can sometimes keep universal tools like MDS2 tied to more accessible, but maybe less state-of-the-art, communication methods. Still, everyone wants to see MDS2 continue as the gold standard for device cybersecurity information sharing between MDMs and HDOs.”
Steven Hughes, FAC-COR FACP/PM VHA-CM, is a VISN 21 Biomedical Engineer with the VA Sierra Pacific Network.




HI MSS recommended a modest delay in the implementing and reporting timeline for the sweeping changes proposed by the Office of the National Coordinator’s (ONC) Health Information Technology (IT) Certification Program.
The delay would accommodate the volume of work required to successfully develop, implement and test these certification changes across the health care ecosystem, without risks to quality, safety and patient privacy. Healthcare Information and Management Systems Society (HIMSS) is a global advocate for digital health transformation.
ONC released the Health Data, Technology, and Interoperability: Certification Program Updates, Algorithm Transparency, and Information Sharing Proposed Rule, widely known as HTI-1, on April 18, 2023. HTI-1 proposed a Dec. 31, 2024, implementation deadline for a wide range of new certification criteria for Certified Health IT, including:
• Raise the baseline version of the United States Core Data for Interoperability (USCDI) from Version 1 to Version 3
• Update existing certification criteria including decision support interventions (DSI); ensure patient requested restrictions in sharing their electronic health information; standardize “Sex for Clinical Use” (SFCU) “Name to Use” and “Pronouns” for documentation; electronic case reporting; and the use of standardized Application Programming Interface (APIs) for patient and population services
• Launch the Electronic Health Record (EHR) Reporting Program or “Insights” for manufacturers of Certified Health IT to maintain their certification, with reporting kicking off in 2025
• Modify exceptions to information blocking regulations.
In a public comment letter submitted to ONC on June 20, 2023, HIMSS stated its support of the proposed new and revised certification criteria, adoption standards, and launch Insights program. These changes will facilitate a nationwide interoperability.
HIMSS, however, called for a modest delay of one year for the implementation deadline for most of the new certification requirements, pushing it to Dec. 31, 2025. This would allow for time to successfully complete the extensive work required to implement and test the new and revised criteria across the health care ecosystem in a manner ensuring that quality, safety and patient privacy are not compromised; and that health care delivery sites caring for underserved communities aren’t left behind.
HIMSS also calls on ONC to delay the adoption of the decision support intervention and predictive decision support to Dec. 31, 2026. This would allow time for sufficient real-world testing and the development of standardized methods for capturing source attribution for DSI by end-users and third-party manufacturers.
Among the key points of the HIMSS response:
• HIMSS supports the adoption of all proposed certification criteria and standards as a means to enable nationwide interoperability.
• HIMSS does not want health care providers acting in good faith to be penalized because of insufficient implementation time because of delays associated with standards development, vendor timelines, and staffing shortages impacting the health care community.
• HIMSS recommends a revised, two-stage implementation timeline for proposed new certification criteria, standards adoption, and reporting:
• All certification criteria, including Fast Healthcare Interoperability Resources 1 (FHIR), and Bulk FHIR and other than DSI, must be implemented in Certi -
fied Health IT no later than Dec. 31, 2025.
• Insight reporting on Stage 1 certification criteria will kick off in October 2026. FHIR and Bulk FHIR certification criteria reporting would be required as a part of 2026 reporting.
• DSI and Predictive DSI certification criterion must be implemented in Certified Health IT no later than Dec. 31, 2026
• Insight reporting on Stage 2 (DSI/Predictive DSI) criteria will kick off in October 2027
This modest delay will allow:
• A thoughtful, complete, and realistic implementation cycle for all health care providers utilizing Certified Health IT consistent with market supplier and health care system capabilities at the present time
• Real-world testing of privacy protections for data elements captured in United States Core Data for Interoperability (USCDI) v3, which are often considered sensitive information, i.e., social determinants of health, sexual orientation and gender identity
• Additional time to convene the health care community to develop a rules-driven, standardized mechanism for delivering the data segmentation required to ensure patient requested restrictions to their electronic health information
• Additional time to develop a standardized approach for capturing source attribution for decision support interventions, especially when submitted by third parties and end-users not subject to certification requirements; and a feasibility study to determine the value of including a user-interface to provide feedback on decision support interventions
• And the adoption of a regimented cadence for publishing additional substantive changes to the Certification program, including assurance that substantive changes will include a minimum 18-month implementation period
• HIMSS calls on the health care community to start the work of implementing these proposed changes as soon as a final rule is published and encourages the industry to be nimbler in adopting future advancements facilitating nation-wide interoperability.
HIMSS also calls on ONC to:
• Collaborate with CMS to incentivize end-user participation in real world testing through scoring bonuses in CMS value-based care and quality reporting programs
• Publish a long-term roadmap for implementing USCDI+, a version of USCDI which includes the nuanced clinical terminology to support the collection of digital quality measures in the future

• Invest in improving processes for standards and implementation guide development
• Note that several sectors, particularly those caring for the underserved and elderly populations are poorly positioned to take on additional cost and regulatory burden associated with the sweeping proposed changes HIMSS is encouraged by ONC’s proposed steps to deliver nationwide interoperability. HIMSS welcomes an ongoing dialogue with ONC to digitally transform the health care ecosystem of the United States through the power of information and technology.
For more information, visit himss.org.
Af ter covering the basis of layer 2 switching hardware, it is important to cover the other layer two connection: wireless communication devices, or WiFi. Most people use Wi-Fi daily without even considering the setup or hardware required to make this technology work. However, as repairers of medical devices, biomedical technicians need to keep in mind the requirements to make wireless medical devices work in a hospital.
Wireless connections function like a hub, but still have a MAC address. It is intended in function to replace a hardwired network switch. It can provide access to both a LAN and Internet traffic. Nearly every device that can work on a hardwired switch can be adapted to work on a wireless network. Technicians can accomplish this by adding an access point to a network and a wireless NIC card to a device. Access points transmit a radio signal, which is picked up by a wireless NIC card on a device. Because a wireless communication can be picked up by anyone with an antenna, Wi-Fi uses passwords to make sure that the communication is only understood by authorized devices.
Since communication happens openly and anyone with an antenna can hear this transmission and security is needed in most wireless transmissions. Since security is dependent on the hardware capability, lets first talk about wireless hardware. Some hardware may be compatible with older devices, but not all wireless devices are to be considered compatible with each other. For this example, consider the most popular hardware in use: the N type Wi-Fi. Older types include A, B, and G.
An N type wireless access point is generally capable of reverse compatibility to the older hardware. This is opposed to the newest communication type, which is the AX standard, also called Wi-Fi 6. It is only reverse compatible to the AC and N standards (Wi-Fi 5 and 4, respectively), but not further. This becomes very important when connecting older medical equipment to newer networks. Always make sure the type of device is compatible with the intended access points. Additionally, the hardware sets the speed of a communication. The speed of Wi-Fi access is set by the highest type of communication both the access point and the wireless device can use. Any Wi-Fi will only function at the highest speed the oldest hardware will operate at.
Modern AX Wi-Fi is capable of speeds at or better than modern hardwired systems whereas N communications are slower. An A or B communication is comparable to early HUB connected networks and their speed is barely functional for modern video communications. Consequently, these are only used as a last resort. Given this, it’s easy to assume that it would be best for everyone to use the most modern hardware all the time. However, that is not cost effective for most users. Medical devices often use older technology, and it is expensive to upgrade all access points when a standard is updated, which occurs about every 5 years. Consequently, we see older hardware used in the medical setting. Very few devices are made with AC or AX networks in mind due to compatibility. Additionally, this affects not only the device speed, but network security as well.
A Wi-Fi password is used to secure the transmission. The techniques using that password have changed over the years with the hardware updates. The earlier WEP encryptions were used in A and B transmissions and resembled a MAC address. WEP was both lengthy and difficult to set up. When G transmissions were introduced, designers incorporated a password in the Wi-Fi Protected Access (WPA)
encryption. It was much simpler to use and became more popular. Its popularity and widespread use made it a target for attacks. To fix this issue, the WPA version 2 standard was quickly implemented for G, N and AC networks. It featured a stronger encryption and proved to be more durable to attacks than WPA. Recently, encryption has been upgraded to the WPA version 3 standard. This is the most secure encryption used with AC and AX networks. However, the hardware must be able to support the speed and encryption. Due to this, the implementation of WPA3 has been slow.
Additional settings for wireless are as follows: The SSID, and the Channel. The name of a network that is visible when connecting to a wireless networking is called the SSID. This SSID does not have to be visible; it can be hidden. Sometimes a hidden network will not show up on certain operating systems as it is assumed the technicians wanted the network to remain private. Devices can still connect to hidden networks, but users must type the name of the network to connect to it. In this way, a hidden SSID acts as a second password. A wireless network can use several access points with the same SSID name to provide for a larger Wi-Fi network. This is what a hospital does to provide the same network on the multiple floors. All the access points use the same eSSID, acting as multiple access points for the same Wi-Fi network. Most biomedical technicians will just have to be aware of these settings and know that a device can still join a hidden network if the correct name is manually entered.
Additional advanced settings for wireless include a channel setting, which are the individual frequencies for broadcast. Channels allow for multiple access points to operate in the same area without interfering with each other. This is due to the frequencies used in the Wi-Fi channels. B, G and N uses a 2.4 GHz range, and the A, N, AC and
AX uses the 5GHz range. The 5 GHz Wi-Fi range has more channels with less overlap, making it the preferred standard for new access points. Newer Wi-Fi uses a much wider set of channels, N and older Wi-Fi uses an older standard with much fewer options for separating channels. This led to more interference in public areas such as hospitals or apartment buildings. N could flex to a newer 5GHz channel setting allowing it to operate more access points in the same area of use. Other advancements also made AC and AX able to coordinate channel usage between access points, allowing for even more Wi-Fi networks to operate in the same area. AC and AX also used the 5GHz range. A site scan will show the number of transmitters in an area. This is an app that is available for most phones. Keep in mind that frequency detections will be limited by the phone hardware.
This may seem to be a bit overwhelming but remember when networking with Wi-Fi, always lookup the hardware capabilities and check the compatibility to intended access points. For example, remember a certain pump with an ABGN card? That was the Wi-Fi that the NIC was compatible with. An ABGN card is a 2.4 GHz, N compatible card that will handle up to WPA2 encryption. In this case, the wireless network needs to use hardware compatible with these networks. This example really solidifies why understanding these settings are of importance to a biomedical technician. It will just happen to come up more and more as medical devices become wireless.
Garrett Seeley, MS, CBET, Biomedical Equipment Support Specialist, VISN 17: VA North Texas Health Care System, Dallas Veterans Affairs Medical Center.












An online resource where medical equipment professionals can find all the information needed to help them be more successful! The easy to navigate Bulletin Board gives you access to informative blogs, expos and events, continuing education opportunities, and a job board. Visit MedWrench.com/BulletinBoard to find out more about this resource. Follow MedWrench on facebook.com/medwrench & linkedin.com/company/medwrench

CONTINUING EDUCATION Visit BulletinBoardMedWrench.com/ for more details and to register for these upcoming classes.

October 2 - 6, 2023 Maull BiomedicalsContrast Injector Service Training


September
October 9 - 20, 2023
Tri-Imaging Solutions - BMET to Imaging 1



October 23-27, 2023
Tri-Imaging SolutionsHologic Dimensions
October
November
Diag nost ic Solut ion s i s a c u stome r se r v ice ba se d pa r t s prov ide r t hat s pe c ia l i ze s i n a l l i mag i ng moda l it ie s a nd manufacturers Created to offer hos pita l s a nd I SO’s a cost ef fe c t ive a nd t i me sav i ng solut ion for or de r i ng i mag i ng r e place me nt pa r t s , e qu ipme nt move s , u lt r a sou nd probe r e pa i r a nd on-site se r v ice.


Contac t u s today, we a re conf ident you w i l l see u s a s T H E Pa r t s Solut ion! diagnostic-solutions.com





J oin us as we celebrate the TechNation community. You - each and every reader, Webinar Wednesday attendee, HTM Jobs user and MD Expo attendee - are the most important part of the TechNation community. Share a photo of yourself, a colleague or the entire biomed team on social media and tag it with #IamTechNation. Then, check each issue of the magazine to see yourself and all of the men and women that are TechNation.
Hanging out with a couple of the bests in the business today at the headquarters of Try-touch Services and The HTM Academy. ZRG Medical is excited to be partnering with Gary Nop and Kevin Davis at ERD Medical Equipment Services on a new pathway to education, hands on, on-site training for those looking to enter the HTM field.



 Photo by Sydney Humes-Carlson
Photo by Zach Johnson
Photo by Sydney Humes-Carlson
Photo by Zach Johnson


