








As a top state for biomedical engineering graduates, Michigan is pioneering the next generation of medicine with advancements in everything from vaccine manufacturing to genomic research. Discover the world-class life sciences talent that make it all possible and seize your opportunity at MICHIGANBUSINESS.ORG

“Innovators and established medical device companies can take can ideas to market without leaving Michigan.”
As one of the leading medical technology states in the U.S. based on the number of medical device facilities and size of the sector’s workforce, Michigan continues to be haven for innovation and manufacturing of medical products.
The state’s medtech cluster includes multi-nationals like Stryker and Terumo Cardiovascular to smaller original equipment manufacturers (like Wareologie and Priorclave featured in this BioMattersTM issue) and early-stage ventures (i.e., Sterile State) that are fed by a fabric of supply chain including contract manufacturers (i.e., Medbio and Sterling Industries), suppliers, and service providers (i.e., Michigan Metrology, Harbor View, and New Life Prosthetics) of every capability. Innovators and established medical device companies can take ideas to market without leaving Michigan.
The past few years have been a whirlwind for the medtech industry, with the rapid mobilization during Covid-19 pandemic, followed by some volatility, especially in supply chains, and now its stabilization. However, mergers and acquisitions by, and of, Michigan medtech companies, as well as site expansions and new startups, that continue to make the news, have driven growth in the statewide cluster.
A recent trip to the Upper Peninsula (UP) was a striking reminder that our medtech sector is deep and geographically dispersed, with assets, skills and talent that is second to none. Along with the larger regional medtech hubs in the Grand Rapids, Kalamazoo, Ann Arbor areas and Oakland County areas, we’re witness to the fact that the world comes to our medtech companies statewide for quality product design, development, and manufacturing.
Coincident with cluster growth is the reliance on skilled talent, whether degreed or not, to work in a highly regulated pre- and post-market product compliance environment. Read here about the new MEng curriculum in Advanced Medical Product Engineering & Development at the University of Michigan (Ann Arbor) and the Bioengineering program at University of Michigan-Dearborn. Similarly, the UP trip revealed how Resolve Surgical, Able Medical and Mount Manufacturing, in partnership with Northern Michigan University, have instituted a Work Scholars program in medical device manufacturing where students receive work-based learning, competitive pay, and academic credit towards certificate, as well as associate and baccalaureate degrees.
All this to say, that Michigan’s medical device industry and its workforce stands at the forefront of innovation, catalyzing groundbreaking advancements that are not only redefining how and where people access everyday medical care, but also shaping the future of medicine.
Sincerely,
STEPHEN RAPUNDALO, PHD President and CEO, MichBio
INNOVATION

CORPORATE CONNECTOR


TECHNOLOGY
INNOVATOR





From groundbreaking discoveries to transformative solutions, together we can achieve extraordinary breakthroughs that redefine industries. Scan the QR codes to learn more about some of the pioneering research being done at MSU or visit our website below to learn how your organization can join forces with our award winning faculty innovators and tap into a wealth of expertise and resources to accelerate your R&D efforts. 2024 Innovation Celebration Faculty Awardees:



The MichBio Preferred Purchasing Program leverages the collective purchasing power of our member companies to obtain steep discounts from our vetted and


















MichBio is the biosciences trade association for the state of Michigan. Our goal is to drive the growth of the state’s bio-industry through advocacy, education, connections, and supportive resources.







Stephen Rapundalo, PhD President and CEO
Emily Brockman Director, Member Relations
Carrie Hranko Manager, Administrative Services
Jamie Krajny Editor Director, Marketing and Industry Engagement
CHAIR
Ken Massey, PhD
Wayne State University
Senior Director, Venture Development Technology Commercialization
VICE CHAIR
Kevin McLeod C2Dx
Founder, President and CEO
MICHBIO
Christy Bigelow
Emergent Biosolutions
Vice President and General Manager, Manufacturing Operations
Sean Callaghan Medbio Inc.
Vice President of Operations and General Manager
Rhonda DeLuca
Terumo Cardiovascular Group
Sr. Vice President Global Human Resources
Robert Donofrio, PhD Neogen Corporation Chief Scientific Officer
Lola Eniola-Adefeso, PhD University of Michigan University Diversity and Social Transformation Professor of Chemical Engineering
Christine Haakenson, PhD
Charles Hasemann, PhD
Michigan State University
Assistant Vice President for Innovation and Economic Development
MichBio
3520 Green Court, Suite 175 Ann Arbor, MI 48105-1175
734-527-9150
info@michbio.org michbio.org
Designer: Daina Fuson Designs
Printer: Progressive Printing
© Copyright Michigan Biosciences Industry Association, DBA MichBio
PRESIDENT AND CEO
Stephen Rapundalo, PhD
MichBio
President and CEO
SECRETARY
Robert Dionofrio, PhD
Neogen Corporation
Chief Scientific Officer
TREASURER
Vacant
Ken Massey, PhD
Wayne State University
Senior Director, Venture Development Technology Commercialization
Kevin McLeod
C2Dx
Founder, President and CEO
Dave Morin
Care Technology Advisors, Managing Member
Stephen Rapundalo, PhD
MichBio
President and CEO
Tom Ross
Grand River Aseptic Manufacturing President and CEO
John J.H. Schwarz, MD
Former U.S. Representative
Uma Sharma, PhD
MMS Holdings Chief Executive Officer







ANEW LIFE PROSTHETICS AND ORTHOTICS
BY KIM CASTEEL, CONSULTANT AND CO-FOUNDER, ANEW LIFE PROSTHETICS
Located in the New Center area of downtown Detroit, Anew Life Prosthetics and Orthotics is a pioneering provider of advanced prosthetic and orthotic solutions.
Specializing in custom artificial limbs, braces, diabetic footwear, custom orthotics, and mastectomy products, Anew Life stands out for its innovative use of technology and its comprehensive, patient-focused approach.
Anew Life caters to a diverse clientele, from underhoused individuals to CEOs and veterans. Their extensive range of services includes home and clinic visits for those unable to travel, ensuring that quality care is accessible to all. The company’s on-site fabrication laboratory allows for the customization of products to meet the unique needs of each patient. This customization is significantly enhanced by the use of cutting-edge digital technologies, such as 3D scanning, CAD/CAM, and 3D printing, which enable the creation of precise, custom-fabricated medical appliances.
The team at Anew Life comprises Board for Certification/ Accreditation (BOC, BOCO, BOCP) and American Board of Certification (ABC, CO, CP) certified prosthetists and orthotists. Utilizing both traditional methods like hand casting and plaster modeling, as well as state-of-the-art digital capture technologies, they fabricate orthoses and prostheses tailored to each patient’s specific needs.
One of the most notable features of Anew Life is its commitment to leveraging advanced technology for patient benefit. The use of 3D scanning technology ensures that prosthetics and orthotics are tailored with a high degree of accuracy, improving fit and comfort. CAD/ CAM and 3D printing technologies enable the efficient production of custom devices, reducing wait times and enhancing the overall patient experience.
The patient care process at Anew Life is meticulous and thorough. It begins with an in-depth evaluation to understand the patient’s lifestyle, expectations, and needs. A prototype is then created using either handcasting techniques or digital scanning and 3D modeling.
After a test fit and necessary adjustments, the final product is crafted, and the team works closely with the patient to ensure they can effectively use their new device.
In their comprehensive approach, Anew Life collaborates closely with patients, their families, caregivers, doctors, referral sources, veterans, and insurance companies, including Humana, BCBS, Meridian, and Medicare. They provide education and training on donning (putting the device on), doffing (taking the device off), care, cleaning, break-in/wearing schedules, and necessary precautions. Their knowledgeable team also offers introductory gait (standing and walking) training to improve step length and gait speed. Additionally, they coordinate with other healthcare practitioners, such as physical (PT) and occupational therapists (OT), encouraging pre- and postoperative care for contracture, long-term therapy, strength training, and rehabilitation.
Anew Life’s dedication to patient education and empowerment is another key aspect of their service. The team ensures that patients fully understand how to utilize their devices, helping them return to their daily activities with confidence and independence. This patient-centric approach has resulted in a consistent 97% satisfaction rate over several years.
In addition to their technological advancements and personalized care, Anew Life is also deeply committed to community involvement. They provide free services to various organizations, including Street Medicine Detroit, Burners Without Borders Detroit, Palestine Children’s Relief Fund, Cabrini Clinic, and the Neighborhood Service Organization. This charitable work highlights their belief that everyone deserves access to quality care, regardless of their financial situation.
Anew Life also fosters a supportive community among its patients through programs like the Amputee Coalition of America’s mentor initiative. This program connects new patients with experienced mentors who have undergone similar journeys, encouraging a pay-it-forward mindset and ensuring ongoing support throughout the recovery process.
Chris Casteel, the founder of Anew Life, brings a wealth of experience and a unique perspective to the company. As an amputee himself, Chris understands the challenges faced by individuals with disabilities and is committed to alleviating these struggles through advanced prosthetic and orthotic solutions. His leadership has driven the company to not only advance prosthetic and orthotic technology but also create a nurturing environment where patients are educated, empowered, and supported.

Anew Life Prosthetics and Orthotics exemplifies the transformative potential of innovation in the MedTech bio industry. By combining state-of-the-art technology with a comprehensive, patient-focused approach, they have set a new standard for prosthetic and orthotic care. Their story is a testament to the practical benefits that advanced technology and personalized care can offer to a diverse clientele, ensuring that every patient can lead a comfortable and independent life.
“We want every amputee or person with limb loss to live their life with strength, courage and confidence and give them the motivation and determination to do whatever they put their mind to.”

BY RICHARD LOVE, FOUNDER AND CEO, HARBORVIEW
Founded in 2013, HarborView located south of Lansing has been a trusted partner for some of the most renowned names in the biotech and medtech industries, including Amgen, Genentech, and GSK. Their team of industry veterans recognized the pressing need for a comprehensive PLM solution tailored specifically for small to midsize biotech and medtech companies.
Drawing from years of experience in regulatory affairs, quality management, and data analytics, HarborView set out to create a platform that bridges the gap between innovation and market readiness. This led to the creation of QMSRpro, HarborView’s advanced platform designed to streamline compliance, enhance data management, and accelerate the product lifecycle from concept to market launch.
At its core, HarborView provides a unified approach to managing the entire lifecycle of a medtech product— from initial concept through regulatory approval and market launch. The platform, QMSRpro, integrates data management and intelligence architecture, ensuring that every piece of critical information is at your fingertips when you need it most.
“One of the major hurdles in medtech product development is ensuring that all regulatory requirements are met without stifling innovation,” explains Richard Love, Founder and CEO at HarborView. “Our platform not only helps companies maintain compliance but also accelerates the development process by providing clear, actionable insights.”
With the FDA’s Quality Management System Regulation (QMSR) set to take effect in 2026, the regulatory landscape for medtech companies is evolving. QMSRpro is designed to facilitate this transition by allowing companies to capture all their QMSR information and records in a global relational database. QMSRpro meets GxP and HIPAA requirements, ensuring comprehensive compliance and providing a robust foundation for managing quality data. This capability reduces the risk of non-compliance and streamlines the path to market.
HarborView is designed to deliver significant value at a competitive cost. For less than the annual salary of a junior quality associate, medtech companies can access a comprehensive PLM solution that streamlines processes, enhances data visibility, and reduces time to market. QMSRpro ensures that every step, from design controls to regulatory submissions, is managed efficiently, allowing companies to focus on innovation.
Since its inception, HarborView has been committed to continuous improvement. We actively solicit feedback from our clients to enhance our platform and services. Each new feature in QMSRpro is designed with the enduser in mind, ensuring that our clients have the tools they need to succeed.
“Our culture is focused on empowering our clients,” Love continues. “We don’t just provide a software solution; we partner with our clients every step of the way. From initial setup to ongoing support, we are there to help them navigate the complex landscape of medtech product development.”
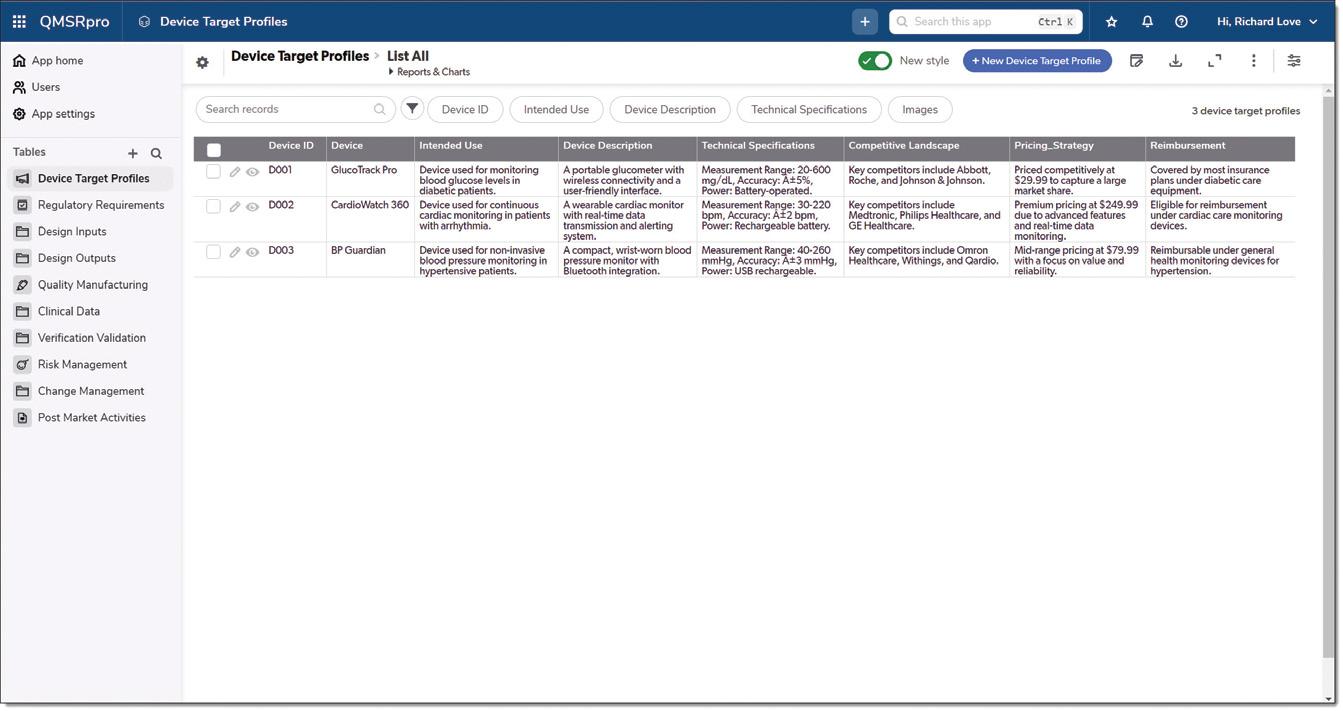
FIG 1 DEVICE TARGET PROFILES: consolidated view of all device target profiles managed in QMSRpro, providing essential information on intended use, device descriptions, and competitive landscapes for strategic decision-making.
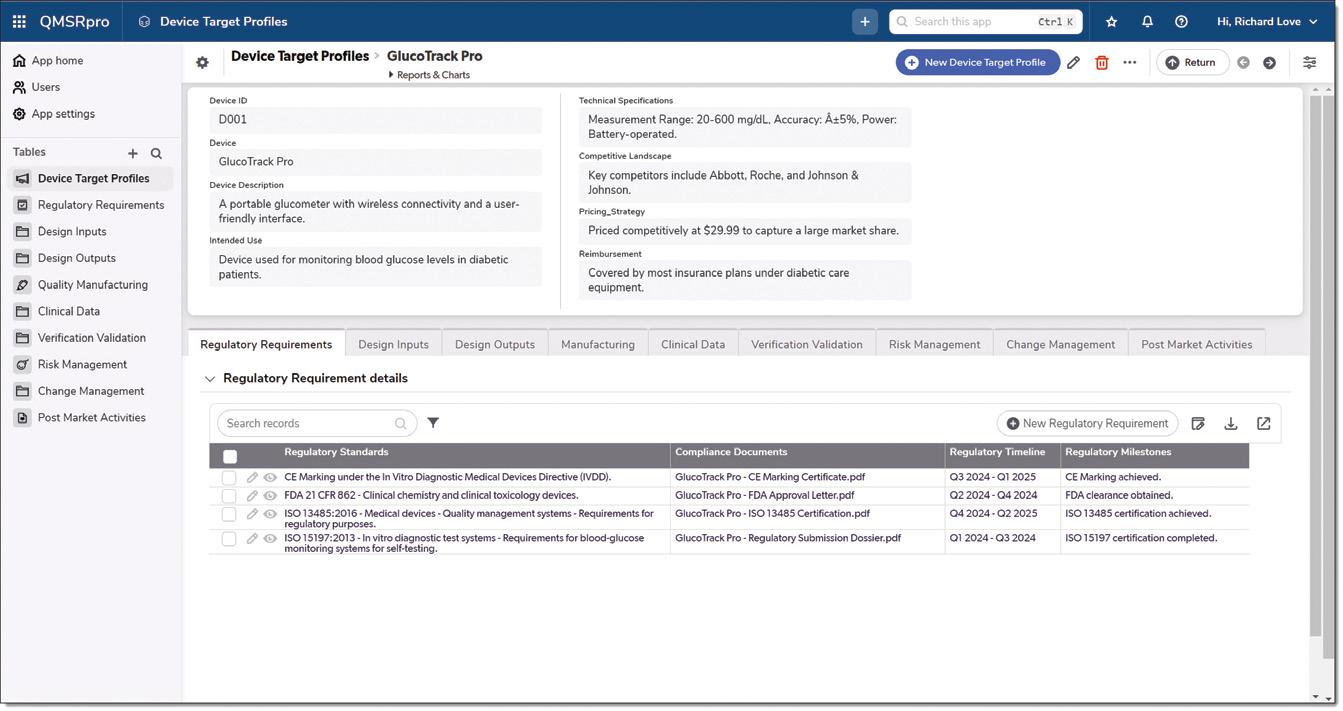
FIG 2 DEVICE TARGET PROFILE FOR GLUCOTRACK PRO: The Device Target Profile for GlucoTrack Pro within QMSRpro, detailing key technical specifications, regulatory requirements, and market strategies, enabling streamlined management of product lifecycle data.
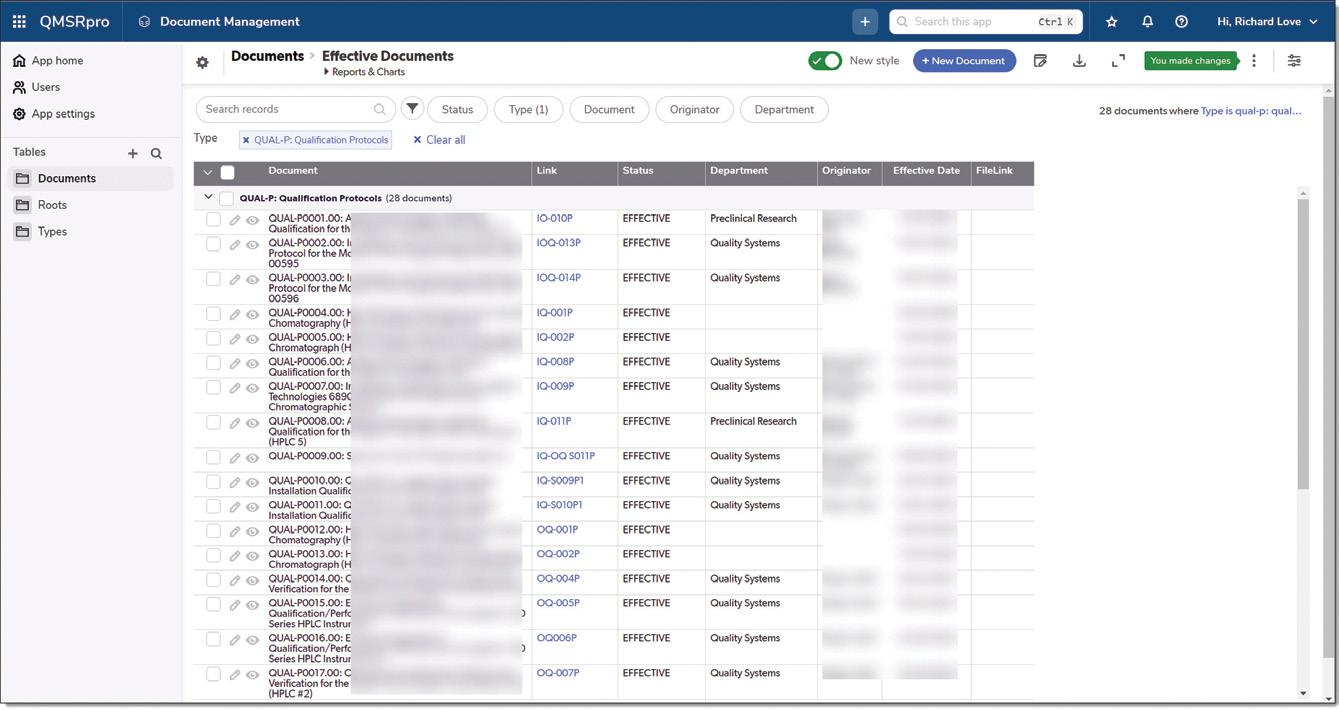
FIG 3 EFFECTIVE DOCUMENTS: A snapshot of the document management interface in QMSRpro, showcasing the effective qualification protocols and their statuses, helping users maintain organized and compliant documentation.
One of the key advantages of QMSRpro is its ability to move data from spreadsheets to a robust, relational database hosted on a GxP and HIPAA-compliant platform. This transition enhances data integrity, improves collaboration, and provides better control and visibility over critical information. By leveraging a relational database, companies can more effectively manage their reference data, ensuring consistency and accuracy across the organization.
QMSRpro also offers advanced business intelligence and AI capabilities to help companies leverage their data. The platform integrates seamlessly with AI tools to analyze trends, predict outcomes, and optimize processes. By harnessing the power of AI, QMSRpro enables medtech companies to make data-driven decisions that enhance efficiency and drive innovation.
HarborView HarborView offers more than just software by providing a suite of services designed to support every aspect of product lifecycle management. The team of experts conducts in-depth consultations and workshops to help medtech companies understand regulatory pathways, design controls, and quality management systems.
Believing in the power of education, they regularly host webinars and training sessions to keep clients informed about the latest industry trends and regulatory changes. By equipping clients with knowledge and best practices, they help them stay ahead of the curve.
As we look to the future, HarborView is committed to expanding our capabilities and enhancing our platform to meet the evolving needs of the medtech industry. Their vision is to be the go-to PLM solution for companies seeking to bring innovative medical devices to market efficiently and compliantly.
“The road to market does not have to be long and challenging,” Love reflects. “With QMSRpro as your guide, you can navigate it with confidence. We are here to help our clients turn their groundbreaking ideas into reality.”

MICHIGAN METROLOGY
BY DON COHEN, MICHIGAN METROLOGY
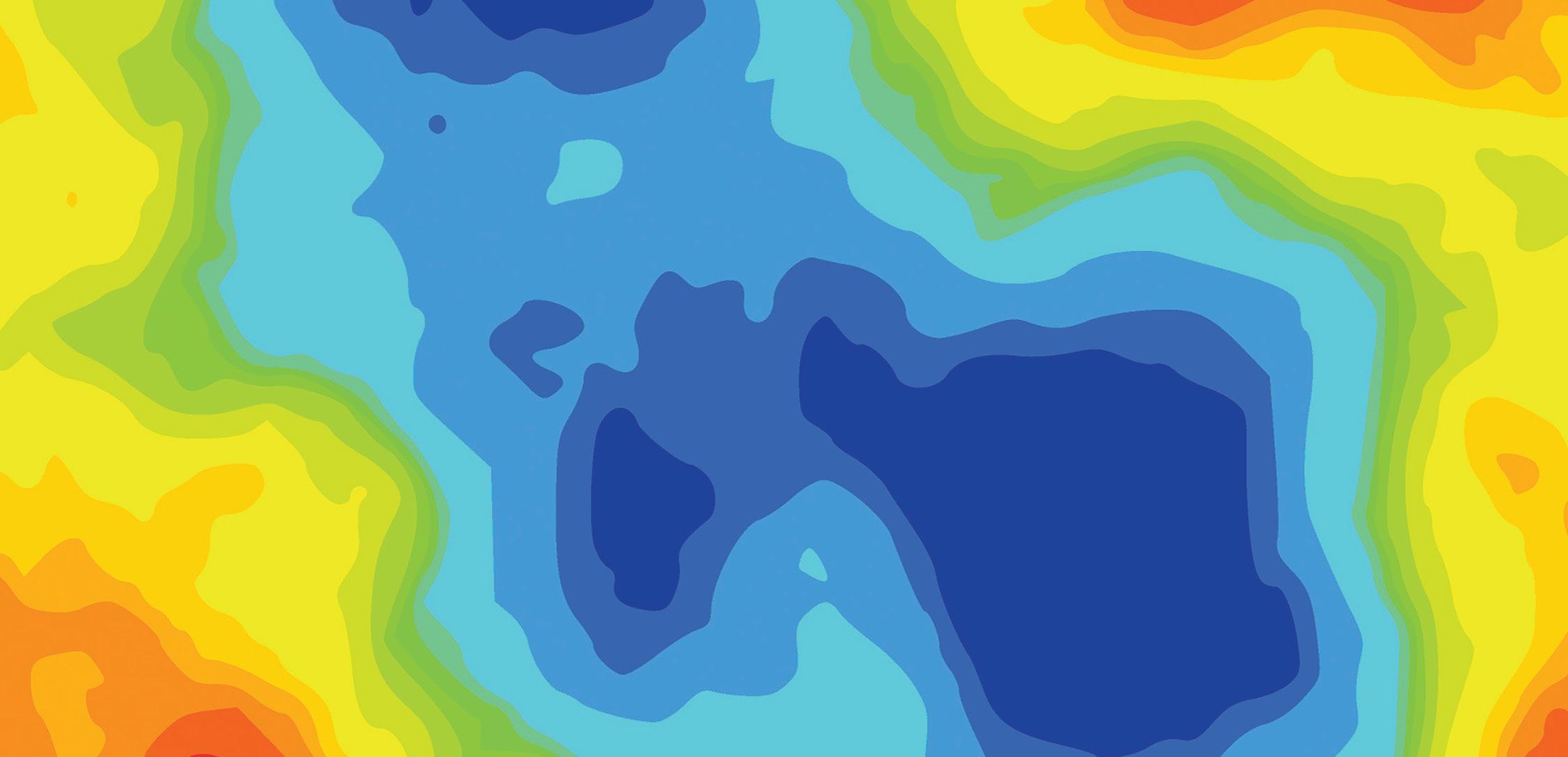
Medical devices are expected to operate efficiently over long periods, often without the opportunity for repair or maintenance. A critical factor in ensuring this longevity and performance is the precise control of surface finish and texture. One key technology that has emerged to meet these demands is 3D optical profiling.
This technology provides 3D, high-resolution maps of surfaces, enabling manufacturers to measure and control surface texture with precision, thereby contributing to high quality and long-lasting medical devices.
The concept of “surface roughness” might imply a uniform, unchanging surface profile. However, in reality, a surface’s microscopic texture consists of diverse features such as flat areas, peaks, valleys, porosity, and tool marks. Traditionally, surface roughness was measured by dragging a diamond stylus across the surface, creating a single, two-dimensional trace that offered limited insight into the overall texture. This method could also damage softer materials, leading to potentially inaccurate results.
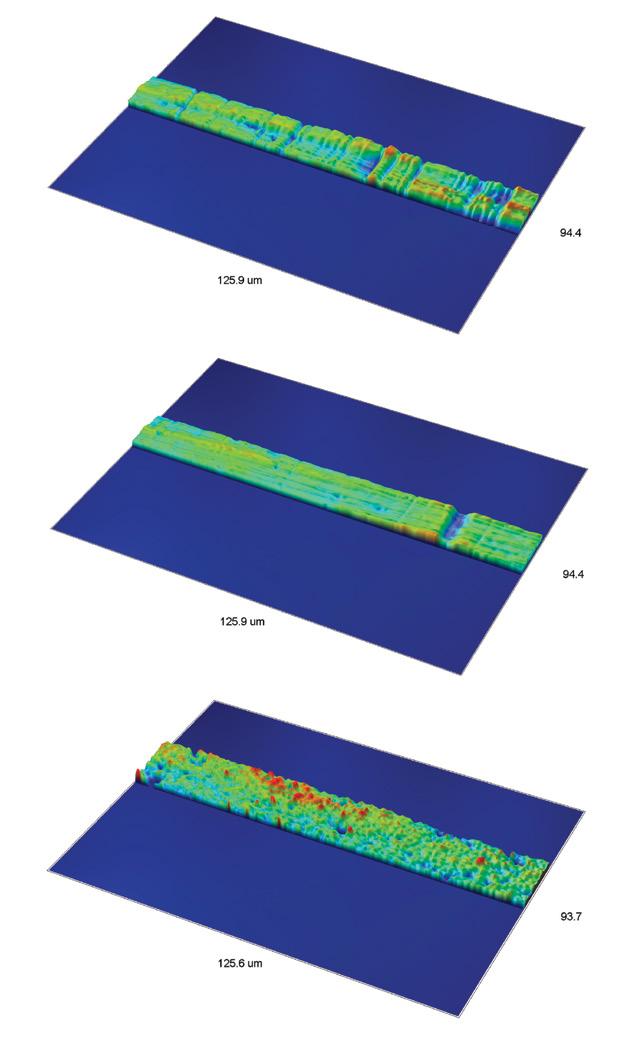
With advancements in optical measurement techniques, 3D, high-resolution, non-contact surface measurements have become possible. A 3D optical profiler can achieve sub-nanometer height resolution and micrometer lateral resolution. It can “stitch” various measurements together into one large assessment, covering extensive surface areas and providing a wealth of data far surpassing that of traditional stylus instruments.
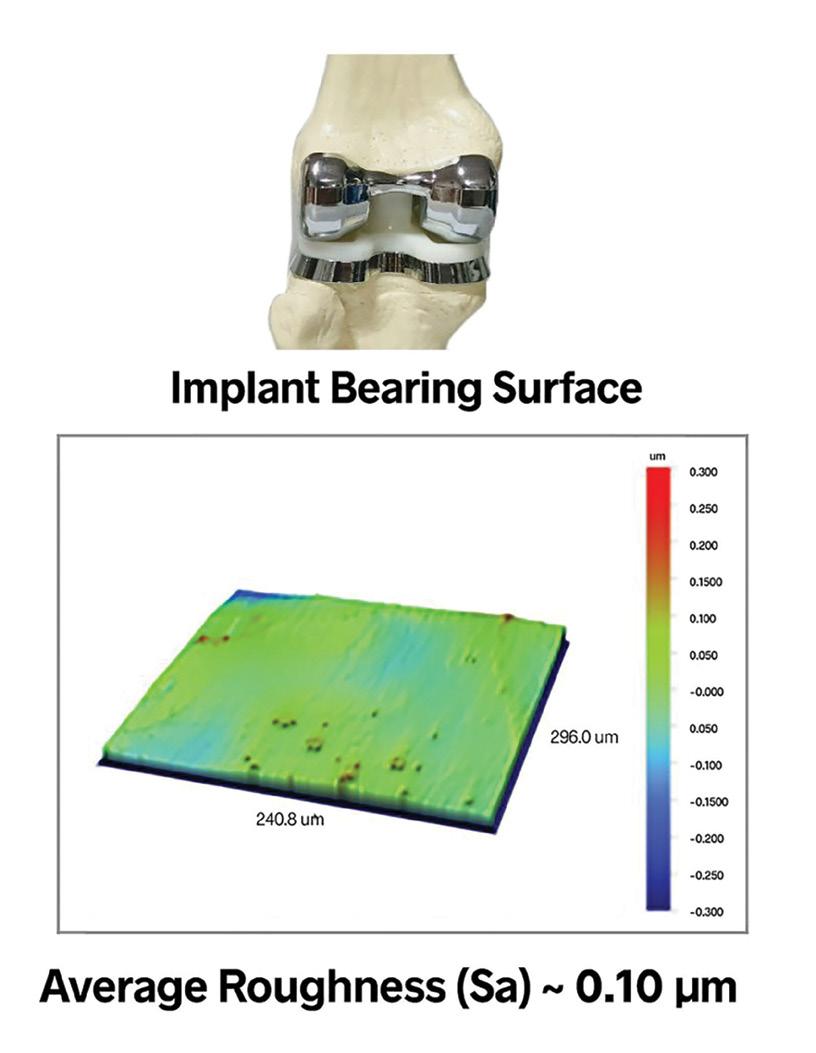
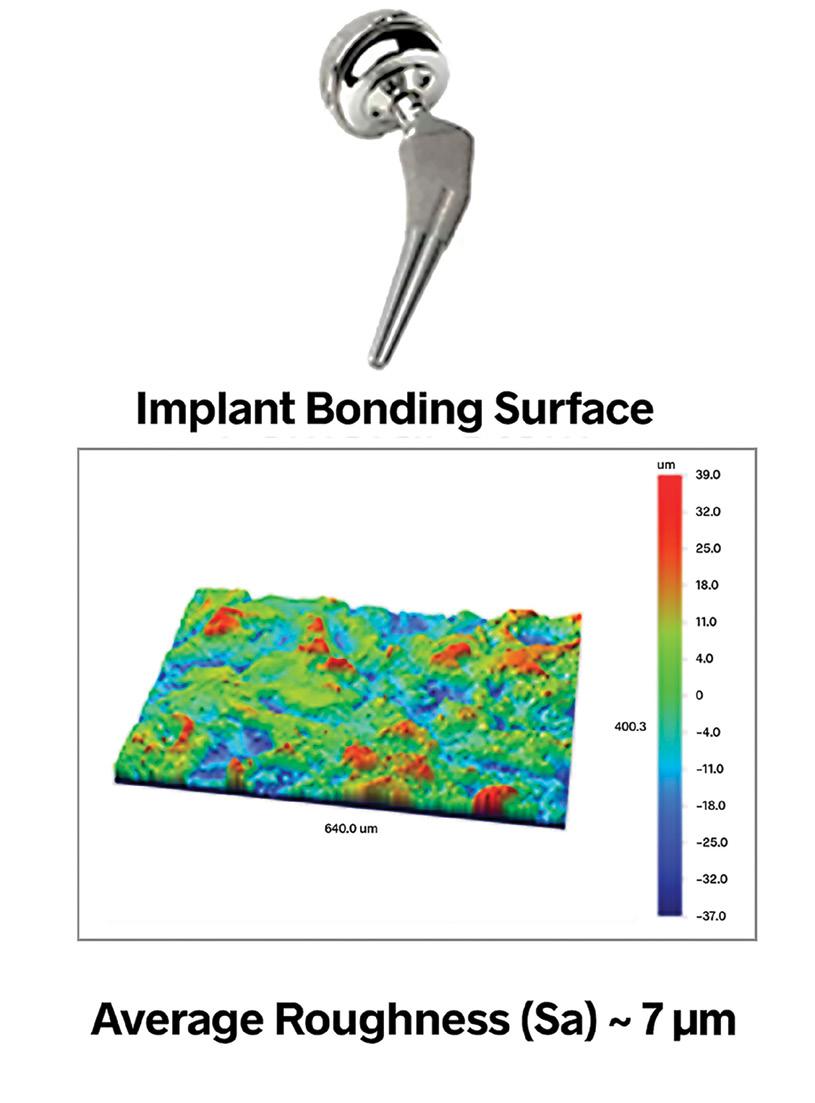
Surface finish specifications often narrow down to a singular texture aspect, such as average roughness, denoted as Ra (or Sa for 3D measurements). While these parameters can hint at changes in manufacturing processes, they don’t fully capture the surface’s overall texture. Different surface textures, even with the same average roughness, can have drastically different functional properties. For instance, a directional texture might impede fluid flow, while a deep notch could lead to fatigue failure. Therefore, understanding and specifying the critical aspects of surface texture are vital for the intended application of medical devices.
Different categories of medical devices require specific surface textures to perform optimally. For bonding surfaces, increased surface area can improve adhesion, which can be achieved through roughening. However, the texture’s nature also matters, as removing directionality can reduce slipping, and specific height and spacing might promote bone growth for implants.
In contrast, bearing surfaces, such as those in joint replacements, may need to be polished to a mirror finish. Although smooth surfaces are generally low friction and advantageous for wear properties, the optimal finish depends on the amount of lubrication available. Some scenarios require a degree of texture to retain lubricants and reduce contact area.

Michigan Metrology’s expertise and dedication to innovation play a significant role in advancing this field, ensuring that medical devices achieve optimal performance, safety, and longevity.
Moreover, devices functioning in the bloodstream must have smooth surfaces to prevent turbulent flow and clot formation. Proteins involved in clotting interact with finely spaced texture, necessitating extremely smooth finishes to prevent blood cell damage.
3D optical profiling proves invaluable in measuring complex surfaces with intricate curves, such as spinal implants. Its ability to reach challenging areas ensures that even the most complicated designs meet necessary specifications.
Michigan Metrology has established itself as a leader in surface roughness measurement and analysis. They provide essential services and training to tackle issues related to wear, surface finish, flatness, sealing, vibration, and noise. The company serves a diverse clientele, from start-ups to Fortune 500 companies in industries like automotive, biomedical, and materials. Their dedication to product development and resolving warranty issues is supported by advanced technology, including the NP Flex 3D Optical Profiler from Bruker Corporation.
Established in 1994 by Dr. Donald K. Cohen, Michigan Metrology benefits from his pioneering work in optical sciences and surface metrology technology. Dr. Cohen’s leadership emphasizes the company’s commitment to continuous learning and innovation, sharing expertise through both in-person and online training sessions. This commitment ensures that their clients remain at the forefront of the latest advancements in surface roughness analysis.
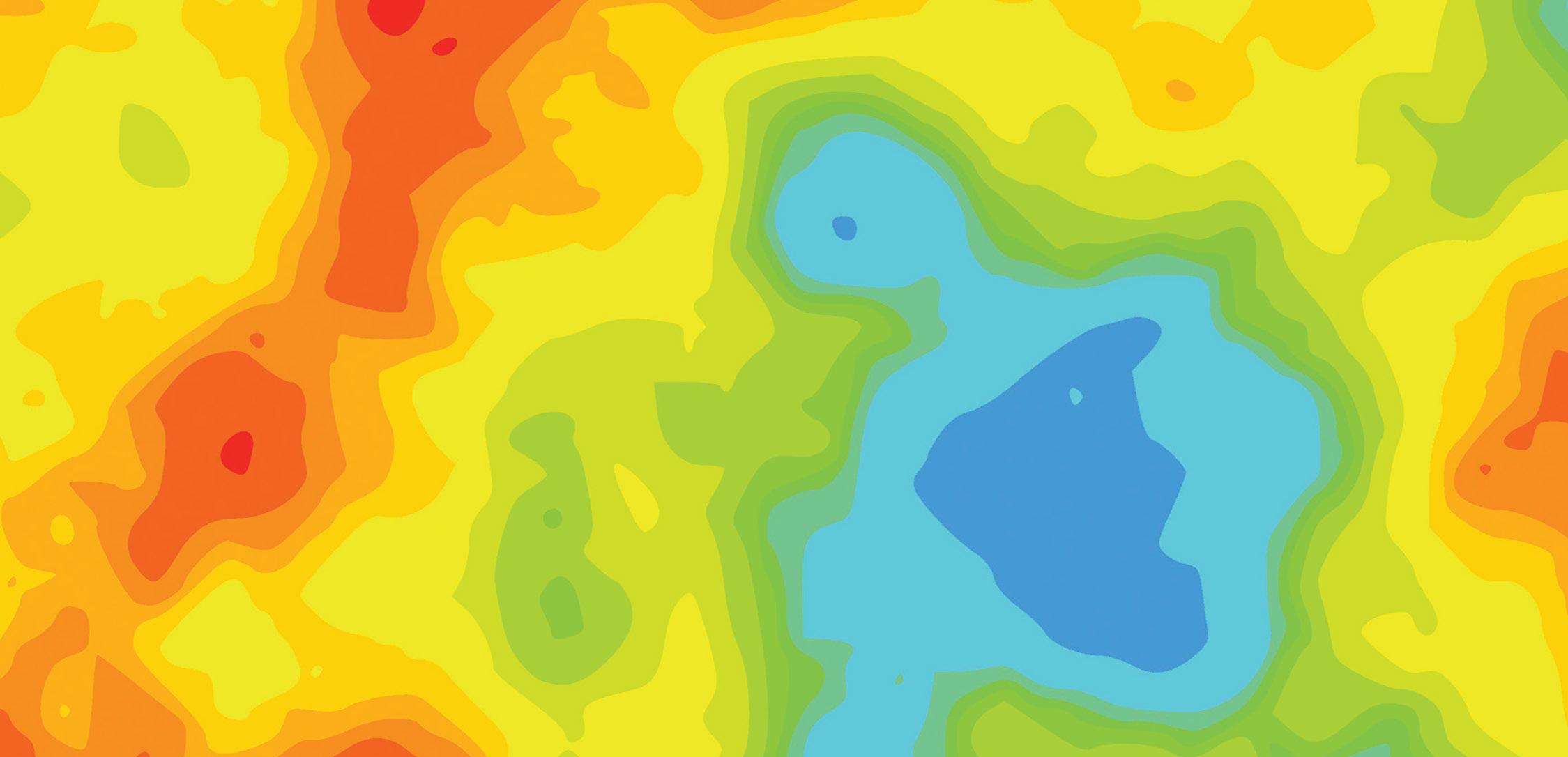
The precise control of surface texture through 3D optical profiling is critical for optimizing medical device performance. This technology allows manufacturers to meet specific functional requirements, improving adhesion, friction levels, and smoothness based on the device’s application. Michigan Metrology’s expertise and dedication to innovation play a significant role in advancing this field, ensuring that medical devices achieve optimal performance, safety, and longevity.

BY JAN P. STEGEMAN, DEPARTMENT OF BIOMEDICAL ENGINEERING, UNIVERSITY OF MICHIGAN
A new professional Master’s program in Biomedical Engineering (BME) at the University of Michigan is aimed at increasing the readiness of engineers to be effective in the medtech industry.
The Advanced Medical Product Engineering and Development (AMPED) program launched last year and the first cohort of students graduated this spring. The program’s creation was motivated by consistent and clear feedback from BME stakeholders in Michigan and across the country that there is a need for a curriculum that better prepares graduates for positions in the medtech industry, with an emphasis on fundamental concepts and skills that are important in medical product development.
The AMPED program leads to a professional Master’s of Engineering degree (MEng) in BME. The curriculum is designed to be completed in two or three academic terms.

• A core design-build-test practicum with a focus on solving current clinical problems by creating new product concepts. Clinicians present their clinical problems to the AMPED cohort, which then vet the need and form into teams to develop novel device-based solutions. There is an emphasis on engineering design control, prototyping, formative testing, and proof of concept.
• Complementary content in quality systems, risk management, and regulatory structures. The class is introduced to the structures and functions of a quality system, and to the processes of risk management that underlie device design. Appropriate regulatory strategies for the team projects are formulated, with an emphasis on current consensus standards and global harmonization.
• Preparation in advanced concepts in the medical product development, with a focus on current topics of interest in the medical technology industry. In the past iteration of the program, topics included medical device cybersecurity, creativity in design, sustainability in medtech, as well as case studies on recent recalls in the medtech space.
• Professional development and leadership training to enhance self-awareness, career preparedness, and cross-functional teamwork. Students are encouraged to explore career options and do a set of informational interviews with engineers in industry.

The first cohort of AMPED students consisted of 25 engineers with a range of backgrounds. Teams worked with clinical consultants who provided guidance and feedback on product concept development. The focus was on vetting, prototyping, and testing of product concepts.
IN 2023-24, SIX PROJECT TEAMS FORMED ACROSS A RANGE OF CLINICAL AREAS:
OB/GYN: wireless diagnostic device for monitoring high risk pregnancies
ORTHOPAEDIC SURGERY: tool to eliminate eschar buildup on electrocautery devices
PEDIATRIC CARDIOLOGY: compression device to achieve hemostasis following catheterization
PLASTIC SURGERY: portless breast tissue expander for breast reconstruction
RADIOLOGY: compression sleeve for screening mammography procedures
THORACIC SURGERY: device to detect air leaks in the lung
Class sessions in the AMPED program use a variety of experiential learning formats, including clinical seminars, workshops, case studies, team reviews, expert lectures, and teamwork sessions. The content is augmented with guest speakers from industry on specific topics related to the course material. The 2023-24 AMPED cohort interacted with over 20 guest speakers from the medtech industry, who joined class sessions to discuss their functional roles and career progression. The 2023-24 AMPED cohort also made site visits to two Michigan medical technology companies: MC3 Cardiopulmonary (Dexter MI) and Terumo Cardiovascular Group (Ann Arbor MI). In each case, the students were provided with an overview of the company and a guided tour of the facility.
The first cohort of AMPED graduates received the MEng degree in May of 2024 (Figure 2) and have gone on to careers in the medical technology industries (see Table 1 for placement data). Employers of AMPED students include some of the largest global healthcare companies, as well as smaller companies, the US government, and academic institutions. The positions span the country and about 30% are located in Michigan. The strong placement data of the AMPED program is particularly notable in context of the wave of layoffs experienced by the medtech industry in 2023-24.
The AMPED program continues to evolve with a focus on enrollment growth, establishing formal guidance from industry partners, and expansion of the curriculum to address the needs of the changing medical technology industries. Applications to the next round of the AMPED program open in mid-September and are due by January 15. Spread the word to those who may be interested in augmenting their education in medical product development. If any readers have feedback on the program or would like to be involved, please contact Jan Stegemann at jpsteg@umich.edu.


BY BARBRA WELLS, PRESIDENT AND CEO, PRIORCLAVE NORTH AMERICA
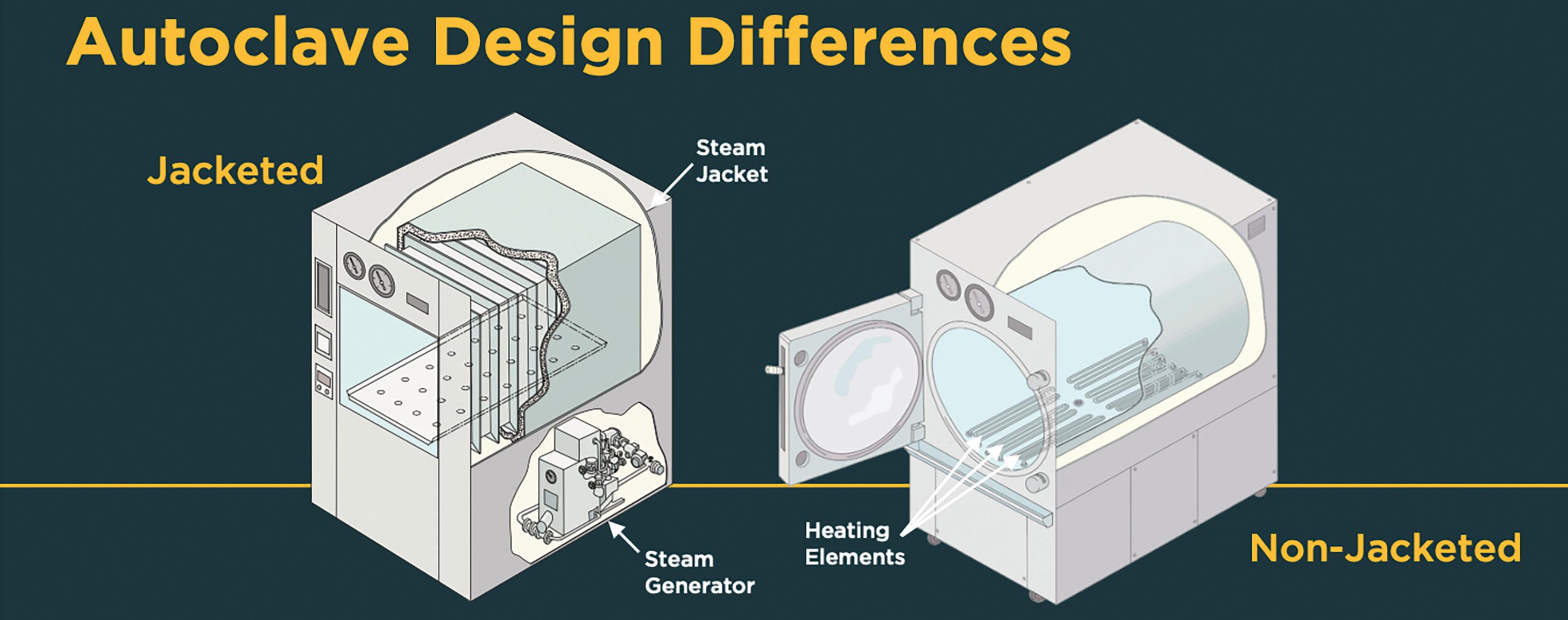
Every lab needs to get more done, more quickly, more cost-effectively, and more sustainably. Since 1988, Priorclave Ltd has been helping them do so, furnishing highefficiency non-jacketed research autoclaves to labs throughout the European Union, Asia, Africa, and the Middle East.
Priorclave North America is bringing this same level of reliability, flexibility, and sustainability to labs in the United States, Canada, Mexico, and the Caribbean.
We all know medical waste poses huge sustainability challenges. By some estimates, modern healthcare facilities produce 10 to 25 pounds of medical waste per day per occupied bed. According to a 2023 study published in the peer-reviewed journal Healthcare, this accounts for nearly two percent of total urban waste globally.
But the solution isn’t as simple as determining which single-use plastics are vital, what tools and supplies must remain disposable, and where we can return to sterilizing reusable items. Advanced electronic medical surgical tools pose a whole new set of challenges.
For example, orthopedic surgeons now use powered reciprocating bone cutting tools. These batterypowered tools safely provide smooth, controllable cuts in the operating room. Most parts of the instrument can go through a hospital’s steam autoclave for sterilization between uses. But the rechargeable lithium battery pack cannot: lithium batteries fail and explode when breached or overheated, making them poor candidates for sterilization processes that rely on high heat and pressure.
A removable battery pack solves the immediate safety problem, but creates new sterilization challenges: if the tool is autoclaved without the battery, there must be a separate procedure to be sure the battery pack has been properly sterilized prior to entering the sterile surgical field. Hospital workflows are already complex. Reusable instruments require more complex workflows with more steps, and more steps mean more opportunities for error.
Meanwhile, as medical device manufacturers create new and more advanced tools, they need to determine the working lifespan of that tool. This must take into account not only the wear and tear that comes with regular use, but the effects of repeated steam sterilization.
“When it’s less work to do tests, you do more testing. When it was me pushing the button…we did the minimum number of tests that our quality group would agree was safe. But now that it’s easy, we find ourselves hitting that minimum number of cycles, then saying ‘Well, it didn’t fail; just keep running it for another week, another two weeks, and see how high we can get.’”
Priorclave North America (PNA) is always eager to help medical device manufacturers develop new tools and sterilization protocols. For example, PNA worked with Blue Belt Technologies (part of Smith & Nephew) during the development of an innovative robotics-assisted suite of tools to improve partial and total knee replacement surgery. These tools included several reusable components, which incorporated electrical sensors in a metal and plastic enclosure assembled with epoxy.
Blue Belt was concerned about how this device would hold up to repeated uses and sterilization. They needed to run prototypes through hundreds of standard hospital sterilization cycles. The solution seemed obvious: get a medical-grade jacketed steam autoclave and repeatedly sterilize their prototypes.
But this quickly proved impractical. Standard hospital sterilizers are intended to reliably run FDA-approved sterilization cycles, not test medical devices for durability. The controls offer little flexibility, and every cycle must be manually initiated. Under these conditions, even a single round of testing took Blue Belt months.
PNA outfitted Blue Belt with a research autoclave capable of repeat cycles, and offering precision control of temperature and pressure ramps. As a result, Blue Belt increased throughput by 500 percent, completing months of testing in mere days.
This isn’t just about making it as easy as possible to do as much testing as possible. It’s also about making sure that research can be done as sustainably as possible. Too many labs find themselves trying to get research done using jacketed hospital-style autoclaves that are wasteful and unreliable under a research workload. By a conservative estimate, PNA’s right-sized non-jacketed autoclaves are currently saving North American labs tens of millions of gallons of water each year, in addition to more than 800 MWh of electricity. That translates to 685,000 fewer pounds of carbon dioxide pumped into our atmosphere annually.
Priorclave North America is headquartered in Michigan, collaborating globally to help labs work toward a sustainable future.

PRIORCLAVE.COM
STERLING INDUSTRIES
BY DAVID VAN SLINGERLAND, CEO, STERLING INDUSTRIES
In the world of medical devices, the ability to pivot isn’t just a nice-to-have—it’s a must-have. Whether you’re a fast-scaling newcomer or a Fortune 500 juggling your product portfolio, the need for a manufacturing transfer is sometimes inevitable. There’s a lot at stake, many moving parts… and a few principles that can guide you to success.
Let’s be real: change is one of the constants in the medtech world. Maybe you’ve outgrown your current manufacturing setup faster than a teenager outgrows shoes. Perhaps you’re eyeing a contract manufacturer with automation or clean room capabilities that can help you grow. Or maybe—let’s be frank—your current CM just isn’t cutting it anymore.
Whatever the reason, manufacturing transfers for medical devices are the bridge between where you are and where you need to be. They’re complex, high-risk endeavors that can make or break your product launch, market expansion, or bottom line. Get it right, and you’re looking at improved scalability, lower unit costs, and a potential edge over the competition. Get it wrong, and... well, let’s just say it’s not pretty.

SO, HOW DO YOU MAKE SURE YOUR MANUFACTURING TRANSFER GOES SMOOTHLY? HERE’S A SIMPLE RECIPE FOR SUCCESS:
PRECISION PLANNING: Map out every detail, from regulatory requirements to supply chain logistics. Take a “stage gate” approach: agree on step A before moving to step B. It’s like a well-choreographed dance—everyone needs to know their moves.

AGILE EXECUTION: Your team needs to be nimble, ready to pivot (there’s that word again) when unexpected challenges pop up. And trust me, they will.
CONTINUOUS COMMUNICATION:
Overcommunication is better than leaving your team or partners wondering, “What’s happening with this transfer?” Keep everyone in the loop, always.
We’ve all seen it in this business. The “it’ll probably be fine” approach to timeline planning. The “we’ll figure it out as we go” strategy for regulatory compliance. The “surely they know what we mean” philosophy of documentation transfer. Spoiler alert: none of these end well.
Common pitfalls include underestimating timeline complexity, overlooking critical quality system requirements, and failing to account for supply chain hiccups. Naïve optimism doesn’t help anyone. Bake risk mitigation strategies into every step of the transfer process. Spot and neutralize threats before they can derail your project.


A manufacturing transfer is only as good as the team behind it. Don’t just assign a project manager and call it a day. Assemble a cross-functional dream team tailored to your specific needs. Think of them as superheroes of manufacturing transfers, but with fewer capes and more cleanroom suits.
YOUR SQUAD SHOULD TYPICALLY INCLUDE:
A VETERAN PROJECT MANAGER with a range of transfers under their belt
QUALITY ASSURANCE EXPERTS who live and breathe regulatory compliance
SUPPLY CHAIN WIZARDS who could source a unicorn if your BOM called for it
ENGINEERING GURUS who translate your design intent into manufacturable reality
OPERATIONS MAVENS who ensure your product flows through the facility like water
Checklists are great for groceries, but they fall short for complex, multi-discipline, timeline-challenged projects. The backbone of successful manufacturing transfers for medical devices is a modern Quality Management System (QMS), and a team that embraces it.
Look for a partner whose staff lives and breathes their QMS. It should guide every decision, from the bigpicture strategy to the smallest component inspection. This systemic approach to quality ensures consistency, traceability, and compliance at every step of the transfer process.
The medical device manufacturing landscape doesn’t stand still, and neither should you. Both you and your manufacturing partner should be committed to Continuous Improvement, always looking for opportunities to enhance quality, consistency, and cost-effectiveness of your devices.
When selecting a contract manufacturer for your transfer, consider the benefits of working with a CM that exclusively focuses on medical devices. Companies tend to live and breathe end-to-end compliance in ways that differ from manufacturers that serve multiple industries.
At Sterling Industries, for example, the transfer process is tuned to assure that clients meet all regulatory requirements, and Sterling handles every medical device specific need, from supply chain validation to clean room assembly to lot tracking.
Remember, in the world of medical device manufacturing, standing still is moving backward. Whether you’re a scale-up preparing for rapid growth or an established OEM looking to worry about one less product line, finding the right partner for your manufacturing transfer can make all the difference. Choose wisely, and happy pivoting!




MICHBIO
MICHIGAN BIO-INDUSTRY QUICK FACTS *

5 th 9th
LARGEST BIOPHARMA STATE BY NUMBER OF ESTABLISHMENTS
LARGEST MEDICAL DEVICE STATE BY NUMBER OF ESTABLISHMENTS
44,340* BIOSCIENCE JOBS
2,429* BIOSCIENCE ESTABLISHMENTS
15 th
9 th
LARGEST AGRI-BIOSCIENCES BY NUMBER OF ESTABLISHMENTS
LARGEST BIOSCIENCE STATE BY EMPLOYMENT
$101,374* AVERAGE BIOSCIENCE SALARY
BIOSCIENCE PERFORMANCE METRICS*
ACADEMIC R&D INVESTMENT
$1.6 billion* VENTURE CAPITAL INVESTMENT $956 million*
million*


Anchored by several large hubs, this representative sample of Michigan life sciences companies shows the breadth of the industry footprint.



SUBMITTED BY MEDBIO
Medbio operates as a full-service ISO 13485:2016 certified contract manufacturer, seamlessly partnering from design through to assembly and packaging.
At Medbio, the team embodies a commitment to efficiency, always eager to invest in cutting-edge technologies that promise superior quality, swifter cycle times, and diminished overhead and labor costs.
Harnessing the power of the latest innovations, including robotics, end-of-arm tooling, cavity separation, and automated packaging, Medbio elevates efficiency and quality as production demands increase. Their capacity flexes to accommodate both large-scale projects and bespoke orders, ensuring scalability that supports rapid expansion. Medbio’s seasoned team is dedicated to their clients’ success, collaborating closely at every juncture to guarantee quality, efficiency, and accelerated market delivery.
Nestled in the charming region of Western Michigan, Medbio’s headquarters in Grand Rapids spans three facilities, collectively offering 115,000 square feet of space. This multifaceted facility integrates engineering, quality, manufacturing, and corporate business departments under one roof. Complementary locations in Clinton Township and Schoolcraft, Michigan, along with Orchard Park, New York, extend Medbio’s reach, melding global expertise with a personal touch for clients.
Designed and built to conform to the rigorous standards demanded by medical and biotech device manufacturing, Medbio’s facilities are impressive. The company features over 100 molding presses, ranging from 17 to 440 tons, within an ISO Class 8 certified cleanroom, and a micro-molding press nestled in a modular, ISO Class 7 certified cleanroom. The headquarters alone boasts four
modular, ISO Class 7 certified cleanrooms, specifically for assembling and packaging finished products.
In an exciting development, Medbio has joined forces with Caplugs Medical, expanding capabilities to 17 global locations, ready to tackle customers’ greatest challenges. With specialized facilities for silicone, diagnostics, and more, Medbio is trusted by over 1500 medical customers and the top 30 medical device manufacturers, presenting a vast array of medical molded solutions and market-leading capabilities.
Caplugs Medical offers a diverse selection of commonly used parts and sizes in the medical industry, available for immediate shipment. From caps and plugs to netting, tubing, containers, and various plastic components, these non-proprietary parts have been crafted to meet prevalent industry needs for swift, cost-effective solutions.
Medbio prides itself on top-tier manufacturing excellence, outstanding customer service, expert program management, rapid turnaround times, and innovative problem-solving. The company consistently surpasses customer expectations, whether launching new products or enhancing manufacturability and part quality in the transfer business.
Medbio delivers comprehensive solutions for today’s contract manufacturing challenges with flair and dedication.


Michigan continues to be a leader in advancing biosciences research and commercialization, thanks to its rich pipeline of intellectual property. A whole new crop of startups are germinating in the state, which is good news for the regional cluster of established companies that are focused on innovation in therapeutics, medical devices, healthcare technologies, clinical diagnostics and agri-/ industrial biotechnology.


For decades medical devices have been sterilized with steam, radiation or ethylene oxide. The existing sterilization methods cause damage to medical devices and ethylene oxide (EO) has been deemed a carcinogen by the EPA and FDA. Multiple lawsuits around the country claim children living vicinity of EO sterilization plants are suffering from blood-born cancers as a result of inhaling EO gas emitted from sterilization plants.
A Michigan research and development company; Sterile State invented and patented a completely safe sterilization method for medical devices without any of the risks posed by radiation or EO. Dr. Megan Frost invented the materials and methods to safely sterilize medical devices without risk of injury to children currently posed by EO. The revolutionary gas sterilization method relies upon nitric oxide, a gas produced by all living cells and is a natural
sterilant when properly created and delivered by the Sterile State System.
A pouch is coated with the patented polymer that releases nitric oxide resulting in a self-sterilizing pouch. No trucking is required to move new devices to a regional plant for sterilization, no EO is required. The manufacturer makes a device and simply places the device into the Sterile State pouch where it will be sterilized and immediately shipped to customers.
The system is currently before the FDA. This system will save time and money for manufacturers, and can eliminate the health risks posed by the now outdated EO method.
BY LOU BLOUIN, WRITER, EXTERNAL RELATIONS, UNIVERSITY OF MICHIGAN-DEARBORN

The University of Michigan-Dearborn is demonstrating it takes more than a hospital to have a thriving bioengineering program.
Bioengineering programs have proliferated in the past decade, and by far, the most common place for them to find a home is a campus with a large medical center. But back in 2010, when the University of Michigan-Dearborn was contemplating starting a program, it didn’t see its lack of a hospital as a hangup. Professor and program director Alan Argento says any bioengineering program worth its salt, after all, centers core engineering skills in the curriculum. UM-Dearborn had that expertise covered, with several historically strong engineering programs — plus a 50year history of industry collaborations. The thought was by rooting its program in mechanical engineering, partnering with other departments for the natural sciences curriculum, building a bench of ambitious young faculty with diverse research interests, and leveraging collaborations with hospitals, medical researchers and industry partners, the university could create an experience that was on par with what students were getting on the big medical campuses.
Fourteen years later, UM-Dearborn’s experiment in bioengineering education is showing how well this model can work. Now housed within a new Engineering Lab Building that features a 3,000-square-foot laboratory suite, the program serves 120 students across its undergraduate, master’s and PhD offerings, which include a popular five-year dual undergrad degree in bioengineering and mechanical engineering and several accelerated master’s options. Argento says beyond its rootedness in core engineering skills, the strength of the program lies in a set of well-thought-out hands-on opportunities that help students navigate this diverse discipline. All but one core bioengineering course has an accompanying lab. Many students do research with faculty, some as early as their freshman years. Faculty have research partnerships with major medical centers at UM-Ann Arbor, the University of Virginia and Henry Ford Health System, as well as global corporations like Toyota and Ford. It’s also not uncommon for bioengineering undergrads to complete multiple paid internships, giving them work experience even before they graduate.
The result is students are often graduating with resumes that stack up to or exceed those of their peers in topranked bioengineering programs. ’24 grad Zoie Mink says



she wanted to major in bioengineering as far back as her freshman year of high school — incidentally based on the experience of an older cousin in the UM-Dearborn program who scored a full ride in Purdue’s doctoral programMink ultimately chose UM-Dearborn over two larger schools on the hunch that she’d have better odds of getting deeper experiences on the smaller campus. That’s exactly how it played out. She worked as a research assistant in Associate Professor Amanda Esquivel’s lab from her freshman year on, doing work focused on wearable sensors and sports injury prevention. Mink then parlayed that experience into two summers of internships at Wake Forest University, where she worked in a lab that developed a mouthpiece-based head injury sensor system and got to travel the country working with NASCAR drivers. That’s on top of a stint in Ford’s safety dummy division. When she applied to PhD programs, Mink picked four, including Stanford, one of the top homes for bioengineering in the country, and got into all of them. She ultimately chose Wake Forest, where the relationship with her mentor meant she got to dictate the subject for her PhD work.
Mink says she never thought of UM-Dearborn’s lack of hospital affiliation as a liability, and it obviously had no impact on her own prospects. Her take, in fact, is that it may give UM-Dearborn students more flexibility. Indeed, today at UM-Dearborn, students can get experience in subjects as diverse as biomechanics, biomaterials, nanotheranostics and microfluidics. And Argento says they’re always working to expand those options. Right now, the department is putting the finishing touches on a program for natural sciences undergrads who are studying biology, biochemistry and biophysics. And soon, they’re hoping to leverage the university’s longtime industrial and manufacturing engineering strength into a biomanufacturing program.
It’s proof that a small program—if you’re willing to get creative — can indeed be a mighty one.




SUBMITTED BY WAREOLOGIE ™
Wareologie™, a bootstrapped startup, has successfully integrated innovative rehabilitation technology into major healthcare systems, culminating in the acquisition of its intellectual property (IP) by a leading global medical distributor.
The healthcare industry faces continuous challenges, including the need for effective patient handling and mobility solutions. Wareologie™, Inc., a woman-owned company based in Southfield, MI, has developed a groundbreaking approach to address these challenges. This article explores how Wareologie™ leveraged emerging technologies, strategic partnerships, and rigorous usercentered design to revolutionize patient care.
Wareologie™ identified a critical $34 billion problem in healthcare related to patient falls, handling, and mobility. The company’s mission is to assist individuals suffering from degenerative illnesses and those recovering from surgeries by designing rehabilitation tools. Their flagship product, portable parallel bars, enables physical therapy in various settings, enhancing patient outcomes with early mobility access in an attempt to reduce the incidence of hospital-induced deconditioning.
The development of Wareologie™’s portable parallel bars was guided by extensive user-experience research and collaboration with clinicians. The design process emphasized safety, portability, and ease of use, resulting in a lightweight, foldable device on wheels. This innovative solution allows therapists to administer standing and balancing treatments safely, anywhere within a healthcare facility or at home. Clinical customers, report feeling significantly safer treating stroke patients. By using the
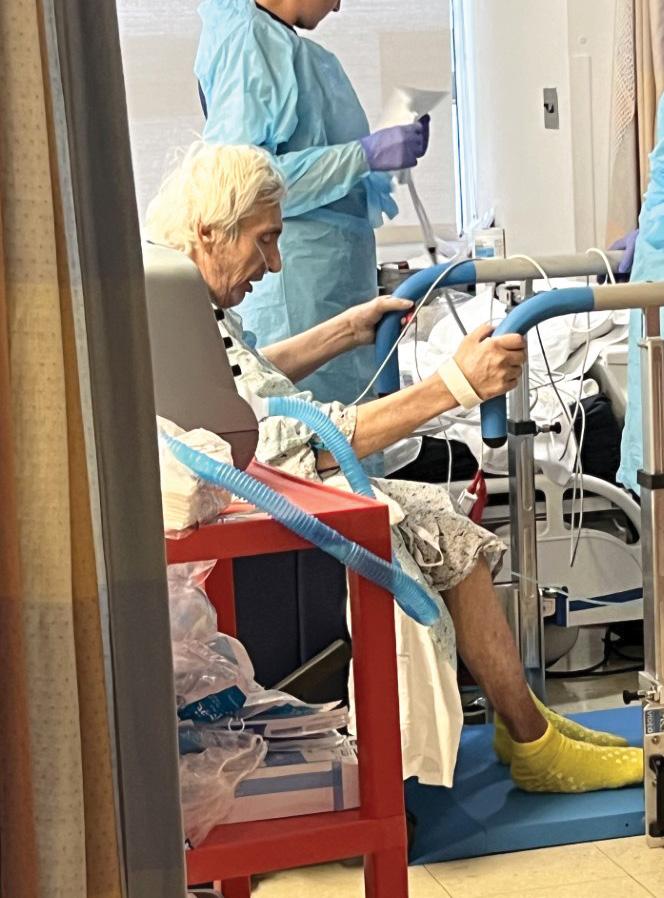

portable parallel bars, clinicians no longer need to stabilize the walker, allowing patients to independently stabilize themselves, thereby enhancing safety and fostering patient empowerment.
KEY TO WAREOLOGIE™’S SUCCESS WAS THE SUPPORT FROM VARIOUS ORGANIZATIONS AND FUNDING SOURCES:
• Veterans Health Administration Innovation Network (VHA iNet) provided a Greenhouse contract and subject matter experts.
• Lawrence Technological University Centrepolis Accelerator and The Bachelor of Science and Nursing School provided initial guidance and resources.
• Michigan Economic Development Corporation (MEDC) Business Accelerator Funding (BAF) grant contributed $50,000.
• Friends and Family (F&F) investments totaling $285,000 facilitated early development stages.
• HA Industries in Sterling Heights, MI, played a crucial role in building the MVP (Minimum Viable Product).
The COVID-19 pandemic spurred Wareologie™ to design the world’s first mobile parallel bar tool, targeting quarantined patients in skilled nursing facilities (SNFs). This initiative led to a Greenhouse contract from the Veterans Health Administration Innovation Network (VHA iNet), accelerating the project’s prototype phase with direct input from physical and occupational therapist subject matter experts.
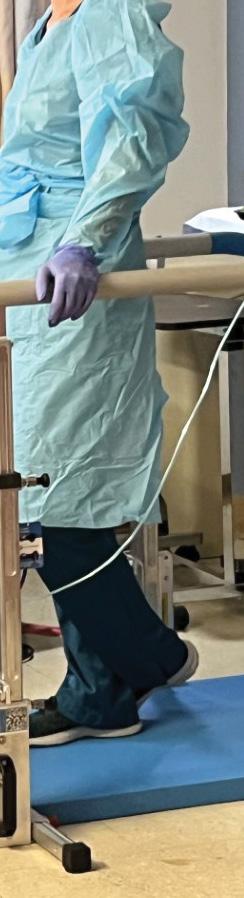
Despite a significant setback when a 100-unit order was pulled by the VA due to budgetary cuts, Wareologie™ secured additional F&F investments. The prototype collaboration with Global Tech Ventures ensured highperformance and aesthetically pleasing initial designs. The company launched the FDA Class 1 exempt portable parallel bars MVP with HA Industries and released the first products in Q1 2023.
Securing patents was crucial for protecting innovative designs, and with the expertise of Aurora Patents, Wareologie™ successfully obtained IP claims across the US, Europe, Canada, and the UAE. This protection facilitated the company’s growth, ultimately leading to the sale of its IP in July 2024.
Wareologie™ effectively marketed its products through a strategic combination of direct outreach, live virtual demonstrations, and comprehensive evaluations, alongside participation in major rehabilitation and Safe Patient Handling conferences. Despite being a small team of two, supported intermittently by contractors, their relentless efforts successfully garnered the interest and trust of clinicians. Consequently, the portable parallel bars have been adopted by prominent healthcare
providers, including the VA, and large private hospital institutions such as Thomas Jefferson Hospital, and Gaylord Specialty Healthcare. This versatile tool is now seamlessly integrated into patient care across various settings, including ICU, acute care, pediatrics, amputee rehabilitation, spinal cord injury (SCI) units, and outpatient facilities.
The journey of bringing a medical device from concept to market is underscroed with challenges and triumphs. Despite difficulties in securing traditional venture capital and state-funded support, Wareologie™ persevered, forming strategic alliances that led to a successful IP acquisition. Its innovations will continue to benefit healthcare providers and patients globally, with a new product version set to launch in Q1 2025..
This achievement would not have been possible without the contributions of LTU Centrepolis Accelerator, MEDC, HA Industries, Global Tech Ventures, Aurora Patents, and VHA iNet. Special recognition goes to the founder, Gina Adams, and teammate Peter Makarov, for their extraordinary perseverance.


Every day, from discovery to delivery, Avantor is an essential partner to the scientific community — pioneers, scientists, innovators and educators — relentlessly focusing on breakthroughs that help solve the world’s most complex challenges.
Our proven expertise and trusted portfolio of products and services, combined with a global reach and ability to provide customized materials of the highest quality for highly regulated applications move science forward.
As a global leader in life sciences, we fulfill our mission: to set science in motion to create a better world
Visit vwr.com or avantorsciences.com

Backed by 40 years of experience in lipid chemistry, synthesis, and analysis, Cayman Chemical is a Michigan-based provider of lipid nanoparticle (LNP) research tools and services. We support the entire LNP research and development process from discovery to bioanalysis.
· Lipids for LNP Formulation
· Custom Lipid Synthesis
· GMP Lipid Production
· LipidLaunch™ Research-Ready LNPs & Reagent Kits
· LNP Formulation, Characterization, & Screening Services
Explore Research Tools for Every Step of LNP Discovery
MichBio
3520 Green Court Suite 175, Ann Arbor, MI 48105
WE INVITE YOU TO BECOME AN ACTIVE MEMBER OF MICHIGAN’S THRIVING BIOSCIENCES COMMUNITY. JOIN MICHBIO. Proudly Serving Michigan’s Bio-Industry Sectors
3520 Green Court, Suite 175 Ann Arbor, MI 48105-1175 734-527-9150