IMPROVING PATIENT CARE & PHARMACY COST CONTAINMENT HEALTH SYSTEM & INFUSION EDITION WINTER 2022 Defying Drug Diversion A Case Study From Medacist pg. 42 20 Unique Products & Services Discover 20 products and services that will help your pharmacy improve patient care or contain costs. Refrigeration Industry Expert Q&A, Market Leaders Buyer’s Guide, and More pg. 58 New Features The Evolving Role of Compounding Pharmacies pg. 66 Trends to Watch as 340B Turns 30 pg. 87 4 Case Studies CPS Solutions, Leiters, Wolters Kluwer Health, and More
~ Allied Health at
Brian O’Neal, PharmD, MS, FASHP Senior Vice President
Children’s Mercy Kansas City
















































to the cold zone! Cold storage brought to you by We offer a broad range of cold storage options that provide practical, effective, affordable solutions to protect temperature-sensitive products for pharmaceutical, dietary and laboratory use anywhere in your facility. From our countertop-sized to large stand-up units and CDC-compliant under counter models, we carry a complete line of lockable refrigerators and freezers to suit all of your cold storage needs. 20132 GoHCL.com • 1.800.848.1633 © Health Care Logistics, Inc. 2022 20162 20434 20154 19299
Welcome

Partner with us to simplify 340B administration, confidently optimize



























Leiters. We Are Compounding Health™
Leiters is a trusted FDAregistered 503B outsourcing provider of compounded sterile preparations committed to providing healthcare professionals and their patients with the highest-quality medications.
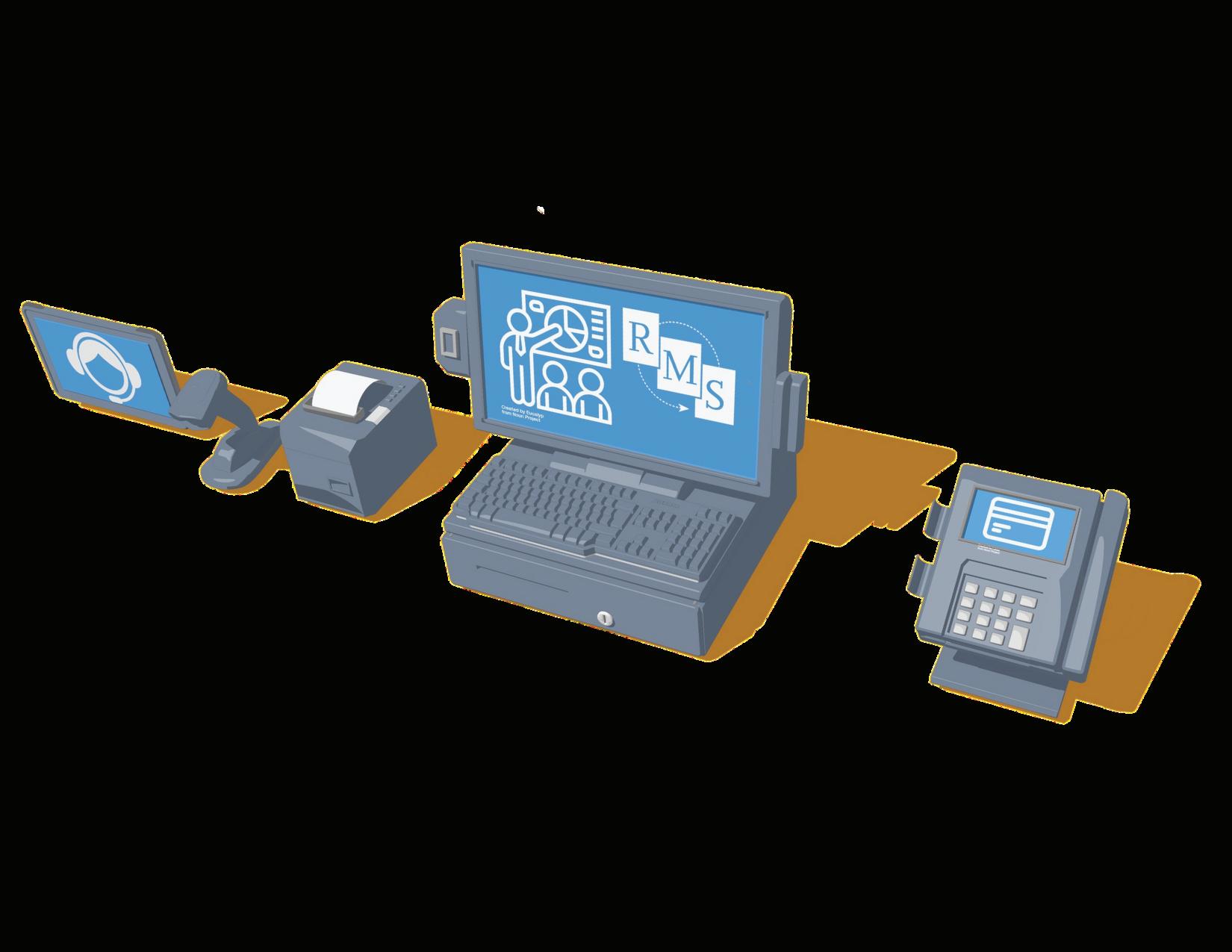
Pharmacy point-of-sale should work for you, not the other way around. Meet the solution that helps you improve patient outcomes, streamline operations, and use your choice of pharmacy management systems.



GX Solutions Pass-Thru Refrigerators Offer Clinical Pharmacy Workflow Efficiency

Case Study:
Drive Inpatient &
3 WINTER 2022 I HEALTH SYSTEM • INFUSION Contents
COMPOUNDING HEALTH™ www.leiters.com 800.292.6772 Helping you deliver better medicine to more people. With increasing regulatory pressure and drug shortages, access to quality medicine is more important than ever. Leiters is an FDA-registered 503B outsourcing provider of high-quality, compounded sterile preparations including: Pre-filled syringes, IV bags and vials ON-Q* Pain Relief System fill services Opioid-free surgical pain services medications Ophthalmology medications and services including FDA-compliant repackaged Avastin® ON-Q* is registered trademark of Avanos Medical, Inc., or its affiliates. Avastin® is registered trademark of Genentech, Inc.
Point-of-Sale Simplify Your Pharmacy Operations — Retail Management Solutions
page 29
customized software turnkey hardware innovative training 24/7 US-based support www.rm-solutions.com MEET YOUR NEXT STAR EMPLOYEE.
— Your Invested
page 31 Verity Solutions
Partner for 340B
federal pricing benefits, and benefit from the dedication and knowledge of our expert team. Results you can believe in. 1.800.581.1378 info@verity340b.com www.verity340b.com 2022 Verity Solutions Group, Inc. Innovative. Invested. Proven. Partner with us so that you can dedicate fewer resources to your 340B program administration and more time to community wellness. SPLIT BILLING CONTRACT PHARMACY VERISAVE SPECIALTY CONTRACT PHARMACY COMPLIANCE MANAGEMENT PURCHASE ANALYTICS VHUB ® for Contract Pharmacies For all healthcare systems who are challenged with the process of selecting the optimal drug that would reduce spend, Verisave automatically selects the best priced product before your order is placed. With patented technology, Verisave reduces tedious manual processes and dramatically decreases your drug spend. page 27 Meeting Your USP <797> Requirements Veltek Makes it Simple CLEANINGAND DISINFECTING PRODUCTS DISINFECTING PRODUCTS ENVIRONMENTAL MONITORING E ENVIRONMENTAL GOWNING Veltek Associates, Inc. 15 Lee Boulevard Malvern, PA 19355-1234 1-888-4-STERILE VAI covers every aspect necessary for full compliance including: ■ Consultation on setting up and maintaining aseptic processes ■ SimpleMix – our easy to use disinfectants in pre-measured containers ■ Easy2Gown sterile garment system ■ Easy to use portable SMA air samplers ■ RTU Sterile & Non-Sterile cleaners & disinfectants available in a large assortment of packaging sizes With VAI’s innovative product line, Meeting USP<797>requirements is just a phone call away…IT’S THAT SIMPLE! Meeting Your USP <797> Requirements Veltek Makes It Simple CLEANING AND DISINFECTING Saturated WiperS GOWNING USP 800 Gown Cleanroom Garments VAI covers every aspect necessary for full compliance including: Consultation on setting up and maintaining aseptic processes SimpleMix® Veltek’s easy to use disinfectants in pre-measured RTU containers Easy2Gown sterile garment system Ready-to-use and saturated Process2Wipe® IPA70 wipes Sterile cleaners & disinfectants available in a large assortment of packaging sizes SCAN HERE USP <797> & <800> Solutions From Veltek Associates, Inc. Combining experience, innovation, performance, GMP manufacturing, GLP testing services, and unrelenting service has propelled VAI® as the ultimate innovative leader in the market.
GX Solutions Medical-grade Cold Storage Pass-thru Refrigerators to stored products enhanced work flows i.Lock™ interlock door technology available to meet USP <797> clean room requirements Extensive testing to provide optimal storage of ambient product loads Best-in-class temperature management of uniformity, stability, and recovery ENERGY STAR® certified and up to 50% more energy efficient than conventional medical-grade refrigerators and freezers Workflow efficiency and regulatory compliance for clinical pharmacies Learn More helmerinc.com/gx-solutions
page 39
Strategic Partnerships
Helmer Scientific offers the only medical-grade pass-thru refrigerator that meets the proposed changes to USP <797> for use in cleanroom applications. page 33 page 34-37 Outpatient Pharmacy
Performance
CASE STUDY STRATEGIC PARTNERSHIPS DRIVE INPATIENT AND OUTPATIENT PHARMACY PERFORMANCE A DECADES-LONG PARTNERSHIP Holzer Health System traces its roots back more than a century to when Dr. Charles E. Holzer Sr. opened the first private hospital in southeastern Ohio. Located in Gallipolis, that small, seven-bed hospital has grown into the multi-facility healthcare system it is today. This includes a medical center and care center in Gallipolis, a medical center in Jackson, and numerous walk-in clinics, retail pharmacies, wellness centers, and post-acute care services, among other resources, across the region. Holzer has remained true to the philosophy set forth by their founder — “The Patient Operating Officer for Holzer, Todd Fowler, to continue building on the foundation of their fruitful partnership with CPS for inpatient and specialty pharmacy management services. Over the 20+ year relationship, CPS has consistently driven value for Holzer through initiatives supporting clinical performance, cost savings, and operational expertise. A RELATIONSHIP THAT CONTINUES TO EVOLVE The partnership between Holzer and CPS, one of the country's largest pharmacy and hospital services providers, reaches back to 1998 when CPS began helping the health system manage its inpatient pharmacy operations. As the system has grown, CPS has continued to deliver incremental value to Holzer’s leadership and staff. Based on these successes, when Holzer chose to streamline their three retail pharmacies in Gallipolis, Jackson, and Athens in 2014, they turned again to CPS as partner. Everything CPS brings to Holzer is focused on growing the impact of their partnership while providing ongoing positive financial and quality outcomes for the health system. One way the company drives value on a continual basis is through its Comprehensive Pharmacy Assessment (CPA), proprietary tool that audits over 450 points of pharmacy's operational health. During the company’s most recent analysis, they found Holzer had successfully improved compliance metrics, decreased regulatory risk, and increased alignment with ISMP best practices. Through the CPA, CPS helps the health system maintain visibility to the performance of its pharmacy in accordance with current Executive Vice President and Chief Operating Officer Holzer Health System REFRIGERATION
From CPS Solutions.
Optimize Outcomes with Specialty Pharmacy Services
As health systems expand outside acute settings, you need a partner that can help to optimize outpatient medication management and drive clinical and business outcomes. Omnicell’s Specialty Pharmacy Services brings together dedicated services to launch, operate and optimize your specialty pharmacy program. This comprehensive, turnkey solution delivers:
A Improved access to specialty medications to enhance care and support patient outcomes

A Financial outcomes through a value-based service model
A Technology, services, and expertise to support medication management from hospital to home
Learn More at Omnicell.com/products/specialty-pharmacy-services

20Ways MISSION
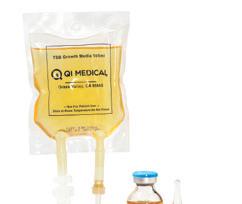
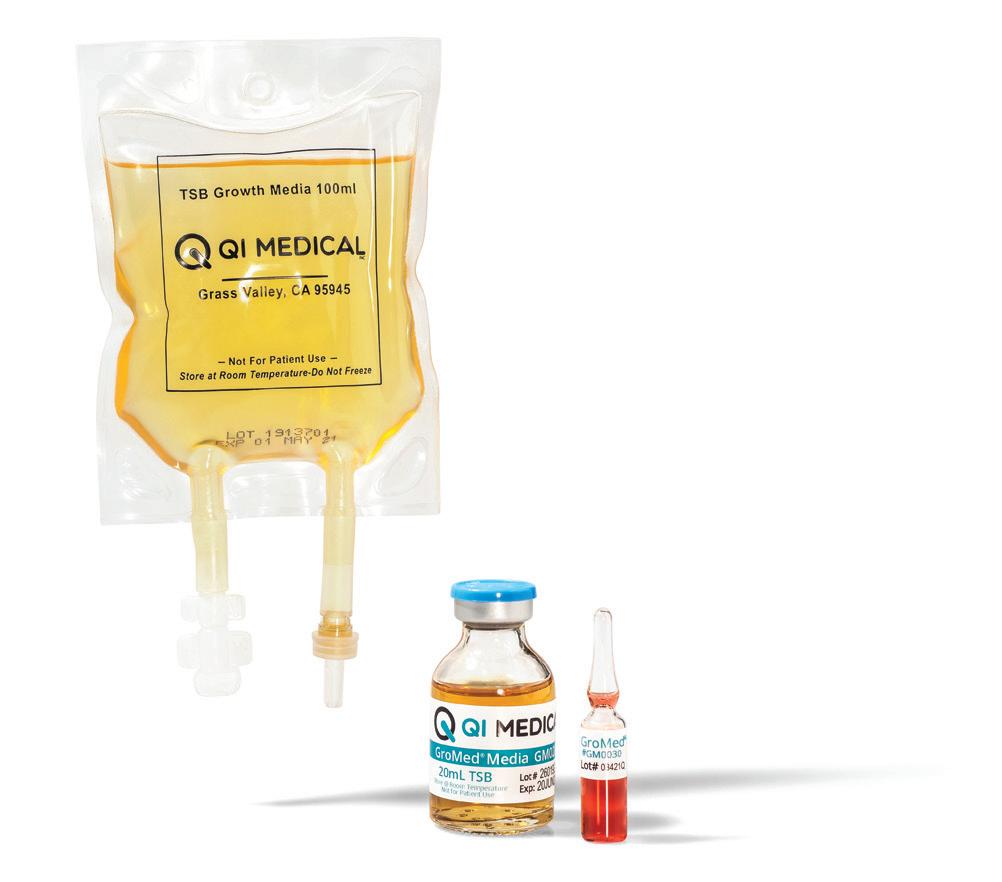
Q.I. Medical, Inc.™ Your Partner in USP <797> Pharmacy Compliance







Providing quality assurance products to hospitals and pharmacies practicing sterile compounding, with a singular focus on pharmacy compliance.















Case Study: Defying Drug Diversion
The pharmacy executive, nurse executive, and drug diversion analyst tell how. Sponsored by Medacist.







Steri-Tamp®






Tamper-Evident

Sterile Seals by Allied Pharmacy Products, Inc.


The only single-use, tamper-evident seal that provides and maintains a 100% sterile barrier. page 47
Capital Inventory, Inc. — The Premier Leader in Pharmacy Inventory Services


Our decades of expertise, working exclusively with pharmacies, makes us uniquely qualified to provide accurate and dependable inventory valuations through a streamlined process. Our clients can trust that we will be transparent, supportive, and reliable in every interaction.

page 49
Sterile & Paper Free (USP 797 Compliant). Maintains a 100% sterile barrier* with 3X greater adhesion. *Tested in Nelson Labs, Salt Lake City, UT Helps prevent contamination of drugs and provides added protection to pharmacists. Will not fall off, even in cold storage conditions (down to -20 degrees centigrade). Patented dual-layer indicates true tamper-evidence, with “OPENED” marking. The Most Innovative Tamper-Evident Seals In Hospital Pharmacy Today steri-tamp.com Visit Our Website to Request Samples Syringe Seals IV Bag Port Seals Contents
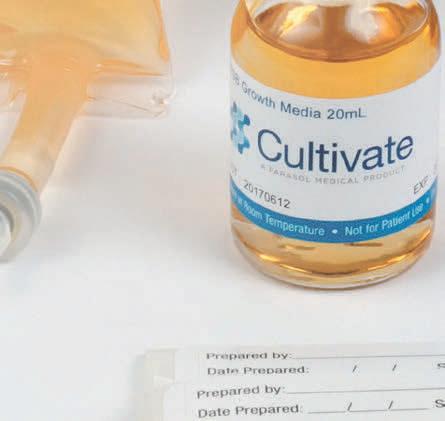
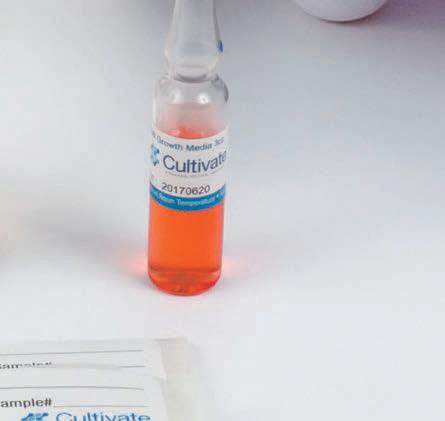
To educate pharmacy management on products and services that serve to improve patient care or improve a pharmacy’s financial bottom line, while distilling and presenting this relevant information via 20 product profiles. 2022 CIRCULATION Issue Focus: Hospital & Infusion Issue Frequency: Summer & Winter Circulation Per Issue: 12,200+ 6,500+ Hospital Directors 2,200+ Clinical Consultants 2,500+ Industry Executives 1,000+ Trade Show Handouts Issue Focus: Retail, Specialty, & LTC Issue Frequency: Spring, Fall, & Winter Circulation Per Issue: 26,500+ 18,000+ Owners (Independents) 1,500+ Long-Term Care Pharmacies 2,500+ Retail Chain Executives 2,500+ Industry Executives 2,000+ Trade Show Handouts QUARTERLY ISSUES Visit 20Ways ONLINE at RXinsider.com/20Ways Health System • Infusion IMPROVING PATIENT CARE & PHARMACY COST CONTAINMENT 20 Unique Products & Services Discover 20 products and services that will help your pharmacy improve patient care or contain costs. Clean Air Technology & Supplies Industry Expert Q&A, Market Leaders Buyer’s Guide, and More pg. 54 New Features Storage Standard Transfer Devices) 3 Case Studies CPS, Liberty Software, Follett Refrigerators are Omnicell FlexLock-Ready 20Ways Profile by Follett pg. 43 Summer 2022 Community • Specialty • LTC Pharmacy Management Software Industry Expert Q&A, Market Leaders Buyer’s Guide, and More IMPROVING PATIENT CARE & PHARMACY PROFITABILITY 20 Unique Products & Services Discover 20 products and services that will help your pharmacy improve patient care or increase profitability. 3 Case Studies McKesson, Liberty Software, 8 Thought Leaders Spring 2022 Community • Specialty • LTC IMPROVING PATIENT CARE & PHARMACY PROFITABILITY 20 Unique Products & Services Discover 20 products and services that will help your pharmacy improve patient care or increase profitability. Pharmacy Automation & Robotics Technology Buyer’s Guide, More 8 Thought Leader Videos The Compliance Team, CPS Zebra Case Study A Success Story with Altru Specialty Pharmacy Pg. 37 Fall 2022 Qimedical.com info@qimedical.com Tel. 800.837.8361 Media Fill Hazardous Drug Handling Validation Surface and Fingertip Testing Sterility Testing USP <797> USP <71> USP <800> Your partner in pharmacy compliance since 1992 FDA Registered | Iso Certified Left: GM7030 PATT2®, Personal Aseptic Technique test kit, 3mL ampules, 20mL vials, 100mL partially lled minibag. Below: ET1000 EnviroTest® TSA with Lecithin & Tween 80 growth media paddles for surface, air, or glove ngertip sampling.
page 42-45
CASE STUDY Receive insights from Senior Vice President of Allied Health, Brian O'Neal, PharmD, Children's Understand how nursing deals with diversion given unprecedented challenges from Retired Learn about analytics when managing a drug diversion program from Retired Drug Diversion Surveillance Analyst, Patricia Penland, RN, at Wake Forest Baptist Health. State Boards of Nursing, approximately 15% of healthcare workers struggle with drug dependence underestimated, undetected, and underreported. And if diversion hasn’t wreaked enough havoc, healthcare professionals have endured upheaval like stress levels, burnout, and staff shortages, which can exacerbate drug diversion. Diversion poses risks to patients, including inadequate pain relief and exposure to infectious However, solutions and strategies exist to combat it, and hospitals are winning the battle. A pharmacy executive, chief nurse officer, and drug diversion analyst share insights on the state of drug diversion and the tools to detect it. Profile: Senior Vice President of Allied Health, Brian O’Neal, PharmD, Children's Mercy Kansas City Q. How does your role relate to preventing diversion and protecting patients? The likelihood is there. Prevention and detection are priority for me. My guidance is to focus on fundamentals, which means consistent quality checks, audits, and understand how informs possible problems and how to pull the right data. Q. Thoughts on the state of drug diversion at hospitals given COVID and staff shortages? Our hospital, like the industry, experiencing shortages, especially in pharmacy technicians, due we are still dealing with all those issues and current prevalence data. We are dealing with gap in scientific evidence. How does Children’s Mercy Kansas City deal with diversion? We developed controlled substance oversight council led by pharmacy and nursing that includes members from security, human resources, and other hospital departments who meet regularly to review transactions and approve policies. DEFYING DRUG DIVERSION Allied Health at Children’s Mercy Kansas City, MO Barbara Jacobs, MSN Retired Chief Nurse Officer Anne Arundel Medical Center, Patricia Penland, RN Retired Drug Diversion Surveillance Analyst Wake Forest Baptist Health, The Pharmacy Executive, Nurse Executive, and Drug Diversion Analyst Tell How Sponsored by an Educational Grant From Medacist page 41 Health System • Infusion & Community • Specialty • LTC IMPROVING PATIENT CARE & PHARMACY PROFITABILITY COMMUNITY, SPECIALTY, LTC EDITION 20 Unique Products & Services Discover 20 products and services that will help your pharmacy improve patient care or increase profitability. 8 Thought Leader Videos Merchant Systems, Surescripts, The Compliance Team, & Noritsu Pharmacy Case Study Automating Long-Term Care Pharmacy Services Pg. 37 Winter 2022/2023 IMPROVING PATIENT CARE & PHARMACY COST CONTAINMENT HEALTH WINTER Defying Drug Diversion Case Study From Medacist pg. 42 20 ProductsUnique & Services Discover 20 products and services that will help your pharmacy improve patient care or contain costs. Refrigeration Industry Expert Q&A, Market Leaders Buyer’s Guide, and New Features The Evolving of Compounding Pharmacies pg. 66 Trends as 4 Case Studies Solutions, Leiters, Wolters Health, and More PharmD, Allied Health Mercy Kansas

Pre-assembled kits and a la carte components for infusion pharmacists. Compliance was never so easy! Hardy Diagnostics is a proud Corporate Member of the National Home Infusion Association. Contact us today for more information about our HardyVal™ products! 800.266.2222 Sales@HardyDiagnostics.com HardyDiagnostics.com/HardyVal Member USP <797> changes are coming. Are you ready?
Praxi Ject SF
0.9% NaCl Prelled Syringes ™







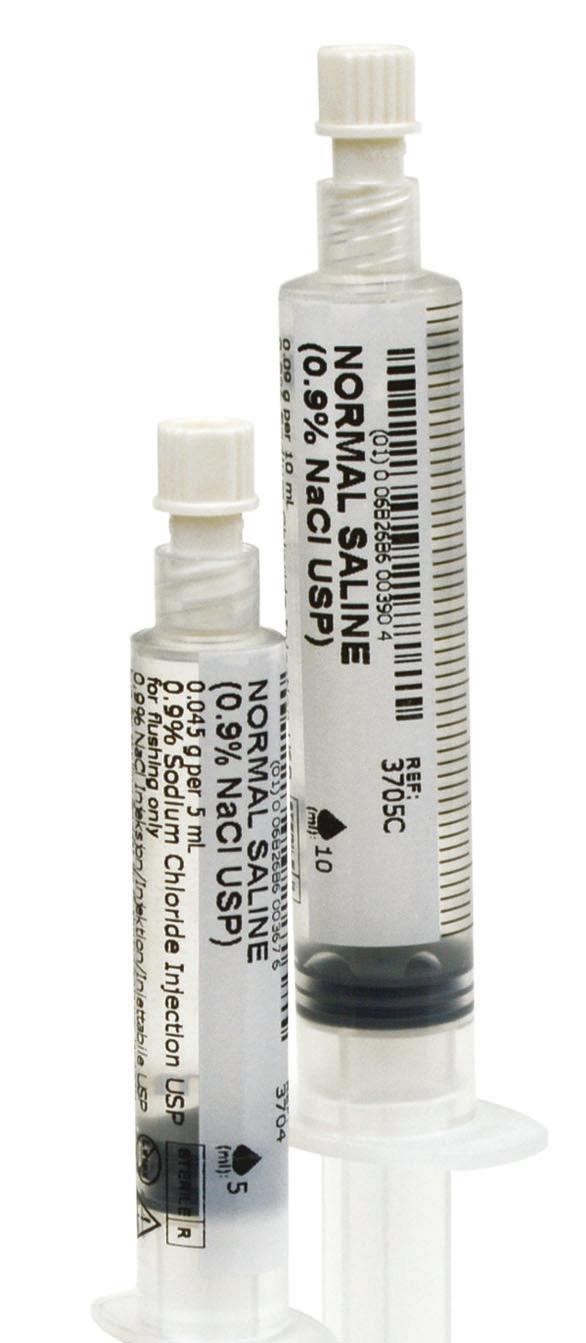
“ ” Syringes that set the standard for quality and safety

Precise graduation
Color coded cap and label
Respects ISO specication

Pharmaceutical grade plunger
Take a closer look at all the benets a PraxiJect™ SF 0.9% NaCl prelled syringe offers you.
PraxiJect™ SF 0.9% NaCl prelled syringes set the standard for quality and safety.
No other company offers you all these advantages in a cost effective product. Manufactured according to internationally recognized technical standards, current Good Manufacturing Practices and ISO 13485 Quality Management System requirements.
Discover the advantage many of the largest hospital institutions have realized work best for them.
Features & Benets
Zero reux design
Designed for use in a sterile eld Precise graduations Meets USP specications
Latex and preservative free
Screw on luer lock cap for additional safety Terminal sterilization, provides the highest degree of sterility available
Easy glide piston. No lock effect or inadvertant solution leaking during priming.
Praxiject.com INNOVATING FOR HEALTH






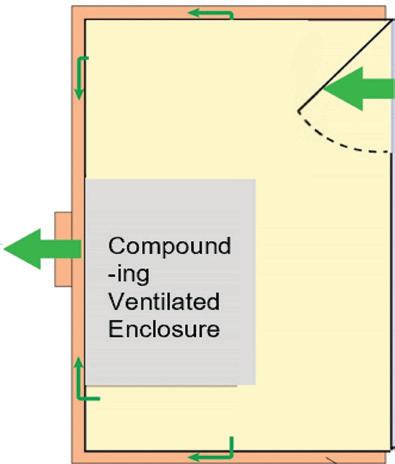
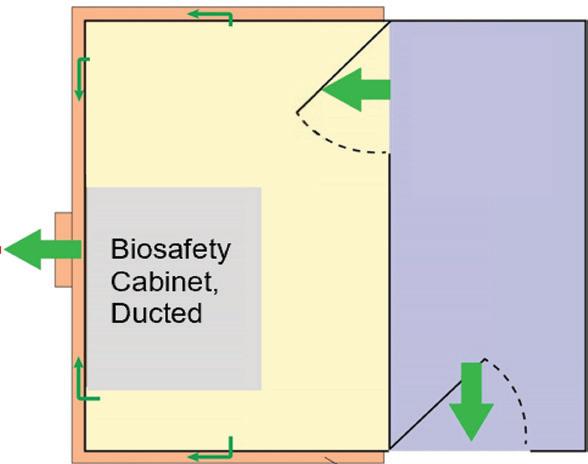
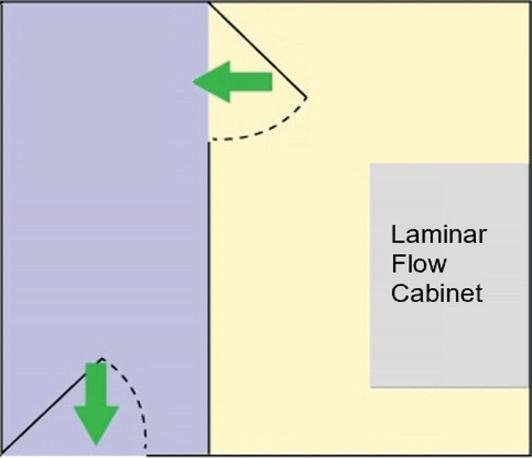
SECONDARY Engineering Control (SEC) For BUD > 12 hrs ENGINEERING CONTROL REQUIREMENTS for USP-797 and USP-800 Compliance ALSO AVAILABLE FROM ESCO Sterile Non-Hazardous Cleanroom Pass Boxes Non-Sterile Hazardous Downflow Booth Sterile Hazardous 503B cGMP Isolators PRIMARY Engineering Control (PEC) Esco Models: LHG (Horizontal): General compounding LVG (Vertical): Put large machine inside Esco Models: PW1 (Compact): Single exhaust HEPA ADC (Full-sized): Double exhaust HEPA Esco Model: DFB: Downflow Booth Esco Models: AC2 (Compact): Low ceiling, Mobile labs LA2 (Full-sized): Best comfort & safety DRUG TYPE USP 797: Applies to Non-hazardous and Hazardous Drugs USP 800: Additional Requirements for Hazardous Drugs Horizontal Laminar Flow Cabinet Powder Weighing Station with Ducting Class II A2 Biosafety Cabinet w/Ducting Esco Models: EPB-SS: Passbox, Full Stainless Steel BIOPASS: Passbox, with H2O2 Decon Esco Models: GPPI: General Processing Isolator ACTI: Aseptic Containment Isolator 215-441-9661 • Horsham, PA, USA • ETI.Admin@escolifesciences.com • www.escolifesciences.com Compounding Ventilated Enclosure Biosafety Cabinet, Ducted Laminar Flow Cabinet ISO 7 buffer 12 ACPH 0.01-0.03 “WG neg. pressure ducted ISO 7 buffer 30 ACPH 0.02 “WG positive pressure ISO 8 ante 20 ACPH 0.01 “WG positive pressure ISO 7 buffer 30 ACPH 0.01-0.03 “WG neg. pressure ducted ISO 7 ante 30 ACPH 0.02 “WG positive pressure

Qimedical.com info@qimedical.com Tel. 800.837.8361 Media Fill Hazardous Drug Handling Validation Surface and Fingertip Testing Sterility Testing Your partner in pharmacy compliance since 1992 USP <797> USP <71> USP <800> A re you properly mimicking your processes? Ask us about a custom media fill solution for your facility. FDA Registered ISO Certified FDA Registered | Iso Certified

The supply chain solutions you need, when you need them. Our distribution solutions will help your business increase operational efficiencies, maximize profitability and improve prescription fill rates. Make Anda a part of your distribution solution. Learn more about our products, pricing and programs. Scan the QR code to request more information. 1-800-331-ANDA (2632) www.andanet.com Follow us @AndaInc: Product Access • Brand, Generic, & Specialty Medications • Seasonal Flu & Disease Preventable Vaccines • Point-of-Care Diagnostic Tests • Vitamins & OTCs • Medical Supplies & PPE • Pet Meds A01-31155-080322
Case Study: Leiters — Mobile Infirmary Pilots Innovative Concentrated Norepinephrine Vials
































Study design and findings — an overview.



American Health Packaging, Leading Manufacturer of Serialized






page 63
Visante — High-Performing Pharmacy is Who We Are
We're in the business of advancing pharmacy. Our consultants work with hospitals and health systems combining a wealth of expertise with personalized and comprehensive support. page 55
Shields Health Solutions — The Premier Specialty Pharmacy Accelerator
The
Contents Health Care Logistics® — Ensure HospitalWide Temperature Accuracy With Stat Temp™
safe ranges,
waste, and
this remote temperature monitoring system
GoHCL.com • 1.800.848.1633 Health Care Logistics, 2022 NO SUBSCRIPTIONS! Online software alert and monitoringsystem included with units. CONFIGURE REAL-TIME ALERT SETTINGS ACCORDING TO FACILITY NEEDS User Friendly & Complete Customization Instant Access to Reporting Peace of Mind – 24 hours a day https://StatTemp.io * CONTACT CUSTOMER SERVICE FOR NIST CERTIFICATE INFORMATION Wireless Temperature Monitoring by Health Care Logistics® page 57
Manage
avoid drug
provide peace of mind with
exclusively from Health Care Logistics.
Evolving
of Compounding Pharmacies
the risks that compounding pharmacies
microbial contamination
page 66-69 While pharmaceutical compounding has existed throughout history, mass production of pharmaceuticals drove down the need for such drugs for much of the 20th century. It wasn't until the 1970s and 1980s, when the demand for chemotherapeutic and total parenteral nutrition regimens increased, that the pharmaceutical compounding industry began to grow. The market has been advancing steadily since — driven by bulk compounding, increases in home infusion therapy, the evolution of personalized medicines, rise in hormone replacement therapy, and more. Compounding pharmacies also serve critical role in providing alternate dosage forms of medications. For example, some patients may need an oral liquid versus tablet or have allergies or dietary restrictions requiring specially formulated drug versions. Despite their unique position in the marketplace, regulations specific to pharmaceutical compounding are relatively recent. An Evolving Regulatory Landscape In September 2012, the CDC, FDA, and local and state health officials began investigating multistate outbreak of fungal meningitis among patients who received contaminated steroid injections. The contaminated medication was traced back to a compounding facility in Massachusetts, which the FDA had previously visited and found sterility issues. The agency lacked the authority to impose or enforce any changes to the site even though facility operations resembled an FDA-regulated drug manufacturer. Instead, due to the regulatory environment at that time, the facility was able to continue operating as pharmaceutical compounder, The Evolving Role of COMPOUNDING PHARMACIES 66 WINTER 2022 HEALTH SYSTEM INFUSION Unit Dose Done Right Unit Dose Done Right Unit Dose, Bar PharmacyCoding, & NursingExperts!Supply Unit Dose, PharmacyCoding, & Cold Seal Tamper-Evident Moisture Resistant Ultraviolet Inhibitant Reduces Cross Contamination Ideal for Meds Covered by USP 800 6 and 12-month Beyond-Use Dating 1-D and 2-D Bar Coding Flexible Label and Report Formatting Multiple Sizes and Shapes to Fit Your Meds & Storage Needs MediDose.com 800.523.8966 Brightly Colored Labels Call A tention to Meds Requiring Special Handling Simple. Reliable. Medi-Dose, Inc./EPS, Inc. Delivers Bar Coding, Packaging, and Labeling Pharmacy Solutions Improve your Solid Oral Unit Dose needs with our comprehensive Bar Coding, Packaging, and Labeling Solutions — designed by healthcare professionals, for healthcare professionals. page 71
Role
Among
face,
has always been a significant concern.
Barcoded Unit-Dose Products
a responsive line of barcoded unit-dose oral solutions, a
liquid unit-dose offering,
Product description Cup delivery Cup strength (cups/case) Diphenhydramine HCl Liquid 25 mg 10 mL Hydrocodone Bitartrate & APAP Oral Solution (CII) 7.25 mg 325 mg Hydromorphone HCl Oral Solution (CII) mg mL Phenobarbital Elixir (CIV)* 20 mg /5 mL dose products from American Health Packaging. Our products quickly deliver safety and e ciency in every dose without the With access to more than 600-unit dose items, including 100+ exclusives, your patients and streamline your operations. Browse our extensive catalog and take advantage of these featured liquid unit dose products from AHP. Browse our full catalog through americanhealthpackaging.com or through your preferred wholesaler today. Scannable bar codes Clear “Tall Man” lettering Extended shelf life No mess.
waste.
problem.
With
growing
as well as individually-wrapped inhalants, American Health Packaging continues to deliver on their commitment to supporting pharmacy efficiency.
No
No
in the Country Transform your specialty pharmacy into a powerful growth engine and elevate performance where it matters most — with ShieldsRx. page
Leverage our dedicated experts, proven collaborative care model and integrated care technologies to help you produce the superior outcomes your patients deserve and the financial results your health system demands. CASE STUDY Study Design and Findings — An Overview PARTNERING TO ENHANCE WORKFLOW EFFICIENCY TO HELP CREATE BETTER PATIENT OUTCOMES Infirmary Health is the largest non-governmental, non-profit healthcare system in Alabama. Serving southern Alabama, the organization's network of award-winning hospitals, physician practices, and affiliates makes it top healthcare system on the Gulf Coast. This includes Mobile Infirmary — Infirmary Health's flagship hospital, which is among the leading hospitals in the state for surgical volume. The hospital houses comprehensive cardiovascular program with hybrid OR/cath lab, the region’s only Bariatric Center of Excellence, a CARF-accredited rehabilitation hospital, renowned cancer program, thrombectomy-capable stroke center, and freestanding emergency department. It's no surprise that Mobile Infirmary's doctors and nurses keep the I.V. room pharmacists and technicians very busy. To help alleviate the stress and strain, Leiters, an FDAregistered 503B outsourcing provider of compounded sterile preparations, reached out to the hospital about participating in a workflow study for its new concentrated vials — Vicky Vega, Pharm.D., says they were very interested. “We always want to be involved in anything that promotes change and advancement,” says Vega. “Especially where that means achieving better patient outcomes.” READY-TO-DILUTE, CONCENTRATED, PRE-FILLED VIALS Leiters' concentrated vials are ready-to-dilute, pre-filled vials of highly used compounded sterile preparations that are not commercially available. Norepinephrine Bitartrate (Norepi) was selected for this workflow study. “We prepare a good many Norepi bags in all milligram strengths — mg, mg, and 16 mg,” says Vega. “We could see how having something readily available in concentrated vials could get medication to patients faster, so we wanted to take part." MOBILE INFIRMARY PILOTS INNOVATIVE CONCENTRATED NOREPINEPHRINE VIALS Nathan Browning, Pharm.D. I.V. Room Supervisor Mobile Infirmary Vicky Vega, Pharm.D. Pharmacy Education Coordinator Mobile Infirmary Roland Naseman, R.Ph. Director of Pharmacy Mobile Infirmary
65
page 50-53
WINTER 2022 I HEALTH SYSTEM • INFUSION 11 Connect with Visante today to learn how we can help you reach your goals. Visit us at visanteinc.com, or call 866-388-7583. We help you achieve extraordinary results in all areas of health system pharmacy Making the most of opportunities to manage is core to our consulting. We help you improve financial performance across your organization. Financial Results Our team of compliance experts will improve ensure patient, worker and community safety. We collaborate with you your organization to peak performance levels. Strategic Planning & Implementation Compliance that Improves Care Pharmacy consulting designed for optimal growth Visante consultants bring you expertise in all areas of hospital and health system pharmacy: • Comprehensive Strategic Assessments • 340B Program Solutions • Specialty Pharmacy Programs • Revenue Cycle and Drug Reimbursement Strategies Supply Chain Strategies Drug Diversion Programs Drug Compounding Excellence
page 58-61 REFRIGERATION Buyer’s Guide REFRIGERATION Buyer's Guide 770 Garrison Avenue Bronx, NY 10474 (718) 893-3900, Ext. 204 accucold.com/pharmacy Aegis Scientific Warminster, PA 18974 (800) 796-2344 aegisfridge.com American BioTech Supply Horizon Scientific, Inc. 125 Varnfield Drive Summerville, SC 29483 (800) 648-4041 americanbiotechsupply.com Arctiko Us Inc. Nashville, TN 37217 (615) 988-7000 www.arctiko-int.com Medical Systems 14560 Bergen Boulevard Suite 200 Noblesville, IN 46060 (888) 456-7099 www.bmedicalsystems.com FFF Enterprises Temecula, CA 92590 (800) 843-7477 fffenterprises.com Follett Products, LLC 801 Church Lane Easton, PA 18040 (610) 252-7301 www.folletthealthcare.com Guardian Medical Systems 409 Edgewood Drive Exton, PA 19341 (484) 872-2500 guardianmed.net Health Care Logistics Circleville, OH 43113 (800) 848-1633 14400 Bergen Boulevard Noblesville, IN 46060 (800) 743-5637 LabRepCo 101 Witmer Road, Suite 700 Horsham, PA 19044 labrepco.com NAFEM The Legacy Companies 3355 Enterprise Avenue, Suite 160 Ft. Lauderdale, FL 33331 (954) 202-7419 thelegacycompanies.com Migali Scientific 1 Triangle Lane Blackwood, NJ 08012 (855) 464-4254 migaliscientific.com PHC Corporation of North America Wood Dale, IL 60191 (800) 858-8442 www.phchd.com/us/biomedical Qingdao Haier Biomedical Co., Ltd No. 280 Feng Yuan Road High-tech Zone, Qingdao 266109 P.R. China Usa.haiermedical.com Stirling Ultracold Athens, OH 45701 (855) 274-7900 stirlingultracold.com Thermo Fisher Scientific, Inc. 81 Wyman Street (781) 622-1000 Industry expert Q&A, Refrigeration Market Leaders Buyer’s Guide, and more. REFRIGERATION WINTER 2022 HEALTH SYSTEM INFUSION REFRIGERATION Q&A The NSF Joint Committee for Vaccine Storage was formed in 2015 to develop a new standard. The committee was responsible for creating a standard that ensures engineering controls are inplacetoassistinsafelystoringvaccinesunder real-world conditions in clinical environments. The NSF/ANSI 456 Vaccine Storage Standard was finalized in May 2021, and equipment manufacturers can submit their products for independent testing and certification against the standard. Q. Why was the new NSF/ANSI 456 Vaccine Storage Standard needed? Up until the release of the NSF/ANSI 456 standard there wasn’t good standard in North America that could be referenced for the performance of a refrigerator or freezer for the storage of vaccines or refrigerated medications. What was available, the CDC Storage and Handling Toolkit, provided guidance on how to store vaccines in refrigerators and freezers, buttherewerestillissuesinthefieldwhereproviderswerelosing vaccines due to poor temperature performance. The providers were seeking something more that focused on performance and how these units were being used in real-world conditions. The committee was established to develop a standard that lookedatoverallperformanceof therefrigeratorsandfreezers. Q. Who created the new NSF/ANSI 456 Vaccine Storage Standard, and what was the driving force? The NSF/ANSI 456 standard was developed by a committee includingmanufacturersof vaccines,directorsfromseveralstate health departments, pediatricians and physicians, members from the Immunization Coalition, National Association of County and City Health Officials (NACCHO), National Institute of Standards and Technology (NIST), and the CDC, as well as multiple equipment manufacturers. It was a very cross-functional group. The decisions on what requirements were needed in the standard were driven by public health members and end users. The key aspects of the requirements were driven by research and data collected from healthcare facilities throughout the US. Health clinics were visited, and usage data collected to understand how these units were being used. The test methods in the standard were developed to provide confidence that the vaccines would maintain their temperature requirements and stay in compliance during these real-world use cases. Q. Can you explain the basics of the standard? TheStandardfollowsthestandardformat anANSIcertified standard. The standard is written to address both refrigerators and freezers and covers design and construction, test methods, performance requirements, and guidance on performing the actual temperature measurements including construction of a vaccinesimulationdevice.NISTprovidedasignificantamount of support for the development of the test methodologies and how to perform the temperature measurements that were written into the standard. The standard itself includes some minimum refrigerator and freezer performance requirements throughout the testing protocols. The test method portion of the standard focused on maintaining temperature performance through frequent door openings and door openings related to loading of the cabinet, as well as how many vaccines are stored in the unit. We looked at two storage scenarios to develop the requirements, which were totally empty cabinet and a totally full cabinet. We simulated the full cabinet by filling the units with standard size boxes to make sure we filled a large percentage of total volume Dennis Smith, Chair of the Use Group NSF Joint Committee for Vaccine Storage Q A& REFRIGERATION





































































DRUG DIVERSION MITIGATION SPECIALISTS

Drug Diversion is a real risk that facilities face. Failure to secure controlled substances can result in millions in fines, court-mandated actions, and serious harm for both patients and employees.
Rxpert Solutions has years of experience in drug diversion mitigation.


• We start with a comprehensive, external audit with gap analysis
• We evaluate and enhance current diversionrelated education
• We assist with remediation
• We help develop an effective drug diversion program
• We offer centralized monitoring with diversion surveillance software
• We assist with investigation discovery and interview preparation
www.rxpert.solutions Call or Text: (760) 705-4078 tvidals@rxpert.solutions Schedule your FREE consultation today! LinkedIn: @terri-vidals
THERESA VIDALS, B.S. PHARM FOUNDER OF RXPERT SOLUTIONS
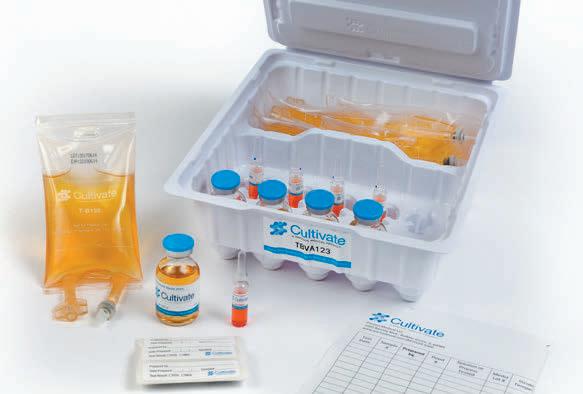
inclusiv® is a comprehensive IV compounding portfolio of integrated technology, software, and service solutions designed to keep your patients safe.

Misterium®
Modular Cleanroom Systems






75 Years of experience, and more than 2 Million ft2 of cGMP cleanrooms designed and built worldwide. inclusiv® modular cleanroom systems and consulting services can help promote safety and compliance in the design, construction, and environmental control of the most stringent sterile compounding settings.





Plasmair™ Mobile Air Decontamination Systems





Increase air quality in existing pharmacy cleanrooms, segregated compounding areas, and temporary cleanrooms. With HEPA-MD cold plasma technology, PLASMAIR™ eliminates viable airborne contamination and optimizes air quality around the clock to protect healthcare providers, your compounded products, and your patients.
Gri-fill® 4
Semi-Automated Compounding System

A semi-automated system for compounding a variety of intravenous mixtures, including chemotherapy doses. Gri-fill® is compact, easy to install and readily adaptable to your IV compounding operation.


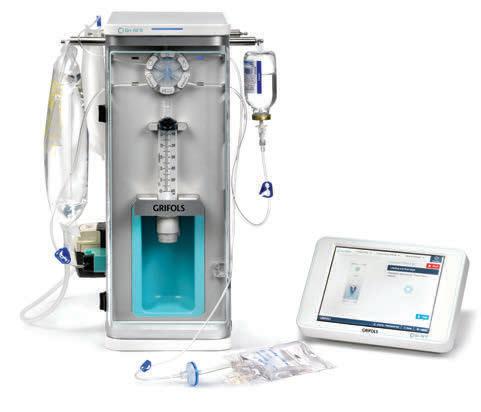
KIRO® Fill

Automated
Compounding System
An automated compounding device designed to enhance patient safety and optimize operational efficiencies during the production of non-hazardous compounded sterile preparations.

KIRO® Oncology Robotic Compounding System
An automated compounding device designed to enhance patient safety and optimize operational efficiencies during the production of hazardous compounded sterile preparations.
© 2022 Grifols All rights reserved November 2022 US-INCP-2200007
Protecting patients is your most important responsibility
Visit grifolsinclusiv.com to learn more. See you at ASHP Midyear 2022 Booth #1123
airinspace and PLASMAIR are registered trademarks. Guardian and Sentinel registered models.
Medication Management Solutions From Swisslog Healthcare Elevate the Way You Work






Deliver exceptional patient care with innovations from the core of the central pharmacy to enhance performance workflow efficiency, improve accuracy, and increase safety.













page 75

page 79
Trends to Watch as 340B Turns 30
This year marked an important milestone for the 340B drug pricing program. On November 4th, we celebrated the 30th anniversary of 340B.
page 86-87

If it Keeps Your Hospital Clean, ISO-MED Keeps it in Stock
It’s never been easier to pull the trigger on contamination because ISO-MED’s unique 0.22-micron filter vented sprayer on our 70% IPA keeps the compounding environment clean and safe. And like all our other products that help you maintain a sterile cleanroom, ISO-MED always has a full supply on hand.
Contents Central Pharmacy Automation Solutions that transform organizations through efficiency Experience exponential cost savings by streamlining supply chain operations Significantly improve fulfillment accuracy and minimize waste with centralized pharmacy inventory management Improve clinical outcomes by spending more time attending to patient needs Contact the Central Fill Pharmacy Experts at swisslog-healthcare.com/ pharmacyautomation 2022 RXinsider Winter 20 Ways Ad.indd
Safeguard Your Organization, Community, and the Environment With Stericycle Solutions
Safeguard Your Community SafeDrop Sharps Mail Back Containers By providing your employees, patients, and community neighbors with a safe and convenient way to dispose of used sharps, you help reduce the risk of injury or misuse MedDrop Drug Collection Kiosks collection kiosks can be placed within pharmacies, hospitals, and law enforcement agencies to ensure safe and convenient way for consumers (including employees) to dispose of unused medications. Seal&Send Medication Envelopes By instituting compliant, safe, convenient, and anonymous prescription drug disposal mail back solutions, you actively demonstrate your organization’s commitment to public health protection and environmental stewardship. Visit Stericycle.com or call 866-783-7422 P H A R M A C E U T C A L W A S T E S O L U O N It’s Time To Protect What Matters Most. With the right tools in place, your organization can take significant steps towards advancing community health, addressing the opioid crisis, and positively impacting the environment.
Stericycle’s Safe Community Solutions provide your patients with safe and convenient disposal of sharps waste and unused medications.
WINTER 2022 HEALTH SYSTEM INFUSION 86 With that increased attention has come increased challenges for 340B covered entities and their patients. We have had to defend 340B on 340B Health, which represents more than 1,400 health systems and member pharmacists, 340B program managers, and other health patients in need. As we wrap up the 30th anniversary year for 340B, we are centered on several key trends and areas of focus that we believe will be pivotal for the future the program. Drug Industry Attacks on 340B The pharmaceutical industry recently has been pushing hard to change the program in ways that diminish the amount of help provides to safety-net providers and the patients with low incomes whom they serve. This not true for all drug companies, as more than Since 2020, the single biggest challenge to 340B has come in the form After years of unsuccessful attempts convince Congress to make discounts covered entities on drugs dispensed at community and specialty pharmacy partners, actions that the Department of Health and Human Services (HHS) has said are illegal. What started with handful of companies has now expanded into major threat 340B. The companies now restricting access to 340B discounts include several of the largest drugmakers in the world that Their actions have caused immense harm to safety-net providers hospitals we conducted in early 2022 found larger, typically urban 340B hospitals reporting average financial losses millions dollars per year because of these restrictions. Smaller, typically more rural hospitals are losing hundreds of thousands of dollars per year on average. These losses are devastating to facilities that already rely on the thinnest of operating margins to stay open. Not surprisingly, the answer rooted in systematic pursuit of profits. and the drugs they are targeting for these restrictions demonstrate two of safety-net providers and patients. The first focuses on denying access to discounts for some of the costliest specialty drugs that physicians are prescribing for patients living with chronic diseases, including various forms of cancer. The second focuses on getting around the federal penalties imposed on them after they raised the price of drugs much faster than the rate of inflation. For such drugs as insulin, for penalties permit 340B providers to pay a nominal amount and use the The unilateral cuts to 340B from these manufacturers keep more dollars in drug company coffers and deprive safety-net hospitals resources they need to fund patient care. Both the Trump and Biden administrations have told the drugmakers they are breaking the law. But instead complying, several companies went to court to challenge the government’s authority. Several federal appeals courts Trends
340B Turns 30 This year marked an important milestone for the 340B drug pricing program. On Nov. 4, we celebrated the 30th George H.W. Bush signed into law, 340B has enabled nonprofit hospitals, health centers, and clinics to serve communities in every corner of our country. Thanks to 340B, patients receive quality care closer to their homes, services and delivery networks needed to sustain a robust social fabric. And all this has been possible because of administrations, 15 Congresses, thousands of communities, and millions of Americans without relying on taxpayer dollars Despite these successes, the stakes have never been and the providers who participate in it. With 340B growing and evolving to meet changing patient health needs, it has ago, we might have had trouble finding people who had even heard of 340B, let alone who were familiar with the Now 340B is appearing regularly in publications throughout the U.S., in the floor speeches of prominent members of Congress, and even in the highest court in the land. Maureen Testoni is the president and CEO of 340B Health, which represents more than 1,400 hospitals participating in the 340B drug pricing program. page 91 ScriptPro’s Endto-End Pharmacy Solutions Drive Health System Success ScriptPro’s game-changing pharmacy solutions enable health systems to create an optimum retail pharmacy business model to deliver outstanding patient care while raising operational performance and profits. page 92-95 Case Study: Streamline Compounding and Medication Compliance With Simplifi+® From Wolters Kluwer. CASE STUDY www.wolterskluwer.com/en STREAMLINE COMPOUNDING AND MEDICATION COMPLIANCE WITH Simplifi+ ® INTRODUCTION AND BACKGROUND The Unites States Pharmacopoeia (USP) serves as book of standards for the protection of the public and has been maintained since 1820 by physicians and pharmacists. compounding and care of the hundreds of medicinal products they handle. Your Doctor relies on them when sickness comes. They are trained in college and by experience to be accurate, and to upon to make in the public welfare entitle them to large measure of the good will of the community they serve.” years — including those for patient safety, medication management, safe handling of hazardous drugs, and compounding, making the pharmacist’s job more complex. And despite an increase in regulations and enforcement, many pharmacies rely primarily on manual processes for everything from I.V. preparation to temperature checks to medication dispensing. At Wolters Kluwer Health, we believe patients deserve the Best Care Everywhere — and that includes anywhere medications are handled or prepared. We aim to simplify the complexities by building software that incorporates not only the minimum regulatory standards, but also the deep domain expertise from our industry, to guide completion of standardized workflows and competencies that deliver consistent compliance habits, excellent outcomes, and peace of mind. USP COMPOUNDING STANDARDS The USP Compounding Standards continue to evolve, with updates to USP <795> can expect increased scrutiny from accrediting and governing agencies such as The Joint Commission, CMS, FDA, and State Boards of Pharmacy that inspect compounding pharmacies for quality control, training, and documentation. As you know, failing an inspection can result in financial and reputational consequences. Annie Lambert, PharmD, BCSCP Clinical Program Manager Simplifi+ Solutions page 89
to Watch as
1220 Graphite Drive, Corona, CA 92881 USA 800-797-1405 sales@iso-med.com www.iso-med.com Product Description Complete filtering of incoming ambient air during use extends sterility. No contamination due to accident or lack of protocol. Compliance with OSHA legal documentation requirements. Compliance with USP 797, NO refilling of bottle. With unique 0.22 micron filter vented sprayer, ISO-MED’s sterile isopropyl alcohol, it is the only product of its kind: 0.22 micron filter vented sprayer. Complete filtering incoming ambient air during use. No contamination due to accident or lack of protocol. Compliance with OSHA legal documentation requirements. Compliance with USP797, no refilling of bottle. Sterile, Pyrogen-free Gamma Sterilized to 10-6 Double-Bagged lot numbers and expiration dates. Certificates of Assurance and Sterility. Product Numbe cription Steril Case Packaging Sterile Call for fast reordering: ISOA16 70% Isopropyl Alcohol steril ISOA16-C 70% Isopropyl 70% Isopropyl 946 mL) trigger spray ISOA128 70% Isopropyl Alcohol steril 1 gallon (3.8 liters) polybottl Isopropyl Alcohol 70% Sterile IPA 12 lybottles 12 lybottles polybottles lybottles 1 uid ounces (473 mL) trigger spray uid ounces (473 mL) flip cap with trigger spra ISO-MED. rights reserved. 800-797-1405 WINTER 2022 I HEALTH SYSTEM • INFUSION 15 page 80-85 Trade Show & Meeting Calendar Thought Leader Video Series page 76-77














storage
optimize your workflow and improve efficiency
creating quality and affordable pharmacy storage solutions for
used
countries. We offer a wide selection of custom-made products including modular storage shelves, security bags, trays, flexible dividers, mobile storage and
Visit us online at www.HHsystem.com or call (800) 477-2123 for more information or a free quote!
Custom-made
solutions to
H+H SYSTEM has been
over 45 years,
in more than 50
more! Created by pharmacists for pharmacists.
ENSURING A HEALTHY WORLD














ACC combines robust reagents, analysis and technical service expertise, to provide you with diverse solutions for endotoxin and glucan testing.


Associates of Cape Cod, Inc. Your Endotoxin & Glucan Experts
Associates of Cape Cod, Inc. - a Seikagaku Group Company
MKT#21-107
OUR TEST, YOUR CURE...
SmartPak® provides a revolutionary


system for these essential drugs, offering faster, easier preparation more economically. It’s also safer. The SmartPak bag system is a convenient alternative to using multiple glass vials. No glass means no breakage during handling. SmartPak saves time and labor with less waste. SmartPak — the smart choice for your pharmacy service.



SMART THINKING. FEATURED PRODUCT Cefazolin 100g
AVAILABLE PRODUCTS Description NDCList# Vancomycin 100 grams 66288-7100-17100 Cefazolin 100 grams 66288-1100-11100 Cefazolin 300 grams 66288-1300-1 1300 Ceftriaxone 100 grams 66288-6100-1 6100 Cefepime 100 grams 66288-8100-18100 www.samsonmt.com Toll Free (877) 418-3600 PO Box 2730, Cherry Hill, NJ 08034 Telephone (856) 751-5051 Fax (856) 751-5044
delivery
Zebra Temperature Monitoring & Sensing Solutions — Monitoring Devices & Integrated Solutions for Medication Shipments, Storage, & Pharmacy Facilities




































Providing the devices and information to accurately monitor temperature sensitive medications.




International Medical Industries — Innovation in Secure Drug Delivery








Increase pharmacy productivity and safety with economical, American-made devices designed to advance the safety and security of your drug products and ultimately your patients.


NOTICE OF POLICY
20Ways is published quarterly by RXinsider LTD, 1300 Division Road, Suite 103, West Warwick, RI 02893. Postmaster: Send address changes to 20Ways/RXinsider, 1300 Division Road, Suite 103, West Warwick, RI 02893. Notification of address change must be made six weeks in advance, including old and new address with zip code.

Editorial – views expressed in articles or profiles in the 20Ways are those of the author(s) and do not necessarily reflect the policies and opinions of RXinsider, our editorial board(s), our advisory board(s), or staff. Advertising – products, services, and educational institutions advertised in 20Ways do not imply endorsement by RXinsider.

Copyright © 2022 RXinsider LTD. All rights reserved. Reproduction without permission is prohibited.
Mobile Pharmacy Compounding Cleanrooms by Modular Devices
Premanufactured mobile cleanroom available in a fraction of the time compared to traditional design/build projects. Fully engineered turn-key mobile cleanrooms designed to provide immediate compliance for USP 797 and USP 800.
Download the 20Ways App
RXinsider.com/20ways
20Ways is an official publication of RXinsider

GENERAL SUBSCRIPTION INQUIRIES
RXinsider LTD c/o 20Ways
1300 Division Road, Suite 103 West Warwick, RI 02893
20Ways Online: www.RXinsider.com/20Ways
General Information: 20Ways@RXinsider.com Circulation: See page 5
IMI designs innovative medical devices that enhance the security of medication from pharmacy to patient. Fifty years of product design exclusively focused on the needs of the compounding pharmacist means exceptional quality, reliability and customer-driven innovation. From Tamper Evident Caps to essential sterile compounding supplies, ensure the integrity and security of your medications with solutions from the leader in secure drug delivery. Over 50 Years Serving The Industry Exceptional Quality & Reliability Enhance Your Drug Security Program Strengthen USP <797> Practices IMIWEB.COM 1.800.344.2554 page 99
Peace of mind for your patients Temperature monitoring for direct-to-patient shipments outgoing medication shipments can help. These inexpensive, singleuse indicators monitor temperature exposures – letting your patients Learn more about how TransTracker cards, manufactured by Temptime, can help you give your patients added peace of mind while helping you save money by avoiding medication replacements. zebra.com MOBILE PHARMACY COMPOUNDING CLEANROOMS Whether you are planning cleanroom renovation or building new cleanroom, Modular Devices’ mobile cleanrooms provides an expedited option that combines modular and prefabricated approach by delivering ready-to-use, turnkey cleanroom that only requires utility connections upon being delivered. Our pharmacy compounding cleanrooms are available to rent or purchase! Experience unmatched quality, state-of-the-art materials your expectations but USP 797/800 as well! » » » » » » » HEPA Filtered Interlocked Passthroughs » » Dedicated Haz and Non-Haz Gowning Rooms USP 800 Negative Pressure Unpack Storage Room Guaranteed Environmental Control Anywhere in U.S. Large Fleet and Nationwide Coverage CALL FOR INFORMATION OR TO REQUEST A QUOTE (317) 489-4616 www.portable-cleanroom.com Full Compliance with USP 797 and USP 800 is one phone call away! Contents page 97 page 101
LTD RXinsider Chairman: Gregory Cianfarani, RPh DESIGN AND PRODUCTION Design & Layout Lora Bourque Multimedia Eric Simmons Marketing and Operations Samantha Roy Alexa DiLuca Kristin Fennessey
PUBLISHER RXinsider,
SALES AND BUSINESS DEVELOPMENT
Chris Kolkhorst, EVP chris.kolkhorst@rxinsider.com Mike Rahme mike.rahme@rxinsider.com Shaun Russell shaun.russell@rxinsider.com Jillian Melly jillian.melly@rxinsider.com Email sales@rxinsider.com Toll-Free Phone 800.972.2083 Fax 646.329.9766 Website www.RXinsider.com
WINTER 2022 I HEALTH SYSTEM • INFUSION 19










THE MOST COMPLIANT AUTOMATED MONITORING SOLUTION Rees Scientific View sensor min/max conditions with the LCD display module Min 2.0 °C Max 8.0 °C Regional Sales and Service teams for superior support Continuous, real time monitoring rate exceeds requirements of CDC Receive alarm noti cation via interactive phone, texts and e-mail Meet compliance for FDA, WHO, USP <797>, USP <800>, VFC, GxP & more Exceeds data logger capabilities Local audio and visual (LED) alarm available Monitor temperature of any cold storage (refrigerators, freezers, ultra-low freezers) from +1300 to -196 °C 609.530.1055 www.reesscienti c.com
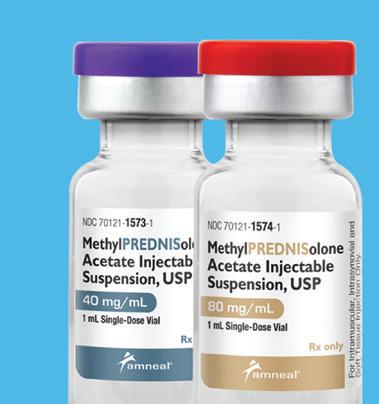
Multi-source Injectable Products from Amneal



















Methylprednisolone Acetate Injectable Suspension, USP Pack NDC Dosage Strength Pack Size 70121-1573-01 40 mg/mL 1 mL Single-Dose Vial 70121-1573-05 40 mg/mL 25 x 1 mL Single-Dose Vials 70121-1574-01 80 mg/mL 1 mL Single-Dose Vial 70121-1574-05 80 mg/mL 25 x 1 mL Single-Dose Vials
At Amneal, we take pride in delivering vital medicines like corticosteroids. Our goal is to get these products into the hands of those who need them most. When they need them most. With over 250 generic products, we provide customers and patients with value, true accessibility, and quality. This is how we make healthy possible. Not made with natural rubber latex
Not made with natural rubber latex Preservative free
Methylprednisolone Sodium Succinate for Injection, USP
Pack NDC Dosage Strength Pack Size
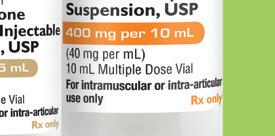
70121-1000-05 40 mg per vial 25 x 1 mL Single-Dose Vials 70121-1001-05 125 mg per vial 25 x 2 mL Single-Dose Vials

Not made with natural rubber latex
Triamcinolone Acetonide Injectable Suspension, USP Pack NDC Dosage Strength Pack Size 70121-1049-02 40 mg/mL 1 x 1 mL Single-Dose Vial 70121-1049-05 40 mg/mL 25 x 1 mL Single-Dose Vials 70121-1168-01 200mg/5 mL (40mg/mL) 1 x 5 mL Multiple-Dose Vials 70121-1169-01 400 mg/10mL (40mg/mL) 1 x 10 mL Multiple-Dose Vials

Available when you need them most amneal.com Order from your wholesaler/distributor or contact Amneal: 866.525.7270 or CustomerRelations@amneal.com Images are for reference only; actual product may vary. © 2022 Amneal Pharmaceuticals LLC. All rights reserved. CORAD-02 05.2022
Our ever-expanding portfolio includes corticosteroids and complex injectables that you can count on.
METHYLPREDNISOLONE ACETATE
TRIAMCINOLONE ACETONIDE
METHYLPREDNISOLONE
SODIUM SUCCINATE

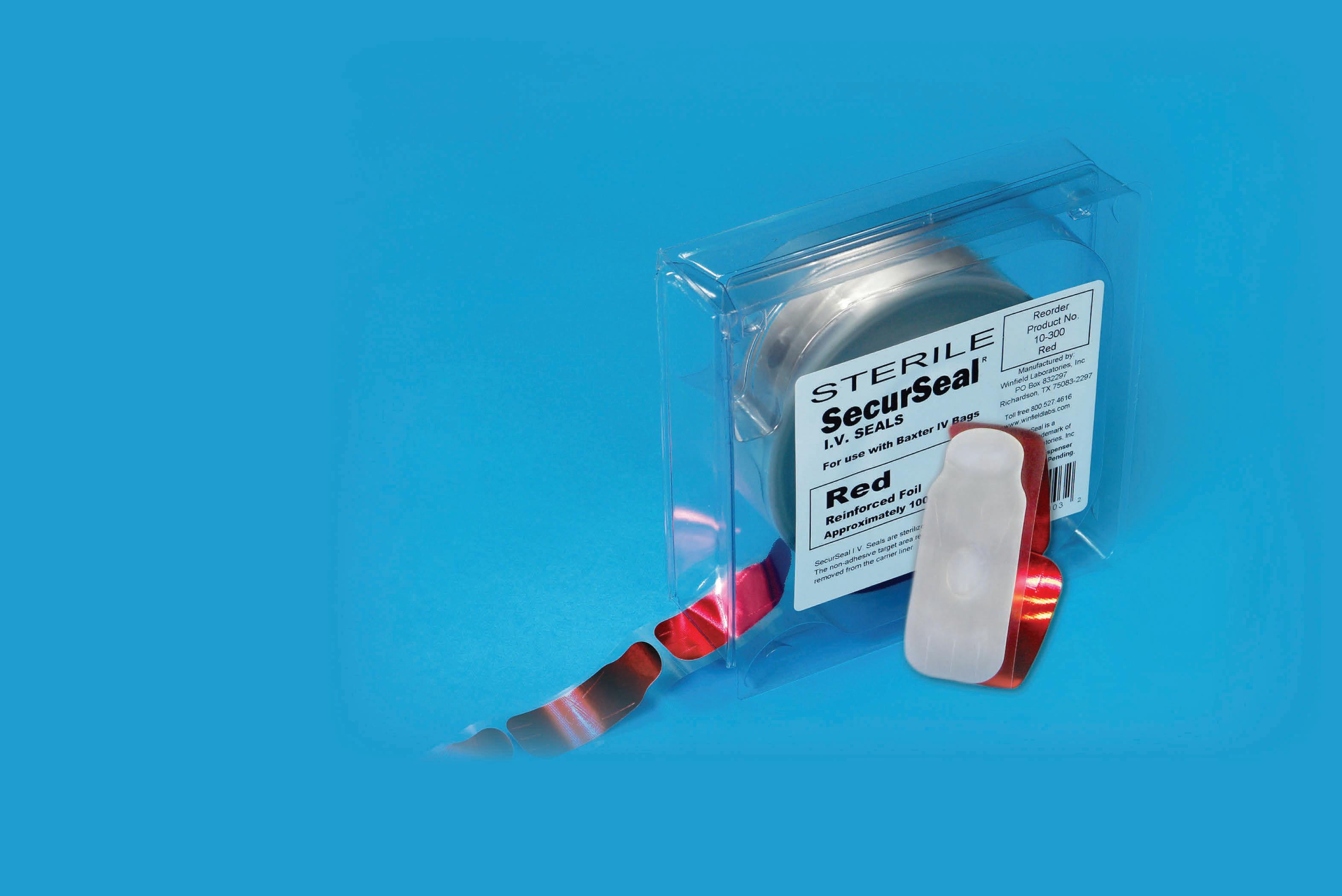



» Adhesive Free Area Eliminates Paper Dots » No Cardboard Insert » Vinyl Label, Not Paper C-it ™ Syringe & Container Seal (You Can See Through It!) Make the Move to Zero Paper IV Seals With SecurSeal ® IV Seals 100% Paper Free Seals, including the dispenser and labeling! ... Because Particulate Matter MATTERS! SM CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEM CHEM CHEM CHEM HEMO HEMO HEMO HEMO HEMO HEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEMO CHEM CHEM CHEM HEMO HEMO CHEMO HEMO CHEMO CHEMO If your wholesaler doesn’t carry SecurSeals®, we will drop ship! New Product! Chemotherapy Seals Complimentary Under the Hood Hanging Racks for our “Zero Paper” Customers! Complimentary Under Hood Hanging Racks & Samples Call (800) 527-4616 or Visit www.winfieldlabs.com Group Contracts Available
USP <800> RESOURCES
Leading Pharmacy Suppliers of USP <800> Compliant Solutions


These leading pharmacy suppliers offer product and service solutions to help your pharmacy and/ or cleanroom achieve USP <800> compliance.
Contact these companies directly for more details on how they can help you meet the mandate of USP <800> compliance. www.RXinsider.com/USP800

© 2022 RXinsider LTD. All rights reserved.
Rees Scientific










Results you can believe in. 1.800.581.1378 | info@verity340b.com | www.verity340b.com © 2022 Verity Solutions Group, Inc. Innovative. Invested. Proven. Partner with us so that you can dedicate fewer resources to your 340B program administration and more time to community wellness. SPLIT BILLING | CONTRACT PHARMACY | VERISAVE TM SPECIALTY CONTRACT PHARMACY | COMPLIANCE MANAGEMENT PURCHASE ANALYTICS | VHUB ® for Contract Pharmacies For all healthcare systems who are challenged with the process of selecting the optimal drug that would reduce spend, Verisave automatically selects the best priced product before your order is placed. With patented technology, Verisave reduces tedious manual processes and dramatically decreases your drug spend.
Verity Solutions — Your Invested Partner for 340B
What Sets Verity Solutions Apart?
Founded: 2015
Employees: 100
Toll-Free Phone: (800) 581-1378 Phone: (425) 947-1922 Address: 12131 113th Avenue NE, #200 Kirkland, WA 98034 Website: www.verity340b.com
Company Background
Verity Solutions is a leader in 340B program administration. Recognized as Best in KLAS: 340B Management Systems for the last five years, our mission is to make every aspect of 340B program management clear and easy to understand. We believe in optimizing program benefits through predictable automation and outstanding support so that every customer can maximize their savings. We partner with integrated healthcare systems, acute-care hospitals, community health centers, federally qualified health centers, pharmacies, and other 340B-eligible covered entities throughout the U.S. who rely on Verity 340B® software and services to successfully manage their 340B program.
Product Overview
Our powerful V340B® cloud-based software platform provides comprehensive solutions for split billing, contract pharmacy, specialty contract pharmacy, compliance management, purchase analytics, and pharmacy network management (VHUB®). Our innovative new product, VERISAVE™, automatically selects the best blended priced products available for your complete order before it is submitted, reducing tedious manual processes and dramatically decreasing your drug spend.
Our depth of in-house technical and software development resources and our highly skilled account management team are closely aligned to swiftly adapt as changes arise in the 340B program. We offer:
• Agile Software Platform: Built and deployed with security, performance, scalability, and agility as primary goals.
The Verity 340B platform is HITRUST certified, demonstrating robust HIPAA compliance.
• Intuitive Application: Designed with our users in mind, we maintain ongoing feedback and collaboration with our clients. This collaboration steers our continual software and services development.
• Responsive Support: Designated account managers provide focused support, training, audit readiness, and regular business reviews to maximize your 340B program success and help you maintain compliance.
We continually invest in our technology and people to ensure 340B program success for our clients. With increasing 340B regulatory complexity and demand for audit preparedness, it's more important than ever to have the right solution for your 340B program management — and the right partner.
n Highlights
• Industry leading core functionality of our V340B® platform with optional, patent-pending add-on modules to enhance all aspects of 340B operations in challenging and unique environments.
• Rapid 120-day average implementation time frame for both split billing and contract pharmacy solutions upon receipt of compliant dataset.
• Access to your own unique 340B program test environment — before and after implementation. Test environment runs continually in parallel to your live system.
• Easy and exportable reporting functionality including detailed data for manufacturer audits, HRSA audits, and UDS reporting.
• New — Advanced Reporting Insights gives users rich data visualization, interactive reports, and performance trends to help drive strategic change.
• Transition from one electronic medical record (EMR) to another without downtime (contingent upon receipt of dispense file from the covered entity).
• Multiple vendor support with controlled substances ordering system (CSOS) — efficiently place orders with both EDI (electronic data interchange) and non-EDI vendors.
• Two-week sprint release cycles ensure timely software updates driven by customer feedback, regulatory changes, and user needs.
• Flexible and “winners only” contract pharmacy pricing models. No true-ups.
• Verity Care Card Program — directly pass 340B savings to uninsured and underinsured patients.
• Referral capture opt-in functionality to compliantly add meaningful savings lift to your 340B program.
• Responsive customer service provided by our in-house staff and the ability to submit and track issues in our online customer portal.
• 80% of our customer support cases are resolved within two hours. 95% are resolved within 24 hours.
WINTER 2022 I HEALTH SYSTEM • INFUSION 27 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
CEO: George Puckett
Partner with us to simplify 340B administration, confidently optimize federal pricing benefits, and benefit from the dedication and knowledge of our expert team.
COMPOUNDING HEALTH™ www.leiters.com | 800.292.6772 Helping you deliver better medicine to more people. With increasing regulatory pressure and drug shortages, access to quality medicine is more important than ever. Leiters is an FDA-registered 503B outsourcing provider of high-quality, compounded sterile preparations including: Pre-filled syringes, IV bags and vials ON-Q* Pain Relief System fill services Opioid-free surgical pain services medications Ophthalmology medications and services including FDA-compliant repackaged Avastin® ON-Q* is a registered trademark of Avanos Medical, Inc., or its affiliates. Avastin® is a registered trademark of Genentech, Inc.
Leiters. We Are Compounding Health™
Additional Information
Founded: 1926
Employees: 215
Toll-Free Phone: (800) 292-6772
Phone: (720) 697-5140 Fax: (408) 288-8252
Address: 13796 Compark Boulevard, Englewood, CO 80112 Website: www.leiters.com
Company Background
Leiters, founded in 1926, is an FDA-registered 503B outsourcing provider of high-quality compounded sterile preparations and pharmacy services. Leiters has a long history of evolving and innovating to meet the latest regulatory requirements and market needs.

All sterile preparations are produced under Section 503B of the FD&C Act (503B Guidance), follow current good manufacturing practices (cGMP) and USP <797>. The Leiters facility consistently upholds all standards based on the audits conducted by the FDA, states of California and Florida Boards of Pharmacy, multiple health systems, group purchasing organizations, and other independent accreditation organizations.
The Leiters team of experts in sterile pharmaceutical manufacturing, repackaging, and compounding provide a sophisticated understanding of what it takes to elevate the quality and consistency of supply in pharmaceutical outsourcing. Leiters combines a highly experienced team, with robust processes, in a new state-of-the-art outsourcing facility to ensure delivery of the highest-quality products and services.
Product Overview
Leiters provides ready-to-use compounded sterile preparations and pharmacy services across the continuum of healthcare, including hospitals, surgery centers, physician offices, and clinics.
n Hospital Products
• Prefilled operating room syringes and I.V. bags.
• PCA (patient-controlled analgesia) prefilled syringes.
• Surgical Pain Services portfolio including opioid free medications.
n Ophthalmology Products
• Prefilled syringes, vials, and dropper bottles including: injections, antibiotics, dilating agents, and topical anesthetics.
• FDA-Compliant† Repackaged Avastin® Service.
n ON-Q* Pump Fill Service
• Pharmacy fill service for the Avanos Medical opioid sparing ON-Q* Pain Relief System.
• Prefill pump services offered for both Bupivacaine HCl and Ropivacaine HCl.
We combine our team, robust processes, and state-of-the-art outsourcing facility to ensure the highest-quality medications for healthcare professionals and their patients. Through three key pillars: people, place, and product, we are elevating the standards in pharmaceutical outsourcing.
Key Business Partners
Leiters is partnered with many market leading innovative healthcare companies that compliment what we do. The products and services offered by these companies may provide additional value to your organization. Our business partners include: Avanos Medical, Besse Medical, Cardinal Health OptiFreight®, CPS Solutions, LLC, Eye Connect International, Hibernation Therapeutics, Kit Check, and Prodigy Health.
Trade Shows/Meetings Attended
Leiters supports and attends various regional and national industry trade shows and conferences. Please visit www.leiters.com to view a list of 2022 events we will attend.
GPO Affiliations
Leiters provides its products and services to many leading health systems, community hospitals, clinics, and physician offices. In addition, we currently have national contracts with: HealthTrust, Intalere, JDJ Consulting, Kaiser, Premier, The Resource Group, US Retina, and Vizient. Please contact us for additional information regarding our contracts.
Ordering Information
Leiters offers four convenient ways to place your order.
1. Toll-Free Phone: (800) 292-6772
2. Fax: (408) 288-8252
3. Email: orders@leiters.com
4. Visit the Leiters Online Ordering Portal: orders.leiters.com
Site Visits
We don’t want to simply tell you about what we do, we want to show you! We invite you to visit our facility to better understand the cGMP regulations, sterile manufacturing processes, and automation we use to elevate the quality of our products and services. Come join the growing list of organizations who have visited our facilities. To schedule a site visit or learn more about how Leiters is Compounding Health™, please visit www.leiters.com.
COMPOUNDING HEALTH™ is a registered trademark of Leiters. Avastin® is a registered trademark of Genentech, Inc. ON-Q* is a registered trademark of Avanos Medical, Inc., or its affiliates. 1BUD is from date compounded. † Mixing, Diluting, or Repackaging Biological Products Outside the Scope of an Approved Biologics License Application Guidance for Industry https://www.fda.gov/downloads/drugs/guidances/ucm434176.pdf
WINTER 2022 I HEALTH SYSTEM • INFUSION 29 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
President & CEO: Robin Smith Hoke
Leiters is a trusted FDA-registered 503B outsourcing provider of compounded sterile preparations committed to providing healthcare professionals and their patients with the highest-quality medications.




customized software turnkey hardware innovative training 24/7 US-based support www.rm-solutions.com MEET YOUR NEXT STAR EMPLOYEE.
Point-of-Sale to Simplify Your Pharmacy Operations — Retail Management Solutions
President & CEO: Brad Jones
Founded: 1998
Toll-Free Phone: (877) 767-1060 Phone: (360) 438-8276
Fax: (360) 438-8284
Address: 4535 Lacey Boulevard SE Lacey, WA 98503 Website: www.rm-solutions.com
Company Background
Founded in 1998 with a mission to help pharmacies understand and use technology-based tools to run a successful business, Retail Management Solutions continues to lead point-of-sale innovation. Giving pharmacies access to tools and technology to improve patient outcomes, streamline operations, and improve profits.
At RMS, we believe that point-of-sale should simplify pharmacy operations and management all while improving the patient experience. Our POS products are designed exclusively for pharmacies with flexibility, ease of use, and scalability in mind.
Product Overview
RMS’ solutions create a more customer-centric patient experience and reduce employee workload through many different avenues.
n At the Register: RMS customers have freedom to choose from over 30 pharmacy system partners, all with integrations that make prescription sales fast, easy, and accurate. Signatures for HIPAA, safety caps, prescription acknowledgment, etc. are captured at the time of sale and are always easily accessible when needed for reporting and audits. Built-in date of birth verification processes and additional prescription for pickup notifications bring added patient safety and convenience to every transaction.
Additionally, every RMS system includes built-in will-call management. Batch multiple prescriptions together into a single bag. Store according to your organizational preferences. Scan a single barcode at checkout. This easy-to-use option improves patient safety and saves time.
Beyond prescription sales, OTC product sales are as simple as scanning the item barcode. Custom items can also be created for easy sale of unique products, gift items, etc. Payroll deduct can be added to any implementation for easy employee sales.
Sales can be processed in many different ways to meet the unique needs of your pharmacy. Whether you need traditional retail lanes, Medsto-Beds technology, an option for drive-thru payments and signatures, curbside pickup solutions, or an easy way to track deliveries. You can mix and match to create the perfect technology scheme.
n Beyond the Basics: Improving patient outcomes and increasing customer satisfaction are goals at the heart of most organizations today.
n Meds-to-Beds: Technology and support help you to align your pharmacy with organizational goals. Point-of-sale tailored for bedside interactions and full transaction processing help you work towards increasing patient compliance and reducing readmittance rates as a result.
n Nutrient Depletion: Notifications at the register help you to improve patient wellness by making supplement recommendations based on prescription induced nutrient depletion.
n Easy Compliance: RMS' credit card processing capabilities include options for validated P2PE, allowing the potential for reduced PCI scope. Processing integrations also include options for EMV, tokenization, NFC, and FSA/HSA card acceptance. Every RMS system includes optional integration with NPLEx for Pseudoephedrine tracking as well as signatures, ID capture, and tracking for HIPAA, safety caps, prescription acknowledgment, etc.
n Headquarters and Management: Point-of-sale applications extend beyond the customer interaction to help you run your pharmacies more efficiently. Enterprise-level multi-location management unlocks powerful tools for policy, pricing, employee, and customer management. Data fed back to the headquarters saves time and resources.
For pharmacies managing retail departments, RMS’ inventory management solutions open the door for advanced management of front-end product with wholesaler interfaces, automated purchasing and receiving, price updates, and integrated signs and labels.

A host of reports, both canned and customizable, are available. From cash management and employee performance to A/R balances, and more. RMS systems give you the data you need to drive important decisions and run pharmacies efficiently.
n When You Need Support: RMS’ solutions are more than point-of-sale software and hardware. You won’t have to hire a team to manage your RMS system. All RMS customers are supported by a team of U.S.-based technical specialists that are available 24 hours a day, seven days a week. Certified trainers help you implement your solution in a timely manner that meets the requirements of your organization. Every RMS customer is also assigned a dedicated customer success manager to help your team stay on track with organizational goals following implementation. The RMS customer center is available at any time to request support, access the customer knowledgebase, and more.
Trade Shows/Meetings Attended
Experience RMS in person this year at one of our many pharmacy industry events. Find out where we’ll be next at www.rm-solutions.com/events.
WINTER 2022 I HEALTH SYSTEM • INFUSION 31 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
Pharmacy point-of-sale should work for you, not the other way around. Meet the solution that helps you improve patient outcomes, streamline operations, and use your choice of pharmacy management systems. All while enjoying 24/7 customer support.





© 2022 Helmer Inc. All rights reserved.
Solutions Medical-grade Cold Storage Pass-thru Refrigerators Access from both sides of the unit for more convenient access to stored products and enhanced work flows i.Lock™ interlock door technology available to meet USP
clean room requirements
testing
provide optimal storage of ambient product loads Best-in-class temperature management of uniformity, stability, and recovery ENERGY STAR® certified and up to 50% more energy efficient than conventional medical-grade refrigerators and freezers Workflow efficiency and regulatory compliance for clinical pharmacies Learn More helmerinc.com/gx-solutions
GX
<797>
Extensive
to
GX Solutions Pass-Thru Refrigerators Offer Clinical Pharmacy Workflow Efficiency
Founded: 1977
Employees: 500
Toll-Free Phone: (800) 743-5637
Phone: (317) 773-9073
Address: 14400 Bergen Boulevard, Noblesville, IN 46060 Website: www.helmerinc.com
Company Background
Helmer Scientific is a U.S.-based manufacturer and worldwide distributor of medical-grade cold storage and laboratory processing equipment. We have over 45 years of experience in providing high-quality temperaturecontrolled environments, with our products used in over 125 countries throughout the world. Precise temperature performance and control are essential to the successful storage of pharmaceuticals and Helmer cold storage products have been designed and developed with these principles.
Product Overview
Pass-thru refrigerators play a key role in providing workflow efficiency and regulatory compliance in clinical pharmacies. With staff shortages and supply chain issues creating challenges across the healthcare continuum, it’s critical to use effective cold storage units that support productivity while still ensuring valuable medications, vaccines, patient samples, and lab media are protected.
Best practices recommend using purpose-built or pharmacy-grade units with forced-air circulation, microprocessor-based controls, and alarms, as well as necessary regulatory and compliance requirements based on application.
GX Solutions Pass-Thru Refrigerators are the only medical-grade pass-thru refrigerators that include innovative technology to support environmental sustainability initiatives and evolving regulatory requirements while maintaining the unique temperature performance requirements required in pass-thru refrigerator applications to reduce the risk of damaging valuable medications and vaccines.
GX Solutions support the clinical pharmacy with enhanced workflow efficiency, allowing access from both sides of the unit for more convenient access to stored products. This is particularly useful when increased efficiency is desired when transferring contents between locations.
GX Solutions Pass-Thru Refrigerators are specifically designed to provide safe and effective storage in three ways:
n OptiCool™ Refrigeration Technology
GX Solutions are powered by OptiCool technology which pairs a variable capacity compressor (VCC) and natural refrigerants to:
• Ensure optimal temperature uniformity, recovery, and stability.
Temperature is maintained within +/-1° C throughout the unit, quickly recovers after prolonged door openings, and creates fewer deviations from the set point avoiding rapid, significant changes in temperature which could put items stored at risk of temperature excursion.
• Efficiently manage energy consumption (up to 50% reduction over traditional medical-grade pass-thru units).
• Reduce noise output from the system (up to three times quieter than traditional medical-grade pass-thru units).
• Meet SNAP, GWP, and EU F-Gas initiatives for sustainability.
n Extended Testing Protocols
In addition to Accelerated Life Testing, GX Solutions Pass-Thru Refrigerators also undergo heavy-use testing to ensure they meet the rigorous daily use cases in the pharmacy. This includes testing ambient temperature loads, frequent door openings, and extended door openings to ensure temperature performance is not compromised while avoiding nuisance alarms.
n USP <797> Sterile Compounding Pharmacy Support
GX Solutions Pass-Thru Refrigerators support compliance requirements related to proposed changes to USP General Chapter <797>.
• Pass-thru refrigerators used in cleanroom applications must be able to prevent cleanroom-side and anteroom-side doors from being opened simultaneously.
GX Solutions Pass-Thru Refrigerators are the only pass-thru refrigerators available with optional iLock™ Interlock Door Technology which electronically locks doors on one side of the unit when the other side is opened. This is especially useful when placed between a cleanroom and an anteroom, limiting access to sterile environments.
Professional, Medical-Grade Performance
• Best-in-class temperature management, including uniformity, recovery, and stability, to safeguard medications and vaccines and supply confidence that contents are stored at the precise temperature regardless of where they are placed within the unit.
• Only pass-thru refrigerator with available electronic interlocking doors to meet proposed USP <797> requirements.
• Avoids rapid, significant changes in temperature to ensure that medications and vaccines are always in an optimal storage environment.
• Designed for noise-sensitive areas such as retail and specialty pharmacies, GX Professional Medical-Grade Refrigerators are quieter than traditional models leading to fewer distractions for healthcare personnel.
• Energy management reduces operating costs and supports sustainability initiatives by utilizing a variable capacity compressor (VCC) system and natural refrigerants to create a highly efficient cooling system.
• i.C3® Information Center provides constant temperature monitoring, multiple information logs which can be exported for regulatory compliance, and security features to protect crucial refrigerator settings.
WINTER 2022 I HEALTH SYSTEM • INFUSION 33 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
President: Bruce King Vice President of Sales & Marketing: Lori Gabrek
Helmer Scientific offers the only medical-grade pass-thru refrigerator that meets the proposed changes to USP <797> for use in cleanroom applications while ensuring the safe storage and efficacy of temperature-sensitive medications and vaccines.
CASE STUDY
STRATEGIC PARTNERSHIPS DRIVE INPATIENT AND OUTPATIENT PHARMACY PERFORMANCE
A DECADES-LONG PARTNERSHIP
Holzer Health System traces its roots back more than a century to when Dr. Charles E. Holzer Sr. opened the first private hospital in southeastern Ohio. Located in Gallipolis, that small, seven-bed hospital has grown into the multi-facility healthcare system it is today.
Todd Fowler Executive Vice President and Chief Operating Officer

~
 Holzer Health System
Holzer Health System
This includes a medical center and care center in Gallipolis, a medical center in Jackson, and numerous walk-in clinics, retail pharmacies, wellness centers, and post-acute care services, among other resources, across the region.

Holzer has remained true to the philosophy set forth by their founder — “The Patient is at the Center of All We Do.” It is this outlook that led Executive Vice President and Chief Operating Officer for Holzer, Todd Fowler, to continue building on the foundation of their fruitful partnership with CPS for inpatient and specialty pharmacy management services.
Over the 20+ year relationship, CPS has consistently driven value for Holzer through initiatives supporting clinical performance, cost savings, and operational expertise.
A RELATIONSHIP THAT CONTINUES TO EVOLVE
The partnership between Holzer and CPS, one of the country's largest pharmacy and hospital services providers, reaches back to 1998 when CPS began helping the health system manage its inpatient pharmacy operations. As the system has grown, CPS has continued to deliver incremental value to Holzer’s leadership and staff. Based on these successes, when Holzer chose to streamline their three retail pharmacies in Gallipolis, Jackson, and Athens in 2014, they turned again to CPS as a partner.
Everything CPS brings to Holzer is focused on growing the impact of their partnership while providing ongoing positive financial and quality outcomes for the health system. One way the company drives value on a continual basis is through its Comprehensive Pharmacy Assessment (CPA), a proprietary tool that audits over 450 points of a pharmacy's operational health. During the company’s most recent analysis, they found Holzer had successfully improved compliance metrics, decreased regulatory risk, and increased alignment with ISMP best practices. Through the CPA, CPS helps the health system maintain visibility to the performance of its pharmacy in accordance with current
34
www.cps.com
CASE STUDY
recommended best practice. CPS supported the implementation of a conversion program that replaces FDA-approved biologics with biosimilars expected to yield over $386,000 in savings annually. Through additional CPS supported operational and clinical initiatives Holzer realized a net financial impact of over $10 million in their last fiscal year.
The health system continues to get positive feedback from both internal and external stakeholders. In 2021, pharmacy staff scored 4.21 out of 5 in the Annual Nurse Survey.
CPS provides staff with continuing education and competency courses to help Holzer pharmacists and technicians build on their knowledge. In 2021, the company delivered more than 180 courses and continuing education credits.
The effectiveness of the partnership has also helped Holzer strengthen its position as a resource in the local community and raise its visibility among other health systems in the region, notes Fowler. “When staff from a large regional cancer center were onsite doing an assessment of our cancer services as we work toward an affiliation, they were very complimentary of our pharmacy — even calling our pharmacist a rock star," he says.
Todd Fowler
Executive Vice President and Chief Operating Officer
~ Holzer Health System
“That speaks volumes not only to what Holzer does but also the value CPS adds in helping us deliver excellent patient care to a county of 30,000 people.”
Part of that has to do with CPS' approach that leverages its collaborative network of pharmacy professionals. “We have a CPS pharmacy director who oversees all Holzer's pharmacy services,” Fowler says. The pharmacy director visits each site weekly and focuses on creating a supportive and cooperative atmosphere. “So even though we have different types of operations, we're not siloed — we're one team.”
“I'm proud that the collaborative efforts between CPS and Holzer result in better care for my community and neighbors which I believe is something we all can count as success,” reflects, CPS Pharmacy Director, Neil Creasey.
HOLZER'S PATH TO SPECIALTY PHARMACY
~ Holzer Health System
The idea for establishing a specialty pharmacy came about like many of Holzer's pharmacy decisions — following a conversation with CPS. “Several of our cancer center patients need infusion therapies,” explains Fowler. “We had limited access to some medications, and those we did have access to were cost prohibitive.” As a result, patients often had to travel up to two hours away for therapies that might need to be administered a few times a week. Fowler asked their onsite system director of pharmacy services from CPS whether they would have better and more affordable access to these types of medications if Holzer had a specialty pharmacy. The answer was yes. “I learned we'd be able to get what we needed at a pay structure that fit into our budget,” he says. “I knew then it was something I wanted to explore so we could continue expanding our ability to provide care to our patients."
Fowler says he knew CPS could help make it happen. That's because theirs has evolved into far more than a vendor-customer relationship. “We've come to consider CPS a strategic partner — and I saw establishing a specialty pharmacy as a strategic step,” he explains.
1 Cost savings are calculated by CPS analysts and pharmacy purchasing best practices that compare CPS spend against benchmarks based on assumptions of what pharmacy spend would be without the strategic partnership in place.

www.cps.com
“ If you want to maximize opportunities in the pharmacy — it's critical to have a true strategic partner on your side, and CPS has proven to me over and over again that they are that partner.”
“When I began to think seriously about establishing a specialty pharmacy, I knew CPS could help us make it happen.”
Todd Fowler Executive Vice President and Chief Operating Officer
35
CASE STUDY
Holzer Specialty Pharmacy opened its doors in February of 2022 – and although still in the early stages, progress has been encouraging. In its first six months, the specialty pharmacy dispensed more than 300 prescriptions and contributed $1 million in new revenue.
“I'm impressed by the number of prescriptions we've dispensed, but also the volume of calls being handled,” says Fowler. One of the things that stands out for him is how involved the specialty pharmacy team is with patients. That's critical as many of the medications require a lot of education and support to ensure proper use. More than that, CPS tracks incoming calls in very much the same way as Holzer's centralized scheduling center, a system that has been nationally recognized. “Like us, they pay close attention to wait times and abandoned call rates — and their statistics are very good.” Calls are answered on average within 8.5 seconds and abandoned calls are less than 1%.
Another area where CPS and Holzer are aligned is in their hiring practices. “When they brought on someone to manage our specialty pharmacy operation, they made sure it was someone local — a person who already knows the culture and community of rural southern Ohio. That's important,” says Fowler. As a result, he adds, the relationships pharmacy and hospital staff have with CPS managers are so seamless — many don't even realize they are not Holzer employees. “That says something about how much a part of our team CPS is.”
LOOKING AHEAD TO
2023 AND BEYOND
Launching the specialty pharmacy and completing accreditations would have taken significantly longer without the efficiency CPS’ range of expertise brings. The same is true for the collaborative practice agreements they’ve been able to establish for ambulatory care, compliance reviews, and auditing for retail locations. That’s why Holzer plans to build on their relationship with the company and explore new opportunities to help them fulfill their longer-term strategy — to focus on quality and patient outcomes. “We have a relatively large outpatient physician practice covering five counties and 15 sites — and we want to optimize how we use our pharmacy services in the clinical settings,” says Fowler. That's why for 2023 and beyond, they are looking at the possibility of implementing new solutions such as telepharmacy and medication therapy management. They're also looking for ways to augment their oncology services with tactics like expanding biosimilar integration and optimize outpatient infusion revenue.
"The strategy and direction of pharmaceutical services are so specialized,” says Fowler. “If you want to maximize opportunities in the pharmacy — it's critical to have a true strategic partner on your side, and CPS has proven to me over and over again that they are that partner," says Fowler. "They know what's happening in the industry and bring ideas to us that meet our strategic vision. Then together, we decide what makes the most sense for Holzer."

www.cps.com
MORE THAN 300 $1 MILLION In NEW Revenue PRESCRIPTIONS CONTR IBUTED DISTR I BUTED 36
Partnerships to Drive Pharmacy Performance Excellence
CPS partners with healthcare leaders to improve inpatient and outpatient pharmacy capabilities and drive financial, operational, and clinical performance. We empower healthcare organizations to advance their standard or care through collaboration:

• Implementing best practices and proprietary technologies to drive value and enhance the patient experience
• Leveraging a deep bench of subject-matter experts
• Continually improving the way healthcare is delivered
• Driving performance to reduce costs, increase revenues, and elevate the status of internal leaders and teams
Let’s discuss opportunities to improve the financial and clinical performance of your pharmacy.
Management TELEpharmacy Specialty &
Pharmacy
Consulting 340B
Solutions PT, OT, ST Rehabilitation
Pharmacy
Ambulatory
Pharmacy
Program
800.968.6962 • contactus@cps.com • cps.com Supply Chain & Materials Management
Therapy Management Software
Specialty

















Meeting Your USP <797> Requirements Veltek Makes it Simple CLEANINGAND DISINFECTING PRODUCTS CLEANINGAND DISINFECTING PRODUCTS ENVIRONMENTAL MONITORING E ENVIRONMENTAL MONITORING E GOWNING GOWNING Veltek Associates, Inc. 15 Lee Boulevard Malvern, PA 19355-1234 1-888-4-STERILE www.sterile.com VAI covers every aspect necessary for full compliance including: ■ Consultation on setting up and maintaining aseptic processes ■ SimpleMix® – our easy to use disinfectants in pre-measured containers ■ Easy2Gown sterile garment system ■ Easy to use portable SMA air samplers ■ RTU Sterile & Non-Sterile cleaners & disinfectants available in a large assortment of packaging sizes With VAI’s innovative product line, Meeting USP<797>requirements is just a phone call away…IT’S THAT SIMPLE! Meeting Your USP <797> Requirements Veltek Makes It Simple CLEANING AND DISINFECTING Saturated WiperS GOWNING USP 800 Gown Cleanroom Garments VAI covers every aspect necessary for full compliance including: Consultation on setting up and maintaining aseptic processes SimpleMix® – Veltek’s easy to use disinfectants in pre-measured RTU containers Easy2Gown sterile garment system Ready-to-use and saturated Process2Wipe® IPA70 wipes Sterile cleaners & disinfectants available in a large assortment of packaging sizes • • • • • SCAN HERE TO CONTACT US HEALTHCARE @STERILE.COM
USP <797> & <800> Solutions
From Veltek Associates, Inc.
Combining experience, innovation, performance, GMP manufacturing, GLP testing services, and unrelenting service has propelled VAI® as the ultimate innovative leader in the market.

President & CEO: Arthur L. Vellutato Jr.
Founded: 1981
Employees: 200+
Phone: (610) 644-8335
Address: 15 Lee Boulevard, Malvern, PA 19355
Website: sterile.com
Company Background
For nearly 40 years, Veltek Associates, Inc. (VAI®) has pioneered the design and manufacture of hundreds of cleanroom solutions that surround contamination control. These innovations, many of them landmarks in the industry’s history, allow our customers to overcome challenges, and reach their business goals. Plus, VAI clients have more than a solutions provider, they have a partner and trusted advisor. With today’s complex healthcare challenges, and increasing regulations, a true partnership is more important than ever.
For us, it’s simple. Innovation is about listening to industry challenges directly from our customers and not stopping until we find the answer. Together with our clients, we have been developing new solutions for the cleanroom industry for more than 40 years attaining over 150 worldwide patents. Our innovations have allowed our clients to do remarkable things — from biotechnology breakthroughs to pharmaceutical discoveries — that help millions of people every day. From our early days of developing the first sterile garments to our latest innovations, VAI develops products that revolutionize and simplify cleanroom operations.
Product Overview
VAI’s Sterile Chemical Manufacturing Division (SCMD) has addressed the needs of the pharmaceutical, biotechnology, and healthcare industries by designing a complete range of sanitizers, sterile disinfectants, sporicides, lubricants, cleaners, and process cleaners for cleanroom environments. VAI’s SCMD products are used at hundreds of healthcare facilities, pharmaceutical, and biotechnology organizations worldwide. SCMD manufactures a complete range of cleaning agents and disinfectants that are used daily in cleanroom operations. VAI capabilities for manufacturing products include the ability to fill aerosol, bulk, and unidose packages in a classified filling operation. Our filling operations are coupled with the validated and proven ability to irradiate a final product. Assurances are taken in every aspect of chemical manufacturing concerning sterility and particulate removal. Our chemical manufacturing operations mirror current GMP’s and enforces the adherence to USP methods and specifications for testing of all manufactured products. VAI is an EPA and FDA registered facility and possesses worldwide registrations.
SCMD has taken another advancing step in product quality assurance by incorporating USP Water for Injection (WFI) into the majority of our products. The validated WFI systems in our chemical manufacturing facility incorporate an added advantage to the use of our products. The mission of VAI’s SCMD is to manufacture top-of-the-line quality products that address any regulatory requirements demanded.
SCMD has chemical manufacturing capabilities to produce both VAI products and contract custom manufacturing designs. VAI’s SCMD uncompromising cGMP manufacturing style and our complete adherence to USP specifications has assured outside organizations that their products will not only be produced and tested as sterile, but moreover, their product will be completely documented and validated.
VAI’s Disposable Products Manufacturing Division (DPMD) DPMD has addressed the needs of the healthcare, pharmaceutical, biotechnology, semi-conductor, medical device, and electronics industries by designing a complete range of sterile and non-sterile disposable garments. Product lines include: sterile disposable garments, sterile and non-sterile face masks, and non-sterile cleanroom apparel.
Disposable garments are packaged in VAI’s, patented, Easy2Gown fold system. The Easy2Gown design is a fold that makes a proper aseptic gowning procedure an easy process instead of a routine challenge. Additionally, VAI offers barrier gowns for use where handling hazardous drugs or chemicals, where exposure to bloodborne pathogens, or where liquid splashes are a concern. USP <800> gowns have been designed and tested specifically for compliance with the USP <800> guidance for handling of hazardous drugs.
VAI offers four face masks with varying levels of barrier capabilities for use in clean and critical environments. VAI’s PF-2 face masks are made of 100% rayon. The PF-2 face masks allows for excellent breathability, comfort, and protection, while maintaining filtration efficiency. VAI’s PF-4 face masks are made up of three layers of non-woven material that includes a soft layer to prevent skin irritation or allergy problems. VAI’s FaceVector masks are made up of three layers of SBPP materials which has soft layers that help prevent skin irritation or allergy problems. Finally, VAI offers a National Institute for Occupational Safety and Health (NIOSH) approved N95 filtering face mask. These N95 masks have a 95% filter efficiency level effective against particulate aerosols free of oil.
VAI offers a complete line of wipers, using various clean substrates and packaging to meet the needs of our diverse types of customers. Our wipers come in multiple chemical saturations, sizes, absorbency, and material. Our wipers are sterile or non-sterile and either saturated or dry. The sterile versions are sterility assured through gamma irradiation at a 10-6 SAL or through aseptic filling. All materials are quality assurance tested and released to specifications defined by IEST and ASTM test methods. Our wipers can be used in both aseptic and non-aseptic wipe downs of filling and packaging machinery, stainless steel, Lexan, polycarbonate, glass, and any critical surface that requires cleaning.
WINTER 2022 I HEALTH SYSTEM • INFUSION 39 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
Left: GM7030 PATT2®, Personal Aseptic Technique test kit, 3mL ampules, 20mL vials, 100mL partially lled minibag. Below: ET1000 EnviroTest® TSA with Lecithin & Tween 80 growth media paddles for surface, air, or glove ngertip sampling.





















Qimedical.com info@qimedical.com Tel. 800.837.8361 Media Fill Hazardous Drug Handling Validation Surface and Fingertip Testing Sterility Testing USP <797> USP <71> USP <800> Your partner in pharmacy compliance since 1992 FDA Registered | Iso Certified
Q.I. Medical, Inc.™ Your Partner in USP <797> Pharmacy Compliance

Providing quality assurance products to hospitals and pharmacies practicing sterile compounding, with a singular focus on pharmacy compliance. Our long-standing regional distribution partners are able to provide exceptional in-person service and training.
Additional Product Lines
Founded: 1992
Toll-Free Phone: (800) 837-8361 Phone: (530) 272-8700
Fax: (530) 272-8702
Address: 1415 Whispering Pines Lane, Suite 150 Grass Valley, CA 95945 Website: www.qimedical.com
Company Background
Q.I. Medical, Inc. was founded in 1992 with a focus on providing the sterile compounding industry with testing kits and products to assure proper technique and quality is maintained in their facility. We have a broad network of distribution partners throughout North America, Canada, and other international markets. These distribution partners provide in-person servicing and education to accounts to comply with USP guidelines.
Product Overview
Our cost-effective, disposable products are available for various needs in the compounding pharmacy. Aseptic technique validation kits are available for low, medium, and high-risk settings. We also sell multiple à la carte sizes of vials, bags, tubes, and syringes of growth media for facilities looking to create a custom media fill test that mimics their day-to-day practice. In regard to environmental monitoring/gloved fingertip sampling, our EnviroTest™ paddle is ideal for both. The rectangle shape and built in hinge allow for testing critical areas such as edges and corners of a hood and behind latch handles. Sterility testing products are available in both USP 71 approved methods of testing. For the full filtration method, the QT Micro™ and QT Junior™ systems work for both small and large volume solutions. For the direct inoculation methods, our TuffTest 2™ product is ideal.
All Q.I. Medical growth media is challenged with a battery of USP specified organisms and lot specific Certificate of Analysis (CofA) are available for download.
All products are sold through regional stocking distributors in order to provide fast local service and support.
Additional products are available for validation of hazardous drug handling, automated compounder manipulation validation, filter integrity testing, and vial adaptors.

n Support Equipment Includes:
• Incubators
• Sterile Filters
• Vial Blocks
• UV Lights
Ordering Information
To learn more about how Q.I. Medical, Inc. can help your facility, please contact us at (800) 837-8361 or email info@qimedical.com.
WINTER 2022 I HEALTH SYSTEM • INFUSION 41 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
President & CEO: Brady K. Schwarz
CASE STUDY
DEFYING DRUG DIVERSION
The Pharmacy Executive, Nurse Executive, and Drug Diversion Analyst Tell How
• Receive insights from Senior Vice President of Allied Health, Brian O'Neal, PharmD, Children's Mercy Kansas City.
• Understand how nursing deals with diversion given unprecedented challenges from Retired Chief Nurse Officer, Barbara Jacobs, MSN, at Anne Arundel Medical Center.
O’Neal, PharmD, MS, FASHP Senior Vice President
~ Allied Health at Children’s Mercy Kansas City, MO
• Learn about analytics when managing a drug diversion program from Retired Drug Diversion Surveillance Analyst, Patricia Penland, RN, at Wake Forest Baptist Health.


Diversion threatens patient safety and a hospital's reputation. According to the National Council of State Boards of Nursing, approximately 15% of healthcare workers struggle with drug dependence at some point in their careers. Drug diversion among healthcare workers is substantially underestimated, undetected, and underreported.
And if diversion hasn’t wreaked enough havoc, healthcare professionals have endured upheaval like never before because of an unprecedented pandemic. Hospitals were already dealing with rising stress levels, burnout, and staff shortages, which can exacerbate drug diversion.
Jacobs, MSN Retired Chief Nurse Officer

~ Anne Arundel Medical Center, Annapolis, MD
Diversion poses risks to patients, including inadequate pain relief and exposure to infectious diseases from contaminated needles and drugs. A healthcare worker’s impaired state also creates an unsafe environment.
However, solutions and strategies exist to combat it, and hospitals are winning the battle. A pharmacy executive, chief nurse officer, and drug diversion analyst share insights on the state of drug diversion and the tools to detect it.
Profile: Senior Vice President of Allied Health, Brian O’Neal, PharmD, Children's Mercy Kansas City

Q. How does your role relate to preventing diversion and protecting patients?
~ Wake Forest Baptist Health, Winston-Salem, NC
My responsibility is to ensure we have the resources needed to stop anyone looking to divert drugs. The likelihood is there. Prevention and detection are a priority for me.
My guidance is to focus on fundamentals, which means consistent quality checks, audits, and reliance on a strong drug diversion system. But software doesn’t run itself. It takes personnel to understand how it informs possible problems and how to pull the right data.
Q. Thoughts on the state of drug diversion at hospitals given COVID and staff shortages?
Our hospital, like the industry, is experiencing shortages, especially in pharmacy technicians, due to the pandemic and job hopping. And how it correlates to diversion is not entirely clear because we are still dealing with all those issues and current prevalence data. We are dealing with a gap in scientific evidence.
Q. How does Children’s Mercy Kansas City deal with diversion?
We developed a controlled substance oversight council led by pharmacy and nursing that includes members from security, human resources, and other hospital departments who meet regularly to review transactions and approve policies.
www.medacist.com
Brian
Barbara
Patricia Penland, RN Retired Drug Diversion Surveillance Analyst
42
Sponsored by an Educational Grant From Medacist
CASE STUDY
I’m involved with The American Society of Health System Pharmacists (ASHP) and apply their strategies to diversion prevention program.
Q What solutions are Children’s Mercy applying today?
I view a successful diversion control solution as having four legs, consisting of:
1. Hardware: The hospital needs an automated dispensing system in various locations, such as the operating room or nursing units.
2. Software: Diversion systems have come a long way in the last 20 years. Health systems have benefited from the accuracy of audits and true positives.
3. Human Resource: The system is only as good as the employee who knows how to use and maximize the capabilities. It benefits significantly from a savvy technician. Make an investment.
4. Culture: Everyone needs to look for warning signs across their system. Red flags exist. And the staff needs to drive out the thought that it wouldn’t happen here because diversion happens.
Q What resources are needed to monitor controlled substances?
At Children’s, our pharmacists or pharmacist technicians are responsible for working with the nursing staff to review the reports. They are the detectives who use the system as their metal detector to find that needle in the haystack.
Brian O’Neal, PharmD, MS, FASHP Senior Vice President
~ Allied Health at Children’s Mercy Kansas City, MO
Q. With the abundance of data analytics available, how do you use the data for decision-making?
From our system, we are hyper-focused on specific reports. We run aggregated reports on practice variables, such as the movement or removal of excessive doses. The system flags high transactions from individuals.
Another critical report for us is the purchase versus receipt. It shows reconciled data from the wholesaler within our controlled inventory. Our system allows us to watch for ordered pharmaceuticals that were not logged into the system.
Q. What is the essential data point in detecting diversion?
One of the most significant causes of concern is when a drug is removed and not accounted for in the system. The starting point to the answer is, “Where should it be?” A successful diversion program needs a robust reconciliation process.
Q. Based on your expertise, what is your guidance on a monitoring system for drug diversion?
A good starting place is the Guidelines on Preventing Diversion of Controlled Substances from the American Society of Health-System Pharmacists. Hospital management will receive a robust blueprint to help focus resources, build capabilities, and implement a collaborative, comprehensive controlled substance diversion prevention program.
Final Thoughts From Brian
One area that needs more attention is analytics to understand and address drug diversion in retail and ambulatory pharmacies. Ample transactions and inventory benefit from robust monitoring and advanced analytics.
Profile: Retired Chief Nurse Officer, Barbara Jacobs, MSN, Anne Arundel Medical Center
Q. How did your role relate to preventing diversion and protecting patients?
I was the chief nurse officer for the 384-bed at Anne Arundel Medical Center, where I guided nursing care throughout the facility, partnering with many experts in medical specialty areas. The care and safety of patients were my responsibilities, which included initiatives to stop drug diversion.
Q. Thoughts on the state of drug diversion at hospitals given COVID and staff shortages?
The Pandemic presented a potential for increased diversion, as did the temporary staffing due to shortages. Nursing is about trusting relationships, and with an influx of travel nurses in the hospital, nursing leadership didn’t have a history with travel nurses nor the time to get to know them.
Nurses' stress level and diversion have always been my concern, and it accelerated during COVID.
www.medacist.com
“Everyone needs to look for warning signs across their system.
Red flags exist. And the staff needs to drive out the thought that it wouldn’t happen here because diversion happens.”
43
CASE STUDY
Q. During your tenure, how did Anne Arundel Medical Center deal with diversion?
During my tenure, we developed a multi-disciplinary approach to drug diversion and a standing committee that manages prevention in multiple ways with input from the pharmacy, nursing, anesthesia, and human resources.
Q. What solutions did Anne Arundel Medical Center apply?
One of the highest risk areas involves someone signing out medication indicating the patient need and the person diverting it. We have an auditing tool from our drug diversion system that makes monitoring the process manageable by frontline leaders. It tracks hundreds of doses given out each day. Specifically, our sophisticated system looks at the medication situation and reports on what a person is doing and what is going on with the medication, informing on withdrawal or wasting and comparing the transaction to others to see it’s different.
Q. What resources are needed to monitor controlled substances?
As the CNO, I felt relief for my staff if a system could free up nurses’ time. A necessary resource is a system that alleviates the burden of impossible amounts of basic auditing and monitoring.
Q. With the abundance of data analytics available, how do you use the data for decision-making? The data on waste, withdraws, and returns inform us of the status of medications. The data can pinpoint if even a small amount was taken or wasted. The analytics tells us if there is more to the story than the patient refused.
Q. What is the essential data point in detecting diversion?
Being accurate is essential for a possible diversion case, so we look to the analytics to provide evidence. I received a monthly report on discrepancies and unresolved items to update me on how we were doing with the practice.
Q. Based on your expertise, what is your guidance on a monitoring system for drug diversion?
My guidance is to establish a multi-disciplinary committee that meets regularly to review protocols and drug diversion performance. I advocate for a strong relationship with human resources because they can guide you with communications and support if a diversion problem occurs.
Jacobs, MSN Retired Chief Nurse Officer
~ Anne Arundel Medical Center, Annapolis, MD
Final Thoughts From Barbara
It’s about the patient, and diversion could mean a patient doesn’t get the needed medication, which is against the nursing mission.
An allegation requires substantial diligence. Diversion is a felony, so getting it right is necessary for the patient, employee, and hospital.
We can’t lose sight of fairness and empathy for an employee. Helping an employee with a drug problem is also a responsibility.
Diversion requires objectivity and analytics. Concrete evidence is a must in any investigation. We sent a strong message to employees and traveling nurses that this hospital prioritizes diversion prevention and that this is not the place if you want to divert.
Profile: Retired Drug Diversion Surveillance Analyst, Patricia Penland, RN, Wake Forest Baptist Health
Q. How did your role relate to preventing diversion and protecting patients?
When I started as the surveillance analyst in 2019, it was to support the head of pharmacy with the new Drug Diversion Prevention and Response Team. Our initial processes for surveillance and investigation were very manual, requiring drilling down through pages of reports. It was very labor intensive. One of my most crucial roles entailed educating employees about preventing drug diversion and collaborating with nursing leadership on all aspects of the program.
Q Thoughts on the state of drug diversion at hospitals given COVID and staff shortages? When we rolled out the education component of our diversion program, there was still a surprise from the staff that drug diversion was happening. We heard, “people do that?” So, some staff
www.medacist.com
“It’s about the patient, and diversion could mean a patient doesn’t get the needed medication, which is against the nursing mission. We sent a strong message to employees and traveling nurses that this hospital prioritizes diversion prevention and that this is not the place if you want to divert.”
Barbara
44
CASE STUDY
didn’t know it was happening. And they didn’t know what to look for or how to address it if they suspected a case.
Diversion wasn’t top of mind at the hospital. It was my job to raise awareness that it is still an issue. We put diversion detection at the forefront of our staff education program.

Q. How did Wake Forest Baptist Health deal with diversion?
While I was the diversion analyst, the chief nursing, pharmacy, and legal officers led an executive oversight committee on diversion. They also bring in communications and compliance to advise on policies and situations. This committee established the Drug Diversion Prevention and Response Team, which regularly informs committee members about the state of diversion, current issues, and possible diversions.
The team continually mitigates diversion risk by analyzing clinical practice workflows because someone who will divert will find weaknesses, permitting the possibility of diverting throughout the system.
Q. What solutions are Wake Forest Baptist Health applying? Are they successful?
As an analyst, technology that allowed me to pull documents into one system and validate the data make my job easier and more successful.
Q. What resources are needed to monitor diversion of controlled substances?
Patricia Penland, RN Retired Drug Diversion Surveillance Analyst
~ Wake Forest Baptist Health, Winston-Salem, NC
Resource need depends on the area in the hospital. Monitoring med surge is different than reviewing controlled substances in general nursing. If you must put a case together, you have to have tools and resources that substantiate the case because we don’t want to accuse people falsely.
Q. With the abundance of data analytics available today, how do you use the data to make an informed decision?
Data allows us to identify trends and pinpoint issues quickly. Analytics is a needed piece of the puzzle when putting a case together. It’s a big benefit to having analytics.
Q. What is the essential data point to detect diversion?
One of the easiest and earliest is to focus on where the highest level of dispensing is occurring. A good start is examining the top five or ten users dispensing narcotics and why they are dispensing. Is it because they work in oncology or the ICU where high usage occurs? It’s about trust and verification.
Q. Based on your expertise, what is your guidance on a monitoring system for drug diversion? This work is not trying to “catch” someone but rather mitigate a problem that could potentially harm the patient. People don’t want to discuss diversion, but you must discuss it.
Final
Thoughts From Patricia
Getting executive buy-in from the start was critical for the diversion prevention team. Leadership put an organized structure around addressing diversion, making it an organizational priority.
“This work is not trying to 'catch' someone but rather mitigate a problem that could potentially harm the patient. People don’t want to discuss diversion, but you must discuss it.”
• Sterile & Paper Free (USP 797 Compliant).




• Maintains a 100% sterile barrier* with 3X greater adhesion.

*Tested in Nelson Labs, Salt Lake City, UT
• Helps prevent contamination of drugs and provides added protection to pharmacists.


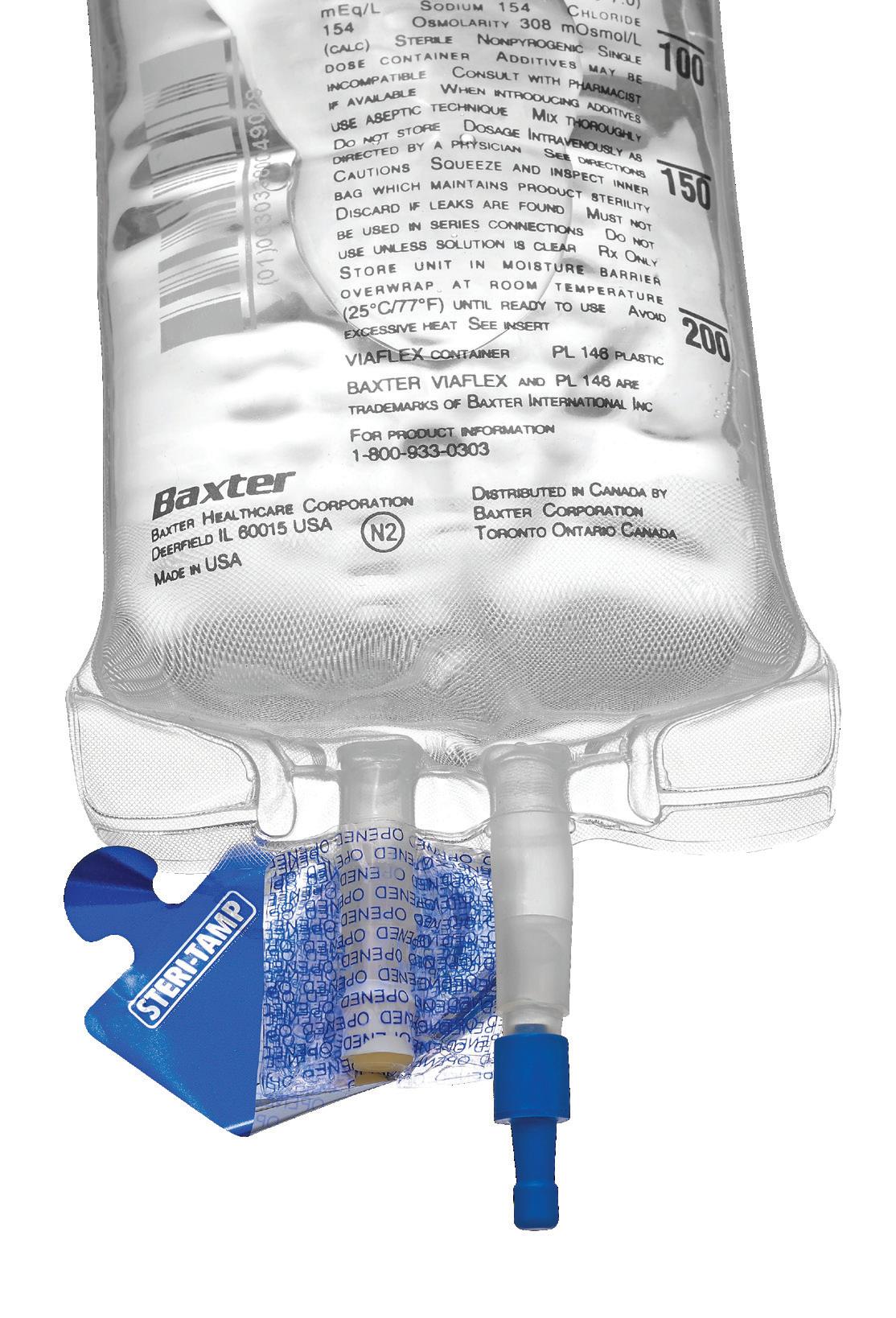
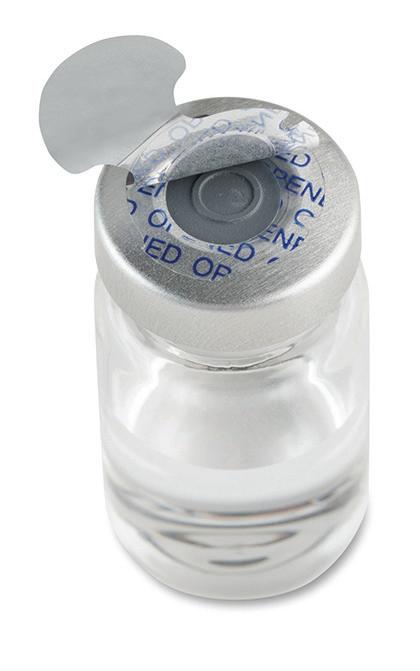

• Will not fall off, even in cold storage conditions (down to -20 degrees centigrade).
• Patented dual-layer indicates true tamper-evidence, with “OPENED” marking.

The Most Innovative Tamper-Evident Seals In Hospital Pharmacy Today VIEW TRAINING VIDEOS HERE steri-tamp.com Visit Our Website to Request Samples Made in the USA Syringe Seals IV Bag Port Seals Belly Button Bag Seal Vial Seals 28mm 20mm 13mm
Steri-Tamp® Tamper-Evident Sterile Seals by Allied Pharmacy Products, Inc.
The only single-use, tamper-evident seal that provides and maintains a 100% sterile barrier.
National Sales Manager: Alex Meadow
Phone: (516) 374-8862
Address: 2905 S. Congress Avenue, Suite A Delray Beach, FL 33445
Website: steri-tamp.com Email: info@steri-tamp.com

Company Background
Allied Pharmacy Products, Inc. was founded in 2010 with the focus on improving the preparation and dispensing of intravenous medication in the hospital pharmacy and compounding settings. Steri-Tamp® was developed by a pharmacist who understood the need for better tamperevidence and sterility in I.V. preparation. Allied Pharmacy Products, Inc. has always been responsive to pharmacists’ requests, which continues to contribute to expanding our product line.
Product Overview
The Steri-Tamp® sterile product line is comprised of seven innovative tamper-evident seals. Steri-Tamp I.V. Bag Port Seals are available in blue, red, and CHEMO (yellow). The vial seals are available in 13 mm (red), 20 mm (silver), and 28 mm (blue). Steri-Tamp Belly Button Bag Seals (green) are the only seals that will adhere to the port of a Hospira/ICU Medical “belly button” bag, as well as the top of a 13 mm vial.
Steri-Tamp's recently released USP 800 and ISMP specific I.V. Bag Port Seals help address labeling recommendations for paralytic agents or hazardous drugs in a single workflow.
Unlike other seals on the market, Steri-Tamp seals provide true tamperevidence. Using dual-layer technology, the seals help to alert hospital staff if a bag or vial has been used or modified. If the top foil layer is removed, the “opened” layer is revealed and the top layer cannot be reapplied. Due to the 3X stronger adhesive, Steri-Tamp is able to provide a 100% sterile barrier.
Steri-Tamp also offers two non-sterile syringe seals. Their increased tensile strength allows you to remove easily from the liner and manipulate over any desired container (syringes, pill bottles, inhalers, EpiPens, insulin pens, medication boxes, etc.) without the seal breaking before it is applied. Our new Tamper-Clear Syringe Seal® will now allow you to see through to the markings on the syringe and scan the barcodes on syringes, inhalers, insulin pens, etc.
Features
n Steri-Tamp® I.V. Bag Port Seals
• Offers the most fool-proof, potentially life-saving way of clarifying whether or not medication has been dispensed.
• Protects the point of entry of I.V. admixture bags from contamination and accidental double dosing.
• Using a simple “twist” method, the seal adheres to itself and the port ensuring no air channels.
• The green Belly Button Bag Seal is the only seal specifically designed to cover the “belly button” port, which is located in the middle of the Hospira bag. The seal can also be used on a 13 mm vial top.

n Steri-Tamp® Vial Seals
• Designed to lay flat on the vial top preventing bacteria from entering the vial. The seal should not overlap the edges.
• Helps to maintain the integrity of the medication.
• Sized specifically to fit 28 mm, 20 mm, and 13 mm vial tops. A seal should only be applied to its corresponding vial size.
n Steri-Tamp® Syringe Seals
• Non-sterile solutions to provide tamper-evidence for syringes and other medical containers.
• The new Tamper-Clear Syringe Seal® provides clear visibility to the barrel of the syringe and allows barcodes to be scanned. Perfect for sealing small oral, pediatric, and NICU syringes.
n Available in 1,000 Seals Per Roll
n All Seals Help to Reduce Waste, Improve Workflow, and Save You Time and Money
Testimonials
“�These seals have improved our workflow — Pharmacy Technician, LA “Love Tamper-Clear and dual layer nature of seals really loved that they don't in fridge.”
— Pharmacy Buyer, MI “We love the Tamper-Clear Syringe Seals — they don’t obscure any information.”
— Pharmacy Inventory Supervisor, OK
Trade Shows/Meetings Attended
ASHP Midyear, EAHP, CPC (United Kingdom), Annual Compounding Pharmacy Compliance, and various ASHP state affiliate pharmacy conferences.
Ordering Information
Available through major wholesalers and distributors. Visit our website, steri-tamp.com, for ordering information or to watch dispensing and application training videos.
WINTER 2022 I HEALTH SYSTEM • INFUSION 47 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT


Capital Inventory, Inc. — The Premier Leader in Pharmacy Inventory Services
Benefits
Founded: 1979
Employees: 75
Toll-Free Phone: (800) 345-0849 Phone: (770) 928-7202 Fax: (770) 928-2287 Address: 9725 Main Street Woodstock, GA 30188 Website: www.capitalinventory.com
Company Background
Capital Inventory, Inc. was founded by William Straub Sr. during the late 1970s in Alexandria, VA. The company relocated to Georgia in the early 1980s and serves the entire nation, including Alaska, Guam, Hawaii, Puerto Rico, and the U.S. Virgin Islands from its office in the Atlanta, Georgia area.
Capital Inventory, Inc. has been in business for over 43 years, providing inventory services to thousands of hospitals, university medical centers, regional medical centers, and health systems. Capital Inventory exclusively services hospital, outpatient, specialty, and infusion pharmacies. Our expert on-site inventory teams conduct pharmacy inventories daily and are employed year-round. Employees of Capital Inventory are employed full-time and receive complete benefit packages. Employees undergo extensive and continuing education including pharmacy practices, drug information, and HIPAA regulations. You can be confident and secure as all of Capital Inventory’s employees undergo a thorough background check including felony, misdemeanor, and sex offender. Our employees are 12-panel drug tested regularly, at least twice a year. Third-party specialty companies conduct all testing. Additionally, our employees are required to be current with all vaccinations including yearly Influenza and TB testing.
Product Overview
Our people are the difference. Our teams speak ‘NDC’ fluently and understand the dynamics of pharmacy operations. The physical inventory process is seamless and unobtrusive as team leaders strategically place the team in locations throughout the pharmacy to ensure accuracy and efficiency. Before completion of an inventory service, our teams actively work with finance and/or independent auditors while on-site to validate the accuracy of the inventory data collected. Beyond the physical inventory, the data is then received, analyzed, and formatted into a report that includes all pertinent information to allow for accurate and precise analysis of the inventory data. Let our team of experts provide your next inventory valuation with precision, accuracy, and confidence.
n Expertise: Our expert inventory teams consist of inventory specialists who speak ‘NDC’ fluently and are accustomed to working with finance and/or external auditors for validation. Our processes are streamlined for minimal interruption to the pharmacy staff and operations.
n Analysis: Our expert analysis team of data specialists average over 15 years of experience, working in pharmacies and with pharmacy ADM data. All client data is analyzed and adjusted as needed to ensure the most accurate representation and valuation of your inventory.
n Reporting: Final inventory data is provided in a clear, concise format across multiple mediums, all designed for maximum inventory management. Our secure online client portal allows you to access, view your data, and create customized reports and graphs.
n Client Care: We provide friendly and personal experiences with highly knowledgeable and professional customer service-oriented personnel. Our pharmacies can trust that Capital Inventory will be transparent, supportive, and reliable every step of the way.
Why choose Capital Inventory for your inventory needs?
• Pharmacies are our natural environment. Our expert on-site inventory teams specialize in conducting pharmacy inventories only and within a few hours.
• Our on-site inventory teams work seamlessly around your pharmacy staff ensuring that the “snapshot” is a true and accurate representation of the inventory on-hand.
• Eliminate the need for overtime, or coordination of staffing, to accommodate the inventory process with minimal interruption to the pharmacy staff and operations.
• Our inventory specialists have the expertise and knowledge to work with finance and/or external audit firms to validate the inventory.
• Our expert data processing team collaborates with the pharmacy to provide an accurate report in a timely manner.
• Receive expert valuation and pricing of the inventory by an independent party.
GPO Affiliations
Premier, The Resource Group, HealthTrust
More Information
To learn more about Capital Inventory and how we can help meet your pharmacy’s objectives with our inventory services, please contact us at (800) 345-0849 or info@capitalinventory.com.
WINTER 2022 I HEALTH SYSTEM • INFUSION 49 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
Founder: William Straub Sr. President: Shannon McArthur
Our decades of expertise, working exclusively with pharmacies, makes us uniquely qualified to provide accurate and dependable inventory valuations through a streamlined process. Our clients can trust that we will be transparent, supportive, and reliable in every interaction.
CASE STUDY
MOBILE INFIRMARY PILOTS INNOVATIVE CONCENTRATED NOREPINEPHRINE VIALS


Study Design and Findings — An Overview PARTNERING TO ENHANCE WORKFLOW EFFICIENCY TO HELP CREATE BETTER PATIENT OUTCOMES
Nathan Browning, Pharm.D.
I.V. Room Supervisor
~ Mobile Infirmary
Vicky Vega, Pharm.D. Pharmacy Education Coordinator
~ Mobile Infirmary


Roland Naseman, R.Ph. Director of Pharmacy
~ Mobile Infirmary
Infirmary Health is the largest non-governmental, non-profit healthcare system in Alabama. Serving southern Alabama, the organization's network of award-winning hospitals, physician practices, and affiliates makes it a top healthcare system on the Gulf Coast. This includes Mobile Infirmary — Infirmary Health's flagship hospital, which is among the leading hospitals in the state for surgical volume. The hospital houses a comprehensive cardiovascular program with a hybrid OR/cath lab, the region’s only Bariatric Center of Excellence, a CARF-accredited rehabilitation hospital, a renowned cancer program, a thrombectomy-capable stroke center, and a freestanding emergency department.
It's no surprise that Mobile Infirmary's doctors and nurses keep the I.V. room pharmacists and technicians very busy. To help alleviate the stress and strain, Leiters, an FDAregistered 503B outsourcing provider of compounded sterile preparations, reached out to the hospital about participating in a workflow study for its new concentrated vials — Vicky Vega, Pharm.D., says they were very interested. “We always want to be involved in anything that promotes change and advancement,” says Vega. “Especially where that means achieving better patient outcomes.”

READY-TO-DILUTE, CONCENTRATED, PRE-FILLED VIALS
Leiters' concentrated vials are ready-to-dilute, pre-filled vials of highly used compounded sterile preparations that are not commercially available. Norepinephrine Bitartrate (Norepi) was selected for this workflow study.
“We prepare a good many Norepi bags in all milligram strengths — 4 mg, 8 mg, and 16 mg,” says Vega. “We could see how having something readily available in concentrated vials could get medication to patients faster, so we wanted to take part."
www.leiters.com
50
CASE STUDY
Leiters
Concentrated Vials
Benefits at a Glance
• Compliant with all I.V. workflow and I.V. compounding software and all vial docking technologies.
• The concentrated vial sterile preparations are in solution, making them easier to dilute with no waiting for reconstitution of a lyophilized powder.
• Inventory space is reduced, waste minimized, and inventory turns increased.
• In the forward positions, vials can be stored in automated dispensing machines (ADMs) for rapid retrieval, dose tracking, and administration based on the hospital's specific vial-tobag adapter and activation processes.
• Pre-labeled vials and boxes include TALLman lettering, barcodes, and color-coding for drug/strength differentiation to help reduce medication errors.
WORKFLOW STUDY: DESIGN
Nathan Browning, Pharm.D., was responsible for designing and tracking the workflow study for Mobile Infirmary. “Vicky and I decided to break the study down into three phases,” he explains.
• In Phase 1, Browning and his team gathered data on the I.V. room's traditional manual compounding processes.
• In Phase 2, they switched to using Leiters' precise dosage Norepi vials for manual compounding.
• In Phase 3, they used the concentrated vials of Norepi from Leiters that use the vial docking technology so require no compounding.
“In each phase, we tracked the number of bags prepared, the staging time, the compounding time, the pharmacist check time, and the time to administration,” says Browning. “We then used that information to calculate the cost per bag in each phase, which included product and supply costs as well as labor expenses.” The study measured 8 mg and 16 mg vials across three and a half weeks.
Leiters was supportive through the entire process. “The team was very responsive and answered my questions or helped me find a solution quickly no matter the challenge,” says Browning. “At one point, we ran out of some of the study drug, and Leiters sent us what we needed overnight so it was there by 7 a.m. the next morning.”
WORKFLOW STUDY: TOPLINE FINDINGS
8 mg Norepi
Phase 1 Phase 2 Phase 3
Number of Bags 23 24 N/A
Staging Time 56 seconds 85.3 seconds N/A
Compounding Time 2.48 minutes 1.58 minutes N/A
Pharmacist Check Time 26.6 seconds 15.3 seconds 9.6 seconds
Time to Administration 174.3 minutes 152 minutes 61.4 minutes
Cost Per Bag $17.52 $16.49 $17.93
16 mg Norepi
Phase 1 Phase 2 Phase 3
Number of Bags 38 66 N/A
Staging Time 47.8 seconds 31.9 seconds N/A
Compounding Time 3.8 minutes 1.34 minutes N/A
Pharmacist Check Time 27.7 seconds 13.6 seconds 9.6 seconds
Time to Administration 246.4 minutes 161.4 minutes 59.2 minutes
Cost Per Bag $31.05 $18.31 $19.89
www.leiters.com
51
CASE STUDY
TIME SAVED GETS MEDICATION TO PATIENTS SOONER
“For us, the crux of the study was to see if we could reduce the time it took to hang a bag on the patient,” says Browning. “Obviously, with the concentrated vials, there's no compounding time since they're ready to use, but we found that even when we spiked them under a hood, the relative prep time was something like 10 seconds compared to two or three minutes or more for compounding.” That's because the concentrated vials require a simple aseptic technique where the top is popped, swabbed, and spiked. “The process saved a lot of time, which got medication to patients faster and freed up our I.V. room to handle other urgent tasks.”
The nurses also liked the concentrated vials because they helped eliminate delays in care for their patients. “Requesting a bag, waiting for it to get made, and then eventually sent up all takes time,” says Browning. Based on Phase 1 data, that was an average of 264 minutes from request to administration for the 16 mg bags.
“With the concentrated vials, when nurses needed a bag — they could just go to the ADM, pull out what they needed, dock it with vial docking technology and activate and hang it,” Browning explains. In Phase 3 of the workflow study, Mobile Infirmary found that Norepi infusion began significantly faster, in 59.2 minutes on average for 16 mg bags.
The result? 100% of nurses answering an internal survey said having the concentrated vials in an ADM made it either a great deal or a lot faster to administer the first dose compared to waiting for the I.V. room to compound the product.
KEY NURSING SURVEY RESULTS
In your unit having the concentrated vials in your automated dispensing machine (ADM), was it quicker to administer the first dose rather than waiting on the pharmacy?
How likely would you support the pharmacy to continue to use the norepinephrine bitartrate in this format?
How likely would you request pharmacy to add additional critically needed drugs in this vial format?
Very Likely Likely Neither Likely, Nor Unlikely
Unlikely Very Unlikely
Very Likely Likely Neither Likely, Nor Unlikely
Unlikely Very Unlikely
Over 80% of nursing respondents stated that they would ‘very likely or likely’ support the pharmacy’s continued use of the Norepi concentrated vial, as well as support other critically needed drugs in the same format. According to Browning, the concentrated vial was also supported by the I.V. room staff. Leiters provides compounded sterile preparations in several different volumes and precise milligrams — Norepi comes in 4 mg, 8 mg, and 16 mg single-use vials. “With our previous product, the vials only came in
www.leiters.com
72.73% 27.27% A Great Deal A Lot A Moderate Amount A Little None at All
63.64% 18.18% 18.18%
63.64% 9.09% 18.18% 9.09%
“
The process saved a lot of time, which got medication to patients faster and freed up our I.V. room to handle other urgent tasks.”
Nathan Browning, Pharm.D. I.V. Room Supervisor
52
~ Mobile Infirmary
CASE STUDY
4 mg, so if we needed a 16 mg bag, we'd have to pull four vials. With Leiters, it's just one," says Browning. This seamless process just adds an extra layer of confidence in the final compounded product, he adds
That's one of the reasons more than 90% of pharmacists and technicians surveyed internally said the concentrated vials were easy to use, took less time to complete a dose compared to a manual compounding processes, and would recommend the format to leadership.
Did the concentrated vials take less time to complete a dose than the manual compounding process?
7.69%
33.33% 8.33%
Nathan Browning, Pharm.D. I.V. Room Supervisor
No
WHY CONCENTRATED VIALS?
Unlikely Very Unlikely
ASHP reports that health system pharmacies are faced with significant staffing shortages – particularly among pharmacy technicians. Reasons cited in a recent survey conducted by the organization include workload, work schedules and pay. Regardless of why, the simple fact is that pharmacy managers are continually looking for ways to do more with fewer resources by adding efficiency.1
Leiters concentrated vials add a lot of efficiency to pharmacy processes. Concentrated vials reduce the time to compound medications by reducing manual steps. For example, manually compounding a 16 mg/250 mL dose of Norepi diluent with commercially available vials takes 34 steps from staging to pharmacist check. With the concentrated vials, this is reduced to just nine steps.
ABOUT LEITERS
Leiters is an FDA-registered 503B outsourcing provider of compounded sterile preparations and pharmacy services. Their team of experts in sterile pharmaceutical manufacturing, compounding, and pharmacy provide a sophisticated understanding of what it takes to elevate quality and consistency of supply in pharmaceutical outsourcing. They combine a deeply experienced team, with robust processes, in a state-of-the-art facility, to ensure delivery of the highest quality medicines.
1 "Hospitals and Health Systems Experiencing Severe Shortage of Pharmacy Technicians," ASHP, March 15, 2022: https://www.ashp.org/news/20 22/03/15/hospitals-and-health-systems-experiencing-severe-shortage-of-pharmacy-technicians
53
www.leiters.com
53.85% 7.69% 38.46%
Were the concentrated vials easy to use? 92.31%
Very Likely Likely Neither Likely, Nor Unlikely Yes
Unlikely Very Unlikely Very Likely Likely Neither Likely, Nor Unlikely 58.33%
How likely would you recommend to your leadership on continuing to use the concentrated vials to save time, make compounding easier and more accurate?
KEY PHARMACY SURVEY RESULTS
“With our previous product, the vials only came in 4 mg, so if we needed a 16 mg bag, we'd have to pull four vials. With Leiters, it's just one.”
~ Mobile Infirmary

























with Visante today to learn
we
your goals.
Transforming healthcare through pharmacy.TM We help you achieve extraordinary results in all areas of health system pharmacy Making the most of opportunities to manage costs and increase revenue is core to our consulting. We help you improve financial performance across your organization. Financial Results Our team of compliance experts will improve confidence, enhance care and ensure patient, worker and community safety. We collaborate with you to deliver innovative solutions that will elevate your organization to peak performance levels. Strategic Planning & Implementation Compliance that Improves Care Pharmacy consulting designed for optimal growth Visante consultants bring you expertise in all areas of hospital and health system pharmacy: • Comprehensive Strategic Assessments • 340B Program Solutions • Specialty Pharmacy Programs • Revenue Cycle and Drug Reimbursement Strategies • Supply Chain Strategies • Drug Diversion Programs • Drug Compounding Excellence © Visante Consulting, LLC, 2021. All Rights Reserved.
Connect
how
can help you reach
Visit us at visanteinc.com, or call 866-388-7583.
Visante — High-Performing Pharmacy is Who We Are

n Pharmacy Revenue Cycle
Founded: 1999
Toll-Free Phone: (866) 388-7583
Address: 101 East 5th Street, #2220 St. Paul, MN 55101 Website: www.visanteinc.com
Company Background
Visante is a specialized consulting firm focused exclusively on helping health systems accelerate strong financial and operational performance through pharmacy. Our team of professionals bring deep, contemporary expertise and innovation to optimizing all aspects of a fully integrated health system pharmacy program, driving significant value quickly. By providing customized solutions to fit the needs of our clients, we deliver sustained financial results through revenue growth, cost savings, and optimal business performance.
Featured Services
n Business Strategy Optimization
We put our extensive experience and knowledge to work to assess your current operations and help you take your pharmacy to the next level. We have the right combination of skills to evaluate all the moving parts and recommend improvements that will help you provide better patient care while improving your bottom line. We listen and work closely with you to support your objectives, delivering a personalized pharmacy plan for optimal growth, including a complete evaluation of your current program; opportunity analysis for safety, clinical, operational, and financial improvements; strategic planning support based on identified opportunities; and program enhancement design and implementation.
n Specialty Pharmacy Services
A clearly defined specialty pharmacy strategy can help improve patient care and greatly improve your bottom line. Developing a specialty pharmacy strategy or expanding your existing services can be a complex process, but our team of experts guides you through each phase and delivers lasting ROI. Our specialty pharmacy consulting capabilities include: operational assessment and pro forma models; multi-year business and strategic plans; facility design including workflow and automation options; implementation and project management; accreditation support (URAC/ACHC/CPPA); contract pharmacy strategy, if applicable; wraparound strategy to include consideration for site of care challenges (infusion); and home infusion, DME strategies, and business planning.
Sustained financial growth is one of the top challenges for even the most successful organizations in the healthcare industry. Effective pharmacy programs can increase revenue by maximizing existing opportunities, creating new programs and services, and reducing costs. Our team of experts is here to help maximize financial performance within your organization through our mastery of both pharmacy and revenue cycle operations. Our consultants bring a wide range of experience to the table to assist each of our clients in a way that best fits them and their financial goals.
n Pharmacy Supply Chain Optimization
Supply chain and utilization management are complex and ever-changing functions of a pharmacy enterprise. To find success in these areas, disciplined focus and alignment must be prioritized. Visante’s supply chain experts help hospitals and health systems achieve reliable, safe, and efficient drug supply chain performance while also realizing significant financial returns.
n 340B Solutions
Visante’s independent, external audit support continues to provide transparency to your 340B processes, allowing you to recognize compliance gaps while also focusing on new opportunities within the program. Visante provides: internal and external audit support, on-site HRSA audit support, and corrective action plan guidance in the event of HRSA audit findings; gap analysis and targeted recommendations, focusing on long-term strategy in the mixed-use and contract pharmacy space to ensure program optimization; program implementation and development of internal oversight structure and maintenance; and split billing RFP guidance, implementation, and program re-designs.
n Sterile and Non-Sterile Compounding Compliance
Safely compounded medications are essential to quality patient care. Hospitals face the challenge of optimizing care while also meeting safety requirements and managing cost. We will prepare you for compliance with USP Chapters <795>, <797>, <800>, and <825>. We’ll also assist you in preparing for compounding related inspections by the CMS, FDA, DEA, and Joint Commission/DNV. Our consulting services include: USP <795>, <797>, <800>, and <825> as well as gap assessment and facility design; 503A, 503B assessment, facility design, and implementation support; and home infusion compounding services.
n Drug Diversion
Our program takes a comprehensive multidisciplinary approach to identify drug diversion risk points in your medication use processes. Minimize risk through assessment of pharmacy operations, informatics, automation, nursing, and perioperative processes to identify and strengthen points of vulnerability. Our drug diversion experts have real-life experience in hospitals and health systems and understand the challenges hospitals face today and in the future.
WINTER 2022 I HEALTH SYSTEM • INFUSION 55 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
We're in the business of advancing pharmacy. Our consultants work with hospitals and health systems combining a wealth of expertise with personalized and comprehensive support to optimize your pharmacy operations and deliver better patient care.
President & CEO: James Jorgenson




























































































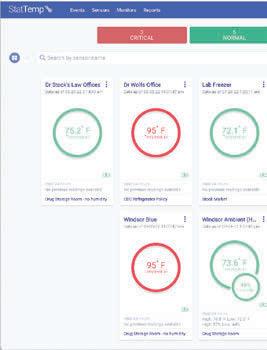
GoHCL.com • 1.800.848.1633 © Health Care Logistics, Inc. 2022 ® NO SUBSCRIPTIONS! Online software alert and monitoringsystem included with units. CONFIGURE REAL-TIME ALERT SETTINGS ACCORDING TO FACILITY NEEDS User Friendly & Complete Customization Instant Access to Reporting Peace of Mind – 24 hours a day https://StatTemp.io * * CONTACT CUSTOMER SERVICE FOR NIST CERTIFICATE INFORMATION Wireless Temperature Monitoring by Health Care Logistics®
President & CEO: Gary Sharpe
Founded: 1978
Toll-Free Phone: (800) 848-1633
Phone: (740) 477-3755
Address: P.O. Box 25 Circleville, OH 43113 Website: GoHCL.com
Company Background
Health Care Logistics® —
Ensure Hospital-Wide Temperature Accuracy With Stat Temp™
Unique and hard-to-find products are the lifeblood of our business. That includes manufactured solutions made by skilled technicians in our central Ohio facilities. By Design is where our manufacturing talent converts customer ideas into highquality cabinet, metal, plastic, or print products made to their exact specifications. Best of all, we deliver those made-to-order solutions in five working days or less.
Product Overview
Our goal is firm — create functional products to improve everyday workflow — there are no limits to our design capabilities. We can turn virtually any idea into reality without outrageous upcharges or extended shipping schedules. Start with a no-obligation, no-cost digital proof for a cabinet, metal, plastic, or print project today!
Product Specifications
n Cabinets by Design
Cabinets, shelving, and storage units for those who want to renovate existing spaces or outfit newly constructed areas with high-quality pieces made to their exact specifications.
The process starts with our free 3D Design Service, which provides a virtual layout of the targeted space paired with unique solutions that showcase specific design goals. We can create performancerich products in multiple sizes, styles, and colors that make it easier than ever to achieve a customized brand.
Cabinets by Design offers the quickest, easiest, and most affordable way to maximize organization and efficiency. All units ship in five working days or less so customers can get the look they want without the wait.
n
Metals by Design and Plastics by Design
One-of-a-kind items manufactured to match individual customer requests. Modifications to stock products are also available. These allow customers to work more efficiently and produce better results.
From stainless steel storage containers, lock boxes, and utility trays to specially sized plastic lock boxes and dividers, we have the engineering and manufacturing capabilities to design and modify products or create affordable replacements that meet the everyday needs of our customers.
We can create and modify functional products using stainless steel, aluminum, acrylic, PETG, polycarbonate, polystyrene, HDPE, and PVC. There are no limits to our design capabilities.
Whether customers want to simplify USP compliance or better manage changing trends, we can design a solution and deliver it quickly.
n Printing and Graphics by Design
From labels, magnets, and clings to banners, signs, and stamps, our print specialists can create solutions for any situation. Prevent the confusion that results from the unknown by communicating a consistent message for all products across all departments. Choose the size, color, and style of your message and our print team will create it.
Kicking off a marketing campaign? We can help with an assortment of fun promotional materials. Our print experts can hot stamp a customer logo, message, or facility information on a variety of products. Our process ensures a professional finish with lasting results. We can print as few as 50 or as many as 1,000+ — and quick!
Ordering Information
It’s easy for customers to begin their design journey. Complete a cabinets, metal, or plastics options request form, view our Print By Design options, or call our product specialists at (800) 848-1633 to discuss options.
WINTER 2022 I HEALTH SYSTEM • INFUSION 57 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
Manage safe ranges, avoid drug waste, and provide peace of mind with this remote temperature monitoring system exclusively from Health Care Logistics.
Q&A
Dennis Smith, Chair of the

Use Group
Storage Q A&
NSF Joint Committee for Vaccine
The NSF Joint Committee for Vaccine Storage was formed in 2015 to develop a new standard. The committee was responsible for creating a standard that ensures engineering controls are in place to assist in safely storing vaccines under real-world conditions in clinical environments.
The NSF/ANSI 456 Vaccine Storage Standard was finalized in May 2021, and equipment manufacturers can submit their products for independent testing and certification against the standard.
from the Immunization Coalition, National Association of County and City Health Officials (NACCHO), National Institute of Standards and Technology (NIST), and the CDC, as well as multiple equipment manufacturers. It was a very cross-functional group.
The decisions on what requirements were needed in the standard were driven by public health members and end users. The key aspects of the requirements were driven by research and data collected from healthcare facilities throughout the U.S. Health clinics were visited, and usage data collected to understand how these units were being used. The test methods in the standard were developed to provide confidence that the vaccines would maintain their temperature requirements and stay in compliance during these real-world use cases.
Q. Can you explain the basics of the standard?
Up until the release of the NSF/ANSI 456 standard there wasn’t a good standard in North America that could be referenced for the performance of a refrigerator or freezer for the storage of vaccines or refrigerated medications. What was available, the CDC Storage and Handling Toolkit, provided guidance on how to store vaccines in refrigerators and freezers, but there were still issues in the field where providers were losing vaccines due to poor temperature performance. The providers were seeking something more that focused on performance and how these units were being used in real-world conditions.
The committee was established to develop a standard that looked at overall performance of the refrigerators and freezers.
Q. Who created the new NSF/ANSI 456 Vaccine Storage Standard, and what was the driving force?
The NSF/ANSI 456 standard was developed by a committee including manufacturers of vaccines, directors from several state health departments, pediatricians and physicians, members
The standard follows the standard format for an ANSI certified standard. The standard is written to address both refrigerators and freezers and covers design and construction, test methods, performance requirements, and guidance on performing the actual temperature measurements including construction of a vaccine simulation device. NIST provided a significant amount of support for the development of the test methodologies and how to perform the temperature measurements that were written into the standard. The standard itself includes some minimum refrigerator and freezer performance requirements throughout the testing protocols.
The test method portion of the standard is focused on maintaining temperature performance through frequent door openings and door openings related to loading of the cabinet, as well as how many vaccines are stored in the unit. We looked at two storage scenarios to develop the requirements, which were a totally empty cabinet and a totally full cabinet. We simulated the full cabinet by filling the units with standard size boxes to make sure we filled a large percentage of total volume
WINTER 2022 I HEALTH SYSTEM • INFUSION REFRIGERATION
58
Q. Why was the new NSF/ANSI 456 Vaccine Storage Standard needed?
inside. Many storage units are packed very full when they first receive their vaccine shipments, so we wanted to make sure that the performance of the unit was the same whether empty or full. In addition to testing the unit with two different storage scenarios the units are tested with multiple and frequent door openings in these storage scenarios. The refrigerators or freezers must maintain a temperature that is specific to the product that would be stored in that particular device during the duration of the test.
The door openings are a critical piece of the requirements. When we were out in the field, we observed two typical use cases. During the daytime there were many short openings when they were getting in and out of the refrigerator to pull vaccines for injection. The other use case was a very long door opening. This is when they’re refilling or restocking the refrigerator and we found that to average around three minutes.
So, the protocol was written to address both cases, a large number of short, sequential door openings and then a long door opening. These protocols are tested on both an empty and a full cabinet.
Q. How is the new standard going to improve public health and safety?
The biggest benefit is that if a vaccine provider purchases a vaccine storage unit that is certified to the NSF standard, they will be confident in the performance of that unit, and that with normal usage, the vaccines will be stored safely within the temperature range specified by the manufacturers of the vaccines. Having an NSF certified unit should lead to less temperature excursions for the provider and improved vaccination effectiveness for the public. Long term, we are hoping to see a higher rate of effective vaccinations across the U.S.
Q. What are the implications of the new standard for vaccine refrigerator and freezer manufacturers?
The new standard will set a new, higher standard for the performance of purpose-built, medical-grade vaccine refrigerators and freezers. From the manufacturer standpoint, they’re going to have to review their portfolio and address or redesign products to be compliant with this standard.
Q. What is next for the NSF Joint committee? Will the standard evolve or change over time?
The standard is considered a living standard. The committee continues to meet to work on continually improving the standard. Anyone can offer recommendations for changes or additions to present to the NSF committee.
Between the CDC Vaccine Storage and Handling Toolkit which makes important recommendation to help protect the safety and efficacy of vaccines, and the new NSF/ANSI 456 Vaccine Storage Standard which further defines appropriate temperature performance for vaccine storage equipment, significant progress has been made to improve the safety and viability of vaccines.
Ongoing efforts to further protect public health via safe and effective vaccine storage will continue to support effective immunization.
Dennis Smith serves as Chair of the Use Group on the NSF Joint Committee for Vaccine Storage. He joined the committee at its inception in 2015 and continues his duties as they work on continuous improvement of the standard. Dennis has been designing equipment for cold chain

for Vaccine Storage
storage for over 30 years and is currently vice president of research and development at Helmer Scientific where he has been instrumental in the development of long-term product portfolio strategy and execution of product and technology road maps.
WINTER 2022 I HEALTH SYSTEM • INFUSION 59 REFRIGERATION Q&A
BIOGRAPHY
Dennis Smith, Chair of the Use Group NSF Joint Committee
* This is an abridged interview. The original feature was published in 20Ways Summer Hospital 2022 issue.
REFRIGERATION
Buyer’s Guide
Buyer's Guide
Accucold
770 Garrison Avenue Bronx, NY 10474 (718) 893-3900, Ext. 204 accucold.com/pharmacy
Aegis Scientific
P.O. Box 2008 Warminster, PA 18974 (800) 796-2344 aegisfridge.com
American BioTech Supply Horizon Scientific, Inc. 125 Varnfield Drive Summerville, SC 29483 (800) 648-4041 americanbiotechsupply.com
Arctiko Us Inc.
1400 Donelson Pike, Suite B5 Nashville, TN 37217 (615) 988-7000 www.arctiko-int.com
B Medical Systems
14560 Bergen Boulevard Suite 200 Noblesville, IN 46060 (888) 456-7099 www.bmedicalsystems.com
FFF Enterprises 44000 Winchester Road Temecula, CA 92590 (800) 843-7477 fffenterprises.com
Follett Products, LLC
801 Church Lane Easton, PA 18040 (610) 252-7301 www.folletthealthcare.com
Guardian Medical Systems
409 Edgewood Drive Exton, PA 19341 (484) 872-2500 guardianmed.net
Health Care Logistics
P.O. Box 25 Circleville, OH 43113 (800) 848-1633 GoHCL.com
Helmer Scientific 14400 Bergen Boulevard Noblesville, IN 46060 (800) 743-5637 Helmerinc.com
LabRepCo 101 Witmer Road, Suite 700 Horsham, PA 19044 (800) 521-0754 labrepco.com
NAFEM The Legacy Companies
3355 Enterprise Avenue, Suite 160 Ft. Lauderdale, FL 33331 (954) 202-7419 thelegacycompanies.com
Migali Scientific
1 Triangle Lane Blackwood, NJ 08012 (855) 464-4254 migaliscientific.com
PHC Corporation of North America
1300 Michael Drive, Suite A Wood Dale, IL 60191 (800) 858-8442 www.phchd.com/us/biomedical
Qingdao Haier Biomedical Co., Ltd No. 280 Feng Yuan Road, High-tech Zone Qingdao, 266109, P.R. China 86-532-88935593 Usa.haiermedical.com
Stirling Ultracold 6000 Poston Road Athens, OH 45701 (855) 274-7900 stirlingultracold.com
Thermo Fisher Scientific, Inc. 81 Wyman Street Waltham, MA 02454 (781) 622-1000 thermofisher.com
REFRIGERATION
WINTER 2022 I HEALTH SYSTEM • INFUSION 60








WINTER 2022 I HEALTH SYSTEM • INFUSION 61 REFRIGERATION Market Leaders Visit RXinsider’s Pharmacy Market BUZZ to Research the Leaders in Refrigeration www.RXinsider.com
No mess. No waste. No problem.
Get maximum quality and convenience with these liquid unit dose products from American Health Packaging. Our products quickly deliver safety and e ciency in every dose without the mess or waste.


With access to more than 600-unit dose items, including 100+ exclusives, you’re sure to find oral liquids, oral solids, and inhalants that can help your patients and streamline your operations. Browse our extensive catalog and take advantage of these featured liquid unit dose products from AHP.
Product description
AHP unit dose products include:
• Scannable bar codes
• Color-coded labels
• Clear “Tall Man” lettering
• Extended shelf life
Cup delivery Cup strength UD size (cups/case) NDC
Diphenhydramine HCl Liquid 10 mL 25 mg / 10 mL 100 UD 60687-0267-56
Diphenhydramine HCl Liquid 10 mL 25 mg / 10 mL 30 UD 60687-0267-08
Hydrocodone Bitartrate & APAP Oral Solution (CII) 15 mL 7.25 mg / 325 mg / 15 mL 50 UD 60687-0417-71
Hydromorphone HCl Oral Solution (CII) 5 mL 5 mg / 5 mL 30 UD 60687-0566-86
Midazolam HCl Syrup (CIV) 2.5 mL 5 mg / 2.5 mL 30 UD 60687-0576-10
Midazolam HCl Syrup (CIV) 5 mL 10 mg /5 mL 30 UD 60687-0576-86
Phenobarbital Elixir (CIV)* 5 mL 20 mg /5 mL 50 UD 60687-0448-67


Theophylline Oral Solution 15 mL 80 mg / 15 mL 50 UD 60687-0258-71

The NDC shown is in the 11-digit format required for the Centers for Medicare & Medicaid Services (CMS) processing, 42 CFR § 447.502 – Definitions.
Browse our full catalog through americanhealthpackaging.com or through your preferred wholesaler today.
*Product contains alcohol
AD202204-0134
American Health Packaging, Leading Manufacturer of Serialized Barcoded Unit-Dose Products
Additional Product Modules
President: Bruce Bennett
Toll-Free Phone: (800) 707-4621
Address: Columbus, OH 43217
Website: americanhealthpackaging.com
Company Background
Located in Columbus, Ohio, American Health Packaging (AHP) is an industry leader in manufacturing serialized, barcoded unit-dose (UD) medications provided for the healthcare marketplace. As a UD manufacturer, AHP’s commercially-available UD products are available to hospital, institutional, and long-term care pharmacies nationwide through partner GPOs and wholesalers.
AHP’s reputation for quality is supported by a 30+ year history of broad manufacturing expertise — operating a facility that is registered with the FDA, fully adherent to cGMP guidelines, and licensed by the DEA to package Schedule II-V controlled substances. Synonymous with unit dose, following years of success and leadership in the production of oral solids, AHP expanded their offering in 2017 to include liquid unit-dose cups and inhalants in 2019.
Product Overview
AHP is committed to supporting pharmacy efficiency through a diverse range of both high-utilization and niche treatments. Producing nearly 600 UD oral solid SKUs for the healthcare marketplace, AHP’s broad selection of products are produced with quality components and printed with legible barcodes that facilitate effective execution of BPOC initiatives. Their wide selection of products reduces the gap between what pharmacies are forced to repackage themselves, and what is commercially available on the market — supporting health systems nationwide in their efforts to create efficiencies throughout the chain of care.
AHP’s tailored offering of UD oral liquids provide similar efficiency, safety, and cost-savings benefits as their oral-solids products. Product features include right-sized packaging, thoughtful tray design, differentiated labelling, and accurate barcodes. AHP’s unit-dose inhalants provide efficiency and feature individually-wrapped vials and pouches barcoded to the dose level. AHP oral solids, liquids, and inhalants include major therapeutic classes and product groups to meet unique pharmacy needs. They are continuously evolving to meet the changing demands of caregivers and staff to support more effective medication procurement strategies.
As facilities nationwide compete to demonstrate they provide the highest quality of care, AHP UD supports caregivers as they strive to promote positive outcomes for patients. Pharmacies simultaneously strive to be cost-effective as they provide necessary resources for caregivers.
AHP UD supports these objectives while providing cost-savings opportunities. Sourcing pre-packaged UD allows pharmacies to obtain adequate supply while mitigating capital expenses, such as those related to repackaging equipment, bulk supply, and labor.
Benefits for Health Systems
n Patient Safety: Ensuring the right medication is given to the right patient at the right time — and in the right strength — is imperative. Pharmacies can facilitate effective execution of these “rights” by providing caregivers with as many products in a pre-packaged UD format as possible. Removing repackaging tasks from the pharmacy eliminates a potential point of failure during the UD process as medications arrive to pharmacies ready to dispense.
n Pharmacy Efficiency: Pharmacies strive to process orders and supply the proper medications to caregivers for their patients as quickly as possible. Adding potentially-complex repackaging steps to the procurement process not only harms the ability of pharmacy to supply caregivers effectively, but also removes clinicians from their core patient care competencies. In addition, pre-packaged UD often allows for products to be sourced more quickly than third-party repackaging can support.
n Cost-Savings Opportunity: Health systems that choose to package on-site must consider all direct costs, such as purchasing capital equipment for packaging areas and paying highly trained clinical professionals to perform, manage, and support non-core work. AHP UD products allow for pharmacies to avoid these costs while also shifting the potential costs associated with packaging errors. The pre-packaged format also prevents additional fees that may result from utilizing thirdparty repackaging services.
n Liability Management: Pharmacy repackaging operations can be subject to distractions from a variety of sources. An active pharmacy environment can encourage lapses in concentration and present opportunities for staff error. Since these errors may vary in gravity and place liability on the facility and caregivers, mitigation of risk is key. Unit dose from American Health Packaging can help shift liability burden away from staff.
Hitting the Mark for BCMA, USP <800> Support
Effective execution of BCMA initiatives require medications that scan correctly at the bedside. With a robust oral-solids portfolio — and growing offering of unit-dose liquids — AHP UD provides reliable access to UD treatments. AHP products promote safety towards BPOC and efficiency in pharmacy while freeing up internal resources. AHP UD supports pharmacies as they strive for compliance with USP General Chapter <800> guidelines. As pharmacies craft effective procurement strategies to meet the needs of their facilities, protecting patients and caregivers alike from potential harm while handling hazardous drugs is a priority. AHP's UD portfolio has a number of NIOSH/USP <800> products already packaged for bedside dispensing which supports compliance to USP <800> handling procedures.
WINTER 2022 I HEALTH SYSTEM • INFUSION 63 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
With a responsive line of barcoded unit-dose oral solutions, a growing liquid unitdose offering, as well as individually-wrapped inhalants, American Health Packaging continues to deliver on their commitment to supporting pharmacy efficiency.
Leverage our dedicated experts, proven collaborative care model and integrated care technologies to help you produce the superior outcomes your patients deserve and the financial results your health system demands.

Shields Health Solutions — The Premier Specialty Pharmacy Accelerator in the Country
CEO: John Lucey
Founded: 2012
Employees: 1,700 Phone: (781) 566-5066
Address: 100 Technology Center Drive, Suite 600 Stoughton, MA 02072 Website: www.shieldshealthsolutions.com

Company Background
At ShieldsRx, improving lives and elevating performance are at the heart of everything we do. That’s why more health system leaders trust ShieldsRx to elevate access, outcomes, and growth within specialty pharmacy — delivering value throughout the entire health system. Transform your specialty pharmacy into a powerful growth engine and elevate performance where it matters most with ShieldsRx — the premier specialty pharmacy accelerator in the country.
Product Overview
n Elevate Performance Where it Matters Most. At ShieldsRx, improving lives and elevating performance are at the heart of everything we do. That’s why more health system leaders trust ShieldsRx to elevate access, outcomes, and growth within specialty pharmacy — delivering value throughout the entire health system. Leveraging our proven Shields Performance Platform, we help you produce the superior outcomes your patients deserve and the financial results your health system demands. We work alongside your team, scaling resources and implementing the processes required to deliver more affordable care, optimize specialty pharmacy services, improve outcomes, retain more patients, and accelerate system-wide growth.
n Make Complex Care More Accessible and Affordable. It all starts by providing your health system with access to the medications, health plans, and financial support that patients with complex and chronic conditions require. That’s why we put our trade relations experts to work for you — securing access to 90% or more of the limited distribution drugs and specialty medications that patients desperately need. Simultaneously, our payer teams open the door to nine out of 10 restricted payer networks most specialty pharmacies are unable to access — expanding your eligible patient opportunity twofold. While our financial professionals work tirelessly to ensure that patients have access to affordable care and support services through your health system.
n Scale Integrated Pharmacy Services and Improve Outcomes. As we are increasing access to affordable care, our clinical pharmacist, patient liaison, and support teams are also implementing the proven Shields Care Continuum to improve therapy management and care coordination
across your health system. These dedicated experts operate as integrated members of your care team — improving patient success by reducing time to therapy to 48 hours or less; optimizing the patient experience by expanding the number of critical care touchpoints four times; and enhancing outcomes by achieving 90% or greater adherence across your entire specialty pharmacy patient population.
n Attract and Retain More Patients. Our Patient Workflow and Care Coordination Solution, along with our data experts also enable you to scale patient engagement and care coordination throughout the hospital. Rapidly identify patients in need and follow them throughout therapy — connecting patients with the medical providers, specialty pharmacists, patient liaisons, and financial support professionals required to deliver the best possible outcomes. This one-of-a-kind solution has proven to increase specialty pharmacy retention by 40-60%; improve patient experience Net Promoter Scores (NPS) from 20 to 80+; and reduce the total cost of care by 13% or more.
n Accelerate System-Wide Growth. While we are laser-focused on helping you produce superior patient outcomes, we also understand that your health system must be financially fit. That’s why we leverage Shields Acceleration Solutions and Services to unlock economic upside as we scale your specialty pharmacy capabilities. Whether it is reducing your total cost of care by double digits, enhancing margins through 340B; increasing specialty pharmacy revenue by 30%; or generating $20M+ in incremental year-over-year net operating income — we will deliver the system-wide financial performance required to accelerate growth and optimize care.
n Transform Specialty Pharmacy Into a Growth Engine. With the foremost leaders in specialty pharmacy on our team; proven success partnering with more than 75 health systems across the country; and a vested interest in delivering measurable results — we are the partner of choice for health system leaders who want to transform specialty pharmacy into a powerful growth engine and produce superior patient outcomes. Together, we will expand payer and drug access; improve therapy management and care coordination; deliver unsurpassed patient experiences; and generate the net operating income you need to accelerate growth.
Key Customers
We are the partner of choice for health system leaders who want to transform specialty pharmacy into a powerful growth engine and produce superior patient outcomes.
Visit our website to see our growing list of health system partners nationwide, including leading academic medical centers, integrated delivery networks, accountable care organizations, and health systems.
WINTER 2022 I HEALTH SYSTEM • INFUSION 65 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
Transform your specialty pharmacy into a powerful growth engine and elevate performance where it matters most — with ShieldsRx.
The Evolving Role of COMPOUNDING PHARMACIES
While pharmaceutical compounding has existed throughout history, mass production of pharmaceuticals drove down the need for such drugs for much of the 20th century. It wasn't until the 1970s and 1980s, when the demand for chemotherapeutic and total parenteral nutrition regimens increased, that the pharmaceutical compounding industry began to grow. The market has been advancing steadily since — driven by bulk compounding, increases in home infusion therapy, the evolution of personalized medicines, a rise in hormone replacement therapy, and more. Compounding pharmacies also serve a critical role in providing alternate dosage forms of medications. For example, some patients may need an oral liquid versus a tablet or have allergies or dietary restrictions requiring specially formulated drug versions.
Despite their unique position in the marketplace, regulations specific to pharmaceutical compounding are relatively recent.

An Evolving Regulatory Landscape
In September 2012, the CDC, FDA, and local and state health officials began investigating a multistate outbreak of fungal meningitis among patients who received contaminated steroid injections. The contaminated medication was traced back to a compounding facility in Massachusetts, which the FDA had previously visited and found sterility issues. The agency lacked the authority to impose or enforce any changes to the site even though facility operations resembled an FDA-regulated drug manufacturer. Instead, due to the regulatory environment at that time, the facility was able to continue operating as a pharmaceutical compounder,
66 WINTER 2022 I HEALTH SYSTEM • INFUSION
subject only to regulation by state boards of pharmacy. As a result of medications being produced under insanitary conditions by the facility, 753 patients were affected across 20 states, and 64 died.
In response, Congress passed the Compounding Quality Act as part of the broader Drug Quality and Security Act in 2013. This legislation defined two categories of compounders — 503A traditional compounding pharmacies and 503B outsourcing facilities.
n 503A: Traditional Compounding Pharmacies
A 503A pharmacy can compound for individual prescriptions only, not bulk production of drugs. They are required to comply with state boards of pharmacy regulations and requirements outlined in USP <795> Pharmaceutical CompoundingNonsterile Preparations, USP <797> Pharmaceutical Compounding-Sterile Preparations, and any standards referenced within those chapters.
n 503B: Outsourcing Facilities
Once a 503B outsourcing facility voluntarily registers with the FDA, it must comply with USP <795>, USP <797>, and any standards referenced within those chapters. In addition, they are also required to follow cGMP regulations specified in the Code of Federal Regulations Title 21 Parts 210 & 211 and are under the jurisdiction of the FDA. Any compounding pharmacy not complying with applicable regulations or failing to register as an outsourcing facility may be subject to rules set forth for drug manufacturers in the Food, Drug, and Cosmetic Act.
The Risk of Microbial Contamination
Among the risks compounding pharmacies face, microbial contamination has always been a significant concern. While licensed pharmacists oversee the actual compounding of medications, they are typically not trained on how to set up, run, and monitor a contamination control program.
One of the most considerable risks for 503A compounding pharmacies producing compounded sterile preparations (CSPs) is the lack of proper training and expertise necessary to implement contamination
control measures. Ineffective microbial control measures are combined with the fact that these facilities generally do not register with the FDA and are not subject to cGMP requirements. Plus, although 503A compounding pharmacies should be following USP <797> when producing
The bottom line for any pharmaceutical compounder is that while the “cost of quality” is not cheap, investing in quality personnel, processes, and procedures is worth it to increase patient safety and ensure business longevity.
Accurate Microbial Identification
Getting an accurate microbial identification is easier said than done, but it's a critical practice. Correct identification of microorganisms improves reporting capabilities, which facilitates the full investigation of root causes for contamination and informed decisionmaking. With accurate data, it is possible to fully understand issues that arise and develop meaningful corrective and preventive actions to reduce production downtime and improve patient safety. However, USP chapters have some variability regarding the degree to which microbes should be identified.
CSPs, they are also under the jurisdiction and quality standards set in state law or policy, and such criteria may differ from state to state.
For 503B outsourcing facilities, the biggest risk lies in the challenge of producing sterile medications themselves. As CSPs become more prevalent, sterility failures can lead to an increase in adverse events.
In both cases, 503A compounding pharmacies and 503B outsourcing facilities need to remain vigilant in understanding the risks of producing CSPs. This calls for hiring highly skilled microbiologists to implement microbial control policies and procedures, train staff, and cover everything from environmental monitoring to cleaning and disinfection to sterility and endotoxin testing (when applicable). Similarly, as the market grows, so will competition.
Producing consistently high-quality, safe, and effective products is an excellent competitive advantage.
In USP <797>, the chapter states isolates should be identified “at least to the genus level.” USP <1116> Microbiological Control and Monitoring of Aseptic Processing Environments indicates that an “appropriate level of identification” should be used. On the other hand, USP <1117> Microbiological Best Laboratory Practices states that isolates should be identified “to species level whenever possible” for risk assessments and that confirmatory tests for laboratory QC microorganisms should be performed “at the level of genus and species.”
n Species-Level Microbial Identification
Overall, species-level identifications will provide the best resolution in microbial data sets compared to genus-level identifications. Species-level identifications add value when investigating an environmental monitoring excursion, a sterility failure, or a potentially objectionable organism in your product. As part of your root cause analysis, the value of species-level identifications is realized when you will be comparing your isolate of interest to historical data obtained from the environmental monitoring of your facility. If your historical data is full of genus-level identifications, it becomes very difficult to track the source of a particular species.
SUMMER 2022 I HEALTH SYSTEM • INFUSION 67 WINTER
The bottom line for any pharmaceutical compounder is that while the “cost of quality” is not cheap, investing in quality personnel, processes, and procedures is worth it to increase patient safety and ensure business longevity.
Additionally, strain-level characterizations may be necessary to provide more evidence linking your investigational isolate to a contamination source.

n Methods for Microbial Identifications
Phenotypic methods, dependent on the analysis of biochemical reactions of a microorganism, can have subjective test results and often rely on very limited or
are the gold standard for microbial identifications. They provide the best chance for accurate and reliable specieslevel identifications but require complex instrumentation and highly skilled personnel, and the throughput is limited. Experienced scientists are also needed to properly analyze the sequence data and phylogenetic trees to ensure accurate
n Audits
Periodic internal audits conducted by quality assurance departments are an excellent way to identify potential issues and engage in continuous quality improvement initiatives.
Addressing issues internally helps ensure that external audits run smoothly with minimal impact on the facility. This is
clinically-focused identification libraries. These methods also rely on ancillary tests, such as Gram stains, prior to analysis, leading to further variability and data integrity concerns.
Proteotypic methods, based on MALDITOF analysis of primarily ribosomal proteins in a microorganism, can be very reliable and have a high sample throughput. However, attention needs to be paid to the breadth (number of species) and depth (number of strains) of the identification library to ensure it is pharma-focused to reproducibly obtain the highest rate of species-level calls.
Genotypic methods (DNA sequencing)
identifications are made. DNA sequencing is usually best left to a trusted outsourcing partner.
Best Practices for Mitigating Risk
Compounders produce drugs under less regulatory pressure while also, paradoxically, being expected to maintain product quality and ensure patient safety to the level at which FDA-approved drugs are held. With that, those looking for quality improvements to mitigate risk may consider implementing processes and procedures often seen in pharmaceutical industry facilities that produce regulated products.
especially critical given that, although some external audits are scheduled or expected within a certain time frame, some are complete surprises.
The types of policies, procedures, or systems being audited depend on the auditing entity. Auditors may be potential clients, current customers, state boards of pharmacy, FDA or DEA, and city or county officials. More detailed audits may include an examination of organizational structures and responsibilities, training and quality policies, corrective and preventive actions, facility operations, equipment qualification, incoming material receipt and testing, finished product testing and
release, and environmental monitoring. Regardless of the audit type, it is essential to be ready.
n Outsourcing
Identifying a trusted, reliable outsourcing partner offers many advantages for a pharmaceutical compounding facility. Most importantly, outsourcing provides compounding pharmacies access to qualified, compliant products and services that help them protect patients and their businesses while improving microbial control programs.
A highly experienced consultant in microbial control can help identify gaps in a facility's quality processes and procedures and define a plan for quality improvement. The cost per reportable result also decreases while the accuracy of identifications increases without having to make capital investments and maintain expensive identification equipment — helping improve operational efficiencies while reducing compliance risk.
One factor often overlooked in the cost analysis of partnering with an outsourcing company is the cost of inaccurate identification. Processes that result in inaccurate, inconsistent, or no identification must be repeated using other methods to get successful identification. In addition to adding time and operational costs, it can lead to misdirected remediation efforts and pose a threat to patient safety. Outsourcing microbial identifications also frees up your quality control microbiology staff to work on other critical tasks.
BIOGRAPHY
What to Look for in an Outsourcing Partner
When choosing an outsourcing partner, it is imperative that you start by conducting a comprehensive supplier verification audit to assess the quality of their products and services. After all, their data is your data, and you must be confident that your chosen outsourcing partner is generating compliant, audit-ready information to support your business.
First and foremost, outsourcing partners should have the industry knowledge and technical expertise to provide value to the organization to ensure the safe supply of compounded medications. Whether you're a 503A pharmacy or 503B facility, look for companies that:
• Are cGMP-compliant, FDA-registered, and maintain one or more ISO certifications for quality testing, such as ISO 17025.
• Have FDA-licensed products used for compliance with compendial test methods.
• Have extensive, quality reference databases with respect to microbial identification that are validated and updated frequently.
• Offer integrated cGMP-compliant data management tools for tracking and trending microorganism information so you can access data whenever needed.
• Provide access to a capable technical support group to assist with everything from routine identifications to microbial investigations
More than anything, however, you should look for an outsourcing partner you feel confident will be with you every step of the way, from sample submission to identification to technical support.
Looking Ahead
The unique position of compounding pharmacies in the drug production and regulatory landscape enables them to help combat supply chain disruptions during catastrophic events.
This was seen most recently in 2017 with Hurricane Maria's impact on pharmaceutical production in Puerto Rico and, of course, COVID-19. During the pandemic, the FDA temporarily allowed commercially available drugs to be compounded by pharmacy compounders not registered as outsourcing facilities. With this, market growth is expected to accelerate as these pharmacies continue to play an increasingly important role in the industry as it relates to the rising costs of commercial drugs, decreasing drug accessibility, and drug shortages.
Dr. Doug Botkin

Technology and Market Development Manager
~ Charles River Laboratories
Dr. Doug Botkin is a Technology and Market Development Manager at Charles River Laboratories. He is a subject matter expert in the Microbial Solutions group, focusing on Accugenix products and services for microbial identifications. Doug has over 24 years of experience in infectious disease research, spaceflight microbiology, and pharmaceutical microbiology. He holds a bachelor’s degree in Biological Sciences from Illinois State University and earned his PhD in Microbiology and Molecular Genetics from The University of Texas Health Science Center at Houston.
SUMMER 2022 I HEALTH SYSTEM • INFUSION 69 WINTER




Unit Dose Done Right Unit Dose Done Right Unit Dose, Bar PharmacyCoding, & NursingExperts!Supply Unit Dose, Bar PharmacyCoding, & NursingExperts!Supply • Cold Seal • Tamper-Evident • Moisture Resistant • Ultraviolet Inhibitant • Reduces Cross Contamination • Ideal for Meds Covered by USP 800 • 6 and 12-month Beyond-Use Dating • 1-D and 2-D Bar Coding • Flexible Label and Report Formatting • Multiple Sizes and Shapes to Fit Your Meds & Storage Needs MediDose.com 800.523.8966 Scan QR Code for more information and pricing. Brightly Colored Labels Call At tention to Meds Requiring Special Handling Simple. Reliable.
Medi-Dose, Inc./EPS, Inc. Delivers Bar Coding, Packaging, and Labeling Pharmacy Solutions


President: Robert Braverman
Founded: 1971
Employees: Private
Toll-Free Phone: (800) 523-8966
Phone: (215) 396-8600
Toll-Free Fax: (800) 323-8966
Address: 70 Industrial Drive Ivyland, PA 18974 Website: www.medidose.com
Company Background
Medi-Dose/EPS was founded in 1971 when Milton Braverman, a former pharmaceutical company territory manager, saw the need for inexpensive, manual unit dose packaging allowing a hospital to convert from traditional dispensing. He developed the Medi-Dose System to package, handle, and dispense predetermined amounts of medication so they would be accessible for one regular dose. Comprehensive bar coding and labeling identification is accomplished using our innovative MILT 4 software. Because of the continued success of the MediDose System, Medi-Dose/EPS expanded its product line to include the TampAlerT System, offering tamper-evident liquid packaging without the need for heat tunnels and accessory sealing equipment, as well as a full line of supplies and disposable products designed specifically for the pharmacy and health care professional.
Product Overview
Medi-Dose provides Tamper-Evidence as well as UV and Moisture Resistance for one-year beyond-use dating. Our MILT® 4 software maintains packaging logs and lets you design and print your Lid-Label® Covers with color, bar codes, tall man lettering, and graphics. You can format the labels any way you want, directly from your own computer and printer!
Medi-Dose works well in any pharmacy operation and can be used with all classifications of drugs (chemo meds, meds covered by USP 800, compounded drugs, controlled substances, etc.). It’s affordably priced for all pharmacy budgets. For the best in manual unit dose packaging, check out Medi-Dose!
Features & Options
n Packaging and Labeling Solutions
• Simple to use — no extensive training needed.
• 1-D and 2-D bar coding — including NDC, lot numbers, and expiration dating.
• Ideal for hazardous medications and USP 800 drugs.
• Tall Man Lettering and dynamic formatting options.
• Built-in NDC lookup database and extensive image library.
• Packaging logs and error reporting.
• Six-month and one-year beyond-use dating.
• UV and moisture resistance.
• Tamper-Evidence.
• 15 styles of blisters to accommodate virtually all meds.
• No machinery or space requirements.
• Inexpensive — no capital outlay required.
Product Specifications
n Accompanying Labeling Software
• MILT 4
• MILT 3.0
• MILT 2.6
• Medi-Dose 2000
Trade Shows/Meetings Attended
ASHP Midyear, ASHP Summer, Joint Forces Pharmacy Seminar, National Pharmacy Purchasing Association, EAHP, IACP, and various state, regional, and pharmacy compounding conferences.
Ordering Information
For additional information, please contact us at (800) 523-8966, visit our website at www.medidose.com, or email us at info@medidose.com.
WINTER 2022 I HEALTH SYSTEM • INFUSION 71 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
Improve your Solid Oral Unit Dose needs with our comprehensive Bar Coding, Packaging, and Labeling Solutions — designed by healthcare professionals, for healthcare professionals.
Why DrReddysDirect.com?




DrReddysDirect.com is designed to provide a convenient platform for customers. Save money and time by placing your order online with us.

Easy ordering and turnaround time You get fast access to a ordable medicines. You can also view and manage your orders online. Visit DrReddysDirect.com today or contact your Sales Rep for more information.


Your
store
your
www.DrReddysDirect.com
virtual
at
fingertips.
©2022 Dr. Reddy’s Laboratories Inc. RDY-0821-364 AVAILABLE
FROM















































EXPLORE OUR CAPABILITIES AND ANYWHERE ELSE YOU CAN IMAGINE US! Our Mission Health Care Logistics
distribution
medical supplies
lines
medical products,
dispensing items,
prevention supplies
materials. Hospital Departments Served • Central Supply • Materials Management • Emergency Room • Nursing • Facilities Engineering • Oncology • ICU • Operating Room • Infection Control • Patient Room • Laboratory • Pharmacy GoHCL.com • 1.800.848.1633 HCL® BY DESIGN HOSPITAL PHARMACY PATIENT ROOM PLASTICS BY DESIGN METALS BY DESIGN
has been a veteran-owned, industry leader in the
and manufacture of
since 1978. We have over 22 extensive
of
which include compounding and
infection
and unit dose packaging
Central Pharmacy Automation Solutions that transform organizations through efficiency — Experience exponential cost savings by streamlining supply chain operations — Significantly improve fulfillment accuracy and minimize waste with centralized pharmacy inventory management — Improve clinical outcomes by spending more time attending to patient needs Contact the Central Fill Pharmacy Experts at swisslog-healthcare.com/ pharmacyautomation
Medication Management Solutions
From Swisslog Healthcare Elevate the Way You Work
CEO: Cory Kwarta
Founded: 1915
Employees: 500-1,000 Toll-Free Phone: (800) 764-0300
Address: 11325 Main Street Broomfield, CO 80020 Website: www.swisslog-healthcare.com
Company Background
Swisslog Healthcare connects the medication supply chain, bringing together automated transport and pharmacy automation solutions in more than 3,000 healthcare institutions worldwide. These connections are made possible through:
• Proven pharmacy robotics.
• Enterprise medication management software.
• The industry’s most trusted pneumatic tube systems. Through an agnostic approach to integration, Swisslog Healthcare enables forward-thinking health systems to create a connected medication ecosystem. Learn more at www.swisslog-healthcare.com.
Product Overview
Hospitals today are held to increasingly higher accountability for the overall patient experience. The timely dispense and delivery of correct medications are critical to improving patient outcomes and fostering a positive experience. Whether on a large or small scale, the right automation can enhance patient safety, reduce medication waste and drug shortages, reduce costs, and enable staff to spend more time focusing on clinical activities. Discover how our comprehensive automation solutions can boost efficiency, improve care, and reduce costs at your healthcare facility.
Product Solutions
n BoxPicker® Automated Pharmacy Storage System provides pharmacies with secure, high-density, modular storage of medications, including varied dosage forms — controlling access, improving pharmacy workflows, and expediting the picking process. BoxPicker uses robots to multitask, and multiple operator stations allow for simultaneous operations, significantly boosting staff efficiency. With the dual access points, one technician can stock medications within the system, while a second technician can pick patient-specific drugs, complete refills for automated dispensing cabinets, or restock returns.
n PillPick® Automated Packaging and Dispensing System decreases human touches in bar coding, packaging, storing, dispensing, and returning unit-dose medications. The patented PickRing® delivers patient-specific medication on a single ring ready for administration, reducing the opportunity for missed medications and speeding up
administration time. With the ultimate automated pharmacy system for patient safety and operational efficiency, nurses can spend more time delivering high-quality care.
n AutoCarousel ® Semi-Automated Pharmacy Storage System , together with medication management software, transforms medication management by reducing labor, drug waste, missing medications, and picking mistakes. AutoCarousel is accurate and reliable, and the vertical design ensures maximal storage in a compact footprint, reclaiming valuable pharmacy space. In addition, AutoCarousel helps pharmacies ensure medication safety with password-protected access and barcode scanning during the stocking and dispensing processes to track the chain of custody and manage all items comprehensively.
n AutoPack™ Automated Oral Solid Packager is an oral solid packager that integrates easily with pharmacy operations to provide fully automated, unit-dose, or multi-dose oral solid medication packaging, ensuring that 100% of medications are scan-ready at the bedside. Barcoded medications make it easier for nurses to do their job well.
n Pharmacy Manager Medication Management Software is designed with growing health systems in mind. It provides the operational framework for your pharmacy. Pharmacy Manager connects the supply chain to patient delivery by controlling inventory movement across the enterprise. Manage as many or as few locations as you need, from large hospital campuses to clinics and satellite pharmacies. Optimize inventory spending, uncover business insights, and control medication movement with Pharmacy Manager.
Testimonial
As a trusted partner, Swisslog Healthcare pharmacy automation plays an essential role in safe, efficient medication administration at the largest acute care center in the greater Toronto area. The state-of-the-art Humber River Hospital features two pharmacy automation solutions from Swisslog Healthcare, the PillPick® Automated Packaging and Dispensing System and the BoxPicker Automated Pharmacy Storage System. As a result, Humber River Hospital reports a reduction of more than 50% in medication picking errors. In addition, the deployment of the PillPick has also led to a reduction of at least 30% in pharmacy technician labor, allowing for the re-deployment of skilled staff to other higher-value and rewarding tasks.
“�The whole ecosystem helps us do our jobs better; this means safety and efficiency enterprise wide. We have a great relationship with our Swisslog Healthcare team.” — Albert Karas, Director, Pharmacy Services, Humber River Hospital
WINTER 2022 I HEALTH SYSTEM • INFUSION 75 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
Deliver exceptional patient care with innovations from the core of the central pharmacy to enhance performance workflow efficiency, improve accuracy, and increase safety.
Video Series
THOUGHT LEADER VIDEO SERIES
THOUGHT LEADER VIDEO SERIES
Advancing the Business of Pharmacy
Join Steve Rough, Senior Vice President, Phil Brummond, Senior Vice President, Jerame Hill, Vice President, Joe Cesarz, Vice President, and Maxie Friemel, Senior Director of Pharmacy Revenue Cycle Services, as they discuss hospitals and health systems, infusion, and pharmacy revenue cycles.
www.visanteinc.com
rxinsider.com/videosTLS.php
THOUGHT LEADER VIDEO SERIES
The Compliance Team



Join Caitlin Warner, Provider Relations, Jack Haire, Director of DMEPOS & Pharmacy Services, Sandy Canally, RN, Founder & CEO, Steve Simmerman, COO, and Scott Muscarella, Vice President of Marketing, as they discuss Exemplary Provider Accreditation in a 12-part video series.














Surescripts
Join Melanie Marcus, Chief Marketing Officer, Cecelia Byers, Specialty Pharmacy Clinical Product Manager, and Tom Skelton, Chief Executive Officer, as they discuss the nation’s most trusted and capable health information network.
surescripts.com

rxinsider.com/videosTLS.php


THOUGHT LEADER VIDEO SERIES
www.thecomplianceteam.org
rxinsider.com/videosTLS.php
Transforming Healthcare Through Pharmacy
Join Jim Jorgenson, CEO, Phil Brummond, Senior Vice President, Joe Lassiter, Senior Vice President, Chief Pharmacy Informatics Officer, Steve Rough, Senior Vice President, Dave Hager, Senior Director, Health Systems, and Alan Yordy, Former President of PeaceHealth, as they discuss transforming healthcare through pharmacy.
www.visanteinc.com
rxinsider.com/videosTLS.php
WINTER 2022 I HEALTH SYSTEM • INFUSION 76
THOUGHT LEADER
THOUGHT LEADER VIDEO SERIES
THOUGHT LEADER VIDEO SERIES
CPS

Join Frank Segrave, Chuck Ball, Michael McCarrell, Joseph Dula, Rabiah Dys, Chris Beebe, Kelly Kolker, Paresh Patel, Keith Cook, Eric Murphy, Lars Ringger, and Tony Callander, as they discuss partnering with hospital and health system leaders to drive performance excellence.

www.cpspharm.com
rxinsider.com/videosTLS.php

THOUGHT LEADER VIDEO SERIES


The Nation’s Premier Pharmaceutical Returns Processor in the Pharmacy Market







Thierry Beckers, President & COO, Gil Kanner, Vice President, Supply Chain Logistics, Hilmer Beckers, Chairman & CEO, and Adam Bottie, Vice President, Sales & Business Development, discuss PharmaLink: The Nation’s Premier Pharmaceutical Returns Processor in a 12-part video series.






Azina
Join Keith Cook, President, Doug Massey, Senior Vice President of Pharmacy Services, John Luebker, Vice President of Payer Strategies, and Susan Trieu, Vice President of Trade Relations, as they discuss a specialty and ambulatory pharmacy solution designed to unleash the powerful potential inside hospitals and health systems.
www.azina.com
rxinsider.com/videosTLS.php
THOUGHT LEADER VIDEO SERIES
www.pharmalinkinc.com
rxinsider.com/videosTLS.php
Accreditation Commission for Health Care (ACHC)

Join Josè Domingos, Matt Hughes, Renee White, Ralph McBride, Jon Pritchett, Megan Reed, and Greg Stowell as they discuss Accreditation Commission for Health Care (ACHC), for providers, by providers.
www.achc.org
rxinsider.com/videosTLS.php
WINTER 2022 I HEALTH SYSTEM • INFUSION 77
THOUGHT LEADER Video Series
It’s
Safeguard Your Community
Time To Protect What Matters Most.
With the right tools in place, your organization can take significant steps towards advancing community health, addressing the opioid crisis, and positively impacting the environment.
SafeDrop™ Sharps Mail Back Containers
By providing your employees, patients, and community neighbors with a safe and convenient way to dispose of used sharps, you help reduce the risk of injury or misuse and protect the environment.
MedDrop™ Drug Collection Kiosks
These versatile and secure medication collection kiosks can be placed within pharmacies, hospitals, and law enforcement agencies to ensure a safe and convenient way for consumers (including employees) to dispose of unused medications.
Seal&Send™ Medication Envelopes
By instituting compliant, safe, convenient, and anonymous prescription drug disposal mail back solutions, you actively demonstrate your organization’s commitment to public health protection and environmental stewardship.
Visit Stericycle.com or call 866-783-7422.

P H A R M A C E U T I C A L W A S T E S O L U T I O N S
© 2022 Stericycle, Inc. All rights reserved. RXINSAD_0922
Safeguard Your Organization, Community, and the Environment With Stericycle Solutions
President & CEO: Cindy J. Miller
Founded: 1989
Employees: 15,000+ Stock Symbol: SRCL
Toll-Free Phone: (866) 783-9816 Phone: (844) 752-6211 Address: 2355 Waukegan Road Bannockburn, IL 60015 Website: www.stericycle.com
Company Background
Stericycle supports the leading role that pharmacists hold as healthcare providers in our communities. For over three decades, Stericycle has introduced innovative products and services for regulated medical waste management and is a pioneer in offering pharmaceutical waste solutions that help drive safety, regulatory compliance, and environmental sustainability.
Product Overview
n Increase Efficiency, Safety, and Your Organization’s Community Stewardship
Stericycle’s Safe Community Solutions increase your patient engagement by offering convenient disposal of unused consumer medication and sharps waste generated by home injectable therapies.
• MedDrop™ Drug Collection Kiosks help prevent prescription drug abuse in your community and reduce the environmental risk that results from improper disposal. Customers and patients appreciate the convenience and anonymity of returning unused medications to the same place they received them. Add additional value to retain customers and promote brand loyalty with Stericycle’s MedDrop Medication Collection Kiosks.
• Seal&Send™ Medication Mail Back Envelopes allow consumers to package and mail their unwanted or expired medications to Stericycle in secure, anonymous, prepaid envelopes via USPS. Seal&Send envelopes are particularly useful for rural and homecare patients to safely and sustainably dispose of unused medications simply by placing them in a mailbox.
• SafeDrop™ Sharps Mail Back Kits include everything needed to properly collect and safely dispose of sharps.
With a convenient auto-replenishment option, Stericycle makes it easy to manage container inventory. SafeDrop provides efficient and compliant disposal of sharps waste generated at your facility and serves as a driving component of successful COVID-19, influenza, and other immunization programs.
Additionally, your patients can use the SafeDrop Mail Back program to safely store and dispose of sharps waste generated through their injectable therapies at home.
Stericycle: Your Trusted Partner
Stericycle has partnered with thousands of pharmacies of all sizes to provide easy-to-use regulated waste disposal services that help:
• Keep employees safe and healthy and protect your brand — by safeguarding your staff, community, and organization through compliant disposal solutions.
• Add customer value in store — by driving foot traffic and brand loyalty through safe in-store disposal solutions.
• Extend customer satisfaction at home — by prolonging enhanced customer experience and extending your brand’s reach through secure in-home disposal solutions.
To learn more, visit www.stericycle.com/en-us/solutions/specialtyservices/consumer-take-back-solutions today. Protect your employees, customers, and community.
WINTER 2022 I HEALTH SYSTEM • INFUSION 79 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
Stericycle’s Safe Community Solutions provide your patients with safe and convenient disposal of sharps waste and unused medications.
& MEETING
Event
Calendar

Event Calendar
Trade Shows, Meetings, & Expositions

RXinsider’s Virtual
Pharmacy Trade Show 24 / 7 / 365 450+ Booths, 89 Aisles www.rxinsider.com
ASHP Midyear Clinical Meeting and Exhibition December 4-8, 2022 Las Vegas, NV ashp.org/Meetings-andConferences/MidyearClinical-Meeting-andExhibition
PDS Super Conference February 16-18, 2023 Disney’s Coronado Springs Resort Orlando, FL Pdsconference.com
American College of Apothecaries Annual Conference & Expo 2023 February 22-25, 2023 Wyndham Grand Rio Mar, Puerto Rico acainfo.org/ace
APhA 2023 March 24-27, 2023 Phoenix, AZ Aphameeting.pharmacist.com
NHIA 2023
March 25-29, 2023 Gaylord National Resort & Convention Center Washington, DC conference.nhia.org
340B Coalition Winter Conference March 27-29, 2023 San Diego, CA 340bwinterconference.org
NACDS Annual Meeting April 22-25, 2023 The Breakers Palm Beach, FL Annual.nacds.org
AXS23 Summit April 30-May 4, 2023 Las Vegas, NV Asembiasummit.com
ESTECH 2023 May 8-11, 2023 Minneapolis/St. Paul, MN iest.org/Meetings/ESTECH
ACVP Veterinary Pharmacy Conference June 1-3, 2023 Denver, CO vetmeds.org/vpc
WINTER 2022 I HEALTH SYSTEM • INFUSION 80
TRADE SHOW
For listing and advertising details, please contact sales@RXinsider.com or call 800.972.2083.
Continued on page 85
We provide support in addressing your 340B operational and compliance challenges
Prepare for a government or manufacturer audit
Stay compliant with changes to Medicare Part B reimbursements
Compare split-billing software vendors
Member Benefits
Advocacy
As your voice in Washington, we encourage you to join our advocacy e orts to protect 340B, which includes creating an Impact Profile document illustrating how your savings support your safety-net mission. Our sta is ready to help your hospital engage in advocacy through the development of op-eds and social media campaigns.
Webinars
You and your sta will be invited to 340B related webinars throughout the year on topics ranging from audit prep and findings to recertification. We host over a dozen webinars each year and encourage your team to join us.
Real-Time 340B Information
Stay in-the-know and up to date through our email alerts, members-only bi-weekly Bulletin, and the 340B Informed blog.
Connect with 340B hospitals around the country
Technical Assistance (TA) Calls
Take advantage of this invaluable resource by speaking with a member of our legal and/or pharmacy teams. Our sta is able to answer questions on topics ranging from audit prep and diversion to adding child sites and drafting policies and procedures.
Individual Membership
Through your hospital’s membership, you are eligible to join this supplemental program which provides professional development and networking opportunities.
340B Health Website
The website has sample forms, letters and P&Ps, as well as policy guides and member best practices. Miss a webinar? No problem, past webinars are housed on our website. Find our audit and compliance resource centers along with a calendar of upcoming events.
The Exchange
Access our secure online member discussion forum 24/7 for answers to your most pressing 340B related questions, hear ideas and best practices, and grow your network.
National Conferences
340B Health, along with our 340B Coalition partners, provides you unparalleled access to information and networking opportunities at our summer and winter conferences. 340B Health and 340B Coalition members receive discounts to both events.
Questions? Contact Shane Kelley at (202) 552-5864 or email shane.kelley@340bhealth.org.
Meet with Customers that Matter to
Your Business ECRM’s Pharmacy & Medical Programs set you up for success with pre-scheduled, one-on-one meetings with your strategic partners. Contact our SVP of Pharmacy & Medical Markets, Michael Castillo to learn how you can take advantage of virtual and in-person opportunities at 440-528-0441 or mcastillo@ecrm.marketgate.com




















T H E INTERNAT I O N A L JOURNAL O F P H ARMACEUT I C A L C OMPOUNDI N G IJPC 19 97 • • OUR COMPOUNDING KNOWLEDGE, Your Peace of Mind® IJPC OUR COMPOUNDING KNOWLEDGE, Your Peace of Mind® 800-757-4572 EXT1 PHARMA CISTS WHO WANT Peace of Mind SUBSCRIBE to IJPC EMAIL US for a FREE SAMPLE COPY at IJPC-SUBSCRIPTIONS@IJPC.COM Subject Line: 20Ways Winter Hospital The ONLY PUBLI CATION focusing on QUALITY PHARMACEUTICAL COMPOUNDING. www.IJPC.com H NTER A A O NA O A MACEU I M OUN G IJPC 19 OUR COMPOUNDING KNOWLEDGE, Your Peace of Mind® IJPC 34 CONSIDERATIONS AND BACKGROUND INFORMATION FOR COUNSELING PATIENTS USING COMPOUNDED SUPPOSITORIES 41 COMPOUNDING STERILE PREPARATIONS WITH CYCLODEXTRINS SHOULD ALL PHARMACISTS COMPOUND? INTERNATIONAL JOURNAL OF PHARMACEUTICAL COMPOUNDING JANUARY FEBRUARY 2022 V.26 OurCompoundingKnowledge, YourPeace Mind Jan_Feb_2022_Covers.indd 1/16/22 AM H N NA OURNA O P A MAC U MPOUN G IJPC OUR Your Peace of Mind® IJPC 34 CONSIDERATIONS AND BACKGROUND INFORMATION PATIENTS COMPOUNDED SUPPOSITORIES 41 COMPOUNDING STERILE PREPARATIONS WITH COMPOUND? INTERNATIONAL JOURNAL OF PHARMACEUTICAL COMPOUNDING JANUARY | FEBRUARY 2022 V.26 Our Compounding Knowledge, Your Peace of Mind 1/16/22 H TERN T O AL O P ARM CE T C MPOU D N IJPC 19 97 OUR COMPOUNDING KNOWLEDGE, Your Peace of Mind® IJPC 34 CONSIDERATIONS AND BACKGROUND INFORMATION COUNSELING PATIENTS USING COMPOUNDED SUPPOSITORIES 41 COMPOUNDING STERILE PREPARATIONS WITH CYCLODEXTRINS PHARMACISTS COMPOUND? INTERNATIONAL JOURNAL OF PHARMACEUTICAL COMPOUNDING JANUARY FEBRUARY 2022 V.26 NO.1 Our Compounding Knowledge, Your Peace of Mind Jan_Feb_2022_Covers.indd 1/16/22 E N RN T O O RN F HA C A C MPO N N IJPC 19 97 OUR COMPOUNDING KNOWLEDGE, Your Peace of Mind® IJPC CONSIDERATIONS AND BACKGROUND INFORMATION FOR COUNSELING PATIENTS USING COMPOUNDED SUPPOSITORIES 41 COMPOUNDING STERILE PREPARATIONS WITH CYCLODEXTRINS PHARMACISTS COMPOUND? INTERNATIONAL JOURNAL OF PHARMACEUTICAL COMPOUNDING V.26 NO.1 OurCompoundingKnowledge, YourPeaceofMind Jan_Feb_2022_Covers.indd 1/16/22 10:29 N NA N U F HA U T C OMPO N IJPC 19 97 OURCOMPOUNDINGKNOWLEDGE, YourPeaceofMind® IJPC CONSIDERATIONS AND BACKGROUND INFORMATION FOR COUNSELING PATIENTS USING COMPOUNDED SUPPOSITORIES COMPOUNDING STERILE PREPARATIONS WITH CYCLODEXTRINS SHOULD ALL PHARMACISTS COMPOUND? INTERNATIONAL JOURNAL OF PHARMACEUTICAL COMPOUNDING JANUARY|FEBRUARY2022 V.26 NO.1 OurCompoundingKnowledge, YourPeaceofMind® Jan_Feb_2022_Covers.indd 1/16/22 10:29AM N ERN T N O RN ARM C COMP U D G IJPC 19 97 OURCOMPOUNDINGKNOWLEDGE, YourPeaceofMind® IJPC INFORMATIONCONSIDERATIONSANDBACKGROUNDFORCOUNSELINGPATIENTS USINGCOMPOUNDEDSUPPOSITORIES PREPARATIONSCOMPOUNDINGSTERILEWITHCYCLODEXTRINS 24 PHARMACISTSSHOULDALLCOMPOUND? INTERNATIONAL JOURNAL OF PHARMACEUTICAL COMPOUNDING JANUARY|FEBRUARY2022 V.26 NO.1 OurCompoundingKnowledge, YourPeaceofMind IJPC.com and CompoundingToday.com Jan_Feb_2022_Covers.indd 1 1/16/22 10:29AM

Every Good Pharmacist ... is a Great Resource.
80+ aisles, the Virtual Pharmacy Trade Show is pharmacy’s one-stop destination to research and connect with leading providers of products and services throughout every pharmacy category and practice setting.
Behind
With
RXinsider.com
Event Calendar

Trade Shows, Meetings, & Expositions
ASHP Summer Meetings and Exhibition
June 10-14, 2023 Baltimore, MD ashp.org/Meetings-andEvents
McKesson ideaShare June 22-25, 2023 Las Vegas, NV Mckessonideashare.com
Cardinal Health RBC
July 19-22, 2023 Boston, MA rbc.cardinalhealth.com
NACDS Total Store Expo
August 12-14, 2023 San Diego, CA tse.nacds.org
NASP 2023 Annual Meeting & Expo September 18-21, 2023 Grapevine, TX naspnet.org/annual-meeting
NCPA Annual Convention
October 14-17, 2023 Orlando, FL ncpa.org/annual-convention
ASCP Annual Meeting & Exhibition
October 26-29, 2023 Kissimmee, FL ascp.com/page/events
ASHP Midyear Clinical Meeting and Exhibition 2023 December 3-7, 2023 Anaheim, CA ashp.org/Meetings-andConferences/MidyearClinical-Meeting-andExhibition

RXinsider.com

WINTER 2022 I HEALTH SYSTEM • INFUSION 85 TRADE SHOW & MEETING
Event Calendar For listing and advertising details, please contact sales@RXinsider.com or call 800.972.2083.
Behind Every Good Pharmacist ... is a Great Resource. The Pharmacy Market BUZZ is a daily news feed, designed to keep the pharmacy community informed on the latest industry news, products, services, and trends.
Behind Every Good Pharmacist ... is a Great Resource. As a complimentary service, RXinsider provides FREE, high-resolution downloads of vintage pharmacy ads and imagery.
RXinsider.com Continued from page 80
Trends to Watch as 340B Turns 30
Written By: Maureen Testoni President & CEO 340B Health
Maureen Testoni is the president and CEO of 340B Health, which represents more than 1,400 hospitals participating in the 340B drug pricing program.

This year marked an important milestone for the 340B drug pricing program. On November 4th, we celebrated the 30th anniversary of 340B. In the three decades since President George H.W. Bush signed it into law, 340B has enabled nonprofit hospitals, health centers, and clinics to serve communities in every corner of our country. Thanks to 340B, patients receive quality care closer to their homes, jobs, and families. Communities have access to vital health services and delivery networks needed to sustain a robust social fabric. And all this has been possible because of a program that has enjoyed bipartisan support across five administrations, 15 Congresses, thousands of communities, and millions of Americans without relying on taxpayer dollars to fund the care.
Despite these successes, the stakes have never been higher for those of us who advocate on behalf of 340B and the providers who participate in it. With 340B growing and evolving to meet changing patient health needs, it has gained more national attention than ever before. Ten years ago, we might have had trouble finding people who had even heard of 340B, let alone who were familiar with the critical role it plays in bolstering the health care safety net. Now 340B is appearing regularly in publications throughout the U.S., in the floor speeches of prominent members of Congress, and even in the highest court in the land.
With that increased attention has come increased challenges for 340B covered entities and their patients. We have had to defend 340B on several new fronts that never were in play before.
340B Health, which represents more than 1,400 health systems and hospitals, advocates on behalf of 340B. We also work to help our member pharmacists, 340B program managers, and other health professionals run effective, compliant programs for the benefit of patients in need. As we wrap up the 30th anniversary year for 340B, we are centered on several key trends and areas of focus that we believe will be pivotal for the future of the program.
Drug Industry Attacks on 340B
The pharmaceutical industry recently has been pushing hard to change the program in ways that diminish the amount of help it provides to safety-net providers and the patients with low incomes whom they serve. This is not true for all drug companies, as more than 700 participate in 340B and continue to abide by the law.
Since 2020, the single biggest challenge to 340B has come in the form of pharmaceutical company restrictions on savings for covered entities. After years of unsuccessful attempts to convince Congress to make cuts to 340B, several major drugmakers started unilaterally restricting discounts to covered entities on drugs dispensed at community and specialty pharmacy partners, actions that the Department of Health and Human Services (HHS) has said are illegal.
What started with a handful of companies has now expanded into a major threat to 340B. The companies now restricting access to 340B discounts include several of the largest drugmakers in the world that collectively brought in more than $600 billion in revenues in 2021. Their actions have caused immense harm to safety-net providers who rely on these savings to treat their patients in need. A survey of hospitals we conducted in early 2022 found larger, typically urban 340B hospitals reporting average financial losses of millions of dollars per year because of these restrictions. Smaller, typically more rural hospitals are losing hundreds of thousands of dollars per year on average. These losses are devastating to facilities that already rely on the thinnest of operating margins to stay open.
Why are these drug companies waging this assault on 340B savings? Not surprisingly, the answer is rooted in a systematic pursuit of profits. Our analysis of the companies that are restricting access to discounts and the drugs they are targeting for these restrictions demonstrate two main strategies companies are using to boost revenues at the expense of safety-net providers and patients. The first focuses on denying access to discounts for some of the costliest specialty drugs that physicians are prescribing for patients living with chronic diseases, including various forms of cancer. The second focuses on getting around the federal penalties imposed on them after they raised the price of drugs much faster than the rate of inflation. For such drugs as insulin, for which the price skyrocketed 1,200% over the past two decades, these penalties permit 340B providers to pay a nominal amount and use the savings to care for more patients.
The unilateral cuts to 340B from these manufacturers keep more dollars in drug company coffers and deprive safety-net hospitals of resources they need to fund patient care. Both the Trump and Biden administrations have told the drugmakers they are breaking the law. But instead of complying, several companies went to court to challenge the government’s authority. Several federal appeals courts heard oral arguments in those cases earlier this fall as part of what we
86 WINTER 2022 I HEALTH SYSTEM • INFUSION
expect to be a protracted legal battle over the drug company actions.
In the meantime, pharmacists and other health providers are continuing to do their utmost to preserve 340B-funded care for patients in need amid the cuts in savings. Many of these providers also are sharing the challenges of doing so with lawmakers, media outlets, and others. Complaints and reports against 340B restrictions have led to bipartisan shows of support for covered entities from members of Congress and state elected officials. It has aided immensely in the federal government’s efforts to pursue strong enforcement actions against the companies and to assert its authority to do so in federal courts. And it has put the drug industry in the position of defending the reputations of companies that are harming the health care safety net by cutting 340B.
The End of Medicare 340B Cuts
The federal litigation process can take a significant amount of time to navigate, and it can have mixed outcomes for the parties involved. But a years-long legal battle over Medicare payment cuts to 340B hospitals recently culminated in a decisive victory for the hospitals.
Starting in 2018, Medicare started cutting its payment rates for outpatient drugs used at most 340B hospitals. A group of hospitals and hospital associations filed a lawsuit against the government challenging the pay cuts. Hospitals said the cuts forced them either to offer fewer comprehensive services to patients in need or to postpone planned expansions of that care. It is rare for a lawsuit to make its way to the U.S. Supreme Court, and it is rarer still for a case to result in a unanimous decision. But earlier this year, all nine justices signed onto a decision stating that Medicare broke the law when it applied the cuts just to 340B hospitals without first conducting a required survey of hospital drug acquisition costs. The high court sent the issue back to a lower court to determine what remedies affected hospitals should receive for the years’ worth of unlawful cuts.
The Supreme Court decision resulted in some long-overdue clarity for 340B hospitals about how much the law requires Medicare to pay them for outpatient drugs that they prescribe
to their senior patients. However, it also raised some new areas of uncertainty that will require further clarification. The most immediate effect of the decision was to prompt the Centers for Medicare & Medicaid Services (CMS) to restore full Medicare outpatient drug payment rates to 340B hospitals starting in late September.
play in the federal court fight over Medicare payments.
Increasing numbers of states have been cracking down on discriminatory payment policies by health insurers and PBMs that effectively divert the benefit of 340B from the providers for whom it is intended. Before 2019, no state had a law banning 340B payment discrimination.
Now nearly half of the states in the U.S. have such statutes on the books, and that number continues to grow every year.
These laws vary in terms of what types of discriminatory payment they ban and what covered entities they protect.
But that development did not settle the question of how 340B hospitals would be made whole for the nearly five years’ worth of cuts that they have sustained under the unlawful policy.
Federal officials and courts will continue to work out the remedy details, a process that we expect could take a significant amount of time to complete. We have told the government that affected hospitals should receive repayments to cover the full amounts for outpatient drugs that they should have received had Medicare set the rates correctly since 2018, plus interest. We are opposing any future attempts to implement new drug payment cuts based on hospital drug acquisition cost data, as this would bring back cuts that are antithetical to the intended 340B benefit for the health care safety net.
The Supreme Court decision remains an important victory for 340B hospitals that goes well beyond Medicare. Private health insurers and pharmacy benefit managers (PBMs) had cited the Medicare policy as a precedent for imposing their own payment cuts on providers purchasing 340B drugs at discounted rates. The unanimous decision eliminates that precedent and makes it much more difficult for payers and PBMs to justify their discriminatory payment rates for covered entities.
Heightened State Activity on 340B
The importance of 340B also has drawn the attention of state officials. Much of the recent attention in the states has focused on the same type of payment discrimination that was at

The typical statute bans payers and PBMs from paying less for a 340B-purchased drug than they pay a non-340B provider for the same drug or from assessing additional fees or chargebacks on providers just because they participate in 340B. Several states ban burdensome claims reporting requirements on covered entities that do not apply to other providers. Some have extended the scope even further than private insurers and PBMs to prohibit drug companies from refusing discounts on drugs dispensed at contract pharmacies, though those protections are facing legal challenges. To assist states considering adding such protections, we have developed model state 340B nondiscrimination legislation for lawmakers to consider.
Looking Forward
All of us in the 340B provider community will continue to track these key trends as we advocate on behalf of the dedicated safetynet health professionals who care for patients in need. 2023 will be bringing more occasions for us to speak out on these topics. A new Congress will mean new opportunities to educate lawmakers on what 340B is and why they should protect it.
Pharmacists, 340B program managers, and other health professionals play important roles in these efforts. They can help advocate for the future of the program by documenting how they use their savings to care for patients in need, as well as communicating to policymakers and the public how important 340B is to their patient care missions. This is what it will take to demonstrate why 340B is a 30-year success story that is worth continuing for decades to come.
SUMMER 2022 I HEALTH SYSTEM • INFUSION 87 WINTER
Product Description
Complete filtering of incoming ambient air during use extends sterility. No contamination due to accident or lack of protocol.
Compliance with OSHA legal documentation requirements. Compliance with USP 797, NO refilling of bottle.
With a unique 0.22 micron filter vented sprayer, ISO-MED’s sterile isopropyl alcohol, it is the only product of its kind:

• 0.22 micron filter vented sprayer.
• Complete filtering of incoming ambient air during use.
• No contamination due to accident or lack of protocol.


• Compliance with OSHA legal documentation requirements.
• Compliance with USP797, no refilling of bottle.
• Sterile, Pyrogen-free Gamma Sterilized to 10-6
• Double-Bagged lot numbers and expiration dates.
• Certificates of Assurance and Sterility.
1220 Graphite Drive, Corona, CA 92881 USA | 800-797-1405 | sales@iso-med.com | www.iso-med.com
Products Number Description Sterile Case Packaging Sterile IPA Call for fast reordering: ISOA16 70% Isopropyl Alcohol, sterile ISOA16-C 70% Isopropyl Alcohol, sterile ISOA32 70% Isopropyl Alcohol, sterile 32 fl uid ounces ( 946 mL) trigger spray ISOA128 70% Isopropyl Alcohol, sterile 1 gallon (3.8 liters) polybottle Isopropyl Alcohol 70% Sterile IPA 12 polybottles 12 polybottles 6 polybottles 4 polybottles 16 fl uid ounces (473 mL) trigger spray 16 fluid ounces (473 mL) flip cap with trigger spray ISOlutions for Cleanroom & Medical Supplies © 2022 ISO-MED. All rights reserved. 800- 797-1405
If it Keeps Your Hospital Clean, ISO-MED Keeps it in Stock
It’s never been easier to pull the trigger on contamination because ISO-MED’s unique 0.22-micron filter vented sprayer on our 70% IPA keeps the compounding environment clean and safe. And like all our other products that help you maintain a sterile cleanroom, ISO-MED always has a full supply on hand.
President: David Lowrie
Founded: 2010
Toll-Free Phone: (800) 797-1405 Fax: (951) 547-1681
Address: 1220 Graphite Drive Corona, CA 92881 Website: www.iso-med.com
Company Background
Since 2011, ISO-MED, INC., a medical supply distributor, has been a trusted hospital supplier with a solid combination of quality, selection, service, and convenience. We supply quality products for pharmacy cleanrooms and other medical industries in the United States.
We provide high-grade supplies for cleanrooms, laboratories, home-care, and more. We strive to achieve and maintain compliant sterile compounding environments.
Product Overview
ISO-MED maintains the largest and broadest portfolio of on-theshelf warehouse supplies.
We offer customized supply plans, a seamless e-commerce customer experience, easy ordering with matching manufacturer, and dependable purchasing/supplier aggregation services.
With one-on-one scheduled training services, we increase the efficiency of your purchasing processes, consolidate your portfolio, and link your on-site supply demand to your inventory management processes.
Offering value-added services, we provide consistent customer service and on-time delivery with a streamlined transaction process. We are driven to help healthcare-oriented organizations save money.
Features & Options
ISO-MED not only has products that help a hospital conform to cleanroom standards, but we also offer the following products for testing cleanliness.
n ICR Plus TSA LT Contact Plates
Environmental monitoring for isolators and cleanrooms (surface and air monitoring).
n Tryptic Soy Agar
Added measure to assure sterility in your environmental monitoring program! Hardy Diagnostics contact plates are recommended for use in the cultivation of microorganisms from environmental surfaces.
Additional Product Modules
n Premier Product Offerings
• PPE Product Selection
• HD Spill Kit
• Chemotherapy Gowns and Supplies
• Cleaning and Disinfection Solutions
For every cleanroom or critical environment cleaning and disinfection program, ISO-MED offers several brands and ISO-MED, INC. formularies.
Markets Served
• Hospital
• Compounding
• Infusion
• Retail
Ordering Information
Contact us by phone at (800) 797-1405 or email sales@iso-med.com to gain access to our exclusive online ordering platform.
WINTER 2022 I HEALTH SYSTEM • INFUSION 89 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
ScriptPro’s End-to-End Pharmacy Solutions Drive Health System Success
President & CEO: Michael E. Coughlin
Founded: 1994
Stock Symbol: Privately Held
Toll-Free Phone: (800) 673-9068 Phone: (913) 384-1008 Address: 5828 Reeds Road Mission, KS 66202 Website: www.scriptpro.com Email: info@scriptpro.com
Company Background
ScriptPro pioneered the use of robotics in community pharmacies with the launch of its SP 200 Robotic Prescription Dispensing System in 1997. Today, ScriptPro’s innovative pharmacy automation and management solutions drive the success of thousands of ambulatory and community pharmacies around the world.
ScriptPro Powers the Business of Pharmacy
Health systems are beginning to recognize that empowering their retail pharmacies can reduce readmissions, increase medication adherence, improve patient satisfaction measures, and generate substantial revenues. To compete in this market, a highly functioning, retail-oriented pharmacy business model is required.
In searching for the correct model, IT leadership may be inclined to set up the retail pharmacy core as an outgrowth of their hospital’s EHR system. This desire to have a single, unified platform ultimately leads down a path that requires interfacing the core with many downstream systems to obtain functionality that is essential for a retail operation. These include POS, charge accounts, mobile apps for staff and patients, IVR, inventory management, class-of-trade pricing management, etc. So instead of avoiding headaches by having a unified platform, they end up with exactly what they were trying to avoid: a collection of vendors and interfaces to manage.
ScriptPro’s comprehensive ambulatory pharmacy platform incorporates all the functionality needed with just a short list of interfaces to synchronize retail operations with the EHR. The financial success of this strategy can be seen at UK HealthCare — its ambulatory pharmacy program has grown to become a huge advantage in patient care and a major underpinning of UK’s powerful growth.
Just as importantly, retail pharmacy should be treated as a separate, entrepreneurial business instead of another hospital cost center such as lab, radiology, and inpatient pharmacy.
Health systems with the most successful retail pharmacy programs have followed the entrepreneurial model and have shown that retail pharmacy can make substantial contributions to covering health system operating expenses and losses from providing below-cost care to populations in need.
As a retail enterprise, ambulatory pharmacy is very different from all other health system business units. Instead of appointment-based, scheduled operations where customers (patients) are captive, pharmacy customers choose when, and if, to use the health system’s retail pharmacy services. Another difference is the complex maze of third party reimbursement sources, which are the lifeblood of retail pharmacies. Retail pharmacy billing challenges are unfamiliar to health system financial staff who work in a world where hospital revenues are reported based on theoretical charges. Hospital finance routinely absorbs contractual allowances (write-offs) equal to 50% or more of charges, and often they lose financial opportunities by outsourcing this business.
Embrace Entrepreneurial Strategies
The most successful health systems have embraced entrepreneurial strategies by utilizing the following guidance from ScriptPro:
• Implement a highly functional, fully integrated ambulatory pharmacy operating platform that supports:
• Prescription dispensing
• Point of sale with staff mobile apps
• Patient charge accounts
• Class-of-trade (340B) management
• Pharmacy benefit administrator functionality
• Inventory management
• Interactive voice response
• Integrated clinical patient case management
• Embed IT talent within the pharmacy team to enable deployment of technologies that are beyond the scope and limits of the health system IT organization.
• Secure contracting staff and/or expert third party contracting support to negotiate PBM and direct to manufacturer agreements.
• Leverage retail pharmacy revenue cycle management services.
Today’s healthcare leaders rely on ScriptPro and our innovative, industryleading solutions to build ambulatory pharmacy programs that are competitive in the retail market, deliver outstanding patient care, and play a critical role in the financial well-being of health systems.
WINTER 2022 I HEALTH SYSTEM • INFUSION 91 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
ScriptPro’s game-changing pharmacy solutions enable health systems to create an optimum retail pharmacy business model to deliver outstanding patient care while raising operational performance and profits.
CASE STUDY
STREAMLINE COMPOUNDING AND MEDICATION COMPLIANCE WITH Simplifi+ ®
INTRODUCTION AND BACKGROUND
The Unites States Pharmacopoeia (USP) serves as a book of standards for the protection of the public and has been maintained since 1820 by physicians and pharmacists.
Annie Lambert, PharmD, BCSCP Clinical Program Manager ~ Simplifi+ Solutions

“The pharmacist must not only know well the standards of the U.S.P., but they must be skilled in the compounding and care of the hundreds of medicinal products they handle. Your Doctor relies on them when sickness comes. They are trained in college and by experience to be accurate, and to judge and appreciate high quality. Their professional services and the sacrifices they are often called upon to make in the public welfare entitle them to a large measure of the good will of the community they serve.” 1
While many of these statements ring true today, additional standards have evolved over the years — including those for patient safety, medication management, safe handling of hazardous drugs, and compounding, making the pharmacist’s job more complex. And despite an increase in regulations and enforcement, many pharmacies rely primarily on manual processes for everything from I.V. preparation to temperature checks to medication dispensing.

At Wolters Kluwer Health, we believe patients deserve the Best Care Everywhere — and that includes anywhere medications are handled or prepared.
We aim to simplify the complexities by building software that incorporates not only the minimum regulatory standards, but also the deep domain expertise from our industry, to guide completion of standardized workflows and competencies that deliver consistent compliance habits, excellent outcomes, and peace of mind.
USP COMPOUNDING STANDARDS
CHALLENGE: The USP Compounding Standards continue to evolve, with updates to USP <795> and <797> recently published and enforcement looming in 2023. As the standards unfold, you can expect increased scrutiny from accrediting and governing agencies such as The Joint Commission, CMS, FDA, and State Boards of Pharmacy that inspect compounding pharmacies for quality control, training, and documentation. As you know, failing an inspection can result in financial and reputational consequences.
www.wolterskluwer.com/en
92
CASE STUDY
Between the electronic, device-based operation and the customizable checklists, we became more efficient and completed the inspections much faster and more reliably. With the option for notifications, we immediately saw our compliance increase.”
Daniel De Arazoza Clinical Pharmacist
~ Nicklaus’ Children’s Hospital
SOLUTION: Simplifi 797® is the country’s leading web-based pharmacy compliance solution designed to meet the requirements of USP <795>, <797>, and <800> and beyond by incorporating industry best practices into easy-to-adopt templates. With policies, training, competency assessment, daily task management, and inspection-ready reporting integrated throughout the software, you can be confident nothing will fall through the compliance cracks. Leverage system standardization tools to reduce variation in competencies and tasks across the health system and monitor performance with system analytics reports.
OUTCOME: Trusted by over 2,000 pharmacies, Simplifi 797’s swift implementation and up-to-date application reduces the effort required to achieve compliance and gives users peace of mind that they will pass inspections easily. Proactive and real-time notifications combined with standard reports available in the analytics platform provide actionable insights for pharmacy leaders to ensure compliance day to day and to close gaps before they become challenges. Says Vice President of Clinical Pharmacy Services for Optum Infusion, Jeff Arquiette, “Simplifi 797 has really helped to standardize our documentation and competencies across our sites. This has been huge and one of the biggest drivers of our success.” For Optum Infusion and other integrated pharmacy networks — whether small, medium, or large in scope — many utilize centralized management of competencies to achieve standardization as well as compare performance across facilities with system analytics reports.
MEDICATION STORAGE AND UNIT INSPECTIONS

CHALLENGE: Pharmacy is responsible for medications stored anywhere in the health system, including nursing units, clinics, carts, and operating rooms. Medication errors and improper medication storage present threats to patients and staff. Routine monitoring of these areas is necessary but time consuming, and a paper-based tracking system rarely delivers meaningful data.
SOLUTION: Simplifi+® MedStorage is a web-based tool that allows pharmacy leaders to create standard workflows for pharmacy staff to complete from a mobile-device — all to meet regulatory standards for storage of medications across units and improve patient safety. Simplifi+ MedStorage serves as a quality management system that helps ensure staff members are following standard checklists essential to meeting quality objectives and improving care. Customized reporting and notifications improve visibility into inspection metrics, making it easy to share data with other health system leaders and enabling administrators to identify trends early.
OUTCOME: The transition to a web-based unit inspection tool was easy for Nicklaus Children’s Hospital. “Between the electronic, device-based operation and the customizable checklists, we became more efficient and completed the inspections much faster and more reliably. With the option for notifications, we immediately saw our compliance increase,” shared Clinical Pharmacist at Nicklaus’ Children’s Hospital, Daniel De Arazoza. Not only was Simplifi+ MedStorage useful in their acute care and surgery sites, but the benefits have also extended to their offsite clinic facilities as well. All inspections are easily compiled and shared with the Board of Pharmacy. Dr. De Arazoza emphasized: “Having data available through the analytics platform is extremely powerful. The MedStorage team was invaluable in helping to develop our analytics and to learn from live data and trends.”
www.wolterskluwer.com/en
“
93
CASE STUDY
EMERGENCY MEDICATION TRAY MANAGEMENT
CHALLENGE: Management and restocking of medication trays is another tedious, yet necessary, pharmacy task. Many organizations have challenges coordinating the restocking workflow, relying on paper logs and binders. Worse yet, they may have little oversight of the process once the tray leaves the pharmacy.
SOLUTION: Simplifi+® MedTrays helps hospital pharmacies standardize emergency medication tray management by removing manual processes and automating the tracking and inventory of emergency medications.
“When looking at the solutions that were available in the market, we saw an opportunity to deliver impact where it matters most — by helping busy pharmacies simplify the process of restocking and managing medication trays. MedTrays offers consistent and efficient workflows to improve accuracy, traceability, and oversight of critical medications distributed throughout the hospital or in other care facilities,” says General Manager, Compliance and Surveillance Solutions, Karen Kobelski.
Simplifi+ MedTrays takes the effort out of emergency cart medication tray management without the cumbersome implementation or high cost of RFID hardware or proprietary software installation. Simplifi+ MedTrays can be customized to fit where it is most beneficial in a system that has facilities of diverse sizes and needs.
Annie Lambert, PharmD, BCSCP Clinical Program Manager ~ Simplifi+ Solutions
OUTCOME: Implementing Simplifi+ MedTrays has brought many efficiencies for Southwest Mississippi Regional Medical Center. According to Director of Pharmacy, Tiffany Poole, “Once we migrated to Simplifi+® MedTrays, we officially retired the paper binders our team used to manage medication tray restocking and management. Streamlining our workflow with MedTrays’ ability to electronically trace medication lot numbers and expiration dates provides a significant value to my team’s time and our ability to ensure overall patient safety.”
Simplifi+ COMPLIANCE SUITE
Wolters Kluwer is known for Simplifi 797 and providing USP compounding guidance and for supporting pharmacies who rely on automating medication storage support. Simplifi+ is a natural expansion of our knowledge of pharmacy challenges and desire to provide Best Care Everywhere. When our team thinks about how to help customers by innovating in this space, we first consider the level of complexity and external factors you are navigating daily. Wolters Kluwer wants to be a partner to help you simplify that complexity where we can make it easier for you. “The tools and reporting available with all our Simplifi+ applications enable pharmacy leaders to focus on the right details at the right time, all so we don’t lose our focus on what really matters — delivering safe medications to our patients every time,” shares Clinical Program Manager for Simplifi+ Solutions, Annie Lambert, PharmD.

With robust analytics and reporting, we go beyond just checking the boxes to provide valuable insights to support continuous quality improvement for your compounding program and to achieve medication management goals.
Now with three compliance solutions available on our SoleSource platform, you can transform evidence-based guidance into practice across a system or in a specific location — optimizing your operations and overall quality.
Streamline compliance, inside the pharmacy and beyond with Simplifi+ solutions.
1 Adapted from “Why Uncle Sam ok’d the Pharmacopeia” historical poster, Images from the History of Medicine. Accessed: http://resource.nlm.nih. gov/101449493 10/10/2022
www.wolterskluwer.com/en
“
The tools and reporting available with all our Simplifi+ applications enable pharmacy leaders to focus on the right details at the right time, all so we don’t lose our focus on what really matters — delivering safe medications to our patients every time.”
94
With Simplifi+, you can customize a system of market-leading pharmacy compliance solutions to solve medication management challenges that risk reputation and patient safety. By choosing one or all of the Simplifi+ solutions, you can transform evidence-based guidance into practice across a system or in a specific location, optimizing your operations and overall quality.

All the tools you need, when and where you need them, to achieve safety and compliance confidence.
Learn more at www.wolterskluwer.com/en/solutions/simplifi-plus-pharmacy-compliance
Simplifi+ ®
complicated emergency medication tray management with
MedTrays Streamline compliance, inside and out of the pharmacy.
Ensure USP compliance confidence with Simplifi 797 Improve medication storage compliance with Simplifi+ MedStorage Automate
Simplifi+
Peace of mind for your patients
Temperature monitoring for direct-to-patient shipments

Getting medication to patients in the right temperature range is critical for reducing unnecessary reshipments. Including a TransTracker® in outgoing medication shipments can help. These inexpensive, singleuse indicators monitor temperature exposures – letting your patients know at-a-glance whether medication has gotten too hot or too cold while in transit.
Learn more about how TransTracker cards, manufactured by Temptime, can help you give your patients added peace of mind while helping you save money by avoiding medication replacements.

TEMPERATURE INTELLIGENCE® zebra.com
zebra.com/transtracker
Zebra Temperature Monitoring & Sensing Solutions — Monitoring Devices & Integrated Solutions for Medication Shipments, Storage, & Pharmacy Facilities
Providing the devices and information to accurately monitor temperature sensitive medications, indicating if they have been exposed to temperature events that could impact their effectiveness in treating patients.
Product Specifications
CEO: Anders Gustafson
Founded: 1969
Employees: 8,800 Phone: (847) 634-6700 Fax: (847) 913-8766
Address: 3 Overlook Point Lincolnshire, IL 60069 Website: www.zebra.com
Company Background
Zebra empowers organizations to thrive in the on-demand economy by making every front-line worker and asset at the edge visible, connected, and fully optimized. With an ecosystem of more than 10,000 partners across more than 100 countries, Zebra serves customers of all sizes — including 94% of the Fortune 100 — with an award-winning portfolio of hardware, software, services, and solutions that digitize and automate workflows. Supply chains are more dynamic, customers and patients are better served, and workers are more engaged when they utilize Zebra innovations that help them sense, analyze, and act in real time. In 2021, Zebra expanded its industrial automation portfolio with its Fetch Robotics acquisition and increased its machine vision and AI software capabilities with the acquisitions of Adaptive Vision and antuit.ai.

Product Overview
Zebra’s portfolio of solutions includes: specialty printing and supplies, barcode scanning, mobile computing and rugged tablets, RFID and real-time location systems (RTLS), intelligent workforce management and execution solutions, data services and prescriptive analytics, support, managed and professional services, intelligent automation systems, and temperature monitoring and sensing solutions. Our comprehensive temperature monitoring and sensing portfolio, manufactured by Temptime, meets the cold chain needs of specialty pharmacies and their patients. This portfolio includes:
• Visual, low-cost, chemically-based heat and freeze indicators for blood products, vaccines, biologics, and other medication during storage.
• Heat and freeze indicators for monitoring medication during shipment.
• Sophisticated electronic, wireless temperature and humidity monitoring systems with cloud-based data storage and sharing.
Zebra is striving to improve supply chain efficiency, global health, and patient care through the effective deployment of devices and solutions that identify temperature excursions and provide information so that action can be taken.
n
Wireless Monitoring Systems
The family of wireless Bluetooth®-enabled sensors monitor and record temperature data 24 hours a day, seven days a week.
The W-200 is designed for facilities and warehouses, monitoring both temperature and humidity in medication storage areas to help with accreditation compliance and regulatory requirements. Data can be accessed remotely using a web-based application and customized alerts notify users instantly if any areas have exceeded temperature limits.
The M-300 and S-400 programmable dataloggers are ideal for monitoring temperatures during transport or storage, letting users:
• View and share temperature data with a free mobile app.
• Customize alarm limits, datalogging intervals, startup options, and more.
• Store unlimited data on the cloud and generate reports with a complementary web app.
n Package Performance Qualification
Testing
For specialty pharmacies, package performance qualification (PPQ) testing ensures that the shipment packaging is able to provide the necessary temperature range for the required period of time during transport of critical and often lifesaving medications to patients. More than that, PPQ testing is also required for certain pharmacy accreditations, such as URAC.
These wireless monitoring devices help specialty pharmacies simplify PPQ testing while providing data and information to optimize packouts. Customized third-party PPQ testing services also help meet the requirements of pharmacy networks that make third-party validation testing a condition of participation.
n
TransTracker® Shipment Indicators
TransTracker® visual temperature indicators monitor medication heat and freeze events during shipment. They are simple to read and understand, so patients can tell at a glance if medication has been handled within the appropriate temperature range. These devices instill a higher level of patient confidence so that unnecessary, costly reshipments due to suspected temperature damage can be reduced or eliminated. These cost-effective, single-use indicators also help specialty pharmacies comply with state regulations.
Testimonial

97% of medication recipients said they prefer a specialty pharmacy that uses the TransTracker® indicator in its shipments.*
* Based on “Experience of Specialty Pharmacies in TransTracker Product-in-Use Patient Research” with over 8,000 patient responses.
WINTER 2022 I HEALTH SYSTEM • INFUSION 97 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
IMI designs innovative medical devices that enhance the security of medication from pharmacy to patient. Fifty years of product design exclusively focused on the needs of the compounding pharmacist means exceptional quality, reliability and customer-driven innovation. From Tamper Evident Caps to essential sterile compounding supplies, ensure the integrity and security of your medications with solutions from the leader in secure drug delivery.


Over 50 Years Serving The Industry Exceptional Quality & Reliability Enhance Your Drug Security Program Strengthen USP <797> Practices © 2022 International Medical Industries, Inc. All rights reserved. Companies and products mentioned herein may be trademarks or registered trademarks of their respective trademark owners. IMI-501-AA-77 R1 MADE IN THE USA IMIWEB.COM | 1.800.344.2554 To See All Our Products and Request Samples Visit
International Medical Industries — Innovation in Secure Drug Delivery
President: Jonathan Vitello
Founded: 1969
Employees: 100+
Toll-Free Phone: (800) 344-2554 Phone: (954) 917-9570 Fax: (954) 917-9244
Address: 2981 Gateway Drive, Pompano Beach, FL 33069 Website: www.imiweb.com
Company Background
Founded in 1967 and exclusively devoted to the needs of the compounding pharmacist, at IMI creating products for the compounding pharmacist is our sole focus. As a result, our customers experience the quality, service, and value that only a specialized partner can offer. Through our partnerships with compounding professionals, we continue to advance our devices to serve the healthcare community. From new product developments to customized packaging configurations IMI’s customer-focused ethos and superior engineering capabilities allow us to be responsive to the needs of our partners and customers. Our capabilities and highly trained teams are the reasons we have remained the industry standard for tamper evident cap technology and how we continue to deliver customer-focused products to enhance pharmacy productivity, safety, and security. All IMI products are manufactured in the United States at our FDA-registered, ISO 13485-certified facility under the strictest quality standards.
Product Overview
n Prep-Lock™ Tamper Evident Caps
Compounded preparations are at their greatest risk when they leave the custody of your pharmacy. The benefits of tamper-evident products to address this risk have been recognized within the standards and guidelines of multiple influential organizations including the American Society of Health-System Pharmacists and the FDA. Tamper-evident products increase overall accountability in the chain of custody of mediation, maintain sterility, prevent leakage, ensure patients receive the full intended dose and reduce the risk of contamination. Experts agree that the use of tamper-evident products increases the confidence of pharmacies, health care workers, and patients. IMI’s Prep-Lock™ line of products provide high-value, high-quality, tamper-evident closures devices for a variety of drug delivery containers, including I.V., enteral and oral syringes, medication cassettes, and I.V. bags.
n Prep-Lock Tamper Evident Caps Featuring RFID
RFID Technology continues to garner adherents in hospital pharmacy systems. The technology enables real-time scanning that optimizes inventory management, efficiency, and medication safety while creating quantifiable pharmaceutical supply chain benefits by providing item level inventory
visibility down to NDC and lot. Recognizing the advantages that RFID technology presents, IMI joined the industry consortium UnitVisID to help increase the interoperability, quality, and performance of RFIDtagged products. Tamper-evident caps with incorporated UnitVisID RFID provide a labor-reducing solution to adding RFID to your drug doses. With a simple twist of a syringe, your CSPs are equipped with renowned tamperevident protection and robust analytic capabilities provided by compatible RFID systems. These two powerful technologies, in combination, enhance workflow efficiencies, eliminate time-consuming manual inventory control processes, provide assistance with growing regulatory demand, and supply a comprehensive strategy to prevent, detect, and resolve drug diversion events. In addition, by incorporating RFID technology into IMI’s Industryleading Tamper Evident Caps, facilities can significantly reduce their cost of RFID implementation with little time-to-live and minimal staff investment, procedural changes, or capital investments.
n Prep-Lock Tamper Evident Additive Port Caps
The tamper-evident additive port cap provides remarkable protection, and protocol assurance to the medication ports of a variety of I.V. bags, including Baxter, B. Braun, Fresenius Kabi, and more. The simple one-handed installation and considerable contributions to the integrity of I.V. compounds have gained acclaim with pharmacists and healthcare professionals. “I find their products to be the best on the market in terms of the device itself, the functionality, and the securement,” says Neil Colby RPh, director of infusion pharmacy services CDRX infusion. These products extend the intention of USP <797> from pharmacy to patient by providing last mile security that strengthens pharmacist and HCP confidence and reduces risk of contamination and diversion.
Testimonial
“�I find their [tamper-evident] products to be the best on the market in terms of the device itself, the functionality, and the securement.”
— Neil Colby RPh, Director of Infusion Pharmacy Services CDRX Infusion
Additional Product Lines
IMI manufactures a variety of high-quality devices for the compounding pharmacist including: Prep-Lock™ Tamper Evident Caps, Prep-Seal™ Caps and Plugs, Prep-Fill™ Sterile Connectors, Rx-Vent™ Filtered Venting Needles, Rx-Tract™ Aspirating Needles.
Trade Shows/Meetings Attended
IMI regularly participates at a variety of industry trade shows and conferences including:
• American Society of Health System Pharmacists (ASHP)
• European Association of Hospital Pharmacists (EAHP)
• National Pharmacy Purchasing Association (NPPA)
• International Health Facility Diversion Association (IHFDA)
• Hospital Pharmacy Conference (Health Connect Partners)
• Visit the event website or contact IMI for dates and booth numbers.
WINTER 2022 I HEALTH SYSTEM • INFUSION 99 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT
Increase pharmacy productivity and safety with economical, American-made devices designed to advance the safety and security of your drug products and ultimately your patients.




MOBILE PHARMACY COMPOUNDING CLEANROOMS Whether you are planning a cleanroom renovation or building a new cleanroom, Modular Devices’ mobile cleanrooms provides an expedited option that combines a modular and prefabricated approach by delivering a ready-to-use, turnkey cleanroom that only requires utility connections upon being delivered. Our pharmacy compounding cleanrooms are available to rent or purchase! Experience unmatched quality, state-of-the-art materials and equipment, and a cleanroom that will not only exceed your expectations but USP 797/800 as well! » Meets and Exceeds USP 797/800 » Hands-Free Interlocked Doors » Continuous Data Logging and Monitoring » 14' Wide x 48' Long » Includes 4' BSC’s and 4' LFH’s » Includes Dedicated Refrigerators » HEPA Filtered Interlocked Passthroughs » Hands-Free Scrub Sinks » Dedicated Haz and Non-Haz Gowning Rooms » USP 800 Negative Pressure Unpack / Storage Room » Hands-Free Flush Mount Intercoms » Guaranteed Environmental Control Anywhere in U.S. » Large Fleet and Nationwide Coverage » Guaranteed Certification CALL FOR INFORMATION OR TO REQUEST A QUOTE (317) 489-4616 www.portable-cleanroom.com Full Compliance with USP 797 and USP 800 is one phone call away!
Mobile Pharmacy Compounding Cleanrooms by Modular Devices
Premanufactured mobile cleanroom available in a fraction of the time compared to traditional design/build projects. Fully engineered turn-key mobile cleanrooms designed to provide immediate compliance for USP 797 and USP 800. Rent one today as an interim solution, or purchase one as your new permanent, state-ofthe-art cleanroom. We make compliance as simple as a phone call.
Product Specifications
President & CEO: Greg Mink
Founded: 1987
Toll-Free Phone: (800) 456-3369 Phone: (317) 489-4616 Address: 6678 Guion Road Indianapolis, IN 46268 Website: www.portable-cleanroom.com

Company Background
Modular Devices is based in Indianapolis, Indiana and offers short and long-term mobile cleanroom solutions for any industry or application that requires controlled environments throughout the USA and North America. Every cleanroom that we offer meets our high-quality standard commitment, has the most state-of-the-art materials and equipment, and includes unmatched customer service. We have all the required expertise under one roof. This includes architects, engineers, construction disciplines, and cleanroom experts that can guide you through the entire project.
As the race continues to become compliant with USP 797 and USP 800, our primary focus as a company is to provide solutions for our clients by solving problems. We are a company that puts your needs first, and that takes care of the many complexities of building a compliant cleanroom so that you can focus on what is most important to you, your patients and staff!
Product Overview
We offer high-quality, cost-effective mobile cleanroom solutions which are pre-engineered and pre-built, then delivered as turnkey compounding cleanrooms ready for use immediately upon delivery. Compared to traditional design/build, our mobile cleanrooms can be built in a fraction of the time as we control the entire production at our facility — eliminating delays and wasted capital, and providing immediate compliance and peace of mind.
What sets MDI/CRD Mobile Pharmacy Cleanrooms apart?
• Pre-certified prior to delivery and guaranteed to pass third-party inspection.
• 14' wide mobile cleanrooms, up to 40% larger than 8' wide trailers.
• Short or long-term rentals, or as permanent additions.
• Cloud-based control and monitoring system with real-time data-logging and alarms.
n Safety
• All rooms have hands-free interlocking doors with red and green light indicators to ensure the integrity of differential pressures.
• USP compliant hazardous unpack/storage room, dedicated gowning rooms, HEPA filtered pass-throughs, and gowning room sinks deep enough for fingertip to elbow scrubbing.
n Productivity
• Our mobile cleanrooms are 14' wide, 6' wider with over 40% more space than other mobile cleanroom trailers (on wheels). This provides a much more functional environment for personnel and workflow.
• Each compounding room comes equipped with two 4' wide biosafety cabinets and/or two 4' wide laminar flow hoods.
n Flexibility
• Two standard size mobile pharmacy cleanroom products.
• Single purpose: Either USP 797 or USP 800.
• Dual-purpose: Larger size engineered for USP 797 and USP 800.
• Turnkey building and HVAC systems designed and engineered to be placed in locations anywhere in the U.S.
• Custom HEPA-filtered pass-through designs include single- and double-door, through-the-wall pass-throughs, cart pass-throughs, and pharmacists’ checking pass-throughs.
n Security
• Real-time data-logging and alarming for out-of-range temperatures, humidity, and pressures.
• Video recording systems and fire protection systems included.
Testimonial
“Thank you for the excellent service Modular Devices has provided to us for the temporary pharmacy compounding cleanrooms for the Staten Island University Hospital Campus. Upon delivery the NYS Board of Pharmacy conducted an inspection and approved the unit for operation in the capacity of compounding both hazardous and traditional I.V. solutions for patients. The unit surpassed our expectations and was able to maintain the high-volume production necessary during the recent COVID-19 outbreak.”
— Otto VonEilbergh, Director — Capital Projects, Corporate Facilities Services, Northwell Health
Ordering Information
To learn how our mobile cleanrooms can assist with your pharmacy upgrade or expansion, or to help meet new USP 797 or USP 800 standards, contact us by phone at (317) 489-4616 or visit our website for a free quote, www.portable-cleanroom.com.
WINTER 2022 I HEALTH SYSTEM • INFUSION 101 20WAYS TO IMPROVE PATIENT CARE & PHARMACY COST CONTAINMENT





Pharmacy Design and Modular Casework that help your pharmacy perform at its best! OUTPATIENT PHARMACY INPATIENT PHARMACY INTERFACING WITH AUTOMATION With over 10,000 completed installations, we have the experience to make your pharmacy project a complete success! PHARMACY SPACE PLANNING · MODULAR PHARMACY CASEWORK (FURNITURE) · PROFESSIONAL INSTALLATION Call us today toll-free, 1 (800) 747-7648. View project gallery and product options at www.rcsmith.com and www.mmisystems.com
COVERALLS
Get the right Coverall for your specific controlled environment, delivered by Cintas.

FROCKS, ESD LAB COATS & SPECIALTY GARMENTS



Frock options to keep your employees in the cleanroom Ready™ .


BOOTS AND SHOE COVERS
The right footwear for your controlled environment.
ACCESSORIES
with your gowning.
LEARN MORE
Additional cleanroom products/services delivered
Resources Gowning Service to Help Keep Your Controlled Environment Ready for the Workday®
Cleanroom




ACCUCOLD DIVISION FELIX STORCH, INC. ISO 9001:2015 CERTIFIED 770 Garrison Avenue, Bronx, NY 10474 USA • TEL 1.888.4-MEDLAB FAX 844.478.8799 • info@accucold.com • accucold.com Ike Goldstein 1.888.4.MEDLAB ext. 204 ikeg@accucold.com Christian Geddes 1.888.4.MEDLAB ext. 268 christiang@acccucold.com IN STOCK NOW FORSTORAGEVACCINE DDLReady Access port & remote contacts allow easy connection to external logging systems ADA Height Option 32” high choices for use under lower ADA compliant counters EcoFriendly Uses environmentally-friendly hydrocarbons for a greener footprint Fast Temperature Recovery Optimized forced air cooling Flexible Interior Ventilated drawers, interior lockers, or adjustable shelves Antimicrobial Handles Silver ion coating to reduce the spread of germs Built-In Display Digital display of the current & high/low temperature with a buffered temperature probe Advanced Temperature Control Intelligent microprocessor controls with a 2-8ºC range & +/-1ºC variation Multiple Alarms Audible alerts for temperature spikes, door open, & power failure Trusted Security Keyed or keyless locks 1 to 46 cu.ft. Accucold products are available through a host of high quality dealers To discuss your storage needs, contact our product specialists:
Behind

Pharmacist
Great
products and services that
pharmacies
patient care, profitability, and cost containment.
our
Every Good
... is a
Resource. Pharmacy Case Studies take an in-depth look at specific
help
improve
Available in
digital library and within each issue of 20Ways.
RXinsider.com




















PATIENT SAFETY FIRST EXPECT MORE FROM YOUR UNIT DOSE PACKAGING PARTNER Communication is the key, now more than ever. Take control of your inventory and use UDS to repackage your unit dosed products. WHY USE UDS? • Reduce Your Workload • Reliable & Responsive Customer Service • Fully Operational Despite of Covid • Eliminate In-House Expenses • Industry Leading Turn-Around Times CALL US TO DISCUSS YOUR OPTIONS. WE’LL SAVE YOU TIME AND MONEY! 866.649.1145 HOSPITAL@UNITDOSEINC.COM NOW YOU CAN TRACK YOUR PRODUCT WITH UDOTS (UNIT DOSE ORDER TRACKING SYSTEM) Place an order with your wholesaler, have it shipped to UDS and in no time you will have it in your facility. At any time, UDOTS will let you know where your order is in our process.



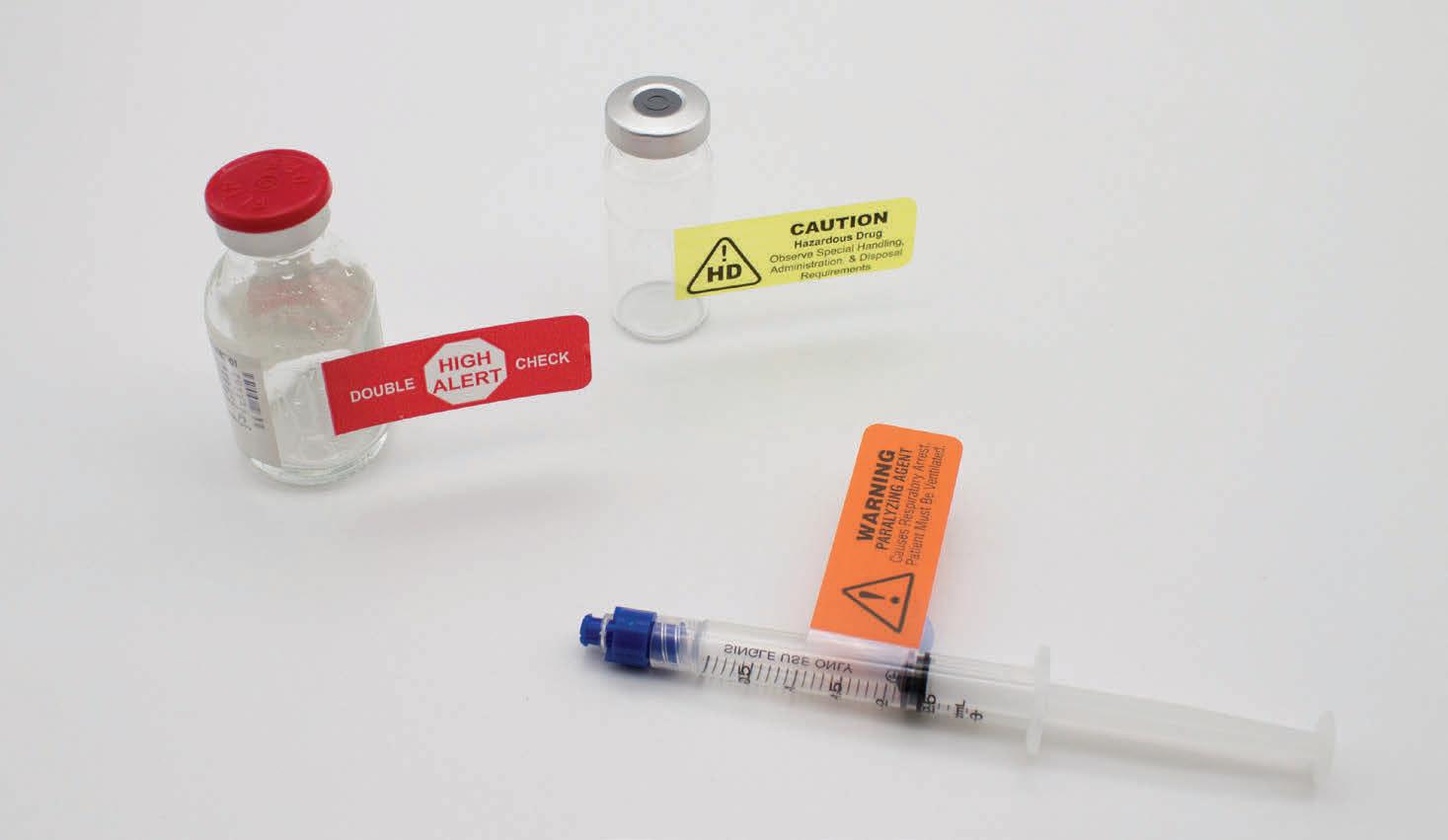



MAXGRIND ® Lite Twist Pill Crusher Expert Products For Expert Care® CONNECT WITH US! (866) 231-1222 | maxpertmedical.com | info@maxpertmedical.com Specialty Medication Preparation & Medication Safety Products Medication Flag Labels Hazardous Drug Transport Bags
It’s a cold, hard fact
When it comes to product temperature accuracy, there is no room for error. Follett upright refrigerators and freezers feature our exclusive plenum air distribution which delivers cold air at multiple levels to ensure consistent top‑to bottom temperatures, even when heavily loaded with product.


See our full line at folletthealthcare.com or call 800.523.9361 for more information

SECURE DRUG DELIVERY INNOVATION IN

PREP-LOCK™ TAMPER EVIDENT CAPS
Strengthen <797> Compliance Ensure the integrity of your compounds with Prep-Lock™ Tamper Evident Caps. Maintain Sterility Evidence of access indicates the potential compromise of content sterility.

Mitigate Diversion Serves as an active deterrent to potential diversion and misuse.
Prep-LockTM Tamper Evident Caps are available for a variety of devices.
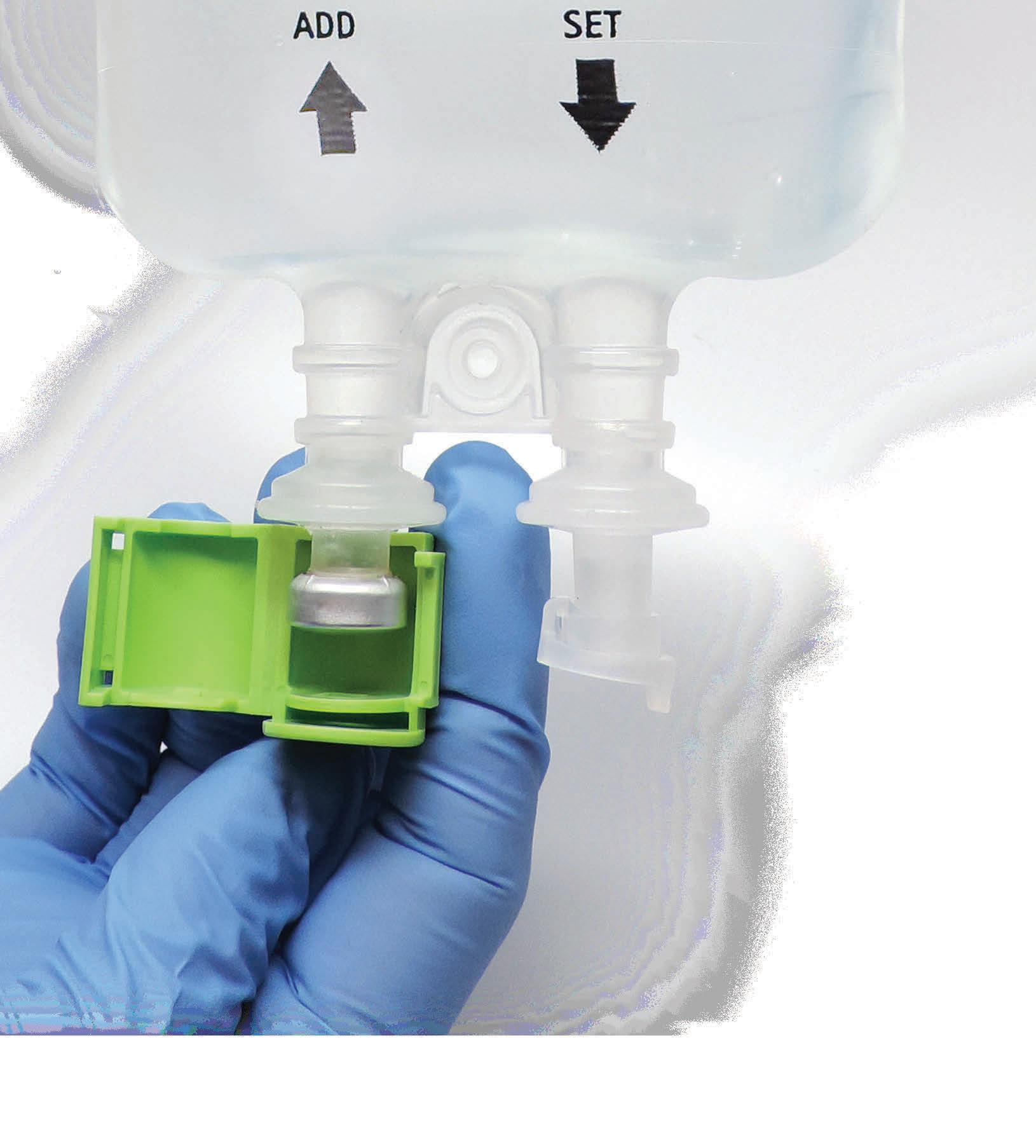
• ENFit® Syringes • CADD Cassettes
Help ensure the safe delivery of your compounds, comply with growing regulations, and enhance patient care. • IV Syringes • Oral Syringes • IV Bags
© 2022 International Medical Industries, Inc. All
reserved. Companies and products mentioned herein may be trademarks or registered trademarks of their respective trademark owners.
IMI-501-AA-67 R1 Contact us for Free Evaluation Samples
rights
MADE IN THE USA IMIWEB.COM | 1.800.344.2554
Fresenius Kabi is pleased to announce the launch of our New Potassium Chloride Injection Products in freeflex IV bags. Our rapidly growing portfolio of IV solutions include several products with 36-month expiry. All freeflex IV bags are non-PVC and non-DEHP, so one IV bag can be used across a facility for the broadest clinical application. Potassium Chloride in 5% Dextrose Injection, USP




WE CAN CARE BETTER
Chloride Injection Products in freeflex® IV Bags
©2022 Fresenius Kabi USA, LLC. All Rights Reserved. 2387-FFX-05-08/22 TOGETHER,
Potassium
Strength Product code NDC code Fill size (mL) Units per Case • 10 mEq Potassium Chloride
1000
• 20 mEq Potassium Chloride
1000 10 Potassium Chloride in Sodium Chloride Injection, USP Strength Product code NDC code Fill size (mL) Units per Case • 20 mEq Potassium Chloride in 0.45% Sodium Chloride
1000 10 • 20 mEq Potassium Chloride in 0.9% Sodium Chloride
1000 10 • 40 mEq Potassium
in 0.9% Sodium Chloride
1000 10 • 36-month expiry products
667110 63323-667-10
10
669110 63323-669-10
683110 63323-683-10
686110 63323-686-10
Chloride
688110 63323-688-10
For more information and to view our growing portfolio of freeflex products, please visit www.freeflexivbags.com. To place an order, contact your IV Therapy Sales Representative or call Customer Service at 1.888.386.1300.
































































































































































































































































































































































































 Holzer Health System
Holzer Health System