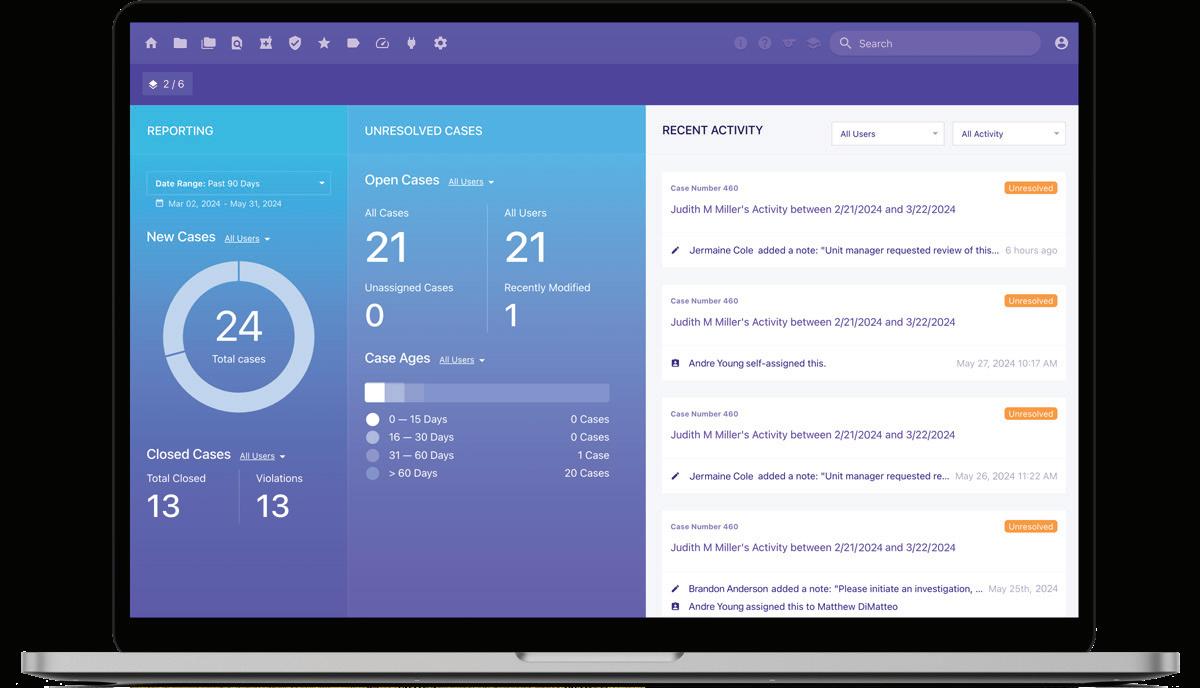
Partner with us to optimize pharmacy purchasing, simplify 340B administration, ensure compliance, and benefit from the dedication and knowledge

subject matter and gain insight through expert videos.









































































































































































Partner with us to optimize pharmacy purchasing, simplify 340B administration, ensure compliance, and benefit from the dedication and knowledge

subject matter and gain insight through expert videos.








INDICATIONS1
Treatment of Hypoprothrombinemia Due to Vitamin K Deficiency or Interference: Phytonadione injectable emulsion is indicated for the treatment of the following coagulation disorders which are due to faulty formation of factors II, VII, IX and X when caused by vitamin K deficiency or interference with vitamin K activity:
· anticoagulant-induced hypoprothrombinemia caused by coumarin or indanedione derivatives;
· hypoprothrombinemia due to antibacterial therapy;
· hypoprothrombinemia secondary to factors limiting absorption or synthesis of vitamin K, e.g., obstructive jaundice, biliary fistula, sprue, ulcerative colitis, celiac disease, intestinal resection, cystic fibrosis of the pancreas, and regional enteritis;
· other drug-induced hypoprothrombinemia where it is definitely shown that the result is due to interference with vitamin K metabolism, e.g., salicylates.
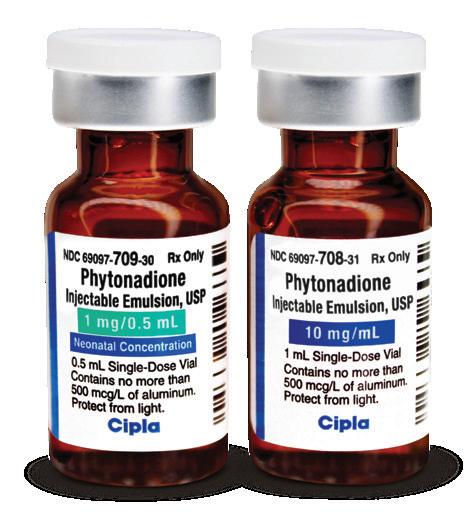
Prophylaxis and Treatment of Vitamin K-Deficiency
Bleeding in Neonates: Phytonadione injectable emulsion is indicated for prophylaxis and treatment of vitamin K deficiency bleeding in neonates.
BOXED WARNING1
WARNING - HYPERSENSITIVITY REACTIONS WITH INTRAVENOUS AND INTRAMUSCULAR USE: Fatal hypersensitivity reactions, including anaphylaxis, have occurred during and immediately after intravenous and intramuscular injection of phytonadione injectable emulsion. Reactions have occurred despite dilution to avoid rapid intravenous infusion and upon first dose. Avoid the intravenous and intramuscular routes of administration unless the subcutaneous route is not feasible and the serious risk is justified.
Please see www.ciplausa.com











Whether planning a cleanroom renovation or building a new cleanroom, Modular Devices’ mobile cleanrooms provide an expedited option combining a modular and prefabricated approach by delivering a readyto-use, turnkey cleanroom that only requires utility connections upon delivery. Our pharmacy compounding cleanrooms are available to rent or purchase! Experience unmatched quality, state-of-the-art materials and equipment, a cleanroom that will exceed your expectations, and USP 797/800!
14' wide x 48' long
Meets and Exceeds USP797 & 800
Large Fleet and Nationwide Coverage
Hands-free Scrub Sinks
Hands-free Interlocked Doors
Continuous Data Logging & Monitoring
Includes 4' BSC’s and 4' LFH’s
Includes Dedicated Refrigerators
HEPA Filtered Interlocked Passthroughs
Dedicated Haz & Non-Haz Gowning Rooms
USP 800 Negative Pressure Unpack/
Storage Room
Hands-free Flush Mount Intercoms
Guaranteed Environmental Control
Guaranteed Certification





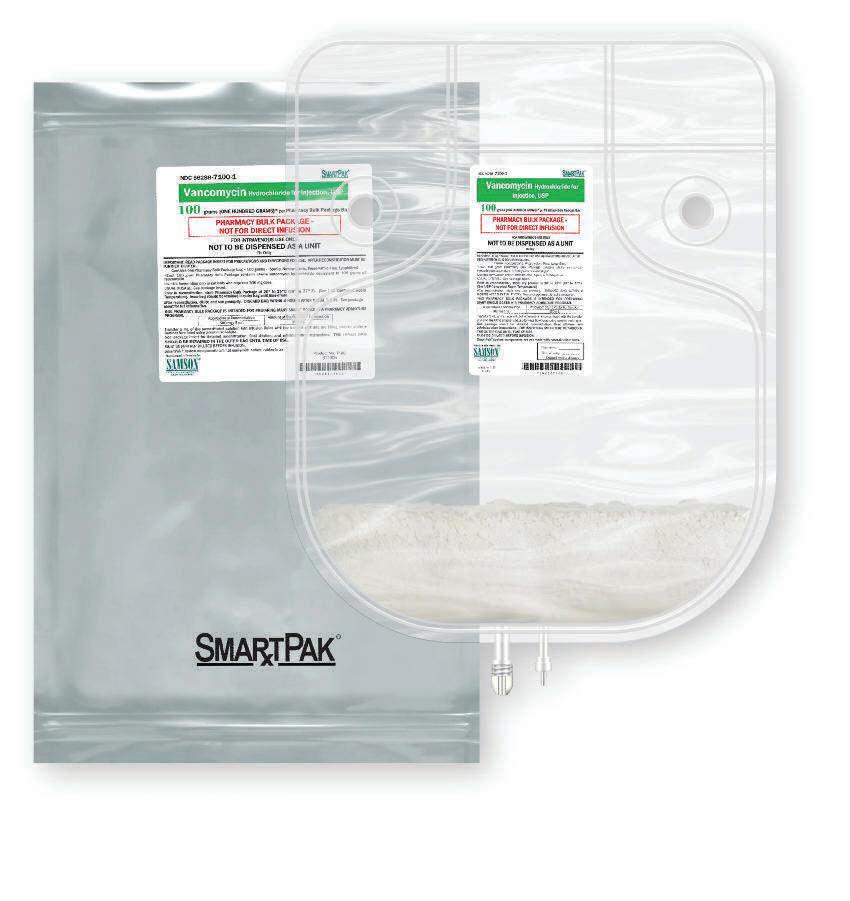


Propofol Injectable Emulsion, USP supplied by Samson Medical Technologies, under the Avet label, is the smart choice for your pharmacy service, offering you the same formulation as Diprivan® , free of benzyl alcohol, sulfites, and latex, with the easy ordering and excellent service that you have come to expect from the providers of SmartPak® .
SmartPak® provides a revolutionary delivery system for these essential drugs, offering faster, easier preparation more economically. It’s also safer. The SmartPak bag system is a convenient alternative to using multiple glass vials. No glass means no breakage during handling. SmartPak saves time and labor with less waste. SmartPak — the smart choice for your pharmacy service.
This important product now in the exclusive delivery system.
important product now in the exclusive delivery system. provides a
This important product now in the exclusive delivery system.
SmartPak® provides a revolutionary delivery system for these essential drugs, offering faster, easier preparation more economically. It’s also safer. The SmartPak bag system is a convenient alternative to using multiple glass vials. No glass means no breakage during handling. SmartPak saves time and labor with less waste. SmartPak — the smart choice for your pharmacy service.

delivery system for this essential drug, offering faster, easier more economically. It’s also safer. The SmartPak bag system is a convenient using multiple glass vials. No glass means no breakage during handling. SmartPak labor with less waste. SmartPak — the smart choice for your pharmacy service
SmartPak® provides a revolutionary delivery system for this essential drug, offering faster, easier preparation more economically. It’s also safer. The SmartPak bag system is a convenient alternative to using multiple glass vials. No glass means no breakage during handling. SmartPak saves time and labor with less waste. SmartPak — the smart choice for your pharmacy service
66288-1100-11100
SmartPak® provides a revolutionary delivery system for this essential drug, offering faster, easier preparation more economically. It’s also safer. The SmartPak bag system is a convenient alternative to using multiple glass vials. No glass means no breakage during handling. SmartPak saves time and labor with less waste. SmartPak — the smart choice for your pharmacy service
SmartPak® provides a revolutionary delivery system for these essential drugs, offering faster, easier preparation more economically. It’s also safer. The SmartPak bag system is a convenient alternative to using multiple glass vials. No glass means no breakage during handling. SmartPak saves time and labor with less waste. SmartPak — the smart choice for your pharmacy service.















Inc./EPS, Inc. Delivers Bar Coding, Packaging, and Labeling Pharmacy Solutions Improve your Solid Oral Unit Dose needs with our comprehensive Bar Coding, Packaging, and Labeling Solutions — designed by healthcare professionals. American Health Packaging (AHP) is a Leading Manufacturer of Serialized Barcoded Unit-Dose Products With a responsive line of barcoded unit-dose oral solutions, a growing liquid unit-dose offering, as well as individually wrapped inhalants.


Leiters Health is a trusted FDAregistered 503B outsourcing provider of ready-to-administer compounded sterile preparations, committed to providing healthcare professionals and their patients with the highest-quality medications.
page 45 page 54-55 page 57 page 51 page 53
and
Ensure your health-system’s compounded drug products and facilities consistently meet quality standards.

Contributed by Clinical Assistant Professor & Clinical Pain/SUD Pharmacist, Mark Garofoli, PharmD, MBA, BCGP, CPE, CTTS, at West Virginia University School of Pharmacy.
As one of the top leading suppliers for pharmacy cleanroom supplies, we provide an unparalleled selection of specialized medical products, globally trusted quality and service, and delivery convenience for meeting USP <797> and USP <800> standards.

Pharmacy Claim Adjudication
Pharmacy Benefit Administration
Self-Funded Employer Services
Hospital & Health System Services Pharmacy Savings Card Programs
Better insights. Bigger returns. By seamlessly integrating 340B and PBM management, PharmaForce unlocks significant cost savings and delivers a holistic and transparent view of your pharmacy operations. Our unique platform empowers informed decision-making with actionable insights. We offer comprehensive reporting, analytics and leakage identification tools to optimize both your 340B program and employee health plans, substantially reducing drug costs across the board.
Not convinced? Give us 30 minutes. We’ll show you real savings (with your actual claims). A PharmaForce specialist will analyze your claims for free, revealing how much you could be saving with our 340B + PBM solution. We’re confident we can deliver better insights and bigger returns. Schedule a consultation.
Let us show you how: www.thePharmaForce.com/demo
Every
Pharmacist ... is a Great Resource.
RXinsider’s Video Series are informative videos comprised of interviews, facility tours, trainings, and case studies throughout the pharmacy profession. Explore niche subject matter and gain insight though hundreds of expert videos.




USP <800> Compliance: Looking Back, Looking Forward.
Contributed by Clinical Program Managers, Annie Lambert, PharmD, BCSCP, and Jennifer Smith, PharmD, BCSCP, at Simplifi+ Pharmacy Compliance Solutions, Wolters Kluwer.












Prudential Cleanroom Services (PCS) — ISO 9001 Certified Quality Management System
PCS specializes in cleanroom garment processing services for aseptic, particulate, and ESD controlled environments.
500 of the most impactful pharmacy supply chain companies and associations in the U.S.
PUBLISHER
RXinsider, LTD
RXinsider CEO Gregory Cianfarani, RPh
DESIGN AND PRODUCTION
Design & Layout
Lora Bourque
Multimedia Eric Simmons
Marketing and Operations
SALES AND BUSINESS DEVELOPMENT
Samantha Roy Alexa DiLuca Kristin Fennessey
Chris Kolkhorst, EVP chris.kolkhorst@rxinsider.com
Mike Rahme mike.rahme@rxinsider.com
Shaun Russell shaun.russell@rxinsider.com
Savannah DaSilva savannah.dasilva@rxinsider.com
Jeff Rackliff jeff.rackliff@rxinsider.com
Email sales@rxinsider.com
Fax 646.329.9766 Website www.RXinsider.com
20Ways is published quarterly by RXinsider LTD, 1300 Division Road, Suite 103, West Warwick, RI 02893. Postmaster: Send address changes to 20Ways/RXinsider, 1300 Division Road, Suite 103, West Warwick, RI 02893. Notification of address change must be made six weeks in advance, including old and new address with zip code.
Editorial – views expressed in articles or profiles in the 20Ways are those of the author(s) and do not necessarily reflect the policies and opinions of RXinsider, our editorial board(s), our advisory board(s), or staff. Advertising – products, services, and educational institutions advertised in 20Ways do not imply endorsement by RXinsider.
Copyright © 2024 RXinsider LTD. All rights reserved. Reproduction without permission is prohibited.
General Information: 20Ways@RXinsider.com Circulation: See page 5

www.RXinsider.com/USP800









Verity Solutions® is a recognized leader in 340B program optimization and innovator of pharmacy purchasing solutions that drive compliance and savings. Our continually improved products and commitment to client satisfaction mean you can dedicate less time to program administration and more focus on patients.



Optimize savings on qualified transactions.
Automatically selects the best priced drug before order is placed.
Extend 340B savings through retail chain and independent pharmacies.
Leverage our preferred Specialty and Infusion partnerships.
Automated replenishment solution that simplifies owned outpatient pharmacy program management and improves savings.
Boost 340B savings with our no risk tools and support services.
Restore access to eligible 340B claims.
Consolidate 340B ordering, reporting and financial functions across multiple third party administrator (TPA) relationships.




*340B program participation not required.

Partner with us to optimize pharmacy purchasing, simplify 340B administration, ensure compliance, and benefit from the dedication and knowledge of our expert team.
CEO: George Puckett
Founded: 2015
Employees: 140
Toll-Free Phone: (800) 581-1378
Phone: (425) 947-1922
Address: 12131 113th Avenue NE, #200 Kirkland, WA 98034
Website: www.verity340b.com
Verity Solutions is a leader in 340B program administration and innovator of pharmacy purchasing optimization. Recognized as Best in KLAS: 340B Management Systems for six years, we are on a mission to create pharmacy excellence for 340B and beyond through predictable automation and outstanding support so that every customer can maximize their savings. We partner with integrated healthcare systems, acute-care hospitals, community health centers, federally qualified health centers, pharmacies, and 340B-eligible covered entities throughout the U.S. who rely on Verity software and services to successfully manage their pharmacy purchasing and 340B program.
Our powerful cloud-based software platform provides comprehensive solutions for split billing, contract pharmacy, specialty contract pharmacy, plus a contract pharmacy gateway solution called VHUB®. Our purchase optimization product, VERISAVE®, automatically selects the best blended priced NDC available for your complete order before it is submitted, reducing tedious manual processes and dramatically decreasing your drug spend. Our depth of in-house technical and software development resources and our highly skilled account management team are closely aligned to swiftly adapt as changes arise in the 340B program. We offer:
• Agile Software Platform: Built and deployed with security, performance, scalability, and agility as primary goals.
The Verity 340B platform is HITRUST® certified, demonstrating robust HIPAA compliance.
• Intuitive Application: Designed with our users in mind, we maintain ongoing feedback and collaboration with our clients. This collaboration steers our continual software and services development.
• Responsive Support: Designated account managers provide focused support, training, audit readiness, and regular business reviews to maximize your 340B program success and help you maintain compliance.
We continually invest in our technology and people to ensure pharmacy program success for our clients. With increasing 340B regulatory complexity and ongoing pressure for improved margins, it’s more important than ever to have the right solution for your 340B and pharmacy program management — and the right partner.
• Industry leading core functionality of our V340B platform with optional, patent-pending add-on modules to enhance all aspects of 340B operations in challenging and unique environments.
• Rapid 120-day average implementation time frame for both split billing and contract pharmacy solutions upon receipt of compliant dataset.
• Access to your own unique 340B program test environment — before and after implementation. Test environment runs continually in parallel to your live system.
• Easy and exportable reporting functionality including detailed data for manufacturer audits, HRSA audits, and UDS reporting.
• Advanced Reporting Insights gives users rich data visualization, interactive reports, and performance trends to help drive strategic change.
• Transition from one electronic medical record (EMR) to another without downtime (contingent upon receipt of dispense file from the covered entity).
• Multiple vendor support with controlled substances ordering system (CSOS) — efficiently place orders with both EDI (electronic data interchange) and non-EDI vendors.
• Two-week sprint release cycles ensure timely software updates driven by customer feedback, regulatory changes, and user needs.
• Flexible and “winners only” contract pharmacy pricing models. No true-ups.
• Verity Care Card Program — directly pass 340B savings to uninsured and underinsured patients.
• Referral capture solutions, self-serve or full service, to compliantly add meaningful savings lift to your 340B program.
• Responsive customer service provided by our in-house staff and the ability to submit and track issues in our online customer portal.
• 80% of our customer support cases resolve within two hours, and 95% within 24 hours.
• 97% highly satisfied rating of account management support.
• Own Use Program for any non-profit hospital to optimize your owned outpatient pharmacy for additional savings.

President & CEO: Nick Culbertson
Founded: 2014
Employees: 110
Phone: (410) 995-8811
Fax: (410) 995-8842
Utilize artificial intelligence and advanced analytics to audit 100% of medication use transactions — reducing the risk to your workforce, organization, and most importantly, your patients.

Address: 1629 Thames Street, Suite 200 Baltimore, MD 21231
Website: www.protenus.com
Founded in 2014, Protenus is an innovative, technology-first company headquartered in Baltimore, MD. Our team of problem solvers, subject matter experts, and support stewards spans the entire country. Protenus was born of a determination to solve problems in healthcare that would provide better patient outcomes and increase patient trust in the healthcare organizations where they sought care.
Although hospitals in the U.S. spend $39 billion per year to maintain compliance, most of it goes towards time-consuming, inefficient manual tasks. We thought the money could be better allocated to improving patient outcomes, so through innovation we began to tackle common healthcare compliance challenges. First focusing on protecting patient privacy, we then broadened our scope to issues of healthcare workers stealing or misusing controlled substances. In the years since, we’ve empowered hundreds of healthcare organizations to reduce risk. We’ve also cultivated the largest collaborative user community of healthcare compliance peers and experts from Protenus, PANDAS, that continues to move healthcare forward. That same innovation, determination, and community are still the pillars of Protenus today and carry us forward in our belief that the delivery of care should be without risk.
It’s important to partner with a vendor who understands current industry drivers and challenges, is aligned with your organization’s compliance and risk strategies, and is focused on providing the guidance and solutions to help you achieve your goals. Our powerful healthcare compliance platform harnesses the power of artificial intelligence (AI) to identify and surface inappropriate behavior that may otherwise go unnoticed as it happens, reducing risk to your workforce, your organization, and most importantly, the patients you serve.
When you choose Protenus as your partner, you are choosing a company that makes a commitment to innovation, determination, and community to better protect your organization, workforce, and patients. When working with Protenus, you will benefit from cutting-edge technology and a team that is dedicated to helping you navigate the complex landscape of healthcare compliance and risk management.
Who We Serve: Our sole focus is on healthcare organizations such as hospitals, health systems, AMCs, and large physician groups, supporting their risk elimination and compliance efforts as well as strategic initiatives like reducing costs, maintaining a stellar organizational reputation, and protecting their patients, workforce, and community at-large.
Our Vision: We believe that the delivery of care should be without risk.
Our Mission: We empower healthcare organizations with AI-powered, scalable risk-reduction solutions built to deliver cohesive and actionable data when and where needed. Ultimately, protecting the delivery of care while challenging the industry to focus on what matters most by driving the safest, highest quality patient outcomes.
Drug Diversion Surveillance, developed by Protenus, identifies and helps prevent clinical drug diversion incidents that occur within healthcare organizations. Awarded 2023 Best in KLAS for Drug Diversion Monitoring, the Protenus solution uses the power of artificial intelligence (AI) to monitor up to 100% of medication use transactions, it identifies unseen behavior as it happens, reducing risk to workforces, organizations, and most importantly, patients.
n Protenus Drug Diversion Surveillance Offers
• Visibility Into Incident Generation: Platform is powered by data covering 100% of medication use transactions to give investigators complete visibility into potential drug diverters’ behavior patterns that may otherwise go unnoticed.
• Time Savings and Efficiencies: Each incident is surfaced along with clear and concise background information to allow investigators to resolve incidents quickly. Customers have reported a five to 15 minute incident evaluation time using the Protenus platform.
• Flexible, User-Friendly Analytics and Dashboards: User-friendly dashboards reduce the need for manual analytics and report creation. Our File and Error Delivery dashboards empower IT end users to monitor and troubleshoot the robust set of data feeds that power the Protenus platform.
• Commitment to Continuous Innovation: Our product and engineering teams partner to receive feedback directly from our customers and deliver maximum value. Customers have forwardlooking visibility into planned feature and functionality enhancements.
The Drug Diversion Checkup [DDC] offers a quarterly or annual review of a healthcare organization’s overall drug diversion surveillance program, answering the question, “how is our program doing and how can we improve?” The DDC is included as part of the Premium Services Package or can be purchased as an add-on.
Key Business Partners/Supplier Contracts
Business Partner: Inmar Intelligence | Supplier Contract: Premier Inc.



Annie Lambert, PharmD, BCSCP, Clinical Program Manager
Topics Include:
• The role of pharmacists: How have they evolved, and what challenges they face.
• Regulatory changes and how pharmacies can stay up-to-date.
• How pharmacy CE requirements have evolved over the last 10 years.
• Effective pharmacy team training.


Erick Siegenthaler, Senior Director of Infusion Strategy, Kristin Fox-Smith, Managing Director 340B ACE, Angela De Ianni, Senior Director, Angie Amado, Director of Specialty Pharmacy Services, and Herolind Jusufi, Consultant
Topics Include:
• Five keys to success with specialty pharmacy.
• Partnering with Visante for infusion strategy.
• 340B optimization.


Nicole Faucher, President, Allison Arant, Senior Vice President of Client Development & Marketing, Mo Kharbat, Vice President of Industry Affairs, Alex Pham, Vice President of Client Strategy, Rusty Atkinson, Vice President of Information Technology, and Daniel Gladwell, Associate Vice President of Finance
Topics Include:
• Clearway Health: Specialty Pharmacy Accelerator.
• Three key elements that establish a healthy and effective partnership.
• How the information technology team impacts patients’ lives.

Jason Dutcher, Director of Strategic Accounts
Topics Include:
• Discussion on the Mundus HD Mini by EQUASHIELD.
• Demo of the Mundus HD Mini.



Immediate access to energy efficient and sustainable ultra-cold storage.

HELMER’S EXCLUSIVE OPTICOOL™ TECHNOLOGY
DELIVERS SUSTAINABLE, ENERGY-EFFICIENT WORLDCLASS TEMPERATURE PERFORMANCE
SUPPORTS TEMPERATURE UNIFORMITY
GX Solutions offer optimized temperature uniformity and recovery
ENERGY EFFICIENT
ENERGY STAR® certified ULTs are 35-45% more energy efficient than conventional medical-grade ULTs.
SUPPORTS SUSTAINABILITY
Environmentally-friendly natural HC refrigerants reduce heat output, decrease the carbon footprint, and decrease global warming potential.
IMMEDIATE ACCESS ULTRA-COLD STORAGE
GX Solutions ultra-low freezers are available for immediate shipment. Why wait?


General Manager: Matt Barga
Sales Leader: Betsy Cox
Toll-Free Phone: (800) 743-5637
GX Solutions ULTs provide immediate access to energy efficient and sustainable ultra-cold storage.
Address: 14400 Bergen Boulevard Noblesville, IN 46060
Website: www.helmerinc.com
Helmer Scientific, now part of Trane Technologies Life Science Solutions, is a U.S.-based manufacturer and worldwide distributor of medical-grade cold storage and laboratory processing equipment. We have over 45 years of experience providing high-quality temperature-controlled environments, with our products being used in over 125 countries. Precise temperature performance and control are essential to the successful storage of pharmaceuticals, and Helmer cold storage products are designed and developed with these considerations.
Product Overview
n GX Solutions ULT Freezers
GX Solutions ULT Freezers are designed and tested to deliver world-class temperature performance. Featuring OptiCool™ Cooling Technology, GX ULTs offer:
• Tight temperature uniformity and fast recovery after door openings.
• Quiet operation.
• Sustainable, natural hydrocarbon refrigerants.
n Efficiency and Sustainability
GX Solutions ultra-low temperature freezers support efficiency and sustainability initiatives without sacrificing performance. Helmer ULTs have been recognized by the U.S. Environment Protection Agency (EPA) as meeting Energy Star® certification requirement for the High-Performance Laboratory Grade Freezer category.
The Energy Star® program helps safeguard the environment by promoting superior energy efficiency across a broad range of products, including testing and recognition of cold storage devices used for clinical applications. This equipment category is used across healthcare and research facilities to store medications, vaccines, and more.
The energy management afforded by the OptiCool™ cooling system means that GX Solutions ULTs are 35-45% more energy efficient than conventional medical-grade ultra-low freezers. By using multi-stage cascade refrigeration technology combined with dual capacity compressors (VCCs) and natural hydrocarbon refrigerants, OptiCool™ efficiently manages both low and highstage cooling loops.
n
The OptiCool™ system uses naturally occurring refrigerants R170 and R290 that are EPA, Significant New Alternatives Policy (SNAP), and EU F-Gas compliant. These natural refrigerants are environmentally friendly, having no impact on ozone depletion and a very low Global Warming Potential (GWP) grade.
The use of natural hydrocarbons:
• Reduces Heat Output — allows facilities to place freezers in small work areas while limiting impact on HVAC systems.
• Decreases Carbon Footprint — uses natural hydrocarbon (HC) refrigerants to dramatically decrease global warming potential.
• Helps meet ever-changing energy standards.
Sustainability in the healthcare and life science industries has become a focus the past several years. While healthcare systems are responsible for treating patients impacted by climate change, they are also major contributors to the overall problem.
Helmer and its parent organization, Trane Technologies, are fully committed to supporting global sustainability initiatives. We have committed to removing a gigaton of greenhouse gas emissions from our customers’ carbon footprints by 2030.
Need an ultra-low freezer but don’t have time to wait? Helmer Scientific GX Solutions are available for immediate shipment.
Contact a Helmer Scientific sales representative at sales@helmerinc.com.



































































































































































































Avoid unwanted outcomes for critical drug products that need to be held at room temperature prior to administration.
President & CEO: Gary Sharpe
Founded: 1978
Employees: 300+
Toll-Free Phone: (800) 848-1633
Phone: (740) 477-3755
Address: P.O. Box 25 Circleville, OH 43113
Website: goHCL.com
What began in 1978 as a garage-based business at the home of HCL® Owner Gary Sharpe now encompasses five well-equipped facilities in central Ohio and reaches customers around the globe. Employment has grown too, with more than 300 employees now dedicated to the company’s mission of providing value with every interaction.
Sharpe discovered early in his career the need for healthcare products in sizes, quantities, and materials not readily available, and was determined to deliver. And has he ever.
Today HCL’s inventory includes more than 9,000 different products all designed to provide “Special Answers to Special Problems.” Most of these items are maintained locally in more than 320,000 sq. ft. of warehouse space and meet the supply needs of hospitals, pharmacies, chain drug stores, pharmaceuticals, and many other facets of healthcare.
Pre-administration vaccine protocol isn’t merely a suggestion, it’s a series of procedures designed to protect patient safety. Our temperature management solutions allow pharmacies to have processes in place that match industry standards so personnel can provide more confident care.
Using Controlled Room Temperature Cabinets, pharmacy staff can safeguard the vaccine supply and avoid unwanted treatment outcomes. Our inventory includes five units that maintain a range of 20ºC - 25ºC (68ºF - 77ºF) to keep specified doses such as smallpox, mpox, and some COVID-19 vaccines within safe parameters when they need to be stored in a freezer, then held at room temperature prior to administration.
Each unit includes a key lock to protect contents as well as audible and visual alarms. Choose from multiple sizes, all with glass doors, to meet a range of unique vaccine storage needs.
Refrigerators and freezers that provide reliable and consistent temperature control are the gold standard in pharmacies of all sizes, but knowing which ones to choose can be a challenge. We’ve taken the guesswork out of the process by filling our inventory with solutions designed to fit any footprint.
From full-size freestanding units to compact countertop models, we’re proud to offer cold storage options that provide these benefits — and many others:
• Proper storage to extend the shelf life of temperaturesensitive medications and reduce waste plus the cost of replacing items.
• Reliable temperature control to maintain drug integrity and minimize the risk for damage or degradation.
• Solid door gaskets to seal out the risk for environmental contaminants that can lead to bacterial growth or crosscontamination.
• Locking doors to protect controlled medications.
• Compliance to assure items are stored in accordance with the regulatory guidelines of agencies such as the CDC, FDA, and JCAHO.
In addition to appliances, we offer a full line of temperature monitoring devices along with vaccine storage and transport containers.
Our additional lines include unit dose; storage; IV accessories and injectables; compounding and dispensing; seals; plastic bags; refrigerators, freezers and accessories; temperature monitoring; infection prevention; carts and accessories; pharmacy supplies; crushers, cutters, and organizers; and error prevention.
We serve central supply/purchasing/MM; emergency department; facilities/engineering/maintenance; infection control/sterile processing; labs/clinical; nursing; oncology/ hematology; infusion; O.R.; pharmacy; and surgery centers.
We offer small package quantities, no order minimums, free samples, and ship most orders the same day. Our hassle-free return policy allows you to return any product, at any time, for any reason. Connect with our live chat team from 8 a.m. to 8 p.m. EST Monday - Friday.
Sponsored by an Educational Grant From Baxter
The right drug with the right diluent for the right dose at the right time has been and remains the ever-present responsibility of a pharmacist. The introduction of IV Workflow Management systems (IVWFM) into the sterile compounding space has significantly reduced the effort and improved the chances of consistently meeting this responsibility. This type of quality assurance verification has been the primary use of IVWFM systems since their introduction into the United States in 2008 when DoseEdge Pharmacy Workflow Manager system (DoseEdge) was introduced. However, the pharmacist’s responsibilities continue to grow with the increasing number and ever more strict regulations and guidelines governing the processes for compounding sterile preparations and the environments they are prepared in. Therefore, pharmacists must consider how these IVWFM systems can be used to support these needs.
Accurate and consistent data from published literature on the incidence of IV compounding errors is difficult to find as most facilities and pharmacists are reluctant to share this type of information. Confounding this is the fact that there is no single recognized definition of what constitutes these types of medication errors. The relatively small number of data sets that are available for error rates at individual facilities or groups of facilities not using any type of IVWFM system (or technology-assisted workflow systems) can range from 0.22%1 to 9%2. These errors can result from pharmacy staff not knowing with specificity what drugs, diluents, and volumes should be used to prepare a compounded sterile preparation (CSP).
Before automation, pharmacists had to verify every CSP dispensed from the cleanroom using manual and sometimes inexact methods. It was not always possible to determine if the correct drug and/or amount was added to
an IV container. Therefore, the true number of compounding errors could not be determined.
The Institute for Safe Medication Practices (ISMP) published a survey in October of 2020 that included 634 respondents, 80% of whom were pharmacists, and another 18% were pharmacy technicians3. The survey results demonstrated some concerning results3:
• 56% of all responders (355) reported always having and following standard operating procedures for the compounding process.
• 48% of pharmacist responders (243) stated that it was always easy to identify with certainty which drugs, diluents, and volumes were used when verifying the preparation of a CSP.
• 57% of respondents (361) were using technologies to support sterile compounding.
• 47% of the 361 respondents using technology (170) were taking advantage of IVWFM systems that included both barcode scanning and image capture to help manage risks.
Additionally, on November 1, 2022, the United States Pharmacopeia (USP) published the revised General Chapter <797> Pharmaceutical Compounding — Sterile Preparations (USP <797>) which provides the new minimum acceptable standards for sterile compounding. In recent years, USP <797> requirements seem to also be garnering more attention from organizations such as The Joint Commission and other accrediting bodies which now conduct inspections according to these requirements.
Many of the updates to USP <797> are relatively minor changes, and the basic principles remain the same. Examples of this are the need to maintain acceptable levels of viable and non-viable particle counts in the classified areas. However, more detail is provided around the performance and frequency of the required sampling. These changes in testing detail don’t affect the basic requirements of how many particles per cubic meter or colony forming units per cubic meter are acceptable as those limits are set by separate ISO standards. However, as with previous revisions of the chapter, some changes in the guidelines are more significant and may require considerable

changes to pharmacy and cleanroom operations. An example of this type of significant change would be how restricted-access barrier systems (RABS) can be used. In the 2008 version of the chapter, the ISO 5 environment that can be achieved with these systems alone was considered sufficient to apply extended Beyonduse Dates (BUDs) for CSPs regardless of where the RABS was located4. However, the 2023 version of USP <797> requires that the RABS be located in an ISO 7 environment to apply Category 2 or 3 beyond-use dating5. Essentially, a RABS in an unclassified space is now equivalent to a segregated compounding area. In any case, whether the standards of the 2008 and 2023 versions of the chapter remain the same, or they receive minor or significant updates, history tells us that they can be challenging to consistently adhere to for many pharmacies. In the 2023 State of Pharmacy Compounding survey published in Pharmacy Purchasing and Products, only 31% of respondents indicated their facilities were in full compliance with standards that will become effective in November of 20236. An even more alarming statistic is that only 76% of respondents indicated their facilities were in full compliance with the 2008 version of USP <797>6
The compounded sterile preparation goals of ISMP and USP are both rooted in ensuring that safe medications are available to patients when they are needed, ISMP with the Guidelines for Sterile Compounding and the Safe Use of Sterile Compounding Technology, and USP with General Chapter <797>. Although, in some ways, the two organizations approach the goals differently, there is overlap in that they both focus on processes to achieve the goals. It’s this process overlap that can allow IVWFM systems with broad functionality such as the DoseEdge system to support the needs of pharmacies in their pursuit of compliance with both organizations’ guidelines.
From an ISMP perspective, the use of technology such as an IVWFM system to improve the safety, efficiency, and prioritization of compounding within the cleanroom is a well-known and frequent topic of discussion. In fact, ISMP includes this in their 2022-2023 Targeted Medication Safety Best Practices for Hospitals and has done so since 20167. These improvements can be achieved by automating potentially error-prone processes that have traditionally been performed manually. These error-prone processes can include researching and following the correct and complete compounding process, manually performing dose calculations and determining appropriate BUDs, prioritizing urgently needed products and using the ‘syringe pull-back’ or ‘proxy’ method for indicating volumes of drugs injected into final containers. Additionally, in 2022, “ISMP Guidelines for Sterile Compounding and the Safe Use of Sterile Compounding Technology” was published. It contains essential technology attributes, safe pharmacy processes, safety gaps, and associated
best practices for various technologies such as automated compounding systems, IV robotics and IVWFM systems.
The ISMP essential attributes for IVWFM systems are listed below. Many align nicely to address the gaps or issues associated with CSP preparation8
IV workflow management systems are interfaced with the electronic health record to eliminate order transcription from one system into another. If a compounded sterile preparation has been discontinued before initiation of the compounding process, the system interface allows for the removal of these products from the queue.
IV workflow management systems allow users to create a master formulation record for nonpatient specific batch, stock solution, and patientspecific compounded sterile preparations.
When master formulation records are created, the IV workflow management system prompts for an independent double check, which is documented in the system.
Master formulation record changes are timestamped, saved, and identify the user who made the modification.
IV workflow management systems provide an electronic log of changes made to the database by users.
IV workflow management systems allow users to customize the incoming order queue to prioritize work.
Machine-readable coding (e.g., barcode, RFID) is used to verify source products, including diluents, during the compounding process.
IV workflow management systems automatically perform calculations or conversions.
IV workflow management systems guide users through essential steps in the compounding process including which steps require video or still images or gravimetric analysis.
Image-capture pictures are clear such that syringe graduation marks, drug and/or diluent names, lot numbers, and expiration dates are easily visible.
System Functionality

IV workflow management systems that use gravimetric analysis prevent users from creating master formulation records for preparations that are outside the system’s tolerance limits, and if staff attempt to weigh a volume outside the integrated scale’s tolerance limit the IV workflow management system alerts the user.
IV workflow management systems document all steps and components of the compounding process (e.g., products used, the practitioner who performed the compounding, the primary engineering control, machine readable code scans, date and time of preparation, alerts or warnings presented during the process, the practitioner who verified the preparation), and the information is available to users in a log and/or report.
IV workflow management systems allow for remote verification using video or image capture, and, when used, gravimetric analysis.
IV workflow management systems track beyond-use dating of opened or reconstituted products to warn practitioners and prevent use of an expired product.
IV workflow management systems allow for customization of labels (e.g., tall man lettering, color print, reverse print, electronic health record compatible barcode).
IV workflow management systems limit the printing of the dispensing label until the compounding process is complete.
Workload (e.g., incoming load) is documented by the technology and captured in a report to inform and facilitate operational improvement.
Close-call compounding events (e.g., wrong drug scans) intercepted by the technology are captured in a report to facilitate compounding error analysis and process improvement. Data in vendor reports are provided in a useful format and do not require significant manipulation by the end user.
When a system update is available, IV workflow management system vendors ensure all customers receive and install the update in a reasonable timeframe.
The use of IVWFM systems to help meet the Chapter <797> standards is discussed far less, if at all. However, given the broad functionality of some of these systems, such as the DoseEdge system, they can influence pharmacy procedures and documentation practices etc. which can directly or indirectly support these requirements.
The following are many of the standards from the 2023 version of USP <797> that could potentially be supported by using IVWFM systems5
USP Chapter <797> Standards How DoseEdge Functionality Could Provide Support
Personal hygiene and garbing are essential to maintain microbial control of the environment. Most microorganisms detected in cleanrooms are transferred from individuals. Squamous cells are normally shed from the human body at a rate of 106 or more per hour, and those skin particles are covered with microorganisms. Individuals entering a compounding area must be properly garbed and must maintain proper personal hygiene to minimize the risk of contamination to the environment and/or CSPs.
Individuals that may have a higher risk of contaminating the CSP and the environment (e.g., personnel with rashes, recent tattoos, oozing sores, conjunctivitis, or active respiratory infections) must report these conditions to the designated person(s).
The designated person(s) is responsible for evaluating whether these individuals should be excluded from working in compounding areas before their conditions have resolved because of the risk of contaminating the CSPs and the environment.
The design of the facility should take into account the number of personnel and their movements.
4.1 Protection from Airborne Contaminants
Proper design and controls are required to minimize the risk of exposure of CSPs to airborne contaminants.
Total airborne particle counts by ISO classification must not be exceeded:
ISO 5 = 3520 particles/m3
ISO 7 = 352,000 particles/m3
Remote verification could help decrease the number of pharmacists required to enter the cleanroom to verify doses including in-process and final checks.
This can be especially beneficial on off-shifts when staffing may be lower or when pharmacist verifiers with higher risks for contamination may be the only pharmacist on duty.
Remote verification can help minimize the number of pharmacist verifiers needing to enter the cleanroom which may be able to affect the overall design. This decrease in staff needing to enter the cleanroom can also be used as a control mechanism to decrease airborne contaminants.

6. MICROBIOLOGICAL AIR AND SURFACE MONITORING
6.2.3 Viable air sampling data evaluation and action levels
Viable airborne particle counts by ISO classification must not exceed actionable levels:
ISO 5 > 1 cfu/m3
ISO 7 > 10 cfu/m3
6.3.3 Surface sampling data evaluation and action levels:
ISO 5 > 1 cfu/media device
ISO 7 > 10 cfu/media device
11.1 Creating Master Formulation Records (MFR)
An MFR is a detailed record of procedures that describes how the CSP is to be prepared. An MFR must be created for all CSPs prepared from nonsterile ingredient(s) or CSPs prepared for more than one patient.
11.2 Creating Compounding Records (CR)
CR documents the compounding of each CSP. A CR must be created for all Category 1, Category 2, and Category 3 CSPs. A CR must also be created for immediate-use CSPs prepared for more than one patient. The CR must be created to document the compounding process. A prescription or medication order or label may serve as the CR. If an ACD, workflow management system, or other similar equipment is used, the required information in the CR may be stored electronically as long as it is retrievable and retains the required information.
12.2 Sterility Testing
For Category 2 CSPs assigned a BUD that requires sterility testing (see Table 13) and all Category 3 CSPs, the testing must be performed.
12.3 Bacterial Endotoxins Testing
Category 2 injectable CSPs compounded from one or more nonsterile component(s) and assigned a BUD that requires sterility testing and Category 3 injectable CSPs compounded from one or more nonsterile component(s) must be tested to ensure that they do not contain excessive bacterial endotoxins.
The decrease in staff needing to enter the cleanroom associated with remote verification can also be used as a control mechanism to decrease viable air and surface contaminants.
Each CSP label must state the date, or the hour and date, beyond which the preparation must not be used and must be discarded (i.e., the BUD). The BUD is determined from the date and time that preparation of the CSP is initiated.
14.2 Parameters to Consider in Establishing a BUD
When establishing a BUD for a CSP, compounders must consider parameters that may affect quality.
The following DoseEdge functionality can be used in aggregate to support most/all the requirements for the contents of an MFR and CR, and help ensure that the MFRs and CRs are followed:
• Customizable Drug Formulary
• Customizable Product Formulary
• Customizable Actions
• Customizable Procedures
• Customizable Scan Events
• Storage capabilities
• Customizable product-specific information fields
Customizable Actions and/or Procedures can be used to remind compounders when sterility and/or bacterial endotoxin testing (or other requirements) needs to be completed.
The BUDs for CSPs are based primarily on factors that affect the achievement and maintenance of sterility, which include, but are not limited to:
• Conditions of the environment in which the CSP is prepared
• Aseptic processing and sterilization method
• Starting components (e.g., sterile or nonsterile ingredients)
• Whether or not sterility testing is performed
• Storage conditions (e.g., packaging and temperature)
14.3 Establishing a BUD for a CSP
The BUD must not exceed the shortest remaining expiration date of any of the commercially available starting components.
14.5 Multiple-Dose Containers
The use of preservatives must be appropriate for the CSP formulation and the route of administration. For example, the preservative must not be inactivated by any ingredients in the CSP, and some preservatives are not always appropriate for the patient (e.g., neonates) or route of administration (e.g., intrathecal or ophthalmic injection).
After a multiple-dose CSP container is initially entered or punctured, the multiple-dose container must not be used for longer than the assigned BUD or 28 days if supported by antimicrobial effectiveness testing results on the CSP, whichever is shorter.
BUDs are automatically calculated from the time the compounding process is initiated. They can be customized and automated to account for the many options that are available between and within the three Compounding Categories
BUDs can be customized at the dose level based on:
• Starting components used in the dose
• Location of preparation (environmental conditions such ISO classified vs SCA/RABS)
• Storage conditions
• Processes used during preparation (including sterility testing)
The BUD of individual doses are compared to the BUDs or expiration dates of the components used in dose to ensure they are appropriate.
With ‘Preservativefree” and “Route of Administration” designations in the Product Formulary, inappropriate component use can be prevented.
“Work in Progress” products and labels can automate BUDs for components used during preparation

15. USE OF CONVENTIONALLY MANUFACTURED PRODUCTS AS COMPONENTS
15.1 Use of Conventionally Manufactured Single-Dose Containers
A conventionally manufactured single-dose container is a container closure system that holds a sterile product for parenteral administration (injection or infusion) that is not required to meet the antimicrobial effectiveness testing requirements. If a single-dose vial is entered or punctured only in an ISO Class 5 or cleaner air, it may be used up to 12 h after initial entry or puncture as long as the labeled storage requirements during that 12-h period are maintained. Opened single-dose ampules must not be stored for any time period.
15.3 Use of Conventionally Manufactured Pharmacy Bulk Packages
A conventionally manufactured pharmacy bulk package is a container of a sterile product for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the sterile preparation of admixtures for infusion or, through a sterile transfer device, for the filling of empty sterile containers. The pharmacy bulk package must be used according to the manufacturer’s labeling (see <659>, General Definitions, Injection Packaging Systems). The pharmacy bulk package must be entered or punctured only in an ISO Class 5 PEC.
18.1 Notification About and Recall of Out-ofSpecification Dispensed CSPs
If a CSP is dispensed or administered before the results of release testing are known, the facility must have procedures in place to:
•Immediately notify the prescriber of a failure of specifications with the potential to cause patient harm (e.g., sterility, strength, purity, bacterial endotoxin, or other quality attributes)
•Recall any unused dispensed CSPs and quarantine any stock remaining in the pharmacy.
•Determine the distribution of any affected CSP, including the date and quantity of distribution.
•Identify patients who have received the CSP.
“Work in Progress” products and labels can also automate BUDs for single dose products, multiple dose products and pharmacy bulk packages used during preparation
The safety, efficiency, and waste-reduction benefits with the use of IVWFM systems are well-established. ISMP has put forth much effort in defining what an effective system should be capable of and recommending their use among other technologies in the cleanroom. Although IVWFM systems are only used in a minority of facilities, that number continues to grow due to an ever-increasing focus on safety and efficiency.
With the updated version of USP <797> effective on November 1, 2023, there is also a heightened interest in what facilities need to do to become compliant before the effective date.
IVWFM systems with broad functionality such as the DoseEdge system, can help support pharmacies in their pursuit of regulatory compliance, optimized workflows, compounding efficiency, and most of all, patient safety.
The DoseEdge System is not intended to replace the knowledge, judgment or expertise of pharmacists and pharmacy technicians in the preparation of IV admixtures or oral doses.
For safe and proper use of the product mentioned herein, please refer to the appropriate Operator's Manual.
Chuck Ferris, R.Ph. Associate Director, Medical Affairs ~ Baxter Healthcare Corporation
Jeff Brittain, PharmD, BCPS Senior Manager, Medical Affairs ~ Baxter Healthcare Corporation
References
Lot number tracking can be used to trace products and components to individual patient doses.
1. Stephen F Eckel, et al. Multicenter study to evaluate the benefits of technology-assisted workflow on i.v. room efficiency, costs, and safety, American Journal of Health-System Pharmacy, Volume 76, Issue 12, 15 June 2019, Pages 895–901.
2. Elizabeth Flynn, et al. Observational study of accuracy in compounding i.v. admixtures at five hospitals, American Journal of Health-System Pharmacy, Volume 54, 15 April 1997, Pages 904-12.
3. ISMP Medication Safety Alert Newsletter October 22, 2020 Volume 25, Issue 21. Pg 1-5
4. Pharmaceutical compounding—sterile preparations (general information chapter 797). In: The United States Pharmacopeia, 35th rev., and the National Formulary, 30 ed. Rockville, MD: The United States Pharmacopeial Convention; 2012: pp 2-38 State of Pharmacy Compounding.
5. Pharmaceutical compounding—sterile preparations (general information chapter 797). In: The United States Pharmacopeia, https://online.uspnf.com/uspnf/document/1_GUIDA4CAAA8B-6F02-4AB8-8628-09E102CBD703_7_en-US35th rev.pp 1-33
6. Pharmacy Purchasing and Products, 2023; 4:1-53 National Survey (Can’t get fill reference info)
7. ISMP Targeted Medication Safety Best Practices for Hospitals, 2022-2023
8. 2022 ISMP Guidelines for Sterile Compounding and the Safe Use of Sterile Compounding Technology
Baxter and DoseEdge are trademarks of Baxter International Inc. US-MD14-230014 v1.0 10/2023

Medication errors can occur at any point in the infusion therapy process.

Help protect patients, from dose preparation to administration.






CEO: Steve Rough
Founded: 1999
Toll-Free Phone: (866) 388-7583
We’re in the business of advancing pharmacy. Our consultants work with hospitals and health systems combining a wealth of expertise with personalized and comprehensive support to optimize your pharmacy operations and deliver better patient care.
Address: 101 East 5th Street, #2220 St. Paul, MN 55101
Website: www.visanteinc.com

Visante is a specialized consulting firm focused exclusively on helping health systems accelerate strong financial and operational performance through pharmacy. Our team of professionals brings deep, contemporary expertise and innovation to optimizing all aspects of a fully integrated health system pharmacy program, driving significant value quickly. By providing customized solutions to fit the needs of our clients, we deliver sustained financial results through revenue growth, cost savings, and optimal business performance.
n Specialty Pharmacy Services
A clearly defined specialty pharmacy strategy can help improve patient care and greatly improve your bottom line. Developing a specialty pharmacy strategy or expanding your existing services can be a complex process, but our team of experts guides you through each phase and delivers lasting ROI. Our specialty pharmacy consulting capabilities include: operational assessment and pro forma models; multi-year business and strategic plans; facility design including workflow and automation options; implementation and project management; accreditation support (URAC/ACHC/CPPA); contract pharmacy strategy, if applicable; wraparound strategy to include consideration for site of care challenges (infusion); and home infusion, DME strategies, and business planning.
n
A comprehensive infusion services strategy can help lower total cost of care, maximize patient outcomes and access to quality care, and optimize organizational financial performance. Visante infusion consulting helps with business planning, design, and implementation of comprehensive infusion care strategies using our deep and specialized expertise in all areas, including home infusion therapy, specialty pharmacy, 340B program management, supply chain strategies, and innovative solutions to drug delivery and therapy administration.
n 340B Solutions
Visante’s independent, external audit support provides transparency to your 340B processes, allowing you to recognize compliance gaps while focusing on new opportunities within the program. Our 340B team
offers unique expertise in supporting 340B ESP™ data submission and price restoration analysis. Our services include: internal and external audit support, on-site HRSA audit support, and corrective action plan guidance in the event of HRSA audit findings; gap analysis and targeted recommendations, focusing on long-term strategy in the mixed-use and contract pharmacy space to ensure program optimization; program implementation and development of internal oversight structure and maintenance; and split billing RFP guidance, implementation, and program re-designs.
n Pharmacy Revenue Cycle
Sustained financial growth is one of the top challenges for even the most successful organizations in the healthcare industry. Effective pharmacy programs can increase revenue by maximizing existing opportunities, creating new programs and services, and reducing costs. Our team of experts are here to help maximize financial performance within your organization through our mastery of both pharmacy and revenue cycle operations. Our consultants bring a wide range of experience to the table to assist each of our clients in a way that best fits them and their financial goals.
n Pharmacy Supply Chain Optimization
Supply chain and utilization management are complex and ever-changing functions of a pharmacy enterprise. To find success in these areas, disciplined focus and alignment must be prioritized. Visante’s supply chain experts help hospitals and health systems achieve reliable, safe, and efficient drug supply chain performance while also realizing significant financial returns.










Left: GM7030 PATT2®, Personal Aseptic Technique test kit, 3mL ampules, 20mL vials, 100mL partially lled minibag. Below: ET1000 EnviroTest® TSA with Lecithin & Tween 80 growth media paddles for surface, air, or glove ngertip sampling.












President & CEO: Brady K. Schwarz
Founded: 1992
Toll-Free Phone: (800) 837-8361
Phone: (530) 272-8700
Fax: (530) 272-8702
Providing quality assurance products to hospitals and pharmacies practicing sterile compounding, with a singular focus on pharmacy compliance. Our long-standing regional distribution partners are able to provide exceptional in-person service and training.

Address: 1415 Whispering Pines Lane, Suite 150 Grass Valley, CA 95945
Website: www.qimedical.com
Q.I. Medical, Inc. was founded in 1992 with a focus on providing the sterile compounding industry with testing kits and products to assure proper technique and quality is maintained in their facility. We have a broad network of distribution partners throughout North America, Canada, and other international markets. These distribution partners provide in-person servicing and education to accounts to comply with USP guidelines.
Our cost-effective, disposable products are available for various needs in the compounding pharmacy. Aseptic technique validation kits are available for low, medium, and high-risk settings. We also sell multiple à la carte sizes of vials, bags, tubes, and syringes of growth media for facilities looking to create a custom media fill test that mimics their day-to-day practice. In regard to environmental monitoring/gloved fingertip sampling, our EnviroTest™ paddle is ideal for both. The rectangle shape and built-in hinge allow for testing critical areas such as the edges and corners of a hood and behind latch handles. Sterility testing products are available in both USP <71> approved methods of testing. For the full filtration method, the QT Micro™ and QT Junior™ systems work for both small and large volume solutions. For the direct inoculation methods, our TuffTest 2™ product is ideal.
All Q.I. Medical growth media is challenged with a battery of USP specified organisms and lot specific Certificate of Analysis (CofA) are available for download.
All products are sold through regional stocking distributors in order to provide fast local service and support.
Additional products are available for validation of hazardous drug handling, automated compounder manipulation validation, filter integrity testing, and vial adaptors.
n Support Equipment Includes:
• Incubators
• Sterile Filters
• Vial Blocks
• UV Lights
To learn more about how Q.I. Medical, Inc. can help your facility, please contact us at (800) 837-8361 or email info@qimedical.com.

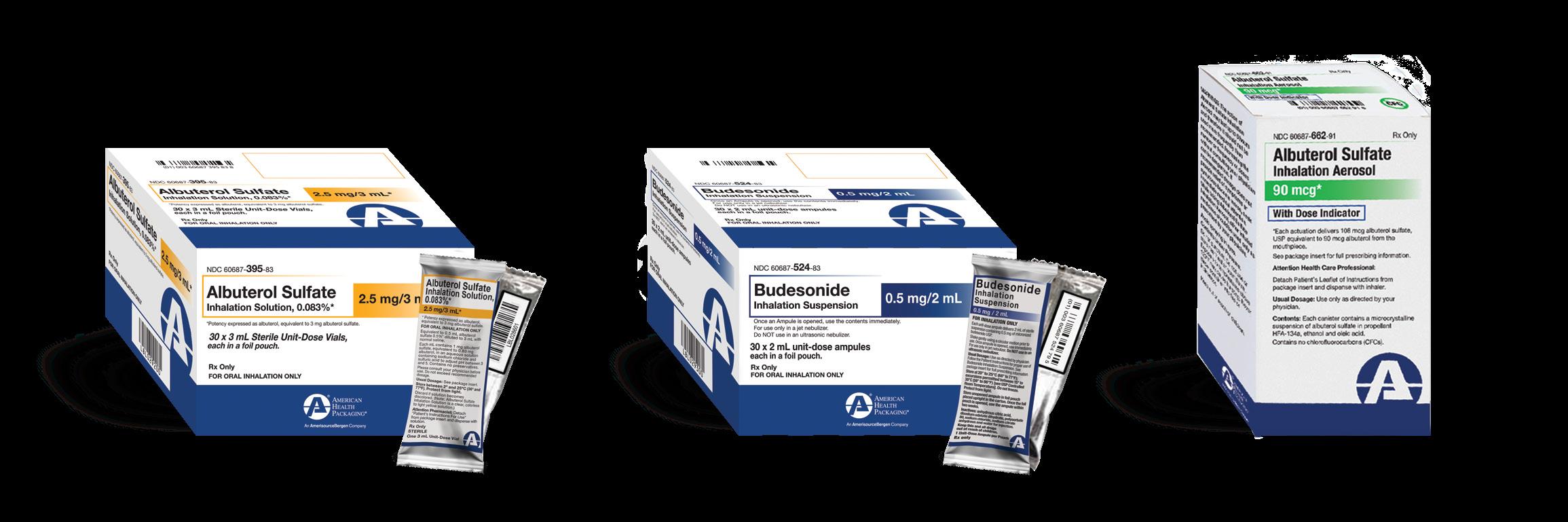
Explore our inhalation line at American Health Packaging
Order inhalation products through your preferred wholesaler and experience the reliability of AHP’s serialized, barcoded unit dose offerings for acute care centers, hospitals, and long-term care facilities.
Exciting new products are on the horizon! Stay tuned for what’s next in our commitment to enhancing patient care.
Inhalation
Metered dose inhaler (MDI)
Click here to explore our inhalation line!
AD202404-0212
With a responsive line of barcoded unit-dose oral solutions, a growing liquid unitdose offering, as well as individually wrapped inhalants, AHP continues to deliver on it’s commitment to supporting pharmacy efficiency.
Senior VP &
General Manager: Sasha Kellerman
Toll-Free Phone: (800) 707-4621
Address: Columbus, OH 43217
Website: www.americanhealthpackaging.com
Located in Columbus, Ohio, American Health Packaging (AHP) is an industry leader in manufacturing serialized, barcoded unit-dose (UD) medications provided for the healthcare marketplace. As a UD manufacturer, AHP’s commercially-available UD products are available to hospital, institutional, and long-term care pharmacies nationwide through partner GPOs and wholesalers.
AHP’s reputation for quality is supported by a 30+ year history of broad manufacturing expertise — operating a facility that is registered with the FDA, fully adherent to cGMP guidelines, and licensed by the DEA to package Schedule II-V controlled substances. Synonymous with unit dose, following years of success and leadership in the production of oral solids, AHP expanded their offering and now includes liquid unit-dose cups, inhalants, and powder/granule oral suspension.
AHP is committed to supporting pharmacy efficiency through a diverse range of both high-utilization and niche treatments. Producing nearly 600 UD oral solid SKUs for the healthcare marketplace, AHP’s broad selection of products are produced with quality components and printed with legible barcodes that facilitate effective execution of BCMA initiatives. Their wide selection of products reduces the gap between what pharmacies are forced to repackage themselves, and what is commercially available on the market — supporting health systems nationwide in their efforts to create efficiencies throughout the chain of care.
AHP’s tailored offering of UD oral liquids provide similar efficiency, safety, and cost-savings benefits as their oral-solids products. Product features include right-sized packaging, thoughtful tray design, differentiated labelling, and accurate barcodes. AHP’s unit-dose inhalants provide efficiency and feature individually-wrapped vials and pouches barcoded to the dose level. AHP oral solids, liquids, and inhalants include major therapeutic classes and product groups to meet unique pharmacy needs. They are continuously evolving to meet the changing demands of caregivers and staff to support more effective medication procurement strategies.
As facilities nationwide compete to demonstrate they provide the highest quality of care, AHP UD supports caregivers as they strive to promote positive outcomes for patients. Pharmacies simultaneously strive to be cost-effective as they provide necessary resources for caregivers.
AHP UD supports these objectives while providing cost-savings opportunities. Sourcing pre-packaged UD allows pharmacies to obtain adequate supply while mitigating capital expenses, such as those related to repackaging equipment, bulk supply, and labor.
n Patient Safety: Ensuring the right medication is given to the right patient at the right time — and in the right strength — is imperative. Pharmacies can facilitate effective execution of these “rights” by providing caregivers with as many products in a pre-packaged UD format as possible. Removing repackaging tasks from the pharmacy eliminates a potential point of failure during the UD process as medications arrive to pharmacies ready to dispense.
n Pharmacy Efficiency: Pharmacies strive to process orders and supply the proper medications to caregivers for their patients as quickly as possible. Adding potentially-complex repackaging steps to the procurement process not only harms the ability of pharmacy to supply caregivers effectively, but also removes clinicians from their core patient care competencies. In addition, pre-packaged UD often allows for products to be sourced more quickly than third-party repackaging can support.
n Cost-Savings Opportunity: Health systems that choose to package on-site must consider all direct costs, such as purchasing capital equipment for packaging areas and paying highly trained clinical professionals to perform, manage, and support non-core work. AHP UD products allow for pharmacies to avoid these costs while also shifting the potential costs associated with packaging errors. The pre-packaged format also prevents additional fees that may result from utilizing thirdparty repackaging services.
n Liability Management: Pharmacy repackaging operations can be subject to distractions from a variety of sources. An active pharmacy environment can encourage lapses in concentration and present opportunities for staff error. Since these errors may vary in gravity and place liability on the facility and caregivers, mitigation of risk is key. Unit dose from American Health Packaging can help shift liability burden away from staff.
Effective execution of BCMA initiatives require medications that scan correctly at the bedside. With a robust, growing, unit-dose portfolio AHP provides reliable access to UD treatments. AHP products promote safety towards BCMA and efficiency in pharmacy while freeing up internal resources. AHP UD supports pharmacies as they strive for compliance with USP General Chapter <800> guidelines. As pharmacies craft effective procurement strategies to meet the needs of their facilities, protecting patients and caregivers alike from potential harm while handling hazardous drugs is a priority. AHP’s UD portfolio has a number of NIOSH/USP <800> products already packaged for bedside dispensing which supports compliance to USP <800> handling procedures.


Dan Cleveland, PharmD, BCPS
System Director of Pharmacy Services
~ Union Health

Kathy Canup, PharmD
Clinical Operations Manager
~ CPS Solutions, LLC/Union Health
n An Integrated Health System Dedicated to Treating the Whole Patient Union Health is an integrated health system serving communities throughout western Indiana and eastern Illinois. The main campus, Union Hospital, is a 350-bed not-for-profit community hospital in Terre Haute, Indiana. The health system also includes Union Medical Group, which is comprised of over 130 providers in 20 specialties practicing at locations throughout the region — making it Wabash Valley’s largest multi-specialty provider group.
Union Health strives to provide patient-centered care using a collaborative, multidisciplinary approach. An essential part of that care is helping patients get affordable access to the medications they need. That’s why Union Health had long operated two outpatient retail pharmacies — one in the main hospital serving discharged patients and employees and another for the general public in their medical office building. However, at that time, the health system did not have its own specialty pharmacy, which left a gap in its ability to provide exceptional patient care for those prescribed complex, expensive therapies to manage cancer, rheumatoid arthritis, HIV, multiple sclerosis, and other chronic conditions.
n Navigating the Complexities of Specialty Medication Access
According to System Director of Pharmacy Services, Dan Cleveland, PharmD, BCPS, for Union Health, without a specialty pharmacy — patients and providers were left to navigate an often confusing process on their own.
“The trouble is that process is not always straightforward,” explains Cleveland. That’s because specialty medications require pre-authorizations, so there can be a lot of back and forth and follow-up required. “We were hearing from providers, especially

“Hospitals our size don’t often have an onsite specialty pharmacy. Being able to help our patients access and manage these complex medications improve their experience and outcomes — and that’s something we couldn’t have done without the help of CPS.”
Dan Cleveland, PharmD, BCPS System Director of Pharmacy Services
~ Union Health
those in the oncology and rheumatology space, that patients were waiting weeks before they started therapy.”
Plus, since specialty medications are often very expensive, most patients need financial assistance above and beyond insurance coverage. “That’s just another process to be managed and can be a barrier to treatment,” says Cleveland. “And while our providers did what they could — they simply didn’t have the dedicated staff necessary to handle all the administration that comes along with specialty medications.” That’s why he decided to explore how Union Health could establish an onsite specialty pharmacy to elevate patient care.
n A Partnership That Paved the Way for Patient-Centered Specialty Pharmacy Services
After conversations with a number of potential partners, Union Health ultimately chose CPS Solutions, LLC (CPS), one of the nation’s largest pharmacy and hospital services providers, to build out and manage their specialty pharmacy. “One of the things that stood out about CPS was their expertise and relationships with payors and manufacturers,” Cleveland says. Most payors require dual accreditation for specialty pharmacies to gain access to their specialty networks and limited distribution drugs — and CPS’ team of experts helps systems like Union Health earn those accreditations rapidly. “I just knew my team and I would not have the time to navigate that piece of the puzzle, so it was important for me.”
While payor access is one of the keys to a successful specialty pharmacy — having the people and processes in place to help patients is also critical. That’s why CPS hires and integrates pharmacists and liaisons in clinics to work directly with staff and providers under Union Health’s brand. The benefit of CPS’ locally-focused approach is that when one of the specialty pharmacy team reaches out to patients — they know they are talking to someone from their community. “We’ve found that having a local Terre Haute patient talking to a Terre Haute-based pharmacist or liaison has made a difference in building trust and helping them understand we have a specialty pharmacy right here onsite,” says Senior Vice President, Zel Skrtic, PharmD, of Onsite Operations for CPS.
Clinical Operations Manager, Kathy Canup, PharmD, who grew up in Wabash County and has been with the health system for 13 years, oversees the specialty pharmacy operations. She and her team take the time to work with patients to get medications into their hands as quickly as possible. “We get prior authorizations and do what we can to make the medication affordable through insurance, copay cards, financial assistance programs, or grants,” Canup says. Beyond that, specialty pharmacists and liaisons support patient needs — walking them through treatment plans, helping them manage refills, and more.
n Fast, Affordable Access to Critical Specialty Medications
Union Health Specialty Pharmacy opened its doors in February of 2021. “Once we signed the contract — it was pretty much plug and play from our standpoint,” says Cleveland. CPS handled everything, including absorbing the upfront risk from a capital and operational staffing perspective. “Hospitals our size don’t often have an onsite

“We’ve found that having a local Terre Haute patient talking to a Terre Hautebased pharmacist or liaison has made a difference in building trust and helping them understand we have a specialty pharmacy right here onsite.”
Zel Skrtic
Senior Vice President of Onsite Operations
~ CPS Solutions
specialty pharmacy,” he explains. “Being able to help our patients access and manage these complex medications improves their experience and outcomes — and that’s something we couldn’t have done without the help of CPS.”
Today, the specialty pharmacy supports Union Health’s patients who are dealing with various cancers, rheumatoid arthritis, neurological disorders, infectious diseases, and respiratory conditions. Canup says having pharmacists and liaisons onsite is a big win for clinic staff. “I didn’t realize the workload nurses were taking on trying to obtain prior authorizations and help patients research and apply for financial assistance ” Now, with dedicated staff to manage the process, provider satisfaction has improved. Moreover, patients are better able to access and afford their therapies. “The specialty pharmacy maintains a 96.7% prior authorization approval rate and has saved patients over $2 6M in financial assistance,” says Canup.
As a result, patient satisfaction has increased, too. Union Health earned a Net Promoter Score (NPS) of 97.7, placing it as the 2024 winner and top performer for patient loyalty in Managed Markets Insight & Technology’s (MMIT) 9th Annual Specialty Pharmacy Patient Choice Awards. This is a significant honor as the MMIT awards recognize health system specialty pharmacies nationwide that provide exemplary patient satisfaction.
n Uncovering New Opportunities
Working with CPS provides Union Health with opportunities to serve that community that would not otherwise have been possible. This includes focusing on programs and initiatives to improve patient adherence and outcomes.
In 2023, the specialty pharmacy team began working with a group of local neurologists to follow their migraine patients closely. “Starting with a patient’s baseline migraines, we track their progress once they’ve started therapies to confirm treatment is effective and migraine days are decreasing each month,” explains Canup. If there isn’t improvement after three months, the pharmacist works closely with the neurologist to adjust and optimize therapy. “And when an office visit is required for further evaluation, we can typically get patients seen faster so they are able to adjust therapies as needed in a more timely fashion.”
Working with the rheumatology clinic, the specialty pharmacy team also conducted a study to determine if self-administered subcutaneous injections could be a more affordable and convenient option for patients with autoimmune diseases being treated with intravenous (IV) therapies. The catalyst for the study was related to the cost of IV therapies, which were becoming unaffordable for many patients, even with financial assistance, as well as the time and expense spent traveling to a medical facility for treatment. Self-administered medications were delivered to the homes of patients who agreed to participate. Union Health specialty pharmacists then interviewed participants using a standardized six-question survey to measure patient satisfaction, ease and convenience of use, and confidence in the self-administered medication. Overall, 87% of participants said the self-administered medication was either extremely convenient or very convenient. With this, the specialty pharmacy team began working with rheumatology patients to

The specialty pharmacy maintains a 96.7% prior authorization approval rate and has saved patients over $2.6M in financial assistance.”
Kathy
Canup,
PharmD Clinical Operations Manager
~ CPS Solutions, LLC/ Union Health
educate them on at-home treatments — helping them transition from IV therapies and secure financial assistance to cover more of the medication costs.
Overall, how would you rate the convenience of receiving your medication with the autoinjector?
Extremely Convenient
Moderately Convenient
Very Convenient
• 80% increase in Rx volume.
• 50% growth in the number of patients serviced.
• 92% patient adherence.
• $2.6 million in financial assistance secured.
• 94% provider satisfaction.
“What we do on a daily basis is very rewarding,” says Canup. “Patients are constantly thanking us for making a complex and scary situation more manageable.” That includes helping them get access to the specialty medications they need, as well as the financial assistance to make treatment more affordable. She also notes that being in the clinics helps doctors and nurses understand exactly what the specialty pharmacy team can do for them. “The CPS team frees clinicians up to do what they are trained to do — build relationships with and care for their patients,” says Skrtic. “That’s why we’ll continue looking for more opportunities to help other specialty providers increase patient satisfaction.”
From Cleveland’s perspective, he believes many directors of pharmacy in smaller hospitals might not think they can embed a specialty pharmacy. “That’s just not the case. I had no idea what opportunity was waiting for us until we decided to establish a specialty pharmacy.” As a result of adding specialty pharmacy services, Union Health’s pharmacy department continues to exceed its operational margin goal. “I think any hospital with 340B access that offers specialty services should explore what is possible working with a partner like CPS.”
For more information on Union Health, please visit their website at www.union.health.
For more information about CPS Solutions, LLC, please visit their website at www.cps.com.


• Cold Seal
• Tamper-Evident
• Moisture Resistant
• Ultraviolet Inhibitant
Dose, Bar PharmacyCoding, & NursingExperts!Supply Unit Dose, Bar PharmacyCoding, & NursingExperts!Supply
• Reduces Cross Contamination

• Ideal for Meds Covered by USP 800
• 6 and 12-month Beyond-Use Dating
• 1-D and 2-D Bar Coding
• Flexible Label and Report Formatting
• Multiple Sizes and Shapes to Fit Your Meds & Storage Needs


President: Robert Braverman
Founded: 1971
Employees: Private
Toll-Free Phone: (800) 523-8966
Phone: (215) 396-8600
Toll-Free Fax: (800) 323-8966
Address: 70 Industrial Drive Ivyland, PA 18974
Website: www.medidose.com
Improve your Solid Oral Unit Dose needs with our comprehensive Bar Coding, Packaging, and Labeling Solutions — designed by healthcare professionals, for healthcare professionals.

Features & Options
n Packaging and Labeling Solutions
• Simple to use — no extensive training needed.
• 1-D and 2-D bar coding — including NDC, lot numbers, and expiration dating.
• Ideal for hazardous medications and USP 800 drugs.
• Tall Man Lettering and dynamic formatting options.
• Built-in NDC lookup database and extensive image library.
• Packaging logs and error reporting.
• Six-month and one-year beyond-use dating.
• UV and moisture resistance.
Medi-Dose/EPS was founded in 1971 when Milton Braverman, a former pharmaceutical company territory manager, saw the need for inexpensive, manual unit dose packaging allowing a hospital to convert from traditional dispensing. He developed the Medi-Dose System to package, handle, and dispense predetermined amounts of medication so they would be accessible for one regular dose. Comprehensive bar coding and labeling identification is accomplished using our innovative MILT 4 software. Because of the continued success of the MediDose System, Medi-Dose/EPS expanded its product line to include the TampAlerT System, offering tamper-evident liquid packaging without the need for heat tunnels and accessory sealing equipment, as well as a full line of supplies and disposable products designed specifically for the pharmacy and health care professional.
Product Overview
Medi-Dose provides Tamper-Evidence as well as UV and Moisture Resistance for one-year beyond-use dating. Our MILT® 4 software maintains packaging logs and lets you design and print your Lid-Label® Covers with color, bar codes, tall man lettering, and graphics. You can format the labels any way you want, directly from your own computer and printer!
Medi-Dose works well in any pharmacy operation and can be used with all classifications of drugs (meds covered by USP 800, chemo meds, compounded drugs, controlled substances, etc.). It’s affordably priced for all pharmacy budgets. For the best in manual unit dose packaging, check out Medi-Dose!
• Tamper-Evidence.
• 15 styles of blisters to accommodate virtually all meds.
• MPB® — Multi-purpose blisters to easily package large medications, compounded drugs, double and triple “0” capsules, unit-of-use packaging, repackaged medications, and suppositories.
• New Ointment Lid-Label Covers to package ointments in all Medi-Cup Blisters.
• No machinery or space requirements.
• Inexpensive — no capital outlay required.
Product Specifications
n Accompanying Labeling Software
• MILT 4
• MILT 3.0
• MILT 2.6
• Medi-Dose 2000
Trade Shows/Meetings Attended
ASHP Midyear, ASHP Summer, Joint Forces Pharmacy Seminar, National Pharmacy Purchasing Association, EAHP, IACP, and various state, regional, and pharmacy compounding conferences.
Ordering Information

For additional information, please contact us at (800) 523-8966, visit our website at www.medidose.com, or email us at info@medidose.com.

*Product shown with optional features
Containment Primary Engineering Control (C-PEC)
Protection: Product, Personnel, & Environmental
Containment Primary Engineering Control (C-PEC)
Optimized for Sterile Hazardous Drug Compounding Systems
Telescoping or Mechanical Auto-Rising Base Stand Options
Optional IV Bar with 3 Height Locations and 6 SST Hooks
Interior Duplex Outlets and Cord Pass-Through Port
Optional Back-Wall Viewing Window for PC Monitor
Up to 12 inch (305 mm) Access Opening
LabGard ES NU-813 Series
Optimized for Non-Sterile Hazardous Drug Compounding
Telescoping or Mechanical Auto-Rising Base Stand Options
Optional Ergonomic Mount for PC Monitor, Keyboard, and Mouse
Double Exhaust Filter with Bag-In/Bag-Out Options
Polycarbonate Viewing Window and Sidewalls
Sidewall Waste Pass-Through Ports
Protection: Personnel & Environmental

*Product shown with optional features
President & CEO: Bill Peters
Founded: 1971
Employees: 265
Toll-Free Phone: (888) 468-2473
Phone: (763) 553-1270
Toll-Free Fax: (800) 328-3352
Address: 2100 Fernbrook Lane Plymouth, MN 55447
Website: www.nuaire.com
Choose Containment Primary Engineering Controls (C-PECs) built around your pharmacy’s workflow to enable you to perform safe, ergonomic, and efficient drug compounding.

a PVC core. One easily monitors workbench performance through the Aeromax™ control system that visualizes pressure in the internal airflow plenum. The work zone comes standard with cord-pass throughs and LED lighting but can be optionally configured with polycarbonate walls, an IV bar, outlets, and service valves to fit your workflow needs. Optional base stands are available with motorized height adjustability and support arms for keyboards and monitors to further support an ergonomic workflow.
n NU-LXXX Custom Biosafety Cabinets
NuAire, Inc. is a family-owned company that has provided airflow equipment to laboratories for over 50 years.
As a small company with a big footprint, NuAire has supplied both public and private laboratories around the world in sectors such as academia, pharmaceuticals, animal research, healthcare, and more. We seek to differentiate our products by minimizing their long-term cost of ownership, maximizing their configurability for each customer’s application, and extending their service life through personalized after sales support and a generous warranty.
n NU-543
The NU-543 LabGard® provides the pharmacy with a Containment Primary Engineering Control (C-PEC) whose core design and configuration options enable one to optimize it for their sterile, hazardous drug compounding workflows. This model features a dual thermistor airflow sensor and alphanumeric display for laboratories requiring a clear measurement of their cabinet’s performance and a choice of three NSF listed work access openings for ergonomic access to the work zone. Built standard with two outlets and a cord pass through for needed devices, the cabinet can optionally be made with a smooth interior instead and always includes a prop up work tray to ease regular cleaning under the work surface. Optional base stands are available with motorized height adjustability and support arms for keyboards and monitors to further support an ergonomic workflow. A separately available exhaust canopy with configurable side air gaps provides the installer with flexibility when connecting the cabinet to an external exhaust system.
n NU-240
Airflow Workbench
The AireGard™ NU-240 creates HEPA-filtered horizontal laminar airflow to provide product protection for sterile but non-hazardous drug compounding in pharmacies. The DC ECM motor draws air through a top pre-filter and propels it through a backwall HEPA filter to create an ISO Class 5 level of air cleanliness inside a work zone surrounded by stainless steel walls and a work surface made of stainless steel with
The ultimate form of configurability is customization of a solution for your specific needs. Whether it is an oversized work zone for automation equipment, a reinforced work surface, support for a camera, or a back wall cutout for a monitor, NuAire can go to the drawing board to offer you a tailor-made solution.
Additional Product Lines
• Class II Type B1 Biosafety Cabinets
• Class II Type B2 Biosafety Cabinets
• Containment Ventilated Enclosures
• Horizontal Laminar Airflow Workbench (Console Style)
• Vertical Laminar Airflow Workbench
• Restricted Access Barrier Systems (RABS)
• CO2 Incubators
• Ultralow Freezers
• Animal Transfer Stations
• CETA, April 19-22, Reno, NV
• Lab Design, May 19-22, Phoenix, AZ
• Pharmacy Futures, June 8-12, Portland, OR
• CALAS/ACSAL, June 22-25, Saskatoon, SK
• ADLM, July 28 - August 1, Chicago, IL
• CABS/ACSB, September 17-19, Saskatoon, SK
• Tradeline Animal, September 30-October 1, Boston, MA
• ABSA, November 1-6, Phoenix, AZ
• AALAS, November 3-7, Nashville, TN
• ASHP, December 8-12, New Orleans, LA
• Vizient, HealthTrust, Premier, Ascension
Ordering Information
Visit our website, nuaire.com.








to include ready-to-use bags.


With consistent supply, proven quality and reliable service, Amneal is in the bag business for the long haul. We’re committed to providing the institutional market with products like multi-source injectables and biosimilars, plus convenient presentations like bags and prefilled syringes, ensuring availability and selection for years to come.
This is how we make healthy possible.

Learn more about new launches and all our products by scanning the QR code or visiting amneal.com.
Order from your wholesaler/distributor or contact sales@amneal.com



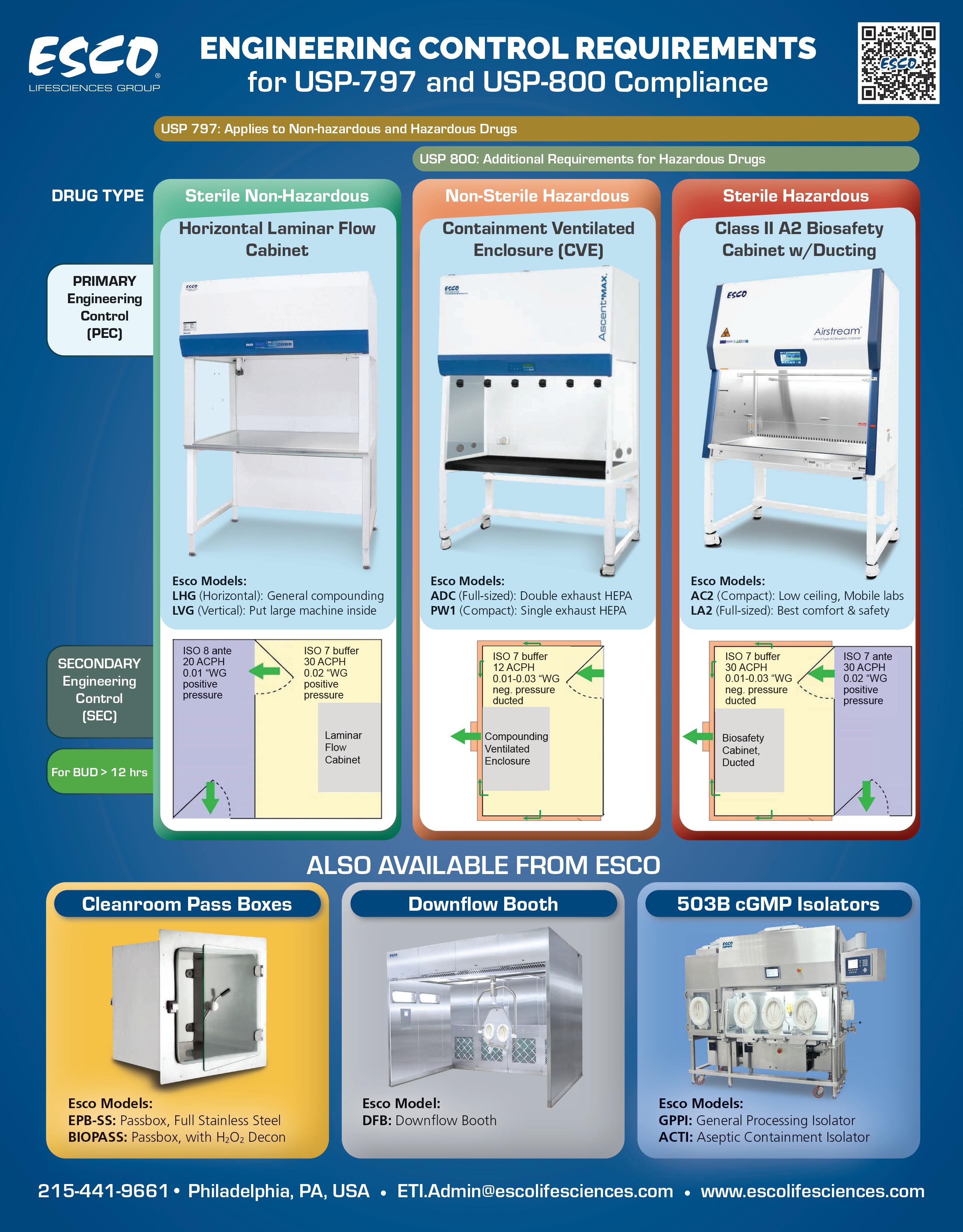
Leiters Health helps our partners gain more control over their medication supply. We serve as an extension of your pharmacy, helping to standardize your portfolio, prevent shortages and determine when to outsource or compound inhouse.
With increasing drug shortages, supply chain disruptions, and staffing concerns, access to quality compounded medicine is more important than ever. Our products include:
Pre-filled IV bags, syringes and vials.


Contact us to learn how we can support your hospital pharmacy and your patients.

Leiters.com | 800.292.6772 | info@leiters.com
Leiters Health is a trusted FDA-registered 503B outsourcing provider of ready-to-administer compounded sterile preparations committed to providing healthcare professionals and their patients with the highest-quality medications.
n Ophthalmic Products and Services
President & CEO: Joseph C. Cosgrove
Founded: 1926
Employees: 375
Toll-Free Phone: (800) 292-6772
Phone: (720) 697-5140
Fax: (408) 288-8252
Address: 13796 Compark Boulevard Englewood, CO 80112
Website: www.leiters.com
Leiters Health, founded in 1926, is an FDA-registered 503B outsourcing provider of high-quality compounded sterile preparations and pharmacy services. We have a long history of evolving and innovating to meet the latest regulatory requirements and market needs.
All sterile preparations are produced under Section 503B of the FD&C Act (503B Guidance), follow current Good Manufacturing Practices (cGMP), and USP <797>. Our facility consistently upholds all standards based on the audits conducted by the FDA, the states of California and Florida Boards of Pharmacy, multiple health systems, group purchasing organizations, and other independent accreditation organizations.
Our team of experts in sterile pharmaceutical manufacturing, repackaging, and compounding provide a sophisticated understanding of what it takes to elevate the quality and consistency of supply in pharmaceutical outsourcing. We combine a highly experienced team, with robust processes, in stateof-the-art outsourcing facilities to ensure delivery of the highest quality products and services.
As part of our ongoing commitment to quality, compliance, and sterile manufacturing excellence, we continue to invest in our state-of-the-art 503B facilities, including two existing facilities in Denver, Colorado, and a new facility in Buena, New Jersey, is planned to be operational in 2025. We stand ready to support the needs of the healthcare community now and in the future.
Leiters Health provides ready-to-use compounded sterile preparations and pharmacy services across the continuum of healthcare, including hospitals, surgery centers, physician offices, and clinics. n Hospital and Surgery Center Products and Services
• Prefilled syringes, vials, and bags.
• Ready-to-administer pain services portfolio to support ERAS and opioid reduction strategies.
• Pharmacy fill service for the Avanos Medical opioid sparing ON-Q* Pain Relief System (bupivacaine HCl and ropivacaine HCl).
• Prefilled syringes, vials, and dropper bottles including: injections, antibiotics, and dilating agents.
• FDA-Compliant Repackaged Avastin® Service. Multiple presentations available based on physician preference.
Leiters Health is partnered with many market leading innovative healthcare companies that compliment what we do. The products and services offered by these companies may provide additional value to your organization. Our key business partners include: Angels for Change, Avanos Medical, Besse Medical, Bluesight, Cardinal Health, End Drug Shortages Alliance, Eye Connect International, Prodigy Health, and The Academy.
Leiters Health provides our products and services to many leading health systems, community hospitals, clinics, and physician offices. In addition, we currently have national contracts with: AmerisourceBergen, Captis, Cardinal Health, EyePro, Healthtrust, Premier, US Retina, and Vizient. Please contact us for additional information regarding our contracts.
Leiters Health is committed to new product development in collaboration with its customers to meet evolving market needs. We work closely with our 10 strategic health system investors to evaluate, create, and deploy solutions that enable pharmacy efficiencies. Pharmacy leaders from each of our investor health systems hold a seat on the Leiters Health Strategic Pharmacy Advisory Board. We meet with this group regularly to understand and nimbly respond to pharmacy challenges with real-world, innovative solutions. This allows us to offer a meaningful portfolio across the continuum of care to all of our customers and ultimately ensure that healthcare providers and their patients have a continuous supply of critically needed drugs.

We don’t want to simply tell you about what we do, we want to show you! We invite you to visit our facility to better understand the cGMP regulations, sterile manufacturing processes, and automation we use to elevate the quality of our products and services. Come join the growing list of organizations who have visited our facilities. To schedule a site visit, please visit www.leiters.com.

PRODUCT TESTING MADE SIMPLE PRODUCT TESTING MADE SIMPLE
PRODUCT TESTING MADE SIMPLE
Appearance
Container Closure USP <1207>
Endotoxin USP <85>
Microbial Limits USP <61>
Particulate Matter USP <788> / <789>
pH USP <791>
Potency
Preservative Effectiveness USP <51>
Sterility USP <71> or Rapid Sterility
Tests for Specified Organisms USP <62>
Water Activity
Ensure your health-system’s compounded drug products and facilities consistently meet quality standards.
President & CEO: Thomas C. Kupiec, PhD
Founded: 1998
Employees: 300+
Toll-Free Phone: (800) 393-1595
Phone: (405) 271-1144
Email: info@arlok.com

Address: 840 Research Parkway, Suite 546 Oklahoma City, OK 73104
Website: www.arlok.com
ARL Bio Pharma is a contract laboratory that provides analytical and microbiological testing for health-system pharmacies. Since 1998, ARL has supported the industry-wide commitment to deliver high-quality drug products by providing guidance and test services for all phases of the product lifecycle following USP and FDA guidelines. ARL is ISO 17025:2017 accredited, FDA-registered and audited, and DEA licensed for Schedules I through V.
USP standards are critical for health-system pharmacies to produce safe and effective compounded medications. Incorporating these standards into compounding practices promotes patient safety and quality preparations, while minimizing the risk of hazardous exposure.
ARL offers comprehensive testing services for health-systems to quickly and easily meet industry standards.
n Analytical Testing: qualifies drug substances, excipients, and drug products to meet pharmacopeia specifications.
n Microbiology Testing: offers the highest quality in USP-required microbiology testing for non-sterile and sterile preparations.
n Personnel and Environmental Monitoring: evaluates plates and media fills for incubation and observation.
n USP 800 Surface Wipe Sampling: measures the level of hazardous drug surface residue to ensure workplace safety.
Health-system pharmacies may want to extend a beyond use date (BUD) on a drug product to increase compounding efficiencies and reduce drug waste. USP 795 and 797 require the BUD assignment to be supported by a stability-indicating analytical method.
ARL’s stability indicating methods meet the requirements set by USP and FDA. To ensure that a product retains its quality characteristics throughout its BUD, it is important to also evaluate the physical, chemical, and microbiological properties. ARL has extensive experience in stability studies, and will guide health-systems through the entire process from understanding goals and helping design the study, to providing results and interpreting the data.
Request A Quote: info@arlok.com
ARL Bio Pharma Client Portal: portal.arlok.com
ARL’s client portal allows users to submit samples online, receive test results in real-time, and analyze test data trends.
• Scientific and Technical Competence: ARL Bio Pharma delivers comprehensive and exceptional analytical and microbiological solutions to help our customers meet quality standards.
• Customer Service: ARL Bio Pharma is committed to understanding our customers’ needs and strives to make every touch point a positive experience.
• Quality: ARL Bio Pharma is committed to compliance with regulatory requirements, laboratory standards of practice, and continuous improvement.
• Integrity: ARL Bio Pharma is committed to honesty, reliability, and transparency.
• Collaboration: ARL Bio Pharma is committed to engaging internal and external stakeholders to produce optimal outcomes.
What are your thoughts on naloxone?
Mark Garofoli, PharmD, MBA, BCGP, CPE, CTTS
Clinical Assistant Professor & Clinical Pain/SUD Pharmacist West Virginia University School
Regardless of your profession, or even beliefs, our opioid overdose crisis is one of the single most discussed and impactful scenarios ever. Naloxone. One word. One medication. So many conversations. In forming one’s “personal facts” (i.e., opinions), it’s best to first review the actual facts with a review of naloxone pharmacology, clinical pearls, and products.
Speaking of pharmacology, can you tell us about naloxone’s pharmacology?
Naloxone’s respective pharmacology includes being highly lipophilic (crossing the blood brain barrier), having low oral bioavailability, and a short duration of action (commonly observed as approximately a half-hour).1
How is naloxone available?
Naloxone opioid overdose reversal medications are generally available via prescription (Rx), over-the-counter (OTC), or harm reduction programs as either injectable or nasal formulations. The injectable formulations doses include 0.4 mg vials (Rx)5, a 5 mg device (Rx)2 , and a 10 mg auto-injector device3 approved only for U.S. military utilization. The nasal formulations doses include a 1 mg assembly-required kit (Rx)5, a 3 mg device (OTC)4, a 4 mg device (Rx & OTC)5, and an 8 mg device (Rx)6. There is also a novel nasal swab formulation 3 mg product under FDA review for OTC status approval.7

Naloxone has been available via prescription through general prescribing, board of pharmacy protocols, and statewide standing orders. The more recent OTC status aimed to increase access and eliminate supply barriers, and although ubiquitous availability beyond pharmacies is a result, cost and administration education remain concerns for many people.
Many have debated what the “perfect dose” of naloxone to be within various circular conversations across our country. In 2016, our FDA conveyed a joint advisory committee meeting which concluded that there was simply no “best” or “perfect” naloxone dose since there are several factors that come into play such as the patient’s past and current opioid tolerance, geography relative to a hospital, and dosage of the opioid causing the overdose (yet completely unknown in illegal products), amongst other concerns. Additionally, one must consider the propensity to reverse the opioid overdose (respiratory depression) without precipitating opioid withdrawal.
A recent study illustrated that a higher naloxone nasal spray dose of 8 mg compared to 4 mg results in no additional benefit, yet with potential additional harm in precipitating opioid withdrawal.8
Easy. The one that’s closest to you. In order to save lives, our society needs naloxone available at a moment’s notice, thus
strategically positioned throughout our communities similar to, if not next to, defibrillators, particularly in rural areas further away from a hospital. That directly means placing naloxone products in malls, libraries, restaurants, and even airplanes.
Can you tell us about recent naloxone expiration date extensions?
Recently, our FDA extended the shelf-life of the brand name naloxone 4 mg nasal spray to four years9, which is appreciably longer than most, if not all, other medications in our country. Once again, emphasizing the importance of this vital medication.
Have you heard of “Narcan Parties” and do they actually exist?
A “Narcan Party” is said to be an event where one would inject heroin with complete disregard for experiencing a deadly overdose since there is naloxone in the vicinity to reverse the opioid overdose respiratory depression and save one’s life.10 Essentially, the reality is that one continually utilizing illicit or diverted opioids or misusing prescribed opioids is aiming to avoid withdrawal (“getting by” rather than “getting high”), and the impending utilization of naloxone to reverse an opioid overdose can precipitate withdrawal, the very thing that one would be looking to stave off in the first place. Illogical, to say the least. Yet, media still sensationalize the concept, even if utilizing headlines that include the word “if” in reference to these events existing.11 Rather unfortunate.
How many is too many for naloxone reversals for a given person?
Cost and coverage of naloxone in the scenario of potentially saving a human life, once or repeatedly is a tough consternation, as it truly is beyond difficult to put a price tag on a human life. Yet, considering the “relapse rate” of asthma (i.e., having an asthma attack while being actively provided maintenance therapy) is more than that of substance use disorder (addiction)12, one should appreciate that folks may experience “curve balls” in life and end up relapsing in their addiction recovery by experiencing an overdose, more than once. When a patient doesn’t utilize their maintenance asthma medications (inhalers) as prescribed and dispensed, yielding a life-threatening asthma attach, is the rescue inhaler ever withheld? When a person chooses an unhealthy diet due to a myriad of reasonable scenarios (cost, transportation, etc.) and then has a heart attack, is the nitro ever withheld?
If your father, mother-in-law, neighbor, best friend, or better yet, if you needed a lifesaving medication more than once, if not 10 times, wouldn’t you want it provided? These answers are best considered for your own point of view right here and right now.
Are there any studies illustrating the lifesaving efforts of pharmacy professionals in respect to providing naloxone?
Naloxone saves lives. Healthcare professionals discussing naloxone education, or even providing naloxone medication products to patients indirectly, if not directly, save lives. One study illustrated that for every eight patients that a community pharmacy provided naloxone and its respective education to, one human heartbeat was saved.13 Considering that many more than eight people a day walk into each and every community pharmacy every day in our country, and certainly an incredible number in each and every hospital, a huge opportunity exists for saving human lives.
Any final thoughts on naloxone?
We are all called to “Do No Harm”. Conflating the pharmacology, societal factors, and stigma, one can easily see that discussions on this one medication really can lead to saving lives by the dozen. One Medication. One heart beat saved. PRN.
References:
1 Clinical Pharmacology Online Database. Accessed April 2024.
2 https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212854s000lbl.pdf
3 https://naloxoneautoinjector.com
4 https://www.fda.gov/news-events/press-announcements/fda-approves-second-overcounter-naloxone-nasal-spray-product
5 https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208411lbl.pdf
6 https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212045s000lbl.pdf
7 https://www.wsj.com/articles/opioid-overdose-naloxone-over-the-counter-11669859905 https://www.biospace.com/article/releases/naxswab-otc-novel-naloxone-nasal-swabsuccessfully-administered-by-children-and-adults-to-potentially-rescue-someone-from-anopioid-overdose-in-study/
8 Payne ER, Stancliff S, Rowe K, Christie JA, Dailey MW. Comparison of Administration of 8-Milligram and 4-Milligram Intranasal Naloxone by Law Enforcement During Response to Suspected Opioid Overdose — New York, March 2022–August 2023. MMWR Morb Mortal Wkly Rep 2024;73:110–113.
9 https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-shelf-lifeextension-naloxone-nasal-spray
10 https://www.vice.com/en/article/qv7jmp/heroin-overdose-parties-are-a-dehumanizingmyth
11 https://www.tennessean.com/story/news/local/cheatham/2019/01/14/cheatham-coems-director-explains-very-very-dangerous-narcan-parties/2572355002/
12 Comparison of Relapse Rates Between Drug Addiction and Other Chronic Illnesses JAMA. 284: 1689-1695, 2000.
13 Katzman JG, Takeda MY, Bhatt SR, Moya Balasch M, Greenberg N, Yonas H. An Innovative Model for Naloxone Use Within an OTP Setting: A Prospective Cohort Study. J Addict Med. 2018 Mar/Apr;12(2):113-118.

President: David Lowrie
Founded: 2010
Toll-Free Phone: (800) 797-1405
Fax: (951) 547-1681
Address: 1220 Graphite Drive Corona, CA 92881
Website: www.iso-med.com
As one of the top leading suppliers for pharmacy cleanroom supplies, we provide an unparalleled selection of specialized medical products, globally trusted quality and service, and delivery convenience for meeting USP <797> and USP <800> standards.

Since 2011, ISO-MED, Inc., a medical supply distributor, has been a trusted hospital supplier with a solid combination of quality, selection, service, and convenience. We supply quality products for pharmacy cleanrooms and other medical industries in the U.S.
Providing high-grade supplies for cleanrooms, laboratories, homecare, and more, we are dedicated to achieving and maintaining compliant sterile compounding environments.
ISO-MED maintains the largest and broadest portfolio of on-theshelf warehouse of supplies.
We offer customized supply plans, a seamless e-commerce customer experience, easy ordering with matching manufacturer, and dependable purchasing/supplier aggregation services.
With one-on-one scheduled training services, we increase the efficiency of your purchasing processes, consolidate your portfolio, and link your on-site supply demand to your inventory management processes.
Offering value-added services, we provide consistent customer service and on-time delivery with a streamlined transaction process. We are driven to help healthcare-oriented organizations save money.
Features & Options
ISO-MED not only has products that help a hospital conform to cleanroom standards, but we also offer the following products for testing cleanliness.
n ICR Plus TSA LT Contact Plates
Environmental monitoring for isolators and cleanrooms (surface and air monitoring).
n Tryptic Soy Agar
Added measure to assure sterility in your environmental monitoring program! Hardy Diagnostics contact plates are recommended for use in the cultivation of microorganisms from environmental surfaces.
Additional Product Modules
n Premier Product Offerings
• PPE Product Selection
• HD Spill Kit
• Chemotherapy Gowns and Supplies
• Cleaning and Disinfection Solutions
For every cleanroom or critical environment cleaning and disinfection program, ISO-MED offers several brands and ISO-MED, Inc. formularies.
Markets Served
• Hospital
• Compounding
• Infusion
• Retail
Ordering Information
Contact us by phone at (800) 797-1405 or email sales@iso-med.com to gain access to our exclusive online ordering platform.
INTRODUCING THE NEW INFINITY SERIES ™ WITH PRECISE, PATENTED TEMPERATURE CONTROL

Variable Speed Temperature Control
Patented infinite speed control technology delivers only the precise amount of refrigeration needed to reduce energy consumption without sacrificing performance.
Durable Design
Our cold storage solutions deliver uncompromising performance and lasting peace of mind over years and years of continuous use.
Flexible Storage
Interior cabinet space can be configured to your exact needs.
Follett high-performance NSF 456-Certified refrigerators provide precise temperature control for safely storing vaccines and medications.
General Manager: Mike Raycher
Founded: 1948
Employees: 500+
Toll-Free Phone: (800) 523-9361
Phone: (610) 252-7301
Fax: (610) 250-0169
Address: 801 Church Lane Easton, PA 18040
Website: www.follettpharmacy.com
Follett Products LLC is a leading manufacturer of innovative equipment for the healthcare market, including medical-grade refrigerators and freezers, ice and water dispensing equipment, and ice machines. Our focus on 100% customer satisfaction results in equipment that provides outstanding innovation and design excellence to meet the specific needs of each healthcare facility.
Follett medical-grade pharmacy refrigerators and freezers maintain precise and consistent temperatures for your valuable medications and vaccines. Powerful forced air refrigeration enables quick recovery after door openings, ensuring your critical products are stored at specified temperatures and reducing the risk of lost product.
n Follett Infinity Series™ upright refrigerators and freezers feature a powerful top-mounted refrigeration module and innovative plenum air distribution system for superior temperature consistency.
• Patented “infinite” variable speed control algorithm delivers only the precise amount of refrigeration needed at any given time, providing a ±1⁰C temperature consistency throughout the storage cabinet while minimizing noise and energy consumption.
• Industry-exclusive ducted air distribution delivers cold air at multiple levels for top-to-bottom temperature uniformity, allowing full utilization of the storage space.
• All models are NSF 456-Certified for safe vaccine storage.
• Intuitive full-color touchscreen user interface features enhanced data management with data downloading capability, providing the ultimate in user convenience.
n Full stainless steel cabinet and high-quality components designed with the user in mind.
• Heavy-duty, self-closing doors provide important energy savings and lock open at 90 degrees to allow easy product loading.
• Interchangeable storage options.
• Choose from epoxy-coated shelves, full extension epoxy-coated “floating” baskets, or full extension stainless steel drawers.
• Interior can be reconfigured at any time to accommodate changing storage needs.
n Follett’s innovative modular refrigeration system provides outstanding serviceability.
• Top-mounted modular design allows the entire refrigeration system to be removed as one unit without cutting refrigerant lines. If needed, a spare system can slide into place, virtually eliminating downtime or the need to relocate product.
n High-performance countertop refrigerators and freezers offer superior temperature performance in space-saving configurations.
• 1 and 2 cu ft models fit on standard 24" deep counters.
• Forced-air cooling provides cabinet-wide temperature consistency and quick recovery after door openings.
• External digital temperature display in user-selectable F or C with integral high/low alarming and display sleep mode.
• Keyless entry via keypad and electronic lock option.
n Powerful undercounter refrigerators and freezers are designed for use below standard and lower ADA-compatible counters.
• Heavy-duty forced air cooling for quick recovery after door openings.
• External digital temperature display in user-selectable F or C with integral high/low alarming and display sleep mode.
• Keyless entry via keypad and electronic lock option.
For more information, contact Follett at (800) 523-9361 or visit www.follettpharmacy.com.
FOLLETT HEALTHCARE is a trademark of Follett Products, LLC. FOLLETT is a registered trademark of Follett Products, LLC, registered in the U.S.

Steven Miller is the vice president of pharmacy services for 340B Health, a nonprofit organization of more than 1,500 hospitals and health systems participating in the federal 340B drug pricing program.
Q. How does the Inflation Reduction Act (IRA) affect the 340B drug pricing program?
The IRA’s changes to the Medicare Part D and B drug reimbursement process will have financial implications for 340B covered entities. They include:
• Medicare’s maximum fair price (MFP) for selected drugs will be the basis for Part D and Part B reimbursement. Medicare negotiates the MFP it will pay starting in 2026 for 10 Part D drugs with the highest total annual Medicare expenditures. The negotiated drug list will increase over time, with an additional 15 drugs in 2027 (Part D) and 2028 (Part B and Part D), and 20 more per year (Part B and Part D) starting in 2029.
• If a covered entity purchases drugs at the 340B price, its 340B savings will be the difference between the 340B price and the MFP.
• If the MFP is less than the 340B price, there will be no savings for the covered entity.
These pricing and reimbursement policies could have significant impacts on the financial health of 340B covered entities, and it is important for entities to understand them. Other IRA-mandated changes in the coming years include:
• Lowered Prescription Drug Costs: The IRA aims to reduce the cost of prescription drugs for millions of Americans, including cancer medications, blood thinners, and insulin.
• Savings for Patients: The IRA authorizes the federal government to set prices on the prescription drugs that represent the highest expense for Medicare, which would result in cost savings for patients.
• Out-of-Pocket Cost Caps: The IRA sets a $2,000 annual cap on what seniors pay for medicines under Medicare Part D and limits the monthly cost of insulin to $35 for seniors.
• Premium Subsidies Extension: The IRA extends enhanced premium assistance for lower-income enrollees to purchase health coverage on HealthCare.gov and state-based marketplaces.
• Free Vaccines: The IRA provides most vaccines for seniors at no additional out-of-pocket cost.
Q. During the COVID-19 public health emergency, the Health Resources and Services Administration (HRSA) allowed covered entities to use 340B drugs in provider-based locations not yet listed on the most recently filed Medicare cost report (MCR). Has anything changed with this policy?
HRSA implemented a policy in 1994 that prohibits new hospital outpatient locations from using 340B drugs until they have appeared on a hospital’s filed MCR and are registered with the agency. During the COVID public health emergency, HRSA started allowing these new locations — also known as child sites — to use 340B immediately for eligible patients of the entity. The agency did not say at the time that this policy would be temporary, and agency contractors informed some hospitals it would be permanent. However, HRSA announced in late October 2023 that the administration would reinstate its preCOVID policy after a short transition period that ended in late January 2024. The policy effectively reinstates delays of eight to 23 months in the use of 340B drugs in new child site locations. That process involves:
1. Inclusion in MCR: A hospital must include new services, departments, or clinics on its most recently filed MCR with reimbursable outpatient costs and charges. Hospitals file cost reports annually, five months after the end of the hospital’s fiscal year.
2. Registration: After the MCR filing, hospitals must register the new services, departments, or clinics with HRSA during an open registration period. The agency only has four two-week registration periods per year.
3. HRSA Approval: Following registration, HRSA imposes a waiting period of three months before approving it.
4. Activation on HRSA Database: Once approved, HRSA lists the new services, departments, or clinics as active on the agency’s database.
5. Usage of 340B Drugs: Only after the completion of these steps may hospitals use 340B drugs for patient encounters in these new locations.
Q. Have HRSA audit findings changed since the November 2023 court decision involving the community health center lawsuit?
The federal court decision stems from a lawsuit by Genesis Health Care, Inc., a federally qualified health center (FQHC) in South Carolina that sued HRSA over audit findings relating to prescriptions that outside providers had written. The court ruled that HRSA exceeded its authority by limiting the health center’s use of 340B to drugs involving care initiated by Genesis. The decision notes that HRSA continues to have statutory authority to audit covered entities for compliance with the 340B statute. Notably, HRSA diversion findings changed in mid-2019 following the Genesis lawsuit filing and a Trump administration decree limiting federal agencies from enforcing guidance. (The Biden administration subsequently withdrew that decree.) HRSA no longer appears to be enforcing a strict requirement that 340B prescriptions originate with the covered entity.
HRSA has released fewer fiscal year 2023 audit reports through March 2024 than it has in prior fiscal years. This delay could suggest that the HRSA Office of Pharmacy Affairs (OPA), which directly oversees audits of covered entities and manufacturers, is considering an audit enforcement policy change around patient definition. When the OPA posts all outstanding audit reports, it might provide more insight into the direction HRSA will take on compliance enforcement. It is important to note that HRSA will continue to audit covered entities to assess compliance with the prohibition against diversion and other 340B compliance requirements. Non-compliant providers might receive audit findings and be subject to enforcement actions, including repayment to drug companies.
Q. How does the recent federal court decision on the Arkansas contract pharmacy law affect covered entities?
In March 2024, a federal appeals court upheld the constitutionality of a state law that Arkansas enacted in 2021 prohibiting drug companies from restricting delivery of 340B-priced drugs within the state. Drug companies and the
Pharmaceutical Research and Manufacturers of America (PhRMA) have challenged the law in federal court under several legal theories. The appeals court rejected the argument that 340B is exclusively federal and that states are not able to legislate in this area. The upholding of the Arkansas statute could encourage other states to adopt similar laws, potentially restoring broader access to 340B pricing. This could greatly benefit hospitals and their eligible patients, particularly those using contract pharmacies.
The financial impact of the drug company restrictions on 340B access is substantial, involving losses of billions of dollars in 340B savings intended for patient care. This court decision could help covered entities staunch some of these losses to the safety net and the patients who rely on it. In the absence of federal 340B protections, states have the authority to legislate protections for covered entities in ways that do not conflict with federal laws.
Q. What is a duplicate discount, and why is it difficult to prevent them?
The 340B statute protects manufacturers from paying both a 340B discount and a Medicaid rebate to a state for a drug billed to Medicaid fee for service (FFS). These duplicate discounts can occur because of the complexities involved in billing Medicaid for 340B drugs. 340B covered entities have important considerations for preventing Medicaid duplicate discounts:
• Notification: Covered entities are responsible for notifying the state if they are billing 340B drugs to Medicaid FFS. HRSA maintains a database called the Medicaid exclusion file (MEF) for this purpose.
• Billing Practices: If any location, department, or service is sending Medicaid claims for 340B drugs, all billing numbers on the claims form must be listed on the HRSA website before submission. This requires communication between different departments within the entity to ensure it has accurately listed all billing numbers with HRSA.
• Carving Out: When entities or locations choose not to use 340B for Medicaid FFS patients, this is called “carving out.” In this case, the entity must not purchase 340B drugs for the areas billing Medicaid.
• Out-of-State Claims: Entities must not bill states for 340B drugs for Medicaid beneficiaries if those states and billing numbers are not listed on the MEF. Entities can apply filters to block out-of-state claims or employ internal reviews and audits of these claims to prevent potential duplicate discounts.
• Non-Compliance: If a review determines an entity erroneously billed Medicaid FFS for 340B claims, it must contact the state to confirm if the state sought a rebate on those claims and work with the affected manufacturers to resolve the issue or offer repayment.
340B Compliance Partners 1310 Cove Lane Road
Roaring Spring, PA 16673 (304) 964-3903 www.340bcompliancepartners.com
340B Health 1101 15th Street, NW Suite 910
Washington, DC 20005 (202) 552-5864 www.340bhealth.org
340BDirect
5241 South State Street Unit #2
Murra, UT 84107 (801) 716-4824 340bdirect.com
Apexus
290 E. John Carpenter Freeway Irving, TX 75062 (469) 299-7380 www.apexus.com
Atlas Health 701 5th Avenue S Suite 42
Seattle, WA 98104 (888) 566-6126 atlas.health
Aventi Health 1433 North Water Street 4th Floor Milwaukee, WI 53202 (414) 792-9673 www.aventihealth.com
CaptureRx
219 East Houston Street Suite 350 San Antonio, TX 78205 (210) 587-3486 capturerx.com
Cardinal Health 7000 Cardinal Place Dublin, OH 43017 (614) 757-5000 www.cardinal.com
Cervey
410 Kay Lane
Shreveport, LA 71115 (913) 568-0455 cervey.com
Clearway Health 1 Boston Medical Center Place Boston, MA 02118 (844) 319-7588 clearwayhealth.com
Cloudmed, an R1 company 1100 Peachtree Street Suite 1550 Atlanta, GA 30309 (615) 612-9562 www.cloudmed.com
Codonics 17991 Englewood Drive Middleburg Heights, OH 44130 (440) 243-1198 www.codonics.com
CPS Solutions, LLC
655 Metro Place South Suite 450 Dublin, OH 43017 (832) 541-2504 cps.com
Health Law Alliance 51 JFK Parkway Short Hills, NJ 07078 (800) 345-4125 www.healthlawalliance.com
Hudson Headwaters 340B
333 Glen Street Floor 7 (Pharmacy Services) Glen Falls, NY 12801 (855) 835-3402 www.hudson340b.com
iA
8888 Keystone Crossing Suite 1550 Indianapolis, IN 46420 (607) 526-0376 iarx.com
Kalderos
625 W. Adams Street Floor 20 Chicago, IL 60661 (844) 930-2322 www.kalderos.com
Liberty Software
3205 E Southlake Boulevard Southlake, TX 76092 (800) 480-9603 libertysoftware.com
Macro Helix (McKesson Corporation)
450 Lindbergh Drive Moon Township, PA 15108 (888) 462-4526 www.macrohelix.com
Maxor National Pharmacy Services, LLC
2805 N Dallas Parkway Suite 500
Plano, TX 75093 (469) 573-7211
maxor.com
Morris & Dickson
410 Kay Lane
Shreveport, LA 71115 (800) 388-3833 www.morrisdickson.com
Nuvem
161 Gaither Drive Suite 201
Mount Laurel, NJ 08054 (609) 541-1300 nuvem.com
Omnicell
4220 North Freeway Fort Worth, TX 76137 (877) 415-9990 www.omnicell.com
PharmaForce
225 Wilmington-WestChester Pike Suite 202
Chadds Ford, PA 19317 (302) 213-6990 thepharmaforce.com
Polsinelli Healthcare Solutions
150 N. Riverside Plaza Suite 3000
Chicago, IL 60606 (312) 463-6338
polsinellihealthcaresolutions.com
PrimeRx
805 RXR Plaza Uniondale, NY 11556 (516) 408-3999 primerx.io
ProxsysRx
1500 Urban Center Drive Suite 530 Birmingham, AL 35242 (214) 808-2700 proxsysrx.com
Ravin Consultants
6322 Glen Abbey Lane Bradenton, FL 34202 (941) 441-6212 www.ravinconsultants.com
RedSail Technologies
201 W. Saint John Street Spartanburg, SC 29306 (800) 845-7558 www.redsailtechnologies.com
Rx|X Advisory Services
(720) 608-3194 rxxconsulting.com
RxPreferred
2520 N Mt. Juliet Road
Mt. Juliet, TN 37122 (888) 666-7271 rxpreferred.com
RxStrategies Inc 1900 Glades Road Suite 350 Boca Raton, FL 33431 (954) 602-0046 rxstrategies.com
ScriptPro 5828 Reeds Road Mission, KS 66202 (913) 403-5128 www.scriptpro.com
Secure340B
245 N Highland Avenue Suite 230 Atlanta, GA 30307 (888) 732-3402 www.secure340b.com
Shields Health Solutions 100 Technology Center Drive Suite 600 Stoughton, MA 02072 shieldshealthsolutions.com
SpendMend Pharmacy 2680 Horizon Drive SE Grand Rapids, MI 49546 (616) 257-6323 www.spendmend.com/solutions/ pharmacy-solutions
SUNRx
10181 Scripps Gateway Court San Diego, CA 92131 (858) 746-1760 www.sunrx.com
The Craneware Group
800 Fairway Drive Suite 400 Deerfield Beach, FL 33441 (614) 843-0992 www.craneware.com
Verity Solutions 12131 113th Avenue NE Suite 200 Kirkland, WA 98034 (425) 947-3790 www.verity340b.com
Virtue 340B Ontario, NY 14519 (585) 329-0280 virtue340b.com
Visante 101 East Fifth Street #2220 St. Paul, MN 55101 (612) 850-0897 visanteinc.com
Wellpartner, Inc.
22 Cortlandt Street 17th Floor New York, NY 10007 (877) 231-6346 www.wellpartner.com
Wilco Data P.O. Box 5202 Midland, TX 79701 (800) 975-8430 www.wilcodata.com















Technology leaders, healthcare professionals, and pharmacy experts all geared toward growing your clinical and financial results. Nuvem is at the forefront of the 340B program. We think ahead so you don’t have to.

340B Technology Pharmacy Management Program Integrity
•Contract Pharmacy
•Split-Billing
• Advanced Referral Program
•In-House Pharmacy Services
•Clinical Programs
•Patient Engagement
•HRSA Mock Audits
•340B Consulting
•
Outsourced 340B Management
We support Covered Entities of all sizes that provide care to communities facing a variety of barriers to access comprehensive medical care and medication. Providers require innovative technology and specialized expertise to manage a successful pharmacy program, remain compliant, and achieve clinical objectives. Nuvem is the solution.
President & CEO: Matt Umscheid
Founded: 2014
Employees: 250+
Toll-Free Phone: (888) 356-6225
Phone: (856) 272-1458
Empowering covered entities to successfully manage all of their patient scripts with one holistic pharmacy solution, positively impacting patient health and financial outcomes.

Address: 161 Gaither Drive, Suite 201 Mount Laurel, NJ 08054
Website: www.Nuvem.com
Nuvem is a leading integrated pharmacy solutions partner that optimizes accessibility and adherence to medication improving clinical care for non-profit healthcare organizations. Through a complete technology suite, integrated service delivery, and elevated 340B expertise, Nuvem’s holistic solution yields enhanced drug inclusion, increased financial performance, and improved patient care.
Nuvem’s story began in 2014 and grew quickly into three separate brands: 340Basics, Assent, and Apovia. Together in improving accessibility and adherence to clinical care, but seemingly independent in operation. As the brands grew and expanded, the value of joining forces became clear. In 2023, Nuvem merged their expertise and resources, bringing one comprehensive solution, streamlined experience, and equitable partnership to medically underserved, non-profit healthcare organizations.
Combining all of our solutions and services represents our commitment to facilitating 340B programs — from compliance and optimization to patient engagement and education — through a fully integrated suite of cloud-based pharmacy solutions.
n Enhance program management, increase savings, and optimize performance.
Nuvem’s proprietary software empowers all eligible organizations to efficiently manage the complexities of the 340B pharmacy program. Our industry-leading technology solutions, knowledge, training, and service offerings enable healthcare organizations to increase claims capture and optimize their programs’ clinical and financial results.
Our 340B technology suite including TPA services such as contract pharmacy, split-billing, and advanced referrals, offers precision and attention to detail, along with the flexibility to customize the needs of each unique healthcare organization.
n Implement a more robust healthcare mission and enhance financial performance.
We offer pharmacy management services to implement and manage in-house pharmacies to improve patient script capture and avoid manufacturer blocks. Nuvem offers top to bottom support in building or managing in-house pharmacies (credentialing, licensing, construction, ordering, hiring, etc.), and guides health organizations through the full process, focusing on providing the best care for their patients.
n Remain compliant, pass audits with confidence, and outsource program management.
Evolving state and federal regulations can often challenge health organization’s program integrity. We use our experience to deliver a broad scope of services, such as HRSA mock audits, 340B consultation, and outsourced 340B management to deliver thorough analyses and recommendations.
Our expert services and consultants bring clarity and compliance to your 340B program for better clinical and financial results.
“Your entire team has a wealth of experience matched with a caring heart and a fierce determination which has enabled you to accomplish so much and I thank you for sharing that well-earned insight.”
— Executive Director, Health Center, Brooklyn, NY

Founder & CEO: Dean Erhardt
Founded: 2008
Phone: (636) 537-7805
Reducing the operational burden is possible. D2’s team of healthcare leaders have designed novel technologies and experience-based consulting solutions that enable hospitals and hospital systems to increase efficiency, minimize risk, and drive access.
Address: 400 Chesterfield Center, Suite 400 Chesterfield, MO 63017
Website: www.d2rx.com
Since 2008, D2 Solutions has helped hospitals and hospital systems across the industry reimagine their processes and reduce the operational burden, arming them with a groundbreaking combination of expert consulting and strategic SaaS solutions. From financial, licensing, regulatory, and accreditation pressures to managing the hassles of payer contracting and declining patient engagement, navigating every detail can be overwhelming. As a team of healthcare leaders, D2 strives to leverage our over 1,000 years of combined industry experience towards changing the narrative and developing scalable solutions that truly enhance operational efficiency, minimize exposure to regulatory risk, and drive patient access. Having collaborated with a variety of hospital and specialty pharmacy providers, ranging from independent establishments to wholesale distributors, supermarket chains, and hospital health systems, our hope is that our expertise can become your peace of mind.
• ComplySuite® Technologies: Automate compliance management, reduce the operational burden, and restore confidence in your compliance.
• UltraTouch® Technologies: Enhance the patient experience and increase speed-to-therapy.
• Specialty Consulting Solutions: Accreditation, licensing, 340B maximization, PBM and health plan mapping, prime vendor contracting, regulatory compliance, overcoming staffing challenges, etc.
• Pharmaceutical Manufacturer Consulting: Market access, contracting, pricing, distribution, compliance, therapy positioning, etc.
For over 12 years, D2 has collaborated with major accreditation bodies, regulatory agencies, and our internal pharmacist consultants to identify gaps and streamline processes for staying ahead of accreditations, monitoring licenses company-wide, and more efficiently managing regulatory alerts. ComplySuite® is comprised of three, web-based platforms:
n AccredComply: A centralized database for accreditation and reaccreditation management.
• Built-in global database of accreditation requirements.
• Automated, customizable, and auditor-ready reporting.
• Roles-based platform designed for cross-departmental communication.
n RegComply: Novel regulatory alert management dashboard that ensures only the right alerts, go to the right people, every time.
• Customizable alert paths and intelligent triaging based on organizational structure.
• Automated alert response documentation and audit-ready reporting.
• Legislation tracking supported by real people.
n LicenseComply: A single database for organizing, monitoring, and tracking licenses so you never miss a renewal.
• License storage with searchable license libraries.
• Live status tracking dashboard.
• Roles-based platform with custom admin views.
UltraTouch® consists of patient engagement, prior authorization, and therapy management solutions designed to enhance the patient experience by integrating with your existing systems, increasing speed-to-therapy, and simplifying communication with patients, payers, prescribers, and team members alike.
n UltraTouch® Verify: Revolutionary prior authorization management supported by real people.
• PAs, patient data, PA statuses, and all communication in one organized dashboard.
• Team efficiency reporting and payer behavior analysis.
• Agile integration with existing systems (EHR, APIs, Epic, FHIR, etc.) and existing PA processes.
n UltraTouch® Engage: Automated, bi-directional patient communication.
• Custom digital outreaches for onboarding and collection of missing information.
• Multi-language compatible without data loss.
• Proven 53% increase in speed-to-therapy.
n UltraTouch® Patient Management: Patient data optimized for accreditation.
• Provides forms and assessments aligned with current accreditation standards.
• Supports disease and drug-specific therapy programs.
• Hosts all patient data points in one place for simple outcome reporting.
To request a demo or learn more about how we partner with organizations like yours, check out our website or email us at connect@d2rx.com.

YOUR SCIENCE & REGULATORY PARTNER
LABORATORY TESTING
CUSTOM CLEANROOM DESIGN
CLEANROOM AND PEC CERTIFICATION
SMOKE STUDIES CALIBRATIONS
ASEPTIC TRAINING
CONSULTING
USP <800> WIPE SAMPLING

ENVIRONMENTAL MONITORING PLATES
EAGLEANALYTICAL.COM/HOSPITALS

President & CEO: Ross Caputo
Founded: 2003
Employees: 100
Toll-Free Phone: (800) 745-8916
Navigating the complexities of regulations can indeed be challenging. But fret not – we’ve got your back! From microbiological to chemistry testing, certification, and calibration, to consulting with a focus on compliance and patient safety, we leave no stone unturned.

Address: 11111 S. Wilcrest Drive, S1000 Houston, TX 77099
Website: eagleanalytical.com
Eagle was established in 2003 to meet the regulatory needs and requirements of the pharmaceutical industry. During that time, Eagle grew and morphed into a full-fledged analytical chemistry and microbiological testing laboratory and consulting firm. In the coming decade, Eagle will continue building greater capabilities so that we can meet new market demands.
Eagle is committed to providing your organization with the tools and knowledge to operate and maintain compliance within a highly regulated environment. Our science and pharmaceutical experts, state-of-the-art equipment, dynamic systems, and advanced procedures allow us to offer superior customized services.
Our desire, dedication, and discipline to ensure that patient safety and product quality are at the forefront of everything we do and our holistic approach to resolving your needs make Eagle a truly unique organization.
Product Specifications
1. Testing
• Microbiology
• Chemistry
• Research and Development
2. Consulting Services With a Focus On
• CGMP
• Quality Control
• Quality Assurance
• USP <797> Sterile Compounding
• USP <795> Nonsterile Compounding
• USP <800> Hazardous Drugs Handling
• GAP Analysis Audit, Environmental Solutions
3. Certification
• Calibration
• Certification
• Qualification
• Facility Design and Review
Markets Served
• 503A
• Compounding Outsourcing Facilities 503B
• CGMP
• Hospital Pharmacies
• Conventional Drug Manufacturers (CMO/CDMO)
• API Manufacturers
• Medical Device
• Cosmetics
• Educational Institutions
• Investigational Drugs and CROs
• Repackagers
TRADE SHOWS, MEETINGS, & EXPOSITIONS
Pharmacy Futures 2024 (Formerly ASHP Summer)
June 8-12, 2024 Portland, OR www.ashp.org/meetings -and-conferences/summermeetings-and-exhibition
ECRM Health System/ Institutional Pharmacy Session
June 10-13, 2024
JW Marriott Starr Pass Resort & Spa Tucson, AZ ecrm.marketgate.com/ Sessions/Category/ HealthSystems
Compounding Pharmacy Compliance 2024
June 18-19, 2024
Revere Hotel Boston Common Boston, MA informaconnect.com/ compounding-pharmacycompliance

McKesson ideaShare 2024
June 23-26, 2024 New Orleans, LA Mckessonideashare.com
340B Coalition Summer Conference
July 8-10, 2024
Gaylord National Resort & Convention Center National Harbor, MD 340bsummerconference.org
AACP Annual Meeting 2024
July 20-23, 2024
Hynes Convention Center Boston, MA www.aacp.org/pharmed24
Cardinal Health RBC
July 24-27, 2024 McCormick Place Convention Center Chicago, IL rbc.cardinalhealth.com
NACDS Total Store Expo
August 17-19, 2024 Boston, MA
Tse.nacds.org
APC’s Compounders on Capital Hill
September 17-18, 2024 Washington, DC a4pc.org/cch




July 8-10, 2024
Gaylord National Resort & Convention Center
National Harbor, MD
Please join us in the nation’s capital for the 340B Coalition Summer Conference, occurring July 8–10, 2024, at the Gaylord National Resort & Convention Center in National Harbor, MD, just minutes from downtown Washington, D.C. This event is the largest gathering of the 340B community— covered entities, pharmacies, service providers, drugmakers, government officials, and others—and it focuses on the 340B issues of greatest importance to those caring for patients in need.
Register to join your colleagues in the 340B community to ensure your continued success in using 340B to serve your patients in need. The conference will enable you to learn best practices, keep you apprised of important 340B and drug pricing developments, connect with your peers, and explore areas for new partnerships.
Behind Every Good Pharmacist ... is a Great Resource
The Pharmacy Market BUZZ is a daily news feed, designed to keep the pharmacy community informed on the latest industry news, products, services, and trends that impact both patient care and the bottom line of a pharmacy.


Explore Sessions Related to:
• Pharmaceuticals
• Contract Packaging
• Health Systems
• Home Health & Diabetes Care
• Pharmacy Technology







... is a
With 80+ aisles, the Virtual Pharmacy Trade Show is pharmacy’s one-stop destination to research and connect with leading providers of products and services throughout every pharmacy category and practice setting.


TRADE SHOWS, MEETINGS, & EXPOSITIONS
Continued from pg. 72
NASP 2024 Annual Meeting & Expo October 6-9, 2024
Gaylord Opryland Resort & Convention Center Nashville, TN naspnet.org/annual-meeting
NCPA Annual Convention
October 26-29, 2024 Columbus, OH ncpa.org/annual-convention
ASCP 2024 Annual Meeting & Exhibition November 7-10, 2024
Gaylord Rockies Resort & Convention Center Aurora, CO Annual.ascp.com
ECRM Pharmacy Technology, Services, Supplies, & Automation Session
November 18-20, 2024
Hyatt Regency Coconut Point Resort & Spa Bonita Springs, FL ecrm.marketgate.com/ Sessions/2024/11/ PharmacyTechnology ServicesandAutomationEPPS
ASHP Midyear Clinical Meeting and Exhibition
December 8-12, 2024 New Orleans, LA www.ashp.org/meetings -and-conferences/ midyear-clinical-meeting -and-exhibition
340B Coalition Winter Conference
February 24-26, 2025 San Diego, CA 340bwinterconference.org

972-2083.

Expertise - Commitment - Integrity
© ©
© TAILORED 340B AUDIT SERVICES
COMMITMENT TO COMPLIANCE
EXPERT 340B CONSULTING
(£iJ EDUCATIVE APPROACH
Stay ahead with proactive adaptations to regulatory uncertainty.


President & CEO: Edward Vargas
Founded: 2022
Employees: 1-10
Phone: (585) 329-0280
Address: Ontario, NY 14519
Virtue 340B ensures your pharmacy optimizes operations and complies with 340B regulations, transforming compliance challenges into strategic advantages for sustained growth and enhanced patient care.

Website: www.virtue340b.com
Founded with a mission to enhance the integrity and efficiency of health systems, Virtue 340B has established itself as a leader in 340B compliance and pharmacy management. Our journey began with a clear vision: empowering healthcare providers by ensuring adherence to 340B regulations, maximizing patient benefit, and organizational sustainability. Over the years, we have expanded our expertise and services, consistently paving the way for innovations in compliance audits and 340B program management. Our deep commitment to healthcare excellence and compliance has helped numerous pharmacies and health centers meet and exceed their operational goals.
Virtue 340B specializes in comprehensive 340B compliance audits and consulting services tailored to the unique needs of hospitals and health centers. Our services are designed to ensure full compliance with all 340B program regulations, minimizing risks and elevating efficiencies. Our consulting solutions cover everything from policy development to implementation strategies, helping pharmacies to optimize their 340B program operations effectively. Our flagship book, authored by our CEO and Founder, Edward Vargas, “340B Mastery: Transforming Risk into Reward in 340B Pharmacy Program Management,” is a resource that provides invaluable insights and strategies, aiding pharmacy managers and health system executives in navigating the complexities of the 340B program.
Choosing Virtue 340B as your compliance audit and consulting partner means securing a dedicated ally who understands the challenges and complexities of the 340B program. Our expertise is in compliance and turning potential risks into substantial rewards. By partnering with us, pharmacies benefit from:
• Customized Compliance Solutions: Tailored auditing and consulting services that align perfectly with your organizational needs and goals.
• Proven Expertise: Vast healthcare experience in pharmacy management and 340B compliance ensures that you receive knowledgeable support that is both effective and transformative.
• Strategic Advantage: Our innovative approaches and thorough understanding of the 340B landscape empower your pharmacy to comply with stringent regulations and thrive by optimizing operational efficiencies and financial outcomes.
• Comprehensive Support: From initial assessment to ongoing support, Virtue 340B stands by your side, ensuring that every aspect of your 340B program is flawless and future-proof. At Virtue 340B, we don’t just manage risks — we transform them into tangible benefits, helping you achieve operational excellence and enhance patient care. Partner with us and let us help you navigate the complexities of the 340B program with confidence and ease.
Explore our comprehensive suite of services designed to enhance your pharmacy’s efficiency and compliance further:
• Independent 340B Compliance Audits
• Consultative 340B Program Support
• 340B Savings Optimization
• Continuous 340B Claims Monitoring








Founder: Walt Schum
Founded: 1959
Phone: (513) 772-9410
Address: 10310 Spartan Drive Cincinnati, OH 45215
Email: sales@so-low.com
Website: www.so-low.com
For over 65 years, So-Low has been manufacturing ULT freezers and medical-grade refrigeration equipment. Our goal is to provide the highest quality cold storage equipment with a personalized customer service experience.

Since 1959, So-Low Environmental has been manufacturing ultralow temperature freezers, and laboratory and medical cold storage equipment. Based in Cincinnati, Ohio, the family owned and operated company has shipped equipment worldwide to over 130 countries. Our goal is to provide the highest quality cold storage equipment with a personalized customer service experience.
Product Overview
So-Low Environmental offers a comprehensive line of ultralow temperature freezers to -85°C, along with medical-grade refrigerators and freezers designed to meet the demanding storage needs of sensitive medications and vaccines. Our refrigeration systems are designed to keep your valued content in a uniform and stable temperature environment. Select models are tested and verified to the Lab Grade Refrigerator and Freezer Energy Star® certification to help minimize operating cost. Our equipment is built to last using the highest quality materials. Precise and userfriendly digital controls have all the alarm features you depend on to be informed when intervention is needed. Built in portholes allow access to the chamber for 3rd party probes for additional data recording and monitoring. We offer upright and chest style equipment to fit your space and storage requirements. Sizes range from small undercounters to large three door units with custom sizes available upon request.
So-Low Environmental is the U.S. distributor of Liebherr Scientific and Healthcare cold storage equipment. We believe all medical and scientific professionals should have access to the highest quality cold storage equipment. Liebherr products grow our catalog to ensure our customers have the highest quality choice for their cold storage.
Product Specifications
n Temperature Ranges
• 2°C to 8°C Refrigerators
• -25°C Freezers
• -45°C Freezer
• -85°C Freezers
n Product Styles
• Undercounter
• Chest
• Upright: Single, Double, and Triple Door
• Passthrough: Single, Double, and Triple Door
• Combination Refrigerator and Freezer
n Safety
• Audible and Visual Alarms
• High and Low Temperature
• Remote Alarm Contacts
• 3rd Party Probe Access Port
• Temperature Recording
n Certifications
• UL, ETL, CSA, Energy Star®, and SNAP Compliant
Ordering Information
To request a quote, please contact our office by phone at (513) 772-9410 or email us at sales@so-low.com.
Contributed by: Clinical Program Managers, Annie Lambert, PharmD, BCSCP, and Jennifer Smith, PharmD, BCSCP, at Simplifi+ Pharmacy Compliance Solutions, Wolters Kluwer
Hazardous Drugs (HDs) are present throughout the healthcare setting, posing risks to the personnel that handle them. While many drugs have therapeutic effects for patients, acute or long-term exposure to staff can cause adverse effects such as nausea or respiratory irritation, reproductive challenges, and even cancer.
The United States Pharmacopeia (USP) published General Chapter <800> Hazardous Drugs — Handling in Healthcare Settings initially in 2016 to set standards for the protection of healthcare workers and the environment. Even with years of evidence and guidance documents, practices to reduce the risk of HD exposure were not widely adopted until the creation of USP <800>. Enforcement was further delayed until USP <800> became compendially applicable as of November 1, 2023i , when it was referenced in both USP <797> Pharmaceutical Compounding –Sterile Preparations and USP <795> Pharmaceutical Compounding – Nonsterile Preparations. USP <800> applies to all facilities and personnel that handle, prepare, or administer HDs, including hospitals, clinics, pharmacies, long-term care facilities, infusion centers, veterinary offices, and other healthcare settings where HDs are handled. The primary goal of USP <800> is to minimize the risk of HD exposure to the environment, patients, and healthcare workers handling these drugs throughout the facility. This includes receiving, storing, compounding, transporting, administration, disposal, and spill cleanup. Following safe handling procedures outlined in USP <800> reduces the risk of HD exposure via prevention and containment.
With all the delays, you may need a refresher on the importance of safe handling of hazardous drugs. This article will provide an overview of USP <800> requirements, considerations for implementation, and future directions for compliance.
HAZARDOUS DRUG LIST: A facility-specific list of HDs must be generated to understand and identify HDs at the facility. HDs are defined by USP <800> based on the criteria established by NIOSH. NIOSH maintains a list of HDs with the most recently published document from 2016, the NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016, also called the NIOSH List. The criteria for a drug to be defined as hazardous and included on the NIOSH List include drugs that are toxic to organs at low doses, carcinogenic, teratogenic, reproductive, or genotoxicii. NIOSH groups HDs into three categories: Table 1, 2, or 3.
Table 1 drugs are antineoplastic drugs that pose significant health risks from exposure.
Table 2 drugs are non-antineoplastic drugs that meet one or more NIOSH HD criteria and pose a potential risk from exposure.
Table 3 drugs meet the NIOSH criteria for reproductive hazards to men or women trying to conceive and women who are breastfeeding.
All Table 1 drugs on the NIOSH List and all HD Active Pharmaceutical Ingredients (APIs) must be handled with all the containment strategies and work practices required by USP <800>. However, the Chapter allows organizations to develop an Assessment of Risk (AOR) for Table 2 and 3 drugs and final dosage forms of compounded HD preparations and HD (including antineoplastic) dosage forms not requiring further manipulation. An AOR approach determines if alternate
containment strategies or work practices may be employed to limit HD exposure instead of the full protective measures described in the Chapter.
FACILITY DESIGN: The requirements for HD facility design focus on containment. This is likely the most challenging component of USP <800> for facilities to achieve since re-design and construction may be required to bring existing facilities into compliance. With the elimination of the “low-volume” HD exemption found in the 2008 version of USP <797>, all HD compounding must occur in a containment secondary engineering control (C-SEC) maintaining negative pressure and external ventilation. Additionally, HDs on NIOSH Table 1 or API of any HD must be stored in a negative pressure environment. When a separate HD storage room is not attainable at the facility, HDs can be stored in C-SECs provided storage does not negatively affect engineering controls or increase the microbiological burden in sterile compounding rooms. The following table summarizes the minimum facility requirements for HD compounding and storage areas.
Containment primary engineering controls (C-PECs) for sterile HD compounding require external ventilation and negative pressure. In contrast, C-PECs for nonsterile HD compounding can be externally vented (preferred method) or have redundant HEPA filters in a series.
PERSONAL PROTECTIVE EQUIPMENT: Ensuring staff compliance with Personal protective equipment (PPE) serves as the last level of control against HDs from exposure. PPE worn when handling HDs must be outlined in standard operating procedures (SOPs) and is based on the activity personnel are performing including all or some of the following PPE:
• ASTM standard D6978iii gloves (single or double and sterile or nonsterile based on activity).
• Long-sleeved impermeable gowns that close in the back and have elastic cuffs.
• Shoe covers (double shoe covers required during HD compounding).
• Head and facial hair covers.
• Face and eye protection.
• Respiratory protection.
Removal of HD PPE and hand hygiene is equally important to prevent crosscontamination after the completion of HD handling activities.
TRAINING & COMPETENCY: All personnel who handle HDs must receive training and demonstrate competency. HD training needs to supplement general education and competency for handling any drugs and occurs in addition to USP <797> and <795> training. When considering the components of an HD training
program, all personnel handling HDs must receive training on identifying HDs, organizational HD SOPs, PPE use, correct use of engineering controls and other devices, response to HD exposure, HD spill management, and proper HD disposal.
DEACTIVATION & DECONTAMINATION: USP <800> introduces the requirement for deactivation and decontamination before cleaning and disinfection of areas where HDs are handled. Deactivation renders the HD inactive, while decontamination removes the residue by transferring it to a disposable material like a low-lint wiper. Deactivation and decontamination occur in addition to the cleaning and disinfection, or sanitation required by USP <797> and USP <795>. Ensure personnel training and organizational SOPs outline PPE worn along with the agents and supplies for each deactivation, decontamination, and cleaning step.
GAP ANALYSIS: The first step to the implementation of USP <800> standards is to assess current practices. Since the standards have been available since 2016, many pharmacies may have started to implement elements of the Chapter but paused progress when enforcement was delayed. Several gap analysis tools are available online such as, readyfor800.com or from state boards of pharmacy.
Also key to the assessment of current practices is knowing what HDs are handled in your facility and where. Begin by reviewing purchasing records or dispensing logs and compare them to the NIOSH List to identify what HDs have been procured. Then follow the HD through the process from receiving through dispensing and administration, even to waste and disposal. This exercise may identify additional gaps or personnel that require training. Understanding what HDs are present will lead to further discussion about how they should be handled to ensure personnel safety.
DESIGNATED PERSON: In addition to conducting a gap analysis, it is also important to identify who will be the point person for USP <800> compliance. The designated person is responsible for oversight of all aspects of the hazardous drug safety program and should have the authority to implement change when needed. This may be a committee or multiple individuals, just be clear to describe the roles and responsibilities to ensure all aspects are assigned.
POLICIES & PROCEDURES: Foundational to any compliance program are policies and procedures outlining how standards will be adopted at the facility level. USP <800> requires entities to maintain SOPs to include at least 16 different topics including receipt and storage to hand hygiene and use of PPE to transport and spill control. SOPs must be reviewed at least every 12 months by the designated person. Developing SOPs can be daunting at first, but maintenance should be minimal once the standards are established.
FACILITY UPDATES: For HD compounding and storage activities, facility modifications and upgrades may be required to achieve the required negative pressure, external exhaust, and air exchanges.
Additional primary engineering controls may also need to be purchased and vented to the outside. Engage with your facilities and engineering team to understand the current HVAC design and capabilities. Ensure these teams realize the minimum USP <800> requirements and collaborate to discern how these align with other environment of care standards, such as those from ASHRAEiv and OSHA.
TRAINING & COMPETENCY: After knowing what HDs are handled in your facility, understanding the types of exposure, and establishing SOPs for the containment of HDs, personnel must receive training based on their job functions. This includes general education such as the risks of handling HDs and proper use of PPE and engineering controls, as well as facility specific training related to the entity’s list of HDs, SOPs, and response to and HD exposure and spill management. Developing an HD training checklist ensures all personnel complete all required HD training initially and annually during employment.
ANNUAL REVIEW: All the aspects described above must be reviewed or repeated at least every 12 months to ensure new information is incorporated and safe handling practices are maintained. Engaging stakeholders in this process is encouraged as frontline personnel are ultimately the ones at greatest risk during their day-to-day responsibilities.
Even with the delays in enforcement of USP <800>, many entities have moved forward with compliance and other aspects of HD safety have continued to evolve. Here are a few topics to have on the radar:
• NIOSH Updates: The current official NIOSH List of Hazardous Drugs in Healthcare Settings was published in 2016. Several drafts have been proposed, but no new updates have been finalized. When the next revision is published, it will include a change in classification of HDs, going from three groups to two groups which may require entities to revise SOPs and approach to HD identification and precautions.
• ASTM Gown Standard: In late 2022, the American Society for Testing and Materials established a testing standard for chemotherapy gowns. ASTM F3267-22v establishes design and performance standards for protection against chemotherapy and other liquid HDs. Similar to ASTM D6978 for gloves, this will help ensure PPE protects workers based on evidence. Look for this testing standard when evaluating PPE.
• Compounding Automation: Technologyassisted workflow solutions continue to evolve to meet the needs of various compounding settings. For facilities that compound a higher volume of HDs, implementing compounding robotics should be considered as a method to increase safety and decrease exposure to HDs.
• HD Wipe Sampling: Testing for HD residue is currently a recommendation in USP <800>, though many organizations have already
implemented this practice. HD wipe sampling can be used as a routine component of a quality assurance/quality control program and/or to verify effective clean-up of an HD spill. Several testing kits and vendors are available, providing both qualitative and quantitative results.
• Medical Surveillance: Monitoring of workers exposed to HDs for health conditions is also a best practice recommendation in USP <800>. Implementation of a medical surveillance program requires a coordinated effort with employee health, human resources, and legal and risk teams. Refer to guidance in USP <800> and from OSHAvi
With nearly a decade since USP <800> was published, the standards may still feel new and overwhelming. The Chapter provides many of the expectations for what is required to protect healthcare workers and the environment. Many of the nuances of how to implement the standards exist in facility SOPs. Organizations further along in the compliance journey may now be encountering annual reviews and adjusting their initial safe handling practices based on feedback from staff and experience with HDs. As enforcement of the standards moves forward, additional technologies and resources will emerge to further reduce the risk of HD contamination.
Ultimately, the USP <800> standards exist to protect healthcare workers and the environment, and reduce exposure to HDs overall. The risks are clear, and the evidence is abundant. Regardless of the practice setting, implement appropriate precautions and strive for consistent compliance with the standards.
Resources
United States Pharmacopeia (USP). General Chapter <795> Pharmaceutical Compounding - Nonsterile Preparations. USP-NF (2023).
United States Pharmacopeia (USP). General Chapter <797> Pharmaceutical Compounding - Sterile Preparations. USP-NF (2023).
United States Pharmacopeia (USP). General Chapter <800> Hazardous Drugs - Handling in Healthcare Settings. USP-NF (2019).
NIOSH [2023]. Managing hazardous drug exposures: information for healthcare settings. National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2023-130
References
i USP General Chapter <800> Hazardous Drugs - Handling in Healthcare Settings Context for Implementation. November 1, 2023
ii NIOSH [2016]. NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016. DHHS (NIOSH) Publication Number 2016-161 (Supersedes 2014-138).
iii ASTM Standard Practice for Assessment of Resistance of Medical Gloves to Permeation by Chemotherapy Drugs. DOI: 10.1520/D697805R23.
iv ASHRAE Standing Standard Project Committee 170, Pharmacy Work Group. HVAC Design of Compounding Pharmacies. 2024.
v ASTM. Standard Specification for Protective Clothing for Use Against Liquid Chemotherapy and Other Liquid Hazardous Drugs. DOI: 10.1520/F3267-22.
vi Occupational Health and Safety Administration (OSHA). Controlling Occupational Exposure to Hazardous Drugs. Available at: https:// www.osha.gov/hazardous-drugs/controlling-occex#resources Accessed 4/25/24.






PCS specializes in cleanroom garment processing services for aseptic, particulate, and ESD controlled environments. All PCS plants fold, package, and hermetically seal each finished item in an ISO Class 3 cleanroom.
President: Chris Welch
CEO: John Clark
Founded: 1932
Employees: 2,000+
Toll-Free Phone: (800) 767-5536
Phone: (949) 250-4850
Address: 1661 Alton Parkway, Irvine, CA
Website: www.prudentialuniforms.com
Since its inception in 1960, Prudential Cleanroom Services (PCS) has been at the forefront of providing superior cleanroom apparel services. What started as a humble endeavor has flourished into a renowned North American entity, serving a wide spectrum of industries such as compounding, bio-science, pharmaceuticals, medical devices, semiconductors, aerospace, and beyond.
At PCS, we specialize in delivering tailored cleanroom solutions to facilities involved in the compounding and preparation of medications and sterile infusions. Our comprehensive services ensure that medications are manufactured under meticulously controlled conditions, mitigating the risk of contamination and maintaining patient safety.
By utilizing PCS’s services, businesses gain access to top-tier sterile gowning and essential ancillary items crucial for maintaining cleanroom standards and ensuring accurate gowning procedures. This not only reinforces cleanroom standards but also bolsters operational efficiency and regulatory compliance.
One distinguishing feature of PCS is our unwavering commitment to excellence. We take immense pride in being the sole national provider with all locations certified to the ISO 9001:2015 standard, all PCS serviced items are finished and packaged in an ISO Class 3 Cleanroom. Our sterile product is all validated at six log reduction through gamma radiation; 1,000,000:1 bioburden risk reduction. With a rich history as industry pioneers, coupled with our relentless pursuit of improvement year after year, PCS offers unparalleled value as your trusted cleanroom garment service partner to deliver unmatched quality and reliability.
n Cleanroom Uniforms and Apparel: Prudential carries both sterile and non-sterile garments for a variety of cleanroom needs. Our products utilize non-linting, high density fabrics that are resistant to liquids and bacteria.
n Non-Garment Reusable Products: From hood mops, floor/ceiling/ wall mops, to goggles, Prudential aims to provide comprehensive support for your cleanroom facilities. We carry both sterile and non-sterile products for a variety of cleanroom needs.
n Cleanroom Fabric Study: Prudential offers the contamination control industry an independent third-party cleanroom fabric study that credibility tests and rates the industry’s most widely recognized fabrics.
n ISO 9001 Certification: PCS, the world leader in cleanroom laundry service systems is ISO 9001 Certified for its Quality Management System by TUV America, Inc. This certification applies to PCS’s corporate headquarters and its national network of cleanroom and industrial processing facilities. The scope of the Quality Management System ISO certification includes providing cleanroom and industrial apparel along with other contamination control products and services for use in controlled environments.
n Garment Tracking System: GTS RFID/Barcode Tracking System provides our customers with a complete account history report for each garment from installation to any repairs. It also tracks usage analysis by wearer, department, and distribution point.
n Stock Garment Program: Our STOCK program provides a service that is unique to the industry. The high-quality services Prudential delivers each week are thanks in part to our one-of-a-kind distribution center. It is the heart of our operations that ensures we remain the leading uniform service, cleanroom, and facility services provider in North America.
n Sterilization Validation Program: The validation activities contained in our validation manual and subsequent reports are based on ANSI/AAMI/ ISO 11137:2015 (Sterilization of Health Care Products — Radiation — Part 1 Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices). The validation is performed to ensure that the products meet PCS sterility requirements.
n Sustainable Gowning Protocol: Help drive sustainability programs by reducing consumable products (disposable coveralls, hoods, boots, masks, goggles, mops, and sleeve covers) all items you throw away and convert them to a reusable solution.
Compounding/Pharmaceutical/Biotechnology; Medical Device; Semiconductors; Aerospace; and other companies manufacturing and operating in controlled environments.
Ordering
Visit www.prudentialuniforms.com. Contact us or your PCS professional for more information.
• Sterile and Paper Free (USP 797 Compliant).
• Maintains a 100% sterile barrier* with 3X greater adhesion. *Tested in Nelson Labs, Salt Lake City, UT
• Helps prevent contamination of drugs and provides added protection to pharmacists.
• Will not fall off, even in cold storage conditions (down to -20 degrees centigrade).
• Patented dual-layer indicates true tamperevidence, with “OPENED” marking.








National Sales
Manager: Alex Meadow
Phone: (516) 374-8862
The only single-use, tamper-evident seal that provides and maintains a 100% sterile barrier.
Address: 2905 South Congress Avenue, Suite A Delray Beach, FL 33445
Website: steri-tamp.com
Email: info@steri-tamp.com
Established in 2010, Allied Pharmacy Products, Inc. was created from a commitment to enhance the preparation and distribution of intravenous medication within hospital pharmacies and compounding environments. The development of Steri-Tamp® stems from the insights of a pharmacist who recognized the imperative for improved tamper-evident measures and sterility in IV preparation. Throughout our journey, Allied Pharmacy Products, Inc. has remained dedicated to listening and responding to the needs of pharmacists. This ongoing responsiveness has been instrumental in our continuous product line expansion.
The Steri-Tamp® product line features seven cutting-edge tamperevident seals. Our IV bag port seals come in blue, red, and yellow (labeled CHEMO) variants. For vial seals, we offer options in 13 mm (red), 20 mm (silver), and 28 mm (blue). Among these, our belly button bag seals (green) stand out for their ability to adhere to both the ICU medical “belly button” bag port and the top of a 13 mm vial.
Steri-Tamp® has introduced specialized IV bag port seals tailored to meet the labeling requirements of USP 800 and ISMP. These seals offer a streamlined solution for handling paralytic agents and hazardous drugs in a unified workflow.
What sets Steri-Tamp seals apart is their true tamper-evident design. Utilizing dual-layer technology, these seals serve as a clear indicator to hospital staff if a bag or vial has been tampered with or used. Removing the top foil layer reveals an “opened” layer, after which it cannot be reapplied. Thanks to a 3X stronger adhesive, Steri-Tamp ensures a 100% sterile barrier.
In addition to our sterile options, we also offer two non-sterile syringe seals. These seals boast increased tensile strength, making them easy to remove from the liner and apply to a wide range of containers, including syringes, inhalers, EpiPens, insulin pens, and more. Our latest innovation, the Tamper-Clear Syringe Seal®, enables visibility of syringe markings and barcode scanning on syringes, inhalers, insulin pens, and other related items.
n Steri-Tamp® IV Bag Port Seals:
• Deliver a foolproof and potentially life-saving method to ascertain medication dispensing.
• Safeguard the IV admixture bag’s point of entry from contamination and accidental double dosing.
• Securely attach to the port through a simple “twist” mechanism, ensuring an airtight seal.
• The green belly button bag seal is purpose-built for the unique “belly button” port on ICU medical bags and can also be used on 13 mm vial tops.
n Steri-Tamp® Vial Seals:
• Designed to lie flat on the vial top, effectively preventing bacteria from entering the vial, without overlapping the edges.
• Preserve the medication’s integrity.
• Specially sized for 28 mm, 20 mm, and 13 mm vial tops, ensuring the right seal for the corresponding vial size.
n Steri-Tamp® Syringe Seals:
• Offer non-sterile solutions to provide tamper-evidence for syringes and other medical containers.
• The innovative Tamper-Clear Syringe Seal® provides clear visibility to the syringe barrel and facilitates barcode scanning, making it ideal for sealing small oral, pediatric, and NICU syringes.
n Available in rolls of 1,000 seals each.
n All our seals contribute to reducing waste, streamlining workflow, and ultimately saving you time and money.
“Tamper-Clear works great! Helps prevent the caps falling off the syringes.”
— Lead Pharmacy Technician, Colorado Springs, CO
“They’re the best seals we use. It’s a must have!”
— Pharmacy Purchasing Specialist, Orangeburg, SC
“Love the syringe seals. Able to remove them without them breaking beforehand. It really is a higher quality seal!”
–– Pharmacy Tech/Purchasing Lead, Houston, TX
Additional Product Lines
Stay tuned for exciting updates to our products set to launch in 2024!
Trade Shows/Meetings Attended
ASHP Midyear, EAHP, NPPA Conference, and various ASHP state affiliate pharmacy conferences.
Ordering Information
Available through major wholesalers and distributors. Visit our “order info” page at steri-tamp.com to find Steri-Tamp® order numbers for your preferred vendor.
Abbott
Academy of Managed Care Pharmacy (AMCP)
Accreditation Commission for Health Care, Inc
Accreditation Council for Pharmacy Education
Accu-Chart Plus Health Care Systems
Accucold
Acute Care Pharmaceuticals
Adheris Health
AdvanceNet Health Solutions, Inc
Advasur LLC
Aegis
Air Link International
AirCare Automation
AirClean Systems, Inc.
AIS Healthcare
Akro-Mils
AlignRX
Alliance for Pharmacy Compounding
Allied Pharmacy Products
Altium Packaging
American Analytics
American Associated Pharmacies (AAP)
American Association of Colleges of Pharmacy
American Association of Health-System Pharmacists (ASHP)
American BioTech Supply
American College of Apothecaries
American College of Clinical Pharmacy
American College of Veterinary Pharmacists
American HealthCare Capital
American Pharmacies, Inc.
American Pharmacists Association (APhA)
American Pharmacy Cooperative, Inc.
American Rx Group
American Society of Consultant Pharmacists (ASCP)
Americorp Financial, LLC
Amneal Pharmaceuticals LLC
Anda Inc.
ANiGENT LLC
Animal Med Express
Apexus LLC
Apothecary Products, LLC.
APS - Advanced Pharmacy Solutions
ARL Bio Pharma
ARxIUM
Asembia
Aspen RxHealth
AssistRx
Associates of Cape Cod, Inc
AssureCare LLC
Asteres Inc.
Atlas Health
Avel eCare
Aventi Health
Avery Weigh-Tronix
AvKARE, LLC
Azurity Pharmaceuticals, Inc.
Azzur Group
B Medical Systems
B. Braun Medical Inc.
BA Sciences
Baxter
BD
Berkshire Corporation
Berry Global Inc.
BestRx
BetterRx
Beutlich Pharmaceuticals, LLC
Biolife Solutions Inc.
Blue Thunder Technologies Inc.
Bluesight
BluPax Pharma
Board of Certification/Accreditation (BOC)
Board of Pharmacy Specialties (BPS)
Bonita Pharmaceuticals
Bureau Veritas
Buy-Sellapharmacy.com
CCAM Commerce Solutions / Celerant Technology
Capital Inventory, Inc
Capital Wholesale Drug Company
Capsa Healthcare
CaptureRx
Cardinal Health
Care Services, LLC.
CarepathRx CarePoint
Carter Health
CEImpact
Cencora / American Health Packaging
Cencora / AmerisourceBergen
Centor
Central Admixture Pharmacy Services, Inc. (CAPS)
Cervey
Change Healthcare
Charles River Laboratories
ChemoGLO
CINTAS Corporation
Cipla, Inc.
Civica
Clean Harbors, Inc.
Cleanetics
Clearway Health
Cloudmed
Codonics
Cold Chain Technologies
ComCo Systems
CompleteRx
ConsortiEX Inc.
Containment Technologies Group, Inc.
Contec, Inc.
CORE Higher Education Group
CORMED
COSHATT
CoverMyMeds (McKesson Corporation)
CPESN USA, LLC
CPS Solutions, LLC
CriticalPoint, LLC
Crocus Medical Inc.
CuraScriptSD
Cypress Software, Inc.
DD2 Solutions
Datarithm
Datascan Pharmacy, Inc.
Dexcom, Inc.
Dickson
Digital Business Solutions, Inc.
DisposeRx, Inc.
DiversifyRx
DoseMe
DosePacker, Inc.
DosePlanner
Dr.Reddy’s Laboratories Ltd.
DrFirst
DRX SOFTWARE LLC
Dycem Ltd
EEagle Analytical Services
Eagle Pharmaceuticals, Inc
Ecolab
ECRM
Elsevier
Emporos
EnlivenHealth
EPIC Rx
Epic Systems Corporation
Epicor Software Corporation
epocrates / Athenahealth
EQUASHIELD
Esco
Euclid Medical Products
Eurofins Analytics
EveryDose
Everyware
EzriRx
EZSCRIPTRx
FFagron
FFF Enterprises, Inc.
FG Clean Wipes
First Databank, Inc.
First Financial Bank
Flash Returns, LLC
FLAVORx
Flexible Pharmacy Services, PLLC
Flowsell
Follett Healthcare
Franklin Eyewear
Fresenius Kabi Group
Frier Levitt
GGenetco Inc.
GeriMed, Inc.
Germfree
Grifols
HH+H System, Inc.
Hardy Diagnostics
Hayslip Pharmacy Brokers Inc.
HealNow
Health Care Logistics, Inc.
Health Connect Partners, Inc.
HealthTrust
HelioMetrics
Hematology/Oncology Pharmacy Association (HOPA)
Hikma Pharmaceuticals
Hudson Headwaters 340B
ICU Medical
iLocalBox
Imprivata / FairWarning
IND Consulting
Independent Pharmacy Cooperative (IPC)
InfiniTrak, LLC
Infinity Laboratories
InfoWerks
Inmar Inc.
Innova Medical Group Inc.
Innovatix, LLC
Inovalon
InsightRX, Inc.
Institute for Safe Medication Practices (ISMP)
InstyMeds
Integrated Medical Systems, Inc. (IMS)
Integrity Pharmacy Consultants
Intelliguard / MEPS Real-Time, Inc.
Interlink AI, Inc.
International Journal of Pharmaceutical Compounding (IJPC)
International Medical Industries
ISO-MED, Inc.
IsoTech Design
JJackson Pharmacy Professionals
Jays Company
JFCRx
Jones Healthcare Group
KKeycentrix, LLC
KeySource
Keystone Folding Box Co.
KNAPP
LLabconco
Lakeland Industries, Inc.
Legacy Pharmacy Group
Leiters Health
Liberty Software
Liebherr
LifeFile
Lincoln Savings Bank
Live Oak Bank
Loopback Analytics
LSPediA, Inc.
Lumistry
Lupin Pharmaceuticals Inc.
MMacro Helix (McKesson Corporation)
Managed Health Care Associates, Inc. (MHA)
Manchac Technologies
Masters Pharmaceutical
MatchRX
Maximum Rx Credit, Inc.
Maxor National Pharmacy Services, LLC
Maxpert Medical
MaxQ
McKesson Corporation
Medi-Dose, Inc./EPS, Inc.
Medical Information Technology, Inc.
Medical Packaging Inc., LLC
Medicine-On-Time, LLC
Medility Inc.
Medisca Inc.
Medivant Healthcare
MediZap
MedSafety Solutions
MedShorts
Medtel Communications
MedXL Inc.
Merative
Mesmerize
Micro Merchant Systems, Inc.
MIDAS Healthcare Solutions
Mideast Delivery Solutions
Migali Scientific
Mobile MediClaim, Inc.
Modern Designs, Inc.
Moderna, Inc.
Modular Cleanrooms, Inc.
Modular Devices
Modular Pharmacy Solutions, LLC
Moog, Inc.
Morris & Dickson
N3PR
NarcX
National Association of Boards of Pharmacy (NABP)
National Association of Chain Drug Stores (NACDS)
National Association of Specialty Pharmacy (NASP)
National Community Pharmacists Association (NCPA)
National Council for Prescription Drug Programs (NCPDP)
National Home Infusion Association (NHIA)
National Pharmaceutical Returns, Inc.
National Pharmacy Purchasing Association (NPPA)
National Pharmacy Technician Association (NPTA)
Nephron Pharmaceuticals Corporation
Net-Rx (an MHA Solution)
Nimble
Noritsu Pharmacy Automation
NuAire, Inc.
Nuvem
OOmega Pharmacy Group
Omnicell
OmniSYS (XiFin Pharmacy Solutions)
Onset Computer Corporation / InTemp
Oracle Health / Cerner
OrderInsite, LLC
OurPharma LLC
Outcomes
Owen Mumford
PP&C Pharma
PAAS National LLC
Paladin Data Corporation
Par Pharmaceutical, Inc.
Paragon Ventures
Parasol Medical, LLC
Parata Systems (BD)
ParcelShield
PCCA
PDM Healthcare
PDR / ConnectiveRx
Pentapack
PEPID, LLC
Perfex Corporation
Pevco
phactMI
Pharma Logistics, LLC
Pharma Source Direct
Pharmacists Mutual Insurance Company
PharmaComplete
Pharmacy Automation Supplies
Pharmacy Brokers
Pharmacy Consulting Broker Services
Pharmacy First
Pharmacy Podcast Network
Pharmacy Quality Alliance (PQA)
Pharmacy Quality Solutions (PQS)
Pharmacy Stars, LLC
Pharmacy Technician Certification Board (PTCB)
Pharmacy Times (MJH Life Sciences)
Pharmacy-Lite Packaging
PharmaForce
PharmaLink Inc.
PharmaPoint, LLC
Pharmathek
PharmCon
PharmEcology
Pharmetric Laboratory
PharmID, Inc.
Pharmpilot
Pharmsource, LLC
PharmWaste Technologies, Inc.
PHC Corporation of North America
Pine Pharmaceuticals
PipelineRx
PK Telehealth (TCE Group)
Plenful
PointClickCare
Powerpak (Postgraduate Healthcare Education)
PreciseRX
Precision Dose, Inc.
Premier
Prescryptive Health, Inc.
Primex Inc.
Prompt Praxis Laboratories
ProPharma Cleanrooms
Protenus, Inc.
ProxsysRx
PRS Pharmacy Services
Prudential Cleanroom Services
Pure Microbiology, LLC
Pyrls (Cosmas Health, Inc)
QQ.I. Medical Inc
QleanAir Scandinavia AB
QRx Solutions
QuicksortRx, Inc.
QuidelOrtho Corporation
QuVa Pharma
RR.C. Smith
R.J. Hedges & Associates
R&S Northeast LLC
RD Plastics Company, Inc.
Real Value Rx
Recovered Health
Redsail Technologies, LLC
Rees Scientific
Relay Robotics, Inc.
Retail Designs Inc.
Retail Management Solutions, LLC
Return Solutions, Inc.
Revelation Pharma
Robotik Technology
Roemer Industries
RPh on the Go
Rx Advisors
RX Destroyer
Rx relief
Rx Return Services, LLC.
Rx Reverse Distributors, Inc.
Rx Systems, Inc.
RxConnexion
RxDispense
RXinnovate Consulting
RXinsider
RxLive, Inc.
RxOwnership (McKesson Corporation)
RxPreferred
RxRise
RxSafe, LLC.
RxScan
RXshelving.com (Surplus Equipment Company)
RxStrategies, Inc.
SSafeChain Solutions, LLC
Safecor Health
Samson Medical Technologies, LLC
Sanford Guide
SCA Pharma
ScriptPro LLC
SDS Rx
SensoScientific, Inc.
Shamrock Labels
Sharps Medical Waste Services
Shepard Medical Products
Shields Health Solutions
SKY Packaging (McKesson Corporation)
Skyline Pharmaceuticals
SmartCells (SATECH, Inc.)
SmartSense (Digi International)
Smith Drug Company
So-Low Environmental Equipment Co.
SoftWriters, Inc
Soliant Health
Source Communications LLP
South Pointe Wholesale, Inc.
SpecializedRx Products, LLC
Specialty Medical Staffing
Spectrum Pharmacy Products
Speed Script
SpendMend
SpotSee
SRS Pharmacy Systems
STACK, LLC
STAQ Pharma, Inc.
Stäubli
STChealth
Stericycle, Inc.
Stratix Labs Corporation
SuiteRx, LLC
Suncrest Solutions
SUNRx
Supplylogix (McKesson Corporation)
SureCost, LLC
Surescripts
Swisslog Healthcare
Sykes & Company, P.A.
TTabula Rasa HealthCare
TEAM Technologies, Inc.
Teknipure
TelNet-Rx
Tension Packaging & Automation
Terso Solutions, Inc.
Texwipe (ITW)
The Baker Company
The Compliance Team, Inc.
The Craneware Group
The Medicine Shoppe Pharmacy
The Relief Products
Thermo Fisher Scientific, Inc.
ThoroughCare, Inc.
TICKERWORKS
TopRx, LLC
Total Pharmacy Supply, Inc.
Total-Shield PLUS (McGowan Industries, Inc.)
TouchPoint Medical
TraceLink, Inc.
TrackTraceRx, Inc.
Trane Technologies
TransferMyRx
Travis CleanAir, Inc.
TRC Healthcare
Trilogy MedWaste, Inc.
TruMed Systems, Inc.
Turbare Manufacturing
U.S. Micro-Solutions, Inc.
Unidoses
United Pharmacy Network (UPN)
UNITED Staffing Network, Inc.
United States Pharmacopeia (USP)
UNIWEB, Inc.
Value Drug Company
Veltek Associates, Inc.
Veracity Group, Inc.
Veridikal
Verity Solutions
VigiLanz Corporation
Vileda Professional
VIP Pharmacy Systems
Virtue Technologies
Visante
VIVID (Scientific Industries, Inc.)
Vizient, Inc.
VMI Care
VPL, Inc.
WWeInfuse, LLC
Well-Served Pharmacy Community (WSPC)
Wellgistics
Wellpartner
Wells Pharma
West Pharmaceutical Services, Inc.
Winfield Laboratories Inc
Wolters Kluwer
Workflow Services
WorkingBuildings
Yardi Systems, Inc.
Yukon Medical, LLC
Yuyama Usa, Inc.
Zebra Technologies Corp.
340B Health
340BDirect | Procuity

American Biotech Supply ensures uncompromised integrity of medical supplies with state-of-the-art cold storage solutions. Cutting-edge technology guarantees precise temperature control, safeguarding the efficacy of vital pharmaceuticals and biological materials. Trusted by leading healthcare institutions, we deliver peace of mind, reliability, and compliance with stringent industry standards.
Ensures medication efficacy, safeguarding patient safety and reducing wastage.
Experienced technical support available for prompt troubleshooting and resolution.
Logistics professionals to manage nationwide freight and installation, including removal of obsolete equipment.
Call Us : (843) 821-8010
On hand inventory for immediate fulfillment of critical needs.
Product experts available to assist with order inquiry and product solutions.
Largest selection of purpose-built cooling options with natural refrigerants and Energy Star certification.