STERILIZATION
AS A STERILIZING AGENT, ETHYLENE OXIDE’S DAYS ARE NUMBERED, AS THE MEDICAL DEVICES INDUSTRY LOOKS SET TO EMBRACE ALTERNATIVE STERILIZING AGENTS SUCH AS CHLORINE DIOXIDE, SAYS EMILY LORCHEIM, PROJECT MANAGER, CLORDISYS SOLUTIONS, INC.
ET0
M
Go Home
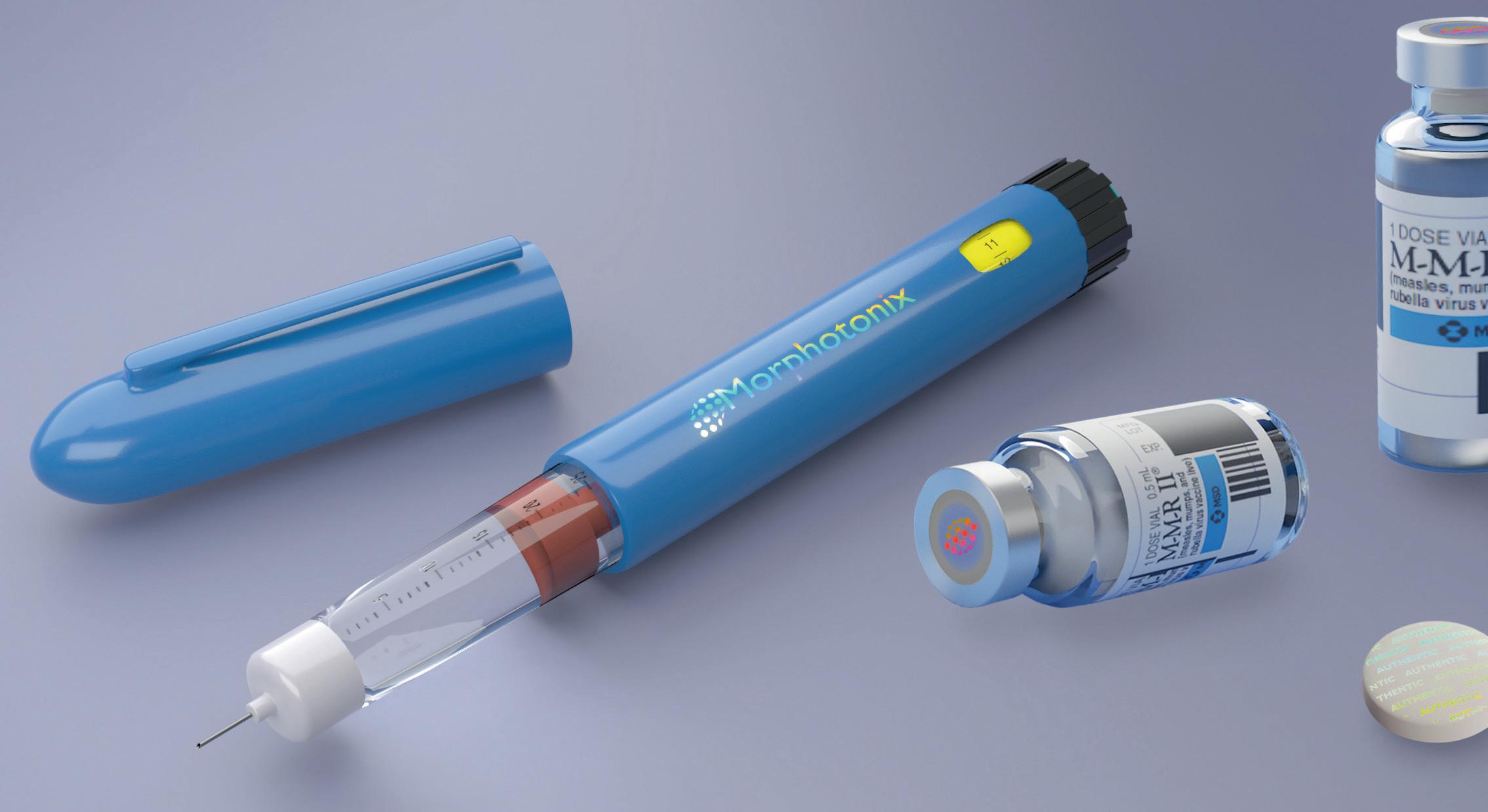
edical device sterilization has seen few advancements compared with most other industries. Even as ethylene oxide (EtO) faces increasing pressure due to potential environmental health hazards, its market share has held steady since the mid-1990s. Much of this is due to the lack of a clear industry standard to innovate and accept new technologies, prolonging the potentially dangerous status quo. Recent events, however, have shown chlorine dioxide (CD) gas to offer promise as a viable low temperature sterilization method, and the first commercially available medical device product sterilized with CD gas has just hit the US market.1 ETO ISSUES In 2006, and after reviewing studies by the National Institute of Occupational Safety and Health (NIOSH) studies, the Environmental Protection Agency (EPA) released a draft of its review of EtO and determined it is a human carcinogen (as published in its Final Report).² In 2018, the EPA released its latest National Air Toxics Assessment based on industry supplied emissions data from 2014.³ The report showed that 109 of the 73,057 census tracts within the US faced cancer risks due to exceeding EtO emission levels according to EPA guidelines. One area with very high EtO levels was Willowbrook, IL. Illinois Attorney General and the County Attorney General sued the main sterilization
company who performs EtO services on medical devices within the city.⁴ Despite attempts to add extra pollution control measures, the city of Willowbrook experienced exceedingly high EtO levels in the air for nearly a year. In February 2019, the Governor ultimately decided to ban the sterilization firm from using EtO at the plant. A legal settlement eventually allowed the company to resume operations in Willowbrook once they installed new equipment to reduce EtO emissions. ADDRESSING THE ROADBLOCKS TO INNOVATION One reason why the use of EtO was allowed to continue was the lack of a suitable alternate method. Guidance existed for the use of established medical device
WWW.MEDICALPLASTICSNEWS.COM
17