
Pathways to get your medical device reimbursed in France
Webinar

Agenda
Introduction
1. Context around medical devices and health insurance policy in France
2. Understanding the reimbursement system for medical devices in France
• Different market access pathways (standard and derogatory)
• Key factors in pricing negotiations with medical device suppliers
3. Market Access case studies
• Medical device without CE marking yet
• Medical device with MDD (directive)
• Medical device with MDR (regulation)
4. Main challenges for the digital health acceleration strategy Q&A
Abbreviations and acronyms
Speakers




Hubert GALMICHE
Head of the Medical Device Assessment Department (SED)
French National Authority for Health (HAS)
Bernard CELLI
Vice-President in charge of Medical Devices
Economic Committee for Health Products (CEPS)
Vincent VERCAMER
Project Director in charge of Market Access of Digital Health Innovations
Delegation for Digital Health (DNS) - Ministry of Health
Thomas RIQUIER
MarketAccess Director
Digital Medical Hub
DBT France: Who Are We?
The Department for Business and Trade (DBT) is a UK Government department focused on promoting economic growth and boosting international trade.
FRANCE OFFICES
DBT France’s main office is located at the British Embassy in Paris. We are also located in Lyon and Bordeaux.
We help businesses to:

1. Grow


2. Invest

3. Trade

VISION
Make the UK the brand of choice in France

Prepare the UK economy for the future
3. Export

Vincent VERCAMER
Project Director in charge Market Access of Digital Health Innovations
Delegation for Digital Health (DNS) - Ministry of Health

What is the context around medical devices and health insurance policy in France?
French health insurance landscape

● In France (67,9M people) health insurance is mandatory for everyone
● 99,9% of population in France is covered by general health insurance



In addition, 95% of French have a complementary health insurance, these voluntary health insurance are provided by :
● mutual organization
● & private insurances (417)
Reimbursement is regulated by the state
Example of reimbursement rates
General health insurance reimbursement rates :
● 70% for office visit
● 60% for medical devices

The remaining fees are covered by complementary health insurance or at 100% for patient with Long Term Disease (ALD ; 12.4M people)
About 3000 hospitals in France

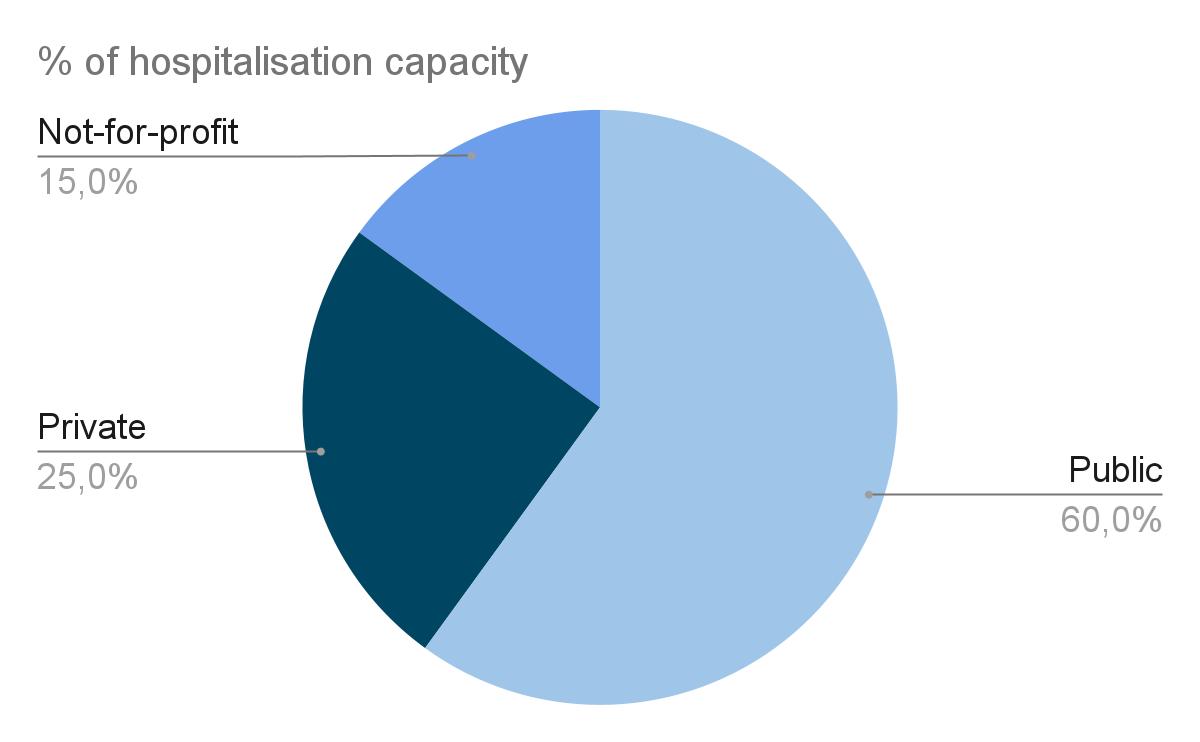
MD market

France is the 2nd largest European MD market
5th world (~240B€), 2nd Europe (~100B€)
Revenue ~ 20 billion € :
● 12 B€ : Ambulatory
● 8 B€ : Hospital
: SNITEM, 2021
source

Hubert GALMICHE
Haute Autorité de santé (French National Authority for Health) (HAS)

What are the different market access pathways (standard and derogatory) ?
HTA in France
MD assessment

 Hubert Galmiche February 2024
Hubert Galmiche February 2024
Three core missions

Assess and appraise pharmaceuticals, devices and procedures for inclusion on the national list of reimbursed products and services
Recommend best practices for health care professionals and elaborate public health guidelines

Measure and improve the quality of care delivered in health and social care organizations
Advance quality in health and social care to serve both individual and collective interests

assessment
12 MD
General rules for MD reimbursement
▪ CE marking is a requirement to be on EU market
▪ To be reimbursed, devices have to be assessed by HAS (if individual use)
▪ Before inclusion on the positive list of reimbursed products (LPPR or LATM)
▪ The reimbursement by the National Health Insurance is essential for the diffusion of a technology
▪ Clinical data should demonstrate the benefit for the patient
Distinction between technical performance and clinical benefit
Company should anticipate the requirements
for the CE marking
▪ and for the reimbursement by National Health Insurance

MD assessment
13
▪
▪
▪
MDs: HAS assessment scope

Technologies without medical purpose ≠ MD

MD et DMD for individual use

MD for professional use
• Assessment based on initiative of manufacturers

• Individualised reimbursement of the device Assessment only through associated medical procedure


MD assessment
14
MD reimbursement in France -
Ambulatory care vs Hospital
Individual use

Funded by the LPP




Ambulatory care In hospital
DMD for remote monitoring
Associated to a procedure

Funded by the LATM

Included in procedure’s prices


MD assessment
Individual use


Associated to a procedure
Exception : Specific funding General rule

Included in hospital fees (DRG)

only for specific devices

Funded by the LPP

15
The 2 types of inscription for reimbursement
Generic lines
Set of products :

Common characteristics called "technical specifications".

Fulfill the same function and have the same indication

Reimbursed by health insurance funds at a single tariff.
Generic Lines re-assessment process is still in progress
⮚ Direct Access to the market, under specific conditions (once line is has been created after HAS assessment ) :
- Code declaration for LPPR
- IT conformity certification for DMD

Brand name
▪ If the device does not meet the criteria of existing generic lines
▪ If there is a ‘plus’ over an existing generic line
▪ If innovative or necessitates specific monitoring
=> Dossier to be submitted to HAS (CNEDiMTS ) : single technology assessment.
=> HTA report (CNEDiMTS appraisal)
⮚ Price negotiation by Economic Committee and Listing for LPPR
⮚ Fixed prices for remote monitoring MD
MD assessment
16
Market access pathway in France - MD

CE marking (medical device)

Innovation funding (optional)
2 pathways for early access authorisation
- PECT for “classic” MDs
- PECAN dedicated to digital MDs (telemonitoring & DTx DMDs) (optional)



Pricing negotiation

Patient access
HTA

MD assessment
Decision on registration

Request for RWD
Re-assessment (optional)

17
General assessment pathway

Request for reimbursement for the MD
Application for conformity certification if Digital MD


Opinion Decision

CNEDiMTS (MDs Assessment Committee)
Health Technology Assessment
Healthcare Products Economic Committee (CEPS) for LPPR
Price / Tariff Negotiation
Social Security Department For LATM


Minister
Listing Decision ⇨ LPPR ⇨ LATM


Product Launching
Vigilance Post-listing studies Reassessment
MD assessment
18
Exemption ways vs regular ways for reimbursement

DMD without therapeutic effect

Temporary funding (PECT)

Digital MD for individual use with therapeutic effect
Digital MD for remote monitoring
Early funding for Digital MD(PECAN)

Exemption way
Innovation package
List of remote medical monitoring activities (LATM) regular way
List of reimbursable products and services (LPPR)

MD assessment 19
General process MDs Assessment Committee






20
MD assessment
Literature
»
»
Dossier from company Review of available data CNEDiMTS Guidance/ Opinion Ministry of health HAS internal assessors ------Healthcare Practitioners « ASSESSMENT » « APPRAISAL
« DECISION
Take home messages
HAS assessment is linked to the funding pathway.
HAS assess MD for individual use.
For MD for professional use : Assessment only through associated medical procedure. There are dedicated assessment pathways for DMD. HAS makes a distinction between technical performance and clinical benefit.
Specific ways can be activated for innovative medical devices (PECT or PECAN).

MD assessment
21


What are the key factors in pricing negotiations with medical device suppliers?
Bernard CELLI Economic Committee for Health Products (CEPS)
CEPS - Economic Committee for Health Products


Tarification

Based on therapeutic value
Fair and reasonable prices
Economic impact of new health products



Attractivity
Ensuring Access to Healthcare
Balance between industrial innovation and economic sustainability

Regulation
Annual price decrease strategy
Cost-effectiveness
Overall economic value
23
3 1 2

Thomas RIQUIER Associate Partner, Market Access Director - Digital Medical Hub

What are the main challenges for the digital MD and other MD for market implementation?

Actions to support the implementation of new MD and Digital MD
Use cases in the French landscape / Maturity level
Tech. & Reg.
Clin./Mkt
with a CE mark
under MDR/IVDR
with a CE mark
under MDD
Hospital Ambulatory settings
Intended use
Associated w/ proc.
Others (RPM)
Individual use
without any CE mark
Structured Not yet…
25

Depending on the maturity of evidence
And specific regulations for France (IT, cyber, interop…)
Check the box!
❏ POC study
❏ Pivotal clinical investigation
❏ HEOR evaluation
❏ Prices & Reimbursement in other countries a/o
“situations” (use cases)
❏ Implementations in clinical routine (recommendations)
❏ Distribution channels
❏ Others:
❏ Carbon footprint
❏ Organisational impact
❏ UX/UI maturity for French HCP
❏ Level of commitment (staff/team): SLA & execution
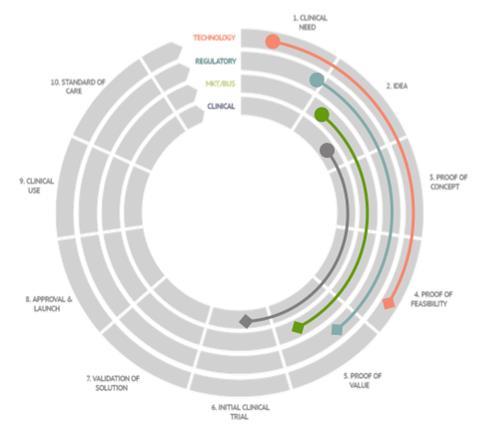

If your solution (DMD or MD or IVD) is under clinical investigations or in need of a renewal Landscape of innovation fast-tracking opportunities
PECAN ≈ DiGA = Opportunity
NOTE. Compared to France, in the UK, no formal reimbursement process for medtech (device, DTx, digital health tech) but policy support mechanisms for national adoption with highly beneficial and innovative products
27

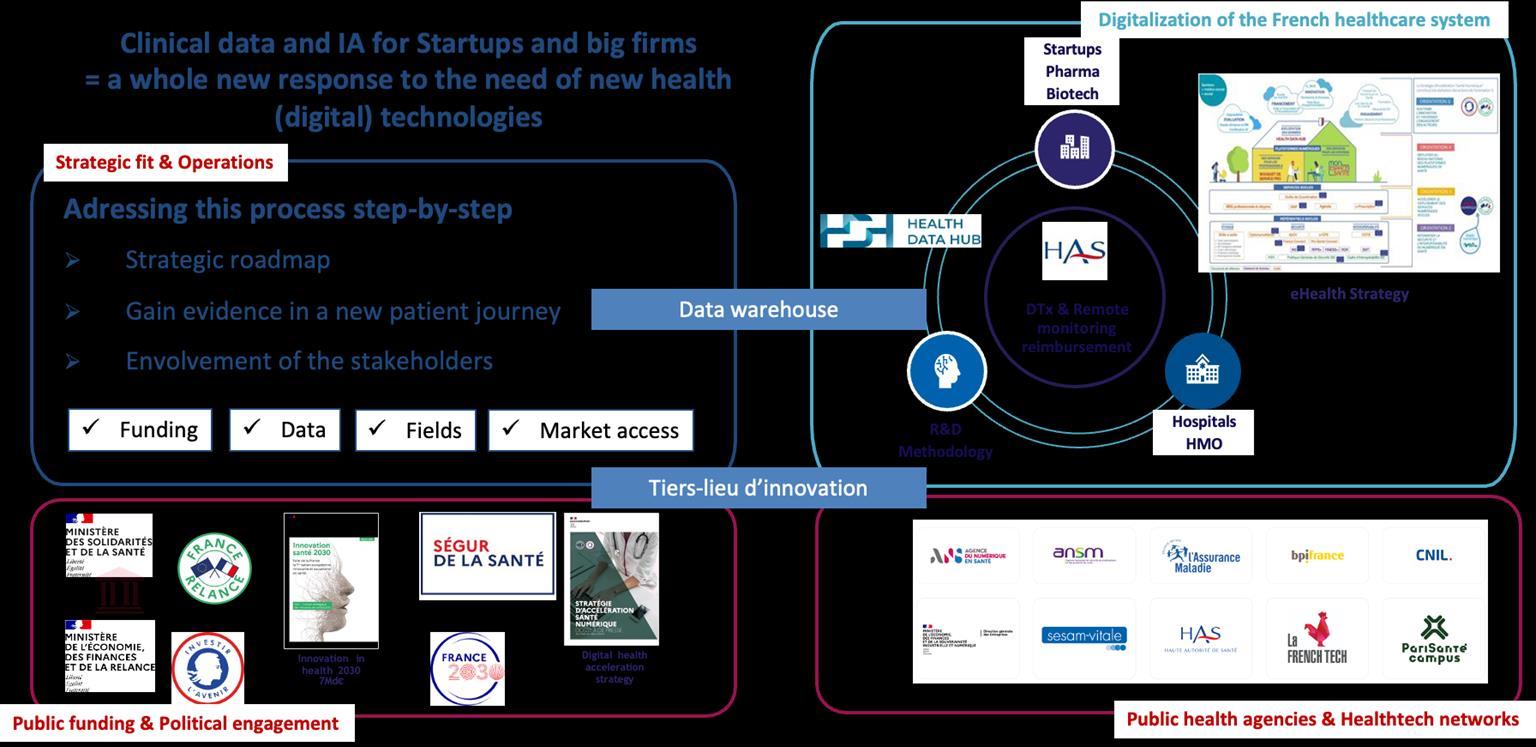
The benefits of a French-German axis strategy for digital MD

Opportunities in France
❏ Strong culture of innovation
❏ Very good clinical data
❏ Low prices for RCT and other clinical investigations
❏ Data integrity with (teaching) hospitals data warehouse
❏ Heal Data Hub
❏ National strategy for ehealth
28

Where are the barriers?
Time-to-market vs market access
What books say…
What market says…

How to deal with it?
Precision and commitment

Why the hospital route as a first strategy?
The most relevant option to start your journey in France: taking purchasing operations as a key to select the best KPI

OPTION: → P4P & VBP
Tenders & Procurement framework → high specificity vs competitors, different criteria including prices but not only the price

Actions to support the implementation of new MD and Digital MD
Use cases in the French landscape: key principles before a full scalability in the French market
SHEP
KOL a/o Practitioners
/ Users involvement
Strategic fit & Product market fit
Competitors & Value
Product & environment
Integration & Deployment
Pricing & Reimbursement
Purchasing & Distribution
Legal & Compliance
ANSM ANS HAS MoH
GIE Sesame CNIL
(+GDPR)
Referencing (ARS, CPAM, tenders)
Communication & Awareness
MedEd
Protocol & Recomme ndations
32

Actions to support the implementation of new MD and Digital MD Use cases in the French landscape: Ambulatory setting
Problem: new wound therapy based on nurse prescription, CE marked, no need for medical procedure covered by national health insurance candidate for reimbursement in France, basic HEOR evidence (UK)

❏ Nurse do not prescribe directly
❏ LPP code does not exist: HAS procedure
❏ CE mark under MDR (class IIb)
❏ Direct purchasing by hospital: price?
❏ Protocol: recommendations?
❏ Evidence: weak in the French context, notably because patients’ journey is not defined properly

❏ Nurse prescribe directly
❏ Reimbursement code exist
❏ CE mark under MDD (class IIb)
❏ Direct purchasing by hospital: included in the patients’ journey
❏ Protocol: national
❏ Evidence: good, including HEOR for pricing purposes
Problem solving approach: Test&Deploy w/ leading hospital (learned society members inside)
Selection of KPI based on: clinical, organisational, budget, care management, HCP management (cooperation protocol), involving local authorities if necessary (budget purposes); i.e. nurses groups dedicated to wound management therapy, PROMs & PREMs data collection and interpretation
→ Scientific publication (open label test, quality/security purpose, other endpoints/criteria: budget)
→ Learned society involvement to create a new medical procedure and ad hoc reimbursement
33

Actions to support the implementation of new MD and Digital MD Use cases in the French landscape: Hospital setting
Problem: new AI based diagnostic tool for cardiovascular diagnostic integrated within Big Player devices for a better qualification of aneurysms (size, potential risks/complications, non contrasted possible)

❏ Radiologist or Surgeons
❏ Medical procedure excludes Surgeons
❏ CE mark under MDR (class IIb)
❏ DRG system: performance? Efficency?
❏ No VBP available: price?
❏ Protocol: recommendations on IA?”black box”?
❏ Evidence: weak on transposability

❏ Fully radiologist-based procedure
❏ no extra payment needed
❏ CE mark under MDR (class IIb)
❏ Direct purchasing by hospital: included in the VBP contract with Big Player
❏ Protocol: national
❏ Evidence: good, including HEOR for pricing purposes
Problem solving approach: Test&Deploy OR small RCT tackling feasability/scalability
KPI: security/feasibility, clinical, ROI (budget) OR Effectiveness study/HEOR evidence generation depending on final users willingness to do/learned society orientations (incl. Reg./ANS compliance, interop, identity + GDPR)
→ No Scientific publication + direct purchasing vs new medical procedure and ad hoc reimbursement (extra-DRG-like system)
34

Actions to support the implementation of new MD and Digital MD Use cases in the French landscape: ambulatory part of hospitalisation
Problem: new DTx solution based on a 3x functionalities (symptom-checker, PROMs, rehabilitation exercices selfadministered in neurological issues) is on the market in UK but does not have any reimbursement code available in the next couple of month

❏ GP are not trained
❏ LPP could exist: HAS procedure
❏ CE mark under MDR (class IIa)
❏ Direct purchasing by hospital: No, DTx
❏ Protocol: recommendations?
❏ Evidence: scarce, French context is close to UK context, strong (I-A) recommandation by EU.based med. soc.

❏ GP/Nurse well trained
❏ No reimbursement scheme in UK
❏ CE mark under MDR (class IIa)
❏ Direct purchasing by HMO-like GP groups
❏ Protocol: recommendations?
❏ Evidence: very good, no HEOR but quality and awareness are strongly demonstrated
Problem solving approach: PECAN/DTx + transposability study into French context (≈ 150 patients) in order to confirm the results → fully reimbursed DTx on LPPR
Question: is rehabilitation a matter of GP OR physiotherapists? - Answer: step-by-step evidence generation and cooperation protocol (scope: regional areas, ±3 years/ARS) based on reimbursement
35

Vincent VERCAMER
Delegation for Digital Health (DNS) - Ministry of Health

What are the main challenges for the digital health acceleration strategy ?

Delegation for Digital Health (DNS)
Part of the French Ministry of Health, DNS manages digital health transformation projects

Regulation & Compliance
Hela
Co-responsible for Digital Health
Technical Board
Europe & International relations

SPIS
Skills, Innovation and Research
Public Service for Health Information
"Ségur Numérique"
Mon Espace Santé
3 7
Ghariani and David Sainati

A strategy to support all levels of a digital health project cycle

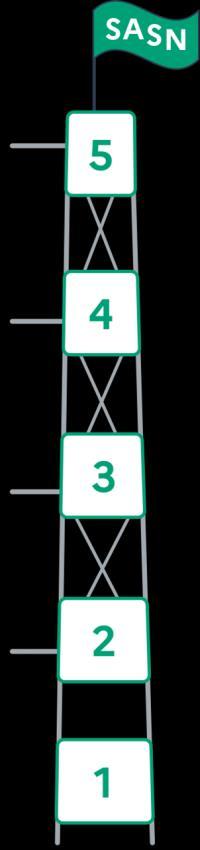
Foster the conditions for the large-scale deployment of successful digital health projects
Sustain the implementation of experimentations in real-life conditions and first industrial steps
Support the development of structural projects to strengthen strategic territorial advantages
Prepare the future generation of key digital health technologies and facilitate rapid transfer mechanisms of research results
Develop stakeholder training, stakeholder trust and professional attractiveness of the sector
3 8
Digital health acceleration strategy (SASN)
Actions to support the life cycle of digital health projects
Exemple of a digital medical device (DMD) reimbursement

Actions

Research, development, innovation

Regulatory diagnostic service for MD
Call for proposals for evaluation support
Clinical evaluation harmonisation
third party digital healthcare solutions
PEPR
Prematuration/ maturation calls
I-Nov call for projects
Health data warehouses
« AMI » call for proposals

Research


Commercialization
CE marking

Clinical investigation
Regulatory support
Technical documentation
Quality Management System
Financial support
Experimentation
Co-design
Maturation
Innovation
Health Data Warehouses
Education


Regulatory support
Clinical or medico-economic research protocols
European collaboration
Harmonisation of clinical evidence methods
Experimentation
Uses
Experimentation
Evaluation
Health professionals, engineers, computer scientists, in-houselawyers, regulatory affairs associates


Experimentation Post Marketing Clinical Follow-up

Clinical
Reimbursement DMD reimbursed
evaluation
39
health acceleration strategy (SASN)
track
DTx DMDs
Digital
PECAN Fast
reimbursement for telemonitoring &
PECAN, an innovative approach to derogatory reimbursement of digital medical devices (DMDs)



• New one-year transitional and temporary reimbursement access scheme
• Launched in April 2023
• First listed solution in October 2023

• Innovative DMDs for individual use
• for therapeutic purposes (DTx)
• Remote Monitoring (TLS)
• With initial elements to assess the potential benefits of the solution
• Running studies with results available before the end of PECAN

Overview Scope Why?
• Rapid access to digital health innovations for patients
• Operational deployment in parallel of procedures to get a standard definitive reimbursement

How?
• Parallel evaluation process by HTA Agency (HAS) & Digital health agency (ANS)
• Authorisation decision by the Minister of Health
• process duration: less than 6 months from application to first patient
40



The requirements of the repository are derived from the doctrine of digital healthcare and the various ANS benchmarks.

Documents available on the Digital Health Agency portal for industrials:
https://industriels.esante.gouv.fr/produits-et-services/dispositifs-medicaux-avec-des-fonctions-numeriques




https://www.hassante.fr/jcms/p_3213810/en/pathway-ofmedical-devices-in-france
Additional online resource

Last CEPS activity report
https://sante.gouv.fr/IMG/pdf/ra_ceps _2022.pdf
Abbreviations and acronyms
HAS= Haute Autorité de santé (French National Authority for Health)
LPPR= Liste des produits et prestations remboursables (List of products and services qualifying for reimbursement
MD=Medical Device
DMD=Digital Medical Device
DRG= Diagnosis-related group
CNEDiMTS=Commission nationale d’évaluation
des dispositifs médicaux et technologies de santé (Medical Device and HealthTechnology Evaluation Committee)
CEPS=Comité économique des produits de santé (French Healthcare Products Pricing Committee)
PECT= mécanisme de prise en charge transitoire (transitional reimbursement mechanism)
PECAN=prise en charge anticipée numérique (Early Access to Reimbursement for Digital Medical Devices.
MDR : medical device regulation (2017/745)
IVDR : in vitro device regulation (2017/746)
MDD : medical device directive (93/42 CE)
Clin/Mkt : Cinical/Market
RPM : remote patient monitoring
POC : proof of concept
HEOR: health economics and outcome research
UX/UI : interface user experience (usability)
HCP: healthcare professional
SLA: service level agreement
Abbreviations and acronyms
RCT: randomized controlled trials
RWD/RWE: real world date real world evidence
BD/L: bus dev and licensing
SHEP/KOL: stakeholder engagement plan/key opinion leaders
P4P/VBP: payment for performance/value based procurement
Meded: medical education
PROMs/PREMs: patient reported outcome/experience
DRG: disease related groups
GP: general practitioners
HMO: health management organisation
Q&A
• Chaymaa.chalf@fcdo.gov.uk
• Julie.lebouleux@fcdo.gov.uk
THANK YOU!
























 Hubert Galmiche February 2024
Hubert Galmiche February 2024


































































