El únic o sistema robótic o en el mundo de radio c irugía p ara todo el cuerp o c on verd a d era p rec isión sub - milimétric a y sinc roniza c ión d e movimiento en tiemp o rea l.
CONTENIDO
CYBERKNIFE ¿QUÉ ES EL SISTEMA CYBERKNIFE? DELEC CIENTÍFICA
5 7 8
A B O C A D O S A L A I N N O VA C I Ó N
9
C O N S U LT O R Í A I N T E G R A L
10
S E R V I C I O T É C N I C O P O S V E N TA
12
¿POR QUÉ ELEGIRLO?
17
S O B R E A C C U R AY Y C Y B E R K N I F E
18
P I L A R E S F U N D A M E N TA L E S
19
DISEÑO ÚNICO
21
MEJOR EXPERIENCIA
22
OPCIONES DEL SISTEMA
25
DIFERENCIAS ENTRE CYBERKNIFE Y OTROS SISTEMAS
26
¿CÓMO ES UNA SESIÓN CON CYBERKNIFE?
29
T R ATA M I E N T O S
31
RADIOCIRUGÍA DE PULMÓN
32
R A D I O C I R U G Í A D E P R Ó S TATA
34
CÁNCER CEREBRAL
34
CÁNCER DE HÍGADO
38
C Á N C E R D E PA N C R E A S
42
RADIOCIRUGÍA SNC
46
ENTRENAMIENTO
51
D I S E Ñ O D E L B Ú N K E R E I N S TA L A C I Ó N
55
PUBLICACIONES CIENTÍFICAS
63
Esta carpeta fue generada por el equipo de consultores de DeLeC Científica. 2021. DeLeC Científica Uruguaya - Representante Regional Exclusivo Fco. García Cortina 2357 – Piso 1. Montevideo - Uruguay Tel: (+598) 2711 4466 DeLeC Científica Argentina – Agente Comercial Local Aráoz 821 -C1414DPQ - Buenos Aires – Argentina. Tel: (+54-11) 4775 5844 2
A lo largo de la historia, las patologías oncológicas han representado uno de los desafíos más importantes a los que se enfrenta la medicina y la industria asociada, a nivel mundial. Con la premisa de lograr controlar estas enfermedades con el mínimo impacto negativo sobre la calidad de vida de los pacientes, las instituciones han destinado cuantiosos recursos a la investigación y el tratamiento oncológico en todas las áreas clínicas involucradas: la cirugía, la quimioterapia y la radioterapia (RT). En el ámbito de la RT, donde la eficiencia, la exactitud y la precisión juegan un papel fundamental, se han logrado numerosos avances con el desarrollo de soluciones tecnológicas realmente innovadoras. La complejidad del proceso radioterapéutico es tal que ha obligado al desarrollo de diferentes hardwares y softwares especializados en cada una de las modalidades y etapas que lo constituyen, de modo que en conjunto se complementan y permiten garantizar la ejecución de procedimientos óptimos y seguros para el mayor número de patologías oncológicas posible. Así, para cada modalidad: RT externa, braquiterapia y RT intraoperatoria, se han logrado grandes avances tecnológicos que se han traducido en el desarrollo de equipos y sistemas versátiles que permiten abandonar la RT convencional y adentrarse a la RT moderna, con todos los beneficios que esto conlleva para los pacientes. En Delec Científica, vamos de la mano de estos avances y nos preocupamos por ofrecer las mejores y más completas soluciones. Entre estas: • CyberKnife: único sistema para radiocirugía robótica intra y extracraneal; • Radixact: único sistema para radioterapia helicoidal; • RayStation: el mejor software de planificación de tratamientos que integra en una misma plataforma aceleradores de cualquier casa comercial;
En este documento les presentamos con detalle el sistema CyberKnife, único equipo de radiocirugía robótica con precisión sub-milimétrica en la entrega de la dosis sobre el tejido tumoral; capaz de tratar enfermedades oncológicas, malformaciones de los vasos sanguíneos, enfermedades funcionales del cerebro, tumores benignos, entre otros; logrando resultados similares o incluso mejores a los de la cirugía sin ser invasivo. Sus prestaciones clínicas van más allá del tratamiento de lesiones intracraneales, es el único sistema de radiocirugía capaz de tratar lesiones en cualquier parte del cuerpo, tales como: lesiones en pulmón, hígado, páncreas, columna y próstata. Este exclusivo sistema consiste en un acelerador lineal transportado por un brazo robótico y un sistema de imágenes de rayos-X que en conjunto con su sistema de seguimiento (conformado por 6 métodos de rastreo diferentes e independientes) permiten monitorear, en tiempo real y durante el tratamiento, los movimientos del paciente para garantizar la máxima exactitud y precisión en la entrega de la dosis. Además, posee tres poderos sistemas de colimación que le dan versatilidad en la definición del haz de tratamiento de acuerdo a cada caso. El brazo robótico tiene un amplio rango de movimiento para dirigir el haz a cualquier parte del cuerpo del paciente logrando una cobertura total de la lesión y evadiendo las estructuras sanas involucradas. Su exactitud y precisión es tan alta que no tiene comparación con otro sistema en el mercado. En DeLeC Científica estamos seguros que el CyberKnife es el equipo más seguro, eficiente y versátil para la entrega de tratamientos de radiocirugía en cualquier parte del cuerpo. Es un paso hacia el futuro en la lucha contra el cáncer para la comunidad médica y científica, y nuestra recomendación más enfática en la decisión de brindar un servicio de radiocirugía con el nivel más alto de excelencia.
• Xoft: primer sistema de braquiterapia electrónica; • Liac HWL: sistema más eficiente para radioterapia intraoperatoria con electrones.
MSc Miguel Yanez Director de División Radioterapia y Radiocirugía DeLeC Científica
3
SISTEMA ROBÓTICO DE RADIOCIRUGÍA PARA TODO EL CUERPO.
6
¿Qué es el sistema CyberKnife?
Actualmente en nuestra región la mayoría de los casos en los que se requiere una radiocirugía a un volumen tumoral sensible a movimientos, son tratados con técnicas que amplían los márgenes de los volúmenes a irradiar para asegurarse de que la dosis llegue al blanco. Esto solo lleva a que el tejido sano circundante sea irradiado con altas dosis. CyberKnife® es una alternativa no invasiva, indolora y no quirúrgica para tratar tumores en todo el cuerpo. Con su avanzado sistema de rastreo, es posible seguir en tiempo real los movimientos que pudieran tener los volúmenes a tratar, lo que evita que los órganos a riesgo reciban altas dosis de radiación que suelen causar importantes efectos secundarios. Su avanzado software brinda la posibilidad de hacer el seguimiento de tumores en constante movimiento, como el caso de algunas lesiones localizadas en los pulmones, además, permite sincronizar la entrega del cabezal del tratamiento con el movimiento del tumor y así asegurarse de irradiar solo el volumen a tratar, disminuyendo la radiación que reciben los tejidos sanos a niveles mínimos. Además, permite que los haces de radiación puedan llegar al tumor desde más de 1.200 posiciones diferentes, concentrando la radiación sobre el objetivo, logrando muy buenas distribuciones de dosis en volúmenes tumorales muy pequeños, mejorando la experiencia del usuario y del paciente.
Es ideal para pacientes que no desean someterse a una cirugía, con tumores inoperables o quirúrgicamente complejos.
7
8
DeLeC Científica, abocados a la innovación
En DeLeC Científica hicimos de la innovación tecnológica el combustible para impulsar la modernización de los sistemas de salud y la calidad de los servicios médicos. Trabajamos acercando las innovaciones tecnológicas más destacadas del siglo
xxi
a los hospitales y
clínicas de la región, desde la consultoría, la comercialización y el desarrollo de programas médicos integrales que permiten garantizar servicios médicos de excelencia. Nuestra firma comercializa la mayoría de sus productos en Argentina, Uruguay, Paraguay y Bolivia, y cuenta con representaciones que alcanzan Chile, Perú, Ecuador y Brasil.
Misión Nos hemos propuesto hacer foco en lo especial y proveer soluciones a problemas de los que nadie se ha ocupado. Por eso aportamos equipamiento y asesoramiento para hacer factibles y seguros los nuevos paradigmas en el ámbito de la salud, como son los tratamientos personalizados, con mayor seguridad y una experiencia más confortable para los pacientes. Nos interesan los procesos y sus resultados. Por eso trabajamos junto a nuestros partners desde el diseño de sus propuestas, con consultorías especializadas, asesoramiento y asistencia técnica oficial, garantizando la ejecución de proyectos exitosos.
9
Consultoría integral
Nuestra experiencia en el ámbito de la innovación tecnológica en salud nos dice que, tan importante como el equipamiento son las etapas de formación, la comprensión de la tecnología, el acompañamiento clínico, el asesoramiento y los objetivos que orientan la práctica. Por eso en DeLeC Científica acompañamos a las instituciones desde el desarrollo de los proyectos, el diseño de nuevas áreas o servicios de salud, el asesoramiento en la adquisición de nuevas tecnologías, el seguimiento clínico con especialistas en radioterapia, los requerimientos normativos y legales, la diagramación logística, el mantenimiento y el monitoreo del uso. Un asesoramiento adecuado es clave para:
Obtener planificaciones que permitan optimizar el tiempo de los proyectos,
Implementar know how para conseguir mejores resultados,
Aplicar estrategias para retorno de la inversión,
Visualizar un camino de crecimiento con fundamentos sólidos y desarrollo de valor.
Con el fin de asesorar, tomando como referencia los máximos estándares de calidad, los consultores de DeLeC nos actualizamos de acuerdo a los programas de formación de las firmas que representamos y participamos de forma activa en la agenda más relevante de la innovación tecnológica médica a nivel global.
10
Acompañamiento desde Aplicaciones Clínicas
DeLeC Científica se destaca por ser la única em-
Nuestro personal de Aplicaciones Clínicas brindará
presa capaz de proveer un servicio de aplicaciones
capacitación y entrenamiento a los equipos de salud,
clínicas completo, que va desde la etapa de consul-
con orientaciones prácticas y teóricas para aprove-
toría hasta la docencia post instalación de manera
char al máximo la potencialidad de la tecnología.
continua. Este equipo conformado por especialistas con una amplia experiencia clínica en radioterapia, capacitados por fábrica y con actualizaciones permanentes, asisten a las instituciones en el diseño de programas médicos de excelencia que mejoran de forma exponencial los flujos de trabajos asocia-
Con este programa de acompañamientos, brindamos a nuestros partners la seguridad de estar alineados con las mejores prácticas de cada especialidad, favoreciendo una mayor seguridad tanto para los usuarios como para los pacientes.
dos a la práctica de la radioterapia. De esta forma, las tecnologías seleccionadas se logran implementar con los mejores resultados, favoreciendo una práctica médica integral que seguramente superará los objetivos clínicos y económicos de los proyectos. El acompañamiento a nuestros clientes no conoce distancias. Implementamos plataformas, videoconferencias y aplicamos un cronograma de visitas para anticiparnos a las necesidades de consultas y actualización.
11
Servicio técnico especializado
Todos nuestros proyectos de consultoría están respaldados por la dirección de Servicio Técnico. El área se compone de ingenieros biomédicos y bio-ingenieros capacitados por las fábricas para brindar asistencia local de alta performance. Trabaje seguro con equipamiento único en el mundo, contando con un grupo de especialistas que le garantizará continuidad de servicio y respaldo los 365 días del año.
Ofrecemos un servicio técnico de alta performance, alineado tanto a las exigencias y estándares de las marcas con las que trabajamos, como a los requerimientos de nuestros clientes.
El equipo técnico asiste en la interpretación de los requerimientos previos (condiciones eléctricas, infraestructura, etc.), se ocupa de la instalación, cuando el equipo lo requiere, y luego monitorea el funcionamiento y el uso para garantizar el desempeño óptimo de la tecnología.
Nuestros ingenieros deben cumplir con un cronograma de formación y capacitación anual, en las casas matrices de las firmas que representamos. Por lo tanto, desde DeLeC Cientíífica ofrecemos una asistencia de instalación y posventa certificada por fábrica.
12
Servicio oficial de instalaciones
Staff
Ofrecemos el servicio de instalación oficial de los
• División de Radioterapia y Radiocirugía: Desarrolla proyec-
equipos de las firmas que representamos en Ar-
tos llave en mano para el tratamiento de tumores malignos y
gentina, Uruguay, Paraguay, Bolivia y Chile. Nues-
benignos, previendo todas las dimensiones vinculadas: consul-
tro servicio cumple con todos los procesos reco-
toría, docencia, comercialización, servicio de Aplicaciones Clí-
mendados por la fábrica.
nicas pos-venta completo. Brinda servicio docente a los usua-
Contar con el certificado y la habilitación de servicio
rios para asegurar su correcta utilización y las buenas prácticas.
La firma cuenta con seis áreas:
oficial garantiza a nuestros clientes seguridad y calidad a lo largo del proceso de instalación de los siste-
• División de Sistemas Médicos: Provee la mejor tecnología de
mas, contemplando los más altos estándares a nivel
punta para cubrir necesidades de equipamiento de diagnósti-
mundial. La formación constante en fábrica de nues-
co. Busca optimizar resultados clínicos y mejorar la calidad de
tros ingenieros se traslada en mejoras continuas en
la experiencia vivida por los pacientes.
los procesos de instalación. El Servicio Técnico de instalaciones combina la mejor tecnología disponible
• División de Simulación Clínica: Pone a disposición de la co-
en la actualidad, respaldo y experiencia.
munidad médica un catálogo de simuladores que abarca desde soluciones sencillas hasta las más completas que existen en el mercado. Esta versatilidad nos permite ofrecer proyectos a medida y escalables. • División de Ingeniería y Servicio Técnico: Lleva a cabo todas las acciones de logística necesarias para la importación de las distintas tecnologías. Asegura que el funcionamiento de los equipos instalados sea igual que el de origen, en fábrica. Controla y monitorea el funcionamiento de la base de instalada, con mantenimiento preventivo y correctivo, y actualización continua. • División de Comunicación y RSE: Genera contenidos para favorecer el conocimiento de las innovaciones tecnológicas que representamos. Asimismo, promovemos eventos de divulgación, demostraciones y acciones para conectar con nuestra audiencia. • División de Administración, Personal y Finanzas: Optimiza los resultados económicos de la empresa, cuidando que haya una distribución equitativa de los recursos entre los proveedores, clientes, personal, accionistas, bancos/inversores y el fisco. Su objetivo principal es velar por una gestión eficiente y ecuánime al momento de crear valor económico produciendo, al mismo tiempo, valor social.
13
Tecnología
Representamos exclusivamente equipos que son seguros y están debidamente certificados y aprobados por los organismos internacionales de control -FDA y CE- y también los nacionales -ANMAT y ARN-. Además, brindamos un soporte pre y post venta de excelencia para garantizar la funcionalidad una vez instalados. Nuestro diferencial es que no sólo proveemos equipos, sino que desarrollamos programas médicos de excelencia, acompañando al cliente desde la etapa embrionaria del proyecto hasta su optimización operativa. Trabajamos codo a codo con las instituciones, haciendo transferencia de tecnología desde el servicio técnico, el acompañamiento clínico, la comunicación y la consultoría integral. Así logramos que las inversiones en equipamiento, se conviertan en mejoras en la calidad de vida de los pacientes.
Nuestro lema es ganar cuando el cliente también gana, cumplir con lo prometido y hacerlo a tiempo.
14
Potenciamos desde la comunicación
En DeLeC no hablamos de clientes sino de partners. Nuestro modelo de innovación y comunicación nos vincula a todas las organizaciones e instituciones que integran la comunidad médica regional. En este marco, brindamos soporte de comunicación y marketing a nuestros parterns. Sabemos que toda innovación tecnológica, para ser capitalizada debidamente, requiere un trabajo de divulgación y comunicación. Por eso nuestro equipo en Argentina y Uruguay genera materiales atractivos de todos los sistemas y equipos que representamos. Desarrollamos eventos propios, workshops, webinars con finalidades formativas y de divulgación. Son abiertos y de fácil acceso. Potenciamos los proyectos a través de diferentes estrategias de comunicación:
•
Marketing digital
•
SEO y datos
•
Contenidos originales
• Videos
•
Ciclos temáticos
•
Eventos y conferencias
•
Proyectos con instituciones
15
Nuestros representados
A Subsidiary of Samsung Electronics Co. , Ltd
16
17
Sobre Accuray y CyberKnife
En 1987 la empresa norteamericana Accuray desarrolló
CyberKnife® es una alternativa no invasiva, indolora y no qui-
el sistema CyberKnife®, el único equipo de radiocirugía
rúrgica para tratar tumores en todo el cuerpo. Con su avan-
robótica basado en un acelerador lineal. En el año 1999
zado sistema de rastreo, es posible seguir en tiempo real los
fue aprobado por la FDA para realizar radiocirugías intra-
movimientos que pudieran tener los volúmenes a tratar, lo
craneal y en el 2001 para tratar tumores en cualquier parte
que evita que los órganos a riesgo reciban altas dosis de ra-
del cuerpo. La precisión alcanzada por este equipo, tanto
diación que suelen causar importantes efectos secundarios.
en la localización como en el tratamiento de tumores, es incomparable y única. Gracias a su brazo robótico, los haces de radiación pueden irradiar el tumor desde más de 1200 ángulos diferentes, logrando una estupenda conformación de la dosis y disminuyendo los efectos secundarios sobre el tejido sano circundante.
Es ideal para pacientes que no desean someterse a una cirugía, con tumores inoperables o quirúrgicamente complejos. Su avanzado software brinda la posibilidad de hacer el seguimiento de tumores en movimiento constante, como el caso de algunas lesiones localizadas en los pulmones, además, permite sincronizar el cabezal que entrega el
Actualmente, más de 100.000 pacientes fueron tratados
tratamiento con el movimiento del tumor y así asegurarse
por el CyberKnife® obteniendo excelentes resultados. En
de irradiar solo el volumen a tratar, disminuyendo la radia-
nuestra región la mayoría de los casos, en los que se re-
ción que reciben los tejidos sanos a niveles mínimos.
quiere una radiocirugía a un volumen tumoral sensible a movimientos, son tratados con técnicas que amplían los márgenes de los volúmenes a irradiar para asegurarse de que la dosis llegue al blanco. Esto solo lleva a que el tejido sano circundante sea irradiado con altas dosis.
18
Además, permite que los haces de radiación puedan llegar al tumor desde más de 1.200 posiciones diferentes, concentrando la radiación sobre el objetivo, logrando muy buenas distribuciones de dosis en volúmenes tumorales muy pequeños, mejorando la experiencia del usuario y del paciente.
Pilares fundamentales
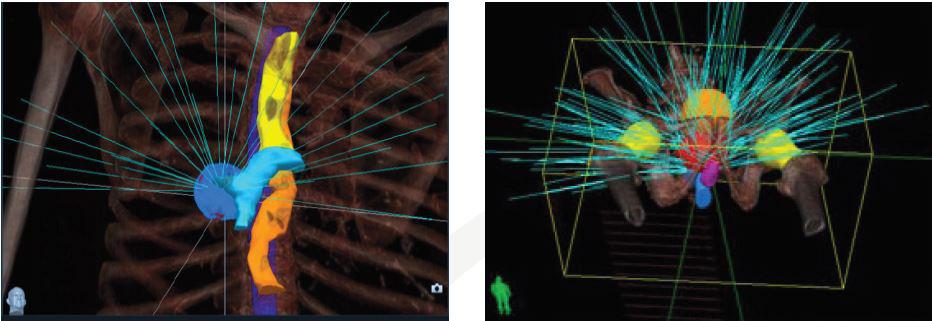
Seguimiento continuo del tumor: Los tumores suelen moverse durante la ejecución del tratamiento de radioterapia, aún estando el paciente inmovilizado. Movimientos involuntarios como el de la respiración o, en el caso de tumores en la próstata, el movimiento de la misma, pueden ocasionar variaciones en la posición del tumor durante la administración de la radiación, generando que el tejido sano circundante sea irradiado. El CyberKnife® posee un software que permite la detección automática del movimiento y facilita el seguimiento continuo del tumor mediante la corrección de la posición del brazo robótico, readecuando la dirección del haz de radiación sin tener que interrumpir el tratamiento o reposicionar al paciente. No es necesaria la colocación de fiduciales ni marco estereotáxico. Tratamiento no coplanar: El CyberKnife®, gracias a su manipulador robótico, permite realizar tratamientos no coplanares sin la necesidad de cambios en la posición de la camilla. Los haces de radiación son entregados desde diferentes planos, pudiendo atacar al tumor desde distintos ángulos.
Imágenes de planificación del tratamiento con CyberKnife que muestran los ángulos potenciales del haz (azul), distribuidos en el espacio de trabajo 3D
Flexibilidad para entregar haces no isocéntricos de radiación: CyberKnife® es el único sistema capaz de ofrecer tratamientos isocéntricos y no isocéntricos sin la necesidad de mover al paciente, proporcionando tratamientos más adecuados a lesiones con formas irregulares, preservando el tejido sano circundante. El CyberKnife® ofrece más opciones de tratamiento, proporcionando una nueva esperanza a los pacientes con tumores inoperables o a aquellos que rechazan la cirugía. Con CyberKnife se pueden planficar radiocirugías en cualquier parte del cuerpo.
19
Efectúa un seguimiento continuo de la posición del tumor, detecta la localización de los mismos y corrige el movimiento del tumor y del paciente a lo largo del tratamiento. Administra una dosis alta de radiación con precisión submilimétrica, minimizando el daño al tejido sano circundante. Este tratamiento es indoloro y no requiere la utilización de anestesia, eliminando eficazmente el riesgo de complicaciones tales como infecciones, hemorragia y otros efectos colaterales de la cirugía tradicional. Menor tiempo de recuperación en comparación con la cirugía abierta tradicional, ya que se realiza de forma ambulatoria. En el caso de los tratamientos intracraneales, los pacientes reciben la radiación de forma cómoda, gracias a la ausencia de un marco estereotáxico, y por lo tanto sin ser invasivos. El paciente puede recibir su tratamiento en un número de sesiones que oscila entre 1 y 5.
Tratamiento indoloro, no invasivo y efectivo.
20
Diseño único
Precisión CyberKnife es el único equipo que posee un acelerador lineal montado en un brazo robótico, es capaz de realizar radiocirugías y radioterapia estereotáctica logrando la mejor distribución de dosis posible, gracias a que maneja el más amplio rango de movimiento para tratamientos radiantes. Además, la precisión con la que logra dichas distribuciones de dosis es submilimétrica, incluso cuando el volumen blanco está en movimiento. Esta capacidad ha demostrado ser fundamental para minimizar los márgenes a los volúmenes de tratamiento, consiguiendo proteger el tejido sano y reducir los efectos secundarios producidos por la radiación.
Sistema de rastreo específico de acuerdo a la anatomía Es capaz de seguir el movimiento de los volúmenes involucrados en el tratamiento, utilizando marcadores fiduciales implantados o simplemente la anatomía del paciente. Imágenes intrafracción, tomadas en intervalos definidos por el usuario, permiten una continua visualización de la posición del volumen blanco. Estos sistemas de rastreo pueden ser usados en el cerebro, el hígado, la columna, el páncreas, los pulmones, entre otros.
Marcadores fiduciales en tratamiento de cáncer de próstata. CyberKnife evita irradiar el tejido sano.
21
Mejor experiencia
Ajuste automático en tiempo real La precisión y flexibilidad del CyberKnife, junto al sistema de rastreo Synchrony Respiratory, proveen el único sistema en la industria que es capaz de seguir en tiempo real el movimiento del tumor y ajustarse automáticamente a esos cambios de posición para poder entregar el tratamiento con la mayor precisión posible e irradiar el menor volumen de tejido sano posible. Gracias a su sistema de seguimiento, CyberKnife no requiere el uso de inmovilizadores invasivos.
Entrega de dosis con precisión submilimétrica El equipo es capaz de lograr tal precisión incluso con tumores en movimiento, esto es gracias a su amplio rango de haces no coplanares, que pueden ser entregados desde más de 1200 ángulos diferentes, y a su adaptación automática al movimiento del tumor, logrando minimizar los márgenes prescritos al volumen tumoral, proteger el tejido sano circundante y minimizar los efectos secundarios producidos por la radiación.
Pacientes cómodos durante el tratamiento El CyberKnife está diseñado pensando en el paciente, y esto es algo que se puede notar en la comodidad experimentada por ellos durante el tratamiento. El diseño del equipo incluye elementos ambientales relajantes y permite que el paciente esté cómodamente acostado en todo momento, además, entrega el tratamiento de forma sencilla y eficiente, eliminando la necesidad de utilizar marcos estereotácticos. Es un procedimiento no invasivo y personalizado.
22
Sistema de colimación Está equipado con tres tipos diferentes de colimadores, respondiendo a todas las necesidades del grupo de profesionales que diseña los planes de tratamiento.
Colimadores fijos
Colimador Iris
Son colimadores secundarios, circulares, de diferentes diá-
Es un colimador de apertura variable que permite haces con
metros (5, 7.5, 10, 12.5, 15, 20, 25 30, 35, 40, 50 y 60 mm)
características virtualmente idénticas a aquellos que se ob-
que permiten que el haz salga con un diámetro mucho
tienen con colimadores fijos. Posee dos partes de seis seg-
menor que con el que llega al colimador. Ellos pueden ser
mentos de tungsteno cada uno, ambos son capaces de crear
cambiados para variar el tamaño del haz dependiendo de
aperturas hexagonales. Los dos están colocados de manera
los requerimientos del plan de tratamiento.
que se encuentren desfasados 30 grados, lo que permite que la apertura resultante tenga forma de dodecaedro. Es capaz de replicar doce tamaños de colimadores fijos.
Imagen: Colimador exclusivo Imagen: iris de CyberKfnife
InCise MLC Es el colimador multiláminas diseñado específicamente para el CyberKnife convirtiéndolo en el único que está montado en un brazo robótico, su precisión y eficiencia permite conformar campos de tratamiento con múltiples formas, de acuerdo a los requerimientos de cada caso, a la vez que se entregan diversos haces no coplanares. Este colimador, integrado con los sistemas de rastreo, le permite al CyberKnife entregar los planes de tratamiento con mucha más flexibilidad y eficiencia. Imagen: colimador multiláminas
23
Sistema de imágenes Es claro que durante cada sesión, debido a movimientos in-
Este sistema está compuesto por dos tubos de rayos X (kV)
voluntarios o no del paciente, los volúmenes involucrados
ubicados en el techo de la sala de tratamiento y un arreglo
en el tratamiento de radioterapia pueden cambiar de posi-
de dos detectores localizados en el piso de la sala, debajo
ción. Para minimizar errores debido a estos movimientos,
de la camilla donde está el paciente, lo que permite tener
CyberKnife está diseñado con un sistema de adquisición
imágenes radiográficas que muestran el movimiento de los
de imágenes intrafracción que permite seguir en tiempo
volúmenes involucrados en el tratamiento. Estas imágenes
real los volúmenes de interés y así asegurar la máxima pre-
sirven de guía al sistema de rastreo para hacer luego las co-
cisión posible a la hora de entregar la dosis prescrita.
rrecciones necesarias en el posicionamiento del brazo robótico y así evitar irradiar tejido sano de manera innecesaria.
Imagen: el sistema de imágenes reconstruye las estructuras biológicas y guía el tratamiento en tiempo real.
24
Opciones del sistema
SISTEMA DE SEGUIMIENTO FIDUCIAL Permite realizar el seguimiento de tumores extracraneales a través del seguimiento de marcadores fiduciales implantados. SISTEMA DE SEGUIMIENTO XSIGHT® SPINE Permite realizar un seguimiento preciso y automático de todas las regiones de la columna vertebral, desde la primera cervical hasta la sacra. SISTEMA DE SEGUIMIENTO RESPIRATORIO SYNCHRONY® Se utiliza para supervisar la respiración del paciente y regular la posición del LINAC, de modo que coincida con el movimiento respiratorio del tumor. SISTEMA DE SEGUIMIENTO XSIGHT® LUNG Se utiliza en conjunto con el XSight® Spine para llevar a cabo la alineación del paciente. Durante el tratamiento es posible utilizarlo con el sistema Synchrony® para realizar un seguimiento de los movimientos del tumor debido a los patrones de respiración del paciente. SISTEMA ADAPTABLE DE ADQUISICIÓN DE IMÁGENES IN TEMPO™ Se ha diseñado especialmente para representar los tipos de movimientos que se encuentra en general al administrar radiación a la próstata. MULTIPLAN MD SUITE Permite a los usuarios realizar una preparación previa a la planificación y una revisión posterior a la planificación de los planes de tratamiento. Proporciona acceso a los datos del registro del paciente desde la base de datos del sistema CyberKnife® para permitir la carga remota de estudios de imágenes y de esta manera facilitar las tareas de planificación. CAMBIADOR ROBÓTICO DEL COLIMADOR XCHANGE® Es un sistema controlado por computadora para intercambiar automáticamente los colimadores del sistema, tanto antes como durante el tratamiento.
25
Diferencias entre CyberKnife y otros sistemas
26
Sistema CyberKnife®
Sistema Gamma Knife® de Elekta
Sistemas IGRT (Varian Trilogy™, Elekta Axesse®)
Sistemas de Radioterapia convencional (Varian Clinac™, Elekta Synergy®)
Exac tud total en el blanco
< 1 milímetro para tumores está cos. < 1.5 milímetros para tumores en movimiento.
< 1 milímetro para tumores está cos.
3 – 20 milímetros.
5 – 20 milímetros.
Aplicaciones
Intracraneal. Extracraneal.
Intracraneal solamente. Capacidades medulares limitadas (Perfexion
™ solamente). Intracraneal. Extracraneal.
Intracraneal. Extracraneal.
Fraccionamiento
Ilimitado.
Limitado a una única fracción debido al empo, recursos y limitaciones por el dolor.
Ilimitado.
Ilimitado.
Guía de Imagen
Guía de imagen con nua a lo largo de todo el tratamiento. Imágenes kV de alta resolución. Automá camente rastrea, detecta y corrige los movimientos del tumor y del paciente.
Ninguna, recae exclusivamente en la posición rela vamente fija de los blancos respecto a los marcos estereotáxicos. Exac tud mecánica del marco puede introducir un error entre 1.2-1.9 mm.
Limitado a la etapa del pretratamiento, solamente para el set-up del paciente. Cone-beam CT (kV o MV).
Típicamente limitada a imágenes MV en un esquema semanal.
Capacidades de entrega no-coplanar
La movilidad robó ca posibilita un enorme espacio de trabajo no-coplanar a par r de sus más de 1200 ángulos de disparo, sin interrupción del tratamiento o la necesidad de reposicionar manualmente al paciente.
Hemiesfera con colimadores fijos que posibilita un espacio de trabajo no coplanar capaz de entregar un máximo de 201 haces con ángulos definidos (190 Perfexion™).
Movilidad de gantry limitada a rotaciones horarias/an horarias, lo cual posibilita un único plano de entrega a par r de 7 haces picamente o menos. Planos adicionales pueden ser alcanzados a par r de rotaciones manuales de la camilla, pero al estar limitada al setup, la guía visual con nua resulta en una disminución de la precisión.
Movilidad de gantry limitada a rotaciones horarias/an horarias, lo cual posibilita un único plano de entrega a par r de 7 haces picamente o menos. Planos adicionales pueden ser alcanzados a par r de rotaciones manuales de la camilla, pero al estar limitada al setup, la guía visual con nua resulta en una disminución de la precisión.
Rastreo de tumores en movimiento
Entrega haces altamente conformados, sincronizados en forma precisa al movimiento del tumor resultando en una mínima exposición del tejido
sano. N/A
U liza técnicas de ga ng / breath-holding que resulta en menores márgenes de contorno y exposición de tejido sano innecesariamente.
U liza técnicas de ga ng / breath-holding que resulta en menores márgenes de contorno y exposición de tejido sano innecesariamente.
Marcos estereotác cos
No se requieren.
Requiere la u lización de marcos en todos los casos.
Se requieren en prác camente la mayoría de los casos intra y extracraneales.
Se u lizan disposi vos de inmovilización. Bajas dosis por fracción disminuye la necesidad de precisión.
27
SUMARIO
CyberKnife S7 Precisión líder en la industria y comodidad para el paciente • Proporciona dosis con precisión submilimétrica, incluso a objetivos en movimiento. • Soluciones de seguimiento y administración de tratamientos específicos de la anatomía para el cerebro, la columna vertebral, los pulmones y la próstata. • Las soluciones de sincronización de movimiento CyberKnife se utilizan de forma rutinaria para enfermedades en otros sitios, como el hígado (incluida la enfermedad metastásica), el páncreas y las indicaciones en la pelvis y el área torácica. • Se ajusta automáticamente en tiempo real a la respiración y otros cambios en la posición del tumor. • Espacio de trabajo mejorado para ampliar la cobertura.
Mayor eficiencia • Reducción del tiempo de tratamiento. • Mejor recuperación del paciente. • Personalización real de las terapias.
Escalabilidad • Opción de colimador InCise Multileaf: SBRT basado en MLC de alta resolución. • Admite sistemas de actualización que permiten escalar su performance, como el colimador InCise Multileaf.
29
Antes del procedimiento
Una vez en la sala de tratamiento.
• El paciente se realiza una tomografía computarizada
• El paciente se posiciona cómodamente en la camilla
de alta resolución, para determinar el tamaño, forma y
de tratamiento.
localización del tumor. • La tomografía digitalizada es transferida a la estación de trabajo del sistema CyberKnife®, donde se planificará el tratamiento, definiendo los volúmenes que serán blanco de la radiación y aquellos volúmenes a riesgo que se desean proteger. • A continuación, un equipo de profesionales calificados utilizan el sistema de planificación CyberKnife® para gene-
• Se adquieren las imágenes de verificación de la posición del paciente y de los volúmenes involucrados en el tratamiento. • Luego se realiza el seguimiento de los volúmenes que estén sujetos a movimientos intrafracción. • El cabezal del equipo se mueve lentamente alrededor del paciente para ubicarse en todas las direcciones planificadas para la entrega del tratamiento.
rar un plan de tratamiento. El plan es diseñado para dirigir
• Cada sesión de tratamiento tiene una duración de entre
la dosis de radiación prescrita por el radioterapeuta al vo-
30 y 90 minutos, dependiendo del tipo de tumor a tratar.
lumen blanco, limitando la exposición de los tejidos sanos circundantes. Este paso permite simular todos los haces de radiación que van a ser aplicados sobre el paciente antes de entregar el tratamiento.
ESCANEÁ Y CONOCÉ EL TESTIMONIO DE LOS PACIENTES
30
31
CÁNCER CEREBRAL El Sistema de Radiocirugía Robótica CyberKnife® fue aprobado por la U.S. Food and Drug Administration en el 1999 para el tratamiento de tumores en la cabeza y la base del cráneo. CyberKnife administra dosis elevadas de radiación directamente a los tumores cerebrales.
El Sistema CyberKnife ofrece a los pacientes una alternativa no invasiva a la cirugía del cáncer, y puede utilizarse en tumores cerebrales que se consideran inoperables debido a su ubicación en la cabeza, en pacientes que no pueden someterse a una operación debido a su mala condición médica o en pacientes que rechazan la cirugía. Además, el Sistema CyberKnife puede tratar tumores benignos o no cancerosos y otros trastornos, como por ejemplo la neuralgia del trigémino y las malformaciones arteriovenosas.
Los tratamientos para el cáncer cerebral con el Sistema CyberKnife se realizan de manera ambulatoria a lo largo de un período de entre uno y cinco días y no requieren noches de hospitalización. La mayoría de los pacientes no experimentan ningún efecto secundario o son mínimos, con un tiempo de recuperación rápido. ¿Qué es el cáncer cerebral? El cáncer cerebral es un crecimiento anormal de células en el cerebro, que provoca la acumulación de un conjunto de células denominado tumor cerebral. Si las células anormales eran originariamente células cerebrales que empezaron a crecer de manera incontrolada, se trata de un tumor cerebral primario. Si las células anormales se originaron en otra parte del cuerpo, como el pulmón o la mama, y fueron transportadas al cerebro por la sangre u otros fluidos corporales, se considera que es un tumor cerebral metastásico.
Tumores cerebrales primarios Hay muchos tipos de tumores cerebrales primarios, como por ejemplo, meningiomas, adenomas hipofisarios, schwannomas y gliomas, que se dividen en astrocitomas, ependimomas, meduloblastomas y oligodendrogliomas. Cada tumor cerebral primario se clasifica en función del tipo de célula cerebral normal en el que tiene su origen y muestra unas características y patrones de crecimiento especiales. Los gliomas representan el 40% de la totalidad de los tumores cerebrales primarios y es frecuente que se extiendan desde el cerebro hasta otras partes del cuerpo. El tipo más agresivo de glioma se denomina glioblastoma multiforme. 32
Tumores cerebrales metastásicos
Radiocirugía
Las células que forman los tumores cerebrales metastásicos llegan al cerebro desde otras partes del cuerpo a través del torrente circulatorio, siguiendo los nervios o en el líquido que rodea la médula espinal y el cerebro. En la mayoría de los casos, estas células tienen su origen en tumores de pulmón, mama, piel o colon, y se depositan en el cerebro, donde crecen hasta formar un tumor.
Durante los últimos 25 o 30 años, la radiocirugía se ha convertido en una alternativa a la cirugía. A diferencia de la radioterapia convencional, durante la cual se administran pequeñas dosis de radiación durante semanas y meses, la radiocirugía puede tratar un tumor en entre una y cinco sesiones mediante la administración de una dosis elevada de radiación con una precisión extrema. Durante la radiocirugía con CyberKnife, se administran centenares de pequeños haces de radiación desde diferentes ángulos que confluyen en el tumor. Este tratamiento permite atacar el tumor con una dosis elevada de radiación sin dañar el sensible tejido cerebral circundante. Para ser eficaz y segura, la radiocirugía debe ser precisa. Para conseguir esta precisión, algunos aparatos de radiocirugía, como el Gamma Knife®, requieren la fijación de un marco estereotáctico rígido a la cabeza del paciente con el fin de que el sistema pueda situar la localización exacta del tumor. Estos marcos se atornillan al cráneo del paciente después de administrarle anestesia local. Muchos pacientes consideran que estos marcos son incómodos y dolorosos. Además, si son necesarias múltiples sesiones terapéuticas, es posible que el paciente deba ser hospitalizado con el marco colocado durante varios días hasta la finalización del tratamiento.
Los tumores cerebrales, tanto primarios como metastásicos, pueden ser muy peligrosos porque pueden comprimir el sensible tejido cerebral y los nervios situados en la cabeza, provocando síntomas como pérdida de visión, pérdida de audición, problemas de equilibrio, dolor o convulsiones. A medida que estos tumores crecen, pueden ser potencialmente mortales porque alteran partes críticas del cerebro responsables de la respiración y otras funciones vitales básicas.
Otros aparatos de radiocirugía, como el Sistema de Radiocirugía Robótica CyberKnife, traen una mejora respecto a otras técnicas de radiocirugía gracias a la eliminación de los marcos estereotácticos. En consecuencia, el Sistema CyberKnife permite a los médicos lograr un alto grado de precisión de manera no invasiva y a los pacientes recibir el tratamiento de manera ambulatoria.
33
¿Cómo trata CyberKnife el cáncer cerebral? El Sistema CyberKnife puede determinar la localización exacta del tumor en tiempo real mediante el uso de imágenes radiográficas obtenidas durante el tratamiento del cáncer cerebral que hacen referencia a las estructuras óseas únicas de la cabeza del paciente. CyberKnife ostenta el récord de eficacia clínica demostrada. Más de 25.000 pacientes con tumores cerebrales y otras lesiones intracraneales han sido tratados con el Sistema CyberKnife. Se utiliza como tratamiento único o en combinación con otros tratamientos para el cáncer cerebral, como la quimioterapia, la cirugía o la radioterapia de todo el cerebro.
¿Qué conlleva un tratamiento con CyberKnife típico? A diferencia de lo que ocurre con otros sistemas de radiocirugía –como Gamma Knife– con el Sistema CyberKnife no es necesario colocar a los pacientes un marco de cabeza rígido e invasivo. En la fase de preparación, el radioterapeuta confeccionará una máscara de malla blanda que se adapta al rostro del paciente. Esta máscara, cómoda y no invasiva, ayuda al paciente a mantener la cabeza y el cuello inmóviles durante el tratamiento. Con la máscara puesta, se llevará a cabo una TC. A continuación el equipo de CyberKnife empleará los datos de esta TC para determinar el tamaño, la forma y la ubicación exactos del tumor. Es posible que también sea necesario utilizar imágenes médicas de Resonancia Magnética, Tomografía de Emisiío de Positrón o Angiografía para visualizar completamente el tumor y la ana-
34
tomía cercana. Una vez obtenidas las imágenes, la máscara facial se retirará y guardará hasta el inicio del tratamiento con CyberKnife. Entonces un físico médico y el médico del paciente utilizan los datos para elaborar el plan terapéutico del paciente. El paciente no tiene que estar presente en ese momento. Durante la fase de planificación del tratamiento con CyberKnife, se descargarán los datos de la TC, RM o TEP al software de planificación de tratamiento del Sistema CyberKnife. El equipo médico determinará el tamaño del área que debe irradiarse y la dosis de radiación. Además, identificarán las estructuras críticas en que debe minimizarse la radiación. Con esta información, el Sistema CyberKnife calcula el plan de administración de la radiación óptimo para tratar el tumor.
El plan de tratamiento aprovechará la maniobrabilidad extrema del Sistema CyberKnife, lo que permite la administración de un tratamiento más seguro y preciso.
administrará radiación al tumor. Lo único que tendrá que hacer el paciente durante el tratamiento es relajarse y permanecer lo más quieto posible.
Una vez elaborado el plan de tratamiento del cáncer cerebral, el paciente volverá al centro CyberKnife para comenzar a tratarse. Los médicos pueden optar por administrar el tratamiento en una única sesión o fraccionarlo en varios días. Normalmente, los tratamientos para el cáncer cerebral se llevan a cabo en un máximo de cinco días. Para la mayoría de los pacientes, el tratamiento con CyberKnife es una experiencia completamente indolora. Los pacientes llevan ropa de calle cómoda y, dependiendo del centro donde se
Una vez finalizada la sesión con CyberKnife, la mayoría de los pacientes retoman rápidamente sus rutinas cotidianas sin apenas interrumpir sus actividades normales. Si el tratamiento se administra por fases, el paciente tendrá que volver para recibir tratamientos adicionales durante los días siguientes según recomienden los médicos. Los efectos secundarios varían de un paciente a otro. Por lo general, algunos pacientes experimentan efectos secundarios mínimos de los tratamientos con CyberKnife, pero éstos suelen desaparecer en una o dos semanas. Antes del
traten, es posible que se les permita llevar música para escucharla durante el tratamiento. Si prefieren, pueden llevar algo para leer, o ser acompañados por un amigo o familiar para que los apoye antes del tratamiento y después del mismo.
tratamiento, el médico comentará con el paciente todos los posibles efectos secundarios que puede experimentar. Además, el médico puede prescribir medicación con el fin de controlar cualquier efecto secundario, si lo hay. Después de terminar un tratamiento con radiocirugía CyberKnife, es importante que se programen visitas de revisión y que el paciente asista a las mismas. Además, debe tener presente que el tumor no desaparecerá de repente. La respuesta al tratamiento varía de un paciente a otro. La experiencia clínica revela que la mayor parte de los pacientes responden muy bien a los tratamientos con CyberKnife. Como seguimiento, los médicos controlarán el resultado en los meses y años siguientes al tratamiento, a menudo con el uso de TC o TEP-TC.
Cuando llega el momento del tratamiento, el paciente se tumba en la mesa mientras le colocan la máscara facial personalizada en su sitio. El robot controlado por ordenador del Sistema CyberKnife se moverá alrededor del cuerpo del paciente hasta las diferentes localizaciones desde las que
35
RADIOCIRUGÍA SNC El CyberKnife® es el primer y único sistema que puede realizar radiocirugía en todo el sistema nervioso central, extendiéndose a todos los niveles de la columna, desde las cervicales hasta las sacras, sin la necesidad de utilizar marco estereotáctico o fiduciales. Permite tratar lesiones benignas, malignas, vasculares e intracraneales funcionales, y periféricas, con una preservación de la visión y de la audición ampliamente mejorada. El CyberKnife® ha revolucionado la radiocirugía intracraneal sin marco estereotáctico, siendo el único sistema con guía por imágnenes continua durante la administración de la radiación, que detecta constantemente el movimiento intracraneal, permitiendo verificar que las dosis prescripta sea entregada en el objetivo de planificación.
36
La columna vertebral puede moverse hasta 4mm durante un tratamiento de 15 minutos, aunque el paciente se encuentre inmovilizado. Mientras que con los sistemas de IGRT (Radioterapia Guiada por Imágenes) los movimientos de los volúmenes a tratar son corregidos ampliando solo los márgenes asociandos al movimiento del tumor, lo que conlleva a irradirar de igual forma tejido sano, el CyberKnife®, con su sistema de rastreo es capaz de seguir el volumen a tratar y corregir la posición del cabezal para brindar exactitud y precisión durante todo el tratamiento.
La dosis correcta en el lugar correcto
¿Cómo trata CyberKnife el cáncer de columna? El Sistema CyberKnife ofrece a los pacientes una nueva opción para el tratamiento del cáncer de columna. A diferencia de la radioterapia convencional, durante la cual se administran dosis bajas de radiación durante semanas y meses, el Sistema CyberKnife puede tratar un tumor en entre uno y cinco días mediante la administración de una dosis elevada de radiación con una precisión extrema. Los tumores de columna suponen un desafío terapéutico porque se mueven con la respiración del paciente. La radioterapia convencional no puede tener en cuenta este movimiento, por lo que la radiación daña el tejido sano circundante. El Sistema CyberKnife es capaz de lograr un alto grado de precisión de manera completamente incruenta, sin el uso de marcos temporales o marcadores fiduciales implantados. Puede determinar la localización exacta de un tumor a tiempo real durante el tratamiento. El Sistema CyberKnife permite al paciente respirar normalmente en la mesa de tratamiento, lo
Sistema de Radioterapia Convencional Tratamiento coplanar de 7 haces
que posibilita que el médico apunte al tumor en movimiento y administre centenares de haces de radiación desde diferentes ángulos que confluyen en el tumor. Con este método, el Sistema CyberKnife es capaz de administrar una dosis elevada de radiación al tumor evitando dañar el sensible tejido de la médula espinal y otras estructuras críticas. En consecuencia, la radiación se administra de una manera más precisa y los tratamientos pueden llevarse a cabo en un período de tiempo más corto. Por lo general, los tratamientos con CyberKnife sólo tienen efectos secundarios mínimos. En ocasiones, los pacientes refieren náuseas leves temporales, sobre todo si recibe el tratamiento la parte inferior del abdomen. Antes del tratamiento, el médico comentará con el paciente todos los posibles efectos secundarios que puede experimentar. Además, el médico puede prescribir medicación con el fin de controlar cualquier efecto secundario, si lo hay.
Sistema CyberKnife® Tratamiento no coplanar de 103 haces
37
RADIOCIRUGÍA DE PULMÓN Los tumores que se mueven con la respiración suelen generar mucha incertidumbre con respecto a la precisión del tratamiento. El Synchrony Respiratory Tracking System permite detectar automáticamente los tumores que se mueven con la respiración, posibilitando un tratamiento continuo mientras que el paciente respira normalmente. De esta manera se evitan las técnicas de compensación para movimientos respiratorios. La radioterapia convencional, denominada radioterapia por haz externo, suele implicar la administración de haces amplios de radiación que abarcan tanto el tumor como una cantidad significativa de tejido sano circundante. Estos haces de radiación son necesarios porque los tumores
Durante este tratamiento, la dosis de radiación es limitada para reducir la toxicidad para el paciente provocada por los daños del tejido pulmonar sano. Por tanto, la radioterapia por haz externo convencional suele administrarse en pequeñas dosis de entre 30 y 40 sesiones durante un período de cuatro a seis semanas. Las tasas de toxicidad varían ampliamente en los estudios publicados, según los cuales la toxicidad grave a corto plazo se sitúa en el 10-30% y la toxicidad grave a largo plazo (neumonitis por radiación) en el 18%; los intentos por aumentar la dosis de radiación administrada mediante los métodos convencionales de radioterapia han desembocado en una toxicidad aún mayor.
se mueven con la respiración del paciente.
PTV CTV
PTV
GTV
CTV
Técnica convencional de compensación respiratoria convencional
• GTV (Gross Tumor Volume) = Contorno visual del tumor
GTV
• CTV (Clinical Tumor Volume) = GTV + margen (incluye la enfermedad microscópica)
CyberKnife®
• PTV (Planning Tumor Volume) = CTV + margen (incluye las inexactitudes de planificación y tratamiento)
Un cáncer de pulmón se puede mover hasta 50 mm
38
¿Cómo trata CyberKnife el cáncer de pulmón? A diferencia de la radioterapia tradicional, el Sistema CyberKnife identifica de manera precisa la localización del tumor mientras el paciente respira normalmente durante el tratamiento y puede utilizarse, en algunos casos, para tratar tumores pulmonares de manera no invasiva. Como parte del diagnóstico, los médicos identificarán la ubicación y el tamaño del tumor pulmonar. En función de estos resultados, algunos pacientes pueden no requerir la implantación de marcadores fiduciales. El Sistema CyberKnife sólo utilizará las características identificadoras del propio tumor para visualizar claramente la lesión en el tórax y seguir su movimiento mientras el paciente respira normalmente. Antes de que puedan iniciarse los tratamientos con CyberKnife, se confeccionará un soporte corporal especial para los pacientes. El soporte está hecho de un material blando que se amolda al cuerpo del paciente y está diseñado para que el tratamiento sea más cómodo y para garantizar
que la posición del paciente sea la misma para cada sesión de tratamiento. Además, el paciente se pondrá un chaleco especial, que se lleva durante el tratamiento con CyberKnife y permite al robot correlacionar el movimiento torácico y los patrones respiratorios con la posición del tumor. Los datos generados con el chaleco permiten al robot CyberKnife seguir de manera precisa el movimiento del tumor mientras éste administra cada haz de radiación, garantizando una entrega de la radioterapia segura y precisa. El Sistema CyberKnife podrá calcular el plan de administración de radiación óptima para tratar el/los tumor(es) de pulmón. El plan de tratamiento personalizado de cada paciente aprovechará la maniobrabilidad extrema del Sistema CyberKnife, lo que permite la administración de un tratamiento seguro y preciso para el cáncer de pulmón. Una vez elaborado el plan de tratamiento, el paciente volverá al centro de radiocirugía para recibir el tratamiento. Normalmente, el tratamiento se administra en entre una y cinco sesiones.
Para los pacientes, el tratamiento con CyberKnife es una experiencia completamente indolora
39
CÁNCER DE HÍGADO Al igual que con el resto de las indicaciones, el Sistema de Radiocirugía Robótica CyberKnife® fue aprobado por la U.S. Food and Drug Administration en el 2001 para el tratamiento de tumores en cualquier lugar del cuerpo, incluyendo el hígado. CyberKnife puede administrar dosis elevadas de radiación directamente a los tumores hepáticos, ofreciendo un tratamiento alternativo mínimamente invasivo para el cáncer de hígado a los pacientes que no pueden someterse a una intervención quirúrgica debido a su mala condición médica o que rechazan la cirugía.
Normalmente, los tratamientos para el cáncer de hígado con CyberKnife se realizan de manera ambulatoria en un período de tiempo de entre uno y cinco días y no requieren noches de hospitalización. La mayoría de los pacientes experimentan ningún efecto secundario o efectos secundarios mínimos con un tiempo de recuperación rápido. ¿Qué es el cáncer de hígado? El cáncer de hígado es el crecimiento anormal de células en el hígado que causa un tumor hepático. Si las células anormales se originaron como células de cáncer de hígado, el grupo de células resultante se denomina tumor hepático primario. Algunos tumores son benignos, lo que significa que no son cancerosos y no se extienden a otras partes del cuerpo. Los tumores hepáticos malignos, en cambio, pueden invadir otros órganos y extenderse por todo el cuerpo. La mayoría de los cánceres de hígado primarios se clasifican como carcinoma hepatocelular o hepatomas. El tumor hepático metastásico es un cáncer de hígado mucho más frecuente. En este caso, las células cancerosas de otra parte del cuerpo son transportadas hasta el hígado por la sangre u otros fluidos corporales. Los tumores hepáticos metastásicos pueden extenderse al hígado desde el colon, el pulmón, la mama, el estómago y el páncreas, entre otros puntos del cuerpo. El Sistema CyberKnife utiliza el Sistema de Seguimiento Respiratorio Synchrony®
40
¿Cómo se trata el cáncer de hígado?
Radioterapia
Una vez detectado el cáncer hepático y determinado su estadio, el médico comentará diferentes opciones terapéuticas. Los tratamientos del cáncer de hígado dependen del tipo y el estadio del cáncer. El cáncer de hígado primario inicial, y algunos tumores metastásicos, pueden tratarse con cirugía, con el objetivo de eliminar la totalidad del tumor. Otra posibilidad es tratar el tumor mediante ablación –o destruirlo allí donde está– mediante uno de varios métodos posibles, como pueden ser la aplicación de una sonda a temperatura elevada (ablación por radiofrecuencia), una sonda a baja temperatura (crioablación), un tratamiento quimioterapéutico dirigido (quimioembolización), radioterapia convencional, inyección local de alcohol o radiocirugía de CyberKnife. En algunos casos, es posible eliminar el hígado entero y sustituirlo por un trasplante de un donante. En casos de cáncer de hígado más avanzado, puede emplearse quimioterapia combinada con algunos de los tratamientos antes mencionados.
La radioterapia convencional, denominada radioterapia por haz externo, suele consistir en la administración de campos amplios de radiación que abarcan tanto el tumor como una cantidad significativa de tejido sano circundante. Estos campos amplios de radiación, administrados en pequeñas dosis durante muchas sesiones en el transcurso de varias semanas, son necesarios para compensar el movimiento del tumor durante la respiración del paciente. Los campos amplios de radiación limitan la dosis total de radiación que puede administrarse en cada sesión debido a la toxicidad del tejido hepático normal que incluyen, lo que obliga a dividir el tratamiento total en entre 30 y 40 sesiones que se administran durante semanas. Los daños del tejido hepático normal provocados por la radiación se han descrito como enfermedad hepática causada por radiación, un síndrome que puede darse en las primeras semanas posteriores a la radioterapia y que, en los casos más graves, puede causar insuficiencia hepática. Informes recientes han puesto de manifiesto que las tasas de supervivencia a un año oscilan entre el 47 y el 95% y, a los cinco años, entre el 11 y el 25%, y que en general los resultados son mejores cuando se tratan tumores pequeños con dosis elevadas.
CyberKnife no requiere inmovilizadores invasivos
Retención de la respiración La retención de la respiración es un procedimiento utilizado con otros sistemas de tratamiento en la cual la radiación se administra cuando se cree
que el tumor se encuentra en una ubicación determinada durante el ciclo respiratorio del paciente. La retención de la respiración da por sentadas varias suposiciones acerca de la ubicación del tumor hepático. Estas suposiciones son que el tumor siempre se encuentra en la misma localización durante un momento específico del patrón respiratorio del paciente; el patrón respiratorio del paciente no cambia durante el tratamiento; y los pacientes respiran durante el tratamiento igual que durante la fase de planificación. En realidad, muchos pacientes respiran de diferentes maneras durante el tratamiento, sobre todo si están nerviosos o se duermen. Estos cambios de los patrones respiratorios pueden provocar errores en la administración de radiación. 41
Apnea inspiratoria Mientras el paciente retiene el aliento, se activa el haz de radiación, que vuelve a desactivarse justo antes de que el paciente empiece a respirar normalmente de nuevo. La apnea inspiratoria da por supuesto que el tumor se encontrará en una ubicación determinada cuando el paciente inspira. Esto puede no ser siempre así, según la profundidad de la respiración del paciente. Además, la apnea inspiratoria puede ser muy difícil para los pacientes con enfermedad pulmonar avanzada. Las técnicas como la retención de la respiración y la apnea inspiratoria han permitido a los médicos la administración de dosis mucho más elevadas de radiación en apenas entre tres y cinco sesiones con un procedimiento denominado radioterapia estereotáctica corporal (SBRT).
Este tratamiento alternativo para el cáncer hepático ha demostrado ser más eficaz que la radioterapia convencional, con un control tumoral que oscila entre el 70 y el 100% 18 meses después del tratamiento y tasas muy bajas de efectos secundarios, generalmente leves. Se ha observado un control tumoral ligeramente más elevado en tumores pequeños9 y lesiones metastásicas. Aunque la SBRT permite a los médicos preservar más tejido hepático normal que los métodos convencionales, normalmente todavía exige grandes márgenes alrededor de los tumores para garantizar que la radiación se administra al tumor y compensar las imprecisiones de la retención de la respiración y la apnea inspiratoria.
CyberKnife puede administrar dosis elevadas de radiación directamente a los tumores hepáticos Radiocirugía Los aparatos de radiocirugía, como por el ejemplo el Sistema de Radiocirugía Robótica CyberKnife®, ofrecen a los pacientes una nueva opción para el tratamiento del cáncer de hígado. El Sistema CyberKnife puede administrar dosis elevadas de radiación con una precisión extrema gracias a la capacidad de seguir la localización de los tumores hepáticos a tiempo real durante el tratamiento. El Sistema CyberKnife utiliza el Sistema de Seguimiento Respiratorio Synchrony® para seguir el movimiento del tumor mientras los pacientes respiran normalmente y ajusta el haz de radiación en consecuencia. El Sistema Synchrony
correlaciona la subida y la bajada del pecho con la ubicación real del tumor en radiografías. Como resultado, durante el tratamiento con CyberKnife, los pacientes pueden permanecer cómodamente tumbados y respirar con normalidad sin ningún tipo de marco y no se les pide que retengan la respiración ni que realicen complicadas maniobras respiratorias. El Sistema Synchrony puede seguir objetivos en movimiento con una precisión de 1 milímetro o superior, lo que permite a los médicos administrar radiación de manera precisa al tumor y limitar la exposición del tejido circundante sano.
¿Cómo trata CyberKnife el cáncer de hígado? El tratamiento del cáncer de hígado con radioterapia es complicado, porque los tumores hepáticos se mueven con la respiración. Además, el tejido que rodea los tumores hepáticos es muy sensible y 42
puede sufrir daños con facilidad. El Sistema de Radiocirugía CyberKnife es capaz de administrar dosis muy elevadas de radiación a tumores hepáticos tanto primarios como metastásicos con una precisión extrema. En conjunción con el Sistema CyberKnife opera el Sistema de Seguimiento Respiratorio Synchrony®, que permite al haz de radiación seguir el movimiento tumoral a tiempo real y posibilita que los pacientes respiren normalmente durante las sesiones de tratamiento. Con el Sistema CyberKnife, los médicos pueden apuntar a un objetivo en movimiento –el tumor hepático– e irradiarlo sin dañar el tejido circundante sano. En consecuencia, el tratamiento con CyberKnife es más cómodo para los pacientes, la radiación se administra con mayor precisión y los tratamientos pueden llevarse a cabo en entre una y cinco sesiones.
RADIOCIRUGÍA DE PRÓSTATA La próstata puede moverse de manera impredecible más de 5mm en solo 30 segundos. El CyberKnife®, con su guía visual continua, permite brindar al paciente tratamientos precisos y seguros en la próstata, evitando afectar órganos críticos como el recto, la vejiga y la uretra. Es el único sistema capaz de administrar a la próstata, de forma no invasiva, dosis equivalentes a braquiterapia HDR (high dose rate, por sus siglas e inglés) con precisión submilimétrica, reduciendo los márgenes que se prescriben a los volúmenes a tratar y asegurando que se minimice el volumen de tejido sano que recibe altas dosis de radiación.
Sistema de Radioterapia Convencional Tratamiento Coplanar de 6 haces
¿Cómo trata CyberKnife el cáncer de próstata? El problema al que se enfrentan los médicos al tratar los tumores de próstata con radioterapia es que este órgano se mueve de manera imprevisible cuando pasa aire por el recto y se vacía y se llena la vejiga. Minimizar los movimientos grandes de la próstata puede ayudar a reducir la irradiación innecesaria del tejido sano circundante. El Sistema de Radiocirugía Robótica CyberKnife® es capaz de solventar este problema gracias a la identificación continua de la ubicación exacta del tumor de próstata durante el transcurso del tratamiento. Durante esta sesión con CyberKnife, el paciente puede permanecer inmóvil y respirar normalmente mientras el médico apunta a un objetivo móvil –la próstata– y la irradia sin dañar las áreas circundantes. En consecuencia, la intervención es más cómoda para los pacientes, la radiación se administra con mayor precisión y los tratamientos pueden llevarse a cabo en entre uno y cinco días. En publicaciones con revisión científica externa, los investigadores de CyberKnife han observado reducciones fiables de los valores de PSA con tasas bajas de efectos secundarios leves durante el seguimiento a corto plazo después de un monotratamiento con CyberKnife, lo que sostiene el beneficio clínico con investigaciones recientes.
CyberKnife® System Plan de tratamiento de próstata
43
CANCER DE PÁNCREAS CyberKnife está aprobado para el tratamiento de tumores en el páncreas y ha demostrado ser el mejor sistema para atender esta patología con el menor riesgo de eventos adversos. Administra dosis elevadas de radiación directamente a los tumores pancreáticos. El Sistema CyberKnife ofrece un tratamiento alternativo mínimamente invasivo para el cáncer de páncreas a los pacientes que no pueden someterse a una intervención quirúrgica debido a su mala condición médica o que rechazan la cirugía. Normalmente, los tratamientos para el cáncer de páncreas con CyberKnife se realizan de manera ambulatoria en un período de tiempo de entre uno y cinco días y no requieren noches de hospitalización. La mayoría de los pacientes experimentan ningún efecto secundario o efectos secundarios mínimos con un tiempo de recuperación rápido.
¿Qué es el cáncer de páncreas? El cáncer pancreático es el crecimiento anormal de células en el páncreas que produce un tumor. Si las células anormales se originaron en el páncreas, se considera un tumor pancreático primario. En este caso, la mayoría de los tumores aparecen en los conductos del páncreas y se denominan adenocarcinomas. Si las células anormales se originaron en el páncreas y fueron transportadas a otras partes del cuerpo por la sangre u otros fluidos corporales, se considera que es un tumor pancreático metastásico. En las primeras fases, el cáncer pancreático no suele causar síntomas. Cuando hay síntomas, normalmente el cáncer está avanzado. Los pacientes pueden experimentar ictericia, dolor y pérdida de peso. Puede emplearse una exploración física y análisis de sangre en el diagnóstico inicial, pero los análisis de sangre no bastan para confirmar la presencia de cáncer de páncreas. Para facilitar el diagnóstico pueden usarse tomografías computarizadas (TC), resonancias magnéticas (RM), ecografías y tomografías por emisión de positrones-TC (TEP-TC). Es posible que haya que realizar una biopsia para determinar la naturaleza de las células cancerosas en los pacientes que no son aptos para cirugía. Entonces los médicos determinan el “estadio” –o extensión de la enfermedad– estableciendo el tamaño del tumor y su grado de extensión.
tratamiento alternativo mínimamente invasivo para el cáncer de páncreas a los pacientes que no pueden someterse a una intervención quirúrgica
44
¿Cómo trata CyberKnife el cáncer de páncreas? El Sistema CyberKnife se ha empleado para tratar el cáncer pancreático en pacientes que no son aptos a intervención quirúrgica, en quienes rechazan la cirugía y en pacientes en quienes han fracasado la cirugía u otros tratamientos. El tratamiento con radioterapia de los tumores situados en el páncreas y sus proximidades es complicado, porque los tumores se mueven con la respiración, lo que dificulta la administración precisa de radiación. En consecuencia, es posible que el tumor no reciba radiación suficiente y el tejido sano que hay cerca de éste
puede sufrir daños. Con otros tipos de radioterapia –con frecuencia denominados radioterapia convencional, por haz externo o de intensidad modulada–la dosis total de radiación se divide en dosis más pequeñas que se administran en entre 25 y 40 sesiones con el fin de minimizar los daños del tejido sano circundante. La radiocirugía CyberKnife está diseñada para destruir el tejido tumoral con dosis elevadas administrada en entre una y cinco sesiones. Para no causar efectos secundarios es necesario un sistema de alta precisión que detecte el movimiento del tumor a lo largo de las lesiones de tratamiento y administre la radiación con una precisión extrema.
Tratamiento con CyberKnife Con el Sistema CyberKnife, los médicos pueden apuntar a un objetivo en movimiento –el tumor pancreático– e irradiarlo sin dañar el tejido circundante sano. En consecuencia, el tratamiento con CyberKnife es más cómodo para los pacientes, la radiación se administra con mayor precisión y los tratamientos pueden llevarse a cabo en entre una y cinco sesiones. Durante el primer paso del proceso terapéutico, se programa una breve intervención ambulatoria durante la cual se insertan entre tres y
cinco fiduciales –diminutos granos de oro del tamaño aproximado de un grano de arroz– en el tumor pancreático con la guía de una TC. El Sistema CyberKnife utiliza estos marcadores como puntos de referencia para identificar la localización exacta del tumor pancreático durante el tratamiento. Una vez implantados los fiduciales, el paciente debe esperar una semana aproximadamente antes de que pueda empezar la planificación del tratamiento con CyberKnife para asegurarse de que el movimiento de los fiduciales se ha estabilizado. 45
Durante la preparación y obtención de imágenes, el paciente se colocará en un soporte corporal adaptado, que está diseñado para que esté más cómodo y garantiza una posición uniforme durante las pruebas de imagen y el tratamiento. Además, el paciente se pondrá un chaleco Synchrony, que se lleva durante el tratamiento con CyberKnife y permite al robot correlacionar el movimiento torácico y los patrones respiratorios con la posición del tumor. Los datos generados con el chaleco permiten al robot CyberKnife seguir de manera precisa el movimiento del tumor mientras éste administra cada haz de radiación, garantizando una administración de la radioterapia segura y precisa.
A continuación, un físico médico, junto con los médicos del paciente, elaborará un plan de tratamiento específico. El paciente no tiene que estar presente en ese momento. Durante la planificación del tratamiento, se descargan los datos de las TC y RM en el software del Sistema CyberKnife. El equipo médico determina el tamaño del área que recibirá la radiación, así como la dosis, e identifica las estructuras críticas en que debe minimizarse la radiación. El plan de tratamiento personalizado de cada paciente aprovechará la maniobrabilidad extrema del Sistema CyberKnife, lo que permite la administración de un tratamiento seguro y preciso para el cáncer de páncreas.
Con el chaleco puesto, el paciente se someterá
Una vez elaborado el plan de tratamiento, el pa-
a una serie de TC, que permitirán al equipo de CyberKnife determinar el tamaño, la forma y la localización exactos del tumor. Es posible que también sea necesaria una RM para visualizar completamente el tumor, el páncreas y la anatomía cercana. Una vez obtenidas las imágenes, el soporte corporal se guardará para el resto de tratamientos con CyberKnife.
ciente vuelve al centro CyberKnife para recibir el tratamiento. Normalmente, el tratamiento del cáncer pancreático se administra en cuatro o cinco sesiones, que en general se llevan a cabo en el plazo de una semana.
46
El tratamiento con CyberKnife es una experiencia completamente indolora. Los pacientes llevan ropa de calle cómoda y, dependiendo del centro donde se traten, es posible que se les permita lle var música para escucharla durante el tratamiento. Además, los pacientes pueden llevar algo para leer o escuchar mientras esperan y tener consigo un amigo o familiar para que los apoye antes del tratamiento y después del mismo. Cuando llega la hora del tratamiento, el paciente se pone el chaleco Synchrony y se tumba en el soporte corporal adaptado. El radioterapeuta se asegurará de que el chaleco está ajustado correctamente y de que el paciente está bien colocado en la camilla de tratamiento.
cotidianas sin apenas interrumpir sus actividades normales. Si el tratamiento se administra por fases, el paciente tendrá que volver para recibir tratamientos adicionales durante los días siguientes según determinen los médicos. Los primeros resultados indican que los pacientes toleran muy bien la intervención con CyberKnife y que ésta no deteriora el funcionamiento del páncreas. Los médicos comentarán todos los posibles efectos secundarios antes del tratamiento. Además, los médicos pueden prescribir medicación para controlar cualquier efecto secundario, si lo hay.
Cuando empieza el tratamiento, se seguirá y detectará de manera ininterrumpida la localización del tumor de páncreas mientras el paciente respira normalmente. El equipo médico observará cada paso del proceso mientras el Sistema CyberKnife sigue el movimiento del tumor de páncreas y le administra radiación de manera segura y precisa. El robot controlado por ordenador del Sistema CyberKnife se moverá alrededor del cuerpo del paciente hasta las diferentes localizaciones desde las que administrará radiación. A cada posición, el robot se detendrá. Entonces, un software especial determinará con precisión donde debe administrarse la radiación correlacionando la ubicación del tumor con imágenes digitales de los fiduciales y la información del chaleco Synchrony. El brazo robótico de CyberKnife ajustará la fuente de radiación automáticamente para seguir el movimiento del tumor pancreático. Lo único que tendrá que hacer el paciente durante el tratamiento es relajarse y permanecer lo más quieto posible. Una vez terminado el tratamiento, la mayoría de los pacientes retoman rápidamente sus rutinas
Después de terminar el tratamiento con radiocirugía CyberKnife, es importante que se programen visitas de revisión y que el paciente asista a las mismas. El paciente debe ser consciente de que su tumor no desaparecerá de repente. La respuesta al tratamiento del cáncer de páncreas varía de un paciente a otro. De hecho, es posible que haya que esperar varios meses o más para determinar la eficacia del tratamiento con CyberKnife. Los médicos controlarán el resultado en los meses y años posteriores al tratamiento mediante pruebas de diagnóstico por la imagen y evaluando los síntomas del paciente.
47
Indicaciones para CyberKnife
REGIÓN
48
PAT O LO G Í A S
CABEZA Y CUELLO
TUM O RE S P RI M ARI O S P EQ UE ÑOS RE I RRAD IACIO NE S & BO O ST
PULMÓN
TUM O RE S P RI M ARI O S E S TAD I O TE M P RANO ME TÁS TAS I S
MAMA
IRRAD I ACIÓN PARCIA L
COLUMNA
TUM O RE S BE NI G NO S MALFORMACIONES ARTEREOVENOSAS METÁSTASIS
HÍGADO
P RI M ARI O S I NO P E RABLE S ME TÁS TAS I S HE PÁTI CAS
PÁ N C R E A S
P RI M ARI O S I NO P E RABLE S BO O S T P RE / P O S T O P E RATO RIO
RIÑONES
TUM O RE S I NO P E RABLE S METÁS TAS I S
GINECOLÓGICO / GENITOURINARIO
O LI G O M E TÁS TAS IS
Radiocirugía robotizada para tratar tumores benignos o malignos en cualquier parte del cuerpo
49
50
51
Cursos que debe realizar el usuario final de CyberKnife √ Cursos en fábrica. √ Curso en una institución a definir, con experiencia en el sistema y equipo en funcionamiento. √ Curso en la institución de destino una vez instalado el equipo. √ Capacitación permanente brindada por personal de Aplicaciones Clínicas de DeLeC.
Cursos en fábrica El usuario deberá determinar el número de asistentes que enviará a cada curso. Se debe considerar que previo a la instalación, al menos un físico debe tener el curso en fábrica finalizado y es recomendable que también tenga completo el curso en un sitio donde ya se encuentra un equipo en funcionamiento. Los cursos en fábrica son 5. En la siguiente tabla se enuncian y se indica además quién debería realizarlos.
Cursos ofrecidos para el Sistema CyberKnife
La capacitación para el personal de la Dirección General de Ingeniería será proporcionada por los ingenieros especialistas acreditados por Accuray localmente en Argentina.
52
Curso de Física para Físicos Médicos Audiencia: Físicos médicos responsables de realizar tareas de puesta en servicio y control de calidad (QA) del sistema CyberKnife. Objetivo: Este curso provee una visión general de la operación clínica con instrucciones en profundidad de los procedimientos para los test de aceptación (ATP), procedimientos de comisionamiento y herramientas prácticas de rutina para QA. Detalles: El plan de estudios del curso combina la instrucción conceptual con ejercicios prácticos para preparar a los físicos para la participación activa en el ATP, así como para realizar pruebas de control de calidad de rutina. Este curso inclu-
tunidad de aplicar las habilidades enseñadas en un contexto práctico de resolución de problemas para prepararlos para operar el sistema en un entorno clínico.
ye una introducción a la planificación del tratamiento y la experiencia práctica con el sistema CyberKnife. La finalización del curso prepara al físico para realizar la puesta en marcha y las pruebas necesarias para garantizar que el sistema esté listo para su uso clínico. Nota: Para los físicos que diseñan planes clínicos de tratamiento se les recomienda alistarse en el curso separado de Planificación de Tratamiento.
tiPlan®. Detalles: El plan de estudios del curso combina la instrucción conceptual sobre la planificación del tratamiento con ejercicios prácticos de laboratorio para permitir que los asistentes desarrollen y apliquen habilidades prácticas. La instrucción avanza desde los fundamentos a través de técnicas avanzadas de planificación. Se abordan los aspectos y consideraciones únicos para planificar los tratamientos del sistema CyberKnife. Atención a diferentes aplicaciones clínicas, incluidos los tratamientos intracraneales y extracraneales, para proporcionar un contexto clínico para las habilidades que se enseñan.
Curso para Operaciones de Entrega de Tratamiento Audiencia: Todos los operadores del sistema CyberKnife. Objetivo: Este curso enseña las habilidades requeridas para la entrega de tratamientos y el desarrollo de tareas básicas de mantenimiento de datos. Detalles: El plan de estudios del curso combina la instrucción conceptual sobre la administración del tratamiento con ejercicios prácticos de laboratorio para permitir que los asistentes realicen pasos específicos en el proceso de tratamiento con CyberKnife. Los asistentes tendrán la opor-
Planificación de Tratamiento Audiencia: Dosimetristas, Físicos Médicos y personal clínico responsable del desarrollo y optimización de los planes de tratamiento clínico utilizando la estación de planificación de TomoTherapy. Objetivo: El curso incluye una descripción general completa de las herramientas y capacidades del sistema de planificación de tratamiento Mul-
Curso de Sistema Nervioso Central para Médicos Audiencia: Médicos que tienen roles principales en la evaluación y aprobación de planes de tratamiento y en la confirmación de la configuración y alineación del paciente para todas las aplicaciones clínicas. Objetivo: Los médicos desarrollarán una comprensión de la radiocirugía de cuerpo completo,
53
incluida la selección de pacientes y las aplicaciones clínicas. Este curso enfatiza el papel del médico en las decisiones que involucran la planificación y la administración del tratamiento. Detalles: El plan de estudios del curso combina la instrucción sobre los conceptos de planificación y administración del tratamiento con laboratorios prácticos. Incluye instrucciones sobre el SNC y los tratamientos extracraneales, como los de próstata y pulmón. Los médicos interesados solo en aplicaciones intracraneales y de columna vertebral pueden optar por asistir solo al módulo CNS realizado el día 1 y el día 2.
cliente no tenga un buen dominio del idioma inglés, Accuray hará los arreglos necesarios para que haya un intérprete disponible para la capacitación correspondiente; dichos intérpretes deberán estar capacitados para realizar interpretación simultánea y estar familiarizados con la terminología involucrada. El cliente notificará con anticipación a Accuray si son necesarios los servicios de traducción. El cliente será responsable de todos los gastos de interpretación y traducción.
Módulo de Radiocirugía de Sistema Nervioso Central Audiencia: Los médicos que tratan los objetivos del sistema nervioso central utilizando el sistema CyberKnife. Objetivo: Los asistentes desarrollarán un entendimiento de la evaluación y aprobación del plan de tratamiento, así como la confirmación de la configuración y alineación del paciente para aplicaciones intracraneales y de columna. Este curso enfatiza el papel del médico en las decisiones que involucran la planificación y la administración del tratamiento. Detalles: El plan de estudios del curso combina la instrucción sobre los conceptos de planificación y administración del tratamiento con laboratorios prácticos. Las fechas se definirán entre el usuario final y Accuray. La capacitación se dicta únicamente en idioma inglés. En caso de que el personal del
ción a coordinar con el personal del usuario final y Accuray, el entrenamiento en un sitio donde ya se encuentra instalado un equipo. Allí se sugiere la participación del personal médico radioterapeuta, físico y/o dosimetrista y técnico del servicio.
54
Curso en institución con experiencia en el sistema Además de los cursos en fábrica para físicos, técnicos y médicos, se realizará en fecha e institu-
Curso en la institución una vez instalado el sistema Finalmente, la última etapa de entrenamiento se coordinará para ser realizada al finalizar la instalación del equipo en el sitio. Se brinda a todo el personal del servicio de Radioterapia. Capacitación permanente brindada por personal de Aplicaciones Clínicas de Delec Una vez finalizados los tres cursos anteriores mandatorios de Accuray, el personal de Aplicaciones Clínicas y Docencia de DeLeC Científica proveerá capacitaciones de forma permanente a la totalidad de los profesionales del servicio.
55
“El sistema CyberKnife es una excelente solución de terapia radiante para el tratamiento y seguimiento de objetivos en movimiento, garantizando una precisión y exactitud extremas”. Dwight E. Heron, MD, FACRO, FACR UPMC Cancer Center
DeLeC y Accuray prestarán asesoría a los profesionales de la institución en la evaluación de las instalaciones y en la interpretación de las especificaciones técnicas para la construcción del búnker, con el fin de que la institución proceda a una adecuada instalación y operación del equipo. En términos generales, el servicio consistirá en una asesoría prestada por parte de los ingenieros de DeLeC y Accuray, en conjunto, a los profesionales que designe la institución, en relación a: la evaluación de las actuales instalaciones; el visto bueno formal de los planos presentados para la obra de construcción del búnker, de forma previa a su inicio; la definición de las especificaciones técnicas para el diseño del mismo y la realización de visitas inspectivas de carácter formal a la obra de construcción de las instalaciones necesarias para alojar al equipo. A continuación, se relaciona un documento orientativo de las disposiciones genéricas que debe tener un búnker para el sistema CyberKnife. Cada caso concreto se tratará de forma puntual y detallada teniendo en cuenta las características particulares de cada proyecto.
Plano típico del búnker para un sistema CyberKnife. A = Sala de tratamiento. B = Sala de equipos. C = Sala de control. D = Sala de planificación de tratamiento.
57
Dimensiones del BÚNKER
A: 300 cm. B: 731,5 cm. C: 640 cm
58
Modelo de documento otorgado por la fábrica formalmente para comenzar con la construcción o modificación del búnker. Cada institución obtendrá esta documentación específica personalizada con las características de cada centro.
59
60
Bibliografía KRESL. J. ET. AL Non-small cell lung cancer. Left upper lung HAN H. ET. AL Non-invasive stereotactic radiosurgical treatment of non-small cell lung cancer COLLINS B. ET. AL Synchronus bilateral stage IA non-small cell carcinoma. MARCHETTI M. ET. AL Bilateral trigeminal neuralgia. FREEMAN D. ET. AL Posterior fossa renal cell metastasis. SPELLBERG D. ET. AL Low-risk organ-confined prostate cancer. FULLER D. ET. AL CyberKnife monotherapy for low-risk prostate cancer: high dose-rate brachytherapy fractionation and dose gradients. PERMAN M. ET. AL Recurrent ovarian carcinoma. MARSHAL J ET. AL Pancreatic adenocarcinoma. YUAN Z ET. AL Medically inoperable primary liver carcinoma. MUACEVIC A. ET. AL C5 malignant peripheral nerve sheath tumor. KRESL J. ET. AL Primary renal cell carcinoma. LARSON D. ET. AL Recurrent multiple myeloma at clivus & C1 COLOMBO F. ET. AL Spinal arteriovenous malformation. VERMEULEN S. AND COTRUTZ C. Partial breast irradiation after lumpectomy. KRESL J.ET. AL Left optic nerve meningioma (who grade 2) WONG D. ET. AL Para-aortic lymph node metastasis. KRESL J. A clinical comparison of cyberknife & gamma knife radiosurgery systems at St. Joseph’s Hospital & Medical Center/Barrow Neurological Institute. COLLINS S. ET. AL CyberKnife radiosurgery in the treatment of complex skull base tumors: analysis of treatment planning parameters. COLOMBO F. ET. AL PCyberKnife radiosurgery for benign meningiomas: short-term results in 199 patients. FARISELLI L. ET. AL CyberKnife Radiosurgery as a first treatment for idiopathic trigeminal neuralgia. WOWRA B. ET. AL CyberKnife radiosurgery for brain metastases. SIO T. ET. AL Comparing gamma knife and CyberKnife in patients with brain metastases. KING C. ET. AL Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. SEPPENWOOLDE Y. ET. AL Accuracy of tumor motion compensation algorithm from a robotic respiratory tracking system: a simulation study.
61
COLLINS B. ET. AL Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. PARTHAN A. ET. AL Comparative cost-effectiveness of stereotactic body radiation therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer. GERSZTEN P. ET. AL Radiosurgery for spinal metastases. VAUTRAVERS-DEWAS C. ET. AL Image-guided robotic stereotactic body ratiation therapy for liver metastases: is there a dose response relationship?
62
63
64
ÍNDICE
Publicaciones científicas 1.Non-invasive stereotactic radiosurgical treatment of non-small cell lung
69
cancer 2.Bilateral trigeminal neuralgia
73
3.Low risk organ confined prostate cancer
77
4.CyberKnife® monotherapy for low-risk prostate cancer: high dose-rate
81
5.Recurrent ovarian carcinoma
85
6.Pancreatic adenocarcinoma
89
7.Medically inoperable primary liver carcinoma
93
8.C5 malignant peripheral nerve sheath tumor
97
9.Spinal arteriovenous malformation
101
10.Partial breast irradiation after lumpectomy
105
11.Left optic nerve meningioma (who grade 2)
109
12.Para-aortic lymph node metastasis
113
Papers clínicos 1.Giant Tumor in the Inferior Vena Cava Treated With CyberKnife
119
2.CyberKnife for Recurrent Malignant Gliomas: A Systematic Review and
125
Meta-Analysis 3.Stereotactic Radiotherapy for Localized External Auditory Canal Carcino-
137
mas: Report of Four Cases 4.Long-term outcomes of 170 brain arteriovenous malformations treated
142
by frameless image-guided robotic stereotactic radiosurgery 5.CyberKnife® radiosurgery in the treatment of complex skull base tumors:
150
analysis of treatment planning parameters 6.CyberKnife radiosurgery for benign meningiomas: short-term results in
160
199 patients. 7.CyberKnife radiosurgery as a first treatment for idiopathic trigeminal neu-
167
ralgia
65
8.Comparing Gamma Knife and CyberKnife in patients with brain
173
metastases 9.Stereotactic body radiotherapy for localized prostate cancer: inte-
186
rim results of a prospective phase II clinical trial 10.Radical stereotactic radiosurgery with real-time tumor motion
192
tracking in the treatment of small peripheral lung tumors. 11.Comparative cost-effectiveness of stereotactic body radiation
299
therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer 12.Radiosurgery for spinal metastases
208
13.Deep brain stimulation and frameless stereotactic radiosurgery in
215
the treatment of bilateral parkinsonian tremor. 14.Atlas-based functional radiosurgery: Early results
66
222
PUBLICACIONES CIENTÍFICAS
A continuación se pone a disposición una selección de publicaciones científicas que avalan las características y beneficios del sistema Radixact, de Accuray. En la selección de estos papers se dio prioridad a los contenidos más recientes y pertinentes, teniendo en cuenta la presentación que se ofrece en la carpeta Radixact confeccionada por DeLeC Científica. Si el lector desea ampliar la información científica sobre los usos del Radixact puede consultar a los sitios www.delec.com.ar, o bien al correo: comunicaciones@delec.com.ar.
MÁS PUBLICACIONES
WWW.DELEC.COM.AR
67
68
CASE STUDY
Non-invasive stereotactic radiosurgical treatment of non-small cell lung cancer
N O N - I N VA S I V E S T E R E O TA C T I C R A D I O S U R G I C A L T R E AT M E N T O F NON-SMALL CELL LUNG CANCER CyberKnife® Team:
10
Radiation Oncologist:
Hoke Han, M.D.
Thoracic Surgeon:
Mark Block, M.D.
Physicists:
Alberto de la Zerda, Ph.D. Elizabeth Bossart, Ph.D.
Dosimetrist:
Vidya Persaud
Therapists:
April Hildebrand Steve Reyes
CyberKnife Center:
CyberKnife Center of Miami
69
N O N - I N VA S I V E S T E R E O TA C T I C R A D I O S U R G I C A L T R E AT M E N T OF NON-SMALL CELL LUNG CANCER
DEMOGRAPHICS Sex: Age: Histology:
Female 72 years Adenocarcinoma
Case History A 72-year-old female former smoker with a history of COPD presented for evaluation of a growing, PET-positive right upper lobe (RUL) lung lesion. The lesion was first discovered four years earlier when she had a chest CT for unclear reasons. At that time it measured 1 cm in diameter and reportedly was not active on PET. No intervention was recommended. No further imaging was performed until she presented with worsening shortness of breath and a cough. Repeat chest CT demonstrated that the lesion had grown, and now measured 1.5 x 1.2 cm. PET-CT two months later identified activity in the lesion with an SUV of 4.9. Biopsy results demonstrated the lesion to be consistent with NSCLC. Given the PET-CT and biopsy results, this patient was diagnosed with Stage 1a non-small cell lung cancer (T1N0M0 NSCLC).
CLINICAL HISTORY Referred by: Past Medical History:
Thoracic Surgeon Smoker (50 pack-year history), COPD (chronic obstructive pulmonary disease), breast cancer, hypercholesterolemia
CyberKnife® Treatment Rationale When possible, Stage Ia NSCLC is treated by primary surgical resection (lobectomy or segmentectomy).1,2 Conventional radiation therapy and chemotherapy have been reserved for patients who refuse surgery or who are deemed medically inoperable because of associated co-morbidities. In recent years improved tumor control with relatively few complications has been achieved using high-dose, hypofractionated stereotactic radiation delivery.3-8 The patient investigated several treatment options for the RUL lesion. Her pulmonary function tests (PFTs) showed a V02 max of 18 ml/kg/min, an FEV1 of 0.85 L (43% of predicted) and a perfusion scan demonstrated the contribution of the right lung to total lung function to be high (65%). Therefore, despite a good V02 max value, clinicians recommended against a lobectomy and recommended the CyberKnife® System as a treatment alternative. Repeat CT at this time showed the lesion to have increased in size to 1.8 x 1.8 cm. Given the relatively poor outcomes associated with conventional radiation therapy,9 the inclusion of large volumes of normal lung tissue within the radiation field, and the patient’s prior history of external beam radiation to the right breast for treatment of her breast cancer in the past, radiosurgery using the fiducial-free Xsight® Lung Tumor Tracking System was chosen to deliver focal, high-dose, hypofractionated radiation treatment that maximized dose to this patient’s lung tumor and minimized dose to surrounding normal tissue. The non-invasive nature of this treatment allows patients to breathe freely as the CyberKnife System tracks tumor motion throughout treatment without the need for implantation of metal fiducials or other markers near the lung tumor.
Pre-treatment diagnostic CT axial image demonstrating 1.8 cm x 1.6 cm x 1.7 cm lesion in the upper lobe of the right lung.
Pre-treatment diagnostic PET-CT axial image demonstrating increased SUV uptake in the right upper lobe lesion.
70
11
N O N - I N VA S I V E S T E R E O TA C T I C R A D I O S U R G I C A L T R E AT M E N T OF NON-SMALL CELL LUNG CANCER
TREATMENT DETAILS Gross Tumor Volume (GTV): Imaging Technique(s): Rx Dose & Isodose: Conformality Index (PTV): Number of Beams: Homogeneity Index:
3.60 cm3 CT, PET-CT 60 Gy to 75% 1.15 148 beams/fraction 1.33
Planning Process The patient’s only preparation for treatment was a planning CT scan to identify the tumor target. Implantation of fiducial markers was not required given the use of the Xsight® Lung technology. Following completion of imaging, the lesion was outlined on the scans resulting in a gross tumor volume of 3.60 cm3. A treatment plan was created to deliver 60 Gy in 3 fractions to the 75% isodose line with 8-mm tumor margins to encompass microscopic extension and targeting uncertainties, using the 20-mm and 30-mm collimators.
Number of Fractions: Tracking Method: Collimator(s): Tumor Coverage:
3 fractions of 20 Gy Synchrony® with Xsight® Lung (No fiducials) 20 and 30 mm 100%
Treatment Delivery The patient underwent CyberKnife® treatment using 148 beams/ fraction. The prescribed dose covered 100% of the tumor volume with a homogeneity index of 1.33 and a conformality index of 1.15 for the PTV. The patient tolerated the procedure well.
Three-dimensional reconstruction of beam geometries.
Axial and coronal treatment planning images showing gross tumor volume (GTV, red) and planning tumor volume (PTV, red). The 75% prescription isodose line is shown in yellow.
12
71
N O N - I N VA S I V E S T E R E O TA C T I C R A D I O S U R G I C A L T R E AT M E N T OF NON-SMALL CELL LUNG CANCER Outcome and Follow-Up • Four months after CyberKnife® treatment PET-CT imaging revealed an interval decrease in the size of the RUL lesion to less than 1 cm in maximum dimension (0.9 x 0.8 x 0.9 mm); there were no noted acute complications - the patient remained asymptomatic with stable post-treatment PFTs • Eleven months after CyberKnife treatment PET-CT imaging demonstrated no evidence of disease with only residual scarring noted and no PET positivity; the patient continued to do well without any noted complications Conclusion and CyberKnife Advantages • This patient had an excellent initial outcome with the CyberKnife System using Xsight® Lung Tracking; the patient was able to undergo treatment completely non-invasively without implanted fiducials or markers • The Xsight Lung Tracking system allows the CyberKnife to track tumor motion continuously throughout treatment, minimizing irradiation of surrounding tissue and maximizing dose to the tumor, thus decreasing the risk of complications such as radiation pneumonitis • No acute side effects or change in-post treatment PFTs were noted; at eleven months posttreatment there was no evidence of disease - patient continued to be without complications • The CyberKnife System provides a completely non-invasive treatment option for selected patients with lung tumors who are poor surgical candidates because of associated medical conditions which make them unable to tolerate any degree of invasiveness in the treatment of their cancer
CYBERKNIFE CENTER OF MIAMI
The CyberKnife Center of Miami, operational since 2003, was the first CyberKnife center on the South-East coast. The center was among the first to implement Xsight Spine and Xsight Lung tracking modalities. Over 700 patients with a wide range of clinical indications in various anatomical locations have been treated successfully. Much of the center’s clinical and research work has been published, including several papers and chapters on lung radiosurgery.6-8,10-11
References 1. Handy JR, Jr., Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 2002;122:21-30. 2. Jones DR, Detterbeck FC. Surgery for stage I nonsmall cell lung cancer. In: Detterbeck FC, Rivera MP, Socinski MA, et al., editors. Diagnosis and treatment of lung cancer: An evidence-based guide for the practicing clinician. Philadelphia: W.B. Saunders; 2001. pp. 177-190. 3. McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys 2005;63:1010-1015. 4. Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-1631. 5. Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-4839. 6. Brown WT, Wu X, Amendola B, et al. Treatment of early non-small cell lung cancer, Stage IA, by image-guided robotic stereotactic radioablation – Cyberknife. The Cancer Journal 2007; 13(2):87-94. 7. Brown WT, Wu X, Fayad F, et al. CyberKnife® Radiosurgery for Stage I Lung Cancer: Results at 36 Months. Clinical Lung Cancer 2007; 8(8): 488-492. 8. Brown WT, Wu X, Wen BC, et al. Early results of CyberKnife image guided robotic stereotactic radiosurgery for treatment of lung tumors. Computer Aided Surgery. Sept 2007;12(5):1-9. 9. Timmerman, R.D., Abdulrahman, R., Kavanagh, B.D., & Meyer, J.L. Lung cancer: A model for implementing stereotactic body radiation therapy into practice. Front Radiat Ther Oncol., 2007: 40; 368-385. 10. Wu X, Fu D, De la Zerda A, et al. Patient Alignment and Target Tracking in Radiosurgery of Soft-Tissue Tumors Using Combined Fiducial and Skeletal Structures Tracking Techniques. Robotic Radiosurgery Volume II: CyberKnife Society Press; 2007; 31-36. 11. Brown WT, Perman M, Wu X, et al. Image-guided robotic stereotactic radiosurgery for treatment of lung tumors. In: Mould RF, Bucholz RD, Gagnon GJ, et al., editors. Robotic Radiosurgery. Vol 1. Sunnyvale, CA: CyberKnife Society Press; 2005. pp. 255-269. 12. Brown WT, Wu X, Amendola B, et al. Initial experience treating lung tumors with the CyberKnife. In: Urschel HC, Kresl JJ, Luketich JD, et al., editors. Robotic Radiosurgery. Vol II. Treating Tumors that Move with Respiration; 2007. pp. 155-163.
www.accuray.com
72
© 2007 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500341.B
13
Bilateral trigeminal neuralgia
CASE STUDY
B I L AT E R A L T R I G E M I N A L N E U R A L G I A
CyberKnife® Team: Neurosurgeon:
Marcello Marchetti, M.D.
Radiation Oncologist:
Laura Fariselli, M.D. Ida Milanesi, M.D. Livia C. Bianchi, M.D.
Medical Physicist:
Lorenzo Brait, Ph.D.
CyberKnife Center:
Department of Neurosurgery, Division of Radiotherapy, Fondazione IRCCS Istituto Neurologico C. Besta, Milano, Italy CyberKnife Center, Centro Diagnostico Italiano, Milano, Italy
73
B I L AT E R A L T R I G E M I N A L N E U R A L G I A
DEMOGRAPHICS Sex: Age: Histology:
Female 85 years Bilateral trigeminal neuralgia
Case History An 85-year-old woman presented with severe and disabling facial pain. The patient had been diagnosed 10 years prior with bilateral idiopathic trigeminal neuralgia. During her first visit to our center in late 2004 she described her pain as paroxysmal, electric shock-like, occurring along the distribution of the first branch of left trigeminal nerve and first and second branch of the right trigeminal nerve. The pain was triggered by tactile stimulation of the face. The patient had a Barrow Pain Intensity Score (BPS) of 4 and no facial numbness. The patient initially experienced a good response to medical treatment, but had since become increasingly resistant to medication.
CLINICAL HISTORY Referred by: Past Medical History:
Neurosurgeon Bilateral cataract surgery, chronic sinusitis, bilateral knee prostheses, cerebello-pontine angle meningioma (stable since 1974)
CyberKnife® Treatment Rationale CyberKnife Radiosurgery for trigeminal rhizotomy was considered the treatment of choice given this patient’s increasing resistance to medication. In addition, the patient was considered to be a poor candidate for surgery due to her advanced age. The left trigeminal neuralgia was treated with the CyberKnife System in October 2004, and the right trigeminal neuralgia was treated in June 2006.
Pre-treatment MRI showed possible neurovascular compression just into the cisternal portion of the left trigeminal nerve. No radiological evidence of neurovascular compression of the right trigeminal nerve was present. The MRI also documented a meningioma in the right cerebello-pontine angle. This meningioma proved to be radiologically stable by comparison to previous diagnostic images taken 30 years prior, and therefore no treatment was pursued for this lesion as it was felt this was not contributing to the patient’s new current symptoms.
74
19
B I L AT E R A L T R I G E M I N A L N E U R A L G I A
TREATMENT DETAILS Imaging Technique(s): Fractions: Rx Dose & Isodose:
63 beams for left-side treatment; 64 for right side Skull 6D tracking system 5 mm 3 head path 800 mm
Number of Beams:
In both cases a 4-mm length of trigeminal nerve was treated T1 Gadolinium MRI and a contrast-enhanced CT scan Single fraction per side 55 Gy to 100%
Target Site:
Tracking Method: Collimator(s): Path Template:
Treatment Planning and Delivery The patient’s bilateral trigeminal neuralgia was treated in two stages, beginning with the left trigeminal nerve. Treatment planning was based on a 1.25-mm slice T1 gadolinium MRI, including fast imaging employing steady-state acquisition (FIESTA), and a contrast-enhanced CT scan. The obtained images were fused to better define the intracisternal portion of the trigeminal nerve and optimize target definition. The target was defined as a segment of 4 mm of trigeminal nerve localized in the cisternal portion, 2 mm anterior to the dorsal root entry zone (see Figure 1, left panel). The prescribed dose was 55 Gy delivered to the 100% isodose line. The dose constraint to brainstem was 10 Gy, and the dose limit for the left 7th and 8th cranial nerves was 6.55 Gy (see Figure 2, left panel). The smallest collimator (5 mm) was used. In June 2006 the patient underwent a second CyberKnife® radiosurgery treatment for the right trigeminal nerve. Treatment of the right nerve was similar to treatment of the left; the target was the 4 mm of trigeminal nerve in the cisternal portion 2 mm anterior to the dorsal root entry zone (see Figure 1, right panel), and 55 Gy was delivered to the 100% isodose line. The dose constraint to the brain stem was 11 Gy, and for the left 7th and 8th cranial nerves it was 8.2 Gy (see Figure 2, right panel).
Figure 1. Plans for the left (left panel) and right (right panel) trigeminal nerve treatments. In both cases 55 Gy was delivered to the 100% isodose line (orange contour).
Volume (%)
Left Trigeminal Nerve
Right Trigeminal Nerve
100
100
80
80
60
60
40
40
20
20
0
0 0
20
40
60
80
100
0
20
40
60
80
100
Dose (%) Target
Brain Stem
Soft Tissues
Figure 2. Dose-volume histograms for both plans. Note high dose to the nerve and sparing of brain stem and nearby soft tissue.
20
75
A
B I L AT E R A L T R I G E M I N A L N E U R A L G I A Outcome and Follow-Up LEFT TRIGEMINAL NERVE • Thirty days after treatment the patient reported complete pain relief on the left side accompanied by mild dysesthesia (which resolved 4 months post-treatment after the patient accidentally fell down and suffered a mild hemorrhagic stroke, from which she recovered completely) • The patient continued on medication during the year-and-a-half period between treatment of the left trigeminal nerve and treatment of the right trigeminal nerve RIGHT TRIGEMINAL NERVE • Twenty days after treatment the patient reported relief of pain to a BPS of 3; two months after treatment the patient reported complete resolution of the pain • Since completing both treatments the patient has been pain-free and medication-free Conclusion and CyberKnife® Advantages • CyberKnife® Stereotactic Radiosurgery is a safe and effective therapeutic modality for the treatment of medication-refractory trigeminal neuralgia • Rapid and durable pain relief was achieved without the risks associated with open surgery or post-operative recovery
CENTRO DIAGNOSTICO ITALIANO (CDI) Centro Diagnostico Italiano (CDI) is a private medical center established in 1975. The mission of CDI is to assure its leadership in healthcare by providing the widest range of preventive, diagnostic and therapeutic services while constantly researching ways to improve our patient care. CDI serves the Milanese territory by providing access to the CyberKnife via the CyberKnife Milan Project.
FONDAZIONE ISTITUTO NEUROLOGICO CARLO BESTA The Fondazione Istituto Neurologico Carlo Besta is internationally recognized as a leading center for neuroscience. The Institute belongs to the Health Promoting Hospitals network, a project of the World Health Organization. The Istituto specializes in neurological disorders, surgical and oncological pathologies, and chronic and rare diseases. Its philosophy is to continually improve the efficacy of treatments by creating a synergy between scientific research and healthcare assistance.
www.accuray.com
76
© 2009 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500633.A
CASE STUDY
LOW-RISK ORGAN-CONFINED P R O S TAT E C A N C E R Naples Urology Associates CyberKnife® Team: Urologist:
David M. Spellberg, M.D., FACS
Radiation Oncologist: Debra Freeman, M.D. Jay Friedland, M.D. Medical Physicist:
26
Mary Ellen Masterson-McGary, M.S.
77
L O W - R I S K O R G A N - C O N F I N E D P R O S TAT E C A N C E R
DEMOGRAPHICS Sex: Age: Histology:
CLINICAL HISTORY
Male 70 Prostate Adenocarcinoma: stage T1c
Referred by: Past Medical History:
Urologist Transurethral resection of the prostate (TURP) for benign prostatic hyperplasia (BPH)
Case History This 70-year-old male with a history of atrial fibrillation, hypertension and benign prostatic hyperplasia (BPH) presented with elevated prostate specific antigen (PSA) of 4.5 ng/ml. He had been followed by his urologist for the previous six years with regular PSA monitoring. He had no family history of prostate cancer and underwent a TURP 2 years prior for BPH. His atrial fibrillation and hypertension were managed by Coumadin, Toprol, Lanoxin and Zestoretic.
CyberKnife® Treatment Rationale The patient was evaluated by Urology and Radiation Oncology for his prostate cancer. Treatment options included surgery, external beam radiation therapy (IMRT, conformal) and CyberKnife® monotherapy. The patient wanted a less invasive and convenient therapy in order to continue his work and day to day activities and therefore elected for CyberKnife monotherapy.
The patient’s symptoms included nocturia times two and a history of erectile dysfunction. Patient denied a history of dysuria, hematuria, urinary incontinence, urinary urgency, urinary frequency or hesitancy. Transrectal ultrasound (TRUS) guided biopsy revealed adenocarcinoma of the prostate in 6 of 12 biopsy cores, all of which were less than 5% positive and a Gleason score of 3 + 3. Tumor was found in both lobes of the prostate, and was staged cT1c by digital rectal examination. A CT scan of the abdomen / pelvis was unremarkable and a bone scan was negative for metastatic disease.
Current literature suggests that prostate cancer will respond favorably to hypofractionated radiotherapy due to its low α/α ratio of prostate cancer.1,2 Several groups have demonstrated that hypofractionation schemes for prostate cancer achieve excellent local control with minimal toxicity to the urethra and rectum.3,4 CyberKnife stereotactic radiosurgery has been shown to decrease prostate tumor volume and decrease PSA levels of human prostate cancer cells in a mouse model.5 Initial studies of CyberKnife monotherapy have shown beneficial effects, including decreased PSA results and low toxicity in patients with organ-confined prostate cancer.6,7
Multiplanar pre-treatment planning images show all 4 fiducial markers placed within the prostate.
78
27
L O W - R I S K O R G A N - C O N F I N E D P R O S TAT E C A N C E R
TREATMENT DETAILS Prostate Volume: Imaging Technique(s): Rx Dose & Isodose: Conformality Index: Tumor Coverage: Number of Beams:
29.5 cc CT 35 Gy to 82% 1.39 95% 130
Treatment Planning Process Four fiducial markers were placed under intravenous conscious sedation in the prostate by the urologist using a TRUS-guided template. Eleven days later a CT study was performed with the patient in the treatment position using a custom immobilization device. The fiducial locations were identified and the prostate and critical structures (rectum, bladder, and urethra) were contoured. The planning target volume (PTV) included the prostate with a 5-mm margin in all directions except for a smaller 3-mm posterior margin to decrease dosage to the rectum. Treatment planning was designed to encompass 95% of the target volume and minimize dose to critical structures.
Fractions: Path Template: Tracking Method: Collimator(s):
5 3 path 900_1000 mm Fiducial 20 mm and 35 mm
Treatment Delivery A few days after treatment planning the patient began treatment. A prescription dose of 35 Gy was delivered in 5 fractions over 5 consecutive days to the 82% isodose line. Two collimator sizes were used and a conformality index of 1.39 was achieved. There were 130 beams from 111 nodes delivered. Following the fourth treatment, the patient experienced nocturia and was given 0.4 mg Flomax with resolution of symptoms. The patient reported mild urinary frequency and mild urgency 5 days after completion of last fraction of radiosurgery and was treated with Pyridium with resolution of symptoms. Overall, the patient tolerated the treatment well.
Inferior-superior 3D of bony anatomy and CyberKnife beam positions showing treated tumor with rectal sparing.
Coronal and axial treatment plans showing the 82% prescription isodose line relative to the prostate (red). Lower percentage isodose lines demonstrate sparing of the rectum (green).
28
Dose-Volume Histogram (DVH) for prostate.
79
L O W - R I S K O R G A N - C O N F I N E D P R O S TAT E C A N C E R Outcome and Follow-Up • The patient responded to CyberKnife® treatment with a decrease in PSA value from 4.5 ng/ml to 1.3 ng/ml at one month following radiosurgery and to 0.3 ng/ml at 8 months • The patient experienced mild acute urinary toxicities which resolved with medication • There were no reported acute rectal toxicities • The patient has now been followed for 3 years; PSA remains stable at 0.3 ng/ml, and the patient has experienced no chronic urinary or rectal toxicities Conclusion and CyberKnife Advantages • CyberKnife monotherapy produced an early and stable reduction in PSA in a patient with low-risk organ-confined prostate cancer with minimal acute urinary toxicities and no noted chronic toxicities to date • CyberKnife treatment provides a convenient, minimally invasive option for patients with early-stage, organ-confined prostate cancer
PSA Results
Low risk organ confined prostate 5 4.5
cancer PSA (ng/ml) (ng/ml) PSA
4 3 2 1.3
1 0
Pre
1
0.3
0.3
0.3
0.3
0.3
0.3
4
8
12
18
24
36
MonthsPost-Treatment Post-Treatment Months
At Naples Urology Associates, we are proud to offer our patients the best treatment options for a wide range of conditions. Our staff provides patients with several cutting edge treatment options depending on their needs. We believe in treating each case according to its unique conditions, so each patient that we see gets a customized treatment plan. We choose the least invasive treatments possible, including CyberKnife Radiosurgery, with the goals of prolonging life and preserving quality of life. Since our CyberKnife program began in 2004, we have treated over 400 patients with prostate cancer. See our website (www.urologyofnaples.com) or call us at 239-434-8565.
References 1. Brenner DJ and Hall EJ. Fractionation and Protraction for Radiotherapy of Prostate Carcinoma. Int J Radiat Oncol Biol Phys 43: 1095-1101, 1999. 2. King CR and Cotrutz C. Hypofractionated Radiotherapy for Localized Prostate Cancer: Therapeutic Rationale and Feasibility of the CyberKnife. Robotic Radiosurgery – Volume 1, 315-323, CyberKnife Society Press, 2005. 3. Martinez A, Pataki I, Edmundson G, Sebastian E, Brabbins D, Gustafson G. Phase II Prospective Study of the Use of Conformal High-Dose Rate Brachytherapy as Monotherapy for the Treatment of Favorable Stage Prostate Cancer: a Feasibility Report. Int J Rad Oncol Phys Biol 49: 61-69, 2001. 4. Yoshioka Y, Nose T, Yoshida K, Oh RJ, Yamada Y, Tanaka E, Yamazaki E, Inoue T, Inoue T. High-Dose Rate Brachytherapy as Monotherapy for Localized Prostate Cancer: a Retrospective Analysis with Special Focus on Tolerance and Chronic Toxicity. Int J Rad Oncol Biol Phys 56: 213-220, 2003. 5. Lotan Y, Stanfield J, Cho C, Sherwood J, Abdel-Aziz KF, Chang C, Forster K, Kabbani W, Hsieh J, Choy H, Timmerman R. Efficacy of High-Dose per Fraction Radiation for Implanted Human Prostate Cancer in a Nude Mouse Model. J Urology 175: 1932-1936, 2006. 6. Jo MK, Park K, Kim KH, Cho CG, Lee C. Feasibility of CyberKnife for the Treatment of Localized Prostate Cancer: Preliminary Results: presented at the American Urological Association annual meeting, Atlanta, GA, May 2006, Abstract # 1167. 7. King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti JC. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 73:1043-1048, 2009.
5
4.5
4.5 4
PSA (ng/ml)
3.5 3
2.8
2.5 2 1.9
1.8
www.accuray.com
1.5
© 2009 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500097.B
1.3
1 0.5 0
80
1/99 1/00 1/01 1/02
1/05
5/05
0.3
0.2
0.2
8/05
12/05
3/06
29
CASE STUDY
CyberKnife® monotherapy for lowrisk prostate cancer: high dose-rate brachytherapy fractionation and dose gradients
C Y B E R K N I F E® M O N O T H E R A P Y F O R L O W - R I S K P R O S TAT E C A N C E R : H I G H D O S E - R AT E B R A C H Y T H E R A P Y F R A C T I O N AT I O N A N D D O S E G R A D I E N T S
CyberKnife® Centers of San Diego CyberKnife Team:
30
Radiation Oncologist:
Donald B. Fuller, M.D.
Medical Physicist:
Haoran Jin, Ph.D.
CyberKnife Center:
CyberKnife Centers of San Diego San Diego, CA
81
C Y B E R K N I F E® M O N O T H E R A P Y F O R L O W - R I S K P R O S T A T E C A N C E R
DEMOGRAPHICS Sex: Age: Histology:
Male 76 years Prostate Adenocarcinoma
Case History This 76-year-old man presented during a routine check-up with a PSA of 7.7 ng/ml. A biopsy four months later revealed adenocarcinoma involving 30% of the submitted tissue with a Gleason score of 3+3=6. A subsequent bone scan and CT of the abdomen and pelvis showed no evidence of metastatic disease. PSA analysis was repeated two months later, and measured 9.4 ng/ml. In his initial evaluation, he had an International Prostate Symptom Score (IPSS) of 4 and Sexual Health Inventory for Men (SHIM) score of 23; the lesion was staged as T2a at the right base. The patient was diagnosed as having low-risk, organ confined prostate cancer.
CLINICAL HISTORY Past Medical History:
None
CyberKnife® Treatment Rationale Several treatment options were discussed with the patient, including radical prostatectomy, external beam radiotherapy, and brachytherapy. The unique radiobiology of prostate cancer suggests that the disease is particularly sensitive to large-dose-per-fraction (hypofractionated) radiation treatment regimens.1 In support of this, good biochemical disease control with few serious side effects has recently been reported for the hypofractionated approach referred to as high dose rate (HDR) brachytherapy.4 The CyberKnife® Centers of San Diego developed a CyberKnife-based hypofractionated prostate treatment that effectively reproduces the dose, dose distribution, and fractionation of HDR brachytherapy.3 This approach has been designed to escalate the dose to the peripheral zone of the prostate, which typically harbors the majority of cancer cells.4 This treatment option allows patients to benefit from HDR brachytherapy dose sculpting while avoiding the invasive aspect of of indwelling catheter placement required of the HDR brachytherapy procedure.
T1-weighted, gadolinium-enhanced MRI treatment planning image; GTV (prostate) defined by white line; planning target volume (PTV) defined by blue line; rectal mucosa defined by yellow and turquoise lines. Note that the 1.5T MRI renders the neurovascular bundle (NVB) visible (red arrow) so that a treatment plan can be constructed that limits dose to this structure.
82
31
C Y B E R K N I F E® M O N O T H E R A P Y F O R L O W - R I S K P R O S T A T E C A N C E R
TREATMENT DETAILS Prostate Volume: Imaging Technique(s): Rx Dose & Isodose: Number of Beams:
55 cm3 CT, 1.5T MRI 38 Gy to 57% isodose line 261
Planning Process CT and MRI imaging were used for the planning process. The MRI images were fused to the CT image set to better define the prostate capsule, rectal mucosa, neurovascular bundle (NVB) and the penile bulb. For both image sets, a Foley catheter was used to fill the bladder with 100 ml of H2O, to aid in identifying the urethra and bladder. The rectum was emptied by administration of a Fleet Enema®. The planning treatment volume was created by expanding the prostate volume in all directions by 2 mm, except posteriorly where the prostate abutted the rectum. In this region the margin expansion was reduced to zero. Constraints provided by the radiation oncologist resulted in the following doses to critical structures: Structure
Dose parameters
Urethra
Dmax – 44 Gy (116%); Median – 38.4 Gy (101%)
Rectum Outer Wall
Dmax – 34.7 Gy (91%)
Rectum Mucosa
Dmax - 25.3 Gy (67%)
Penile Bulb
Dmax – 22.7 Gy (60%); D50 – 7.3 Gy (19%)
Neurovascular Bundles
Steep gradient: 48 Gy (126%) - 26.8 Gy (71%)
Fractions: Path Template: Tracking Method: Collimator(s):
4 Single collimator long path 6D fiducial tracking 15 mm
Treatment Delivery Four fiducials were implanted transperineally into the prostate prior to the planning CT scan. A treatment plan was constructed based on co-registered CT/MRI scans. The patient was treated with a total dose of 38 Gy delivered in 4 equal fractions occurring on consecutive days.
Axial planning image demonstrating extreme conformality, which results in the potential to spare the NVB from the full dose zone in selected low-risk patients. The prescription isodose line is shown in orange (57%). Note that the regions of high dose within the prostate correspond approximately to the peripheral zone.
Three-dimensional rendering of beam trajectories to the PTV. Sagittal planning image demonstrating a very sharp dose gradient sparing the rectum and urethra. Prescription dose line is orange (57%).
32
83
C Y B E R K N I F E® M O N O T H E R A P Y F O R L O W - R I S K P R O S T A T E C A N C E R Outcome and Follow-Up Two weeks post-treatment, the patient experienced increased frequency of bowel movements (BM) and acute dysuria, and was prescribed Flomax® One month after the treatment, the patient’s dysuria and increased BM frequency improved; PSA dropped to 4.7 ng/ml from a pretreatment level of 9.4 ng/ml At the 2-month follow-up, the patient’s dysuria and increased BM frequency had resolved; patient’s IPSS and SHIM score were at baseline levels; PSA continued to decline, measured at 2.5 ng/ml During the 18-month follow-up period, the patient’s PSA level continued to decline steadily, reaching 0.5 ng/ml (see figure); the patient’s IPSS was 5 and his SHIM score was 16
Dose-volume histogram showing escalation of dose to the peripheral zone of the prostate (lavender line) and sparing of nearby organs at risk.
PSA Results
PSA (ng/ml)
Conclusion The CyberKnife® System successfully reproduced an HDR-like dose distribution, delivering treatment in a minimally invasive fashion Treatment-related toxicity was mild (grade I - II urinary symptoms) and resolved over time in this first 18 months of follow up; further follow-up is required to assess chronic toxicity in this patient A significant reduction in PSA levels followed treatment throughout the first 18 months of follow-up
10 9 8 7 6 5 4
9.4
4.7
3
2.5
2
1.7
1 0
Pre
1
2
4
1.3
6
0.7
0.5
12
18
0.1
24
Months Post-Treatment
CYBERKNIFE CENTERS OF SAN DIEGO (www.sdcyberknife.com)
CyberKnife Centers of San Diego opened their first office in a patient friendly, centrally located San Diego location in June of 2006. To serve increasing demand from patients and referring physicians alike, the second CyberKnife Centers of San Diego office was opened in North Coastal San Diego County in November 2007. Prostate and lung cancer have been the two most prevalent CyberKnife applications to date in this practice, with the physicians and staff believing CyberKnife radiosurgery to represent paradigm-shifting technology for these and other cancer indications. To contact a CyberKnife Center of San Diego, call 858-505-4100 or 800-470-1256.
References 1. King CR, Lehmann J, Adler JR, Hai J. CyberKnife radiotherapy for localized prostate cancer: rationale and technical feasibility. Technol Cancer Res Treat. 2003;2:25-30. 2. Grills IS, Martinez AA, Hollander M, Huang R, Goldman K, Chen PY, Gustafson GS. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol. 2004;171:1098-1104. 3. Fuller DB, Naitoh J, Lee C, Hardy S, Jin H: Virtual HDR(SM) CyberKnife Treatment for Localized Prostatic Carcinoma: Dosimetry Comparison With HDR Brachytherapy and Preliminary Clinical Observations. Int J Radiat Oncol Biol Phys 70:1588-1597, 2008. 4. McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12:897-906.
www.accuray.com
© 2008 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500433.B Other copyrights: Fleet Enema® C.B. Fleet Co., Inc. Flomax® Boehringer Ingelheim Pharmaceuticals, Inc.
84
33
CASE STUDY
Recurrent ovarian carcinoma
RECURRENT OVARIAN CARCINOMA North Florida Regional CyberKnife® Team: Radiation Oncologists:
Mark Perman, M.D. Cherylle A. Hayes, M.D.
Gynecologist:
Kelli C. Ross, M.D.
Chief Medical Physicist: Donald Dubois, Ph.D. Medical Physicist:
Howard Salmon, Ph.D.
Medical Dosimetrist:
Eric Larsen, CMD, RTT
CyberKnife Therapists:
Jeanne Wilson, RTT Scott McGee, RTT
Director of North Florida Radiation Oncology Center: Gail Suarez, MSHSA, RT(R)(T) CyberKnife Center:
North Florida Regional Medical Center Gainesville, FL
85
RECURRENT OVARIAN CANCER
DEMOGRAPHICS Sex: Age: Histology:
Female 60 years Poorly differentiated serous papillary ovarian carcinoma
Case History A 60-year-old female with a history of Stage IV ovarian carcinoma presented with rising CA-125 levels (21.1 U/ml to 34.4 U ml), signifying a probable ovarian carcinoma recurrence. Subsequent MRI showed a 2.8 x 2.5 cm nodule in the region of the left upper vaginal cuff which was confirmed by bimanual examination. Initial diagnosis of Stage IV ovarian carcinoma was made six years earlier when the patient presented with right-sided pleural effusion and CA-125 of 1482 U/ml. An exploratory laparotomy was done and an omental cake, peritoneal studding and a nodule adjacent to the rectosigmoid were resected. Pathology showed poorly differentiated serous papillary ovarian carcinoma. Over the next four years the patient underwent multiple rounds of chemotherapy as well as stem cell transplant and additional surgical resection in the effort to control her disease. The patient’s latest chemotherapy was prematurely discontinued when she experienced a severe anaphylactoid reaction. At that time the decision was made to follow her closely with imaging and CA-125 measurements.
86
CLINICAL HISTORY Referred by: Past Medical History:
Gynecologist Stage IV ovarian carcinoma
CyberKnife® Treatment Rationale Epithelial ovarian cancer is the leading cause of death among women with gynecologic malignancies and is the fifth most common cancer in women in the US. More than 70% of women with epithelial ovarian cancer have Stage III or IV disease at the time of diagnosis.1 Adjuvant chemotherapy is recommended for all patients with advanced stage ovarian cancer after appropriate surgery. However, more than 70% of patients relapse, with a median time to progression of less than 2 years.2 Secondary surgery for recurrent patients has not significantly improved survival. Radiation therapy has improved survival in a subset of patients with chemotherapy-refractory disease, particularly those patients with minimal residual or relapsed disease to the pelvis, and has provided good palliation in patients with local abdominopelvic symptoms.3 This patient presented with a recurrent ovarian cancer in the left vaginal cuff. Due to the patient’s medical history, she was not a candidate for further chemotherapy or surgery. Nevertheless, the left vaginal cuff recurrence was and had always been the only site of recurrence in this patient, therefore aggressive definitive care was indicated. High dose rate brachytherapy was considered, but was declined due to its invasive nature and associated risks. External beam radiation was considered an inferior option due to the inability to limit the dose to adjacent critical structures with increased risks of bladder, vaginal and rectal toxicities. The use of stereotactic body radiotherapy as an alternative to brachytherapy for gynecologic tumors has been reported to achieve excellent local control rates with minimal toxicities.4 The CyberKnife® Radiosurgery System offered a minimally invasive method for delivering hypofractionated radiation to the left vaginal cuff of a patient who had failed two surgical resections and numerous cycles of chemotherapy.
35
RECURRENT OVARIAN CANCER
TREATMENT DETAILS Treatment Volume: Imaging Technique(s): Rx Dose & Isodose: Dose and Fractions: Number of Beams: Number of Fiducials:
42.53 cc CT 30 Gy to 69% 10 Gy x 3 fractions 114 4
Planning Process The patient was prepared for treatment planning by implanting four fiducials into the periphery of the tumor by the gynecologist. A planning CT scan was obtained 7 days later. The fiducials were identified and the lesion was outlined on the scans. The GTV was defined as the tumor and the PTV was defined as the GTV plus a 3-mm expansion resulting in a treatment volume of 42.53 cc. The final plan was created to deliver 30 Gy in 3 fractions to the 69% isodose line. The patient began treatment 6 days after the planning CT images were taken.
Axial treatment plan showing isodose curves to the tumor while sparing the rectum and bladder (outlined in yellow). The orange line indicates the prescription dose to the 69% isodose line providing 94.9% coverage of the PTV.
Number of Nodes: Conformality Index: Tracking Method: Collimator(s): Tumor Coverage:
140 1.25 Fiducial 35 mm 94.9%
Treatment Delivery The patient received 30 Gy delivered in three fractions over four days. The treatment was delivered to a volume of 42.53 cc using 114 beams and a 35-mm collimator. The 69% isodose line provided 94.9% coverage of the PTV, with a conformality index of 1.25. The maximum rectal dose was 30.33 Gy. Less than 0.22 cc of the rectum received greater than 28.8 Gy, meeting the rectal constraint of less than 1 cc of the rectum receiving that dose. The maximum bladder dose was 31.8 Gy with less than 0.47 cc of the bladder receiving greater than 30 Gy. The bladder constraint of less than 10 cc of the bladder receiving greater than 30 Gy was easily met.
Sagittal view of the treatment plan showing isodose curves to the tumor while sparing the rectum and bladder (outlined in yellow). The orange line indicates the prescription dose to the 69% isodose line.
3D image of the bony anatomy and CyberKnife beam positions delivered to the left vaginal cuff.
36
87
RECURRENT OVARIAN CANCER Outcome and Follow-Up • One month following the CyberKnife® treatment a CT scan demonstrated a decrease in size of the left vaginal cuff lesion, which measured 1.86 cm x 2.14 cm • Eight months after the CyberKnife treatment the mass could not be seen and only minimal fullness in the left vaginal wall could be detected on CT imaging • The patient experienced minimal toxicities following CyberKnife treatment, which included occasional loose stools and mild vaginal discharge that resolved six weeks post-treatment without medications, as well as mild urinary urgency and fatigue which completely resolved by five months post-treatment without medications • Eight months after CyberKnife treatment, CA-125 levels decreased to 7.9 U/ml
2 months pre-treatment
first CyberKnife treatment day
2 months post-treatment
5 months post-treatment
8 months post-treatment
Conclusion and CyberKnife Advantages This patient had an excellent initial outcome with the CyberKnife System for the treatment of a recurrent ovarian carcinoma located at the vaginal cuff The CyberKnife System provided a convenient and minimally invasive treatment option for this patient with recurrent ovarian carcinoma who had failed previous surgery and chemotherapy A benefit of the CyberKnife System’s capacity to target lesions accurately is the potential for low toxicity to nearby critical organs such as the bladder, rectum, urethra and vagina
T1 and T2 MRI of 28 mm x 25 mm recurrent ovarian mass located at the left vaginal cuff prior to CyberKnife treatment.
CT image demonstrating no evidence of the left vaginal cuff mass or recurrence 8 months after CyberKnife treatment.
NORTH FLORIDA REGIONAL MEDICAL CENTER
The CyberKnife Center at North Florida Radiation Oncology opened its doors on August 1, 2006 and treated more than 170 patients in its first year. The cases consist of 71% extracranial, 23% intracranial and 6% spinal treatments. HCA and North Florida Regional Medical Center, a 325-bed community hospital, are working together to develop a comprehensive cancer community oncology program to provide patients with quality care supported by state of the art technologies such as the CyberKnife. Contact the CyberKnife Center at North Florida Radiation Oncology at 1-800-621-0575 or 352-333-5643. References 1. Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2006. CA Cancer J Clin 56: 106-130, 2006. 2. McGuire WP, Hoskins WJ, Brady MF et al. N Engl J Med 334: 1-6, 1996. 3. Fein DA, Morgan LS, Marcus RB et al. Int J Rad Oncol Biol Phys 29: 169-176, 1994. 4. Molla M, Escude L, Nouet P, et al. Int J Rad Oncol Biol Phys 62: 118-124, 2005.
www.accuray.com
88
© 2007 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500431.B
37
CASE STUDY
Pancreatic adenocarcinoma
PA N C R E AT I C A D E N O C A R C I N O M A
Georgetown University Hospital CyberKnife® Team: Medical Oncologist:
John Marshall, M.D.
Radiation Oncologist: Gregory J. Gagnon, M.D. Medical Physicists:
Michael S. Lundsten, M.Sc. Sonja Dieterich, Ph.D.
Radiation Therapists:
Sosena Asrat, R.T.(T.) Gerard Elie, R.T.(T.)
CyberKnife Center:
Georgetown University Hospital Washington, DC
38
89
PA N C R E AT I C A D E N O C A R C I N O M A
DEMOGRAPHICS Sex: Age: Histology:
Male 54 years Unresectable, pancreatic adenocarcinoma
Case History The patient was in good health until he developed generalized pruritis and jaundice with a bilirubin level in the 5-7 mg/dl range. An elevated gastrointestinal cancer antigen (CA19-9) serum level of 189 units/ml was obtained. A CT scan with 3D reconstruction of the pancreas revealed a 2.5-cm mass AP within the uncinate process abutting the superior mesenteric artery (SMA) medially and the comon bile duct (CBD) laterally. A 2 x 1 cm lymph node was identified within the portacaval space. An ultrasound-guided fine needle aspiration (FNA) revealed adenocarcinoma. A cholecystectomy was performed. Another 3D scan 3 days later revealed involvement of the superior mesenteric vein (SMV) with occlusive thrombus and tumor involving approximately 180 degrees of the SMA, although the vessel remained patent.
CLINICAL HISTORY Referred by: Previous Treatment:
Self referral External beam radiation of 54 Gy in multiple fractions, plus chemotherapy
CyberKnife® Treatment Rationale The patient was not felt to be a resection candidate due to the involvement of the SMA and the SMV. He was treated with chemotherapy − continuous-infusion 5-FU, cisplatin (30 mg/ m2) and interferon alpha throughout radiotherapy (see Previous Treatment, above). Radiotherapy was followed by postoperative chemotherapy − gemcitabine, Taxotere and Xeloda − which caused bone marrow suppression issues. Post-standard RT treatment, the pancreatic mass increased in size to 2.8 cm AP (compared to the previous 2.5 cm). CyberKnife radiosurgery was felt to be a reasonable treatment which might render the disease inactive or perhaps allow for subsequent surgery. ®
Pretreatment CT showing the pancreatic mass, which measured 2.5 cm AP. A biliary stent is seen in place. The tumor was deemed unresectable because of involvement of the SMA and SMV. Pretreatment PET obtained on showing a positive FDG uptake in the pancreas.
90
39
PA N C R E AT I C A D E N O C A R C I N O M A
TREATMENT DETAILS Tumor Volume: Imaging Technique(s): Rx Dose & Isodose: Conformality Index: Tumor Coverage: Number of Beams:
33.31 cc CT 24 Gy to 81% 1.56 97.2% of PTV 278
Planning Process and Goals The 81% isodose line represents the prescribed dose of 24 Gy to the tumor. The treatment plan provided a 1.56 conformality index. Tumor coverage was 97.2% of the planning treatment volume. The tumor and the critical structures (duodenum, stomach, bowel, aorta and inferior vena cava (IVC)) were contoured for dose calculation purposes. An optimized inverse treatment plan was created such that the 81% isodose contour provided a conformal index of 1.56 while minimizing dose to the critical structures.
Fractions: Path Template: Tracking Method: Collimator(s):
3 3 path 900_1000 mm 6D fiducial tracking 15 mm
Treatment Delivery The patient was treated with 24 Gy to the 81% isodose line in three daily fractions. This treatment was five months after completion of initial radiation therapy and chemotherapy and seven months after initial symptoms. The planning CT for the CyberKnife System revealed that the prior standard RT and chemotherapy had not reduced the size of the tumor, though the size appeared to have stabilized. The patient tolerated his treatment with no morbidity other than some fatigue.
Rendering of the CyberKnife’s beam positions for the treatment of the pancreas from an anterior view point.
Axial and sagittal planning images with the tumor, isodose curves, and critical structures reconstructed from 1 mm sections. Note the highly conformal dose distribution to the pancreas avoids the aorta (violet), IVC (blue), duodenum (green), stomach (light blue) and bowel (pink and coral).
40
Dose volume histogram (DVH) for tumor and critical structures.
91
PA N C R E AT I C A D E N O C A R C I N O M A Outcome and Follow-Up • Follow-up PET scan is negative in the pancreatic bed and the mass is not clearly identified • Tumor demonstrated shrinkage on CT scan acquired 11 months post-CyberKnife® radiosurgery • Patient has no pain, 13 months post-CyberKnife treatment, 22 months after initial symptoms • Patient has survived beyond the 10 - 12 months commonly obtained for locally advanced disease3 Conclusion and CyberKnife Advantages • Pancreatic cancer presents many problems, including significant nutritional problems, chronic pain, difficulties with local control, frequent systemic metastases, especially to liver, intraperitoneal metastatic spread, and a dismal survival rate; an improvement in local control will be a necessary but not sufficient step to improve on the current therapeutic outcomes • The CyberKnife is well-poised to improve the local control rates−this is accomplished with high doses per fraction delivered via a highly conformal approach1-5; improvements in systemic therapy are also necessary, which can easily be sequenced into the treatment as the CyberKnife treatment is of short duration • Therapeutic expectations of this disease are largely palliative, but systemic improvements could lead to survival improvements • CyberKnife radiosurgery alone may produce survival gain in a small number of patients as suggested by this case
CT 11 months post-radiosurgery shows reduction in the tumor size compared to both the initial CT (p. 2) and the planning CyberKnife CT (p. 3) scans. No tumor recurrence has been observed.
Follow-up FDG PET-CT scan 11 months post-radiosurgery is negative in the pancreatic bed. The mass has significantly reduced in size since the CyberKnife radiosurgical procedure.
CYBERKNIFE AT GEORGETOWN UNIVERSITY HOSPITAL (www.georgetownuniversityhospital.org)
Georgetown University Hospital’s (GUH) CyberKnife Robotic Radiosurgery System, installed in 2002, was the first system on the East Coast. The Synchrony® Respiratory Tracking System was added in 2004 and Xsight® Spine Tracking in 2006. The CyberKnife System allows GUH physicians to provide a targeted, minimally invasive alternative to open surgery and a treatment option for certain tumors that are otherwise untreatable. GUH physicians and the Radiation Oncology Department have created a multi-disciplinary approach to provide their patients with the most comprehensive diagnosis and treatment possible. Over 400 patients were treated in 2006, with a clinical workload of 45% intracranial, 20% spine and 35% extracranial non-CNS.
References 1. Murphy MJ, Adler JR, Bodduluri M, Dooley J, Forster K, Hai J, Le Q, Luxton G, Martin DP, Poen J: Image-guided radiosurgery for the spine and pancreas. Computer Aided Surgery 5(4):278-88, 2000. 2. Murphy MJ, Martin D, Whyte R, Hai J, Ozhasoglu C, Le Q: The Effectiveness of Breath Holding to Stabilize Lung and Pancreas Tumors During Radiosurgery. International Journal of Radiation Oncology Biology Physics 53(2):475-482, 2000. 3. Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, Ford J, Poen, J, Gibbs IC, Mehta VK, Kee S, Trueblood W, Yang G and Bastidas JA: Phase I Study of Stereotactic Radiosurgery in Patients with Locally Advanced Pancreatic Cancer, Int. J. Radiation Oncology Bio. Phys. 58(4):1017-1021, 2004. 4. Perman M, Bellairs EE, Wu X, Schwade, JG: Cancer of the pancreas with special reference to epidemiology & radiosurgery. In Mould RF et al. (eds.): Robotic Radiosurgery, Vol 1, Sunnvale, CA: CyberKnife Society Press, 2005. 5. Goodman KA, Koong AC: CyberKnife radiosurgery for pancreatic cancer. In Mould RF et al. (eds.): Robotic Radiosurgery, Vol 1, Sunnvale, CA: CyberKnife Society Press, 2005.
www.accuray.com
92
© 2008 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500103.C
41
CASE STUDY
M E D I C A L LY I N O P E R A B L E PRIMARY LIVER CARCINOMA
Tianjin Medical University, Cancer Institute and Hospital CyberKnife® Team:
42
Radiation Oncologist:
Zhiyong Yuan, M.D.
General Surgeon:
Ming Gao, M.D.
CyberKnife Physicists:
Yang Dong, Ph.D. Jingsheng Wang, Ph.D.
CyberKnife Center:
Tianjin Medical University, Cancer Institute and Hospital Tianjin, China
93
M E D I C A L LY I N O P E R A B L E P R I M A R Y L I V E R C A R C I N O M A
DEMOGRAPHICS Sex: Age: Histology:
Male 65 years old Hepatocellular Carcinoma
Case History A 65-year-old man with history of cirrhosis due to alcohol abuse was found to have a solitary lesion in the dome of his liver consistent with hepatocellular carcinoma (HCC). A PET-CT scan suggested hepatic dome carcinoma without evidence of extrahepatic disease. Biopsy by ultrasound-guided percutaneous needle puncture confirmed the diagnosis of HCC. The patient was also noted to have an elevated alphafetoprotein (AFP) level of 193.6 μg/L at that time. The patient’s liver disease was classified as Child-Pugh class A, and the staging of the patient’s HCC was T1N0M0.
CLINICAL HISTORY Referred by: Past Medical History:
General Surgeon 20-year history of alcohol abuse
CyberKnife® Treatment Rationale The patient was not a candidate for surgery due to his advanced level of cirrhosis. Furthermore, the patient had tumor recurrence after two lines of TACE; an additional TACE treatment was not a viable option. External beam radiation therapy was rejected for this patient because clinicians felt it would be difficult to meet dose-volume constraints with liver reserve compromised by cirrhosis. CyberKnife® treatment was chosen because it is a non-invasive, ablative treatment that offers excellent tumor control while minimizing damage to the cirrhotic liver and adjacent organs.
The patient underwent transcatheter arterial chemoembolization (TACE). Despite two rounds of treatment with TACE over the next two months, AFP levels continued to increase to 619 μg/L. The patient was readmitted to the hospital and a PET-CT scan revealed a higher SUV uptake at the tumor site, highly suggestive of tumor progression.
Figure 1. PET-CT scan demonstrates persistent high SUV uptake by the tumor despite two lines of transcatheter arterial chemoembolization.
94
43
M E D I C A L LY I N O P E R A B L E P R I M A R Y L I V E R C A R C I N O M A
TREATMENT DETAILS Gross Tumor Volume (GTV): 35.8 cc Tumor Diameter: 2.9 cm by 4.4 cm Imaging Technique(s): CT (1.25 mm thickness) and PET-CT Rx Dose & Isodose: 50 Gy to 75% isodose line Fractions: Five fractions
Treatment Planning The patient was prepared for treatment planning by implanting one fiducial within the tumor under ultrasound guidance. A CT scan (1.25 mm thickness) was obtained with the patient resting in a custom-fit vacuum cushion also used during treatment. PET-CT imaging was fused to the CT scan in the MultiPlan® planning system software. The hepatic tumor was contoured with a 3-mm expansion from the GTV to CTV and a 2-mm additional expansion from CTV to PTV. The normal liver, kidney, bowel, esophagus, stomach and spinal cord were contoured as critical structures; dose constraints for these structures are listed in Table 1.
Body 900-1000 126 non-isocentric, non-coplanar beams Synchrony® System 35 mm
Path Template: Number of Beams: Tracking Method: Collimator:
Treatment Delivery The patient received 50 Gy to the 75% isodose line delivered in five equal fractions using 126 beams per fraction and a 35-mm collimator. Synchrony® fiducial tracking was used to track and correct for tumor motion during each fraction.
Critical Structure
Dose Constraints 50% limited to < 8 Gy
Normal liver
75% < 8 Gy
Kidneys
50% < 8 Gy, Max dose < 20 Gy
Bowel & stomach
Max dose < 8 Gy
Spinal cord
Table 1. Dose constraints to critical structures.
100
Volume (%)
80
60
40
20
0 0
20
40
60
80
100
Dose (%)
Spinal Cord
Figure 2. CyberKnife treatment. The patient received 50 Gy to the 75% isodose line delivered in five fractions using 126 beams per fraction.
44
Esophagus
Liver
PTV
Figure 3. CyberKnife treatment. Dose-volume histogram shows the PTV and the rapid dose fall-off for normal liver, spinal cord and esophagus.
95
M E D I C A L LY I N O P E R A B L E P R I M A R Y L I V E R C A R C I N O M A Outcome and Follow-Up • One month after treatment liver function tests remained stable as compared to pretreatment values; AFP remarkably decreased to 75 ug/L • Two months after CyberKnife® treatment there was no visual evidence of the tumor on PET-CT nor was there any residual abnormal metabolic activity noted in the previous location of the tumor (Figure 4) • Five and nine months after CyberKnife treatment PET-CT scans continued to demonstrate no evidence of disease; liver function continues to be stable
Conclusion and CyberKnife® Advantages • The patient tolerated the CyberKnife treatment well and no acute liver toxicity was noted • After 9 months the tumor continued to be PET-CT negative and there were no noted side effects from the treatment
Figure 4. Two months after CyberKnife treatment. PET-CT scan demonstrating no evidence of activity in the treated region of the liver tumor.
Medically inoperable primary liver carcinoma
TIANJIN MEDICAL UNIVERSITY CANCER INSTITUTE AND HOSPITAL CyberKnife Center, Tianjin Cancer Hospital is located in Tianjin City, China. Tianjin Cancer Hospital is considered the birthplace of Oncology in China. It has become one of the largest and most famous modernized cancer hospitals and is also one of the largest bases for cancer prevention, treatment, training and research in China. The center treats over 220,000 cancer patients a year, many of these patients with liver and lung cancer. http://www.tjmuch.com/english/
www.accuray.com
96
© 2009 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500717.A
45
C5 malignant peripheral nerve sheath tumor
CASE STUDY
C5 MALIGNANT PERIPHERAL NERVE S H E AT H T U M O R European CyberKnife® Center (Munich) Team:
46
Neurosurgeons:
Alexander Muacevic, M.D. Berndt Wowra, M.D.
Medical Physicists:
Christian Drexler, M.Sc. Sibylle Staerk, M.Sc.
Technical Assistant:
Stefanie Himmerich
CyberKnife Center:
European CyberKnife Center Munich, Germany
97
C 5 M A L I G N A N T P E R I P H E R A L N E R V E S H E AT H T U M O R
DEMOGRAPHICS Sex: Age: Histology:
F 56 Malignant peripheral nerve sheath tumor (MPNST)
Case History Malignant peripheral nerve sheath tumors (MPNST) are rare spindle-cell sarcomas derived from Schwann cells or pluripotent cells of the neural crest. The estimated incidence of MPNST in patients with Neurofibromatosis Type 1 (NF1) is 2-5% compared with 0.001% in the general population. MPNST originate from peripheral nerve root trunks, extremities, and the head and neck region. The tumor can be located intraspinally, foraminally, and extend extraforaminally. These tumors pose specific therapeutic challenges as they recur locally after surgery. Adjuvant conventional radiation therapy improves survival rate or local control in some series but not in others.1,2,3
CLINICAL HISTORY Referred by: Past Medical History:
Sheba Medical Center, Israel Surgery, Radiotherapy, Chemotherapy
CyberKnife® Treatment Rationale There were no further treatment possibilities as surgery, conventional radiation therapy and chemotherapy were already attempted without preventing tumor growth. It was hoped that additional robotic radiosurgery targeting only the tumor mass could help to prevent further tumor progression and to reduce cervical pain.
We present here a rare case of a recurrent spinal non-NF1 MPNST treated by spinal radiosurgery. This 56-year-old female patient was transferred for progressive gait disturbances accompanied by severe neck pain. She was on morphine medication for 8 weeks. Incomplete surgery for a cervical nonNF1 MPNST at the level of C5 was performed 6 months prior to admission. After surgery the patient underwent a course of conventional fractionated radiotherapy (40 Gy) covering the whole spinal canal. Magnetic resonance imaging (MRI) of the cervical spine on admission revealed a large contrast enhancing extradural mass compressing the ventral spinal cord at C5. T2-weighted pre-treatment MRI scan. The lesion is clearly visualized at C5 significantly compressing the ventral aspect of the cervical spinal cord resulting in severe neck pain.
98
47
C 5 M A L I G N A N T P E R I P H E R A L N E R V E S H E AT H T U M O R
TREATMENT DETAILS Tumor Volume: Imaging Technique(s): Rx Dose & Isodose: Conformality Index: Tumor Coverage: Number of Beams:
1.8 cm3 CT, MRI 13.5 Gy to 80% 1.46 91.4% 311
Treatment Planning Process Treatment planning utilized CT, T1- and T2-weighted MRI scans +/- Gd with the patient positioned supine. These data sets were transferred to the MultiPlan® Treatment Planning System and registered using an algorithm based on normalized mutual information. The target volume, spinal cord, and other structures were defined mainly using the T2 MRI data. A treatment plan was generated using an iterative inverse planning algorithm. A dose of 13.5 Gy was prescribed to the 80% isodose, and delivered in a single fraction.
Fractions / Treatment Time: Path Template: Tracking Method: Collimator(s): Homogeneity Index:
1 @ 105 minutes 3 paths 900_1000 mm Xsight™ spine tracking 5 mm 1.25
Treatment Delivery The patient was positioned supine and fitted with a thermoplastic mask only. Treatment was delivered using the XsightTM fiducial-less spine tracking system. With this system the position of the spinal vertebrae are calculated automatically from orthogonal X-ray images acquired during treatment. These are compared with digitally reconstructed radiographs (DRRs) calculated from the pre-treatment CT. The offset required to maintain the alignment of each individual beam to the target volume is calculated by a deformable registration algorithm which also compensates for any non-rigid change in patient position between pre-treatment imaging and treatment delivery. By repeating this process throughout treatment, intra-fraction patient motion, which can be significant in spinal treatments, is effectively negated.4
Dose Volume Histogram (DVH) for all key structures.
Mid-sagittal (top) and axial (bottom) isodose distributions: The red structure is the target volume and the green structure the spinal cord. A very steep dose gradient was obtained posteriorly in order to spare the spinal cord.
48
99
C 5 M A L I G N A N T P E R I P H E R A L N E R V E S H E AT H T U M O R Outcome and Follow-Up The patient was seen for multiple follow-up evaluations after completing CyberKnife® Stereotactic Radiosurgery. • One week after therapy the patient was able to stop pain medication • Neck pain completely resolved after two weeks and walking improved considerably • MRI follow-up scan four weeks after therapy showed tumor shrinkage, with considerable reduction in the spinal cord compression • Three months after treatment she continued to be off pain medication and pain free • There were no adverse treatment effects during the follow-up period Conclusion and CyberKnife Advantages Robotic radiosurgery with the Xsight™ fiducial-less spine tracking system was shown for this patient, to be a safe and effective tool to treat spinal lesions, either as the primary treatment modality or within the framework of a multidisciplinary treatment strategy. This technique might be particularly valuable for recurrent tumors after conventional treatments such as surgery and/or radiation therapy.
T2-weighted MRI scan acquired four weeks post-treatment. There was significant tumor shrinkage. The compression to the spinal cord is considerably reduced.
CYBERKNIFE AT EUROPEAN CYBERKNIFE CENTER, MUNICH (www.cyber-knife.net)
The European Cyberknife Center in Munich is a new medical center operated in collaboration with the University Hospital, Munich. The center is dedicated to providing high precision robotic radiosurgery and is the first Cyberknife facility to open in Germany. The medical team has extensive radiosurgical experience, having previously treated 3200 patients over 12 years. On the basis of this experience they opted to open a new center dedicated to CyberKnife radiosurgery, and in July 2005 the system was installed. By May 2007 over 800 patients had been treated for a variety of central nervous system indications, of which approximately 20% have been spinal treatments.
References: 1. Baehring et al. Malignant peripheral nerve sheath tumor: The Clinical Spectrum and Outcome of Treatment. Neurology. 61:696-698, 2003. 2. Celli P, Trillo G, Ferrante L. Extrathecal Intraradicular Nerve Sheath Tumor. J Neurosurg Spine. 3:1-11, 2005. 3. Baek WS, Pytel P, Undevia SD, Rubeiz H. Spinal Cord Metastasis of a Non-Neurofibromatosis Type-1 Malignant Peripheral Nerve Sheath Tumor: an Unusual Manifestation of a Rare Tumor. J Neurooncol. 74:183-5, 2005. 4. Murphy MJ et.al. Dosimetric Effect of Intra-fraction Motion During Spinal Radiosurgery Proceedings – 7th International Stereotactic Radiosurgery Society Congress, 11-15 Sept 2005, Brussels.
www.accuray.com
100
© 2007 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500095.A
49
CASE STUDY
SPINAL ARTERIOVENOUS M A L F O R M AT I O N San Bartolo Hospital CyberKnife® Team:
58
Neurosurgeons:
Frederico Colombo, M.D. Leopoldo Casentini, M.D.
Medical Physicists:
Paolo Francescon, Ph.D. Carlo Cavedon, Ph.D. Stefania Cora, Ph.D. Paolo Scalchi, Ph.D.
Biomedical Engineer:
Joseph Stancanello, Ph.D.
CyberKnife Center:
San Bortolo Hospital Vicenza, Italy
101
S P I N A L A R T E R I O V E N O U S M A L F O R M AT I O N
DEMOGRAPHICS Sex: Age: Histology:
Male 37 years T10-T11 Spinal AVM
Case History This 37-year-old male presented with progressive paraparesis together with impaired bladder control and sexual function. A 0.8 cm3 spinal arteriovenous malformation (AVM) was diagnosed at the T10-T11 vertebral levels. Three attempts at embolization were made without success. The patient suffered two bleeding episodes, one as a complication following embolization. In each case, the patient’s symptoms worsened leading to paraplegia and partial recovery in one case.
CLINICAL HISTORY Referred by: Past Medical History:
Neurosurgeon None
CyberKnife® Treatment Rationale The patient was considered to be inoperable, and was referred by the neurosurgeon for radiosurgery using the CyberKnife® Robotic Radiosurgery System. Treatment of intracranial AVMs was one of the original radiosurgical applications, and radiosurgery has proven an effective modality in this field. Until recently, treatment of spinal AVMs has been impossible because of the limitations of rigid frame-based systems. By providing frameless stereotactic alignment, the CyberKnife System makes it possible to extend radiosurgery to extracranial targets, and its application to spine lesions has been previously reported.1,2,3 Patients are usually referred on the basis of unsuitability for conventional surgery, or when conventional surgery is refused, and in some cases (as in the present one) embolization will have been attempted prior to radiosurgery.
Pretreatment MR angiogram showing the nidus (arrow) and spinal vertebrae.
102
59
S P I N A L A R T E R I O V E N O U S M A L F O R M AT I O N
TREATMENT DETAILS Tumor Volume: Imaging Technique(s): Rx Dose & Isodose: Conformality Index: Number of Beams:
0.8 cc CT, 3DRA, MR Angiography 18.2 Gy to 70% 1.56 130
Treatment Planning and Delivery Four fiducials were implanted in the vertebral bodies above and below the nidus without complication. One week later pre-treatment images were acquired including 3D rotational angiography (3DRA), MR angiography, and contrast-enhanced CT scanning. These image-sets were registered using a normalized mutual information algorithm, and the target volume was defined using the 3DRA images. The patient was positioned
Fractions / Treatment Time: Path Template: Tracking Method: Collimator(s):
4 / 40 minutes average 3 path 900_1000 mm Fiducial tracking 5 mm
supine in a standard vacuum conformed immobilization device. The supine position is preferred over prone because this minimizes the uncertainty due to respiratory motion. Inverse planning was used to generate a plan of 130 beams. A dose of 18.2 Gy was prescribed to the 70% isodose and delivered in 4 daily fractions using a 5-mm collimator. Fiducial tracking was used, and each outpatient session lasted 40 minutes including setup.
Isodose lines superimposed on multiplanar 3DRA fused images. Note contrast enhancement within the nidus.
3D reconstruction of the CT dataset showing the non-isocentric, non-coplanar beam arrangement.
60
103
S P I N A L A R T E R I O V E N O U S M A L F O R M AT I O N Outcome and Follow-Up Follow-up MR angiography was performed at 6 and 12 months post-treatment. Progression of neurological symptoms was apparent for the first six months but then stabilized at 12 months post-treatment. Twelve months post-treatment the AVM nidus volume had decreased in size. MR angiography was repeated at 24 months, and showed further reduction in the nidus volume. The patient has remained clinically stable with no bleeding episodes throughout this follow-up period, and there was no chronic treatment-related toxicity. At 36 months MR angiography indicated complete obliteration and this was confirmed by selective digital subtraction angiography (DSA). The patient’s symptoms had also improved with reappearance of normal knee reflexes and he can walk with a cane.
Conclusion and CyberKnife® Advantages CyberKnife® radiosurgery was successfully applied to obliterate this intramedullary spinal AVM. 3DRA images were used to accurately define the AVM. Highly conformal dose delivery with steep falloff avoided radiation-induced neuropathy despite the location of the nidus within the spinal cord. The CyberKnife System allowed delivery of a non-invasive, painless treatment which resulted in hemorrhage-free follow-up period and ultimately in complete nidus obliteration within 36 months.
Angiographic images acquired pre-treatment (top left) and 24 months post-treatment (top right) showing significant nidus volume reduction. Complete nidus obliteration was achieved at 36 months (bottom left).
Spinal arteriovenous malformation
CYBERKNIFE AT SAN BORTOLO HOSPITAL
The CyberKnife System was installed in January 2003, and was the first installation in Europe. By March 2007 it has been used to treat nearly 1200 patients. Radiosurgery for AVMs had been performed in this center since 1984 using a conventional linear accelerator technique with a rigid head-frame4. Since 2003 these treatments have been transferred to the CyberKnife System. By March 2007 almost 220 intracranial and spinal AVMs have been treated using the CyberKnife System. Professor Colombo and his team have been pioneers in linac-based radiosurgery over 20 years. Their CyberKnife patient population is approximately 76% intracranial and 24% extracranial.
References 1. Ryu SI, Chang SD, Kim DH, Murphy MJ, Le QT, Martin DP, Adler JR Jr.: Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery 49(4):838-846, Oct 2001 2. Gerszten PC, Ozhasoglu C, Burton SA, Vogel WJ, Atkins BA, Kalnicki S, Welch WC: CyberKnife frameless stereotactic radiosurgery for spinal lesions: Clinical experience in 125 cases. Neurosurgery 55(1):89-98, Jul 2004 3. Stancanello J, Cavedon C, Francescon P, Cerveri P, Ferrigno G, Colombo F, Perini S: Development and validation of a CT-3D rotational angiography registration method for AVM radiosurgery. Medical Physics 31(6):1363-1371, June 2004 4. Colombo F, Benedetti A, Pozza F, Zanardo A, Avanzo RC, Chierego G, Marchetti C: Stereotactic radiosurgery utilizing a linear accelerator. Appl Neurophysiol 48:133-145, 1985
www.accuray.com
104
© 2008 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500300.B
61
Partial breast irradiation after
Case study
lumpectomy
Pa r t i a l B r e a s t i r r a d i at i o n after lumPectomy
swedish radiosurgery center at swedish medical center Seattle, Washington cyberKnife® team Sandra Vermeulen, M.D. Cristian Cotrutz, Ph.D.
62
105
Pa R t I a L B R e a s t I R R a d I at I O N a f t e R L u M P e C t O M y
deMOGRaPHICs sex: age: Histology: Past Medical History:
Female 44 years old Infiltrating ductal carcinoma (IDC) Breast cysts, Graves disease No prior surgeries Never pregnant
Case History A 44-year-old woman presented with a density in the upper outer quadrant of her left breast detected on screening mammogram. An MRI confirmed this lesion to be the only suspicious mass in either breast. An ultrasound-guided core biopsy was consistent with an infiltrating ductal carcinoma. The patient underwent a lumpectomy and left axillary sentinel node dissection. Final pathology revealed a 0.8 cm, high-grade tumor with a Bloom-Richardson score of 8/9. No vascular/ lymphatic space invasion was identified. DCIS of high nuclear grade without necrosis, estimated size of 0.8 cm, was present. Surgical margins were free of both invasive and noninvasive disease components. The infiltrating tumor was ER/PR negative and HER2/Neu 2+, as well as FISH positive for low level gene amplification. There was no evidence of metastasis in the dissected sentinel lymph nodes. The patient’s postoperative course was uneventful.
CLINICaL HIstORy
family History:
Pathologic stage:
Unspecified cancers on mother’s side, breast cancer on father’s side. Patient is of Ashkenasi Jewish heritage. t1bN0Mx infiltrating ductal carcinoma and ductal carcinoma in situ (dCIs).
CyberKnife® treatment Rationale After surgery, the patient was seen in the Radiation Oncology Clinic. She was informed about the current standard of care, which includes about 6 weeks of daily radiation to the involved breast, as well as the accepted risks of side effects. At the time, accelerated partial breast irradiation (APBI) was not considered a suitable option based on the patient’s age, BRCA 1/2 status, and extensive intraductal component (EIC). Instead of opting out of radiation treatment completely, the patient chose to pursue CyberKnife treatment. CyberKnife is being used by multiple centers to treat early stage breast cancer. The ability of the CyberKnife System, using Synchrony® Respiratory Tracking, to track the tumor as it moves with respiration and automatically correct the beam aim in realtime allowed the diameter of the treated region to be reduced from 2.5 cm, used in APBI, IMRT and 3DCRT, to 1.8 cm or less with CyberKnife. This could decrease the risk of side effects by eliminating more normal tissue from the high-dose volume.
Screening mammography revealed new density in the outer upper quadrant of the left breast.
Post-lumpectomy MRIs (pre-Cyberknife treatment).
106
63
Pa R t I a L B R e a s t I R R a d I at I O N a f t e R L u M P e C t O M y
tReatMeNt detaILs target Volume:
Imaging technique(s): Rx dose & Isodose:
CTV = 55.5 cm3 PTV = 92.1 cm3 Lumpectomy cavity = 16 cm3 CT + MRI 30 Gy delivered to the 70% isodose line
fractions: Path template: tracking Method: Collimator: Number of Beams:
5 fractions Short Synchrony® Respiratory Tracking 25 mm 112
treatment Planning The treatment plan was developed using the MultiPlan® Treatment Planning System, and was based on a singlepath, fiducial tracking algorithm. Four fiducials were implanted during the lumpectomy and were identified on the CT images. Treatment volumes were created by first outlining the resection cavity, resulting in a volume of 16 cm3. The resection cavity was expanded 15 mm isotropically to create the clinical target volume (CTV), except posteriorly where no expansion was applied because the cavity abutted the chest wall. The planning target volume (PTV) was created by expanding the CTV by 3 mm (except towards the chest wall, where no expansion was applied) to account for remaining uncertainty in target position. The final plan consisted of 112 non-zero beams and used one fixed collimator of 25 mm. treatment delivery The patient was treated with the CyberKnife® System, which precisely targeted multiple non-isocentric, noncoplanar beams at the tumor bed. A large dose of radiation was delivered to a small field while sparing lung and cardiac tissues, as well as other critical structures. A prescription dose of 6.0 Gy per fraction was delivered in 5 fractions for a total dose of 30 Gy to the 70% isodose line. Synchrony Respiratory Tracking was used to track and correct the motion of the target due to respiration. The patient tolerated the treatment well, and reported no complaints due to treatment.
Axial planning image showing the lumpectomy bed, lung, heart and isodose curves.
LT BREAST PTV LT LUNG HEART CTV SPINAL CORD RT LUNG RT BREAST
Dose-volume histogram (DVH) showing the dose delivered to the lumpectomy cavity and organs at risk.
MRI 5 months post-CyberKnife treatment.
64
107
Pa R t I a L B R e a s t I R R a d I at I O N a f t e R L u M P e C t O M y
Outcome and follow-up • Almost 3-1/2 years after treatment the patient has had no complaints referable to treatment. She specifically denied any breast tenderness, chest wall pain, or overlying skin changes. There was no lymphedema of the arm and/or breast. • The patient’s mammogram and MRI scans, 41 months after treatment, were negative for cancer recurrence. Morphologic appearance of the breast parenchyma, on MRI, appeared relatively unchanged as compared to prior examinations. No abnormal internal mammary or axillary lymph nodes were identified. • Physical exam revealed an excellent cosmetic result, with no palpable abnormalities in either breast, no discharge from either nipple, and no peripheral lymphadenopathy.
Conclusion • Upon completion of the treatment no skin toxicity was noted. • Almost 3-1/2 years after Cyberknife® treatment the patient had no complaints referable to treatment; specifically no skin toxicities or lymphedema were noted. • CyberKnife radiosurgery, in conjunction with Synchrony® Respiratory Tracking, precisely delivers the prescription dose over a much shorter course of treatment than conventional radiotherapy.
SwediSh RadioSuRgeRy CenteR, Seattle, wa (www.swedish.org/radiosurgery) Swedish Radiosurgery Center, formerly Seattle CyberKnife, was the first center in the Pacific Northwest to offer the CyberKnife radiosurgery option. The team of experienced physicians, physicists, and clinical staff has been instrumental in developing disease-specific treatment protocols, including protocols for breast and prostate cancer. The ability to track movement in real-time offers patients precise radiation delivery with outstanding results. to contact the Swedish Radiosurgery Center, call 206-320-7130.
www.accuray.com
108
©2013 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony and MultiPlan are are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 501034.A
65
Left optic nerve meningioma (who grade 2)
CASE STUDY
LEFT OPTIC NERVE MENINGIOMA (WHO GRADE 2) Barrow Neurological Institute CyberKnife® Team: Radiation Oncologists: John Kresl, M.D., Ph.D. Surgeon:
Randal Porter, M.D.
Medical Physicist:
Raymond Rodebaugh, Ph.D.
Radiation Therapist:
Nancy Berstein, R.T.(T.)
Additional Contributors: Debra Traylor CyberKnife Center:
66
Saint Joseph Hospital Barrow Neurological Institute Phoenix, AZ
109
LEFT OPTIC NERVE MENINGIOMA (WHO GRADE 2)
DEMOGRAPHICS Sex: Age: Histology:
Male 75 years Meningioma
Case History History of progressive loss of visual acuity and peripheral vision. Patient was referred for resection. Patient complained of post-surgical retrobulbar pain and discomfort.
CLINICAL HISTORY Referred by: Previous Treatment:
Neurosurgery Previously resected
CyberKnife® Treatment Rationale This lesion is prone to recurrence so a recommendation to perform adjunctive radiosurgery to the resection bed was made by the neurosurgeon. Due to the risk of recurrence and intimate association of the lesion with the optic nerve, the CyberKnife System was selected. SRS will reduce the risk of encroachment into the optic chiasm and risk to the sighted eye. Studies report hypofractionation to be safer and effective for optic nerve menigiomas.1 ®
Pre-treatment CT image showing target lesion.
110
Pre-treatment MR image showing target lesion.
67
LEFT OPTIC NERVE MENINGIOMA (WHO GRADE 2)
TREATMENT DETAILS Tumor Volume: Imaging Technique(s): Rx Dose & Isodose: Conformality Index: Tumor Coverage: Number of Beams:
3.1 cc CT, MRI 25 Gy to 75% 1.3 95.7% 170
Planning Process and Goals The treatment plan that was developed covered 95.7% of the tumor target volume with the 75% isodose line. The patient underwent thermoplastic immobilization and simulation, followed by CT and MR imaging localization for CyberKnife treatment planning purposes.
Fractions / Treatment Time: Path Template: Tracking Method: Collimator(s):
5 / 50 minutes per fraction 3 path 800 mm 6D Skull Tracking 10 mm
Treatment Delivery The prescription was set at 25 Gy, delivered in five fractions of 5 Gy each. The treatment was delivered without difficulty. The patient tolerated the procedure well with some vague mild complaints of left frontal sinus irritation. He specifically denied any headaches, nausea, vomiting, reduced vision in the left eye, or cranial neuropathies.
Non-isocentric beam geometry of the solution.
Fused (MR/CT) - Axial and Coronal view of target and dose distribution.
68
Dose Volume Histogram for target volume and critical structures.
111
LEFT OPTIC NERVE MENINGIOMA (WHO GRADE 2) Outcome and Follow-Up MRI follow-up was completed three months post-treatment. The film review showed the tumor is stable.
3D representation of target volume, critical structures, and prescription isodose volume.
3 month follow-up MRI.
CYBERKNIFE AT BARROW NEUROLOGICAL INSTITUTE / ST. JOSEPH’S HOSPITAL (www.thebni.com) The Barrow Neurological Institute (BNI) is an internationally renowned medical center that offers care for people with brain and spine diseases, disorders and injuries. Dr. Robert Spetzler, one of the world’s leading neurosurgeons, is the Director of the Institute. There are 4,000 neurological procedures performed at BNI each year including up to 500 radiosurgical procedures. CyberKnife radiosurgery began at BNI in September 2003. The Center’s CyberKnife population has been 71% intracranial, 21% spine and 8% whole body. The CyberKnife System is used on those patients for whom traditional radiosurgery is not possible or in situations where patients specifically request this procedure over other treatment options.
References 1. Pham CJ, Chang SD, Gibbs IC, Jones P, Heilbrun MP, Adler Jr, JR: Preliminary Visual Field Preservation after Staged CyberKnife Radiosurgery for Perioptic Lesions. Neurosurgery, 54(4):799-812, 2004.
www.accuray.com
112
© 2007 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500100.A
69
Para-aortic lymph node metastasis
CASE STUDY
PA R A - A O RT I C LY M P H N O D E M E TA S TA S I S CyberKnife® Team: Radiation Oncologists: Douglas Wong, M.D., Ph.D., MPH Urologist:
Greg Rainwater, M.D.
Physicist:
Georg Weidlich, Ph.D.
CyberKnife Therapists: Jeff Wallace, RTT Valerie Cano, RTT CyberKnife Coordinator: Laura Valenzuela, RN, BSN, OCN CyberKnife Center:
Community Regional Medical Center Fresno, CA
70
113
PA R A - A O R T I C LY M P H N O D E M E TA S TA S I S
DEMOGRAPHICS Sex: Age: Histology:
CLINICAL HISTORY
Male 66 years Metastatic Renal Cell Carcinoma
Case History A 66-year-old male with a history of Stage IV renal cell carcinoma, treated with a left nephrectomy one year prior, presented with progressive left flank pain and findings of a large confluent left para-aortic lymph node mass on follow-up imaging studies. A CT-guided biopsy of the left para-aortic lymph node revealed metastatic carcinoma consistent with high-grade renal cell carcinoma. PET-CT scans revealed focal hypermetabolism in a region of confluent left para-aortic adenopathy, measuring 9.0 cm x 4.5 cm x 5.0 cm, with invasion of the adjacent psoas muscle (see Figure 1). At the time of the initial diagnosis of the renal cell carcinoma (Stage III, pT3N1M0) a radical nephrectomy had been performed to remove a 2-cm lesion involving the lower pole of the left kidney and a 5-cm mass in the mid-kidney that extended focally through the left renal capsule involving the perinephric fat. Lymph node sampling at the time of nephrectomy revealed one of four para-aortic lymph nodes positive for metastatic renal cell carcinoma. At the time a decision was made to follow these findings with PET-CT imaging.
Referred by: Past Medical History:
Medical Oncologist Stage IV renal cell carcinoma, treated by radical nephrectomy one year prior
CyberKnife® Treatment Rationale Renal cell carcinoma has typically been treated primarily with surgical resection and has not responded well to treatment by conventional radiation therapy because of the inability to deliver high, tumoricidal doses of radiation to the tumor safely. The location of this large para-aortic mass as well as its intimate relationship with adjacent vasculature made surgical resection an unlikely option in this case. Treatment was attempted with multiple tyrosine kinase inhibitors to shrink the tumor but this therapy was not tolerated. CyberKnife® radiosurgery was chosen to allow delivery of high doses of radiation in a conformal manner to spare the adjacent critical structures while still delivering doses high enough to achieve tumor control. The CyberKnife System has successfully treated primary renal cell carcinomas,1 as well as spinal metastases from renal cell carcinoma,2 while preserving the surrounding tissue and critical structures.
Figure 1. Pretreatment PET-CT scans revealed high FDG uptake in the region of the confluent left para-aortic adenopathy measuring 9.0 cm x 4.5 cm x 5.0 cm, with invasion of the adjacent psoas muscle.
114
71
PA R A - A O R T I C LY M P H N O D E M E TA S TA S I S
TREATMENT DETAILS Treatment Volume: Imaging Technique(s): Rx Dose & Isodose: Dose and Fractions: Number of Beams:
PTV=99.9 cm3 CT 24 Gy to 80% 8 Gy x 3 fractions 174
Planning Process The patient was prepared for treatment planning by implanting five fiducials into the periphery of the tumor. A vac lock bag and knee sponge were used for immobilization. Contours of the GTV, PTV, right kidney, adjacent bowel and spinal canal were generated on the imaging studies. To protect adjacent small bowel, the PTV was deliberately made smaller than the GTV, accepting a dose gradient across the anterior rim of the GTV. A conformal treatment plan was developed using the MultiPlan® Treatment Planning System to cover 97.7% of the PTV and 86.2% of the GTV (see Figure 2).
Number of Fiducials: Treatment Time: Tracking Method: Collimator(s): Tumor Coverage:
5 120 minutes/fraction Synchrony 25 mm PTV=97.7%
Treatment Delivery The patient received 24 Gy delivered in three fractions of 8 Gy over three days. The treatment was delivered to a volume of 99.9 cm3 (PTV), using 174 beams and a 25-mm collimator. The 80% isodose line provided 97.7% coverage of the PTV. The three outpatient treatments were accompanied by mild nausea that was relieved with oral compazine.
Figure 2. Left panel shows an axial view of the treatment plan developed using the MultiPlan® System. The 80% prescription isodose line is shown in green. Note high conformality and sparing of critical structures. The right panel shows a 3-D rendering of treatment beams as selected by the optimization algorithm.
72
115
PA R A - A O R T I C LY M P H N O D E M E TA S TA S I S • Two months following CyberKnife® treatment, a PET scan demonstrated a decrease in size of the para-aortic mass and decreased SUV from 7.5 to 2.2 (see Figure 3) • Four months after CyberKnife treatment, a repeat PET scan revealed further decrease in SUV (from 2.2 to 1.7) within a decreasing left para-aortic nodal mass; no new areas of PET positivity SUV uptake were noted • Fourteen months after CyberKnife treatment, a PET-CT scan revealed continued decrease in the size of the left para-aortic mass with SUV of 2.3 (Figure 4); the patient remains pain-free and enjoys normal activities without limitation
Conclusion and CyberKnife® Advantages • This patient had an excellent initial outcome with the CyberKnife System for the treatment of a metastatic renal cell carcinoma to a para-aortic lymph node • The CyberKnife System provided a convenient and minimally invasive treatment option for patients with metastasis to para-aortic lymph nodes
Figure 3. PET-CT scans obtained 2 months after CyberKnife treatment demonstrating a significant reduction in the FDG uptake in the region of the confluent left para-aortic adenopathy.
Figure 4. PET-CT scans obtained 14 months after CyberKnife treatment demonstrating a continued reduction in the FDG uptake in the region of the confluent left para-aortic adenopathy.
CYBERKNIFE CENTER AT COMMUNITY REGIONAL MEDICAL CENTER
The CyberKnife Center at Community Regional Medical Center in Fresno, California entered clinical service in the fall of 2005. Community’s cancer program is one of the few in California with teaching-hospital level accreditation from the American College of Surgeons (ACOS) Commission on Cancer. At Community Regional in downtown Fresno we operate the only combined burn and Level 1 trauma center between Sacramento to Los Angeles, the only high-risk pregnancy unit in the region, and the state’s largest and second busiest emergency department.
To contact us please visit our website at www.communitymedical.org or call (559) 447-3591. References 1. Ponsky LE, Crownover RL, Rosen MJ, et al. Initial evaluation of Cyberknife technology for extracorporeal renal tissue ablation. Urology 2003;61:498-501. 2. Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine 2005;3:288-295.
www.accuray.com
116
© 2007 Accuray Incorporated. All Rights Reserved. Accuray, the stylized logo, CyberKnife, Synchrony, Xsight, Xchange and RoboCouch are among the trademarks and/or registered trademarks of Accuray Incorporated in the United States and other countries. 500334.A
74
PAPERS CLÍNICOS
A continuación se presentan algunos de los papers y estudios clínicos más representativos de los tratamientos con CyberKnife realizados en prestigiosas entidades e instituciones alrededor de todo el mundo. Entre otros trabajos se resaltan los realizados en Flushing Radiation Oncology, en Nueva York, en Georgetown University Hospital, en Stanford University School, en S. Bortolo City Hospital, Vicenza, Italia y en ErasmusMC, Holanda. En estos papers encontrarán resultados interesantes como los referidos por Kantz, para próstata donde se obtienen en todos los grupos de riesgo altas tasas de control tumoral local evidenciada mediante1 bajos niveles de PSA después de 2-3 años de seguimiento, el potente efecto biológico se logró
PAPERS CLÍNICOS
con la entrega total por el CyberKnife® de 35-36.25 Gy en 5 fracciones y los resultados son comparables a los obtenidos con HDR hipofraccionada. Kresl, por su parte muestra cómo CyberKnife, superando las limitaciones de la tecnología tradicional, amplía las posibilidades en radiocirugía para un amplio rango de volúmenes en diferentes locaciones anatómicas, CyberKnife extrapola las posibilidades clínicas a partir de la experiencia obtenida con Gamma Knife. También se resalta el estudio de Sean P Collins2 para tumores complejos en base de cráneo, donde se trataron 80 pacientes con lesiones en base de cráneo y se clasificaron en simple o complejo basado en la proximidad a estructuras críticas. Se indica que los planes obtenidos con CyberKnife manifiestan una excelente homogeneidad, conformación y una cobertura porcentual suficiente en los casos más complejos. Los parámetros de tratamiento obtenidos para los casos complejos incluyen3 un nuevo índice de conformación de dosis, de homogeneidad y de cobertura que no difieren significativamente con respecto a los casos simples.
1 Ver en la sección Datos clínicos – Papers clínicos: CyberKnife® Radiosurgery for Prostate Cancer, Katz. J. 2 Ver en la sección Datos clínicos – Clinical Comparison of CyberKnife & Gamma Knife Radiosurgery Systems, kresl J. 3 Ver en la sección Datos clínicos – Papers clínicos: CyberKnife® radiosurgery in the treatment of complex skull base tumors: analysis of treatment planning parameter, Sean P Collins, Nicholas D Coppa, et all. 75 75 117
Para el caso de meningiomas sobresale el estudio de Colombo4 con 199 pacientes donde muestra que el uso del CyberKnife permitió extender la indicación a un 30% de los pacientes los cuales no hubiera sido posible realizar con técnicas de radiocirugía de una sesión. Las mejoras observadas en los pacientes fueron mayores con el CyberKnife que con los aceleradores lineales. Adicionalmente se incluyen una comparación costo-efectividad entre la stereotactic body radiation therapy (SBRT), la Intensity Modulated Radiation Therapy (IMRT) y la protonterapia (PT) en cáncer de próstata localizado. Los autores5 muestran que asumiendo que los resultados de cada modalidad de tratamiento a largo plazo son efectivos, SBRT es la técnica más costo-efectiva resultando en una mejora en la sobrevida respecto a las otras técnicas para el caso concreto de cáncer localizado de próstata.
4 Ver en la sección Datos clínicos – Papers clínicos: Comparative costeffectiveness of stereotactic body radiation therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer 5 Ver en la sección Datos clínicos – Papers clínicos: Cyberknife Radiosurgery for benign Meningiomas: Short-Term Results in 199 Patients
118
Open Access Case Report
DOI: 10.7759/cureus.13620
Giant Tumor in the Inferior Vena Cava Treated With CyberKnife Yuko Harada 1 , Shinichiro Miyazaki 2 , Toshiaki Kunimura 3 1. Cardiology, Kawasaki Municipal Ida Hospital, Kawasaki, JPN 2. Radiation Oncology, CyberKnife Center, ShinYurigaoka General Hospital, Kawasaki, JPN 3. Clinical Pathology, Yamato Tokushukai Hospital, Yamato, JPN Corresponding author: Yuko Harada, adayuko1219@yahoo.co.jp
Abstract Renal cell carcinoma (RCC) is a slow-progressing cancer that may cause tumor embolism in the inferior vena cava (IVC) and has a high mortality rate. Treatment for IVC metastasis of RCC is basically surgical resection often requiring cardiopulmonary bypass. RCC has been regarded as a radio-resistant tumor; however, stereotactic radiotherapy (SRT) has proven effective in recent years. We present a case of advanced RCC in which CyberKnife radiotherapy was successful in saving and preserving quality of life. An 81-year-old male presented with severe edema in both legs. Contrast CT scan displayed giant tumor in IVC and bilateral mediastinal lymphadenopathy. The cancer appeared to originate from the lower pole of the right kidney. The tumor protruded into the right atrium, and surgical resection with pump oxygenator was impossible due to patient’s age. CyberKnife SRT was performed for tumor in the IVC. Biopsy for hilar lymph node revealed clear cell RCC, and the second CyberKnife treatment was performed. The patient is surviving over three years without any symptoms. CyberKnife was successful in preserving patient’s quality of life for advanced stage IV RCC.
Categories: Internal Medicine, Radiation Oncology, Urology Keywords: ivc tumor, cyberknife, radiotherapy, clear cell renal cell carcinoma
Introduction Renal cell carcinoma (RCC) is a slow-progressing cancer. The mortality rate of RCC is not considered high; however, RCC could be fatal if it invades large vessels. The first choice of treatment is surgical resection. Radiotherapy is not considered effective. Molecular targeted drugs are used for inoperative cases. Treatment options are very limited for elderly patients with advanced stage RCC. Patients notice symptoms usually at advanced stage. Most patients are elderly and cannot tolerate surgery or chemotherapy. In recent years, radiotherapy has attracted attention as a promising treatment for advanced RCC. CyberKnife (Accuray Incorporated, Sunnyvale, CA, USA) is a robotic radiosurgery system that provides highly precise stereotactic radiotherapy (SRT). SRT with CyberKnife is minimally invasive and is usually completed within several days. Thus, CyberKnife is the preferred option for palliative treatment of advanced cancer.
Review began 02/05/2021 Review ended 02/21/2021 Published 02/28/2021 © Copyright 2021 Harada et al. This is an open access article distributed under the terms of the
In the case presented here, giant tumor of RCC in the inferior vena cava (IVC) was successfully treated with CyberKnife. SRT by CyberKnife demonstrated successful to sustain patient’s life.
Creative Commons Attribution License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Presentation An 81-year-old male visited our hospital with remarkable edema in both legs, particularly in his right leg. Vascular ultrasound on lower extremities revealed thrombus in the left popliteal vein; however, no thrombus was found in the right leg. Initial laboratory workup revealed mild kidney dysfunction with urea nitrogen level of 28.7 mg/dL, creatinine level of 1.27 mg/dL, and estimated glomerular filtration rate of 42 mL/min. Ddimer was elevated to 8.4 μg/L. Abdominal ultrasound revealed hyper-echoic lesion filled in the IVC from the kidney level to the right atrium. This revealed low-echoic lesion of 23.2 x 23.0 mm in the lower pole of the right kidney, which suggested tumor. Cardiac ultrasound and CT scan of the abdomen with intravenous contrast medium revealed IVC tumor protruding into the right atrium (Figures 1, 2). However, contrast enhancement in kidneys was not clear. FDG-PET (fluorodeoxyglucose-positron emission tomography)/CT scan revealed tumor in the lower pole of the right kidney, which continued into the right renal vein through the IVC (Figure 3). A right mediastinal lymph node and left hilar lymph node also showed FDG uptake. Thus, the patient was diagnosed with IVC tumor embolism possibly of RCC with mediastinal metastasis.
How to cite this article Harada Y, Miyazaki S, Kunimura T (February 28, 2021) Giant Tumor in the Inferior Vena Cava Treated With CyberKnife. Cureus 13(2): e13620. DOI 10.7759/cureus.13620
119
FIGURE 1: Contrast CT scan at diagnosis. Massive IVC tumor (red arrow) is protruding into the right atrium. IVC, inferior vena cava
FIGURE 2: Cardiac ultrasound showing tumor. Four-chamber view from the apex. Red arrow denotes IVC tumor protruding into the right atrium (23.6 x 22.4 mm in size). IVC, inferior vena cava
2021 Harada et al. Cureus 13(2): e13620. DOI 10.7759/cureus.13620
120
2 of 6
FIGURE 3: FDG-PET/CT scan before and after treatment. (A) FDG uptake is shown in red spot, revealing IVC tumor, arising from the lower pole of the right kidney, and two lymph node metastases. (B) One year after treatment. FDG uptake is not shown in IVC or kidneys. FDG-PET, fluorodeoxyglucose-positron emission tomography; IVC, inferior vena cava
Transperitoneal biopsy was avoided as tumor in the right kidney was small and in the lower pole. Even if biopsy was performed to confirm the diagnosis of RCC, surgical treatment was nearly impossible due to the patient’s age. The patient and his family requested palliative care or minimally invasive treatment to preserve quality of life. CyberKnife was chosen because it is a minimally invasive short-term treatment. Multisession SRT was performed using CyberKnife G4 system. Tumors were tracked with spine-tracking algorithm. Gross tumor volume (GTV) was defined as visible tumor on enhanced CT scan with images merged for target definition. GTV was considered the same as clinical target volume (CTV). Planning target volume (PTV) included CTV with a 1.2-mm margin. Giant IVC tumor (GTV 211.3 cm3) was treated with a prescription dose of 40 Gy in 10 fractions and prescription isodose line 89%. Second CyberKnife treatment was performed four months afterward for left hilar lymphadenopathy (GTV: 22.6 cm 3) with a prescription dose of 40 Gy in five fractions and prescription isodose line 57%. Twelve months after initial treatment, FDG uptake was not observed in IVC or left hilar lymph node. However, right mediastinal lymphadenopathy remained. The patient presented allergic reaction to the contrast medium, thereby precluding its usage. Biopsy was performed by bronchoscope, which revealed clear cell RCC (Figure 4). Eleven months after the second CyberKnife treatment, the third CyberKnife treatment was performed for right mediastinal lymphadenopathy (GTV: 11.7 cm3) with a prescription dose of 45 Gy in 10 fractions and prescription isodose line 73%. Cardiac ultrasound did not reveal any mass in the right atrium, which indicated that the tumor in the IVC had shrunk and cancer cells were destroyed over time by radiotherapy.
2021 Harada et al. Cureus 13(2): e13620. DOI 10.7759/cureus.13620
3 of 6
121
FIGURE 4: Pathological findings of lymph node biopsy (magnification x20). (A) Hematoxylin-eosin staining. (B) Positive staining with A/E staining. (C) Positive staining with CD 10 staining. (D) Positive staining with Vimentin. These findings are compatible with clear cell renal cell carcinoma metastatic to lymph node.
All three treatment courses were performed on outpatient basis. Edema in both legs disappeared 18 months after initial treatment. The patient reports no symptoms thereafter. Three years and three months have passed since initial treatment, with FDG-PET indicating cancer remission for IVC tumor and mediastinal lymphadenopathy (Figure 5). The patient is enjoying a normal life without any treatment-related toxicity.
FIGURE 5: FDG-PET/CT scan three years and three months after initial treatment.
2021 Harada et al. Cureus 13(2): e13620. DOI 10.7759/cureus.13620
122
4 of 6
Coronal sections from anterior to posterior are displayed from left to right. FDG uptake in IVC tumor and two lymph node metastases have disappeared. FDG-PET, fluorodeoxyglucose-positron emission tomography; IVC, inferior vena cava
Discussion RCC with IVC thrombus above hepatic veins is technically complex, often requiring cardiopulmonary bypass and resulting in a major complication rate of 34% and mortality rate of 10.8% [1]. As tumor thrombi in the IVC protruded into the right atrium, cardiopulmonary bypass was mandatory. RCC has traditionally been regarded as a radio-resistant tumor based on preclinical data and negative clinical trials using conventional fractionated radiotherapy [2]. However, radiotherapy delivered in few fractions with high single-fraction and total doses may overcome RCCs radio-resistance; thus, SRT has been successful showing high local control and low toxicity [2]. Robotic radiosurgery system is more precise, as Staehler et al. reported that single-fraction radiosurgery using 25 Gy with CyberKnife system resulted in a local control rate of 98% after nine months [3]. In 2018, a multicenter study revealed that single-fraction (median dose of 25 Gy) and multi-fraction (median dose of 40 Gy delivered in 2-10 fractions) stereotactic ablative radiotherapy resulted in a local control rate of 97.8% and a cancer-specific survival rate of 91.9% at four years [4]. Therefore, SRT for RCC is now considered an excellent treatment option for inoperable cases. In 1987, Didier et al. reported that primary neoplasm causing tumor thrombi of IVC were RCC, adrenal tumors, retroperitoneal tumors, and hepatic tumors [5]. Shuch et al. also reported that vascular invasion commonly occurs in RCC and that the mortality rate of tumor embolism was 75% [6]. The patient survived over one year after urgent life-saving CyberKnife treatment for IVC tumor of unknown origin. Subsequent lymph node biopsy confirmed RCC. Therefore, CyberKnife radiotherapy proved successful in life-saving treatment of massive IVC tumor embolism of RCC. There have been only two reports of radiotherapy for IVC tumor thrombosis of RCC. One is a case report of intensity-modulated radiotherapy (56 Gy/24 fractions of one fraction per day) that stabilized a tumor embolus in clear cell RCC [7]. Another is the only existing report of SRT for IVC tumor thrombus of RCC by Hannan et al., which showed only partial remission at two years follow-up and 18-month survival [8]. There is a choice of performing radiotherapy without biopsy or performing chemotherapy according to RCC treatment guidelines because of a higher probability of RCC. CyberKnife radiotherapy was preferable due to its non-invasivity and patient’s poor prognosis. CyberKnife radiotherapy for this patient was appropriately adjusted to treat such a large tumor on an outpatient basis. In standard CyberKnife radiosurgery, a fiducial may be inserted into the tumor to monitor respiratory movement, and fractions are usually three to five. In our case, we avoided insertion of the fiducial into the IVC due to a high risk of bleeding. Instead of inserting the fiducial, treatment plan settings were adjusted to minimize damage by radiation as follows: larger margin for PTV and increased fractions (10 fractions instead of five) (Figure 6). IVC tumor was very closely located to the spine and thus CyberKnife spine-tracking worked efficiently.
FIGURE 6: Treatment plan
2021 Harada et al. Cureus 13(2): e13620. DOI 10.7759/cureus.13620
5 of 6
123
Conclusions We reported a successful treatment of advanced RCC with massive IVC tumor embolism and lymph node metastases by CyberKnife radiotherapy. The patient is now over 84 years old, surviving without any symptoms, and enjoying normal life. SRT with CyberKnife radiotherapy was successful in treating massive IVC tumor embolism of RCC, which was considered to have a poor prognosis. CyberKnife radiotherapy thereby demonstrates a favorable treatment for elderly patients with IVC tumor thrombus who may otherwise not be good surgical candidates.
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements We would like to acknowledge Dr. Paul William Chin, Jr. for discussions and contribution.
References 1.
2. 3. 4.
5. 6.
7. 8.
Abel EJ, Thompson RH, Margulis V, et al.: Perioperative outcomes following surgical resection of renal cell carcinoma with inferior vena cava thrombus extending above the hepatic veins: a contemporary multicenter experience. Eur Urol. 2014, 66:584-92. 10.1016/j.eururo.2013.10.029 Ruhle A, Andratschke N, Siva S, Guckenberger M: Is there a role for stereotactic radiotherapy in the treatment of renal cell carcinoma?. Clin Transl Radiat Oncol. 2019, 18:104-12. 10.1016/j.ctro.2019.04.012 Staehler M, Bader M, Schlenker B, et al.: Single fraction radiosurgery for the treatment of renal tumors . J Urol. 2015, 193:771-75. 10.1016/j.juro.2014.08.044 Siva S, Louie AV, Warner A, et al.: Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: a report from the International Radiosurgery Oncology Consortium for Kidney (IROCK). Cancer. 2018, 124:934-42. 10.1002/cncr.31156 Didier D, Racle A, Etievent JP, Weill F: Tumor thrombus of the inferior vena cava secondary to malignant abdominal neoplasms: US and CT evaluation. Radiology. 1987, 162:83-9. 10.1148/radiology.162.1.3024211 Shuch B, Larochelle JC, Onyia T, et al.: Intraoperative thrombus embolozation during nephrectomy and tumor thrombectomy: critical analysis of the University of California-Los Angeles experience. J Urol. 2009, 181:492-8. 10.1016/j.juro.2008.10.036 Gong ZH, Yan LJ, Sun JG: Postoperative radiotherapy to stabilize a tumor embolus in clear cell renal cell carcinoma: a case report. Oncol Lett. 2014, 8:1856-8. 10.3892/ol.2014.2421 Hannan R, Margulis V, Chun SG, et al.: Stereotactic radiation therapy of renal cancer inferior vena cava tumor thrombus. Cancer Biol Ther. 2015, 16:657-61. 10.1080/15384047.2015.1026506
2021 Harada et al. Cureus 13(2): e13620. DOI 10.7759/cureus.13620
124
6 of 6
SYSTEMATIC REVIEW published: 29 March 2021 doi: 10.3389/fonc.2021.652646
CyberKnife for Recurrent Malignant Gliomas: A Systematic Review and Meta-Analysis Lucio De Maria 1*, Lodovico Terzi di Bergamo 2, Alfredo Conti 3†, Kazuhiko Hayashi 4†, Valentina Pinzi 5†, Taro Murai 6†, Rachelle Lanciano 7†, Sigita Burneikiene 8†, Michela Buglione di Monale 9, Stefano Maria Magrini 9 and Marco Maria Fontanella 1 Unit of Neurosurgery, University of Brescia and ASST Spedali Civili, Brescia, Italy, 2 Institute of Oncology Research, Bellinzona, Switzerland, 3 Unit of Neurosurgery, Alma Mater Studiorum University of Bologna and IRCCS Istituto delle Scienze Neurologiche, Bologna, Italy, 4 Unit of Radiation Oncology, Osaka University Graduate School of Medicine, Suita, Japan, 5 Unit of Neurosurgery, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy, 6 Unit of Radiology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan, 7 Philadelphia CyberKnife/Crozer Health, Havertown, PA, United States, 8 Boulder Neurosurgical Associates, Boulder, CO, United States, 9 Unit of Radiation Oncology, University of Brescia and ASST Spedali Civili, Brescia, Italy 1
Edited by: Christine Marosi, Medical University of Vienna, Austria Reviewed by: Birgit Flechl, MedAustron, Austria Pierina Navarria, Humanitas Research Hospital, Italy *Correspondence: Lucio De Maria l.demaria@unibs.it These authors have contributed equally to this work
†
Specialty section: This article was submitted to Neuro-Oncology and Neurosurgical Oncology, a section of the journal Frontiers in Oncology Received: 12 January 2021 Accepted: 15 March 2021 Published: 29 March 2021 Citation: De Maria L, Terzi di Bergamo L, Conti A, Hayashi K, Pinzi V, Murai T, Lanciano R, Burneikiene S, Buglione di Monale M, Magrini SM and Fontanella MM (2021) CyberKnife for Recurrent Malignant Gliomas: A Systematic Review and Meta-Analysis. Front. Oncol. 11:652646. doi: 10.3389/fonc.2021.652646
Frontiers in Oncology | www.frontiersin.org
Background and Objective: Possible treatment strategies for recurrent malignant gliomas include surgery, chemotherapy, radiotherapy, and combined treatments. Among different reirradiation modalities, the CyberKnife System has shown promising results. We conducted a systematic review of the literature and a meta-analysis to establish the efficacy and safety of CyberKnife treatment for recurrent malignant gliomas. Methods: We searched PubMed, MEDLINE, and EMBASE from 2000 to 2021 for studies evaluating the safety and efficacy of CyberKnife treatment for recurrent WHO grade III and grade IV gliomas of the brain. Two independent reviewers selected studies and abstracted data. Missing information was requested from the authors via email correspondence. The primary outcomes were median Overall Survival, median Time To Progression, and median Progression-Free Survival. We performed subgroup analyses regarding WHO grade and chemotherapy. Besides, we analyzed the relationship between median Time To Recurrence and median Overall Survival from CyberKnife treatment. The secondary outcomes were complications, local response, and recurrence. Data were analyzed using random-effects meta-analysis. Results: Thirteen studies reporting on 398 patients were included. Median Overall Survival from initial diagnosis and CyberKnife treatment was 22.6 months and 8.6 months. Median Time To Progression and median Progression-Free Survival from CyberKnife treatment were 6.7 months and 7.1 months. Median Overall Survival from CyberKnife treatment was 8.4 months for WHO grade IV gliomas, compared to 11 months for WHO grade III gliomas. Median Overall Survival from CyberKnife treatment was 4.4 months for patients who underwent CyberKnife treatment alone, compared to 9.5 months for patients who underwent CyberKnife treatment plus chemotherapy. We did not observe a correlation between median Time To Recurrence and median Overall Survival from CyberKnife. Rates of acute neurological and acute non-neurological side effects were
1
March 2021 | Volume 11 | Article 652646
125
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
3.6% and 13%. Rates of corticosteroid dependency and radiation necrosis were 18.8% and 4.3%. Conclusions: Reirradiation of recurrent malignant gliomas with the CyberKnife System provides encouraging survival rates. There is a better survival trend for WHO grade III gliomas and for patients who undergo combined treatment with CyberKnife plus chemotherapy. Rates of complications are low. Larger prospective studies are warranted to provide more accurate results. Keywords: CyberKnife, stereotactic radiosurgery, malignant gliomas, recurrence, HGG, high-grade gliomas, glioblastoma, anaplastic astrocytoma
INTRODUCTION
(PRISMA) guidelines (17). A comprehensive literature search of the databases PubMed, Ovid MEDLINE, and Ovid EMBASE databases was designed and conducted by an experienced librarian with input from the authors. The keywords “glioblastoma”, “anaplastic astrocytoma”, “malignant glioma”, “high-grade glioma”, “HHG”, “recurrence”, “recurrent malignant glioma”, “brain”, “CyberKnife”, “CK”, “stereotactic radiosurgery”, “SRS”, and “stereotactic radiotherapy” were used in “AND” and “OR” combinations. The search was limited to articles published between 2000 and 2021. The following inclusion criteria were used: 1) English language, 2) case series reporting greater than 5 patients 3) studies reporting exclusively histologically proven World Health Organization (WHO) grade IV gliomas or WHO grade III gliomas of the brain (18), 4) studies reporting recurrence, and 5) studies reporting retreatment with the CK System at recurrence. The exclusion criteria were: 1) case series reporting fewer than 10 patients and case reports, 2) brain lesions other than MGs, 3) lesions not located in the brain (e.g. gliomas of the spinal cord), 4) studies reporting only newly diagnosed MGs, 5) studies reporting on irradiation techniques other than the CK System, 6) studies not reporting survival data. Two authors determined the inclusion and exclusion criteria for the studies in the literature search. In studies with overlapping patient populations written by the same author/institution, we only included the largest or most complete dataset. In cases where outcomes were separated by WHO grade or CMT at recurrence, we abstracted outcomes separately to perform our subgroup analyses. Missing baseline data and outcomes information was requested from the authors via email correspondence. The authors of six included studies replied and the information provided was integrated into the data abstraction process.
The majority of malignant brain tumors are represented by gliomas (70%) (1). The standard management of newly diagnosed malignant gliomas (MGs) is maximal resection followed by radiotherapy (RT) with concomitant and adjuvant chemotherapy (CMT) (2). Although a solid treatment strategy has been established for MGs, recurrence still occurs in almost all patients within 2 years after initial treatment (3–5). Possible treatment strategies for recurrent malignant gliomas (rMGs) include second-line CMTs, surgery with or without adjuvant therapies, and RT (2, 6, 7). Reirradiation appears to be an efficacious and safe treatment modality, providing survival benefits with acceptable risk (8, 9). Among different reirradiation modalities, hypofractionated stereotactic radiotherapy (HFSRT) has shown promising results as it allows delivery of a large total dose, in a precise target volume and short treatment duration (10, 11). Nowadays, various HFSRT and stereotactic radiosurgery (SRS) machines are available and their usage has been gradually increasing. All systems have excellent accuracy with targeting areas close to 1 mm (12–14). Among those, the CyberKnife® (CK) is a frameless image-guided radiotherapy system mounting a 6-MV linear accelerator on a highly maneuverable robotic arm (15). The CK System is a non-invasive and pain-free treatment strategy that requires a customized thermoplastic face mask, reducing patient discomfort associated with other frame-based radiosurgical systems. Unlike other SRS techniques, the CK does not require general or local anesthesia still ensuring a comparable level of accuracy (12). Particularly, the CK was found to have clinically relevant accuracy of 0.7 +/- 0.3 mm, minimizing normal brain radiation exposure and allowing for high doses of radiation to targeted areas (12, 16). Given its recent development, few case series have been reported on CK for rMGs of the brain, and indications are still debated. We hereby conducted a systematic review of the literature and a meta-analysis to provide physicians awareness about the efficacy and safety of CK treatment for rMGs.
Data Extraction For each study, we abstracted the following baseline information: number of patients; median age at CK treatment; gender; median Karnofsky Performance Status (KPS) at CK treatment; WHO grade and histotype at recurrence. Regarding treatment at initial diagnosis we collected information about: the extent of resection (EOR), i.e. gross total resection (GTR, resection of more than 99% of the preoperative tumor volume), subtotal resection (STR, 95%–99% resection); partial resection (PR, < 95% resection), and biopsy (B) (19); the number of patients who underwent
MATERIALS AND METHODS Literature Search The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis
Frontiers in Oncology | www.frontiersin.org
126
2
March 2021 | Volume 11 | Article 652646
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
conventional radiation therapy (CRT); and the number of patients who underwent CMT. About the recurrence interval, we abstracted the Time To Recurrence (TTR, the time span between initial treatment and CK) (20, 21). As for treatment at recurrence, we gathered the following data: median planned target volume (PTV); the median number of fractions; total radiation dose in Gray (Gy); the number of patients who underwent CMT.
selected items from the scale, focusing on the following questions: 1) Did the study include all patients or consecutive patients versus a selected sample? 2) Was the study retrospective or prospective? 3) Was clinical follow-up satisfactory, thus allowing ascertainment of all outcomes? 4) Were outcomes clearly reported? 5) Were there clearly defined inclusion and exclusion criteria?
Objectives
We estimated each cohort’s cumulative prevalence and 95% confidence interval for each outcome. Event rates were pooled across studies using a random-effects meta-analysis. Heterogeneity across studies was evaluated using the I2 statistic. An I2 value of >50% suggests substantial heterogeneity. Metaregression was not used in this study. For some outcomes it was not possible to estimate the standard errors, therefore a standard error of 0 was used in the meta-analysis. Pearson’s correlation was used to correlate median TTR and median OS from CK treatment. Statistical analyses were performed using OpenMeta [Analyst] (http://www.cebm.brown.edu/openmeta/) and R statistical package v3.4.1 (http://www.r-project.org).
Statistical Analysis
Our primary endpoints were median Overall Survival (OS), median Time To Progression (TTP), and median ProgressionFree Survival (PFS). As for OS, we extracted data from initial diagnosis (i.e. time-length from the date of initial diagnosis to death from any cause) and from CK (i.e. time-length from the date of the start of CK treatment to death from any cause) (22). Concerning TTP and PFS, we abstracted data from CK. The former was defined as the time elapsed between the start of CK treatment to Beside Recurrence (BR, new lesion developed after 4 weeks beside or inside the prescribed marginal isodose line of previous CK treatment) or Progressive Disease (PD, more than 25% growth of Gd−enhanced area within 4 weeks after CK treatment) (21). The latter was defined as the time elapsed between the start of CK treatment to any disease recurrence or death from any cause (23). For our subgroup analysis, we were able to abstract median OS from initial diagnosis and from CK treatment for WHO grade IV gliomas versus WHO grade III gliomas separately and for CK plus CMT versus CK treatment alone separately. Besides, we analyzed the relationship between median TTR and median OS from CK treatment. The secondary endpoints were Local Response (LR), New Lesion (NL), and complications. The LR was assessed with Gdenhanced Magnetic Resonance Imaging (MRI) at 1 month after CK treatment and was classified into the following categories: Complete Response (CR, Gd−enhanced area disappears and no regrowth is recognized for at least 4 weeks after treatment), Partial Response (PR, Gd−enhanced area is reduced by more than 50% and maintains this state for at least 4 weeks after treatment), No Change (NC, less than 50% reduction or less than 25% growth of Gd−enhanced area, maintained for at least 4 weeks after treatment) and PD (24). The development of NLs following initially controlled disease (i.e. CR, PR, NC), was divided into BR and Remote Recurrence (RR, lesion located remotely from the prescribed marginal isodose line of previous CK treatment) (25). Regarding complications, we extracted the number of acute neurological and non-neurological side effects, corticosteroid dependency (the onset of neurological deficits and/or cephalalgia requiring daily doses of dexamethasone > 4 mg for more than 8 weeks), radiation necrosis, and other toxicities (26).
RESULTS Literature Review A total of 1420 papers were identified after duplicates removal. After title and abstract analysis, 67 articles were identified for full-text analysis. Eligibility was ascertained for 12 articles (20, 21, 24–26, 28–34). The remaining 55 articles were excluded for the following reasons: 1) irradiation techniques other than the CK System (19 articles), 2) improper study design (12 articles), 3) studies reporting only on newly diagnosed MGs or not reporting survival data (9 articles) 4) studies reporting on brain lesions other than MGs (7 articles), 5) case series reporting fewer than 10 patients (5 articles), and 6) studies in other languages (3 articles). All studies included in the analysis had at least one or more outcome measures available for one or more of the patients’ groups analyzed. Figure 1 shows the flow chart according to the PRISMA statement (17).
Study and Patients Characteristics Our meta-analysis included a total of 398 patients. The smallest study included 13 patients (32) and the largest included 128 patients (33). The median age at CK treatment was 54.5 years. There was a male predominance (1.6:1). The median KPS at CK treatment was 80. Six studies (50%) reported on WHO grade IV and III gliomas and other 6 studies (50%) reported on WHO grade IV gliomas. The histotype was available in 9 studies (75%): six studies (67%) reported on glioblastomas (GBMs) and 3 studies (33%) reported on GBMs and anaplastic astrocytomas (AAs). At the time of initial diagnosis, most of the patients underwent STR (131, 33%), followed by GTR in 112 patients (28%), B in 30 patients (8%), and PR in 12 patients (3%). Post-operative CRT was undertaken in 343 patients (86%) and the median dose was 60 Gy.
Study Risk of Bias Assessment We modified the Newcastle-Ottawa Quality Assessment Scale to assess the methodologic quality of the studies included in this meta-analysis (27). This tool is designed for use in comparative studies; however, our analyzed studies did not have control groups, therefore, we assessed the study risk of bias based on
Frontiers in Oncology | www.frontiersin.org
3
March 2021 | Volume 11 | Article 652646
127
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
FIGURE 1 | PRISMA flow-diagram depicting the literature search process.
Primary Outcomes
Post-operative CMT was undertaken in 275 patients (69%) and Temozolomide (TMZ) was the CMT regimen reported in most studies (193 patients, 48%). Patients were followed-up with a Gdenhanced MRI performed every 1 to 3 months. The median TTR was 14 months (range 1-171). At recurrence, the GTV was defined as the MRI Gd-enhanced area and the PTV was reconstructed adding 0 to 3 mm margin to the GTV. The median target volume (PTV) was 12.1 ml. The median number of fractions was 3 (range 1-6) and the median dose was 24.5 Gy (range 13.9-48.8). The prescribed marginal isodose ranged from 78% to 91%. Half of the patients (203, 51%) underwent CMT at recurrence. Although TMZ was the most reported CMT regimen (66 patients, 17%), other therapies were undertaken, particularly Bevacizumab (BEV) in 22 patients (5%) and Interferon in 16 patients (4%). Administration of CMT was concomitant and/or after CK treatment in 199 patients (98%) and before CK treatment in 4 patients (2%). The latter received BEV-based salvage therapy prior to CK treatment. A summary of the included studies is provided in Table 1.
Frontiers in Oncology | www.frontiersin.org
128
Median OS from initial diagnosis and CK treatment was 22.57 months (95%CI=17.56-27.58) and 8.56 months (95%CI=6.6510.47) respectively. Figure 2 shows the median OS forest plots. Median TTP and median PFS from CK treatment were 6.68 months (95%CI=2.13-11.22) and 7.05 months (95%CI=1.3012.79) respectively. Concerning the WHO grade, the median OS from initial diagnosis was 19.88 months (95%CI=17-22.76) for WHO grade IV gliomas, compared to 48.35 months (95%CI=15.72-80.98) for WHO grade III gliomas. Median OS from CK treatment was 8.4 months (95%CI=6.35-10.45) for WHO grade IV gliomas, compared to 11 months (95%CI=5.12-16.88) for WHO grade III gliomas. About the treatment, median OS from initial diagnosis was 25.4 months (95%CI=16.97-33.83) for patients who underwent CK plus CMT treatment, compared to 16.05 months (95% CI=13.99-18.11) for patients who underwent CK treatment alone; median OS from CK treatment was 9.52 months (95%
4
March 2021 | Volume 11 | Article 652646
No. of Patients
Median Age; range
Frontiers in Oncology | www.frontiersin.org
5
Lé vy, Cancer/Radiothé rapie, 2017 (32)
Pinzi, Neurol Sci, 2015 (33)
Torok, Technol Cancer Res Treat, 2011 (25)
Villavicencio, Radiosurgery, 2010 (34)
Yazici, J Neurooncol, 2014 (21)
7
8
9
10
11
12
6
Yoshikawa, Minim Invasive Neurosurg, 2006 (24)
Greenspoon, Onco Targets Ther, 2014 (20) Hasan, Front Oncol, 2015 (31)
5
3 4
2
Adachi, Anticancer Research, 2019 (28) Conti, Acta Neurochirurgica, 2012 (26) Ekici, J BUON, 2014 (29) Glavatskyi, Neuro-Oncology, 2014 (30)
1
P
R
R
R
R
R
R
P
R R
P
R
25
37
26
14
128
13
19
31
27 26
23
29
54; 28-78
37; 22-69
55.5; 36-82
58; 39-76
51; 18-79
55
56; 29-79
53; 36-75
NA NA
58
53
13:12
18:19
18:8
7:7
80:48
11:02
13:6
NA
NA NA
13:10
19:10
77.3; 30-90
60-100
90; 50-100
NA
60-100
85; 65-100
80; 40-100
80; 60-90
70-100 NA
80
75
Median KPS; range
M:F
Prospective/ Retrospective
No.
Author, Journal, Year
Baseline Data
Study
TABLE 1 | Summary of studies.
IV-III
IV
IV
IV
IV-III
IV-III
IV
IV
IV-III IV-III
IV
IV-III
WHO Grade
GBM-AA
GBM
GBM
GBM
GBM-AA
NA
GBM
GBM
NA GBM-AA
GBM
NA
Histotype
9 PR (36), 1 B (4)
13 STR (35), 1 B (3) 3 GTR (12), 12 STR (48),
9 STR (34), 2 B (8) 23 GTR (62),
3 PR (21) 15 GTR (58),
77 STR (60), 13 B (10) 11 GTR (79),
3 STR (23), 4 B (31) 38 GTR (30),
7 GTR (37), 8 STR (42), 4 NA (21) 6 GTR (46),
NA 8 GTR (30), 9 STR (35), 9 B (35) NA
NA
NA
GTR/STR/ PR/B (%)
14 (56)
37 (100)
26 (100)
14 (100)
128 (100)
13 (100)
31 (100) 19 (100)
29 (100) 23 (100) NA 9 (35)
CRT (%)
1st Treatment
14 (56)
37 (100)
25 (96)
14 (100)
84 (67)
13 (100)
19 (100)
0 (0)
29 (100) 23 (100) NA 17 (65)
CMT (%)
NA
15; 5-45
13; 5-89
8; 1-28
15; 6-171
11
16; 2-122
NA
NA NA
NA
NA
Median TTR (months); range
Recurrence Interval
19.1
NA
7
7
6.5
23.6
20.9
12.1
NA NA
15.7
4.1
Median PTV (ml)
3
5
2
3
2
NA
5
5
NA NA
2
1
CK Median No. of Fractions
20.3
30
NA
24
19
30
25
NA
NA NA
20
25.5
Median Dose (Gy)
2nd Treatment
16 (64)
11 (30)
5 (19)
12 (86)
61 (48)
11 (85)
31 (100) 15 (79)
29 (100) 12 (52) NA 0 (0)
CMT (%)
De Maria et al. CyberKnife for Recurrent Malignant Gliomas
March 2021 | Volume 11 | Article 652646
129
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
FIGURE 2 | Forest plots showing median OS from initial diagnosis (below) and CK treatment (above).
18.8% (95%CI=10.0-27.6), while the overall rate of radiation necrosis was 4.3% (95%CI=2.1-6.6). Rates of other toxicities were reported in 267 patients. The overall rate was 1.1% (95%CI=0.42.7) and these were hematological toxicities. Rates of LR after CK treatment at recurrence were reported in 84 patients. The overall rate of PD was 37.9% (95%CI=26.549.3), followed by overall rates of 29.2% for NC (95%CI=15.742.7), 27.7% for PR (95%CI=18.2-37.2), and 2% for CR (95% CI=1.0-4.9). Rates of NLs developed following CK treatment were reported in 61 patients, and the overall rate was 88.4% (95% CI=80.5-96.4). Rates of BR or RR were reported in 50 patients. The overall rate of BR was 75.9% (95%CI=64.3-87.6), compared to 17.7% for RR (95%CI=1.5-33.8). The secondary outcomes are summarized in Table 3.
CI=7.78-11.25) for patients who underwent combined treatment, compared to 4.44 months (95%CI=0-9.46) for patients who underwent CK treatment alone. Primary outcomes are reported in Table 2. We did not observe a positive correlation between median TTR and median OS from CK.
Secondary Outcomes Rates of acute neurological and non-neurological side effects after CK treatment at recurrence were reported in 287 patients. The overall rate of the former was 3.6% (95%CI=1.5-5.7), while 13% for the latter (95%CI=0-26.1). Acute neurological effects included worsening of pre-existing symptoms, dizziness, nausea/ vomiting, and neurological deterioration. Acute nonneurological effects included alopecia, fatigue, asthenia, and clinical deterioration. Figure 3 shows the acute neurological side effects forest plot. Rates of corticosteroid dependency and radiation necrosis were reported in 271 patients and 306 patients respectively. The overall rate of corticosteroid dependency was
Frontiers in Oncology | www.frontiersin.org
130
Study Heterogeneity
I2 values were <50% indicating a lack of substantial heterogeneity for all the outcomes.
6
March 2021 | Volume 11 | Article 652646
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
TABLE 2 | Primary outcomes.
TABLE 3 | Secondary outcomes. Months (95%CI)
Median Overall Survival From initial diagnosis WHO grade IV WHO grade III CK alone CK plus CMT From CK treatment WHO grade IV WHO grade III CK alone CK plus CMT
Overall % (95%CI) Local Response Complete Response Partial Response No Change Progressive Disease Recurrence New Lesion Beside Recurrence Remote Recurrence Complications Acute Neurological Side Effects Acute non-Neurological Side Effects Corticosteroid Dependency Radiation Necrosis Other Toxicities
22.57 (17.56-27.58) 19.88 (17.00-22.76) 48.35 (15.72-80.98) 16.05 (13.99-18.11) 25.40 (16.97-33.83) 8.56 (6.65-10.47) 8.40 (6.35-10.45) 11.00 (5.12-16.88) 4.44 (0-9.46) 9.52 (7.78-11.25)
Median Time To Progression From CK treatment
6.68 (2.13-11.22)
Median Progression Free Survival From CK treatment
7.05 (1.30-12.79)
2.0 (1.0-4.9) 27.7 (18.2-37.2) 29.2 (15.7-42.7) 37.9 (26.5-49.3) 88.4 (80.5-96.4) 75.9 (64.3-87.6) 17.7 (1.5-33.8) 3.6 (1.5-5.7) 13.0 (0-26.1) 18.8 (10.0-27.6) 4.3 (2.1-6.6) 1.1 (0.4-2.7)
female ratio of 1.6:1 has been previously reported for MGs, with greater incidence in men (1, 37). The prevalence of MGs in males appeared to be related mainly to genetic dissimilarities and not only to the presence of sex hormones (38). Gender differences can be pivotal for developing tailored approaches to MGs and pursuing studies are taking into account sex differences for innovative treatment strategies (37).
DISCUSSION Findings The treatment strategy for patients harboring rMGs is still debated and no clear consensus has been achieved yet. Treatment modalities include surgery, CMT, RT, and combined treatments. Reirradiation with SRS can provide survival benefits with acceptable risks. Among diverse SRS machines currently available, we focused on the CK System. Our study’s primary aim was to establish the efficacy of CK treatment for rMGs, concerning survival and time to disease progression. Our secondary aims were to establish the local disease response, recurrence of disease, and toxicities. We performed a systematic review and meta-analysis of published studies on CK for rMGs and found several interesting findings.
Primary Outcomes Median OS of rMGs without any treatment has been reported to range between 3 and 6 months (5). Reoperation of recurrent GBMs provides 3 to 5 months median survival, without a significant increase in morbidity and mortality, and is still limited to 10-30% of patients due to the infiltrative nature of the disease and the involvement of eloquent areas (39–42). Over the past years, reirradiation has been increasingly proposed as an alternative treatment strategy with successful results (43, 44). Among different reirradiation modalities, HFSRT and SRS have been reported to provide a median OS ranging from 8.6 to 18 months with acceptable side effects (45). Our meta-analysis on CK System revealed a median OS of 8.6 months (95%CI=6.65-10.47) from SRS treatment and 22.6 months (95%CI=17.56-27.58)
Patients Characteristics In our meta-analysis, we observed a male predominance (1.6:1). Recent evidence suggested that sex-associated biological features can play a role in MGs incidence, regardless of the age, race, and geographic location of patients (1, 35, 36). An average male-to-
FIGURE 3 | Forest plot showing rates of acute neurological side effects following CK treatment.
Frontiers in Oncology | www.frontiersin.org
7
March 2021 | Volume 11 | Article 652646
131
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
either before or after CK treatment (31). Palmer et al. reported a slightly higher survival for patients with recurrent GBMs treated with HFSRT before BEV rather than BEV before HFSRT (13.9 vs 13.3 months) but stressed the importance of a randomized multiinstitutional trial for more definite conclusions (61). We did not observe a positive correlation between median TTR and median OS from CK. Likewise, Greenspoon et al. did not find a statistical difference in OS or PFS when stratifying by TTR (<12 months or >12 months) (20). Conversely, Yazici et al. reported improved survival for patients with a TTR of more than 12 months (21).
considering survival from initial diagnosis. Median TTP and median PFS after CK treatment were comparable (6.7 vs 7.1 months), with a slightly longer median PFS as this outcome only differs for the inclusion of remote recurrence or death from any cause (23). Barbagallo et al. reported a similar mean PFS for patients with rMGs undergoing second surgery (7.7 months) (46). Randomized controlled trials (RCTs) are needed to provide more definitive answers about differences in particular treatment strategies for rMGs. Regarding the grade of the disease, WHO grade IV rMGs showed a shorter median survival from CK treatment (8.4 months), compared to WHO grade III rMGs (11 months). Notably, Murai et al. reported a 3-year survival rate of 38% for re-irradiated patients with recurrent WHO grade III anaplastic ependymomas (AEs) and a median OS from CK treatment of 31.5 months (47). Therefore, treatment of recurrent AEs with CK System is a promising alternative, especially for deep-seated lesions or lesions located adjacent to eloquent areas (47–50). The subgroup analysis of treatment strategy revealed a longer survival for patients undergoing CK plus CMT treatment (9.5 months) compare with patients undergoing CK treatment alone (4.4 months). Hu et al. previously reported that HFSRT combined with CMT confers a slight survival improvement for patients with rMGs compared with HFSRT alone (8.23-23.0 months vs 3.9-12.0 months) (51). In their meta-analysis including 388 patients, 3 out of the 7 selected studies presented statistically significant differences (P < 0.05) between these two treatment approaches, and 3 out of the 4 remaining studies showed a favorable survival for patients treated with combined therapy rather than HFSRT alone. Likewise, our meta-analysis suggests a longer survival for patients who undergo combined treatment, but we cannot ascertain the absence of confounding bias between the two groups and stratified RCTs would be needed for ultimate conclusions. Moreover, we were unable to perform qualitative subgroup analyses of the systemic agents used and the time of systemic therapy sessions with respect to CK treatment. Among the different agents used in the included studies, TMZ was the most reported CMT regimen (66 patients, 16%), followed by BEV in 22 patients (5%) and Interferon in 16 patients (4%). Administration of CMT was concomitant and/or after CK treatment in 199 patients (98%) and before CK treatment in 4 patients (2%). The latter received BEV-based salvage therapy prior to CK treatment (31). The most commonly used systemic therapies for rMGs include TMZ, nitrosoureas, and BEV (52–54). The combination of lomustine with BEV has shown improved PFS but not OS, and a higher toxicity rate compared with lomustine alone (55). Bevacizumab alone or in combination with chemotherapy agents such as lomustine or irinotecan has demonstrated a median survival time from recurrence around 9 months and radiographic response rates of approximately 30 to 40 percent (55, 56). Few reports described the combination of bevacizumab with HFSRT for recurrent GBMs with safe and effective results (57–59). This treatment strategy is under study in an ongoing larger randomized trial (60). Among the studies included in our meta-analysis, Hasan et al. showed a better survival for patients with recurrent GBMs treated with BEV
Frontiers in Oncology | www.frontiersin.org
132
Secondary Outcomes Our meta-analysis shows that CK is a relatively safe and effective treatment modality for rMGs. Rates of complications were relatively low. Corticosteroid dependency had the highest rate among the complications (18.8%), followed by acute nonneurological side effects (13%, including fatigue, alopecia, and clinical deterioration), and by radiation necrosis (4.3%). Notably, the authors of the included studies included steroid use among side effects only when requiring daily doses of dexamethasone > 4 mg for more than 8 weeks. However, we must acknowledge that current guidelines mention steroid use as a side effect from basic prescription (62). Larger re-irradiated tumors (maximum diameter greater than 4 cm) are more inclined to develop radiation necrosis (33, 63). Indeed, a crucial factor in developing radiation necrosis is the volume of the irradiated normal brain, which is relative to the tumor volume (64, 65). Radiation necrosis is known to occur in the normal brain when the normalized total dose (NTD) is greater than 100 Gy (66). Other authors reported that using a fractionated scheme aimed to maintain a normalized total dose (NTD)<100 Gy can reduce the risk of radionecrosis in larger tumors (26, 33). Conversely, rates of acute neurological effects (3.6%) such as worsening of pre-existing symptoms, dizziness, nausea/vomiting, neurological deterioration, and rates of hematological toxicities (1.1%) were the lowest. Acute side effects were higher in patients treated with large single fraction volumes, supporting the hypothesis that fractioned schemes may be safer for tumors larger than 4 cm in maximum diameter or proximal to eloquent areas (33). Hematological toxicities such as leukopenia and thrombocytopenia were mainly reported for patients who underwent CK treatment plus CMT (26). Although we meta-analyzed the side effects reported by the authors, it was not possible to grade toxicity because of a lack of uniformity among studies. Future trials should report the side effects according to standardized grading systems to enhance uniformity and facilitate interpretation of results (62, 67). The analysis of LR at 4 weeks after CK treatment showed disease progression in 37.9% of cases, stability in 29.2%, reduction in 27.7%, and complete disappearance in 2%. Yoshikawa et al. reported a higher control rate (i.e. CR, PR, NC) for GBM patients than AA patients (63.6% vs 45.5%) (24). However, LR after CK was reported in a small overall cohort (84 patients), and this outcome should be validated by more extensive analyses. Moreover, true progression may often be indistinguishable from pseudoprogression (21). Pseudoprogression is a subacute effect of
8
March 2021 | Volume 11 | Article 652646
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
each study provided the confidence intervals and/or standard deviations did not allow the use of the standard errors for some of the outcomes in the meta-analysis. In such cases, a standard error of 0 was adopted for each study. This led to an imperfect approximation of the meta-analyzed outcome and its confidence interval. While we were able to perform subgroup analyses based on WHO grade and CMT at recurrence as well as analyzing the relationship between TTR and OS, we were unable to perform more granular analyses stratifying outcomes by other relevant variables such as the histotype, the PTV, irradiation dose, the number of fractions, the patients’ age and KPS. Moreover, we were unable to perform qualitative subgroup analyses of the CMT agents used and the time of CMT sessions with respect to CK treatment at recurrence. The assessment of LR was reported in a small cohort and differential diagnosis of lesions developed post-CK treatment can be misleading. Therefore, this outcome should be validated by more extensive analyses and future studies should focus on discrimination of lesions developed following CK treatment. Nonetheless, to the best of our knowledge, this is the first meta-analysis providing helpful conclusions on the treatment of rMGs with the CK System and a potential start point for future studies.
radiotherapy observed in the first 12 weeks after treatment, first described by Hoffman et al (68). It was pathologically defined by Chamberlain et al. as necrosis without evidence of tumor and appears as increased contrast enhancement following radiotherapy (69). These imaging findings are consequences of disruption of the blood-brain barrier and represent proof of radiation’s efficacy rather than progression or toxicity, indeed correlate with longer OS (21, 70, 71). Diagnosis of pseudoprogression is made during follow-up when stabilization or improvement of clinical and radiographic findings is observed (21). Instead, true progression within the first 12 weeks after radiotherapy, can only be defined if the majority of new enhancement is outside the radiation field or if there is pathological confirmation of PD (72). The overall rate of NLs was considerably high (88.4%), with a greater rate of BR rather than RR (75.9% vs 17.7%). However, the rate of NLs was reported in only 61 patients, and the location of recurrence in only 50 patients overall. Therefore, this outcome needs to be corroborated by larger studies as well. Although it is known that recurrence of MGs appears mainly within 2 cm of the enhancing edge of the original tumor, Yoshikawa et al. reported the development of BR despite an initial high control rate (63.2% for GBM and 42.9% for AA controlled patients) (73). Despite surgery plays a key role in GBM recurrence, most of all for large volumes, CK radiosurgery has shown good results with a low rate of toxicity. Some aspects though remain unclear, such as radiation dose and fractionation. A focus on the quality of life (QoL) is imperative given the poor prognosis and short life expectancy of patients with a diagnosis of rMGs. The QoL of MG patients is most often affected by the development of CMT/RT side effects, changes in physical functioning, and global health status (74). Unlike surgery and other SRS techniques, the CK treatment can be delivered without sedation and as an outpatient, which would help maximize the QoL. The primary and secondary end-points of our meta-analysis were based on outcomes reported by authors of the included studies. Therefore, we were unable to meta-analyze the effect of CK treatment on KPS, cognitive function, and QoL. However, Greenspoon et al. reported on the benefit of BEV in preventing toxicity and improving QoL of patients undergoing CK plus TMZ (20). Quality of Life after HFSRT for rMGs patients has been previously reported to remain stable for a median follow-up of 9 months (75). A subsequent study on high-dose reirradiation in selected patients with recurrent/progressive MGs found a stable QoL and improvement of activities of daily living (ADL) over a 1-year time period (76). Future studies should include KPS and QoL among their primary outcomes to evaluate the impact of CK treatment in life-limiting diseases such as rMGs.
CONCLUSIONS Reirradiation of rMGs with the CK System has reasonable efficacy and provides encouraging survival rates. There is a better survival trend for WHO grade III lesions and for patients who undergo combined treatment with CK plus CMT. Treatment of rMGs with CK is a safe alternative, considering the low rates of complications. Larger and well-designed prospective studies are warranted to provide more accurate results.
DATA AVAILABILITY STATEMENT The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
AUTHOR CONTRIBUTIONS LDM: Writing - Original Draft, Term, Methodology, Validation, Resources, Project administration, Formal analysis, Investigation, Data Curation. LTB: Investigation, Resources, Data Curation, Formal analysis. AC: Resources, Writing - Review & Editing. KH: Resources, Writing - Review and Editing. VP: Resources, Writing - Review and Editing. TM: Resources, Writing - Review and Editing. RL: Resources, Writing - Review and Editing. SB: Resources, Writing - Review and Editing. MBM: Writing - Review and Editing. SMM: Writing - Review and Editing. MMF: Conceptualization, Validation, Writing - Review and Editing, Supervision, Project administration. All authors contributed to the article and approved the submitted version
Limitations Despite the significant number of patients included in our study, this meta-analysis was based primarily on a few single-center case series and thus has limitations inherent to single-center retrospective studies. Based on the data abstracted from the articles and provided by the authors of the included studies, we could not ascertain the number of patients undergoing repeat surgery and the EOR at recurrence. The different ways in which
Frontiers in Oncology | www.frontiersin.org
9
March 2021 | Volume 11 | Article 652646
133
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
REFERENCES
21. Yazici G, Cengiz M, Ozyigit G, Eren G, Yildiz F, Akyol F, et al. Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J Neurooncol (2014) 120:117–23. doi: 10.1007/s11060-014-1524-0 22. Fetcko K, Lukas RV, Watson GA, Zhang L, Dey M. Survival and complications of stereotactic radiosurgery: A systematic review of stereotactic radiosurgery for newly diagnosed and recurrent high-grade gliomas. Medicine (2017) 96:e8293. doi: 10.1097/MD.0000000000008293 23. Lamborn KR, Yung WKA, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, et al. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol (2008) 10:162–70. doi: 10.1215/15228517-2007-062 24. Yoshikawa K, Saito K, Kajiwara K, Nomura S, Ishihara H, Suzuki M. CyberKnife Stereotactic Radiotherapy for Patients with Malignant Glioma. Minim Invasive Neurosurg (2006) 49:110–5. doi: 10.1055/s-2006-932183 25. Torok JA, Wegner RE, Mintz AH, Heron DE, Burton SA. Re-irradiation with Radiosurgery for Recurrent Glioblastoma Multiforme. Technol Cancer Res Treat (2011) 10:253–8. doi: 10.7785/tcrt.2012.500200 26. Conti A, Pontoriero A, Arpa D, Siragusa C, Tomasello C, Romanelli P, et al. Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir (2012) 154:203–9. doi: 10.1007/s00701-011-1184-1 27. Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/ programs/clinical_epidemiology/oxford.asp (Accessed December 8, 2020). 28. Adachi K, Hayashi K, Kagawa N, Kinoshita M, Sumida I, Akino Y, et al. Feasibility of Salvage Re-irradiation With Stereotactic Radiotherapy for Recurrent Glioma Using CyberKnife. Anticancer Res (2019) 39:2935–40. doi: 10.21873/anticanres.13423 29. Ekici K, Ozseker N, Mayadagli A, Erdogan Kocak M, Olmezoglu A. Efficacy of stereotactic radiotherapy as salvage treatment for recurrent malignant gliomas. J BUON (2014) 19:1029–34. 30. Glavatskyi O, Buryk VM, Kardash KA, Pylypas OP, Chebotaryova TI. P13.11 * USAGE OF CYBER KNIFE HYPOFRACTIONATED RADIOSURGERY IN HIGH GRADE GLIOMAS COMPLEX TREATMENT. Neuro Oncol (2014) 16:ii68–8. doi: 10.1093/neuonc/nou174.257 31. Hasan S, Chen E, Lanciano R, Yang J, Hanlon A, Lamond J, et al. Salvage Fractionated Stereotactic Radiotherapy with or without Chemotherapy and Immunotherapy for Recurrent Glioblastoma Multiforme: A Single Institution Experience. Front Oncol (2015) 5:1–11. doi: 10.3389/fonc.2015.00106 32. Lé vy S, Chapet S, Scher N, Debbi K, Ruffier A, Bernadou G, et al. Reirradiation of gliomas under stereotactic conditions: Prognostic factors for survival without relapse or side effects, a retrospective study at Tours regional university hospital (France). Cancer Radiother (2017) 21:759–65. doi: 10.1016/j.canrad.2017.05.006 33. Pinzi V, Orsi C, Marchetti M, Milanesi IM, Bianchi LC, DiMeco F, et al. Radiosurgery reirradiation for high-grade glioma recurrence: a retrospective analysis. Neurol Sci (2015) 36:1431–40. doi: 10.1007/s10072-015-2172-7 34. Villavicencio AT, Burneikienė S, Romanelli P, McNeely L, Lipani JD, Fariselli L, et al. Survival following Stereotactic Radiosurgery for Newly Diagnosed and Recurrent Glioblastoma Multiforme: A Multicenter Experience. Radiosurgery (2010) 7:288–99. doi: 10.1159/000288740 35. Ostrom QT, Gittleman H, Stetson L, Virk SM, Barnholtz-Sloan JS. Epidemiology of gliomas. Cancer Treat Res (2015) 163:1–14. doi: 10.1007/ 978-3-319-12048-5_1 36. Gould J. Breaking down the epidemiology of brain cancer. Nature (2018) 561: S40–1. doi: 10.1038/d41586-018-06704-7 37. Matteoni S, Abbruzzese C, Villani V, Malorni W, Pace A, Matarrese P, et al. The influence of patient sex on clinical approaches to malignant glioma. Cancer Lett (2020) 468:41–7. doi: 10.1016/j.canlet.2019.10.012 38. Dunford A, Weinstock DM, Savova V, Schumacher SE, Cleary JP, Yoda A, et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat Genet (2017) 49:10–6. doi: 10.1038/ng.3726 39. Barbagallo GMV, Jenkinson MD, Brodbelt AR. ‘Recurrent’ glioblastoma multiforme, when should we reoperate? Br J Neurosurg (2008) 22:452–5. doi: 10.1080/02688690802182256 40. Montemurro N, Perrini P, Blanco MO, Vannozzi R. Second surgery for recurrent glioblastoma: A concise overview of the current literature. Clin Neurol Neurosurg (2016) 142:60–4. doi: 10.1016/j.clineuro.2016.01.010
1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol (2018) 20: iv1–iv86. doi: 10.1093/neuonc/noy131 2. Nayak L, Reardon DA. High-grade Gliomas. Continuum (Minneap Minn) (2017) 23:1548–63. doi: 10.1212/CON.0000000000000554 3. Kortmann R-D, Jeremic B, Weller M, Plasswilm L, Bamberg M. Radiochemotherapy of malignant glioma in adults. Clinical experiences. Strahlenther Onkol (2003) 179:219–32. doi: 10.1007/s00066-003-1027-y 4. Kirkpatrick JP, Sampson JH. Recurrent malignant gliomas. Semin Radiat Oncol (2014) 24:289–98. doi: 10.1016/j.semradonc.2014.06.006 5. Martı́nez-Carrillo M, Tovar-Martı́n I, Zurita-Herrera M, Del Moral-Á vila R, Guerrero-Tejada R, Saura-Rojas E, et al. Salvage radiosurgery for selected patients with recurrent malignant gliomas. BioMed Res Int (2014) 2014:657953. doi: 10.1155/2014/657953 6. Seystahl K, Wick W, Weller M. Therapeutic options in recurrent glioblastoma–An update. Crit Rev Oncol Hematol (2016) 99:389–408. doi: 10.1016/j.critrevonc.2016.01.018 7. Birk HS, Han SJ, Butowski NA. Treatment options for recurrent high-grade gliomas. CNS Oncol (2017) 6:61–70. doi: 10.2217/cns-2016-0013 8. Rades D, Witteler J, Leppert J, Schild SE. Re-Irradiation for Recurrent Glioblastoma Multiforme. Anticancer Res (2020) 40:7077–81. doi: 10.21873/ anticanres.14735 9. Kaul D, Pudlitz V, Böhmer D, Wust P, Budach V, Grün A. Reirradiation of High-Grade Gliomas: A Retrospective Analysis of 198 Patients Based on the Charité Data Set. Adv Radiat Oncol (2020) 5:959–64. doi: 10.1016/ j.adro.2020.06.005 10. Gzell C, Back M, Wheeler H, Bailey D, Foote M. Radiotherapy in Glioblastoma: the Past, the Present and the Future. Clin Oncol (R Coll Radiol) (2017) 29:15–25. doi: 10.1016/j.clon.2016.09.015 11. Vordermark D, Kölbl O, Ruprecht K, Vince GH, Bratengeier K, Flentje M. Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer (2005) 5:55. doi: 10.1186/1471-2407-5-55 12. Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP. An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery (2003) 52:140–6; discussion 146-7. doi: 10.1097/ 00006123-200301000-00018 13. Yu C, Main W, Taylor D, Kuduvalli G, Apuzzo MLJ, Adler JR. An anthropomorphic phantom study of the accuracy of Cyberknife spinal radiosurgery. Neurosurgery (2004) 55:1138–49. doi: 10.1227/01.neu. 0000141080.54647.11 14. Yan H, Yin F-F, Kim JH. A phantom study on the positioning accuracy of the Novalis Body system. Med Phys (2003) 30:3052–60. doi: 10.1118/1.1626122 15. Home. CyberKnife. Available at: https://cyberknife.com/ (Accessed October 11, 2020). 16. Pantelis E, Moutsatsos A, Antypas C, Zoros E, Pantelakos P, Lekas L, et al. On the total system error of a robotic radiosurgery system: phantom measurements, clinical evaluation and long-term analysis. Phys Med Biol (2018) 63:165015. doi: 10.1088/1361-6560/aad516 17. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Rev (2015) 4:1. doi: 10.1186/20464053-4-1 18. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. Available at: https://publications.iarc. fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHOClassification-Of-Tumours-Of-The-Central-Nervous-System-2016 (Accessed July 19, 2020). 19. Southwell DG, Birk HS, Han SJ, Li J, Sall JW, Berger MS. Resection of gliomas deemed inoperable by neurosurgeons based on preoperative imaging studies. J Neurosurg (2017) 129:567–75. doi: 10.3171/2017.5.JNS17166 20. Greenspoon JN, Sharieff W, Hirte H, Overholt A, Devillers R, Gunnarsson T, et al. Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: a prospective cohort study. Onco Targets Ther (2014) 7:485–90. doi: 10.2147/OTT.S60358
Frontiers in Oncology | www.frontiersin.org
134
10
March 2021 | Volume 11 | Article 652646
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
58. Cabrera AR, Cuneo KC, Desjardins A, Sampson JH, McSherry F, Herndon JE, et al. Concurrent Stereotactic Radiosurgery and Bevacizumab in Recurrent Malignant Gliomas: A Prospective Trial. Int J Radiat Oncol Biol Phys (2013) 86:873–9. doi: 10.1016/j.ijrobp.2013.04.029 59. Shapiro LQ, Beal K, Goenka A, Karimi S, Iwamoto FM, Yamada Y, et al. Patterns of Failure After Concurrent Bevacizumab and Hypofractionated Stereotactic Radiation Therapy for Recurrent High-Grade Glioma. Int J Radiat Oncol Biol Phys (2013) 85:636–42. doi: 10.1016/j.ijrobp.2012.05.031 60. Radiation Therapy Oncology Group. Randomized Phase II Trial of Concurrent Bevacizumab and Re-Irradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma(2020). Available at: https:// clinicaltrials.gov/ct2/show/NCT01730950 (Accessed February 24, 2021). clinicaltrials.gov. 61. Palmer JD, Bhamidipati D, Song A, Eldredge-Hindy HB, Siglin J, Dan TD, et al. Bevacizumab and re-irradiation for recurrent high grade gliomas: does sequence matter? J Neurooncol (2018) 140:623–8. doi: 10.1007/s11060-0182989-z 62. RTOG Foundation. Available at: https://www.rtog.org/ (Accessed March 2, 2021). 63. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys (2000) 47:291–8. doi: 10.1016/s0360-3016(99) 00507-6 64. Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol (2005) 23:8863–9. doi: 10.1200/JCO.2005.03.4157 65. Hall WA, Djalilian HR, Sperduto PW, Cho KH, Gerbi BJ, Gibbons JP, et al. Stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol (1995) 13:1642–8. doi: 10.1200/JCO.1995.13.7.1642 66. Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys (2008) 70:1350–60. doi: 10.1016/j.ijrobp.2007.08.015 67. Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. Available at: https://ctep.cancer.gov/protocol development/electronic_applications/ctc.htm (Accessed March 2, 2021). 68. Hoffman WF, Levin VA, Wilson CB. Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg (1979) 50:624–8. doi: 10.3171/ jns.1979.50.5.0624 69. Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol (2007) 82:81–3. doi: 10.1007/s11060-006-9241-y 70. de Wit MCY, de Bruin HG, Eijkenboom W, Sillevis Smitt P a E, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology (2004) 63:535–7. doi: 10.1212/ 01.wnl.0000133398.11870.9a 71. Brandes AA, Bartolotti M, Franceschi E. Second surgery for recurrent glioblastoma: advantages and pitfalls. Expert Rev Anticancer Ther (2013) 13:583–7. doi: 10.1586/era.13.32 72. Ellingson BM, Wen PY, Cloughesy TF. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics (2017) 14:307–20. doi: 10.1007/s13311-016-0507-6 73. Brady LW, Perez CA, Wazer DE. Perez & Brady"s Principles and Practice of Radiation Oncology. Alphen aan den Rijn Netherlands: Lippincott Williams & Wilkins (2013). 74. Weitzner MA, Meyers CA. Cognitive functioning and quality of life in malignant glioma patients: a review of the literature. Psychooncology (1997) 6:169–77. doi: 10.1002/(SICI)1099-1611(199709)6:3<169::AIDPON269>3.0.CO;275. Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Survival and quality of life after hypofractionated stereotactic radiotherapy for recurrent malignant glioma. J Neurooncol (2007) 81:287–94. doi: 10.1007/ s11060-006-9231-0 76. Maitre P, Gupta T, Maitre M, Goda J, Krishnatry R, Chatterjee A, et al. Prospective Longitudinal Assessment of Quality of Life and Activities of Daily Living as Patient-Reported Outcome Measures in Recurrent/Progressive Glioma Treated with High-dose Salvage Re-irradiation. Clin Oncol (Royal Coll Radiol) (2021) 33:1–4. doi: 10.1016/j.clon.2020.08.011
41. Park JK, Hodges T, Arko L, Shen M, Dello Iacono D, McNabb A, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol (2010) 28:3838–43. doi: 10.1200/JCO.2010.30.0582 42. Park C-K, Kim JH, Nam D-H, Kim C-Y, Chung S-B, Kim Y-H, et al. A practical scoring system to determine whether to proceed with surgical resection in recurrent glioblastoma. Neuro Oncol (2013) 15:1096–101. doi: 10.1093/neuonc/not069 43. Nieder C, Andratschke NH, Grosu AL. Re-irradiation for Recurrent Primary Brain Tumors. Anticancer Res (2016) 36:4985–95. doi: 10.21873/anticanres. 11067 44. Clarke J, Neil E, Terziev R, Gutin P, Barani I, Kaley T, et al. Multicenter, Phase 1, Dose Escalation Study of Hypofractionated Stereotactic Radiation Therapy With Bevacizumab for Recurrent Glioblastoma and Anaplastic Astrocytoma. Int J Radiat Oncol Biol Phys (2017) 99:797–804. doi: 10.1016/ j.ijrobp.2017.06.2466 45. Hu Y, Chen D, Zhang L, Chen J. Efficacy and Safety of Hypofractionated Stereotactic Radiotherapy for Recurrent Malignant Gliomas: A Systematic Review and Meta-analysis. World Neurosurg (2019) 127:176–85. doi: 10.1016/ j.wneu.2019.03.297 46. Vincenzo Barbagallo GM, Certo F, Di Gregorio S, Maione M, Garozzo M, Peschillo S, et al. Recurrent high-grade glioma surgery: a multimodal intraoperative protocol to safely increase extent of tumor resection and analysis of its impact on patient outcome. Neurosurg Focus (2021) 50:E20. doi: 10.3171/2020.10.FOCUS20744 47. Murai T, Sato K, Iwabuchi M, Manabe Y, Ogino H, Iwata H, et al. Reirradiation of recurrent anaplastic ependymoma using radiosurgery or fractionated stereotactic radiotherapy. Jpn J Radiol (2016) 34:211–8. doi: 10.1007/s11604-015-0511-5 48. Iqbal MS, Lewis J. An overview of the management of adult ependymomas with emphasis on relapsed disease. Clin Oncol (R Coll Radiol) (2013) 25:726– 33. doi: 10.1016/j.clon.2013.07.009 49. Rodrı́guez D, Cheung MC, Housri N, Quinones-Hinojosa A, Camphausen K, Koniaris LG. Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973-2005). J Surg Res (2009) 156:340–51. doi: 10.1016/ j.jss.2009.04.024 50. Bouffet E, Hawkins CE, Ballourah W, Taylor MD, Bartels UK, Schoenhoff N, et al. Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys (2012) 83:1541–8. doi: 10.1016/j.ijrobp.2011.10.039 51. Hu Y, Zhang L, Ding C, Chen D, Chen J. Hypofractionated stereotactic radiotherapy combined with chemotherapy or not in the management of recurrent malignant gliomas: A systematic review and meta-analysis. Clin Neurol Neurosurg (2019) 183:105401. doi: 10.1016/j.clineuro.2019. 105401 52. Chamberlain MC. Salvage therapy with lomustine for temozolomide refractory recurrent anaplastic astrocytoma: a retrospective study. J Neurooncol (2015) 122:329–38. doi: 10.1007/s11060-014-1714-9 53. Taal W, Oosterkamp HM, Walenkamp AME, Dubbink HJ, Beerepoot LV, Hanse MCJ, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol (2014) 15:943–53. doi: 10.1016/S1470-2045(14)70314-6 54. Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA (2015) 314:2535–43. doi: 10.1001/jama.2015.16669 55. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med (2017) 377:1954–63. doi: 10.1056/NEJMoa1707358 56. Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol (2009) 27:740–5. doi: 10.1200/JCO.2008.16.3055 57. Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys (2009) 75:156–63. doi: 10.1016/j.ijrobp.2008.10.043
Frontiers in Oncology | www.frontiersin.org
11
March 2021 | Volume 11 | Article 652646
135
De Maria et al.
CyberKnife for Recurrent Malignant Gliomas
access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Copyright © 2021 De Maria, Terzi di Bergamo, Conti, Hayashi, Pinzi, Murai, Lanciano, Burneikiene, Buglione di Monale, Magrini and Fontanella. This is an open-
Frontiers in Oncology | www.frontiersin.org
136
12
March 2021 | Volume 11 | Article 652646
Open Access Case Report
DOI: 10.7759/cureus.14499
Stereotactic Radiotherapy for Localized External Auditory Canal Carcinomas: Report of Four Cases Yoshimasa Mori 1, 2, 3, 4 , Shinichiro Mizumatsu 5 1. Radiology and Radiation Oncology, Aichi Medical University, Nagakute, JPN 2. Neurological Surgery, Ookuma Hospital, Nagoya, JPN 3. Neurological Surgery, Aoyama General Hospital, Toyokawa, JPN 4. Radiation Oncology and Neurological Surgery, Shin-Yurigaoka General Hospital, Kawasaki, JPN 5. Cyberknife Center, Aoyama General Hospital, Toyokawa, JPN Corresponding author: Yoshimasa Mori, yoshimmori@yahoo.co.jp
Abstract External auditory canal carcinoma (EACC) is sometimes diagnosed at an early stage because it arises superficially in the ear canal and may cause ear obstruction symptoms early. In addition, in the early stage of EACCs, involvement of lymph nodes or distant metastases are reported less frequently. And so, stereotactic radiotherapy (SRT) concentrating high-dose radiation on the primary tumor may be an effective option. The aim of this study is to evaluate the preliminary results of upfront SRT for early-stage localized EACCs. Four cases (four females, 84 to 98 years old) with EACC of N0M0 (=no lymph node involvement and no distant metastasis) were treated. All four tumors (0.30 - 11.1 ml in volume) were diagnosed as squamous cell carcinoma histologically. A total dose of 24 - 33 Gy in 3 - 5 fractions (D95 [dose delivered to 95% of the target volume]=100% dose) was delivered by SRT using CyberKnife. All four cases were alive at the end of the follow-up period of 19 to 106 months. In three cases (tumor volume, 0.3 - 3.5 ml) the treated tumors had regressed or disappeared by the end of the follow-up period of 106, 28, and 19 months respectively. In the remaining one case, the treated tumor (11.1 ml) regrew and cervical lymph node metastasis occurred, and both were treated with SRT again 6 months and 20 months after the initial SRT respectively. The tumors were still stable at 39 months after the initial SRT. In conclusion, in three cases the small tumors had regressed or disappeared without lymph node involvement or distant metastasis. In the remaining case, additional SRT was performed for recurrent tumors, after which the patient’s condition remained stable. SRT may be an effective option for early-stage EACCs.
Categories: Otolaryngology, Radiation Oncology, Oncology Keywords: head and neck malignancies, hearing impairment, stereotactic radiotherapy, radiosurgery, squamous cell carcinoma, external auditory canal carcinoma, temporal bone, early stage
Introduction External auditory canal carcinoma (EACC) is sometimes diagnosed at an early stage because it arises superficially in the ear canal and may cause ear obstruction symptoms early. In addition, in the early stage of EACCs, involvement of lymph nodes or distant metastases are reported less frequently [1,2]. Therefore, stereotactic radiotherapy (SRT) concentrating high-dose radiation on the primary tumor may be an effective option. The aim of this study is to evaluate the preliminary results of CyberKnife® (CK) (Accuray, Inc., Sunnyvale, USA) SRT for early-stage localized EACCs instead of surgical extirpation.
Received 03/05/2021 Review began 03/08/2021 Review ended 04/13/2021 Published 04/15/2021 © Copyright 2021 Mori et al. This is an open access article distributed under the terms of the Creative Commons Attribution License CC-BY 4.0., which permits unrestricted
This study was approved by the Ethical Committee Board of Shin-Yurigaoka General Hospital (20190520-2) and Aoyama General Hospital (19-02). The need for patient consent was waived.
use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Presentation Four cases (four females, 84 to 98 years old) with EACC of N0M0 (=no lymph node involvement and no distant metastasis) were treated (Table 1).
How to cite this article Mori Y, Mizumatsu S (April 15, 2021) Stereotactic Radiotherapy for Localized External Auditory Canal Carcinomas: Report of Four Cases. Cureus 13(4): e14499. DOI 10.7759/cureus.14499
137
Case
Age / Sex
Side
Tumor vol. (ml)
Prescription dose
Repeat SRT
FU (mos.)
Results
1
84/F
left
0.3 (T1)
24 Gy/ 3 fx.
(-)
106
CR
2
85/F
left
2.3 (T1)
33 Gy/ 3 fx.
(-)
28
MR
3
98/F
right
3.5 (T1)
30 Gy/ 5 fx.
(-)
19
CR
4
85/F
left
11.1 (T2)
33 Gy/ 3 fx.
primary (6 mos), LN (20 mos)
39
PG
TABLE 1: Four cases of early-stage external auditory canal carcinomas treated by CyberKnife hypofractionated stereotactic radiotherapy FU=follow-up, mos=months, Gy=Gray, fx.=fraction, LN=lymph node involvement, T1=tumor diameter <2 cm, T2=tumor diameter >2 cm and <4 cm, CR=complete response, disappearance of the tumor, MR=minor response, tumor volume reduction by <25%, PG=tumor progression
All four tumors (0.30 - 11.1 ml) were diagnosed as squamous cell carcinoma (SCC) histologically. A total dose of 24 - 33 Gy in 3 - 5 fractions (D95 [dose delivered to 95% of the target volume]=100% dose) was delivered by CK-SRT. All four cases were alive at the end of the follow-up period of 19 to 106 months (Table 1). In three cases (tumor volume, 0.3 - 3.5 ml) the treated tumors had regressed or disappeared by the end of the respective follow-up period of 106, 28, and 19 months. In the remaining one case, the treated tumor (11.1 ml) regrew and cervical lymph node metastasis occurred, and both were treated with CK-SRT again 6 months and 20 months after the initial CK-SRT respectively. The tumors had been stable until 39 months after the initial CK-SRT. All four cases had hearing disturbance before CK-SRT. In two (Case 1 and Case 2) of four, after CK-SRT, the obstruction of the external ear canal was released and hearing improved by half one year after CK-SRT.
Illustrative cases Case 1: 84-Year-Old Female Squamous cell carcinoma was identified by biopsy (Figure 1). A D95 (dose covering 95% of the tumor volume) prescription dose of 24 Gy in 3 fractions was delivered to a small tumor located at the external auditory canal by CK-SRT. After the treatment, complete remission was maintained until 9 years after the treatment.
2021 Mori et al. Cureus 13(4): e14499. DOI 10.7759/cureus.14499
138
2 of 5
FIGURE 1: Dose Planning for Case 1 Axial image of iodine enhancement computed tomography (CT) on CyberKnife MultiPlan (Accuray, Tokyo, Japan) workstation showed excellent conformity for a small external auditory canal tumor (arrow). A prescription dose of D95%=24 Gy/ 3 fractions was adopted. After treatment, complete remission was obtained and maintained for 9 years.
Case 3: 98-Year-Old Female Squamous cell carcinoma was identified by biopsy (Figure 2). A D95 (dose covering 95% of the tumor volume) prescription dose of 30 Gy in 5 fractions was delivered to a tumor located at the right external auditory canal by CK-SRT. After the treatment, complete remission was maintained until 19 months after the treatment. Otoscopy revealed a patent external auditory canal at 19 months after the treatment.
2021 Mori et al. Cureus 13(4): e14499. DOI 10.7759/cureus.14499
3 of 5
139
FIGURE 2: Dose Planning for Case 3 and Follow-up Axial (left) and coronal (right upper) images of iodine enhancement CT on MultiPlan workstation showed a small external auditory canal tumor (arrow). A prescription dose of D95%=30 Gy/ 5 fractions was adopted. Complete remission was obtained and maintained until 19 months after the treatment. Otoscopy (right lower) revealed patency of the external auditory canal 19 months after the treatment.
Discussion Of various head and neck cancers, it is reported that the early-stage nasal and paranasal carcinomas [3-5] and early-stage external auditory canal carcinomas (EACCa) [1,2] rarely develop regional LN metastasis or cervical lymph node metastases [6]. Such patients might be good candidates for stereotactic radiosurgery (SRS)/SRT as an upfront therapy instead of surgical extirpation as the initial therapy [6]. Recently, Shinomiya et al. [1] reported the good results of surgical treatment of early-stage EACC in 33 cases (T1, 14; T2, 19). All were SCC and were operated on with sleeve resection or lateral temporal bone resection. In four of 33 patients, the surgical margin was positive and postoperative radiotherapy was added. The fiveyear overall and disease-specific survivals were 95% and 100% respectively. They described that potential parotid LN metastasis rates of T1 and T2 were 0% (0/14) and 5% (1/19) respectively. Regional recurrence in a parotid LN in a single case was successfully salvaged by total parotidectomy. They concluded that prophylactic superficial parotidectomy or neck dissection is not mandatory. Nam, et al. [7] also reported the results of surgery for EACCs. Locoregional recurrence occurred in four of 18 cases of T1 and T2. Favorable results have been reported with SRT as the first-line therapy for auditory canal and middle ear cancers by Murai et al. [8]. These included T1 (n=3) and T2 (n=7). Doses of 37.5 Gy in 3 fractions or 40 Gy for 5 fractions were delivered as first-line therapy. The three-year overall survival rate and local control rate for T1/T2 disease were 69% and 70% respectively. Facial nerve function was preserved in all cases. In our present study, in all three cases of small tumors (T1) regression or disappearance without lymph node involvement or distant metastasis was achieved. In the other case (T2) additional SRT was performed for recurrent tumors, with the patient’s condition remaining stable. Adverse effects, defined as the deterioration of symptoms without tumor progression, were not observed. SRT may be an effective option for early-stage EACCs. Regarding the prescription dose, a more conservative regimen of 30 Gy in 5 fractions was adopted in Case 3, as we wanted to avoid toxicities considering her very old age (98 years old). In addition, a little reduced dose of 24 Gy in 3 fractions was adopted in Case 1 who was treated initially. In the other two cases (Case 2 and Case 4), we gave 33 Gy in 3 fractions, which was close to that reported by Murai et al [8]. The organs at risk were the brainstem, brain, facial nerve, ipsilateral cochlea (if the hearing was expected to preserve), and skin. We considered dose constraints (max point dose) provided by Timmerman [9], which were 20 to 24 Gy in 3 fractions. Except for the skin dose, they were easily achieved, as the tumors were not very large.
Conclusions In all three cases of small tumors (T1), regression or disappearance without lymph node involvement or distant metastasis was achieved. In the remaining case, additional SRT was performed for recurrent tumors and the patient’s condition remained stable. SRT may be an effective option for early-stage EACCs. A shorter
2021 Mori et al. Cureus 13(4): e14499. DOI 10.7759/cureus.14499
140
4 of 5
treatment period of SRT would be beneficial, especially for elderly patients. Indications and treatment planning, including optimal prescription dose, fraction schedule, and field decision, will have to be established in future studies.
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. The Ethical Committee Board of Shin-Yurigaoka General Hospital and that gf Aoyama General Hospital issued approval 20190520-2 and 19-02. This study was approved by the Ethical Committee Board of Shin-Yurigaoka General Hospital (20190520-2) and Aoyama General Hospital (19-02). The need for patient consent was waived. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References 1. 2.
3.
4.
5.
6. 7.
8. 9.
Shinomiya H, Uehara N, Teshima M, Kakigi A, Otsuki N, Nibu KI: Clinical management for T1 and T2 external auditory canal cancer. Auris Nasus Larynx. 2019, 46:785-9. 10.1016/j.anl.2019.02.004 Yoon M, Chougule P, Dufresne R, Wanebo HJ: Localized carcinoma of the external ear is an unrecognized aggressive disease with a high propensity for local regional recurrence. Am J Surg. 1992, 164:574-7. 10.1016/s0002-9610(05)80709-3 Cantù G, Bimbi G, Miceli R, et al.: Lymph node metastases in malignant tumors of the paranasal sinuses: prognostic value and treatment. Arch Otolaryngol Head Neck Surg. 2008, 134:170-7. 10.1001/archoto.2007.30 Pezner RD, Moss WT, Tong D, Blasko JC, Griffin TW: Cervical lymph node metastases in patients with squamous cell carcinoma of the maxillary antrum: the role of elective irradiation of the clinically negative neck. Int J Radiat Oncol Biol Phys. 1979, 5:1977-80. 10.1016/0360-3016(79)90948-9 Kondo M, Ogawa K, Inuyama Y, et al.: Prognostic factors influencing relapse of squamous cell carcinoma of the maxillary sinus. Cancer. 1985, 55:190-6. 10.1002/1097-0142(19850101)55:1<190::aidcncr2820550130>3.0.co;2-2 Mori Y, Kida Y, Matsushita Y, Mizumatsu S, Hatano M: Stereotactic radiosurgery and stereotactic radiotherapy for malignant skull base tumors. Cureus. 2020, 12:e8401. 10.7759/cureus.8401 Nam GS, Moon IS, Kim JH, Kim SH, Choi JY, Son EJ: Prognostic factors affecting surgical outcomes in squamous cell carcinoma of external auditory canal. Clin Exp Otorhinolaryngol. 2018, 11:259-66. 10.21053/ceo.2017.01340 Murai T, Kamata SE, Sato K, et al.: Hypofractionated stereotactic radiotherapy for auditory canal or middle ear cancer. Cancer Control. 2016, 23:311-6. 10.1177/107327481602300315 Timmerman RD: An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008, 18:215-22. 10.1016/j.semradonc.2008.04.001
2021 Mori et al. Cureus 13(4): e14499. DOI 10.7759/cureus.14499
5 of 5
141
Medicine
®
Observational Study
OPEN
Long-term outcomes of 170 brain arteriovenous malformations treated by frameless image-guided robotic stereotactic radiosurgery Ramathibodi hospital experience Pritsana Punyawai, MDa, Nicha Radomsutthikul, MDb, Mantana Dhanachai, MDc, Chai Kobkitsuksakul, MDd, ∗ Ake Hansasuta, MDe, Abstract
This study was conducted to report long-term outcomes of the frameless robotic stereotactic radiosurgery (SRS) for brain arteriovenous malformation (AVM) at Ramathibodi Hospital. Retrospective data of patients with brain AVM (bAVM), who underwent CyberKnife SRS (CKSRS) at Ramathibodi Hospital from 2009 to 2014, were examined. Exclusion criteria were insufficient follow-up time (<36 months) or incomplete information. Patients’ demographics, clinical presentation, treatment parameters, and results were analyzed. Excellent outcome was defined as AVM obliteration without a new neurological deficit. Risk factors for achieving excellent outcome were assessed. From a total of 277 CKSRS treatments for bAVM during the 6 years, 170 AVMs in 166 patients met the inclusion criteria. One hundred and thirty-nine cases (81.76%) presented with hemorrhages from ruptured bAVMs. Almost two-thirds underwent embolization before radiosurgery. With the median AVM volume of 4.17 mL, three-quarters of the cohort had single-fraction CKSRS, utilizing the median prescribed dose of 15 Gray (Gy). In the multisession group (25.29%), the median prescribed dose and the AVM volume were 27.5 Gy and 22.3 mL, respectively. An overall excellent outcome, at a median follow-up period of 72.45 months, was observed in 99 cases (58.24%). Seven AVMs (4.12%) ruptured after CKSRS but 1 patient suffered a new neurological deficit. Two patients (1.18%) were classified into the poor outcome category but there were no deaths. Negative factors for excellent outcome, by multivariate regression analysis, were the male sex and multisession SRS delivery, but not age, history of AVM rupture, previous embolization, or AVM volume. Despite relatively larger bAVM and utilizing a lower prescribed radiation dose, the excellent outcome was within the reported range from previous literature. This study offers one of the longest follow-ups and the largest cohorts from the frameless image-guided robotic SRS community. Abbreviations: ARE = adverse radiation effects, AVM = arteriovenous malformation, bAVM = brain arteriovenous malformation,
CI = confidence interval, CKSRS = CyberKnife stereotactic radiosurgery, CT = computerized tomography, GBM = glioblastoma multiforme, GK = Gammaknife, Gy = Gray, IQR = interquartile range, LINAC = linear accelerator, ml = milliliter, mm = millimetre, MRA = magnetic resonance angiography, mRBAS = modified radiosurgery-based score, MRI = magnetic resonance imaging, N/A = not applicable, NS = not specified, SD = standard deviation, SRS = stereotactic radiosurgery. Keywords: arteriovenous, CyberKnife, frameless, malformation, radiosurgery, robotic, stereotactic
Editor: Jianxun Ding. The authors report no conflicts of interest. The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request. a
Chakri Naruebodindra Medical Institute, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Samut Prakan, b Department of Surgery, Division of Neurosurgery, King Narai Hospital, Lopburi, c Department of Diagnostic and Therapeutic Radiology, Division of Radiation Oncology, d Department of Diagnostic and Therapeutic Radiology, Division of Interventional Neuroradiology, e Department of Surgery, Division of Neurosurgery, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. ∗
Correspondence: Ake Hansasuta, Department of Surgery, Division of Neurosurgery, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand (e-mail: ake.han@mahidol.ac.th). Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc. This is an open access article distributed under the Creative Commons Attribution License 4.0 (CCBY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. How to cite this article: Punyawai P, Radomsutthikul N, Dhanachai M, Kobkitsuksakul C, Hansasuta A. Long-term outcomes of 170 brain arteriovenous malformations treated by frameless image-guided robotic stereotactic radiosurgery: Ramathibodi hospital experience. Medicine 2021;100:19(e25752). Received: 9 June 2020 / Received in final form: 22 March 2021 / Accepted: 8 April 2021 http://dx.doi.org/10.1097/MD.0000000000025752
1
142
Medicine
Punyawai et al. Medicine (2021) 100:19
1. Introduction
selected for the single-fraction SRS plan whereas 4 to 6 Gy/ fraction for 5 consecutive fractions was applied for the multisession regimen. The prescribed dose was typically assigned to the 50% to 75% isodose line. A ray-tracing algorithm was exercised for dose calculation.
Arteriovenous malformation (AVM) of the brain is a common cause of spontaneous intracranial hemorrhage in the young. Current treatment options for brain AVM (bAVM) are observation, surgical excision, endovascular embolization, and stereotactic radiosurgery (SRS).[1,2] Numerous publications, from the GammaKnife (GK) and linear accelerator (LINAC) SRS series, demonstrated the efficacy and safety of radiotherapy for bAVMs.[3–13] In contrast, data from CyberKnife (Accuray, Sunnyvale, CA) (CK) frameless image-guided robotic SRS centers were much less abundant. Colombo et al reported early results in 279 patients, treated with CK robotic SRS, with a median followup of 31 months. Subgroup analysis of the 102 cases, who had a follow-up duration of ≥36 months, showed an overall 71.5% rate of AVM obliteration.[14] Apart from this report, other studies by Gupta et al, Ding et al, Wowra et al, Oermann et al, and Feutren et al comprised 9, 11, 20, 26, and 48 subjects, respectively.[15–19] Thus, to furnish results from a greater number of patients with a longer follow-up period, a database of patients undergoing AVM treatment by CyberKnife SRS (CKSRS) at Ramathibodi Hospital was evaluated.
2.3. Outcome assessments After the CKSRS treatment, appointments for clinical evaluation were scheduled at a 6-month interval. MRI and MRA scans at 24 or 36 months were typically obtained to determine complete obliteration of the AVM. At that time, those with apparent nidus or remaining flow, evident by early draining vein(s), were examined by annual neurologic tests, MRI, and MRA. In patients with nondetectable AVM nidus and no venous outflow by MRI/ MRA, cerebral angiography would have been performed except in the event of patient refusal. For post-CKSRS assessment of outcomes, the included cases must have had at least 36 months of follow-up duration. The outcome of each patient, at his/her latest follow-up, was determined by the classification described by Pollock and Flickinger as follows: excellent, good, fair, unchanged and poor outcome, and death. Complete obliteration of the AVM without a new neurological deficit was defined as excellent outcome. Patients with AVM obliteration were classified into good outcome if they had minor deficit, and into fair outcome if they suffered major deficit that resulted in a decline of their functional status. If the AVM was not obliterated, the unchanged outcome was given to those without a new deficit while poor outcomes were patients who sustained a new deficit. Death was the last category of outcomes if it was believed to be directly related to the AVM or the SRS treatment.[21] The percentage of the aforementioned outcomes were stratified into groups based on the ranges of mRBAS,[20] as described earlier. Owing to the nature of this retrospective chart review, the clinical and radiographic outcomes, documented by multiple examiners and radiologists, were not blinded.
2. Materials and methods 2.1. Data collection After approval by the Institutional Review Committee, a retrospective study of patients with bAVMs who underwent CKSRS from 2009 to 2014 was undertaken. Cases with incomplete data were excluded. Pre-SRS demographics of each patient such as age, sex, history of hemorrhage, previous AVM treatment (surgery, embolization, or radiotherapy), neurological status and clinical presentation were recorded. In those with unruptured AVMs, their initial symptom(s), such as seizure or incidental finding, were documented. Patients’ age, AVM volume, by milliliter, and location were taken into account for the calculation of modified radiosurgery-based score (mRBAS) as per the following equation: AVM score = (0.1 volume [mL]) + (0.02 age [year]) + (0.5 location [0 or 1]). One point was assigned for deep locations (brainstem, basal ganglion, and thalamus), whereas 0 points were given for the non-deep areas.[20] The mRBAS were arranged into ranges of scores, 1, 1.01 to 1.5, 1.51 to 2 and >2, for further examination. The CKSRS treatment parameters from the included AVM were retrieved.
2.4. Data analysis Patient, treatment, and outcome data would be presented as mean ± standard deviation (SD) or median (interquartile range [IQR]), where appropriate, for continuous variables and as percentage for categorical variables. To investigate patient and treatment factors influencing an excellent outcome, the data were compared using the Student t test or the Mann–Whitney U test for continuous variables, and the x2 or Fisher exact test for categorical variables. Univariate and multivariate analyses were utilized to identify predictors for excellent outcomes by the logistic regression model with odds ratios and 95% confidence intervals (CI) computation. Kaplan–Meier survival graph of the proportion of AVM obliteration over time would be generated by linear regression analysis. All statistical tests were performed with Stata version 14 software (StataCorp, College Station, TX). Statistical significance was considered with a P value <.05.
2.2. Radiosurgery planning and treatment technique A moldable plastic, custom-made, mask was individually fitted for each patient before obtaining the CKSRS protocol, with 1.2 mm cuts, and a contrast-enhanced computerized tomography (CT) scan. In addition, selected series of magnetic resonance imaging (MRI) consisting of thin-sliced (1–3 mm) gadoliniumenhanced T1, proton-density, and contrast-enhanced magnetic resonance angiography (MRA) were uploaded to the CK planning station. Subsequent integration of the MRI/MRA with the CT was done. The target (AVM nidus) as well as critical structures were delineated before treatment planning. For bAVMs with a diameter <30 mm, or volume <15 mL, a single-fraction SRS was employed. For larger targets, a multisession, in 5 daily deliveries, SRS regimen was utilized. An appropriate prescribed radiation dose, 15 to 20 Gray (Gy), was
3. Results From 2009 to 2014, there were 277 bAVM patients who underwent CKSRS at our institute. The excluded subjects were 22 patients whose data could not be retrieved and another 89 cases with insufficient follow-up (<36 months). This resulted in a study cohort of 166 patients, harboring 170 AVMs. Two cases 2
143
Punyawai et al. Medicine (2021) 100:19
www.md-journal.com
Table 1
Table 3
Demographics of the 166 patients, 170 AVMs, who underwent CyberKnife SRS.
Overall AVM obliteration and outcomes in the patients who underwent CKSRS.
∗
Demographics
∗
No. (%)
No. (%)
Age, y median (IQR) Sex Female Male Presentation Hemorrhage (ruptured AVM) Asymptomatic Seizure Previous treatment Previous embolization 1 Time 2 Times 3 Times ≥4 Times Previous surgery Previous SRS/radiotherapy
26.5 (17–39)
Follow-up time, mo, median (IQR) Overall AVM obliteration AVM obliteration in single fraction CKSRS treatment group AVM obliteration in 5-fraction CKSRS treatment group Time to AVM obliteration, mo, median (IQR) Post-CKSRS AVM hemorrhage New neurological deficit Glioblastoma multiforme Outcomes Excellent Good Fair Unchanged Poor Death
94 (55.63) 72 (43.37) 139 2 29 118 109 53 30 13 13 7 14
(81.76) (1.18) (17.06) (69.41) (64.12) (48.62) (27.52) (11.93) (11.93) (4.12) (8.24)
72.45 99 86 13 39.4 7 1 1
(60.7–91.8) (58.24) (67.72) (30.23) (24.6–60.8) (4.12) (0.59) (0.59)
99 (58.24) 0 0 69 (40.59) 2 (1.18) 0
AVM = arteriovenous malformation, CKSRS = CyberKnife stereotactic radiosurgery, IQR = interquartile range, m = month. ∗ Number with percentage in brackets unless specified otherwise.
AVM = arteriovenous malformation, IQR = interquartile range, SRS = stereotactic radiosurgery, y = year. ∗ Number with percentage in brackets unless specified otherwise.
With the median follow-up duration of 72.45 (60.7–91.8) months, 99 patients (58.24%) had complete AVM obliteration, confirmed by cerebral angiography in 72 (72.73%), and by MRI/ MRA in 27 (27.27%) cases. The median time to obliteration was 39.4 (24.63–60.8) months (Table 3). The actuarial AVM obliteration rates from the single-fraction cohort, at 3, 5, 8, and 10 years, were 32.28%, 50.04%, 75.99%, and 75.99%, respectively. Patients with multisession CKSRS did not achieve the same level of success, evident by Log-rank test (Fig. 1), as the single-fraction treatment group (P < .001). Its actuarial post-SRS AVM obliteration rates were 9.3%, 11.75%, 31.6%, and 43%, at 3, 5, 8, and 10 years, respectively. No patient with AVM eradication suffered a new neurological deficit; hence, the rate of excellent outcome was maintained at 58.24% and none was classified into good or fair categories. Among the nonobliterated AVMs, 69 patients had no new deficit, resulting in the proportion of 40.59% for the unchanged outcome group. After CKSRS, 7
underwent CKSRS twice during the study period. Both of them had a substantial volume reduction in their AVMs but did not achieve complete obliteration. The other 2 subjects had 2 AVM nidi at different locations. A summary of the patients’ demographics is shown in Table 1. Most of the patients (81.76%) suffered AVM rupture with intracranial hemorrhage. One hundred and nine AVMs (64.12%) had endovascular occlusion before CKSRS. Approximately half of them underwent ≥2 sessions of embolization. Table 2 summarizes the treatment characteristics of the reviewed cases. The majority (74.71%) of the bAVMs received single-fraction CKSRS with the median prescribed and maximum radiation doses of 15 (15–16) and 25.4 (22.9–27.6) Gy, respectively. The median AVM volume for single-fraction treatment was 4.17 (2.19–9.2) mL. Forty-three patients (25.29%) received a hypofractionated regimen, in 5 sessions. Their median prescribed and maximum doses were 27.5 (25–28) and 43.6 (38.2–45.3) Gy, respectively. The median AVM volume in this multisession cohort was 22.3 (13.39–37.86) mL. Table 2 Stereotactic radiosurgery treatment characteristics of the AVM. ∗
Single fraction
∗
5 Fractions
∗
Overall
No. (%) 127 (74.71) 43 (25.29) 170 (100) Treatment parameters AVM volume, mL 4.17 (2.19–9.2) 22.3 (13.39–37.86) 7.365 (2.53–12.71) AVM location†: Deep, n (%) 28 (22.05) 9 (20.93) 37 (21.76) Non-deep, n (%) 99 (77.95) 34 (79.07) 133 (78.24) Prescribed dose, Gy 15 (15–16) 27.5 (25–28) N/A Maximum dose, Gy 25.4 (22.9–27.6) 43.6 (38.2–45.3) N/A Isodose line (%) 60.63 ± 6.11 64.19 ± 6.11 61.52 ± 6.32 AVM coverage (%) 94.82 ± 0.72 94.82 ± 0.64 94.82 ± 0.70 Conformity index 1.3 (1.21–1.48) 1.18 (1.14–1.22) 1.23 (1.17–1.37) AVM = arteriovenous malformation, Gy = Gray, N/A = not applicable. ∗ The parameters are presented in median (interquartile range) or mean ± standard deviation unless specified otherwise. † Based on modified radiosurgery-based scoring system (deep AVM locations = brainstem, basal ganglion, and thalamus).[20]
Figure 1. Kaplan-Meier curves demonstrating significantly different rates of arteriovenous malformation obliteration after single- vs 5-fraction CyberKnife stereotactic radiosurgery (CKSRS) treatments.
3
144
Medicine
Punyawai et al. Medicine (2021) 100:19
obliteration with a trivial proportion of complications. Despite advances in imaging studies and radiation delivery techniques, AVM obliteration rates remained relatively unchanged. However, newer technologies appeared to have lowered the overall sequelae of radiotherapy.[22] Thanks to the frameless immobilization, CyberKnife SRS permits the option of administering treatment, by either single- or multisession, for varying sizes of AVMs. However, unlike ample data by GK and LINAC series, the literature search, for full-text documents published in the English language, produced just <10 publications from CK series.[14–19] Moreover, there was only 1 publication that had a median follow-up duration >60 months. Unfortunately, only 9 patients comprised this published data by Gupta et al.[15] Our study, therefore, included both the large number of AVM subjects and the long follow-up time. The overall AVM obliteration rate in this study was in line with the previously reported range of 50% to 90%,[3,4,8,11,12,23,24] albeit on the lower end of the spectrum, possibly due to the lesser-than-average prescribed dose (15 Gy) along with the larger target volume. The overall rate of post-SRS hemorrhage was also within the reported 1% to 5% range.[2,7,14,17–19] Recognized negative factors, for AVM eradication by SRS, include patient factors, such as age, deep location, history of hemorrhage or large AVM volume, and treatment factors, that is, prescribed dose.[7,8,25] This study concurred with previous publications with regard to the AVM volume and multisession SRS. It should reflect the substantial magnitude of targets that automatically mandated fractionation of radiotherapy rather than the poor selection of treatment options. On the other hand, the excellent outcome was not inversely affected by age, history of AVM rupture and deep location, as previous studies validating radiosurgery-based AVM scoring systems might suggest.[26–28] Although most of the SRS series did not find sex to be associated with outcomes, this study, in a multivariate analysis, identified the male sex as an independent negative predictor. Frager et al published a similar observation[29] and Bir et al found that female patients had a higher proportion of AVM obliteration.[30] On the contrary, Yang et al found the male sex to be one of the protective factors against the post-SRS rebleeding[31] and Liscak et al[3] demonstrated that male patients achieved a higher percentage of AVM obliteration. In addition to the above-mentioned negative factors, it is largely well-known that pre-SRS embolization hinders the probability of AVM obliteration.[32,33] At Ramathibodi Comprehensive Neurovascular Center, there has always been a significant proportion of AVM patients whose pre-SRS embolization were necessary. In contrast, our statistical analysis contradicted those facts. Similar findings, of no untoward effect from prior embolization, were previously published by few centers.[34,35] Oermann et al noted that the previously embolized AVMs had a significantly worse rate of obliteration after SRS. However, upon multivariate analysis, it failed to prove the case but, instead, the AVM architectural complexity was the actual negatively-affecting variable.[36] We have not explored this particular matter in the present study. By not including abstract-only information, an English language literature search for full-text, from the PubMed, Scopus, and Google Scholar databases, returned 4 CKSRS for bAVM series with at least 20 cases.[14,17–19] Despite the largest number of subjects in their study, Colombo et al performed the assessments of outcomes from 102 patients who had at least 36 months of follow-up.[14] Therefore, with 170 AVMs and the
Figure 2. Cumulative incidence of arteriovenous malformation (AVM) bleeding after CyberKnife stereotactic radiosurgery (CKSRS) treatment.
overall occurrences of AVM hemorrhage (4.12%), in 6 patients, were recorded. The events arose at as early as 6, but no later than 67 months after treatment (Fig. 2). Of the 6 patients, only 1 suffered a new neurological deficit with declined functional status. Apart from the hemorrhagic events, a 14-year-old girl developed glioblastoma multiforme (GBM), at the irradiated AVM region, 4 years after embolization and multisession CKSRS of her ruptured AVM. Surgical excision of the tumor was performed with subsequent chemotherapy and radiation. Although she did not have post-SRS hemorrhage or sustained a new neurological deficit, this patient and the aforementioned post-SRS hemorrhage victim were sorted into the same category, making the total number of 2 cases (1.18%) in the poor outcome group. Apart from the mentioned patients, the rest of the study cohort had no documented adverse radiation effect or death. Table 4 details the patients’ and treatment’s variables for excellent outcome. The significant factors were the male sex (P = .018), AVM volume (P = .021), multisession SRS (P < .001), isodose line (P = .018), and time to AVM obliteration (P < .001). In contrast to many publications, we did not find a history of AVM rupture (P = .672) or previous embolization (P = .632) to correlate with untoward results. Moreover, the age (P = .908) and deep location (P = .338) were not associated with excellent outcome, although the mRBAS appeared to be related (P = .013). Further evaluation by uni- and multivariate analyses for independent predictors of excellent outcome was performed. The AVM volume (95% confidence interval [CI] = 0.98–1.04, P = .488), mRBAS (95% CI = 0.27–2.31, P = .675), and isodose line (95% CI = 0.91–1.02, P = .272) were insignificant, by multivariate examination, whereas the male sex (95% CI = 0.25–0.99, P = .048) and multisession SRS (95% CI = 0.06–0.57, P = .003) were confirmed to negatively affect the outcome (Table 5).
4. Discussion Stereotactic radiosurgery is an established treatment modality for bAVM.[1,2] Ideal SRS ought to yield high rates of AVM 4
145
Punyawai et al. Medicine (2021) 100:19
www.md-journal.com
Table 4 Analyses of variables for excellent outcome by SRS treatment of brain AVM. ∗
Total, n AVMs Patient factors Sex Female Male Age, y, mean ± SD Presentation: Hemorrhage (ruptured AVM) Seizure Asymptomatic Previous surgery No Yes Previous radiotherapy No Yes Previous embolization No Yes AVM volume, mL, median (IQR) AVM location: Deep (brainstem, basal ganglion, and thalamus) Non-deep mRBAS, median (IQR) mRBAS: mRBAS 1 mRBAS 1.01–1.5 mRBAS 1.51–2 mRBAS >2 Treatment factors SRS fractionation Single fraction 5 Fractions Single-fraction prescribed dose, Gy, median (IQR) Single-fraction maximum dose, Gy, median (IQR) 5-Fraction prescribed dose, Gy, median (IQR) 5-Fraction maximum dose, Gy, median (IQR) Isodose line, % mean ± SD AVM coverage, %, mean ± SD Conformity index, median (IQR) Time to AVM obliteration, mo, median (IQR)
∗
Non-excellent outcome, n (%)
∗
Excellent outcome, n (%)
170
71 (41.76)
99 (58.24)
97 73
33 (46.48) 38 (53.52) 34.28 ± 14.42
64 (64.65) 35 (35.35) 34.03 ± 13.72
139 29 2
57 (80.28) 12 (16.90) 1 (1.41)
82 (82.83) 17 (17.17) 1 (1.01)
163 7
69 (97.18) 2 (2.82)
94 (94.95) 5 (5.05)
156 14
65 (91.55) 6 (8.45)
91 (91.92) 8 (8.08)
61 109 7.4 (2.5–12.7)
24 (33.80) 47 (66.20) 9.2 (3.1–16.3)
37 (37.37) 62 (62.63) 4.9 (2.2–11.5)
37 133 1.4 (0.9–2.0)
18 (25.35) 53 (74.65) 1.6 (1.1–2.3)
19 (19.19) 80 (80.81) 1.2 (0.8–1.9)
15 15 22 19
32 33 10 24
P — .018
.908 .672 .963 .999 .701
.931
.632
47 48 32 43
(21.13) (21.13) (30.98) (26.76)
.021 .337
.013 .003
(32.32) (33.33) (10.10) (24.24) <.001
127 43 15 (15–16) 25.4 (22.9–27.6) 27.5 (25–28) 43.6 (38.2–44.8) 61.52 ± 6.32 94.82 ± 0.70 1.2 (1.2, 1.4) 60.4 (30.5–75.2)
41 (57.75) 30 (42.25) 15 (15–16) 24.2 (22.4–27.3) 26.7 (25–28) 43.6 (37.9–45.3) 62.87 ± 6.76 94.88 ± 0.85 1.2 (1.2, 1.3) 76.0 (60.8–106.1)
86 (86.87) 13 (13.13) 15 (15–16) 25.4 (23.3–27.7) 27.5 (27–28) 43.1 (42.2–44.8) 60.56 ± 5.83 94.77 ± 0.57 1.2 (1.2, 1.4) 39.4 (24.6–60.8)
.997 .254 .145 .853 .018 .347 .320 <.001
AVM = arteriovenous malformation, Gy = Gray, IQR = interquartile range, m = month, mRBAS = modified radiosurgery-based AVM score[20], N/A = not applicable, SD = standard deviation, SRS = stereotactic radiosurgery, y = year. ∗ Number with percentage in brackets unless specified otherwise.
Table 5 Regression analyses of the predictors for excellent outcome by SRS treatment of brain AVM. Univariate analysis Odds ratio (95% CI) Patient factors Sex, male Age AVM volume Deep AVM locations mRBAS Previous hemorrhage (ruptured AVM) Previous embolization Treatment factors Multisession SRS Isodose line
P
Multivariate analysis Odds ratio (95% CI)
(0.25–0.88) (0.97–1.02) (0.95–0.99) (0.34–1.45) (0.62–0.95) (0.54–2.59) (0.45–1.62)
.019 .908 .023 .338 .019 .672 .632
0.505 (0.25–0.99) — 1.010 (0.98–1.04) — 0.796 (0.27–2.31) — —
.048
0.206 (0.09–0.43) 0.942 (0.89–0.99)
<.001 .020
0.185 (0.06–0.57) 0.969 (0.91–1.02)
.003 .272
0.474 0.998 0.973 0.699 0.770 1.184 0.855
CI = confidence interval, mRBAS = modified radiosurgery-based AVM score,[20] deep AVM locations = brainstem, basal ganglion, and thalamus, SRS = stereotactic radiosurgery.
5
146
P
.488 .675
Medicine
Punyawai et al. Medicine (2021) 100:19
Table 6 CKSRS for brain AVM series with at least 20 cases including the present study. Colombo et al,[14] 2009
Wowra et al,[17] 2009
Oermann et al,[18] 2014
Feutren et al,[19] 2018
Present study
279 267 102 31
20 20 NS 25
26 26 NS 25
48 48 33 41
277 255 166 Patients, 170 AVMs 72.45
34 45 (44.12)†
33.4 9 (45)
41 14 (54)
32 19 (39.58)
26.5 139 (81.76)
50†
30
42.3
85.42
64.12
71.5† 1.41† 17 (16.67)† 37 (36.27)† 30 (29.41)† 18 (17.65)†
67 1.35 NS NS NS NS
57.69 NS NS NS NS NS
68 1.24 14 (29.2) 26 (54.2) 7 (14.5) 1 (2.15)
58.24 1.4 (mRBAS) 47 (27.65) 48 (28.24) 32 (18.82) 43 (25.29)
79.41†
100
100
100
74.71
∗
Parameters No. of patients treated with CKSRS No. of patients with follow-up No. of patients with follow-up >36 mo Follow-up duration, mo Patient characteristics Age, y Patients with AVM rupture (hemorrhage) , n (%) Proportion of patients with previous embolization (%) Overall AVM obliteration rate (%) RBAS (median) <1, n (%) 1.01–1.5, n (%) 1.51–2, n (%) >2, n (%) Treatment parameters Proportion of patients treated with single-fraction CKSRS (%)
AVM volume, mL Prescribed dose, Gy Maximum dose, Gy IDL (%) CI
1.95 18.7 25.5 NS NS
1.8 22 30.3 67 NS
Single-fraction
5 Fractions
4.17 15 25.4 60 1.3
22.3 27.5 43.6 64 1.18
1.62 19 NS 80 NS
2.6 NS 25 NS NS
NS NS NS NS NS 0 3 Patients with motor deficit 1 (3.8)
NS NS NS NS NS 0 2 Patients with grade 4 symptomatic ARE NS
Outcome (%) Excellent Good Fair Unchanged Poor Death Remarks Overall post-CKSRS hemorrhage (%)
NS NS NS NS NS 1 1 Patient with new deficit 8 (3)‡
NS NS NS NS NS 0 1 Patient with new deficit 1 (5)
58.24 0 0 40.59 1.18 0 1 Patient with GBM and 1 patient with new deficit 7 (4.12)
ARE = adverse radiation effect, AVM = arteriovenous malformation, CKSRS = CyberKnife stereotactic radiosurgery, GBM = glioblastoma multiforme, m = month, mL = milliliter, mRBAS = modified RBAS,[20] NS = not specified, RBAS = radiosurgery-based arteriovenous malformation score[21]. ∗ The parameters are presented in median unless specified otherwise. † Outcome report from Colombo et al[14] derived from 102 patients with follow-up >36 months. ‡ Post-CKSRS hemorrhage from Colombo et al[14] was calculated from 8 incidences of 267 patients.
post-SRS AVM rebleeding. None detailed their results based on RBAS or mRBAS systems; hence, this study was the first, among CKSRS cohorts, to stratify results by standardized method. One patient with GBM was observed in the study. She was the second case who developed this malignancy after CKSRS for bAVM, after the first patient report from Xhumari et al.[37]
median follow-up duration of 72.45 months, our study represents the largest cohort demonstrating long-term results among the published series of frameless image-guided robotic SRS for bAVM (Table 6). Although the overall AVM obliteration from our study, compared with others, appeared to be relatively low, there are several conceivable explanations for it. First, the median AVM volume of 4.17 mL in the single-fraction group was the largest among the CKSRS series. In addition, this study comprised a higher proportion of larger AVMs than other CK series, with the median volume of 22.3 ml. Due to sizeable AVMs, the prescribed doses for single- and multisession CKSRS were relatively lower than in other studies. Considering this, lower than average obliteration rates were rather predictable. Nevertheless, the incidence of post-SRS AVM hemorrhage appeared to be within the reported range.[14,17–19] Unfortunately, because different bodies of literature described various but not standardized outcomes, it was rather difficult to directly compare complication rates among the CKSRS series, other than the
5. Study limitations The presented study has some limitations. First, the “criterion standard” cerebral angiography to determine complete obliteration was not used in all cases. Due to the fact that some patients refused to take part in the post-SRS cerebral angiographic study, the outcome assessment is less than ideal because of the nonuniform post-treatment radiographic evaluation. Another constraint was the exclusion of 111 cases (39%) for lack of data or insufficient follow-up duration. It could have affected the overall obliteration rate or the incidence of complications as 6
147
Punyawai et al. Medicine (2021) 100:19
www.md-journal.com
shown by Heffez et al.[38] However, these limitations are common hindrances associated with retrospective reviews.
[11] Bollet MA, Anxionnat R, Buchheit I, et al. Efficacy and morbidity of arctherapy radiosurgery for cerebral arteriovenous malformations: a comparison with the natural history. Int J Radiat Oncol Biol Phys 2004;58:1353–63. [12] Raffa SJ, Chi YY, Bova FJ, et al. Validation of the radiosurgery-based arteriovenous malformation score in a large linear accelerator radiosurgery experience. J Neurosurg 2009;111:832–9. [13] Fokas E, Henzel M, Wittig A, et al. Stereotactic radiosurgery of cerebral arteriovenous malformations: long-term follow-up in 164 patients of a single institution. J Neurol 2013;260:2156–62. [14] Colombo F, Cavedon C, Casentini L, et al. Early results of CyberKnife radiosurgery for arteriovenous malformations. J Neurosurg 2009;111: 807–19. [15] Gupta R, Moore JM, Amorin A, et al. Long-term follow up data on difficult to treat intracranial arteriovenous malformations treated with the CyberKnife. J Clin Neurosci 2019;61:120–3. [16] Ding C, Solberg TD, Hrycushko B, et al. Multi-staged robotic stereotactic radiosurgery for large cerebral arteriovenous malformations. Radiother Oncol 2013;109:452–6. [17] Wowra B, Muacevic A, Tonn JC, et al. Obliteration dynamics in cerebral arteriovenous malformations after cyberknife radiosurgery: quantification with sequential nidus volumetry and 3-tesla 3-dimensional time-offlight magnetic resonance angiography. Neurosurgery 2009;64(2 suppl): A102–9. [18] Oermann EK, Murthy N, Chen V, et al. A multicenter retrospective study of frameless robotic radiosurgery for intracranial arteriovenous malformation. Front Oncol 2014;4:298. [19] Feutren T, Huertas A, Salleron J, et al. Modern robot-assisted radiosurgery of cerebral angiomas-own experiences, system comparisons, and comprehensive literature overview. Neurosurg Rev 2018;41: 787–97. [20] Pollock BE, Flickinger JC. Modification of the radiosurgery-based arteriovenous malformation grading system. Neurosurgery 2008;63: 239–43. discussion 243. [21] Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg 2002;96: 79–85. [22] Patibandla MR, Ding D, Kano H, et al. Effect of treatment period on outcomes after stereotactic radiosurgery for brain arteriovenous malformations: an international multicenter study. J Neurosurg 2018;1–10. [23] Zabel A, Milker-Zabel S, Huber P, et al. Treatment outcome after linacbased radiosurgery in cerebral arteriovenous malformations: retrospective analysis of factors affecting obliteration. Radiother Oncol 2005;77:105–10. [24] Tayebi Meybodi A, Lawton MT. Modern radiosurgical and endovascular classification schemes for brain arteriovenous malformations. Neurosurg Rev 2020;43:49–58. [25] Vlaskou Badra E, Ermis E, Mordasini P, et al. Radiosurgery and radiotherapy for arteriovenous malformations: outcome predictors and review of the literature. J Neurosurg Sci 2018;62:490–504. [26] Pollock BE. Development and testing of a radiosurgery-based arteriovenous malformation grading system. Prog Neurol Surg 2013; 27:58–66. [27] Starke RM, Yen CP, Ding D, et al. A practical grading scale for predicting outcome after radiosurgery for arteriovenous malformations: analysis of 1012 treated patients. J Neurosurg 2013;119:981–7. [28] Pollock BE, Storlie CB, Link MJ, et al. Comparative analysis of arteriovenous malformation grading scales in predicting outcomes after stereotactic radiosurgery. J Neurosurg 2017;126:852–8. [29] Frager MJ, Glazener EM, Rahimian J, et al. A comparative outcomes analysis of patients treated for arteriovenous malformation with LINACbased stereotactic radiosurgery by a standard frame-based technique or a frameless technique utilizing 3-dimensional rotational angiography. J Clin Neurosci 2020;77:185–90. [30] Bir SC, Ambekar S, Maiti TK, et al. Clinical outcome and complications of gamma knife radiosurgery for intracranial arteriovenous malformations. J Clin Neurosci 2015;22:1117–22. [31] Yang W, Luksik AS, Jiang B, et al. Venous stenosis and hemorrhage after radiosurgery for cerebral arteriovenous malformations. World Neurosurg 2019;122:e1615–25. [32] Russell D, Peck T, Ding D, et al. Stereotactic radiosurgery alone or combined with embolization for brain arteriovenous malformations: a systematic review and meta-analysis. J Neurosurg 2018;128: 1338–48.
6. Conclusion These results, with a considerable number of patients and extensive follow-up duration, confirmed the efficacy and safety of the frameless image-guided robotic stereotactic radiosurgery for brain AVM. Identified risk factors hindering achievement of excellent outcome were the male sex and multisession treatment.
Acknowledgments The authors thank Miss Suraida Aeesoa for her assistance in statistical analyses of the study and Dr. Nattaphong Rattanavirotkul for manuscript corrections.
Author contributions Conceptualization: Pritsana Punyawai, Mantana Dhanachai, Chai Kobkitsuksakul, Ake Hansasuta. Data curation: Pritsana Punyawai, Nicha Radomsutthikul. Formal analysis: Pritsana Punyawai, Ake Hansasuta. Investigation: Pritsana Punyawai, Nicha Radomsutthikul. Methodology: Pritsana Punyawai, Mantana Dhanachai, Ake Hansasuta. Project administration: Ake Hansasuta. Supervision: Mantana Dhanachai, Chai Kobkitsuksakul, Ake Hansasuta. Validation: Pritsana Punyawai, Ake Hansasuta. Writing – original draft: Pritsana Punyawai, Ake Hansasuta. Writing – review & editing: Mantana Dhanachai, Ake Hansasuta.
References [1] van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA 2011;306:2011–9. [2] Solomon RA, Connolly ESJr. Arteriovenous malformations of the brain. N Engl J Med 2017;376:1859–66. [3] Liscak R, Vladyka V, Simonova G, et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery 2007;60:1005–14. discussion 10151006. [4] Jokura H, Kawagishi J, Sugai K, et al. Gamma knife radiosurgery for arteriovenous malformations: the Furukawa experience. Prog Neurol Surg 2009;22:20–30. [5] Paul L, Casasco A, Kusak ME, et al. Results for a series of 697 arteriovenous malformations treated by gamma knife: influence of angiographic features on the obliteration rate. Neurosurgery 2014;75:568–83. dicussion 582-563; quiz 583. [6] Cohen-Inbar O, Lee CC, Xu Z, et al. A quantitative analysis of adverse radiation effects following Gamma Knife radiosurgery for arteriovenous malformations. J Neurosurg 2015;123:945–53. [7] Starke RM, Kano H, Ding D, et al. Stereotactic radiosurgery for cerebral arteriovenous malformations: evaluation of long-term outcomes in a multicenter cohort. J Neurosurg 2017;126:36–44. [8] Pollock BE. Gamma knife radiosurgery of arteriovenous malformations: long-term outcomes and late effects. Prog Neurol Surg 2019;34:238–47. [9] Friedman WA, Bova FJ, Mendenhall WM. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg 1995;82:180–9. [10] Schlienger M, Atlan D, Lefkopoulos D, et al. Linac radiosurgery for cerebral arteriovenous malformations: results in 169 patients. Int J Radiat Oncol Biol Phys 2000;46:1135–42.
7
148
Medicine
Punyawai et al. Medicine (2021) 100:19 [33] Zhu D, Li Z, Zhang Y, et al. Gamma knife surgery with and without embolization for cerebral arteriovenous malformations: A systematic review and meta-analysis. J Clin Neurosci 2018;56:67–73. [34] Lee CC, Chen CJ, Ball B, et al. Stereotactic radiosurgery for arteriovenous malformations after Onyx embolization: a case-control study. J Neurosurg 2015;123:126–35. [35] Izawa M, Chernov M, Hayashi M, et al. Combined management of intracranial arteriovenous malformations with embolization and gamma knife radiosurgery: comparative evaluation of the long-term results. Surg Neurol 2009;71:43–52. discussion 52-43.
[36] Oermann EK, Ding D, Yen CP, et al. Effect of prior embolization on cerebral arteriovenous malformation radiosurgery outcomes: a case-control study. Neurosurgery 2015;77:406–17. discussion 417. [37] Xhumari A, Rroji A, Enesi E, et al. Glioblastoma after AVM radiosurgery. Case report and review of the literature. Acta Neurochir (Wien) 2015;157:889–95. [38] Heffez DS, Osterdock RJ, Alderete L, et al. The effect of incomplete patient follow-up on the reported results of AVM radiosurgery. Surg Neurol 1998;49:373–81. discussion 381–374.
8
149
CyberKnife® radiosurgery in the
Radiation Oncology treatment of complex skull base tumors: analysis of treatment
Methodology
BioMed Central
Open Access
planning parameters of complex skull base CyberKnife® radiosurgery in the treatment tumors: analysis of treatment planning parameters
Sean P Collins†2, Nicholas D Coppa†1, Ying Zhang3, Brian T Collins2, Donald A McRae2 and Walter C Jean*1,2 Address: 1Department of Neurosurgery, Georgetown University Hospital, USA, 2Department of Radiation Oncology, Georgetown University Hospital, USA and 3Biostatistics Unit, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, USA Email: Sean P Collins - mbppkia@hotmail.com; Nicholas D Coppa - coppan@georgetown.edu; Ying Zhang - yz9@georgetown.edu; Brian T Collins - collinsb@gunet.georgetown.edu; Donald A McRae - mcraed@georgetown.edu; Walter C Jean* - wcj4@georgetown.edu * Corresponding author †Equal contributors
Published: 16 December 2006 Radiation Oncology 2006, 1:46
doi:10.1186/1748-717X-1-46
Received: 05 August 2006 Accepted: 16 December 2006
This article is available from: http://www.ro-journal.com/content/1/1/46 © 2006 Collins et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract Background: Tumors of the skull base pose unique challenges to radiosurgical treatment because of their irregular shapes, proximity to critical structures and variable tumor volumes. In this study, we investigate whether acceptable treatment plans with excellent conformity and homogeneity can be generated for complex skull base tumors using the Cyberknife® radiosurgical system. Methods: At Georgetown University Hospital from March 2002 through May 2005, the CyberKnife® was used to treat 80 patients with 82 base of skull lesions. Tumors were classified as simple or complex based on their proximity to adjacent critical structures. All planning and treatments were performed by the same radiosurgery team with the goal of minimizing dosage to adjacent critical structures and maximizing target coverage. Treatments were fractionated to allow for safer delivery of radiation to both large tumors and tumors in close proximity to critical structures. Results: The CyberKnife® treatment planning system was capable of generating highly conformal and homogeneous plans for complex skull base tumors. The treatment planning parameters did not significantly vary between spherical and non-spherical target volumes. The treatment parameters obtained from the plans of the complex base of skull group, including new conformity index, homogeneity index and percentage tumor coverage, were not significantly different from those of the simple group. Conclusion: Our data indicate that CyberKnife® treatment plans with excellent homogeneity, conformity and percent target coverage can be obtained for complex skull base tumors. Longer follow-up will be required to determine the safety and efficacy of fractionated treatment of these lesions with this radiosurgical system.
Background
Lesions of the base of skull are typically slow growing, but potentially morbid tumors [1]. They rarely metastasize
making local control the primary determinant of longterm survival [2]. Although surgical resection may still be the treatment "gold-standard" [3,4], radiosurgery is an Page 1 of 10 (page number not for citation purposes)
90 150
Radiation Oncology 2006, 1:46
appropriate treatment option for many patients [5]. However, single-fraction radiosurgical treatment may be difficult because of the potentially large size and irregular shapes of these tumors. Their proximity to critical structures also leads to a risk of radiation-induced, long-term, neurological complication [6]. The CyberKnife® is a newly FDA approved radiosurgical devise for the treatment of brain lesions. Unlike the LINAC and Gamma Knife, the CyberKnife® is an imageguided, frameless radiosurgery system. Treatment is delivered by a linear accelerator mounted on a flexible robotic arm. Several-hundred treatment beams are chosen out of a repertoire of greater than one thousand possible beam directions using inverse treatment planning. These beams are delivered in a non-isocentric manner via circular collimators of varying size without intensity modulation. Non-isocentric treatment allows for simultaneous irradiation of multiple lesions. The lack of a requirement for the use of a head-frame allows for staged treatment. Since the planning system has access to a large number of potential non-isocentric beams, the CyberKnife® should theoretically be able to deliver a highly conformal, uniform dose with steep dose gradients [7]. Therefore, treatment with the CyberKnife® radiosurgical system should minimize toxicity to surrounding structures. When compared to commonly used radiosurgical devices, such as the Gamma Knife, linear-accelerator based stereotactic radiosurgery systems with multiple arcs (LINAC), or intensity modulated radiation therapy, dosimetric studies of ellipsoid phantoms have shown that the CyberKnife® radiosurgical system has the best homogeneity within the target volume and comparable conformity [8]. A dose-volume histogram (DVH) is the tool most commonly used to compare radiosurgical plans. Unfortunately, the large volume of data in these histograms does not allow for simple differentiation between multiple plans and systems [9,10]. Thus, an effort has been made to determine simple measurements for plan optimization. A conformity index is a single measure of how well the treatment dose distribution of a specific radiation treatment plan conforms to the size and shape of the target volume. In general, the conformity index of a given radiosurgical plan is dependent on target shape [11], target volume [9], collimator size [12], type of collimation (circular vs multileaf) and radiosurgical delivery system. The new conformity index (NCI) and homogeneity index (HI) allow for the quick and simple comparison of different radiosurgical treatment plans, whether within the same radiosurgical system, or across diverse systems such as between the LINAC and Gamma Knife [13]. Conformity indices have been reported in the literature, ranging from 1.0 to 3.0 for varying radiosurgical systems [14-18].
http://www.ro-journal.com/content/1/1/46
Typically, multiple iso-center plans generated with the Gamma Knife have homogeneity indices (HI) of 2.0 to 3.0 while the LINAC plans generate homogeneity indices (HI) of 1.0 to 1.2 [17]. The significance of these differences between systems is controversial. We determined the NCI and HI for the first 82 base of skull lesions treated at Georgetown University Hospital using the CyberKnife® radiosurgical system (Accuray, Sunnyvale, CA). We undertook this study to determine the effect of target shape, target volume and proximity to critical structures on radiosurgical treatment parameters. This is the first study that we are aware of that investigates these parameters in patients treated with the CyberKnife® radiosurgery system.
Patients and methods
Patient population We performed a retrospective review of 262 patients with intracranial tumors, who were treated with CyberKnife® stereotactic radiosurgery at Georgetown University Hospital between March 2002 and May 2005. Eighty-one patients were classified to have tumors of the skull base resulting in a total of 84 treated lesions. Thirty-three percent of these lesions had been previously irradiated. One patient was excluded from analysis because two tumor volumes were treated simultaneously making it impossible to calculate indices for each individual lesion.
Of the remaining lesions, 46 were categorized into the complex, skull base tumor group. A complex skull base tumor was defined as one that completely encircles, partially circumscribes, or directly contacts the brainstem, optic chiasm, hypophysis, or cranial nerves with meaningful remaining function. This complex tumor group consisted of 18 men and 26 women, with a median age of 53 (range: 29 – 88). These tumors were further categorized by histopathology as follows: 21 meningiomas, 6 metastatic tumors, 8 schwannomas, 7 pituitary adenomas, 1 chordoma, 2 sarcomas, and 1 glioma. The median tumor size was 7.27 cc (range: 0.62 – 98.3 cc) (Table 1 &2). The data from the group with complex skull base tumors were compared with data from two control groups. The first group consisted of 36 patients with skull base tumors that were classified as simple. Although still located in the region of the skull base, tumors in this group had at least a 2 mm separation from the nearest critical structure. This group consisted of 16 men and 20 women, with a median age of 55 (range: 17 – 18). These tumors were also categorized by histopathology as follows: 5 meningiomas, 13 metastatic tumors, 10 schwannomas, 3 pituitary adenomas, 1 chordoma, 2 sarcomas, and 2 malignant gliomas. The median tumor size in this group was 8.83 cc (range: 0.19 – 206.3 cc) (Table 1 &2).
Page 2 of 10 (page number not for citation purposes)
15191
Radiation Oncology 2006, 1:46
http://www.ro-journal.com/content/1/1/46
Table 1: Patient Characteristics
Control Group I (simple)
Control Group II (metastases)
Study Group (complex)
36 36 16 20
43 43 23 20
44 46 18 26
17 81 53 55
21 85 57 58
29 88 55 53
Number of Patients Number of Lesions Male Female Age Min Max Mean Median
A second control group used for comparison consisted of 43 patients with metastatic tumors of the cerebral and cerebellar hemispheres. These lesions represented volumes that were spherical, with smooth borders, and relatively distant from critical neurovascular structures. This group consisted of 23 men and 20 women, with a median age of 58 (range: 21 – 85). These tumors were further categorized by histopatholgy as 33 metastatic carcinomas and 10 melanomas. The median tumor size in this group was 1.43 cc (range: 0.12 – 66 cc) (Table 1 &2).
Radiosurgical treatment planning The basic technical aspects of CyberKnife® radiosurgery for cranial tumors have been described in detail (CyberKnife® Radiosurgery, A Practical Guide). Briefly, the patient was placed in a supine position on a vacuum bag and a malleable thermoplastic mask was molded to the head and attached to the head support. Thin-sliced (1.25 mm) high-resolution CT images were obtained through the region of interest with the patient in the treatment position. Target volumes and critical structures were deline-
Table 2: Skull Base Tumor Characteristics Control Group I (simple) (n = 36)
Control Group II (metastases) (n = 43)
Study Group (complex) (n = 46)
0.19 206.3 45.61 8.83
0.12 66 4.87 1.43
0.62 98.3 12.6 7.27
Histology Carcinomas Chordoma Gliomas Malignant Gliomas Melanoma Meningioma Pituitary Adenoma Sarcomas Schwannoma (not VIII) Vestibular Schwannoma
13 1 0 2 0 5 3 2 0 10
33 0 0 0 10 0 0 0 0 0
4 1 1 0 2 21 7 2 4 4
Location Cavernous Sinus CP Angle/IAC Foramen Magnum Nasopharynx Orbital Apex/Parasellar Paranasal Sinus Petroclival Sellar Cerebral Hemishpere Thalamus/Hypothalamus Cerebellum Other*
2 12 0 4 3 4 3 3 1 0 0 4
0 0 0 0 0 0 0 0 34 2 7 0
15 6 4 0 5 0 7 7 0 1 0 1
Volume (cc) Min Max Mean Median
* Pons, mandible, infratemporal fossa
Page 3 of 10 (page number not for citation purposes)
152 92
Radiation Oncology 2006, 1:46
ated by the treating neurosurgeon. The treating neurosurgeon and radiation oncologist determined the minimal tumor margin dose of the target volume and the treatment isodose. This discussion was influenced by various factors, including previous radiation to the area, tumor volume, and extent of contact and compression of critical neurological structures. In most cases, the dose was prescribed to the isodose surface that encompassed the margin of the tumor. Twelve collimator sizes are available with the CyberKnife® radiosurgical system ranging from 5 mm to 60 mm. In general, a collimator size less than the maximum length of the prescribed target volume (PTV) was chosen for treatment planning [12]. An inverse planning method with non-isocenteric technique was used for all cases. The treating physician and physicist input the specific treatment criteria, limiting the maximum dose to structures such as the optic chiasm and brainstem. The majority of the treatments were given in five fractions. In general, for non-previously treated cases, treatment plans were deemed acceptable if the maximum dose to critical structures was less than 2000 cGy in five fractions. Nonanatomical dose constraint structures were commonly incorporated to aid the optimization process in minimizing the dose to critical structures. The planning software calculated the optimal solution for treatment. The DVH of each plan was evaluated until an acceptable plan was generated. Treatment planning parameters Target volume Target volume was defined as the volume contoured on the planning CT scan by the treating neurosurgeon. No margin was added to the target volume. In this study, there was no limit set on the treatable target volumes. Homogeneity Index The homogeneity index (HI) describes the uniformity of dose within a treated target volume, and is directly calculated from the prescription isodose line chosen to cover the margin of the tumor:
HI =
(maximum dose) (prescription dose)
New Conformity Index The new conformity index (NCI) as formulated by Paddick [13], and modified by Nakamura [16] describes the degree to which the prescribed isodose volume conforms to the shape and size of the target volume. It also takes into account avoidance of surrounding normal tissue. NCI =
[(treatment volume) × (prescription isodose volume)] ume)2 ( volume of the target covered by the prescription isodose volu
http://www.ro-journal.com/content/1/1/46
Percent Target Coverage PTC = The percentage of the target volume covered by the prescription isodose. Radiosurgical treatment delivery Image-guided radiosurgery was employed to eliminate the need for stereotactic frame fixation. Using computed tomography planning, target volume locations were related to radiographic landmarks of the cranium. With the assumption that the target position is fixed within the cranium, cranial tracking allows for anatomy based tracking relatively independent of patient's daily setup. Position verification was validated several times per minute during treatment using paired, orthogonal, x-ray images. Statistical analysis Chi-square test or two-sample t-test was used to test the distributions of the characteristics between the simple and complex groups. To assess the association between radiation treatment parameters and the tumor volume, simple linear regressions on tumor volume for each of the three indices were performed. The estimates of the slopes and their 95% confidence intervals were determined. Pearson's correlation coefficients and their 95% confidence intervals were calculated for the whole cohort.
Results
Patient and tumor characteristics The characteristics of the two treatment groups including their gender, age, tumor histology and locations are detailed below and summarized in Tables 1 and 2. The simple group was composed predominantly of malignant lesions and vestibular schwannomas, while the complex group consisted primarily of cavernous sinus meningiomas and pituitary adenomas. Overall radiosurgical parameters: effect of tumor shape Overall, compared to previously reported conformity indices for LINAC and GammaKnife systems, the CyberKnife® radiosurgical system compared favorably with a mean NCI of 1.6–1.8 and a mean HI of 1.2–1.3 (Table 3). The standard percentage target coverage of 95% was not compromised to obtain these values.
Base of skull lesions commonly have irregular, non-spherical shapes due to the presence of dural tails and the anatomy of the region. To determine the effect of tumor shape on radiosurgical parameters, a group of spherical cerebellar and cerebral hemisphere metastases were analyzed for comparison (Control Group II (metastases)). The calculated indices for this group were similar to the indices obtained for the base of skull lesions: mean NCI of 1.73 and a mean HI of 1.21 (Table 3). These data suggest that the CyberKnife® radiosurgical system generates conformal and homogeneous plans independent of tumor shape.
Page 4 of 10 (page number not for citation purposes)
15393
Radiation Oncology 2006, 1:46
http://www.ro-journal.com/content/1/1/46
Table 3: Radiosurgery Treatment Plan Control Group I (simple) (n = 36)
Control Group II (metastases) (n = 43)
Study Group (complex) (n = 46)
900 3500 2301 2500
1500 3000 1905 1900
1500 3500 2387 2500
Treatment Stages Min Max Mean Median
3 10 5.2 5
1 5 1.5 1
1 5 4.7 5
Homogeneity Index Min Max Mean Median
1.11 1.49 1.26 1.25
1.11 1.54 1.21 1.19
1.07 1.67 1.24 1.25
New Conformity Index Min Max Mean Median
1.04 2.59 1.66 1.57
1.04 3.11 1.73 1.64
1.27 2.27 1.67 1.57
Percent Target Coverage (%) Min Max Mean Median
82.5 99.9 95.9 97.5
79.6 100.0 97.0 99.1
80.2 99.9 94.3 94.7
Dose (cGy) Min Max Mean Median
Comparison of radiosurgical parameters between complex and simple base of skull lesions Complex base of skull lesions were defined as one that completely encircles, partially circumscribes, or directly contacts the brainstem, optic chiasm, hypophysis, or cranial nerves with meaningful remaining function (see Figure 1 for example). All other lesions were classified as simple base of skull lesions (see Figure 2 for example). Table 4 gives the distribution of tumor volume, homogeneity index, new conformity index, and percentage target coverage for the simple and complex groups, respectively.
between the simple and complex groups (p = 0.0059) (Table 4). For the simple group, the mean tumor volume was 45.6 cm3. The mean tumor volume for the complex group was smaller at 12.5 cm3. Hence, we explored the relationship between target volume and radiosurgical indices using the CyberKnife® treatment planning system. To assess the association between the three radiosurgical treatment parameters (new conformity index, homogeneity index, and percentage of tumor coverage) and the target volume, scatterplots were constructed from the data obtained from all skull base tumors (Figure 3, 4, 5).
Overall, there is no statistically significant difference in homogeneity index, new conformity index and percentage target coverage between the two groups at the 5% level. There was a trend towards lower percent target coverage in the complex group, however this was not statistically significant. These data suggest that the CyberKnife® radiosurgical system generates acceptable plans independent of the proximity of adjacent critical structures to the target volume.
Simple linear regressions on the tumor volume for each of the three indices were then performed. The estimates of the slopes are given in Table 5. The estimated slopes for all indices are near zero. Pearson's correlation coefficients were also calculated as seen in Table 5. All Pearson correlation coefficients were less than ± 0.4 suggesting a poor correlation between the examined variables. Therefore, tumor volume does not appear to markedly effect radiosurgical parameters when using the CyberKnife® radiosurgical treatment planning system in our patient population.
Relationship between tumor volume and radiosurgical parameters Previous radiosurgical series have shown that radiosurgical indices can be influenced by target volume [9]. In our study, the mean tumor volumes differed significantly
Discussion
The CyberKnife® radiosurgical system has several advantages over conventional radiosurgical systems. Cranial
Page 5 of 10 (page number not for citation purposes)
154 94
Radiation Oncology 2006, 1:46
1A
http://www.ro-journal.com/content/1/1/46
1B
Figure (A) A 511year old woman presented with progressive hearing loss (A) A 51 year old woman presented with progressive hearing loss. An axial MRI of the brain After gadolinium administration demonstrated a left cerebellopontine angle acoustic neuroma. (B) Planning CT scan with IV contrast. The patient was treated with 2500 cGy to the 79% isodose line in five stages.
tracking, using skeletal anatomy to position the radiation beam, is as precise as frame-based approaches and eliminates the need for headframes [19]. In phantom studies, the system's precision has been shown to compare favorably to frame-based systems [20]. Its sub-millimeter clinical accuracy is due both to improvements in radiation delivery and target localization [21,22]. In addition, most LINAC and Gamma Knife systems use forward planning with user-selected arcs and beams. The CyberKnife® radiosurgical system employs inverse planning algorithms based on specific constraints to critical structures. In theory, inverse planning should allow for easily obtainable, optimized plans. The appropriate measure(s) of plan optimization is still debated [9]. Assessment of success in radiosurgery requires time for data to mature. But treatment-planning parameters, including conformity and homgeneity, can be assessed much earlier. In this study, we demonstrate that the CyberKnife® radiosurgical system generates plans with excellent conformity and homogeneity. Theoretically, improvements in conformity should improve local control and decrease complications in the treatment of skull base lesions with adjacent critical structures. These general
principles have found acceptance in the treatment of other sites with radiation therapy [23,24]. When irradiating complex skull base tumors that abut or displace critical normal structures the dose constraints to those normal structures may cause areas of under-dosing within the target volume. Of particular concern is that the resulting low dose regions within the tumor volume will increase the rate of local failure. In two radiosurgical series, the majority of local failures were due to tumor progression just outside the prescribed isodose volume [25,26]. At least one report in the literature has documented that increased conformity is paradoxically associated with poorer outcomes [27]. It has been suggested that improved conformity may lead to underdosing microscopic disease, not visible with current imaging modalities. However, in the study cited above, the poorer outcomes were likely due to the fact that conformity improves with increasing size of the lesion and is not related to an intrinsic and pure relationship between conformity and outcome. As logic dictates, increased rate of local failure is predicted to be dependent on both the dose minimum and the volume of this dose. Currently, percent target coverage is used as a surrogate for quantifying these
Page 6 of 10 (page number not for citation purposes)
15595
Radiation Oncology 2006, 1:46
http://www.ro-journal.com/content/1/1/46
2A
2B
Figure (A) A 772year old woman presented ten years after craniotomy for acoustic neuroma resection with deafness (A) A 77 year old woman presented ten years after craniotomy for acoustic neuroma resection with deafness. An axial MRI of the brain after gadolinium administration demonstrated radiographic progression of disease within the left internal acoustic meatus. (B) Planning CT scan with IV contrast. The patient was treated with 2500 cGy to the 84% isodose line in five stages.
low dose areas. In this study, percent target coverage was maintained across all groups. Longer follow-up is required to judge the effectiveness of this system in terms of local tumor control.
Dose homogeneity is a second measure by which radiosurgical plans are compared. The homogeneity index (HI), the maximum dose within the target volume divided by the prescription isodose (MDPD), is a com-
Table 4: Statistical Analysis Control Group I (simple) (n = 36)
Study Group (complex) (n = 46)
Difference of the means (95% CI)
p Value
Volume (cc) Mean Median
45.61 8.83
12.60 7.27
---
0.0059a
Homogeneity Index Mean Median
1.26 1.25
1.24 1.25
0.019 (-0.024, 0.063)
0.38a
New Conformity Index Mean Median
1.66 1.57
1.67 1.57
-0.007 (-0.155, 0.142)
0.93a
Percent Target Coverage (%) Mean Median
95.9 97.5
94.3 94.7
1.581 (-0.304, 3.466)
0.10b
a = t-test b = t-test for log transformed percent target coverage
Page 7 of 10 (page number not for citation purposes)
96 156
Radiation Oncology 2006, 1:46
http://www.ro-journal.com/content/1/1/46
NCI vs volume
Percent age tu mor coverage vs vo lume
NCI = 1.6857-0.0006*volume
% tumor coverage = 95.5292-0.0179*volume
2.8
102
2.6
100
2.4
98
% tumor coverage
new conformity index
96
2.2 2.0 1.8 1.6
94 92 90 88 86
1.4
84
1.2
82 80
1.0
78 -20
0.8 0
40
80
120
160
200
20 0
60 40
100 80
140 120
180 160
220 200
volume (cc)
volume (cc)
Figure New relation conformity 3analysis index versus volume scatter plot with corNew conformity index versus volume scatter plot with correlation analysis.
Figure 5analysis Percent relation target coverage versus volume scatter plot with corPercent target coverage versus volume scatter plot with correlation analysis.
monly used measure of dose homogeneity. The importance of dose homogeneity in radiosurgical outcomes is controversial. Inhomogeneous high central doses achieved with some radiosurgical treatment systems may provide improved local control [28]; however, this increased local control may come with an increased risk of
neurologic complications [29]. A homogeneity index of less than 2.0 is felt to balance the risk of local failure and neurologic injury (RTOG guidelines) [28]. Homogeneity indices less than 2.0 are especially important in treating large tumors or tumors in close proximity to critical structures [29]. Even though we did not place limitations on target volume or proximity of critical structures, we were able to obtain homogeneity indices less than 2.0 for every plan. Homogeneity of dose distributions for the CyberKnife® was favorable compared with devices using multiple isocenters which are typically 2.0. In the opinion of the authors, allowable target volumes and proximity to critical structures need to be determined in the context of the homogeneity index. Larger target volumes and smaller separation from critical structures may be acceptable for systems that consistently generate low homogeneity indices [5].
HI vs volume HI = 1.2375+0.0005*volume 1.7
homogeneity index (CI)
1.6 1.5 1.4 1.3
Abbreviations
1.2 1.1 1.0
0
40
80
120
160
200
volume (cc)
Figure Homogeneity tion analysis 4 index versus volume scatter plot with correlaHomogeneity index versus volume scatter plot with correlation analysis.
FDA, Federal Drug Administration; LINAC, Linear Accelerator; DVH, Dose Volume Histogram; NCI, New Conformity Index; HI, Homogeneity Index; PTV, Planning Treatment Volume; PTC, Percent Target Coverage; MRI, Magnetic Resonance Imaging; CT, Computed Tomography.
Competing interests
The author(s) declare that they have no competing interests.
Page 8 of 10 (page number not for citation purposes)
15797
Radiation Oncology 2006, 1:46
http://www.ro-journal.com/content/1/1/46
Table 5: Linear Regression Analysis: Radiosurgical Indices as a Function of Lesion Volume
Homogeneity Index New Conformity Index Percent Target Coverage (%)
y-intercept
Slope
Pearson's Correlation Coefficient
1.2377 1.6995 95.52
0.00054 -0.00076 -0.00030
0.3715 -0.1365 -0.3875
Authors' contributions
SC: Drafted the manuscript and participated in data analysis, prepared the manuscript for submission, created tables and results section NC: Drafted the manuscript and participated in data analysis, prepared the manuscript for submission, created tables and results section
11.
12.
13.
YZ: Biostatistical analysis
14.
BC: Participated in treatment planning and manuscript revision
15.
DM: Extracted data from treatment planning systems; manuscript revision WJ: Participated in treatment planning and manuscript revision; corresponding author
References 1. 2.
3. 4.
5.
6.
7. 8.
9. 10.
Van Havenbergh T, Carvalho G, Tatagiba M, Plets C, Samii M: Natural history of petroclival meningiomas. Neurosurgery 2003, 52(1):55-62; discussion 62-4. Condra KS, Buatti JM, Mendenhall WM, Friedman WA, Marcus RB Jr., Rhoton AL: Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys 1997, 39(2):427-436. Mathiesen T, Lindquist C, Kihlstrom L, Karlsson B: Recurrence of cranial base meningiomas. Neurosurgery 1996, 39(1):2-7; discussion 8-9. De Jesus O, Sekhar LN, Parikh HK, Wright DC, Wagner DP: Longterm follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery 1996, 39(5):915-9; discussion 919-20. Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA: Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys 2003, 55(4):1000-1005. Stafford SL, Pollock BE, Leavitt JA, Foote RL, Brown PD, Link MJ, Gorman DA, Schomberg PJ: A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2003, 55(5):1177-1181. Webb S: Conformal intensity-modulated radiotherapy (IMRT) delivered by robotic linac--testing IMRT to the limit? Phys Med Biol 1999, 44(7):1639-1654. Yu C, Jozsef G, Apuzzo ML, Petrovich Z: Dosimetric comparison of CyberKnife with other radiosurgical modalities for an ellipsoidal target. Neurosurgery 2003, 53(5):1155-62; discussion 1162-3. Lomax NJ, Scheib SG: Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys 2003, 55(5):1409-1419. Theodorou K, Platoni K, Lefkopoulos D, Kappas C, Schlienger M, Dahl O: Dose-volume analysis of different stereotactic radio-
16.
17.
18.
19. 20. 21. 22.
23.
24. 25.
26.
27.
therapy mono-isocentric techniques. Acta Oncol 2000, 39(2):157-163. Wagner TH, Yi T, Meeks SL, Bova FJ, Brechner BL, Chen Y, Buatti JM, Friedman WA, Foote KD, Bouchet LG: A geometrically based method for automated radiosurgery planning. Int J Radiat Oncol Biol Phys 2000, 48(5):1599-1611. Hamamoto Y, Manabe T, Nishizaki O, Takahashi T, Isshiki N, Murayama S, Nishina K, Umeda M: Influence of collimator size on three-dimensional conformal radiotherapy of the cyberknife. Radiat Med 2004, 22(6):442-448. Paddick I: A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 2000, 93 Suppl 3:219-222. Nedzi LA, Kooy HM, Alexander E 3rd, Svensson GK, Loeffler JS: Dynamic field shaping for stereotactic radiosurgery: a modeling study. Int J Radiat Oncol Biol Phys 1993, 25(5):859-869. Kubo HD, Wilder RB, Pappas CT: Impact of collimator leaf width on stereotactic radiosurgery and 3D conformal radiotherapy treatment plans. Int J Radiat Oncol Biol Phys 1999, 44(4):937-945. Nakamura JL, Verhey LJ, Smith V, Petti PL, Lamborn KR, Larson DA, Wara WM, McDermott MW, Sneed PK: Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys 2001, 51(5):1313-1319. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N: Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2000, 47(2):291-298. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr.: Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004, 363(9422):1665-1672. Adler JR Jr., Murphy MJ, Chang SD, Hancock SL: Image-guided robotic radiosurgery. Neurosurgery 1999, 44(6):1299-306; discussion 1306-7. Yu C, Main W, Taylor D, Kuduvalli G, Apuzzo ML, Adler JR Jr.: An anthropomorphic phantom study of the accuracy of Cyberknife spinal radiosurgery. Neurosurgery 2004, 55(5):1138-1149. Chang SD, Gibbs IC, Sakamoto GT, Lee E, Oyelese A, Adler JR Jr.: Staged stereotactic irradiation for acoustic neuroma. Neurosurgery 2005, 56(6):1254-61; discussion 1261-3. Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP: An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery 2003, 52(1):140-6; discussion 146-7. Leibel SA, Fuks Z, Zelefsky MJ, Hunt M, Burman CM, Mageras GS, Chui CS, Jackson A, Amols HI, Ling CC: Technological advances in external-beam radiation therapy for the treatment of localized prostate cancer. Semin Oncol 2003, 30(5):596-615. Ozyigit G, Yang T, Chao KS: Intensity-modulated radiation therapy for head and neck cancer. Curr Treat Options Oncol 2004, 5(1):3-9. Hakim R, Alexander E 3rd, Loeffler JS, Shrieve DC, Wen P, Fallon MP, Stieg PE, Black PM: Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery 1998, 42(3):446-53; discussion 453-4. Stafford SL, Pollock BE, Foote RL, Link MJ, Gorman DA, Schomberg PJ, Leavitt JA: Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery 2001, 49(5):1029-37; discussion 1037-8. DiBiase SJ, Kwok Y, Yovino S, Arena C, Naqvi S, Temple R, Regine WF, Amin P, Guo C, Chin LS: Factors predicting local tumor
Page 9 of 10 (page number not for citation purposes)
158 98
Radiation Oncology 2006, 1:46
28.
29.
http://www.ro-journal.com/content/1/1/46
control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 2004, 60(5):1515-1519. Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, Martin L: Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 1993, 27(5):1231-1239. Shaw E, Scott C, Souhami L, Dinapoli R, Bahary JP, Kline R, Wharam M, Schultz C, Davey P, Loeffler J, Del Rowe J, Marks L, Fisher B, Shin K: Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05). Int J Radiat Oncol Biol Phys 1996, 34(3):647-654.
Publish with Bio Med Central and every scientist can read your work free of charge "BioMed Central will be the most significant development for disseminating the results of biomedical researc h in our lifetime." Sir Paul Nurse, Cancer Research UK
Your research papers will be: available free of charge to the entire biomedical community peer reviewed and published immediately upon acceptance cited in PubMed and archived on PubMed Central yours — you keep the copyright
BioMedcentral
Submit your manuscript here: http://www.biomedcentral.com/info/publishing_adv.asp
Page 10 of 10 (page number not for citation purposes)
15999
INTRACRANIAL RADIOSURGERY CyberKnife radiosurgery for benign meningiomas: short-term results in 199 patients.
CYBERKNIFE RADIOSURGERY FOR BENIGN MENINGIOMAS: SHORT-TERM RESULTS IN 199 PATIENTS Federico Colombo, M.D. Stereotactic Radiosurgery Center, S. Bortolo City Hospital, Vicenza, Italy
Leopoldo Casentini, M.D. Stereotactic Radiosurgery Center, S. Bortolo City Hospital, Vicenza, Italy
Carlo Cavedon, Ph.D. Department of Medical Physics, S. Bortolo City Hospital, Vicenza, Italy
Paolo Scalchi, Ph.D. Department of Medical Physics, S. Bortolo City Hospital, Vicenza, Italy
Stefania Cora, Ph.D. Department of Medical Physics, S. Bortolo City Hospital, Vicenza, Italy
Paolo Francescon, Ph.D. Department of Medical Physics, S. Bortolo City Hospital, Vicenza, Italy Reprint requests: Federico Colombo, M.D., Stereotactic Radiosurgery Center, S. Bortolo City Hospital, Viale Rodolfi 37, 36100 Vicenza, Italy. Email: federico.colombo@ulssvicenza.it Received, April 4, 2008. Accepted, October 10, 2008. Copyright © 2009 by the Congress of Neurological Surgeons
OBJECTIVE: To present initial, short-term results obtained with an image-guided radiosurgery apparatus (CyberKnife; Accuray, Inc., Sunnyvale, CA) in a series of 199 benign intracranial meningiomas. METHODS: Selection criteria included lesions unsuitable for surgery and/or remnants after partial surgical removal. All patients were either symptomatic and/or harboring growing tumors. Ninety-nine tumors involved the cavernous sinus; 28 were in the posterior fossa, petrous bone, or clivus; and 29 were in contact with anterior optic pathways. Twenty-two tumors involved the convexity, and 21 involved the falx or tentorium. One hundred fourteen patients had undergone some kind of surgical removal before radiosurgery. Tumor volumes varied from 0.1 to 64 mL (mean, 7.5 mL) and radiation doses ranged from 12 to 25 Gy (mean, 18.5 Gy). Treatment isodoses varied from 70 to 90%. In 150 patients with lesions larger than 8 mL and/or with tumors situated close to critical structures, the dose was delivered in 2 to 5 daily fractions. RESULTS: The follow-up periods ranged from 1 to 59 months (mean, 30 months; median, 30 months). The tumor volume decreased in 36 patients, was unchanged in 148 patients, and increased in 7 patients. Three patients underwent repeated radiosurgery, and 4 underwent operations. One hundred fifty-four patients were clinically stable. In 30 patients, a significant improvement of clinical symptoms was obtained. In 7 patients, neurological deterioration was observed (new cranial deficits in 2, worsened diplopia in 2, visual field reduction in 2, and worsened headache in 2). CONCLUSION: The introduction of the CyberKnife extended the indication to 63 patients ( 30%) who could not have been treated by single-session radiosurgical techniques. The procedure proved to be safe. Clinical improvement seems to be more frequently observed with the CyberKnife than in our previous linear accelerator experience. KEY WORDS: CyberKnife, Meningioma, Stereotactic radiosurgery Neurosurgery 64:A7–A13, 2009
T
DOI: 10.1227/01.NEU.0000338947.84636.A6
oday, single-session stereotactic focused irradiation (radiosurgery) represents a generally accepted treatment alternative for intracranial meningiomas that are not suitable for surgical removal. Since its introduction into clinical practice in 1985, the procedure has proved to be safe and reliable. Reported results, in terms of clinical stabilization and tumor growth control, seem to be relatively independent of the machine used (either gamma knife or modified linear accelerator [LINAC]); many reports point to 5-year control rates of more
www.neurosurgery-online.com
than 95%, with a low or very low treatmentrelated complication rate. This rate of success represents the procedure’s “gold standard” (10–13, 17, 19–23, 26, 27, 31, 32, 40). Although the procedure has proved to be effective, some limitations and drawbacks are still present today. As a common feature of single-session radiosurgery, large tumors cannot be irradiated, owing to the increased risk of treatment-related complications. Moreover, long-lasting tumor control seems to be more difficult to attain (8, 21).
ABBREVIATIONS: CI, conformality index; CT, computed tomographic; DMAX, maximum dose; DPI, dose at the prescription isodose; HI, homogeneity index; LINAC, linear accelerator; mCI, modified conformality index; MRI, magnetic resonance imaging; PIV, prescription isodose volume; 3D, 3-dimensional; TV, tumor volume
NEUROSURGERY
160 100
VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT | A7
COLOMBO ET AL.
The CyberKnife (Accuray, Inc., Sunnyvale, CA) is a dedicated radiosurgery apparatus using a radiation source (a 6-MV LINAC) mounted on a frameless, image-guided, computer-operated robot (1, 2, 4, 5, 30). We thought that this apparatus could afford significant improvements in the procedure, and we used it in meningioma radiosurgery. The aim of this article is to describe the results obtained with the CyberKnife in intracranial meningioma radiosurgery and to discuss possible advantages connected to the peculiar features of this robotic radiosurgery system.
PATIENTS AND METHODS From January 2003 to December 31, 2007, 218 patients affected by extracerebral tumors of meningeal origin were treated in our center. This report deals with 199 patients affected by intracranial benign meningiomas that were deemed to be World Health Organization Grade I (16). Patients with Grade II (atypical) and III (malignant) meningiomas were excluded. In 85 patients, the diagnoses of benign meningiomas were based only on imaging appearance and clinical evolution (10, 21). The intended goals of the treatment were long-term prevention of tumor growth, maintenance of patient function and quality of life, and prevention of new neurological deficits (19, 22). Entry criteria for treatment were lesions unsuitable for surgery and/or remnants after partial surgical removal. All patients were either symptomatic and/or harboring growing tumors. Radiosurgery was offered to patients who refused open surgery (if feasible) and who asked for a less invasive treatment procedure. Radiosurgery was considered contraindicated in the presence of cerebral edema with significant mass effect and/or acute symptomatic compression of brainstem or optic pathways. For these patients, microsurgical decompression or removal was strongly suggested. Radiosurgical treatment was also considered futile in patients with long-lasting stable disease without any sign of clinical or radiological progression. Treatment planning was performed on contrast-enhanced magnetic resonance imaging (MRI) fused to the computed tomographic (CT) scan. Automatic target delineation was routinely used, whereas automatic delineation of critical structures (motor cortex and language areas) was used in 5 critically located meningiomas. In meningioma radiosurgery, the definition of target outline and nearby critical structures requires contrast-enhanced MRI; this imaging modality was routinely used except in patients with claustrophobia or other contraindications to the exposure of magnetic fields or gadolinium. Contrast-enhanced MRI is coregistered to the CT scan used for image guidance, and it is used for treatment planning with an original procedure that allows the evaluation of coregistration accuracy. The procedure was developed for using 3-dimensional (3D) rotational angiography in arteriovenous malformation radiosurgery (39). It can also be used for other pathologies, allowing fusion between different types of 3D imaging modalities, such as MRI, CT-positron emission tomography, and functional MRI (38). Once registered data sets have been imported into the CyberKnife treatment planning system, automatic delineation of the tumor contour can be performed, slice by slice, on axial sections of MRI scans and/or contrast-enhanced CT scans, using an automatic contouring tool with an appropriate threshold on voxel values that immediately delineate the target boundaries and reconstruct the tumor volume in 3D space. The same image registration procedure can be used for implementing functional MRI in treatment planning and for automatic contouring of critical regions (motor strip, language cortical areas, and so forth). The radiation dose to be delivered to the target volume and dose limits to critical structures are then decided, according to general radiosurgery experience. The optimal collimator dimension is selected accord-
A8 | VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT
ing to the volume and shape of the target, usually depending mainly on the minimum target cross sectional dimension. Finally, an inverse planning optimization procedure can be started. The CyberKnife G3 robotic arm can move the LINAC to 100 fixed positions (nodes), evenly spaced in a virtual hemisphere surrounding the target. From each of these nodes, 12 radiation beams with different angular directions can be used (a total of 1200 radiation beams). We used this apparatus until August 2007, when we upgraded our system to the G4 level. The CyberKnife G4 has 130 nodes, and the total number of usable beams is increased proportionally. In both apparatuses, inverse treatment planning determines the radiation dose and the angular direction of each individual radiation beam that are adequate for satisfying the dose prescriptions (1–5). Treatment planning is evaluated by standard radiobiological indexes (29, 35): CI TV/TVPIV (where CI is the conformality index, TV is the tumor volume, and TVPIV is the volume of the target covered by the prescription isodose); mCI TV PIV/TVPIV2 (where mCI is the modified conformality index, and PIV is the prescription isodose volume), and HI DMAX/DPI (where HI is the homogeneity index, DMAX is the maximum dose, and DPI is the dose at the prescription isodose). After physician evaluation and acceptance of the proposed solution, the irradiation procedure can be undertaken. The patient is fixed in the treatment position using a standard thermoplastic face mask immobilization system that limits the displacement of the head into the range in which the image-guided robot can correct for involuntary movements during the procedure. In cases of fractionated treatments, once the session is terminated, the face mask is stored and used again for subsequent fractions (1, 2, 4). Treated target volumes varied from 0.1 to 64 mL, and radiation doses ranging from 11 to 25 Gy were delivered at treatment isodoses of 70 to 90%. Single-session irradiation was used in patients with small tumors, at a safe distance ( 3 mm) from the brainstem or optic pathways. In patients with lesions larger than 8 mL and/or close to important critical structures, the dose was administered in 2 to 5 daily fractions. Prescription doses were calculated to be roughly equivalent to 11 to 12 Gy in a single fraction, considering an α/β ratio of 3 for meningiomas (1, 36). We always tried to reduce the dose absorbed by any portion of the anterior optic pathways to less than 7 Gy per session. In patients in whom the adjacent critical structure was impossible to locate, the structure was considered to be exposed to radiation doses equal to those delivered to the periphery of the tumor. The location of the point of maximal dose was always checked, and when it was found to be close to the surface of the tumor where the critical structure could be located, the plan was modified to move it to a more safe, central tumor location. Treatment planning for recurrences after failed radiosurgery was undertaken, taking into account the previous irradiation. Recurrences outside the previous target volume (outfield recurrences) were treated with the same doses as those used in this series. Tumors displaying failure of growth control inside the previously irradiated target (in-field recurrences) were treated with a slightly lower dose (15–20% less than that considered for first-time radiosurgery). Our follow-up protocol dictates MRI evaluation at 3, 6, 12, 18, and 24 months after treatment, and yearly controls thereafter. The clinical picture and visual field examination (for perioptic lesions) were assessed every 6 months after irradiation for the first 2 years, and every year thereafter. Tumor control was considered attained until the appearance of tumor volume increase and/or clinical worsening.
RESULTS The male-to-female ratio for this series was 56:143. The ages of the patients ranged from 15 to 83 years (mean, 55.8 years). Before CyberKnife treatment, 114 patients (57.3%) underwent
www.neurosurgery-online.com
161 101
CYBERKNIFE RADIOSURGERY FOR BENIGN MENINGIOMAS
CyberKnife radiosurgery for benign partial microsurgical removal. All patients were either symptomeningiomas matic and/or harboring growing tumors. Eight patients refused open surgery and asked for radiosurgery. Eleven recurrences after failed previous radiosurgery were also treated; 6 patients displayed unrestrained tumor progression after multiple-arc LINAC radiosurgery. Two patients had undergone previous gamma knife radiosurgery elsewhere. Three patients in the present series who showed tumor progression were re-treated 14, 18, and 30 months after the first CyberKnife radiosurgery. Regarding tumor location, large tumors frequently involved more than a single region, and precise anatomic landmarks were often trespassed. Nevertheless, the principal or initial site of growth was considered to be the cavernous sinus in 99 tumors; 28 grew from the dura of the posterior fossa, petrous bone, or clivus; and 29 abutted the anterior optic pathways coming from the dura of the orbit, planum sphenoidale, tuberculum sellae, or anterior clinoid process. Twenty-two tumors involved the dural convexity, and 21 involved the falx or tentorium. Treated target volumes varied from 0.1 to 64 mL (mean, 7.5 mL; median, 6.8 mL), and radiation doses ranging from 11 to 25 Gy (mean, 18.5 Gy) were delivered at the treatment isodose. Single-session irradiation was used in 49 patients with small tumors, at a safe distance ( 3 mm) from the brainstem or optic pathways. In 150 patients with lesions that were larger than 8 mL and/or close to important critical structures, the dose was administered in 2 (32 patients), 3 (76 patients), 4 (18 patients), and 5 (24 patients) daily fractions (Table 1). Treatment planning was evaluated by radiobiological indexes, as described in the previous section. The conformality index ranged from 1.01 to 1.48 (mean, 1.18), the modified conformality index ranged from 1.14 to 1.52 (mean, 1.29), and the homogeneity index ranged from 1.18 to 2.01 (mean, 1.35). Follow-up ranged from 1 to 59 months (mean, 30 months; median, 30 months). Clinical data are available for 191 patients (111 patients with follow-up longer than 24 months and 142 patients with follow-up longer than 12 months). We did not observe any kind of acute treatment-related neurological complications. Tumor volume decreased in 36 patients; in most patients, the observed decrease of volume was in the range of 10 to 25%. In only 3 patients was there an exceptional ( 50%) volume decrease; the most striking case is displayed in Figure 1. Tumor volume was unchanged in 148 patients and increased in 7
(tumor progression). Three patients underwent repeated radiosurgery, and 4 patients underwent operations. From a clinical point of view, 154 patients were unchanged. In 34 patients, a significant improvement in clinical symptoms (proptosis in 4, headache in 7, visual function in 2, oculomotor function in 10, and trigeminal pain in 9) was obtained. The onset of improvement appeared from 3 to 18 months after irradiation. In 6 patients, clinical deterioration was observed. Two patients with a superficial lesion had transient limited hair loss. One patient with tumor progression displayed new cranial deficits (facial nerve impairment Grade II and trigeminal dysesthesia). In 2 patients, diplopia worsened (1 with increased tumor volume). Two patients had visual field reduction (both with increased tumor volume), and 1 patient had worsened headache (Table 2). In all patients, except in the patient with new cranial nerve deficits, the worsening of the clinical picture could be related to direct radiation effects (0.5% complication rate). Treatment failures seem to be unrelated to the fractionation scheme (3 progressions in 49 patients treated with single-session irradiation, and 4 of 150 patients treated with 2–5 fractions) or to tumor dimensions (3 patients with failed control in tumors larger than the median, and 4 patients with tumors smaller than the median tumor volume of the entire series, 6.8 mL). Time to tumor progression was coded at the time of the first imaging study that showed tumor volume increase. KaplanMeier evaluation of the progression-free survival (15) demonstrated a 93.56% control rate at the 5-year follow-up point (Fig. 2).
DISCUSSION Indications for Surgery/Radiosurgery Although meningiomas have some characteristics that make them ideal targets for stereotactic radiosurgery (clear-cut, infiltration-free boundaries, ideal visibility on contrast-enhanced examinations, slow progression that allows time for radiosurgery effect), they were not considered among the possible indications by the pioneers of the method. At that time, surgery was considered the only way to deal with these benign tumors, and complete surgical resection with the associated dural base was considered the goal in every patient with relevant morbidity. The first meningioma patient treated by radiosurgery (an angioblastic meningioma of the cavernous sinus, recurrent after surgery) was reported by our group in 1985 (6, 7).
TABLE 1. Fractionation distributiona No. of fractions
a
No. of patients
Total dose, range (Gy)
BED, α/β 2 (Gy)
BED, α/β 3 (Gy)
1
49
11–13
71.5–97.5
51.3–69.3
2
32
14–17
63–89.2
46.6–65.1
3
76
16–20
58.4–86.6
44.2–64
4
18
18–23
58.5–89.1
45–67.8
5
24
19–25
64.1–87.5
49–66.7
BED, biological equivalent dose.
NEUROSURGERY
162 102
VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT | A9
COLOMBO ET AL.
A
B
FIGURE 1. A 28-year-old woman presented with a postoperative recurrence of a cavernous sinus meningioma (volume, 36 mL). The patient experienced diplopia and visual field reduction. Treatment was 20 Gy, conformal plan, delivered in 5 fractions. A, computed tomographic scans showing treatment
It was after publication of the landmark article by Kondziolka et al. (20) in 1991 that this indication became gradually more popular, and meningiomas now represent one of the more frequent indications for stereotactic irradiation. Interest in the use of stereotactic radiosurgery is proven by a large mass of publications dealing with the different aspects of this practice, starting with indications and finishing with results that are obtainable. Today, there are excellent articles on long-term experience in dealing with several hundreds of patients, the vast majority of them treated by gamma knife groups, but some of them treated by modified LINACs (10–13, 17, 19–23, 26, 27, 31–33, 40). The most controversial point of this practice, the relative indications for surgery and radiosurgery, has been the object of a long debate. Two of the most appreciated grading systems introduced by general neurosurgeons for predicting the extent of resection and the outcome of surgery strongly supported the view of as complete a removal as possible, and they defined, as limitations to this achievement, vessel encasement, cranial nerve involvement, unfavorable imaging, and, of course, previous radiosurgery (24, 32). Recently, an open attitude seems to be more prevalent (14).
A10 | VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT
plan. Isodose lines: 80% (prescription isodose), orange; 60%, violet; 30%, light blue. B, magnetic resonance imaging scans 24 months after treatment showing normal clinical appearance.
Regarding relative indications for microsurgical removal and radiosurgery and/or possible combination strategies using the 2 treatment modalities, a large number of reports have been presented. Today, there is a general agreement on a strategy of complete microsurgical removal when a low probability of complications exists and a judicious combination of microsurgery and radiosurgery when complete removal would likely be accompanied by a high rate of complications (18, 25). Good indications for surgery/radiosurgery have been defined as follows: for minimal lesions ( 2 cm) that are clinically and radi-
TABLE 2. Clinical evolution after radiosurgery Symptoms Ocular movements
No. of patients
Improved
Unchanged
Worsened
90
12
76
2
Visual function
65
2
61
2
Exophthalmos
36
4
32
0
Pain
37
16
20
1
www.neurosurgery-online.com
163 103
CYBERKNIFE RADIOSURGERY FOR BENIGN MENINGIOMAS
FIGURE 2. Graph showing progression-free survival of the entire series (199 patients) evaluated using the Kaplan-Meier method.
ologically stable: observation; for small ( 3 cm) tumors that are confined to the cavernous sinus and distant from the optic pathways and brainstem: primary radiosurgery; for large tumors ( 3 cm) that are attached to or compressing the optic pathways: radical surgery or decompresion, followed by radiosurgery; and for larger tumors associated with optic nerve encasement, neural cavernous infiltration, cranial base dural spreading (Sekhar Grades 4–6), or likely malignancy: surgical debulking, followed by adjuvant treatments (mainly fractionated radiation therapy) (25, 26). The introduction of the CyberKnife significantly impacts the tenets of radiosurgery indications. In the absence of symptomatic compression of the brainstem or optic pathways or acute mass effect, hypofractionated radiosurgery can safely be applied to lesions larger than 3 cm in diameter ( 13.5 mL). In our series, we treated a group of 63 patients who had lesions of more than 13.5 mL (34 patients), were lacking a safe distance ( 3 mm) from the optic pathways (29 patients), or both. In our opinion, it would have been impossible to treat these patients by means of our previous single-session, frame-based procedure without accepting a high risk of complications. With increasing experience, fractionated schemes were extended to tumors close to critical structures other than the optic pathways (brainstem; cranial nerves; and motor, visual, and language cortex). By use of 2 to 5 fractions, we have treated lesions measuring up to 65 mL without neurological complications and with a probability of consistent tumor control that is not significantly different from that obtainable in small ( 3 cm) meningiomas. The possibility of extending the limit on the volume of lesions treatable with radiosurgery may also simplify the task of the surgeon, who today must perform a potentially dangerous removal until only a small remnant is left, but tomorrow may be allowed to leave tumors of up to 4 to 5 cm to radiosurgery, once efficient debulking and critical structure decompression have been attained.
Tumor Control Long-lasting tumor control has been the main goal in meningioma radiosurgery since the beginning and, in the absence of
NEUROSURGERY
164 104
characteristic imaging modifications, the only way to assess the success of the treatment. Usually, progression-free survival, ascertained using the Kaplan-Meier method (15), has been used to plot the probability of tumor control in relation to time elapsed since radiosurgery. Recently, Nicolato et al. (28) proposed induced modifications in functional imaging (single-photon emission computed tomography with a 111In-labeled somatostatin analog) as a way to ascertain a positive response to treatment, but their interesting suggestions have not been followed thus far. As the 5- and 10-year progression-free percentage remains the main index for measuring radiosurgery success, 5-year control in recent literature seems to range from 87 to 98%, according to different authors (10–13, 17, 19–23, 26, 27, 31–33, 40). A selection of these outcomes is summarized in Table 3. Because of the restricted length of our follow-up periods, we can present only initial short-term results. With meningiomas, a longer follow-up period would be necessary to definitively ascertain treatment efficacy. However, the Kaplan-Meier evaluation of our series seems to point to 93% progression-free survival at the time of the 5-year follow-up evaluation. In these terms, the progression-free fraction seems to be only slightly inferior to the best gamma knife series. On the other hand, the CyberKnife has the obvious advantage of being a less invasive procedure, allowing the same precision as a frame-based technique (1, 2). Regarding tumor control, 2 factors must be taken into account to make an objective comparison. First of all, we usually treat lesions that are larger and closer to clinical structures than those usually selected for gamma knife treatment. Target volume seems to be the most important factor for predicting meningioma response to radiosurgery (8, 19). In all of our patients, the volumes of the targets were evaluated in 3D imaging data sets (usually contrast-enhanced MRI scans), and measurements were made on tumors delineated by the automatic method described previously (9). The automatic method avoids any operator variability and, in our experience, usually measures volumes that are smaller than those delineated manually. In our series, a relatively large number of patients harbored lesions greater than 10 mL in volume; such lesions are usually not considered good candidates for radiosurgery. DiBiase et al. (8) found 68% progression-free survival in patients with lesions of more than 10 mL, versus 91% in those with target volumes of less than 10 mL, and this difference was found to be statistically significant. In contrast, our results seems to be uninfluenced by tumor volume. The second issue to be taken into account is the fact that a large fraction (43%) of our patients were treated on a diagnosis of benign meningioma based only on imaging studies; consequently, histological verification was not available in these patients. The lack of histological verification may obscure the results of the procedure. This limitation, however, may only lead to a bias against radiosurgery, because of the presumption that all treated tumors were benign and that, on the other hand, patients harboring more aggressive tumors could not have been identified and excluded from evaluation. As demonstrated by Flickinger et al. (10), tumor control probability in imaging-diagnosed patients may be lower than that obtained in surgically verified benign meningiomas.
VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT | A11
COLOMBO ET AL.
TABLE 3. Summary of recently published series for meningioma radiosurgerya Series (ref. no.)
a b
System
No. of patients
Follow-up (mo)
PFS 5 y
Morbidityb
Roche et al., 2000 (32)
GKS
92
30 median
94%
2%
Nicolato et al., 2002 (27)
GKS
122
48 median
96%
2.5%
Pollock, 2003 (31)
GKS
330
43 mean
94%
8%
Kreil et al., 2005 (22)
GKS
200
94 median
98.5%
4.5%
Malik et al., 2005 (26)
GKS
309
96 median
87%
3%
Hasegawa et al., 2007 (13)
GKS
115
62 median
94%
5.5%
Kollová et al., 2007 (17)
GKS
368
60 median
97.9%
5.7%
Kondziolka et al., 2008 (21)
GKS
972
48 median
97%
7.7%
60 median
95%
9%
39 mean
96%
2.3%
Villavicencio et al., 2001 (40)
LINAC
56
Friedman et al., 2005 (11)
LINAC
210
Selch et al., 2004 (34)
FSRT
45
36 median
97.4%
2.1%
Hamm et al., 2008 (12)
FSRT
183
36 median
96.9%
3.8%
Adler et al., 1999 and 2006 (1, 2)
CKS
27
Current series
CKS
199
49 mean 30 median
— 93.5%
1% 0.5%
PFS, progression-free survival; GKS, gamma knife; LINAC, linear accelerator; FSRT, fractionated stereotactic radiotherapy; CKS, CyberKnife. Radiation-related permanent morbidity.
In 2 reported series of patients treated with fractionated stereotactic radiotherapy (with standard fractionation and the use of a relocatable frame), tumor control at 5 years seems to be slightly higher than that we were able to obtain in our patients (Table 3) (12, 34). In those series, large tumor volumes of up to 75 mL were treated (12). On the other hand, the frequency of reported adverse effects in those studies was also higher than what we observed in our series. At least a part of the complications could be a result of the inferior precision of relocatable head frames in comparison to that provided by standard head frames. Accurate positioning is also influenced by sustained, long-lasting patient cooperation. The correct position is measured at the beginning of each fraction, and there is no online verification during the treatment session, such as that provided by CyberKnife continuous image guidance and robot adjustments. Minor target shifts occurring during treatment cannot be ascertained and corrected.
Optic Tolerance The reported incidence of radiation-induced optic neuropathy in routine radiosurgical treatment of meningiomas may vary between 1.1 and 1.4% when a cutoff exposure dose of 11 Gy/ 15 mm3 in adults, and 8 to 9 Gy in children, is adopted (3, 37). This is confirmed by studies on fractionated radiation therapy isoeffective doses, given an α/β factor of 2.7 to 3.8 (36). The introduction of the CyberKnife may represent an important issue regarding generally accepted indications for radiosurgery. Cranial tracking, using skeletal anatomy to position the radiation beam, is as precise as frame-based approaches and eliminates the need for a head frame (1, 2). Consequently, it is possible to revert to hypofractionated regimens without deterioration of spatial accuracy and, in our
A12 | VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT
experience, without influence on the probability of tumor control. Fractionation has been proposed to decrease the risk of complications, especially in perioptic lesions, allowing the treatment of tumors that are in direct contact with optic pathways in patients in whom they are not clearly identifiable in images used for treatment planning (2). The small number of fractions possible with the CyberKnife (up to 5) has been advocated by Shrieve et al. (36) as an effective way to decrease visual complications in stereotactic radiosurgery of parasellar meningiomas. They calculated that using equivalent biological doses in a small number of fractions continues to exceed optic tolerances until at least 25 fractions are applied. In contrast to these data, Adler et al. (1) reported a clinical experience of 49 patients affected by perioptic lesions, 27 of them being meningiomas. With a mean follow-up period of 4 years ( 24 months in 2 patients), 38 patients (78%) remained stable, 8 patients (16%) improved, and 3 patients (6%) worsened, 2 of them for tumor progression (incidentally, both tumors were meningiomas). The real rate of treatment-related complications is consequently 2% (1 case). Our results seem to confirm that hypofractionation can not only decrease the risk of visual complications, but also improve, in a limited but significant number of patients, visual function, a result seldom observed after single-session radiosurgery.
CONCLUSION In relation to isocentric, single-session LINAC radiosurgery, which we used until 2003, the use of the CyberKnife expanded the indications for radiosurgery to include more than 30% of patients who could not have been treated by our frame-based procedure. From a clinical point of view, the follow-up period was too short to definitively evaluate the efficacy of the
www.neurosurgery-online.com
165 105
CYBERKNIFE RADIOSURGERY FOR BENIGN MENINGIOMAS
method. However, some conclusions can be drawn. We observed very few treatment-related complications (only 1 neurological), even in large tumors. Moreover, clinical improvement (pain, ocular movements, and, in a small number of patients, visual function) seemed to be more frequently observed. CyberKnife radiosurgery for meningiomas proved to be effective and safe. The tumor control rate was not significantly different for small and large tumor volumes or singleand multiple-session treatments.
Disclosure The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES 1. Adler JR Jr, Gibbs IC, Puataweepong P, Chang SD: Visual field preservation after multisession CyberKnife radiosurgery for perioptic lesion. Neurosurgery 59:244–254, 2006. 2. Adler JR Jr, Murphy MJ, Chang SD, Hancock SL: Image-guided robotic radiosurgery. Neurosurgery 44:1299–1307, 1999. 3. Carvounis PE, Katz B: Gamma knife radiosurgery in neuro-ophthalmology. Curr Opin Ophthalmol 14:317–324, 2003. 4. Chang SD, Adler JR Jr: Treatment of cranial base meningiomas with linear accelerator radiosurgery. Neurosurgery 41:1019–1027, 1997. 5. Collins SP, Coppa ND, Zhang Y, Collins BT, McRae DA, Jean WC: CyberKnife radiosurgery in the treatment of complex skull base tumors: Analysis of treatment planning parameters. Radiat Oncol 1:46, 2006. 6. Colombo F, Benedetti A, Pozza F, Zanardo A, Avanzo RC, Chierego G, Marchetti C: Stereotactic radiosurgery utilizing a linear accelerator. Appl Neurophysiol 48:133–145, 1985. 7. Colombo F, Pozza F, Chierego G: Linear accelerator radiosurgery: Current status and perspectives, in Lunsford LD (ed): Stereotactic Radiosurgery Update. New York, Elsevier, 1992, pp 37–46. 8. DiBiase SJ, Kwok Y, Yovino S, Arena C, Naqvi S, Temple R, Regine WF, Amin P, Guo C, Chin LS: Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 60:1515–1519, 2004. 9. Feigl GC, Samii M, Horstmann GA: Volumetric follow-up of meningiomas: A quantitative method to evaluate treatment outcome of gamma knife radiosurgery. Neurosurgery 61:281–287, 2007. 10. Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD: Gamma knife radiosurgery of imaging diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys 56:801–806, 2003. 11. Friedman WA, Murad GJ, Bradshaw P, Amdur RJ, Mendenhall WM, Foote KD, Bova FJ: Linear accelerator radiosurgery for meningiomas. J Neurosurg 103:206–209, 2005. 12. Hamm K, Henzel M, Gross MW, Surber G, Kleinert G, Engenhart-Cabillic R: Radiosurgery/stereotactic radiotherapy in the therapeutical concept for skull base meningiomas. Zentralbl Neurochir 69:14–21, 2008. 13. Hasegawa T, Kida Y, Yoshimoto M, Koike J, Iizuka H, Ishii D: Long-term outcomes of gamma knife surgery for cavernous sinus meningiomas. J Neurosurg 107:745–751, 2007. 14. Heros RC: Radiosurgery and neurosurgeons. J Neurosurg 103:203–205, 2005. 15. Kaplan E, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481, 1958. 16. Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK: The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61:215–229, 2002. 17. Kollová A, Liscák R, Novotný J Jr, Vladyka V, Simonová G, Janousková L: Gamma knife surgery for benign meningiomas. J Neurosurg 107:325–336, 2007. 18. Kondziolka D, Flickinger JC, Perez B: Judicious resection and/ or radiosurgery for parasagittal meningiomas: Outcomes from a multicenter review. Neurosurgery 43:405–414, 1998.
NEUROSURGERY
166 106
19. Kondziolka D, Levy EI, Niranjan A, Flickinger JC, Lunsford LD: Long term outcomes after meningioma radiosurgery: Physician and patient perspectives. J Neurosurg 91:44–50, 1999. 20. Kondziolka D, Lunsford LD, Coffey RJ, Flickinger JC: Stereotactic radiosurgery of meningiomas. J Neurosurg 74:552–559, 1991. 21. Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, Niranjan A, Flickinger JC: Radiosurgery as definitive treatment of intracranial meningiomas. Neurosurgery 62:53–60, 2008. 22. Kreil W, Luggin J, Fuchs I, Weigl V, Eustacchio S, Papaefthymiou G: Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry 76:1425–1430, 2005. 23. Lee JY, Kondziolka D, Flickinger JC, Lunsford LD: Radiosurgery for intracranial meningiomas. Prog Neurol Surg 20:142–149, 2007. 24. Levine ZT, Buchanan RI, Sekhar LN, Rosen CL, Wright DC: Proposed grading system to predict the extent of resection and outcomes for cranial base meningiomas. Neurosurgery 45:221–230, 1999. 25. Linskey ME, Davis SA, Ratanatharathorn V: Relative roles of microsurgery and stereotactic radiosurgery for the treatment of patients with cranial meningiomas: A single surgeon 4-year integrated experience with both modalities. J Neurosurg 102 [Suppl]:59–70, 2005. 26. Malik I, Rowe JG, Walton L, Radatz MW, Kemeny AA: The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg 19:13–20, 2005. 27. Nicolato A, Foroni R, Alessandrini F, Bricolo A, Gerosa M: Radiosurgical treatment of cavernous sinus meningiomas: Experience with 122 treated patients. Neurosurgery 51:1153–1161, 2002. 28. Nicolato A, Giorgetti P, Foroni R, Grigolato D, Pasquin IP, Zuffante M, Soda C, Tomasini A, Gerosa M: Gamma knife radiosurgery in skull base meningiomas: A possible relationship between somatostatin receptor decrease and early neurological improvement without tumor shrinkage at short-term imaging follow-up. Acta Neurochir (Wien) 147:367–375, 2005. 29. Paddick I: A simple scoring ratio to index the conformity of radiosurgical treatment plans. J Neurosurg 93 [Suppl]:219–222, 2000. 30. Pham CJ, Chang SD, Gibbs IC, Jones P, Heilbrun MP, Adler JR Jr: Preliminary visual field preservation after staged CyberKnife radiosurgery for peri-optic lesion. Neurosurgery 54:799–812, 2004. 31. Pollock BE: Stereotactic radiosurgery for intracranial meningiomas: Indications and results. Neurosurg Focus 14:e4, 2003. 32. Roche PH, Régis J, Dufour H, Fournier HD, Delsanti C, Pellet W, Grisoli F, Peragut JC: Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg 93 [Suppl]:68–73, 2000. 33. Saberi H, Meybodi AT, Rezai AS: Levine-Sekhar grading system for prediction of the extent of resection of cranial base meningiomas revisited: Study of 124 cases. Neurosurg Rev 29:138–144, 2006. 34. Selch MT, Ahn E, Laskari A, Lee SP, Agazaryan N, Solberg TD, CabatanAwang C, Frighetto L, Desalles AA: Stereotactic radiotherapy for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 59:101–111, 2004. 35. Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, Martin L; Radiation Therapy Oncology Group: Radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 27:1231–1239, 1993. 36. Shrieve DC, Hazard L, Boucher K., Jensen RL: Dose fractionation in stereotactic radiotherapy for parasellar meningiomas: Radiobiological considerations of efficacy and optic nerve tolerance. J Neurosurg 101 [Suppl]:390–395, 2004. 37. Stafford SL, Pollock BE, Leavitt JA, Foote RL, Brown PD, Link MJ, Gorman DA, Schomberg PJ: A study on radiation tolerance of the optic nerve and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 55:1177– 1181, 2003. 38. Stancanello J, Cavedon C, Francescon P, Causin F, Avanzo M, Colombo F, Cerveri P, Ferrigno G, Uggeri F: BOLD fMRI integration into radiosurgery treatment planning of cerebral vascular malformations. Med Phys 34:1176– 1184, 2007. 39. Stancanello J, Cavedon C, Francescon P, Cerveri P, Ferrigno G, Colombo F, Perini S: Development and validation of a CT-3D rotational angiography registration method for AVM radiosurgery. Med Phys 31:1363–1371, 2004. 40. Villavicencio AT, Black PM, Shrieve DC, Fallon MP, Alexander E, Loeffler JS: Linac radiosurgery for skull base meningiomas. Acta Neurochir (Wien) 143:1141–1152, 2001.
VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT | A13
FUNCTIONAL RADIOSURGERY CyberKnife radiosurgery as a first treatment for idiopathic trigeminal neuralgia
Laura Fariselli, M.D. Division of Radiotherapy, Fondazione Istituto Neurologico C. Besta, and Centro Diagnostico Italiano, Milan, Italy
Carlo Marras, M.D. Department of Neurosurgery, Fondazione Istituto Neurologico C. Besta, Milan, Italy
Michela De Santis, M.D. Division of Radiotherapy, Fondazione Istituto Neurologico C. Besta, Milan, Italy
Marcello Marchetti, M.D. Department of Neurosurgery and Division of Radiotherapy, Fondazione Istituto Neurologico C. Besta, Milan, Italy
Ida Milanesi, M.D. Division of Radiotherapy, Fondazione Istituto Neurologico C. Besta, Milan, Italy
Giovanni Broggi, M.D. Department of Neurosurgery, Fondazione Istituto Neurologico C. Besta, and Centro Diagnostico Italiano, Milan, Italy Reprint requests: Marcello Marchetti, M.D., Fondazione Istituto Neurologico C. Besta, Via Celoria 11, 20133 Milan, Italy. Email: marchettimarcello@gmail.com Received, May 25, 2008. Accepted, November 6, 2008. Copyright © 2009 by the Congress of Neurological Surgeons
CYBERKNIFE RADIOSURGERY AS A FIRST TREATMENT FOR IDIOPATHIC TRIGEMINAL NEURALGIA OBJECTIVE: To report the level of effectiveness and safety, in our experience, of CyberKnife (Accuray, Inc., Sunnyvale, CA) robotic radiosurgery as a first-line treatment against pharmacologically refractory trigeminal neuralgia. METHODS: We treated 33 patients with the frameless CyberKnife system as a monotherapy. The retrogasserian portion of the trigeminal nerve (a length of 4 mm, 2–3 mm anterior to the root entry zone) was targeted. Doses of 55 to 75 Gy were prescribed to the 100% isodose line, according to a dose escalation protocol. The patients were evaluated for the level of pain control, time to pain relief, hypesthesia, and time to pain recurrence. RESULTS: The median age was 74 years. All but 2 patients (94%) achieved a successful treatment outcome. The follow-up period was 9 to 37 months (mean, 23 months). The Barrow Neurological Institute Pain Intensity Scale (BPS) score before radiosurgery was III in 2 patients (6%), IV in 8 patients (24%), and V in 23 patients (70%). The time to pain relief was 1 to 180 days (median, 30 days). No facial numbness was observed. Only 1 patient developed a transitory dysesthesia of the tongue. After treatment, the BPS score was I, II, or III in 31 patients (97%). Pain recurred in 33% (11 patients) at a mean of 9 months (range, 1–43 months). Three patients with recurrences had low pain control by medication (BPS score, IV), and 1 patient (BPS score, V) needed a radiofrequency lesioning (BPS score, I at 12 months). CONCLUSION: CyberKnife radiosurgery for trigeminal neuralgia allows pain relief at safe doses and is suggested for pharmacologically refractory trigeminal neuralgia. Higher prescribed doses were not associated with improvement in pain relief or recurrence rate. KEY WORDS: CyberKnife, Facial pain, Radiosurgical rhizotomy, Stereotactic radiosurgery, Trigeminal neuralgia Neurosurgery 64:A96–A101, 2009
DOI: 10.1227/01.NEU.0000341714.55023.8F
T
rigeminal neuralgia (TN) is a chronic, episodic, and disabling facial pain syndrome affecting approximately 4.3 per 100 000 people annually (9). Most patients are treated initially with a variety of medications (e.g., carbamazepine, phenytoin, gabapentin, lamotrigine) up to the maximal dose they can tolerate. Pharmacological therapy is considered the appropriate initial modality; however, some patients do not respond or respond poorly. For those who do not receive adequate pain relief from medications or who cannot tolerate the side effects, surgical intervention is a feasible option, although the designation of the best surABBREVIATIONS: BNS, Barrow Neurological Institute Facial Numbness Scale; BPS, Barrow Neurological Institute Pain Intensity Scale; MRI, magnetic resonance imaging; TN, trigeminal neuralgia
A96 | VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT
www.neurosurgery-online.com
gical treatment for TN in the general population remains controversial. Surgical treatment options include craniectomy with microvascular nerve decompression or percutaneous procedures such as balloon compression, glycerol rhizotomy, or thermal radiofrequency rhizotomy. The application of radiosurgery to treat TN dates back to the 1950s when Lars Leksell (21) first used stereotactic radiosurgery to target the gasserian ganglion in humans, with encouraging results (28). Nowadays, stereotactic radiosurgery with the gamma knife (Elekta AB, Stockholm, Sweden) technique is a well-established option for the treatment of TN, and the reported series confirm the efficacy and safety of this modality (18, 24, 26, 28). The development of CyberKnife (Accuray, Inc., Sunnyvale, CA) radiosurgery technology, starting in 1994, introduced a new and effective
www.neurosurgery-online.com
167 107
CYBERKNIFE RADIOSURGERY FOR IDIOPATHIC TRIGEMINAL NEURALGIA
TABLE 1. Pretreatment pain distribution in a series of 33 patients Distribution
Score
No. of patients (%)
Description
I
No trigeminal pain, no medication
V1
1 (3%)
II
Occasional pain, not requiring medication
V2
4 (12.1%)
III
Some pain, adequately controlled with medication
V3
4 (12.1%)
IV
Some pain, not adequately controlled with medication
V1–V2
8 (24.3%)
V
Severe pain/no pain relief
V2–V3
9 (27.3%)
V1–V2–V3
7 (21.2%)
therapeutic option (1, 8). CyberKnife is an image-guided, frameless stereotactic radiosurgery system. It consists of a compact linear accelerator mounted on a 6-axis robotic arm that accurately delivers high doses of radiation and minimizes damage to nearby healthy or sensitive tissue. For intracranial targets, digitally reconstructed x-rays in concert with frequently acquired in situ x-ray images allow the tracking of head movements during the entire procedure without any invasive head immobilization (30). CyberKnife radiosurgery provides a homogeneous, nonisocentric, and conformal delivery of the radiation doses and permits the treatment of irregularly shaped targets, such as the trigeminal nerve. A small series reported on CyberKnife radiosurgery for TN showed a short-term response rate of 70% (29). Thus far, the long-term efficacy and the choice of appropriate doses necessary to obtain safe and stable pain control have not been established. On the basis of 3 sequential dose escalation protocols, the aim of the present study was to prospectively evaluate the efficacy and side effects of CyberKnife radiosurgery performed as first-line treatment of idiopathic drug-resistant TN.
PATIENTS AND METHODS Patients In the period between September 2004 and March 2006, 46 patients affected by idiopathic TN underwent CyberKnife radiosurgery. For this prospective study, we selected 33 patients who had not undergone previous surgical treatment. All of these patients were refractory to medication or experienced persistent side effects at the effective dose. The mean age at the time of treatment was 74 years (range, 56–90 years). The male-to-female ratio was 9:24. The Karnofsky Performance Scale score was 100 in 89% of patients and between 80 and 90 in 11%. The mean duration of symptoms was 6 years (range, 0.5–20 years). Twenty-two patients (67%) had right-sided TN, and 11 patients (33%) had left-sided TN; no patients had bilateral neuralgia. The pain distribution is reported in Table 1. Patients whose magnetic resonance imaging (MRI) scans suggested neurovascular compression were excluded. These data were always confirmed before treatment by MRI scans that included a fast imaging employing steady-state acquisition sequence and T1-weighted images with gadolinium.
Pretreatment Evaluation Before the CyberKnife treatment, all patients were clinically evaluated to determine their intensity of pain and facial numbness. Data on
NEUROSURGERY
168 108
TABLE 2. Barrow Neurological Institute pain intensity scorea
a
Pain intensity was assessed in relation to the patient’s drug consumption.
TABLE 3. Barrow Neurological Institute facial numbness scorea Score
a
Description
I
No facial numbness
II
Mild facial numbness, not bothersome
III
Facial numbness, somewhat bothersome
IV
Facial numbness, very bothersome
Facial numbness was assessed according to the patient’s subjective discomfort.
type, doses, and side effects of medications were also collected. Pain intensity and facial numbness were assessed according to the Barrow Neurological Institute Pain Intensity Scale (BPS) score (Table 2) and the Barrow Neurological Institute Facial Numbness Scale (BNS) score (Table 3). BPS and BNS scoring was performed before radiosurgery and at each follow-up examination (28). At presentation, 94% of the patients had BPS scores of IV or V. None of the patients had trigeminal hypesthesia. MRI and computed tomographic studies were performed to exclude neurovascular compression, demyelinating disease, or cerebellopontine lesions.
Follow-up Examinations After radiosurgery, patients were evaluated for pain intensity, time to pain relief, presence of facial numbness, or anesthesia dolorosa. Followup assessments were performed every 3 months after radiosurgery as outpatient visits or by telephone interviews. The first follow-up examination took place at 3 months, and the last available follow-up was referred to as the long-term follow-up. Clinical response was classified as excellent (pain-free or occasional pain without medication), good (pain relief with medication requirement), or mild (pain inadequately controlled by medication but improved after treatment). Recurrence was defined as a worsening of pain compared with baseline.
Treatment Planning All patients underwent 1.25-mm-thickness MRI scanning, including fast imaging employing steady-state acquisition and T1-weighted gadolinium sequences, as well as contrast-enhanced computed tomographic scans. The obtained images were fused to better define the intracisternal portion of the trigeminal nerve and to optimize the target definition. The target volume was usually defined as a 3- to 5mm segment of the trigeminal nerve (4 mm in 85% of cases), localized in the cisternal portion, 2 to 3 mm anterior to the dorsal root entry zone. Patients were treated in a uniform fashion according to 3 consecutive protocols with escalating dose regimens. The first 6 patients were treated with a maximum dose of 55 Gy (minimum tar-
VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT | A97
FARISELLI ET AL.
Clinical Response All but 2 patients achieved a complete or successful outcome (94%) (BPS score, I, II, or III) after treatment. An excellent response was observed in 27% of the patients at both the initial and long-term followup examinations. A good response was obtained in 61 and 42% of the patients at the first and last follow-up examinations, respectively. The rate of mild response was 6% at the first follow-up examination and 24% at the last follow-up examination (Fig. 2).
Overall Pain Relief Pain control (BPS score, I–III) had been achieved in 67% of the patients at the 2year follow-up examination.
Latency Period Time to pain relief ranged between 1 and 180 days (median, 30 days; 95% confidence interval, 21–39 days). Four patients (12%) reported immediate pain relief ( 24 hours). Seven patients (21%) in this series obtained pain reduction within the first week.
FIGURE 1. Radiosurgery isodose distribution including the anterior portion of the trigeminal nerve. The nerve close to the root entry zone was not included in the target volume. get dose, 44 Gy). As no adverse events were encountered in the 6 months after these first treatments, the maximum dose was increased to 65 Gy (minimum target dose, 52 Gy). Ten patients were then treated with 65 Gy, and no early or delayed side effects were observed. After September 2005, the prescription dose was increased to 75 Gy (minimum target dose, 60 Gy), and the next 17 patients were treated according to this protocol. The target was encompassed by the 80% isodose line, and the dose to brainstem was limited to 14 Gy (18–26% of the prescribed dose). The radiation dose prescription was calculated using an inverse treatment planning algorithm. A single-fraction treatment was delivered using a 5-mm collimator (Fig. 1).
Pain Recurrence Recurrence was defined as a worsening of the pain compared with baseline. Pain recurrence was experienced by 11 patients
Statistical Analysis Pain control response rate, recurrence, latency to pain relief, and toxicity were analyzed and correlated with the maximum dose. The probability of freedom from pain and median time to pain relief were estimated by Kaplan-Meier analysis, and pointwise confidence intervals were calculated.
RESULTS The follow-up period after CyberKnife radiosurgery ranged from 9 to 37 months (median, 23 months). One patient died from unrelated causes 10 months after treatment. One patient was lost to follow-up after 9 months. Before treatment, the BPS score was III in 2 patients (6%), IV in 8 patients (24%), and V in 23 patients (70%).
A98 | VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT
FIGURE 2. Graph showing clinical response after CyberKnife radiosurgery. The analysis was performed at 3 months and at the last available follow-up (minimum, 12 mo). FU, follow-up.
www.neurosurgery-online.com
169 109
CYBERKNIFE RADIOSURGERY FOR IDIOPATHIC TRIGEMINAL NEURALGIA
(33%). The median time to recurrence was 9 months (range, 1–43 months). Three of the 11 patients in the recurrence group had poor control of their pain by painkillers (BPS score, IV), and in 1 case (BPS score, V), percutaneous radiofrequency lesioning was needed to relieve the pain (BPS score, I at 12 months).
Toxicity Only 1 patient who was treated with the maximum dose of 75 Gy developed a transitory, partial dysesthesia of the tongue.
Univariate Analysis No correlation between pain control and the use of any of the protocols was found. The rate of freedom from posttreatment pain was 78 and 67% at 12 and 24 months, respectively (Fig. 3).
FIGURE 3. Graph showing percentage of pain-free patients after CyberKnife rhizotomy, according to Kaplan-Meier method.
DISCUSSION TN is a severe and frequently disabling facial pain syndrome. Pain may be confined to a small area or may spread throughout the distribution of 1 or more divisions of the trigeminal nerve. The features of the pain that characterize TN, other than its location, are its severity and lancinating or electric shock-like quality. Many procedures, including surgery and radiosurgery, have been used in an attempt to alleviate TN in patients in whom pharmaceutical treatment has failed to control the pain or has induced side effects. Surgical treatment is successful in 90% of patients, with a low rate of side effects and recurrences (2, 3). Surgical options include microvascular decompression, performed by suboccipital retrosigmoid craniectomy, and percutaneous procedures on the nerve at the level of the foramen ovale (balloon compression, glycerol rhizotomy, or thermal radiofrequency rhizotomy) (4–7, 16, 17). Radiosurgery is a noninvasive procedure based on the principle that large numbers of cross-fired beams target and injure the planned anatomic area specifically and selectively. In 1949, Leksell performed the first radiosurgical treatment of TN and reported positive long-term results (20–22). Since then, it has been possible to define the efficacy and safety of gamma knife stereotactic radiosurgery, and an increasing number of institutions have started to adopt this procedure to treat TN (18, 33). In the literature, the data on TN treatment by gamma knife show complete pain control in 35 to 76% of patients and recurrence rates of 6 to 34% 30 days after the procedure. Hypesthesia and numbness are the most common complications (in 10–50% of patients) and are related to the dose, the length of nerve treated, and the distance from the brainstem (19, 29, 31, 33).
NEUROSURGERY
170 110
Target definition in the radiosurgery of TN is still controversial and must take into account the pathophysiology of the disease, side effects related to irradiation of the midbrain, and the length of the treated trigeminal nerve (26, 32). Many radiosurgery groups have targeted the trigeminal segment close to the root entry zone; the rationale for this choice is based on both the concept of compression of the trigeminal nerve by a vascular structure at the root entry zone, and the higher radiosensitivity of this portion of the nerve (11, 14). Percutaneous surgical procedures have targeted the gasserian ganglion, which is situated 3 to 4 mm anterior to the root entry zone; the good results obtained using this procedure suggested that the root entry zone was not always involved and the whole retrogasserian portion could be considered to be involved in the pathophysiology of TN. To reduce the involvement of the brainstem, Kondziolka et al. (18, 19) recommended irradiating a target 3 mm anterior to the root entry zone, and the reported results showed a low rate of sensitivity deficit. This hypothesis was not, however, accepted by the entire radiosurgery community (13, 15) but nevertheless was followed by some CyberKnife users with encouraging results (29). One of the gamma knife’s radiosurgical limitations is its requirement of the stereotactic frame. CyberKnife radiosurgery, despite being a stereotactic and nonisocentric treatment modality, does not require skeletal fixation and allows easier treatment in multiple fractions of nonspherical structures such as the trigeminal nerve. Moreover, with gamma knife and ordinary linear accelerator systems, the target dose is heterogeneous and has to be shaped by the dosimetric overlapping of spherical volumes (1, 8, 10). CyberKnife treatment of TN is still recent, but the group of patients treated with CyberKnife is comparable to those patients treated with the gamma knife (23, 29). In our 3 sequential protocols of dose escalation, the dose ranged from 55 to 75 Gy at the reference isodose line of 100%; the targeted nerve length was 4 mm, and the posterior portion was 2 to 3 mm or more away from the root entry zone. In our series, the median time between CyberKnife radiosurgery and pain control was 30 days, and the rate of pain relief was similar to that reported by other groups. Moreover, the interval between gamma knife radiosurgery and pain control was similar to our results (19, 23, 32). Our data also showed that pain relief was not associated with trigeminal nerve impairment. In the Stanford series (23), pain relief was achieved within 24 to 72 hours in 70 to 93% of patients treated with CyberKnife radiosurgery. Furthermore, these results appeared to be stable after 1 year of follow-up. Lim et al. (23) reported a series of 41 patients affected by TN of various etiologies, treated by a dose ranging between 60 to 79.5 Gy to the 80% isodose line. The authors observed an onset of numbness in 51% of patients and a dose-dependent increase in this side effect. Three months after treatment, 96.6% of our patients had a positive response, and this result was independent of the prescribed dose. In this study, the univariate analysis power was limited by the small number of patients for each group, but this
VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT | A99
FARISELLI ET AL.
result is also in agreement with the experience of the Stanford group, which reported no statistically significant relationship between the prescribed dose and pain relief or recurrence rate. We also report a favorable set of long-term results; 1 and 2 years after treatment, pain control was achieved in 78 and 67% of the cases, respectively. The pain recurrence rate in our series was low (33%), and only 3 cases were identified as refractory to medication; no correlation with the prescribed dose was observed in these cases. The dosimetric values and the target planning performed by our group allowed treatment without early or delayed side effects; with this approach, we were able to treat the trigeminal fibers with less involvement of the brainstem and the root entry zone, which have been shown to be more radiosensitive (12). Our results with regard to side effects and complications are in contrast to those of earlier reports. All gamma knife series reported hypesthesia and numbness in 10 to 50% of patients (19, 29, 31). These side effects could be related to the target volume, the dose distribution homogeneity, and the use of 2 overlapping isocenters. The absence of hypesthesia and numbness in our series could be related to the fact that the length of the trigeminal nerve treated was less than 5 mm. Lim et al. (23) reported a statistically significant increase in the numbness score if the length of the trigeminal nerve treated was increased from 6 mm to a median of 8 mm. The absence of this common side effect in our patient cohort could be also related to the absence of any previous surgical treatments; thus far, this is the first prospective study on a consecutive drug-resistant series of patients who underwent CyberKnife radiosurgery as first treatment. Historically, radiosurgery has been indicated for patients who failed previous treatments or were not suitable for undergoing anesthesia. In our institution, radiosurgery is used in patients with pain that is refractory to medication or for whom surgical procedures are contraindicated. Currently, in our institution, microvascular decompression remains the “gold standard” treatment; however, the minimally invasive nature of radiosurgery seems more and more attractive for an increasing number of patients of all ages who are seeking surgical relief from pain. Our data and those of other groups (14) show that patients receiving radiosurgery as the first ablative intervention achieve good pain relief. Some other authors have also reported better pain relief after stereotactic radiosurgery as the first approach, as compared with results in patients who underwent previous surgical procedures. These results could be explained by a higher efficacy of radiosurgery on an intact nerve (25, 27). To confirm these hypotheses, a larger group of patients and longer follow-up periods are required. However, the data suggest the indication of CyberKnife radiosurgery in younger patients, particularly when MRI studies do not show any vascular compression of the trigeminal nerve (usually reported in 15% of cases) (34).
CONCLUSION We found CyberKnife radiosurgery to be safe and effective as a primary treatment for drug-resistant TN. CyberKnife radio-
A100 | VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT
surgery for TN allows pain relief at safe doses and is recommended for pharmacologically refractory TN.
Disclosure The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES 1. Adler JR Jr, Murphy MJ, Chang SD, Hancock SL: Image-guided robotic radiosurgery. Neurosurgery 44:1299–1307, 1999. 2. Apfelbaum RI: Advantages and disadvantages of various techniques to treat trigeminal neuralgia, in Rovit RL, Murali R, Jannetta PJ (eds): Trigeminal Neuralgia. Baltimore, Williams & Wilkins, 1990, pp 239–250. 3. Barker FG 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD: The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med 334:1077–1083, 1996. 4. Broggi G, Ferroli P, Franzini A, Galosi L: One size does not fit all: Choosing a treatment strategy for trigeminal neuralgia. Clin Neurosurg 52:283–291, 2005. 5. Broggi G, Ferroli P, Franzini A, Nazzi V, Farina L, La Mantia L, Milanese C: Operative findings and outcomes of microvascular decompression for trigeminal neuralgia in 35 patients affected by multiple sclerosis. Neurosurgery 55:830–839, 2004. 6. Broggi G, Franzini A, Giorgi C, Servello D, Brock S: Trigeminal neuralgia: New surgical strategies. Acta Neurochir Suppl (Wien) 58:171–173, 1993. 7. Broggi G, Franzini A, Lasio G, Giorgi C, Servello D: Long term results of percutaneous retrogasserian thermorhizotomy for “essential” trigeminal neuralgia: Consideration in 1000 consecutive patients. Neurosurgery 26:783–787, 1990. 8. Chang SD, Adler JR: Robotics and radiosurgery: The CyberKnife. Stereotact Funct Neurosurg 76:204–208, 2001. 9. Chang JW, Chang JH, Park YG, Chung SS: Gamma knife radiosurgery for idiopathic and secondary trigeminal neuralgia. J Neurosurg 93 [Suppl 3]: 147–151, 2000. 10. Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP: An analysis of the accuracy of the CyberKnife: A robotic frameless stereotactic radiosurgical system. Neurosurgery 52:140–147, 2003. 11. Dandy WE: Concerning the cause of trigeminal neuralgia. Am J Surg 24:447–455, 1934. 12. Flickinger JC, Kondziolka D, Lunsford LD: Dose and diameter relationships for facial, trigeminal, and acoustic neuropathies following acoustic neuroma radiosurgery. Radiother Oncol 41:215–219, 1996. 13. Gorgulho AA, De Salles AA: Impact of radiosurgery on the surgical treatment of trigeminal neuralgia. Surg Neurol 66:350–356, 2006. 14. Gorgulho A, De Salles AA, McArthur D, Agazaryan N, Medin P, Solberg T, Mattozo C, Ford J, Lee S, Selch MT: Brainstem and trigeminal nerve changes after radiosurgery for trigeminal pain. Surg Neurol 66:127–135, 2006. 15. Goss BW, Frighetto L, DeSalles AA, Smith Z, Solberg T, Selch M: Linear accelerator radiosurgery using 90 Gray for essential trigeminal neuralgia: Results and dose volume histogram analysis. Neurosurgery 53:823–830, 2003. 16. Hamlyn PJ: Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia. Review of the literature and development of a new method of vascular injection-filling in cadaveric controls. Clin Anat 10:371–379, 1997. 17. Jannetta PJ: Treatment of trigeminal neuralgia by micro-operative decompression, in Youmans JR (ed): Neurologic Surgery. Philadelphia, WB Saunders, 1990, ed 3, pp 3928–3942. 18. Kondziolka D, Lunsford LD, Flickinger JC, Young RF, Vermeulen S, Duma CM, Jacques DB, Rand RW, Régis J, Peragut JC, Manera L, Epstein MH, Lindquist C: Stereotactic radiosurgery for trigeminal neuralgia: A multi-institutional study using the gamma unit. J Neurosurg 84:940–945, 1996. 19. Kondziolka D, Perez B, Flickinger JC, Habeck M, Lunsford LD: Gamma Knife radiosurgery for trigeminal neuralgia: Results and expectations. Arch Neurol 55:1524–1529, 1998. 20. Leksell L: A stereotaxic apparatus for intracerebral surgery. Acta Chir Scand 99:229–233, 1949.
www.neurosurgery-online.com
171 111
CYBERKNIFE RADIOSURGERY FOR IDIOPATHIC TRIGEMINAL NEURALGIA
21. Leksell L: Stereotactic radiosurgery in trigeminal neuralgia. Acta Chir Scand 102:316–319, 1951. 22. Leksell L: A stereotaxic method for radiosurgery of the brain. Acta Chir Scand 37:311–314, 1971. 23. Lim M, Villavicencio AT, Burneikiene S, Chang SD, Romanelli P, McNeely L, McIntyre M, Thramann JJ, Adler JR: CyberKnife radiosurgery for idiopathic trigeminal neuralgia. Neurosurg Focus 18:E9, 2005. 24. Longhi M, Rizzo P, Nicolato A, Foroni R, Reggio M, Gerosa M: Gamma knife radiosurgery for trigeminal neuralgia: Results and potentially predictive parameters—Part I: Idiopathic trigeminal neuralgia. Neurosurgery 61:1254– 1261, 2007. 25. Lopez BC, Hamlyn PJ, Zakrzewska JM: Stereotactic radiosurgery for primary trigeminal neuralgia: State of the evidence and recommendations for future reports. J Neurol Neurosurg Psychiatry 75:1019–1024, 2004. 26. Massager N, Lorenzoni J, Devriendt D, Desmedt F, Brotchi J, Levivier M: Gamma knife surgery for idiopathic trigeminal neuralgia performed using a far-anterior cisternal target and a high dose of radiation. J Neurosurg 100:597–605, 2004. 27. Petit JH, Herman JM, Nagda S, DiBiase SJ, Chin LS: Radiosurgical treatment of trigeminal neuralgia: Evaluating quality of life and treatment outcomes. Int J Radiat Oncol Biol Phys 56:1147–1153, 2003. 28. Rogers CL, Speiser BL: Gamma Knife radiosurgery for trigeminal neuralgia: The initial experience of the Barrow Neurological Institute. Int J Rad Oncol Biol Phys 47:1013–1019, 2000.
29. Romanelli P, Heit G, Chang SD, Martin D, Pham C, Adler J: CyberKnife radiosurgery for trigeminal neuralgia. Stereotact Funct Neurosurg 81:105– 109, 2003. 30. Shaya M, Jawahar A, Caldito G, Sin A, Willis BK, Nanda A: Gamma knife radiosurgery for trigeminal neuralgia: A study of predictors of success, efficacy, safety, and outcome at LSUHSC. Surg Neurol 61:529–535, 2004. 31. Smith ZA, De Salles AA, Frighetto L, Goss B, Lee SP, Selch M, Wallace RE, Cabatan-Awang C, Solberg T: Dedicated linear accelerator radiosurgery for the treatment of trigeminal neuralgia. J Neurosurg 99:511–516, 2003. 32. Urgosik D, Liscak R, Novotny J Jr, Vymazal J, Vladyka V: Treatment of essential trigeminal neuralgia with gamma knife surgery. J Neurosurg 102 [Suppl]: 29–33, 2005. 33. Young RF, Vermeulen SS, Grimm P, Blasko J, Posewitz A: Gamma Knife radiosurgery for the treatment of trigeminal neuralgia: Idiopathic and tumor related. Neurology 48:608–614, 1997. 34. Zakrzewska JM, Lopez BC: Quality of reporting in evaluations of surgical treatment of trigeminal neuralgia: Recommendations for future reports. Neurosurgery 53:110–122, 2003.
Acknowledgments We thank Achille Bergantin, Ph.D., Livia Corinne Bianchi, M.D., Lorenzo Brait, Ph.D., Luisa Fumagalli, Ph.D., Anna Merlotti, M.D., and Marco Possanzini, M.D., for their care in the treatment of the patients.
INFORMATION FOR SUBSCRIBERS NEUROSURGERY (ISSN 0148-396x), a peer-reviewed journal, is a program of the Congress of Neurological Surgeons and is published monthly by Lippincott Williams & Wilkins, 16522 Hunters Green Parkway, Hagerstown, MD 21740 -2116. Business offices are located at 530 Walnut Street, Philadelphia, PA 19106-3621. Periodical postage paid at Hagerstown, MD, and at additional mailing offices. Copyright © 2007 by the Congress of Neurological Surgeons. POSTMASTER: Send address changes to NEUROSURGERY, P.O. Box 1550, Hagerstown, MD 21740. CNS MEMBERSHIP SERVICES Congress of Neurological Surgeons 10 North Martingale Road, Suite 190 Schaumburg, IL 60173 TEL: 847/240-2500; FAX: 847/240-0804 TOLL-FREE: 877/517-1CNS EMAIL: info@1cns.org
EDITORIAL CORRESPONDENCE Managing Editor, Rodrick A. Faccio NEUROSURGERY 1420 San Pablo Street, PMB A-106 Los Angeles, CA 90033 TEL: 323/442-3001; FAX: 323/442-3002 EMAIL: neurosurgery-journal@hsc.usc.edu
ANNUAL SUBSCRIPTION RATES UNITED STATES—$496.00 Individual, $715.00 Institution, $247.00 In-training; REST OF WORLD—$728.00 Individual, $969.00 Institution, $430.00 In-training; Single copy rate $71.00. (All prices include a handling charge.) Subscriptions outside of North America must add $23.00 for airfreight delivery. United States residents of AL, CO, DC, FL, GA, HI, IA, ID, IN, KS, KY, LA, MD, MO, ND, NM, NV, PR, RI, SC, SD, UT, VT, WA, WV add state sales tax. The GST tax of 7% must be added to all orders shipped to Canada (Lippincott Williams & Wilkins’ GST Identification #895524239, Publications Mail Agreement #617687). Subscription prices outside the United States must be prepaid. Prices subject to change without notice. (Visit us online at: www.lww.com ) Members of the Congress of Neurological Surgeons receive the journal as a benefit of membership. Please indicate in-training status and name of institution. Institutional rates apply to libraries, hospitals, corporations, and partnerships of three or more individuals. Subscriptions will begin with currently available issue unless otherwise requested. Copies will be replaced without charge if the publisher receives a request within 90 days of the mailing date. Individual and in-training subscription rates include print and access to the online version. Institutional rates are for print only; online subscriptions are available via Ovid. Institutions can choose to purchase a print and online subscription together for a discounted rate. Institutions that wish to purchase a print subscription, please con-
NEUROSURGERY
172 112
tact Lippincott Williams & Wilkins, 16522 Hunters Green Parkway, Hagerstown, MD 21740-2116; TEL: 800/638-3030 (outside the United States 301/223 -2300); FAX: 301/223-2400. Institutions that wish to purchase an online subscription or online with print, please contact the Ovid Regional Sales Office near you or visit: www.ovid. com/site/index.jsp and select Contact and Locations. ADDRESS FOR NON-MEMBER SUBSCRIPTION INFORMATION, ORDERS, OR CHANGE OF ADDRESS: Lippincott Williams & Wilkins, P.O. Box 1580, Hagerstown, MD 21741-1580; TEL: 800/638-3030 (outside the United States 301/223-2300); FAX: 301/223-2400. IN JAPAN, CONTACT: LWW Igaku-Shoin Ltd., 3-23-14 Hongo, Bunkyo-ku, Tokyo 113-0033; TEL: 81/3-5689-5400; FAX: 81/3-5689-5402. IN BANGLADESH, INDIA, NEPAL, SRI LANKA, AND PAKISTAN, CONTACT: Globe Publications Pvt. B-13, 3rd Floor, A Block, Shopping Complex, Naraina Vihar, Ring Road, New Delhi 110028, India; TEL: 91/11-579-3211; FAX: 91/11-579-8876. REPRINTS: Individual articles are available only from the authors. Reprints in large quantities, for commercial or academic use, may be purchased from the Publisher. For information and prices, call 800/341-2258.
BOUND VOLUMES FOR VOLUMES 58 & 59 will be available in early 2007 to subscribers only (U.S.—$253.00; FOREIGN—$271.00). Orders must be received by the Publisher before December 1, 2006. DISCLAIMER The statements and opinions contained in the articles of NEUROSURGERY are solely those of the individual authors and contributors and not of the CNS or LWW. The appearance of advertisements in the Journal is not a warranty, endorsement, or approval of the products or their safety. The CNS and LWW disclaim responsibility for an injury or property resulting from any ideas or products referred to in the articles or advertisements. INDEXING ABSTRACT SERVICES:The Journal is currently included by the following services in print and/or electronic format: Index Medicus, Current Contents/Life Sciences, Clinical Medicine, and Science Citation Index. COPYRIGHT & PERMISSIONS NEUROSURGERY is copyrighted by the CNS. No portion(s) of the work(s) may be reproduced without written consent from LWW. Permission to reproduce copies of articles for noncommercial use may be obtained from the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923; TEL: 978/7508400. Copyright © 2007 by the Congress of Neurological Surgeons.
VOLUME 64 | NUMBER 2 | FEBRUARY 2009 SUPPLEMENT | A101
JournAL oF APPLIEd CLInICAL MEdICAL PHYSICS, VoLuME 15, nuMBEr 1, 2014
Comparing GammaComparing Knife and CyberKnife in patients with brain metastases Gamma Knife
Terence T. Sio,1a Sunyoung Jang,2 Sung-Woo Lee,3 Bruce Curran,3 S. Sternick3 Anil P. Pyakuryal,4 Edward and CyberKnife
Department of Radiation Oncology,1 Mayo Clinic, Rochester, MN; Princeton Radiation Oncology,2 Monroe, NJ; Department of Radiation Oncology,3 Rhode Island Hospital, in patients with Providence, RI; Department of Physics,4 University of Illinois at Chicago, Chicago, IL Sio.Terence@mayo.edu
brain metastases
Received 21 June, 2012; accepted 12 August, 2013 The authors compared the relative dosimetric merits of Gamma Knife (GK) and CyberKnife (CK) in 15 patients with 26 brain metastases. All patients were initially treated with the Leksell GK 4C. The same patients were used to generate comparative CK treatment plans. The tissue volume receiving more than 12 Gy (V12), the difference between V12 and tumor volume (V12net), homogeneity index (HI), and gradient indices (GI25, GI50) were calculated. Peripheral dose falloff and three conformity indices were compared. The median tumor volume was 2.50 cm3 (range, 0.044–19.9). A median dose of 18 Gy (range, 15–22) was prescribed. In GK and CK plans, doses were prescribed to the 40–50% and 77–92% isodose lines, respectively. Comparing GK to CK, the respective parametric values (median ± standard deviation) were: minimum dose (18.2 ± 3.4 vs. 17.6 ± 2.4 Gy, p = 0.395); mean dose (29.6 ± 5.1 vs. 20.6 ± 2.8 Gy, p < 0.00001); maximum dose (40.3 ± 6.5 vs. 22.7 ± 3.3 Gy, p < 0.00001); and HI (2.22 ± 0.19 vs. 1.18 ± 0.06, p < 0.00001). The median dosimetric indices (GK vs. CK, with range) were: RTOG_CI, 1.76 (1.12–4.14) vs. 1.53 (1.16–2.12), p = 0.0220; CI, 1.76 (1.15–4.14) vs. 1.55 (1.18–2.21), p = 0.050; nCI, 1.76 (1.59–4.14) vs. 1.57 (1.20–2.30), p = 0.082; GI50, 2.91 (2.48–3.67) vs. 4.90 (3.42–11.68), p < 0.00001; GI25, 6.58 (4.18–10.20) vs. 14.85 (8.80–48.37), p < 0.00001. Average volume ratio (AVR) differences favored GK at multiple normalized isodose levels (p < 0.00001). We concluded that in patients with brain metastases, CK and GK resulted in dosimetrically comparable plans that were nearly equivalent in several metrics, including target coverage and minimum dose within the target. Compared to GK, CK produced more homogenous plans with significantly lower mean and maximum doses, and achieved more conformal plans by RTOG_CI criteria. By GI and AVR analyses, GK plans had sharper peripheral dose falloff in most cases. PACS number: 89.20.-a Key words: Gamma Knife, CyberKnife, dosimetry, dosimetric comparison, brain metastases I.
IntroduCtIon
Brain metastases significantly shorten the lives of cancer patients, with the majority of primary tumors originating from lung, breast, skin (melanoma), kidney, and gastrointestinal organs. It represents a significant clinical burden, with an incidence of at least 40% in advanced-stage cancer patients, and directly responsible for an estimated 20% of cancer deaths.(1) Economically, brain metastases represent a significant burden in total health-care expenditure for cancerrelated treatments.(2) a
14
122
Corresponding author: Terence T. Sio, Department of Radiation Oncology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA; phone: (507) 284 2511; fax: (507) 284 0079; email: Sio.Terence@mayo.edu 14
173
15
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
15
Brain metastases occur more commonly than primary brain tumors in adults.(3) A metropolitan study reported that 19.9% of lung cancer patients developed brain metastases, followed by melanoma (6.9%), renal (6.5%), breast (5.1%), and colorectal cancer (1.8%).(4) Stereotactic radiosurgery (SRS) is effective for palliating intracranial metastases, even from radio-resistant tumors such as melanoma.(5) Prognosis for patients with brain metastases remains very poor, typically with median survival ranges from 2.3–7.1 months.(6) Treatment options include expectant medical management, systemic chemotherapy, biological agents, surgery, whole-brain radiotherapy (WBRT), and local boost with SRS.(7) In patients with single brain metastasis, adding adjuvant WBRT after surgery decreased the rate of local recurrence.(8) However, up to 10% of patients receiving WBRT may experience cognitive deterioration, short-term memory loss, and radiation-induced dementia.(9) Increasingly, radiation oncologists and neurosurgeons prefer using local techniques, such as SRS and surgery, as first-line treatments in patients with oligometastatic brain tumors, while deferring WBRT as a salvage option. For patients with reasonable performance status and life expectancy, the American Society for Radiation Oncology (ASTRO) supports the use of WBRT with a radiosurgery boost to control up to four brain metastases. The combination of WBRT and SRS significantly improves survival in patients with single brain metastases.(10) For selected patients with good performance status and limited metastatic burden, treatment with SRS alone is a reasonable option. Stereotactic boosts can be carried out in several modalities, such as Gamma Knife (GK) (Elekta AB, Stockholm, Sweden), CyberKnife (CK) (Accuray Inc., Sunnyvale, CA), and various linac-based systems such as Novalis (BrainLAB, Feldkirchen, Germany). Other modalities include tomotherapy, proton radiotherapy, and volumetric-arc modulated therapy can also deliver SRS. Regardless of modality choice, RTOG 9005 established dose escalation schedule for brain metastases, based on diameter.(11) Doses vary from 15 to 24 Gy, and are inversely related to size (up to 40 mm) in order to minimize possibility of radiation necrosis. SRS also has a role in treatment of previously resected cavities of brain metastases.(12) In this study, two common SRS modalities (GK and CK) will be dosimetrically compared. Gamma Knife is probably the most well-known SRS system in the world. Brain metastases typically represent more than 50% of GK cases at any institution. The radiological concept of the GK system is fairly simple: it utilizes 201 concentrically placed Cobalt-60 energy sources to concentrate beams from different angles into a precisely defined spot inside the skull. The patient’s position is fixed by a rigid metal headframe, which allows for accuracy in beam delivery from many directions and a focused radiation dose. CK first obtained their FDA approval for therapeutic use in humans in 2001. Since then, there has been an expanding use of this versatile system worldwide. CK utilizes 6 MV photon beams produced by a compact linear accelerator, which in turn is mounted on a robotic arm with six degrees of translational and rotational freedom for spatial beam introduction. Stereotactically, CK relies on new assistive and adaptive technology called image-guided radiotherapy (IGRT) for tracking its target(s) both in space and time. Compared to GK, this technology allows CK to introduce a frameless treatment option for patients with brain metastases. CK delivers nonisocentric beams with a highly conformal dosing schedule and gives precision of beam delivery at submillimeter range by IGRT technologies.(13) As CyberKnife is still a relatively new technology, few direct comparison studies with other SRS systems have been published in the literature.(14) A recently published case-controlled study reported a detailed dosimetric comparison between the two modalities in patients with single brain metastases, but their survival analysis was confounded due to the CK patients receiving more modern chemotherapies.(15) In another retrospective series,(16) 25 patients with brain metastases from non-small cell lung cancer were either treated with GK or CK. A total of 56/58 (97%) lesions were successfully controlled.
Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
174
123
16
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
16
II. MAtErIALS And MEtHodS The authors performed a head-to-head, quantitative comparison of dosimetric profiles between the Leksell Gamma Knife C and CyberKnife robotic radiosurgery systems. Dual treatment plans based on 15 patients with 26 existing brain metastases were created and compared according to dosimetric parameters and indices. Difference in conformity, dose homogeneity, and peripheral dose falloff was also evaluated. This study explored the relative merits of dosing capacities and capabilities between the two SRS systems. From 5/27/2008 to 2/3/2010, 15 patients previously treated on the Leksell Gamma Knife C radiosurgery system were selected. The Institutional Review Board (FWA-00001230, IRB Registration #0004624) approved the study. These patients all had a deliverable GK plan produced by Leksell GammaPlan 8.3 (Elekta). The GK plans were generated according to an institutional protocol, with adherence to the RTOG guidelines and respecting critical organ constraints such as the optic chiasm and brain stem. For comparison, the Accuray treatment planning system MultiPlan DTS 3.0 was used for reproducing treatment plans previously delivered by the GK system. Identical stereotactic MRI images were transferred to CK, including weighted T2 & FLAIR sequences (5 mm thick), a T1-weighted sequence (5 mm thick), and axial/coronal 3D-MPRAGE sequences (2 mm thick). Computed tomography (CT) series was also acquired, which was fused with MR by manual seed point registration and algorithm-assisted translational and rotational steps (see Fig. 1). After quality fusion images were created, critical organ structures (also called volume of interest (VOI)) including spinal cord, brainstem, eyes, lens, optic nerve tracts, optic chiasm, and pituitary gland, were outlined in axial CT/MR images. These critical organ constraints were respected and must be met in the dose optimization process. The gross tumor volume (GTV) (also a VOI) was designated as target which matched the GK’s volume. The GTV’s location, target size, shape, and convexity were well-matched (< 5% volume difference in all duplicated lesions). A clinical objective list and relaxed convergence values for each individual step were then decided and carried out in a temporal order as predefined by a user’s script. Two additional hollow contour sets (shells), 3 mm and 30 mm away from the
Fig. 1. Overall, axial, coronal, and sagittal views (clockwise from top left) of CT/MR fusion in CK MultiPlan. Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
124
175
17
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
17
GTV, were created to optimize the dose distribution to normal tissues. The prescription isodose percentage was applied to optimize the GTV coverage to 97%–100%. The ray-tracing algorithm generated an initial beam set and began the sequential optimization process. Identical marginal dose prescription was given in each pair of comparative CK and GK plans. A high-resolution calculation step was performed in the evaluate step to finalize the CK treatment plan. In both GK and CK systems, the minimum, mean, and maximum doses were calculated and compared. The homogeneity index (HI) measured as the ratio of maximum dose over prescription dose, was also reported. V12 was the tissue volume receiving at least 12 Gy, and V12net was the difference between V12 and tumor volume. GI50 was the ratio of prescription isodose volume (PIV) to the isodose volume receiving half of the prescription dose, which is a commonly used index in comparing various rival plans.(17) PIV represents the three-dimensional volume which receives the prescription dose or more, as enclosed by the prescription isodose contour at that level. Dose-volume histogram (DVH) tables were extracted from both GK and CK planning software programs for peripheral dose falloff calculations. Conformity and homogeneity indices were calculated for all GK and CK plans. The Appendix summarizes the theory and dosimetric concepts employed in this study. III. rESuLtS Table 1 summarizes the patients’ demographics and tumor characteristics in this study. The median age was 63 years old. The primary tumor sites included lung (SCLC and NSCLC), breast, colorectal, skin (melanoma), and non-Hodgkin’s lymphoma. Most patients had an excellent Karnofsky Performance Score (KPS) at the time of receiving SRS (13 patients had documented KPS 80 or above, with one inpatient case having a KPS of 50). Five patients received prior surgical resections, with six resection cavities ranging 2.9–19.9 cm3. Three patients (two received total gross resections, one subtotal resection) underwent GK as a postoperative boost, three to four weeks after their initial surgeries. Two other patients received salvage GK as local recurrence developed. The 15 patients altogether presented with a total of 65 lesions, which were all treated by GK accordingly. Three patients had a total of 12, 12, and 9 lesions, respectively. Twenty-six Table 1. Demographics in the GK and CK comparison study (15 patients). n(%) or Median (range) Male
5 (33%)
Age
63 (30-80)
Primary Site SCLC NSCLC Colorectal Breast Melanoma NHL
1 (6.7%) 6 (40%) 2 (13%) 3 (20%) 2 (13%) 1 (6.7%)
WBRT prior to SRS
2 (13%)
Surgery prior to SRS Total resection Subtotal resection
4 (27%)a 1 (6.7%)
Number of brain metastases Karnofsky performance score
2 (1-12) 90 (50-100)
a Two cases presented as recurrence, for salvage consideration with SRS. GK = Gamma Knife; CK = CyberKnife; SCLC = small cell lung cancer; NSCLC = non-small cell lung cancer; NHL = non-Hodgkin’s lymphoma; WBRT = whole brain radiation therapy; SRS = stereotactic radiosurgery.
Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
176
125
18
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
18
representative lesions (including all surgically resected cases) were then selected and replanned in CK. They were well-distributed in both cerebral and cerebellar hemispheres, representing typical pattern of brain metastases (see Table 2). The excluded lesions were generally small (< 1 cm in diameter) and of less dosimetric interest. Special cases included one lesion in the right internal auditory canal and another one in the brainstem. Most of these lesions resembled spheres in shape, except for the resected cavities which appeared more irregular. A balanced distribution of tumor sizes was achieved: < 1 cm, 4 (15%); 1–1.99 cm, 11 (42%); 2–2.99 cm, 9 (35%), and 3–4 cm, 2 (8%). Basic parameters for both GK and CK dosimetric plans are shown in Table 3. All GammaPlan tumor volumes were reproduced well in CK’s MultiPlan. The shape and position of these tumors were well-preserved, with a pair-wise volume difference of no more than 5%. Same prescription dose was given to each pair of plans. The median prescription dose was 18 Gy (range 15–22 Gy), all delivered in 1 fraction. Due to intrinsic difference between GK’s isocentric and CK’s nonisocentric planning, prescription isodose percentage must be altered when cases were re-simulated in CK. In GK, radiation oncologists typically prescribe to an isodose line of 45%–55% for brain metastases. This is agreed by our data, which had a median prescription isodose percentage of 45%. For CK, nonisocentric planning allows user to prescribe to a higher percentage of isodose line. In our CK plans, the median prescription isodose level reached 86% (range 77%–92%), while a high level of coverage (median 98%, range 96%–100%) was maintained. This level of coverage by CK was comparable to GK in all cases. On average, eight isocentric shots per lesion were used in GK, while 75 beams per lesion were needed for a nonisocentric CK plan. The median values for minimum dose were 18.2 Gy and 17.6 Gy for GK and CK, respectively (p = 0.40, not significant). The median values for mean dose were 29.6 Gy (GK) and 20.6 Gy (CK); for maximum doses, 40.3 Gy (GK) and Table 2. Summary of tumor locations (26 lesions). n(%) Frontal
Left Right
4 (15%) 4 (15%)
Temporal
Left Right
3 (11%) 0 (0%)
Parieto-occipital
Left Right
1 (3.8%) 2 (7.7%)
Cerebellar
Left Right
3 (12%) 5 (19%)
Vermis
3 (12%)
Brain stem
1 (3.8%)
Table 3. Comparison of GK and CK plan parameters (26 lesions). GK median (range) (cm3)
Planned tumor volume Prescription dose (Gy) Prescription isodose % GTV coverage (%) Minimum dose (Gy) Mean dose (Gy) Maximum dose (Gy) Homogeneity index (HI)
CK median (range)
2.50 (0.044-1.99) 2.49 (0.053-20.0) 18.0 (15.0-22.0) 45 (40-50) 86 (77-92) 100 (96-100) 98 (96-100) 18.2 (12.8-26.7) 17.6 (13.0-21.4) 29.6 (19.9-38.5) 20.6 (16.1-25.8) 40.3 (29.4-51.0) 22.7 (16.5-28.6) 2.22 (2.00-2.50) 1.18 (1.09-1.30)
p value 0.94 N/A < 0.00001 0.99 0.40 < 0.00001 < 0.00001 < 0.00001
GK = Gamma Knife, CK = CyberKnife, N/A = not applicable, GTV = gross tumor volume. Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
126
177
19
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
19
22.7 Gy (CK). The differences for both mean and maximum doses reached statistical significance (p < 0.00001). The homogeneity index (HI) measures the ratio of maximum dose to prescription dose. Consequently, it often favors nonisocentric planning as employed by CK, as it yields a lower maximum dose and makes the overall plan more homogenous. HI is also inversely correlated to the prescription isodose percentage. The median values for HI were 2.22 and 1.18 for GK and CK plans, respectively (p < 0.00001). One lesion was excluded from this series of conformality index (CI) analyses (one patient had a central brainstem metastasis and did not tolerate wholebrain radiotherapy). He was given a palliative dose by GK, and his lesion was not covered entirely (coverage 82% only). Table 4 summarizes the various conformity indices applied in this study. From “loose” to “rigorous,” these indices may be ranked in this order: RTOG_CI, CI, and nCI. As more than 80% of evaluable lesions reached 100% coverage in GK plans, the three GK-related indices all had a median value of 1.76. For CK, an isodose line which yielded a coverage value of 97% was typically prescribed. From RTOG_CI to nCI, the CI values slowly increased (becoming less conformal), as coverage was taken into consideration. As a result, the CI and nCI comparisons were statistically insignificant (p ≥ 0.05), while the RTOG_CI index did reach statistical significance (p = 0.022). Adjusted for coverage, the CK’s conformity advantage diminished and became negligible in CI and nCI. Averaged volume ratio (AVR) and gradient index (GI) methods were calculated to evaluate dose falloff, which was commonly used in comparing various SRS modalities.(18) For both AVR and GI, the calculated percentages were normalized with respect to the prescription dose. With the same prescription dose, CK prescribes to a higher isodose line percentage. For example, an equivalent plan may prescribe 20 Gy to an 80% isodose line in CK, versus 50% isodose line in GK. The normalized 90%, 80%, 60%, 50%, 40%, 20%, and 10% isodose lines were calculated. Table 5 shows the AVR of different isodose volumes in relation to the prescribed isodose volume. Table 4. Summary of three comparative conformity indices.
RTOG CI CI nCI
GK median (range)
CK median (range)
p value
1.76 (1.12-4.14) 1.76 (1.15-4.14) 1.76 (1.59-4.14)
1.53 (1.16-2.12) 1.55 (1.18-2.21) 1.57 (1.20-2.30)
0.022 0.050 0.082
GK = Gamma Knife; CK = CyberKnife; RTOG CI = Radiation Therapy Oncology Group conformity index; CI = conformity index; nCI = new conformity index. Table 5. Summary of GK and CK peripheral dose falloff. Percenta [GTV] 100 90 80 60 50 40 20 10
Gamma Knife SRS median (range) AVR 2.50 (0.04-19.9) 4.40 (0.20-37.8) 5.35 (0.22-46.0) 6.40 (0.28-56.7) 9.75 (0.44-88.3) 12.9 (0.58-110.1) 17.0 (0.83-133.4) 35.8 (2.80-172.7) 49.6 (5.90-178.7)
0.57 1 1.21 1.48 2.30 2.99 4.05 8.03 11.05
CyberKnife SRS median (range) AVR 2.49 (0.05-20.0) 3.75 (0.08-42.3) 5.62 (0.13-81.5) 7.69 (0.22-112.2) 12.8 (0.57-173.7) 17.3 (0.90-213.0) 25.2 (1.54-264.9) 80.9 (5.56-522.3) 183.3 (14.4-1058.2)
0.66 1 1.55 2.15 3.80 5.21 7.59 23.49 64.88
p value 0.94 N/A < 0.00001 < 0.00001 < 0.00001 < 0.00001 < 0.00001 < 0.00001 < 0.00001
a
Percent refers to normalized levels (compared with 100) in relation to prescription dose, not actual isodose line percentage. SRS = stereotactic radiosurgery; GTV = gross tumor volume; AVR = averaged volume radio; N/A = not applicable. Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
178
127
20
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
20
As isodose line percentage decreases, more normal tissue will be included in the irradiating volume. In CK, a slower rate of falloff was observed. This difference appeared more significant at lower normalized isodose levels. For example, at normalized 20% isodose line, AVR was found to be 64.88 in CK (versus 8.03 in GK). Compared to GK, 15.46 more times of PIV were included by CK at this level. These differences were statistically significant across all levels (for normalized 90%, 80%, 60%, 50%, 40%, 25%, 20%, and 10%, p < 0.00001). Table 6 summarizes the results of V12, V12net, GI50, and GI25. Similarly, GK generated better plans compared with CK. GI50 and GI25 both reached statistical significance (p < 0.00001). There was a trend of smaller V12 and V12net volumes favoring GK. Additionally, a wide range of indices was noted in CK. For example, there is a wider range for CK’s GI50 (3.42–11.68) compared to GK (2.48–3.67). This effect was likely multifactorial, and may not be generalizable. The median “beams-on” times for GK and CK (extrapolated from monitoring unit) were 107 and 220 minutes, respectively. Table 6. Summary of GK and CK peripheral dose falloff.
V12 (cm3) V12net (cm3) GI50 GI25
GK median (range)
CK median (range)
p value
7.09 (0.44-64.60) 5.04 (0.40-44.70) 2.91 (2.48-3.67) 6.58 (4.18-10.20)
11.12 (0.57-127.5) 8.64 (0.52-107.7) 4.90 (3.42-11.68) 14.85 (8.80-48.37)
0.13 0.077 < 0.00001 < 0.00001
V12 = volume covered by 12 Gy isodose; V12net = volume covered by 12 Gy isodose, excluding gross tumor volume; GI = gradient index.
IV. dISCuSSIon Recently, a German study(15) reported a matched-pair analysis between 423 GK and 73 CK patients with single brain metastases. Compared to GK, the authors reported significantly lower numbers strongly favoring CK, including minimum dose, maximum dose, isodose line percentage used, PIV, CI (equivalent to RTOG_CI), HI, V10, and V10net. In contrast, we did not find a significant difference in minimum dose in our study. Also, our V12 and V12net (analogous to V10 and V10net) were higher in CK, not lower. A different range of CK isodose prescription percentage was used in the German study (67% ± 5%) vs. ours (85% ± 4%), which may account for some of the differences observed. All other dosimetric results and findings were similar or the same. Investigators have previously examined results from multicenter randomized trials which involved stereotactic radiosurgery as a boost. However, these conclusions were limited to subgroup analyses. In the RTOG 9005 final report, the authors found that patients treated with linac had a 2.84 times higher risk of local tumor progression, as compared to patients being treated with GK. This observation led them to suspect that “(GK) may have effectively boosted the central, hypoxic, more radioresistant portion of the tumor, accounting for the better local control … one possible explanation lies in the inherent inhomogeneity that exists in the dosimetry of GK radiosurgery”.(19) However, this was not seen in a later trial (RTOG 9508), which also included SRS boosts. No significant difference was observed in progression-free survival between GK and linac choices.(20) A multi-institutional analysis of 502 patients(21) also similarly concluded that GK versus linac did not seem to matter — a SRS boost increased median survival, regardless of modality choice, compared with patients only treated with WBRT alone (16.1, 10.3, and 8.7 vs. 7.1, 4.2, and 2.3 months for RPA classes I, II, and III, respectively, p < 0.05). CK was not included as a SRS option, as it is a relatively new modality. Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
128
179
21
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
21
Through measurement of various indices, CK appeared to produce more conformal plans in our series as compared to GK. The difference in RTOG_CI index was statistically significant. Modified (CI) and new conformality (nCI) indices barely missed statistical significance; however, due to limited sample size, the post hoc statistical power was low (47.2%). Some studies showed that SRS dosimetric conformality may relate to clinical outcome. For example, according to the Stanford experience with resected cavities,(12) the authors observed that higher conformality indices correlated with lower rate of local tumor recurrence. As a result, they recommended the use of a 2 mm margin when treating brain metastases postoperatively. Regardless of modality choice, an important motivation for optimizing gradient index is to prevent SRS complications. In the RTOG 9005 study, higher rates of CNS toxicity were noted in patients with larger size of tumors, which was the most important predictor for radionecrosis.(19) Other risk factors included increased volume receiving 10 Gy or more, higher radiation dose, repeated radiosurgical treatments to the same tumor, and increased size of erroneously irradiated normal brain tissue (i.e., a less conformal plan).(22-24) For GK, tissue volume enclosed by the 12 Gy isodose line also correctly predicted complication risk in patients with AVMs and other non-AVM intracranial tumors.(25,26) Our results showed significantly different falloff profiles between GK and CK, as evident in gradient index, AVR, V12, and V12net calculations. Recently, a joint study by UCSF and Princess Margaret Hospital (Canada) observed nearly identical dose falloff profiles among the GK, CK, and Novalis systems.(18) While our data matched well to that of their GK system, significant GI difference was observed in the CK series. The UCSF researchers noted a GI of 2.88 ± 0.82 with a prescription range of 49–78% (n = 10), while in our series, a GI of 4.95 ± 0.91 was observed with a prescription range of 77–92% (n = 26). We suspected that the use of a higher prescription isodose percentage may account for this difference. A more fundamental understanding of CK dosimetric properties will be needed in the future. Compared to GK, CK’s GI50 was poorer in our series. Two other studies from University of Southern California also observed that CK had a slower falloff as compared with GK.(27) However, in absolute scale, this difference was small and may not be clinically significant. For a hypothetical 2 cm diameter spherical target, the additional falloff distance as incurred by CK is only 2.6 mm in any direction at GI50. Magnitudes of V12 and V12net (or V10/V10net) correlated with chance of developing radionecrosis, an uncommon but feared complication of SRS.(22,24,26) V12 and V12net values are a function of homogeneity, conformity, prescription dose, and peripheral falloff. In our series, GK appeared slightly better, but it did not reach statistical significance. Our study has the following limitations: 1) did not address hypofractionation, which is possible for both modalities but more cumbersome for GK due to headframe immobilization; 2) was not designed to examine the clinical outcome and long-term control of brain metastases by various SRS modalities; 3) was not planned for analyzing the influence of patient and tumor characteristics on the conformality of the treatment modalities. V. ConCLuSIonS In patients with brain metastases, we showed that CK can create dosimetrically equivalent plans, as compared to GK. With similar coverage and minimum dose, both modalities effectively irradiated the entire tumor volume. Compared to GK, CK produced more homogenous plans with statistically lower mean and maximum doses. There is also a trend for CK being more conformal by RTOG_CI, CI, and nCI indices, with RTOG_CI reaching statistical significance. GK had a faster rate of dose falloff to the periphery in our series, as suggested by AVRs, V12, V12net, and GI. We suspect that this may due to a higher prescription isodose percentage in our planning with CK; further dosimetric investigation is warranted. Our results showed that ideal conformity and dose falloff may not always be easily and simultaneously achieved, which call Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
180
129
22
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
22
for further investigation. For example, a combined index taking into account of both conformity and dose gradient effect has recently been proposed.(17) In this project, we built a dosimetric foundation for systematically comparing various SRS modalities, which may be correlated with clinical outcome when combined with future studies. Our study provided preliminary insight in guiding future research and interdepartmental collaboration in considering whether GK or CK may be more suitable for the individual cancer patient with disabling brain metastases. Other factors, such as number and location of these lesions, patient’s preference for SRS and also whole brain radiotherapy, personal history of previous intracranial irradiation, and functional status of the patient, will also need to be considered. Future work may include multimodality dosimetric comparison, and also a detailed economic analysis in comparing GK, CK, a linac-based system with other emerging technologies such as RapidArc,(28) and proton therapies. rEFErEnCES 1. Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78(8):1781–88. 2. Bennett CL, Tigue CC, Fitzner KA. The economics of brain metastases. Cancer Treat Res. 2007;136:23–29. 3. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533–40. 4. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vignear FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–72. 5. Samlowski WE, Watson GA, Wang M, et al. Multimodality treatment of melanoma brain metastases incorporating stereotactic radiosurgery (SRS). Cancer. 2007;109(9):1855–62. 6. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–51. 7. Bradley KA and Mehta MP. Management of brain metastases. Semin Oncol. 2004;31(5):693–701. 8. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–89. 9. DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–96. 10. Mehta MP, Tsao MN, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005;63(1):37–46. 11. Shaw E, Scott C, Souhami L, et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05). Int J Radiat Oncol Biol Phys. 1996;34(3):647–54. 12. Soltys SG, Adler JR, Lipani JD, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70(1):187–93. 13. Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP. An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery. 2003;52(1):140–46. 14. Andrews DW, Bednarz G, Evans JJ, Downes B. A review of 3 current radiosurgery systems. Surg Neurol. 2006;66(6):559–64. 15. Wowra B, Muacevic A, Tonn JC. Quality of radiosurgery for single brain metastases with respect to treatment technology: a matched-pair analysis. J Neurooncol. 2009;94(1):69–77. 16. Mould R, Schulz R, Bucholz R, et al. Robotic radiosurgery, Vol 1. Sunnyvale, CA: The CyberKnife Society Press; 2005. p.104. 17. Paddick I and Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 2006;105 Suppl:194–201. 18. Ma L, Sahgal A, Descovich M, et al. Equivalence in dose fall-off for isocentric and nonisocentric intracranial treatment modalities and its impact on dose fractionation schemes. Int J Radiat Oncol Biol Phys. 2010;76(3):943–48. 19. Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–98. 20. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–72. 21. Sanghavi SN, Miranpuri SS, Chappell R, et al. Radiosurgery for patients with brain metastases: a multi-institutional analysis, stratified by the RTOG recursive partitioning analysis method. Int J Radiat Oncol Biol Phys. 2001;51(2):426–34. 22. Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94(6):899–904. Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
130
181
23
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
23
23. Nakamura JL, Verhey LJ, Smith V, et al. Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys. 2001;51(5):1313–19. 24. Valery CA, Cornu P, Noel G, et al. Predictive factors of radiation necrosis after radiosurgery for cerebral metastases. Stereotact Funct Neurosurg. 2003;81(1-4):115–19. 25. Borden JA, Mahajan A, Tsai JS. A quality factor to compare the dosimetry of gamma knife radiosurgery and intensity-modulated radiation therapy quantitatively as a function of target volume and shape. Technical note. J Neurosurg. 2000; 93 Suppl 3:228–32. 26. Korytko T, Radivoyevitch T, Colussi V, et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64(2):419–24. 27. Yu C, Jozsef G, Apuzzo ML, Petrovich Z. Dosimetric comparison of CyberKnife with other radiosurgical modalities for an ellipsoidal target. Neurosurgery. 2003;53(5):1155–62. 28. Clark GM, Popple RA, Young PE, Fiveash JB. Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. Int J Radiat Oncol Biol Phys. 2010;76(1):296–302. 29. Lomax NJ and Scheib SG. Quantifying the degree of conformality in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys. 2003;55(5):1409–19.
Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
182
131
24
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
24
APPEndICES Appendix 1: technical Summary of Conformity and Gradient Indices This session briefly summarizes the theories and rationale of using dosimetric indices in comparison of rival SRS plans. It involves the construction and application of two major concepts: conformity and gradient indices. Delivering conformal irradiation is a cornerstone of good radiotherapy practice. Medical physicists developed clinical and physical techniques in ensuring that the planned volume receiving the prescription dose can be best geometrically shaped in adapting to the target or tumor volume delineated on CT or MR imaging. This is especially important for stereotactic radiosurgery, for both ensuring accurate and efficacious radiation delivery (“hit the target”) and protecting the surrounding healthy tissues from excessive radiation injury (“do not harm the innocent”). The goal of stereotactic radiosurgery is to deliver extremely focused radiation beams to the chosen target, with submillimeter accuracy. It is possible to capture and compare the level of conformity quantitatively. In 1993, The Radiation Therapy Oncology Group (RTOG) proposed the first version of conformity index (also called the “RTOG CI”):
/
CIRTOG = PV TV
Eq.(1)
where PV is the total tissue volume which receives the prescription isodose, and TV is the actual tumor volume (equals to GTV when no additional margin is added). If CIRTOG is greater than 1, the prescription isodose line includes healthy tissue other than the tumor volume. If CIRTOG is less than 1, the tumor volume is under-irradiated. By RTOG standards, a treatment plan is acceptable if CI is between 1 and 2. A conformity index between 2 and 2.5, or 0.9 and 1, is a “minor” violation; an index less than 0.9 or more than 2.5 is considered a “major” violation. Researchers, however, soon discovered that this definition failed to take into account the level of overlapping, or spatial interaction, of the two volumes involved. For example, two identical volumes (PV, TV) which do not overlap with each other at all (i.e., target is entirely missed) will still receive a CI of 1, a number misleadingly indicating perfect conformation. It then became clear that information regarding coverage must be integrated. Here, coverage (CO) is volumetrically defined as
/
CO (Coverage) = TVPV TV × 100%
Eq. (2)
where TVPV is the volume of the tumor receiving at least the prescription dose or more. Traditionally, coverage is expressed in percentage. In order to accurately account for target coverage in conformity evaluation, a new index called healthy tissue conformity index (HTCI) was proposed by the Lomax and Scheib group.(29) The inverse of this index is reported by the CyberKnife’s MultiPlan. This CI is defined as:
/
CI = PV TV PV
Eq. (3)
which is sometimes also called the modified conformity index (mCI). However, this conformity index cannot detect under-treatment (e.g., a prescription volume to 1 cm sphere completely included in a 2 cm TV sphere will produce a deceptively perfect CI of 1, yet the coverage is only 12.5% by volume). A new CI was then used by CyberKnife, which could correct for this problem (the new CI can also be easily adapted to other stereotactic systems as well). This new CI may represent an improvement to the CI as defined in Eq.(3) because it balances the Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
132
183
25
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
25
proportion of the TV covered by the PV (TVPV/TV, an “under-treatment” ratio), with the proportion of the PV inside the tumor volume (TVPV/PV, an “over-treatment” ratio). The new CI can then be calculated as:
/
nCI = (PV ⋅ TV ) PV 2 TV
Eq. (4)
Here, the nCI is essentially the reciprocal product of the popular Ian Paddick’s index.(17) An algebraic examination reveals that the three conformality indices above can be interconverted through calculation of the coverage factor (Eq. (5)), which is a piece of information readily available in the normal tissue dose-volume histogram (DVH). This becomes the basis for numerical conversion, as GammaPlan does not automatically calculate CI or nCI. In particular, when coverage equals 100% (which were the majority of brain metastasis cases in GammaPlan), the three indices essentially become equal in value. This relationship can then be summarized as:
(/ )
(/ )
nCI = CI × 1 CO = CIRTOG × 1 CO
2
Eq. (5)
Finally, a volume-based gradient index (GI) can also give us more information about the quality of a SRS plan. An important goal of stereotactic radiosurgery is to ensure that the surrounding tissue gets as little-to-no radiation dose as possible (i.e., a rapid gradient of dose falloff must be created at the margin of the prescription isodose line). Ideally, all radiation should be contained within the prescription volume, and nothing else outside of the PV to periphery (i.e., no radiation “spilling”). A gradient index (GI)(17) provides a convenient method in measuring this intrinsic property of a SRS plan, which is independent of tumor volume and shape. The formula is: GI50 = V(p/2)%
/V
Eq. (6)
p%
where Vp% is the tissue volume enclosed by the prescription isodose line, and V(p/2)% is the volume enclosed by half (50%) of the prescribed dose. The advantages of using Eq. (6) are that: 1) these volumes are easily extractable from the DVH; and 2) it allows fair comparison across different rival plans which may not prescribe to same percentage of isodose lines. An optimal plan should enclose small volume even at their lower isodose lines. An analogous gradient index (GI25) at 25% prescription isodose line can then also be defined. GI25 is the ratio of V(p/4)%, the volume enclosed by one fourth of the prescription dose to Vp%. The homogeneity index (HI) is also a common measure for comparing rival SRS plans. HI is the ratio of the maximum dose to the prescription dose (maximum dose is always at 100% isodose in both CK and GK planning). Figure 2 gives a pictorial illustration of the dosimetric volumes as utilized in this project.
Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
184
133
26
Sio et al.: dosimetric comparison of Gamma Knife vs. CyberKnife
26
Fig. 2. A graphical presentation of dosimetric nomenclatures.
Journal of Applied Clinical Medical Physics, Vol. 15, no. 1, 2014
134
185
Int. J. Radiation Oncology Biol. Phys., Vol. 73, No. 4, pp. 1043–1048, 2009 Copyright 2009 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/09/$–see front matter
doi:10.1016/j.ijrobp.2008.05.059 Stereotactic body radiotherapy for localized prostate cancer: interim results of
a prospective phase II clinical trial CLINICAL INVESTIGATION
Prostate
STEREOTACTIC BODY RADIOTHERAPY FOR LOCALIZED PROSTATE CANCER: INTERIM RESULTS OF A PROSPECTIVE PHASE II CLINICAL TRIAL CHRISTOPHER R. KING, PH.D., M.D.,* JAMES D. BROOKS, M.D.,y HARCHARAN GILL, M.D.,y TODD PAWLICKI, PH.D.,* CRISTIAN COTRUTZ, PH.D.,* AND JOSEPH C. PRESTI, JR., M.D.y Departments of *Radiation Oncology and y Urology, Division of Urologic Oncology, Stanford University School of Medicine, Stanford, CA Purpose: The radiobiology of prostate cancer favors a hypofractionated dose regimen. We report results of a prospective Phase II clinical trial of stereotactic body radiotherapy (SBRT) for localized prostate cancer. Methods and Materials: Forty-one low-risk prostate cancer patients with 6 months’ minimum follow-up received 36.25 Gy in five fractions of 7.25 Gy with image-guided SBRT alone using the CyberKnife. The early (<3 months) and late (>6 months) urinary and rectal toxicities were assessed using validated quality of life questionnaires (International Prostate Symptom Score, Expanded Prostate Cancer Index Composite) and the Radiation Therapy Oncology Group (RTOG) toxicity criteria. Patterns of prostate-specific antigen (PSA) response are analyzed. Results: The median follow-up was 33 months. There were no RTOG Grade 4 acute or late rectal/urinary complications. There were 2 patients with RTOG Grade 3 late urinary toxicity and none with RTOG Grade 3 rectal complications. A reduced rate of severe rectal toxicities was observed with every-other-day vs. 5 consecutive days treatment regimen (0% vs. 38%, p = 0.0035). A benign PSA bounce (median, 0.4 ng/mL) was observed in 12 patients (29%) occurring at 18 months (median) after treatment. At last follow-up, no patient has had a PSA failure regardless of biochemical failure definition. Of 32 patients with 12 months minimum follow-up, 25 patients (78%) achieved a PSA nadir #0.4 ng/mL. A PSA decline to progressively lower nadirs up to 3 years after treatment was observed. Conclusions: The early and late toxicity profile and PSA response for prostate SBRT are highly encouraging. Continued accrual and follow-up will be necessary to confirm durable biochemical control rates and low toxicity profiles. 2009 Elsevier Inc. Prostate cancer, Stereotactic body radiotherapy, PSA, Hypofractionation.
INTRODUCTION
fractionated radiotherapy course (i.e., large dose per fraction) over a conventionally fractionated one (i.e., 1.8–2 Gy). The first study to suggest that prostate cancer possesses a radiobiology uniquely different from other cancers showed that one could quantify the sensitivity of prostate cancer to dose per fraction by comparing the dose response with permanent low-dose-rate brachytherapy to that from fractionated external beam (2). Using a standard radiobiologic model of dose response (the linear quadratic model), this study showed that prostate cancer possessed an unusually low a/b ratio of �1.5 Gy (i.e., a high sensitivity to dose per fraction). This a/b ratio is low compared with the value of �10 Gy for other cancers, and is also remarkably lower than that of late effects for normal tissues, where it is �3–5 Gy (3). The implications of such a high sensitivity to dose per fraction were
In the late 1960s through early 1980s, motivated primarily by economy of resources, a clinical program was open in the United Kingdom delivering hypofractionated radiotherapy for prostate cancer (36 Gy in six fractions over 3 weeks). Although staging was limited (this was the pre–prostatespecific antigen [PSA] era), radiotherapy techniques were simple (this was the pre–computed tomography era) and many of these patients had high-risk features by today’s criteria (e.g., bulky palpable disease or high grade), the update of that clinical experience with 22 years’ follow-up confirmed the long-term safety and potential effectiveness of this treatment (1). Modern understanding of the radiobiology of prostate cancer now offers a biologic rationale in favor of such a hypo-
Acknowledgments—The authors thank Dr. Deep Patel and Dr. Wendy Hara for their help with data collection during their residency in the early phases of this trial, Dr. John Adler for his exuberant encouragement over the years, and Dr. Jack Fowler and Dr. Dan Kapp for insightful discussions on radiobiology. Received April 4, 2008, and in revised form May 19, 2008. Accepted for publication May 27, 2008.
Reprint requests to: Christopher R. King, Ph.D., M.D., Department of Radiation Oncology, Stanford University School of Medicine, Stanford Cancer Center, 875 Blake Wilbur Drive, Stanford, CA 94305. Tel: (650) 736-0698; Fax: (650) 725-8231; E-mail: crking@stanford.edu Conflict of interest: none. 1043
186
135
immediately recognized, being that hypofractionation would be a more effective dose regimen for prostate cancer (4). Numerous studies have since followed the initial report of a low a/b ratio for prostate cancer. A recent review of 17 such studies estimated a mean a/b ratio of 1.85 Gy (5). There are four contemporary clinical series using external beam hypofractionated regimens, with dose per fraction ranging from 2.5 to 3.1 Gy (6–9) and one using a linac-based stereotactic body radiotherapy (SBRT) technique delivering 5 daily fractions of 6.7 Gy (10). There are also several series using high-doserate brachytherapy combined with conventionally fractionated external beam with dose-per-fraction ranging from 5.5 Gy to 11.5 Gy (11, 12) and one with high-dose-rate brachytherapy monotherapy delivering eight to nine fractions of 6 Gy each (13). These clinical series have uniformly demonstrated excellent biochemical control rates and low rectal and bladder toxicities with the use of hypofractionated radiotherapy. Fowler et al. (14) proposed several hypofractionated dose regimens for prostate cancer based on the assumption of a low a/b ratio. They showed that a significantly higher therapeutic ratio (i.e., simultaneous higher rates of tumor control rates and lower incidence of toxicities) could be achieved with these dose regimens. Although none is proposed as optimal, the gain in therapeutic ratio is proportional to the doseper-fraction size. In this report, we present our preliminary experience of an ongoing prospective Phase II clinical trial using SBRT for localized low-risk prostate cancer that delivers 36.25 Gy in five fractions of 7.25 Gy, focusing on the early and late rectal/bladder toxicities as well as the initial patterns of PSA response. METHODS AND MATERIALS Patient eligibility In December 2003, we began an institutional review board–approved Phase II clinical trial of hypofractionated SBRT for lowrisk prostate cancer. This is an ongoing prospective clinical program whose end points are the early and late urinary and rectal toxicities, quality of life measure, and patterns of PSA response. Eligible patients were newly diagnosed with biopsy proven prostate cancer presenting with low-risk features. The criteria for low-risk were a pre-biopsy PSA of 10 ng/mL or less, a biopsy Gleason Grade of 3+3 or lower, and a clinical T-stage T1c or T2a/b based on the American Joint Committee on Cancer, 6th edition (15). We allowed for a Gleason Grade of 3+4 if present in fewer than 2 of 10–12 core biopsies and involving less than 5 mm in aggregate tumor length. All biopsy grading was obtained at our institution. Patients with prior treatment (hormone therapy or transurethral resection of prostate) were excluded. An interim analysis was planned for when at least 40 patients had achieved a median follow-up >24 months with a minimum of 6 months. As of May 2008, there were 56 patients enrolled in this trial, with 41 satisfying the criteria for an interim analysis and who form the study population of this report. The median age was 66 years (range, 48–83 years). The median initial PSA was 5.6 ng/mL (range, 0.7–10; 1 patient enrolled with a PSA of 15.6 with Stage T1c, Gleason grade 3+3 involving 2 mm in 1/12 cores). There were 30 patients with clinical Stage T1c, 10 were T2a and one T2b. Biopsy Gleason grade was 3+3 in 29 patients and 3+4 in 12 patients.
136
Volume 73, Number 4, 2009
Treatment specifics The Cyberknife (Accuray Inc., Sunnyvale, CA) was used to deliver image-guided SBRT. Three gold fiducials were placed in the prostate via transrectal ultrasound guidance. A same-day computed tomography scan was obtained with patients in the supine position and in an alpha cradle, at 1.25-mm slice thickness and indexing. Anatomic contouring of the prostate, seminal vesicles, rectum, bladder, penile bulb, and femoral heads were done. Dose was prescribed to the planning target volume that consisted of a volumetric expansion the prostate by 5 mm, reduced to 3 mm in the posterior direction. For the prescription dose to cover 95% of the planning target volume, normalization was required to the 89–90% isodose line (i.e., the resulting dose heterogeneity was 10–11%). In Fig. 1, we show a typical dose–volume histogram. In arriving at an optimal treatment plan, great care was taken to respect the rectal tolerance, which is particularly important when delivering hypofractionated radiotherapy. Our rectal dose–volume histogram goals were <50% rectal volume receiving 50% of the prescribed dose, <20% receiving 80% of the dose, <10% receiving 90% of the dose, and <5% receiving 100% of the dose. The course of radiotherapy consisted of five fractions of 7.25 Gy for a total dose of 36.25 Gy. Treatments were given over 5 consecutive days for the first 21 patients and 3 times per week subsequently. From the linear quadratic equation, one can derive the equivalent biologic dose when given at 2 Gy per fraction (EQD2) from that of a hypofractionated course for any tissue or tumor type by the simple relationship: EQD2 = D[a/b +d]/[a/b+2], where D is the total dose given at dose d, the dose per fraction. Our hypofractionated dose regimen corresponds to a tumor EQD2 of 90.6 Gy (assuming an a/b of 1.5 Gy), a normal tissue late effect EQD2 of 74.3 Gy (assuming an a/b of 3 Gy), and an acute toxicity EQD2 of 52.2 Gy (assuming an a/b of 10 Gy).
Follow-up and toxicity scoring Patients were followed every 3 months with PSA and quality of life (QOL) questionnaires. All patients have baseline QOL data. The International Prostate Symptom Score(16) and Expanded Prostate Cancer Index Composite (17) validated questionnaires were used. Toxicity was also scored on the Radiation Therapy Oncology Group (RTOG) urinary and rectal toxicity scale (18). Available data are at baseline and are categorized as early (at 3 months) and as late (6 months and later).
100 80 Prostate PTV
Bladder
% Volume
I. J. Radiation Oncology d Biology d Physics
1044
60 Rectum
40
Rt femur
20
Lt femur 0
0
20
40
60
80
100
120
% Normalized Dose
Fig. 1. Dose–volume histogram achieved with the CyberKnife for a typical prostate cancer patient. Dose is normalized to cover the 95% of the prostate planning target volume.
187
Stereotactic body radiotherapy for localized prostate cancer d C. R. KING et al. 8
10 8
6
6
PSA (ng/mL)
Median PSA ± SEM
1045
4 2
4
2
0 0
6
12
18
24
30
36
42
48
Months after RT
Fig. 2. Prostate-specific antigen (PSA) response plotted as median PSA as a function of time after SBRT. The error bar indicates 1 standard deviation from the mean (SEM).
RESULTS PSA response The median follow-up was 33 months (range, 6–45 months). The patterns of PSA response after completion of SBRT show a gradual decline. Figure 2 shows the median PSA as a function of time after radiation therapy. To date, no patient has experienced a PSA failure regardless of the biochemical failure definition used. The median PSA nadir was 0.32 ng/mL (range, 0.03–2.65). We present in Table 1 the proportion of patients achieving a given PSA nadir threshold at 1, 2, and 3 years after RT. We note that 25 of 32 patients (78%) with 12 months’ minimum follow-up achieved a PSA nadir #0.4 ng/mL. It is also worth noting that a greater proportion of patients continue to achieve a given nadir threshold as a function of time up to 3 years. A benign PSA bounce (defined here as a PSA rise of 0.2 ng/mL or more above its previous nadir with a subsequent decline to that nadir or lower) was observed in 12 patients (29%). The median time to PSA bounce was 18 months (range, 12–33 months) after RT and the median bounce height was 0.39 ng/mL (range, 0.2–2.47). We present in Fig. 3 the typical pattern of PSA for 3 patients experiencing a benign bounce. Note that two to three bounces occur during the follow-up time. Urinary/rectal toxicity and QOL In Table 2, we summarize the patient self-reported urinary and rectal QOL score at baseline, 3 months, 1 year, and 2 Table 1. Proportion of patients achieving a given PSA nadir threshold as a function of time after SBRT % achieving PSA nadir by follow-up time PSA nadir
At 1 y (32 patients)
At 2 y (17 patients)
At 3 y (15 patients)
#1 ng/mL #0.6 ng/mL #0.4 ng/mL #0.2 ng/mL
53% 31% 19% 9%
70% 70% 53% 6%
93% 87% 67% 40%
Abbreviations: PSA = prostate-specific antigen; SBRT = stereotactic body radiotherapy.
188
0
0
6
12
18
24
30
36
42
48
Months after RT
Fig. 3. Benign prostate-specific antigen (PSA) bounce as seen in 3 representative patients. Note that a PSA bounce can occur several times during the available follow-up.
years after completion of SBRT. Two patients reported ‘‘terrible’’ (QOL score 6) urinary symptoms at 3 months and were those patients who reported ‘‘mostly dissatisfied/unhappy’’ (QOL score 4–5) at baseline. We note that, although the urinary QOL scores deteriorated somewhat at 3 months, they recovered and in fact improved over baseline at 1 and 2 years, with more than 90% of patients reporting QOL scores below 3. The rectal QOL became worse at 3 months, but never reached the ‘‘big problem’’ QOL score. With 89% of patients reporting ‘‘no problem’’ (QOL score 1) at baseline, about half were still reporting ‘‘very small/small problem’’ (QOL score 2–3) at 1 and 2 years, suggesting a residual of long-term, lowlevel rectal symptoms. In Table 3, we report the late urinary and rectal toxicities on the RTOG scale. We note that 2 patients (5%) experienced Table 2. Urinary QOL from IPSS and rectal QOL from EPIC, as a function of time after SBRT % with given QOL score, urinary* QOL score (IPSS) 0–1 2–3 4–5 6
Baseline
3 months
1y
2y
51% 41% 8% —
37% 58% — 5%
44% 52% 4% —
92% 8% — —
% with given QOL score, Rectal** QOL score (EPIC) 1 2–3 4 5
Baseline
3 months
1 year
2 years
89% 11% -
37% 48% 16% -
46% 50% 4% -
45% 45% 9% -
Abbreviations: QOL = quality of life; IPSS = international prostate symptom score; EPIC = expanded prostate cancer index composite; SBRT = stereotactic body radiotherapy. * Urinary QOL (IPSS) scale: 0–1 (delighted/pleased); 2–3 (mostly satisfied/mixed); 4–5 (mostly dissatisfied/unhappy); 6 (terrible). ** Rectal QOL (EPIC) scale: 1 (no problem); 2–3 (very small/ small problem); 4 (moderate problem); 5 (big problem)
137
1046
I. J. Radiation Oncology d Biology d Physics
Table 3. Late urinary and rectal toxicity on the RTOG scale after prostate SBRT RTOG grade 0
I
II
III
IV
Urinary, late toxicity 30% (11) 41% (15) 24% (9) 5% (2) — % (no. patients) Rectal, late toxicity 51% (20) 33% (13) 15% (6) — — % (no. patients) Abbreviations: RTOG = radiation therapy oncology group; SBRT = stereotactic body radiotherapy.
Grade 3 urinary toxicity and no patient suffered any Grade 4 urinary toxicity. There were no Grade 3 or 4 rectal toxicities. Finally, we compare the late urinary and rectal QOL among patients treated over 5 consecutive days (QD) with those treated QOD in Table 4. Having noted a mildly disappointing, albeit still acceptable, rate of rectal toxicity with a QD regimen among the first 21 patients, we altered our trial to be delivered in an every-other-day (QOD) regimen. Although this issue was not part of the intended analysis, it does provide an interesting point for discussion and a possible future trial design. Although there were no significant differences in low-level urinary or rectal QOL at baseline, we note that the QD group had more patients who scored small problems compared with the QOD group. A significant improvement in high-grade rectal toxicities was noted in the QOD group. No patient in the QOD group reported a QOL score 4–5, for either any individual rectal symptom or for the overall QOL, whereas in the QD group 8 patients (38%) reported a score of 4–5 for any individual rectal symptom (p = 0.0035) and 5 patients (24%) reported a score of 4–5 for the overall QOL (p = 0.048). For the urinary QOL, there was no significant difference between the QD and QOD groups (p = 0.34), although 4 patients reported a score of 4–6 for the QD group compared with 1 for the QOD group. DISCUSSION Urinary/rectal toxicity and QOL The outcomes from this clinical trial demonstrate that a hypofractionated course of stereotactic radiotherapy for local-
Volume 73, Number 4, 2009
ized prostate cancer is associated with urinary and rectal toxicities that are of the expected nature and severity as those experienced with conventionally fractionated courses of external beam radiotherapy. There was no severe urinary toxicity (RTOG Grade 4), and the 2 patients who experienced the worst problems were those who at baseline had described their urinary QOL as ‘‘mostly dissatisfied/unhappy.’’ Interestingly, after peaking at around 3 months, most patients returned to near-baseline levels of urinary satisfaction, and many have in fact improved above baseline levels at 2 years. An increase in the use of medications (e.g., alpha-blockers) could be an explanation for this observation, although we cannot exclude the possibility that it results from a late response from hypofractionated radiation therapy. We have not prospectively tracked the usage and timing of alpha-blockers in relation to urinary symptoms to be able to draw a more definitive conclusion. Our trial has now been modified to do so. We compare our late urinary and rectal toxicities with that from the MD Anderson (MDA) dose-escalation trial that delivered 78 Gy in 2 Gy per fraction using three-dimensional conformal techniques (19). These toxicity rates are summarized in Table 5 for comparison. Although the incidence of low-grade (RTOG Grades 1 and 2) late urinary toxicity is about double that observed from the MDA dose-escalation trial, it has not resulted in a significant degradation of patients’ urinary QOL. An evolving refinement of our technique to improve the dosimetry with the CyberKnife by using a urethral ‘‘tuning’’ structure to limit the dose heterogeneity from encroaching onto the urethra will likely lessen this toxicity in the future. In comparing our results, we note that the MDA data are of a much longer median follow-up time of 8.7 years. In addition, their study showed an actuarial increase in toxicity with time achieving a plateau at around 5 years (19). Because our follow-up is much shorter, we must remain cautious about the interpretation of our late urinary and rectal toxicities because it is fully expected that these will continue to appear at least up to 5 years after treatment. We also note that comparison with the MDA results assumes that our patients had a similar baseline QOL profile as theirs. There were no severe rectal toxicities (RTOG Grade 3 or 4) observed. A decline in patients’ rectal QOL score appears to
Table 4. Comparison of late urinary and late rectal toxicity from EPIC between consecutive daily treatments (QD) and those delivered every other day (QOD)
Urinary Overall urinary QOL score 4–6 (‘‘mostly dissatisfied’’ or ‘‘unhappy’’ or ‘‘terrible’’) Baseline urinary QOL score 3 (‘‘mixed’’) Rectal Any itemy score 4–5 (‘‘moderate’’ or ‘‘big problem’’) Overall rectal QOLz score 4–5 (‘‘moderate’’ or ‘‘big problem’’) Baseline rectal QOL score 2 (‘‘very small problem’’)
QD
QOD
19% (4/21) 38% (8/21)
5% (1/20) 15% (3/20)
38% (8/21) 24% (5/21) 19% (4/21)
0% (0/20) 0% (0/20) 0% (0/20)
p value* 0.34 0.16 0.0035 0.048 0.11
Abbreviations: QOL = quality of life; EPIC = expanded prostate cancer index composite. * Fisher’s exact test (two-tailed). y Urgency, frequency, control, bloody stool, pain. z Bowel habits (at 6 months or later).
138
189
Stereotactic body radiotherapy for localized prostate cancer d C. R. KING et al.
Table 5. Late urinary and rectal toxicity on the RTOG scale from the University of Texas MD Anderson (MDA) doseescalation trial, 78 Gy at 2 Gy per fraction group (19) RTOG grade 0
I
II
III
IV
Urinary, late toxicity 76% (114) 14% (21) 7% (11) 3% (5) — % (no. patients) Rectal, late toxicity 47% (71) 28% (42) 19% (28) 7% (10) — % (no. patients) Abbreviation: RTOG = radiation therapy oncology group.
plateau around 3 months after RT, persisting at the ‘‘very small/small problem’’ up to 2 years after radiation therapy. No significant difference in the incidence of low-grade rectal toxicity (RTOG Grades 1 and 2) was observed when compared with the MDA dose-escalation trial. QD vs. QOD Our data allowed us to study differences in late toxicities between patients treated over 5 consecutive days (QD) and those treated every other day (QOD). A significant improvement was observed for late rectal problems when treatment was given QOD, where 0/20 patients reported a score of 4 or 5 compared with 8/21 patients when treated QD (p = 0.0035) for any rectal symptom, and 0/20 vs. 5/21, respectively, for overall rectal QOL (p = 0.048). Although fewer patients experienced a QOL score 4–6 for late urinary problems with QOD vs. QD treatment, 1/20 vs. 4/21, it was not significant (p = 0.34). The apparent improvement in rectal toxicity with QOD vs. QD regimen, if real, is interesting for what it suggests about the repair kinetics of hypofractionated radiation damage to the rectum. The data for late bladder and rectal toxicity suggests a repair half-life of 1 h (e.g., reference 20). Thus after 24 h, the repair of sublethal damage is complete (it should be nearly complete after five half-lives) and no further gain (or reduced toxicity) would be observed with a longer interval between dose fractions. One possible explanation of our observations is for a much longer repair half-life, on the order of at least 8 h, because repair is incomplete by 24 h but approximately complete by 48 h. This seems unlikely because it is inconsistent with previous data on repair kinetics. Other possible explanations are that either we are seeing the effects of normal tissue repopulation of rectal mucosa or that late damage actually results from vascular injury. Although these are only hypotheses, it is possible
1047
that either a separate mechanism of repair for late rectal effects or a different nature of radiation damage is present with hypofractionation. We are cautious about overinterpreting this data, but given our observations, we favor treating with a longer interval between fractions for hypofractionated dose regimens. We note that only a randomized trial would be able to properly study differences between QD and QOD SBRT regimens. PSA response The patterns of PSA response from our trial are highly encouraging. It is interesting to note the high proportion of patients (78%) with 12 or more months of follow-up achieving a low PSA nadir of 0.4 ng/mL. It is also worth noting that the PSA nadir achieved is progressively lower as time goes by, up to 3 years. This continued late PSA response after radiation therapy for prostate cancer is well-known and is consistent with the radiation biology of prostate cancer behaving similarly to that of late effects in normal tissues. What if our radiobiologic hypothesis for prostate cancer is wrong and that it in fact possesses an a/b ratio that is similar to other tumors (i.e., 10 Gy)? In that case, the tumor dose from our hypofractionated regimen, EQD2 = 52 Gy, would be seriously inadequate. An estimate of the 5-year biochemical control rate based on the dose response for low-risk prostate cancer is predicted to be only 40% for 52 Gy, as opposed to 90% for 78 Gy. Although our follow-up time is relatively brief, we have not observed a biochemical failure so far. We have also shown that a benign PSA bounce was present after hypofractionated radiation therapy at roughly the same frequency, timing, and magnitude as has been described after permanent brachytherapy (21) or after external beam radiotherapy (22). CONCLUSION This study suggests that hypofractionated radiotherapy for localized prostate cancer has an early and late toxicity profile no worse than with dose-escalated radiotherapy delivered at conventional fractionation. The favorable biochemical response observed supports the radiobiologic assumption on which the rationale for prostate cancer hypofractionation is based. Continued pursuit of this trial seems warranted but with reasonable caution however, because longer follow-up will be necessary to confirm durable biochemical control rates and low late toxicity profiles.
REFERENCES 1. Lloyd-Davies RW, Collins CD, Swan AV. Carcinoma of prostate treated by radical external beam radiotherapy using hypofractionation. Twenty-two years’ experience (1962–1984). Urology 1990;36:107–111. 2. Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999; 43:1095–1101. 3. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys 2004;60:1013–1015.
190
4. Duchesne GM, Peters LJ. What is the a/b ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 1999;44:747–748. 5. Dasu A. Is the a/b value for prostate tumours low enough to be safely used in clinical trials? Clin Oncol 2007;19:289–301. 6. Yeoh EE, Holloway RH, Fraser RJ, et al. Hypofractionated vs. conventionally fractionated radiation therapy for prostate carcinoma: Updated results of a phase III randomized trial. Int J Radiat Oncol Biol Phys 2006;66:1072–1083.
139
1048
I. J. Radiation Oncology d Biology d Physics
7. Lukka H, Hayter C, Julian JA, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol 2005;23:6132–6138. 8. Kupelian PA, Willoughby TR, Reddy CA, et al. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys 2007;68:1424–1430. 9. Livsey JE, Cowan RA, Wylie JP, et al. Hypofractionated conformal radiotherapy in carcinoma of the prostate: Five-year outcome analysis. Int J Radiat Oncol Biol Phys 2003;57: 1254–1259. 10. Madsen BL, Hsi RA, Pham HT, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized prostate disease: First clinical trial results. Int J Radiat Oncol Biol Phys 2007;67: 1099–1105. 11. Demanes DJ, Rodriguez RR, Schour L, et al. High-dose rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California Endocurietherapy’s 10-year results. Int J Radiat Oncol Biol Phys 2005;61: 1306–1316. 12. Martinez A, Gonzalez J, Spencer W, et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J Urol 2003;169:974–979. 13. Yoshioka Y, Nose T, Yoshida K, et al. High-dose rate brachytherapy as monotherapy for localized prostate cancer: A retrospective analysis with special focus on tolerance and chronic toxicity. Int J Radiat Oncol Biol Phys 2003;56:213–220.
140
Volume 73, Number 4, 2009
14. Fowler JF, Ritter MA, Chappell RJ, et al. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys 2003;56:1093–1104. 15. Greene FL, Page DL, Flemming ID, et al. AJCC manual for staging cancer. 6th ed. New York: Springer Verlag; 2002. p. 309–313. 16. Barry MJ, Fowler FJ Jr., O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549–1564. 17. Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899–905. 18. Common toxicity criteria. National Institutes of Health publication (version 2.0) 1998. 19. Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M.D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67–74. 20. Guerrero M, Li XA. Halftime for repair of sublethal damage in normal bladder and rectum: An analysis of clinical data from cervix brachytherapy. Phys Med Biol 2006;51:4063–4071. 21. Crook J, Gillan C, Yeung I, et al. PSA kinetics and PSA bounce following permanent seed prostate brachytherapy. Int J Radiat Oncol Biol Phys 2007;69:426–433. 22. Horwitz EM, Levy LB, Thames HD, et al. Biochemical and clinical significance of the posttreatment prostate-specific antigen bounce for prostate cancer patients treated with external beam radiation therapy alone: A multiinstitutional pooled analysis. Cancer 2006;107:1496–1502.
191
Radiation Oncology
BioMed Central
Radical stereotactic radiosurgery with real-time tumor motion tracking in the
treatment of small peripheral lung tumors Research
Open Access
Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors Brian T Collins*1, Kelly Erickson1, Cristina A Reichner2, Sean P Collins1, Gregory J Gagnon1, Sonja Dieterich1, Don A McRae1, Ying Zhang3, Shadi Yousefi4, Elliot Levy4, Thomas Chang4, Carlos Jamis-Dow4, Filip Banovac4 and Eric D Anderson2 Address: 1Department of Radiation Medicine, Georgetown University Hospital, Washington, DC. USA, 2Division of Pulmonary, Critical Care and Sleep Medicine, Georgetown University Hospital, Washington, DC. USA, 3Biostatistics Unit, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC. USA and 4Division of Vascular & Interventional Radiology, Georgetown University Hospital, Washington, DC. USA Email: Brian T Collins* - collinsb@gunet.georgetown.edu; Kelly Erickson - kellyterickson@gmail.com; Cristina A Reichner - reichnerc@aol.com; Sean P Collins - mbppkia@hotmail.com; Gregory J Gagnon - gagnong@georgetown.edu; Sonja Dieterich - sd84@georgetown.edu; Don A McRae - mcraed@gunet.georgetown.edu; Ying Zhang - yz9@georgetown.edu; Shadi Yousefi - shadiyousefi@yahoo.com; Elliot Levy - levye@gunet.georgetown.edu; Thomas Chang - tcc@gunet.georgetown.edu; Carlos Jamis-Dow - jamisdoc@gunet.georgetown.edu; Filip Banovac - fb2@gunet.georgetown.edu; Eric D Anderson - andersoe@gunet.georgetown.edu * Corresponding author
Published: 22 October 2007 Radiation Oncology 2007, 2:39
doi:10.1186/1748-717X-2-39
Received: 18 June 2007 Accepted: 22 October 2007
This article is available from: http://www.ro-journal.com/content/2/1/39 © 2007 Collins et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract Background: Recent developments in radiotherapeutic technology have resulted in a new approach to treating patients with localized lung cancer. We report preliminary clinical outcomes using stereotactic radiosurgery with real-time tumor motion tracking to treat small peripheral lung tumors. Methods: Eligible patients were treated over a 24-month period and followed for a minimum of 6 months. Fiducials (3–5) were placed in or near tumors under CT-guidance. Non-isocentric treatment plans with 5-mm margins were generated. Patients received 45–60 Gy in 3 equal fractions delivered in less than 2 weeks. CT imaging and routine pulmonary function tests were completed at 3, 6, 12, 18, 24 and 30 months. Results: Twenty-four consecutive patients were treated, 15 with stage I lung cancer and 9 with single lung metastases. Pneumothorax was a complication of fiducial placement in 7 patients, requiring tube thoracostomy in 4. All patients completed radiation treatment with minimal discomfort, few acute side effects and no procedurerelated mortalities. Following treatment transient chest wall discomfort, typically lasting several weeks, developed in 7 of 11 patients with lesions within 5 mm of the pleura. Grade III pneumonitis was seen in 2 patients, one with prior conventional thoracic irradiation and the other treated with concurrent Gefitinib. A small statistically significant decline in the mean % predicted DLCO was observed at 6 and 12 months. All tumors responded to treatment at 3 months and local failure was seen in only 2 single metastases. There have been no regional lymph node recurrences. At a median follow-up of 12 months, the crude survival rate is 83%, with 3 deaths due to comorbidities and 1 secondary to metastatic disease. Conclusion: Radical stereotactic radiosurgery with real-time tumor motion tracking is a promising welltolerated treatment option for small peripheral lung tumors.
Page 1 of 7 (page number not for citation purposes)
152 192
Radiation Oncology 2007, 2:39
Introduction
Treatment options for medically inoperable patients with lung cancer are limited. Poor outcomes with protracted conventionally fractionated radiotherapy approaches prompted researchers in the last decade to explore ways of delivering high doses of radiation in shorter periods of time [1]. Utilizing a body frame and abdominal compression to limit lung motion, small mobile lesions have been treated with relatively tight margins (10 mm) [2]. This enhanced accuracy has facilitated the safe, swift delivery of highly effective doses of radiation to small discrete peripheral lung tumors such as stage I lung cancer and pulmonary metastases [3-13]. Recently updated outcomes of a Phase I stereotactic body radiotherapy (SBRT) dose escalation study confirm that abbreviated radiosurgery treatment courses, in which doses in the range of 45 Gy to 60 Gy are delivered in less than 2 weeks, result in durable local control rates ranging from 70 to 90% [14]. Such favorable outcomes establish thoracic stereotactic radiosurgery as a new radical treatment option for small peripheral lung tumors. The CyberKnife frameless image-guided robotic radiosurgery system (Accuray Incorporated, Sunnyvale, CA) has been successfully employed at Georgetown University Hospital to treat stationary extracranial tumors since early 2002 [15]. With the introduction of the Synchrony motion tracking module, in mid 2004, tumors that move with respiration have been treated without potentially uncomfortable methods to compensate for respiratory movement, such as stereotactic body frames with abdominal compression devices and respiratory gating techniques [16]. Synchrony, an automated CyberKnife imageguidance subsystem, continuously points the robotmounted linear accelerator at lung tumors as they move with uninhibited respiration during radiation delivery [17]. We report preliminary clinical outcomes from 24 consecutive patients with single small peripheral lung tumors radically treated using Synchrony real-time tumor motion tracking.
Methods and materials
Eligibility This study was approved by the hospital institutional review board and all participants provided informed written consent. The Georgetown University Hospital multidisciplinary thoracic oncology team evaluated patients. Mandatory baseline studies included CT scans of the chest, abdomen and pelvis with IV contrast, PET imaging and routine pulmonary function tests (PFTs). Patients with small peripheral pathologically confirmed inoperable Stage I lung cancer or single pulmonary metastases were treated. Tumors were considered small if the maximum diameter measured less than 4 cm and peripheral if radical treatment was feasible without exceeding conserv-
http://www.ro-journal.com/content/2/1/39
ative maximum point dose limits to critical central normal tissues derived from historical data (Table 1). Conventional thoracic irradiation was permitted if it was delivered more than one year prior to stereotactic radiosurgery and directed to a different lobe of the lung and/or the extrapulmonary thoracic lymphatics (i.e., hilar, mediastinal and supraclavicular lymph nodes). Concurrent and salvage systemic therapies other than gemcitabine were also permitted. Fiducial placement Tracking based on translational and rotational target information requires that a minimum of 3 non-collinear fiducials be placed in such a way that they do not obscure each other on the orthogonal images of the CyberKnife xray targeting system. Therefore, 3 to 5 gold fiducials measuring 0.8–1 mm in diameter by 3–7 mm in length (Item 351-1 Best Medical International, Inc., Springfield, VA) were placed in or near the tumors under CT-guidance as recently described [18]. Treatment planning Fine-cut (1.25 mm) treatment planning CTs were obtained 7–10 days after fiducial placement during a full inhalation breath hold with the patient in the supine treatment position. This short delay prior to imaging allowed procedure-related hemorrhage to resolve and limited post-CT fiducial migration. Gross tumor volumes (GTV) were contoured utilizing lung windows. All critical central thoracic structures (Table 1) and the lungs were contoured. A treatment plan with a 5-mm margin on the GTV for contouring and tracking uncertainty was generated using the TPS 5.2.1 non-isocentric, inverse-planning algorithm with tissue density heterogeneity corrections for lung based on an effective depth correction. Radical doses of 45 to 60 Gy in three equal fractions of 15 to 20 Gy were prescribed to an isodose line that covered at least 95% of the planning treatment volume (PTV = GTV + 5 mm). In general, total doses closer to 45 Gy were prescribed when concerns about the radiation tolerance of adjacent critical structures arose and when patients were felt to have severe cardiopulmonary dysfunction. The percentage of the total lung volume receiving 15 Gy or more (V15) was limited to less than 15% in order to decrease Table 1: Critical central thoracic structure point dose limits
Critical Structure
Spinal cord Left ventricle Esophagus Main bronchus Trachea Aorta
Maximum Point Dose Limit (Gy) (total for 3 fractions) 18 18 24 30 30 30
Page 2 of 7 (page number not for citation purposes)
153 193
Radiation Oncology 2007, 2:39
the risk of clinically significant radiation pneumonitis or pulmonary dysfunction. Treatment delivery The treatment course was completed in less than two weeks. Prior to the initial treatment, each patient was evaluated with fluoroscopy to verify that the motion of the fiducials chosen for tracking correlated with tumor motion. Prophylactic corticosteroids were not administered. Patients were placed supine and unrestrained on the treatment table with their arms at their sides. They wore a form-fitting vest upon which 3 red light-emitting markers were attached on the surface of the patient's anterior torso in the region of maximum respiratory excursion of the chest and upper abdomen. These markers projected to an adjustable camera array in the treatment room. Precise patient positioning was accomplished utilizing the automated patient positioning system. Fiducials were located using orthogonal x-ray images acquired with ceiling-mounted diagnostic x-ray sources and corresponding amorphous silicon image detectors secured to the floor on either side of the patient.
Immediately prior to treatment delivery, an adaptive correlation model was created between the fiducial positions as periodically imaged by the x-ray targeting system and the light-emitting markers as continuously imaged by the camera array [17]. During treatment delivery the tumor position was tracked using the live camera array signal and correlation model, the linear accelerator was moved by the robotic arm in real time to maintain alignment with the tumor during uninhibited respiration. Fiducials were imaged prior to the delivery of every third beam for treatment verification and to update the correlation model [16]. If fiducials were misidentified by the software or the correlation model error exceeded 3 mm in two consecutive paired x-ray images, treatment was discontinued and the correlation model rebuilt. Follow-up studies Patients were followed with physical examinations, CT imaging and routine PFT's at 3, 6, 12, 18, 24 and 30 months. Complete response was defined as resolution of the tumor on CT imaging and partial response as a decrease in the tumor volume relative to the treatment planning CT. Local and regional tumor recurrence was defined as unequivocal tumor progression on CT imaging within the treated lobe or regional lymph nodes, respectively. Biopsy was recommended for pathologic verification. Toxicity was scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0 [19].
http://www.ro-journal.com/content/2/1/39
Statistical analysis Follow-up was determined from the date of the last treatment. Two-sided Wilcoxon signed-ranks tests were used to assess statistical significance (α = 0.05) of post-treatment changes in forced expiratory volume in 1 sec (FEV1), total lung capacity (TLC) and diffusing capacity of the lung for carbon monoxide (DLCO) at 6 and 12 months.
Results
Patient and tumor characteristics Twenty-four consecutive patients (10 men and 14 women) were treated over a 2-year period extending from July 2004 to July 2006 (Table 2). The median follow-up time among survivors is 12 months (range, 6–30 months). No patients were lost to follow-up. Seventeen percent of patients received prior conventional thoracic radiation. All but one patient had stopped smoking in the distant past (> 3 years) or had never smoked. Nonetheless, pulmonary dysfunction was the primary rationale for non-surgical treatment among the stage I lung cancer patients and 3 such patients required supplemental home oxygen prior to receiving treatment. Sixty-seven percent of the tumors involved the upper lobes. Fifteen were inoperable primary lung tumors (adenocarcinoma 7, NSCLC not otherwise specified 5, squamous cell carcinoma 2 and typical carcinoid tumor 1) and 9 were single lung metastases (NSCLC 5, esophagus 1, small bowel 1, renal 1 and cutaneous basal cell carcinoma 1). The mean maximum tumor diameter was 2 cm (range, 0.9 – 3.5 cm). Treatment characteristics Three equal fractions of 15 to 20 Gy were delivered in an average of 7 days (Table 3). Treatment plans were composed of hundreds of pencil beams shaped using a single 20, 25 or 30-mm diameter circular collimator. The percentage of the total lung volume receiving 15 Gy or more was low despite the radical treatment intent. On average, 55 paired x-ray images were taken each day to confirm the accuracy of the correlation model. Twenty-five percent of the patients received concurrent systemic therapy as previously described [20].
Table 2: Patient and Tumor Characteristics
Mean (Range) Age (years) Weight (lbs) FEV1 (L) % predicted FEV1 % predicted TLC % predicted DLCO Maximum Tumor Diameter (cm) Gross Tumor Volume (cc)
70 (50 – 82) 160 (118 – 285) 1.47 (0.53 – 2.62) 61 (26 – 121) 94 (69 – 136) 61 (44 – 96) 2.0 (0.9 – 3.5) 8 (1 – 14)
Page 3 of 7 (page number not for citation purposes)
154 194
Radiation Oncology 2007, 2:39
http://www.ro-journal.com/content/2/1/39
Table 3: Treatment Characteristics
Mean (Range) Dose (Gy) Biologic Effective Tumor Dose (BED Gy10) Prescription Isodose Line (%) Planning treatment volume coverage (%) Number of beams per fraction Number of paired x-ray verification images per fraction Beam-on time (minutes) Treatment course (days) % Total lung volume receiving 15 Gy or more
54 (45 – 60) 150 (110 – 180) 80 (75 – 90) 97 (95 – 100) 164 (87 – 270) 55 (29 – 90) 82 (53 – 120) 7 (3 – 11) 7 (3 – 11)
Complications Pneumothorax either during or immediately following fiducial placement was seen in 30% of patients, and 17% of all patients required tube thoracostomy to correct clinically significant pneumothorax. All patients completed treatment without interruption. Following treatment, acute toxicity consisting of mild brief fatigue was reported in the majority of patients. Transient chest wall discomfort, typically lasting several weeks, developed in 7 of 11 patients with lesions within 5 mm of the pleura.
Grade III pneumonitis was observed in 2 patients (8%). One of the patients received concurrent Gefitinib treatment. She developed an infiltrate corresponding to the high dose stereotactic radiosurgery volume and dyspnea requiring temporary supplemental oxygen 4 weeks after completing CyberKnife treatment. Her symptoms resolved quickly with steroids and the discontinuation of Gefitinib. The second patient, who had a history of extensive conventional esophageal irradiation, was treated for a single lung metastasis. He developed symptomatic infiltrates largely confined to the conventional radiation volume following the initiation of salvage experimental systemic therapy 10 months after radiosurgery. His symptoms resolved over several weeks on steroids and he discontinued supplemental oxygen. Post-treatment pulmonary status Among the entire group, no change was seen in FEV1 and TLC at 6 and 12 months. A statistically significant decline of 8% (from 61% to 53%; p = 0.002) and 10% (from 61% to 51%; p = 0.01) in the mean % predicted DLCO was seen at 6 and 12 months, respectively. Tumor response All tumor volumes were reduced on CT imaging at 3 months. Six-month CT scans were available for all 24 patients. Fourteen lesions continued to respond to treatment, three of which had resolved completely. Ten lesions were obscured by radiation fibrosis at 6 months and were not clearly evaluable. At 12 months, 16 patients' CT scans
were available for review. Four of the evaluable lesions had responded completely, two exhibited an excellent partial response to treatment and eight, or 50% of the evaluable lesions, were obscured by radiation fibrosis which corresponded with the planned high-dose treatment volume and consistently encompassed the fiducials (Figure 1). Despite the development of significant radiation fibrosis with time, it was clear that two single lung metastases had progressed locally per CT imaging at 12 months (Table 4). Therefore, with a median follow-up of 12 months, the crude local control rate for the group is 92%. Consistent with other reports, local control was 100% for stage I tumors and lower (78%) for single lung metastases (Table 5) [21]. Disease spread and survival Regional lymph node failure was not observed in early follow-up. Four patients with locally controlled single lung metastases developed additional metastatic sites and received salvage systemic therapy. Despite treatment one patient died of progressive metastatic disease at 8 months. A second single lung metastasis patient died of a myocardial infarction at 11 months without evidence of local or systemic disease. No stage I lung cancer patient developed metastatic disease. However, 2 stage I lung cancer patients died of comorbid illnesses (1 secondary to progressive congestive heart failure at 6 months and 1 secondary to progressive emphysema at 9 months). Therefore, with a median follow-up of 12 months, the crude survival rate for the group is 83%, with 3 deaths due to co-morbidities and 1 secondary to metastatic disease. As expected, the crude survival rate for patients with single lung metastases was lower (Table 5) [21].
Discussion
In mid-2004 we initiated a frameless image-guided highdose fractionated stereotactic radiosurgery treatment protocol for patients with medically inoperable small peripheral stage I lung cancer and single small peripheral lung metastases. Continuous tracking of respiratory tumor motion with Synchrony and highly accurate beam alignment throughout treatment with the CyberKnife prompted us to deliver dose distributions with tighter margins than historically feasible (5 mm) [2]. Hundreds of beams were used to produce a relatively high central tumor dose and dose gradients that conformed closely to the shape of the tumors [22]. Twenty-four patients have been treated in 24 months without notable discomfort during the treatment procedure. With a median follow-up of 12 months the crude local control rate is 92% and there have been no severe (grade IV) treatment-related complications or mortalities. Thus, we conclude that radical stereotactic radiosurgery with real-time tumor motion tracking and continuous beam correction utilizing the CyberKnife system is a feasible, well-tolerated and highly
Page 4 of 7 (page number not for citation purposes)
195 155
Radiation Oncology 2007, 2:39
http://www.ro-journal.com/content/2/1/39
A
B
C
D
show Right ment Figure upper volume progressive 1 lobe is shown clinical fibrosis instage orange in the IAtreated NSCLC and thevolume 30 treatment Gy isodose thatplanning ultimately line in CTblue), impedes (A), and planned CT CTevaluation at radiation 6 and 12 of dose months tumor distribution response post-treatment (B: the planning (C and treatD) Right upper lobe clinical stage IA NSCLC treatment planning CT (A), planned radiation dose distribution (B: the planning treatment volume is shown in orange and the 30 Gy isodose line in blue), and CT at 6 and 12 months post-treatment (C and D) show progressive fibrosis in the treated volume that ultimately impedes CT evaluation of tumor response.
effective treatment option for small peripheral lung tumors. Despite promising early results, critical issues concerning the evaluation of treatment efficacy and the possibility of late complications have yet to be fully addressed. Highdose radiation delivered precisely to small peripheral pulmonary nodules will cause focal lung parenchyma fibrosis that complicates interpretation of tumor response. At 3 months all tumors had responded to treatment, as seen by a decrease in volume on CT imaging. However, at 12 months half of the lesions were obscured by radiation fibrosis conforming to the high-dose radiation volume, Table 4: Tumor response per CT imaging
Complete Response Partial Response Obscured by Fibrosis* Local Progression * no evidence of progression
6 months (%)
12 months (%)
12 46 42 0
25 13 50 12
making further CT tumor response assessment difficult [23,24]. In our experience, PET activity within irradiated regions does not reliably indicate tumor recurrence because the radiation response in the lung is itself PET avid. Therefore, PET imaging was not routinely used to follow patients in this study. Although biopsy could aid response assessment, it was not recommended in these typically frail patients in the absence of frank CT tumor progression given the limited salvage treatment options available. Consequently, when treated tumors appeared to be obscured by radiation-induced fibrosis on serial CT images (Figure 1), the tumors were considered locally controlled and patients were observed with the understanding that the documentation of local recurrence might be delayed. High-dose thoracic radiotherapy delivered to small pulmonary nodules, no matter how accurate, results in limited peritumoral lung damage and dysfunction. In the absence of validated radiation pneumonitis risk parameters for stereotactic radiosurgery, we chose to simply limit the volume of lung receiving 15 Gy or greater. Although we were able to limit this volume (V15 ranged from 3% to
Page 5 of 7 (page number not for citation purposes)
196156
Radiation Oncology 2007, 2:39
http://www.ro-journal.com/content/2/1/39
Table 5: Crude Local Control and Survival Rates at a Median Follow-up of 12 months
Stage I Lung Cancer Single Lung Metastases Overall
Crude Local Control Rate (%)
Crude Survival Rate (%)
100 78 92
87 78 83
11% of total lung volume), Grade III pneumonitis occurred in two patients, one at 4 weeks post-treatment and the other at 10 months post-treatment. In both cases pneumonitis onset was correlated with systemic therapy, and one patient had had prior extensive conventional thoracic irradiation. Both patients recovered with steroid treatment. No patients died of pneumonitis, lung fibrosis or local recurrence; deaths in this trial were due to comorbid illness or preexisting metastatic disease progression. Limited data are available evaluating the impact of stereotactic radiosurgery on pulmonary function in patients with small peripheral lung tumors (< 4 cm). Furthermore, available findings are difficult to interpret because a large fraction of lung cancer patients stop smoking just prior to treatment; any deleterious effects of radiosurgery may be offset by the early beneficial effects of smoking cessation [25]. Ninety-five percent of the patients in the current trial discontinued smoking in the distant past (>3 years prior to treatment) or had never smoked. The mean percentage of the total lung volume receiving a minimum of 15 Gy was 7%. As might have been anticipated given the relatively small volumes of peripheral lung irradiated to doses capable of causing local lung dysfunction, small but statistically significant 8% and 10% declines in the mean % predicted DLCO were seen at 6 and 12 months, respectively [26]. Regardless of the decline, no adverse clinical effect was observed. Furthermore, the negative impact of radiosurgery on diffusion capacity may be overestimated in the current study as this effect is expected to be greater in patients treated with prior conventional thoracic irradiation or concurrent systemic therapy [27]. Critical central structure toxicity was not observed in this trial. It is likely that toxicity was absent because we strictly adhered to conservative maximum point dose limits for critical central structures (Table 1). However, transient mild-to-moderate chest wall pain typically lasting several weeks was seen following treatment in the majority of patients with lesions within 5 mm of the pleura. These patients were treated conservatively with non-steroidal anti-inflammatory medications or opioid analgesic combinations. Although it is tempting to limit the dose delivered to the chest wall in these patients, this would likely
result in additional local failures and is not recommended at this time. The current CyberKnife treatment approach requires the implantation of fiducials to permit tumor targeting and tracking. Fiducial placement results in a delay in therapy while awaiting the resolution of procedure-related hemorrhage and fiducial fixation. Furthermore, the procedure may result in pneumothorax, sometimes requiring tube thoracostomy and a brief hospital stay [28]. Our institution has developed a technique for placing fiducials in or near central and larger peripheral tumors via bronchoscopy reducing the risk of pneumothorax [29]. However, for the small peripheral tumors treated in this study sophisticated navigation systems would be required to place fiducials precisely in this manner. Fortunately, ongoing research evaluating fiducial-less tracking will likely result in technology that obviates the need for peripheral fiducial placement in the near future [30].
Conclusion
Small peripheral lung tumors may be radically treated with the CyberKnife frameless image-guided robotic radiosurgery system, resulting in encouraging early local control rates (92%) and minimal toxicity. The delivery of hundreds of beams while continuously tracking respiratory tumor movement and adjusting beam directions allows for highly conformal dose distributions with tight margins (5 mm). It is likely that such treatment will result in superior long term tumor control with acceptable toxicity and overall better treatment outcomes.
Abbreviations
BED Gy10: biologic effective tumor dose; CT: computed tomography; DLCO: diffusing capacity of the lung for carbon monoxide; FEV1: forced expiratory volume in 1 sec; GTV: gross tumor volume; Gy: Gray; NSCLC: non-small cell lung cancer; PET: positron emission tomography; PFT: pulmonary function tests; PTV: planning treatment volume; TLC: total lung capacity; V15: total lung volume receiving 15 Gy or more.
References 1. 2.
3.
4.
Qiao X, Tullgren O, Lax I, Sirzen F, Lewensohn R: The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer 2003, 41(1):1-11. Lax I, Panettieri V, Wennberg B, Amor Duch M, Naslund I, Baumann P, Gagliardi G: Dose distributions in SBRT of lung tumors: Comparison between two different treatment planning algorithms and Monte-Carlo simulation including breathing motions. Acta Oncol 2006, 45(7):978-988. Fukumoto S, Shirato H, Shimzu S, Ogura S, Onimaru R, Kitamura K, Yamazaki K, Miyasaka K, Nishimura M, Dosaka-Akita H: Small-volume image-guided radiotherapy using hypofractionated, coplanar, and noncoplanar multiple fields for patients with inoperable Stage I nonsmall cell lung carcinomas. Cancer 2002, 95(7):1546-1553. Hoyer M, Roed H, Hansen AT, Ohlhuis L, Petersen J, Nellemann H, Berthelsen AK, Grau C, Engelholm SA, von der Maase H: Prospective study on stereotactic radiotherapy of limited-stage non-
Page 6 of 7 (page number not for citation purposes)
197 157
Radiation Oncology 2007, 2:39
5.
6.
7.
8.
9.
10.
11.
12.
13.
14. 15.
16. 17.
18.
19. 20.
21.
small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006, 66(4 Suppl):S128-35. McGarry RC, Papiez L, Williams M, Whitford T, Timmerman RD: Stereotactic body radiation therapy of early-stage nonsmall-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys 2005, 63(4):1010-1015. Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, Sakamoto M, Mitsumori M, Shibuya K, Araki N, Yano S, Hiraoka M: Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005, 63(5):1427-1431. Nyman J, Johansson KA, Hulten U: Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer--mature results for medically inoperable patients. Lung Cancer 2006, 51(1):97-103. Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, Yamashita T, Niibe Y, Karasawa K, Hayakawa K, Takai Y, Kimura T, Hirokawa Y, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada K: Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004, 101(7):1623-1631. Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J: Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006, 24(30):4833-4839. Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, Williams M: Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003, 124(5):1946-1955. Uematsu M, Shioda A, Suda A, Fukui T, Ozeki Y, Hama Y, Wong JR, Kusano S: Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys 2001, 51(3):666-670. Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M: Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004, 60(1):186-196. Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y, Li P, Chang JY: Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006, 66(1):117-125. Timmerman RD, Park C, Kavanagh BD: The North American experience with stereotactic body radiation therapy in nonsmall cell lung cancer. J Thorac Oncol 2007, 2(7 Suppl 3):S101-12. Degen JW, Gagnon GJ, Voyadzis JM, McRae DA, Lunsden M, Dieterich S, Molzahn I, Henderson FC: CyberKnife stereotactic radiosurgical treatment of spinal tumors for pain control and quality of life. J Neurosurg Spine 2005, 2(5):540-549. Schweikard A, Shiomi H, Adler J: Respiration tracking in radiosurgery. Med Phys 2004, 31(10):2738-2741. Sayeh S, Wang J, Main WT, Kilby W, Maurer CR: Respiratory Motion Tracking for Robotic Radiosurgery. In Robotic Radiosurgery: Treating Tumors that Move with Respiration Edited by: Urschel HC, Kresl JJ, Luketich JD, Papiez L, Timmerman RD. Berlin , Springer-Verlag; 2007:15-29. Banovac F, McRae D, Dieterich S, Wong K, Dias L, Chang T: Percutaneous Placement of Fiducial Markers for Thoracic Malignancies. In Robotic Radiosurgery: Treating Tumors that Move with Respiration Edited by: Urschel HC, Kresel JJ, Luketich JD, Papiez L, Timmerman RD. Berlin , Springer-Verlag; 2007:15-29. Program CTE: Common Terminology Criteria for Adverse Events, Version 3.0. 2006. Malik SM, Erickson K, Collins S, Reichner C, Jamis-Dow C, Banovac F, Anderson ED, Smith FP, Hwang J, Collins BT: CyberKnife Highdose Fractionated Stereotactic Radiosurgery with Tumor Tracking: An Effective Non-surgical Treatment Alternative for Single Small Peripheral Lung Tumors: June 1-5; Chicago, Illinois. ; 2007. Le QT, Loo BW, Ho A, Cotrutz C, Koong AC, Wakelee H, Kee ST, Constantinescu D, Whyte RI, Donington J: Results of a phase I dose-escalation study using single-fraction stereotactic radi-
http://www.ro-journal.com/content/2/1/39
22. 23.
24.
25.
26. 27.
28.
29.
30.
otherapy for lung tumors. Journal of Thoracic Oncology 2006, 1(8):802-809. Papiez L, Timmerman R, DesRosiers C, Randall M: Extracranial stereotactic radioablation: physical principles. Acta Oncol 2003, 42(8):882-894. Aoki T, Nagata Y, Negoro Y, Takayama K, Mizowaki T, Kokubo M, Oya N, Mitsumori M, Hiraoka M: Evaluation of lung injury after three-dimensional conformal stereotactic radiation therapy for solitary lung tumors: CT appearance. Radiology 2004, 230(1):101-108. Takeda T, Takeda A, Kunieda E, Ishizaka A, Takemasa K, Shimada K, Yamamoto S, Shigematsu N, Kawaguchi O, Fukada J, Ohashi T, Kuribayashi S, Kubo A: Radiation injury after hypofractionated stereotactic radiotherapy for peripheral small lung tumors: serial changes on CT. AJR Am J Roentgenol 2004, 182(5):1123-1128. Ohashi T, Takeda A, Shigematsu N, Kunieda E, Ishizaka A, Fukada J, Deloar HM, Kawaguchi O, Takeda T, Takemasa K, Isobe K, Kubo A: Differences in pulmonary function before vs. 1 year after hypofractionated stereotactic radiotherapy for small peripheral lung tumors. Int J Radiat Oncol Biol Phys 2005, 62(4):1003-1008. Mehta V: Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005, 63(1):5-24. Gopal R, Starkschall G, Tucker SL, Cox JD, Liao Z, Hanus M, Kelly JF, Stevens CW, Komaki R: Effects of radiotherapy and chemotherapy on lung function in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003, 56(1):114-120. Yousefi S, Collins BT, Reichner CA, Anderson ED, Jamis-Dow C, Gagnon G, Malik S, Marshall B, Chang T, Banovac F: Complications of thoracic computed tomography-guided fiducial placement for the purpose of stereotactic body radiation therapy. Clin Lung Cancer 2007, 8(4):252-256. Reichner CA, Collins BT, Gagnon GJ, Malik S, Jamis-Dow C, Anderson ED: The placement of gold fiducials for CyberKnife stereotactic radiosurgery using a modified transbronchial needle aspiration technique. Journal of Bronchology 2005, 12(4):193-195. Fu D, Kahn R, Wang B, Wang H, Mu Z, Park J, Kuduvalli G, Maurer CR: Xsight Lung Tracking System: A Fiducial-less Method for Respiratory Motion Tracking. In Robotic Radiosurgery: Treating Tumors that Move with Respiration Edited by: Urschel HC, Kresl JJ, Luketich JD, Papiez L, Timmerman RD. Berlin , Springer-Verlag; 2007:15-29.
Publish with Bio Med Central and every scientist can read your work free of charge "BioMed Central will be the most significant development for disseminating the results of biomedical researc h in our lifetime." Sir Paul Nurse, Cancer Research UK
Your research papers will be: available free of charge to the entire biomedical community peer reviewed and published immediately upon acceptance cited in PubMed and archived on PubMed Central yours — you keep the copyright
BioMedcentral
Submit your manuscript here: http://www.biomedcentral.com/info/publishing_adv.asp
Page 7 of 7 (page number not for citation purposes)
198 158
ORIGINAL RESEARCH ARTICLE
published: 20 August 2012 doi: 10.3389/fonc.2012.00081
Comparative cost-effectiveness of stereotactic body radiation therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer Anju Parthan 1 *, Narin Pruttivarasin 1 , Diane Davies 2 , Douglas C. A. Taylor 1 , Vivek Pawar 3 , Akash Bijlani 2 , Kristen Hassmiller Lich 4 and Ronald C. Chen 4 1 2 3 4
Comparative cost-effectiveness of stereotactic body radiation
Health Economics and Outcomes Research, OptumInsight Accuray Incorporated Bayer (Formerly with OptumInsight) University of North Carolina at Chapel Hill
therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer
Edited by: Brian Timothy Collins, Georgetown University Hospital, USA Reviewed by: Sean Collins, Georgetown University Hospital, USA Debra Freeman, Naples Radiation Oncology, USA *Correspondence: Anju Parthan, Health Economics and Outcomes Research, OptumInsight, 425 Market Street, 12th Floor, San Francisco, CA 94105, USA. e-mail: anju.parthan@optum.com
Objective: To determine the cost-effectiveness of several external beam radiation treatment modalities for the treatment of patients with localized prostate cancer. Methods: A lifetime Markov model incorporated the probabilities of experiencing treatment-related long-term toxicity or death.Toxicity probabilities were derived from published sources using meta-analytical techniques. Utilities and costs in the model were obtained from publicly available secondary sources. The model calculated quality-adjusted life expectancy and expected lifetime cost per patient, and derived ratios of incremental cost per qualityadjusted life year (QALY) gained between treatments. Analyses were conducted from both payer and societal perspectives. One-way and probabilistic sensitivity analyses were performed. Results: Compared to intensity-modulated radiation therapy (IMRT) and proton beam therapy (PT), stereotactic body radiation therapy (SBRT) was less costly and resulted in more QALYs. Sensitivity analyses showed that the conclusions in the base-case scenario were robust with respect to variations in toxicity and cost parameters consistent with available evidence. At a threshold of $50,000/QALY, SBRT was cost-effective in 75% and 94% of probabilistic simulations compared to IMRT and PT, respectively, from a payer perspective. From a societal perspective, SBRT was cost-effective in 75% and 96% of simulations compared to IMRT and PT, respectively, at a threshold of $50,000/QALY. In threshold analyses, SBRT was less expensive with better outcomes compared to IMRT at toxicity rates 23% greater than the SBRT base-case rates. Conclusion: Based on the assumption that each treatment modality results in equivalent long-term efficacy, SBRT is a cost-effective strategy resulting in improved quality-adjusted survival compared to IMRT and PT for the treatment of localized prostate cancer. Keywords: prostate, cancer, radiation, cost-effectiveness, localized
INTRODUCTION The National Cancer Institute estimates that 241,740 new cases of prostate cancer will be diagnosed in 2012, along with 28,170 deaths (National Cancer Institute, 2012). The Prostate Cancer Outcomes Study (PCOS), which was initiated by the National Cancer Institute in 1994, noted that 88% of the newly diagnosed prostate cancer cases will be localized disease. Based on a review of the current published literature, there are several options for patients diagnosed with clinically localized prostate cancer including active surveillance, surgery, and radiation therapy. In 2010, the Agency for Healthcare Research and Quality (AHRQ) concluded that insufficient evidence exists to fully assess the superiority of one type of radiation therapy over another for the treatment of localized prostate cancer (Agency for Healthcare Research and Quality, 2010). The objective of this study is to address this gap by developing a decision analysis model that integrates currently available published evidence on the post-treatment incidence of
www.frontiersin.org
long-term toxicities to compare the incremental cost-effectiveness of three modern external beam radiation therapies for localized prostate cancer: intensity-modulated radiation therapy (IMRT), proton beam therapy (PT), and stereotactic body radiation therapy (SBRT). Intensity-modulated radiation therapy, which is a commonly used treatment for patients with localized prostate cancer, involves the external delivery of multiple beams of radiation that conform to the shape of the tumor, and where the intensity of each beam can be modulated in order to spare surrounding healthy tissue. IMRT therapy is typically delivered in 40 fractions (i.e., treatment sessions) and requires 8–9 weeks of treatment. PT is delivered externally to a predefined depth, potentially with little radiation delivered beyond that point, thus sparing surrounding healthy tissue. Like IMRT, PT typically is delivered in 40 fractions and requires 8–9 weeks of treatment. A study conducted by Konski et al. (2007) found that PT was not a cost-effective
August 2012 | Volume 2 | Article 81 | 1
159 199
Parthan et al.
treatment option for localized prostate cancer when compared to IMRT. Stereotactic body radiation therapy is the use of accurate and image-guided radiation therapy to treat tumors using up to five intense radiation treatments (Martin and Gaya, 2010). Because a higher dose is given in each fraction, image guidance during treatment and an ability to adjust for tumor/target motion is important in order to minimize treatment-related toxicity. Recent studies have indicated that SBRT toxicity levels are comparable to those of other radiation treatment options (Sanda et al., 2008; Chen et al., 2009; Townsend et al., 2011), with long-term (5-year) progression-free survival at 93% (Freeman and King, 2011). In the current paper we present a Markov model developed to estimate the comparative long-term incremental cost-effectiveness of IMRT, PT, and SBRT. The analyses are conducted using a lifetime horizon from both the perspective of a health care payer as well as a societal perspective that includes the value of patients’ time spent in treatment.
MATERIALS AND METHODS OVERVIEW
Decision-analytic techniques involved analysis of a lifetime cohort Markov model, programmed in Microsoft® Excel, to assess the cost-effectiveness of three radiation treatment modalities for localized prostate cancer: SBRT, IMRT, and PT. Brachytherapy was not included as a comparator in the model because the method of delivery (i.e., requiring anesthesia and invasive) was considered to be significantly different from SBRT, IMRT, and PT. Furthermore, because of the anesthesia requirement, some studies have shown that brachytherapy is more likely to be used with younger men when compared with external beam radiation (Sanda et al., 2008; Chen et al., 2009). Depending on the treatment selected, patients in the model are at risk of experiencing one or more of three types of treatmentrelated long-term toxicity, or death. Long-term toxicities included in the analysis were gastrointestinal (GI), genitourinary (GU), and sexual dysfunction (SD). Long-term toxicity is defined as adverse events = grade 2, using the Radiation Therapy Oncology Group (RTOG) scale ≥12 months following treatment. The model
Cost effectiveness of prostate radiation
assumes that patients can experience treatment-related long-term toxicity within the first year following treatment and are at risk to continue to experience it throughout their lives. Figure 1 illustrates the Markov states and transitions that can occur in the model. The model includes eight health states that reflect all possible combinations of the three long-term toxicity states (no toxicity, GI only, GU only, SD only, GI & GU, GI & SD, GU & SD, GI & GU & SD). Since data were not available regarding the duration of side effects, the assumption in the model is that during each Markov cycle patients remain in the same Markov health state or die. Measures of utility (an overall quality of life measure on a 0 to 1 scale) and cost are assigned to each state. Treatment-specific mortality and long-term annual mortality rates with localized prostate cancer are based on published sources and data from SEER, respectively. The model assumes that the long-term disease control is comparable across the treatments, which is consistent with currently available literature. In the base-case we assume that all toxicities are associated with a one-time cost. Analysis of quality-adjusted survival with the Markov model involves tracking patients according to whether or not they experience each of the types of long-term toxicity, and weighting their years of survival by the utility associated with their corresponding toxicity state. The model calculates the qualityadjusted life years (QALYs) and costs per patient over the course of a lifetime. Analyses were performed from a healthcare payer perspective. An additional analysis was conducted from a societal perspective that included the forgone value of patients’ time spent in initial radiation treatment. MODEL PARAMETERS AND DATA SOURCES
The model population consists of 65-year-old males with localized prostate cancer who declined or were ineligible for surgery and are eligible for external radiation therapies. Model parameters and data sources are summarized in Tables 1–5, and are described below. Long-term toxicity and mortality
The probability of experiencing each type of treatment-related long-term toxicity was derived using meta-analytic techniques.
FIGURE 1 | Markov model. GI, gastrointestinal; GU, genitourinary; SD, sexual dysfunction.
Frontiers in Oncology | Radiation Oncology
200 160
August 2012 | Volume 2 | Article 81 | 2
Parthan et al.
Cost effectiveness of prostate radiation
A search was conducted in MEDLINE, EMBASE, PsycINFO, and PubMed for studies published between 1998 and 2010 that reported GU, GI, and SD toxicities over a given period of time. Table 1 displays the characteristics of the 10 studies that were chosen for the meta-analysis. Due to a lack of randomized controlled trials, all 10 studies were either clinical trials with no control group or observational studies with a single cohort. The meta-analysis used a random-effects model to minimize heterogeneity across studies due to clinical or methodological differences. Furthermore, the meta-analysis used the normally distributed log odds ratio as the outcome variable, after which the pooled log odds ratio was converted back to an annual rate. Table 2 displays the treatment-related probabilities of experiencing the various types of long-term toxicity and mortality. A
recent paper published by Coen et al. (2012) suggests that PT toxicity has changed very little in the last 10 years. Also, in the absence of long-term SD toxicity rates for PT, in the base-case, the SD rates were assumed to be equal to IMRT because the method of delivery over time was similar – i.e., small doses over a long period of time. An age-specific mortality rate for prostate cancer patients was applied to all patients in the model using SEER data (Altekruse et al., 2010). The risk of dying from treatment (IMRT, SBRT, and PT) was assumed to be zero. Health-related quality of life
Quality-adjusted life expectancy associated with each treatment was estimated using utilities assigned to each health state in the model (Table 3). Utilities are weights that quantify health-related
Table 1 | Characteristics of the 10 studies chosen for the meta-analysis. Treatment
Study
Sample size
Follow-up
Age (range)
Toxicity studied
time (months) SBRT
Friedland et al. (2009)
112
24
Mean 70 (55–87)
SD
Katz et al. (2010)
206
17 (dose 36.25)
Mean 69 (45–88)
GU, GI, SD
30 (dose 35)
IMRT
PT
King et al. (2009)
41
33
Median 66 (48–83)
GU, GI
Wiegner and King (2010)
20
35.5
Median 68 (57–83)
SD
Kirichenko et al. (2006)
928
36
NA
GU, GI
Zelefsky et al. (2002)
772
36
Median 69 (46–86)
GU, GI, SD
Zelefsky et al. (2006)
561
96
Median 68 (46–86)
GU, GI, SD
Schulte et al. (2000)
870
36
NA
GU, GI
Slater et al. (1998)
643
36
NA
GU, GI
Slater et al. (1999)
315
36
NA
GU, GI
GI, gastrointestinal; GU, genitourinary; IMRT, intensity-modulated radiation therapy; NA, not available; PT, proton beam therapy; SBRT, stereotactic body radiation therapy; SD, sexual dysfunction.
Table 2 | Treatment-related mortality and long-term toxicity. Probabilities
Base-case default value
SE
Source
TREATMENT-RELATED MORTALITY SBRT
0.000
–
Assumption
IMRT
0.000
–
Assumption
PT
0.000
–
Assumption Friedland et al. (2009), Katz et al. (2010), King et al. (2009)
LONG-TERM TOXICITIES – SBRT GU
0.040
0.023
GI
0.027
0.010
Katz et al. (2010), King et al. (2009)
SD
0.159
0.088
Friedland et al. (2009), Katz et al. (2010), Wiegner and King (2010)
LONG-TERM TOXICITIES – IMRT GU
0.035
0.016
Kirichenko et al. (2006), Zelefsky et al. (2002), Zelefsky et al. (2006)
GI
0.013
0.008
Kirichenko et al. (2006), Zelefsky et al. (2002), Zelefsky et al. (2006)
SD
0.272
0.232
Zelefsky et al. (2002), Zelefsky et al. (2006) Schulte et al. (2000), Slater et al. (1998), Slater et al. (1999)
LONG-TERM TOXICITIES – PT GU
0.019
0.004
GI
0.015
0.006
Schulte et al. (2000), Slater et al. (1999)
SD
0.272
0.232
Assume the same as IMRT
GI, gastrointestinal; GU, genitourinary; IMRT, intensity-modulated radiation therapy; PT, proton beam therapy; SBRT stereotactic body radiation therapy; SD, sexual dysfunction; SE, standard error. An RTOG (Radiation Therapy Oncology Group) scale was used to measure toxicity in all but the Kirichenko et al., 2006; scale not reported) and Zelefsky et al., 2006; CTCAE scale) studies.
www.frontiersin.org
August 2012 | Volume 2 | Article 81 | 3
201 161
Parthan et al.
Cost effectiveness of prostate radiation
quality of life (HRQoL) on a scale from 0 to 1, where 0 reflects death and 1 reflects perfect health. The QALYs associated with each of the long-term toxicity health states were estimated using utility weights derived from Stewart et al. (2005). The utility value for not experiencing treatment-related long-term toxicity was assumed to be 1, and the utility value for death was 0. QALYs in each Markov state and cycle were determined by multiplying the utility
Table 3 | Model utility inputs. Utility parameters
Base-case
SE
associated with each of the long-term toxicities by a baseline agespecific background utility for males in the general population (Hamner et al., 2006). Resource utilization
Table 4 displays the base-case resource utilization parameter values for each treatment, including the numbers of routine and toxicity-related office visits and treatments, and the number of patient hours lost due to treatment. The resource utilization parameter values were used to estimate treatment-related costs in the model.
Source
Costs
default value No long-term toxicities
1.00
0.00
Assumption
GU
0.83
0.02
Stewart et al. (2005)
GI
0.71
0.02
Stewart et al. (2005)
SD
0.89
0.01
Stewart et al. (2005)
GU & GI
0.70
0.04
Stewart et al. (2005)
GU & SD
0.79
0.03
Stewart et al. (2005)
GI & SD
0.57
0.04
Stewart et al. (2005)
GU & GI & SD
0.45
0.04
Stewart et al. (2005)
Death
0.00
0.00
By definition
GI, gastrointestinal; GU, genitourinary; SD, sexual dysfunction; SE, standard error; QALYs in each Markov state and cycle are determined by the product of the baseline age-specific background utility for males (Hamner et al., 2006) and the utility associated with long-term toxicities.
Table 5 displays the unit costs per unit of resource utilization that were used in the model. All unit costs in the table reflect Medicare payments. The SBRT treatment cost was based on nationally unadjusted Medicare rates using codes for robotic SBRT, and the remaining treatment costs were based on published sources. For the societal perspective, the age-specific cost per hour of time lost in treatment was based on 2011 estimates from the Bureau of Labor Statistics (2011). Additional patient costs, such as transportation costs, are not included in the societal perspective. From a lost work time perspective, we assumed that this wage value reflects the opportunity cost of time for both working and retired persons of the same age. The cost of routine monitoring and toxicityrelated costs was applied annually for GU and SD whereas for GI a one-time cost was applied assuming that GI symptoms are less
Table 4 | Model resource utilization inputs. Utilization parameters
Value
Source
TREATMENT-RELATED (PER TREATMENT) SBRT Work-time lost (hours per treatment)
10
Expert opinion
90
Ollendorf et al. (2009)
100
Ollendorf et al. (2008)
IMRT Work-time lost (hours per treatment) PT Work-time lost (hours per treatment) ROUTINE MONITORING (PER YEAR) Office visit (1st year)
2
Expert opinion
Office visit (subsequent years)
1
Expert opinion
PSA
2
Expert opinion
TOXICITY-RELATED GU toxicity (per year) Routine office visit Pharmacologic treatment (oxybutynin) Cystoscopy
2 365
Expert opinion Daily dosage based on package insert
1
Expert opinion
Routine office visit
2
Assumption
Enema (additional colonoscopy and sigmoidoscopy if needed)*
1
Assume one-time treatment for GI toxicity
GI toxicity (one-time utilization)
SD toxicity (per year) Pharmacologic treatment (sildenafil)
22
Cooke et al. (2005)
GI, gastrointestinal; GU, genitourinary; IMRT, intensity-modulated radiation therapy; PSA, prostate specific antigen; PT, proton beam therapy; SBRT, stereotactic body radiation therapy; SD, sexual dysfunction. *Patients experiencing late GI toxicity were first treated with a 6-month course of enema in 70% of the cases. The remaining patients were assumed to undergo a colonoscopy followed by an average of three sigmoidoscopies and an additional 6-month course of enema. The cost is calculated as a one-time weighted average cost.
Frontiers in Oncology | Radiation Oncology
202 162
August 2012 | Volume 2 | Article 81 | 4
Parthan et al.
Cost effectiveness of prostate radiation
Table 5 | Model cost inputs. Cost parameters
Value
Source
SBRT
$20,889
Medicare rates – data on file, Accuray Inc.
IMRT
$28,805
Konski et al. (2006), MAG Mutual (2011)
PT
$65,250
Ollendorf et al. (2008)
$20
Bureau of Labor Statistics (2011)
Urologist
$177
MAG Mutual (2011)
Office visit
$102
MAG Mutual (2011)
PSA
$103
MAG Mutual (2011)
Daily pharmacologic treatment (oxybutynin)
$1
Red Book (2010)‡
Cystoscopy
$214
MAG Mutual (2011)
$259†
MAG Mutual (2011), Red Book (2010)‡
$12
Red Book (2010)‡
TREATMENT COST
Work-time lost (cost per hour) ROUTINE MONITORING
TOXICITY-RELATED GU toxicity
GI toxicity Enema (additional colonoscopy and sigmoidoscopy if needed)* SD toxicity Daily pharmacologic treatment (sildenafil)
GI, gastrointestinal; GU, genitourinary; IMRT, intensity-modulated radiation therapy; PSA, prostate specific antigen; PT, proton beam therapy; SBRT, stereotactic body radiation therapy; SD, sexual dysfunction. *Patients experiencing late GI toxicity were first treated with a 6-month course of enema in 70% of the cases. The remaining patients were assumed to undergo a colonoscopy followed by an average of three sigmoidoscopies and an additional 6-month course of enema. The cost is calculated as a one-time weighted average cost. †
Assumes one-time treatment cost.
‡
Costs are inflated to 2011 values based on the Consumer Price Index (Bureau of Labor Statistics, 2011).
likely to be chronic. All costs were based on published sources and costs used in the analysis are expressed in 2011 US dollars, with costs and utilities discounted at 3.0% annually. ANALYSES
Base-case model
Lifetime costs and QALYs per patient were calculated for each treatment. Lifetime costs were calculated by summing all costs associated with each health state that patients spend time in, and QALYs are calculated by summing the product of utilities associated with each health state and the time spent in the health state. The incremental cost-effectiveness ratio (ICER) value was calculated by first rank-ordering the treatment regimens by increasing cost and then comparing each strategy to the next less costly strategy by dividing the additional cost by additional benefit (QALY).
The probability estimates for toxicity and utility were assumed to follow a beta distribution [see Tables 2 and 3 for the standard errors (SE)], and cost parameters were assumed to follow a gamma distribution and the SE was calculated as 50% of the base-case default value. An alternative cost scenario was tested by applying an annual cost for treating GI toxicity, instead of the one-time costs used in the base-case. In the absence of a SD toxicity rate for PT, an alternative toxicity scenario was tested by setting the SD toxicity for PT equal to SBRT. Finally, in threshold analyses, the toxicity of SBRT was varied from the base-case assumption to assess the impact of alternate toxicity rates on base-case results.
RESULTS BASE-CASE MODEL
Table 6 displays the results of the cost-effectiveness analyses from the payer and societal perspectives.
Sensitivity analyses
One-way sensitivity analysis was conducted to determine the parameters to which the ICER is most sensitive. One-way sensitivity analyses were performed by varying selected model parameters based on the 95% confidence interval (CI) of the base-case estimate, where available, while keeping all other parameters constant. CIs were not available for the costs used in the model, and so they were varied through a range of 75%–125% of the base-case estimate while keeping all other parameters constant. Probabilistic sensitivity analysis was also conducted to assess uncertainty in the cost-effectiveness analysis by determining the probability of a treatment being cost-effective at a threshold of $50,000/QALY. www.frontiersin.org
Payer perspective
SBRT was the least expensive option in terms of lifetime costs ($24,873), followed by IMRT ($33,068) and PT ($69,412). IMRT and PT were both more costly and yielded fewer QALYs when compared with SBRT (total QALYs: 8.11, 8.05, 8.06 for SBRT, IMRT, PT, respectively). Societal perspective
When the value of lost time in treatment was included, SBRT remained the least expensive treatment option ($25,097), followed by IMRT ($35,088) and then PT ($71,657). Once again, IMRT and August 2012 | Volume 2 | Article 81 | 5
203 163
Parthan et al.
Cost effectiveness of prostate radiation
Table 6 | Cost-effectiveness results for all comparator treatments for
Table 7 | Probability of SBRT being cost-effective at the $50,000/QALY
the base-case.
threshold.
Total per patient
Incremental
ICER Costs/
SBRT Comparator
Payer perspective
Societal perspective
$50,000/QALY
$50,000/QALY
IMRT
75.1%
75.1%
PT
94.1%
95.5%
QALY gained Costs
QALYs
Costs
QALYs
PAYER PERSPECTIVE SBRT
$24,873
8.11
–
–
Reference
IMRT, intensity-modulated radiation therapy; PT, proton beam therapy; QALY,
IMRT
$33,068
8.05
$8,195
Dominated*
quality-adjusted life year; SBRT, stereotactic body radiation therapy.
PT
$69,412
8.06
$44,539
−0.062 −0.047
Dominated
SOCIETAL PERSPECTIVE† SBRT
$25,097
8.11
–
–
Reference
IMRT
$35,088
8.05
$9,991
Dominated
PT
$71,657
8.06
$46,560
−0.062 −0.047
Table 8 | Alternative sensitivity analysis with proton therapy toxicity set equal to SBRT toxicity.
Dominated
Total per patient
Incremental
ICER Costs/ QALY gained
ICER, incremental cost-effectiveness ratio; IMRT, intensity-modulated radiation therapy; QALY, quality-adjusted life year.
Costs
QALYs
Costs
QALYs
NOTE: Results are estimated for a patient 65 years old using Medicare reimburse-
PAYER PERSPECTIVE
ment rates. *Dominated – higher cost and lower QALY. †
Societal perspective also includes productivity costs owing to time spent in
treatment.
SBRT
$24,873
8.11
–
–
Reference
IMRT
$33,068
8.05
$8,195
Weakly dominated*
PT
$69,094
8.17
$44,221
−0.062 0.057
$13,755,207
SOCIETAL PERSPECTIVE†
PT were both dominated by SBRT because they were more costly and yielded fewer QALYs when compared with SBRT (total QALYs: 8.11, 8.05, 8.06 for SBRT, IMRT, PT, respectively).
SBRT
$25,097
8.11
–
–
Reference
IMRT
$35,088
8.05
$9,991
Weakly dominated
PT
$71,339
8.17
$46,242
−0.062 0.057
$14,383,693
ICER, incremental cost-effectiveness ratio; IMRT, intensity-modulated radiation
SENSITIVITY ANALYSES
therapy; PT, proton beam therapy; QALY, quality-adjusted life year; SBRT, stereo-
When the toxicity parameters were varied based on their CIs, and the costs by ±25%, the results did not change from the base-case in both payer and societal perspectives; SBRT was the dominant strategy, being less expensive with more QALYs compared to IMRT and PT. The probabilities of SBRT being cost-effective at $50,000/QALY from both the payer and societal perspective are presented in Table 7. The probabilities of SBRT being cost-effective compared to IMRT and PT are 75.1% and 94.1%, respectively, from the payer perspective; 75.1% and 95.5%, respectively, from the societal perspective. Figure 2 displays the probabilities of SBRT being cost-effective compared to IMRT and PT, from the payer (Figure 2A) and societal (Figure 2B) perspective. The alternative cost scenario analysis using an annual cost for treating GI toxicity, instead of the one-time costs used in the basecase model, produced results that were similar to the base-case. Both IMRT and PT remained more costly and yielded fewer QALYs when compared with SBRT from either perspective. Also, Table 8 displays the results of an alternative toxicity scenario where SD toxicity for PT was set equal to SBRT, in contrast to the base-case model which set PT equal to IMRT. When the SD toxicity for PT was set equal to SBRT, then SBRT weakly dominates IMRT and no longer dominates PT. In threshold analyses, when all three toxicity rates for SBRT were increased by 23% from the base-case, the total lifetime costs for SBRT was lower than for IMRT ($25,037 versus $33,068), and the QALYs for SBRT were slightly higher than for IMRT (8.051 versus 8.049). If all three toxicity rates for SBRT were increased by ≥24%, the QALYs for SBRT are lower than for IMRT. Furthermore, the threshold analyses showed that IMRT was only cost-effective
tactic body radiation therapy.
Frontiers in Oncology | Radiation Oncology
204 164
NOTE: Results are estimated for a patient 65 years old using Medicare reimbursement rates. *Dominated – higher cost and lower QALY. †
Societal perspective also includes productivity costs owing to time spent in
treatment.
compared to SBRT at a $100,000/QALY threshold when all three toxicity rates for SBRT were increased by 54% from the base-case rates, from a payer’s perspective. Similarly, from a societal perspective, IMRT was only found to be cost-effective at a $100,000/QALY threshold when the toxicity rates of SBRT were increased by at least 61% from the base-case rates. Among the three toxicities included in the model, the SD toxicity rate for SBRT was found to be lower than for IMRT. If the SD toxicity rate for SBRT is increased by more than 43% from the basecase rate, then the QALY for SBRT was lower than for IMRT. From a payer’s perspective, the threshold analyses showed that IMRT was only cost-effective compared to SBRT at a $100,000/QALY threshold when the SD toxicity rate for SBRT was increased by 97% from the base-case rate. Similarly, from a societal perspective, IMRT was only cost-effective compared to SBRT at a $100,000/QALY threshold when the SD toxicity rate for SBRT was increased by 109% from the base-case rate.
DISCUSSION In this study, we found that SBRT was less expensive than both IMRT and PT, with SBRT patients expecting to have a better quality of life owing to its more favorable toxicity profile. The August 2012 | Volume 2 | Article 81 | 6
Parthan et al.
Cost effectiveness of prostate radiation
FIGURE 2 | Probability of SBRT being cost-effective when compared to IMRT and Proton Therapy, from the Payer (A) and Societal (B) Perspective. Note: the cost-effectiveness acceptability curve shows the percentage of iterations in the probabilistic sensitivity analysis that are cost-effective at a certain threshold.
probabilistic sensitivity analyses from both the payer and societal perspectives showed that, compared to the other treatments, SBRT is cost-effective at a $50,000/QALY threshold in 75%–96% of model simulations. There is a continued trend of increasing utilization of more costly treatments for localized prostate cancer. From 2000 to 2008, the use of IMRT over the older 3D conformal radiation has increased from 28.7% to 81.7% (Nguyen et al., 2011). Since 2008, there has been an exponential increase in the number of proton facilities built in the United States – each of which costs approximately $200 million – with a corresponding increase in its use for prostate cancer treatment. Cost-effectiveness is one way to examine the “value” of different treatment options. Consistent with prior findings by Konski et al. (2007), we also demonstrate that proton therapy is unlikely to be cost-effective. Importantly, we find that newer technology does not always equate to costlier treatment. SBRT, because of an ability to shorten radiation treatment to five fractions, results in cost savings compared to IMRT and PT. If longer-term follow-up continues to demonstrate favorable toxicity and disease control outcomes for SBRT,
www.frontiersin.org
then this study provides important data which may have policy implications. This study has several limitations worth mentioning. There are limited data on SBRT for localized prostate cancer. In fact, since this study began, reports from five additional SBRT studies, each with varying numbers of patients and months of follow-up, have been published (Aluwini et al., 2010; Bolzicco et al., 2010; Kang et al., 2011; Townsend et al., 2011; Jabbari et al., 2012). None of these studies reached conclusions that differ substantially from the assumptions used in the present analysis (i.e., toxicity remains relatively low and disease control mirrors that obtained with other radiation therapy options). Further, the published literature on SBRT of localized prostate cancer has consisted mostly of patients treated with the CyberKnife Robotic Radiosurgery System (Accuray Incorporated, Sunnyvale, CA, USA; Friedland et al., 2009; King et al., 2009, 2012; Katz et al., 2010; Freeman and King, 2011). Two studies have been published which used conventional linear accelerators to deliver prostate SBRT (Madsen et al., 2007; Boike et al., 2011), but both used lower doses compared to current SBRT standards and therefore were not included in the model estimates.
August 2012 | Volume 2 | Article 81 | 7
205 165
Parthan et al.
Cost effectiveness of prostate radiation
Inclusion of toxicity results from studies that used lower doses of SBRT may bias our results further in favor of SBRT. There is also a lack of data on the median time to resolution of long-term toxicity. Therefore, in the model, patients who develop long-term toxicity were assumed to remain in a health state reflective of their toxicity until they die. In conclusion, based on the assumption that each treatment modality results in equivalent long-term disease control, results from this study suggest that SBRT is cost-effective, resulting in cost savings and improved quality-adjusted survival compared to IMRT and PT for the treatment of localized prostate cancer. Additional studies are needed to directly examine the comparative effectiveness of the different radiation treatments for localized prostate cancer. Given the lack of randomized trial data, this study provides important and novel information based on currently available published evidence, and contributes to an understanding of the comparative value of three
REFERENCES Agency for Healthcare Research and Quality. (2010). Comparative Evaluation of Radiation Treatments for Clinically Localized Prostate Cancer: An Update. Technology Assessment Report ID: CANT1209. Altekruse, S. F., Kosary, C. L., Krapcho, M., Neyman, N., Aminou, R., Waldron, W., Ruhl, J., Howlader, N., Tatalovich, Z., Cho, H., Mariotto, A., Eisner, M. P., Lewis, D. R., Cronin, K., Chen, H. S., Feuer, E. J., Stinchcomb, D. G., and Edwards, B. K. (eds). (2010). SEER Cancer Statistics Review, 1975-2007. Bethesda, MD: National Cancer Institute. Aluwini, S., van Rooij, P., Hoogeman, M., Bangma, C., Kirkels, W. J., Incrocci, L., and Kolkman-Deurloo, I. K. (2010). Cyber knife stereotactic radiotherapy as monotherapy for low- to intermediate-stage prostate cancer: early experience, feasibility, and tolerance. J. Endourol. 24, 865–869. Boike, T. P., Lotan, R., Cho, L. C., Brindle, J., DeRose, P., Xie, X. J., Yan, J., Foster, R., Pistenmaa, D., Perkins, A., Cooley, S., and Timmerman, R. (2011). Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediaterisk prostate cancer. J. Clin. Oncol. 29, 2020–2026. Bolzicco, G., Favretto, M. S., Scremin, E., Tambone, C., Tasca, A., and Guglielmi, R. (2010). Image-guided stereotactic body radiation therapy for clinically localized prostate cancer: preliminary clinical results. Technol. Cancer Res. Treat. 9, 473–477. Bureau of Labor Statistics. (2011). Usual Weekly Earnings of Wage and Salary
Workers, ed. M. D. Levi (Bureau of Labor Statistics). Chen, R. C., Clark, J. A., and Talcott, J. A. (2009). Individualizing qualityof-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J. Clin. Oncol. 27, 3916–3922. Coen, J. J., Paly, J. J., Niemierko, A., Weyman, E., Rodrigues, A., Shipley, W. U., Zietman, A. L., and Talcott, J. A. (2012). Long-term quality of life outcome after proton beam monotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 82, e201–e209. Cooke, C. E., Wong, W., and Lee, H. (2005). Utilization and cost of sildenafil in a large managed care organization with a quantity limit on sildenafil. J. Manag. Care Pharm. 11, 674–680. Freeman, D. E., and King, C. R. (2011). Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat. Oncol. 6, 3. Friedland, J. L., Freeman, D. E., Masterson-McGary, M. E., and Spellberg, D. M. (2009). Stereotactic body radiotherapy: an emerging treatment approach for localized prostate cancer. Technol. Cancer Res. Treat. 8, 387–392. Hamner, J., Lawrence, W. F., Anderson, J. P., Kaplan, R. M., and Fryback, D. G. (2006). Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-oflife scores. Med. Decis. Making 26, 391–400. Jabbari, S., Weinberg, V. K., Kaprealian, T., Hsu, I. C., Ma, L., Chuang,
Frontiers in Oncology | Radiation Oncology
206 166
types of external beam radiation treatments for localized prostate cancer.
AUTHORS’ CONTRIBUTIONS Anju Parthan: Primary investigator, Narin Pruttivarasin: Primary economic modeler, Douglas C. A. Taylor: Study director, Vivek Pawar: Secondary economic modeler, Diane Davies: Health economist and author, Akash Bijlani: Health policy/economic specialist and author, Kristen Hassmiller Lich: Health policy specialist and author, Ronald Chen: Radiation oncologist and senior author.
ACKNOWLEDGMENTS We would like to thank Grace Yang, MPA, MA, for her help with conducting the meta-analysis. We would also like to thank Virginia M. Rosen, PhD, who provided medical writing assistance on behalf of OptumInsight which was contracted by Accuray Incorporated to produce this manuscript. C., Descovich, M., Shiao, S., Shinohara, K., Roach, M. III, and Gottschalk,A. R. (2012). Stereotactic body radiotherapy as monotherapy or post-external beam radiotherapy boost for prostate cancer: technique, early toxicity, and PSA response. Int. J. Radiat. Oncol. Biol. Phys. 82, 228–234. Kang, J. K., Cho, C. K., Choi, C. W., Yoo, S., Kim, M. S., Yang, K., Yoo, H., Kim, J. H., Seo, Y. S., Lee, D. H., and Jo, M. (2011). Image-guided stereotactic body radiation therapy for localized prostate cancer. Tumori 97, 43–48. Katz,A. J., Santoro, M.,Ashley, R., Diblasio, F., and Witten, M. (2010). Stereotactic body radiotherapy for organconfined prostate cancer. BMC Urol. 10, 1. doi:10.1186/1471-2490-10-1 King, C. R., Brooks, J. D., Gill, H., Pawlicki, T., Cotrutz, C., and Presti, J. C. Jr. (2009). Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 73, 1043–1048. King, C. R., Brooks, J. D., Gill, H., and Presti, J. C. Jr. (2012). Longterm outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 82, 877– 882. Kirichenko, A. V., Ruth, K., Horwitz, E. M., Buyyounouski, M. K., Feigenberg, S. J., Chen, D. Y., and Pollack, A. (2006). Intensity modulated radiation therapy for prostate cancer: preliminary results on treatment morbidity compared to 3D conformal radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 66, S326.
Konski, A., Speier, W., Hanlon, A., Beck, J. R., and Pollack, A. (2007). Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J. Clin. Oncol. 25, 3603–3608. Konski, A., Watkins-Bruner, D., Feigenberg, S., Hanlon, A., Kulkarni, S., Beck, J. R., Horwitz, E. M., and Pollack, A. (2006). Using decision analysis to determine the costeffectiveness of intensity-modulated radiation therapy in the treatment of intermediate risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 66, 408–415. Madsen, B. L., His, R. A., Pham, H. T., Fowler, J. F., Esagui, L., and Corman, J. (2007). Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical results. Int. J. Radiat. Oncol. Biol. Phys. 67, 1009–1105. MAG Mutual. (2011). 2011 MAG Mutual Physicians’ Fee and Coding Guide. Atlanta: MAG Mutual Healthcare Solutions, Inc., 2010. Print. Martin, A., and Gaya, A. (2010). Stereotactic body radiotherapy: a review. Clin. Oncol. 22, 157–172. National Cancer Institute. (2012). Bethesda, MD: NIH, DHHS. Available at: http://www.cancer.gov/ cancertopics/types/prostate [accessed May 24, 2012]. Nguyen, P. L., Gu, X., Lipsitz, S. R., Choueiri, T. K., Choi, W. W., Lei, Y., Hoffman, K. E., and Hu, J. C. (2011). Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J. Clin. Oncol. 29, 1517–1524.
August 2012 | Volume 2 | Article 81 | 8
Parthan et al.
Ollendorf, D. A., Hayes, J., McMahon, P., Kuba, M., Tramontano, A., and Pearson, S. D. (2008). Brachytherapy & Proton Beam Therapy for Treatment of Clinically-Localized, LowRisk Prostate Cancer. Boston: Institute for Clinical and Economic Review. [Final appraisal document]. Ollendorf, D. A., Hayes, J., McMahon, P., and Pearson, S. D. (2009). Active Surveillance & Radical Prostatetomy for the Management of Low-Risk, Clinically-Localized Prostate Cancer. Boston: Institute for Clinical and Economic Review. [Final appraisal document]. Red Book. (2010). Pharmacy’s Fundamental Reference. Montvale, NJ: Thomson Reuters (Healthcare) Inc. Sanda, M. G., Dunn, R. L., Michalski, J., Sandler, H. M., Northouse, L., Hembroff, L., Lin, X., Greenfield, T. K., Litwin, M. S., Saigal, C. S., Mahadevan, A., Klein, E., Kibel, A., Pisters, L. L., Kuban, D., Kaplan, I., Wood, D., Ciezki, J., Shah, N., and Wei, J. T. (2008). Quality of life and satisfaction with outcome among prostate-cancer
www.frontiersin.org
Cost effectiveness of prostate radiation
survivors. N. Engl. J. Med. 358, 1250–1261. Schulte, R. W., Slater, J. D., Rossi, C. J. Jr., and Slater, J. M. (2000). Value and perspectives of proton radiation therapy for limited stage prostate cancer. Strahlenther. Onkol. 176, 3–8. Slater, J. D., Rossi, C. J. Jr., Yonemoto, L. T., Reyes-Molyneux, N. J., Bush, D. A., Antoine, J. E., Miller, D. W., Teichman, S. L., and Slater, J. M. (1999). Conformal proton therapy for earlystage prostate cancer. Urology 53, 978–984. Slater, J. D., Yonemoto, L. T., Rossi, C. J. Jr., Reyes-Molyneux, N. J., Bush, D. A., Antoine, J. E., Loredo, L. N., Schulte, R. W., Teichman, S. L., and Slater, J. M. (1998). Conformal proton therapy for prostate carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 42, 299–304. Stewart, S. T., Lenert, L., Bhatnagar, V., and Kaplan, R. M. (2005). Utilities for prostate cancer health states in men aged 60 and older. Med. Care 43, 347–355. Townsend, N. C., Huth, B. J., Ding, W., Garber, B., Mooreville, M.,
Arrigo, S., Lamond, J., and Brady, L. W. (2011). Acute toxicity after cyberknife-delivered hypofractionated radiotherapy for treatment of prostate cancer. Am. J. Clin. Oncol. 34, 6–10. Wiegner, E. A., and King, C. R. (2010). Sexual function after stereotactic body radiotherapy for prostate cancer: results of a prospective clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 78, 442–448. Zelefsky, M. J., Chan, H., Hunt, M., Yamada, Y., Shippy, A. M., and Amols, H. (2006). Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J. Urol. 176(4 Pt 1), 1415–1419. Zelefsky, M. J., Fuks, Z., Hunt, M., Yamada, Y., Marion, C., Ling, C. C., Amols, H., Venkatraman, E. S., and Leibel, S. A. (2002). High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int. J. Radiat. Oncol. Biol. Phys. 53, 1111–1116.
Conflict of Interest Statement: This study was funded by Accuray, Inc.
Received: 20 June 2012; paper pending published: 04 July 2012; accepted: 12 July 2012; published online: 20 August 2012. Citation: Parthan A, Pruttivarasin N, Davies D, Taylor DCA, Pawar V, Bijlani A, Lich KH and Chen RC (2012) Comparative cost-effectiveness of stereotactic body radiation therapy versus intensitymodulated and proton radiation therapy for localized prostate cancer. Front. Oncol. 2:81. doi: 10.3389/fonc.2012.00081 This article was submitted to Frontiers in Radiation Oncology, a specialty of Frontiers in Oncology. Copyright © 2012 Parthan, Pruttivarasin, Davies, Taylor, Pawar, Bijlani, Lich and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
August 2012 | Volume 2 | Article 81 | 9
207 167
SPINE Volume 32, Number 2, pp 193–199 ©2007, Lippincott Williams & Wilkins, Inc.
Radiosurgery for Spinal Metastases Clinical Experience in 500 Cases From a Single Institution Peter C. Gerszten, MD, MPH, Steven A. Burton, MD, Cihat Ozhasoglu, PhD, and William C. Welch, MD, FACS Radiosurgery for spinal metastases
Study Design. A prospective nonrandomized, longitudinal cohort study. Objective. To evaluate the clinical outcomes of singlefraction radiosurgery as part of the management of metastatic spine tumors. Summary of Background Data. The role of stereotactic radiosurgery for the treatment of spinal lesions has previously been limited by the availability of effective target immobilization and target tracking devices. Large clinical experience with spinal radiosurgery to properly assess clinical experience has previously been limited. Methods. A cohort of 500 cases of spinal metastases underwent radiosurgery. Ages ranged from 18 to 85 years (mean 56). Lesion location included 73 cervical, 212 thoracic, 112 lumbar, and 103 sacral. Results. The maximum intratumoral dose ranged from 12.5 to 25 Gy (mean 20). Tumor volume ranged from 0.20 to 264 mL (mean 46). Long-term pain improvement occurred in 290 of 336 cases (86%). Long-term tumor control was demonstrated in 90% of lesions treated with radiosurgery as a primary treatment modality and in 88% of lesions treated for radiographic tumor progression. Twenty-seven of 32 cases (84%) with a progressive neurologic deficit before treatment experienced at least some clinical improvement. Conclusions. The results indicate the potential of radiosurgery in the treatment of patients with spinal metastases, especially those with solitary sites of spine involvement, to improve long-term palliation. Key words: CyberKnife , image-guided surgery, robotic surgery, spine metastases, spine tumors, stereotactic radiosurgery. Spine 2007;32:193–199
In the past decade, surgical spinal oncology has focused on new surgical approaches to the spine, the application of new instrumentation to spinal reconstruction, various forms of radiation delivery systems, and, most importantly, complication avoidance. Patients with metastatic spine tumors are usually debilitated and at a high risk for surgical morbidity. For patients with limited life expectancies from their underlying disease, high surgical comFrom the Departments of Neurological Surgery and Radiation Oncology, University of Pittsburgh Medical Center, Pittsburgh, PA. Acknowledgment date: September 25, 2005. First revision date: December 13, 2005. Second revision date: February 22, 2006. Third revision date: April 7, 2006. Acceptance date: April 10, 2006. The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication. Professional Organizational funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. Address correspondence and reprint requests to Peter C. Gerszten, MD, MPH, Department of Neurological Surgery, Presbyterian University Hospital, Suite B-400, 200 Lothrop Street, Pittsburgh, PA 15213; E-mail: gersztenpc@upmc.edu
plication rates with a subsequent decrease in quality of life are most unacceptable. The role of radiation therapy in the treatment of metastatic tumors of the spine is well established and is often the initial treatment modality.1–7 The goals of local radiation therapy in the treatment of spinal tumors have been palliation of pain, prevention of pathologic fractures, and halting progression of or reversing neurologic compromise.8 Surgery is usually reserved for spinal instability or subluxation, in patients with neurologic deficits, despite other forms of therapy, and those with intractable pain attributable to an isolated lesion.9 –12 Studies have previously determined the clinical efficacy of single-fraction therapy for painful bone metastases.13,14 A primary factor that limits radiation dose for local vertebral tumor control with conventional radiotherapy is the relatively low tolerance of the spinal cord to radiation. Conventional external beam radiotherapy lacks the precision to deliver large single-fraction doses of radiation to the spine near radiosensitive structures such as the spinal cord. It is the low tolerance of the spinal cord to radiation that often limits the treatment dose to a level that is far below the optimal therapeutic dose.1,15,16 Radiotherapy may provide less than optimal clinical response since the total dose is limited by the tolerance of the spinal cord. Precise confinement of the radiation dose to the treatment volume, as is the case for intracranial radiosurgery, should increase the likelihood of successful tumor control at the same time that the risk of spinal cord injury is minimized.16 –24 In stereotactic radiosurgery, a high dose of radiation is delivered in a single fraction to a well-defined intracranial or extracranial target.25 Radiosurgery has been shown to be very effective for controlling intracranial malignancies.26 –32 Stereotactic radiosurgery has been demonstrated to be an effective treatment for brain metastases, either with or without whole-brain radiation therapy, with an 85% to 95% control rate. The emerging technique of spinal radiosurgery represents a logical extension of the current state-of-the-art radiation therapy. Since Hamilton et al33 first described the possibility of linear-accelerator based spinal stereotactic radiosurgery in 1995, multiple centers have attempted to pursue largefraction conformal radiation delivery to spinal lesions using a variety of technologies.16,19 –24,33– 41 Recent technological developments, including imaging technology for 3-dimension localization and pretreatment planning, the advent of intensity modulated radiated therapy and a higher degree of accuracy in achieving target dose conformation while sparing normal surrounding tissue have allowed clinicians to 193
168 208
194 Spine • Volume 32 • Number 2 • 2007
expand radiosurgery applications to treat malignant vertebral body lesions within close proximity to the spinal cord and cauda equina. Researchers have shown the feasibility and clinical efficacy of hypofractionated stereotactic body radiotherapy for metastases to the spine.16,18 –24,42– 45 Stereotactic radiosurgery for tumors of the spine has more recently been demonstrated to be accurate, safe, and efficacious.16,19,20,22–24,33–38 Others have demonstrated the effectiveness of protons for spinal and paraspinal tumors.46 There has been a rapid increase in the use of radiosurgery as a treatment alternative for malignant tumors involving the spine.6,8,19,28,46 The purpose of this study was to evaluate the clinical outcomes of radiosurgery for the treatment of metastases to the spine to see if such a radiosurgery technique parallels the efficacy that has been demonstrated for the treatment of intracranial metastases. Materials and Methods This study involved the prospective evaluation of 500 lesions of histologically proven metastases to the spine that were treated using the CyberKnife Image-Guided Radiosurgery System with the Dynamic Tracking System 3.0 software. This represented a total of 393 patients; some patients had a second lesion subsequently treated. All cases were analyzed individually, addressing the outcome of that particular lesion treated. All patients were treated at the University of Pittsburgh Medical Center, Pittsburgh, PA, and University of Pittsburgh’s institutional review board approved the protocol. Computed tomography (CT) and/or magnetic resonance imaging was used to diagnose spinal metastases. Table 1 summarizes the characteristics of the treatment group, including the primary indications for spinal stereotactic radiosurgery that were used for patient selection for this study. Table 2 summarizes the primary sites. Information regarding the first 95 patients in this series was previously published.47 No acute radiation toxicity or new neurologic deficits occurred in that series. These same patients were further followed and included in the current series. Their inclusion allowed for longer-term follow-up of both the safety as well as the long-term efficacy of spinal radiosurgery. Three hundred forty-four lesions had previously undergone external beam irradiation that precluded further conventional
Table 1. Characteristics of the Treatment Group (n 500) Characteristic Previous external beam irradiation Primary indications for radiosurgery treatment Pain Primary treatment modality Tumor progression Progressive neurologic deficit Postsurgical treatment Radiation boost Levels treated Cervical Thoracic Lumbar Sacral Skull tracking Fiducial tracking Mean/median tumor volume (range) Mean maximum dose (range) Mean volume of spinal canal dose 8 Gy
No. Cases 344 336 65 51 32 9 7 73 212 112 103 68 432 46/29 cm3 (0.20–264) 20 Gy (12.5–25) 0.6 cm3
Table 2. Lesion Histopathologies (n 500) No. Renal cell Breast Lung Melanoma Colon Sarcoma Prostate Multiple myeloma Unknown primary Squamous cell (laryngeal) Thyroid Other
93 83 80 38 32 26 24 18 14 12 11 69
irradiation to the involved level. In these 344 cases, prior irradiation was delivered using fractionation schedules ranging from 3 Gy 10 to 2.5 Gy 14. Radiosurgery was felt indicated in order to limit further radiation dose to the neural structures. Tumor dose was not decreased in a uniform manner in these previously irradiated patients. Instead, the maximum dose to the spinal cord or cauda equina was more strictly limited, constrained by the CyberKnife’s inverse treatment planning system. The combination of a steep dose gradient and high conformality of the CyberKnife treatment allows for such high doses to be delivered so close to the adjacent critical structures (e.g., the spinal cord). Except in a single case, patients with myelopathy or cauda equina syndrome from direct tumor progression were not felt to be candidates for radiosurgery treatment. Exclusion criteria for CyberKnife treatment were: (1) evidence of overt spinal instability or (2) neurologic deficit resulting from bony compression of neural structures. For evaluation of pain relief, a 10-point visual analog scale with an intensity description was administered to all patients before radiosurgery and one month after radiosurgery. Pain scores range from (0 no pain) to 10 (10 worst imaginable pain). This score was recorded in each patient’s clinical chart as well as within a prospectively collected database. Furthermore, subsequent evaluations of pain were obtained directly from the patient on subsequent follow-up visits to either the oncology, radiation oncology, or neurosurgical service as part of the continued multidisciplinary cancer management at the University of Pittsburgh Cancer Institute. This last point of contact was used as the last date of follow-up. The last pain score referable to the treated lesion was used to determine long-term pain control. Pain improvement was operationally defined as a pain score improvement of 3 points. Analgesic usage was also documented to ensure that subsequent pain improvement was not offset by an increase in the amount or type of analgesic usage. Radiographic tumor control was determined by direct comparison by at least 2 of the authors of the most recently obtained images to the immediate pretreatment images. The final written radiology report was also referenced. The CyberKnife consists of a 6-mV compact linear accelerator that is smaller and lighter in weight than linear accelerators used in conventional radiotherapy.48 –51 The smaller size allows it to be mounted on a computer-controlled 6-axis robotic manipulator that permits a much wider range of beam orientations than can be achieved with conventional radiotherapy devices.16,34,52,53 Two diagnostic radiograph cameras are positioned orthogonally (90° offset) to acquire real-time images of the patient’s internal anatomy during treatment. The images
209 169
Radiosurgery for Spinal Metastases • Gerszten et al 195
Figure 1. A representative case of a 42-year-old man with a painful melanoma metastasis of the T3 vertebral body. He had not received prior irradiation. The treatment plan was designed to treat the tumor with a prescribed dose of 18 Gy that was calculated to the 80% isodose line; the maximum tumor dose was 22.5 Gy. The tumor volume was 16.8 cm3 and the spinal cord received a maximum dose of 10 Gy. Notice the conformality of the isodose line around the spinal cord. are processed to identify radiographic features (skull bony landmarks or implanted fiducials) and then automatically compared to the patient’s CT treatment planning study. The precise tumor position is communicated through a real-time control loop to a robotic manipulator that aligns the radiation beam with the intended target.13 An analysis of the accuracy of the CyberKnife radiosurgery system found that the machine has a clinically relevant accuracy of 1.1 0.3 mm using a 1.25-mm CT slice thickness.53 The CyberKnife spinal radiosurgery treatment consists of 3 distinct components: (1) CT image acquisition based on skull bony landmarks or implanted bone fiducials, (2) treatment planning, and (3) the treatment itself.54 All cervical lesions down to C7 were tracked relative to skull bony landmarks. All patients with cervical lesions were fitted with a noninvasive molded Aquaplast facemask (Aquaplast Corp., Wyckoff, NJ) that stabilized the head and neck on a radiographically transparent headrest. All other lesions were tracked relative to fiducial markers placed within the bone adjacent to the lesion. Because these implanted fiducials have a fixed relationship with the bone in which they are implanted, any movement in the vertebrae would be detected as movement in
Figure 2. A representative case of a 66-year-old woman with an isolated painful T6 metastasis previously treated with 30 Gy external beam irradiation in 10 fractions. Sagittal and axial projections of the isodose lines of the treatment plan (A and B). The 80% isodose line represents the prescribed dose of 16 Gy, the tumor volume is 10.3 cm3, and 0.3 cm3 of the spinal cord received greater than 8 Gy. The patient experienced pain relief within 1 month.
170 210
the fiducials, and this movement is detected and compensated for by the CyberKnife . For cervical spine lesions, CT images were acquired using 1.25-mm thick slices from the top of the skull to the bottom of the cervical spine. All other lesions underwent fluoroscopically guided percutaneous placement of 4 – 6 gold fiducial markers ( -Omega Services, Inc., Bellflower, CA) into the pedicles immediately adjacent to the lesion to be treated using a standard Jamshidi Bone Marrow Biopsy Needle (Allegiance Healthcare Corp., McGraw Park, IL), as previously described.54 The fiducial placement procedure was performed in the operating room in an outpatient setting before undergoing the planning CT. The patient was placed in a supine position in a conformal alpha cradle during CT imaging as well as during treatment. CT images were acquired using 1.25-mm thick slices to include the lesion of interest, as well as all fiducials and critical structures. The second component of the CyberKnife treatment is the development of the radiosurgical treatment plan. In each case, the radiosurgical treatment plan was designed based on tumor geometry, proximity to spinal cord, and location (Figures 1 and 2). The tumor dose was maintained at 12.5–25 Gy contoured to the edge of the target volume (mean 20 Gy). The prescription dose was chosen based on currently used intracranial radiosurgery doses as well as the limitation of the maximum dose to the spinal cord as the primary critical structure for each treatment plan. The planning treatment volume was defined as the gross tumor volume with no margin. The dose was prescribed to the 80% isodose line, which covered the planning treatment volume in all cases. The prescription dose was independent of the tumor volume. For each case, the spinal cord and/or cauda equina was outlined as a critical structure. At the level of the cauda equina, the spinal canal was outlined. Therefore, at the level of the cauda equina, the critical volume is the entire spinal canal and not actual neural tissue. The maximum dose was defined as the dose delivered to a single pixel. Given their relative radiosensitivity, a limit of 2 Gy was set as the maximum dose received by each of the kidneys. The third component of the CyberKnife treatment is the actual treatment delivery. All treatments were performed using a single-fraction technique. The patients were placed on the CyberKnife treatment couch in a supine position with the appropriate immobilization device. Preoperative analgesics, sedation, or steroids were not routinely given. During the treatment, real-time digital radiograph images of the implanted fiducial markers were obtained. The location of the vertebral body being treated was established from these images and is used to determine tumor location as previously described. Closed circuit television was used to observe the patient
196 Spine • Volume 32 • Number 2 • 2007
Table 3. Summary of Pain and Radiographic Outcome for the 4 Most Common Histopathologies (n 294) Long-term pain improvement All patients Renal cell Breast Lung Melanoma Long-term radiographic control All patients Renal cell Breast Lung Melanoma
86% 94% 96% 93% 96% 88% 87% 100% 100% 75%
throughout the treatment. The mean treatment time (patient on the couch) is approximately 90 minutes.
Results Table 1 provides a summary of the clinical characteristics and treatment of the patient cohort. There were 251 women; ages ranged from 18 to 85 years (mean 56). Follow-up ranged from 3 to 53 months (median 21). Sixty-eight cases (i.e., all lesions limited to the cervical spine) were treated using bony landmarks for image guidance. The remaining 432 cases (thoracic, lumbar, and sacral cases) were treated using fiducial tracking. Tumor volume ranged from 0.2 to 264 cm3 (mean 46). During a follow-up ranging from 3 to 53 months (median 21), there were no clinically detectable neurologic signs that could be attributable to the acute or subacute radiation-induced spine cord injury. Posttreatment magnetic resonance imaging failed to reveal any changes suggestive of radiation induced spinal cord toxicity. The most frequent indication for the treatment of spinal tumors is pain, and pain was the primary indication for spinal radiosurgery in 336 cases (67%). Spinal radiosurgery was found to be highly effective at decreasing pain in this difficult patient population, with an overall long-term improvement of pain in 290 of the 336 cases (86%), depending on primary histopathology (Table 3). Long-term pain improvement was demonstrated in 96% of women with breast cancer, 96% of cases with melanoma, 94% of cases with renal cell carcinoma, and 93% of lung cancer cases.55,56 Sixty-five cases (13%) underwent spinal radiosurgery as their primary treatment modality (meaning no prior irradiation to the lesion). When used as a primary treatment modality, long-term tumor control was demonstrated on follow-up imaging in 90% of cases (in all breast, lung, and renal cell carcinoma metastases, and 75% of melanoma metastases).55,56 Spinal radiosurgery was used to treat radiographic tumor progression in 51 cases (10%). These lesions had already received irradiation with significant spinal cord doses. Currently, spinal radiosurgery is often employed as a “salvage” technique for those cases in which further conventional irradiation or surgery is not appropriate. Overall long-term radiographic tumor control was 88%
for all cases (Table 3). Radiographic tumor control differed based on primary pathology: breast (100%), lung (100%), renal cell (87%), and melanoma (75%). Seven cases with radioresistant tumors (e.g., renal cell carcinoma, melanoma, sarcoma) were treated with spinal radiosurgery after conventional irradiation, with or without intensity modulated radiotherapy for a “boost” treatment with equal long-term radiographic control. In this series, there were no cases of tumor progression at the immediate adjacent levels. Thirty of 35 cases (85%) with progressive neurologic deficits before treatment experienced at least some improvement based on independent physical examination by 2 of the authors. The 5 patients (renal cell carcinoma 3, lung 2) who failed to improve after radiosurgery had all received prior conventional irradiation. In all 5 of these cases, open surgical decompression was precluded because of medical comorbidities. In 3 cases, the neurologic status stabilized; the remaining 2 progressed to paraplegia. In these 2 cases, imaging revealed clear tumor progression and spinal cord compression; neurologic impairment was felt not to be due to radiationinduced spinal cord injury. Discussion Standard treatment options for spinal tumors include radiotherapy alone, radionuclide therapy, radiotherapy plus systemic chemotherapy, hormonal therapy, or surgical decompression and/or stabilization followed by radiotherapy.7,57 The role of radiation therapy in the treatment of metastatic tumors of the spine is well established and is often the initial treatment modality.1–7,45 The goals of local radiation therapy in the treatment of spinal tumors have been palliation of pain, prevention of pathologic fractures, and halting progression of or reversing neurologic compromise.8 During the past 2 decades, several clinical trials have compared the relative efficacy of various dosefractionation schedules in producing pain relief.14 The idea of single-fraction radiotherapy for symptomatic bone metastases is not new. Several studies, including a Radiation Therapy Oncology Group Phase III trial as well as a metaanalysis, found no significant difference in complete and overall pain relief between single-fraction and multi-fraction palliative radiation therapy for bone metastases.13,14 Most of these trials used 8 Gy in a single fraction. However, none of these trials were specifically evaluating spinal metastases. In addition, the prescribed doses that were delivered in our study were far greater than 8 Gy (median dose of 19 Gy), possibly translating into a more durable symptomatic response as well as local control. Furthermore, the issue of re-irradiation could not be analyzed by the metaanalysis. The spinal radiosurgery program at the University of Pittsburgh Medical Center began in 2001 with the implementation of extracranial image-guided radiosurgery technology. Our institution’s experience currently represents the largest spinal radiosurgery series in the
211 171
Radiosurgery for Spinal Metastases • Gerszten et al 197
world.22–24,26 This new modality was initially introduced into the treatment paradigm for spinal tumors to a subset of our institution’s oncology patient population that did not meet the criteria for other forms of therapy, including conventional radiotherapy and the latest in open surgical techniques. The indications for spinal radiosurgery at our institution have evolved over time and will continue to evolve as clinical experience increases. This is similar to the evolution of indications for intracranial radiosurgery that occurred in the past. There is no large experience to date with spinal radiosurgery or hypofractionated radiotherapy that has previously developed optimal doses for these treatment techniques. Other centers, using intensity modulated, near-simultaneous, CT image-guided stereotactic radiotherapy techniques have used doses of 6 –30 Gy in 1–5 fractions.20,21,24,43– 45 We initially chose to use a singlefraction radiosurgery technique as opposed to fractionate therapy because of our background of intracranial radiosurgery principles using the Leksell Gamma Knife. Given the lack of adverse consequences to normal tissue, including the spinal cord, we have continued to employ a single-fraction treatment paradigm for our spinal radiosurgery program. In our series, maximum tumor dose was maintained at 15–22.5 Gy delivered in a single fraction. The appropriate dose for spinal radiosurgery for metastatic tumors to the spine has not been determined. In this series, a maximum tumor dose of 20 or 16 Gy to the tumor margin appeared to provide a good tumor control, with no radiation-induced spinal cord or cauda equina injury. Spinal radiosurgery was found to be safe at doses comparable to those used for intracranial radiosurgery without the occurrence of radiation-induced neural injury. In the current series, there was no clinically or radiographically identifiable acute or subacute spinal cord damage attributed to the radiation dose with a follow-up period long enough to have seen such events were they to occur.2,58 – 63 In this series, pain was the primary indication for radiosurgery treatment. Radiation is well known to be effective as a treatment for pain associated with spinal malignancies. This, of course, is different than the primary indication for intracranial radiosurgery for brain metastases. Eighty-six percent of cases were found to have long-term improvement in their pain after radiosurgery treatment accounting for level of pain medication use. This is similar to the success reported by others using hypofractionated radiotherapy techniques.18 –22,42– 45 Conventional external beam irradiation may provide less than optimal pain relief since the total dose is limited by the tolerance of adjacent tissues (e.g., spinal cord). In some cases, posttreatment imaging revealed pathologic fractures, likely the cause of pain and the reason for radiosurgical failure. Such fractures require either open or closed internal fixation to alleviate the pain due to spinal instability. Nevertheless, single-fraction spinal radiosurgery achieved rapid and durable pain control, as
212 172
well as radiologically documented tumor control in the majority of this patient cohort. Overall long-term radiographic tumor control was found to be 88% for all cases. When used as a primary treatment modality, long-term tumor control was demonstrated on follow-up imaging in all breast, lung, and renal cell carcinoma metastases, and 75% of melanoma metastases (overall 90%). Spinal radiosurgery was more frequently employed to treat lesions that had previously been treated with other forms of irradiation. The current status of spinal radiosurgery at the present time as it is used at many centers is as a “salvage” technique for patients in which further conventional irradiation or surgery is not appropriate. As greater experience is gained, the technique will likely evolve into an initial upfront treatment for spinal metastases in certain cases (e.g., oligometastases). This is similar to the evolution that occurred for the treatment of intracranial metastases using radiosurgery that occurred over the past decade. Nine cases in this series (2%) were treated as a postsurgical treatment. Fiducials were implanted at the time of open surgery. Given the steep falloff gradient of the target dose, such treatments can be given early in the postoperative period as opposed to the usual significant delay before standard external beam irradiation is permitted by the surgeon. With the ability to perform effectively spinal radiosurgery, the current surgical approach to these lesions might change. Open surgery for spinal metastases will likely evolve in a similar manner in which malignant intracranial lesions are debulked in such a way as to avoid neurologic deficits and minimize surgical morbidity. The spinal tumors can be removed away from neural structures allowing for immediate decompression, the spine can be instrumented if necessary, and the residual tumor can be safely treated at a later date with radiosurgery, thus further decreasing surgical morbidity. We have found that anterior corpectomy procedures in certain cases can be successfully avoided by posterior decompression and instrumentation alone, followed by radiosurgery to the remaining anterior lesion. One concern that has been raised regarding radiosurgery for spinal metastases is that adjacent levels are not included in the radiation field. One possibility is that the tumor can progress within the adjacent levels. In this series, there were no cases of tumor progression at the immediate adjacent levels, justifying the treatment of the involved spine only. Other authors have also found this not to be the case.37 Further experience with spinal radiosurgery and careful patient follow-up will better define the clinical efficacy of this new treatment modality. There are several theoretical advantages to using a stereotactic radiosurgery technique as a primary treatment modality for spinal tumors. Early treatment of these lesions before the patient becomes symptomatic and the stability of the spine is threatened has obvious advantages.22 Conformal radiosurgery avoids the need to irradiate large segments of the spinal cord. Early stereotactic radiosurgery treat-
198 Spine • Volume 32 • Number 2 • 2007
ment of spinal lesions may obviate the need for extensive spinal surgeries for decompression and fixation in these already debilitated patients. It may also avoid the need to irradiate large segments of the spinal column, known to have a deleterious effect on bone marrow reserve in these patients. Avoiding open surgery as well as preserving bone marrow function facilitate continuous chemotherapy in this patient population. Furthermore, improved local control such as has been the case with intracranial radiosurgery could translate into more effective palliation and potentially longer survival. An advantage to the patient of using single-fraction radiosurgery is that the treatment can be completed in a single day rather than over the course of several weeks, which is not inconsequential for patients with a limited life expectancy. The technique may be useful to capitalize on possible advantages of radiosensitizers. In addition, cancer patients may have difficulty with access to a radiation treatment facility for prolonged, daily fractionated therapy. A large single fraction of irradiation may be more radiobiologically advantageous to certain tumors such as sarcomas, melanomas, and renal cell metastases compared to prolonged fractionated radiotherapy. Clinical response such as pain or improvement of a neurologic deficit might also be more rapid with a radiosurgery technique. Finally, the procedure is minimally invasive compared to open surgical techniques and can be performed in an outpatient setting. Conclusions In the largest clinical series to date, this study demonstrated that single-fraction spinal stereotactic radiosurgery for metastases is both safe and clinically effective. Spinal radiosurgery represents a logical extension of the current state-of-the-art radiation therapy. The major potential benefits of radiosurgical ablation of spinal metastases are relatively short treatment time in an outpatient setting combined with potentially better local control of the tumor with minimal risk of side effects. Such an outcome could translate into better palliation of symptoms and a longer survival period while avoiding the significant morbidity associated with open surgical intervention. In addition, this technique allows for the treatment of lesions previously irradiated using conventional external beam irradiation. Spinal radiosurgery offers an important new therapeutic modality for the treatment of spinal metastases. Further experience with higher irradiation doses as well as improved tumor imaging will likely lead to even better clinical outcomes. Key Points ● Single-fraction radiosurgery was used to safely treat 500 spinal metastases. ● Overall long-term pain improvement occurred in 86% of cases.
● Overall long-term radiographic tumor control was demonstrated in 88% of cases.
References 1. Faul CM, Flickinger JC. The use of radiation in the management of spinal metastases. J Neurooncol 1995;23:149 – 61. 2. Kim YH, Fayos JV. Radiation tolerance of the cervical spinal cord. Radiology 1981;139:473– 8. 3. Markoe AM, Schwade JG. The role of radiation therapy in the management of spine and spinal cord tumors. In: Rea GL, ed. Spine Tumors. Park Ridge, IL: American Association of Neurological Surgeons; 1994:23–35. 4. Shapiro W, Posner JB. Medical vs surgical treatment of metastatic spinal cord tumors. In: Thompson R, ed. Controversies in Neurology. New York, NY: Raven Press; 1983:57– 65. 5. Sundaresan N, Krol G, Digiacinto CV, et al. Metastatic tumors of the spine. In: Sundaresan BSH, Schiller AL, Rosenthal DL, eds. Tumors of the Spine. Philadelphia, PA: Saunders; 1990:279 –304. 6. Sundaresan N, Digiacinto GV, Hughes JEO, et al. Treatment of neoplastic spinal cord compression: Results of a prospective study. Neurosurgery 1991; 29:645–50. 7. Gerszten PC, Welch WC. Current surgical management of metastatic spinal disease. Oncology (Williston Park) 2000;14:1013–36. 8. Lu C, Stomper PC, Drislane FW, et al. Suspected spinal cord compression in breast cancer patients: A multidisciplinary risk assessment. Breast Cancer Res Treat 1998;51:121–31. 9. Ghanayem AJ. Metastatic tumors of the spine. In: Vaccaro AR, Betz RR, Zeidman SM. eds. Principles and Practice of Spine Surgery. Philadelphia, PA: Mosby; 2003:213–22. 10. Krishnaney AA, Steinmetz MP, Benzel EC. Biomechanics of metastatic spine cancer. Neurosurg Clin N Am 2004;15:375– 80. 11. Perrin RG, Laxton AW. Metastatic spine disease: Epidemiology, pathophysiology and evaluation of patients. Neurosurg Clin N Am 2004;15:365–73. 12. Wedin R, Bauer HC, Rutqvist LE. Surgical treatment for skeletal breast cancer metastases. Cancer 2001;92:257– 62. 13. Bruner D, Winter K, Hartsell W, et al. Prospective health related quality of life valuations (utilities) of 8 Gy in 1 fraction vs 30 Gy in 10 fractions for palliation of painful bone metastases: Preliminary results of RTOG 97–14. Int J Radiat Oncol Biol Phys 2004;60:S142. 14. Wu JK, Wong R, Johnston M, et al. Meta-analysis of dose-fractionated radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys 2003;55:594 – 605. 15. Loblaw DA, Laperriere NJ. Emergency treatment of malignant extradural spinal cord compression: An evidence-based guideline. J Clin Oncol 1998; 16:1613–24. 16. Ryu S, Chang S, Kim D, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery 2001;49:838 – 46. 17. Shiu AS, Chang EL, Ye J-S. Near simultaneous computed tomography image-guided stereotactic spinal radiotherapy: An emerging paradigm for achieving true stereotaxy. Int J Radiat Oncol Biol Phys 2003;57:605–13. 18. Amendola B, Wolf A, Coy S, et al. Gamma knife radiosurgery in the treatment of patients with single and multiple brain metastases from carcinoma of the breast. Cancer J 2000;6:88 –92. 19. Benzil DL, Saboori M, Mogilner AY, et al. Safety and efficacy of stereotactic radiosurgery for tumors of the spine. J Neurosurg 2004;101:413– 8. 20. Bilsky MH, Yamada Y, Yenice KM, et al. Intensity-modulated stereotactic radiotherapy of paraspinal tumors: A preliminary report. Neurosurgery 2004;54:823–30. 21. Chang EL, Shiu AS, Lii M-F, et al. Phase I clinical evaluation of nearsimultaneous computed tomographic image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys 2004;59: 1288 –94. 22. Desalles AA, Pedroso A, Medin P, et al. Spinal lesions treated with Novalis shaped beam intensity modulated radiosurgery and stereotactic radiotherapy. J Neurosurg 2004;101:435– 40. 23. Milker-Zabel S, Zabel A, Thilmann C, et al. Clinical results of retreatment of vertebral bone metastases by stereotactic conformal radiotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2003;55:162–7. 24. Ryu S, Yin FF, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer 2003;97:2013– 8. 25. Yu MK, Buys SS. Medical management of skeletal metastasis. Neurosurg Clin N Am 2004;15:529 –36. 26. Auchter R, Lamond J, Alexander E. A multinstitutional outcome and prog-
213 173
Radiosurgery for Spinal Metastases • Gerszten et al 199
27. 28.
29.
30. 31. 32.
33.
34. 35. 36.
37. 38. 39. 40. 41.
42.
43.
214 174
nostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys 1996;35:27–35. Chang S, Adler J, Hancock S. The clinical use of radiosurgery. Oncology 1998;12:1181–91. Flickinger J, Kondziolka D, Lunsford L. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys 1994;28:797– 802. Kondziolka D, Patel A, Lunsford L. Stereotactic radiosurgery plus whole brain radiotherapy vs radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427–34. Loeffler J, Alexander EI. Radiosurgery for the Treatment of Intracranial Metastases. New York, NY: McGraw-Hill; 1993. Loeffler JS, Kooy HM, Wen PY, et al. The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol 1990;8:576 – 82. Sperduto P, Scott C, Andrews D. Stereotactic radiosurgery with whole brain radiation therapy improves survival in patients with brain metastases: Report of radiation therapy oncology group phase III study 95– 08. Int J Radiat Oncol Biol Phys 2002;54:3a. Hamilton A, Lulu B, Fosmire H, et al. Preliminary clinical experience with linear accelerator-based spinal stereotactic radiosurgery. Neurosurgery 1995;36:311–9. Chang S, Adler J. Current status and optimal use of radiosurgery. Oncology 2001;15:209 –21. Gerszten PC, Welch WC. Cyberknife radiosurgery for metastatic spine tumors. Neurosurg Clin N Am 2004;15:491–501. Medin P, Solberg T, DeSalles A. Investigations of a minimally invasive method for treatment of spinal malignancies with LINAC stereotactic radiation therapy: Accuracy and animal studies. Int J Radiat Oncol Biol Phys 2002;52:1111–22. Ryu S, Rock J, Rosenblum M, et al. Patterns of failure after single-dose radiosurgery for spinal metastasis. J Neurosurg 2004;101:402–5. Yin FF, Ryu S, Ajlouni M, et al. Image-guided procedures for intensitymodulated spinal radiosurgery. J Neurosurg 2004;101:419 –24. Colombo F, Pozza F, Chierego G. Linear accelerator radiosurgery of cerebral arteriovenous malformations: An update. Neurosurgery 1994;34:14 –21. Hitchcock E, Kitchen G, Dalton E, et al. Stereotactic LINAC radiosurgery. Br J Neurosurg 1989;3:305–12. Pirzkall A, Lohr F, Rhein B, et al. Conformal radiotherapy of challenging paraspinal tumors using a multiple arc segment technique. Int J Radiat Oncol Biol Phys 2000;48:1197–204. Song DY, Kavanagh BD, Benedict SH, et al. Stereotactic body radiation therapy rationale, techniques, applications and optimization. Oncology 2004;1419 –36. Klish MD, Watson GA, Shrieve DC. Radiation and intensity-modulated radiotherapy for metastatic spine tumors. Neurosurg Clin N Am 2004;15: 481–90.
44. Rock JP, Ryu S, Yin FF. Novalis radiosurgery for metastatic spine tumors. Neurosurg Clin N Am 2004;15:503–9. 45. Yamada Y, Lovelock M, Yenice KM, et al. Multifractionated image-guided and stereotactic intensity modulated radiotherapy of paraspinal tumors: A preliminary report. Int J Radiat Oncol Biol Phys 2005;62:53– 61. 46. Isacsson U. Potential advantages of protons. Radiother Oncol 1997;45: 63–70. 47. Gerszten PC, Ozhasoglu C, Burton S, et al. CyberKnife frameless stereotactic radiosurgery for spinal lesions: Clinical experience in 125 cases. Neurosurgery 2004;55:89 –99. 48. Adler JR, Cox RS, Kaplan I, et al. Stereotactic radiosurgical treatment of brain metastases. J Neurosurg 1992;76:444 –9. 49. Guthrie B, Adler J. Computer-assisted preoperative planning, interactive surgery, and frameless stereotaxy. Clin Neurosurg 1991;38:112–31. 50. Murphy M, Cox R. The accuracy of dose localization for an image-guided frameless radiosurgery system. Med Phys 1996;23:2043–9. 51. Murphy MJ, Cox RS. Frameless radiosurgery using real-time image correlation for beam targeting. Med Phys 1996;23:1052–3. 52. Adler J, Murphy M, Chang S, et al. Image-guided robotic radiosurgery. Neurosurgery 1999;44:1– 8. 53. Chang SD, Main W, Martin DP, et al. An analysis of the accuracy of the CyberKnife: A robotic frameless stereotactic radiosurgical system. Neurosurgery 2003;52:140 –7. 54. Gerszten PC, Welch WC. Cyberknife radiosurgery for the spine. Techniques in Neurosurgery 2003;9:232– 41. 55. Gerszten PC, Burton S, Ozhasoglu C, et al. Stereotactic radiosurgery for spine metastases from renal cell carcinoma. J Neurosurg Spine 2005;3: 288 –95. 56. Gerszten PC, Burton S, Welch WC, et al. Single fraction radiosurgery for the treatment of breast metastases. Cancer 2005;14:2244 –54. 57. Bilsky MH, Boakye M, Collignon F, et al. Operative management of metastatic and malignant primary subaxial cervical tumors. J Neurosurg Spine 2005;2:256 – 64. 58. Abbatucci JS, Delozier T, Quint R, et al. Radiation myelopathy of the cervical spinal cord: Time, dose and volume factors. Int J Radiat Oncol Biol Phys 1978;4:239 – 48. 59. Boden G. Radiation myelitis of the cervical spinal cord. Br J Radiol 1948; 21:464 –9. 60. Hatlevoll R, Host H, Kaalhus O. Myelopathy following radiotherapy of bronchial carcinoma with large single fractions: A retrospective study. Int J Radiat Oncol Biol Phys 1983;9:41– 4. 61. McCuniff AJ, Liang MJ. Radiation tolerance of the cervical spinal cord. Int J Radiat Oncol Biol Phys 1989;16:675– 8. 62. Phillips TL, Buschke F. Radiation tolerance of the thoracic spinal cord. AJR Am J Roentgenol 1969;105:659 – 64. 63. Wara WM, Phillips TL, Sheline GE, et al. Radiation tolerance of the spinal cord. Cancer 1975;35:1558 – 62.
Acta Neurochir (2011) 153:1069–1075 DOI 10.1007/s00701-011-0962-0
CASE REPORT
Deep brain stimulation and frameless stereotactic radiosurgery in the treatment of bilateral parkinsonian tremor: target selection and case report of two patients Angelo Franzini & Marcello Marchetti & Lorenzo Brait & Ida Milanesi & Giuseppe Messina & Elisabetta Forapani & Giovanni Broggi & Laura Fariselli
Received: 4 November 2010 / Accepted: 31 January 2011 / Published online: 20 February 2011 # Springer-Verlag 2011
Abstract Considerable positive experience in functional radiosurgery has been reported since Leksell’s first experience in 1951, but the development of frameless radiosurgery was been limited because of the difficulty of identifying invisible functional targets. In this paper we report on two cases of bilateral parkinsonian tremor successfully treated with DBS on one side and with frameless radiosurgery on the contralateral side. We focus on the methodology developed to define the three-dimensional target coordinates for frameless radiosurgery. Two patients suffering from a disabling upper-limb parkinsonian tremor underwent frameless radiosurgical thalamotomy. To accurately identify the treatment target the CT gantry was treated as a stereotactic frame; a rototranslation between the origin of the screen and the origin of the stereotactic atlas allowed us to obtain atlas-registered 3D coordinates of each point on the CT axial brain slices. Both patients achieved complete bilateral tremor control by unilateral radiosurgery and contralateral A. Franzini : M. Marchetti : G. Messina (*) : E. Forapani : G. Broggi Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico C. Besta, Via Celoria 11, 20133 Milan, Italy e-mail: giusmex@gmail.com M. Marchetti : I. Milanesi : L. Fariselli Division of Radiotherapy, Fondazione IRCCS Istituto Neurologico C. Besta, Milan, Italy L. Brait : G. Broggi : L. Fariselli CyberKnife Center, Centro Diagnostico Italiano, Milan, Italy
DBS. We developed a method for determining the 3D coordinates of a known functional target to treat with frameless radiosurgery. Based on the initial experiences, frameless radiosurgery appears to be an alternative treatment for Parkinsonian upper limb tremor in the presence of increased surgical risks for DBS placement. Keywords Deep brain stimulation . Parkinson's disease . Stereotactics radiosurgery . Tremor . Cyberknife
Introduction Stereotactic lesioning of the thalamus and basal ganglia for treatment of tremor is a well-known procedure that, prior to the introduction of deep brain stimulation, or DBS, was usually achieved using stereotactic surgical procedures [16]. Considerable positive experience in functional radiosurgery using the gamma knife or linear accelerators has been reported since Leksell’s first report in 1951 [4, 8, 11, 13, 14, 17–19, 21, 24–27]. The CyberKnife (CK, Accuray Inc., Sunnyvale, CA) allows effective frameless stereotactic radiosurgery of visible intracranial targets such as arteriovenous malformations, tumours, and trigeminal neuralgia [1–3, 7, 9, 23]. Radiosurgery on invisible targets to treat movement disorders and intractable pain is still the domain of framebased procedures because of the need for a solid reference system registered to the anterior commissure-posterior commissure (AC-PC) line, which allows the use of stereotactic atlases. In this report we describe a mathematical method that uses atlas-derived stereotactic coordinates
215
1070
to perform radiosurgery of invisible targets with the frameless CyberKnife methodology. The methodology and long-term results in two consecutive patients are reported with particular regard to the future applications of frameless radiosurgical treatment of upper limb and hand tremor in elderly patients. No similar cases have been reported in the literature.
Methods Two patients who had previously undergone successful DBS for parkinsonian tremor on one side developed a severe tremor on the contralateral, untreated side. Both patients had contraindications or considerable risk factors for a second DBS surgery on the side contralateral to the previous DBS implant. Their cases are detailed below. CyberKnife radiosurgical procedure The CK is a stereotactic, frameless, image-guided radiosurgery system capable of delivering non-isocentric radiation distributions to a target with submillimetric precision [1, 2, 6, 27]. It consists of a miniaturized, lightweight 6-MV LINAC coupled with a six-degree-of-freedom robotic arm. The robotic arm provides a large number of noncoplanar beam trajectories (over 1,200 trajectories for the G3 version). Image guidance is based on the match between digitally reconstructed radiographs (DRRs) of the skull and real-time digital radiographs acquired during the treatment. A CT center is defined in order to establish a stereotactic coordinate system. Two x-ray imaging devices positioned on either side of the patients and two corresponding amorphous silicon sensors provide an accurate 3D reconstruction of the cranial volume. The system dynamically and automatically adjusts the LINAC position in order to keep the skull position aligned to the treatment planning. Target definition and stereotactic atlas registration of the CT images During CT scanning it is critical that the patient’s head remains in a fixed position in order to avoid movement artefacts. For the patients reported here, high-quality images were obtained by restraining the patient’s head using a standard thermoplastic mask and acquiring the images very rapidly, always taking less than 40 s, with the CT equipment in our possession (Light Speed Ultra, General Electric, Fairfield, CT). Mild sedation may be necessary for some patients with head tremor. In more severe cases, in which body and/or head movements could prevent an optimal image acquisition, administration of a low dosage of Midazolam under anaesthesiological control
216
Acta Neurochir (2011) 153:1069–1075
is mandatory. If the head is immobile the CT gantry behaves like a solid reference system with fixed relationships to the brain structures. In other words the CT screen may be seen as a bidimensional stereotactic frame, and each pixel of the CT screen represents a discrete part of the brain identified by X lateral and Y anteroposterior coordinates in relation to the screen origin. The slice containing the anterior commissure (AC) is arbitrarily assigned to depth=0 (Z coordinate); the depth of each slice is calculated relative to this point (the slices are 1.25 mm thick). In this system we can calculate the AC X, Y, and Z coordinates (Z=0) and the coordinates of the posterior commissure (PC) where Z is the distance in mm from slice zero. In cases in which the AC and PC lie on the same slice, AC and PC Z coordinates are both equal to zero, and the calculations are easier. Finally, the values in pixels are converted into millimetres based on the matrix/FOV ratio of the CT screen. In other words the X and Y values of each pixel of the brain image on the CT screen are obtained, and Z is derived as the depth of the slice measured as the vertical distance from slice 0. Finally, we calculate the coordinates of the AC-PC midpoint, which is the origin of the stereotactic atlas, and a simple rototranslation between the origin of the screen and the origin of the stereotactic atlas allows us to obtain atlas-registered X, Y, and Z coordinates in millimetres of each point on the CT axial brain slices (Fig. 1). Target coordinates of the Voa/Vop complex (X=+/−12 mm , Y=2 mm , Z=2 mm) derived from the stereotactic atlases registered to the midcommissural point are easily transposed onto the corresponding CT slice, and the target is drawn on the treatment planning system (Multiplan, Accuray Inc.). In other words, the roto-translation of the axes between the CT screen and the commissural system of the patient allows the use of atlas-derived stereotactic coordinates to make the invisible functional target visible. The CT images may be fused with MRI to obtain more details about the anatomical structures surrounding the estimated target. High quality control of CT couch movements is of course mandatory for the above-described procedure, and possible undesired movements of the CT couch during the examination could affect the precision of the Z coordinate; even if the Voa/ Vop complex is relatively close (slice+2 mm) to the slice containing the anterior commissure (slice 0), possible errors must be taken into account. Dose definition The aim of this procedure is to cause a lesion confined to the estimated target. The first patient was treated with a dose of 70 Gy to the 100% isodose line. Due to the lack of side effects and considering the small size of the obtained lesion, in the second case the dose was escalated to 90 Gy to the 100% isodose line.
Acta Neurochir (2011) 153:1069–1075
1071
Fig. 1 Right: Schematic drawing of the CT slice sequences: the CT containing the AC-PC plane is considered Z=0 mm as in the stereotactic atlases. The origin of the bidimensional coordinate system (Xt, Yt) of each CT slice is the ac-pc midpoint (0). Left: Rototranslation between the CT screen coordinate system (Xs, Ys) and the ac-pc registered coordinate system of the patients (Xt, Yt) where the commissural line midpoint is the origin (0 ac-pc); beta represents the angle between the ac-pc line and the screen when the
patient’s head is rotated within the CT gantry. The algorithms that allow us to transform the screen coordinates (Xs, Ys) into stereotactic coordinates registered to the commissural system and vice versa (Xt, Yt) are seen at the bottom of the figure. The obtained coordinates are converted from pixels to millimetres and each point of the CT may be transposed to stereotactic atlases, and thus becomes visible. Conversely, any target may be transposed from the stereotactic atlas to the patient CT and utilised for CyberKnife lesioning
Case 1
Unified Parkinson’s Disease Rating Scale (UPDRS) motor score was 45 (see Table 1 for tremor items) [20, 21]. After neurological and neuroradiological investigations the patient underwent bilateral implantation of DBS electrodes within the VOA-VOP complex connected to two subclavicular implantable pulse generators (IPGs; Soletra Medtronic Inc., Minneapolis, MN). The patient showed a consistent improvement in tremor in both upper arms, maintained by high-frequency stimulation (185 Hz; 60 μs; 1.5 V). Unfortunately, because of a subgaleal infection, removal of the system became necessary on the left side, and tremor on the right side reappeared with the same intensity and characteristics as before the DBS implant. To restore the control of tremor on the right side, in January 2006 the patient, after signing an ad hoc informed consent, underwent CyberKnife radiosurgery for a left VOA-VOP thalamotomy using the target identification methodology described above. The
The first patient is a 71-year-old man. Parkinsonian signs started at the age of 69, with rest and postural tremor of the right upper limb and subsequent major motor impairment of the right hand. He began levodopa treatment [levodopa equivalent daily dosage (LED) up to 400 mg] with improvement in akinesia but without a significant benefit for tremor control. One year later he began to complain of worsening of rest and postural tremor with further impairment of his capacity to manipulate common objects and severe difficulty in carrying out his daily living activities. He came to our attention in September 2005. Upon observation, the patient had a typical tremorigenous parkinsonian picture with prevalence on the right hand side, four limb rigidity, and gait disturbance with a limited response of the tremor to dopaminergic treatment. The
Table 1 Pre-/post-treatment scale scores (UPDRS, FTMTRS) and LED Patient 1
Pre-cyberknife
Post-cyberknife
UPDRS motor score Tremor items Trem. rest up. Limb Trem. rest low. limb Action trem.
45 Dx 4 1 3
34 Dx 1 1 1
LED FTMTRS
400 94
Sx 2 1 2
400 27
Sx 1 1 1
Patient 2
Pre-DBS STN sx
Pre- cyberknife
Post-cyberknife
33 Dx 3 2 3
26 Dx 2 1 0
18 Dx 1 0 0
1,490 69
Sx 2 1 2
1,380 −
Sx 1 2 1
Sx 0 1 1
1180–1,500 12
217
1072
total dose was 70 Gy to the 100% isodose line. The treatment volume was 22.5 mm³, and a 5-mm collimator was used. One month after treatment excellent tremor control was achieved on the right side. At 41 months’ follow-up, tremor control was still preserved and the patient’s independence maintained. Because the major source of this patient’s disability was tremor, we administered the Fahn, Tolosa, Marin Tremor Rating Scale (FTMTRS) before and after the radiosurgical procedure to evaluate clinical and functional changes in the patient’s life. This scale assesses specifically and accurately the severity, loss of function, and disability related to tremor [21]. Tremor items measured with the FTMTRS decreased from 94 preoperatively to 27 postoperatively (percentage reduction 71.3%). LED intake was left unchanged (400 mg) to control the non-tremor Parkinsonian symptoms. A few days after CyberKnife treatment a brain MRI (performed with a 0.5-T apparatus because of the presence of a contralateral DBS electrode) revealed a hypointense spot on T2-weighted images (nearly matching the imaging expression of the removed electrode). Three months later, an MRI control showed a new small hyperintense volume on T2-weighted images within the thalamus. We believe this to be the correlate on MRI of the efficacy of radiosurgery treatment. The last available MRI, at 1-year follow-up, confirmed the presence of this hyperintense volume without further evolution.
Case 2 The second patient first came to our attention in 2005 when he was 73 years old. He had suffered from Parkinson’s disease since 1998. Initially, the patient presented a typical tremorigenous parkinsonian picture, mainly affecting the right arm. The UPDRS motor score at the time of the first presentation was 33, and the total sum of tremor items was 15 (see Table 1). The LED was 1,490 mg. Medical therapy failed to control his symptoms, so in July 2005 the patient underwent implantation of a DBS electrode within the VOA-VOP complex. Postoperatively the patient improved both in tremor and akinesia. His UPDRS motor score was 26, the total sum of tremor items was 7, and the LED was reduced to 1,380 mg. In September 2007 the patient returned to our attention because of worsening of the tremor on the untreated side. He had become a poor surgical candidate because of a chronic vascular disease, and so a radiosurgical approach was considered. In January 2008 a radiosurgical lesioning of the right VOA-VOP was planned. The total dose was 90 Gy to the 100% isodose line administered in a single fraction with a 5-mm collimator. The treatment volume was 12 mm³. Immediately after the treatment the patient reported a significant improvement in his left upper limb tremor.
218
Acta Neurochir (2011) 153:1069–1075
Eighteen months after radiosurgery he showed very good tremor control of the left limbs. His UPDRS motor score was 18, the total sum of tremor items was 3, and the LED was 1,180 mg. He regained independence in most of his daily living activities, as shown by the variation in FTMTRS scores (pre-treatment 69 vs. post-treatment 12, a reduction of 82.6%). Two months later the patient had a moderate clinical worsening as his non-tremorigenous symptoms advanced. This required a therapy adjustment (LED 1,500 mg), while the tremor control persisted. No side effects were observed. At 6 months’ follow-up, no lesion was detected on MRI, but at 19 months, MRI showed the appearance of a consistent lesion in the irradiated area (Fig. 2). The lesion was characterised by central necrosis, peripheral-edge contrast enhancement, and perilesional oedema. Fusion of the treatment planning images with this postoperative MRI confirmed the correct localisation of the lesion. The lesion was still evident 2 months later, morphologically and dimensionally unchanged, and did not show any clinical correlate.
Discussion The methodology we used to plan CyberKnife procedures on “invisible” functional targets is based on the concept that the CT gantry can be viewed as a stereotactic frame and the mathematical roto-translation (manually performed or embedded in software programs) allows the transfer of targets from any stereotactic atlases that locate structures relative to the commissural system (e.g., the Talairach, Schaltenbrand, and May atlases all have this property). An absolute requirement for stereotactic accuracy is that the head must be immobile during the CT acquisition, which on our CT scan equipment took less than 40 s. In this preliminary experience we chose a safe target for the radiosurgical lesion; the VOA is anterior and medial to the posterior limb of the internal capsule (Fig. 3). The capsule itself can be transposed from the stereotactic atlas to the CyberKnife CT plan according to its known relationships to the commissural reference system. The choice of VOA (12 mm lateral to the midline, 2 mm anterior to the midcommissural point, and 2 mm superior to the commissural plane ) was intended to lessen the risk of anatomical variability in these patients. In fact, this target is relatively far from the motor fibres running in the posterior limb of the internal capsule and far from other “eloquent” nuclei. The lack of intraoperative neurophysiological confirmation of the target (e.g., microrecording, microstimulation, and macrostimulation) is the main limitation of the described procedure. In fact, nowadays, this procedure cannot be applied when the functional target is affected by individual anatomic variability (i.e., subthalamic nucleus, STN). Image fusion between CT (with the marked target)
Acta Neurochir (2011) 153:1069–1075
1073
Fig. 2 Patient’s MRI merged with the planned treatment. The 18-month post-treatment MRI shows a lesion surrounded by oedema overlapping the 10% isodose line
and MRI may be utilised to refine the targeting procedures with more anatomic individual details. In the cases reported here, however, MRI image fusion did not modify the original CT plans. This procedure resulted in tremor control in the two cases presented, with significant improvement in daily living activities and quality of life, thus restoring or maintaining the patients’ independence. From a clinical point of view this report describes an unconventional therapeutic option, the combination of DBS with radiosurgical lesioning to treat bilateral parkinsonian tremor in fragile patients in whom double or staged DBS implantation carries a double surgical risk [5, 12, 22]. We observed that neither patient complained of speech impairment after the procedure. Of course, two patients are not enough to state that the risk of speech impairment is lower with this “hybrid” procedure versus bilateral DBS, but because
successful bilateral thalamic lesions often cause speech difficulty, the absence of speech impairment observed here is encouraging. If compared to frame-based radiosurgery, frameless radiosurgery is a pain-free procedure that offers the advantage of better patient compliance, avoiding local anaesthesia and the discomfort of wearing the frame for what can be a long time period. Moreover, the reported patients were over 70 years old, and the risks of DBS including skin erosion and recurrent infections cannot be underemphasised in aged patients. Anyway, the aim of this report is not to demonstrate that frameless is preferable to frame-based radiosurgery , but instead to demonstrate the safety and the feasibility of frameless functional radiosurgery in the treatment of drugrefractory tremor.
219
1074
Acta Neurochir (2011) 153:1069–1075
Fig. 3 Patient 2 treatment planning with the relative dose volume histograms. In our preliminary experience we chose a safe target for radiosurgical thalamotomy (VOA is anterior and medial to the
posterior limb of the internal capsule). The capsule itself is contoured; it can be transposed from a stereotactic atlas
With just two patients treated, we can only speculate on the appropriate radiation dose for this procedure. The literature on functional radiosurgery shows that a mean dosage of 140 Gy has been used, but we used lower doses in both patients (70 and 90 Gy) [8, 10, 15, 25]. Both patients showed long-term control of tremor, but postoperative MRI and CT only showed minimal changes at the target in the 70-Gy case and a consistent lesion for the 90-Gy case. CyberKnife radiosurgery has been shown to be effective for trigeminal neuralgia when a maximum dose of about 75 Gy is delivered to the fifth nerve, so we can argue that this dosage is not ineffective on nervous tissue [3]. The absence of complications in the first patient, treated with up to 70 Gy, and the high doses found in published reports encouraged us to deliver a somewhat higher dose (90 Gy) to the second patient. A large lesion appeared at
19 months post-treatment at the estimated target, with surrounding oedema. Even if this lesion was clinically uneventful (mostly because of the target location), it suggests that lower doses should be delivered in elderly patients, particularly in the presence of neurovascular diffuse changes. In our opinion, the size of the expected radiosurgical lesion cannot be accurately predicted, expecially in aged parkinsonian patients where locoregional vascular factors play a major role in the mechanisms and phenomenology of the actual size but, as was the case for trigeminal neuralgia, a standard dose needs to be determined statistically on a larger series of patients. Long-term control of parkinsonian tremor in these patients was obtained. This may suggest that radiosensitivity is greater in patients of advanced age, but it is also possible that DBS and the radiosurgery lesion interacted. There may have been
220
Acta Neurochir (2011) 153:1069–1075
reciprocal strengthening between VOA DBS and VOA lesion. In fact, turning off the IPG resulted in recurrence of tremor on both sides, and tremor on the side contralateral to DBS reappeared earlier and was much more intense. Turning on the IPG restored the control of tremor on both sides in few minutes.
Conclusions The interest in radiosurgical procedures for functional disorders may be renewed in light of the frameless, one-stage procedure reported here. At this point the procedure should be limited to elderly patients with a previous DBS implant who are at increased risk for undergoing a contralateral implant because of previous infection or age. In the future, if dosage issues have been resolved, this application may result in a new non-invasive tool to treat other diseases such as cancer pain (CM thalamotomy), tremor in multiple sclerosis patients (VOA-VOP thalamotomy), or spasticity (dentatotomy). Aknowledgements The authors wish to thank Dr. David W. Schaal, Accuray Incorporated, for technical and editorial assistance. Disclosure The authors have no financial interest in the instruments presented in this manuscript.
Conflicts of interest None.
Reference 1. Adler JR Jr, Chang SD, Murphy MJ, Doty J, Jeis P, Hancock SL (1997) The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg 69:124–128 2. Adler JR Jr, Murphy MJ, Chang SD, Hancock SL (1999) Image guided robotic radiosurgery. Neurosurgery 44:1299–1307 3. Adler JR Jr, Bower R, Gupta G, Lim M, Efron A, Gibbs IC, Chang SD, Soltys SG (2009) Nonisocentric radiosurgical rhizotomy for trigeminal neuralgia. Neurosurgery 64(2 Suppl):A84–A90 4. Alvarez L, Macias R, Lopez G, Alvarez E, Pavon N, Rodriguez-Oroz MC, Juncos JL, Maragoto C, Guridi J, Litvan I, Tolosa ES, Koller W, Vitek J, DeLong MR, Obeso JA (2005) Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain 128(Pt 3):570–583 5. Benabid AL, Chabardes S, Mitrofanis J, Pollak P (2009) Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol 8(1):67–81 6. Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP (2003) An analysis of the accuracy of the Cyberknife: a robotic frameless stereotactic radiosurgery system. Neurosurgery 52:140–147 7. Colombo F, Cavedon C, Casentini L, Francescon P, Causin F, Pinnna V (2009) Early results of CyberKnife radiosurgery for arteriovenous malformations. J Neurosurg 111:807–819 8. Duma CM, Jaques DB, Kopyov OV, Mark R, Copcutt B, Farokhi H (1998) Gamma knife radiosurgery for thalamotomy in parkinsonian tremor: a five years experience. J Neurosurg 88:1044–1049
1075 9. Fariselli L, Marras C, De Santis M, Marchetti M, Milanesi I, Broggi G (2009) CyberKnife radiosurgery as a first treatment for idiopathic trigeminal neuralgia. Neurosurgery 64(2 Suppl):A96–A101 10. Friedman DP, Goldman HW, Flanders AE, Gollomp SM, Curran WJ Jr (1999) Stereotactic radiosurgical pallidotomy and thalamotomy with the gamma knife: MR imaging findings with clinical correlation–preliminary experience. Radiology 212(1):143–150 11. Frighetto L, De Salles A, Wallace R, Ford J, Selch M, Cabatan-Awang C, Solberg T (2004) Linear accelerator thalamotomy. Surg Neurol 62 (2):106–113, discussion 113-4 12. Hariz MI, Rehncrona S, Quinn NP et al. (2008) Multicentre advanced Parkinson’s disease deep brain stimulation group. Multicenter study on deep brain stimulation in Parkinson’s disease: an independent assessment of reported adverse events at 4 years. Mov Disord 23(3):416–21 13. Leksell L (1951) The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 102(4):316–319 14. Lindquist C, Kihlstrom L, Hellstrand E (1991) Functional neurosurgery–a future for the gamma knife? Stereotact Funct Neurosurg 57(1–2):72–81 15. Mathieu D, Kondziolka D, Niranjan A, Flickinger J, Lunsford LD (2007) Gamma knife thalamotomy for multiple sclerosis tremor. Surg Neurol 68(4):394–399 16. Oh MY, Hodaie M, Kim SH, Alkhani A, Lang AE, Lozano AM (2001) Deep brain stimulator electrodes used for lesioning: proof of principle. Neurosurgery 49(2):363–367 17. Ohye C (2006) From selective thalamotomy with microrecording to gamma thalamotomy for movement disorders. Stereotact Funct Neurosurg 84(4):155–161 18. Ohye C, Shibazaki T, Ishihara J, Zhang J (2000) Evaluation of gamma thalamotomy for parkinsonian and other tremors: survival of neurons adjacent to the thalamic lesion after gamma thalamotomy. J Neurosurg 93(Suppl 3):120–127 19. Ohye C, Shibazaki T, Sato S (2005) Gamma knife thalamotomy for movement disorders: evaluation of the thalamic lesion and clinical results. J Neurosurg 102(Suppl):234–240 20. Stacy MA, Elble RJ, Ondo WG, Wu SC, Hulihan J (2007) Assessment of interrater and intrarater reliability of the FahnTolosa-Marin Tremor Rating Scale in essential tremor. Mov Disord 22(6):833–838 21. Svennilson E, Torvik A, Lowe R, Leksell L (1960) Treatment of parkinsonism by stereotactic thermolesions in the pallidal region. A clinical evaluation of 81 cases. Acta Psychiatr Scand 35:358–377 22. Tripoliti E, Zrinzo L, Martinez-Torres I, Tisch S, Frost E, Borrell E, Hariz MI, Limousin P (2008) Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Mov Disord 23(16):2377–2383 23. Villavicencio AT, Lim M, Burneikiene S, Romanelli P, Adler JR, McNeely L, Chang SD, Fariselli L, McIntyre M, Bower R, Broggi G, Thramann JJ (2008) Cyberknife radiosurgery for trigeminal neuralgia treatment: a preliminary multicenter experience. Neurosurgery 62 (3):647–655 24. Young RF, Vermeulen SS, Grimm P, Posewitz AE, Jacques DB, Rand RW, Copcutt BG (1995) Gamma Knife thalamotomy for the treatment of persistent pain. Stereotact Funct Neurosurg 64(Suppl 1):172–181 25. Young RF, Shumway-Cook A, Vermeulen SS, Grimm P, Blasko J, Posewitz A, Burkhart WA, Goiney RC (1998) Gamma knife radiosurgery as a lesioning technique in movement disorder surgery. J Neurosurg 89(2):183–193 26. Young RF, Jacques S, Mark R, Kopyov O, Copcutt B, Posewitz A, Li F (2000) Gamma knife thalamotomy for treatment of tremor: long-term results. J Neurosurg 93(Suppl 3):128–135 27. Yu C, Main W, Taylor D, Kuduvalli G, Apuzzo LJ, Adler JR, Wang MY (2004) An anthropomorphic phantom study of the accuracy of cyberknife radiosurgery. Neurosurgery 55(5):1138–1149
221
Atlas-based functional radiosurgery: Early results J. Stancanelloa
Politecnico di Milano, Bioengineering Department and NEARlab, Milano, Italy 20133 and Siemens AG, Research and Clinical Collaborations, Erlangen, Germany 91052
P. Romanelli
Functional Neurosurgery Deptartment, Neuromed IRCCS, Pozzilli, Italy 86077
E. Pantelis
CyberKnife Center, Iatropolis, Athens, Greece 15231
F. Sebastiano and N. Modugno
Functional Neurosurgery Deptartment, Neuromed IRCCS, Pozzilli, Italy 86077
Received 16 July 2008; revised 7 November 2008; accepted for publication 4 December 2008; published 14 January 2009 Functional disorders of the brain, such as dystonia and neuropathic pain, may respond poorly to medical therapy. Deep brain stimulation DBS of the globus pallidus pars interna GPi and the centromedian nucleus of the thalamus CMN may alleviate dystonia and neuropathic pain, respectively. A noninvasive alternative to DBS is radiosurgical ablation internal pallidotomy IP and medial thalamotomy MT . The main technical limitation of radiosurgery is that targets are selected only on the basis of MRI anatomy, without electrophysiological confirmation. This means that, to be feasible, image-based targeting must be highly accurate and reproducible. Here, we report on the feasibility of an atlas-based approach to targeting for functional radiosurgery. In this method, masks of the GPi, CMN, and medio-dorsal nucleus were nonrigidly registered to patients’ T1-weighted MRI T1w-MRI and superimposed on patients’ T2-weighted MRI T2w-MRI . Radiosurgical targets were identified on the T2w-MRI registered to the planning CT by an expert functional neurosurgeon. To assess its feasibility, two patients were treated with the CyberKnife using this method of targeting; a patient with dystonia received an IP 120 Gy prescribed to the 65% isodose and a patient with neuropathic pain received a MT 120 Gy to the 77% isodose . Six months after treatment, T2w-MRIs and contrast-enhanced T1w-MRIs showed edematous regions around the lesions; target placements were reevaluated by DW-MRIs. At 12 months post-treatment steroids for radiation-induced edema and medications for dystonia and neuropathic pain were suppressed. Both patients experienced significant relief from pain and dystonia-related problems. Fifteen months after treatment edema had disappeared. Thus, this work shows promising feasibility of atlas-based functional radiosurgery to improve patient condition. Further investigations are indicated for optimizing treatment dose. © 2009 American Association of Physicists in Medicine. DOI: 10.1118/1.3056460 Key words: functional radiosurgery, internal pallidotomy, medial thalamotomy I. INTRODUCTION Lesioning or neuroprostethic stimulation1 of selected targets in the thalamus and basal ganglia are widely accepted procedures aimed towards the symptomatic improvement of a variety of neurodegenerative brain disorders. The subthalamic nucleus STN is the region most commonly targeted today.2 Deep brain stimulation DBS of the STN is a procedure commonly offered to patients with advanced Parkinson’s disease PD who have developed motor complications related to dopaminergic replacement on-off phenomena, dyskinesias, freezing, etc. . DBS is a safe procedure that can produce substantial motor benefit in patients undergoing chronic stimulation.3 The main advantages of STN DBS include a remarkable improvement of off-medication motor functioning scores, substantial reduction in the need for dopaminergic drugs, and abolition of drug-related side effects such as dyskinesias. On the other hand, several side 457
222
Med. Phys. 36 „2…, February 2009
effects have been observed after STN DBS, including cognitive decline,4 apathy and other affective disorders,5 and hypophonia.6 While DBS rarely induces permanent brain damage, lesioning of the STN induces a permanent ablation of brain tissue. Nevertheless, the results of STN lesioning by radiofrequency thermal ablation or by radiosurgery appear to be equivalent to those induced by DBS.7–9 In addition, lesioning techniques are less expensive than DBS, and their use is spreading in countries where the cost of DBS is prohibitive. Gene therapy targeting the STN,10 which is functionally modified to produce a gabergic instead than glutamatergic output, is a novel therapy being currently investigated in clinical trials.11 The globus pallidus is another common stereotactic target in the basal ganglia. The target lies in the postero-ventro-lateral part of the internal globus pallidus12 GPi where the sensorimotor region is located. GPi DBS induces motor improvements in patients affected by PD which are grossly equivalent to those associated with
0094-2405/2009/36„2…/457/7/$25.00
© 2009 Am. Assoc. Phys. Med.
457
458
Stancanello et al.: Atlas-based functional radiosurgery: Early results
STN DBS, but to date has not resulted in substantial reduction of dopaminergic drugs.13 On the other hand, GPi DBS has a safer profile in terms of cognitive and psychiatric complications associated with chronic stimulation.14 GPi DBS is also used to treat dystonias, with remarkable improvement seen in primary dystonias such as those characterized by the presence of the gene DyT1.15 GPi lesioning has been widely used until recently, providing good symptomatic improvement in PD patients.16 The lesioning technique most widely used is microelectrode-guided postero-ventro-lateral pallidotomy, and reports of radiosurgical pallidotomy are also available.17 Another less common basal ganglia target is the putamen, where stem cells producing dopamine have been implanted.18,19 Other important stereotactic targets are located in the thalamus. The lateral region of the thalamus is part of the sensorimotor circuit which returns to the cortex the inputs originated from the sensorimotor cortex and transmitted to the thalamus by the basal ganglia. The nucleus ventralis intermedius VIM is an excellent target to control tremor,20 while the nucleus ventralis oralis pars anterior and pars posterior are effective to control focal dystonias such as writer’s cramp.21 Again, there is wide experience lesioning the VIM,22 and recently VIM DBS has gained acceptance to treat essential tremor.20 DBS implantation of the nucleus ventralis posterolateralis of the thalamus has been used to treat neuropathic pain.23 Another thalamic target used to treat neuropathic pain is the centromedian nucleus CMN , which is part of the intralaminar complex.24 A recent report suggests a promising effect of DBS implantation of the CMN and the anterior nucleus of the thalamus to treat selected cases of medically refractory epilepsy.25 Correct target identification is crucial for the success of each of these procedures. Image guidance is essential and includes modalities such as MRI, CT, and ventriculography. MRI allows direct localization of several structures; STN and GPi can be easily identified on T2 FSE imaging.26 3 T MRI offers enhanced visualization of basal ganglia and thalamic targets, allowing identification of targets such as the VIM not otherwise visible using most 1.5 T MRI scanners.27 Ventriculography is a long-practiced invasive technique that permits the targeting of specific nuclei on the basis of a fairly fixed relation with a line passing through the anterior and posterior commissure AC-PC line .28 For DBS implantation, microelectrode recording is frequently used to obtain final confirmation that a specific target is reached, a technique that is effective because the target nuclei have a characteristic electrophysiologic activity.29–31 Nevertheless, volumetric reconstruction of basal ganglia and thalamic targets has been recently described as an additional tool to improve target localization.32 Our recent efforts have been devoted to the development and validation of an accurate technique for threedimensionally mapping a few nuclei relevant in functional neurosurgery and radiosurgery, such as the STN, the GPi and globus pallidus pars externa GPe , the CMN, and the red nucleus.33 The technique has been developed and validation has been published;34,35 we believe three-dimensional atlasbased identification of such nuclei could significantly im-
458
prove the accuracy of radiosurgery treatment. Until now, however, it had not been applied to real cases. The aim of this work was to demonstrate the clinical feasibility of functional radiosurgery using atlas-based target identification. II. MATERIALS AND METHODS Two patients affected by functional brain disorders were selected for functional radiosurgery. The first patient, 53 years old, suffered from facial neuropathic pain. He had undergone several surgeries to drain an infected right on the right maxillary sinus developing over time a lancinanting pain over the distribution of the second and third branch of the trigeminal nerve. This pain proved to be refractory to medical therapy and to several procedures including partial section of the right trigeminal nerve which elicited a severe constant burning overlapping the original shooting pain . He underwent CyberKnife Accuray Incorporated, Sunnyvale, CA radiosurgery targeting the cisternal segment of the nerve 57 Gy prescribed to the 70% isodose volume and left motor cortex stimulation. The second patient, 41 years old, developed post-anoxic focal dystonia secondary to prolonged cerebral hypoxia experienced during heart valve surgery. The patient was in an anoxic coma for about 72 hours after surgery and then in a barbiturate coma for about 15 days. He subsequently developed dysarthria, bilateral blepharospasm and severe and painful dystonia of the right arm and leg. Eventually, according to the pathologies, the two patients underwent medial thalamotomy MT and internal pallidotomy IP , respectively, delivered by CyberKnife radiosurgery. Radiosurgical MT was offered due to the severity of pain, lack of response to any other non-destructive procedure and to CT-PET findings indicating bilateral hypermetabolism of the medial thalamus. Radiosurgical IP was offered to relieve dystonia because he was on anticoagulant therapy. Targets were selected and spatially defined on the patients’ MRIs by an expert functional neurosurgeon, aided by atlas-based computerized identification providing volumetric reconstruction of selected targets. For the neuropathic pain patient, 18F-FDG-PET ECAT PET, Siemens Medical Solutions, Malvern, PA , using reconstructed transversal spatial resolution and slice thickness equal to 2 and 2.4 mm, respectively, was also considered as a primary source of information for target delineation in the nonselective MT, a lesioning technique involving many thalamic nuclei: this exam showed increased metabolism in the medial thalamus and intralaminar complex, including the CMN and centrolateral nucleus. The accuracy of target localization was aided by reference to Talairach and Tournoux and Montreal Neurological Institute MNI atlases. Medio-dorsal MD and CMN nuclei were conferred onto the images of the neuropathic pain patient, and GPi and GPe were conferred onto the dystonia patient’s images. Both patients were scanned using T1-weighted-MRI T1w-MRI and T2-weighted-MRI T2w-MRI , acquired with standard MRI at 1.5 T with a Genesis Signa GE Medical Systems, Waukesha, WI for the IP patient and at 3 T with a TrioTim Siemens Medical Solutions, Malvern, PA for the MT patient. Acquisition and reconstruction param-
Medical Physics, Vol. 36, No. 2, February 2009
223
459
Stancanello et al.: Atlas-based functional radiosurgery: Early results
FIG. 1. Superposition of the atlas-based identification of the GPi and CMN on the T1w-MRIs of the IP upper panel and MT lower panel patients, in axial, coronal, and sagittal views.
eters for T1w- and T2w-MRIs were the same as in Ref. 34. T1w-MRIs were used for automatic local non-rigid registrations of the MNI electronic atlas onto patient volumes, according to the method described in Ref. 33. T2w-MRIs were acquired for manual identification of the target nuclei by an expert functional neurosurgeon, whose delineation was aided by automatic atlas-based identification. MD, CMN, GPi, and GPe masks were nonrigidly registered33 to the patient T2wMRI see Fig. 1 and integrated into the planning CT. The target delineation for the MT was based on the hypermetabolic zone identifiable in the 18F-FDG-PET, which was registered to the patient CT. The CMN was superimposed on the 18F-FDG-PET-based target, to be sure it was entirely enclosed within the target limits. Additionally, the MD nucleus mask was superimposed on the 18 F-FDG-PET-based target to determine that extent to which it was actually involved. In this case no modification was applied to the 18F-FDG-PET-based target because the CMN
459
and MD were judged by the functional neurosurgeon to be adequately within the 18F-FDG-PET-based target. The coarse target delineation for the IP was initially based on anatomical landmarks. The GPi and GPe masks were then superimposed on the anatomical-landmark-based target, which was substantially refined using the three-dimensional information from the two masks in order to target the postero-ventro-lateral part of the GPi. The MultiPlan™ treatment planning system was used to delineate the multimodality-imaging-based targets and create conformal treatment plans for the IP and MT see Fig. 2 . For the IP, the critical structure of the optic tract was delineated; it was much farther from the target than the reported error of the atlas-based identification method.35 During the optimization of the treatment plan, the dose delivered to the optic tract was kept as low as possible. Additional artificial critical structures were delineated to limit the spread of the low percentage isodose curves, which given the high doses used in functional radiosurgery may still be associated with a high absolute dose. A strategy to minimize radiation delivery time for the dystonic patient was investigated due to severe dystonia and generally poor clinical condition. After comparing conformality and treatment time for IP treatment plans generated using the 5- and 7.5-mm collimators with the trigeminal path, we chose to accept the slightly lower plan conformality associated with the 7.5-mm collimator to reduce the amount of time the patient was under anesthesia during treatment delivery. Therefore, the plan using the trigeminal path with 7.5-mm collimator i.e., 6.1 mm field size at 650 mm source-to-axis distance was selected. Six- and twelve-month follow-up exams consisted of contrast-enhanced CE- T1w- and T2w-MRIs T1w-MRI and T2w-MRI reconstructed transversal spatial resolution and slice thickness equal to 0.47 and 1.33 mm, respectively; both of them acquired by the same 1.5 T scanner used for planning the IP . Additionally, MR diffusion-weighted images DWIs were acquired to distinguish the lesion from
FIG. 2. 3D beam distribution upper-left , axial upper-right , sagittal lower-left , and coronal lower-right views of the IP a and MT b treatment plans visualized on the patients’ T2w-MRIs. Medical Physics, Vol. 36, No. 2, February 2009
224
460
Stancanello et al.: Atlas-based functional radiosurgery: Early results
TABLE I. Treatment parameters for MT and the IP. Treatment parameter Target volume mm3 Path Collimator s No. of beams MUs Prescription dose Gy Prescription isodose % Coverage % CI nCI HI Treatment time min
MT 240.5 Even 5 / 7.5 mm 207 67 946 120 77 95 1.3 1.37 1.3 156
IP 215.3 Trigeminal 7.5 mm 164 35 423 120 65 77 1.49 1.94 1.54 102
radiation-induced edema reconstructed transversal spatial resolution and slice thickness equal to 1 and 5 mm, respectively; b value equal to 1000 s / mm2 . For the neuropathic pain patient, a 6-month follow-up 18F-FDG-PET was acquired reconstructed transversal spatial resolution and slice thickness equal to 2.34 and 3.27 mm, respectively, scanned by a Discovery ST scanner, GE Medical Systems, Waukesha, WI . All follow-up exams were registered on the pretreatment T1w-MRI. III. RESULTS AND DISCUSSION Table I summarizes the parameters of the treatment plans. In both cases target volumes as delineated by the expert functional neurosurgeon with the support of the automatic atlas-based method were relatively large: this limits the potential misidentification of the nuclei due to the 2-mm maximum error of the atlas-based method.35 In fact, considering the cubic root of the volumes was about 6 mm in both cases, this length is significantly larger than the potential atlasbased localization maximum error. These cases therefore represent good practical examples for application of the atlasbased identification method, where correct localization is not compromised by the potential error in the atlas-based identification of the nuclei. The relatively small number of beams and total monitor units MUs in the dystonic patient was achieved using the trigeminal path. Although the prescription radiation dose was equal for both patients, the prescription isodose was significantly different, generating a much higher absolute dose maximum in the IP. This higher dose was selected on the basis of the poor success rate reported in the literature,36 combined with the clinical goal of alleviating dystonia-related problems in the patient. While the MT plan coverage with the prescription isodose was high equal to 95% , the IP plan was significantly lower and resulted in a less homogenous treatment as quantified by the homogeneity index defined as the ratio between the maximum dose value inside the target and the prescription dose . The small difference between the conformity index CI defined as the ratio between the total volume enclosed by the prescription dose surface and the vol-
460
ume of tumor enclosed by the prescription dose surface and the new conformity index37 nCI indicates the prescription isodose closely encompasses the target in the MT plan. Conversely, in the IP plan both the nCI and the CI are much greater than 1.0, suggesting a lower target volume coverage by the prescription isodose. Compared to the results of Nakamura et al.,37 in our cases the CI for the MT is lower than the 25th percentile of the CI obtained using the Gamma Knife 1.38 while the CI for the IP is lower than the median value 1.67 ; the nCI for the MT is lower than the 25th percentile 1.51 while the nCI for the IP is higher than the median value 1.78 . This comparison shows again the high conformality achieved by using the CyberKnife in the MT, while suggesting that a more conformal solution could have been applied to the IP using the 5-mm collimator, which in this case was rejected in favor of a shorter treatment duration given the clinical condition of the patient. From a comparative perspective, because of its isocentric nature, the GammaKnife is likely to produce dose distributions with steeper gradients in the midlow isodose range than that obtainable by the CyberKnife used in the nonisocentric mode while producing less homogeneous dose distributions within the target. We attempted to limit the spread of the low-dose percentage curves by applying artificial critical structures during the treatment plan optimization. For the dose delivered in functional radiosurgery it is imperative to limit as much as possible the spread of low dose percentage curves. In fact, in radiosurgery the volume of normal tissue within or immediately adjacent to the prescribed isodose surface is generally highly correlated with the risk of complications.38 This means that complication probability rises as CI and nCI increase above 1.0. This correlation was found in the study by Nakamura et al.,37 where more serious complications from mild side effects to those requiring corticosteroids or surgical resection for radionecrosis, to mortality were associated with higher CIs and nCIs. On the other hand, in the same study no correlation was found when lesions were smaller than 1 mL, as were the IP and MT lesions described in this study. The fact that both cases experienced the same time course of corticosteroids independently of the CI and nCI values is in agreement with the results provided by Nakamura et al.37 The maximum dose to the optic tract in the IP was about 15 Gy delivered to a small volume, and the minimum dose was about 2 Gy. Treatments lasted longer than 100 min in both cases, even when using the trigeminal path. Thus, high-dose functional radiosurgery is a time-consuming technique, especially when dealing with patients in low tolerance conditions. The inevitable arrival of high dose-rate LINACs39 will significantly increase the speed of functional radiosurgery for example, the latest CyberKnife system version has a dose-rate of 800 MU/ min . Figure 3 shows the pretreatment T1w-MRI a , the 6-month follow-up T2w-MRI b and DWI c , and the atlasbased targets GPi d and GPe e . The 6-month follow-up T2w-MRI shows a large area of radiation-induced edema which prevents the identification of the actual lesion. The
Medical Physics, Vol. 36, No. 2, February 2009
225
461
Stancanello et al.: Atlas-based functional radiosurgery: Early results
461
FIG. 3. Pretreatment patient T1w-MRI a , 6 months follow-up T2w-MRI b , 6 months follow-up DWI c , and GPi d and GPe masks reported on the pretreatment T1w-MRI of the IP patient. All the other volumes apart from the masks were rigidly registered to the pretreatment patient T1w-MRI.
FIG. 4. Pretreatment patient T1w-MRI a , 6 months follow-up CE-T1wMRI b , 6 months follow-up DWI c , MD d and CMN e masks reported on the pretreatment T1w-MRI of the MT patient. All the other volumes apart from the masks were rigidly registered to the pretreatment patient T1w-MRI.
6-month follow-up DWI distinguishes lesion from edema, yet one cannot assess the accuracy of lesion location relative to GPi and GPe masks due to the edema-related geometric distortion. Edema internally deformed most of the anatomical region of the lesion in the 6-month follow up T2w-MRI. Given that the registration of the 6-month follow-up T2wMRI to the pretreatment T1w-MRI is based on a global rigid transformation, the localized deformed part does not affect the parameter optimization. A registration based on a global affine transformation of the subvolumes containing the lesion in the two MRIs would probably improve the accuracy of the match, but would not be sufficient for an evaluation of the accuracy of the lesion placement compared to the GPi mask. On the other hand, a local nonrigid registration would fail due to the hyperintense signal related to the edematous area in the 6-month follow-up T2w-MRI not present in the pretreatment MRIs. However, the correspondence of the lesion to the deformed GPi mask suggests some level of agreement. The patient was scanned again 18 months after treatment and the edema was negligible data not shown . The exam indicated an accurate location of the lesion in the postero-ventrolateral zone of the GPi. Figure 4 shows the same analysis for the MT. The 6-month control MRI was a CE-T1w-MRI b , where the lesion rim is clearly visible; the internal portion identifiable on the CE-T1w-MRI corresponds to the lesion highlighted in the 6-month follow-up DWI c . Figures 4 d and 4 e show the deformed MD and CMN masks, respectively, inside the
FIG. 5. Pretreatment patient 18F-FDG-PET a , 6 months follow-up 18 F-FDG-PET b , 12 months follow-up DWI c , 12 months follow-up CET1w-MRI d , CMN e and MD f masks reported on the pretreatment T1w-MRI of the MT patient. All the other volumes apart from the masks were rigidly registered to the pretreatment patient T1w-MRI.
Medical Physics, Vol. 36, No. 2, February 2009
226
462
Stancanello et al.: Atlas-based functional radiosurgery: Early results
462
FIG. 6. Time course of medication reduction for pain- and dystonia-related problems for the IP left and MT right patients before, 6 and 12 months after the treatment.
lesion. However, direct evaluation of the accuracy of the lesion location is not possible because the actual target was chosen primarily based on the 18F-FDG-PET-based hypermetabolic zone incorporating other nuclei as well and, as in the IP case, edema severely limited evaluation of clinical accuracy. Figure 5 shows, for the MT patient, the pre-treatment 18 F-FDG-PET a , the 6-month follow-up 18F-FDG-PET b , the 12-month follow-up DWI c , the 12-month follow-up CE-T1w-MRI d , and the deformed MD e and CMN f masks. All these volumes were registered to the pretreatment T1w-MRI. Radiosurgical MT resulted in a clear suppression of hypermetabolism, as visible on the follow-up 18 F-FDG-PET crosshair region . Still, radiation-induced edema was present 6 months after treatment; 12 months after treatment, edema was negligible. Figure 6 shows the time course of the reduction in painand dystonia-related medications for the IP a and the MT b patients. For the IP case, all medication was terminated 12 months post-treatment; for the MT patient, all medications save neurontin were terminated 6 months posttreatment, and neurontin was terminated 12 months posttreatment. In both cases high doses of steroids, especially at 3 and 6 months post-treatment, were necessary for edema mitigation. Pain relief after the IP was evaluated using a visual analog scale VAS and dystonia was evaluated by means of the unified dystonia rating scale UDRS . The pretreatment VAS value was 10/ 10, and decreased to 0 / 10 in the 12-month post-treatment evaluation; the pretreatment UDRS value of 42.5/ 44 fell to 29/ 44 in the 12-month post-treatment evaluation. Quality of life was evaluated in this patient with the activities of daily living ADL index, specifically assessing the need for help in the daily activities, and using the functional independence measure FIM to assess motor and cognitive activities. The pretreatment ADL was 0 / 6 and in-
creased to 1 / 6 in the 12-month post-treatment evaluation; the pretreatment FIM was 29/ 91 and rose to 32/ 91 in the 12-month post-treatment evaluation. Only pain relief was evaluated in the MT patient. The pretreatment VAS was 10/ 10, and 12 months post-treatment it had decreased to 0 / 10. All these evaluations showed significant improvements in pain and dystonia and slight improvement in independence for activities of daily living. Of course, further clinical investigations are needed for optimizing dose assessing the success of treatment, and detecting complications. IV. CONCLUSIONS This proposed approach for functional radiosurgery resulted in lesions being accurately located and improvements in quality of life in the first two treated patients. Additionally, this work shows how atlas-based identification can be used not only in preoperative scenarios, but also in combination with post-therapeutic follow-up exams such as DWIs, for comprehensive correlation between lesion position on threedimensional atlas-based reconstructions and patient outcomes. Further investigations are needed to understand if dose reduction is possible without compromising treatment efficacy. ACKNOWLEDGMENTS Dr. Himanshu Shukla, Dr. David Schaal, Dr. Warren Kilby, and Dr. Francesco Lena are gratefully acknowledged for their help with the development of the manuscript. a
Present address: Siemens AG, Research and Clinical Collaborations, Erlangen, Germany. Electronic mail: joseph.stancanello@polimi.it 1 E. Y. Uc and K. A. Follett, “Deep brain stimulation in movement disorders,” Semin Neurol. 27 2 , 170–182 2007 . 2 S. Breit, J. B. Schulz, and A. L. Benabid, “Deep brain stimulation,” Cell Tissue Res. 318 1 , 275–288 2004 . 3 K. E. Lyons and R. Pahwa, “Deep brain stimulation in Parkinson’s disease,” Curr. Neurol. Neurosci. Rep. 4 4 , 290–295 2004 .
Medical Physics, Vol. 36, No. 2, February 2009
227
463
Stancanello et al.: Atlas-based functional radiosurgery: Early results
4
M. Tir, D. Devos, S. Blond, G. Touzet, N. Reyns, A. Duhamel, O. Cottencin, K. Dujardin, F. Cassim, A. Destée, L. Defebvre, and P. Krystkowiak, “Exhaustive, one-year follow-up of subthalamic nucleus deep brain stimulation in a large, single-center cohort of parkinsonian patients,” Neurosurgery 61 2 , 297–305 2007 . 5 S. Takeshita, K. Kurisu, L. Trop, K. Arita, T. Akimitsu, and N. P. Verhoeff, “Effect of subthalamic stimulation on mood state in Parkinson’s disease: Evaluation of previous facts and problems,” Neurosurg. Rev. 28 3 , 179–187 2005 . 6 J. G. Zhang, K. Zhang, Y. Ma, W. H. Hu, A. C. Yang, J. S. Chu, S. T. Wu, M. Ge, Y. Zhang, and Z. C. Wan, “Follow-up of bilateral subthalamic deep brain stimulation for Parkinson’s disease,” Acta Neurochir. Suppl. Wien 99, 43–47 2006 . 7 M. F. Keep, L. Mastrofrancesco, D. Erdman, B. Murphy, and L. S. Ashby, “Gamma knife subthalamotomy for Parkinson disease: The subthalamic nucleus as a new radiosurgical target. Case report,” J. Neurosurg. 97 5 Suppl , 592–599 2002 . 8 P. Blomstedt and M. I. Hariz, “Are complications less common in deep brain stimulation than in ablative procedures for movement disorders?,” Stereotact. Funct. Neurosurg. 84 2–3 , 72–81 2006 . 9 O. Vilela Filho and D. J. da Silva, “Unilateral subthalamic nucleus lesioning: A safe and effective treatment for Parkinson’s disease,” Arq. Neuropsiquiatr. 60 4 , 935–948 2002 . 10 S. Muramatsu, H. Tsukada, I. Nakano, and K. Ozawa, “Gene therapy for Parkinson’s disease using recombinant adeno-associated viral vectors,” Expert Opin. Biol. Ther. 5 5 , 663–671 2005 . 11 A. Feigin, M. G. Kaplitt, C. Tang, T. Lin, P. Mattis, V. Dhawan, M. J. During, and D. Eidelberg, “Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson’s disease,” Proc. Natl. Acad. Sci. U.S.A. 4; 104 49 , 19559–19564 2007 . 12 T. Schubert, J. Volkmann, U. Müller, V. Sturm, J. Voges, H. J. Freund, and D. Y. Von Cramon, “Effects of pallidal deep brain stimulation and levodopa treatment on reaction-time performance in Parkinson’s disease,” Exp. Brain Res. 144 1 , 8–16 2002 . 13 J. Volkmann, “Deep brain stimulation for the treatment of Parkinson’s disease,” J. Clin. Neurophysiol. 21 1 , 6–17 2004 . 14 V. C. Anderson, K. J. Burchiel, P. Hogarth, J. Favre, and J. P. Hammerstad, “Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease,” Arch. Neurol. 62 4 , 554–560 2005 . 15 M. G. Cersosimo, G. B. Raina, F. Piedimonte, J. Antico, P. Graff, and F. E. Micheli, “Pallidal surgery for the treatment of primary generalized dystonia: Long-term follow-up,” Clin. Neurol. Neurosurg. 110 2 , 145– 150 2008 . 16 E. J. Herrera, J. C. Viano, M. Cáceres, G. Costello, M. Suárez, and J. C. Suárez, “Posteroventral pallidotomy in Parkinson’s disease,” Acta Neurochir. Wien 142 2 , 169–175 2000 . 17 R. F. Young, S. Vermeulen, A. Posewitz, and A. Shumway-Cook, “Pallidotomy with the gamma knife: A positive experience,” Stereotact. Funct. Neurosurg. 70, 218–228 1998 . 18 G. V. Sawle et al., “Transplantation of fetal dopamine neurons in Parkinson’s disease: PET 18F 6-L-fluorodopa studies in two patients with putaminal implants,” Ann. Neurol. 31 2 , 166–173 1992 . 19 P. Piccini, N. Pavese, P. Hagell, J. Reimer, A. Björklund, W. H. Oertel, N. P. Quinn, D. J. Brooks, and O. Lindvall, “Factors affecting the clinical outcome after neural transplantation in Parkinson’s disease,” Brain 128 Pt 12 , 2977–2986 2005 . 20 P. Blomstedt, G. M. Hariz, M. I. Hariz, and L. O. Koskinen, “Thalamic deep brain stimulation in the treatment of essential tremor: A long-term follow-up,” Br. J. Neurosurg. 21 5 , 504–509 2007 . 21 C. Fukaya, Y. Katayama, T. Kano, T. Nagaoka, K. Kobayashi, H. Oshima, and T. Yamamoto, “Thalamic deep brain stimulation for writer’s cramp,” J. Neurosurg. 107 5 , 977–982 2007 . 22 C. Ohye and T. Shibazaki, “Lesioning the thalamus for dyskinesia,” Ste-
Medical Physics, Vol. 36, No. 2, February 2009
228
463
reotact. Funct. Neurosurg. 77 1–4 , 33–39 2001 . M. I. Hariz and A. T. Bergenheim, “Thalamic stereotaxis for chronic pain: Ablative lesion or stimulation?,” Stereotact. Funct. Neurosurg. 64 1 , 47–55 1995 . 24 R. F. Young, S. S. Vermeulen, P. Grimm, A. E. Posewitz, D. B. Jacques, R. W. Rand, and B. G. Copcutt, “Gamma Knife thalamotomy for the treatment of persistent pain,” Stereotact. Funct. Neurosurg. 64 Suppl 1, 172–181 1995 . 25 F. Velasco, A. L. Velasco, M. Velasco, F. Jiménez, J. D. Carrillo-Ruiz, and G. Castro, “Deep brain stimulation for treatment of the epilepsies: The centromedian thalamic target,” Acta Neurochir. Suppl. Wien 97 Pt 2 , 337–342 2007 . 26 X. L. Zhu, W. Hamel, B. Schrader, D. Weinert, J. Hedderich, J. Herzog, J. Volkmann, G. Deuschl, D. Müller, and H. M. Mehdorn, “Magnetic resonance imaging-based morphometry and landmark correlation of basal ganglia nuclei,” Acta Neurochir. Wien 144 10 , 959–969 2002 . 27 R. Spiegelmann, O. Nissim, D. Daniels, A. Ocherashvilli, and Y. Mardor, “Stereotactic targeting of the ventrointermediate nucleus of the thalamus by direct visualization with high-field MRI,” Stereotact. Funct. Neurosurg. 84 1 , 19–23 2006 . 28 J. D. McKean, P. B. Allen, L. J. Filipow, and J. D. Miller, “CT guided functional stereotaxic surgery,” Acta Neurochir. Wien 87 1–2 , 8–13 1987 . 29 N. A. Hamid, R. D. Mitchell, P. Mocroft, G. W. Westby, J. Milner, and H. Pall, “Targeting the subthalamic nucleus for deep brain stimulation: Technical approach and fusion of pre- and postoperative MR images to define accuracy of lead placement,” J. Neurol., Neurosurg. Psychiatry 76, 409– 414 2005 . 30 J. L. Vitek, R. A. Bakay, T. Hashimoto, Y. Kaneoke, K. Mewes, J. Y. Zhang, D. Rye, P. Starr, M. Baron, R. Turner, and M. R. DeLong, “Microelectrode-guided pallidotomy: Technical approach and its application in medically intractable Parkinson’s disease,” J. Neurosurg. 88, 1027–1043 1998 . 31 A. L. Benabid, “Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease,” Mov Disord. 17 suppl 3 , S145–S149 2002 . 32 P. St-Jean, A. F. Sadikot, L. Collins, D. Clonda, R. Kasrai, A. C. Evans, and T. M. Peters, “Automated atlas integration and interactive threedimensional visualization tools for planning and guidance in functional neurosurgery,” IEEE Trans. Med. Imaging 17 5 , 672–680 1998 . 33 J. Stancanello, P. Romanelli, N. Modugno, P. Cerveri, G. Ferrigno, F. Uggeri, and G. Cantore, “Atlas-based identification of targets for functional radiosurgery,” Med. Phys. 33 6 , 1603–1611 2006 . 34 J. Stancanello, P. Romanelli, F. Sebastiano, N. Modugno, A. Muacevic, P. Cerveri, V. Esposito, G. Ferrigno, F. Uggeri, and G. Cantore, “Direct validation of atlas-based red nucleus identification for functional radiosurgery,” Med. Phys. 34 8 , 3143–3148 2007 . 35 J. Stancanello, A. Muacevic, F. Sebastiano, N. Modugno, and P. Romanelli, “3 T MRI accuracy evaluation of atlas-based subthalamic nucleus identification,” Med. Phys. 35 7 , 3069–3077 2008 . 36 C. M. Duma, “Movement disorder radiosurgery—Planning, physics and complication avoidance,” Prog. Neurol. Surg. 20, 249–266 2007 . 37 J. L. Nakamura, L. J. Verhey, V. Smith, P. L. Petti, K. R. Lamborn, D. A. Larson, W. M. Wara, M. W. McDermott, and P. K. Sneed, “Dose conformity of gamma knife radiosurgery and risk factors for complications,” Int. J. Radiat. Oncol., Biol., Phys. 51, 1313–1319 2001 . 38 V. Smith, L. Verhey, and C. F. Serago, “Comparison of radiosurgery treatment modalities based on complication and control probabilities,” Int. J. Radiat. Oncol., Biol., Phys. 40 2 , 507–513 1998 . 39 J. E. Bayouth, H. S. Kaiser, M. C. Smith, E. C. Pennington, K. M. Anderson, T. C. Ryken, and J. M. Buatti, “Image-guided stereotactic radiosurgery using a specially designed high-dose-rate linac,” Med. Dosim. 32 2 , 134–141 2007 . 23
I N N OVAC I Ó N TE C N O LÓ G I CA APLICADA A LA M E D I C I NA
DELEC CIENTÍFICA
D E LE C C I E NTÍ FI CA U R U G UAYA
Fco. García C Montevideo, Uruguay. Tel. (+598) 2711 4466 Móvil: (+598) (0) 93 507 500 delecuruguay@delec.com.uy
www.delec.com.uy
DELEC CIENTÍFICA ARGENTINA
Aráoz 821 - C1414DPQ C.A.B.A - Ar Tel. (54-11) 4775 - 8544 Móvil: (54-9 11) 6209 - 1924 consultas@delec.com.ar
www.delec.com.ar
229