




n recent years, the field of medical retina has witnessed remarkable innovations that have revolutionized the diagnosis and treatment of various retinal conditions. These advancements have significantly improved patient outcomes and expanded our understanding of retinal diseases.
The obvious game-changers—anti-vascular endothelial growth factor (anti-VEGF) therapies—have transformed the management of wet agerelated macular degeneration (AMD), diabetic macular edema (DME), retinal vein occlusion (RVO) and diabetic retinopathy (DR). There are currently multiple players in the market, each offering their own approved treatment regimens.
Biosimilars are also up and coming, making remarkable advancements and offering hope for improved outcomes for patients facing a range of vision-threatening conditions. Even geographic atrophy (GA), previously without available treatment, now has two FDA-approved treatments, this year alone.
Innovations are unstoppable, and as imaging technology becomes increasingly sophisticated, it offers invaluable insights into retinal structure and pathology, undoubtedly contributing to personalized treatment plans.
While this issue mainly focused on the ‘medical retina’ side of developments, innovations in imaging and retinal surgery techniques play a crucial role as well. As artificial intelligence and machine learning are increasingly being integrated into medical retina practice, these technologies contribute to early disease detection, image analysis, and eventually, treatment optimization.
Overall, these breakthroughs offer patients with earlier diagnoses, more effective treatments, and improved quality of life—marking a promising era in the fight against retinal diseases.
Best regards,
Gloria D. Gamat Chief Editor, Media MICE PIE, CAKE and COOKIE magazines





Dr. Alay S. Banker
Banker’s Retina Clinic and Laser Centre
Ahmedabad, India
alay.banker@gmail.com
Prof. Gemmy Cheung

Singapore National Eye Centre (SNEC) Singapore
gemmy.cheung.c.m@singhealth.com.sg
Dr. Hudson Nakamura
Bank of Goias Eye Foundation Goiânia, Brazil
hudson.nakamura@gmail.com
Dr. Barbara Parolini

Eyecare Clinic
Milan, Italy
parolinibarbara@gmail.com
Arunodaya Charitable Trust (ACT)
Prof. Mark Gillies

University of Sydney Sydney, Australia
mark.gillies@sydney.edu.au
Dr. Saad Waheeb

King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia
saadwaheeb@hotmail.com
Asia-Pacific Vitreo-retina Society
Orbis Singapore Subthreshold Ophthalmic Laser Society



He Eye Specialist Hospital

Retinawesome Retina & Vitreous International

Vitreo-Retinal Society - India




The Royal Australian and New Zealand College of Ophthalmologists

ASEAN Ophthalmology Society

Ophthalmology Innovation Summit
Young Ophthalmologists Society of India ( YOSI ) World Ophthalmology Congress


GA cannot be cured, the drugs are meant to slow the progression of the disease.
IZERVAY is given as an injection in the eye once a month for up to 12 months. It works by targeting excessive activation of the complement system, which is the immune system’s early response to harmful pathogens and is implicated in the development of GA.
In clinical trials, IZERVAY was shown to reduce the rate of GA lesion growth by blocking excess production of the C5 protein, believed to play a key role in the development and growth of scarring and vision loss associated with GA.
By targeting this protein, the drug reduces the activity of the complement system, which causes the degeneration of retinal cells.
SYFOVRE, which was the first FDAapproved treatment for geographic atrophy, was also shown to slow the progression of the disease with monthly injections. It targets a different protein that is part of the immune system, protein C3.3-5 It is approved for patients with GA, with dosing flexibility every 25 to 60 days.
by Hazlin HassanWhile doctors cautiously welcome the approval of the two drugs for geographic atrophy, SYFOVRE® and IZERVAY™, questions have been raised over safety following reports of rare side effects with the former. We sat down with renowned retinal specialists Dr. Kenneth Fong (Malaysia) and Prof. Gemmy Cheung (Singapore) to weigh in on the latest developments in the geographic atrophy saga.
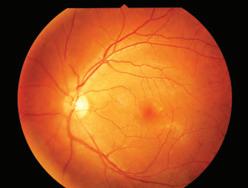
Geographic atrophy (GA) is an advanced and irreversible form of age-related macular degeneration (AMD) and a leading cause of blindness worldwide, impacting more than one million Americans and five million people across the globe.
Approximately 75% of people living with GA in the United States are believed to be undiagnosed. And there is no cure for the disease.
However, two new drugs have been approved this year by the US Food and Drug Administration (FDA)
to treat the condition, namely SYFOVRE® (pegcetacoplan injection) developed by biopharmaceutical company Apellis Pharmaceuticals, Inc. (Massachusetts, USA) in February and IZERVAY™ (avacincaptad pegol intravitreal solution) by biotechnology company Iveric Bio (New Jersey, USA) in August.1,2

The FDA’s decision to greenlight IZERVAY comes about six months after it approved SYFOVRE. Although
Following reports of rare side effects, including a potentially blinding condition known as occlusive retinal vasculitis, which has cropped up with the commercial use of SYFOVRE, Apellis Pharmaceuticals, Inc. issued an update on August 22 on the injection kits supplied by the company. They also issued an update on the cases of retinal vasculitis reported in real-world treatment with SYFOVRE for GA secondary to AMD.6

“As part of the comprehensive investigation into the real-world safety events, internal structural variations were identified in the specific 19-gauge x 1½-inch filter needle included in certain injection kits,” the firm said.
Filter needles are used to withdraw treatment from the vial when preparing for an injection procedure. However, the company said that a causal relationship has not been established between the structural
variations in this 19-gauge filter needle and the rare events of retinal vasculitis in the real world.
It recommended that “practitioners immediately discontinue use of any injection kits that contain the 19-gauge filter needle and use injection kits with the 18-gauge filter needle, which are already in distribution.”
While injection kits previously contained one of two types of filter needles (either 18- or 19-gauge), Apellis is now exclusively distributing injection kits with the 18-gauge filter needle.
“Based on the findings from our investigation, we believe it is prudent that practitioners only use the kits with the 18-gauge filter needle, which are already in distribution. This recommendation is out of an abundance of caution as patient safety is our top priority,” said Dr. Caroline Baumal, chief medical officer of Apellis, in a press release.
“To date, more than 100,000 vials have been distributed for commercial use and administration in clinical trials, and the events of retinal vasculitis continue to be very rare at an estimated real-world rate of 0.01% per injection. We believe SYFOVRE is an important medicine for patients living with this chronic disease and are committed to providing patients with a meaningful and safe treatment,” Dr. Baumal continued.
Over 100,000 SYFOVRE vials have been distributed in the real world and for administration in clinical trials. This includes over 78,000 vials distributed since its launch, including commercial vials shipped and sample vials distributed to physician practices. Over 26,000 vials have been distributed in the third quarter to date.
Approximately 24,000 SYFOVRE injections have been administered in clinical trials to date.
In total, eight events of retinal vasculitis (five occlusive, three non-
occlusive) have been confirmed. The last confirmed event of retinal vasculitis occurred on June 20, based on a review of adverse events reported to the company.
Two of the patients had their SYFOVRE injection in April, three in May, and three in June. All events of retinal vasculitis were observed after the first injection of SYFOVRE.
One patient remained stable at baseline vision, two patients have recovered vision nearly back to baseline, two patients have severe vision impairment which is unlikely to be resolved, and three patients’ outcomes are still pending.
The most common adverse reactions (incidence ≥5%) are ocular discomfort, neovascular age-related macular degeneration, vitreous floaters, and conjunctival hemorrhage.
progression at the 12-month primary endpoint across two Phase 3 clinical trials,” he said.
According to Dr. Dugel, in each registrational trial (GATHER1 and GATHER2), over a 12-month period, the primary analysis showed a statistically significant reduction of GA growth in patients treated with IZERVAY compared to sham.
“IZERVAY slowed loss of photoreceptors and disease progression as early as six months with up to a 35% reduction in the first year of treatment,” he added.
Across the GATHER clinical trial program7,8, the most common adverse reactions (≥ 5%) reported at 12 months in patients who received IZERVAY 2 mg were conjunctival hemorrhage (bleeding beneath the clear lining of the eye: 13%), increased intraocular pressure (increased fluid pressure of the eye: 9%), and blurred vision (8%).
The safety of IZERVAY was evaluated in over 700 patients with AMD.
“Across the GATHER clinical trial program, IZERVAY had a consistent safety profile and was well tolerated. There have been no reported cases of retinal vasculitis, retinal occlusive vasculitis or hemorrhagic occlusive retinal vasculitis,” said Dr. Dugel.

Iveric Bio was completely acquired by Astellas Pharma, Inc. (Tokyo Japan) on 11 July 2023.9
On the other hand, Dr. Pravin U. Dugel, president of Iveric Bio, shared with PIE magazine that its drug showed good results across two Phase 3 clinical trials and had a consistent safety profile with no reported cases of retinal vasculitis.

“IZERVAY, a new complement C5 inhibitor, is the only approved GA treatment with a statistically significant reduction in the rate of GA
“The approval of SYFOVRE and IZERVAY is a ray of hope for the millions of patients who are facing irreversible blindness,” said Dr. Kenneth Fong, managing director and consultant ophthalmologist at OasisEye Specialists, Malaysia.
SYFOVRE is a complement factor C3 inhibitor and was approved based on the OAKS and DERBY trials, which showed a 17% reduction of GA lesion growth over 2 years, he continued.
IZERVAY is a complement factor C5 inhibitor and was recently approved as a treatment for GA. It blocks the complement factor cascade further
downstream compared to SYFOVRE. The GATHER1 and GATHER2 trials showed a 35% reduction in GA lesion growth over 6 months.
“For the reporting of GA clinical trials, we can no longer use visual acuity improvement and OCT macula thickness as markers of drug efficacy. Modern retinal practices now need to acquire imaging tools like scanning laser ophthalmoscopes that are able to perform fundus autofluorescence imaging that can accurately measure the GA lesion size at the macula and track its progression,” he explained.
“Intraocular inflammation is one of the feared adverse events from intravitreal injection and postapproval monitoring of patients receiving new medications is essential,” he added.
While such adverse events were not highlighted in the pre-approval OAKS and DERBY trials, 8 cases of retinal vasculitis after SYFOVRE injection were reported by the American Society of Retinal Specialists (ASRS), he pointed out.
“The cause is uncertain but the manufacturers suggest that this may be due to the 19 g filter needle that was supplied with the medication. This is a disappointing event as the drug itself requires a dosing schedule of every 25 to 60 days for at least
a year to have some effect to slow progression,” he said.
Patients will not expect to see any improvement in vision with administration of either drug, he noted.
Prof. Gemmy Cheung, head and senior consultant of the Medical Retina Department at Singapore National Eye Centre, said the two drugs were not yet available in Singapore.
“Of course, it is exciting to have treatment approved for GA for the first time. Although there appears to be fewer patients with GA in Asia, there are definitely some patients who could benefit from these therapies,” she said.
At least the ASRS, however, expressed caution due to the reported cases of vasculitis linked to SYFOVRE, and said that this may affect doctors’ decisions in prescribing the drug.10
According to studies, GA is uncommon in Asian populations compared with those of European ancestry. Even within Asia, geographic differences in GA prevalence were seen, with prevalence in South Asia compared with East Asia.
1. Apellis Press Release. 17 February 2023: Available at: https://investors.apellis.com/newsreleases/news-release-details/fda-approves-syfovretm-pegcetacoplan-injection-first-and-only
2. Astellas Pharma Inc. Press Release. 05 August 2023. Available at: https://www.astellas. com/en/news/28281
3. Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology. 2020;127(2):186-195. [Epub 2019 Jul 16]
4. Liao DS, Metlapally R, Joshi P. Pegcetacoplan treatment for geographic atrophy due to agerelated macular degeneration: a plain language summary of the FILLY study. Immunotherapy. 2022;14(13):995-1006. [Epub 2022 Jul 21]
5. Hoy SM. Pegcetacoplan: First Approval. Drugs. 2021;81(12):1423-1430.
6. GlobeNewsWire. Apellis Press Release. 22 August 2023. Available at: https://www. globenewswire.com/news-release/2023/08/22/2730020/0/en/Apellis-Provides-Updates-onInjection-Kits-and-Rare-Safety-Events-with-SYFOVRE-pegcetacoplan-injection.html
7. Jaffe GJ, Westby K, Csaky KG, et al. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology. 2021;128(4):576-586.
8. Patel SS, Lally DR, Hsu J, et al. Avacincaptad pegol for geographic atrophy secondary to agerelated macular degeneration: 18-month findings from the GATHER1 trial. Eye (Lond). 2023; Mar 24 [Online ahead of print]
9. Astellas Pharma, Inc. Press release. 12 July 2023. Available at: https://www.astellas.com/en/ news/28116
10. ReST Committee Update on Intraocular Inflammation (IOI). Available at: https://www.asrs.org/ content/documents/asrs-rest-committee-update-on-intraocular-inflammation-after-ivi-2023.pdf
Accessed on 29 September 2023.
A version of this article was first published on piemagazine.org
Dr. Kenneth Fong is recognized as an ophthalmologist in the United Kingdom, Australia and Malaysia. He graduated with a medical degree from the University of Cambridge in 1998 and trained to be an eye surgeon in London. Dr. Fong then spent two more years training in the U.K. and at the Royal Perth Hospital in Australia to subspecialize in retina. After 18 years of working in the U.K. and Australia, he returned to Malaysia in 2009 to serve as associate professor, consultant ophthalmologist and retinal surgeon at the University of Malaya in Kuala Lumpur. He is currently the managing director of OasisEye Specialists in Kuala Lumpur. Dr. Fong is the president of the Malaysian Society of Ophthalmology and serves as a council member for the APVRS.

ken.fong@oasiseye.my
Prof. Gemmy Cheung is currently a professor at Duke-NUS Medical School, National University of Singapore, and head of the Medical Retina Department at Singapore National Eye Center (SNEC). Her research focuses on retinal diseases, including age-related macular degeneration (AMD), polypoidal choroidal vasculopathy (PCV) and myopic macular degeneration, as well as risk factors for these conditions that may be unique to Asian populations. Prof. Cheung has more than 200 peer-reviewed publications and serves on the editorial boards of several journals, including the American Journal of Ophthalmology, Retina and Eye.
gemmy.cheung.c.m@singhealth.com.sg

 by Andrew Sweeney
by Andrew Sweeney
Anti-VEGF treatment is one of the most valuable tools ophthalmologists use to treat age-related macular degeneration (AMD). But what happens if your patient shows resistance to this treatment? We delve a little deeper into the progress made over the past decade and the alternative tools clinicians have at their disposal to treat AMD.

Imagine traveling back in time, even just a few decades, and informing elderly patients with vision problems that there would be a way to help arrest the progression of AMD. Despite telling them that the disease cannot be fully cured, the prospect of halting its progression and ensuring that their current eyesight could be preserved would be fantastic news.
However, they might be a little less excited when you explain to them that successful treatment relies on frequent injections directly into the eye, and that despite diligently adhering to this regimen, there remains a reasonable chance of treatment failure.
AMD is one of the most common causes of blindness throughout the world, affecting over 200 million people globally. As many countries continue to experience aging populations, cases will grow exponentially year on year.
Currently, AMD is most commonly treated by targeting vascular endothelial growth factor (VEGF), a potent endothelial-specific mitogen that promotes angiogenesis and vascular hyperpermeability in response to hypoxia.1
These processes are associated with both retinal neovascularization and choroidal neovascularization. The resulting treatment, anti-VEGF, is designed to inhibit VEGF, blocking the growth of this detrimental factor.1
Non-compliance is one of the most prominent issues with anti-VEGF treatment, as nobody likes to get injections in the eye. In fact, this topic is commonly discussed during ophthalmology conferences around the world.
That’s not surprising as only a masochist of the highest order would
think this a jolly experience. However, maintaining a regime of treatment is absolutely crucial when it comes to anti-VEGF treatment. There’s no shortage of literature out there about how to maintain a good treatment schedule, which interval regime is most ideal, and how to encourage recalcitrant patients when they begin to slip, among others.
What happens when your patient is anti-VEGF resistant?
But perhaps a more critical issue arises—and it’s one that is less discussed in AMD literature: What do you do when anti-VEGF treatment encounters resistance and fails to achieve the desired result, even if the patient is fully compliant? After all, the success rate of anti-VEGF treatment is not 100%.
While the treatment does offer significant functional and anatomical advantages for most patients, 20% to 40% of eyes fail to respond, or exhibit only a partially positive response.2
The search for a solution to this problem goes back well over 10 years, and it’s interesting to examine the progress that’s been made over the years. For instance, one research paper that was published during the 70th All India Ophthalmology Conference (AIOC) in 2012, titled Why Does Anti-VEGF Treatment Fail In Age-Related Macular Degeneration AMD?, examined the issue of treatment failure in more detail. It’s worth noting that anti-VEGF was only approved by the US Food and Drug
The study examined 52 eyes that received anti-VEGF therapy and showed a poor response (>2 line loss) one year afterward, with nonresponders defined as having no change in fluid/vision after three loading injections.3
The main finding of note was that all patients involved were primarily diagnosed to have subfoveal occult choroidal neovascularization, and that 11 eyes were identified to have tachyphylaxis. The Indian researchers focused on the latter issue, noting that there was “some evidence to suggest that tachyphylaxis may be partially alleviated by combining medical therapies with different mechanisms of action.” The studied called for further studies to investigate the possibility of using anti-VEGF in combination with photodynamic therapy (PDT).3
Given the growing issue of rapidly aging populations and the increasing rates of AMD, we have a ticking time bomb on our hands. Were our friends from the AIOC right after all?
Another study, Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options, published by two researchers based in the US, found that anti-VEGF resistance was associated with platelet-derived growth factor, fibroblast growth factor, placental growth factor, interleukins and transforming growth factor.2
The authors added that “given the origins of anti-VEGF therapy are in oncology, resistance in cancers may provide insight into the culprits of anti-VEGF resistance in retinal diseases2,”—an interesting point for further research.
To counteract tachyphylaxis resistance, as covered by the Indian researchers, the American paper said that numerous researchers have proposed switching agents to a different anti-VEGF, which may offer improved responsiveness.
In one study cited by the paper, of 63 treatment-naive eyes with AMD initiating anti-VEGF therapy, 14 eyes (22.2%) were unresponsive to initial anti-VEGF medication, and eight eyes (12.7%) developed tachyphylaxis during the one-year follow-up period. In these cases, treatment with ranibizumab was noted to be effective, with 34% of eyes in one study exhibiting an increase of five or more letters after the switch in therapy.3
In another eyebrow-raising finding, the American researchers said that while PDT has fallen out of favor and has been eclipsed by antiVEGF, it should be considered in cases of resistance. That’s because in one study where 34 patients required ‘rescue PDT’ as a result of resistance, their collective visual acuity improved significantly at three months, withmacular thickness doing the same at all follow-ups. This treatment, which still included anti-VEGF injections, highlights that a hybrid approach could be effective.

All this may be a blast from the past, but it is one that validates the accuracy of the AIOC research paper. One thing we’d like to emphasize, echoing the concluding statements of every paper examined for this article: More research is required.
So please, if you’re looking for a new paper to write for an upcoming performance, give dealing with antiVEGF resistance a go.
1. Tan CS, Ngo WK, Chay IW, et al. Neovascular Age-Related Macular Degeneration (nAMD): A Review of Emerging Treatment Options. Clin Ophthalmol. 2022;16:917-933.
2. Wallsh JO, Gallemore RP. Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells. 2021;10(5):1049.
3. Manoj S, Unnikrishnan Nair R, Ramachandran Nair KG. Why Does Anti-VEGF Treatment Fail In Age-Related Macular Degeneration AMD? 70th All India Opthalmology Conference. Available at http://proceedings.aios.org/ aiosedu/uploads/BOB19.pdf. Accessed on July 05, 2023
Innovations in medical retina are rapidly evolving, and all are aimed at providing safe and optimal care to retina patients. These include advancements in imaging that allow us to see the retina with unprecedented detail, precise treatment strategies of previously untreatable sight-threatening diseases and delivering novel and familiar agents in new ways.

While reports indicate that the first intravitreal injection (of air) was administered in 1911, significant progress in the intravitreal treatment of retinal diseases didn’t really pick up steam until about 25 years ago. Case in point: The US Medicare billing code for ‘intravitreal injection of a pharmacologic agent’ was submitted approximately 2,300 times in 1993, compared to 3.4 million times in the past few years.
Keep in mind this was solely based on Medicare billing—the real number of injections is estimated to be closer to 7 or 8 million in the US alone. To keep up with the demand for treating a wide range of retinal conditions that can be managed by intravitreal injections. There are reports of some retinal specialists performing over 50 injections each day!
Don’t get me wrong, you won’t hear the retina community complaining. Having treatment options where none existed before is wonderful— especially for those who have been practicing both before and since the anti-vascular endothelial growth factor (VEGF) era. However, the growing number of patients –primarily those with age-related retinal disease and the increasing prevalence of diabetic-related complications – coupled with the need for frequent treatments, is squeezing retina clinics into a major capacity challenge. It’s like needing to wear roller skates to keep up!
In order to meet this demand, researchers and innovators are taking their best shots to develop products that are safe, efficacious and durable.
So what are the main forms of the innovation pipeline? New agents include new molecules with new modes of action as well as some familiar agents with new formulations or dosing regimens. Although safety is the highest priority, efficacy and durability are key to reducing the number of patient visits. This all lessens the burden on patients and caregivers, and reduces the stress on
retina specialists and their bursting clinics.
Dr. Arshad M. Khanani, a vitreoretinal specialist, managing partner and director of clinical research and director of fellowship at Sierra Eye Associates, as well as a clinical associate professor at the University of Nevada at Reno, has been on the frontline of many of these innovations as a principal investigator for numerous clinical trials.
Dr. Khanani has been witness to how anti-VEGF therapies have revolutionized the management of neovascular age-related macular degeneration (nAMD) and other VEGF-driven ocular diseases, significantly improving visual outcomes.
However, he also recognizes that there is still work to do. “Unfortunately, treatment burden continues to be a major concern for many patients, their caregivers and the healthcare system. For most of the current agents, some patients are still requiring eye injections as frequently as every month, and outcomes outside of clinical trials tell us that we can still improve with longer-acting and more durable therapies.”
Dr. Khanani notes that the approval of faricimab (Vabysmo; Genentech, California, USA) has provided a welcome addition to the retinal armamentarium. “It is the first bispecific drug approved for retina diseases and results in simultaneous and potent inhibition of VEGF-A and angiopoietin-2, which play an important role in vascular stability and permeability. [This] delivers the potential for sustained efficacy through extended durability, which, importantly, can help reduce treatment burden for our patients.”
Dr. Benjamin Bakall is a retina specialist at Associated Retina Consultants in Phoenix, Arizona and a clinical assistant professor at the University of Arizona College of Medicine in Phoenix. “The recent approval of intravitreal Vabysmo for both neovascular AMD and diabetic macular edema has definitely been an exciting addition to current treatment options,” he agreed. “Because it is a dual antibody with two binding sites for both VEGF-A and Ang-2, it delivers
that additional benefit over the current treatments we have available.”


A challenge often arises when new agents are released into the real world. Outcomes may differ from those published in clinical trials. Patients seen in the clinic are not necessarily represented by trial participants. Some have been previously heavily treated, some have been non-responders and others switched from other agents.
Dr. Khanani was the lead author of the recently published TRUCKEE study, describing the real-world treatment and outcomes of 376 patients treated with faricimab.1
This group, with varied treatment history, demonstrated positive results in both visual and anatomical parameters, including maintenance of their visual acuity and significant improvements in their anatomy, via central subfield thickness (CST) and improvement of intraretinal fluid (IRF), subretinal fluid (SRF), and pigment epithelium detachment (PED)—even after just one injection. The treatment was well-tolerated with a low incidence of intraocular inflammation.
Improvements continued after three injections, which may indicate the need for loading doses or multiple injections to achieve maximum benefit—an important tip from realworld data.
High-dose anti-VEGF agents are looking to be the option for those pesky treatment-resistant cases, and recent clinical studies have shown promising results.
Most notably, high-dose aflibercept (8 mg, as compared to 2 mg)* has been evaluated in patients with nAMD in the PULSAR trial, with exciting results.2 At
48 weeks, both highdose treatment arms (8 mg every 12 weeks and 8 mg every 16 weeks after three initial monthly aflibercept injections) met the primary endpoint of non-inferior BCVA gains compared to aflibercept 2 mg, and additionally demonstrated superiority in improving anatomic outcomes of retinal fluid. Significantly, in relation to the treatment burden, 83% receiving high-dose aflibercept maintained ≥12-week treatment intervals.
Another old friend, bevacizumab, the off-label workhorse of the retina, may be receiving a makeover after nearly 20 years. Outlook Therapeutics (New Jersey, USA) is working to develop and launch the first FDAapproved ophthalmic formulation of bevacizumab ONS-5010 / LYTENAVA™ (bevacizumab-vikg) for use in retinal indications.
An ophthalmic-specific formulation means that it will adhere to the specific manufacturing standards and purity requirements for agents designed to be administered into the eye.
Current therapies are focused on blocking VEGF-A by binding to VEGF receptor 2. However, new therapies are looking at whether binding to other receptors, such as VEGF receptor 3 (VEGFR-3), and blocking VEGF-C and VEGF-D might enhance treatment efficacy, especially for those who are not achieving optimal response to current therapy.
OPT-302 (Opthea; Ontario, Canada) targets and sequesters VEGF-C and VEGF-D, so when paired with our trusty VEGF-A blocker, ranibizumab, the combination provided statistically significant superior gain in visual acuity compared to ranibizumab alone.3 Two phase 3 trials are currently underway.
Innovation can be most impactful when there are newly available treatments for devastating

eye diseases. Until only a few months ago, people with advanced dry agerelated macular degeneration with geography atrophy had no options but to go home and go blind.
Dr. Bakall and many others in the medical retina community are now relieved to be able to offer treatment solutions to their patients with geography atrophy.
“The FDA approvals of IZERVAY™ (avacincaptad pegol intravitreal solution; Iveric Bio, New Jersey, USA), and SYFOVRE® (pegcetacoplan injection; Apellis Pharmaceuticals, Massachusetts, USA) are game changers. Both have demonstrated that they can effectively slow down the progression of geographic atrophy in order to prevent vision loss,” he explained.
Recently, there has been a growing interest in the role of tyrosine kinase inhibitors (TKIs) in the treatment of retinal disease. TKIs have the potential to be a maintenance treatment for patients with retinal diseases.
Dr. Khanani explained: “While anti-VEGF therapies target VEGF molecules in the extracellular space, selective TKIs can work intracellularly to stop the downstream effects of all the isoforms of VEGF as well as platelet-derived growth factor (PDGF), potentially improving therapeutic efficacy by inhibiting multiple components of angiogenesis,” he said.
“Early trial results have shown great promise in this space, and I’m excited to see the continued development of TKIs with multiple novel delivery platforms, aimed at promoting sustained delivery,” he continued.
Some TKIs to watch include Vorolinib (Eyepoint Pharmaceuticals, Massachusetts, USA), a TKI that targets all VEGFRs and PDGFRs and comes in the Durasert sustained delivery platform. In the Phase 1 DAVIO trial, 53% and 35% of wet AMD patients did not require a rescue anti-VEGF injection at six and 12 months, respectively, and there were no significant safety concerns.4 The Phase 2 study is ongoing.
TKIs are also taking the suprachoroidal route. Axitinib is a selective TKI for both VEGFR and PDGFR and is being investigated both intravitreally (OTX-TKI; Ocular Therapeutix, Massachusetts, USA) and suprachoroidally (CLS-AX; Clearside Biomedical, Massachusetts, USA) with both routes demonstrating important reductions in treatment burden in early studies.
Also making big waves in the treatment of retinal disease is gene therapy. In the near future, perhaps not all gene therapy requires a trip to the operating room.
In addition to spending his days in the clinic treating the heavy hitters of medical retina, age-related macular degeneration and diabetic retinopathy, Dr. Bakall has a special interest in diagnosing, treating, and providing genetic counseling to patients with inherited retinal diseases.
He shared his thoughts on the most impactful innovation in the retina in the past five years. “Finally having an approved treatment for early onset retinal dystrophy caused by mutations in the RPE65 gene has been impactful for me and, importantly, my patients,” Dr. Bakall said. “Although LUXTURNA (voretigene neparvovec-rzyl; Spark Therapeutics, Pennsylvania, USA) is delivered by subretinal injection in the operating room, outside the purview of most medical retina specialists, this approval has stimulated new research and development into alternative, some even office-based, treatments for retinal diseases caused by different genetic mutations. Current clinical trials are underway investigating intravitreal or suprachoroidal delivery systems.”
Gene therapy does not just stop at the door of inherited retinal disease. This innovation utilizes a nonintegrating viral vector that carries an encoded genetic message to make a novel protein, specifically an anti-VEGF protein. This translates into sustained VEGF suppression, which is very exciting if the current studies are positive, investigating in-
office intravitreal and suprachoroidal delivery of gene therapy for nAMD and diabetic retinopathy.
Dr. Khanani explained how gene therapy may be the next big game changer in nAMD and DR. “Once the cells produce their own anti-VEGF protein, this would effectively allow for a more permanent therapeutic effect compared to other long-lasting delivery platforms. Potentially being a one-time treatment for our patients, or certainly reducing the burden of frequent intravitreal injections,” he said.
So how can gene therapy change the landscape of retinal disease management? “Emerging data from intravitreal and suprachoroidal programs have highlighted the potential of a single in-office treatment to have great durability and disease control for patients with nAMD and DR. Gene therapy has the potential to be a paradigm shift for patients with retinal diseases in the next five to 10 years,” he shared.


* Regeneron Pharmaceuticals, on 18 August 2023, announced that the US FDA has approved EYLEA HD (aflibercept) Injection 8 mg for the treatment of patients with wet AMD, DME and DR.
1. Khanani AM, Aziz AA, Khan H, et al. The real-world efficacy and safety of faricimab in neovascular age-related macular degeneration: the TRUCKEE study - 6-month results. Eye (Lond). 2023 May 12. [Online ahead of print]

2. Regeneron. Aflibercept 8 mg positive pivotal results in diabetic macular edema and wet age-related macular degeneration presented at AAO. News release, September 30, 2022. Available at https://investor.regeneron.com/ news-releases/news-release-details/aflibercept8-mg-positive-pivotal-results-diabetic-macularedema#. Accessed on July 22, 2023.
3. Jackson TL, Slakter J, Buyse M, et al; Opthea Study Group Investigators. A Randomized Controlled Trial of OPT-302, a VEGF-C/D Inhibitor for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2023;130(6):588-597.
4. EyePoint Pharmaceuticals. EyePoint Pharmaceuticals Announces First Patient Dosed in Phase 2 DAVIO 2 Clinical Trial of EYP-1901 for the Maintenance Treatment of Wet AMD. News Release, August 1, 2022. Available at https://investors.eyepointpharma. com/news-releases/news-release-details/ eyepoint-pharmaceuticals-announces-firstpatient-dosed-phase-2. Accessed on July 22, 2023.
REGENXBIO (Maryland, USA) is currently conducting Phase 2 trials of suprachoroidal gene therapy delivery of RGX-314 to treat wet AMD and diabetic retinopathy.
Researchers and innovators, we salute you! In the not-too-distant future, we are hoping that patients will have access to treatment alternatives that are less demanding on their part, consequently taking some of the pressure off our retina clinics.
Maybe one day soon patients can use their home-based OCT and
Dr. Benjamin Bakall , MD, PhD, is a retina specialist at Associated Retina Consultants in Phoenix, Arizona, and a clinical assistant professor at the University of Arizona, College of Medicine Phoenix. Dr. Bakall diagnoses, treats, and provides genetic counseling to patients with a range of retinal disorders, including inherited retinal diseases, age-related macular degeneration, and diabetic retinopathy. He completed his medical degree and PhD at Uppsala University in Sweden, focusing on identifying and analyzing the gene causing macular dystrophy. He continued his laboratory research at the University of Arizona as a post-doctoral fellow, where he also did his ophthalmology residency training. He completed a medical retina and inherited retinal disease fellowship at the University of Iowa. Dr. Bakall is also involved in dozens of clinical trials for retinal diseases. He has written several research manuscripts and book chapters on retinal diseases. He is the founder and president of the Retina Research Foundation of America. He has been frequently invited speaker on retinal diseases, including at the Foundation Fighting Blindness Visions conference, and has been the medical chair for the Arizona Vision Walk.

bbakall@gmail.com
electronically send the scans to you, no matter where you are—whether you are at the clinic or enjoying some beachfront downtime. And perhaps you can let them know that it is time for them to come back for that 12-month retreatment.
Dr. Arshad M. Khanani , MD, MA, FASRS, is a managing partner, director of clinical research and director of Fellowship at Sierra Eye Associates, and clinical associate professor at the University of Nevada, Reno School of Medicine. Dr. Khanani founded the clinical research department at Sierra Eye Associates, which is now one of the leading clinical research centers in the country. He has served as a principal investigator for over 100 clinical trials and has been a top enroller in the country for multiple Phase 1-3 trials. In addition, Dr. Khanani has been the first one to perform surgical procedures in multiple surgical clinical trials dealing with sustained delivery and gene therapy. He has over 90 scientific publications. Dr. Khanani also serves as a member of national and international clinical trial steering committees as well as scientific advisory boards to bring new treatment options for patients with retinal diseases. He is frequently invited as a guest speaker at national and international meetings. Dr. Khanani is an elected member of the Macula Society, Retina Society and has received numerous awards of distinction. In 2019, he received the Nevada Business Magazine Healthcare Heroes Physician of the Year award for his continued dedication to the field of ophthalmology. He received the Senior Honor Award from the American Society of Retina Specialists (ASRS) and was also awarded the prestigious ASRS Presidents’ Young Investigator Award in 2021. In 2023, Dr. Khanani was invited to give the prestigious 2023 Ernst Bodenheimer Memorial Lecture at the Wilmer Eye Institute at Johns Hopkins University School of Medicine.
arshad.khanani@gmail.com
nature of most nanoparticles eliminates the need for surgical implantation and subsequent removal after the release of the payload.1
When it comes to the latest advancements in posterior segment treatment, we often focus on anti-vascular endothelial growth factor (anti-VEGF), imaging and lasers. However, there are so many other areas worth exploring. Case in point: Gels and nanoparticles. We sat down with Dr. Hudson Nakamura to talk about the role these new technologies might play in shaping the future of posterior segment patient outcomes.
With so many developments in ophthalmology these days, it’s quite surprising that we only tend to focus on a few key advancements— often overlooking some truly fascinating new techniques and technology. Innovative uses of antiVEGF therapies for numerous conditions continue to generate attention, as do advancements in imaging techniques. Of course, lasers, too, will always capture our attention, not only due to their cool factor but also because of their effectiveness.
Needless to say, it’s easy to see why certain technological developments tend to garner more interest in publications and among ophthalmologists. However, several significant breakthroughs could be slipping under our radar, and we don’t want that!
We spoke with a friend of PIE magazine, Dr. Hudson de Carvalho Nakamura, a vitreoretina specialist from Brazil, to get his expert opinion about these new advances.
“Treating retinal diseases could become a challenge if you do not consider delivery systems,” Dr. Nakamura said. “Considering the effectiveness of using nanoparticles, with the advantage of their biodegradable nature, and thus avoiding the need for removal due to their biodegradability, is highly beneficial.”
Take nanocarrier-mediated retinal drug delivery, for example. This technology uses tiny nanoparticles to deliver drugs to areas of the body that can be very resistant to foreign substances. To overcome this barrier, frequent intravitreal injections are currently used to achieve high drug concentrations in the vitreous and retina. But as is known, using these repetitive injections may result in several side effects.
A potential solution could involve utilizing the aforementioned nanoparticles as, compared to the majority of commercially available ocular implants, the biodegradable

“The nanoparticles’ size and their highly modifiable surface properties are characteristics that make them very good candidates for intraocular drug delivery – even those nanocarriers aiming at treating genetic diseases,” continued Dr. Nakamura.
Three researchers based at the University College Cork in Ireland, carried out a review of scientific literature on the efficacy of nanoparticle drug delivery. They examined most studies available on the issue and found that “nanotechnology-based drug delivery systems possess great potential to resolve the shortcomings of existing strategies for drug delivery to the retina.” In particular, they pointed to Ozurdex, manufactured by Allergan, Inc. (Dublin, Ireland), as being particularly promising for clinicians, adding that there’s room for improvement across the board when it comes to low drug loading and possible toxicity issues.1
Keeping the theme of intraocular drug delivery going, we also looked into the biocompatible reverse thermoresponsive polymer for ocular drug delivery. This novel technique could have several applications in age-related macular degeneration (AMD).
As AMD is one of the most pervasive conditions in modern ophthalmology, with thousands of new patients emerging every year as global populations age, finding new treatments is a major priority for medicine today.
This issue becomes even more evident considering anti-VEGF, the current gold standard for treating the disease, relies on frequent injections directly into the eye (which is not ideal for patient compliance) and is only effective at arresting the progression of the disease.2
“It is very important to rely on intraocular drugs for the treatment
of major retinal diseases, such as diabetic retinopathy and AMD, especially with the latter where the primary complication involves choroidal neovascular membranes. It’s just as important to achieve an optimal result with fewer injections and using a better vehicle to deliver the medication to the patient’s eye,” Dr. Nakamura added.

A group of intrepid researchers from Australia also caught Dr. Nakamura’s eye for their investigation of the suitability of a novel reverse thermoresponsive polymer (RTP) as an ocular drug-delivery vehicle. They examined RTP as it has a polylacticco-polyglycolic acid backbone, which is structured to have linear polymeric backbones, resulting in a loosely bound gel structure due to weak intermolecular attractions and entanglements with less solid content. This prevents the long-term encapsulation of drugs within the gel, characterized by initially high burst release and faster ongoing release rates, thereby reducing the window of release period.2
During trials, approximately 67% of bevacizumab, an anti-VEGF, was released during the first 14 days as compared to 10% of aflibercept 2 mg for the same period. Overall, approximately 95% of bevacizumab and 25% of aflibercept were released over the 183-day period covered. The Australian researchers said that the high initial burst release of bevacizumab can be beneficial in ocular drug release, allowing for a rapid increase in intravitreal drug concentration to a therapeutic level. Depletion of the drug in the matrix is a potential concern. However, in their concluding remarks, they stated that RTP is well-suited for use as a sustained drug delivery vehicle in the eyes2—a sentiment that Dr. Nakamura echoed.
“RTP could actually improve the release curves of drugs such as bevacizumab and aflibercept, while the appropriate biocompatibility of the device could result in the improvement of visual acuity. This should help resolve AMD’s complications using such a good delivery method. There are already good signs for the success of this technique,” Dr. Nakamura enthused.
Another interesting development in the field posterior segment that deserves our attention is the use of hydrogel. Similar to other techniques covered in this article, hydrogels have the potential to get through those pesky barriers within the eye that make treatment more challenging.
Hydrogels are three-dimensional networks of synthetic or natural polymer chains crosslinked by physical or chemical bonds and are believed to offer numerous advantages compared to other treatments. These include their tissue-mimicking properties, desirable soft nature, and ability to absorb large amounts of water (up to 99% of their weight) while maintaining their structure—thanks to the crosslinks between the hydrophilic polymer chains.3
So what does Dr. Nakamura think?
“In the era of intraocular injections, hydrogel formulations may make it more feasible for intraocular drugs to reach the retina and act in maintaining and sustaining therapeutic levels without the need for too many injections. Diseases such as diabetic retinopathy, agerelated macular degeneration and others could be of great benefit for that purpose,” Dr. Nakamura said.
The research3 supports Dr. Nakamura’s position in terms of the potential of hydrogels. However, there are numerous challenges faced by scientists in bringing the technology to full efficacy. There are, for example, numerous problems with initial gel viscosity, hydrogel turbidity, crosslinking strategies, sterilization procedures, storage conditions, and long-term intraocular safety. Also, the continuous down-regulation of VEGF in the eye due to the prolonged release of anti-VEGF from the hydrogel depot might, in the long run, cause undesired side effects.3
However, the same study that points out the above issues surrounding hydrogels also highlights that injectable hydrogels are attractive tools for sustained protein delivery to the back of the eye. More research for hydrogel products is in the
pipeline, showing great potential in this area.4-6
Thus, with two proven techniques in the (capsular) bag as it were, and a promising one in the pipeline, there’s a lot to be excited about in the realm of posterior segment drug delivery innovations.3
1. Alshaikh RA, Waeber C, Ryan KB. Polymerbased Sustained Drug Delivery to the Ocular Posterior Segment: Barriers and Future Opportunities for the Treatment of Neovascular Pathologies. Adv Drug Deliv Rev. 2022;187:114342.
2. Balachandra A, Chan EC, Paul JP, et al. A Biocompatible Reverse Thermoresponsive Polymer for Ocular Drug Delivery. Drug Deliv. 2019;26(1):343-353.
3. Ilochonwu BC, Urtti A, Hennink WE, Vermonden T. Intravitreal hydrogels for sustained release of therapeutic proteins. J Control Release. 2020;326:419-441.
4. Wu Y, Tao Q, Xie J, et al. Advances in Nanogels for Topical Drug Delivery in Ocular Diseases. Gels. 2023;9(4):292.
5. Rafel D, Guerrero M, Marican A, et al. Delivery Systems in Ocular Retinopathies: The Promising Future of Intravitreal Hydrogels as Sustained-Release Scaffolds. Pharmaceutics. 2023;15(5):1484.
6. Gabai A, Zeppieri M, Finocchio L, Salati C. Innovative Strategies for Drug Delivery to the Ocular Posterior Segment. Pharmaceutics. 2023;15(7):1862.
Dr. Hudson Nakamura is an ophthalmologist specializing in the retina and vitreous. He completed his medical degree from School of Medicine at the Federal University of Goiás, UFG and residency from the Base Hospital of the Federal District, Brasília, DF. Presently, Dr. Nakamura is a member of the AAO, Brazilian Council of Ophthalmology, Canadian Society of Ophthalmology and ARVO. He currently works as a professor in the Department of Retina and Vitreous Course of Medical Residency in Ophthalmology at the Bank of Goias Eye Foundation. Dr. Nakamura holds a vitreoretinal disease fellowship from the University of Toronto Canada and the Brazilian Center for Eye Surgery.
hudson.nakamura@gmail.com
AI tools make the workflow more effective for eye care specialists.
The eye care industry produces billions of medical images each year. For good reason, too: Treatment success often depends on the accurate interpretation of these medical images by eye care specialists. Artificial intelligence (AI) tools are available to offer support in interpreting fundus photos and optical coherence tomography (OCT) scans. However, a significant number of ophthalmologists are still skeptical about these advancements.
Progress in the healthcare industry is unstoppable. And soon, AI tools that analyze medical images will be more prevalent in eye care practices for various reasons.
Here are four key factors highlighting the importance of artificial intelligence in medical image analysis:
AI tools enable better patient triage.
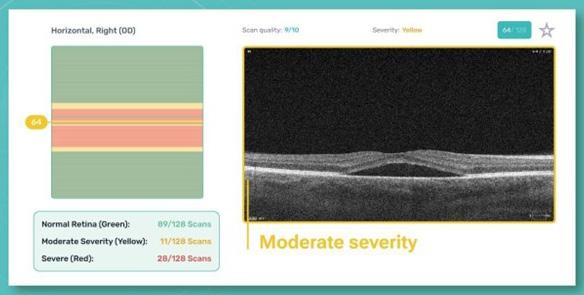
For instance, Altris AI (Illinois, USA), one of the tools for automated OCT scan analysis, has an AI severity module that separates B-scans into three categories: low-severity, medium-severity, and high-severity B-scans. If all scans are graded as green, then there is no pathology detected.
This can help to quickly screen
patients to see if they are healthy, or if the scans need more in-depth analysis. As a result, ophthalmologists can avoid wasting time on nonpathological scans.
If any pathology is detected, then the severity module will highlight any B-scans with pathology and grade the level of severity of the pathology. This creates a more efficient way to determine the presence of pathology and its specific location within the OCT exam. This, in turn, reduces the time needed for interpretation and more time to discuss the results with the patients, or potentially to see more patients in the clinic.
After all, what modern ophthalmologists need is time for diagnostic methods and communication with patients.
AI tools help to timely detect life-threatening conditions.

Many companies are already using the potential of AI to provide early detection and diagnostic advice for eye care specialists.
AI Severity Module: This module differentiates between high, medium and low severity b-scans and helps eye care professionals to pay attention to those that need urgent referral only.
For instance, Eyenuk (California, USA)—AI for fundus photo analysis— aims to screen every eye in the world to ensure timely diagnosis of life- and vision-threatening diseases, including diabetic retinopathy, glaucoma, agerelated macular degeneration and others.
Altris AI—AI for OCT analysis—pays special attention to screening agerelated conditions, genetic diseases and diabetic retinopathy, as these conditions lead to blindness.
Artificial intelligence tools can help direct attention to red flags when examining the patient. For instance, macular holes, macular telangiectasia (MacTel), vascular diseases, central retinal vein occlusion (CRVO), and central retinal artery occlusion (CRAO) should be studied in more detail.
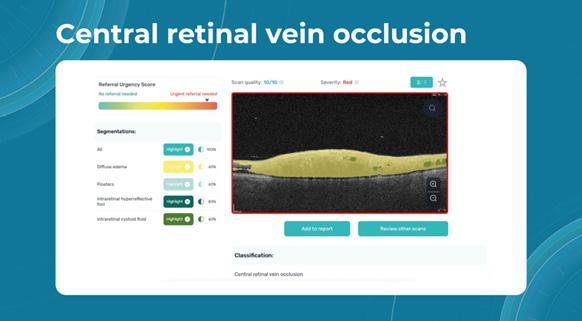
Here is an example of how AI detects CRVO:
Detecting early signs of conditions that may lead to blindness is of utmost importance for the healthcare industry. Given the aging population trend, the workload for ophthalmologists is bound to increase.
AI helps in enhancing patient education through comprehensive visual reports.
Patient education plays a critical role in patient outcomes, as well. When patients truly understand their
condition, they are more likely to undergo regular eye checkups and adhere to the treatment plan.
Medical images can sometimes seem intimidating and puzzling to patients. However, AI tools often use color coding for detecting pathologies, allowing patients to understand medical images and reports better. Patients can even trace the progress of certain pathologies with the help of AI reports.
There are several AI tools for OCT and fundus photo analysis that are CE-certified and have FDA clearance, proving their efficiency and safety. As eye care practitioners, one of our most important tasks today is
to eliminate skepticism surrounding these new tools.
After all, these tools are not intended to replace the work of ophthalmologists in any way. Instead, they are meant to automate routine operations and serve as decision-making support systems for ophthalmologists.
On 18 September 2023, Altris, Inc. announced that it has received FDA 510(k) clearance for the Altris IMS, the company’s image, and data management platform.
Maria Znamenska , MD, PhD, is the chief medical officer of Altris Inc, the company that developed an AIpowered platform for retina pathologies detection on OCT scans (74 pathologies including wet AMD, DR and glaucoma). The system (Altris AI) is CE-certified and is widely used at optometry centers and eye clinics in the United Kingdom. Dr. Znamenska owns an ophthalmic clinic in Kyiv, Ukraine.
mznamenska@altris.ai



Driven by a passion and motivation for transforming lives through vision preservation and restoration, Dr. Sashwanthi Mohan has been making a mark as a young and accomplished ophthalmologist in her native country of India and, more recently, in Dubai. More than anything, she is committed to making a positive impact on people’s lives.
Dr. Mohan received her training with outstanding performances at two prestigious ophthalmic institutes. She did her residency at L.V. Prasad Eye Institute in Hyderabad under the National Board of Examinations, where she was awarded a gold medal for the highest score in Ophthalmology. And for her Vitreoretinal fellowship at Sankara Nethralaya, Medical Research Foundation in Chennai, she was recognized as the Best Outgoing Vitreoretinal Fellow.
“Working in those two top-notch institutes has truly shaped me into a better ophthalmologist. The exposure to advanced techniques and the guidance of exceptional mentors have significantly helped me enhance my skills and knowledge,” said Dr. Mohan, adding that the diverse range of patient cases she encountered has been instrumental in her growth and development.
Growing up in Chennai, Tamil Nadu, India, as a thirdgeneration ophthalmologist, Dr. Sashwanthi Mohan has inherited a profound passion for the field.
“Both my parents are respected ophthalmologists, and their dedication has been a constant source of inspiration,” shared Dr. Mohan, who, at only 32, is currently a specialist ophthalmologist and vitreoretinal specialist at Medcare Eye Centre in Al Safa, Dubai, under the Dubai Health Authority (DHA).
However, she confessed that at first, ophthalmology wasn’t really on her radar. “With both my parents being ophthalmologists, I wanted to venture into something different to carve out my own niche. I eventually found myself drawn to ophthalmology due to my genuine interest in its combination of medical expertise and surgical skills. It seemed like the perfect balance for me, allowing me to explore both fields simultaneously,” she noted.
“I also had the opportunity to work as a consultant at Rajan Eye Care in Chennai, alongside my parents. It was a valuable experience that further enriched my skills and provided me with practical exposure in the field of ophthalmology,” she added.
During her residency, Dr. Mohan was instantly fascinated by the retina, especially when she got her first glimpse of it using an indirect ophthalmoscope.
“This fascination kept growing throughout my residency and ultimately led me to choose this field,” she enthused. “The delicate nature of the retina, with its intricate layers and the vital role it plays in our vision, truly intrigued me. Witnessing the profound impact that

retinal conditions can have on patients’ lives, combined with the challenging yet rewarding nature of retinal procedures, solidified my decision to specialize in this field,” she shared.
For Dr. Mohan, the opportunity to closely engage with patients, diagnose various retinal disorders, and provide comprehensive management while restoring and enhancing vision is both intellectually stimulating and deeply gratifying.
After completing her fellowship in vitreoretinal practice in July last year, Dr. Mohan embarked on her professional journey. Initially, she started performing surgeries at Rajan Eye Care, her parent’s hospital in Chennai. However, earlier this year, she relocated to Dubai, where she is currently in the process of establishing herself as an ophthalmologist specializing in cataract and vitreoretinal diseases.
“Starting my career in Dubai meant embarking on a journey to build my reputation as an ophthalmologist from scratch,” she said. “While my current focus is on medical retinal practice and cataract surgeries, I have yet to venture into vitreoretinal surgery in this new setting. I aspire to do that in the near future.”
Initially, the young ophthalmologist would see a modest number of five to six patients a day. “However, with unwavering effort and dedication, my patient load gradually increased over the span of two to three months,” she shared.
Through perseverance and a steadfast commitment to providing quality care, Dr. Mohan is steadily growing her practice and making a positive impact on the lives of her patients.
“I am excited about the opportunities and challenges that come with building my practice in this new environment, and I look forward to making a meaningful contribution to the field of ophthalmology in the region,” she added.
Dr. Mohan’s passion for education is evident in her dedication to sharing her knowledge through various educational initiatives. In December 2019, she launched Ophthalmobytes, a comprehensive ophthalmology website and Instagram page, which aims to create an all-encompassing atlas of ophthalmology.
Her primary objective was to provide concise and accessible educational content through visually appealing images and succinct text. Her sister, Dr. Madhuvanthi Mohan, who is currently pursuing a fellowship in cornea, is in charge of the website, playing a pivotal role in ensuring regular updates with high-quality content.
The updates provided on Ophthalmobytes have been incredibly useful to ophthalmic professionals. “We have endeavored to build a resource that simplifies ophthalmology education and offers practical insights,” she shared.
“Working in those two top-notch institutes has truly shaped me into a better ophthalmologist. The exposure to advanced techniques and the guidance of exceptional mentors have significantly helped me enhance my skills and knowledge.”
Dr. Mohan has also initiated the #Retina101bySM on LinkedIn, where she actively shares information and insights related to the vitreoretinal field.
In addition, Dr. Mohan also actively participates in webinars organized by renowned organizations, such as the Ophthalmology Foundation, All India Ophthalmological Society, Cybersight by Orbis, India Vision Institute, iTeach, and Medisage, among others.
“These opportunities allow me to contribute to the educational community and expand my reach to a wider audience. I also serve as a virtual education and research associate for Rajan Eye Care Hospital in Chennai. In this role, I actively contribute to postgraduate training programs and regularly conduct educational classes,” she noted.
Dr. Mohan believes in the power of continuous learning and strives to foster a culture of ongoing education and improvement. “By sharing insights, collaborating with colleagues, and staying updated with the latest advancements, I aim to contribute to the advancement of ophthalmology and ultimately enhance patient care,” she enthused.
Working and living in Dubai is quite a stark contrast to her previous work experiences in India, particularly when it comes to healthcare services.
“One prominent distinction is the substantial role insurance plays in Dubai’s healthcare system. Unlike in India, where access to medical services was readily available without significant delays, navigating the insurance landscape in Dubai has become an integral aspect of my practice here,” shared Dr. Mohan.
For her, understanding the intricacies of the insurance system in country has proved to be quite challenging, and required a dedicated effort to familiarize herself with the processes, policies, and requirements involved. “Adapting to the insurancedriven model has been a learning curve, as it necessitates effective communication with insurance providers, adhering to their protocols, and ensuring optimal patient care
within the framework of coverage,” she added.
Having said that, Dubai has become a home away from home for Dr. Mohan. She is enjoying being part of its vibrant and diverse community and finds a unique blend of familiarity and novelty in a city that provides the best of both worlds.
Dr. Mohan has a strong drive for continuous learning and staying up to date with the latest advancements.
“Collaborating with fellow professionals to push the field forward is something I’m really passionate about. The personal fulfillment I get from using my skills to improve the lives of others keeps me dedicated and excited about ophthalmology and its ongoing growth,” she remarked.
What advice does Dr. Mohan have for aspiring ophthalmologists?
“Continuously strive to expand your knowledge, be open to making mistakes and learning from them, connect with experienced mentors, engage with professional societies, embrace technological advancements, prioritize the wellbeing of the patient, maintain a healthy work-life balance, and contribute to community initiatives,” she replied.
In the future, Dr. Mohan hopes to see ophthalmology thriving through increased collaboration.
“Ophthalmologists should be empowered to work together towards breakthrough discoveries,” she said. “I hope to see joint research, interdisciplinary initiatives, innovative solutions, and partnerships with government and nonprofit sectors to revolutionize patient care.”
She also hopes to see conferences and digital platforms serving as vibrant hubs for collaboration, fostering rapid progress, and ensuring widespread access to the latest advancements. “I also hope to see ophthalmological services reaching every corner of the world, ensuring access to quality eye care for all,” she added.
Dr. Mohan considers herself incredibly fortunate to have had the opportunity to train under amazing mentors who have played a pivotal role in shaping her journey in ophthalmology with their guidance and mentorship.

“I owe this incredible journey to the amazing support system I have—my parents, my husband, my sister, my mentors, family, and friends. They have always believed in me and cheered me on every step of the way,” she concluded. “I’m so grateful for their unwavering encouragement, which keeps me motivated to make a real difference in ophthalmology.”
Dr. Sashwanthi Mohan is a licensed specialist ophthalmologist and a vitreoretinal specialist at Medcare Eye Centre in Al Safa, Dubai, under the Dubai Health Authority (DHA). She also remotely serves as the education & research associate at Rajan Eye Care Hospital in Chennai, India. She completed her DNB Ophthalmology at L.V. Prasad Eye Institute in Hyderabad, India, where she was awarded the Dr. G. Venkataswamy Gold Medal by the National Board of Examinations. She then pursued a vitreoretinal fellowship at Sankara Nethralaya, Medical Research Foundation in Chennai, where she was recognized as the Best Outgoing Vitreoretinal Fellow and Lady Fellow. She is skilled in performing cataract surgeries, retinal lasers, intravitreal injections, and vitreoretinal surgeries. She has a keen interest in research and has published many peerreviewed articles. She is a Fellow of the International Council of Ophthalmology (FICO) and a Member of the Royal College of Surgeons, Edinburgh (MRCS). She is also a speaker and editorial board member of the Ophthalmology Foundation (OFEC) and a lecturer on Cybersight by Orbis International.
sashu23@gmail.com

Sophi is the first batterypowered phaco device which offers the surgical team a high mobility and flexibility. It wins through its slim, reduced design. Nonetheless, everything needed for both ergonomic and safe usage is in place.

at the RAI) will foster collaboration, communication and contact directly between industry, regulators, investors, researchers and physicians involved (or interested) in the retinal innovation space.

Hold on to your handlebars: EURETINA 2023 is coming to Amsterdam! From October 5 to 8, delegates from across the globe will convene in the picturesque city known for its canals, art, history and stroopwafels to exchange the latest updates in retinal practice. Here’s what you need to know.
Taking place daily in the Grand Auditorium, these 15 EURETINAsessions capture some of vitreoretina’s key topics and discussions, including vitreoretinal surgery, diabetic retina, imaging, tumors, central serous chorioretinopathy, myopia, age-related macular degeneration, inherited retinal disease and much more.
On Thursday, October 5, from 8:30 to 18:00, delegates can enjoy a deep dive into a key condition with“Posterior Uveitis: The Essentials.” Broken up into several talks throughout the day, this subspecialty session gives doctors the keys to one or more of informative presentations, including Uveitis I: Diagnosis, Work-up and Investigational Procedures; Uveitis II: Main Entities – Non-infectious; and Uveitis III: Treatment, Complications and Scleritis.
There are four prestigious keynote lectures taking place at EURETINA 2023:
#1 Euretina Lecture: “OncoVR—A new subspecialty?”
The 23rd EURETINA Congress will take place at RAI Amsterdam, and this year’s hybrid event is filled with all of the usual (and exciting) meeting suspects. From a top-notch scientific program and fascinating keynote lectures to the exhibition hall and after-hours networking events, we’ve got your preview and can’t-miss guide to EURETINA 2023.

With so many interesting sessions and symposiums featured in this
year’s scientific program, delegates will be faced with tough choices when it comes to deciding which to attend. Below, we’ve earmarked a few highlights—although we recommend thoroughly exploring the final program before making your final selections (so many sessions, so little time!).
Pre-show: Euretina Innovation Spotlight
The fun starts even before EURETINA officially does. On October 4, attend the inaugural EURETINA Innovation Spotlight (EIS). This new half-day program (from 13:00-19:00 CEST
Given by Prof. Bertil Damato (UK) on Friday, October 5, at 16:15 CEST during the Opening Ceremony; followed by the August Deutman Awards
Prof. Damato’s research focuses on ocular oncology—particularly the treatment of uveal melanoma—as well as survival prognostication and quality of life of patients with these tumors. He has served as president of the European Ocular Oncology Group, president of the European Vision and Research Association, and president of the International Society of Ocular Oncology.
#2 Ophthalmologica Lecture: “Beyond Atrophy: Investigating the Leading Disease”
Given by Dr. Maximilian Pfau (Switzerland) on Saturday, October 7, at 13:00 CEST
Dr. Pfau’s research focuses on retinal imaging and functional testing, particularly in degenerative retinal diseases. He is the head of an innovative research lab (Pfau Group) in Basel with a coherent focus on disease mechanisms in inherited retinal degenerations and age-related macular degeneration, as well as applications of artificial intelligence.
#3 Kreissig Award Lecture: “Uveitis: Management with Vitreoretinal Surgery?”
Given by Prof. Dr. Shwu-Jiuan Sheu (Taiwan) on Saturday, October 7, at 14:00 CEST
Prof. Dr. Sheu is well-known for her expertise as a retinal surgeon both in Asia-Pacific and around the world. She is the director and a professor of ophthalmology at Kaohsiung Medical University in Taiwan. And since 2021, she has served as president of the Taiwan Ocular Inflammation Society.
#4 Gisbert Richard Lecture: “OCT at the Point of Care, A Surgeon’s Perspective”
Given by Dr. Cynthia Toth (USA) on Saturday, October 7, at 14:30 CEST
For more than 25 years, Dr. Toth has collaborated with Prof. Joseph Izatt and colleagues to develop integrated
OCT imaging for use in retinal surgery. Her research has also been groundbreaking in the field of infant ocular OCT imaging by enabling the first FDA-cleared handheld system for neonates in 2012. Dr. Toth divides her time between her university appointment and serving as chief medical officer and co-founder of Theia Imaging in Durham, North Carolina.
The city is well-known for having a rather vibrant and lively party atmosphere. So after a long day, make room for a bit of time to play. Celebrate Day 1 in the Exhibition Hall with Welcome Drinks and Networking on Thursday, October 5, from 17:15 to 18:30.
And for a bigger party… not to be outdone by its host city’s reputation, EURETINA is throwing a little bash of its own. On the evening of October 7, delegates are invited to party at the iconic De Hallen Studios in Amsterdam. Because, after all, everyone deserves a little time to unwind and socialize late into the evening.
In 2020, as COVID-19 caused the world to fall apart, Media MICE held down the (virtual) fort, independently producing the CAKE & PIE Post Our team covered ESCRS and EURETINA, reporting on both anterior and posterior news, and digitally delivering these updates during a time when they were truly needed most. This virtual trend carried through to 2021 with another independent PIE Post for EURETINA as the Congress stayed virtual.
Here at Media MICE, we’ve been fortunate to support EURETINA both in-person and online for the last several years. We really got things going at the 2018 Congress in Vienna, where two colleagues (Matt Young and Rob Anderson) embraced a bit of the city’s history (and famous past residents) by dressing in resplendent Mozart costumes.

But we’re more than just fancy dress. During the Congress, Media MICE also independently published and distributed PIE Post, our show daily focusing on the vitreoretinal space, which covered all of the latest news from the meeting. From that point on—cemented by our scientific prowess in print (and the tight leggings and questionable violin playing by our staff)—our fate was sealed and we were sold on EURETINA.
Finally, in 2022, we were back inperson for the 22nd EURETINA Congress in Hamburg, Germany. We took full advantage of the hybrid show, covering sessions both inperson and online to produce another independent PIE Post
Now in 2023, Media MICE is back at EURETINA!
So, look for us in Amsterdam… we probably won’t catch your eye with fancy costumes this time around— but our content sure will. Make sure to follow us on Facebook, LinkedIn and Twitter for live updates from the show!

