FRONTIERS OF SCIENCE






MEDC is here to help bring great ideas to life, right here in Michigan. We’ll help you gain access to investors, expose you to SmartZones, Michigan’s network of regional tech incubators, and connect you to local university resources.
Whether you’re a one-person startup or already established, MEDC offers programs and access to capital that can help your business grow.
Learn more at michiganbusiness.org/entrepreneurship

Michigan’s life sciences industry continues to thrive. From established global leaders to fast-growing companies to numerous early-stage ventures, the state’s life sciences community is diverse.
As this latest issue of BioMatters™ clearly demonstrates, Michigan is uniquely positioned as a global life sciences powerhouse, a home to discoveries and innovation around novel treatments and medical technologies whose development is moving the state’s economy forward.
Our strong life sciences innovation ecosystem is pushing the frontiers of medicine and science broadly. It all begins with a robust basic research cluster, led by the University Research Corridor that includes the University of Michigan (the #1 federally funded R&D public research institution in the U.S.), Michigan State University and Wayne State University, along with other academic and clinical research centers. Together they are conducting cuttingedge research that serves as the substrate necessary for company formation and commercialization, and spurring entrepreneurship across the state.
A network filled deep and wide with manufacturing suppliers, contract research and manufacturing organizations, life science professional services, and more, exist to support private company growth. Similarly, a bevy of government-supported programs and initiatives, a collaborative environment, an engaged investment community, and a can-do spirit, are there to provide the necessary resources to ensure company success.
As a result, Michigan’s life sciences industry continues to thrive. From established global leaders to fast-growing companies to numerous early-stage ventures, the state’s life sciences community is diverse. This growth is rooted in a rich scientific legacy spanning over 150 years, a nationally leading STEM talent pool and a desirable business climate.
The statewide life sciences cluster continues to evolve as well, with the growing need for telemedicine and digital health technologies, preventative and personalized treatments and tests, and being prepared against future public health challenges. We see hives of activity bringing support to inspirational visionaries and promising projects that are pushing the frontiers of scientific knowledge.
The race is on around the globe to lead the bio-industry’s rapid evolution. Here in Michigan – with a plethora of opportunities, assets, capabilities, and momentum – our life sciences cluster is at the forefront of that pursuit.
Sincerely,
 STEPHEN RAPUNDALO, PHD President and CEO, MichBio
STEPHEN RAPUNDALO, PHD President and CEO, MichBio
INNOVATION

CORPORATE CONNECTOR


TECHNOLOGY
INNOVATOR





From groundbreaking discoveries to transformative solutions, together we can achieve extraordinary breakthroughs that redefine industries. Scan the QR codes to learn more about some of the pioneering research being done at MSU or visit our website below to learn how your organization can join forces with our award winning faculty innovators and tap into a wealth of expertise and resources to accelerate your R&D efforts. 2024 Innovation Celebration Faculty Awardees:



The MichBio Preferred Purchasing Program leverages the collective purchasing power of our member companies to obtain steep discounts from our vetted and





















MichBio is the biosciences trade association for the state of Michigan. Our goal is to drive the growth of the state’s bio-industry through advocacy, education, connections, and supportive resources.
Stephen Rapundalo, PhD President and CEO
Emily Brockman Director, Member Relations
Carrie Hranko Manager, Administrative Services
Jamie Krajny Editor Director, Marketing and Industry Engagement
CHAIR
Ken Massey, PhD
Wayne State University
Senior Director, Venture Development Technology Commercialization
VICE CHAIR
Kevin McLeod C2Dx
Founder, President and CEO
Christy Bigelow
Emergent Biosolutions
Vice President and General Manager, Manufacturing Operations
Sean Callaghan Medbio Inc.
Vice President of Operations and General Manager
Rhonda DeLuca
Terumo Cardiovascular Group
Sr. Vice President Global Human Resources
Robert Donofrio, PhD Neogen Corporation Chief Scientific Officer
Lola Eniola-Adefeso, PhD University of Michigan University Diversity and Social Transformation Professor of Chemical Engineering
Christine Haakenson, Ph,D Integrated DNA Technologies Director of Operations
Charles Hasemann, PhD Michigan State University
Assistant Vice President for Innovation and Economic Development



MichBio
3520 Green Court, Suite 175
Ann Arbor, MI 48105-1175
734-527-9150
info@michbio.org michbio.org
Designer: Daina Fuson Designs
Printer: Progressive Printing
© Copyright Michigan Biosciences Industry Association, DBA MichBio
PRESIDENT AND CEO
Stephen Rapundalo, PhD
MichBio
President and CEO
SECRETARY
Vacant
TREASURER
Vacant
Ken Massey, PhD
Wayne State University
Senior Director, Venture Development Technology Commercialization
Kevin McLeod
C2Dx
Founder, President and CEO
Dave Morin
Care Technology Advisors, Managing Member
Stephen Rapundalo, PhD
MichBio
President and CEO
Tom Ross
Grand River Aseptic Manufacturing
President and CEO
John J.H. Schwarz, MD
Former U.S. Representative
Uma Sharma, PhD
MMS Holdings Chief Executive Officer





Century Surgical Training Education is Driving Medical Device Success Encoris
the Urgent Need for Ovarian Cancer Research
Ovarian Cancer Alliance
Adds 25% More cGMP
of
the Gap for Infertility Innovation
New GCIONM Program Launches for Aspiring Intraoperative Neuromonitoring Professionals Lawrence
Reputation for Solving Tough
Patients Around the World
As medical science continues developing cutting-edge implant technologies, Encoris Group Corporation stands out as a leader of innovation in surgical training education— an indispensable part of launching and accelerating the surgical learning experience and the adoption of new medical devices.
Headquartered in Holland, MI, Encoris is a leading designer and manufacturer of 21st-century medical models and surgical trainers. The company’s approach and commitment to excellence in surgical training education is renowned globally, preparing surgeons, fellows, and medical device companies for the challenges of today’s healthcare environment. Incorporating the latest technologies into its simulated surgical training products will significantly expand its portfolio.
When launching a new device, anatomical accuracy and speed to market are of utmost importance. Encoris’ expertise in CAD technologies accelerates product development and manufacturing processes, producing accurate products with the fastest lead times in the industry.
Encoris’ 20+ years of industry knowledge are highly respected and appreciated among medical institutions and corporate heavyweights like Stryker, Johnson & Johnson, Zimmer, Medtronic, and many up-and-coming leading medical device companies. Keen market insight drives Encoris’ innovative spirit, bringing innovative differentiation to its medical device clientele to ensure they remain at the forefront.
With an increasing demand for products that enhance technical skills development, proficient surgical training education instills greater competence and confidence, promoting higher-quality surgical procedural outcomes.
Encoris contributes significantly to elevating the standards of healthcare services and patient healing overall. A welltrained surgeon performs safer surgeries, significantly reducing surgical complications and time in the OR.
Encoris’ surgical education products are not a one-size-fits-all product.
Key to the company’s success are customized trainers that capture the user experience and the unique features and benefits of medical devices.

Cadavers are the primary means of surgical training. Still, they are limited in supply, costly, biohazardous, and do not produce favorable anatomic results - a system of training that continues to burden minimally invasive surgical (MIS) innovation.

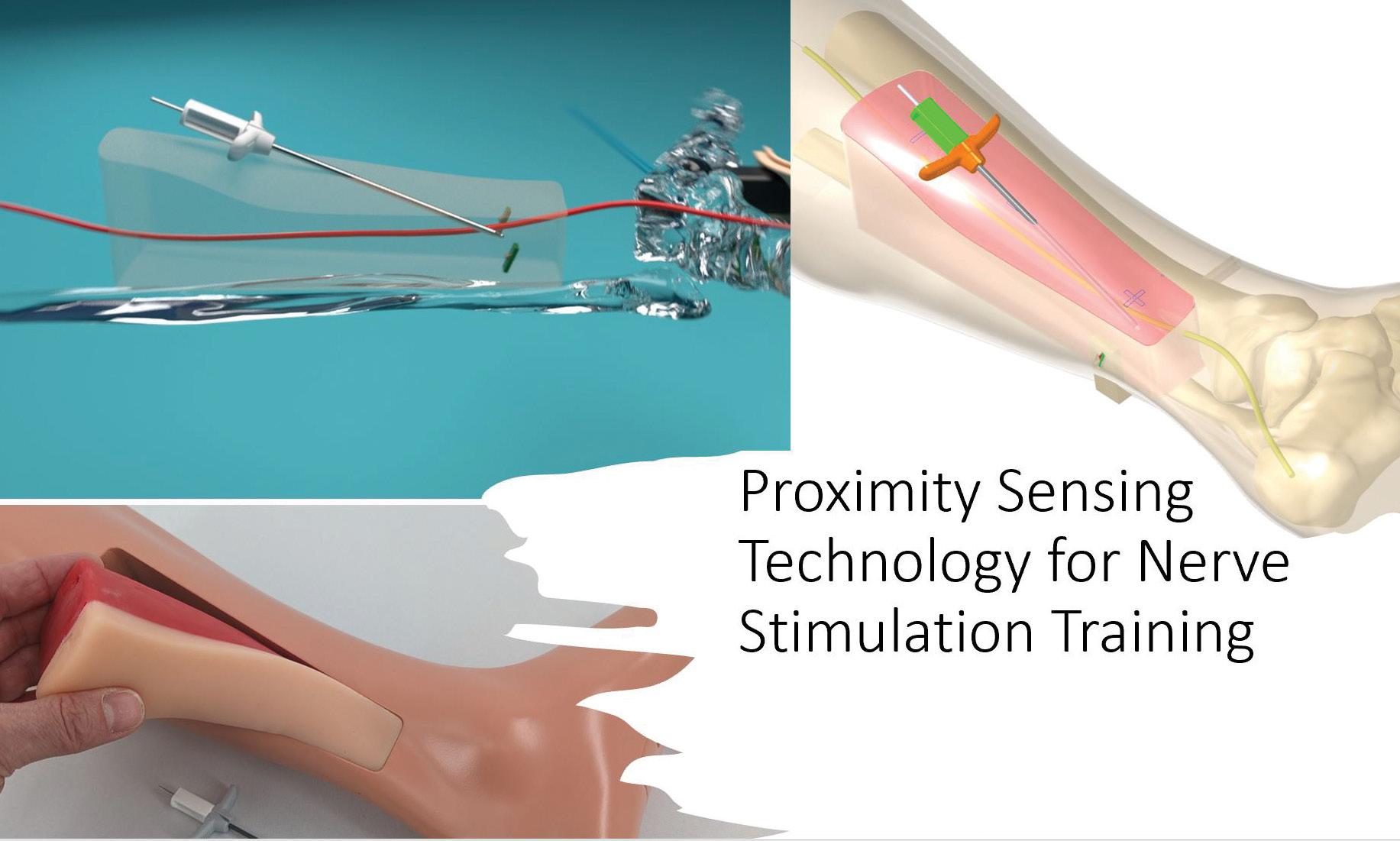
“By understanding a medical device company’s unique educational goals and challenges, Encoris can meticulously design and craft training models that meet specific implant requirements, no matter how elusive. ”
Encoris’ cutting-edge product development capabilities, using the latest technologies like 3D printing, sensory feedback, radiation-free X-ray imagery, and augmented Reality (AR), coupled with pathology-specific anatomy, provide greater experiences for trainees to practice and refine intricate surgical techniques outside traditional cadaver labs.
By understanding a medical device company’s unique educational goals and challenges, Encoris can meticulously design and craft training models that meet specific implant requirements, no matter how elusive. This personalized approach ensures that a medical device company’s medical education objectives are focused, maximized, and refined when introducing new medical devices.
Encoris’ services benefit implant product development and new regulatory compliance. Integrating medical models elevates the assessment of engineering usability, aligning seamlessly with BS EN 62366:2015 +A1 2020 principles. This standard delineates a comprehensive process for manufacturers to scrutinize, specify, develop, and evaluate the usability of medical devices, prioritizing safety. Usability engineering, also referred to as human factors engineering (HFE), plays a pivotal role in designing user interfaces that foster efficient and error-free interactions. Encoris’ models integrate into the usability engineering process, offering manufacturers a dynamic platform to simulate and assess user-device interactions. The iterative nature of medical modeling ensures the final product aligns with contemporary usability engineering concepts, per the updated BS EN 62366:2015 +A1 2020 standards.
Encoris Group Corporation is working with high-tech partners and universities, in collaboration with NASA, to prepare for surgical operations for the moon and Mars missions and long stays on the International Space Station (ISS). Encoris’ models help companies develop instrumentation specific to space travel, test spatial awareness, and the ease and ability to locate organs and deal with fluids in zero gravity.
Nursing and doctor shortages and lack of training are of utmost concern. Encoris is collaborating with Allied Health to help solve this looming problem -We’re bridging the gap between the virtual and physical worlds by creating training products with simultaneous access to the web and HoloLens 2 or Apple Vision Pro devices. Imagine a scenario where a remote instructor can provide live support during a medical procedure. The HoloLens 2 wearer will have access to the procedure program and embedded media of the surgical instruments, with a physical model and instruments to perform the procedure. They will view everything the instructor wants to share. From the instructor’s point of view, they will have access to a screen showing the full context of the procedural steps, advanced live on a physical model. Full access to an audio/ video goggle stream, Encoris’ web-enabled stream, and any additional A/V remote instructor will all bring about an incredibly immersive training experience.

Encoris Group Corporation’s pursuit of excellence in surgical training education has made it an indispensable part of the medical device industry. The company’s model development and surgical training products resonate globally, making it a cornerstone in the ever-evolving field of medical devices.

Reflecting on the progress made in cancer research and treatment, it is important to acknowledge that not all types of cancer have benefited equally.
While there have been significant improvements in overall cancer statistics throughout the past 30 years, ovarian cancer remains a formidable challenge. Despite being the fifth leading cause of cancer deaths among women, ovarian cancer tends to receive less funding and attention compared to other cancers.
There is currently no reliable early detection test that can be used to screen for ovarian cancer and symptoms of the disease can be difficult to recognize or attributed to other conditions. A woman’s risk of getting ovarian cancer during her lifetime is about 1 in 87. The American Cancer Society estimates that in the U.S. nearly 19,680 women will receive a new diagnosis of ovarian cancer, and 12,740 women will die from ovarian cancer in 2024.
The Michigan Ovarian Cancer Alliance (MIOCA) was founded in 2011 because of these disparities and exists today because the community impacted by this cancer continues to rally for change. Through advocacy, education, research funding and awareness initiatives, this network

strives to create visibility of the disease and its symptoms and the urgent need for further progress in the field.
Desiree Swiney, a registered nurse and advocate member of the nationwide Ovarian Cancer Research Alliance (OCRA), was diagnosed with stage 3C ovarian cancer in November 2018. Despite having more than 16 years of experience in healthcare upon diagnosis, she was unaware of what her symptoms could be pointing to. Now, she works with both MIOCA and OCRA on programming that educates future healthcare professionals, political representatives and the general public on issues around ovarian cancer.
“We need to really get that information out there,” says Swiney who is a regular presenter through the Survivors Teaching Students program aimed to educate those entering the medical field. “Talking to that audience is an important thing for me because I know information is lacking.” She has found having those conversations to be a healing part of her cancer journey and looks forward to opportunities where she can educate a wider community.
Her experience of finding out about ovarian cancer is not uncommon. In fact most of those individuals connected to MIOCA did not know about ovarian cancer until they or someone they knew were diagnosed with the disease.


MIOCA was co-founded by Pam Dahlmann and her mother, Geri Fournier, who was diagnosed with stage 3C ovarian cancer in September 2009. Both nurses, the pair recognized the need for greater awareness and treatment options. But by experiencing this cancer first hand and not finding many resources along the way, Geri knew another crucial need of patients was connection and a sense of community.
“There wasn’t any support for her on her journey so she didn’t want another woman to go through an ovarian cancer journey alone.” says Dahlmann.
Together, she and her mother connected with existing out-of-state groups and networked with others in the area working to bring awareness to ovarian cancer. They actively worked to engage Michiganders across the state by partnering for events, presentations and fundraisers to get the word out about MIOCA as a resource for all those impacted by the disease.
Geri passed away in March of 2011 but her memory lives on in the work of MIOCA and in the spirit of all those who engage in the movement toward progress and support. Today, MIOCA serves those impacted by ovarian cancer by providing resources, education and opportunities to connect with a network of people dedicated to a shared cause.

On May 8 for World Ovarian Cancer Day, we urge people in Michigan and beyond to educate themselves and others on ovarian cancer risks and symptoms, understand their family history, and consider genetic testing—a crucial step in detecting ovarian cancer. We also invite those members of the bioscience community to use their unique expertise and perspectives to explore concepts that could improve the future landscape of ovarian cancer.
“There’s more work to be done and the community can help by being part of the change that has yet to be made,” says Swiney. By working together, we can achieve a future where individuals are diagnosed with ovarian cancer early and have access to support, resources and effective treatment. Please join us in our mission and help as we work collaboratively to drive innovation in the field.
 MIOCA.ORG
MIOCA.ORG
Operational excellence and innovation are at the core of Grace’s operations—the recent expansion of their flagship facility in South Haven, MI allows them to advance in both these realms.
Taking almost two years to complete, the expansion initiative introduces a new HASTELLOY® centrifuge and three 4,000-gallon multi-use chemical reactors to the plant situated southeast of Lake Michigan.
Being a full-service current Good Manufacturing Practice (cGMP) custom manufacturing facility, the South Haven location engages over 200 local employees and is fully capable of designing and producing custom drug intermediates and APIs. As a North American contract development and manufacturing organization (CDMO), Grace’s Fine Chemical Manufacturing Services (FCMS) cater to diverse customer requirements through their two interconnected U.S.-based sites—in South Haven and Tyrone, PA.

With a focus on placing the right resources in the right locations, the South Haven facility supplies premium active pharmaceutical ingredients (APIs) to prominent drug developers across the U.S., supporting therapies for oncology, diabetes, cardiovascular, and antiviral treatments. Equipped with the necessary tools and knowledge, the plant covers the API development process from pre-clinical stages to commercialization. By having manufacturing capabilities within North America, Grace’s FCMS aids in mitigating the typical challenges associated with international sourcing.
Among the upgrades brought by the expansion is a new 4,000-gallon reactor train, crafted from glass-lined carbon steel and stainless-steel components. Furthermore, the expansion allocates space for an additional HASTELLOY® centrifuge on-site, ensuring clients have access to top-tier equipment for product isolation from the new reactor setup. Renowned for its superior corrosion resistance and durability, the HASTELLOY® centrifuge can manage a higher number of processes compared to regular stainless steel alternatives. This substantial investment in a highly valuable asset highlights Grace’s dedication to enhancing manufacturing flexibility at the South Haven facility, enabling them to cater to a diverse clientele with superior equipment quality.

EXPERIENCE: With a proven track record of scale-up success, Grace has the right scientific and operational personnel in place to quickly move projects from concept to production. The South Haven site has proudly been part of Michigan’s pharmaceutical industry for over 40 years—this expansion further cements our commitment to the community and the industry as a whole.
COLLABORATION: You’ll have a dedicated team assigned to your project, enabling frequent and open communication with our long-tenured team of engineers, chemists and analysts. Designated program managers and quality specialists help facilitate a streamlined flow of communication for project reviews as well as adherence to project timelines and quality standards.
CAPABILITIES: Our teams can support your entire project, from initial screening, to lab development, to first scale-up, all the way through to commercialization. In addition to highly flexible and highly capable equipment, the facility is also home to a cGMP kilo lab and pilot plant.
MANUFACTURING EXCELLENCE: All Grace sites focus on continuous improvement in productivity, quality and efficiency. The South Haven facility holds certifications from the Michigan Voluntary Protection Program (MVPP), American Chemistry Council’s Responsible Care Management System (RCMS) and GMP (21 CFR part 211 cGMP).
PROCESS TECHNOLOGY: Our team is experienced in the principles of Quality by Design and has the resources and process knowledge necessary to ensure successful tech transfer at every step of the project life cycle. Because Grace does not outsource any design or method development work, and all project work is performed at a single site, products can move smoothly through the process.

The March 18 ribbon cutting celebration brought together South Haven employees, Grace executives, local officials and members of the Michigan biopharmaceutical community to mark this important milestone.
For over 40 years, the South Haven site has served the pharmaceutical drug substance market, and now with 25% more capacity, we’re entering a new era of possibilities. Grace, a Standard Industries company, is a leading global supplier of catalysts, engineered materials, and fine chemicals.
GRACE.COM/PHARMA

In 1958, Kalsec was founded by Paul Todd Jr., a visionary who believed in the power of science to transform the food industry.
Headquartered in Kalamazoo, MI, Kalsec is a global company at the forefront of providing high-quality, natural food and beverage ingredients for savory food and brewed beverage applications. Sixty years after its founding, Kalsec remains a family-owned business committed to providing tailored solutions that meet the evolving needs of food manufacturers and their consumers.
Todd’s passion for innovation laid the groundwork for what would become a worldwide leader in natural food protection, colors, and flavors. Driven by dedication to scientific excellence and commitment to “hold nature in awesome respect” Kalsec embarked on a journey of discovery, pioneering groundbreaking technologies and solutions for natural ingredients.
Under Todd’s leadership, Kalsec began its journey of innovation with Vegetone® Color Systems, renowned for creating unique, natural color combinations. This legacy continued with pioneering techniques like the use of gasliquid chromatography for analyzing spice compounds to better understand flavor and aroma. In 1961, the Kalsec plant breeding program was launched, focusing on resilient crop development while minimizing environmental impact. Through innovative farming methods and partnerships with sustainability-focused farmers, Kalsec has elevated the cultivation of capsicum and rosemary plants to ensure a reliable supply of these key ingredients. This hands-on approach embodies Kalsec’s commitment to quality and innovation that shapes the company’s culture to this day.

Continued early innovations laid the foundation for future success, positioning Kalsec as a trusted partner for food manufacturers seeking cutting-edge solutions. In 1977, Tetralone® Hop Extract was introduced, revolutionizing bitterness and light stability in a way that made beer in clear glass bottles possible for the first time. Building on this success, Kalsec developed Aquaresin® waterdispersible extracts in 1978, providing new ways for food manufacturers to add natural flavors, colors, and antioxidants to food.
In 1987, Herbalox® Rosemary Extract made its debut, establishing a new standard for natural shelf-life extension in savory foods. This marked a significant milestone in Kalsec’s journey, showcasing their expertise in rosemarybased antioxidants. This focus was further underscored with the launch of Duralox® Oxidation Management Systems in 1994, an innovative approach to synergistically blend different antioxidants and amplify their power to naturally extend shelf-life and reduce food waste.




In 1995, Kalsec expanded internationally with the establishment of Kalsec Europe, enhancing its support in international markets. Embracing collaboration, Kalsec continued to work closely with partners and farmers to develop customized offerings addressing specific challenges facing food manufacturers globally.
In 2000, Kalsec revolutionized the culinary landscape with the introduction of Clearcap® Super Soluble Capsicum. This breakthrough colorless and odorless capsicum extract became a key solution in the company’s heat management portfolio. Continuing cutting-edge innovation in capsicum, HeatSync® Heat Systems were introduced in 2012, offering unique combinations of spice ingredients to deliver targeted sensory performance. By presenting a versatile solution that seamlessly integrates into various applications, Clearcap and HeatSync enhances the culinary experience letting heat levels be precisely dialed in without impacting flavor or sensory appeal.


In 2023, Kalsec saw the culmination of years of planning with large projects both in Michigan and abroad. The company opened a new distribution center in Kalamazoo, MI to increase capacity and operational efficiency (and reduce lead times for customers?). Continuing its global growth, Kalsec opened the Singapore finishing center, enhancing support for customers in the region. Additionally, the launch of the Savory Products Innovation Center of Excellence in Wageningen, the Netherlands, further solidifies Kalsec’s commitment to innovation and customer success on a worldwide scale.
Since its inception, Kalsec has been a trailblazer, consistently delivering tailored solutions and unmatched expertise to its global audience. Through collaborative partnerships and a relentless pursuit of innovation, Kalsec has earned a reputation as a trusted ally in the everevolving marketplace. In 2023, Kalsec was recertified as a B Corp, this is a testament to its longstanding dedication to environmental and social responsibility and commitment to use its business as a force for good. With a steadfast focus on collaboration, expertise, and innovation, Kalsec remains true to its purpose of unlocking the potential of people, nature, and science to nourish the world.


MICHBIO
MICHIGAN BIO-INDUSTRY QUICK FACTS *

5 th 9th
LARGEST BIOPHARMA STATE BY NUMBER OF ESTABLISHMENTS
LARGEST MEDICAL DEVICE STATE BY NUMBER OF ESTABLISHMENTS
44,340* BIOSCIENCE JOBS
2,429* BIOSCIENCE ESTABLISHMENTS
15 th
9 th
LARGEST AGRI-BIOSCIENCES BY NUMBER OF ESTABLISHMENTS
LARGEST BIOSCIENCE STATE BY EMPLOYMENT
$101,374* AVERAGE BIOSCIENCE SALARY
BIOSCIENCE PERFORMANCE METRICS*
ACADEMIC R&D INVESTMENT
$1.6 billion* VENTURE CAPITAL INVESTMENT $956 million*
million*


Anchored by several large hubs, this representative sample of Michigan life sciences companies shows the breadth of the industry footprint.
TRAVERSE CITY MUSKEGON BATTLE CREEK/JACKSON KALAMAZOO GRAND RAPIDS HOLLAND MID-MICHIGAN
ANN ARBOR
GREATER DETROIT
LANSING
OAKLAND/MACOMB/ ST. CLAIR
MID-MICHIGAN
ANN ARBOR
GREATER DETROIT
LANSING
OAKLAND/MACOMB/ ST. CLAIR
Everything is on track. You have your career, you are married, and now you are ready to start a family, only to discover that you cannot conceive. A feeling of shock sets in, as you have done everything right.

 DENOVO FERTILITY
DENOVO FERTILITY

Devastated by the news, you go to your medical provider, who refers you quickly for In vitro fertilization (IVF) You go to your consultation, feeling like this isn’t real, listening to statistics, and learning about the invasive procedures you must now undergo to have your miracle baby.
This happens to 1 in 5 couples trying to conceive. Malefactor infertility impacts up to 40% of infertility cases, not ruling out a female factor as well. It can take 3-6 cycles of IVF to conceive, with an incredible toll on a woman’s body, both mentally and physically. On their own, couples are researching alternatives to IVF, hoping to find some answers. Multitudes of online groups, Reddit threads, and social media communities are filled with tens of thousands of people, asking what natural methods will improve their fertility: What foods, lifestyle measures, and supplements will help them?
Integrative medicine providers are working diligently to answer these questions. Denovo Fertility’s Founder and CEO, Jessica Preston, is one of them. With over 20 years in conventional medicine in Obstetrics & Gynecology with a focus on fertility, over 10 of which focused on integrative medicine, along with her infertility issues, she found the same struggle and decided to do something about it.
setting. Pharmaceutical-grade supplements are available, along with a home lab for hormone monitoring. This “VIP’ program is customized for those who have experienced IVF without success or who would like to pursue additional assistance in concert with IVF treatments.
Working hand in hand with medical providers, Denovo Fertility is assisting with their patients, providing education, coaching, and emotional support. These relationships are bidirectional, with Denovo Fertility seeking partnerships throughout the country to which they can refer when a client doesn’t have a medical provider for their care. Here, technology and integrative medicine complement and work alongside conventional medicine, assisting where lifestyle modifications can decrease infertility rates.
Denovo Fertility is working with OB/Gyns, REI (Reproductive Endocrinology and Infertility) specialists working in IVF, primary care providers, concierge medicine specialists, direct patient care specialists, nonprofit organizations, and working toward exploring relationships with self-insured plans.
Munther Alaiwat, MD, one of Denovo Fertility’s referring OB/Gyns, is a great supporter of the programs, “I wish a program like this was available when I started practicing 25 years ago.
Patients I’ve referred to Denovo (Fertility) have had excellent results.”

The answer was found in building an easy-to-access appbased technology platform, to which medical providers who are unable to take the time to educate their patients about lifestyle changes can refer. Here, men and women can find evidence-based methods they may undertake to naturally assist in improving their fertility. This HIPAAcompliant easy-to-use system focuses on simplifying how to embrace changes and apply tools in everyday life in the areas of nutrition, stress management, sleep, exercise, and personal relationships.

Denovo Fertility is not simply a femtech platform: it is a system bringing together scalable technology to sustain the ever-growing needs of the infertility market while bridging the gaps often overlooked: conventional medicine with the integrative medicine and wellness space; technology meeting with the consistency of human touch, empathy, and care.
Denovo Fertility has programs ranging from a simple 5-week self-directed program incorporating basic changes for those who are looking to improve their fertility, to a full-service coaching experience with weekly community meetings involving a team of experts who help guide couples on their path to parenthood. This personalized experience focuses on nutrition, health coaching, biofeedback training, hormone monitoring, and highlevel male and female support groups, all in a community
Denovo Fertility’s focus is on getting people pregnant. Still, they are finding that their methods are improving other areas of wellness including alleviating the depression and anxiety that are common with infertility, correcting gastrointestinal issues, reversing insulin resistance, resolving PCOS, and alleviating fatigue and insomnia. As this was anticipated, they are following these trends as well, for future program development.
Our current goals center around raising awareness of the success of integrative and functional medicine modalities to improve fertility in our medical communities at large.

LAWRENCE TECHNOLOGICAL UNIVERSITY
SUBMITTED BY LAWRENCE TECHNOLOGICAL UNIVERSITY
Lawrence Technological University (LTU) is proud to announce the launch of its new Graduate Certificate in Intraoperative Neuromonitoring (GCIONM) program, set to provide healthcare professionals with specialized knowledge and skills in this rapidly evolving field.
As we step into the future of medical technology, LTU is at the forefront, offering a program that integrates theory, simulation, and hands-on experience to prepare students for successful careers in Intraoperative Neuromonitoring (IONM).
Overview of GCIONM Program:
Launching in the summer of 2024, the GCIONM program at Lawrence Technological University is a comprehensive certification designed to meet the increasing demand for skilled professionals in the field of IONM. As technology continues to play a crucial role in surgical interventions, the need for trained individuals who can monitor and interpret neural function in real-time has never been more critical.
1. Cutting-edge Simulation Labs:
The GCIONM program at LTU places a strong emphasis on practical application through cuttingedge simulation labs. Students will engage in realistic, hands-on scenarios that replicate surgical procedures, enabling them to practice and refine their neuromonitoring skills in a controlled environment.
2. Integration with Neuroanatomy and Physiology:
The GCIONM program at LTU provides a comprehensive neuroanatomy and physiology education specifically curated to focus on IONM relevant aspects of neurological A&P. This neuroanatomical and physiological understanding provides the foundation from which students will make complex connections between the patient, and the collection and analysis of neurological data.
3. Collaboration with Healthcare Professionals:
The program fosters interdisciplinary collaboration, recognizing the importance of effective communication and teamwork within the healthcare setting. Students will gain insights into the roles and responsibilities of various healthcare professionals involved in spine and craniotomy surgeries.
4. Hands-on Experience with IONM Modalities:
With a focus on modalities such as SSEP, EEG, EMG, TCMEP, NAP, Cortical Mapping, and ABR, students will engage in hands-on simulations and analyses both in the Sim Lab and in the Operating room. This practical experience will enhance their ability to apply theoretical knowledge to real-world scenarios.
5. Ethical Considerations in IONM:
The GCIONM program addresses ethical considerations unique to the field of IONM. Students will explore patient advocacy, informed consent, and the ethical responsibilities associated with real-time neuromonitoring during surgeries.
6. Preparation for Certification Exams:
Recognizing the importance of professional certification, the program provides thorough preparation for the ABRET IONM certification exam. Students will receive guidance on exam requirements and best practices for success in the certification process.
1. Innovation in Education:
LTU is renowned for its commitment to innovation and cutting-edge education. The GCIONM program reflects this commitment by incorporating the latest advancements in IONM technology and techniques.
2. Experienced Faculty:
Students will learn from a faculty of experienced professionals and experts in the field of IONM. The program combines academic rigor with practical insights gained from faculty members actively engaged in neuromonitoring research and practice.
3. Career Opportunities:
Graduates of the GCIONM program at LTU will be well-equipped for fulfilling careers in healthcare, contributing to surgical teams and improving patient outcomes.
4. Hands-on Learning Environment:
LTU’s state-of-the-art facilities and collaboration, providing access to simulation labs and other equipment provide an immersive learning environment where students can develop and refine their skills in a safe and controlled setting.
In conclusion, the launch of Lawrence Technological University’s Graduate Certificate in Intraoperative Neuromonitoring marks a significant milestone in the education and training of healthcare professionals. This program sets the stage for a new generation of skilled IONM professionals, ready to make a meaningful impact in the evolving landscape of surgical interventions.
As we embrace the future of healthcare technology, LTU invites aspiring professionals to join this pioneering program and become leaders in the field of Intraoperative Neuromonitoring.
LTU.EDU
SUBMITTED BY AAPHARMASYN
AAPharmaSyn was founded in 2006 by several ex-Pfizer scientists. In the early days, the company struggled to gain its footing and relied heavily on existing relationships.
As AAPharmaSyn’s reputation for solving tough problems grew, the company started onboarding new clients and expanding its service offerings. In 2020, the ownership of the company was transitioned to a strategic investor focused on further strengthening company thought and execution leadership, investing in long-term growth and contributing to helping build a vibrant early-stage pharma ecosystem in greater Ann Arbor / Detroit area.
AAPharmaSyn provides superior synthetic chemistry services to global and regional pharmaceutical and biotechnology clients. The company’s scientists are frequently faced with tough questions that contain a hierarchy of unknown unknowns. Applying a rational problem-solving framework is the difference between solving the puzzle in several weeks or several months. For some clients, this means everything. In this respect, AAPharmaSyn takes the approach to problem solving seriously and, by and large, finds it more important than pure play experience, proprietary knowledge and shear raw effort. The company’s framework for dealing with unknown unknowns is what makes AAPharmaSyn different.
Within a synthetic organic chemistry paradigm there is a significant degree of covariation and a high sensitivity to initial conditions. This means that many theories do not have a significant predictive generalizable power. There are many ways to deal with this reality. At one extreme, propensity toward action bias is underpinned by a global belief that shear numbers and “shots at the goal” will increase the technical probability of success. Literature precedence is incorporated, albeit in a superficial capacity, and most effort is expended toward conducting the experiments. “Failed” experiments are quickly discarded unless they yield obvious methodological flaws and little time is spent triangulating hypotheses, overall strategy and empirical data.
At another extreme there is a demonstrated tendency to overanalyze and overextrapolate. Much discussion ensues about which experiments are to be conducted and why. Ubiquitously, personal bias is injected into discussions as a justification for a specific argument. Not surprisingly, much time and emotive energy can be spent without having anything to show for it. Moreover, once the experiment is performed the results are analyzed to the extreme degree frequently generating far-fetched conclusions based on confounded assumptions.

Through trial and error, AAPharmaSyn came to appreciate the utility of a context-based approach that generates empirical results without succumbing to guesswork. The company believes it is critically important to establish what is unknown and what can be done to shed some light on these knowledge gaps. However, in doing so, care must be made to consistently pay attention to natural decisionmaking tendencies.
In the course of everyday life, one’s decisions are consistently influenced by a wide range of biases such as ease of recall, retrievability, insensitivity to base rates, insensitivity to sample size, confirmation trap, anchoring, overconfidence and many others. If left unchecked, these biases cause suboptimal decision making resulting in a lower probability of achieving desired outcome. This is also just as true within professional settings where time pressures and immediate needs come ahead of objective evaluation of various hypothesis connected to causal beliefs.
Given how important the results of our work are, AAPharmaSyn feels the weight of responsibility to ensure that the most rational decisions are made to achieve the best outcome for clients in the shortest timeframe possible. This means that for every project the company remains keenly aware of what is true and what remains to be discovered or confirmed without succumbing to biases that unnecessarily prolong the duration of the project. AAPharmaSyn staff often arrive at unorthodox solutions that require the development of competencies generally unobserved within the company’s domain.
For example, AAPharmaSyn resorted to making its own silicas because off the shelf solutions were not robust enough. On a different project, the company invested in the development of proprietary late-stage functionalization methodologies because abandoning route was an expensive proposition.
Invariably, the culture at AAPharmaSyn is the most important determinant of the ability to problem solve. The company’s core belief is that client success comes above all else and the staff holds each other accountable, and never stops learning and challenging their own thinking on how to meet clients’ objectives efficiently and effectively.
Beyond making immediate profit, the company is committed to helping its clients to succeed in bringing new medicines to patients around the world. Whether it is an academician running a laboratory, a recent graduate with an idea that can eradicate a disease, early stage biopharma company with a lead molecule or a publicly traded corporation, AAPharmaSyn encourages everyone looking for solution to tough chemistry problems to engage with the company.

COMMUNICATION
Transparency
• Presentation of all available information.
• Auditable systems.
Integrity
• Evidence guided discussion.
• Clearly and unambiguously articulated goals.
Humility
• Continued focus on hypothesis refinement and challenging our assumptions
PROBLEM SOLVING EXPERTISE AND CAPABILITIES
Team members with depth and breadth of synthetic organic chemistry experience
Extensive proprietary experience with synthetically challenging targets
• In-house developed methods for separation/ purification using internally developed proprietary custom silicas and blends.
• Proprietary methodologies for synthesis of acid / base / moisture sensitive compounds.
• Proprietary methodologies for synthesis of acid / base / moisture sensitive compounds.
Searchable repository of accumulated knowledge
Comprehensive framework for problem solving
• Decision trees.
• Regression analysis.
• Practical methodology for weighing patent / academic literature.
CLIENTS ARE ENGAGED EITHER THROUGH FEE FOR SERVICE OR FULL TIME EQUIVALENT CONTRACTS.
AAPharmaSyn’s core services include:
• Structure-based design
• Hit to lead and lead optimization
• High-throughput screening outcome triage / SAR development
• Route scouting and selection
• Process development
• Stable isotope labeling
• Scale up (up to 5 kg)
• Process impurities
• Lipids
• Carbohydrates
• Flavonoids

Michigan continues to be a leader in advancing biosciences research and commercialization, thanks to its rich pipeline of intellectual property. A whole new crop of startups are germinating in the state, which is good news for the regional cluster of established companies that are focused on innovation in therapeutics, medical devices, healthcare technologies, clinical diagnostics and agri-/ industrial biotechnology.

M3D offers a variety of real-time radiation cameras and technology for use in healthcare facilities around the world. Their RAVIN Cam assists radiation safety teams in the cleanup, storage and disposal of radioactive material.
Their Lympho Cam is a surgical cart system with articulating arm that provides real time images to surgical oncologists, both pre-op and intraoperatively while performing sentinel lymph node biopsies. It forms high resolution images quickly and can even provide the depth of the involved nodes prior to any incision.
M3D also offers a variety of cameras and tools for nuclear medicine professionals, helping to monitor and alert personnel of any potential extravasations. Additionally, M3D is developing and testing tools to provide dose measurement during radionuclide treatments enabling

nuclear medicine physicians to develop a much more accurate understanding of the dose delivered to the tumor and surrounding organs.
The company is based in Ann Arbor, Michigan and is a spinout from H3D, who sells the world’s leading portable gamma camera to customers in the defense, homeland security and energy markets around the globe. Their technology was developed at the University of Michigan over 30 years and is the product of over $35M in funded research.
SUBMITTED BY GRAND RIVER ASEPTIC MANUFACTURING
Grand River Aseptic Manufacturing (“GRAM”) is a leading sterile injectable drug manufacturer that provides biologic, small molecule, and vaccine capabilities for liquid and lyophilized vials, syringes, and cartridges.
GRAM’s 300,000 sq. ft. of sophisticated GMP space supports pharmaceutical and biotech customers’ drug products with advanced equipment, innovative technology, and a breadth of experienced team members.
GRAM is known for its state-of-the-art technology, world-class facilities, customer-centric team, and regulatory compliance. GRAM manufactures customer drug products by taking the product’s formulation and transferring it into syringes, cartridges, or vials, then packages based on client needs. Current industry trends focus on drug delivery systems, such as on-body devices, to enhance patient care. GRAM stays at the forefront by providing capabilities that support advances in healthcare.

2010 Company founded; first facility on Front Avenue in Grand Rapids.
2013 1st commercial product FDA approved.
2017 Arlington Capital Partners becomes the new majority owner, offering capital for expanding capacity and continuing to invest in best-in-class technology.
2018 Large-scale filling expansion began to increase capacity and serve more pharma and biotech clients.
2020 Large-scale filling expansion complete.
Selected by the U.S. Government for Operation Warp Speed.
Partnered with Johnson & Johnson to manufacture COVID-19 vaccine.
2021 FOYA Award Winner for Special Recognition for Operational Agility: COVID-19 Impact.
Grand opening of new 200,000+ sq. ft. inspection, finishing, and warehouse center.
2022 Phase 2 filling expansion complete, reaching over 50 million units per year with space to serve a greater variety of pharma and biotech customers.
Selected by the U.S. Government for domestic Medical Countermeasures Capacity partnership for future pandemic preparedness.
Partnered with Bavarian Nordic to manufacture JYNNEOS vaccine.
2023 7-time Inc 5000 Fastest-Growing Private Companies in America.
GRAM’s authenticity and transparency with our partners and component manufacturers allow us to serve intentionally and continue our growth. We are proud to have products that positively impact the health of people around the world. With over 100 million units of capacity, GRAM is ready to support pharma and biotech customers further.

5- time West Michigan’s Best and Brightest Companies to Work For®.
2023 Best and Brightest Companies to Work For® in the Nation.
Contributed over 840 hours of paid volunteer time off serving local charities through GRAM’s GoBeyond program.
2024 90,000 sq. ft. expansion of inspection, finishing, and warehouse center complete.
Introduced Wellness Time Off to GRAM’s 440+ employees.
Employ a diverse team of talent ranging from high school diplomas to advanced postgraduate degrees
Occupy 6 facilities in Grand Rapids.

“Money is raining from the sky!” have only a few startups said since the beginning of time.
However elusive it may be, capital is critical for startups looking to get to their minimum viable product and first customer. Advocacy groups and legislators in Michigan are recognizing this need and have introduced a Research and Development (R&D) Tax Credit bill to the Michigan Legislature to help Michigan businesses of any size recoup costs spent on R&D. While in the works, the legislative road is long. In the meantime, what can startups do to acquire at least a fraction of the capital needed to move the needle toward revenue-positive?
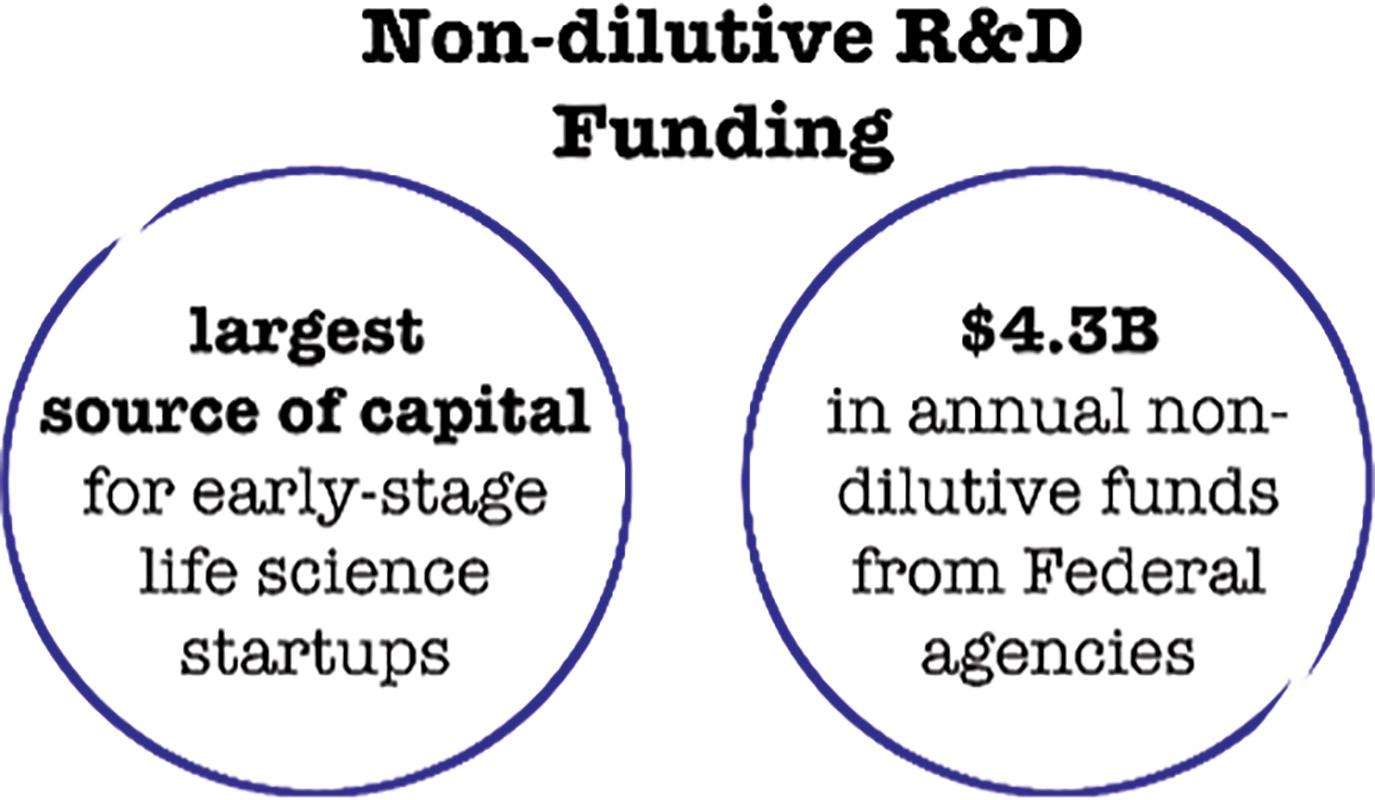
Many startups turn towards the seemingly free money offered by government grants, regulated by The Bayh-Dole Act of 1980. The Small Business Technology Transfer (or STTR)and Small Business Innovation Research(or SBIR) grants are the largest source of early-stage capital for life science startups in the United States, combining to provide over$4billionannuallyinsupportfromfederalagencies. While generally positive,these grants come with some potential strings attached.
Government paid-up license. If the invention was conceived of or first reduced to practice under the government grant, a government grant statement will need to be included in the utility patent application claiming the invention, indicating that the government has certain rights to the invention. Further, for elected inventions, the government has a nonexclusive, non-transferable, irrevocable, paid-up license to practice the invention throughout the world. To the surprise of many, this can even include royalty-free use of patents obtained before applying for the grant! So, if the government could be one of your customers, you may need to think twice about using government grants. The paid-up license provision was recently upheld by the Federal Circuit in University of South Florida Board of Trustees v. United States, 222248 (Fed. Cir.February9,2024).In short, a government contractor used transgenic mice patented by the University of South Florida (USF) without authorization. Unfortunately for USF, the patented invention (the transgenic mice) was first actually reduced to practice under a government grant…so the court concluded that the government – and its contractors– have a paid-up license for the mice.
Sub contractors cannot assign invention rights. Sneakier still,if your company hires a subcontractor to perform some of the work under the grant, any invention (conceived of or first actually reduced to practice) from the subcontractor cannot be assigned to your company. The subcontractor retains IP ownership.
March-in rights. The government can require licensing of any patent that results from federally-supported research. This is a provision that causes some confusion and alarm. As discussed, when you use government grant money, that often comes with a requirement to license your patent to the government for free. This doesn’t prevent you from licensing to others for profit and doesn’t have to impact your ownership rights – it just means that Uncle Sam gets free use in exchange for your now federally-supported research. March-in rights, however, can take that one step further and would allow the government to demand that
you license – or even assign your invention – to another entity, potentially even outside of the government! March-in rights have largely been an empty threat since the inception of the Bayh-Dole Act,but times could be changing. The Biden Administration announced, in December 2023, a draft framework that sets forth factors that may be considered when deciding whether to exercise march-in rights pursuant to the Bayh-Dole Act. The framework includes a first-time-ever provision that price could be a factor in determining that a drug(or any other government grant-funded invention!) is not accessible to the public. This could open the proverbial floodgates to new march-in rights petitions based on the cost of goods, effectively opening the door to government price controls for innovations that receive federal dollars.
Perilous oversights. Other provisions can result in lost ownership rights if you fail to commercialize or neglect to file the correct paperwork on time.
Considering funding sources is one of the most important decisions startups face, and non-diluting capital can be an essential source when trying to get your innovation off the ground. When compared with investor money that comes with the loss of equity and/or control – and family and friends’ money that may come with the risk of strained relationships, government grants may seem like a better option. For many, they can be, but like with any other funding source, it’s essential to understand the strings.

Sources: https://seed.nih.gov/sites/default/files/SEED-SBIR-STTR.pdf https://www.highergov.com/news/sbir-awards-reach-record-high-2023/#:~:text=The%20total%20 value%20of%20SBIR,and%20approximately%203%2C700%20different%20awardees https://www.publicspendforum.net/blogs/public-spend-forum-2-2-2/2023/05/17/sbir-sttrprogram-alignment-to-critical-emerging-technology-priorities/
SUBMITTED BY DNA SOFTWARE
DNA Software, Inc. (DNAS) is a Michiganbased life science company located in Plymouth, MI at the Michigan Life Sciences Innovation Center (MLSIC) facility. DNA Software offers state-of-the-art software for the design of nucleic acid diagnostics, particularly in the polymerase chain reaction (PCR) market.
Before COVID, PCR wasn’t necessarily the household term it has become today. Awareness for PCR rapidly increased throughout the pandemic as PCR based testing for disease is recognized as the “gold standard” in diagnostics. Suddenly an acronym that was foreign to most was in the daily news, on the bottom of the sports-ticker and became a new requirement for travel. At a $7B market size pre-COVID in 2019, molecular diagnostics today is a $25B industry in 2024 which will grow to greater than $50B in 2030.
DNA Software has always been uniquely positioned in the PCR and molecular diagnostics space, however, now finds itself at an inflection point in its 23rd year of business. Demand for PCR-based testing from healthcare providers continues to grow. This includes multiplexed PCR based detection methods as multiplex based testing enables many samples to be tested at once to save costs. Multiplexed PCR assays allow for the most accurate detection of many diseases, lowering costs and increasing throughput. “All multiplex roads in molecular diagnostics run through DNA Software” shares Dr. John SantaLucia, founder and CEO of DNA Software, Inc. “Whether licensing software, our services, or our assays, our expertise shines when designing multiple PCR tests simultaneously because of the foundational science discovered at my academic lab at Wayne State University (Detroit,MI) where I was a professor for nearly 30 years”.
PCR tests are not only limited to COVID and respiratory illness (such as FluA/FluB/RSVA/RSVB) but include everything from the flu and strep throat. This also

includes a multitude of panels including UTIs (urinary tract infections), STIs (sexually transmitted infections) GI (gastrointestinal) along with many other commonly ordered pathogen tests. Customer benefits include detecting many pathogens simultaneously, superior results and limit of detection while decreasing per sample test costs along with a higher sample throughput.
DNA Software has provided “best in class” software to leading biotech firms for FDA approved molecular diagnostics. DNAS worked on custom projects with Moderna to characterize modified nucleotide behavior in the mRNA vaccine delivery. During the onset of COVID, the Department of Defense contacted DNAS to design SARSCoV-2 assays along with biodefense panels. DNAS has long been recognized as an industry leader for its software, R&D and client services and was the 2023 recipient for the Frost & Sullivan “best in class” award for multiplex solutions provider in the industry.
Through a “COVID Awakening”, DNA Software realized that R&D teams throughout the molecular diagnostics field relied on its science, algorithms, software and models for diagnostic design. Having provided software and client services to a “who’s who” list of biotech organizations including BD, Cepheid, Roche, Qiagen, Hologic, Thermo Fisher and Eli Lilly, DNAS moved to the MLSIC facility in late 2020 to manufacture its own line of PCR kits and assays through its new subsidiary, wwww.pcrassays.com. DNAS is uniquely positioned to leverage its technology to build high performing multiplexed PCR assays.

To capture more of the value they create, DNAS launched a new brand, PCRassays, which directly provides multiplexed RUO qPCR kits. These assays detect human pathogens, food borne pathogens, and are used for wastewater surveillance and research-based applications. PCRassays offers flexible multiplex configurations. Through its unique automated design platform (DNA Software), these “plug


To capture more of the value they create, DNAS launched a new brand, PCRassays, which directly provides multiplexed RUO qPCR kits.
and play” assays are adaptable for ever changing market dynamics. PCRassays has transformed the firm’s digital assets into physical assets as the organization now ships reagents throughout the world for a variety of applications.
The DNAS subsidiary PCRassays has grown with several Michigan based partners. This includes the State of Michigan Department of Health and Human Services (DHHS) (Lansing, MI) to develop a novel Hepatitis A assay. Recent outbreaks in MI of Hepatitis A brought about the need for the rapid development of a qPCRbased assay and the State of MI elected to work with DNAS and PCRassays for their expertise in the development of such an assay.
The portfolio of infectious disease qPCR assays has grown to nearly 200 pathogens, >50 in multiplex. PCRassays has focused on growing channel partners and distribution to increase its adoption. Michigan based partnerships include enzyme manufacturer Fortis (Formerly Empirical Biosciences in Grand Rapids, MI). Fortis custom formulated an enzyme that optimizes PCRassays kits and its product performance overall.
DNAS has also partnered with Cayman Chemical, (Ann Arbor, MI) a global leading supplier of research tools for the life science community, to widen their portfolio to include molecular-based detection kits through a strategic partnership. To build inroads into the clinical market, DNAS partnered and signed a distribution agreement with Horiba Medical (Canton, MI) to make its assays available to the Horiba team of 80 international distributors.
PCRassays has customers in Michigan that include Great Lakes Medical Laboratories (Farmington, MI), Biogenetics (Southfield, MI) and World Wide Labz (Detroit, MI), Henry Ford Health System (Detroit, MI) and Wayne State University (Detroit, MI).


REPRODUCTIVE STRESS INC.
SUBMITTED BY REPRODUCTIVE STRESS INC.
The enigma of human embryo development after fertilization persists, with approximately 70% of embryos typically lost to miscarriage, underscoring the urgency to understand underlying causes and predict risks.
Reproductive Stress Inc. addresses this challenge by employing pioneering technologies, drawing from four Nobel Prize-winning methodologies.
Historically, knowledge of environmental factors affecting reproduction has been skewed toward sperm due to its ease of collection. Conversely, the collection of oocytes and early embryos is arduous and costly, leaving a dearth of understanding regarding their susceptibility to internal factors like stress hormones and nutrition levels and external factors like environmental pollution, pharmaceuticals, diet supplements, cosmetics, and manufactured compounds. The European Union has led the way in making international trade in pharma and manufactured compounds undergo necessary toxicity testing, creating a market that Reproductive Stress Inc. transformative assays aim to enter.
CEO Elizabeth Puscheck, MBA, MD MS emphasizes that Reproductive Stress Inc’s patented assays for placental and embryonic stem cells accurately categorize into strong-, weak-, and non-embryotoxic compounds— first standardized by a European multi-lab effort to validate an embryonic stem cell cytotoxicity test. Some examples are penicillin is very safe, but retinoic acid (used in anti-acne treatments) is very risky to both stem cell types in the early embryo.
To bridge this gap in knowledge of oocytes and embryos, Reproductive Stress Inc. pioneers two areas of re- search utilizing the components of early embryos: embryonic and placental stem cells. Leveraging four Nobel Prize-winning advancements, two for embryonic stem cell research and one each for, in vitro fertilization (IVF) methodologies and fluorescence protein discovery, our assays dissect the effects of internal and external factors on growth, stemness, and differentiation balances of the stem cells. This enables the prediction of miscarriage and birth defects of embryos.
Through high-throughput screening (HTS) facilitated by automated microscopy and AI-driven analysis pipelines, our living fluorescent stem cell assays provide risk assessments for clients in pharmaceuticals, agribusiness, compound manufacturing, and IVF. These assessments of medicines, environmental contaminants, nutrition supplements, etc., can pinpoint adverse-effect doses that impede growth and induce differentiation, culminating in miscarriage risk prediction.
Reproductive Stress Inc’s principal investigator, Maurice Recanati, MD MS, leads the company lab and is interested in the application of the embryonic stem cell assay to ensure that the novel pharmaceuticals being developed to promote fetal lung maturity are not toxic to the placenta or baby.
Additionally, our assays assign adverse effects doses to facilitate specific follow-up molecular analyses, uncovering intricate signaling changes indicative of birth defect risks. We call these signaling changes “stress finger- prints,” which are unique to each stress and dose. By examining the effects on critical markers in the forebrain and heart development, these analyses predict birth defects. They also predict doses that suppress precursor cells for egg and sperm production, underscoring the profound implications for future generations.
Founder and CSO, Dr. Dan Rappolee, PhD a recipient of several non-diluted funding sources; two business grants from the National Institutes of Health (NIH) for HTS development, an NIH pilot grant for follow-up molecular analyses, and NIH Build/Detroit ReBuild for funding three underserved students to work on assay development, the Barber foundation for two grants to optimize the placental stem cell HTS and the Michigan Emerging Technologies fund for equipment.
Reproductive Stress Inc.’s transformative assays not only promise to revolutionize developmental toxicology and the IVF market but also represent a significant leap forward in understanding and safeguarding reproductive health.


Effective operations are essential for life science companies to meet their customer’s needs. Critical Point Consulting’s mission is to provide the expertise and leadership required to ensure our client’s operations are compliant, reliable, and efficient. With a unique combination of biotech and pharmaceutical manufacturing, engineering, operational excellence, and financial expertise, we meet our client’s needs at all stages of their product life cycle.
Critical Point Consulting supports client’s capital projects by serving as user representatives, driving operational readiness efforts, and managing capital portfolios. For ongoing operations, our deep expertise leading and improving manufacturing operations allows us to quickly assess and then improve the operations of our clients through application of the appropriate operational excellence tools. Additionally, we have experience
partnering with Quality Assurance teams to drive facility, equipment, and operational improvements to correct regulatory deficiencies. When needed, we can leverage our significant direct leadership experience and take on interim leadership roles.
Our industry experience and broad skill set allow us to bring world-class ideas and best practices to our clients. This experience is coupled with a flexible approach that allow us to quickly respond to our customer’s specific needs. Whether you are an established player in the life science industry looking to take your organization to the next level or an up-and-coming company navigating the launch of your first commercial product, Critical Point Consulting has the expertise to ensure your success.
CRITICALPOINT.US

Every day, from discovery to delivery, Avantor is an essential partner to the scientific community — pioneers, scientists, innovators and educators — relentlessly focusing on breakthroughs that help solve the world’s most complex challenges. Our proven expertise and trusted portfolio of products and services, combined with a global reach and ability to provide customized materials of the highest quality for highly regulated applications move science forward. As a global leader in life sciences, we fulfill our mission: to set science in motion to create a


Backed by 40 years of experience in lipid chemistry, synthesis, and analysis, Cayman Chemical is a Michigan-based provider of lipid nanoparticle (LNP) research tools and services. We support the entire LNP research and development process from discovery to bioanalysis.
· Lipids for LNP Formulation
· Custom Lipid Synthesis
· GMP Lipid Production
· LipidLaunch™ Research-Ready LNPs & Reagent Kits
· LNP Formulation, Characterization, & Screening Services
Explore Research Tools for Every Step of LNP Discovery
MichBio
3520 Green Court Suite 175, Ann Arbor, MI 48105
WE INVITE YOU TO BECOME AN ACTIVE MEMBER OF MICHIGAN’S THRIVING BIOSCIENCES COMMUNITY. JOIN MICHBIO.
