REIMBURSEMENT FOR 3D-PRINTED DEVICES
HIGHLIGHTS FROM THE 2024 AOPA NATIONAL ASSEMBLY
MEET A FUTURE-FOCUSED O&P COMPANY OWNER

REIMBURSEMENT FOR 3D-PRINTED DEVICES
HIGHLIGHTS FROM THE 2024 AOPA NATIONAL ASSEMBLY
MEET A FUTURE-FOCUSED O&P COMPANY OWNER
New CMS rule presents opportunities for postmastectomy and lymphedema care P.14



THE PREMIER MEETING FOR ORTHOTIC, PROSTHETIC, AND PEDORTHIC









September 3–6, 2025, for an ideal combination of top-notch education and entertainment at the 108th AOPA National Assembly in Orlando, FL, at the Orange County Convention Center (OCCC).










EXHIBITS EDUCATION NETWORKING








Facilities that wade beyond traditional O&P care to offer postmastectomy and lymphedema products and services benefit from a new revenue opportunity while serving underserved patient bases. Plus: Learn how Medicare coverage for lymphedema products has improved with implementation of the Lymphedema Treatment Act.
BY CHRISTINE UMBRELL
Cole Carlson, PE, owner and chief executive officer at Tamarack


The 107th AOPA National Assembly drew thousands of O&P stakeholders to take part in a future-focused keynote address; educational presentations on the latest O&P research, advancements, and business practices; exciting networking opportunities; and an exhibit hall packed with the latest products and technologies. Our photo feature from the September event features popular sessions and activities from Charlotte.


President
Mitchell Dobson, CPO
Hanger Clinic, Austin, TX
President-Elect
Rick Riley
O&P Boost, Bakersfield, CA
Vice President
Kimberly Hanson, CPRH Ottobock, Austin, TX
Treasurer
Chris Nolan
Össur, Foothills Ranch, CA
Immediate Past President
Teri Kuffel, JD
Arise Orthotics & Prosthetics, Spring Lake Park, MN
Executive Director/Secretary Eve Lee, MBA, CAE
AOPA, Alexandria, VA
Arlene Gillis, MEd, CP, LPO International Institute of Orthotics and Prosthetics, Tampa, FL
Adrienne Hill, MHA, CPO(L), FAAOP Kennesaw State University, Kennesaw, GA
John “Mo” Kenney, CPO, LPO, FAAOP Kenney Orthopedics, Lexington, KY
James Kingsley
Hanger Clinic, Oakbrook Terrace, IL
Lesleigh Sisson, CFo, CFm OrthoPro of Carson City Carson City, NV
Matt Swiggum Proteor, Tempe, AZ
Linda Wise
Fillauer Companies, Chattanooga, TN
Shane Wurdeman, PhD, CP, FAAOP(D) Research Chair
Hanger Clinic, Houston Medical Center, Houston, TX
AMERICAN ORTHOTIC & PROSTHETIC ASSOCIATION (AOPA)
330 John Carlyle St., Ste. 200 Alexandria, VA 22314
Office: 571-431-0876
Fax: 571-431-0899
AOPAnet.org
EXECUTIVE OFFICES
Eve Lee, MBA, CAE, executive director, 571-431-0807, elee@AOPAnet.org
Akilah Williams, MBA, SHRM-CP, director of finance and strategic operations, 571-431-0819, awilliams@AOPAnet.org
HEALTH POLICY AND ADVOCACY
Joe McTernan, director of health policy and advocacy, 571-431-0811, jmcternan@AOPAnet.org
Devon Bernard, assistant director of coding and reimbursement services, education, and programming, 571-431-0854, dbernard@AOPAnet.org
Morgan Fabber, MPH, manager of state and federal advocacy, 571-431-0814, mfabber@ AOPAnet.org
MEETINGS & EDUCATION
Ashley Vande Bunte, CMP, director, meetings and education, 571/431-0860, avandebunte@AOPAnet.org
Kelly O’Neill, CEM, assistant director, meetings and exhibitions, 571-431-0852, kelly.oneill@AOPAnet.org
MEMBERSHIP & COMMUNICATIONS
Joy Klapp, director of communications and membership, 571-431-0817, jklapp@AOPAnet.org
Betty Leppin, senior manager of member services, 571-431-0810, bleppin@AOPAnet.org
Madison McTernan, coordinator of membership and communications, 571-431-0852, mmcternan@AOPAnet.org
AOPA Bookstore: 571-431-0876
Reimbursement/Coding: 571-431-0833, LCodeSearch.com
O&P ALMANAC
Eve Lee, MBA, CAE, executive director/publisher, 571-431-0807, elee@AOPAnet.org
Josephine Rossi, editor, 703-662-5828, jrossi@contentcommunicators.com
Catherine Marinoff, art director, 786-252-1667, catherine@marinoffdesign.com
Bob Heiman, director of sales, 856-520-9632, bob.rhmedia@comcast.net
Christine Umbrell, editorial/production associate and contributing writer, 703-662-5828, cumbrell@contentcommunicators.com
PUBLISHER EVE LEE, MBA, CAE
EDITORIAL MANAGEMENT CONTENT
COMMUNICATORS LLC
ADVERTISING SALES RH MEDIA LLC
DESIGN & PRODUCTION MARINOFF DESIGN LLC
PRINTING SHERIDAN
O&P Almanac (ISSN: 1061-4621) is published monthly, except for combined issues in June/July and November/ December, by the American Orthotic & Prosthetic Association, 330 John Carlyle St., Ste. 200, Alexandria, VA 22314. To subscribe, contact 571-431-0876, fax 571-431-0899, or email info@aopanet.org. Yearly subscription rates: $59 domestic, $99 foreign. All foreign subscriptions must be prepaid in U.S. currency, and payment should come from a U.S. affiliate bank. A $35 processing fee must be added for non-affiliate bank checks. O&P Almanac does not issue refunds. Periodical postage paid at Alexandria, VA, and additional mailing offices.
Postmaster: Send address changes to: O&P Almanac, 330 John Carlyle St., Ste. 200, Alexandria, VA 22314.
Copyright © 2024 American Orthotic and Prosthetic Association. All rights reserved. This publication may not be copied in part or in whole without written permission from the publisher. The opinions expressed by authors do not necessarily reflect the official views of AOPA, nor does the association necessarily endorse products shown in the O&P Almanac. The O&P Almanac is not responsible for returning any unsolicited materials. All letters, press releases, announcements, and articles submitted to the O&P Almanac may be edited for space and content. The magazine is meant to provide accurate, authoritative information about the subject matter covered. It is provided and disseminated with the understanding that the publisher is not engaged in rendering legal or other professional services. If legal advice and/or expert assistance is required, a competent professional should be consulted.
Share your message with AOPA membership— approximately 9,000 orthotic and prosthetic professionals, facility owners, and industry personnel. Contact Bob Heiman at 856-520-9632 or email bob.rhmedia@comcast.net. Learn more at bit.ly/24AlmanacMediaKit.


The new Icon microprocessor knee features responsive sensors, streamlined setup, and the intuitive Stride Studio app. Our team has developed patented technology to put the Icon in a league of its own—providing remarkable accuracy, response time, and weight savings. Equipped with a long-lasting battery and IP68 rating, the Icon is the versatile solution for low to high activity users.


“I was surprised by the smoothness of the Icon’s microprocessor while walking. My favorite feature is that it’s waterproof in fresh water. During the summer months, I am always in and out of lakes, so being able to just jump right in without thinking twice is so nice.”
-Jake, Icon User




In 2024, 32.8 million people are enrolled in a Medicare Advantage plan, accounting for 54% of the eligible Medicare population.
SOURCE: “MEDICARE
A new study by University of Toronto researchers finds that lower-limb prosthesis users may benefit from a wearable rhythmic auditory stimulation (RAS) system that facilitates “walking to a beat” during rehabilitation to regain mobility. The protocol involves having lower-limb prosthesis users leverage wearable technology integrated with RAS-based biofeedback.
Study participants were equipped with two systems: a biofeedback system designed to provide RAS and a wearable sensor-based motion capture system to gather spatial and kinematic gait parameters. The researchers tested both open-loop and closed-loop feedback strategies for RAS: The open-loop approach does not consider the user’s performance, but the closed-loop approach takes user performance into account via a progressive adaptive-based strategy.
Researchers found that RAS induced changes in temporal gait symmetry via both open-loop and closed-loop approaches; participants were able to maintain the beat and improve temporal symmetry immediately after the biofeedback trial.
“Compared to open-loop, closed-loop was able to improve temporal symmetry by an additional 2%, on average,” reported the research team. “This highlights the potential benefits of using a closed-loop strategy, where the system ensures that the user achieves the given target before introducing a substantially different target from their baseline gait.”
Future investigations should focus on adaptable systems that cater to individual needs and incorporate long-term assessments across diverse gait symmetries, according to the researchers. The study was published in IEEE Access in August. CODING CLARIFICATION
In the Reimbursement Page article titled “PDAC Primer” on page 10 of the September 2024 O&P Almanac, it was stated that customfabricated inserts fabricated in-house do not need to be PDAC verified by the Pricing, Data Analysis, and Coding (PDAC) contractor. That rule only applies to the A5513 and not the A5514. We apologize for any confusion the information may have caused.

To explore the physical impacts of pregnancy on women with amputation, a team of Canadian researchers conducted semistructured interviews with 19 women with limb loss who had experienced a pregnancy in the past 10 years. Led by Brittany Pousett, MSc, CP(c), head of research at Barber Prosthetics Clinic, the team sought to understand how pregnancy affects mobility, prosthesis fit, and prosthesis use.
The researchers found variation in the experience of women’s symptoms and prosthesis use. They noted that physical symptoms were similar to—but more substantial than—those experienced by women without limb loss. Most participants reported that they leveraged both
self-management techniques and prosthetist adjustments to manage changes in residual limb volume. The researchers noted that pregnancy impacted the way in which women were mobile and the activities they chose to participate in, and some devised “creative mobility solutions” to complete activities, including prosthesis use, assistive equipment, and adaptive movement.
“Women with lower-limb absence and their healthcare providers must be aware of the wide range of experiences women face during pregnancy and treat each pregnancy uniquely,” the researchers reported. “Planning ahead and working with a healthcare team can mitigate many of these challenges.” The research was published in July in Disability and Rehabilitation
The American Heart Association (AHA) and its partners published a map of the United States that indicates rates of lower-limb amputations by region. The interactive graphic demonstrates the risk of leg, foot, or toe amputations due to peripheral artery disease (PAD) by locale.
According to the map, which pulled data from Medicare and the U.S. Census Bureau, residents of Southern states face the most significant risk of lower-limb amputation: Rates were highest in Mississippi, followed by Texas, Louisiana, Alabama, and South Carolina. The map also indicates that rural residents, people with fewer resources, and Black and Native American people are at greatest risk of PAD-related amputation.
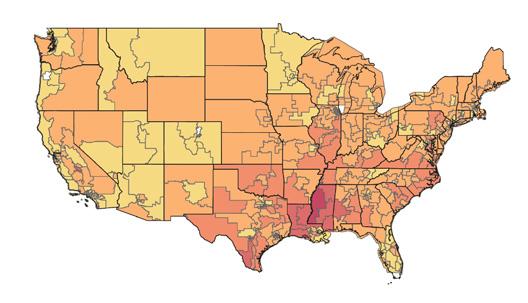
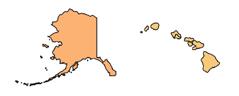
“Much of the amputation data we are seeing is not surprising, yet the map helps us confirm and illustrate what we suspected in terms of where the biggest issues are,” said Marc Bonaca, MD, chair of cardiovascular research at University of Colorado School of Medicine, in an AHA press release. “When patients with PAD and their health teams advocate for themselves, we can share a firm message that this is a public health issue, and it is preventable.”

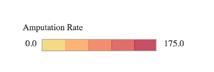
Visit “Nontraumatic Lower-Extremity Amputations By Congressional District Heat Map” to access information on your region.
Team USA prevailed during the Paralympic Games in Paris, accumulating a total of 105 medals: 36 gold, 42 silver, and 27 bronze.
Members of Team Össur and Össur Ambassadors delivered medal-winning performances in several long jump, high jump, sprinting, para triathlon, and para cycling contests, earning a total of 11 gold, seven silver, and four bronze medals.

Fleur Jong of The Netherlands defended her Tokyo Paralympic sprinting and long jump gold medals with another double gold medal-winning performance in Paris. Össur athletes completed a sweep of the women’s T64 long jump event, led by Jong, with a 6.53-meter jump, followed by silver medalist Marlene van Gansewinkel of The Netherlands and bronze medalist Beatriz Hatz of the United States.
Germany’s Markus Rehm earned his fourth consecutive Paralympic gold medal in the men’s long jump T64 category with a distance of 8.13 meters, followed


by first-time U.S. Paralympic competitor Derek Loccident, who earned the silver medal.
Team USA’s Hunter Woodhall, who was featured in the June/July issue of O&P Almanac, earned his first-ever Paralympic gold medal in the men’s 400-meter sprint.
“The Paris Paralympics set new standards for excellence, inclusivity, and audience enthusiasm, and our athletes more than rose to the occasion,” said Sveinn Solvason, president and chief executive officer of Embla Medical, Össur’s parent company.
Ten children lose one or both legs each day in the Gaza Strip as a result of the Israel-Hamas war, according to senior officials of the United Nations Relief and Works Agency for Palestine Refugees. “Gaza is home to the largest cohort of child amputees in modern history,” noted one official during the October 9 United Nations Security Council meeting.
O&P ATHLETICS
PARALYMPIC ATHLETES FACE INJURY RISK
Paralympic athletes had a 1.79 times greater risk of injury and 2.44 greater risk of illness than Olympic athletes, according to a study of 300 Team USA athletes led by the U.S. Olympic & Paralympic Committee.
O&P facilities that offer these products and services meet the needs of an important patient demographic
U.S. WOMEN IMPACTED BY BREAST CANCER
310,720
New cases of invasive breast cancer diagnosed in 2024
4 Million
Breast cancer survivors in the United States
13%
Percentage of women who will be diagnosed at some point in their lives
62
Average age when diagnosed
PERCENTAGE OF O&P FACILITIES THAT OFFER POSTMASTECTOMY AND LYMPHEDEMA PRODUCTS
Read tips for offering postmastectomy and lymphedema care at O&P facilities on page 14.

The American Board for Certification in Orthotics, Prosthetics, and Pedorthics Inc. (ABC) has awarded the William D. Beiswenger Volunteer Award to John H. Reynolds, CPO, FAAOP. Reynolds was chosen by the ABC Board of Directors to receive the award for his volunteer work supporting ABC’s mission and for embodying the spirit of the award.

The Beiswenger Award was established in 2000 to honor its first recipient, William Beiswenger, CPO, FAAOP, for his record of volunteerism and his commitment to the ABC mission. The award recognizes volunteers who demonstrate dedicated service to ABC and the promotion of its mission to provide the highest standards of excellence in patient care.
Reynolds began his service to ABC in 1988 as an examiner for the Clinical Patient Management (CPM) examinations. He has volunteered for several ABC committees and served as Long-Range Examination Advisory Committee chair, Professional Credentialing Committee chair, Prosthetic Exam Development Committee member, Practice Analysis Task Force member, and ABC Board of Directors member, as well as president of the board in 2001. Reynolds continues to serve as an orthotic CPM examiner and chair of the Scope of Practice Committee.
“I have had the pleasure of working with John for many years as a CPM examiner and have seen him mentor many other ABC volunteers,” said Billy Lester, CPO, ABC president. “His long-time commitment to serving the profession is commendable, and he has certainly demonstrated the leadership and volunteerism that the Beiswenger Award represents.”
Sara Peterson-Snyder, PhD, CPO, and Kyle Leister, PhD, CPO, have been awarded a 2024 Early Career Research Grant by the Orthotics and Prosthetics Foundation for Education and Research. The grant, which supports clinically relevant, investigator-initiated research in O&P, is a one-year award in the amount of $30,000 that is intended to help early career investigators initiate lines of research that will be competitive for larger funding opportunities.


Peterson-Snyder, a research prosthetist and consultant for O&P Clinical Innovations, and Leister, an assistant professor and program director for the Master of Science in O&P Program at East Tennessee State University, were awarded for their work on “Detecting Change in Muscle Parameters, Pain, and Function With Neuromuscular Electrical Stimulation Treatment in Individuals Living With Transtibial Amputation.” Peterson-Snyder is the principal investigator and Leister is the co-investigator on the project.

Cascade Orthopedic Supply and Ottobock have agreed that Cascade will redeem Ottobock’s ownership interests in Cascade. The signing of this transaction took place Sept. 13, 2024, and the closing is expected to take place during the next few weeks. At the closing, Cascade will move forward as an independently held company. Cascade and Ottobock have continued to operate autonomously, and their working relationship as supplier and distributor will remain unchanged. Cascade will maintain distribution of Ottobock’s orthotic and prosthetic devices and uphold a strong supplier partnership.
“I am elated to have the opportunity to redeem all outstanding ownership interests of Ottobock by Cascade. Our time at Ottobock allowed us to strengthen the core competencies of the business and achieve growth despite challenging market conditions. I am grateful for those who have worked with us along the way, and excited to bring independent innovative solutions to our customers,” said Jeff Collins, chief executive officer of Cascade Orthopedic Supply.
“We are proud of the progress Cascade made as part of Ottobock. Under Jeff Collins’ leadership, the team achieved significant growth and refined their core competencies during a dynamic period,” said Scott Schneider, head of government/medical affairs and future development at Ottobock North America. “We are confident in their future success as an independent entity. We look forward to seeing the innovative solutions they will continue to bring to the O&P community, and we remain committed to advancing our shared mission of improving patients’ lives through innovation and collaboration.”
The Northwestern University Prosthetics-Orthotics Center (NUPOC) has announced a new scholarship: The Hittenberger Legacy Fund Scholarship. The scholarship was established after an initial lead gift from Tina Hittenberger, CO(E), who comes from a family of O&P professionals. The C.H. Hittenberger Co. was established in 1902 by Hittenberger’s grandfather. Hittenberger hopes that the scholarship will generate awareness of the growing number of women in the O&P profession, and that the scholarship will encourage others to support a new generation of practitioners. The first Hittenberger Legacy Fund Scholarship will be awarded in the 2024-2025 school year.
BY JOE MCTERNAN

The O&P profession relies on innovation and advances in technology to keep patients active and confident that their prosthesis or orthosis will meet their needs and demands. Stronger and lighter materials and technologies—such as microprocessor control, artificial intelligence, and enhanced fabrication techniques—are just some of the ways that advancements are changing how O&P practitioners treat their patients. O&P is not immune to the challenges that face other professions when technology outpaces the ability of the coverage and coding system to adjust accordingly.
Managing these challenges effectively is important so that innovators continue to see value in pursuing new technology that improves the lives of the patients we serve.
One area where these challenges are becoming more relevant is the advent and increasing commercial viability of 3D printing in the fabrication of complete orthoses and prostheses and O&P components. This month’s Reimbursement Page examines how 3D printing is generating exciting discussions within the O&P profession and discusses ways in which it can be brought into the arena of coverage, coding, and reimbursement.

For years, when the topic of 3D printing was discussed, the narrative was dominated by visions of large, expensive, and slow machines that took hours to create tiny plastic trinkets, such as figurines. 3D printing was cool to watch, and it was fun to touch the end product of the process—but it was cost prohibitive and overly time consuming to be much more than a conversation piece.
The days of the 3D-printed novelty item are well behind us as advances in
technology have led to more efficient, cost-effective, and commercially viable 3D-printing techniques with applications across the manufacturing spectrum, including the fabrication of prostheses, orthoses, and their individual components.
The use of 3D printing as a fabrication technique is no longer in question, but multiple challenges remain for the O&P profession. Common questions include: Is there a place for 3D printing within the existing O&P coding structure? Are 3D-printed prostheses considered exoskeletal or endoskeletal prostheses? How does 3D printing fit within Appendix C of the Medicare Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Quality Standards? Do addition/feature codes apply to 3D-printed prostheses and orthoses?
For many, if not all, of these questions, the answer is certainly not easy and—depending who you ask—may not be consistent. AOPA is actively collaborating with manufacturers, engineers, clinicians, and researchers to determine how 3D printing can be effectively accommodated—whether within the existing coding, coverage, and reimbursement hierarchy; through development of new codes and coverage policies; or some combination of both.
Traditionally, the first branch of the decision tree regarding proper coding of a prosthesis was whether it utilized an endoskeletal or exoskeletal design. This decision would allow the clinician to choose the appropriate base code for the general prosthetic design and then choose appropriate addition codes to describe specific components and features of the finished prosthesis that made it unique in design and function. Essentially, if the prosthesis used a pylon to connect the socket to the foot, it was considered endoskeletal; and if it used a rigid, shin-like shell to distribute forces between the socket and the foot, it was considered exoskeletal.
3D-printed prostheses blur those lines significantly as these devices often use a hybrid design that distributes forces—for example, a shin component of an exoskeletal prosthesis but provides support and flexibility similar to an endoskeletal pylon. 3D printers are used to create lattice-like structures that provide the benefits of both an exoskeletal and an endoskeletal prosthesis in a single design. The natural question is: How is this type of prosthesis coded?
Addressing coding challenges for 3D-printed orthoses may not be as difficult as it is for prostheses because 3D-printed orthoses are not required to be classified as exoskeletal or endoskeletal.
The coding system that has been in place for more than 40 years intentionally relies on the use of a handful of “base” procedure codes and multiple “addition” codes to describe a complete prosthesis or orthosis. This modular system keeps the total number of unique procedure codes manageable but allows for virtually endless combinations of codes that can be used to describe highly customized O&P items.
While the coding system generally works well, it lacks the ability to quickly accommodate significant changes in technology, such as 3D printing. The current system requires classification of a prosthesis as either exoskeletal or endoskeletal and does not include an “other” option, other than using miscellaneous procedure codes. Also known as “99” codes, miscellaneous codes
work well to describe small nuances but are difficult to use when describing a prosthesis or orthosis that is so different that it cannot be classified under an existing base procedure code. Because 3D-printed prostheses do not clearly meet the definition of an endoskeletal or an exoskeletal prosthesis, assigning an appropriate base procedure code is difficult, if not impossible. So, what do we, as a profession, do?
Whenever emerging technology creates a coding scenario that cannot be met within the existing system, there are essentially two options: Either new codes must be created, or existing codes must be modified to accommodate the technology. Both processes take time and must be undertaken with careful consideration of unintended consequences.
Getting a new code is never as simple as just asking. New codes must be supported by data, research, and documentation of need. Existing codes may be able to be modified to include 3D printing as a design feature but still include the challenge of whether that design is exoskeletal or endoskeletal in nature.
Addressing coding challenges for 3D-printed orthoses may not be as difficult as it is for prostheses because 3D-printed orthoses are not required to be classified as exoskeletal or endoskeletal. 3D printing of orthoses is much more of a different way to fabricate an orthosis that is already described by the existing Healthcare Common Procedure Coding System (HCPCS) code set. This was reinforced when the Pricing, Data Analysis, and Coding (PDAC) contractor and the durable medical equipment Medicare administrative contractors (DME MACs) issued a joint Correct Coding Bulletin in February 2024 that confirmed that 3D-printed orthoses could be described using custom-fabricated orthotic HCPCS codes as long as the fabrication process is compliant with the requirements of Appendix C of the DMEPOS Quality Standards.
It is clear that proper coding of 3D-printed prostheses will require the creation of at least some new codes, and profession-wide consortia have been convened to discuss the best ways to accomplish this goal. 3D-printed orthoses are more likely to fit within the existing code set, as indicated by the publication of formal coding guidance from the PDAC and DME MACs, and work has been started to help O&P businesses and practitioners effectively navigate the process. Another equally important challenge involves compliance with the DMEPOS Quality Standards, specifically Appendix C.
The PDAC/DME MAC Correct Coding Bulletin clarifies the use of customfabricated codes to describe 3D-printed orthoses, but there is an entire subset of custom-fabricated HCPCS codes that require the creation and use of a positive model of the patient’s anatomy in the actual code language. This is where the Quality Standards come into play.
When the DMEPOS Quality Standards were established 20 years ago, digital











models and additive manufacturing processes were barely on the radar and certainly were not part of the equation. The first time there was a need to amend the Quality Standards involved the use of direct carving of total contact diabetic inserts. Technology was developed that would allow a practitioner to create a digital model of the patient’s foot using a sophisticated scanning device that would then be used to direct a milling machine to carve a total contact diabetic insert from a block of material. This created a conflict with the DMEPOS Quality Standards, which required a physical model of the patient’s foot to be created in order to meet the definition of “molded to patient model.” While it took some time, CMS eventually agreed to amend Appendix C of the DMEPOS Quality Standards to include this process.
This is precedence—and may be used as a basis to argue for additional amendments to the Quality Standards to better accommodate 3D printing as a complete custom-fabrication process, regardless of whether a physical or digital model is used to achieve it.
3D printing continues to expand as a popular fabrication option for the full
























spectrum of orthotic and prosthetic services. While technology always comes with growing pains, work is already under way to make sure that it remains a viable way to provide the best possible care for your patients. It is exciting to see what the future will bring.

Joe McTernan is director of health policy and advocacy at AOPA. Reach him at jmcternan@AOPAnet.org
In the Reimbursement Page article titled “PDAC Primer” on page 10 of the September 2024 O&P Almanac, it was stated that custom-fabricated inserts fabricated in-house do not need to be PDAC verified by the Pricing, Data Analysis, and Coding (PDAC) contractor. That rule only applies to the A5513 and not the A5514. We apologize for any confusion the information may have caused.












































It actually feels like you have a leg. Feels a lot lighter, very comfortable. When I stand still, I balance very easily. It’s like a part of you and you’re not scared.”


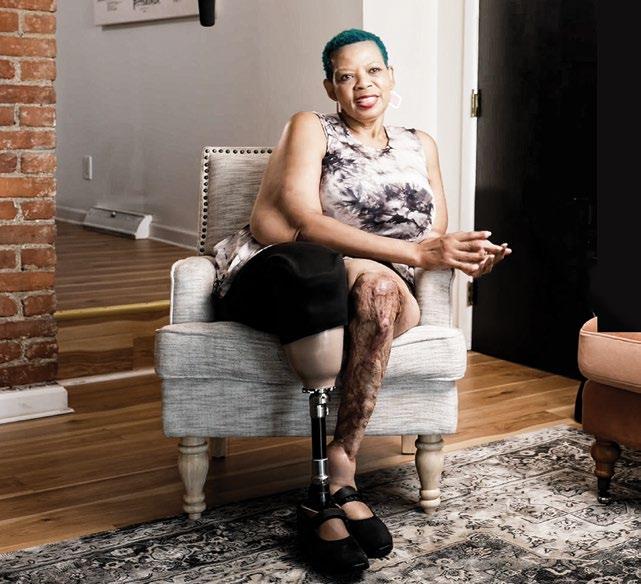
For the first time in all the different prostheses and feet I have tried, and I have tried so many, it felt like I had my leg back again. The Kinterra is life changing. It has inspired me.”

BY CHRISTINE UMBRELL

Branching out to offer products and services beyond traditional orthotic and prosthetic devices provides benefits to patients and can potentially improve patient-care facilities’ bottom lines. Recently, some facilities that offer mastectomy and lymphedema products have experienced a boost in sales of lymphedema garments due to CMS’s decision to cover a new benefit category as part of Medicare Part B: S04 lymphedema compression treatment items. The coverage, which began January 2024 as part of the Lymphedema Treatment Act (LTA), is geared toward patients diagnosed with primary or secondary lymphedema who have a prescription for compression supplies. Since January, some facilities have seen increased sales of lymphedema
f Offering postmastectomy and lymphedema care can boost revenues while meeting a significant patient need as many Americans struggle to find facilities that offer these services.
f Via the Lymphedema Treatment Act, which took effect in January, Medicare Part B covers a new benefit category, S04 lymphedema compression treatment items, for patients diagnosed with lymphedema who have a prescription for compression supplies.
and mastectomy products to individuals affected by tissue swelling or breast cancer. Breast cancer accounts for 30% of all new female cancers each year, with more than 300,000 new cases of invasive breast cancer diagnosed annually, according to the American Cancer Society. Up to 30% of breast cancer survivors acquire debilitating lymphedema; other individuals acquire lymphedema secondary to other cancers, radiation treatment, surgery, trauma, or other comorbidities. Approximately one in 1,000 Americans are impacted by secondary lymphedema, according to the National Institutes of Health. Primary lymphedema, which is inherited or congenital, is far rarer, affecting one in 100,000.



f Between 20% and 30% of breast cancer patients will develop secondary lymphedema, so pairing lymphedema care with mastectomy care makes sense for O&P facilities.
f When adding mastectomy or lymphedema services, O&P business owners and fitters can work closely with manufacturers of these products and lean on their resources and network of support to select the right product mix and options for their patient base.
f O&P facilities can boost awareness of their postmastectomy and lymphedema offerings by networking with local doctors and surgeons and marketing to cancer centers, physical therapy and lymphedema therapists, hospitals, and medical groups.




All of these patients need appropriate care so they can live their best lives. “Severe lymphedema can be as impactful to patients as losing a limb due to the pain, discomfort, and complexities of care,” says Lesleigh Sisson, CFo, CFm, vice president and general manager of O&P Insight.
Patients diagnosed with lymphedema have traditionally struggled to receive Medicare and other payor coverage for compression garments, according to Sisson. “The LTA passed by Congress in December 2022 now provides patients with the coverage they need to manage their chronic disease,” she explains. “Since being passed, the hope is for this specialized care to be recognized by private payors. This also signifies a new opportunity for facilities to expand their services, recognize a new billing opportunity, and create additional support networks for this underserved community.”
Medicare lymphedema coverage “represents a significant victory for patients struggling with lymphedema and the healthcare providers dedicated to their care,” says Matthew Gruskin, MBA, BOCO, BOCPD, CDME, chief operating officer of the Board of Certification/Accreditation (BOC). BOC recently launched a lymphedema/ compression accreditation program.

Matthew Gruskin, MBA, BOCO, BOCPD, CDME
O&P facilities that were already accredited by BOC could submit a form to add Product Category S04 to their existing accreditation. BOC also offers a “New Supplier DMEPOS” enrollment option for companies that previously did not offer DMEPOS services but wanted to offer lymphedema services in accordance with the new rules. “More than 350 BOC-accredited facilities have added lymphedema accreditation” this year, says Gruskin.
The American Board for Certification in Orthotics, Prosthetics, and Pedorthics (ABC) has added the new DMEPOS product category for all facilities that have the provision of lymphedema items within their scope of practice, until they have been reaccredited to determine whether they provide that code or not, according to Tammi Richards, director of facility accreditation services at ABC.
Coverage of lymphedema products is important given the significant population who suffers from the disease. Lymphedema refers to tissue swelling caused by an accumulation of proteinrich fluid that’s usually drained through the body’s lymphatic system, according to the Mayo Clinic. It most commonly affects the arms or legs but can also occur in the chest wall, abdomen, neck, and genitals.
Severe cases of lymphedema can impact the ability to move the affected limb, increase the risks of skin infections and sepsis, and lead to skin changes and breakdown. Treatment may include compression bandages, massage, compression stockings, sequential pneumatic pumping, careful skin care, and even surgery to remove swollen tissue or to create new drainage routes.
Between 20% and 30% of breast cancer patients will develop secondary lymphedema, so pairing lymphedema care with mastectomy care makes sense for O&P facilities. “Often, mastectomy patients can benefit from lymphedema compression garments” because there is no cure for lymphedema, says Sisson. “It is also important to note that lymphedema is not always related to mastectomy, and that specific patient population is often overlooked.”

Accredited O&P facilities already offer dozens of DMEPOS product categories, and incorporating lymphedema requires adding just three specific categories—“so it’s not that heavy a lift to add,” says Gruskin. “O&P providers already understand this patient base much better than a drugstore” or other sources of compression garments.
Although there are no certifications required to fit compression items, O&P facilities need to add products to their accreditation and Medicare 855S application, explains Sisson. “This new service offering requires a well-trained team of administrative, billing, and fitting staff that are informed on the selection of compression products available, and the associated coding, billing, and reimbursement.”
Adding lymphedema care at an O&P facility provides “continuity of care” for patients, says Gruskin. “For providers, you reach patients you haven’t been able to reach before,” and position your facility as “a resource to the community.”
Profit margins may be smaller than for orthoses and prostheses, but there is a lot of opportunity for O&P facilities that add these products. “Reimbursement for compression garments can be pretty good,” says Gruskin. While lymphedema pumps are only prescribed once failure of compression garments has been documented, reimbursement for pumps is favorable, Gruskin says. He recommends that facilities consider state laws and requirements about disbursement of lymphedema pumps before adding this category of products.
One facility that has seen an uptick in patients seeking lymphedema care since the LTA took effect is Sierra ProstheticsOrthotics in Northern California.

Sierra P&O has offered mastectomy, lymphedema, and pedorthic care in addition to standard O&P care since the facility was launched 30 years ago. The offerings comprise about 15% of the facility’s business, says Tanya Baer, CFom, office manager and daughter of the company’s founder. While not quite as lucrative as more traditional O&P offerings, mastectomy and lymphedema care serve as a much-needed “community service” because Sierra P&O is in a rural area without many healthcare providers. “It’s a bonus for our patients,” Baer says, and leads to word-of-mouth referrals.
Turbomed’s premium foot drop or thosis sits on the outside of the shoe to facilitate maximized ankle movement while reducing the risk of injury
New MONOCORE TechnologyTM for a drill-less, one-piece design
New height-adjustable calf culf with pressure screws





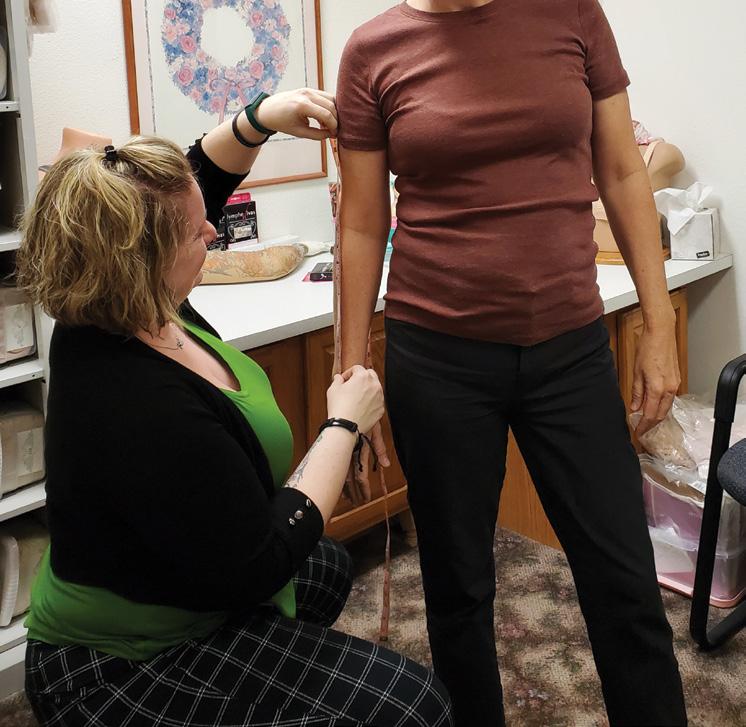
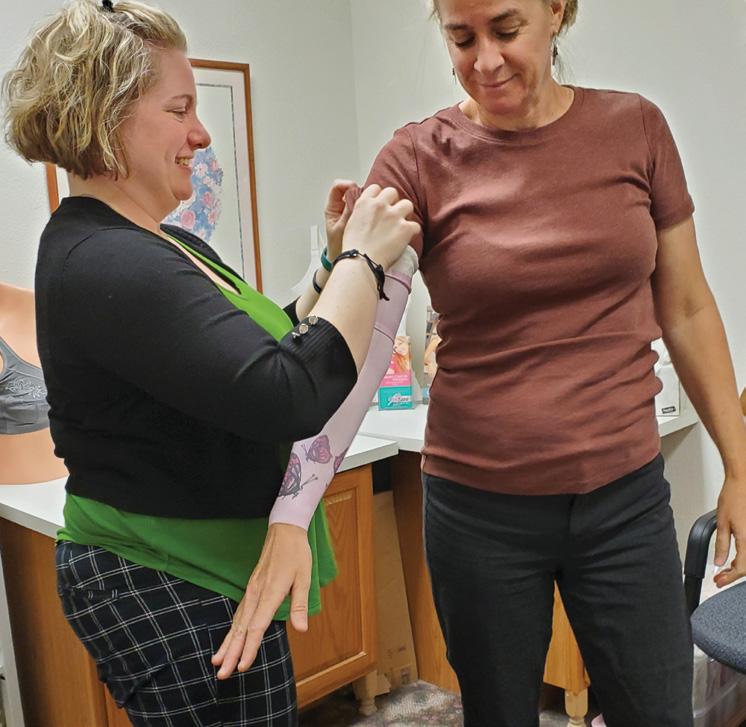



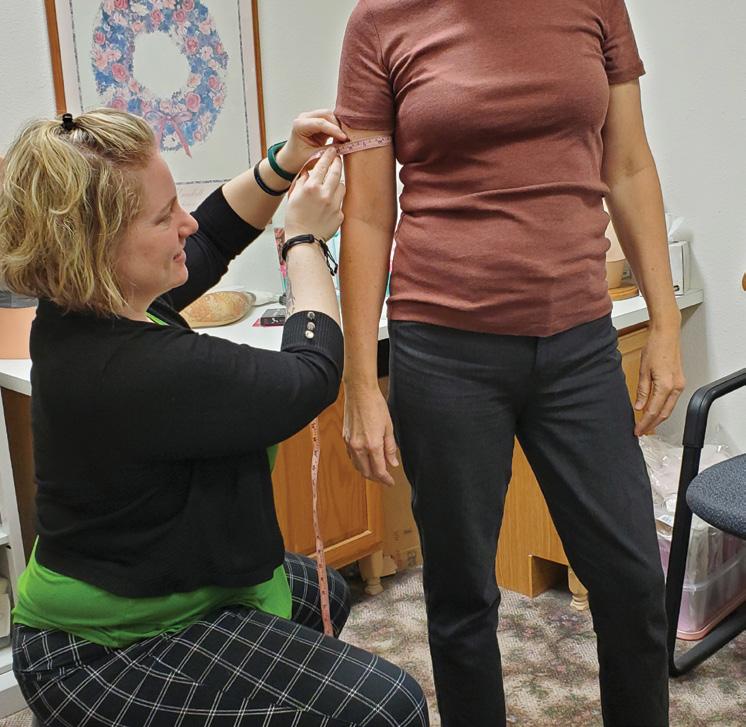
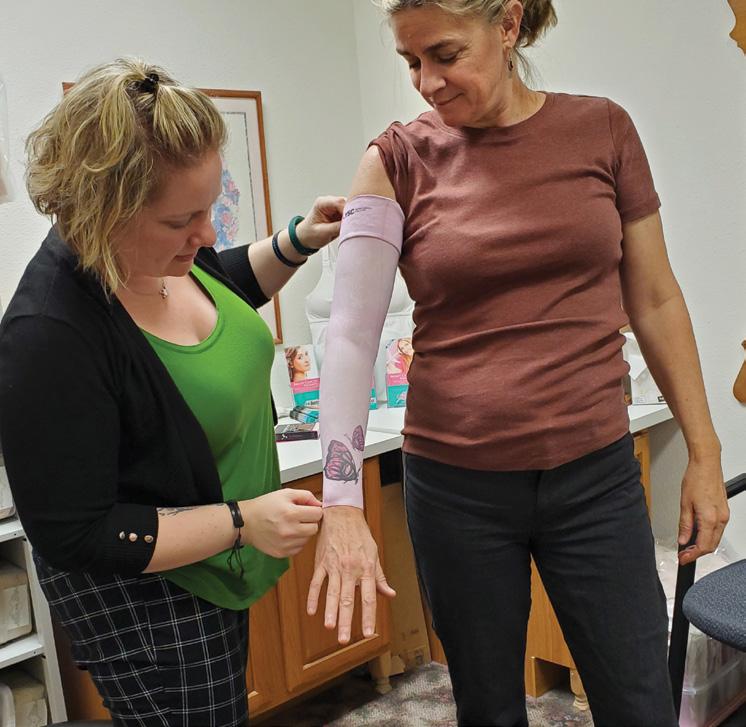
Patients appreciate that Sierra P&O offers a full spectrum of services. “We do mastectomy and over-the-counter compression stockings, sleeves, and compression bras,” says Baer. “Sometimes they’ll come to us for mastectomy care, then decide to get diabetic shoes,” Baer notes. And some women who had lumpectomies come in for lymphedema care, then notice the mastectomy products and decide to purchase partial breast prostheses.
Baer notes that these types of services are increasingly in demand as many boutiques that previously offered these products are closing. She believes that boutique facilities may have struggled with the documentation required for reimbursement from payors— but this is an area where O&P traditionally shines. “In an O&P clinic, you’re already used to doing the documentation and charting,” so adding these services is not much more complicated, says Baer.
Valley Institute of Prosthetics and Orthotics (VIPO) offers both mastectomy services—customized bras, breast forms, and camisoles—and lymphedema products at its locations in California. VIPO’s

two certified mastectomy fitters, Lesly Garcia, CFo, CFts, CFm, and Emma Domke, CFo, say that the coverage changes in January have had a significant positive impact on many of their patients. Some patients who previously had to pay out-of-pocket for compression garments now have coverage for those garments.
Preparing an O&P facility to add mastectomy or lymphedema services isn’t complicated, says Sisson. Facilities can work closely with manufacturers of these products and lean on their resources and network of support to select the right product mix and options for their patient base.
“Manufacturers offer great classes on their products and how to fit them,” agrees Gruskin. “BOC encourages people to take some sort of training” before fitting patients, he says. He also encourages O&P providers to consider taking educational courses on lymphedema as a disease to understand the etiology.
In terms of equipping an O&P facility appropriately, “I recommend keeping a select inventory of prefabricated items like sleeves and stockings, as well as having the ability to measure and scan for custom-fabricated items,” Sisson says.
For those facilities with limited space, Baer notes that “it’s OK to limit your inventory,” although it’s important to stock the minimum required by the accrediting bodies, such as ABC or BOC.
At VIPO, Garcia’s location is spacious, so she keeps many samples on hand. Domke, who is also an O&P resident, works in smaller satellite offices with minimal space for expanded services. “We can’t keep much in stock. But we do keep a wide range of sizes in a couple of products,” Domke says. “I also rely on [print and online] catalogs” to show patients additional options.
Some facilities choose to dedicate a patient room specifically for mastectomy and lymphedema services, but such a space is not required—a shared room can work as well, say sources.
How can an O&P facility market its mastectomy and lymphedema products and services and encourage sources to refer business?




As of Jan. 1, 2024, Medicare Part B covers a new benefit category: S04 lymphedema compression treatment items—for patients diagnosed with lymphedema who have a prescription for compression supplies. CMS created 57 new HCPCS codes for lymphedema compression treatment items.
Highlights of the new coverage rules include the following:
• Covers both prefabricated/standard and custom-fabricated daytime gradient compression garments and/or wraps with adjustable straps. Those with higher levels of compression, ranging from 18 mmgh to more than 40 mmgh. Patient may receive three daytime garments per affected body part every six months.
• Covers both prefabricated/standard and custom-fabricated nighttime gradient compression garments. Those that offer milder compression and are less snug against the skin.
Patient may receive two nighttime garments per affected body part every two years.
• Covers compression bandaging systems and supplies.
• Covers accessories, such as zippers, linings, paddings, or fillers, necessary for the effective use of a gradient compression garment/wrap.
At VIPO, much of their mastectomy and lymphedema business comes from word of mouth. Garcia and Domke have spent time educating patients on the new rules and explaining the frequency with which they are now allowed to be fit with new garments and bras, says Domke.
The Medicare coverage for lymphedema care is comprehensive, says Baer. The new law covers three compression garments per affected body part every six months for daytime usage and two per affected body part every two years for nighttime usage (see the sidebar above)—but many patients are unaware of these benefits.
Baer works with local cancer centers “to let them know these types of services are available.” In rural areas, many mastectomy patients choose not to have reconstructive surgery, she says— but they appreciate the partial breast forms or bras available at Sierra P&O. Baer also works with a local small business that provides wigs for cancer patients in a cross-marketing effort. When new doctors begin practicing in the area, “we introduce our facility and services,” she says.
Sisson recommends getting involved with local support groups—both breast cancer and lymphedema—as well as women’s health centers, cancer centers, physical therapy and lymphedema therapists, hospitals, medical groups, and surgeons.
“It also would be beneficial for businesses to provide marketing materials that showcase their range of services, the selection of products, and how to connect with them,” Sisson says. “Additionally, it can be helpful to work with manufacturers
Under the new rules, if a patient has a diagnosis of lymphedema and a physician or other qualified practitioner orders compression garments, you could bill for custom or standard lymphedema compression garments/wraps for more than one body part or area per patient, and you could bill for both a daytime and nighttime garment/wrap for the same body part or area per patient.

Note that the provision of compression items to treat lymphedema includes all aspects of providing the items and care, including taking all appropriate measurements of the patient’s affected body area(s); providing all fitting services; training the patient on how to don and doff the garments/wraps; training the patient on proper care of the wraps/garments; and providing any needed adjustments.
For details, see the Reimbursement Page article, “New Year, New Rules,” in the January 2024 O&P Almanac
on cooperative education for referral sources, case managers, or anyone else involved in a patient’s lymphedema treatment.”
Mastectomy and lymphedema care are “kind of a niche,” says Domke, “but they’re very important—particularly because the number of facilities that offer these services are few and far between.” Some patients drive more than an hour to be fit with mastectomy or lymphedema products by Domke and Garcia. As more O&P facilities add these services, it will become easier for patients to access them.
“It’s a good service,” agrees Baer. She believes mastectomy and lymphedema care are integral to being a “full-service” clinic. “When a patient who has had a lot of health issues comes in, then gets fit with something that makes them feel ‘normal’ again, they light up,” she says. “It’s great to be a part of that.” This type of care can help boost the mental and emotional well-being of patients.
Facilities that add or expand mastectomy and lymphedema products are graced with the opportunity to serve an underserved community, adds Sisson. “This can provide facilities with a new revenue opportunity and access to a larger breadth of patients,” she says. “As O&P professionals, we are uniquely qualified to evaluate, treat, and deliver these services because of the incredible care we offer to our prosthetic and orthotic patients.”
Christine Umbrell is a contributing writer to O&P Almanac Reach her at cumbrell@contentcommunicators.com


Nearly 2,000 O&P stakeholders celebrated the 2024 AOPA National Assembly in Charlotte, North Carolina, Sept. 12-15. With a theme of “Ignite 24,” the Assembly provided the perfect opportunity for O&P stakeholders from all parts of the industry—clinicians, manufacturers, suppliers, educators, students, and more—to catch up with colleagues, exchange ideas, debate hot topics, and experience new products and services during the many educational sessions and exhibit hall activities at the Charlotte Convention Center.



Highlights of the event included an energetic keynote presentation by Chuck Gallagher, CSP, on “The AI Revolution: Unlocking the Future of Business,” and educational sessions focusing on clinical care, digital care, business, technical, and pedorthic topics. Several prominent O&P professionals were awarded high honors in the form of the AOPA Lifetime Achievement Award, the Thranhardt Clinical Education Awards, the Hamontree Business Education Award, the Hans Georg Näder Digital Education Award, the Legislative Advocacy Awards, and more.
As always, an exuberant crowd took part in this year’s Professional Women in O&P Luncheon.
Attendees flooded to the exhibit hall floor to view cutting-edge products and technologies from O&P manufacturing companies. The exhibit hall also served as home base for several social and networking events, including the Welcome to Charlotte Reception, the Friday night Happy Hour, and lunch and coffee breaks.
Virtual content from this year’s Assembly will be available online through Nov. 30 for registered participants. Mark your calendar now for next year’s Assembly, slated for Sept. 3-6, 2025, in Orlando, Florida.


Professor Hans Georg Näder Digital Education Award
Joshua Steer, PhD
Orthotic and Prosthetic Business Management Certificate
Johnson
Timothy

Opening General Session and Keynote Address
Chuck Gallagher, CSP, keynote speaker
“The AI Revolution: Unlocking the Future of Business”




Student Poster Award Winners
Edwin and Kathryn Arbogast Student Poster Award: Abrar Alamri, MSPO, PT, University of Pittsburgh, “Prosthetic Users’ Survey (OPUS) Scores in Lower-Limb Prosthetic Users Across Multiple Follow-Ups: A Retrospective Analysis” (above left)
Otto and Lucille Becker Student Poster Award: Caitlin Bowman Martwinski, MSPO, Children’s Healthcare of Atlanta /University of Pittsburgh, “Development of a Children’s Book Intervention to Increase Pediatric Orthotic Device Compliance and Improve Peer Attitudes Toward Disability for 3-12 Year Olds” (above right)

Achievement Award
Thranhardt Lecture Series Award Winners
“Reduction in Falls and Fall-Risk With Increased Walking Speed Found Following 1 Year of C-Brace® Use: Interim Results From the C-Brace® Registry”
Tyler Klenow, MSPO, MBA, CPO, FAAOP
Russell Lundstrom, MS
“Assessing Outcomes With Microprocessor Knee Utilization in a K2 Population (ASCENT K2): Findings From a Clinical Trial of 107 Individuals With Above-Knee Amputation”
Andreas Kannenberg, MD (GER), PhD
Shane Wurdeman, PhD, CP, FAAOP(D)


Sam E. Hamontree Award Winner
“Prevalence of Limb Loss and Limb Difference in the United States: Implications for Public Policy”
Ashlie White, MSHLS, MA



Exhibitor-Sponsored Happy Hour



“Administrative Law Judge Trial—Simulation Proceedings”
Special Session: “So Every BODY Can Move Panel”
“Every Choice Has a Consequence—Ethics, Integrity, and the Power of Choices in Life and Business”







Professional Women in O&P Luncheon
The ninth annual Professional Women in O&P event featured a keynote presentation by Jenn Andrews.


Several presenters spoke on the importance and relevance of taking and aggregating outcomes measurements in
AOPA Membership Meeting


One of the most popular Assembly events, the Digital O&P Showcase is a hands-on event marked by a patient case where exhibitors present a fabricated mold, device, or 3D print as a solution. Brent Wright, CP, BOCO; Corey Baum, CO, LO; Jeff


Manufacturing firm owner prioritizes innovation and conservation in O&P


The Fresh Faces column introduces readers to prominent O&P professionals who are making an impact with their contributions to the orthotics and prosthetics profession. This month, we speak with Cole Carlson, PE.
Cole Carlson, PE, is an owner, engineer, and chief executive officer for Tamarack Habilitation Technologies, headquartered in Blaine, Minnesota. Tamarack—a manufacturing company that offers O&P products to improve comfort and mobility for people with ankle-foot orthoses, limb loss, and wheelchairs, as well as athletes—was founded in 1990 by Carlson’s parents, J. Martin “Marty” Carlson, MS, CPO, FAAOP, and Peggy Carlson. Carlson worked as an engineering consultant for several years before joining his parents at Tamarack in 2021. He took over executive leadership duties when his father retired last year.
O&P Almanac: What brought you to a career in O&P?
Cole Carlson, PE: My parents founded Tamarack Habilitation Technologies when I was 2, so I grew up around the company and all the conversations and experiences that come with a small family-owned business. That childhood background instilled in me a set of personal and professional values—authenticity, compassion, concerns about equality and accessibility, the importance of active lifestyles—that align very closely with the O&P industry.
I obtained degrees in physics and mechanical engineering and began my career working for several consulting firms,

but my professional experiences left me feeling that something was missing. I was fortunate to be able to carve out enough time for self-reflection that I realized I wanted to be involved in O&P somehow, and the timing of that realization serendipitously aligned with my parents’ desires to begin stepping away from day-to-day executive management of Tamarack. We carefully developed a plan for me to assume a primary leadership role, and working alongside them these past few years has been very special and incredibly educational.
My primary responsibilities are to establish Tamarack’s main company vision and priorities, and to cohesively manage the executive operations, strategic partnerships, and growth opportunities. I’m also responsible for managing our marketing department, environmental/sustainability goals, and some of our research initiatives. What I enjoy most about my job is the challenge of my various responsibilities and
their respective potential to significantly and positively impact people’s lives. Engaging with and mentoring the next generation of O&P professionals is another highlight of my work!

O&P Almanac: What are your priorities at Tamarack for the next five to 10 years?
Carlson: Tamarack has begun working diligently to be a better steward of the environment in as many areas as possible. We have always implemented recycling, waste

reduction, and lean manufacturing procedures at our facilities, but we are starting to go way beyond that with renewable energy sources for 100% of our energy consumption, minimizing our use of plastic packaging, and reformulating our product lines to move away from persistent, bioaccumulative, and toxic chemicals (PBTs).
Another key strategic initiative for Tamarack is to create more compelling case studies and value propositions for products, technologies, and practices focused on prevention. Hopefully, this may one day lead to reimbursement codes that support these effective, forward-thinking approaches.
O&P Almanac: What roles do sustainability and corporate social responsibility play at your company?
Carlson: This goes back to my parents, Marty and Peggy, who have always humbly led by example without any need for recognition. They began Tamarack with the conviction that everyone is deserving of equal respect, healthcare, and opportunities. From as far back as I can remember, they were instilling in me these same values, and at Tamarack we’ve never deviated from that philosophy.
As an organization that has internalized equal rights for all, we are constantly reflecting on ways we can do even more in this regard. Our manufacturing procedures, many of them developed as part of our ISO 13485 certification, are a great example of how Tamarack has incorporated these aspects into its daily operations.
O&P Almanac: What technological advancements will have the greatest impact on the O&P industry?
Carlson: The transition to digital workflows and 3D printing is already in full swing and is an obvious choice here. But I hope the O&P industry can begin making earnest technological advancements in its utilization of materials, fabrication techniques, and manufacturing processes

that are recyclable, biodegradable, and generally less environmentally detrimental. In doing so, I suspect the industry will discover many new ways to produce and provide devices that are better for users and the planet.
O&P Almanac: How are you involved in advocacy for O&P patients and practitioners?
Carlson: From the very beginning, Tamarack has been on the front lines of advocacy for people with mobility challenges. In terms of clinical services and, later, product innovation, we have always been driven to solve mobility problems for the people who benefit from the products we provide. But that advocacy has taken other forms, too, including the fight for equitable reimbursement codes, definitions, and policies to sustain the equipment and care for those who need it.
CMS ought to be doing much, much more to encourage and support innovation across the entire spectrum of the healthcare industry. This spirit—and the refusal to settle for the status quo within the healthcare
system—really began with my parents and very much defines how we approach our business model today.
I also want to say that I’m so very impressed with the So Every BODY Can Move campaigns, and want to thank all those individuals who have been working diligently behind the scenes to move those bills toward becoming law.
O&P Almanac: What volunteer/ charitable efforts are you involved in?
Carlson: It’s important for all of us to give back and contribute to our communities in one way or another. I choose to volunteer on an advisory board for a local organization I feel strongly about. In addition, I recently applied to serve on one of the AOPA committees and hope to get more involved in helping guide the industry forward.
For Tamarack, charitable activities have looked like a lot of things over the years— volunteering at food shelves, picking up shifts at the Ronald McDonald house to help take care of families who need a place to stay while their children get medical care,
doing litter cleanups at parks, donating to nonprofits whose work we believe in, and more. The value of education is also very important to us, and Tamarack does what it can to help ease the financial and material burdens on O&P students and educational programs around the world.
O&P Almanac: What are the biggest challenges and opportunities for the future of O&P?
Carlson: Value-based healthcare. We all know that promoting and enacting preventative healthcare measures has the potential to help people enjoy happier, healthier, and longer lives. We also know that doing so presents a significant opportunity to reduce the expense and hardship that result when we treat proactively rather than reactively.
The O&P community needs to be an integral part of shifting the healthcare industry to a value-based model where providers, caregivers, patients, and insurers alike are rewarded for preventing disease and improving outcomes. Otherwise, we’ll likely continue facing the same high costs, challenging reimbursement amounts, and less-than-optimal care cycles for a very long time.
O&P Almanac: What else would you like to share with O&P Almanac readers?
Carlson: Increasingly, I am convinced that collaboration and a heavy dose of persistence are the keys to effecting change within a healthcare system that we all know can be discouraging and bureaucratic. We need to be creative and seek out strategic partnerships that can provide life-changing solutions to the problems that disrupt the lives of so many.
As Tamarack’s collaborations strengthen and grow, our hope is that overall public awareness will increase for the challenges we are addressing, and that the shared brainpower, determination, and voices of the partners we join with will lead to more influence and larger, more positive outcomes.




FACILITY:
Tegerstrand Orthotics & Prosthetics



OWNER: Justin Tegerstrand, CPO
LOCATIONS: Redding, Chico, and Mt. Shasta, California HISTORY: 33 years
facility values hands-on care and in-house fabrication
When Justin Tegerstrand, CPO, talks about his business, Tegerstrand Orthotics & Prosthetics, he speaks with pride.
“We’re a true mom-and-pop shop,” he says. “It’s more than a small business—it’s a family legacy, built on decades of commitment to personalized care.”
The story began in 1991, when his father opened Ray Tegerstrand Orthopedic Appliances in a modest 3,000-square-foot building in Redding, California. Ray’s journey into the world of O&P was inspired by love, says Justin Tegerstrand: love for the children he encountered while visiting his wife, Becky, at her job, where she worked with students with orthopedic disabilities.
After earning a degree in orthotics and prosthetics, Ray moved his family from Los Angeles to the small Northern California town where he would launch his practice. Ray handled clinical work, while Becky worked in the front office, ensuring that patients felt like family as well.
“I grew up steeped in this environment of compassion and innovation, and I knew from an early age that I wanted to follow in my father’s footsteps,”
Justin Tegerstrand says. “I spent every school holiday helping around the place, and I fell in love with it.” He enjoyed art and creativity, and realized there was something beautiful about making things that improve people’s lives. By the time he was a teenager, he was already working as a technician and immersing himself in the technical and clinical aspects of the business.
“We have participated in quite a few of AOPA’s events and Assemblies. It’s also a great resource for help with coding and paperwork.”
—Justin Tegerstrand, CPO
When Ray passed away in 2013, Tegerstand was ready to step into the leadership role, continuing the tradition of hands-on care his parents had established. One of his first moves as owner was to rebrand the company to Tegerstrand Orthotics & Prosthetics.
Tegerstrand O&P has grown over the years and now occupies a modern facility in Redding, with satellite offices in Chico and Mt. Shasta, California. Tegerstrand’s team of 13 includes certified clinicians Deanna Hansen, CO, and Daniel Brown, CPed, CFo, who share his commitment to high-quality, personalized care.
Staples for the facility are pediatric orthoses and some pediatric prostheses. Like so many

O&P practices, vascular amputations account for the majority of adult prosthetic care. Although Tegerstrand O&P provides a full range of devices, lower-extremity prostheses are far more common.
What sets the facility apart is its in-house fabrication, says Tegerstrand. He has done some work with 3D scanning companies but says, “I feel the control you gain with building and modifying devices yourself is something that can get lost in our industry these days.”
For Tegerstrand, the most rewarding part of his job is the patients. He recalls a particularly memorable success story involving a 70-year-old above-knee patient who worked hard at rehabilitation, eventually qualifying for a microprocessor-controlled knee. “It took a lot of time and hard work, but eventually he qualified as a K3. Once he got the microprocessor knee, his life changed dramatically,” says Tegerstrand. “He was building cars, fixing his daughter’s roof—he even started applying for construction jobs.”
As he looks to the future, Tegerstrand envisions expanding the facility’s services while maintaining the family-oriented culture that has been the facility’s hallmark. “We’re thinking about adding more CPOs and extending hours at our satellite locations,” he says. “But no matter how we grow, we’ll always keep the personal touch. We love the family feel, and we plan to keep it that way.”
Deborah Conn is a contributing writer to O&P Almanac. Reach her at deborahconn@verizon.net

CUSTOM: FOOT ORTHOTICS • AFO’S • RICHIES
One size does not fit all. At Hersco, our team of professionals works to fabricate orthotics from your scans and casts to match your patients’ specific needs. Customer service is at the heart of everything we do and we work endlessly to help you be as effective and efficient as possible. When you want the job done quickly and accurately, Hersco is here to help.

BY DEBORAH CONN
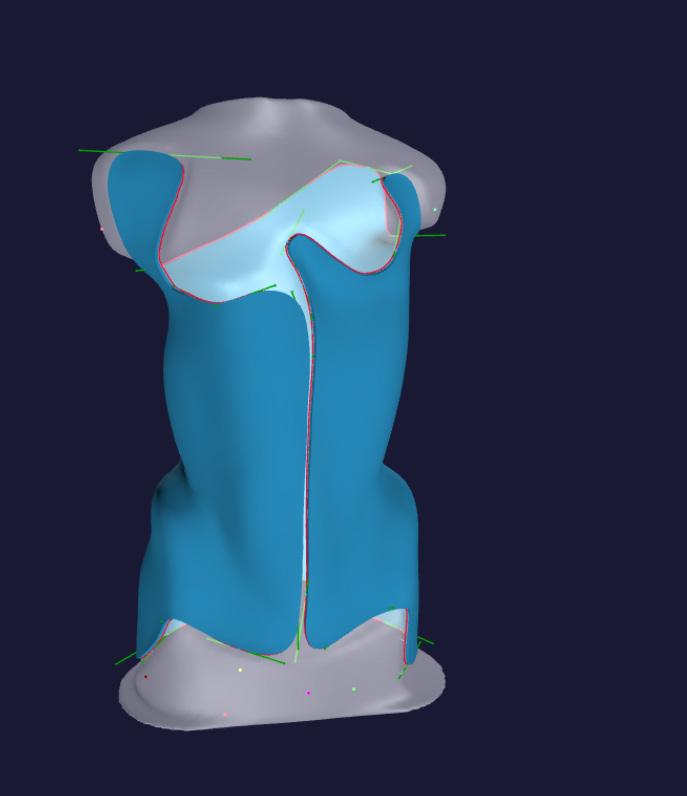
LeoShape offers software to digitize the manufacture of spinal braces, top, and prosthetic sockets.
“We joined AOPA because we want to signal our involvement in the industry and our commitment to O&P innovation. Our membership allows us to stay in touch with the people who are involved in individual transformation, and it helps us understand market needs so we can provide better solutions.”
—Roland Mànyai
OWNER:
Roland Mànyai, LeoShape chief executive officer; Zoltan Karpati, Leopoly chief operating officer
LOCATIONS: Budapest, Hungary; and San Mateo, California
HISTORY: Nine years
LeoShape is a subsidiary of Leopoly, a Budapestbased company that specializes in 3D and extended reality technology. Launched in 2015, LeoShape brings expertise to help O&P practices and others in the medical industry automate the manufacture of medical devices. The company’s flagship products include orthotic insoles and ankle-foot orthoses, and it recently launched software to digitize the manufacture of spinal braces and prosthetic sockets.
LeoShape is based in Budapest, with another location in San Mateo, California. The company works with more than 300 O&P clinics in the United States to fabricate customized orthoses and prostheses; it also partners with such companies as HP, Formlabs, EdserLabs, and WikySolutions.
Prospective clients can conduct a free trial of the web-based software. LeoShape offers its web-based editor as a standalone solution or integrated into an end-to-end solution.
“We like to understand our clients’ needs, so we usually kick off with a personal introductory call to understand which solution they are looking for, and then we provide the demo,” explains Roland Mànyai, chief executive officer. The end-to-end workflow solution handles patients’ data management, scanning, design, and some order management, while the standalone is just for the design, he says. “In both cases, they’ll end up having a 3D file, which they can print or send to a service bureau.” If facilities don’t want to invest in printers or simply prefer to outsource that function, LeoShape can help them find a service bureau to print the devices for them.
One of the benefits of the software to clinicians, says Mànyai, is precision. “Using a scan of the patient’s actual foot gives a specific, personalized outcome,” he notes. “It’s simple and quick, and it’s also easy to follow up at a later date to track how things have changed and if adjustments need to be made.” The software is “device specific, easy to use, online, and no design and engineering skills are needed,” according to Mànyai.
Additive manufacturing offers advantages: “3D printers can create multiple insoles at the same time, and it’s about five times faster and half as expensive compared to traditional insole production,” Mànyai
says. “Not only that, but fully customized products create from 70% to 90% less scrap waste than conventional manufacturing techniques.”
LeoShape customers pay for each use of the software or may purchase a prepaid package with several tokens. “They don’t have to sign up for a huge package,” says Mànyai. “They pay after each download, which gives them even more flexibility. As does our modular design, so users can select the components that best support their practice.”
Although the company’s market in the United States continues to grow, creating orthoses digitally is not yet as popular in Hungary. “This is partly because implementing such technologies would require some financial background and funding that most clinics are not prepared for,” Mànyai explains.
As a result, LeoShape is working through medical universities in Hungary and has set up a research institute to explore digital processes. “We provide research institutions with free software and help them outsource the manufacturing to our partners during the pilot, getting insoles fabricated through our manufacturing partners elsewhere,” he says. “Once they see the results of the technology, we are hoping that people in the professional world will be convinced to work toward innovation.”
As time goes on, Mànyai expects that digitization will lead to better quality O&P products, with advancements in scanning, 3D printing, and materials. “Software will be a critical element, with more automation and precision in customization processes,” he predicts. “Global demand is growing for O&P devices, and LeoShape aims to play a significant role in this evolution by providing scalable, efficient digital solutions.”
Deborah Conn is a contributing writer to O&P Almanac. Reach her at deborahconn@verizon.net
The AOPA Hub, AOPA’s new member portal, went live Sept. 30. AOPA Hub has been designed to improve the AOPA member experience and provide more convenient access to key resources and tools. AOPA Hub offers a range of features that will make managing your membership and access to AOPA benefits easier than ever:
1. User-friendly app-based dashboard: Navigate through your account with ease, accessing your membership information, event registrations, education, and more, all in one place
2. Enhanced capabilities: Quickly find the resources, contacts, and information you need in the app-based structure
3. Secure access: As always, your data is protected with security features, ensuring that your information is safe and secure
4. Improved experience: Enjoy the ability to easily access all of your member benefits and AOPA resources in one place.
Be assured your information from the former member portal has been transferred to the AOPA Hub. You can access your account and

Did you miss the 2024 AOPA National Assembly in Charlotte, North Carolina? Did you attend the Assembly but miss some key sessions and want to learn more? AOPA brings the education to you— virtually—starting Oct. 1 and ending Nov. 30. Learn on your own schedule—access is included with Full Conference Registration. Visit aopaassembly.org
AOPAversity on AOPA Hub now. AOPA will be rolling out additional updates over the next few weeks.
Visit AOPA’s website and go to the AOPA Hub to access your account. If you have any questions or need assistance, email info@aopanet.org or call 571-431-0876.
Level up your O&P business acumen! Core courses for the Certificate in O&P Business Management are now open. AOPA and the University of Hartford Barney School of Business have partnered for the past several years to offer this certificate. The comprehensive program offers a series of business and management courses to provide O&P business owners and managers, clinicians, manufacturers, and distributors an opportunity to explore crucial business challenges. Topics include finance, sales and marketing, business operations, reimbursement policies, and management.
In 2025, all of the core courses, run by the University of Hartford, will be available online, asynchronously. They opened Aug. 26 and close Dec. 11. Electives, offered through AOPA, are available online, on demand. Electives are available in all areas of learning, with more courses expected to be added in 2025.
Graduates of the certificate program in 2025 will be awarded their certificates during the 2025 AOPA National Assembly Awards Ceremony in Orlando, Sept. 3-6, 2025, and will be publicly recognized in O&P Almanac
This unique leadership experience provides an opportunity to gain fresh insights, learn new tools, and acquire proven techniques for developing better business practices. Learn more and enroll in the Certificate in O&P Business Management program at aopanet.org/certificate-in-op-business








The officers and directors of the American Orthotic & Prosthetic Association (AOPA) are pleased to present these applicants for membership. Each company will become an official member of AOPA if, within 30 days of publication, no objections are made regarding the company’s ability to meet the qualifications and requirements of membership.
Strive Orthotics & Prosthetics, LLC
50714 Van Dyke Ave Shelby Township, MI 48317-1363 www.striveop.com
Melissa and Matthew McEwin Patient Care Facility






















































KiddieFLOW™, Allard USA’s extension to our pediatric AFO line, was introduced in response to clinician requests for an orthosis with more foot plate flexibility. KiddieFLOW™ allows for better control of foot positioning in late swing, which aids in stability during stance. FLOW models offer increased range of motion in the sagittal plane and a smoother transition (flow) throughout the gait cycle.
For more information, contact customer service at 888-678-6548 or info@allardusa.com and request your free teddy bear tape measure!


Hersco is delighted to offer HP’s advanced 3D-printing technology for custom orthotics. 3D printing has unique design capabilities not possible with other methods—reducing landfill waste by 90%! The accuracy of 3D is unparalleled, specs exceed direct-milled polypro, and manual plaster fabrication. Among the benefits: a 90% reduction in landfill waste, many new design possibilities for posting, and the ability to vary thickness and flexibility across the shell. The PA-11 polymer is a biobased renewable material that has been tested and proven in research and industry.



The new Aura polycentric knee was designed to offer stability and relief to low-impact users. Like all College Park knees, adjustments are quick and easy. Featuring a unique push-button lock and full waterproof capabilities, the lightweight Aura goes beyond mere physical support. It can open doors for mobility goals and newfound peace of mind.
For more information, contact college-park.com
Join a dynamic online support group specifically for finger and partial-hand loss.



Engineered to exceed expectations, giving you more of what you need to live the life you want. PROTEOR QUATTRO™ MPK is designed to be MORE INTUITIVE for elevated security and dependability with patented valve technology for effortless transitions between activities, hyperresponsive sensors read body movements, and exclusive stumble recovery determines appropriate resistance to recover more naturally. MORE PERSONALIZED with up to 20 customizable modes and the shortest MPK available allows a wider selection of ankles/feet. MORE ADAPTABLE for fewer interruptions with a 2-3+ day battery life, PLUS Battery Booster allows charging ON THE GO. Visit us.proteor.com

Turbomed’s leading line of foot drop ankle-foot orthoses sit completely outside the shoe for an invisible, painless support that will follow you as long and as far as you want. Their unique design acts as an exoskeleton to the impaired limb, keeps the foot at 90 degrees, and provides the user with unparalleled levels of function. Each model takes minutes to assemble and is easily transferrable to most shoes, boots, and sandals through an innovative lace clip design.
The Xtern Summit is the lightest model, has the most dorsiflexion power, and features a see-through design. The Xtern Frontier was designed for patients with reduced hand dexterity and requiring front leg support. Visit turbomedusa.com, and think outside the shoe!


Contact Bob Heiman at bob.rhmedia@comcast.net

October 1–November 30
Virtual 2024 AOPA National Assembly Education Available. Visit aopaassembly.org

October 18–19
PrimeFare Central. Renaissance Hotel and Convention Center, Tulsa OK. In-Person Meeting. Contact Cathie Pruitt at 901-359-3936, primecarepruitt@gmail.com, or visit primecareop.com
November 1–30
ABC: Application Deadlines, Exams Dates, O&P Conferences, and More! Check out ABC’s Calendar of Events at abcop.org/calendar for the latest dates and event details, so you can plan ahead and be in the know. Questions? Contact us at info@abcop.org; abcop.org/contact-us
November 18–19
AOPA In-Person Coding & Billing Seminar. Alexandria, VA. To register, visit aopanet.org


The Pedorthic Footcare Association: Diabetic Wound Prevention, Management, and Healing Program. 10-session online education program series. Approved CEs by ABC and BOC, monthly classes are 1.5 hours each. For more information and to register, visit pedorthics.org/page/.Diabetic_Series_LMS_List
September 3–6
AOPA National Assembly. Orlando. For more information, visit aopanet.org

For Job Seekers:
Job searching is easy with the pane-view job search page. Set up job alerts, upload your resume or create an anonymous career profile that leads employers to you.
For Employers:
Reach 4,500+ O&P professionals through the Job Flash™ email. Ensure high visibility for your open positions through this highly engaging email.
Advertise O&P events for maximum exposure with O&P Almanac. Contact Bob Heiman at bob.rhmedia@comcast.net or learn more at bit.ly/24AlmanacMediaKit. Announcement and payment may also be sent to O&P Almanac, Calendar, P.O. Box 34711, Alexandria, VA 22334-0711 or emailed to jburwell@AOPAnet.org along with VISA or MasterCard number, cardholder name, and expiration date. Make checks payable in U.S. currency to AOPA. Note: AOPA reserves the right to edit calendar listings for space and style considerations.
Finding your next job or hire just got easier with the AOPA Career Center.

If you are interested in participating in the
More than 100 attendees are expected to attend the 2024 California Orthotic and Prosthetic Association (COPA) Symposium Nov. 7-8 at Loma Linda University in Southern California. Highlights of the event include two keynote speakers, a Women in O&P event, a robust exhibit hall, and opportunities to discuss issues that affect O&P businesses in California. Registration information is available on the COPA website at californiaoandp.com
Senate Bill 357, introduced in early 2024, would have required that state-regulated commercial and public plans provide coverage for limb salvage treatment. The bill also would have required limb salvage protocols to be established by the Georgia Diabetes Control Grant Program, the State Board of Podiatry Examiners, and hospitals and ambulatory surgical centers in the state. The bill was referred to the Senate Health and Human Services Committee, but it failed to advance and was not passed; however, its introduction paved the way for future efforts in this area.
The Michigan Orthotic & Prosthetic Association (MOPA) will hold its annual Continuing Education Seminar in Bay City, Michigan, June 5-7, 2025. Registration information is available on the MOPA website at m-opa.com/continuing-education-seminarevent-promotion
Efforts continue in New York to require New York Medicaid managed-care organizations (MCOs) to reimburse O&P providers at rates consistent with rates established by the traditional
Medicaid agencies in the state. This has been a long process, with primary opposition coming from MCO lobbying interests. While slow, progress continues to be made, and advocates in the state remain enthusiastic.

AOPA has been honored to be entrusted with serving the So Every BODY Can Move (SEBCM) initiative, the amazing advocates on the ground, and the founding partners as the administrative arm over these first two critical years of this initiative. AOPA led the creation of the infrastructure that has enabled the initiative to see the success it has. After careful consideration, the partners have determined that the Amputee Coalition, as the primary patient advocacy organization, would best serve as the backbone organization for SEBCM
The Amputee Coalition, along with the SEBCM founding partners, are excited to have Nicole Ver Kuilen continue her leadership role in the SEBCM movement.
Ver Kuilen will join the senior leadership team at the Amputee Coalition as impact campaign director, So Every BODY Can Move lead. Kyle Stepp will continue his role as a key advisor and consultant working with Ver Kuilen in this position for SEBCM
The Amputee Coalition will assume responsibility as the administrative arm for SEBCM, hosting fundraising and key staff as well as executing on plan and budget. Priority setting and strategic direction will remain with the four founding partners: Amputee Coalition, AOPA, National Association for the Advancement of Orthotics and Prosthetics, and American Academy of Orthotists and Prosthetists.
This transition marks an exciting moment of growth for SEBCM as we look to the next phase and iteration of the initiative. The founding partners are proud to have SEBCM as a disability-led initiative, uplifting the voices and leadership of those most impacted by access to this medically necessary care.
Are you ready to take your career and your business expertise to new heights?
The Certificate in O&P Business Management can help you do just that!












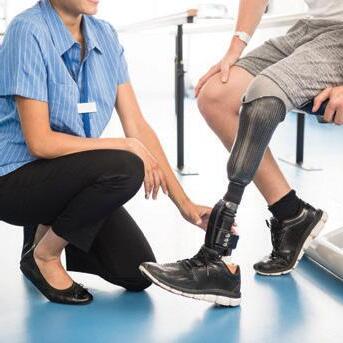



This joint certificate program will provide you with:
• Basic business acumen
• Practical knowledge to apply immediately to your work
• Techniques for developing better business practices
• How to think about improving your company’s returns
A comprehensive certificate program for business owners, managers, and practitioners of O&P patient care facilities, O&P manufacturers and distributors to explore crucial business challenges as they relate to O&P.
To complete the certificate program, you must register and complete one core course and one elective course from each of the four areas of learning within a four-year period


Certificate in O&P Business Management

The new X4 is the next revolutionary step in advanced knee technology. Its unmatched functionality and cutting-edge digital ecosystem provide a whole new mobility experience for users.


Learn more about this groundbreaking MPK!
#WeEmpowerPeople shop.ottobock.us