














































































Need





P.12 SPOTLIGHT
p.12 Professional of the Month: Ashley O’Mara, MS, CCE
p.14 Department of the Month: The AHN West Penn Hospital Clinical Engineering Department
p.16 Next Gen: Josh Ablian
p.18 Association of the Month: Association of Medical Engineering of Kenya (AMEK)
P.22 INDUSTRY UPDATES
p.22 MD Expo New England Among the Best Ever
p.25 News & Notes
p.32 AAMI Update
p.34 Ribbon Cutting: UptimeServices
p.36 ECRI Update


P.39 THE BENCH
p.39 Tools of the Trade
p.40 Biomed 101
p.42 Webinar Wednesday
P.46 FEATURE ARTICLES
p.46 Roundtable: Infusion Pumps
p.50 Cover Story: The Real Value of Real-Time Location Systems
P.57 EXPERT ADVICE
p.57 Career Now
p.58 SPONSORED: Innovatus Imaging
p.60 Networking Notes
p.62 Cybersecurity
p.64 Right to Repair
p.67 The Future
p.68 Health ISAC
PUBLISHER John M. Krieg
VICE PRESIDENT Kristin Leavoy
VICE PRESIDENT Jayme McKelvey OF SALES
EDITORIAL John Wallace
CONTRIBUTORS Roger Bowles
K. Richard Douglas
Jim Fedele
Joie Marhefka
Manny Roman
Steven J. Yelton
ACCOUNT Megan Cabot
EXECUTIVES Emily Hise
ART DEPARTMENT Karlee Gower
Taylor Hayes
Alicia Brown
DIGITAL SERVICES Cindy Galindo
Kennedy Krieg
Haley Harris
EVENTS Kristin Leavoy
WEBINARS Linda Hasluem
HTMJOBS.COM Kristen Register Sydney Krieg
ACCOUNTING Diane Costea
CIRCULATION Joanna Manjarrez
Rob Bundick, Director HTM & Biomedical Engineering, ProHealth Care
Carol Davis-Smith, CCE, FACCE, AAMIF, Owner/ President of Carol Davis-Smith & Associates, LLC
Jim Fedele, CBET, Senior Director of Clinical Engineering, UPMC
Bryant Hawkins Sr., Site Manager, Children’s Hospital of New Orleans
Benjamin Scoggin, MBA, MMCi, Director, Clinical Engineering | Biomedical Operations, Equipment Distribution, Clinical IT, DHTS, Duke Health Technology Solutions
Allison Woollford, Biomedical Equipment Specialist at Duke University Health System
Bryant Hawkins Sr., HTM on the Line, Trimedx Site Manager at Children’s of New Orleans
Dr. Brian Bell, HTM Workshop, Faculty Biomedical Engineering at St. Petersburg College in St. Petersburg, Florida
Carlos Villafane, BMET Latino, Certified Biomedical Engineering Technician III, Baycare Health Systems
Chace Torres, Bearded Biomed, Lead Technician SPBS, Dallas-Fort Worth Metroplex

p.71 Biomed Brainbuster
p.72 MedWrench Bulletin Board
p.73 CEAI Scrapbook
p.74 MD Expo New England Scrapbook
p.76 Preferred Vendors
p.82 #HTMLife
p.78 Service Index
p.81 Alphabetical Index







As
As we look forward to a new year, now is the perfect time to plan your next career move. CBET’s online programs in Healthcare Technology Management are designed for anyone eager to gain essential skills and secure a future in this high-demand field. Join a growing community of professionals, access flexible learning, and start strong in 2025.

With

BY K. RICHARD DOUGLAS
As hley O’Mara is the VA New England Healthcare System Chief of Healthcare Technology Management (HTM). She is also president of the New England Society of Clinical Engineering .
O’Mara’s interest in the HTM field was fueled during her undergrad studies when she did volunteer work at a local hospital.
“I attended an education session on clinical engineering as a profession during my undergraduate studies. After learning about this job field, I reached out to a local hospital and volunteered during my last year of undergraduate studies while I applied for a master’s in clinical engineering. I loved the idea of working closely with clinical staff to solve problems and implementing the latest and greatest medical technologies,” she says.
She took night classes to earn her master’s degree while working at a hospital 30-plus hours a week.
“During this training program I worked alongside highly trained clinical engineers and biomedical equipment technicians,” O’Mara says.
After graduating with a master’s degree in clinical engineering, O’Mara worked as a staff biomedical engineer at a hospital in Buffalo, New York for two years. She then took a promotional opportunity to work as a supervisory biomedical engineer at a hospital in North Florida/South Georgia for three years.
“A wonderful opportunity to relocate to [the] Northeast and become the chief biomedical engineer in Connecticut, which was closer to family as I started to consider growing
our family. Currently, I’ve been in the VA New England Healthcare System Chief HTM position for 7-plus years and oversee eight medical centers’ HTM program and HTM staff,” she says.
With the exodus of retirement-age baby-boomer biomeds, any effort to replenish the field with qualified replacements is a noble undertaking. O’Mara helps by spearheading one of the efforts to recruit new HTM talent.
“I serve as the co-chair for the National HTM Biomedical Engineering Recruitment and Retention Workgroup which focuses on developing guides/tools for the field to aid in facility recruitment/retention strategies,” she says.
She says that the Biomedical Engineering Recruitment and Retention (BERR) Workgroup is the principal advisory body to the VHA Healthcare Technology Management (HTM) Program Office (PO) for the development of recruitment and retention processes and practices to attract and keep highly qualified biomedical engineering support specialists (BESS) and biomedical engineers (BME).
“This group will focus on three critical positions for recruitment and retention: (1) BESS; (2) BME; and (3) Technical Career Field (TCF) BME trainees. The overall BERR workgroup, along with three (3) subgroups, each focusing on one of the critical roles within the organization (i.e., BESS, BME and TCF trainees), aim to make VHA the employer of choice for professionals in the field,” O’Mara says.
The BERR Workgroup is responsible for developing recommendations in tandem with field feedback for improving recruitment and retention for biomedical engineering positions: (1) BESS; (2) BME; and (3) TCF trainees.

Ashley O’Mara co-presents a session for “VA HTM 403 Leadership Training.” This is a three-day course for new chief/ supervisory biomedical engineers and supervisory biomedical equipment support specialists.
O’Mara says that they also formulate and document best practices for highly effective recruiting methods, support the professional development of our existing biomedical engineering staff and recognize high performing members of the biomedical engineering professional community, recruit TCF trainees and additional high-qualified personnel from the industry-atlarge for roles within VHA.
Some projects that O’Mara has been involved in have helped guide her team to update and manage the assets they manage.
“When I began in the regional HTM chief role, the team and I outlined and wrote a comprehensive region-wide medical equipment management program (MEMP) which allowed for us to update one document annually with changes. Though it does allow for local variation when needed, any variation is documented in their local plan.
She says that they also rolled out a new HTM CMMS system during calendar year 2023 which involved the team reviewing, mapping, validating data on top of their normal duties as HTM professionals.
“Thankfully our regional MEMP was able to be updated with all the CMMS changes versus eight separate MEMPs (this system change affected over 30 percent of our SOPs),” O’Mara says.
Away from work, she enjoys running and coaching.
“I recently took up running and finished my first 5k! I am coaching my daughters’ ‘Girls on the Run’ group in our local town,” O’Mara says.
“I lead third- to fifth-grade girls through a bi-weekly physical activity-based positive youth development program developed/designed by ‘Girls on the Run (GOTR).’ We use running and other physical activities to teach life skills. This group also selects a community impact project that helps make the world a better place, so I’m excited to see what ideas the girls have this year. At the end of the season, the teams participate in a 5k to
celebrate all they’ve learned and the progress they’ve made over the eight-week program,” she adds.
On the home front, O’Mara has been married almost 15 years and has a 10-year-old daughter.
In November of 2021, she was named the 2021 “VHA Supervisory Biomedical Engineer of the Year.” While serving as NESCE president, the society received AAMIs “2020 HTM Association of the Year” award.
“I am passionate about the healthcare technology management profession. I have a fantastic team that works tirelessly in the medical centers to ensure safe, effective medical equipment for their clinicians and veterans,” O’Mara says.
She says that she stays involved in both VA national initiatives and external organizations like ACCE Board member-at-large and planning the 2024 ACCE/AAMI mini symposium program topic and format as well as planning quarterly webinars, symposiums and education opportunities for the New England Society of Clinical Engineering (NESCE).
“This involvement allows me to learn from my colleagues and share experiences/resources that we’ve developed along the way,” O’Mara says.
As a very active member of leadership, both on the job and in the greater HTM community, O’Mara has devoted her time and talent to bettering the field.

BY K. RICHARD DOUGLAS
The Allegheny Health Network (AHN) operates 14 hospitals in western Pennsylvania, serving counties in portions of New York, Ohio and West Virginia. One of those hospitals is the West Penn Hospital in Pittsburgh.
West Penn hospital is a 356-bed facility, primarily offering oncology and women’s health services. It was named the 30th best hospital in the nation for obstetrics and gynecology by U.S. News, according to Brad Klauss, clinical engineering manager in AHNs clinical engineering department.
“West Penn Hospital and Clinical Engineering are both part of Allegheny Health Network, a system of 14 hospitals and numerous offsite locations in Western Pennsylvania. Clinical
Engineering is responsible for repairs and maintenance of medical equipment at all of these locations,” Klauss says.
In addition to Klauss, the CE team is made up of Network Director Dave Petrosky, five biomeds and two rad techs.
“All our techs are assigned to special areas,” Klauss says.
He says that Jake Kerlin primarily takes care of the NICU department and infant respiratory equipment.
“As one of the largest NICU departments in Pennsylvania, we have a substantial amount of specialty equipment. Everything from infant specific transport beds to multiple types of infant ventilators,” Klauss says.
Joe Shybloski is responsible for anesthesia.
“This involves PMs and repairs on 27 gas machines in addition to patient monitoring. He assists Glen with the OR PMs as well. He also has patient monitor floors that he is responsible for,” Klauss says.
He says that Dave Herron primarily takes care of vital signs machines and patient monitoring throughout the hospital. This

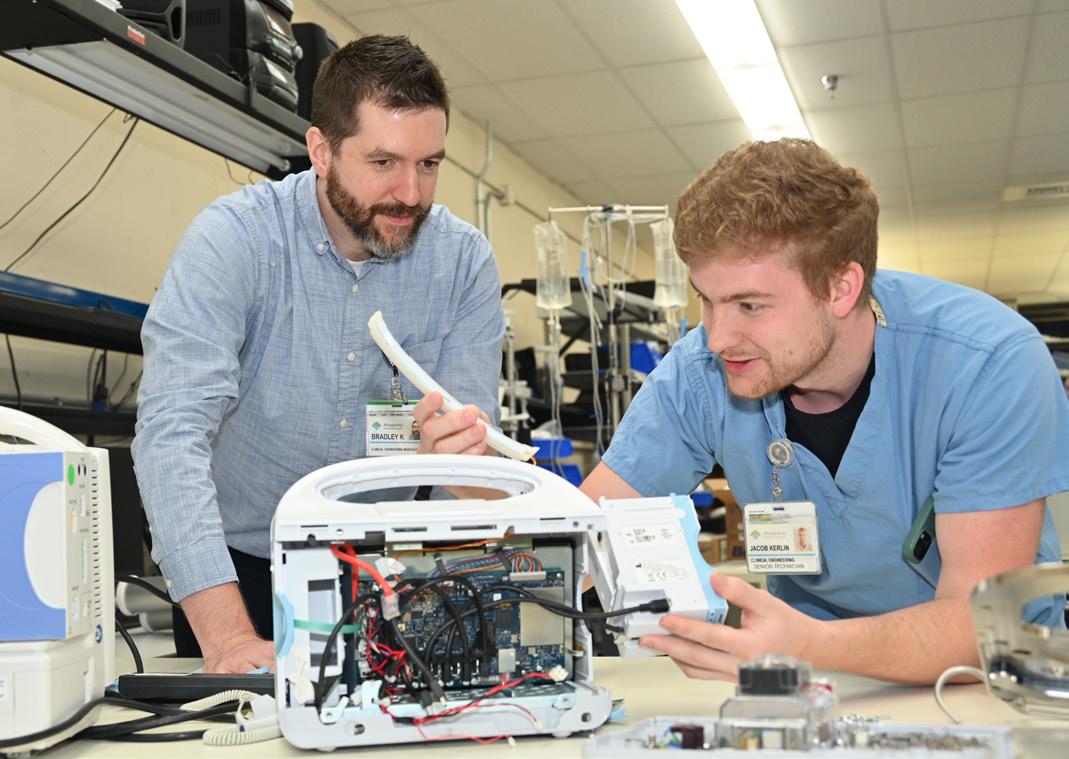
year he will start working on our adult respiratory equipment.
“Nick Hwostow is one of our rad techs. He has a hand in all our rad equipment repairs and will be specializing in angio equipment soon,” Klauss says.
He says that another biomed is responsible for the OR, which has 18 main rooms. He works on all the OR equipment such as ESUs, surgical towers, surgical tables, and surgical lights. He also assists Joe Shybloski with anesthesia machines.
“Dan Quast was a former biomed tech for us until earlier this year. He used to be responsible for respiratory and anesthesia equipment and has at some point worked on pretty much everything we have in the hospital,” Klauss says.
He says that the department also has one open clinical engineering technician position and that the team includes an intern from the PSU (Penn State University) biomed program, which started in August.
“Clinical Engineering and IT are separate departments but work closely with each other. Together, we are responsible for getting vitals to go from our devices to our EMR. With the increased importance of cyber security, we work together to identify and remediate any network vulnerabilities and reduce the risk of cyber-attacks,” Klauss explains.
He says that service contracts are reviewed on a system wide basis.
“As a cost saving initiative, AHN Clinical Engineering has determined the use of single service agreements are less cost efficient. We have determined that utilizing network wide master service agreements prove the greatest cost savings,” Klauss says.
He also states that the team collaborates with procurement to utilize the technology life cycle for all assets.
“We use our CMMS TruAsset to organize and analyze this data to decide capital equipment purchases,” Klauss adds.
The department plays an important role in capital purchases of the assets they manage along with reorganizing departments.
“West Penn Hospital is the oldest secular hospital in Pittsburgh, originally founded in 1848. With this comes a lot of old infrastructure. Our department plays a critical role in prioritizing updates to our equipment. We’ve recently been instrumental in deciding updates to equipment in our radiology department as well as updates to our OR surgical lights,” Klauss says.

He says that the team has become extremely good at reorganizing departments.
“As an example, during COVID, we were crucial in changing our unused pediatric department into a 16-bay expansion to our ICU and a 20-bay stepdown unit in an old, unused floor. This involved reconfiguring all patient monitors, work with IT to ensure all vitals are captured by our EMR, and guaranteeing each room is properly equipped to use ventilators,” Klauss says.
He says that now that the pandemic is past, they have used these skills to open and rearrange units throughout the hospital.
“When our NICU was at capacity, we were tasked with quickly converting the COVID ICU back into the peds unit. The same goes with other floors as well,” Klauss adds.
In addition to dealing effectively with equipment and infrastructure changes, the team has proven adept at problem solving.
“Jake identified an issue with the replacement fans we were receiving for our incubators. The fans being produced by the vendor were no longer compatible with the incubators. He worked with the vendor, who sent a design engineer on site to assess the issue. As a result, the vendor changed their design for all fans they were producing,” Klauss says.
He says that there have been issues with the wiring for the telemetry network.
“Dan, during his time as a biomed tech, poured over the schematics and traced the issue to a single splitter in one of our network closets. This is notable because our telemetry network covers approximately 150,000 square feet,” Klauss says.
“However, I believe since every tech works closely with their departments to help with equipment problems, purchases, or upgrades we avoid many ‘major’ issues. We do have our share of equipment malfunctions, but overall, because of the diligence of our technicians we do not run into many catastrophic issues. This includes maintaining regulatory compliance to meet the Joint Commission standards,” Klauss adds.
He says that the CE team works closely with local biomed educational programs.
“Staffing is an issue across the nation, and we’ve developed close relationships with both the PSU BMET and RMU biomedical programs. We regularly host interns as part of both programs,” Klauss says.
Josh Ablian, CBET, is a senior biomed with UCHealth (University of Colorado). TechNation recently caught with him and learned more about his HTM journey.
Q: Where did you grow up?
A: Lee’s Summit, Missouri
Q: Where did you receive your HTM training/ education?
A: On the job and from good mentors.
Q: How did you first discover HTM?
A: My parents who work in health care told me about HTM when I was trying to find a different job other than software development.
Q: Why did you choose to get into this field?
A: Out of college, my first job was for a big health care software corporation. I couldn’t stand sitting in a drab cubicle all day and wanted something more hands on but still in health care. HTM it was!
Q: What do you like most about your position?
A: Creating a positive vibe with the team around me while bettering patient care.
Q: What interests you the most about HTM?
A: The future of AI technology with medical equipment, it’s so interesting and could truly change how we do what we do.

Q: What has been your greatest accomplishment in your field thus far?
A: Collaborating with my team and anesthesiologists during COVID to retrofit anesthesia machines to be ready for multiple patients at one time. This was a scary time and the vent shortage across the U.S. was tough.
Q: What goals do you have for yourself in the next 5 years?
A: I want to be in an HTM leadership role that allows me to mentor others, grow the field and be a changemaker.
FAVORITE HOBBY: Snowboarding
FAVORITE SHOW OR MOVIE: Transformers “Autobots ROLL OUT!”
FAVORITE MEAL: Burnt ends and fries
WHAT WOULD YOUR SUPERPOWER BE: Super speed
ONE THING ON YOUR BUCKET LIST: Attend an Olympics. Summer or winter, doesn’t matter
SOMETHING YOUR CO-WORKERS DON’T KNOW ABOUT YOU:
I grew up a farmboy; drove tractors and showed animals at the fair.



BY K. RICHARD DOUGLAS
Home to the African elephant, the East African lion, the Cape buffalo and the black rhinoceros, the country of Kenya, on Africa’s east coast, offers endless vistas within its national park and game reserves. The country sits on the Indian Ocean and borders Uganda, Tanzania, Somalia and South Sudan.
A place known for rhinos, elephants and safaris may not be the first place thought of when it comes to biomed associations, but Kenya also has the capital city of Nairobi, with its national park, the third largest sub-Saharan economy, a strong agriculture sector and 12,375 public and private hospitals.
The hospitals fall under six categories according to guidelines in the country’s Health Act.
The Association of Medical Engineering of Kenya (AMEK) brings biomeds across the country together in one organization.
“The Association has now grown from a small number of members of less than 50 members at the start to now over 1,500 registered members, but with at least 300 members who are active (fully paid members),” says the group’s chairman, the Honorary Symon Mbakah.
Mbakah is the group’s sixth chairman.
He says that the Association of Medical Engineering of Kenya (AMEK) is a national professional association registered under Cap.108 section 10 of the laws of Kenya on July 9, 1998 and officially launched in November of 1998.
“The Association was started in Mombasa by the then head of Medical Engineering Department at the then
Mombasa Polytechnic, now Technical University of Mombasa (TUM) by the name Andrew Oscar Obura who was also made the first chairman. His vice chairman was Philip Amoko, currently doing private practice and later became the second chairman of the Association after the death of Oscar Obura,” Mbakah says.
The group provides many benefits to members including conferences and workshops, increasing member’s knowledge, including virtual training through partners, professional development and reporting and management tools review.
“AMEK has eight types of meetings which may be virtual or physical,” Mbakah says
He says that this includes full council meetings every quarter, executive meetings monthly, an annual general meeting for all members, special general meetings for all members; held when there is need to pass an agenda, usually with only one agenda item.
There are also planning meetings, which are held to organize an event such as for training, workshop, conference or any other matter as guided by the constitution.
There are some specialty events as well.
“Women in Biomedical Engineering Day symposium; specifically for women to empower and motivate them in the profession,” Mbakah says. He says that this symposium usually occurs annually on June 23.
There is also the World Biomedical Engineering Symposium for all members and held in December every year as well as the Global Clinical Engineers’ Week.
“This is an activity carried out globally and AMEK participates in the activities in October every year,” Mbakah adds.
The group works with local training programs to help new biomeds enter the field.


“The association collaborates with Kenyatta University (KU), Technical University of Mombasa (TUM), Kenya Medical Training College (KMTC) and North Coast Medical Training College (NMTC),” Mbakah says.
The group has very specific goals and initiatives during its annual meetings. These initiatives aim to increase the competency, professionalism and standards within the biomed profession countrywide.
“The Association usually holds one scientific conference on a yearly basis. The association has partnered with other associations in East Africa as part of the Federation of East Africa Healthcare Engineering Associations (FEAHEA) which organizes rotational conferences referred [to] as [the] East Africa Regional Conference (EARC). AMEK was the first to hold the first EARC in 2008. This year, Tanzania will be hosting the 7th EARC in Mwanza, Tanzania, on November 20 to 22, 2024,” Mbakah says.
He says that during the first African Regional Biomedical Engineering Conference and Healthcare Technology event hosted by AMEK in 2023, there were several resolutions decided on.
regulated and now the association has taken an initiative to develop a biomedical engineering profession bill to regulate training, registration, licensing and practice,” Mbakah says.
The group has also engaged in a review of reporting and management tools.
“The biomedical engineering professional management tools were developed over 20 years ago and since then there are many issues that have emerged such as oxygen ecosystem management, artificial intelligence, noting that technology is not dynamic. Therefore, there is a need to have these tools improved to enhance capturing the necessary data during maintenance of the various medical devices,” Mbakah explains.
“Women in Biomedical Engineering Day symposium; specifcally for women to empower and motivate them in the profession.”
“There is need to harmonize and regulate programs and professional training for [the] biomedical engineering profession in Africa. Advocacy for countries to adhere to World Health Organization (WHO) staffing norms for biomedical engineering in Africa. Promote translation of local innovations/inventions to market-level in African countries and strengthening healthcare technology management, Health Technology Assessment (HTA), and regulations of medical devices in African countries,” Mbakah says.
The group is working on a professional regulatory bill.
“Currently [the] biomedical engineering profession is not
He points out that the COVID-19 pandemic highlighted the important work that biomeds do every day.
“The pandemic in one way or the other opened avenues for biomedical engineering professionals. This is because their role in health care service delivery came into the limelight and many organizations, managers and institutions became appreciative [of] the role biomedical engineers play in health care service delivery,” Mbakah says.
He says that in a nutshell, the pandemic changed the way biomedical engineers operate because now there is a lot of involvement in planning and managing the health care sector.
“Additionally, the association has also been able to carry out capacity building to its members through various partners as a result of the pandemic,” Mbakah says, referring to staffing.
Kenya is much more than safaris; it is home to a vibrant and proactive biomed association that is guiding the profession to be the best it can be.


All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.
ON-DEMAND:
FSI’s Flow Tool Tools of the Trade demo

Solve the 6 Toughest Problems in HTM + HFM – The CMMS in 2025

How AI location intelligence can aid PAR level automation and Equipment Distribution with RTLS (Real-Time Locating Systems)
TUNE IN TO ANY LIVE WEBINAR TO WIN A $100 LOWE’S GIFT CARD
LIVE:
DEC. 4 | Pronk Technologies Tools of the Trade

DEC. 11 | Kontakt.io
Save the date for this live webinar.



sponsored by Healthmark
AI and Automation Inspection for Sterile Processing & Endoscopy
sponsored by MMS
Zen and The Art of Medical Equipment Maintenance
sponsored by MMS
The Key to Growth in the HTM Industry







New England • October 8-10, 2024

early 1,000 HTM professionals came together for amazing networking events, ACIapproved education, an exhibit hall packed with the latest solutions and more.
The Mohegan Sun was the perfect venue with lots of space, restaurants and entertainment options. The MD Expo was proudly supported by the New England Society of Clinical Engineering (NESCE).
The 2024 MD Expo New Englad Fall conference has it all. “MD Expo New England continues to set the standard for conferences in the HTM industry,” MD Publishing President John Krieg said. “We were fortunate enough to be able to bring our world-class show to a part of the country that is ripe for continuing education, networking, and displaying the latest in medical equipment technology.”
“By partnering with the incredible team at NESCE, we brought together over 800 clinical engineers, students and directors of the leading health care facilities in the country, for three days of personal and professional
growth,” Krieg added. “We look forward to returning to New England as part of our regular MD Expo rotation.”
MD Publishing Vice President Kristin Leavoy, CMP, said the local support for MD Expo was amazing.
“It had been eight years since MD Expo was held in New England, so seeing so many new faces was great! Support from the local hospitals, the VA and the New England Society of Clinical Engineering (NESCE) was tremendous! We will be sure to return to Connecticut sooner rather than later!” she shared.
One of the many highlights was the first-ever H.O.T. Workshop (hands-on training workshop). In this workshop, Glenn Schneider, chief service officer with Elite Biomedical Solutions, showed attendees how to disassemble, inspect, reassemble and test the MX-40 patient monitor. A review of the options, parts availability, common failures and proper maintenance were presented. Schneider introduced a patentpending tool that can be used to open and work on the MX-40. Attendees were also invited to get their hands inside the device.
Another highlight was a keynote panel discussion on the Right to Repair medical devices. Mike Busdicker, Binseng Wang, Nathan Proctor and Rob Kerwin took the stage as Chris Nowak moderated the discussion. After prepared remarks, the panel fielded questions from the more than 200 HTM professionals in attendance at the
session sponsored by Zopec Medical.
Attendees praised the MD Expo venue and complimented TechNation magazine and MD Publishing for providing top-tier education and networking. Recordings of the educational sessions are available for on-demand viewing at MDExpoShow.com
Nicole Ruffin, BMET II, described MD Expo as “inspiring and educational.” She added that three words she would use to describe MD Expo are “community, support and diversity.”
Luis Pimentel, a BMET with CME, said this was his first MD Expo.
“The MD Expo is the perfect event for professionals in the field who want to expand their knowledge and find their calling,” he said. “The most valuable aspect of the conference for me was the networking opportunities and the educational sessions. I also enjoyed the keynote speakers.”
“What inspired me the most during my time was the passion and expertise shared by industry leaders and fellow professionals,” he added.
When asked to describe MD Expo in three words, Pimentel said “innovative, collaborative, educational.”
Bob Larkin, the director of HTM at University Hospitals Healthcare/Sodexo HTM, described MD Expo as “immersive, productive, fun.” However, he could not stop with just a threeword description.
Larkin said MD Expo is “an immersive experience into our HTM universe and its many facets, functions and services.”
He explained that MD Expo presented an opportunity to network and have “direct contact with vendors I use every day but didn’t know face-to-face.”
Hartford Hospital Biomed Technician
Dominic Cianciolo’s three words to describe MD Expo are “educational, fun, exciting.”
“Fun” is a word that comes up often when discussing MD Expo and the finale party sponsored by USOC is one reason why. The party featured food, drink, games, fire pits and live music!
Next up, is MD Expo SoCal in April. Sign up for the newsletter at MDExpoShow.com.




The Insulation tester, Leak Tester Tester, Shaver Leak Tester and Cable Continuity Tester are easy-to-use devices for verifying the functionality of equipment for safety
McGan Insulation Tester
Detect & locate defects such as pinholes, cracks and bare spots in the jacket or coating of laparoscopic and bi-polar electrosurgical instruments

Cable Continuity Tester
Test the functionality of automated & handheld endoscope leakage testers with healthmark’s new Leak Tester Tester.

Test the quality of monopolar and bipolar cords with this user-friendly, durable device. A green light noti es the user that the cable passed testing.

Shaver Leak Tester

Designed for pressure testing arthroscopic shavers to help identify leaks caused by failing seals that degrade over time from repeated use and processing.
For more of Healthmark’s intelligent solutions for instrument care & infection control, visit hmark.com hmark.com | 800.521.6224 | healthmark@hmark.com


The North Carolina Biomedical Association is actively raising funds to support local communities impacted by Hurricane Helene. The NCBA is accepting donations toward a Hurricane Helene relief fund. The 2024 Board of Directors voted unanimously to donate to the recovery efforts in North Carolina. All the funds collected will be donated to hurricane relief efforts in the area!
“We invite you to join us in this vital mission by making a
Frazier Healthcare Partners, a Seattle-based, healthcarefocused investment firm, has completed the acquisition of DirectMed Imaging, a leading provider of aftermarket parts and component repairs for diagnostic imaging equipment, from NMS Capital.
The partnership will leverage Frazier’s growth-oriented investment experience and health care industry expertise to build upon DirectMed’s success as a leading solution provider in the aftermarket imaging parts and repairs market. DirectMed supports provider organizations by extending the life of medical imaging equipment to improve patient access, reduce waste, and lower healthcare costs. The company will continue to invest in its people and capabilities organically and through acquisition as it expands its expertise in existing and new imaging modalities.
“Frazier is an ideal partner for DirectMed, and we are fortunate to be working with a firm that has 30 years of experience building companies,” said Brad de Koning, CEO of DirectMed. “We looked for a partner with deep healthcare experience and a proven ability to bring resources that will allow us to accelerate our growth initiatives and better serve our customers. This partnership marks the beginning of an
donation. Your contribution will directly assist families and organizations in need of essential resources such as food, shelter, medical supplies, and recovery support. Every dollar you donate helps us provide hope and relief to those affected by this disaster. Together, we can make a meaningful difference in the lives of our neighbors during this challenging time,” the NCBA said.
Donations can be made at tinyurl.com/ys8mcuah

exciting chapter for our organization.”
Tanner LoRusso, co-founder and vice president of sales, said “We are excited to partner with Frazier to build our capabilities in interventional radiology, mammography, and ultrasound modalities, reinforcing our commitment to becoming the one-stop supplier of diagnostic imaging solutions for customers around the world.”
“Brad, Tanner, and the DirectMed leadership team have built a differentiated Company in an increasingly important part of the healthcare ecosystem, and we are thrilled to have the opportunity to partner with them in the company’s next phase of growth,” said Kent Berkley, partner at Frazier. “We look forward to supporting the DirectMed team’s vision with incremental capital and experienced resources, including several members of our Center of Excellence team, to accelerate growth and further solidify its leadership position in the market.”

UptimeHealth, in collaboration with the College of Biomedical Equipment Technology (CBET), kicked off the Dental Fix Summit 2024 in early October at the Embassy Suites by Hilton Nashville Airport. This four-day event is designed to equip independent dental equipment repair technicians and biomedical technicians with the latest technical training, business strategies, and networking opportunities in the dental industry.
CBET President Richard “Monty” Gonzales was among the speakers at the opening session. He discussed health care silos and the need to eliminate them. He shared a vision where biomeds and dental repair technicians work together to create an expert who can work on all health care equipment including dental equipment.
“The military long ago got rid of the distinction between dental and medical equipment,” Gonzales said.
He explained that CBET is working on the creation of a dental repair technician training program.
He also expressed a desire to work with AAMI and ADA to create a Certified Dental Repair Technician (CDRT) certification. Danielle McGeary from AAMI, who was in attendance at the
conference along with AAMI Chief Learning and Development Officer Robert Burroughs, said the CDRT is something being considered but that it will take time to develop.
At least one HTM insider in attendance at the summit said he expects the new certification to be available in 2025.
The Dental Fix Summit 2024 was an opportunity for technicians to advance their skills and knowledge through hands-on workshops, insightful sessions, and interaction with industry leaders. Attendees had access to OEM technical training and business solutions tailored to the needs of independent service organizations. The event also offered a chance to earn continuing education units (CEUs), enhancing the professional growth of participants.
“We are thrilled to bring together such a dynamic group of professionals for the Dental Fix Summit 2024,” said Darrine Miller, vice president of operations at UptimeServices, in a press release leading up to the event. “This event is all about empowering technicians with the tools, knowledge, and connections they need to thrive in the industry. We look forward to seeing the impact this summit will have on their businesses and careers.”

As hospitals across the United States navigate the challenges of delivering patient care in increasingly high-acuity environments, a new partnership between US Med-Equip (USME), a leading provider of medical equipment rentals and services, and Sentec, a global leader in respiratory monitoring technology, is set to help more clinicians with real-time insights into the conditions of critically ill respiratory patients, including newborns in Neonatal Intensive Care Units (NICUs).
Under the new partnership, USME added Sentec’s transcutaneous monitoring (TCM) devices as part of its extensive portfolio of life-saving medical equipment. Sentec’s TCM devices provide continuous, non-invasive monitoring of a patient’s carbon dioxide levels and oxygen saturation to help clinicians make better-informed decisions in real-time – particularly vital in the care of patients, such as those in the NICU, where precise monitoring of respiratory function is crucial.
In addition to transcutaneous monitoring, Sentec’s Intrapulmonary Percussive Ventilation (IPV) technology will also be available through this partnership. IPV therapy supports the front-line care of critically ill respiratory patients as a management tool to restore lung function and mobilize secretions.
By adding TCM and IPV technology into USME’s rental and service offerings, health care providers gain greater access to non-invasive equipment often preferred by clinicians, especially in intensive care units, emergency departments, and during transport of critically ill patients.
“Partnering with US Med-Equip enables us to better support our shared hospital customers dedicated to providing the highest quality care for respiratorycompromised patients, especially during periods of high patient census and urgent capital needs,” Sentec CEO Bob Cormier said.
This partnership reflects a growing trend in health care, where the integration of advanced technology with on-demand service is increasingly essential. Under the new partnership, US Med-Equip, known for its rapid response and high-quality service, is offering Sentec’s devices with the level of support that has made them a trusted partner for hospitals nationwide.
“Sentec shares our unwavering commitment to supporting clinicians in delivering exceptional patient care,” Greg Salario, CEO of USME, said. “This collaboration enhances our ability to provide health care partners with access to the most advanced patient monitoring solutions, precisely when they need them most.”

The U.S. News recently released its 100 Best Jobs list for 2024.
“No single job suits all of us, but many of the best ones have a few attributes in common: They pay well, challenge us year after year, match our talents and skills, aren’t too stressful, offer room to advance throughout our careers, and provide a satisfying work-life balance. Job seekers also often consider whether a position is in demand. U.S. News used these qualities to rank the 100 Best Jobs of 2024. You can also explore the Best-Paying Jobs and other more specific career rankings,” the website states.
Biomed, which is listed as Medical Equipment Repairer is #1 in Best Maintenance and Repair Jobs and #26 in 100 Best Jobs.
Nurse Practitioner tops the list with an overall score of 7.4 out of 10. It is listed as the #1 in 100 Best Jobs, #1 in Best STEM Jobs, #1 in Best Health Care Jobs and #23 in Best-Paying Jobs.
Physician Assistant is #5 in the Best Jobs list and #2 in Best Health Care Jobs, #4 in Best STEM Jobs, and #21 in Best-Paying Jobs.
Medical and Health Services Manager is #2 in Best Business Jobs, #5 in Best STEM Jobs and #6 in 100 Best Jobs.
EQ2 HEMS – is focused entirely on healthcare maintenance management
Management and Technicians achieve more in less time
Integrate with all of your existing systems
Compliance and AEM made easier with the right tools and reports
Our system helps you keep your devices protected
Data and information that shows leadership how your department saves the hospital money


ISS SOLUTIONS INC. ACHIEVES ISO 13485:2016
CERTIFICATION
ISS Solutions Inc., a leading provider of customized technology life cycle services for health care and other mission-critical industries, recently announced its achievement of ISO 13485:2016 certification. This internationally recognized certification demonstrates the company’s commitment to the highest standards of quality management systems (QMS) specific to medical devices and related services.
ISO 13485:2016 is the globally accepted standard for organizations involved in the design, production, installation, and servicing of medical devices. By achieving this certification, ISS Solutions affirms its dedication to consistently meeting customer expectations, regulatory requirements, and ensuring the safety and reliability of the medical devices it services.


“Achieving ISO 13485:2016 certification is a significant milestone for ISS Solutions,” said Barbara Maguire, vice president quality and healthcare technology management, ISS Solutions. “This accomplishment underscores our unwavering commitment to delivering the highest quality services to the healthcare industry while ensuring the safety of patients and caregivers. We are proud to provide the peace of mind that comes with adhering to internationally recognized best practices.”
The certification process involved a comprehensive review and audit of ISS Solutions’ quality management systems, operational processes and commitment to continuous improvement. Achieving this certification positions ISS Solutions as a trusted partner for health care providers looking to enhance their medical device management and ensure compliance with the latest industry standards.
With this certification, ISS Solutions continues to strengthen its role as an industry leader, offering a wide range of medical equipment services, including repair, preventive maintenance, and asset management, all backed by a proven commitment to quality and regulatory compliance.
TechNation magazine turns 15 in 2025! And, who better to celebrate with than the readers?
TechNation invites readers old and new to help celebrate its 15th anniversary with 15 prizes and a monthly time capsule in the magazine.
Enter the contest and help TechNation celebrate. Visit 1TechNation.com/contest and fill out the short form for a chance to win. Additional entries to win can be acquired by sharing on LinkedIn or submitting a photo.
Each month, a winner will be selected and featured in TechNation magazine!



An expert panel convened for an informative discussion regarding medical Right to Repair at the MD Expo New England. Mike Busdicker, Binseng Wang, Nathan Proctor and Rob Kerwin took the stage as Chris Nowak moderated the discussion.
After prepared remarks, the panel fielded questions from the more than 200 HTM professionals in attendance at the session sponsored by Zopec Medical.
A brief history of Right to Repair and landmark moments were shared by panel members.
Overall, the key takeaways were how HTM professionals need to raise their voice to be heard in the nation’s capital while at the same time educating and encouraging C-suite level decision
makers at hospitals to also ring the bell.
Collaboration among ISOs, in-house biomeds and OEMs was another hot topic during the discussion.
It was pointed out that many of the OEMs stated concerns in the United States do not seem to be a hinderance in the European Union or in the middle of a pandemic. Questions were raised as to why that is the case.
References were also made to FDA, FTC and other decisions and communications that indicate that third-party repairs are not a safety issue.
The January 2025 issue of TechNation will include a cover story on the Right to Repair with additional insights from industry experts.

MD Expo introduced hands-on-training workshops, or H.O.T. Workshops, at the New England conference.
The inaugural H.O.T. Workshop: Patient Monitors, approved for 6 CEUs by ACI, was presented by Glenn Schneider, chief service officer with Elite Biomedical Solutions.
In this workshop, he showed attendees how to disassemble, inspect, reassemble, and test the MX-40 patient monitor. A review of the options, parts availability, common failures, and proper maintenance were presented. Attendees were also invited to get their hands inside the device.
Schneider also introduced a patent-pending tool that can be used to open and work on the MX-40.
Kristin Leavoy, vice president at MD Publishing, said the H.O.T.
Workshop was the result of feedback from MD Expo attendees and TechNation readers.
“We heard from attendees and they want more in-depth training on equipment,” Leavoy said. “We worked with our vendor partners who would have enough equipment for attendees to work on.”
James Dubois said the H.O.T. Workshop was interesting to him because “we get to tear the thing apart, so that’s good.”
Larry Lamourine from MGB-Cooley Dickinson Hospital enjoyed the hands-on aspect of the training and said it was a “great experience.”
He said everyone learned from the presenter and from each other during the session.
MD EXPO SOCAL DATES ANNOUNCED
The next MD Expo is set for Southern California this spring!
MD Expo SoCal will be held April 15-17 at Pechanga Casino & Resort in Temecula, California.
The spring event is already shaping up to be a can’t-miss
conference for HTM professionals with H.O.T. Workshops, Leadership Summit, Keynote, Exhibit Hall, Young Professionals at MD Expo, 7 hours of education and a finale party! Find


The Association for the Advancement of Medical Instrumentation (AAMI) has signed a memorandum of understanding with the Global Clinical Engineering Alliance (GCEA). The agreement establishes a joint effort from AAMI and GCEA to strengthen the healthcare technology management (HTM) field, known globally as clinical engineering (CE).
AAMI’s core mission is to promote the safe and effective use of healthcare technology. To that end, AAMI produces industry standards related to medical devices and their use, and strives to elevate, standardize, and promote the HTM field. GCEA was founded to support and empower the clinical engineering profession across the world and to contribute to international harmonization efforts.
This MOU is a natural fit for the missions of both organizations and is expected to yield multiple new initiatives that will support the HTM/CE community around the globe. This will include:
• Co-marketing of events like AAMI eXchange and the International Clinical Engineering and Health Technology Management Congress (ICEHTMC).
• Creation, translation, and distribution of educational materials, guides, and other resources for HTM/CE professionals.
• Expanding the network of HTM professionals internationally.
After the signing of the MOU, AAMI’s Vice President of HTM Danielle McGeary stated, “The signing of this MOU with the
Global Clinical Engineering Alliance is a key opportunity to bring AAMI’s HTM work to an international audience with the goal of creating international HTM standards and guidance that will further harmonize the field globally.”
According to GCEA President Yadin David, “This collaboration creates a powerful framework for advancing healthcare technology innovation, safety, and management on a global scale. Through this agreement, both organizations can strategically align their expertise and resources, enabling the development of global guidelines, best practices, and educational programs in clinical engineering across the world. Ultimately, this partnership will ensure that the global health care community benefits from improved medical technologies, elevated standards, and enhanced health care delivery systems. Our shared commitment to safety, innovation, and education will play a pivotal role in improving patient outcomes and enhancing health equity worldwide.”
A statement of work from AAMI and GCEA related to forthcoming collaborations between AAMI and GCEA is expected later this year.
As artificial intelligence (AI) becomes more integrated into the health care sector, there is a pressing need for industry standards that will protect patients and promote best practices.
The Association for the Advancement of Medical Instrumentation (AAMI) has signed a memorandum of understanding with the Consumer Technology Association (CTA) that will enable the production of new industry standards related to the use of AI and machine learning (ML) in health care products.
“The signing of this MOU is a key opportunity to bring AAMI’s HTM work to an international audience with the goal of creating international HTM standards and guidance that will further harmonize the field globally.”
The new MOU establishes a framework for AAMI and CTA to collaborate on efforts like the production of new standards documents. Any forthcoming projects will promote the safe, effective use of AI and ML-enabled tools and are expected to impact medical device manufacturers and end users, and the health IT field.
Pat Baird, senior regulatory specialist at Philips, and longtime member of both associations said, “Standards are a great way to share good practices and warn about common pitfalls for a particular topic; I’m happy that AAMI and CTA are starting down a path where even more people can share their perspectives, experiences, and ideas of what good looks like.”
The MOU states that AAMI and CTA intend to coordinate standardization activities related to digital health products and solutions that have AI or ML capabilities. This may include:
1. Post-market surveillance methodology for over the counter (OTC) health devices.
2. Evaluation and assessment criteria for AI and MLenabled products.
3. Other activities such as mutual recognition of standards, information sharing, and workshops.
4. The potential for additional collaborative projects.
Collaboration with CTA and its members is an opportunity for AAMI to advance its core mission of producing regulatory ready standards. Since 1967, AAMI has used its voluntary industry standards to ensure medical devices and products promote optimal patient outcomes. This new relationship with CTA is a continuation of that mission.
According to Matt Williams, Vice President of Standards at AAMI, “This MOU with CTA is a major milestone in AAMI’s ongoing work related to artificial intelligence. I look forward to the opportunities that this joint endeavor will bring to both our organizations in the future.”
“As health technologies develop at an unprecedented rate, industry-driven standards will be crucial to ensuring the efficacy, safety, and innovation of these products and services,” said Kerri Haresign, Sr. Director of Technology and Standards at CTA. “For CTA, collaborating with AAMI marks an important step in creating the standards needed to guide the responsible development of AI in health care.”
AAMI members interested in the specifics of the new relationship can learn more by reaching out at standards@aami.org.




UptimeServices was created to support technicians by providing them with job opportunities, education and career development. Its technician education program is one of the most exciting offerings, providing hands-on training and certifications to help technicians sharpen their skills and grow in their careers. Additionally, the platform offers software that helps technicians manage their jobs, schedules, certifications and career progression seamlessly.
UptimeServices CEO and Founder Tamara Dhanda recently took some time to share more information about the company.
Q: HOW DOES YOUR COMPANY STAND OUT IN THE MEDICAL EQUIPMENT FIELD?
DHANDA: We stand out by focusing on empowering technicians with the tools and resources they need to succeed. UptimeServices bridges education and career advancement through job placement, ongoing learning opportunities, and intuitive software that makes it easier for techs to manage their work. Our approach puts the technician at the center of everything we do, helping them thrive in their roles.
Q: WHAT IS ON THE HORIZON FOR YOUR COMPANY?
DHANDA: We aim to expand our Technician School and strengthen partnerships with health care facilities to provide more job opportunities for technicians. We’re also working on enhancing our software to further streamline how technicians manage their tasks and career growth, providing an even better experience.
Q: IS THERE ANYTHING ELSE YOU WOULD LIKE TECHNATION READERS TO KNOW?
DHANDA: UptimeServices is committed to the success of medical equipment technicians. We offer education, career support and powerful tools that help techs stay ahead in the fast-paced healthcare environment.
For more information, visit uptimehealth.com/uptimeservices.


BY MARC SCHLESSINGER, RRT, MBA, FACHE, LSSGB
In the rapidly changing field of health care, the planning and procurement of capital medical devices are critical for hospitals and other medical facilities. Capital equipment planning in health care enhances workforce and patient safety and reduces preventable harm by providing reliable, up-to-date tools that improve care quality. Biomedical engineers play a vital role in this process, ensuring that medical devices are not only effective but also align with the institution’s needs, budget and regulatory requirements.
Given the experience and training of biomedical engineers, no one is more qualified to assist in this planning. This article will explore how biomedical engineers can assist with the capital equipment planning process for medical devices and highlight their contributions to the process in several key areas.
Capital equipment planning involves the strategic and objective assessment of a medical facility’s current and future needs for medical devices and equipment. This process includes identifying the types of equipment required, estimating costs, analyzing vendor options, understanding cybersecurity risks, and ensuring compliance with health regulations. These tasks are essential for maintaining a facility’s operational efficiency and quality of care.
The first step in capital equipment planning is to conduct a comprehensive needs assessment. Biomedical engineers collaborate with clinical stakeholders to understand the specific requirements of different departments. This might involve:
• Identifying Gaps: Biomedical engineers should assess current equipment and identify any gaps in functionality or capacity. For instance, if a hospital’s imaging department lacks newer 3D breast tomography units, engineers can pinpoint this need based on available current technology and diagnostic requirements.
• Futureproofing: They should also consider future trends in health care, such as emerging technologies, to ensure that the planned equipment will meet long-term clinical needs.
Once needs are identified, biomedical engineers can assist in performing a TCO analysis of potential equipment purchases. Cost of ownership for a medical device refers to the comprehensive assessment of all costs associated with acquiring, operating, and maintaining that device over its entire life cycle. This goes beyond just the initial purchase price and includes several key components including:
• Acquisition Costs: The upfront cost of purchasing the medical device, including any taxes, shipping and installation fees.
• Operational Costs: Ongoing expenses related to the device’s use, such as:
• Supplies and Consumables: Items required for the device to function (e.g., syringes, sensors).
• Energy Costs: Electricity or other energy sources needed to operate the device.
• Maintenance and Support: Costs associated with regular maintenance, repairs and technical support. This includes:
• Preventive maintenance
• Unforeseen repair costs
• Warranty and service contracts
• Training Costs: Expenses related to training staff to use the device effectively, which can include time, materials and external training programs.

Choosing the right vendor is crucial in capital equipment planning. Biomedical engineers should play a significant role in this selection process by:
• Conducting Hands-On Evaluations: The technical specifications of available medical devices need to be researched, ensuring that they meet the facility’s clinical needs. This should involve detailed comparisons of features, performance metrics and human factor considerations.
• Facilitating Demos and Trials: Biomedical engineers often coordinate equipment demonstrations and trials, allowing clinical staff to evaluate equipment firsthand. Their technical expertise helps in analyzing performance data during these trials.
• Explore Product Evaluations: ECRI provides firsthand deep-dive evaluations of various medical devices. These evaluations are objective and provide industry leading information useful to both the biomedical engineer and clinician.
Health care facilities must adhere to stringent regulations regarding medical devices. Biomedical engineers are essential in navigating these complexities by:
• Ensuring Compliance with Standards: They can ensure that all equipment meets regulatory standards set by organizations such as the Food and Drug Administration (FDA). This involves verifying that devices have the necessary certifications and have undergone proper testing.
• Risk Assessment: Biomedical engineers conduct risk assessments to identify potential safety concerns associated with new equipment. This proactive approach helps mitigate risks that could impact patient safety or lead to costly legal issues. Searching for recall information on the device in addition to exploring the FDA’s MAUDE (https://www. accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm) database to help determine reported incidents.
The integration of new medical devices into existing systems is another critical phase where biomedical engineers contribute significantly. Their involvement includes:
• Interoperability Assessment: Ensuring that new equipment can seamlessly integrate with existing systems, such as electronic health records (EHR) or other medical devices, is crucial for efficient operations. Biomedical engineers can analyze compatibility and recommend solutions to any integration challenges.
• Training and Support: They provide training sessions for clinical staff, ensuring that users are comfortable and competent in operating new devices. This training is vital for maximizing the benefits of the new technology and minimizing the risk of errors, thereby improving patient care.
Biomedical engineers help manage the life cycle of medical devices, from maintenance schedules to replacement planning. By keeping track of a device’s performance over time, including repair history and costs, they can recommend upgrades or replacements when necessary.
Biomedical engineers are integral to the capital equipment planning process for medical devices in health care settings. Their expertise ensures that medical facilities can make informed decisions that enhance patient care, reduce preventable harm, comply with regulations and optimize costs. As the health care landscape continues to evolve, the role of biomedical engineers will become even more crucial in navigating the complexities of medical devices, cybersecurity and equipment planning.

Marc Schlessinger, RRT, MBA, FACHE, LSSGB, is a principal consultant and investigator with ECRI.










PartsSource PRO® is the only comprehensive, clinical management platform built for the specialized workflow and procurement needs of clinical engineering and supply chain teams. Discover why over 1,600 health systems across the country rely on PartsSource PRO® to maximize their time and cost savings.









Pronk Mobilize is a multi-level solution that provides flexibility to streamline and automate medical device testing. Mobilize is easy to use and can be adapted to fit specific testing and documentation needs.
Pronk’s Mobilize App connects with Mobilize-compatible test equipment – automated electrical safety, vital signs simulation, IV pump, defibrillator, and ventilator testing – individually or in combination. Testing protocols/procedures can be generated in an intuitive and user-definable way.
Mobilize includes automated step-by-step electronic procedure features. It includes user-controllable options to adapt procedures according to the particular configuration of the medical device and test equipment at hand.
A third operating mode provides the ability for a biomed to create a library of test protocols that can be loaded instantly for executing automated testing with wireless connectivity to the test equipment.
Mobilize also has the capability of conducting automated tests directly from the CMMS in Direct Control Mode. This provides the added feature of capturing the test results live into the CMMS. Mobilize provides a complete automation solution as well as providing the capability of integration to a wide range of CMMS to transfer all the electronic reports right into a work order.
For more information, visit pronktech.com
BY ZACHARY SILAS
The year 2023 was a big journey for me. I was supposed to start my job as a site manager with TRIMEDX on Jan. 3, but I found out during my relocation that my cousin was reported to have committed suicide. As the co-executive producer of Ellen DeGeneres Show, he was a very public figure. But to me, he was a family member who I admired and appreciated. To be so genuine and humble while navigating from Montgomery, Alabama all the way to Los Angeles while maintaining your roots. He was just remarkable. It was a huge blow to find out.
During my grieving process, the first order of business was doing something I had never done and really exposing myself to therapy in a way that required vulnerability. It also helped me work through some life changes, like my new role as a site manager. In the midst of everything, I was trying to build a team and motivate others, but I realized you also have to be motivated yourself.
I realized my leadership was an extension of Stephen’s legacy and what he was able to accomplish in this world. I want people I meet, regardless of the relationship, to feel empowered in some way. I want them to be better than they were before. I felt that way prior to Stephen, but I think it’s more intentional now. I had a friend who would ask “Are you happy?” instead of “How are you?” and it always got a more truthful response. I think that’s the one question I wish I was more intentional about asking Stephen. I wholeheartedly believe he was happy when we were together, but I wish I had asked him how he felt when he wasn’t surrounded by loved ones. We assume because you have goals and aspirations, you’re still moving towards something. We assume because you’re moving, you’re in a good place, but that might not be the case.

Even more than before, I think my purpose is to shed positive light on the people I encounter. I took that into my new role as a site manager. Since the account is in a rural location, we have had to get creative with hiring some people who didn’t have any background in the medical field whatsoever. With such a new team, we really had to focus on team building with lunches, team activities and ways to just get to know each other outside of the shop. We try to be creative. We have had team lunches where we each bring in dishes traditional to our ethnicities, which is always a fun way to learn about others. This past Halloween, we needed some last-minute costumes and one of the team members loves drawing. He came up with the Ghostbusters idea, so we got some OR bunny suits and made the designs. Sometimes it’s important to do something fun and memorable for the team to look back on and laugh. That’s really the dynamic the team has formed over time and it’s cool to see.
To any new incoming leader, I would offer the following advice:
1. Be patient. This phase is about learning, not necessarily doing.
2. Trust the process. When you make yourself available, the training and exposure will make you capable. Availability is sometimes as valuable as capability.
3. Become familiar with how you are being measured internally and externally and prioritize often.
4. Determine a plan that will deliver results while giving the entire team ownership. The approach to goal attainment should be sustainable, so no cutting corners. Do the right thing.

Illinois.














WORLD’S LARGEST RTLS DEPLOYMENT: HCA’S 75% REDUCTION IN RENTAL
The Webinar Wednesday session “World’s Largest RTLS Deployment: HCA’s 75% reduction in rental fleet” was sponsored by Kontakt.io. Presenters Rom Eizenberg, Kontakt. io’s CRO, and Noel Hodges, HCA’s South Atlantic Supply Chain CEO, shared valuable information and insights during the webinar.
What if you could reduce your medical device fleet and CapEx? Give nurses more bedside time and generally operate more efficiently, responding in real time?
That’s exactly what HCA accomplished in just a few months. With Kontakt.io’s asset management solution, HCA increased their equipment utilization and reduced their active rental fleet by 75% with 7x annualized ROI.
Attendees walked away with details about the HCA success storyas well as how health systems, big and small, can leverage enterprise technology at a price point that achieves 7x annualized ROI. Attendees also learned about best practices from a real-world example of optimizing care operations and cutting costs.
Winston Henry, a Sodexo program support manager in Ohio won a $100 Amazon gift card as part of Webinar Wednesday’s 10th anniversary during the session.
Attendees were asked “How much new information did you receive from today’s webinar?”
“I was familiar with the RTLS applications mentioned. What I learned was building upon the technology to make sure we have the people, technology and processes to ensure a successful program,” said Winston Henry, Program Support Manager, HTM, Sodexo.
“Great bit of information about rental fleets,” said Alec Hadley, Field Service Manager, ReNew Biomedical.
Watch these webinars on-demand
Webinar Wednesday’s session “Enhancing Device Management and Extending Cyber Protections with Cynerio + AiRISTA” presented by Tim Bloomer, Cynerio director of sales engineering, and Vince Grove, AiRISTA vice president of product marketing, is eligible for 1 CE credit from the ACI. Registration was free and a recording is available for on-demand viewing thanks to sponsor Cynerio.
Cybersecurity and asset management have become inextricably linked in the complex landscape of modern health care. As biomedical engineering teams grapple with the challenges of tracking assets, they are increasingly tasked with contributing to the prevention of escalating cyber threats. The result is a much-needed unified approach to asset management and security implementation. This webinar explored how the integration of AiRISTA Flow and Cynerio empowers health care organizations to effectively manage medical devices, mitigate risks and ensure patient safety.
Bloomer and Grove provided a guided tour of the powerful combination of these solutions. Attendees were able to discover how a real time location system (RTLS) can revolutionize asset management by providing real-time visibility, status updates and location tracking. They illustrated how this enhanced visibility directly impacts patient experience, improves operational efficiency and helps meet stringent compliance mandates. The duo also fielded questions from attendees.
The webinar was a hit with attendees, especially Dakota Brown-Goodwin, CBET, in California. He won a $100 Amazon gift card as part of the Webinar Wednesday 10th anniversary celebration!
Attendees were asked “Excluding CE credits, why do you attend Webinar Wednesday?”
“I enjoy the new learning experience,” ReNew Biomedical Field Service Manager Alec Hadley said.
“Learn about new things in our field,” said Jason Chaffin, the lead BMET at St. Luke’s Vintage.


The Webinar Wednesday Tools of the Trade Live Demo featuring the Fluke Biomedical IDA-6 Infusion Device Analyzer was presenter Justin Ross, a Fluke Biomedical sales engineer. The webinar is eligible for 1 CE credit from the ACI. The webinar was sponsored by Fluke Biomedical.
The IDA-6 Infusion Device Analyzer is the next generation of infusion device analyzers.
The IDA-6 elevates medical device testing, providing simplicity and efficiency. Ross discussed how biomeds can embrace the transformative power of the IDA-6 Infusion Device Analyzer, equipped with the exclusive OneQA workflow automation software. This groundbreaking analyzer is the solution to common pain points including extended periods dedicated to testing and documenting results. With IDA-6, biomeds can effortlessly modify pre-set procedures. Plus, the results are automatically recorded on the device and seamlessly synced to a PC, making PM completions a breeze with its user-friendly interface. Recognized worldwide for its excellence in infusion pump testing for decades, the IDA-6 allows biomeds to focus on protecting lives. Ross invited attendees to incorporate the IDA-6 Infusion Device Analyzer into their organization and experience the change.
More than 100 people attended the live presentation and a recording of the webinar is available for on-demand viewing at WebinarWednesday.live.
Attendees were asked, “What was your single biggest takeaway from today’s product demo?”
“The ability to accurately test the pumps and individually calibrating of the tester,” said Helen Brazen, biomedical manager with Blount Memorial Hospital.
“The ability to store any tests that were done internally/within the IDA-6 using either the work order or asset number of the device. This is handy for me as I visit multiple sites where I have to service infusion pumps, specifically from Baxter,” shared Bruce Wan, biomedical technologist, Ottawa Hospital, Civic Campus.
“That Fluke is putting a lot of thought into the future of medical equipment testing,” said Robert Wentworth, vice president of biomedical operations for VIKAND Solutions Inc.
All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.
The Webinar Wednesday session “HHS’ Top 3 Cyber Attack Vectors: HTM’s New Compliance Challenge” sponsored by Claroty delivered valuable insights. The webinar was presented by Ty Greenhalgh, industry principal-healthcare, Medigate by Claroty, and Skip Sorrels, senior director healthcare at Claroty.
This webinar explored the top 3 cybersecurity attack vectors for the health care industry and their critical impact on medical device cybersecurity. Health care experienced a 156% increase in records breached from 2022 to 2023. The experts broke down health care’s 3 primary attack vectors, what they mean for HTM teams, and how upcoming regulations will require proactive risk reduction.
Attendees were able to learn actionable strategies for mitigation and remediation, tailored specifically for health care environments. This session is a must for anyone involved in HTM, healthcare IT, or medical device security.
Attendees also had the opportunity to ask the experts questions during the Q&A portion of the webinar.
Common Spirit Health’s James Whitaker won a $100 Amazon gift card as part of the ongoing Webinar Wednesday 10th anniversary celebration.
Attendees were asked “How did today’s webinar meet your expectations?”
“Excellent updated information,” said David Banister, a field service technician with Clinical Engineering Services.
“I was particularly interested in hearing more about the cyber risk with medical devices,” shared Vince DiFranco, CEO with WJ Mangold Memorial Hospital.
“Provided great insight into potential HTM attack vectors,” said Jalil El, a biomedical equipment support specialist with Edward Hines Jr. VA Hospital.
“By providing a peek beyond the usual cyber posture, this expanded the scope from a holistic approach,” said Lee Woodruff, regional operations senior manager, Genesis Healthcare.
“Being in the Clinical Engineering world I appreciate the way the information is relayed and explained,” said Summa Health System HTM Manager Sandra Munsey.
Featured Employers: Agiliti, Renovo Solutions, TRIMEDX, SP Associates, Erbe USA Incorporated, and more!
htmjobs@mdpublishing.com for posting inquiries htmjobs.com to register today
A niche job board for the HTM and imaging communities
350+ open job opportunities
Single-job postings to 12-month unlimited memberships
3200+ biomedical and imaging candidates
Infusion pumps are everywhere in health care facility. Infusion pumps and patient monitors might be the most common and abundant devices when it comes to health care delivery. Maintaining infusion pumps in good working order is a huge task for HTM departments everywhere. TechNation reached out to HTM experts for the tips and advice regarding infusion pumps in this month’s Roundtable article.
Participants in the article are:
• Soma Tech Intl Biomedical Engineer Schleshin Molly David
• MMS Regional Service Manager Jose Jimenez
• ECRI Senior Project Officer Mukui Mutunga
• USOC IV Pump Lead Technician Jeremy Sommer
• ECRI HII-Tech Consultant Dustin Telford
Q: WHAT IS MOST COMMON ISSUE BIOMEDS ENCOUNTER WHEN IT COMES TO INFUSION PUMPS?
DAVID: The most common issue biomeds face with infusion pumps is occlusion alarms, which indicate a blockage in the flow of fluids and often require troubleshooting. Battery failures or charging problems are also frequent, especially in older or heavily used devices, leading to interruptions in operation. Additionally, calibration errors and software issues can result in inaccurate flow rates and dosages, necessitating maintenance or software updates.
JIMENEZ: Flow rate, low battery life, air-in-line (AIL) codes, and cosmetic damage. The bad battery problems are usually cause the AIL and flow rate.
MUTUNGA: Field serviceability: a. Some infusion pumps on the market are not easy to repair in-house. b. Parts are difficult to obtain from the manufacturer.
SOMMER: Insufficient training of staff. With the constant advancement of IV technologies, device compatibility and operation can be confusing. While the user interface or design may only have minor changes, these changes can easily shake up the confidence of the most seasoned staff member. Training is key to avoiding this.
TELFORD: User errors and usability issues are common challenges, often causing unnecessary alarms or improper setups. While mechanical problems like occlusion alarms and battery failures are frequent, poorly designed interfaces can make these issues worse. Simplifying user workflows and keeping firmware up to date helps reduce both mechanical and user-driven problems.
Q: WHAT TOOLS OR TECHNOLOGIES ARE INDISPENSABLE WHEN IT COMES TO INFUSION PUMPS?
DAVID: Flow and pressure sensors are essential for monitoring fluid delivery and detecting occlusions or blockages in infusion pumps. Battery management systems are also crucial, as they ensure uninterrupted operation, making rechargeable batteries and efficient charging
circuits vital for mobile or long-term use. Additionally, software and wireless connectivity play a key role in updating pump settings, tracking performance, and integrating with hospital systems for real-time monitoring and alerts.
JIMENEZ: There are a few main aspects to infusion pumps that need to be tested. These are flow rate and pressure of occlusion. The flow rate can be determined with a scale and the occlusion for the upper and low lines can be analyzed with a pressure meter.
MUTUNGA: 1) Wireless firmware and software updates/ upgrades – reduce the need for physical interaction with each device to update.
2) Log extraction and interpretation – infusion pump logs that can be extracted through a wired connection and wirelessly, and that are easy to understand (without consulting the IFU for error codes), make the troubleshooting process faster and aid in accident investigations.
3) Built-in asset tracking – especially across hospital enterprises that consist of smaller hospitals transferring patients to larger hospitals in the network.
4) Unified software for multiple types of infusion pumps (e.g., syringe and large volume) – reduces the burden on biomeds to maintain multiple software licenses. Provides a centralized location for tracking all infusion pumps across a network.
SOMMER: Quite possibly a cliché, but a calm and clear thought process. Taking a step back and analyzing an issue carefully and methodically almost always ensures a successful outcome. Besides that, access to the endless available amount of information on the Internet.
TELFORD: Precision tools such as feeler gauges, force and pressure gauges, and syringe jigs are crucial for maintaining infusion pumps. Air-in-line injection methods and diagnostic software help verify pump performance and ensure safety. Additionally, precision and torque drivers play a critical role in ensuring accurate repairs.
Q: HOW HAVE ADVANCEMENTS IN TECHNOLOGY CHANGED THE WAY YOU APPROACH REPAIRS?
DAVID: Advancements in diagnostic tools and software have streamlined the process of identifying and troubleshooting issues, reducing repair times and improving accuracy. Remote monitoring and connectivity features also enable technicians to access device data and perform firmware updates without needing to be on-site, which boosts efficiency. Meanwhile, the introduction of more complex electronic components and automated systems has increased the demand for specialized training and expertise to handle sophisticated repairs.
JIMENEZ: Before the use of scales, the flow rate was determined with graduated cylinders. This was fine for the time being, but not entirely accurate due to the measurement being taken at the curve of the water in the cylinder. The change to
the scale has given a much more accurate reading.
SOMMER: Biomeds have now become junior IT personnel. With more and more IV pumps connecting to hospital networks and the Internet, troubleshooting has gone from simple mechanical to a full spectrum of wireless issues.
TELFORD: Modern infusion pumps now have diagnostic tools that allow biomeds to track usage patterns and flag issues before they become critical. This proactive approach helps prevent breakdowns by identifying problems early through built-in logs and usage data. These advancements allow for more effective preventive maintenance, minimizing downtime.
DAVID: Regular preventive maintenance, such as cleaning, calibration and battery checks, is crucial for ensuring proper functionality and identifying issues before they lead to failures. Routine software updates are also essential to keep infusion pumps running efficiently and to address potential bugs or performance concerns. Additionally, proper user training on the correct handling and operation of the pumps can minimize user-induced errors and help extend the equipment’s lifespan.
JIMENEZ: Staff training has always been a big concern amongst biomeds. Regular reminders to staff to keep it plugged in when in use, proper cleaning with recommended chemicals, and preventing abuse of the device such as forced closure of the door.
MUTUNGA: Adhere to the manufacturer’s recommendations for preventative maintenance and performance verification testing. Most infusion pumps require a 2-year PM cycle. Infusion pumps should not go longer than 2 years without undergoing PM.
SOMMER: PM! PM! PM! Preventative maintenance is an absolute key to minimizing downtime and the need for significant repairs to be performed. I’ve pushed the idea of thinking of performing a PM like filling a cavity or getting a deep cleaning at the dentist. It’s not fun and it takes some time, but it’s sure a lot better than a root canal cost and time.
TELFORD: Optimizing preventive maintenance is essential, starting with using high-quality parts and following standardized procedures. Regular analysis of performance data allows for continuous improvement in maintenance practices. Sharing insights with the HTM community and OEMs helps foster iterative improvements across the field.
Q: HOW CAN A BIOMED STAY UPDATED ON THE LATEST EQUIPMENT AND REPAIR TECHNIQUES?
DAVID: Attending industry conferences and training workshops enables biomeds to learn about the latest equipment and repair techniques directly from experts and manufacturers. Subscribing to medical technology publications, newsletters, and online





forums offers regular updates on new advancements, troubleshooting tips and best practices. Additionally, participating in manufacturer certification programs ensures that biomeds receive hands-on training on the newest models and repair procedures.
JIMENEZ: The tried-and-true method that stands strong to this day is to read the service manual of the pump. The next best thing is to dive a little deeper into how electronics work and/or with an experienced technician. Most biomeds are willing to pass on knowledge to someone else.
MUTUNGA: Manufacturers generally do not allow the use of third-party or non-OEM parts to repair their devices. Therefore, the best way for biomeds to stay updated is to take any training course offered by the manufacturer. Consult the most up-to-date version of the IFU for the devices you are servicing. Keep in mind, these may not always be the versions that came with the devices.
SOMMER: By reading fine publications like this one of course! Web forums can also be indispensable fountains of knowledge on the latest and greatest information pertaining to repairs, known issues, and fixes available.
TELFORD: Staying current requires a mix of manufacturer and equivalent training, along with engaging in biomed associations and online platforms. Resources like MedWrench, TechNation’s webinars, and YouTube channels such as The Better Biomed and HTM Workshop offer valuable learning opportunities. Many test equipment companies, OEMs, and ISOs also provide excellent online training.
Q: WHAT ELSE SHOULD TECHNATION READERS KNOW ABOUT INFUSION PUMPS?
DAVID: Infusion pumps are vital devices in health care settings, requiring consistent maintenance to ensure reliable
performance. When it comes to purchasing refurbished infusion pumps, it’s essential to choose a reputable and reliable vendor. Investing in equipment from a trusted source guarantees that health care providers receive dependable pumps that are safe for patient use. Here at Soma Tech Intl, we pride ourselves on our meticulous refurbishment process, ensuring each pump meets stringent quality standards.
JIMENEZ: Always read the manual. It covers everything from the approved chemicals that are recommended for cleaning the unit to the most minor of calibrations if needed. The approved chemical is a big issue. I understand facilities try to save money on supplies where they can, but the improper chemical can eat at the plastic on pumps which can turn it brittle and/or weak. This can cause issues with flow rate, free flow and occlusion pressure.
SOMMER: Even with an ever-changing industry, most IV pumps stay fairly the same. Yes, user interface, compatibility and operation may vary a little from model to model and manufacturer to manufacturer but essentially, they all do the same thing to varying degrees. It may take some research but finding an IV pump that fits the needs of your facility, no matter how big or small, is not a difficult task in today’s industry.
TELFORD: Infusion pump maintenance is critical, with 60-80% of hospital or ER patients on pumps during stays longer than two hours. Both junior and senior biomeds are essential in ensuring these pumps operate safely and reliably. The work is more than routine – it’s vital for patient care and hospital operations.












Lessons
BY: K. RICHARD DOUGLAS

“Where is it? I need one stat, but I can’t locate it.”
Those words are heard in hospitals across the globe every day as medical devices and equipment go missing, are misplaced or become difficult to locate. This is a regular and vexing problem for clinicians; but it doesn’t have to be.
Nearly every device in a hospital can be tracked and located through technology and the problems created by a device’s disappearance, or running out of vital devices, doesn’t have to occur. Devices, along with staff and patients, can be tracked in real-time along with location. Location infrastructure can be added or modified to enhance these capabilities.
Like everything from DIY how-to videos on YouTube to evidence-based medicine, the experience and results achieved by others can be used as a guide to take on a project. In this case, it would be a real-time location system (RTLS).
At the Orlando MD Expo, in October of 2023, Michele Manzoli, CCE, and Jaspinder Singh Ratth, MS, both colleagues in the clinical engineering department at Cedars-Sinai Medical Center, presented on their own experience setting up one of these systems after a successful pilot.
Manzoli is manager of clinical engineering and Ratth is a clinical engineer. The presentation was titled: “The Real Value of Real-Time Location Systems: Lessons Learned.”
The clinical engineering department first initiated a pilot program, followed by a full implementation of an RTLS system encompassing more than 14,000 assets, and the guidance from the CE engineers is informative.
The engineers kicked off their presentation by highlighting the benefits of an RTLS system. At Cedars-Sinai, the CE team worked with leadership in device integration and nursing to form a steering committee. This group directed progress on the project, along with other stakeholders.
Any CE project at Cedars-Sinai is a big project, in a hospital with 889-beds and 15,000 employees, the CE department is made up of 30 team members. The RTLS project was no exception.
Manzoli explained that the incentive for installing an RTLS system was to reduce time searching for equipment, reduce
staff frustration and improve morale and improve workflows and productivity.
Much was learned during the pilot phase of the project. There were lessons learned firsthand during this phase that allowed the CE team to plan accordingly, while using the same devices.
Would you recommend using the same equipment/ devices during that phase?
“During the pilot phase, we learned the importance of choosing the optimal tag location: some devices didn’t adhere well to the tags, so we experimented with zip-tying tags to equipment power cables. We also encountered defective tags, highlighting the need for thorough quality checks before deployment. Additionally, if accessories like adhesives or cradles/brackets are involved, you would want to have a generous amount on hand. We recommend using the same devices during the pilot, as it serves as a valuable trial run to identify areas for improvement before full deployment,” Manzoli says.
One challenge of the pilot phase was that it began in March of 2021 during the COVID-19 pandemic with a go-live near the end of April. The pilot phase included approximately 700 tags and various devices including infusion pumps, cardiac chairs, transport monitors, vein finders, glucometers and others.
The entire project was rolled out in phases. Phase one took place during January through March of 2022, phase two during September and October of 2022 and phase three started in May of 2023. Phase three was still ongoing at the time of the MD Expo presentation.
The CE team focused on room-level accuracy for the med/ surg and ICU floors during phase one, as well as area-level accuracy in other spaces in the facility’s main building. The goal for the team was to tag approximately 11,000 assets during that time.
During phase two, they tagged approximately 400 gurneys, 600 multiparameter modules and 650 pumps/ patient warmers. Approximately 2,400 devices in total. Phase three saw 1,100 beds tagged and need-based tagging in the OR, cath lab and GI spaces, which included approximately 400 tags.
Another consideration that was discovered by the team was the need to check tag functionality right out of the box.
“Depending on the tag type, the activation of the device consists in holding a button for three seconds or removing a thin seal. The functionality verification requires our team to

enter the tag ID into the RTLS application and confirm the location displayed,” Manzoli says.
The team decided on several standardized naming conventions that included asset names and asset groups. An abbreviation for the floor and department is part of the standard, followed by an agreed-upon name for the device. Within asset groups, an abbreviated unit name, followed by an abbreviation for the device name.
To provide some useful guidance for the project, stakeholders from CE, materials management, transportation, nursing, imaging, EVS, periop services, pharmacy, lab and respiratory therapy were involved.
Department representatives shared information about the challenges of locating devices; the CE team kept all involved appraised of the progress of the project and there was a review of usage along with success stories to keep all parties motivated.
A video provided end-user training. End-users fill out an online electronic tagging request form, to make CE aware of all the particulars.
The other benefit of the system is loss prevention. Low frequency exciters are placed near linen rooms, so that an alert goes out to the charge nurse and unit secretary.
At Cedars-Sinai, much of the infrastructure was already in place to accommodate existing systems, such as infrared emitters, and both low-frequency and ultra-high-frequency components in inpatient spaces. The system that the CE team installed used infrared emitters and tags that can communicate with Wi-Fi or ultra-high frequency.
Using Wi-Fi, packets are sent out every five minutes that allow for the triangulation of device locations. Wi-Fi is an
optional pathway where battery-operated infrastructure cannot be installed.
Not all health care environments have the benefit of an existing infrastructure. How difficult would it be to add in infrastructure in environments that are not already as built-out as the facilities at Cedars-Sinai? What might need to be added to modernize any existing infrastructure?
“If a facility is looking to add room-level accuracy, they would need to allocate funds and resources for additional infrastructure. Depending on the specific RTLS vendor, the infrastructure could consist in both battery-powered beacon devices using infrared or ultrasound signals and networked proprietary access points,” Manzoli says.
He says that if structural and financial limitations are in the way, the facility can also take the “Wi-Fionly” approach. This would leverage the existing access points that support the hospital wireless network. Depending on the specific RTLS vendor, this approach may work only with certain RTLS tags and would generally result in less granular accuracy.
To get best results from a RTLS system, the process of mapping needs to be accomplished by the project team or by the vendor they are working with. What produces best results?
“Cedars-Sinai leveraged the RTLS vendor to assist with map creation. Areas with room-level accuracy required the custom build-up of the map using MS Visio or similar application. Areas with zone-level accuracy leveraged the architectural drawing that were already available to the project team,” Manzoli says.
There are two levels of maps; one room-by-room which allowed the vendor to draw up maps using Visio. This is the more accurate approach. A second type of map is a Wi-Fi map and it has a zone-level accuracy. This has a lower level of accuracy.
Battery management and back-end support are important components of an RTLS system. This was a focus of the CE team.
“Battery life on tags can vary, with some depleting early and others lasting beyond two years. After our pilot, we began annual battery replacements during PM for all equipment maintained by clinical engineering. We also generate low-battery reports twice weekly, with a dedicated team member handling replacement. This approach keeps our compliance at 99 percent,” Manzoli says.
Post implementation support is a key consideration and much of this will be based on the size of the facility and the numbers of assets. The pilot revealed the need to budget for one or two FTEs needed for on-site support and one FTE for system/application support. On-site support would include battery and tag management. There was also the need for server support, back-end support, expansion and reports creation.
“At our facility, we currently have two FTEs to support our RTLS and EM (environmental monitoring) system. One
FTE focuses on infrastructure and EM services, while the other handles new tagging requests and routine battery maintenance. The overall FTE requirement depends on the infrastructure and on the number of assets the facility plans to tag and maintain,” Manzoli says.
The criteria for tagging included several conditions. The asset had to have a value of at least $500. The asset should also satisfy at least one of several criteria, including that the tag can protect against accidental loss of small items, facilitate maintenance workflows, improve clinical workflows and facilitate patient transport, among others. Was CE satisfied with the chosen criteria?
“Yes, we are satisfied,” Manzoli says.
training for new staff members,” Manzoli says.
Finally, naming conventions help keep all on track. The terms decided on are a good way to align all stakeholders. The initial choices that the CE team went with have proved to be adapted by all.
Nearly every device in a hospital can be tracked and located through technology and the problems created by a device’s disappearance, or running out of vital devices, doesn’t have to occur.
Setting up a functioning system is just a portion of the challenge. Clinical staff need to be made aware of how the process works and there needs to be a means to provide training. The CE team and the steering committee addressed these requirements.
Manzoli says that some of the components of a staff awareness campaign can include creating visuals such as posters and flyers that can be circulated, which has been effective to promote RTLS throughout the facility.
“These posters are often shared during larger nursing education meetings, mass distributed through leadership listservs, and occasionally even added to the hospital website announcements page,” he says.
They made clinician training available through the hospital education portal.
“We encourage department leaders to have their staff enrolled in the course that provides the basics of how to navigate the RTLS application. It is prerecorded, which allows staff to rewatch any sections they need to. Our team is always available to assist and perform an in service for any departments that request so,” Manzoli adds.
Another benefit to keeping all clinical staff aligned with the use and issues of the system is a workflow committee.
“The need for workflow committee meetings on a regular schedule is important to reinforce best practices. It serves as a refresher for end users as well as

“We have not made any changes to naming conventions. We believe the current setup allows both our department and the end users to easy find and track equipment in the RTLS application,” Manzoli says.
During the course of the project, Manzoli and his colleagues have discovered several benefits besides tagging pharmacy and/or phlebotomy carts or infusion pumps. The tags allowed for the determination of how long the carts remained in targeted areas. This also allowed for the monitoring of time spent in each area by phlebotomists.
“We have found tagging all loaner equipment very helpful. These devices are intended to be at the medical center for a limited time and locating them without RTLS would be problematic in a facility as large as ours,” he says.

Feedback from CE colleagues, as well as clinicians, imaging, surgical floor, critical care and central transport has all been positive. The average time savings from surveys has been about 20 minutes a day per user. That equates to 8,500 hours saved annually. This time can be better allocated to value-added activities.
Loss prevention figures were impressive also with wound vac rental unit losses reduced by 80 percent in a 12-month period, with savings exceeding $65,000. The system helps with remediations and recalls. The system also helps with inventory optimization with devices like infusion pumps.
Future RTLS systems will continue to evolve and the technology will become even more precise. AI and machine learning will no doubt play a role in the future of these systems.


The experience and results of the Cedars-Sinai CE team can be instructive in determining considerations for an RTLS system, potential cost and time savings and other less-thought-of benefits.
Chief among the benefits will be fewer CE and clinical staff complaining: “Where is it?”
An on-demand recording of the MD Expo presentation is available at tinyurl.com/yaddjwar.











Objectively develop a systematic, multi-year capital budget replacement plan with help from ECRIʼs expert team on your timetable that meets strategic goals, controls maintenance costs, and minimizes risk.
Uncover the hidden costs of aging technology
Mitigate chances of sudden device failure


Identify potential hazards with existing devices
Learn more at home.ecri.org/predictive-replacement-planning or contact us today at 610-825-6000, x5891.













Check out our Youtube channel for more information and exclusive content!
BY VICKI SALEMI
hether your company is going through a reorganization, your industry is constantly changing or you’re always learning new technology, there’s no denying that change is happening all around us. How can you navigate it when it feels out of your control?
About 2,500 years ago, the Greek philosopher Heraclitus was onto something when he said to the effect, “The only constant in life is change.”
For starters, expect change so you’re not blindsided by it. It’s inevitable. This can be in many forms – the boss you respect may suddenly announce their resignation, your company may be merging, your job may be outsourced or down sized.
Whatever the situation, remind yourself that everything is temporary, both the good job and company you enjoy working for, toxic situations and everything in between. Be proactive by learning new skills, staying on top of industry trends, keeping your networking connections alive, and learning how your job, company and industry at-large can get ahead of automation (especially as it relates to how AI can impact your job – we’ll cover this more closely on an ongoing basis in future columns).
Make your mental health a priority – talk to therapists, practice yoga, meditate and exercise regularly to stay physically healthy and move to keep your mind off matters that are out of your control so you can focus on accomplishments you own like the achievement of finishing an intense workout or training for a half-marathon.
Dealing with change requires you to flex and build your resilience. Adriana L. Cowdin, executive career coach and founder, Be Bold Executive Coaching, said, “The first step is accepting that some aspects of business are uncontrollable. However, you can control how you respond by focusing on adaptability and upskilling. Flexibility is key, and those who learn to embrace change and look for opportunities within it tend to fare better. Learning new tools or gaining certifications can also improve your value and confidence in uncertain times.”

Additionally, by being solutions-oriented rather than dwelling on problems, you can focus on positive aspects like opportunity and growth. “This shifts the mindset,” said Cowdin. Another mindset shift involves reframing the situation; change doesn’t always mean it’s leading to something worse.
Brian Solis, author of “Mindshift: Transform Leadership, Drive Innovation, and Reshape the Future,” said, “Shift your perspective on change and accept that change is a natural, inevitable part of life, from nature to technology to society at large. When you see every new experience as an opportunity to gain knowledge or grow, you’ll be more receptive to change. Always ask, ‘How can this experience make me better?’”
Instead of focusing on what’s out of your control, focus on what you can control. Solis said, “Shifting from reactive behavior to proactive behavior will bring a sense of empowerment. It’s also important to adopt a growth mindset. Without losing touch with reality, a mind shift means challenging our negative self-talk and reframing negative thoughts into proactive thoughts and behavior. When facing unemployment or career stagnation, practice reframing them into positive affirmations. By adopting strategies like developing resilience, focusing on self-development and cultivating emotional flexibility, you can turn uncertainty into a period of transformation and personal growth.”

Vicki Salemi is a career expert for Monster, an author, a speaker and consultant, TV commentator and former corporate recruiter. Send your questions to hello@vickisalemi.com.

BY TED LUCIDI, CBET
We’re going to continue in our series focusing on the key components within an ultrasound probe. This month, we’ll examine the wiring harness and cable jacket.
After the acoustic array, the most failed component on an ultrasound probe is the wiring harness or system cable. After all, the cable is flexed hundreds of thousands of times during a probe’s life cycle. It is also subjected to accidental pulls, roll-overs and pinches. Wiring failures are something with which most of us are familiar, they are not just limited to ultrasound probes. Knowing this, one might suggest that OEMs develop wireless ultrasound probes. Well, they have. But, wireless probes have their own challenges. They can be

easily dropped, hindered by excessive RF interference, experience poor signal-to-noise, and range/distance limitations. And, yes, they have even been lost or discarded into the trash bin. It will be sometime before wireless ultrasound probes become mainstream.

The graph shows about 2-years of data only including standard probes (omitting TEE and mechanical 3D/4D probes). Spanning just under 10,000 standard probe repairs, over 50% presented with array failures, while about 37% presented with wiring or cable failures. Failures to ONLY the lens, the strain relief, cosmetics, and electronics consumed only 13%.
In legacy probe models, and even in some of today’s more-simple designs, the wiring harness may contain less than 100 micro-coaxial wires. That’s right, ONLY less than 100. Today’s more-complex probe designs have wire counts of up to almost 200. Traditionally, the
purpose of the wiring harness was to carry the transmit and receive signals to and from the scanner/probe, and for most, it still is. Typically, there was one micro-coaxial wire for every acoustic element in the array.
More complex probe models, such as the Philips VL13-5, L12-5 50mm, and L18-5 increase the element counts in the acoustic array to 192, 256, and 288 respectively, yet still use a wiring harness containing about 170 wires. In these models, both the scanner and the probe electronics use multiplexers. The harness carries, not only the T/R signals, but also power and signals to control the Mux’s. Newer live-3D volumetric probe models, such as the Philips X5-1c and GE 4Vc-D have over 3,000 acoustic array elements, yet still only use a harness with less than 200 wires.
Barring physical damage, when the cable is cut, pinched, rolled over, or pulled, wiring failures occur as a normal part of almost every probe’s life cycle. Wiring failures usually lead to service calls being placed for non-descript problems or general terms, such as noise artifacts. Depending upon the level of detail provided by the customer, wiring failures may be challenging to reproduce in the field without a consistent test process. Our transducer assessment guide provides step-by-step instructions on a consistent testing process. More information about the guide is below.
Most wiring failures begin to present as intermittent, vertical streaks of color in color Doppler mode or as intermittent static or sharp “crackling” sounds in CW Doppler mode. As the wires continue to degrade, intermittent black, vertical, lines of dropout may appear in 2D mode. For probe models, such as the L12-5 mentioned above, if one or more of the wires that are used for control or power to the Mux’s fails, users may be presented with a double image or partial image. Sometimes, on more advanced probe models, only an error code is displayed.
There are multiple methods that service providers use to address wiring intermittencies.
• One frequently used approach is to cut-back and re-terminate one end of the wiring harness … attempting to remove the intermittent section of wires. The challenge with this approach is that the system cable becomes shorter and shorter with each repair attempt. Most times, sonographers and end-users notice the shorter cable and are less than satisfied.
• Another solution is to harvest used cables from other defective probes. The difficulty with this solution is
that you don’t truly know what you’re getting. A cable is designed to only flex x-number of times before degrading. How worn is the cable on your last exchanged probe? The supplier is betting that the life cycle of their cable will be longer than their warranty period. Is that something you’re willing to risk?
• One, final, ‘typical” solution is to replace your probe’s OEM cable with a generic, one-size-fits-all, replacement cable from an overseas supplier. Although this solution may get your probe working again, it bypasses the design controls of the OEM. Performance, safety and efficacy might become compromised.
The ideal solution to address any cable intermittency is to replace the cable with a new one. The challenge is that OEMs do not sell replacement parts for ultrasound probes to ANY independent service provider. The next-best solution would be to fabricate a replacement cable proven to be OEM-equivalent. It’s not an easy task. Most of the wires are classified as micro-coaxial, and can be as fine as 48-guage (thinner than a human hair). GE’s 4Vc-D utilizes 52-gauge wires. They are the finest that we’ve seen in ultrasound probe designs. Items to be considered when designing a wiring solution in an ultrasound probe are the length, outside diameter, inside diameter, jacket material, dielectric type, solid or stranded core, magnetic permeability, shielded or unshielded, capacitance, inductance, and resistance. As an FDA-registered manufacturer of ultrasound probes, Innovatus has unparalleled expertise to test, classify, and design an entire cable harness. We currently fabricate over 100 unique cable assemblies. Each solution is verified and validated against the OEM intended design. We continue to repair probes for several OEMs based upon our deep understanding of the full device and track-record for sustainable results. As a result, we are able to deliver sustainable repairs and performance that can renew the product’s life cycle at a fraction of the cost of exchanging the probe.
For more information on this topic, or to request our transducer assessment guide, reach out to training@ innovatusimaging.com

Ted Lucidi, CBET, is the director of commercial operations and business analytics at Innovatus Imaging.
BY GARRETT SEELEY
The effects of quality documentation have always impressed me. A lot of techs would see this as tedious and, in truth, it can be. However, keeping adequate records is one main tasks of a healthcare technology management professional. This has become a bit of a double-edged sword when balancing workload and the need to document it. It is often hard to find the time. Regardless, it is important, not just in work orders and inventory, but also in networking. To illustrate, I would like to present a model that was given to me by a past shop manager. Before I begin, let me state that this it is an earlier model from before technologies such as Cisco ISE. We covered some of the possibilities of networking software in the Unified Threat Management (UTM) articles and it is superior to this technique. Please consider the following as a launching point; a beginning thought as to what to look for in complete networking documentation. This will give a starting point as to what to ask the UTM systems to provide.
When I was taught about networked device documentation, the presenter gave the following scenario: if there was an emergency, say a vulnerability in an operating system that required an immediate response from the HTM shop, how would anyone know what machines are affected? What if it was just a certain version of an operating system that is affected, would anyone know where those devices are? What if it was just a certain patch? For example, if this vulnerability only affected the Windows 10 Operating System, OS builds 1803 and earlier, and these devices must be unhooked from all networks immediately what inventory numbers are those units and where are they located? This is a hypothetical situation that illustrates exactly what was asked of people during a cyber-attack. In such a case, we may have to find and isolate vulnerabilities quickly. The challenge is, how do we do that? This scenario drives home
the value of knowing what is on the network, what OS it has, and where it is physically located.
To help in these situations, use a list. This list can help serve as a guide regarding what to keep track of pertaining to networked medical devices. As best practices, every biomed should have access to something like this in their hospitals:










All hospitals have on site inventories, and therefore specific numbers for each machine. This is specific to each site in their work order clinical maintenance management system (CMMS). I find it important to describe what to look for by saying, for example, Ultrasound, GE, Logiq P9. This helps to know exactly what we are looking for. Sometimes a location, room number, or department can be added as an additional guide to find a unit. We really need to know the VLAN information. This can be a number, such as 101. It helps see how the network is segmented and will help if the device moves to another VLAN within the hospital (see articles on network subnetting). MAC and IP addresses, operating systems and build information are critical for assessing a vulnerability in a pinch. I suggest also having a SBOM link, if possible. We covered SBOM in a previous article; it is a list of software loaded on the device. This could be on the web, but it could be a PDF file in a local shared folder. This is a great item to be able to reference in a hurry as not all vulnerabilities are operating system related.
One of the main goals of this type of list is consistency and searchability, and this leads to another important concept. We have not covered this term before, but the
term for database consistency is “normalization.” Frankly, there is a lot of information regarding normalization and how the problems with inconsistent language use can be avoided. Consider a computer’s a search function. If a user searches for my employer, the computer thinks the terms VA, Veterans Affairs, and Veterans Administration are all different things. To a person, they are all the same thing, even though the last one is the wrong term for the Veterans Affairs Healthcare System. This is an example of how a computer requires exactness whereas our minds think in terms of “it’s close enough.” Believe it or not, when referencing information, small details such as correct spelling are extremely important. Computers don’t think in terms of close enough. This effect is vastly important for searching through information. The act of fixing all these little inconsistencies in personal data entry is called normalization. For example, CPT and ICD10 codes are a normalization used in health care. We had to use such things because the inconsistencies of medical language use, statistically around 40%. That means the co-workers entering the same thing without cut and paste or some form of template to help will use different wording 4 out of 10 times. Therefore, have a uniform stated language to make the data easier to search in an emergency. This is not just useful for
networking documentation, but consistent wording in work orders lends itself to easily quantifying our workday. This helps ensure that our work orders are consistent and easily searchable, and clear.
These are some basics of documentation for networked medical devices. Thankfully, there are companies that produce software to help, that is however, another article. Regardless of the method used, encourage HTM professionals to compile this information. “You’ll thank yourself later if you document now.” It’s a quote from myself written in my planner 25 years ago. It has become a personal motto of sorts. I use this as a personal reminder that we are paid to be meticulous. After all, when it comes to medical equipment, it is literally a matter of life and death. It can be a matter of litigation. In such a crisis, there is no substitute for adequate documentation.

Garrett Seeley, MS, CBET, is a biomedical equipment support specialist-imaging with VISN 17: VA North Texas Health Care System at the Dallas Veterans Affairs Medical Center.
BMES specializes in refurbished equipment, specifically transmitters, featuring premium brands like GE and Philips.
Every item undergoes a rigorous quality control process, ensuring reliability and top performance. Each unit includes our industry-leading warranty, offering you peace of mind with every purchase.
When you choose BMES refurbished equipment, you can trust it’s been expertly serviced by our skilled biomedical technicians.



BY NADIA ELKAISSI, CHTM
As the holidays are approaching, many of us are busy decking the halls and preparing for festive celebrations. But in hospitals, there’s no time to let down our guard when it comes to cybersecurity. With so many hightech medical devices in use, keeping our digital defenses strong is especially crucial. Understanding the differences between credentialed and non-credentialed scans for medical equipment, and knowing the important questions to ask vendors, can make all the difference in securing a hospital’s network. In this article, we’ll explore why these types of scans matter, and how to ensure you’re evaluating equipment correctly to get the necessary information.
Before we dive into the why, let’s break down the what. Scans are essential for assessing the security posture of medical devices, such as MRI machines, infusion pumps, and patient monitors, that often connect to the hospital’s network. These scans detect vulnerabilities and ensure that devices are not easy targets for cyber threats.
1. Credentialed Scans: A credentialed scan is a thorough scan performed with valid user credentials, allowing in-depth access to the medical device’s internal system. A credentialed scan simulates what an authorized user might see, providing comprehensive information about installed software versions, configurations and
potential security risks. This level of access reveals more vulnerabilities and gives HTM a clear view of how to patch weaknesses.
2. Non-Credentialed Scans: These scans are performed without any special access or credentials, simulating an attack by an external threat actor. While they can identify some vulnerabilities, they often miss internal issues such as outdated software version or insecure configurations. Non-credentialed scans provide a limited picture of the device’s security posture, focusing more on perimeter defenses.
Unfortunately, medical devices in hospitals often come with their own set of unique challenges when it comes to cybersecurity. One of the major issues HTM professionals are facing, is that many medical systems are not able to do credentialed scans. So, we must ask the question, why do we need to push for credentialed scanning, if it is not approved for some medical devices? For many medical devices, the risks associated with vulnerabilities goes beyond data breaches. Patient care is at risk if devices such as infusion pumps or ventilators are compromised. In addition, medical devices typically run specialized software, often with outdated operating systems because of regulatory constraints or compatibility requirements. Credentialed scans would provide a detailed view of vulnerabilities and identify outdated software and configurations. These would both help mitigate risk and prioritize patches and updates.
Now, you are probably thinking: Great! Why don’t we just mandate credentialed scans for all medical equipment? While credentialed scanning should always be considered

during the procurement process, it is important to understand some medical devices simply cannot have credentialed scans. Medical devices may have restrictions that limit the extent of what can be scanned, either because of the vendor’s proprietary systems or regulatory requirements. In some case, performing a credentialed scan may void warranties or violate service agreements. Additionally, medical equipment can be sensitive to intrusive scans, risking interruptions to essential functions. In these cases, non-credentialed scans, though less comprehensive, may be more suitable where minimal system interaction is necessary.
Vetting equipment and asking the hard questions are crucial, especially during the procurement process or when planning a cybersecurity assessment. Here are some targeted questions to help you fully understand the scanning capabilities and limitations of medical devices:
1. Confirm the scanning techniques that are supported. Question the vendor on whether the device supports both credentialed and non-credentialed scans and which scanning methods are recommended to avoid potential disruptions.
2. Ask if there are any restrictions on credentialed scans. Vendors may place restrictions on performing credentialed scans, citing reasons such as warranty concerns or potential interferences with the device’s normal functions. You need to understand what is permitted and if there are any associated risks you need to be aware of.
3. Determine the recommended scanning schedule and frequency. For instance, many medical systems are scanned after hours to reduce patient care disruption. Make sure to clarify any associated impact.
4. Understand what mitigation options are available if vulnerabilities are found. You need to understand how to address identified vulnerabilities, including if the vendor will provide support for patches, software updates or configuration changes.
Just as Santa checks his list twice during the holidays, HTM professionals must continue to check the cybersecurity list for medical equipment. Understanding the difference between credentialed and non-credentialed scans and knowing the right questions to ask vendors of how to best secure your systems can help you get the best insight into the security posture of your medical devices without compromising clinical functionality. By ensuring a layered approach to scanning and collaborating closely with vendors, HTM professionals can unwrap the gift of cybersecurity this holiday season.

Nadia ElKaissi, CHTM, is a biomedical engineer with Healthcare Technology Management, VA Central Office (19HTM).
BY NATHAN PROCTOR
The Open Repair Alliance released a new report in October, written by the Restart Project, which examines data collected about more than 200,000 repairs conducted at community repair events around the world.
Part of the global coalition fighting for your Right to Repair, the Open Repair Alliance helps repair advocates keep track of what people are trying to fix, whether or not repairs are successful, and why.
Repair events are quite obviously a different context than fixing medical devices in a hospital or other medical setting. But there are lessons in this huge data set.
Community repair events – including Repair Cafes, Fixit Clinics and Restart Parties – have grown considerably over the last 15 years. The report estimates more than 4,000 community repair groups, operating in 31 countries, now host events that offer free repair help to anyone who wants it, performing an estimated 190,000 successful repairs each year. People not only donate their time to help neighbors fix products, but also catalog hundreds of thousands of repair attempts. That serves as the data for this new report.
People everywhere are passionate about fixing stuff.
People are better at fixing things than you might think. Of all the electronic products that get brought to community repair events, 53% get fixed at the event. Furthermore, many of the people whose products are not fixed at the event, make a plan to complete the repair (which might include ordering a part or visiting a repair shop with the needed equipment). Onequarter of the products are deemed “end of life,” without a realistic or cost-effective way to complete the repair.
You might think that older item you have is probably dead …
but it might not be. You should bring it to a local repair event and see if a tinkerer can revive it – the odds are pretty good that someone can.
COULD REMEDY MOST OF THE REASONS WHY THINGS DON’T GET FIXED.
Right to Repair reforms – which require manufacturers to provide fair and reasonable access to spare parts, service manuals and repair tools – can help us fix more of our stuff.
Looking at the tens of thousands of products that repair hobbyists could not fix, no spare parts were available for 25%, another 18% had parts available but they were cost-prohibitive, while another 12% lacked repair information, meaning repair might be possible, but technicians were unable to obtain the documentation or instructions they needed.
These are exactly the issues Right to Repair reforms are intended to remedy.
THINGS LAST A LOT LONGER THAN MANUFACTURERS TEND TO THINK – AND PEOPLE NEED THOSE OLDER PRODUCTS.
Data from thousands of repair events shows that people hold on to most products for far longer than five years, regardless of intended support. On average, the age of coffee makers, laptops and printers brought in for repairs was seven years old; power tools and hair dryers averaged 10 years old; hi-fi audio systems were 18 years old on average; and projectors (which includes digital, film and slide projectors) averaged an impressive 28 years old.
I think this data is important to our work in medical Right to Repair because it represents the paradigm shift we need to understand. A diverse, distributed system of repair is critical to community resilience – just as it is for a hospital’s resilience. What a manufacturer might consider an “edge case” for repair is viewed pretty differently when that edge case is happening to some product you are relying on.

Nathan Proctor is senior director of the U.S. PIRG Campaign for the Right to Repair.








BY JOIE N. MARHEFKA, PH.D.
As I write this, during the fall 2024 semester, I am seeing some encouraging signs from our students on campus. I see students attending events on campus in numbers that I haven’t seen since March of 2020. I’m seeing similar trends with professional organizations and events. Our Women In Bio Pittsburgh chapter just hosted an event with nearly 100 attendees. I believe that people’s priorities and routines shifted during the pandemic, and it seems to me that things are finally getting back to normal. Therefore, I want to explore opportunities to get involved.
For students, the opportunities to participate in school activities are numerous. One option is to join a club at school. Some schools have major specific clubs, such as a BET or HTM club, or perhaps a more general STEM club, as we do. Clubs offer opportunities to participate in activities, meet other students, and perhaps sharpen leadership skills. Students can always start a new club if one doesn’t already exist. Another opportunity available to many college students is working on a research project with a professor. It’s a great way to explore some aspects of the field more deeply. It is also a nice thing to put on a resume and a way to get a good letter or recommendation. Outside of school, students can get involved in the HTM field by joining AAMI as a student member, watching HTM webinars and attending conferences. These are all opportunities to learn more about the field and can be helpful when searching for a job. As a professional, it may take a little more effort to find ways to get involved in the field. Some hospitals and

companies have committees or various organizations that employees can join. Outside of work, joining AAMI or other professional organizations can provide a chance for networking, professional development and leadership. I am currently a member of AMMI, the Society of Women Engineers, and Women In Bio. I have held various leadership roles within the Pittsburgh chapter of Women in Bio. With AAMI, I regularly attend and present at conferences and am a member of a standards committee. Attending and presenting at conferences is a great way to share your expertise, learn about new technologies and trends in HTM, and network with other professionals. Participating in standards committees is a way to help shape the industry and a good volunteer opportunity.
Finally, for people who live near a school that offers HTM or a related major, there are opportunities to participate in various activities that help shape the future of HTM. We have an advisory board for our program that provides feedback to ensure that our curriculum meets industry needs. We also have a number of professionals who volunteer to give guest lectures to our classes. I’m sure other schools are in need of volunteers for these types of activities. While I certainly value work-life balance, I have found spending just a little bit of my free time participating in the types of activities described above to be rewarding. I believe most of my students would agree. I encourage all of you to look into opportunities like these and to find a way to get involved in an HTM related activity (if you aren’t already).

Joie N. Marhefka, Ph.D., is an Associate Teaching Professor, Biomedical Engineering Technology Program Coordinator with Penn State New Kensington.

BY PHIL ENGLERT
Food Network fans may be familiar with Guy Fieri’s classic greasy spoon road trip across America known as “Diners, Drive-Ins and Dives.” The program focuses on small, independent eateries, regional styles or ethnic specialties.
Cybersecurity has its own Triple D – Design, Default and Demand. Design, Default and Demand are three concepts that HTM professionals can apply to the medical device environment to help ensure the equipment needed to provide patient care is available and safe. Secure by Design is an approach to software and product development that prioritizes security from the beginning of the design process. Secure by Default is a security principle that ensures products are configured with the most secure settings. Secure by Demand is a principle that empowers software customers to ensure that the products they procure are designed with security as a core consideration. Let’s review each principle and explore what implementation can look like.
Products designed with Secure by Design principles prioritize customer security as a core business requirement rather than merely treating it as a technical feature. Instead of treating security as an afterthought or a feature to be added later, it is integrated into every stage of the product life cycle. This method aims to identify and mitigate potential vulnerabilities before the product is released. What does that look like? Consumers can look for foundational principles when researching new medical devices.
When dealing with companies with a mature secure-bydesign posture, you should feel that security is part of their culture and not just a program. Security will come across as a core value and responsibility at all levels of the organization. You will understand that security requirements are incorporated in the initial design and development
phases. Your investigation should reveal that tailored threat models anticipate and address potential security threats during development and that secure coding standards prevent common vulnerabilities. Product literature will reveal defense-in-depth strategies to provide multiple layers of security and the fact that automated tools are continuously utilized to test for security issues throughout the development process. Finally, look for a robust program to manage and address vulnerabilities as they are discovered both in the development cycle and after the product is in your environment.
Three key implementation traits will help you better understand a company’s commitment and maturity to security by design principles. First, look for evidence of executive ownership demonstrating that security is a priority at the executive level and that there is accountability for security outcomes. Next, look at the level of transparency and accountability. Does the company embrace or resist sharing key information about vulnerabilities and security practices? A culture of willing transparency is evidence of a company with a higher maturity level and commitment to a better partnership in maintaining the cyber posture of medical technology. Finally, look for evidence of continuous improvement through regular updates and a roadmap for improving security measures based on new threats and vulnerabilities. Threat actors constantly find new ways to exploit vulnerabilities and attack organizations through technologies in their environment. By following these principles and steps, organizations can create products that are inherently more secure, reducing



the risk of cyber-attacks and improving overall trust in their technology.
Secure by Default ensures that products are secure and out of the box, with secure configurations enabled by default. This approach minimizes the need for users to make additional security configurations, thereby reducing the risk of vulnerabilities due to misconfigurations or oversight. A few key traits to look for are that default settings prioritize security, such as disabling unnecessary services and features that could introduce vulnerabilities. Look for products designed to be secure without requiring extensive setup or configuration changes to apply security controls. Default passwords should be unique and strong, and instructions should include how to change them. User authorization should incorporate the concept of least privilege. Set-up instructions should identify changes that could reduce security and are communicated to the user, ensuring they understand the potential risks. This includes clear instructions and warnings when users attempt to change settings that could compromise security. If practical, look for systems configured to automatically receive security updates, reducing the exposure window to known vulnerabilities.
To implement Secure by Default in medical devices, start by determining which settings and configurations are critical for security on the device and within your network environment. Focus on authentication, access controls, network configurations and data protection. Ensure that default user accounts have minimal privileges. Enable encryption, use strong passwords and disable unnecessary services. Implement automated updates for your software to ensure it receives the latest security patches without user intervention using secure channels to deliver updates and prevent tampering. By following these principles and steps, organizations can significantly enhance the security of their products, making it easier for users to maintain a secure environment.
Secure by Demand empowers software customers to ensure that the products they procure are designed with
security as a core consideration. This approach involves customers actively demanding and verifying that medical device manufacturers prioritize security throughout the product development life cycle. Customers are crucial in driving security by explicitly requiring it in their procurement processes. Demand transparency about security practices and use the Healthcare Sector Coordinating Council’s Model Contract Language for Medtech to identify and assign responsibilities and to hold suppliers accountable for security outcomes. Key steps during the procurement process is to clearly define and communicate security requirements in the procurement documentation and request security artifacts such as a Software Bill of Materials (SBOM), vulnerability disclosure policies, and security roadmap for identified vulnerabilities and missing or weak controls. How does the manufacturer handle security patches and updates? Are they automated and easy to apply? What is the patch and update cadence? Include security requirements as part of the contractual obligations. This can involve specifying the need for regular security updates, vulnerability management and secure coding practices. Provide feedback to the software manufacturer and work collaboratively to address security issues.
Technology buyers can enforce Secure by Demand principles by integrating these specific security requirements and practices into their procurement processes. Technology buyers can ensure that their software vendors prioritize security, thereby reducing the risk of vulnerabilities and enhancing the overall security of their technology ecosystem. HTM professionals can use the Triple D principals when evaluating, selecting, implementing and maintaining medical devices in the connected health care ecosystem.

Phil Englert is the vice president of medical device security for Health-ISAC Inc.
• My computer
• My multimeter
• The tool bag
• A notepad for my reminders
• Sani-Cloth wipes
• Label scraper

Submit your bench to be featured in TechNation at 1technation.com/my-bench/. You could win a $25 Amazon gift card via the “What’s On Your Bench” Contest!

An online resource where medical equipment professionals can find all the information needed to help them be more successful! The easy to navigate Bulletin Board gives you access to informative blogs, expos and events, continuing education opportunities, and a job board. Visit MedWrench.com/BulletinBoard to find out more about this resource.
Follow MedWrench on Facebook & LinkedIn


Ben Calibrating, or Ben C. for short, is the face of MedWrench! While he may just be a small lego figure, you can find him all over the world.
At MedWrench we have an ongoing contest to see where Ben C. is traveling to and what he’s been up to! Here’s how you can participate:
STEP 1: Like the MedWrench Facebook & LinkedIn pages

#Ben C. in Northern California
STEP 3: Post a funny caption with your picture telling us what Ben C. is up to
STEP 2: Post your picture of




BY RAYMOND ALI
The field of biomedical engineering is quite a fascinating one. Not only is it an engineering field that has significantly grown over the past few decades, but it is one that will continue to evolve and expand as the limits of technology are pushed. Biomedical engineering has changed the landscape of patient care in ways that were previously unheard of. When Willem Einthoven invented the first ever practical ECG in 1901, it was a 600-pound device that took up 2 rooms. It included a massive electromagnet, a continuous-flow water jacket to prevent overheating, and needed 5 people to operate it. The patient needed to have both of their hands and 1 foot submerged in a saline solution which were used as electrodes. This ‘string galvanometer’ as it was called was the first device capable of taking electrocardiograms. At the time, this was the absolute pinnacle of medical technology.
STEP 4: Make sure you tag @MedWrench in your post so our team can see it

The Clinical Engineering Association of Illinois (CEAI) recently held its annual meeting as a means to meet its objectives. The objectives of CEAI are to promote cooperation, education, formal/informal exchange of ideas and technical information related to clinical engineering. It will help promote better patient care and reliable maintenance, operation and application of clinical equipment. The annual meeting was a success with attendees and vendors coming together to network, learn about products and services as well as attend continuing education presentations. For more information, visit ceaiweb.org.
1. Jaka Jusu receives this year’s $1,000 scholarship to continue his HTM journey.
2. The conference closed out with a night of fun at Puttshack!
3. The 2nd day keynote was a Q&A session with these HTM experts.
4. The volunteer CEAI leadership team with Danielle McGeary (AAMI).
5. The 2-day vendor expo was a hit!
6. Another successful conference with attendees from all over the Chicagoland area!




5 6 3 4


New England • October 8-10, 2024
MD Expo New England surpassed expectations with close to 1,000 HTM professionals in attendance at the Mohegan Sun in Connecticut. Biomeds representing every skill level joined the top solution providers for three days of educational sessions, networking, exhibit hall and more. The New England Society of Clinical Engineers proudly supported the MD Expo with many members in attendance at the event.


1 3 5 6 4


2


1. Members of the Women In Leadership (WIL) Society gathered for a Mimosas and Mingling event during the New England conference.
2. Glenn Schneider, chief service officer with Elite Biomedical Solutions, led attendees during the inaugural H.O.T. Workshop: Patient Monitors.
3. MD Expo’s Reverse Expo gives vendors valuable face-to-face time with key decision makers from leading healthcare organizations.
4. The Leadership Summit gave the best and brightest in the industry an opportunity to gain invaluable knowledge to help their departments grow and prosper.
5. Over 100 Young Professionals at MD Expo (YP at MD) gathered for a fun event, sponsored by Prescott’s.
6. Employees of CME Corp enjoyed the conference bags, sponsored by Tenacore.
7. An expert panel convened for an informative discussion regarding medical Right to Repair. (from left) Rob Kerwin, Mike Busdicker, Binseng Wang, and Nathan Proctor took the stage as Chris Nowak (not pictured) moderated the discussion.
8. Kevin McGowen, senior project manager with Tenacore, explains Tenacore Vending Service to intrigued attendees.
9. Attendees were all smiles while collecting stickers from vendors inside the exhibit hall. Over 25 prizes were given away during door prize drawings.
10. Leadership from FSI Software were on hand to greet guest at the Leadership Summit Dinner.




11. Two industry icons pose for a photo. Bryant Hawkins Sr. gifted MD Publishing President John Krieg with a B-Hawk hat.
12. The finale party, sponsored by USOC Medical, provided a biergarten-themed atmosphere where attendees could relax around fire pits, connect with friends, or make new acquaintances.
13. One of the biggest benefits attendees receive from MD Expo is the opportunity to earn CE credits while learning from some of the industry’s top leaders. MD Expo New England was pre-approved for up to 8 CEUs from the AAMI Credentials Institute (ACI). 7 9 11 12 10 8 13



Need a powerful way to elevate your brand and connect with your target audience?
Becoming a Preferred Vendor attracts attention to your products or services, ensuring that your ad reaches potential customers who are genuinely interested. Plus, you are able to enhance your brand credibility and position your company as an industry leader through additional highlight in our monthly e-newsletter and preferred vendor playlist on TechNation.TV.























USOC Medical usocmedical.com
Installs/Deinstalls
KEI Medical Imaging Services keimedicalimaging.com • 512-477-1500
Innovatus Imaging innovatusimaging.com • 844-687-5100
International X-Ray Brokers internationalxraybrokers.com • 508-559-9441 55
KEI Medical Imaging Services keimedicalimaging.com • 512-477-1500
Tri-Imaging Solutions triimaging.com • 855-401-4891
Diagnostic Solutions diagnostic-solutions.com • 330-296-9729
International X-Ray Brokers
PartsSource, Inc. partssource.com • 877-497-6412 5,38
Oxygen Blender
Tenacore LLC tenacore.com • 800-297-2241
BC
Prescott’s, Inc. https://prescottsmed.com/ • 833-423-3786
Pronk Technologies, Inc. pronktech.com
Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010










“It

Our training program at Tri-Imaging transcends traditional education by instilling skills that will resonate throughout your professional journey for years to come. Unlike conventional training experiences focused solely on facts and figures, we prioritize imparting practical, applicable skills.
Our commitment extends to fostering a deep understanding of the intricate inner workings of various devices and machines that our students will service in their careers. Here at Tri-Imaging we believe in nurturing individual growth, and to that end, we provide one-on-one assistance for each student.
Step into the future of education with Tri-Imaging, where our training program transcends boundaries to equip you with skills that stand the test of time.







