A CALL TO ACTION

















































































































































It seems like just yesterday we were discussing if the biomed community could use a new voice, news source—a new “nation” if you will. That was 2010!
Well, 15 years later, I could not be more proud of the TechNation brand we have built.
It’s important to remember your roots, and for those who may not remember, here’s a quick trip down memory lane.
We started Medical Dealer magazine in 1997, as a secondary resource for pre-owned medical equipment, parts and service. I was 30 years old, had a marketing/publishing background, and was as green as it gets to understanding this industry.
I remember early morning phone calls with my old buddy Jim Fedele who was instrumental in teaching me the ins and outs of the biomed department. Manny and Ruth Roman, whom I met at the NCBA conference in Raleigh, NC (that was how long it’s been), taught me how to treat others with kindness, be humble and show gratitude and always do the right thing. We wouldn’t be where we are today without these pillars in our community.
As Medical Dealer grew, we saw an increase in biomeds reading the magazine, always looking for alternatives to help save their facilities time and money. A real audience emerged. That helped launch the TechNation we are today:
• 2002 inagural MD Expo
• 2009 inagural spring MD Expo
• 2010 launched TechNation magazine
• 2014 debut of Webinar Wednesday
• 2019 YPs (Young Professionals) networking community unveiled
• 2020 HTM Jobs launched
• 2020 HTM Mixers introduced
• 2023 debut of TechNation TV
• 2023 premier of TechChoice Awards (The Wrenchies!)

What we are most proud of is being able to help grow and prosper an incredible group of heroes called biomeds. Who day in and day out, maintain the equipment and devices to help save lives and keep patients safe. We are honored to recognize these phenomenal people, their clinical engineering departments, and HTM associations. Our dedication to highlighting these efforts will never waver.
Of course, a huge shout out to my Vice President Kristin Leavoy, who has been there every step of the way, helping forge our way ahead to become the industry leader we are today. And my vice president of sales, Jayme McKelvey who has been instrumental in keeping TechNation at the forefront of the industry.
And of course, none of this could have been accomplished without the continued support of our loyal clients, advertisers, and sponsors. Connecting these clients with our TechNation audience has been our ultimate goal, and the ability to help them gain new prospects and nurture their existing customers is something we are most proud of.
Be on the lookout each month in 2025 as we share highlights and take a fun look back through the years. (See Page 66.)
Thank you from the entire team at MD Publishing, it’s been 15 years, and we are just getting started! Cheers!
John Krieg President of MD Publishing




















P.5 LETTER FROM THE PRESIDENT
P.12 SPOTLIGHT
p.12 Department of the Month: Durham VA Healthcare Technology Management Department
p.14 Professional of the Month: Jake Calhoun
p.16 Next Gen: Ranjita Shrestha
p.18 Shifting Gears: Time Off to Make a Difference
P.21 INDUSTRY UPDATES
p.21 News & Notes
p.29 Ribbon Cutting: ZRG Medical
p.30 AAMI Update
p.32 ECRI Update


P.34 THE BENCH
p.34 Biomed 101
p.37 Tools of the Trade
p.39 MedWrench Shop Talk
p.40 Webinar Wednesday
P.42 FEATURE ARTICLES
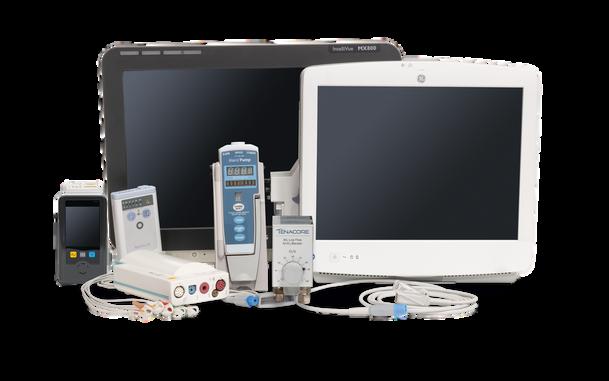
p.42 Roundtable: Patient Monitors
p.48 Cover Story: A Call to Action: Expanding the Right to Repair Beyond Third-Party Initiatives
P.52 EXPERT ADVICE
p.52 Careers Now
p.54 SPONSORED: Soma Tech Intl
p.56 Networking Notes
p.59 The Future
p.60 Right to Repair
p.62 Health-ISAC
PUBLISHER John M. Krieg
VICE PRESIDENT Kristin Leavoy
VICE PRESIDENT Jayme McKelvey OF SALES
EDITORIAL John Wallace
CONTRIBUTORS Roger Bowles
K. Richard Douglas
Jim Fedele
Joie Marhefka
Manny Roman
Steven J. Yelton
ACCOUNT Megan Cabot
EXECUTIVES Emily Hise
ART DEPARTMENT Karlee Gower
Taylor Hayes
Alicia Brown
DIGITAL SERVICES Cindy Galindo
Kennedy Krieg
Haley Harris
EVENTS Kristin Leavoy
WEBINARS Linda Hasluem
HTMJOBS.COM Kristen Register Sydney Krieg
ACCOUNTING Diane Costea
CIRCULATION Joanna Manjarrez

p.65 Biomed Brainbuster
p.66 Time Capsule
p.74 HTM Mixer Lexington Scrapbook
p.68 Preferred Vendors p.70 Service Index
p.73 Alphabetical Index
Dr. Brian Bell, HTM Workshop, Faculty Biomedical Engineering at St. Petersburg College in St. Petersburg, Florida
Rob Bundick, Director HTM & Biomedical Engineering, ProHealth Care
Carol Davis-Smith, CCE, FACCE, AAMIF, Owner/ President of Carol Davis-Smith & Associates, LLC
Jim Fedele, CBET, Senior Director of Clinical Engineering, UPMC
Bryant Hawkins Sr., Site Manager, Children’s Hospital of New Orleans
Benjamin Scoggin, MBA, MMCi, Director, Clinical Engineering | Biomedical Operations, Equipment
Distribution, Clinical IT, DHTS, Duke Health Technology Solutions
Allison Woollford, Biomedical Equipment Specialist at Duke University Health System










Start 2025 Strong with an

Make 2025 the year you invest in your future with a career in Healthcare Technology Management. CBET offers 100% online programs tailored to fit your life, equipping you with the expertise to succeed in this rapidly evolving industry. Whether you're starting fresh or enhancing your current role, our programs empower you to reach new heights.

Whether you're a
professional seeking to enhance your expertise or a military veteran making the transition to a civilian career, our VR labs bring real-world scenarios to life, allowing you to practice and perfect your skills in a controlled, risk-free environment. With CBET, you’re not just earning a certification—you’re gaining a competitive edge that will propel your career to new heights.


BY K. RICHARD DOUGLAS
The nomination for this month’s Department of the Month stated that the department is “a paragon of operational excellence.”
The nomination went on to say that the department had improved several “key performance indicators (KPI) that the shop and facility were monitoring.”
“The KPI metrics included improving customer satisfaction, preventative maintenance completion rates and reducing the turnaround time for corrective maintenance. The shop was able achieve a 100 percent overall composite score, making them one of the top VHA HTM departments in the nation. Meeting these KPIs ensures the safety, reliability, security and availability of medical equipment and medical systems across the Veterans Health Administration,” the nomination stated.
The department that garnered this impressive praise is the Durham VA Healthcare Technology Management Department.
“The Healthcare Technology Management Department at
the Durham Veterans Healthcare Administration supports a comprehensive medical center supporting 151 operating beds and a 100-bed, five-star rated community living center, managing a wide range of specialized medical modalities including dialysis, laboratory services, pharmacy, surgery, patient monitoring, radiology [and] dental care,” says Bob Gunshefski, imaging equipment support specialist.
He says that the HTM team is made up of a total of 21 positions, structured with an HTM chief, biomedical engineering supervisor, two biomedical engineers, two imaging biomedical equipment support specialists, two biomedical information systems biomedical equipment support specialists, 12 biomedical equipment support specialists and a facility service assistant.
“Some key roles like the HTM chief, biomedical engineering supervisor and a biomedical engineer are currently vacant. In addition to their primary responsibilities, the team supports 11 off-site clinics across multiple counties, ensuring that their expertise reaches a wide geographic area. Many staff members are former military personnel, or have family members who are veterans, adding a unique depth of experience and dedication to the department. This background helps the team uphold their responsibility as efficient





stewards of government resources, taking pride in their role of taking care of veterans,” Gunshefski says.
The department utilizes the (VA) CMMS system known as VISTA (Veterans Health Information System Technology Architecture) to document work orders, manage equipment inventory and track the entire life cycle of medical devices.
“The HTM department leverages the power of Power BI to monitor and manage key performance indicators (KPIs) effectively. By utilizing this advanced data analytics tool, the VA can aggregate, visualize and interpret vast amounts of data in real-time. The department has a dedicated Power BI dashboard to provide the biomedical engineering staff, as well as leadership, the ability to review various operational metrics, enabling the ability to track performance, identify trends and make data-driven decisions,” Gunshefski says.
He says that the department works closely with its IT colleagues to support the Vas Medical Device Protection Program, which is a comprehensive security initiative intended to better safeguard networked medical devices.
“The Durham HTM department integrates the Medical Device Protection Program throughout the entire life cycle of medical devices. During the procurement process, HTM reviews all equipment proposals to ensure that only devices with updated operating systems and robust encryption methods for data-at-rest and data-in-transit are purchased,” Gunshefski says.
He says that this ensures that procured devices comply with medical and technology requirements.
“Post-procurement, HTM assists with the implementation and deployment phases by securing Virtual Local Area Networks (VLANS), applying Access Control Lists (ACLs) to network interfaces, and integrating the modalities with the EHMR records. This exemplifies the seamless integration of HTM with IT in the hospital,” Gunshefski adds.
The team has kept busy with projects and has been successful at addressing a potential problem in a resourceful manner.
“Noteworthy projects include supporting the new cancer center to house two linear accelerators and a CT, installation of a new hybrid OR with integrated fluoroscopy system and the implementation of a new Wake County clinic. Moreover, the

department has been instrumental in managing the Cherry Point Marine Corps Air Station Clinic,” Gunshefski says.
He says that the Cherry Point Marine Corps Air Station Clinic is the first VA clinic for Durham VAMC that has been located on Department of Defense property.
“Located approximately 160 miles from Durham’s main campus. The Veteran Patient Aligned Care Team (PACT) clinic poses specific problems with such distance. Remote interfacing with/among DOD personnel and multiple Durham clinical and engineering sections utilizing TEAMS for communication, sharing documentation and floor plans and even employing video chat walkthroughs,” Gunshefski says.
The team has also proven its mettle to solve problems.
“In the biomedical engineering department of the Durham VA Healthcare System, a team faced a critical challenge while planning the installation of telemetry cabling for real-time patient monitoring. The original plan involved routing the cables through existing conduits, but these were unexpectedly congested with old wiring and utilities, and an even more concerning issue arose: asbestos was found in the conduit space,” Gunshefski says.
He says that recognizing the need for an alternative solution to avoid delays and potential health hazards, the team proposed routing the new cables along the hallways’ ceilings, concealed by decorative molding. This approach bypassed the congested and hazardous conduits and allowed for easier access and maintenance.
“By identifying this alternative route, the team provided the contractor with the capability to develop a new design for implementation. This new plan not only mitigated the risks associated with the original conduits but also ensured that the installation could proceed without significant disruptions. The administration praised the team’s ingenuity and adaptability, seeing the revised design as a promising step forward. While the project has not yet been implemented, the groundwork laid by the team demonstrates a proactive and innovative approach to problem-solving in biomedical engineering,” Gunshefski adds.
When any group of employees can be described as “a paragon of operational excellence,” there is nothing more to say. The HTM team at the VA Durham is setting the standard.

BY K. RICHARD DOUGLAS
MercyHealth Javon Bea Hospital, in Rockford, Illinois, is a 563,000-square-foot, 194-bed hospital with advanced operating and surgical suites. The facility includes comprehensive cardiac, vascular, peripheral and neurovascular interventional laboratory and a rooftop healing garden.
The hospital’s HTM department includes Tech 3 Jake Calhoun. Calhoun garnered an extensive amount of electronics experience before entering the HTM field.
“I saw an ad for an open position in biomed at the hospital, after working 17 years on component level repairs. I
knew it would be a lot of testing of equipment, verification of operations than actual component level repair,” he says.
Through his work in other industries, Calhoun had obtained substantial on-the-job training and experience.
“I was an electronics technician for an automatic door rebuild facility for 11 years started in 2003, then repaired, built and assembled conveyor belt electronic scales for six years. I started as a biomedical technician I in 2020 and am now a biomed tech 3,” he says.
Calhoun’s areas of expertise include OEM-training on patient lifts, medical lasers, ultrasound, X-ray and fluoro-radiology equipment. He attended Gadsden State Community College with additional training through GE, RSTI, DirectMed and Laser Training Institute.
Calhoun’s involvement over the years working with electronics has presented several challenges and has
contributed to his ability to be resourceful.
“The biggest challenge in working on electronics was engineering changing software that required board modifications. Initially we had to hand solder half inch wires to .5mm spacing pins on a processor that took half hour per board. I was able to work with a company that made preformed traces that allowed this process to be two minutes to modify,” he says.
“With the help of the software sales technician, we were able to build an automated testing system for the belt scales, reducing the time from half hour to five minutes and allowed zero human errors,” Calhoun adds.
He says that this saved the company $90,000 for just the automated testing system and increased production.
“In the medical world, it’s finding exact components for equipment to save thousands of dollars when needed. Recently, just found the exact buck boost chip for six video converters that has saved the company $12,000,” Calhoun says.
Electronics has always been in Calhoun’s blood. In high school, he won second-place in the Alabama State Skills USA competition in electronics. While in college in 2003, he received an “outstanding achievement award” trophy.
“I enjoy working on electronics and learning new things about electronic circuits. It started well before taking courses in high school with curiosity of electronics. I took apart things at home, that were broken attempting to repair them. I took two years in high school, learning about the basics of electronic theory, completed my associate degree at Gadsden State Community College, in Gadsden, Alabama.
He says that he has been actively learning about electronics since then.
“I moved states and landed my first job at Addison Automatics Inc. where over 200 different models of electronic door controllers were available to build my troubleshooting skills. I left there to work at a company called Belt-way Scales Inc. It was the first and only OEM job I’ve had,” Calhoun says.
He worked there for six years, calibrating A/D circuits, flashing boot loaders and assembling the electronic boxes.
“From there, I landed a role at Mercyhealth, which has catapulted me into harder and harder equipment. I’ve been able to learn with others and share my learnings with newer technicians. I do greatly enjoy working in the medical field. I also enjoy showing others about electronic repair as well to broaden their knowledge. The biomedical world has opened up many avenues in learning other equipment that most people wouldn’t use day to day. I enjoy working with staff daily in getting solutions to their needs,” Calhoun says.
He says that over the years; to supplement his income, he was able to create a business helping many in his area, as well as globally.
“My YouTube channel has 11.5 million views from people sourcing out repairs for their products. It is a good feeling being able to help out others,” Calhoun says.
Away from work, Calhoun enjoys kayaking and repairing electronics at home.
“I share these repairs on YouTube, helping others throughout the world. I also like to design and assemble my own circuit boards,” he says.
He has been married to his wife, Teresa, for 19 years. Calhoun proves that an accumulated electronics background serves a biomed very well. He is an asset to his employer and the patients who benefit by his knowledge, as well as the YouTube community who learn from his expertise.

FAVORITE BOOK: Anything electronic circuit related
FAVORITE MOVIE: “Predator” or “Pay It Forward”
HIDDEN TALENT:
I can juggle 3 balls and wiggle my ears.
FAVORITE FOOD: Cheese
FAVORITE PART OF BEING A BIOMED?
Making staff’s bad day be better.
Ranjita Shrestha
Ranjita Shrestha holds a Bachelor of Science in Biomedical Engineering from Louisiana Tech University and is pursuing M. Eng in Biomedical Engineering at UConn. She serves as the Chief Biomedical Engineer at Gulf Coast Veterans Health Care System in Biloxi, Mississippi. TechNation recently had an opportunity to get to know this high-achieving HTM professional.
Q: WHERE DID YOU GROW UP?
A: I grew up in Nepal. Moved to the U.S. for higher education and pursued my degree in biomedical engineering.
Q: WHERE DID YOU RECEIVE YOUR HTM TRAINING/ EDUCATION?
A: I graduated from Louisiana Tech University with BSBME in 2014. After that, I started my HTM journey at a third-party medical equipment supplier company, where I got an opportunity to get hands-on technical experience on different types of medical equipment from infusion pumps and bedside monitors to life support equipment like defibrillators and ventilators. Currently, I am employed with VHA, which has a very robust HTM community and great resources on HTM trainings and education.
Q: HOW DID YOU FIRST DISCOVER HTM?
A: I have always been interested in the medical sector, and I wanted to get an engineering degree. I stumbled upon the BME program at LaTech when I was doing a random Google search on engineering degrees in the medical field. Back then biomedical engineering was a relatively new field, and when I learned about the program in Louisiana Tech University, I was immediately fascinated and decided to pursue the program. So, I would say I was destined to become an HTM professional.
Q: HOW DID YOU CHOOSE TO GET INTO THIS FIELD?
A: It was new and interesting at that time, at the same time it


seemed like it would be a challenging and fulfilling career and, indeed, it is.
Q: WHAT DO YOU LIKE MOST ABOUT YOUR POSITION?
A: I like collaborating with different stakeholders and helping them solve issues they are facing in delivering patient care.
Q: WHAT INTERESTS YOU THE MOST ABOUT HTM?
A: There is never a boring day. New things to learn and new challenges to overcome every day.
Q: WHAT HAS BEEN YOUR GREATEST ACCOMPLISHMENT IN YOUR FIELD THUS FAR?
A: With my previous employer, I was able to get a new regional support center ISO 9001:2015 certified with zero non-conformances or findings.
Q: WHAT GOALS DO YOU HAVE FOR YOURSELF IN THE NEXT 5 YEARS?
A: To graduate with master’s degree in biomedical engineering and I would also like to get CCE certification.
FAVORITE HOBBY:
Traveling, hiking, playing with my new pet.
FAVORITE SHOW OR MOVIE: “Madame Secretary”
FAVORITE MEAL: Momo (Nepalese version of Dumplings)
WHAT WOULD YOUR SUPERPOWER BE?
Reading minds
1 THING ON YOUR BUCKET LIST:
Travel to Greece
SOMETHING YOUR CO-WORKERS DON’T KNOW ABOUT YOU: I can speak three languages.

BY K. RICHARD DOUGLAS
Many lives have been saved in developing countries because of the availability of modern medical equipment. Often, this equipment would not be obtainable by the hospitals and health clinics that receive it. Several U.S.-based charitable organizations have restored and provided used medical equipment to developing countries for many years.
One of those organizations is SOS International, founded in 1993 by Dr. Norton Waterman. Waterman realized that unused medical supplies were discarded based on protocol. This resulted in six million pounds of medical waste annually. Why not, instead, provide used medical equipment and unused supplies to those in need?
Since that time, the organization has provided medical needs to health care facilities in 108 countries. The organization depends on volunteers to help accomplish this mission. As a provider of recovered medical equipment, there is a need for biomeds who have the skills to repair and maintain that equipment.
“The company I work for partners with SOS International (and many other volunteer programs). When the organization has a bulk of equipment come in, or is in need of something to go out as soon as possible, they reach out and ask for volunteers,” says Brandon Plank, a biomedical technician
with TRIMEDX in Louisville, Kentucky.
Plank says he was given the opportunity to volunteer at SOS Louisville.
“I took personal time off and spent three days learning how the organization works. I was very humbled to see all the volunteers come together to receive, sort and ship all of the sterile supplies and equipment received. When I arrived, there was equipment ranging from oxygen concentrators, infusion pumps, ultrasounds, defibrillators and ESUs. They asked us to work on two infant warmers that came in and needed repair, which was a stat request due to the severity of need of infant warmers overseas,” Plank says.
He says that it felt great to volunteer his skills to help support those in need.
Plank says that SOS International accepts medical equipment, sterile supplies and other disposable items. His experience highlights why biomeds are needed.
“The volunteers work hard to organize and ship the supplies, but when it comes to the equipment, they need assistance. The biomed is expected to perform a full functional checkout and ensure the device has basic leads/ hoses. The shop has rooms full of patient leads/hoses/cuffs so it wasn’t difficult to locate missing pieces,” he says.
When Plank volunteered, the organization was in particular need of baby warmers.
“The program really opens your eyes to the number of countries in need of basic medical equipment and sterile supplies. When I was first contacted about the opportunity, I was informed the location had two broken baby warmers and those items were in tremendous demand. They were able to be fixed on site and shipped out the next day,” Plank says.

What skills would a biomed need to be able to do this kind of volunteer work?
“The tech should already have a solid understanding of medical equipment basics such as infusion pumps, electrosurgical generators, ventilators and pediatric/neonatal equipment. Being able to approach any medical device and run a basic check out is a must,” Plank says.
He points out that the industry has changed a lot since the generation of equipment that is often donated and requires a biomed to navigate through older menus. “The equipment that comes in is typically quite old and at end of life by OEM, so even those who are familiar with newer medical equipment would have difficulty working on the dated equipment,” Plank points out.
Plank’s career as a biomed began in 2008 when he graduated from Vincennes University with an A.A.S in biomedical electronics.

job in sterile processing at an orthopedic hospital. I figured if I can’t get into the field right away, I can at least learn the OR,” he says.
Plank says that it took two years before a BMET opportunity came along.
“The program really opens your eyes to the number of countries in need of basic medical equipment and sterile supplies. When I was first contacted about the opportunity, I was informed the location had two broken baby warmers and those items were in tremendous demand. They were able to be fixed on site and shipped out the next day.”
“The economy crashed during that time and hiring freezes were across the country. Without a chance for a new technician getting a BMET job before tenured techs, I took a
“The next position was an in-house biomed at a small hospital in Indiana. I worked for a third-party company and that was my assigned site. I stayed there for five years until a field service position opened up in Indianapolis within the same company. I stayed there another three years until I decided I wanted to work for an OEM and see the other side of medical equipment; the manufacturing and engineering,” he says.
He says that he took a position with an OEM for two years until a large hospital group in Indianapolis had a position he really wanted, which is where he is now.
There are very likely two babies thriving in a developing country somewhere today thanks to Plank’s skill set and efforts. This is a worthwhile use of a biomed’s spare time, knowing the contribution makes a big difference.

Our training program at Tri-Imaging transcends traditional education by instilling skills that will resonate throughout your professional journey for years to come. Unlike conventional training experiences focused solely on facts and figures, we prioritize imparting practical, applicable skills.
Our commitment extends to fostering a deep understanding of the intricate inner workings of various devices and machines that our students will service in their careers. Here at Tri-Imaging we believe in nurturing individual growth, and to that end, we provide one-on-one assistance for each student.
Step into the future of education with Tri-Imaging, where our training program transcends boundaries to equip you with skills that stand the test of time.






It’s a staggering fact that 400 million people around the world have no access to basic health care and every two seconds, an adult dies from a noncommunicable disease. Without lifesaving equipment found routinely in most hospitals in the U.S., these diseases often go without detection and end in mortality.
That’s where Colorado-based nonprofit Project C.U.R.E. steps in. Project C.U.R.E. is the largest distributor of donated medical equipment to communities in 135 countries, including underserved hospitals in the U.S. In the wake of poverty, natural disasters, war and violence and other environments that foster health care inequality, Project C.U.R.E. shows up to provide life-saving medical equipment to ensure doctors have the resources they need to properly treat the patients they see.
Much of the equipment Project C.U.R.E. ultimately sends on to hospitals comes in as donated, second-hand devices that need repairs. Trained technicians are needed to breathe life back into this highly technical medical equipment.
Earlier this month, a group of Crothall Healthcare Technology Solutions (HTS) technicians were onsite at Project C.U.R.E.’s Woodbridge, Ill. warehouse, volunteering their time to repair the donated equipment. Using their own tools and testing equipment, the Crothall HTS team repaired 150 medical
devices – including EKGs, ECGs, patient monitors, defibrillators, infant warmers, infant scales, anesthesia machines and ventilators.
“Some of the donated equipment had been on-site for months, awaiting repair. The Crothall HTS team was able to get the equipment into peak working order so that it can be used at its next destination to save lives,” said Tajwar Khan, regional director of operations for Crothall HTS. “This volunteer opportunity is a natural extension of what we as HTS technicians have committed our professional lives to do – support the cause of saving lives.”
The volunteers consisted of HTS associates who work at Methodist Hospitals in Gary and Merrillville, Indiana; Marion General Hospital, Marion, Indiana, and Lurie Children’s Hospital and the Shirley Ryan AbilityLab in Chicago.
A group of 14 Crothall HTS technicians also volunteered last year with Project C.U.R.E. in lieu of doing a usual holiday dinner. At that time, 50 medical devices were serviced. The volunteer event so successful, that the team has decided to make it an annual opportunity. Crothall HTS also works with its partner hospitals to donate their unused medical equipment to Project C.U.R.E.
The 2025 HTMA-OH Conference & Expo is set for February 6-7 at Renaissance Columbus Westerville Hotel (409 Altair Parkway Westerville, OH 43082). The conference features a leadership seminar, a Top Golf networking event, top-notch educational sessions, lunch buffet, keynote address and an exhibit hall filled with companies who can help HTM professionals do their job better.
The Ohio Clinical Engineering Association started in 1974 as a group of hospital medical equipment engineers who were a part of a joint Safety Committee group of the Greater Cleveland Hospital Association. These engineers had a disagreement with the City of Cleveland over electrical testing laboratory requirements for medical equipment that was in use in the city. After this disagreement was resolved, these engineers continued to meet on a regular basis and the organization Clinical Engineering Technology Association (CETA) was formed.
In 1980, the words “North Coast” were added to the name to designate the geographic area of Northern Ohio near Lake Erie and NCCETA was created.
Hospital Corpsman 1st Class Kirk Ashley Young from Naval Medical Research Unit (NAMRU) EURAFCENT was announced as the Navy Medicine Research and Development (NMR&D) 2024 Sailor of the Year (SOY), in October. Young, the command’s leading petty officer, was selected amongst the eight NMR&D commands.
“This is a big accomplishment for the command,” said Young. “It is very rewarding and motivating that all our hard work is recognized by our leaders.”
“NAMRU EURAFCENT is one of the best commands I have ever worked at. Military and civilians, they are the most dedicated, hardworking, and most approachable people. I am very grateful for my command for giving me the opportunity to learn, to fail, and to be better,” he added.
Young, who is stationed at NAMRU EURAFCENT’s Sigonella, Italy-based headquarters is a biomedical equipment technician and a command equipment manager. He is responsible for managing medical equipment and ensuring operational readiness at NAMRU EURAFCENT and its remote lab sites, which support missions across 22 countries in Africa Command (AFRICOM), Central Command (CENTCOM), and European Command (EUCOM) areas of responsibility.
“This is a fantastic recognition of both his character and competence, as well as the achievements that he has been able to accomplish as part of this team,” said Capt. Virginia Blackman, commanding officer, NAMRU EURAFCENT.
“Thanks not only to HM1 [Young] for his consistent excellence in performance, but also to Chief [Gene] Nuevo for
In the 1990s other organizations came into existence in Ohio. There was the Toledo Biomed Association, the Central Ohio Medical Equipment Technicians Association, and the Dayton Biomed Association. The Ohio Clinical Engineering Association is a combination of all of these Ohio associations and we are still in the process of collecting information about these older groups. HTMA-OH is seeking help with this collecting process so if you have further details about these early organizations please email info@htma-oh.org.
In 2002, after being dormant for a few years, NCCETA was brought back into existence. In 2003, the decision was made by the 2003 Operations Committee to make the organization statewide and the new name Ohio Clinical Engineering Association (OCEA) as the acronym was selected for the new and larger organization.
Today, HTMA-OH carries on the missions of its predecessors.
Find more information and register today at htma-oh.org/expo.

mentoring and guiding him, to Ms. Marian Mikhail for her partnership and collaboration on equipment maintenance and management, to Lt. Cmdr. [Robert] Hontz for facilitating his participation in the board from Ghana, and to all who have helped him achieve,” added Blackman.
As part of other recognitions, Young was also named Sailor of the Quarter (2nd Quarter) in 2021 while stationed at U.S. Navy Medicine Readiness and Training Command Guam, formerly known as the U.S. Naval Hospital Guam.
The “Sailor of the Year” is a time-honored tradition introduced by Chief of Naval Operations, Adm. Elmo Zumwalt and Master Chief Petty Officer of the Navy John Whittet in 1972. This annual competition is held to recognize the superior performance of individual Sailors, who excel in personifying the ideals of the Navy Core Values.
Recently, the U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) announced a communications pilot to enhance the medical device recall program.
This pilot is intended to improve the timeliness of communications about corrective actions being taken by companies that the FDA believes are likely to be the most serious type of recalls, but where the FDA may not yet have determined that the actions meet the regulatory definition of a recall. These actions may include when companies remove products from the market, correct products, or update instructions for using products due to potentially high safety risks.
This effort aims to increase transparency and minimize the time between the FDA’s initial awareness of and public communication of potentially high-risk medical device removals
The InterMed Group recently announced several leadership positions. Stacy Williams is the CFO. Todd Hilehoffer is senior vice president of technology and Michelle Williamson is vice president of client success. Also, BJ Cumbie recently posted on LinkedIn “I’m happy to share that I’m starting a new position as Manager2 at The InterMed Group!”
Stacy Williams is a results-driven finance leader with extensive experience in operational and financial management. She excels in improving financial performance, streamlining acquisitions, and optimizing processes. Most recently, she was COO for a national pathology lab services provider. Previously, she held leadership roles as both COO and CFO and began her career in public accounting, gaining experience in audit, tax, and support services. Known for her collaborative leadership, she motivates teams to achieve organizational goals. Williams holds a BS in Accounting from Pennsylvania State University and is a Certified Public Accountant. She resides in Pennsylvania with her husband Tim and son Timmy.
Todd Hilehoffer has over 25 years of IT experience, with a background in software development and leadership. He began as a software support specialist and progressed into roles such as Solution Architect and IT team leader. Before joining InterMed, Hilehoffer was a Solution Architect at FM Global, where he contributed to the digital transformation of a multi-billion-dollar enterprise. With expertise in lean product development, Hilehoffer leads teams to deliver Minimum Viable Products (MVPs) that add customer value. His leadership fosters collaboration and continuous
or corrections, providing more timely communication to consumers and health care providers. This pilot follows the commitments noted in our 2024 Safety Report and recommendations from the Patient Engagement Advisory Committee, to enhance our medical device recall program.
Specifically, the pilot will provide early alerts of potentially high-risk device removals or corrections removals or corrections related to: Cardiovascular, Gastrorenal, General hospital, Obstetrics and gynecology, and Urology.
There is no change to any other recall process or recall communication timelines for other areas at this time.
This pilot demonstrates the important role of reflecting patient input in our regulatory efforts. The FDA takes seriously our role in communicating both the benefits and risks of medical devices, to support an informed public and strong health care system.


improvement, helping companies harness technology for growth. Todd holds a BA in Sociology from the University of Richmond. Hilehoffer currently lives in Pennsburg, PA, with his wife Laura and two children, George and Kate, and their dog Coco.
Michelle Williamson is a service-oriented leader with over 11 years of experience in healthcare technology management. She has held various leadership roles in both field operations and corporate settings, excelling in operational oversight, implementation, and organizational growth. Most recently, Williamson served as Network Director for a large academic health system, where she standardized operations across multiple sites and led a team of over 85 associates. Williamson holds a BS in Biomedical Engineering from Marquette University and an MBA from UNC Kenan-Flagler Business School. Michelle currently resides in Chapel Hill, NC.
In its continued effort to promote and applaud the men and women of healthcare technology management (HTM), TechNation magazine launched the Tech Choice Awards. The Tech Choice Awards (also known as the Wrenchies) shine a spotlight on the heroes of HTM. Finalists for the awards were selected during a month-long nomination process.
The Tech Choice Award categories are:
• Professional of the Year – sponsored by 626 Holdings
• Department of the Year – sponsored by USOC Medical
• Young Professional of the Year – sponsored by Prescott’s
• ISO Employee of the Year – sponsored by MedWrench
• Director/Manager of the Year – sponsored by MW Imaging
• Lifetime Achievement Award – sponsored by Renovo Solutions
• Military BMET of the Year – sponsored by College of Biomedical Technology
• Educator of the Year – sponsored by Maull Biomedical
• Humanitarian Award – sponsored by Pronk Technologies
• Women in Leadership Award –sponsored by Avante Health Solutions
• Vendor of the Year
• Association of the Year – sponsored by MultiMedical Systems
• Industry Influencer of the Year –sponsored by Soma Tech Intl
Visit 1technation.com/ techchoiceawards to vote for your favorites. Voting ends January 10, 2025.


Welcome to GMED ONE, where we have been setting the gold standard in biomedical repair services since 2011. As a leader in the field, we pride ourselves on our reputation for excellence, trust, and unparalleled service.
Surgical Equipment:
• Rigid Scopes
• Flexible Scopes
• Cameras
• Instruments
• Ortho Power
• Fiber Optic Cables
Biomedical Equipment:
• Ultrasound Probes/TEE
• Patient Monitors
• MRI Coils
• On-Site QA Probe Testing
Our mission is to provide fast, reliable repairs on a wide range of medical equipment, ensuring that healthcare professionals can continue to deliver the highest quality care to their patients.

The 2024 HTM Mixer in Lexington, Kentucky, was a success! More than 300 people attended the HTM Mixer Lexington, proudly supported by KAMI, November 15-16 at the Marriott Resort Griffin Gate.
The HTM Mixer featured networking opportunities, ACI-approved educational sessions, an exhibit hall filled with industry-leading solutions providers and more!
Mixer offered everything an MD Expo offers HTM professionals just on a smaller scale.
It is a hybrid event that combines the best of a state association annual meeting and an MD Expo.
MD Publishing President John Krieg was excited to partner with KAMI to offer this educational and networking event to the HTM community.
KAMI President Tom Bledsoe said, “It was a total success, and we are so proud of what was accomplished.”
“We were overwhelmed with the attendance numbers and had great success electing our new BOD (Board of Directors),” he added. “It surpassed our expectations, and I believe the social media attention helped us round the corner and exceed the expected numbers.”
“KAMI would like to extend a heartfelt ‘Thank You’ to the MD Publishing team for helping us put on a wonderful event. What a way for the sitting BOD to end on such a high note. And a great way to welcome new membership and a new BOD. We are still getting positive feedback from all vendors and attendees,” he said.
Harrison County Hospital BMET Earl Morris Jr. is one of many attendees impressed by the two-day conference.
TechNation magazine, along with MD Publishing, announces exciting conferences for healthcare technology management (HTM) professionals in 2025.
HTM professionals from throughout the United States will have ample opportunities to continue their education, network and meet with the industry’s leading companies at two MD Expos and two HTM Mixers this year. Imaging service engineers will also want to check out the Imaging Conference & Expo (ICE) set for next month in sunny Orlando, Fla. Find out more at AttendICE.com.
The spring MD Expo is set for Southern California beginning April 15. The fall MD Expo kicks off Nov. 10 in Dallas, Texas.
MD Expo strives to provide HTM pros a one-of-a-kind intimate and rewarding conference. Clinical engineers, biomedical technicians, directors, managers, procurement/asset managers and others responsible for medical technology are invited to come together in a warm and welcoming environment to earn continuing education credits, network with peers and visit with the top HTM
“The HTM Mixer was great,” Morris said. “It was everything you could imagine how an event could go. There was bourbon, horses, great conversations, and great synergy. Lots of great people with the same mindset of moving the HTM world further than it has ever been.”
“The highlight of the mixer for myself would be that I was elected to the KAMI Board of Directors,” he added.
When asked how the HTM Mixer compares to other HTM conferences Morris said, “I think the HTM Mixer really gives off an intimate setting versus the bigger events, what I mean by that is you can have more of a one-on-one with the vendors or other HTM professionals. I know from the bigger events I have been to it is almost overwhelming versus the Mixers.”
Tarik Zecevic, a director of clinical engineering with Crothall Healthcare, described the HTM Mixer with one word, “Amazing.”
He added that “everything and everyone was professional.” Indiana Army National Guard Recruiter Clarissa Walls said the HTM Mixer was “Lots of fun, very easy to network in a relaxing atmosphere.” She said that she “would 100% go again.”
“Each session I attended was illuminating because I am completely new to the industry,” Peter Hrabak said.
Landon Dale Orr, a clinical systems engineer with Renovo Solutions LLC at UK Health Care described the HTM Mixer as follows, “A wonderful experience for HTM members and vendors.”
“I am excited for the next HTM Mixer,” he added.
companies – all under one roof!
Find out what everyone has been talking about; this is one event you can’t afford to miss!
For the latest details, and to register, visit MDExpoShow.com.
The HTM Mixers are small versions of an MD Expo with elite networking and educational opportunities supported by state HTM associations.
The HTM Mixer Denver is set for May 15-16. It is proudly supported by the Colorado Association of Biomedical Equipment Technicians (CABMET).
HTM Mixer Milwaukee kicks off July 31 in Milwaukee. It is backed by the Wisconsin Biomedical Association (WBA).
Find out more at HTMmixer.com.











JANUARY 22
SPONSORED BY

TOOLS OF THE TRADE
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
FEBRUARY 5
SPONSORED BY

TOOLS OF THE TRADE
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
FEBRUARY 12
SPONSORED BY
WEBINAR WEDNESDAY
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.


All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.
SPONSORED BY
“The Key to Growth in the HTM Industry and MMS sponsored Bringing together Women Leaders in HTM”
SPONSORED BY

“Solve the 6 Toughest Problems in HTM + HFM – The CMMS in 2025”
SPONSORED BY

“How AI location intelligence can aid PAR level automation and Equipment Distribution with RTLS”
SPONSORED BY
TOOLS OF THE TRADE “Pronk Mobilize Wireless Solution – Powering Service with User-defined Procedures at Your Fingertips”
SPONSORED BY

“Going beyond “Where’s my thing?” Asset Management that increases utilization and automates PAR”

ZRG Medical has been built on asset disposition and recycling services as well as used and refurbished equipment sales.
“Over the last year, we have developed and solidified relationships with OEM manufacturers and other suppliers to expand our product offerings,”
ZRG Medical eCommerce & Marketing Coordinator Dylan Wade explains. “We are now able to offer OEM parts, systems and service contracts from over 200 manufacturers. These manufacturers include companies like Zoll, Oakworks, Mindray, GE Healthcare, Philips, Siemens, Canon, Fuji, Konica Minolta and many more.”
“We have rebranded ourselves with a new updated logo and a completely revamped website,” Wade adds. “ZRG Medical values our relationships with health care partners and will continue to remove decommissioned medical equipment for them, test and refurbish medical equipment, and sell quality pre-owned medical equipment. We are very excited about the addition of products and services and are looking forward to our business evolving even further.”
TechNation found out more about ZRG Medical via a Q&A session with Wade.
Q: HOW DOES YOUR COMPANY STAND OUT IN THE MEDICAL EQUIPMENT FIELD?
A: ZRG Medical offers a full end-to-end solution for our customers. We work with our customers to get them the
exact right equipment for their capital purchases as well as removing, purchasing and refurbishing their old equipment. Customers no longer need to seek out multiple companies to purchase the parts and equipment they need. In addition, customers can come to us when services are needed on their existing equipment, whether it’s still on warranty or not. We work with our suppliers to give our customers piece of mind when purchasing.
Q: WHAT IS ON THE HORIZON FOR YOUR COMPANY?
A: We are continuing to add manufactures and suppliers to our already lengthy list. Our goal is to be the go-to solution for all types of health care facilities in the Southwest. Our sights are set on providing this solution country-wide.
Q: IS THERE ANYTHING ELSE YOU WOULD LIKE OUR READERS TO KNOW?
A: We are a certified woman-owned small business. Our CEO and owner, Mimi Lively, helped create the biomedical engineering program at MiraCosta College. Our team is very involved in the HTM community. Our President, Tony Lively, has been an active member of the California Medical Instrumentation Association (CMIA) for years. Our involvement gives us a unique insight into the pain points that BMETs encounter on a day-to-day basis. With this understanding, we can build out our company offerings to meet the needs of the BMET community.
For more information, visit ZRGMedical.com.

AAMI and the College of Biomedical Equipment Technology have exciting news regarding our ongoing collaboration with HOSA – Future Health Professionals. We are in the process of establishing a national program that will teach high school students concrete healthcare technology management (HTM) skills.
Following their promotion of the HTM field by a poster campaign, AAMI, the College of Biomedical Equipment Technology, and HOSA are working together to build a national HTM educational track for students. The forthcoming educational track is the second part of a nationwide campaign meant to inspire students to explore high-demand career opportunities in HTM. Once launched, it will expose students across the country to professional opportunities in the HTM field. AAMI’s hope is that this initiative yields a new generation of dedicated, involved HTM professionals and spreads awareness about the field in general.
The program will be piloted during the 2026-2027 academic year and will be structured like a science bowl competition. Students will gain concrete skills and knowledge applicable to the HTM field. The curriculum will combine AAMI’s BMET 101 course with online, interactive VR training modules from the College of Biomedical Equipment Technology, where students will learn to troubleshoot an infusion pump. Each student will be provided with a step-by-step procedure for troubleshooting the pump, which they must learn and practice.
All HOSA students who choose the HTM track at their high school will follow this exact curriculum. Students who excel in the program will advance to HOSA competitions at the regional and state levels, showcasing their HTM knowledge and skills.
In the competition, Round 1 will feature a written exam based on BMET 101 content to assess their HTM knowledge. High-scoring students will advance to Round 2, where they will demonstrate hands-on skills by troubleshooting a VR-simulated infusion pump. Top-performing students will qualify to compete in the HTM track at the HOSA National Competition. According to AAMI Vice President of HTM Danielle McGeary, this collaboration is the perfect match of existing organizational capabilities. She said, “The College of Biomedical Equipment Technology and AAMI are coming together to provide crucial HTM information to students, and HOSA has the capability to build educational tracks with an emphasis on hands-on learning. This collaboration is sure to provide information on HTM careers to students all around the U.S.” Richard L. “Monty” Gonzales, Ed. D., President of the College of Biomedical Equipment Technology, agreed, stating, “The HOSA-Future Health Professionals is a global program designed to inspire high school students considering careers in healthcare. The establishment of an HTM career path competitive program, jointly sponsored by AAMI and the College of Biomedical Equipment Technology, directly addresses the pressing national shortage of qualified technicians in healthcare. By fostering interest in STEM fields among high school students, this initiative aims to develop the skilled workforce necessary to meet critical industry needs.”
A Call to Action: AAMI and the College of Biomedical Equipment Technology will need the field’s help when the HOSA HTM track is fully established. For this track to be a success, HOSA relies on volunteers to help judge the student’s skills during these regional and state level competitions. As the HTM track is rolled out nationally, we hope that you will consider getting involved!
Stay tuned for future updates from AAMI regarding the program! If you have questions about program details or how to get involved, please contact htm@aami.org.








In 2023, health care facilities saw a shift from advisory best practices in water quality for medical device processing to enforceable standards with the release of AAMI ST108:2023. This new standard is more than just a set of recommendations –it’s a comprehensive guide to managing water quality and steam purity, ensuring patient safety through proper water system design, monitoring, and maintenance. Let’s break down what these new standards mean for health care organizations.
One of the most significant updates in AAMI ST108 is the requirement for health care facilities to establish a multidisciplinary water management team. Why is this important? It ensures that water quality isn’t handled in silos – every aspect of water management benefits from the expertise of individuals from various departments. This team should include:
• Senior organizational leadership
• Facilities and engineering personnel
• Infection prevention and control specialists
• Medical device processing staff
• Clinical engineering professionals
• Surgical suite and procedure room staff
• Water treatment experts By pulling together a team with diverse skills, health care facilities can create a more thorough and effective approach to managing water quality, with each member playing a role in maintaining a safe environment for patient care.
AAMI ST108 introduces updated water quality categories that directly impact the medical device reprocessing workflow:
• Utility Water: This is water from the tap, which might need additional treatment. It’s typically used for tasks like flushing, washing, and the initial rinsing of medical devices.
• Critical Water: This water undergoes extensive treatment to remove microorganisms and contaminants. It’s primarily used for final rinsing and generating steam for sterilization.
• Steam: This is water heated into vapor, used for sterilizing medical devices.
Along with these categories, the standard defines specific water quality parameters such as pH levels, bacteria, endotoxin presence, and ionic contaminants. These guidelines ensure water safety throughout different stages of medical device processing.
Effective water system design is crucial under AAMI ST108. Health care facilities need to tailor their systems to meet the specific demands of their environment. Key factors to consider include:
• The quality of local feedwater
• Treating municipal water to meet the standard for utility water (e.g., through filtration or softening)
• Employing systems like reverse osmosis (RO), deionization (DI), or distillation to produce critical water
Water treatment isn’t just about meeting the basic requirements – proper system design ensures long-term functionality and compliance. Facilities must also validate their systems with operational protocols, ensuring everything works smoothly from day one.
One of the more challenging aspects of AAMI ST108 is the
enhanced focus on ongoing monitoring and testing. Regular checks are not just recommended; they are now required to keep the water quality up to standard. The responsibility primarily falls on engineering and water maintenance teams, but sterile processing staff also need to be aware of the implications when water quality falters.
Contaminated water can lead to serious issues such as:
• Corrosion and staining of medical instruments
• Elevated risk of microbial transmission
Regular testing helps prevent these problems, ensuring that water used in medical device reprocessing is always up to par.
Implementing AAMI ST108 means more than just updating policies – it requires active engagement from all parts of the health care facility. Here are a few next steps:
• Partner with infection preventionists to understand your current water treatment systems.
• Conduct regular laboratory testing to assess water quality for processing.





• Audit and monitor existing water management plans – making adjustments as needed to meet the new standards.
• Educate staff on the updates to ensure compliance. In some cases, facilities may need to invest in infrastructure upgrades, especially if existing water systems don’t meet the new requirements. Not doing so could lead to serious consequences, including failure to pass sterilization tests, or worse, posing health risks to patients due to improperly sterilized equipment.
The new AAMI ST108:2023 standard represents a significant leap forward in health care facility management of water quality for medical device processing. By adopting these standards, facilities can ensure a higher level of patient safety and operational efficiency. The key is to start now – build your multidisciplinary team, assess your systems, and take proactive steps to stay compliant.
For further details and resources, visit ECRI.org.

• MEDICAL DEVICE REPAIR & MAINTENANCE
Onsite and 24x7 on-call services for most medical devices, surgical and lab equipment.
• SHORT AND LONG TERM BIOMEDICAL STAFFING
On-demand BMET/HTM Technician(s) & Manufacture Remediation/Recall Support
• ENDOSCOPE REPAIRS
Repair estimate within 48 hours, quick turnaround time & extensive list of Loaners available at no charge
• INFUSION DEVICE ANNUAL PREVENTATIVE MAINTENANCE
MMS will deploy a team of technicians to your facility to complete Annual Periodic Maintenance on your Infusion Pumps
• EQUIPMENT SALES
All types of medical equipment- new or refubished

BY BOYD CAMPBELL
As someone who attends numerous biomedical conferences, I often hear discussions about how new technologies have improved patient safety. I agree that innovations in medical technology have dramatically enhanced diagnostic capabilities and introduced devices that make health care safer. Over my 37 years in the biomedical field, I’ve witnessed significant shifts – AC defibrillators replaced by lower energy Biphasic units, bouncing ball patient monitors now have arrythmia detection and data storage, and film processors have been replaced by more advanced digital solutions giving the physician instant retrieval and comparison capabilities.
Beyond the marvels of technology, we cannot overlook the crucial role that government regulations have played in enhancing patient safety. Many biomedical technicians in this field recall the landmark Safe Medical Device Act (SMDA) of 1990, which mandated that medical device users report serious injuries and deaths to the FDA and manufac-
turers. The Patient Safety and Quality Improvement Act of 2005 took things a step further, encouraging broader reporting of patient safety incidents. Alongside these regulations, organizations like AAMI, ANSI, ECRI, JCAHO, and DNV have set standards that reflect the ever-changing landscape of health care.
Yet, while we often hear about the role of new technologies and regulation in making patient care safer, we rarely give enough credit to the professionals working behind the scenes – biomedical technicians. In my experience, our primary role has always been to ensure the safety of both patients and staff when it comes to medical devices. Though we may not have been present when laws like the SMDA were drafted, it is often biomedical professionals who identify trends, report issues, and enforce compliance, sometimes having to be the enforcer.
Without us standing our ground would all these improvements be made. I recall a technician that worked for us informed a customer that a new O2/NO2 mixer failed. It was replaced and the second failed in the same manner. Once again it was replaced and the third one failed as well. At this point the physician was sure that the technician was obviously the problem. How could three brand new devices all be defective? After an uncomfortable discussion with the physician and the manufacturer, reviewing his testing
methods many times, the manufacturer determined that their testing methods were flawed, and a recall was issued for all the units already in the field. Why did this safety issue get resolved? It was due to one technician who stood his ground under the scrutiny of both the health care provider and manufacturer.
Let’s think for a moment about what happens when we do not have the proper enforcement of regulations. Under 21 CFR certain manufacturers are required to provide complete service manuals with calibration and adjustment procedures. This is a battle that many of us have faced when requesting these and being told they are proprietary and will not be provided. While this is clearly written in the Code of Federal Regulation, without enforcement what is motivation for compliance?
In addition, as biomedical technicians we have demanded improvements in test devices to keep up with the changes in technology. When Biphasic defibrillators first hit the market all we had were defib analyzers that were designed for monophasic units. These analyzers gave readings that were close, but we required that changes be made in order to make sure what we were measuring was absolutely correct. Another improvement that we demanded are test devices that are more efficient with the use of automation which still is being improved upon today.
While we view ourselves in the present day as making health care safer, let’s not forget the contribution of those that have gone before us and accept the challenge to be the change that takes medical device safety into the future. How do we do this?
The first step is to cultivate a mindset focused on continuous improvement. This mindset is essential for health care as a whole. Many companies, especially those striving for ISO certification, must document and demonstrate continuous improvement. However, simply continuing to do what has always been done is insufficient in an ever-evolving industry like health care.
We, as biomedical professionals, must actively look for areas where processes can be improved. A key part of this is clear communication. As technicians, we cannot be afraid to speak up when we notice risks or identify potential areas for improvement. Speaking up may not only involve identifying problems but also asking questions to gain a deeper understanding of processes and devices. Fear of asking questions should never be a barrier when the purpose is greater understanding because this curiosity is what ultimately leads to improvements.
Another vital aspect of improvement is offering input. While one person may not have all the answers, sharing ideas and suggestions can lead to significant breakthroughs. Often, the best solutions arise from collaborative efforts. When a few people contribute different perspectives or ideas, those contributions can evolve into a practical solution that benefits the entire organization or field as a whole.

Boyd S. Campbell, CBET, CRES, CHTM, is co-owner of Southeastern Biomedical Associates Inc.

Alarms Errors
Calibration












JAN. 22 at 2PM ET
Registration on WebinarWednesday.live. Eligible for 1 CE credit from the ACI.
Claroty xDome is the industry’s leading health care cyber-physical systems protection platform – enabling health care organizations to safely deliver connected care while enhancing efficiencies across the clinical environment. Claroty xDome spans the entire health care cybersecurity journey regardless of the scale or maturity of your environment through asset inventory, exposure management, network protection, threat detection and operational efficiency.
As a modular solution, Claroty xDome is suited for organizations at any stage in their health care cybersecurity journey, regardless of their scale, staffing, or program maturity. The solution consists of platform essentials, offering foundational capabilities across all core areas mentioned above, as well as advanced modules that provide increased value and enhanced programmatic capabilities.
For more information, visit claroty.com.












Objectively develop a systematic, multi-year capital budget replacement plan with help from ECRIʼs expert team on your timetable that meets strategic goals, controls maintenance costs, and minimizes risk.
Uncover the hidden costs of aging technology
Mitigate chances of sudden device failure


Identify potential hazards with existing devices
Learn more at home.ecri.org/predictive-replacement-planning or contact us today at 610-825-6000, x5891.



MedWrench is a product focused support network where medical professionals, purchasing administrators, manufacturers, dealers and industry experts can provide opinions, share ideas, and gather relevant information on medical technology and equipment. The following are examples of how the MedWrench community members help each other in the website’s forums.
Q: MY CANDELA GENTLE YAG DEVICE FAILED TO HEAT UP TODAY. I CHECKED THE INPUT VOLTAGE OF THE HEATING ROD AND IT IS AC 175V (IT WAS 220V BEFORE). WHAT IS THE REASON? IS IT THE AC DISTRIBUTION BOARD THAT IS FAULTY? DOES IT NEED TO BE REPLACED AS A WHOLE?
A: Please check the fuse on the AC distribution board, also verify the fuse holder to ensure it has not failed. this is a common problem
Q: MY TOSHIBA APLIO TESTS POSITIVE TO PSA-N FE LED (POWER SUPPLY ISSUE) IT LOOKS LIKE THE PROTECTORS CUT OFF POWER FROM THE MAIN CIRCUIT TRIGGERING AN ABRUPT SHUTDOWN ANY ONE HAS A CONCISE SOLUTION TO THIS PROBLEM?
A: The PSA-N FE LED issue on the Toshiba Aplio 500 ultrasound system is typically related to a power supply malfunction, as indicated by the LED alert. This specific issue suggests that the power supply unit’s protectors are cutting off power due to a fault, causing an abrupt shutdown of the machine. Here’s a concise troubleshooting approach:
Steps to Resolve the PSA-N FE LED Power Issue:
1. Power Supply Check:
First, ensure the main power supply to the unit is stable and within the proper voltage range (typically 100-240V, 50/60 Hz).
Verify that the facility’s power supply isn’t experiencing voltage fluctuations or surges, as this can trigger the protective circuits.
2. Inspect the Power Supply Unit (PSU):
Open the system casing and inspect the PSA-N power supply board.
Look for any obvious signs of damage, such as burnt components, blown fuses, or bulging capacitors.

If any components seem damaged, they should be replaced. If you’re not comfortable with board-level repairs, replacing the entire PSU may be necessary.
3. Check Internal Cables and Connections:
Ensure all power connections inside the unit are firmly seated. Loose or intermittent connections can cause power issues and trigger protective cutoffs. Check the connections between the PSA-N and other critical components like the mainboard and the front-end processing unit.
4. Test the Battery Backup (if applicable):
Some Toshiba Aplio models use a small internal battery for backup power. If this battery is depleted or faulty, it could cause power instability. Test and replace it if necessary.
5. Cooling and Ventilation:
Overheating can sometimes cause the PSU to shut down as a protective measure. Make sure the system’s ventilation is clear, and the fans are working correctly.
6. Software and Firmware Update:
In some cases, a power supply issue can be related to firmware bugs. Check if there are any firmware updates available for the Aplio 500 that address power-related issues.
7. Replacement: If none of the above steps work, the PSA-N unit may need to be replaced entirely.
Q: I HAVE NOTICED THE MM/S IS NOT ADJUSTABLE NOR DOES THE GENERAL SETUP SAVE THE CM/MIN ON RESTART. WHAT I FOUND OUT SO FAR IS THE CM/MIN CAN BE SET FROM 1 TO 3 CM/MIN. THE “PAPER ADVANCE” BUTTON IS SET AT 40 CM/MIN. MY GOAL IS TO CHANGE THE DEFAULT SETTINGS TO EITHER 50 MM/S OR 3 CM/MIN WITHOUT HAVING TO CHANGE THE GENERAL SETUP ON RESTART. TRYING TO FIND A SOLUTION FOR THIS GOAL/PROBLEM.
A: Try setting it and then go into Install Options 2 screen and select Store Current to Hospital (assuming the Default Settings is Hospital). That should save the setting.
For more FREE forum information, visit medwrench.com/forums.


All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.
“HOW AI LOCATION INTELLIGENCE CAN ENHANCE PAR LEVEL AUTOMATION AND EQUIPMENT DISTRIBUTION WITH RTLS”
The Cognosos-sponsored Webinar Wednesday session “How AI location intelligence can enhance PAR level automation and Equipment Distribution with RTLS” is eligible for 1 credit from the ACI.
Cognosos Director of Product Marketing-Healthcare Jeff Stiffler gave an overview of how AI can be used for location intelligence in RTLS platforms. Attendees received information on PAR leveling and best practices for transitioning their asset management system from locating equipment to effective equipment distribution.
Key takeways included:
• How AI can be used for location intelligence in RTLS platforms.
• Defining PAR leveling as it pertains to mobile medical equipment in hospitals.
• The pathway of transitioning RTLS asset management system from locating to effective equipment distribution.
Stiffler also answered questions from attendees provided additional insights that HTM professionals can use to do their jobs better.
The webinar received 99 individual registrations with 56 online for the live session. A recording of the webinar is available for on-demand viewing at WebinarWednesday.live.
Attendee Scott Neal, Biomed II with Bryan Health in Nebraska won a $100 Lowes gift card during the webinar!
Attendees were asked, “How did today’s webinar meet your expectations?”
“Better insights into this topic,” said Alejandro Chavez, Manager, CommonSpirit Health.”
“Very good topic and presentation!” shared David Banister, Field Service Engineer, Clinical Engineering Services LLC.
“I like how it expanded the role of RTLS into PAR Levels,” said Andrew Krittofek, Senior BMET, Baylor Scott & White All Saints.
“It was a great presentation, we learned a lot of the new applications of AI, and the most efficient ways to maximize the productivity of the equipment,” said Cesar Ivan Gonzalez Perez, Biomedical Engineer, HAL.
“Excellent introduction to understanding AI location systems,” Corewell Health Biomed Justin Cozadd said.
Attendees were able to learn more about the Tool of the Trade featured in the November issue of TechNation magazine – the FSI Flow Tool –during an information Tools of the Trade Demo webinar on November 6. FSI Solutions Engineering Manager Mike Zimmer shared three PDFs from sponsor FSI. He also answers several submitted questions during the live webinar. A recording of the webinar is available for on-demand viewing at WebinarWednesday.live.
Zimmer shared how to achieve a new level of operational efficiency from a CMMS with the timesaving, error-reducing automation capabilities introduced with FSI’s new Flow tool.
FSI’s Flow tool combines added automation with the configurability that FSI users benefit from throughout the platform. Flow enables users to create custom automations, streamlining common workflows from a lengthy multitude of clicks to one press of a button.
For an additional level of automation, FSI has partnered with Fluke Biomedical to integrate with OneQA workflow automation software, empowering technicians to perform testing using their OneQA equipment and systematically capturing the test results on the PM within FSI’s CMMS – thus streamlining PM completion.
Attendees shared positive feedback regarding the ACI-approved webinar worth 1 CEU. One lucky attendee was Latoya Beard from UAB. She answered a trivia question to win a pair of AirPods as part of Webinar Wednesday’s 10th anniversary!
Attendees were asked, “What was your single biggest takeaway from today’s product demo?”
“Enhanced my experience and knowledge,” said Yousri M.F. Okasha, senior clinical engineer, King Faisal Hospital, Egypt.
“Well thought out integration with automation of testing. Also knowledgeable of TJC requirements. Some vendors don’t have such empathy for our needs,” said Jay W. Hall, owner, Medical Equipment Planning and Solutions.

bcgroupintl.com
TechNation’s Webinar Wednesday series teamed up with BC Group to present the “Patient Sim Testing” session in October. Eligible for 1 credit from the ACI the session featured BC Group Engineering Manager Lucio Simoni.
Simoni shared tips on how to address patient sim testing. He demonstrated how to test a patient monitor using an NIBP-1040 and FSX-1101. He also demonstrated ECG, Respiration, NIBP, IBP, Temperature, Cardiac Output, and SpO2 simulations. Simoni shared his expertise using myBC Mobile.
Simoni also took time to field questions from attendees. He provided thoughtful answers to better share wisdom about testing.
More than 100 people registered for the webinar recording of the session is available for on-demand viewing at WebinarWednesday.live.
Attendees were asked, “What does Webinar Wednesday provide the industry?”
“Up-to-date information about innovations in industry,” shared Rafik Mesropyan, CBET, at Loma Linda University.
“This event provides a great support system for HTM employees to stay active in the industry and the AAMI standards,” ReNew Biomedical Field Services Manager Alec Hadley said.

“SOLVE THE 6 TOUGHEST PROBLEMS IN HTM + HFM – THE CMMS IN 2025”
The Webinar Wednesday session “Solve the 6 Toughest Problems in HTM + HFM – The CMMS in 2025” presented by Margaret Nardini, senior product manager, and Israel Ortiz, senior director of product strategy & innovation for Accruent’s TMS, is eligible for 1 credit from the ACI.
More than 100 individuals registered for the live presentation and a recording of the webinar is available for on-demand viewing at WebinarWednesday.live. A special “thank you” goes out to Accruent for sponsoring the webinar.
The presenters said every workday should include the following:
• Your team is on top of its asset management.
• Work orders are auto-prioritized.
• Equipment is working, about to be working, or being replaced.
• You’re confident in your data (and that it’s protected).
• Your documentation is people-friendly and compliance-oriented.
• Your costs are under control and trending down overall.
The duo from Accruent explained that the company worked backwards from this scenario, and the need to solve these 6 problems first. Then, Accruent’s 30 years of experience in health care led to today’s TMS: a CMMS for how health care works – now and into the future. They also fielded questions from attendees which provided additional expert insights.
Attendees were asked “How much new information did you receive from today’s webinar?”
“I learned about new features and what is being done to ensure information is secure,” said Angele Tanner, an application administrator with Denver Health.
“Interesting to see the TMS transformation,” shared Pronk Senior Field Application Engineer Jeff Goldstein.
One lucky attendee during the live presentation was Biomed/Equipment Distribution Director Jeff Grubb. He won a $100 Lowe’s gift card during the webinar.

The recent Webinar Wednesday session “How Leaders Can Combat Biomed Burnout” was eligible for 1 credit from the ACI.
In today’s climate of more work and fewer workers, leadership needs to address and alleviate biomed burnout within their teams with proven techniques that include teamwork, ownership, and compassion.
GE HealthCare’s Sharon Steeley, lead services specialist, and Colleen Haugen-Ortiz, HTM quality specialist, broke down what burnout is and how to recognize the signs of burnout within oneself, colleagues and team. Attendees gained knowledge on how to apply techniques such as: Implementation Intentions, Habit Stacking, Sharing of Responsibilities, Changing of Scenery, and Putting Meaning Behind Work.
More than 100 HTM professionals registered for the session. Attendees were asked “Excluding CE credits, why do you attend Webinar Wednesday?”
“Acquiring up to date knowledge related to the field that I am interested in,” states Rafik Mesropyan, CBET, Kaiser Permanente.
“To learn from others within the HTM field,” shares Robert Wentworth, vice president biomedical operations, VIKAND Solutions Inc.
“To expand my knowledge,” says Justin Christoffel, director, Renovo.
“Great opportunities to address current HTM issues and best practices,” states Sam Ghannam, clinical engineering supervisor, Kingman Regional Medical Center.
is a customer service based parts provider that specializes in all imaging modalities and manufacturers. Created to offer hospitals and ISO’s a cost effective and time saving solution for ordering imaging replacement parts, equipment moves, ultrasound probe repair and on-site service.
Contact us today, we are confident you will see us as THE Parts Solution!
330.296.9729 diagnostic-solutions.com CONTACT US TODAY!

TechNation kicks off the new year with a roundtable discussion about patient monitors. TechNation Editor John Wallace invited several industry leaders to participate in this month’s informative article.
Participants are:
• Elite Biomedical Solutions’ Glenn Schneider
• Integrity Biomedical Services Owner S. Michele Shahbandeh
• Soma Tech Intl Biomedical Engineer Mousumi Roy Dutta
• Tenacore Biomedical Service Manager Juan Ortiz
• USOC Medical General Manager Thomas Wang
Q: WHAT ARE THE MOST COMMON TYPES OF REPAIRS OR ISSUES YOU ENCOUNTER WITH PATIENT MONITORS, AND HOW DO YOU TYPICALLY ADDRESS THEM?
DUTTA: The most common issues with patient monitors typically fall into several categories. First, inaccurate parameter readings often require calibration. If the problem persists, we address it by replacing parameter
modules, defective boards (e.g., ECG, SPO2, NIBP, IBP, TEMP), or the related cables and sensors. The second main issue is power supply failure, which is diagnosed by checking the power sources and testing components such as fuses, transformers, rectifiers, and diodes to ensure functionality. Third on the list are display problems, including touchscreen calibration, keypad issues, or trim knob malfunctions, which may require repairs or adjustments. Lastly, failures in anesthetic gas modules and recorders are addressed through calibration, fan filter replacement, or recorder maintenance as needed.
ORTIZ: The most common issue we encounter with monitors is failure to boot up. To troubleshoot this, we start by checking the power supply, then the on/off switch, and finally the main board. Another common problem we encounter is the NIBP (non-invasive blood pressure) not reading or not inflating properly. In this case, you may need to replace either the module or the pump, depending on the specific type of monitor.
SCHNEIDER: The most common repairs we encounter are physical damage to transport monitors and
mechanical failures (pumps, etc.) in monitors and physiological modules. We typically replace the defective parts with new or re-certified OEM parts and occasionally use aftermarket parts that meet our rigid quality standards. Once repaired, we run the device through a complete PM as outlined by the OEM.
SHAHBANDEH: We receive patient monitoring equipment with broken screens, components not soldered correctly, damaged boards, and some units that are intermittent. We have the skills and time to reassure the biomed that their units have been running for 24 hours. And, since we are a component-level troubleshooting company, we change the components and not the boards.
WANG: The most common type of repair issues we encounter are related to power, display problems, sensor malfunctions, alarm failures, ECG connectivity, software errors, and physical damages. At USOC Medical, we address these issues by first evaluating the issues, identify potential root causes, implementing the appropriate solutions. The key is to accurately diagnose the issue through careful calibration and testing of the unit followed up with swift, effective repairs to minimize customer downtime.
Q: HOW OFTEN DO PATIENT MONITORS REQUIRE ROUTINE MAINTENANCE, AND WHAT TASKS DO YOU PRIORITIZE DURING SERVICING TO ENSURE OPTIMAL PERFORMANCE AND PATIENT SAFETY?
DUTTA: Patient monitors typically require routine maintenance at least once a year to ensure they remain safe and reliable. During servicing, we prioritize functionality testing, complete performance checks, and preventive maintenance to detect and address potential issues early. The calibration of all critical parameters is essential to guarantee accurate readings and optimal performance. We also focus on replacing wear-and-tear components (such as cables or connectors) and securing all connectors to maintain operational integrity. We conduct electrical safety inspections, including checking for hazards or malfunctions in the power supply, and ensuring proper grounding and surge protection, are critical steps to safeguard the equipment and protect patient data.
ORTIZ: Most monitors require annual maintenance, which includes a full performance test to verify that the device is functioning correctly and meeting OEM specifications. Additionally, we recommend calibrating the NIBP during this service to ensure accurate readings.
SCHNEIDER: From my experience, monitors can be separated into two categories; with physiological inputs or displays. The monitors that have the inputs from the
patient need to be inspected on a routine basis, depending on the hospital’s Medical Equipment Management Plan. Display monitors that receive inputs from other monitors can be on a much less frequent schedule (possibly no schedule) since they just replicate what the physiological monitor is measuring. All configured inputs must be verified as accurate or calibrated to OEM required tolerances. We perform those tests after every repair our technicians perform, as well as an electrical safety test.
SHAHBANDEH: We thoroughly go through each device that is sent in. We do not just repair and send it back without going through the complete unit with QC and burn in time.
WANG: Patient monitors typically require routine maintenance every 6 to 12 months to inspect and calibrate sensors, test the monitor’s alarms, and maintain components to ensure consistent performance. At USOC, we take every step of the repair process seriously from initial inspection, calibration, physical repair, software update, cleaning, and quality control. At USOC Medical, we follow a thorough inspection, repair, and quality control process on all customer units with a first-pass yield of 98% for the year. Our technicians are thoroughly trained to protect our patients’ safety by ensuring all repaired units pass our quality testing before shipping them out to the customers.
Q: HAVE YOU NOTICED ANY PARTICULAR CHALLENGES WITH NEWER MODELS OF PATIENT MONITORS, SUCH AS SOFTWARE RELIABILITY, COMPATIBILITY WITH OTHER DEVICES, OR EASE OF REPAIR?
DUTTA: Newer models of patient monitors often come equipped with advanced software features that significantly benefit health care facilities. However, upgrading to the latest software versions can sometimes present challenges, as it requires careful consideration of compatibility and system requirements. Service manuals are invaluable resources for addressing these challenges, as they provide detailed guidance on installation and troubleshooting. While these advancements enhance functionality, they can also complicate repairs, making technical expertise and manufacturer support crucial for effective maintenance.
ORTIZ: Yes, we notice that some of the newer models have issues with the NIBP readings being high. It appears to be a software problem, as the readings remain elevated even after calibration.
SCHNEIDER: The software on newer models seems to be very reliable. At times, the challenge is software and hardware compatibility conflicts. As the device ages and





updates/modifications are made to them, versions can force incompatibility and require replacement of something that is not defective. For example, if a software update is needed, it may require a PCB to be replaced, even if it operated fine on the previous software version.
SHAHBANDEH: If we need a CPU that has the current software and we don’t have it in stock, we let the customer know and it is their decision to let us do the repair by offering to get it from the OEM and just charge what the OEM would charge us, with the labor to put it in.
WANG: Yes, newer models of patient monitors have challenges such as software glitches, frequent software updates, and more complex software architecture. Compatibility issues can arise with these newer patient monitors despite their advancements in accuracy, connectivity, and functionality. While the improvements on new models can be significant, they also bring higher costs, increased software reliability concerns, and potential compatibility issues. We recommend that health care facilities ensure proper training, establish robust maintenance protocol, and communicate with the OEM and service providers to ensure smooth integration of newer models.
Q: HOW DO YOU HANDLE THE CALIBRATION AND ACCURACY TESTING OF PATIENT MONITORS, AND WHAT STEPS DO YOU TAKE TO ENSURE THAT THE EQUIPMENT PROVIDES RELIABLE READINGS?
DUTTA: Calibration is critical for maintaining the precision and reliability of your monitors. To ensure accurate and reliable readings, we use OEM-certified calibrated simulators. The calibration process involves adjusting and verifying measurement parameters, as well as calibrating sensors and transducers. If the initial calibration does not match the desired result, a recalibration is needed. Furthermore, we meticulously record all calibration data and maintain proper documentation to ensure compliance and facilitate future servicing.
ORTIZ: We follow all calibration steps outlined in corresponding service manuals and use the recommended test equipment. We also ensure that all calibration and test equipment is functioning properly and recalibrated according to manufacturer recommendations in order to maintain accurate readings.
SCHNEIDER: We test every device serviced against the MFR tolerances and all parameters as a minimum. Our ISO certification has traceability requirements on all repaired/ PMed devices as well as test equipment. The technicians are required to document the test equipment used as well as its next due date on each work order. So, verification within the required recalibration period is confirmed.
SHAHBANDEH: Each of our technicians has their own devices that are calibrated and tested each year. We have provided the equipment and training to use each device.
WANG: At USOC Medical, we know reliable and accurate readings are critical for patient care. We carefully review the OEM’s guidelines closely before performing any calibration or testing. We then follow established testing procedures to verify the monitor’s performance against our calibrated equipment to ensure that it provides accurate readings according to a known standard. Additionally, we conduct accuracy testing validation process to ensure that the monitor provides consistent, accurate readings under varying conditions. All of this is supported by thorough documentation and routine maintenance at our facility for process consistency.
Q: WHAT ARE SOME OF THE MOST IMPORTANT CONSIDERATIONS FOR HEALTH CARE FACILITIES WHEN SELECTING A PATIENT MONITOR, PARTICULARLY REGARDING SERVICEABILITY, COST, AND LONG-TERM RELIABILITY?
DUTTA: When selecting a patient monitor, health care facilities should prioritize a balanced cost-to-performance ratio to ensure they receive high value without
compromising quality. Rapid and readily available service options are equally important, as timely maintenance and repairs minimize downtime and ensure continuity of care. Long-term reliability is critical, with consistent performance over time being a key factor in both patient safety and operational efficiency. By considering these elements, facilities can make informed decisions that meet both clinical and budgetary needs.
ORTIZ: We recommend choosing a reputable brand for the particular health care setting in which it will be utilized, ideally a manufacturer that makes their parts available to the end-user for serviceability. Some manufacturers do not offer replacement parts, or make it very difficult to obtain them, which can make repairs challenging and more costly.
SCHNEIDER: Do your homework. Software compatibility between the monitoring and IT portions of the systems make things very complicated. My approach has always been to contact the hospital HTM department referred to by the OEM, as well as organizations that have the systems you’re considering, but not referred to by the OEM. Do a five-to-ten-year cost of ownership (COO) evaluation on each brand. Be aware of tactics used by sales to sway the clinicians. I’m aware of at least one vendor that tells them that software upgrades are free. What they fail to divulge is that many times a costly hardware upgrade is required to move to a new software revision. Every organization has specific needs for clinical monitoring but has a limited budget.
SHAHBANDEH: Ensure you have a service provider at least ISO 9001-2015 certified, which assures them the company they choose to send their equipment adheres to those standards. Your equipment will need service, the cost matters, the warranty matters, and if they are reliable, you can count on your patient monitoring to be reliable.
WANG: When selecting patient monitors, health care facilities should consider the total cost of ownership of the units, product reliability/durability, user-friendliness and, most importantly, serviceability and maintenance needs. It’s essential to identify a reliable third-party service partner because a good service partner is the most cost-effective way to maintain your equipment in the long term. At USOC Medical, we pride ourselves on our high-quality repairs, exchanges, rentals, and we demonstrate this with some of the best warranties in this industry.
Q:
DO YOU THINK TECHNATION READERS NEED TO KNOW ABOUT PURCHASING AND SERVICING PATIENT MONITORING DEVICES?
DUTTA: TechNation readers should evaluate the total cost of ownership when purchasing patient monitoring
devices, including the initial investment, maintenance expenses, and potential software or hardware upgrades. Service support options are another critical consideration, as reliable and accessible support is essential for maintaining functionality and accuracy. Compatibility with existing systems should also be assessed to prevent integration challenges. Additionally, the device’s ease of use can significantly impact training time and overall patient care quality. At Soma Tech Intl, our dedicated sales, customer support, and operations teams are ready to assist with every step, from purchasing to shipping. For more information, call 800-438-7662.
ORTIZ: There are a few factors to keep in mind when purchasing and servicing patient monitoring devices. First, compatibility and integration are important as you will need the equipment to work seamlessly with existing systems in the facility Second, consider opting for recertified devices which can offer all the performance needed at a fraction of the cost without compromising quality. Lastly, choose a brand that supports the servicing of their products by offering training, documentation such as service manuals, and parts availability to the end user.
SCHNEIDER: HTM plays a major supporting role and should provide all information available related to projected support costs (including staff training) to clinical and hospital leadership when deciding to purchase a patient monitoring system. It’s the most pervasive system, after the Electronic Medical Record (EMR), the hospital will purchase.
SHAHBANDEH: Service companies have a core competency, know who you are sending your patient monitoring to. Make sure they are the ones doing the job.
WANG: Having a reliable partner like USOC Medical is critical because we will ensure all your needs, whether it be repairs, parts, or replacement units are met consistently. At USOC Medical, we take pride in providing our customers with comprehensive and robust solutions to our customers’ patient monitoring needs. Our customer service team is available from 5 a.m. to 4:30 p.m. PST to support your inquiries, and we employ Lean and continuous improvement methodologies to ensure efficient, fast turnaround times on repairs. Above all, our commitment to quality and customer satisfaction makes us a trusted, well-established partner that stands by our work with an industry-leading warranty.

• A niche job board for the HTM and imaging communities powered by TechNation.
• 3300+ actively looking biomedical and imaging professionals.
» Completely free and confidential registration and application process.

Featured Employers: Agiliti, Renovo Solutions, TRIMEDX, Erbe USA Incorporated, Middlesex Health, SP Associates, and more!
htmjobs@mdpublishing.com for posting inquiries htmjobs.com to register today
• The fastest growing HTM talent network in the country.
• 350+ open opportunities throughout the United States.
» A variety of posting options ranging from single-job postings to 12-month unlimited memberships.
BY K. RICHARD DOUGLAS

More than seven decades ago, the right to repair movement was born out of the frustration that manufacturers put certain controls, impediments and barriers to repairs and maintenance by anyone other than their technicians.
These restrictions have hampered the efforts of professionals and home DIY’ers from doing self-repair or maintenance; allowing for quicker repairs, emergency repairs or saving money.
The question in the right-to-repair issue has always been: Why can’t the owner/purchaser have the right to
repair/modify or upgrade an item they have purchased instead of relying on the manufacturer? Why does a consumer have to purchase a replacement because they are not able to repair or upgrade what they have?
Push-back against these restrictions have mostly originated with organizations founded to represent the rights of consumers, self-employed repair technicians and independent service organizations (ISOs).
Only through legislation would OEMs be compelled to release schematics and manuals or other documentation, make parts, diagnostic tools or software available to independent service businesses, qualified technicians or to consumers.
When the home auto mechanic, with a consumer-level OBD-ll scanner attempts to diagnose an issue with their vehicle, the data needed from the onboard computer may be restricted, and only read, by the auto manufacturers’ specialty scanners.
By restricting access to all of the available data, the manufacturer is assured that only their technicians can fully diagnose problems with their products. The independent service shop, or the home auto mechanic, is left without other options.
This problem has impacted farmers who often need to get farm equipment back into service as soon as possible. The problem has also obstructed HTM professionals from moving ahead with repairs that might need to be expedited.
Organizations that are consumer-advocates, along with ISOs within the medical equipment repair and maintenance business, have traditionally been advocates for change. These groups have brought the issue before state legislatures and the U.S. Congress to promote change in the laws.
The effort has seen small victories in some states. But it hasn’t been enough to put the effort and achievements solidly into the win-column.
The start of 2025 and beyond is a time that the biomed community, the C-suite and HTM state associations should come out en masse and push the issue over the finish line. If legislators at the state and federal levels heard from thousands of HTM professional, state associations and societies, along with ISOs, the importance of the problem would be driven home. New legislation would be passed and a new day would dawn.
“I worry that we haven’t yet been able to ‘crack the code’ of fighting for medical right-to-repair in state legislatures. We have had only some action led by legislators with hospital administration experience – Illinois and Delaware – but that alone hasn’t been enough to get bills beyond the filing stage. It takes commitment from legislative leadership – party doesn’t matter – and leadership won’t fight battles they don’t believe they can win; catch 22,” says Gay Gordon-Byrne, executive director of The Repair Association.
townhall discussion at the April 2024 Las Vegas MD Expo.
But, has the issue been demanding enough to lead to a widespread mobilization of the biomed community?
“I don’t think the average in-house biomed sees/feels the debate quite the same way that the primary players do. Most do not support the OEM side of the debate as that side is seen as interference and restriction to what they do. However, when a paycheck comes from the health system itself, there is possibly a sense of false comfort that they are outside of the real debate itself – almost to the point where they don’t feel that they are going to be affected by it – not at least in the same way that an ISO/third party or an individual entrepreneur would,” says Perry Kirwan, executive, clinical engineering, eQuip-Center for Clinical Technology Management at Sutter Health.
He says that added to this is the introverted nature of most biomeds.
“With the exception of a few vocal and dynamic leaders, the profession is largely composed of individuals that will talk about issues around the water cooler but that doesn’t necessarily translate into activism. One has to put oneself out there with activism and the gravity of the debate hasn’t quite hit a trigger to motivate a majority to action. Sprinkle a little bit of the ‘my vote doesn’t really matter’ mentality and that’s a good recipe for passivism,” Kirwan says.
“One has to put oneself out there with activism and the gravity of the debate hasn’t quite hit a trigger to motivate a majority to action. Sprinkle a little bit of the ‘my vote doesn’t really matter’ mentality and that’s a good recipe for passivism.”
- Kirwan
There are thousands of biomeds who have been impacted by the right-to-repair issue in recent decades. With the ever-evolving high-tech nature of medical equipment, the intensity and frustration of the struggle can be experienced even more in the future.
“I definitely hear hospital-based biomeds talking about how important it is to get access to the repair materials as a condition of starting the bidding process for new equipment. Clearly, this is where the hospitals have leverage, with the bigger hospitals having more purchasing power. Two different attendees to the keynote talk at MD Expo raised this point,” says Nathan Proctor, senior director at U.S. PIRG Campaign for the Right to Repair, referring to a
He believes that state associations can be a good way to motivate people because there is strength in numbers.
“If individuals can be around other like-minded individuals that educate on the topic, what’s important in the debate, and what’s at risk if owners lose their ability to make maintenance and support decisions around the equipment they own, they might be a little more motivated to act,” Kirwan says.
He says that is the primary reason that people tend to respond to things that are bigger than just themselves. He added that it also helps to feel supported and that they are not alone.
“Associations solve that by being a place where individuals network with other individuals in the same industry. One opportunity for state associations is to align with other associations outside of HTM, but hopefully aligned in the interests of quadruple aim – most of which is affordability. The American Hospital Association (AHA) is a much larger lobbying group than the state association and each state has their own chapter,” Kirwan says.
He says that getting the state AHA involved provides access and a voice to local and state legislators as well as federal equivalents.
“State legislators are much more important to persuade along the lines of this argument as state laws that favor right-to-repair may be attenable to achieve while the much slower federal process grinds along,” Kirwan adds.
He says that in addition, there is an emerging but growing trend of HTM leaders becoming members of the American College of Healthcare Executives (ACHE).
“This organization provides HTM leaders access to administrative and C-suite members that lead healthcare delivery organizations who would see impact should right-to-repair be lost to their organizations. These forums are invaluable in informing decision makers of what’s at stake and how those decisions would impact patient care, safety and affordability,” Kirwan says.
The HTM community – from the individual biomed to health care systems and state associations – can make a difference and encourage lawmakers to push forward legislation that promotes right to repair.
“Biomeds can provide the facts, but we have to first make sure legislators are interested in listening to them. A good crisis would be helpful, such as the OEM flubs on ventilators during the pandemic, but that momentum has dissipated,” Gordon-Byrne says.
Third parties and ISOs have led the fight for right-to-repair in past years, but they could use the help of the HTM community to further gains.
“If I could waive a magic wand and speak to every biomed, or rad tech, and the associations which represent them, I would ask that they request that those in charge of purchasing/ procurement insist when purchasing equipment from a manufacturer that they include in the procurement offer an obligation for the winning manufacturer to provide service access information for good safety practices and to advance right to repair,” says Robert Kerwin, general counsel at the International Association of Medical Equipment Remarketers and Servicers (IAMERS).
He says that the 2024 FDA Remanufacturing Guidance sets forth on Page 21 a good description of what is needed to service the equipment and conduct periodic maintenance.
“When/if there is pushback or some unwelcome tactics (i.e., conditioning updates and upgrades or cybersecurity protections on the
hospital’s agreement for a long-term servicing contract) let IAMERS know. We consider these kinds of tactics as a competition concern. Remember: when the hospital has choices, the hospitals and their patients are better off,” Kerwin adds.
IAMERS is a trade organization that actively participates in regulatory and legislative advocacy in the U.S., E.U. and worldwide.
“I think it’s an excellent idea to try and secure access to repair materials at the bidding stage, but I did offer a word of warning. Some hospitals, such as small rural hospitals, don’t have the kind of purchasing power that moves the needle, first of all. The other is that this problem will not be solved unless there are rules to protect all equipment owners. We have a lot of experience in all manner of marketplaces, and ‘voting with your dollars’ doesn’t work very well in highly consolidated markets,” Proctor says.
He says that for some equipment, hospitals have a range of vendors, but others are limited in terms of choice.
“In-house HTM departments need to ensure the word is out and everyone is educated on the issue. This includes the HTM caregivers, but just as important and maybe more importantly, they need to educate organizational leadership on the issue. I would expect that every organization has a leadership council of some sort; legal counsel, and government relations. These personnel need to know and be educated on the issue,” says Mike Busdicker, MBA, CHTM, AAMIF, FACHE, senior director of clinical engineering at Intermountain Health.
He says that state associations should be involved at the local politician level along with bringing the HTM caregivers in the area together.

“They should be actively engaged with an education effort at the state political level and at the same time encouraging the local HTM caregivers to be involved within their sites. This could be somewhat of a delicate issue if they have non-inhouse or ISOs organizations involved in their local association,” Busdicker says.
Those who have been consumeradvocates also look to biomeds for support to change the status quo.
“So, what is the best way hospitals can help reach a more open repair ecosystem for equipment? They can speak up for rules like the right-to-repair, that require all manufacturers to provide access to the parts, tools and information needed to fix equipment. I fundamentally believe that selling someone equipment, and then preventing them from repairing it without paying more for a special contract, is an unfair practice,” Proctor says.
Which equipment should we allow to be sold unfairly, he asks?
Proctor says that over the last year, right-to-repair has continued to make progress.
“We passed three more laws in 2024, a consumer Right to Repair law in Oregon and a consumer and business electronics repair law in Colorado and a new wheelchair Right to Repair law in California. So far, making progress on medical repair issues has been challenging, which is why it is so important for hospitals to engage with the process,” he adds.
Gordon-Byrne suggests holding meetings with the top hospital administration and impress upon them the excessive costs that limit their budgets without any improvement in quality of care.
“Money does talk, once quality of care is out of the picture. If hospital admins/leadership wants Right to Repair – its far more likely to happen than without. Large hospital chains have the power of the purchase order and can at least try to demand better terms and conditions in exchange for a large P.O. The larger the chain; the bigger their leverage,” she says.
Gordon-Byrne says that legislators react to consumers as voters, and that’s where we’ve had results. If we can drill
down to the cost per person in excessive medical costs which drive personal insurance rates, that’s another way we can push voters to complain to their legislators.
“It is crucial that health care system leaders, facility leaders, physician leaders and other influencers within the industry understand what ‘right to repair’ is, how it can impact their organization, and how it can impact health care. Also, leaders should understand the findings of the 2018 FDA Report on Medical Device Servicing, what states have introduced and/or passed legislation and the organizations involved with the process,” Busdicker says.
He says that biomeds can participate in moving this issue forward by ensuring that they are educated on the issue and understand how their support can drive the process forward.
“They need to understand how to become involved, why their involvement is so important, and we need to provide them with materials and information that makes the process easier for them,” Busdicker adds.
Gordon-Byrne says that “maybe we should be asking biomeds to tell their horror stories to us and allow us to use our media friends to get consumers angry. We can take anonymous complaints on our complaint collector repair.org/repair-complaints.”
It’s time for HTM to unite, organize and lend its collective voice to the right-to-repair issue. Some in the field have done so for years, but when lawmakers hear from voters in large numbers, and when associations and organizations decide to provide focus to an issue, mountains can be moved. The time is ripe.
BY VICKI SALEMI
It is a new year, post-election watercooler conversations are still buzzing and life may seem more hectic overall, it can seem harder to concentrate on work. How can you stay focused to block out the noise?
You’re not alone! It can feel very easy to be distracted at work.
Both leaders and workers can do their part to stay engaged at the task at hand. Ethan Kross, author of “Shift: Managing Your Emotions So They Don’t Manage You,” said, “Leaders can provide their workers with engaging activities that capture their attention. Such activities point their mental spotlights away from election-related anxiety and towards vital workplace tasks.”
When it comes to workers, Kross said they can “proactively find tasks that sustain their attention; for example, throwing themselves into a report or important projects that require their complete focus.”
Also, remove cues that trigger you – one simple change to reduce distractions so you can immerse yourself in work involves shutting off notifications on your phone.
This includes reducing voracious appetites of consuming social media, especially before working on an important task. Lauren Farina, LCSW, psychotherapist and founder of Invited Psychotherapy and Coaching, said, “While it’s important to stay informed, the practice of ‘doom scrolling’ is known to rob us of time and energy. What’s more is that the content we consume is often sensationalized in order to exploit the emotion centers of the brain. In general, too much time on screens can stress the brain and leave us in what’s known as a dopamine deficit – a brain state in which low levels of the key neurotransmitter leave us feeling depressed, fatigued and unmotivated.”
Another way to stay engaged involves time blocking. Phil
Libin, co-founder and CEO of video communications app mmhmm, and co-founder and former CEO of note taking and task management app Evernote, suggested evaluating the work itself.
“If you’re really having trouble focusing on work, first I’d have a hard think about whether this work was sufficiently important to you. Then, I’d start trying to open up severalhour-consecutive-blocks every day to either look for different work, or focus on the current one. The best way to do that is to shift some things from ‘synchronous’ to ‘asynchronous.’ ”
Libin mentioned if your team operates this way, sometimes asynchronous work and communication is best. “Adjust your work and personal life to use time in ways that make sense for you, when you personally can be most productive.”
Above all, it’s important to stay on top of it rather than having everything else manage us. Kross reminded us about our powerful abilities to stay focused, even as we toggle between devices and constant outside noise.
“Being able to strategically deploy our attention is the foundation of our ability to be happy and successful. As a species, we are unique in that we can deploy our attention where we want – toward the things we want or need to do and away from the distractions that impede progress toward our goals,” said Kross. “The more we hone this skill, the more capable we are of being successful. All of us – from CEOs to mid-level management to entry-level workers – need to strategically deploy attention to accomplish our goals.”

Vicki Salemi is a career expert for Monster, an author, a speaker and consultant, TV commentator and former corporate recruiter. Send your questions to hello@vickisalemi.com.



IIn health care, medical equipment is essential to providing optimal patient care, from diagnostics to critical life-saving procedures. The performance and reliability of these devices is non-negotiable, as lives depend on them. Whether new or refurbished, medical equipment must function flawlessly, as intended by the original manufacturer. This highlights the crucial role that quality assurance plays, particularly in refurbished medical equipment, where attention to detail is essential.
Unlike new medical devices, where FDA regulations dictate performance standards, there are no formal guidelines governing the refurbishment process. This lack of regulation means that the quality of refurbished equipment can be significantly different between providers. It also places the responsibility on decision makers to do their research and make sure they’re purchasing from a reputable company. Creating a comprehensive refurbishment process that meets high standards is essential to make sure refurbished equipment is reliable and safe to use on patients. Without a standardized process, refurbishing companies
may adopt different methods, some of which may not fully restore the equipment to its original condition. This variability introduces risks to patient safety and complicates clinical workflows. Health care providers should choose partners who adhere to comprehensive quality assurance protocols that make sure refurbished devices meet the same performance standards as new equipment.
The stakes are high when it comes to medical equipment performance. A failure in a clinical setting could have tragic, even fatal, consequences. Quality assurance isn’t just important – it’s essential. A comprehensive refurbishment process ensures that equipment performs as well as it did when it first left the manufacturer. This gives health care providers confidence that refurbished devices will deliver safe and effective patient care. Quality assurance in refurbished medical equipment goes beyond simply ensuring that the device powers on. It includes a thorough assessment of the equipment’s functionality, safety features and reliability. For example, ventilators and anesthesia machines are life-support systems in critical care environments. The refurbishment process should extend beyond powering the unit on and performing a ventilation check. This involves measuring the volume and pressure accuracy of gases, checking the gas mixture, ensuring that


backup systems work in case of failure, and verifying that anesthetic gases are not exposed to health care staff.
Additionally, this process must inspect and identify parts that are on the verge of failure, which might not always be visible through standard OEM preventive maintenance procedures. The refurbishment should also restore the device’s original cosmetic appearance, avoiding contamination during the process, and undergo a re-check to ensure that performance parameters are retained. After this process, the system should easily pass the biomedical pre-check in an operating room. A comprehensive quality assurance process simulates real-world usage to ensure the equipment is patient ready.
ISO 13485:2016 is an internationally recognized certification that demonstrates a medical equipment company’s commitment to stringent quality management standards. Achieving and maintaining this certification is a rigorous process that involves thorough audits and continuous improvement processes. While medical refurbishment companies aren’t required to get this certification, Soma Tech Intl consistently re-certifies, proving that their facility, products, and production process meet the highest standards of safety and performance.
This certification not only reflects Soma Tech Intl’s dedication to quality assurance, but also provides health care providers confidence that their refurbished equipment is safe and reliable. By adhering to ISO 13485:2016, Soma ensures that their products are refurbished under the same standards as newly manufactured medical equipment.
At Soma Tech Intl, the refurbishment process is designed to restore each unit to its original performance and safety standards. It starts with a technical refurbishing process where our highly trained and certified biomedical engineers, many with OEM experience, disassemble the system and check each area, replacing components as per OEM recommendations. This includes individual circuit board testing and repair, resolution of any pending recalls and upgrading any necessary software. Engineers take a preventative approach to identify parts that might seem functional but are on the verge of failure.

This is followed by replacement of necessary parts, assembly and calibration of the system.
An operational test of the system then follows, during which individual components are assessed. This includes testing the speakers, indicators, switches, and other critical elements. Additionally, it checks whether the software routines are functioning as intended. Any detected failures are promptly corrected and retested to ensure proper operation.
A functional test is also conducted to assess various parameters and the overall operation of the system, followed by an electrical safety inspection. All readings are thoroughly documented to ensure complete transparency throughout the process. These results are compiled into a detailed engineering testing report. The parameter testing and electrical safety inspection further validate that the device meets the manufacturer’s performance specifications, ensuring it is safe for use in patient care.
In addition to ensuring the equipment works flawlessly, Soma tech Intl also restores its appearance. The cosmetic restoration process includes repairing or replacing broken panels, handles and pieces. As well as cleaning, sanding, painting, and adding new decals to bring the device back to its original look. This not only enhances the aesthetic appeal of the equipment but also ensures its durability in a clinical setting.
Before any unit is labeled “patient-ready,” it undergoes a final round of testing and inspection. This step includes re-testing the performance of the devices, verifying the accuracy of the engineering report, and checking the physical appearance one last time. Only then is the unit approved for shipment, complete with a warranty.
Quality assurance in refurbished medical equipment isn’t just a matter of compliance; it’s a commitment to patient safety and effective healthcare delivery. By choosing a refurbishing company that prioritizes quality, like Soma Tech Intl, healthcare providers can ensure that their equipment is both reliable and safe, capable of performing at the highest level when it matters most. In an industry where there are no formal regulations, a well-executed process stands as a testament to a company’s dedication to quality, providing healthcare providers with reliable, safe, and effective equipment.
BY TODD BRITTON
Atrend has emerged in healthcare technology management (HTM), which is to become a High Reliability Organization (HRO). Documentation is a key component of high reliability, and the heart of good documentation is meaningful data – lots, and lots of data. Admittedly, documentation can be tedious and time-consuming. However, improved health care system documentation facilitates higher productivity, improves patient care and, ultimately, saves lives.
Consider receiving repeated requests for service regarding the same issue. Nothing is more effective than consistent information indicating the root cause of a problem. It can potentially indicate a new approach. It shows where action should be taken to permanently resolve the issue. For example, a customer reports that they briefly lost network connectivity on a system. They claim that this happens all the time and no one seems to fix the problem. They bring up the dreaded fact that “this is adversely affecting patient care.” The equipment history doesn’t appear to support their claim, since there is only one other record of this issue. During a follow-up conversation with the customer, they mention that when the problem happens, they typically pick up the phone and call a specific technician, and the problem goes away. The technician, says that he is aware of the issue, and he takes care of the customer every time they call. He tells you not to worry because he simply resets the network switch in the telecom closet and the issue is resolved. This situation occurs every day across all industries. Now try to picture an alternative scenario unfolding after the initial event occurred. Instead of finding the work order history mostly undocumented, the service history shows that the customer has been losing connectivity every Wednesday morning for three months
and that it occurs around the same time each day. Coincidently, this is the exact time that the hospital tests the emergency generator. Let’s say that for this example, the telecom closet network switch was connected to the wall outlet, and not to the rack UPS, which is what is causing the issue. Problem solved. This actually plays out weekly in the field and it is an example of how good documentation guides troubleshooting.
Consider a system so old that local schools and museums are offering to pay to take it off your hands. One of the best ways to support replacing old and worn-out technology is by utilizing documentation. A list of service events occurring over time can show that a system is no longer economical to repair. Instead of believing that a request for a replacement system isn’t justified, management is listening, since the information is being presented in a way they understand and appreciate. The desired result is a direct outcome of a strong commitment to documentation. Good documentation shows that a $80k service agreement for maintenance is better than a $200k unexpected CT tube replacement. Management wants proof that they are getting their monies worth. Trying to explain to leadership why the $220k unexpected expense wasn’t included in your yearly budget can be a difficult conversation, but an even worse situation is having to explain to staff why there will not be any Christmas bonus this year.
Joint Commission inspections can evoke fear and their visits are not typically considered a fun time. Does this always have to be the case, or are there things we can do to alleviate some of the discomfort? The following example happened recently. During a JC inspection, an inspector asked to see the preventative maintenance (PM) history for every medical imaging device belonging to the radiology department. Now I work at one of the largest VA hospital networks in the United States. I confess, the first thought that popped into my head was retrieving that much maintenance information was an impossible task. We performed a database search and within minutes had a record of every imaging system, every PM and all work order
notes. But then, we noticed that one PM was apparently missed. It caused panic. However, after reading the detailed notes on the original work order (Hooray for Documentation!), we discovered that there were extenuating circumstances. The PM was performed off-schedule. The off-schedule work order had the original PM work order number, with a detailed explanation of the situation. The inspector was happy. The technician’s thorough documentation not only showed their commitment to excellence, but they also helped navigate a stressful situation. This reflected positively on our department, our clinic and our entire organization.
As a biomedical engineering supervisor, I am looking for the following in documentation:
• Complete sentences with accurate spelling and punctuation. Anything less can reduce the effectiveness of the message, and negatively influence how the information is received. Everyone makes mistakes, but good documentation should not be characterized by errors. Your extra effort will influence the reader to believe in you and increase the credibility of the information.
• Be truthful, concise and to the point. Do not attempt to manipulate people by obscuring the truth. It rarely has a positive outcome. Maintain the trust of your intended audience, so they continue to listen to what you have to say. Include just the facts and leave out your personal
feelings. Take a moment to ask yourself how the message would be received if it was read by the company CEO.
• Be strategic by understanding the purpose of the information you are providing, and document with that goal in mind. What are you trying to accomplish? Is the reader getting inundated with unnecessary information that interferes with their ability to clearly understand your message? Are you providing the key information that potential readers are needing? Too much irrelevant information can simply create more issues.
In conclusion, documentation is a great equalizer. You might not be the greatest technician in the world, but if you are able to reliably record an accurate picture of events regarding the history of a medical device, you are a hero. Your work is instrumental to the success of any organization. Good documentation makes everyone who prioritizes it an extremely important part of a team, that exemplifies high reliability, great customer service, accountability, compliance and, ultimately, the best possible patient care.

Todd Britton is a biomedical engineering supervisor at Veterans Health Administration North Texas.









Columbus, Ohio

FEBRUARY 6
FEBRUARY 6
Leadership Development Course
Leadership Development Course
Top Golf Social Outing
Top Golf Social Outing

FEBRUARY 7
FEBRUARY 7
12 education courses including
12 education courses including
• Imaging training by RSTI
Keynote Speaker Ray Dalton HTM Entrepreneur
- Imaging training by RSTI
• MX40 repair


- MX40 repair
• Anesthesia troubleshooting
• Sterilizer
- Anesthesia troubleshooting
• Contrast injectors
- Sterilizer
- Contrast injectors
• Med Device Integration
• 3 Leadership courses
- Med Device Integration
- 3 Leadership courses
Women in HTM Roundtable Session
Women in HTM Roundtable Session
Mentorship in HTM Roundtable Session
Mentorship in HTM Roundtable Session
Food, Prizes and Happy Hours
Food, Prizes and Happy Hours



BY ROGER A. BOWLES, MS, EDD, CBET
No, I’m not retiring from Texas State Technical College, at least not in the immediate future, or until other plans come to fruition. I’m still teaching classes. But, as many of you know, I have been involved in several endeavors over the years and some of them are taking more and more of my time. I hope to perhaps even change roles in the near future. The holidays are upon us as I write this and it is a great time for reflection. By the time this column comes out, it will be a new year, and for a column called “The Future,” it seems like a good time for me to pass the baton to a fresher face and a more modern perspective. So, this will be my last “The Future” column.
I can’t recall the first article I wrote for TechNation, but I think it was over 20 years ago now. I have long appreciated the opportunity MD Publishing and TechNation have provided me over the years. I have met so many inspiring people at MD Expos and I hope to continue to attend a few of these great events and others in the HTM world. Thank you to John Krieg, John Wallace, and all of the TechNation team for allowing me to promote the field through this column. Most of all, a big thank you to the readers who have been my biggest inspirations and have often sent me texts and emails about the column.
One thing is for sure, I am confident that HTM will continue to be an excellent career choice for those with a technical flair who decide to make a difference in health care. As of today, there are many, many opportunities out there for graduating students. Many of the hiring managers who come to us are graduates of our program and that makes me feel good to have contributed a little to this field. It is a great feeling to have children of graduates enrolling in and graduating from the program.
Our biomedical equipment technology program at TSTC has changed quite a bit over the years. There have been some solid improvements in a couple of areas and I’m proud of those. I’m happy I am still able to contribute a little bit of value to this program. As many of you know, all of our faculty
were once full-time biomedical equipment technicians or medical imaging specialists. Almost all of them graduated from our program and went out and gained many years of experience before coming back to teach. We survived the pandemic shutdown, and despite the somewhat continuous chaos and uncertainty, we continue to attract students.
As I mentioned in a previous column, we are in the midst of a massive building renovation with constant leadership and personnel changes. This has been disruptive and sometimes these changes have not been for the better and will challenge certain aspects of our reputation in the future. Despite these challenges, I am hopeful this program will survive for many, many years to come. As with my colleagues, for me, being in the classroom and teaching in this program is still a thrill and is the best part of the job for me. If you graduated from this program and have not connected with me on LinkedIn, please do, I enjoy keeping up with your successes.
Speaking of success … if you are a BMET looking to complete a bachelor’s degree, East Texas A&M has some excellent opportunities in Organizational Leadership, Applied Arts and Sciences, Healthcare Administration, General Studies, and Technology Management. Many of these are competency-based programs and can be completed online. Many of your Associate of Applied Science Degree credits can be transferred in. I am already teaching one class for them online. Check them out at this website: tamuc.edu/college-of-innovation-and-design/. I look forward to seeing you at future events and seeing your contributions to this evolving occupation. I will continue to promote HTM and biomedical equipment technology every chance I get.

Roger A. Bowles, MS, EdD, CBET, is a biomedical equipment technology/medical imaging technology instructor at Texas State Technical College-Waco.

Veterans who have struggled with repair restrictions while serving their country are calling for change
BY NATHAN PROCTOR
U.S. armed forces veterans have launched a new sign-on campaign in support of reforms that would “ensure military service personnel have the Right to Repair the equipment they rely on.”
I spoke with retired Master Sgt. Wesley Reid about why he is supporting this letter. During his 20 years in the Army, he found that repair restrictions around medical devices put soldiers’ lives at risk. He described his experiences fixing equipment for the Army, including while deployed in Afghanistan.
When a soldier is wounded in the line of duty, triage to determine the severity begins immediately, starting what is referred to as the “Golden Hour.” You have roughly one hour to get that person to a higher echelon of medical care if you want to save their life. “That hour is everything,” Reid says. In many of these situations, a CT scan is critical. It can detect internal bleeding, or shrapnel the field medic could
have missed. Reid’s job was to make sure the CT in his hospital was working. It was one of only two U.S. CT scanners in Afghanistan at the time, from 2007-2008. At some point during Reid’s 14-month deployment, he lost access to a critical diagnostic feature used to keep the scanner running. It had a microcontroller that would allow access for him to use the diagnostic program to address artifacts and errors in the scan without much downtime. These controllers were designed to last one year and then deactivate. Normally, the Army would order a new one, but while Reid was deployed, the manufacturer said it would not send a new controller because the device was too old. Instead, the manufacturer told Reid to “buy a new device.” Meanwhile, wounded service members who needed a scan that could save their lives kept arriving at Reid’s medical facility. Reid did what he could to keep the equipment running, though it was a lot harder without the microcontroller.
Reid recalls that he received special training from the manufacturer on the device, and that the Army had paid for the highest level of support. However, the way the equipment was built required the manufacturer’s permission to keep using the repair tool every year, and
one day, it decided not to.
“My mental health really struggled,” Reid told me, recalling what a challenge it was to keep the CT scan working and how many lives were at stake. “I was fixing that equipment with one hand tied behind my back.”
Another service member I spoke with, who I will call Peter (he asked me not to share his name for fear of retaliation), has a similar story of working in Bagram Air Field. A six-year veteran of the Army, he was based at the largest overseas U.S. military field hospital in Landstuhl, Germany, and also in Kabul, Afghanistan.
Peter recalls that his MRI machine and CTs required special software service keys to access critical repair and diagnostic functions, such as reading the error logs, adding a new doctor’s network designation to send patient scans, or run diagnostic self-tests. Unfortunately, these service keys expired periodically, and sometimes it would take several days or longer to restore access. These delays often meant the equipment went down, and people couldn’t get the scans they needed.
“It was broke all the time there. It was a disgrace, honestly,” Peter added.
Making things more difficult, manufacturers’ contracts forbid trained Army technicians from training other field technicians, requiring everyone to attend the manufacturers’ (pricey) trainings. Peter wasn’t allowed to even share manuals with other technicians.
“It’s ridiculous,” said Peter, when describing the hoops they were forced to jump through. “The military school is the hardest … Everyone who is deployed is training. We should give them everything they need.”
Master Sgt. Reid believes that when we ask our service members to go to battle, we make an implicit promise to take care of them. The medical service we provide is a big part of that. “It’s important to me to keep that promise,” he said.
Peter agreed, and expressed frustration recalling the impact of equipment downtime. “Who knows how people’s lives were affected?”
Are you a current or former service member who wants to support the military Right to Repair? Check out the letter and add your name.

Nathan Proctor is senior director of the U.S. PIRG Campaign for the Right to Repair.
EQ2 HEMS – is focused entirely on healthcare maintenance management
Management and Technicians achieve more in less time
Integrate with all of your existing systems
Compliance and AEM made easier with the right tools and reports
Our system helps you keep your devices protected
Data and information that shows leadership how your department saves the hospital money




BY PHIL ENGLERT
In an era of increasingly sophisticated and prevalent cyber threats, health care providers face unique challenges in protecting sensitive patient data and maintaining the integrity of their systems. One powerful tool in the fight against cybercrime is participation in the Health Information Sharing and Analysis Center (Health-ISAC). This collaborative organization makes health care providers less susceptible to hacks and breaches.
As we begin a new year, I wanted to highlight how participation in an ISAC builds resilience across the sector. Information Sharing and Analysis Centers (ISACs) began to form in 1999 in response to the U.S. Presidential Decision Directive-63 (PDD-63), which was signed in 1998. PDD-63 laid the groundwork for subsequent policies and initiatives to enhance the security of critical infrastructures. It highlighted the need for a coordinated approach to cybersecurity and infrastructure protection, which has continued to evolve in response to emerging threats. The directive asked critical infrastructure sectors to establish organizations to share information about threats and vulnerabilities. The National Council of ISACs (NCI) was formed in 2003 to coordinate and collaborate between the various ISACs. The NCI comprises 23 sector-based ISACs that collect and analyze cyber and physical threat intelligence.
Health-ISAC, founded in 2010, is a nonprofit organization that serves as a central hub for sharing cybersecurity information among health care
organizations. Its mission is to foster collaboration and information sharing to enhance the sector’s overall cybersecurity posture. By participating in Health-ISAC, health care providers gain access to a wealth of resources, including threat intelligence, best practices and a community of peers facing similar challenges.
One of the most significant benefits of Health-ISAC membership is access to real-time threat intelligence. Cyber threats evolve rapidly, and having up-to-date information is critical for effective defense. Health-ISAC collects and disseminates information about emerging threats, vulnerabilities and attack vectors. This intelligence allows health care providers to address potential risks before malicious actors can exploit them proactively. For example, if a new ransomware strain is detected targeting health care systems, Health-ISAC can quickly alert its members, providing details on the threat and recommended mitigation strategies. This rapid dissemination of information can be the difference between a minor incident and a significant breach.
Cybersecurity is not a solitary endeavor. Health-ISAC’s collaborative nature enables health care providers to learn from each other’s experiences and develop more robust defense strategies. Members share indicators of compromise, so other members can detect similar attack techniques. Members also share insights about successful security measures, lessons learned from past incidents and innovative approaches to emerging threats. This collective knowledge helps health care organizations to implement more effective security protocols. For instance, if one hospital successfully thwarts a phishing attack using a particular technique, it

can share this information with other members, who can adopt similar measures to protect their systems.
Health-ISAC provides its members with access to a wide range of expert resources. These include detailed reports on cybersecurity trends, technical analyses of specific threats and guidance on regulatory compliance. Additionally, Health-ISAC hosts webinars, workshops, and conferences where members can learn from industry experts and network with peers. This access to expert knowledge is invaluable for health care providers, who may not have extensive in-house cybersecurity expertise. By leveraging the resources provided by Health-ISAC, organizations can enhance their understanding of complex cybersecurity issues and implement more effective defenses.
On the medical device front, Health-ISAC is the only organization that brings together medical device manufacturers and health delivery organizations to support the security of medical devices within health care, thus supporting patient safety. This collaboration is done through the Medical Device Security Information Sharing Council, which has over 480 participants from 180 different organizations.
Participation in Health-ISAC also helps to build a culture of
cybersecurity within health care organizations. By regularly engaging with the community and staying informed about the latest threats and best practices, health care providers can foster a proactive approach to cybersecurity. This cultural shift is essential for creating an environment where security is prioritized and integrated into all operations.
In conclusion, participating in Health-ISAC offers numerous benefits for health care providers looking to enhance their cybersecurity posture. From real-time threat intelligence and collaborative defense strategies to access to expert resources and incident response support, Health-ISAC provides the tools and knowledge needed to protect against cyber threats. By fostering a culture of cybersecurity and leveraging the collective strength of the community, health care organizations can become less susceptible to hacks and breaches, ensuring the safety and privacy of patient data.

Phil Englert is the vice president of medical device security for Health-ISAC Inc.
The words can appear horizontally, vertically, diagonally, and may be spelled forwards or backwards.
Kari Lawrence
CBET, MSHM
• Water cup
• Inventory validation stickers
• Label peeler
• Daily planner
• S’mores granola bar.
Submit your bench to be featured in TechNation at 1technation.com/my-bench/.
You could win a $25 Amazon gift card via the “What’s On Your Bench” Contest!
1. Jake Calhoun
2. Professional of the Month
3. Biomed 4. Healthcare 5. Department of the Month 6. Durham VA Medical Center
7. YP at MD
8. I am Technation
9. Fifteen Years
10. Roundtable
11. Patient Monitors
12. Right to Repair
13. Call to Action
14. Wrenchies
15. HTMJobs


ACROSS
1 Tool that combines two functions: used for bolting, holding, pressing and bending: _____ wrench
4 Mechanical device consisting of a toothed wheel
8 It’s used with a drill
9 Six-sided
11 Digital laser ___: used to test medical lasers
13 Smooth
14 For each one
15 Scanning equipment, for short
16 Bolted down
17 PC “brain”, abbr.
18 Rapid 20 Enclose
21 It can be measured by a dose meter, 2 words
24 Check carried out to measure the effectiveness of a dielectric in resisting the flow of electrical current, goes with 27 down 27 Firm, briefly 28 Time of arrival guess, abbr.
29 “Send help!” signal
Tweaks
34 Prefix with surgery or transmitter
35 Metal used in many medical devices, symbol 37 Drill a hole
38 Attaches to a support
12 Hospital for critical medical cases, abbr.
____ scan 18 “Just take care of the problem!”, 2 words
Go across
Wander
Face shields
Doze (off)
26 See 25 across 29 Diagnostic test
30 Surgeon’s domain, abbr. 32 Slang for an injection 33 Type of camera
36 ___ getting a new set of tools!






















The inaugural issue of TechNation included a feature article on the Indiana Biomedical Society and the first of many columns submitted by industry guru Manny Roman.




Need a powerful way to elevate your brand and connect with your target audience?
Becoming a Preferred Vendor attracts attention to your products or services, ensuring that your ad reaches potential customers who are genuinely interested. Plus, you are able to enhance your brand credibility and position your company as an industry leader through additional highlight in our monthly e-newsletter and preferred vendor playlist on TechNation.TV.









...Since you are still flipping through this magazine, it simply means you still have not found an


















CM Parts Plus cmpartsplus.com •
Innovatus Imaging
Metropolis International, LLC Metropolismedical.com
Integrity Biomedical Services integritybiomed.com
Pronk Technologies, Inc. pronktech.com • 800-609-9802
Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010
Tenacore LLC tenacore.com
800-297-2241
USOC Medical usocmedical.com
855-888-8762
Refurbish
Innovatus Imaging innovatusimaging.com • 844-687-5100
Rental/Leasing
J2S Medical www.j2smedical.com • 844-342-5527
BC
Interlight
BMES
Every item undergoes a rigorous quality control process, ensuring reliability and top performance. Each unit includes our industry-leading warranty, offering you peace of mind with every purchase.
When you choose BMES refurbished equipment, you can trust it’s been expertly serviced by our skilled biomedical technicians.




The 2024 HTM Mixer in Lexington, Kentucky, was a hit. Approximately 300 people attended the HTM Mixer, proudly supported by KAMI, November 15&16. HTM professionals were treated to networking opportunities, ACI-approved educational sessions, an exhibit hall filled with industry-leading companies and more!
Visit HTMmixer.com for information about upcoming events in Colorado and Wisconsin.
1. The 2025 Kentucky Association of Medical Instrumentation Board poses for a photo after announcing election results.
2. Tom Bledsoe, 2024 KAMI President, addresses the audience prior to the panel discussion on test equipment.
3. Attendees enjoy networking and reconnecting at the Bluegrass Kick-off Party.


1 3 5 6 4


4. Bourbon tasting and live bluegrass music were a couple of highlights of the Bluegrass Kick-off Party.
5. Attendees visit exhibits like the J2S Medical booth during exhibit hall hours.
6. Save the date for HTM Mixer events in 2025 which will take place in Denver, CO, May 15-16, and Milwaukee, WI, July 31- Aug.1.




When your ultrasound equipment is down, so is your business. Don’t miss our Solutionist Series videos on Ultrasound Equipment Support where you’ll learn how to service your equipment, keep your business up and running and lower your total cost of ownership.
In this 10-episode virtual training course on the Philips Epiq ultrasound platform, Larry Nguyen, Summit Imaging’s Founder and CIO, identifies common failures and provides solutions for:
• Image quality — 2D grayscale, color, continuous wave or pulsed wave
• Power subsystem — power module and power regulator board
• External interfaces — control panel, touch panel, trackball, monitor and external IO
• Transducer types and applications — Doppler, linear, curved, endo-cavity and more.
• Transducer parts — a breakdown of standard and TEE
• Transducer parts and operational failures — from lens failure to cut or creased cords to CW noise and error codes

Larry Nguyen addresses lots more in our 10-episode Solutionist Series, so be sure to tune in.


