

MONEY DOESN’T INSPIRE GREAT IDEAS. GREAT IDEAS INSPIRE MONEY.
ENTREPRENEURSHIP & INNOVATION

MEDC is here to help bring great ideas to life, right here in Michigan. We’ll help you gain access to investors, expose you to SmartZones, Michigan’s network of regional tech incubators, and connect you to local university resources.
Whether you’re a one-person startup or already established, MEDC offers programs and access to capital that can help your business grow.
Learn more at michiganbusiness.org/entrepreneurship
The high concentration of life sciences talent in these hubs creates a critical mass of infrastructure that supports the 15th largest life sciences economy in the nation, with the 5th largest medtech sector, and 9th largest biopharma and agricultural biotechnology sectors.
PRESIDENT’S MESSAGE

Home to a population known for its grit and work ethic, Michigan has an extensive educational and research network, a willingness to invest in future technologies, and unsurpassed expertise in manufacturing, all fueling a drive to innovate that has been part of the state’s DNA since the beginning. Together, those attributes have created a durable and diverse life sciences industry that has continued to grow – at a pace greater than the national average - despite the many challenges of the last several years.
Life sciences hubs throughout the state – from Detroit to Marquette - are centers of innovation. The high concentration of life sciences talent in these hubs creates a critical mass of infrastructure that supports the 15th largest life sciences economy in the nation, with the 5th largest medtech sector, and 9th largest biopharma and agricultural biotechnology sectors.
With an extensive network of SmartZones and business accelerators, small businesses can grow and thrive alongside the many international brands –organizations coming to Michigan will find no shortage of both public and private support programs, and partnership opportunities.
This issue of BioMatters provides a glimpse of Michigan’s life sciences ecosystem with companies like Akadeum Life Sciences and Upkara pioneering new technologies that have the potential to revolutionize drug delivery and distribution, while Phenomics Health and CSL Behring are focused on pushing the boundaries of precision medicine, and Excel Medical and Sterling Industries are contributing to medtech growth. Alongside these companies, Michigan’s patient advocate organizations like Lupus Detroit and the Blood Cancer Foundation of Michigan are bringing awareness to the challenges faced by patients that impact the efficacy of the treatments, technologies, and cures developed in the state. Together, these, and other, industry and patient organizations work to advance the resources that are available to patients, as well as the technologies that impact agriculture, support sustainability, and improve lives the world over.
MichBio has spent the past 30 years representing Michigan’s life sciences industry, we invite you to learn more about our great state and are ready to connect you to the organizations and people you need to be successful.
Sincerely,
STEPHEN RAPUNDALO, PHD President and CEO, MichBio
Michigan’s life sciences industry stands as an example of what can be accomplished with the right combination of resources and talent.
DELIVER CONFIDENCE
WITH CAYMAN’S CANNABIS & HEMP ANALYTICAL STANDARDS
Cayman provides analytical reference standards and ISO 17034-produced Certified Reference Materials (CRMs) to identify and quantitate the components found in Cannabis or hemp products with accuracy and confidence.
EXPAND YOUR CANNABIS TESTING SERVICES
From single standards to multi-component mixtures designed to improve accuracy and streamline workflows, we offer a diverse and ever-expanding collection of Cannabis and hemp analytical standards.

Major & Minor Phytocannabinoids
Novel Compounds
Terpenes
Contaminants
Degradants & Byproducts
Try Cayman’s Phytocannabinoid CRMs for the first time with a 75% discount.
Available to new users of Cayman’s single- or multi-component phytocannabinoid CRMs (US only). Learn more at caymanchem.com/runthecaymancomparison
EXPLORE OUR CANNABIS TESTING PRODUCTS & RESOURCES AT CAYMANCHEM.COM/CANNABISTESTING
Email sales@caymanchem.com to discuss your specific project needs
PREMIUM MEMBERS
MichBio is the biosciences trade association for the state of Michigan. Our goal is to drive the growth of the state’s bio-industry through advocacy, education, connections, and supportive resources.
Stephen Rapundalo, PhD President and CEO
Alisha Brown Editor Director, Marketing and Communications
Carrie Hranko Manager, Administrative Services
MICHBIO BOARD OFFICERS
CHAIR
Ken Massey, PhD
Wayne State University
Senior Director, Venture Development Technology Commercialization
VICE CHAIR
Kevin McLeod
C2Dx
Founder, President and CEO
MICHBIO BOARD DIRECTORS

Kate Bekasiak Stryker Medical Vice President, Human Resources
Christy Bigelow Emergent Biosolutions
Vice President and General Manager, Manufacturing Operations

Sean Callaghan Medbio Inc.
Vice President of Operations and General Manager
Robert DeRyke, MBA
Terumo Cardiovascular Group President and CEO
Lola Eniola-Adefeso, PhD University of Michigan
University Diversity and Social Transformation Professor of Chemical Engineering
Charles Hasemann, PhD Michigan State University Assistant Vice President for Innovation and Economic Development
Anna Langerveld, PhD Genemarkers, LLC President and Chief Scientific Officer
Ken Massey, PhD Wayne State University
Senior Director, Venture Development Technology Commercialization
MichBio
3520 Green Court, Suite 175 Ann Arbor, MI 48105-1175 734-527-9150
info@michbio.org michbio.org
Designer: Daina Fuson Designs
Printer: Progressive Printing
© Copyright Michigan Biosciences Industry Association, DBA MichBio
PRESIDENT AND CEO
Stephen Rapundalo, PhD MichBio President and CEO
SECRETARY
Robert Donofrio, PhD Neogen Corporation Chief Scientific Officer TREASURER Vacant
Kevin McLeod C2Dx
Founder, President and CEO
Emily Miner, MS Terumo Cardiovascular Group Senior Vice President, Global Quality and Regulatory
Fredrick Molnar, MBA Michigan Economic Development Corporation
Vice President, Entrepreneurship and Business Development
Dave Morin Care Technology Advisors, Managing Member
Stephen Rapundalo, PhD MichBio
President and CEO
Tom Ross
Grand River Aseptic Manufacturing President and CEO


John J.H. Schwarz, MD
Former U.S. Representative
Uma Sharma, PhD
MMS Holdings
Chief Executive Officer
MICHBIO PREFERRED



The MichBio Preferred Purchasing Program leverages the collective purchasing power of our member companies to obtain steep discounts from our vetted and endorsed vendor portfolio. Members have saved upwards of 900 times their annual membership dues.












Unchaining Innovation to Advance Health Equity
SUBMITTED BY KRISTEN POOR, DIRECTOR OF MARKETING PROGRAMS, INNOSIGHT AND LAURA BRONSART, DVM, PHD, SENIOR DIRECTOR OPERATIONS AND PRODUCT DEVELOPMENT, UPKARAUpkara decreases cold chain reliance with no-freeze stabilization technology.
Imagine waiting for a lifesaving medication or vaccine only for it to arrive damaged after sitting in a hot shipping container for too long. Picture years of research and hard-earned grant funding being wasted because materials spoiled due to a delay on the tarmac. Though it isn’t always obvious, billions of people worldwide are affected by the inefficiencies of cold chain logistics.
Each year, the pharmaceutical industry alone spends approximately $18.6 billion on cold chain support to store and transport temperaturesensitive products. Losses related to cold chain failures add up to an additional $35 billion annually. Such costs are also challenging for universities, researchers, and reagent manufacturers.
But what if it was possible to stabilize fragile biomolecules without the use of freezing? Thanks to cutting-edge technology, it is.
Upkara is a diverse, forward-thinking biotechnology company headquartered in Ann Arbor, Michigan. It was founded by Pravansu Mohanty, Ph.D., as a spin-off of the Somnio think tank whose mission was to address global unmet needs. Upkara’s focus is biomolecule stabilization. Their novel technology, unlike the current stabilization technique of lyophilization or freeze drying, is rapid, cost-effective, and broadly applicable.
Company CEO and Chairman, Alan Turfe, MBA, says, “Upkara eliminates the cold-chain requirements of reagents, therapeutics, and vaccines using our innovative, patented biopreservation technology, with the ultimate aim of improving global health equity.”
The company has successfully used this technology to stabilize antibodies, enzymes, small molecules, and mRNA.
Laura Bronsart, DVM, Ph.D., Senior Director of Operations and Product Development says, “We fully anticipate that our technology can stabilize the same products that lyophilization is currently used for. However, our technology does not freeze the product to achieve stabilization.”
This creates massive cost savings for multiple industries by decreasing cold-chain operation needs and increasing yield. Stabilized products can be stored and shipped at ambient temperatures, have less risk of damage, and have extended shelf lives. Additionally, Upkara’s technology requires significantly less processing time–just one hour versus the 12-72 hours required for lyophilization.
Upkara is currently focused on stabilizing temperaturesensitive reagents used ubiquitously in all research and development while future applications of this technology will be aimed at therapeutics and vaccines. The technology has the promise to positively impact patients globally as improving access to analytical reagents opens the door for countless innovations and increases the affordability of materials needed for groundbreaking research.
Upkara is working with reagent manufacturers to identify ways its technology can be utilized to produce stabilized products. “Analytical reagents are used across a wide variety of institutions, including academia, government, and industry. They are the foundation upon which all new knowledge and biological advancements are built, and many are susceptible to damage due to thermal stress,” says Dr. Bronsart.

A driving factor behind Upkara’s success is the real-world experience and diversity among its employees. The team is comprised of a unique mix of biologists and engineers who are passionate about their work and the company’s mission.



Jamal Mosallam, CFO, says, “We are very proud of our company’s diversity. The science and product development are being led by two female scientists. Our lab and technical staff include people from many nationalities–all of whom have witnessed the negative impact of having limited or unreliable access to cold chain storage and transportation.”
Of cold chain difficulties facing the biotechnology industry, Mosallam says, “The status quo has not solved this problem, and we are hoping some of the brightest, most optimistic, and collaborative minds in the industry will.”

“The status quo has not solved this problem, and we are hoping some of the brightest, most optimistic, and collaborative minds in the industry will.”
Upkara strives to create a world with greater health equity through products that decrease reliance on cold chain logistics and improve access to therapeutics and research tools.
ASSOCIATION FOR MIGRAINE DISORDERS
Bringing Community and Awareness to Migraine Disease
BY KYLIE PETRARCA, ASSOCIATE PROGRAM DIRECTOR, ASSOCIATION FOR MIGRAINE DISORDERSDid you know that one in seven people live with migraine disease? Migraine is a complex neurological disorder that is NOT just a headache.
The typical symptoms of a migraine attack include head pain, nausea, vomiting, and light and/or sound sensitivity. However, many people experience additional symptoms such as dizziness, vertigo, ringing in the ears, fatigue, neck pain, yawning, difficulty concentrating, sinus pain or pressure and more. People may be diagnosed with episodic migraine which means they have less than 15 headache days per month or they may have a chronic form, meaning they have 15 or more headache days per month and on at least 8 days of the month, the person has migraine-like features. Migraine alone is the second leading cause of disability in the world and the number one leading cause of disability in women who are 15-49 years old.
The Association of Migraine Disorders is a non-profit organization, founded in 2012, with a mission to expand the understanding of migraine by supporting research, education and awareness. The organization is dedicated to being a trusted and comprehensive resource for people living with migraine and clinicians who diagnose and treat people with migraine, and
provides educational content via videos, blogs, webinars, printed materials, and so much more.
To raise awareness about migraine disease, the Association of Migraine Disorders created Shades for Migraine, a global awareness campaign. Each year people living with migraine, along with their friends, families, significant others, and coworkers post pictures on social media wearing sunglasses on (and around) June 21. Participants use the hashtag #ShadesForMigraine and challenge others to take part to make the campaign go viral.
Sunglasses are a visual representation of photophobia, also known as light sensitivity, that many people with migraine experience. This program is an opportunity for people to share their connection to migraine disease, in the hopes of spurring more conversation. After its launch in 2017, Shades for Migraine quickly caught on around the world and people from all 50 states and at least 59 countries have participated.
To foster engagement and fun competition, each year the Association for Migraine Disorders have a photo contest where its followers can vote for their favorite images from people who participated in the campaign. Besides bragging rights, there are prizes for the top 10 most popular images.

In 2020, the organization began a Community Leader program where volunteers educate and connect with people in their community to raise awareness about migraine and introduce people to a world of resources. The Community Leaders help distribute sunglasses and materials at workplaces, healthcare centers, schools, churches, sporting events, or beaches, talk to the local media, help fundraise, and more!
The Association for Migraine Disorders has some exciting events planned for Shades for Migraine this year - it will be attending the FEI World Cup Finals in Omaha, Nebraska and the International Balloon Festival in Albuquerque, New Mexico!
If you or someone you know lives with migraine, you can visit the organization at an event near you, follow it on social media or become a Community Leader and help pass out sunglasses to people in your school, community or headache center.



“This is such an amazing cause and being an ambassador made me feel like part of something bigger which after 14 years of chronic illness taking things away was nice to have back.” —
LEARN MORE ABOUT MIGRAINE DISEASE, VISIT MIGRAINEDISORDERS.ORG
TO STAY UP TO DATE ON SHADES FOR MIGRAINE, VISIT SHADESFORMIGRAINE.ORG

“Wearing those purple sunglasses filled me with pride knowing I can help make a difference in people’s lives just by sparking a conversation.” — NICOLE
DANNIELLE
Accelerating Drug Discovery in Michigan
BY PETER TOOGOOD, DIRECTOR OF MICHIGAN DRUG DISCOVERY AND RESEARCH & ASSOCIATE PROFESSOR, COLLEGE OF PHARMACY, UNIVERSITY OF MICHIGANWhen University of Michigan (U-M) scientist Isin Cakir, Ph.D. discovered a link between an enzyme called histone deacetylase 6 (HDAC6) and obesity in mice, he knew the next step in his research would be identifying molecules that could potentially block that same enzyme safely in humans.
To pursue that investigation, he turned to Michigan Drug Discovery (MDD), U-M’s university-spanning program, to help coordinate and fund drug discovery projects across campus. By working with MDD, Cakir was able to optimize his compounds, which were recently disclosed in a 2022 patent application, for testing relevant disease models.
The ability to capitalize on fundamental new discoveries in biology, by applying the tools of drug discovery, is at the core of what MDD was established to achieve. Academic research programs are replete with opportunities to translate fundamental studies of human health and disease into new methods of treatment. However, while university faculty are specialists in their fields, they may have less expertise or fewer resources for translating basic science research into new medicines – which is where the MDD plays an important role.
Once associated with a project, MDD is a forcemultiplier that can bring critical experience and resources to early-stage drug discovery projects by connecting faculty with relevant experts on campusincluding scientists who have experience bringing new treatments to the market in the pharmaceutical industry. By providing faculty with funding to conduct important proof-of-concept experiments, along with mentorship and guidance, MDD helps researchers identify and optimize novel drug candidates. The program supports research teams seeking new therapies for a wide range of diseases, including cancer, heart disease, opioid addiction, pain, and bacterial and viral infections.
The staff at MDD select projects to support through a competitive review process that includes input from industry experts. Top projects are teamed with one or more of several research core facilities on campus that specialize in high throughput screens, drug repurposing, medicinal chemistry, structural biology, natural products, or pharmacokinetics. Most projects rely on multiple cores to progress and MDD helps to coordinate resources while providing advice on prioritization and pivotal experiments.
Since its launch in 2012 (as the Center for the Discovery of New Medicines), MDD has invested over $3.5 million in drug discovery research at U-M. These investments have gone on to garner more than $48 million in external grant support, a 14-fold return on investment. In addition, the work supported by MDD has contributed to more than 80 publications and patent applications, and several projects have been licensed or formed the basis of new company formation.
The benefits of working with MDD are not limited to U-M faculty. MDD, and its associated research cores, also work with external partners, including faculty from other universities and researchers at biotech companies. For example, the high throughput screening lab recently completed projects for groups at the Van Andel Institute in Grand Rapids and for a biotech company in San Diego.
Although the considerable investment necessary to advance basic science projects to the clinic, and ultimately to patients, can be daunting, MDD see’s multiple opportunities to germinate and grow these projects in U-M labs. New ways to identify drug targets, better screening technologies, and the application of machine learning models enable smarter decisionmaking at all stages of drug discovery and are expected to trim costs while increasing the probability of success. These developments help set the stage for U-M to make major contributions to the discovery and advancement of new medicines that will address the unmet medical needs of patients around the world.
Success stories
Dr. John Traynor and Dr. Andy Alt are conducting work on modulators of the mu opioid receptor seeking ways to combat the devastating effects of the opioid epidemic. This work has been supported by MDD, and recently,the team was rewarded with an STTR grant in conjunction with the U-M startup company, Eleven. Current funding will support the identification of a lead compound for toxicology studies.


In 2020, investigators from the University of Michigan and Michigan State University were fairly certain that they had identified a new way to treat fibrosis. The researchers had discovered through in vitro experiments that the protein pirin plays a critical role in the onset of fibrosis. They were close to validating pirin as a potential drug target, but they needed to conduct one more critical experiment to convince potential partners of their discovery. With a timely grant from MDD the team was able to secure the final piece of data needed to attract a development partner.

The benefits of working with MDD are not limited to U-M faculty. MDD, and its associated research cores, also work with external partners, including faculty from other universities and researchers at biotech companies.
Scaling Your Medical Device Production
 BY DAVID VAN SLINGERLAND, CEO, STERLING INDUSTRIES
BY DAVID VAN SLINGERLAND, CEO, STERLING INDUSTRIES
Benefits of Partnering with a Contract Manufacturer
Whether you work for a large Medical Device OEM or a fast-growing device startup, scaling production marks a new phase in your product’s journey. You’ve already identified the opportunity, designed the solution, validated demand, iterated prototypes… perhaps you’ve received FDA approvals and sold some units into the market. Now it’s time to grow a profitable business by delivering a quality medical device, over-and-over again, without compromise.
Historically, many device makers assumed they needed to become a high-volume manufacturer, building out a facility and production line, hiring the expertise required, securing inputs and managing the supply chain, handling packaging and logistics. Even for a large OEM with established products, building a new production line is a daunting and capital-intensive task. For fast-growing medical device ‘scale-ups’, it can be paralyzing. It’s no wonder that partnering with an experienced Medical Device Contract Manufacturer is becoming an increasingly popular path to success.
Do What You’re Best At
What is at the heart of your business’ success? For most growing medtech companies, it’s product innovation, marketing and sales… not managing a complex, compliant manufacturing operation. Growing the business is your organization’s primary focus; for an experienced Medical Device Contract Manufacturer, it’s efficiently managing the thousands of details and disciplines required to consistently produce medical devices that meet stringent industry regulations.
It Starts With People
A successful, modern manufacturing operation requires a highly-skilled, experienced team of professionals:
Design for Manufacturing Experts to ensure optimal scaling and efficiency; Manufacturing and Assembly Engineers to design innovative solutions, as well as procure and configure the right technologies; Supply Chain Specialists to secure consistent inputs; Quality Experts to monitor and optimize repeatability and reliability; …and that’s all before hiring and managing the skilled machinists and assembly line workers
In today’s competitive labor markets, one of the biggest advantages of working with an established, Medical Device Contract Manufacturer: they tend to attract and retain the industry’s top talent, by offering them a steady supply of cutting-edge, innovative medical device projects to work on.

Design for Manufacturing
As the demand for your medical device grows, so does the chance to boost profits while simultaneously enhancing quality, reliability, and repeatability, if you are able to avoid costly supply chain issues. Top-tier Medical Device Contract Manufacturers with Design for Manufacturing (DFM) and supply chain expertise can have a massive impact on the product’s business case.
The Supply Chain Challenge
When new medical device scale-ups attempt to scale their own manufacturing in-house, one of the most common and surprising challenges faced is managing supply chain. The seemingly straightforward task of obtaining inputs and components can quickly become a major risk to both the business and staff when faced with unexpected shortages or price increases. Securing reliable sources, managing price fluctuations, and having effective contingency plans requires both meticulous planning and maintaining strong relationships with a wide range of suppliers. This is where long-established Contract Manufacturers have an advantage: because they produce a broad range of products, they typically have strong relationships with a wide range of reliable suppliers.
Technology and Facilities
For medtech ‘scale-ups’ and OEMs, the most obvious advantage of partnering with a Contract Manufacturer is the lower capital requirements. The idea of constructing a suitable facility — especially when demand growth and profitability are still unclear — typically involves significant capital investment and betting on uncertain projections. By contrast, a good Contract Manufacturer will typically own much of the advanced technology required for producing your medical device; and any capability they don’t have in-house, they can often obtain through trusted subcontractors.
Certification, Compliance and Quality
Lastly, in this highly regulated and scrutinized industry, it’s crucial for medtech companies to safeguard their reputation. Clients of top-tier Medical Device Contract Manufacturers benefit from using their FDA registered facilities, ISO certification, and robust quality control systems — all respected industry standards that are expensive and resource-intensive to maintain. STERLINGINDUSTRIES.COM
FOR ANY TEAM TASKED WITH SCALING
PRODUCTION OF A MEDICAL DEVICE —
whether from a well-established OEM or a fast-growing scale-up — in-house manufacturing presents a capitalintensive and resource-consuming challenge that can pose significant risk to the business. Device makers are increasingly turning to medtech-exclusive contract manufacturers — like Sterling Industries — that have a track record of helping medical device makers increase quality and profitability, while reducing risk.
If you’re looking for a partner to help manage production and free up resources for business growth, don’t hesitate to reach out to learn more about how Sterling Industries can help your medical device succeed in the marketplace.



Bringing Next Level Intellectual Property to the Cannabis Industry
BY JESSICA WEBBER, DIRECTOR OF OPERATIONS, DEHYDR8Dehyd8 LLC has licensed the rights of a pharmaceutical technology that provides pharma grade bioavailability cannabis to the Cannabis Industry.

Based in Science - Backed by StudiesSupported by Data
Dehydr8 develops all products based on a SEDDS technology (Self Emulsifying Drug Delivery System). SEDDS’s are scientifically proven to enhance oral bioavailability and are used in high-end pharmaceutical products. This elegant technology can be applied to the cannabinoid particles and active ingredients that are not water soluble as DehydraTECH™.
This patented process enhances oral bio-delivery of DeHydr8’s cannabis and hemp-based products, allowing the end user to reduce dosage without reducing efficacy, and provides for more consistent absorption and protection of the cannabinoids from the hostile environment in the gastrointestinal tract. The delivery process safely introduces cannabinoids into the system through the lymphatic system reducing or avoiding first pass liver metabolism. In a nutshell, the process ensures absorption of cannabinoid molecules directly into the bloodstream within minutes of ingestion or as applied to the skin, ensuring a faster and cleaner uptake though dermal, buccal, and sublingual exposure.
DeHydraTECH™’s quick absorption helps to solve the consumer issues of low bioavailability and solubility of many hydrophobic active pharmaceutical ingredients (API’s). Limitations in solubility lead to less solubilization in the gastrointestinal tract and absorption by the body. Many solutions, within the industry, have been used to overcome solubility
concerns including the use of surfactants, lipids, permeation enhancers, micronization, salt formulations, cyclodextrins, nanoparticles and solid dispersions. Dehydr8 offers technology that does not need to rely on any of this, therefore creating a product that is clean, fast absorbing, and consistent.
Predictable and Enjoyable - What Consumers Want is What DehydraTECH Delivers
DehydraTECH™ has been developed to withstand the test of time; internal quality control used to infuse products have been produced repetitively with strict adherence to protocols. The company’s products meet strict accuracy and precision requirements measured, recorded, and monitored to ensure the consumer receives a predictable cannabis experience every time.
In addition to safe, fast, and predictable, the delivery system creates a more enjoyable experience for consumer without the weedy taste or smell that’s often found in cannabis products within the marketplace. The process almost completely masks unwanted organoleptic properties of both hemp and cannabis. Not only is the technology patent protected but flavor profiles are patent protected as well. DeHydr8’s technologically enhanced suite of SKU’s (over 500) are designed to provide consumers with all the benefits cannabinoids offer in a superior format.
The latest trend in cannabinoid delivery methods is “nano emulsion”. Nano technology essentially seeks to reduce the size of the cannabinoids particle and when combined with an emulsion, enhances dissolvability. But - does it enhance bioavailability? The answer is no.
“Nano technology simply makes things smaller. We make things simply effective”.*When looking to find products that utilize the DehydraTECH™ technology look for this logo and “Powdered by DehydraTECH™
The emulsion of choice or current industry standard uses a nano technology that usually employs a medium chain triglyceride (MCT) as a carrier. When the nano cannabinoid and emulsion combination break apart in the stomach, the cannabinoid is directed to the liver. The liver metabolizes up to between 85% and 95% of the cannabinoid, greatly affecting bioavailability of a nano product. Some nano technologies use Polysorbate 80, an emulsifier that has serious and known adverse effects on the mucous lining of the stomach among other undesirable side effects. Nano technology creates smaller particles in the manufacturing process, but once in the stomach these particles recombine to randomly different sizes and the rate of bio-absorption in the body depends on how much fat is in the consumer’s diet. Additionally, nano particles have been reported to create significant health concerns in areas of the body not designed for such wholesale particle size reduction.

In short, Nano-Tech products that include the addition of substrates and excipients are often inconsistent, unpredictable, and possess undesirable side effects.
DehydraTECH™ is not a Nano technology delivery system. Nano technology simply makes things smaller - DeHydr8 makes things simply effective with a SEDDS delivery system that enhances oral bioavailability, and gives the consumer a better tasting, more predictable, and faster-acting experience every time.

A Competitive Advantage in Cannabis Delivery
Dehydr8 LLC, with its extensive R&D, substantial cannabis knowledge and the licensed technology (IP), has led to the development of a multitude of form factors (and over 500 SKU’s). A comprehensive understanding of (ADME) Absorption, Delivery, Metabolism and Elimination has allowed DeHydr8 to master and develop a superior, technologically advanced drug delivery process creat6ing precision in (ADME), and consistency of dose that consumers can trust in.
Simply put, the pharmacokinetic technology found in all Dehydr8 products aids in delivering a host of competitive advantages including a clean product that creates a safer more effective and predictable cannabis experiences for consumers than what is currently found in the cannabis market.
MEET METRIC: A Leading Biotech Marketing Agency, Based Right Here in Michigan

When thinking of the best location for startups, marketing, or lifestyle most people immediately think of the coasts. Rarely do you expect someone to say, “The best company to work with is in the Midwest.”
But as a born-and-raised Midwesterner, I think that’s the beauty of it. We all live, work, and have laid our foundations in what I believe is the greatest place in the nation: the state of Michigan.
From fiber optics to automobiles to penicillin, Michigan is home to some of the most world-changing innovations, and if you are in the biotech industry, you are likely among them. But as a recognized thought leader in digital marketing, I often see that creating the technology itself is not enough. In fact, getting your technology or innovative products out into the world doesn’t happen on its own. From formation to funding to development to acquisition, the pathway is often riddled with difficulties beyond getting new technologies to work.
We have found that, in order to succeed, you need a team that not only understands what you do and cares about why it matters, but also knows how to reach your target market. In fact, one of the most common questions we get asked by our biotech clients is how to get their revolutionary technology from infancy and little market awareness to maturity and market recognition as a leading product.
As a leading biotech digital marketing agency and recognized as one of the fastest-growing companies in the Midwest by Inc. Magazine, we are passionate to work at the forefront of innovation. It’s an exciting place to be, knowing there’s an incredible discovery just waiting to be revealed to the world, and having the power to get it in front of anyone that needs to know about it.
What’s Different About Metric?

In our work, we have come to believe that the most innovative biotechnologies should have the best-in-class digital marketing behind them. Because of this belief and our desire to help innovative companies, we have worked to become the best at what we do: biotech digital marketing.

By implementing a series of hard-earned insights, our clients are consistently top ranked in Google for their key search terms, leading to increased visibility—which means more sales, partnerships, and even investment opportunities.




How do we drive such stellar results? At our core, we’re marketers first. It’s important to comprehend the science but understanding the intricacies of digital channels and algorithms is key. Just like science, it involves a lot of experimentation, data analysis, and expertise.
Not only do we apply our learnings to ourselves and to our clients, but we teach digital marketers how to do this as well. As a marketing trainer, I have educated over 2,000 digital marketing professionals, many of whom work in the life sciences. These specialists learn how to apply the same principles to their companies, including teams from Thermo Fisher, STEMCELL, and BD.
How Does Metric Partner with Biotech Companies?
In short, we ensure our clients show up online in front of the people they’re trying to reach—and continue to market to them after the first engagement. This is accomplished through a variety of methods that are tailored to each company and its goals. No client has the exact same plan or combination of efforts, but we implement strategies that include search engine optimization (SEO), content marketing, website development, paid advertising, email marketing, and even CRM management, which is key to ensuring our efforts are tracked so we can learn which ones are driving the most targeted engagement.

Success is never overnight, but through continued analysis and implementation, we’re able to increase rankings and traffic like these real client results seen here, leading to more customers, sales, and stakeholder interest.
BLOOD CANCER FOUNDATION OF MICHIGAN

No One Has To Walk Their Blood Cancer Journey Alone
BY JENNIFER RAAB, MARKETING AND BRAND MANAGER, BLOOD CANCER FOUNDATION OF MICHIGANEvery 90 minutes, someone in Michigan learns they have blood cancer. For many, the physical battle is only the start of the hurdles they’ll face.

A cancer diagnosis can empty one’s emotional reserves, make it hard to stay engaged socially, and can place a large burden on a family’s finances. Fortunately, The Blood Cancer Foundation of Michigan (BCFM) has spent the last 70 years making sure no local patient ever has to walk their blood cancer journey alone. Anyone dealing with a blood cancer diagnosis in the state of Michigan is eligible for BCFM’s comprehensive, holistic support services. BCFM’s Master’s-level Patient Support Specialists customize a plan for each patient and family, ensuring they’re equipped with the very best resources to navigate their journey, take back control of their lives, and achieve long-term stability.
BCFM is keenly aware that local patients and families are facing a unique set of challenges. They’ve observed many patients and families
struggling with the ongoing strain of this postpandemic world. Prior to diagnosis, 77% of reporting patients and families lived below the ALICE (Asset Limited Income Constrained Employed) guidelines set by the United Way. These families are one crisis away from falling deeper into Michigan’s social safety net, currently costing the state $25 billion per year. Blood cancer is one of the costliest cancers to treat, with many patients requiring lifelong treatment. The financial struggle for patients has been made even greater due to increased fuel, inflation, and treatment costs.
Financial challenges are only one example of how the pandemic has affected BCFM’s patient base. As a result of measures taken to curb the spread of COVID-19, a greater number of patients missed regular doctor visits and screenings, often resulting in more advanced stages of disease at the time of diagnosis. Additionally, due to overwhelmed healthcare systems, BCFM referral rates dropped, leaving a greater number of the newly diagnosed without outside support. Social and emotional strain
is an inevitable byproduct of battling illness, but that feeling of isolation was exacerbated when folks weren’t able to see loved ones for long periods of time. These vulnerable patients need BCFM’s financial, emotional and social assistance now more than ever.


Over the last year, BCFM has seen a dramatic increase in referrals and expects those numbers to continue to grow. They have taken the lessons learned from the pandemic to bolster resources, ensure quality services, and innovate outreach. They’ve hired a full-time community liaison to build and maintain relationships with their key healthcare and community partners. Barriers caused by rising transportation costs have been addressed by increasing patient reimbursement for travel to treatment. While continuing to provide high-quality care and programming for existing patients, BCFM has also created additional reserves to guarantee that they never have to turn patients away in the future. These steps illustrate BCFM’s full commitment to ensuring that anyone in Michigan coping with a blood cancer journey will know they have a partner on this road.
If you, or someone you love, is facing a battle with blood cancer, the Blood Cancer Foundation of Michigan is here to help. BCFM is focused on giving power back to patients during a time when they feel most out of control. When families are able to count on support from BCFM, they can keep their eyes fixed on healing and a happier future.

BLOODCANCERFOUNDATIONMI.ORG

CAYMAN CHEMICAL
Expanding Toolbox for Drug Discovery

 BY MELISSA PARSEY, SCIENTIFIC CONTENT DEVELOPER, CAYMAN CHEMICAL COMPANY
BY MELISSA PARSEY, SCIENTIFIC CONTENT DEVELOPER, CAYMAN CHEMICAL COMPANY
Cayman Chemical grows its drug discovery toolbox with an integrative approach when opportunity knocks.
LNPs: The Next Frontier of Drug Discovery Hardship brings people together. The COVID-19 pandemic brought about an unprecedented common adversary that inspired a collective effort to overcome and persevere. Amidst the suffering and untold disruptions caused by the COVID-19 pandemic, a new wave of innovation was born.
The COVID-19 vaccines surged a monumental interest in lipid nanoparticles (LNPs). In development for nearly 20 years as protective and directed drug delivery carriers, the COVID-19 pandemic served as an opportunity for LNPs to jump suddenly from pilot scale to massive worldwide application. The increased awareness highlighted the versatility of LNPs to deliver RNA/DNA, antibodies, vaccines, and cancer treatments with great precision.

Simple and inexpensive lipids like cholesterol and egg lecithin comprise most of the protective fatty soap bubble that is an LNP. The key lipids, those that direct small RNA or DNA strands to pack into the
inside or place an antibody navigator on the outside of the LNP, make up a small fraction of the total lipid composition. Cayman was one of only a handful of companies with the experience necessary to bring to market research-grade versions of the same lipids used in the COVID-19 vaccines. Now, Cayman is the premier source of ionizable and cationic lipids, one of the key LNP components. The company also offers services for custom lipid synthesis and a number of ancillary lipid-related services, including analytical testing and full-scale GMP production of novel lipids.
Today, Cayman is bringing an LNP-based drug discovery service to Michigan. Headquartered in Ann Arbor, Michigan, Cayman has grown to be a formidable presence as one of the leading suppliers of lipids and lipid-related services for biomedical research. Operations are underway to offer an LNP formulation service by mid-2023. Once complete, clients will have their choice of options for the design, formulation, and analysis of LNPs – all from a single-source partner.
Innovation Driven by Integration
Cayman is more than just lipids. After 42 years of growth, Cayman has built integrated teams of experienced scientists that fuel the discovery of new medicines across many therapeutic areas.
Cayman’s drug discovery program was born from opportunity. Having already established a core team of synthetic chemists, the departure of Pfizer’s Ann Arbor R&D facility in 2006 opened up the chance for Cayman to tap into the pool of now displaced medicinal chemists who brought years of expertise and Big Pharma experience to Cayman’s growing portfolio of products and services.
An expanded synthetic toolbox for medicinal chemistry that improves the quality of drug candidates and shortens drug discovery times is made possible at Cayman. By leveraging the synergy between medicinal and synthetic chemistry, Cayman brings the collective
Cayman is more than just lipids. After 42 years of growth, Cayman has built integrated teams of experienced scientists that fuel the discovery of new medicines across many therapeutic areas.
wisdom of its expert chemists to client projects. Custom building blocks or commercially unavailable starting materials can be synthesized in-house, increasing the number of substrates available and producing more structurally diverse compound libraries. Cayman’s drug discovery chemists have extensive experience performing a variety of complex, multi-step organic syntheses. When problems arise, they can troubleshoot reaction chemistries and work through the challenges of synthetic roadblocks.
These early ventures in drug discovery set the foundation for a drug discovery program at Cayman. Now, the company offers a thriving suite of integrated contracted services, from novel drug design and hit-to-lead optimization to assay development, high-throughput screening, and bioanalytical mass spectrometry.


A Drug Discovery Success Story
Drug discovery is a long, costly, and risky endeavor. At Cayman, this investment is paying off. The success of the company has brought about the opportunity for Cayman to pursue its own internal drug development program. It has been known for decades that the E-series prostaglandins promote bone growth. Many unmet needs in medicine, including osteoporosis, dental implants, spinal fusions, and complex bone fractures awaited a solution that was selective and mitigated side effects. In 2015, Cayman discovered and patented a series of EP4 receptor agonists highly selective for
osteogenic activity. KMN-159 was the lead compound that emerged from this series. Today, it is being assessed in an equine proof-of-concept study to evaluate its feasibility for bone growth and repair.
This success was made possible by the drug discovery team at Cayman. What can Cayman help you discover? CAYMANCHEM.COM
•
•
•
•
Predicting and Measuring Medication Effectiveness to Improve Patient Outcomes
SUBMITTED BY RACHEL KINGSLEY, OPERATIONS SPECIALIST, PHENOMICS HEALTH
Phenomics Health Inc. is a precision health company based in Plymouth, MI that aims to lead the wave of precision prescribing and monitoring patient treatment outcomes.
Phenomics Health is the only company worldwide that offers both pharmacogenomic (PGx) and pharmacometabolomic (PMx) testing for a wide variety of healthcare settings, including clinical practice and research, to improve patient care and encourage clinical studies in the field. As a precision health company, Phenomics combines the value of PGx testing with comprehensive PMx testing to provide another dimension for precision medicine.
The growth of PGx shows that medication therapy cannot use a one-size-fits-all approach. Each person and their unique genomic profile require prescribing based on the individual needs of the patient’s genetics. When patients have comorbid medical conditions, their medication lists can grow to considerable lengths, resulting in

polypharmacy and a host of adverse effects from drugdrug interactions (DDIs). While those lists may show the possible medications the patients are currently taking, it will not tell the story of how they got there and all the failed medications to reach their best treatment. The implementation of PMx and PGx together can shorten the path each patient will require to find their optimal therapies and alleviate the burdens that can accumulate along the way.
Phenomics Health PrecisMed® PMx products are the sole technology available to detect and measure over 200 commonly used drugs in a patient’s circulation. This can be done with a serum sample collected by venipuncture, or a plasma sample collected with a fingerstick and a few drops of blood. The patient sample results in a report from one of four different PrecisMed tests, each focusing on a certain specialty such as neuropsychiatry, pain, or polypharmacy. This allows providers and researchers to quantify the drugs in their patients and assess their drugs’ pharmacokinetic properties are being affected. While this
can be done for various individual analytes, PrecisMed offers this information for a wider net of targets together.
The PredictScript™ products offer comprehensive PGx information to interested providers and their patients. It integrates guidelines from organizations like the FDA and CPIC along with independent Phenomics research to provide one of the most comprehensive PGx tests on the market. PredictScript Poly covers up to 140 drugs across the therapeutic areas of neuropsychiatry, pain, cardiovascular, gastrointestinal, and diabetes. The prediction provided by the tests offers a chance to avoid the trial-and-error burden that can be involved in drug therapy. Rather than start a new drug and wait days to weeks to observe its safety and efficacy in the patient, providers can eliminate some inappropriate choices and narrow down the selection among better options.
Both PMx and PGx technology has been used separately in for both clinical and research applications, but Phenomics has brought the two innovations together


into six commercial products. Expanding and combining the two technologies has allowed Phenomics to make precision medicine more precise by enabling the potential to both predict and measure the drug therapy.
These tools can reduce healthcare costs, adverse drug reactions, and many more issues plaguing the healthcare system. The goal is to empower providers to make stronger informed decisions for the benefit of their patients.

PHENOMICSHEALTH.COM

EXCEL MEDICAL PRODUCTS
Celebrating Twenty Years of Medical Device Manufacturing in Michigan

 BY BRIAN STERN, DIRECTOR OF SALES AND MARKETING, EXCEL MEDICAL PRODUCTS
BY BRIAN STERN, DIRECTOR OF SALES AND MARKETING, EXCEL MEDICAL PRODUCTS
This year, Excel Medical Products will celebrate its 20th year in business as a medical device OEM and contract manufacturer.

The medical device industry landscape is now significantly different due to regulatory changes, and advances in medicine and technology. Nevertheless, the core values, commitment to quality and continuous improvement, and customer-centric focus of the company remains the same.
Excel founded its business upon the manufacture of a line of OEM hemostasis valves, which are commonly used in various diagnostic and interventional procedures. To this day, the hemostasis valves continue to be molded and assembled in Excel’s cleanroom manufacturing facility in Wixom, MI. Over the years, the product offering has been expanded and Excel has set itself apart in the hemostasis valve market because of the capability and willingness to customize the product according to customer specifications, for a variety of applications.

As the company grew its hemostasis valve and PTCA accessory business, opportunities developed to perform contract manufacturing services for other OEM firms. As an FDA registered and ISO 13485 certified manufacturer, Excel already had the facility and quality system in place to expand its service offerings. Beginning with just a few small run projects with startups, Excel developed experience and expertise in helping customers launch new products by collaborating on design for manufacturing and packaging, performing various types of qualifications, and coordinating sterilization validations.
Excel continued to expand its cleanroom injection molding, assembly, packaging, testing, and distribution capabilities, and gradually developed longer term contract manufacturing relationships based on consistent, cost-effective quality, on time delivery, and responsive customer service. Some customers were initially attracted to Excel because they were turned away by larger contract manufacturing firms. However, many of those customers have stayed with Excel as their
projects have grown to be successful because they have come to feel that the team at Excel treats their product as if it were their own. The company continues to take on small startup projects, but now performs contract manufacturing for some of the largest medical device companies in the world.
The last twenty years of growth have not always been smooth. Like companies in just about every other industry, the last three years have presented considerable challenges for Excel. At times, the supply chain and labor market disruptions made it nearly impossible to produce and deliver as planned. Communication, adaptation, and flexibility were required to overcome these obstacles, and because of the passion and resilience of its team, Excel was able to come out of the pandemic era stronger than ever.
Although the supply chain and labor market continue to present challenges, Excel continues to grow and invest in the future. The company has acquired new injection molding machines and added to its prototyping services with enhanced 3D printing capabilities for precision fabrication of parts in a range of sizes. Excel has also been fortunate to find new talented and dedicated members for its team.
The future looks bright for the company. Here’s to another twenty years of medical device manufacturing in Michigan!

Phase 3 Expansion of Kalamazoo Facility
BY PAOLA CLERICI, COMMUNICATIONS MANAGER, DIPHARMAThroughout its history, innovation has always been at Dipharma’s core.
The Dipharma Group is a global Contract Development and Manufacturing Organization (CDMO) and a leading manufacturer of Active Pharmaceutical Ingredients and Intermediates for generic and contract manufacturing markets.
As part of the global pharmaceutical industry, it serves human health and, therefore, operates to the highest standards of quality and safety with proven experience in handling complex and hazardous processes.
Dipharma was founded in 1949 some 100 km northeast of Venice, Italy, by Mario Biazzi, who created an innovative, and much safer, continuous process to manufacture nitroglycerin. It has expanded its global presence, with more than 500 skilled and highly committed employees, serving over 200 customers in more than 60 countries, including biotech, biopharma and leading pharmaceutical companies.
The company’s fully equipped R&D centers, in the U.S. and Italy, foster development of robust chemical synthesis, to further secure the manufacturing continuity of the Drug Substance in our 4 cGMP plants while manufacturing sites located in U.S. and Italy and are equipped to supply quantities suitable from preIND to market, covering the entire lifecycle of a drug.



Full compliance with the highest GMP quality standard has been confirmed, since 1970, by an extensive list of successful inspections conducted by major Regulatory Agencies, such as the FDA, Italian Health Authorities and PMDA.
Nowadays the Dipharma Group is a third-generation family-owned company with an outstanding history of stability and financial soundness, and a strong commitment to integrating the principles of Corporate Social Responsibility into its business and all its corporate activities.
Dipharma established its presence in the US market in 2012, with the opening of a new office, Dipharma Inc., and, 6 years later, further reinforced it with the acquisition of the Kalamazoo-based Kalexsyn, Inc., a world-class Contract Research Organization (CRO) founded in 2003. This purchase represented a complement to Dipharma’s existing business and in accordance with its strategic decision to strengthen its position in the CDMO market and leverage its presence in the United States, in 2020 Dipharma inserted the missing tile of the puzzle with the completion of a brandnew cGMP suite in Kalamazoo site, that will mostly provide clinical supplies across Phase 1 and early Phase 2. This enhanced the role of the Kalamazoo site from CRO to CDMO and therefore enabled the Dipharma Group to cover the whole lifecycle of a molecule, from discovery to commercialization.

The state-of-the-art QC laboratory is designed and equipped according to current pharmaceutical quality standards, with full 21CFR Part 11 Electronic Data Integrity compliance including automated monitoring of environmental systems.
The Company recently completed the second phase of its expansion of the Kalamazoo facility. The additional 2000 ft2 of space has been designed to complement the cGMP Kilolab and quality control laboratories, and to support further expansion of the site. This new space triples the size of its cGMP quality control laboratory and significantly increases the warehouse spaces.

The state-of-the-art QC laboratory is designed and equipped according to current pharmaceutical quality standards, with full 21CFR Part 11 Electronic Data Integrity compliance including automated monitoring of environmental systems.



The new, larger warehouse spaces are fully-climate controlled and allow for greater material storage with increased material segregation.
Furthermore, the Kalamazoo-based R&D department is adding analytical method development services to its portfolio to aid in bringing pre-clinical projects into the clinical realm. This additional tool is completing the broad portfolio of services that Dipharma can provide to its customers, including development of chromatographic methods, expertise in solid-state properties, safety, elemental impurities, potentially genotoxic impurities, IP, and commercialization services.
Up next for the Kalamazoo facility is the installation of a larger second line of reactors for the cGMP Kilolab production suite. This third phase of the expansion will add two ca. 82 L reactors to the Kilolab, allowing the Kalamazoo group to better implement early-phase deliveries where processes may not yet be fully optimized for commercial delivery. This expansion is currently underway and is scheduled for completion in summer 2023.
This expansion mirrors investments the Dipharma Group is making in its Italian facilities, adding to R&D laboratories and doubling of pilot plant capacity, with the aim to further strengthen Dipharma’s capacity and support the growing demand for its contract development and manufacturing activities.
All these investments strengthen Dipharma’s offerings and allow the Group to offer a full range of contact services, ensuring continuity across the whole lifecycle of pharmaceutical development, from preclinical to commercial deliveries.

MICHIGAN'S BIO-INDUSTRY
Anchored by several large hubs, this representative sample of Michigan life sciences companies shows the breadth of the industry footprint.
 TRAVERSE CITY
MUSKEGON
BATTLE CREEK/JACKSON
KALAMAZOO
GRAND RAPIDS HOLLAND
TRAVERSE CITY
MUSKEGON
BATTLE CREEK/JACKSON
KALAMAZOO
GRAND RAPIDS HOLLAND
OAKLAND/MACOMB/ ST. CLAIR
LANSING
ANN ARBOR
GREATER DETROIT


DISTRIBUTION OF MICHIGAN BIOSCIENCES INDUSTRY *

AFTER THE
ELEVATOR PITCH

Adlore, Inc is a Kalamazoo-based start-up developing a smart boot to improve diabetic foot ulcer (DFU) healing rates and reduce treatment cost.

The company’s founding idea is based on research conducted by Daryl Lawson PT, MPT, DSc – one of the company’s founders. Dr. Lawson has treated chronic, non-healing wounds with a combination of electrical stimulation and heat to reduce inflammation and increase blood flow. His approach has improved non-healing DFU healing rates up to 70% vs. 25% when using the standard of care.
The device Adlore is developing – SenLoreTM - will combine the current standard of care – offloading weight from the DFU - with electrical stimulation, heat and sensors to monitor wound conditions and patient compliance. Monitoring wound conditions expands potential treatment efficacy for patients who don’t live near a wound care center.
Adlore has developed a prototype boot and conducted a pilot study on diabetic patients to confirm that the data match the past clinical studies by Dr. Lawson. The pilot study data demonstrated significantly increased blood flow vs. baseline, and was comparable to 6 peer-reviewed clinical studies done in the past. An additional study focused on healing rates showed wound size was reduced 54% after 4 weeks vs. 25% with the standard of care. Proof-of-concept has been demonstrated, and patents have been issued. Adlore is now focused on developing a marketable device, complete process/product verification and validation, completing clinical studies, and obtaining FDA 510(k) approval. The business model is to sell Adlore to a wound care company following FDA approval.

Michigan continues to be a leader in advancing biosciences research and commercialization, thanks to its rich pipeline of intellectual property. A whole new crop of startups are germinating in the state, which is good news for the regional cluster of established companies that are focused on innovation in therapeutics, medical devices, healthcare technologies, clinical diagnostics and agri-/industrial biotechnology. Renewal is crucial to sustaining and growing an innovation cluster. All the way around, Michigan bioscience businesses are truly improving the quality of life for patients and consumers.
BioMEMS Diagnostics, Inc. is a Michigan-based in vitro diagnostics startup developing a breakthrough testing platform to deliver high-need, high-complexity diagnostics at point of care, at home or at point of need.

The firm has developed a biomarker-agnostic, biofluidagnostic, biomedical microelectromechanical sensor array that vastly improves upon the processing speed and sensitivity of immunoassays and nucleic probes. Instead of designing an expensive, single-purpose chip or sensor for specific diagnostic targets, the hybrid BioMEMS test platform can accept and optimize 200,000+ commercially available, clinically relevant antibodies or probes, with the potential to quickly develop hundreds of new tests while exponentially driving down the cost of manufacture and administration. This contemplates a massive shift in the creation and deployment of POC and OTC diagnostics.
The company is actively pursuing collaborations, project funding, strategic partnerships, and co-ventures to demonstrate the capabilities of its patent-pending


diagnostics platform. The R&D effort is led by Chief Science & Technology Officer Michael J. Pugia, Ph.D. The prospective applications include infectious disease, metabolomics, fertility, healthy aging, nutritional biomarkers at point of care, at home or public health screening.
BioMEMS has also formed an affiliate entity – NOXBOX, Inc. to develop and market a 5-minute concussion test that can be administered at point-of-need – a sporting event, accident scene, nursing home, ambulance, industrial clinic or ER, with definitive results immediately available and ported to any paired smart device.
Founder & Executive Chairman Andrew A. Dahl is managing the capital campaign and corporate relationships for BioMEMS and NOXBOX.
Denovo Fertility is a virtual wellness program that nurtures couples with fertility issues through a dynamically guided coaching approach. Through evidence-based education regarding nutrition and hydration, stress management, sleep, joyful movement, and relationships, members of the program meet in a supportive online community allowing for an understanding that fertility IS achievable.


The company’s unique team-based approach includes coaches specializing in different areas to meet members where they are on their wellness journey. Not only are the ‘basics’ taught, but special attention has also been placed upon how to measure progress with devices and apps, provided to members within the company’s global program. Methods of how to utilize these new skills are reviewed and different success stories are shared within their support groups weekly in a positively reinforced environment. As it is well known that community support
assists a body’s perception of being safe and out of ‘fight or flight’ mode, this is a mainstay for every member.
In addition to weekly classes and community groups, a virtual community is also available to all members in order to keep the community together throughout the week between classes.
Denovo Fertility is working toward its long-term goal of positively impacting transgenerational health by making these changes attainable in this safe and impactful community. After conception, Denovo Fertility’s allencompassing programs extend through pregnancy as well as post-partum for both moms and dads. The company is driven to provide a complete wellness model and is currently planning for it’s scaling due to anticipated growth.
GeneToBe, a University of Michigan gene therapy startup, seeks cures for genetic diseases. GeneToBe has two gene therapy approaches. The first is a next generation CRISPR/ Cas9 platform, known as Mi-Cas9, a highly efficient and accurate gene-editing tool that allows disease-altering modifications by changing existing DNA in a living organism. AAV, viral-like particles, and liposomes for Mi-Cas9 delivery are being evaluated in proof-of-concept studies of gene-editing therapy.
The second technology is precision-medicine small interfering RNA (siRNA). siRNA targets undesirable mRNA, preventing protein translation. Two variants of Patatin-Like Phospholipase Domain-Containing Protein 3 (PNPLA3); a desirable (I), and an undesirable (M) form exist. In humans, 34% are heterozygous (M/I), and highly susceptible to fatty liver disease, alcohol-associated hepatitis, cirrhosis and liver cancer. A precise siRNA drug
only targeting the M isoform PNPLA3 mRNA has been designed. Transgenic PNPLA3 human M form mice, when treated with the precision siRNA drug candidate prevents fatty liver formation. IND-enabling studies for the siRNA program are planned.

GeneToBe also creates translational animal models of human diseases, used for preclinical evaluation of therapeutic agents. Rabbit models of two deafblindness diseases, Usher Syndrome Type 2A and Type 3A, and defective and deficient Cystic Fibrosis have been created. These models are used to understand disease natural history, develop therapies, and identify surrogate markers that may be valuable in the clinical setting. GeneToBe seeks partners for existing programs and to develop future gene-editing targets and animal models.

Innsightful is an early-stage health tech company focused on improving access and efficacy of mental health care.

Innsightful provides an inexpensive, integrated endto-end digital health solution to help people manage stress and prevent stress-induced mental health issues. The company’s solution utilizes off-the-shelf wearables for biomonitoring accompanied by its app to detect users’ hourly levels of stress, anxiety, and depression. The app facilitates a short AI-based dialog with users to determine the causes of stress and their associated emotions. Innsightful then provides the user with
Aer Sol E
Inspire Rx, LLC
According to the World Health Organization, over 100,000 healthcare workers died between Jan 2020 and May 2021 due to COVID-19 in the U.S. and millions died alone as their loved ones were not allowed by their side. To prevent transmission of virus, at the height of Covid-19 pandemic in 2020, Inspire Rx LLC, in collaboration with clinicians at the University of Michigan developed a novel device, called AerosolVE™ BioHelmet, to protect health care workers or anyone in the vicinity of a patient with respiratory infectious disease while enabling non-invasive (sparing ventilators) means to provide supplemental oxygen via nasal cannula or CPAP. In early April 2020, the Aerosolve BioHelmet was successfully used at the University of Michigan Hospital System to treat Covid-19 patients noninvasively without ventilators.
The BioHelmet is a personal, compact, portable, and inexpensive negative pressure barrier environment enabling treatment in field hospitals, ambulances, etc. Worn by the patient, the AerosolVE™ BioHelmet, using
just-in-time (JIT) therapy to reduce the effects of their stressors immediately. These results can be reported to a care team on a daily or weekly basis for intervention.

Using an off the shelf wearable, Innsightful has been able to create AI algorithms that provide measurements of anxiety and depression that are highly correlated with clinical measures of anxiety and depression. Innsightful will be submitting a presubmission letter to the FDA for determining their requirements for a Class 2 submission for a wearablebased screening device for anxiety and depression.

negative pressure, directs exhaled air through a HEPA filter that contains infectious particles, preventing transmission to front-line workers and others, while sparing negative pressure rooms and ventilators. Serving as a “PPE for the Patient”, the BioHelmet enables “quarantine in plain sight”. The BioHelmet is designed to provide safe transportation via ambulance, ship, aircraft, or helicopter, allowing critical staff to maintain station. It permits personal mobility and reduces infrastructure downtime and decontamination costs.
The Aerosolve BioHelmet would be an invaluable therapeutic adjunct for any respiratory infectious disease such as tuberculosis, SARS, MERS etc. or a future pandemic.
MAFEREB LLC
MAFEREB LLC is an African American woman owned life sciences start-up with a long-term goal of commercializing an efficacious, safe pharmaceutical formulation that provides targeted delivery of therapeutic molecules for chronic kidney disease (CKD) management, thus leading to reduction in morbidity and mortality rates of chronic kidney disease nationally and possibly globally. Chronic Kidney Disease is currently considered a progressive disease with no cure, and in some patients will eventually lead to End-Stage Renal Disease (ESRD), thus necessitating dialysis or a kidney transplant. It is being proposed that the formulation would stop disease progression, treat chronic kidney disease, and possibly reverse the disease process. The key differentiator of MAFEREB’s technology is that it will provide targeted delivery of therapeutic molecules in novel drug delivery systems (NDDS).
MAFEREB LLC is located in Michigan Life Sciences and Innovation Center (MLSIC) in Plymouth, Michigan and SPARK East Innovation Center in Ypsilanti, Michigan. Founder & CEO Joan Onyebuchi Erebor, PhD, MPhil, BPharm, CAPM, PMP has combined experience of over 20 years in the pharmaceutical industry, academia, pharmaceutical science research, retail pharmacy, and formulation. Co-founder/CFO Peter Omoruyi Erebor, MBA, PGDCompSci, BSc, CDPSE, has over 20 years of combined experience in accounting, Information technology and business administration. He has been involved in various start-ups and management of small businesses.

MAFEREB LLC proposes that if its therapeutic formulation is commercialized, it could lead to a paradigm shift in how chronic kidney disease will be managed in the US and possibly globally, thus saving lives.
Nitric oxide (NO, not N2O the laughing gas) is a critically important molecule found throughout the body. It functions as a virucide, bactericide, angiogenic, vasodilator, and antithrombotic. NOTA plans to enter the market with its light-activated LANORTM System that uses a simple chemical film strip to generate NO on-demand when exposed to its incorporated LED lights. This system will launch in 2025 for the FDA approved Persistent Pulmonary Hypertension in the Newborn (PPHN) indication, which is not expected to require clinical trials. LANOR’s price point, that is ¼ to 1/10 of competitive systems, its incredibility small size, long-lasting cartridges, and ease of use, make it ideal for both hospital and ultimate home use (COPD).
Today’s NO products are offered on a rental basis and currently generate ~$800M in annual revenues. While the market continues to grow at a solid 7.4% CAGR, there are currently over 400 active clinical trials that will cause the market to explode in the next 3-5 years.
NOTA has been financed by over $6M of NIH grants and by a $2.5M investment from Pegasus Tech Ventures of San Jose, CA. The company has also received a Term Sheet for it’s next $6M round it plans to close by May. In addition, NOTA closed on a corporate partnership with NGK Sparkplug’s Medical Division in 2022, who are assisting with engineering and in-home distribution. NOTA is also working with Prof. Robert Bartlett’s at UofM on NO’s application in open-heart surgery and to extend the viability of donor organs.


JOIN MICHIGAN’S MEDTECH DIRECTORY
WHY JOIN
WHY JOIN? SIGN UP
As the 5th largest MedTech state - measured by number of establishmentsMichigan has a robust infrastructure of OEM’s, contract manufacturers, service providers, and others who support the development and distribution of Michigan made medical devices and technologies.
In partnership with the Michigan Economic Development Corporation (MEDC), MichBio is cataloging that infrastructure. The information collected - including company type, accreditations or certifications, and capabilitieswill be used to connect in state MedTech partners, and market the sector to out-of-state investors.

Example Matrix
Submit
AKADEUM
Flotation-Based Bioprocessing Platform Expands to Cell & Gene Therapy
SUBMITTED BY ADITI HARSH, ACCOUNT EXECUTIVE, UPROAR PRAkadeum is moving into C> applications with its latest product release, growing IP portfolio, team, GMP compliant manufacturing, and technology
Since Akadeum’s Series B in late 2021, the company has significantly advanced and focused its flotationbased bioprocessing platform to cell & gene therapy applications. The focus of the platform was enabled by a scale up of its cell isolation technology from 200M cells at a time to over 10 billion. This scale up could not have come at a better time as the need for improved processing of billions of cells has never been greater. In fact, the company received numerous requests from cell therapy companies for a product line that could isolate and activate T cells as part of the cell therapy manufacturing process.
After speaking directly with dozens of scientists working within the C> space, Akadeum uncovered major issues in isolating and activating specific immune cells—
issues like long and laborious processes, low throughput capability and handling T cells in a way that exhausts them. This discovery resulted in the first flotation-based bioprocessing product family of its kind—a more gentle and efficient way to conduct isolation and activation for critical treatments such as CAR T therapy.
By implementing Akadeum’s patented buoyancy-based cell sorting (BACS ™), the first product in this family, the Microbubble Leukopak Human T Cell Isolation Kit, was released at the end of 2022. The kit is able to isolate pristine T cells out of 12.5 billion cells and the results show 4X higher throughput and up to 20% higher yields than other products on the market.


“Cell isolation advancement has been minimal over the last four decades even though needs have been rapidly evolving,” stated Will Plentl, Chief Operating Officer of Zen-Bio. “Akadeum’s BACS™ Microbubbles are a rare and refreshing innovation in cell separation. With the Microbubble Leukopak Human T Cell Isolation Kits, we have been able to isolate more cells in a way that’s faster and easier than ever before.”
The isolation of cells from leukopaks is a critical first step in developing CAR T therapy. Akadeum’s method provides a less labor-intensive and more efficient method for recovering targeted cells. The Microbubble Leukopak Human T Cell Isolation Kit recovered highpurity T cells at an average of over 96% with up to 20% higher yield as compared to industry standards, and enabled users to go from a leukopak bag to purified cells in just one hour, compared to three or more hours.
In August 2022, Akadeum announced the release of its Dead Cell Removal Kit. The kit is an important step forward for scientists and researchers working in cell sorting, because it allows them to separate viable cells from dead cells, eliminating the detrimental effects of the dead cells within a sample that would otherwise be usable for cell culture experiments, function studies or single-cell applications. It also removes the need for costly and less-effective materials like magnetic columns. The kit is able to retain more than 85% of viable cells compared to 20-40% through traditional methods and requires only half the number of cells to start with.
The Dead Cell Removal Kit has a positive impact on researchers by giving them more flexibility in the types and the number of experiments that can be performed and was developed to meet the needs of this community, providing them with more testable samples and additional time.
Currently, Akadeum is preparing to launch an activation/expansion kit, which is another critical step in cell therapy that takes place after isolation. In parallel, the company is also preparing to launch its first GMPgrade kit.

These important developments are part of the company’s commitment to producing the best quality of products, ensuring customers that all products are reliable and manufactured under conditions and practices required to be GMP compliant. Additionally, Akadeum has other best-in-class products in the pipeline that enable closed system processing, expanded cell selection, and additional activation capability.

Empowering Lupus Warriors –That is Our Goal
A PERSONAL STORY FROM LUPUS DETROIT’S FOUNDER, SHARON HARRIS


I am Sharon Harris, and I have been a Lupus Warrior for more than 20 years. The mission of Lupus Detroit is a personal one for me.
Some time ago, I was employed at a (now defunct) lupus organization. Part of my role was conducting research – research that led me to understand that Lupus is a disease more prevalent in underserved or minority populations – African American, Hispanic, and Asian especially. In fact, African American women are three times more likely to get Lupus than Caucasian women. To me, that meant we should be providing services directly to those communities, and I asked leadership of that organization if we could create a Lupus chapter in Detroit. I was met with an emphatic “no”.
Frustrated by the lack of action for this critical need community, I began to think of ways that I could fill the gap for Lupus patients in Detroit. In 2012, I created Lupus Detroit, located in the heart of the city. Lupus Detroit (LD) is a 501©(3) charity and is a communitybased, voluntary health organization dedicated to eliminating lupus as a major health problem through education, advocacy, and service.
Lupus is an incurable and non-contagious disease that can affect any organ in the body. Systemic Lupus Erythematosus (SLE) symptoms include prolonged or extreme fatigue, pained joints, fever, butterfly shaped rash across the face and nose, hair loss. SLE can affect any part of the body. Discoid Lupus Erythematosus (DLE) is chronic scarring and inflammation of the skin. While the disease is challenging for all “Lupus Warriors” (those with the condition), African Americans and Hispanics/Latinos
tend to develop the disease at a younger age and have more symptoms at diagnosis (including kidney problems) – emphasizing the need for an organization dedicated to that community.
Any service organization can offer brochures, speakers and lupus presentations, but Lupus Warriors needed financial assistance as well. Warriors need money for medications, bills that are in arrears, hardware to help increase their mobility, among other things. To date, Lupus Detroit has awarded close to $75,000 to assist 200 Warriors.
LD generates its funds through annual Lupus Walks and gala/luncheon – which have garnered significant interest from African American activists and celebrities, as well as many others with an interest in supporting the Lupus Warrior community. Comedian Roy Wood Jr as Master of Ceremonies at the fifth-year gala. Grammy nominee and 90’s rapper YoYo was the keynote speaker at 2022’s gala. Detroit Lion’s player Tavon Wilson participated in our walks. The Lupus community in Detroit strong – and important.
Many Warriors have been ostracized by some of their friends and family, even doctors. Warriors receive comments about being lazy and not “looking sick.” At Lupus Detroit, Warriors have a community that can offer encouragement and knows exactly what each other is facing on a daily basis.
We also offer zoom support group meetings monthly. At each, an expert presents information on topics important to Lupus Warriors such as trauma, boundaries, dermatology and balancing a checkbook.
In addition to the general financial assistance LD provides, since 2017, twelve $1,000 educational scholarships have been awarded. The Jetuan Perkins Scholarship Fund is made possible by her husband, Attorney Todd Perkins, to continue her legacy. Jetuan transitioned from lupus in April of 2016 and this partnership assists university, college-bound, trade school students of any age. The only requirements are that the recipient must a Michigan lupus warrior and be a graduate of a Michigan high school.

Despite supporting Lupus Warriors with financial awards, Lupus Detroit does not have a paid staff - only a Board of Directors, all of whom are passionate about supporting lupus warriors. As the Executive Director of the organization, I have never received a paycheck. Lupus Detroit doesn’t have an office, dedicated phone line, a xerox or fax machine – it’s address is to a UPS P.O. box. UPS store staff tell us that the looks they get when telling visitors that Lupus Detroit is not physically there, are priceless.

As the leader of the organization, I have even been surprised by the impact of Lupus Detroit. Initially, I thought that I created Lupus Detroit as a group to offer financial assistance, but it has proven to be so much more. Through our events, there is camaraderie that has developed and grown. Throughout the years, members have gotten together outside of Lupus Detroit and went on trips, enjoyed spa days and just loved on one another. They have found siblings that they never knew that they needed.

I also never expected people to respond to my leadership. Many of our members have never seen the light in themselves, but at Lupus Detroit, their demeanor changes. Our members are certified chefs, boutique owners, photographers, and advocates despite their autoimmune disease diagnosis – and this organization that I get to lead is a factor in their ability to see themselves as more than a Warrior. Lupus Detroit believes in showcasing abilities even though the word “disability” is often associated with our community.


Our ministry is glorious and awe-inspiring. We exude dignity, compassion and respect to every person with lupus that we meet. That is our goal. Join us.


Bringing New Perspectives to Life Science Commercialization
BY SARAH MURCHISON, MANAGING DIRECTOR, NEW PERSPECTIVES, INCNew Perspectives, Inc. is a women-owned pharmaceutical and biotech product commercialization consulting firm that provides small and mid-sized companies senior-level involvement at every step of the product development journey.


Our team includes practice expertise in Sales, Marketing, Analytics, Trade, Market Access, Government Affairs, Compliance, HR, Business Development and Executive Leadership.
Whether through a go-to-market launch strategy, a license or acquisition opportunity, New Perspectives guides the process to ensure the strongest possible value proposition for the product, maximizing access and facilitating uptake with patients, physicians and payers to ensure the product’s potential value.
WAYS TO WORK WITH US
Whether it is a specific project or providing extra resources to fill a role on the product development or commercial team (FTE), New Perspectives collaborates with pharmaceutical and biotech clients to strategically optimized the product for licensing and marketing by the time the phase III clinical trial has ended.
With a suite of opportunity assessment, project management tools and dashboards, New Perspectives customizes an approach based on the company’s needs – whether the product is being developed for licensing or commercialization.
NEWPERSPECTIVES-US.COM
Insight Perspective®
Features situation analysis, market access, reimbursement assessment, product positioning and strategy as well as product business plan.
Valuation Perspective®
Fundraising, licensing and partnering, forecasting and budget development and commercial scenario building are featured in this toolkit.
Product Development Team & Launch Perspectives® Blueprint

Commercial-minded planning, functional team integration and commercial strategy are integrated into this tool
Launch Readiness Perspective®
This toolkit guides launch team building, Key Opinion Leader (KOL) development, advocacy and policy development.



Operational Perspective®
Process and system set up, registrations and state licenses as well as distribution coordination are featured in this toolkit.
Acquisition Perspectives®
Consulting and project management services that support a seamless integration of a product acquisition into a life science organization.


MEMBERSHIP VALUE
Why Should Your Organization Engage With MichBio?
1
Elevate Michigan’s Life Science Innovators and Organizations to: Attract Investment, New Businesses, and Talent Retain Homegrown Resources, Innovations, Businesses, and Talent Encourage Business-Friendly Policies and Positive Public Perception
2 3
WHAT WE DO IN EXCHANGE
Connect Essential Stakeholders Throughout the Life Science Ecosystem to: Drive Growth Through the Development of Relationships, Partnerships, and Mentoring Remain Knowledgeable on Industry Needs, Challenges, and Trends Develop Strategies and Resources that Best Serve the Industry
Galvanize Industry Resources, Accelerators, and Partners to: Unify the Messages from the Industry Stimulate Collaboration, Synergy, and Ideation Cooperate on Strategic Initiatives and Exchange Information
for partnering with us on long-term industry growth e ortsby joining as a member - we can o er immediate and tangible benefits in the form of:
Savings on Business Essentials
Education on Navigating the Industry, Best Practices, and Industry Trends
Partners can take advantage of the collective knowledge of our network to gain best practice insight, how-to information and industry reporting, peer review of materials, mentorship, and more.

Connections to Potential Partners, Mentors, and Customers
Partners enjoy lower prices on business-essential products and services through our network of preferred vendors, as well as discounted admission to industry events, and more. 1 2 3
Partners gain access to potential customers, mentors, investors, and more by attending industry events, requesting introductions, participating in committees, and more, while also taking engaging MichBio to partner on your behalf or amplify your messages.
Learn more at michbio.org.
Changing the Medical Field With Tritium Batteries
SUBMITTED BY ISABELLE OWENS, CONTENT MANAGER, METRIC MARKETINGCity Labs Is Changing the Medical Field With Tritium Batteries
Very few among us would trust the batteries in our TV remotes to keep our hearts functioning for 10+ years. And the good news is that we don’t have to.
Dr. Peter Cabauy, founder and CEO of Florida’s City Labs Inc.,—and a University of Michigan graduate—recognizes the importance of a sustainable, long-term power solution for challenges faced by the medical industry. City Labs has developed betavoltaic battery cells—powered by processed tritium—that can power medical implants. Among other uses, these new batteries will help address long-standing issues with cardiac pacemakers.
Lithium-based batteries are the current standard for powering leadless cardiac pacemakers (LCP). As it stands, lithium LCPs are limited by their size, output, and lifespan. Decreasing the size of a lithium battery will subsequently decrease its energy density. Therefore, these chemical batteries must exceed a certain volume to supply the necessary charge.
To house these batteries, leadless pacemakers are often built to be ~1 cc. This limits the possible locations for
the LCP implant and the number of implants a person can have. LCPs are not always removable, and if there is limited room for another device within the heart’s chambers, the patient will need to undergo high-risk surgery to survive.
Additionally, lithium-based LCP batteries have a projected lifespan of only 7-12 years. Depending on usage levels, these pacemakers may not even last a full decade, limiting their benefit to older populations.
Harnessing Betavoltaic Batteries For Leadless Pacemakers
Tritium betavoltaic technology harnesses radioactive decay’s natural process to produce steady and lasting energy. The energy density of tritium does not change when the size is scaled up or down, meaning that very small tritium samples can still output sufficient power. Tritium betavoltaic batteries for leadless pacemakers will be 1/6 of the volumetric size of lithium’s technological limit and are capable of outputting ≥3.8 microwatts for 20 years. This is more than twice the energy capacity and lifetime of current lithium batteries—and at a fraction of the size.
These characteristics will greatly increase the potential use cases for pacemakers. The smaller volume will allow LCPs to be implanted in younger patients who may have smaller heart chambers. It will also increase the number of LCPs a person can receive in their lifetime without requiring the removal of the old device.

Leadless pacemakers already use a titanium outer shell that prevents any radiation from leaking into the body. City Labs uses an additional package that will fit inside the titanium shell for double encapsulation. Tritium is a relatively benign isotope that does not give off strong enough radiation to penetrate the double barrier.
When it comes to applications for tritium batteries, LCPs are only one of the many options. City Labs’ betavoltaic cells can be used to power industrial sensors, structural health monitoring, IoT devices, space satellites, and more.
City Labs is the only commercial U.S. laboratory with the ability to process tritium at high pressures and temperatures and is the only facility with a regulatory manufacturing and distribution license for tritium batteries. Tritium is an isotope of hydrogen with two neutrons and one proton and a half-life of approximately 12.3 years. Produced as waste from nuclear power plants, this substance is in no short supply. Tritium is already used commercially to illuminate products like exit signs and watch faces, but its capabilities are much greater.
Bringing City Labs back to its Michigan roots, the company is actively contracted with the University of Michigan for battery components and has collaborated with UM researchers for projects with the National Institutes of Health.


If you’re curious about how tritium technology is revolutionizing sustainable batteries for low-power devices, you’re not alone. Visit citylabs.net to learn about these batteries, explore the technology, and stay updated on relevant news in the microelectronics industry.
CITYLABS.NET

Gene Therapies Shifting the Cost and Care Paradigm
 SUBMITTED BY CSL BEHRING LLC
SUBMITTED BY CSL BEHRING LLC
For eligible adult patients with hemophilia B, a new one-time gene therapy treatment offers life changing possibilities.
HEMGENIX® (etranacogene dezaparvovec-drlb), the world’s first FDA approved gene therapy for appropriate people with hemophilia B, launched by CSL Behring, is changing the conversation on how to manage this rare bleeding disorder.

Currently, there are many effective treatments for hemophilia B. However, people with the condition could still need up to 156 intravenous infusions per year to manage their condition with available treatments today,1 which can be burdensome. In addition, these patients may still experience spontaneous and/or traumatic bleeding into their muscles, internal organs, and joints, which could be life-threatening and possibly lead to permanent physical debility.1
The life-long effects of living with and managing hemophilia B aren’t just physical. More than 40% of people living with hemophilia B experience depression, anxiety, or other psychological disorders.2 Healthcare costs can be 25 times higher for a person living with hemophilia B than a person without a bleeding disorder, which amounts to a total lifetime cost of more than $20 million per person.3,4
HEMGENIX has demonstrated the ability to reduce the mean adjusted annualized bleeding rate of all bleeds by 54 percent seven to 18 months post. In addition, 94 percent (51 out of 54) of patients treated with HEMGENIX discontinued use of prophylaxis and remained free of previous continuous routine prophylaxis therapy.5 As new and advanced gene therapies continue to be developed, it’s critical to ensure people have access to these new innovative treatments.
With high healthcare costs due to the need for treatments throughout a person’s life, an innovative onetime therapy such as HEMGENIX can both reduce the treatments needed throughout a patient’s life and the cost on the healthcare system.
The Institute for Clinical and Economic Review (ICER), which is an independent institute for clinical and economic review, used multiple cost-effectiveness scenarios to determine the value of HEMGENIX, and their base case demonstrated that the therapy would be cost-effective at $9.9 million and could offer significant savings to the overall healthcare system of approximately $6 million over the lifetime of a patient at the hypothetical price of $4 million for treatment.6
ICER determined that HEMGENIX is incredibly valuable through their ability to deliver cost-effective therapies for both patients and the entire healthcare system.6 The organization highlighted the potential long-term impacts of HEMGENIX and the value that eliminating the need for expensive prophylactic treatments could bring to the healthcare system.6
Gene therapy treatment continues to build on the revolutionary progress that has already been made in hemophilia B treatment. This treatment will shift the treatment paradigm for people with hemophilia B by addressing the cause of the condition and reduce the financial burden for patients and the healthcare system.
HEMGENIX is manufactured by uniQure Inc. and distributed by CSL Behring LLC. HEMGENIX® is a registered trademark of CSL Behring LLC.
References:
1. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020 26(Suppl. 6):1-158.
2. Buckner TW, Batt K, Quon D, et al. Assessments of pain, functional impairment, anxiety, and depression in US adults with hemophilia across patient-reported outcome instruments in the Pain, Functional Impairment, and Quality of Life (P-Fi) study. Eur J Haematol. 2018;100 Suppl 1:5-13. doi:10.1111/ejh.13027Li et al. Adult lifetime cost of hemophilia B management in the US... J Med Econ. 2021 (v1.0) - 24(1):363–372.
3. Li N, Sawyer EK, Maruszczyk K, et al. Adult lifetime cost of hemophilia B management in the US: payer and societal perspectives from a decision analytic model. J Med Econ. 2021;24(1):363-371.
4. Buckner TW, Bocharova I, Hagan K, et al. Health care resources utilization and cost burden of hemophilia B in the United States. Blood Advances. 2021 April; Vol 5, Issue 7, page 1954-1962.
5. HEMGENIX Prescribing Information.
6. Tice JA, Walton S, Herce-Hagiwara B, Fahim SM, Moradi A, Sarker J, Chu J, Agboola F, Pearson SD, Rind DM. Gene Therapy for Hemophilia B and An Update on Gene Therapy for Hemophilia A: Effectiveness and Value; Evidence Report. Institute for Clinical and Economic Review, November 2, 2022. https://icer.org/assessment/hemophilia-a-and-b-2022/
BRIEF SUMMARY OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HEMGENIX safely and effectively. See full prescribing information for HEMGENIX. HEMGENIX (etranacogene dezaparvovec-drlb) suspension,for intravenous infusion
Initial U. S. Approval: 2022
-----------INDICATIONS AND USAGE-----------
HEMGENIX is an adeno-associated virus vector-based gene therapy indicated for treatment of adults with Hemophilia B (congenital Factor IX deficiency) who:


• Currently use Factor IX prophylaxis therapy, or
• Have current or historical life-threatening hemorrhage, or
• Have repeated, serious spontaneous bleeding episodes.
-----------CONTRADICTIONS-----------
None.
-----------WARNINGS AND PRECAUTIONS-----------
• Infusion reactions: Monitor during administration and for at least 3 hours after end of infusion. If symptoms occur, slow or interrupt administration. Re-start administration at a slower infusion once resolved.
• Hepatotoxicity: Closely monitor transaminase levels once per week for 3 months after HEMGENIX administration to mitigate the risk of potential hepatotoxicity. Continue to monitor transaminases in all patients who developed liver enzyme elevations until liver enzymes return to baseline. Consider corticosteroid treatment should elevations occur.
• Hepatocellular carcinogenicity: For patients with preexisting risk factors (e.g., cirrhosis, advanced hepatic fibrosis, hepatitis B or C, non-alcoholic fa liver disease ( AFLD), chronic alcohol consumption, non-alcoholic steatohepattis (NASH), and advanced age), perform regular (e.g., annual) liver ultrasound and alpha-fetoprotein testing following administration.
• Monitoring Laboratory tests: Monitor for Factor IX activity and Factor IX inhibitors.
-----------ADVERSE REACTIONS-----------
The most common adverse reactions (incidence 5%) were elevated ALT, headache, blood creatine kinase elevations, flu-Iike symptoms, infusion-related reactions, fatigue, malaise and elevated AST.
To report suspected adverse reactions, contact CSL Behring at 1-866-9156958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-----------USE IN SPECIFIC POPULATIONS-----------
No dose adjustment is required in geriatric, hepatic, or renal impaired patients
Founded in 2005, E4 Bioscience is a Michigan based consulting firm that helps investors and lab teams plan, fund, design, equip, license, staff, and accredit analytical laboratories.
The Science of Talent Acquisition
BY SHAUN R. OPIE, PHD, MANAGING PARTNER, E4 BIOSCIENCECannabis analytical laboratories face a particularly difficult set of challenges when it comes to hiring and retaining top talent. The cannabis laboratory industry is highly specialized and has a limited pool of qualified candidates. Additionally, the pressure to keep pace with rapidly changing regulations, advancing technology, decreasing reimbursement, and fierce competition means that finding and retaining the right employees is mission critical. Unfortunately, many pre-hire employee selection methods (ESMs) used by cannabis laboratory hiring managers are much less effective than they believe and directly contribute to low productivity and morale, high
turnover, and loss of institutional knowledge within the first year. To help all analytical laboratories improve their hiring success rate, E4 Bioscience uses validated, proprietary, evidence-based ESMs that quantitatively measure job eligibility, job suitability, personal traits, to predict job success.


Quantitative Testing
The ability to test for future job performance is dependent upon correctly identifying the appropriate factors for the role. Assessing job eligibility through a resume alone represents a comparatively small portion of the critical factors to predict performance.
When job suitability (behavioral competencies including work preferences, emotional intelligence, and personality) is also quantitatively measured, a high degree of accuracy is attained to predict individual performance in specific jobs. However, job suitability cannot be determined from a resume or CV and standard interviews are generally ineffective at revealing key factors. By combining eligibility and suitability test results using a validated mathematical formula, a combined score indicating how closely the individual comes to having the ideal job behavior patterns is generated. The numerical result provides an easy comparison between multiple potential job candidates.

In addition to laboratory job eligibility and suitability, the assessment independently scores 24 “paradoxical” traits that can support or hinder a candidate’s work behaviors. Paradoxical traits are pairs of traits which appear to be contradictory and derail job performance but are in fact complementary and synergistic. For example, if a candidate is strong in two paradoxical traits, frankness and diplomacy, both will be genuine strengths when communicating. However, if person only has evidence of one trait, the other will necessarily be related to a counter-productive behavior and be a potential hiring red flag. Continuing the example, a candidate with strong frankness and weak diplomacy will have an innate tendency to be disruptively blunt. This can lead to hurt feelings, lack of trust, and employee turnover. Alternatively, a person with strong diplomacy and weak frankness will tend to be evasive when communicating about laboratory needs, leading to confusion, misalignment, and unresolved issues. If a person is weak in both frankness and diplomacy, it can be expected that they will avoid communication. Any candidate without balanced diplomacy and frankness is a risky hire for any laboratory leadership position and should be thoroughly vetted before offering a position.

Employee Turnover & Performance

Turnover rates and actual job performance correlate closely with predictive job suitability testing. In a study with 341 employees, each employee completed a pre-hire assessment and overall performance was rated by supervisors scoring attendance, productivity, service quality, and safety at 6 months. Of the employees predicted to have probable success using pre-hire assessment testing, 91% were successful.
Of the employees predicted to fail using pre-hire testing, 75% were identified as poor performers or did not complete 6 months in the job.
Galvanizing and Mobilizing the Rare Disease Community in Michigan

 BY KORTNEY LEE, FOUNDER, MICHIGAN RARE
BY KORTNEY LEE, FOUNDER, MICHIGAN RARE
Michigan Rare is an up-and-coming patient advocacy organization, founded to enrich the lives of those impacted by rare diseases living in Michigan. Michigan Rare will support patients in their advocacy efforts, raise public awareness about the challenges that people with rare diseases face, and create a community of belonging for patients and their caregivers.
Michigan Rare’s founder, Kortney Lee, draws from her experience in navigating the rare disease community on her son Liam’s behalf. Liam was born with a genetic condition called SATB2 Associated Syndrome. When Liam was newly diagnosed, Kortney struggled to find support and programs to help Liam and their family. After issues and obstacles in obtaining Michigan’s Children’s Special Health Care Services, Kortney wrote her elected state representative on her struggles and for assistance. It was from this point that Kortney’s advocacy journey began - she began to wonder what others were experiencing and if they knew they had the ability to turn to their elected officials.
Kortney currently serves as the regional representative of Michigan for the SATB2 Gene Foundation. In this role, she serves as the point of contact for those in Michigan - hosting gatherings, making connections and assisting where possible. She hopes to prevent others from experiencing what she did and wishes to expand those services beyond one condition.
Michigan Rare is a dual service organization which will hold a 501(c)(3) designation upon approval, as well as offer an affiliated 501(c)(4) organization to further promote and expand upon the advocacy goals for the rare disease community of Michigan.
The first service that Michigan Rare plans to offer is community development by acting as a “one stop shop” for those in the rare disease community to learn about the state and federal programs are available - such as social and health services programs, grants, and programs offered by other organizations. With the creation of an index of patient organizations, Michigan Rare also hopes to help build connections for the individuals and families within the rare community.
The organization also plans to create opportunities for the rare community to come together at events where they can make connections and develop a sense of belonging. There is no better way to identify the common needs of the rare disease community of Michigan and collaborate to become better advocates together.
By bringing together patients, caregivers, and other patient organizations, the aim is to become the state’s standardized data collection agency for the rare disease community. This will make data collection easier, simpler, and more streamlined - a huge benefit to community partners working in research.
The second service Michigan Rare will focus on is advocacy. Michigan Rare believes that advocacy is the single most powerful tool to be used in meeting the needs of the rare disease community because empowering the community to be self-advocating is the best way to convey to lawmakers the urgency and importance of the issues faced.
Advocacy efforts will also include offering support for legislation that helps the needs of the rare community. This will be done through community and partner connections and in private and public formats such as email, social media, and other public outlets. It is important to build connections and relationships between elected officials and the rare disease community and give the community a voice. As the state data collection agency of the rare disease community, Michigan Rare will also be able to impact advocacy efforts through information sharing – with both the community and legislators and partner organizations.
Michigan Rare’s policy priorities include supporting the passage of a Rare Disease Advisory Council (RDAC). This will allow Michigan to work in partnership with them on policy change impacting the rare community while also providing data and other forms of support.



Michigan Rare is in the exciting beginning stages of forming this organization and are welcoming volunteers from anywhere in the state of Michigan who are interested in getting involved.
FOR MORE INFORMATION OR TO BECOME A MICHIGAN RARE PARTNER find Michigan Rare on Facebook, Instagram, Twitter, or connect with Michigan Rare through email at michiganrare@yahoo.com
PURE GREEN PHARMA

Accelerating the Pace of CBD Drug Discovery
BY STEVE GOLDER, CEO, PURE GREEN PHARMA WITH DRAFT ASSISTANCE BY AI CHAT TECHThe foundation for medical practice is “do no harm”. Which is why the team of experts at Pure Green Pharma, a worldclass cannabinoid company in Southfield, Michigan, is focused on providing CBDbased pain relief therapy for millions of patients with diabetic neuropathy.
“When your first focus is providing medical care that ‘does no harm’ it allows you to place strict conditions on the active ingredients you investigate,” says Steve Goldner, CEO of Pure Green Pharma. “Your active ingredients must have large safety margins, must be non-habit forming, and must use receptors in the body that are not life-critical, among other things. CBD is perfect – it provides significant intrinsic metabolic advantages with none of the pharmacologic disadvantages of d9-THC for medical applications.”
Pure Green’s approach has paid off with clinical trials of its water soluble, sublingual tablet technology –which is suitable for diverse cannabinoid molecules - demonstrating significant efficacy in reducing chronic pain from diabetic neuropathy while maintaining remarkable safety – all completed in a faster timeframe than other treatments.
“Pharmaceutical scientists love to solve problems and they are smart, flexible, self-starters,” says Steve. “I needed to develop an operations strategy that harnessed the collective power of those pharmaceutical scientists. From day one, I’ve focused on creating an environment that facilitated collaborations.”
Pure Green Pharma uses a mentorship model that brings senior mentors together with younger scientists to work toward a common goal. Dr. Deb Kimless – a
30-year Board Certified Anesthesiologist, including 10 years treating more than 400 patients with medical cannabinoid preparations – was the first to join the team.
Dr. Kimless wanted a reliable, consistent cannabinoid dosage form for her patients, and Pure Green – with its mentorship model and FAST TRACK management process utilizing parallel instead of sequential-gate thinking - provided an avenue to create one.
Dr. Kimless provided clinical trial leadership during the COVID-19 pandemic and allowed PG Pharma to capitalize on the expanded availability of clinical trial participants who were forced to spend more time at home with their devices. In fact, decentralized trial methods tripled the expected enrollment of women and minorities – Pure Green put clinical operations right in front of patients every day and gathered real-world information in real time.
Chief Science Officer, Ara Karakosyan, D.Sci., Ph.D., built research teams across North America with senior scientists and newly minted NIH post-docs. “We built collaborations with Department Chairs from Rutgers Medical, MD Anderson, and UCSD Medical and additional senior-level collaborators from University of Michigan, University of Wisconsin, Texas Tech University, and the Cleveland VA Hospital,” says Dr. Karakosyan. “Those collaborators, and more than a dozen younger scientists working in their own labs, combined to create a sizable research engine at the right time to respond to the additional availability of clinical trial participants. In truth, everything came together to build the strong collaborations between industry and academia I’ve always wanted to create.”
The pace of discovery has been significant with a management strategy that accelerates growth, strong financial controls, access to trial participants, diverse
research teams, cloud computing and other technologies, all with a mission stakeholders coalesced around. “We’ve got enough strong lead compounds for a dozen drug discovery companies,” says Steve, “but we are completely focused on gaining FDA approval to launch PGP-001 and provide pain relief for millions of patients and value for our investors.”


Pure Green Pharma is primed to bring its sublingual tablet technology to the underserved $8 billion pain relief market for diabetic neuropathy treatment. The ‘A’ Investment Round produced a very strong business valuation. Now a non-dilutive funding round is underway via multiple SBIR NIH grant applications to power phase III pivotal clinical trials and expand label indications via mechanistic and metabolomics preclinical studies.
“The FDA and NIH are on board and have helped to provide the regulatory road map to rapid New Drug Approval for PGP-001,” says Steve. “We are looking forward to the next phase and to building value for the investors who put their faith in us – including a Capital Investment Group I previously worked with, Michiganbased family offices and individuals who have seen the promise of PG Pharma.”
He continued, “I believe a company succeeds or fails based on the strength of its people – they are the true gems who work tirelessly to solve massive global problems. They embody our powerful core mission and are why we have so many willing collaborators, partners, and investors.”






Interactome Bio, a Michigan-based biotechnology company, has been expanding its process development and production facility inside the WMED Homer Stryker MD School of Medicine Innovation Center.


The organization has developed a process to isolate primary progenitor cells from tissue using a xenofree process, which forms the foundation for its therapeutic nanoparticle production facility. The company plans to continue optimizing the process to comply with Good Manufacturing Practice (GMP) standards and its planned ISO 9001 audit certification in the Summer of 2023.
Interactome Bio is now expanding its presence by opening a new research and development facility at Portal Innovations in Chicago’s West Loop. The new facility will support the company’s manufacturing headquarters in Kalamazoo, Michigan. The company aims to increase its exosome production capacity, expand its portfolio of protected exosome engineering technology, and enhance its industry and institutional collaborations, utilizing its AI-driven software tools and services.
Interactome Bio’s expansion comes at a time when exosome-based therapeutics are gaining momentum, and the shift from nascent technology to real clinical outcomes is on the horizon. The company is focusing on nanoparticle development and production, specifically creating scalable, batch-to-batch consistent, and specific sub-classes of exosomes tailored for targeted pipelines.
The new research and development facility at Portal Innovations will help Interactome Bio to attract top life science talent and expand biopharma partnerships. The company also aims to optimize its local collaborative projects with academic institutions. The CEO of Interactome Bio, Adam Koster, said that the company’s production home in Kalamazoo and the new facility in Chicago’s West Loop will play a key role in accelerating its current pipeline via local collaborations and taking advantage of the growing talent pool in the Midwest.
INTERACTOMEBIO.COM



Your Full-Service Biotech Marketing Partner
Don’t waste time and money with ineffective marketing. At Metric Marketing, we implement proven online strategies for amazing results.
But don’t just take our word for it. Metric Marketing is the marketing agency that biotech companies trust to grow their business and help them get unstuck in their marketing strategies. Check out how we’ve helped our biotech client, Akadeum Life Sciences, rise to the top.
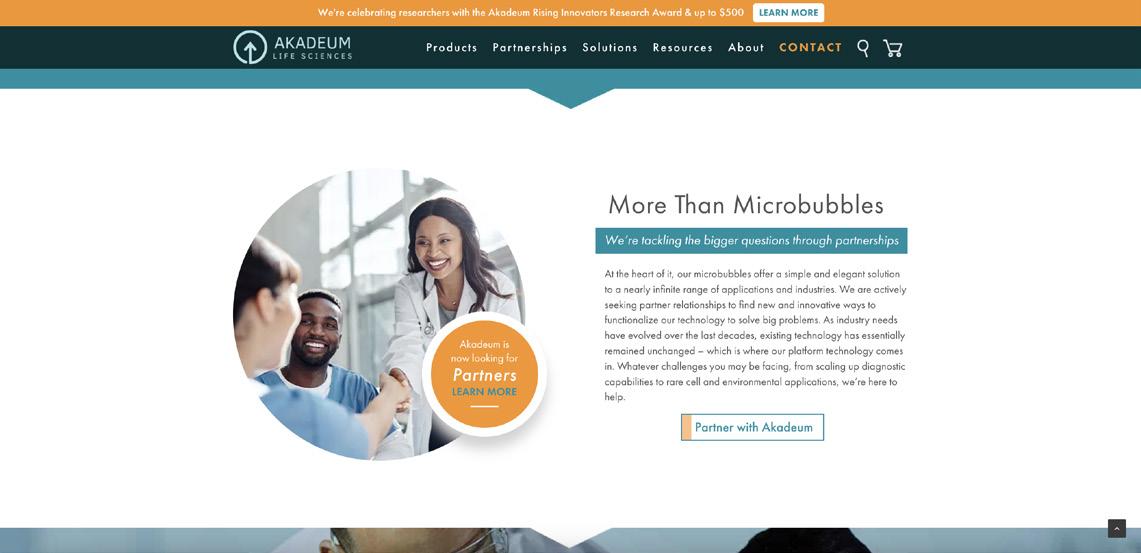
Tritiumisabeta-(electron)emittingby-productofcertain nuclearpowerplants(e.g.CanDUReactors),whichCityLabs implementsinasafeandeffectivepower-harvestingbattery. Tritiumisthemostbenignofradioactiveisotopesandisa technologyalreadyusedasanilluminationsourceforExit signscommonlyfoundinschools,theatres,commercial buildingsandcommercialaircraft.

• Website development
• Search engine optimization
• Paid search advertising

• Social media marketing
• Copywriting



• Content marketing
• Branding
• Graphic design
CAR-TGENERATION
Withourproprietarytechnology, abletoproducemoreclinically CAR-Tcellsreportedtolive andcontinuetodeliveranti-cancer effects.Ourproductis effective,andusesless cellsthancompetitor

• Scientific illustration




• Marketing strategy
• Marketing automation



• CRM integration
“I’ve been working with the team at Metric on SEO, paid search & social, and content marketing campaigns. Without a doubt, their guidance and work-product has been a major boost to our commercialization efforts. As a direct result, we have seen consistent gains in web traffic, conversions, and happy customers. Having worked with other firms in the past, it’s obvious that Metric excels at leveraging modern digital marketing tools in the most capital-efficient way.”
Michael Maloney, Akadeum Life Sciences





