

MD EXPO KEYNOTE BINSENG WANG EXHIBIT HALL BIOMED COLLEAGUES COLLEAGUES SEMINAR RTLS BOOS & BREWS FREE RIGHT TO REPAIR CAREER IT WOMEN IN HTM WOMEN IN HTM PEERS PEERS TOOLS OF THE TRADE LIVE! EDUCATION FLORIDA DR. LARRY EXPERT VIP CBET FUN FBS SEMINAR GROWTH YP’S MEEKS & SCHULTE HTM B-HAWK FBS 140+ EXHIBITS OCTOBER OCTOBER CARIBE ROYALE ORLANDO CARIBE ROYALE REVERSE EXPO REVERSE EXPO TOOLS OF THE TRADE LIVE! BIOMEDS ROCK HTM ACI ACI VENDORS LEADERSHIP DOOR PRIZES DR. LARRY DR. LARRY 140+ 36 CEU’S 36 CEU’S CE ACI DEMOS VIP PASS KNOWLEDGE KNOWLEDGE BIOMEDS ROCK KNOWLEDGE EXPERIENCE TOOLSCZAJKA TOOLS EXPERTS SHARE JOBS CE ENGAGE ACI CBET HTM ACI FBS YP’S SUN SUN SUN YP’S FREE! ENGAGE YP’S PATIENT SAFETY NETWORKING VENDORS FREE! REGISTER VENDORS MEDICAL NETWORKING EQUIPMENT SERVICE FINALE PARTY RTLS TOOLS CONFERENCE CONFERENCE CONFERENCE SUN ATTENDEES MEDICAL CONNECT CONNECT COMPLIANCE COMPLIANCE TECHNOLOGY TECHNOLOGY TECHNOLOGY GROWTH GROWTH GROWTH CARIBE ROYAL ORLANDO SOLUTIONS EQUIPMENT SERVICE BOOS & BREWS BOOS & BREWS Orlando, Florida OCTOBER 29-31, 2023 APPROVED FOR 36 CEU CREDITS BY THE ACI. Vol. 14 1technation.com ADVANCING THE BIOMEDICAL / HTM PROFESSIONAL OCTOBER 2023 See page 32 for more info.
SEE THE BMET PACK IN ACTION!
















Contact Us Today for All Your Device Needs! ISO 9001:2015 & 13485:2016 Certified 20 MORGAN, IRVINE, CA 92618 US 333 NORTHPARK CENTRAL DR, HOUSTON, TX 77073 CALL: 855-888-8762 VISIT: USOCMEDICAL.COM USOC300168425 Philips M3001A MMS Main Board USOCX220901 Philips X2 M3002A Main Board USOC66402 Philips MP20 Main Board with Red connector (SW H and Higher) USOC68450 Philips MP50 Main Board Old Style (SW A to G) USOC64105411 MP50 New Hardware Rev, SW goes from H and higher USOC HAS YOU COVERED. From Boards to plastic pieces, USOC is stocked and ready to ship. NEED PARTS?

We provide authentic imaging and biomedical parts through our OEM allegiant relationships. sebiomedical.com | (888) 310-7322








BIOMED SHOP YOUR Rentals • Sales • Service infusion pumps patient monitors Surgical Microscopes – Patient Monitors – Infusion Pumps You need it? We sell it. | Need extra? We rent it. | Is it broken? We fix it. Oh, and enjoy the beef jerky (you’ll see). SURGICALMICROSCOPES.COM PIOBIO.COM ADEPTOMED.COM
ONE STOP
You need to know it’s right. Who’s in your corner?
Our technicians know what’s at stake and are trained on a wide variety of OEM equipment. Our rigorous standards and inspections ensure that pre-owned equipment meets final approval before being labeled “T.Certified” and heading out the door. We understand the needs of healthcare professionals and work to provide the most efficient, detail-oriented solutions.




We’re in YOUR corner. Call today to schedule repairs or to purchase parts and equipment.

800.297.2241 | Tenacore.com
Monitors | Modules | Telemetry Infusion Pumps | Defibrillators | ESU




OEM NE W BOOTH 804 Come Visit Us

Centers of Excellence for Ultrasound Probe and MRI Coil Repair, and Design & Manufacturing When You Are Down... Is your current ultrasound probe repair provider costing you or saving you time and money? Scan here to see how our clients are optimizing their uptime and budget with Innovatus Imaging. 844-687-5100 customercare@innovatusimaging.com
P.12 SPOTLIGHT
p.12 Professional of the Month: Richard L. Roettger, CHTM, CBET
p.14 Company Showcase: IMT Analytics
p.18 Department of the Month: Samaritan’s Purse World Medical
Mission Technical Support Department
p.20 Next Gen: Katherine Navarro
p.24 Shifting Gears: Tyler’s Hope
P.28 INDUSTRY UPDATES
p.28 News & Notes
p.34 Ribbon Cutting: Cynch!
p.36 AAMI Update
p.39 ECRI Update
P.40 THE BENCH
p.40 Biomed 101
p.43 Tools of the Trade
p.45 Webinar Wednesday
50
P.46 FEATURE ARTICLES
p.46 Roundtable: Ultrasound Probes
p.50 Cybersecurity Hygiene: Updated Guidance for HTM

P.57 EXPERT ADVICE
p.57 Career Center
p.58 [Sponsored Content] Avante Health Solutions
p.61 The Future
p.62 [Sponsored Content] Innovatus Imaging
p.64 [Sponsored Content] ReNew Biomedical
P.66 CONNECTED
p.66 Cybersecurity
p.68 Health-ISAC
p.70 Networking Notes
p.74 HIMSS
p.75 Get Connected Company Directory
TechNation (Vol. 14, Issue #10) October 2023 is published monthly by MD Publishing, 1015 Tyrone Rd., Ste. 120, Tyrone, GA 30290. TechNation magazine is dedicated to providing medical equipment service professionals with comprehensive, reliable, information concerning medical equipment,
service and supplies. It is published monthly by MD Publishing, Inc. Subscriptions are available free of charge
qualified individuals within the United States. Publisher reserves the right to determine qualification for a free subscriptions. Every precaution is
to
information,
and
in the articles and advertisements
parts,
to
taken
ensure accuracy of content; however, the
opinions,
statements expressed
herein are those of the writer and/or advertiser, and not necessarily those of the publisher. ©2023 CONTENTS
46
October 2023 | TechNation 9
PUBLISHER John M. Krieg
VICE PRESIDENT Kristin Leavoy
VICE PRESIDENT Jayme McKelvey OF SALES
EDITORIAL John Wallace
CONTRIBUTORS
Roger Bowles
K. Richard Douglas
Jim Fedele
Joie Marhefka
Manny Roman
Connor Walsh
David Witt
Steven J. Yelton

ACCOUNT
EXECUTIVES
ART DEPARTMENT
Megan Cabot
Emily Hise
Karlee Gower
Taylor Hayes
Kameryn Johnson
DIGITAL SERVICES
Cindy Galindo
Kennedy Krieg
Haley Wells
EVENTS Kristin Leavoy
WEBINARS
HTMJOBS.COM

ACCOUNTING
CIRCULATION
Linda Hasluem
Kristen Register
Sydney Krieg
Diane Costea
Joanna Manjarrez
EDITORIAL BOARD
Rob Bundick, Director HTM & Biomedical Engineering, ProHealth Care
Carol Davis-Smith, CCE, FACCE, AAMIF, Owner/ President of Carol Davis-Smith & Associates, LLC
Jim Fedele, CBET, Senior Director of Clinical Engineering, UPMC
Bryant Hawkins Sr., Site Manager, Children’s Hospital of New Orleans
Benjamin Scoggin, MBA, MMCi, Director, Clinical Engineering | Biomedical Operations, Equipment Distribution, Clinical IT, DHTS, Duke Health Technology Solutions
Allison Woollford, Biomedical Equipment Specialist at Duke University Health System
MD Publishing / TechNation Magazine 1015 Tyrone Rd., Ste. 120, Tyrone, GA 30290 800.906.3373 • Fax: 770.632.9090 Email: info@mdpublishing.com www.mdpublishing.com Proud supporters of
P.79 BREAKROOM p.79 [Contest] What’s on Your Bench? p.81 TechNation Poll p.83 Biomed Brainbuster p.85 GBIS Scrapbook p.87 NCBA Scrapbook p.89 Where in the World is Ben C.? p.94 #IamTechNation p.90 Service Index p.93 Alphabetical Index 94 Like us on Facebook, facebook.com/TechNationMag Follow us on LinkedIn, linkedin.com/company/iamtechnation Subscribe to TechNation TV, youtube.com/@TechNation_TV 10 TechNation | October 2023
FALL 2 COHORT BEGINS ON 30 OCTOBER 2023
WINTER 1 COHORT BEGINS ON 8 JANUARY 2024

PROFESSIONAL OF THE MONTH Richard L. Roettger, CHTM, CBET
BY K. RICHARD DOUGLAS
ounded in 1870, Ohio State University (OSU) was first known as the Ohio Agriculture and Mechanical College. Today, OSU is nationally known for its NCAA Division I sports teams, its research and its endowment.
The university has a college of medicine and operates both cancer and heart hospitals. Its two primary teaching hospitals include the Ohio State University Wexner Medical Center and the Nationwide Children’s Hospital. Both are nationally ranked.
Richard L. Roettger, CHTM, CBET, is a manager in the OSU Wexner Clinical Engineering Department. When Roettger first considered the field, he didn’t even know that biomed was a career choice.
“Originally, I had no clue that this was even a career field or that medical equipment even broke or required regular maintenance. My senior year of high school, I went to enlist in the United States Army. I wanted to do anything with computers; primarily computer programming,” he says.
Roettger says that the liaison plugged his test scores into the computer and began showing him jobs that he qualified for.
“When Biomedical Electronics Technician popped up, the liaison got very excited talking about the job and that it was rare that it even came up, especially for a high school student. She showed me an old 1970s video on what the job and training entailed. It was definitely a career field I could do once I left the service, so I enlisted as a 35G Biomedical Equipment Specialist,” he adds.
Like many biomeds, Roettger received his training in the military.
“My formal biomed training was at Fitzsimmons
Army Medical Center at the United States Army Medical Equipment and Optical School (USAMEOS). I was in Class 02-95G, which started in October of 1994. We started with 17 soldiers and two Navy sailors. We graduated in September of 1995 with 19 freshly minted biomedical equipment technicians, but only about 50 percent from the original starting class,” Roettger remembers.
He says that back then, they would actually build circuits on a bread board and then test them out in normal operation and then introduce failures to see how it affected readings on the multimeter and oscilloscope.
“I was fortunate enough in 2004 to go back and be an Instructor at the Tri-Service School at Sheppard Air Force Base in Wichita Falls, Texas,” Roettger says.
Today, Roettger manages the clinical engineering department for five hospitals and 16 technicians.
BIG PROJECT MAKES FOR A LEARNING EXPERIENCE
Roettger is involved in a building project; a 25-story smart hospital which is scheduled to go-live in 2026.
“This project started about five years ago with just a concept and many brainstorming sessions, with what a hospital of the future should be, and how we could leverage technology to meet the needs of future patients,” he says.
Roettger says that the project received input from various departments and employees from clinicians to support staff and even patients on current challenges and what would make their experiences more enjoyable.
“It has definitely been a learning experience working with project managers and designers to ensure clinical engineering’s needs are met so we can best support all the medical devices in the new hospital but also working with clinical leadership to understand their work flows and ensure that equipment selections and building layouts meet their needs as well. The greatest challenge I have had is reviewing
SPOTLIGHT
12 TechNation | October 2023
F
architectural drawings to ensure things like network drops for the patient monitoring network are captured,” he says.
He says that the project is the largest project he has ever worked on.
“I am looking forward to the final stages of this project and actually installing and validating all the new medical devices for this new hospital,” he adds.
Roettger says he has two amazing children. His daughter, Makayla, is part way through her sophomore year at OSU studying criminology.

“My son, Noah, is starting his sophomore year in high school, where he has been taking an engineering college course. He currently wants to be an aeronautical engineer,” Roettger says.
Away from the workplace, Roettger enjoys watching movies and listening to music and collecting both.
“I think I have owned ‘Star Wars’ on every media format known to mankind. I love spending time with my kids and engaging in activities and interests they have,” he says.
He says that the success he has had in HTM has not been an individual achievement.
“I have always been surrounded by amazing technicians that I have learned a great deal from, to include my current team at the Ohio State University Wexner Medical Center. I have also been blessed with amazing leaders who have pushed me, put me in uncomfortable situations and then allowed me the freedom to work through solutions,” Roettger says.
“Those have always been the greatest learning experiences for me,” he says.
BIOMETRICS
FAVORITE BOOK: “Dune”
FAVORITE MOVIE:
“Dead Poets Society.” It is a great movie about carpe diem, seizing the day and chasing the dream of what you are passionate about.
HIDDEN TALENT:
I have an unusual ability to remember unnecessary details about unimportant things. I probably won’t remember a person’s name quickly, but I can tell you every detail of a tourniquet machine I repaired in 1995 to include what shelf the literature was on at Madigan Army Medical Center.
FAVORITE FOOD:
Grilled salmon. I typically get this at any restaurant that serves it. I have learned though that seafood in Ohio is not the same as seafood on the coast.
WHAT’S ON MY BENCH?
On my bench I have a Microsoft Surface Pro that I keep notes on everything and all my cheat sheets. It beats the hundreds of half-filled notebooks I used to have. Tons of sticky notes with things I need to remember to do, a bottle of Smart Water because I need all the help I can get, a bag of chips, and my Zen Frog which was a gift from my team to have serenity now during pump PM season.
FAVORITE PART OF BEING A BIOMED?
Teaching and mentoring. In the Army, I realized my legacy was not my individual accomplishments, but the impact I had on the people around me and the knowledge I was able to pass on to them.
October 2023 | TechNation 13
COMPANY SHOWCASE
IMT Analytics Strengthens Its Position in U.S.
IMT Analytics is a high-tech company founded in 1999 headquartered in Switzerland. It specializes in developing, manufacturing, selling and servicing biomedical testing devices.

“Our high-precision gas flow and pressure measurement instruments are used to evaluate and calibrate a wide variety of medical devices, such as life-supporting mechanical ventilators and anesthesia machines,” IMT Analytics Inc. Managing Director Daniel Benz explains. “In addition, IMT Analytics offers test lungs for cost-effective and safe patient simulation; FlowLab, our easy-to-use comprehensive PC software; and, most recently, flow meters for both industrial and medical flow measurement applications.”
The IMT brand is well known globally for dependable, high-quality products and services.
“We’ve been selling our products through our worldwide network of partners and distributors for over 20 years and in some cases your readers may have used our products that were private labeled for companies like Fluke Biomedical, BC Group International and Rigel Medical,” Benz says. TechNation recently found out more about IMT Analytics Inc. via a question-and-answer session with Benz.

Q: WHAT ARE YOU MOST EXCITED ABOUT RIGHT NOW?
BENZ: We are super excited to announce the opening of our new U.S. sales and service facility in Saint Petersburg, Florida this year! IMT Analytics is expanding its operations into the U.S. with our new entity opening in Q4/2023. We will now be able to support our U.S. distributors and end users even better with locally stocked goods and services including calibrations and repairs.

Q: WHAT ARE SOME ADVANTAGES THAT YOUR COMPANY HAS OVER THE COMPETITION?
BENZ: We are a R&D driven company that is agile and responsive to the needs of our customers. Our full focus is on flow and pressure measurement instruments and with continuous innovation as one of our core values, we are committed to the development of new products and services with an emphasis on offering our customers a better user experience. Good examples of this are integrated apps which are available on our CITREX H5 and FlowAnalyser PRO models. These testing apps offer our users a guided and semi-automated testing solution for a growing set of medical devices. Entire test sequences are displayed with images and texts and measurements are taken automatically. The test results are recorded in a PDF report, that can be signed directly on the screen.
Q: WHAT ARE SOME CHALLENGES THAT YOUR COMPANY FACED LAST YEAR?
BENZ: As with all other manufacturing companies we struggled with supply chain challenges, and huge demands during the pandemic. We’ve learned from those experiences and in some ways profited from them. As a result, we’ve improved many of our processes and relationships to make our supply chain more robust. We invested heavily in new product development and in improving our existing products and services.
Q: CAN YOU EXPLAIN IMT ANALYTICS’ CORE COMPETENCIES AND UNIQUE SELLING POINTS?
BENZ: IMT Analytics was born from the need for better test devices for use in developing ventilators and other medical devices. Our designs are created by people who use them in real world situations and demand a better user experience than they can get from other commercially available products. We have decades of experience in developing medical and biomedical test devices and can draw from our engineering sister company IMT Information Management Technology for additional resources when necessary. This allows us to adapt to user needs promptly and develop new innovative products quickly. Paired with our exceptional customer service, we provide our customers with a unique level of support.
SPOTLIGHT
14 TechNation | October 2023
IMT Analytics Inc. Managing Director Daniel Benz
Q: WHAT IS ON THE HORIZON FOR THE COMPANY?
BENZ: We’ll continue to innovate and expand as we have been. We’ve recently launched new products on both ends of our product portfolio and we’re looking for new ways to improve efficiencies and workflows for our customers through collaborations and testing data management.
The FlowAnalyser PRO is our new flagship ventilator and anesthesia testing instrument. It’s a complete redesign of our long-time industry standard, the FlowAnalyser PF-300. We’ve kept the best of the look and feel of the PF-300 series and combined them with the popular user interface of our CITREX H5, then cranked up the capabilities and features to a whole new level.


On the other end of our product line, we have the brand new and easy-to-use FlowMeter series consisting of two models, the F1 and F2, with their exciting new Bluetooth capability and mobile app.
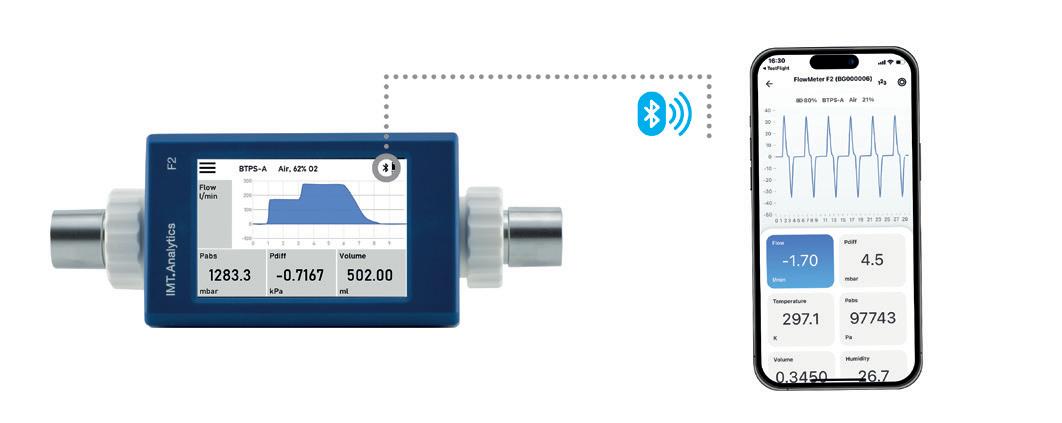
Again, we are very excited about our U.S. expansion. Our new sales and service entity is opening in St. Petersburg, Florida later this year. Its opening will allow us to provide enhanced support to our U.S. distributors and end users. It will be equipped with a calibration laboratory offering domestic calibration and repair services for all our products.
Q: CAN YOU TELL US ABOUT ANY RECENT CHANGES TO YOUR COMPANY, INVENTORY, SERVICES, ETC.?
BENZ: We recently released a completely new product line with our brand-new FlowMeter models. These easy-to-use, battery-powered, compact and versatile, high-precision flow and pressure measurement devices are used in a wide range of medical and industrial applications. As with all our products, we paid special attention to maximizing the customer experience. That’s why we equipped these mobile devices with built-in batteries for up to 10 hours of independent use, made its user interface highly customizable, and featured it with comprehensive data recording. To further elevate the usability, we equipped them with Bluetooth modules and developed an iOS and Android app that we are offering for free to all FlowMeter users.
Q: CAN YOU SHARE A COMPANY SUCCESS STORY WITH OUR READERS?
BENZ: We recently had a customer who was looking for ways to standardize the testing of his medical devices across his service organization. Thanks to the “test sequence” feature of our FlowLab PC software, we were able to offer him a fully customizable solution to create standardized tests that can now be run by his service engineers with our flow analyzers. This has not only helped him to improve quality, by reducing the risk of human errors, but also to significantly increase testing efficiency, since the software takes measurements automatically. The cherry on top is the software creates a PDF test report they can easily attach to their work orders.
Q: CAN YOU DESCRIBE YOUR COMPANY’S FACILITY?
BENZ: Our headquarters are located in Buchs in Switzerland. It’s where all our products are designed and manufactured. It also hosts our ISO 17025 accredited calibration laboratory. We also have a Customer Service office in Singapore, giving us a local presence in the Asia Pacific region, allowing us to ideally support these important markets.
Q: WHAT IS YOUR COMPANY’S MISSION?
BENZ: At IMT Analytics, our mission is to provide our customers with innovative and reliable solutions for their testing and analysis needs. We strive to continuously improve our products and services to meet the evolving demands of the industry, while maintaining a commitment to exceptional customer service and satisfaction.
Q: IS THERE ANYTHING ELSE YOU WANT READERS TO KNOW ABOUT YOUR COMPANY?
BENZ: Yes, we would like readers to know that we would love to hear what challenges they are facing in their daily work as biomedical engineers and how we as an innovative and agile company can support them in overcoming those challenges. We are happy to talk about opportunities. Please don’t hesitate to contact us through the contact form on our website. We are looking forward to hearing from you!
For more information, visit IMTanalytics.com.
SPONSORED CONTENT October 2023 | TechNation 15
ON-DEMAND:
sponsored by RTI Group
“Significant Time Savings When Performing X-ray Testing in the Hospital Environment. A Real-life Case Study.”
sponsored by BC Group


“Tools of the Trade LIVE Demo: Infusion Pump Testing with the IPA-3100”

sponsored by Kontakt
“The Kontakt.io Playbook: Expert Insights on Successful RTLS Deployments in Hospitals”

sponsored by Chronos Imaging

“Is My CT Tube Arcing? How to Diagnose and Troubleshoot”
sponsored by Phoenix Data
“Maintaining Proficiency of a CMMS at All Levels of an Organization”


LEARN, GROW AND BE INSPIRED.
LIVE:
OCT 4 | Crothall
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
OCT 18 | Claroty
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
OCT 26 | Tools of the Trade LIVE with Fresenius Kabi

Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
PODCASTS:
sponsored by MMS
“The Intersection of Technology and Patient Safety”
sponsored by MMS
“Unpacking Congress’ Biggest Bill: What You Need To Know And How To Prepare “
All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.


webinarwednesday.live
CELEBRATING9
Y !SRAE

DEPARTMENT OF THE MONTH
Samaritan’s Purse World Medical Mission Technical Support Department
BY K. RICHARD DOUGLAS
From providing emergency supplies to Sudanese refugees to supplying farming resources to poor farmers in Syria to bringing much-needed aid to the victims of floodwaters in Highland Falls, New York, the Christian organization Samaritan’s Purse is always at the standby.
And those are examples of a few recent events. The organization has been providing help, funding and emergency supplies to those in dire circumstances for more than 53 years. Led by Franklin Graham, the son of evangelist Billy Graham, the organization goes anywhere in the world where help is needed.
For those who fall victim to earthquakes, hurricanes, wars or famine, Samaritan’s Purse has provided aid and comfort, which includes a medical mission. The organization runs 50 hospitals and many smaller clinics and outreach programs. With all these medical facilities, the not-for-profit organization needs HTM professionals to manage, repair, maintain and calibrate equipment.
Samaritan’s Purse World Medical Mission’s Technical Support team is made up of 22 members. Team members include Assistant Director and Technical Support Supervisor David Bucklin; Assistant Supervisor Monte Oitker, CRES; eight full-time biomedical technicians; six “on-call” biomedical technicians; as well as four laboratory, one electrical and one mechanical support staff.
Bucklin explains that Samaritan’s Purse International Relief is a Christian humanitarian aid organization with several programs aimed at meeting the spiritual and physical needs of those who fall victim to war, disease, natural disasters and poverty. Though Samaritan’s Purse is not a permanent health care facility, one of its unique services involves a team of biomedical equipment technicians.
“The catalyst for adding biomeds, in the World Medical
Mission arm of Samaritan’s Purse, came as a result of numerous volunteer doctors describing their experiences working at mission hospitals. Most had little equipment to work with, and chances are it did not work properly, if at all. The need was prevalent, so Samaritan’s Purse hired a biomed and began accepting donations of medical equipment and supplies. Sea containers were loaded with the items and shipped abroad with the intent of improving health care services beyond just the scope of recruiting doctors to come practice,” he says.
Bucklin says that today, this team’s scope of biomedical services expands far beyond their shop in North Wilkesboro, North Carolina.
“Their work is divided between their international ‘customers’ and their own colleagues. For the mission hospitals that Samaritan’s Purse partners with, they offer shipments of supplies and equipment in addition to remote and on-site services. This includes installation/repair/training and consulting. The volume of equipment they are able to source has drastically increased as well. No longer do they rely only on donations, but now have a budget by which they can purchase new and used equipment specific to their clients’ needs,” he says.
Everything that enters their warehouse goes through a double check-out procedure by two different technicians: the first one performs any preventative maintenance (PM) and places it in inventory as “operational.” The second technician performs a functional check and makes sure the device has any necessary accessories and documentation before shipment. The other group this biomed department supports resides within the organization itself. Several medical outreaches have developed as more health care professionals are looking for opportunities to volunteer in short-term missions. The biomedical department therefore prepares equipment and travels internationally with the emergency field hospital, cleft lip and palate, orthopedic, cataract and other surgical teams. The year 2022 saw the biomed team process over 3,000 pieces of medical equipment for mission hospitals and perform PMs on
SPOTLIGHT
18 TechNation | October 2023
approximately 450 pieces [of equipment] for the emergency field hospital and surgical specialty teams.
Data collection is managed through an internally developed inventory and tracking systems.
Also, the establishment of contracts is a little different.
“Our department is unique; we do not work on a contract basis. We provide services as our resources allow,” Bucklin says.
GLOBAL RESPONSIBILITY
With a worldwide backdrop, the biomed team faces challenges and projects that are unique to the organization it supports.
“We have many projects that our team consults, designs and builds. One project we are presently working on is a multi-million dollar, state-ofthe-art cardio-thoracic surgical center at Tenwek Hospital in Bomet, Kenya,” Bucklin says.
During the COVID-19 pandemic, Samaritan’s Purse deployed an emergency field hospital to numerous sites, first in Cremona, Italy, and then various sites in the United States.
“Providing adequate oxygen for use in a respiratory response became critical. We were blessed to have liquid O2 available on most sites but put in motion a project to develop a deployable oxygen generating system with higher output capacity than small portable units. Our field hospital utilized a POGS 33 system in past deployments, but this was not adequate. Our team purchased
EDOCS-120 units (5) and devised a control system that would generate 600LPM for use in future field hospital deployments, if necessary,” Bucklin says.
In 2019, the biomed team began offering a summer internship program which offers two undergraduate students and one international biomed technician the opportunity to spend a couple months at its facility in North Wilkesboro.

“These interns have the opportunity to be exposed to a wide variety of medical devices, perform repairs and PMs and potentially travel abroad to a remote mission hospital to carry out equipment repairs in an environment that provides challenges and where ingenuity is required,” Bucklin says.


While most biomeds serve the needs of a hospital or group of hospitals in their general geographic area, the team at Samaritan’s Purse has oversight over medical devices in every corner of the world.
Nearly 40 years after the first biomed joined the organization, the department has experienced meaningful growth as it strives to keep up with the number of mission hospitals (now more than 50 partner and 100 affiliate locations) and other medical programs that have formed within the organization.
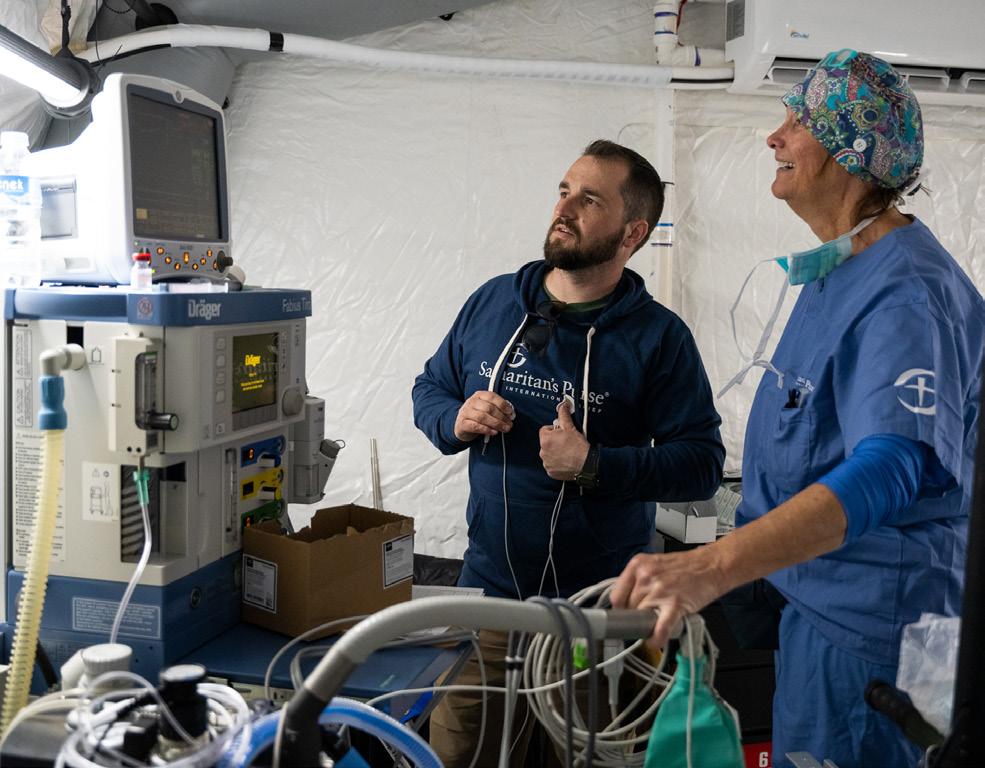
“Our department is unique; we do not work on a contract basis. We provide services as our resources allow.”
October 2023 | TechNation 19
- David Bucklin
NEXT GEN POWERED BY YP AT MD
Katherine Navarro
atherine Navarro earned a Bachelor of Science in Biomedical Engineering from Texas A&M University and currently serves as a biomedical engineer in HTM Operations for the VHA Office of Healthcare Technology Management (HTM).
TechNation recently found out more about her via a question-and-answer session.

Q: WHERE DID YOU GROW UP?
A: Growing up, my dad was in the Army, so we moved around every few years. I lived in Huntsville, Alabama; Fort Hood, Texas; White Sands Missile Range, New Mexico; and Washington, D.C.; but I lived the longest in Huntsville, so that is what I consider my “hometown.”
Q: WHERE DID YOU RECEIVE YOUR HTM TRAINING/EDUCATION?

A: I attended Texas A&M University where I received a Bachelor of Science degree in Biomedical Engineering, and after graduation, I was accepted into the VA’s Technical Career Field (TCF) training program for biomedical engineers. I trained under an experienced biomedical engineer for two years at the Central Texas Veterans Health Care System in Temple, Texas before moving to a staff biomedical engineer position at the South Texas Veterans Health Care System in San Antonio, Texas.
Q: HOW DID YOU FIRST DISCOVER HTM?
A: I first discovered biomedical engineering at a college day at Texas A&M before I started my freshmen year. I had originally been accepted to attend the university with a major in biomedical science, hoping to go to vet school, but after talking with the biomedical engineering representatives, I was so fascinated with the research and medical technology they were involved in that I immediately changed my major. During college, though, my career track was in device design and manufacturing. It was not until I attended a BMES meeting where the Central Texas VA HTM staff presented that I learned about the HTM career. I loved the idea of working in a hospital and applying my biomedical engineering expertise to health care.
Q: WHY DID YOU CHOOSE TO GET INTO THIS FIELD?
A: Growing up, I was always interested in the medical field, and after learning about biomedical engineering and HTM, I thought it was the perfect career for me to apply my love of math, science and problem solving to the medical field. I chose to join the VA because I was raised with an appreciation for serving our country, and I wanted to give back to the veterans like my dad and help provide safe, high-quality health care for them.
Q: WHAT DO YOU LIKE MOST ABOUT YOUR POSITION?
A: I love the mission of the VA to care for our nation’s veterans, and I love all the people I get to work with, especially now at the national level. I meet so many different people who are passionate about what they do. I love being able to serve and support them in projects and initiatives to improve and expand the care and services in the VA.
SPOTLIGHT
20 TechNation | October 2023
K
VERIFY THE INTEGRITY OF EQUIPMENT
The Insulation tester, Leak Tester Tester and Cable Continuity Tester are easy-to-use devices for verifying the functionality of equipment for safety

McGan Insulation Tester


Detect & locate defects such as pinholes, cracks and bare spots in the jacket or coating of laparoscopic and bi-polar electrosurgical instruments
Cable Continuity Tester
Leak Tester Tester
Test the functionality of automated & handheld endoscope leakage testers with healthmark’s new Leak Tester Tester.
Test the quality of monopolar and bipolar cords with this user-friendly, durable device. A green light notifies the user that the cable passed testing.
For more of Healthmark’s intelligent solutions for instrument care and infection control, visit HMARK.COM
INTELLIGENT SOLUTIONS FOR INSTRUMENT CARE & INFECTION CONTROL
HMARK.COM | 800.521.6224
4 11 18 25 2 16 23 30 13 20 27 4 11 18 25 1 15 22 29 12 19 26 11 18 25 8 15 22 29 6 13 20 27 Principles of Servicing Diagnostic X-Ray Systems (Phase 1) Principles of Servicing Diagnostic X-Ray Systems (Phase 1) - eLearning Apr 1-5 Apr 8-12 Advanced Radiographic Systems Maintenance (Phase 2) Advanced Digital Imaging Systems Maintenance (Phase 3) Advanced Diagnostic Imaging Systems Analysis (Phase 4) PACS Engineer/Administrator Certification (Phase 1) eLearning Sep Jan 29- May PACS Engineer/Administrator Certification (Phase 2) eLearning Sep Feb PACS Troubleshooting (3 days) eLearning C.A.M. (Capital Asset Management) Intro to Diagnostic Imaging & PACS For Managers & Sales Professionals 14-16 May 6-10 GE Optima/Discovery/Definium DR Family: Optima XR640/XR646, Discovery XR650/XR656, Definium 6000/8000 2-6 Feb 2 May 20-24 GE Precision 500D R/F 12-16 GE Precision 600FP Mar 1 GE Proteus Oct 30Philips Easy Diagnost Eleva Sep Mar Philips Digital & Bucky Diagnost Oct Jan Apr Siemens AXIOM Luminos TF Siemens Luminos Agile 9-13 Apr 2-5* Shimadzu RADspeed/RADspeed Pro May May Private Practice X-Ray Systems (CPI CMP200 & Quantum Odyssey HF) Injector Systems May GE AMX Portable (IV, IV+) GE Optima XR200/XR220/XR240, & Brivo XR285 Digital Portables Shimadzu MobileDaRt Evolution Portables (Evolution EFX, MX7) 23-27 22-26 Canon RadPro Digital 16-20 Apr 29May 3 Philips MobileDiagnost Digital Apr 29Fuji FDR Go & Go Plus Nov May Multi-Product C-Arm OEC 9800/9900 OEC 9900 C-Arm Sep Feb 26OEC Elite (CFD) Flat Panel C-Arm Philips Veradius C-Arm 16-20 May 3 Philips BV Pulsera C-Arm GE Innova/IGS/Optima Family (21/31/4100, 3XX/5XX/6XX) Philips Allura FD Family (FD10/FD20) Lorad Multicare Platinum Breast Biopsy System GE Digital Mammography (1-week ESSENTIAL only) Hologic Selenia Digital Mammography Hologic Dimensions 3D Tomo Digital Mammography 2-6 Feb 2 Hologic MG Products: Affirm, SecurView, R2, ATEC Sapphire 9-13 5-9 Siemens Inspiration or Novation Apr 29Multi-Vendor Bone Densitometry Dec Apr Principles of Servicing US Systems (2.5 days) Sep Dec Mar Philips Epiq 5 & Epiq 7 (2.5 days) Sep Mar Philips iU22/iE33 (2.5 days) GE Logiq E9, Vivid E9 (2.5 days) 11-13 Principles of Servicing Multivendor CT Systems GE Optima, Brivo, VCT, LS, BS CT Family GE Revolution & Discovery CT Oct Philips Brilliance Family Siemens Sensation Family Siemens Definition Family Servicing Multivendor MRI Systems Siemens MRI Family (Aera, Skyra, Espree, Avanto) GE Excite & GEMS MRI Family (X) Principles of Servicing Nuclear Medicine Systems Nov 27- Feb 26Siemens Symbia Family Dec CRES CERTIFICATION CRES Certification Prep Oct Apr Mar 18-29 Mar 11-22 Apr 8-19 MD Expo Orlando, FL Jan 15-26 Oct 16-27 Sep 13-15 Duluth, MN Sep 6-7 Chicago, IL Dec 11-22 T H A N K S G I V I N G Sep 25-Oct 6 Oct 2-13 L A B O R D A Y N E W Y E A R S C H R I S T M A S May 6-17 NOVEMBER DECEMBER Nov 27-Dec 8 Nov 6-17 Jan 8-19 Feb 5-16 Mar 4-15 Jan 22-Feb Tues M E M O R I A L D A Y SEPTEMBER OCTOBER JANUARY FEBRUARY MARCH APRIL MAY MRI TRAINING COURSE TITLE EXHIBITS, PRESENTATIONS & NOTES WOMEN'S HEALTH NUCLEAR MEDICINE CT C-ARMS NETWORKING & DIGITAL INFO X-RAY CERTIFICATE SERIES MANAGEMENT PRODUCT SPECIFIC CARDIAC PORTABLES ULTRASOUND Apr 8-19 Apr 15-26 May 6-17 Dec 11-22 Mar 18-29 Sep 11-22 Sep 25-Oct 6 Oct 16-27 Oct 30-Nov 10 STATE OF OHIO REGISTRATION NO 93-09-1377T WWW.RSTI-TRAINING.COM/REGISTER CHECK OUT OUR COURSE CALENDAR! rsti-training.com | registration@rsti-training.com 2023-2024 Course Schedule Register online at rsti-training.com/register or call 440-349-4700 Improving the quality of diagnostic imaging service through knowledge, education and Fully Engaged Hands-on Learning™. ISO 9001:2015 Certified (IQC Certificate No. Q-1158); State of Ohio Reg. No. 93-09-1377T
SHIFTING GEARS
Tyler’s Hope
BY K. RICHARD DOUGLAS
even-year-old Tyler was like so many boys his age; he loved sports and played several. While practicing basketball, his dad noticed that Tyler’s arm was shaking uncontrollably. That was in 2005 and Tyler’s parents took him to many specialists who all failed to make a diagnosis of this mysterious condition.
Eventually, while in the hospital, Tyler was examined by Dr. Michael Okun. Okun was able to diagnose Tyler with dystonia. Because Tyler had acquired dystonia at such a young age, the form of the condition was early-onset.
Dystonia is a condition characterized by involuntary muscle contractions, twisting of specific body parts such as an arm or a leg, rhythmic tremors and other uncontrolled movements. A gene mutation is most commonly the cause. There currently is no cure for the disorder. There are a half-million people affected with the disorder in North America.
Because the condition is linked to a gene mutation, that means that other siblings can also carry that same gene mutation. That turned out to be the case. Tyler’s sister, Samantha, was diagnosed with generalized dystonia and there is a chance that Tyler’s younger brother could eventually show some symptoms of the condition as well.
For most parents, getting a diagnosis and any available treatments would be the scope of their response. Tyler’s parents took their response a big step further.
Rick Staab, CHTM, chief executive officer of The InterMed Group and his wife established the Tyler’s Hope Foundation, a 501(c)(3) organization, to advance research for a cure, discover effective treatments and to bring awareness and education of dystonia. The organization also supports the annual Tyler’s Hope Summit on Dystonia Research.
“Our journey began in 2005 when our oldest son, Tyler, was diagnosed with dystonia and received care at the University of Florida. We quickly realized that dystonia research
received very little funding, despite the presence of a known gene mutation and protein associated with the condition,” Staab says.
He says that understanding the significance of targeted interventions, they took on the responsibility to raise substantial funds and accelerate the timeline for finding a cure, not only for their children but also for others affected by dystonia.
“The dedication of Dr. Michael Okun and Dr. Kelly Foote, who are my children’s neurologist and neurosurgeon, respectively, along with their patient-centric approach and commitment to translational research, has gained widespread recognition. Their motto, ‘The patient is the sun, and the health care team should orbit around the patient’s needs,’ has resonated throughout the medical community,” Staab says.
He says that they designated the University of Florida as their first Center of Excellence in recognition of the university’s groundbreaking research.
“As part of this designation, the center established a fellowship program, which we fund, resulting in one of the largest movement disorder fellowships in the country, if not the largest. These fellows have since moved on to other institutions, furthering their research and expanding the field,” Staab says.
He says that by closely monitoring dystonia research publications and proactively reaching out to researchers and institutions, they are able to establish connections and offer support and funding whenever possible.
“Our successful partnership with the University of Florida and the extraordinary return on investment in terms of scientific advancements and breakthroughs have cemented their status as a leading institution in dystonia research. While there may be competing funding priorities and various conditions requiring research, our passionate pursuit of a cure coupled with the exceptional work of talented scientists has garnered attention and support for dystonia research,” Staab adds.
THE HOPE WEEKEND
The efforts of the Staabs and the researchers and medical professionals becomes even more focused once a year
SPOTLIGHT
24 TechNation | October 2023
S
with The Hope Weekend every October. The 2023 Hope Weekend is October 19-21.
“Preparation for The Hope Weekend involves the formation of a planning committee consisting of our executive director, board members and dedicated volunteers. The committee begins by creating a budget, securing event locations, confirming vendors, recruiting speakers and developing marketing materials. We also proactively approach companies to donate items for the auction, aiming to offer a diverse range of items and maximize funds raised for research,” Staab says.
He says that at the same time, they coordinate with entertainment providers, such as live music and bagpipers, and organize swag bags, awards and various marketing initiatives like PSAs, banners and radio/TV spots.
“Throughout the planning process, we strive to keep the mission of celebrating dystonia progress and raising funds for a cure at the forefront of everyone’s minds,” Staab says.



The Hope Weekend requires year-round planning and preparation with planning for the next event starting just after the conclusion of the current one.
“We utilize the months of October and November to initiate preparations for the following year’s event. Given the scale of the undertaking, we require ample time to ensure its success. Tasks during this period include reserving space for appreciation dinners, setting golf tournament dates, sending Save the Date reminders to sponsors and potential participants. It’s essential to remind people early on about the upcoming Hope Weekend,” Staab says.
The Summit brings together important stakeholders within the dystonia research community.
“The Tyler’s Hope for a Dystonia Cure Summit on Dystonia Research is a collaborative effort between the Tyler’s Hope Foundation and the University of Florida Health, where our first Dystonia Center of Excellence is located. We primarily extend invitations to renowned researchers in the field of dystonia research and care, including Ph.D. students, new researchers and fellows with an interest in dystonia research. Additionally, we actively participate in neurological and disease-related conferences to stay informed about ongoing research efforts. We believe it is essential to incorporate diverse perspectives and ideas, even from those studying other diseases and disorders,” Staab says.
He says that originally inspired by the concept of the Manhat-
tan Project, where top researchers were brought together to solve a problem, they organized the Dystonia Summit as a think tank.
“While we cannot physically confine participants like in the Manhattan Project, our aim is to gather the world’s leading scientists, break down institutional silos and foster collaboration. We emphasize that at the Summit, we unite to find a cure for children and individuals impacted by dystonia, urging everyone to set aside their egos and focus on the greater cause,” Staab says.
The Summit has been active for 14 years and has attracted researchers worldwide who share their work, learn from others and contribute to the growing attendance numbers each year.
Many of the functions of Tyler’s Hope and The Hope Weekend could not be possible without the efforts of volunteers.

“Tyler’s Hope for a Dystonia Cure is led by a remarkable volunteer board, surrounded by individuals who generously dedicate their time and energy to our cause. Many of the InterMed teammates actively volunteer for various Tyler’s Hope events. Prior to each event, we reach out to local individuals to enlist their volunteer support. While some may initially have limited knowledge about our cause, they often become deeply connected once they understand our mission,” Staab says.
In March, the Tyler’s Hope Center of Excellence at Duke University joined the Dystonia Center of Excellence at the University of Florida.

A 7-year-old’s diagnosis in 2005 has led to a movement to find a cure for this vexing condition. The perseverance of the Staabs, volunteers and researchers will eventually pay off.
Donations can help support research and education for Tyler’s Hope for a Dystonia Cure.
For more information, visit www.tylershope.org.
Donations: bit.ly/3Eguqrh
Volunteer registration: www.tylershope.org/volunteer-registration.html
October 2023 | TechNation 25
THANK YOU! TO OUR 165,000+ USERS
- Ben C. REGISTER NOW!
is a product focused support network where medical professionals, purchasing administrators, manufacturers, dealers and industry experts can provide opinions, share ideas, and gather relevant information on medical technology and equipment. We want to say thank you for all of our users over the years. MedWrench wouldn’t be here if it wasn’t for our users!
















The manufacturers listed are the holders of their respective names and/or trademarks, and are not to be taken as an endorsement or a liation with AIV, Inc. Infusion Pump Support & Power Solutions 776C Infusion Pump Support Fixed or Flat Rate Repair Options on Many Popular Pumps AIV-Manufactured Replacement Parts PowerMATE Family AIV’s Special Purpose Relocatable Power Taps Check out the PowerMATE -CM with current monitoring! Visit our Website! Come visit AIV in Booth #301 for all your infusion pump and power needs!
NEWS & NOTES
Updates from the HTM Industry
PIEDMONT RECOGNIZED BY LOWN INSTITUTE, SEEKS BIOMEDS
Piedmont Healthcare, Georgia’s largest healthcare provider with locations that reach 80% of the state, has been certified as a Great Place to Work and in 2023, Piedmont has earned recognition from Newsweek as one of America’s Greatest Workplaces for Diversity and also as one of America’s Greatest Workplaces for Women. In 2022, Forbes ranked Piedmont on its list of the Best Large Employers in the United States.
Piedmont has been recognized by the Lown Institute for outstanding social responsibility with five of its hospitals – Piedmont Athens, Piedmont Cartersville, Piedmont Fayette, Piedmont Eastside, and Piedmont Newnan – receiving an “A” grade on the 2023-24 Lown Institute Hospitals Index. The Lown Institute is a nonpartisan think tank that generates bold ideas for a better system of health.
Piedmont achieved this honor through strong performance across metrics of health equity, patient outcomes, quality and safety, and value of care, out of more than 3,600 hospitals nationwide.
CYNERIO, CHECK POINT TEAM UP
Cynerio and Check Point Software Technologies have announced a partnership to provide health care organizations with comprehensive security for medical IoT devices. Cynerio’s 360 platform will provide functionality critical to securing health care IoT devices including device discovery, patch guidance, microsegmentation and attack detection. By pairing this functionality with threat prevention and remediation technologies provided by Check Point Quantum IoT Protect, health care organizations will receive complete device security protection.
“The global health care industry continues to see increasing attacks resulting in impacted patient care, multi-million dollar recovery costs, and exposed patient records numbering in the tens of millions,” said Cynerio CEO Leon Lerman. “We are excited to partner with Check Point to provide health care organizations with a comprehensive solution that will secure their medical IoT devices. This partnership joins Cynerio’s deep expertise in medical IoT security and Check Point’s market-leading threat prevention technology. Together, we will help health care organizations protect their patients, data and operations from cyber threats.”
Piedmont Athens ranked first out of 99 hospitals in Georgia and 131 nationally on the Social Responsibility metric. According to the Index, the hospital also received “A” grades in value of care, cost efficiency, and was named a Top Hospital for Fair Share Spending. Piedmont Athens also ranked a Top 5 in Georgia for Clinical Outcomes, including quality and safety, and received “A” grades for patient outcomes and clinical outcomes.
“We are honored to be recognized as Georgia’s No. 1 hospital for social responsibility,” said Piedmont Athens Regional CEO Michael Burnett. “Piedmont Athens is committed to improving the health of our entire community and investing in programs and services that reach all patients in our service areas.”
Piedmont is looking for qualified candidates for its in-house biomedical engineering and diagnostic imaging services team. Interested candidates can email a resume to Dan.harrison@piedmont.org.
HDO SIGNS CONTRACT FOR SECURITY ROBOT
Knightscope Inc., a developer of autonomous security robots and blue light emergency communication systems, recently announced that a California-based health care organization signed a contract for its K5 Autonomous Security Robot (ASR) service at a San Francisco Bay Area hospital.
The client is a national leader in health care quality with more than 20 hospital locations and over 50,000 employees serving health care needs across urban, suburban and rural communities. Health care organizations across the U.S. have partnered with Knightscope for many years to make advancements in various critical areas of physical security and in patient satisfaction. Knightscope is improving the patient experience in a variety of ways and it is aiding health care organizations in maintaining or increasing their HCAHPS scores as referenced in a recent blog highlighting the use of its technologies in hospitals and health care facilities.
INDUSTRY UPDATES 28 TechNation | October 2023
FSI COMPLETES SOC 2 TYPE 1 AUDIT
FSI, a health care CMMS provider, has completed a System and Organization Controls (SOC) 2 Type 1 audit.
“The SOC 2 audit is one of the highest recognized standards of information security compliance in the world. Achieving a certification of this level signifies FSI’s dedication to maintaining data security and integrity at the highest level,” a press release states.

Developed by the American Institute of CPAs (AICPA) to allow a third-party auditor to validate a service company’s internal controls with respect to information security, the SOC 2 Audited Report is the auditor’s opinion on how an organization’s security controls meet the SOC 2 criteria.
“Successfully achieving compliance shows that our policies, procedures and infrastructure meet or exceed the SOC 2 criteria,” expressed Derek Smith, director of technology at FSI. “Our diligence ensures our customers can trust and rely on us for security and data privacy, which is becoming more important every day. This proves our commitment to improving our information security program for our customers’ success.”
To obtain an audited SOC 2 Report, a third-party auditor
reviewed internal controls, including policies, procedures and infrastructure regarding data security, firewall configurations, change management, logical access, backup and disaster recovery, security incident response and other critical areas of FSI’s business.
A Type 1 audit pinpoints a specific timeframe in which to assess security processes, while Type 2 assesses these processes over a period of six months. FSI has completed the first phase and is seeking a Type 2 audit next.

October 2023 | TechNation 29
PROCUREMENT ‘AN INTEGRAL PART’ OF ARMY MEDICAL MAINTENANCE MISSION
When working on highly complex medical devices, the repair bills for new parts can rack up quickly.
Parts for computed tomography or portable X-ray machines, for example, can soar past $25,000, which U.S. Army regulations state require additional oversight at the command level for purchasing.
It’s a common occurrence for the U.S. Army Medical Materiel Agency’s medical maintenance teams across the country, including at the agency’s Medical Maintenance Operations Division at Tracy, California, or MMOD-Tracy.
“For us specifically at Tracy, we often saw that problem with the $25,000 threshold because of a lot of the parts for imaging machines that we specialize in put us over that limit,” MMOD-Tracy Chief of Operations Ian McNesby said. “It was definitely a problem for us.”
To simplify and streamline the process, leaders at USAMMA’s Medical Maintenance Management Directorate, or M3D, created the Maintenance Procurement Office, or MPO, in 2016.
The MPO, made up of 13 civilian and contractor personnel, centralizes the ordering practices for repair parts and services that support all three of USAMMA’s MMODs across the U.S., as well as different medical materiel supplies located at Army Prepositioned Stocks sites around the globe.
The team functions largely behind the scenes and maintains constant contact with leaders at each of the MMODs as they work together to provide valuable sustainment-level maintenance support to operational Army units.
“We are one of those teams in the background,” said Newt Oliphant, one of the MPO team leads. “But we do matter and the warfighter is better able to perform their duties and survive because of what we do.”
M3D Director Jorge Magana likened the MPO’s role to one leg of a three-legged stool, providing resources in the form of medical materiel, Class VIII repair parts and supplies to execute the directorate’s mission.
“The MPO’s work is important as it consolidates, tracks and manages efforts for the entire directorate,” Magana said. “They support the entire maintenance program, which includes the three MMODs, the Medical Materiel Readiness Program and all three APS sites. That is a global presence in supporting medical device readiness.”
USAMMA is a direct reporting unit to Army Medical Logistics Command, the Army’s Life Cycle Management Command for medical materiel.
The MPO, functioning under M3D, supports the agency’s overall mission to deliver medical materiel readiness, synchronizing and integrating strategic sustainment, supply support and maintenance capabilities to enable global health care operations.
Comprised of supply management officers and specialists, equipment specialists and contract administrative support specialists, the team executes purchase orders, bulk purchasing agreements and other procurements through prime vendors and various contracts.
“Without the MPO executing their function, both its assigned tasks and those as needed without additional resources, AMLC, USAMMA and M3D would cease to operate as a whole, and thus, the entire operational force would suffer,” said Jesus Tulud, a retired chief warrant officer five and current M3D contract employee.
Since its creation, the MPO has continued to evolve to better meet the medical maintenance needs of the operating force.
Prior to 2016, MPO functions were essentially split between the MMODs and USAMMA’s contract management section, which “was not medical maintenance-friendly,” Oliphant explained, often resulting in longer wait times.
Among several other responsibilities, the contract office would step in to handle larger purchases, with the smaller procurement operations happening at the MMOD level directly.
“The ability to purchase the vast array of repair parts needed was restrictive and difficult to execute,” Oliphant said.
“We could buy stuff that we needed, but only if it didn’t exceed $25,000,” McNesby said of past operations. “Now, each of the MMODs typically have the attention of at least one of the purchasing agents at headquarters who are there to support our needs. They’ve done a great job.”
Oliphant said M3D saw tremendous improvements in efficiency and turnaround times following the creation of the MPO. Revisions to regulations also made it easier to purchase repair parts to keep the MMODs stocked as needed to meet operational schedules and lessen wait times, promoting high levels of readiness for medical units that rely on their services.
Additionally, the MPO works to improve and maintain vendor relationships, as well as perform a host of administrative tasks, including processing security requirements for contractors, database management, training tracking and customer support.
In short, the office is dedicated to and promotes the medical materiel readiness mission.
“They’re definitely important for not just our operation, but for the warfighter as well,” McNesby said. “They are an integral part of M3D.”
INDUSTRY UPDATES 30 TechNation | October 2023
THE JOINT COMMISSION SHARES CYBERATTACK TIPS
The number of cyberattacks and information system breaches in health care has grown steadily, escalating from isolated incidents to widespread targeted and malicious attacks, according to a 2021 BI&T article.
In 2022, 707 data breeches occurred, exposing more than 51.9 million patient records, according to data from the Department of Health and Human Services (DHHS).
To help health care organizations address this growing patient safety concern, The Joint Commission has issued a new Sentinel Event Alert, “Preserving patient safety after a cyberattack.” The alert focuses on risks associated with cyberattacks and provides recommendations on how health care organizations can prepare to deliver safe patient care in the event of a cyberattack.
The alert stresses that preparing for a cyberattack should not only concern hospital IT staff, but instead all hospital staff. Every staff member must prepare to operate during a cyber emergency. Actions suggested in The Joint Commission alert include:
• Evaluate hazards vulnerability analysis (HVA) findings and prioritize hospital services that must be kept operational and safe during an extended downtime.
• Form a downtime planning committee to develop preparedness actions and mitigations, with representation from all stakeholders.
• Develop and regularly update downtime plans, procedures and resources.
• Designate response teams. Create an interdisciplinary team to mobilize during unanticipated downtime events.
• Train team leaders, their respective teams and all staff on how to operate during downtimes, including specific incidents that would cause downtime to go into effect.
• Establish situational awareness with effective communication throughout the organization and with patients and families.

• After an attack, regroup, evaluate and make necessary improvements. Take steps to recover and protect systems.
“Cyberattacks cause a variety of care disruptions – leading to patient harm and severe financial repercussions,” says David W. Baker, MD, MPH, FACP, executive vice president for healthcare quality evaluation and improvement, The Joint Commission.
“Taking action now can help prepare health care organizations to deliver safe patient care in the event of future cyberattacks. The recommendations in the Sentinel Event Alert, as well as The Joint Commission’s related requirements on establishing and following a continuity of operations plan, disaster recovery plan and more, can help health care organizations successfully respond to a cyber emergency.”
The Sentinel Event Alert also reviews related Joint Commission requirements and provides resources and references. The full alert is available on The Joint Commission and The Joint Commission Journal on Quality and Patient Safety websites.
October 2023 | TechNation 31
MD EXPO APPROACHES ATTENDANCE RECORDS
MD Publishing’s fall MD Expo is set for sunny Florida this October with support from the Florida Biomedical Society (FBS). The MD Expo will be held October 29-31 in Orlando.
Healthcare technology management’s (HTM) continued growth means the next MD Expo could be the most-attendees ever! One reason for the popularity of the event is its top-notch continuing education sessions. It has been approved for 36 CEU credits on a range of pertinent topics hand-picked for HTM professionals.


Registration is FREE for hospital employees, active members of the military and students at MDExpoShow.com.

The biannual healthcare technology management conference also includes an exhibit hall filled with the companies offering ideal products and solutions for biomeds. Don’t forget about the event’s signature networking events – including a Halloween bash with a costume contest!
Advocate Health’s Greg Czajka encourages biomeds to attend.
“MD Expo is a great experience to network and learn from others in the HTM industry! I’ve made new connections each time I attended MD Expo and always walked away with something meaningful,” he said. “I highly recommend attending and getting out of your comfort zone!”
Shawn Molloy, who serves as the network director of clinical engineering at University of Chicago Medical Center, agrees.
“An essential experience to reinforce the support structure and backbone of our career field,” Molloy said about MD Expo. “It gives you the true visibility of the scope of our career field. I compare it to when astronauts see the Earth for the first time and their feeling of one humanity. That is the same feeling I get
when I attend the MD Expo and it revitalizes my engagement in the field.”
The venue for MD Expo is the Caribe Royale Orlando – a destination that offers all the connections you could ever imagine right within reach. Located only a mile and a half from Walt Disney World Resort, this is a conveniently central location to experience all the fun Orlando has to offer.

Find hotel reservation information, including a code for a discounted room rate, at mdexposhow.com/location.
To recap, MD Expo strives to provide healthcare technology management professionals with a unique, intimate and rewarding conference second to none. Clinical engineers, biomedical technicians, directors and managers, procurement/asset managers and others responsible for medical technology will gather in a one-ofa-kind welcoming environment to network with peers, learn the latest technologies and advances in HTM.
Highlights include:
• Industry-leading speakers covering the hottest topics in HTM, compliance, IT, cybersecurity, management and equipment service
• The industry’s most unique networking events to help you connect and share best practices with other leading HTM professionals
• World-class exhibit hall with the latest technology, products and services
For more information about the MD Expo, visit MDExpoShow.com.
INDUSTRY UPDATES
32 TechNation | October 2023
Orlando, FL • October 29-31, 2023


































































































NATIONWIDE 1.877.254.7086 Service@QRS-Solutions.com www.QRS-Solutions.com PRECISION CALIBRATIONS & BIOMEDICAL TEST EQUIPMENT Dependable Support Wherever You Need Us. ECAT Supplier Accredited to ISO/IEC 17025:2017 & National Standard ANSI/NCSL Z540-1-1994 (R2002) and Certified to ISO 9001:2015
RIBBON CUTTING Cynch!

ynch is an asset management platform offering computerized maintenance management system (CMMS) plus purchasing, inventory management, subcontracting and warranty claims. Assets are tracked from purchase through every maintenance or repair until final disposition for a complete and real-time audit trail. The platform is designed for easy addition of new assets on the fly to orders, customers and locations.
Cynch CEO Dan Sallis Jr. recently spoke with TechNation about the company.
Q: WHAT ARE SOME OF THE SERVICES AND PRODUCTS YOU OFFER?
A: Cynch is the best-in-class SaaS CMMS for managing PMs, repairs and assets. Biomeds use Cynch for managing and invoicing PMs and bench orders. Hospitals use Cynch for managing assets and facilities, and for managing PMs and repairs with their in-house biomed staff or third-party biomeds. The solution also smoothly manages inventory, purchasing, vendors, subcontracting, warranties and payments.
Cynch is built on modern technology for lightning-fast performance.
The company is excited to announce that FDA recalls will be implemented Q4 2023!
Q: HOW DOES YOUR COMPANY STAND OUT IN THE MEDICAL EQUIPMENT FIELD?
A: Cynch is laser focused on providing the absolute best technology for managing assets in the medical field to maximize equipment uptime. Because the solution is built using only modern technology, Cynch is fully extensible to match the specific requirements of each customer.
Q: WHAT IS ON THE HORIZON FOR YOUR COMPANY?
A: Although Cynch has been using AI and machine learning for years, we are excited to be rolling out new functionality built on AI to further automate tedious, manual processes.
Q: IS THERE ANYTHING ELSE YOU WOULD LIKE OUR READERS TO KNOW?
A: We have a backlog of implementations! Please contact us right away to be sure your implementation will be completed before the end of the year!
For more information, visit cynch.me.

INDUSTRY UPDATES
C 34 TechNation | October 2023


SOLUTIONS Unlock your potential. ISO 13485:2016 certified Become an Imaging Engineer. Start an exciting career as a Medical Imaging Service Engineer with the Apprentice Program at Tri-Imaging! We have world-class hands on training facility at our facility in Madison, TN. WEEKS 2 BMET to Imaging 1 training WEEKS 2 BMET to Imaging 2 training WEEKS 8 Technical Services 12 WEEK APPRENTICE PROGRAM Visit triimaging.com/training/training-program.html to see our 2023 Training Calendar! 855.401.4888 Located in Nashville, TN
AAMI UPDATE
Technological Intervention to Improve Alarm Management in Acute Care Telemetry Units
BY CORA R.
Telemetry monitoring is intended to improve patient safety and reduce harm. However, excessive monitor alarms may have the undesired effect of staff ignoring, silencing or delaying a response because the alarm is falsely believed to be a “nuisance alarm.” Nuisance alarms are defined as monitoring device alerts that may be either false or true but are nonactionable. Research has shown that the majority of monitor alarms are nuisance alarms and can occur hundreds of times per day.
Using alarms safely has been a priority of The Joint Commission (TJC) since it published a sentinel event alert in 2013 outlining the severity of alarm fatigue in the United States. Of every 98 alarm-related sentinel events, 80 resulted in patient deaths. TJC subsequently implemented National Patient Safety Goal 6 on clinical alarm systems and began auditing hospital compliance in January 2016.
Research has found that between 72% and 99% of monitor alarms do not require intervention and are nuisance alarms that contribute to alarm fatigue. Bonafide et al. found that nursing staff had slower response times to true alarm events among patients who had a larger number of nuisance alarms preceding a true alarm. Research supports excessive alarms as being an important contributing factor to registered nurses (RNs) failing to respond to critical alarms in a timely fashion.

A major contributing factor to excessive monitor alarms is patient outliers. Patient outliers are those patients whose monitoring devices generate the most alarms. In two studies, only a few patients generated greater than 50% of total telemetry monitor alarms. Yeh et al. noted that two patients caused 54% of all alarms in 24 hours, with 91% of those alarms being due to low heart rate. A team of experts assembled by
the National Coalition for Alarm Management Safety found a consistent problem across health settings: Many of the alarms on a given unit were caused by only a few patients. When data were analyzed on a large scale across multiple health systems within this group, it was found that telemetry units had the largest quantity of alarms compared with both intermediate and intensive care unit settings, with high and low heart rate alarms being the most common. In one of the reported practice settings, three patients were responsible for generating 83% of the 719 monitor alarms on a unit. In another, 78% of alarms were attributed to four of 45 patients. Alarm customization, personalized for patient-specific parameters (e.g., heart rate), is suggested as a best practice to reduce the quantity of preventable nuisance alarms. By customizing and adjusting alarm parameters appropriately, the total quantity of nuisance alarms can be reduced.
Alarm technologies (e.g., middleware) are effective at improving alarm notification and response times. Middleware technology allows for customization and filtering of alarms between the primary alarming device and the second receiving device. Using alarm escalation rules within middleware can reduce false alarms and disable alarm escalation after clinical staff acknowledge the alert on the receiving device. In one study in which middleware technology was used to filter alarm tones, the positive predictive value improved, demonstrating a reduction in staff response time to critical alarms.
In many health systems, acute care telemetry units may have the largest quantity of generated monitor alarms; however, limited research exists regarding alarm management in these settings. Most alarm research has occurred in critical care settings. RNs on acute care telemetry units are prone to experiencing the effects of alarm fatigue because they have an increased number of patients, and these patients are more susceptible to frequent false alarms because of increased mobility and care activities. Alarm fatigue was found to be the contributing factor that led to a delayed response to a patient event at the project facility. When RNs were surveyed before
INDUSTRY UPDATES
36 TechNation | October 2023
LEHET, DNP, RN, CNL; JULIE A. LOPEZ, DNP, RN, NE-BC, FACHE; ROBERT J. FRANK; AND MARIA CVACH, AND DNP, RN, FAAN
project implementation, most responded they “usually/always” become indifferent to alarms when they sound repeatedly. Alarm data from the project facility indicated that patient outliers may be a contributing factor leading to alarm fatigue.


This quality improvement (QI) project took place between August 1, 2021, and December 31, 2021, in the department of medicine (DOM) at a large academic medical center after review by the hospital’s institutional review board.

Alarm data analysis demonstrated a statistically significant reduction in overall alarm duration across the four DOM acute care telemetry units. These data suggest that the technological intervention was a beneficial alarm management strategy. Targeting patient outliers by notifying RNs through their mobile phone when patients exceeded the unit’s average alarms per bed per day empowered them to customize alarm threshold adjustments for patients. Literature supports that nuisance alarms often are caused primarily by only one or two patients and that nuisance alarms are an important cause of alarm fatigue. The four DOM acute care telemetry units’ RNs recommended continued use of this intervention as a method to reduce alarm duration, and plans are in motion to expand to other units within the organization.
An unexpected finding, based on chi-square analysis comparing the pre- to postintervention periods, was that an increase in alarm frequency occurred rather than a decrease. A decrease in alarm frequency was predicted based on the

for the increase in alarm frequency was the Omicron variant surge in COVID-19 patients admitted to the four DOM acute care telemetry units during the postintervention data collection period (December 2021). The patients may have required increased monitoring. However, when SpO2 data were excluded from the data set, a statistically significant increase in alarm frequency persisted. Considering the significant reduction in overall alarm duration, paired with an increase in alarm frequency, any alarm duration reduction was viewed as a meaningful finding. Alarm frequency data analysis should be reassessed at a time point not affected by a COVID-19 surge.


In the QI project described here, a technological intervention was implemented with the goal of heightening RN awareness of patient outliers – or those patients responsible for most monitor alarms – and thereby empower RNs to adjust alarms in a meaningful way. The QI project demonstrated a reduction in alarm duration but an increase in alarm frequency. This may have been due to the timing of the project, which occurred during a surge in COVID-19 cases. More research is needed to determine if the technological intervention described here can reduce alarm duration and frequency.
Reference: https://array.aami.org/doi/10.2345/0899-820557.2.67#i0899-8205-57-2-67-fig4
Features: • Simple to use – Accurate and reliable • Customizable Touch Screen • Wi-Fi and USB Computer Connectivity • Report Generation DAP For further details: Contact us at +1 (626) 357-7921, sales@radcal.com or www.radcal.com Need to check the performance of X-ray machines? Then the Radcal Touch meter is your tool of choice. Radcal Touches the World! Visit us at MD Expo Orlando #900, 10/29-10/31 vc_TechNation_Clr Ad_7x4.5_23Aug23.pdf 1 7/21/23 4:54 PM October 2023 | TechNation 37















You asked for an end-to-end imaging equipment solution at a value. 626 delivers it. Acquisitions to expand our services to our customers The world moves fast. We move faster. - The right choice as a partner ISO 13485:2016 Certified 90% We are dedicated to quality management and we ensure best practice in everything we do of our customers would recommend our services to a colleague Consistent YoY Growth Strategic company acquisitions to expand our services to our customers include Walsh Imaging in 2017, ISS in 2019 and Innovatus, CR-DR business in 2020. Recognized by Broward Health Medical Center for corporate community giving Florida Biomedical Society recognizes as an industry leader in 2019 Walsh Imaging recognized as tier Del Medical dealer We are your multi-modality, multi-manufacturer contrast injector service and support solution. We want
your first
call WeAre626.com We are your multi-modality, multi-manufacturer service and support solution. Our number of expert, full-time engineers on staff ready for service +130 Remote diagnostic solution CMMC-1 Cyber certified (800) 516-0990 | weare626.com ISO 13485:2016 CERTIFIED
to be
— and last —
ECRI UPDATE
Keys to Optimizing CMMS Value
BY TOM TOCZYLOWSKI, ASSISTANT DIRECTOR – ALERTS
Discover the challenges associated with legacy equipment asset information and why so many healthcare technology management groups are seeking ECRI’s help with standardization and normalization of CMMS data.
Over the years, healthcare technology management (HTM) teams have leveraged computerized maintenance management systems (CMMS) to enable more efficient work order management aimed at reducing asset downtime and improving maintenance workflows. As workforce challenges have grown and technology has advanced, it is driving an even greater need for automation and operational efficiency.
Many hospitals have looked at upgrading and modernizing these systems and are struggling with how to consolidate, organize and clean the data before migration. Often, inconsistent equipment naming conventions and descriptions, and variable device classifications can impact predictive replacement planning, management of preventive maintenance procedures and work orders associated with cybersecurity threats, recalls and field corrections that could impact the safety of patients and staff. Maintaining a clean and organized CMMS can lead to greater operational efficiencies and improved patient outcomes, especially as health systems become more complex and continue to grow.
Having clean and normalized data is becoming the new strategic imperative; however, it can be an arduous and time-intensive process.
• A well-organized CMMS system starts with consistent naming conventions for the appropriate vendor or OEM, which may change over time as companies merge or are acquired.
• A clean and consistent model name or number across the various assets can also be a challenge as product brands change and different users enter variations of descriptions or
models used to describe the family or brand of device.
• Underlying these are the basic classifications of devices, such as ECRI’s Universal Medical Device Nomenclature System (UMDNS), which drive downstream reporting and processes informed by the CMMS, such as work orders or PM procedures.
Inconsistencies across any of these parameters – vendor, device or classification – can threaten the integrity of your life cycle analysis, preventive maintenance, equipment replacement planning, critical field corrections and recalls.
As consistency is built into the CMMS data, there is the opportunity for improved business processes and efficiencies. As work orders are assigned, whether it be for preventive maintenance or corrective maintenance resulting from recalls or incident reports, the process will run smoother with normalized data in the system. In addition, if there are assets under alternative equipment maintenance (AEM) or advanced analytics and reports being run, the documentation in the CMMS can more easily be organized and tracked with a clean data set. Consistent CMMS data contributes to better outcomes in your patient care, support operations and regulatory compliance audits.
ECRI has seen first-hand the benefits of standardized data and how it has helped many hospitals to improve their HTM operations while mitigating risk and enhancing regulatory compliance. This has been evident in the matching of recalls to inventory for departments that are using ECRI’s Alerts Workflow enterprise solution for recall management. After implementation and integration of the CMMS standardization and normalization, clients reported significant time savings associated with prior efforts to match incoming recalls with medical asset inventory to flag impacted products. Automatic matching, based on a standardized data set, facilitated more expedient attention and closure of work orders associated with impacted inventory.
Learn more about how ECRI can help you optimize the value of your CMMS investment at ECRI.org.

INDUSTRY UPDATES
October 2023 | TechNation 39
BIOMED 101
Technical training and its disappearance in offerings for product
BY MARK NEWELL
ne of the key tools that aid a health care organization in controlling its medical equipment support costs is by having its in-house healthcare technology management (HTM) team attend technical service training for the products it purchases.
This training, provided by a manufacturer or in some cases an independent service company, helps to address concerns around operating costs and total cost of ownership, timeliness of support and flexibility in when planned or unplanned service can be performed. It also helps to provide options for how to extend the life of equipment when the original manufacturer may limit or end support. This access to technical training is an important part of the right to repair movement, and enables health care organizations to have better control of their overall equipment and related support costs.
The real challenge is when access to this tool is taken away, such as when a manufacture uses the premise of a new product release as the rationale for them to discontinue offering any technical service training to customers — even though that type of training was readily available for the last model product. Some manufacturers may even mention how a recent company merger, or the acquisition of a new product line prevents them from offering technical service training. In practice, what those manufacturers fail to mention is that they still have a process to train their own factory field service staff, especially when they want a health care organization to still buy its products. They may also not inform the health care organization that they will train an authorized distributor or service provider, but not the employees of the health care system that is buying the equipment.
The arguments typically presented by manufacturers generally revolve around how they are “suddenly in flux for their customer-facing training program due to restructuring” or “how they are moving to a new support model for customers due to a shift in corporate business strategy.” What this usually means is that they are no longer offering technical training as there is no longer any compelling competitive advantage for them to do so, especially if they absorbed their competition and they now hold the market share for a particular product type. In health care, when a product is established in the industry as the standard, physicians are not likely to consider other options, in turn leaving health care organizational leadership with zero room for demanding or negotiating something different.
One common problem that you may see is that many manufacturers will try to do a “divide and conquer” approach with customers. They will try to manage the interests of the physician and/or clinical user separately from that of the supply chain team, and what seems to happen more and more, separate both groups from the HTM team. End of the day, a clinical user is using the equipment to treat the patient, and in turn let the health care organization generate revenue. If capital pricing is not negotiated in conjunction with software and service offerings, to include technical training, then the post-purchase support options and costs over the term of ownership generally end up unfavorable to the health care organization.
Presenting and maintaining a unified front is the ongoing challenge that many health care organizations face. Some do much better than others, thankfully. Many health care organizations are working more and more with their respective Group Purchasing Organizations to help gain leverage when dealing with manufacturers and, in turn, raise awareness regarding this issue.
Over the past 20 years, the intertwining of medical equipment with network connectivity and software is driving the need for ensuring compatibility with network infrastructure
THE BENCH
O 40 TechNation | October 2023
and applications, cybersecurity needs, and the ongoing cost of software support and product licenses needed for interfacing with software applications. The unified effort of a health care organization’s supply chain, healthcare technology management and information services departments is becoming ever important.

Sales and service have to be addressed concurrently for a health care organization to have the most purchasing power and control the total cost of ownership. For HTM, as more organizations bring that team to the negotiating and capital planning table, the simple message to share with manufacturers is that, “the simpler and easier it is to support a product means the easier it is for healthcare technology management to be the champion for recommending one vendor over another during the final selection process.” The health care organization holds the funds to buy the new equipment. It’s during the time of evaluating and comparing manufacturer products and service offerings that the health care organization has the most leverage; it’s also when the manufacturers will make the most concessions to win the sale and the related down-stream revenues.
Some manufacturers are starting to listen to this message and, in turn, are starting to reconsider offering technical training, while other manufacturers don’t appear to care. This seems especially true for manufacturers who have overwhelming market share and see their customers as a captive
audience. In those situations, the manufacturer sets itself to be a price-setter versus a price-taker.
A health care organization having cost and time effective options on how it supports and maintains its patient care equipment is very critical. The right to repair is a right all health care organizations need to fight for, be it when doing a competitive bid between manufacturers while assessing total cost of ownership, when replacing old equipment with newer models, and when talking with its own government liaison staff for raising awareness with state and federal officials.
Eventually insurance providers and the state and federal government may wake up and realize they too are paying too much for procedure reimbursements when a health care provider cannot readily and economically service the equipment it owns. Maybe that is when real change may happen. Until then, HTM professionals need to keep raising awareness around the value of technical service training and the right to repair.

October 2023 | TechNation 41
Mark Newell is a clinical engineering-operations support specialist in the Advocate Health Clinical Engineering Department.


Call our SVP Mike McRoberts today –let us know what you need, and we will create a custom solution. Welcome to the Café! HTM S O LU T I O N S M E N U ON DEMAND BMET STAFFING INFUSION PUMPS TEAMS ENDSOCOPY REPAIR SURGICAL DEVICE REPAIR MEDICAL DEVICE/EQUIPMENT SALES INVENTORY VERIFICATIONS N E W S O LU T I O N S BMET RECRUITING AGREEMENTS PM s /REPAIR AGREEMENTS ON: PATIENT BEDS STRETCHERS GURNEYS 888-492-3400 multimedicalsystems.com mike.mcroberts@multimedicalsystems.com
TOOLS OF THE TRADE
Fresenius Kabi
Ivenix Infusion System
Meet the Ivenix Infusion System, a state-of-the-art technology changing the game in delivering intravenous medications to patients. An IV pump that pumps differently.
The Ivenix Infusion System can be fully EMR-integrated and provides accurate and safe delivery of medications to patients. The system uses advanced pneumatic pumping technology to measure and control the flow of medication, helping to ensure that patients receive the right dose at the right time. The system also has a broad range of safety features aimed at helping prevent errors and adverse events, such as medication errors, air embolism and over-infusion.
According to the World Health Organization, medication errors are a major cause of harm to patients, with one in 10 hospitalized patients experiencing an adverse drug event. These errors can have serious consequences, including prolonged hospital stays, disability and loss of life.
One of the key features of the Ivenix Infusion System is its advanced flow control technology. The system uses a patented, high-precision flow-sensing system that monitors the medication delivery rate in real time. This technology helps ensure that the medication is delivered consistently and accurately, regardless of the infusion site relative to the fluid bags, the flow rate and other variables that may impact legacy infusion pumps. This may help to reduce the risk of over-infusion or under-infusion, which can lead to serious adverse
events such as fluid overload, low blood levels of critical medications and electrolyte imbalances.
Another key feature of the Ivenix Infusion System is its intuitive and user-friendly touchscreen. The system is easy to use and can be customized to meet the needs of different users, such as nurses, pharmacists, anesthesiologists and biomeds. The system also provides real-time data and alerts, which can help clinicians make informed decisions about patient care.
It would be remiss of us not to address the financial impact of the system, as it requires no annual PM and there are no scheduled reasons for the infusion pump to be collected for the duration of its use. All updates to the drug library, wireless network configurations or general pump settings are delivered wirelessly and do not interrupt the nurse. Operating system updates occur during normal clinical use of the pumps.
A game changer in nurse and hospital support in IV therapy, the Ivenix Infusion System is a valuable technology aimed at improving patient safety, reducing medication errors and enhancing the efficiency of hospital operations.
For more information, visit Ivenix.com.
Please see the Instructions for Use for a full list of warnings and precautions associated with the Ivenix Infusion System.
THE BENCH
LIVE DEMOOCTOBER 26 AT 2 PM ET Register at WebinarWednesday.live SPONSORED CONTENT October 2023 | TechNation 43

Top 10 Health Technology Hazards for 2023 Prevent dangerous device hazards and improve patient safety SPECIAL REPORT Download Now www. ecri.org/2023hazards
“Reporting from the Frontlines: A Journalist’s Perspective of the Cyberwar Raging in Healthcare” claroty.com

As a senior editor at SC Media, Xtelligent Media, and Healthcare IT News, Jessica Davis was at the forefront in the cyberwar on health care. In this webinar, Ty Greenhalgh and Davis uncovered the latest threats, trends and best practices to protect patient safety and secure critical clinical systems. Davis’ unique perspective, gained from years of analyzing and reporting on health care cybersecurity challenges, equips attendees with practical solutions and proactive measures to safeguard their organizations against evolving threats. Attendees provided feedback via a survey that also asked, “Was today’s presentation worth your time?”
• “Absolutely! Great data to view and assess.”
- Sal Cruz, Director of Clinical Engineering, Cook Children’s Medical Center
• “Yes, Cybersecurity is a real problem these days.”
- Clinton Anderson, Area Clinical Technology Manager, Kaiser Hospital
“Infusion Pump Testing with the IPA-3100” bcgroupstore.com

BC Group Engineering Manager Lucio Simoni demonstrated the features and functions of the BC Biomedical Single Channel Infusion Pump Analyzer starting with a general overview of the device. He also shared how to use the touch screen to set up menus and prepare the unit for testing of an infusion pump. The webinar also featured a demonstration of how to conduct a flow test and occlusion test with and without the Bluetooth/myBC mobile. Simoni fielded questions during the webinar and provided insightful answers with additional knowledge for attendees. Lucio has been with BC Group for 28 years and has been instrumental in the development of most of the BC Biomedical brand testers and analyzers. BC Group International is a biomedical test equipment manufacturer and distributor for over 75 brands of products. Attendees provided feedback via a survey that included the question, “Why did you attend today’s webinar?”
• “BC Group has a lot of products we use for our biomed shops across the USA and Canada. It is always good to see new products or learn something totally new like the myBC mobile app.”
- Mark O’Neal, HTM Technical Training Instructor, GE HealthCare-College of HTM
htmjobs.com

HTM Jobs Recruiting Specialists Sydney Krieg and Kristen Register discussed ways in which HTMjobs.com can alleviate the hiring difficulties in the HTM industry while also sharing helpful tips for job candidates and employers. They shared why candidates should use the platform for a job search. They also explained how HR departments and hiring managers benefit from getting their open listings in front of the website’s talent network. The presenters also answered some great questions during an informative Q&A session. Attendees were asked “What was your single biggest takeaway from today’s webinar?”
• “HTM Jobs is the place to go for candidates and employers.” - James Gebhardt, biomed tech III, WVU Medicine Reynolds Memorial Hospital
• “A place to go when I’m looking for a HTM position.” - Mark Heintzelman, biomed III, GE Healthcare
watch these webinars on-demand IN CASE YOU
IT
MISSED
“HTM Jobs: The Future of HTM Careers”
October 2023 | TechNation 45
ROUNDTABLE Ultrasound Probes
This month, TechNation reached out to several healthcare technology management and imaging professionals for insights regarding ultrasound transducers/probes. Three companies shared knowledge from one of their staff members including Avante Health Solutions Clinical Applications Manager Todd Dennis, BS, RVT, RDCS; MW Imaging Senior Service Director Tom Hanak; and Innovatus Imaging Vice President of Sales and Marketing Matt Tomory.
Q: WHAT ARE SOME OF THE NEWEST ULTRASOUND SOFTWARE APPLICATIONS HEATH CARE FACILITIES SHOULD CONSIDER?
DENNIS: Several cutting-edge ultrasound applications are currently being adopted and deserve consideration, particularly artificial intelligence in ultrasonic image analysis. The primary AI applications within ultrasonic image analysis
encompass detection, diagnosis or classification, and segmentation. These advancements empower clinicians to conduct more precise image analyses. While AI integration holds the potential to significantly reduce examination time, enhance accuracy and image quality, and elevate patient care standards, it is crucial to emphasize that it should not substitute human oversight.
HANAK: When it comes to the newest ultrasound software applications, health care facilities should consider factors that are important to the individual department goals. Advancements in software applications include features to improve workflow for individual exams which can speed up exam times and allow for more patients to be scanned. Ultimately, meaning more revenue for the hospital. Many OEMs are starting to include the use of AI to assist with improved and repeatable workflow. The inclusion of AI in the software applications is adding to increased diagnostic capabilities such as recognizance standard views and automating measurements.
ROUNDTABLE
46 TechNation | October 2023
TOMORY: Artificial intelligence, which can assist with diagnosis, annotation and reporting, is becoming widely available and greatly increases accuracy of exams and reduces exam times. You also want to ensure software updates are regularly performed for keeping your system up to date as well as ensuring the latest cybersecurity patches and enhancements are installed. Remote diagnosis and service capabilities will reduce downtime as well.
Q: HOW IS THE INCREASING USE OF ULTRASOUND IMPACTING THE MAINTENANCE OF PROBES AND TRANSDUCERS?
DENNIS: On average, a probe/transducer is utilized approximately 20 to 30 times per day. Due to this frequency of use, the most significant challenge arises from the human factor. Ultrasound technology has now become integral to nearly every medical specialty. As a result, within a hospital environment, ultrasound systems are no longer confined to just one or two departments; they are dispersed throughout the entire facility. Consequently, the responsibility for handling ultrasound probes/transducers has extended beyond the traditional role of sonographers. Today, a diverse range of medical professionals, from endocrinologists to physicians, can serve as end users. However, this expanded usage also leads to increased wear and tear, resulting in higher service requirements.
HANAK: The increased use of ultrasound will directly impact the maintenance of ultrasound transducers. On the transducer side we see a lot of lens wear and strain relief issues as well as fluid infiltration. There is a higher probability of physical damage such as dropping the probe or improper cleaning of TEE and endocavity transducers. Making sure your central sterile processing team is up to date on proper disinfecting is also a must. We see a lot of TEE probes that have been submerged during this process causing corrosion within the control housing.
TOMORY: Ultrasound has expanded throughout hospitals but one thing remains constant: Probes are a Class II medical device and have very specific care instructions regarding use, cleaning, storage and testing. Chemical damage is a leading cause of premature probe failure and adherence to the OEM user manual’s recommended/approved cleaners and disinfectants is critical. Innovatus Imaging performs accelerated chemical testing on all materials including plastics, lenses, cable sheathes and even adhesives to ensure compatibility with OEM-qualified chemicals.
Q: WHAT ARE THE PROS AND CONS OF BUYING BRAND NEW VERSUS BUYING REFURBISHED PROBES/TRANSDUCERS?
DENNIS: When opting for a new purchase, the most significant advantage lies in acquiring cutting-edge, state-of-the-art probes/transducers complete with the latest features and advancements. On the flip side, the drawback is the OEM pricing. Furthermore, once your initial contract or point of sale warranty expires, you might find yourself left without support and subjected to exorbitant service charges. In addition to the OEM option of purchasing new, Avante provides an alternative. We offer new probes for many models, often leading to extended warranties and competitive pricing. In contrast, by choosing refurbished options from Avante, you have the opportunity to acquire a certified restored probe that’s offering nearly all the capabilities of a brand-new probe, at a significantly reduced cost. This not only enables you to maximize your budget but also provides the possibility of expanding your inventory with additional systems or probes. As an added benefit, you’ll get our built-in warranty and service, plus Avante offers loaner probes to minimize downtime when your probe is being repaired.
HANAK: The pros of buying a brand new transducer include OEM image quality, the possibility of a longer warranty and the probe will be new from a cosmetic standpoint. The cons include a much higher price and in most cases the warranty may be the same length. The pros of buying a refurbished transducer is that the price is much cheaper and warranty time could be the same as a new probe if not greater. Depending on where it was purchased, the cons could be the image quality is not as good compared to a brand new probe, there may be wear and tear inside the probe cable that could cause elements to short down the road, and cosmetically the probe may not look new.
TOMORY: When you purchase new transducers, you know you are getting a fully qualified device with factory warranty. With a refurbished probe, how do you know the device meets OEM form, fit and function and is safe and effective? A properly restored probe can provide excellent savings and a warranty which may be longer than the OEM, but it is imperative you qualify the repair provider.
Q: WHAT CRITERIA SHOULD BE USED TO COMPARE AND SELECT A PROBE/TRANSDUCER REPAIR PROVIDER WHETHER IN-HOUSE, ISO OR OEM?
DENNIS: The majority of OEMs typically opt to replace the probe/transducer rather than repairing it, often
October 2023 | TechNation 47
resulting in the purchase of a completely new probe. Even if you are enrolled in a service contract that offers a new probe at a discounted rate, this can sometimes involve inflated service contract costs. When choosing a third-party ISO like Avante, it becomes essential to assess their extensive experience and profound understanding of various manufacturers and models. As a medical device provider, we understand the critical importance of ensuring the safety, effectiveness and reliability of our products. That is why we continuously invest in our quality management system, which is certified according to the internationally recognized and rigorous ISO 13485:2016 standards.
HANAK: When purchasing a refurbished or new transducer it is good practice to purchase from an ISO 13485:2016 certified company, this ensures they have a quality management system in place and they are dedicated to providing the highest quality products and services. They should be able to provide evidence of thorough testing and images to confirm the probe meets OEM specifications.
TOMORY: There are many questions you should be asking your probe repair provider including ISO 13485:2016 certification (ensure probe repair is within scope of certification), device history records, materials qualification data, testing, standards, etc. The single best thing you can do is ask for a tour of the repair facility, either in person or virtual, and ask for proof of these items. Innovatus Imaging provides virtual and in-person tours for our clients several times per month where the answers to these questions are demonstrated. Teams and Zoom allow for fast, inexpensive and effective virtual tours.
Q: WHAT ELSE DO YOU THINK TECHNATION READERS NEED TO KNOW ABOUT PURCHASING AND SERVICING ULTRASOUND PROBES/TRANSDUCERS?
DENNIS: Ensure that the individuals utilizing the probe/ transducer are involved or consulted when making


purchasing decisions. This approach guarantees a clear understanding of the specific requirements. Unfortunately, it’s quite common for transducer purchases to be made by individuals who won’t be the end users, leading to misunderstandings about ultrasound technology and how the probe will function in practice. Given the substantial financial investment that goes into acquiring probes, it’s paramount to ensure that the right selections are made. Furthermore, it’s important to inform the reader that Avante’s ARTS (Advanced Repair Technical Solutions) department is actively enhancing its proficiency in repairing and servicing many of the latest transducer models such as the X5-1c Philips Transducer, which has recently transitioned out of warranty coverage. Our commitment to mastering the intricacies of this state-of-the-art technology reflects our dedication to staying ahead in this dynamic field. This pursuit aligns with our aim to continually advance our capabilities and provide unmatched services.

HANAK: When servicing or completing preventive maintenance on your ultrasound equipment always perform a physical inspection of the transducers as well. Look for issues with strain relief, housing separation or cuts on the cable. Transducer cables can get run over and cause damage to the cable and internal wires, all of these issues could affect the safety and/or image quality of the probe. When purchasing a transducer make sure to partner up with a company that is ISO 13485:2016 certified.
TOMORY: There are many options for purchasing and repairing probes – just Google both and it will be apparent. These devices can be repaired so they just work again or they can be restored to be as safe and effective as when they left the factory. As mentioned earlier, they are Class II medical devices so ask the right questions to ensure you and your patients are being served properly.
ROUNDTABLE
Tom Hanak MW Imaging
Matt Tomory Innovatus Imaging
Todd Dennis Avante Health Solutions
48 TechNation | October 2023
PROBO MEDICAL
Your Ultrasound Partner
Your Ultrasound Partner
FREE TECH SUPPORT
FREE TECH SUPPORT
QUALITY, TESTED PARTS
QUALITY, TESTED PARTS
ULTRASOUND SERVICE TRAINING
ULTRASOUND SERVICE TRAINING
PROBE REPAIR WITH FREE LOANERS
PROBE REPAIR WITH FREE LOANERS


NEXT DAY RENTALS
NEXT DAY RENTALS
SERVICE ACROSS THE US
SERVICE ACROSS THE US
Mention this ad for 10% OFF your next part or repair.
Mention this ad for 10% OFF your next part or repair.
HAVING PROBLEMS WITH YOUR ULTRASOUND PROBE?
HAVING PROBLEMS WITH YOUR ULTRASOUND PROBE?


Scan the QR code and get an instant quote by using our free ultrasound probe evaluation tool.
Scan the QR code and get an instant quote by using our free ultrasound probe evaluation tool.
WWW.PROBOMEDICAL.COM
WWW.PROBOMEDICAL.COM
PROBO MEDICAL
Cybersecurity Hygiene Updated Guidance for HTM
 By K. Richard Douglas
By K. Richard Douglas
50 TechNation | October 2023
review of worldwide media during any given week will reveal reports of cyberattacks on institutions of higher learning, corporations, government agencies and health care organizations.
These attacks have a financial toll and disrupt important operations within these organizations.
Recently, the University of Hawaii gave into cybercriminal demands and paid a ransom reported to be over $200,000. The hackers threatened to release private, non-public information on 28,000 current and former students and employees.
An important government website in Kenya, used by the public to access more than 5,000 government services, was hacked by cybercriminals. The website, eCitizen, is used for passport applications and renewals, issuing driver’s licenses, health records and identification cards and e-visas. The hackers claimed to have obtained passport information as a result of the incursion.
One of the concerning offshoots of this cyberattack is that an estimated 76 percent of Kenyans use mobile money. The mobile-money service M-Pesa was impacted by the attack, leaving many Kenyans unable to make payments or purchases. This is a drawback of digital currency in an age of cyberattacks and one more reason why this type of activity can harm the U.S. as the move towards a digital dollar becomes a reality. No network is un-hackable.
While the Constitution mandates that the federal government protect U.S. borders, that protection responsibility has gained a wider scope with the advent of the Internet and cyber-criminals. There is much more traversing the border digitally.
The FDA has stated that “cybersecurity threats to the health care sector have become more frequent, more severe and more clinically impactful” and includes a cybersecurity focus during the premarket submission process for medical devices.
In addition to the cybersecurity measures exercised by the FDA, the government’s Health and Human Services (HHS) agency includes the HHS 405(d) group, which is a program started as a congressional mandate under the Cybersecurity Act of 2015 (CSA), Section 405(d).
The group’s webpage states: “The 405(d) Program is a collaborative effort between industry and the federal government to align health care industry security practices to develop consensus-based guidelines, practices and methodologies to strengthen the health care and public health (HPH) sector’s cybersecurity posture against cyber threats.”
A government-corporate collaborative is necessary to fight the organized cybercriminals and the ever-evolving approaches they develop to breach secure networks.
In health care, medical devices must be available for use.
This isn’t only a major goal of every biomed department, but it is also one of the leading hazards of a cyberattack. If the attacker can knock out a device, or an entire network, they deprive clinicians of much-needed devices.
The challenge for biomeds is the same challenge for all those entrusted with a cybersecurity duty; the need for constant hyper-vigilance is essential because the bad actors never sleep.
“I think of an analogy with codebreakers and code makers. For every newly created code (i.e., digital system), there is always someone working to break the code (i.e., hack the system). It is a never-ending leapfrogging situation. There is no perfect code or cybersecurity; it is only a matter of time and ingenuity before a new hack or new security solution is created,” says Scott Trevino, senior vice president of cybersecurity at TRIMEDX.
He says that this is why it is critical to be innovative, always learn, and stay on top of the evolution of new security solutions and techniques. It’s also important to stay aware of what the bad actors are doing, so you are ready to defend against their latest methods of attack.
“This can and should be done through a multifaceted approach including course work, certifications, conferences and continuing education. There is also value in simply being curious and reading as much as possible,” Trevino says.
Ali Youssef, director of medical device and emerging tech security for Henry Ford Health says that staying ahead of sophisticated cybercriminals requires an unrelenting commitment to continuous learning and adaptation.
He shared some key strategies:
• Pursuing ongoing technical education through certifications, conferences, training exercises and hands-on practice.
• Building connections with other cybersecurity experts to collaborate and share intelligence.
• Proactively monitoring cybercriminal forums, malware sites and threat feeds for early warnings on emerging attacks.
• Adopting an authentic lifelong learning mindset, utilizing all available resources, and establishing a strong web of professional relationships are critical to matching and exceeding the capabilities of the bad actors.
“Curiosity, vigilance and collaboration are the best defense,” Youssef adds. The need for cooperation and information sharing among those in health care and beyond, as cyberthreats are discovered is one necessity in hardening the entire universe of vulnerable networks.
“The unique challenge in cybersecurity is how quickly things can evolve and change, often faster than formal educational programs can catch up. Therefore, networking across the industry and developing relationships with peers in other organizations and building partnerships with vendors and government agencies is crucial,” says Axel Wirth, CPHIMS, CISSP, HCISPP, AAMIF, FHIMSS, chief security strategist at MedCrypt.
This effort to collaborate and share information needs to occur expeditiously.
A October 2023 | TechNation 51
“Breaches have traditionally been detected after some damage is done. At that point, efforts are largely focused on limiting the scope of damage, restoring normal operations, reporting to government and affected parties, and taking steps to stop future compromises of this type,” says Stephen L. Grimes, FACCE, FHIMSS, FAIMBE, AAMIF, principal consultant, Strategic Healthcare Technology Associates LLC in Swampscott, Massachusetts.
He says that there are public and private organizations designed to rapidly share known vulnerabilities and their mitigations among industry members in order to help others avoid the same compromises.
“There have also been improvements in safeguards that can more quickly detect attempted compromises, prevent and limit the effects of the compromise. It is a continually evolving process and requires on-going attention,” Grimes says.
In 2021, Grimes and Wirth wrote an article titled: “The Case for Medical Device Cybersecurity Hygiene Practices for Frontline Personnel,” which was published in AAMI’s BI&T publication and was recognized with the best article award from AAMI that year. The article presented 20 best cyber-hygiene practices for clinicians and tech support. It is available at https://array.aami.org/doi/10.2345/0899-8205-55.3.96.
REAL MEASURES FOR BIOMEDS
Grimes says that biomeds need to think outside the box and consider the larger universe of vulnerable devices around them.
“The first thing to recognize is that, while networked devices may be particularly vulnerable to cyber compromises, cyber-vulnerable devices are not limited to only those on networks. The fact is that any device that has a microprocessor or that can execute commands in software/firmware is vulnerable,” he says.
Grimes says that it is critical for members of the HTM community to familiarize themselves with cybersecurity and consider the implications that cybersecurity compromises can have on their organizations in regard to the delivery of safe and effective care. Familiarization may take the form of courses offered by AAMI and other expert sources. It may also involve searching literature and reading articles related to medical device security.
“Simultaneously, HTM staff should be going through their
inventories and identifying all devices and systems that are microprocessor or software/firmware-based. This inventory examination will help HTM staff to focus their risk analysis and mitigation efforts in a manner that prioritizes and first addresses issues that may have the greatest impact,” he adds.
Trevino points out that close collaboration with the IT department is key and sorting out responsibilities is important.
“Too often, health systems are especially vulnerable to cyberthreats because of siloed IT and biomedical equipment technician teams. IT teams have the cybersecurity expertise but often don’t deal with the medical devices every day. Biomed teams know the medical devices but may lack cybersecurity knowledge. In this increasingly connected world, the two teams need to share expertise and work together to develop a strategy and plan of action for medical device cybersecurity,” he says.
Trevino says to clearly establish roles and responsibilities, a health system should put together a well-defined Responsible, Accountable, Consulted and Informed (RACI) chart. This responsibility matrix should be designed to help clarify roles and responsibilities across departments.
“When implementing their cybersecurity strategy, health systems should also be sure to use proper technology to improve efficiency, accuracy and reproducibility of processes,” he adds.
Wirth agrees that the two departments need to have a good relationship.
“HTM and IT have to have a good working relationship as often issues need to be addressed that go further than just the network plug in the wall — responding to a cybersecurity incident would be one example,” he says.
Youssef says that there are three basic steps that are great starting points for biomeds seeking to harden their systems:
• Deploy/use a medical device security management tool to quickly identify network anomalies, and hone in on vulnerabilities with connected medical devices.
• Enforce strong access controls to limit access to biomed systems and data to only authorized users.
• Provide cybersecurity awareness training to biomed staff on things like phishing, strong passwords, social engineering, etcetera.
Wirth points out that best practices should be implement-

“There have also been improvements in safeguards that can more quickly detect attempted compromises, prevent and limit the effects of the compromise. It is a continually evolving process and requires on-going attention.”
52 TechNation | October 2023
- Stephen L. Grimes
ed in securing wireless networks.
“Wireless networks require special attention to assure that they meet the desired level of security. They are not inherently insecure but there are many opportunities to mis-configure or mis-design a wireless connection or infrastructure. Hence, it is strongly advised to follow implementation best practices for medical wireless networks as they may be provided by the device manufacturer or industry organizations, such as AAMI (aami.org/medical-device-connectivity),” he says.
SHORTER DISTANCE TECHNOLOGIES AND CONCERNS
The evolution of wireless technologies has resulted in smart homes, dependence on smartphones, hot spots and any number of controllers and linked devices. That potpourri of wireless devices often travels with the consumer outside the home as well and can land in the health care setting. These devices can be brought into the health care environment by clinicians who utilize their capabilities.
“Wi-Fi, Bluetooth, Zigbee, Z-wave are examples of wireless technologies that connect devices together wirelessly. By virtue of the wireless nature of their communications, devices using these technologies can potentially receive data from, and transmit data to, unintended devices and recipients. This has implications for data integrity as well as confidentiality. Effective hand-shaking between devices attempting to send or receive data wirelessly, as well as encryption of that data as it is wirelessly transmitted, are important security safeguards,” Grimes says.
Wirth says that since Bluetooth device connections are typically designed by the device manufacturer, the best an HTM can do is require proof from the manufacturer that the Bluetooth protocol is implemented securely and that known vulnerabilities and common design mistakes are mitigated.
“Some of the challenges specific to Bluetooth include: Eavesdropping — Bluetooth signals can be intercepted by third-party devices within range, allowing hackers to access sensitive information. To prevent eavesdropping, it is essential to use strong encryption when transmitting sensitive data and to disable Bluetooth when not in use,” Trevino says.
Another cybersecurity concern would be man-in-the-middle attacks.
“Bluetooth connections can be intercepted by a hacker who poses as a legitimate device to gain access to sensitive information. To prevent man-in-the-middle attacks, it’s important to verify the authenticity of devices before connecting to them. It’s also vital to use secure authentication protocols,” Trevino says.
He says another concern would be unauthorized access: Bluetooth devices can be configured to be discoverable, making them vulnerable to unauthorized access. To prevent unauthorized access, it is vital to configure devices to be non-discoverable and to use strong passwords or PINs to secure them.
“Because of the unique challenges Bluetooth devices present, it’s crucial for health systems to use the latest version of Bluetooth, update patches, use encryption, verify device authenticity and disable when not in use,” Trevino adds.
Youssef says that core recommended cybersecurity best practices include migrating all wireless traffic to using encrypted protocols such as WPA2 or WPA3, establishing unique, sophisticated passphrases, segmenting wireless networks, disabling client-to-client communication, and implementing wireless intrusion detection systems to monitor for threats.
“Additionally, biomedical engineering should ensure regular patching and upgrades to wireless infrastructure,” he adds.
ZERO-TRUST ENVIRONMENT
The “Zero Trust” framework is an approach that makes the assumption that no device, individual or service can be trusted. Everything that is within an organization’s network, outside the network or within the near-proximity of the network presents a vulnerability.
“Supporting a Zero Trust networking architecture should be a requirement that is articulated and verified during the device purchasing process. This includes support of zero trust on the hospital network but also to any eternal network access, e.g., by the manufacturer for the purpose of remote service and maintenance,” Wirth says.
Trevino says that biomeds should work with IT and determine the current security model and collaborate to determine the best way to adhere to that model.
“It’s critical for the two teams to work together to guard against threats. If they are working under a Zero Trust model, teams should be sure to consider the seven factors of Zero Trust: workforce security, device security, workload security, network security, data security, visibility and analytics, automation and orchestration,” he says.
Grimes says that HTM professionals need to be familiar with medical device cybersecurity best practices and consider those practices during acquisition, installation/ configuration, operation and disposal.
“Among these practices are guidelines from AAMI, NIST, MITRE, UL/EMERGO and other organizations. It would be useful to obtain an MDS2 (containing a description of security features and vulnerabilities) from manufacturers on each of their products, and to attempt to get devices with security certifications (e.g., UL/EMERGO, US Cyber Trust Mark),” he says.
He adds that HTM professionals need to recognize that, while manufacturers can take these steps to help facilitate the security of their devices, ultimately those devices will be placed in diverse environments which will have their own unique impact on the security of each device and that security will need to be managed by responsible individuals working on behalf of that organization.
With knowledge that can be implemented to harden the health care network surface, the insights from these experts can provide a framework for HTM’s contributions to cybersecurity. Knowledge without action is only knowledge though. Along with IT, HTM plays an important role is securing their environments from cyberthreats.
October 2023 | TechNation 53

TECHNATION TECHNATION Breaking News: THE FUTURE OF HTM JOBS IS HERE. htmjobs@mdpublishing.com for posting inquiries htmjobs.com to register today • A niche job board for the HTM and imaging communities powered by TechNation. • 2600+ actively looking biomedical and imaging professionals. » Completely free and confidential registration and application process.
• 300+ open opportunities throughout the United States.


» A variety of posting options ranging from single-job postings to 12-month unlimited memberships.
“My HR department advertised on various government sites and our web site but we did not get a single applicant in over 120 days. Fairbanks Alaska is hard to recruit for but I took out an ad on HTM Jobs and got two good applicants in less than 30 days. I am hiring them both. Thanks HTM Jobs.”

TECHNATION TIMES TECHNATION TIMES
- D. Anderson, Tanana Chiefs Conference
• The fastest growing HTM talent network in the country.
Featured Employers: Agiliti, InterMed, TRIMEDX, Kaiser Permanente, Renovo Solutions, SPBS, Inc., Universal Medical Resources, Inc. and more!










MEDICAL The one-of-a-kind FOBI NOW app saves you the time and frustration of researching parts or service solutions. Simply, open the app, request what you need and let us find you a solution while you get back to the important tasks at hand. Requesting parts and services has never been easier! Click on the QR Code to download It's quick, easy and free Locate FOBI Now! in the app store or point your phone's camera at the QR code above. From your phone's main screen, simply launch FOBI Now! and enter your contact information. Launch Download Request Now you're ready-to-go! Just send us your request! 3 2 1
CAREER CENTER
Want your resume to stand out?
BY KATHLEEN FURORE
Irecently read that QuillBot spoke to employment experts around the country to find out what makes a resume stand out. To me, one of the most interesting findings was that using common buzzwords like “passionate” won’t work if you want to set yourself apart. That made me wonder: What’s wrong with being “passionate”? And how can job seekers describe themselves without being trite?
“[Buzzwords] just put you in the same category as others without any evidence of reasons to select you,” interview coach Graeme Jordan told QuillBot. “Phrases and words such as ‘passionate,’ ‘motivated’ and ‘hard-working’ are things employers just expect as a baseline anyway, and by using them they become white noise within your application.”
That doesn’t necessarily mean the actual words are the problem, according to Milosz Krasinski, the managing director at Chilli Fruit Web, who works in the realm of digital public relations and deals with messaging, perception and storytelling every day.
“The same principles that apply to effective public relations are instrumental when crafting a resume,” Krasinski says. “While it may seem tempting to avoid words like ‘passionate,’ ‘driven’ or ‘dedicated’ due to their perceived overuse … the flaw here isn’t the word itself; it’s the absence of a story or evidence that gives it life.”
His advice: “In a resume or cover letter, just as in a good PR campaign, storytelling is key. Instead of merely stating that you’re passionate about your field, why not weave a narrative that illustrates this passion?”
When interviewing for a digital marketing job, for example, Krasinski says to go beyond saying you’re passionate about the field. “Share an anecdote about a project where you went above and beyond to achieve success or discuss a challenge you faced and how your dedication helped you overcome it,” he suggests.
“Use the resume to showcase the results that your passion has driven, whether it’s remarkable growth in social media engagement, innovative campaigns that broke the mold or mentoring junior staff to reach their full potential.”
Other business pros echo Krasinski’s comments.
“From a product and customer-centric perspective, I believe that words like ‘passionate’ are not necessarily ineffective, but they need to be framed correctly,” says Kamil Rejent, CEO at Survicate.
“In the tech industry, what really matters is how you apply your passion and talents. Do you innovate? Do you solve real problems for customers? Don’t just state that you are passionate; show how you have used that passion to create something new or enhance a product. Include links to your projects, or detail how your passion led to growth in customer satisfaction. Focus on the outcomes of your passion, rather than the passion itself.”
Shirley Borg, head of human resources at Energy Casino, who sees “countless resumes come through every day,” agrees that adjectives like “passionate” — which “may have been striking once” — have become overused. She stresses that job seekers must remember that recruiters are looking for evidence to back up any claims.
“Instead of just saying you’re passionate, demonstrate it. Talk about specific projects or initiatives you have spearheaded,” Borg advises. “Include tangible results and statistics that showcase your dedication. Actionable examples always speak louder than vague adjectives. In this way, your resume will truly reflect your skills and drive.”
As Krasinski concludes, “Generic buzzwords can be transformed into powerful descriptors if they are embedded in real, tangible experiences. It’s all about creating a connection with the reader, making your resume resonate with authenticity.”
Kathleen Furore is a Chicago-based writer and editor who has covered personal finance and other business-related topics for a variety of trade and consumer publications. You can email her your career questions at kfurore@yahoo.com.

EXPERT
October 2023 | TechNation 57
ADVICE
BY KEITH HAMM, REGIONAL SERVICE MANAGER

ULTRASOUND IMAGE QUALITY TROUBLESHOOTING TIPS D
uring our fieldwork on ultrasound calls, my team and I frequently encounter a prevalent issue – artifacts in the image. These abnormalities can be frustrating for both end users and HTM professionals, as they are often intermittent and caused by various factors. Moreover, they can impede our customers’ capacity to conduct a thorough diagnostic examination. To pinpoint the root cause of an image artifact problem, we focus on three key factors: the environment, the transducer and the ultrasound system.
The environment in which the ultrasound system is situated significantly influences image quality. When tackling image artifacts in the field, it’s important to consider your surroundings and ensure that no other medical systems are in close proximity. The emergence of external interference from other medical equipment is becoming more frequent as facilities broaden their diagnostic imaging capabilities. Fortunately, addressing this concern is often straightforward. By relocating the ultrasound from its current setting to an area away from CT or MRI systems that might lead to interference, you can readily resolve this issue.
Beyond the environment, malfunctions within the ultrasound equipment often stand as the primary culprits behind artifacts. Image artifacts usually elude detection by modern test tools and error logs in contemporary systems. Modifying system variables presents an uncomplicated method to navigate around these issues, allowing for troubleshooting and resolution. Begin by shifting the transducer to another available connection on the system, observing any alterations in the scan field. If you have access to an alternative ultrasound system, transfer the
transducer to that system and observe whether the artifacts persist. If the artifact remains present when the transducer is used with another connection or system, then the likelihood is high that the problem lies within the ultrasound transducer.
The transducer, or probe, undoubtedly plays a role in influencing image quality. Artifacts could potentially originate from various crucial components of the probe. Conduct a thorough inspection of the probe, covering from the scanhead to the connector, while searching for any physical or diagnostic irregularities. Begin your examination with the lens – run your fingers across it to detect any delamination or separation of the lens material from the crystal. Additionally, scrutinize the lens for the presence of air bubbles or material bunching. Another critical focus area is the array or crystal. Examine it closely for signs of physical damage and any regions within the diagnostic scan field where information might be missing.
Given that the cable often contributes to artifacts due to the presence of numerous individual wires, it’s crucial to engage in both physical and visual examinations of the cable’s entire length to identify any signs of damage. Among the more challenging areas to inspect are the wires nestled within the cable. To scrutinize these wires, carefully flex the cable from side to side along the probe’s length and watch for any alterations within the diagnostic scan field. Furthermore, conduct a thorough inspection of transducer connectors for signs of broken, bent, damaged, or missing pins or contacts. Should any pins or contacts be soiled, cleaning them with a blast of air, contact cleaner or alcohol is recommended. Poor probe connection can also give rise to image artifacts. Thankfully, resolving this issue is straightforward – simply reseating the probe can often rectify it.
If the artifact remains unchanged after the initial transducer test, this might indicate a hardware defect within the system. Such malfunctions could involve the transducer interface or the front-end board. To ascertain whether the error indeed stems from hardware, I suggest trying a different probe of the same model to determine if the
EXPERT ADVICE
58 TechNation | October 2023
artifact persists across the ultrasound machine itself. For your convenience, many probe repair facilities, like Avante, offer loaner probes that can aid in troubleshooting or repair procedures.
Image problems can also arise from corrupted user-defined presets. To prevent this, it’s advisable to consistently rely on factory default presets during troubleshooting. Additionally, when conducting tests on ultrasound systems in the field, it’s imperative to bear in mind that image quality is subjective and hinges on various factors. These include user perception, skill level, custom presets, the patient’s body type and the complexity of the procedure. It’s essential to account for these variables diligently while troubleshooting and strive to eliminate as many contributing components as possible.
For more information about Avante’s field service and probe repair capabilities, call one of our experts at 800-979-6742 or visit avantehs.com.

Keith Hamm is a Regional Service Manager servicing the East Coast for Avante Health Solutions, with over 14 years of experience as an ultrasound field service engineer.

SPONSORED CONTENT TV ANNOUNCING THE LAUNCH OF Check out our Youtube channel for more information and exclusive content! October 2023 | TechNation 59
NEW REPLACEMENT PARTS

At Elite, we believe that accessibility should never come at the cost of quality. Our cutting-edge, in-house manufacturing process combines innovation with precision, bringing you replacement parts that are not only durable, but also rigorously tested for compliance.
With years of experience in the healthcare industry, our team of experts understands the complexities of healthcare systems. Ensuring that our replacement parts meet the specific quality requirements of the industry.

Don't settle for less when you can have Elite.

THE FUTURE Artificial Intelligence in the Biomedical Equipment Technology Classroom?
BY ROGER A. BOWLES, MS, EDD, CBET
Maybe it is just me, but it seems like all I’ve been hearing about in the news lately (at least the non-political type) is artificial intelligence (AI) and how fast it is growing. Usually, I am behind the curve in learning new technology and I have been for a very long time.
When I was starting out as a biomedical equipment technician in 1991, our hospital only had one “computer guy” and he worked in our shop. He would work on computers, printers, fax machines and just about every other related device. He was self-taught. He didn’t have a degree. But, he was always reading computer-related magazines. He was always trying to show me articles about Windows 3.0 or 3.1, I can’t remember which but I never really paid attention. I should have.
As computers became a household item, I had to learn on the fly. I did learn, but I would have done better to pay attention sooner. This brings me to the AI revolution, or so it has been called. From what I’ve read, there will be people who work on AI, people who work with AI and people who live with AI. Sort of like computers back in the day. Many of us here are taking steps to learn how to work with it because it isn’t going away.
Starting with ChatGPT, I have used it to help students develop a resume. Starting with just developing a job description for an entry-level BMET or a field service technician, ChatGPT does a pretty good job of using key words and formatting. I’ve also experimented with developing lesson plans on certain topics. There it does a fair job and definitely better than I expected.
The school uses it to determine students’ likelihood of dropping out by monitoring certain variables like attendance, grades, engagement with the learning management system and other factors.
This column was inspired by the use of ChatGPT by asking it to write an article about the benefits and
shortfalls of using AI in training entry-level BMETs. One benefit is personalized learning experiences. AI-powered learning platforms can gauge a student’s strengths and weaknesses and tailor topics to meet that student’s needs. Other benefits include real-time feedback and assessment, virtual simulations, intelligent tutoring systems (apparently Oxford and Cambridge already use this in place of many traditional classroom experiences) and data-driven curriculum design. Our school and program are already moving in this direction through the performance-based learning design. Although, I believe the full implementation of this approach is a few years away because of its complexity and also from the resistance from many older faculty members.
Shortfalls of AI in training entry-level BMETs included lack of human interaction and guidance. The experience and expertise of former BMETs training BMETs is hard to replicate. AI may struggle to provide the same level of encouragement and empathy as a human instructor. Another shortfall that was identified is limited real-world context. AI-powered simulations and virtual laboratories are great in that they can replicate real-world scenarios and some equipment, but they lack in some of the nuances and sense activation that working on real devices provides.
This is a fairly short list of the benefits and shortfalls given by ChatGPT. I guess the main question many are asking is, “Will AI replace human instructors?” The short answer, in my opinion, is no, at least not completely. However, I do believe AI will enhance the education and training of future technicians. It will enable instructors to do more in less time. So, in essence, maybe less instructors will be required in the future. Nevertheless, faculty will need to understand AI and be able to use it. Several of us are taking online tutorials and classes on it. We are trying to understand how to better the service we provide our students.
Roger A. Bowles, MS, EdD, CBET, is a biomedical equipment technology/ medical imaging technology instructor at Texas State Technical College-Waco.

EXPERT ADVICE October 2023 | TechNation 61
Taking a closer look: Micro-damage
BY TED LUCIDI, CBET
really enjoy working with Innovatus clients. One area that I particularly enjoy is sharing failure analysis data and helping clients understand root cause to potentially prevent future recurrences. It can be frustrating to HTM teams, and end-users, to learn that their ultrasound probe that was only sent in to address a damaged strain relief may require a more invasive repair. Two of the most-common failures with ultrasound probes are intermittent or broken wires in the cable harness and damaged elements in the acoustic array. Let’s take a look at the latter.

The acoustic array of an ultrasound probe might be considered a magical component to the layperson. Its piezoelectric properties enable it to convert electrical energy into sound energy AND reverse the process by converting sound energy into electrical energy. The array combines micro-electronics and precision mechanics. It’s a highly precise component manufactured to very detailed specifications and specifications are measured in microns.
Innovatus is an FDA-registered manufacturer of ultrasound probes, so we have the in-house talent, expertise, and specialized tools and instruments needed to construct acoustic arrays as well as finished-goods-quality ultrasound probes for several prominent OEMs. Let’s look at the design of an acoustic array.
Think of the array as a sandwich that consists of multiple layers. At the core of the array are the acoustic elements which are commonly referred to as the “crystals.” Despite the size and weight of the entire array, the elements themselves, are extremely small. The height or
thickness of a single element may be less than 0.4mm. Their thickness is typically compared to that of less than a hair.
Beneath the array is a backing material to which the acoustic elements are bonded. The backing material serves two purposes:
To dampen the high frequency vibrations of the elements and provide a crisp clean signal. Think of a single element as a bell. Ring a bell and the tone will continue until either A) it loses energy or B) someone grasps the bell to stop it from ringing. The backing material stops the acoustic elements from “ringing” so that they can be “rung” again … and again … and again, with high frequency ultrasound pulses, and
To direct the energy created by the array in the appropriate direction … Out of the probe, through the lens, and into the patient. Without backing material, the acoustic energy would emanate in all directions, throughout the entire scan head, minimizing that which enters the patient.
On top of the array elements are acoustic matching layers, and, ultimately, the rubber-like acoustic lens. Matching layers are designed to maximize energy transfer from the array into the patient. The scan gel, used on a probe during patient studies, serves as a matching layer. Without the use of scan gel, it’s hard to couple the acoustic energy to the body. The latest matrix array probes from Philips utilize seven different materials on top of one another as matching layers. They’re quite complex.
Although the acoustic lens serves as another matching layer, it’s more than just that. Being a rubber-like material, it serves as an electrical insulator against the several hundred volts applied to the array elements. It’s
20/20 IMAGING
INSIGHTS
EXPERT ADVICE I
62 TechNation | October 2023
also a chemical barrier, sealing the internal components from potential liquid entry, such as chemical disinfectants and body fluids. The lens is also a precision component. It has a very defined thickness and shape, and is constructed of a specific material set. Lens designs typically employ a curvature to enable mechanical focusing of the sound beam to a particular distance away from the probe.

Special Note: Repairs made to ultrasound probes need to be performed using adhesives and materials consistent with the OEM design and must be ISO 10993 compliant. However simple the process of replacing a lens or strain relief may seem, repairs should not be made using standard, over-the-counter type materials such as caulking, epoxy, super glue or standard RTV materials (which we see on a regular basis).
ROOT CAUSE ANALYSIS
After 30-plus years of repairing probes, we can say with confidence that a strong majority of probes sent-in for repair have presented with image dropout due to traumatic array damage, yet there is no, or very little, evidence of an impact to the probe itself. How can this be? Knowing that the ceramic-like acoustic elements are extremely thin and fragile, and knowing that the scan head weighs about a pound … when a probe falls to the floor or is accidentally dropped, the impact shatters a portion of the elements. The elements no longer resonate at the designed frequency, and they fail in a certain pattern. There’s a visual signature in image quality that is easily recognized by our evaluation team. Although, acoustic and electrical testing can be used to confirm the damage, a tissue mimicking phantom and a properly configured scanner are more than sufficient.

Today’s software laden scanners are capable of

performing image quality “miracles.” Software enhancements, such as harmonic imaging, compound imaging, multiple focal zones, speckle reduction imaging, etc., make it possible to mask, or even hide, deficiencies in system or probe performance. Simply put, it’s possible through all of the system software, to NOT visualize image quality problems on a probe with a damaged array or intermittent wiring. Conversely, it’s actually possible to create a very poor image using a healthy probe, connected to a healthy system. Innovatus has created a how-to guide for testing image quality. It provides guidance for properly performing standard image quality tests and presents disabling software corrections.
WHAT’S ALL THIS MEAN FOR YOU
For years, a damaged acoustic array translated to a probe’s replacement. Since 2008, Innovatus Imaging has had the ability to surgically repair or fully replace the acoustic array on over 100 of the most popular probe models. By being able to repair or replace the array, fully replace the wiring harness, and performing complex electrical repairs, Innovatus is able to restore your probe’s performance … not just get it working again. And, we do so at a fraction of the cost of a replacement. For more information on how to identify array damage or to request a copy of our how-to guide, reach out to TedL@innovatusimaging.com.

SPONSORED CONTENT October 2023 | TechNation 63
Ted Lucidi, CBET, is the director of commercial operations and business analytics at Innovatus Imaging.
EMS STRETCHERS: RELIABLE RESTORATION
BY WESLEY JONES, CABT
ur priority at ReNew Biomedical is delivering top-of-the-line service to our customers and their equipment. We specialize in servicing, calibrating and repairing emergency medical equipment, but one area of equipment renewal stands out: emergency cots and stretchers. Our extensive stretcher repair process includes an industry-leading overhaul that cosmetically makes the stretcher almost indistinguishable from new.
Many biomedical depots will inspect your stretcher to see if it still works. but don’t put the effort to ensure its performance and make it look brand new. Our stretcher team knows the importance of a structurally restored unit, but also holds themselves to a high standard of cosmetic repairs. Our refurbishing process confirms your stretcher is fully functional and looks as good as new.
Our stretcher restoring process is extensive. We go through each stretcher with a fine-tooth comb, removing all signs of wear and tear, such as rust, stains and fading. We then clean every part, removing accumulated debris or buildup over time. Next, we carefully scour the stretcher frame to remove any rust or damage that has occurred. Once the frame is clean and smooth, we powder coat it to ensure a durable and long-lasting finish. This process improves the look of the stretcher and helps protect it from the wear and tear of daily use. We then clean the stretcher using chemical and mechanical cleaning methods, ensuring no chemicals or harmful organisms remain.
In addition to cleaning and removing any signs of wear, we also replace all consumable parts on the stretcher. This includes new belts, stickers, plastic guards, rubber bumpers and storage flats. Instead of putting your used and worn parts back on the stretcher, we opt for new parts to ensure your stretcher returns in the best possible condition.
The unique component of our refurbishing process is our focus on the aesthetics of the stretcher. We understand that a good-looking stretcher can provide comfort and confidence to patients and health care professionals alike.
To EMS providers, a stretcher is one of the most valuable tools. They rely on it to transport patients safely and securely from the scene of an emergency to the hospital. But as with any equipment used frequently, wear and tear can take its toll over time. Frequent wear and tear is why it’s so important to have your stretcher serviced, repaired and renewed by a biomedical company you trust.
At ReNew Biomedical, our stretcher repair and refurbishing process is streamlined and efficient. We take pride in our work and customer satisfaction. We understand a stretcher is a critical piece of equipment in emergencies, and we want you to have the peace of mind that comes with knowing that your stretcher is reliable and in like-new condition. Request a quote online or call today to learn more!
Biomedical.

EXPERT ADVICE
Wesley Jones, CABT, is a stretcher specialist with ReNew
O
SPONSORED CONTENT 64 TechNation | October 2023







October 2023 | TechNation 65 Continue your free subscription of TechNation magazine! SUBSCRIBE TODAY! 1technation.com ADVANCINGTHEBIOMEDICAL HTMPROFESSIONAL MARCH 2022 RighttoRepairUpdate AMOVEMENTGAININGMOMENTUM PAGE56 ProfessionaloftheMonth:MDExpoCelebrates20Years GainingOrganizationalBuyInforRTLSAssetTracking 71 TheOtherSide YouDon’tKnowWhatYou Don’tKnow 2203_TN_Mag.indd 1 2/7/22 12:29PM PROTECTING PATIENTS ANSI/AAMIST91 INCLUDESHTM RESPONSIBILITIES 1technation.com ADVANCINGTHEBIOMEDICAL HTMPROFESSIONAL FEBRUARY 2022 ACCREDITATION SURVEY TIPS L PREPARATION RELIEVES ANXIETY 1technation.com ADVANCINGTHEBIOMEDICAL HTMPROFESSIONAL PAGE 52 Publishing1015 Tyrone Ste. Tyrone, 30290 PRSRT PostagePAID MD Publishing Address Service Requested 12 Professional of the Month Francesca Fam 14 Department of the Month ThePiedmontAtlantaHospitalBiomedical EngineeringDepartment 18 Shifting Gears The Bearded Biomed Podcaster 48 Roundtable AEDs/Defibrillators APRIL 2022 2204_TN_MAG.indd 1technation.com ADVANCINGTHEBIOMEDICAL HTMPROFESSIONAL PublishingMD 120Ste.Rd.,Tyrone1015 30290GATyrone, STDPRSRT PostageU.S. PAID PublishingMD RequestedServiceAddress 12 Professional of the Month AllisonWoolford 14 CompanyShowcase J2SMedical 38 AAMI GuidetoAAMIeXchange 52 Roundtable IVPumps MAY2022 Examining eqUipment expensesTIPSFOR ESTIMATINGLIFE CYCLECOSTS, SUPPORTPAGE58 2205_TN_MAG.indd 4/4/22 12:53 1technation.com/subscribe
CYBERSECURITY Rubber
Ducks, Dolphins and Bunnies, Oh My!
How Bad USBs Can Get Past Even the Best Cybersecurity Program
BY NADIA ELKAISSI AND JANE LACSON
i cture this: a few healthcare technology management (HTM) interns have just come back from a cybersecurity conference and brought with them a handful of free USBs (universal serial bus) phone charging cables they found on a vendor table. They decided to be generous and hand them out to HTM staff at the hospital. Several were excited for the merchandise and immediately started plugging them into their computers to charge their phones. Suddenly, they started noticing their computers reboot one by one. What they did not realize was that the charging cables included another chip embedded within them. The chip was loaded with a malware code which executed the code on the computers to pull patient data. This simple method is known as “juice jacking” and is one type of cyber-attack where malware is installed onto a device using a charging port that doubles as a data connection, typically over USB. With some strict policies and processes in places, this hack may have been preventable.
To fully comprehend how a bad USB works, we must first understand what a USB is and the technology behind it. A USB is an industry standard for short-distance digital data communications. It’s typically used as a bridge for transferring and sharing data between
devices and can be used to charge devices. This type of technology can provide several benefits for hospitals in that it provides a quick data transfer, is portable, provides ease for backing up data offline, and is compatible with most technology. Although the devices can offer speed, efficiency and accuracy with data transfer, they also pose risks. These seemingly innocent tools can act as Trojan Horses, bringing malicious threats to a health care environment.
The method described in the scenario is particularly deceptive, because typically the charging cable will function as expected, while in the back end transferring data or installing a malicious code on the system. USB malware can come in several forms and each poses its own unique threat and require its own defense strategies. Many can be categorized into the following sections: viruses, worms, trojans, spyware, ransomware, rootkits and BadUSB. Some of the more known hacking tools are the Rubber Ducky, Flipper Zero and the Bash Bunny.
1. Rubber Ducky: The “Rubber Ducky” is a hacking tool that mimics a USB flash drive but acts as a type of Human Interface Device (HID) such as a keyboard when plugged into a device. Once plugged in, it can start “typing” pre-programmed commands.
The DuckyScript does not require drivers and

P CONNECTED 66 TechNation | October 2023
therefore can bypass several security measures. In addition, the scripts run very quickly and often make detection difficult.
2. Flipper Zero: The ”Flipper Zero” uses an RF frequency to mimic an IR remote control which allows it to interact with several IR-controlled devices, TVs and even air conditioners. It has the capabilities for both RID and NFC, which can pose potential threats to devices such as access control systems or payment systems. The tool has a built-in “Cyber dolphin” that responds and evolves based on the user’s interaction.

Similar to the Rubber Ducky, it can also mimic a keyboard and mouse.
3. Bash Bunny:
The “Bash Bunny” looks just like a regular USB flash drive and has a small computer to perform cyber-attacks when plugged in. It can mimic several different forms of USBs and contains onboard storage. The storage will allow for the exfiltration of any data, and the small computer will allow for code to be quickly executed. This tool can be very hard to detect before codes have been completely executed.
Each of these tools can pose a significant risk to patient care. Hospitals and health care institutions must prioritize cybersecurity, ensuring they have a robust system and protocols in place to defend against and respond to cyber threats.
Protecting against BadUSB attacks requires a multi-layer approach. Policies should be developed;


expectations should be set and best practices should be followed. Organizations must implement USB use policies to ensure only authorized USB devices are to be used within the network. This can be enforced by creating a whitelist of approved devices and blocking all other devices. Another option is to disable the autorun feature on all USB devices. This feature automatically runs a program when a USB device is inserted into a system. Disabling this feature can prevent malicious code from executing automatically. In addition, an effective vendor management policy needs to be in place to ensure the use of USB device verification. Whenever a vendor or outside source brings in a USB that needs to be plugged into a networked medical device, it should be scanned and verified in a controlled environment. A good practice is using a type of media scanning stations unplugged from the network before scanning. The scanning station should be updated with the latest virus/ malware scanning software by Information Technology (IT). Using a scanning tool and following proper policies will allow for an attack to be isolated from the health care network and can allow proper authorities to start the remediation process without impacting operations.
 Jane Lacson, CCE, CHTM, is a biomedical engineer in the Healthcare Technology Management VA Central Office (19HTM).
Jane Lacson, CCE, CHTM, is a biomedical engineer in the Healthcare Technology Management VA Central Office (19HTM).
October 2023 | TechNation 67
Nadia ElKaissi, CHTM, biomedical engineer in the Healthcare Technology Management VA Central Office (19HTM).
HEALTH-ISAC New Regulations Impact Cybersecurity Management
 BY PHIL ENGLERT
BY PHIL ENGLERT
f ew weeks ago, I was on a discussion panel with Dr. Suzanne Schwartz, MD, Director - Office of Strategic Partnerships & Technology Innovation (OST), Center for Devices & Radiological Health (CDRH), FDA. The conversation revolved around the recent regulatory changes granting FDA statutory authority over managing cybersecurity risks of medical devices.
A question came in from the audience asking for an operational perspective of how the new regulations translated into managing risks for health care providers. In that moment, I had a flash that FDA’s cybersecurity guidance was very similar in approach to The Joint Commission’s (TJC) risk management approach found in the Environment of Care (EoC) guidelines. In that aha! moment, the similarities clarified, and I’d like to explore that thread a bit farther.
My response included the observation that TJC guidelines for any of the six Environments of Care follow a consistent framework. The TJC EoC framework outline is: 1) you must have a risk management program specific to the environment. An example is medical equipment, 2) the program must adequately address specified elements, 3) there must be evidence through measurement and reporting that the program is being followed and risk management effectiveness assessed, and 4) the program will identify and pursue opportunities for improvement. More succinctly, “EC.01.01.01 requires an individual or individuals to manage risk, coordinate risk reduction activities in the physical environment, collect deficiency information, and disseminate summaries of actions and results.”
In contrast, FDA’s April 2022, Cybersecurity in
Medical Devices: Quality System Considerations and Content of Premarket Submissions
, draft guidance states FDA “will assess the adequacy of a devices security” based on five security objectives and the submissions should include information that “describes how the above security objectives are addressed by and integrated into the device design.” The Joint Commission (TJC) and the FDA’s 2020 draft premarket guidance share some similarities in their approach to managing risk within healthcare delivery organizations, particularly in the context of cybersecurity risks introduced by medical devices in the health care market.
Both the Joint Commission and the FDA’s draft guidance adopt a risk-based approach to manage risks. This means that instead of applying a one-size-fits-all approach to all aspects of health care delivery or medical device cybersecurity, they focus on identifying and prioritizing risks based on their potential impact on patient safety and overall quality of care. This is essential given the variety of health care delivery environments and the vast spectrum of medical device technologies. The safety risks of medical devices present in an ambulatory surgery center (ASC) and a trauma center are not intrinsically different but the patient tolerance for care interruption is typically higher in an ASC than the more severe cases which may present at the trauma center. While the risks may not differ significantly, outcomes due to environmental risks may vary drastically.
The TJC and FDA frameworks each emphasize the importance of identifying and assessing risks. The Joint Commission’s environment of care standards requires health care organizations to perform risk assessments related to various aspects of patient care, including the use of medical devices. Similarly, the FDA’s draft guidance on premarket cybersecurity for medical devices requires manufacturers to assess and document the potential cybersecurity risks associated with their
CONNECTED
68 TechNation | October 2023
A
products. The FDA expects manufacturers to consider the risks their products introduce into the health care environment including the risks associated with the transactions between those devices and systems with other end points and applications.




























Both approaches encourage the implementation of mitigation strategies to address identified risks effectively. The FDA’s draft guidance emphasizes the importance of implementing appropriate cybersecurity controls and measures during the design and development of medical devices to mitigate potential risks. The Joint Commission expects health care organizations to have risk management plans that outline how they will address identified risks and prevent potential adverse events.
Both the Joint Commission and the FDA’s draft guidance promote a culture of continuous improvement when it comes to risk management. The Joint Commission expects health care organizations to monitor and evaluate their risk management strategies regularly and make necessary improvements as needed. Similarly, the FDA encourages medical device manufacturers to continuously monitor and update their cybersecurity practices throughout the device’s life cycle, considering new threats and vulnerabilities that may emerge.



Ultimately, the main goal of both approaches is to enhance patient safety and ensure the delivery of high-quality health care services. This includes the safe operations, the availability for treatment, and the integrity of the treatment and data. By addressing risks associated with medical devices and health care delivery processes, these frameworks aim to minimize the potential harm to patients and improve overall care outcomes.

While The Joint Commission’s environment of care standards and the FDA’s draft premarket guidance for medical device cybersecurity have their specific scopes and nuances, they share a fundamental risk-based philosophy that centers on patient safety and the delivery of effective health care services. I’m grateful for the gentlemen’s question that brought me to the realization that the regulatory drivers for cyber risk management of medical devices is closely aligned for both manufacturer and health care delivery. Hopefully, this commonality can improve patient safety and health care sector resilience.






SWBIOMED.COM 800.880.7231 “We Put The ACE In Spacelabs!” RECORDERS ACCESSORIES • FREE TECHNICAL SUPPORT • FREE REPAIR EVALUATIONS BEDSIDE & CENTRAL MONITORING • MODULES • TELEMETRY FLAT PANELS SPACELABS • DRAGER • SCOTTCARE DATASCOPE • GE • PHILIPS WWW.SWBIOMED.COM • 800.880.7231 “We Put The ACE In Spacelabs!” • NIHON KOHDEN • BLEED 11” TRIM 10.75” SAFETY 10.25” FULL PAGE PROOF APPROVED CLIENT SIGN–OFF AD SIZE NOTES PLEASE CONFIRM THAT THE FOLLOWING ARE CORRECT MEDICAL DEALER TECHNATION ORTODAY BUYERS GUIDE OTHER PUBLICATION MONTH J F M A M J J A S O N D SPACELABS • DRAGER • SCOTTCARE NIHON KOHDEN • DATASCOPE GE • PHILIPS THE SEARCH IS OVER. WE WILL REPAIR YOUR SPACELABS EQUIPMENT. October 2023 | TechNation 69
Phil Englert is the director of medical device security for Health-ISAC.
NETWORKING NOTES Router and Layer Three Hardware Tips
BY GARRETT SEELEY AND CLINT WILLIAMS
In past articles, we discussed the purpose and use of layer two networking hardware such as switches and wireless access points. Continuing with networking discussions, let’s evaluate layer three hardware. This is a very useful set of hardware because layer three of the OSI model uses the IP address.
The following hardware will use an IP address in its settings: routers, gateways, DNS servers, firewalls, VPNs, access control lists and cybersecurity appliances. Recall that the letters IP literally mean Internet protocol, it is the setting to access the Internet. The most important additional concepts are the gateway and Domain Name Servers (DNS). These settings are in routers used in home networks and servers in hospitals.
device using an IP to communicate to a device outside of the LAN. It allows for communication to the Internet, which is a wide area network or WAN. If this is confusing, look at the graphic.

To get to the Internet, traffic from the computers must leave the LAN through the router. The router functions as a gateway and provides Internet sharing. One connection on the router connects the entire network to the Internet. Technically, this does not have to be done by a router. It could be accomplished with a server and two different NIC adaptors; one for each network. The pictured router has a built-in gateway server, as well as a switch for the three devices connected to it. It also has a wireless access point because it has two integrated antennas. Most home routers will perform these functions. Look at a home network for a live view of this network flow. Keep in mind the gateway had two IP addresses, one for the LAN side and one for the WAN/Internet side. These are often marked on the router as separate network ports. The switch and access point share this connector to the attached devices. This is the purpose of a gateway, and all routers are gateways.
The settings for Internet access were discussed in the article on TCP/IP and in the article on advanced subnetting. To recap, all Internet devices use an IP, subnet, gateway and DNS. IP address is a location of a device on a Local Area Network (LAN), and the subnet is a description of the scope of the network. For home networks, the gateway and DNS settings are usually the local router IP. To view these settings, go to the command prompt. To run this, search “cmd” in any Windows based machine. This will bring up the command prompt. From there type, “IPCONFIG/ALL” to see the IP settings. The two settings not previously explained are the gateway and the DNS. A gateway is just as it sounds. A gate is a way to leave somewhere. A gateway allows a
The hard part of understanding routers isn’t the integrated hardware of services, it is the way it sorts data. Picture a scenario where two people are on the same website. They are both accessing the same search from the Internet. Their local network router sends data between the computer and the laptop, sorted because they each have a different IP, let’s say 192.168.1.101 and 192.168.1.102 respectively. Assume they are both looking at 142.250.191.206. The computers are both doing different things on the same server. The Network Address Translation table, or NAT, in the router knows which answer from 142.250.191.206 is intended for 192.168.1.101 and which is for 192.138.1.102. It does this by using the ports as a separator. If this is not clear, do not worry. A NAT is just something a biomed technician should be aware of, and full understanding is not required. Just know that the router will keep the communications separate, even when receiving replies from the same WAN server for devices on the same LAN.
The DNS setting in a home network is usually the router as well, but it does not have to be. The DNS is like a phone book for the Internet. The Internet does not use names. It uses numbers. However, people use names for websites.
CONNECTED
70 TechNation | October 2023
For example, pinging Google.com may give an answer from the IP: 142.250.191.206. A DNS is the server matching human given names to an IP number. It is like a global phonebook for the Internet. It describes to LAN devices how to get to the IP that represents the name. The best machine on a home LAN to give these numbers for the names on the Internet is the router. If the router does not know, it will ask the router of DNS it attaches to, and so on and so forth. Eventually, a DNS router or server that knows the answer gives back a number. This is sufficient for a small network. However, larger networks have too many DNS requests for a router to focus on this task. For this reason, hospitals will run a separate DNS server. This also allows for internal DNS names. A LAN server can be given a name by a local DNS as if it were an Internet web address. Other devices can use this LAN name instead of an IP. Because of the convenience of this feature, most hospitals use a separate DNS server on their LAN. In a hospital setting, it is almost never the router or gateway.

There are other things that routers can do. For example, they can work together. They can be used inside a LAN to separate the subnets and restrict access from the larger LAN. This creates an issue called “port forwarding.” Just know that rules can be set to allow communication through a router to another subnet on a LAN. This is also programable using routing protocols. Again, like NAT, it is important to be aware of these things. Thankfully, it is not required that the average biomedical technician know how to set these things up. There is a whole separate degree for this work called network administration. Just be aware that routers can be used inside a LAN as well, usually on the divisions of subnet to isolate LAN traffic. This gives better control of LAN access to that subnet. Future articles will discuss the use of firewalls, port forwarding and permissible traffic. This is really where networking takes off and warrants its own article. Unfortunately, biomed technicians are being pulled into dealing with firewalls, port forwarding, VPN access, ACL lists and even basic concepts of routing protocols. More will have to follow on this subject.
For now, just look at the router at home. It’s a marvel performing thousands of operations per second. Look at its integrated switch, LAN and WAN ports, and wireless access points. Perhaps it even has integrated USB for file and print server functions. If not done already, download the router manual and read on its settings and capabilities. The best lab to learn this stuff is right at home. Get to it and enjoy exploring it.
Diagnostic Solutions is a customer service based parts provider that specializes in all imaging modalities and manufacturers. Created to offer hospitals and ISO’s a cost effective and time saving solution for ordering imaging replacement parts, equipment moves, ultrasound probe repair and on-site service.

 Garrett Seeley, MS, CBET, Biomedical Equipment Support Specialist, VISN 17: VA North Texas Health Care System, Dallas Veterans Affairs Medical Center.
Clint Williams is a Biomedical Information Systems Specialist at North Texas Veterans Administration.
Garrett Seeley, MS, CBET, Biomedical Equipment Support Specialist, VISN 17: VA North Texas Health Care System, Dallas Veterans Affairs Medical Center.
Clint Williams is a Biomedical Information Systems Specialist at North Texas Veterans Administration.
Contact us today, we are confident you will see us as THE Parts Solution! 330.296.9729 diagnostic-solutions.com CONTACT US TODAY! We’ve got the solution! October 2023 | TechNation 71

Electrical Safety Patient Simulation Defibrillator Testing Performance Analyzers & performance test equipment by Rigel Medical Scan QR code to find out more or visit rigelmedical.com/TN10



Call or text Dan at (720) 939 8467 | Send an email to hello@cynch.me *See FDA recalls Easily add a new asset on the fly See the current status See all PMs in one place. Last performed, next due, overdue, etc. See all open / unresolved problem reports BEST IN CLASS SOFTWARE for managing PMs, repairs, & assets Actual screenshot of the asset list
HIMSS23
Informa Markets, HIMSS to Expand the HIMSS Global Health Conference & Exhibition
nforma Markets and the Healthcare Information and Management Systems Society (HIMSS) recently announced a landmark partnership to propel the growth and evolution of the HIMSS Global Health Conference and Exhibition. Informa Markets will take on management of the HIMSS Exhibition, while HIMSS will continue to oversee developing expert content and programming.
Recognized as the most influential healthcare technology event of the year, the HIMSS Global Health Conference and Exhibition brings together the global health ecosystem to network, discover, learn and innovate. As one of the largest health care events in North America, it draws 40,000 health professionals, technology leaders, providers and governmental organizations from around the world. Informa Markets is the world’s leading exhibition organizer, connecting communities across diverse sectors ranging from fashion to engineering, health and wellness to construction, and more, with a portfolio encompassing over 450 brands.
This partnership will bring together the scale, expertise and resources of the world’s leading exhibitions organizer with the unparalleled thought leadership that HIMSS has established in the health tech community. Participants will continue to benefit from HIMSS’ excellence in curating content and programming that showcases the latest advancements and innovations in health information and technology, while drawing on Informa Markets’ unrivaled expertise in delivering world-class event experiences. With a strong presence in the health care and medtech space through brands such as FIME, Arab Health and IME, Informa Markets is an ideal partner to elevate the HIMSS Exhibition to new heights. Under Informa Markets’ leadership, the 2024 version of the HIMSS Conference and Exhibition will provide fresh
opportunities and enhance the customer experience for HIMSS members, featuring improved digital features, enhanced registration processes, marketing tools and cutting-edge product discover applications.
“The HIMSS 2023 Global Health Conference and Exhibition was an outstanding success. We are determined to continuously advance this conference and exhibition, delivering ever greater value to everyone who attends: speakers, sponsors, exhibitors and health care professionals,” said Hal Wolf, president and CEO of HIMSS. “With the remarkable growth that the global conference has reached, it’s the right time to partner with North America’s leading exhibitions organizer, Informa Markets, who shares our vision to make the HIMSS Global Health Conference and Exhibition the best it can be for the entire ecosystem.”
After a successful 2023 conference hosted in Chicago, the 2024 HIMSS Global Health Conference and Exhibition will move to Orlando, where Informa’s South Florida Ventures team has recently added Art Miami, the Miami International Boat Show and Premiere Beauty to its rapidly growing luxury lifestyle portfolio in the region, under the leadership of Ken McAvoy.
“We are thrilled to expand our work connecting the health care community with the addition of the HIMSS Global Health Conference and Exhibition,” said McAvoy, president, South Florida Ventures at Informa Markets. “Drawing on our extensive experience in organizing some of the largest and most successful events in the U.S., combined with HIMSS’ unparalleled expertise in the digital health space, we are excited to collaborate and leverage our resources to deliver even greater benefits and value to the HIMSS community and its members.”
The 2024 HIMSS Global Health Conference and Exhibition, the inaugural edition under this new partnership, will take place in Orlando, Florida, on March 11-15.
CONNECTED
I 74 TechNation | October 2023











Operate Efficiently, Perform Real Time, Simplify Compliance Simplify the PM Process www.truasset.com 214-276-1280 sales@truasset.com Our GET CONNECTED Directory is a great place to find contact information from companies who specialize in software, asset management, cybersecurity, CMMS, etc. LOCK DOWN YO CYBERSEC THREA RENOVO1.COM 844-473-6686 Get a free evaluation networked m device Wireless automation has arrived! Automated Electrical Safety, Simulation & IV Pump Test Sequences P Complete control + data capture P Run user-defined / manufacturers’ checklists P Generates a complete electronic test report CONNECTED COMPANY DIRE CTORY LET’S GET October 2023 | TechNation 75




BETABIOMED.COM RELIABLE DURABLE ACCURATE AFFORDABLE COMFORTABLE Striving for Perfection Through Discovery and Innovation ISO 13485 ACCREDITED AND FDA REGISTERED CUSTOM CABLES AVAILABLE! WE REPAIR AND REPLACE!













Looking to lower repair cost? Need a FREE loaner and FREE evaluation? SakoMed is here to help, offering 2-3 days turn around time on all repairs. REFURBISHED MEDICAL EQUIPMENT SALES AND SERVICES 27751 LA PAZ RD. STE A LAGUNA NIGUEL, CA • 92677 (844) 433-SAKO • INFO@SAKOMED.COM • Anesthesia Machines • Respiratory Ventilators • Electro Surgical Units • Patient Monitors & Telemetries • Defibrillators and AEDs • Parts and Accessories Check out our Live Inventory List www.sakomed.com PRINT YOUR FREE SHIPPING LABELS HERE


BIOMEDICAL When It Comes To CENTRIFUGES, One Name Stands Out • Free Tech Support • Depot Repair • Rental Units • Re-manufactured Parts • New Parts • Exchanges Your Centrifuge Solutions Center www.ozarkbiomedical.com 800-457-7576 birdrf.com 78 TechNation | October 2023
WHAT’S ON Y UR BENCH?
• “#1 in California” coffee mug
• fruit
• dark chocolate
• more pens than I need
• laptop

• 2 computer monitors

• Web camera
Michele Manzoli
CCE, is a manager of Clinical Engineering at Cedars-Sinai in Los Angeles. Manzoli grew up in Italy. Submit
your bench to be featured in TechNation at 1technation.com/my-bench/. BREAKROOM October 2023 | TechNation 79

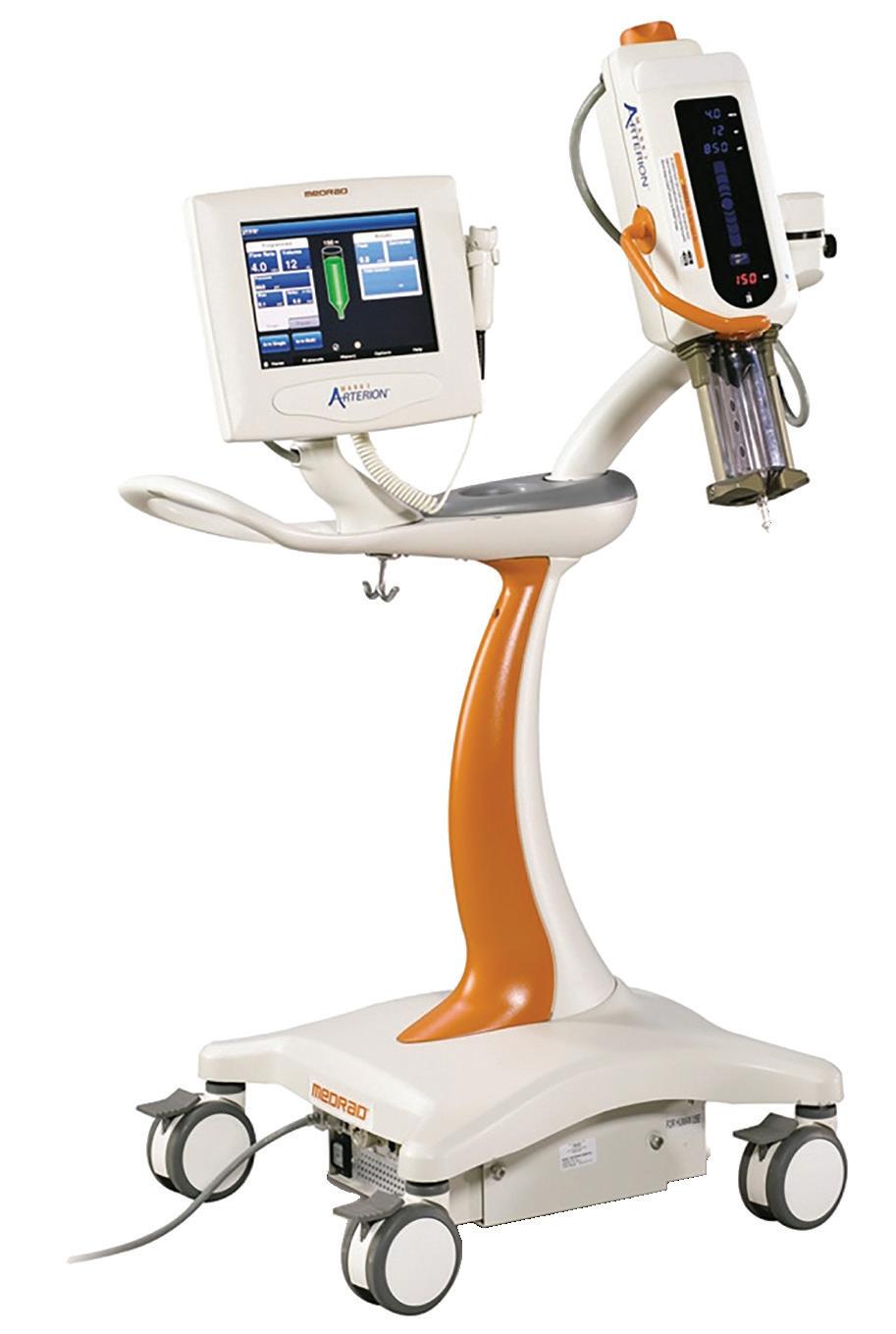


Contrast Injector Training • 100s of Error Codes not found in OEM Lit • Online and Onsite Training Available • Training BMETs since 2008 • Up to $5,500 in FREE Parts, PM Kits and Service • Massive Troubleshooting Library WWW.MAULLBIOMEDICAL.COM | 440-724-7511 | STEVE@MAULLBIOMEDICAL.COM ON-SITE AVAILABLE 80 TechNation | October 2023
TECHNATI N P LL
What is your favorite candy bar?
PAY DAY 18% SNICKERS 51%
Connect with TechNation on LinkedIn at linkedin.com/company/iamtechnation to participate in our next poll: What is your favorite part about attending a MD Expo?
LET OUR CMMS STREAMLINE YOUR WORKFLOW
“HEMS One” by EQ2: A CMMS and company matched to the needs of hospitals...
• Upload equipment information easily with just a couple of clicks

• Search and replace multiple data fields at once with impact preview

• Create or edit your own PM Procedures
• Convenient quick links to reports
• Department Manager Dashboard: KPI summaries for specific areas such as surgery, nursing, ICU, etc.
“HEMS One” empowers HTM / Biomed teams to do their best work! Schedule your demo of HEMS today: 888-312-4367 or EQ2LLC.com
BREAKROOM
BABY RUTH 12% MILKY WAY 19%
OF INNOVATIO N Years October 2023 | TechNation 81



82 TechNation | October 2023

BIOMED BRAINBUSTER Visit 1technation.com/crossword for an interactive puzzle. THIS MONTH’S CROSSWORD SPONSOR: ACROSS 1 Volumetric infusion system, 2 words 5 Sanitize 8 Slang name for an analgesic 9 Pioneer in radiation dose measurements 11 Break open 13 Vital tool for a BMET 18 Cheerleading cry 19 Conductor of electric current in one direction 20 Electronic display, abbr. 21 __ de plume (pen name) 22 Number in PSA testing 24 Pre-owned 27 Leader in the supply and repair of medical equipment 31 Label 33 Conduct, with out 34 An endocrine gland DOWN 1 Sub atomic particle with an electric charge used in some scans 2 Put into operation 3 Lady referred to 4 Young kid 5 Protection from hacking and malware 6 Otologist’s subject 7 Hair cover 10 GPS suggestion, abbr. 12 Visit 13 They hold plugs firmly in place 14 Medical imaging branch of medicine 15 Reporter’s question, often 16 Heart part 17 Absorbed-dose units 23 Incident 25 Dover’s state 26 Software provider enabling electronic health record storage 28 Keep out 29 Auto 30 Earlier 32 A patient provides this on admittance, abbr. BREAKROOM October 2023 | TechNation 83





Ask Us AboutReferralClient Program thesoaringhearts.com NOW YOU DO! Do you know any company that can REMOTELY diagnose, and possibly repair your contrast injector issues? • Step into the 21st century with our tablet powered assistant, remote support, speech to text, image capture, reporting, and integration into your maintenance system. • Call us today for a FREE welcome kit. This is everything you need to connect your tablet/laptop with the injector. Including cables, adapters, and software. • Now offering our 30+ years of injector experience to maintain and repair your devices. Services include PMs, repairs, installs, de-installs, upgrades, training, and sales. • We use our advanced tools to provide you with a superior service event. • Get OEM quality service at the lowest cost in the industry. Callfortoday our $1099.00 PM Special. 724-782-0227 contrastinjectors.com 84 TechNation | October 2023
GBIS SCRAPBOOK





The Georgia Biomedical Instrumentation Society (GBIS) is a professional association of biomeds, manufacturers, hospitals and colleges who are interested in furthering the knowledge, professionalism and interaction of these unique and important individuals. The GBIS 2023 Conference had an estimated 75 attendees, 6 instructors, 6 classrooms and 13 vendors. It was a successful conference with continuing education, networking and more. For more information, visit gbis.wildapricot.org.



1 2 3 4 5 6 7
1: An award in honor of the late Dr. MIke O’Rear was presented at the 2023 GBIS Conference.
2: Glen Stone, an instructor at Southeastern Technical College, presents “CBET Review for Electronics.”
3: Children’s Healthcare of Atlanta Vice President of Facilities Carlos Ruiz answers a question about the new Arthur Blank Hospital.
4: An anesthesia servicing session is lead by Melba Lawrence, Ken Stephens, Stan Guthrie and Ralph Mitchell.
5: GBIS Executive Director Horace Hunter addresses attendees.
6: Pictured from left to right are GBIS’s Michael Cameron with biomeds Margarita Alvarado, Santos Gonzalo and Justin Barbour.
BREAKROOM October 2023 | TechNation 85
7: GBIS attendees are seen attending an ultrasound preventive maintenance session.
NCBA SCRAPBOOK
The 2023 North Carolina Biomedical Association (NCBS) hosted around 400 attendees and 80 vendor booths at its annual symposium. Healthcare technology management professionals in attendance had access to 30 classes, two keynote speakers and a business lunch with awards. The annual symposium is part of the NCBA’s mission of enhancing biomedical professionals via classes, networking opportunities, vendor hall, awards, scholarships, prizes and more.
The 2024 NCBA symposium will be held at Harrah’s Cherokee Casino & Resort. The four-day event will feature the annual Mike McCoy Golf Tournament. Vendor registration for next year’s symposium is open.

6: USOC sponsored the annual NCBA business lunch during the symposium. This event brings together association members for updates on board elections and events.
5:
available.
7: TechNation loves hosting an annual networking event during NCBA. Attendees and vendors meet up to continue conversations and make new connections.







1 2 3 4 5 6 7
1: The RSTI team (left to right) Todd Boyland, Kim Rowland, Jason Whitfield, Dale Cover and Terry Speth had a busy couple of days at the NCBA symposium, participating in networking events, leading education and hosting a booth in the exhibit hall. This team does it all!
2: Jayme McKelvey (center) greets TechNation advertisers Amy Hobbs of USOC and Kerwin Sanger of IMT Analytics as they arrive to TechNation’s networking happy hour at the Pinehurst Brewery.
3: Tenacore was one of 80 vendors exhibiting inside the NCBA exhibit hall.
4: 626 Holdings CRO Kevin Gill and son Nick Gill, vice president of strategic accounts, spoke with NCBA attendees about the expansive knowledge and experience 626 brings to HTM.
Attendees enjoyed the packed exhibit hall, visiting with vendors to learn about the newest technologies
BREAKROOM October 2023 | TechNation 87



88 TechNation | October 2023 PUTTING CUSTOMERS FIRST SINCE 1987 TOSHIBA • GE • PHILIPS • SIEMENS AND MORE! Call: 508.730.9544 or 508.559.9441 www.InternationalXrayBrokers.com admin@intxray.com INTERNATIONAL X-RAY BROKERS IS NOW AN AUTHORIZED DISTRIBUTOR FOR CUSTOM BUILT SURGICAL TABLES! ALL MANUFACTURES & MODALITIES WE ALSO BUY AND SELL PRE-OWNED MEDICAL IMAGING EQUIPMENT. Tech Choice Awards Introducing the 2024 (The Wrenchies) The 13 Tech Choice Awards are: 1. Professional of the Year 2. ISO Employee of the Year 3. Department of the Year 4. Director/Manager of the Year 5. YP of the Year 6. Lifetime Achievement Award 7. Humanitarian Award 8. Women in Leadership Award 9. Ingenuity Award 10. Outstanding Vendor of the Year 11. Association of the Year 12. Industry Influencer of the Year 13. Military BMET of the Year TheWrenchies NOMINATE SOMEONE TODAY! nominations are accepted through November 3rd
WHERE IN THE WORLD IS BEN C?









Show
Step
us what Ben Calibrating has been up to!
MedWrench on Facebook at facebook.com/medwrench & on LinkedIn at linkedin.com/company/medwrench
Follow
y’all!
1: Like the MedWrench Facebook or LinkedIn company page. Step 2: Post your picture of Ben C. to Facebook or LinkedIn & tag MedWrench in your photo. Step 3: Post a funny caption with your picture. Step 4: Use #BenC It’s fall
Ben C.loves pumpkin spice lattes.
Ben C.is off to the pumpkin patch.
Ben C. enjoys a hike.
Ben C.is ready for football season.
BREAKROOM October 2023 | TechNation 89
SPONSORED BY:
Company Info AD PAGE PARTS SERVICE TRAINING Company Info AD PAGE PARTS SERVICE TRAINING SERVICE INDEX Anesthesia A.M. Bickford www.ambickford.com • 800-795-3062 31 P SakoMED sakomed.com • (844) 433-7256 77 P P Soma Technology, Inc www.somatechnology.com • 1-800-438-7662 79 P USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P Asset Management Capital i capitali.us • 417-708-2924 80 Cynch cynch.me • 73 EQ2 www.eq2llc.com • 888-312-4367 81 Renovo Solutions www.renovo1.com • 844-4RENOVO 41,75 P P Cables BETA Biomed Services www.betabiomed.com/ • 800-315-7552 76 iServe Biomedical iservebiomedical.com • 281-741-0849 93 P Medical Equipment Doctor, INC. www.medicalequipdoc.com • 800-285-9919 17 PM Biomedical pmbiomedical.com • 800-777-6468 83 P P Calibration Bird RF birdrf.com • 866-695-4570 78 Rigel Medical, Seaward Group www.seaward-groupusa.com • 813-886-2775 72 Cardiac Monitoring Cardiotronix cardiotronixhealth.com • (855)-4DEFIBS 21 P P Jet Medical Electronics Inc www.jetmedical.com • 714-937-0809 65 P P Soaring Hearts Group soaringheartsinc.com • 855.438.7744 84 P P Cardiology Soaring Hearts Group soaringheartsinc.com • 855.438.7744 84 P P Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010 4 P P Southwestern Biomedical Electronics, Inc. www.swbiomed.com/ • 800-880-7231 69 P P CMMS Capital i capitali.us • 417-708-2924 80 Cynch cynch.me • 73 EQ2 www.eq2llc.com • 888-312-4367 81 TruAsset, LLC www.truasset.com • 214-276-1280 59,75 Computed Tomography AllParts Medical www.allpartsmedical.com • 866-507-4793 7 P P Diagnostic Solutions diagnostic-solutions.com • 330-296-9729 71 P P International X-Ray Brokers internationalxraybrokers.com/ • 508-559-9441 88 RSTI www.rsti-training.com • 800-229-7784 23 P P P Tri-Imaging Solutions www.triimaging.com • 855-401-4888 35 P P P Consultancy Health Tech Talent Management, Inc. www.HealthTechTM.com • 757-563-0448 82 NVRT Labs Inc. nvrtlabs.com • 29 Contrast Media Injectors 626 Holdings weare626.com • 800-516-0991 38 Contrast Injector Tools contrastinjectors.com • 724-782-0227 84 P P Maull Biomedical Training www.maullbiomedicaltraining.com • 440-724-7511 80 P Defibrillator Cardiotronix cardiotronixhealth.com • (855)-4DEFIBS 21 P P iServe Biomedical iservebiomedical.com • 281-741-0849 93 P P SakoMED sakomed.com • (844) 433-7256 77 P P Diagnostic Imaging 626 Holdings weare626.com • 800-516-0990 38 P Diagnostic Solutions diagnostic-solutions.com • 330-296-9729 71 P P International X-Ray Brokers internationalxraybrokers.com/ • 508-559-9441 88 Probo Medical www.probomedical.com • 3174947872 49 P P Renovo Solutions www.renovo1.com • 844-4RENOVO 41,75 P P Soma Technology, Inc www.somatechnology.com • 1-800-438-7662 79 P Tri-Imaging Solutions www.triimaging.com • 855-401-4889 35 Education/Training 626 Holdings weare626.com • 800-516-0990 38 P College of Biomedical Equipment Technology www.cbet.edu • 866-866-9027 11 P ECRI Institute www.ecri.org • 1-610-825-6000. 44 P Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6703 60 P NVRT Labs Inc. nvrtlabs.com • 29 Probo Medical www.probomedical.com • 3174947872 49 Renovo Solutions www.renovo1.com • 844-4RENOVO 41,75 P P RSTI www.rsti-training.com • 800-229-7784 23 P 90 TechNation | October 2023
Company Info AD PAGE PARTS SERVICE TRAINING Company Info AD PAGE PARTS SERVICE TRAINING Webinar Wednesday www.triimaging.com • 855-401-4888 16 P Employment/Recruiting Health Tech Talent Management, Inc. www.HealthTechTM.com • 757-563-0448 82 HTM Jobs www.htmjobs.com • 54 Endoscopy Healthmark Industries hmark.com • 800-521-6224 22 Medical Equipment Doctor, INC. www.medicalequipdoc.com • 800-285-9921 17 Multimedical Systems www.multimedicalsystems.com • 888-532-8056 42 P PM Biomedical pmbiomedical.com • 800-777-6471 83 P P SakoMED sakomed.com • (844) 433-7256 77 P P ESUs SakoMED sakomed.com • (844) 433-7257 77 P P Fetal Monitoring iServe Biomedical iservebiomedical.com • 281-741-0849 93 P P Multimedical Systems www.multimedicalsystems.com • 888-532-8056 42 P General Bird RF birdrf.com • 866-695-4571 78 P P Health Tech Talent Management, Inc. www.HealthTechTM.com • 757-563-0448 82 SalesMaker Carts salesmakercarts.com • 800-821-4140 65 Infection Control Healthmark Industries hmark.com • 800-521-6224 22 Infusion Pumps Adepto Medical adeptomed.com • 833-423-3786 5 AIV aiv-inc.com • 888-656-0755 27 P P Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6701 60 P P FOBI www.FOBI.us • 888-231-3624 56 P P iServe Biomedical iservebiomedical.com • 281-741-0849 93 P P Multimedical Systems www.multimedicalsystems.com • 888-532-8056 42 P SakoMED sakomed.com • (844) 433-7258 77 P P Soma Technology, Inc www.somatechnology.com • 1-800-438-7662 79 P USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P Installs/Deinstalls Tri-Imaging Solutions www.triimaging.com • 855-401-4889 35 Labratory Ozark Biomedical www.ozarkbiomedical.com • 800-457-7576 78 P P Mammography International X-Ray Brokers internationalxraybrokers.com/ • 508-559-9441 88 RSTI www.rsti-training.com • 800-229-7784 23 P P P Monitors/CRTs Medical Equipment Doctor, INC. www.medicalequipdoc.com • 800-285-9925 17 PM Biomedical pmbiomedical.com • 800-777-6467 83 P P Tenacore Holdings, Inc www.tenacore.com • 800-297-2241 6 P P USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P MRI CM Parts Plus www.cmpartsplus.com • 877-267-2784 88 P P Diagnostic Solutions diagnostic-solutions.com • 330-296-9729 71 P P Innovatus Imaging www.innovatusimaging.com • 844-687-5100 8 KEI Medical Imaging www.keimedicalimaging.com • 512-477-1500 21 Online Resource HTM Jobs www.htmjobs.com • 54 MedWrench www.MedWrench.com • 26 Webinar Wednesday www.webinarwednesday.live • 800-906-3373 16 P Oxygen Blender FOBI www.FOBI.us • 888-231-3624 56 P P iServe Biomedical iservebiomedical.com • 281-741-0849 93 P P PACS RSTI www.rsti-training.com • 800-229-7784 23 P Patient Monitors AIV aiv-inc.com • 888-656-0755 27 P P BETA Biomed Services www.betabiomed.com/ • 800-315-7551 76 P P Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6701 60 P P iServe Biomedical iservebiomedical.com • 281-741-0849 93 P P Jet Medical Electronics Inc www.jetmedical.com • 714-937-0809 65 P P SakoMED sakomed.com • (844) 433-7259 77 P P Soma Technology, Inc www.somatechnology.com • 1-800-438-7662 79 Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010 4 P P Southwestern Biomedical Electronics, Inc. www.swbiomed.com/ • 800-880-7231 69 P P Tenacore Holdings, Inc www.tenacore.com • 800-297-2241 6 P P October 2023 | TechNation 91
Company Info AD PAGE PARTS SERVICE TRAINING
USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P Refurbish AIV aiv-inc.com • 888-656-0755 27 SakoMED sakomed.com • (844) 433-7261 77 P P Repair Cardiotronix cardiotronixhealth.com • (855)-4DEFIBS 21 P P Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6702 60 Jet Medical Electronics Inc www.jetmedical.com • 714-937-0809 65 P P SakoMED sakomed.com • (844) 433-7262 77 P P Soaring Hearts Group soaringheartsinc.com • 855.438.7744 84 P P Replacement Parts Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6701 60 P P iServe Biomedical iservebiomedical.com • 281-741-0849 93 P KEI Medical Imaging www.keimedicalimaging.com • 512-477-1500 21 SakoMED sakomed.com • (844) 433-7263 77 P P Respiratory A.M. Bickford www.ambickford.com • 800-795-3062 31 P FOBI www.FOBI.us • 888-231-3624 56 P P Software Cynch cynch.me • 73 EQ2 www.eq2llc.com • 888-312-4367 81 NVRT Labs Inc. nvrtlabs.com • 29 TruAsset, LLC www.truasset.com • 214-276-1280 59,75 Surgical Healthmark Industries hmark.com • 800-521-6224 22 Telemetry AIV aiv-inc.com • 888-656-0755 27 P P Elite Biomedical Solutions elitebiomedicalsolutions.com • 855-291-6701 60 P P iServe Biomedical iservebiomedical.com • 281-741-0849 93 P Multimedical Systems www.multimedicalsystems.com • 888-532-8056 42 P SakoMED sakomed.com • (844) 433-7260 77 P P Southwestern Biomedical Electronics, Inc. www.swbiomed.com/ • 800-880-7231 69 P P Company Info AD PAGE PARTS SERVICE TRAINING Tenacore Holdings, Inc www.tenacore.com • 800-297-2241 6 P P USOC Bio-Medical Services www.usocmedical.com • 855-888-8762 3 P P Test Equipment A.M. Bickford www.ambickford.com • 800-795-3062 31 BC Group International, Inc www.BCGroupStore.com • 314-638-3800 BC P P Bird RF birdrf.com • 866-695-4569 78 P P iServe Biomedical iservebiomedical.com • 281-741-0849 93 P Pronk Technologies, Inc. www.pronktech.com • 800-609-9802 2,75,82 QRS Solutions www.qrs-solutions.com/ • 877-254-7086 33 P P Radcal Corporation www.radcal.com • 800-423-7169 37 Rigel Medical, Seaward Group www.seaward-groupusa.com • 813-886-2775 72 SakoMED sakomed.com • (844) 433-7264 77 P P Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010 4 P P Tubes/Bulbs Tri-Imaging Solutions www.triimaging.com • 855-401-4888 35 P P Ultrasound AllParts Medical www.allpartsmedical.com • 866-507-4793 7 P P Innovatus Imaging www.innovatusimaging.com • 844-687-5100 8 Medical Equipment Doctor, INC. www.medicalequipdoc.com • 800-285-9936 17 MW Imaging www.mwimaging.com • 877-889-8223 86 P P P Probo Medical www.probomedical.com • 3174947872 49 P P Ventilators iServe Biomedical iservebiomedical.com • 281-741-0849 93 P P X-Ray AllParts Medical www.allpartsmedical.com • 866-507-4793 7 P P Innovatus Imaging www.innovatusimaging.com • 844-687-5100 8 International X-Ray Brokers internationalxraybrokers.com/ • 508-559-9441 88 RSTI www.rsti-training.com • 800-229-7784 23 P P P Tri-Imaging Solutions www.triimaging.com • 855-401-4888 35 P P P 92 TechNation | October 2023
SERVICE INDEX CONTINUED








ALPHABETICAL INDEX
You Can Count On. At iServe Biomedical, we strive to provide only the best quality replacement parts and accessories on the market. We offer an extensive selection of OEM Compatible replacements for top brands such as Philips, GE Healthcare, Nellcor, Masimo, Nihon Kohden, Spacelabs, Draeger, B.D/Carefusion/ Alaris, GE/Corometrics, Baxter, and more. Our products are designed to meet the highest standards of quality and reliability, ensuring that healthcare professionals can provide the best possible care for their patients. Whether you need replacement parts for your medical equipment or accessories to enhance their functionality, iServe Biomedical is your one-stop-shop for all your needs. Contact us today for a quote! iservebiomedical.com Info@iservebiomedical.com 281-741-0849 626 Holdings 38 A.M. Bickford ……………………… 31 Adepto Medical 5 AIV 27 AllParts Medical 7 BC Group International, Inc BC BETA Biomed Services 76 Bird RF 78 Capital i 80 Cardiotronix 21 CM Parts Plus 88 College of Biomedical Equipment Technology 11 Contrast Injector Tools …………… 84 Cynch 73 Diagnostic Solutions 71 ECRI Institute 44 Elite Biomedical Solutions 60 EQ2 81 FOBI 56 Health Tech Talent Management, Inc. 82 Healthmark Industries 22 HTM Jobs 54 Innovatus Imaging 8 International X-Ray Brokers 88 iServe Biomedical 93 Jet Medical Electronics Inc 65 KEI Medical Imaging 21 Maull Biomedical Training 80 Medical Equipment Doctor, INC. 17 MedWrench 26 Multimedical Systems 42 MW Imaging 86 NVRT Labs Inc. 29 Ozark Biomedical 78 PM Biomedical …………………… 83 Probo Medical 49 Pronk Technologies, Inc. 2,75,82 QRS Solutions ……………………… 33 Radcal Corporation 37 Renovo Solutions 41,75 Rigel Medical, Seaward Group 72 RSTI 23 SakoMED 77 SalesMaker Carts 65 Soaring Hearts Group 84 Soma Technology, Inc 79 Southeastern Biomedical, Inc 4 Southwestern Biomedical Electronics, Inc. 69 Tenacore Holdings, Inc 6 Tri-Imaging Solutions 35 TruAsset, LLC 59,75 USOC Bio-Medical Services 3 Webinar Wednesday ……………… 16 October 2023 | TechNation 93
The Service Team
#IamTechNation


J oin us as we celebrate the TechNation community. You - each and every reader, Webinar Wednesday attendee, HTM Jobs user and MD Expo attendee - are the most important part of the TechNation community. Share a photo of yourself, a colleague or the entire biomed team on social media and tag it with #IamTechNation. Then, check each issue of the magazine to see yourself and all of the men and women that are TechNation.

Greetings from South Dakota #BMETLIFE
 Photo by Gary Nop
Photo by Gary Nop
BREAKROOM
Day 2 at CABMET Symposium
Photo by Gary Nop
Day 2 at CABMET Symposium
Photo by Hamidsha Shahudeen
Eng. Shibu Shanker deeply engrossed in the installation of an Elekta Radiotherapy (LINAC) equipment.
94 TechNation | October 2023




POWERED BY: CALL FOR PRESENTERS To apply, visit mdexposhow.com/call-for-presenters/



































































































































































































































 By K. Richard Douglas
By K. Richard Douglas
















































 Jane Lacson, CCE, CHTM, is a biomedical engineer in the Healthcare Technology Management VA Central Office (19HTM).
Jane Lacson, CCE, CHTM, is a biomedical engineer in the Healthcare Technology Management VA Central Office (19HTM).
 BY PHIL ENGLERT
BY PHIL ENGLERT



























 Garrett Seeley, MS, CBET, Biomedical Equipment Support Specialist, VISN 17: VA North Texas Health Care System, Dallas Veterans Affairs Medical Center.
Clint Williams is a Biomedical Information Systems Specialist at North Texas Veterans Administration.
Garrett Seeley, MS, CBET, Biomedical Equipment Support Specialist, VISN 17: VA North Texas Health Care System, Dallas Veterans Affairs Medical Center.
Clint Williams is a Biomedical Information Systems Specialist at North Texas Veterans Administration.































































































 Photo by Gary Nop
Photo by Gary Nop







