HEALTH, LIFE & HIV

Participating in HIV clinical trials
A SMART+STRONG PUBLICATION JULY/AUGUST 2024 POZ.COM $3.99
Alicia Diggs
Follow Your Heart
IMPORTANT FACTS FOR BIKTARVY®
This is only a brief summary of important information about BIKTARVY® and does not replace talking to your healthcare provider about your condition and your treatment.
MOST IMPORTANT INFORMATION ABOUT BIKTARVY
BIKTARVY may cause serious side e ects, including:
` Worsening of hepatitis B (HBV) infection. Your healthcare provider will test you for HBV. If you have both HIV-1 and HBV, your HBV may suddenly get worse if you stop taking BIKTARVY. Do not stop taking BIKTARVY without fi rst talking to your healthcare provider, as they will need to check your health regularly for several months, and may give you HBV medicine.
ABOUT BIKTARVY
BIKTARVY is a complete, 1-pill, once-a-day prescription medicine used to treat HIV-1 in adults and children who weigh at least 55 pounds. It can either be used in people who have never taken HIV-1 medicines before, or people who are replacing their current HIV-1 medicines and whose healthcare provider determines they meet certain requirements.
BIKTARVY does not cure HIV-1 or AIDS. HIV-1 is the virus that causes AIDS.
Do NOT take BIKTARVY if you also take a medicine that contains:
` dofetilide
` rifampin
` any other medicines to treat HIV-1
BEFORE TAKING BIKTARVY
Tell your healthcare provider if you:
` Have or have had any kidney or liver problems, including hepatitis infection.
` Have any other health problems.
` Are pregnant or plan to become pregnant. It is not known if BIKTARVY can harm your unborn baby. Tell your healthcare provider if you become pregnant while taking BIKTARVY.
` Are breastfeeding (nursing) or plan to breastfeed. Talk to your healthcare provider about the risks of breastfeeding during treatment with BIKTARVY. Tell your healthcare provider about all the medicines you take:
` Keep a list that includes all prescription and over-thecounter medicines, antacids, laxatives, vitamins, and herbal supplements, and show it to your healthcare provider and pharmacist.
` BIKTARVY and other medicines may a ect each other. Ask your healthcare provider and pharmacist about medicines that interact with BIKTARVY, and ask if it is safe to take BIKTARVY with all your other medicines.
POSSIBLE SIDE EFFECTS OF BIKTARVY
BIKTARVY may cause serious side e ects, including:
` Those in the “Most Important Information About BIKTARVY” section.
` Changes in your immune system. Your immune system may get stronger and begin to fight infections that may have been hidden in your body. Tell your healthcare provider if you have any new symptoms after you start taking BIKTARVY.
` Kidney problems, including kidney failure. Your healthcare provider should do blood and urine tests to check your kidneys. If you develop new or worse kidney problems, they may tell you to stop taking BIKTARVY.
` Too much lactic acid in your blood (lactic acidosis), which is a serious but rare medical emergency that can lead to death. Tell your healthcare provider right away if you get these symptoms: weakness or being more tired than usual, unusual muscle pain, being short of breath or fast breathing, stomach pain with nausea and vomiting, cold or blue hands and feet, feel dizzy or lightheaded, or a fast or abnormal heartbeat.
` Severe liver problems , which in rare cases can lead to death. Tell your healthcare provider right away if you get these symptoms: skin or the white part of your eyes turns yellow, dark “tea-colored” urine, light-colored stools, loss of appetite for several days or longer, nausea, or stomach-area pain.
` The most common side e ects of BIKTARVY in clinical studies were diarrhea (6%), nausea (6%), and headache (5%).
These are not all the possible side e ects of BIKTARVY. Tell your healthcare provider right away if you have any new symptoms while taking BIKTARVY. You are encouraged to report negative side e ects of prescription drugs to the FDA. Visit www.FDA.gov/medwatch or call 1-800-FDA-1088.
Your healthcare provider will need to do tests to monitor your health before and during treatment with BIKTARVY.
HOW TO TAKE BIKTARVY
Take BIKTARVY 1 time each day with or without food.
GET MORE INFORMATION
` This is only a brief summary of important information about BIKTARVY. Talk to your healthcare provider or pharmacist to learn more.
` Go to BIKTARVY.com or call 1-800-GILEAD-5.
` If you need help paying for your medicine, visit BIKTARVY.com for program information.
(bik-TAR-vee) BIKTARVY, the BIKTARVY Logo, GILEAD, the GILEAD Logo, and KEEP BEING YOU are trademarks of Gilead Sciences, Inc., or its related companies. © 2024 Gilead Sciences, Inc. All rights reserved. US-BVYC-0411 03/24

People featured take BIKTARVY and are compensated by Gilead.





















#1 PRESCRIBED HIV TREATMENT*




Ask your healthcare provider if BIKTARVY is right for you. NOW THERE’S




MORE TO LOVE.


BIKTARVY® is now approved for more people than ever before.
BIKTARVY is a complete, 1-pill, once-a-day prescription medicine used to treat HIV-1 in certain adults. BIKTARVY does not cure HIV-1 or AIDS.
*Note: This information is an estimate derived from the use of information under license from the following IQVIA information service: IQVIA NPA Weekly, for the period week ending 04/19/2019 through week ending 05/19/2023. IQVIA expressly reserves all rights, including rights of copying, distribution, and republication.
Scan to learn more about the latest BIKTARVY update.
Please see Important Facts about BIKTARVY, including important warnings, on the previous page and at BIKTARVY.com.
EXCLUSIVELY ON POZ.COM
POZ BLOGS

Our roster of bloggers spans the diversity of the HIV community. Go to poz.com/blogs to read varying points of view from people living with the virus as well as from HIV-negative advocates. Join the conversation in the comments section. Visit the blogs to nd hope and inspiration from others.
D
POZ OPINIONS

Advocates, researchers, politicians, thought leaders and folks just like you all have ideas worth sharing. Go to poz.com/ opinions to read about topics such as living with HIV, improving care and treatment, increasing prevention e orts and ghting for social justice.
#UNDETECTABLE

The science is clear: People who have an undetectable viral load don’t transmit HIV sexually. In addition to keeping people healthy, e ective HIV treatment also means HIV prevention. Go to poz.com/undetectable for more.
POZ DIGITAL
Scan the QR code (le ) with your smartphone camera or go to poz.com/digital to view the current issue and read past issues online.

Long-term survivor Alicia Diggs resides in North Carolina.

24 HEART OF THE MATTER People living with HIV are crucial to clinical trials related to the virus. BY JAY LASSITER
28 GIVING IT A TRY Studies of new therapies, prevention methods and comorbidity management are key to improving quality of life for people with HIV. BY LIZ HIGHLEYMAN
3 FROM THE EDITOR Heart of Gold
4 POZ Q & A
Paul Edmonds is one of a handful of people cured of HIV and cancer after a stem cell transplant.
6 POZ PLANET
A new home for Lifebeat • HIV cannot spread in pools • Grindr allegedly shared HIV status • cosmetic injection transmits HIV • long COVID data easier to access • viral hepatitis deaths increase • POZ Stories: Ian Bicko • Everyday: HIV milestones
10 VOICES
Harold Phillips, NMAC’s deputy director of programs, takes us down memory lane • social justice advocate Matthew Rose helps us understand syndemics
14 SPOTLIGHT
GMHC’s 2024 AIDS Walk
16 NUTRITION & FITNESS
Chocolate tahini bars • add lemon to water
18 BASICS
HIV and your lungs
20 CARE & TREATMENT
More PrEP leads to fewer HIV diagnoses
• HIV reservoirs and viral rebound
• treatment for fatty liver disease • older people with HIV have unmet needs
22 RESEARCH NOTES
Ultra-long PrEP • delayed treatment • CRISPR disappoints • inadequate sleep
32 HEROES
HIV and U=U researcher Alison Rodger
CONTENTS POZ (ISSN 1075-5705) is published monthly except for the January/February, April/May, July/August and October/November issues ($19.97 for an 8-issue subscription) by Smart + Strong, 157 Columbus Avenue, Suite 525, New York, NY 10023. Periodicals postage paid at New York, NY, and additional mailing offices. Issue No. 277 POSTMASTER: Send address changes to POZ/Smart + Strong, 157 Columbus Avenue, Suite 525, New York, NY 10023. Copyright © 2024 CDM Publishing, LLC. All rights reserved. No part of this publication may be reproduced, stored in any retrieval system or transmitted, in any form by any means, electronic, mechanical, photocopying, recording or otherwise without the written permission of the publisher. Smart + Strong® and POZ® are registered trademarks of CDM Publishing, LLC.
COVER AND THIS PAGE: (DIGGS) NATALIA WEEDY; (MEGAPHONE AND SPEECH BUBBLES) THINKSTOCK; (MAGNIFIER) ISTOCK
EDITOR-IN-CHIEF
ORIOL R. GUTIERREZ JR.
MANAGING EDITOR
JENNIFER MORTON
DEPUTY EDITOR
TRENT STRAUBE
SCIENCE EDITOR
LIZ HIGHLEYMAN
COPY CHIEF
JOE MEJÍA
EDITORIAL ASSISTANT
LAURA SCHMIDT
ART DIRECTOR
DORIOT KIM
ART PRODUCTION MANAGER
MICHAEL HALLIDAY
CONTRIBUTING WRITERS
SHAWN DECKER, OLIVIA G. FORD, ALICIA GREEN, MARK S. KING, TIM MURPHY, MATHEW RODRIGUEZ, CHARLES SANCHEZ
CONTRIBUTING ARTISTS
JOAN LOBIS BROWN, LIZ DEFRAIN, ARI MICHELSON, JONATHAN TIMMES, BILL WADMAN
FOUNDER
SEAN STRUB
LEGACY ADVISER MEGAN STRUB
ADVISORY BOARD
A. CORNELIUS BAKER, GUILLERMO CHACÓN, SABINA HIRSHFIELD, PHD, KATHIE HIERS, TIM HORN, PAUL KAWATA, NAINA KHANNA, DANIEL TIETZ, MITCHELL WARREN
PRESS REQUESTS NEWS@POZ.COM
SUBSCRIPTIONS HTTP://ORDER.POZ.COM
UNITED STATES: 212-242-2163
SUBSCRIPTION@POZ.COM
FEEDBACK
EMAIL WEBSITE@POZ.COM OR EDITOR-IN-CHIEF@POZ.COM
SMART + STRONG
PRESIDENT AND COO
IAN E. ANDERSON
EDITORIAL DIRECTOR
ORIOL R. GUTIERREZ JR.
CHIEF TECHNOLOGY OFFICER
CHRISTIAN EVANS
VICE PRESIDENT, INTEGRATED SALES DIANE ANDERSON
INTEGRATED ADVERTISING MANAGER
JONATHAN GASKELL
INTEGRATED ADVERTISING COORDINATOR
SARAH PURSELL
SALES OFFICE
212-938-2051; SALES@POZ.COM
CDM PUBLISHING, LLC
CEO
JEREMY GRAYZEL
CONTROLLER
JOEL KAPLAN


Heart of Gold
IRECENTLY SWITCHED HIV
medications. My doctor had been suggesting I make this change for a while now. I was reluctant to do so due to my adherence to the “If it ain’t broke, don’t fix it” perspective. Over the years, that way of thinking has mostly served my health well.

Although nothing was technically “broke,” certain considerations finally surfaced that convinced me to switch. As another saying goes, “An ounce of prevention is worth a pound of cure.” Maintaining my undetectable viral load while also possibly staving off the potential for a problematic side effect was an irresistible combination.
I’m grateful to be living in a time when the choices for effective HIV treatment are manifold. I’m also humbled to have the privilege of access to excellent health care. The result is that making this switch has been, frankly speaking, boring. That said, I prefer uneventful to the hand-wringing that so many of us once faced.
Much of the credit for our current treatment choices goes to the countless people who’ve participated in clinical trials. Without such studies, we would literally have no effective HIV treatments, let alone improvements in such medications. Researchers and pharmaceutical companies usually get the spotlight, so kudos to trial participants.
This special issue, which highlights the latest in HIV treatment, takes a closer look at clinical trials. Alicia Diggs, our cover subject, is a great example of the link between people living with HIV and clinical trials. She’s a long-term survivor working toward a PhD in public health education. Go to page 24 to read about her advocacy.
Most recently, Diggs participated in the REPRIEVE trial, which tested statin use for people living with HIV who are at low to moderate risk for cardiovascular disease, a group that wouldn’t usually be prescribed statins. The results led the Department of Health and Human Services to recommend statin use by this group, a major change.
That’s just one of the latest outcomes from related clinical trials. Go to page 28 to read how studies of new treatments, prevention methods and comorbidity management are key to improving quality of life for people living with HIV or at risk for the virus.
One of the most consequential results from recent HIV-related clinical trials is the confirmation of the Undetectable Equals Untransmittable (U=U) message. Alison Rodger, a physician and a researcher, led the study that put the argument to rest—people living with HIV on effective treatment do not transmit the virus via sex. Go to page 32 for more.
The holy grail of HIV research remains finding a widely applicable cure. A handful of folks have been cured, but the process is too risky and costly to be practical. Nonetheless, the people who are now living virus-free are an inspiration to the rest of us hoping for our own cure. Go to page 4 to read our Q & A with Paul Edmonds, one of the few who are cured.
For the latest roster of HIV medications, go to the back of this issue for our annual quickreference chart comparing current options.


ORIOL R. GUTIERREZ JR. EDITOR-IN-CHIEF editor-in-chief@poz.com Want to read more from Oriol? Follow him on Twitter @oriolgutierrez and check out blogs.poz.com/oriol.
FROM THE EDITOR
poz.com JULY/AUGUST 2024 POZ 3 (GUTIERREZ) JOAN LOBIS BROWN; (ILLUSTRATION) ISTOCK POZ.COM/X POZ.COM/FACEBOOK POZ.COM/INSTAGRAM

SHARING THE HOPE
Paul Edmonds is one of a handful of people cured of HIV and cancer after a stem cell transplant.
PAUL EDMONDS, 68, OF DESERT HOT SPRINGS, CALIFORNIA, is one of only five people to be cured of HIV after a stem cell transplant for cancer treatment from a donor with a rare genetic mutation called CCR5-delta32 that prevents the virus from entering cells.
Edmonds is the oldest person—and the longest living with HIV—to receive this type of transplant. His case was first presented at the 2022 International AIDS Conference. At the time, he chose to remain anonymous and was known as the City of Hope Patient. Today, his leukemia is still in remission, and he remains free of HIV three years after stopping antiretroviral therapy.
The stem cell transplant procedure is too risky for most people living with HIV who do not have cancer and are doing well on antiretroviral treatment, but the handful of functional cures offer clues for researchers working to develop more widely applicable approaches.
You were diagnosed with HIV in 1988. What was it like living with HIV in the early years of the epidemic?
It was very scary. Around 1980, people began getting sick with what was called the “gay cancer.” People were afraid of each other. It was very stigmatizing. There were many nights of marches and protests to get the government to do something. I received an AIDS diagnosis because of my low T-cell count. At the time, most people were living no longer than two years after diagnosis.
My husband, Arnie House, who I met in 1992, is also HIV positive. We’ve taken care of each other over the years, accompanying each other to doctor appoint-
ments, collaborating on healthy diets and exercise regimens and discussing the pros and cons of different medications. I tried almost every drug that came out, including AZT, but the side effects of the early meds were awful. I tried to stay strong, live day-to-day and not allow myself to think about the worst-case scenario. I started combination treatment when it became available in the mid-1990s, and my HIV viral load stayed undetectable for many years.
How did you find a stem cell donor with the rare CCR5-delta32 mutation?
I was diagnosed with acute myeloid leukemia (AML) in 2018. I think I was diagnosed early because I saw my HIV doctor every three months, and he saw my bloodwork plummet suddenly. I felt like I had been here before with a near-fatal diagnosis. I was referred to City of Hope, which pioneered stem cell transplants for people with HIV. I had heard of Timothy Ray Brown,
4 POZ JULY/AUGUST 2024 poz.com
Left to right: Adam Castillejo, Marc Franke and Paul Edmonds
BY LIZ HIGHLEYMAN POZ Q & A VINA CRISTOBAL, UNIVERSITY OF HAWAI’I JOHN A. BURNS SCHOOL OF MEDICINE
the Berlin Patient, who had the same type of leukemia and was the first person cured of HIV after a stem cell transplant from a donor with the CCR5-delta32 mutation. My doctors at City of Hope told me from the beginning that they would look for a donor with the mutation. They searched the Be the Match donor registry and found someone within a month. But first, my cancer had to go into remission, which took three rounds of chemotherapy. By that time, the original donor was no longer available. Fortunately, they found a second donor with the same mutation. I felt as if I had won the lottery.
At the time, Brown was the only person who had been cured of HIV with this kind of transplant, but I had a lot of trust in my doctors. While I was in the hospital getting the AML in remission, the news about the London Patient [later identified as Adam Castillejo], a second person cured with a CCR5-delta32 transplant, came out.
What was it like receiving the stem cell transplant?
I got the transplant in February 2019. The transplant itself was a simple infusion. Unlike Brown, I received reduced-intensity chemotherapy and no radiation because of my age. I had heard from many people how difficult chemotherapy was, but it wasn’t as bad as I thought it could be—certainly not as bad as the years on early HIV meds. I started feeling good rather quickly, but I stayed at a hotel near the hospital because I lived too far away should something go wrong. Arnie and friends from across the country came to stay with me during these two months because I couldn’t be by myself. I have a great support system. I had only minor graft-versus-host disease [when donor cells attack the recipient’s body], with mouth sores and dry eyes. Overall, I’ve been very fortunate.
How did you and your doctors decide to stop antiretroviral treatment?
In March 2021, my AML was still in remission, and my HIV remained suppressed, so we decided to stop my antiretroviral therapy. We were planning to do
so one year after the transplant, but then the COVID-19 pandemic hit. I wanted to wait until I got my COVID vaccine, and there was some talk early on that HIV meds might be offering some protection against COVID. This decision was completely mine. I felt no pressure to stop antiretroviral therapy until I was ready.
In the beginning, City of Hope sent someone to my house to do lab tests every week. Now I get my HIV viral load monitored every three months. Repeated tests have shown that I have no detectable HIV in peripheral blood cells [a marker of the latent viral reservoir] or gut biopsies from colonoscopies. A year and a half later, I still had not experienced viral rebound, and my doctors presented my case at the International AIDS Conference in July 2022.

How did you decide to go public with your cure?
In the spring of 2023, I appeared on Good Morning America and in The Washington Post, New York Post and ABC News. I had always planned to go public with my story if the stem cell transplant was successful. I grew up in a small town in Georgia and had a difficult time coming out as gay. I thought if I stayed anonymous, it would feel like going back into the closet—and I will never return to the closet. I remember how I felt when the news about Timothy Brown came out. For the first time, I felt like a cure for HIV might be possible. My story is too important to keep to myself. The researchers and people affected by HIV deserve to hear a story of hope and resilience.
What do you want to accomplish as one of the few people cured of HIV? When I was diagnosed with HIV in 1988, I thought it was a death sentence. I never thought I would live to see the day that I no longer have HIV. But I’ve lived for half of my life with HIV. I’m still very connected to the community, and I feel like I always will be. I am going to be a strong advocate for HIV cure research. I also want to encourage people to sign up to be stem cell donors with Be the Match.
I’ve met two of the other people cured after stem cell transplants, Adam Castillejo [the London Patient] and Marc Franke [the Düsseldorf Patient]. We have Zoom get-togethers, and we were all together in public for the first time at the Hawai’i to Zero Conference in
Honolulu last September. I sit on the community advisory board of RID-HIV, one of the 10 Martin Delaney Collaboratories for HIV Cure Research. I have been invited to join NMAC’s HIV 50+ Strong and Healthy Program to advocate for people aging with HIV, and I will be attending the International AIDS Society conference in Munich this summer, along with Adam and Marc, and the U.S. Conference on HIV/AIDS in New Orleans in September.
I want researchers to study me and the others who were cured, like they did with Timothy Brown, so we can find a cure that’s accessible to all. We need to challenge stigma and ideologies that hinder progress and work as a global community, transcending borders to eradicate this virus. Q
poz.com JULY/AUGUST 2024 POZ 5
COURTESY OF CITY OF HOPE
Paul Edmonds
A NEW HOME FOR LIFEBEAT
The program is now part of the Elizabeth Taylor AIDS Foundation.
Music was the message at the Elizabeth Taylor AIDS Foundation’s (ETAF) second annual New York Dinner, held May 8 at the legendary Rainbow Room at Rockefeller Plaza.

The event honored Arthur Fogel, who heads global touring and concerts at Live Nation Entertainment, and marked the relaunch of Lifebeat as an HIV program of ETAF.
Founded in 1992 as Lifebeat—The Music Industry Fights AIDS, the nonprofit raised awareness about HIV and safer sex among young people via music events, fundraisers, public service announcements, digital programs and more.
In December 2023, ETAF announced that Lifebeat would become a program of the foundation.
ElizabethTaylorAIDSFoundation.org describes Lifebeat as follows:
“On the ground at major tours, special events and festivals, and within broadcast, digital, social, and print campaigns, we engage at-risk youths about safe sex, HIV prevention and the services that support them regardless of status, gender, or sexuality.”
Model, actress and author Dominique Jackson hosted

Rosie Perez (le ) and Arthur Fogel
the evening, while actress Rosie Perez presented Fogel with the special Lifebeat tribute. Sponsored by Gilead Sciences, the evening featured a musical performance by Talia Rae.
“Arthur Fogel is no stranger to orchestrating unforgettable experiences, having helped to mastermind awe-inspiring tours for artists such as Beyoncé, U2, Sting, Lady Gaga and Madonna. However, tonight, we spotlight Arthur for his dedication to the fight against AIDS, particularly through his steadfast support of Lifebeat—acting on their board for a period of time and supporting financially in various ways,” said Alex Kalomparis, Gilead’s senior vice president of public affairs, in an ETAF press release. “In honoring Arthur Fogel, we not only celebrate his remarkable achievements but also acknowledge his impact on the journey toward eradicating AIDS.”
“I hope that tonight has delivered some meaningful financial support to the foundation [and to] Lifebeat programs going forward,” added Fogel. —Trent Straube
HIV CANNOT SPREAD IN POOLS
Online comments in Texas shared misinformation.
Some Texans recently took to Facebook to amplify rumors about AIDS spreading in a community swimming pool.
To be clear, no cases of HIV transmission via swimming pools have ever been recorded, according to the University of Rochester Medical Center.
The Facebook post read: “HOA [homeowners association] Pool Infected with AIDS. 4th person positive. 4th person tested positive this week. Make sure everyone that visited this pool gets checked. The pool wasn’t cared for and chemicals were not used.”
While the exact location was not disclosed, the area circled on a map is in Arlington, Texas.
The Texas Department of State Health Services received a complaint on April 29 about an HOA pool’s alleged link to positive HIV results, according to Reuters, which factchecked the allegation. An inspection found no unsanitary conditions in the pool water or on the pool deck.

Misinformation surrounding HIV transmission has circulated since the epidemic emerged in the early 1980s. In the United States, HIV is most commonly passed from person to person via sexual contact involving the exchange of certain body fluids. Untreated HIV can progress to AIDS.
Not only is HIV not transmitted via swimming pools, but the chemicals added to the water help protect against the spread of other pathogens.
“Chlorine kills germs found in blood (such as hepatitis B and HIV),” says the Centers for Disease Control and Prevention (CDC) on its website. “CDC is not aware of any instances in which a person has become infected with blood-borne germs after being exposed to a blood spill in a pool.”
HIV can neither survive for very long nor reproduce outside the body. —Laura Schmidt
UPDATES ON HIV & AIDS POZ PLANET 6 POZ JULY/AUGUST 2024 poz.com
(PEREZ/FOGEL) COURTESY OF EUGENE GOLOGURSKY/GETTY IMAGES FOR THE ELIZABETH TAYLOR AIDS FOUNDATION (POOL) ISTOCK
Grindr Allegedly Shared HIV Status
The gay dating app company was sued in London.
A lawsuit filed in London’s High Court alleges that gay dating app Grindr shared users’ personal information, including HIV status and date of most recent HIV test, The Guardian reports. According to the lawsuit filed by the firm Austen Hays, Grindr shared the private data with two advertising companies without users’ consent.
About 670 people in the United Kingdom signed on to the data protection lawsuit, but thousands more may join the case. The alleged breaches of personal data took place between April 2018 and April 2020.
In a statement published in Reuters, a Grindr spokesperson said, “Grindr has never shared user-reported health information for ‘commercial purposes’ and has never monetized such information.”
“We are committed to protecting our users’ data and complying with all applicable data privacy regulations, including in the U.K.,” a Grindr spokesperson told The Guardian. “We are proud of our global privacy program and take privacy extremely seriously. We intend to respond vigorously to this claim, which appears to be based on a mischaracterization of practices from more than four years ago, prior to early 2020.”
Austen Hays managing director Chaya Hanoomanjee is leading the lawsuit. “Our clients have experienced significant distress over their highly sensitive and private information being shared without their consent, and many have suffered feelings of fear, embarrassment and anxiety as a result,” Hanoomanjee said. “Grindr owes it to the LGBTQ+ community it serves to compensate those whose data has been compromised and have suffered distress as a result, and to ensure all its users are safe while using the app, wherever they are, without fear that their data might be shared with third parties.”
Launched in 2009 and based in Los Angeles, Grindr describes itself as “the largest social networking app for gay, bi, trans and queer people. We have millions of daily users who use our location-based technology in almost every country in every corner of the planet.”
In March 2023, Grindr made POZ headlines when it teamed up with the Centers for Disease Control and Prevention’s Together TakeMeHome program to deliver 1 million free home HIV tests. —TS

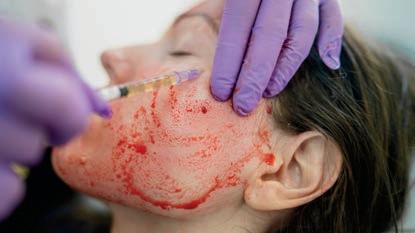
Cosmetic Injection Transmits HIV
Cases linked to a New Mexico spa mark
the first
documented instances.
A cluster of HIV cases from 2018 has been linked to vampire facials, a type of microneedling procedure, at a now-shuttered medical spa in Albuquerque. At least three women contracted HIV in what federal health researchers say is the first documented instance of HIV transmission through cosmetic injection, according to findings in Morbidity and Mortality Weekly Report (MMWR), a publication of the Centers for Disease Control and Prevention (CDC).
The vampire facial HIV story first made headlines in 2019, when the New Mexico Department of Health (NMDOH) notified the public that a woman tested positive for HIV but had no known risk factors except for exposure to needles during a vampire facial the previous year at VIP Spa.
A vampire facial is a cosmetic procedure that involves injecting nutrient-rich plasma into the skin on the face to achieve a more youthful appearance. The plasma is typically collected from an individual’s own blood before the facial.
The spa had closed in September 2018 because of unsafe practices and didn’t keep precise records. Nonetheless, NMDOH and CDC health experts cross-checked available documentation to identify cases and offered former spa clients free and confidential testing. In April 2019, NMDOH announced that two clients had been diagnosed with the same strain of HIV.



In the 2024 MMWR report, CDC officials provided more details about the investigation. In 2021, the New Mexico Attorney General’s office filed 24 felony charges against the former owner of VIP Spa. In July 2023, New Mexico health officials announced that a third case of HIV had been linked to vampire facials at the Albuquerque establishment. Officials also reminded former clients to get tested for HIV as well as hepatitis B and C.
A properly performed vampire facial shouldn’t expose individuals to HIV, hepatitis or other bloodborne diseases. But if the equipment used during the procedure, such as a microneedling pen, is not disposed of correctly or sterilized between facials, the risk of exposure to such diseases is high. —TS

poz.com JULY/AUGUST 2024 POZ 7 ALL IMAGES: ISTOCK (MODEL USED FOR ILLUSTRATIVE PURPOSES ONLY)

Long COVID Data Easier to Access
Deidentified data on thousands of adults are now available.
Secure data from more than 14,000 adults who participate in National Institutes of Health (NIH) observational research on long COVID are now available to authorized researchers through BioData Catalyst (BDC). BDC is a cloud-based ecosystem developed by the National Heart, Lung, and Blood Institute (NHLBI), part of the NIH, to accelerate research on heart, lung, blood and sleep disorders.
The research on long COVID—broadly defined as signs, symptoms or conditions that persist or develop for at least four weeks after an infection with SARS-CoV-2, the virus that causes COVID—is provided through the NIH Researching COVID to Enhance Recovery (NIH RECOVER) Initiative.
By giving researchers access to secure data, analysis tools and resources, the BDC ecosystem aims to spur scientific innovation, collaboration and discovery, while providing a platform for sharing data and validating results. The addition of RECOVER data to BDC can help investigators identify and explore long COVID connections that may benefit from or inform future studies.
Authorized researchers can now request access to a subset of data on adults in the observational RECOVER cohort. These data include information from more than 92,000 study visits collected between October 29, 2021, and September 15, 2023, at 79 locations throughout the United States. New RECOVER data, including data from other studies, will be added to BDC at regular intervals.
As investigators seek to better understand, diagnose and treat long COVID, many critical questions remain. By making RECOVER data more accessible by adding it to a central ecosystem, experts aim to find answers sooner. —NIH
VIRAL HEPATITIS DEATHS ARE INCREASING
Worldwide, hepatitis B and C claim 3,500 lives each day.
According to the World Health Organization (WHO) 2024 Global Hepatitis Report, the number of lives lost due to viral hepatitis is increasing. Viral hepatitis is the second leading infectious cause of death globally— with 1.3 million deaths per year, the same as tuberculosis, a top infectious killer.
The report, released at the World Hepatitis Summit, highlights that despite better tools for diagnosis and treatment and decreasing product prices, testing and treatment coverage rates have stalled. But reaching the WHO’s hepatitis elimination goal by 2030 should still be achievable, if swift actions are taken now.
New data from 187 countries show that the estimated number of deaths from viral hepatitis increased from 1.1 million in 2019 to 1.3 million in 2022. Of these, 83% were caused by hepatitis B, and 17% by hepatitis C. Every day, 3,500 people die globally due to hepatitis B and C infections.
“This report paints a troubling picture: Despite progress globally in preventing hepatitis infections, deaths are rising because far too few people with hepatitis are being diagnosed and treated,” said WHO Director-General Tedros Adhanom Ghebreyesus, PhD. “WHO is committed to supporting countries to use all the tools at their disposal—at access prices—to save lives and turn this trend around.”
Updated WHO estimates indicate that 254 million people were living with hepatitis B and 50 million with hepatitis C in 2022. Half the burden of chronic hepatitis B and C infections is among people 30 to 54 years old, with 12% among children under 18 years of age. Men account for 58% of all cases.
New incidence estimates indicate a slight decrease compared to 2019, but the overall incidence of viral hepatitis remains high. In 2022, there were 2.2 million new infections, down from 2.5 million in 2019.
These include 1.2 million new hepatitis B infections and nearly 1 million new hepatitis C infections. More than 6,000 people are getting newly infected with viral hepatitis each day.
The revised estimates are derived from enhanced data from national prevalence surveys. They also indicate that prevention measures such as immunization and safe injections, along with the expansion of hepatitis C treatment, have contributed to reducing the incidence.
—WHO





UPDATES ON HIV & AIDS POZ PLANET BOTH IMAGES: ISTOCK 8 POZ JULY/AUGUST 2024 poz.com
The Best Medicine
Finding out I had HIV forced me to look at myself in the mirror and make real changes.
By Ian Bicko
I found out I was HIV positive in February 2017 in my last semester at the University of South Alabama in Mobile. Ironically, I was taking a class on global health and chose HIV/AIDS as my research topic. I chose it before I knew about my own status. Life is funny. The research was good because it forced me to confront some realities and learn my new normal. I think I reached a place of acceptance earlier than I would have otherwise.
It took at least nine months to find out that I was living with HIV. In the spring of 2016, the guy I had been seeing decided to cut off our relationship. I thought we were exclusive. Meanwhile, I had a three-month research/mission trip to Haiti planned that summer.
Within my first couple of weeks in Haiti, I experienced what I now believe to have been a pretty terrible experience with seroconversion. I was very, very sick for over a week, but I assumed I had some form of malaria or food poisoning. I eventually recovered, and the thought of having been exposed to HIV never
EVERYDAY
July
crossed my mind. Fast-forward to early 2017, and I was experiencing mild symptoms. I generally got tested regularly but hadn’t since I returned from Haiti.
I’ll never forget receiving the news. I don’t know how long I sat in that cramped room at the Mobile Public Health Department. The windows were frosted glass, and I just stared at the blank window for I don’t know how long. They called me and told me to come in to go over my results. I knew that wasn’t good because usually, I got a quick phone call to tell me my results were clear. I sat down, and I was handed a yellow piece of paper: syphilis. My heart dropped; I was so relieved. I didn’t see the pink piece of paper that the man still held in his hand.
As I look back, I feel like, in many ways, the day I was diagnosed is when I began living. Before my diagnosis, I was barely out of the closet. I didn’t like myself. Hated myself even. I didn’t know how to reconcile my sexuality with my faith. I was a very scared and, honestly,
depressed person. Being Christian and a closeted gay man made me an excellent liar. I was never actually myself in my first 25 years of life. Finding out I had HIV forced me to look at myself in the mirror and make real changes. I found volleyball, and it ended up being the best medicine. At first, it was where I could be myself. I could forget about my diagnosis. I could be competitive and work out all of my emotions. I’ve continued to pursue beach volleyball to the highest level I can, and I’ve gotten pretty far. Recently, I was in a tournament where I beat a U.S. Olympian and multiple top 10 players in the country—my best showing. Seven years ago, I never would have thought I’d have reached this level of success.


I’m finally in a place where I want to combine my sport with my advocacy work. I’d love to use my sport as an avenue to reduce HIV stigma. I’m currently based in New Orleans and serve as an ambassador with a local initiative called Bounce to Zero. I’ve loved being involved locally, but I’m ready for what’s next— whatever it is.
Read other POZ Stories or share your own at poz.com/stories.
These dates represent milestones in the HIV epidemic. Visit poz.com/aidsiseveryday to learn more about the history of HIV and AIDS. BY JENNIFER
GMHC (Gay Men’s Health Crisis) publishes a newsletter to address questions relating to GRID (gayrelated immune deficiency). (1982)


1 13 11
MADONNA performs a benefit concert at Madison Square Garden in New York City and raises $400,000 for amfAR, the Foundation for AIDS Research. The show, an addition to her Who’s That Girl summer tour, commemorates her friend Martin Burgoyne. (1987)






4 20 August 21 25

BACK ON BOARD: GREG LOUGANIS, a documentary about the Olympic diver’s life, premieres on HBO. (2015)


Louganis presents
Greg Louganis presents RYAN WHITE the gold medal he won in the 3-meter springboard at the Pan American Games. (1987)










poz.com JULY/AUGUST 2024 POZ 9 (BICKO) COURTESY OF SARAH DEMUTH; (MADONNA) WIKIPEDIA UPDATES ON HIV/AIDS EVERYDAY
SOUTHERN HIV/AIDS AWARENESS DAY NATIONAL FAITH HIV/AIDS AWARENESS DAY
MORTON
ZERO HIV STIGMA DAY
Ian Bicko

MEMORY LANE
In an opinion piece titled “Harold Phillips Takes Us Down Memory Lane,” NMAC’s new deputy director of programs looks back on his HIV advocacy and urges for renewed efforts in 2024. Below is an edited excerpt.
WHEN I RETURNED TO NMAC in April a er exactly 30 years, I went down memory lane. April 1994 was a busy time for me. I had turned in my master’s thesis at the University of North Carolina at Chapel Hill and was running the review process for the state health department’s first Housing Opportunities for Persons With AIDS grant applicants and studying for finals, and my friend Phil from Maryland had died of an AIDS-related illness.
I felt compelled to find time and make the journey from Chapel Hill to Washington, DC, for his memorial service. Phil’s service was one of several that I and others attended in the 1990s, the early days of the epidemic before effective HIV treatment was available.
While in DC that weekend in April 1994, I saw an ad in the Washington Blade. The National Minority AIDS Council (now known as NMAC) was hiring technical assistance specialists to provide capacity-building assistance to community-based organizations providing HIV prevention and treatment services for people of color.
Between the last week of class and final exams, I returned to DC for an interview with Paul Kawata, NMAC’s executive director, and Pablo Manuel
Magaz, who was NMAC’s director of technical assistance. It changed my life and altered my journey into HIV, public health and health policy.
What I remember most about working at NMAC from 1994 to 1997 was the pace and the passion. We were the Fast and the Furious before it was a movie franchise: fast-paced because we knew lives were at stake and furious and passionate because all of us had lost friends and lovers, like Phil, Dennis, Marc, Janet and Tracey.
The loss was enormous. We felt the injustice and knew they deserved better access to HIV research, testing, prevention, care and treatment. Dr. Martin Luther King Jr. once said, “Of all the forms of inequality, injustice in health care is the most shocking and inhumane.” We saw the inequality and demanded humane responses from the government and society with a sense of urgency, o en without apology.
Leaving NMAC in 1997, I joined the Health Resources and Services Administration and remained in the federal government until January 2024 to advance HIV programs and policy. My decades of fast-paced and passionate work in and with the government focused on creating and making systems work better for our communities—in memory
of those we lost and those harmed by the government and society.
While leading the development of our National HIV/AIDS Strategy, I included a sense of urgency, passion and a need to address injustice in this national plan. Our current national strategy calls for us to accelerate our efforts, use science and data and focus on priority populations. It acknowledges that we cannot end the HIV epidemic if we do not address the HIV disparities among communities of color.
As I began a new, yet familiar, journey at NMAC in April, we convened the eighth annual Biomedical HIV Prevention Summit in Seattle to highlight the implementation of biomedical tools in our prevention and treatment efforts. Centered on research, science and data, the summit’s goal was to bring science back to the community and create access to the biomedical tools to help end the HIV epidemic.
I am excited to be back at NMAC. I look forward to working with government, academia, the private sector and the community in this new role. I am older and wiser, but, 30 years later, I have a greater sense of urgency, passion and fury at the injustices and health disparities that still persist. I hope you will join me. Q
BLOGS AND OPINIONS FROM POZ.COM VOICES ISTOCK 10 POZ JULY/AUGUST 2024 poz.com

SYNDEMIC APPROACHES
In a post titled “Understanding Syndemics: A Pathway to Ending the HIV Epidemic,” POZ blogger and social justice advocate Matthew Rose underscores that multiple challenges lie ahead. Below is an edited excerpt.
LET’S DELVE INTO A CONCEPT that holds profound implications for our lives: syndemics. O en discussed in public health, syndemics refer to the co-occurrence of two or more health or social issues that collectively impact individuals or communities. Simply put, it’s not just one challenge we’re facing but a tangled web of interconnected issues affecting our well-being.
Imagine living in a community where HIV rates are high, but access to health care and stable housing is low. These factors aren’t isolated. Recognizing these interconnected challenges is crucial for cra ing effective programs and policies that address the root causes of health disparities.
In recent years, there has been a growing emphasis on syndemic approaches in public health, particularly in the context of combating HIV. Governments and organizations are recognizing that tackling HIV requires more than just focusing on the virus itself—it demands a holistic understanding of the social, economic and environmental factors that influence health outcomes.
So why should you care about syndemics? Understanding syndemics is critical to understanding the broader context of health and wellness. By
recognizing the interconnectedness of health issues, we can develop more effective strategies for everyone.
Take, for example, the COVID-19 pandemic and its impact on HIV. Both are public health crises that have disproportionately affected vulnerable populations. By adopting a syndemic approach, policymakers can identify shared vulnerabilities and design interventions that address multiple challenges simultaneously.
Understanding syndemics is also about finding solutions. We can create more equitable care and support systems by addressing the underlying social and structural factors contributing to health disparities. This means investing in affordable housing, expanding access to health care and combating stigma and discrimination.
At the heart of the syndemic approach is recognizing that our health is shaped by more than just biology— it’s influenced by a complex interplay of social, economic and environmental factors. We can build healthier, more resilient communities for all.
ADVOCATING FOR SYNDEMIC FRAMEWORKS
So how can you get involved in advocating for syndemic approaches at the
local and state levels? Here are a few ways to make an impact:
1. Raise awareness. Start by educating yourself and others about syndemics and their impact on health outcomes. Share information with your community and encourage discussions about the interconnected nature of health issues.
2. Advocate for policy change. Contact your elected representatives and urge them to support policies that address the social determinants of health, such as affordable housing, access to health care and antidiscrimination measures.
3. Support community-based organizations. Get involved with local groups working to address syndemic challenges in your community. Your support can make a difference through volunteering, fundraising or advocacy.
4. Promote health equity. Advocate for policies and programs that prioritize populations disproportionately affected by syndemics, including communities of color, LGBTQ individuals and people living in poverty.
By coming together to address syndemics, we can create communities where everyone has the opportunity to thrive. Let’s work together to end the HIV epidemic and build a future where health equity is a reality for all. Q
poz.com JULY/AUGUST 2024 POZ 13 ISTOCK BLOGS AND OPINIONS FROM POZ.COM VOICES
AIDS WALK NEW YORK
On May 19, 2024, the 39th annual AIDS Walk New York brought together 10,000 people in Central Park to raise money for primary beneficiary GMHC (formerly Gay Men’s Health Crisis) as well as other HIV and AIDS service organizations in the New York, New Jersey and Connecticut tristate area. Presented by sponsor ViiV Healthcare and featuring premier sponsor Gilead Sciences, the event featured a four-mile run as well as a walk, performances and rousing speeches. The walk’s theme this year was “Stride Against Stigma.”
The 2024 AIDS Walk took place as the city is poised to cut $5.3 million in spending on HIV education and outreach programs to comply with Mayor Eric Adams’s directive to slash the New York City Department of Health & Mental Hygiene’s budget by $75 million overall in fiscal year 2025. In addition to affecting GMHC, this decision would impact other stalwart HIV service providers, including Housing Works and Planned Parenthood. Despite the looming news—or perhaps in bold defiance of it—diverse groups and individuals of all ages (young people seemed especially well represented this year) donned their walking shoes and brightest smiles to fight HIV stigma and raise money during what is arguably one of the most visible and enduring AIDS fundraisers in the world.




1. At Central Park’s bandshell, GMHC team members proudly announce the total amount raised by this year’s walk. 2. Wearing a Keith Haring–inspired dress she made famous on an episode of RuPaul’s Drag Race during which she came out as HIV positive, contestant Q poses with (left to right) New York City Council members Erick Bottcher and Gale Brewer and New York State Assemblyman Tony Simone . 3. Kecia Lewis , a star of Alicia Keys’s musical Hell’s Kitchen, sings Keys’s “Author of Forever,” as Larry Mass, MD, one of the six gay men who founded GMHC in 1981, looks on. 4. Members of AIDS Walk team Caribbean Equality Project promote the message “Knowing Matters,” underscoring the importance of getting tested for HIV.
BY JOE MEJÍA SPOTLIGHT 14 POZ JULY/AUGUST 2024 poz.com
3 2 4 1



5. GMHC’s Luna Ortiz and AIDS Healthcare Foundation’s Jomil Luna are all smiles as they walk while wearing GMHC’s signature colors. 6. Members of the Legendary Gorgeous House of Gucci repped the house and ballroom community. 7. Brothers of La Unidad Latina Lambda Upsilon Lambda Fraternity show are united against AIDS. 8. He might have died in 2020, but that didn’t stop GMHC founding member Larry Kramer from looming large at the AIDS Walk. 9. Francine Goldstein has raised nearly a million dollars since first participating in the fundraiser in 1988, the year her best friend died of AIDS. This year, Goldstein raised more money than any other individual: a whopping $82, 858.


(1) GMHC/AIDSWALK/INSTAGRAM; (2) TONYWSIMONE/INSTAGRAM; (3) STEPHEN NACHAMIE/FACEBOOK; (4) CARIBBEANEQUALITYROJECT/INSTAGRAM; (5) JOMIL LUNA/FACEBOOK; (6) RANAREEVES/INSTAGRAM; (7) LUL-PACE/INSTAGRAM; (8) SLO.REEDER/INSTAGRAM; (9) CBSNEWS.COM
Send your event photos to POZ at website@poz.com or tag us on Facebook, Instagram or Twitter. For a list of community events, visit poz.com/calendar. poz.com JULY/AUGUST 2024 POZ 15 6 9 7 5 8


CHOCOLATE TAHINI BARS
These treats are incredibly easy to throw together.
A SINGLE SERVING OF THESE RICH, filling little treats will seriously satisfy your sweet tooth while nourishing you. Not only do the toasted sesame seeds in the tahini make the bars deliciously nutty-tasting, but they also add an abundance of nutrients and minerals.
SERVINGS: 12 / INGREDIENTS: 6 / PREP: 15 MINUTES
INGREDIENTS
1½ cups graham cracker crumbs
DIRECTIONS
¾ cup confectioner’s sugar
1 cup tahini
¼ cup coconut oil, melted
1. Grease an 8-by-8-inch glass baking dish.
1 cup dark chocolate chips
1 cup heavy cream
2. In a large bowl, mix together graham cracker crumbs, confectioner’s sugar, tahini and coconut oil. Pour the mixture into the dish and flatten into an even layer.
3. Place chocolate chips into a large bowl. In a small pot, bring cream to a simmer. Pour the cream over the chocolate and stir the chocolate until melted and smooth. Pour the chocolate over the tahini mixture and spread into an even layer.
4. Cover with plastic wrap and place in the refrigerator for about 45 minutes, until the chocolate is set.
5. Cut into 12 squares and serve.
NUTRITION FACTS (per serving)
Calories: 380; fat: 31 g; fat: 14 g; polyunsaturated fat: monounsaturated fat: carbohydrates: 23 g; fiber: 4 g; protein: 5 g; sodium: 81 mg

©2024 Fred Hutchinson Cancer Research Center, a ����F�����QRQSURƓW� organization. Used with permission.
ADD LEMON TO YOUR WATER
By Craig Ramsay
Here are some slim-down tips:
• Adding lemon to your water has many benefits. It helps burn more calories. It also relieves many digestion problems.
• Lemon’s antiaging properties make it great for the skin.
• Have a toothache or bad breath? Lemon water can help.
• If you have tonsil issues, it’s good to gargle lemon water frequently.
• Lemons are high in potassium, which can help control high blood pressure.
• Lemons can also help with respiratory issues, such as asthma and other such problems.




• Lemons are a natural diuretic and can flush out bacteria and toxins.
Craig Ramsay is a fitness expert, an author and a winner of season 8 of The Amazing Race Canada . Follow him on Instagram at @craigramsayfit.





16 POZ JULY/AUGUST 2024 poz.com (TAHINI BARS) COURTESY OF COOK FOR YOUR LIFE; (TAHINI AND CHOCOLATE) ISTOCK; (BOARDS) ISTOCK; (RAMSAY) COURTESY OF CRAIG RAMSAY
ADVICE ON DIET AND EXERCISE NUTRITION & FITNESS

HELP CANCER PATIENTS MAKE NEW MEMORIES FOR YEARS TO COME Stand Up To Cancer, with support from Visit Myrtle Beach, is working to push WYVNYLZZ�MVY^HYK�[V�ÄUK�UL^�HUK�IL[[LY treatments so cancer patients can thrive. Join this mission at StandUpToCancer.org
Matthew McConaughey Stand Up To Cancer Ambassador
Photo by John Russo Background
STAND UP TO CANCER IS A 501(C)(3) CHARITABLE ORGANIZATION.
Photo by Bobby Altman
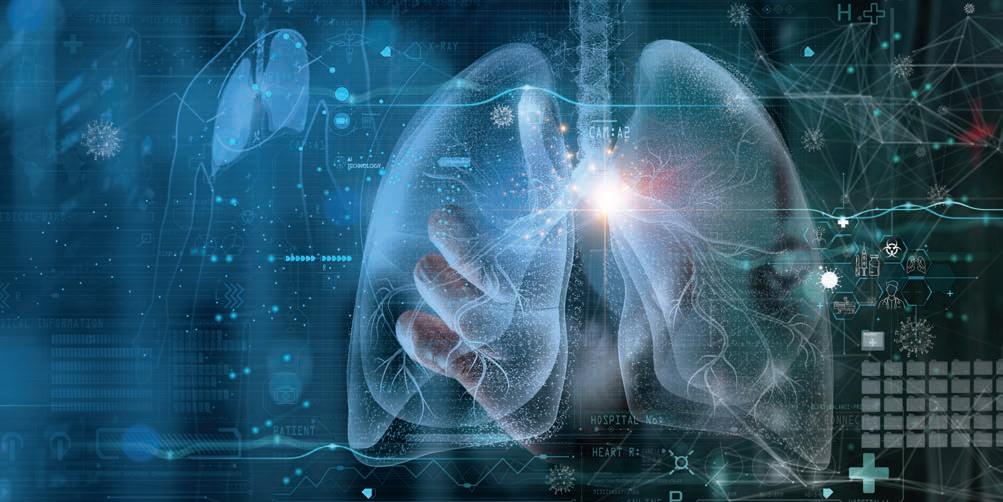
HIV AND YOUR LUNGS
Smoking cessation, exercise and vaccines can help keep your lungs healthy.
HEALTHY LUNGS ARE A KEY to good quality of life. With e ective antiretroviral therapy, people who stay on treatment and achieve viral suppression are no longer at high risk for AIDS-de ning opportunistic infections (OIs) that a ect the lungs, such as Pneumocystis pneumonia. However, people with a low CD4 T-cell count remain susceptible. Even with good treatment, people living with HIV have higher rates of certain lung diseases, such as chronic obstructive pulmonary disease (COPD). COPD, including emphysema and chronic bronchitis, causes blockage of air ow and makes breathing more di cult. COPD risk increases with age, and cases are rising among HIV-positive people as they live longer. Chronic in ammation and a higher smoking rate contribute to the elevated risk.
Research shows that people with HIV are more likely to smoke and less likely to quit than their HIV-negative peers. In addition to COPD, smoking also raises the risk for cardiovascular disease, cancer and other health problems.
Unlike some malignancies, lung cancer does not appear to be directly related to immune suppression, but some studies have found that lung
cancer incidence is higher among people living with HIV.
Here are some steps you can take to maintain good lung health.
Smoking cessation: The best thing you can do for your lungs is to stop smoking. But nicotine is addictive, and quitting can be a challenge. Some people nd nicotine gum or patches, prescription medications or support groups helpful. Vaping is less detrimental than smoking, but the inhaled aerosols also contain harmful chemicals.
The sooner you stop smoking, the better, but quitting is bene cial at any age. The health bene ts of smoking cessation begin soon a er stopping, but the risk for lung cancer remains elevated compared with never smokers. Annual lung cancer screening is recommended for longtime heavy smokers starting at age 50, but some research suggests HIV-positive people may bene t from starting sooner.
Aerobic exercise: Physical activity that gets your heart pumping and leaves you out of breath improves cardiovascular tness and can increase lung capacity. This includes brisk walking, running, bicycling, swimming and dancing. Guide-
lines recommend at least 150 minutes of moderate-intensity activity—ideally, 30 minutes on ve days—or 75 minutes of vigorous activity per week, along with muscle strengthening exercises.
Vaccines: Viruses, bacteria and fungal infections can cause pneumonia and other lung diseases. People with HIV should receive an annual u shot (not the FluMist nasal spray vaccine) and COVID-19 vaccines and boosters when eligible. HIV-positive people should also receive a pneumococcal vaccine to prevent bacterial pneumonia. Preventive vaccines are recommended for everyone, but they’re especially important for people living with HIV.
People with HIV should receive regular care to monitor their overall health. Antiretroviral therapy is the best way to keep HIV in check and allow the immune system to recover, but people with a low CD4 count may need additional medications to prevent or treat OIs. Minor upper respiratory infections are common and usually nothing to worry about, but contact your health care team if you have di culty breathing, a persistent cough or other ongoing respiratory symptoms. Q
BY LIZ HIGHLEYMAN BASICS ISTOCK 18 POZ JULY/AUGUST 2024 poz.com










HEALTH BASICS





















FOFREE R FOFFICE!YOUR O Have questions about COVID-19? Visit COVIDHealth.com your trusted source for coronavirus prevention, vaccine and treatment news. A SMART + STRONG UBLICATION Award-winning consumer Follow us on: POZ TIPS 1. CONNECT TO CARE maintain your health and greatly prolong your life—as long as you access care and treatment. 2. ASK FOR HELP food or other types of assisreferral to consult with a social 3. FIND SUPPORT and friends who will be there for you, in good times and bad. Support groups and online discussion boards can also help. 4. LEARN AS MUCH AS YOU CAN the better you’ll understand why care and treatment a important. POZ.com is a great place to start. For more info on living with HIV, visit
keeps me informed and always gives me options and ideas to improve my health care. I’m healthier than I’ve ever been.” Richard Schieffer r Positive since 2011 You
Your Doctor Managing HIV involves teamwork. A good relationship with your health care providers is key to good health. Here are a few tips to help you get the most out of your doctor visits. Visit us onsocial media BE PREPARED Between trips to the doctor, keep a running list of questions you have about your health on a notepad or on your smartphone. That way, you’ll maximize your time once you’re face-to-face with your doctor—and you won’t forget to bring up any of your concerns. ASK QUESTIONS Don’t be afraid to let your doctor know when you don’t understand something. Your doctor may be busy, but you have the right to ask questions and to state your needs, concerns and fears. A good doctor, even a busy one, will hear you and respond in kind. Your health care provider should also be available between visits if you have urgent issues or queries. BE HONEST Have you been depressed? Not sleeping well? Have you missed doses of your meds? Be honest with your doctor about what’s going on in your life. It’s the only way he or she can really assess your health and help you. TAKE NOTES Write down any instructions or information your doctor gives you during your appointment. Or ask your doctor if it’s OK to record notes on your smartphone. MEMBER OR FRIEND Some people find it helpful to bring along a family member or friend for support or to take notes and help you remember what was discussed. OTHER MEDS HIV meds can have bad interactions with other drugs, so be sure to tell your doctor about anything else you’re taking, including overthe-counter medications, vitamins, herbs, supplements, alcohol or recreational drugs. SUPPORT STAFF Your doctor is only one member of your health care team. Keep in mind that nurses and pharmacists can also be good sources of information. Questions about this poster? Contact us at or visit POZ.com/poster DO YOU OFFER HIV SERVICES? GET A FREE POZ HEALTH INFORMATION POSTER FOR YOUR WAITING ROOM. VISIT POZ.COM/POSTER
and
MORE PrEP LEADS TO FEWER HIV DIAGNOSES
U.S. states with the highest pre-exposure prophylaxis (PrEP) coverage saw the largest declines in new HIV diagnoses, a recent analysis shows. PrEP use has risen steadily since Truvada (tenofovir disoproxil fumarate/emtricitabine) was approved for HIV prevention in 2012. But while PrEP uptake has been high among urban white gay and bisexual men, coverage is lagging for women and Black and Latino gay men.
A team led by Patrick Sullivan, MD, of Emory University and AIDSVu, analyzed the population-level impact of PrEP on trends in new HIV diagnoses from 2012 through 2021 in all 50 states and Washington, DC.
Average PrEP coverage during 2012–2021 ranged from 5.8% in the 10 states with the lowest coverage to 15.7% in the 10 jurisdictions with the highest coverage. Looking at specific states, coverage ranged from 3.8% in West Virginia up to 22.2% in New York. Over the same period, the HIV diagnosis rate increased by 1.7% in the states with the lowest PrEP coverage while declining by 8.0% in the jurisdictions with the highest coverage, a er controlling for viral suppression rates.
“In addition to HIV testing and PrEP referral programs, Medicaid expansion and PrEP Drug Assistance Programs have been found to be associated with higher equity in PrEP use, which is important to maximize the prevention benefits of PrEP,” Sullivan and colleagues concluded.

HIV Reservoirs and Viral Rebound
Several studies presented at the Conference on Retroviruses and Opportunistic Infections (CROI) shed light on HIV persistence and strategies for long-term remission. Antiretroviral therapy (ART) keeps HIV replication suppressed, but the virus inserts its genetic blueprints into the DNA of human cells and establishes a longlasting reservoir that antiretrovirals can’t reach.
Scientists at the University of California San Francisco analyzed posttreatment viral control in a complex cure trial that combined a therapeutic vaccine regimen, two broadly neutralizing antibodies (bnAbs) and a TLR9 agonist to coax HIV out of hiding. At last year’s CROI, they reported that seven of the 10 participants experienced delayed viral rebound to lower-than-expected levels a er ART interruption. Two maintained a viral load below 1,000, and one remained in remission for 18 months.
This year, investigators presented further data showing why this might have occurred. One study found that posttreatment control was not consistently associated with bnAb exposure or susceptibility, which suggests that changes
in host immune function play a key role in viral control.
In a second analysis, people who maintained partial control of HIV a er stopping a standard ART regimen or the combination intervention showed stronger CD8 killer T-cell responses to reactivated virus.
“CD8 T cells are responding more robustly as virus rebounds in people who are able to control,” says Michael Peluso, MD.
Another study looked at sex differences in the viral reservoir. Analyzing more than 4,000 viral genomes from 34 men and 30 postmenopausal women, the researchers found that women were more likely than men to have intact HIV DNA inserted in transcriptionally silent parts of their chromosomes where they were unable to reactivate. “The HIV reservoir in women is associated with features of deeper latency,” wrote Toong Seng Tan, MD, of the Ragon Institute of Mass General, MIT and Harvard, and colleagues.
“Therefore, women may be primed to achieve a state of HIV control, and the inclusion of women in cure studies should be a priority.”
BY LIZ HIGHLEYMAN CARE & TREATMENT 20 POZ JULY/AUGUST 2024 poz.com
Treatment for Fatty Liver Disease
Metabolic dysfunctionassociated steatotic liver disease (MASLD)— the new name for nonalcoholic fatty liver disease (NAFLD)—and its more severe form, metabolic dysfunctionassociated steatohepatitis (MASH), are responsible for a growing share of advanced liver disease.
Linked to obesity and diabetes, fatty liver disease is increasingly recognized as a metabolic condition. Over time, the buildup of fat in the liver can lead to inflammation, cirrhosis and liver cancer. Until the Food and Drug Administration approved Rezdiffra (resmetirom) for MASH in March, management relied on lifestyle changes such as exercise and weight loss.
ated semaglutide for HIV-positive people with MASLD. Better known by the brand names Ozempic and Wegovy, semaglutide is a GLP-1 agonist used to treat type 2 diabetes and obesity. The pilot study enrolled 51 adults on antiretroviral therapy who had insulin resistance or prediabetes, a large waist circumference and overweight or obesity. They self-administered semaglutide injections once weekly for six months. At that point, 29% showed complete MASLD resolution, and 58% had at least a 30% relative decrease in liver fat. This was accompanied by improvements in weight, waist circumference and glucose and triglyceride levels.
The prevalence of fatty liver disease among people with HIV appears to be somewhat higher than that of the population at large. One recent study found that about half of HIV-positive people with overweight or obesity and even 20% of lean people had liver steatosis.
At the Conference on Retroviruses and Opportunistic Infections, Jordan Lake, MD, of the University of Texas, reported findings from the SLIM LIVER study, which evalu-
“What we saw were really great, clinically significant reductions in liver fat even over that short period of time,” Lake told reporters.
One potential drawback of semaglutide is that it can lead to the loss of lean body mass along with fat. But another SLIM LIVER analysis found that although study participants saw a decrease in psoas muscle volume, muscle function did not decline—and it may have even improved.
OLDER PEOPLE WITH HIV HAVE UNMET NEEDS
Nearly 40% of older people with HIV have at least one unmet need, according to a recent study. Today, more than half of HIV-positive people in the United States are ages 50 or older. Like the general population, people with HIV are more prone to comorbidities as they age, and they may face challenges such as reduced mobility, cognitive decline and financial instability.
Researchers with the Centers for Disease Control and Prevention assessed the need for ancillary services among 2,391 HIV-positive people ages 55 and older using data from the Medical Monitoring Project collected between June 2019 and May 2021.
They found that 17% had at least one unmet need for HIV support services, such as medication payment assistance, adherence support, case management, patient navigation or HIV peer group support. Further, 27% had an unmet need for non-HIV medical services such as dental care, mental health care or substance use counseling, and 27% had an unmet need for subsistence services, including transportation, food or assistance with shelter or housing.
“Additional concerted efforts by state and local health departments, federal entities, private providers and community partners are necessary to reduce unmet needs,” the researchers concluded.

(VIRUS) NIAID; ALL OTHERS: ISTOCK (MODEL USED FOR ILLUSTRATIVE PURPOSES ONLY)

Ultra-Long-Acting PrEP PREVENTION
An ultra-long-acting formulation of cabotegravir may offer an HIV pre-exposure prophylaxis (PrEP) and treatment option that could be administered three times a year. The approved formulation of cabotegravir for PrEP (Apretude) is administered by a health care provider every other month, while cabotegravir plus rilpivirine (Cabenuva) for treatment is given monthly or every other month. In a Phase I trial, the new formulation—dubbed CAB-ULA— given by subcutaneous or intramuscular injection achieved comparable drug exposure levels but lasted longer in the body. Its pharmacokinetic profiles were flatter, indicating slower absorption. Pharmacokinetic modeling predicted that intramuscular CAB-ULA given at least four months apart would achieve higher drug exposure than the current formulation given every two months. CAB-ULA was generally well tolerated. Intramuscular CAB-ULA will now progress to late-stage trials, and more testing of the subcutaneous formulation—which could potentially be self-administered—is planned.

TREATMENT
Delayed Treatment
People who delay antiretroviral therapy continue to be at higher risk for complications and death years later, apparently due to greater inflammation. The START trial randomly assigned newly diagnosed individuals to begin treatment either immediately or when their CD4 count fell below 350 or they developed AIDS symptoms. People who started immediately had a 57% lower risk of AIDS-related events, serious non-AIDS events or death. The randomized portion of the trial was halted in 2015, but follow-up continued. A START sub-study measured biomarkers of inflammation and blood coagulation. During the randomized study period, the delayed treatment group had higher levels of IL-6 and D-dimer. During 2016–2021, the delayed group had about a 30% higher risk of complications or death despite starting treatment. What’s more, participants with the highest IL-6 and D-dimer levels in both the immediate and delayed groups had about double the risk. These findings emphasize the importance of prompt diagnosis and early treatment.
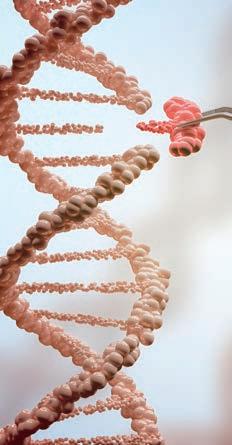
CURE
CRISPR Disappoints
A CRISPR-based gene therapy was safe and well tolerated in a Phase I study, but it did not prevent viral rebound a er stopping antiretroviral therapy (ART). Antiretrovirals can keep HIV suppressed, but the virus inserts its genetic blueprints into human cells and establishes a long-lasting reservoir that makes a cure nearly impossible. EBT-101, from Excision BioTherapeutics, acts as “molecular scissors” to cut viral DNA out of cells. The study enrolled people on ART with an undetectable viral load. They received a single infusion of EBT-101. Three people who maintained viral suppression 12 weeks later started an analytic treatment interruption. Unfortunately, all three experienced viral rebound. This likely occurred because the gene therapy did not reach all cells harboring latent HIV. However, one recipient was able to maintain viral suppression for four months, longer than it typically takes for the virus to rebound a er stopping antiretrovirals. This suggests that CRISPR-based therapies like EBT-101 might play a role in a combination functional cure strategy.
Inadequate Sleep CONCERNS
Inadequate sleep can contribute to increased inflammation in people living with HIV. Researchers looked at the effects of short-term sleep deprivation on immune activation and inflammation and assessed the function of the adenosine pathway, a compensatory mechanism that reduces inflammation and increases the urge to sleep. Twenty people on stable antiretroviral therapy with viral suppression first had one week of regulated sleep and then stayed awake for 24 hours. Blood samples were collected to measure biomarkers of immune activation, inflammation, cell cycling and adenosine pathway activity. CD8 killer T-cell activation increased a er sleep deprivation, and there was a trend toward greater monocyte and macrophage activation, but no differences in levels of IL-6, TNF-alpha or soluble CD14. Plasma adenosine levels were similar before and a er sleep deprivation, indicating that the compensatory pathway did not kick in. These findings suggest that getting enough sleep may be particularly important for people living with HIV.
ALL IMAGES: ISTOCK (MODEL USED FOR ILLUSTRATIVE PURPOSES ONLY)
BY LIZ HIGHLEYMAN RESEARCH NOTES 22 POZ JULY/AUGUST 2024 poz.com
AIDS 2024, the 25th International AIDS Conference, will take place in Munich, Germany, and virtually from 22 to 26 July.


Register now at aids2024.org Join
first
the HIV response!
Visit this diverse
vibrant space where communities from all over the
connect, share
other. Entrance, as always, is free!
AIDS. Experience the AIDS 2024
Village
July!
us to put people
in
and
world
and learn from each
Join the world’s largest conference on HIV and
Global
from 21

PEOPLE LIVING WITH HIV ARE CRUCIAL TO CLINICAL TRIALS RELATED TO THE VIRUS.
BY JAY LASSITER PHOTOGRAPHY BY NATALIA WEEDY
ALICIA DIGGS, MPH, IS A BUSY WOMAN. CURRENTLY wrapping up her PhD in public health education, she’s driven by a desire to improve the health care system for women and people of color.
A Philadelphia native now residing in Burlington, North Carolina, Diggs splits her time between her academic pursuits and cutting-edge scientific collaborations with some of the most brilliant doctors and clinicians in the game.
Along the way, she’s helping change the status quo that gives rise to race- and gender-based disparities in the American health care system.
In the past decade alone, Diggs has participated in “roughly a dozen” research studies and clinical trials. Diagnosed HIV positive in December 2001, she participated in her first research trial in 2007.
“It’s important that we— we, meaning women—are in these studies,” says Diggs. “Years ago, when HIV treatment started to come out, there weren’t many women in a lot of those clinical trials—[it was] predominantly
24 POZ JULY/AUGUST 2024 poz.com
 Alicia Diggs is a long-term survivor and a clinical trial advocate.
Alicia Diggs is a long-term survivor and a clinical trial advocate.
just white men. Medications were being developed, but we were not in those studies, especially Black women. So we’re taking medications but aren’t sure how the side effects are going to affect us differently than our male counterparts.”
She also notes the historical medical trauma women and people of color have faced—for example, the infamous Tuskegee experiment in which Black men with syphilis were left untreated so physicians could study the progression of the sexually transmitted infection.
Diggs also cites J. Marion Sims, long considered the “father of gynecology” in America.
“He was a Caucasian physician back in slavery times doing experiments on Black women and children with no anesthesia,” Diggs says. “These women were in pain. And this continued. Some women died. Some children died. But the experiments were being done on Black bodies with no anesthesia.”
This history helps explain why recruiting more women, especially Black women, for clinical trials can be an uphill battle.
“But today, we have a voice that researchers and scientists would like to hear from,” Diggs says. “There are a lot of consent documents that are in place to protect us. You can sign up for a study and then in the middle of it decide you don’t want to do it anymore. And that’s OK.”
Diggs cites “free health care” as the best reason to sign up for clinical trials. (Most clinical trial costs are covered by the trial sponsor.) She’s also keen to propel science forward for humanity, which she says is “rewarding and meaningful.”
“I’m not there for the $50 gift card,” she quips. “It is not about the money. It’s about making sure that there’s a diverse population participating. It’s to make sure that there are people who look like me taking part.”
CLINICAL TRIALS ARE USEFUL FOR EXAMINING gender-based health care disparities.
The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) study demonstrated that women living with HIV face a higher risk for adverse cardiovascular events than HIV-negative women. REPRIEVE was a randomized trial studying the heart health of participants living with HIV. Diggs was a participant in the REPRIEVE study.
Markella V. Zanni, MD, of Harvard University, was one of REPRIEVE’s lead researchers.
“There were 7,769 participants overall, among whom 2,419 (31%) had female sex at birth,” says Zanni.
“REPRIEVE results suggest that traditional risk calculators may underestimate heart disease risk among women with HIV,” she says. “For this reason, it’s important for women with HIV to discuss their heart disease risk with clinical care providers and strategize about ways to reduce risk.” That’s the bad news.
The good news is, neither a cure nor a vaccine are needed to address the disparities revealed by REPRIEVE. For instance, we already know that diet and exercise can mitigate the increased heart disease risk that HIV-positive women face. There’s also medication.
“Through REPRIEVE, we learned that statin therapy re-

“IT MAKES ME WORK HARDER. IT MAKES ME STAY IN THE FIGHT.”

duces heart disease events to the same extent among women with HIV as among men with HIV,” Zanni says. All it took was a clinical trial that prioritized female participation to achieve this very consequential conclusion. (For more on the REPRIEVE trial, please see “Give It a Try” on page 28.)
IN GENERAL, THE MISCONCEPTIONS THAT BLACK people face when seeking care are extensive, says Diggs. These include the belief that Black people’s skin is actually thicker than white people’s, that Black people’s blood coagulates differently and that Black people’s nerve endings are less sensitive than those of their white counterparts. None of these are true, but the myths endure and affect how clinicians perceive and treat Black people. Especially when it comes to treating Black people in pain.
“So for us as, Black and brown communities, especially women, when we go to the doctor and we say that we’re in pain, we’re not treated like our white counterparts when it comes to that pain,” Diggs says.
What’s more, Black women confront health care disparities, especially during pregnancy and childbirth, even after controlling for factors such as income and access to care.
Diggs cites as an example Serena Williams, arguably the greatest champion in the history of tennis. Williams won the 2017 Australian Open while 10 weeks pregnant. She is one of the most physically gifted humans ever to wield a racket. And yet she nearly died during childbirth.
More recently, the Olympic gold medal–winning sprinter Tori Bowie died last year during childbirth. Once quite literally the fastest woman on the planet, Bowie went into labor early and died at home, alone, from childbirth complications. Her premature child was stillborn.
Does Diggs find these examples demoralizing? Quite the
26 POZ JULY/AUGUST 2024 poz.com

contrary. “It makes me work harder. It makes me stay in the fight. It truly makes me stay in the fight,” she says.
Diggs knows she’s fighting HIV stigma. She’s also fighting to get more women—in particular, women of color— into clinical trials, which starts by setting an example.
“This work around HIV advocacy can be stressful,” she explains. “It can be tiring. It is hard. It is heavy. But when you know there are other people dealing with unfair treatment or unfair access or lack of access, it makes you want to continue to fight. Because I am the person that I am—someone who speaks up because I have a voice—I think about those who haven’t found their voice yet. I truly feel like it is my duty to stand up for and represent my peers who don’t have the same access or who don’t have the same voice as I do.”
Diggs says clinicians also have a duty to communicate better with trial participants as well as those who aren’t selected for trials.
“Researchers need to explain to the participants clearly why they can’t participate,” Diggs says. “People feel like they’re being rejected because of race and gender. I hear women say, ‘Well, I was rejected from a study, and I know it’s because I’m Black, and I’m a woman.’ But what I’ve learned over time is if there are other health issues going on, it is safer for the person to not participate versus participating. Like if you have anemia, it’s not good to be in a study that has large blood draws. Years ago, people were rejected because of race or gender, but now it is primarily about safety for the participants.”
A far cry indeed from the days when unethical medical experiments on vulnerable populations were common.
WHEN WE DO HAVE A REPRESENTATIVE NUMBER OF
women enrolled in clinical trials, we learn interesting and important things, such as that HIV-positive women are at
Diggs is completing a PhD in public health education.
greater risk for heart health issues than women who are HIV negative.
According to Diggs, the first step in encouraging others to participate might be acknowledging the cultural and systemic barriers women of all races—especially mothers and caregivers—face.
“Most times, women are not focusing on themselves because they’re taking care of other family members or they’re working,” she explains. “Transportation could also be a huge issue. There are so many different factors and barriers for women.”
Zanni, the REPRIEVE researcher, says her colleague Sara Looby, PhD, was “instrumental in developing and launching the “Follow Your Heart” campaign to recruit more women into clinical trials and research studies.”
The number of women in the REPRIEVE study, roughly a third of participants, might seem low considering women make up half the population. But women constitute slightly less than a quarter of America’s HIV-positive population. So in this instance, women were in fact robustly represented.
Diggs says the trials she’s involved with usually have a patient advisory board, something she strongly recommends joining.
“Being a part of the advisory board consisted of just listening to some of the new writings that the researchers were doing to get our opinion, especially on screening questions,” Diggs explains. “When some of the questions or the surveys were being generated, we had buy-in. It was really just asking us as a community to make sure that they were delivering in a way that was equal, if you will.”
Community advisory boards help craft eligibility questionnaires to be more culturally competent and relevant so that future studies don’t just enroll a cohort of white men.
“It’s really important that our voices are there so that we have input and changes can be made that are favorable for us,” Diggs says. “A lot of times I am encouraging my peers to at least understand research studies and get a little bit more information and talk to your physician, social worker or someone who is involved with these studies and trials to get more information.”
Diggs also advises would-be participants to connect with others who are involved and who have success stories. “Such as myself,” she says.
Alison Rodger, MD, of University College London, echoes the sentiment that gender parity is a must. Rodger’s groundbreaking research underpins the Undetectable Equals Untransmittable message (U=U), which states that people on effective HIV treatment don’t transmit the virus via sex. (Go to page 32 for a profile of Rodger.)
“We know that sex and gender are major determinants of health outcomes and responses to medical treatments and vaccines,” Rodger says. “The REPRIEVE study demonstrated yet again that it is critical to ensure adequate recruitment of women to clinical trials to be able to effectively inform evidence-based care.” Q
poz.com JULY/AUGUST 2024 POZ 27

A Guide to Clinical Trials


STUDIES OF NEW TREATMENTS, PREVENTION TOOLS, COMORBIDITY MANAGEMENT AND CURE STRATEGIES ARE KEY TO BETTER QUALITY OF LIFE FOR PEOPLE LIVING WITH OR AT RISK FOR HIV.
BY LIZ HIGHLEYMAN
MOST OF WHAT WE KNOW ABOUT HIV PREVENtion, treatment and care comes from clinical trials. There are many types of research studies, and they all add to our knowledge in different ways. Joining a trial can be a good way to gain access to cutting-edge therapies and contribute to science, but it’s important to weigh the pros and cons.
“We really need rigorous clinical trials,” says Jeanne Marrazzo, MD, MPH, director of the National Institute of Allergy and Infectious Diseases (NIAID). “They’re not perfect, and they’re not the real world. They don’t always give us the answers we want, but I do think they give us the answers we need.”
TRIAL BASICS
The gold standard for research is the double-blind randomized controlled trial. In these studies, participants are randomly assigned to receive an experimental intervention or a comparison—for example, current standard care or a placebo. This minimizes bias and helps ensure that the groups are otherwise similar. Double-blind means neither the researchers nor the participants know who is in which group; if these assignments are known, the study is open-label.
Randomization means that any given participant might not be initially assigned to the experimental group. But modern antiretroviral therapy trials do not include inactive placebos, and often, participants will be offered the new treatment once the randomized part of the study ends.
For ethical and practical reasons, some studies can’t use randomization, controls or blinding. Observational studies— such as the large Multicenter AIDS Cohort Study and Women’s Interagency HIV Study, which were combined in 2019— also yield valuable information. These studies don’t test specific interventions but rather monitor real-world outcomes over time.
A good trial design ensures that a study can provide useful data. Trials should include enough participants and last long enough to produce statistically significant results, meaning that the findings are unlikely to be due to chance alone. What’s more, it’s important to include participants who reflect the full population that will use an intervention in the real world, including people with more advanced disease or coexisting health problems and people of all genders, ages and racial and ethnic groups.
NEW TREATMENT TRIALS
The most familiar clinical trials assess new treatments in a stepwise manner (see “Clinical Trial Stages,” page 30). The process of developing new medications is lengthy and expensive, and only a small fraction of experimental therapies ever
make it from the laboratory to pharmacy shelves. The fact that a new drug shows activity in a test tube or a mouse doesn’t necessarily mean it will work in humans.
Early in the epidemic, AIDS activists were instrumental in speeding up access to new therapies. Thanks to these efforts, the Food and Drug Administration (FDA) can grant accelerated approval of experimental drugs based on surrogate markers, such as viral suppression, rather than waiting to see whether study participants get sicker or die.
In the early years, treatment trials were riskier. Scientists often didn’t know whether investigational drugs would work or what side effects they might cause. Nonetheless, people with no good options were eager to enroll. Once effective combination antiretroviral regimens were developed in the mid-1990s, the focus turned to reducing side effects. Today, many trials aim to make treatment easier—for example, by testing drugs that can be taken less often.
Now that many people with HIV are doing well on treatment, there’s less incentive to join a trial to find something better. But some people still need other options, such as those who can’t take daily pills and long-term survivors who tried less effective medications and developed extensive drug resistance. For the latter group, new options include the HIV entry inhibitors ibalizumab (Trogarzo) and fostemsavir (Rukobia) and the HIV capsid inhibitor lenacapavir (Sunlenca), which work differently than other antiretrovirals and remain active against highly resistant virus.
Nelson Vergel, who was diagnosed with HIV nearly 40 years ago, found himself in this situation, struggling to achieve viral suppression. In 2013, he joined a clinical trial of ibalizumab, a monoclonal antibody that blocks HIV from entering CD4 cells. Within two months, his viral load became undetectable for the first time. “I was able to restart my life again,” he previously told POZ.
But looking back, Vergel is more wary. “I joined too many studies in the past that used functional monotherapy, and on each one, I developed more resistance. But I didn’t have many options,” he says. “Everybody should be told that if you join a study, you may not be eligible for other interventions down the line. Now that we have more options, we have to be more transparent about what people give up when they join studies.”
TREATMENT STRATEGIES
Clinical trials don’t end when new drugs are approved. Some studies look at treatment strategies to learn how best to use them.
When HIV medications were less well tolerated, some
poz.com JULY/AUGUST 2024 POZ 29 BOTH IMAGES: ISTOCK
experts thought starting antiretrovirals later or taking periodic treatment breaks might reduce toxicity. But two large treatment strategy trials put these ideas to rest. The START study showed that people with a CD4 T-cell count above 500 who were randomly assigned to start antiretrovirals immediately had a lower risk for AIDS-related events, serious non-AIDS complications and death than those who delayed treatment until their CD4 count fell below 350. Likewise, the SMART study showed that participants randomized to stay on continuous treatment had better outcomes than those who took medication breaks when their CD4 count rose above 350. These and other studies helped inform current guidelines that recommend continuous treatment starting as soon as possible after HIV diagnosis.
Once drugs are approved, researchers can conduct pilot studies to try out innovative strategies. For example, longacting Cabenuva (injectable cabotegravir and rilpivirine) is currently approved only as a switch option for people with an undetectable viral load. Researchers at San Francisco General Hospital’s Ward 86 HIV clinic conducted a pilot study of Cabenuva, given in the context of extensive support, for people who were unable to maintain viral suppression on daily pills. Of the 57 people with a detectable viral load who received Cabenuva injections, all but two achieved viral suppression, many for the first time. Based on these findings, the International Antiviral Society–USA recently updated its treatment guidelines to say that Cabenuva may be considered for some people with detectable virus who are unable to take oral meds consistently.
“For those of us treating HIV on a daily basis, we know that some patients have challenges taking pills, including substance use, housing and food insecurity and stigma,” says Ward 86 medical director Monica Gandhi, MD,
CLINICAL TRIAL STAGES
Preclinical: Laboratory and animal studies are done prior to human trials.
Phase I: Early feasability trials, typically including 10 to 100 participants, assess safety and collect pharmacokinetic and dosing data.
Phase II: Mid-stage trials, typically including a few hundred participants, evaluate safety in a larger group and gather preliminary e cacy data.
Phase III: The largest and longest trials, typically including hundreds or thousands of participants, evaluate e cacy and side e ects in a population re ecting patients who will use a new therapy.
Phase IV: Post-marketing studies conducted a er approval and commercial availability assess how well a therapy works in the real world.
MPH. “We saw high success rates that were equivalent to those in clinical trials.”
PREVENTION TRIALS
Clinical trials for new prevention methods, such as preexposure prophylaxis (PrEP) and HIV vaccines, follow a similar pathway, but the safety bar is higher. While side effects may be acceptable for HIV-positive people whose health would worsen without treatment, PrEP is used by a large number of healthy HIV-negative people.
Beyond testing specific prevention tools, large trials can also shed light on the bigger picture. The PARTNER studies, for example, showed that people with an undetectable viral load do not transmit HIV via sex (see “Heroes,” page 32).
As with treatment trials, it is important for prevention studies to include all populations that will use an intervention in the real world—and the FDA can take a hard line if they don’t. Tenofovir alafenamide/emtricitabine (Descovy) was approved as PrEP for men and transgender women in 2019, but it has not yet been approved for cisgender women and others exposed to HIV through vaginal sex because they weren’t included in a pivotal trial.
While both men and women respond similarly to antiretroviral treatment, studies of HIV prevention methods have yielded more variable results. Initial studies suggested that daily tenofovir disoproxil fumarate/emtricitabine (Truvada or generic equivalents) did not protect cisgender women as well as gay men and transgender women. Further analysis shows that protection depends on adherence, not biology. A recent analysis of real-world data from post-marketing studies found that daily PrEP pills were highly effective for women who took at least four doses per week—but less than 40% achieved this level of adherence.
“Oral PrEP is working for women, but uptake remains very low, and HIV incidence hasn’t really budged,” Marrazzo told POZ. “Women want different choices. Whether that’s a longer-acting product, a vaginal product, a shot—those are things we still need to look at. There’s a big gap between what we have and what we need.”
In contrast to the remarkable success of antiretroviral treatment and PrEP, large vaccine trials have led to a string of disappointments, as study after study has failed to show that traditional vaccines can prevent HIV. But according to Larry Corey, MD, of Fred Hutchinson Cancer Center, these trials shouldn’t be considered a failure: “We did get an answer, but it wasn’t the one we wanted,” he says.
What’s more, the benefits of clinical research are not confined to a single field. “Advances in HIV vaccine science have literally paved the way for SARS-CoV-2 vaccines,” says former NIAID director Anthony Fauci, MD. “What goes around comes around, and I expect this to feed back into HIV vaccine development.”
Indeed, researchers are now studying more sophisticated HIV vaccine approaches in small trials, including some that utilize the same messenger RNA technology as COVID vaccines. But testing HIV vaccines has become more challenging
30 POZ JULY/AUGUST 2024 poz.com
now that highly effective oral and injectable PrEP is widely available. Ethics require that study participants be offered the best available existing prevention tools, and it will be difficult to show that vaccines add to the already high level of protection provided by PrEP.
COMORBIDITY STUDIES
Clinical trials can help people with HIV lead healthier lives beyond new antiretrovirals. In the era of effective treatment— and given that more than half of HIV-positive people are now ages 50 or older—HIV care has largely shifted to managing coexisting health conditions.
TRIAL PROS AND CONS
PROS:
• Early access to new therapies
• Free drugs and health monitoring
• Expert doctors and leading medical centers
• Financial incentives
• Satisfaction of helping others
• Advancement of science
CONS:
• Time commitment
• Associated unreimbursed costs
• May need to stop or forgo other treatments
• Might not receive experimental therapy
• Intervention might not work
• Potential side effects
Compared with the population at large, studies have shown that people living with HIV are at greater risk for many chronic conditions, including cardiovascular, liver and kidney disease and certain cancers. Comorbidity trials may evaluate new types of treatment for these conditions, novel care strategies or behavioral interventions, such as exercise or smoking cessation.
The REPRIEVE trial, for example, tested pitavastatin (Livalo) for HIV-positive people at low to moderate risk for cardiovascular disease, a group that ordinarily would not be prescribed statin medications.
The study showed that people randomly assigned to receive the statin had a 35% lower risk for heart attacks, strokes and other major cardiovascular events. Based on these findings, the Department of Health and Human Services recently updated its treatment guidelines to recommend statins for HIV-positive people ages 40 to 75 who are at low to intermediate risk for cardiovascular disease.
While testing the statin, REPRIEVE also shed new light on cardiovascular disease in people with HIV. Nearly a third of the participants were women, enabling the study to see how cardiovascular disease manifests differently in the two sexes. One finding was that traditional cardiovascular risk scores for the general population underestimate risk for HIV-positive people, and this is especially true for women. (REPRIEVE participant Alicia Diggs describes her experience in the “Heart of the Matter,” page 24).
Another trial showed that doxyPEP—a single dose of the antibiotic doxycycline taken within 72 hours after sex—lowers the risk of chlamydia and syphilis for gay men and trans women, though it was less effective against gonorrhea, and a similar study of cisgender women did not see the same benefit. Other recent comorbidity studies have shown that weight-loss drugs appear to work well for people living with HIV, and that screening and early treatment can reduce the risk of anal cancer among HIV-positive people.
CURE TRIALS
HIV cure trials can be more complex and may involve greater risks. Unlike new antiretrovirals, researchers are not so sure whether interventions such as therapeutic vaccines, broadly neutralizing antibodies or gene therapy will work or what adverse events they might cause.
To determine whether a cure strategy leads to long-term remission, participants may need to interrupt antiretroviral therapy, which could lead to disease progression and HIV transmission. What’s more, while these studies add to our understanding and may help other people with HIV down the line, they generally are not expected to cure current trial participants.
Here, too, the inclusion of women is crucial. Some research suggests that women may be more likely to be elite controllers or to achieve long-term HIV remission after stopping antiretrovirals, perhaps due to differences in their immune response.
“We are still in the exploratory phases of cure research. This will be a very tough nut to crack—but one that is essential to see the end of the HIV pandemic,” says Sharon Lewin, MD, of the Peter Doherty Institute at the University of Melbourne. “Participation in early clinical trials is absolutely essential to understand what might or might not work. I tell potential participants that it’s very unlikely that they personally will have any benefit from an early phase cure trial. However, their participation will have a major impact on the field.”
JOINING A TRIAL
If you are thinking about joining a clinical trial, HIV care providers, advocates, support groups and the National Institutes of Health’s ClinicalTrials.gov website are good sources of information about available studies.
When considering a trial, learn all you can about the intervention being tested, what other options are available and the potential risks and benefits (see “Trial Pros and Cons,” above). Don’t be afraid to ask questions! Before agreeing to join a study, participants must sign an informed consent document, but this is not a contract—you have the right to withdraw at any time for any reason.
Studies of new antiretroviral medications, prevention methods, comorbidity management and cure strategies don’t offer guarantees, and researchers can’t rule out unforeseen adverse events. But despite this uncertainty, clinical trials can be a gateway to better care for people living with or at risk for HIV, now and in the future. Q
poz.com JULY/AUGUST 2024 POZ 31
LASSITER


Consequential Scientist
Alison Rodger, MD, is one of the most consequential HIV scientists in the history of the disease. Her research, much of it done at University College London, demonstrated definitively that a person with an undetectable viral load does not transmit HIV via sex.
Anecdotal evidence from serodiscordant couples had already suggested that people with undetectable viral loads don’t transmit HIV. Rodger and her colleagues proved that Undetectable Equals Untransmittable (U=U) via rigorous adherence to the scientific method. By proving that U=U, she helped to usher in a new era in the battle against the virus.
“We’ve been building the evidence for about two decades, and we know that a person’s viral load is the biggest determinant of risk,” says the Scottish-born Rodger. “But the PARTNER studies that I did were kind of the tipping point in our approach to messaging. Those studies gave people the confidence to be definitive about U=U. Actually, the risk is zero.”
Rodger’s collaboration with U=U evangelist Bruce Richman, founding executive director of the Prevention Access Campaign, has been especially fruitful.
“Alison’s revolutionary research provided overwhelming evidence for the U=U movement,” Richman says. “She didn’t stop at research. She’s worked tirelessly to ensure the science reaches the field, enabling millions of people living with HIV to live and love without fear. It’s been one of my life’s greatest joys to work with Alison to translate her research into real-world impact.”
In addition to saving lives and combating stigma, the science behind U=U is helping to roll back laws from the 1980s and ’90s that made it a crime not to disclose your HIV status to sexual partners, regardless of whether HIV was transmitted. A punitive response to a heavily stigmatized disease, these laws criminalized the sex lives of people with HIV. And by amplifying the stigma and discouraging testing and treatment, these reactionary laws made the AIDS crisis even more lethal.
Currently, Rodger’s research is helping activists around the world to repeal those laws.
“The PARTNER data challenges these laws because there is no risk,” she says. “And there’s been a lot of repeal of these laws because of U=U. I think one of the strengths of U=U is actually the evidence behind it. It’s not simply an activist or community rallying cry. It’s based on such robust evidence, and I think that’s what convinces you.”
If Rodger remains modest about the scientific and political impact of her work, it’s because to her, the real heroes in her remarkable journey are the people who volunteer for clinical trials and research studies. It’s their sacrifice that propels the science, a fact she’s keen to note whenever she presents her research.
(Go to page 28 for the pros and cons of clinical trials.)
“My final slide [in presentations] is always a huge thanks to people who generously took part in these studies, people with HIV who so generously took part just to push the science a little bit further. They’re the real heroes of all these studies.” Q
JON ATTENBOROUGH 32 POZ JULY/AUGUST 2024 poz.com
HEROES BY JAY
Alison Rodger’s research proved U=U.
Treatment helps prevent the spread of HIV
If you're living with HIV, a major goal is to get your viral load to undetectable. This means that there is so little virus in the blood that a lab test can't measure it. Current research shows that taking treatment as prescribed and getting to and staying undetectable prevents the transmission of HIV through sex. This is also known as U=U.
Why U=U matters
U=U means undetectable=untransmittable. It helps destigmatize living with HIV, raises awareness that today's medications can be e ective, and reminds people of the importance of continuing to take treatment as prescribed. Stay empowered to live a longer and healthier life.
Speak with your healthcare provider
It's important to be open and honest with your healthcare provider to find the right treatment for you and your routine. No questions are o limits when you meet with them.
HEALTH, LIFE & HIV
ATRIPLA *

efavirenz + tenofovir disoproxil fumarate + emtricitabine
One tablet once a day. Each tablet contains 600 mg efavirenz + 300 mg tenofovir disoproxil fumarate + 200 mg emtricitabine. Take on an empty stomach. Dose should be taken at bedtime to minimize dizziness, drowsiness and impaired concentration.

BIKTARVY
bictegravir + tenofovir alafenamide + emtricitabine
One tablet once a day. Each tablet contains 50 mg bictegravir + 25 mg tenofovir alafenamide + 200 mg emtricitabine. Take with or without food.
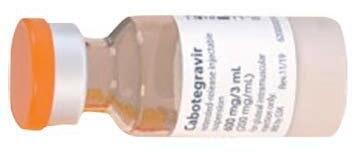
CABENUVA
cabotegravir + rilpivirine
A long-acting injectable regimen administered as two intramuscular injections every four weeks or eight weeks. A one-month lead-in period with Vocabria (cabotegravir) + Edurant (rilpivirine) pills is optional. Take with food.

COMPLERA
rilpivirine + tenofovir disoproxil fumarate + emtricitabine
One tablet once a day. Each tablet contains 25 mg rilpivirine + 300 mg tenofovir disoproxil fumarate + 200 mg emtricitabine. Take with a meal.

DELSTRIGO
doravirine + tenofovir disoproxil fumarate + lamivudine
One tablet once a day. Each tablet contains 100 mg doravirine + 300 mg tenofovir disoproxil fumarate + 300 mg lamivudine. Take with or without food.

DOVATO
dolutegravir + lamivudine
One tablet once a day. Each tablet contains 50 mg dolutegravir + 300 mg lamivudine. Take with or without food.

GENVOYA
elvitegravir + cobicistat + tenofovir alafenamide + emtricitabine
One tablet once a day. Each tablet contains 150 mg elvitegravir + 150 mg cobicistat + 10 mg tenofovir alafenamide + 200 mg emtricitabine. Take with food.
This quick-reference chart compares antiretroviral (ARV) options for the treatment of HIV, including adult dosing and dietary restrictions. Visit poz.com/drugchart for more info.
*GenericversionavailableintheU.S. (Pillsnotshownactualsize)

CIMDUO
tenofovir disoproxil fumarate + lamivudine
One tablet once a day. Each tablet contains 300 mg tenofovir disoproxil fumarate + 300 mg lamivudine. Take with or without food.

DESCOVY
tenofovir alafenamide + emtricitabine
One tablet once a day. Each tablet contains 25 mg tenofovir alafenamide + 200 mg emtricitabine. Take with or without food.

EMTRIVA * emtricitabine (also known as FTC)

EVOTAZ atazanavir + cobicistat
One tablet once a day. Each tablet contains 300 mg atazanavir + 150 mg cobicistat. Take with food.
KALETRA * lopinavir + ritonavir

dolutegravir + rilpivirine
One 200 mg capsule once a day. Take with or without food.

EPIVIR * lamivudine (also known as 3TC)
One 300 mg tablet once a day, or one 150 mg tablet twice a day. Take with or without food. Also approved for the treatment of hepatitis B virus but at a lower dose. People living with both viruses should use the HIV dose.

EPZICOM * abacavir + lamivudine
One tablet once a day. Each tablet contains 600 mg abacavir + 300 mg lamivudine. Take with or without food. Should be used only by individuals who are HLA-B*5701 negative.

TEMIXYS
tenofovir disoproxil fumarate + lamivudine
One 300 mg tablet twice a day, or two 300 mg tablets once a day. Take with or without food. Should be used only by individuals who are HLA-B*5701 negative. Nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NRTIs, or nukes)
One tablet once a day. Each tablet contains 300 mg tenofovir disoproxil fumarate + 300 mg lamivudine. Take with or without food.

TRUVADA *
tenofovir disoproxil fumarate + emtricitabine
One tablet once a day. Each tablet contains 300 mg tenofovir disoproxil fumarate + 200 mg emtricitabine. Take with or without food.
VIREAD *

tenofovir disoproxil fumarate
One 300 mg tablet once a day. Take with or without food.
ZIAGEN * abacavir

Protease Inhibitors (PIs)
Two tablets twice a day, or four tablets once a day, depending on HIV drug resistance. Each tablet contains 200 mg lopinavir + 50 mg ritonavir. Take with or without food.

PREZCOBIX
darunavir + cobicistat
One tablet once a day. Each tablet contains 800 mg darunavir + 150 mg cobicistat. Take with food.
PREZISTA
darunavir

One 800 mg tablet, or two 400 mg tablets plus one 100 mg Norvir tablet once a day, or one 600 mg tablet plus

REYATAZ * atazanavir Two

ISENTRESS
raltegravir
Two 600 mg Isentress HD tablets (shown) once a day for those who are treatment naive or whose virus has been suppressed on an initial regimen of Isentress. One 400 mg Isentress tablet twice daily for people with HIV treatment experience. Take with or without food.
TIVICAY
dolutegravir

One 50 mg tablet once a day for those rst starting ARV therapy or for those who have not used an integrase inhibitor in the past. One 50 mg tablet twice a day for people with treatment experience who have HIV that is resistant to other integrase inhibitors and when taken with certain ARVs. Take with or without food.
VOCABRIA
cabotegravir

One 30 mg tablet taken once a day with once-daily Edurant for a month as an optional lead-in regimen before switching to Cabenuva injections or for short-term treatment. Take with food.
JULUCA
or non-nukes) EDURANT rilpivirine One 25 mg tablet once a day. Take with food.
200 mg capsules once a day, or one 300 mg capsule plus one 100 mg Norvir tablet once a day. Take with food.
one 100 mg Norvir tablet twice a day, depending on drug resistance. Take with food.
NORVIR * ritonavir Norvir is usually taken to boost the levels of other ARVs in the Boosters Complete Regimens Integrase Inhibitors
elvitegravir + cobicistat + tenofovir alafenamide + emtricitabine
One tablet once a day. Each tablet contains 150 mg elvitegravir + 150 mg cobicistat + 10 mg tenofovir alafenamide + 200 mg emtricitabine. Take with food.

JULUCA
dolutegravir + rilpivirine
One tablet once a day. Each tablet contains 50 mg dolutegravir + 25 mg rilpivirine. Take with a meal.

ODEFSEY
rilpivirine + tenofovir alafenamide + emtricitabine
One tablet once a day. Each tablet contains 25 mg rilpivirine + 25 mg tenofovir alafenamide + 200 mg emtricitabine. Take with a meal.
STRIBILD

elvitegravir + cobicistat + tenofovir disoproxil fumarate + emtricitabine
One tablet once a day. Each tablet contains 150 mg elvitegravir + 150 mg cobicistat + 300 mg tenofovir disoproxil fumarate + 200 mg emtricitabine. Take with food.

SYMFI AND SYMFI LO efavirenz + tenofovir disoproxil fumarate + lamivudine
One tablet of either Sym or Sym Lo once a day. Each tablet of Sym contains 600 mg efavirenz + 300 mg tenofovir disoproxil fumarate + 300 mg lamivudine. Each tablet of Sym Lo (shown) contains 400 mg efavirenz + 300 mg tenofovir disoproxil fumarate + 300 mg lamivudine. Take on an empty stomach. Dose should be taken at bedtime to minimize dizziness, drowsiness and impaired concentration.

SYMTUZA
darunavir + cobicistat + tenofovir alafenamide + emtricitabine
One tablet once a day. Each tablet contains 800 mg darunavir + 150 mg cobicistat + 10 mg tenofovir alafenamide + 200 mg emtricitabine. Take with food.
TRIUMEQ
dolutegravir + abacavir + lamivudine
One tablet once a day. Each tablet contains 50 mg dolutegravir + 600 mg abacavir + 300 mg lamivudine. Take with or without food. Should be used only by individuals who are HLA-B*5701 negative.
ZIAGEN * abacavir

One 300 mg tablet twice a day, or two 300 mg tablets once a day. Take with or without food. Should be used only by individuals who are HLA-B*5701 negative.

EDURANT
rilpivirine
One 25 mg tablet once a day. Take with food.

INTELENCE
etravirine
One 200 mg tablet twice a day. Take with food.
PIFELTRO
doravirine
One 100 mg tablet once a day. Take with or without food.

SUSTIVA *
efavirenz
One 600 mg tablet (shown) once a day, or three 200 mg capsules once a day. Take on an empty stomach or with a low-fat snack. Dose should be taken at bedtime to minimize dizziness, drowsiness and impaired concentration.

RUKOBIA fostemsavir
Inhibitors
Entry
VOCABRIA cabotegravir
One 30 mg tablet taken once a day with once-daily Edurant for a month as an optional lead-in regimen before switching to Cabenuva injections or for short-term treatment. Take with food.

PK Boosters
Capsid Inhibitors
NORVIR * ritonavir
Norvir is usually taken to boost the levels of other ARVs in the blood. Take with food.

TYBOST
cobicistat
One 150 mg tablet once a day in combination with ARVs that require boosting. Used only to boost other drugs. Take with food.


SUNLENCA lenacapavir
Sunlenca tablets are taken as a loading dose, with injections once every six months therea er. Take with or without food.
These antiretroviral medications are rarely prescribed and no longer recommended:


APTIVUS tipranavir
COMBIVIR * zidovudine + lamivudine
CRIXIVAN indinavir
One 600 mg tablet twice a day for people with HIV treatment experience. Take with or without food.
SELZENTRY
maraviroc

One 150 mg, 300 mg (shown) or 600 mg tablet twice a day, depending on other meds used, for people with HIV treatment experience. Take with or without food.

TROGARZO
ibalizumab
A long-acting injectable administered intravenously as a single loading dose of 2,000 mg followed by a maintenance dose of 800 mg every two weeks for people with HIV treatment experience.
Visit poz.com/drugchart-prevention for a list of ARV options to prevent HIV.







FUZEON enfuvirtide
INVIRASE saquinavir
LEXIVA fosamprenavir
RETROVIR * zidovudine (AZT)
TRIZIVIR abacavir + zidovudine + lamivudine
VIRACEPT nelfinavir

VIRAMUNE nevirapine
ZERIT stavudine (d4T)
Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs, or non-nukes)
Complete Regimens
Treatment prevent spread
If you're living with viral load to undetectable. little virus in the blood it. Current research prescribed and getting prevents the transmission also known as U=U
Why U=U matters
U=U means undetectable=untransmittable. It helps destigmatize living with HIV, raises awareness that medications can be e ective, and reminds people importance of continuing to take treatment as prescribed. Stay empowered to live a longer and healthier
your
It's important to be healthcare provider and your routine. meet with them.
Speak
healthcare provider GILEAD and the GILEAD Logo are trademarks of Gilead Sciences, Inc. (SS�V[OLY�THYRZ�HYL�[OL�WYVWLY[`�VM�[OLPY�YLZWLJ[P]L�V^ULYZ�� ������.PSLHK�:JPLUJLZ��0UJ��(SS�YPNO[Z�YLZLY]LK��<:�<5)*�� �������� There is power in maintaining your sexual health HIV doesn't have to stop you from being you. Discover helpful tips and support to keep living your authentic life. It's important to start HIV treatment as soon as possible and stick with it. Remember to take your treatment as prescribed and stay engaged in care. Taking care of yourself is a great way to help you live well with HIV. You can live well with HIV Find more helpful information and resources at HelpStopTheVirus.com

If you are living with HIV, talk to your healthcare provider about treatment options. Help Stop the Virus provides resources and information that can help you stay engaged in your health. Visit HelpStopTheVirus.com SEE INSIDE with HIV, a major goal is to get your undetectable. This means that there is so blood that a lab test can't measure research shows that taking treatment as getting to and staying undetectable transmission of HIV through sex. This is U=U. be open and honest with your provider to find the right treatment for you routine. No questions are o limits when you Treatment helps prevent the spread of HIV matters helps that today's people of the prescribed. life. Speak with healthcare provider Learn how to look after your health Start HIV treatment ASAP
Model portrayal





































































































































 Alicia Diggs is a long-term survivor and a clinical trial advocate.
Alicia Diggs is a long-term survivor and a clinical trial advocate.











































