



Company: www.international-pharma.com Volume 15 Issue 1 Peer Reviewed
the Future
Next-generation Small Molecules
the Potential of Drug R&D
Predictions for 2023
Drug Delivery Device Development
Are We At?
Net Zero
to Initiate Realistic and Lasting Supply Chain Sustainability Efforts in Pharma
Sponsor
Into
With
Fulfilling
Six
Connected
Where
Achieving
How










See our auto-adjust plunger technology in action Find out more by scanning the QR code or visiting ompharmaservices.com/ipi-march2023 *In addition to an air bubble and overfill Aidaptus® is a registered trademark of Owen Mumford Ltd, ©️2023 OMPS/ipi/ad/ob/0123/7 Available now Now in collaboration with Your fill volume may change, with Aidaptus® auto-adjust plunger technology your auto-injector doesn’t need to Accommodates both 1mL and 2.25mL glass syringes in the same device Versatile design intuitive delivery 0.3mL - 1mL* 0.5mL - 2.0mL* Auto-adjust plunger technology Auto-adjust plunger technology 1mL 2.25mL
DIRECTOR: Mark A. Barker
BUSINESS DEVELOPMENT: Eliza Sarfaraz eliza@senglobalcoms.com
EDITORIAL: Virginia Toteva virginia@senglobalcoms.com
DESIGN DIRECTOR: Jana Sukenikova www.fanahshapeless.com
FINANCE DEPARTMENT: Akash Sharma accounts@senglobal.com
RESEARCH & CIRCULATION: Jessica Chapman jessica@senglobalcoms.com
COVER IMAGE: iStockphoto ©
PUBLISHED BY: Senglobal Ltd.
Unit 5.02, E1 Studios, 7 Whitechapel Road, E1 1DU, United Kingdom
Tel: +44 (0) 2045417569
Email: info@senglobalcoms.com www.international-pharma.com
All rights reserved. No part of this publication may be reproduced, duplicated, stored in any retrieval system or transmitted in any form by any means without prior written permission of the Publishers.
The next issue of IPI will be published in Summer 2023. ISSN No.International Pharmaceutical Industry ISSN 1755-4578.
The opinions and views expressed by the authors in this journal are not necessarily those of the Editor or the Publisher. Please note that although care is taken in the preparation of this publication, the Editor and the Publisher are not responsible for opinions, views, and inaccuracies in the articles. Great care is taken concerning artwork supplied, but the Publisher cannot be held responsible for any loss or damage incurred. This publication is protected by copyright.
2023 Senglobal Ltd./Volume 15 Issue 1 – Spring – 2023
REGULATORY & MARKETPLACE
08 Intellectual Property Matters for Economic, Social, Technological, Scientific, and Industrial Development
The African Regional Intellectual Property Organisation (ARIPO) is an intergovernmental organisation established on 9 December 1976 under the Lusaka Agreement signed in Lusaka, Zambia. It facilitates cooperation among Member States in intellectual property matters to pool financial and human resources and seek technological advancement for economic, social, technological, scientific, and industrial development. IPI Speaks with experts at ARIPO about its Vision to be Africa's leading intellectual property organisation that promotes socio-economic development & to foster creativity and innovation for the socio-economic growth of our Member States through an effective intellectual property system.
10 IPI Speaks with Experts at PharmaLex on Integrated Product Development
Introducing a new drug to market requires a complex interplay of multiple activities and disciplines. For a successful launch, drug developers need a partner that offers holistic solutions and collaborative expertise to help them overcome rising R&D costs and growing regulatory complexities. That’s where a forward-looking approach, such as Integrated Product Development (IPD), can make the difference. IPI Speaks with experts at PharmaLex about helping clients establish an understanding of what is needed to get their product through development and build a strong foundation for commercial success.
REGULATORY & MARKETPLACE
12 How Are Emerging Biotechs Harnessing Strategic Partnerships to Strengthen Their Journey to Commercialization?
If anyone had suggested only a few years ago that it is a realistic proposition to float a psychedelics company on any of the London markets that suggestion would likely have been met with derision. Psilocybin research is still at a relatively early stage, and, at present, only a very limited number of global regulators approve the use of psychedelics to treat mental health conditions. However, as Nigel Gordon at Fladgate LLP discusses, the “direction of travel” in the thinking about psilocybin is towards a conclusion that it may form the basis of the development of much-needed novel treatments for mental health conditions and it might well, therefore, be the case that there will be a change in the view of regulators in future.
14 Navigating the Data-driven Future of Life Sciences Regulatory Process: Who Will Take the Lead?
Generis hosted a live video discussion with a panel of industry thought leaders from BioNTech, Bayer, Iperion – a Deloitte business, and Beczek.COM to discuss the critical next steps for the Life Sciences industry as it embraces a future of data-driven information management and business processes. The panel was chaired by Max Kelleher Chief Operating Officer at Generis, who led the discussion to determine who is ultimately responsible for the quality and consistency of the data and how it can be achieved.
16 Data Transformation to Accelerate Time to Market and Address Product Shortages in Life Sciences Post-Pandemic
Last year was another challenging one for the Life Sciences industry. On top of residual pandemic-related challenges and supply chain issues, the ever-adapting Regulatory environment has continued to set new standards and enforce new requirements. Those companies that have managed to keep pace with the changes can expect to start reaping some of the rewards of their efforts over the coming year. Frits Stulp of Iperion, a Deloitte business, predicts that a wave of data-driven transformation that began in 2022 will enhance insights and decision-making, allowing life sciences companies to gain a competitive edge and provide better patient experiences.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 1 www.international-pharma.com Contents
06 Editor’s Letter
DRUG DISCOVERY, DEVELOPMENT & DELIVERY
18 Navigating the Changing Oral Solid Dose Landscape
Oral solid dose (OSD) forms have accounted for the lion’s share of the pharmaceutical market for decades, thanks to their easy administration leading to patient convenience benefits, not to mention their shelf-life advantages. Philippe Gorria, and Dr. Uwe Hanenberg, of Recipharm, explore the trends shaping the OSD space and discuss the development pitfalls facing the developers of new OSD products and how to overcome them.
22 Fulfilling the Potential of Drug R&D: Six Predictions for 2023
From enabling patient choice during clinical trials to strengthening vital partnerships across the quality ecosystem, connected data will become the lifeblood that enables Life Science teams to collaborate efficiently and effectively in 2023. Chris Moore and Veeva’s industry experts share their predictions about how breaking down silos across clinical, regulatory, safety, and quality teams will benefit patients.
24 Into the Future with Next-generation Small Molecules
Advances in the field of biotechnology have tended to excite interest in their potential as targeted therapies. Vaccines, monoclonal antibodies, cell and gene therapy and other biotechnology innovations have been researched and developed to offer patients targeted treatments. Small molecules continue to be the mainstay in disease treatment and increasingly are gaining the attention of traditional biotech and specialty companies looking to build their therapeutic portfolio and provide a wide range of treatment options for patients in need. Dr. Patrick Larcier at PharmaLex, explains that there is an urgent need for innovation to address diseases and small molecules potentially hold the key.
CLINICAL & MEDICAL RESEARCH
26 How Decentralised and Hybrid Clinical Trials Can Support Subject Recruitment and Retention to Support Clinical Trial
Success
Decentralised and hybrid clinical trials are delivering benefits for sponsors in several ways - delivering quality data quicker to reduce time to market, achieving higher levels of compliance, increasing the diversity of patients participating in trials, and importantly, recruiting and retaining more subjects. Encouragingly, there has been significant progress in decentralised and hybrid clinical trials in the last few years. Laney Preheim at ICON explains that, with the global COVID-19 pandemic serving as a catalyst that brought major shifts in how we approach the execution of clinical trials.
28 Revolutionising Life Sciences Research and Delivery with a New Approach to Modelling Complexity
In life sciences, the adoption of new standards such as SDTM and ADaM is proving critical for efficient and effective data management and sharing. SDTM provides a new way of organising human clinical and nonclinical study data tabulations, which is required for data submission to regulatory bodies, while ADaM defines dataset and metadata standards for clinical trial statistical analyses. Pharma data expert Dr. Alexander Jarasch at Neo4j reveals why knowledge graphs have the potential to accelerate life sciences research and delivery in multiple ways.
TECHNOLOGY
30 Intelligent Innovations for the QA/QC Labs Of the Future
Errors come in all shapes and sizes. While human errors are usually accidental, these mistakes can have far-reaching consequences in the pharmaceutical industry, including risks to patient safety. Even when the damage is minimal, errors cost time – and time costs money. Lab users often struggle to identify errors, and most current liquid chromatography instruments cannot flag them proactively. Current systems generate huge amounts of metadata that also must be manually checked for errors.
In this article, Mike Wilson at Waters UK reveals whether is possible to overcome these obstacles and improve outcomes in the QA/QC environment.
32 The Adoption of Artificial Intelligence and Big Data in the Sector
As the Life science sector evolves, being on top of new trends and regulations is essential for success. According to Jane Lyons at PharmaLex Ireland, by navigating the complexities of risk-based systems, leveraging cutting-edge technology such as artificial intelligence (AI), and prioritizing their supply chains, companies in Ireland can remain at the forefront of the industry in 2023.
MANUFACTURING
34 How to Take a Safety-first Approach When Harnessing the Power of HPAPIs
The international high potency active pharmaceutical ingredient (HPAPI) market is growing rapidly. This surge has predominantly been driven by the potential these ingredients have for enhancing the efficacy of new drugs for patients. In this article, Michael Avraam at ChargePoint Technology explores the challenges of handling HPAPIs safely and discusses how to comply with safety regulations. He explains how the use of nextgeneration containment technology, including single-use components can support pharma manufacturers in harnessing the potential of HPAPIs while ensuring the safest possible working environment for employees.
36 Speed and Precision in Vial Handling
Freeze drying is the preservation method of choice for many pharmaceuticals that are supplied in vials for transfer to syringes in hospitals and doctors’ offices. This requires that the small vials are filled with the active ingredient and dried partially sealed in a freeze dryer. In this case study, Sonderanlagenbau GmbH, a company based in the small town of Lohra, southwest of Marburg in Hessen, Germany, details its SIRIUS robot-assisted loading and unloading system, which HOF unveiled for the first time at Achema 2022.
38 Pharmaceutical Processing Equipment Moves are Made Easier by Capturing the Power of Air
A proven option to optimize production efficiency is now working its way through the pharma and biotech world: air casters. This technology, similar to air hockey pucks, uses compressed air to ‘float’ tanks, casks, and columns on cleanroom floors with potentially no damage to the floor, equipment, or the cleanliness of the environment. AeroGo, the manufactures of innovative load moving equipment explains how they are utilizing hovercraft technology, to move heavy, awkward, delicate, or sensitive loads in manufacturing. Medical-grade manufacturing processes are made easier by capturing the power of compressed air.
PACKAGING
46 Local Lung Delivery for Small and Large Molecules via Dry Powder Inhaler
Inhalation is a familiar route of delivery for many drugs designed to treat asthma and related conditions. Asthmatics routinely carry some form of metered dose inhaler around with them to relieve the symptoms of attacks, and many take preventative medicines such as steroids via inhalers, too. However, small molecule APIs are not the only drugs used to treat lung disease. Kimberly Shepard at Lonza reveals more about the Local lung delivery for small and large molecules.
46 Just What is a Manufacturing Ecosystem and Why are They Growing?
Deloitte shows that a massive 88% of manufacturers agree it is important to work with outside partners and vendors to reach their smart manufacturing and digital goals. With supply chains becoming ever more complex, it’s clear to see that collaboration is at a defining
2 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Contents
In collaboration with FUJIFILM Wako Chemicals U.S.A. Corporation
• Compact and fully integrated endotoxin testing system

• Excellent performance using a fraction of FDA-licensed turbidimetric LAL reagent


• Quantitative results are provided in under half the time of traditional tests
• Minimal user input is needed beyond the introduction of the sample
• On-board FDA part 11 ready software package ensures data integrity
The CMD αBETTM system is a fully integrated endotoxin testing system providing users with a rapid and sustainable solution to endotoxin testing without compromising on sensitivity or performance.
CMD also offers an ISO/IEC 17025 accredited endotoxin testing service covering routine testing of liquid pharmaceutical products, method development & validation for new products, and low endotoxin recovery studies for more complex products including biopharmaceuticals.




FUJIFILM Wako Chemicals U.S.A. Corp.
www.wakopyrostar.com
wkuspyrostarinfo@fujifilm.com
Cotton Mouton Diagnostics Ltd
Your endotoxin testing partner
Cotton Mouton Diagnostics Ltd Unit 8 Cae Gwyrdd Greenmeadow Springs Business Park Cardiff CF15 7AB Tel: +44 (0)29 22 406 366 Email: info@cm-dx.com
© 2022 FUJIFILM Wako Chemicals USA
moment across the manufacturing industry. Bob Tilling, VP of Global Sales at Kallik, explains the value and challenges of developing a shared ecosystem particularly across highly regulated markets such as pharmaceutical, food and beverage and consumer packaged goods (CPG), and how something so simple as accurate labelling plays a critical role in collaborative success.
48 Connected Drug Delivery Device Development: Where are We at?
The market for parenteral drug-device combination products has evolved rapidly over the last 15–20 years and is expected to grow at a CAGR of 24.3% in the next eight years. One area driving this growth is connected devices, with their ability to collect a host of data relating to drug administration. These devices aim to deliver enhanced healthcare outcomes – by enabling remote patient monitoring and promoting treatment adherence – however, there remains room for improvement in several areas to ensure the data captured is being exploited to its fullest. Michael Earl at Owen Mumford lays out the opportunities and challenges currently facing the connected drug delivery device market.
50 Reducing Medical Device Packaging Waste and Improving Information Provision Through E-labelling
E-labelling offers patients and healthcare professionals the convenience of always having up-to-date information on their products, such as the latest safety updates. It also provides regulators with greater oversight and assurance that manufacturers are providing accurate and timely information. Dr. Jutta M. Hohenhörst, from Schlafender Hase, explores the benefits of e-labelling for the medical device sector and provides some practical tips for getting ahead with processes, procedures and solutions that deliver efficiencies and add value for patients.
52 Challenges and Development Perspectives of Primary Packaging for Parenteral Drugs
When talking about parenteral drugs, we usually refer to the administration of drugs by injection through the integument or directly into the circulation. The parenteral route could be intradermal, subcutaneous, intramuscular, or intravenous and indeed allows a rapid effect, the administration of orally inactivated drugs, rapid intervention in emergencies, and the administration of nutritional solutions to patients who cannot feed themselves normally. Andrea Sentimenti at Bormioli Pharma gives his opinion as to why forward-thinking is increasingly required when it comes to parenteral packaging as a system, integrating additional functionalities thanks to a structured and controlled innovation process.
SUSTAINABLE SOLUTIONS
54 Thin Paper: A Solution for Sustainability Challenges within the Pharmaceutical Industry
In the present age, there is extensive pressure on the ecosystems and biodiversity of the world, and consumers are becoming increasingly aware of environmental issues, which spurs demand for sustainably produced products and services. Anne Tammimäki of PharmaLex Finland, Kaija Rinne of DRA Consulting Oy & Outi Hemmo of PharmaLex Finland discuss why thin paper is a promising option for printed package leaflets. Its unique qualities, combined with specific printing techniques, allow reducing the ecological footprint of package leaflets while maintaining excellent print quality and manageability.
58 Achieving Net Zero – How to Initiate Realistic and Lasting Supply Chain Sustainability Efforts in Pharma
Pharmaceutical manufacturers are rightly taking steps to improve their sustainability, but equally important is that the pharmaceutical supply chain matches these efforts. Against the backdrop of a looming climate emergency, there has never been such commitment to finding more sustainable ways of doing business. Inevitably, the pharmaceutical industry, which spans the entire globe and has a profound impact on our daily lives, is at the vanguard of change. Richard Peck of Tower Cold
Chain explores how sustainability can be impacted through the choice of a temperature-controlled container.
LOGISTICS & SUPPLY CHAIN MANAGEMENT
60 Emerging Quality Considerations Across the Global Life Sciences Supply Chain

New waves of innovation in Life Sciences, and a redoubled effort by regulators around the world to maintain the highest standards of safety, are placing increased pressure on drug and device manufacturers to assure the consistent standards of their manufacturing processes, systems and supply-chain partnerships. During 2022, several important trends emerged or deepened, which are having a significant impact on the sector – ranging from practical supply chain issues to medicinal/device innovation. 2023 is set to be another milestone year, with more regulatory changes afoot linked to process digitalisation and automation. Dr. Eduard Cayón at REPHINE sets out the key Quality considerations global LS manufacturers need to be on top of.
64 Packaging: The First Line of Defence in Life Science Logistics
Temperature-controlled packaging plays a vital role in life science logistics. Packaging has multi-faceted role in supporting cold chain logistics to ensure transit times are met and the shipment is protected during handling. Unique packaging solutions have been developed to transport anything from sensitive biological materials during a clinical trial to lifesaving medicines and cell and gene therapy treatments. Robert Pagan at Biocair explains that greater investments and developments in tracking technology will create more robust supply chains and improve patient safety as the industry grows.
68 Fast-moving Pharma Organisations Prescribe Gamified Training to Keep Coaching and Management Skills Scalpel-sharp
In an industry where new technologies and techniques are advancing all the time, cutting-edge coaching training is key to helping people keep pace with change. As the pharmaceutical industry continues its post-pandemic recovery, experts predict sector’s value will grow to US$2.4 trillion by 2029. Thomas Andersen of Attensi and Jennifer Quist of Boehringer Ingelheim discuss the value of effective coaching, which can help individuals identify and overcome obstacles, develop new skills and knowledge, and achieve their personal and professional goals.
72 Evolution of the Pharmaceutical Cold Chain
Pharmaceutical products require a dependable and unbroken cold chain at all stages, including strict, often extreme storage temperatures. The rising demand has also put the spotlight on the sustainability aspect. Logistics companies, while needing to comply with the stringent requirements specific to the pharma industry, have paid attention to how transport and storage can impact their sustainable practice goals, carbon emission reduction requirements etc. Muge Suner at Thermo King explains how the pharma transport industry is evolving rapidly, driven by the demand for more sustainable, patient-centric, and efficient supply chains.
4 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Contents































INTERNATIONAL PHARMACEUTICAL INDUSTRY 5 www.international-pharma.com KahleAutomation.com We’ve merged with BBS Automation Our ideas just got a whole lot brighter Announcing a partnership in automation equipment that brings a whole new light to medical and pharmaceutical device manufacturing. Visit www.KahleAutomation.com or contact Kahle@KahleAutomation.com U.S.A. | ITALY | CHINA Kahle® is dedicated to providing custom automation machinery solutions for the Medical Device, Pharmaceutical, and Healthcare Industries around the world.
Small molecules have consistently enabled medical breakthroughs and tackling unmet medical needs, thus saving countless lives. Moreover, small molecules have been vital as chemical probes in biomedical research, aiding understanding of disease biology. Traditional small-molecule drugs have been the dominant modality in drug research over the past century. However, newer modalities, such as proteolysis-targeting chimeras (PROTACs) and RNA-targeting small molecules (RSMs), as well as biological approaches, such as antibodybased therapy and cell and gene therapy, have been added to the drug discovery toolbox. Most big pharmaceutical companies are now embracing drug research in a more modalityagnostic manner. Structure-based drug design has been widely used in target-based drug discovery, serving as a powerful strategy to design small-molecule drugs. Therefore, structures of targets are always explored at the early stage of a drug discovery project. When a target is identified, the structure of the target can be obtained via different strategies and utilized to predict a ligand binding site, which is critical for understanding molecular interactions and selecting hits using computational methods, such as docking. Structures of target proteins can be obtained through several methods, such as X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, cryogenic electron microscopy (Cryo-EM), homology modelling and structure prediction from protein sequences. X-ray crystallography plays critical roles in determining structures of proteins and their complexes at a high resolution. NMR spectroscopy is able to provide both structural, dynamic and ligand-binding information of a target in solution. In recent years, the development of Cryo-EM makes it possible to determine structures of high molecular weight proteins and complexes which is a great contribution in drug discovery. As the number of protein structures has increased
Editorial Advisory Board
Bakhyt Sarymsakova, Head of Department of International Cooperation, National Research, Center of MCH, Astana, Kazakhstan

Catherine Lund, Vice Chairman, OnQ Consulting
Deborah A. Komlos, Principal Content Writer, Clarivate
Diana L. Anderson, Ph.D president and CEO of D. Anderson & Company
Franz Buchholzer, Director Regulatory Operations worldwide, PharmaNet development Group
Francis Crawley. Executive Director of the Good Clinical Practice Alliance – Europe (GCPA) and a World Health Organization (WHO) Expert in ethics
Rick Turner, Senior Scientific Director, Quintiles Cardiac Safety Services & Affiliate Clinical Associate Professor, University of Florida College of Pharmacy
dramatically, homology modelling can provide reliable structures of many targets.
“Into the Future with Next-generation Small Molecules” is the cover story of this issue of IPI. Advances in the field of biotechnology have tended to excite interest in their potential as targeted therapies. Dr. Patrick Larcier at PharmaLex, explains that there is an urgent need for innovation to address diseases and small molecules potentially hold the key.
To start the year off with the first issue of IPI, we have two exciting interviews. The first with the African Regional Intellectual Property Organisation on Intellectual Property Matters in Africa. This gives a fascinating insight into ARIPO’s vision to be Africa's leading intellectual property organisation that promotes socioeconomic development & to foster creativity and innovation for the socio-economic growth of our Member States through an effective intellectual property system.
The following interview is with experts at PharmaLex about helping clients establish an understanding of what is needed to get their product through development and build a strong foundation for commercial success.

In the present age, there is extensive pressure on the ecosystems and biodiversity of the world, and consumers are becoming increasingly aware of environmental issues, which spurs demand for sustainably produced products and services. In our new section called Sustainable Solutions, we bring you some exciting articles where Anne Tammimäki of PharmaLex Finland, Kaija Rinne of DRA Consulting Oy & Outi Hemmo of PharmaLex Finland discuss why thin paper is a promising option for printed package leaflets. Miroslav Skorepa of Hive-Zox explains that by providing real-time information and automated responses, cMaaS can help logistics providers optimise their supply chain, reduce costs, and ensure the safety and efficacy of pharmaceutical products, and Richard Peck of Tower Cold Chain explores how sustainability can be impacted through the choice of a temperature-controlled container.
I hope you all enjoy this edition of IPI. We look forward to meeting some of you at many exhibitions and conferences coming up.
Look out for our summer edition, where my team and I will bring you more thought-provoking articles and features.
Virginia Toteva, Editorial Manager – IPI
Georg Mathis Founder and Managing Director, Appletree AG
Jagdish Unni, Vice President – Beroe Risk and Industry Delivery Lead – Healthcare, Beroe Inc.
Jeffrey Litwin, M.D., F.A.C.C. Executive Vice President and Chief Medical Officer of ERT
Jeffrey W. Sherman, Chief Medical Officer and Senior Vice President, IDM Pharma
Jim James DeSantihas, Chief Executive Officer, PharmaVigilant
Mark Goldberg, Chief Operating Officer, PAREXEL International Corporation
Maha Al-Farhan, Chair of the GCC Chapter of the ACRP
Stanley Tam, General Manager, Eurofins MEDINET
(Singapore, Shanghai)
Steve Heath, Head of EMEA – Medidata Solutions, Inc
Patrice Hugo, Chief Scientific Officer, Clearstone Central Laboratories
Heinrich Klech, Professor of Medicine, CEO and Executive Vice President, Vienna School of Clinical Research
Robert Reekie, Snr. Executive Vice President Operations, Europe, Asia-Pacific at PharmaNet Development Group
Sanjiv Kanwar, Managing Director, Polaris BioPharma Consulting
Stefan Astrom, Founder and CEO of Astrom Research International HB
T S Jaishankar, Managing Director, QUEST Life Sciences
6 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Editor's Letter

INTERNATIONAL PHARMACEUTICAL INDUSTRY 7 www.international-pharma.com ROBOTICS Discovering new pharma automation solutions Robots for Life Sterile or standard environments, high-end or routine tasks, Stäubli robots deliver clean, consistent performance ensuring the highest levels of product hygiene, safety, flexibility and productivity. Benefit from our know-how and discover the new automation possibilities of intelligent and safe robot technology. Interpack May 4 – 10, 2023 Booth 15C37 Stäubli – Experts in Man and Machine www.staubli.com Stäubli Tec-Systems GmbH, Tel. +49 (0) 921 883 0, sales.robot.de@staubli.com
Intellectual Property Matters for Economic, Social, Technological, Scientific, and Industrial Development
Q: SPL – Please tell us about ARIPO. Why was ARIPO formed and what are your core functions and visions of ARIPO?
A: The African Regional Intellectual Property Organisation (ARIPO) is an intergovernmental organisation established on 9 December 1976 under the Lusaka Agreement signed in Lusaka, Zambia. It facilitates cooperation among Member States in intellectual property matters to pool financial and human resources and seek technological advancement for economic, social, technological, scientific, and industrial development.
ARIPO's Vision is to be Africa's leading intellectual property organisation that promotes socio-economic development. The mission is to foster creativity and innovation for the socio-economic growth of our Member States through an effective intellectual property system.
Membership in the Organisation is open to all the States members of the United Nations Economic Commission for Africa (UNECA) or the African Union (AU). Currently, there are twenty-one Member States, namely, Botswana, Kingdom of Eswatini, The Gambia, Ghana, Kenya, Kingdom of Lesotho, Liberia, Malawi, Mauritius, Mozambique, Namibia, Rwanda, São Tomé and Príncipe, Seychelles, Sierra Leone, Somalia, Sudan, United Republic of Tanzania, Uganda, Zambia, and Zimbabwe. The Republic of Cabo Verde is to become ARIPO’s 22nd Member State. On the 7th of January 2022, the Cape Verdean Parliament approved the country’s accession to the Lusaka Agreement, Banjul, and Harare Protocols. On the 27th of January, 2022, the Parliament further approved the accession to the Swakopmund Protocol. The Instruments of Accession were deposited with the ARIPO Director General on 14 July 2022 at a ceremony held at the Embassy of Cape Verde in Geneva, Switzerland, in the margins of the 63rd WIPO General Assemblies.
Substantive activities of the Organisation are implemented through three treaties, each focusing on a specific field of intellectual property. These treaties are the Harare Protocol on Patents and Industrial Designs,
the Banjul Protocol on Marks, and the Swakopmund Protocol on the Protection of Traditional Knowledge and Expressions of Folklore. The other treaties are the Arusha Protocol for the Protection of New Varieties of Plants and the Kampala Protocol in Voluntary Registration of Copyright and Related Rights. The two Protocols are yet to enter into force.
The protocols were established to form legal frameworks to supplement national frameworks. Currently, the Harare and Banjul Protocols are active, thus, the article will seek to explore the usage of the Harare and Banjul Protocols.
The Harare Protocol empowers ARIPO to grant patents and register industrial designs and utility models on behalf of the contracting States. All Member States of ARIPO, except for Somalia and Mauritius, are party to this Protocol. The Harare Protocol also incorporates other international treaties of relevance, such as the Paris Convention and the Patent Cooperation Treaty (PCT), enabling applicants from the African region and elsewhere to file international applications and obtain the protection of their intellectual property rights. The Harare Protocol has also been linked to the Budapest Treaty, which enables applicants to provide information on new micro-organisms claimed in patent applications.
The Banjul Protocol empowers ARIPO to register marks for goods and services on behalf of the contracting States, which are: Botswana, Kingdom of Eswatini, The Gambia, Kingdom of Lesotho, Liberia, Malawi, Mozambique, Namibia, São Tomé and Príncipe, The United Republic of Tanzania, Uganda, and Zimbabwe.

The Harare Protocol and the Banjul Protocol provides a centralised system of granting and registration of IP rights and provides a mechanism for the ARIPO system to co-exist with the national systems of the contracting States of the Protocols. Thus, an applicant can choose to seek protection with a national Office for protection limited to that country or may elect to use the ARIPO route in which case the application should designate at least one contracting State party to the Protocols.
An ARIPO application may be made by the owner or by a representative. Any resident or anyone with a place of business in any of the Member States can file an application without necessarily seeking the services of an agent. This was deliberately put in place to reduce filing costs for residents of the Member States. Filing can be by personal delivery, post, email, courier, or registered mail, whichever method is convenient to the applicant. Since 2015, filing can be done online through the ARIPO e-service platform. By the end of 2021, 96% of all patent applications were online.
The patent granted by ARIPO, or the utility model, industrial design or mark registered by ARIPO shall in each contracting state for which it is granted or registered, as the case may be, have the effect of and be subject to the same conditions as, a national patent granted, or utility model, industrial design and mark registered, by that State.
Q: Most African countries have incorporated intellectual property protection in their domestic laws, including the granting of patent protection on medicines. There are now 53 countries on the African continent, of which 42 are members of the WTO, and liable to comply with its rules, notably the TRIPS Agreement. At least half of this number consists of the least developed countries, who are eligible to apply the WTO waiver regarding providing patent protection to pharmaceuticals. Regrettably, countries have not taken advantage of this important flexibility. However, some of ARIPO member States have incorporate some flexibility in their national legislation. Can you explain how ARIPO addresses this situation.
A: On the issues of flexibility, each member State exercises their right to choose options made available in international treaties to meet its domestic policy objective. A government makes choices from the various options and implements those choices under the national legislation.
Talking Point
Spring 2023 Volume 15 Issue 1
ARIPO is a Member States driven Organization, which means if a Member State chooses to implement TRIPS flexibilities in its national legislation, ARIPO complies with the laws of the country. Currently, some of our Member States, Uganda and Rwanda, have implemented the specific option of flexibilities in their national legislation by excluding pharmaceutical products from patentability subject matter. Although Section 1(3) of the Harare Protocol provides that the patent granted by ARIPO shall, in each contracting State for which it is granted, have the effect of, and be subject to the same conditions as, a national patent granted by that State, in this case, if ARIPO grants a patent on a pharmaceutical product, such patent will have no effect these countries.
The issue of some countries to not take advantage of this flexibility is a topical issue currently discussed in the WIPO Standing Committee on the Law of Patents. Several constraints encountered by governments at the stage of national implementation of flexibilities have been identified, such as Constructive ambiguity of international treaties, Complexity of practical implementation, Practical operation of law, Institutional capacity, National governance and internal coordination and Extrinsic influence, where political and economic pressure from some industrialized countries and/ or pharmaceutical industries.
Q: For Patent examination, majority of countries do not have a system of substantive patent examination, there being a mere registration process of approving the formalities for applications. How does ARIPO seek to overcome these challenges?
A: One of the objectives of establishing ARIPO was pooling together human resources. At ARIPO, we have qualified and experienced patent examiners who have extensive training and experience in patent examination. A bulk of patent applications seeking protection in ARIPO Member States are filed through ARIPO under the Harare Protocol.
Once the application complies with formal requirements, it will notify the applicant, and the applicant will be required to request substantive examination (Form 13A) of the patent application within three
years from the date of filing. The request shall be deemed to have been filed when the search and examination fees have been lodged. Where no request is made within the prescribed period, the application shall be deemed to have lapsed.
When receiving the request for substantive examination, the patent application will be assigned to an examiner to conduct search and substantive examination and issue a search and examination report which will be sent to the applicant.
As for the case where the applicant files a patent application directly to any of our Member States and that Member State has no capacity to substantively examine the patent application, ARIPO provides assistance by examining the application on behalf of the Member State/s.
Q: “Anti-counterfeiting” legislation in The East African Community (EAC) and several countries have wither adopted, or are in the process of adopting, legislation purportedly to regulate the serious problem of substandard and falsified medicines. The main criticism levelled against these measures is that they conflate quality and safety issues (the responsibility of drug regulators) with intellectual property enforcement (that of private law enforcement). ‘Counterfeit,’ in these laws, has come to be so widely defined as to attack legitimate generics. The issue is discussed in the section on TRIPS-plus initiatives. Where does your company stand regarding this topic?
A: In an effort to curb counterfeiting, ARIPO has been taking part in different initiatives to raise awareness of the consequences counterfeiting has on safety and the economy. In collaboration with the World Intellectual Property Organisation (WIPO), Interpol and the World Customs Organisation, several initiatives, including a training of trainers’ programmes for police academies, designed to introduce IP modules and courses to help authorities better understand the topic. However, more work needs to be done, such as vigorous campaigns to educate people about the negative impact of counterfeiting; border measures must be strengthened; seizure and destruction of counterfeit goods must be encouraged in many cases, and legislation must be improved.
Q: Your website, despite having multiple different sections for topics such as resources, publications, and media, does not mention explicitly ARIPO's impact within the pharmaceutical industry. What plans has ARIPO made regarding the pharmaceutical industry?
A: The ARIPO website shows the services offered by the organisation as per the Lusaka Agreement and the mandates under the Protocols. Pharmaceutical companies are considered part of the diverse clients ARIPO has. We have pharmaceuticals that have used the ARIPO IP system.
ARIPO is conscious of the pharmaceutical industry. Two critical factors limit access to medical treatment: the high prices of medicines, particularly those that are protected by patent, and the lack of medicines to treat neglected diseases, a consequence of a lack of Research and Development.

A robust IP legal framework is fundamental for innovation to thrive and address the challenges relating to the development of affordable medical technologies. On the other hand, countries can take advantage of flexibilities available under the WTO TradeRelated aspects of IP Rights (TRIPS), especially by incorporating them into their national laws.
The African Regional Intellectual Property Organization (ARIPO) is an intergovernmental organization that grants and administers Intellectual Property (IP) titles on behalf of its 22 Member States and provides IP information to its clientele in search services, publications, and awareness creation. The IP titles granted under the Harare and Banjul protocols are for patents, industrial designs and marks. Membership is open to all Member States of the African Union (AU). The Secretariat is based in Harare, Zimbabwe.
Email: communications@aripo.org

Talking Point
Aripo
INTERNATIONAL PHARMACEUTICAL INDUSTRY 9 www.international-pharma.com
IPI speaks with experts at PharmaLex on Integrated Product Development
Q: Can we start with a brief history of PharmaLex, what are your key offerings into the industry?
A: PharmaLex was established 25 years ago with a vision to make a difference to how the industry interacts with regulatory authorities. Today, we support more than 600 clients worldwide with an expert approach to compliance. PharmaLex offers six solution areas that cover the entire product lifecycle: Post-Launch Outsourcing, Local Affiliate Services, Business and Portfolio Mergers and Acquisitions, Strategic and Scientific Consulting, Innovations to Market, and Integrated Product Development.

I’m a solution lead for our Integrated Product Development (IPD) area. A major part of that is strategic product development, which is about helping clients establish an understanding of what is needed to get their product through development and build a strong foundation for commercial success. It’s the centrepiece of what I do. We have experts in all major regions around the globe, including a team of eight here in the US. Our subject matter experts know what it takes to bring products to the patients who need them. They also have a strong working knowledge of the various functional activities that need to be integrated seamlessly in order to ensure the most time- and cost-efficient development program, with a high probability of success. We take a bench-to-bedside view across the product lifecycle, from designing a preclinical program that will meet regulatory approval to designing a global clinical/regulatory strategy and helping to guide commercial success. Given the complexity of development and the multi-year programs, a significant challenge for many companies is understanding what questions they should be asking or answering and when. That’s how we can help.
Q: We hear a lot about the need for integration in drug development – why is this important to the future of the industry, especially as it relates to small molecules?
A: It’s always been important, but now it’s even more so, because it helps to move drugs faster and more efficiently through the development process. It’s quite simple. Consider an activity such as preparing for a meeting with the Food and Drug Administration (FDA). If this activity is not well planned out, with all of the major contributors in alignment, the meeting may be delayed and critical feedback from the agency will not be available when needed to advance the program further into development. This could lead to a delay in the clinical program, for example, which would ultimately lead to a delay in product launch. For a product expected to return US$400 million in the first year of sales, each day the program is delayed would mean lost revenue of more than US$1 million.
All the functional activities needed to bring the product to the market are intricately interconnected. Understanding how the various puzzle pieces fit together is critical to efficient planning of the development phase.

When focusing on single activities or only on the next milestone, it’s common for development programs to go through fits and starts, for example having to repeat a study in a different geography because the input of regulators in various regions was not sought or incorporated into the development plans.
So, thinking from an integrated point of view, instead of just getting to the next milestone or the next dataset, taking a holistic view can prevent a lot of that rework and hassle that goes along with it. This is especially true for innovative small molecules, some of which are developed using artificial intelligence (AI) and/or machine learning (ML). The higher the quality of the data being fed into AI/ML, the greater the chance of success in hitting a druggable target with beneficial clinical outcomes.
Q: How can an integrated product development strategy drive speed and innovation across biopharma?
A: Taking an integrated and holistic approach can help reduce cost and white space between key activities. For example, in a typical relationship between service providers and companies, the sponsor company will wait until data is available and then approach the service provider with a protocol for the next study. Of course, this delays study startup, not only due to the wait for the data, but also because the provider will inevitably have questions and input into the protocol, leading to additional startup time. Engaging with a service provider to be a partner in development very early on would allow work to begin on the next study protocol and execution several months ahead of the typical time frame.
Q: What is the key role an integrated product development strategy can play in the drug development process?
A: The key thing you need is a development lead who understands what is needed to bring the drug to the market and also has a strong working knowledge of how the various activities need to come together, and when. This is a unique skill set, as it requires a deep understanding of drug development as opposed to being a subject matter expert in just one functional area.
Q: What prevalent culture issues within pharma companies are holding back strategy?
A: Working and making decisions in siloes and not empowering project teams are two of the prevalent perspectives that prevent the most efficient path through development. Communication across functions and, more importantly, program decision-making in a cross-functional manner can enable efficient development. In contrast, many companies enable each function to make their own
Talking Point Spring 2023 Volume 15 Issue 1
decisions on how to support the development program.
It’s important to empower project teams to make certain decisions along the way. These teams have deep knowledge of the program, its history and challenges and are best positioned to decide how to move the program forward most efficiently. One approach to this would be for senior leadership to enter into a “contract” of sorts with the development team revolving around development milestones. The team presents a plan and, if senior management agrees, it commits to providing support for that program. At each milestone, the team reports on progress, describes the key activities to the next milestone and requests resources to support those activities. Teams then are free to work through the program to the next milestone as long as resources and timelines agreed in the “contract” are maintained, within reason.
Q: In general, how has the efficiency and productivity of pharmaceutical companies increased/evolved over the past 20 years and how much of this evolution is due to the knowledge and experience contract development and manufacturing companies bring to the industry?

A: Some things have changed for the better. For example, COVID-19 taught us that we can do more things in parallel and take informed risks as development proceeds. An important part of integrated thinking is to ask “what do you need to know and when do you need to know it?” Answering these questions can help in designing the most efficient and informative development program. It is also important to explore what activities can be done in parallel rather than treating development more sequentially. So, I think those are the things that have changed. Unfortunately, for the most part, this siloing and the risk-averse culture really hasn’t changed all that much.
As for having contract development and manufacturing companies, they are part of the larger ecosystem of service providers. Utilising service providers that can provide a broader set of solutions and services can help evolve development to be more efficient. When working with multiple providers there are often challenges in communication, confusion over accountability, decisionmaking and delays in timelines as deliverables are handed from one provider to the next. With the multi-vendor approach there is a
loss of institutional memory. Every time a new provider is brought on board there is time lost while they get up to speed. When using fewer providers, the need for this ramp-up time is eliminated or reduced.


Q: You prioritise helping organisations hit their product development milestones. We understand how critical this is, not only for the success of the product, but also to meet the next value inflexion point. Can you explain how you do this, and what are the key points our readers should take away?
A: It's focusing on the goal, and the goal is to get to the market, not just in terms of approval, but to ensuring patient access and ultimately commercial success. Planning key milestones and the activities that support them with a sharp focus on that ultimate goal is critical to success.
Then, the second thing is to remember that there are few, if any shortcuts in drug development. Efficiencies can be gained of course, but the efficacy, safety, and market access hurdles to be considered remain the same. Ultimately, the program must generate adequate data to support approval, patient access and commercial success.
Q: It has been widely publicised that innovations in biomedical sciences and technology fuel the opportunity to transform R&D for new drug development holistically, sometimes 500 days faster, better tailored to patient needs, and 25 percent cheaper. Can you explain how this is possible, and how does PharmaLex assist in this development?
A: The numbers you state here stem from an article on the importance of transforming the traditional approach to drug development. While innovative science is paving the way for medicines that could potentially cure many diseases, the type of innovation that can speed development and reduce costs is a process rethink. It’s about drug designs that are centred on patients and clinicians, processes that ensure cross-functional collaboration, leveraging digital technologies to automate repetitive tasks, leveraging advanced analytics to improve decisionmaking, and adopting more agile ways of working. This aligns with our approach to
integrated product development. It is about designing the most efficient and informative development program. It’s about empowering project teams and ensuring decisions aren’t made in silos.
Mark Lane, Ph.D., is Vice President of Development Consulting and Scientific Affairs at PharmaLex, where he draws on his experience with leading product development teams and functions accountable for product development across all phases. Mark’s expertise combines program, project and portfolio management, a deep understanding of drug development, and a strong working knowledge of cross-functional activities combined with business and scientific acumen.
PharmaLex
PharmaLex is one of the largest specialised providers of Development Consulting, Regulatory Affairs, Quality Management & Compliance and Pharmacovigilance, Epidemiology & Risk Management worldwide. Our GLOCAL (GLObal reach and loCAL presence) teams of experts can take you through early strategic planning activities and non-clinical requirements to clinical development, through regulatory submission processes and finally guide you to market approval and product maintenance post-launch activities.

Talking Point
INTERNATIONAL PHARMACEUTICAL INDUSTRY 11 www.international-pharma.com
Mark Lane
How Are Emerging Biotechs Harnessing Strategic Partnerships to Strengthen Their Journey to Commercialisation?
Forecasted to reach $108 billion by 2023, the global pharmaceutical services outsourcing market is currently undergoing incredible levels of growth.
Organisations that are reaping the benefits include contract development and manufacturing organisations (CDMOs), contract research organisations (CROs), as well as strategic commercialisation partners that are helping companies ensure their products achieve their full market potential.
This expansion is the result of a number of key drivers. The impact of the COVID-19 pandemic led to a greater need for expanded capacity across pharmaceutical companies as they sought to meet the demand for vaccines, as well as continue the production of their non-COVID projects.
Growth can also be attributed to the rise in the number of small emerging startup and scale-up biopharmaceutical companies bringing new levels of innovation to the industry. These companies are increasingly seeking support from strategic partners to help them launch their drug products.
But how can these emerging biotech companies work with their partners more effectively to ensure they maximise the value of their assets? Jay Janus, Senior Account Director at Inizio Biotech, explains the rise in strategic partnerships to offer greater support for emerging biotechs in overcoming challenges to thrive in the future pharma landscape.
Current Biotech Market Growth
Expected to reach $3.4 trillion by 2030, the global biopharmaceutical market is set to reach unprecedented levels of growth. Emerging biotechs are responsible for a considerable portion of this expansion. An increasing number of new products are now coming from these companies, with 33% of new drug approvals coming from emerging biotechs in the last few years.
High growth levels for emerging biotech companies come as a result of greater demand for more specialised treatments. Populations in many advanced and emerging
economies are aging, meaning there is a greater need for specialised medicines to treat age-related illnesses. These illnesses include cancers, chronic, rare, and orphan diseases, and many emerging biotechs are striving to meet patient needs by bringing innovative new treatments to market.

Biotechs often have an edge over large pharma companies, as they have greater
agility and ability to advance their therapies through trials to treat patients with often rare and complex diseases.
Emerging biotechs are in a strong position to advance these treatments due to their innate entrepreneurialism and dynamic company structures. Not only does this allow them to mobilise investment funds faster than larger companies, but they also
12 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Regulatory & Marketplace
have the flexibility to innovate at speed by focusing on one project at a time.
The promise of the biotechs' innovations, combined with their dynamism in advancing them quickly, makes them more attractive to venture capital companies.
In fact, venture capital companies invested in 3,100 biotech startups in 2021, and biotech companies raised over $34 billion. This is more than double the investment secured by biotechs in 2020, highlighting how keen investors are to support these innovators in their journey to market.
The Challenges Facing Emerging Biotechs
However, it takes more than a simple investment to reach commercial success. Emerging biotechs must repay the faith their investors have shown in their business and innovation, by maximising the value of their assets. This can be especially challenging to achieve for a small company that is working alone.
While biotechs may have agility and dynamism on their side, they do have disadvantages in realising the full potential of their innovations compared with their larger peers.
Larger companies with years of experience and investment have far deeper resources, making them more resilient when unforeseen challenges delay their projects. They also have the in-house expertise to optimise the efficiency of their product’s development journey. Emerging biotechs must constantly monitor their cash spending, regardless of how much investment they may have secured, to ensure it is being used wisely. They may not always have the expertise within the business to plan an effective development strategy.
The product journey from development through to commercialisation is unique for every biotech. However, many emerging biotechs delay considering their commercial strategies until the later stages of their product launch journey.
This is because many of these companies are hesitant to spend their capital on anything other than product and trial delivery during their early phases, as these are critical to ensuring their asset is truly viable.
Some biotechs decide to delay investing their capital into commercialisation until a fully scaled medical affairs organisation is
needed pre-launch, often leaving it too late to have an optimal effect.
By focusing on commercialisation earlier, emerging biotechs are better positioned to demonstrate the value of their assets to investors, highlight the sustainability of their company, and showcase their readiness for launch.
The Benefits of Working with a Strategic Partner
Engaging with an external commercialisation partner with the expertise and capabilities in a chosen therapeutic area gives biotechs a strategic edge across all of these critical milestones.
These partnerships provide access to specialised expertise and support across their therapeutic area and allow biotechs to focus on their core competencies. Biotechs can also access the necessary skills and resources without incurring the cost and time of building them in-house.
By harnessing the specialised services and support of these partners across the clinical development and commercialisation journey, emerging biotechs are able to personalise and coordinate their activities to meet their specific needs and hit their strategic goals.
Working with a strategic partner, biotechs can optimise their launch activities at the right time to help their assets reach their full potential.
Ensuring the success of these activities requires a dedicated partner that not only helps them deliver on their objectives, but also provides the guidance, expertise, and validation required to avoid potential pitfalls throughout their journey and ensure success.
Engaging with Strategic Partners at the Right Time to Maximise Value
Identifying the right strategic partner to work with at the right point in their commercialisation journey is of paramount importance for emerging biotechs.
Many of the areas and activities that strategic partners can add value to need to be done in Phase II to be effective.
These Phase II activities include:
• Mapping the patient journey
• Prioritising key opportunities
• Developing a platform for scientific communications
• Conducting value pricing and access work
Completing these activities in Phase II is key to derisking an asset, communicating its potential value, and differentiating it to a wide range of audiences. This phase is also one that typically experiences a high level of demand from investors, making it a critical milestone to maximise value.
By working with the right strategic partners at this decisive moment, emerging biotechs can harness their specialist expertise, years of experience, and readily available resources, rather than having to recruit for and build this in-house.
As a result, this will help to lay the groundwork for the success of further milestones and strengthen their appeal to investors.
Finding the Right Partnerships for Successful Commercialisation
The biopharmaceutical market remains highly competitive, and emerging biotechs must adopt a strategic approach as early as possible in their journey to achieve a successful launch.
Engaging a specialist partner as early as Phase II will allow emerging biotech companies to avoid last-minute pressures to hit their milestones in later stages. This support can also help demonstrate the full value of an asset to potential investors, helping to acquire further funding.
With the market becoming increasingly competitive, partnering with a strategic partner is no longer a ‘nice to have’. These partnerships are now an absolute necessity for emerging biotechs to achieve commercial success in getting their innovative drug products to patients who need them.
Jay Janus, Senior Account Director with Inizo Biotech has spent the last half decade advising emerging market biotech companies. Specifically offering fit for purpose solutions, shepparding sponsor partnerships with outside consultancy to help companies power their clincial programs forward.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 13 www.international-pharma.com
Jay Janus
Regulatory & Marketplace
Navigating the Data-driven Future of Life Sciences Regulatory
Processes: Who Will Take the Lead?
Generis hosted a live video discussion with a panel of industry thought leaders from BioNTech, Bayer, Iperion – a Deloitte business, and Beczek. COM to discuss the critical next steps for the Life Sciences industry as it embraces a future of data-driven information management and business processes. The panel was chaired by Generis COO Max Kelleher, who led the discussion to determine who is ultimately responsible for the quality and consistency of the data, and how it can be achieved.
Trends in Regulatory Data
The panel began by reflecting on the current state of data management, particularly around regulatory data, in Life Sciences –and where this is all moving.
Frits Stulp (FS), Iperion – a Deloitte business: “The visibility of the Life Sciences industry has been elevated tremendously over the last three years, due to COVID and the direct and immediate value of emerging medicine to quality of life for many people. But the pandemic also shone a light on archaic registration processes which today still involve the exchange of PDFs. Stakeholders across the industry have begun to see that there is a better way to manage this.
“If the financial services sector was subject to the same rate of innovation that we apply to Life Sciences regulatory processes, people still might not even be using an ATM at this point. In other words, we have a lot of work to do as an industry. Yes, there is a huge passion for the science, but because it's such a regulated environment we tend to avoid risk and need to be told what to do. The trouble is that regulators are not necessarily innovators: that's not their job.”
Who Will Ensure Data Quality?
As processes become more data driven, though, who will drive all of the necessary rigour around data quality? The regulators, via compliance?
FS: “A joint innovation initiative would be most productive. Regulators are not
necessarily there to drive innovation, but do need to pave the way for new ways of working. Despite a great deal of drive by the industry, there can be paralysis when new initiatives centre around a legal mandate. It makes sense for both parties to work together.”
Vada Perkins (VP), Bayer: “From an industry perspective, the regulator’s role is more about fostering opportunities. Look at what pharmaceutical industry is doing with investments in AI to support novel ways of doing things and advance the pipeline. There’s a lot of momentum in the industry; what we require from the authorities is that they are open to this kind of innovation.”
Melanie Ruppel (MR), BioNTech: “There needs to be greater coordination too. Although there’s a lot of expertise and guidance, it isn’t currently focused into Centres of Excellence. In terms of data governance, we’re in a very fluid environment too. Creating something more concrete will require both sides setting out where we want to be in terms of exchanging data in the future – so that everyone can get started as soon as possible.”
Max Kelleher (MK), Generis: “How free are companies to innovate with those processes, given that the initial hurdle of compliance in data management is so high? Is it hard to know where best to focus – for instance, developing great products, improving your processes and as part of that managing your relationship with compliance?”
Structuring Master Data Management
MK: “A recent Gens & Associates report on Life Sciences companies’ innovation priorities found that one of top responses was master data management and how companies can improve that. The missing link is how we structure this data, and bring in applications to allow different areas of the business to own or manage it.”
Preeya Beczek (PB), Beczek.COM: “It’s important not to lose sight of the patient in all of this, too. Ultimately, everything we’re trying to advance now is with a view of improving the speed of patient access to the treatments they need. If we keep that in
mind, the conversation about data becomes more meaningful – right across the value chain. Today’s processes might be outdated, but they are standardised and driven by the current regulations we have. So we need to get better at managing those processes – whether that’s transitioning from phase one to phase two, or on to phase three then registration and delivery.
“It's often at an operational level where companies get lost; where there are disagreements about who owns and leads what process and therefore who owns the data coming out of that process. Certainly, there’s a real opportunity to think about having fewer data handoffs. Other issues relate to who owns the data. Usually it’s seen as ‘Regulatory’ data because those teams are the ones who submit to the health authority. But that’s not where the data originated; there’s a data supply chain – spanning CMC, Quality, Clinical, etc. The function that generates the data owns it.
“Ultimately, data needs to be correct and consistent wherever and however it appears. That starts with a culture of making sure that data is right first time, every time; a sense of commitment to the quality, completeness and consistency of that data; and an appreciation of what that means for the patient.”
Cross-functional RegOps and Informatics
MR: “We’re moving beyond data being a Regulatory Affairs preoccupation, towards more of a general ‘informatics’ concern for companies. It isn’t necessarily a new role, but additional tasks and abilities may need to be developed now.”
PB: “Really now, someone from Regulatory Operations should be part of a cross-functional data governance or data committee, alongside representatives of the functions that are the sources of the data. Yes, Regulatory will be at the centre of this to an extent, but the burden of responsibility shouldn’t fall to Regulatory Operations.”
FS: “I see the Regulatory function acting almost as the editor of a newspaper: ensuring that the people submitting content have done their research properly and checked their work.”
14 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Regulatory & Marketplace
PB: “Some organisations are hiring Chief Data Officers – someone who sits at the level of the CFO – which feels the right way to go.”

MR: “For me the Regulatory function should serve more as the trigger to those at the data source – translating what is required by the authorities to the contributing teams, and how to collect this data in the right format to meet the requirements. In this sense, Regulatory takes on more of an educational role.

“I see RegOps remaining independent, but I also see the teams working in Regulatory Operations having the opportunity to develop into data governance/cross-functional roles. And certainly there needs to be thought about acquiring additional talent and expertise linked to trending topics – systems, data collection and so on. It would certainly be very helpful to have the ‘informatics’ point of view included in Regulatory Operations.”
Regulatory & Marketplace




VP: “I know of a medium-sized company which has renamed its RegOps function ‘Regulatory Informatics’, which is an interesting move. To be effective, such teams do need to work closely with other groups. But what an opportunity for those experts to augment their skill set in this data space, complementing the technology being deployed for tomorrow.”
The panel concluded that the Life Science industry is facing a bright future, thanks to its commitment to embracing a data-first approach. Despite the challenges that lie ahead, the panel was optimistic that the industry is taking the steps needed to ensure a successful and prosperous future.
A fuller report of the debate is available to download from Generis's web site at www.caralifesciences.generiscorp.com/ post/the-big-debate-the-future-will-bedata-driven-white-paper
Max Kelleher has worked in the Regulated Data Management space for 8 years at Generis, working first in implementation and configuration of the CARA Platform, before moving into business development and eventually becoming COO. Along with his father, CEO James Kelleher, Max's vision is to enable the digital transformation of processes in regulated industries with the CARA Platform. In his own words, his goal is "helping hundreds of thousands of people to not hate their work-life with outrageously good software."
Email: max.kelleher@generiscorp.com
INTERNATIONAL PHARMACEUTICAL INDUSTRY 15 www.international-pharma.com
Max Kelleher
Dr. Melanie Ruppel
Dr. Melanie Ruppel, Senior Director Global Regulatory Affairs Regulatory Operations, BioNTech. Vada
A. Perkins
Vada A. Perkins, Executive Director, Regulatory Policy & Intelligence and Head of Regulatory Intelligence, Bayer Pharmaceuticals. Frits Stulp
Frits Stulp, Managing Director, Iperion –a Deloitte business.
Preeya Beczek
Preeya Beczek, Director & Independent Regulatory Affairs Expert, Beczek.COM
Data Transformation to Accelerate Time to Market and Address Product Shortages in Life Sciences Postpandemic
Frits Stulp of Iperion, a Deloitte business, predicts that a wave of data-driven transformation that began in 2022 will enhance insights and decision-making, allowing life sciences companies to gain a competitive edge and provide better patient experiences.
Last year was another challenging one for the Life Sciences industry. On top of residual pandemic-related challenges and supply chain issues, the ever-adapting Regulatory environment has continued to set new standards and enforce new requirements. Those companies that have managed to keep pace with the changes can expect to start reaping some of the rewards of their efforts over the coming year. Others still have work to do, but as new waves of digital transformation promise new process efficiency burdens should start to reduce and improved patient experiences will gradually become a reality.
Here's a roundup of some of the most notable developments of the last year, and what’s likely to be a growing focus in 2023.
Streamline Processes Across the EU
In terms of drug development and clinical trials, EU Clinical Trial Regulation and more specifically the Clinical Trial Information System (CTIS) presented one of the main practical changes of 2022, and the transition to the new registration system has begun in earnest now. From February this year all new clinical trials applications must be submitted via the new portal yet a good many companies are not yet well set up for this, leaving work still to do.
The aim with the EU developments is to harmonise and streamline processes across the diverse region, making it a less daunting location for conducting clinical trials. Persistent anomalies between countries continue to trigger enquiries however, and this year all kinds of companies will be trying to figure out how to navigate the new requirements and overcome any residual complexity.
Data Governance is Key
Supply chain issues, driven by the pandemic, continue to present problems – a situation
that has been exacerbated during the winter, when demand has been at an all-time high. Even though the worst of the recent crisis appears to be over, there is now an extended mandate – certainly in Europe – to monitor and manage any shortages of medicinal products and medical devices. That’s both at an industry level, and by EU member states.

The onus is on the industry now, to capture and provide the right data during major events and public health emergencies. This may require formal data mapping – to identify where the relevant data sits within a company, and who can provide it. Given that efficiency and accuracy of these insights are critical when public health is at stake, it is essential that the industry is prepared.
Forward-thinking companies will see this as an opportunity to review existing data governance, determining where the relevant data sits within their organisations, for instance, and how it might be provided to the EMA most efficiently.
The increased speed of regulatory processes seen during the pandemic has set a precedent, and the only way to maintain that pace over the longer term is to modernise. ISO IDMP standards remain pivotal to the expanding and transforming role of data.
But this requires more proactive data governance, if companies are to truly harvest the power of their data, and it’s something they’ll need to navigate this year alongside
16 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Regulatory & Marketplace
other internal and external pressures –alleviating the increasingly critical but hugely labour-intensive burden of data management.


Challenging Norms
Necessity being the mother of invention, much faster regulatory processes materialised during the pandemic – from rapid access to scientific advice and rolling reviews, to accelerated assessments and other possibilities under compassionate-use programmes. Improvements were also seen in the drug development process itself.

Ultimately, the pandemic forced outof-the-box thinking and helped identify weaknesses in existing systems. We’ve seen old norms being challenged in clinical trials, for instance, as the result of issues that peaked in the pandemic, including those linked to subject recruitment. These issues have helped inspire alternative approaches to trials and their design – including decentralised trials, faster data sharing, and increased collaboration across parties.
Having an agreed model for data that multiple stakeholders and collaborators can work with is potentially transformational. The

European project on substances (the EU-SRS database), which went live on January 24, illustrates what’s possible here. Using the same data model, software and also scientific standards per substance class enables increased exchange with FDA, WHO and other regulators.
Data is the Answer
Applying the lessons of the past few years to reduce the time to patients of the latest advances in Life Sciences is key. As escalating cost and resourcing pressures threaten safe access to healthcare for all, the drive for new care models is strong.
Data lies at the heart of many of the proposed solutions. These include increasingly sophisticated patient self-care propositions (using devices for condition monitoring and management), and first-line care provision by high-street pharmacies.
High quality, standards-based data will underpin operational efficiency and increase automated processes,. There is no time to wait for regulators to take the lead on data strategy and/or process innovation. And there is certainly no reason to delay data-driven




transformations that will ultimately benefit patients.



Frits Stulp is managing director of Iperion – a Deloitte business, and Life Sciences partner within Deloitte where he leads a team of regulatory/IDMP experts to deliver value to both pharma companies as well as regulators. With a background as Program Manager and Advisor in information management, process design and regulatory compliance, he is an expert in the implementation of both XEVMPD and ISO IDMP requirements and the digitalization of regulatory data and processes. Frits works within the broader Life Sciences/risk division of Deloitte in the Netherlands. This article combines the latest opinions and insights from across that team.
Email: fstulp@deloitte.nl








INTERNATIONAL PHARMACEUTICAL INDUSTRY 17 Pressure regulators Control valves Pipeline ancillaries Steam traps Special equipment
adca@valsteam.pt www.valsteam.com +351 236 959 060 Zona Ind. da Guia, Pav. 14 - Brejo 3105-467, Guia PBL PORTUGAL PRODUCTS MANUFACTURED IN PORTUGAL AV 001 IN EN 01.20
STEAMING SOLUTIONS FOR ALL INDUSTRIES
Frits Stulp
Regulatory & Marketplace
Drug Discovery, Development & Delivery
Navigating the Changing Oral Solid Dose Landscape

Oral solid dose (OSD) forms have accounted for the lion’s share of the pharmaceutical market for decades, thanks to their easy administration leading to patient convenience benefits, not to mention their shelflife advantages. As a mature market, it has an unfair reputation for stasis. Much of the innovation spotlight has been on other dosage forms as they have evolved to enhance their own useability, or as they have risen to prominence as a means of enabling the delivery of new therapies, such as biologics.
Despite this, the segment is not standing still. There is considerable innovation going on behind the scenes to provide an everimproved patient experience and to open the oral delivery route to an ever-expanding array of treatments and molecules, including those not traditionally able to be administered via the GI route.
So, what does the future hold for OSD forms? What challenges face pharmaceutical companies as they try to harness the patient experience benefits of OSD in the future?
Philippe Gorria, Senior Director Formulation Development and Sales, and Dr. Uwe Hanenberg, Head of Product Development at Recipharm, explore the trends shaping the OSD space, discuss the development pitfalls facing the developers of new OSD products, and how to overcome them.
OSD Trends Under the Spotlight
A number of trends are shaping the OSD space at the moment, and look set to transform the segment over the coming years. These include:
• The Rise of Fixed-dose Combination products to Optimise Patient Centricity – this has been a trend for several years now, and there is no sign it will be diminishing in importance in the foreseeable future. These formulations allow multiple active pharmaceutical ingredients (APIs) to be combined in a single dosage form, such as a tablet or capsule. They harness modified release
technologies to stagger or delay the release of each API into the bloodstream to minimise side effects or maximise the duration of therapeutic action. As a result, it is possible to reduce the number of doses a patient has to take each day, enhancing convenience and boosting patient adherence.
• Changing Patient Demands – by 2030, 1 in 6 people in the world will be aged 60 years or over.1 As global populations continue to age, patient demands are evolving, requiring OSD drug developers to respond. Older patients present administration challenges, from poor dexterity to issues swallowing. As such, OSD products need to adapt to ensure they continue to meet patient needs –tablets need to be smaller and easier to swallow for example, and packaging needs to be easier for elderly patients to open. Alternatives to tablets and capsules are being explored by drug developers to meet these needs, such as easy-open stick packs containing powdered drug products, designed either to be dissolved in water or swallowed dry. The older cohort isn’t the only demographic OSD drug developers need to consider. Younger patients also have unique administration needs to support adherence. For instance, bitter-tasting medicine can be particularly unpleasant for young patients to take – drug developers increasingly need to consider the flavour of their child-centric drug products to optimise patient adherence.
• Biopharma Continues to Grow – the global biologics market size is expected to be worth around $719.84 billion by 2030, expanding growth at a CAGR of 7.8% from 2022 to 2030.2 This growth is continuing to impact OSD. Traditionally, biologics had to be administered parenterally, despite the relative inconvenience to patients – the sensitive nature of the active ingredients made them unsuitable for delivery via the gastrointestinal tract. However, advances in OSD technology means that it is possible for some biologics, such as insulin, to be developed for oral delivery, allowing the biopharma space to take advantage
of the patient convenience benefits of OSD. Nevertheless, formulating to enable oral delivery of large molecule products poses challenges that drug developers need to overcome.
• Small-batch Manufacturing to Support Orphan Drug Production – The global rare diseases treatment market size was valued at $119.6 billion in 2021 and is expected to expand at a CAGR of 12.8% from 2022 to 2030.3 This is an exciting area for innovation, but it poses challenges for drug developers – by their nature, these diseases are rare, affecting a small number of people worldwide. As such, new commercial paradigms and manufacturing processes are required to make them financially viable. 3D printing of OSD forms is one option that drug developers are increasingly considering as a means of manufacturing small batches of these, often personalised, medicines as cost-effectively as possible.
The Challenge of Adapting to these Trends
These trends are exciting and offer a wide range of opportunities for OSD drug developers to differentiate themselves from competitors and to deliver drug products that meet the changing needs of patients around the world.
However, developing and manufacturing effective formulations to address these trends does pose challenges for drug developers that need to be overcome.
Formulation Development Challenges
Whether reformulating existing APIs or developing formulations for new molecules, it is crucial that the final OSD formulation meets the specific needs of the active pharmaceutical ingredient to ensure it offers optimum therapeutic performance.
Above all, it is important to understand the physical/powder characteristics of the API, such as particle size, morphology, and solubility. For instance, more and more new Chemical Entities (NCEs) have solubility challenges – if these aren’t addressed, along with questions of how to carry drug substance to the right location along the intestinal tract, there can be bioavailability issues
18 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
that undermine the ability of the NCE to be delivered orally. The physical characteristics of APIs also drive the selection of excipients and as well as the specific parameters for the final manufacturing process.
Fixed-dose combination formulations can also be challenging. It is important to understand how the different APIs interact with each other, and to understand their therapeutic performance needs to identify potential formulation solutions to achieve optimum effect. One formulation option that is fast gaining popularity as a means of addressing this issue is to use mini-pellets and coated pellets contained within a capsule. These can allow controlled release of multiAPIs to eliminate compatibility issues.
For the new generation of oral biologics, there are two major challenges. The first is ensuring stability of the active in the gastrointestinal tract. The other is absorption into the body, particularly for larger biologic molecules. Actives over 8-12kDa molecular weight cannot be absorbed naturally by the human body, so require active transport systems or tight junction openers to ensure successful oral delivery.
Infrastructure Issues Trying to Adopt New Forms and Packaging Formats
Whether adopting OSD drug products for the first time, or changing to a new OSD form, such as a powder in a stick pack, upgrading manufacturing and packaging lines is crucial. This can take considerable time and financial investment depending on the nature of the line and the needs of the new drug product, as well as its packaging. This can be challenging for pharmaceutical companies to deliver on their own, especially at speed.

Regulatory Difficulties
When formulating a drug product for OSD for the first time, there is a complex range of regulatory challenges that need to be addressed in order to gain approval. Documentation must be able to show that issues such as solubility and bioavailability, as well as API stability have been addressed to ensure optimum therapeutic performance – a particular challenge for OSD forms. Complicating the issue is the lack of harmonisation between different regulatory frameworks – local regulatory knowledge is needed to gain approvals for multiple markets. Drug developers must ensure they
define their regulatory strategy alongside their development approach and deliverables as early in the development journey as possible.
Capacity Issues
Finally, it can be challenging for drug developers to find or build the capacity they need to manufacture high-volume OSD drug products reliably and cost-effectively, particularly if they are doing so for the first time. The new generation of OSD treatments for rare or orphan diseases also face capacity issues, due to their small batch sizes - it can be difficult to find the infrastructure needed to produce at low volumes cost-effectively, undermining the commercial viability of the project.
Overcoming Challenges
These are significant issues that need to be addressed for pharmaceutical companies to develop effective OSD products that offer the convenience and therapeutic performance that patients expect. Harnessing new OSD formulation technologies and presentations can be particularly challenging, particularly for those companies exploring them for the first time.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 19 www.international-pharma.com Drug Discovery, Development & Delivery
However, there are expert contract development and manufacturing organisation (CDMO) partners available that can help pharma companies to overcome common OSD development challenges and harness the benefits of the new generation of technologies.
For example, CDMOs with a strong track record in the OSD space can provide dedicated formulation expertise, including insight into the latest techniques, to allow the development of effective orally administered biologics. They can also support in identifying new presentations that may help existing orally administered drug products to stand out in a competitive market. CDMOs with a global footprint can help provide local knowledge about regulations or patient demands to ensure that products meet the requirements of the markets they are intended for.
CDMOs can also support pharma companies in overcoming the infrastructure challenges presented by manufacturing OSD products for the first time, whether at high or low volumes. Those that specialise in the segment will have dedicated manufacturing and packaging infrastructure already in place, which can help pharma companies begin commercial manufacturing with minimal delay.
To meet small-volume project needs, a growing number of CDMOs are investing in small scale manufacturing solutions. E.g., 3D printing is one such option, which can allow the rapid and cost-efficient creation of small numbers of tablets containing precise and consistent doses of API. These tablets can be designed to encapsulate one or more APIs within a complex matrix to control their release, according to the needs of the treatment. Working with partners that offer these advanced technologies, pharma companies can be confident that they have the support they need to deliver small batches or even personalised OSD drug products to treat rare or orphan diseases.
Looking Ahead
As a segment, OSD will continue to grow for the foreseeable future. Their useability and unique patient convenience benefits are compelling, meaning we can expect more and more drug products to be developed with innovative dispersing/delivery devices combined and fine-tuned around the oral route of administration.
Nevertheless, adapting to the changing OSD landscape can be daunting, especially for those drug developers that are harnessing new technologies, or seeking to formulate oral versions of traditionally parenteral drugs. Working with an expert development partner is crucial if pharma companies want to adapt



and thrive in the future pharma climate, and fully harness the unique benefits of OSD.
REFERENCES
1. https://www.who.int/news-room/fact-sheets/ detail/ageing-and-health#:~:text=At%20 this%20time%20the%20share,2050%20to%20 reach%20426%20million.
2. https://www.globenewswire.com/en/newsrelease/2022/04/20/2425668/0/en/BiologicsMarket-Size-to-Worth-Around-US-719-84-Bnby-2030.html
3. https://www.grandviewresearch.com/industryanalysis/rare-diseases-treatment-market-report
Philippe is Senior Director, Formulation Development at Recipharm. In his role Phillipe is responsible for the development and formulation of oral dosage forms using controlled drug delivery systems with his team. With over 15 years' experience in the pharmaceutical industry, and almost 25 years in life sciences, he has held a number of both scientific and managerial positions within start-ups and pharma companies. He has a degree in physics and a PhD in physicalchemistry from the Bordeaux University in France.
Uwe
Hanenberg
Dr. Uwe Hanenberg, Head of Product Development, Oral Solid Dose (OSD) at Recipharm. Uwe is responsible for implementing and executing the OSD Product Development strategy that assures science driven, timely development of new products or services. Uwe has 25 years of experience in the pharmaceutical industry with Bayer, Altana, Grünenthal and Catalent. He has held several leading positions in quality, manufacturing and packaging, product development, project management within the Science & Technology sphere. His areas of expertise are oral formulation development, oral manufacturing technologies, stick pack technologies and pharmaceutical contract services and project management.
20 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Drug Discovery, Development & Delivery
Philippe Gorria
Vials with Exceptional Inner Surface Durability For Diluents
For Biotech
Less interactions of drug molecules and formulations with the inner glass surface
•
Optimized lyophilization process (less fogging)
•
Reduced risk of glass delamination
Water for injection | Aqueous NaCl solution

Lower pH shift
•
Reduced risk of glass delamination
NIPRO PHARMAPACKAGING INTERNATIONAL Blokhuisstraat 42, 2800 Mechelen, Belgium | pharmapackaging@nipro-group.com | www.nipro-group.com
Fulfilling the Potential of Drug R&D: Six Predictions for 2023
From enabling patient choice during clinical trials to strengthening vital partnerships across the quality ecosystem, connected data will become the lifeblood that enables Life Science teams to collaborate efficiently and effectively in 2023.

Veeva’s industry experts share their predictions about how breaking down silos across clinical, regulatory, safety, and quality teams will benefit patients.
Data Will Unlock Clinical and
Economic
Value in Europe’s Challenging Landscape
Launching a new drug has always been fraught with risks, and the pandemic raised the stakes. Emerging and mid-sized
companies launching products in Europe are also navigating a healthcare ecosystem that is complex and fragile due to regulatory and cost pressures. With no margin for error, teams will need to rethink their data strategy to quickly unlock clinical and economic value.
Chris Moore, President, Veeva Europe, predicts that “in 2023, successful companies will be more deliberate in how they access, manage, and learn from data ahead of a product launch. In R&D, data ownership will be critical to understanding the market landscape as fast as possible. Sponsors will insource data management even as they outsource trials, so they can improve how they track and use trial execution data. On the commercial side, teams will seamlessly integrate proprietary, external, and public
data sources to identify the potential patient population for a new treatment.”
“Clinical, commercial, and market access teams will start working together sooner to share insights and accelerate the launch process. With an early view of market viability, leading companies will be able to monetise new products quickly and at scale across Europe.”
Simplicity and Technology Intersect to Streamline Drug Development
Jim Reilly, Vice President, Development Cloud Strategy, believes that “the intersection of operational simplification and technology advancement will create cross-functional efficiency across clinical, regulatory, quality, and safety. This will enable biopharmas to create a more stream-
22 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Drug Discovery, Development & Delivery
lined drug development process rooted in lean process execution and higher-quality data.”

“Connected data across the development lifecycle will enable different functions to coordinate decisions, and a common technology framework will eliminate duplicate data capture and inefficient processes. Automated workflows, data reuse, common training, and a simpler technology experience will help companies to adapt quickly to changing market conditions and deliver products more efficiently to the market.”
“By connecting disparate teams, processes, and systems into one operating model, organisations will deliver new products to patients with greater speed and efficiency.”
Patient Choice Will Push Sponsors Toward Operational Excellence

Umbrella, adaptive, and platform trials are now mainstream ways to show treatment success, but they’ve made protocol design increasingly complex. And even with technology to facilitate data collection from diverse sources, companies still struggle to manage non-conventional data at scale.
“In 2023, the volume of data collected from patients will increase – and border on the intrusive,” believes Richard Young, Vice President, Strategy, Vault CDMS. “Sponsors running oncology and diabetes trials, for example, will ask for more information on patients’ real-life movements to demonstrate their quality of life, as well as efficacy and safety.”
“We’re entering a new era of patient choice, and it will be for data management to balance scientific endeavour with operational excellence. Leading companies will help patients understand how their data contributes to the greater good and give
them real-time access to their own health data insights during studies.”
“Patients will choose how to participate in studies from one day to the next, whether in person or through digital methods, based on their daily health status and personal preference, in addition to age, location, and condition. Companies committed to patient-centric trials will reassess their technology and processes to achieve the dual objectives of advancing digital clinical trials and managing data holistically.”
Safety will create a single source of truth for content management.
Kelly Traverso, Vice President, Veeva Vault Safety Strategy and Consulting, predicts that “in 2023, companies will broaden their focus beyond safety data to include all the content and documents that come with it. Teams will develop, review, approve, and track content and documents more closely than ever, right down to version history and user access. Improved control reduces the risk of misinformation, or having teams send outdated or incorrect information to partners.”
“Having a single source of truth, not only for data but for documents and content, will enable more content sharing and review to occur in real time. As safety’s sphere of influence grows, this approach will put content ownership and control directly in safety’s hands. It will result in better, faster, and more informed decisions, not only in safety but in areas where safety collaborates with quality, clinical, and regulatory functions.”
Technology and Data Access Control Will Strengthen Quality Partnerships
As companies began collaborating more closely with contract partners, a pioneering few invited preferred CDMOs into their quality networks. Others were too concerned about security and data access to take this approach. Ashley Wentworth, Senior Director, Vault Quality Strategy, predicts that “this will change.”
“Encouraged by technical innovations that permit seamless access and granular control over information, more biopharmas and CDMOs will invite suppliers to participate in key processes within their quality management systems. CDMOs will also increasingly invite their customers directly into their QMS, quality documentation, and training systems for better visibility and realtime sharing of information.”
“This approach will make it easier for smaller innovators to accept products, sign off and approve batches, review quality events, and share quality data. Over time, companies will develop distinct informationsharing strategies, training programs, and new processes tailored to each partner.”
Regulatory Pressure Will Drive Innovation in Data Management and Submissions
Marc Gabriel, Vice President, Veeva Vault RIM, anticipates that “increasing regulatory pressure will lead to more agile, connected data approaches that allow companies to ensure global compliance. For example, one company that implemented new digital approaches to manage its regulatory data had 40 small RIM system releases in one year – up from one big change every five years before implementation.”
“In addition, the long-anticipated shift from document to data-based regulatory submissions will soon take place, starting with new FDA and industry initiatives that will use data management approaches for manufacturing CMC submissions. In 2023, more companies will focus on developing the systems, infrastructure, and skill sets required to work with data-based submissions. Far from a trivial effort, it will require a whole new operating model and significant organisational change.”
Chris Moore
As president of Veeva Europe, Chris is responsible for growing the business in the region. A 29-year veteran of the life sciences industry, Chris started his career at ICI Pharmaceuticals (now AstraZeneca). Chris then joined a start-up called Kinesis, building a team delivering document management solutions for pharmaceutical companies. Through a series of mergers and acquisitions, Kinesis ultimately became PwC; Chris made partner with PwC in 2001. Chris went on to run both European and US (West Coast) life sciences business for IBM before leading the IBM global life sciences consulting Business Analytics and Optimization unit. Most recently, Chris was the lead partner for life sciences for Europe, the Middle East, and Africa at EY.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 23 www.international-pharma.com Drug Discovery, Development & Delivery
Into the Future with Next-generation Small Molecules
Heightened interest in small molecules is likely to continue in 2023 and beyond as several biotech companies look beyond biologics to bring medicines to patients in need.
Advances in the field of biotechnology have tended to excite interest in their potential as targeted therapies. Vaccines, monoclonal antibodies, cell and gene therapy and other biotechnology innovations have been researched and developed to offer patients targeted treatments.
Despite the high-profile of these therapies, small molecules continue to be the mainstay in disease treatment and increasingly are gaining the attention of traditional biotech and specialty companies looking to build their therapeutic portfolio and provide a wide range of treatment options for patients in need.
In fact, the majority of US Food and Drug Administration (FDA) approvals of new molecular entities (NMEs) continue to be small molecules. In 2022, 22 out of 37 novel drugs approved by the FDA were small molecules.
What is particularly notable is a trend amongst traditional biotech companies to expand their research and development portfolio into the area of small molecules. These innovative products are more complex than traditional small molecules but with fewer of the regulatory and manufacturing challenges faced by more innovative biologic products, such as cell and gene therapies.
Biotech Expands its Portfolio
Among the companies turning their attention to small molecules are Amgen and Biogen. In January 2022, Amgen announced a collaboration with Arrakis Therapeutics focused on research into RNA degrader therapeutics against a range of difficultto-drug targets in multiple therapeutic areas. This is a new class of RNA degraders consisting of small molecule drugs, which “selectively destroy RNAs encoding diseasecausing proteins by inducing their proximity to nucleases.”1
The company has been exploring a range of small molecule products across its oncology platform. For example, its product Lumakras® (sotorasib) is a small molecule indicated to treat adults with KRASG12Cmutated locally advanced or metastatic non-small cell lung cancer (NSCLC).
At Biogen, small molecules are part of the research arsenal aimed at treating neurological diseases. The company has several small molecules in phase 3 studies, including for depression and stroke, as well as research in phase 2 for multiple sclerosis and various other neurological conditions. In relapsing MS, the company is studying orelabrutinib, a small molecule Bruton’s tyrosine kinase inhibitor (BTKi) which researchers believe will help to reduce damage caused by BTK cells.2
It’s not only the biotech heavyweights that have been getting into small molecules. Mirati, a small biotech company from California, in December 2022 received breakthrough therapy designation from the FDA for its small molecule product Adagrasib (MRTX849) in combination with cetuximab for patients with KRASG12C-mutated advanced colorectal cancer.3 In November 2022, biopharmaceutical innovator Agios Pharma was granted marketing authorisation for its small molecule product Pyrukynd to treat PK deficiency in adults suffering from chronic anemia.
Small Molecules of the Future
The next generation of small molecules are structurally diverse and complex, which is paving the way for these NMEs to be targeted at underserved therapeutic areas, such as in cancer and neurological conditions such as Alzheimer’s disease, MS and Parkinson’s disease.
There has been promising research into targeting RNA structures with small molecules to alter the way RNA functions. Several small molecules that bind RNA structures have been shown to modulate a range of biological processes.
The FDA has already approved risdiplam, the first small molecule splicing modifier drug, for the treatment of spinal muscular
atrophy. Marketed by Roche as Evrysdi®, the product, which was approved for adults and children 2 months of age and older, was shown to improve motor function in people living with SMA over a broad spectrum of ages and levels of disease severity.4
Another large pharma company carrying out research in this area is Bayer, which is drawing on its library of more than 4 million compounds and next-generation cell biology technologies to create a platform targeting RNA to develop innovative small molecules to treat diseases with high unmet need.5
Many other smaller companies are also pursuing research into RNA-targeted small molecules, including Accent Therapeutics, which is developing small molecule therapies in the field of epitranscriptomics – a collection of RNA-modifying proteins (RMPs) that control many aspects of RNA biology – to develop cancer medicines.6 Drug discovery and development company Epics Therapeutics is focused on small molecule drugs targeting RNA epigenetic mechanisms involved in cancer development.7
Another small biopharma company developing small molecules to correct RNA expression is Skyhawk Therapeutics. The company has several programs underway targeting autoimmune disease, cancer, neurodegenerative conditions as well as neuromuscular disorders.8 There are many other small companies pursuing RNA targets through small molecules, paving the way for many more breakthroughs and advances in the near future.
These treatments tackle inflammatory conditions in a different way to established treatments, offering hope of both symptom relief and slowing the progression of disease.
Small molecule Janus kinase (JAK) inhibitors are also emerging as innovative new treatment options. JAK inhibitors to treat several chronic inflammatory disorders such as rheumatoid arthritis, ulcerative colitis and atopic dermatitis have recently been approved. These include Pfizer’s tofacitinib (Xeljanz®) for the treatment of arthritis and ulcerative colitis and Eli Lily’s baricitinib
24 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
& Delivery
Drug Discovery, Development
(Olumiant®), which the European Medicines Agency approved in 2017 for rheumatoid arthritis, with the FDA approving a lower dose of the drug a year later.
There are several companies focused on next-generation JAK inhibitors with the goal of lessening adverse side effects.
Navigating Health Crises with Small Molecules

With pressing healthcare issues, such as antimicrobial resistance, there is a specific need for new antibiotic and antibacterial agents. Since small molecules act at the cell level they are potentially more efficacious for targeting these types of pathogens. The World Health Organization (WHO) and global governments have highlighted the need for investment in treatments for fungal or bacterial diseases and several studies indicate the potential of small molecules to tackle these challenges.10
With many countries facing aging populations, treatment for diseases such as Alzheimer’s is a priority and there is some promise with small molecule innovation in this field. In animal studies, KARI 201, a dualaction small molecule, was found to improve neuropathological features of Alzheimer's disease in mice, suggesting its potential as a multifaceted drug to treat Alzheimer’s.11
Other areas where small molecules will play an important role in managing health challenges include autoimmune disorders such as Type 1 diabetes. With studies showing an increase in autoimmune disorders over the past 25 years,12 there is an urgent need for innovation to address these diseases and small molecules potentially hold the key.
REFERENCES
1. Amgen and Arrakis Therapeutics announce multi-target collaboration to identify novel RNA degrader small molecule therapeutics, Amgen, Jan 2022. https://www.amgen.com/ newsroom/press-releases
2. https://www.biogen.com/science-and-innovation/ pipeline.html
3. Mirati announces Adagrasib (KRAZATI™) Receives Breakthrough Therapy Designation from FDA for Patients with Advanced, KRASMutated Colorectal Cancer, Dec 2022, Mirati. https://ir.mirati.com/press-releases
4. FDA approves Roche’s Evrysdi (risdiplam) for treatment of spinal muscular atrophy (SMA) in adults and children 2 months and older, Aug 2020. https://www.roche.com/investors/ updates/inv-update-2020-08-10b
5. RNA-Targeting Small Molecules, Bayer. https:// www.bayer.com/en/pharma/rna-targetingsmall-molecules
6. https://www.accenttx.com/our-scientificfocus/
7. https://www.epicstherapeutics.com/
8. https://skyhawktx.com/pipeline/
9. Antibacterial Activity of Small Molecules

Which Eradicate Methicillin-Resistant Staphylococcus aureus Persisters, Feb 2022. https://www.frontiersin.org/articles/10.3389/ fmicb.2022.823394/full
10. A small molecule that mitigates bacterial infection disrupts Gram-negative cell membranes and is inhibited by cholesterol and neutral lipids, Dec 2020. https://pubmed. ncbi.nlm.nih.gov/33290418/
11. Discovery of a dual-action small molecule that improves neuropathological features of Alzheimer's disease mice, Proc Natl Acad Sci USA, Jan 2022. https://pubmed.ncbi.nlm.nih. gov/35027452/
12. Research round-up: autoimmune disease, Nature, July 2021. https://www.nature.com/ articles/d41586-021-01834-x
Dr. Patrick Larcier, Senior Director at PharmaLex, has worked in drug development and regulatory affairs for 30 years, at biotech companies, at CROs and in consulting. Until April 2022, he led drug development and pharmacovigilance activities for PharmaLex France and Benelux; he now provides support for PharmaLex’s growing EU and US activities in these areas.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 25 www.international-pharma.com Drug Discovery, Development & Delivery
Dr. Patrick Larcier
How Decentralised and Hybrid Clinical Trials can Support Subject Recruitment and Retention to Support Clinical Trial Success
Decentralised and hybrid clinical trials are delivering benefits for sponsors in a number of ways – delivering quality data quicker to reduce time to market, achieving higher levels of compliance, increased diversity of patients participating in trials, and importantly, recruiting and retaining more subjects.
Encouragingly, there has been significant progress in decentralised and hybrid clinical trials in the last few years, with the global COVID-19 pandemic serving as a catalyst that brought major shifts in how we approach the execution of clinical trials. Although the recent uptake has been considerable, decentralised clinical trial components have been successfully implemented and ongoing for quite some time, and we have seen different phases and scales of adoption across the board. More organisations are rolling out pilot programs, implementing hybrid trials more extensively and overall delving deeper into decentralisation to explore the benefits it offers.
Therefore, at this point in the shift to decentralised and hybrid studies, we are getting valuable real-world experience that enables us to drive continuous improvement. Sponsors now have a clearer view of critical success factors such as early planning to ensure optimal engagement with subjects and sites, selecting the right in-home clinical services, wearables or sensors, and staying close to the patient throughout the study.
I’ll explore these lessons in this article, in addition to outlining some of the key elements that need to be in place in a DCT setting to ensure good levels of patient retention.
Enhanced Convenience Drives Enhanced Recruitment
In any clinical trial, be it decentralised or not, the priority is always the patient. We conducted patient voice surveys in 2019 and again in 2020 to better understand attitudes toward decentralised or hybrid trials. The findings indicate that patients are open to decentralised clinical trial approaches, and
overall, they want the options and flexibility they provide.
Decentralised and hybrid clinical trials overcome two common hurdles for recruitment and retention: accessibility and convenience. Typically, subjects that participate in clinical trials live within 50 miles of the site. In our most recent survey in 2021, 90% of respondents were not willing to travel more than one hour to participate in a clinical trial. The physical parameters and burden of travel associated with traditional trials exclude significant populations of patients that are either incapable or unwilling to travel to the site. Providing hybrid and fully decentralised clinical trial options eases the burden of travel and delivers a more positive and convenient patient experience.
Furthermore, the removal of geographic constraints unlocks the potential to recruit participants from more locations, and from more diverse backgrounds. Increasing the diversity of subjects that are participating in clinical research is an important goal we are all working towards in the industry. Our experience of designing and conducting decentralised and hybrid trials to date certainly indicates that by increasing the number of patients we reach, we also achieve increased diversity.
Reducing Patient Burden Through Consistent Support
Reducing patient burden is perhaps even more important in a DCT setting as the flexibility of decentralised methods and technology can potentially replace one challenge with another, for example, using the technology devices themselves.
So, how to approach this? Using the right technology devices and maintaining sight of patients throughout the trial are critical elements.
Selecting the right digital health technology devices is paramount. The sheer range of possibilities can make this challenging. Fundamentally, selected devices must tick boxes such as operational excellence, safety, storage and visualisation, and privacy and security. Study objectives
will feed into these considerations. Device selection also depends on the therapeutic area involved, study duration, endpoints, patient-burden assessments, whether the trial is blinded or not, and whether it involves passive monitoring or active assessment. And just as important are patient centricity and device useability. Consideration must be given to participants’ levels of technology familiarity and comfort. The more comfortable they are in using the selected devices, the more likely they will remain in the trial.
Maintaining sight of subjects in the home setting is also hugely important from a retention perspective. By increasing touchpoints with trial participants, sponsors can make sure patients are filling out their eDiaries or generating ePROs, for example, on a scheduled basis. And so, while it may seem obvious to state, staying close to and supporting the patient is an absolute must.
Dedicated concierge services are important to ensure patient support is fully embedded in decentralised or hybrid trials. The expertise to fully support patients in setting up connectivity to digital platforms, devices and wearables should be considered where digital health technologies are included in the clinical trial. And while assisting patients with technical support is essential, ensuring retention requires engagement beyond technical support.
Direct inbound and outbound communication with patients improves overall engagement, compliance and retention throughout the trial by onboarding the patient and being a central contact for any challenges they might come up against. Customised patient programs ensure the support is aligned to the patient profile and challenges, expected in the disease or indication.
How does this patient support look throughout the clinical trial? This is best outlined by looking at the patient journey.
One of the ways these services might start the support journey during a trial is by reaching out to a patient, after screening, to schedule visits – telehealth, site visit or
26 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Clinical and Medical Research
a combination. This support will include the downloading of digital study apps (eDiaries, ePROs, etc) if they are using their own mobile devices.
If a mobile device is sent to the patient, once they receive their device, an outbound call welcoming the patient into the study will be placed, walking them through logging into the digital study app, setting up, and making sure the patient understands how to use the technology and ensure information is being transmitted correctly. At this time, the coordinator will review study instructions with the patient, Investigational Product delivery and go over dosage instructions.
When the patient has everything that they need to begin their study, the service shifts into a monitoring and oversight phase. For example, if a study requires patients to do daily eDiaries on their medication adherence, the coordinator will review the entire study population and perform outreach to those who have missed their
Clinical and Medical Research
is the patient’s central point of contact to help support them and ensure the patientphysician relationship is really focusing where it should be, on the treatment and care, and less on the day to day demands of the study. This all supports the objective of removing the burden from the patient, ultimately supporting increased retention.
The benefits that can be delivered through decentralised and hybrid clinical trials are compelling for sponsors. Incorporating these approaches into clinical research opens the possibility for reduced timelines and cost savings while increasing the accuracy of safety and efficacy evaluations of investigational medical products. Of course, before these benefits can be realised patients need to be recruited and remain in the trial until its conclusion, which is another area where decentralised and hybrid trails are delivering for sponsors also.
eDiary entries and assist them with any issues that have arisen.
In this monitoring and oversight phase, anomalies in reporting can also be identified. The patient coordinator will track deviations in timeframes or missing data – and when the data goes back to the informatics team, they are able to review the anomalies. This real-time monitoring of progress means that issues are dealt it when they happen rather than affecting data quality and compliance when it comes to close out.
As the patient gets to the end of the study, the patient coordinator will provide instructions on the return of any equipment, medication destruction, generally ensuring that the logistics behind closing the study are all dealt with efficiently.
Overall, the concierge support service informs patients what they should be doing, reminds them when they need to do it and assists them along the way. The coordinator

Laney Preheim, Vice President of Concierge Services at ICON, has over 20 years in the high-tech industry, with the majority of her career focused on the unique intersection between healthcare and technology. She leads the global Concierge Services team at ICON, which includes offerings such as direct to patient services, site support, device logistics and patient safety surveillance and reporting. Laney's team drives services that deeply consider and improve the patient journey and site staff study experiences, across the clinical trial process. She joined ICON in 2021 through the acquisition of PRA Health Sciences. Prior to this, Laney was a key member in the formation of the executive leadership team at Care Innovations, a unique remote monitoring start-up. She previously held a variety of positions at Intel Corporation in the Digital Health and Information Technology organisations, with focus on procurement, ecosystem enablement, product management and strategic planning where she has been at the forefront of managing organisational operations for the development of innovative strategies. She earned a Bachelor of Business Administration degree from the University of California, Davis.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 27 www.international-pharma.com
Laney Preheim
Clinical and Medical Research
Revolutionising Life Sciences Research and Delivery with a New Approach to Modelling Complexity
In life sciences, the adoption of new standards such as SDTM and ADaM is proving critical for efficient and effective data management and sharing. SDTM provides a new way of organising human clinical and nonclinical study data tabulations, which is required for data submission to regulatory bodies like the FDA and PMDA, while ADaM defines dataset and metadata standards for clinical trial statistical analyses, ensuring efficient generation, replication, and review of data. CDISC 360 is another important initiative that aims to implement standards as linked metadata to support metadata-driven automation across the entire clinical research data lifecycle, making it easier for researchers to analyse and share their findings.
So, the digital transformation of pharma industry regulatory processes has started to make data a key tool. There’s a price for this advance – with the increasing volume and complexity of data generated in drug discovery and clinical research, life sciences R&D practitioners need better ways of organising, structuring and exploiting their data, and at scale.
To address this, the sector is increasingly adopting an innovative data structure approach, the knowledge graph. Graph databases can tackle complex problems in drug discovery, multiomics, and clinical research by allowing researchers to store and analyse complex interconnected data such as relationships between genes, proteins, cells, and tissues, as well as help the sector get better at meeting standards like SDTM and AdaM.
The main advantage knowledge graphs offer is their basic design. Unlike traditional SQL databases that use fixed tables with rows and columns to store data, knowledge graphs represent data as interconnected ‘nodes’ (or entities) linked by ‘edges’ (or relationships).
This network (a graph is a mathematical name for a network) of interconnections
holds the key to unlocking breakthrough insights. The power of knowledge graphs is evident in their ability to represent complex data relationships. In the Panama Papers work, for example, a knowledge graph helped uncover an intricate network of opaque offshore accounts, shell companies, and individuals allowing investigators to connect the dots and uncover hidden relationships. These insights would have been difficult to detect using traditional data analysis methods.
Owing to their ability to represent intricate data, knowledge graphs have many applications beyond financial investigations. One such area is biological science, where knowledge graphs can capture the intricate interconnections and correlations among diseases, genes, environment, diet, behaviour, and other factors.
Analysis of such connections and correlations leads to a more profound understanding of the domain, enabling faster and more significant deductions. And with the advent of modern native graph databases, cross-comparisons involving billions of connections can be carried out at scale, facilitating the identification of hidden patterns and connections. This ability has the potential to revolutionise biotech and medicine.
AI Algorithms Can be Applied to Patient Data
To take one example, AstraZeneca is leveraging the power of knowledge graphs to facilitate reaction and synthesis prediction, streamlining the development of novel organic molecules and even demonstrating the potential of knowledge graphs in reaction and synthesis prediction during drug discovery. The firm is working with a nine-million-node graph featuring 33 million relationships to do this, with the graph helping identify areas in the chemical space where new reaction networks can be formulated.
According to the firm, graphs are useful in drug discovery because chemical reactions naturally form networks. When a reaction occurs, the product can lead to other reactions, resulting in a graph structure. By utilising
path queries between two molecules, data scientists can understand the connections between reactions. This information can help train new lead prediction algorithms, enabling scientists to predict how different molecules will react and improve drug discovery efforts. And AstraZeneca’s application of graph technology is being supplemented with data visualisation tools so that scientists can recognise important molecules and reactions they want to investigate more quickly.
AstraZeneca is not the only pharma brand benefiting from knowledge graphs. GSK, for example, finds graph techniques and tools are reducing the manual effort required to validate analysis to ‘nearly zero’ and ensuring compliance with GDPR on informed consent so that the patient's details disappear from downstream renderings of the data.
In addition to using knowledge graphs to enhance GSK’s clinical reporting workflows and address emerging regulatory standards, GSK aims to proactively perform riskbased monitoring. Here, the GSK team has developed a Google-like questionand-answer system that enables users to obtain rapid answers from their clinical trial data. GSK is also employing powerful AI algorithms originally developed for preclinical data sets which can be applied to patient-level clinical data. To manage the dataset effectively, the company has opted for a clinical knowledge graph that provides a patient-centric data model, which integrates all domain silos and enables everyone involved to understand the clinical data. GSK is on the way to achieving this, says the team, and while this project isn’t yet a full industrial process, early results are consistently strong.
Both AstraZeneca and GSK say graph technology was the natural fit for this problem space.
One of the main benefits of knowledge graphs is that they are not restricted by particular data schema or formatting requirements. They can work with native data structures, and queries can be conducted by asking relevant questions. Moreover, these queries can be executed at lightning-fast speeds, often up to 3,000
28 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
times faster than SQL database queries, and across dense networks of knowledge. Such speed can enable rapid pinpointing of the best doctor to target for a clinical trial's success, considering not only their area of expertise but their current capacity, access to the necessary equipment, and whether they may be working with a competitor.
Clinical trials can benefit greatly from knowledge graphs in the case of rare conditions where small patient populations can make it difficult to achieve statistical significance. For example, in diabetes research, knowledge graphs can aid in phenotype mapping, where researchers want to understand the relationship between different observable phenotypes in both humans and animals. This can be particularly challenging when the clinical parameters
Clinical and Medical Research
Novartis's historical and image data in a knowledge graph. The team then uses graph algorithms to identify desired triangular node patterns, allowing them to find data linked by the desired node pattern and arrange the triangles according to a metric that gauges the associated strength between each node in each triangle.
Knowledge Graphs: Vital Role in Pharma R&D
Overall, the Novartis R&D team has found that using knowledge graphs has allowed it to navigate its vast amounts of data more flexibly, helping to accelerate drug discovery and develop the next generation of medicines.
Novartis, AstraZeneca and GSK are far from being alone. As society faces increasingly demanding, complex clinical challenges, understanding the value of relationships between data with the help of advanced tools like a graph-based knowledge graph is emerging as important as the data points themselves. Without the ability to mine correlations for new insights, even the most promising innovations may lack context and researchers may struggle to make much headway.
Based on these and other Life Sciences use cases, it’s becoming ever-more evident that, with their ability to uncover insights from complex data sets, knowledge graphs are starting to play an increasingly vital role in pharma R&D.

and observations used to measure these phenotypes are not directly comparable between species.
Another use case that shows more of the benefits of knowledge graphs is Novartis, which is using knowledge graphs to connect and navigate the vast amounts of data it has accumulated over the years. By using graph technology to create a central database of biological data, the firm is starting to be able to link genes, diseases, and compounds in patterns that allow researchers to quickly identify and investigate correlations between them.
To take one example, text mining is used at the beginning of the drug development pipeline to extract relevant text data from PubMed, which is then combined with
Dr. Alexander Jarasch, the Technical Consultant for Pharma and Life Sciences at native graph database leader Neo4j. He was previously Head of Data Management and Knowledge Management at Germany’s National Center for Diabetes Research (DZD).
He is a visionary speaker on the future of clinical investigation, plus AI and data management in the pharma and healthcare space — in particular the potential to advance pharmaceutical analytics and unlock terabytes of hardto-parse research/trial data by revealing data relationships for better predictive accuracy.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 29 www.international-pharma.com
Dr. Alexander Jarasch
Intelligent Innovations for the QA/QC Labs of the Future
Errors come in all shapes and sizes. While human errors are usually accidental, these mistakes can have far-reaching consequences in the pharmaceutical industry, including risks to patient safety. Even when the damage is minimal, errors cost time – and time costs money.
Lab users often struggle to identify errors, and most current liquid chromatography instruments cannot flag them proactively. Current systems generate huge amounts of metadata that also must be manually checked for errors. This can be a significant burden for two reasons: (1) increasing amounts of time are spent searching for errors; and (2) these mistakes can cause a significant chain reaction before they are detected. Random errors and mistakes that only occur in very specific circumstances are even harder to spot. Many errors will also be detected at the review stage; these reviews require expert attention and time, and cause bottlenecks in many quality control (QC) labs, where speed and consistency are vital.
Is it possible to overcome these obstacles and improve outcomes in the QA/QC environment? Movement toward intelligent instruments and automated processes could make a big difference. But how can augmented intelligence help us to detect – and even prevent – errors? How will these capabilities (and others) impact daily life in the lab? And what difference could all of this make for patients?
Errors in the Age of COVID-19
Prior to COVID-19, big changes were already happening in labs, with one key example being a shift toward remote working. When the pandemic struck, however, remote work became essential, staff turnover increased, and team presence in the lab (including that of supervisors and senior managers) was vastly reduced. The way labs worked was transformed overnight and adapting to the restrictions was no small feat.
Staff onboarding also became increasingly complex. Under usual pre-COVID
circumstances, training a new employee could take months, with complicated elements of the job like diagnosing and troubleshooting problems taking longer still to learn. During the pandemic, new employees (who were likely to be unfamiliar with the existing systems) entered the lab with limited opportunity to receive inperson training. Under these circumstances, it’s highly likely that mistakes will be made, and go unrecognised for an extended period of time.
Common mistakes in the QC lab might include:
• Mislabelling of vials
• Incorrect sample preparation
• Incorrect selection of HPLC columns
• Failure to correctly follow standard operating procedures (SOPs)
• Incorrect loading of HPLC well plates with samples.
The Associated Cost
Small errors in the lab may not represent a cause for panic, but their management is crucial. This is particularly true in the QA/QC environment. And while errors are often the result of individual mistakes, their mismanagement is usually a product of organisational environments that do not encourage employees to report and manage errors in the proper fashion. The result: an anti-quality culture, in which analysts feel pressured to hide their mistakes.
One consequence of laboratory errors that are not addressed in an appropriate manner is the Food and Drug Administration (FDA) warning letters/483 observations, which are used to communicate concerns and document observations made by FDA representatives during a facility investigation. Although 483s do not come with a financial penalty, the findings are made public. Introduction of a company’s mistakes into the public sphere in this way can have damning – and lasting –consequences. Yet, many labs are struggling to identify the tools needed to identify and reduce errors.
While there are many ways to minimise and combat errors in the lab (examples
include maximising the time that employees can dedicate to tasks and training for competence rather than compliance),1 the advent of intelligent analytical technologies and robotic systems may be one of the most promising opportunities for the future. Systems that actively flag errors –rather than creating data files that must be mined – can reduce the burden for labs while streamlining everyday operations and freeing up the time of staff members for tasks that add most value and make best use of their expertise.
WANTED: Smart Systems
In the digital age of smart technology, there’s an expectation that lab equipment should also be adequately intelligent. Everyday consumer technologies boast many user-friendly features, such as training programs and warning messages that can be easily interpreted. Couple this with an intuitive interface, and users are good to go. Today’s lab instruments should follow a similar path, meaning that users can interact with the software, with minimal training. In this day and age, such features are not only ideal, but should become commonplace.
Instruments could even be designed to initiate training programs themselves (an important consideration in the case of complex technologies or for on-going learning or troubleshooting). This training could also provide essential information on lab SOPs, and be available as a video to watch from home when working remotely. Such ways of working are a welcome departure from the hefty user manuals of the past, but what many of today’s labs want is software that is so simple that next-tono training is required. Software that talks the user through its processes in real-time is one potential solution. Such software could create an environment in which users bypass the need to learn how or why the technology works at all. After all, users only need the skills necessary to execute the assay or workflow required to get the job done!
Some intelligent systems are already equipped with features such as system health and suitability checks, which can prevent errors before they happen. As
30 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Technology
an example, a system might be designed to assess the void volume in columns before running a sample, or to flag if the amount of solvent available on the system is insufficient to complete the analysis. Systems could even be specifically designed and developed with the ability to predict future errors before they happen. When systems are suitable for analysis, smart software could also warn users about basic mistakes (think incorrect columns and such) before samples are run – and ultimately wasted. Automating these processes could push labs forward by supercharging their efficiency and ensuring consistent QA/QC outputs with minimal mistakes.
Automating Lab Processes and Procedures
Automation has already made its way into labs.2 In these environments, automation reduces the work burden on staff by improving workflow integrity. In sample preparation, robotic arms have been programmed to assist lab workers in their most mundane and repetitive tasks. But automation conveys many advantages. Now the industry must focus on other key steps in the workflow, such as HPLC column selection and chromatography data review, to employ automation to its maximum – and hopefully reduce error frequency.
It is already possible to set up samples, evaluate the system suitability, and process reports automatically. The next step will be to and enable notifications that eliminate the need for physical visits to the machines (all in the name of sparing the valuable time of scientists). Ways to automatically review
instrument methods would also contribute to eliminating transcription errors. Truly integrated automation would also help labs by:
• Identifying samples within trays

• Guiding users in which solvents or pipettes are appropriate
• Adding consistent sample information and ensuring data integrity
• Reviewing data
• Extracting pertinent results
• Assessing instrument health via trending data
Such advances have clear benefits for compliance: opportunities to interfere with the data are significantly reduced when working within a predefined and automated workflow. However, achieving appropriate levels of traceability could represent a challenge when harnessing automation in lab instruments, so ways of working in individual labs must be adapted accordingly. Including an element of traceability through electronic notebooks or similar devices adds value while preventing errors associated with the need to scribble notes while running experiments.
Then there are the considerations for more complex processes that happen away from the bench. These might include processes that use multiple pieces of lab equipment or processes that happen across multiple laboratories. In these cases, the software must be smart enough to react to situations dynamically and adapt to changing circumstances. Another hurdle is the need to gather metadata to feed
into and train intelligent systems. How can such metadata be provided to systems in a way that drives them to make the correct decisions in the complex and highly regulated environment of pharmaceutical manufacturing?

A View to the Future
Companies are dipping their toes in the water of assistive intelligence in the lab. After all, opportunities to keep regulators at bay and avoid recalls are investments well made. And, while the most immediate benefits are likely to be error identification and prevention, questions remain as to what the ideal future state may look like, and whether labs will allow intelligent products to make the crucial decisions in the QA/QC setting. The answers to such questions will come with time – and experience.
However, human input is still required. Deeper and more theoretical decisions, and regulatory sign off, will continue fall to lab managers and other senior members of the lab team. But working alongside intelligent instrumentation could alleviate some of the practical burden of testing, particularly in the (still difficult to navigate) post-pandemic world. Practical elements of these intelligent systems such as intuitive interfaces could make their impact even more profound – and more immediate.
To learn more about how the role of smart technology can mitigate human error in the lab, listen to this webinar.
REFERENCES
1. Bodmann K, et al. Chimia (Aarau) 2016;70(9): 610–5.
2. Waters Corporation 2021. Case Study: Liquid Handling Automation System Streamlines Sample Preparation for Nutritional Analysis.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 31 www.international-pharma.com
Mike Wilson
Mike Wilson obtained both his Master's and PhD in Chemistry from the University of York, UK. After a period working in the pharmaceutical industry, he joined the Waters UK field service team in 2009. Since then, he has held various product management and product marketing roles at Waters, and now supports the QA/QC team in Milford, USA.
Technology
The Adoption of Artificial Intelligence and Big Data in the Sector
The Life Science Sector has been undergoing an immense transformation, driven by the adoption of artificial intelligence (AI), machine learning (ML), natural language processing (NLP), and big data. These new technologies are enabling companies to restructure their business models, reduce human error, increase efficiency and performance, and bring innovative products to the market much faster. This has led to the emergence of a new “pharmaceutical intelligence” that has allowed the industry to move away from traditional, slow-moving, and costly processes. AI is increasingly important in drug discovery and development as well as clinical trials, operations, pharmacovigilance, and many other areas.
The life science sector is increasingly leveraging technology across its operations and this trend will continue in 2023 with increased collaborations and partnerships between pharma, biotech and medical device companies and IT vendors. Through these collaborations, advanced technologies such as AI and big data are being employed to develop personalised and targeted medicines, ultimately leading to more effective treatments for diseases like cancer and immune deficiencies. This technology will also allow companies to utilise advanced computational models to find better treatments more quickly while also reducing costs associated with drug development. This trend has already begun and is only likely to grow as companies continue to invest in advanced analytics and data-driven decisions.
Blockchain Technology: The Future of Data Transparency
Blockchain technology is quickly becoming one of the most important trends in the sector, offering a secure and easy way to share data across organisations. This technology provides all parties with an up-to-date and accurate view of the supply chain and other data sets, which is essential for maintaining robustness in the manufacturing process. While blockchain is commonly connected to cryptocurrencies, its uses extend far beyond financial transactions, and the technology is
being adopted across the sector to increase transparency and security.
One of the most valuable aspects of blockchain is that transactions on a blockchain network are stored permanently and are viewable by all participants, making it difficult for fraudulent activities to go unnoticed. This helps to ensure that sensitive data, such as patient information or drug safety standards, remain secure and trustworthy. The technology also offers a more efficient and cost-effective way to handle transactions confidentially. In addition, increased R&D in personalised medicines is driving more demand for blockchain technology. With personalised medicines, the need for data accuracy and integrity is paramount, as decisions are made with individual patients’ health in mind. These factors highlight that the need for secure data sharing has become more important than ever in the sector.
Bringing Innovations to Market
The digital revolution in the sector is poised to take a huge leap forward in 2023. We have already seen how the adoption of digital technologies into clinical trials enables decentralised readouts and mobile phone applications for patient participation, allowing for more efficient and cost-effective clinical trials that span multiple regions. This could drastically reduce the time, cost, and resources associated with clinical trials. At the same time, Industry 5.0 will build upon the principles of Industry 4.0 to take production processes to a new level of human-centricity. This could lead to more personalised medicines tailored to individual patient needs and help companies develop more effective treatments for certain patient groups.
At the same time, the sector is closely following advancements in digital health technology and its applications in healthcare. Wearable technologies such as fitness trackers, heart rate monitors, and smartwatches are becoming increasingly popular and allow users to track their health more accurately than ever before. Wearables also have the potential to revolutionise healthcare in clinical trials, providing data that is more accurate and reliable than traditional methods of monitoring patients.
Another component is “connected health,” which refers to the use of digital technologies for healthcare, including mobile health (mHealth) and telehealth applications. Connected health has a wide range of potential applications, from providing patients with more convenient access to care to enabling healthcare providers to better monitor patients’ health. In 2023, technologies that power wearable devices and support connected health are likely to become increasingly popular as companies continue to invest and explore the possibilities of leveraging them for healthcare purposes from remote monitoring to clinical decision support.
Ensuring Safety and Efficacy with CSA and CSV
Computer Software Assurance (CSA) and Computer Systems Validation (CSV) are two distinct areas of compliance that companies must consider when developing new computer systems for use in drug manufacturing and other related processes. CSA is a set of standards that ensure the safety and efficacy of computer systems used in drug manufacturing, while CSV involves validating the computer systems themselves. We expect to see a rise in CSA and CSV as companies continue to invest in computerbased technologies and strive towards meeting regulatory standards.
The adoption of cloud computing has also enabled companies to bring their products to market quickly while maintaining compliance and quality standards. This has allowed for the acceleration of innovation in the sector and is expected to become even more prominent in 2023.
An Evolving Regulatory Landscape
The regulatory environment is becoming increasingly interdependent and globalised. Companies are looking to leverage overseas approvals to increase efficiencies while ensuring quality and safety standards are met. This is especially true as the trend toward incorporating AI, cybersecurity, and data analytics into healthcare products is rapidly growing. In response, new regulations have been created to evaluate the quality, safety and efficacy of these products.
32 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Technology
In particular, companies must pay attention to Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). For example, the IVDR sets out specific requirements for software used as a medical device. These regulations stipulate the necessary steps for the design, development, and fulfillment of Software as a Medical Device (SaMDs), from product concept to market entry.
The release of new guidance from the Medical Devices Coordination Group (MDCG) and any transitional arrangements must also be considered. The US Food and Drug Administration (FDA) has introduced an electronic Submission Template And Resource (e-STAR) program to support companies in developing and reviewing new medical devices, which provides an interesting opportunity.
New Requirements for Safety and Quality
The continued interest in RNA-based therapies such as gene therapy and nanoextracellular vesicles is driving innovation within CMC biologics. CMC, short for chemistry, manufacturing, and control, defines the characteristics, safety, and consistency between batches of a pharmaceutical product. Ultimately, the goal of CMC within the biologics space is to ensure that pharmaceutical products can meet or exceed specifications throughout any step of the production process.
This goes hand in hand with revisions to EU pharmaceutical legislation are providing opportunities to improve the supply chain and reduce drug shortages. The use of electronic Product Information (ePI) and the web-based Human Variation electronic Application Form (eAF) on the Product Lifecycle Management (PLM) portal is also becoming more prevalent, particularly for Centrally Authorised Products (CAPs). Finally, the mandatory use of the Clinical Trials Information System (CTIS)
for clinical trial applications in the EU is increasing, ensuring that safety standards are met.
At the same time, the newly revised EudraLex Annex 1, published in August 2022, has brought a much-needed shift in regulatory expectations. Instead of simply providing what is considered acceptable and not acceptable, companies must now provide risk-based rationales for decisions made with patient safety when it comes to manufacturing processes. This brings with it several challenges for organisations that are not at the same level of maturity when it comes to Quality Risk Management (QRM) systems.
To ensure readiness by the August 2023 deadline, companies should first evaluate their current level of compliance with Annex 1 regulations and identify any gaps between current status and the regulatory expectations. This assessment should include all areas of sterile manufacturing and should involve a cross-functional team to ensure everyone involved is aware of how the new Annex 1 will impact their areas of expertise.
Early Phase Modeling to Inform Strategic Decisions
By 2023, early phase modeling (EPM) is expected to become a standard component within companies' development process. The EPM process involves key evidence gathered from the early stages of product development to support and inform decisions around pricing and reimbursement as well as market positioning. This helps to ensure that companies understand their product's potential before releasing it to the public, increasing the chances of successful reimbursement and anticipated pricing.
EPM not only helps to keep companies informed of their product's potential but also allows them to become more aware of any

gaps that may need attention. The process provides information on how a product will be accepted in the market, helping to predict whether or not there are likely to be any regulatory or health technology assessment (HTA) questions or concerns. This is especially helpful for the developers of more complex therapies and medical devices, as timely evidence gathering can help to demonstrate a product’s potential to investors and encourage further investment in ATMPs.
Preparing for 2023
The rise of advanced technology such as AI will revolutionise how the life science sector manages supply chains in 2023. Companies will be able to leverage AI-driven analytics to predict customer demand and proactively adjust supply chain operations. Companies must prioritise sustainability in their supply chain operations. This means investing in green production methods and ethical sourcing – an area where PharmaLex can provide guidance and assistance.
In 2023, the sector will be shaped by advances in technology, globalisation, and quality compliance. Companies that fail to prepare for these challenges will find themselves left behind by their more nimble competitors. With its unique expertise in compliance-related services, PharmaLex is ideally positioned to help companies navigate these changes and be on the cutting edge of trends within the sector in 2023.
Jane Lyons

INTERNATIONAL PHARMACEUTICAL INDUSTRY 33 www.international-pharma.com Technology
Jane Lyons is the European Regional Coordinator for QMC VDC & Country Manager for PharmaLex Ireland.
How to Take a Safety-first Approach When Harnessing the Power of HPAPIs
In this article, Mike Avraam at ChargePoint Technology, explores the challenges of handling HPAPIs safely, and discusses how to comply with safety regulations. He explains how the use of next-generation containment technology, including single-use components, can support pharma manufacturers in harnessing the potential of HPAPIs while ensuring the safest possible working environment for employees.
The international high potency active pharmaceutical ingredient (HPAPI) market is growing rapidly. According to Kenneth Research, it is forecast to be worth $33.15 billion by 2025, up from $16 billion in 2016, more than doubling in size in less than 10 years.
This surge has predominantly been driven by the potential these ingredients have for enhancing the efficacy of new drugs for patients. This is particularly the case for treatment of serious or chronic conditions, including therapies for cancer, cardiovascular diseases, and diabetes.
Despite the separate categorisation of HPAPIs, all active pharmaceutical ingredients have pharmacological potency and each can be categorised on a scale of potency from low, to moderate, potent and highly potent. HPAPIs elicit a more targeted pharmacological effect at a lower concentration when compared to traditional alternatives.
In addition, for many potential drug product applications, HPAPIs can receive fasttrack designation or accelerated approval to treat the unmet needs of patients often with serious, life-threatening conditions. This means often they pave the way for an expedited route to market, so patients can enjoy the benefits of new, more effective treatments faster.
However, the potent nature of HPAPIs poses many challenges when it comes to their safe handling during the manufacturing process. Careful consideration needs to be taken in the design of production lines,
as well as carrying out reliable testing to ensure containment systems effectively minimise the potential for line operatives to come into contact with the materials.
Maintaining Safety
Commensurate with how potentially hazardous HPAPIs are to work with, regulations and guidance surrounding their handling are growing more robust. Regulators are concerned with ensuring a focus on mitigating cross-contamination and that proper facility design is followed for those involved in multi-product operations.
HPAPIs usually are categorised using an occupational exposure band (OEB) strategy, with compounds placed in bands 3, 4 and 5 requiring a variety of special handling and isolation practices. There must also be careful consideration taken in when it comes to understanding and applying the variation of banding criteria from one manufacturer to another.
Limiting handler exposure to these compounds necessitates effective containment technologies. When exposed to higher levels of HPAPIs than what is deemed safe, employees would be at risk of undesired health effects, as the compounds can be carcinogenic, mutagenic, or clastogenic.
Where manufacturing processes involve manual intervention, the process of ensuring an effective containment system within a pharmaceutical environment is especially difficult. Compounding the difficulty of maintaining safety is the necessity for containment solutions to maintain operability and keep productivity of the employees at normal levels.
Regulatory Considerations
There are specific regulations and guidelines that pharmaceutical companies must comply with. COSHH 2002 regulations, for example, call for exposure quantification and worker protections through risk assessments, continuous improvement, collective protection measures, and health and safety precautions.
Smart Factory Technology (SFT) is one way manufacturers can achieve this with the
addition of mechanical handling devices to their facilities. By preventing direct handling of product containers or split butterfly valves (SBVs), factories of the future will become less reliant on operator intervention.
SFTs can also monitor the health of production line components and identify where maintenance is required without affecting containment integrity while providing documented audit trails. As a result, it removes the need for manual monitoring of production line equipment, boosting efficiency, removing room for human error and reducing contamination risks.
Containment Technology
To ensure all of these factors are taken care of, from safety to operability, manufacturers must carefully select the correct equipment for their needs, outline processes and procedures, and utilise the appropriate containment technologies. The use of isolators, restricted access barrier systems and SBVs, which function to separate drug products from operators, has grown in recent years. The reason for this is that closed transfer technologies, such as the SBV, are able to limit manual intervention and reduce the risk of cross-contamination.
These types of containment technology have been proven to effectively manage the risk of exposure from airborne dust particulate. This is due to the fact that they have been developed to improve containment for processes where there is a risk of airborne exposure, including during all powder transfer stages. The valves, when integrated with isolators and other process equipment, allow for material to be transferred whilst minimising the risk of it escaping.
Single-use Components
Demands placed on manufacturers by regulations and guidance inevitably increase the need to carry out additional cleaning and validation requirements. This has led many companies involved in HPAPI manufacture to adopt or evaluate the possibility of introducing single-use (SU) technology or systems into the process.
SU technology allows companies to deploy manufacturing solutions that are comparable
34 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Manufacturing
in performance but less expensive to operate than traditional systems. In specific use cases, the benefits of SU solutions are the ability to dispose of the materials used rather than employing the labour-intensive cleaning process that traditional systems rely on. The disposability of SU technology is able to reduce cost through various factors, for instance, the initial capital investment is obviously far lower when compared to traditional equipment. However, the ability to reduce washing and cleaning validation activities, alongside the lowered risk of cross contamination, presents other benefits.
It is also now possible to utilise disposable SBV technology within the HPAPI manufacturing environment, which allows for a method of contained powder transfer within a facility, as well as to be used as primary packaging and container closure for transportation to another facility. This enables the manufacturer to eliminate double handling thereby reducing risk while keeping costs down.
Validation Process
Once the handling and containment systems have been selected, it is crucial that they are then tested for efficacy. The International Society for Pharmaceutical Engineering’s
(ISPE) SMEPAC (Standardised Measurement of Equipment Particulate
Airborne Con-centration) guideline is often used as a best practice approach for testing to compare different methodologies. The SMEPAC details the validation methodologies for various technologies and processing equipment, which are focused on determining the efficacy of a system containing particulate matter. It provides methodologies manufacturers can use to derive performance data, which are essential for risk assessments.
Rather than being a list of requirements, the SMEPAC is a guideline but one that
is able to provide an effective means of identifying potential risks. What this guideline does not provide, given that the instructions and test methods are conducted in controlled conditions in the laboratory, is an exact replication of how the handling and containment systems will operate in real-world manufacturing. Companies must utilise the data generated during validation to understand this.
Data Assessment and Interpretation
The second stage of the containment technology validation process centres on the containment performance data and its interpretation. It is at this point that manufacturers must identify that testing protocols in the SMEPAC document allow for a degree of inconsistency. Manufacturers can look at containment data and then use it to qualify containment technology; however, it should be noted that variability in the testing methods is possible, and this must be recognised before decisions are made before qualification.
The importance of validating the whole manufacturing process must also be understood, with this involving all of the containment technologies used for the production of the HPAPI. This means that every step where there is a potential for exposure must be validated and a risk assessment carried out. This extends to testing of the impact of operator intervention, especially where a containment device is dependent on operator technique for performance.
Continuous Operational Monitory and Industry 4.0

As should be clear, the requirements for ensuring an effectively contained HPAPI manufacturing process are extensive, timeconsuming and complex. Industry 4.0 is changing the pharmaceutical manufacturing process: a continuous monitoring approach

with internet-connected equipment that enables real-time evaluation of performance by aligning digital and physical environments.
It is a growing trend to select containment technologies based on their capacity for continuous monitoring and their ability to communicate with other systems on the manufacturing line.
The ability to continuously monitor equipment operational data allows for the revalidation of an existing line, or the validation of lines using duplicate technologies, to be carried out expeditiously. The data generated is also able to indicate potential wear and tear of equipment, which can then be combined with containment testing results to determine a safe operating period or usage cycles for equipment before performance begins to be compromised. This type of containment technology allows preventative maintenance to be carried out preemptively – allowing for the safety and integrity of the manufacturing process for HPAPIs to take place on a more secure level than previously possible.
The innovations happening within the HPAPI manufacturing space are geared to making the process as safe and efficient as possible. The correct choice of containment technology and handling procedures is essential in this process. A securely contained process allows the product to be safely manufactured by employees, which enables the HPAPI to itself to reach the end recipient, the patient, as quickly as possible.
REFERENCES
1. https://health.ec.europa.eu/system/files/ 2022-08/20220825_gmp-an1_en_0.pdf
INTERNATIONAL PHARMACEUTICAL INDUSTRY 35 www.international-pharma.com Manufacturing
Michael Avraam
Michael Avraam is the Director of Applications and Solutions Engineering at ChargePoint Technology. His previous position at the business was as Head of solution engineering where he was instrumental in the evolution of ChargePoint's split butterfly valve technology, including containment performance testing as well as developing new products and technologies.
Speed and Precision in Vial Handling
HOF Sonderanlagenbau GmbH took the opportunity of the Achema 2022 trade show to unveil a new loading and unloading system for freeze drying vials in pharmaceutical production – an innovation that epitomizes the finest in conveying and handling technology. The combination of magnetically propelled “movers” and two high-precision, ultrafast Stäubli Stericlean robots is truly impressive.
Freeze drying is the preservation method of choice for many pharmaceuticals that are supplied in vials for transfer to syringes in hospitals and doctors’ offices. This requires that the small vials are filled with the active ingredient and dried partially sealed in a freeze dryer.
Core Competence: Freeze Drying of Pharmaceutical Products
For the past 30 years, freeze dryers for pharmaceutical production have been the core competence of HOF Sonderanlagenbau GmbH, a company based in the small town of Lohra, southwest of Marburg in Hessen, Germany. HOF freeze dryers have been installed by many prominent pharmaceutical and biotechnology companies around the world, and the scope of supply often includes peripheral loading and unloading systems developed in-house.
As a technology leader in the highly specialised and sophisticated field of customengineered pharmaceutical production systems, HOF continues to set the benchmark for performance and degree of automation in freeze drying as well as associated loading and unloading systems. A recent example of this is the SIRIUS robot-assisted loading and unloading system which HOF unveiled for the first time at Achema 2022.
Fast, Flexible and Gentle on the Product
Just one look at a SIRIUS system in operation reveals that the HOF design team has developed something fundamentally new. The vials arrive from the bottling line in large numbers, with their caps only loosely attached. Magnetic movers then gently yet rapidly funnel them in nests of five into an
oval circuit positioned in front of the freeze dryer, where two compact Stäubli TX260 Stericlean six-axis robots with special grippers developed by HOF wait to pick up 50 vials at a time.
When 10 movers, each with five vials, have parked themselves at the loading station, the robots take turns lifting the 50 vials from the movers and placing them on the shelf of the freeze dryer. It’s a breathtaking spectacle: If just one of the approximately 13,000 vials per shelf were to topple over, it would trigger a disastrous domino effect, entailing costly product loss. Fortunately, however, this does not occur; the vials do not even touch each other, thanks to HOF’s engineering expertise and Stäubli’s highprecision robots.
From Magnetic Mover to Robot Gripper and Back Again
This reliability applies both to loading the freeze dryer and to the unloading stage, which is assisted by a pusher mechanism. Here, the TX2-60 Stericlean also unerringly picks up 50 closely packed vials and places them quickly and precisely onto the movers, which then transport them to the crimping machine for sealing.
The manufacturer specified that the vials should not touch each other at any point in the transport stage, and not just for fear of them knocking against each other and spilling their high-value contents. As Peter Schneider, Sales Management Loading and Unloading Systems points out, “Unsealed vials with sensitive active pharmaceutical ingredients are being handled here. That’s why we work in an aseptic area – in an isolator or a RABS (Restricted Access Barrier System) – and why we have to avoid any particle generation. This includes glass contact.”
Up to 400 Vials Per Minute Under Aseptic Conditions
For this very reason, the robots must also meet all aseptic requirements for working in a Class A cleanroom environment. They do indeed comply, and because HOF chose the Stericlean model, they achieve a long service life despite the intensive cleaning processes prevalent in pharmaceutical production.
The performance of the SIRIUS system, particularly the magnetic movers and the two robots, is truly remarkable. “The target set for the design team was a loading and unloading capacity of up to 400 two-millimeter (2R) vials per minute,” says Schneider. “Consequently, one loading/unloading cycle had to take no longer than 14 seconds. We met our target.”
Exacting Demands in Safety and Flexibility
One prerequisite for achieving this target is the precision with which the robots place the vials on the shelf of the freeze dryer and subsequently retrieve them. The TX2-60 Stericlean has a repeatability rate of ± 0.02 mm, so everything is within the green zone here.
But the paramount factors are safety and reliability. “A safe and reliable process, both for the product and the operator, is the most important requirement,” says Schneider. “In addition to machine and workplace safety, this includes reproducibility and, of course, pharmaceutical cleanroom compatibility.” The senior management at HOF also appreciated the support Stäubli provided during the development phase in the form of advice and suggestions for optimisation based on simulations and feasibility studies.
Readily Adaptable to Different Types of Vials
Another primary consideration in the design of the system was flexibility. As Schneider explains, “There are many different formats and standards for vials, ranging from 2R to 100H. SIRIUS is able to handle all of them.”
This is facilitated in part by the Stäubli robots’ CS9 controller, which stores the format-specific movement paths and points. In the case of SIRIUS, the CS9 is connected to a Siemens PLC over a PROFINET (Process Field Network). The direct programming of the robot’s movements is carried out using VAL3 software; the robot control system receives current product-specific data from the PLC. The operating status is displayed clearly for the operator.
Company Standards for Robotics
The choice of robot was easy for the HOF designers here, and indeed in all of their other projects. Because robots are integrated
36 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Application Note
Winter 2022 Volume 14 Issue 4
Application Note
into many of the systems they supply, the company has defined standards for robotic applications and, with a view to the requirements of the pharmaceutical industry, has opted for the Stericlean models from Stäubli.





HOF can use these robots in any pharmaceutical application, no matter how challenging, because they have been specially developed to operate in GMP (Good Manufacturing Practices) Class A aseptic environments, where they have proven their worth time and time
again. All the designers have to do is specify the appropriate model plus any optional features.
With its clear advantages, ultra-compact form and modular configuration, the SIRIUS system sets a new standard in fast and reliable loading and unloading of freeze dryers with vials under cleanroom conditions. Even before making its debut at Achema 2022, the prototype had completed extensive tests under practical conditions. The feedback from potential customers was excellent. SIRIUS surely has a great future ahead of it.
For more information, please contact:
Sonja Koban Marketing Manager & Division Business MarCom Manager
STÄUBLI TEC-SYSTEMS GMBH ROBOTICS
95448 Bayreuth / DE
Phone: +49 (0)921 883 3212
Fax: +49 (0)921 883 3444
Email: s.koban@staubli.com
Stäubli
Stäubli is a global provider of mechatronics solutions with four dedicated activities: Electrical Connectors, Fluid Connectors, Robotics and Textile. We are an international group operating in 29 countries and represented in 50 countries on 4 continents. Our global workforce of over 5,700 employees is committed to maintaining a collaborative relationship with customers in nearly every industry to provide comprehensive solutions and long-term support. Originally founded in 1892 as a small workshop in Horgen/Zurich, CH, Stäubli is today an international group with headquarters in Pfäffikon, Switzerland.
staubli.com/de/en/corp.html
Stäubli Robotics
Stäubli Robotics is a leading global player in robotics, consistently delivering engineering as effective and reliable as our service and support. A complete solutions provider for digitally networked production, Stäubli offers a broad range of 4- and 6-axis robots including robotic arms designed specifically for sensitive environments, autonomous mobile robots, driver-less transport systems (AGVs) and cobots for human-robot collaboration.
staubli.com/de/en/robotics.html
linkedin.com/company/staubli-robotics
INTERNATIONAL PHARMACEUTICAL INDUSTRY 37 www.international-pharma.com
Pharmaceutical Processing Equipment Moves Are Made Easier by Capturing the Power of Air


A proven option to optimize production efficiency is now working its way through the pharma and biotech world: air casters. This technology, similar to air hockey pucks, uses compressed air to ‘float’ tanks, casks, and columns on cleanroom floors with potentially no damage to the floor, equipment, or the cleanliness of the environment. This move system can contribute to continuous manufacturing initiatives, increase the potential for achieving EMA-approved clean room compliance, and facilitate ergonomic goals to meet the needs of employee safety. A case study regarding chromatography columns pinpoints key reasons to consider air casters when moving loads up to 13–18 metric tons.
The goal in pharmaceutical production is to consistently reduce human error, enable more flexible product tracking and tracing, and optimise production efficiency and throughput. An integral
part of any manufacturing and testing workflow is regularly relocating equipment. Chromatography columns, for example, must be regularly cleaned and repacked. This logistical element of the of the workflow offers ample opportunity for improvement that can yield overall improved efficiency and throughput.
Unfortunately, maneuvering heavy equipment through cleanroom, production, or test lab environments is a challenge for pharmaceutical and biotech firms. Choosing the optimal material handling system relies on factors including facility characteristics, production process, and the equipment to be moved.
One innovative option that can satisfy a range of needs should float to the top of anyone’s list: hovercraft technology that can move heavy, awkward, delicate, and/or sensitive loads with ease – even in dense manufacturing environments or pristine cleanroom areas. Medical-grade manufacturing processes are made easier by capturing the power of compressed air
to ‘float’ equipment, casks, chromatography columns, tanks, tools, production and test equipment with ease and efficiency.
What Are Air Casters?
Originally developed for use in the aerospace industry, air casters are inflatable, donutshaped bags that use compressed air to create a thin film upon which multi-ton loads can float. Once the bags have inflated, excess air escapes underneath and creates lift. This film of air, no thicker than a business card, reduces the friction coefficient of the load to around one percent, so air casters require only about one-tenth the force to move as wheeled casters. A 2.26 metric ton column, for example, would need only 2 to 11 kg of force to move, something even a single operator can manage. A wheeled caster, by contrast, would require as much as 136 kg of force to move across most floor surfaces.
As a result, air casters enable operators to move even giant equipment such as chromatography columns like pucks on an air hockey table. Air caster equipped systems enable multi-ton loads to be moved easily,
38 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Application Note
Winter 2022 Volume 14 Issue 4
precisely, and safely by even just a single operator. This material handling method is inherently safe to operators because of an exceptionally low lift height and an exceptionally low chance of strain due to reduced friction.
How Air Casters Fit into Pharmaceutical Applications
They Meet Cleanroom Standards
Cleanroom facilities for pharmaceutical operations must protect against contamination that can jeopardize product quality and pose safety hazards to staff or end-users, so the material handling system in a cleanroom must itself preserve the environment’s cleanliness and purity. That need eliminates most motorised or mechanised systems that require lubricants or fuel due to potentially dangerous fumes, vapors, or other volatile organic compounds that can be released into the environment. Specifically, air casters help to eliminate:
• Off-gassing: Unlike motorised wheels, air casters do not require fuel or chemicals to operate.
• Particulates: Wheels that move or grind against the floor can release dust, paint flakes, and other particulates.
• Other issues: Medical-grade air casters, made from food-grade plastics and stainless steel, can be easily cleaned.
They Protect Floor Surfaces
Unlike wheels or rollers, air casters do not create pits, scrapes, or other damage to expensive cleanroom floors. Epoxy coatings and other special floor surface treatments in particular can be compromised by a wheeled cart carrying a multi-ton load. The floor loading at the wheel can exert more than 3000 psi at each point of contact with the floor surface, which is more than enough to ruin the coating and gouge or scrape the floor.
The ideal material handling methods to reduce floor damage are those that prevent the load from touching the floor as the load moves. Cranes are one solution; air casters are another. By floating the load above the surface, air casters exert only 30 to 60 psi of floor loading, effectively distributing weight across the entire footprint of the load and preventing damage to the floor.
They Protect the Equipment Being Moved
Pharmaceutical equipment floated on air casters is nearly vibrationless, so it protects


calibrated equipment and reduces the need for post-move recalibration.
That smooth movement can especially help with chromatography columns, which are often packed with specialised media at a precise specification to control flow velocity and ensure optimal performance. Vibration from rolling across the floor coupled with shock loads from hitting bumps or gaps can upset the carefully packed media.
The most effective way to isolate the column from vibration or shock loads is to separate the load from the floor surface.
Air casters effectively function as a shock absorber, shielding the equipment against vibration while moving. When the column arrives at its destination, the air casters slowly and gently deflate until the column is once again resting on the floor.
They can Maneuver even through Challenging Environments.
Tuggers are another very common option for pharma and biotech firms to move heavy equipment, but in terms of maneuverability, they have a big limitation: they have the net effect of increasing the size of the load being moved. Because the steering mechanism
INTERNATIONAL PHARMACEUTICAL INDUSTRY 39 www.international-pharma.com Application Note
extends out from the platform on which the chromatography column or other machinery sits, they add to the total footprint and require more space to operate. In fact, they require a huge amount of open area just to make turns because their turning radius is so extensive. Since tuggers rely on human power, they are also highly vulnerable to problems related to momentum and inertia.
Air casters fit entirely underneath the object being moved, so they reduce the total footprint to the minimum necessary size. They can even be stored under the component when not in use. That way, they can free up more space in a clean room and make it possible to maximise utilisation and place as much productive equipment into the space as is possible.
Since they are floating, they are also very easy to slow or stop as needed. Unlike wheel caster systems and rollers, no additional force is required when you change direction during an air caster move.
The Impact of Air Casters on Pharma Operations
Consider chromatography columns. These are large pieces of equipment, weighing upwards of 18 metric tons, that can be laborious and inefficient to move by traditional methods. Unfortunately, their operation means they must be moved regularly in order to be cleaned and repacked with material. Inefficiency multiplied by frequent moves means productivity losses can stack quickly.
With air casters, these moves can be simplified. For example, one pharmaceutical company uses air casters to move chromatography columns through various workstations to refine and concentrate the proteins used to produce insulin. The columns weigh around 14 metric tons. They use a lowprofile stainless-steel transport with an airpowered drive system to move the columns as follows:
1. Operators slide the transporter under the column, inflate the air bags, and then navigate the machine through the corridors of the cleanroom to its interim destination.

2. There, the air caster bags are deflated, and the column gently settles into its new position.
3. Once it is ready to be returned to the production line, the air casters are reinflated, and the operators simply push the machine into position. The omnidirectional movement inherent to air casters makes it possible to position the machine with extreme precision.
4. Finally, the air bags are deflated again, and the air caster system can be tucked out of the way until it is needed again.
In this case, the actual machine move requires only a few minutes of time and never more than a single operator with a spotter or two.
This kind of move speed and simplicity has several implications for pharmaceutical operations:
1: Facilitating Continuous Production
Continuous manufacturing is key to maximising throughput and has become a popular approach in the pharma/biotech world for producing drugs and other bioproducts faster and at lower cost. Janssen Therapeutics, a pharmaceutical company of Johnson & Johnson, reduced production time for its HIV medicine Prezista (darunavir) from 2 weeks to 3 days after it switched to continuous manufacturing.1
2: Increasing Productive Space
The sheer maneuverability and compactness of air caster-based solutions can even enable more efficient production facilities and layouts. For example, an engineering firm tasked with designing a new pharmaceutical production facility realised air casters required far less space than wheeled solutions. They were able to reduce the width of hallways through the environment by around six inches, which translated into millions of pounds in construction cost savings.
3: Increasing Throughput
Most material handling systems can be surprisingly slow. Cranes, for example, require operators to painstakingly attach the load, wait for a certified operator to become available, carefully make the move, and gently unload the equipment. A wheeled vehicle may need to move even more slowly to avoid risk to the equipment, the facility, or other personnel in the area. Air casters require minimal setup and can be pushed with little fear of tipping, tripping, or collisions. They can move in minutes what might take a crane and other systems half an hour. That can speed up production processes enough to increase overall throughput.
4: Enhancing Flexibility
Because air casters don’t require any kind of permanent installation, they also improve the overall flexibility of the facility. If an operation based on cranes or conveyors wanted to reconfigure the production layout, they wouldn’t be able to. Facilities using air casters can reconfigure their flow as often as they like. That way, the operation can continue to use air casters no matter how their production processes change or evolve over time.
How to Pick the Right Air Caster for Your Application
Air caster systems are often customized and tailored to fit specific needs, but pharmaceutical manufacturers generally utilise one of two broad styles of air casters for use with chromatography equipment, with
40 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Application Note Winter 2022 Volume 14 Issue 4
a small number of alternative solutions also available.
1: The Air Caster Transporter
The Air Caster Transporter is ideal for chromatography equipment weighing only a few tons. Shaped like a pallet jack with either a solid flat surface or two forks to accommodate the structure of the machine, the transporter slides under the column and fits almost entirely within the footprint; it does not extend out to the sides at all. Since air casters can move omnidirectionally and rotate 360 degrees in place, the operator uses a handle to maneuver the machine including the ability to back up and navigate even 90 degree turns with ease.




2: The Air Caster Drive System
The Air Caster Drive System is for heavier chromatography columns up to 13.6 metric tons that require more force than one or two operators can safely provide. In this situation, an air caster system with a built-in throttle and drive enables operators to propel the column from point to point on air, without requiring muscle power alone to actually move the equipment. Integrated controls

help the operator maintain control over move direction and speed at all times, improving maneuverability.
Operationally, the drive system functions much like the human-powered transporter: it slides underneath the column and lifts it up. Even with the drive system incorporated, the air caster is not much bigger than the column itself and requires less overall space when compared to other solutions, like tuggers.
3: The Embedded Air Caster System
Alternatively, it’s also possible to integrate air casters directly into your pharmaceutical equipment for the capability to adapt to everchanging manufacturing configurations. The embedded systems serve as permanent rigging for on-demand capability to reposition machinery. The system includes air bearing modules and an integrated control console for adjustable air control for offset loads. Design engineers are offered immediate access to online models at aerogo.com/ products/embedded systems.
Ultimately, too many pharmaceutical and biotech firms get stuck on a particular move
system because it’s what they already have or what they are already familiar with, not because it is the best option. If the overall goal is to maximise floor space, increase efficiency and enable a safe move for the operator and the equipment, air casters are not just a better way to move machines from one place to another. They have proven themselves essential to operating an optimally cost-effective, continuous manufacturing process in chromatography and pharmaceutical facilities.
For more information and detail, download and read “Buying Guide for Pharma Handling Equipment,” a white paper that details considerations specific to the pharmaceutical, biotech, and medical industries.
AeroGo, Inc. is a proven world leader in the engineering, design, manufacturing, and support of material transport systems. Pharmaceutical, medical and biotech industries around the globe depend on AeroGo movement systems. AeroGo products comply with ISO 9001 quality standards. AeroGo standard products are CE compliant. AeroGo Aero-Casters meet ASME B30.1 requirements. AeroGo offers a full complement of support services throughout the world. To discuss your specific application, contact a product application engineer at 1-800-426-4757 or email info@aerogo.com.
Live chat is available along with other case studies online at www.aerogo.com/ applications/pharmaceutical-biotech/
REFERENCES
1. https://www.biopharmadive.com/news/ pharmas-slow-embrace-of-continuousmanufacturing/532811/
AeroGo
AeroGo manufactures innovative load moving equipment, utilizing hovercraft technology, to move heavy, awkward, delicate or sensitive loads in manufacturing. Medical-grade manufacturing processes are made easier by capturing the power of compressed air to 'float' chromatography columns, tanks, tools, test equipment and columns with ease and efficiency. Companies large and small benefit from a worldwide dealer network, experienced product specialists, and skilled engineers. We work with you to find a load moving solution that is safe, efficient, and costeffective.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 41 www.international-pharma.com
Application Note
Local Lung Delivery for Small and Large Molecules Via Dry Powder Inhaler
Inhalation is a familiar route of delivery for many drugs designed to treat asthma and related conditions. Asthmatics routinely carry some form of metered dose inhaler around with them to relieve the symptoms of attacks, and many take preventative medicines such as steroids via inhalers, too. Nebulisers are also used to deliver asthma medications directly to the lungs, often for those who find using a metered dose inhaler difficult (such as children and the elderly).
The growing incidence of chronic obstructive pulmonary disorders (COPD) has led to a rise in drug treatments for respiratory conditions being delivered by inhalation, but other lung diseases also benefit from direct delivery to the lungs. Mannitol, for example, can be given in a dry powder inhaler (Chiesi’s Bronchitol) to help cystic fibrosis patients clear mucus from their lungs. And for pulmonary arterial hypertension iloprost (Ventavis, Janssen) can be delivered directly to the lungs with using a mesh nebuliser. This greatly aids its efficacy, as the drug only has a half-life of about half an hour; multiple doses are required every day, and newer nebuliser types have since helped reduce the treatment burden.
Delivering treatments for lung diseases by inhalation is an obvious choice, as there are significant advantages when diseased tissue or an infection is within the lung itself. Pulmonary administration also circumvents first pass metabolism in the liver. On account of the high surface area of the lung, the onset of action can be faster, as is clearly the case for beta-agonists such as salbutamol/ albuterol when treating acute exacerbations of asthma.
However, small molecule APIs such as these are not the only drugs used to treat lung disease. Monoclonal antibodies (mAbs) and other proteins, peptides and oligonucleotides have all been investigated as both systemic and local treatments for lung diseases. For example, at least five FDA-approved mAb treatments are available for asthma today, all of which are administered by injection.
In 2022, a mAb was approved in the EU for the prophylactic prevention of respiratory syncytial virus (RSV) in children. And during the Covid-19 pandemic, mAb treatments were approved in the EU and the US for severe cases of the disease. Some of these combined more than one agent in the same treatment.
As with small molecule APIs, there can be significant advantages in delivering biotherapeutics locally to the lung, including tissue-specific targeting, which can improve side-effect profiles. There are significant manufacturing challenges involved in formulating sensitive biotherapeutics as dry powder for inclusion in an inhaler device, and most investigations into inhaled delivery of biologics rely on devices such as mesh nebulisers. Yet DPIs are much preferred by patients and caregivers because of their ease of use, portability, and shelf stability.
Spray-dried Biologics
However, while there remain challenges, it is possible to formulate antibodies and other biotherapeutics as stable dry powders via a carefully designed spray drying process. In spray drying, the API and the excipients are dissolved together in a volatile solvent; this will be water or a buffer for most biotherapeutics. The solution is passed through an atomiser which breaks it up into small droplets, and then sprayed into a drying chamber.
As the droplets encounter a heated drying gas within the chamber, they dry rapidly and form solid, engineered particles whose size distribution is tightly controlled. For direct delivery to the lung, the particles will be engineered to have aerodynamic diameters of about 1–5µm, which is the most appropriate size for pulmonary delivery.
Biotherapeutic molecules need to be handled carefully, as many are sensitive to thermal, shear and dehydration stresses. Instinctively, this makes spray drying appear to be a risky process that might damage or destroy the molecules. Steps are taken in both the process and the formulation to ameliorate these risks. Protective excipients, including sugars such as trehalose, can help to stabilise the delicate molecules as they dry. Furthermore, the evaporative cooling
that occurs in the liquid droplets reduces the exposure of the material to high temperatures during the drying process. Several recent review articles outline progress in pulmonary delivery of spray dried biotherapeutics; the references are listed below.

Cancer Treatment Via Inhalation
A recent case study using the anticancer agent bevacizumab highlights the potential of spray drying a mAb to facilitate the local treatment of lung cancer. The vascular endothelial growth factor (VEGF) inhibitor is approved for the treatment of various forms of cancer, including non-small cell lung cancer (NSCLC), and works by inhibiting angiogenesis, reducing the ability of the tumour to create new blood vessels.
In NSCLC, it is given by intravenous infusion in combination with chemotherapy in threeweek cycles. However, the risk of unexpected bleeding prevents many of the patients who might benefit being treated with the antibody. If it were delivered locally to the lungs rather than dosed systemically, the exposure of healthy tissue would be much lower, likely cutting the chances of these adverse events. Likely, it would also improve patient compliance, as it could be administered in a home setting. In addition, the need for cold chain storage would also likely be eliminated.
When embarking on the development of a formulation for a DPI, it was important that the biological activity of the antibody was preserved; this could be assessed using a cell-based assay. The powder needed to have the correct aerosol properties for delivery to the deep lung. It also, ideally, needed to have good physical stability at 25°C.
The answer lay in the careful selection of excipients. The non-reducing sugar trehalose, commonly used to stabilise proteins in both liquid and solid formulations, was one component. The other, the amino acid L-leucine, is commonly used in formulations for inhalation to improve powder dispersibility. Initial studies indicated that a spray-dried powder that was 40% bevacizumab by weight, 40% trehalose and 20% leucine, the highest loading evaluated, met all the requirements for key performance attributes.
42 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Packaging
CONTRACT MANUFACTURING AND PRIVATE LABEL PRODUCTS
CONTRACT MANUFACTURING OF LIQUID DRUGS AND MEDICAL DEVICES

•BAG-ON-VALVE
•NASAL SPRAY PUMPS
•DERMAL SPRAY PUMPS
•AIRLESS SPRAY PACKAGING

•PureHale®
CE-MARKED PRIVATE LABEL PRODUCTS FOR YOUR BRAND

•SPARKLING SALINE NASAL SPRAY



•SALINE/SEAWATER NASAL SPRAY
•EAR CLEANSING SPRAY
•WOUND WASH SPRAY
•EYE WASH SPRAY
•EMOLLIENT SPRAY
•ADHESIVE REMOVER SPRAY
•BURN GEL
INTERNATIONAL PHARMACEUTICAL INDUSTRY 43 www.international-pharma.com
• contact@aurenalabs.com
www.aurenalabs.com
LET’S COLLABORATE TO CREATE YOUR NEXT PRODUCT. CONTACT AURENA TODAY.
Fortunately, the activity of the spray dried powder against VEGF remained comparable to the as-received antibody. Furthermore, there were no traces of fragmentation or aggregation of the protein in size exclusion chromatography and dynamic light scattering experiments. And, importantly, a solution of the powder in phosphate buffer remained optically transparent.
The powder’s aerosol properties were also tested. With a fine particle fraction of 81%, and a mass median aerodynamic diameter of 2.2µm, the particles fell well within the required specifications for delivery to the deep lung. An ICH stability study where it was packaged with desiccant and stored for a year at both 5°C and 25°C showed that the only noticeable change was a small increase in the powder’s water content. All its other aerosol and physical properties remained the same at both temperatures, as did its bioactivity.
The next step was in vivo testing in a rat model of NSCLC, carried out in conjunction with Lovelace Biomedical. Rats were given weekly treatments, and the lung weight at 12 weeks was determined as a measure of the tumour burden. Three different drug regimens were included in the study: injected bevacizumab plus cisplatin (standard of
care), inhaled bevacizumab both alone and in conjunction with injected cisplatin, plus an untreated group acting as a negative control. The inhaled dose was a tenth of the size of the injected dose.
All three different regimens gave a significant reduction in tumour burden when compared to the untreated group. Importantly, the inhaled mAb in conjunction with cisplatin was as good at reducing the tumour burden as the regimen where both were injected, despite the 10-fold reduction in antibody dose. Even when administered alone, the inhaled bevacizumab reduced the tumour burden, albeit by a smaller amount than when it was given with cisplatin. This combination of efficacy and stability indicate that the formulation may have huge potential in treating patients with NSCLC in a less burdensome way.
Combination Inhaled Treatments
It would be even less burdensome if the other elements of a complex NSCLC treatment regimen could also be delivered via inhalation. Chemotherapy, other receptortargeting drugs, and immunotherapies are all commonly administered alongside bevacizumab. Might it be possible to include more than one drug in the same inhaler?
We therefore looked at whether it would be possible to create an inhaled formulation that comprised bevacizumab plus one of the chemotherapies cisplatin and paclitaxel, or the EGFR inhibitor erlotinib. Unsurprisingly, this posed a significant manufacturing challenge –small molecule drugs would commonly simply be micronised to create the powders, but this is not appropriate for a protein drug. Again, the answer lay in spray drying.
This posed a real challenge. Bevacizumab must remain in an aqueous solution to preserve its activity, but the solubility of both paclitaxel and erlotinib in water is poor. Cisplatin is a little more soluble in water, but its chemical stability in aqueous solution is poor. Add in the excipients, and it was simply impossible to imagine an appropriate solvent that is suitable for all the components.
Instead, we turned to simultaneous spray drying. In a simul-spray process, two separate solutions are spray dried into dry powders in the same spray dryer at the same time via separate atomisers. The two different powders form an intimate mixture in the collection container, ready for further testing and formulation. A scanning electron microscope image of the mixture can be seen in Figure 1.

44 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Packaging
Figure 1: Scanning electron microscope image of simul-spray cisplatin and bevacizumab powders. Bevacizumab particles have a smooth surface with a collapsed sphere shape, and cisplatin particles have a rough surface and nearly spherical shape.
The first step of testing involved making individual dry powder formulations of each of the drugs. That for bevacizumab was the same as had been used in the single agent powder study. Paclitaxel and erlotinib included leucine to improve dispersibility, and as well as leucine, trehalose was included in the cisplatin powder to improve its stability.
Combination formulations of bevacizumab plus each of the three other drugs were then

created, in several different ratios. Scanning electron microscope images of both the single API formulations and the simulspray combinations showed that they were uniformly blended powders, with the two different types of particles distinguishable by eye. The drug concentration in the powders was also assessed.
Activity could not be assessed in a cellbased assay because of the cytotoxic nature
Packaging
of both paclitaxel and cisplatin. Instead, the formulations were reconstituted, incubated with VEGF, and then the amount of unbound VEGF measured via ELISA. It remained similar to non-spray-dried bevacizumab in all cases. The aerosol properties were then also checked, with the particle size distributions of the two types of particle being similar in each different combination. Median aerodynamic diameters fell in the range 1.8–2.9µm, again suitable for inhaled delivery to the deep lung.
The Outlook
Elsewhere, there are other positive signs. The commercial approvals of two inhaled insulin formulations, in 2006 (Exubera) and 2014 (Afrezza), paved the way for future work. Two antibody-based drugs formulated as DPIs have already completed Phase 1 clinical trials, both in asthma: abrezekimab, an antiIL13 antibody fragment from UCB/Vectura, and ecleralimab, an anti-thymic stromal lymphopoietin (TSLP) antigen binding antibody fragment from Novartis. Both included trehalose as a stabilising excipient. Phase 2 studies in both uncontrolled asthma and COPD have also begun for ecleralimab.
It remains early days for the inhaled delivery of monoclonal antibodies via dry powder inhaler as none have yet reached the market. But the signs are looking promising. In the coming years, patients may well be able to benefit from the local delivery of biologic drugs for lung diseases via a convenient dry powder inhaler device.

Kimberly Shepard
Kim Shepard is an Associate Principal Engineer in the Research group at Lonza's site in Bend, Oregon, USA, where she has worked since 2015. She leads projects focused on developing new technologies for bioavailability enhancement and pulmonary delivery. Kim's areas of expertise include the formulation and manufacturing of spray-dried dispersions for inhalation and oral delivery, as well as the physics of polymers and amorphous materials. Kim received her Bachelor and and Master's degrees in Chemical Engineering from Stevens Institute of Technology and her Ph.D. in Chemical Engineering from Princeton University.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 45 www.international-pharma.com
Just What is a Manufacturing Ecosystem and Why Are They Growing? The Benefits and Challenges of Collaborative Manufacturing Partnerships Across Regulated Industries
Deloitte shows that a massive 88% of manufacturers agree it is important to work with outside partners and vendors to reach their smart manufacturing and digital goals. With supply chains becoming ever more complex, it’s clear to see that collaboration is at a defining moment across the manufacturing industry.
Bob Tilling, VP of Global Sales at Kallik, explains the value and challenges of developing a shared ecosystem particularly across highly regulated markets such as pharmaceutical, food and beverage and consumer packaged goods (CPG), and how something so simple as accurate labelling plays a critical role in collaborative success.
A recent analysis of Fortune 500 manufacturers identified that companies with more than 15 strategic alliances as part of a manufacturing ecosystem registered twice the revenue growth, compared with companies with fewer than 15 alliances.
Deloitte sees an ecosystems approach as forming a core part of ‘smart manufacturing’ into the future. A manufacturing ecosystem can be simply defined as “different entities coming together in meaningful ways to solve shared challenges and meet shared objectives.”
Ecosystems and Partnerships Growing Across Pharma, Food and CPG

The manufacturing supply chain is already a complex network of companies and stakeholders, especially in highly regulated sectors such as pharmaceutical, food and beverage and consumer packaged goods. Any manufacturing process and supply chain becomes more complex and intricate when more than one manufacturing party is involved. For example, the Covid-19 vaccine needs to be supported by one of the most sophisticated supply chain ecosystems in the industry – due to there being over 40 Pfizerowned sites and over 200 suppliers globally.
On the food and beverage side, take an international food and beverage organisation
specialising in making and selling a variety of cheeses. The company, as part of its growth plans, has very probably acquired other wellknown organisations owning many brands within the same sector. Consequently, the organisation will have grown in size and complexity, with potentially thousands of suppliers globally.
Managing any such complex manufacturing ecosystem requires a structured system to enable accurate updates and changes to vital processes, and here’s where labelling has a crucial role to play. Ingredient formulations and label artwork must be shared with the right people in the ecosystem at the right time
in a seamless and timely manner in order for the ecosystem to live up to its role of a collaborative partnership of organisations with shared goals.
Regulation and Compliance Add Yet Another Layer of Complexity
The already heightened complexity of manufacturing ecosystems is further exacerbated by regulation. Food and beverage for example, is now tightly controlled by regulations similar to those applied to pharmaceuticals. Regulatory bodies such as the FDA now require food products to be tracked throughout the manufacturing and distribution processes. Adding more players as part of an ecosystem
46 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Packaging
increases the complexity of building regulations into every stage of the manufacturing process for a particular recipe or formulation.
Managing Label and Artwork Information Across a Vast Ecosystem
Controlled real time access and centralised data are essential ingredients of any efficient and safe manufacturing environment. This is never more critical than when managing the accuracy of the information printed on product artwork, labelling and packaging. Keeping this information organised and accurate across the ecosystem of manufacturers and partners is vital.
There are four key areas of a manufacturing ecosystem where key parties will require varying levels of access to critical label and artwork information in order to ensure products continue to be manufactured quickly, safely and compliantly:

1. Laboratory and Key Ingredient Information
The need to share information such as ingredients and recipes while retaining information secrecy is vital in the manufacturing ecosystem. The challenge faced by many pharmaceutical and food and beverage processors is the lack of specific technology that tailors to their operational and industry specific needs such as ingredient confidentiality.
Throughout the labelling and artwork process, a sophisticated Label and Artwork Management (LAM) system is needed in order to ensure that the most up to date and accurate ingredient information is shared to only the necessary individuals in order to create labels and artwork that is accurate.
2. Translation Agencies
Translation agencies are integral to the manufacturing ecosystem, they ensure that the necessary product information can be shared to an international audience across often hundreds of product lines. These agencies need access to LAM software where they can upload information and translations but would only ever be able to see information intended to be translated such as warning phrases. LAM solutions ensure a translation agency see only the information and suggestions they are required to change, and not for example have access to other recipe formulations.
3. Packaging Designers
The use of different materials such as plastic and cardboard during the packaging process
can be incredibly complex – however, using a single LAM solution can aid the organisations in this partnership to complete design and packaging processes or reflect any changes efficiently. The LAM solution ensures that if each manufacturer is sent the correct information that they require – no information exchange between different designers and packagers in the chain would be necessary.
4. Sub-manufacturers
Ensuring that each sub-manufacturer involved in the end-to-end process in any industry is kept up to date with accurate information and artwork is essential to a smooth-running ecosystem. A LAM solution allows the artwork supplier to update artwork and information at any time and will pass this downstream, to provide only the intended recipients with the most up-to-date version.
Any changes made to any artwork or labelling information will be recorded in a log, as required by EU FDA guidelines that require electronic signatures.
Software Providing Visibility and Traceability
LAM solutions keep a single history of the complete lifecycle of the labelling and artwork manufacturing process from translations and packaging design to label and ingredient changes of the product. Barcodes featured on the packaging can then provide further traceability once it’s placed in a consumerfacing position.
LAM solutions provide the manufacturer with complete control of how their critical product information is shared and hidden from different individuals in different processes. From laboratory technicians being able to view the entire recipe formulation to translation agencies only seeing safety guidelines or packaging manufacturers viewing the label artwork – manufacturers can customise specific windows of information.
LAM Solutions Play a Vital Part in Seamless Manufacturing Ecosystems

The key element though, is all this information is housed in one solution, managed by the parent or lead manufacturing organisation. Through various rules and access controls, each player in the manufacturing ecosystem can update packaging, label and artwork information individually – but all changes are visible and auditable down to the last detail.
The growth and development of manufacturing ecosystems is inevitable in the current manufacturing landscape. It is also desirable to enable businesses to meet their sustainability and smart manufacturing goals. At the heart of these ecosystems is collaboration – and that means sharing. LAM solutions have an essential and central role to reduce the potential for errors in accuracy and version control throughout the end-to-end manufacturing process tenfold – while ensuring confidentiality and security throughout the entire manufacturing ecosystem.
Bob Tilling is the VP of Global Sales at Kallik, an enterprise labelling and artwork management company. He has a wealth of knowledge when it comes to the life sciences industry, particularly regarding medical devices. Bob helps businesses in highly regulated industries begin their journey of transforming their labelling and artwork management.
Email: bob.tilling@kallik.com
Linkedin: ww.linkedin.com/in/bob-tilling
INTERNATIONAL PHARMACEUTICAL INDUSTRY 47 www.international-pharma.com
Bob Tilling
Packaging
Connected Drug Delivery Device Development: Where Are We At?
The market for parenteral drug device combination products has evolved rapidly over the last 15–20 years, and is expected to grow at a CAGR of 24.3% in the next eight years.1 One area driving this growth is connected devices, with their ability to collect a host of data relating to drug administration. These devices aim to deliver enhanced healthcare outcomes – by enabling remote patient monitoring and promoting treatment adherence – however there remain room for improvement in several areas to ensure the data captured is being exploited to its fullest.
Drawing from Owen Mumford Pharmaceutical Services’ own experience, as well as conversations with industry experts and counterparts, this article lays out the opportunities and challenges currently facing the connected drug delivery device market.

Flexibility: A Key Concept
The unprecedented pace of change in connected device technology means that the landscape is constantly shifting. What was recently cutting edge is replaced by newer technologies, and opinions on the different configurations and functionality available change as devices and market requirements evolve.
In addition, markets vary significantly across the globe with regard to regulatory approval paths, and the same can be said for patient and healthcare providers’ preferences. As a result, optimal devices are those that offer greater flexibility, allowing pharmaceutical companies a wider choice of potential markets and segments. In many cases this may mean selecting platform devices that work with or without added connectivity, as well as making the choice between reusable or disposable, single-use devices depending on the therapy area, target market and patient needs.
Data for Device Improvement
The data collected by connected devices could be key to their further development and improvement, creating a feedback loop
that leads to enhanced device usability and functionality. Real world usability data – for instance the angle a patient is holding the device or the pressure applied during the injection – could be gathered during clinical trials and would be a valuable supplement to the data gathered in human factors studies. Post-launch, this data could be helpful for resolving complaints and issues, enabling root cause analysis to determine which part of the system requires corrective action. This information could also be relayed to device manufacturers so that they can remedy the issues and make improvement to the device design.
A Rapidly Evolving Industry
The COVID-19 pandemic changed the face of healthcare as we know it, accelerating its digitisation and the expansion of telemedicine.2 Both patients and healthcare professionals are increasingly open to the possibilities of remote health monitoring; seizing the benefits offered by connected devices is therefore crucial.
The changing face of drug delivery, with larger volumes, longer intervals between injections and the increasingly important role of self-administration, presents a significant opportunity for connected devices as the device itself has the capability to provide training information and support to the patient. Step-by-step guidance throughout the injection process and sensory feedback to ensure devices are being handled and
used correctly – such as visual feedback to help confirm dose delivery or rotate injection sites – help patients manage their condition and enhance the user experience, which in turn may increase treatment adherence. In an environment increasingly focused on managing costs, data on treatment adherence can be used to prove the value of the product to payors, thus driving reimbursement.
The Role of Apps and Smart Devices
The role played by smartphone apps within the connected device space is also changing. Until now, they have been key to the success of connected devices, as they provide the means for data upload and allow patients to track and manage their own therapy. However, with the emergence of 5G, connected devices will be able to send data directly to the cloud, removing the need for secondary applications or devices. This real-time data transfer will result in an improved user experience and may increase the appeal to older patient groups who may be less familiar with digital technology; at the same time, it may offer a commercial and competitive advantage to pharmaceutical companies. In the future, stand-alone digital health apps are also likely to gain traction, particularly in markets like Germany where digital apps (DiGAs) have been eligible for prescription via the German statutory health insurance system since 2020. Europe-wide frameworks are anticipated, with the establishment of a European Taskforce for Harmonised Evaluation of Digital Medical Devices in 2022.3
48 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Packaging
Additionally, the growth of smart consumer tech devices and the wealth of physiological and broader lifestyle data they capture – for instance, around sleep and exercise patterns – present a potential opportunity to integrate this data. This in turn would allow healthcare professionals to build a more holistic view of the patient, tailoring the therapy directly to the patient and their environment. For example, a patient with asthma could receive a notification to increase their daily dose of inhaled corticosteroid in locations with high air pollution. Patients are also more aware and more comfortable using apps and tracking their behaviour, with clear benefits for adherence and monitoring, although this comes hand in hand with higher expectations in terms of the user experience.

Data Security, Interoperability and Integration

Data security for connected devices and associated apps is also a critical consideration, although this is now a well-known and well documented field, with clear regulations and guidance in place. Big Cloud providers are very familiar with and aware of data requirements and the standards for data privacy and security are very high in this field. The topic will continue to be a key area of focus and importance, particularly as users become more aware of their rights as data subjects and regulation continues to tighten.
Currently, despite the potential of connected devices, the data they capture is siloed and not combined with other data sources, such as electronic patient health records. Lack of interoperability is a key barrier along with lack of data integration within the healthcare setting. There are several reasons for this, including regional differences in healthcare systems and data privacy laws, and complex regulatory pathways. New standards are needed to streamline communication protocols and facilitate data capture and exploitation at a regional or global level.
While this is slowly changing as progress is made with regard to interoperability, there is still huge potential for connected device data to be better integrated in healthcare systems. Tackling this challenge is likely to be a key focus in the future as connected devices become more commonplace and healthcare providers place more focus on remote patient monitoring and management of population health.
Conclusion
There is likely to be significant further innovation within the connected device
space, fuelled by advances in technology and external factors such as sustainability requirements and regulatory change. Where this innovation is grounded in market need, connected devices can offer a competitive and commercial advantage for pharma companies, allowing them to address key issues relating to therapy management, or enhance the patient experience in a meaningful way.
Despite rapid progress in the field of connected drug delivery device solutions, the complexity of the field means that some challenges remain. Cooperation between pharmaceutical companies, device manufacturers and regulatory bodies will be essential if the immense potential of this emerging area of healthcare is to be unlocked.
REFERENCES
1. Allied Market Control. (2022). Allied Market Research, Connected Drug Delivery Devices Market by Type (Injectable, Inhalation Devices), by Technology (Bluetooth, NFC, Others), by Enduse (Homecare, Hospitals): Global Opportunity Analysis and Industry Forecast , 2021–2030). https://www.alliedmarketresearch.com/ connected-drug-delivery-devices-marketA26017
2. About Digital Health. (2022). Digital Health Trends 2023. https://aboutdigitalhealth.com/
2022/11/24/digital-health-trends-2023/
3. Haute Autorité De Santé. (2022). Towards a European evaluation framework for digital medical devices (DMDs) in the European Union - Launch of a European taskforce. https://www. has-sante.fr/jcms/p_3382241/en/towards-aeuropean-evaluation-framework-for-digitalmedical-devices-dmds-in-the-europeanunion-launch-of-a-european-taskforce
Michael Earl joined Owen Mumford as Director of Pharmaceutical Services in November 2020. He was previously the Commercial VP at Bespak, leading the commercial team there to drive growth in their substantial medical devices business. Prior to that, he worked for a number of pharma, biotech and device companies. In a career spanning more than 35 years, he has been responsible for all aspects and stages of drug and device development and commercialisation. Michael has also completed a substantial number of commercial, licensing and M&A transactions.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 49 www.international-pharma.com
Michael Earl
Packaging
Reducing Medical Device Packaging Waste and Improving Information Provision Through E-labelling
E-labelling offers patients and healthcare professionals the convenience of always having up-to-date information on their products, such as the latest safety updates. It also provides regulators with greater oversight and assurance that manufacturers are providing accurate and timely information. Dr. Jutta M. Hohenhörst, from Schlafender Hase, explores the benefits of e-labelling for the medical device sector and provides some practical tips for getting ahead with processes, procedures and solutions that deliver efficiencies and add value for patients.
In the consumer world, it’s now common practice to access product information online. For detailed instructions or information about manufacturing practices, safety advice and so on, users can scan a QR code or go to a web address. This will take them to the latest details, in an easily digestible format – often including audio and video options now, for maximum accessibility.
Although the life sciences industry has some way to go to match these ‘e-labelling’
experiences, regulators are making moves to change this through new directives and guidance.
E-labelling Benefits
E-labelling makes externally-facing product information more dynamic and immediately accessible online or through an app. That could be as a downloadable document, as shorter-form searchable text, or in alternative formats such as audioor video-based instructions. The idea is to make important information easier for users (patients, caregivers or healthcare providers) to access and digest – while also ensuring that the guidance is as up to date as possible.
Paper inserts (instructions for use –IFUs; or patient information leaflets – PILs) present a number of practical challenges that can be readily solved in the digital age. These include the risk of costly printed instructions being misplaced or damaged; poor accessibility due to very small print because the leaflets have to try to cover all bases; safety information being superseded; potential regulatory Requirements: Section 508 (https://www.section508.gov/create/ pdfs/) and European Accessibility Act.1
Regulatory Change Focused on Safety
Across many developed markets, the medical device industry is already subject to considerable regulatory change, geared to improving device traceability and patient safety. These measures have been driven by high-profile safety events, such as the PIP breast implant scandal in Europe; and by advances in technology which have seen a growth in both smart implanted and wearable devices, and in combination products (devices and pharmaceutical products that work in concert).
EMA’s Medical Device Regulation and its in-vitro equivalent, IVDR, are among the updated sets of requirements designed to provide fit-for-purpose safety controls.
And specific guidance is being added all the time, around e-labelling. In December 2021, the European Commission adopted a new Implementing Regulation (EU) 2021/2226 for the use of e-IFUs for medical devices, with application from January 2022. The Regulation adapts the conditions and requirements for going ‘paper free’ for manufacturers of medical devices – including software covered by EMA MDR/IVDR.
The term ‘e-labelling’ potentially understates the scale of change that will be involved. Appreciating this, EMA is phasing in e-labelling by target user group in Europe. For now, the provision to provide IFUs in an electronic format instead of paper form is limited to certain medical devices and accessories intended for use under specific conditions.

As medical device manufacturers prepare for a digital-first future, it is vital to ensure that content is correct, controlled and compliant at source, that content is transformed accurately across print and online formats. A pragmatic approach to this is likely to involve the application of intelligent proofreading software, which can identify any anomalies swiftly and reliably.
Developing an Effective E-labelling Strategy
There are five key steps to create and execute a coordinated e-labelling strategy and plan:
50 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Packaging
1. Understand the Regulatory Landscapes & Timeframes
Consider the current and future devices in scope and their target user groups.






2. Map the Existing Labelling Process






Assess and map the existing labelling landscape, procedures and vendors to understand the company’s readiness for electronic labelling. Create a gap analysis to feed into any business case.
3. Scope the E-labelling Project
Scope the e-labelling project to align with any immediate needs, focusing on current and future target markets and the devices sold into those regions. Also consider developing foundations for the future, and start with that vision in mind: including patient interaction with online resources to boost the safe use of the device. Look to more established markets (e.g. Asian and Australia) for examples of emerging best practice and target benefits.
4. Create a High-level Project Plan
Produce a high-level project plan, in-

cluding key champions and stakeholders. This should include a crossfunctional team to mirror the scope of e-labelling impact on a manufacturer. Build training and knowledge transfer into the resource planning.
5. Build & Present an ROI/Business Case Build the business case, highlighting the benefits of the e-labelling initiative to patient safety and the business, including wider environmental sustainability gains, making sure to address the four strategic priorities of any manufacturer: financial, patient, operational, and growth.
It is important to define the scope, objectives and key performance results and monitor these on an ongoing basis. Finally, communicate and celebrate all wins, to sustain momentum.

By getting ahead now, companies can take early advantage of processes, procedures and solutions that provide a digital platform for the future, deliver efficiencies across today’s labelling landscape, and add new value for patients.
REFERENCES
1. https://ec.europa.eu/social/main.jsp? catId=1202#:~:text=The%20European%20 accessibility%20act%20is,EU%20leading%20 to%20costs%20reduction
Dr. Jutta Hohenhörst, Regional Sales Director at Schlafender Hase, has over 22 years’ experience in the pharmaceutical business. She has experience in different departments from Supply Chain Management to Strategic Marketing in F. Hoffmann-La Roche HQ, AstraZeneca and Fresenius HQ. While working for IQVIA during 2005 and 2012 she broadened her knowledge through strategic consulting projects in the pharma, medical device and hospital sector.

Email: jutta.hohenhoerst@sh-p.de


INTERNATIONAL PHARMACEUTICAL INDUSTRY
Dr. Jutta Hohenhörst
Packaging
HIGH PURITY. HIGH DEMANDS. Pressure regulators Control valves Pipeline ancillaries Special equipment Steam traps Safety valves High purity equipment for clean steam adca@valsteam.pt www.valsteam.com +351 236 959 060 Zona Ind. da Guia, Pav. 14 - Brejo 3105-467, Guia PBL PORTUGAL PRODUCTS MANUFACTURED IN PORTUGAL AV 001 AP EN 01.20
Challenges and Development Perspectives of Primary Packaging for Parenteral Drugs
When talking about parenteral drugs, we usually refer to the administration of drugs by injection through the integument or directly into the circulation.
The parenteral route – it could be intradermal, subcutaneous, intramuscular or intravenous – indeed allows a rapid effect, the administration of orally inactivated drugs, rapid intervention in emergencies, and the administration of nutritional solutions to patients who cannot feed themselves normally.
The most common parenteral drugs are injectables, therapy drugs administered by professional users in healthcare facilities, such as vaccines or IVs, and infusionals, mostly complementary solutions used as an adjuvant for such therapies.
When it comes to packaging to contain such drugs, it is composed by an integrated system featuring the primary packaging properly said, a vial or a bottle, a rubber stopper, and a basic or tear-off aluminum gear.
Vials are manufactured in glass, with different material specs and manufacturing technologies available, and different features that can better adapt the drugs characteristics and challenges.
Glass specs depends from the characteristics the material should have to better contain drugs: Type I is a borosilicate glass, featuring an enhanced mechanical resistance and a high degree of hydrolytic stability, making it ideal to contain all types of injectable products with an acid, neutral or slightly alkaline pH.
Type II is a soda-lime glass that achieves the performances required by the international pharmacopeia for parenteral applications through appropriate surface treatment, and it is particularly suitable for packaging injectable and non-injectable preparations with acidic and neutral solutions.
Finally, Type III is a low alkaline sodiumcalcium glass with good hydrolytic resistance,
particularly when it comes to sudden temperature changes. These features make it ideal for non-aqueous or powdered injectable preparations, excluding drugs that must undertake a freeze-drying process.
As said, glass used to contain parenteral drugs might come from two different manufacturing technologies: premium molded glass and tubing glass.
The first ones are obtained from an improved molding process known as pressand-blow. Glass is melted into a furnace at over 1600°C, extruded and then blowed into a high precision mold by using mechanical tools (a piston) and a jet of hot air. In just one single process, the melted glass is transformed into a parenteral vial ready to be supplied to pharmaceutical companies or CMOs.
This process is intended to the production of premium borosilicate glass, the golden standard for parenteral and injectable pharmaceutical application.
On the other hand, tubing glass containers require a two-step process. They are produced by transforming a glass pipe into a vial by means of localised flames and through a number of steps to give the desired shape.
Given these different possibilities, the pharma manufacturer might choose the better option for any application, considering multiple elements such as:
• The heat used when forming the vials directly affects the level of extractables at the surface due to the vaporisation of the more volatile elements of glass and to the condensation of vapors on the inner and colder surface, which produces “rough spots”, chemically different from the rest of the surface
• Premium molded vials are mechanically and chemically superior, while tubing vials perform better in terms of the aesthetic quality of the surface. Differences in other tested aspects are negligible.
• The bigger the format in which the drug must be contained, the more molded glass vials are to be considered
as an option due to its mechanical performances; the smaller, the more tubing glass vials are to be preferred
Primary packaging for parenteral use also has extensive variation when it comes to format sizes, with the injectables championing in smaller formats and infusionals that require the bigger ones. More in detail, size spans from 2 to 1,000 milliliters.
The bigger formats could also be marketed with level marks print, and with a special design allowing a perfect fit with the infusion bottle hangers used in the hospitals.
Additional services could be provided to parenteral primary packaging upon client request, such as the ready-to-use and the ready-to-sterilise options that could be chosen in order to let the pharmaceutical manufacturer concentrate on its own core activities, or additional controls to address every customer need, up to the visual inspection on 100% of production and customised post-production second quality check.
Moreover, in-depth, data-based information could be provided to streamline the customer validation procedures, as well as additional, certified analysis about extractables testing and helping companies to address different regulatory frameworks.
The packaging integrated system is completed by a rubber stopper, manufactured with highest quality rubber able to minimise interaction with the drug, and by aluminum classic or tear-off gears.
While classic aluminum gears mostly rely on a mechanism similar to the ones used in soft drink cans, tear-off gears are composed of a pre-drilled aluminum closure together with a plastic cap, providing usability advantages for professional users, as well as customisation capabilities to enhance the drug distinctiveness in a healthcare facility.
This is what a primary packaging for parenteral drugs looks like today. While longterm future could see a convergence towards wearable devices, especially when it comes to chronic diseases treatments requiring a continuous therapy administration, the
52 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Packaging
short-term future could see an evolution of packaging engineering according to multiple directions: as for the improvement of container features, the driver of innovation is primarily the characteristics of the raw material: the glass.
More specifically, the continuous improvement of the treatments used on the internal surface of the glass containers, allowing for low levels of hydrolytic cutoffs, making the bottles ideal for drugs that are administered through an IV.
A second area of improvement is the enhancement of container resistance and, in particular, of the external glass surface through continuous research into new technologies, such as – among others – the use of layers of silicon, CVD and plasma. As containers are subject to intense processes such as sterilisation and depyrogenation, the aim is to reduce the risk of breakage along the production chain due to mechanical and thermal stress, as well as impacts and scrapes.
Thirdly, the identification of new technologies and processes to reduce leachables and extractables, with continuous tests being run to closely analyse the elements that are extracted to address the need for stability with drug formulations that are becoming increasingly chemically aggressive. This need for enhanced stability also applies to closures, searching for innovative, cuttingedge treatments for primary closures aimed at enhancing compatibility with drug formulations.
Another important pillar is the development in terms of materials, with the set-up of brand-new generation of glass bottles that are capable of offering superior chemical neutrality, shock absorption and assembly line flow characteristics.

Yet our innovation goes far beyond R&D for the improvement of materials. It also applies to a general rethinking of containers as complex systems, integrating brand new
characteristics and features. For example, allowing for enhanced integration within the pharmaceutical value chain, or making administration easier for professional users, such as doctors and nurses.
The Covid-19 pandemic has shown us that caregivers and professional users may face sudden, unprecedented workloads and pressure, which consequently enhance margins for error and injury risks. Packaging can make their jobs a little easier, through improved usability, guaranteeing simpler, faster and safer drug administration.
Since 2019, several products or concepts have been presented that address these emerging needs and facilitating parenteral drug reconstitution and administration by professional users, enhancing their safety thanks to luer-lock systems and minimising product waste. These are applicable to both multi dose or single dose containers. Pharmaceutical companies have shown a growing interest in these products and the first tests to scale up their industrial production are underway.
The second pillar of this redesign process is the transformation of single containers to ensure enhanced traceability across the supply chain. This is a huge theme in the pharmaceutical sector and investigating new solutions to track and trace drugs through primary packaging could be a real breakthrough, particularly when it comes to high value-added drugs.

According to the WHO, the counterfeit medicines market is worth 73 billion euros per year and causes serious reputational risks for companies and health risks for patients in terms of safety and therapy efficiency. That’s why a non-modifiable, noncounterfeitable, technologically-advanced tracking solution is increasingly required by pharmaceutical companies. Put into practice, containers would be marked with a unique code, providing new ways of marking complex codes into glass, with multi-layer information
stored with blockchain technologies that can then be verified throughout the supply chain.
Now more than ever, the new challenges brought about by the Covid-19 pandemic call, on one hand, for an increasingly scientific approach to supporting the evolution of single packaging elements in order to anticipate the needs of new drug formulations and guarantee a more enhanced, measurable and stable level of performance. On the other hand, forward thinking is increasingly required when it comes to parenteral packaging as a system, integrating additional functionalities thanks to a structured and controlled innovation process.
In order to develop these processes smoothly and efficiently, new, closer partnerships between pharma companies and packaging manufacturers are required. Continuous dialogue and long-term planning are key to making these new partnerships effective, as is a new shared responsibility model, in which both parties strongly commit to ensuring the success of the entire system and, as a result, of the therapeutic drugs on the global market.
Andrea Sentimenti has been appointed Marketing and Innovation Director of Bormioli Pharma in September 2019. Before joining Bormioli Pharma he was Executive Vice President and Global Marketing Director for Vibac Spa. Previously, he was Chief Marketing & Strategy Officer of Gruppo Fabbri Vignola S.p.a. for more than 10 years. He obtained a Master degree in Applied Physics and Nanotechnology from Università di Bologna and a Master’s in Business Administration (MBA) from Bologna Business School.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 53 www.international-pharma.com
Andrea Sentimenti
Packaging
Thin Paper: A Solution for Sustainability Challenges within the Pharmaceutical Industry
In the present age, there is an extensive pressure on the ecosystems and biodiversity of the world, and consumers are becoming increasingly aware of the environmental issues, which spurs demand for sustainably produced products and services. Therefore, sustainability is an essential part of manufacturing and business strategies across all industries. Despite the extensive regulatory framework, even the pharmaceutical industry has to accommodate this demand. Thin paper is a promising option for printed package leaflets. Its unique qualities combined with specific printing techniques allow reducing the ecological footprint of package leaflets while maintaining excellent print quality and manageability.
Sustainability in the Paper Industry
Only recently have many leading companies begun to work on real indicators of reduction of their impact on the environment. Examples of indicators of sustainable production specifically related to the environment include percent of raw material from renewable resources, acidification potential measured in SO2 equivalent, kilograms of emissions to the air, amount of energy used per unit of product or service produced. However, indicators of sustainable production should include not only production measures but also measures of the relationship between the production and the economic, social, and environmental systems within which it exists.1
Sustainability has become an essential part of the current manufacturing and business strategies in the paper industry. Sustainability is based on three interdependent pillars: environmental, economic and social.2
To be sustainable, the paper industry must fulfill the following conditions:2
• reduce the use of materials and energy in products and their production process,
• close material loop systems, to conserve resources and prevent waste,
• minimize or avoid waste,
• reuse and recycle products,
• dispose of non-recyclable products or production waste in an environmentally acceptable way,
• design products which are easy to repair, adaptable, durable and with longer lifetime,
• minimize transportation needs,
• utilize clean production technologies and procedures throughout the product life cycle and improve process technology,
• research and develop environmentally sound technologies.
Environmental considerations must be integrated into the corporate culture and business planning at all levels of design, manufacturing, distribution, and disposal. Achieving sustainable development will require changes in the industrial processes, in the type and quantity of resources used, in the treatment of waste, in the control of emissions, and in the products produced.3
There are multiple environmental certification systems within the wood processing industries. Within the printing industry, the prevailing environmental certificate is the FSC certificate issued by the Forest Stewardship Council for products made with sustainably grown wood. The FSC is an international non-governmental organization that promotes sustainable use of world’s forest resources. The FSC certificate criteria were originally created regarding tropical forestry, but currently
it is the most comprehensive forest certification system in the world. Each link in the certification chain must be certified. For example, the certification chain for printable goods comprises the forest owner, the forestry company, the paper mill, the printing house, and finally, the company using the printed products.4

Thin Paper and its Market
Wood-free thin paper is based on a chemical pulp in which lignin, the natural binding agent in wood, as well as hemicelluloses, the branched short carbohydrate polymers, are degraded into small water-soluble molecules and subsequently washed away from the cellulose fibers in a chemical process. Consequently, the pulp retains long and undamaged cellulose fibers, giving strength and durability to the paper. Bleaching lignin away in the chemical pulping process has the added benefit that thin paper does not turn brown with time. Thin paper is frequently used for printing in a variety of industries across the world.5
80% of the manufacturers use boreal forest wood as a source for bleached softwood kraft pulp (BSKP), and the largest manufacturers use wood from northern boreal forests. Among others, Finland is an important player in producing bleached softwood kraft pulp of northern boreal forest wood. There are several categories of BSKPs. However, because the northern trees have grown in extreme climate conditions, the cellulose fibers of Scandinavian soft-
54 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Sustainable Solutions
wood are longer and stronger than those obtained from South American wood. These long fibers give optimal mechanical properties to paper, particularly to the lowweight thin paper.6
Globally, there are approximately 20 key players on the thin paper market. The market size was valued at 1.20 billion US dollars in 2021, and the total revenue is expected to grow at 5.9% through 2022 to 2029, approaching total value of 1.89 billion US dollars. Currently, the largest market share is held by the printing paper segment. The rising popularity of digitally printed folding cartons and paper is likely to give a substantial opportunity to the global printing paper market in the coming years. Growing usage of thin sheets for printing and publishing, packaging, and labeling in a variety of sectors such as food and beverages, pharmaceuticals, and cosmetics throughout the world are likely to drive the market's growth. Moreover, increasing environmental awareness and interest in more sustainable products among consumers, as well as the advantageous qualities of thin paper such as ease of use, compactness, manageability, recyclability, reusability, and cost-effectiveness further increase the demand of thin paper.3,5
Printing on thin paper utilizes special techniques that take into account the manageability and reduced opacity of thin papers. The thin printing paper types may be either coated or uncoated. Suitable paper grades for the printing of e.g., package inserts and small booklets range from 29 g/ m² to 40 g/m².
Regulatory Requirements for the Package Leaflets of Medicinal Products
Package leaflets (PLs) of medicinal products are a key component of the labeling of medicinal products, providing information on the appropriate use and precautions of medications. The content should be written and designed to be clear and understandable so that after reading the leaflet, the patient can use their medicine safely and appropriately. PLs are an important source of medical information particularly for the patients, but they also benefit the health care professionals. In the United Kingdom, patients value the PL more highly than any other source of information excluding physicians and pharmacists (7). For many patients, the PL is the only written information they will have about the medicinal products they are taking.
The key requirements for the product information texts are provided in the Directive 2001/83/EC.8 In addition, when preparing the product information texts, the pharmaceutical companies should consider several specific guidelines given by the EU competent authorities. The structure of the PLs is defined in a so-called quality review of document templates issued by the EU competent authorities, aiming at a wellorganized layout with harmonized structure.9
The articles 593 and 611 of Directive 2001/83/EC require that the package leaflet of the medicinal products shall reflect the results of consultations with the target patient groups to ensure that it is legible, clear, and easy to use, considering not only the complexity of language but also
the layout and the font type and size.10 Importantly, for the compliance and the best outcome of the medication, the design of the leaflet should be visually appealing and it should make it easy for the patients to find the information they need. As the amount of information required for the PLs is increasing, more printing space is needed to maintain adequate readability.11

Multi-country packages for medicinal products are intended for distribution across multiple countries. Pharmaceutical companies can use them to streamline distribution and reduce costs, especially in small markets and territories. Multi-country packages for medicines must comply with the regulations of each country where they are distributed, and the PLs must be translated into the official language of each country. Multilingual packages are especially useful for products approved via the EU centralized procedure or mutual recognition and decentralized procedures as the content of the PLs is fully harmonized in the concerned Member States. There are successful procedures and guidelines already in place to facilitate multilingual packages, especially in the Nordic and Baltic countries.12,13 Multilingual packages are useful also because they help ensure the availability of medicinal products, particularly in small language areas. Therefore, they are favored by the regulatory authorities. It is also possible to design PLs in multiple languages even though only one or two languages would be printed on the outer package.
In addition to comprehensible language and clear layout, also the quality of paper affects the usability of the PLs. The manageability of large folded paper sheets or booklets depends on the quality of the material. Thin paper serves this purpose particularly well as it is durable due to the long cellulose fibers that are preserved in the manufacturing process. The high opacity of thin paper as well as the special techniques employed in printing on this type of paper ensure an optimal print quality.
Ecological Footprint of the PLs
While the PLs are an essential source of information for the patients, they nevertheless have an impact on the environment. Electronic PLs are currently being introduced in the European Union. However, an electronic PL can only complement the printed PL, not replace it. Therefore, PLs will still be printed on paper and distributed with the medicinal product.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 55 www.international-pharma.com Sustainable Solutions
Consequently, a substantial amount of paper is used for the PLs each year, eventually resulting in a significant amount of paper waste. Furthermore, careless disposal of PLs with mixed household waste contributes to landfills and wasted resources. Some pharmaceutical companies have started to reduce the environmental impact of the PLs by providing electronic copies or by reducing the size of the paper leaflets.
As sustainability is currently a top priority for the pharmaceutical industry and increasingly crucial for the business, the use of thin, lightweight paper is beneficial to both the environment and the balance sheet. Considering the tight regulatory requirements imposed on the pharmaceutical industry, the fact that pharmaceutical companies take into account the origin and the weight of the paper used in labeling demonstrates how closely tied sustainability and economy have become.
Potential of Thin Paper in Reducing the Ecological Footprint of the PLs
The use of coated wood-free low-weight thin paper (grades ranging from 29g/m2 to 40 g/ m2) in the PLs would offer several potential benefits over conventional printing paper. Firstly, the use of thin paper reduces the environmental impact of printed material. As the production process of wood-free thin paper is based on chemical pulp, the manufacturing of thin paper does not require the energy-consuming mechanical grinding process used in the production of conventional printing paper. Moreover, printing on thin paper means lower
consumption of material and other resources per each leaflet, including lower energy and water consumption and, ultimately, smaller amount of waste. The production chain can be optimized for both high quality and low carbon footprint if the wood harvested from boreal coniferous forest is processed to thin paper in a local paper mill and finally printed in a nearby printing house specialized in thin paper techniques. This type of production chain also facilitates tracking the sustainability of the entire supply chain.
Secondly, thin paper is less expensive than thicker paper, especially if ordered in large quantities, which favorably impacts the cost of labeling materials. Leaflets made

of thin paper weigh less than conventional leaflets, and the reduction of leaflet size permits smaller and lighter packages, cutting the cost and ecological footprint of transportation. In addition to cost savings, thin paper allows multi-country PLs and packages that add flexibility to supply chains and, thus, improve the availability and the security of supply in cases of shortages.
Thirdly, thin paper provides an excellent printing surface. High printing quality ensures good readability of the information content, which potentially improves treatment compliance and adherence and, consequently, leads to better health outcomes. The possibility to print PLs laid out as small-size booklets could also help pharmaceutical companies to meet the regulatory requirements for package leaflets.
Possible Drawbacks
It is important to note that the use of thin paper for the PLs should not compromise the legibility or durability of the leaflet. The package leaflet must be clear, readable, and durable enough to withstand handling and storage, otherwise it would jeopardize the patient safety. Careful selection of the printing paper ensures that the material meets both the cost and quality requirements.
At present, digital PLs are being introduced in Europe. They will allow, for example, a speedy implementation of new information in the PLs, accessibility to patient groups with impaired sight or poor literacy, and a simpler management of product information during regulatory processes. However, digital
56 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Sustainable Solutions
Sustainable Solutions
8. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use (Consolidated text) https://eur-lex.europa.eu/legal-content/EN/ TXT/?uri=CELEX%3A02001L0083-20220101. Visited March 27, 2023.
9. https://www.ema.europa.eu/en/humanregulatory/marketing-authorisation/productinformation/product-information-templateshuman. Visited on March 27, 2023.
10. Guideline on the readability of the labelling and package leaflet of medicinal products for human use https://health.ec.europa.eu/system/ files/2016-11/2009_01_12_readability_guideline_ final_en_0.pdf. Visited on March 27, 2023.
11. Fuchs J., Kutscha M. How best to assess paper quality for package leaflets – weight or opacity? Pharm. Ind. 77, 1380-1383 (2015).
12. Guideline on Nordic packages https://www. lakemedelsverket.se/493148/globalassets/ dokument/tillstand-godkannande-ochkontroll/forsaljningstillstand/produkt information/qna-nordic-packages.pdf . Visited March 27, 2023
13. Common Baltic Package Procedure https:// www.ravimiamet.ee/en/media/971/download. Visited March 27, 2023
14. https://www.ipcc.ch/report/ar6/syr/downloads/ press/IPCC_AR6_SYR_PressRelease_en.pdff. Visited on March 27, 2023.
PLs do not currently supersede printed PLs, and thus, there is a continuous need for environmentally and economically sound solutions for paper leaflets.
Conclusions
As Hoesung Lee, the chairman of the Intergovernmental Panel on Climate Change (IPCC), stated in the recently published IPCC report, mainstreaming effective and equitable climate action not only reduces losses and damages to the nature and the people but also provides wider benefits. Greenhouse gas emissions can be reduced by changes in the food, electricity, transport, industry, building and land use sectors. Simultaneously, these changes can facilitate leading low-carbon lifestyles, further improving health and wellbeing. With every increment of global warming, climate resilient development becomes progressively more challenging. Consequently, the choices made in the next few years will have a critical impact on our future and that of the generations to come.14
All in all, the use of thin paper reduces costs and improves the sustainability in the production chain of medicinal products. Some printing houses specialized in thin paper printing are located close to thin paper manufacturers. Utilizing these geographically favorably positioned services would allow rationalization of the product manufacturing chain and reduce the environmental burden.
The use of thin paper not only supports sustainable forest management and recycling initiatives, but also actively helps preserve natural resources and reduces the carbon footprint of the final product, simultaneously cutting down production costs. The thin paper production and printing technologies are already well established. Therefore, pharmaceutical companies should promptly take the initiative towards thin paper PLs.
REFERENCES
1. Veleva, V., Hart, M., Greiner, T. & Crumbley, C.: Indicators of sustainable production, J Clean Prod. 9, 447-452 (2001).
2. Fortună, M. E., Simion, I. M. & Gavrilescu, M. Indicators for sustainability in industrial systems case study: Paper manufacturing. Scientific Study & Research, Chemistry and Chemical Engineering, Biotechnology, Food Industry. 12, 363-372 (2011).
3. Krajnc, D., Glavic, P.: Indicators of Sustainable Production. Clean Techn Environ Policy. 5, 279–288 (2003).
4. https://connect.fsc.org/certification/forestmanagement-certification Visited on March 27, 2023.

5. https://www.maximizemarketresearch.com/ market-report/global-thin-paper-market/ 47848/. Visited on March 27, 2023.
6. PDL Papeteries du Lémon, personal notification by Stéphane Barbereau.
7. https://assets.publishing.service.gov.uk/ government/uploads/system/uploads/ attachment_data/file/946602/Best_practice_ guidance_on_patient_information_leaflets.pdf. Visited on March 27, 2023.
Ph.D. (Pharm.), Manager, Medical Affairs, PharmaLex Finland. More than 20 years' experience in medical writing at academia and as consultant. Kaija Rinne

Senior Advisor, DRA Consulting Oy. Senior Advisor in market access of pharmaceuticals, strategic management in sustainability, societal and health care issues. Outi
M. Sc. (Pharm.), Director, Regulatory Affairs, PharmaLex Finland. More than 25 years' experience in regulatory affairs at competent authority and as consultant.


INTERNATIONAL PHARMACEUTICAL INDUSTRY 57 www.international-pharma.com
Anne Tammimäki
Hemmo
Achieving Net Zero – How to Initiate Realistic and Lasting Supply Chain Sustainability Efforts in Pharma
Pharmaceutical manufacturers are rightly taking steps to improve their sustainability – but equally important is that the pharmaceutical supply chain matches these efforts. Richard Peck, Global Strategic Advisor of Tower Cold Chain explores how sustainability can be impacted through the choice of a temperature-controlled container.
Against the backdrop of looming climate emergency, there has never been such commitment to finding more sustainable ways of doing business. Inevitably, the pharmaceutical industry – which spans the entire globe and has a profound impact on our daily lives – is at the vanguard of change.
A 2019 study revealed that the pharmaceutical industry emits more greenhouse gases than the automotive sector. So it’s hardly surprising that, according to a survey conducted in 2021 by GlobalData, the environment is the clear priority when it comes to ESG (environmental, social and governance) planning.
Happily, this awareness is being translated into tangible actions; the world’s largest pharmaceutical manufacturers have embarked on ambitious plans to become more sustainable. For example, Pfizer has pledged to become carbon neutral by 2030 and announced a raft of measures to achieve this, from reducing direct emissions to purchasing 100% of electricity from renewable sources.
Novartis is taking similar steps, but interestingly has separated its deadlines for carbon neutrality – 2025 across its own operations, but 2030 throughout its value chain.
That’s a practical assessment of the complexities of achieving real and measurable change. A huge number of steps, involving dozens of different companies, are required to ensure pharmaceuticals reach patients safely and on-time. Each of these processes and partners must be carefully considered to find the optimum balance between operational efficiency, product protection and sustainability.
How can We Optimise TemperatureControlled Containers for Sustainability?
This article focuses on one aspect of the pharmaceutical supply chain: the temperaturecontrolled containers that are vital for shipping drug consignments around the world.
Fundamentally, every solution available on the market does the same thing. The use of temperature-controlled containers ensures palletised delivery of pharmaceutical shipments, in a way that keeps the product safe from physical damage and temperature excursions.
Yet there are important distinctions between suppliers, and various choices that a pharmaceutical manufacturer must make – in terms of the technology, materials, and design of any given container.
Each option will have its benefits and drawbacks, but what happens if we judge containers solely through the prism of sustainability? Where can the greatest reductions in environmental impact be made, without – of course – compromising on the critical function of the container.
Passive vs Active Containers
The first thing to decide when choosing a temperature-controlled pharmaceutical container is whether you prefer an active, or a passive packaging system.
An active container is a system that uses mechanical and electric systems powered by an energy source, combined with thermostatic control in order to maintain proper product temperatures.
In comparison, passive packaging systems consist of materials intended to keep the internal contents of the package within a specific temperature range for a defined period of transport without any means of mechanical assist. Passive packaging systems generally comprise two main components, which are insulation and coolants.
From the pharmaceutical brand’s viewpoint, the critical issue is reliability, and some understandably take comfort in having a powered solution. Yet, as advances in passive technology have progressed,
containers can comfortably maintain internal temperatures in excess of the industry-standard 120 hours.
This comparative quality then puts the onus on other considerations. From a sustainability viewpoint, it makes sense to eliminate a reliance on electricity during transit. And for a global industry where containers may be delayed in remote locations – for example, in areas prone to power cuts – there’s a practical benefit to passive systems, too.
PCM vs Dry Ice
However, the choice of a passive system isn’t quite that simple – answering one question leads logically to the next, which is to consider what is used as the coolant.
Cooling systems typically use either dry ice or phase change materials (PCMs). PCMs are commonly used in a rigid moulded polymer plate and can be conditioned to a particular temperature on multiple occasions, making them flexible and easy to use for product temperature ranges typically from -25°C through to +25°C.
PCMs are used to store and release thermal energy during the process of melting and freezing (changing from one phase to another). When such a material freezes, it releases large amounts of energy in the form of latent heat of fusion, or energy of crystallisation. Conversely, when the material is melted, an equal amount of energy is absorbed from the immediate environment as it changes from solid to liquid. By using materials with appropriately designed melting and freezing points, the internal temperature of the packaging system can be kept within a defined range for a period of time.
Dry ice (the solid form of CO2) is customarily used for temperatures ranging from -80°C to -60°C and is normally enclosed in a reusable or cardboard container within the outer packaging layer. Airlines have limits on how much dry ice can be shipped, therefore it makes sense to use PCM for most applications and only add dry ice for pharmaceuticals that must be kept at the coldest temperatures.
58 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Sustainable
Solutions
Either way, the insulation material used in the packaging system is also critical to the overall performance, by slowing down any effect of external temperature extremes. This allows the coolant to maintain the required temperature range, therefore extending the overall performance duration of the system.
Reusable vs Single-use
A clear advantage when it comes to sustainability is the choice of reusable containers rather than single-use alternatives. This should be a key consideration for any pharmaceutical manufacturer seeking to reduce their carbon footprint.
Reusable containers are a compelling proposition, simply by the fact that they stay in market circulation for a long time – and thus minimise the impact of carbon generated during manufacture. Inevitably, reusable solutions must also be robust to withstand years of freighting, thereby decreasing packaging waste produced in comparison to single-use solutions which are discarded after one journey.
Research demonstrates that reusable passive shippers afford the lowest cost solution when compared to alternative systems. In the White Paper ‘The Total Cost of Shipping,’ reusable passive shippers were compared to single-use passive shippers and active shippers, on a range of cost factors. It was, respectively, 9% and 34% less expensive than the alternatives.
Volumetric Optimisation and Lightweighting
One trap it is easy to fall into is to overengineer solutions for greater security that pharmaceutical shipments are being transported at a consistent temperature. Unfortunately, this peace of mind comes at the expense of inefficiencies in terms of container weight and volumetric space.
Imprecision in the design of the container can greatly increase the overall weight of

a package, making for heavier cargo loads, or superfluous void space that reduces the number of products that can be shipped in one container. Either way, the result is greater CO2 emissions.
Clever product design not only ensures the protective strength to achieve robustness; it also helps to achieve consistent (often sub-zero) temperatures; and optimises the usage of internal space for greater volumetric efficiency.
If the system is modular, even better –because the same structural components can produce multiple shipper sizes to fit differing aircraft types and ISO pallet combinations, requiring just one size of PCM plate regardless of configuration.
Providing Proof
Beyond the container itself, it’s also important to look at the values of the supplier itself. A commitment towards sustainability and wider ESG targets should be embedded throughout an organisation.
Has the provider of temperaturecontrolled containers pledged to become carbon neutral, in line with the requirements



of pharmaceutical customers? Are they taking steps towards using renewable and/or zero emission energy sources? Can they demonstrate measurable progress, independently verified through recognised standards such as EcoVadis or ISO:14001?
Ultimately, though, it comes back to the container itself. Transporting pharmaceuticals requires a solution, optimised for temperature control. Said container needs to be robust enough to withstand the rigours of international air freight. It should be as lightweight as possible, to minimise its impact on the overall cargo payload. And it should maximise volumetric capacity, to enable as many products as possible to be shipped at one time.
All of these points – light weighting, volumetric efficiency, minimising wasted product – play a significant role in sustainability. By factoring in other variables (choosing passive to reduce reliance on electricity, prioritising a reusable solution) it becomes possible to make the selection of a temperature-controlled container a key part of the journey towards sustainability in the pharmaceutical supply chain.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 59 www.international-pharma.com
Richard Peck
Richard Peck, Global Strategic Advisor of Tower Cold Chain. He has significant experience within the pharmaceutical sector, most recently at Clover Biopharmaceuticals and AstraZeneca in leading and developing processcontrolled logistics globally. He specialises in the pharmaceutical temperaturecontrolled supply chain to ensure patient safety, ensuring products reach patients with the same quality and efficacy as when they were first manufactured.
Sustainable
Solutions
Emerging Quality Considerations Across the Global Life Sciences Supply Chain
New waves of innovation in Life Sciences, and a redoubled effort by regulators around the world to maintain the highest standards of safety, are placing increased pressure on drug and device manufacturers to assure the consistent standards of their manufacturing processes, systems and supply-chain partnerships. During 2022, a number of important trends emerged or deepened, which are having a significant impact on the sector –ranging from practical supply chain issues to medicinal/device innovation. 2023 is set to be another milestone year, with more regulatory changes afoot linked to process digitalisation and automation. REPHINE’s Dr. Eduard Cayón sets out the key Quality considerations global LS manufacturers need to be on top of.
If ever the Life Sciences industry needed a robust manufacturing and supply chain Quality backbone, it is now. The reinvention of medicine continues apace, global supply chains are being restructured, and regulators are extending their controls over critical processes in digitalised and automated form.
Change and the need for consistency, fluidity and predictability mean that manufacturers need reliable controls and proof of their attention to detail. Even as they go deeper with digital transformation, they must consider the IT systems and automation tools they are harnessing, designing in quality from the outset and ensuring that everything is validated and will stand up to the scrutiny of an audit inspection.
There’s a lot to unpack, but success starts with awareness and a plan.
Disruption is Everywhere
As a result of the pandemic, and further aggravated by the war situation in Europe, the problem of shortages of some medicines persisted in 2022. The continued/repeat closure of China and the difficulties in the supply of raw materials presented a major problem, forcing pharma companies to rethink their reliance on far-flung markets
for APIs and other raw materials. To ensure supply security, manufacturers have had to review their sourcing options and, in many cases, opt for alternatives closer to home –despite the associated expense. This activity continues, and with it the need to review the quality performance and compliance records of new suppliers or alternative manufacturing sites in Europe or North America.
At a more strategic level, we’re seeing a whole raft of innovation and new medicines focused on macromolecules from biotech processes, or adaptation of existing medicines through the addition of medical devices and/or app-based digital controls and data exchanges. As the regulatory environment strives to adapt its quality and safety requirements, parameters for compliance are becoming stricter and more all-encompassing, putting pressure on manufacturers to keep updating their controls and monitoring more of their activity. We are seeing new harmonised standards and guidelines being published all the time, whose changes are based on the need for better scientific and technical knowledge of the processes, as well as risk-based management.
Fostering Safe Innovation Through GxP Rigour
Developed markets such as Europe and North America are already well used to strict controls and audits, but standards are being raised right around the world now – from South America to the Middle East and Asia. The upshot is that manufacturers must be able to vouch for every aspect of the product manufacture and delivery lifecycle, wherever the different touchpoints are, with GxP standards (recognised good manufacturing/ distribution practices) as their framework and guide.
Keeping on top of all of the requirements, and maximising opportunities, requires that Life Sciences companies are agileboth in resolving immediate problems and adapting to changes that arise as the regulatory environment continues to evolve and modernise. Having access to specialist capabilities is becoming essential, if companies are to be confident of leaving no gaps in their level of GMP compliance.
Lessons from the Covid-19 Chapter
Aside from the direct impact on production lines and supply chains, the pandemic has also left a legacy in the form of new channels and formats for auditing, most notably increased use of remote methodologies for at least some element of inspections. Certainly, there is continued potential for remote auditing activity, which can help ensure best use of auditors’ time during an inspection –as long as all stakeholders appreciate and respect the limitations of the medium.
Remote sessions between inspectors and auditees can add value in the preparation of audits in telematic meetings; the review of CAPAs (corrective and preventive actions); the clarification or extension of information; and even for performing some audits where 360º vision is not fundamental. The realistic scope for remote activity should be assessed via a risk analysis before and after an audit. For any critical auditing activity, however, on-site inspections remain essential.
Targets for 2023: New Cleanroom Measures & Vigilance Around Tech Validation
2023 is set to be another milestone year in Life Sciences, triggering calls for help with quality and compliance related help.
Sterile drugs are currently regaining prominence on the pharmaceutical manufacturing map, for instance. In Europe this has given rise to adaptations to EU GMP. Revised Annex 1 requirements related to the Manufacture of Sterile Medicinal Products were issued last August, starting the clock for compliance. All but one of the new provisions must be fulfilled by August 2023. The updated requirements, designed to protect and increase confidence in the sterility of these products, are significant and wide-ranging, spanning the Quality system and the manufacturing process itself.
To ensure they are ready, affected manufacturers must now perform gap assessments and build a good action plan, or move forward with designated activities as part of that roadmap.
Across the board, the opportunities presented by digital transformation, and regulators’ growing expectations for digital
60 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Logistics & Supply Chain Management
Development and manufacturing
Thermal Blankets for the temperature protection of pharmaceuticals and healthcare products in airfreight (+15°C +25°C) and (+2°C +30°C)

Qualifications & Validations

- Multilayer thermal blanket for PMC -ULD – Euro and Block pallets
- Temax-4000 blanket with multiple reflection technique
- Stress tested in summer (+46°C) and winter ( -15°C) profiles
- Tarmac tested on solar power and greenhouse effects
Ecological notes
- Recyclable + Low ecological footprint
- NON-laminated composition = easy to dismantle
- Re-manufacturing of recycled compounds

INTERNATIONAL PHARMACEUTICAL INDUSTRY 61 www.international-pharma.com
worldwide at freight forwarders
- TEMAX Group Development and manufacturing Belgium - Europe Phone +32-11.26.24.20
Email info@krautz.org Temax Americas LTD Hanover park – Illinois – USA ISO-9001:2015 certified
Available
KRAUTZ
Website www.krautz.org
advancement, mean that manufacturers will need to understand both the capabilities of the new technologies and the intricacies of the processes to be digitalised. This isn’t just about getting the most out of new systems, but also about taking care when redesigning and optimising processes, choosing the best
digital platforms, and implementing and validating them, with quality embedded right from the initial design.
That means planning well for all potential target outcomes, rather than trying to add new capabilities at the eleventh hour and
potentially undermining system validation, while sending implementation costs soaring. The new Annex 11 to EU GMP covers the associated changes currently being discussed by Medicines for Europe’s Quality working group. In the US, the FDA has also issued new guidance, based on Computer Software Assurance for Production and Quality System Software, and the ISPE GAMP guidance has been updated to GMP 5 2° edition.
Making Quality Developments Pay

Harnessing digital transformation and Quality system improvements should always be with a view to delivering something more than compliance, of course. Planned properly, any improvements to quality process ought to serve as an aid to operational effectiveness and efficiency.

Tangible, optimised progress will rely on up-to-date knowledge of new chemicalpharmaceutical processes and technologies, to guarantee quality at all stages of drug development, from pre-clinical studies and clinical trials to the development of new treatments or manufacture of medical devices.
Whatever 2023 has in store, the onus is on pharma manufacturers to be ready, by accessing appropriate expertise and reviewing all of the available options in good time.
Dr. Eduard Cayón is the VP of Audit Services at REPHINE, which provides bespoke technology and manufacturing supply chain compliance consultancy and third party auditing. REPHINE offers services all around the world from three primary locations – Stevenage in the UK, Barcelona in Spain, and Shanghai in China. Dr. Cayón, who holds a Ph.D. in Organic Chemistry, is a deeply experienced pharmaceutical industry consultant and auditor. He has more than 25 years of professional experience developed in Chemical Research, Pharmaceutical Analytical Development, Pharmaceutical and Food Quality Control, Validation, Auditing and Quality Assurance for the Pharmaceutical industry.
Email: eduard.cayon@rephine.com
62 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Dr. Eduard Cayón
Logistics & Supply Chain Management
PROTECT YOUR PHARMA PRODUCT WITH OUR QUALIFIED REFRIGERATED AND DEEP FREEZER CONTAINERS.
REDUNDANT REFRIGERATED & -70°C DEEP FREEZER CONTAINERS FOR PHARMA PRODUCTS

Klinge Corporation has been safely transporting and storing pharmaceuticals for nearly 40 years.
Avoid Temperature Excursions with Redundant Reefer/ Freezer Containers

Keep pharma products at the required temperature from +25°C down to -70°C
Meet international transport regulations
Safeguard pharma products with temperature recorder & online temperature tracking

inquiry@klingecorp.com

www.klingecorp.com
USA: + 1-717-840-4500
Packaging: The First Line of Defence in Life Science
Logistics
Temperature-controlled packaging plays a vital role in life science logistics.

Packaging has a multi-faceted role in supporting cold chain logistics to ensure transit times are met and the shipment is protected during handling. Unique packaging solutions have been developed to transport anything from sensitive biological materials during a clinical trial to life-saving medicines and cell and gene therapy treatments.
The appropriate packaging solution will ensure internal temperatures are maintained throughout global supply chains and that consignments remain viable to their final destination.
As more advanced treatments such as cell and gene therapy have become more complex and prevalent, there is an increased need for comprehensive packaging solutions that can maintain the integrity of a shipment in a wide range of conditions and, most importantly, alert supply chain specialists to any breach in the cold chain.
It is the first line of defence against temperature breaches and damage sustained during handling or tampering.
Industry Challenges
The global rollout of the COVID-19 vaccine demonstrated the need for robust cold chain logistics solutions in the face of a pandemic, with successful delivery and treatment reliant on packaging and transportation for a vast range of terrains and temperatures.

Research from the World Health Organization (WHO) underlines the global challenges the industry needs to overcome. WHO reports approximately 50% of vaccines globally are wasted, including the COVID-19 vaccine, and a proportion of those are damaged during transit and storage due to cold chain malfunction.
A greater risk of future pandemics, the climate crisis and an increasing reliance on personalised treatments, such as cell and gene therapies, are accelerating the need for innovative, sophisticated and validated packaging solutions that can help to mitigate these issues.
Packaging may be the first line of defence against temperature excursions, but how can specialists ensure thermal performance is maintained throughout the consignment’s journey?
As global temperatures rise, and supply chains become more complex, cold chain packaging will inevitably have to work harder.
The nine years from 2013 through 2021 rank among the 10 warmest years on record, according to data from the National Centres for Environmental Information.
Identifying where cold chain malfunction occurs is the key to reducing the industry's biggest issues, including the occurrence of temperature excursions.
Cold Chain Packaging
Packaging for cold chain life science shipments is evolving to meet the growing
64 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Logistics & Supply Chain Management






































INTERNATIONAL PHARMACEUTICAL INDUSTRY www.international-pharma.com
Visibility
Never lose
again!
Logistics & Supply Chain Management
demand for specialist treatments. Vacuuminsulated packaging and phase change materials are just some of the complex packaging components that are being used to maximise the thermal performance of cold chain packaging during transit.
Packaging is carefully selected based on each consignment’s unique requirements, with temperature profile, transit time and shipping lane data all analysed and evaluated to identify the right packaging requirement. Every packaging option must also be GDP compliant and ISTA7D tested –the temperature test for transport packaging managed by the International Safe Transit Association (ISTA).
Solutions have been developed to meet and sustain a comprehensive range of temperatures, from cryogenically frozen (-150°C and below) to refrigerated (+2°C to +8°C) and every temperature in between, while also providing protection from external factors like shock.
Tracking and Data
Increased connectivity through monitoring and tracking devices, as well as clear communication between supply chain partners and logistics coordinators, are one of the best tools the life science supply chain has in understanding where temperature excursions occur and how to overcome them.
Location tracking and the 5G wireless network allow for comprehensive live monitoring of a shipment, identifying delays on route that may increase transit time, and therefore, contribute to consignment damage.
Where do temperature excursions occur?
What external factors have impacted the performance of the packaging? These are important questions that only data can answer.
Having full sight of a shipment during transit enables specialists to intervene at the time of an excursion or take preventative measures, while improved customer visibility means increased customer confidence in the security and On-Time In-Full (OTIF) arrival of their materials.
This level of detail enables the logistics team to develop solutions that reduce or prevent the likelihood of an excursion happening by identifying preferred transit lanes and making informed decisions when selecting airlines and couriers.
Complex location tracking and condition monitoring in conjunction with predictive modelling technologies are the future of life science logistics.
These tools will become an essential component of life science packaging in the near future, as they can provide clarity where human communication falls short.
Ultimately, increasing industry-wide availability and use of condition monitoring technologies will help to prevent cold chain malfunction and increase OTIF rates to improve patient safety.

Closer Collaboration
Some of the biggest issues the life science community industry needs to overcome to improve OTIF rates and reduce product loss are related to disputations and delays in the commercial airline industry.
The COVID-19 pandemic severely disrupted the air transport sector, grounding 66% of global air fleets at its peak and reducing air freight capacity by 25% from 2019 to 2020. This major reduction in capacity resulted in air cargo rates increasing by 500% according to Dan Morgan-Evans, global cargo director at Air Charter Service (ACS) and placed additional pressure on the life sciences sector to balance transit speeds and spiralling costs.
Consignments are most at risk before and during airline transit. Airport security checks can present hazards for consignments. Clear communication and full customs clearance are vital to prevent fragile consignments such as cell and gene therapies from being x-rayed, which can make materials unviable and may result in serious repercussions for patients relying on life-saving treatments.
Improvements to packaging, such as the inclusion of security seals and eye-catching labelling, only go so far in preventing these types of exposure and potential damage to consignments.
66 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Logistics & Supply Chain Management
Closer collaboration and training provide a short-term solution to overcoming these issues, but there is a bigger part for data to play in improving decision-making for logistics coordinators.
Tracking technology can highlight delays in transit, but while sophisticated condition monitoring technology has been developed, greater availability and reliability across the industry is required to confirm the viability of a consignment throughout transit.
Currently, technology exists to provide this level of detail, which could help specialists to intervene to prevent a consignment being delivered that is unsuitable for use, but it is not being widely used across the industry.
Condition monitoring devices with sensors that confirm exposure to light and shock are a game changer for the industry and for patients relying on biopharma treatments.
Resource and cost efficiency remains the priority for organisations, but greater investments in these technologies and global uptake will enable the whole industry to accurately plan and identify the safest shipping routes for life science materials.
Supply chain specialists will also be empowered to take remedial action where consignments may have been exposed and potentially damaged and intervene to replace shipments during transit.
Sustainability
Like all industries, life sciences logistics managers are facing increased pressure to reduce their environmental impact and improve sustainability.
Some packaging types have gained negative connotations in recent years, with discussions focused on the global requirement to reduce plastic and singleuse packaging.
The association of less packaging with sustainability has become synonymous across most industries. In the life science sector, this presents huge challenges, as less typically means reduced protection and a reduction in transit times.
New developments in packaging materials that are smaller or lighter, but can perform to the same standards, are slow.
A move towards smaller packaging is likely to be detrimental to the consignments
being transported, with a loss of thermal performance increasing the likelihood of shipments arriving damaged.
So, in an industry where a reduction in materials is likely to have a detrimental effect, how do you make strides towards sustainability?
By prioritising viability.
We believe one of the industry’s primary sustainability focus lies with optimising OTIF rates.
By not compromising on packaging, supply chain specialists can ensure that consignments arrive in a viable condition.
Delivering damaged materials presents serious issues for patients, but it also presents environmental issues, as any consignment that cannot be used requires re-shipping.
Product loss doubles the use of packaging and coolants, such as liquid nitrogen or dry ice, and emissions from couriers and airlines.
The type of accurate and real-time data that full condition monitoring could provide is the key to improving sustainability in the life science logistics industry and highlighting ways to mitigate product loss before it occurs.
Building Resilience

Data is central to the future of life science and biopharma packaging, and its ability to increase resilience.

WHO data on damaged vaccines underlines the need for greater insight into the issues facing the cold supply chain and the role that packaging can play in improving transit times to help reduce wastage and improve patient treatments.
Climate change and its impact on global temperatures will place more strain on the cold supply chain, meaning that packaging will need to undergo more extreme testing if
it is to deliver the same thermal performance in the future.
Predictive technologies that support at the pre-planning stage will become vital in forecasting what will happen during a shipment and where the biggest risks lie.
Digital models that can analyse temperature in real-time and predict the impact of extreme conditions will enable specialists to calculate a more accurate picture of how different packaging solutions will perform.
Adopting increased data gathering will also lead to digital decision-making, with programs designed to evaluate red flags and re-route where it predicts issues are likely to occur.

Having the technology to predict delays, high traffic, or even grounded flights due to adverse weather, would provide greater insight and improved decision-making to identify the shortest route with the least risk.
Overall, greater investments and developments in tracking technology will create more robust supply chains and improve patient safety as the industry grows.
Robert
is a Packaging
Engineer at Biocair, focusing on identifying new and efficient cold chain packaging solutions and condition monitoring tracking technologies for life science logistics. Prior to his role at Biocair, Robert served in the US Army as a Combat Medic Sergeant with secondary disciplines in clinical research, medical logistics management for satellite laboratories in foreign regions, cold chain management and storage of biological samples.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 67 www.international-pharma.com
Robert Pagan
Pagan
Solutions
Fast-moving Pharma Organisations Prescribe Gamified Training to Keep Coaching and Management Skills Scalpel-sharp


In an industry where new technologies and techniques are advancing all the time, cutting-edge coaching training is key to helping people keep pace with change. Big pharma organisations, like Boehringer Ingelheim and Merck, have turned to simulation training for the solution…
As the pharmaceutical industry continues its post-pandemic recovery, experts predict the sector’s value will grow to US$2.4 trillion by 2029.
This rapid growth is fuelling accelerated change across the industry, with new medicines, technologies and regulations, making it crucial that employee training keeps pace in an environment of constant transformation.
Big pharma companies, including Boehringer Ingelheim and Merck, are seeking fresh ways to ensure that their training is
flexible, fit-for-purpose and fast to respond to ever-shifting needs.
That’s why pharma is increasingly turning to gamified simulation training to keep their teams’ expertise and know-how scalpel-sharp at all times. Computer game-style simulations place people in true-to-life scenarios where they can practise skills in situations that replicate job conditions. These simulations are combined with gaming elements that increase motivation, attention and learning.
Immersive training has proved particularly effective in improving employee skills in two core areas:
1. Coaching and Development
2. Relationship Management
1. Coaching and Development –Helping Managers Lead From the Front Coaching is a vital aspect of success in the pharma industry, where first-line managers (FLMs) have finite opportunities to observe their direct reports interacting with customers.
Many FLMs have advanced from being successful sales representatives, but that does not guarantee that they have the skills or knowledge to effectively coach and develop their team members.
Holding effective coaching conversations is a skill that needs to be learned and regularly practised.
Boehringer Ingelheim took an innovative approach using psychology and gaming mechanics and created a simulation training solution. It had to be mobile-first, easily accessible and shaped into bite-sized modules that employees could easily fit into their already busy schedules.
The simulation encourages effective coaching behaviour in FLMs to help themselves and others grow so that they can be game-changing leaders for their direct reports.
As a part of this project, Boehringer Ingelheim identified the key capabilities required by
68 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1 Logistics & Supply Chain Management






















INTERNATIONAL PHARMACEUTICAL INDUSTRY 69 www.international-pharma.com For further information europe.thermoking.com Find your nearest dealer on dealers.thermoking.com TEMPERATURE CONTROLLED SOLUTIONS FOR THE PHARMA COLD CHAIN TK PharmaSolutions offers both transport and cold storage solutions to ensure vaccines reach their destination quickly and at the right temperature. Our road, air, ocean transport and temporary storage solutions can maintain temperatures from -70°C to +30°C. Safe. Secure. No Compromise. VISIT US AT LOGIPHARMA • Booth 69 25-27 APRIL 2023 LYON, FRANCE
both customer-facing (CF) employees and their FLMs.
Coaching that Counts
Of the capabilities identified for FLMs, coaching (for performance and development) was the top skill that had the potential to make the biggest impact on performance. Effective conversations with great coaches can significantly improve performance, increase motivation, and enhance job satisfaction for individuals being coached. They also have the potential to foster a positive and productive team culture, and to develop future leaders.
Additionally, effective coaching can help individuals identify and overcome obstacles, develop new skills and knowledge, and achieve their personal and professional goals.
To meet Boehringer Ingelheim’s needs, a solution called Coach to Grow was developed, and recently launched in India and Canada. The primary goal of the simulation is for FLMs to gain a clear understanding of coaching methodologies, the EGROW framework and when to use coaching over other leadership skills such as feedback, mentoring or direction.
Learning Through Games
The game-based training, which can be accessed on mobile, desktop or tablet, consists of five modules, focusing on: coaching and leadership skills; leading employee development discussions; engaging in difficult
Chain Management
discussions; and customer engagement. In each of these modules first line managers are faced with true-to-life scenarios that they will typically encounter in their day-to-day roles.
Set in realistic environments, these managers engage in dialogues with avatar colleagues who are all encountering their own set of workplace challenges. FLMs are faced with a range of scenarios including helping team members understand the value of coaching, creating development goals using the EGROW model and navigating difficult conversations. How employees answer each of the questions determines their unique journey through the modules. Employees earn points based on their answers and are also provided with feedback as they work their way through the simulations.
Reaching Peak Performance
The early response from Boehringer Ingelheim’s users is overwhelmingly positive with employees seeing their knowledge improve from 30% to 92%, repeating the training three times on average.
After engaging in the training, a Boehringer Ingelheim employee said: “The options in the game are not inherently obvious, making you pause and work through the process of coaching. I also like that you can pick up non-verbal cues as part of your coaching simulation.”
Virtual World. Real-world Impact
Jennifer Quist, Global Capability Owner –
Leadership Development & Coaching, said: “Simulation training is already having a real-world impact. Because employees are playing in a virtual world, they can practise and repeat the training in a safe-to-fail environment until they feel confident in their coaching skills.
“The engagement and early results are something that we would have not achieved through traditional methods, like role play or classroom-based training,” said Quist. “It’s been a game-changer for us – and we look forward to rolling out this simulation training to 25 countries as part of a wider competency training initiative.”
That reflects the fact that the organisation needs to keep people’s skills sharp across the board. Even experienced managers need refresher training, and FLMs everywhere nurse a genuine desire to be better coaches.
2. Relationship Management –Skills Which Score Big in Every Land and Language
Global pharmaceutical company Merck was tasked with upskilling 650 frontline healthcare sales managers from 65 countries in seven different languages.
To create training at scale, Merck rolled out game-based 3D simulations with Attensi, ensuring that each of its sales managers received the same level of instruction whatever their location.

70 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
& Supply
Logistics
Logistics & Supply Chain Management
Just like a computer game, the training simulations were accessed on computer, tablet or mobile. They immersed managers in realistic scenarios and settings (doctor’s office, conference room, office, a train) that they typically encountered in their day-today roles.

The initiative involved creating gamified simulation training for a range of topics, including coaching and performance reviews, goal-setting and problem-solving.
In each of the 40 simulations, Merck’s managers were faced with working through a range of scenarios and dialogues with avatars representing internal and external stakeholders.
Motivated Managers
Tasks included managing relationships with the organisation’s sales representatives who faced their own unique challenges, including difficulties securing client meetings, navigating sales conversations or handling a data breach.
In each of the scenarios, managers had to evaluate the situation before selecting the most appropriate course of action. Their responses shaped their progress through the game and affected how the narrative ended. Managers saw the sum of their choices play out with realistic results. This highlights a stark difference with traditional training methods which offer only right or wrong answers in an artificial training environment.
These gameplay mechanics motivated managers to keep playing until they achieved mastery in each of the modules. With the simulations providing carefully tailored feedback on managers’ choices, they under-stood exactly what they could have done differently – and were ready to repeat the training in a safe-to-fail environment.
The Story of Success
The simulation scenarios involved narratives depicting common work situations. This is a key element in promoting durable learning.
As recent research explains: “Narrative provides not only meaning but also a mental framework for imbuing future experiences and information with meaning, in effect shaping new memories to fit constructs of the world and ourselves.” Learning theorists call this “active retrieval”.
When people must respond to diverse circumstances, learning involves the ability to retrieve a relevant model or scenario, and
this ability is extended with each iteration. In fact, there’s some evidence that this process reshapes neural pathways over time. In other words, engaging storylines promote durable learning.
VP Hails Effectiveness of New Approach Chetak Buaria, Vice President Global Commercial Operations for Merck’s Healthcare Business, told Harvard Business Review: “The training was a phenomenal success which significantly improved the effectiveness of Merck’s sales leadership team.”
Compared to training in a classroom, Buaria said this new approach “reduced the training time by 70%, saving over 2,000 working days of frontline managers’ time”.
Another benefit was that busy sales managers repeated the training outside work – this wasn’t down to the organisation pushing them to work beyond contracted hours, but because the training drove the motivation for individuals to improve their score and increase their knowledge.
Dedicating this much time to training is rarely achieved with conventional methods, which are often used as tick-box, assurance exercises.
Flexible and Accessible
As the training is available anywhere, anytime, managers dipped into modules at their convenience. For example, if a manager required a quick recap on best practice for navigating a sales conversation, they could play through the relevant module to refresh their memory and increase their confidence ahead of a real-life meeting. The training is also scalable.
Should training be required in another language or an entirely new module developed to keep pace with changing pharmaceutical regulations, this can quickly be created and rolled out simultaneously to all relevant markets.
With an ever-increasing rate of change across the industry, keeping pace with the sector’s innovation is vital.
To do this, organisations require new training technologies, like gamified simulations, that match the market’s demands, patient needs, medical developments and regulatory requirements.
Investing in flexible and accessible training tools will ready individuals and
organisations for a constantly growing and evolving industry.
Thomas Andersen is the VP for Health and Pharma at Attensi. He has been with Attensi since 2019. Thomas has over 20 years' experience in marketing, and commercial and business development in the pharmaceutical and biotech industry. Prior to joining Attensi, Thomas held the position of CEO at Nextera for 6 years. He has also held various commercial roles at Pronova Biopharma and AstraZeneca. Thomas holds a Bachelor in Medical Laboratory Sciences (Bioengineer) from Oslo University College and a Master of Management from BI Norwegian Business School.

Jennifer Quist is the Global Capability Owner of Customer Facing Team Learning, Training & Coaching at Boehringer Ingelheim. She has been with Boehringer Ingelheim since 2005 and has over 20 years' experience in sales, training and management in the pharmaceutical industry. Jenn is an LCI Certified Coach from the Leadership Coach Intensive, and also holds a Bachelor with Honours in Biology and Biological Sciences from Brock University and a Masters in Neurogenetics from the University of Toronto.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 71 www.international-pharma.com
Thomas Andersen
Jennifer Quist
Logistics & Supply Chain Management
Evolution of the Pharmaceutical Cold Chain
The unprecedented scale of the COVID-19 vaccination campaigns has made the world more aware of the essential role cold chain and refrigeration technologies play in the pharma supply chain. Before the pandemic, close to 5 billion doses of vaccines were injected worldwide each year, while more than 7 billion of only COVID-19 vaccines have been pre-ordered1 during the vaccination campaigns.
An Unbroken, Sustainable Cold Chain
Pharmaceutical products require a dependable and unbroken cold chain at all stages, including strict, often extreme storage temperatures. The rising demand has also put the spotlight on the sustainability aspect. Logistics companies, while needing to comply with the stringent requirements specific to the pharma industry, have paid attention at how transport and storage can impact their sustainable practice goals, carbon emission reduction requirements etc.
Evolution is under way, from more environmental-friendly packaging solutions to technological innovation and electrification in transport refrigeration. Electrification can not only sizeably reduce direct vehicle carbon emissions, but also offers more consistent regulation of temperature.
With the deadline for the end of internal combustion engine vehicle sales looming on the horizon, it’s inevitable that all cold chain fleets will begin accelerating the move to electric vehicles. The refrigerated transport industry is strongly moving the needle on reducing carbon emissions. In fact, low carbon refrigerated transport technology already exists for last mile deliveries, vans, truck and trailer vehicles powered by virtually any fuel source. The technology allows to completely avoid emissions from refrigeration systems, and the transport industry is ready for the transition to fully electric cargo transportation in and beyond the pharmaceutical supply chain.
Digital Transformation and Improving Visibility of the Cold Chain
Advanced use of technology for transparency
and real time monitoring; More personalized and patient-centric medicines; Omnichannel distribution – are the trends shaping the industry, with increased focus on digital transformation and improving visibility.
Greater visibility of the flow of pharmaceutical products, using real-time supply chain information systems and traceability tools makes it possible to track shipments in real time. Real-time monitoring capability is especially important with pharmaceutical products, because without it there is no visible indication when a product has deviated from a set temperature. It is one of the key safeguards to provide confidence that the efficacy and integrity of the product have remained intact.
Adoption of Advanced Technologies
The pandemic has accelerated the adoption of advanced technologies like blockchain, artificial intelligence, and telematics that help ensure end-to-end protection for life-saving medicines throughout the cold chain.
Blockchain technology has emerged as a powerful tool for ensuring the endto-end protection of pharmaceutical products in the cold chain. Blockchain allows secure, transparent, and tamperproof recording of data, which help to ensure the integrity and authenticity of data related to the movement and storage of pharmaceutical products every step of the supply chain, from the manufacturing facility to the end user. Each transaction recorded on the blockchain is verified and encrypted, ensuring that the data cannot be tampered with or altered. This creates an immutable record of the entire supply chain, from manufacturing to distribution and storage to delivery, providing complete transparency and traceability.
Artificial intelligence (AI) is another powerful tool for analyzing large amounts of data and identifying patterns and insights that can help to improve the efficiency, accuracy, and reliability of the pharmaceutical cold chain. AI can help to optimize logistics, predict potential disruptions and identify areas for improvement in the supply chain. One of
the benefits of AI is its ability to predict temperature excursions. By analyzing data from sensors and other sources, AI algorithms can identify patterns and trends in temperature fluctuations and other environmental conditions, and alert stakeholders to potential problems before they occur. All this can help reduce costs, improve efficiency and enhance the overall quality of the supply chain.
Telematics plays a crucial role in preserving the end-to-end pharmaceutical cold chain providing real-time monitoring and tracking of vehicles, goods, and equipment. By combining GPS tracking, temperature and other sensors, telematics can provide real-time insight into shipments and ensure that the temperature and other conditions are maintained within the specified range. It enables to detect any deviations from the specified temperature range and take corrective action immediately, preventing spoilage and ensuring the efficacy and integrity of pharmaceutical products.
In addition, telematics can also help to optimize the route planning and scheduling of pharmaceutical shipments, reducing the risk of delays and disruptions. By tracking the location of vehicles and shipments in real-time, logistics managers can make informed decisions about routing and scheduling to avoid congested areas or adverse weather conditions that could affect the temperature or condition of the product. Last but not least, telematics can also help to ensure compliance with regulatory requirements for the transportation and storage of pharmaceutical products, providing the necessary data to demonstrate compliance with various regulations.
Importance of the Good Distribution Practices
The pandemic has further strengthened the importance of the Good Distribution Practices (GDP) Regulations. Even the best equipment can give poor results if it is not used correctly. The GDP standards require compliance with a set of rules designed to ensure the quality, safety and security of medicines – not only the COVID-19 vaccines
72 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Logistics
Supply Chain Management
– throughout the entire supply chain, making the application of good practices essential.
The objective of Good Distribution Practice (GDP) guidelines is to ensure that the high level of product quality, determined by good manufacturing practices, is maintained throughout the distribution chain. This extends beyond the transport vehicles used to take pharmaceuticals (such as APIs) and medical components from the manufacturing facility to distributors and wholesalers. It must also ensure compliant delivery to hospitals and pharmacies.
It is highly recommended for distributors to comply with the European Union guidelines on good distribution practice of medicinal products for human use.
Expert companies can address the precise needs of the pharmaceutical industry by delivering advice and a broad spectrum of
services and solutions purposefully covering the four stages outlined in the GDP Guidelines:
• Design Qualification (DQ)
• Installation Qualification (IQ)
• Operations Qualification (OQ)
• Performance Qualification (PQ)
In conclusion, the pharma transport industry is evolving rapidly, driven by the demand for more sustainable, patientcentric and efficient supply chains. Advanced technologies are available and become increasingly important to achieve these goals, ensuring that pharmaceutical products are delivered safely and efficiently to patients around the world.


Muge Suner is Senior Pharma Business, Key-Strategic Accounts Manager for Thermo King in Europe, Middle East, and Africa. Based in Brussels, Belgium, she has been leading the Thermo King PharmaSolutions operations since 2019. Following her studies at the Ankara University in Turkey she began and continued her career in major forwarding and supply chain companies such as Cosco, DB Schenker, and DHL, where she was responsible for Strategic account management for temperature controlled solutions in life sciences, healthcare, and pharma.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 73 www.international-pharma.com
Muge Suner
&
REFERENCE
1. United Nations Environment Programme 21Brief_PHARMACEUTICAL APPLICATION_ENG. indd (unep.org)
Thermo King: End-to-End Pharma Transport Solutions
Current Trends in Pharma Production and Manufacturing
Through a series of exclusive interviews and a survey of industry insiders, Connect in Pharma identifies the top trends in pharmaceutical manufacturing for 2023.

A new report just published from Connect in Pharma highlights a range of forces shaping the future of the pharmaceutical manufacturing and drug-delivery industry in Europe, from machine learning and connected devices to eco-friendly strategies. According to the report, which drew on both exclusive interviews and a survey of industry leaders, manufacturers need to invest in robotics and artificial intelligence, train and hire to ensure workers have the new skills needed and build new partnerships to continue to survive and thrive in the future of pharma production.
The report lists the following trends expected to make the most impact in the coming year:
1. Manufacturing is Still Playing Digital Catchup: Almost three quarters of respondents indicated that AI and other digital tools are not yet part of their production lines. “In some aspects, pharma is behind other sectors because it sees itself as within the R&D sector, not the manufacturing sector. It’s not behind in the digitisation of R&D, but it is when it comes to manufacturing,” said Henrik Von Scheel, who is widely regarded as the originator of Industry 4.0.
2. The industry is seeing an explosion of advances within the field of drugdelivery devices: Three areas facing marked innovation and posed for market expansion include the inhalable biologics market, long-acting injectable formulations and connected devices that aim to improve the effectiveness and adherence of medications. Half of the survey respondents confirmed that their company is or will be involved in developing, manufacturing, or packaging drugs that are inhaled, with dry powder inhalers and nasal delivery
devices revealed as the most popular formulations.
3. Sustainability: The survey revealed that two-thirds of companies are or will be involved in developing more sustainable practices. Of these, nearly all identified company-led strategy as a key driver, with regulations, individual or depart-mental initiatives and longterm cost savings also driving change.
Katherine Dixon, a partner at Bain & Company who specialises in energy transition, was one of eleven specialists interviewed for the report. “Companies in the pharmaceutical sector really need to be thinking about how their market is going to change, because it's going to change radically, whether it's driven by customer behaviour, technology transition, or a huge pile of a policy that's coming down the track. The world is moving. Understanding how their business is set up to manage the different future scenarios is going to be critical,” said Dixon.
Industry insiders said companies in the sector can remain competitive by forging new partnerships and collaborations, growing their investments in tech-driven innovations, and renewing their focus on patients.
“These trends are addressed in detail in this new report, and they will be further explored in even greater detail at the 2023 Connect in Pharma event,” said Renan Joel, Divisional Managing Director at Easyfairs. “Industry leaders can use this free report to get a sense of how they stack up against others in the industry, and then network and learn from experts by attending the event in Geneva.”
Methodology
Respondents to the survey included 56 individuals based in 13 different countries (including Switzerland, Germany, France, Spain, Italy, Portugal, Ireland, Romania, the United Kingdom, Brazil and India) representing a range of companies, including AstraZeneca, Catalent Pharma
74 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Events
Solutions, Gerresheimer, Kisco Pharma Outsourcing Solutions, Korber Pharma Software, and Unither Pharmaceuticals. Exclusive interviews were conducted in January 2023 with 11 leaders, innovators, strategists and futurists across the pharmaceutical, technological and digital landscape. Their insights were instrumental in giving context to the survey findings. Sciad Communications designed the survey, conducted the interviews and provided the analysis and writing.
Extract: Digitalisation Within Manufacturing
The rapid explosion in digital capabilities – from automated robotics to machine learning and now ChatGPT – is reshaping a range of industries. However, pharma has been relatively slow to embrace digital developments.
“In an industry that faces heavy regulations and high stakes, most pharmaceutical companies see themselves as conservative and change-averse," said Bertalan Meskó, Director of The Medical Futurist Institute. “But pharmaceutical executives must embrace the changes that are now rapidly coming in order to stay competitive.”
Change is happening, but how quickly are companies incorporating artificial intelligence (AI) and other digital tools into the pharmaceutical manufacturing and packaging process? Only 28% of respondents to the survey indicated their companies have developed or are actively developing such tools. While this is not yet an industry-wide revolution, it indicates AI is no longer just a future concept but a real industry practice happening on the ground.
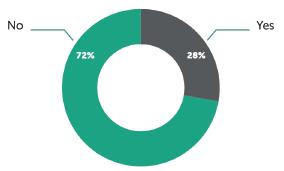

Of those who indicated their companies are engaged in adopting AI, almost all recognised the important role digital tools are playing in improving data collection and reducing manufacturing down time. AI and machine learning are furthering continuous manufacturing practices, which is seen as a key trend to reduce waste, improve efficiency, and increase the speed of drug production.
Others recognised the important role these tools are playing in reducing human error and improving precision fill and finish, workflow integration, and anticounterfeiting. A substantial minority also noted how digital tools improve their company’s ability to record and share supply chain data with regulators.
“I see opportunity for tech across the entire pharma supply chain,” said Fausto Artico, Global R&D Tech Head and Product Director of Innovation and Data Science, GSK. “No part of the supply chain isn’t touched by automation. Tech is an important global enabler,” he added.
However, with almost three-quarters of respondents claiming AI and other digital tools aren’t yet part of their production lines, the question looms as to whether the sector is moving fast enough.
“In some aspects, pharma is behind other sectors because it sees itself as within the R&D sector, not a manufacturing sector. It’s not behind in the digitisation of R&D, but it is when it comes to manufacturing,” said author and advisor Henrik Von Scheel, who is widely regarded as the originator of Industry 4.0.
Andreas Raabe, CEO of Munich-based CDMO Adragos Pharma, echoes this sentiment: “In its core, pharma is of course highly innovative, yet it is also the slowest industry when it comes to digital adoption, and within manufacturing, it is a laggard when it comes to innovation,” he said.
Survey data revealed that the most common barriers to digitalisation were ensuring compliance with regulations and ensuring data integrity and security. Others noted issues in identifying the right software, financial costs, and a lack of leadership at a company-wide level.
“Directors are too comfortable in their position to embrace change. They haven’t considered the benefits digitalisation can have for their business model,” said Christian Baudis, Founder of My Digital and former managing Director of Google Germany.
Artico agrees that people are the ratelimiting factor: “Businesses are run by humans, and humans are the biggest barrier
– they don’t want to try new practices if they don’t understand the benefits or fear losing their jobs.”
Raabe envisions a shakeup in Europe for pharma manufacturers who fail to make investments in state-of-art technology and innovation, due in part to ever-growing regulatory requirements. He points to the example of Annex 1, which is a list of regulatory requirements that stipulates how aseptic filling is to be undertaken to assure no risk of contamination for sterile products. “Automation in fill-finish can help to address some challenges in meeting these requirements, while at the same time providing better efficacy in production,” Raabe said. However, “there are players in the industry who might struggle to have solutions in place in due time or cannot stem the investments needed,” he added. “Some may have to exit the field.”
Furthermore, Raabe predicts that investments in digitalisation, automation and robotics will help to better interface with customers and reduce the need for human intervention. “It will lead to smoother interactions, automated updates, less phone calls and emails, a better flow of information, and greater transparency. It’s nothing revolutionary; it’s just similar to what exists in other industries already,” Raabe said.
Access the complete version of ‘The Future of Pharma Production, Manufacturing and Packaging Trend Report’ by Connect in Pharma at connectinpharma.com.
www.connectinpharma.com/connect-365/ trend-report-2023/
Connect in Pharma
After a successful launch in 2022, Connect in Pharma returns on 14 & 15 June to bring together the community of professionals shaping the future of drug delivery, manufacturing, outsourcing and packaging. Connect in Pharma aims to drive innovation, business and new partnerships in four key areas: innovative packaging, drug delivery systems, subcontracting, and manufacturing. Taking place in Geneva – the centre of a major pharmaceutical and biopharma cluster –Connect in Pharma is the perfect place to meet key influencers from leading pharmaceutical groups, biopharma, industry clusters and suppliers.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 75 www.international-pharma.com
Events
Figure 1: Prevalence of digital technology in pharma production
Media and Communications
IPI

Peer Reviewed, IPI looks into the best practice in outsourcing management for the Pharmaceutical and BioPharmaceutical industry.

www.international-pharma.com
JCS
Peer Reviewed, JCS provides you with the best practice guidelines for conducting global Clinical Trials. JCS is the specialist journal providing you with relevant articles which will help you to navigate emerging markets.

www.journalforclinicalstudies.com
IAHJ
Peer Reviewed, IAHJ looks into the entire outsourcing management of the Veterinary Drug, Veterinary Devices & Animal Food Development Industry.
www.international-animalhealth.com

IBI
Peer reviewed, IBI provides the biopharmaceutical industry with practical advice on managing bioprocessing and technology, upstream and downstream processing, manufacturing, regulations, formulation, scale-up/technology transfer, drug delivery, analytical testing and more.
www.international-biopharma.com

PNP
Pharma Nature Positive, is a platform for all stakeholders in this industry to influence decision making by regulators, governments, investors and other service providers to achieve Nature Net Positive Results. This journal will enable pharma the ability to choose the right services to attain this goal.

www.pharmanaturepositive.com
PHARMA’S DNA
Listen to industry experts on the latest in drug discovery, development, research, industry regulations and much more at Pharma,s DNA, the podcast channel by Senglobal Ltd., available on Sound Cloud, Spotify, iTunes and YouTube.
senglobalcoms.com


INTERNATIONAL PHARMACEUTICAL INDUSTRY 77 www.international-pharma.com Asia’s premier pharmaceutical event 19-21 June 2023 | Shanghai New International Expo Center To register please visit www.cphi.com/china Contact salesoperations@informa.com China’s pharmaceutical market is forecast to expand from CNY1.07trn (USD155.1bn) in 2020 to CNY1.3trn (USD203.8bn) by 2025. 50,000+ Pharmaceutical professionals 3,000+ Exhibiting companies 80+ Conferences & activities
19-21 April 2023
Tokyo, Japan

78 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Your gateway to the Japanese market
More information at cphi.com/japan CPHI Japan is the ideal business platform for international pharma professionals to join in order to grow business in the rapidly changing Japanese pharmaceutical market. 20,000+ Attendees 90+ Countries in attendance 420+ Exhibitors

Page 38–41
AeroGo
Page 8 & 9 African Regional Intellectual Property Organization
Page 43 Aurena Laboratories
Page 79 Connect in Pharma
Page 77 CPHI China
Page 78 CPHI Japan
Page 3 FUJIFILM Wako Chemicals U.S.A. Corporation
Page 65 Hive-Zox International SA
Page 5
Kahle Automation
Page 63 Klinge Corporation
Page 61 Krautz Temax
OBC Natoli Engineering Company
Page 21 Nipro
IFC Owen Mumford
Page 76
Senglobal Ltd.
Page 7 Stäubli International AG
Page 69 Thermo King
IBC Thinprinting

Page 17 & 51
Valsteam ADCA
I hope this journal guides you progressively, through the maze of activities and changes taking place in the pharmaceutical industry
IPI is also now active on social media. Follow us on:
or email info@senglobalcoms.com
80 INTERNATIONAL PHARMACEUTICAL INDUSTRY Spring 2023 Volume 15 Issue 1
Subscribe today at www.international-pharma.com
Advertisers Index
www.twitter.com/ipimediaworld www.facebook.com/ipimediaworld www.plus.google.com/+ipimediaworldmagazine www.ipimediaworld.tumblr.com
Thin paper really means thin paper! With our specialized printing technology, we can use paper grades from 29 g/m2 up to 40 g/m2

Lower paper substance and caliper also mean less space and weighat. With the Booklet your package is lighter and smaller in size.
All-in-one solution. All language versions of your IFU documents, warranty information and legal texts are printed in one Booklet that can be easily slipped into the product package.

UNIQUE ALL-IN -ONE BOOKLET
This picture tells it all: One stitched booklet vs. four folded leafletsboth with a space of 1,2 m2.
Streamlined packaging process: our Booklet works excellently on automated high-speed packaging lines.
• Only one stock item number for your Booklet – meaning less warehousing space, faster inventory cycle and no unnecessary reserves. The tied-up capital will be released for your other needs.
Lighter and smaller package means significant savings in shipping costs and increased sustainability of your business.
Heikki Suomala Director, Sales & Marketing Tel. +358 440 350268 info@thinprinting.fi


YOUR THINPRINTING SPECIALIST
Olli Kivijärvi Sales Manager Tel. +358 440 350269
With almost 40 years of experience, we are one of Europe’s leading printing houses specialized in printing on thin and ultrathin paper. We use only high-quality, totally wood free thin print paper from reliable manufacturers in Finland, and together with our solid logistics network, we guarantee fast and timely deliveries all over the world. Visit www.thinprinting.com
BENEFITS CONTACT
info@thinprinting.fi
With our solution you will have IFU and warranty documents in all languages printed in one thin paper booklet – leading to less warehousing space, simplified packaging process and significant cost savings in logistics.
Natoli Multi-Tip Tooling

Dramatically increase your production without adding a new tablet press or extending press run time. With innovative tooling designs, superior customer service, and incomparable construction, Natoli is your number one choice.

scan the QR to receive your free digital copy. Request your Multi-tip Tooling Guide Today,

natoli.com





















































































































































































































































































