DIGITAL
COATING UPDATE
spy DEVICE UPDATE
www.medovate.co.uk
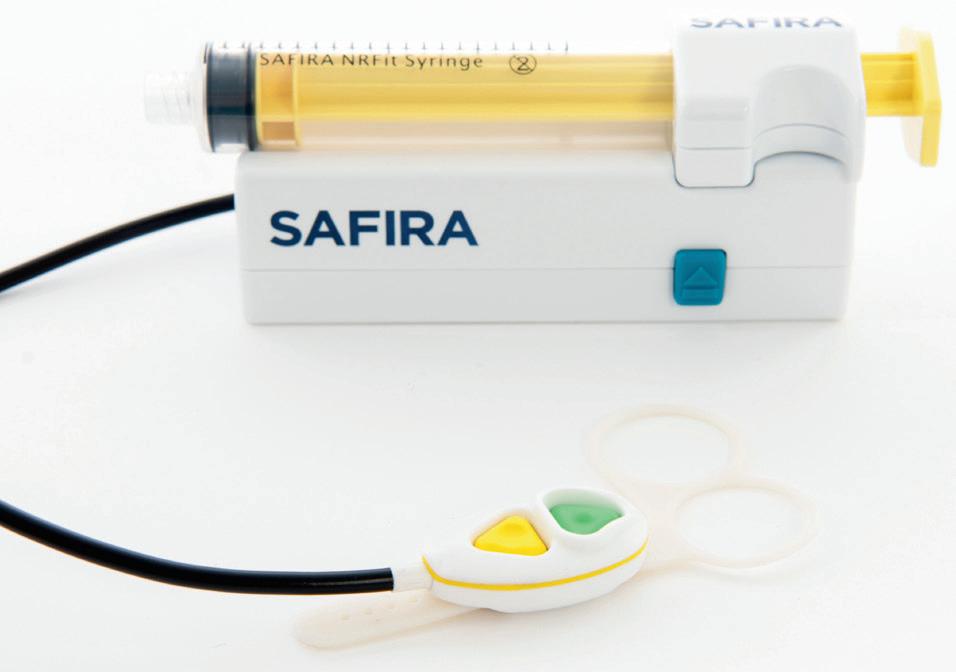
CE Mark regulatory approvals for Medovate’s SAFIRA range
M
edovate has successfully secured additional CE Mark regulatory approvals for its SAFIRA (SAFer Injection for Regional Anaesthesia) technology to include an NRFit syringe and a palm operator. Within the last year, the company has achieved CE Mark regulatory approval covering five devices in its SAFIRA range: infusion driver, foot pedal, luer syringe, NRFit syringe and palm operator. These latest product range additions are intended to further improve patient safety and
offer greater control to anaesthetists. The NRFit syringe provides a second option to the universal luer connection syringes with which the system was originally introduced. With the provision of a brand-new Palm Operator, Medovate hopes to offer anaesthetists more versatility and choice. The palm operator uses the same colour coding as the original foot pedal operator, which remains a key part of the product range for the infusion and aspiration of anaesthetics.
www.amcor.com
Amcor to extend CR27 heat seal coating technology to customers in Europe
S
ince the acquisition of Bemis Company on 11 June 2019, global packaging company Amcor has achieved a milestone in healthcare packaging. By adapting its approach to product development and innovation, and deploying know-how and processes gained in the acquisition, Amcor can now provide unrestricted to customers in Europe key healthcare packaging solutions that were part of the Bemis portfolio. Amcor has long-standing experience with high-quality, heat-sealing adhesives for medical-grade papers and Tyvek material. Adding to that is the well-known, high-performance
Tyvek adhesive and the CR27 heat seal coating technology developed by Bemis, which will now be available in Europe for the first time under the Amcor brand and will be produced in Winterbourne, UK. Amcor’s customers, including medical device manufacturers customers, will be offered to experience the benefits of CR27 heat seal coating technology for medical, sterile barrier applications. Such benefits include enhanced strength and excellent porosity — without compromising on barrier properties; it is also air permeable and suitable for EtO and gamma irradiation sterilisation.
M&A UPDATE
https://atp-ag.com/en/
COMBINING STRENGTHS AND MARKET POSITIONS
A
dhesive tape specialist CCT Coating & Converting Technologies is becoming part of water-based specialty adhesive tapes manufacturer ATP Adhesive Systems Group (ATP) based in Switzerland. The combination
6
strengthens the market position of both companies and allows them to offer a wider range of tailormade solutions to its customers. ATP is a specialist in highperformance adhesive tapes, exclusively focusing on water-
based technology. The company offers tailor-made solutions through its in-house R&D capabilities. With production sites in Germany and the UK, as well as headquarters and a development centre in Switzerland, the
WWW.MEDIC ALPL ASTICSNEWS.COM
company employs approximately 400 staff. US-based CCT Coating & Converting Technologies has provided coating and converting capabilities of specialised adhesive tapes during the past 20 years. CCT’s founder Robert Dempsey as well as CEO Rich Hipp together with their team will continue to run the firm’s operations from their Philadelphia location. The management teams of both companies expect to significantly increase its service offering especially to its US customers.