College-Level
Organic Chemistry
www.AudioLearn.com
TABLE OF CONTENTS Preface........................................................................................................ 1 Chapter 1: Chemical Bonding in Organic Chemistry .................................... 5 Carbon-Based Chemistry ................................................................................................. 5 Orbital Theories ............................................................................................................... 7 Carbon Hybridization .................................................................................................... 10 d Orbital Hybridization.................................................................................................. 12 Multiple Bonding ........................................................................................................... 12 Writing Organic Molecular Structures .......................................................................... 13 Bonding Trends in Organic Chemistry .......................................................................... 14 Constitutional Isomers .................................................................................................. 16 Organic Molecular Charges ............................................................................................17 Resonance Chemistry .................................................................................................... 19 Key Takeaways ............................................................................................................... 22 Quiz ................................................................................................................................ 23 Chapter 2: Basic Organic Molecular Structures ......................................... 27 IUPAC Nomenclature .................................................................................................... 27 Alkyl Halides .................................................................................................................. 30 Alkenes and Alkynes ...................................................................................................... 30 Alcohols .......................................................................................................................... 31 Ethers ............................................................................................................................. 32 Aldehydes ....................................................................................................................... 32 Ketones ........................................................................................................................... 34 Carboxylic Acids ............................................................................................................. 35
Esters .............................................................................................................................. 36 Amines ........................................................................................................................... 36 Functional Groups ......................................................................................................... 37 Stereochemistry and Isomers ........................................................................................ 38 Diastereomerism ............................................................................................................ 39 Enantiomer .................................................................................................................... 40 Key Takeaways ............................................................................................................... 42 Quiz ................................................................................................................................ 43 Chapter 3: Organic Solvent Chemistry ...................................................... 46 Types of Solvents ........................................................................................................... 46 Nonpolar Solvents.......................................................................................................... 47 Solvation......................................................................................................................... 52 Key Takeaways ............................................................................................................... 54 Quiz ................................................................................................................................ 55 Chapter 4: Alkanes, Alkenes, and Alkynes ................................................. 58 Alkanes ........................................................................................................................... 58 Alkyl Groups................................................................................................................... 59 Alkoxides or Alkoxy Groups .......................................................................................... 60 Chemical Properties of Alkanes ..................................................................................... 61 Cycloalkanes................................................................................................................... 62 Alkenes ........................................................................................................................... 63 Physical Properties of Alkenes ....................................................................................... 66 Alkynes ........................................................................................................................... 67 Alkyne Reactivity ........................................................................................................... 68
Key Takeaways ............................................................................................................... 70 Quiz .................................................................................................................................71 Chapter 5: Aldehydes, Ketones, and Carboxylic Acids ............................... 75 Naming Aldehydes ......................................................................................................... 77 Naming Ketones ............................................................................................................. 78 The Carbonyl Group.......................................................................................................80 Reactivity of Aldehydes and Ketones ............................................................................ 82 Natural Occurrence of Ketones and Aldehydes............................................................. 84 Carboxylic Acids ............................................................................................................. 85 Fatty Acids...................................................................................................................... 87 Properties of Carboxylic Acids .......................................................................................88 Key Takeaways ............................................................................................................... 89 Quiz ................................................................................................................................ 90 Chapter 6: Aromatic Compounds .............................................................. 93 Introduction ................................................................................................................... 93 Nomenclature of Aromatics ........................................................................................... 94 Benzene Chemistry ........................................................................................................ 98 Aromatic Reactions .......................................................................................................101 Halogenation of Benzene ............................................................................................. 102 Nitration of Benzene .................................................................................................... 103 Sulfonation of Benzene ................................................................................................ 104 Friedel-Crafts Reaction ................................................................................................ 105 Key Takeaways ............................................................................................................. 108 Quiz .............................................................................................................................. 109
Chapter 7: Alcohols and Alkyl Halides ...................................................... 113 Nomenclature of Alcohols ............................................................................................ 113 Reactivity of Alcohols.................................................................................................... 117 Alcohol Dehydration ..................................................................................................... 118 Oxidation of Alcohols ................................................................................................... 120 Reactivity of Alkyl Halides ........................................................................................... 120 Glycols .......................................................................................................................... 122 Key Takeaways ............................................................................................................. 123 Quiz .............................................................................................................................. 124 Chapter 8: Ethers, Epoxides, and Esters.................................................. 128 Ethers ........................................................................................................................... 128 Physical Properties of Ethers ....................................................................................... 129 Reactions with Ethers .................................................................................................. 130 Epoxides ....................................................................................................................... 132 Esters ............................................................................................................................ 134 Ester Reactions .............................................................................................................137 Polyester Formation .................................................................................................... 140 Saponification .............................................................................................................. 140 Key Takeaways .............................................................................................................. 141 Quiz .............................................................................................................................. 142 Chapter 9: Enols and Enolates ................................................................ 146 Introduction to Enols and Enolates ............................................................................ 146 Enolate reactions ......................................................................................................... 150 Acidic Alpha Halogenation of Ketones and Aldehydes ............................................... 150
Basic Alpha-Halogenation of Ketones and Aldehydes ................................................. 151 The Haloform Reaction ............................................................................................... 152 Alkylation of Enolates .................................................................................................. 153 The Aldol Reaction of Aldehydes ................................................................................. 154 The Aldol Reaction of Ketones .....................................................................................155 Conjugate Reactions .................................................................................................... 156 Michael Addition Reaction ...........................................................................................157 Key Takeaways ............................................................................................................. 158 Quiz .............................................................................................................................. 159 Chapter 10: Sulfur-Containing Organic Compounds................................ 163 Nomenclature of Sulfur Compounds ........................................................................... 163 Oxidation of Alcohols using DMSO ............................................................................. 166 Thiols ............................................................................................................................ 167 Sulfides ......................................................................................................................... 168 Synthesis of Sulfides .................................................................................................... 169 Key Takeaways .............................................................................................................. 171 Quiz ...............................................................................................................................172 Chapter 11: Nitrogen-containing Organic Molecules ................................. 176 Nomenclature of Amines ............................................................................................. 176 Physical Properties of Nitrogenous Compounds......................................................... 178 Alkylation of Ammonia ................................................................................................ 180 Reduction of Nitrogenous Compounds ........................................................................ 181 Reductive Amination via Imines ................................................................................. 183 Amine Reactions .......................................................................................................... 183
Preparing Amides ........................................................................................................ 184 Nitrosation of Amines .................................................................................................. 185 Key Takeaways ............................................................................................................. 187 Quiz .............................................................................................................................. 188 Chapter 12: Carbohydrates in Organic Chemistry .................................... 192 Nomenclature and Naming of Carbohydrates ............................................................ 192 Glycosides .................................................................................................................... 195 Reducing Sugars .......................................................................................................... 196 Substituted Sugars ....................................................................................................... 197 Alpha and Beta Isomers ............................................................................................... 197 Reactions of Carbohydrates ......................................................................................... 198 Alkylation of Carbohydrates ........................................................................................ 198 Acylation of Carbohydrates ......................................................................................... 199 Reduction of Carbohydrates ........................................................................................ 199 Oxidation of Carbohydrates ......................................................................................... 199 Hydrolysis of Sugars ................................................................................................... 200 Glycosidic Bond Formation ........................................................................................ 200 Key Takeaways ............................................................................................................. 201 Quiz ..............................................................................................................................202 Chapter 13: Amino Acids, Proteins, and Peptides in Organic Chemistry .. 206 Amino Acids .................................................................................................................206 Amino Acid Stereochemistry .......................................................................................209 Amino Acid Synthesis ..................................................................................................209 Reactions of Amino Acids ............................................................................................ 210
Terminology for Proteins and Peptides ........................................................................ 211 Amide Bond................................................................................................................... 211 Disulfide Bonds ............................................................................................................. 211 Sequencing Amino Acids ............................................................................................. 212 Protein Structure ......................................................................................................... 212 Alpha Helices and Beta Pleats ..................................................................................... 213 Peptide Synthesis ......................................................................................................... 213 Key Takeaways ............................................................................................................. 215 Quiz .............................................................................................................................. 216 Chapter 14: Lipids in Organic Chemistry ................................................. 220 Fatty Acids and Triglycerides ......................................................................................220 Saturated Fatty Acids ................................................................................................... 221 Unsaturated Fatty Acids (all have 18 carbons)............................................................ 221 Micelles ........................................................................................................................ 222 Phospholipids............................................................................................................... 223 Prostaglandins ............................................................................................................. 224 Terpenes ....................................................................................................................... 225 Steroids ........................................................................................................................ 226 Key Takeaways ............................................................................................................. 228 Quiz .............................................................................................................................. 229 Chapter 15: Nucleic Acids and Nucleosides in Organic Chemistry ............ 233 Nucleic Acids ................................................................................................................ 233 Pyrimidines and Purines.............................................................................................. 233 Nucleosides .................................................................................................................. 234
Nucleotides................................................................................................................... 236 Nucleic Acids ................................................................................................................ 236 Nucleic Acids as Bioenergetic Molecules..................................................................... 237 DNA (Deoxyribonucleic Acid) ..................................................................................... 239 Key Takeaways ............................................................................................................. 242 Quiz .............................................................................................................................. 243 Summary ................................................................................................ 247 Course Questions and Answers ................................................................251
PREFACE In this course, we attempt to bring the basics of organic chemistry to the student needing to understand the nomenclature, chemical properties, and reactivity of carbonbased molecules. While there are far too many organic molecules in nature to discuss in any organic chemistry course, there are specific ways to clearly identify these molecules as well as certain trends in how these various molecules behave in chemical reactions. By the end of the course, you will understand how to identify and name organic molecules, their physical properties, and how they chemically interact with one another in a variety of types of chemical reactions. Chapter one in the course begins the study of organic chemistry by introducing how organic molecules are put together. There really isn’t any difference between the way the atoms in organic molecules are put together and the way other chemical molecules are put together but it is worth reviewing, even if you have studied chemistry in the past. This chapter will focus on orbital theory and the particulars of organic molecular bonding as well as the shorthand involved in writing out organic molecular structures. Finally, the chapter talks about resonance chemistry as it applies to organic molecules. Chapter two in the course covers the basics of nomenclature in identifying organic molecules. Because there are innumerable organic molecules and because they are based on just a few different types of atoms, there needs to be a way to identify what each molecule looks like by name alone. This leads to a discussion of the IUPAC nomenclature and coverage of the different functional groups in organic chemistry. You will need to understand how to name the different molecules you see in organic molecules, which is covered in this chapter. In addition, there will be a discussion of stereochemistry as it applies to organic molecules. The topic of Chapter three in the course is organic solvent chemistry. For students who have participated in regular chemistry experiments, the solvent has typically been water. In organic chemistry, the solvent may or may not be water because many aspects of organic chemistry involve nonpolar substances that do not dissolve in water. Solvents
1
may or may not participate in chemical reactions themselves but are important to the chemistry of different molecules. Issues regarding solvation and solutions in organic chemistry are also covered in this chapter. Chapter four begins a series of chapters on the different organic compounds and their properties. You will have learned about alkane, alkene, and alkyne nomenclature in previous chapters so the focus of this chapter is to learn more about these compounds and how they interact with one another and with other organic compounds. These substances can have a variety of different configurations, which need to be discussed as part of this chapter. The focus of Chapter five in the course is the chemistry of aldehydes, ketones, and carboxylic acids. This is the first time the chemistry of oxygen comes into play in this course. Aldehydes and ketones are discussed together because they have very similar chemistry and reaction types. Carboxylic acids are also oxygen-related because they have a COOH side chain as their defining characteristic. They also have great reactivity and are seen in nature as fatty acids and other biochemically-important molecules. In all cases, you will come to understand their nomenclature, their physical properties, and some of the most important chemical reactions associated with these molecules. Chapter six introduces the structure and chemistry of aromatic compounds. All aromatic compounds consist of a cyclic compound that carries resonance. The most common aromatic compound is benzene, which is very stable and has chemistry unique to the molecule. In this chapter, the nomenclature and chemistry of aromatic compounds will be covered as well as the different reactions that are seen in organic chemistry with these types of molecules. The topic of Chapter seven in the course is the chemistry of alcohols and alkyl halides. Alcohols are organic compounds that have a hydroxyl group as its major functional group, often represented with the general formula of ROH, where R can be any number of organic chemistry alkyl groups. The hydroxyl group is highly reactive so that there are any number of reactions that can occur at this functional group. The chapter also covers the chemistry of alkyl halides, which are alkyl groups that have one or more
2
halogen side chains attached to it. The halogen is also highly reactive, with many possible chemical reactions associated with it. Chapter eight in the course focuses on the organic chemistry associated with ethers, epoxides, and esters. Ethers and epoxides are related to one another in that certain types of cyclic ethers are referred to as epoxides. In both types of molecules, the general formula is ROR’, involving a variety of R side chains. These are molecules commonly seen in perfumes, industrial compounds, waxes, oils, and dyes. Esters are also commonly used in industry, being a part of the making of many products—the most common of which are the polyesters. The topic of Chapter nine is the structure and chemistry of enols and enolates. Enols are also referred to as alkene alcohols, which are alkenes that have an alcohol group added to one of the carbon atoms. These are first alkenes but, chemically-speaking, they should be considered important for their electron-donating capacity. Enols can be mixed with alkali substances to make enolates, which are the conjugate bases of enols. Both of these types of molecules are best known for the many different types of reactions they participate in, which are covered in this chapter. Chapter ten in the course changes nomenclature and reactions in organic chemistry to include molecules that contain sulfur. Sulfur compounds are somewhat similar to oxygen-containing molecules in that they belong to the same group but sulfur is a great deal larger than oxygen, leading to slightly different chemical reactivity unique to these molecules. The nature and chemistry of thiols and sulfides is discussed as part of this chapter. Chapter eleven places a focus on the different nitrogen-containing molecules in organic chemistry. These types of molecules are not only important in basic organic chemistry; they are also important in numerous biochemical processes. The main type of molecule discussed will be the amine compounds, which are considered organic derivatives of ammonia. Like ammonia itself, amine compounds will have a certain degree of basicity, which leads to nucleophilicity of the nitrogen compounds in organic compounds.
3
Chapter twelve in the course begins to make sense of what you will learn in prior chapters on simpler molecules and applies it to more complex biochemical molecular structures. Sugars and carbohydrates are basically organic molecules that come from the phrase “carbon hydrates”. They contain only carbon, oxygen, and hydrogen atoms and have a specific generic formula, based on whether they are simple sugars, disaccharides, or more complex polysaccharides. The main focus of this chapter is to use organic molecular principles that will make sense after you know the basic reaction types involved. The focus of Chapter thirteen in the course is to bring on more of the biochemistry involving organic chemistry principles by talking about the organic chemistry of amino acids, oligopeptides, and proteins. These are molecules that have nitrogenous compounds as the basis of their chemistry and that, like carbohydrates, exist as monomer units and polymers or polypeptides. These will also have reactions at their functional units, which involve a variety of different side chains and parts of the parent chain. Chapter fourteen in the course studies the organic chemistry associated with lipids. The term “lipid” is a broadly reaching term that applies to a wide variety of molecules that are called lipids because of their biochemical nature and their lack of solubility in water. They can range from fatty acids to triglycerides to more complex molecules that are complicated to synthesize and even to understand how they are synthesized in body systems and in organic chemistry models. Lipids have poor solubility in water but are much more soluble in chloroform, benzene, ether, and acetone, which are either nonpolar or weakly polar. The focus of Chapter fifteen and the final chapter of the course is the organic chemistry and biochemistry of nucleic acids, which are deoxyribonucleic acids and ribonucleic acids, commonly called DNA and RNA. These are molecules that contain ribose or deoxyribose sugars, phosphate groups, and nucleic acid bases, which are seen in paired form with DNA and sometimes with RNA. The transcription of nucleic acids and the translation to proteins is covered as part of the chapter as these are not generally made synthetically in an organic chemistry laboratory.
4
CHAPTER 1: CHEMICAL BONDING IN ORGANIC CHEMISTRY This chapter begins the study of organic chemistry by introducing how organic molecules are put together. There really isn’t any difference between the way the atoms in organic molecules are put together and the way other chemical molecules are put together but it is worth reviewing, even if you have studied chemistry in the past. This chapter will focus on orbital theory and the particulars of organic molecular bonding as well as the shorthand involved in writing out organic molecular structures. Finally, we will introduce resonance chemistry as it applies to organic molecules.
CARBON-BASED CHEMISTRY As we have discussed, organic chemistry is carbon-based chemistry. There are many features of carbon that make organic chemistry relatively unique when it comes to molecular structures. Carbon-carbon bonds are completely strong. Consider that when it comes to carbon in diamond form, there is a tightly-bound diamond lattice that provides the substance its strength. Graphite is also carbon but in a different shape. Figure 1 shows the difference between diamond and graphite.
Figure 1
5
What makes carbon unique among the many other atoms that can bond with each other is that it has the capacity to bond strongly with itself as well as with other atoms, making a multitude of possible molecules—with all of the bonds being potentially strong. The backbone of organic chemistry is hydrocarbons, which are strongly-bonded carbon atoms—both with each other and with hydrogen atoms. Why is carbon so versatile in its ability to bond with such a wide variety of elements (including metal salts, halogens, oxygen, and hydrogen)? Part of it is because it is such a small element and part of it is because it has four valence electrons around it. Its choices are that it could lose four electrons or gain four electrons (which would cause too much repulsion of so many electrons) if it were to make a salt. Instead, it shares its valence electrons with other atoms. This is what it “prefers” to do. Figure 2 shows what it does with methane and ethane:
Figure 2.
By sharing electrons with other atoms, there is a decrease in repulsions between electrons of the valence shell that are compensated for by the positive charge of the atom it is associated with (in the case of ethane and methane, this will be hydrogen or another carbon atom). It should be noted that the electrons are not necessarily shared equally, even in situations where the bond is technically “covalent”. More of the negative charge, for example will be on the fluorine atom when it bonds to carbon because the fluorine atom is more electronegative. What this means is that there is a gradual shift from purely ionic to purely covalent bonding with carbon bonding with other atoms being generally somewhere in between. Molecules like lithium hydride are purely ionic, while molecules like H2 are purely covalent (because there is equal sharing of electrons). Lithium hydride becomes a salt6
like ionic compound with a high melting point, while methane, being non-salt-like, has a boiling point of -161 degrees Celsius. Methane or CH4 is mainly a covalently-bonded molecule. There is little electrostatic attraction between methane molecules (they are nonpolar) so that they boil at a very low temperature to become a gas. Hydrogen fluoride boils, on the other hand, 200 degrees higher than methane gas. This is because it is more ionic and has connections between the Hydrogen and Fluorine atoms through not only ionic bonding but also with hydrogen bonding between the HF molecules in chains and rings. This makes the boiling point higher. Because methane is nonpolar, it is inert to almost all reagents that could remove the hydrogen ion under anything but the most extreme conditions. It is therefore difficult to generate a CH3+ or CH3- ion, as these would be very reactive and just wouldn’t last long. In other words, you couldn’t make CH4 plus HF to make CH3F plus H2. This would require a cation of CH3+, which is not very stable in nature.
ORBITAL THEORIES While there are many ways to describe and write covalent bonds (which will be described later in this chapter), there are some concrete theories about how covalent bonds exist in organic molecules. While there is ionic bonding in organic chemistry, the most important bonding in this type of chemistry involves covalent bonds. You may have learned that covalent bonding involves the sharing of electrons but you may not know exactly how this happens. This introduces the topic of the valence bond theory, which describes how bonding happens in covalent molecules. According to this theory, there are two atomic orbitals around a pair of atoms—each orbital of which contains one electron. In sharing orbitals, the orbital pair will contain a stable set of two electrons. The H2 molecule is the simplest case of a covalently-bonded molecule. There are two spherical orbitals (1s orbitals) in each hydrogen atom, each with one electron in it. In the bonding of the two atoms, the electrons no longer are anywhere within the sphere but spend more time in that part of each sphere between the two nuclei, which holds the
7
atoms together. According to quantum chemistry, the two electrons must occupy a shared orbital space in order to form a bond. The hydrogen atoms are too far apart, the 1s orbitals cannot overlap in order to form a covalent bond. As they overlap, a bond will begin to form, lowering the potential energy of the system. There will be attraction of the opposite electron to the opposite nucleus. They cannot, however, get too close or there will be repulsion of the nuclei with each other, which increases the potential energy of the system. A balance is had when there is an optimal distance between the nuclei. In a sense, there is a defined “optimal” distance between two bonding nuclei, which is when the potential energy is the lowest, meaning that the combined repulsive and attractive forces add up to the greatest amount of attraction. This optimal internuclear distance is referred to as the “bond length”. The difference between the optimal “bonded” energy and the separated energy is the “bond energy”. Every covalent bond has a certain strength and bond length. As an example, the carboncarbon bond is 1.5 Angstroms long with an Angstrom being 10-10 meters in length. Bonds aren’t the straight sticks as they are depicted in drawing chemical bonds. Instead, they are more like springs, which have the ability to bend, extend, and compress. According to the valence bond theory, there are two assumptions about bonds: 1) the strength of a given bond is directly proportional to the degree of overlap of the bond (in other words, the greater the overlap, the more stable is the bond). 2) An atom is able to use different combinations of atomic orbitals in order to maximize the overlap of the orbitals used by bonded atoms. Maximum overlap occurs between orbitals of the same spatial orientation and similar energies. Bonding can take place between beryllium and hydrogen to make BeH2 which, according to the periodic table of the elements in figure 3, has an atomic number of 4:
8
Figure 3
According to the atomic number and the atomic orbital approach, beryllium has a 1s22s2 electron configuration. It has apparently filled its 2s orbital subshell, leaving behind no apparent reason why it should want to overlap with the singly occupied 1s orbital associated with the hydrogen atom. How do these two atoms overlap to make BeH2? One way to do this is to excite a 2s electron on beryllium to allow for a partially empty 2p orbital that absolutely could bind with hydrogen. Doing this is called “promotion”. What this looks like is seen in figure 4:
Figure 4.
9
In this excited state (having an unmatched 2s1 and 2p1 orbital), Beryllium could certainly bind with hydrogen in two unmatched orbitals according to this theory. Research, however, has shown that these two bonds are identical. How can this be? Basically, the only way this makes sense is through the process of hybridization. What this involves is combining orbitals that are not equivalent but that are properly oriented to form bonds. These new combinations are called hybrid orbitals because they are made by hybridizing two or more atomic orbitals from the same atom. This leads to a unique hybrid orbital in beryllium that has this energy pattern, as shown in figure 5:
Figure 5.
This obviously leads to the ability of the beryllium to bind with the hydrogen atom in order to make BeH2. This will produce a linear BeH2 molecule. Both promotion and hybridization require an input of energy; however, this is made up for when beryllium bonds with 2 hydrogen atoms. It means that, in situations not associated with beryllium, some compounds are so unstable because they have such a high energy necessary to make the hybrid orbital. This energy is not made up for by the energy recouped in the bonding effort.
CARBON HYBRIDIZATION Carbon has six electrons, with a 2s22p2 configuration. Based on this, one would expect that it would be likely to bond with just two other atoms; we know, on the other hand, that it bonds with four other atoms to make covalent bonds. On the second level, there is one 2s orbital and 3 2p orbitals, which can be hybridized to make four degenerate sp3 10
hybrid orbitals that contain a single electron available for bonding. Figure 6 shows what this hybridization looks like:
Figure 6.
The energy of the hybridized orbitals is somewhere in the middle of the ground state and the excited state of carbon. This leads to orbitals that are in the shape of a tetrahedron with 109.5-degree angles between them. These are equivalent in energy and allow for a tetrahedral molecule, which is the exact shape of methane. The amount of energy released in any hybrid orbital situation increases with the number of bonds formed. In the case of carbon, more energy is released in the formation of four bonds versus just two, making CH4 so much more stable than CH2 or CF2. Intermediates like CH2 are so reactive that they only form temporarily under certain experimental circumstances. Valence bond theory also explains why molecules such as NH3 and H2O are formed. With nitrogen, the 2s22p3 electron configuration becomes hybridized to make four sp3 hybrid orbitals. One of the orbitals is completely occupied with a pair of electrons, while the remaining three are single and are available for bonding.
11
D ORBITAL HYBRIDIZATION Hybridization is not restricted to the s and p atomic orbitals. In period three, the 2d plus 3s and 3p orbitals can be used to create a hybrid orbital, such as is seen in molecules such as SF6 and PF5. In such cases, the five hybrid orbitals created are not the same. Three will form a triangular array of orbitals that are 120 degrees from each other. The other two are at 90 degrees to the first three and are at 180 degrees to each other.
MULTIPLE BONDING So, what about multiple bonding situations, such as is seen in ethylene (C2H4)? What happens is that, out of a 2s22p2 situation, we can promote one of the 2s electrons to a 2p orbital and then create three hybridized sp2 orbitals that each have an electron in it and the promoted 2p orbital. This, as you can see by figure 7, requires energy.
Figure 7.
12
Figure 8 shows what the ethylene molecule looks like from an orbital perspective:
Figure 8.
The two CH bonds come from the sp2 + s orbitals and the double bond comes from the sp2 + Sp2 orbital set. This leaves two 2p orbitals that have a single electron each. They overlap to form a pi-bond which, together with the C-C bond will make a double bond. This pi-bond is also seen in figure 8. As you know, carbon can form triple bonds with itself, using a similar pattern to make acetylene (C2H2). In such cases, the ground state gets promoted as in the case of ethylene but hybridizes to make 2 sp hybrid orbitals and has two remaining 2p orbitals. These 2p orbitals will connect with the 2p orbitals on the opposite carbon atom two make 2 pi-bonds along with the C-C bond it already makes. This leads to a triple bond and a bond left over for a hydrogen atom on each side. The tight bonding makes for a shorter carbon-carbon bond length and a higher bond energy than is seen for single and double-bonded carbon structures like ethane and ethylene.
WRITING ORGANIC MOLECULAR STRUCTURES Hopefully, you now understand a little bit about how organic molecules bond and you understand the periodic table from previous chemistry courses. The good news is that 13
knowledge of the entire periodic table is not necessary to easily recall those things seen commonly with organic molecules. The harder part of organic chemistry is determining how to write out these molecules.
BONDING TRENDS IN ORGANIC CHEMISTRY Using the typical methods used for identifying organic molecules does not work very well, especially when the molecules are large and when there are basically strings of carbon atoms in varying arrangements. For this reason, there are things you need to know in order to write the different organic molecules. All organic molecules are carbon-based and, as you know, it has the ability to form four separate bonds. There are different ways to write this using the chemical abbreviation for carbon. Figure 9 shows what it looks like to write carbon in smaller molecules:
Figure 9.
Hydrogen is also relatively easy. It has one electron and can only have one bond and will have no formal charge. The exception is the single proton H+, and the hydride ion, H-. The hydride ion is a proton plus two electrons. These are highly reactive molecules that do not exist in nature but are important in discussing the chemistry of organic molecules. Next comes oxygen. This exists in one of three ways. It can exist in a neutral way with two bonds and two lone pairs of electrons. It can also become single-bonded with a negative-one negative charge. Lastly, it can come triple-bonded with a formal positive-
14
one charge. A formal charge of negative-one is called a hydroxide ion; a formal charge of positive-one is called a hydronium ion. Nitrogen has two major patterns of bonding. It can exist as a neutral nitrogen atom with three separate bonds, a double bond and a single bond, or a triple bond. Nitrogen can also be positively-charged with four bonds to other atoms. These can be four single bonds, a double bond and two single bonds, or a triple bond and a single bond. There is no double “double-bonding situation” in nitrogen and no formal negative charge on nitrogen, except in highly unstable situations. Two other atoms should be noted as they apply to organic molecules. The first is sulfur and the second is phosphorus. Sulfur generally follows the same pattern as oxygen, while phosphorus is almost always seen in the form of the phosphate ion or PO4 with a three-negative charge. Phosphorus has five bonds, no lone pairs and a zero formal charge. Because it is a larger molecule, it has an expanded valence number with dorbitals in which the octet rule does not apply. Figure 10 shows the phosphate molecule and its charges:
Figure 10.
The halogens, like chlorine, fluorine, iodine, and bromine, are important in organic chemistry but aren’t seen in organic molecules as much as the molecules already discussed. Halogens will have a formal charge of zero and will have a single bond. Rarely, such as in the case of bromine, there will be a three-membered ring with carbon and a formal charge of plus one.
15
Because it is relatively clear that carbon has specific bonding patterns and that other atoms have specific bonding patterns, you can condense the way that organic molecules are written. They can be written in a condensed form, such as CH3CH2CH2OH, as well as in simple line form, in which there is no “C” written for carbon and no “H” written for hydrogen. These are called “line structures” as they look like zig-zagged lines. Only the hydrogens attached to molecules other than carbon are listed and all other atoms are listed in other than line form. Ring structures also do not have a special listing of the atoms separately but is shown as in figure 11:
Figure 11.
These line and ring structures are pared down in a way that it makes it easier to see the bonding and the way the structures are laid out. It tends to work best for larger and more cumbersome molecules; in the case of smaller structures, it is best to write them out without doing a line structure.
CONSTITUTIONAL ISOMERS Constitutional isomers are the same as “structural isomers”. These are molecules that can be written the same way when writing the different carbon, hydrogen, and oxygen molecules but that look quite different. A typical example is fructose and glucose, which are sugar molecules. Both can be written as C4H12O6. How do you write this? Because these are two different molecules, you can imagine that they are different molecules. Figure 12 shows what these two molecules look like when drawn out:
16
Figure 12.
ORGANIC MOLECULAR CHARGES You should also have an understanding of what it means to be a charged molecule. This is relatively easy to understand when it comes to ionized molecules like sodium, which becomes Na+, and chlorine, which becomes Cl- when ionized. What you need to know now is that organic molecules can also be charged. An example of this is methanol, which is CH3OH, which can be neutral. It can also lose an H+ ion to make CH3O- (an anion) or can gain a hydrogen ion to become CH3OH2+. In these cases, the positive charge or negative charge is on the oxygen atom. This can be further categorized as the “formal charge”, which is the charge specifically on the oxygen atom rather than on the molecule as a whole or on any other part of the molecule. To find the formal charge on different atoms of an organic molecule, it is necessary to add up the valence electrons. In the case of oxygen, an unbound oxygen atom has six valence electrons. When it is bound in the methanol molecule, there are two electrons used to make the bonds to carbon and hydrogen and four left over. These four electrons left over are all “owned” by the oxygen molecule. The bound electrons with hydrogen are “half-owned”. Taking the total valence electrons and subtracting the unbound electrons around the atom and half of the bound electrons, gives a net charge of 0 on a methanol molecule. Figure 13 demonstrates the formal charge of -1 on the oxygen atom in the methanol anion:
17
Figure 13.
In the case of methane cation, the extra electron given by hydrogen makes six bonding electrons and two nonbonding electrons. Half of six bonding electrons is three and the two electrons not involved in bonding makes 5 in total. This leads to a +1 charge. The formula for determining the formal charge on oxygen is to take the total valence electrons minus the unpaired electrons and minus half of the bound electrons. Taking six minus five, gives a plus-one charge on the oxygen. Added to the issue is the fact that there can be both negative and positive formal charges on different atoms of a larger molecule. This is the issue with amino acids, in particular. There are zwitterions that have both positive and negative charges on the molecule. The total charge on the molecule is going to be zero. Even though there is a net zero charge on the molecule, it is necessary to show the location of the positive and negative charges on each atom. Formal charges can help to determine which molecules are more stable than others. Let’s look at two possible ways to write CO2 to see which is most stable: In CO2, the C is less electronegative, so that it is the central atom. It has four valence electrons and oxygen has six valence electrons, for a total of 16 valence electrons. The binding of carbon and oxygen gives an O-C-O molecule with double bonds between the two. This would not work with a single bond because it leaves too many unpaired electrons. It can also not be written with a single bond on one side and a triple bond on the other side. It must be symmetric. Figure 14 shows the Lewis dot structure on CO2.
18
Figure 14.
As you can see, the first CO2 structure is more stable because it has a zero formal charge on all atoms, while the second leaves an unacceptable charge on the two oxygen atoms. In general, the Lewis structure with the set of formal charges closest to zero will be the most stable.
RESONANCE CHEMISTRY A discussion of resonance chemistry can be had by looking at the CO3(2-). This has carbon in the middle, a double bond with one oxygen and a single bond with 2 oxygens and an overall charge of 2-. The big question is this: Which oxygen molecule gets the double bond and which get a single bond? This can be explained in terms of resonance. When more than one Lewis dot structure can be drawn for the same molecule, this is said to have “resonance”. What this means is that, for CO3(2-), the charge is rapidly shifting from one place to another in a sort of blur that gives each CO bond equal stability, so that a third of the time, the double bond will be on one of the three oxygen atoms, so that there is equal sharing of this double bond. Instead of a -1 charge on two of the atoms, there will be a 2/3 charge on each oxygen atom on average. Figure 15 shows the resonance of CO3(2-).
19
Figure 15.
The doubles-sided arrow will show that these structures are equivalent. There is not a true -2/3 charge on each atom but, experimentally at least, there is no difference between structures A, B, and C and the experiment to find these structures indicates that these structures as written do not exist. You can draw resonance structures by drawing all of the possible structures and charges on a molecule; however, this can be cumbersome. The only difference between the Lewis structures of these molecules is the placement of the electrons. The atomic position is exactly the same. One can use a curved arrow in order to indicate that an electron shifts between one atom or another. In the example shown in figure 16, there is a curved arrow that indicates the shifting of an electron pair in the ethylene molecule. This is called “electron pushing”.
Figure 16.
20
In drawing resonance structures, all structures must have the same number of valence electrons with no destruction or creation of electrons. The octet rule needs to be obeyed at all times. In other words, you cannot have five bonds around carbon or more than one bond associated with hydrogen. Nuclei cannot change positions in a resonance structure (only the actual electrons). Ozone is a typical resonance structure. It involves three oxygen molecules together that have a double bond between one oxygen and a single bond between the other oxygen. This, however, does not stay stable and the double bond shifts from one oxygen molecule to another. Figure 17 shows the ozone molecule in its resonance arrangement:
Figure 17.
You can try this yourself as you discover that, like ozone, molecules like NO2- will have a resonance structure as well. In short, resonance structures are a simplified way of describing molecular orbitals that extend to cover more than two atoms. The electrons will shift from one place to the next and will be “averaged out” over the course of two molecular bonds.
21
KEY TAKEAWAYS •
Organic chemistry involves carbon, hydrogen, and oxygen, with several other molecules less likely seen.
•
Carbon has four valence electrons, hydrogen has one valence electron, and oxygen has six valence electrons.
•
Covalent bonding involves the promotion of electrons and the hybridization of orbitals so that they take on a unique molecular bonding orbital.
•
The formal charge depends on the number of valence electrons, the number of bonded electrons, and the number of unbonded electrons.
•
The total charge on a molecule in nature will be zero.
•
The simplest way to write an organic molecule is to use line drawings for carbon atoms and all hydrogen atoms not attached to carbon atoms.
22
QUIZ 1. What is the number of valence electrons around the carbon atom? a. One b. Two c. Three d. Four Answer: d. There are four valence electrons around the carbon atom. Rather than taking on electrons entirely, which would make for a crowded state of repulsing electrons, the carbon atom will share these four valence electrons with another atom so that there can be a balance between the shared electron and the positively-charged atom it shares the electrons with. 2. What is the number of valence electrons around the hydrogen atom? a. One b. Two c. Three d. Four Answer: a. Hydrogen has a single electron with only one available for bonding; this means that it has one valence electron in total. 3. Sending an electron to a higher orbital from a lower orbital in order to have an electron able to bind with another atom is called what? a. Elevation b. Hybridization c. Promotion d. Energization Answer: c. Promotion involves sending an electron to a higher orbital from a lower orbital in order to have a free electron available to bind with another atom.
23
4. Creating a new orbital out of two other orbitals in order to have a stable orbital for bonding is called what? a. Elevation b. Hybridization c. Promotion d. Energization Answer: b. Hybridization involves making a new orbital out of two other orbital types in order to have an orbital available for bonding. This takes energy but it is made up for by the energy “saved” in the bonding. 5. Which of the following carbon-based molecules has a triple bond of carbon to carbon? a. Methane b. Ethane c. Ethylene d. Acetylene Answer: d. Acetylene is C2H2 with a triple bond between the two carbon atoms. 6. When methanol becomes charged, where is the formal charge located? a. Oxygen atom b. Hydrogen atom c. Carbon atom d. CH3 side chain Answer: a. When methanol becomes charged, the formal charge of the molecule is located on the oxygen atom rather than on any other part of the molecule.
24
7. What is the charge on a hydronium atom? a. Negative 2 b. Positive 1 c. Positive 2 d. Negative 2 Answer: b. The charge on the hydronium atom or “hydronium ion” is a positive one charge. Hydroxide, on the other hand, is a negative one charge. 8. What situation with nitrogen bonding in organic molecular situations does not exist? a. A triple bond with another molecule b. A double bond and a single bond with two other molecules c. Four single bonds with four other molecules d. Two double bonds with two other molecules Answer: d. The situation of two double bonds with two other molecules with nitrogen just doesn’t exist in nature. 9. In doing line structures, hydrogen is not listed as a separate atom if it is attached to what other atom? a. Hydrogen b. Carbon c. Oxygen d. Nitrogen
25
10. Answer: b. Hydrogen attached to carbon does not have either atom listed separately; however, the hydrogen atom will be listed if it has attached to any other atom. a. What is considered a structural isomer of C6H12O6? b. Deoxyribose c. Ribose d. Fructose e. Lactose Answer: c. Both glucose and fructose are structural isomers that can be written a C6H12O6 but are different structural isomers.
26
CHAPTER 2: BASIC ORGANIC MOLECULAR STRUCTURES This chapter covers the basics of nomenclature in identifying organic molecules. Because there are innumerable organic molecules and because they are based on just a few different types of atoms, there needs to be a way to identify what each molecule looks like by name alone. This leads to a discussion of the IUPAC nomenclature and coverage of the different functional groups in organic chemistry. You will need to understand how to name the different molecules you see in organic molecules, which is covered in this chapter. In addition, there will be a discussion of stereochemistry as it applies to organic molecules.
IUPAC NOMENCLATURE Suffice it to say that there is a lot of memorizing that needs to be done in understanding organic chemistry. Not only are there lots of carbon atoms that come together to form a variety of chemical compounds; there also are a number of ways these carbon atoms can come together. Besides memorizing the smaller compounds, you will need to know the IUPAC nomenclature. This nomenclature is a way of determining what a chemical molecule needs to look like or should be named. IUPAC stands for “International Union of Pure Applied Chemistry”, which has set up a standardized way of writing organic molecules. The first thing you need to start with is how to name the alkanes. We will talk more about alkanes in a later chapter but, for now, you need to name them. The first part of naming any organic molecule is to identify the parent chain. This can be any length of carbon atoms and is generally the longest chain in the molecule. The alkanes are all saturated carbon atoms in a line. You will need to memorize the names of all alkanes based on their carbon number. Any bonding that happens in an alkane happens with hydrogen or another carbon atom. If the chain becomes a
27
functional group, the “ane” ending becomes a “yl” ending. Memorize these and refer back to them using figure 18: Number of Carbons
Chemical Name
1
Methane
2
Ethane
3
Propane
4
Butane
5
Pentane
6
Hexane
7
Heptane
8
Octane
9
Nonane
10
Decane
11
Undecane
12
Dodecane Figure 18.
28
There are some branched chains that have special names that you need to know about and memorize. These are shown in figure 19:
Figure 19.
The first thing to do in any identification system with organic molecules is to identify the longest carbon chain. This is referred to as the “parent chain”. Look then for all of the substituent groups, which replace hydrogen in the parent chain. Number the parent chain starting with the end that gives the carbon atom with the substituent the lowest possible number. For example, you wouldn’t have 9-methyl decane but would instead have 2-methyl decane. If two or more side chains are in equivalent positions, choose the numbering system that will list the one that would come first in the name to have the lowest number. The side chain that comes first is the one that is in the earliest alphabetical order. If two of the same side group occur, the location of each side group is listed numerically with the prefix “di”, “tri”, “tetra”, etc. chosen in order to reflect the number of identical side chains. As mentioned, they are otherwise listed in alphabetical order (not counting the prefixes). The prefix “iso” is not considered a typical prefix (but sec- and tert- are considered prefixes). If there are equal chains that can be considered parent chains, the parent chain with the least number of side chains predominates over the others. Any cyclic ring goes by the prefix “cyclo”, which is not part of alphabetic order. Figure 20 shows some examples of how things are named:
29
Figure 20.
ALKYL HALIDES These are simply halogens added as a side chain to an alkyl carbon chain. It is given no greater or lesser “rank” when considered as a side chain. The halogens are represented as “F” for fluoro, “Cl” for chloro, “Br” for bromo, and “I” for iodo.
ALKENES AND ALKYNES Anytime there is a double bond in the carbon chain, it ceases to become an “alkane” and will become an “alkene”. If there is more than one double bond, it becomes an “-adiene” or “-atriene”. All triple-bonded carbon chains are designated with the ending “-yne”. The position of the multiple bonds can be listed by putting a number on the first carbon of the multiple bond. The rules are lengthy. It starts with a parent chain that is numbered so that the multiple bonds have the lowest number. When there is a choice in numbers, the double bonds are given the lowest numbers. When there are both double and triple bonds, there is an
30
-enyne suffix. The branching nomenclature is done so that the parent chain is the longest chain that contains the most double and triple bonds. If there is still something unclear, the parent chain gets a numbering system that gives the side groups the lowest number at the first point of reference. Some examples are listed in Figure 21:
Figure 21.
ALCOHOLS Alcohols stem from alkanes but have an OH attached to them. This turns the methane molecule to the methanol molecule. The ane ending becomes “anol”. If there is more than one hydroxyl group, it is called an anediol or anetriol, etc. This would lead to an example of 1,3,4-octanetriol, which would be octanol that has three OH groups added to it. In naming the alcohol, the hydroxyl group takes precedence over alkyl groups, halogen side groups, and double bonds in the numbering process. The presence of a double bond takes the -ene from the double bond and the OH from the alcohol to make an enol. The location of the side groups is placed between the en and the -ol. This would lead to a molecule called penten-1-ol. The hydroxyl takes priority in the numbering of the parent chain. The choice of number is given so that the lowest number is given to the first side chain. Figure 22 shows a few examples of alcohols: 31
Figure 22.
ETHERS Ethers, for the most part, need to be memorized by their common name. The basic structure of an ether is two alkane side chains that are connected by an oxygen molecule. An example would be ethyl methyl ether, which is CH3OCH2CH3 and dimethyl ether, which is CH3OCH3. The prefix di is used when there are two equal side chains.
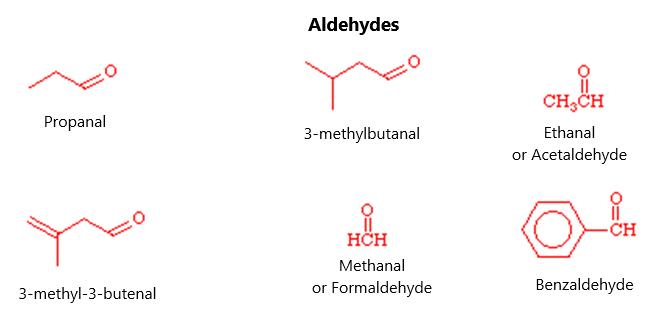
ALDEHYDES These replace the suffix “ane” with the suffix “anal”. It will have a CHO group on it (or more than one). There can be only two of these CHO side chains because they must occur at the end of the molecule. It will be called an anedial if it has a CHO side chain at both ends. The carbon with this configuration will automatically be labeled the first carbon atom in the numbering. Figure 23 shows what some aldehydes look like:
32
Figure 23.
In the numbering system, the carbonyl group takes precedence over any alkyl group and halogen substitutions, as well as any double bonding. If there is a double bond, the en suffix follows the parent chain and the al is last. This makes en-al. If there is any question, number the carbon atoms that give the side chains the lowest number at the first change in the saturated side chains. Figure 24 shows some common examples of common aldehydes. Note that methanol is the same name as the common name called “formaldehyde”:
33
Figure 24.
KETONES In such cases, ketones are named by replacing the “ane” with “anone”. If there is more than one CO group in the chain, it is referred to as anedione or anetrione. The locations of the CO carbon are numbered in the name. The rules include the fact that the carbonyl group takes precedence over double bonds, alkyl groups, and halogens in the numbering of the parent chain. If there are double bonds, the “en” suffix is added after the parent chain and before the “one” suffix, such as 4-penten-2-one. Note that the numbering precedence gives the lowest number to the carbonyl group. The suffix “dione” is used when there are two carbonyl groups. Figure 25 names some ketones:
Figure 25.
34
CARBOXYLIC ACIDS Carboxylic acids start with finding the parent chain, replacing the -ane suffix and adding -anoic acid to the end of the chain. This will have a COOH group. In the case of having two COOH groups, the name will be -anedioic acid. For example, pentanedioic acid will be HOOCCH2CH2CH2COOH. The COOH will be at the end of the side chain. The carboxyl group will take precedence over double bonds, alkyl groups, and halogen substitutes. If the carboxyl group is attached to a ring, the suffix carboxylic acid is added to the molecule. If there is a double bond, the suffix will be enoic acid without numbering as the C1 will be the COOH molecule. Figure 26 shows what common carboxylic acids:
Figure 26.
35
ESTERS Esters have a unique design. This is the RCOOR’ structure, similar to carboxylic acids, but with an R’ side chain instead of a hydrogen atom. The RCO group is called the acyl group, while the R’ group is the alkyl group. The alkyl group name will end in a -yl similar to any side chain. This is then followed by a space and a “carboxylic acid name” but with an “ate” at the end of it instead of carboxylic acid. Figure 27 shows a few examples of what this looks like:
Figure 27.
AMINES Amines will have common names and IUPAC names; however, the common name is what is used in most cases. They are similarly named like ethers with di- and tri- used if two or three of the alkyl groups are the same. They go by the designation RR’NH with the nitrogen group being the “central atom”. Figure 28 shows what some of these amines look like:
Figure 28.
36
FUNCTIONAL GROUPS Functional groups have already been discussed to some degree as the side chains represent functional groups in many cases because they impart reactivity of the molecule. A functional group can be described as a side chain on the molecule that gives the organic substance some type of reactivity. They can be listed as prefixes or suffixes. The suffixes have been talked about but the prefixes haven’t been mentioned. The following is a list of functional groups and the way they are named: •
Carboxylic acids—these have no prefixes but end in -oic acid.
•
Aldehydes—these have no prefixes but end in -al as a suffix.
•
Ketones—these have no prefixes but end in -one as a suffix.
•
Alcohols—the prefix is “hydroxy”, while the suffix is -ol.
•
Amines—the prefix is “amino”, while the suffix is “amine”.
•
Ethers—the prefix is “alkoxy”, while the suffix is “ether”.
•
Halogens—these have no suffixes but only have the prefixes “fluoro”, “chloro”, “bromo”, and “iodo”.
A functional group can be a single atom or a group of atoms, such as Cl or COOH. You should know that many compounds have both a common name and an IUPAC “systematic name”. For example, methanal and ethanal are “formaldehyde” and “acetaldehyde”, respectively. With aromatic rings, these start with “benzo” or end with “benzene”. The chain is numbered so that the lowest numbers are used, for example, if there are any side chains, they are called 1R or, 1-methylbenzene, 1,2-dimethylbenzene, or 1,3, dimethylbenzene, etcetera. Common naming uses “ortho” to describe 1,2 placements, “meta” to describe 1,3 placements, and “para” to describe 1,4 placements of side chains. This is further described in figure 29:
37
Figure 29.
STEREOCHEMISTRY AND ISOMERS In describing isomers of different types of organic molecules, you need to know that there are different types of isomers. Isomers, by definition, have different shapes and arrangements but the same number of atoms per molecule. The one thing that isn’t an isomer is when a molecule rotates on an axis. This happens all the time with organic molecules but doesn’t necessarily represent a different molecule. Molecules can be long and unwieldy but aren’t actually different if they are in different rotational shapes. Structural isomers have completely different orders of molecules. The most obvious is the “chain” isomer. This involves different types of branching in the molecule. For example, C4H10 can be a single chain of carbon atoms to make butane. It can also involve a single carbon atom with a hydrogen on one side and three methyl (CH3) side chains on the other 3 sides. Position isomers have the basic carbon skeleton left unchanged; however, important groups are moved around on the skeleton. An example is C3H7Br. This is propane with a bromine atom attached. The bromine atom can be attached to the end, making it 1bromopropane. It can also be attached to the middle of the chain, making it 2bromopropane. The carbon atoms are still in the same position but the bromine has shifted around. The same thing happens with an alcohol of a chain. The chain stays the
38
same but the OH side chain shifts. When you add position isomerism to chain isomerism, you can get a lot of isomers. There is also functional group isomerism. This involves changing the functional group on the molecule. An example is C3H6O, which could be a ketone (propanone) or an aldehyde (propanal). These are completely different molecules that can be shown in figure 30:
Figure 30.
It is also possible to have an alkene and an alcohol group making up the same molecule in a linear fashion to make CH2CHCH2OH.
DIASTEREOMERISM The phrase diastereomers can also be referred to as diastereoisomers. These are isomers that are structurally the same but with different appearances that are not considered mirror images. When they differ at one stereocenter, they are referred to as “epimers”. D-threose and D-erythrose are structurally the same but look different from a molecular standpoint. As you can see in figure 31, they are not mirror images.
39
Figure 31.
The difference between diastereomers and enantiomers is that enantiomers differ in all of the stereocenters. This makes enantiomers mirror images of one another. Diastereomers will have different physical properties and different chemical properties. If there is a single bond involved, there can be rotation around the carbon atom so that the molecule is the same, regardless of the rotational status.
ENANTIOMER Enantiomers are mirror images that cannot be superimposed upon one another. It is the equivalent of the left hand and the right hand of a person. The thumb makes them not able to be directly superimposed. The mixture of two enantiomers is called a racemic mixture if they have the same concentration. There are S and R stereoisomers, which stand for “left” and “right”, respectively. Enantiomers are important in biochemistry because they act differently in biological systems. Drugs, especially, can be left-handed or right-handed. The general idea is that one handedness will have the primary pharmacological activity, while the other handedness will not be as pharmacologically active. Besides the R/S system, there can be a +/system and a d- and l- system. The D/L system stands for dextro and levo, respectively (right and left-handed). One example is the drug called thalidomide, which was a sedative. One of the enantiomers had sedative properties, while the equally prevalent enantiomer did not have sedative properties but caused birth defects.
40
Another enantiomer drug pair is that of escitalopram and citalopram. These are antidepressants, of which only the escitalopram has antidepressant activity. The drug citalopram is a racemic mixture of S-citalopram and R-citalopram. S-citalopram is a pure enantiomer that makes up the drug escitalopram—given at half the dose of the racemic mixture.
41
KEY TAKEAWAYS •
The IUPAC nomenclature identifies the different organic chemicals based on a few simple rules.
•
There are some organic molecules that must be memorized as being common molecules, although they will have IUPAC nomenclature as well.
•
Diastereomers and enantiomers are the same chemical organization but will have different chemical and physical properties.
42
QUIZ 1. Which is the name for the alkane having three saturated carbon atoms? a. Methane b. Pentane c. Propane d. Ethane Answer: c. Propane has three saturated carbon atoms in a row. It goes by the molecular name of C3H8. 2. What is the name for the alkane that has five saturated carbon atoms? a. Methane b. Pentane c. Propane d. Ethane Answer: b. Five saturated carbon atoms in an alkane is called pentane. It is a C5H12 molecule when written out completely. 3. How does the parent carbon chain get chosen, according to IUPAC rules? a. It is the first in alphabetical order. b. It is the one with the least number of side chains. c. It is longest chain of carbon atoms. d. It is the one that does not have a double bond. Answer: c. It is the longest chain of carbon atoms that is most likely to be chosen as the parent compound. 4. Which side chain will get chosen first if all other things are equal? a. Methyl b. Sec-butyl c. Pentyl d. Isopropyl
43
Answer: b. Iso is not considered a prefix for alphabetizing purposes; however, sec- is considered a prefix. For this reason, “butyl” will be considered to be alphabetically the first side chain chosen. 5. What is the suffix when there is a double bond and a triple bond in the parent compound? a. Enyne b. Adiene c. Yne d. Al Answer: a. The Enyne suffix, which is often broken up to have a number between “en” and “yne” is what a parent compound is called when it contains both a double bond and a triple bond. What this looks like is 4-octen-1-yne. 6. What is added to an organic molecule to get an alcohol? a. CH3 b. Chlorine c. COOH d. OH Answer: d. The OH molecule is added to an alkane to lead to an alcohol from the alkane. 7. Which alcohol has two hydroxyl side groups? a. 2-methyl-1 pentanol b. 1,3 pentanediol c. 2-cyclopenten-1-ol d. 1-cyclohexanol Answer: b. 1,3-pentanediol is a five-carbon pentane molecule with an OH side chain at the one and three position.
44
8. What is the key feature of an ether? a. It has a COOH side chain b. It has an isopropyl side chain c. It has two side chains with an oxygen in the middle d. It has an O side chain with a double bond on the oxygen molecule Answer: c. It has two side chains with an oxygen in the middle, such as diethyl-ether, which is a molecule that is a CH3CH2OCH2CH3 configuration (two ethyl groups attached by a single bonded oxygen molecule. 9. Which aldehyde has a ring structure associated with it? a. Formaldehyde b. Acetaldehyde c. Benzaldehyde d. Propanal Answer: c. Benzaldehyde has a ring structure, which has a benzene ring attached to a carbonyl group as a side chain. 10. Which ketone has two carbonyl subunits? a. Acetone b. 2,4-Pentanedione c. 2-pentanone d. Propanone Answer: b. Anytime there is a dione suffix, it means that there are two carbonyl subunits. If there is any question as to their location, they are numbered.
45
CHAPTER 3: ORGANIC SOLVENT CHEMISTRY The topic of this chapter is organic solvent chemistry. For students who have participated in regular chemistry experiments, the solvent has typically been water. In organic chemistry, the solvent may or may not be water because many aspects of organic chemistry involve nonpolar substances that do not dissolve in water. Solvents may or may not participate in chemical reactions themselves but are important to the chemistry of different molecules. Issues regarding solvation and solutions in organic chemistry are also covered in this chapter.
TYPES OF SOLVENTS A solvent is any liquid that serves as the medium for a chemical reaction. There are two possibilities for a solvent. It can be “non-participatory”, in which it dissolves the reactions only. The theory of “like dissolves like” applies with solvents so that the hydrocarbons in organic chemistry become solvents for nonpolar organic reactions. A solvent can be participatory, and can act as an acid, base, or nucleophile. It is important, too, to recognize the difference between “polar” and “nonpolar” solvents. Polar solvents have large dipole moments, such as with water, which contain bonds between the hydrogen of one molecule and the oxygen of another molecule. This leads to a “partial charge” on the oxygen and hydrogen molecule in water as a solvent. Nonpolar solvents contain bonds between atoms of similar electronegativities, such as carbon and hydrogen in gasoline. These will not be liquids with a great many molecules with dipole moments, leading to a nonpolar solvent. Polarity is measured through understanding the “dielectric constant” of the solvent. The greater the known dielectric constant, the greater is the polarity. One can also measure the dipole moment of the solvent, so that the greater the dipole moment, the greater the polarity.
46
Solvents can be protic or aprotic. A protic solvent is one that has an OH or NH bond. The importance of these is that they can participate in hydrogen bonding between molecules and can donate protons or H+ molecules. Aprotic solvents can have hydrogen in them but, because they do not have an OH or NH bond, they cannot participate in hydrogen bonding and cannot donate a H+ atom. Protic solvents will decrease the reactivity of nucleophiles in substitution reactions, while polar aprotic solvents do not. There are three types of solvents based on what you have just learned. There are nonpolar aprotic solvents, polar aprotic solvents, and polar protic solvents. You should know that there is no such thing as a nonpolar protic solvent.
NONPOLAR SOLVENTS A low dielectric constant is considered a constant of less than five. A solvent with this characteristic will not be good for charged species such as anions. The following is a list of nonpolar solvents; only chloroform and diethyl ether have dielectric constants approaching 5 and have a minimal dipole moment: •
Pentane—this has no dipole moment and a dielectric constant of 1.8
•
Hexane—this has no dipole moment and a dielectric constant of 1.9
•
Cyclohexane—this has no dipole moment and a dielectric constant of 2.0
•
Benzene—this has no dipole moment and a dielectric constant of 2.4
•
Toluene—this has a dipole moment of 0.36 and a dielectric constant of 2.3
•
Chloroform (CHCl3)—this has a dipole moment of 1.04 and a dielectric constant of 4.8.
•
Diethyl ether—this has a dipole moment of 1.15 and a dielectric constant of 4.3.
There is another classification of solvents, called “borderline” polar aprotic solvents. These have dielectric constants of between 5 and 20 and intermediate polarity. They do not have an NH or OH bond so they don’t participate in any reactions. These include the following:
47
•
Dichloromethane (CH2Cl2)—this has a dipole moment of 1.6 and a dielectric constant of 9.1.
•
Tetrahydrofuran or THF—this has a dipole moment of 1.75 and a dielectric constant of 7.5.
•
Ethyl acetate—this has a dipole moment of 1.78 and a dielectric constant of 6.0.
Polar aprotic solvents (not those that are borderline) have large dielectric constants of greater than 20 but do not participate in hydrogen bonding because they have no OH or NH bonds. They can dissolve those solutes that have CN- or OH- bonds, which have more reactivity in these types of solvents because of their high polarity. Examples of these types of solvents include the following: •
Acetone (CH3COCH3)—this has a dipole moment of 2.88 and a dielectric constant of 21.
•
DMF (N, N-dimethylformamide)—this has a dipole moment of 3.82 and a dielectric constant of 38.
•
Acetonitrile (CH3CN)—this has a dipole moment of 3.92 and a dielectric constant of 37.
•
DMSO (dimethyl sulfoxide)—this has a dipole moment of 3.96 and a dielectric constant of 47.
Polar protic solvents have high dielectric constants and high dipole moments. They possess OH and NH bonds and participate in hydrogen bonding. They act as weak acids because they donate protons and are weak nucleophiles that form bonds with strong electrophiles. They often become the solvent for their weak conjugate bases (such as ethanol or EtOH acting as the solvent for EtO-) and water acting as the solvent for OH-. Polar protic solvents include the following: •
Ammonia—this has a dielectric constant of about 25.
•
t-Butanol—this has a dielectric constant of 12.
48
•
n-Propanol—this has a dielectric constant of 20.
•
Methanol—this has a dielectric constant of 33.
•
Ethanol—this has a dielectric constant of 35.
•
Acetic acid—this has a dielectric constant of 6.2.
•
Water—this has a dielectric constant of 80.
These types of solvents are by far the most likely of all solvents to participate in reactions. They can act as nucleophiles when strong electrophiles are present. For the purposes of this course, the solvent will be liquid but it can be a solid, gas, or supercritical fluid. The quantity of solute (which can also be a solid, liquid, or gas) that can dissolve most depends on the temperature of the solvent/solid conditions. When a substance is dissolved into large quantities of another, this is called a solution. The ingredients in a solution are, by definition, evenly distributed in a single phase called a solvate (complexes of solvent and solute). The ability of one compound to be dissolved in another is called “solubility”. If it occurs in all proportions of solvents and solutes, this is called being “miscible”. Molecules of the solvent will arrange around the molecules of the solute. There will be a transfer of heat and an increase in the entropy (or disorder) of the system. There will be the thermodynamic stability of the solution that is greater than the solvent and solute separately, made possible by things like the dipole moments of the molecules, their polarizability, and their hydrogen bonding. With solvation, there is a coordination complex formed that will allow for the stability of the solution. The dielectric constant is a direct measure of polarity of a solvent. Less than 5 is nonpolar, while 5-15 is considered “borderline” polar. Those dielectric constants greater than 15 are definitely polar. The dielectric constant is a measure of the tendency of the solvent to partially cancel out the field strength of the electric field of a charged particle immersed in it. It is the ability of the solvent to dissolve ionic compounds, such as salts. Interestingly, the dielectric constant is not the only measure of a substance’s polarity. There are a few others that are less commonly used, including these:
49
•
The Grunwald-Winstein Y scale is a measure of a solvent’s ability to influence to buildup of positive charge of a solute in a chemical reaction.
•
Kosower’s Z scale is a measure of the solvent’s influence on UV-absorption maxima of a salt.
•
Hildebrand parameter is a measure of the cohesive energy density of a solvent, which is useful in measuring the impact of nonpolar compounds.
•
Reichardt’s dye, which is a dye that changes color in response to the polarity of the solvent.
In general, the polarizability, dipole moment, polarity, and hydrogen bonding of a particular solvent will determine what type of solutes it can dissolve and which liquids are miscible with it. The rule of “like dissolves like” means that polar dissolves polar and nonpolar dissolves nonpolar. Things that are not miscible with one another include water and hexane, vinegar and vegetable oil, and octane and acetic acid. These represent polar and nonpolar substances. Protic solvents can easily solvate anions because they have the strong ability to engage in hydrogen bonding. Water is the best-known protic solvent, while aprotic solvents like dichloromethane and acetone are polar but do not donate hydrogen ions. In organic chemistry, there are pure solvents and many multicomponent solvents that are used and that have mixed characteristics because of their chemical differences. These must be miscible with one another so that they can be conceived of as a solvent. An example is called “solvent 645”, which contains differing amounts of toluene, butyl acetate, ethyl acetate, butanol, and ethanol. Another example is a “thinner” such as Thinner RKB-1, which is 50 percent butanol and 50 percent xylene.
50
Figure 32 shows some polar and nonpolar solvents, according to their chemical structure:
Figure 32.
Most organic solvents will have lower density than water, meaning that they will form a layer above water when mixed with it rather than actually mixing with it. The exceptions are the halogenated solvents, such as dichloromethane and chloroform, which sink to the bottom of the container. Knowing these things is crucial to separating substances in reactions. Those that sink to the bottom and are nonpolar will separate out those solutes first in a separatory funnel, while those that rise above water will collect in the nonpolar solvent and will be isolated after draining the water out first. Often specific gravity being greater or lesser than one will indicate whether the solvent will sit above or below the water in a mixture. Most organic solvents will also be considerably more flammable than water, depending on how volatile they are. The major exceptions are things like chloroform and dichloromethane, which are both chlorinated compounds. Other organic compounds have the capacity to explode in air because they have dense vapors that can go many distances nearly undiluted. Flash fire hazards are possible so empty containers should be stored both open and upside down. Solvents like diethyl ether and carbon disulfide are particularly dangerous because of low autoignition temperatures that greatly increase the fire risk. They can ignite and burn even in the presence of steam pipes, light bulbs, and hotplates because they are 51
ignitable under 100 degrees Celsius. Other solvents, like methanol, will burn invisibly until they catch other materials on fire. Certain ethers, like tetrahydrofuran and diethyl ether, can form organic peroxides with light and oxygen exposure. These organic peroxides are very explosive. This can happen even with a minimum of light involved. Because they have a high boiling point, they can concentrate during distillation and can form a crystalline substance that will explode if disrupted, such as when turning the jar lid as it precipitates on the lid of a jar. This is a bigger problem in laboratories that do not use up the parent solvent quickly. Ethers need to be stored in an airtight container away from light and air.
SOLVATION This describes the interaction of a solvent with whatever dissolves in it. When the solvent is water, ionized substances interact strongly with the solvent with the characteristics of the solution based on the fact that the solvent and solute are polar. Ions are surrounded with a concentric shell of solvent into “solvation complexes”. There is bond forming (as in ionic bonding), van der Waals forces, and hydrogen bonding that happens in solvation. Solvation in water is known as hydration. Solvation is different from solubility. Solvation is a kinetic process that is quantified by its rate, while solubility helps to quantify the dynamic equilibrium state that is achieved when the rate of precipitation is the same as the rate of dissolution. The dissolution rate is listed in moles per second, while the units for solubility is moles per liter, milligrams per milliliter, etcetera. The different types of bonding that can take place in solvation include hydrogen bonding, ion-dipole connectivity, and the different van der Waals forces, which include dipole-induced dipole interactions, dipole-dipole interactions, and induced dipoleinduced dipole interactions. Exactly which forces take place depend on the nature of the solute and solvent. Less polar solutions of course do not have ion-dipole bonding as a general rule because there are no ions; solvents without dipole moments do not engage much in van der Waals forces.
52
The polarity of the solvent is the most important factor in determining how well it solvates a particular solute. From basic chemistry, you should remember that the polarity is determined by the dipole moment, which is the partial “charge” of different aspects of the molecule because of different electronegativity in different parts of the molecule. Something with carbon and hydrogen atoms only that is symmetric will have no “pull” on the part of some of the molecule and will have a zero dipole-moment. Polar molecules as solvents can solvate polar solutes, including ions, creating a solvation shell around each aspect of the solute. Remember that, while water is best studied, other polar solvents can dissolve polar solutes, especially solvents like methanol, ethanol, acetonitrile, acetone, and DMSO. Polar solvents can easily solvate ions; however, nonpolar solvents cannot solvate these ionic substances. Water is a good solvent for any type of acid or base because it can either donate or accept hydrogen ions. Solvation is favored only if the Gibbs free energy of the solution is less than the free energy of the separate solvent and solute. The free energy of the system will be a negative value because of the change in enthalpy and entropy of the solution versus the solvent/solute separately. Solvation involves multiple steps with different energy consequences. There needs to be “space” in the solvent to have the solute enter it—a process that requires energy. Then there needs to be an interaction between the solvent and solute that is favorable in order for the solution to occur. There will be a gain in entropy (or disorder) of the system but this is offset by the favorable interaction between the solute and solvent. Gases tend to be less soluble at high temperatures, which is not the case with solids and liquids in solution.
53
KEY TAKEAWAYS •
Solvents can be polar or nonpolar, depending on the dielectric constant.
•
Solvents can be aprotic or protic, depending on whether or not it can donate a hydrogen ion.
•
The dielectric constant is one way to determine if a solvent is polar or nonpolar.
•
In solvents, the phrase “like attracts like” involves the tendency of polar substances to dissolve in polar solvents and nonpolar substances to dissolve in nonpolar solvents.
•
Solvation involves the dissolution (gradually or quickly) of a solute into a solvent.
54
QUIZ 1. Benzene is what type of solvent? a. Polar and participatory b. Nonpolar and participatory c. Polar and non-participatory d. Nonpolar and non-participatory Answer: d. Benzene is a nonpolar molecule because there are no major dipole moments. It does not add anything to the reactions, making it a non-participatory molecule. 2. Acetic acid is what type of solvent? a. Polar and participatory b. Nonpolar and participatory c. Polar and non-participatory d. Nonpolar and non-participatory Answer: a. Acetic acid is a polar molecule that is also an acid, making it a participatory molecule in the reactions that take place within it. 3. The dielectric constant will determine the polarity of a solvent. What is the “cutoff” for a nonpolar versus polar solvent? a. 1 b. 3 c. 5 d. 20 Answer: c. A dielectric constant of 5 or less is considered a nonpolar solvent.
55
4. What can be donated in a protic solvent? a. H+ ion b. OH- ion c. NH4+ ion d. H20 Answer: a. A protic solvent is considered “protic” because it can donate a proton or H+ ion in an organic chemistry reaction. 5. Which solvent has the highest dielectric constant? a. DMSO b. Acetic acid c. Ammonia d. Water Answer: d. Water has the highest dielectric constant of 80—much higher than any other type of polar solvent. 6. Which type of solvent is most likely to be participatory in a chemical reaction? a. Polar aprotic b. Polar protic c. Nonpolar protic d. Nonpolar aprotic Answer: b. Polar protic solvents have the greatest ability to participate in chemical reactions by virtue of being able to donate a hydrogen ion to a reaction that takes place within it. It should therefore be included in the writing of most reactions as they are participatory. 7. Which solvent does not have a dipole moment of zero? a. Toluene b. Benzene c. Pentane d. Hexane
56
Answer: a. Toluene has a slight dipole moment of 0.36, making it nonpolar; however, it does not have a dipole moment of zero, which is true of the other solvents. 8. Which solvent is most likely to have a density and specific gravity greater than water? a. Toluene b. Pentane c. Chloroform d. Benzene Answer: c. Each of these will be less dense than water except for the halogenated solvents, of which chloroform is one. Chloroform will not mix with water but will sit below it. 9. Which is the least polar solvent? a. Acetone b. Ethanol c. Methanol d. Toluene Answer: d. While there are different ways to determine the polarity of solvents, the dielectric constant is a good measure of polarity. The dielectric constant of toluene is much less than the dielectric constant of the other (polar) solvents. 10. Van der Waals bonding in solvation involves all but what kind of bonding interactions? a. Dipole-dipole interactions b. Dipole-induced dipole interactions c. Ionic-ionic interactions d. Induced dipole-induced dipole interactions Answer: c. Van der Waals bonding in solvation involves several types of interactions but it does not involve ionic-ionic interactions.
57
CHAPTER 4: ALKANES, ALKENES, AND ALKYNES This chapter begins a series of chapters on the different organic compounds and their properties. You’ve learned about alkane, alkene, and alkyne nomenclature in previous chapters so the focus of this chapter is to learn more about these compounds and how they interact with one another and with other organic compounds. These substances can have a variety of different configurations, which need to be discussed as part of this chapter.
ALKANES Alkanes are organic molecules that are made entirely of single-bonded carbon and hydrogen atoms without any functional groups. The general formula is CnH(2n+2). They can be linear (completely straight), branched, or cycloalkanes. They are all completely saturated, meaning that as many hydrogen atoms that can be attached ARE attached in the hydrocarbon. Their saturation makes them the least active of all the hydrocarbons. Their common use is in lubrication and gasoline industries as well as in organic chemistry solvents. Although they are considered nonreactive, there is a great deal of energy stored in the carbon-carbon and carbon-hydrogen bond so that, when oxidized, they give off a great deal of heat in combustion. You should memorize the names of the alkanes up to at least twelve carbon atoms but particularly methane (CH4), ethane (C2H6), propane (C3H8), and butane (C4H10). Those that have an n- in front of the name mean that they are a single non-branched hydrocarbon chain. There may not be anything necessarily different about the way they are written out; however, n-hexane, for example will have different properties from its isomers. When a carbon atom makes four separate bonds, it becomes tetrahedral in nature. In such geometry, only two bonds can occupy a given plane at the same time. The other two bonds are in front of or in back of the plane. In methane, it looks like what is seen in figure 33:
58
Figure 33.
In the drawing, two hydrogens and a carbon atom are in the same plane, the solid wedge shows the forward hydrogen atom, and the dotted wedge shows the hydrogen atom behind the plane. While this is accurate, it is not practical to write out every carbon atom and hydrogen atom in larger molecules. This leads us to the skeletal structure, which shows the bonds but not the carbon or hydrogen atoms. In a saturated and linear molecule, the molecule looks like a zigzag line. The skeletal structure form of propane is shown in figure 34:
Figure 34.
ALKYL GROUPS An alkyl group is an alkane that is a side chain of another group. The formula is CnH(2n + 1).
The ending changes from -ane to -yl but with the same prefix. In such cases, methane
becomes methyl, propane becomes propyl, and butane becomes butyl. Alkyl groups have their own set of common prefixes that have to be memorized. For example, iso means that there is a methyl group on the 2 position of the hydrocarbon. This means that isopentane is 2-methylbutane and isobutane is the same as 2-methylpropane.
59
The prefixes sec- (for secondary) and tert- (for tertiary) should also be memorized. Secmeans that a carbon atom is attached to two other carbons, while tert- means that the carbon is attached to three other carbons. A quaternary carbon is attached to four other carbons. Examples include 4-sec-butylheptane and 4-tert-butyl-5-isopropylhexane. Neo- refers to as a side chain on the second-to-last carbon atom of the chain being trisubstituted. This means that it has three methyl groups attached to it. This leads to molecules, such as neopentane, shown in figure 35:
Figure 35.
ALKOXIDES OR ALKOXY GROUPS Alkoxides are an organic group that is attached to a negatively charged oxygen atom, with the basic structure of RO-. This leads to methoxy-, which is CH3O- and ethoxy, which is CH3CH2O-. The three main principles of naming include first finding the longest, most substituted chain containing a functional group. Second, make sure that the carbon bonded to the first functional group has the lowest possible number. Third, make sure that the side groups and/or functional groups are written in alphabetical order.
60
CHEMICAL PROPERTIES OF ALKANES Alkanes have little opportunity for reactivity because their carbon atoms have their octet of electrons and four covalent bonds with a valence number of 4. This leads to 4 sigma bonds and no pi-bonds around the carbon atom, which are extremely stable. What this means is that, in order to make a reaction occur, heat energy is needed. Gasoline is a mixture of hydrocarbons that can last for long periods of time. It only combusts with the addition of flame, which provides energy for its breakdown. Because it is a hydrocarbon, it will float above the surface of the water it sits on. Alkanes are nonpolar solvents that are miscible only in other nonpolar solvents. They have weak dipole-dipole bonds with combustion producing mainly carbon dioxide and water. They do not release as much heat when combusted when compared to other hydrocarbons. They can also be referred to as paraffins, with branched-chain alkanes called isoparaffins. Methane through butane are very flammable gases at standard temperature and pressure (STP). Pentane is an extremely flammable liquid at STP but boils at 36 degrees Celsius. The boiling points increase with increased alkane length. The first alkane that is solid at room temperature is octadecane, which has 18 carbon atoms. Candle wax has between 20 and 25 carbon chains. Polyethylene is an alkane that has infinite length. All alkanes are virtually inert to acids and bases, having a pKa of greater than 50. This inertness has led to the fact that alkanes remain unchanged in the ground for millions of years. They do undergo redox reactions with halogens and oxygens, so that reactions with oxygen lead to smokeless combustion and halogenation or substitution when mixed with halogens. Free radicals will react with alkanes to create shorter-chain alkanes and branched-chain isomers. Branched alkanes and cycloalkanes have different bond angles from the optimal 109.5 so that there will be increased reactivity and strain on these molecules.
61
CYCLOALKANES These are alkanes that form a ring structure. They have similar physical properties to straight-chain alkanes but will have greater numbers of London forces, causing higher densities, melting points, and boiling points than their straight counterpoints. These are saturated molecules and are nonpolar. Many are used in natural gas, kerosene, diesel, and motor fuels. There are four groups of cycloalkanes: •
Small rings—less than or equal to 4 carbon atoms, such as cyclopropane and cyclobutane
•
Common rings—5-7 carbon atoms, such as cyclopentane, cyclohexane, and cycloheptane
•
Medium rings—8-12 membered rings
•
Large rings—these are thirteen carbon atoms or higher
The chain can have alkyl groups attached to it. Cis-isomers will have the alkyl side chain on the same side of the chain, while trans isomers will have alkyl side chains on the opposite side of the chain. Like regular alkanes, the trend toward increasing boiling points and melting points increases with the number of carbon atoms. London dispersion forces are the attractive or repulsive forces between molecules or between parts of the same molecule. For cycloalkanes, these London dispersion forces refer to the repulsive forces between the molecules that cause ring strain. Ring strain occurs because the bond angle of 109.5 degrees, which is considered ideal, does not happen. Cyclopropane has the greatest ring strain because of a tight bond angle between carbon atoms (at 60 degrees to make a triangular molecule). The heat of combustion or delta H combustion will increase with the number of carbon atoms on the molecule. This change in heat of combustion reflects the amount of London dispersion forces in the molecule. The heat of combustion per CH2 will decrease as the molecular size increases in cycloalkanes because of a decrease in the ring strain.
62
There are several types of strain in cycloalkanes. There is angle strain as a result of the smaller than desirable angle between carbon atoms. There is also torsional strain, which involves hydrogen atoms being closer together than they would like to be. The combination of angle strain and torsional strain is referred to as ring strain. The ring strain decreases as the size of the ring increases so that, with cyclohexane, there is no ring strain at all. Any rings higher than that will not be seen generally in organic chemistry. Even with no ring strain, cyclohexane is not a straight planar molecule. It can be in two different shapes in nature: the chair conformation and the boat conformation. These are the most desirable shapes. The chair conformation is more desirable than the boat conformation because it has less interference between the hydrogen atoms, called “transannular strain”. Only the chair conformation has no angle strain because it has no “eclipsing strain” and a small amount of “steric strain” (which is crowding of hydrogen atoms). Steric strain occurs when two atoms are in close proximity to one another. A summary of the different types of strain involves the following: •
Transannular strain—this is the crowding of two side groups in a ring.
•
Eclipsing strain—this is also called torsional strain, which is strain between molecules because of the bond interaction between two eclipsed atoms or groups.
•
Bond angle strain—this is when there is poor overlap between carbon atoms that allows overlapping of the atomic/hybrid orbitals.
ALKENES As the molecular formula grows in size and number of carbon atoms, the number of possible structures increases. For example, with C5H8, what’s clear is that the hydrogen saturation is 4 less than is acceptable. This means that there may be a triple bond, a couple of double bonds, or a ring structure with a double bond, and two rings, among others. This means that there needs to be a consistency in the nomenclature that defines these types of molecules.
63
Things you need to know about determining alkene names include the following: •
Use the suffix “ene” to indicate an alkene or a cycloalkene.
•
The longest chain listed in the root chain must include both carbon atoms of the double bond.
•
The numbering of the carbon atom is done so that the double bond has the lowest number.
•
If the double bond is in the middle, the numbering is so that the side chain has the lowest number.
•
The double bond locator carbon atom is the smaller number of the two carbon atoms.
•
The term diene or triene is used to determine the number of double bonds if there is more than one, with each double bond assigned a locator number.
•
There are side chains labeled with “common names”, including the vinyl group (which is CH2CH- side group) and the CH2CHCH2- or allyl side group. Figure 36 shows the vinyl and allyl side groups written out:
1.
Simple common alkenes include the following, which are very similar to the names of the alkanes: •
Ethene is C2H4
•
Propene is C3H6
•
Butene is C4H8
64
•
Pentene is C5H10
•
Hexene is C6H12
•
Heptene is C4H14
•
Octene is C8H16
•
Nonene is C9H18
•
Decene is C10H20
As you can see, the ratio of carbon atoms to hydrogen atoms in a single double-bonded molecule is 1:2. You need to number the double bond unless the double bond is at the far end of the chain structure, which would be something like 1-pentene. This is not necessary; however, 2-pentene is a possible type of alkene name. Another way of saying it is pent-2-ene. The only time it is necessary to say 1 before the molecule is if there is a side group, such as 2-ethyl, 1-pentene or 2-ethyl pent-1-ene. If there is more than one double bond in an alkene, all of the bonds should be numbered in the name of the alkene, even if there is a terminal double bond. An example would be deca 1,5 diene. Conjugated double bonds are double bonds separated by a single bond, which adds stability to the molecule. This makes making a conjugated double bond more favorable than other types of double, double bonds. Double bonds that are separated by a single carbon atom are called “cumulated”. An isolated diene has two double bonds separated by a greater distance from each other. Double bonds can have isomers using the cis-trans designation or the E-Z designation. The cis-form means that the side groups are on the same side of the double bond, while the trans-form means that the side groups are on the opposite side of the double bond. The E-Z system is based on the German words for “entgegen” or opposite side and “zusammen” or the same side. In this way, E means trans and Z means cis. The priority of the groups is based on the atomic mass of the attached atoms and not on the size of the group. There can be two types of cyclic bonds in cyclic alkenes. The first is the endocyclic double bond, in which both carbons are within the ring structure. The second is the exocyclic double bond, in which only one carbon of the bond is within the ring structure. 65
There is, for example, cyclopentene, which is a pentene ring with an endocyclic double bond and methylene cyclopentane, in which the double bond is exterior to the ring but has one carbon as part of the ring. When the side chain has a double bond, for example, with ethene, the name becomes “ethenyl” to make it a side chain. As a common name, ethenyl becomes vinyl, which is more commonly used. The conjugated diene molecule can be written as a resonance structure. What this looks like is seen in figure 37, in which a partial charge is necessary to have the bond shift from the 1 and 3 carbon atoms of 1,3-butadiene to the central carbon pair:
PHYSICAL PROPERTIES OF ALKENES The carbon-carbon double bond of alkenes changes the physical properties of the substance. Alkenes can be solid, liquid, or gas at room temperature. Cis isomers will have lower melting points when compare to trans isomers. There are also weak dipoledipole interactions because of the electron-attracting capabilities of the carbon atoms. Ethene (C2H4) and propene (C3H6) are the two smallest alkenes and do not have isomers. Everything larger than that will have an isomer of some sort. Because the carbon-carbon double bond does not allow any rotation about it, there are cis and trans isomers that will not rotate. At STP (standard temperature and pressure), ethene, propene, and butene are colorless gases. This means they have a low boiling point with respect to STP. Pentene, hexene, and heptene have a higher boiling point, existing as liquids at room temperature. Anything with fifteen carbons or more will be solid. These are denser than water and are, of course, insoluble in water because they are nonpolar.
66
The boiling point will be similar to the alkanes of the same length with higher boiling points and melting points at greater molecular masses but with slightly lower boiling points than the corresponding alkanes. Only Van der Waals dispersion forces apply in the stability of the liquid and solid forms. The cis isomers will have a lower melting point than the trans isomers and will have a greater dipole moment because the side chains are on the same side, creating an “unbalanced” molecule.
ALKYNES Alkynes are organic molecules made with at least one carbon-carbon triple bond. Every n number of carbon atoms associated with an alkyne has 2n – 2 hydrogen atoms linked to it. If there is a double bond and a triple bond in the molecule, the first multiple bond gets a lower number. If either bond can be assigned the same number, the molecule is called an “n-ene-n-yne” with the double bond root name preceding the triple bond root name. An example is 2-hepten-4-yne. These are always linear because the carbon atom has nothing to bind to except a hydrogen atom. The names are similar to the alkanes and alkenes except that ethyne (C2H2) mainly goes by the common name of acetylene. The others that are named go directly along with the corresponding alkanes, such as these: •
Propyne—C3H4
•
Butyne—C4H6
•
Pentyne—C5H8
•
Hexyne—C6H10
•
Heptyne—C7H12
•
Octyne—C8H14
According to the IUPAC rules, the longest chain is one that contains the triple bond. The carbon number is done so that the triple bond is closest to “1”. A 1-alkyne is called a terminal alkyne with an alkyne at any other point along the chain being called “internal alkynes”. The side chains or substituents are listed according to their alphabetical order
67
with di, tri, and tetra used for two, three, and four side chains, respectively (with the prefix not taken into account alphabetically). If there is an alcohol in the molecule, the alcohol group takes priority over the triple bond so that the numbering assures that the alcohol side chain has the lowest numbered carbon atom. In such cases, the suffix is “ynol” because this becomes an alcohol. If there are two triple bonds, the suffix becomes “diyne”. An alkynyl side chain is any side chain that contains a triple-bonded aspect. There is, for example, an ethynyl, a 2-propynyl, and a 2-butynyl side chain. An alkenyne is a double and triple-bonded molecule, numbered with the system that gives the functional group the lowest number. Again, the triple bond is given the lower number over the double bond. These are almost never cyclic molecules such that the smallest possible cyclic alkyne is one that has ten carbon atoms associated with it.
ALKYNE REACTIVITY Alkynes can react with electrophiles because of the formation of a pi-complex, in which the electrophile temporarily and weakly bonds to the multiple bonded carbon atom. These reactions do not happen very quickly but are more exothermic than alkene reactions of a similar size. The addition of HCl to acetylene (ethyne) gives rise to molecules like vinyl chloride. The addition of hydrocyanic acid (HCN) to acetylene gives rise to acrylonitrile. The addition of acetic acid to acetylene gives rise to vinyl acetate. Each of these is a useful synthetic molecule. Mercury and copper salts are used as catalysts in these types of reactions. When it comes to acidity, the weakest Bronsted acids in organic chemistry come from the alkanes, with an estimated pKa of about 48 associated with ethane. The pKa is decreased slightly with ethene at a pKa of 44. The pKa of acetylene or ethyne is just 25, making it 1023 times more acidic than ethane. This acidity allows acetylene to react with soluble silver salts and copper salts to make insoluble metal compounds combined with acetylene. Alkenes can be made by reducing alkynes as long as a catalyst is used. The catalyst will not only turn an alkyne into an alkene but it will also determine what side chains are 68
added to the newly-made alkene molecules. In addition, alkynes are quick to undergo additions because they have 2 pi bonds. They can become completely hydrogenated to make an alkane when mixed with hydrogen gas and using platinum, finely dispersed nickel, and palladium-on-carbon catalysts. There are also catalysts that can turn an alkyne into a cis- or trans- alkene. A special catalyst called Lindlar’s catalyst can be used to make an alkene and to stop the hydrocarbon from hydrogenating all the way to an alkane.
69
KEY TAKEAWAYS •
Alkanes are fully hydrogenated hydrocarbons without any multiple bonds.
•
Alkenes have one or more double bond.
•
Alkynes will have one or more triple bond.
•
Each of the hydrocarbons follows typical IUPAC rules but will have many common names that are used in organic chemistry.
•
In terms of reactivity, alkanes are less reactive than alkenes, and alkenes are less reactive than alkynes.
•
Any alkane, alkene, and alkyne of sufficient length will form a cyclic compound but there is bond strain associated with smaller molecules, making them impossible at certain small sizes (which varies according to the type of molecule).
70
QUIZ 1. If there are n carbon atoms on an alkane, how many hydrogen atoms are there on the molecule? a. 2n b. 2n + 2 c. 2n + 4 d. 3n Answer: b. If there are n carbon atoms, there will be 2n + 2 hydrogen atoms on the alkane. This best indicates a completely saturated hydrocarbon molecule. 2. What can be said specifically about n-hexane as opposed to other types of hexane? a. It doesn’t have a double bond while other types of hexanes do. b. It is branched, while other types of hexanes are not branched. c. It is not branched, while other types of hexanes are branched. d. It is much more combustible than other forms of hexane. Answer: c. The n in front of the alkane, as in n-hexane, means it isn’t branched, while other forms of the alkane are possibly branched. 3. A carbon atom with four CH3 molecules attached to it is called what? a. Isobutane b. Tert-pentane c. Sec-butane d. Neopentane Answer: d. Neopentane is a carbon atom with four CH3 molecules attached to it. It is a cross-shaped molecule that has a total of 5 carbon atoms but an unusual shape.
71
4. If you have an ethyl group on the third carbon and a methyl group on the fourth carbon atom of a seven-carbon chain of an alkane, what is this called? a. 3,4 ethyl methyl heptane b. 3-ethyl, 4-methyl heptane c. Iso methyl, 3-ethyl heptane d. 4-methyl, 5-ethyl heptane Answer: b. The lowest numbers should be used and the molecule’s side chains should be in alphabetical order. This means that the molecule is a heptane and that 3 should be used to describe the ethyl group and 4 should be used to describe the methyl group. 5. Which cycloalkane has the greatest ring strain associated with it? a. Cyclooctane b. Cycloheptane c. Cyclobutane d. Cyclopropane Answer: d. Cyclopropane has the greatest ring strain associated with it because it has just a 60-degree angle between carbon atoms, which greatly differs from the ideal 109.5 degree-angle desired by the sp3 hybrid bond. 6. What is the smallest cycloalkane that has no ring strain associated with it? a. Cyclopropane b. Cyclopentane c. Cyclohexane d. Cyclooctane Answer: c. Cyclohexane has a bond angle that will be close to the desirable bond angle of 109.5 degrees; this will lead to no ring strain. In fact, there are no cycloalkanes greater than this size seen commonly in organic chemistry.
72
7. What is the smallest possible alkene molecule? a. Pentene b. Butene c. Ethene d. Hexene Answer: c. The smallest possible alkene molecule is C2H4 or ethene. It is impossible to have an alkene molecule with less than 2 carbon atoms to make the required double bond. 8. When the alkene molecule has side chains on opposite sides of the double bond, what can the designation be in order to describe this? a. L-alkene b. E-alkene c. Z-alkene d. Cis-alkene Answer: b. The term E-alkene refers to the German word “entgegen”, which means “opposite” using the E-Z designation. There is also a cistrans designation but, in alkenes, there is not an L-D designation. 9. What happens to the melting point of alkenes as they get bigger or longer? a. The melting point will be greater if it is a cis molecule versus a trans molecule. b. The melting point is much higher than corresponding alkanes. c. The melting point will be lower with increased molecular weight. d. The melting point will increase with increased molecular weight. Answer: c. The melting point will increase with increased molecular weight and will be lower in a cis versus a trans molecule.
73
10. Alkynes with n carbon atoms will have how many hydrogen atoms associated with it? a. 2n b. 2n – 2 c. 2n + 2 d. 2n – 4 Answer: b. If the number of carbon atoms is n, there will be 2n – 2 hydrogen atoms associated with it in any alkyne.
74
CHAPTER 5: ALDEHYDES, KETONES, AND CARBOXYLIC ACIDS The focus of this chapter is the chemistry of aldehydes, ketones, and carboxylic acids. This is the first time the chemistry of oxygen comes into play in this course. Aldehydes and ketones are discussed together because they have very similar chemistry and reaction types. Carboxylic acids are also oxygen-related because they have a COOH side chain as their defining characteristic. They also have great reactivity and are seen in nature as fatty acids and other biochemically-important molecules. In all cases, you will come to understand their nomenclature, their physical properties, and some of the most important chemical reactions associated with these molecules. Aldehydes and Ketones Like alkenes, aldehydes and ketones have double bonds; however, the double bonds in aldehydes and ketones include the oxygen atom. Because oxygen is highly electronegative, the carbonyl group that defines these compounds has a dipole moment. Because of this, these compounds will have higher boiling points when compared to alkenes of the same length. They are also somewhat more water soluble. Figure 38 illustrates the dipole moment of a ketone molecule:
Figure 38.
75
An example of the changes in features of alkenes and ketones is the difference between (CH3)2CO and (CH3)2CH2. This is described in figure 39:
Figure 39.
The fact that the carbonyl group is polar makes it much more polar when compared to the double bonding of alkenes. Water can easily add to the carbonyl group whereas it cannot easily add to alkenes. The bond energy of the carbon double bond is 146 kcal per mole. The sigma part of this bond is 83 kcal per mole; however, the pi-bond adds 63 kcal per mole. The totality of the energy of the C-O double bond in an aldehyde or ketone is between 170 and 180 kcal per mole with increasing energy when there is an R side group on the molecule versus a hydrogen bond. Just the sigma bond is 86 kcal per mole, which is slightly higher than the C-C sigma bond. This means that the addition of the pi-bond on this linkage adds nearly 100 kcal per mole—much higher than the pi-bond energy of the alkene double bond. What this suggests is that any addition reactions to the carbonyl bond is energetically not favorable. Adding water to an alkene gives rise to an alcohol. This requires a catalyst because, although it is exothermic, it has a high activation energy. The reverse activity (making an alkene from an alcohol) is even slower and is endothermic. The reaction, in contrast, of adding water to a ketone or aldehyde is extremely fast and exothermic—leading to a geminal-diol (with two OH side chains attached to the same carbon atom).
76
NAMING ALDEHYDES Aldehydes have an RCOH configuration with the carbonyl group being an important functional group. Other names for the carbonyl group in an aldehyde are the methanoyl or formyl group. The name “aldehyde” stems from the dehydration of alcohols. The main difference between an aldehyde and a ketone is that there is a hydrogen ion attached to the carbonyl carbon in an aldehyde and an R chain attached in a ketone. Technically, the hydrogen atom can be attached on both sides of the carbonyl carbon atom in “methanal” or formaldehyde. Figure 40 shows the characteristic structure of these important molecules:
Figure 40.
The ending “al” is the designation given to aldehydes. The carbon chain is numbered from the carbonyl atom and the numbering proceeds to identify the longest chain possible that contains the carbonyl group. There are many common names for aldehydes, which will be discussed. Rather than name the carbon atoms by number in smaller aldehydes, they are referred to by Greek letters. This means that the carbon atom next to the carbonyl atom is called alpha, followed by beta, gamma, delta, and so on. If the CHO functional group is attached to a ring, the ring carbon is called “number one” and the aldehyde is referred to as a “carbaldehyde”. Otherwise, for chain aldehydes, it would be wise to remember the alkane side names as these are used in describing aldehydes.
77
These are some common aldehydes: •
H2CO—this is methanal or formaldehyde, the smallest aldehyde
•
CH3CHO—this is ethanal, commonly referred to as acetaldehyde
•
CH3CH2CH2CHO—this is butanal as it has four carbon atoms, commonly called butyraldehyde
•
CH3CH2CH2CH2CHO—this is pentanal as it has five carbon atoms, commonly called valeraldehyde
•
Cyclohexane plus a CHO group is referred to as cyclohexane carbaldehyde
•
Benzene plus a CHO group is referred to as benzene carbaldehyde or simply benzaldehyde
These basic aldehydes have common names and should be memorized.
NAMING KETONES The suffix “-one” is used to define ketones. A ketone is similar to an aldehyde but it has two R groups on either side of the carbonyl group—neither of which is hydrogen. Because it isn’t at the end of the chain, it needs to be numbered. Numbering starts at the carbon atom that gives the carbonyl group the lowest possible number. Propanone and phenylethanone don’t require a locator number because the carbonyl chain can only happen at one spot in the molecule. Another way to name a ketone is just to say the names of the two side groups attached to the carbonyl group and then add “ketone” to the end of the name. The R chains are listed alphabetically. There are common names, such as “acetone”, which is the name for propanone. Another example is something like 3-hexanone, which can also be called ethyl propyl ketone (named for the R and R-prime side chains). Figure 41 shows some ketones that should be memorized:
78
Figure 41.
What should you do when there are aldehydes and ketones within the same molecular structure? In such cases, the aldehyde takes precedence over the ketone group, so it is called an aldehyde. The ketone group is called an “oxo” side group. The aldehyde, of course, does not need to be numbered; however, the “oxo” group must be numbered and named accordingly. The carbonyl group of the aldehyde is given the number “one” so that 2-methyl-3-oxo-butanal has 4 carbon atoms in the main chain, a methyl group at carbon number two and another carbonyl group at carbon number 3. It is entirely possible to have diketones and dialdehydes. The aldehyde doubled would have a carbonyl group at either end and would end in “-dial” to reflect two aldehyde groups. Butanedial has 4 carbon atoms, including two that participate in the aldehyde ends of the molecule. In a “dione” molecule, there are two ketone carbonyl groups— both of which need numbering. The molecule 2, 4, pentanedione is a five-carbon chain that has a carbonyl group on the second and fourth carbon atoms. It is also possible to have a cyclic ketone molecule. The number one carbon atom in the ring is the carbonyl carbon, going around the ring to have the lowest possible numbers. Of course, cyclo is added before the parent chain to indicate a cyclic molecule. The suffix “-one” is added to indicate that the molecule is a ketone. This would lead to molecules like cyclopentanone—a five carbon molecule with a carbonyl group as part of a ring structure. Dione is the suffix given when two carbonyl groups are in the cyclic molecule. This would lead to a molecule like 1,2-cyclopentanedione. In the case of the aldehyde or ketone plus an alcohol side chain, the carbonyl group takes precedence over the alcohol group. It is named as a ketone or aldehyde with a
79
numbered “hydroxy” group in the name of the molecule. This would lead to molecules like 4-hydroxybutanal and 4-hydroxy, 3-methyl-butanal. In the same way, an aldehyde or ketone carbonyl group will take precedence over an alkene functional group. Any carbonyl group will be given the lowest possible number. In order to name the molecule, find the longest chain that contains the alkene and use the “enal” or “enone” ending, depending on whether it is a ketone or an aldehyde. The carbonyl carbon gets the lowest possible number and, because it is an alkene, the designation cis or trans needs to be given. The molecule trans-3-pentenal is an aldehyde with 5 carbon atoms and a double bond between the third or fourth carbon. The CHO fragment is referred to as the formyl fragment, particularly when associated with something that is not an alkane or other carbon group, such as formyl iodide. The acetyl fragment or acety fragment refers to the CH3CO- side chain. This could lead to a molecule called acetyl chloride, which has an acetyl group and a chloride group associated with it.
THE CARBONYL GROUP The carbonyl group itself is the CO double-bonded fragment. The carbon and oxygen are bonded with sp2 hybridized bonds. While this is a double bond like the C-C double bond, it is much different in character. The oxygen atom has two lone pairs of electrons associated with it—one in the 2s orbital and the other in the 2p orbital. These lead to electronegativity of the oxygen atom in the carbonyl group, which is very different from the carbon-carbon double bond situation. The double bond is a regular sigma bond and a pi-bond between oxygen and carbon. Figure 42 shows the bonding structure of the carbonyl bond:
80
Figure 42.
The strength of the carbonyl bond is about 176-179 kilocalories per mole. The longer the bond length, the lower is the polarity of the bond. The bond length of this bond is about 1.2 Angstroms. The polarization leads to partial positivity of the carbon atom and partial negativity of the oxygen atom. The Greek letter delta is used to describe a partial charge (positive or negative) over the appropriate atom. The polarization also leads to water solubility but only up to 6 carbons in length as larger ones are considered insoluble in water. The carbonyl group is prone to additions and to nucleophilic attack because of the partial charges of the carbon and oxygen atoms. Protons or hydrogen atoms are the most likely attacking atom with regard to the electronegative oxygen in the group, while nucleophiles are looking for positive charges, such as exist on the carbon side of the bond. The nucleophile will “take” one of the bonds away from the carbonyl group and this leads to a negative charge on the oxygen, which can be covered with a proton in the form of the hydrogen ion. Figure 43 describes how this takes place:
81
Figure 43.
The nucleophile will be a negatively charged ion or a molecule that has a lone electron pair like ammonia or NH3. As you can see, the first thing that happens in the reaction is that the double bond gets broken with an addition reaction happening after that. Aldehydes, in particular, are easily oxidizable because of the hydrogen attached to the carbon atom. This just doesn’t happen easily in ketones unless there are powerful oxidizing agents involved.
REACTIVITY OF ALDEHYDES AND KETONES Small aldehydes, like methanal and ethanal will be gaseous or will boil close to room temperature. Larger molecules will have higher boiling points and will be liquid at room temperature. As the molecule gets bigger, there will be more electrons as part of it and there will be increased van der Waals attraction forces. This would be true even if it weren’t an aldehyde or ketone. The addition of polarity means that there will be dipole-dipole attraction forces, leading to higher boiling points than the corresponding alkane. Consider the 3-carbon alkane, which has a boiling point of -42 degrees Celsius; this increases to 21 degrees Celsius in aldehydes because of polarity. Alcohols will have hydrogen bonding, further increasing the boiling point. As mentioned, solubility in water with aldehydes and ketones depends mainly on chain length. The small aldehydes and ketones are fully miscible with water because, while they can’t hydrogen bond with themselves, they can hydrogen bond with water. This can be hydrogen bonding between the lone pairs of electrons on the carbonyl oxygen
82
molecule with hydrogen on the water molecule. All of this falls apart when the chain length gets larger and there is more nonpolarity in the molecule. A ketone can be reduced through a process called dehydrogenation. Under certain “reduction conditions”, the RCOR molecule can be reduced to RCH2R plus H2O, completely reducing the carbonyl group to make an alkane or similar molecule plus water. The Wolff-Kishner reduction reaction involves treating an aldehyde or ketone with hydrazine (N2H4), which causes a hydrazone derivative species that, when mixed with heat and a base will lead to a hydrocarbon. This can lead to the making of a hydrocarbon from an aldehyde or a ketone. It essentially hydrogenates the ketone to make a hydrocarbon. Another reduction reaction is the Clemmensen reduction reaction. This involves the use of zinc and an acid like HCl, which again takes a ketone or aldehyde and removes the carbonyl oxygen. The carbon atom of the carbonyl group has a relatively high oxidation rate already. It can, however, be further oxidized by converting the RCHO molecule to an RCOOH molecule, which is a carboxylic acid. There are several reagents, such as bromine and potassium permanganate, that will help oxidize the aldehyde. Even exposing the aldehyde to air will slowly oxidize it. Saturated ketones, on the other hand, will be generally inert to the oxidation process. The alpha carbon, which is the carbon atom next to the carbonyl group, is particularly susceptible to substitution in substitution reactions. They can be mixed with Chlorine or Bromine gas along with an acid or base catalyst in order to halogenate the alpha carbon of the ketone or aldehyde. In ketones, the alpha carbon can be on both sides of the carbonyl carbon—both of which are technically reactive. The substitutions involve the loss of a hydrogen atom and the replacement of it with a halogen. Water can be added to the carbonyl carbon, leading to a geminal-diol, which is relatively unstable. The main exception to this is formaldehyde, which forms a stable geminaldiol. A similar reaction involves the activity of alcohols to aldehydes and ketones to make an unstable hemiacetal. Figure 44 shows the formation of a hemiacetal and an acetal when mixing a ketone with an alcohol:
83
Figure 44.
The reaction that makes an acetal requires an acid catalyst and requires the removal of water from the equation.
NATURAL OCCURRENCE OF KETONES AND ALDEHYDES Aldehydes occur to a great extent in nature and play a particular role in the taste and smell of food. Figure 45 shows several important aldehydes in food:
Figure 45.
84
There are also complex aldehydes and ketones in hormonal structures seen in animals. These include hormones like testosterone, progesterone, and cortisone—all complex ketone structures.
CARBOXYLIC ACIDS The carboxylic acid has a carbonyl group and a hydroxyl group together, making a COOH functional group. This leads to increased activity compared to aldehydes and ketones. Because of this reactivity, it is considered a distinct functional group. The parent chain remains the same but the ending becomes “anoic acid”. The carboxylic acid group is always at the end of the chain, leading to this being the number one carbon atom. Similar to aldehydes, the carbon atoms are considered not by numbers but by the alpha, beta, gamma system set up by the Greeks. There are many common names of carboxylic acids that you should memorize. This includes the following: •
Formic acid—this goes by the IUPAC name of methanoic acid
•
Acetic acid—this has the IUPAC name of ethanoic acid and comes from vinegar
•
Propionic acid—this has the IUPAC name of propanoic acid and comes from milk
•
Butyric acid—this has the IUPAC name of butanoic acid and comes from butter
•
Valeric acid—this has the IUPAC name of pentanoic acid and comes from valerian root
•
Caproic acid—this has the IUPAC name of hexanoic acid and comes from goats
•
Enanthic acid—this has the IUPAC name of heptanoic acid
•
Caprylic acid—this has the IUPAC name of octanoic acid
•
Pelargonic acid—this has the IUPAC name of nonanoic acid
•
Capric acid—this has the IUPAC name of decanoic acid and comes from goats
85
Carboxylic acids can be seen in a ring structure, in which it simply has the suffix “carboxylic acid” attached to it. Wherever it attaches to the ring, the carbon atom is given the number one designation. There can be cis and trans molecules. Figure 46 shows the structure of some cyclic carboxylic acids:
Figure 46.
The salts of carboxylic acids are called carboxylates. This is the case with common endings and IUPAC endings so that sodium acetate becomes the salt of acetic acid. It is made with the loss of the hydrogen ion, the presence of a negative charge, and the addition of a salt. Carboxylic acids are given the highest nomenclature priority using the IUPAC system. The carboxyl group is given the lowest possible number with alcohol groups called “hydroxy” groups in the nomenclature setting. This would lead to things like 3hydroxypentanoic acid, which is a five-carbon chain, a COOH at the end, and a hydroxyl group on the third carbon atom. If an amine functional group is present, this is called an amino group, leading to molecules like 2-aminobutyric acid, also called 2aminobutanoic acid. The addition of an alkene leads to an “enoic acid”, such as 3pentenoic acid, which has a double bond at the 3-position (remember that cis and trans or E and Z are necessary for alkenes). Of course, there can be carboxylic acid functional groups on both ends of the molecule, leading to a dicarboxylic acid. This is then referred to as a “dioic acid”, such as butanedioic acid—a four carbon dicarboxylic acid.
86
FATTY ACIDS Carboxylic acids are found in nature in many forms. Fatty acids are long-chain fatty acids in nature. Long-chain fatty acids are named by their common name, particularly from the C12 to C20 chains. Some of these saturated fatty acids include the following: •
C12—lauric acid
•
C14—myristic acid
•
C16—palmitic acid
•
C18—stearic acid
•
C20—arachidic acid
Most naturally-occurring fatty acids have an even number of carbon atoms, even though it is possible to make the odd-numbered fatty acids synthetically. Unsaturated fatty acids are generally all cis fatty acids. Some of these are commonly seen in nature as linoleic acid, linolenic, arachidonic and oleic acid. Other naturally-occurring carboxylic acids include malic acid (a dicarboxylic acid), pyruvic acid, niacin, and citric acid (a tricarboxylic acid). Figure 47 shows these common carboxylic acids:
Figure 47.
87
PROPERTIES OF CARBOXYLIC ACIDS As you know, the carbon and oxygen in the carbonyl group are both sp2 hybridized bonds, which give the group its trigonal shape. The hydroxyl group is also sp2 hybridized, leaving one of its lone pair of electrons to combine with the pi-bond, which leads to a resonance structure. Figure 48 shows this resonance structure:
Figure 48.
The melting point of carboxylic acids is not easily memorized as it does not vary with molecular size. The boiling point, however, will increase with molecular weight. When it comes to the melting point of fatty acids, the melting point of saturated fatty acids will be generally greater than that of unsaturated fatty acids so that fatty acids will be solid at room temperature and unsaturated fatty acids will be liquid. This is because of the way the solids are packed in unsaturated fatty acids versus saturated fatty acids. Carboxylic acids are weak acids but are much stronger than their comparable alcohols. The acidity is increased when there are electronegative side chains near the carboxyl group, such as chlorine or bromine, which make more acidic compounds. This becomes acidic because of the resonance of the carboxyl group, which leads to a negative charge on the hydroxyl oxygen molecule at the end of the carboxylic acid.
88
KEY TAKEAWAYS •
Ketones, aldehydes, and carboxylic acids all have a carbonyl group, which is a CO double bonded functional group.
•
Of the different functional groups, the carboxylic acid side group takes precedence.
•
The carbonyl group is polar because of the electronegativity of the oxygen atom.
•
There are several common names for the aldehydes and the carboxylic acids, which should be memorized.
•
Many common molecules are aldehydes and carboxylic acids, including many fatty acids in animal cells.
89
QUIZ 1. What is not a characteristic feature of a ketone? a. Carbonyl group b. Resonance between carbon and hydrogen c. Acidity d. Double bonding Answer: c. These are all features of ketones, with the exception of acidity. Ketones are generally not acidic unless they have a particular functional group on them. 2. What is not a difference between the carbonyl double bond in a ketone and the carbon-carbon double bond in an alkene? a. The ketone involves a pi bond, while the alkene double bond does not. b. The ketone double bond is polar, while the alkene double bond is not. c. There is greater energy in the double bond of the ketone compared to the C-C double bond. d. The reaction with water proceeds more rapidly in a ketone than in an alkene. Answer: a. Both of these bonds involve both a sigma bond and a pi bond, making for a relatively stable double bond. 3. What is the common name for the aldehyde called ethanal? a. Butyraldehyde b. Formaldehyde c. Metaldehyde d. Acetaldehyde Answer: d. Acetaldehyde is a two-carbon aldehyde that contains a methyl group and the carbonyl group. It can be made from acetic acid.
90
4. How many carbon atoms exist in the butyraldehyde molecule? a. Three b. Four c. Five d. Six Answer: b. Butyraldehyde is also referred to as butanal, which is a fourcarbon chain aldehyde. 5. Which of these descriptive terms is not the same as the others? a. Acetone b. Dimethyl ketone c. Propanone d. Methyl phenyl ketone Answer: d. Each of these is the same molecule, representing CH3COCH3, except for methyl phenyl ketone, which is another molecule that needs to be memorized. 6. What is not true of a combination ketone and aldehyde molecule? a. The ketone takes precedence over the aldehyde b. The molecule has the term “oxo” in it. c. The number one carbon atom is the aldehyde carbonyl carbon. d. The substance is referred to as an aldehyde. Answer: a. In the molecular name, the ketone does not take precedence over the aldehyde but the aldehyde instead takes precedence and the term has “oxo” in it to identify the second carbonyl group. 7. The molecule 5-hydroxy-3-pentenal has all but what type of features? a. Ketone carbonyl group b. Alcohol side chain c. Alkene double bond d. Aldehyde carbonyl group
91
Answer: a. This is clearly an aldehyde by the “al” ending and has an “enal” ending because of the alkene double bond. The alcohol does not take precedence but is present at the 5th carbon atom. 8. In the carbonyl functional group, the oxygen is considered electronegative because it has how many unbonded electrons associated with it? a. One b. Two c. Three d. Four Answer: d. There are two pairs or “four” unbonded electrons associated with the oxygen molecule in the carbonyl functional group, which leads to the polarity of the carbon-oxygen bond. 9. A carboxylic acid is known to contain what functional group? a. CHO b. CH2O c. COOH d. CH3CHO Answer: c. The COOH side chain is characteristic of a carboxylic acid. This tends to be more reactive than the aldehyde and ketone carbonyl group. 10. There are a great many carboxylic acids to memorize. Which is the smallest carboxylic acid? a. Formic acid b. Acetic acid c. Propionic acid d. Capric acid Answer: a. Formic acid is very small, consisting of HCOOH, making it the smallest possible carboxylic acid.
92
CHAPTER 6: AROMATIC COMPOUNDS This chapter introduces the structure and chemistry of aromatic compounds. All aromatic compounds consist of a cyclic compound that carries resonance. The most common aromatic compound is benzene, which is very stable and has chemistry unique to the molecule. In this chapter, the nomenclature and chemistry of aromatic compounds will be covered as well as the different reactions that are seen in organic chemistry with these types of molecules.
INTRODUCTION Most of the discussions of the chemistry around aromatic compounds begin with the basic benzene ring. Benzene is a six-carbon ring that has three double bonds. It is a C6H12 ring structure involving conjugated double bonds. Figure 49 shows what this structure can look like when written:
Figure 49.
It should be remembered that cyclohexane is not the same thing as benzene. Cyclohexane has no double bonds in its structure. Cyclohexane is a C6H12 structure. Benzene, as you’ll see, acts differently from a regular alkene structure because of its unique ringed characteristics.
93
Benzene’s structure fits as a stable structure when one thinks of a bond as being pairs of shared electrons. With benzene, there is one sigma bond making up a covalent bond as well as half of a pi bond between each of the two carbon atoms. Each bond has the same number of electrons and each is the same bond length. This resonance explains why benzene does not typically undergo addition reactions easily. It is basically because there are no simple pi bonds to connect to. The other aspect of resonance is that the structure tends to be more stable than the molecule without resonance. This lowering of energy, which is about a third as much as in a typical covalent bond in the case of benzene, is important in the types of reactions associated with this molecule. It means that, when benzene does react, it usually means that the benzene ring persists. According to Huckel rule, there is only automaticity when the number of pi electrons equals 4n + 2, where n = any integer at or above zero. Benzene has six pi electrons, in which n = 1. Remember that the number of pi electrons is two for every double bond. This is why cyclooctatetraene is not a stable resonance structure because there isn’t an integer that can be made into 8 using the 2n + 2 rule. The same is true of cyclobutadiene. In addition, there cannot be resonance if there is an interruption of the resonance feature. The p orbitals that make up an unbroken ring of p orbitals can be associated with atoms that are not carbon. Furan, which contains five carbon atoms and an oxygen atom in a ring, and pyrrole, which is a five-carbon ring and an NH in the ring, are both stable molecules. This resonance can also be created be for a negatively charged carbon atom.
NOMENCLATURE OF AROMATICS For all practical purposes, the term “aromatic” in organic chemistry refers to the benzene ring structure. There are other aromatic hydrocarbons, some of which involve more than one ring, which will be discussed later in this chapter. Benzene is a planar molecule consisting of hexagonal rings of sp2-hybridized carbon atoms along with the unhybridized p orbitals, which stick up perpendicular to the ring. The sigma bonds contain three electrons (two with the neighboring carbon atoms and 1 94
with the hydrogen atom linked to it). The fourth valence electron is associated with the unhybridized p orbital making up the pi bonds. Benzene has many derivatives. The hydrogen atom can be replaced by many different side chains. What you will see is that benzene and other aromatic compounds more readily participate in substitution reactions than in addition reactions. There are many possible aromatic compounds that can be made with benzene as the parent compound, some of which must be memorized. Figure 50 shows several of the most common benzene derivatives:
Figure 50.
Benzene has a zero-dipole moment when it has no side chains. The presence of a side chain can result in a dipole moment that will increase the intermolecular forces. This results in a greater melting point and boiling point for these molecules. An example is 1,4-dichlorobenzene, which has a melting point of 52.7 degrees Celsius, compared to a melting point for benzene, which is only 5.5 degrees Celsius. Benzene is 150 kilocalories per mole more stable than would be expected if there were just three separate double bonds. This is because of the resonance factor of benzene, which is an arene molecule and not a true “alkene”. They are not good nucleophiles and instead have multiple types of electrophilic substitution reactions. This involves a process starting with the addition of an electrophile to the pi system of the benzene molecule, called “a carbo-cation”. What this looks like is depicted in figure 51:
95
Figure 51.
It would be difficult to describe all of the potent substances that can be made with a benzene ring. Substances as diverse as aspirin, amphetamine, ibuprofen, and adrenaline are ultimately benzene molecules with extensive side chains. These represent the common names of benzene derivatives, although there are scientific names associated with each of them. Benzene is a ring that can have any number of substitutions. It does not have to be numbered if there is just one side chain associated with it. If there is more than one side chain of the same type, they are numbered and named as di, tri, etcetera, with the term “benzene” added as a separate word. Chlorobenzene or bromobenzene, for example, are simple substitutions that will not have to be numbered. NO2 added to the side chain gives the molecule the name of nitrobenzene. The side chains start with the number 1 carbon atom and proceed so that the lower numbers are followed preferentially. In addition, they are listed alphabetically so that “chloro” precedes “ethyl” and not the other way around. There are side chains that take precedence by virtue of the type of side chain they are. The terms ortho-, meta-, and para-, which have already been described, can be used to describe the side chains on these molecules. Incidentally, the ortho, meta, and para configuration does not have to apply to the same side chain. It is possible to have, for example, o-nitrochlorobenzene and m-nitrochlorobenzene. Benzene isn’t the only name and aromatic compound that you need to memorize. An example is phenol, which is the benzene molecule with a hydroxyl group or OH group attached. An example would be o-chlorophenol, which is a chlorine side chain and a hydroxyl side chain attached in a 1, 2-carbon fashion. It can also be called 2-
96
chlorophenol, which gives the hydroxyl group the number one position. Figure 52 indicates some other common molecules based on benzene that you need to memorize:
Figure 52.
You should know for the purposes of nomenclature that, besides phenol, benzaldehyde and benzoic acid are retained in the IUPAC nomenclature. Others that can be used in the nomenclature system include styrene, toluene, phenanthrene, and naphthalene. The use of others can be recognized in the IUPAC nomenclature but is generally discouraged. One such term is TNT, which is trinitrotoluene—an explosive. The true IUPAC name is 2-methyl-1,3,5-trinitrobenzene. The phenyl group is what benzene is called when it is a side chain. It is named “phenyl chloride” for example rather than chlorobenzene, etcetera. This is the preferred usage of the term when the phenyl group is attached to at least six alkane carbons, as in the molecule 3-phenyl hexane. There is also the “benzyl group”, which is essentially toluene with a side chain on its methyl group, or the Phenyl-CH2-R molecule, also referred to by the initials “Bn-R”.
97
This can lead to molecules like benzyl chloride or benzyl alcohol. In such cases the CH3 side chain is part of the molecule and an R side chain is attached to it. Figure 53 shows benzyl chloride and benzyl alcohol:
Figure 53.
This can lead to a number of side chains that are also attached to the benzyl ring. This can lead to 2,4-fluorobenzyl chloride. The numbering system leads to the lowest number assigned to side chains as possible on the benzene ring.
BENZENE CHEMISTRY Benzene is considered relatively unreactive because of the stability of its aromaticity, making it inert to compounds like bromine gas and hydrochloric acid. As you remember, this aromaticity comes from delocalization of the p-orbital carbons on the sp2 hybridized carbons. This creates a “doughnut”-shaped singular orbital above and below the carbon atoms that can have electrons anywhere in it. This planar molecule has a bond angle between carbon atoms of about 120 degrees. The bond length is 1.39 Angstroms, which is between that of a single and a double bond. If there is a great deal of temperature or a catalyst, substitution reactions can occur rather than addition reactions that are seen in alkenes. This is directly related to the fact that this is a stable resonance molecule. The addition of hydrogen to make cyclohexane leads to a release of heat amounting to 28.6 kilocalories per mole. This is about 35 kilocalories per mole more stable than expected.
98
As an aromatic structure, it must meet four criteria to be considered aromatic: •
It must be cyclic
•
It must be planar
•
It must have fully conjugated double bonds
•
It must have 2n + 2 pi electrons
According to the 2n + 2 rule (Huckel’s rule), there are six pi-electrons in the pi bond, leading to six delocalized electrons that “travel” throughout the perpendicular pi bonds of the benzene ring structure. This is because each double bond (with a pi bond) always contributes 2 pi electrons for a total of six electrons in benzene. Huckel’s rule also applies to ions as long as there are 4n + 2 pi electrons, it doesn’t matter if there is a charge on the ion. This means that cyclopentadienyl anion is aromatic. This is a pentene ring with two double bonds and a carbon atom that is negatively charged without a hydrogen ion. It has an unbound pair of electrons that, along with the four donated electrons in the pi bonds of the other carbon atoms, has 6 pi electrons, which satisfies the 4n + 2 rule. Figure 54 shows the cyclopentadienyl anion structure:
Figure 54.
99
As we have discussed, there are many aromatic cyclic compounds that do not just contain carbon atoms. There is, as you remember, furan, which contains an oxygen atom. It is aromatic because one of the lone pairs of electrons is sp2 hybridized. The other pair is in the p orbital. Because it has a pair in the sp2 hybridized orbital, it is considered resonance. It has two electrons in the sp2 hybridized orbital and four carbon atoms, leading to 6 total electrons contributing to resonance structure that looks very similar to figure 54 but with an oxygen atom instead of a carbon atom at the top. This also means that it is a planar molecule with a trigonal planar shape of the oxygen atomic orbitals. There are aromatic heterocycles, which are unsaturated cyclic compounds that are considered aromatic. There are several compounds that do not have the typical benzene compound associated with them. These include the molecules listed in figure 55:
Figure 55.
In imidazole, for example, there are six pi-electrons: 3 from the carbon atom and three from the nitrogen atoms, making a total of six, which fits the 2n + 2 pi-electron rule. With this molecule, one nitrogen has an H electron added to it with a lone pair adding to the sexted, while one nitrogen is free of a hydrogen so only one electron contributes to the aromaticity. As a general rule, if a compound has the possibility of being aromatic, it will be because of the stability and energy-saving features of aromatic compounds. Benzene rings will join together and fuse to give larger polycyclic aromatic compounds that are stable. There is some localization of the pi-electrons, leading to an unevenness of the carbon-carbon bond. There are six benzene rings in the substance called coronene, which is a planar molecule. Figure 56 shows some of these polycyclic aromatic compounds:
100
Figure 56.
AROMATIC REACTIONS The important thing to remember about aromatic reactions is that the six pi electrons really want to remain in the stable aromatic structure. It means that it is not practical to assume that there will be a simple addition reaction in which something like chlorine is added to the structure without substituting the chlorine atom with a hydrogen atom. This loses the aromatic nature of the structure. Figure 57 shows a reaction that just does not “prefer” to happen, while it also shows a preferred substitution reaction:
Figure 57.
101
Common reactions include chlorination and bromination, which are the most common halogenation reactions involving electrophilic substitution. Others that will react this way include those things that are electrophilic as well. Some of these are included as follows: •
Halogenation—the addition of heat and chlorine or bromine gas (plus a catalyst) to yield bromobenzene or chlorobenzene
•
Nitration—this involves the addition of heat and HNO3 to yield nitrobenzene (C6H5NO2) plus water
•
Sulfonation—this involves heat plus SO3 and H2SO4, leading to benzenesulfonic acid (C5H5SO3H).
•
Alkylation (the Friedel-Crafts reaction)—this takes heat plus an R-Cl molecule to lead to an arene or alkyl side chain added to a benzene molecule plus HCl.
•
Acylation (the Friedel-Crafts reaction)—this takes heat plus RCO-Cl to lead to an aryl ketone, which is a benzene molecule plus a C-OR side chain.
How do these types of electrophilic substitution reactions occur? It is likely a two-step process in which the electrophile forms a sigma bond with the benzene ring first. Secondly, a proton is removed from the benzene molecule, leading to a substitution of an electrophile with hydrogen. There is a benzenonium intermediate that has both the hydrogen atom and the electrophile attached for a brief period of time. This will have a temporary higher energy state that will become lower when the hydrogen ion is lost.
HALOGENATION OF BENZENE While halogenation works for bromine and chlorine gas, it does not work as well for iodine and fluorine. Iodine is unreactive with benzene unless it is mixed with copper (II) chloride, making iodine more electrophilic. Fluorine is too reactive, on the other hand, so it cannot be used to fluorinate benzene directly. There are chemicals containing fluorine that work better than fluorine alone.
102
In the halogenation of benzene, it will not work if just the halogen gas or liquid is mixed with the benzene. They need to be acted on by Lewis acid catalysts in order to activate the halogen. These can be iron (III) halides or aluminum (III) halides. Aluminum bromide, for example is a Lewis acid that can mix with bromine gas to make a Br+ ion. This is much more electrophilic than bromine gas alone. The catalyst will polarize the bromine-bromine bond so that there can be a positive bromine end and a negative bromine end. It is the Br+ ion that will ultimately react with benzene. This will leave behind AlBr4- as an end product ion. This ion allows for the hydrogen ion to be “pulled off” the benzene compound, effectively substituting bromine and hydrogen. Figure 58 shows this reaction:
Figure 58.
As you can see, Aluminum bromide isn’t consumed in this reaction but is regenerated as a catalyst that will be useful for other halogenation processes in the reaction. The reaction involving bromine is exothermic but not as exothermic as fluorine (which is explosive in nature). The electrophilicity decreases as one goes down the group of halogens so that this will be endothermic when iodine is the halogen involved.
NITRATION OF BENZENE The reaction yielding nitrobenzene (nitration of benzene) doesn’t happen without the help of sulfuric acid. It takes the sulfuric acid to make the NO2+ ion from nitric acid in order to make it electrophilic enough to bind with benzene. Interestingly, when nitrobenzene is mixed again with iron and reduced, it makes benzene with an amine group attached.
103
As with halogenation, there needs to be something that makes nitric acid more electrophilic—in other words, to create the nitronium ion. HNO3 is a nitrogen atom with three oxygens around it, one of which is attached to a hydrogen ion. When mixed with sulfuric acid, the NO2 made by pulling the OH group off of the nitric acid as is seen in figure 59:
Figure 59.
This electrophile (NO2) is positively charged and is apt to bind to benzene. The sulfuric acid ion is strong enough now to pull off the extra hydrogen ion on benzene, leaving behind sulfuric acid (completely reconstituted) and nitrobenzene.
SULFONATION OF BENZENE The end result of the sulfonation of benzene is benzenesulfonic acid. In such cases, a catalyst is necessary (as is the case with all electrophilic substitution reactions). Sulfur trioxide (SO3) is used up in the equation, while hot sulfuric acid (H2SO4) is necessary to make the reaction occur. In the reaction, sulfur trioxide is mixed with sulfuric acid to make a mixture called “oleum”. The sulfur in SO3 is considered electrophilic because it is partially charged by the electronegative oxygen atoms. When it combines with benzene, the SO3- ion on the benzene molecule will take the hydrogen off the benzene molecule. What this looks like is seen in figure 60:
104
Figure 60.
This is reversible so that, by heating benzenesulfonic acid, the sulfuric component can be drawn off the molecule in the presence of dilute sulfuric acid, and benzene is produced once again.
FRIEDEL-CRAFTS REACTION This is a type of alkylation reaction that involves the replacement of a hydrogen ion with an alkyl group. There are four limitations you need to know about with regard to this type of reaction: •
It cannot be done with vinyl and aryl halides
•
There cannot be a strong deactivating group on the benzene ring (such as NH2, NH with a side chain, or Nitrogen plus two side chains) because they deactivate the catalyst necessary for the reaction to take place.
•
More than one alkylation can take place with this type of reaction.
•
Carbo-cation rearrangements can occur in any reaction that involves a carbocation. This leads to isomers of the alkyl side chain attaching to the benzene molecule.
In this reaction, benzene is mixed with an alkyl chloride plus Aluminum (III) chloride (AlCl3) to make benzene plus an alkyl group attached to it. The catalyst aluminum chloride acts very similar to the halogenation process. The aluminum chloride makes a carbo-cation, which is an intermediary step allowing for the carbon atom on the alkyl side chain to be electrophilic. It looks like this as seen in figure 61:
105
Figure 61.
Alkylation ability increases as one goes up the halogen group on the periodic table. In the Friedel-Crafts alkylation process, catalysts like BF3, SbCl5, AlCl3, and AlBr3 are commonly used as these are Lewis acids that make the alkyl group more electrophilic. As you can see, there can be rearrangements of the alkyl group when adding a carbon chain greater than two carbons in length. This can lead to the alkyl group being an isomer of unbranched alkyl chain. In addition, a deactivating nitric oxide group or other nitrogen group cannot be also attached to the benzene ring. This is because the lone pair of electrons on the nitrogen will react with the catalyst (such as aluminum chloride), forming a molecule that will not allow the alkylation reaction to occur. Finally, when one alkyl side chain is attached to the benzene ring, this activates the benzene ring, making it increasingly likely to add still more alkyl groups (called polyalkylation). This doesn’t happen in a Friedel-Crafts reaction involving an acyl group (RCO side chain). The addition of the acyl side chain deactivates the ring, preventing polyacylation. Figure 62 shows the Friedel-Crafts reaction as it applies to acylation.
106
Figure 62.
So far, we have talked about the activation and deactivation of the benzene ring. Things that activate the ring make it more likely to add more side chains, while the deactivation of the ring makes a ring that is less likely to add more side chains. One can determine whether something is activating or deactivating by looking at the dipole moment created between the benzene ring and the side chain. Electron-donating side chains have a dipole moment that points toward the benzene ring. These will activate the ring because it will make it more susceptible to electrophilic attack. Activating side chains include CH3, OCH3, OH, and NH2—all of which have a dipole moment that makes the benzene ring more electrically negative. Electron-receiving side chains have a dipole moment that points toward the side chain, making the benzene ring less likely to be susceptible to electrophilic attack. These include NO2, CN, CO2CH3, and Cl. These will effectively “deactivate” the substituted benzene ring.
107
KEY TAKEAWAYS •
The most commonly-recognized aromatic is the benzene ring.
•
The key to aromaticity is resonance between the carbon atoms in a ring structure.
•
It takes 2n + 2 electrons in the p-orbitals to create a resonant structure.
•
The major reaction that takes place with aromatics is the electrophilic substitution involving many different substances attaching to the benzene ring.
•
There are activating and deactivating side chains that will increase or decrease the chances of further substitution reactions taking place with the benzene ring.
108
QUIZ 1. How many carbon atoms and how many double bonds are there in the benzene ring? a. Five carbon atoms and two double bonds b. Six carbon atoms and two double bonds c. Six carbon atoms and three double bonds d. Eight carbon atoms and three double bonds Answer: c. Benzene is a simple six-carbon ring associated with three double bonds that resonate with one another in what is a relatively stable molecule. 2. Which carbon atoms in the benzene structure have resonance? a. The first and second carbon atoms b. The first and fourth carbon atoms c. None of the carbon atoms d. All of the carbon atoms Answer: d. Resonance exists between all of the bonds of the benzene molecule so that the entire molecule’s carbon atoms have resonance. 3. A benzene molecule is considered an ortho molecule. Where are the side chains located? a. On the 1 and 2 carbon atoms b. On the 1 and 3 carbon atoms c. On the 1 and 4 carbon atoms d. On the 1 and 5 carbon atoms Answer: a. There is an ortho, meta, and para system that dictates where the side chains are located. The 1, 2-carbon side chains on the benzene molecule indicate an ortho configuration.
109
4. Benzene with a methyl group attached to it is called what? a. Xylene b. Toluene c. Phenol d. Benzonitrile Answer: b. Toluene is a benzene molecule that has a methyl group attached to it. This is one of the benzene derivatives that needs to be memorized. 5. Which molecule is least likely to comply with IUPAC nomenclature as a common name that also becomes an IUPAC name? a. Phenol b. Benzoic acid c. Benzaldehyde d. Trinitrotoluene Answer: d. The term trinitrotoluene is not preferred in the IUPAC nomenclature as it needs to be written out, rather than referring to it as TNT. The others have been adopted by the IUPAC system as names that can be used to describe benzene derivatives. 6. Benzene is a stable molecule because it has aromaticity; it is what shape and has what bond angles between carbon atoms? a. Planar with 120-degree angles b. Planar with 109.5-degree angles c. Tetrahedral with 109.5-degree angles d. Tetrahedral with 120-degree angles Answer: a. The molecule is planar with a 120-degree angle between carbon atoms, making benzene a very stable molecule.
110
7. What type of reaction is most preferred when an aromatic compound undergoes a reaction? a. Addition b. Subtraction c. Nucleophilic addition d. Electrophilic Substitution Answer: d. The preferred reaction is an electrophilic substitution reaction, which does not involve the loss of aromaticity. Any reaction that would threaten the aromaticity of the compound does not easily occur. 8. In making nitrobenzene, you need an electrophilic substance to add to the benzene molecule. What electrophilic substance is added to benzene in the nitration process? e. HNO2 f. NH3 g. HNO3 h. NH4+ Answer: c. Nitric acid will be electrophilic, adding an NO2 molecule to benzene with the help of sulfuric acid, leading to the benzene molecule with an NO2 side chain or nitrobenzene. 9. The Friedel-Crafts reaction involves the addition of what side chain to benzene? a. Halide b. Nitric oxide c. Sulfonic acid d. Alkyl side chain Answer: d. The Friedel-Crafts reaction involves the addition of an alkyl side chain to a benzene molecule.
111
10. What is an appropriate catalyst in the Friedel-Crafts reaction? a. Sulfuric acid b. Nitric acid c. Aluminum chloride d. Ammonium acetate Answer: c. Aluminum chloride acts to cause a positive partial charge on an alkyl chloride molecule, resulting in the alkyl group being attached to the benzene molecule via electrophilic substitution. An end product is HCl.
112
CHAPTER 7: ALCOHOLS AND ALKYL HALIDES The topic of this chapter is the chemistry of alcohols and alkyl halides. Alcohols are organic compounds that have a hydroxyl group as its major functional group, often represented with the general formula of ROH, where R can be any number of organic chemistry alkyl groups. The hydroxyl group is highly reactive so that there are any number of reactions that can occur at this functional group. The chapter also covers the chemistry of alkyl halides, which are alkyl groups that have one or more halogen side chain attached to it. The halogen is also highly reactive, with many possible chemical reactions associated with it.
NOMENCLATURE OF ALCOHOLS Alcohols are extremely uncommon in nature, seen in alcohol beverages in the form of ethanol (CH3CH2OH) and methanol (wood alcohol), which is CH3OH. There are common names for the many small alcohols, as shown in figure 63:
Figure 63.
113
The typical IUPAC nomenclature for alcohols is to take the alkane compound and add ol to the end of it. According to the rules, the longest chain containing the OH group is chosen as the parent chain with the carbon having the OH group being number one (or the lowest number). In cyclic compounds, the OH carbon is still number one but it is not listed unless it is necessary to clear up the name. If more than one alcohol group is present, the substance is listed as a diol or triol. In some cases, there can an alcohol side chain that is not at the end of the molecule, such as 2-methyl-2-butanol. You should use the rules unless there is a commonly recognized common name. There are three types of alcohols, which depend on the number of carbon atoms attached to the specific carbon atom that is attached to the OH group. These include the following: •
Primary alcohol—in this case, the carbon atom is attached to one other carbon atom with a formula being RCH2OH.
•
Secondary alcohol—this is one in which the carbon atom in the alcohol has two other side chains besides hydrogen, written as R2CHOH.
•
Tertiary alcohol—this is an alcohol in which the carbon atom has three side chains and no hydrogen atoms attached to it. This is written as R3COH.
You need to know that there are common names using sec- for secondary and tert- for tertiary that are not a part of the IUPAC nomenclature. Taking butanol or butyl alcohol as an example, you can get several different types, listed in figure 64:
114
Figure 64.
Some names you need to memorize include the following common and IUPAC names: •
Methyl alcohol or methanol (one carbon primary alcohol)
•
Ethyl alcohol or ethanol (two carbon primary alcohol)
•
Propyl alcohol or propanol (three carbon primary alcohol)
•
Isopropyl alcohol or 2-propanol (which is a secondary alcohol with three carbon atoms)
•
Butyl alcohol or 1-butanol (which is a 4-carbon primary alcohol)
•
Cyclohexyl alcohol (which is a secondary alcohol that contains cyclohexane)
Any compound with a benzene ring and an alcohol group is called a phenol. These are unique among alcohols in that they are slightly acidic. They will combine with a base to
115
make a phenol salt. An archaic name for this phenol compound is carbolic acid (because of its acidic character). It has a high melting point and is solid at room temperature. Phenols can cause burns when applied to the skin but are strong antiseptics in that they can kill microorganisms on living tissue and are strong disinfectants (killing microorganisms on inanimate objects). Because it has side effects in humans, it has been replaced with safer antiseptics and disinfectants. An example is 4-hexylresorcinol (4-hexyl-1,3-dihydroxybenzene). This is a safer phenol-based disinfectant used in throat lozenges and some mouthwashes. Phenols can be described with the ortho-, meta-, and para- designation. Alcohols can also be described as being derivatives of water, written as ROH, in which the ROH molecule is just as bent as the water molecule. Because these are polar substances, they are often soluble in water, particularly with smaller alcohols. Because of the OH group, it will participate in hydrogen bonding. In looking at the physical properties of substances, you need to look at the different types of intermolecular forces between the molecules, which determine things like boiling point and melting point. Some typical boiling points include the following: •
Methane—boiling point -164 degrees Celsius
•
Methanol—boiling point 65 degrees Celsius
•
Water—boiling point 100 degrees Celsius
•
Ethane—boiling point -89 degrees Celsius
•
Ethanol—boiling point 78 degrees Celsius
•
Propane—boiling point -42 degrees Celsius
•
Propanol—boiling point 97 degrees Celsius
Alkanes are nonpolar and have only weak dispersion forces. Small alkanes (those with less than 5 carbon atoms) will be gaseous at room temperature. Contrast that to methanol, which has one carbon atom and is liquid at room temperature. This is because of the hydrogen bonding between the hydroxyl group and hydrogen atoms in
116
the molecule. The boiling point will also increase somewhat with increased molecular size. Alcohols can also have hydrogen bonding with water, making alcohols with one to three carbon atoms miscible with water. As the length of the carbon atom chain increases, the solubility decreases so that alcohols like 1-decanol will be completely insoluble with water.
REACTIVITY OF ALCOHOLS Methanol synthesis can occur by mixing hydrogen gas (H2) and carbon monoxide (CO). This will create CH3OH using zinc oxide and chromium oxide as catalysts. Methanol is used in automotive fuel (in racing cars and gasoline additives). Others are made by the hydration of alkenes. This is how ethanol is made; ethylene is made through hydration with sulfuric acid (H2SO4) as a catalyst. Similarly, propylene or propene can be hydrated to make isopropyl alcohol through hydration. As you may know, there is another way to make alcohol or ethanol. It is made through the fermentation of starches or sugars from different sources, including rice, wheat, corn, and potatoes. There are enzymes that break down sugar starches into glucose that is further broken down enzymatically into two molecules of ethanol (CH3CH2OH) plus 2 CO2 molecules. Methanol, unlike ethanol, is extremely toxic to humans with a fatal dose of between 100 and 150 milliliters. This is toxic because of the presence of liver enzymes that oxidize methanol into formaldehyde, which is more toxic than the methanol itself. Formaldehyde coagulates proteins, with lethal consequences. You should know that ethanol is also oxidized by liver enzymes to make acetaldehyde. This gets further oxidized to acetic acid and finally to CO2 and water. Chemical reactions with alcohols generally occur with respect to the OH functional group. There are three major types of reactions that occur with alcohols; these are oxidation, dehydration, and esterification.
117
Oxidation only happens in the presence of K2CrO7 and an acid solution and only happens in primary and secondary alcohols. Oxidation is not possible with tertiary alcohols. While oxidation of a primary alcohol forms an aldehyde (and subsequently a carboxylic acid), the oxidation of a secondary alcohol forms a ketone. It is not possible to have a tertiary alcohol become oxidized because it has three R side chains that do not have the ability to lose a hydrogen ion to form a further oxidized molecule. Dehydration of an alcohol in the presence of acids and bases will form either an ether or an alkene. It takes heat and high concentrations of H2SO4 (sulfuric acid) to make an alkene. In the presence of high concentrations of heated ROH molecules and heat (plus concentrated sulfuric acid not in excess), there will be the predominance of ether formation. Esterification happens in a carboxylic acid solution, leading to an ester.
ALCOHOL DEHYDRATION Dehydration of alcohols will lead to alkenes by losing water. This can be accomplished by heating an alcohol in the presence of a strong acid (like phosphoric or sulfuric acid). It takes a great deal of heat to generate these alkenes. The amount of heat necessary to force the reaction forward depends on whether it is a primary, secondary, or tertiary alcohol. Heating in the range of 170 to 180 degrees is necessary to generate an alkene from a primary alcohol. Lesser heat (in the range of 100-140 degrees) is necessary to make an alkene from a secondary alcohol and even less heat (25-80 degrees) is necessary to make an alkene from a tertiary alcohol. Alcohols are considered to be amphoteric molecules in that they can act as an acid or a base. The lone pair of electrons available on the oxygen atom will make the hydroxyl group basic, particularly in the presence of a strong acid, which gives rise to an OH2 side chain to make ROH2+, which is extremely acidic. The reason that alkene formation happens at all is because of this basic characteristic of the alcohol molecule. ROH plus a strong acid, gives rise to the alkyloxonium ion (which is ROH2+). ROH plus a strong base gives rise to the alkoxide ion (which is RO-). There are two mechanisms that can happen with the dehydration of alcohols, called the E1 mechanism and the E2 mechanism. These will be discussed when we talk about
118
elimination reactions. The ability to dehydrate is easiest with methanol, followed by primary alcohols, secondary alcohols, and tertiary alcohols. With primary alcohols, sulfuric acid donates a hydrogen ion to make the alkyloxonium ion ROH2+. The HSO4- ion that donated the hydrogen ion in the first place will remove it from the adjacent carbon atom, resulting in the formation of water and a double bond, resulting in the alkene. Figure 65 describes how this happens:
Figure 65.
Secondary and tertiary alcohols will dehydrate similarly but undergo the E1 mechanism of dehydration. This involves protonation of the OH side group to make the alkyloxonium ion. In such cases, there can be more than one product of reaction with trans alkenes preferred over cis alkenes. In addition, the preference is for more substituted alkenes, which also dictates the location of the double bond. This means that the trans substituted alkene is more likely to occur over all other possible alkenes in these types of reactions. Under the right conditions, of excess ethanol, heat and concentrated sulfuric acid, two ethyl alcohol molecules can be dehydrated to make diethyl ether, which is CH3CH2OCH2CH3. Both dehydration and hydration reactions are commonly associated with cellular metabolism in animals, using enzymes to accomplish this.
119
There is the well-known Embden-Meyerhof pathway, in which an alcohol is enzymatically dehydrated to make an alkene.
OXIDATION OF ALCOHOLS This, as we’ve discussed, happens in the liver using enzymes that turn methanol and ethanol into aldehydes. What we have also alluded to is the idea that aldehydes aren’t the most oxidized molecule possible and that the aldehyde can be further oxidized to make a carboxylic acid. This only happens with primary alcohols because secondary alcohols form ketones, which cannot oxidize further. The oxidation of isopropyl alcohol will give rise to the simplest ketone (which is acetone). This is a reaction that involves potassium dichromate (K2Cr2O7). In this reaction, CH3CHOHCH3 will lose two hydrogen atoms to make a double bond between the middle carbon and oxygen atoms to make acetone (CH3)CO(CH3). Ketones do not oxidize any further so the ketone is the end product of these types of reactions. The reaction requires chromate because it is needed to provide oxygen in order to make water as an end product when the alcohol is oxidized.
REACTIVITY OF ALKYL HALIDES Alkyl halides or haloalkanes involve the presence of a halogen on an alkyl group. The possibilities of halogens in these types of reactions include fluorine, bromine, chlorine, and iodine. The presence of these atoms in place of the hydrogen ion will affect the bond length, bond strength, electronegativity, and molecular size of the molecule. The most significant thing that happens is that the bond becomes more polar because the halogen is electronegative with respect to carbon, leading to a bond with a dipole moment that points toward the halogen. If you’ll remember from general chemistry, electronegativity increases as one moves up the halogen group on the periodic table so that, in order of least to most electronegativity, the list goes from Iodine, to Bromine, to Chlorine, and finally, to Fluorine.
120
There is a difference in bond length, bond strength, and molecular size. The molecular size is lowest with fluorine and highest with iodine. Bond strength is strongest with fluorine and weakest with iodine, while bond length is shortest with fluorine and longest with iodine. Because of the increased surface of the alkane that is halogenated, there will be an increase in London dispersion forces. These forces will naturally increase with increased molecular surface area. With higher London dispersion forces, there will be higher boiling points. What this means is that an iodinated alkane will have the greater change in boiling point versus a fluorinated alkane. The dipole moment will affect the boiling point as well. A higher dipole moment means a greater polarity to the bond and a higher boiling point for the alkane of the same carbon size. The end result is that the boiling point effect will be greater with a greater dipole moment in the bond because of dipole-dipole interactions between the molecules. Collectively, this means that the higher boiling point will be higher with iodinated compounds versus those that are brominated, chlorinated, or fluorinated. In the alkyl halide, the bond between the halogen and the carbon atom is reactive with the halogen being nucleophilic (electronegative) and the carbon atom being electrophilic (as it will carry a partial positive charge). The strongest bond strength will be that between carbon and fluorine. This strong bond strength is what makes the alkyl halide a stable molecule, particularly when it comes to the fluorinated alkane. Even so, alkyl halides will undergo elimination and substitution reactions. The carbon attached to the halogen is called the alpha carbon, while the carbon next to the halogenated carbon is called the beta carbon. The most common reaction that can happen is the replacement or substitution of the halogen on the alpha-carbon by a nucleophilic molecule. As a general rule, if the beta hydrogen on the alkyl halide has no hydrogen atoms on it (as in it is completely bound to other carbon atoms), an elimination reaction cannot occur. Fluorinated alkanes are so stable that they don’t break easily. Most reactivity happens with the iodinated alkane because it is the least stable of all of the alkyl halides.
121
GLYCOLS Glycols are alcohols that have two OH groups on them. The most important of these is ethylene glycol, which is 1,2-ethanediol, which goes by the simple formula of HOCH2CH2OH. Another common one is propylene glycol, which is 1,2-propanediol. In nature, the most common related compound is glycerol or glycerin, which is actually a trihydroxy alcohol with the IUPAC name of 1,2,3-propanetriol, having a hydroxyl group on all three carbon atoms. This is the molecule that fatty acids attach in order to make triglycerides. Ethylene glycol is sweet but it is very toxic. Similar to methanol and ethanol, there are liver enzymes that contribute to its toxicity. It forms an oxalate ion with no hydrogen atoms whatsoever, which binds with calcium to form a calcium oxalate precipitate. This results in kidney failure and death. Propylene glycol is nontoxic—used in certain drugs and as a “food moisturizer”. It gets oxidized by liver enzymes to form “pyruvate”, which is an intermediary in cellular metabolism.
122
KEY TAKEAWAYS •
There are primary, secondary, and tertiary alcohols, based on the number of hydrogen atoms on the alpha carbon.
•
Alcohols can undergo several different types of reactions, most commonly oxidation, dehydration, and esterification.
•
The type of molecule that comes out of the oxidation of an alcohol depends on whether it is primary, secondary, or tertiary.
•
Alkyl halides will have different properties, depending on whether it is halogenated with iodine, bromine, chlorine, or fluorine.
•
Glycols are alcohols that have more than one hydroxyl functional group associated with them.
123
QUIZ 1. You are naming a compound that has a number of side chains. Which side chain takes the lowest number? a. CHO b. OH c. CH3 d. Br Answer: a. The alcohol should be given the lowest possible number unless the side chain is an aldehyde or CHO, which takes priority over the rest of these and would be carbon number 1. 2. What is the basic formula for a tertiary alcohol? a. RCH(OH)2 b. R(OH)3 c. R3OH d. R2CHOH Answer: c. A tertiary alcohol has three R side chains attached to a carbon atom that has an OH also attached to it. 3. Which alcohol is acidic enough that it will form a salt when mixed with a strong base? a. Isopropyl alcohol b. Sec-butanol c. Phenol d. Methanol
124
4. Answer: c. Phenol is a mild acid that, when mixed with a strong base like sodium hydroxide, will form a sodium-phenol salt. a. Which alcohol is a good antiseptic for mouthwashes? b. Methanol c. 4-hexyl-resorcinol d. Phenol e. Sec-butyl alcohol Answer: b. 4-hexyl-resorcinol is a phenol-based di-alcohol with a 4hexyl group. The other alcohols are considered unsafe; however, 4hexyl-resorcinol is a good and safe antiseptic used in mouthwashes and throat lozenges. 5. In making an alcohol from scratch, what is the best substrate to use in the hydration process that makes an alcohol? a. Alkane b. Alkene c. Aldehyde d. Ketone Answer: b. Alkenes can be hydrated to make alcohols, using sulfuric acid as a catalyst for the hydration process. 6. When a primary alcohol is oxidized, what is the initial molecule made through the oxidation process? a. Aldehyde b. Glycol c. Ketone d. Ether Answer: a. The oxidation of an alcohol like CH3CH2OH first leads to an aldehyde like CH3CH2CHO. Further oxidation is possible to make a carboxylic acid.
125
7. What is the reactivity of alcohols to strong bases and strong acids? a. They will react with strong acids but not strong bases. b. They will react with strong bases but not strong acids. c. They will react poorly with both strong acids and bases. d. They will react with either strong acids or strong bases. Answer: d. Because alcohols are amphoteric molecules, they will react with strong acids and strong bases (acting as weak acids or weak bases themselves). 8. When a secondary alkene is made from the dehydration of alcohols, there are “preferences” for what happens in the reaction. Which type of alkene is most preferred? a. Substituted trans alkene b. Non-substituted cis alkene c. Substituted cis alkene d. Non-substituted trans alkene Answer: a. The preference because of stability is for a substituted trans alkene. These are more stable than substituted cis alkenes and nonsubstituted alkenes. 9. The halogenated alkane will have an increased boiling point when compared to the non-halogenated alkane. Which halogen has the greatest effect on the boiling point of the alkane when it halogenates it? a. Bromine b. Iodine c. Fluorine d. Chlorine Answer: b. There will be an increased molecular size (meaning increased London dispersion forces) and increased dipole moment on the bond with iodine when it attaches to an alkane, making the boiling
126
point of the iodinated alkane the greatest when compared to the other halogens. 10. Which halogenated alkane is considered the most thermodynamically and chemically stable? a. Brominated alkane b. Iodinated alkane c. Fluorinated alkane d. Chlorinated alkane Answer: c. The fluorine-carbon bond has the greatest strength, leading to fluorinated alkanes being the most stable molecule.
127
CHAPTER 8: ETHERS, EPOXIDES, AND ESTERS This chapter focuses on the organic chemistry associated with ethers, epoxides, and esters. Ethers and epoxides are related to one another in that certain types of cyclic ethers are referred to as epoxides. In both types of molecules, the general formula is ROR’, involving a variety of R side chains. These are molecules commonly seen in perfumes, industrial conditions, waxes, oils, and dyes. Esters are also commonly used in industry, being a part of the making of many products—the most common of which are the polyesters.
ETHERS Ethers can be aliphatic, aryl, or both. An aliphatic ether is one in which there is just a linear or cyclic alkane on either side of the oxygen molecule; an aryl group has a benzene ring on either side of the oxygen molecule. It can be an aliphatic molecule if the benzene ring is not directly attached to the oxygen molecule. There is no suffix that definitely indicates that a substance is an ether. Instead, there are “alkoxy” prefixes that are used to define an ether. These are relatively easy to remember as they are based on alkyl groups you already know. Some examples you should memorize include the following: •
Methoxy—this is a methyl group with oxygen attached.
•
Ethoxy—this is an ethyl group with oxygen attached.
•
Isopropoxy—this is an isopropyl group with oxygen attached.
•
Tert-butoxy—this is a tert-butyl group with oxygen attached.
•
Phenoxy—this is a phenyl group (a benzene molecule with an oxygen attached).
According to the nomenclature, the shorter alkyl group becomes the alkoxy side chain and the longer alkyl group becomes the base. Each of the alkyl carbons will be numbered separately, with the numbering priority given to the carbon closest to the
128
oxygen molecule. There are common names for ethers, with the most obvious being diethyl ether, which is colloquially known as “ether”. Others are named for each side chain plus “ether” at the end of the term (like butyl methyl ether). These involve using the alkanes in alphabetical order. Cyclic ethers or heterocycles have at least one carbon atom replaced by an oxygen atom. In naming these molecules, the prefix “oxa” is used to indicate that oxygen is replacing a carbon atom. They are numbered starting with the oxygen molecule as being “1” and rotating around the ring. An example is 1-oxacyclopentane, which is a 4-carbon, 1oxygen ring. The reference “1” in the name is not truly necessary because the oxygen molecule is automatically going to be number one. When it comes to priority, alcohol has priority over the ether as well as over any halide. The ether also has priority over the halide with the numbering starting with the end that has the side chain at the highest priority. The ring structure with two carbon atoms and an oxygen molecule is called 1-oxirane or oxirane.
PHYSICAL PROPERTIES OF ETHERS Ethers have oxygen atoms but no hydrogen atom associated with them. This leads to less intermolecular hydrogen bonding between molecules; it means that the boiling points tend to be low—nearly the same as that of alkanes. Boiling point increases with molecular mass but does not increase much because the oxygen alone does not cause an increase in intermolecular bonding. Because ether molecules have an oxygen atom, there will be hydrogen bonding with water molecules. This leads to an ether that has about the same solubility in water as the comparable alcohol. It means that ethanol and dimethyl ether have about the same solubility in water; however, diethyl ether has four carbon atoms associated with it and is nearly insoluble in water.
129
REACTIONS WITH ETHERS One way to make an ether is to prepare them from alcohols or their conjugate bases. The Williamson Ether Synthesis reaction involves the mixing of an alkoxide nucleophile with an alkyl halide. It becomes more difficult when an asymmetric ether is being made as some side chains are preferred to be made over others. In this reaction, a RO-Na+ salt is mixed with a brominated alkane, causing a combination of the two to make an ether. The acid-catalyzed dehydration of small primary alcohols is a special way of making symmetrical ethers. It the reaction, sulfuric acid (H2SO4) plus ethanol (CH3CH2OH) at 110-130 degrees Celsius will make diethyl ether. If you’ll remember this type of reaction at high temperatures (over 150 degrees Celsius), the reaction favors the making of the alkene instead. This type of reaction doesn’t work for mixed ethers (asymmetrical ethers) because it gives more than one product. Cleavage of ethers can occur with the addition of an acidic halide molecule, like HI, HBr, or HCl. This will result in an alcohol formation plus an alkane. This works well in situations of aliphatic ethers but does not work as well with aromatic (aryl) ethers. If a phenoxy-aliphatic ether is treated with these acidic molecules, phenol is always made because it is difficult to cleave the oxygen atom from the phenyl (benzene) side chain. What this looks like is seen in figure 66:
130
Figure 66.
Ethers that have a benzene group on either side will not be able to be cleaved by the addition of acids. Overall, however, the most common reaction of ethers is the cleavage of the CO bond by adding strong acids. The intermediary seen is the conjugate acid of the ether molecule. Typical reactions involve mixing a halide acid and getting a breakdown product that includes the alkyl halide plus water. Figure 67 shows this reaction type:
Figure 67.
131
EPOXIDES Epoxides are known as oxiranes, and are three-membered ring structures in which there are two carbon atoms and an oxygen in the middle. There can be side chains on any of the carbon molecules but, of course, there are no side chains on the oxygen molecule. The simplest and most common oxirane is ethylene oxide, which is made by the catalytic oxidation of ethylene with oxygen in air. Further chemical treatment of this molecule will make ethylene glycol, used as antifreeze and used to make polyester and plastic bottles. Figure 68 shows ethylene oxide synthesis:
Figure 68.
More complex epoxides can be made by adding an OH group and a Chlorine atom to an alkene and then adding a strong base. This takes the hydrogen and chloride off of the molecule to create an oxirane. This is shown in figure 69:
Figure 69.
There are other cyclic ethers that have common names that you need to memorize. One of these is furan, which is a five-membered ring that has an oxygen as the fifth member and two double bonds. In common usage, chemicals like 2,5-dimethyltetrahydrofuran, which is made from furan. In determining this molecule, you have to know that this can
132
be a cis or trans molecule, depending on the position of the methyl groups. Another is pyran, which is a five-carbon, six-membered ring that has an oxygen as the sixth member and two double bonds. Some of these molecules are shown in figure 70:
Figure 70.
In the nomenclature of epoxides, the terms oxirane, furan, dioxane, and pyran can be used to describe the more complex molecules that can be made from these basic molecules. These tend to be made by the oxidation of alkenes, making the common term “alkene oxide” used to describe these molecules. The epoxy functional group is the two carbon atoms with the oxygen in the middle of the molecule. Sometimes the nomenclature includes the term “epoxy” to describe the functional group, as in the molecule trans-4,5 epoxydecane, which is a decane molecule that has the fourth and fifth carbon atoms made into a cyclic compound with oxygen in a trans-fashion. A crown ether is a unique ether that consists of a minimum of four oxygen atoms. All crown ethers have a central space that can take on a metal ion effectively protected by the ring of carbon atoms. They are named using both the total number of atoms in the ring plus the total number of rings. As you will see in figure 71, the middle of the crown ether is electronegative, having electron pairs that will have affinity to accommodate a large metal cation, such as potassium:
133
Figure 71.
Figure 71 also shows a cryptand molecule, which is made so that it nearly completely hides the metal ion molecule. Instead of a crown ether, which contains just carbon, hydrogen, and oxygen atoms, the cryptand consists of a nitrogen atom as well, which adds to the electronegativity of the center of the molecule along with the oxygen molecule. Both cryptands and crown ethers will help solvate a metal ion because they allow the metal ion to have a place to dissolve.
ESTERS Esters are well-known in nature in that they are responsible for the smells of different organic molecules. The structure of the ester molecule is RCOOR’. These are formed through the mixture of an acid and an alcohol with water as an elimination product. In an earlier chapter, we discussed the oxidation and dehydration of alcohols; this reaction refers to the esterification of alcohols. The naming of esters involves the alkyl chain being the one that contains the carboxylic acid component with the opposite alkane attached as a side chain. The main chain has the “-e” removed and “-oate” added as an ending. Methyl ethanoate is made by the carboxylic acid CH3COOH plus the methyl group attached instead of the hydrogen atom. Instead of ethanoic acid (a carboxylic acid), the methyl group is attached. When an ester group is attached to a ring, the ester is named as a side chain on the ring.
134
The most common ester talked about in organic chemistry circles is ethyl ethanoate. This is not a symmetrical molecule in that one side is ethanoic acid and the other side is the ethyl or “ethane” side chain. As others are named besides this, they are named so that the side chain is first in the molecule and the parent chain (with the carboxylic acid) is always second. Esters are important because animal and vegetable oils and fats are made from complex, long-chain esters. Some are in liquid form and others are solid at room temperature. Consider the complex number of molecules that can be made from glycerol or propane1,2,3-triol. This can be “esterified” to make three ethanoate groupings. When the carboxylic acid is a very long chain and when all three hydroxyl groups on the glycerol molecule are esterified, this is called a triglyceride. This is looked at in the following example structure seen in figure 72:
Figure 72.
The triglyceride in figure 72 combines three molecules of octadecanoic acid with glycerol. The chemical name is propane-1, 2, 3-triyl trioctadecanoate or glyceryl tristearate. This is considered a saturated triglyceride or saturated fatty acid. Any time that a carbon-carbon bond is unsaturated one time, it is called a monounsaturated fatty acid and if there is more than one carbon double bond, it is called polyunsaturated. You should know that omega-3 and omega-6 fatty acids can be esterified to make a triglyceride; however, they are not named chemically correctly. The omega fatty acids are unsaturated with the name omega-6 referring to a carbon-carbon double bond at the
135
sixth carbon from the methyl end and not from the carboxylic acid end. The same is true of omega-3 fatty acids. Esters are like aldehydes and ketones in that they are polar molecules that have both dipole-dipole interactions and van der Waals dispersion forces. There are no ester-ester hydrogen bonds unlike carboxylic acids so they have lower boiling points than acids of the same number of carbon atoms. The smaller esters will be soluble in water; however, the larger ones are not as soluble in water. They will not be able to hydrogen bond with each other but will hydrogen bond with water, making them somewhat soluble in water. As the length increases, however, the molecule becomes more hydrocarbon-like and will be less soluble in water. Fats and oils are so long-chained that they aren’t soluble in water. When it comes to fats and oils, there are factors that determine their solidity or liquidity at room temperature. Fats tend to be called “fats” because they are saturated chains of fatty acids. This saturation allows for greater van der Waals dispersion forces so that there is more energy required to break their solid structure. The greater the degree of unsaturation, the lower the melting point. These unsaturated molecules have more disordered packing, resulting in a lesser number of forces between different molecules. Cis-fatty acids are highly disordered because of the cis bonding. These are liquid at room temperature. Trans-fatty acids are generally solid at room temperature. This is because the molecules are more tightly packed and behave more like saturated fats. Figure 73 shows a cis and trans fatty acid triglyceride.
Figure 73.
136
ESTER REACTIONS One reaction that can take place in esters is called hydrolysis. This is done with a dilute acid, such as hydrochloric acid or sulfuric acid, plus heat. This is a reversible reaction that needs a lot of water to push the direction of the reaction toward hydrolyzed esters. The reaction with ethyl ethanoate having the chemical structure of CH3COOCH2CH3 with dilute acid will add water or “hydrolyze” the molecule, giving rise to a carboxylic acid (acetic acid) and ethanol (an alcohol)—effectively separating the molecule at one of the oxygen molecules. The reverse reaction does not happen the same way as the forward reaction. In the reaction, a hydrogen ion is added to the double-bonded oxygen on the ester as is shown in figure 74. This starts a multistep process that leads to acetic acid and ethanol:
Figure 74.
As you will see, the charge shifts from one place to another in the molecule because electrons are delocalized and will “travel” from place to place on the molecule so that the hydrolysis can take place. The acid acts as a catalyst and is not used up in the reaction.
137
The different steps happen as follows: •
Protonation of the carbonyl group
•
Nucleophilic attack on the carbonyl carbon atom by water
•
Proton transfer to the alcohol group
•
Leaving of the alcohol from the molecule
The ester molecule can be made into two primary alcohols, using Lithium aluminum hydride (LiAlH4). The general reaction is to take the RCOOR’ molecule and hydrating the carbonyl group twice to make an RCH2OH molecule and an R’OH molecule. The reaction looks like that seen in figure 75:
Figure 75.
This is a multistep process, involving these steps: •
Nucleophilic attack on the carbonyl carbon by the hydride
•
Alcohol R’ group leaves with lithium as a salt
•
Nucleophilic attack of the new aldehyde to make an alcohol (alkoxide) salt with lithium and AlH3
•
Protonation of the alkoxide salt to make an alcohol
As you have seen, esters are chemically related to carboxylic acids. They are also related to acid halides, acid anhydrides, and amides. These are shown in figure 76, where you can see the relationship between these molecules:
138
Figure 76.
The molecules all have a carboxylic acid group with a “leaving group” that has a different degree of electronegativity and a different ability to be attacked in nucleophile substitution reactions. The nucleophile attacks the carbonyl carbon and replaces the leaving group in several different steps: •
Nucleophile (negatively charged) attacks the carbonyl carbon, leaving a tetrahedral intermediate.
•
The leaving group is removed with a negative charge.
•
A hydrogen atom protonates the leaving group.
As you will note, the ketone and aldehyde molecules also have a carbonyl group but their chemistry is different from the carboxylic acid and ester. This is because the leaving group on the aldehyde is hydrogen and the leaving group on the ketone will not easily leave. These molecules will undergo nucleophilic additions but will not undergo substitutions as in carboxylic acids and esters. The increasing order in which the functional group “wants” to leave the carbonyl group in a substitution reaction is this: •
Amide
•
Ester
•
Acid anhydride
•
Acid chloride
139
POLYESTER FORMATION Polyester formation is an example of condensation polymerization, which is different from “addition” polymerization. In condensation polymerization, a molecule is lost in the process, which is not the case in addition polymerization (because nothing gets lost). Polyesters are made by joining an acid that has two carboxylic acid groups as well as an alcohol with two hydroxyl groups. A common polyester is benzene 1,4-dicarboxylic acid (the carboxylic acid molecule) plus ethylene glycol, which is ethane-1,2-diol. These alternate with one another in a long chain with the loss of water as a result. In the polymerization stage, the temperature is heated at about 260 degrees Celsius using a catalyst like antimony (III) oxide. Polyesters cannot be broken down in the same way as simple esters. The simple esters can be easily hydrolyzed with dilute acids or bases but polyesters need alkalis to dissolve (less much so with acids). Alkalis added to polyester will break the ester linkage with ethylene glycol and a salt of the carboxylic acid made.
SAPONIFICATION Saponification is the organic chemical reaction in which an ester is broken down by water and an alkali to make a carboxylic acid and an alcohol. Because of the basic conditions of the reaction, a salt of the carboxylic acid (a carboxylate) is made in the process rather than a true carboxylic acid. The reaction proceeds in the following order: •
Nucleophilic attack by the hydroxide ion
•
Leaving group removal
•
Deprotonation of the carboxylic acid to make an alcohol and a carboxylate ion.
Saponification of the complex fat (which is a complex ester) will yield a salt of the carboxylic acid with a long alkane chain, which is what is used in cleaning.
140
KEY TAKEAWAYS •
Ethers are ROR’ molecules, where either side chain can be aliphatic or aromatic side chains.
•
Both ethers and esters involve an alkoxy side chain, which is a RO side chain.
•
There are several cyclic ethers that involve the substitution of a carbon atom with an oxygen atom.
•
Esters are carboxylic acids connected to an alcohol.
•
Triglycerides are basically tri-esters in biological systems.
•
There are various ways with acids or alkali that esters can be divided at the carbonyl group.
•
Polyesters are di-carboxylic acids and di-alcohols that are connected together.
141
QUIZ 1. Which is the correct way to describe an ether? a. RCOOR’ b. ROR’ c. RCOR’ d. RCOH Answer: b. The basic structure of the ether molecule is ROR’, in which two side chains are connected by an oxygen molecule. 2. Which of the following is the correct name for an ether, according to the IUPAC nomenclature? a. Ethoxy-methane b. tert-butoxy-ethane c. Isopropoxy-ethane d. Methoxy-ethane Answer: d. The only correct naming structure for an ether comes when the shorter alkane is listed as the oxy group or alkoxyl side chain and the longer alkane is considered the parent compound. 3. What can be said about the boiling point of ethers? a. It decreases with molecular mass and approaches that of alcohols of the same size. b. It decreases with molecular mass and approaches that of alkanes of the same size. c. It increases with molecular mass and approaches that of alcohols of the same size. d. It increases with molecular mass and approaches that of alkanes of the same size. Answer: d. Boiling point will increase with molecular mass in all organic molecules; as there are no hydrogens attached to the oxygen
142
atoms, there will be less intermolecular binding so the boiling point is close to that of the alkane and not the alcohol of the same size. 4. There is a reaction with sulfuric acid and ethanol that will produce an ether. What factor most drives this type of reaction? a. Low heat b. Catalyst addition c. Sulfuric acid concentrations d. Very high heat Answer: a. This reaction will make diethyl ether in the presence of sulfuric acid as long as the temperature is low. If the temperature is too high, the same substrates will favor an elimination reaction that will result in an alkene formation. 5. A six-membered ring that is a cyclic ether can be referred to as what molecule? a. Oxirane b. Furan c. Tetrahydrofuran d. Pyran Answer: d. Pyran is a six-membered ring that is a cyclic ether. It has two double bonds that can be substituted to make a more complex ringed molecule. 6. The term epoxy can be used to describe a functional group on an alkane that consists of what? a. The cyclic arrangement of two carbon atoms plus an oxygen molecule in the chain. b. The connection of two carbon atoms by an oxygen molecule between them. c. The presence of two ketone molecules side-by-side. d. The addition of a benzene ring with an oxygen molecule attached to a carbon atom on the alkane.
143
Answer: a. The term epoxy refers to the cyclic arrangement of two carbon atoms plus an oxygen molecule on the chain or side chain, effectively making an oxirane out of the chain. 7. Triglycerides are what type of molecule? a. Tri-esters b. Tri-aldehydes c. Tri-ketones d. Tri-alkenes Answer: a. These molecules are tri-esters, combining long-chain carboxylic acids or “fatty acids) with a glycerol molecule, which is a trialcohol molecule having three carbon atoms. 8. Which boiling point will be lowest, based on having the same number of carbon atoms? a. Alcohol b. Carboxylic acid c. Ester d. Glycerol Answer: c. The ester will have the lowest boiling point because the others will have hydrogen bonding added to the dipole-dipole bonding and the van der Waals dispersion forces. Esters do not have the hydrogen associated with the oxygen so the boiling point will be lower. 9. Which leaving group is most likely to leave the carbonyl group in an electrophilic substitution reaction because of its electronegativity? a. Chloride or halide b. Amide c. Acid anhydride d. Ester
144
Answer: a. In an acid chloride or acid halide, the chloride leaving group is the most electronegative, making it more likely to act as a leaving group when the carbonyl carbon atom is acted on by a nucleophile. 10. What is the pair of molecules necessary to make a polyester in manufacturing? a. Alcohol plus an ester b. Two different ester molecules c. A di-alcohol and a diester d. A di-alcohol and a di-carboxylic acid Answer: d. The polyester is identified as a di-alcohol interspersed between di-carboxylic acid molecules with a loss of water in the chemical process.
145
CHAPTER 9: ENOLS AND ENOLATES The topic of this chapter is the structure and chemistry of enols and enolates. Enols are also referred to as alkene alcohols, which are alkenes that have an alcohol group added to one of the carbon atoms. These are first alkenes but, chemically-speaking, they should be considered important for their electron-donating capacity. Enols can be mixed with alkali substances to make enolates, which are the conjugate bases of enols. Both of these types of molecules are best known for the many different types of reactions they participate in, which are covered in this chapter.
INTRODUCTION TO ENOLS AND ENOLATES Enols go by the chemical symbol of RCHOH, which are alkene enols. They form enolates when mixed with bases; enolates are anions of the enol compounds, similar to the alkoxide ions of alcohols that have been discussed in the previous chapters. Alkenes are already nucleophiles that are made even more reactive than simple alkenes by the addition of the OH (hydroxyl) group. You may ask which of these is more nucleophilic. This is determined by looking at the electronegativity of the oxygen added to the carbon atom and by the charge. Figure 77 shows the relative nucleophilicity of the different molecules:
Figure 77.
146
As you will see from the study later of nitrogen-containing compounds, the nitrogen compound will have nucleophilicity but because it is less nucleophilic than oxygen, it will be less nucleophilic than an enolate. Each of these molecules will be more nucleophilic than an alcohol compound. Enols are by nature unstable compounds, being in natural equilibrium with a carbonyl group, which is the more favorable molecule. This equilibrium process is known as tautomerism. Tautomers are considered isomers of a molecule which differ only in the position of the protons and electrons. The carbon skeleton of the molecule is unchanged but there is a simple hydrogen ion transfer in the molecule. This transfer of protons and electrons is referred to as tautomerism. Figure 78 shows the tautomerism of an enol and carbonyl group—a process that is facilitated by both acids and bases:
Figure 78.
As you can see by the arrows, the carbonyl group is considered more stable; however, in certain circumstances, the enol form is more available for reactivity. Consider phenol, for example, this is an enol compound made from benzene and a hydroxyl group. The 1,3-dicarbonyl compound (with two acetone groups with a carbon in between), and the addition of a hydrogen onto one of the oxygen groups leads to hydrogen bonding between the two oxygen molecules, making this stable, as is seen in figure 79:
147
Figure 79.
The advantage of enolates is that they can be nucleophiles at either the oxygen atom or the carbon atom, taking on a positively charged molecule that needs a pair of electrons. Figure 80 shows the oxyanion and carbanion—either of which can be negatively charged and highly reactive molecules:
Figure 80.
While one would consider the oxygen anion to be more reactive than the carbanion (carbon) anion, this isn’t necessarily the case and, in fact, be prepared to recognize that most reactions occur at the carbanion group. You need to know that, similar to other compounds, there is something called the alphahydrogen. In the carbonyl group of a ketone, there are no hydrogen atoms associated with it because the carbon is tied up with two other carbon bonds and an oxygen double bond. The only hydrogen atoms are those associated with the next carbon over. These are called “alpha hydrogens”. You should remember that there are beta and gamma carbons and hydrogens as well. You should know how many alpha hydrogens are around a given carbonyl group, whether it is an aldehyde, ketone, or carboxylic acid.
148
These alpha hydrogens are considered more acidic than other carbons and can be more easily donated or removed by bases, which can be an OH- group or a RO- group. The removal of the hydrogen proton leaves an enolate that will transfer the negative charge between the oxygen molecule and the alpha carbon molecule as is seen in figure 81. There is, in a very real sense, resonance between the two adjacent bonds. The pKa can be used to describe acidity. The lower the pKa, the more acidic a substance is. Some examples include the following pKa values: •
Alkane pKa = 50 (not very acidic at all)
•
Enolate/Ketone pKa = 19
•
Aldehyde pKa = 17
•
Alcohol pKa = 16
•
Carboxylic acid pKa = 5 (most acidic)
The more stable the resonance pattern of the negatively charged molecule, the more acidic is the parent compound and the more stable is the conjugate base (which exists after the loss of the proton). In some cases, there could be hydrogen atoms next to two carbonyl groups (in which there are two ketones next to each other). This means that there is enhanced resonance stabilization of the resultant anion because the charge can be delocalized to two oxygen atoms (both of which are electronegative). This leads to a compound that is even more acidic and has an even lower pKa. The chemical name for these types of compounds is “active methylene” as is seen in figure 81:
Figure 81.
149
ENOLATE REACTIONS Enols and enolates are highly reactive, being sources of nucleophilic carbon atoms that, by necessity, react with electrophiles. The basic types of reactions we will discuss include the following: •
Acid halogenation of enolates
•
Basic halogenation of enolates
•
Alkylation of enolates
•
Aldol reaction
•
Michael reaction
ACIDIC ALPHA HALOGENATION OF KETONES AND ALDEHYDES This involves the halogenation of the alpha carbon of a ketone or aldehyde using nucleophilic substitution and chlorine or bromine gas or iodine liquid. Figure 82 shows the basic reaction:
Figure 82.
These reactions happen under acidic conditions, involving the di-halogens chlorine, bromine, or iodine. The byproduct, as you can see is the acidic hydrogen halide. There are several steps that make sense when you see them in order:
150
•
Make a tautomer of the carbonyl structure to make it an enol tautomer. This puts the hydrogen atom on top of the oxygen and an alkene out of the carbonyl carbon and the alpha carbon.
•
Then pull the halogen apart, attaching it to the nucleophilic alpha carbon atom. This leaves behind a halogen anion that “needs” a hydrogen ion to make it a complete acid.
•
Then pull the hydrogen atom off the OH molecule on the original enol, making the molecule now a halogenated ketone plus the acidic hydrogen halide molecule.
BASIC ALPHA-HALOGENATION OF KETONES AND ALDEHYDES We have just discussed the acidic alpha halogenation of ketones and aldehydes. As it turns out, the same thing can be done under basic circumstances. The biggest problem with this reaction is that it is not limited to monohalogenation and frequently polyhalogenation occurs. This is because the addition of an electronegative halogen atom makes the enolate more stable and more halogenation occurs. The rates of halogenation are the same for bromine, iodine, and chlorine. The reaction goes as is seen in figure 83:
Figure 83.
This reaction proceeds as follows: •
An acid-base reaction occurs, removing the acidic hydrogen atom by virtue of its attachment to a basic hydroxyl group. This leaves the enolate behind plus water, which is not seen in the acidic version of this reaction.
151
•
The enolate is highly reactive with a negative “charge” on the alpha carbon that is nucleophilic. This draws off the halogen from the di-halogen molecule.
•
The halogen forms a salt from the sodium or potassium originally supplied in the beginning of the reaction (or else it proceeds to add more halogen atoms onto the alpha carbon).
THE HALOFORM REACTION In the haloform reaction, the beginning reactant must be a methyl ketone. This is because, under basic conditions with a di-halogen, the entire alpha carbon (CH3 molecule) can get pulled off and halogenated three times to yield, for example, a CHCl3 or CHBr3 molecule plus a carboxylate (RCOO-) molecule. Figure 84 describes this reaction:
Figure 84.
This reaction is of the nucleophilic substitution type. The end result is a trihalomethane molecule or a haloform, such as chloroform. There is replacement of halogens that have increasing affinity for the alpha carbon until all three of the hydrogen atoms have been replaced. This is the polyhalogenation we recently talked about. The CX3 molecule leaves the main molecule and is substituted by hydroxide. Hydrogen adds to the CX3 in order to make a trihalomethane molecule.
152
The mechanism happens like this: •
An acid-base reaction pulls a hydrogen off of the methyl side group (the alpha carbon group), leaving behind an enolate.
•
The halogen attaches to the enolate.
•
The reaction is repeated twice more with the eventual production of a trihalogenated ketone.
•
The hydroxide ion attacks the tri-halogenated molecule, replacing it to make an oxygen anion and a trihalomethane molecule (that gets protonated for stability). The tri-halogen molecule will be so electronegative that it pulls the remaining hydrogen off of the carboxylic acid to make a carboxylate.
ALKYLATION OF ENOLATES Enolates can undergo a nucleophilic substitution reaction in which the alpha hydrogen gets pulled off in the presence of a base and an alkyl halide. This alkyl group replaces the alpha hydrogen in the ketone molecule. The reaction works best with methyl halides. This reaction starts with an acid-base reaction that pulls off the alpha hydrogen, leaving behind the enolate. Then the nucleophilic enolate attacks the alkyl halide, pulling off the alkyl group and leaving behind the halide anion. This is seen in figure 85:
Figure 85.
153
THE ALDOL REACTION OF ALDEHYDES So far, we have been talking about mechanisms that affect ketones; however, some of these reactions have also applied to aldehydes as the R chain talked about could also be hydrogen. In the aldol reaction of aldehydes, the alpha carbon can be acted on with a base, leaving behind an enolate that can form a beta-hydroxyaldehyde, as is shown in figure 86:
Figure 86.
This is done under sodium hydroxide or potassium hydroxide basic conditions. This environment is necessary to make the enolate out of the aldehyde. In such cases, there needs to be at least one alpha hydrogen molecule on the aldehyde, which gets pulled off to make an enolate. This is highly neutrophilic and binds with another molecule of aldehyde to make a beta hydroxyaldehyde, in which the new beta carbon atom is also an alcohol. In such cases, the carbonyl carbons on those aldehydes not already an enolate become good electrophiles, binding to the nucleophilic alpha enolate carbon atom. The end product, as mentioned is a beta-hydroxyaldehyde but, because it is an alcohol and an aldehyde, it is called an aldol. The simplest reaction is that of ethanal (CH3CHO) and itself. Condensation/dehydration happens to these aldols under usual circumstances to yield an unsaturated aldehyde, with a double bond between the alpha and beta carbon atom and water as an end product. This is referred to as an enal. Figure 87 describes an enal molecule:
154
Figure 87. In the dehydration reaction, hydroxide pulls the alpha hydrogen atom off of the betahydroxyaldehyde to make water. This, creates a double bond and results in the pulling off of the OH group off of the beta-carbon atom, resulting in an enal or alkene aldehyde. This results in a regeneration of the OH ion at the end of the reaction.
THE ALDOL REACTION OF KETONES This is a similar reaction of the previously mentioned aldol reaction of the aldehyde under basic conditions. In this reaction, an alpha hydrogen gets pulled off to make an enolate that ultimately becomes a combined molecule. This reaction doesn’t happen as easily as an aldehyde reaction because there are a lot of side chains that impair the attachment of the ketone enolate to another ketone. These beta-hydroxyketone molecules, when they do occur, become dehydrated easily to become an “enone” molecule. So, what favors the dehydration of aldol products? First, there needs to be an alphahydrogen between the hydroxyl group and the carbonyl group. Heating the reaction favors the dehydration process and the presence of the reaction in non-aqueous solutions will drive the reaction forward. This will immediately remove water from the reaction process so that continued dehydration will occur. Of course, there can be a “mixed aldol” reaction that involves different aldehydes or ketones involved in the reaction process. The carbonyl and alcohol sides do not come from the same molecule. So that there is not a complete mixture of enones and enals, some things must be true:
155
•
Only one of the reactants can form an enolate in this reaction.
•
The other reactant must have no alpha-hydrogen atoms.
•
One reactant must be more electrophilic than the other, acting as the carbonyl component.
•
Aldehydes are more effective as electrophiles than ketones.
Intramolecular aldols can be part of this reaction process with the necessity that there needs to be a dicarbonyl compound so that the carbonyl component and the enolate component come from the same molecule. This molecule must also be sizeable enough to create a stable cyclic aldol product. By necessity there needs to be four carbon atoms “between” the two carbonyl groups so that a stable five-membered ring can be created in this type of reaction. Remember, too, that the aldehydes will be more electrophilic than ketones so the reaction will proceed with the aldehyde being the electrophile and the ketone being the nucleophile.
CONJUGATE REACTIONS In the case of enone or enol, there can be reaction of the carbon atom on the far side of the double bond, called conjugate reactions. The reaction involves a nucleophile that attacks the conjugate or beta carbon atom, resulting in the addition of the nucleophile. What this looks like is seen in figure 88:
Figure 87.
This can involve the use of organocopper reagents under acidic circumstances. Organolithium cuprates or R2CuLi can result in the addition of the R chain to the beta carbon atom in an enone or enal molecular situation. There are two R groups associated 156
with both lithium and copper, which are made from the combination of an alkyl lithium compound and copper halide (such as iodine, bromine, or chlorine). These become rather “ionic” in character with the R chain being negative and both copper and lithium being positive. In such cases, the carbon atom associated with the copper lithium compound is nucleophilic and it attaches to the beta-carbon atom on the “far side” of the double bond (that is, the conjugate carbon atom). This will shift the electrons to the electronegative oxygen molecule to make an intermediate enolate. This reaction proceeds as is seen in figure 89:
Figure 88.
MICHAEL ADDITION REACTION Another conjugate addition reaction takes a ketone and an enone or enal, causing the addition of the ketone to the conjugate carbon atom of the enone or enal. This is done under basic circumstances in order to pull off an alpha hydrogen from the ketone, attaching the ketone that is now effectively negatively charged (nucleophilic) to the conjugate carbon atom, which is slightly electrophilic. It relies on the slight acidity of the alpha carbon next to the carbonyl group. The reaction is otherwise the same as in figure 89 except that it is done under basic conditions with water donating the necessary alpha hydrogen atom to make the molecule saturated rather than a hydrogen ion.
157
KEY TAKEAWAYS •
Enols are basically alkene alcohols that have special reactivity because of their relative instability.
•
Enolates are the conjugate bases of enols and are particularly reactive molecules.
•
Many reactions of aldehydes and ketones involve the relative acidity of the alpha carbon molecule.
•
Acid and base halogenation can occur at the alpha carbon atom.
•
Aldol reactions can occur with ketones and aldehydes, making enals and enones as end products.
•
The various conjugate addition reactions involve the alkyl addition to the beta carbon or conjugate carbon atom of an enone or enal, facilitated by enolate intermediaries.
158
QUIZ 1. What is the most basic chemical representation of an enol? a. RCOOH b. RCHOH c. RCH2OH d. RO Answer: b. The most basic chemical representation of an enol is RCHOH representing an alkane to which a double bond is added as well as an alcohol group on the alkene. The actual name is alkene alcohol, which has been shortened somewhat to “enol”. It can also be represented as RR’COH, in which there are two side chains on the carbon atom. 2. What is the closest molecule chemically to the enolate molecule? a. Ketone b. Carboxylic acid anion c. Alkoxyl group anion d. RR’OO anion Answer: c. The chemical representation of the enolate anion is the RCHO anion or RR’CO, which is similar to the RCH2O alkoxyl group, both of which have the property of being nucleophilic by virtue of having a negative charge on the oxygen molecule, although the enolate molecule is more nucleophilic than the alkoxyl side chain because of the connected double bond. 3. What type of molecule can be considered the least acidic? a. Enol b. Carboxylic acid c. Alkane d. Alcohol
159
Answer: c. Each of these will have acidic properties to some degree; however, alkanes have a pKa of 50, which makes them highly unlikely to give up a hydrogen ion. 4. How many alpha hydrogens are there on 1,3-diketone (diacetone), which is a molecule that is CH3COCH2COCH3? a. 3 b. 5 c. 6 d. 8 Answer: d. These are unique molecules that have 8 alpha carbons because all of the hydrogen atoms are associated with a carbon atom that is next to a carbonyl group. Each of these will be an alpha hydrogen that has an increased acidity compared to a typical hydrogen atom because of a possible resonance structure within the molecule. 5. In the basic alpha halogenation of ketones via the reaction of the enolate, what happens to the halogen ion that is left over after the reaction occurs? a. It forms a di-halogen molecule again b. It binds with hydrogen to form a halogen halide c. It binds to the oxygen molecule on the halogenated ketone d. It forms a salt Answer: d. The basic conditions require the “using up” of hydroxide ions in sodium or potassium hydroxide. The hydroxide ions participate in water formation and the halogen forms a halogenated salt solution as a byproduct. 6. What is not an end-product of the haloform reaction? a. Aldehyde b. Carboxylate c. Tri-haloform d. Water
160
Answer: a. This is a reaction that starts with a methyl ketone and ends with a carboxylate, a tri-haloform, and water. 7. What type of reaction is the aldol reaction which takes an aldehyde and combines it to make a beta-hydroxyaldehyde? a. Nucleophilic subtraction b. Electrophilic substitution c. Nucleophilic substitution d. Nucleophilic addition Answer: d. This is a nucleophilic addition reaction in which two aldehydes combine to make a beta-hydroxyaldehyde molecule (adding one molecule to the next). 8. The aldol reaction specifically starts with what type of molecule as a reactant? a. Aldehyde b. Carboxylate c. Ketone d. Alkyl halide Answer: a. The aldol reaction combines two aldehyde molecules using an enolate as an intermediary molecule. 9. In an aldol reaction, there will be some things that drive the reaction to make an enal or an enone. Which is not one of these? a. Acidic environment b. The addition of heat c. The presence of an alpha hydrogen d. A nonaqueous environment Answer: a. The aldol reaction first makes a beta-hydroxy molecule that will become an enal or an enone in the presence of heat and in nonaqueous environments that take water out of the dehydration equation. The presence of an alpha hydrogen is a necessity; however, it is not driven forward in an acidic environment.
161
10. If an intramolecular aldol reaction takes place in a dicarbonyl compound, what is the minimum number of carbon atoms between the carbonyl groups to make a stable compound after that? a. 2 b. 3 c. 4 d. 5 Answer: c. There can only be stability in a five to six-membered carbon ring if there are four or more carbon atoms between and not counting the two carbonyl carbons. This will leave behind a five-membered ring.
162
CHAPTER 10: SULFUR-CONTAINING ORGANIC COMPOUNDS This chapter changes nomenclature and reactions in organic chemistry to include molecules that contain sulfur. Sulfur compounds are somewhat similar to oxygencontaining molecules in that they belong to the same group but sulfur is a great deal larger than oxygen, leading to slightly different chemical reactivity unique to these molecules. The nature and chemistry of thiols and sulfides is discussed as part of this chapter.
NOMENCLATURE OF SULFUR COMPOUNDS Any molecule that has an SH side chain is called a thiol or mercaptan. In the basic representation of a thiol, the alkyl group is named, followed by the word “thiol”. If the SH group is a side chain rather than a main part of the molecule, it is called a sulfhydryl group. The molecule naming can be more complicated than that with different terms used to mean the same thing. An example is the CH3SH molecule that can be called methanethiol, sulfhydryl methane, or methyl mercaptan. There are a few common names you should know about, including thiophenol, which is a sulfhydryl group instead of a hydroxyl group on a benzene ring. Sulfides, on the other hand, are similar to ethers but with a sulfur atom in the place of oxygen. They are represented by the symbol RSR’. The two alkyl groups are listed alphabetically with the term “sulfide” as the last word, such as butyl methyl sulfide. The numbering of carbons starts at the end of each alkyl chain that gives the sulfur group the lowest possible number, such as 2-butyl 1-propyl sulfide, in which the sulfur group is attached to the second carbon atom on the butyl side chain. Another common name you should remember is the aromatic compound called “thiophene”. It has four carbon atoms and a sulfur atom, making a five membered aromatic ring. Two-ethyl thiophene is a thiophene ring with an ethyl group on the carbon atom next to the sulfur atom so that the sulfur atom is counted as “one”. 163
Disulfides can be named more readily by naming each side group separately and then adding disulfide to the name. The general terminology of a disulfide is RSSR’. The side chains go alphabetically with the numbering going so that the disulfide chain gets the lowest number on each chain. An example would be ethyl 1-propyl disulfide, which adds the SS chain between the two alkyl groups. In addition, there will be sulfoxide molecules. These are named by naming the alkyl groups plus the term “sulfoxide”. It goes by the structure RSOR’, in which the S molecule has four bonds with the alkyl chains plus a double bond between an oxygen molecule. Figure 90 shows some typical sulfur containing compounds:
Figure 90.
Sulfonic acids have three oxygen atoms associated with them plus an alkyl group. The molecule will be RSOOOH with six bonds around the sulfur molecule. It is named by naming the alkyl group plus adding the term “sulfonic acid”. You should know that the RSH molecule is considered more acidic than the ROH molecule. This greater acidity leads to greater nucleophilicity on the part of these molecules. The molecule hydrogen sulfide or H2S is ten million times more acidic than H2O, this means that thiols are also stronger acids. It means that thiolate salts or conjugate bases are more easily made and there are reactions that can easily happen, such as RS-sodium plus R’Br going to RSR’ plus sodium bromide. Because of the nucleophilicity of the sulfur versus oxygen, there are more useful electrophilic substitution reactions of sulfur that aren’t seen in oxygenated molecules. Sulfides can react with alkyl halides in order to make ternary sulfonium salts. There are several other reactions that can be made by sulfur containing compounds that don’t
164
happen as easily as they would in related oxygen compounds. Figure 91 shows one of these reactions:
Figure 91.
The main difference you can see between oxygen and sulfur is that there can be different oxidation states of sulfur that aren’t seen in oxygen. Oxygen has a -1 to -2 oxidation state, with a preference for the -2-oxidation state. Sulfur has oxidation states ranging from -2 to +6. There are many oxidation states that are stable in sulfur. The most common oxidation states are -2 and 0: •
Minus two—H2S, thiols, sulfides, and sulfonium ions, which are R3S+ compounds.
•
Minus one—disulfides
•
Zero—elemental sulfur, sulfoxides, and sulfenic acids (RSOH).
•
Plus two—sulfones and sulfinic (RSOOH) acids
•
Plus four—sulfonic acids and sulfite esters (ROSOOR)
•
Plus six—sulfate esters ROSOOOR
As a third period element, the sulfur has five empty 3d-orbitals that can be used for p-d bonding similar to pi bonding, which is p-p bonding. You need to know that the shape of a sulfoxide is a fixed pyramidal shape with sulfur at the center. This means that, if two different alkyl groups are on the sulfur molecule, the side chains will be chiral. It is possible to isolate different enantiomers of sulfoxides (RSOR’ molecules).
165
With alcohols, the oxidation of primary and secondary alcohols to make aldehydes and ketones will change the oxidation state of the carbon atom but not the oxygen molecule. The oxidation of sulfur compounds will change the oxidation state of the sulfur atom but not the carbon atom. Mild oxidation will turn a thiol into a disulfide. This does not happen with oxygen molecules because the O-O bond is not as strong as the S-S bond, while the OH bond is stronger than the SH bond. This means that peroxides (having an O-O bond) aren’t as stable as a disulfide bond. Figure 92 shows the oxidation of thiols with mild oxidizing agents:
Figure 92.
There can be a carbon-sulfur double bond, indicated by the word “thione”. Anytime there is a sulfur in a carbon ring rather than an oxygen molecule, the molecule has the prefix “thia”.
OXIDATION OF ALCOHOLS USING DMSO Making an aldehyde or ketone by oxidizing a primary or secondary alcohol is important in organic chemistry. Basically, it is a dehydrogenation reaction, losing H2 in the process. Oxygen must be present in the reaction to stabilize the H2 to make water. One good source of this oxygen is the sulfoxide solvent known as DMSO or dimethyl sulfoxide or CH3SOCH3. The alcohol becomes oxidized and DMSO gets reduced to Dimethyl sulfide with water taken up by the electrophilic substance. It is so exothermic it has to be done at extremely low temperatures with cosolvents because the freezing temperature of DMSO is 18 degrees. An example of this is the oxidation reaction shown in figure 93:
166
Figure 94.
THIOLS Much of the chemistry of thiols and related compounds can be predicted from a knowledge of their oxygen-containing analogues. The SH group can be called a sulfhydryl group or a mercapto group. Sulfides, as you’ll remember have the RSR’ configuration, while the disulfide compound is referred to as the RSSR’ molecule. The sulfate ion goes by the chemical name of SO4(two minus), while thiosulfate is the S2O3 (two-minus). The thiolate anion is the RS- anion, similar to the alkoxy- anion. The trialkylsulfonium ion is referred to as R3S+ anion, similar to the hydronium ion (H3O+) ion as shown in figure 94:
Figure 95.
Thiols are made by using alkyl halides and the hydrosulfide anion (SH anion), which acts as a nucleophile in the reaction to create a thiol. The hydrosulfide anion attacks the carbon atom on the alkyl halide, displacing the bromine or chlorine to make the thiol and a bromine or chlorine anion. The problem with this reaction is that the sulfide side product can be formed by continuation of the reaction. The way around this is to use thiourea as the nucleophile with hydrolyzing the intermediate in a basic solution as is seen in figure 95: 167
Figure 95.
The disulfide bond or disulfide bridge is important in proteins. The amino acid cysteine has a sulfhydryl group on it. This can make double bonds with other cysteine sulfhydryl groups to make a disulfide bridge that forms the three-dimensional structure of the protein. This is a redox reaction, in which the thiol is in the reduced state and the disulfide bond is in the oxidized state. The loss of the hydrogen from this reaction is why it is called “oxidized” even though no oxygen is involved. In biochemistry, a complex coenzyme called glutathione is used to create and break down these proteins. It, too, has a sulfhydryl group on it and comes in a reduced “GSH” and an oxidized “GSSG” form. It comes into the reaction oxidized and is reduced to GSH in the redox reaction that oxidizes the cysteine SH group to create a double bond. Disulfide bridges are seen in proteins located outside the cell because glutathione reductase in the cell keeps GSSG in the reduced GSH state, driving the reaction toward the absence of disulfide bridges.
SULFIDES Sulfur analogues of ethers, as you know, are referred to as sulfides or thioethers. These are similar in some ways but different in other ways than ethers. This is because of the acidity of thiols compared to alcohols and phenols. This means that thiolate conjugate bases more easily form to make nucleophiles in reactions between the thiolate and alkyl halides and tosylates. The basicity of ethers is a hundred times greater than sulfides, which means that the sulfur atom is more nucleophilic. If the complex sulfide formula is referred to, it is not called a sulfide but the term “alkylthio” is used instead of “alkoxy”. This leads to more complex names like 3168
(methylthio)cyclopentene, which is a cyclic pentene molecule with a methylthio group on the third carbon of the ring. Sulfides are easily oxidized using hydrogen peroxide. In the reaction, the RSR molecule becomes R2SO, which is a sulfoxide. This can be further oxidized to produce a sulfone using a peroxyacid. As you remember, the sulfone molecule is R2SO2. Figure 96 shows the oxidation of a sulfide to make a sulfoxide and a sulfone:
Figure 96.
The sulfoxide you should memorize is DMSO or dimethyl sulfoxide, in which the two R groups are methyl groups. This is considered a polar aprotic solvent. Other things you should know about a sulfide is that the C-S bonds are longer than the similar C-O bonds, in part because sulfur is larger.
SYNTHESIS OF SULFIDES Sulfides must be synthesized by the nucleophilic substation reaction using thiols. The reaction starts with the addition of sodium hydroxide or NaOH, which pulls off the hydrogen ion, leading to the thiolate anion. This gets acted on by a primary halide, in particular, methyl halide, which acts similarly to the making of ethers. The methyl group on the methyl halide gets attached to the thiolate anion, giving a sulfide. This reaction is described in figure 97:
169
Figure 97.
170
KEY TAKEAWAYS •
Sulfur acts similarly to oxygen in the creation of many organic molecules.
•
The oxidation number of the sulfur atom can be as low as negative two or as high as positive six.
•
The thiol molecule, disulfide molecule, sulfide molecule, and sulfoxide molecule are the most common sulfur compounds used in organic chemistry.
•
Sulfur is important in protein biochemistry because, with cysteine, it can create a disulfide bridge that helps form the 3D characteristic of a protein molecule.
171
QUIZ 1. When the SH (sulfur hydrogen) side group is a part of an organic molecule, what is the term specifically used for this? a. Sulfhydryl b. Thiol c. Sulfide d. Sulfone Answer: a. The sulfhydryl group is what the SH side group is called when it is attached to a larger organic molecule. 2. What name for a molecule is not the same as the other three? a. Sulfhydryl methane b. Methanethiol c. Methyl mercaptan d. Methyl sulfide Answer: d. Each of these is the name appropriate for the CH3SH molecule except for methyl sulfide, which is chemically not the same. 3. What is the chemical structure of a disulfide molecule? a. RSH b. RSR’ c. HSRSH d. RSSR’ Answer: d. The chemical structure of the disulfide molecule is RSSR’, which means there are two alkyl side chains with a double S-S bond between the two alkyl groups, which may or may not be different. The term is alkyl chain one, alkyl chain two (in alphabetical order) plus “disulfide”.
172
4. What is the chemical structure of a sulfoxide molecule? a. RSOR’ b. RSOOH c. RSH d. RSR’ Answer: a. The main structure of a sulfoxide molecule is the RSOR’ structure. This has four bonds around the sulfur molecule that is double-bonded molecule to oxygen like a ketone but with a sulfur atom in place of the carbonyl carbon. 5. What is the chemical structure of a thiolate? a. NaSR b. RSR’ c. RSOOH d. RSOONa Answer: a. NaSR is the chemical structure of a thiolate, which is the conjugate base of a thiol, named as the salt (like sodium or potassium), the alkyl group, and the word “thiolate”. 6. Sulfur compounds have a wide range of oxidation states. What is the range of oxidation states in sulfur? a. -1 to +6 b. -2 to +8 c. -2 to zero d. -2 to +6 Answer: d. Because of the larger size of sulfur, it can have a negative or positive oxidation state. This leads to a possible oxidation status of -2 to +6.
173
7. The formation of the CS double bond makes the molecule called what type of molecule? a. Sulfone b. Thione c. Mercaptan d. Sulfonate Answer: b. A thione molecule has a double C=S bond, which is similar to a ketone molecule using sulfur instead of oxygen. 8. What is referred to as the mercapto group in sulfur chemistry? a. SOOH b. SOH c. SH d. SOOOH Answer: c. The mercapto group is what is also referred to as a sulfhydryl group, found on molecules also referred to as “thiols”. 9. Which type of reaction creates a disulfide bridge when glutathione reacts with two cysteine molecules in biochemical reactions? a. Addition reaction b. Acid-base reaction c. Redox reaction d. Alkylation reaction Answer: c. This is a simple redox reaction in which glutathione gets reduced and the cysteine molecules get oxidized.
174
10. What is the chemical makeup of a sulfone? a. R2SO b. R2S c. R2O3 d. R2SO2 Answer: d. The sulfone molecule is highly oxidized, consisting of two R groups, plus two double-bonded oxygen groups that give a +2oxidation number to the sulfur atom.
175
CHAPTER 11: NITROGEN-CONTAINING ORGANIC MOLECULES This chapter places a focus on the different nitrogen-containing molecules in organic chemistry. These types of molecules are not only important in basic organic chemistry; they are important also in numerous biochemical processes. The main type of molecule discussed will be the amine compounds, which are considered organic derivatives of ammonia. Like ammonia itself, amine compounds will have a certain degree of basicity, which leads to nucleophilicity of the nitrogen compounds in organic compounds.
NOMENCLATURE OF AMINES Amines are considered to be derivatives of ammonia in which at least one hydrogen atom has been replaced by an aromatic side chain or an alkyl group. There are specific terms to describe primary, secondary, and tertiary amines, which are different from the terms used to describe alkyl halides and alcohols. A primary amine has one alkyl or aryl group attached. A secondary amine has two alkyl or aryl groups attached, and a tertiary amine is one that has three side chains attached. This can lead to a variety of isomers so that it is difficult to determine the structure of an amine molecule given the basic number of carbons, hydrogens, and nitrogen atoms in the molecule. If there is a fourth alkyl group attached, it is called a quaternary ammonium cation. An example of this would be tetramethylammonium cation, which would have a single positive charge. The confusion increases when one looks at the different ways to name amine compounds. There are IUPAC names, Chemical Abstract Service names (or CA names), and common names. An example might be tert-butylamine, which is a common name for a tert-butyl group attached to an NH2 group. The IUPAC name would be twoamino-two-methylpropane, and the CA name would be 2-methyl-2-propanamine. Each of these would be correct.
176
There are several common names that you should probably memorize. These are names of cyclic nitrogenous compounds that are in common use in chemistry and biochemistry. Figure 98 shows these common compounds:
Figure 98.
The common system makes use of the term “amine” for these compounds. Notice that none of the common names listed in figure 98 end with amine; these should be memorized nevertheless. If the group is not the entire compound but is just an amine group on a larger compound, the prefix “amino” is used. There are many nitrogenous compounds in nature; these are called “alkaloids”. Figure 99 shows many of these compounds, which do not have to be memorized. Just know that these are common nitrogen-containing compounds you might find in any discussion of natural organic chemistry:
177
Figure 99.
You do not need to memorize the structures in figure 99 but you do need to have a knowledge of those in figure 98. Many of those seen in aromatic rings will have planar configuration with sp2 hybridization (pi-bonding) or will be in a shallower trigonal pyramidal shape. Those in singular bonded form with three hydrogen atoms or up to three alkyl groups will be in a trigonal pyramidal shape.
PHYSICAL PROPERTIES OF NITROGENOUS COMPOUNDS The NH bond is polar by nature of the fact that there are electronegative differences between the two atoms. This leads to hydrogen bonding with itself and with other molecules, such as water. What this means is that there will be a higher melting point, high boiling points, and higher water solubility. There will be a higher partial positive charge on the hydrogen atom and a partial negative charge on the nitrogen atom in this bond. The amine bond leads to an amine nitrogen atom that is a Lewis base. The alkyl ammonium has a pKa of about 10, while the aryl ammonium is more acidic, with a pKa of about 5, owing to the delocalization of electrons in the aromatic ring, making the nitrogen atom less likely to donate an electron pair. Because of the electronegativity of the nitrogen atoms in these types of bonds, they can act as bases or nucleophiles at the nitrogen atom. Removal of the proton, leads to the amide bond. The NH group itself is a
178
poor leaving group, which must change to a better leaving group before the nitrogen can be removed. Ammonium ions of whatever sort leads to a nitrogen atom that is not nucleophilic. It has groups attached to it all over and it sits in the center of the molecule, leading to a low degree of nucleophilicity. It has no electrons to donate in this case. Because of the nature of NH3 as a possible bound group on an alkyl structure, it makes a good leaving group—as opposed to the NH2 molecule, which is infinitely more stable. Amines are considered more basic than alcohols. The pKa of RH3+ is around 10, while the pKa of ROH2+ is about -3. Any factor, such as electronegativity and resonance, that affects the availability of the lone pair of electrons will affect the basicity of the nitrogen atom. Nitrogen is less electronegative than oxygen and will therefore be a better electron donor. As mentioned, aryl amines are stronger acids, less basic, than an alkyl or non-aromatic heterocyclic amine. The order in which this goes in terms of increasing basicity is ROH3+, aromatic amines, heterocyclic amines, and finally alkyl amines. You should know that, when deprotonated, the amine group becomes the amide ion, which is NH2-. These are important bases in organic chemistry, made by the reaction with sodium or potassium, which pulls off the hydrogen ion to give an amide group. You should know that the basicity of the amine group on the benzene ring will be increased by the presence of electron donating side chains on the aromatic compound, which will counteract the delocalization of the lone pair of electrons on the nitrogen molecule into the pi-system of the ring. It will be increased by electron withdrawing substances, especially ortho or para substituents as is seen in figure 100:
179
Figure 100.
ALKYLATION OF AMMONIA This involves taking an alkyl halide and, through nucleophilic substitution, adding the alkyl group onto the ammonia (NH3) molecule. This leaves behind RNH2 plus ammonium halide. It is referred to as a nucleophilic substitution reaction because nitrogen acts as a nucleophile and substitutes the halide for itself, removing a hydrogen from an intermediary by the remaining ammonia molecule. The reaction looks like that seen in figure 101:
Figure 101.
180
The biggest problem with this is that the RNH2 molecule is also nucleophilic so that it reacts with more alkyl halide to give a secondary or tertiary amine molecule. The end result is that there will be a mixture of primary, secondary, and tertiary amines, and quaternary ammonium salts unless a large excess of ammonia is used (which favors a primary amine). Note, this reaction cannot be done with aryl halides because they do not undergo simple nucleophilic substitution.
REDUCTION OF NITROGENOUS COMPOUNDS First of all, what is an azide? It is a RN3 molecule, in which an alkyl chain is attached to a nitrogen, that is attached to another nitrogen, that is attached to still another nitrogen. This will have a positive charge on one nitrogen and a negative charge on the other but it is still unstable and easily reduced to a RNH2 molecule through the reduction process shown in figure 102:
Figure 102.
Figure 102 shows the reduction of nitriles as well, which also gives rise to a primary amine molecule. These are oxidation-reduction reactions that get reduced through the addition of different reagents. With RN3, the reducing agent is either LiAlH4 (lithium aluminum hydride) or catalytic hydrogenation with hydrogen gas and palladium.
181
Nitriles, which are RCN molecules can be reduced to a primary amine, adding four hydrogen ions to make RCH2NH2. This will also take a hydrogenating compound such as lithium aluminum hydride or a combination of hydrogen gas and palladium as catalysts. The alkyl nitrile itself can be made by substituting a CN- cyanide ion onto an alkyl halide molecule. In addition, it is possible to reduce an aryl nitrile to an aryl amine. It is also possible to reduce a nitro compound, which is a RNO2 molecule, to make a RNH2 molecule. This can be accomplished with the R side chain being an aryl group. It is a simple oxidation-reduction reaction. The reducing agents can be iron/H+, selenium/H+, or catalytic hydrogenation using H2 gas plus palladium as a catalyst. This is called the reduction of nitroarenes. Nitroarenes themselves are prepared by the nitration of aromatics with HNO3 (nitric acid) in H2SO4. The ArNH2 molecule is called an aniline. An amine compound can also be made by mixing lithium aluminum hydride with an amide, which is RCONH2. This can remove the oxygen and add two hydrogen molecules to the carbonyl carbon in a nucleophilic acyl substitution plus a nucleophilic addition reaction, the summary of which is seen in figure 103:
Figure 103.
This is accomplished by reducing the CO double bond, which starts in an amide with a +3-oxidation number and reduces it to a -1-oxidation number (which is -2 from the hydrogen atoms and +1 from the nitrogen atom). It involves the leaving of oxygen rather than nitrogen because oxygen is a better leaving group when compared to nitrogen. You should know also that there can be primary, secondary, or tertiary amides, depending on the number of side chains on the nitrogen atom. The example in figure
182
103 is that of a primary amide because it just has the one side chain (RCO) on the nitrogen atom.
REDUCTIVE AMINATION VIA IMINES This creates an amine group, albeit a complex one, from an aldehyde or ketone. The reaction proceeds through the reaction described in figure 104:
Figure 104.
This reaction starts with an aldehyde or ketone and has an imine intermediate, which replaces the oxygen with an amine group via a double bond. This can also give rise to an enamine, which is a fully substituted amine group attached to an alkene at the alpha carbon atom. Figure 105 describes an imine and enamine molecule.
Figure 105.
AMINE REACTIONS The alkylation of amines can be done in several ways. It can be done with a nucleophilic substitution reaction. The starting point is an alkyl halide and a primary amine. The end product is a secondary amine with the alkyl group affiliated with it. The biggest
183
problem with this reaction is that the secondary amine is still nucleophilic so that a mixture of amines in various states of alkylation result, including the quaternary salt compound, which binds to the halide in a salt. This can be demonstrated in figure 106:
Figure 106.
This is similar to starting with ammonia and adding an alkyl group via an alkyl halide. The process just keeps on alkylating the nitrogen group.
PREPARING AMIDES There are a couple of ways to make an amide compound, which is a carbonyl group attached to an R chain and a nitrogen group. It can first be done through an acid chloride molecule, which is an RCOCl molecule—a molecule that can be seen as able to add an acyl group (an RCO group) to a nitrogen compound (an amine) in basic conditions, yielding an amide. A second way is through the addition of an amine to an acid anhydride, also under basic conditions. How these can be done is shown in figure 107:
Figure 107.
184
These reactions are referred to as nucleophilic acyl substitution.
NITROSATION OF AMINES This involves the addition of a primary amine with nitrous acid (HNO2). The reaction will yield a diazonium ion (RNN+) plus water. It can also involve the nitrosation of a secondary amine to yield N-nitrosamine, which is RR’NNO plus water. This is demonstrated in figure 108:
Figure 108.
This type of reaction involves sodium nitrite and an acid, yielding nitrous acid. These can be mixed with aryl amines, in particular to give aryl diazonium salts, which are used to make substituted benzene molecules. Nitrous acid will lose water to make an NO (nitrosyl) cation, which is the actual reactant in this reaction. When this is done with an aryl amine, it yields and aryl-NN molecule (which is highly unstable) that gives off N2 gas and an aryl plus cation, which can easily be substituted to make a substituted benzene group. There are many things that can be made by making first an aryl diazonium ion. It can be mixed with copper chloride or copper bromide to make benzyl chloride or benzyl bromide, respectively. It can be mixed with phenol to make an azo compound. Any halide, in fact, can be added to the aryl diazonium ion to make a halogenated benzene compound, and phenol itself can be made because of the carbo-cation made from the
185
aryl diazonium molecule. Copper cyanide can be mixed with the aryl diazonium ion to make an aryl cyanide compound. This addition of copper cyanide or copper halide to make the substituted benzene compound is referred to as a “Sandmeyer reaction”.
186
KEY TAKEAWAYS •
Amine compounds can be primary, secondary, tertiary, or quaternary, based on the number of alkyl groups substituting for the hydrogen atom on the nitrogen molecule.
•
Nitrogen has a -1-oxidation number, which will affect the oxidation number of the carbon atoms near it.
•
The nitrogen atom has various degrees of nucleophilicity in amine compounds, which affect the reactivity of amine compounds.
•
There are many nitrogenous compounds in nature, some of which need memorizing and others just noting as being important nitrogenous molecules.
•
The carbon-nitrogen bond is polar, affecting the physical and chemical properties of these molecules in comparison to the corresponding alkane.
187
QUIZ 1. An amine called dimethyl ethylamine is what type of amine compound? a. Primary amine b. Secondary amine c. Tertiary amine d. Quaternary amine Answer: c. This amine clearly has three alkyl groups associated with it, making it a tertiary amine. A primary amine has one alkyl or aryl group, while a secondary amine has two alkyl or aryl groups attached to it. 2. What would the charge be on tetramethylammonium, which has four alkyl groups attached? a. +1 b. +2 c. +3 d. 0 Answer: a. This would be an ammonium ion that would have a single positive charge associated with it, which would essentially be no different from a regular NH4+ ammonium cation. 3. Which amine compound is charged? a. Primary amine b. Secondary amine c. Tertiary amine d. Quaternary amine Answer: d. A quaternary amine has four alkyl groups around a nitrogen compound. Because neutral nitrogen compounds have three hydrogen atoms or up to three alkyl groups, having a fourth would mean there is a positive charge on the compound.
188
4. What is something that will not happen to a compound by virtue of having an N-H bond versus an alkane of the same size? a. Decreased water solubility b. Increased melting point c. Increased boiling point d. Increased hydrogen bonding Answer: a. This NH bond will be polar, leading to increased hydrogen bonding, increased melting point, increased boiling point and increased water solubility. 5. In the nucleophilic substitution reaction that makes up an alkyl amine, what is the reactant besides ammonia? a. Alkane b. Alcohol c. Carboxylate d. Alkyl halide Answer: d. The alkyl halide group is the easiest to substitute with the amine group, with the nitrogen being the nucleophile and the alkyl carbon being the electrophile. 6. How can the reaction of an alkyl halide and ammonia yield mostly a primary amine versus a mixture of amines? a. Limit the ammonia b. Have a large excess of ammonia c. Make the reaction in basic circumstances d. Make the reaction in acidic circumstances Answer: b. The reaction will yield a majority of a primary amine if the reaction is done with a large excess of ammonia in the reaction.
189
7. What is the oxidation state of the carbon atom in the RCH2NH2 molecule? a. +3 b. 0 c. -1 d. b. -3 Answer: c. The hydrogen atoms are positive, leading to a -2-oxidation state. This is added to the +1 for the nitrogen-carbon bond, leading to a total of -1 oxidation state for the carbon molecule. 8. What is a good reducing agent for the reduction of azides and nitriles? a. H2SO4 b. H2 gas c. HCl d. LiAlH4 Answer: d. The best reducing agent for these types of reactions is LiAlH4, which provides a great number of hydrogen ions. H2 gas can do this but it requires a palladium catalyst. 9. What can be added to an amine molecule to make an amide molecule? a. Ketone b. Acid chloride (acyl chloride) c. Aldehyde d. Imine Answer: b. This takes an acid chloride (acyl chloride) or alternatively an acid anhydride. These will be able to be separated in an acyl nucleophilic substitution reaction to make an amide molecule. These reactions are possible because of the nucleophilicity of the nitrogen molecule when compared to that of the oxygen and chloride molecule in acid chlorides and acid anhydrides.
190
10. The nitrosation of aryl compounds will lead to aryl diazonium cations, which are unstable and lead to an aryl group that can be substituted with a variety of compounds. What is the reactant in the nitrosation reaction of primary amines besides the amines? a. NH4+Cl b. HNO3 c. HNO2 d. Alkyl nitrate Answer: c. The reactant is an aryl amine plus the HNO2 molecule or “nitrous acid”. This gives rise to the diazonium cation, an unstable molecule that forms a carbo-cation (a positively-charged carbon atom), also an unstable molecule that can be substituted.
191
CHAPTER 12: CARBOHYDRATES IN ORGANIC CHEMISTRY This chapter begins to make sense of what you have learned in previous chapters on simpler molecules and applies it to more complex biochemical molecular structures. Sugars and carbohydrates are basically organic molecules that come from the phrase “carbon hydrates”. They contain only carbon, oxygen, and hydrogen atoms and have a specific generic formula, based on whether they are simple sugars, disaccharides, or more complex polysaccharides. The main focus of this chapter is to use organic molecular principles that will make sense now that you know the basic reaction types involved.
NOMENCLATURE AND NAMING OF CARBOHYDRATES While carbohydrates are complex, they can be referred to as polyhydroxy aldehydes or polyhydroxy ketones. The chemistry of these molecules involves the chemistry of the functional groups you already know about. While there are a lot of different carbohydrates, there are just a few that you should probably memorize. These tend to be important in biochemical circles and are found as ring structures and as linear molecules. Figures 109 and 110 describes some molecules you should know:
Figure 109.
192
Figure 110.
You should also be familiar with the disaccharides: sucrose, which is glucose and fructose, lactose, which is glucose and galactose, and maltose, which is glucose and glucose. Common polysaccharides are starch (glucoses), glycogen (glucoses), cellulose (glucoses), and chitin (glucoses). Other things you need to know include the following: •
An acetal is the product of the reaction with an aldehyde and excess alcohol, leading to a carbon molecule that has two OR groups on it. It can be a complex molecule that must have two oxygen molecules attached to a carbon molecule.
•
An aldose is a carbohydrate that is in the open-chain form with an aldehyde group associated with the end of it. It will have alcohol groups on the other carbons.
•
The anomeric center is the carbon atom that has two Carbon-Oxygen single bonds associated with it. It is the carbon atom most linked to an acetal molecule.
•
The anomeric effect is the preference for polar side chains to be axial at the anomeric center.
•
Anomers are two stereoisomers that differ in the side chain arrangement at the anomeric center of the molecule.
•
A disaccharide is a two-monomer carbohydrate with two simple carbohydrate molecules connected together.
193
•
D-sugar is a stereochemistry definition that describes the chirality of the saccharide relative to the chirality of glyceraldehyde.
•
A furanose is any sugar that, in cyclic form, describes a five-membered ring.
•
A glycoside is a carbohydrate that has been substituted at the anomeric center with an OR group rather than an OH group. The glycosidic bond is the bond linking the side chain to the anomeric center.
•
A Hexose is any carbohydrate that consists of six carbon atoms, regardless of its configuration.
•
A Pentose is any carbohydrate that consists of five carbon atoms, regardless of its configuration.
•
A Tetrose is any carbohydrate that has four carbon atoms, regardless of its configuration.
•
A Ketose is any carbohydrate that, in its open form, makes a ketone at one of its carbon atoms.
•
A Pyranose is the cyclic form of a six-membered sugar with five carbon atoms and an oxygen molecule.
•
Saccharide is another name for sugar; a disaccharide has two monomers; an oligosaccharide has 3-10 sugars; a polysaccharide has more than 10 sugars or monomers associated with it.
You should also know these structures as they are important in the organic chemistry of carbohydrates. These are shown in figure 111:
Figure 111.
194
A hemiacetal is the product of a reaction between an aldehyde and an alcohol. A hemiketal is the product of the reaction between a ketone and an alcohol. A ketal is the product of a reaction between a ketone and a large amount of alcohol. Carbohydrates are determined by the number of sugar monomers associated with them. While we talk about just a few, there are more than two hundred known monosaccharides. Disaccharides, oligosaccharides, and polysaccharides are made by the hydrolysis of different monosaccharide monomers together. The disaccharide sucrose, for example, is made from a glycosidic bond between glucose and fructose.
GLYCOSIDES Glycosides are important in sugar chemistry. It takes a hemiacetal sugar and alcohol under acidic conditions to make an acetal or a glycoside. It is in simplified form in figure 112 but is very complex when you remember that a sugar molecule consists of many alcohol groups that can act as the “alcohol” and alcohols basically have a hemiacetal group at the anomeric carbon:
Figure 112.
In such cases, the ROH is another OH bond attached to another sugar, leading to a disaccharide that can go on to add more and more sugars to make oligosaccharides and polysaccharides. In the case of sucrose, both anomeric carbons are involved in an acetal or glycosidic bond, so that the molecule is not self-replicating as the anomeric carbon is “used up” on both the glucose and fructose molecules, making no other anomeric carbon available for bonding. Figure 113 shows the sucrose molecule:
195
Figure 113.
Furanoses are five-membered rings and pyranoses are six-membered rings. These are relatively stable cyclic compounds because they are relatively free of ring strain. These are similar to the molecules seen in organic chemistry, such as tetrahydrofuran (a fivemembered carbon and oxygen ring that is fully saturated) and tetrahydropyran (a sixmembered carbon and oxygen ring that is fully saturated). Fructose is considered a furanose and glucose is considered a pyranose because of the number of carbons in the chain and the fact that they are not saturated. In addition, Dglucose is referred to as an aldose or aldohexose, while fructose is called a ketose or ketohexose. To be an aldose, the sugar needs to have an aldehyde group on it; to be a ketose, the sugar needs to have a ketone group.
REDUCING SUGARS Reducing sugars are those sugars that will reduce copper from copper (II) to copper (I)—effectively reducing the oxidation number of the copper ion from +2 to +1. They must contain an aldehyde group that itself is oxidized to carboxylic acid. So, when it comes to sugars, the sugar needs to be an aldehyde sugar that opens to make a reactive aldehyde. This also means that the sugar must be hemiacetal, which rules out glycosides that cannot be hemiacetals (these are acetals). Ketoses can also be reducing sugars because they can isomerize to become aldoses through the enediol intermediate.
196
SUBSTITUTED SUGARS Substituted sugars can be many types of things in nature. A hydrogen atom can be the substituted group, making a deoxy sugar. An amino sugar has the NH2 group attached to it or a more substituted amino molecule. Finally, any R chain can be substituted for the OH group. By definition, a substituted sugar is substituted at the OH side chain.
ALPHA AND BETA ISOMERS Cyclic sugars can exist in alpha or beta forms, based on the position of the side group on the anomeric carbon. These are isomers referred to as anomers because it strictly involves side chain positioning on the anomeric atom. The alpha sugar has the RO- or HO- side chain on the opposite side of the CH2OH group. The beta sugar has the ROor HO- side chain on the same side as the CH2OH group. These will rapidly change from one to another so they can’t always be isolated from one another. Basically, you need to look at the oxygen group that is part of the cyclic molecule. On one side will be the CH2OH group and on the other side will be the RO or HO group. If the side chains are on the same face side of the ring, it is called a beta sugar; if the side chains are on the opposite face side of the ring, is called an alpha sugar. Figure 114 shows the difference between the two sugars in alpha and beta form:
Figure 114.
197
The preference in rings that have an oxygen in them that also have electronegative side chains is that the side chain be axial rather than equatorial. Axial, if you remember, means the side chain is perpendicular to the ring, while equatorial means the side chain is in the same plane as the ring. The concept of mutarotation involves the interconversion of alpha and beta anomers. These anomers will be stable in solid form but, in aqueous solutions, there will be a mixture of alpha and beta forms with a slight preference over the beta form. This happens because the ring opens and closes, shifting the position of the RO or HO group, forming a carbonyl group in between. Fischer projections of carbohydrates are done by writing the carbon atoms out vertically and placing the carbonyl carbon atom closest to the top of the molecular structure. The S form of glyceraldehyde, a simple three-carbon sugar has the hydroxyl group on the left, while the R form has the hydroxyl group on the right. The S form is the minus form and the R form is called the positive form. In carbohydrate chemistry, the S or minus form is referred to as the L form, while the R or plus form is called the D form. All other determinations for other carbohydrates are based on the chirality of glyceraldehyde.
REACTIONS OF CARBOHYDRATES There can be several different reactions of carbohydrates—all of which happen at the functional group. The reactions occur at the alcohols, aldehydes, ketones, ketals, and acetals. What you already know about organic chemistry can be applied to carbohydrates. What follows are examples of reactions of the functional groups of carbohydrates.
ALKYLATION OF CARBOHYDRATES Alkylation of carbohydrates can occur on one or all hydroxyl groups. There are five hydroxyl groups on any given hexose sugar, any of which can be alkylated. Under basic conditions (which pulls off the hydrogen ion, an alkyl halide can be used in a nucleophilic substitution reaction to alkylate the hydroxyl groups. Nothing happens to the cyclic ring. Silver oxide is used as a catalyst. Under aqueous acid conditions, the 198
glycoside bond can be broken, giving back the cyclic monomer from a polysaccharide after blocking the rest of the OH side chains with a methyl (alkyl) group.
ACYLATION OF CARBOHYDRATES This type of reaction occurs at the hydroxyl site using acid chloride, acid bromate, or acid anhydride as reactants along with the carbohydrate. If pushed in the forward direction, it adds an RCO side chain to the hydroxyl groups on the carbohydrate, yielding multiple esters. This is usually an ethanoate side chain or “acetic”. This acts as a protecting group, which is the same thing that happens with alkylation, leaving the glycosidic bond intact. As before, acid conditions can uncover the hydroxyl group again from a glycosidic-bonded sugar.
REDUCTION OF CARBOHYDRATES This reaction acts on the ketone or aldehyde (carbonyl) carbon of the carbohydrate molecule. It makes use of sodium borohydride (NaBH4) and the carbonyl carbon to make an alcohol group, taking the carbon oxidation number from +2 to 0 in a ketone group and from +1 to -1 in an aldehyde group. The word to describe the side products is “alditols”. This is a nucleophilic addition reaction, reducing aldoses to primary alcohols and ketoses to secondary alcohols. Normally, LiAlH4 (lithium aluminum hydride) would be used in these types of reactions but it is not polar and does not dissolve well in the solvents that dissolve carbohydrates.
OXIDATION OF CARBOHYDRATES This has been discussed before when talking about reducing sugars. The reaction takes copper II and reduces it to copper I, oxidizing the open-chain form of an aldehyde sugar, resulting in a carboxylate molecule on the sugar. The sugar must be an aldehyde sugar like galactose, glucose, glyceraldehyde, fructose, ribose, and xylose. Note that fructose is a ketose sugar but it can tautomerize to make an aldehyde.
199
HYDROLYSIS OF SUGARS This is basically the reaction of polysaccharides, disaccharides, or oligosaccharides to make monomer monosaccharides. The OR bond gets cleaved, with replacement by an OH or hydroxyl group plus an alcohol of the RO molecule to make and ROH. This will result in a mix of alpha and beta isomers and is actually an acetal or ketal hydrolysis reaction.
GLYCOSIDIC BOND FORMATION This uses acid catalysts and an alcohol group ROH to make a glycosidic linkage out of the hemiacetal or hemiketal in a sugar. It adds the RO side chain to the sugar molecule at the anomeric carbon atom, effectively adding one sugar to the next. It pulls off the HO group to make ROH plus water, making a glycosidic linkage. What this looks like is seen was shown in figure 112. This leads to an acetal as an end product.
200
KEY TAKEAWAYS •
There are more than 200 monomer sugars, only a few of which are important in organic chemistry.
•
Sugars are carbon hydrates because every carbon atom but one has a hydroxyl group associated with it.
•
You should know the different common monosaccharides, disaccharides, and polysaccharides in nature.
•
The reactions of sugars involve the functional groups, mainly the hydroxyl group or the carbonyl group, which ultimately becomes an acetal or hemiacetal in most carbohydrate molecules.
201
QUIZ 1. Which is not considered a polysaccharide but is instead a disaccharide? a. Cellulose b. Chitin c. Maltose d. Glycogen Answer: c. Each of these is a polysaccharide made from different connections of glucose to each other with the exception of Maltose, which is also made from glucose but is a disaccharide. 2. Which six-carbon simple carbohydrate forms a five-membered ring? a. Fructose b. Galactose c. Glucose d. Ribose Answer: a. Fructose is a six-carbon sugar that is made into a fivemembered ring because of the location of its carbonyl group, which is a ketone and is not an aldehyde. Both galactose and glucose are sixmembered rings and ribose is a five-carbon sugar. 3. The product of a reaction of an aldehyde with an equivalent number of alcohol units is called what? a. Acetal b. Hemi-acetal c. Ketal d. Ketose Answer: b. A hemiacetal is a molecule that represents the reaction between an aldehyde and an equivalent number of alcohol molecules. The structure can be complex but is basically RCHOROH.
202
4. How many oxygen atoms, by definition, are around the anomeric carbon center? a. Zero b. One c. Two d. Three Answer: c. The anomeric carbon has, by definition, a bond with two oxygen atoms at the same time. 5. What best describes glucose as a molecule? a. Pentose b. Aldohexose c. Furanose d. Ketohexose Answer: b. Glucose can be named many things. It is a hexose sugar, an aldohexose, an aldose, and a pyranose. It is not a pentose because it has six carbon molecules; it is not a ketohexose because it does not have a ketone group; it is not a furanose because it is a pyranose, a cyclic six-membered ring. 6. What type of sugar cannot possibly be a reducing sugar, being unable to reduce the copper molecule? a. Hemiacetal b. Ketose c. Acetal d. Aldose Answer: c. The sugar that becomes or is a reducing sugar must be able to be an aldehyde in its open form. This means that it can be a hemiacetal and, because of tautomerization, it can be a ketose sugar but it cannot be an acetal because it cannot be oxidized into anything.
203
7. In the glucose or hexose molecule, where does mutarotation occur? a. At the carbon that has a hydroxyl group b. At the terminal carbon atom c. At the hydroxyl group d. At the anomeric carbon atom Answer: d. Mutarotation refers to the change between the alpha and beta forms that occurs through the carbonyl intermediate. The carbonyl carbon is the same thing as the anomeric carbon atom. 8. What form is different from the rest when it comes to stereochemistry of sugars? a. L form b. Plus form c. S form d. Minus form Answer: b. These are confusing ways of describing sugars. The forms most commonly used are L and D. The L form has the OH side chain on the left of the molecule when written in Fischer form. L, minus, and S are the same thing but the “plus form” is the D or R form. 9. What is the substrate used to alkylate the hydroxyl groups on a carbohydrate in carbohydrate chemistry? a. An alkyl hydride b. Another alcohol c. An aldehyde d. An ether Answer: a. An alkyl hydride under basic conditions can cover each of the hydroxyl groups with an alkyl group, effectively blocking these groups from further reactivity, forming multiple ethers with the same side chain.
204
10. In the acyl addition reaction to carbohydrates, what type of product is formed? a. Aldehyde b. Ester c. Ether d. Ketone Answer: b. These will use acid anhydride or acyl halides to add the acyl group onto the alcohol, leading to multiple esters on the hydroxyl side chain.
205
CHAPTER 13: AMINO ACIDS, PROTEINS, AND PEPTIDES IN ORGANIC CHEMISTRY The focus of this chapter is to bring on more of the biochemistry involving organic chemistry principles by talking about the organic chemistry of amino acids, oligopeptides, and proteins. These are molecules that have nitrogenous compounds as the basis of their chemistry and that, like carbohydrates, exist as monomer units and polymers or polypeptides. These will also have reactions at their functional units, which involve a variety of different side chains and parts of the parent chain.
AMINO ACIDS Amino acids are called as such because they must contain an amine group and a carboxylic acid group. These will be joined through amide bonds to yield oligopeptides and polypeptides. A polypeptide is called a protein if it has at least fifty amino acids. These are important structural and biochemical molecules seen in all forms of life. While there are more than 700 naturally occurring amino acids, only 20 or so are seen in protein formation in living things. The simplest amino acid is glycine, which is NH2CH2COOH. This is referred to as an alpha amino acid because it has the NH2 amino group on the alpha carbon of the carboxylic acid. There can be a beta amino acid involving a nitrogen compound on the beta group, such as beta-alanine. There is also a gamma amino acid, involving an amino group on the gamma-carbon of the carboxylic acid. The most important amino acids in nature are referred to as alpha-amino acids because it is the alpha group that has the amine group on it, giving it the formula RCHNH2COOH. These lead to the 20 amino acids found in proteins, which differ only in their side chain. You should know that the close proximity of the amine group and the carboxylic acid group leads to a positive charge on the amine group and a negative charge on the carboxylic acid group.
206
Amino acid R groups can be nonpolar, polar, acidic, or basic. Proline is unique among all amino acids in that it is the only amino acid in which the amine group is not a primary amine as you will see when we show the figure of the amino acids. Nonpolar amino acids have alkane and related hydrophobic side chains. These will not be wholly soluble in water and will become facing inward when the amino acids form proteins. This is referred to as the “hydrophobic effect”. Figure 115 shows the different amino acids seen in nature:
Figure 115.
Note that proline is a cyclic amine compound that is not a primary amine. Methionine and cysteine have sulfur in them, although only cysteine has a thiol component that can form a disulfide bond. Phenylalanine, tryptophan, tyrosine, and histidine have aromatic rings but only phenylalanine and tryptophan are nonpolar. Amino acids with polar side chains are important in hydrogen bonding. Cysteine will form the disulfide bond. Polar side chains will have amine groups or hydroxyl groups associated with them. These include tyrosine, which has a benzene ring with a hydroxyl group on it, making it polar. Acidic amino acids include aspartic acid and glutamic acid, 207
which has a carboxyl group as its side chain as well as at its carboxyl end. These are, in a sense, dicarboxylic acids. Basic amino acids include lysine, arginine, and histidine, which have amine groups as part of their side chains. All amino acids have acidic and basic components as they each will have a carboxylic acid and an amine group. These are considered amphoteric as they can behave as acids or bases. As you probably remember from general chemistry, the rate the reaction of HA going to H+ and A- or the dissociation of an acid is called the Ka. The pKa of a reaction is the negative log of the Ka so that the lower the pKa, the stronger is the acid. Simple carboxylic acids have a pKa of around 5 and simple ammonium compounds (RNH3+) have a pKa of about 9. This means that, at normal physiological pH levels, the amino acid will exist as a zwitterion with charged carboxylic acids and charged amino groups. Figure 116 shows this zwitterion form:
Figure 116.
The isoelectronic point or iso-ionic point is the pH at which the amino acid doesn’t migrate in an electric field. This is the pH at which the amino acid is neutral or when the zwitterion form is dominant. Amino acids will vary significantly with regard to their isoelectronic point. You need to consider that there can be neutral side chains, acidic side chains, or basic side chains. The neutral side chains will have a pKa1 and a pKa2 for the carboxylic acid and the amine group, respectively. The isoelectronic point, is the average of the pKa1 and the pKa2. For the simplest amino acid, glycine, the pKa1 is 2.34 and the pKa2 is 9.6, with an average of these being the pI or isoelectronic point of 5.97. In low pH conditions, there will be a net positive charge on the amino acid; in high pH conditions, there will be a net negative charge on the amino 208
acid; at a neutral pH, between the pKa1 and the pKa 2, the net charge will be zero, with the zwitterion produced. There will be a third pKa called the pKa3 when the R side chain has its own acid or base. In acidic side chains, the pI will be lower because of an extra acidity to the amino acid. In basic side chains, there will be a higher pI because of the extra basicity of the amino acid. In such cases the pI is halfway between the pKa1 and the Pka3 (with acidic amino acids) and halfway between the pKa2 and pKa3 (with basic amino acids). You do not need to memorize the different isoelectronic points but you should know that the one with the lowest pI is aspartate/aspartic acid at 2.77 and the one with the highest pI is arginine at 10.76. Only five amino acids have either acidic or basic side chains, making the chosen pI or isoelectronic point not being the average of the pKa1 and pKa2.
AMINO ACID STEREOCHEMISTRY There is stereochemistry associated with amino acids as well, with both D- and L- forms you should know about. In the Fischer projections of amino acids, the carboxylic acid or most oxidized form of carbon atom is on the top. The chiral center in the amino acid is the alpha center. The naturally-existing amino acids will have the NH3+ side chain on the left of the molecule, making it an L-form (similar to the glyceraldehyde molecule with the OH on the left side). L-amino acids are the ones seen in nature, although, of course, D-amino acids also exist.
AMINO ACID SYNTHESIS Amino acids can be synthesized in several ways. First, there can be a nucleophilic substitution reaction at the alpha carbon of the carboxylic acid. This is only the case if the alpha carbon is brominated or chlorinated. It can be mixed with ammonia to make ammonium bromide or ammonium chloride plus the amino form of the alpha carbon (an amino acid).
209
Second, there can be a Strecker synthesis, in which an aldehyde is mixed with ammonium chloride and sodium cyanide to make an intermediate that is then heated and acidified to make a carboxylic acid as is shown in figure 117:
Figure 117.
In the Strecker synthesis, there is nucleophilic addition first and then nucleophilic acyl substitution—essentially two parts to the reaction. It starts with ammonium chloride and sodium cyanide, which add an ammonium group and a cyanide group to an aldehyde. This gives rise to an amino nitrile intermediate. Hydrolysis then converts the nitrile molecule into the carboxylic acid molecule to give rise to the alpha amino acid.
REACTIONS OF AMINO ACIDS These are reactions that take place on the amine side chain and the carboxylic acid side chain. These are not much different from reactions that we have already covered in this course. The most important reactions that take place are those that happen in the course of making peptides and protein molecules. When it comes to amines, there is acylation to form amides that has already been talked about. The R2NH molecule is acted upon by acid chlorides or acid anhydrides under basic circumstances to make an amide compound. This has already been shown in figure 107 in a previous chapter. This is how amides get formed in the making of peptides.
210
TERMINOLOGY FOR PROTEINS AND PEPTIDES Amines can react with carboxylic acids to form amides, which are RCONH2 molecules. Proteins are basically amino acids linked by amide bonds, making proteins basically polyamides. Peptides are short proteins less than fifty amino acids in length. The peptide bonds in proteins are basically the same thing as the amide bond. A dipeptide has two amino acids, while a tripeptide has three amino acids. The amino acid sequence is the way proteins are ordered, written with the amino acid end on the left and the carboxyl group on the right.
AMIDE BOND There is some resonance with the C=O bond and the C-N bond in the amide molecule. This is because there is a planar sp2 N system in alignment with the pi-bond system of the C=O bond. Two pairs of electrons essentially flow between the NCO bond pairs. This makes the bonding structure of the CN bond less flexible and this CNO structure in a planar fashion. The amide bond will be somewhat unreactive when compared to other bonds. This lowers the general reactivity of proteins.
DISULFIDE BONDS We’ve talked about disulfide bonds that exist with cysteine in protein systems. Cysteine has a thiol group that is reactive through oxidation to form a disulfide bond, which is a RSSR bond, making a protein molecule a better three-dimensional molecule. This is one of the main ways that proteins hold their shape as these are relatively stable bonds. The disulfide bond can be within the same protein chain or intermolecularly—between different chains. Hydrogen bonding is the bonding of hydrogen and electronegative atoms, such as oxygen or nitrogen in the amino acids. Hydrogen is electron-poor and is attracted to the lone electron pairs on the nitrogen or oxygen atoms, being an attractive force that adds to the three-dimensional shape of the protein molecule. The hydrogen on the NH group will have hydrogen bonding with the carbonyl oxygen of the amino acid.
211
SEQUENCING AMINO ACIDS The way to find the sequence of amino acids in a protein is to hydrolyze the protein with heat and acid. This will break all the bonds unselectively and the amino acids can be separated using chromatography techniques with a ninhydrin reaction used to determine how much of each amino acid is present. The enzymatic hydrolysis using proteases is more selective and will break the protein into fragments that can help determine the order of the amino acids in the chain. Examples of proteases include trypsin, which cleaves lysine or arginine at the C=O bond of these amino acids. Chymotrypsin will cleave the C=O bond where the amino acid is phenylalanine, tyrosine, and tryptophan—all three having aromatic side chains. Carboxypeptidases will only cleave the protein at the C-terminus. These can be used together to piece together the structure of the protein.
PROTEIN STRUCTURE There are four different protein structures involved in identifying proteins. These include the following: •
Primary structure—this is the amino acid sequence of the protein or polypeptide molecule. These are the covalent bonds between the different amino acids.
•
Secondary structure—this is the conformational relationship of nearby amino acids, which are controlled by the planarity of the amide bond and the hydrogen bonding between C=O and NH subunits within the peptide structure.
•
Tertiary structure—this the chain folding of proteins that make them fibrous or globular and are determined by hydrogen bonding, hydrophobic effects, and disulfide linkages between cysteine units.
•
Quaternary structure—this is the “holding together” of different subunits that together make a whole protein structure. It involves different interactions,
212
like van der Waals forces, hydrogen bonding, disulfide bonding, and ionic bonding that hold two polypeptide structures together as a whole unit.
ALPHA HELICES AND BETA PLEATS Alpha helices are protein structures that are made because the amide backbone of the peptide is arranged in a right-handed spiral. The side chains are sticking out away from the spiral. This type of helix is named by hydrogen-bonding between the carbonyl oxygen and the amide subunits situated exactly four amino acid residues away. Beta pleats are named because the amide backbone of the peptide is arranged in a zigzag fashion. The side chains exist on either side of the sheet with stabilization of the protein by hydrogen bonding with carbonyl units on one strand with an amide NH unit on another strand.
PEPTIDE SYNTHESIS In order to control the way two amino acids get bonded together, there needs to be a protecting group on the amine group and the carboxylic acid group of opposite amino acids in order to guarantee that just two amino acids get attached to one another and that they get attached in a specific order. After putting on a protecting group and binding the amino acids together, the protection can be removed. What kinds of protecting groups are used? For amine side chains, there is the benzyloxycarbonyl side chain attached to the amine group by the addition of benzyloxycarbonyl chloride to the amine group. This can be removed with catalytic hydrogenation (involving H2 gas and palladium), which makes methylbenzene, CO2, and an unprotected amine group. It can also be removed with hydrogen bromide (HBr) that will brominate the methylbenzene group, leaving behind CO2 and an unprotected amine group. In addition, a tert-butoxycarbonyl chloride molecule can protect the NH2 side chain. It can be removed with hydrobromic acid in the same way as the benzyloxycarbonyl side chain. As for the carboxylic acid protection, a variety of ester formation reactions can be
213
used to protect the carboxyl side group. These can also be removed when necessary to recreate a carboxylic acid end that will be able to be added to in further reactions. It is not effective to react the amine and the carboxylic acid components of amino acids because these do not react well together. A coupling agent is necessary to activate the process. One common coupling agent is DCCI, which is N,N’-dicyclohexylcarbodiimide. This is a complex molecule that contains a N=C=N bond between two cyclohexane molecules. It will form an intermediary that helps combine the amino acid amine group and the carboxylic acid side group. Other reactive esters can be used to combine carboxylic acids and amine groups. There is an important method of protein synthesis called the Merrifield method. It involves attaching the N-terminus amino acid to a polymer that does not react and adding one amino acid at a time until the full protein or polypeptide can be created, with the ability to wash off the intermediates between addition reactions.
214
KEY TAKEAWAYS •
There are hundreds of possible amino acids but only twenty seen in the proteins seen in nature.
•
Amino acids are zwitterions at physiological pH values but can be neutral, basic, or acidic when it comes to the amino acid R side chains.
•
The isoelectronic point is the point at which the amino acid is considered neutral in charge. It is based on the pKa of the different side chains.
•
Proteins are groups of amino acids that make amide bonds between the amino acids.
•
There are primary, secondary, tertiary, and quaternary structures with regard to proteins.
215
QUIZ 1. What is the amino acid cutoff, above which a polypeptide becomes a protein? a. 10 b. 20 c. 50 d. 100 Answer: c. A protein is a polypeptide that will be at least fifty amino acids in total length. 2. What is considered the simplest amino acid? a. Glycine b. Lysine c. Isoleucine d. Alanine Answer: a. Glycine is the simplest amino acid, consisting only of NH2CH2COOH, which serves to have the most basic definition of an amino acid, having an amino group and a carboxylic acid side chain. 3. What type of amino acid makes up the twenty amino acids seen in proteins? a. All alpha amino acids b. All beta amino acids c. All gamma amino acids d. A mixture of alpha and beta amino acids Answer: a. All amino acids seen in nature are consisted of alpha amino acids, which means that the amino group is attached to the alpha carbon or the carbon just adjacent to the carboxylic acid carbon. It means that the NH2 side chain becomes NH3+ and the COOH side chain becomes COO-.
216
4. Which amino acid is not basic in nature? a. Arginine b. Histidine c. Lysine d. Aspartate Answer: d. Aspartate is a carboxylic acid amino acid, making it acidic and not basic. The rest of them have an amine side chain, making them basic in nature. 5. Which amino acid has the highest isoelectronic point? a. Lysine b. Glycine c. Histidine d. Arginine Answer: d. While both arginine and histidine are basic amino acids, arginine will have the greatest isoelectronic point at 10.76. 6. In the Fischer projection of a carbohydrate or amino acid, which is not true of these diagrams? a. The most reduced form of carbon is on the top. b. The longest chain is written vertically. c. The aldehyde, ketone, or carboxylic acid is at the top. d. There can be L- or D- forms written with these diagrams. Answer: a. What is true is that the most oxidized form of the amino acid or carbohydrate is on the top of the diagram with the longest chain written vertically and D or L forms written out.
217
7. In making amino acids, there can be nucleophilic substitution reactions at the chiral center, which is the alpha carbon. Which is the molecule at the alpha carbon that must be there to have the NH2 group take the place of? a. Hydrogen b. Hydroxyl c. NO2 d. Halide Answer: d. The alpha carbon must have a halide atom attached to it in order to act as a leaving group so that the NH2 or NH3+ side chain can be substituted there. 8. The Strecker synthesis of amino acids forms an intermediary, which is an amino nitrile. The end result is an amino acid by the addition of what to the amino nitrile molecule? a. Ammonia b. Base c. H3O+ d. HCl Answer: c. The amino nitrile compound has a CN side chain and an amino side chain. What needs to happen is the transformation of CN into COOH. This can only take place with heat and the addition of H3O+. 9. Which type of bonding systems is least reactive? a. Amide bond b. RCN (cyanide bond) c. Amine bond d. Carboxylic acid bond Answer: a. The amide bond is a relatively inactive bond because of the resonance of the CNO bonding system in an amide. This makes sense
218
because proteins in biological systems need to be relatively inactive when compared to other types of bonding systems. 10. What bonding does not help in the forming of the three-dimensional shape of a protein molecule? a. Hydrophobic attractions b. Covalent bonding c. Disulfide bonding d. Hydrogen bonding attractions Answer: b. Each of these attraction forces or bonding contributes to the three-dimensional shape of a protein except for covalent bonding between R chains. There is no covalent bonding that takes place between R chains except for disulfide bonding.
219
CHAPTER 14: LIPIDS IN ORGANIC CHEMISTRY This chapter studies the organic chemistry associated with lipids. The term “lipid” is a broadly reaching term that applies to a wide variety of molecules that are called lipids because of their biochemical nature and their lack of solubility in water. They can range from fatty acids to triglycerides to more complex molecules that are complicated to synthesize and even to understand how they are synthesized in body systems and in organic chemistry models. Lipids have poor solubility in water but are much more soluble in chloroform, benzene, ether, and acetone, which are either nonpolar or weakly polar.
FATTY ACIDS AND TRIGLYCERIDES The basis of the fatty acids seen in biological systems is the three-carbon molecule called glycerol, which has three hydroxyl groups as well. A triglyceride is a storage-form of fatty acids, with alkane or alkene groups attached to each hydroxyl group in a form of ester or “trialkyl tri-ester”. Figure 118 describes glycerol and what a triglyceride looks like:
Figure 118.
With triglycerides, long-chain fatty acids, which are alkane or alkene-based carboxylic acids, may not be the same long chain molecule. These are linked to the glycerol molecule via an ester linkage. Because they are large enough to have high melting
220
points, they are referred to as fats. Fats are what fatty acids are referred to that come from animal sources. Those molecules that come from plants solidify or melt at low temperatures, causing them to be called oils rather than fats. In general, oils have a higher proportion of unsaturated fatty acid groups, often with multiple double bonds per chain. Triglycerides such as beef fat and other animal fats are generally saturated, having no alkene parent chains. Fatty acids have an even number of carbon atoms, usually 16 or 18 carbon atoms. Those found in nature that are unsaturated have a cis-configuration of the alkene chain. The more carbon-carbon double bonds, the lower is the melting point. The listing of fatty acids that you should remember include the following, which are the ones most commonly seen in nature:
SATURATED FATTY ACIDS •
Twelve carbons—lauric acid
•
Fourteen carbons—myristic acid
•
Sixteen carbons—palmitic acid
•
Eighteen carbons—stearic acid
•
Twenty carbons—arachidic acid
UNSATURATED FATTY ACIDS (ALL HAVE 18 CARBONS) •
Oleic acid (one double bond)
•
Linoleic acid (two double bonds
•
Linolenic acid (three double bonds)
There are several important reactions in triglyceride chemistry—most of which we have also talked about. There is the catalytic hydrogenation of the alkenes, which is a major reaction in the saturation process of unsaturated fats. There is also the ester hydrolysis, which is the simple hydrolysis of triglycerides to get fatty acids and glycerol, which is
221
also referred to as saponification. Saponification involves using alkali like sodium hydroxide to get sodium salts of carboxylates (which are collectively referred to as soap) along with glycerol. The process of transesterification is used to make biodiesel. This takes triglycerides and makes methyl or ethyl esters of fatty acids. These can be used for fuel, making them good alternatives to the petroleum products used in fossil fuels.
MICELLES Fatty acid salts have an ionic polar group at the head and a long nonpolar alkane or alkene tail. Theoretically, the polar end will be soluble in water, while the nonpolar tail will be insoluble in water. The polar group is considered to be hydrophilic, while the nonpolar group will by hydrophobic or “lipophilic”. In aqueous solutions, these compounds will form micelles and lipid bilayers. The micelles will be globules of fatty acids that are hydrophilic on the outside and hydrophobic on the inside. The lipid bilayer will be a layer of fatty acids that are polar on the outside and nonpolar on the inside. This is the structure seen for soaps and detergents. Figure 119 shows what a micelle looks like:
Figure 119.
222
PHOSPHOLIPIDS Phospholipids represent unique structures in lipid organic chemistry; they are structurally related to the triglycerides in that they have a polar head and a long polar tail. The main difference is that there is a phosphoric acid group at the polar end of the molecule. The hydroxyl groups on the phosphoric acid subunit can be made into a phosphodiester bond. These will have a phosphatidic acid linkage that will be a precursor molecule in the synthesis of triglycerides. Figure 120 shows a molecule of phosphatidyl choline, which is called “lecithin”:
Figure 120.
The phospholipid bilayer is what is seen in biological systems, such as cell membranes. This will be similar to micelles except that there will be phosphate groups on one side as well as on the opposite side of the membrane with a lipid component sandwiched in between. Figure 121 shows what a lipid bilayer looks like:
223
Figure 121.
PROSTAGLANDINS Prostaglandins are a family of important chemicals in biology and biochemistry—found in many biological structures and tissues. These are a 20-carbon system of atoms that contain a cyclopentane group, a carboxylic acid functional group, and multiple hydroxyl groups. They are made from 20-carbon fatty acids, particularly arachidonic acid. There are several types of prostaglandins. One of these is shown in figure 122:
224
Figure 122.
TERPENES These are mainly found in plants and are what give many plants their fragrance. Terpenes used to be considered strictly hydrocarbons but there are those that are substituted as well. The isoprene unit or C5H8 is the building block of terpene molecules. These are assembled to give rise to terpenes, usually by a head to tail configuration. Figure 123 is the structure of isoprene and the structure of a simple terpene:
225
Figure 123.
Terpenes may be cyclic or linear with more than 23,000 terpenes known in nature. The simple terpenes like myrcene have a molecular formula of C10H15 with multiples of five carbons added to make C15, C20, C25, etcetera, as numbers of carbon atoms per terpene. Terpenes do not have to have double bonds and can be substituted with ketones and hydroxyl groups. Menthol, limonene, and camphor are examples of terpenes. Certain molecules in plants, such as vitamin A and beta-carotene, are considered terpenes. Figure 124 shows terpenes that are fragrant in nature:
Figure 124.
STEROIDS Steroids are lipids by virtue of being lipid soluble and hydrophobic. These are essentially specific types of terpenes that have a common polycyclic framework. Figure 125 shows what a numbered steroid ring looks like with the rings labelled A, B, C, and D:
226
Figure 125.
In biological systems, the most important and common steroid in the human body will be cholesterol. This is relatively flat structure from the side view in three dimensions. Figure 125 shows the numbered steroid, which is cholesterol in this case. Note it has just one hydroxyl group, five methyl groups, and one double bond. There are many different steroids that come from cholesterol, such as cortisol, estradiol, progesterone, testosterone, and norethindrone. The functional groups will react as all the functional groups do in organic molecules. The cholesterol/steroid backbone seems complex but it is just a series of ring closure reactions that take a long-chain fatty acid and close the molecule to make rings. It starts with a squalene molecule, which is a terpene. This undergoes enzymatic oxidation to make an epoxide intermediate. Once folded and enzymatically acted upon, it gives rise to lanosterol, which is a steroid/cholesterol precursor molecule.
227
KEY TAKEAWAYS •
Lipids are diverse but they share the features of being hydrophilic.
•
Fatty acids combine with glycerol to make triglycerides.
•
Molecules with polar ends and nonpolar ends will make micelles or lipid bilayers.
•
Phospholipids will have a phosphate group at one end of it.
•
Terpenes are made from isoprene and make molecules of multiple isoprene molecules.
•
Steroids are a multi-ringed terpene molecule based on a molecule of squalene.
228
QUIZ 1. What type of molecule is a triglyceride? a. A tri-alcohol b. An alcohol aldehyde c. A tri-aldehyde d. A tri-ester Answer: d. Triglycerides are fatty acids linked with an ester linkage three times to the same alcohol-based molecule. 2. What type of molecule is glycerol? a. A diester b. A tri-alcohol c. A tri-ester d. An alcohol Answer: b. Glycerol is a tri-alcohol molecule that is used extensively in lipid biochemistry even though it is not a lipid molecule itself and is otherwise polar in organic chemistry. 3. About how many carbon atoms are there in a fatty acid typically seen in nature? a. 6-8 b. 10-12 c. 12-20 d. 24-28 Answer: c. Almost all fatty acids seen in nature have 12-20 carbon atoms to the fatty acid chain. There are several different fatty acids to remember that exist in nature.
229
4. Which fatty acid is considered the longest among the known and common fatty acids? a. Stearic acid b. Arachidonic acid c. Palmitic acid d. Myristic acid Answer: b. The longest naturally-occurring fatty acid is arachidonic acid, which is a 20-carbon carboxylic acid. All of them are between 12 and 20 carbon atoms in length. 5. What is the substance known as biofuel or biodiesel? a. Aldehydes of unsaturated fatty acids b. Ethers made from combining fatty acids c. Methyl or ethyl esters of fatty acids d. Alkanes or alkenes made from fatty acids Answer: c. Biodiesel or biofuel is the methyl or ethyl esters of fatty acids, made through the transesterification process. 6. What is not true of a micelle? a. It is hydrophobic on the inside and hydrophilic on the outside. b. It is polar on the outside and nonpolar on the inside. c. It is the side chain that is the nonpolar end. d. It is the carboxylic acid end that is nonpolar. Answer: d. It is the carboxylic end that is polar and the long side chain that is the nonpolar end. The micelle will be hydrophobic on the inside and hydrophilic on the outside. This translates to polar on the outside and nonpolar on the inside. These micelles are seen in soaps.
230
7. What is the fatty acid that makes up prostaglandins? a. Linoleic acid b. Linolenic acid c. Arachidonic acid d. Oleic acid Answer: c. Arachidonic acid is a 20-carbon atom molecule that has multiple double bonds that is used to make prostaglandins. 8. The terpenes are ultimately made by adding together which molecule? a. Butane b. Pentene c. 2-pentenol d. Isoprene Answer: d. Isoprene is the molecule that terpenes are made from. Isoprene goes by the IUPAC name of 2-methyl 1,3-butadiene. 9. What is not true of the structure of a steroid? a. It contains 3 hexane rings b. It contains one pentane ring c. It is considered a terpene molecule d. It has a resonant ring Answer: d. These are molecules that contain three hexane rings, one pentane ring, but no resonant ring associated with it, although it can have double bonds associated with the ring in some form.
231
10. Numbering a steroid molecule is difficult. By convention, how are these molecules numbered? a. Starting at the pentane ring b. Starting at the top of the A hexane ring c. Starting at the first substituent or side chain d. Starting from the right-hand side of the molecule as written Answer: b. The numbering system starts at the top of the A hexane ring, and goes in a specific order until all carbons are numbered, ending at the far right-hand side of the molecule as written.
232
CHAPTER 15: NUCLEIC ACIDS AND NUCLEOSIDES IN ORGANIC CHEMISTRY The focus of this final chapter of the course is the organic chemistry and biochemistry of nucleic acids, which are deoxyribonucleic acids and ribonucleic acids, commonly called DNA and RNA. These are molecules that contain ribose or deoxyribose sugars, phosphate groups, and nucleic acid bases, which are seen in paired form with DNA and sometimes with RNA. The transcription of nucleic acids and the translation to proteins is covered as part of the chapter as these are not generally made synthetically in an organic chemistry laboratory.
NUCLEIC ACIDS Nucleic acids are found in the nuclei of all cells as well as in the interior of viruses, being the main component in the transmission of genetic information. These are complex molecules that consist of a carbohydrate (ribose or deoxyribose) component, a phosphate ester linkage, and a heterocyclic aromatic base molecule. The two types of nucleic acids are ribonucleic acids (RNA) or deoxyribonucleic acids (DNA).
PYRIMIDINES AND PURINES These are the nitrogenous bases that make up a key component of nucleic acids. These are planar structures, which becomes important when you come to understand the structure of the nucleic acids in their totality. There are two basic nitrogenous bases: purines and pyrimidines. While purine and pyrimidine themselves are not the nitrogenous bases seen in nucleic acids, it is the derivatives of these two resonant structures that make up the “purines and pyrimidines” seen in nucleic acids. Figure 126 shows the structure of purine and pyrimidine, as well as the structures of the DNA and RNA bases that are derivatives of these bases:
233
Figure 126.
You should know the difference between a purine derivative and a pyrimidine base and remember that pyrimidines are cyclic, while purines are heterocyclic. The derivatives uracil, thymine, cytosine, and guanine are not resonant as they are shown but, because they have a carbonyl side chain as part of the ring, they do show resonance with a negative charge on the oxygen and a positive charge on one of the nitrogen atoms, leaving a neutral molecule. Hence, they are resonant bases.
NUCLEOSIDES A nucleoside is a glycoside (carbohydrate) and a nitrogenous base attached by means of the purine or pyrimidine nitrogen atom bonded to the anomeric carbon of the carbohydrate molecule. In RNA, the carbohydrate is ribose (D-ribofuranose) while in DNA, the carbohydrate is deoxyribose (2-deoxy-D-ribofuranose), which does not have the hydroxyl group at the C2 carbon. The two molecules of sugar involved are shown in figure 127:
234
Figure 127.
The base pairs are also different in RNA and DNA. The bases in RNA are adenine, cytosine, guanine, and uracil, while the bases in DNA are adenine, cytosine, guanine, and thymine. The sugars will all be beta-glycosides, meaning that the CH2OH group and the base are on the same side of the sugar face (which will be roughly planar. The side groups on the nitrogenous base will be facing away the furanose (ribose or deoxyribose). Figure 128 shows a molecule of adenosine, which is an adenine base and a ribose sugar bound together:
235
Figure 128.
NUCLEOTIDES Nucleotides are the nucleoside plus a 5’-phosphate ester. The nitrogenous base is attached to the anomeric carbon of the sugar, while the sugar is in the “middle” attached to phosphate ester. Remember that the only thing that is the same on DNA and RNA is the phosphate part. The nitrogenous bases and the sugar will be different. The phosphate ester is on the terminal carbon of the sugar molecule.
NUCLEIC ACIDS So far, we have discussed nucleosides and nucleotides. A nucleic acid is nothing more than a polynucleotide molecule. They connect at the phosphate groups, coupled at the 3’ and 5’ ends. As mentioned, the nucleotide attaches the phosphate at the 5’ or terminal end of the sugar molecule but, as you can see, they also attach to the 3’ end or the third carbon of the next ribose molecule. This is how a long-chain molecule starts
236
with the base being a part of the molecule but not attached as part of the chain. Figure 129 shows these structures laid out:
Figure 129.
In the figure, P stands for the phosphate molecule, C stands for the carbohydrate molecule, and N stands for the nitrogenous base.
NUCLEIC ACIDS AS BIOENERGETIC MOLECULES Bioenergetics is the study of the thermodynamics associated with biological molecules. There will be changes in free energy, called the Gibbs free energy or delta-G that determines the favorability of a reaction, similar to the change in enthalpy or delta-H that is seen in organic chemistry situations. If the delta-G is negative, the reaction will be favorable and will be exothermic or exergonic (will give off heat). Unfavorable reactions are endothermic or “endergonic”. Nucleotides play a role in the biochemistry of many biological systems. This includes adenosine triphosphate or ATP, which is adenosine plus three phosphate molecules connected together and attached to the terminal 5’ carbon atom of ribose. This is a highly energetic molecule, more energetic than ADP, AMP, and adenosine. Figure 130 shows the structure of adenosine triphosphate:
237
Figure 130.
In biochemistry, reactions that add phosphate molecules to the adenosine molecule, called phosphorylations, are catalyzed by kinases, which are specific enzymes. ATP is considered the energy currency of the cell, with a third of the glucose energy used in glycolysis (the partial breakdown of glucose) going to the making of the ATP molecule from ADP.
238
DNA (DEOXYRIBONUCLEIC ACID) The two things that happen to the DNA molecule is the replication of itself to make a copy of DNA and transcription, which makes RNA from the DNA template. As mentioned, DNA has the pyrimidine bases thymine and cytosine and the purine bases guanine and adenine. The structure of DNA was discovered in 1962 as being the double helix. Figure 131 shows the double helix of DNA as well as the related structure of RNA:
Figure 131.
239
There are three types of DNA: A-DNA, which is compact DNA, B-DNA, which is common DNA, and Z-DNA, which is a left-handed double helix. B-DNA is the normal DNA seen; it has two strands of nucleic acids twisted in a right-handed fashion with the two strands being anti-parallel. The two strands of the double helix are connected and stabilized by hydrogen bonding between atoms of the nitrogenous bases. The bases are stacked within the molecular structure about 3.4 Angstroms apart. There will be a major groove in the DNA helix and a minor groove between the strands. The amounts of Adenine and Thymine are about equal and the amounts of Cytosine and Guanine are about equal because these are the bonds that connect the two strands. A pyrimidine always hydrogen bonds with a purine base. A always equals T and G always equals C in the DNA molecule. So, how does DNA replication occur? As you might imagine, there are millions of combinations of A, G, T, and C, making DNA the perfect genetic code. By virtue of the fact that A and T will hydrogen bond, and G and C will hydrogen bond, the molecule can split apart and rebuild a matching segment that can effectively replicate the DNA molecule. After replication, the strand that is made will rebuild the initial strand separately by connecting the base pairs so that an actual duplicate of itself can be made. Figure 132 shows this replication strategy:
Figure 132.
240
DNA also gets transcribed to make RNA. There are three types of RNA: messenger RNA (mRNA), transfer RNA (tRNA), or ribosomal RNA (rRNA). Of these, only messenger RNA is the RNA that directly carries the DNA “message” from the nucleus to the ribosomes in order to make proteins. Transcription is the process of taking the DNA message and making a corresponding mRNA molecule. It isn’t the whole strand of DNA copied—just one gene at a time, consisting of 500-6000 nucleotides. There is a promotor sequence that starts the transcription process, a transcribed gene sequence, and a terminator sequence that turns off the transcribing of the gene. Only about ten base pairs get unwound at a time using the enzyme RNA polymerase to add to the mRNA molecule. The 5’-end gets transcribed first with a phosphate ester linkage that uses ATP, GTP, CTP, or UTP, depending on the base that is being added. There are four bases that make up a triplet of bases called a codon. There are 64 combinations of triplets, most of which code for a specific amino acid, although there are some amino acids that have more than one triplet associated with it. The codon AUG is considered the “start” codon, while either UAA, UAG, or UGU act as “stop” codons or off switches. Translation occurs in the cytoplasm of the cell. This is when RNA becomes proteins in the ribosome. The ribosome itself is made from rRNA and protein, acting as the factories to make proteins. Transfer RNA or tRNA is the molecule type that adds the amino acid to the growing protein chain. It does so by “carrying” an amino acid and transferring it to the growing protein chain, led by the codons that determine which amino acid gets added. The tRNA base triplet is referred to as an anticodon. The protein gets added from the N-terminus to the C-terminus (the carboxylic acid).
241
KEY TAKEAWAYS •
Nucleic acids are the genetic material of life, made up of DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
•
Nitrogenous bases get attached to ribose or deoxyribose to make a nucleoside and to a phosphate molecule to make a nucleotide.
•
The nucleic acid molecule is connected by the sugars and phosphates, with the phosphate located at the 3’ and 5’ carbon atom of the sugar molecules.
•
The nitrogenous bases will connect with one another as pyrimidines and purines that make specific base pairs using hydrogen bonding.
•
Bioenergetic molecules in the cell comprise of molecules like adenosine that have up to three phosphate atoms attached to it, making for a high energy molecule.
242
QUIZ 1. What is not a component of a nucleic acid? a. Heterocyclic resonant ring base b. Phosphate molecule c. Disulfide linkage d. Pentose molecule Answer: c. A nucleic acid consists of a heterocyclic resonant base ring molecule, a phosphate molecule, and a pentose molecule. It does not have a disulfide linkage. 2. What is the carbohydrate seen in DNA? a. Deoxyribose b. Glyceraldehyde c. Glucose d. Ribose Answer: a. DNA consists of a deoxyribose molecule, which is a ribose sugar that has a substituted hydrogen on one of the five carbons in the ring. 3. Which is not a pyrimidine base? a. Uracil b. Adenine c. Thymine d. Cytosine Answer: a. The adenine molecule is a purine base, while the others are pyrimidine bases. You need to know the difference between a purine and a pyrimidine.
243
4. Where does the sugar molecule and the nitrogenous base connect with respect to the sugar in a nucleoside? a. A nitrogen on the base connects with the C2 carbon on the sugar b. A carbon on the base connects with the anomeric carbon on the sugar c. An oxygen on the base connects with the terminal carbon on the sugar d. A nitrogen on the base connects with the anomeric carbon on the sugar Answer: d. The connection between the sugar and the base in a nucleoside involves a nitrogen in the base attaching to the anomeric carbon in the sugar molecule. 5. In a nucleotide, what would be a part of this molecule that is not a part of the nucleoside? a. Phosphate b. Ribose c. Nitrogenous base d. Deoxyribose Answer: a. Remember that the phosphate is the added molecule in a nucleotide molecule that is not a part of the nucleoside. In other words, a nucleotide is a nucleoside plus a phosphate ester. 6. In a nucleotide, where is the phosphate ester located on the ribose or deoxyribose sugar? a. Attached to the anomeric oxygen b. Attached to the second hydroxyl group c. Attached to the third hydroxyl group d. Attached to the terminal hydroxyl group Answer: d. The phosphate ester is a linkage between the phosphate molecule and the terminal oxygen on the ribose or deoxyribose sugar.
244
7. Which molecule has the most free-energy associated with it, making it the highest-energy molecule? a. Adenosine b. Adenosine monophosphate c. Adenosine diphosphate d. Adenosine triphosphate Answer: d. Adenosine triphosphate is the highest energy molecule; it lowers in energy as phosphate molecules are removed in reactions. Adenosine triphosphate goes by the shorthand name of ATP. 8. Which nucleotide is considered the precursor molecule to the energy currency of the cell in biochemistry circles? a. Adenosine b. Guanine c. Cytosine d. Thymine Answer: a. While there are molecules of CMP and GMP, these are not the energy currency molecules of the cell. The molecule that is the precursor to the energy currency of the cell is adenosine, which goes to make ATP or adenosine triphosphate, which is the main energy currency of the cell. 9. In the DNA molecule, which base pair hydrogen bonds with the adenine base pair? a. Cytosine b. Guanine c. Thymine d. Uracil Answer: c. The thymine molecule will always hydrogen bond with the adenine molecule, forming the double strand that makes up the DNA infrastructure.
245
10. When proteins are being made, which of the following molecule types get the DNA message first in the process of transcription? a. Transfer RNA b. Ribosomal RNA c. Another DNA molecule d. Messenger RNA Answer: d. The natural trend of things is for DNA to be transcribed into messenger RNA. This gets transcribed with the help of other RNA molecules (like transfer RNA and ribosomal RNA) to make proteins in the process of translation.
246
SUMMARY Throughout this course, we attempted to bring the basics of organic chemistry to the student needing to understand the nomenclature, chemical properties, and reactivity of carbon-based molecules. While there are far too many organic molecules in nature to discuss in any organic chemistry course, there are specific ways to clearly identify these molecules as well as certain trends in how these various molecules behave in chemical reactions. By the end of the course, you should now know how to identify and name organic molecules, their physical properties, and how they chemically interact with one another in a variety of types of chemical reactions. Chapter one in the course began the study of organic chemistry by introducing how organic molecules are put together. There really isn’t any difference between the way the atoms in organic molecules are put together and the way other chemical molecules are put together but it was worth reviewing, even if you have studied chemistry in the past. This chapter looked at orbital theory and the particulars of organic molecular bonding as well as the shorthand involved in writing out organic molecular structures. Finally, the chapter talked about resonance chemistry as it applies to organic molecules. Chapter two in the course covered the basics of nomenclature in identifying organic molecules. Because there are innumerable organic molecules and because they are based on just a few different types of atoms, there needs to be a way to identify what each molecule looks like by name alone. This led to a discussion of the IUPAC nomenclature and coverage of the different functional groups in organic chemistry. You will need to understand how to name the different molecules you see in organic molecules, which was covered in this chapter. In addition, there was a discussion of stereochemistry as it applies to organic molecules. The topic of Chapter three in the course was organic solvent chemistry. For students who have participated in regular chemistry experiments, the solvent has typically been water. In organic chemistry, the solvent may or may not be water because many aspects of organic chemistry involve nonpolar substances that do not dissolve in water. Solvents
247
may or may not participate in chemical reactions themselves but are important to the chemistry of different molecules. Issues regarding solvation and solutions in organic chemistry were also covered in this chapter. Chapter four began a series of chapters on the different organic compounds and their properties. You have learned about alkane, alkene, and alkyne nomenclature in previous chapters so the focus of this chapter was to learn more about these compounds and how they interact with one another and with other organic compounds. These substances can have a variety of different configurations, which needed to be discussed as part of this chapter. The focus of Chapter five in the course was the chemistry of aldehydes, ketones, and carboxylic acids. This was the first time the chemistry of oxygen comes into play in this course. Aldehydes and ketones were discussed together because they have very similar chemistry and reaction types. Carboxylic acids are also oxygen-related because they have a COOH side chain as their defining characteristic. They also have great reactivity and are seen in nature as fatty acids and other biochemically-important molecules. In all cases, you came to understand their nomenclature, their physical properties, and some of the most important chemical reactions associated with these molecules. Chapter six introduced the structure and chemistry of aromatic compounds. All aromatic compounds consist of a cyclic compound that carries resonance. The most common aromatic compound is benzene, which is very stable and has chemistry unique to the molecule. In this chapter, the nomenclature and chemistry of aromatic compounds was covered as well as the different reactions that are seen in organic chemistry with these types of molecules. The topic of Chapter seven in the course was the chemistry of alcohols and alkyl halides. Alcohols are organic compounds that have a hydroxyl group as its major functional group, often represented with the general formula of ROH, where R can be any number of organic chemistry alkyl groups. The hydroxyl group is highly reactive so that there are any number of reactions that can occur at this functional group. The chapter also covered the chemistry of alkyl halides, which are alkyl groups that have one
248
or more halogen side chain attached to it. The halogen is also highly reactive, with many possible chemical reactions associated with it. Chapter eight in the course focused on the organic chemistry associated with ethers, epoxides, and esters. Ethers and epoxides are related to one another in that certain types of cyclic ethers are referred to as epoxides. In both types of molecules, the general formula is ROR’, involving a variety of R side chains. These are molecules commonly seen in perfumes, industrial conditions, waxes, oils, and dyes. Esters are also commonly used in industry, being a part of the making of many products—the most common of which are the polyesters. The topic of Chapter nine was the structure and chemistry of enols and enolates. Enols are also referred to as alkene alcohols, which are alkenes that have an alcohol group added to one of the carbon atoms. These are first alkenes but, chemicallyspeaking, they should be considered important for their electron-donating capacity. Enols can be mixed with alkali substances to make enolates, which are the conjugate bases of enols. Both of these types of molecules are best known for the many different types of reactions they participate in, which were covered in this chapter. Chapter ten in the course changed nomenclature and reactions in organic chemistry to include molecules that contain sulfur. Sulfur compounds are somewhat similar to oxygen-containing molecules in that they belong to the same group but sulfur is a great deal larger than oxygen, leading to slightly different chemical reactivity unique to these molecules. The nature and chemistry of thiols and sulfides was discussed as part of this chapter. Chapter eleven placed a focus on the different nitrogen-containing molecules in organic chemistry. These types of molecules are not only important in basic organic chemistry; they are important also in numerous biochemical processes. The main type of molecule discussed were the amine compounds, which are considered organic derivatives of ammonia. Like ammonia itself, amine compounds will have a certain degree of basicity, which leads to nucleophilicity of the nitrogen compounds in organic compounds.
249
Chapter twelve in the course began to make sense of what you studied in prior chapters on simpler molecules and applies it to more complex biochemical molecular structures. Sugars and carbohydrates are basically organic molecules that come from the phrase “carbon hydrates”. They contain only carbon, oxygen, and hydrogen atoms and have a specific generic formula, based on whether they are simple sugars, disaccharides, or more complex polysaccharides. The main focus of this chapter was to use organic molecular principles that made more sense after you studied the basic reaction types involved. The focus of Chapter thirteen in the course was to bring on more of the biochemistry involving organic chemistry principles by talking about the organic chemistry of amino acids, oligopeptides, and proteins. These are molecules that have nitrogenous compounds as the basis of their chemistry and that, like carbohydrates, exist as monomer units and polymers or polypeptides. These will also have reactions at their functional units, which involve a variety of different side chains and parts of the parent chain. Chapter fourteen in the course studied the organic chemistry associated with lipids. The term “lipid” is a broadly reaching term that applies to a wide variety of molecules that are called lipids because of their biochemical nature and their lack of solubility in water. They can range from fatty acids to triglycerides to more complex molecules that are complicated to synthesize and even to understand how they are synthesized in body systems and in organic chemistry models. Lipids have poor solubility in water but are much more soluble in chloroform, benzene, ether, and acetone, which are either nonpolar or weakly polar. The focus of Chapter fifteen and the final chapter of the course was the organic chemistry and biochemistry of nucleic acids, which are deoxyribonucleic acids and ribonucleic acids, commonly called DNA and RNA. These are molecules that contain ribose or deoxyribose sugars, phosphate groups, and nucleic acid bases, which are seen in paired form with DNA and sometimes with RNA. The transcription of nucleic acids and the translation to proteins was covered as part of the chapter as these are not generally made synthetically in an organic chemistry laboratory.
250
COURSE QUESTIONS AND ANSWERS 11. What single carbon compound is the most stable? e. CH4 f. CH3Li g. CH3F h. NaCH3 Answer: a. CH4 is highly stable and nonpolar so that it would be very unlikely to generate a cation or anion of CH3. This would require extreme conditions in order to occur. 12. What is the general measurement unit for bond length in organic chemistry? a. Nanometer b. Angstrom c. Picometer d. Centimeter Answer: b. The general measurement unit for bond length in organic chemistry is the Angstrom. This will lead to a carbon-carbon bond length of about 175 Angstroms in distance between the atoms. 13. According to the hybridization of carbon, there are four sp3 orbitals made. This leads to what shape of the methane molecule? a. Tetrahedral b. Planar c. Cuboid d. Pyramidal Answer: a. The shape of the orbitals dictates the shape of the molecule. As the orbitals are equal, they must be tetrahedral in order to separate the electrons as far apart as possible.
251
14. According to valence bonding theory, there are 4 sp3 orbitals made from a single 2s orbital and three 2p orbitals in the nitrogen molecule. How many of these four orbitals are available for bonding with nitrogen? a. 1 b. 2 c. 3 d. 4 Answer: c. There are four sp3 orbitals made at the second level with nitrogen and five electrons. Two are paired, giving three left for bonding. 15. What is the total charge on a zwitterion? a. It is generally positive b. It is generally negative c. It can be positive or negative d. It will be zero Answer: d. The total charge on a zwitterion will be zero, even if there are positive and negative charges on the molecule in separate “parts” of the molecule. 16. What is the single positively charged nucleus plus two electrons called? a. Hydrogen atom b. Helium atom c. Hydride anion d. Proton cation Answer: c. Hydride is a single proton and two electrons or a negativelycharged hydrogen ion. It is highly reactive and reacts with many different molecules because of its instability.
252
17. Which atom in organic chemistry does not follow the octet rule? a. Oxygen b. Chlorine c. Carbon d. Phosphorus Answer: d. Phosphorus is a large molecule that has d-orbitals so that it does not have to follow the octet rule. 18. What atom most closely resembles the bonding structures and patterns as oxygen in organic molecules? a. Sulfur b. Nitrogen c. Chlorine d. Fluorine Answer: a. Sulfur is the atom that most mimics the activity of oxygen in its binding abilities and patterns in organic chemistry. 19. When drawing the most stable form of an organic molecule, what formal charges are you looking to have? a. The most negative charge overall b. The most positive charge overall c. The charges don’t matter as long as they add up to zero d. A zero charge on as many atoms as possible Answer: d. The most stable form or structure is going to be the one that has a zero charge on as many atoms as possible. 20. Write out the formula for SCN and determine its formal charge. What is the net formal charge? a. 0 b. b.-1 c. c. +1 d. -2 253
Answer: b. The carbon atom will be in the middle and will bind with a double bond between both the sulfur and nitrogen atom. The nitrogen will prefer a triple bond but, with a double bond, this leaves a formal charge on the nitrogen atom of -1. The formal charges on sulfur and carbon will be zero. This leaves a net formal charge of -1. 21. Given that CO3(2-) is a resonance structure, what is the charge on each oxygen atom? a. -1 on each oxygen atom. b. -2 on one oxygen atom and 0 on the other two. c. -1/3 on each oxygen atom d. -2/3 on each oxygen atom Answer: d. As this is a resonance structure, the charge is equal on the oxygen atoms. The total of -2 must lead to a charge of -2/3 on each of three oxygen atoms. 22. When drawing a resonance structure, what changes between the different Lewis structures of an organic molecule? a. The position of the electrons b. The position of the side chains c. The position of the hydrogen atoms d. The charge on the carbon atom Answer: a. The position of the electrons is the only thing that changes when drawing the different Lewis dot structures on an organic molecule. The rest of the structure remains the same. 23. What is a resonance structure? a. Methane b. Ethane c. Ozone d. Oxygen gas
254
Answer: c. Ozone is a resonance structure because it can shift electrons between the three oxygen molecules in alignment with one another. In other words, the electrons will shift from one place to another without a change in the alignment of the oxygen atoms. 24. How many carbon atoms are there in the isopropyl side chain? a. One b. Two c. Three d. Four Answer: c. The isopropyl side chain is a CH3CH3CH- side chain, which contains three carbon atoms and 7 hydrogen atoms. It is the side chain that contributes to isopropyl alcohol or CH3CH3CHOH. 25. Which alkane has 10 carbon atoms in a single chain? a. Nonane b. Decane c. Undecane d. Dodecane Answer: b. Decane is the name for an alkane (a saturated carbon chain) that has ten carbon atoms with a molecule that is C10H22. 26. What ending is most likely to indicate that the carbon chain has a triple bond? a. Ane b. Adiene c. Atriene d. Yne Answer: d. The ending -yne is an indication that the bond is a triple bond.
255
27. What ending is most indicating that there are three double bonds in the carbon chain? a. Ene b. Adiene c. Atriene d. Yne Answer: c. The ending -atriene means that the carbon chain has three double bonds. Nonatriene would be a molecule that has three double bonds. 28. When an alcohol has more than one hydroxyl group added to it, what is the ending if the OH number is 2? a. iol b. anol c. anediol d. anetriol Answer: c. The ending is anediol when there are two OH molecules on the alkane. The example would be 1,3-pentandiol. 29. In naming an organic compound, which side group takes precedence in the naming of the compound? a. OH b. Double bond c. Alkyl side group d. Halide side group Answer: a. The OH or alcohol side group takes precedence over the rest of the side chains and double bonding.
256
30. The aldehyde will have what type of side chain by definition? a. Hydroxyl b. Carboxyl c. Ethyl d. Carbonyl Answer: d. The carbonyl group or CHO is typical of an aldehyde group. Hydroxyl groups are on an alcohol, while a carboxyl group is seen on a carboxylic acid. 31. In numbering the aldehyde carbon chains, the carbon atom that takes precedence in the numbering process is what? a. The carbonyl group b. The hydroxyl group c. The double bond d. The halide group Answer: a. The carbonyl group always takes precedence over the other side chains, making this the first carbon atom. 32. What side chain is seen in a carboxylic acid molecule? a. COOH b. b CHO c. c. CO d. d. CH3 Answer: a. The carboxylic acid side chain is the COOH molecule, which is actually a part of the main chain. 33. What is not a common name for a carboxylic acid? a. Oxalic acid b. Acetic acid c. Formic acid d. Benzoic acid
257
Answer: d. Each of these is considered a common name for a carboxylic acid, except for benzoic acid, which is an IUPAC name. 34. Which functional group has both a prefix and a suffix when naming it? a. Ketones b. Carboxylic acids c. Alcohols d. Aldehydes Answer: c. An alcohol has both a prefix and a suffix when naming it. It ends in “ol” when it is a suffix and begins in “hydroxy” when it is a prefix. The others will have a suffix only but have no prefixes. 35. The phrases “ortho”, “meta”, and “para” refer to side chains on what types of compounds? a. Benzene rings b. Esters c. Ethers d. Aldehydes Answer: a. The phrases are distinguishing characteristics that define the position of side chains on the benzene ring. Ortho is a 1,2placement situation; meta is a 1,3-placement situation; para is a 1,4placement situation. 36. Why couldn’t you have 3-bromo,3-methyl ethane? a. You cannot have two side chains on the same carbon atom. b. Ethane does not have enough carbon atoms to be the main chain. c. The number should be two and not three because of the “lowest number possible” rule. d. It is an alkyl halide that should end in bromate. Answer: c. There are four carbon atoms with the “lowest number possible” rule indicating that the name should be 2-bromo, 2-methyl ethane.
258
37. Which solvent type does not exist in organic chemistry? a. Polar aprotic b. Polar protic c. Nonpolar protic d. Nonpolar aprotic Answer: c. Nonpolar protic does not exist because there is no nonpolar solvent that will donate an H+ ion by virtue of having an OH or NH side chain. 38. What type of solvent is water? a. Polar aprotic b. Polar protic c. Nonpolar protic d. Nonpolar aprotic Answer: b. Water is polar and protic because it technically has an OH “side chain” and because it has a large dielectric constant and a high dipole moment. 39. Which is not considered a nonpolar solvent? a. Toluene b. Ethyl acetate c. Benzene d. Pentane Answer: b. Ethyl acetate is aprotic but is polar, having a dielectric constant of 6.0. This makes it a borderline polar aprotic solvent that will dissolve polar solutes but will not participate in the reaction.
259
40. What is considered a polar aprotic solvent because of a dielectric constant that is high (basically above 20)? a. Acetic acid b. Water c. DMSO d. Ammonia Answer: c. Each of these is a polar protic solvent except for DMSO, which is a polar aprotic solvent, which does not have an OH or NH bond. 41. The ability of a solute to dissolve in a solvent at any proportion is called what? a. Solvation b. Solubility c. Miscibility d. Polarity Answer: c. Being miscible or having miscibility means that the solute can interact with the solvent at any proportion. 42. Which solvent type is most likely to be able to dissolve an ionic compound? a. Polar aprotic b. Polar protic c. Nonpolar protic d. Nonpolar aprotic Answer: b. Polar protic solvents are more likely to dissolve an ionic compound as these tend to have the highest dielectric constant, which is a measure of the solvent’s ability to negate the charge of the solvent. 43. What is not true of solvation? a. It is the same as solubility. b. It involves polar and nonpolar substances. c. It is the interaction between solute and solvent. d. It involves different types of bonding between solvent and solute. 260
Answer: a. Solvation is not the same as solubility but is the interaction between solute and solvent, with both nonpolar and polar solvents. It involves different types of bonding between solvent and solute molecules. 44. What type of bonding is not seen in polar solutions between solvent and solute? a. Hydrogen bonding b. Covalent bonding c. Ionic bonding d. Van der Waals forces Answer: b. Covalent bonding is not seen between solvents and solute in polar solutions; however, the other types of bonding are commonly seen. 45. Which is considered a polar protic solvent? a. Acetone b. Toluene c. Diethyl ether d. Formic acid Answer: d. In order to identify a protic substance, it is necessary to look for a substance that can give a hydrogen ion. Of these, only formic acid can give a hydrogen ion, making it a polar protic solvent. 46. What is the best description of a dipole moment in an organic solvent? a. It involves partial charges on a given molecule because of differences in electronegativity of atoms. b. It involves an ionic interaction in primarily those solvents that can be divided into separate ions. c. It is directly related to the ability of some solvents to give up hydrogen ions.
261
d. It is directly related to the ability of some solvents to give up OH- ions in order to be able to dissolve acids. Answer: a. The dipole moment of a solvent or any molecule involves partial charges within the molecule because of differences in the electronegativity of the atoms. 47. If there are n carbon atoms in an alkyl group, by definition, how many hydrogen atoms will there be? a. 2n b. 2n + 2 c. 3n d. 2n + 1 Answer: d. In order to make an alkyl group from an alkane, there needs to be 2n + 1 hydrogen atoms attached to it, which would be lost from the end so that carbon can attach to another carbon atom or to something like oxygen or a halide. 48. An alkoxide is considered what? a. An alkane with a negatively-charged oxygen atom attached. b. A branched chain alkane c. A less than completely hydrogenated alkane d. An OH group attached to the alkane Answer: a. An alkane with a negatively-charged oxygen atom attached to it is called an alkoxide, which is a side chain as it is not stable in its RO- form. 49. At STP (standard temperature and pressure), which is considered a solid? a. Pentane b. Octane c. Octadecane d. Dodecane
262
Answer: c. Pentane is the first liquid (and not gaseous) hydrocarbon at 25 degrees and 1 atmosphere, while octadecane is the first solid at this temperature and pressure. The increased carbon length will increase the melting point and boiling point of the hydrocarbon so that things like candle wax have at least 20 carbon atoms in their length. 50. Which molecule has the least amount of reactivity? a. Straight-chain alkane b. Branched-chain alkane c. Cycloalkane d. Alkene Answer: a. The straight-chain alkane is the most stable of all alkanes so that it has the least degree of reactivity. The others will have changes in optimal bond angle and will have an increase in reactivity because of that. 51. What type of strain in cycloalkanes involves overlapping of sp3 orbitals? a. Angle strain b. Torsional strain c. Transannular strain d. Eclipsing strain Answer: a. Angle strain or bond angle strain occurs when the angle of the carbon atoms is different from the acceptable 109.5-degree angle that is desirable for the sp3 orbitals in the carbon atom. This leads to overlapping orbitals that are not energetically favorable. 52. What is the number of hydrogen atoms in an alkene that contains n carbon atoms? a. n b. 2n c. 2n + 2 d. 2n + 4
263
Answer: b. The ratio will be 2:1 so that, with n carbon atoms, there will be 2n hydrogen atoms in an alkene molecule. 53. What is the common name for the side chain also called “ethenyl”? a. Vinyl b. Allyl c. Iso d. Propenyl Answer: a. The vinyl side group is the common name for “ethenyl”. This is preferred over the scientific name as is the “allyl” side group. 54. Two double bonds that are specifically separated by a single carbon atom in an alkene Is called what? a. Diene b. Conjugated diene c. Isolated diene d. Cumulated diene Answer: d. A cumulated diene is a diene in which the double bond is separated by a single carbon atom. It is less stable than a conjugated diene, in which the double bonds are separated by a single bond. 55. Acetylene is a common alkyne that goes by which scientific name? a. Ethyne b. Propyne c. Butyne d. Pentyne Answer: a. Ethyne is the C2H2 molecule that goes by the more commonly-used name of acetylene.
264
56. In naming alkanes, alkenes, or alkynes, which prefix is not used scientifically? a. Dib. Tric. Tetrad. BiAnswer: d. Each of these is the prefix associated with a multiple bond situation or a multiple side chain structure except for “bi”, which is not used for two of the same of any side chain or bond. 57. The molecule octynol has what features? a. A situation with two double bonds and an OH side chain b. A situation with two triple bonds and a methyl side chain c. A situation with a single triple bond and an OH side chain d. A situation with saturated carbon atoms and a methyl side chain Answer: c. Octynol is an alcohol that has a single triple bond and an OH side chain. It becomes an alcohol first and a triple-bonded molecule second. 58. Alkynes can be fully hydrogenated using each of these catalysts, except for which one? a. Platinum b. Lindlar’s catalyst c. Palladium on carbon d. Finely dispersed nickel Answer: b. Each of these will fully hydrogenate an alkyne into an alkane; however, Lindlar’s catalyst only partially hydrogenates alkynes to make certain types of alkenes rather than alkanes.
265
59. What is the smallest aldehyde possible? a. Valeraldehyde b. Acetaldehyde c. Butyraldehyde d. Formaldehyde Answer: d. These are all aldehydes with formaldehyde being the smallest one, having the chemical structure of H2CO. It consists of just one carbon atom and is referred to as “methanal”. 60. A cyclic compound attached to a carbonyl group is referred to as what? a. Ringed aldehyde b. Carboxaldehyde c. Carbaldehyde d. Benzaldehyde Answer: c. The generic name for a cyclic compound that is attached to a carbonyl group is a carbaldehyde. This applies to any ringed hydrocarbon and not just to the benzene ring. 61. What is a molecule that ends in “-one”? a. Ketone b. Ether c. Ester d. Aldehyde Answer: a. A ketone is a molecule that is given the designation “-one”. 62. What is the simplest ketone called? a. Pentanone b. Phenylethanone c. Propanone d. Benzophenone
266
Answer: c. Propanone is the scientific name for acetone, which is CH3COCH3. It is the simplest ketone with just two methyl groups on either side of a carbonyl group. Because the molecule has a carbonyl group, it is considered polar with a dipole moment between the carbon and oxygen of the carbonyl group. 63. The term “dial” as a suffix means what about a specific molecule? a. It has a ketone and an aldehyde aspect. b. It has a benzene ring and an aldehyde group. c. It has a carbonyl group at both ends of the molecule. d. It is an alcohol and an aldehyde. Answer: c. The term “-dial” means that the molecule has a carbonyl group at both ends of the molecule. It is essentially a double aldehyde. 64. If a number of side chains are present in the molecule, what takes the greatest priority? a. Ketone carbonyl group b. Aldehyde carbonyl group c. Alcohol group d. Alkene carbon atoms Answer: b. In such cases, the aldehyde carbonyl group takes precedence. The molecule is referred to as an aldehyde with numbered ketone carbonyl groups, numbered alkene carbons, and numbered hydroxy side chains. 65. What most contributes to the higher boiling temperature of aldehydes versus alkenes? a. van der Waals dispersion forces b. Dipole-dipole interactions c. Ion-ion interactions d. Hydrogen bonding
267
Answer: b. The polarity and dipole-dipole moment interactions of aldehydes is not present in alkenes and alkanes, leading to a higher boiling point. 66. The alpha carbon of an aldehyde is particularly sensitive to substitution. What gets substituted and what does it normally get substituted with? a. The carbon atom gets substituted with nitrogen b. The hydrogen atom gets substituted with an OH side chain c. The carbon atom gets substituted with an alkyl group d. The hydrogen atom gets substituted with a halogen Answer: d. In such cases, the hydrogen atom at the alpha carbon gets substituted with a halogen of any sort, including chlorine, bromine, or iodine, using a catalyst and the di-halogen compound. 67. Which carboxylic acid comes ultimately from vinegar? a. Formic acid b. Acetic acid c. Propionic acid d. Capric acid Answer: b. Acetic acid is the carboxylic acid that makes up vinegar. It has the chemical formula of CH3COOH. 68. When a carboxylic acid becomes a carboxylate, what atom or ion gets lost from the functional group? a. OHb. Carbon c. H+ d. COOH Answer: c. The carboxylic acid loses the hydrogen ion, becoming negatively charged so that it can bind with a positively-charged ion, such as sodium or potassium.
268
69. If a carboxyl group, a hydroxyl group, an amine group, and a carbonyl group are on the same molecule, which group takes precedence in naming the molecule? a. Carboxyl group b. Hydroxyl group c. Amine group d. Carbonyl group Answer: a. The carboxyl group takes precedence, in which case it is called a carboxylic acid or anoic acid. The amine group is called an amino group, the carbonyl group is called an oxo group, and the hydroxyl group is called a hydroxy group—all added to the carboxylic acid. 70. Which of the following is an unsaturated fatty acid (an alkene carboxylic acid)? a. Arachidic acid b. Palmitic acid c. Stearic acid d. Linoleic acid Answer: d. Linoleic acid is an unsaturated fatty acid, having a single alkene bond in the alkane chain. 71. According to Huckel’s rule, which number of electrons does not indicate a resonance structure? a. 6 b. 8 c. 10 d. 14 Answer: b. The structure that is resonant must have 2n + 2 carbon atoms, where n is an integer. This rule, called Huckel’s rule, does not support 8 electrons in a resonance structure.
269
72. The presence of side chains on the benzene molecule leads to what changes in the melting point and boiling point of these molecules? a. Increases the boiling point and increases the melting point b. Increases the boiling point and decreases the melting point c. Decreases the boiling point and increases the melting point d. Decreases the boiling point and decreases the melting point Answer: a. Benzene itself has a zero-dipole moment. When a side chain is added, there is a dipole moment that increases intermolecular forces so that the boiling point and melting point is increased in these types of molecules. 73. A benzene molecule with an NO2 molecule associated with it once is called what? a. Nitrous benzene b. 1-nitrobenzene c. ammonium benzene d. Nitrobenzene Answer: d. The molecule is called nitrobenzene; it has a nitro group attached to it and does not have to be numbered if there is just one group associated with it. It would otherwise have to be numbered if there are multiple side chains on the molecule. 74. Four substituted groups exist around the benzene molecule. Which side group takes the number one position? a. Methyl b. Ethyl c. Bromo d. Chloro Answer: c. The groups are started with the number one carbon atom with the one chosen in this case being the one that takes alphabetical
270
priority. The methyl group would therefore be last in the naming convention. 75. Benzene that is also a carboxylic acid is called what? a. Phenol b. Benzaldehyde c. Xylene d. Benzoic acid Answer: d. The molecule that is both a benzene ring and a carboxylic acid is called benzoic acid. It has a carboxyl group attached to the benzene ring. 76. Benzene that has a hydroxyl group attached to it is called what? a. Phenol b. Benzaldehyde c. Xylene d. Toluene Answer: a. Phenol is the name of the benzene molecule that has a hydroxyl group attached at the 1 position. If it is called phenol, the OH group is, by definition associated with the first carbon atom so it does not have to be specified that way. 77. How many carbon atoms attached to a benzene ring begins to change the name from “benzene” to “phenyl” being a side chain? a. Three b. Five c. Six d. Eight Answer: c. Having six or more carbon atoms attached to a benzene ring makes the ring called phenyl rather than a benzene ring.
271
78. The benzyl group is indicated by what? a. The benzene ring alone b. The benzene ring and a CH2 attached c. The benzene ring opened up d. The benzene ring and an O- attached Answer: b. The benzene ring plus a CH2 attached leads to the benzyl group that is associated with many other additional molecules. 79. What is not a feature of an aromatic compound? a. It must be a ring structure b. It must be tetrahedral c. It must have conjugated double bonds d. It must have 2n + 2 pi-electrons Answer: b. Each of these is a feature of the aromatic compound; however, it must be planar and not tetrahedral. 80.How many delocalized pi electrons are there in the benzene molecule? a. Two b. Four c. Six d. Twelve Answer: c. Each carbon atom donates an electron to the pi bond so that there are six pi-electrons in the benzene six-carbon ring. 81. A three-carbon, 2-nitrogen ring that is aromatic, making a 5-membered ring is known as what? a. Imidazole b. Thiophene c. Pyridine d. Azulene
272
Answer: a. Each of these is a heterocyclic aromatic compound. Imidazole is a five-membered ring that has three carbon atoms and nitrogen atoms. 82. Polycyclic aromatics are different from heterocyclic aromatics. What atom is seen in a heterocyclic aromatic as well as a polycyclic aromatic? a. Nitrogen b. Hydrogen c. Oxygen d. Sulfur Answer: b. The main difference between polycyclic aromatics and heterocyclic aromatics is that, in polycyclic aromatics, there is carbon and hydrogen atoms only; these are the only two atoms that are seen in both heterocyclic aromatics and polycyclic aromatics. 83. In the halogenation of benzene, which halogen is considered the least reactive and more difficult to halogenate the benzene molecule than the others? a. Iodine b. Bromine c. Fluorine d. Chlorine Answer: a. Iodine is the least electrophilic and does not easily react with benzene unless there is a catalyst that makes it more electrophilic. 84. Why is a catalyst necessary for the halogenation of benzene? a. To provide a source of the halogen for the benzene molecule b. To decrease the electrophilicity of the halogen c. To make the halogen into a nucleophile for the reaction d. To polarize the di-halogen molecule in the reaction process Answer: d. As a catalyst, the substance is not consumed but is available to polarize the di-halogen molecule so that one aspect of the molecule
273
can be electrophilic enough to react with benzene and attach to a carbon atom. 85. In the nitration of benzene, what acts as the catalyst for this reaction? a. Nitrous acid b. Ferrous nitrate c. Sulfuric acid d. Aluminum nitrate Answer: c. It takes a strong acid like sulfuric acid to draw off an OH ion from nitric acid, making NO2+, a strong electrophile that will react with benzene to make nitrobenzene. 86. Which electrophilic substitution reaction with benzene is considered reversible? a. Bromination of benzene b. Sulfonation of benzene c. Nitration of benzene d. Chlorination of benzene Answer: b. Of these, the sulfonation of benzene is reversible by mixing benzenesulfonic acid with dilute sulfuric acid and adding heat. 87. In the acylation of benzene, what side chain gets attached to the benzene ring? a. RCO b. RCH2 c. RCOOH d. RNH Answer: a. An acyl group is an RCO group that can be attached via the carbon atom to the benzene ring via the Friedel-Crafts reaction.
274
88. Which side chain is most likely to deactivate the benzene ring when attached to it? a. CH3 b. NH2 c. Cl d. OH Answer: c. Chlorine is a side chain with a dipole moment pointing away from the benzene ring. This makes the benzene side chain more deactivated by drawing electrons away from the ring. 89. Which side chain is most likely to activate the benzene ring when attached to it? a. CN b. NH2 c. NO2 d. Cl Answer: b. NH2 is a side chain that is more electropositive than the benzene ring. It draws electrons away from it and toward the benzene ring so that electrophilic attack is more likely to happen. This activates the benzene ring. 90. What is the basic formula for a primary alcohol? a. RCH(OH)2 b. RCH2OH c. R3OH d. R2CHOH Answer: b. A primary alcohol is an alcohol th3at has a simple RCH2 attached to an alcohol side chain. This makes the carbon atom attached to the alcohol being carbon one.
275
91. What is the basic formula for a secondary alcohol? a. RCH(OH)2 b. RCH2OH c. R3OH d. R2CHOH Answer: d. A secondary alcohol has a carbon atom with an alcohol group and two alkyl side chains also attached to it with the formula R2CHOH. 92. Which is the smallest alcohol in organic chemistry? a. Ethanol b. Methanol c. Butanol d. Isopropyl alcohol Answer: b. Methanol is a very small organic chemical alcohol, being just CH2OH. It is basically methane with a side chain of OH, making it a primary alcohol. 93. Which is the smallest branched chain alcohol in organic chemistry? a. Isopropyl alcohol b. Sec-butyl alcohol c. Tert-butyl alcohol d. Glycerol Answer: a. Isopropyl alcohol is a secondary alcohol that is a threecarbon alcohol with the OH group on the middle carbon atom. Glycerol has three carbon atoms but is a tri-alcohol, making it a bigger molecule.
276
94. What can be said of the boiling point of alcohols related to size and their corresponding alkane? a. The boiling point increases compared to alkanes and decreases with molecular size b. The boiling point decreases compared to alkanes and decreases with molecular size c. The boiling point decreases compared to alkanes and increases with molecular size d. The boiling point increases compared to alkanes and increases with molecular size Answer: d. There are hydrogen bonding forces that increase the boiling point markedly compared to alkanes. This boiling point will also increase somewhat with molecular size. 95. Which alcohol will be least soluble in water? a. Methanol b. Butanol c. Propanol d. Ethanol Answer: b. The greater the molecular side chain size, the least soluble in water the substance will be. This means that butanol, with four carbon atoms will be least soluble in water. Even longer chains will act more like hydrocarbons than alcohols and will not be soluble in water at all. 96. What substrate will most likely lead to ethanol as an end product? a. Ethylene b. Acetylene c. Carbon monoxide d. b. Methylene
277
Answer: a. Ethylene is a substrate that is mixed with sulfuric acid and water to make ethanol as an end product in the synthesis or “hydration” of ethylene. 97. What substrate will most likely lead to methanol as an end product? a. Methylene b. Methane c. Carbon monoxide d. Ethanol Answer: c. Carbon dioxide and hydrogen gas will lead to the end product of methanol by hydrogenating the carbon dioxide molecule. 98. What is not one of the three main functional group reactions that can occur to the alcohol molecule? a. Esterification b. Dehydration c. Oxidation d. Alkene formation Answer: d. Each of these is a reaction that occurs in the functional group of the alcohol molecule except for alkene formation, which does not affect the functional group. 99. Which alcohol reaction does not occur? a. Oxidation of primary alcohols b. Oxidation of secondary alcohols c. Oxidation of tertiary alcohols d. Dehydration of primary alcohols Answer: c. Each of these reactions can occur except for the oxidation of a tertiary alcohol; this reaction does not occur under any circumstances because there are three R side chains on the alcohol.
278
100.
You are trying to make an alkene from an alcohol. If this is a primary
alcohol, what is necessary to make this? a. 70 degrees and sodium hydroxide b. 180 degrees and phosphoric acid c. 25 degrees and sulfuric acid d. 100 degrees and chromium phosphate Answer: b. It takes a great deal of heat in order to make an alkene from a primary alcohol with lesser amounts of heat necessary to make a secondary and tertiary alcohol. Phosphoric acid is a necessary component of this reaction as well. 101.
You are trying to make an alkene from a tertiary alcohol. The
circumstances necessary to make this reaction happen is what? a. 70 degrees and sodium hydroxide b. 180 degrees and phosphoric acid c. 55 degrees and sulfuric acid d. 100 degrees and chromium phosphate Answer: c. The necessary features to make an alkene from a tertiary alcohol is sulfuric or phosphoric acid plus heat in the range of 25-80 degrees. More heat is necessary to make an alkene from a primary or secondary alcohol. 102.
What happens when a secondary alcohol is oxidized?
a. The alcohol becomes an aldehyde b. The alcohol becomes a ketone c. The alcohol becomes a carboxylic acid d. The alcohol cannot be oxidized Answer: b. The secondary alcohol will oxidize to become a ketone but will not oxidize any further as ketones are resistant to oxidation.
279
103.
When a primary alcohol is oxidized fully, what is the end product?
a. Ketone b. Aldehyde c. Carboxylic acid d. Ether Answer: c. A fully oxidized primary alcohol will become an aldehyde and subsequently a carboxylic acid, which further resists any oxidation. 104.
Which halogen is the most electronegative when it comes to an alkyl halide
bond? a. Fluorine b. Bromine c. Chlorine d. Iodine Answer: a. The fluorine atom is the smallest and most electronegative of the halogens, making the bond length shorter and stronger than with the other halogens. 105.
Which halogen most affects the size of the alkyl halide molecule when it
substitutes for the hydrogen atom? a. Fluorine b. Bromine c. Chlorine d. Iodine Answer: d. The iodine molecule is the largest, affecting the molecular size the most when it is attached to the alkyl group in an alkyl halide.
280
106.
Which type of molecule is the least toxic among the multiple alcohol
substances? a. Propylene glycol b. Ethylene glycol c. 1,2-ethanediol d. Glycerol Answer: d. Glycerol is a normal part of human metabolism, being a trialcohol that is involved in the synthesis of triglycerides, being completely nontoxic. Of these, the most toxic is ethylene glycol or 1,2ethanediol, which forms calcium oxalate crystals, resulting in kidney failure. 107.
What is the IUPAC chemical name for two propane molecules attached to
each other with an oxygen group? a. Dipropane ether b. Propane ether propane c. Propoxy-propane d. Propanoxy-propane Answer: c. Propoxy-propane is the name of two propane molecules attached by an oxygen molecule to each other. 108.
How does the carbon numbering work with the naming of ethers?
a. The numbering starts at the far-side of the longest carbon chain and proceeds to the end of the shortest side chain. b. There is separate numbering of the alkyl chains that start with the oxygen and work outward. c. Only the longest chain is numbered from the oxygen-containing carbon atom and working outward. d. The numbering starts at the far side of the shortest chain and proceeds to the end of the longest parent chain.
281
Answer: b. When it comes to numbering, the two side chains are numbered separately with the number one carbon on both sides being closest to the oxygen molecule. 109.
Which is the chemical name for the substance commonly referred to as
“ether”? a. Methoxy methane b. Methoxy ethylene glycol c. Ethoxyethane d. Ethyl butane Answer: c. Ethoxyethane or diethyl ether is commonly referred to as “ether”, being a very commonly-used ether clinically. 110.
How many carbon molecules exist in the molecule known as 1-
oxacyclohexane? a. 2 b. 5 c. 6 d. 7 Answer: b. The molecule looks much like a hexane molecule but has an oxygen molecule in place of one of the carbon molecules, leaving 5 carbon atoms left in the six-membered ring. 111.
Which type of reaction will not be possible?
a. The making of an alkyl halide after acid addition to an ether. b. The making of phenol from a phenoxy-ether. c. The making of a phenyl halide from a diphenoxy-ether. d. The making of an alcohol from the addition of water and an acid. Answer: c. A diphenoxy-ether will not be attackable by a halogenated acid because it is unresponsive to cleavage with these strong acids. 112.
How many carbon atoms are in a simple oxirane?
282
a. 2 b. 3 c. 5 d. d.6 Answer: a. There are 2 carbon atoms in a simple oxirane, which is a three-membered ring with two carbon atoms and an oxygen atom. 113.
The combination of ethylene and oxygen in an oxidation reaction will form
what molecule? a. Ethylene dioxide b. Diethyl oxide c. Dicarboxylic acid d. Ethylene oxide Answer: d. Ethylene dioxide is a three-membered ring with two carbons and an oxygen molecule forming a triangle. The molecule has no side chains and is a simple oxirane. 114.
A four-carbon, one oxygen, five-membered cyclic ether is called what?
a. Oxirane b. Furan c. Tetrahydrofuran d. Pyran Answer: b. Furan is a cyclic ether that consists of a five-membered ring that has two alkene components to the molecule. It can be substituted in order to make a molecule without double bonds, like tetrahydrofuran.
283
115.
What is the atom seen in the cryptand molecule that is not seen in the
crown ether molecule, even though these molecules function similarly? a. Sulfur b. Potassium c. Nitrogen d. Oxygen Answer: c. The cryptand molecule contains nitrogen and a crowned structure, similar in other respects to a crown ether, both of which help to solvate metal cations. 116.
What is the chemical structure of the ester compound?
a. RCOOH b. RCOOR’ c. RCOR’ d. ROR’ Answer: b. An ester goes by the chemical structure of RCOOR’, which makes it similar to a carboxylic acid except for the fact that the R’ molecule is aliphatic or aromatic and not a hydrogen atom. 117.
The chemical methyl propanoate is an ester that is made from which
alcohol and which carboxylic acid? a. Propanol and methanoic acid b. Propanol and methanol c. Propanoic acid and methanol d. Acetic acid and methanol Answer: c. The mixture of propanoic acid (or propionic acid) plus methanol will yield methyl propanoate, which is a carboxylic acid.
284
118.
Esters are most closely associated with what other chemical compound
type? a. Ethers b. Cyclic ethers c. Ketones d. Carboxylic acids Answer: d. Esters are highly related to carboxylic acids because they simply represent a combined carboxylic acid and a side chain rather than a hydrogen atom. 119.
Why do cis-fatty acids have a lower melting point than trans and
unsaturated fatty acids? a. There is less hydrogen bonding between molecules b. There are fewer van der Waals dispersion forces c. There is greater packing of the fatty acid molecules in cis-fatty acids d. There is less packing of the fatty acid molecules in cis-fatty acids Answer: d. Cis-fatty acids are more disordered with less fatty acid packing; this leads to a lower melting point in these molecules. 120.
In hydrolyzing ethyl ethanoate, what are the end-products?
a. Two molecules of ethanol b. Acetic acid and ethanol c. Diethyl ketone and water d. Two molecules of acetic acid Answer: b. The end products add water to the ethyl ethanoate molecule, giving rise to acetic acid and ethanol.
285
121.
The action of lithium aluminum hydride onto the ester molecule creates
what type of molecule? a. Two alcohols b. An aldehyde and an alcohol c. An alkene and an alcohol d. Two aldehydes Answer: a. This molecule of lithium aluminum hydride provides hydrogen atoms that create an RCH2OH alcohol plus an R’OH molecule. This leads to, in the case of ethyl ethanoate, to two molecules of ethyl alcohol. 122.
When a carboxylic acid is attached to another carboxylic acid by joining at
the oxygen group, what is this called? a. Acyl halide b. Diketone c. Acid anhydride d. Ester Answer: c. This type of molecule is called an acid anhydride because it joins two different carboxylic acids together with a common oxygen molecule between them. 123.
Saponification takes an ester and acts on it with an alkali, making what
type of molecules? a. Carboxylic acid and alkane b. Carboxylate and alcohol c. Carboxylic acid and aldehyde d. Carboxylate and aldehyde Answer: b. The reaction creates a carboxylate ion and an alcohol by the nucleophilic attack of the carbonyl carbon by the hydroxide ion.
286
124.
What type of molecule is the substrate for the saponification process?
a. Cholesterol b. Long chain alkane c. Triglyceride d. Glycerol Answer: c. Triglycerides are long chain tri-esters that can be broken down in the saponification process to make a long chain carboxylate salt that acts as a soap. 125.
Which molecule is more nucleophilic?
a. Enolate b. Enol c. Enamine d. Alcohol Answer: a. The enolate anion is the most nucleophilic molecule because oxygen is nucleophilic and because it is a negatively charged molecule. 126.
What changes between molecules that are considered tautomers?
a. Stereoselectivity b. Carbon side chains c. Electrical charge d. Proton and electron position Answer: d. The thing that changes is the position of the protons and electrons, making molecules that are considered isomers of one another. 127.
Acidic halogenation of a ketone or aldehyde uses what source for the
halogen? a. The di-halogen molecule b. Hydrogen halide molecule c. Sodium halide molecule d. Halogen atom 287
Answer: a. The reaction starts with the di-halogen molecule and results in the halogenation of the ketone or aldehyde molecule at the alpha carbon. 128.
Acidic halogenation of the ketone or aldehyde molecule leaves what
molecule aside from the halogenated ketone or aldehyde? a. The di-halogen molecule b. Hydrogen halide molecule c. Sodium halide molecule d. Halogen atom Answer: b. The end result is not the halogen atom because it would be unstable. The halogen is electronegative, pulling off the hydrogen atom from the hydroxyl group of the unstable intermediary, leaving behind the hydrogen halide acidic molecule. 129.
What molecule is an end product of the basic alpha halogenation of
ketones that isn’t present in the acidic alpha halogenation? a. di-halogen b. Halogenated enolate c. H2O d. Hydrogen halide Answer: c. You have to remember how this reaction starts. The alpha hydrogen is pulled off by the basic conditions, leaving behind water. This creates an enolate, which strongly reacts to pull halogen onto the molecule. 130.
What is not a necessary reactant in the haloform reaction?
a. Methyl ketone b. Di-halogen c. Hydroxide ions d. Halogen hydride
288
Answer: d. It is important to remember that this is a reaction that begins with an acid-base reaction of a methyl ketone such that it is necessary to have hydroxide ions that pull off the hydrogen atoms from the alpha carbon. This is a basic reaction and not acidic so that a hydrogen halide would not be a part of it. 131.
In the haloform reaction, how many times does the methyl ketone get
halogenated? a. 1 b. 2 c. 3 d. 4 Answer: c. This reaction is fraught with polyhalogenation but it stops at three halogen molecules, which pull off a hydrogen ion from the resultant carboxylate molecule, leading to a tri-halogenated molecule. 132.
In the alkylation of ketones, what is the substrate that the alkyl group
comes from? a. Alkane b. Alkyl halide c. Alcohol d. Carboxylic acid Answer: b. The alkyl group is derived from an alkyl halide; the alkyl group gets pulled off the halide because of the more electronegativity (nucleophilicity) of the enolate molecule. 133.
Which is the simplest aldehyde that will react in an aldol reaction?
a. Methanal b. Ethanal c. Propanal d. Butanal
289
Answer: b. The smallest aldehyde must be ethanal because there needs to be an alpha hydrogen. Methanal is smallest but it doesn’t have an alpha hydrogen. It is simply a CH2O molecule without an alpha carbon or an alpha hydrogen. 134.
What best represents what an enal molecule is like?
a. It is a double ketone molecule b. It is an unsaturated ketone molecule c. It is an aldehyde and an alkene d. It is a ketone and an alkene Answer: c. An enal is an alkene and an aldehyde, which is made from the dehydration of a beta-hydroxyaldehyde. 135.
What is the end product of the aldol reaction with ketones under basic
conditions? a. Beta-hydroxyketone b. Di-ketone c. Dialdehyde d. Enone Answer: d. The reaction with ketones will become briefly betahydroxyketone but will rapidly dehydrate to make an enone molecule, which is an alkene ketone molecule with a double bond between the alpha and beta carbon atoms. 136.
How many alpha hydrogens are there in 2-pentanone, which goes by the
chemical structure of CH3C=OCH2CH2CH3? a. Zero b. Two c. Three d. Five Answer: d. In a ketone, it is important to look on either side of the carbon atom that has the carbonyl group. In this case, there are three
290
hydrogens on the methyl group and two alpha hydrogens on the propyl group, leading to a total of five alpha hydrogen atoms. 137.
How many alpha hydrogen atoms are there on the aldehyde called
phenylaldehyde or PhCHO? a. Zero b. Two c. Three d. Five Answer: a. There is a phenyl group and an aldehyde group, which means that, next to the aldehyde is a fully bonded carbon atom in the benzene ring. This has no alpha hydrogen atoms associated with it, making it impossible to make an enol or enolate. 138.
Organocuprate molecules can be used in certain types of enolate-mediated
reactions. What type of reaction does this involve? a. Alpha carbon alkyl addition reaction b. Conjugate addition reaction c. Electrophilic substitution reaction at the carbonyl group d. Aldol reaction of a ketone Answer: b. This involves the addition of an alkyl side chain on the conjugate carbon atom of an enone or enal. The beta carbon is attacked by a nucleophile and this attaches the nucleophilic R alkyl chain. 139.
In the Michael reaction, what type of molecule gets added to what?
a. A ketone gets added to a ketone b. An aldehyde gets added to an alcohol c. A ketone gets added to an enone or enal d. An alcohol gets added to a ketone Answer: c. The Michael reaction involves the conjugation addition reaction of a ketone, which has a slightly acidic alpha hydrogen atom to
291
the beta carbon or conjugate carbon, which is electrophilic, of an enal or enone molecule under basic circumstances. 140.
What oxygen-containing molecule is most like a thiol?
a. Ketone b. Alcohol c. Ether d. Aldehyde Answer: b. The molecule most represented by the thiol molecule but in oxygen form is the alcohol. These will behave somewhat similarly. 141.
What oxygen-containing molecule is most like a sulfide?
a. Ketone b. Alcohol c. Ether d. Aldehyde Answer: c. The molecule that most resembles a sulfide molecule is an ether. They will be called similar to an ether with both side chains named as side chains and the word sulfide afterward. 142.
Which sulfur containing molecule has the most oxygen atoms associated
with it? a. Sulfoxide b. Thiol c. Sulfate ester d. Disulfide Answer: c. The chemical structure of a sulfate ester is complex. It has two side chains with oxygen molecules attached to it and two double bonded oxygen molecules so that there are four oxygen molecules are attached to a sulfur atom.
292
143.
Which is the more correctly-named sulfoxide molecule?
a. Methyl ethyl sulfoxide b. 2-butyl ethyl sulfoxide c. 2-propanyl ethyl sulfoxide d. Methyl 1-butyl sulfoxide Answer: b. The appropriate name is 2-butyl ethyl sulfoxide because the side chains are in alphabetical order. 144.
Which sulfur compound has the highest oxidation state for sulfur atoms?
a. Thiol b. Sulfonic acids c. Sulfite esters d. Sulfate esters Answer: d. Sulfate esters are highly oxygenated with oxygen molecules surrounding the sulfur atom—two in double bonding and two in single bonding. The oxygens have -2 and -1 oxidation statuses, leaving a +6oxidation status for sulfur in these types of molecules. 145.
Mild oxidation of a thiol forms what type of molecule?
a. Disulfide b. Sulfone c. Sulfonic acid d. Sulfate ester Answer: a. The disulfide molecule RSSR is what happens when there is mild oxidation of a thiol, increasing the oxidation state of the sulfur molecule rather than the carbon molecule.
293
146.
When oxidizing sulfur compounds, what happens to the oxidation number
of the compound? a. The carbon oxidation number is increased but the sulfur oxidation number is unchanged. b. The carbon oxidation number is decreased but the sulfur oxidation is increased. c. The carbon oxidation number is unchanged and the sulfur oxidation number is decreased. d. The carbon oxidation number is unchanged and the sulfur oxidation number is increased. Answer: d. In the oxidation of sulfur compounds, the carbon oxidation number is unchanged but the sulfur oxidation number is increased. This leads to the oxidation of RSH to make RSSR, which is more oxidized. 147.
Which amino acid contains a crucial sulfhydryl group used in disulfide
bridges in proteins? a. Glycine b. Cysteine c. Histidine d. Leucine Answer: b. Cysteine is a unique amino acid that has a sulfhydryl group which forms disulfide bonds, forming the three-dimensional shape of the protein. 148.
What word would not fit with the others?
a. Thioether b. Alkylthio c. Sulfoxide d. Sulfide
294
Answer: c. The terms are basically similar except that the sulfoxide does not refer to the same term, which is represented as RSR’. With “alkylthio” as a term, the term is used when the thioether component is not the main parent part of the molecule. 149.
What is true of the difference between oxygen and sulfur in organic
compounds? a. Oxygen has fewer oxidation states than sulfur. b. Oxygen is more nucleophilic than sulfur. c. Oxygen is larger than sulfur. d. The oxygen-carbon bond is longer than the sulfur-carbon bond. Answer: a. Each of these is an untrue statement, except that oxygen has fewer oxidation states than sulfur, mainly because of the d-orbitals on the sulfur molecule that does not exist in the case of oxygen. 150.
What is a sulfide usually synthesized from?
a. Disulfide b. Thiol c. Sulfone d. Sulfoxide Answer: b. Thiols can be used in basic environments to make sulfides. The reaction proceeds with a hydrogen atom being pulled off the acidic thiol to make a thiolate anion that adds the R’ alkyl group using an alkyl halide molecule. 151.
What is the most reduced molecule in terms of the sulfur atom?
a. Sulfone b. Disulfide c. Thiol d. Sulfide
295
Answer: c. The thiol molecule or the RSH molecule is fully reduced, giving the lowest possible oxidation number for sulfur, which is negative two. 152.
Which name for an amine compound is not the same as the others?
a. Butaneamine b. 1-aminobutane c. Butylamine d. 1-methylaminopropane Answer: d. There are many confusing names for amine compounds in organic chemistry—from common names to CA names and IUPAC names. Each of these names is the same except that 1methylaminopropane is a secondary amine, while the others are primary amines. These all are, however, structural isomers. 153.
Which of the following is the common suffix for a nitrogenous organic
compound? a. Nitrate b. Amine c. Indole d. Imidine Answer: b. The common name for these types of compounds is an “amine” compound. Nitrates can be organic compounds but these are not unique. 154.
Which of the following is not a six-membered ring containing nitrogen and
carbon atoms? a. Pyridine b. Pyrimidine c. Pyrrole d. Piperidine
296
Answer: c. These are all six-membered rings with carbon and nitrogen, except for pyrrole, which is a five-membered ring. 155.
Which compound in nitrogen chemistry is not considered aromatic?
a. Aniline b. Pyridine c. Indole d. Piperidine Answer: d. These are all aromatic compounds except for piperidine, which is cyclic but is not aromatic. 156.
What is the charge on the nitrogen atom in a CH3NH2 molecule?
a. Partial positive charge on the nitrogen b. Partial negative charge on the nitrogen c. Negative one charge on the nitrogen d. Positive one charge on the nitrogen Answer: b. This is a polar molecule with no formal charge but there will be a partial negative charge on the nitrogen molecule due to the polarity of the bond and a pull of electrons toward the nitrogen atom. 157.
When nitrogen is bonded to three hydrogens or to up to three alkyl groups,
what is the shape of the molecule? a. Planar b. Linear c. Trigonal pyramidal d. Tetrahedral Answer: c. The molecule, when bonded singly to these side chains, will form a trigonal pyramidal shape, with nitrogen considered to be at the “top” of the molecule.
297
158.
When nitrogen is bonded in an aromatic ring, what is the shape of this
bond within the structure? a. Planar b. Linear c. Trigonal pyramidal d. Tetrahedral Answer: a. This will have a planar structure, much like the planar structure seen in a benzene aromatic ring. It will be a sp2 hybridized structure. 159.
Which molecule will be least basic or more acidic?
a. Heterocyclic amine b. Aryl amine c. Alcohol d. Alkyl amine Answer: c. While there are differences between the aryl, heterocyclic, and alkyl amines, the biggest difference comes from the difference in electronegativity between the oxygen and nitrogen molecule, with the alcohol being more acidic. 160.
What is the chemical structure of an azide?
a. RCN3 b. RCN c. RN3 d. RNR Answer: c. An azide molecule is an unusual RNNN or RN3 molecule that can be reduced to make an amine molecule.
298
161.
What is the chemical structure of a nitrile compound?
a. RCN3 b. RCN c. RN3 d. RNR Answer: b. The chemical structure of a nitrile compound is RCN with a triple bond between the carbon and terminal nitrogen compound. 162.
How many hydrogen atoms must be provided in order to fully reduce a
nitrile RCN compound to make a primary amine compound? a. 1 b. 2 c. 3 d. 4 Answer: d. This needs four hydrogen atoms in order to be fully reduced. In order to do this, it takes a hydrogenating compound that adds two hydrogen atoms to the carbon and two hydrogen atoms to the nitrogen to make an RCH2N2 molecule (which is an amine). 163.
In the alkyl nitrile compound of RCN, what is the oxidation state of the
carbon atom on the nitrile substituent? a. +3 b. +2 c. 0 d. -3 Answer: a. This is a +3-oxidation state because the carbon-carbon bond is 0 and the three-carbon-nitrogen bond (triple bond) is adding a +3 to the carbon atom because the oxidation state of the nitrogen bond is +1 for each bond.
299
164.
From what molecule is an aniline made?
a. Alkyl nitrate b. Aryl amine c. Nitroarene d. 1,6-diamine hexane Answer: c. An aniline molecule is an aryl-amine as you may remember from the list of complex amine compounds. This is made by the reduction of a nitroarene, which is an ArNO2 molecule. 165.
What is the oxidation number of the carbonyl carbon on the amide
molecule, which is an R chain with a carbonyl CO group and an amine group (RCONH2)? a. -1 b. +2 c. 0 d. +3 Answer: d. The carbon atom is highly oxidized, with a +3-oxidation number, which is 0 from the R group, +2 from the oxygen double bond, and +1 from the nitrogen group. This leads to an oxidation number of +3. When fully reduced, it leads to an oxidation number of the amine group of -1. 166.
Which oxygen-containing compound is closest to an imine compound?
a. Ketone b. Alkene c. Carboxylic acid d. Ether Answer: a. The imine compound is a double bonded compound with nitrogen instead of oxygen. The main difference is that the nitrogen compound must bond with something else, either a side chain or a hydrogen atom.
300
167.
You are making a substituted amine compound from primary amines.
What is the reactant that can do this? a. An alcohol b. A carboxylic acid c. An alkyl halide d. Another primary amine Answer: c. This reaction must take place with an alkyl halide because the halide is electronegative enough to be separated from the carbon atom by the nucleophilic nitrogen atom on the primary amine. The end result is a multiply alkylated amine. 168.
The Sandmeyer reaction involves the substitution of substances onto the
benzene carbo-cation, which is itself made from the nitrosation of an aryl amine. What cannot be added to this benzene molecule as a result of this reaction? a. Bromine b. Chlorine c. Cyanide d. Hydroxyl group Answer: d. The Sandmeyer reaction begins with the aryl amine and ends with the use of copper bromide, copper chloride, or copper cyanide—adding these to the benzene ring, which is unstable as a carbo-cation. 169.
Which sugar is smallest in that it contains the fewest carbon atoms
associated with it? a. Fructose b. Galactose c. Glucose d. Ribose
301
Answer: d. Ribose is a five-carbon sugar, while the rest are considered six-carbon sugars. The fructose makes a five-membered ring similar to ribose but it actually consists of six carbon atoms. 170.
What is the cutoff number, above which number represents a
polysaccharide versus an oligosaccharide? a. 2 b. 5 c. 10 d. 20 Answer: c. The oligosaccharide is a molecule between 3 and 10 saccharides, while a polysaccharide has more than ten saccharides or sugars associated with it. 171.
Sucrose is made from what two monosaccharide units of what monomers?
a. Two glucose molecules b. Glucose and galactose molecules c. Galactose and fructose molecules d. Glucose and fructose molecules Answer: d. Glucose and fructose together will mark the presence of a sucrose molecule in which the glycosidic bond is located at the anomeric carbon of both molecules. 172.
What does not describe fructose?
a. Aldose b. Ketose c. Ketohexose d. Furanose Answer: a. Fructose is a furanose because it is a five-membered ring, while it can also be referred to as a ketose or ketohexose. It is not an aldose because it is not based on an aldehyde molecule.
302
173.
When talking about a substituted sugar molecule, what part of the
molecule gets substituted to make these sugars? a. Hydroxyl group b. Hydrogen atom c. Keto group d. Aldehyde group Answer: a. The substitution of the sugar molecule is the substitution of the hydroxyl group on the molecule. The substitution can be anything, from a hydrogen atom to a complete alkyl side chain. 174.
Alpha and beta sugars can be most specifically called what?
a. Tautomers b. Stereoisomers c. Anomers d. Structural isomers Answer: c. These are isomers and are specifically called anomers because they are isomers that are different only in the position of the side chains around the anomeric carbon atom and the side chain opposite the oxygen molecule in the ring. They are not structural isomers, are not strictly stereoisomers, and are not tautomers. 175.
What molecule’s chirality is the basis for all the D or L forms of
carbohydrate molecules? a. Glucose b. Ribose c. Glyceraldehyde d. Glycerol Answer: c. The molecule that all other carbohydrates’ chiralities are based on is glyceraldehyde, a three-carbon aldehyde that has a hydroxyl group on the left in the L form on a Fisher projection or on the right in the D form on a Fisher projection.
303
176.
Carbohydrates are known for their many hydroxyl groups. How many
hydroxyl groups are there on a cyclic hexose hemiacetal sugar? a. 3 b. 4 c. 5 d. 6 Answer: c. These are heavily hydroxylated molecules with a ketone or aldehyde group as part of the molecule—the oxygen molecule of which becomes the hemiacetal or hemiketal. There are five hydroxyl groups on these molecules. 177.
Reduction of carbohydrates involves what reactant acting as the reducing
substance? a. Lithium aluminum hydride b. H3O+ c. Sodium hydride d. Sodium borohydride Answer: d. The two major reducing agents seen in these types of reactions include lithium aluminum hydride and sodium borohydride; however, carbohydrates only dissolve in polar substances, which do not easily dissolve LiAlH4 but will dissolve NaBH4, leading to this being the preferred reducing substance. 178.
What group gets reduced in the reduction of carbohydrates?
a. Aldehyde group b. Ether group c. Ester group d. Aldehyde or ketone group Answer: d. The aldehyde or ketone group will be reduced in the reduction of carbohydrates, leading to reductions in carbonyl carbon from +2 to 0 in a ketone or from +1 to -1 in an aldehyde.
304
179.
Which sugar cannot be a reducing sugar?
a. Sucrose b. Glucose c. Galactose d. Fructose Answer: a. Sucrose cannot be a reducing sugar because it does not open up to make and aldehyde or a ketone (which can tautomerize to make an aldehyde). It is this part of the molecule that gets oxidized, thereby reducing copper from copper II to copper I. 180.
Which carbohydrate reaction can break down polysaccharides into
monosaccharides? a. Acylation of carbohydrates b. Reduction of carbohydrates c. Hydrolysis of carbohydrates d. Oxidation of carbohydrates Answer: c. The hydrolysis of polysaccharides turns the glycosidic bond into the OH groups of both molecules in a disaccharide under slightly acidic conditions. 181.
Which reaction of carbohydrates does not react directly on the OH bond?
a. Acylation of carbohydrates b. Alkylation of carbohydrates c. Glycoside formation of carbohydrates d. Oxidation of carbohydrates Answer: d. These will react at the OH bond, leading to a variety of reactions, except for the oxidation of carbohydrates, which happens at the aldehyde group to make a carboxylic acid.
305
182.
What type of bond makes an amino acid to amino acid bond to make a
protein? a. Ester b. Amide c. Ether d. Glycosidic Answer: b. The bond in an amino acid is classically an amide bond, and combines the carboxylic acid component and the amino component from one amino acid to another. 183.
What is the chiral center of the amino acid?
a. The alpha carbon b. The carbonyl carbon c. The R side chain d. The amino acid side chain Answer: a. The chiral center is no different from that of glyceraldehyde. It is located at the alpha carbon of the amino acid. 184.
What is the isoelectronic point of an amino acid?
a. The same as the pKa of the carboxylic acid b. The same as the pKa of the amino group c. The average of the pKa of the carboxylic acid and the amino groups d. The pH of the amino acid in solution Answer: c. The isoelectric point is the average of the pKa of the carboxylic acid and amino groups of an amino acid or the pH at which the zwitterion predominates. 185.
Which amino acid has the lowest isoelectronic point?
a. Lysine b. Aspartic acid c. Histidine d. Arginine 306
Answer: b. Aspartic acid has three groups that contribute to the isoelectronic point—two of which are acidic. This leads to an isoelectronic point of 2.77, which is far below that of many of the others. 186.
In the Fischer projection of the amino acids existing in nature, which
statement is not true? a. The R chain is at the bottom. b. The COOH side chain is at the top. c. The NH2 side chain is on the right. d. The chiral center is the alpha carbon. Answer: c. These are all true statements, except for the fact that the NH2 side chain is on the left at the chiral center (the alpha carbon), making naturally-occurring amino acids “L-amino acids”. 187.
Which amino acid is achiral—having no chiral center?
a. Lysine b. Glycine c. Isoleucine d. Leucine Answer: b. The only amino acid that has no chirality is the glycine molecule because its R chain is the hydrogen atom so it cannot have a left or right form. 188.
In order to make an amide bond from an amine in amino acid reactions in
order to make an amino acid-amino acid bond, what can be added to an amine group? a. Aldehyde b. Acid chloride or acid anhydride c. Carboxylic acid d. Ketone
307
Answer: b. The combination of acid chloride or an acid anhydride to an amine group can be used to make an amide bond in the synthesis of polypeptides. 189.
How are protein structures and sequences written out?
a. From the C terminus to the N terminus b. From the smallest amino acid to the largest amino acid c. From the largest amino acid to the smallest amino acid d. From the N terminus to the C terminus Answer: d. Because the molecules are written from the least to the greatest priority of the functional group and because the carboxylic acid group has the highest priority, the N terminus is on the left and the C terminus (carboxylic acid group) is on the right. 190.
In a protein molecule, there is hydrogen bonding in particular between the
amino group on the amino acid and what other structure? a. The hydroxyl group of a carboxylic acid b. The nitrogen on another amino group c. The carbonyl oxygen on the carboxylic acid d. The carbon atom on the alpha carbon Answer: c. The carbonyl oxygen on the carboxylic acid molecule will have a lone pair of electrons that has attraction to the hydrogen on the amino group on the amide part of the protein. 191.
Which amino acid is not aromatic in nature?
a. Tyrosine b. Phenylalanine c. Tryptophan d. Isoleucine Answer: d. Each of these has an aromatic side chain except for isoleucine, which has an aliphatic side chain.
308
192.
Which structure of proteins is made from the actual bonding of the amino
acids together? a. Primary structure b. Secondary structure c. Tertiary structure d. Quaternary structure Answer: a. The primary structure will be determined by the different arrangements of amino acids. 193.
Which structure of proteins is most affected by disulfide bonding between
cysteine subunits? a. Primary structure b. Secondary structure c. Tertiary structure d. Quaternary structure Answer: c. The tertiary structure is made from hydrophobic interactions, hydrogen bonding, and disulfide bonding that affect the three-dimensional shape of the protein structure. 194.
What is the backbone molecule of a triglyceride?
a. Glycerol b. Pyruvate c. Glyceraldehyde d. Propyl alcohol Answer: a. Glycerol is the backbone molecule of a triglyceride, being the structure that the fatty acids combine with in an ester linkage. The triglyceride can be hydrolyzed to make fatty acids, which are carboxylic acids and the tri-alcohol called glycerol.
309
195.
What is the IUPAC name for glycerol?
a. 1, 2, 3-hydroxypropane b. 1, 2-hydroxyethane c. 2-hydroxypropane d. 2, 3-hydroxypropanoate Answer: a. Glycerol is referred to as 1, 2, 3-hydroxypropane because it is based on a propane molecule that has three hydroxy groups on each of the three carbon atoms of the parent chain. 196.
Which is the more unsaturated fatty acid in nature?
a. Oleic acid b. Linoleic acid c. Stearic acid d. Linolenic acid Answer: d. Linolenic acid is a tri-alkene fatty acid that is 18 carbon atoms long. The other two unsaturated fatty acids commonly seen in nature are oleic acid (one double bond) and linoleic acid (two double bonds). Stearic acid is an unsaturated fatty acid. 197.
What would be a major reactant in the saponification of triglycerides?
a. Sodium hydroxide b. Potassium iodide c. Hydrochloric acid d. Sulfuric acid Answer: a. The saponification of triglycerides leads to the making of sodium-fatty acid combinations, which is soap. It relies on the use of sodium hydroxide to break the ester bonds in fats or triglycerides.
310
198.
What is true of a phospholipid bilayer?
a. The phosphates must bind together to create this structure b. There will be phosphate groups on both sides of the bilayer c. The phosphate is attached to the choline molecule in the bilayer d. The fatty acid side chain will be attracted to the phosphate molecule Answer: b. There will be phosphate groups on both sides of the bilayer but there will not be an actual bonding between the phosphate groups, nor will they necessarily be attached to choline. There is no attraction of the fatty acid layer and the phosphate component. 199.
Prostaglandins contain all but what aspects?
a. A cyclohexane ring b. Twenty carbon atoms c. Double bonds d. A carboxylic acid end Answer: a. These contain a cyclopentane end but not a cyclohexane end. There is a carboxylic acid end and at least one double bond. These are twenty carbon atoms long. 200. Terpene molecules can be described using all but which statement? a. They are molecules with ether linkages b. They can be highly fragrant molecules c. They are made from multiples of five carbons each d. They can be cyclic molecules Answers: a. These tend not to be ether linkages, although they can contain double bonds, alcohol groups, and ketones. 201.
How many rings are associated with a steroid molecule?
a. 2 b. 3 c. 4 d. 5 311
Answer: c. Steroids have four separate but interconnected rings that share at least one side in common with another ring. 202. What is the most common steroid in biological systems in animals? a. Cholesterol b. Cortisol c. Progesterone d. Estradiol Answer: a. Cholesterol is the basic steroid in nature, making up the most common steroid in animal systems. It contains one hydroxyl group, one double bond, and five methyl side chains with the characteristic four-ring system. 203.
Which molecule does not contain the typical steroid ring?
a. Cortisone b. Lanosterol c. Beta-carotene d. Testosterone Answer: c. Each of these will contain the typical steroid ring, with betacarotene being another complex terpene molecule that does not contain this type of ring structure. 204. What is the carbohydrate seen in RNA? a. Deoxyribose b. Glyceraldehyde c. Glucose d. Ribose Answer: d. The sugar in RNA is a ribose sugar, which is a five-carbon sugar that forms a five-carbon ring.
312
205.
Which is not a base seen in an RNA molecule?
a. Uracil b. Adenine c. Pyrimidine d. Cytosine Answer: c. Pyrimidine is the base that makes up some of the bases seen in an RNA molecule but isn’t itself one of the bases. It is instead a precursor base molecule. 206. Which nitrogenous base is not seen in DNA? a. Adenine b. Thymine c. Uracil d. Guanine Answer: c. Uracil is the base that isn’t seen in DNA. Instead it involves a molecule of thymine. 207.
Which nitrogenous base is not seen in RNA but is instead seen in DNA?
a. Cytosine b. Thymine c. Uracil d. Guanine Answer: b. RNA does not have thymine as a nitrogenous base but replaces all thymine with uracil, particularly when it “reads” the DNA molecule in the process of transcription. 208. There is a substitution of the hydroxyl group with hydrogen in the deoxyribose molecule. At which carbon atom does the substitution occur? a. Second carbon b. Third carbon c. Fourth carbon d. Fifth carbon 313
Answer: a. Deoxyribose is 2-deoxyribose, with a substitution of the hydroxyl group on the second carbon atom of the ribose molecule with hydrogen. 209. When the phosphate molecule connects the nucleic acid, it attaches to which two carbon atoms on the ribose or deoxyribose sugar? a. Second and fifth b. Third and fourth c. Third and fifth d. First and fourth Answer: c. The third and fifth carbon atom will connect the nucleic acid together via phosphodiester linkages. 210.
What is not considered true of B-DNA or common DNA?
a. It has anti-parallel coordination of two strands b. The different strands are connected via phosphate linkages c. The molecule is in a double helix structure d. The molecule is a right-handed twisting shape Answer: b. The strands are not connected to one another via phosphate linkages, which are linked instead with hydrogen bonding between the base pairs.
314
www.AudioLearn.com