
NOVEMBER/DECEMBER 2022 PMMI Media Group | www.HealthcarePackaging.com + Breaking: Q&A on Potential Foil Tariffs + Experts' DSCSA Traceability Concerns + 6 Last Mile Considerations for Rare Disease Therapies + IoPP’s 2022 AmeriStar Award Winners Zenni Optical Eyes Robotics For Order Fulfillment

No matter where you are in your DSCSA journey, we’ve got you covered! Are you DSCSA-ready? The 2023 interoperability deadline is fast approaching. It’s time to turn to a trusted partner. Systech provides a complete DSCSA compliance solution for everyone in the supply chain ecosystem. We’re here to help you: •Exchange EPCIS compliant data •Implement credentialing •Deploy a seamless VRS solution •Comply with tracing requirements •Build and share aggregation data with wholesalers Scan to download our infographic: How to comply with the DSCSA SystechOne.com/Pharma
19 Digitization For a Digital World, Packaging Data Needs to Speak a Common Language
Packaging and supply chains are fraught with inefficient data sharing systems and methods. Even simple things like timestamps in differ ent formats can cause inefficiencies.

22 Logistics/Regulatory Experts Still Concerned About Exceptions Under DSCSA
From HDA’s 2022 Traceability Seminar: Data issues may cause double the product holdup when a mismatch of product and data are shipped.
24 Traceability/Labeling Labels: Practically as Important as the Medicine Itself
In becoming compliant with interoperable data exchange require ments under DSCSA, don’t overlook the pharmaceutical label itself. Missing case labels or faded inks can cause holdups.
26 Package Design
From
30 Contract Packaging
A PMMI industry report reveals what’s driving growth in machinery sales to the CoMan/CoPack market.
CONTENTS November/December 2022 • Healthcare Packaging | 3
IoPP’s
AmeriStar
2022
Awards: Achievements in Healthcare Packaging
monomaterials to source reduction, sustainability drove many new innovations. User friendliness, style, and inclusivity also scored among winners.
CPGs
Move More Manufacturing and Packaging Lines to Contract Facilities
32 Raw Materials Q&A: Foil Tariffs Pose Imminent Threat to Healthcare Costs, Lead Times
we
with PAXXUS’
supply chain continuity implications
packaging community
36 Automation Automated Stacker, Bander Picks Up Manual Slack for OTC Manufacturer Reese Pharmaceutical improves their packaging line productivity by 25% through stacking and banding automation. COLUMNS AND DEPARTMENTS 05 KEREN SOOKNE’S PERSPECTIVE 06 QUICK HITS 07 QUOTABLES/BY THE NUMBERS 08 NEWS 10 COLD CHAIN CORNER 13 OEM APPLICATION NOTE 17 MATERIAL DEVELOPMENTS 38 NEW PRODUCTS ↑ pp.13 Cover Story: Zenni Optical Eyes Automation for Order Fulfillment
In this Q&A,
talk
Dwane Hahn about the serious
that loom as the
awaits an expected December 15, 2022, decision.
www.healthcarepackaging.com
Editorial
EDITOR-IN-CHIEF Keren Sookne ksookne@pmmimediagroup.com
SENIOR DIRECTOR, CONTENT & BRAND GROWTH Mike Prokopeak CONTRIBUTING EDITOR Melissa Griffen CONTRIBUTING EDITOR Tim Hayes
Art
ART DIRECTOR Jonathan Fleming UX/UI DESIGNER Maggie Wilson CREATIVE DIRECTOR Dave Bacho
Publishing
PUBLISHER Elizabeth Tierney, 815.861.2992 MANAGER, CUSTOMER EXPERIENCE & SALES SUPPORT Courtney Nichols AD SERVICES/PRODUCTION MANAGER George Shurtleff
Audience & Digital
VICE PRESIDENT, DIGITAL Elizabeth Kachoris DIRECTOR, DIGITAL MEDIA Jen Krepelka
Advertising
VICE PRESIDENT, SALES Wendy Sawtell wsawtell@pmmimediagroup.com SENIOR DIRECTOR, CLIENT SUCCESS & MEDIA OPERATIONS Kelly Greeby
PMMI Media Group
PRESIDENT Dave Newcorn DIRECTOR, MARKETING Sharon Taylor SENIOR MARKETING MANAGER Amber Miller FINANCIAL SERVICES MANAGER Janet Fabiano FOUNDING PARTNER AND EXECUTIVE VP, INDUSTRY OUTREACH, PMMI Joseph Angel FOUNDING PARTNER Lloyd Ferguson
PMMI Media Group 401 N. Michigan Ave., Suite 1700 Chicago, IL 60611 p: 312.222.1010 | f: 312.222.1310 www.pmmimediagroup.com
PMMI The Association for Packaging and Processing Technologies 11911 Freedom Drive, Suite 600, Reston, VA 20190 p: 703.243.8555 | f: 703.243.8556 | www.pmmi.com
PLEASE RECYCLE THIS MAGAZINE
Remove inserts or samples before recycling.
Healthcare Packaging® (ISSN # 21543666) is a registered trademark of PMMI, The Association for Packaging and Processing Technologies. Healthcare Packaging® is published bi-monthly by PMMI with its publishing office, PMMI Media Group, located at 401 N. Michigan Ave, Suite 1700, Chicago, IL 60611; 312.222.1010; Fax: 312.222.1310. Periodicals postage paid at Chicago, IL, and additional mailing offices. Copyright 2022 by PMMI. All rights reserved. Materials in this publication must not be reproduced in any form without written permission of the publisher. Applications for a free subscription may be made online at www.healthcarepackaging.com/ subscribe. Paid subscription rates per year are $55 in the U.S., $80 Canada and Mexico by surface mail; $130 Europe, $200 in all other areas. Single copy price in U.S. is $20. Free digital edition available to qualified individuals outside the United States. POSTMASTER; Send address changes to Healthcare Packaging®, 401 N. Michigan Avenue, Suite 1700, Chicago, IL 60611-3789. PRINTED IN USA by Quad. Volume 16, Number 4 The opinions expressed in articles are those of the authors and not necessarily those of PMMI. Comments, questions and letters to the editor are welcome and can be sent to: editors@healthcarepackaging.com. Mailing List: We make a portion of our mailing list available to reputable firms. If you would prefer that we don’t include your name, please write us at the Chicago, IL address.
PUBLICATIONS MAIL AGREEMENT NO. 40064408 • USPS #25469
Robotics, Contract Services Get a Boost








As consumers seek variety, conve nience, and seemingly more eco-friendly products, brand owners are turning to contract manufacturers and packagers more and more. As Sean Riley reports on pp. 30, rather than investing in complete, dedicated in-house lines, many are moving capital to contract service organizations for flexibility, particularly for over-the-counter pharmaceuticals, nutra ceuticals, and supplements.
But some are still choosing to automate tasks in the plant now that robots are picking up speed. Check out our cover story from Matt Reynolds on pp. 13 for a deep dive into Zenni Optical’s robotic order fulfillment automation. The company is looking to shift


repetitive steps from humans to robots in the form of three robotic bagging systems, which offer dexterity, precision, and visual acuity in packing eyeglass cases for e-commerce shipments.








Down the line on the logistics front: DSCSA compliance was on everyone’s mind at HDA’s 2022 Traceability Seminar. Check out our stories (pp. 22 and 24) where experts discuss their current concerns on exceptions handling and missing case labels, which both have the potential to tie up product in quarantine.
If you rely on light-gauge foil for packaging your medical devices, diagnostics, or pharmaceuticals, take a look at pp. 32 for a Q&A with a converter about the effects of a potential foil tariff. The packaging community awaits a determination from the U.S. government on whether tariffs will apply. Stay tuned to our LinkedIn for updates.
























KEREN SOOKNE is the Editor-in-Chief of Healthcare Packaging She may be reached at ksookne@pmmimediagroup.com or at linkedin.com/in/kerensookne

PERSPECTIVE Doubl ck 0.01g ccuracy W g Q 0-250 10x More Accurate Than Other Check Weighers High Pe f nce Check Weighe Check weigh ll y u high v lue p duc s f he igh es qu li y c n l Q 1.833.4PAXIOM | info@paxiom.com P xi G up P xi Xpe ience Le n e paxiom.com NEW Our Automation Solutions Pre-roll Weighing Filling BaggingWrappingCappingSealingLabelingCartoningFormingPackingPalletizing LAS VEGAS • MONTREAL • MIAMI • TORONTO • MILWAUKEE • SCHIO, ITALY
In an effort to ease labor woes and add flexibility, some brand owners invest outside the facility while others look to automate as many steps in-house as possible.
1‘Click’ Chemistry Could Help Dogs with Cancer
A recent Show Me Mizzou article discussed how “click” chemistry could lead to more efficient delivery of radioactive cancer treatments to re duce side effects. In Sept., the Nobel Prize in Chemistry was awarded to researchers who developed the concept, in which molecules snap together like LEGOs. A researcher at the Univ. of Missouri successfully used click chemistry to efficiently deliver drugs to tumors in large dogs with bone cancer. Antibodies are typically used to deliver the radioactive payload to attack tumors, but they are large molecules that circulate in the bloodstream, and can negatively impact organs, bone marrow, and the liver. The successful canine tests earned the MU College of Veterinary Medicine over $14 million in federal research funding from the NIH, and give hope for a human application.
2A Flexible Catheter Inspired by Wasps
Standard catheters are relatively rigid, which makes reaching certain parts of the brain safely and effectively difficult. A recent Medgadget article cov ered a team of researchers at Imperial College London aiming to improve brain catheters, and found inspiration from a specific species of parasitic wasp with a unique organ used to lay eggs in the bark of a tree. The organ is com posed of interlocking parts that slide over each other. Applying similar structure, the result is a flexible catheter that works with a robotic arm, artificial intelli gence, and optical fibers to help surgeons navigate the delicate tissues of the brain.
3DIY
Microneedle Tattoos Are Now a Thing
Per a New Atlas article, a team of researchers at Georgia Tech created a microneedle patch that can administer a painless tattoo onto skin in just a few minutes. The medical technology has been used for years for everything from insulin delivery to measuring biomarkers, but expert Mark Prausnitz saw the potential for accessible skin art. Each tiny needle delivers tattoo ink, and acts as a single pixel of a larger image that is pressed into the skin. People could one day design and print their own tattoo and self-administer it. Temporary forms of ink can also be used for reversible tattoos.

4
Vial Breakage Causes Nationwide Recall
A recent FDA announcement discussed the nationwide recall of sodium bicarbonate injections from Exela Pharma Sciences. The product poses a threat as the glass vial packaging is prone to breakage when pressur ized during administration preparation. The company has received five reports of injury from flying glass, but no sterility issues. The affected lots are 50mL vials in 20-count cartons distributed to wholesalers, distributors, and other customers between December 16, 2021, and August 10, 2022.
5 Conductive Thread Creates Wearable Sensors for Garments
A recent Medgadget article discussed PECOTEX, a new conduc tive thread that can be embroidered into commercially produced textiles to add smart functions. The thread was developed by Imperial College London researchers who made it ma chine-washable, and stronger and more conductive than previous versions. The fact that it’s compatible with industrial computerized embroidery machines means that it can be easily and inexpen sively incorporated into a large range of garments such as t-shirts or face masks. The new thread, costing just 15 cents per meter, was created by cross-linking multiple materials with cotton thread with the use of divinyl sulfone.
6First Human Trial for Lab-Grown Blood
New lab-grown blood could ease the pressure on donors. Per BBC Health, a clinical trial brings together British researchers and the NHS Blood and Transplant. Starting with a normal pint of donated blood, they use magnetic beads to remove flexible stem cells that can become red blood cells. The stem cells grow, and are guided to become red blood cells before being filtered down at the right stage of development for transplant. Healthy volunteers receive two dona tions of 5 to 10mL at least four months apart–one of which is normal blood, and the other lab-grown. The blood cells are tagged with a radioactive sub stance for monitoring time in the body.
To keep up with the latest news bits from around the world visit healthcarepackaging.com to subscribe and get Quick Hits sent right to your inbox.
QUICK HITS
6 | Healthcare Packaging • November/December 2022 TIM HAYES, CONTRIBUTING EDITOR, HCP
BY THE NUMBERS
32%
THE NUMBER of pharma manufacturers currently sending some serialized data to distributors; most of those manufacturers are sending serialized data for less than 150 SKUs.
Source: HDA Research Foundation's Serialization Readiness Survey
$14 BILLION
THE ESTIMATED VALUE of the medical device sterilization market by 2029. It was $7.2 billion in 2021.
Source: Data Bridge Market Research
Dec. 15, 2022
THE EXPECTED DATE that the U.S. Dept. of Commerce will announce its preliminary determination on foil tariff circumvention. For more, see pp. 32.
4,700
THE APPROXIMATE number of industrial composting facilities in the U.S. These handle bioplastics, biosolids, food scraps, and more.
Source: USDA
“Before redistribution, you combined returned capsules from various lots into new batches with new lot numbers. These new lots did not have uniform character and quality, including capsules that would expire in less than three years. However, you then assigned these comingled batches new lot numbers and granted them an additional three-year shelf life.”
—FDA, IN A 2022 DRUG MANUFACTURING WARNING LETTER
“
the job descriptions themselves, if
want more applicants, you have to make the first two sentences really good. Why? Because our research shows… the first two sentences often determine if somebody will read the rest of the job description. Well, that’s massive because the first two sentences are often terrible, right?”
JASON
PODCAST
—LYDIA THE, MCKINSEY & CO, 'HOW AI COULD REVOLUTIONIZE DRUG DISCOVERY'
November/December 2022 • Healthcare Packaging | 7 QUOTABLES
“We call it 'pilot purgatory': companies focus on one pilot, and they see the returns in a single pilot, but they don’t establish that approach and way of operating across their organization.”
In
you
—
DORSEY, PRESIDENT OF THE CENTER FOR GENERATIONAL KINETICS, ON PMMI’S UNPACKED
Zipline, Intermountain Healthcare Begin Drone Deliveries in Salt Lake Valley
In the fall, Zipline began drone deliveries of prescriptions and medical products to select Intermountain Healthcare patients’ yards in the Salt Lake Valley area. Zipline’s Utah delivery system will expand gradually over five years. Drone delivery capability depends on factors including a home’s yard size, location, and surrounding airspace. The aircraft operate quietly and release packages with parachutes to a patient’s yard, in an area about the size of a couple of parking spaces. According to company estimates, each flight produces about 30 times less CO2 emissions per mile than an average electric vehicle and up to 98% less CO2 emissions than a combustion engine vehicle.
 —Keren Sookne
—Keren Sookne
FDA’s Proposed Change to NDC Codes
In July 2022, the FDA announced a proposed rule, Revising the National Drug Code Format and Drug Label Barcode Requirements, with comments due in November. National drug codes (NDCs) are standards for identifying drugs marketed in the U.S., composed of a labeler code, product code, and package code. The FDA is proposing to change the NDC to 12 digits in length with three distinct and consistent segments and one uniform format. The NDC proposal is intended to minimize the impact of running out of 10-digit NDCs by adopting a single, uniform 12-digit format for FDA-assigned NDCs. The FDA is likely to begin assigning only six-digit labeler codes five years after the regulation is finalized, followed by a likely three-year transtition period.
 —Keren Sookne
—Keren Sookne
IoPP’s Inaugural ‘Fundamentals of Medical Device Packaging’ Course a Success
Developed through IoPP’s Medical Device Packaging Technical Committee (MDPTC), the first “Fundamentals of Medical Device Packaging” course was held at McCormick Place during PACK EXPO International (Oct. 24 through 26) in three half-day sessions.

A team of packaging professionals vetted by the MDPTC delivered the course to a packed house of approximately 40 students. The goal was to deliver essential knowledge around this complex segment of the packaging industry for both new and veteran device packaging professionals, whom often no longer receive the same on-the-job training they once did. “We wanted to reach as many people as possible while keeping the class size small. There are varying levels of knowledge among students, and it’s intensive on multiple levels,” says course founder Jennifer Benolken. “I’m excited it’s been well-received. The hands-on, on-the-show floor portion really helps—students get to see things like sealing inspection up close. We want to create a pipeline to encourage students to commit to med device packaging and make this a place they want to be.”
The course will be offered again at MD&M West in February 2023. Follow the IoPP MDPTC on LinkedIn at: www.linkedin.com/company/iopp-mdptc
—Keren Sookne
NEWS 8 | Healthcare Packaging • November/December 2022
Voices of Women in Packaging and Processing




SCAN TO DOWNLOAD


This resource provides insight on 5 core skills, each presented through expert advice as well as personal experience.





CONNECT, SHARE, INSPIRE: Voices of Women in Packaging and Processing 2022 COMMUNICATIONS EMOTIONAL INTELLIGENCE NEGOTIATION SKILLS BUSINESS ACUMEN NETWORKING
SHARE,
CONNECT,
INSPIRE:
collaboration between PPWLN and the OpX Leadership Network aims to attract women into this field and give them tools to succeed.”
“This
Editor-in-Chief
OEM magazine
Stephanie Neil,
of
pmmi.org/ppwln
6 Last Mile Considerations for Rare Disease Therapies
KEREN SOOKNE, EDITOR-IN-CHIEF
Many patients with rare diseases use temperature-controlled ther apies to manage symptoms. With smaller patient populations and batch volumes, special handling is often required to ensure these expensive drugs arrive safely. Brandon Salke, Pharm.D, is Pharma cist-in-Charge at Optime Care, a specialty pharmacy, distribution, and patient management organization focusing on rare, orphan and ultra-orphan products. He discusses that while not all patients in these populations have mobility issues, these therapies have differ ent last mile and two-way communication practices compared to traditional drugs.
1. Shipment communication
Salke highlights the extra care in delivering to patients with rare and orphan diseases. They call the patients to be sure they’ll be home to sign for packages and let them know they can ship to their work or a relative’s home if that’s more convenient. “We can’t have those packages sitting out on the doorstep–you don’t want to leave a $50,000 pack out in 100° Arizona heat,” he says. “The challenge is making sure that the product is getting to the patient on time, intact.
We go through such depth to ensure the patient will actually be there because launching an exception, calling FedEx for reattempt deliveries—it becomes very cumbersome, and not streamlined for the patient whatsoever.”

For those with mobility issues, communication with the courier is critical. “We have to give instructions to FedEx, because if some one is in a wheelchair and they can’t get to their door quickly, we have to make sure FedEx will wait for them to answer versus a quick door knock, waiting three seconds, and heading back to their truck because they’re trying to finish their route. We take the time to ensure instructions are disseminated on what we need from them out of a delivery to best serve the patients,” he says.
2. Dry ice handling by patients
Generally, cell and gene therapy manufacturers are acutely aware of the unique packaging and logistics requirements associated with shipping temperature-controlled lots. They’re often very involved in how a drug is transported through the supply chain, including the last mile.
COLD CHAIN CORNER 10 | Healthcare Packaging • November/December 2022
A specialty pharmacist discusses the added communication and support— including manufacturers working with patient groups—that foster adherence among smaller patient populations.
“But sometimes what gets lost—specific to cell and gene therapy, and we even saw it with COVID vaccines—is they had to be stored at such cold temperatures, sub 60 or even sub 80, which has impli cations both for how you’re packing that medication and what that means for the patient when they receive it. If I’m shipping somebody dry ice, they have to be very careful with handling when they receive it,” Salke says. “The quality or supply chain team may not be putting their patient cap on as much as they’re putting their product integ rity cap on, and they might not think about how a dry ice shipment is going to affect the patient. I think the patient opening the ship ment is the consideration that might get overlooked in some cases.”
3. Traceability and authentication understanding
Dispensers are brought into the DSCSA discussion more regu larly now, but the patients themselves are largely an afterthought in discussions of supply chain security.
“DSCSA traceability has a whole slew of rules that are still coming down the pipeline. In DSCSA conversations, everyone is worried about ‘How do we do it? How do we maintain it?’ Patients probably aren’t even aware of these activities and why a serial number is there. I think the explanation will give a patient peace of mind that they’ve received an authentic product,” Salke notes. “And this is especially the case when you see drug recalls because, for example, a batch had shards of glass and it was manufactured outside of the U.S. News like that gives people pause, and that makes them hesitant and ques tion the drug manufacturing system as a whole. They’re taking rare drugs that, quite frankly, many have never heard of. They’re hesi tant already. Traceability will give peace of mind, especially when it comes to these small patient populations.”
Speaking of recalls, Salke sees that more granular traceability will make recalls faster and more efficient. Dispensers will know who received what serial number and contact them to get it back, meaning they won’t have to send blanket recalls for multiple lots and worry patients who are unaffected.
4. Patient education on the therapy
Treatment in the home setting continues to grow, meaning patients or caregivers without healthcare backgrounds are admin istering drugs, often biologics via a medical device. At Optime Care, they follow a patient-first philosophy. “Everything we do—all the decisions, all the parts in the process, we do it with the patient in mind. When the Optime team performs intake with a patient and they’re new to a medication, a dose, or a specific delivery device, we take the time to educate that patient or caregiver until they feel competent that they understand why they’re on it, how to use it, and when there could be issues or problems,” Salke says.
Should an issue arise when the office is closed, they also offer on-call services 24/7 where a pharmacist is paged to get back with the patient quickly and resolve any issues or problems. He adds, “By having a robust education system in place, we find that the educa tion gaps for patients are very minimal. But we do have fallbacks for the patient should they ever need something. We’re always available to answer them.”
5. Getting involved with patient populations
In a traditional pharmaceutical setting, most patient communica tion takes place between the pharmacy and the patient, and not the manufacturer that works with the wholesaler. But Salke says when you’re talking about rare and orphan diseases, life science manufac turers understand there is a need for some hand-holding—high-ser vice, high-touch experiences for the patients.
“A lot of manufacturers want the best-in-class service models, and work with a pharmacy in lockstep to create robust care and significantly impact the patients in rare communities,” he says. “If a manufacturer wants to be even more involved with patients, one way to show their support for a disease state is to work with the patient advocacy group, such as the U.S. Hereditary Angioedema Association (HAEA), which is a big one.”
Getting involved with an organization not only shows the manu facturer’s commitment to their patients, but it allows them to communicate on a different level, which patients usually appreci ate. “It shows that they’re being cared for. They’re just not another number. Generally, for a manufacturer to do that, they have to spend a bit to get the benefit back,” Salke notes. “Manufacturers can also consider setting up an advocacy group of their own. We’ve seen manufacturers set up a team of nurse advocates or pharmacist advo cates. If the manufacturer has a consented program—meaning the patient has opted in to share personal information with the manu
The Value of QR codes
Whether a package has QR codes or other links is up to indi vidual manufacturers. “But we’ve shipped things in the pack age like brochures and flyers with QR codes that allow patients to scan and get linked up to an educational website or the advo cacy group for their specific disease,” Salke says. “QR codes are obviously everywhere in our society. There’s a lot of benefit to using them from an education standpoint, and even to take them to a survey to help report disease-specific outcomes. We’ll send that back to the pharmacy to capture that informa tion and make good decisions when working with the manufac turer or prescribers on that therapy.”
COLD CHAIN CORNER November/December 2022 • Healthcare Packaging | 11
facturer—these advocates can reach out to the patient and talk about their journey and challenges. That’s just another level of support that manufacturers can provide.”
Along with advocate programs, Salke says mentoring programs exist where the manufacturer can connect newly diagnosed patients (or patients newer to the medication) to patients more experienced with the disease state. “These are a few ways manufacturers can help communicate with their patients so that they feel supported. In small patient populations, that’s what the patients and caregivers really want to feel… like somebody there has their back and cares about them,” he says. “I think certain manufacturers have been a lot quicker on the uptake. Over the next probably five or 10 years, you’re going to see more manufacturers go this route where they want to sell on services to support these patients.
“At the end of the day, if you’re serving a rare disease, you only have so many patients in the United States. At some level, we have to talk about the dollars and for the manufacturer, they can’t afford to lose a patient. So, they need to make sure that they have done all they can to support them.”
Trend Watch
Software and machinery as a service have been growing for years. In a way, this is parallel to what’s happening with patient populations. It’s not enough to sell machinery or platforms to pharma manufacturers—or medication to patients—there’s much more need for collaboration and support.
6. Avoid the maze: touchpoints and handling
We’ve all heard the motivational quotes eschewing the mindset of “We’ve always done it this way.” And Salke says this is some thing to look out for in pharma. “Specifically, when you’re talking about rare and orphan diseases, small patient populations or small drug volumes, the biggest pitfall we see manufacturers fall into is when they do things traditionally,” he explains. “They have multi ple vendors and systems set up. They create what we call a maze for patients—it’s a system where there are 20 or 30 touchpoints along the way. It causes a lot of confusion, and it’s not streamlined. Finan cially, it’s disadvantageous for manufacturers because they spend on extra vendors that they don’t need. That is the biggest pitfall I see in regard to rare and orphan patient populations.”
He recommends manufacturers for rare disease therapies look for a partner with a high-service level model that can provide end-toend solutions for 95% of what’s needed. “I leave that 5% off because there are some things that a specialty pharmacy can’t do, that a manufacturer will need to work through. But as it pertains to the
patient, having a full end-to-end solution is what’s going to set you apart and offer the most positive impact on this patient population.”
For a patient, navigating the various systems to get what they need in the U.S. can be exhausting. In a traditional model, there’s typically a hub for enrollment, which can take two to three weeks to contact the patient, work through a prior authorization, and get everything approved to then send to a pharmacy. The pharmacy has to perform intake on the patient, followed by a benefits investigation to make sure everything is authorized before they do a coordination of care.
He adds, “To get a refill, a patient might have to call back, go through a prompter, and not get the same person. Nothing seems personal. Those kinds of things and delays in trying to get medica tion, the process is very cumbersome.”
This process can differ if the manufacturer works with a single pharmacy. “In our case, we receive the prescription, we handle everything in-house, including the investigation and prior authori zation, to streamline everything. We have KPIs to support getting medications turned around within 48 hours. We’ve reduced the time and the burden for the patient,” he says.
Optime has care coordinators regionally aligned to state zip codes, so patients and their prescribing doctors talk to the same person every single time. Dealing with one person over the course of many interactions lets them build a good reputation with the patient. “The patient feels like they have somebody there that’s supporting them. A lot of our manufacturers will even work with us to create bio cards with care coordinator pictures, sometimes on a magnet. When we send a shipment out to a new patient in a given territory, we put a bio of Stephanie in there if that’s her patient. They put it on their fridge with her number. They know who she is, a few details about her, and it organically fosters a good relation ship,” Salke says.
Coordinators also don’t have time quotas on calls, so time on the phone can be tailored to the patient’s needs.
Beyond the service, Salke notes that this model can deliver better data—it’s coming from one source vs. different pharmacies, and a hub might report differently to different aggregators. “That data allows better decisions for the manufacturer. Maybe we see patients are dropping off at month four because of certain adverse events,” he says. “So, manufacturers can work with the pharmacy to get some kind of intervention to help these patients get through that and figure out the challenge. The patient communication, the data, the support, it all keeps a more compliant patient. Especially when it comes to a rare and orphan disease, it may be genetic in nature and the patient will have it for life. We have to move forward making sure the patient is compliant, because that is going to yield the best possible outcome in their disease state and in their life.”
COLD CHAIN CORNER 12 | Healthcare Packaging • November/December 2022
D2C Zenni Optical Eyes Robotic Order Fulfillment Automation
Packaging operations at popular D2C brand and platform Zenni Optical are getting a boost from a six-axis robotic arm designed to pack eyeglass cases into poly bag mailers in the place of repetitive labor, cutting human operator tasks by more than half.

 MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD
MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD
It’s always fun to follow fast-growing new consumer brands as they level-up on their packaging technology. Just three years ago, Packaging World first reported on packaging operations at Zenni Optical, a challenger eyeglass brand with a unique Direct to Consumer (D2C), e-comm-based business model.
The company was born in 2003 when its founders identified the outlandish difference between manufacturing cost and unit price among existing prescription eyewear channels and sought to inhabit an as-of-yet unoccupied niche. The result was a volume supported D2C model with low entry prices. The entry price for prescription glasses is

OEM APPLICATION NOTE November/December 2022 • Healthcare Packaging | 13
↙ Three OSARO robotic bagging systems will take on the responsibility of readying eyewear orders for shipment to Zenni’s U.S. customers.
↑ Zenni Director of Distribution and Facilities Simon Goh (right), talks about the deployment now underway with OSARO’s Bryan Yong, a Senior Solutions Engineer at the AI robotics company’s San Francisco Robot Lab.
$6.95, with select styles on the higher end of the scale at $49.95. Children’s glasses begin at $6.95 and go up to $45.95.
Back in in 2019, semi-automatic poly baggers from both PAC Machinery and Sharp Packaging Systems by Pregis were the latest and greatest in packaging tech at Zenni’s San Francisco Bay-Area fulfillment center, and that equipment is still going strong.
What we thought deserved an update has been the company’s recent plunge into robotics and its announced partnership with OSARO, a robotics developer that deploys advanced robotics for e-commerce.
The project will automate the “last meter” of Zenni’s fulfillment center in Novato, CA. Three OSARO robotic bagging systems will take on the responsibility of readying eyewear orders for shipment to U.S. customers.
According to OSARO, the deployment is the first time a robot will be assigned the responsibility of working with an automated mechanical bagging system to ensure a customer’s unique order is placed into the correct bag for shipment. OSARO has partnered with Pregis, who already has bagging equipment in the Zenni facil ity, to deploy the robotic system. According to Zenni, OSARO’s superior vision and grasping technology enables the robot to rise
to the challenge of this piece-picking task, which is important to the company since each eyeglass order is associated with a customer’s prescription.

“We are focused on quality and innovation,” says Simon Goh, Director of Distribution and Facilities at Zenni, which has surpassed 45 million pairs of eyeglasses sold over 19 years of growth. “With a mountain of online orders and a persistent shortage of labor, we looked to OSARO to take us to the leading edge of technology to be sure our customers receive fast and accurate processing.”
“The stakes are high here: special-order, high-value items that must be bagged and labeled correctly to ensure they are sent to the right customer,” adds Derik Pridmore, CEO of OSARO. “Our exten sive experience in other e-commerce production environments, coupled with our machine-learning capabilities, enables our robotic bagging system to meet customer production targets and to deploy workers onto more important tasks.”
Break-even and beyond
Goh has always had his antennae up for new packaging automa tion, hence the addition of poly baggers three years ago. But when he looked at his packaging equipment fleet, it still bugged him that
OEM APPLICATION NOTE 14 | Healthcare Packaging • November/December 2022
↑ Zenni Optical customers create custom D2C eyeglass orders via an online platform. Orders are delivered direct to consumers’ doorsteps using the e-comm channel.
he was only able to automate away about half of the human labor tasks needed to get an order out, from the time when they were fully manual.
Goh is a numbers guy, and his personal metrics for continuous improvement and building efficiency into a process revolve around reducing friction and reducing the number of steps needed before an operator starts using the packaging machine. Prior to the packag ing equipment, that constituted a total of nine steps—nine discrete tasks an operator needed to accomplish, correctly and in a certain order, to successfully send out an order. Once Zenni switched over to use the poly bagging packaging equipment, the company reduced required human labor to only five steps per order.
Now with this OSARO robotic cell, he expects to reduce human tasks down to only two steps. But at Zenni’s workflow speeds, this tech wasn’t ready for prime time until recently.
“We intend to bring it all the way to full automation,” he says. “So, we never stop looking; we always check out the new technol ogy every year. But three years ago, the technology wasn’t ready. I saw robotic solutions in the past, and everything was so slow; it just couldn’t keep up with our speed requirements. But every year, I check. And we have bagging machines from Sharp Packaging Systems, who is working with OSARO, too. Sharp made the intro duction, OSARO came here to visit us, and we realized how far the technology had come.”
Crunching the ROI numbers, Goh’s cost calculation reflected that the OSARO robotic installation would hit a break-even point when compared to the existing cost of human labor performing five steps at speed. Zenni could do it either way—with people or with robot ics—and still spend the about same amount. But the cost of labor is pointed up, and the cost of technology is pointed down, so the break-even point for Goh was the time to strike and make a move. And as robotics manufacturers like to point out, a robot never takes a sick day or a vacation. So while they aren’t saving money currently, they see costs going down over time.
The project will optimize the use of a pick-and-place robot to perform a complex task that demands visual acuity, precision, and dexterity. Zenni has a team of workers capable of handling 10 orders/ min and accuracy is key to making sure that the right order gets to the right customer. Deploying robots to handle e-commerce processing also mitigates labor turnover and staff shortages that are challenges in the rapidly growing e-commerce fulfillment sector.
“We knew we needed an integrator like OSARO for this kind of project anyhow, because we cannot program a robot to do what we need to do, so there’s no way we can work directly with the robot manufacturer,” Goh says. “And OSARO is a company where the speed and the accuracy are pretty impressive, so it made sense. They
can pack our cases with the accuracy, the position, and the speed we require for our operation. Also, the Sharp baggers are actually inte grated with the OSARO robots, and our software, too.”
From five steps to two Under the current system, each online order generates a pair of eyeglasses to be manufactured and associated with a barcode in China, then shipped to the Zenni facility in Novato. Each morning, operators open their shipment of freshly manufactured glasses, scan the bar codes, and begin completing the order. This involves first placing the glasses in a protective case, then closing the case and placing it in a poly bag via one of the PAC Machinery or Sharp Packaging Systems baggers.
“Right now the five steps are handled by a human operator,” Goh says. “But when we deploy the robot, we will have an operator to put the eyeglasses inside a case, and then put the cases into a bin. But then, the robot will work with the bagger, a Sharp MaxPro-18, to take care of the other three steps… actually, that’s now four steps, because we are adding more marketing material into the packaging.” So, the robot will take care of an extra step that the operators did not have to do before.
Once the eyeglasses are loaded into cases and collected in batches in bins, the robot will pick up each protective case and carry it to a dimensional scanner, to identify each order by barcode.
“Once the barcode is scanned, a shipping label will be printed by the packaging equipment and the robot will drop the case with glasses into the [poly] bags. And then there will be some mechanism to drop a few marketing materials, like inserts,” Goh says.
Notably, Zenni sometimes co-markets with aligned brands—for instance with meal kit producers like Blue Apron or Hello Fresh. Zenni also has its own marketing materials and value-adds for customers. For instance, the company includes a pupil distance [PD] ruler for consumers to check their unique distance from pupil to pupil, a distance that maps onto and determines the fit of their glasses. The robot will have a mechanism to drop this kind of marketing material into the poly bag. All told, that’s four distinct steps that human operators don’t have to do.
Poly bagged mailers with shipping labels are then dropped onto a conveyor belt for an operator to collect and prepare for mailing. Goh and Zenni are considering adding another conveyor belt to consolidate all of the ready-to-ship packs, and have them dropped into a post office container.
Robot specs and changeover
The robot used for this application is a six-axis robotic arm that operates with a tool changer kit, built by OSARO, carrying different
OEM APPLICATION NOTE November/December 2022 • Healthcare Packaging | 15
vacuum-based end effectors for optimal gripping geometry.
“We chose the FANUC six-axis model M10iD/12 robot arm because it had the greatest reach with the smallest footprint and plenty of payload capacity to effectively operate in a robotic bagging system cell,” says Bryan Yong, Senior Solutions Engineer at OSARO. “It also integrates easily with our vision and control software.”
“This robot actually can change the [end effector] head, and the changing speed is really fast,” Goh adds. “The robot has the vision to see what’s in the bins. It determines what kind of material it needs to pick up, and it selects the head that is best for picking up that kind of material.”
At the moment, the Zenni eyeglass cases being picked and bagged by the end-of-arm tool (EOAT) will all be made of the same case material. That said, marketing material substrates to be co-bagged with the eyeglass cases will certainly vary. Also, the brand may shift to a limited run special offer with another case style or material, or, down the road, Zenni may launch a differently dimensioned SKU for eyeglass cases designed to carry two pairs of glasses, or sunglass clip-ons alongside prescription glasses. If (or when) any of these occasions occur, the robotic cell will have tool-changing capabili ties already on board.
“But what’s impressive with the robot is that it changes [the end-of-arm tool] on the fly,” Goh says. “And it takes only about maybe one or two seconds. The robot will determine the next tool each time. It simply goes to the tool changer, picks the right tool, and then it will do it again for the next pick. I mean, I saw quite a lot of robots at trade shows over the years, like PACK EXPO Las Vegas in 2019, and it took a long time for the robot to determine what tool to use. But when I was visiting OSARO, I could see that this robot
can swap out up to about 10 different tool heads, all designed for different kinds of material. Just pick, change your head, bag your next product, then do it again. It’s very good, and very fast.”
What’s next
“From my perspective, what OSARO is doing that makes them stand out is the speed,” Goh says, noting that other robots he’s seen can do the same tasks, but not as quickly. “The vision equipment and software can see the style, and determine what kind of material, and also the way they’re designed, they can actually pick anything with the right tool. So the speed is making the difference.”
With increasing demand for D2C eyeglasses by Zenni, contin uous improvement is always on his mind. With labor getting scarcer and more expensive, Goh is already thinking about his next forays into automation.
“Even with this new instal lation, we still need a human to pack the eyeglasses into cases. And this is still about two steps. If there’s a way, I really want to automate that piece also,” Goh says. “We started as fully manual, progressed to half auto mated, and now are almost 80 or 90% automated. One day we will be 100%.”
Watch a video of OSARO’s end-toend automated bagging solution, which can pack and ship more than 350 parts per hour. See also the tool-changer kit in detail.

OEM APPLICATION NOTE 16 | Healthcare Packaging • November/December 2022
↑ The project will optimize the use of an OSARO pick-and-place robot to perform a complex task that demands visual acuity, precision, and dex terity. Deploying robots to handle Zenni’s e-commerce processing mitigates labor turnover and staff shortages.
Thermoformer Boosts Antibiotic and Tranquilizer Delivery in Deer
After designing a new animal tranquilizer dart that is free from the problems with those already on the market, this deer farmer then revisited the secondary issue with existing darts: a poor user-experience with packaging.
SEAN RILEY, SENIOR NEWS DIRECTOR, PMMI MEDIA GROUP
Kevin Grace is a long-time deer farmer/rancher at the Whitetail Sales & Services deer farm in Eldon, MO. He found one part of his job was a constant cause of frustration, not to mention a waste of money. When his deer needed tranquilizing, or needed to be given an antibiotic, the standard delivery method was and continues to be via darts fired from a distance away. And Grace had significant issues with the darts that were widely available in the marketplace.
The number one problem Grace and his fellow farmers and ranch ers experienced was never knowing if the animal received the total dose of medicine. He saw a need in the market for a better product and took the bull (or in this case, buck) by the horns and built one himself. After coming up with and tinkering on multiple design iterations, he worked with a machinist to create a prototype dart.
“I just had the idea. I didn’t know how to do it,” Grace says. “Once we figured out what we needed, I said, ‘This is what you need to build me.’ So, they built me a completely automated machine that builds the dart from A to Z.
“We wanted a dart you could fill right when you get ready to use it,” Grace adds. “That way, you know what level of drug you’re using. Or if you’re going to use an antibiotic, which antibiotic to use.”
After tackling various conspicuously named challenges like “bounce outs” and “tissue plugs,” Grace’s ClearDart developed a
needle and hub partnership that eliminates these issues and allows for a more accurate medicinal dosage. The result offered a clear chamber tube so the contents would be visible, and features a fillport in two different sizes.
“We have what we call a medicating size, which would be a bigger dart with a larger chamber so it can give more drugs at a time,” he says. “And then we also make a dart that we call the petite dart, which would be used more for the small game industry like the deer or the antelope species, and that would be tranquilizing them.”
With his initial problem solved, Grace turned towards his second biggest frustration with the dart products currently available on the market: the packaging. His main competition distributed darts in clamshell packs of five, which he says aren’t ideal for a farm/ranch environment.

Recognizing that other devices were not user-friendly with their packaging, Grace envisioned a pack format that not only kept darts individually wrapped right up to the point of use, but also supplied farmers with a lot more than five darts at a time. According to Grace, farmers/ranchers typically buy large quantities of up to 100 darts at a time, especially cattle ranchers.
He preferred something like the standard banana-peel blister package that medical syringes might come in. This allowed the user
↙ The patented sideport needles eliminate “bounce out” during detonation and keeps the needle clear of tissue plugs, reducing animal stress.
MATERIAL DEVELOPMENTS
November/December 2022 • Healthcare Packaging | 17
to tear off a strip of the number of darts they needed, put them in their pocket, and head out into the field. The rest stay clean and ready to go back at the farm, to be used as needed.
“With our packaging, you can wad them up—three, four, ten darts, whatever—stick them in your back pocket, and open them up as you need them,” he says.
Alicia Shoulders, Regional Sales Manager–Midwest, HarpakUlma Packaging, LLC, is Grace’s sales representative from the company and was present recently when a TFS 400 thermoformer with a Bellmark Intellijet TS printer was installed at his farm. Grace found two machines he liked that could make a package that met his needs, but ultimately went with Harpak-Ulma since they offered a Midwest representative in nearby Kansas City.

“I always found it to be such a cool story because Kevin essen tially saw a need in the market for a better product than what was commercially available, so he built the entire product from the ground up,” Shoulders says.
Grace was so ambitious that he had the packaging machine deliv ered and installed before he was completely prepared with a prod uct to run.

“I had a three-year payment plan, and it’s now paid off,” he says with a chuckle. “I kind of ordered it three years early.”
parts parts

MATERIAL DEVELOPMENTS 715 Church Road, Elmhurst, IL 60126 • 630-530-2203 • www.guk-vijuk.com …on our PAC K AG I N G L I N E l e a f l e t f o l d e r s and f e e d e r s n Call today to optimize production! phone support
training
n Factory-trained technicians are available for service n Parts are stocked at our plant, ready for overnight shipment n Restore your folder with a recondition kit n Thorough training onsite or in our plant n for questions or help with leaflet production problems
service service
training
↑ Deer and cattle darts are placed in two-cavity thermoformed trays, three trays across, prior to lidding on the Harpak-Ulma equipment.
For a Digital World, Packaging Data Needs to Speak a Common Language
 MELISSA GRIFFEN, EDITOR-IN-CHIEF, CONTRACT MANUFACTURING & PACKAGING
MELISSA GRIFFEN, EDITOR-IN-CHIEF, CONTRACT MANUFACTURING & PACKAGING
TOP THREE TAKEAWAYS
1. Packaging and supply chains are fraught with inefficient data sharing systems/methods.
The ISTA Forum 2022, including TransPack and TempPack, took place in San Diego in April and featured the latest in technical topics related to logistics and temperature-con trolled performance packaging.
Matthew Wright, Founder and CEO, Specright, a cloud-based platform designed to manage specification data across the supply chain, presented on the urgency of transitioning packaging to a digital platform using data.
The packaging space and its supply chain have become increasingly dynamic through the years, and are now plagued with ineffi cient data sharing systems and methods, such as email, which are unsustainable as future-proofed solutions. Wright mentioned a survey put together by his company that
2. Even simple things like timestamps in differ ent formats can cause inefficiencies.
gleaned results explaining that it generally takes 600 emails or more to get a product to market. He also said that though Excel has been a saving grace in the world of business as far as digital spreadsheets go, it too is not a solution for the future.
“What we have to do is we have to think and embrace technology in a different way. We cannot digitize our data and then map it the same way we do today. There’s no way with technology that you should have to do 120 steps to get product to the market safely and on time,” Wright said. “The answer is not email. And yet we find that the vast majority of data transfers for industrial products are still done on email. I don’t want to see people create 600 emails to create data structures.”
The future lies, he said, in interweaving
3. Numerous platforms can be kept, running a top-level process over them for data analysis.
technology with each company’s data struc ture using a common language, or in other words, a common system, which would also simplify mergers and acquisitions. Apps, print technology, digital tools, and various software all need to connect and commu nicate. As the world stands right now, even intracompany data uses different languages, which is in part to blame for those 120 steps.
Wright provided an analogy in which 10 of the smartest people in the world are placed in a room to solve a problem, but progress is hindered as they each speak a different language. The industry used to believe their data structures, or systems, were to be safe guarded secrets, but Wright called out the issues surrounding this perspective, insist ing we need to set a standard language and data structure that everyone follows and
DIGITIZATION November/December 2022 • Healthcare Packaging | 19
which can be easily shared. Proper safety would be put in place to keep everyone’s data secure. Wright referred to this as spec management.
He said, “I firmly believe in an open API world where everybody’s sharing data and nobody’s really worried about what system it’s on and we provision to protect that data however you want it to be protected. I firmly believe if everybody’s data is on the same language, provisioned correctly, and shared appropriately in this room, we could do some amazing things quickly.”
Don’t build tech in a silo Companies struggle to communicate among different languages—for proper spec management, the industry must develop a data stream that computers and technology will understand. One reason systems have not worked in the past is because the indi viduals creating the data streams did not understand how things come to market, how computers interact, and/or the language of the future.
Systems cannot be built in a silo—they also need to be built considering future

technology coming down the pipeline. A company Wright talked with a few years back said they had put a lot of hours, time, and energy into building a phenomenal system that worked really well for corru gated. But they didn’t see clamshells coming in the near future, which rendered their system useless.
“You have to break the data down to the raw level of product, the DNA level of prod uct. And then you have to build that back up to create finished product. If you don’t do that and you jump to the end, just try to manage the BOM, you are never going to get anywhere. It’s just not going to help you with all the challenges we have today,” said Wright.
Referring to the pandemic, Wright suggested that a data structure that allows companies to quickly and easily see when problems in the world occur and what areas of the supply chain are affected through instant alerts would save companies not only the time it takes to chase down data but also reduce some of the kinds of monetary losses that ensued during 2020 and 2021. This kind of system could also provide the data structures needed to share with global partners and other pertinent parties.
Wright calls this process of progres sion the “upgrade cycle,” which demands change when the previous way of work ing is no longer sustainable. Our current upgrade cycle is in industrial technology and the legacy systems that have been used to collect data—such as email, spread sheets, PDFs, file cabinets, etc.—which are not sustainable when talking about digiti zation. Yet all the millions of specs coming from this legacy system must be digitized so that they can be incorporated into that shareable, open API system. Companies can pick a system or partner to help with this digital transformation—only ensure the system is fluid and user-friendly. A good data structure, building data for the future, will enable 3D modeling, 3D digitization, and 3D PDF-style specification.

DIGITIZATION 20 | Healthcare Packaging • November/December 2022
↑ Matthew Wright, Founder and CEO, Specright, speaking at the ISTA Forum.
↑ Wright said COVID-19 highlighted lack of visibility into supply chains with existing technology.
Convincing those at the top
The concept of the upgrade cycle can help champions appeal to departments and partners, explaining the importance of taking this next step in data management. “You have to get everyone believing,” said Wright. The world can’t remain driving a horse and buggy, as he explained, but you have to convince the ones driving buggies that the automobile, though new and different, is the better way.
Wright reports that more and more departments are willing to help and jump on board, including the executive level managers (C-suite), and the likely inducer is the COVID-19 pandemic, when it caused failure upon failure within the supply chain. But with proper spec management, company’s outcomes can be changed. Specright helps companies with this shift and offers a number of resources for the future of supply chain and other pertinent topics, including a book and audiobook.
Methods to convince your company to invest in the upgrade cycle include:
• In the midst of a massive failure event, a champion in the company takes initiative and promotes a method to the C-suite that will avoid such a failure in the future.
• The viral community effect propels an effort, where companies jump on board as they see other companies taking the leap.
• Companies choose to adopt the data structure they’re getting from their partners as they see its efficiency and worth.
“But I think you have to have a champion,” said Wright. “Every one of our hundreds of customers started with a champion that believed that they had to digitize and then picked one of those three routes to take it.” To become the change agent, or champion, in one’s company, Wright suggested first learning all you can about digitization and futureproof spec data management systems, then conversing with successful champions from other companies. There is no one-size-fits-all approach to starting the conversation within a company, but learning from successful stories will set a champion up for their own success.
Sustainability and SKU proliferation within spec management
Though sustainability may have taken a backseat during the pandemic, it is roaring back and with it will come an increase in regu lations. Wright explained that companies cannot truly be sustain able unless they know what they have, which requires proper data management, and this will further enable sustainability goals to be more easily achieved on time.
Wright further emphasized that sustainability is not an event, but a continuum. We cannot know how sustainability will be defined in coming years and the rules may change dramatically as methods to dispose of or recreate products shifts. The industry must have the data to keep up with the rapid changes that could and will come.
Data Formatting Hurdles Affect Sustainability, Efficiency
It’s not enough to simply digitize data, which continues to be a hurdle in life sciences. Product and packaging data needs to be formatted in a standardized way that computers understand—and that can be understood across the various platforms and soft ware—in order to truly be actionable.
There are still issues where a company can’t crunch numbers across a variety of platforms because, for example, one timestamp is HH:MM while one is HH:MM:SS, or temperature is formatted with different numbers of significant figures.
This has sustainability implications—how can a company say they’re improving when they don’t have true visibility into “what they have” in packaging, supply chain, etc. across the organiza tion? Wright noted that Gartner predicts 90% of public sustainable packaging commitments won’t be met by 2025, and that “it’s not for lack of desire or trying. It’s lack of data. If we don’t address the data issues, we won’t jump the chasm to be sustainable.”
Wright also noted that companies can still keep the numerous platforms and systems they’ve established but run a top-level process over them for data analysis and visualization.
Keren Sookne
The same is applicable to the proliferation of SKUs, which should not be looked at as an event or a project. A near decade ago, Kellogg’s and other companies began addressing the over-proliferation known as SKUzilla, trying to consolidate SKUs as they were negatively impacting the bottom line and supply chain partners.
However, Wright reported that there are more SKUs today than there were back when SKUzilla was first addressed. When seen as less of a project and more of a continuum, companies can begin to think of how the issue of SKU increase can be managed automat ically. “The reduction of SKUs and data is critical and you can do it,” said Wright.
Key takeaway
Duplicate data slows the speed to market. Wright noted that companies coming to Specright with 50,000 specs, in reality, may have ten useful specs. A current customer with 600,000 will prob ably have a hundred.
“Ultimately going forward, the two worlds of product and package have to be connected, and they have to be connected through data, and you have to be connected with your partners… I don’t think we can stop and hide from this anymore. I think we have to go through the difficulty of digitization, get to the other side, and do amazing things,” said Wright.
DIGITIZATION November/December 2022 • Healthcare Packaging | 21
Experts Still Concerned About Exceptions Under DSCSA
KEREN SOOKNE, EDITOR-IN-CHIEF
1. Data issues may cause double the product holdup when a mismatch of product and data are shipped.
Throughout HDA’s
TOP THREE TAKEAWAYS
2. Processes for exception handling need to be real-world ready, including SOPs for after-hours issues.
2022 Traceability Seminar in Washington
D.C., experts expressed concerns over undecided exception management processes that could lead to pharmaceutical products in limbo and disrupted supply chains under the Drug Supply Chain Security Act (DSCSA).



There are a number of ways that operations can go awry as saleable units, aggregated cases, and their associated data move through the supply chain. (Check out HDA’s Exceptions Guidelines for the DSCSA at hcpgo.to/406) Often receiving top billing in industry conversations: “Product, no data” exceptions.
As the name implies, this scenario occurs when a trading partner receives a product shipment without data. It may mean that the majority of the shipment and data match, and maybe a single case’s data is missing, or it could be a larger volume issue where an entire truckload (or more) arrives without corresponding data.
In a roundtable on exceptions in the afternoon, Jeff Primeau, VP

3. Experts have run into labels with inks that have faded in time, which can’t be scanned/ read properly.
Business Solutions at ICS, offered an example. An employee scans Box A before getting distracted by a coworker, and then inadvertently picks up Box B and places it on the pallet when they turn back around. This would result in the downstream trading partner receiving Box B physically, but with Box A’s data.
As Primeau noted, any exception like this will technically result in double the affected product. Not only is Box B held up at the trading partner’s site, but Box A now can’t be shipped anywhere as it scans as “shipped” in the system. One error—two boxes hamstrung. It may not seem like a big deal when talking about two units in the example, but at scale, this could tie up a serious amount of product with millions of units moving around the globe every day. This issue becomes more acute for products requiring temperature control or delicate handling, and therapies in short supply.
Manufacturers and distributors must consider how exception notification and resolution will be handled. This sparked a number
LOGISTICS/REGULATORY 22 | Healthcare Packaging • November/December 2022
↙ From left at the HDA 2022 Traceability Seminar: Moderator Anita T. Ducca, HDA; Matt Sample, AmerisourceBergen Corporation; Arthi Nagaraj, Sanofi US; Julie Malone, Value Drug Company; and Michael Mazur, Pfizer Inc.
of questions that must be worked out by the life science logistics community:
• In the example above, if the trading partner sends an image of Box B’s label to the manufacturer confirming that it matches the manufacturer’s data for Box B, can the box somehow be marked as “shipped” in the system (and subsequently marked “received” by the trading partner)?
• Will data correction of this nature be possible, or does it go against the spirit of the DSCSA to alter a product’s data/status?
• Would the trading partner have to send Box B back? Would Box A need to be located and shipped? If the issue is discovered days or weeks later, how will Box A be located in a crowded warehouse?

• Who will perform such a search? With labor already stretched thin, where will manufacturers come up with headcount to deal with the investigation and resolution?
• Can solution providers come up with a process to help auto mate the resolution of these issues? What tools could the indus try benefit from to deal with lot numbers that don’t match, and
other
exception scenarios?
In a panel discussion, Matt Sample, Senior Vice President, Manufacturer and Replenishment Operations, AmerisourceBer gen Corporation, also reminded attendees that any processes for exception handling need to be real-world ready. “This can happen at one o’clock in the morning. My national distribution center is 24/6. How are you doing off-hour support?” he asked, cautioning manufacturers that if someone ships seven trucks to a centralized hub and he doesn’t receive data, he can’t have his team unload them.
Sample also mentioned a labeling issue heard mere weeks prior at an AIM webinar (for more, see pp. 24). They’re running into “ink degradation, meaning it was probably good when it left the line six months ago, but now it’s faded,” and can’t be scanned/read properly.
Parting advice from panelists
Michael J. Mazur, Director, Trade Operations, Pfizer Inc., said they have 500 manufacturers to onboard and they have asked to get them engaged early. “2016 is the first time we did EPCIS exchange— it takes time to get them onboarded. So, we’re managing inbound, trying to get our partners to recognize you can’t wait until October 2023. In fact, if your project plan says, ‘October 23 go-live,’ you need go back.”
Arthi Nagaraj, Manager, Serialization, Analytics and Special Projects, Sanofi US, said, “Get involved. There are a lot of workgroups… HDA, PDG, GS1, everyone is working toward a common purpose. Mike [Mazzur] once told me, ‘You’re not alone, we’re all in this together’ and that’s what I’ll tell you guys. We are in this together. Every company has a slightly different process or a slightly different system, but your company is going through the challenges we’re all going through. Reach out to your trading partners.”
Julie Malone, Regulatory Affairs Manager, Value Drug Company, noted, “We’re going to have a crunch time right now. We’re working as hard as we can,” before going on to thank HDA and key members of the community. “It’s an awesome membership—take advan tage of that fully and the resources that are out there… Don’t operate in fear. I think a lot of people are operating in fear and misun derstanding, so you have to be proactive in the process. My advice is educate, educate, educate, and implement.”
LOGISTICS/REGULATORY November/December 2022 • Healthcare Packaging | 23
↑ If wholesalers are unable to read serial numbers or case label information, that product will need to be quarantined, researched, and verified, causing a supply chain disruption.
Labels: Practically as Important as the Medicine Itself
KEREN SOOKNE, EDITOR-IN-CHIEF
1. In becoming DSCSA-compliant, don’t over look item and case labels themselves.
TOP THREE TAKEAWAYS
2. Missing case labels or faded inks on serial ized products can lead to quarantined product.
While the pharmaceutical packaging community scram bles to comply in the home stretch of DSCSA regula tions coming into force in Nov. 2023, many are focused on “what’s digital”—interoperable systems, data storage, format ting, and sharing.

But at a recent AIM Virtual Summit panel on auto ID technolo gies and supply chain traceability, experts reminded attendees not to overlook something more tangible: the case label itself. [Editor’s note: some responses have been edited for brevity.]
As Brian Schmidt, Principal IT Generalist at McKesson , explained, pharmaceutical manufacturers spend a lot of time making
3. Labels can offer a wealth of item-level infor mation including cold chain excursions.
sure that the unit-of-use bottles are good-looking and durable to last the test of time to avoid disruptions, but they often pay less atten tion to the case labels. “As the cases or pallets of cases move through the supply chain, each one of those case labels is just as significant as the label that’s printed on the unit-of-use bottles,” he said.
Currently, if they’re not able to read those labels, they can place an internal label on the package to move that package through the supply chain. “But going forward, if we’re not able to read those serial numbers and the information on the case, there’s a disrup tion,” he said. “That product needs to be quarantined, researched, and looked at. That’s the next thing that we’re going to need to pay
TRACEABILITY/LABELING 24 | Healthcare Packaging • November/December 2022
attention to as an industry.”
























He said there have been conversations about what happens when this label falls off, and not only will this issue prevent them from moving product, but they’ve even had instances where the label remains but the color fades. “You have marketing get involved with some of the packaging labels—and they’re really innovative with special colors of pink and light sienna and other colors—and we’ve seen packages that have been serialized early on that their labels are starting to fade and we just can’t read them anymore. These kinds of things are what we, as an industry, need to continue to keep at the forefront, and to improve upon as we move forward,” he said.
Lori Bitar, Market Development Manager, Medical Device and Pharma at FLEXcon (global manufacturer of pressure-sensitive film products), has discussed the important issue of label failure with colleagues, particularly with regulations and changes in materials and container types. Being prepared ahead of time is key. “It’s very important to identify all of that upfront, because that’s the trunk of the tree that holds all of this other great technology,” she said.
Tony Fonk, President and CEO of SpotSee (provider of indicator technology and monitoring devices/services), agreed that while the label has historically been a bit of an afterthought, it’s now becom ing the “center of everything” as a result of track-and-trace require




































ments and new technologies coming online.












“What’s amazing to me is it’s not only identification, it’s verifi cation. There’s a lot of other information that you can get from it. It’s no longer just the printing of a serial number,” he said. He high lighted the use of QR codes for patients, who can access dosage for their particular product and videos on appropriate administration. “There’s just so much more power in what we describe as a label beyond just identification and track-and-trace.”


Looking forward, Fonk gets most excited about where technolo gies can be applied to help manufacturers identify whether the cold chain remained intact, where excursions took place, etc. “We’ve talked about ‘beyond the wholesaler,’ it gets really fragmented really fast. There’s a lot of variables when it comes to temperature, time, traffic, etc., that can cause a problem. We want to bring that intel ligence to the label, as well. The label is becoming this wonderful brain of any shipped item-level device or pharmaceutical. And I think there’s plenty more to be discovered.”

Echoing the sentiments above, Fonk added that with DSCSA regulations, if you lose a product’s identification label, it’s as good as gone. “You really can’t do much with that medication if you can’t turn around and re-verify that. So, [the label] is as important as the medicine itself, which is pretty amazing.”
Whether it’s Rx, OTC, or Medical Device filling, it’s hard to match our speed, capabilities, and know how. So, bring us your BoV filling or formulation challenge. We’d love to help. Contact us today: (727) 373-3970 websales@formsol.com | FormulatedSolutions.com





TRACEABILITY/LABELING
Bag on Valve Filling for Consumer Healthcare, We’re the Best in the Business!
Turnkey Concept-to-Consumer CDMO Solutions INNOVATE | FORMULATE | CREATE
IoPP’s 2022 AmeriStar Awards: Achievements in Healthcare Packaging
 KEREN SOOKNE, EDITOR-IN-CHIEF, AND MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD
KEREN SOOKNE, EDITOR-IN-CHIEF, AND MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD
TOP THREE TAKEAWAYS
Twenty-one professional and six student winners were selected as recipients of the AmeriStar Award, a design competition in the packaging industry produced by the Institute of Packaging Professionals (IoPP).
A roster of 21 judges from various packaging industry segments evaluated this year’s entries and examined each for product protection, packaging innovation and performance, economics, marketing, and environmental impact. Additional evaluations for 2022 coincided with introducing two new categories: design excellence and sustainability.
Judges reviewed package entries across the 19 categories for professional and student awards and the program’s prestigious Best in Show. Here, we profile winners from drug/pharmaceutical, medical device, and personal care product categories. See images of all the 2022 AmeriStar Award winners at IoPP’s site (QR code on pp. 29).
Drug and Pharmaceutical
Vital Proteins Collagen Peptides Powder Induction Liner from Selig Group and Closure from Pano Cap has an interrupted thread closure that allows for material reduction and less distortion of the thread when stripped from the mold. This pack-

PACKAGE DESIGN 26 | Healthcare Packaging • November/December 2022
2. User
3.
1. From monomaterials to source reduction, sustainability drove many new innovations.
friendliness, style, and inclusivity also scored among winners.
A first aid booklet and all-gender personal care won from among student entries.
↑ Medical Device: 3-Web Pouch Design from Oliver Healthcare Packaging and SeaSpine, Inc ↑ Medical Device: CapSure from Becton Dickenson-Surgery
age not only has a tamper band but has the added benefit of a heat induction liner, providing two levels of tamper protection. The container, scoop, and cap are fully recyclable HDPE, and the induction seal is of a “clean peel” variety, leaving no unsightly or non-recyclable material on the container land area.
Medical Device
The 3-Web Pouch Design from SeaSpine, Inc. and Oliver Healthcare Packaging (pp. 26) was designed with the end user in mind, offering a “multipack” configuration without increasing the shipping footprint. The surface treatment applied to the product exponentially increases patient safety and has sensitivities to contact materials and
environmental conditions. The packaging system needed to accommodate these variables while providing a user-friendly sterile barrier that is easy to manufacture.

CapSure™ from Becton Dickenson-Surgery (pp. 26) is used for a minimally invasive hernia repair procedure. With a miniature-sized retainer called SEEkeyMD®, this 1-g retainer allows additional support to the base tray to reduce sheet thickness from 40 mils to 30 mils. The 25% plastic reduction— equal to 295,417 16-oz. water bottles a year— and associated cost savings advanced the project.


Sustainable Packaging




AmSky™ Thermoform Blister System from




↑

PACKAGE DESIGN November/December 2022 • Healthcare Packaging | 27
Amcor Flexibles Healthcare North
Sustainable Packaging: Colgate Keep Toothbrush from Colgate-Palmolive
↑ Student Winner: OUCH! First Aid Kit from the University of Cincinnati
↑ Student Winner: Rinse from the University of Cincinnati






The most reliable and complete online directory of packaging and processing technology suppliers in North America – designed from the buyer’s perspective. Features include: You’re looking for... Are you looking for packaging or processing solutions and you don’t know where to start? • Plain-language filters so it’s easy to find what you need. • Type-ahead keyword search. • Visual navigation by machine or desired package type. • Packaging-specific filters that are tailored to each product category. ProSource.org
America takes a unique approach to recycle-ready blister packaging by using high-density polyethylene (HDPE) in a proprietary formulation to create both the formed blister and lidding, demonstrating a new usage of existing materials. This packaging system presents end users with a seamless, single-stream option for end-oflife disposal.
Colgate Keep Toothbrush from Colgate-Palmolive has a first-of-its-kind handle, made with aluminum instead of plastic–eliminating 80% of the plastic waste from a typical toothbrush (pp. 27). Keep’s
100% paper-based package allows consumers to recycle the packaging in a single waste stream and eliminates new plastic from being introduced into the environment. Colgate-Palmolive leveraged its expertise in thermoforming to deliver a package that is now fully recyclable at scale and operates well with established packaging lines.
Recyclable Monomaterial Pump – Future from Aptar Beauty + Home & Dermalogica is a fully recyclable monomaterial pump consisting of a plastic spring that makes it possible to recycle the bottle and pump as one unit. Because this Future pump is made from polyethylene (PE) only, which aligns with the most common materials used to make bottles (PE and polyethylene terephthalate [PET]), the complete packaging, including pump and bottle, is more efficiently recycled.
Health and Beauty Aids
Head & Shoulders CLINICAL Scalp Calming Treatment Tube from Viva Healthcare Packaging (Canada) Ltd. was developed to facilitate the precision application of its formula and provide a striking impact on the shelf ( this page ). The applicator head is injection molded, then welded onto the cut shoulders of an injection-molded polypropylene tube, the lowest density of commodity plastics. The applicator is made from polypropylene, maintaining a monomaterial design. The in-mold labels provide full coverage artwork from shoulders to end of crimp, 360 degrees around the tube.

Cosmetics
Repeat Refillable Airless from FusionPKG has a POM-free pump engine that is thoughtfully integrated into its refill cartridge, allowing users to refill the product without exposing the formula to air and providing users with a new pump engine at every refill. When a user engages the pump, the actuator on the outer component connects to the pump engine on the inner cartridge to initiate dispensing and
precision dosing.
Industrial/Commercial
TerpLoc® Automated Curing & LongTerm Storage Cannabis Pouch from Kinzie Advanced Polymers LLC dba Grove Bags takes a new approach to the idea of modified atmospheric packaging and allows industrial cannabis facilities to maintain up to 37% more terpenes and up to 7% more THC by using the natural moisture of the cannabis plant to support the internal microclimate, and keeps packaged product moisture loss below 1% variance.
Student Winners
OUCH! First Aid Kit from the University of Cincinnati is a first aid kit in book form (pp. 27). When opened, the left side is a home for tools, each in a designated pouch, and the right side is divided into pages of usage instructions on the top and items on the bottom. When the first aid book is closed, it takes the shape of a box to provide more structure and protection.
Rinse from the University of Cincinnati (pp. 27) is a collection of all-gender, sustainable personal care products geared toward bridging the gap between products specified for a single gender, intended to reduce the amount of both manufacturing and purchasing needs and result in a more inclusive and sustainable system. See images of all the 2022 AmeriStar Award winners at IoPP’s website:
PACKAGE DESIGN November/December 2022 • Healthcare Packaging | 29
↑ Health and Beauty: Head & Shoulders
CLINICAL Scalp Calming Treatment Tube from Viva Healthcare Packaging (Canada) Ltd.
CPGs Move More Manufacturing and Packaging Lines to Contract Facilities
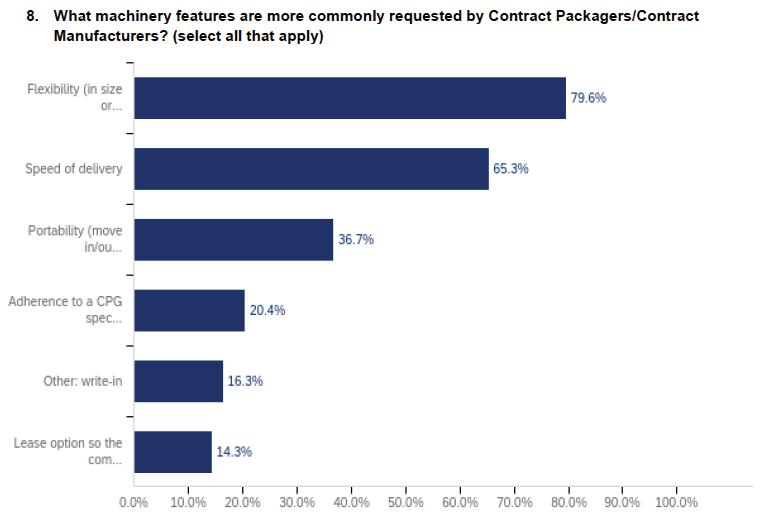
 SEAN RILEY, SENIOR NEWS DIRECTOR, PMMI MEDIA GROUP
SEAN RILEY, SENIOR NEWS DIRECTOR, PMMI MEDIA GROUP
TOP THREE TAKEAWAYS
1. Consumers’ desires are evolving; they seek convenience, variety, and more eco-friendly items.
2. Brand owners are shifting away from inhouse ops and moving to contract facilities to meet these needs.
The needs of brand owners and the machinery required from OEMs to churn out goods were already experiencing a sea change before COVID-19 completely turned supply and demand on its head. To keep up with these changes, many consumer packaged goods companies (CPGs) employed more contract manufac turers and packagers (CM&Ps) to stay ahead of the evolving whims of consumers seeking convenience, variety, and more eco-friendly items.
“[CM&P] business has grown drastically at an average growth rate of 10.2% over the last five years. We have segments growing much faster than that, but overall, every body’s experiencing a very high growth rate,” says Ron Puvak, Executive Director of The Association for Contract Packagers & Manufacturers (CPA).
Some of that is attributable to COVID-19, like in food and beverage, which are tradi tionally the most significant segments of CM&Ps. Still, the numbers indicate that the spike remained once CM&Ps met the imme diate need.
“CM&Ps haven’t lost that business,” Puvak says. “There has been a conversion [by CPGs] away from doing it in-house, or with dedicated lines, to moving it out to contract facilities.”
Another reason Puvak projects this growth to continue: once it was common for CM&Ps to have short-term contracts
3. Nutraceutical and over-the-counter (OTC) pharmaceuticals are a popular market for con tract service providers.
of six months or less, but many are now signing longer-term agree ments. Others are turning to CM&Ps as risk management against future supply chain issues. Rather than dedicate complete in-house lines, CPGs and processors can be more flexible and put the capital
CONTRACT PACKAGING 30 | Healthcare Packaging • November/December 2022
investment in the hands of the CM&Ps.
To understand these new business dynamics, PMMI Business Intelligence surveyed PMMI members on the current state of machinery sales to the CoMan/CoPack market to determine what areas are experiencing the most growth and if the growth is here to stay.
Food and beverage dominate the largest markets for equipment sold by responding OEMs with beverages, including alcohol (17%) as the largest market, proteins (11%), and confectionery (9%), joining medical devices (13%) in the top four. Nutraceuticals and supple
ments are the most popular market among respondents, with over half (53%) selling into that sector, followed by baked goods and confectionery at 41% each, beverages including alcohol and over-thecounter pharmaceuticals rounding out the top five, each at 39.3%.
When PMMI asked OEMs about the automation or technology needs CM&Ps are asking for, the most popular solutions are inte grated lines which would align with Puvak’s belief that some CPGs are moving entire processes to CM&Ps.
Where single pieces of machinery are needed, conveying, feed ing, and handling equipment (34%), bagging, pouching, and wrap ping equipment (32%), and cartoners/ casepackers (31%) were sold the most to CM&Ps. Puvak indicates that these numbers are typical as end of line equip ment traditionally dominates CM&P equipment purchases. He adds, however, that many CM&Ps are seeing opportuni ties to address labor issues via machinery purchases.
Variety packs, for example, are some thing that CM&Ps might have done previ ously with manual labor, but as robotics become more affordable and the strain on the labor force continues, he has seen an increase in incorporating OEM solutions to replace these repetitive functions.
According to Puvak, CM&Ps are looking for flexibility and asking themselves: “Can I purchase something that I can use a vari ety of ways and for a variety of products?”
In addition to flexibility, PMMI’s survey asked OEMs to note some specific demands and needs that CM&Ps are requesting when they purchase machines, and the following were the most common:
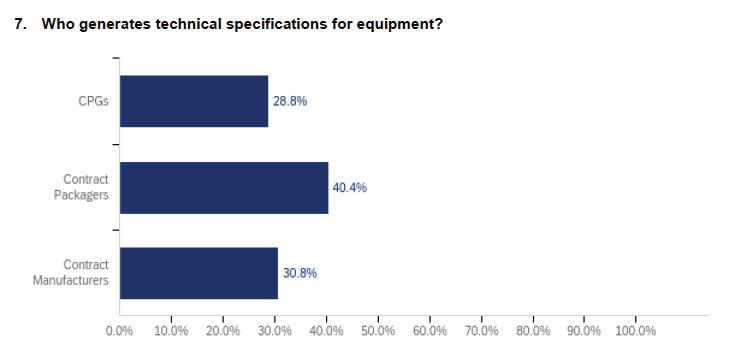

• Quick changeovers
• Predictive maintenance and produc tion monitoring
• Versatility for different package formats
Read this story on why high-mix, low-volume (HMLV) manufacturers are adopting flexible automation. Because many HMLV manufacturers are subcontractors, the reshoring of manufacturing operations has benefited them. Particularly in industries such medical device and automotive manufacturing, there is growing interest in working with suppliers who are as geographically close as possible—not only to shorten lead times, but to avoid supply chain disruptions and maintain better oversight of quality.
• Lines need to be built around new labeling requirements
• Easy-to-operate machinery
• Turnkey systems for secondary packaging
• Sophisticated vision systems and robotics
• Process automation
CONTRACT PACKAGING November/December 2022 • Healthcare Packaging | 31
Q&A: Foil Tariffs Pose Imminent Threat to Healthcare Costs, Lead Times
KEREN SOOKNE, EDITOR-IN-CHIEF
1. Serious supply chain continuity implications loom for light-gauge foil.
TOP THREE TAKEAWAYS
2. The packaging community awaits a sched uled decision from the Dept. of Commerce.
Aluminum foil is a critical packaging material for pharma ceuticals, medical devices, foods, personal care, consumer goods, and more, providing needed barrier protection from oxygen, light, moisture, and bacteria.
Unfortunately, U.S. converters are now facing extreme tariffs on light-gauge foil used for packaging medical devices, diagnostics, and pharmaceuticals, which are poised to impact costs and lead times. One of the most distressing components of the tariffs is the threat of retroactive price increases for foil already sold and consumed in 2021 and 2022.
What happened?
In 2018, the United States Department of Commerce (DOC) imposed duties on imports of Chinese aluminum. While the goal was to make U.S.-manufactured foil more competitive, domestic

3. Tariffs could be retroactively applied to goods sold in 2021 and 2022.
supply has only decreased since this regulatory action.
One foil mill shut down their converter-grade aluminum foil oper ations in January 2020. With no domestic sources for thin-gauge aluminum foil (< 0.001”) or for wide web aluminum foil available, U.S. manufacturers moved from China-sourced aluminum foil to aluminum foil from other countries, including the Republic of Korea and Thailand.
As FPA president and CEO Alison Keane stated to the House Ways and Means Committee in June 2020, “At a time when sterile packaging for food, health and hygiene, and medical equipment is more important than ever, and as U.S. manufacturers are suffering from the worst economy in decades, the Adminis tration should be looking at ways to alleviate supply chain burdens, not increase them.”
RAW MATERIALS 32 | Healthcare Packaging • November/December 2022
Then in July 2022, the DOC announced a self-ini tiated investigation of aluminum foil duties target ing light-gauge foil that has been processed to final gauge in Korea and Thailand. The DOC’s concern is that companies are engaging in circumvention of the tariffs because both countries may be import ing inputs (base metal and foil stock) from China.
What’s next?
This is a breaking story, with schedules accu rate as of press time. Follow Healthcare Packaging on LinkedIn to stay up to date:
On December 15, 2022, the DOC is scheduled to announce its preliminary determination. If they determine circumvention did take place, they may announce duty rates, with payments possibly being collected a mere 10 days later on December 25 (Christmas Day). This may include retroactive duties for product imported as far back as Nov. 4, 2021. On May 14, 2023, the DOC is scheduled to issue a final determination at which point rates may be further adjusted.
The Flexible Packaging Association (FPA) is coordinating lobby ing efforts around this investigation, in parallel with individual supplier and end user lobbying efforts. We sat down with PAXXUS’ Chief Strategy Officer Dwane Hahn to discuss this time-sensitive issue, the implications of the DOC’s upcoming decision, and the need for packaging suppliers and end users to make their voices heard.
Keren Sookne (KS): How has the market changed since the U.S. imposed these duties on Chinese aluminum foil imports? Let’s talk about global thin-gauge foil sources that do not use inputs from China.
Dwane Hahn (DH): To be specific, there are no U.S. suppliers of thin-gauge foil, so we do not have the option to source from the U.S. even if we wanted to, even at five or 10 times the price.
Back in 2018, when the China tariffs were implemented, every company in the U.S. tried to move to acquire their foil needs outside of China. This caused a ripple effect that pushed lead times out and drove up pricing—basic supply and demand economics—because China was obviously a significant supplier of aluminum foil to many U.S. converters like PAXXUS.
Fast forward to July of this year, when the DOC entered into a self-initiated circumvention investigation—they became concerned over the source of foil sourced from South Korea and Thailand. If these tariffs are deployed to these two countries, that would mean that the supply chain is going to be even further constrained. There is not enough capacity in the world to accommodate what people are buying from Thailand and South Korea.
Converter lead times are going to increase dramatically, along
with prices. We’re already seeing that impact in the price of foil, which is broken up into two things: raw aluminum ingots and the manufacturing of the aluminum foil itself, rolling it back and forth to thin it out. That price has gone up significantly.
KS: Can you elaborate on the financial impacts to manufacturers who rely on thin-gauge aluminum foil for their packaging?
DH: The financial impact is severe. If they’re passed, the duty rates will be dependent upon the source and currently range from 78.03% to 124.36% of import value. The People’s Republic of China (PRC)-wide rate is 108.43%.
The thicker the foil, the higher the percentage increase. It ranges depending on the final lamination from 20% up to a 45% increase for 2 mil foil laminates.
KS: Beyond the financial issue, is there potential for a public health crisis because companies can’t get the materials they need for packaging products in time?
DH: Yes, it’s causing a lot of fear. There is the potential to cause a medical device supply crisis, which is definitely not what any of us want.
KS: This is largely speculative, but is there any chance that the lack of domestic foil sources will play into the DOC’s action?
DH: It’s a good question, and it’s hard to answer. The origin of the tariffs back to 2018 was to try and drive investment into the U.S. aluminum foil market. It just didn’t happen to work.
If you’re familiar with this issue, and the way it’s written right now, the action is essentially driving manufacturing away from the U.S. MDMs [medical device manufacturers] may decide they’d rather purchase fully laminated foil from countries without tariffs, instead of from local U.S. converters. That would be driving manu facturing and business away from the U.S. While the DOC action was definitely well-intentioned, the potential impacts are the inverse of the real objective.
The DOC wants to have aluminum foil manufacturing in this country, which is awesome, and we agree. And over the last year, our customers have been extremely focused on what we’re doing to reshore or manufacture materials close to their plants. Everyone wants to de-risk their supply chains. This is why I understand what the DOC is doing—we’re all trying to do the same thing. But we should go about it a little differently.
RAW MATERIALS November/December 2022 • Healthcare Packaging | 33
KS: This policy action will obviously impact end users—MDMs and pharma manufacturers—if the duties are imposed. What’s been the reception when you explain this to your customers?
I can’t tell you how many calls I’ve had to explain the situation to the high-level folks at our customers. At first, they may not under stand how it affects them. Then I explain what it means to us: “We’re not drawing a line in the sand. We’re digging a trench, and we’re digging in, because our existence depends on how we manage this.”
Imagine your cost of goods sold as an organization goes up by 50% on December 15, and you potentially owe the government all that money on Christmas Day, 10 days after the determination. And it could be retroactive for a year. How do you deal with that? What if your house payments or rent went up by 50%, retroactive for a year, and it’s due in 10 days? It’s a really serious issue.
KS: So what are packaging suppliers doing in response? What’s your desired outcome?

DH: At PAXXUS, we have filed a separate brief with the DOC, and Dhuanne Dodrill, our CEO, is actively meeting with the offices of Illinois senators. We’re participating in lobbying efforts on The Hill, with priority placed on demonstrating the impact on medical device packaging. The Flexible Packaging Association (FPA) has also


organized lobbying efforts among suppliers to educate legislators on the impact of these potential duties. [Get involved by contacting FPA President and CEO Alison Keane. —KS
The real ask of the DOC is to just stop the action altogether. If the determine circumvention occurred, a delay on the tariffs for some period of time would be the next best outcome. If we have time, we could work with our customers to manage this. It’s this retroactive piece that’s the scariest. We’re hiring people to manage this because there are so many moving pieces and it’s very compli cated. For example, the Department of Commerce could come back

Contact Your Elected Officials
PAXXUS is urging customers to get involved and explain to offi cials:






+ The U.S. does not have sufficient capacity or even capability to meet needs domestically.
+ A global shortage of aluminum foil has already extended lead times by several months.

+ The action will further exacerbate lead time challenges and potentially result in stock-outs of critical products.

+ Prices will continue to escalate at non-impacted mills (specifi cally the fabrication portion of the aluminum foil price). This will further reinforce inflationary pressures.




34 | Healthcare Packaging • November/December 2022
and say, “For Mill A in Thailand, the duty goes back to July at 100%. For Mill B, it goes back to November at 80%. For South Korea, Mill C goes back to August at 82.1%.”
We know that lobbying has worked in the past. There was a circumvention investigation in the solar industry, and it was found in the affirmative, meaning the DOC said that yes, tariffs do apply. However, the industry was successful in lobbying, and the tariffs were deferred for two years, and no retroactive tariffs or duties were applied.

KS: Is there anything MDMs and pharma manufacturers can do at this point?

DH: Definitely. We’re asking our customers to get involved and lobby on The Hill. Heavyweight companies have government affairs teams, and many are already hard at work with lobbying efforts.
Only Interested Parties (i.e., importers of record) were able to submit a formal response to the DOC itself, but any company can go to The Hill and lobby their representative. [See sidebar for tips. —KS] We’re asking manufacturers to approach their senators and house representatives and demonstrate the implications of this action on the market, so the elected officials can then go to the DOC.
There are also efforts among policy organizations that repre sent some of our customers. That’s another route for manufactur ers to go, especially if they’re smaller and don’t have the time and resources for their own government affairs teams. There’s strength in numbers.
In general, for all manufacturers, the goal is to be loud and to make your voice heard in whatever vehicle you have.
KS: I know many in the industry are extremely worried about the outcome. What’s giving you hope?
DH: Fortunately, it’s been somewhat heartwarming. Many of the conversations with our customers, once they understand the exact situation, it goes from not understanding to, “What can we do to help you? This isn’t acceptable.”
In the beginning, I think some of us felt like we were on an island, dealing with this alone, and not sure what was going to happen. But the industry is banding together.
It’s still a really bad issue. Ultimately, it will be passed to the consumer if the tariffs are passed. It’s a bad policy and a major chal lenge, but the industry is united up and down the supply chain on trying to minimize the risk to everyone.
Filling and Capping Solutions









RAW MATERIALS (815) 363-3524 | dispenseworks.com | cap-fill.com | info@dispenseworks.com Dispense Works Inc
Streamline your Production with a Complete Solution for Automated Assembly From compact benchtop designs to full scale production machines. We have the equipment to fit your needs! Pharmaceutical • Food• Healthcare Chemical • Industrial and many other Industries Large Capacity Benchtop Models Programmable Torque Filling Capping Different Size Vials & Bottles Production Models Ring Dex Models
Made in the USA
Automated Stacker, Bander Picks Up Manual Slack for OTC Manufacturer
MELISSA GRIFFEN, EDITOR-IN-CHIEF, CONTRACT MANUFACTURING & PACKAGING
TOP THREE TAKEAWAYS
1. Reese Pharmaceutical improved its packag ing line productivity by 25% through stacking and banding automation.

2. The AP Stacker can provide standalone stacking or be coupled with a bander (as well as with a CE Auto Roll Change.)
In early 2020, understaffing was a challenge for many manufac turers as the COVID-19 pandemic began to spread like wildfire. But even before this worldwide situation, everyday staff ing issues were already leading to productivity hurdles for Reese Pharmaceutical Company—a privately held manufacturer of OTC branded and private label products selling to national and regional chains, food and grocery stores, drug wholesalers, co-ops, and inde pendent pharmacies.
In 2019, the Cleveland-based company’s secondary packaging line was fully automated, except for the end of the line, where finished product bundling and shrink-wrapping were still done manually by a two- to three-person team. Each bundle was oriented, stacked, and banded by a human technician. This forced the line to operate at 60% to 70% of its throughput capacity versus the 80% to 85% that was possible if the line could run at the full speed afforded by automation. Three main issues caused this decrease:
• A bottleneck in the manual carton stacking component, which couldn’t keep up with the 18,000 to 20,000 unit per day production, resulting in frustrating downtime
• Changes to usual staffing arrangements due to illness, position changes, and other staffing issues, leading to understaffing of the manual component
• Realizing the huge importance of solving these problems, Reese researched possible equipment solutions, but struggled initially to find anything already on the market
Assuming there was no existing solution, they turned to the more expensive and headache-inducing option of arranging a custom solution that could automate the processes involved and integrate with the existing line—a process Reese’s Operations Manager Jeff Reese refers to as, “a big mess.”
“Going custom would have been tough and expensive. We didn’t have any machines like the one we needed,” he says. “There would
3. The machine eliminated bottleneck issues and changes to usual staff arrangements to accommodate order increases.
↑ Cartons are properly oriented before stacking.
have been a lot of head-scratching and legwork to design and build something from the ground up. It would have put a lot back on our plate given how much we’d have to be involved.”
Not only would this process have been slow and demanding, but there would be no guarantee that the custom machine would oper ate successfully once installed.
Integrated system solves customization dilemma
While looking into custom solutions, Reese was introduced to Controls Engineering, and its Madison Banders Array Pack (AP-25). This automated stacking and banding system is capable of fully integrating with the Reese line, making it the right fit for the company’s needs.
The AP-25 provides automated carton stacking, indexing, and
AUTOMATION 36 | Healthcare Packaging • November/December 2022
banding for both vertical and horizontal stacked cartons. It has been designed with enough flexibility to interchange quickly between various bundle configurations as well. Jason Kenney, President at Controls Engineering, reports that changeover typically takes five minutes, thanks in part to synchronization of the Festo C Model CMMP four-axis servo-controlled motors for quick changeover between package and stack configurations, with less stress on the motors than typically found in multi-motor solutions. The system can handle items from 1- x 1- x 3- in. to 12- x 11- x 4-in. View the machine in action at: hcpgo.to/ap25
The AP Stacker can:
• Provide standalone stacking
• Be coupled with the bander
• Be coupled with the bander and CE Auto Roll Changer
After speaking with Controls Engineering and determining that the AP-25 was indeed the solution Reese had been looking for, the company decided to implement the Array Pack as a more or less turnkey stacking and banding solution. The AP-25 effectively elim inated the bottleneck and further automated Reese’s secondary packaging line.
Success leads to additional purchases
Following their first Array Pack installation in March of 2019, the immediate impact and sustained improvement in productivity prompted Reese to procure two additional machines in an effort to reach higher productivity levels on other lines as well.

“As far as I know, there wasn’t anything like the Array Pack on the market. We went to the trade shows. We did our homework. There was nothing else out there,” explains Reese. “If we went custom, we would have obviously incurred way more costs. It would have taken way more time, and honestly, it probably wouldn’t be anywhere near the Array Pack in terms of capability. It perfectly fit our processes.
We met the company—Jason Kenney and the customer service were incredible. I was sold pretty much instantly.”
The company’s second Array Pack installation followed in May of 2020. A third installation took place in August of 2020. Reese has plans to purchase additional machines from Controls Engineering.
Benefits from increased productivity to increased customer satisfaction
Since their first implementation in March of 2019, Reese has seen a 20% to 25% increase in throughput productivity. The company has also witnessed a 50% reduction in labor costs as the personnel required on the line has been cut in half. “Almost immediately, we were able to reassign positions and reduce staff on our line from four people down to two. In some instances, we’ve taken it from six people down to three, depending on the job that we’re running,” Reese says.
Furthermore, with a variety of change-out parts provided as part of every install, Reese’s frontline technicians are able to save each configurability setting it uses across its various product lines directly into the machine. This enables technicians to save time switching over, which minimizes downtime.
Reese notes that the configurability is a great feature in that there are “just a couple change-out parts and then changing the setting. It takes very little time and our basic technicians can do it with ease. That time savings really adds up as we grow and add more products to the line.”
Another benefit Reese has seen is better customer satisfaction with the convenient stretch film bundles. While not easily quanti fied, the company reports a more consistent, user-friendly bundle from the Array Pack compared to the manual bundles it produced before.
“The bundles are more consistent and definitely improved aesthetically. They’re just nicer to look at and handle. Without the human element, the cartons are being oriented one way and one way only. When our customers get our packages, you know that it’s a lot cleaner, they’re a lot neater, a little more consistent. That might seem like a small detail, but it’s important to us because it’s really important to them,” he says.
Reese highlights the importance of the strong customer service experience that has served to strengthen and expand the relation ship. “I personally haven’t dealt with a company that’s been as recep tive and helpful as Controls Engineering,” he says. “Anytime I can recommend them to somebody else that is dealing with the same issues that we were, I do. Just because of the experience and the relationship that we have been able to have with Controls [Engi neering]—I really don’t have a bad thing to say. And I couldn’t ask for anything more from a partner than what they’ve been able to offer us. They’ve made our lives easier in more ways than one.”
AUTOMATION November/December 2022 • Healthcare Packaging | 37
↑ Reese’s first installation was followed by two more machines.
1 Tablet Press Solution
SYNTEGON PACKAGING
TECHNOLOGY
+ The automatically adjustable pow der feeder and Automated Process Development (APD) software for tablet presses allow users to determine ideal process parameters for each product.

+ The APD tool is suited for development and for optimizing existing processes.
2
Prefilled Syringes With RFID Labels

SCHREINER MEDIPHARM AND SCHOTT PHARMA
+ Combined syringes/RFID labels opti mize processes in hospital inventory management, patient care, and docu mentation, as well as the digital identi fication of drugs and med devices.
+ The RFID labels combine convention al product information and unique, digital identifiers for each unit; digital first-opening indication can be incor porated for syringe integrity.
3 Paratubing Solutions
TEKNIPLEX HEALTHCARE
+ Paratubing can be produced in up to eight tube configurations, including cus tom-colored, textured, or striped tubes to distinguish between flow paths.

+ Multiple single lumen tubes are bonded together for operations such as arterial drills or biopsies, and med devices for wound management, laparoscopic, neuro vascular, and ophthalmic procedures.
4 Recycle-ready Laminate
CONSTANTIA FLEXIBLES
+ Perpetua Alta mono-polymer is the first full polypropylene recyclable solution with high resistance against chemically aggressive prod ucts, such as pharma liquid and gel composi tions, for stick packs, sachets, and more.

+ It offers protection from oxygen, water vapor, and light at a reduced weight.
5 Connected Printer Services
MARKEM-IMAJE
+ Using a tested and proven IoT platform, Mi Vista™ connected printer services streamline and improve daily coding operations across an entire printer fleet.

+ The services provide real-time visibility and printer alerts; advanced remote diagnostics, predictive analytics, and automated improve ment alerts; and proactive prevention advice.
6 Nasal Pump
SILGAN
+ Offering over 40 different spray config urations, the Gemini™ BE nasal pump is adaptable to match specific formulations for performance and quick time-to-market.
+ The pump has the capability to match existing reference listed drug devices across a range of formulations and the ability to modify spray characteristics, such as DSD, plume geometry, and spray pattern.

38 | Healthcare Packaging • November/December 2022
NEW PRODUCTS
PMMI Media Group, 401 North Michigan Ave., Suite 1700 Chicago, IL 60601
Elizabeth Tierney, 401 North Michigan Avenue, Suite 1700 Chicago IL 60611

Keren Sookne, 401 North Michigan Avenue, Suite 1700 Chicago IL 60611 PMMI
Worldgate Drive, #200, Herndon VA 20170
Dispense Works Inc. 35 www.dispenseworks.com
Formulated Solutions LLC 25 www.formulatedsolutions.com G&K-Vijuk Intern. Corp. 18 www.guk-vijuk.com Jadex 40 www.jadexinc.com Markem-Imaje 2 www.SystechOne.com/Pharma Paxiom Group 5 www.paxiom.com PMMI ProSource 28 www.prosource.org Voices of Women in
AD INDEX 39 | Healthcare Packaging • November/December 2022
Statement of Ownership, Management, and Circulation (Requester Publications Only) 1. Publication Title 2. Publication Number 3. Filing Date 4. Issue Frequency 5. Number of Issues Published Annually 6. Annual Subscription Price (if any) 8. Complete Mailing Address of Headquarters or General Business Office of Publisher (Not printer) 9. Full Names and Complete Mailing Addresses of Publisher, Editor, and Managing Editor (Do not leave blank) Publisher (Name and complete mailing address) Editor (Name and complete mailing address) Managing Editor (Name and complete mailing address) 10. Owner (Do not leave blank. If the publication is owned by a corporation, give the name and address of the corporation immediately followed by the names and addresses of all stockholders owning or holding 1 percent or more of the total amount of stock. If not owned by a corporation, give the names and addresses of the individual owners. If owned by a partnership or other unincorporated firm, give its name and address as well as those of each individual owner. If the publication is published by a nonprofit organization, give its name and address.) 11. Known Bondholders, Mortgagees, and Other Security Holders Owning or Holding 1 Percent or More of Total Amount of Bonds, Mortgages, or Other Securities. If none, check box. PS Form 3526-R July 2014 [Page 1 of 4 (See instructions page 4) PSN: 7530-09-000-8855 None 7. Complete Mailing Address of Known Office of Publication (Not printer) (Street, city, county, state, and ZIP+4 ®) Contact Person Telephone (Include area code) Full Name Complete Mailing Address Complete Mailing Address Full Name 12. Tax Status (For completion by nonprofit organizations authorized to mail at nonprofit rates) (Check one) Has Not Changed During Preceding 12 Months Has Changed During Preceding 12 Months (Publisher must submit explanation of change with this statement.) The purpose, function, and nonprofit status of this organization and the exempt status for federal income tax purposes: PRIVACY NOTICE: See our privacy policy on www.usps.com. Healthcare Packaging Magazine 2 1 5 4 3 6 6 6 Oct 1, 2022 6x a year 6 N/A PMMI Media Group, 401 North Michigan Ave., Suite 1700 Chicago, IL 60601 George Shurtleff 312-205-7890
Packaging and Processing 9 www.pmmi.org.ppwin
11911
PS Form 3526-R, July 2014 (Page 2 of 4) Extent and Nature of Circulation Average No. Copies Each Issue During Preceding 12 Months No. Copies of Single Issue Published Nearest to Filing Date 15. 14.Issue Date for Circulation Data Below 13. Publication Title a.Total Number of Copies (Net press run) In-County Paid/Requested Mail Subscriptions stated on PS Form 3541. (Include direct written request from recipient, telemarketing, and Internet requests from recipient, paid subscriptions including nominal rate subscriptions, employer requests, advertiser’s proof copies, and exchange copies.) d. Nonrequested Distribution (By mail and outside the mail) b. Legitimate Paid and/or Requested Distribution (By mail and outside the mail) c.Total Paid and/or Requested Circulation (Sum of 15b (1), (2), (3), and (4)) Outside County Paid/Requested Mail Subscriptions stated on PS Form 3541. (Include direct written request from recipient, telemarketing, and Internet requests from recipient, paid subscriptions including nominal rate subscriptions, employer requests, advertiser’s proof copies, and exchange copies.) (1) (2) (4) Requested Copies Distributed by Other Mail Classes Through the USPS (e.g., First-Class Mail®) Sales Through Dealers and Carriers, Street Vendors, Counter Sales, and Other Paid or Requested Distribution Outside USPS® (3) Nonrequested Copies Distributed Outside the Mail (Include pickup stands, trade shows, showrooms, and other sources) (4) (1) Outside County Nonrequested Copies Stated on PS Form 3541 (include sample copies, requests over 3 years old, requests induced by a premium, bulk sales and requests including association requests, names obtained from business directories, lists, and other sources) (2) In-County Nonrequested Copies Stated on PS Form 3541 (include sample copies, requests over 3 years old, requests induced by a premium, bulk sales and requests including association requests, names obtained from business directories, lists, and other sources) (3) Nonrequested Copies Distributed Through the USPS by Other Classes of Mail (e.g., First-Class Mail, nonrequestor copies mailed in excess of 10% limit mailed at Standard Mail ® or Package Services rates) Total Distribution (Sum of 15c and e) f. Total Nonrequested Distribution Sum of 15d (1), (2), (3) and (4)] e. Copies not Distributed (See Instructions to Publishers #4, (page #3)) g. Total (Sum of 15f and g) h. Percent Paid and/or Requested Circulation (15c divided by 15f times 100) i. *If you are claiming electronic copies, go to line 16 on page 3. If you are not claiming electronic copies, skip to line 17 on page 3. Healthcare Packaging Magazine Sept/Oct 2022 18,933 19,981 16,830 16,843 0 0 0 0 0 0 16,830 16,843 0 0 0 100 0 0 0 2183 100 2,183 16,930 19,026 1,100 955 18,030 19,981 99% 89% Statement of Ownership, Management, and Circulation (Requester Publications Only) 16.Electronic Copy Circulation Average No. Copies Each Issue During Previous 12 Months No. Copies of Single Issue Published Nearest to Filing Date a.Requested and Paid Electronic Copies b.Total Requested and Paid Print Copies (Line 15c) + Requested/Paid Electronic Copies (Line 16a) c.Total Requested Copy Distribution (Line 15f) + Requested/Paid Electronic Copies (Line 16a) d.Percent Paid and/or Requested Circulation (Both Print & Electronic Copies) (16b divided by 16c Í 100) I certify that 50% of all my distributed copies (electronic and print) are legitimate requests or paid copies. 18.Signature and Title of Editor, Publisher, Business Manager, or Owner Date I certify that all information furnished on this form is true and complete. I understand that anyone who furnishes false or misleading information on this form or who omits material or information requested on the form may be subject to criminal sanctions (including fines and imprisonment) and/or civil sanctions (including civil penalties). 17.Publication of Statement of Ownership for a Requester Publication is required and will be printed in the issue of this publication. Healthcare Packaging Magazine 2,002 1,976 18,832 18,819 18,932 21,002 99% 90% December 2022 George Shurtleff PMMI Media Group Production Manager 630-329-1967 10/1/2022






































































 —Keren Sookne
—Keren Sookne
 —Keren Sookne
—Keren Sookne













 MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD
MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD







 MELISSA GRIFFEN, EDITOR-IN-CHIEF, CONTRACT MANUFACTURING & PACKAGING
MELISSA GRIFFEN, EDITOR-IN-CHIEF, CONTRACT MANUFACTURING & PACKAGING





























 KEREN SOOKNE, EDITOR-IN-CHIEF, AND MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD
KEREN SOOKNE, EDITOR-IN-CHIEF, AND MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD






















 SEAN RILEY, SENIOR NEWS DIRECTOR, PMMI MEDIA GROUP
SEAN RILEY, SENIOR NEWS DIRECTOR, PMMI MEDIA GROUP
















































































