
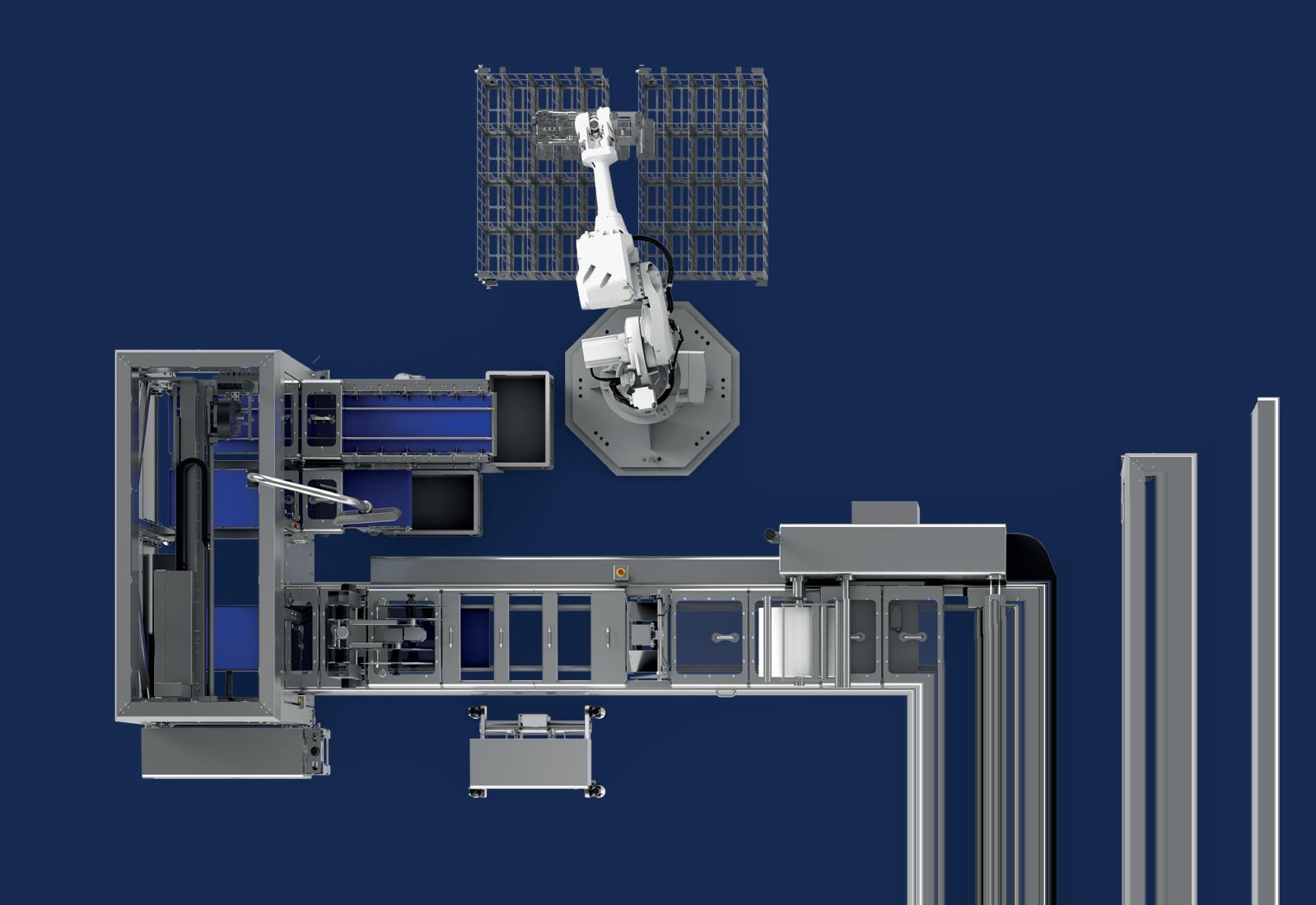
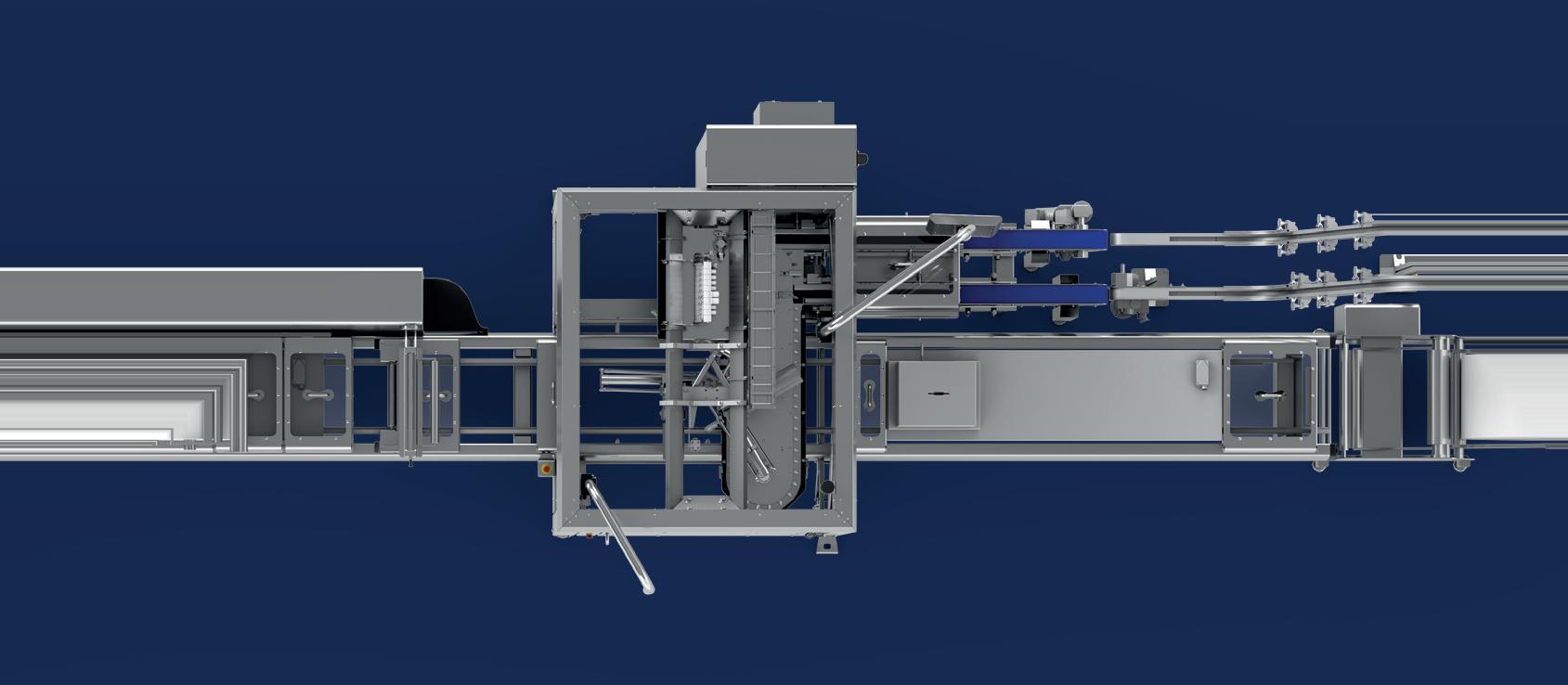
Medical Heat Sealers



A ll Packworld USA Medical Heat Sealers come equipped with TOSS Technology and the advanced PIREG ® Series temperature controllers.

The PIREG controller monitors the resistance on the heatseal band and responds in milliseconds to assure precision temperature control over the entire heatseal band without the use of thermocouples.

Heat Sealing Experts”
PW5548 Touch Screen
• Touch Screen Interface
• Bag Stretcher
• Vacuum (optional)
5 Star Rating
Truly are the EXPERTS AT IMPULSE HEAT SEALING! 15 minutes with a TOSS engineer and I learned more about impulse heat sealing than I have in my 30 years of designing V/F/F/S machines... THANKS TOSS!!
Technology is simply Fantastic. The PIREG ® Temperature Controller controls the time and temperature of the impulse heat seal band flawlessly. Truly performs as advertised…
• Touch Screen Interface
• Horizontal Orientation
• Stand Mounted (as shown)
• Vacuum (optional)
All sealers meet the standards of ISO 11607-2 and the Touch-Screen line is compliant to 21 CFR Part 11.












precision controlled Medical Heat Sealers with






Impulse Booth# 222 Sep 17-20, 2023 National Harbor MD

©2023 PackworldUSA · Nazareth, PA 18064 USA · 610-746-2765· PackworldUSA.com or TossHeatSeal.com
SEAL PERFECTION validatable - repeatable - consistent
HEAT
available worldwide
PW3416 Touch Screen
That’s our Promise
Heat Seal Controls & Components Featuring PIREG ® Temperature Controllers 1,67�7UDFHDEOH�&HUWLÀ�FDWLRQ�DYDLODEOH�XSRQ�UHTXHVW� “We are...
Screen See the complete line of
PIREG
GENUINE TOSS Alloy-20 ® impulse heat seal bands. Booth# 6407 Sep 11-13, 2023 Las Vegas NV
® Temperature Control.

16 PACKAGE DESIGN Serena Williams-Co-founded Recovery Line Delivers Daily Relief with Packaging
A modern take on packaging signals a mindset shift for pain and athletic recovery products. The design serves up hands-free delivery, portability, and sporty shelf appeal.
22 SERIALIZATION Op-ed: Phased Approach to DSCSA an Alternative to Nov. 27 Disruptions
The pharmaceutical supply chain is not where it needs to be as the DSCSA deadline approaches. HDA opines on a possible new approach to ensure the safe and secure delivery of medicines come November.
24 MED DEVICE/STERILIZATION Breakthrough Med Device Sterilization Technology Uses Nitric Oxide
Not to be confused with laughing gas, this “molecule inside a polymer” has potential to offer safe sterilization of medical devices to kill microorganisms without the same environmental issues brought by EtO sterilization.
28 BUSINESS INTELLIGENCE Pharma and Medical Device Companies Optimistic Despite Investment Decline
The pharmaceutical and medical device industry is mellowing after a sharp increase in scheduled projects in 2022, but growth continues.
30 PACK EXPO PREVIEW Sustainability Meets Innovation at PACK EXPO Las Vegas
Keeping pace with a growing industry, PACK EXPO Las Vegas 2023 will be the most comprehensive packaging and processing show in North America. Check out the new sustainability features.
37 REGULATORY Annex 1 Implementation Tips for CDMOs, ATMP Producers, and Sterile Manufacturers
Experts from PDA’s Annex 1 task force provide insight on compliance with the new requirements and suggestions for delayed implementation dates.
40 AUTOMATION/WORKFORCE 7 Takeaways from Women on the Future of Robotics
Help a colleague today via sponsorship. Says one expert, “…to have someone who’s not only talking with you, but talking to others about you. That really opens up connections and opportunities that a lot of underrepresented groups don’t have.”
CONTENTS 2 | Healthcare Packaging • Fall 2023
COLUMNS AND DEPARTMENTS 05 KEREN SOOKNE’S PERSPECTIVE 06 QUOTABLES/BY THE NUMBERS 08 COLD CHAIN CORNER 10 NEWS 12 QUICK HITS 13 MATERIAL DEVELOPMENTS 44 NEW PRODUCTS pp. 24 Profiling med device sterilization using nitric oxide.
Image: Sterile State LLC.

Where formulations take ight. 45 years of expertise in contract lling sensitive formulations into unique, single-dose ampoule packaging. CALL 908-362-9266 JAMES-ALEXANDER.COM See us at PACK EXPO Booth SL-6745
QUICKLY. SAFELY. CLEANLY.

Editorial
EDITOR-IN-CHIEF Keren Sookne ksookne@pmmimediagroup.com
SENIOR DIRECTOR, CONTENT & BRAND GROWTH Mike Prokopeak
CONTRIBUTING EDITOR Melissa Griffen
CONTRIBUTING EDITOR Tim Hayes
Art
ART DIRECTOR Jonathan Fleming

CREATIVE DIRECTOR Dave Bacho
Publishing
MANAGER, CUSTOMER EXPERIENCE & SALES SUPPORT Courtney Nichols
AD SERVICES/PRODUCTION MANAGER Lara Krieger
Audience & Digital
VICE PRESIDENT, DIGITAL Elizabeth Kachoris
DIRECTOR, DIGITAL MEDIA Jen Krepelka
Advertising
VICE PRESIDENT, SALES John Schrei jschrei@pmmimediagroup.com
ACCOUNT EXECUTIVE ElizabethTierney
SENIOR DIRECTOR, CLIENT SUCCESS & MEDIA OPERATIONS Kelly Greeby
PMMI Media Group
PRESIDENT Dave Newcorn
DIRECTOR, MARKETING Sharon Taylor
SENIOR MARKETING MANAGER Amber Miller
FINANCIAL SERVICES MANAGER Janet Fabiano
FOUNDING PARTNER AND EXECUTIVE VP, INDUSTRY OUTREACH, PMMI Joseph Angel
FOUNDING PARTNER Lloyd Ferguson
PMMI Media Group 401 N. Michigan Ave., Suite 1700 Chicago, IL 60611 p: 312.222.1010 | f: 312.222.1310
www.pmmimediagroup.com
PMMI The Association for Packaging and Processing Technologies 11911 Freedom Drive, Suite 600, Reston, VA 20190 p: 703.243.8555 | f: 703.243.8556 | www.pmmi.com






PLEASE RECYCLE THIS MAGAZINE Remove inserts or samples before recycling.
www.healthcarepackaging.com Healthcare Packaging® (ISSN # 21543666) is a registered trademark of PMMI, The Association for Packaging and Processing Technologies. Healthcare Packaging® is published bi-monthly by PMMI with its publishing office, PMMI Media Group, located at 401 N. Michigan Ave, Suite 1700, Chicago, IL 60611; 312.222.1010; Fax: 312.222.1310. Periodicals postage paid at Chicago, IL, and additional mailing offices. Copyright 2022 by PMMI. All rights reserved. Materials in this publication must not be reproduced in any form without written permission of the publisher. Applications for a free subscription may be made online at www.healthcarepackaging.com/ subscribe. Paid subscription rates per year are $55 in the U.S., $80 Canada and Mexico by surface mail; $130 Europe, $200 in all other areas. Single copy price in U.S. is $20. Free digital edition available to qualified individuals outside the United States. POSTMASTER; Send address changes to Healthcare Packaging®, 401 N. Michigan Avenue, Suite 1700, Chicago, IL 60611-3789. PRINTED IN USA by Quad. Volume 15, Number 6 The opinions expressed in articles are those of the authors and not necessarily those of PMMI. Comments, questions and letters to the editor are welcome and can be sent to: editors@healthcarepackaging.com. Mailing List: We make a portion of our mailing list available to reputable firms. If you would prefer that we don’t include your name, please write us at the Chicago, IL address.
PUBLICATIONS MAIL AGREEMENT NO. 40064408 • USPS #25469
spee-dee.com | 877.375.2121
FILL NUTRACEUTICAL POWDERS INTO RIGID CONTAINERS OF MANY SIZES, SHAPES & MATERIALS WITH OUR COMPACT ROTARY FILLER. PACK EXPO Booth: C-4809
Patient Adherence in a Modern Package


Our cover story this issue focuses on package design for a line of athletic recovery and pain management products (pp. 16). While the aesthetics and the high-profile co-founder— the Serena Williams—might be the first thing readers notice, it’s ultimately a story about adherence. In their research, WILL PERFORM
found that many people shy away from recovery products because of implications around what it means to be in pain. They used input from Williams, who knows sports pain inside and out, to make a modern, counter-worthy product line that hit shelves in December.
Switching gears to sterile manufacturing and packaging, we showcase a new technology using nitric oxide as a medical device steril-


ant. With so many concerned about risks related to ethylene oxide sterilization, check out pp. 24 for details on the process and safety features. Next, Melissa Griffen offers tips for sterile pharma manufacturers and CDMOs from PDA experts on the Annex 1 document (pp. 37), the requirements of which came into force in August. The story features insights on compliance and suggestions for delayed implementation dates.
If you’re looking to update your packaging, equipment, or logistics solutions, join us in September at the Healthcare Packaging Pavilion at PACK EXPO Las Vegas to see new machines and technologies, sustainable packaging ideas, and plenty more—our show preview is on pp. 30. There’s even a bit of Cirque du Soleil at PACK gives BACK™ (tickets sold separately). See you there!
KEREN SOOKNE is the Director of Editorial Content of Healthcare Packaging. She may be reached at ksookne@pmmimediagroup or at linkedin.com/in/kerensookne

PERSPECTIVE Performance, Packaged Safe. Reliable. Customizable. 252-446-6177 • www.ossid.com Healthcare and medical packaging solutions for your products. Visit us in Pack Expo Booth #3033 Ossid 8000M From entry level flow wrappers to highly engineered and customizable horizontal form fill seal machines, Ossid has a solution to suit your product needs.
Sporty design and delivery aim to offer a mindset shift for pain products. Plus, check out new tech at PACK EXPO Las Vegas’ Healthcare Packaging Pavillion.
77 BILLION
THE ESTIMATED VALUE that the diabetes drug market is expected to reach by 2030.
Source: Adroit Market Research 16
THE NUMBER of interactive, 45-minute educational sessions taking place at The Forum at PACK EXPO Las Vegas (Sept. 11-13), providing collaboration on solutions to widespread issues such as digitalization, cybersecurity, sustainability, automation, and integration of cobots into manufacturing.
13
WORKER DEATHS in America have decreased from around 38 worker deaths per day in 1970 to 13 a day in 2020, per OSHA. Worker injuries and illnesses are down—from 10.9 incidents per 100 workers in 1972 to 2.7 per 100 in 2020.
7/31/23
THE DATE the NIH launched phase 2 clinical trials to evaluate at least four potential treatments for long COVID, with additional clinical trials to test at least seven more treatments expected in the coming months.
Source: NIH
6 | Healthcare Packaging • Fall 2023
QUOTABLES BY THE NUMBERS
“But analysts say UPS’ competitors won’t be able to absorb all the backlog created by a strike. UPS delivered an average of 24.3 million packages per day in 2022—more than any competitor can take on.”
—DANIELLE KAYE, NPR, ON WHAT A UPS STRIKE WOULD HAVE MEANT
“When it comes to setting up recycling programs, first is finding partners, and there are also regional considerations that need to be accounted for. One, where is the resource being generated? I hope you notice I didn’t use the term ‘waste.’ I think we need to get away from that because waste means it doesn’t have any value. So how do we change that mindset and think of packaging as a resource?”
—NICK PACKET, TYVEK® HEALTHCARE PACKAGING IVM, DUPONT
“With 4 in 5 Americans taking vitamins and supplements as part of their self-care routine, 75% of those surveyed have forgotten to take them in the past year, with 2 out of 5 people admitting they forget to take them at least once a week.”
—CVS HEALTH, ON ITS RECENT CVS PHARMACY SURVEY






INNOVATIVE PACKAGING. IT’S IN OUR DNA. No matter what your packaging challenge, we’ll provide a ʍ Ȉɧʍljӗ� ʍ ʰӸȈ ɽljǼɨƃɽljǁ�ɰ ʍɽȈ �ɽȃƃɽ�ɥljɨ ljƺɽ ʰ�ˎɽɰ�ʰ ʍɨ� ljljǁɰӝ� Fairfield, New Jersey 973-808-8185 mgamerica.com See us at Pack Expo Booth SL-6534 Cartoning Liquid Filling/Capping Thermoforming
Temp-controlled Cancer Treatment Trial Benefits from Specialty Label
EDITED BY MORGAN SMITH, CONTRIBUTING EDITOR
A number of clinical trials are currently investigating the systematic use of oncolytic viruses in cancer therapies. Virotherapy is an immunotherapy in which patients receive injections with oncolytic viruses—these viruses infect tumor cells, selectively destroying malignant cells.
Shanghai Yuan Song Biotechnology is among the companies conducting virotherapy clinical trials. In their trial, the active pharmaceutical ingredient (API) for its virotherapy cancer treatment is especially sensitive, posing labeling challenges. The API must be stored and transported at low temperatures and is subsequently thawed into a liquid before being administered. These temperature differences place high demands on the adhesion of the marking and documentation label attached to unit-dose vials.
In addition, prior to administering the drug, healthcare staff visually check that there are no flaws in the medication and that the liquid is clear. A transparent inspection window must be integrated
into the label for that purpose. The label also must provide adequate space for important information, and contain two removable parts for documenting the administration of the medication in the patient and hospital files for the clinical trial.
For the trial, Schreiner MediPharm’s customized Pharma-Comb IL label was a clear choice. Capable of withstanding temperatures from -20°C to -70°C, the specialty label enables the permanent marking of unit-dose vials, even at very low temperatures, while its transparent inspection window allows for a vial liquid check prior to administration. (Visit hcpgo.to/421 to view an image.)
Plus, two removable documentation labels have tabs for easy, efficient peel-off and attachment to patient files for accurate documentation of the clinical trial.
Combined, these features enable reliable and clear product marking from storage to transportation to dispensing, helping to optimize the clinical trial process and support patient safety.
Why booklets?…


t�&BTJFS�UP�PSHBOJ[F�JOGPSNBUJPO
t�&BTJFS�UP�PSHBOJ[F�JOGPSNBUJPO
t�&BTJFS�GPS�DPOTVNFST�UP�öOE�GBDUT�BOE�EJSFDUJPOT
t�&BTJFS�GPS�DPOTVNFST�UP�öOE�GBDUT�BOE�EJSFDUJPOT
t�(VJEFMJOFT�DBO�CF�CPPLNBSLFE�GPS�GVUVSF�SFGFSFODF
t�(VJEFMJOFT�DBO�CF�CPPLNBSLFE�GPS�GVUVSF�SFGFSFODF
Make spine-glued booklets… t�#PPLMFU�JOTFSUT�BOE�POTFSUT t�&YUFOEFE�DPOUFOU�MBCFMT t�"WPJE�TIBSQ�NFUBM�TUJUDIFT
t�#PPLMFU�JOTFSUT�BOE�POTFSUT t�&YUFOEFE�DPOUFOU�MBCFMT t�"WPJE�TIBSQ�NFUBM�TUJUDIFT
COLD CHAIN CORNER
715 Church Road, Elmhurst, IL 60126 t 630-530-2203 t www.guk-vijuk.com Book
into the packaging industry! See more possibilities at Booth B645 řDQG�VWDUW�ERRNLQJ�SURƓWV� Call today to get the advantage!
it…










OSHA Launches Program to Reduce, Prevent Workplace Hazards
Under the new three-year national emphasis program, OSHA will conduct comprehensive safety inspections in the distribution centers of warehouses and processing facilities, focusing on hazards related to powered industrial vehicle operations, material handling and storage, walking and working surfaces, means of egress, and fire protection. In addition, inspections focused on storage and loading areas will be conducted in retail establishments with high injury rates; an inspection’s scope may be expanded when evidence shows violations may exist in other areas. OSHA also will assess heat and ergonomic hazards. To find out more about the new program, go to hcpgo.to/418 —Morgan Smith
ISTA Publishes ATP Best Practice Guideline
The ISTA Pharma Committee has published the technical paper “ISTA PCG-04 | Thermal Packaging System Ambient Temperature Profile (ATP) Best Practice Guideline.” Developed by members of the ISTA Pharma Committee, the document was established to align industry best practices for the selection, development, and comparison of ATPs for use in the thermal qualification testing of thermal packaging to transport temperature-sensitive medicinal products. The collaborative effort between life science companies and solution providers has resulted in a mutually beneficial, harmonized best practices guideline to aid the industry in comparing, investigating, and implementing agile compliant solutions. Download at hcpgo.to/419 —Morgan Smith

Also in the News
+ FDA issued draft guidance for designing psychedelic research, clinical trials.
+ GS1 US published a new guideline on healthcare industry use of RFID.
+ Sanofi Consumer Healthcare North America earned B Corp Certification.

+ Chiesi, Kindeva, and H&T Presspart are partnering on a production line for dose counter & dose indicator devices.
HPRC Releases Medical Waste Recycling Case Study
The Healthcare Plastics Recycling Council (HPRC) has released a case study that explains how the University Medical Center Utrecht (UMC Utrecht), a hospital located in the Netherlands, has overcome the recycling medical waste challenges that many members of the healthcare sector face due to a wide range of barriers, such as economic and legislative obstacles. The study also details why the University Medical Center Utrecht serves as a best practice example for hospital waste management and shares the knowledge that the healthcare network has gained. To read the entire case study and/or watch an accompanying video, go to hcpgo.to/420
—Morgan Smith
+ Oliver Healthcare Packaging acquired EK-Pack Folien, adding in-region film and foil production.
+ TekniPlex Healthcare will open a new 200,000-sq-ft production facility in Wisconsin.
+ FDA approved a new Bristol Myers Squibb CAR T manufacturing facility in Devens, MA.
+ AstraZeneca and Vanguard Renewables have partnered to reduce greenhouse gas emmisssions.
+ UPS opened a healthcare logistics facility in Singapore.
Get more at healthcarepackaging.com/news

NEWS 10 | Healthcare Packaging • Fall 2023













r
v yda
v y
1
FDA Approves First OTC Birth Control
CNN reported that the FDA has granted approval for the oral contraceptive Opill to be available over-the-counter, making it the first non-prescription birth control pill in the U.S. Expected to be on shelves only in early 2024, Opill is a “mini-pill” that uses progestin and does not contain estrogen, with an effectiveness rate of about 98% when taken as directed. (Opill differs from combination birth control pills, which contain both progestin and estrogen.) While Opill may have some side effects like irregular bleeding and headaches, progestin-only formulas are considered safer for certain medical conditions.
2
New Patch Detects Intestinal Leaks

Per Medgadget, scientists at EMPA have developed a surgical sealant for post-gastrointestinal surgery. The sealant is a polymer patch that reacts to pH changes in leaked intestinal fluid, producing bubbles within its structure. This physical change can be visualized using ultrasound or CT scans, providing early warning of potential leaks. The patch is flexible and can also change shape in response to intestinal fluid, allowing clinicians to identify leaky stitches in a non-invasive way to monitor patients. The patch can also release drugs, such as antibiotics, if necessary.
3
DuPont to Acquire Spectrum Plastics
According to a recent Medical Device Network article, DuPont agreed in May to acquire Spectrum Plastics Group, a specialty medical devices manufacturer, from AEA Investors for $1.75 billion. Spectrum serves major medical device OEMs in various markets including electrophysiology, structural heart, surgical robotics, and cardiovascular applications. The acquisition is expected to strengthen DuPont’s position in the medical market and contribute about 10% to the company’s overall revenue. The deal is subject to regulatory approvals and is anticipated to be completed by the end of Q3 2023.
4
FDA Holds Hearing on Delayed CBD Regs
Marijuana Moment reported that the U.S. House Oversight and Accountability Subcommittee on Health Care and Financial Services held a July 27 hearing to investigate the FDA’s delay in creating regulations for hemp-derived CBD products. The lack of regs has caused industry uncertainty, and has raised concerns about consumer safety and restricting the availability of credible CBD products. The bipartisan Hemp Access and Consumer Safety Act has been reintroduced to remove barriers that hinder CBD sales in food and as dietary supplements, while providing clear rules for consumer safety.
5 Recall of 250,000+ Catheters
According to a recent Cardiovascular Business article, the FDA issued a Class I recall for over 250,000 catheters manufactured by Arrow International, a subsidiary of Teleflex. The recall is due to the risk of device separation and leakage, which can lead to serious injuries or death. The affected product is the Arrow Endurance Extended Dwell Peripheral Catheter System, commonly used for short-term access to a patient’s peripheral vascular system during procedures like blood sample collection or fluid administration. So far, there have been 83 complaints, including 18 reported injuries, but no deaths. Medical facilities and distributors are advised to quarantine and return the recalled catheters, a process Teleflex has promised to assist with.
6Tornado Ravishes NC Pharma Plant
A powerful tornado heavily damaged a section of a large Pfizer pharmaceutical plant in Rocky Mount, North Carolina—part of a string of extreme weather events striking the U.S. on the same day, per a recent CBS Austin article. The plant suffered significant destruction to a large complex causing a reported 50,000 pallets of medicine to be scattered and damaged by the storm; manufacturing lines were not affected. Thankfully, Pfizer stated there were no reports of serious injuries at the facility. In a July 21 statement, FDA Commissioner Robert Califf said they are working closely with Pfizer to assess the impact and supply of products.
To keep up with the latest news bits from around the world visit healthcarepackaging.com to subscribe and get Quick Hits sent right to your inbox.
QUICK HITS
12 | Healthcare Packaging • Fall 2023 TIM HAYES, CONTRIBUTING EDITOR, HCP
NeoPharm Cuts Product Loss, Boosts Reputation with Protective Paper System
Motivated by aesthetics and ESG concerns, the medical skincare company moved from plastic air pillows to paper pads for transport packaging.
South Korea-based NeoPharm is a cutting-edge global skincare company with a 20-year history of growth and innovation in barrier technology against irritants and allergens. The company produces a number of successful brands, including Atopharm skincare for infants and toddlers with sensitive skin, and Zeroid medical skincare products.
Leading the skincare market for infants and children in South Korea, NeoPharm places high value on premium packaging both for presentation and damage prevention. But with the growing importance of the ESG movement in South Korea and abroad—and a company focus on circular economy—they decided to replace their legacy plastic air pillows used in transit packaging. Alongside a commitment to ensuring healthy skin, the company is seeking to play a part in creating a healthy environment for everyone. For NeoPharm, cutting down on plastic waste was a priority both for aesthetics and sustainability.
Conveying the right message
Parents are concerned with the presentation of the products that they order for their children. Taking this concern seriously, NeoPharm believed that plastic pillows conveyed the wrong impression for a business that supports future generations. “Reducing plastic was important for the brand because of their focus on products for babies. Representing the future, it’s important to be stewards of the environment for the next generation and cut down on environmental pollution— including microplastic particles, which have been studied to impact babies as well as adults,” says Jin Yoo, Ranpak’s Senior Account Manager for South Korea.
In addition, packaging presentation in the South Korean market is a big factor in how consumers evaluate products, particularly with the popularity of unboxing videos on social media.
Reducing the amount of in-transit damage was another goal. Not only were plastic air pillows conveying the wrong message to customers about sustainability, but they were also not as effective as needed during transit, leading to high-value product loss. With pressure on both material and labor costs, NeoPharm also sought a more efficient and cost-effective packaging line.
Given these priorities, any solution that would replace plastic air pillows needed to provide equal or greater levels of product protec-
tion while also being attractive to look at, easily recyclable, and made from renewable materials.
Switching to paper pads
NeoPharm turned to Ranpak because of their reputation in paper packaging systems. After close consultation, NeoPharm opted to replace their existing plastic airbag packaging line with PadPak LC (Light Cushioning) converters.

While Ranpak’s Geami was originally considered, NeoPharm preferred the look of PadPak for their in-the-box cushioning needs. Geami still holds potential for other applications at NeoPharm since it’s suited for packaging smaller, high-value items in retail settings either within a warehouse or at a cash-wrap station.
The PadPak LC systems use kraft paper, fan-folding it into light pads that are well suited to cushioning, blocking-and-bracing, and void filling. Offering visually pleasing aesthetics and high levels of in-the-box protection, the pads are created using a patented folding and stitching process, with paper specific to Ranpak machines. Both batches and individual pads can be made via five different modes of operation.
Two LC machines were installed at the end of NeoPharm’s product packaging line. The machines comfortably handle the requirements of the facility while maintaining a low physical profile in
Fall 2023 • Healthcare Packaging | 13 MATERIAL DEVELOPMENTS
KEREN SOOKNE, EDITOR-IN-CHIEF
↑ The PadPak LC systems use kraft paper, fan-folding it into light pads that are well suited to cushioning, blocking-and-bracing, and void filling.
the packing area. “The PadPak works on a razor-razorblade model. NeoPharm does not own, but rather leases the machines while paying instead for their required packaging materials,” Yoo explains.
The packing process is performed manually, with picked items being placed into the box along with precut pads manually placed by employee packers before being moved to labeling operations. By using precut pads, the



packing line can move very quickly— the precise number of required pads are produced on demand to be available during packing. The packing configuration features items within the box at an angle so that they are both cushioned and blocked in place by the paper pads. Ranpak’s team was on-site to help install and offer hands-on training to employees on the new systems, facilitating a quick learning process. NeoPharm experienced a productivity boost thanks to bins where workers could access the pre-cut paper pads, replacing the multiple storage areas that had formerly held plastic air bubbles.
Improved look, protection, and worker reception











Implementing the PadPak system helped NeoPharm with a variety of challenges. Switching to plastic-free transport packaging is in line with their ESG commitment to use 100%-recyclable packaging material. It’s also had the benefit of improving customer experience and boosting brand reputation with curbside-recyclable packaging. By using paper packaging, the company notes it is also better placed to comply with forthcoming changes in regulations.
Fewer packages are now damaged in transit thanks to the PadPak cushioning pad, which is designed to offer improved shock absorption. The company reports that no more than one or two packages out of every 100 are now damaged, thanks to the protective pads formed directly around the products.
Logistics and stockroom requirements are simpler because one material (kraft paper) now meets all transport packaging requirements, replacing the previous two types of pre-made airbags. Their original plastic air bubble solu-



14 | Healthcare Packaging • Fall 2023 MATERIAL DEVELOPMENTS
tions took up considerable space, with the inflated materials being bulky and requiring large amounts of storage area. Since switching to Ranpak paper, now a centralized bin system is used to store paper pads which are formed on-demand, saving both time and valuable space within the packing area. The system also eliminated the step of tearing plastic from the machine while producing air bubbles thanks to the integrated cutting blades within the PadPak solution, saving a step in the process and improving packaging efficiency.






Though packers had initially been hesitant to learn a new packaging process as their former system had become familiar to them, they quickly came to appreciate the new system and its increased efficiency. With the legacy air pillows, workers who were using large amounts of plastic packaging daily reported feeling guilty about the impact of these materials on the environment. They were impressed by paper’s strength compared to the previous plastic bubbles, while being a renewable material and easily recycled. “The reception on the part of packers went from initially skeptical about new paper solutions to enthusiastic thanks to the sustainability improvements and the increased productivity and room within the packing area,” shares Yoo,
Make plans to check out PACK EXPO Las Vegas’ Sustainability





Central, a new show floor destination taking an expansive look into packaging sustainability and what it means to brands, including expert speakers, interactive content, and a look at actionable, sustainable innovations in manufacturing, materials, design, and more. For info on the show, visit packexpolasvegas.com
who was also frequently on site during the transition.
Says Yangsu Kim, CEO of NeoPharm, “Ranpak’s eco-friendly packaging was ideal for our ESG and social responsibility commit-

ments. Their consultation was very helpful, and our packaging is now more protective and cost-efficient. It’s good to have a partner who helps with solutions that meet both our environmental and business needs.”

Fall 2023 • Healthcare Packaging | 15 MATERIAL DEVELOPMENTS sales@formostfuji.com • 425-483-9090 • www.formostfuji.com
Fuji
equipment SEE OUR PACKAGING SOLUTIONS IN PERSON Pack Expo Las Vegas 2023 September 11-13 BOOTH C-4000 Register for FREE
Formost
packaging
Serena Williams-Co-founded Recovery Line Delivers Daily Relief with Packaging
KEREN SOOKNE, EDITOR-IN-CHIEF
With Serena Williams in its court as co-founder, this active recovery line’s packaging is designed for top notch performance.
Launched in December 2022, WILL PERFORM is offering a refreshing take on athletic recovery with muscle care and pain management. “Our brand is very much about the idea that recovery is bigger than just the reactive pain relief. Recovery should be something that we think about every day, especially for people like Serena, who are working incredibly hard and need to make sure that they can perform the next day,” says Alexia Lundberg, Ph.D., Chief Product Officer at WILL PERFORM, and biochemist by training.
As Lundberg explains, co-founders Eric Ryan and Serena Williams knew each other through her investments in OLLY (Ryan
is co-founder of several recognizable brands including Olly, Method, and Welly). “They came together with a shared interest in redefining the athletic recovery category. Serena wanted to create something that felt authentic to her, with products she wanted to use. That, combined with Eric’s idea that Millennials think about health and wellness as a lifestyle pursuit and want products to support that, led to the start of the brand,” Lundberg says. Ryan brought in Hank Mercier as the CEO of WILL PERFORM and its third co-founder.
The line consists of six products with different benefits for recovery—pain relief and muscle care—with two roll-ons, three lotions, and a spray format.

Entering the pain relief and muscle care categories, WILL PERFORM is combining modern aesthetics and ease of use. All

PACKAGE DESIGN 16 | Healthcare Packaging • Fall 2023
1. A modern take on packaging signals a mindset shift for pain and athletic recovery products.
2. The design serves up hands-free delivery, portability, and gender-inclusive shelf appeal.
3. For formats containing lidocaine, child-resistant cap designs were a must.
TOP THREE TAKEAWAYS
WILL PERFORM launched on D2C, at Target.com, and on Target endcaps in December.
of the formulations are intended to have a high level of efficacy and an elevated product experience, with a focus on feel, smell, and delivery. Lundberg explains, “Recovery and rest are obviously top of mind for professional athletes, but for a lot of people they’re not. The Rest and Soothe lotions are our introduction to what we call Performance Care and it’s a mindset shift in thinking about recovery and performance daily, intended for day and nighttime use.”
Of course, certain benefits help fit a new product into a familiar consumer routine. With many women used to applying lotion after a shower, they added ceramides and vitamin E to the Soothe lotion for antioxidant and skin barrier protection, along with magnesium for muscles.
Reducing the stigma of pain relief and reaching women were key goals. “With traditional pain relief products there can be an association with ‘You’re older, so you hurt.’ We also talked to a lot of women as we were designing this brand, and no woman thinks that there’s a product in this category that is made for them,” Lundberg says. “So that’s the other piece. We want to reach every athlete, but there is a miss with women in this category and we want to make sure we’re speaking to them. This is one of the reasons our cartons feature images of men and women.”
The product line launched online at WILL PERFORM and Target websites on December 8, and in Target stores on endcaps on December 22. After some time on endcaps, they moved in-line at the end of March via eye-catching displays with a picture of Williams and education about the products.
Challenges
WILL PERFORM had a number of challenges to solve for as they iterated on the industrial design and graphics.
The three Relieve products in roll-on, lotion, and spray formats contain lidocaine. Lundberg explains, “It’s a highly effective ingredient and allows us to create elegant
products, but it requires a child-resistant [CR] cap. Child-resistant closures are a highly regulated area, so we knew we had to design something that could pass the required testing but still look unique. Not easy at all!”
They also wanted hands-free delivery options, so consumers didn’t have to touch the products if they didn’t want to. To accomplish this, they designed applicator
heads to optimize the product experience. Maintaining a cohesive aesthetic across a range of formulations and delivery mechanisms was also top of mind.
Structural design and HDPE
For the primary packaging of all products except the aerosol, the team selected 100% HDPE bottles because they wanted to offer consumers the ability to recycle. “Rest is a
↙ For launch, they chose a five-panel carton design (pictured), later shifting to a four-panel carton.

PACKAGE DESIGN Fall 2023 • Healthcare Packaging | 17
very viscous lotion—squeezability was something we had to keep in mind. At the same time, we wanted recyclability,” Lundberg says. “So we played around with blends of LDPE and HDPE. But knowing that that can pose issues with recycling, we leaned into HDPE. We worked on creating a package where we could thin out the sides in certain areas to make the sides squeezable, and then redistribute plastic in other places to create an element of squeezability that you need to get the product out, but that still lets us use HDPE.”
Since three of the products in the HDPE bottles are over the counter (OTC) drug products, the brand needed to find a way to label them with all the required information on a small primary package. They chose a multi-panel pressure-sensitive label applied to the back of the package to solve for this. All other artwork is screen printed.
“We added design elements like debossed grooves and pops of color to create an aesthetic that felt sporty and fun,” she says. “When designing the packaging, we were very conscientious about how an athlete would use the product. Hands-free application and a loop to allow the product to be clipped to a gym bag are examples of how we kept the athlete in mind during the industrial design process.”
Child-resistant closures
With custom packaging, designing a CR cap can be risky. “We knew we wanted to do custom packaging for most of the products, because we believe that design is one of the most impactful ways to build a brand. We did look at stock to make our lives easier, but
Product Line
+ WILL Relieve Pain Relief Roll-on and Spray contain 4% lidocaine and botanicals in a thin, gel-like formula delivered by a triple rollerball in a 3-oz bottle and a 4-oz aerosol, respectively.
+ WILL Cool Cooling Pain Relief Roll-on contains a blend of menthol, camphor, and botanicals in a thin, gel-like formula that is delivered by a triple rollerball system in a 3-oz bottle.
+ WILL Rest Nightly Muscle Recovery Lotion is an emollient lotion with lavender and geranium to help with calm and sleep. With magnesium and Vitamin D, the 3-oz squeezable bottle features an angled applicator for a hands-free delivery.
+ WILL Soothe Daily Muscle Soothing Body Lotion is a lotion with a viscosity that allows for easy application and massage. It contains magnesium, ceramides, and Vitamin E to help skin and muscles daily and is delivered in a 6-oz squeezable bottle.
+ Launched in late March, WILL Relieve Pain Relief Lotion contains 4% lidocaine and botanicals in a creamy lotion formula, with a hands-free angled applicator in a 3-oz bottle.
we always landed back at doing something custom,” Lundberg says.
In designing for child-resistance, the team opted to bring in consultants who’d forged the path with other brands and had overcome similar challenges. “It took a lot of work to be able to land an aesthetic that we really liked, while also creating functionality. There was a lot of pilot work as we were iterating, testing at the same time. That was probably one of the biggest risks we had throughout the entire development process,” she notes.
For the lidocaine products, the CR cap is a squeeze-and-turn mechanism, with a triple click. On either side is a small divot with instructions. Lundberg says, “Throughout the entire process, not a single kid was able to open it. To be honest, our biggest challenge when we were developing this was making sure adults could open it.”
For the spray, they did opt for a stock child-resistant closure (CRC) because designing a custom aerosol bottle with a CRC didn’t seem like a viable option.
While contract manufacturing or packaging suppliers couldn’t be named, Lundberg explains that they use one contract manufacturer for processing and another for packaging. They sought a partner they could trust to produce topical OTC products and the ability to do CRC.
Convenient delivery mechanisms
WILL PERFORM sought hands-free delivery for a few reasons. “Serena brings that athlete’s perspective to say, ‘Here are things that I really like or don’t like.’ Many people don’t participate in this category, either due to strong smells or they don’t think about it because there’s nothing that feels like it’s for them. So we wanted to create something that people actually want to use,” she adds.
The application of lidocaine and menthol benefit from being as hands-free as possible. Says Lundberg. “With lidocaine and menthol there are a few reasons why you don’t want to touch them with your fingers. Not that it’s bad to touch, but it can create other issues such as when you rub your eyes or brush your teeth. So we wanted the pain relief products to be hands-free.”
With the Rest and Relieve lotions, the team created an angled applicator that could be applied directly to the body so people can choose whether to apply via applicator or by hand.
The roll-on Relieve and Cool products use a triple rollerball design to dispense product. “We liked the idea of a triple rollerball because not only does it look cool, but we felt it could also be a massage tool and it adds to the cooling effect of our WILL Cool Pain Relief product. With the single plastic rollerballs, there can be a lot of product that ends up coming out, and so the idea with the triple rollerball is you can use it for massage without soaking yourself in product. Also, some people feel a spray isn’t as effective for targeted products. It’s not necessarily true, but it’s just a consumer perception,” she explains.
PACKAGE DESIGN 18 | Healthcare Packaging • Fall 2023
Double cap and cohesive aesthetic
A key focus for WILL PERFORM was creating a cohesive product family across the various formats and delivery mechanisms that will look good at-shelf. In this case, the inner and outer cap worked in their favor. “It’s a two-component cap and it works because it allows you to do both a CRC and non-CRC that look exactly the same from the outside,” Lundberg explains. “The spray is the outlier only in the sense that it’s not a plastic bottle, but we still have the same graphical treatment. We wanted to create an iconic look, where people are able to identify this brand by the way the package looks and create continuity across all products.”
For portability, the brand sought to bring in texture and functionality so they created a loop on the cap made from TPE (thermoplastic elastomers), so the product could easily be strapped to gym bags for on-the-go use.

The cap consists of an inner and an outer component composed of polypropylene for both form and function. “The translucent outer cap allowed us to show through to a pop of color on the inner cap, which was designed in a color that complemented the color of the TPE ‘loop.’ The two-cap system also allowed us to create visual continuity in the cap design whether or not the cap needed to be child-resistant,” she says.
Moving from five-panel carton to four
The team initially wanted to stick to primary packaging because they loved the bottles so much, but they opted to add a carton. Among other considerations, a main driver was that consumers wanted education on their products. “When we were first thinking about it, we thought we’d be able to put all the drug facts and information on this tiny little bottle, and there was just no way to include that in addition to the educational content” she says.
The team designed their cartons to fit the bottles snugly with no inserts. Per Lundberg, “We right-sized the cartons for each bottle
so there were no inserts used. It wasn’t that challenging because three of our products are the exact same size, and then the aerosol and Soothe are almost the same size. So we ended up with just two carton sizes, which wasn’t overly complex from an operational standpoint.”
For launch via D2C (direct to consumer) and endcaps at Target, they chose a five-panel carton design and placed images of Williams—who lends a lot of credibility—and everyday athletes on the extra panels. It was important for WILL PERFORM to show that these products were designed for every athlete, not just the best of the best.
WILL PERFORM has since phased-in a four-panel carton (next page), with the front panel image focused on the primary package. “We ended up cutting off that wing, and now we’re going to be in a four-panel. We continue to learn and optimize, and with the fourpanel the key information is right in front of you and more focused. All the feedback we’ve gotten… everybody loves the bottle itself. Maybe eventually we get out of a carton altogether—there’s other things we have to solve for related to that,” she says.
Launch and learn
With the recent launch, Lundberg says she’s incredibly happy with the packaging: “With anything new there are opportunities to learn and improve and we will certainly do that, however, we are very proud of how our primary package turned out. A theme that runs through all of Eric Ryan’s companies is to create a package that is counter-worthy. This helps with compliance, which is important for efficacy, and can take away the stigma that can be associated with a product.”
In the case of pain relief products, Lundberg reiterates the stigma around using these products and differentiating their industrial and graphic design from the current pain relief category. “Many consumers don’t enter this category, despite needing to, because they don’t
PACKAGE DESIGN Fall 2023 • Healthcare Packaging | 19
↘ From left, a cooling pain relief roll-on, a muscle recovery lotion with angled applicator, and a daily muscle soothing body lotion (not to scale).
believe the products were made for them. Our packaging design and messaging is one way we can help change that. As much as we love our primary packaging, we felt it was important to use a carton to help the consumer understand
what the products do and when to use them. This is particularly important for WILL Rest and WILL Soothe, which are all about daily muscle care—something that is new for many consumers.


“We love the look. But there are always things that you want to change. Our philosophy with any new brand is that the first year is ‘launch and learn.’ You put it out there, go with your gut, and figure out what you think is best, but quickly, you’re going to learn,” she says. “So when it comes to packaging, whether it’s messaging or the fifth panel or other things, we just plan that we’re going to be iterating, iterating, iterating… listening to customers and making adjustments accordingly.”
Get the latest in package design and e-commerce solutions at PACK EXPO Las Vegas (Sep. 11-13, 2023; Las Vegas Convention Center). Get the info at packexpolasvegas.com








PACKAGE DESIGN WWW.PALLETIZING.COM / 800 628 4065 LEARN MORE THE ULTIMATE IN FLEXIBILITY! A single Columbia palletizer can handle all of these products and more with ease. Visit Us Booth C-2838 LAS VEGAS
← A four-panel carton has been phased in, with primary package imagery.














©2023 ProMach Inc. 5600 Kieran Montreal, QC H4S 2B5 PHONE 514-337-6990 EMAIL info@NJMPackaging.com WEB NJMPackaging.com Complete Pack aging Lines for Oral Solid Dosage Complete Solid Dose Lines including Tamper Evidence ProMach Collaboration, ProMach Performance On display at Pack Expo Booth C-3225
Op-ed: Phased Approach to DSCSA an Alternative to Nov. 27 Disruptions
PATRICK KELLY, EXECUTIVE VICE PRESIDENT, GOVERNMENT AFFAIRS & ELIZABETH A. GALLENAGH, GENERAL COUNSEL AND SENIOR VP, SUPPLY CHAIN INTEGRITY, HEALTHCARE DISTRIBUTION ALLIANCE
Throughout the first half of 2023, pharmaceutical trading partners have been steadily making progress toward the November 27 Drug Supply Chain Security Act (DSCSA) implementation deadline. By that date, manufacturers, distributors, and dispensers must begin to interoperably and electronically exchange data identifying each prescription drug package purchased and sold.
However, manufacturers and distributors still face very real issues in connecting their organizations’ data systems. The Healthcare Distribution Alliance’s (HDA) final EPCIS Implementation Benchmarking Survey reaffirmed that despite companies’ best efforts, many supply chain trading partners are struggling to establish EPCIS connections, test systems, and onboard trading partners.
The package-level data exchange Congress envisioned in 2013 is interdependent for each supply chain segment and becomes effective for all trading partners at the same time. This interdependency means that the ability of wholesale distributors and dispensers to purchase and resell needed medicines from manufacturers is dependent upon a manufacturer’s provision of this package-level data. There is a complexity surrounding this requirement and the development of necessary systems. Due to many factors, including the problem of how to handle exceptions, numerous trading partners will not be able to send accurate, package-level data to their customers.
Time is not on the side of the pharmaceutical supply chain. In February, HDA flagged several implementation issues while making recommendations to the agency to help keep the supply chain on
track for compliance. Given that the industry is still not where it needs to be as fall approaches—and that the U.S. already faces persistent drug shortages—a new approach is needed to ensure the continued safe and secure delivery of medicines come November 27.
A workable solution toward DSCSA compliance
On June 2, HDA wrote to FDA recommending that the DSCSA’s final requirements be implemented in phases to build capacity and stabilize these complex processes. The phased approach would include a limited FDA grant of enforcement discretion to certain DSCSA requirements and trading partners, with full implementation phased in over a period of two years. Trading partners would continue current business practices needed to move medicines to patients safely and securely while also continuing the push toward the package-level tracing and enhanced supply chain security Congress envisioned.
HDA believes a phased approach is the best option to avoid drug shortages due to the interdependency of the data exchange. When the final phase of the DSCSA goes into effect, the ability of wholesale distributors and dispensers to purchase and resell needed medicines from manufacturers is dependent upon a manufacturer’s provision of the serialized data.
Without receipt of the requisite serialized data from its supplier, a wholesale distributor may not lawfully accept ownership of a covered drug product package or resell the package, which will lead to delivery disruptions to dispensers and even shortages of needed medicines. The only alternative would be to accept and distribute a
SERIALIZATION 22 | Healthcare Packaging • Fall 2023
1. The pharmaceutical supply chain is not where it needs to be as the DSCSA deadline approaches.
2. A new approach may be necessary to ensure the safe and secure delivery of medicines come November.
3. HDA shares a potential phased approach for final requirements, while urging FDA to maintain pressure on trading partners.
TOP THREE TAKEAWAYS
drug package without the required serialized data, thereby risking enforcement action for non-compliance with DSCSA. Both scenarios have the potential to cause significant disruptions, delays, and prevent access for patients across the U.S.
HDA believes the phased approach would mitigate these disruptions, with the following segments culminating in full compliance with the DSCSA’s package-level data requirements by November 2025:
Phase 1, November 27, 2023–November 26, 2024:

• During the first phase, manufacturers would provide package-level data to wholesalers, or obtain an exemption from FDA. All companies will continue to share lot-level data with each prescription transaction to distributors. Distributors and dispensers, meanwhile, will maintain their current DSCSA-compliant practices.
Phase 2, November 27, 2024–May 26, 2025
• In the second phase, manufacturers must comply with all DSCSA requirements or obtain an exemption from FDA and continue to provide lot-level data to customers to ensure prescription drugs move in the supply chain. Wholesale distributors will begin providing package-level data to dispensers with each prescription drug transaction, while also continuing to provide lot-level data. Dispensers would continue to maintain their current DSCSA business processes.
Phase 3, May 27, 2025–November 26, 2025
• Manufacturers and wholesale distributors must comply with
all DSCSA requirements or obtain an exemption from FDA. In this final phase, dispensers will work on stabilizing their business processes for receipt of package-level data from wholesale distributors and manufacturers.

We urge FDA to maintain the pressure on trading partners to ensure full implementation of the DSCSA. Broad enforcement discretion, without conditions and oversight, is likely to result in even more delay.
Continued industry collaboration and education are key
No matter FDA’s decision on the impending deadline, DSCSA compliance efforts and progress throughout the supply chain should not stop. Countless pharmaceutical wholesale distributors, manufacturers, and other supply chain partners have invested years of work and millions of dollars to reach the requirements set forth in the DSCSA and will continue to do so. Likewise, those companies that have not started their implementation must focus their attention on these critical requirements now.
While a phased approach to product serialization may mean the implementation of DSCSA takes longer, it is important to ensure that this law is implemented in a manner that Congress intended while ensuring the stability of the supply chain on which patients depend. The time is now to forge a workable path forward.
Additional resources, including exceptions handling guidance, are available at HDA.org/pharmaceutical-traceability
SERIALIZATION Fall 2023 • Healthcare Packaging | 23
HDA believes a phased approach is the best option to avoid drug shortages due to the interdependency of the data exchange under DSCSA.
Breakthrough Med Device Sterilization Technology Uses Nitric Oxide
KEREN SOOKNE, EDITOR-IN-CHIEF
Billions of medical devices are sterilized with ethylene oxide (EtO) each year. While EtO has made headlines and caused facility closures for unsafe environmental exposure and resulting health risks, for many years, there was no readily available process to serve as an alternative for medical device sterilization.
In 2019, the FDA set up innovation challenges to identify new sterilization methods and reduce EtO emissions. With limited facilities and significant device volumes, EtO sterilization providers become impacted giving rise to long lead times for sterilization.
At the[PACK]out conference in Austin, TX, in May, a new technology for medical device sterilization was unveiled from speakers Megan Frost, PhD, and Kurt Yockey, both of Sterile State LLC

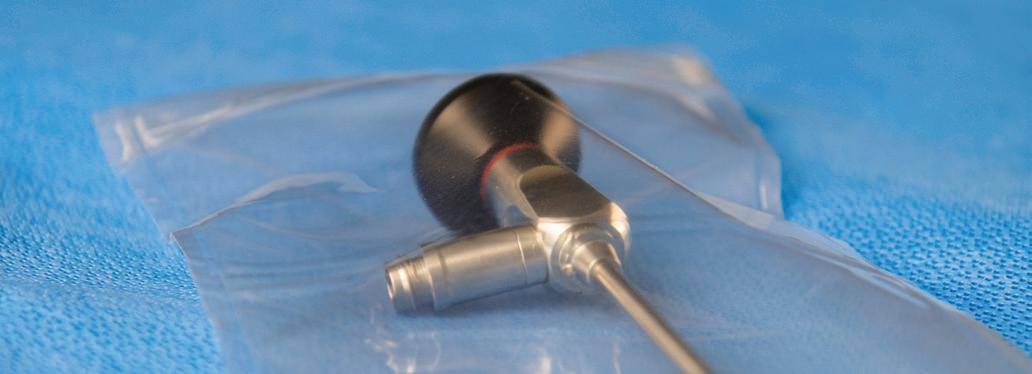
Yockey has worked with hospitals, nurses, medical device companies, and the Joint Commission is his tenure. “I’m here to introduce that our system is really very simple. Devices are placed into an enclosure, it’s sealed, and then shipped immediately to customers. It’s that simple,” he said. “There are no regional sterilization facilities, no sterilizer fees, no logistics, no delays, no waiting for sterilization, and it’s completely safe.”
This technology, nitric oxide (NO) inside a polymer, was announced May 9. If successful, it could help assuage industry fears over having sufficient sterile medical devices while reducing risks to environments and worker safety.
Dr. Frost, CTO at Sterile State LLC, has worked on the technology for 20 years, enhancing the sterilant and delivery system: “I started out using this technology to make implanted medical devices. The reason I’m telling you that is because it’s a really safe technology, and it was developed to be at a standard much safer than what we use for packaging devices that aren’t actually going to be implanted within the human body.”
The problem Sterile State set out to solve is a large and multi-faceted one. 20 billion med devices sold in the U.S. are sterilized with EtO, which is toxic at certain exposure levels. For years, the EPA and the FDA have asked the medical device industry to come up alternatives to EtO sterilization. Visit hcpgo.to/FDAeto
Nitric oxide and unique delivery
Beyond the unveiling of nitric oxide as a sterilant, Frost explained, “The second component to this innovation is the packaging. The way that we deliver the nitric oxide as a sterilant is through the packaging itself.”
↑ As Kurt Yockey explained at the[PACK]out, a device is placed into an enclosure, it’s sealed, and then shipped immediately to customers, precluding the need for regional sterilization facilities, sterilizer fees, and associated logistics.
MED DEVICE/STERILIZATION 24 | Healthcare Packaging • Fall 2023
1. EtO sterilization is effective but brings with it environmental and safety concerns.
2. This “molecule inside a polymer” may offer safe sterilization of medical devices.
3. Clinically, nitric oxide was initially used in maturing neonatal lungs in babies.
TOP THREE TAKEAWAYS
Frost is well-versed on the topic, having worked with the molecule for decades. “The most common question I get is, ‘Is that laughing gas?’ Nitric oxide is not nitrous oxide, which is laughing gas. Nor is it NO2, nitrogen dioxide, which is what’s used in some other sterilization systems,” she explained. “Nitric oxide itself is a free radical gas. It’s produced by virtually every cell in the human body, and what we’re doing is mimicking the way the human body actually eliminates microorganisms in living systems. So this is a natural system, it’s a natural sterilant, and it’s safe to tissues and organs in the human body.”

At its core, the technology works with nitric oxide embedded in a polymeric system—a polymer carries the molecule as a solid-state material that, under appropriate conditions, will release the nitric oxide. This means the nitric oxide is safely stored, and then safely released within the sterilization system after an activation period. “Because the carrier for our sterilant is a polymer, what we want to do then is apply that sterilant to the packaging that we’re going to use to encase medical devices,” she said.
• Activation: There are two activation modalities—room temperature or light. The light that is necessary is broadband white light.
• Compatibility: In terms of device compatibility, where initial development time has been spent, the process has been shown to be gentle on electronics, epoxies, and more. Sterile State has not found polymers that are incompatible as of press time.



• Sterility and shelf-life: Sterility is maintained as long as the package barrier is maintained. As a relatively new application, the package itself has a one-year shelf-life prior to the device entering the system. That shelflife study is ongoing.
• Polymers: Sterile State has developed approximately 27 different proprietary polymers that carry nitric oxide. These can be tooled to an MDM’s preference, and Frost noted that they are open to discussing an MDM’s specific applications and answering further questions. With any new sterilization technology,

it’s normal to question how an MDM can prove efficacy. As she explained, the sterilization efficacy of nitric oxide has been tested for log six reduction against all gram-positive and gram-negative E. coli, and spore formers. “When you look at the hierarchy of what the FDA requires to prove sterilization, the hardest species to kill are the spore formers. So that’s used as the biological indicator to prove that we have actually reached sterilization. So we use the same biological indicator that’s used with EtO: the spore former. When we put it in our system, we are able to eliminate it,” she said.

MED DEVICE/STERILIZATION Fall 2023 • Healthcare Packaging | 25
At the[PACK]out, Dr. Megan Frost discussed that nitric oxide is not to be confused with nitrous oxide (laughing gas).
In 2019, the FDA set up innovation challenges to identify new sterilization methods and reduce EtO emissions. Image: Sterile State LLC
During the presentation, Frost shared an image of paper strips in two petri dishes infused with bacteria. One dish was exposed to NO while the control was not. “Where you see the yellow halos means the bacteria is still growing after the four-hour exposure time. And the picture on the right-hand side where there is no halo indicates that the bacteria was completely eliminated…NO really is killing these infectious agents. We don’t rely on a qualitative test to do this. We do quantitative microbiology, and if anybody has questions about this, I would be happy to go through how we do this. But we’ve proven quantitatively that we do reduce full log-6 reduction that’s required,” she explained.
Frost also showed a chemiluminescence detection measurement, which is a measurement of nitric oxide within the packaging system. Upon activation there is a visible spike showing NO, which is sustained for the time at which it’s exposed, and then it stops releasing.
Practical use
NO sterilization in packaging demands that two main conditions be met.
• As a gas sterilization technique, the device you want to sterilize must have gas contact (as it does with EtO). No box or chamber
is needed—MDMs simply seal the package, and sterilization will take place by sitting in the sealed packaging.


• The NO must be confined within the package, meaning that a gas impermeable membrane must encase the outermost layer of the package.

Frost explained that an MDM will purchase packaging materials from their packaging supplier. “The intention is that there will be no to very little change in machinery. We are using smarter materials with no change from the user’s perspective,” she added.


NO could be placed in a number of spots in the package to cause sterilization to take place, including within a tray if there’s gas contact for the device, or an inner membrane in a double package system if the outer membrane is gas impermeable. “There’s a lot of configurations we can use to take this polymer and make these device packages to allow us to sterilize these devices,” Frost said. “We can make soft packages that also enclose and encase the device we want sterilized, and it will become sterile. There are a lot of alternatives and options for how you can design the package to contain the NO release of polymer as long as we can find the polymer and allow gas contact with the device.”
EtO sterilization is confirmed via chemical and biological indicators. Sterile State has developed a chemical indicator that changes
MED DEVICE/STERILIZATION
from a light color to a dark green. “So for my Michigan State friends, I always say to Kurt, this is your Spartan green indicator. As soon as it turns dark green, it’s good to go. So green means go,” said Frost. “When the device has been sterilized, it reaches the appropriate dark green color, and you can see that sterilization has taken place. So the end result of this—here’s the solution that we’re proposing to answer the EtO challenge from the EPA and from the FDA—[is that] you create a package that contains a polymer, you simply put your device in that package, you seal the package, and that device is ready to be shipped to a customer who wants to have that available for use.”
Safety and benefits
Frost pointed out that nitric oxide is safe for handling. “You are not required to use any special PPE when you’re handling this. In fact, nitric oxide in its initial clinical application was for use in maturing neonatal lungs in babies. So it’s safe enough that we can breathe it and be around it,” she said. “The sterilization takes place under ambient conditions, so you don’t need special temperature or pressure to sterilize the equipment that you’re using. There’s no aeration as is needed with EtO. So the total sterilization time takes about four hours to complete as opposed to shipping it to an external source, sending it through the EtO system that takes 16 to 24 hours, and then returning it or shipping it to whoever’s going to be using it.”
There are obvious environmental benefits to this—the sterilization cycle is shorter, there are no special environmental conditions needed, and the need to ship to and from a sterilization facility is eliminated. Frost noted that there are also no special chemical disposal requirements necessary.
During the Q&A, when asked why the technology hasn’t taken off yet, Frost noted, “The technology is new—we just developed it. This is the first time we’ve actually spoken about it publicly. The challenges with it are things like
getting to the FDA—we are before the FDA right now with an application on this. We’re working with a handful of medical device manufacturers who have different needs that we’re looking at.”
PHARMACEUTICAL INSPECTION & FOREIGN OBJECT DETECTION

• CEIA high sensitivity metal detection; ferrous, non-ferrous, stainless steel

• Ishida X-ray for foreign object and product defects


• Ishida precision checkweighing for [IMKLX�ZIVMƼ�GEXMSR
• Powders, capsules, tablets, liquids
Heat and Control® offers a complete line of metal detectors, checkweighers, and X-ray inspection systems for pharmaceutical products from the leading manufacturers, Ishida and CEIA®. Technical support, demonstration, and testing is available in North, Central and South America.



Sep. 11-13, 2023 Booth C-1623, Central Hall Las Vegas Convention Center Las Vegas, NV USA


MED DEVICE/STERILIZATION Fall 2023 • Healthcare Packaging | 27 92 91 97 98 34 108 94 6 116 17 20 33 32 99 36 26 42 0842 ~2 info@heatandcontrol.com | heatandcontrol.com 3 Ensure your products match the prescription. Quality Control. Inspection and foreign object detection systems that offer unparalleled sensitivity for pharmaceutical products to protect your consumer and your brand. LOOKING BACK. PRESSING FORWARD. ALWAYS INNOVATING.
Pharma and Medical Device Companies Optimistic Despite Investment Decline
CASEY FLANAGAN, DIGITAL EDITOR, PMMI MEDIA GROUP
This year’s pharmaceutical and medical device investments are a far cry from the success of 2022, but the industry continues to grow at a slower pace.
That’s according to PMMI Business Intelligence’s 2023 report, Purchasing Plans and Priorities. The report analyzes packaging industry sectors through a purchasing index that measures expected investments, scheduled projects, and the number of SKUs manufactured, with an index of 50 representing the midpoint or no change.
Pharmaceutical and medical device companies are optimistic about market trends, with expected increases in investments at an index of 69.1, scheduled projects at 71.4, and the number of SKUs at 73.8. Those numbers show industry expansion, but compared to 2022, these companies anticipate a significant deceleration in the number of scheduled projects. The prior PMMI purchasing index measurement for scheduled projects was 91.7.
The pharma and medical device sector is trending lower than the overall industry in every category. The total industry’s 2023 index is 75.6 for equipment investments, 77.5 for scheduled projects, and 76.8 for the number of new SKUs.
Pharmaceutical and medical device industry concerns
Inflation, higher interest rates, decreased availability of research and development funding, and supply chain problems were among pharmaceutical and medical device companies’ concerns in a survey conducted for the report.
Within this sector, 48% of companies expressed supply chain improvements, while 19% indicated it is worsening.
European (mostly German, Italian, and Swiss) machinery companies have traditionally had strong penetration in this sector, with a smaller representation of Japanese and American companies. Users reported problems finding suppliers in Europe able to commit to delivery dates.
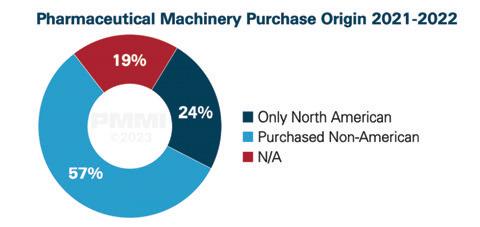
Pharmaceutical machinery purchase origins
The trend of buying machinery from outside the U.S. has continued in recent years in the sector. Over half (57%) of survey respondents indicated they purchased machinery from outside the U.S. between 2021 and 2022. Another 19% of respondents were not sure where their machinery was manufactured, leaving about a quarter (24%) of respondents that have only bought North American machinery in recent years. Safety and flexibility ranked higher as operational priorities than in any other industry, and those topics were frequently discussed in post-survey interviews.
“Pharma and medical device companies need to comply with Good Manufacturing Practices (GMPs) and industry-specific regulations,” one survey interviewee said. “Most have controlled environments and strict documentation and quality control requirements. Thus, only a few pre-qualified vendors can participate in our purchasing processes.”
Survey respondent recommendations for packaging machinery companies in this sector were diverse, ranging from providing spare parts and lowering prices, to reducing total cost of ownership, and improving service. The most common recommendation was to provide faster spare parts delivery. Some indicated they would like to see faster response times when they require a part or component that is not considered part of a regular maintenance program.
Download the free executive summary at hcpgo.to/plans23
BUSINESS INTELLIGENCE 28 | Healthcare Packaging • Fall 2023
1. The industry is mellowing on projects, but growth continues.
2. Over half of respondents purchased machinery from outside the U.S. in recent years.
3. Faster spare parts delivery remains a common request.
TOP THREE TAKEAWAYS


2346 Success Drive Odessa, FL 33556 PHONE 727-232-8200 EMAIL Sales@Pharmaworks.com WEB Pharmaworks.com ©2023 ProMach Inc. ProMach Collaboration, ProMach Performance Integrated Blister Line Solutions On display at Pack Expo Booth C-3225
Sustainability Meets Innovation at PACK EXPO Las Vegas
Keeping pace with a growing industry, PACK EXPO Las Vegas 2023 will be the most comprehensive packaging and processing show in North America. Read on for new sustainability features.
CASEY FLANAGAN, DIGITAL EDITOR, PMMI MEDIA GROUP
As the premier destination to explore cutting-edge packaging and processing solutions, PACK EXPO Las Vegas (Sept. 11–13, 2023; Las Vegas Convention Center) will feature new show-floor destinations along with old favorites.
Produced by PMMI, The Association for Packaging and Processing Technologies, the show is the most comprehensive packaging and processing event in North America with over 2,000 suppliers showcasing diverse innovations for more than 40 vertical markets.
“Our industry is stronger than ever and positioned for unprecedented growth, making the connections and innovations that come from a world-class trade show more crucial than ever,” says Jim Pittas, President & CEO, PMMI. “PACK EXPO Las Vegas offers unrivaled opportunities to explore and learn about packaging and processing solutions. It is the best place to gain market-trend insights, forge relationships, and see the latest technologies— all in one place.”
Attending PACK EXPO Las Vegas is the most efficient way to move projects forward by discovering new solutions and expanding professional knowledge and networks. Attendees will gain insight from 100+ educational sessions and have access to more solutions under one roof than available anywhere else in North America. A multitude of familiar and new features make the show a must-see for all packaging and processing professionals.
Notable New Features at PACK EXPO Las Vegas
Sustainability Central: This interactive destination takes an expansive look into what sustainability means and provides actionable sustainable solutions in manufacturing, materials, and design.

Sustainability Stage: Attendees will hear from experts on a range of packaging sustainability topics and learn how to make brands more sustainable in the future.

Industry Speaks Stage: Experts from the PACK EXPO Part-
ner Program, covering multiple industry verticals, will address hot topics and industry trends like sustainability, remote access, supply chain solutions, augmented reality, and operational efficiency.
To learn more about these programs and destinations, and to register, visit:
New Pavilion in Las Vegas
The Logistics Pavilion: With the boom in e-commerce, The Logistics Pavilion is an important addition to the show that will feature targeted solutions related to the supply chain, including warehousing, fulfillment, distribution logistics services, and transportation providers.
PACK EXPO PREVIEW 30 | Healthcare Packaging • Fall 2023


1256 North Church Street Moorestown, NJ 08057 PHONE 856-273-3377 EMAIL WLS@ProMachBuilt.com WEB Weilerls.com ©2023 ProMach Inc., Background image taken from NBC News. Vials | Bulk syringes | Bottles | Ampules & cartridges Autoinjectors | Non-pharmaceutical The most advanced pressure sensitive labeling and label printing solutions available Pressure sensitive labeling Label printing | Serialization Vial coding | Inspection Highest speed Best local service Compact footprint Simplicity & autonomy For: Booth C-3225 & SL-6501 Visit Us at
Additional Can’t-Miss Show Offerings
The Healthcare Packaging Pavilion: Life sciences is an important focus for PACK EXPO Las Vegas, and this “show within a show”
will house innovations for pharmaceuticals, biopharmaceuticals, nutraceuticals, and medical devices.


The Processing Zone: Currently 44% larger than in 2021, The Processing Zone supports
Partner With the Product Inspection Experts
Offering a full suite of solutions to boost productivity, increase product safety, protect brand reputation, and reduce recalls.
See Live Demonstrations at Pack Expo Booth C1814 September 11-13, 2023 in Las Vegas

• Metal Detection
• Checkweighing
• X-ray Inspection
• Vision Inspection
• Serialization Solutions

• Data Management and Connectivity
• Customized Material Handling
• Global Field-based Service
the integration of processing and packaging and will showcase front-of-theline solutions like homogenizing, heat treating, forming/sizing, and coating.
Additional Pavilions: PACK EXPO Las Vegas will showcase pavilions focused on various aspects of packaging and processing: The PACKage Printing Pavilion, The Containers and Materials Pavilion, The Reusable Packaging Pavilion sponsored by the Reusable Packaging Association, The Workforce Development Pavilion, and The Association Partner Pavilion
Innovation Stages: Free, 30-minute seminars on breakthrough technologies and best practices are presented daily by suppliers and subject matter experts on the show floor. The Processing Innovation Stage will offer show-floor education focused on breakthroughs in processing.
The Forum: Returning to PACK EXPO Las Vegas as an interactive industry knowledge exchange, The Forum offers free, 45-minute interactive learning sessions. Leading organizations holding sessions at The Forum include: OpX Leadership Network; CPA, The Association for Contract Packagers and Manufacturers; The Organization for Machine Automation and Control (OMAC); and PMMI Business Intelligence.
Reusable Packaging Learning Center: Here experts will share strategies on reusable packaging system implementation, which can improve material-handling performance, reduce operating costs, create new economic value, and lower supply chain environmental impact.
Technology Excellence Awards: This program recognizes exhibitors’ brand-new innovations never-before displayed at a PACK EXPO trade show.
PACK EXPO PREVIEW 32 | Healthcare Packaging • Fall 2023
�www.mt.com/pi
The Showcase of Packaging Innovations®: Sponsored by WestRock , this attendee favor -

Focus on Sustainability
Exhibitors showcasing sustainable solutions via new materials, technology, or strategies are easily identified with a PACK EXPO Green icon (next page). These include sustainable processes and machines; renewable packaging; source reduction and lightweighting; recyclable and recycled-content materials; and innovations that reduce carbon footprint. The PACK EXPO Green icon also highlights educational sessions focused on sustainability. For more, visit packexpolasvegas.com/green
The show’s official Sustainability Partner, Dow, will contribute its expertise in promoting sustainable practices by speaking on the Sustainability Stage, participating in Sustainability Central, and sponsoring all the recycling on the show floor. Increasing the number of recycling bins on the show floor provides an opportunity to not only divert as much waste as possible from landfills but also educate attendees and exhibitors on the importance of recycling.
PMMI Media Group has created the PACK EXPO Sustainability Solutions Finder (sustainability.packexpo.com), a targeted, curated list of sustainable solutions that exhibitors will be displaying at the show.
Exhibitors can participate in a post-show donation program benefiting local organizations in the Las Vegas area by donating any unwanted food, electronics, or booth materials. PMMI also will donate or recycle any remaining carpet after the show.
ite displays award-nominated and innovative packaging solutions.
Networking and Special Events
PACK EXPO Las Vegas is flush with
networking opportunities, including:
• The First-time Attendee Reception, sponsored by ProMach.
• Packaging & Processing Women’s Leadership Network (PPWLN) Break-
PACK EXPO PREVIEW Fall 2023 • Healthcare Packaging | 33
fast, sponsored by BW Packaging, Emerson, ID Technology, Morrison, Septimatech, SMC Corp, and WestRock. Hear from award-winning entrepreneur Manjit Minhas as she shares her extraordinary journey of transforming a simple idea into a colossal beer empire. Minhas will generously share her insights, strategies, and secrets to conquering the challenges of a male-dominated industry.
• Young Professionals Networking Event, sponsored by Beckhoff Automation
Don’t miss PACK gives BACK™—Private “O™” by Cirque du Soleil® Performance . PACK gives BACK features an all-new format this year—jumpstart the PACK EXPO Las Vegas 2023 show on Sunday evening, September 10, by gathering with colleagues and customers to enjoy a private performance of “O” by Cirque du Soleil at the Bellagio Hotel & Casino. Rockwell Automation is the title sponsor and proceeds benefit the PMMI Education Foundation. Tickets are available for single purchase or in blocks of 10, and include general admission seating and drinks/snacks at the theater.
Student Opportunities
PACK EXPO Las Vegas offers many programs and activities aimed at students that generate excitement about careers in packaging and processing. Students take center stage at the Future Innovators Robotics Showcase, which features local high school robotics teams that have designed and built robots. The Amazing Packaging Race, sponsored by Emerson, invites teams from colleges, trade schools, and universities across the U.S. to race around the PACK EXPO Las Vegas show floor and complete tasks or solve problems at the booths of participating exhibitors. And students looking for career resources can use Career Link to connect with companies with packaging and processing professional opportunities.
PACK EXPO Las Vegas 2023 will be the largest in its history and is not to be missed. Be sure to download the free mobile app to develop your show plans. You can also use My Show Planner to schedule your show and plan your routes. For more information on all these programs and to register now, visit packexpolasvegas.com

The PACK EXPO Green icon highlights both exhibitors showcasing sustainable solutions and educational sessions focused on sustainability.

PACK EXPO PREVIEW 34 | Healthcare Packaging • Fall 2023
CLEANING HEADS S.N.C. DI Manfredi Dante E Fabrizio.SL-6723
Fall 2023 • Healthcare Packaging | 35 BOOTH LISTING Current exhibitors as of July 11th 3CK Srl SL-6851 Accura Pharmaquip Pvt. Limited SL-6846 ADE, Inc. SL-6738 AIC / Muller USA SL-6553 Airnov Healthcare Packaging SL-6516 Alconox Inc. SL-6920 All Packaging Machinery Corp. C-3709 Alltrista Plastics LLC SL-6835 Amcor Flexibles North America SU-7254 Amcor Healthcare Packaging SL-6635 AMETEK MOCON SL-6853 Anritsu - Product Inspection & Detection SL-6163 Antares Vision Group N-9729 Ascend Packaging Systems, LLC SL-6741 Asia Eastern Media Company (Asia Package) SL-6937 AstroNova Product Identification SL-6517 Atlanta Stretch Spa SL-6653 Automatic Liquid Packaging Solutions (ALPS) SL-6924 Autonics SL-6748 Avery Dennison Corporation N-11315 AWS Bio-Pharma Technologies, LLC SL-6823 Aylward Enterprises, LLC SL-6703 Bastian Solutions N-10343 BAUSCH Advanced Technology Group SL-6622 Belden SL-6943 Bell-Mark Sales Company C-1602 BERGAMI USA SL-6619 BOLONDI
Brentwood Medical SL-6906 Brevetti Angela Srl SL-6851 Brevetti C.E.A. SL-6722 Brightly Software SL-6608 bXperts (BEXP) SL-6529 Chase-Logeman Corporation C-4636 Cilicant Inc. SL-6732 Colamark USA SL-6963 Colbert Packaging Corporation SL-6833 Columbia Machine, Inc., Columbia/Okura LLC C-2838 Com-Pac International, Inc. SL-6636 COPA-DATA SL-6961 Countec Co., Ltd. SL-6627 Cremer North America SL-6509 CVC Technologies SL-6831 Dara Pharmaceutical Equipment SL-6501 DDL SL-6752 Delta Modtech C-1446 Dorner Mfg. Corp. C-1455 DPSS Lasers Inc. SL-6519 Drug Plastics & Glass Company, Inc. SL-6731 EAGLE Certification Group SL-6941 EBAC Industrial Products SL-6603 ECM SL-6917 EFP, LLC. SL-6729 Elmach Packages PVT. LTD. SL-6703 Esco Technologies SL-6721 Esko | Brand Solutions SL-6839 ESS Technologies, Inc. SL-6604 Famic Technologies, Inc. SL-6946 Fette Compacting America, Inc. SL-6507 Fluke Reliability SL-6923 Formost Fuji Corporation C-4000 Franke Gmbh SL-6638 G&K-Vijuk Intern. Corp. N-9952 GEMCO SL-6736 groninger SL-6614 Harpak-ULMA Packaging, LLC SL-6101 Heat and Control, Inc. C-1623 HERMA US, Inc. SL-6503 HICOF SL-6850 Hitachi Global Air Power N-9520 Hiwin Corporation SL-6640 I Jang Industrial Co., Ltd SL-6934 Igb Srl SL-6922 Ilsemann Corp. USA / Heino Ilsemann Gmbh SL-6929 IME Automation SL-6919 Jadex Inc. SL-6835 James Alexander Corporation SL-6745 Jekson USA Inc SL-6825 Jones Healthcare Group SL-6911 JY Plastic Products Inc. SL-6837 Keneng Limited SL-6552 Key International, Inc. SL-6525 Keystone Package Testing SL-6521 KISICO GmbH SL-6948 Klöckner Pentaplast SL-6954 KOCH Packaging Systems, Inc. SL-6509 Körber Pharma SL-6715 KORSCH America Inc. SL-6629 Kraemer US LLC SL-6914 L.B. Bohle SL-6628 Lebal Packaging Machinery Co., Ltd SL-6932 Lifoam SL-6835 Liveo Research Inc. SL-6925 LX Pack Mexico S.A. DE C.V. SL-6927 M.O. Industries, Inc. SL-6506 M&O Perry Industries SL-6931 M3 Automation SL-6535 MACTEC Packaging Technologies SL-6907 Magnetic Products, Inc. SL-6530 Marchesini Group USA Inc. SL-6701 Markem-Imaje C-1528 METTLER TOLEDO C-1814 MG America, Inc. SL-6534 MGS Machine Corporation C-4400 MHI - Maruho Hatsujyo Innovations SL-6808 Morrison Container Handling Solutions C-1651 MRP Solutions N-9159 Multivac, Inc. SL-6601 New Age Industrial Corp SL-6830 Newman Labeling Inc. SL-6850 Company Name Booth Number Company Name Booth Number
Here we feature a selection of companies exhibiting in the Healthcare Packaging Pavilion and beyond. For full booth listings, visit packexpolasvegas.com

36 | Healthcare Packaging • Fall 2023
NJM Packaging SL-6501 Nordic Cold Chain Solutions SL-6528 OMG THERMOFORMING SL-6913 OPTIMA Machinery Corporation SL-6717 Ossid C-3033 PACK3000 SL-6536 Packaging Digest SL-6958 Packworld USA, Ltd. SL-6407 Pacmac Solution Pvt Ltd SL-6710 Pacteon Group SL-6604 Paradigm Solutions SL-6962 Paxiom Automation, Inc. C-5006 Paxxus SU-7622 Pelton Shepherd Industries SL-6942 Peoria Production Solutions N-11345 Perfex Corporation SL-6077 Perlen Packaging SL-6712 Pester USA Inc. SL-6726 Pfeiffer Vacuum SL-6812 Pharmaceutic Litho & Label Company SL-6921 PharmaMed SL-6809 Pharmapack North America SL-6617 Pharmaworks LLC SL-6501 Phoenix Wrappers CORP SL-6604 Pilz Automation Safety LP SL-6754 PKB SL-6734 Planar Motor Inc. SL-6854 Plexpack Corp. C-3036 Pluemat SL-6949 Polyplex USA LLC SL-6719 PR PACKAGINGS LTD SL-6819 Preco, LLC SL-6910 Premium Label & Packaging Solutions N-10044 Probandas America LLC N-11014 Promarksvac Corporation SL-6840 Prosys Fill LLC C-2638 PTI - Packaging Technologies & Inspection C-1841 QT9 Software SL-6508 QuickPouch SL-6714 Qwik Pack Systems SL-6645 Rasco Industries, Inc. SL-6520 Romaco Group SL-6708 RxSafe, LLC SL-6847 Sanko Kikai SL-6550 Sanner of America, Inc. SL-6740 SATO America N-9716 Scanware Electronic GmbH SL-6811 Schneider Packaging Equipment Co., Inc. SL-6604 Schubert North America LLC SL-6820 SEA Vision USA SL-6901 Sepha Ltd. SL-6711 Serpa Packaging Solutions SL-6501 Shakespeare Company, LLC SL-6835 Shanghai Baixin Material Co.Ltd SL-6938 Shawpak SL-6807 Shibuya Hoppmann SL-6005 Showy Industrial Co., Ltd. SL-6936 SIGNODE C-4814 Sonoco SL-6725 Sonoco Alloyd SL-6604 Sonoco TEQ, LLC SL-6725 Span Tech, LLC SL-6125 Spee-Dee Packaging Machinery, Inc. C-4809 Spookfish Innovations Private Limited SL-6904 Starview Packaging Machinery Inc. C-3600 Staubli Corporation SL-6951 Steelco Group - Pharma Division SL-6501 Sterimed Inc. SL-6637 STERIS Corporation SL-6957 Steven Label & Robinson Printing SL-6909 Superior Gearbox Company SL-6950 Systech SL-6649 Technipaq Inc. SL-6750 Technoflex SL-6903 Tedelta North America LLC SL-6527 TekniPlex Healthcare SL-6620 Totani America, Inc. SL-6549 Tzaw Bao Co., Ltd SL-6935 Uhlmann Packaging Systems L.P. SL-6509 Universal Machine and Engineering Corporation SL-6540 UPM Raflatac SL-6945 VALMATIC, SRL SL-6654 Viscotec SL-6621 VMI SL-6739 VORTEX SALES GROUP, LLC SL-6915 VTEC / VMECA SL-6848 WestRock C-2023 Wipotec SL-67502 Wisesorbent Technology LLC SL-6939 WLS SL-6501 Wrapade Packaging Systems, LLC SL-6522 Zhoutai Pouch Machine (Zotech) SL-6743 ZHUS CAPITAL LLC SL-6969 Company Name Booth Number Company Name Booth Number
BOOTH LISTING
Annex 1 Implementation Tips for CDMOs, ATMP Producers, and Sterile Manufacturers

MELISSA GRIFFEN, EDITOR, CONTRACT MANUFACTURING & PACKAGING
TOP THREE TAKEAWAYS
The finalized EU GMP Annex 1 Manufacture of Sterile Medicinal Products came into force August 25, 2023. The Annex 1 document came to fruition as the Parental Drug Association (PDA) coordinated 11 professional associations in Europe, working together under the umbrella of the European Federation of Pharmaceutical Industries and Associations (EFPIA) to clarify regulations and requirements for the manufacturing of sterile medicinal products.
PDA, along with the European Medicines Agency (EMA) and the Inspectors Working Group, took the lead on the discussion of the Annex 1 topic and its drafting. To download the Annex 1 document, visit the European Commission’s official website.
As manufacturers and contract development and manufacturing organizations (CDMOs) prepare to meet the implementation date, PDA is hard at work writing “Points-to-Consider” (PtC) technical documents. Among the topics the documents will cover are break-
ing down the Annex 1 requirements to the science and technology behind them, insights on how to implement the requirements, and efforts to clarify nuanced interpretations that have risen.
Machinery and filing line changes most common among manufacturers complying with Annex 1 requirements include:
• Additions or adoptions of barrier systems, such as RABS and isolators, to separate operators from the products and processes.
• Equipment and instrumentation changes, additions to active air pass-throughs, continuous total particulate air monitoring, and PUPSIT assemblies.
Most larger companies put in place gap assessments and actions to implement these changes before the approval of the revised Annex 1, but some companies have taken on a wait-and-see attitude, delaying their identification of definitive corrective action.
“The Annex 1 document has been drafted and revised more than
REGULATORY Fall 2023 • Healthcare Packaging | 37
1. The finalized EU GMP Annex 1 came into force August 25, 2023.
2. PDA is hard at work writing “Points-to-Consider” (PtC) technical documents.
3. Experts from the Annex 1 task force provide suggestions for delayed implementation dates.
15 times before becoming final. So, it’s been a moving target. Typically, CDMOs are more on the waiting side because they need to be extremely careful with their investments,” says Gabriele Gori, Head of Global Pharmaceutical Quality Assurance for Zambon, a multinational company based in Italy, and co-chair of the PDA Task Force in charge of providing comments to the revised version of Annex 1.
With implementation expected to take around one year, companies should be well on their way to becoming compliant with Annex 1 requirements. With the deadline right around the corner, any last-minute implementations may take months with writing procedures, conducting training, performing qualification, and even ordering of new instrumentation or equipment. The final version of Annex 1 is also four or five times the volume of the 2008 version, according to task force member Hal Baseman, Chief Operating Officer at ValSource Inc., a consulting firm and validation contracting group based in the U.S. Considering the increased volume of the document and the need to interpret the regulators’ intentions within each section correctly before making changes, acting late is likely to lead to a lack of compliance for some companies.
Quick tips for late implementation
Gori and Baseman suggest the following to all manufacturers and CDMOs but especially those that are behind on their implementation timeline:
• Ensure that you attend as many discussion forums led by qualified individuals as possible and participate actively in the debate that PDA is offering in order to better interpret the requirements.
• Have a conversation with your regulators and health authorities to ensure you are in line with their interpretations of the Annex 1 requirements. This is especially important in Europe, as despite best efforts, not all countries are on the same page as far as interpreting the requirements. Having a conversation earlier on with your health authorities will also allow you to see what they find most important and learn about potential possibilities for your contamination control strategy.
• If you’re a CDMO, have a conversation with key customers to come to an understanding of what has to be done and an agreement on timelines.
• Develop a plan for potential delay in implementation that includes the following three actions. First, articulate the reason for the delay and show that those reasons were beyond your control as the manufacturer or CDMO. Second, show a commitment to the proposed alternative implementation date—the regulators may not accept it, but at least they might see your company is committed to meeting requirements within a reasonable amount of time. Third and most importantly, complete an assessment of the risks posed by the delay
in implementation along with a list of controls in place to mitigate the risks and ensure patient safety.
Contract service organizations coming into compliance
CDMOs have extra pressure placed on them to have an in-depth understanding of where responsibilities for compliance fall and to properly translate the new requirements into their processes to support their clients’ compliance. If contract operations are not compliant, their reputations can suffer, which will impact their business. And as Baseman highlights, CDMOs occupy such a prominent part of the supply chain and are relied upon so heavily by the industry, that if these contractors are not compliant, the final implication would be drug shortages.
Part of the challenge to compliance, however, is that CDMOs must make both regulators and customers happy, and their customers may not always interpret regulations in the same ways. To this, Gori suggests that CDMOs create a sound plan discussed with their health authorities, and then have the confidence to defend their position and explain to their customers how they are in compliance.
“Do this rather than try to satisfy each and every client’s interpretation, because you first have to comply to your health authority,” says Gori. “You are responsible for your facility, you are the one who has the GMP certificate, not your client.”
A level of transparency and open discussion on the CDMO side will also build confidence in the customer that the company knows how to control its processes. The customers can also help the CDMOs on a practical level through well-defined roles of responsibility and quality agreements to avoid any finger-pointing and develop more than a commercial relationship but a CDMO-client partnership.
Where do ATMPs fit in?
Though an impressive step forward for industry regulations, some sections of Annex 1 can be misinterpreted or unclear. One such aspect is where Annex 1 fits with advanced therapy medicinal products (ATMPs). Baseman says it is yet unclear where regulators stand on the topic as Annex 1 is not necessarily a compliance document for ATMP manufacturers. However, if it includes aseptic processing, the principles in Annex 1 should apply.
PDA concludes that regardless of whether the Annex 1 document becomes a strict requirement for ATMPs, the principles presented in the document for aseptic processing are the same principles that should be followed by any drug manufacturer, regardless of product type.
ATMP manufacturers should therefore make themselves aware of the document and follow many of the requirements and recommendations.
REGULATORY 38 | Healthcare Packaging • Fall 2023
Benefits of implementation
Though the Annex 1 document has room for improvement and clarification, it is proof of a partnership among health authorities and industry, providing direction and ultimately benefits to public health. Implementation of Annex 1 requirements will lead to increased harmonization of requirements across the world, according to Gori and Baseman. It will also provide input to better understand manufacturing and control processes, which will result in higher process reliability and equipment modernization in sterility assurance.
The Annex 1 requirements have forced manufacturers and CDMOs to assess their processes and strive to better control them by evaluating what is important. This leads to the industry gaining knowledge and potentially more control over the processes, which could increase authorities’ confidence in the industry. Gori expresses a hope that the common ground created by the Annex 1 document will also lead to health authorities providing and heeding feedback from one another.
The document also reflects how technologically-driven the industry is with a more modern approach to sterile product manufacturing in a more collaborative effort between regulators, industry, and technology owners.
McKinsey & Co reports that the global sterile manufacturing market is expected to grow by more than 50% by 2028. Capacity growth is slowed by long installation times and a shortage of sterile manufacturing talent, but McKinsey notes that “tremendous untapped potential, however, remains in operational excellence.” Visit hcpgo. to/422 to view the report.

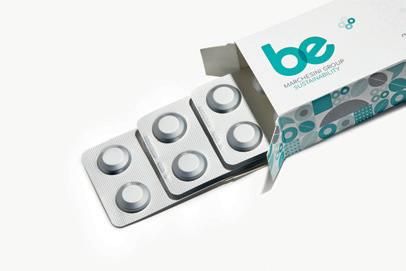

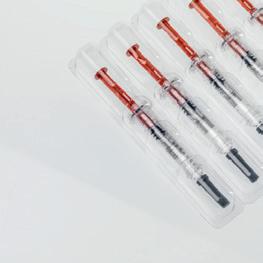
“There were several iterations of the revision and sets of comments and discussions that went into this document. I am impressed with how well the health authorities listened to the comments of the industry. What comes
out of this is not just the regulators telling us what to do, but this open dialogue [which generated] a very rich and useful document that’s more comprehensive and modern,” says Baseman.
REGULATORY Fall 2023 • Healthcare Packaging | 39
Booth 6701 Visit us at:
Environmentally responsible packaging machinery solutions marchesini.com/be
7 Takeaways from Women on the Future of Robotics

 KEREN SOOKNE, EDITOR-IN-CHIEF
KEREN SOOKNE, EDITOR-IN-CHIEF
At ATX West 2023, a panel convened to discuss women’s perspectives on the path forward for robotics. Here are seven key takeaways on boosting colleagues’ careers, innovation in the industry, and finding the next generation of automation experts. [Editor’s note: some answers have been edited for brevity.]
1. Embrace the power of sponsorship
Moderator Morgan Zandonella, Engineering Program Manager at Medtronic Neurovascular, highlighted sponsorship—an act that differs from more widespread mentorship—that can make a major impact in a person’s career. “Something that’s come up a lot recently is the difference between mentorship and sponsorship. I’m a huge
believer in sponsors,” Zandonella said. “The difference there is being that advocate when that person is not in the room. I’ve had the pleasure of having that happen—unbeknownst to me—to have someone who’s not only talking with you, but talking to others about you. That really opens up connections and opportunities that a lot of underrepresented groups don’t have. ... So to be a sponsor to advocate for others is a really powerful thing that we can all ask for and recognize.”
2. Don’t overlook mentorship
The value of mentorship is also undeniable. As Laura Armstrong, Robotics Application Engineer at Archytas Automation, noted, “I

AUTOMATION/WORKFORCE 40 | Healthcare Packaging • Fall 2023
1. Help a colleague today via sponsorship, which differs from mentorship.
2. Safety must remain paramount as robots become more friendly-looking.
3. Don’t overlook community college graduates to fill the need for robotics personnel.
TOP THREE TAKEAWAYS
can speak for myself that I wouldn’t be in the position I’m in without mentors. And I think that it’s really important—it goes back to representation. If you can see it, you can be it. One really important piece of mentorship is showing up for someone in a way that they feel seen, that they feel heard, and that they feel encouraged to keep going in this career.”
Groups like SWE [Society of Women Engineers] do valuable outreach to mentor girls/women in school, along with industry partners who pitch in with scholarships and funding, said Vinita Palaniveloo, Senior Software Engineer at Miso Robotics

All genders can add value via mentorship. “I also don’t want to discount the value of male mentors in the workforce, as Laura mentioned,” added Zandonella. “Having a mentor, regardless of their diversity identifier, means a lot to have someone that believes in you, that supports you, because as we all know, the field of engineering is very challenging, there are going to be mistakes—we’re trying to solve problems that haven’t been solved before. So, to have a mentor that can really support and walk someone through that, whether it’s someone younger or older or a peer, I think it’s incredibly valuable.”
3. Stay vigilant about safety
Courtney Fernandez, Application Engineer at Universal Robots, is optimistic about all the different ways she’s seeing robots utilized now, far beyond the widely adopted pick-and-place robot for manufacturing. “I’ve programmed robots that were part of a dance production. I’ve seen robots playing with toys. At this point, I’m curious to see what somebody thinks of to do with a robot, because it’s not just restricted to this industry anymore,” she said.
But she also reminded attendees not to become complacent around safety: “The other thing I would be cautious of is as these
robots become friendlier looking… we have these humanoid robots, and I work for a company that makes a collaborative robot arm. But we can’t get complacent around this machinery because it’s still machinery. So it looks friendly, it’s getting easier to use, and it’s becoming more accessible for everybody. But it’s still a robot. If you go willy-nilly around any piece of machinery, it has the potential to cause a problem if you don’t treat it with respect. So as things get easier to use, and more friendly looking, it’s still a machine, and we still need to have training and appropriate respect for it.”
4. Implement work-life policies that benefit all Statistics for women working in engineering roles are often quite low. Palaniveloo hopes to see increased participation by women in the field, and noted recent statistics that show that 30% of the engineers who graduate are women, but they only make up approximately 14% of the engineering workforce. Many drop out after they graduate or leave the profession. “It all starts with the employer, creating a place to express and bring [employees’] creativity out. The second thing that’s important to me is that I go back to a study by Kenan Institute of Private Enterprise, which highlights the need for female-specific work benefits,” she said. Though not everyone chooses to have children, these include maternity leave, and flexibility for childcare and parenting emergencies.
Zandonella added that “those types of benefits benefit all employees, including the men that are fathers of those kids. A company policy that gives paternity leave is allowing that woman to also be empowered and be able to go back to work. So I feel that by offering those benefits, maybe at the urging of fellow women colleagues, it’s really going to benefit the entire workforce. Because those benefits again are being applied favorably to everyone.”
5. Recognize the business value of inclusion
While conversations around promoting diversity in engineering and manufacturing often jump ahead to solutions, it’s important to frame why diversity and inclusion are so important (aside from the obvious: being ethical).
Armstrong noted that when you have a diverse team, you have more creativity, knowledge, and experience. She added, “If we all look the same, we all are the same, we’re only going to have a very narrow experience and vision for what we could create or could do together.”
Those differing perspectives can be key to viewing problems in a more solvable way.
AUTOMATION/WORKFORCE Fall 2023 • Healthcare Packaging | 41
IME Panelists from left: Laura Armstrong; Courtney Fernandez; Vinita Palaniveloo; and moderator Morgan Zandonella.
“I’m sure everyone has seen the meme where you’re looking at a cylinder from one side, and from the other side, it looks like a square. Perspective matters. If you have multiple viewpoints... to be able to look at a problem, you can solve it from multiple angles, which I think is really important,” said Fernandez. “Regardless of race, age, gender, we all have these different skillsets that can be utilized. And it’s not like we’re walking around wearing a label of what those skillsets are.” She said you won’t know that skillset until you’ve bothered to extract it from someone, or challenged the person to use it.
Armstrong added that no one skill is better than another and that it’s important to acknowledge that you’re all on the same playing field when tackling a challenge, regardless of title or role.
An organization will be far more successful when a variety of voices are being respected and included. Zandonella said, “I think that’s why so often we see the words ‘diversity’ and ‘inclusion’— how they get lumped together—it’s because it’s one thing to have diversity. But if you don’t have that inclusion piece of valuing that opinion, of having space in that environment where you feel safe to share your ideas, that diversity is lost. It doesn’t add value then because you’re not seeking it and truly using it to its full potential.”
6. Invest in education and don’t overlook community colleges
Prior to her work at Archytas, Armstrong spent most of her career in education, most recently teaching high school math and engineering. While Archytas has engineers that work on the primary business of integrating robotics for small- and medium-sized business, she is dedicated to developing educational content in her role. “My bosses are very supportive. Part of why they hired me was because of my educational background… we are primarily working with the community college level right now. We actually did a survey last year for hiring managers within manufacturing and 87% of them said that they wish that their new employees had more robotics experience. And 71% of them said they’re looking to the community college level for those new hires. That was higher than for four-year degrees, so associate’s degrees, two-year degrees,” she said. “When we talk with educators, we find that they know that but they’re having a hard time getting students. They’ve told us that there are companies that have already hired students before the student even arrives on campus, that’s how much they are in need of somebody to work on a robot. And so that is a part of my job, but I will say I’m very, very lucky in that sense, because I do think education sometimes becomes a philanthropic thing and I’m fortunate to work for bosses who support education. It’s not an afterthought, it’s leading the way.”
Universal Robots also invests in education, offering an education discount, and an Edu kit for purchase for technical colleges. “I spend one week a month doing training courses for people using these robots. We work with another instructor that teaches classes that are
only other educators. So [attendees] won’t be end users of robots, but teachers who will buy a robot and use it in their classrooms,” said Fernandez. “[UR] also has free online academy training—hours of totally free training. When I entered the industry about 11 years ago, any training I could get for under $500, I was all over it, so I think they do a really good job of making training affordable for people.”
7. Bolster complementary skills
Palaniveloo explained she’s optimistic about the job opportunities that robotics will open up. “What I like to be cautious about is we want women to get into robotics and engineering, and there’s no doubt about the capability to do it. But we need to train them on other skills, such as training them how to handle the intellectual property… they might be creating a lot more of that. They can be entrepreneurs, so let’s educate them on that,” she said.
Where is automation shifting?
Palaniveloo said she envisions more autonomous manufacturing taking place in the coming few years, and drew attention to the recent publication, Research on Structural Performance and Assembly of Led Lamps Based on Automatic Manufacturing, which is published in the Journal of Interactive Design in Manufacturing. “These robots are going to be very affordable, and they are going to be very agile in that you can program them for one multifaceted operation,” she explained. “So in my perception, instead of having different workstations being specialized to perform one function, you’re going to have one unit that can actually pick-and-place, assemble, and then label, pack, and send it so it’s going to be more like the Amazon warehouse style of automated manufacturing.”
As robotics are implemented more and more in manufacturing, Armstrong said, “I’m passionate about education. For me, personally, I like to focus on that in a way of ‘Who’s going to fix those robots when they break? Who’s going to design the new technology? Who’s going to use these robots to integrate into systems? How are they going to be programmed?’ So I think a big part of this, too, should focus on education and the training of humans to do these jobs. You know, there’s a lot of myths out there, one being that robots are going to replace humans. Well, they’re not going to really replace humans if these humans are using the robots in applications, in integration, and in learning how to fix the broken robots. So a big part of this involves education, training, and the engineering side of it.”
For the latest in robotics solutions, including show-floor demos and interactive discussion, make plans to visit PACK EXPO Las Vegas (Sept. 11-13, 2023; Las Vegas Convention Center). Also, save your spot for the Packaging & Processing Women’s Leadership Network breakfast on Sept. 12 at the show. To register for the show and the breakfast, visit packexpolasvegas.com
AUTOMATION/WORKFORCE 42 | Healthcare Packaging • Fall 2023







































































Discover your next packaging or processing solution fast on the free PMMI ProSource directory. Find validated suppliers by using the Package Type lter, such as Flexible Bags or Rigid Bottles. Browse categories, such as Filling, Capping & Closing or Processing Equipment. Narrow down your search using lters within a category, such as Capabilities or Fill Type d li t d li Search by package types: Liquid Fillers (11 companies) Entry-level/budget models available Hot Fill r (8) Fill Type Capabilities Fill by time (6) Fill by weight (7) Hot fill (11) Volumetric (9) Entry-level/budget models available (11) IIoT ready (6) Tool-less changeover (8) www.prosource.org
1 Decontamination Tunnel

IMA Group
+ Using aerodynamic containment of highly concentrated vapor phase hydrogen peroxide, the IMA Nebula achieves 6-log decontamination over the entire surface.
+ The system allows for continuous, high-speed tub introduction, up to 6 tubs/min.
2 Capsule Filling Machine






Syntegon

+ Filling up to 360,000 capsules/hr, the GKF Capsylon 6005 has 48 segment bores in four rows and a click system that ensures faster changeovers.



+ Covering the segment bores from below during filling, a slide-gate allows pellets or granules to be processed on the same dosing station.


3 Pharmaceutical Glass Vials


Corning
+ Corning Viridian vials use 20% less glass material than conventional glass vials, decreasing waste. A low-friction external coating minimizes cracks, breaks, and cosmetic rejects.




+ The reduction in material lowers manufacturing and transportation-related emissions by up to 30%.



NEW PRODUCTS Automation From
CONVEYING WEIGHING/ COUNTING FILLING CAPPING LABELING CARTONING PACKING PALLETIZING paxiom.com 1.833.4PAXIOM | info@paxiom.com ¿ƝŪ PaxiomGroup } PaxiomXperience Visit us in Las Vegas, Miami, Montreal or Toronto Over 30 years of experience delivering automated packaging systems. to see our solutions in action. Our team is ready for your next project.
a Single Source ng
4 Blister Cutter
Azco





+ Integrating with new or existing equipment, the Azco blister cutter provides different sizes of blister cards without expensive tooling, a punch station, or long changeovers.
+ An optic sensor tracks the blisters, ensuring cross-cuts are in the proper location.


5 Pouch Seal Inspection System

PTI
+ The PTI Seal-Sensor PQX seal inspection and handling system is adjustable for different sizes of pouches.

+ The fully automated unit incorporates ultrasonic technology to automatically scan and inspect final pouch seals 100% online and non-destructively.
6 Recycle-ready Flexible Film


Charter Next Generation (CNG)
+ GreenArrowRx flexible forming film for the healthcare market is built from readily available, recycle-ready resins.



















+ The film delivers seamless validation and fast qualification on existing flexible packaging lines; it is designed to help ease supply issues often associated with traditional resins.





Experience tamper evidence like never before. Top TabTM 2 TamperSeal o ers a half-moon tab, the industry standard in consumer convenience, which eliminates the need for unsanitary tools or finger punctures. The secondary liner ensures the packaging can be resealed providing leak protection and enhanced freshness.
No more compromising on consumer convenience. Our advanced technology guarantees a consistently robust hermetic seal, complemented by a generous induction process window for hassle-free application.
With Top TabTM 2 TamperSeal, you can trust that your products remain protected and your customers have peace of mind.
Choose the future of tamper-evident packaging today.














NEW PRODUCTS Introducing Top Tab 2 TamperSeal The
TM
Ultimate Tamper-Evident Solution
www.seliggroup.com/solution/toptab
COMPANY PROFILES










This special section of company profiles includes many companies who will be exhibiting at PACK EXPO Las Vegas, Sept. 11–13, 2023. These profiles highlight the companies, the markets they serve, and the packaging equipment, products, and services they offer.

Columbia Machine, Inc. 47 Delta ModTech 48 ESS Tecnologies, Inc. 49 Formost Fuji Corporation 50 G&K- Vijuk International 51 Heat and Control, Inc. 52 HERMA US Inc. 53 James Alexander Corporation 54 Klockner Pentaplast, Pharma, Health & Protection and Durables 55 Marchesini Group USA 56 METTLER TOLEDO Product Inspection 57 MG America 58 MULTIVAC 59 NJM, A ProMach Product Brand 60 Ossid LLC 61 Packworld USA 62 Paxiom/WeighPack Systems, Inc 63 PAXXUS - Global Flexible Healthcare Packaging 64 Pharmaworks, A ProMach Product Brand 65 ProSys Fill LLC. 66 Selig Group 67 Spee-Dee Packaging Machinery, Inc. 68 STARVIEW PACKAGING MACHINERY, INC. 69 Weiler Engineering, Inc. 70 WLS, a ProMach Product Brand 71
PROFILES
Reduce Contamination in Production Areas
The Load Transfer Station (LTS) product line, offered by Columbia Machine, helps to reduce contamination from entering production areas, streamlines operations & reduces costs. Columbia manufactures a complete line of pallet transfer solutions including stand-alone stations to fully automatic solutions that allow pallet load transfer to be completed in less than one minute, without requiring the operator to leave the safety of the forklift.
Industry Leading Standard Safety Features
Columbia Machine’s LTS solutions come standard with the latest safety features, including Category 3 electrical safety components, up-stacker guarding with A-B SensaGuard RFID interlocked door switches, multiple emergency stops, and muted light curtains on the auto -
mated systems. Every Columbia Machine LTS exceeds the requirements of pharmaceutical processors working to meet FSMA regulations.
Flexible Product Handling

Columbia’s LTS is capable of transferring products that are packaged in cases, super sacks, glass vials, pails, barrels, drums and bags from one pallet type to another, including Plastic, Chep and GMA pallets that are commonly used in both receiving and shipping applications.
Columbia Machine, Inc.


The Load Transfer Station (LTS) product line is part of the Palletizer Division of Columbia Machine, a leading American palletizer manufacturer. For more than 80 years, Columbia has manufactured complete palletizing and material handling solutions.

The mission at Columbia Machine is to be the preferred supplier of engineered product solutions in the targeted markets we serve.
We provide exceptional customer value through strategic marketing, innovative product development and unparalleled customer service. We value safety, integrity, trust, fairness, professionalism and teamwork in relationships with our customers, employees, business partners, suppliers and shareholders.
We “always” see our business through “the eyes of our customers,” and provide them with superior solutions through innovation, quality, reliability and continuous improvement.
PHONE EMAIL WEBSITE
www.loadtransfer.net pallsales@colmac.com 360.694.1501
BOOTH C-2838 See our ad on page20 107 Grand Blvd., Vancouver, WA 98661 Fall 2023 • Healthcare Packaging | 47
Columbia Machine, Inc.
Delta ModTech
We deliver web converting and packaging systems for the most complex and demanding jobs in the medical, pharmaceutical, label, RFID and cosmetic industries. Our systems are designed to meet your application requirements, improve your profitability, and reduce your risk.
contact@deltamodtech.com
www.deltamodtech.com
For over 40 years Delta ModTech has been a leader in web converting and packaging innovation. We are dedicated to providing flexible automation solutions for converters and manufacturers worldwide.


Delta ModTech systems feature a variety of processes including heat-seal pouching, rotary die cutting, laser cutting, tight tolerance registration, printing, laminating, coating and drying.
Seal to print registration — The packaging solution for process applications where speed, flexibility and consistent seal quality are important. For the manufacturing of:
• eat-seal/Cold-seal pouches
• Resealable pouches
• Li uid dispense pouching
• Sachet filling
Improve your capability — We’ve developed several solutions to meet the pouching needs of our customers and their end users.
• Mod-Track Vision Inspection – Part-inpouch, closed loop registration, date/bar code reading, rejecting
• Re ect conve ors/marking e uipment
• Part handling stacking, sorting, etc.
• Printing for date, lot and bar code printers
• Case packing and cartoning integration
Packaging & Pouching Machine Specifications

• Web Width mm , mm , mm , Custom Widths Available
• Max speed – Reciprocating Packager ft/min m/min , ft/min . m/min
• Seal Repeat – Up to mm
• Delta ModTech INTELLI-MOD controls
• Drive S stem Servomotor Control
• User Controls Touchscreen MI Pendant Arm
• Welded Steel Frame, Aluminum Front Plate
Service and Support — Serving our customer’s has always been Delta’s highest priority. We focus on quick, efficient solutions to keep your machines running to their full potential. To back up this philosophy, we have a full-service staff on call, made up of Engineers and Technicians. We have the process knowledge and proven modules to ensure the longevity of your machine.
EQUIPMENT SOLUTIONS FOR A VARIETY OF PRODUCTS:
Diagnostics, Drug Delivery, Ostomy, Wound Care, Personal care, Electronics, Battery
See our ad on page 34

PHONE EMAIL WEBSITE
. . • . .
BOOTH C-1446
48 | Healthcare Packaging • Fall 2023
8445 Bunker Lane Blvd. NW, Ramsey MN
ESSTechnologies, a Pacteon Company

540.961.5716
info@esstechnologies.com
www.esstechnologies.com
ESS Technologies, a Pacteon company, designs, manufactures, and installs automated packaging lines for pharmaceuticals, diagnostics, medical devices, animal health, cosmetics, nutraceuticals, and consumer packaged goods. Pacteon Group provides one source for endof-line packaging solutions. As an authorized FANUC system integrator, ESS designs, installs, and supports all your end-of-line automation needs. ESS also designs custom robotic end-ofarm-tooling (EOAT), which allows the precise handling of components and packaging materials. Our packaging line solutions include:
o Horizontal Cartoners
o Vertical Cartoners
o Tray Erectors and Loaders
o RSC Case Packers
o Wrap Around Case Packers
o Robotic Pallet Cells
o TaskMate Robotic Systems®

ESS robotic systems, cartoners, case packers, and palletizers are engineered to meet all applicable standards and production requirements. ESS is a UL-certified builder of electrical panels. We do not lock any portion of the PLC or robotic code in our controls, and ESS provides all OEM part numbers in the Bill of Materials. ESS engineers work closely with our customers to understand the level of automation required, physical limitations, OSHA requirements, flexibility, and future expandability. The result is an ergonomic and cost-effective production line that requires minimal operator training and can be easily retrofitted for future applications. ESS has extensive experience integrating serialization systems to meet pharmaceutical serialization mandates.

ESS and all the Pacteon Group companies bring integrity, pride, accountability, customer focus and teamwork to every packaging line we install. This is our iPact: Integrity - Build trust through honesty and transparency.

Pride - Committed to our Pacteon Promise for our customers, selves, owners, communities.
Accountability - Say what we do and do what we say.
Customer Focus - We listen with purpose, respond with urgency, and deliver with quality.
Teamwork - Together everyone accomplishes more.
The Pacteon Promise is “We make it right.”
See our ad on page 26
PHONE
EMAIL WEBSITE
BOOTHSL-6604
Fall 2023 • Healthcare Packaging | 49
3160 State Street Blacksburg, VA 24060
Formost Fuji Corporation
First and Formost, we strive to put you the customer first. Meeting and exceeding your expectations is our gold standard. Whether it be in design, manufacturing, or service, you can expect Formost Fuji to deliver a solution you are proud of. Throughout 55 plus years in this business our ideals have remained the same; build it right and deliver on time, all at a value to our customers.

www.formostfuji.com
As a manufacturer of horizontal flow wrap machines and horizontal bagging machines, Formost Fuji has earned an outstanding reputation for equipment quality, dependable parts and service, and engineering know-how. Formost Fu i flow wrappers FFS are designed to operate efficiently while providing a strong, dependable seal. The Formost horizontal bagging machines have been the most reliable on the market for nearly 60 years.
Our orizontal Flow Pack Wrappers are built with an eas -to-operate MI that includes graphics and step-b -step instructions. The MI can be customized for simplicit with icons or photos representing each product for a one-touch changeover. This innovative flow wrapper has a simple-to-thread film process that saves time and a shortened film route that saves money. The center fin seal unit tilts down allowing easy access for sanitation and maintenance.
B Box Motion End Seal Technolog offers four times the sealing pressure and longer dwell time with improved design. This provides high performance hermetic seals on difficult-to-seal films.
The Formost Bagging Machine provides performance proven technology with speed, versatilit and dependabilit . Built to run up to 90 bags per minute, it is ideal for gently bagging a wide range of products including medical drapes.

We provide sustainable and efficient packaging solutions for the healthcare industry, including masks, IV bags, inhalers, blister packs, syringes, tube sets, and many other medical and pharmaceutical products, which are wrapped and bagged on performance proven Formost Fuji equipment.

Our team of experienced people listen to you, and understand your needs.

• orizontal Wrappers
• Baggers
• Automation Solutions
• Special Applications
PHONE EMAIL WEBSITE
sales@formostfuji.com 425.483.9090
BOOTH C-4000 See our ad on page 15 905 80th Street SW, Everett WA 98203 50 | Healthcare Packaging • Fall 2023
G&K—VIJUK INTERNATIONAL

630.530.2203
info@guk-vijuk.com
www.guk-vijuk.com

To save time and labor, invest in GUK packaging-line equipment that runs on the packaging line’s drive. GUK–Vijuk Cartonac Leaflet Folders enable you to fold leaflets inline and immediately insert them into cartons along with the product. The servo-driven GUK Cartonac 2005 has a hinged feeding table for easy access to the fold plates and rollers. Improved suction rollers and air blasts effects efficient feeding of products— up to 3 thicknesses. The PLC controlled Cartonac 2003DS sets up easily and quickly and is servo driven for better control and speed.
Pre-folded leaflets can be fed in-line for insertion into cartons with GUK PA21 Leaflet Feeders. The PA15 model can rotate leaflets 90º for feeding, while mounted parallel to the packaging line to save space. These may also be used with gluing or tabbing equipment in offline leaflet bundling systems.


GUK–Sigma Pick & Place Stations precisely tip flat or 3-dimensional items onto products in
production lines.
Make spine-glued booklet leaflets on the GUK FA53 Folder. Fold large med-guides on sheets up to 28-3/4 inch wide on the GUK FA73 Folder. Choose from a number of G&K–Vijuk Outsert Systems with varying capabilities — from folding outserts with up to 90 panels, to up to 350 panels.
Meet TRACK & TRACE requirements with the G&K–Vijuk CTM Coding and Serializing Station. Print unique or standard 1- and 2-dimensional codes on outserts. A quality verification system logs all results, good and bad, for a tab on missing numbers in a series.
Save time and labor with the GUK–Sigma PPM Auto Stacker, which automatically collects and packs outserts compactly into trays.
GUK RS Roll-Fed Folders save paper, cutting, and labor costs—and the probability of leaflet mix-up.
World leader in outsert-producing machinery, G&K–Vijuk has been specializing in miniature-leaflet folding solutions for over 40 years.
Member of the GUK Group headquartered in Germany (manufacturer of folding machinery since 1949), we continue to assess the needs of the pharmaceutical industry, as shown by recent developments in machinery to fold a greater number of panels and fold wider sheets—both for more print space on a single leaflet; and machinery for bundling leaflets for dispensing even more information on two to four leaflets.
GUK recently acquired MB Bäuerle, manufacturer of automated folding and inserting systems in Germany, and Sigma Engineering, manufacturer of pick and place product-handling machinery in the Netherlands to further our goal to provide innovative, time-saving equipment to improve our customers’ efficiency.
PHONE EMAIL WEBSITE
BOOTH XX-00000
our ad on page 8 715 Church Road, Elmhurst, Illinois 60126 Fall 2023 • Healthcare Packaging | 51
See
Heat and Control, Inc.
800.227.5980

info@heatandcontrol.com
www.heatandcontrol.com
Anywhere along the line, protect your consumer and your equipment. Efficient detection of foreign objects is critical to consumer safety, brand survival, and will also protect machinery and prevent downtime. We offer a complete line of X-ray inspection systems, metal detectors, and checkweighers from our strategic partners: CEIA® and Ishida.

X-Ray
The photon counting dual energy IX-PD series X-ray machine employs an alternative sensor and accompanying image processing technology to give our highest sensitivity and accuracy of lowdensity and minute foreign object contaminant detection. This technology differentiates with high accuracy between product and foreign objects, reducing the rate of erroneous detection.
Metal Detection
CEIA, is a world leading innovator of industrial metal detection systems for products such as powders, capsules, tablets, and liquids. Quality control is at the core of CEIA development of the most advanced electronic and mechanical technologies for detection of contaminants accidentally present in products

Weighing
The new Ishida CCW-AS (Advantage Series) multihead weigher’s advanced automation raises the bar in high-speed challenging weighing applications, delivers unbeatable value, improves manufacturing and packaging efficiencies to assure a fast return on investment, and improves product quality and safety. The latest technology developments result in higher accuracy, reduced giveaway, faster speeds—improving volume output, and gentler handling for better product quality and pharmaceutical safety.
Secondary Rising to the challenge, advancing processes, and helping to bring the best products to the world using science, technology, and creative thinking.
We are process and product technologists committed to advancing pharmaceutical and other industries with science and imagination. Whether you measure success by efficiencies, improvement, or innovation, count on us to deliver results.
Providing sales, service and spare parts expertise across the globe for metal detection, X-ray and checkweighing anywhere along a production line, Heat and Control works closely with strategic partners to bring new technologies and solutions that meet the strictest of quality standards.
To view the entire line up of pharmaceutical solutions offered please visit: www.heatandcontrol.com/solutions/ pharmaceutical

PHONE EMAIL WEBSITE
BOOTH C-1623 See our ad on page 27
52 | Healthcare Packaging • Fall 2023
21121 Cabot Blvd. Hayward, CA 94545
973.521.7254
www.herma.us info-usa@herma.com

RECONFIGURED HIGH-SPEED LABELER MEETS EVOLVING PHARMA PRODUCTION NEEDS
At Pack Expo, HERMA US will debut a redesigned, updated version of its flagship 132M Wraparound Labeler. Faster and more aligned with evolving pharma production lines, the 132M HC includes a sophisticated yet simplified touchscreen HMI, improved ergonomics, and an output ceiling of 600 products/min – a 50% uptick. Primarily designed for glass vials and round pharma containers, the labeler offers rapid, tool-free changeover of rotary tables, electronic position indicators and format sets, and the HERMA 500 label applicator combines labeling speed with exacting precision.

The reconfiguration exemplifies HERMA’s effort to streamline its equipment portfolio. Dubbed Clean Design, the approach encompasses several manufacturing-centric elements, from faster ordering and greater modularity to easier cleaning and enhanced sustainability.
Add-ons like printer integration, side belts and wraparound rollers now come standard on many HERMA labelers; units also are easier to transport between lines and, once moved, flexibly engage with differing production scenarios.
ULTRA COMPACT LABELER FOR SMALL-BATCH & BIOPHARM PRODUCTION

The HERMA 211 HC Wraparound Labeler meets demand for fully FDA-compliant labeling in a highly compact footprint. The semi-automatic unit is particularly helpful in the transition from clinical trials to full production, and for smaller-batch manufacturing typically found in biopharmaceuticals settings. The labeler is suitable for a wide range of cylindrical products including syringes, tubes, glass vials and ampoules. Capable of applying approximately 30 labels per minute, the HERMA 211 HC can handle webs as wide as 80mm, and products ranging in diameter from 10-120mm.
HERMA US Inc. is a subsidiary of HERMA GmbH, a Germany-based provider of labeling machinery and self-adhesive labels and materials to the global packaging marketplace. HERMA GmbH’s comprehensive range of products spans the labeling production process to include labeling machinery, a variety of adhesive materials, and finished self-adhesive products.

In the United States, HERMA is best known for its equipment. HERMA’s flexible labeling machines are designed, developed, and built for integration into industrial processes, while its self-adhesive paper and film compounds are manufactured with unsurpassed precision. The company’s range of finished adhesive products includes labels for a broad set of industries, including healthcare and pharmaceuticals, automotive and electrical, chemicals, food, cosmetics and logistics. The company’s three divisions comprise nearly 1,000 personnel.
See our ad on page 1
PHONE EMAIL WEBSITE
BOOTHSL-6503
39 Plymouth Street,
300,
NJ 07004 Fall 2023 • Healthcare Packaging | 53
HERMA US, Inc.
Suite
Fairfield,
James Alexander Corporation


908.362.9266
James Alexander Corp. has added 18,000 square feet to its warehousing capacity, providing more space for servicing pharmaceutical (OTC & Rx), medical devices, health & beauty products, first aid and diagnostics sectors.
JAC’s patented single-use plastic ampoules are available in a variety of colors and applicators, offering singlehanded activation in a customizable format. JAC’s glass ampoules can be filled and assembled in single-use swab or dropper packages, and its novel winged device, THE ACTIVATOR™, provides easier activation for these glass formats. Other services include autoclave sterilization for glass ampoules, blister packaging and formula compounding.
PLASTIC UNIT-DOSE DISPENSING SYSTEMS
James Alexander Corp.’s plastic ampoule combines style and ease of use through singlehanded activation. With a gentle squeeze, the inner membrane ruptures, allowing contents to

be dispensed. Available in sizes up to 5ml, and a range of colors and applicators.
UNIT-DOSE GLASS SWABS
James Alexander Corp.’s unit-dose swabs offer the stability of glass in one- or two-part systems for convenient application of pharmaceuticals and health aids. JAC also produces single-use glass ampoules for inhalation and dropper tip assemblies for liquid dispensing.
THE DUODISPERSION SYSTEM® TANDEM PACKAGE
Safe and easy to use, the DuoDispersion System® tandem dropper or swab can hold two separate liquids, or a powder and a liquid; each formula is sealed in its own ampoule until the point of application. It can hold a combined volume of 1.2mls. Two versions are available: a dropper tip and a swab for topical application.

Located in northern New Jersey, James Alexander Corporation (JAC) is a leading contract manufacturer and custom filler of single-use crushable glass and plastic ampoules. Founded in 1976 by Francesca Fazzolari and Alexander Davidson, JAC is a privately-owned, ESOP company that still services several of the same customers it originated with 45 years ago.
JAC’s manufacturing facility features unique, company-designed equipment and produces its patented plastic ampoules, among other products. The company makes great efforts to ensure that most of its components are made in the USA, aligning with its goal of investing in local communities, regional job markets and the American manufacturing sector at large.
Currently, James Alexander is developing new and innovative materials for JAC’s plastics ampoules. Among other aims, the goal is to make the containers compatible with alcohol-based liquids.
See our ad on page 3
PHONE EMAIL WEBSITE
www.james-alexander.com info@james-alexander.com
BOOTHSL-6745
Route 94, Blairstown, NJ 07825 54 | Healthcare Packaging • Fall 2023
845
Klockner Pentaplast, Pharma, Health & Protection & Durables


540-832-3600
kpinfo@kpfilms.com
www.kpfilms.com
Focused on delivering its vision: The Sustainable Protection of Everyday Needs, kp is a global leader in rigid and flexible packaging and specialty film solutions, serving the pharmaceutical, medical device, and shrink sleeve label markets, amongst others. With a broad and innovative portfolio of packaging and product films and services, kp plays an integral role in the customer value chain by safeguarding product integrity, protecting brand reputation, and improving sustainability. kp’s “Investing in Better” sustainability strategy solidifies its commitment to achieving ten clear targets for longterm improvement by increasing recycling and recyclability of products, cutting carbon emissions and continuous improvement in employee engagement, safety, and diversity, equity and inclusion. kp has earned a gold rating from EcoVadis, the leading platform for environmental, social, and ethical performance ratings, putting kp in the top 3% of companies rated in the manu-
facturing of plastics products sector. Founded in 1965, kp has 31 plants in 18 countries and employs over 5,700 people committed to serving customers worldwide in over 60 locations
The sustainable protection of everyday needs, is not just a slogan to kp, it is a way of life. We have invested heavily across all the market segments we service, in the development and production of recyclable and recycled content packaging films. Chief among these markets is the pharmaceutical blister films market. We are proud that our kpNext® R1 pharmaceutical blister film was the first to market as a completely recyclable blister film in the RIC 1 recycling stream and remains the top choice for many major brands globally! We have since expanded the kpNext® family to include a certified recyclable polypropylene blister film with enhanced barrier characteristics called kpNext® RB5. When it comes to sustainable packaging for pharmaceuticals, think of kp and kpNext® brand of products.

PHONE EMAIL WEBSITE
BOOTHSL-6954
See our ad on page 11
Fall 2023 • Healthcare Packaging | 55
3585 Klockner Road, Gordonsville, VA 22942
Marchesini Group USA

973.575.7445
sales@marchesiniusa.com
www.marchesini.com

We are a leading supplier of primary and secondary packaging machines and lines to the pharmaceutical, biotech and cosmetic industries. Established in 1992, our 23,000-squarefoot North American Headquarters supports the USA, Puerto Rico, and Canada and is backed by 2,500 Marchesini staff members worldwide. This modern facility is equipped and staffed to meet the ever-increasing demands of today’s sophisticated and quality-conscious packaging clients.

Our Group provides stand-alone machines and complete lines to handle the entire packaging process of pharmaceuticals and cosmetics. The finished product is a combination of craftsmanship integrated with robotics and new technology.
Thanks to 30 years of experience, our team of experts comprehensively analyzes and defines the customer’s packaging requirements to translate technical challenges into effective, unique solutions. By combining the most-advanced
technology with our knowledge and understanding of the pharmaceutical, cosmetic and consumer products industries, we have made solving packaging challenges our specialty.
All precision packaging machinery available through Marchesini Group USA has been manufactured to meet the highest level of quality certification using established production benchmarks and testing procedures. Our solutions are characterized by high quality, reliability, flexibility and customization.
At Marchesini Group, customer service is not a department, it is a commitment to our company philosophy and a realization that our steady growth can only be maintained through customer care. Whether our customer’s packaging solution requires a new installation, a modification or retrofit, we are here to help optimize productivity and maximize uptime to get the best results and the highest output.
“It has been almost fifty years since my father, Massimo, developed the first cartoning machine branded Marchesini. He started his business in his garage at home, in Pianoro, just outside Bologna, but soon expanded thanks to the abilities of a man who had worked for years as a skilled technician for a leading manufacturing company of automated machines.
Over time, we have grown without giving up the quality that characterizes the made in Italy production. Today we export 90% abroad, thanks to our employees, collaborators, suppliers and to the entire supply chain.
The packaging industry is indeed one in continuous evolution and to remain competitive we have to constantly deal with technological innovation. We are the right partner where customers will find the best cutting edge solutions for their specific needs.”
Maurizio Marchesini, President

PHONE EMAIL WEBSITE
BOOTHSL-6701
our ad on page 39
56 | Healthcare Packaging • Fall 2023
See
43 Fairfield Place, West Caldwell, NJ 07006-6206
METTLER TOLEDO Product Inspection

PHONE EMAIL WEBSITE

813-889-9500
www.mt.com/pi pi.marketing@mt.com
METTLER TOLEDO is your single source for product inspection solutions offering metal detectors, x-ray inspection systems, checkweighers, machine vision systems, Track & Trace, serialization, and aggregation solutions. Our broad product inspection line ranges from very basic and economical systems to sophisticated, state-of-the-art systems with customized material handling solutions. Systems can ensure perfect product presentation, create codes, verify package and label integrity, ensure weight range compliance, provide tamper-evident sealing, detect physical contaminants and inspect contents inside the closed package.
PCE Track & Trace systems help you comply with regulations including the new DSCSA Act. Comprehensive solutions are available for Track & Trace programs with serialization and aggregation software, hardware and product handling using stations or integrated kits for existing pack-
aging machinery. Scalable and module solutions offer serialization of saleable item and aggregation to bundles, case and pallet. Serialization data is captured and recorded for each line and for the site with completion of production orders and communicated to MES/ERP/Cloud systems to enable full traceability across the packaging line and site as well as traceability across the supply chain.
CI-Vision machine vision systems perform accurate label and package quality inspections on all types of packaging, including label quality, 1D, 2D, and alphanumeric text, proper sealing, cap and lid placements, and tamper band presence.


Safeline metal detection systems are extremely sensitive, easy to use, and prevent costly recalls by ensuring your products are free of ferrous, non-ferrous and stainless-steel
contaminants which can be introduced during processing.
Safeline X-ray inspection systems can detect contaminants including stone, glass, and ferrous, non-ferrous, and stainless-steel metals, as well as detect mass and check for missing or damaged product inside closed packages.
Hi-Speed checkweighers are available in a wide range of sizes and configurations to handle nearly any container type or package design at high speeds to ensure accurate product delivery, reduced giveaway, and an optimized production process.
We also offer connectivity and data management solutions and global service support for increased productivity and profits brand protection, and regulatory compliance.
See our ad on page 32
BOOTH C1814
1571
Fall 2023 • Healthcare Packaging | 57
Northpointe Parkway, Lutz, FL 33558
MG America
31 Kulick Rd, Fairfield, NJ 07004
PHONE
EMAIL
WEBSITE
973-808-8185
sales@mgamerica.com
www.mgamerica.com
Headquartered in Fairfield, New Jersey, MG America is a subsidiary of MG2 of Bologna Italy, a company that was founded in 1966 and today is one of the world’s three leading manufacturers of capsule filling equipment.

MG America is a leading supplier of an innovative family of precision-crafted processing and packaging machinery that includes capsule fillers, material handling, primary packaging equipment, secondary packaging equipment, checkweighing/weight control systems, tablet & capsule inspection, and line integration solutions. From sales, field service and spare parts to machine trials and local service/support representation, MG America offers a true “Partnership for Success.”
Packaging equipment from MG America can be found throughout North America in industries such as pharmaceutical, medical device, diagnos-


tics, nutritional products, and OTC products. Our lineup of premier, European-made machinery has earned a global reputation for reliability, precise performance, and superior craftsmanship.
BIN BLENDING
WASHING/DRYING
CAPSULE FILLING
CAPSULE WEIGHT CONTROL
VISION INSPECTION
CHECKWEIGHING
FILLING/CLOSING FOR:
VIALS, BOTTLE, TUBES
STICK PACKING
SACHET PACKING
DEEP-DRAW THERMOFORMING
CASE PACKING
TOP-LOAD/SIDE-LOAD CARTONING
TRAY FORMING/LOADING
SERIALIZATION
PALLETIZING
LINE INTEGRATIONS
See our ad on page 7

BOOTHSL-6534
58 | Healthcare Packaging • Fall 2023
MULTIVAC

11021
800.800.8552
info@multivac.com
www.multivac.com/us

The thermoform packaging equipment with MULTIVAC Clean Design™ are solutions for sensitive pharmaceutical and biotech products, such as combination packs, pre-fill syringes, ampoules, vials, and injectors. This GMP-compliant equipment concept separates the process area and equipment for maximum control and reliable packaging.

The thermoform packaging equipment with MULTIVAC Clean Design™ provides a high level of flexibility in formats, packaging materials, and process validation. A simple format change can quickly convert the equipment for small and medium batch sizes. The modular construction makes a high level of flexibility possible in the equipment design. The innovative equipment concept allows the process area to be strictly separate from the equipment. Transparent enclosures with large-area doors guard against direct access and environmental influences.
The thermoform packaging equipment with Clean Design™ has the proven MULTIVAC IPC control to guarantee reliable and reproducible packaging outcomes. The aim of the design of all equipment with MULTIVAC Clean Design™ is maximum visibility and the avoidance of crosscontamination. Accordingly, particular emphasis was placed on equipment design with the following features:
• Strict separation between the product processing area and the equipment technology area
• Visibilit of all process-related areas
• Deflector plates inside e uipment
• Widest possible avoidance of hidden voids
• Minimum gap dimensions

• Cable & pipework routing in enclosed ducts
MULTIVAC is passionately focused on contributing to our customer’s success by delivering innovative, reliable solutions and superior support services.
MULTIVAC is the global leader in thermoforming packaging equipment. Our equipment can package a wide range of products efficiently and cost-effectively, including medical and pharmaceutical products, food, industrial and consumer goods. Our product range is the broadest in size, performance, and equipment: compact equipment for small volumes, high-speed equipment for large volumes, and specialized machines for producing applications, including FormShrink and MultiFresh™ packaging.
Our sales & technical service professionals are committed to helping our customers achieve their goals with the most innovative and reliable packaging solutions.
PHONE EMAIL WEBSITE
BOOTHSL-6601
See our ad on page 9 N Pomona Avenue, Kansas City, MO 64153 Fall 2023 • Healthcare Packaging | 59
NJM, A ProMach Product Brand
800.811.6990
info@NJMPackaging.com
www.NJMPackaging.com
NJM Products Include:

Line Integration – NJM can integrate a tablet, powder or liquid packaging line. As part of this service, we provide equipment and then integrate turnkey systems at the customer’s plant, and offer in-plant training, documentation and validation support.

Unscramblers – NJM unscrambling equipment is compact and ideal for packagers. Our unscrambler is designed for round, rectangle, square or oval bottles.
Cappers & Retorquers – NJM cappers include inline belt, inline disc and rotary continuous models. Our compact design makes our cappers easy to incorporate into your packaging operation. We also supply retorquers of inline belt or inline disc type.
Cottoners – NJM cottoners are simple yet precise, with a guillotine tearing device as standard equipment, and options such as missing cotton detection and wisp detection.
Labelers – NJM labelers are customized for labeling applications, and all are built in the same way: robust, reliable and versatile, with electronic controls and up-to-date container handling features.
Liquid Filling & Closing – Aseptic liquid filling & closing systems for the pharmaceutical liquid dose industries are offered by our partner, Dara Pharmaceutical. NJM is Dara’s exclusive sales partner in the USA and Canada.
Tablet Counters – NJM offers tablet counters from our partner, Cremer. Cremer counters feature thoughtful design, robust construction, quality and accuracy. Cremer tablet counters are also simple to take apart for cleaning, without tools.
Washing, Sterilization, & Decontamination
Equipment – NJM is the exclusive North American representative for Steelco’s Pharma and Biopharma products.

NJM, part of ProMach Pharma Solutions, offers a broad range of technologies and applications, specializing in the needs of pharmaceutical, nutraceutical/vitamin, and personal care product packagers. Complementing NJM’s manufacturing and integration expertise, we supply quality packaging line equipment from other leading manufacturers. The full range of NJM’s services – manufacturing, representation of NJM’s distributed brands, and integration –makes NJM a one-stop packaging solutions provider offering expert knowledge and experience from the earliest stages of planning through implementation and production. NJM’s extensive portfolio includes unscramblers, tablet counters, bottle filling, cottoners, cappers, liquid aseptic processing/filling equipment, labelers, printers and coders, end-of-line equipment, as well as custom solutions and software. NJM is a premier single source solutions provider.
See our ad on page 21

PHONE EMAIL WEBSITE
BOOTH C-3225, SL-6501
5600
60 | Healthcare Packaging • Fall 2023
Kieran, Montreal, QC H4S 2B5 Canada
Ossid LLC, a ProMach brand, is committed to providing our customers with a superior line of medical packaging machines.

Working with customers in the medical industry, Ossid provides sterile, safe, and effective packaging equipment solutions. There’s nothing standard about medical devices. Ossid understands the unique and complicated requirements of the medical industry and strives to meet those needs with precision and safety at the forefront of the design. That’s why Ossid’s line of thermoform fill and seal machines are flexible to fit your specialized needs, no matter the specifications. If you can imagine it, our team of engineers, with a combined 75 years of experience, will work to design it.
Ossid’s medical device thermoformers are versatile; packaging both flexible and rigid medical package types and are built in accordance with UL 508A standards.
These machines offer many features and

benefits including an auto web aligner, static eliminator, hinged upper tooling, servo actuated presses, and a robust framework and guarding package. These machines are ideal for packaging medical devices, dental kits, syringes, pharmaceuticals, and other products.
In addition to thermoformers, Ossid is also a master distributor for the Reepack brand of flow wrappers. With four styles to choose from, flow wrappers can provide a packaging solution ideal for products requiring a hermetic seal or a simple dust covering. Let our team guide you to the best packaging solution for your application.
Our comprehensive customer service program, including service technicians, parts and training teams know how to help you keep your equipment running at maximum efficiency. Ossid helps its packaging customers protect and grow the reputation and trust of their consumers. ProMach is performance, and the proof is in every package.

Our mission at Ossid is to provide our customers with cost-effective packaging and labeling solutions. Ossid works to give our customers a complete flexible packaging solution. Providing customers with excellent service nationwide, we can respond quickly to customers experiencing downtime needs and offer preventive maintenance programs to reduce overall downtime concerns. Our goal is to build long term relationships with our customers. Our committed sales, service, and aftermarket parts teams work collectively to quickly and effectively assist customers with their needs.
ProMach can offer solutions for any customer project or application needs by providing best in class stand-alone equipment from industry leading product brands, partial line integrations, and complete large multi-brand turnkey packaging line integrations.
PHONE EMAIL WEBSITE
www.Ossid.com Ossid@ProMachBuilt.com 252.446.6177 Ossid LLC BOOTH C-3033 See our ad on page 5 4000 College Rd, Battleboro, NC 27809 Fall 2023 • Healthcare Packaging | 61
Packworld USA
From the start, Packworld USA’s sole mission has been to redefine conventional impulse heat sealing by employing advanced, Variable Resistance Controlled (VRC), TOSS® Technology in every machine it builds. Today our entire company is committed to the design and engineering of precision VRC impulse heat sealing machines that are validatable, repeatable, and consistent - capable of producing “Perfect Seals… Every Time”.
Packworld USA offers a complete line of precision-controlled heat-sealing equipment engineered with the advanced TOSS Technology – The Optimum Sealing System. Designed specifically for today’s medical device, biotech, and pharmaceutical markets, Packworld USA machines produce validatable, repeatable, and consistent seals on all types of medical pouches and related polymeric products.

All Packworld USA sealers come equipped with advanced TOSS® Technology with PIREG® Heatseal Temperature Control. Unlike other sealing methods, only the PIREG® controller uses Variable Resistance Controlled (VRC) technology to monitor the resistance on the heat seal band responding in milliseconds to adjust temperature precisely over the full length and width of the heat-sealing element accurately up to 500°C.
Packworld USA’s focus is dedicated to designing heat seal machines meeting today’s stringent validation requirements of the FDA/GMP/NIST
demanded by the medical, pharmaceutical, and biotech industries.

Packworld USA continues to develop advanced features. The touchscreen interface provides a graphical display of time/temperature/pressure, password protection, recipe storage, data logging, multi-point calibration, and the ability to run in 21 CFR Part 11 compliance. Vacuum/purge, automatic knife cut, safety gates, electronically adjustable stand heights, and automatic bag stretching are all possibilities on Packworld USA precision heat sealers.
Along with its line of validatable pouch sealers, Packworld USA also specializes in the design and manufacturing of custom heat seal tooling for applications requiring contoured-shaped heating elements. Packworld USA is so confident in the quality of their machines their entire line is backed with a 30-Month Warranty.
Packworld USA’s entire team is driven by founder Charles H. Trillich’s core values: quality, trust, honesty, integrity, respect for the individual, teamwork, partnerships, and a striving for excellence.
Added all together, Packworld USA, with their complete line of benchtop, vacuum/purge, and seal/cut heat sealers has become one of the world’s most recognized brands of high-quality heatsealing machines in today’s medical device, biotech, and pharmaceutical markets.


PHONE EMAIL WEBSITE
www.packworldusa.com sales@packworldusa.com
610.746.2765
BOOTHSL-6407 See our ad on page IFC 539 South Main Street Nazareth, PA 18064 62 | Healthcare Packaging • Fall 2023
Paxiom / WeighPack Systems, Inc


(702) 450-0808
info@paxiom.com
www.paxiom.com
Paxiom Automation is a leading provider of primary, secondary, and end-of-line packaging machines with more than 30 years of industry expertise. We are an industry-leading provider of primary, secondary and end-of-line packaging machines. Designed and manufactured through our WeighPack Systems, EndFlex, and ValTara brands, and supported by Paxiom Service, our state-of-the-art packaging solutions are engineered to fit a wide variety of production requirements and budgets. Our group has delivered over 7,000 packaging machines and fully automated systems throughout the world while creating long-lasting relationships built upon hard work, integrity, and exceeding customer expectations.
Our packaging machine technology includes net weigh filling, multi-head weighing, auger filling, optical counting, vertical/horizontal form fill & seal bagging, premade pouch bagging,

flow wrapping, container unscrambling, filling, capping, labeling, cartoning, case erecting, tray forming, case packing, sealing and robotic palletizing.
We manufacture packaging solutions to automate all the stages of your healthcare packaging processes including container filling, capping, induction sealing, labeling, weighing & bagging, wrapping, check weighing, cartoning, case and tray forming as well as box erecting, robotic case/ tray packing, and palletizing for retail display, merchandising and distribution within the nutraceutical and pharmaceutical industries.

Visit our website Paxiom.com to watch all our real-life application videos and customer installs.
Come visit any of our Xperience Centers to see, test, and operate our packaging machines in person.
Our new compact PKR Delta pick & place cell will automatically top-load your flexible bags and packages into cases or trays. Featuring gentle product handling by either vacuum or mechanical means, your product integrity and packing consistency are assured. And we can configure the PKR to accommodate a wide variety of case/tray sizes and pack patterns.
Our mission is to customize complete turnkey solutions to meet almost any production need. We have a deep lineup of automated packaging machines from upstream weigh fillers and baggers to downstream case packers and palletizers.
We engineer and manufacture the machines you need to fully integrate your product packaging line, giving you the peace of mind that comes from one vendor taking complete line responsibility. Let’s start your system today!
See our ad on page 44
PHONE EMAIL WEBSITE
BOOTH C-5006
E Maule Ave, Las Vegas, NV 89119 Fall 2023 • Healthcare Packaging | 63
2037
PAXXUS - Global Flexible Healthcare Packaging

630.628.1700
Hello@PAXXUS.com

www.PAXXUS.com
PAXXUS is the leading supplier of engineered flexible materials for the global healthcare market and dedicated to supporting the complex regulatory requirements of the medical device, pharmaceutical, diagnostic, and life sciences industries across the globe. As a vertically integrated manufacturer with locations on three continents, PAXXUS provides tailored material structures to meet your specific packaging requirements. Our packaging formats include pre-made pouches, die-cut lidding, form fill seal, flowrap, rollstock, and more.

Through constant innovation, PAXXUS has been recognized and awarded for cutting-edge advancements in clear high-barrier films, aluminum foil composites, peelable solutions, and chemically-resistant materials. In addition to best-in-class material science, our partners can expect agile and responsive support from design to validation and beyond.
Stop by our booth to see our engineered flexible materials in person and talk to our team of experts who are waiting to provide you with personalized guidance for your next packaging project. We understand the unique challenges of the healthcare industry, and we are committed to working closely with our customers to develop customized solutions that meet their specific requirements.
Don’t miss the opportunity to learn more about PAXXUS and our innovative solutions for the global healthcare market. We look forward to meeting you and discussing how we can help achieve your packaging goals.
We are committed to improving the quality of life – now and for future generations – through engineered flexible materials. Our flexible healthcare solutions are designed to ensure that your product stays protected so it performs exactly as intended. Positively impacting patient lives is at the center of everything we do.
See our ad on page 33

PHONE EMAIL WEBSITE
BOOTHSU-7622
64 | Healthcare Packaging • Fall 2023
320 South Stewart Ave, Addison, Illinois 60101
Pharmaworks, A ProMach Product Brand


Pharmaworks Products Include:

• TF Blister Machine – A simple and cost-effective blister packaging solution. The TF blister machine is perfect for entr -level packagers where budget matters.
• TF e Blister Machine – Features a compact footprint, quick changeover, and the latest in servo technolog . The TF e is perfect for small, medium, or clinical production.
• TF pro Blister Machine – The latest in blister packaging technolog , the TF pro is built for today’s demanding cGMP and changeover requirements.

• TF Blister Machine – A medium output workhorse that is great for deep-draw medical devices and consumer products.
• TF Blister Machine – All the features expected in a high output machine.
• BlisterMate – A standout semi-automated blister machine that is great for R&D, low volume type production.
• Vision S stems – Scanware blister and print vision inspection systems.
• Feeding S stems – Pharmaworks provides a variety of feed systems.
• Blister Machine and Cartoner Rebuilds –Pharmaworks is the clear leader with over 20 years of experience rebuilding and upgrading third-party equipment.
• Tooling & Change Parts – Pharmaworks is the go-to source for tooling for any supplier’s equipment.
Pharmaworks, part of ProMach Pharma Solutions, is the premier supplier of blister packaging machinery for North America and has a worldwide installed base. Backed by the power of ProMach, Pharmaworks can turnkey any size blister project from our expanded production facility in Odessa, FL. For 20 years, Pharmaworks has set ourselves apart from other OEMs by not only selling our line of blister machines and equipment, but by rebuilding and upgrading other OEM brand equipment. This uniquely positions Pharmaworks to be a full-service provider for our packaging customers.
Industries served:
• Pharmaceutical
• Medical/ Medical Device
• Animal ealth
• Nutraceutical & Vitamin
• Personal Care
• Consumer Products
PHONE EMAIL WEBSITE
Sales@Pharmaworks.com . .
www.Pharmaworks.com
BOOTH
See our ad on page 29 2346 Success Dr., Odessa, FL 33556 USA Fall 2023 • Healthcare Packaging | 65
C-3225, SL-6501
BOOTH C-2638
ProSys Fill LLC.

422 East Fountain Road, Webb City, MO 64870
PHONE
EMAIL
WEBSITE
info@pros sfill.com
ProS sFIll.com

ProS s is a premier manufacturer of semiautomatic and full automatic e uipment for filling, S ueeze Tubes, S ringes, Airless Pumps, Cartridges, ars, Containers & ot Melt applications for the Pharmaceutical industr . A global supplier of filling e uipment since with U.S. sales, manufacturing and customer service facilities located in Southwest Missouri.
FEATURES & BENEFITS
• Fill Accurac of /- . b Volume
• Turnke & Custom Designs
• Air-Free Vertical Bottom-Up Filling
• Custom Mix Solutions Eliminates Batching
• Drum & Pail Presses
• SERVO Solutions
• Explosion Proof Controls
Class Division & , ATE &
• Tool-free Release S stem for Simple Changeovers
• Digital Readout Indicators for Fast & Accurate Ad ustments
• Multiple Service Technicians for Less Down Time & Preventive Maintenance
• On-line Service & Support
• Recipe Storage & Recall
• Creams, Lotions & Viscous Pastes to Million Centipoise

• Designed & Built in the U.S.A.
MA OR MAR ET LOBAL INSTALLS
• Pharmaceutical
• Cosmetic
• Chemical
• Adhesive/Sealant
• Lubricant
• Food
Building Quality, Integrity & Value in Our Team, With Our Customers, & in the Equipment We Design, Build & Deliver.
FLEXIBLE COMBINATION SYSTEMS
• Plastic & Metal Tube Filling S stems
• Tube & Airless Pump Filling S stems
• Tube & Cartridge Filling S stems
• , & oz. Cartridge Filling S stems
S ueeze Tube Filling Machines speeds from to per minute.

S ringe Filling Machines speeds from to per minute.
Airless Pump Filling Machines speeds from to per minute.
Cartridge Filling Machines speeds from to per minute.
Custom Filling Systems
See our ad on page 14
. . . .
US
.
Onl
66 | Healthcare Packaging • Fall 2023
Selig Group

815.785.2100
sales@seliggroup.com
www.seliggroup.com
Selig Group is a leading global provider of innovative packaging solutions. The company is the leading global supplier of innovative, technically differentiated container sealing and venting solutions for food, beverage, pharmaceutical, healthcare, personal care, and industrial applications. Selig also manufactures a range of technical laminates and flexible packaging products in Europe for these applications. The company’s products are designed to serve customers’ needs for ensuring freshness, providing packaging integrity, extending shelf life, providing tamper evidence, expanding in e-commerce, and protecting brand identity. Selig is headquartered in Naperville, Illinois, USA with manufacturing and distribution locations worldwide.

Selig’s History:
1890: Selig established as a container sealing solutions company.
1972: The Selig name introduced, focusing on container sealing with compression-sealed liners
and induction sealing technology.
2008: Unipac joins Selig, adding Lift ‘n’ Peel™ technology for improved seal integrity and convenience.
2011: ISCO joins Selig, expanding into flexible packaging and enhancing manufacturing capabilities.
2021: PSI joins Selig, contributing venting technology expertise for unique sealing solutions.

2022: MGJ joins Selig, bringing industry-leading foam-based sealing solutions to the product lineup.
Selig’s strength and experience began in 1890 as a container sealing solutions company. Many successful decades followed, and the liner business was introduced as Selig in 1972. Several acquisitions and technology developments have strengthened our portfolio, expanded our footprint allowing us to serve customers globally.
WHY CHOOSE SELIG?
Customer Focus
We will continually improve our products and processes by listening to our customers. We provide both our customers and brand owners with products and services that exceed their current and future needs.
Focus on Safety
Selig’s policy provides safe working conditions and follows operating practices that will safeguard employees, our products, customers, and the general public. There is nothing of greater importance than the health and well-being of our employees.
Supplier Involvement
Selig suppliers are part of the quality team. Our selection of suppliers is based on their proven ability to continually supply Selig with high-quality products and services at competitive prices.
See our ad on page 45

PHONE EMAIL WEBSITE
184 Shuman Blvd., Suite 310, Naperville, IL 60563 Fall 2023 • Healthcare Packaging | 67
AUGER FILLERS - Servo technology eliminates time-consuming manual maintenance. Ideal for dry fill applications such as powdered drink mixes, sports nutrition, nutraceuticals and more.



ROTARY FILLERS - Fill rigid containers with ease and accuracy. Changeovers are a breeze with patented magnetic funnels and all tool-less change parts. An open design allows 360 degree access. Ideal applications include nutraceutical gummies and powders. Stream gummies without bridging, guaranteeing an accurate fill, every time, with our patented streaming system.
PLC-BASED CHECKWEIGHERS - ElectroMagnetic Force Restoration (EMFR) weighing technology is 10X more accurate than strain gauge models. Tool-less belt changes, USB port for easy data collection and calibration by your technician - all in a small footprint. Sanitary, heavy-duty and inclined models available.
VOLUMETRIC CUP FILLERS - Quick-change cups are easily inserted in minutes, cleaning and maintenance are straightforward and user-friendly. Automatic or manual fine tuning available to adjust the fill weight for volume and weight accuracy. Designed to handle a variety of applications from simple hand-fills to interfacing with automatic equipment. Speeds up to 140 fills per minute.
CANNABIS FILLING SYSTEMS - Unique tare/ gross weighing system that tares the jar weight and verifies the gross weights. Easily integrates with a variety of multihead weighers. Reach speeds up to 40 jars per minute as you fill jars with a range of 1 to 28 grams of cannabis flower.
Spee-Dee Packaging Machinery, Inc. supplies filling solutions and checkweighers for the food, pharmaceutical and nutraceutical industries. Designed for specific applications with simplicity in mind, SpeeDee systems achieve accurate, reliable filling and weighing, and are backed by a commitment to service and quality. Since 1981, companies around the world have trusted Spee-Dee fillers and checkweighers to improve efficiency, productivity and profitability.

PHONE EMAIL WEBSITE
www.spee-dee.com info@spee-dee.com 877.375.2121
BOOTH C-4809 See our ad on page 4 1360 Grandview Pkwy, Sturtevant, WI 53177, USA 68 | Healthcare Packaging • Fall 2023
Spee-Dee Packaging Machinery, Inc.
STARVIEW PACKAGING MACHINERY, INC.


514-920-0100 ext.310
sales@starviewpackaging.com
www.starviewpackaging.com
Starview Packaging Machinery, Inc. is the leading manufacturer of packaging machinery for high-visibility packaging with over 30 years of supplying standard and custom packaging systems to our customers. Providing the Medical Device and Pharmaceutical Industry with innovative packaging machines for:

• MedicalDevicePackaging
• PharmaceuticalPackaging
• Blister&ClamshellPackaging
• CustomizedPackagingE uipment
• Integrated AutomatedS stems
We design, engineer, and manufacture a comprehensive line of manual, semi-automatic, and automatic sealing machines. Available in shuttle, rotary, carousel, and inline conveyor configurations, a variety of standard and custom sealing areas are available to meet the specific re uirementsofcustomersandmaximize
productivity. Machines are configured to suit the specific application such as carded packages, sterile medical device packages, or pharmaceutical wallet packages.
Our distinct competitive advantage is in providing a complete range of both standard andcustomized ualit packagings stems backed by solid machine designs, robust machine construction, and superior service. Starview offers many value-added features for our machines such as product sensing, printing and/or verification, hydro-pneumatic cold seal presses, robotic product loading, automatic packaging materials loading, automatic inline fold-over, finished package unloading with reject features. Quick-change mechanisms for tooling sets, on-screen sealing press adjustments, machineperformancetracking,andANSIClass 4 safety make Starview machines an excellent choice.
The owners, management, and staff of Starview are dedicated to designing and manufacturing packaging machines in North America with the highest quality fit, and finish backed up with industry leading customer service.

Starview’s directive is to produce a full range of sealing machines for medical device packaging, pharmaceutical packaging and retail high visibility packaging. We offer standard machines with an array of custom and in demand options to provide our clients with machines to match their manufacturing requirements.
We are fast, efficient and client focused. We invite you to come and experience the Starview Advantage.
PHONE EMAIL WEBSITE
BOOTH C-3600
See our ad on page 72
Fall 2023 • Healthcare Packaging | 69
1840 ST. REGIS BLVD. DORVAL, QC H9P 1H6 CANADA
Weiler Engineering, Inc.

PHONE
WEBSITE
Weiler Engineering, Inc., a leading provider of aseptic custom Blow/Fill/Seal li uid packaging e uipment for pharmaceutical and healthcare applications, is committed to the highest standards of excellence and to expand products and s stems to enhance patient care. Weiler s proprietar ASEP-TEC B/F/S packaging machines produce shatterproof, durable, asepticall -packaged products in one uninterrupted operation. This hands-free manufacturing process ensures that parenterals, ophthalmic solutions, and respirator drugs reach the marketplace sterile, in the most cost-effective manner possible - ever time. The ASEP-TEC S stem is the culmination of ears of innovation in machine design and sterile process development, producing the most advanced aseptic li uid packaging process machiner available toda .
The Weiler design incorporates the threestep process of blow molding, aseptic filling, and hermetic sealing of li uid products in one
se uential operation on a compact machine frame. Weiler s patented electronicall controlled fill s stem, automatic sterilization s stem with integral data collection, and filter integrit test s stem are provided as standard e uipment for each machine configuration. Each machine is also e uipped with a EPA air shower to ensure a Class environment under d namic conditions in the nozzle shroud area.
Weiler s latest innovation is the NEW compact ASEP-TEC LAB Blow/Fill/Seal machine, which is ideal for Stabilit and Clinical batches for pharmaceutical products and/or small development batches using advanced aseptic technolog . This revolutionar small footprint design focuses on ease of changeover and product range flexibilit .


ASEP-TEC Blow/Fill/Seal machines are proudl made in the USA, designed and built b Weiler Engineering, Inc. in a , ft , stateof-the-art plant. Weiler s facilities and corporate offices are located near Chicago s O are Airport.
INNOVATION DRIVEN BY SCIENCE!
FACTS:
• Recognized as an advanced aseptic technolog b the USFDA
• ears serving global markets
• Experience gained from ears operating a captive pharmaceutical CMO
• Close operation with regulator authorities – compliance is ke
• Qualit Operational now-how Integrit

GOALS:
• Focus on the science of the technolog for maximum customer benefit
• Simplicit of design to maximize product flexibilit and minimize footprint
• Optimum service support throughout the markets we serve high customer satisfaction
www.weilerengineering.com . .
See our ad on page OBC 1395 Gateway Drive, Elgin, IL 60124 USA 70 | Healthcare Packaging • Fall 2023
WLS, a ProMach Product Brand
1256 N. Church Street, Moorestown, NJ 08057 USA
PHONE EMAIL WEBSITE

856.273.3377
WLS@ProMachBuilt.com
www.WeilerLS.com
Rotary Labelers – Pressure-sensitive labelers for labeling a variety of product shapes vertically.
Vertical In-line Labelers – Pressure-sensitive labelers for labeling a variety of cylindrical products vertically.
Horizontal In-line Labelers – Pressure-sensitive labelers for labeling a variety of cylindrical products horizontally.
Label Heads – Pressure sensitive label heads with label print and inspection options including serialized codes and RFID tags.
Label Coders – For stand-alone or integrated high-speed coding of labels, including serialized codes.
Label Printers – Autonomy® drop-on-demand digital ink label printers with UV curing and full label inspection.
Vial Coders – For code printing on vials or bottles using ink jets or lasers.
Documentation & Certifications – DDS, FAT, SAT, IQ/OQ and Trace Matrix documents as well as UL, Seismic and CE certifications.
Field Service – US and European field service team also offering equipment training courses and maintenance contracts.
ProMach Pharma now offers sales and service capabilities through our European office.

WLS, part of ProMach Pharma Solutions, is an industry-leading designer and manufacturer of high-speed rotary and in-line labeling machines and serialization, coding and label printing solutions for the pharmaceutical and medical packaging markets as well as the food, beverage, personal care, and consumer markets. With over three decades of experience, our mission is to improve our customer’s labeling capabilities and ensure that our labelers provide them with the highest possible OEE.
Industries Served:
• Pharmaceutical
• Medical/Medical Device
• Nutraceutical & Vitamin
• Food
• Beverage
• Consumer Products
Learn more about WLS at WeilerLS.com
See our ad on page 31

BOOTH C-3225, SL-6501
Fall 2023 • Healthcare Packaging | 71

AD INDEX Columbia Machine, Inc. www.palletizing.com 20, 47 Delta ModTech www.deltamodtech.com 43, 48 ESS Technologies www.esstechnologies.com 26, 49 Formost Fuji Corporation www.formostfuji.com 15, 50 G&K-Vijuk International. Corp. www.guk-vijuk.com 8, 51 Heat and Control, Inc. www.heatandcontrol.com 27, 52 HERMA US Inc. www.herma.us 1, 53 James Alexander Corp. www.james-alexander.com 3, 54 Klöckner Pentaplast, PHD. www.kpfilms.com 11, 55 Marchesini Group USA Inc. www.marchesini.com 39, 56 Mettler Toledo North America www.mt.com/pi 32, 57 MG America, Inc. www.mgamerica.com 7, 58 Multivac Inc. www.multivac.com 9, 59 NJM Packaging www.njmpackaging.com 21, 60 Ossid www.ossid.com 5, 61 PACKWORLD USA www.packworldusa.com IFC, 62 Paxiom Group www.paxiom.com 44, 63 Paxxus www.paxxus.com 33, 64 Pharmaworks LLC www.pharmaworks.com 29, 65 PMMI, The Association for Packaging and Processing Technologies www.pmmi.org 43, 73 Prosys Innovative Packaging Equipment Co. www.prosysfill.com 14, 66 Selig Group www.seligsealing.com 45, 67 Spee-Dee Packaging Machinery www.spee-dee.com 4, 68 Starview Packaging Machinery www.starviewpackaging.com 69, 72 Weiler Engineering www.weilerengineering.com 70, OBC WLS www.weilerls.com 31, 71
prepare to be wowed
Explore Every Possible Solution
In the life sciences industry, you’re challenged by multiple market forces, so attend the show that offers comprehensive solutions. PACK EXPO Las Vegas is where you can delve into innovations from specialized suppliers and discover surprising crossover solutions from other industries.

2,000 exhibitors
40+ vertical markets
100+ free show floor sessions
Endless networking opportunities
Featuring REGISTER NOW at packexpolasvegas.com









































































































































































































































 KEREN SOOKNE, EDITOR-IN-CHIEF
KEREN SOOKNE, EDITOR-IN-CHIEF






































































































































































































































