

Editor’s Office and Advertiser Information: Florida
1402 Emerald Lakes Drive
Clermont, FL 34711
Phone: 352-241-6006
Email: Editorial, editor@fwrj.com
Display and Classified Advertising, ads@fwrj.com
Business Office: 1402 Emerald Lakes Drive, Clermont, FL 34711
Web: http://www.fwrj.com
General Manager: Michael Delaney Editor: Rick Harmon
Graphic Design Manager: Patrick Delaney Mailing Coordinator: Buena Vista Publishing

Published by BUENA VISTA PUBLISHING for Florida Water Resources Journal Inc.

President: Richard Anderson (FSAWWA) Peace River Manasota Regional Water
Vice President: Jamey Wallace (FWEA) Jacobs
Treasurer: Rim Bishop (FWPCOA) Seacoast Utility Authority
Secretary: Mish Clark Mish Agency
Moving?
The Post
Florida
1402 Emerald Lakes Drive, Clermont,
Membership Questions
FSAWWA: Casey Cumiskey – 407-979-4806 or fsawwa.casey@gmail.com
FWEA: Karen Wallace, Executive Manager – 407-574-3318
FWPCOA: Darin Bishop – 561-840-0340
Training Questions
FSAWWA: Donna Metherall – 407-979-4805 or fsawwa.donna@gmail.com
FWPCOA: Shirley Reaves – 321-383-9690
For Other Information
DEP Operator Certification: Ron McCulley – 850-245-7500
FSAWWA: Peggy Guingona – 407-979-4820
Florida Water Resources Conference: 407-363-7751
FWPCOA Operators Helping Operators: John Lang – 772-559-0722, e-mail – oho@fwpcoa.org
FWEA: Karen Wallace, Executive Manager – 407-574-3318
Websites
Florida Water Resources Journal: www.fwrj.com
FWPCOA: www.fwpcoa.org
FSAWWA: www.fsawwa.org
FWEA: www.fwea.org and www.fweauc.org
Florida Water Resources Conference: www.fwrc.org
Throughout this issue trademark names are used. Rather than place a trademark symbol in every occurrence of a trademarked name, we state we are using the names only in an editorial fashion, and to the benefit of the trademark owner, with no intention of infringement of the trademark. None of the material in this publication necessarily reflects the opinions of the sponsoring organizations. All correspondence received is the property of the Florida Water Resources Journal and is subject to editing. Names are withheld in published letters only for extraordinary reasons. Authors agree to indemnify, defend and hold harmless the Florida Water Resources Journal Inc. (FWRJ), its officers, affiliates, directors, advisors, members, representatives, and agents from any and all losses, expenses, third-party claims, liability, damages and costs (including, but not limited to, attorneys’ fees) arising from authors’ infringement of any
or trademark, or other right of any person, as applicable under the laws of the State of
property,
Water Resources Journal
Supply Authority
Office will not forward your magazine. Do not count on getting the Journal unless you notify us directly of address changes by the 15th of the month preceding the month of issue. Please do not telephone address changes. Email changes to changes@fwrj.com or mail to
Water Resources Journal,
FL 34711
intellectual
copyright
Florida. Florida
Water
Resources Journal, USPS 069-770, ISSN 0896-1794, is published monthly
by Florida Water Resources Journal,
Inc., 1402
Emerald Lakes Drive, Clermont,
FL 34711, on behalf of the Florida Water & Pollution Control Operator’s Association, Inc.; Florida Section, American
Water Works
Association;
and the Florida
Water Envi ronment Association. Members of all three associations receive the publication as a service of their association; $6 of membership dues support the Journal. Subscriptions are otherwise available within the U.S. for $24 per year. Periodicals postage paid at Clermont, FL and additional offices. POSTMASTER: send address changes to Florida Water Resources Journal, 1402 Emerald Lakes Drive, Clermont, FL 34711 News and Features 24 Contractors Roundup—Owners: We Want to be Your Partner—Matthew Allen 58 Florida Student Receives AWWA Woodard & Curran Scholarship 58 News Beat Technical Articles 14 Integration of a Distribution System Tracer Study Into a Water Quality Model to Control Disinfection Byproducts in a Potable Water System—Greg Taylor, Benjamin Yoakum, and Curtis Wade 42 Case Study to Reduce Lead and Copper Corrosion Through Water Quality Optimization and Control of Nitrification—Richie Angley, GJ Schers, Peter Davis, and Rich Giani Education and Training 13 CEU Challenge 26 FSAWWA Fall Conference Schedule 27 FSAWWA Fall Conference Registration 28 FSAWWA Fall Conference Chair’s Reception and BBQ Challenge 29 FSAWWA Fall Conference Poker Night and Happy Hour 30 FSAWWA Fall Conference TopGolf 31 AWWA Scholarship Program 40 Florida Water Resources Conference 45 FWPCOA Training Calendar Columns 23 Test Yourself—Donna Kaluzniak 32 FWEA Focus—Sondra W. Lee 34 FWEA Chapter Corner—FWEA Southeast Chapter: We’re Back in Action!—Isabel Botero 36 C Factor—Patrick “Murf” Murphy 38 FSAWWA Speaking Out—Emilie Moore Departments 59 Display Advertiser Index 60 Classifieds ON THE COVER: The American flag, show here at sunrise, symbolizes pride, sacrifice, opportunity, hope, strength, and freedom. The salute to veterans begins on page 4. (photo: Google Images) Volume 73 November 2022 Number 11 Florida Water Resources Journal • November 2022 3 Salute to Veterans in the Water and Wastewater Industry 4 Happy Veterans Day! 4 Governor’s and Mayor’s Challenges to Prevent Suicide Among Service Members, Veterans, and Their Families 10 Turn the Tide: Veterans and the Future of Water—Isaiah Moss 12 Central Florida Veterans Memorial Park: Remembering Those Who Served
HAPPY VETE NS DAY!
Welcome to the magazine’s fifth annual celebration of military veterans who work in the water industry.
We’re honored to acknowledge these brave men and women who proudly served their country, both here and abroad, and who are again serving American citizens by working as water professionals.

Along with medical personnel, police officers, firefighters, and first responders, those who work in the water industry provide a vital service and help to protect the health
and well-being of the community. They are especially vital in times of crisis, as was recently shown in Florida after Hurricane Ian, and they will be on the job for the recovery efforts that follow in the weeks and months ahead.
Water is a precious resource—one we can’t live without—and all water workers play a vital role in ensuring that everyone has all of the clean, safe water they need every day.
This section includes updated information on the governor’s and mayor’s
O O O O
challenges program to prevent suicide among service members, veterans, and their families, which was first highlighted here last year; an article by a veteran who now works in the water industry; and a story about a Florida veterans memorial.
To those selfless veterans who are and will soon be our colleagues: we thank you and salute you!
O

Governor’s and Mayor’s Challenges to Prevent Suicide Among Service Members, Veterans, and Their Families
The Substance Abuse and Mental Health Services Administration (SAMHSA) has partnered with the U. S. Department of Veterans Affairs (VA) to bring the Governor’s and Mayor’s Challenges to Prevent Suicide Among Service Members, Veterans, and their Families (SMVF) to states, territories, and communities across the United States.
There are 52 states and territories taking part in the challenge and working to develop and implement statewide suicide prevention best practices for SMVF using a public health approach.
For the Mayor’s Challenge, 22 communities were originally engaged as part of the challenge. Currently, 19 of those teams are still actively participating and sixteen of the communities are within participating Governor’s Challenge states.
The SMVF’s Technical Assistance (TA) Center is providing assistance for these initiatives.

Challenge Objectives
The objectives of the program are as follows:
S Convene a state/territory (Governor’s Challenge) or city/community (Mayor’s Challenge) interagency military and civilian team of leaders to develop an implementation
plan to prevent suicide among SMVF that will advance the VA’s “National Strategy for Preventing Veteran Suicide” and incorporate evidence-based strategies from the Centers for Disease Control and Prevention (CDC) program, “Preventing Suicide: A Technical Package of Policy, Programs, and Practices.”
S Engage with city, county, territory and state stakeholders to enhance and align local and statewide suicide prevention efforts.
S Understand the issues surrounding suicide prevention for SMVF.
S Increase knowledge about the challenges and lessons learned in implementing best policies and practices by using state/territory-to-state/ territory and community-to-community sharing.
S Implement promising, best, and evidencebased practices to prevent and reduce suicide at the local level.
S Define and measure success, including defining assignments, deadlines, and measurable outcomes to be reported.
Team Composition
Each state/territory or city/county will select team members with a long-term commitment to developing and implementing a strategic plan to enhance access to SMVF suicide prevention services and best practices. The interagency team should be comprised largely of military and
civilian individuals with the ability and authority to impact and implement state/territory or city/ county-level policy changes. Technical assistance is provided primarily through site-visit meetings and academies. All team members are encouraged to attend, including:
S A team leader, appointed by the governor, who serves as the point of contact throughout the policy academy process, and throughout the process of implementing the action plan. A team may also elect to have a coleader if desired.
S Mayor’s Challenge team leader(s), if applicable.
S Senior-level suicide prevention and behavioral health representatives from:
• State/territory agencies responsible for mental health, substance abuse (e.g., single state authority), and state VAs.
• National Guard (i.e., the adjutant general or his/her representative)
• Medicaid and/or Social Security Administration
• VA Integrated Service Network (VISN) serving a state/territory (chief mental health officer and/or VISN suicide prevention lead).
S Leadership from SMVF caregiver organizations.
S Private-sector provider and peer support leadership from programs serving the health and behavioral health needs of service members, veterans, and their families.
4 November 2022 • Florida Water Resources Journal
O
O O O O
Continued on page 6






Florida’s Original On-Line Tank Cleaning Service Since 1982 Settled Solids Management WWTP Tank Cleaning Eliminate Your Grit: visit SSM.Hydro-int.com or call 407.322.0330 or email SSM@Hydro-int.com Inventors of Sand Dragon Technology State Funding Available to Pay for Cleaning - Contact us for Details! WE REMOVE GRIT WHILE YOUR PLANT REMAINS IN SERVICE & FULL OF WATER
S Data and evaluation lead.
Teams are encouraged to consider the needs of SMVF in selecting other members of the team.
Examples include leadership from:
S Federally recognized tribes
S Reserve Affairs
S Community Veterans Engagement Board (CVEB)
S VA community engagement and partnership program (community engagement and partnership program manager [CEPPM] or community engagement and partnership coordinator [CEPC])
S Crisis response system lead
S Law enforcement
S Public and private sectors in labor/ employment, criminal justice, housing/ homelessness, primary care, substance abuse and mental health services (including suicide prevention), and child/family issues
S Academic partners from colleges and universities
S SMVF advocacy groups and social/public health organizations or coalitions
S Faith-based communities
S Public school systems
S State and/or local legislators
S Outreach or public information/public affairs representatives
S State/territory medical boards and/or licensing authorities (social work, nursing, mental health professions, etc.)
S Members from diverse populations, including historically underrepresented groups (women, ethnic minority advocacy organizations, LBGTQ+ organizations, etc.)
S Military spouse organizations, caregiver support organizations, etc.

This cross section of military and civilian agencies allows the teams to effectively plan for increased coordination of efforts and integration of SMVF into their existing state/territory/local suicide prevention plans. Creating a representative team is a critical phase of work. It builds collaborative working relationships across sectors that are too often isolated. This process also allows for initiatives to be championed, coordinated, and disseminated across many sectors.

Framework for Planning: Ensuring a Comprehensive Approach
The VA’s “National Strategy for Preventing Veteran Suicide” provides a framework for integrating and coordinating suicide prevention activities across multiple sectors. The Governor’s and Mayor’s Challenges advance the principles of the national strategy by facilitating policy-topractice implementation plans. These plans will serve as instruments of change, providing a bestpractice public health model that demonstrates meaningful results in suicide prevention.
The four interconnected strategic directions of the national strategy are as follows:
1. Healthy and Empowered Veterans, Families, and Communities

2. Clinical and Community Preventive Services
3. Treatment and Support Services
4. Surveillance, Research, and Evaluation

From the national strategy, Suicide Prevention (SP) 2.0, “Community-Based Interventions for Suicide Prevention” (CBI-SP) was developed. The CBI-SP model aims to reach veterans through multiple touchpoints. The CBI-SP initiatives include the Governor’s Challenge, together with “Veterans and Community Engagement and Partnership for Suicide Prevention,” which involves a comprehensive strategy to hire and train qualified CEPCs and communitybased interventions program managers, who collaborate at the community, regional, and state levels, to support community coalition building for evidence-informed suicide prevention interventions specific to each locality’s veteran population. This model strengthens the VA’s focus on high-risk individuals in healthcare settings, while embracing cross-agency collaborations and community partnerships to meet veterans where they live and work.
In addition, CDC’s “Preventing Suicide: A Technical Package of Policy, Programs, and Practices” is used to help teams incorporate evidence-based strategies and best practices into their planning.
Governor’s Challenge Process
The Governor’s Challenge is an intensive process that takes each state/territory team through the stages of both a policy academy model and an implementation
6 November 2022 • Florida Water Resources Journal
Continued on page 8 Continued from page 4
FIT
for more than 90 years—Lakeside





Florida Water Resources Journal • November 2022 7 Raptor® Screening Products Fine Screen Micro Strainer Rotating Drum Screen Septage Acceptance Plant Septage Complete Plant Complete Plant Multi-Rake Bar Screen FalconRake® Bar Screen Rotary Strainer Screen Wash Press WE TAILOR OUR SCREENING EQUIPMENT TO
YOUR NEEDS. All wastewater treatment plants are not alike. That’s why plant designers prefer our Raptor® line of screening products, the innovative all-in-one units that screen, wash, convey and dewater screenings efficiently, capturing more fine solids and long fibers than other available screens. Raptor® products are adaptable to a wide range of configurations, giving you more choices for better performance in your unique application. They are preferred among plant operators for their simple operation, ease of use, and minimal maintenance. When performance counts, count on the industry leader
Equipment Corporation. All trademarks owned by Lakeside Equipment Corporation. © 2022 Lakeside Equipment Corporation. Cleaner Water for a Brighter Future® Speak to one of our experts at 630.837.5640, email us at sales@lakeside-equipment.com, or visit www.lakeside-equipment.com for more product information. REPRESENTED LOCALLY BY: Florida Panhandle Only T: 205.424.7570 www.eshelmancompany.com Trippensee Shaw, Inc. T. 863.382.2101 www.TrippenseeShaw.com
academy model. These models offer a proven process and foundation for bringing policyto-practice change in state/territory systems. Technical assistance is provided by SAMHSA’s SMVF TA Center throughout this process.
The process descriptions are included here. Note that the graphics and descriptions are intended only as guidelines to be considered through the process. Actual sequencing of events may be modified as a result of situational and funding factors.
The purpose of the policy academy model is to provide an introduction to the Governor’s Challenge process, support states and territories in selecting and inviting their team members, and begin the planning process. This includes conducting environmental scans of current efforts; analyzing strengths, weaknesses, and
opportunities; and developing logic models and action plans that can be implemented in subsequent stages of the process.
These efforts are provided through statespecific site visits with SMVF TA Center facilitators, a session introducing the RAND Corporation’s Getting To OutcomesTM framework, and a multistate policy academy that provides an opportunity for state-to-state sharing and support from subject matter experts and national leaders.
The next stage moves forward to the implementation academy model, with the purpose of supporting the established teams in planning for the implementation of pilot projects or efforts within their suicide prevention action plans.
To this end, teams work to define and measure success, create milestones with assignments and timelines, and report outcomes to key stakeholders. These efforts will also include state
and territory-specific site visits with SMVF TA Center facilitators, a second session with RAND on specific implementation of the Getting to Outcomes framework, and another multistate and territory implementation academy that will help teams initiate the implementation process.
RAND Prep Session

The RAND prep session serves to introduce the Getting To Outcomes framework for comprehensive planning, implementation guidance, and evaluation of programs and community initiatives. The virtual session is led by a subject matter expert from RAND specifically discussing best practices in the implementation of prevention practices and providing examples and guidance on how to build an evaluation plan for each strategic priority area.

Key Efforts and Accomplishments

Key accomplishments for the Governor’s and Mayor’s Challenge teams include the following:
S Reducing suicide among service members, veterans, and their families.
S Increasing access to services and support.
S Expanding state and territorywide capacity to engage SMVF in public and private services.
S Enhancing provider and SMVF peer and best practices.
S Forming cross-system military and civilian consensus on priorities and plans for action.
S Identifying critical data elements to measure impact and quality of care.
S Strengthening the continuum of care.

S Transferring knowledge on evidence-based practices, policies, and strategies that are effective across teams.
Contact Information
S For technical assistance inquiries, email smvftacenter@prainc.com.
S For help with interagency coordination and federal efforts, email Stacey Owens, SAMHSA military and veteran affairs liaison, at Stacey. Owens@samhsa.hhs.gov.
S Contact SAMHSA at www.samhsa.gov for questions about its other programs and services.
S Subscribe to “Topics in the News,” a monthly e-newsletter with the latest information in behavioral health for service members, veterans, and their families.
8 November 2022 • Florida Water Resources Journal
Year One: Planning Stages Technical Assistance Events Year Two: Implementation Stages Technical Assistance Events Governor’s Challenge: Focused Priority Areas Continued from page 6






Florida Water Resources Journal • November 2022 9 The P6 Di erence Zero Pump Maintenance 10-Year Wear Warranty Reduce Polymer Increase Cake Solids Optimize System CONTACT: Stephen Gerber PHONE: 407 834 9104 EMAIL: sales@gerberpumps.com PATENTED www.p6polymix.com www.gerberpumps.com Want More from Your Belt Press? (Also Centri f uges and Screw Presses)
Turn the Tide: Veterans and the Future of Water
Isaiah Moss
Do you have a veteran in your life? Are you close to a person dedicated to a career driven by purpose in the military? Do you know a veteran who is looking for a new start, moving from the military to the civilian workforce? These are a few questions I would like you to remember as you read this article.

As a veteran, I know that all veterans are connected in their own way. We are brothers and sisters in arms. Many of us share some of the same experiences and can relate to one another. Though we come from all walks of life we all took an oath to protect and defend the United States.
The brotherhood among veterans is a strong one. For example, while I was traveling recently, I met a fellow veteran at a rest stop. We both asked each other about previous duty stations and our branch of service. These are some of the common questions we veterans all ask each other.
Veterans who are now in the water industry are very similar to this. I’m currently enrolled in a master’s degree program and I have come across fellow operators at the university who are also veterans. We engage in discussions about the water industry and exchange advice on how to deal with issues we have encountered in our water treatment operations. Both veterans and those in the water industry serve to protect the public, so veterans are looking to be part of this type of environment once they leave the service.
Here is the Problem: Challenges to Entering the Industry

When veterans seek employment in the water industry, frequently state and local governments do not accept their water-related experience from the service. After years of training and performing water operations, some veterans are told they do not qualify because they do not have the certification to

become operators. Since the experience in the service does not transfer to civilian experience it prevents the applicant from being considered for a position within the water industry. Some utilities and districts have policies that disallow the hiring of trainees, discouraging veterans from even trying to enter the industry. Why is there no path for veterans with water experience to be considered once they have reentered the civilian world?
In terms of employment, the water industry is shrinking, and in some places, it is only remaining stagnant in terms of people entering the field to replace those who are retiring. Many people have little to no knowledge about the water industry as a career, which is also true when it comes to veterans. Even though there are some attempts to change this situation, there is a large disconnect between the military and the water industry.
Law enforcement and the trucking industry make a conscious effort of seeking out veterans. They have programs and policies in place for veterans once they leave the service where they will have some, if not all, of the requirements needed to obtain a position. Why do we not have the same in the water industry? We need to find
a way to unite current and former military water operators with the present needs and demands in water utilities, both here in Florida and across the U.S.
Florida and many other states do not have policies in place that recognize a veteran’s experience as an operator, which could assist veterans with getting their license certifications. This extends to engineers, chemists, environmental scientists, and other professions within the water industry.
Here is the Solution:
Veterans Have What it Takes
The skills that veterans have can be valuable to countless water utilities. The qualities veterans bring with them from the service are unique. While in the service veterans must update and maintain their knowledge of military skills, tasks, and drills, meaning that veterans must be experts at their jobs at all times.
Since the location of a mission could change at any time, veterans must be good at adapting their skills to different situations. I once received orders to aid in a particular mission. The location was Poland and I was given a three-day
10 November 2022 • Florida Water Resources Journal
O O O O O O O O O O
notice before I went. My mission was to conduct a reconnaissance of an area where I would be responsible for supplying water to both U.S. and allied forces. Keeping up with the tasks for this mission called for was only half of the work; the other half was researching the water in Poland and knowing what equipment I would need. Now that I’m in the civilian water profession, I must also focus on the knowledge of my chosen field.
Veterans can experience a culture shock once they reenter the civilian world. Their thought processes are trained to be different from that of most people. For instance, to veterans, remaining on the job until a task is completed comes naturally—they do not question it. Veterans will not leave until their mission is complete. Veterans will work nights, weekends, and holidays to get the job done. The simple fact of being in the same country with their family for at least part of the holidays, or being able to go home soon after the work is done, is a great incentive for them, as opposed to being away from family far away in another country.
There are many more examples like this. Veterans are self-motivated, which ties into getting the mission done completely and effectively. Most veterans will view a company’s organizational chart as their chain of command. They tend to look for orders from above much more than the average employee. Make no mistake, though—in the absence of orders they will take the initiative and see the job through.
Reaching Veterans
The water industry should try to seek out these unique qualities that veterans have to offer. To reach these individuals, consider posting positions in locations where veterans are likely to visit:
S Veterans of Foreign Wars (VFW)
S Disabled American Veterans (DAV)
S American Legion
S Local veteran organizations
The water industry should make itself known to veterans that this is where they can start the next chapter in their careers, and even seek out veterans before they leave the service.
The water industry could help veterans, once they return to civilian life, by offering training in resume writing, interviewing skills, and other workplace-related needs that will help them be the most qualified for a position.
Veterans have a different approach when being interviewed. Many may see their demeanor as defensive, and how they sit during the interview may be seen as rigid. To the veteran, however, this is how you show attentiveness and courtesy. They will respond to questions very directly and respectfully. Some people do not like to be referred to as “sir” or “ma’am,” but veterans are trained to refer to people in authority in this way. The hiring teams that are aware of this will be able to understand the perspective from the veteran’s point of view. This should not be considered an obstacle to overcome, but, in today’s parlance, a “diversity” that should be acknowledged and celebrated.
Service members leaving the military must go through a two-week training period to prepare them for the civilian world. Many times they will have speakers from various fields of employment speak to the group. It would be beneficial for them to have someone speak on behalf of the water industry. And, once a representative from a Florida utility or other water-related company came to talk, the entire state, in terms of employment, would be open to them. You see, veterans are used to moving a lot, so the location of a job may not be a big deal to them.
Hiring a Veteran
Now that you have a clearer picture of the qualities veterans will bring to an organization, let’s focus on who does the hiring. As I mentioned before, some municipalities require full certification to be hired. We should start with training hiring teams with the skills needed to assess a veteran’s experiences. A veteran’s record of service (DD 214) will have a briefing on the job titles the veteran held while in the service. These documents do not give a full detail of that veteran’s specific duties, but some will have past evaluations showing the level of responsibility held during the time of service. At www.military. com there is a military-to-civilian translator for people to better understand how jobs in the service will transfer to the civilian world.
Many fields look to hire veterans and do a great job at marketing for veterans; as I mentioned before, law enforcement and truck driving, but also commercial busing, teaching (troop-to-teachers programs), and construction all seek to hire veterans at a high rate.
When I retired from the service I had over 12 years of water operations experience in several counties. I was turned down for many positions I
knew I was capable of performing. The reason for this was that my experience was not recognized by the hiring teams and according to the state where I was a trainee. I was finally granted hours after I petitioned the Florida Department of Environmental Protection. This was a good outcome for me, but this is not always the case for many veterans trying to enter the water field as an operator, because for most, the issue of recognizing a veteran’s experience is still there.
Moving forward, it would a plus to see more human resource and hiring managers getting more training on identifying veterans that are attempting to enter the water industry. Advertising that merely says “we hire vets” or other veteran-friendly slogans should evolve into a more proactive approach. Water industry managers should face this challenge head-on and be ready to add a veteran to their ranks.
There is currently a joint effort with members ranging from the Florida Water Pollution and Control Operators Association (FWPCOA), American Water Works Association (AWWA), and some member governments to write a policy that would help veterans gain entry into the water industry. The policy would grant veterans hours toward their water license as they integrate into civilian life. For right now, the policy is called the “Veterans Initiative.” The ultimate goal is to write a policy into law signed by the governor of Florida.
Initiate the Conversation
At the start of this article, I asked you to keep in mind a few questions:

S Do you have a veteran in your life?
S Are you close to a person dedicated to a career driven by purpose in the military?
S Do you know a veteran who is looking for a new start moving from the military to the civilian workforce?
Next time you talk to a veteran, inquire if they are aware of the water industry. Let them know the similarities that the military and the water industry have in common. Both veterans, and those in the water industry, answer the call to serve and protect.
Isaiah Moss is the vice chair for Region XII of FWPCOA, a Florida Gateway College graduate, and an East Central University graduate student. Jarome Madigan and Marta Madigan contributed to this article.
Florida Water Resources Journal • November 2022 11
Central Florida Veterans Memorial Park: Remembering Those Who Served
The Central Florida Veterans Memorial Park honors those who left the central Florida community in the uniform of the United States in theaters of conflict around the world and never returned, and is a place of healing for their family and friends.

The memorial is located adjacent to the new Orlando Veterans Administration Medical Center (VAMC) overlooking a tranquil lake and a short walk to the campus chapel. It truly is a special place to both remember the names of central Floridians who died in declared conflicts while serving their country and to mourn their loss. This memorial is also a peaceful place for patients, families, and friends to heal, pray, relax, reflect, and connect with their loved ones.
It honors 1,186 veterans from Orange, Lake, Brevard, Osceola, Seminole, and Volusia counties who made the ultimate sacrifice. The six counties located in central Florida are home to 400,000 veterans, and there are more former military personnel over 65 years of age living here than in any other place in the U.S. Also, there are more veterans that are over 50 percent disabled living here than anywhere else. Central Florida is the number one destination for combat veterans, and the VAMC attracts them from across the country.
A Joint Community Project

This was a major project involving approximately $3 million in combined construction costs and an endowment for maintenance of the memorial park.
This memorial was a joint project, with the use of land provided by Lake Nona Land Development, and with control of use, design, monument specifications, and events to be retained by the Central Florida Memorial Park Foundation Inc. From Winter Park, RLF Architects contributed substantial design and engineering-related services on a nofee basis. Wharton-Smith Inc., contributed construction project management, also at no fee. No member of the foundation has received compensation for services. The memorial


dedication ceremony was held on Nov. 11, 2013.
To the left and right of the Eternal Arch are smaller more-private areas with granite monuments with the names of those in uniform who have fallen in specifically named wars, starting with World War I through to the present day. Each private area has shaded seating and is beautifully landscaped to coordinate with the adjacent properties.
Contributing to the Cause
Please consider helping to continue to honor those who left the community and never returned. An endowment fund was established for perpetual maintenance of the memorial and it continues to be funded by donors. Major outright gifts of cash or stock qualify for naming a gift in honor or in memory of a loved one. In addition, some donors prefer setting up a trust that provides them income during their lifetime and a contribution to the memorial as well. Donors who contribute $1,000 or more are honored on the donation walls. Please consult a tax advisor before making such gifts. For more information, contact COL DeLloyd Voorhees, USA (Ret.), president, at de.voorhees@cfvmpf.org. S
12 November 2022 • Florida Water Resources Journal O O O O O O O O O O
Operators: Take the CEU Challenge!
Case Study to Reduce Lead and Copper Corrosion Through
Quality Optimization and Control of Nitrification
Angley, GJ Schers, Peter Davis, and Rich Giani
CEU =
DW/DS02015412)
Integration of a Distribution System Tracer Study Into a Water Quality Model to Control Disinfection Byproducts in a Potable Water System
Greg Taylor, Benjamin Yoakum, and Curtis Wade (Article 2: CEU = 0.1 DW/DS02015413)
Florida Water Resources Journal • November 2022 13
Members of the Florida Water and Pollution Control Operators Association (FWPCOA) may earn continuing education units through the CEU Challenge! Answer the questions published on this page, based on the technical articles in this month’s issue. Circle the letter of each correct answer. There is only one correct answer to each question! Answer 80 percent of the questions on any article correctly to earn 0.1 CEU for your license. Retests are available. This month’s editorial theme is Water Treatment. Look above each set of questions to see if it is for water operators (DW), distribution system operators (DS), or wastewater operators (WW). Mail the completed page (or a photocopy) to: Florida Environmental Professionals Training, P.O. Box 33119, Palm Beach Gardens, Fla. 33420-3119. Enclose $15 for each set of questions you choose to answer (make checks payable to FWPCOA). You MUST be an FWPCOA member before you can submit your answers! SUBSCRIBER NAME (please print) Article 1 LICENSE NUMBER for Which CEUs Should Be Awarded Article 2 LICENSE NUMBER for Which CEUs Should Be Awarded Article 3 LICENSE NUMBER for Which CEUs Should Be Awarded If paying by credit card, fax to (561) 625-4858 providing the following information: ___________________________________ (Credit Card Number) (Expiration Date) EARN CEUS BY ANSWERING QUESTIONS FROM PREVIOUS JOURNAL ISSUES! Contact FWPCOA at membership@fwpcoa.org or at 561-840-0340. Articles from past issues can be viewed on the Journal website, www.fwrj.com. 1. In combination with the recarbonation system, the new ___________ chemical feed system mitigated calcium carbonate filter precipitation and increased finished water alkalinity. a. sodium hydroxide b. calcium hydroxide c. calcium bicarbonate d. magnesium hydroxide 2. A 2016 Water Research Foundation study demonstrated that iron has the affinity to absorb a. lead. b. copper. c. manganese. d. arsenic. 3. Calcium carbonate precipitation potential values between ___________ mg/l as CaCO3 will cause a light noticeable scale to form. a. 1 and 4 b. 5 and 10 c. 11 and 15 d. 16 and 20 4. Manganese can cause the same type of discolored water event at concentrations _____ times lower than iron. a. 2 b. 2.5 c. 4 d. 4.5 5. _____________ is the natural ability of the water to maintain a stable pH throughout the distribution system. a. Alkalinity b. Hardness c. Buffer intensity d. Langelier Saturation Index
Water
Richie
(Article 1:
0.1
1. The tracer used in this study was a. food grade dye. b. sodium chloride. c. potassium bromide. d. sodium hypochlorite. 2. Which of the following is not identified as an effective treatment technique for reducing natural organic matter? a. Stripping b. Granular activated carbon c. Ion exchange d. Membrane treatment 3. Results of the tracer study indicated that free chlorine residual in the University of Central Florida (UCF) system decayed to the regulatory minimum in approximately _____ hours. a. 12 b. 24 c. 36 d. 72 4. The regulatory maximum contaminant level (MCL) for total trihalomethanes (TTHMs) is _____ parts per billion. a. 40 b. 60 c. 80 d. 100 5. Which of the following was not among the recommended im provements to reduce TTHMs? a. Change disinfectants b. Additional autoflushing stations c. Add treatment process to reduce precursors d. Construct additional distribution system loops
Integration of a Distribution System Tracer Study Into a Water Quality Model to Control Disinfection Byproducts in a Potable Water System
Greg Taylor, Benjamin Yoakum, and Curtis Wade
The University of Central Florida (UCF) owns and operates its potable water system, which supplies water to UCF’s main campus and some outlying areas. From 2016 to 2020, UCF distributed approximately 0.733 mil gal per day (mgd) of potable water to campus facilities, classrooms, and student residences. The majority of UCF’s water supply comes from four Upper Floridan aquifer (UFA) source wells that are permitted through the St. Johns River Water Management District (SJRWMD). This raw source water is treated at UCF’s water treatment plant (WTP), which aerates water to remove hydrogen sulfide and then chlorinates the water for primary disinfection and residual disinfection prior to pumping into UCF’s distribution system.
The UCF utilizes sodium hypochlorite to disinfect the water and provide residual disinfection in the distribution system. Two regulated groups of disinfection byproducts
(DBPs) form when natural organic matter (NOM) in source water comes into contact with this disinfectant: total trihalomethanes (TTHMs) and a group of five haloacetic acids (HAA5s). Historically, compliance with TTHM regulations has been challenging for UCF during periods of the year when the university is not in session and water demand decreases.
Figure 1 shows historical TTHM compliance results for UCF’s four monitoring sites. Over the evaluated time period UCF has been out of compliance for TTHMs in one quarter in 2014 and one quarter in 2018.

Both TTHMs and HAA5s form when organic matter naturally found in groundwater is oxidized during disinfection with free chlorine. The amount of TTHMs and HAA5s that form is dependent on the following:
S Chlorine dose – The higher the chlorine dose, the greater the DBP formation.
Greg Taylor, P.E., is senior project manager at Wright-Pierce in Orlando. Benjamin Yoakum, P.E., Ph.D., is research and innovation project manager with Orange County Utilities in Orlando. Curtis Wade is utilities director with the University of Notre Dame in Notre Dame, Ind. At the time the article was written, Benjamin Yoakum was a project engineer at Wright-Pierce in Orlando and Curtis Wade was utilities and energy services senior director with the University of Central Florida in Orlando.
S Type and concentration of NOM in the source groundwater – The greater the concentration of NOM, the greater the DBP formation.
S The amount of time the disinfectant is in contact with NOM – The longer chlorine is in contact with NOM, the greater the DBP formation.
S Temperature of water – The higher the temperature, the greater the DBP formation.
S pH – The higher the pH, the lower the formation of HAA5s, but the higher the formation of TTHMs.
S Bromide – The higher the concentration of bromide, the higher the DBP formation.
There are treatment options and operational strategies that can be implemented to reduce TTHM and HAA5 formation. These strategies include:
S Reducing the amount of chlorine used during disinfection. The reduction in chlorine dose is limited by the requirement to maintain a minimum free chlorine residual of 0.2 mg/L within the potable water distribution system.
S Modifying the treatment process to utilize chloramines for residual disinfection in lieu of free chlorine can reduce the DBP growth in the distribution system.
14 November 2022 • Florida Water Resources Journal
FWRJ Figure 1. Historical total trihalomethane concentrations for each of University of Central Florida’s compliance monitoring sites.
S Reducing the amount of NOM, specifically DBP precursor matter, prior to disinfection. Reduction of precursor matter can be accomplished with a variety of treatment options, including granular activated carbon (GAC), ion exchange, and membrane treatment.
S Reducing the amount of time water containing NOM is in contact with the disinfectant. This contact time can be reduced by looping dead end or low-use distribution mains or implementing a flushing program. Flushing is limited by cost and consumptive use permit (CUP) considerations.
S Aeration of chlorinated water can strip formed TTHMs from treated water where the contaminant is transferred from the liquid to the air. This treatment process does not appreciably remove HAA5s and does not prevent the reformation of TTHMs postaeration.
Given the historical difficulties complying with TTHM regulations, UCF has implemented two of these strategies to reduce the concentration of DBPs in its distribution system:
1) Potable Water Flushing: UCF installed automated flushing stations throughout the potable water distribution system. These stations are automated to flush potable water at a set flow rate for a selected duration. Operations can adjust the timing and quantity of flow at each flushing location. Potable water flushing is limited by the UCF’s CUP and the total water withdrawn from UCF’s permitted wells cannot exceed a set value listed in the CUP.
2) Spray Aeration: UCF installed a spray aerator and tank mixer in its ground storage tank (GST) to aerate chlorinated water and keep the GST well mixed. This aeration process strips TTHMs from the water and reduces their concentration in the finished water. This results in a reduced concentration of TTHMs leaving the WTP; however, they can continue to form in finished water after they are stripped, so if water resides in UCF’s distribution system for an extended period of time, TTHMs can reform and exceed 80 parts per bil (ppb) at maximum contaminant level (MCL).
To optimize its flushing program, UCF performed an evaluation to quantify two variables that, in conjunction, could determine if TTHMs would be expected to exceed regulated levels in the distribution system. The two variables were the rate of DBP formation and the detention time of the water in the distribution system.
Table 1. Water Quality Testing Instruments and Analysis Location
Test Instrument Analysis Location pH/Temperature HACH HQ40d Field
Conductivity HACH HQ40d Field
Free Chlorine HACH Pocket Colorimeter II Field
TTHM / HAA5 Gas Chromatograph Certified Laboratory*
Figure 2. University of Central Florida’s tracer study sampling locations.
The overall goal of the evaluation was to better understand TTHM formation in the distribution system to reduce flushing and associated maintenance costs. The rate of DBP formation in the distribution system was determined by performing a tracer study. The detention time of water throughout the distribution

system was predicted by the development of a hydraulic model. Integrating tracer study results with hydraulic modeling can produce a water quality model capable of assessing TTHM concentrations throughout UCF’s distribution system under various flow regimes.

Florida Water Resources Journal • November 2022 15
*Orlando Utilities Commission Water Quality Laboratory
Continued on page 16
Methods and Materials
Distribution System Tracer Study
A tracer is a substance that is injected into a system that can be tracked (or traced) as it travels through the system, over time, and it does not react with other water quality parameters, nor degrades over time. For UCF’s tracer study, the selected tracer was table salt (sodium chloride) and the system was UCF’s potable water distribution system. Dosing salt into water leaving the WTP and entering the distribution system increased the conductivity of water from its baseline value. Conductivity in the distribution system can be measured as the water travels throughout
the system. If the conductivity remains at the baseline value, the analyst knows the salt tracer has not made it to that location in the distribution system. Conversely, if the conductivity increases to above the baseline value the analyst knows the tracer has made it to the distribution system location being sampled. Using an initial timestep as the point at which the tracer is injected into the system, the age of the water can be ascertained when the conductivity increases above the baseline value.
The remainder of this subsection describes the protocol performed during UCF’s tracer study.
Prior to the tracer study, UCF contacted and received written approval from the Florida Department of Environmental
Protection (FDEP) to perform such a study in UCF’s public water system. Pursuant to FDEP approval, UCF posted a public notification describing the study prior to initiating study activities.
On the morning of the tracer study, an initial batch of the salt dosing solution was produced by mixing food grade table salt with finished water from UCF’s point of entry (POE) tap in a food grade 55-gal drum. Throughout the duration of the study additional batches of the salt solution were produced to refill the drum. A National Science Foundation (NSF) 61-compliant chemical feed pump was used to dose the salt solution into the suction header pipe of the WTP’s high-service pumps. These pumps helped to mix the salt solution with finished water before the water entered the distribution system.
Figure 2 shows the monitoring route where the tracer was tracked as it flowed through UCF’s distribution system. Prior to dosing, the hydrant at the end of the monitoring route (Hydrant J1660; see Figure 2) was opened and the flow was set at approximately 150 gal per minute (gpm). This artificial flow helped pull fresh water containing the salt tracer through the distribution system, along the monitoring route and to this terminal location. After the hydrant was opened and flowing at approximately 150 gpm, dosing at the WTP commenced.


Once the tracer entered the distribution system it was traced through the system from POE to the terminal hydrant. The following describes the procedure used at the first monitoring location in UCF’s distribution system: Sample Location No. 2 (see Figure 2).
Upon arriving at Sample Location No. 2 the hydrant was flushed, and then left to continually flow at approximately 5 to 10 gpm. The conductivity of the water was measured continually using a probe until there was a measured rise in conductivity, which indicated the tracer had reached the location. After the tracer had reached the sample location, a water sample was collected and measured for free chlorine residual. Then, another water sample was collected and quenched for TTHM and HAA5 analyses at a certified laboratory. This process continued at subsequent locations until the terminal hydrant was reached.
After the tracer reached the terminal hydrant the free chlorine residual was measured and TTHM and HAA5 samples were quenched for laboratory analysis. Then, water was flushed from the hydrant for an additional 15 minutes. After that time, the hydrant was closed and the chemical feed
16 November 2022 • Florida Water Resources Journal
Figure 3. Classroom Building I diurnal potable water demand. Figure 4, Classroom Building I potable water demand (weekday versus weekend). Continued from page 15 Continued on page 18

pump dosing the tracer was turned off. Water with a known age was now captured in the terminal pipeline and could be sampled over the following days. Note that selection of a terminal pipeline that has only minimal flow is required to ensure that fresh water is not pulled into the terminal pipeline over the next several days of testing.
Over the next several days an analyst would return to the terminal hydrant to sample water. For each sampling event, samples were collected from the terminal hydrant, free chlorine residual was measured, and samples were quenched for TTHM and HAA5. An analyst would continue to return to the terminal hydrant and sample until either: 1) the chlorine residual at the location had decayed to a value of less than 0.2 mg/L (the minimum allowable limit); or 2) the conductivity at the terminal location decreased back to the baseline value, which would indicate that the water from the day of dosing was no longer in the terminal pipeline that feeds the terminal hydrant.
Table 1 presents the water quality testing instruments used during the study and identifies where samples were analyzed.
Metering Data Preparation for Modeling
Modeling of water flow in UCF’s distribution system was performed by assigning a set quantity of potable water flow to each building or facility that demands potable water and then describing how the water flow (i.e., demand) varied over a time period. How potable water demand varied over a period of time for a specific facility or group of facilities can be described nominally as a demand pattern.
In a traditional potable water system that serves residential, commercial, and industrial customers, water demand varies throughout the day. For residential customers, there is typically a peak in water demand in the morning hours when people are waking up and preparing for work, and a subsequent peak in the evening after people are returning from work and preparing meals. During midday there is normally moderate water demand,
and then during the night there is typically low water demand while people are asleep. For commercial customers, there is typically low demand in the morning and evening, but a peak during midday when people are shopping, or at offices and restaurants.
The UCF potable water system aligns more with the commercial demand pattern, with relatively low demands in the early morning and evening hours, and then a large peak in water demand during the midday period. This is due to a large portion of the demand being attributed to students, faculty, and staff who live off campus, but are on campus during the day to attend classes, eat at on-campus restaurants, or work at the university. This can be seen in Figure 3, which presents the diurnal demands for Classroom Building I, which is served by UCF’s potable water system.
The data used to create Figure 3 came from hourly flow totals, captured every hour at the building from May 1, 2016, to May 1, 2019, representing approximately three years of data. This large diurnal flow variation is important when evaluating the water flow through the distribution system.
There is a fairly unique potable water demand at UCF throughout the week as well. During the standard workweek, when most classes are in session (i.e., Monday through Friday), there is significantly more potable water demand than on the weekend. Figure 4 shows the demand pattern difference between the workweek and weekend for Classroom Building I.
The data used to create Figure 4 were the same data used to create Figure 3; however, only data from weekdays were used to create the “weekday” line in Figure 4 and only data from weekends were used to create the “weekend” line. As shown in the figure, the “weekday” hourly flow values for each building are significantly higher than the “weekend” hourly flow values. This reflects a wide variation from the average daily flow when considering all seven days of a week. This large variability indicates the need to differentiate the potable water demand in modeling for both weekdays and weekends to accurately reflect the system demand. The water age will be significantly higher after a weekend of little to no demand. Using an average of all seven days to reflect weekend flow would show a lower water age, which is not accurate.
In addition, UCF has a unique potable water demand during periods of time when the university is in session (i.e., classes are being held, referred to as “in session”) when compared to periods of time when the

18 November 2022 • Florida Water Resources Journal
Demand Scenario Day of the Week (Average1/Weekdays/Weekends) University Academic Period (Average1/In Session/Out of Session) 1 Average Average 2 Weekdays Average 3 Weekends Average 4 Average In Session 5 Weekdays In Session 6 Weekends In Session 7 Average Out of Session 8 Weekdays Out of Session 9 Weekends Out of Session (1) Average represents that the criteria category is not split in the scenario (e.g., average for day of the week evaluates all seven days of the week. Figure 5. Classroom Building I potable water demand (in session versus on break). Table 2. Evaluated Potable Water Demand Scenarios Continued from page 16
university is out of session (i.e., classes are not being held, referred to as “on break”). Figure 5 shows this difference for Classroom Building I. As depicted in Figure 5 there is significantly more flow in the building when the university is in session compared to when it’s on break. This large variability requires a differentiation between time periods when the university is in session and time periods when it’s on break, rather than simply taking the average flow for all time periods.
As a result of these findings, these demand criteria are important to incorporate into hydraulic modeling to appropriately represent different time periods so that water age during these periods can be evaluated; therefore, nine demand scenarios were created to represent each combination of demand criteria. These demand scenarios are presented in Table 2.
Figure 6 shows the potable water demand for each of the nine scenarios for Classroom Building I. Note that in the figure, the highest potable water demand occurs during weekdays when the university is in session and the lowest demand occurs during weekends when it’s out of session. So, UCF expects to find the lowest water age in its distribution system on weekdays, when school is in session (highest flow scenario), and conversely, expects to find the highest water age in its system on weekends when school is not in session (lowest flow scenario).

The UCF has close to 200 potable water meters, and if every meter had nine demand patterns developed for each unique meter (representing each demand scenario), then over a thousand demand patterns would need to be developed. This would result in a model that would be hard to manage; therefore, meters were further categorized by specific use type to create a manageable data set that could be efficiently modeled, while at the same time remain representative of the data set.
Meters were sorted into one of five categories/groups; the UCF operations staff was consulted to ensure accurate categorization. These categories, a description of each category, and the total number of meters sorted into each category are presented in Table 3. Water demand associated with the meter categories of Classroom, Student Residence, Facility, and Other is not controlled by UCF operations, but rather is based on the daily potable water use in each building. The UCF operators do control the flow through the meter category of Autoflusher, which is used to flush water from the distribution system to maintain water quality through reduced water age. As a result, demand patterns were not developed for this group of meters.
Figure 6. Classroom Building I potable water demand scenario results.
Table 3. Meter Categories and Associated Descriptions
Meter Category Number of Meters
Description of Meter Category Classroom 37
Student Residence 46
Facility 68
Other 28
Autoflusher 6
The meter serves a building that is primarily used only during days when the university is in session and in most cases is used as a classroom.
The meter serves a building that students live in while on campus. Note: this category includes both "term only" residences and "full year" or "12 month" residences.
The meter serves a UCF nonclassroom and nonresidence building that house offices or operations facilities.
The meter serves a building that does not fit into one of the above categories.
The meter has a defined flushing pattern that can be set by UCF potable water utility operators.
Prior to creating the potable water demand patterns, the average, maximum, and minimum daily flow for each meter was calculated using monthly metering data and dividing by the number of days for that month. Most meters had monthly meter data for every month from May 2016 through March 2019, representing 35 records for each meter.
Thirty-six demand patterns were developed using hourly flow data from meters that were active during the evaluated time period. These 36 demand patterns equal the number of different combinations of the nine unique demand scenarios and the four meter groupings developed. Each demand pattern consists of 24 hourly peaking factors representing the flow through a meter for a specific hour of the day (e.g., 2 a.m.) for a specific scenario (e.g., Scenario 5: Only days
that are weekdays and only days when the university is in session). The peaking factor compares flow through a meter at a specific hour to the overall average daily flow. The resulting table of peaking factors is presented in Table 4.
The last row of Table 4 calculates the average peaking factor for each demand pattern. This value represents the ratio of flow for the demand pattern when compared to the overall average flow through that meter group. For this reason, the first four demand patterns have an average peaking factor equal to 1, as these demand patterns represent the average meter data for every day. The other remaining scenarios and corresponding demand factors have average peaking factors greater than or less than 1. This is due to the fact that the peaking factors under these
Florida Water Resources Journal • November 2022 19
Continued on page 20
scenarios only use a portion of the flow data used for developing the demand patterns.
When the average demand peaking factor is greater than 1, it means that the average flow for that time period is higher than average over an entire week. For example, in Table 4, Demand Pattern 5 has an average peaking factor of 1.281; this represents that, for this demand, the meters assigned to the Classroom category and during the weekdays (in and out of session), will have 28.1 percent more flow than the overall average flow for meters in this group. Conversely, an average peaking factor of less than 1 represents a lower average daily flow compared to meters in this group.
To model a water demand scenario each potable water meter needed to be assigned a flow rate for each hour of the day. This process was accomplished in the manner that follows.
For Classroom, Student Residence, Facility, or Other meters:
1. One of the nine water demand scenarios is selected.
2. Each meter is assigned a meter category.

3. The average flow for each meter is assigned to the meter and then divided by 24 to get an average hourly flow.
4. Each meter is assigned a demand pattern based on the selected demand scenario (Step 1) and assigned meter category (Step 2). The assigned demand pattern dictates the hourly peaking factors based in Table 4.
5. The flow rate for each hour of the day for each meter is calculated by multiplying the average hourly flow rate for a meter (Step 3) by the peaking factor for that hour (Step 4).
For autoflushing meters, hourly flow is assigned by the user. Typically, at UCF autoflushing meters are run in the early morning hours when system demand is low at a set rate; for example, from 2 to 4 a.m. at 50 gpm. This would result in an assigned flow of 3000 gal per hour (gph) for hours 2 and 3 and an assigned flow of 0 gph for hours 0, 1, and 4 to 23 for this meter.
Metering data are then loaded into a
model to assess water age throughout the system.
Hydraulic Model Development
For systems as extensive as the UCF potable water distribution system, a computerized modeling program becomes an essential tool. The selected software for the modeling efforts was the InfoWater® platform by Innovyze™. InfoWater uses the ArcGIS platform where AutoCAD and geographic information system (GIS) shapefile features can be uploaded using the drawing’s scale, metadata, and other features. InfoWater is also capable of importing and exporting data to be used with other spreadsheet, database, and modeling software, such as Excel® and Access®.
There are two primary hydraulic modeling timestep options available to develop model scenarios:
1. Steady-State (SS) – An SS timestep captures a specific instance in time (snapshot) for the hydraulic model and does not look at
Table 4. Diurnal Demand Patterns and Hourly Peaking Factors
20 November 2022 • Florida Water Resources Journal
Continued from page 19
how the system reacts to changes. The SS modeling is the traditional option selected for master planning, as the focus is typically system pressures in relation to a change in potable water demand. The SS modeling is acceptable for predicting future pressures and hydraulic grade lines (HGLs) and estimating the sizes and routes of future pipes.
2. Extended Period Simulation (EPS) – The EPS modeling evaluates how a system behaves over a period of time. This model adjusts to system changes over time and can reflect how the system reacts. This type of model is used for evaluating tank drain and fill cycles, monitoring water age and DBP formation, and evaluating how pumping systems ramp up and down on variable frequency drives (VFDs) or turn on and off in reaction to system flow changes.
Either of these analysis options can be employed in the system analyses, but are dependent on the purpose of the modeling and/or evaluation. The EPS model analysis option was selected to evaluate water age throughout the distribution system over a period of several days. This analysis would provide insights related to water age and associated DBP concentrations throughout the distribution system.
The UCF potable water model contained 1,253 pipes and 1,184 junctions/nodes. The 183 potable water flow meters were represented in the GIS system as discrete points. The next step in allocating demands to meters was assigning an average flow and meter classification to each geolocated meter. This was done by importing average flow values for each meter, assigning meter classifications for each flow meter, and matching these values to each meter using the meter identifications. Each of the 183 meters was then assigned to the closest node in the hydraulic model that also corresponded to the actual pipeline that the meter was connected to. Most meters were assigned to a dedicated node; however, there were some closely clustered meters that were assigned to a singular node, with an additive sum.
The final step in allocating demands was importing demand patterns into the model. Each of the scenarios from Table 2 was incorporated into the model with the associated demand patterns for each node. The demand patterns were assigned to each meter by importing peaking factors found in Table 4 and then assigning them to each meter based on the meter’s classification and the selected demand scenario.
Figure 7. Tracer study results of distribution system total trihalomethane formation and free chlorine decay.

Results and Discussion
Tracer Study Results: Total Trihalomethane Formation Within the University’s Distribution System
Figure 7 presents the TTHM formation in the distribution system and the associated free chlorine decay measured from the same tracer study samples. This figure represents the best available data for how TTHMs form and free chlorine residual decays in UCF’s potable water distribution system. The following can be inferred from Figure 7:
S Based on the free chlorine dose at UCF’s WTP on the day of testing, TTHMs are expected to exceed the regulated MCL after approximately 18 to 24 hours in UCF’s distribution system.
S Given the free chlorine dose at UCF’s WTP on the day of testing, the free chlorine residual is expected to decay to below the regulated limit (0.2 mg/L) after approximately 72 hours in UCF’s distribution system.
S Based upon historical data that UCF has not exceeded the regulatory MCL for TTHMs, the practical water age target is 48 to 72 hours.
The UCF operators have the option of reducing the chlorine residual at the POE by decreasing the chlorine dose at the WTP; however, this would result in chlorine decaying below the regulated level of 0.2 mg/L in a shorter period of time (i.e., in less than 72 hours). It’s
expected that water resides in UCF’s system for at least three days (72 hours), and as a result, this is not an appropriate DBP control strategy.
Modeling Results – Part 1: Simulated Water Age in the University’s Distribution System
InfoWater was used to simulate water age in UCF’s distribution system for each developed demand scenario. Figure 8 shows the results of modeling Demand Scenario 1: Average Weekly Demand. This figure shows the water age at each model node in UCF’s distribution system. Results showed that only 18.7 percent of the modeled distribution system nodes had a water age of less than 24 hours without any potable water flushing A similar modeling effort was performed for each of the other eight demand scenarios.
Modeling Results – Part 2: Simulated Total Trihalomethane Concentrations in the University’s Distribution System
The next modeling step was to integrate the TTHM tracer study results into water age modeling results. This was performed by importing the distribution system TTHM formation curve developed during the tracer study into the InfoWater model. InfoWater can then model TTHM concentrations, over time, for each water demand scenario. It should be noted that this additional modeling step can be omitted if desired.
If the goal is to assess if TTHMs cross a threshold value—for example, the 80-ppb MCL—the user can look up the associated
Florida Water Resources Journal • November 2022 21
Continued on page 22
TTHM concentration in the tracer study results and identify the associated critical water age. In UCF’s case, TTHMs were expected to exceed the MCL after 18 to 24 hours; therefore, modeling showed that water age could be used as a surrogate to assess anticipated compliance with TTHM regulations. In this evaluation, any node that had a water age of above 24 hours was color-coded to identify areas that were expected to have water with TTHM concentrations at or above the MCL.
The final step in modeling was to determine the amount of flushing required under each demand scenario to reduce TTHM concentrations in the system to below regulated levels (80 ppb, which corresponded to a water age of 18 to 24 hours). Figure 8 shows UCF’s five existing autoflushing stations within its
station was proposed at the location identified as “Barbara Ying.” For each scenario, the flow rate and duration of flushing at each station was adjusted. In general, if the water age around an autoflushing station was above the determined critical water age of 18 to 24 hours, then additional flushing at the nearest autoflushing station was needed. In some instances, it was prudent to add a line loop in the distribution system. The result of these efforts showed how optimal flushing could be accomplished under each demand scenario.
Autoflushing becomes cost- and CUPprohibitive if an excessive amount of water is flushed to reduce water age. During certain periods of the year, specifically when the university is not in session, UCF is required to flush a significant amount of potable water to remain in regulatory compliance. As a result, it
process that will remove DBP precursor matter, which would result in a reduction in TTHM formation potential. This would allow water to reside in the distribution system for a longer period of time without forming TTHMs in excess of the regulated MCL. In addition, this treatment addition would allow UCF to significantly reduce its need to flush potable water.
Findings Summary
A distribution system tracer study was performed with food grade sodium chloride to assess the TTHM formation potential and free chlorine residual decay in UCF’s potable water distribution system. The study results found that TTHMs reached the U.S. Environmental Protection Agency (EPA) MCL of 80 ppb after approximately 18 to 24 hours in the distribution system and free chlorine residual decayed past the minimum regulatory level of 0.2 mg/L after approximately 72 hours. While UCF has not exceeded the MCL for TTHMs, these are general guidelines for long-term planning with the hydraulic model.
Historical water usage data were used to develop nine unique potable water demand scenarios that characterize the potable water demand of UCF’s system during different periods of time throughout the year. These demand scenarios were imported into an InfoWater model that contained UCF potable water assets, including distribution system piping and potable water meters. A hydraulic model was developed that could estimate water age throughout the distribution system for each developed demand scenario. Modeling results showed that water age in several areas of the system exceeded 96 hours.
Tracer study results were integrated into the hydraulic model to predict TTHM concentrations throughout the distribution system. The goal of subsequent modeling efforts was to optimize flushing to reduce the amount of potable water being flushed, while also maintaining compliance with TTHM regulations. Model runs were completed to determine optimal flushing regimes for each developed demand scenario.
Recommended short-term improvements, elucidated through modeling, included the addition of several line loops in the distribution system and the construction of an additional autoflushing station. The recommended longterm improvement was to add a treatment process that will remove DBP precursor matter, which would result in a reduction in TTHM formation potential. This improvement would greatly reduce the concentration of TTHMs throughout UCF’s distribution system and significantly reduce potable water flushing in the system.

22 November 2022 • Florida Water Resources Journal
SFigure 8. Water age modeling in demand scenario 1 showing average weekly demand. Continued from page 21
Test Yourself
What Do You Know About Water/Wastewater Staffing and Classification?
Donna Kaluzniak

1. Per Florida Administrative Code (FAC) 62-699, Treatment Plant Classification and Staffing, classification and staffing requirements for each water treatment plant are determined using a two-step procedure. First, the plant’s category is determined by the treatment processes. Then the classification and staffing requirements within that category are determined by using the plant’s permitted
a. annual average daily flow. b. maximum-day operating capacity. c. peak daily demand. d. peak hourly capacity.
2. Per FAC 62-699, an onsite computerized system with sensors and programs that can adjust and control domestic wastewater or water treatment plant equipment and processes over the normal range of expected operating conditions without operator assistance is a(n)
a. automatic control system. b. electronic surveillance system. c. consecutive control system. d. process control system.
3. Per FAC 62-699, a 6-mil-gal-per day (mgd) wastewater treatment plant using the activated sludge process (not extended aeration), with filtration would require the lead/chief operator to be a Class A. The plant would also need to be staffed by a Class C or higher operator for how many hours per day?
a. 8 b. 12 c. 16 d. 24
4. Per FAC 62-699, for water and wastewater treatment plants that are under an electronic surveillance system, automatic control system, or electronic control system, staffing requirements can be reduced for new treatment plants and for existing treatment plants that have been in compliance with water quality
standards and operation and maintenance requirements for the past a. six months. b. year. c. two years. d. five years.
5. Per FAC 62-699, classification and staffing requirements for distribution systems are determined with a two-step procedure using the highest-classification treatment plant to which they are connected and the
a. number of persons served directly by the distribution system.
b. maximum treatment plant capacity. c. peak hourly demand over the last year. d. peak daily demand.
6. Per FAC 62-699, suppliers of water shall employ only those appropriately licensed to be in onsite charge of any water distribution system operation or maintenance activity that may affect
a. customers utility bills. b. the water treatment process. c. the utility’s budget. d. water quality or quantity.
7. Per FAC 62-699, a Class C, 0.5-mgd conventional filtration water treatment plant would require staffing by Class C or higher operator: 6 hours per day for five days per week. On the weekend it would require an onsite examination to ensure that equipment is functioning properly, that chemical supplies are sufficient, and to record the quantity or quality of drinking water being treated and other relevant information. This is defined as a(n)
a. abbreviated workday. b. drive-by. c. short shift. d. visit.
8. Per FAC 62-610, Reuse of Reclaimed Water and Land Application, minimum staffing requirements for domestic wastewater treatment plants that provide reclaimed water to a reuse system permitted under Part III is a Class C or higher operator 24 hours per day, seven days per week.
The lead/chief operator shall be at minimum Class B, or higher if required by FAC 62-699. The minimum staffing requirement, however, can be reduced in conjunction with provisions for increased facility reliability or a. if an automatic control system is installed. b. if acceptable quality reclaimed water is
diverted to the reuse system only when operators are present.
c. if additional filtration is added to the system. d. if additional disinfection is provided.
9. Per FAC 62-640, Biosolids, the level of operator staffing at biosolids treatment facilities is determined by the type of facility, based on design capacity in dry tons per year and
a. class of biosolids based on pathogen reduction.
b. type of biosolids treatment.
c. level of volatile solids reduction. d. level of vector attraction reduction.
10. Per FAC 62-699, an operator meeting the lead/ chief operator class for a treatment plant and an operator meeting the lead/chief operator level or class for the water distribution system shall be available
a. 8 hours per day, five days per week. b. 8 hours per day, seven days per week. c. 12 hours per day, seven days per week. d. during all periods of treatment plant or distribution system operation.
Answers on page 62
References used for this quiz:
• Florida Administrative Code 62-699, Treatment Plant Classification and Staffing: https://www.flrules.org/gateway/ChapterHome. asp?Chapter=62-699
• Florida Administrative Code 62-610, Reuse of Reclaimed Water and Land Application: https://www.flrules.org/gateway/ChapterHome. asp?Chapter=62-610
• Florida Administrative Code 62-640, Biosolids: https://www.flrules.org/gateway/ChapterHome. asp?Chapter=62-640
Send Us Your Questions
Readers are welcome to submit questions or exercises on water or wastewater treatment plant operations for publication in Test Yourself. Send your question (with the answer) or your exercise (with the solution) by email to: donna@h2owriting.com
Florida Water Resources Journal • November 2022 23
Owners—We Want to be Your Partner
Matthew Allen
Traditional design-bid-build delivery methods
employed by owners across the Sunshine State are increasingly being replaced by various integrated project delivery methods leveraging the inherent collaboration among team members. These delivery methods characteristically lend themselves to a more relationship-based approach, encouraging collaboration from day one, while taking into consideration each key stakeholder’s goals and their individual definition of project success, with a focus on the best value for all parties. This approach, however, doesn’t need to be limited solely to collaborative project delivery methods.
Ensuring Successful Projects
Unsuccessful design and construction projects traditionally are rooted in poor communication, not clearly understanding or appreciating all parties’ project objectives, and a general lack of trust among members of the project team. All of this has the potential to create an adversarial environment, resulting in strained relationships, delayed project delivery, and eroded margins.
Together, we have the ability and power to set the course for each project on how we are going to do business. We can develop tools to establish relationships that can help to resolve many of these common issues for our projects. Simply investing time at the beginning of a project to communicate our collective project goals, making a personal commitment to our team on how
we’re going to resolve issues as they arise, and committing to an established process throughout the project duration to mitigate potential issues—these are, in essence, partnering.

The formal partnering process enhances collaboration and mitigates potential conflict through shared goal setting and accountability. It leverages the value-added services of each party to best achieve shared project objectives through the principles of teamwork, proactive communication, trust, and honesty, while developing a win-win mentality. Formal partnering has become more common on some projects, but it’s still not being implemented to the degree it could be.
Often, a project may begin with a formal partnering meeting with the greatest of intentions by all parties, but it’s soon abandoned at the first sign of conflict, indicating there was never a good partnering plan to begin with. Implementation and follow-through are frequently hindered by internal barriers, a reluctance to change the way it’s always been done, and a lack of understanding of how to best employ and manage a successful partnering relationship.
Partnering Process Model
The Construction Industry Institute (CII) established a partnering process model that consists of five phases, much of which relies upon the owner to initiate, but requires commitment from executive, management, and craft-level participants throughout each organization.

The following is a high-level overview of each phase of this process.
Owner’s Internal Assessment
This includes evaluation of longterm business drivers, the different types of partnering and associated benefits of each, and the cost and effort required to implement and manage the process through the project life cycle. Proactive planning will increase the likelihood of success with the lowest cost.
Owner Creates the Partner Selection Process

While cost is often driving the selection criteria, it doesn’t always produce the best partner or best project results. The owner should spend the time now to develop the project-specific selection criteria that result in the optimum partner, as well as forming a competent selection team.
Planning and Implementation
Following selection of a partner and award of a contract, this phase involves alignment of the partnering relationship and development of each partner’s objectives in the relationship and the incentives that may support these objectives.
Developing Project-Specific Objectives
Through workshops/partnering alignment sessions and team building events, each partner must first determine and understand their own criteria for success and then align this to mutually beneficial project objectives.
Management of the Partnering Relationship at All Organizational Levels
This final phase extends through the duration of the project and involves communicating the partnering message throughout each organization’s successive levels (from executive to craft) so it’s
24 November 2022 • Florida Water Resources Journal
CONTRACTORS ROUNDUP
ingrained in the work processes of all team members.
Maintaining a Partnering Relationship
There are several keys to successfully implementing and managing a true partnering relationship throughout the lifecycle of the project.
1. Establish trust between both organizations, from the top level of management down.


2. Offer full support and involvement in the implementation and management at all levels of the project.
3. Recognize and address internal barriers within each organization that may hinder the partnering process. These may include:
a. Personality conflicts
b. Lack of training on partnering process at all levels
c. Reluctance to change
d. Cultural barriers
e. Lack of recognition
4. Identify a champion to direct the process and team members with a partnering mentality. This could be a third-party
facilitator who specializes in developing a partnering relationship, but there must also be a champion at every level.
5. Develop measures, linked to each shared objective, so there are clear roles and responsibilities and means to measure success.

6. Empower individuals to resolve disputes at the lowest level and establish a formal dispute resolution process.
7. Establish win-win objectives and provide recognition or rewards when objectives are achieved.



It’s been proven that, by successfully implementing and managing partnering on our projects, there have been improved results across all project metrics when compared to traditional relationships on design and construction projects. This includes:
S Long-term relationships between firms
S Improved moral and pride of projects
S Cost reduction
S Schedule duration reduction and increased compliance
S Improved safety statistics
S Improved quality statistics
S Claims significantly reduced

We Want to be Your Partner
To all our owners, from a “contractor’s perspective,” we want to be part of the team and contribute to your project’s success at every level. Partnering can work with any project delivery method and any project size, but the process starts with you; initiate this partnering approach and require this commitment in your solicitations. Be an advocate and reap the benefits of improved project performance and development with trusted long-term partner relationships.
References
• Construction Industry Institute, RR10211. The Partnering Process: Its Benefits, Implementation, and Measurement (Paul Thompson, Travis Crane, Steve Sander –Clemson University). September 1996.
• International Partnering Institute, 2020.

Matthew Allen is Miami area sponsor with Kiewit Water Facilities Florida Co. S

Florida Water Resources Journal • November 2022 25
Factory Trained Technicians - Emergency Repair Services - PM Service/Plans Gas Feed Systems Dry Chemical Feed Systems Peristaltic Pumps Fiberglass Enclosures Metering Pump Skids Tablet Feeders Analyzers Scale Systems Serving the Southeast since 1976 800–826-7699 watertc@watertc.com watertc.com
PRELIMINARY SCHEDULE OF
November 27 to November 30,
Hyatt Regency Grand Cypress
Windsong 1-4 Grand Cypress Foyer Windsong 1-4
and Response Resources for Utilities Workshop 2C: Automation & SCADA Workshop 2D: GIS/Asset Management Regions / Council Chairs Lunch Meeting
Opening General Session
Exhibit Hall Meet & Greet
BBQ Challenge & Incoming Chair's Reception Poker Tournament
Registration
Exhibit Hall Open (closed 11:30 AM -1:30PM)
Continental Breakfast Session 1A: Potable Reuse
1B: Management of Pipelines Session 1C: PFAS Treatment Session 1D: Water Treatment – Membrane Applications
FWRC/FWRJ Board Meeting
High School Academy Students Session
STEAM Ahead Youth Program Meeting
Equation Committee Meeting
Backhoe
Ductile
Technical
Session
“Best
FL2051
Water
Meeting
Competition
Lunch
Committee Meeting
Council Meeting
for Organizations
Grand Cypress Foyer Regency Hall 1 Regency Hall 2 Regency Hall 3 Regency Hall 4 Regency Hall 9 Regency 8 Magnolia Regency Hall 5 Regency Hall 6 Regency Hall 7 Regency Hall 8 Regency Hall 1 Regency Hall 2 Regency Hall 3 Regency Hall 4 Poinciana Windsong 1-2 Grand Cypress Portico Patio La Coquina & Alcove
Grand Cypress Foyer Grand Cypress Grand Cypress Regency Hall 1 Regency Hall 2 Regency Hall 3 Regency Hall 4 Poinciana Windsong 6 TBA
Greet
Registration
Continental
Council Meeting
Meeting
Bowl
Taste Contest
Session
Division Meeting
Magnolia Regency Hall 5 Self-Parking Lot Self-Parking Lot Windsong 1-2 Regency Hall 5 Magnolia Self-Parking Lot Regency Hall 1 Regency Hall 2 Regency Hall 3 Regency Hall 4 Grand Cypress Poinciana Grand Cypress Foyer Grand Cypress Foyer Poinciana Grand Cypress Regency Hall 5 Grand Cypress
Grand Cypress Foyer Grand Cypress Grand Cypress Regency Hall 1 Regency Hall 2 Regency Hall 3 Regency Hall 4 Grand Cypress Windsong 1-2 Grand Cypress Regency Hall 5 TopGolf
Sunday, November 27, 2022 Monday, November 28, 2022 Tuesday, November 29, 2022 Wednesday, November 30, 2022
EVENTS Aging Well- Protecting Our Infrastructure 10:00 AM 12:00 PM 1:00 PM 7:00 AM 8:30 AM 8:30 AM 8:30 AM 8:30 AM 8:00 AM 9:00 AM 9:00 AM 10:00 AM 10:00 AM 11:00 AM 11:00 AM 12:00 PM 12:00 PM 12:00 PM 12:00 PM 11:30 AM 2:30 PM 4:00 PM 6:00 PM 9:00 PM 7:00 AM 8:00 AM 8:15 AM 8:30 AM 8:30 AM 8:30 AM 8:30 AM 9:00 AM 9:00 AM 9:00 AM 9:30 AM 10:00 AM 10:00 AM 11:00 AM 11:30 AM 1:00 PM 1:00 PM 1:00 PM 1:30 PM 1:30 PM 1:30 PM 1:30 PM 2:00 PM 2:00 PM 3:00 PM 3:00 PM 4:00 PM 4:00 PM 4:00 PM 4:00 PM 7:00 AM 8:00 AM 8:15 AM 8:30 AM 8:30 AM 8:30 AM 8:30 AM 9:00 AM 12:00 PM 12:00 PM 2:00 PM 5:00 PM ––––––12:00 PM 5:00 PM 5:00 PM 5:00 PM 11:00 AM 11:00 AM 11:00 AM 11:00 AM 5:00 PM 10:00 AM 11:00 AM 11:00 AM 11:00 AM 12:00 PM 12:00 PM 2:30 PM 2:30 PM 2:30 PM 2:30 PM 1:00 PM 4:00 PM 6:00 PM 9:00 PM 12:30 AM 5:00 PM 6:00 PM 9:15 AM 11:30 AM 11:30 AM 11:30 AM 11:30 AM 11:00 AM 1:00 PM 4:00 PM 10:30 AM 11:00 AM 11:00 AM 12:00 PM 1:00 PM 2:00 PM 2:00 PM 2:30 PM 4:30 PM 4:30 PM 4:30 PM 4:30 PM 3:00 PM 4:00 PM 4:00 PM 6:00 PM 5:00 PM 5:00 PM 5:00 PM 6:00 PM 12:00 PM 12:00 PM 9:15 AM 11:30 AM 11:30 AM 11:30 AM 11:30 AM 11:00 AM 2:00 PM 6:00 PM 4:00 PM 9:00 PM Executive Committee Meeting Registration Board of Governors Meeting Registration Workshop 1A: Engineering Laws and Rules Workshop 1B: Navigating the State and National Regulatory Landscape Workshop 1C: Cybersecurity Insights, Resources, and Best Practices Workshop 1D: Growth and New Development Considerations Workshop 1E: Utility Systems Symposium - “The Intelligence of Water” Top Ops & Operators / Maintenance Council Meeting Member Engagement & Development Council Meeting Distribution Division Meeting Public Affairs Council Meeting Finance and Rates Committee Meeting Automation Committee Meeting Workshop 2A: Source Water Protection (Farm Bill 2018) Workshop 2B: Emergency Preparedness
Session
Full
Water
Young Professionals
Rodeo
Iron Tap
Students / Young Professionals
Contaminants
& Educational
Fun Tap Competition
2A: Managing Your Finances Session 2B: Reclaimed Water Solutions Session 2C: Emerging Contaminants Session 2D: Where are the Workers the New Normal
Students/Young Professionals Water
Water Utility
of the Best” Tap Water
Students/Young Professionals Poster
Committee
Meter Challenge
Quality & Resources
Meet &
Exhibits
Breakfast Session 3A: Lead and Copper Rule Compliance Session 3B: Less Conventional Water Treatment Session 3C: Hydraulic Modeling of Piping Systems Session 3D: Water Conservation Symposium Hydrant Hysteria Competition Annual Business Lunch & Awards Ceremony Tear Down / Move Out Water Use Efficiency Division Meeting Golf Tournament
2022
Member REGISTRATION Online Registration Deadline: November 15, 2022 fsawwa.org/2022fallregistration First Name: ___________________________________________________________ Last Name: ____________________________________________ Name to Appear on Badge: ___________________________________________________ AWWA Member No: ________________________________ Organization: ________________________________________________________________ Position | Title: ____________________________ Address: ______________________________________________________________________________________________________________________ City: _________________________________________________________ State: _____________________________ Zip: _____________________ Phone: (______) ________-__________ Fax: (______) ________-__________ Email: _______________________________________________ CEU|PDH Registration: We offer 0.1 CEU and 1 PDH per hour of participation in Technical Sessions, Symposiums, and Workshops. Fall Conference Course # 05100590 I am a Florida Professional Engineer, No: ____________________________________ I am a Florida Licensed Operator, No: _______________________________________ Please select one: Plant Distribution Collection Full Registration (3 days): Includes: Monday Workshops, Opening General Session, BBQ, Technical Sessions, Exhibits, Meet & Greet (Does not include Laws/Rules, Utility Systems Symposium, and Business & Awards Luncheon) Daily Registration: Workshops, Technical Sessions and Exhibits Please select a day: Monday (Includes all Monday events, except Laws/Rules & Utility Systems Symposium) Tuesday Wednesday Special Registration: (Must register) Speaker (One-day only)* Select day: Mon Tue Wed Free with full or one-day registration Retired AWWA member Select day: Mon Tue Wed Spouse (Lunch not included) Mon Tue Wed Operator Competitions Tue Wed Students - Free (Registration required if attending) Exhibit Hall Only Mon Tue Wed Engineering Laws | Rules 2.0 PDH Monday AM Utility Systems Symposium Monday 8-5 (Includes lunch) 0.8 CEU | 8.0 PDH Students/Young Professionals Lunch Tuesday PM FSAWWA Business & Awards Luncheon Wednesday PM Free Events: (You will need to register for an accurate count if you are attending the following events) Opening General Session Monday AM BBQ after Exhibits Meet & Greet Monday PM Water Conservation Symposium (No Credits) Wednesday AM Thank you for your interest in the FSAWWA. Looking forward to seeing you at the Hyatt Regency Grand Cypress on November 27 to November 30, 2022. Aging Well- Protecting Our Infrastructure Register online is strongly recommended at: www.fsawwa.org/2022 fallregistration Questions: Peggy Guingona | peggy@fsawwa.org Note: A 30% service fee will be retained on any cancellation by Nov. 1. No refunds after Nov. 2, 2022. Hotel Accommodations Host hotel is Hyatt Regency Grand Cypress. For more information, visit fsawwa.org/2022hotel Member Non-Member By Nov. 1 After Nov. 1 By Nov. 1 After Nov. 1 $375 $200 $200 $100 $30 $50 $50 $35 $ 0 $50 $70 $120 $30 $50 $0 $0 $0 $425 $250 $250 $150 $30 $50 $50 $35 $ 0 $50 $70 $120 $30 $50 $0 $0 $0 $495 $275 $275 $175 $30 $50 $50 $35 $ 0 $50 $70 $190 $30 $50 $0 $0 $0 $545 $325 $325 $200 $30 $50 $50 $35 $ 0 $50 $70 $190 $30 $50 $0 $0 $0 Non$475 $275 $275 $175 $30 $50 $50 $35 $ 0 $50 $70 $150 $30 $50 $0 $0 $0 $595 $375 $375 $250 $30 $50 $50 $35 $ 0 $50 $70 $220 $30 $50 $0 $0 $0 On-Site Registration Member
















Monday, November 28, 2022 6:00pm | Hyatt Regency Portico Patio (Beer/Wine No Charge | Cash Bar for Liquor) INCOMING CHAIR’S RECEPTION & CHALLENGE Opportunities to Sponsor Bar Sponsor | $1200 Company Logo on banner by the bars Food Sponsor | $750 Company Name on banner by the side items Combined Sponsor | $1500 Company Name on banners at both locations Looking forward to seeing you at the Hyatt Regency Grand Cypress on November 27 to November 30, 2022 Thank you for your interest in the FSAWWA. Come join the festivities which include the BBQ, networking, entertainment and toast to Greg Taylor, our 2023 Section Chair. BBQ is serious business! BBQ teams from utilities and firms from around the state compete in Culinary Abilities with beef brisket, chicken, pork ribs, and pork butt. 1st place trophies for each category, and of course, BRAGGING RIGHTS for Overall Champion! To sponsor, please contact: Courtney Dantone, Kiewit Water Courtney.dantone@kiewit.com (312) 339-1306 or use this link: www.fsawwa.org/2022BBQ The FSAWWA Contractors Council is seeking your support by sponsoring the FSAWWA's Fall Conference Annual BBQ Competition and Incoming Chair's Reception.
tgullett@neptunetg.com
The Roy Likins Scholarship
Fund
Opportunities to Sponsor

Straight | $50
• One of four at a game table sponsors
• Logo on a prominently displayed sponsor board at the registration table
Full House | $150
• One of two at a game table sponsors
• Logo on a prominently displayed sponsor board at the registration table

• 2 Blackjack or 2 Poker Buy-ins
Royal Flush | $250
• Sole game table sponsor
Logo on a prominently displayed sponsor board
4 Poker Buy Ins or 5 Blackjack Buy-ins

Any
the
purchased at the door
play
check, or
first hour
a
Space is limited so pre-purchase
ensure that you have a chance to win. Entry tickets and chips have no cash value. Once they are purchased no refunds will be given. Only paid entries
sponsors will be allowed access to the
Join us at poker after the BBQ and continue the networking. Your participation will benefit the Likins Scholarship Fund, Water Equation, and Water For People. Register Today! fsawwa.org/2022poker It is not necessary to participate in the tournament in order to be a sponsor. Please send Terry Gullet at
a pdf or jpeg version of your company logo for all sponsorships. Pre-Paid Buy-ins: Blackjack Buy in | $20.00 (2000 in chips) Poker Buy In | $40.00 (5000 in chips) At the Door Buy-ins: Blackjack Buy in | $30.00 (2000 in chips) Poker Buy In | $50.00 (5000 in chips) Grand Prize: 50” HDTV! Monday, November 28, 2022 8:30 - 11:30pm Hyatt Regency Grand Cypress Poker Night & Happy Hour Looking forward to seeing you at the Hyatt Regency Grand Cypress on November 27 to November 30, 2022.
contribution of prizes is greatly appreciated for
worthwhile cause. Pre-purchase Buy-In and Table Sponsorships through Conference Registration. Buy-Ins may be
during
of
with
credit card, personal
cash.
to
and
hall.
•
•
Aging Well- Protecting Our Infrastructure
Benefiting
The Roy Likins Scholarship Benefiting Fund


Recognized
1 bay with
Recognition at the
Birdie Sponsor

Recognized
1 bay with up to
Recognition at the

ceremony.
at
ceremony.
ceremony.
Join us at TopGolf. Have fun and network with water industry professionals. Your participation will benefit the Likins Scholarship Fund, Water Equation, and Water For People. Register Today! fsawwa.org/2022golf Location: TopGolf Orlando 9295 Universal Blvd. Orlando, FL 32819 (407)218-7714 | topgolf.com/us/orlando Entry: Entry Fee includes: Entry into the tournament, buffet food, 2 drink tickets per person, and fellowship with conference attendees Individual/Additional Registration | $125 Individuals will be placed into bays with other registrants Individual Utility Operator Registration | $50 Individuals will be placed into bays with other registrants Social Attendee | $50 Come mingle with friends and colleagues (Food and 1 Drink Ticket included) Wednesday, November 30, 2022 Registration starts at 5:00 pm Event: 6:00 - 9:00 pm Par-Tee at TopGolf Opportunities to Sponsor Eagle Sponsor | $850 • Your company’s logo streaming on all TVs of the tournament bays. •
with signage at bay. •
up to 6 entries. •
awards
| $750 •
with signage
bay. •
6 entries. •
awards
Food & Beverage Sponsor | $500 • Recognized with signage. • Recognized on rotating bay displays. • Recognition at the awards
It is not necessary to participate in order to be a sponsor. Please send Chase Freeman a pdf or jpeg version of your company logo for all sponsorships. Email: Cfreeman@spiritgroupinc.com Rain or shine, let’s play golf for a great cause. Aging Well- Protecting Our Infrastructure
Jessica Cormier University of Central Florida


Holly
Paula Campesino University of Central Florida 2022 Woodard & Curran recipient

Apply today for a scholarship through the AWWA Scholarship program! $200,000 in funding available for students pursuing a career in the water community Investing in the Water Workforce Water Equation
2021
A. Cornell Jacobs recipient AECOM Scholarship • American Water Scholarship • Arcadis Diversity Scholarship • Brown & Caldwell Dave Caldwell Scholarship • Carollo Engineers Bryant L. Bench Scholarship • CDM Smith Scholarship • Denver Water Centennial Scholarship • Gannett Fleming Forces of Change Scholarship • Hazen & Sawyer Scholarship • HDR One Water Institute Scholarship • Jacobs Holly A. Cornell Scholarship • AWWA Larson Aquatics ResearchDoctoral • AWWA Larson Aquatics Research-Masters• Mueller Water Products • Neptune Technology Group Scholarship • AWWA Immediate Past President Scholarship • J & L Presidential Scholarship for HBCU students • Raftelis Leadership Scholarships • Roberts Filter Group Charles “Chick” Roberts Scholarship • Dr Philip C. Singer Scholarship • Stantec Scholarship • SUEZ Vernon D. Lucy III Scholarship • Dr. Abel Wolman Fellowship • Woodard & Curran Scholarship
Being Thankful
Sondra W. Lee, P.E. President, FWEA
One of things I became very thankful for after Hurricane Michael was volunteers. At first it was the volunteers who showed up from other parts of Florida to help clear off our streets, followed by groups like the Red Cross that came out to make life a little more bearable during our recovery.
Water Industry Organizations
of FlaWARN and WATER Tracker. These two networks match utility needs to those with resources available to help. To learn more, visit the FlaWARN site at www.flawarn.pwd.aa.ufl. edu, which also provides a link to WATER Tracker. In 2018 the City of Tallahassee first received aid through FlaWARN; then, once the city’s system was up and running, Tallahassee’s crews provided aid to other communities.
There are times when we become thankful for the simple things. Before Hurricane Ian ripped through southwest Florida, I had planned to write about volunteer engagement and thank all of those who take the time and effort to volunteer for FWEA or for other organizations. I’d still like to do that, but perhaps from a different angle.
Volunteers Are Key to Helping Communities


Overcome Difficult Times
The damage from Hurricane Ian reminds me of the devastation from Hurricane Michael in 2018. At the time I was living in a small coastal community south of Tallahassee that had suffered a lot of storm damage, but nothing like Mexico Beach just to the west of us, which certainly was not physically able to withstand the Category 5 hurricane. After storms such as these, we often stop and realize how thankful we are for our lives, family, and friends. Then, you may become thankful for the availability of water and power once they get restored.
Helping Behind the Scenes
In addition to the public volunteers, there was the mutual aid that came to Tallahassee to help us get our collection system up and running. It was a joy to work with the teams from other utilities during a time that was so stressful for me personally. I cannot express how much the various organizations and individuals that came out had really helped make the recovery at work and at home more manageable.
Times like these remind me of the value that our water industry organizations bring, not only to our careers, but to our utilities and communities. I know that all of us understand how much work goes into storm preparation and recovery, but the public is often unaware of out efforts. We really should work on effective public messaging, like the power industry, to let our communities understand that our crews work extra hours before, during, and after the storms, while many are dealing with their own personal storm damage.
Hopefully all utilities are taking advantage
Consider Volunteering With Some Organization at Least Once a Year
I encourage everyone to consider taking on some sort of volunteer work, even if it’s just an hour with a professional organization, like FWEA, or any kind of group outside of work. Not sure where to help out? Churches often have the need for volunteers. You can also take a look at some of your hobbies. The first time I volunteered for a running race, I was amazed at the amount of work that takes place behind the scenes and developed a new level of respect for the volunteers at different events. Then there are sites like Volunteer Match that may be useful. A little word of warning: sometimes volunteering makes you feel so good, you might catch yourself overvolunteering, so be sure to keep it balanced!
I have one last thought of encouragement for this Thanksgiving season. I hope you can take the time to thank those who work and volunteer behind the scenes to keep our worlds functioning seamlessly. S
32 November 2022 • Florida Water Resources Journal
FWEA FOCUS



Complete Visibility in Full Wastewater Tanks SediVision®️ technology delivers unprecedented high-resolution image mapping of full wastewater tanks. Eliminate probing. Know with accuracy, where and how much material is under dark water. 844-765-7866 ©️2022 USST HOLDINGS, LLC ussubmergent.com sedivision.com
Welcome to the FWEA Chapter Corner! The Member Relations Committee of the Florida Water EnvironmentvAssociation hosts this article to celebrate the success of recent association chapter activities and inform members of upcoming events. To have information included for your chapter, send details to Melody Gonzalez at gonzalezm@bv.com. Melody Gonzalez

FWEA Southeast Chapter: We’re Back in Action!
Iam so happy to report that the Southeast Chapter of FWEA has activated inperson and hybrid events for our members. We held our first chapter meeting offering a technical presentation in Deerfield Beach on Oct. 27, 2021. We had Hardeep Anand, director of one water strategy for
Miami-Dade County, address our audience and we also included a biosolids technical presentation. This was our first hybrid event, and our members had the option of participating in person or virtually.

We held a networking event in West Palm Beach on March 10, 2022. Our members had the opportunity to practice their golf skills, while interacting with local professionals, at Drive Shack. This was probably a good warmup in preparation of
our annual golf tournament held later in the summer.
On July 15, 2022, the chapter gathered for a day of fun and fundraising as we held our annual golf tournament. The tournament was held at Eagle Trace Golf Club in the City of Coral Springs, with participation from multiple local utilities


34 November 2022 • Florida Water Resources Journal FWEA CHAPTER CORNER
Isabel Botero
Events Held in 2021 and 2022
Welcoming attendees to the golf tournament. Luncheon presentation after the tournament.
FWEA Southeast Chapter participants at the annual golf tournament.
Chapter Board and Volunteer Recruitment
Our current board is comprised of the - Isabel Botero, Black & Veatch - Alexander Kramer, Thermal



- Daryl Hauquitz-Ellison,
S Utility Liaison - Manuel Moncholi, Stantec




We are always looking for new professional and student members interested in volunteering and aquiring valuable leadership skills. For more information please contact me at boteroi@bv.com.

Florida Water Resources Journal • November 2022 35
Hardeep Anand gives the keynote presentation at the October 2021 meeting. Chapter members and their families participate in the beach cleanup.
After Hurricane Ian: Essential Personnel Will Bring Us Back
Patrick “Murf” Murphy President, FWPCOA
y heart is not in this article. I waited too long to work on what would have been the subject matter for the magazine’s editorial calendar, and now that I’m one day away from the deadline for submittal, we’re in the aftermath of Hurricane Ian crawling across the state of Florida.
The FWPCOA is an organization of volunteers who provide training to the operators in the water industry. Yes, I believe that we provide excellent training, but I’m not sure that any amount of training can prepare our “unsung heroes” for horrific and catastrophic disasters that hurricanes making landfall here deal out. Our thoughts and prayers go out to all the citizens who suffered losses during this event.

As the death toll continues to climb, one can’t help but wonder if some of that group was part of the dedicated (and maybe stubborn) operators who were hunkered down at their jobs while the hurricane hit, with the goal to protect the health of the citizens and preserve the functionality of treatment facilities and infrastructure.
No part of being “essential personnel” means you should be forfeiting your life. First responders don’t go out until the winds die down and the storm has mostly passed.
Cost of Water
As water scarcity is witnessed from California to Texas, there seems to be no way to get across the importance of how much water should cost. In Florida, the citizens see water everywhere, so they have a hard time realizing that we need to be proactive in taking steps not to follow in the footsteps of other states. With 48 percent of wastewater being recycled in Florida (we’re leading the nation in this effort), we are on a better track than most states.
Will Hurricane Ian open our eyes to the fact that water can easily be made
unavailable, that wastewater plants aren’t equipped to handle such flows, and that stormwater infrastructure is unsatisfactory? We had already been experiencing long lead times on equipment and supplies, shortages of chemicals and other essential products, and price increases associated with these shortages. Our chemicals have seen an average price increase of 30 percent this year.
Money must be poured into our facilities and infrastructure. Operators should be paid more than what they’re making. If I hear one more time that there is an operator shortage, my head may blow up! There are shortages in every faction of business, and we are faced with people who think that working from home—or not at all—is okay.
Increasing rates is not something anyone wants to do. Your ratepayers aren’t going to like it; hence, management and governing entities aren’t going to either. How much more money will (or can) the government provide? The American Rescue Plan Act of 2021 provided a substantial infusion of resources to eligible state, local, territorial, and tribal governments to help reverse the negative impacts of the pandemic, address its economic fallout, and lay the foundation for recovery, but it can’t last forever.
Are there statistics on how much of that money went to necessary plant and infrastructure rehabilitation, or for the essential personnel that keep the water and wastewater compliant versus throwing it at new developments and infrastructure for
growth? Will enterprise fund employees be decoupled from general fund employees so that they are paid for providing safe and palatable water and keeping lumpy water off the streets, or will everyone continue to pay the weedwhacker guy the same as an operator trainee or distribution/collection system operator?
Will utilities ever realize that the key to success for having a stream of good operators is to support them, provide training (and budget for it), invest in their activities, and give them the pay and kudos they deserve? It’s mind boggling to me that there are not a lot of employers that want to hire trainees. Why? All that I can assume is that they aren’t willing to train folks (yes, it’s an investment), or that they aren’t willing to staff sufficiently and just get away with the bare minimum that meets plant staffing requirements. I know some of those facilities, and they run the employees hard and put them away wet.
Long Road Back
One would have to think that the devastation caused by Hurricane Ian will put priority on the facilities that were hit the hardest. That will have an impact on projects that have been on the table to be started, new projects that might have been critical for a utility or government facilities.
There used to be a force majeure clause in the governor’s emergency declaration; I don’t remember which governor was in office, or what year it was removed from the declaration, but it was. It scares me to think that facilities might be levied with fines, in addition to the cost of the work that will need to be done to rebuild. It takes years to recover from such a catastrophic event.
I realize that I’ve wobbled more than the eye of Ian, and veered off my original track, so to close: Be safe out there! Think of and record what you need to do to plan for the next event, incorporating lessons learned and any improvements that can be made to the smallest detail. Start planning and budgeting now.
Thank you everyone for your service and for what you have been doing every single day, but especially for the things you will be doing to right the ship after this disastrous event! S
36 November 2022 • Florida Water Resources Journal
C FACTOR

Florida Water Resources Journal • November 2022 37
Critical Resources and Connections When You Need Them
Emilie Moore, P.E., PMP, ENV SP Chair, FSAWWA

assist the impacted water and wastewater system as quickly as possible by whatever means necessary until such time that a permanent solution to the devastation may be implemented.

FlaWARN’s mission is to:
S Prioritize, assign, and track scarce utility resources.
The outpouring of response to help our friends in southwest Florida after the unwelcome arrival of strong Category 4 Hurricane Ian on Sept. 28, 2022, is truly heartwarming and bolsters my belief that no matter what manmade or natural disaster comes our way, we always band together to aid those in need. Our utilities, statewide and beyond, are always at the ready to help those utilities affected by these disasters.
Bringing disaster-impacted water and wastewater utility infrastructure back online is crucial to the recovery of a ravaged community. The Florida Water/Wastewater Agency Response Network (FlaWARN) is an essential support entity helping Florida utilities with their recovery (www.flawarn. org). FlaWARN is a formalized system of “utilities helping utilities” that provides mutual aid during emergency situations.
FlaWARN’s History
FlaWARN was created in April 2005 to facilitate timely response to Florida utilities during emergencies. FlaWARN’s purpose is to get personnel the necessary tools and equipment that can both assess and
S Coordinate with state and federal agencies as additional resources become available.
S Provide training to members.
S Provide assistance and protection to water supplies as a top priority.
S Help minimize wastewater spills that can contaminate water supplies and cause widespread disease.
FlaWARN uses a secure web-based data bank of available resources and a practical mutual aid agreement appropriate for emergency response activities.

The history of FlaWARN dates to Florida’s hurricane season of 2004 when, in August and September of that year, hurricanes Charley, Frances, Jeanne, and Ivan ravaged the state and the formation of a statewide utility network was accelerated.
Prior to FlaWARN being established, Florida utilities had difficulty getting assistance without a formalized agreement for reimbursing the responding utility’s efforts. FlaWARN resolved this challenge by offering member utilities a standardized mutual aid agreement outlining terms and conditions of reimbursement prior to requesting and receiving assistance. FlaWARN was modeled after California’s emergency response program, CalWARN (California Water/ Wastewater Agency Response Network).
A steering committee, which meets as
needed, provides leadership for FlaWARN and is comprised of representatives from the Florida Section American Water Works Association (FSAWWA), Florida Water Environment Association (FWEA), Florida Water Pollution Control Operators Association (FWPCOA), Florida Rural Water Association (FRWA), Southeast Desalting Association (SEDA), three at-large members, and a representative of the Florida Department of Environmental Protection (FDEP). FlaWARN is funded by FDEP and the University of Florida Center for Training, Research, and Education for Environmental Occupations (UF/TREEO) implements the program.
The agencies supported include, but are not limited to, FDEP, county and state departments of emergency management (DEM), U.S. Army Corps of Engineers (USACE), and U.S. Environmental Protection Agency (EPA).
FlaWARN uses the Florida WATER (Water Assistance Tracking and Emergency Response) Tracker website for utilities to report event-related status and to submit needs and request resources (www.flwatertracker. com). The WATER Tracker was developed after Hurricane Michael in 2018 and has even been used by utilities to locate hard-to-find equipment during nonemergency situations. Additionally, a listing of certified laboratories is included in WATER Tracker.

FlaWARN in Action
The benefits that FlaWARN provides to our water and wastewater utilities have been front and center since Hurricane Ian recovery efforts began. Utilities immediately jumped into action to support our colleagues in southwest Florida as the standardized mutual aid agreements outlining terms and conditions of reimbursement were already in place prior to requesting and receiving assistance. Utilities worked with the FlaWARN regional coordinators and used
38 November 2022 • Florida Water Resources Journal FSAWWA SPEAKING OUT
the centralized system to list and track needs and available resources.
As our state keeps growing in population and tropical storms continue to impact the state, FlaWARN will serve as the go-to “utility helping utility” resource in response to emergencies.
Connecting at the FSAWWA 2022 Fall Conference
The FSAWWA 2022 Fall Conference will be held November 27-30, 2022, at the Hyatt Regency Grand Cypress in Orlando. The theme for the conference is “Aging Well – Protecting Our Infrastructure.” I look forward to the Fall Conference as it gathers our partners in utilities, manufacturing, consulting, regulations, academia, and other water-focused professions to collaborate and demonstrate our skills in all things water.
There is something for everyone, with a mixture of technical sessions, operator competitions, workshops, exhibits, and networking events; fundraising for the Roy Likins Scholarship, Water For People, and
Water Equation; and our annual business lunch and awards ceremony.
Technical sessions and workshops will highlight the following topics:
S Cybersecurity
S Regulatory Landscape
S Asset Management and Geographic Information Systems (GIS)
S Growth and Development
S Source Water Protection

S Emergency Preparedness and Response
S Automation and Supervisory Control and Data Acquisition (SCADA)
S Potable Reuse
S Pipelines Management
S Per- and Polyfluoroalkyl Substances (PFAS) Treatment
S Membrane Treatment Applications
S Financial Management
S Reclaimed Water Solutions
S Emerging Contaminants
S Workforce Development
S Lead and Copper Rule Compliance

S Innovation Water Treatment
S Hydraulic Modeling
S Water Conservation
Check out the conference webpage at pheedloop.com/FSAWWA2022/site/home and plan to join us for a great conference later this month.
I look forward to seeing you there and sharing experiences that continue to improve our water industry. S
Florida Water Resources Journal • November 2022 39





40 November 2022 • Florida Water Resources Journal www.fwrc.org THE NEW WEBSITE IS LIVE! Our new website is live and open for registrations! May 31 - June 3, 2023 @ Gaylord Palms in Kissimmee, FL ABSTRACT DEADLINE EXTENDED TO NOV. 15 RESERVE YOUR BOOTH BOOK YOUR 2023 SPONSORSHIP

Florida Water Resources Journal • November 2022 41 ATTENDEE REGISTRATION Attendee registration opens December 1, 2022. Prices valid until April 30, 2023. May 31 - June 3, 2023 @ Gaylord Palms in Kissimmee, FL Ticket Type Includes Exhibit Hall Includes Technical Sessions Includes FWRC &/or FWEA Lunch(s) Price (valid thru 4 30 23) Full Registration YES YES YES MEMBER: $425 NONMEMBER: $500 RETIRED: $100 SPOUSE: $100 Exhibit Hall Only YES NO NO $15 1 Day Registration (Thur OR Fri) YES YES Thursday: FWRC Friday: FWEA MEMBER: $275 NONMEMBER: $325 1-Day Contestant (Thur OR Fri) YES NO NO $105 1-Day Speaker (Thur OR Fri) YES YES NO $90 Booth Staff (4 free/booth) YES NO NO FREE $10 EACH ADD'L TIX. Student Tickets YES NO NO FREE www.fwrc.org
Case Study to Reduce Lead and Copper Corrosion Through Water Quality Optimization and Control of Nitrification
Richie Angley, GJ Schers, Peter Davis, and Rich Giani
Thisarticle presents a methodology to approach and optimize corrosion control in a distribution system following a logical sequence of data gathering, investigative work and additional water quality sampling, bench- or pilot-scale testing, and full-scale implementation and monitoring. This methodology is considered best practice, and the same structured method can be used for other water systems to optimize corrosion control and compliance with the Lead and Copper Rule (LCR) from 1991 and Lead and Copper Rule Revisions in 2021.
The utility’s system for this case study, located in City of Cocoa, is supplied by one water treatment plant (WTP), which includes a groundwater treatment process, a surface water treatment process, and an aquifer storage and recovery (ASR) well system; finished water can be a blend of treated waters from these three sources. Chloramines are utilized as the secondary disinfectant in the system.
The groundwater source is characterized by high hardness and alkalinity, moderate levels of organics and other minerals, and low levels of dissolved metals. Production wells draw water from both the Intermediate and Upper Floridan
aquifers, with some distinct differences in water quality. The groundwater treatment process includes forced draft aeration for stripping hydrogen sulfide; softening in the solids contact clarifiers with the addition of lime, soda ash, and potato starch; and recarbonation with carbon dioxide and media filtration.
The surface water source is characterized by low hardness, alkalinity, and other minerals, and high levels of organics and color. The source water quality varies during the year, with less stormwater flow into the reservoir during the winter. The surface water treatment process includes clarification in lamella clarifiers, with the addition of ferric sulfate, liquid calcium hydroxide, and polymer, followed by ozonation and media filtration.
Water recovered from the ASR wells is very similar in terms of water quality to the finished water; however, native water from the aquifer blends with the finished water and the recovered water quality varies based on the ASR well and the recovery stage.
There are some distinct differences in the treated water chemistry of the three water sources, and therefore, finished water chemistry is dependent upon the blend ratio of these sources. Understanding the differences in water chemistry between source waters and varying blend ratios is important for forecasting finished water quality and pipe scale stability on water

Richie Angley, P.E., is a professional engineer with Jacobs in Orlando. GJ Schers, PMP, is a principal water treatment technologist with Jacobs in Fort Lauderdale. Peter Davis is a project engineer with the City of Cocoa. Rich Giani is a discipline leader for corrosion control with CDM Smith in Atlanta.
mains, as well as understanding the potential for increased corrosion rates and possible drinking water discoloration. In addition, high detention time and nitrification are known to be significant issues in certain portions of the system, especially during the warmer months of the year.

Background
Formerly, the final location to adjust pH in the groundwater treatment process was recarbonation upstream of the media filters. The optimal pH in the system to promote chloramine stability would create precipitation of calcium carbonate in the filters; therefore, the utility installed a new chemical feed system with liquid calcium hydroxide to adjust the pH of the filtered groundwater. In combination with the recarbonation process upstream of the filters it also mitigated the calcium carbonate
42 November 2022 • Florida Water Resources Journal
FWRJ Figure 1. Percentage of total distribution system pipe length for various pipe materials. Figure 2. Median iron, manganese, and calcium concentrations (along with color and turbidity levels) found in each type of pipe material collected from high-velocity samples. Median is used to identify the prevalence of a measured parameter in the specific type of pipe. Note the Y-axis is in logarithmic format. Continued on page 44
Figure
Figure
and
distribution



from high flow distribution system samples.

Florida Water Resources Journal • November 2022 43
3. Correlation between iron, manganese, and calcium versus color
turbidity in the
system.
4. Correlation between iron and manganese concentrations and correlation between iron and calcium concentrations
precipitation in the groundwater filters and increased the alkalinity of the finished water. The increased total alkalinity increased the buffer intensity and thus aided in control of impacts of nitrification in the system.

During the potable water supply construction permitting process for the new liquid calcium hydroxide feed system, the change in finished water quality and impacts on corrosion control treatment and regulated water quality parameters (WQPs) were discussed with the Florida Department of Environmental Protection (FDEP). The utility and FDEP agreed that the utility would conduct additional sampling for a one-year period following implementation of the new chemical feed system. In addition, the utility would conduct an optimal corrosion control treatment (OCCT) analysis using the sampling results to determine new optimal WQPs.
The OCCT under the LCR requires that the utility select and implement the most effective corrosion control practice, while continuing to meet other regulatory and nonregulatory water quality requirements. The goal of OCCT is to reduce the concentrations of metals in the drinking water. Effective corrosion control requires understanding and management
Table
of the common mechanisms that may cause metal release, such as changes to source water conditions, treatment practices, and distribution system operation and maintenance practices.
Historical Water Quality Data Collection and Analysis
Water quality data were collected from historical compliance sampling records and from the utility’s electronic databases that store operational records. Data were collected on source water quality (both surface water and groundwater), treated water quality (both surface water and groundwater), ASR water quality, and distribution system water quality. Parameters of interest included alkalinity, calcium, magnesium, pH, temperature, chlorides, iron, manganese, sulfate, aluminum, sodium, fluoride, nitrate, phosphate, barium, aluminum, oxidation reduction potential (ORP), total dissolved solids (TDS), and conductivity. Collection of historical compliance sampling data included primary and secondary national drinking water standards sampling events, unregulated contaminant monitoring rule (UCMR) sampling events, LCR sampling events, and surface water iron sampling events. Operational data on historical chemical use at the WTP were also collected.
The data collected were used to define water quality for the various water sources (groundwater, surface water, and ASR water). The water quality data were then used in predictive water quality models to predict finished water quality for varying ratios of the different source waters.
Distribution System Assessment
An assessment of the system’s water quality was conducted in August 2020 to identify the nature of the current scales on interior walls of the water mains and to develop a general evaluation of system water quality. The data collected, in combination with historical water quality data, were used to predict how pipe scales may react to changes in water quality resulting from changes in source water or treatment. This information was used as a basis for establishing recommended water quality ranges to minimize destabilization of existing pipe scales.
The utility serves potable water to more than 80,000 customer connections, or an estimated population of approximately 200,000, across a service area of more than 250 sq mi. The system is supplied from the utility’s single WTP, which includes three water sources as described previously. Within the system, the utility operates one elevated storage tank and three booster stations consisting of ground storage tanks (GSTs), high-service pumps, and sodium hypochlorite storage and feed systems.
#9 #19 #26 MCL1
10
100
13002
The distribution system contains more than 1,300 mi of pipeline. Data on the potable water system piping were obtained from the utility’s geographic information system (GIS) database and included pipe material, pipe size, pipe length, and installation date. The system is predominantly comprised of polyvinyl chloride (PVC) and asbestos cement (AC) pipe. Ductile iron (DI) pipe, prestressed concrete cylinder pipe (PCCP), and cast iron (CI) pipe are also present in the system, while other pipe material types

44 November 2022 • Florida Water Resources Journal
Table 1. Accumulated Metals Found at Locations With Highest Iron and Manganese Depositions Location ID
Arsenic, µg/L 4.9 5.8 ND
Barium, µg/L 28.9 29.7 13.6 2000 Chromium, µg/L 3.1 6.6 ND
Copper, µg/L 17.3 4 3.7
Lead, µg/L 5.3 8.1 1.4 152 Titanium, µg/L 9.5 5.9 2.2 NA Notes: 1MCL is measured at the POE and does not apply to distribution samples.2Action level where 90 percent of samples must be below this value at a customer’s tap after stagnating for at least 6 hours.
1. Accumulated Metals Found at Locations With Highest Iron and Manganese Depositions Figure 5. Correlation between oxygen reduction potential and total chlorine in the distribution system. Figure 6. Nitrification
cycle from the addition of chloramine.
Continued from page 42 Continued on page 46

FWPCOA TRAINING CALENDAR Please go to the FWPCOA website www.fwpcoa.or g for the latest updates on classes Course registration forms are available at http://www.fwpcoa.org/forms.asp. SCHEDULE YOUR CLASS TODAY! November November 14-18 Reclaimed Water Field Inspector Winter Garden $350/380 November 14-17 Backflow Tester Course .............................................................................. Deltona ............. $375/405 The course includes classroom instruction and hands-on training that utilizes a backflow prevention assembly wet lab where students will field test a double check assembly, reduced pressure principle assembly, and pressure vacuum breaker. November 17 Backflow Tester Recertification and Exams............................................. Deltona ............. $85/115 December December 5-7 Backflow Repair Course Deltona $275/305 December 7 Backflow Tester Recertification and Exams............................................. Deltona ............. $85/115 December 13-16 Water Distribution 2 Deltona $325/325 March March 13-17 2023 SPRING SHORT SCHOOL You are required to have your own calculator at state short schools and most other courses. For additional information on these courses or other training programs offered by the FWPCOA, please contact the FW&PCOA Training Office at (321) 383-9690 or training@fwpcoa.org. Florida Water Resources Journal • November 2022 45
are limited. Figure 1 presents the percentage of each type of pipe material in the system by total length.
A calibrated distribution system hydraulic model that was previously developed was used to predict water age under different demand scenarios. Water quality in the system can vary with water age. Distribution system assessment sample locations were selected to provide a range of pipe materials, pipe ages, and water ages in the system. A wide variety of sample locations improves the likelihood of capturing the various scales and water quality conditions that may be present within the system. For example, different pipe materials can result in the formation of different scales within the system.
The 27 sample locations were identified, including 10 sample locations for PVC pipe, 10 for AC pipe, three for DI pipe, three for CI pipe, and one sample at the point of entry (POE) at the WTP. Selected sample locations targeted pipe diameters of 6 to 8 in. Large-diameter pipes were not sampled because of the inability to obtain samples by flushing at high flow velocities; therefore, no PCCP pipes were sampled because these pipes in the system are at least 24 in. in diameter.
Sample locations included some closer to the WTP, where the water age is low, and some
at the far ends of the system, including dead end pipes, where the water age is high. Sample locations covered the ends of the system in the north, south, and east. Some sample locations were intended to target older DI and CI pipes, while other locations targeted more-recentlyinstalled PVC pipes.
The system assessment consisted of a series of water samples collected from fire hydrants during low- and high-velocity flushing of selected water mains. The hydrants were first flushed at a flow of 30 to 50 gal per minute (gpm) for a few minutes to remove sediment accumulation in the hydrant service line. After a few minutes of flushing, the low-velocity samples were collected under the 30- to 50-gpm flow condition. The low-velocity samples are generally indicative of water quality in the bulk water and can be used to identify the sensitivity of certain pipe scales.
After the low-velocity samples were collected, the hydrant valve was opened to increase the velocity in the water main; typical high-velocity flows ranged from 300 to 500 gpm. The goal of the high-velocity sampling was to purposely remove loose pipe scales to assess the composition of the scaled material and the potential for water discoloration from physical and chemical water quality changes in the system. It should be noted that these samples contained higher sediment and metal concentrations and
were not indicative of concentrations found in the bulk water flowing through the pipes at the normal, lower-flow velocities.

Samples collected under low flow conditions were analyzed for pH, temperature, ORP, total chlorine, color, turbidity, conductivity, free ammonia, and nitrite. Samples collected under high flow conditions were analyzed for color, manganese, iron, aluminum, turbidity, alkalinity, total calcium hardness, and dissolved calcium hardness. Select samples collected under high flow conditions were also analyzed for chloride and sulfate. Samples containing high levels of iron and manganese were also analyzed for natural source metals that could have accumulated on the pipes over decades, including mercury, antimony, arsenic, barium, beryllium, cadmium, chromium, copper, lead, selenium, and titanium.
Distribution System Assessment Results
The utility’s distribution system contained a light to moderate coating of calcium-based scales in pipes closer to the WTP; pipes located further out in the system had a light to nonexistent coating of calcium scales. Iron was found throughout the system, with moderate concentrations in the middle of the system and significant concentrations in the system’s further reaches. Iron deposits were most prevalent in older mains. Significant concentrations of manganese were found in areas with high concentrations of iron scales.
The CI pipes are the oldest pipes and had pockets of the highest concentrations of iron and manganese, as would be expected for this material. Metal deposition was more prevalent in the AC pipe, most likely because the pipes are old. This was identified by the median concentration of each metal broken down by pipe material, as shown in Figure 2.
Calcium concentrations in the distribution system samples did not correlate well with color and turbidity, and therefore, calcium scales

46 November 2022 • Florida Water Resources Journal
Figure 7. Nitrite concentrations versus total chlorine residuals in the distribution system. Figure 8. A correlation exists between nitrification and pH. As total chlorine residuals decrease and nitrite concentrations increase, the pH is depressed. Continued from page 44
are unlikely to be the cause of discolored water events. Iron and manganese concentrations correlated well with color and turbidity, meaning that discolored water events are likely to be related to iron and/or manganese release and are most likely to occur in the cast iron and asbestos cement pipes because they had the most accumulated deposition. Figure 3 presents individual correlations between iron, manganese, and calcium versus color and turbidity.
Iron in the water can originate from the source water, but contributions can also come from iron-based water mains. Manganese in the water typically originates from the source water. Based on historic source water data, several of the utility’s wells contain iron and manganese concentrations above the secondary maximum contaminant level (MCL) of 0.3 mg/L and 0.05 mg/L, respectively; however, data from the POE in 2018 show iron and manganese concentrations well below the secondary MCL and also below the concentration of distribution accumulation of 0.15 mg/L and 0.020 mg/L, respectively.
Iron-based scales can adsorb and accumulate manganese from source waters or from byproducts of treatment chemicals, such as ferric sulfate. Thus, a correlation is expected to exist if both metals have been present in the distribution system for some time. A linear correlation exists between iron and manganese concentrations in the distribution system pipe scales, as illustrated in Figure 4. The figure also verifies that there is no relationship between iron and calcium in the pipe scales.
The results indicate that manganese has adsorbed onto the iron scales in the system. It’s likely to have occurred over decades, starting with the unlined CI pipe, where manganese adsorbed onto existing iron pipe scales. The relationship also exists in the AC pipe, which is the second oldest pipe in the distribution system; however, original AC pipe lining is cement and contains no iron. Iron deposition on these pipes must have originated from the source water and accumulated over many years. Iron deposits were greatest further out in the distribution system, indicating that lower water velocity may also play a role in accumulation in addition to pipe age.
Iron and manganese can also accumulate other heavy metals, such as arsenic and lead, if they exist in low concentrations in the source water. Like iron and manganese, over the years these metals can accumulate to concentrations above the MCL in iron- and manganese-based pipe scales. Based on the results of a study conducted by the Water Research Foundation (Friedman et al., 2016), iron has an affinity to adsorb arsenic, while manganese may adsorb lead. This would infer that pipe scales containing iron and manganese could contain arsenic and lead, and possibly some other heavy metals.
Figure 9. Monthly average of nitrite and free ammonia in the distribution system.

Table 2. Average Water Quality Entering the Distribution System Between 2015 and 2018
Table 2. Average Water Quality Entering the Distribution System Between 2015 and 2018
Average Historic Treated Water Quality
Average Treated Groundwater Water Quality After Calcium Hydroxide System
Average Treated Surface Water Quality
Predicted Blended 70% Groundwater 30% Surface Water
Predicted Blended 50% Groundwater 50% Surface Water
TDS, mg/L 379 379 100 295 240 Calcium (Total), mg/L 33.0 33.0 43.0 36.0 37.9 Total Alkalinity, mg/L as CaCO3 33 41 21 35 31
pH 9.10 9.00 9.20 9.05 9.00 Water Temperature, C 23 23 23 23 23 Chloride, mg/L 112 120 28 92 74 Sulfate, mg/L 107 107 26 83 80 Magnesium, mg/L 7.0 7.0 5.0 6.4 4.7 Corrosion Indices
DIC, mg/L as C 7.1 9.2 4.5 7.8 7.0 Aggressive Index (AI) 12.5 12.5 12.5 12.5 12.4 Langelier Saturation Index (LSI) 0.61 0.60 0.68 0.63 0.58
Calcium Carbonate Precipitation Potential, mg/L as CaCO3 (CCPP) 5.4 4.4 3.7 4.3 3.4 Buffer Intensity 0.136 0.123 0.101 0.116 0.095 Alk/(Cl + SO42 ) (Larsons Ratio) 0.1 0.1 0.3 0.2 0.2
Copper II, mg/L at Field Temp 0.01 0.01 0.01 0.01 0.01 Lead II, mg/L at Field Temp 0.09 0.106 0.084 0.100 0.1
Chloride to Sulfate Mass Ratio (CSMR) 1.05 1.12 1.08 1.12 0.92
Release of iron and manganese can not only cause discolored water complaints, but can also co-release other metals at concentrations higher than their respective regulatory entry point levels in customer taps that are close to the scale release event.
Three samples collected during the assessment that contained high concentrations
of iron and manganese were sent to a certified laboratory for heavy metal analysis. Table 1 shows the detected metals and their respective concentrations. Two of the samples contained arsenic concentrations at 5 and 6 µg/L, which is half of the MCL (at the POE), and lead concentrations between 5 and 8 µg/L, which is Continued on page 48
Florida Water Resources Journal • November 2022 47
also almost half of the lead action level (from 90 percent of stagnated samples collected at customer taps).
The ORP identifies the level of oxidation capacity applied by a body of water, which can have a major influence on pipe scale formation in the distribution system. The ORP was measured in the system during the field sampling of the distribution assessment. Distribution systems containing chloramine typically have an ORP ranging from 250 to 500 millivolts (mV), where
the lower end of the spectrum is chloraminated groundwater or water with higher water age, and the higher end is preozonated surface water with chloramine and lower water age. During the assessment, water leaving the WTP was a blend of approximately 70 percent treated groundwater and 30 percent surface water, with no water supply from the ASR wells.
In distribution systems that have free chlorine, there is a distinct correlation where higher free chlorine residuals have higher ORP values. This correlation may not be as distinct

in chloraminated systems, but it was visible in the system from samples collected during the system assessment (Figure 5). The ORP levels ranged from 290 to 449 mV, with an average of 398 mV. These values are typical of blended chloraminated groundwater and surface water systems. For those samples where ORP was measured, total chlorine residuals ranged from 0.03 to 2.62 mg/L with an average residual of 1.37 mg/L.
Nitrification can occur in systems that add chloramine as a secondary disinfectant or systems that have natural ammonia in the source water. When chloramine is produced at the WTP, free ammonia can enter into the system. The breakdown of chloramine in the distribution system can also contribute to free ammonia, which is a primary source of food for ammonia-oxidizing bacteria (AOB). The AOB are ubiquitous in distribution systems and will convert ammonia to nitrite, which in turn becomes food for nitrogen-oxidizing bacteria (NOB). To be simplistic, this creates a symbiosis community that will continue to grow and break down chloramine, utilizing the ammonia to spark what’s called the nitrification cycle (Figure 6). Under full nitrification, chloramine disinfectant can decay rapidly, leaving very little disinfectant downstream and causing pH levels to decrease in low buffered water.
Figure 10. Lead and copper results from Lead and Copper Rule monitoring events expressed as a percentile.

Nitrification parameters were also measured during the distribution system assessment, as nitrification can have an impact on pipe corrosion and scale stability, mostly because the process can depress pH. Nitrification was prevalent throughout the system and nitrite concentrations collected in the system that are greater than 0.05 mg/L typically indicate
Continued on page 50
Figure 11. Pourbaix diagram showing that the current lead species is predicted to be lead (II) hydroxide (shown in blue). This compound is rarely formed in water distribution systems and is most likely hydrocerussite, a combination of lead carbonate and lead (II) hydroxide.
Figure 12. Lead (II) hydroxide solubility chart. The dashed line is the estimated solubility of hydrocerussite. In general, as pH increases, the solubility of lead decreases.

Figure 13. Pourbaix diagram showing that the current copper species is predicted to be cupric oxide, shown in blue.

48 November 2022 • Florida Water Resources Journal
Continued from page 47
Why choose an Or-Tec Micro Bar Screen?




than
have data to

✓ It’s the Greatest Value: The Micro Bar Screen is the most valuable screening technology because it removes more screenings than any other screening technology without the cost of wash water ✓ Best Screenings Capture: Better screenings capture
center-flow band screen, a perforated plate drum screen or escalating perforated plate screen. For example, 2 mm spaced bars (without washwater) provide superior screening to 2 mm holes (with washwater) and we
prove it. ✓ It has the Highest Capacity: Due to low headloss, this screen can achieve a higher flow capacity than its competition while also providing superior screening ✓ Uses No Washwater or Brushes: The elimination of wash water saves money, prevents debris scalping and minimizes maintenance and operational costs ✓ Has No Screen Bypass Points: This screen has no moving seal to wear and cause debris to get past the screen Special Features • Screening down to 1 mm, with in tank units available • Requires no wash water or brushes • Unique triangular bar design eliminates clogging • Excellent for handling fats, oils and grease • Very low headloss with the BEST capture of any 2mm screen • Self-aligning and self-tensioning rakes with very short teeth • All stainless steel, 304SS or 316SS • Simple to install, simple to O&M • Over 2,500 screens installed worldwide! The Micro Bar Screen catches a majority of the smaller particles such as large grit or sand, and excess build up in the channel is seldom a problem.” -Alan Drake, Plant Operator, Kingsland, GA This is three days of screening at 1.5 MGD!! The teeth on the rake are very short and extremely strong. They keep the screen clean with no wear areas. The secret sauce of the MBS is the reverse, triangular shaped bars. They don’t clog, have minimal headloss, and allow for shorter teeth that do not break! 101 Devant Street #804 770 742 3321 Fayetteville, GA 30214 info@cornerstoneh2o.com www.cornerstoneh2o.com Installation at Kingsland, GA.
localized nitrification in the pipe. Average nitrite concentrations found in the system during the assessment was 0.238 mg/L. Higher nitrite concentrations correlated well with lower total chlorine residuals (Figure 7).

Higher total chlorine residuals correlated well with higher pH levels. Higher nitrite concentrations did not correlate as well with pH compared to pH and total chlorine (Figure 8), but still correlated, indicating that nitrification and biological activity have an impact on internal pipe scale corrosion and scale stability. During the distribution assessment, the pH leaving the WTP was 8.8 and ranged between 7.65 and 8.64 in the distribution system, with an average of 8.4. As would be expected, the distribution system assessment results indicated that higher water age was associated with higher nitrite concentrations, lower total chlorine residuals, and lower pH values.

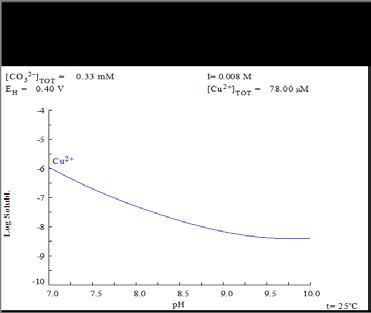
The evidence of nitrification observed in the distribution system assessment was supported by the collected historical data. Figure 9 shows the monthly average nitrite concentrations and the monthly average free ammonia concentrations in the distribution system over the past four years. The monthly data points are derived from the average of 150 samples collected from 75 locations in the system each month. During this time, monthly average nitrite concentrations have increased, while monthly average free ammonia concentrations have decreased due to the increased breakdown of monochloramine from microbiological activity and oxidation of nitrite. Increases in nitrite concentrations and decreases in free ammonia concentrations are typical indicators that nitrification has been increasing in the distribution system. Sample data also showed a clear relationship between nitrite concentrations and total chlorine concentrations, similar to what is shown in Figure 7.
Water Quality Evaluation
Distribution system water quality data were collected to better understand current conditions. The data collected in the distribution system assessment were combined with the collected historical data and used to predict how pipe scales may react to changes in water quality resulting from changes in source water or treatment. For example, increased use of the surface water source, which has lower dissolved inorganic carbon (DIC) compared to the groundwater source, can impact corrosion and the stability of existing pipe scales. Water quality data were input into WaterPro, a water quality modelling software, to calculate water quality indices, such as the Langelier Saturation Index (LSI) and buffer intensity. Geochemical models were used to predict the theoretical speciation of lead, copper, iron, and manganese based on the water quality observed in the distribution system.
Treated groundwater is the utility’s primary source of finished water; however, the utility began increasing the use of the surface water source in recent years. During the distribution system assessment, the treated surface water contributed approximately 30 percent of the total finished water flow, but it can contribute up to 50 percent. In addition, the utility has ASR wells that can provide as much as 30 percent of the flow.
Table 2 shows the average historic treated water quality entering the distribution system between 2015 and 2018; average treated groundwater quality after the installation of the calcium hydroxide system; average treated surface water quality; and a predicted blend of treated 70 percent groundwater, 30 percent surface water, and a 50 percent blend of each. Corrosion indices were calculated using the WaterPro model. These data represent “historic”
data that can help identify the original pipe scales on the water mains in the distribution system before the addition of the calcium hydroxide system to the groundwater treatment process.
With respect to corrosion indices, the water blends are similar; however, the buffer intensity in the treated surface water is lower compared to the treated groundwater. This could cause wider pH swings in the distribution system unless additional alkalinity is added to the blended water, especially if the use of the surface water source is increased.
The water recovered from the ASR wells is similar to the historic treated water, but the chloride concentrations increase in the water recovered from ASR wells when they are operated for consecutive days. Based on historic treated water quality data, the average chloride concentration is 112 mg/L when the ASR wells are operating, which is not significantly different from historic averages when they are not operating. Over run times of several weeks, chloride concentrations could increase in the treated water.
There have been case studies that reported increases in customer red water complaints (iron release) when chloride concentrations increased greater than 50 mg/L (on average) leaving the POE, and this scenario could occur if chloride concentrations increase to approximately 160 mg/L leaving the WTP. In addition, if sulfate concentrations do not increase as chloride concentrations increase, then the chlorine-to-sulfate mass ratio (CSMR) will also increase, causing the potential for higher lead concentrations at customer taps.
With respect to predicting pipe scale and metal deposition speciation, the differences in water quality between the blended water and groundwater (with and without calcium hydroxide) are marginal. The key water quality
Figure 14. Cupric oxide solubility chart. In general, as pH increases, the solubility of copper decreases.
Figure 15. Iron Pourbaix diagram showing ferric oxide as the only species predicted to be found in the distribution system.
Figure 16. Ferric oxide solubility chart. A pH of 7.9 is the optimal pH for ferric oxide solubility.
50 November 2022 • Florida Water Resources Journal
Continued from page 48
parameters from blended water containing 30 percent surface water were used in the geochemical models, which generate Pourbaix diagrams that are useful in identifying the primary metal species that would theoretically form on the pipe wall.

Understanding the current species can help predict how a pipe scale may react with a change in water quality or treatment in the future. In addition, the diagrams can predict if the metal speciation will change, and any changes in metal species in many instances can create periods of pipe scale destabilization. The data from the Pourbaix diagrams can then be used to create solubility charts of the identified metal species based on key water quality parameter changes.
In addition to DIC, other key water quality parameters that can have an impact on pipe scale speciation and solubility are pH, alkalinity, calcium, and ORP (total chlorine residuals).
Historic lead and copper data can help assess the impacts that changes in source water quality and treatment can have on pipe scales in the distribution system mains and customers’ premise plumbing. Lead in the water distribution system can be found within lead solder in the premise plumbing from homes built before the lead ban in the 1980s and within some fixtures. The LCR compliance samples were analyzed from the past three sampling events.
Figure 10 presents lead and copper results from LCR monitoring events expressed as a percentile. With regard to lead, concentrations slightly increased from 2014 to 2017 and remained similar in 2020, while copper concentrations remained similar and extremely low during all three sample events.
Lead corrosion is primarily affected by pH, alkalinity, DIC, and ORP, but can also be impacted by a change in CSMR. The ORP is primarily dependent on the type of disinfectant used and the disinfectant residual; in this instance, the type of disinfectant was chloramine in the presence of groundwater or a groundwater/surface water blend. Total chlorine residual concentrations ranged between 0.03 and 2.62 mg/L in the distribution system during the distribution assessment. The ORP range was 290 to 449 mV, with an average of 389 mV. The pH range was between 7.65 and 8.64, with an average of 8.37.
Pourbaix diagrams and solubility curves were developed using the SPANA geochemical model. Figure 11 shows a Pourbaix diagram for theoretical lead speciation based on the water quality observed in the distribution system. The blue box represents the water quality range expected with groundwater only and blended ground/surface water in all areas of the distribution system. According to Figure 11, the
Figure 17. Manganese Pourbaix diagram showing both hausmannite and manganese (III) oxide as the dominant species in the distribution system. Hausmannite is more likely to form in areas of lower chlorine residuals and is not as stable as manganese (III) oxide.
Figure 18. Manganese solubility chart. As pH increases, manganese solubility will decrease.
Figure 19. Buffer intensity. Points above the minimum alkalinity line indicate an adequate alkalinity and pH combination to maintain a stable pH in the distribution system.
current lead compound is predicted to be lead (II) hydroxide (Pb[OH]2) in the entire distribution system; however, this compound is rare to form in reality and is more likely a combination of lead carbonate and lead hydroxide known as hydrocerussite (PbCO3·2Pb[OH]2). Figure 12 shows the solubility of lead (II) hydroxide and hydrocerussite (pH below 7.5). These models are only theoretical models and cannot develop a solubility curve for hydrocerussite; however, the dashed line on Figure 12 is an estimated solubility curve.
Hydrocerussite is not as stable as lead (II) hydroxide and is more likely to leach higher concentrations of lead into the water. Based on these data, it’s expected that higher lead concentrations at the tap would be found where the pH in the distribution system is lower. This means that nitrification has an impact on corrosion solubility as it’s the main cause for pH depression in the system and minimizing nitrification can reduce pH depression.

According to Figure 12, lead levels could be
reduced by as much as 37 percent (estimated) if the pH were increased from 8.3 to 8.7 on average throughout the entire distribution system.
Increases in CSMR above a value of 0.6 have caused an increase in lead release in some systems. Historically, The CSMR was 1.1 and remains the same for groundwater treated with calcium hydroxide and for the groundwater/surface water blend leaving the WTP. As previously mentioned, the ASR wells increase chloride concentrations over time of operation, but recent data from December 2019 to January 2020 show little change in chloride concentrations leaving the WTP when the ASR wells are blended with groundwater.
The CSMR calculated from the distribution system during the assessment had slightly higher values than historic entry-point ratios, ranging between 1.2 and 1.3. These values indicate that a slight increase in lead release could potentially occur, but most likely would be insignificant with regard to the 90th percentile value (under

Florida Water Resources Journal • November 2022 51
Continued on page 52
the LCR). If the surface water treatment process were to switch coagulants from ferric sulfate to a chloride-based coagulant, such as ferric chloride, the CSMR could increase enough to cause noticeable lead release.
Copper can be found in customers’ premise plumbing inside the home and in some service lines copper corrosion is affected primarily by pH, DIC, ORP, and chlorides. Copper speciation is primarily dependent upon pH and DIC. Figure 13 is a Pourbaix diagram that indicates cupric oxide as the expected copper species in customers’ homes.
Similar to most copper species, cupric oxide solubility will decrease as the pH increases and the DIC decreases. Figure 14 shows the solubility of cupric oxide. Although copper concentrations (collected from LCR sampling events) are well below the action level, according to the WaterPro model, copper II concentrations can decrease by as much as 64 percent if the pH were to remain 8.7 throughout the entire distribution system. The solubility curve generated by the geochemical models (Figure 14) indicates a lower cupric oxide solubility at a pH of 8.7 compared to a pH of 8.4.
Increase in copper solubility has also been associated with increases in chloride
concentrations. With respect to copper corrosion, chloride concentrations in the blended groundwater/surface water are lower than groundwater concentrations and should not have an impact on copper solubility when switching between the two sources, but copper solubility could increase when the ASR wells are operating and if chloride concentrations increase by as much as 50 mg/L when leaving the WTP.
The distribution system contains a small percentage (2 percent) of CI pipe, which typically was not manufactured with a protective lining. There is a larger percentage of DI pipe (7 percent), but this type of pipe typically contains a mortar lining. The majority of pipe is PVC and AC, with AC pipe being older. From the assessment, there are portions of the distribution system that contain moderate to significant iron deposition. The AC pipe contains a higher deposition compared to PVC, mostly because it’s older pipe. This deposition could have come from upstream CI pipe, but most likely has come from the treated source water that has accumulated over several decades. Iron deposition was significant near the ends of the system.
With regard to water quality parameters, iron is primarily affected by pH, ORP, DIC, and chloride. The Larson’s Ratio (LR) is the ratio of concentration of alkalinity in moles to the sum of chloride and sulfate concentrations in moles in a water sample. The LR is used to qualify the water’s aggressiveness to iron pipe scales and the LR values less than 5 are typically aggressive to iron. Based on the WaterPro model, the average LR in the distribution system was 0.1, which is considered extremely aggressive to exposed iron scales, such as cast iron pipes and galvanized pipe, where the zinc coating has worn away. With these values, considerable tuberculation would be expected; however, this effect has most likely been dampened by the calcium layer found in most areas containing CI mains.
Changes to water quality can cause discolored water complaints in areas with significant and moderate accumulation of iron. Figure 15 presents an iron Pourbaix diagram, which indicates that the iron species in the distribution system is ferric oxide. This compound is commonly found in water
distribution systems. The ferric oxide solubility is slightly dependent on pH (Figure 16), with the lowest (most optimal) solubility occurring around pH 7.9. The average pH for all locations that had significant iron deposition was 8.3. If the pH were to stabilize and increase from an average of 8.3 to 8.7, according to the solubility chart (Figure 16), iron solubility would increase by approximately 37 percent. This could potentially be enough to cause an increase in discolored water complaints in areas where iron deposition is significant. It should be noted that areas with significant iron deposition at the ends of the distribution system do not have the calcium layer that is on the CI pipes; therefore, there would be no calcium layer to help dampen iron release.
Similar to copper, an increase in chloride concentrations can impact iron release, but the blended water has a lower concentration of chloride compared to the groundwater and is therefore not expected to have an impact on iron scales and deposits. Like for copper, chloride concentrations should be monitored at the POE when the ASR wells are in operation. Concentrations nearing 160 mg/L could cause noticeable iron release that could cause widespread discolored water complaints.
Manganese was found in low concentrations throughout most of the system, but moderate to significant concentrations were found on CI pipe in the older section of the system and on some of the AC pipe. Although manganese concentrations were lower than iron concentrations, manganese can cause the same type of discolored water event at concentrations two and a half times lower than iron concentrations.
Based on the Pourbaix diagram in Figure 17, manganese can primarily exist as two species: manganese (III) oxide (Mn2O3) and hausmannite (Mn3O4). Manganese (III) oxide is more likely to form in areas with higher chloramine residuals. The average pH and ORP in areas with moderate to significant manganese deposition was 8.2 mV and 363 mV, respectively, indicating that hausmannite dominates these areas. Hausmannite is not as stable as manganese (III) oxide and is much more susceptible to causing discolored water complaints. Reducing nitrification will most likely increase pH and chlorine residuals (ORP), which will then most likely form manganese (III) oxide in the areas of concern. As shown in Figure 18, increasing pH from an average of 8.3 to 8.7 can reduce manganese solubility by as much as 96 percent.

The average calcium carbonate precipitation potential (CCPP) in these areas was -0.1 mg/L as calcium carbonate (CaCO3), indicating that there is little to no calcium coating in these areas; therefore, no “dampening” effect exists in these areas to further reduce manganese release.

52 November 2022 • Florida Water Resources Journal
Table 3. Optimal Water Quality Parameters
Figure 20. A simplified diagram of what pipe scales may look like in older portions of the distribution system that contain both iron and manganese.
Continued from page 51 Continued on page 54
Water & Wastewater Process Treatment & Pumping Equipment


Florida Water Resources Journal • November 2022 53
SINCE Maintenance & Repair Service Available Copyright ©2021 Tencarva Machinery Company. All Rights Reserved. TM.FL.FWRJ.MU.11.16.2021
Calcium deposition was highest in distribution system locations closest to the WTP with moderate scale formation; further out, calcium scales were minimal to nonexistent. A logical explanation would be, as pH is depressed from nitrification, the potential to precipitate calcium decreases. The CCPP is a calculation that can predict localized calcium precipitation potential based on key water quality parameters. The CCPPs were calculated using the WaterPro model. The average CCPP leaving the WTP with groundwater only and blended groundwater/surface water was 4.4 mg/L and 4.3 mg/L as CaCO3, respectively. The median for the distribution CCPPs was 0.5 mg/L as CaCO3, with a maximum CCPP of 6.2 mg/L as CaCO3.
The CCPP values between 1 and 4 mg/L as CaCO3 will form a light microscopic layer over several years, while values between 5 and 10 mg/L as CaCO3 will cause a light noticeable scale to form. The CCPP values greater than 10 mg/L as CaCO3 can cause a thicker calcium layer that can create friction and pipe-clogging issues. These theoretical values were in line with the true values obtained from the distribution system, which had a median of 3 mg/L as CaCO3.
The AC and DI pipes contain a cement lining that is primarily made from calcium; positive CCPP values between 1 and 10 mg/L are beneficial in protecting these linings. The aggressive index (AI) is used to determine the water’s aggressiveness to cement lining. Values greater than or equal to 12 indicate that the water is nonaggressive to the lining. The median AI was 11.4, with a range between 11.1 and 12.5. Values between 11.5 and 12 are considered slightly aggressive to the cement lining; values between 11 and 11.5 are considered moderately aggressive. The more-moderately aggressive
values were found in the further sections of the distribution system, which is primarily PVC pipe and has no lining. Some AC pipe exists in these areas, which would indicate that the calcium layer on the pipe wall is slowly dissolving. Stabilizing the pH to 8.7 throughout most of the distribution system will reduce the slow dissolving of the cement lining layer and further protect the life of the pipe.
Buffer intensity is the natural ability of the water to maintain a stable pH throughout the distribution system, and water’s natural pH stability is influenced by both pH and alkalinity. As the buffer intensity decreases, changes in pH can be more easily influenced by factors such as biological activity and metal scales in the distribution system. Large swings in pH can cause an increase in corrosion rates of all metal scales. Water’s natural buffer intensity is lowest between a pH of 8.2 and 8.4; in this range, a high concentration of alkalinity is required to maintain adequate pH stability.
In general, a buffer intensity greater than 0.1 millimolar per pH unit (mM/pH) will typically result in a stable pH in the distribution system (American Water Works Association [AWWA] Manual M58, Internal Corrosion Control in Water Distribution Systems). Figure 19 illustrates an acceptable pH and alkalinity combination to maintain a buffer intensity value of 0.1 mM/pH. The pH and alkalinity combinations that are above the minimum alkalinity line in the graph are considered adequate to maintain a stable distribution system pH.
The average buffer intensity value leaving the WTP with groundwater only since 2015 was 0.12 mM/pH at a pH of 9 and an alkalinity of 33 mg/L as CaCO3. When blended with 30 percent surface water the predicted buffer intensity remained the same at 0.12 mM/pH. These values are right at the minimal level to maintain pH stability. During the assessment,
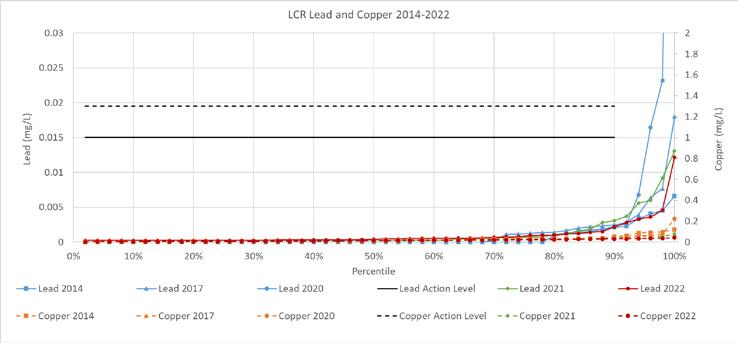
the water leaving the WTP had a pH of 8.8 and an alkalinity of 37 mg/L as CaCO3, which calculates to a buffer intensity of 0.088 mM/ pH. This allowed nitrifying bacteria to depress the pH to as low as 7.6. If the blended water were to increase to 50 percent surface water, the predicted buffer intensity would decrease to 0.10, which could be a concern in areas where nitrification is occurring.
Due to the elevated nitrification activity in the distribution system, it’s recommended to maintain a buffer intensity greater than 0.15. This can be accomplished by adding additional alkalinity in the water leaving the WTP using existing soda ash, carbon dioxide, and calcium hydroxide feed systems. At a pH of 9, obtaining an alkalinity of 50 mg/L will accomplish a buffer intensity of 0.15 mM/pH in both the treated groundwater and blended groundwater containing 30 percent surface water. Under this combination, pH depression should lessen; however, the CCPP will increase from 4 to 7 mg/L as CaCO3, meaning the calcium layer would be slightly thicker, but should not cause friction or clogging issues. If the percent surface water were to increase to 50 percent, then the soda ash, carbon dioxide, and calcium hydroxide doses will also need to increase to achieve a finished water alkalinity of 50 mg/L.
Another option to reduce the impact of nitrification is to conduct an annual temporary switch to free chlorine in the distribution system. Switching to free chlorine (turning off the ammonia) for approximately three weeks should reduce nitrification and pH depression. This would then only require a buffer intensity of 0.12 mM/pH, which would be similar to the water quality currently leaving the WTP. Increasing chloramine residuals at booster stations could also reduce nitrification, provided that chloramine residuals remain consistently above 2 mg/L throughout the entire distribution system.
The utility occasionally receives discolored water complaints. Based on the distribution assessment data, most noticeable discolored water events are the result of the simultaneous release of iron and manganese. In areas where manganese is nonexistent (newer portions of the distribution system) iron release can cause “light” discolored water events. During these releases, some arsenic and lead that accumulated on these metals from adsorption may also release.
Figure 20 shows a simplified diagram of a pipe scale containing both iron and manganese. Iron has the ability to adsorb both manganese and arsenic, while manganese can further adsorb additional lead. In this situation, iron is considered the most sensitive metal, meaning that if iron were to release, it would
54 November 2022 • Florida Water Resources Journal
Figure 21. Lead and copper results from Lead and Copper Rule monitoring events expressed as a percentile.
Continued from page 52
simultaneously release all the other metals that have coprecipitated on the pipe, including arsenic and lead if they exist within the pipe scale. This can happen in both the distribution mains and customers’ premise plumbing.
Optimal Corrosion Control Treatment
The primary chemical treatment strategies commonly used by utilities to achieve corrosion control are pH adjustment, alkalinity (DIC) adjustment, and application of corrosion inhibitors, such as orthophosphates. The goal of these strategies is to form and maintain an insoluble, uniform, nonporous barrier layer between the drinking water and the pipe material to prevent or limit corrosion. Adjustment of pH and/or alkalinity achieves corrosion control by reducing the solubility of most pipe materials and the compounds that create a protective barrier along the pipe wall (for example, lead precipitates like hydrocerussite). In other words, the goal of pH and alkalinity adjustment is to create water quality conditions conducive to a stable pipe scale that will not leach metals into the drinking water.
Optimizing finished water quality to minimize lead and copper release in the drinking water is the primary focus because they are regulated metals, but iron and manganese must also be considered because they most likely have additional lead and arsenic within their scales that have been adsorbed and accumulated over time.
One of the primary concerns was the large decrease in pH by more than 1 pH unit (from 8.8 to 7.7) observed in the far reaches of the utility’s distribution system. A pH variance of this magnitude makes it difficult to control pipe and scale corrosion. The buffer intensity in the distribution system is right at, or at times slightly below, the recommended minimum value of 0.1 mM/pH.
The depression in pH correlated well with other indicators of nitrification, which is the common cause of pH suppression in lowbuffered waters. This becomes a cyclic process because as nitrification causes pH depression, it also causes monochloramine to become less stable and break down easier, releasing more free ammonia as food for the AOB. These AOB produce nitrite, which is food for the NOB. These NOB produce nitrate and hydrogen ion, which cause further acidification and a pH reduction in the drinking water.

Based on the WaterPro model used to simulate water quality conditions in the distribution system, increasing the alkalinity to 50 mg/L as CaCO3 at a pH of 9 will form a calculated buffer intensity of 0.15 mM/pH, which
would provide additional resistance to a pH change from nitrification. If pH values were to stabilize where the minimum pH was 8.3 and the average pH was near 8.7 in the system, it should further decrease lead and copper solubility (release) into the tap water from customers’ homes, while possibly increasing the stability of monochloramine, thus reducing nitrification. This will also further stabilize manganese, making it more difficult to release on its own in the distribution system.
The weakest link is iron scale and deposits. If the pH were to stabilize and increase from an average of 8.3 to 8.7, iron solubility would increase by approximately 37 percent based on the geochemical model. This could potentially be enough to cause an increase in discolored water complaints in areas where iron deposition is significant and a calcium layer is nonexistent, and areas with elevated manganese deposition. As the iron releases, it will also release the manganese that has adsorbed to it, along with other heavy metals that have coprecipitated. To minimize scale release, it’s recommended to physically remove the iron in the locations that have significant iron scales and deposition by either swabbing the pipe walls or using unidirectional flushing (UDF) methods.
Controlling nitrification in the distribution system will improve pH and alkalinity, thus reducing the corrosion of lead, copper, and manganese. Reducing lead and copper corrosion will also improve LCR compliance sampling results. Reducing manganese corrosion will decrease discolored water complaints and additional release of lead into the bulk water; therefore, an additional recommendation to improve corrosion control is to reduce nitrification in areas with high
water age by increasing the monochloramine residual.
The utility plans to modify the booster stations to include ammonium sulfate storage and feed systems, in addition to existing sodium hypochlorite storage and feed systems to boost monochloramine residual further out in the system. Maintaining monochloramine residuals consistently above 2 mg/L throughout the system would help control nitrification, increase pH and alkalinity, and reduce corrosion.
In addition to monochloramine booster stations, the utility was recommended to perform a free chlorine burn where the secondary disinfectant is switched to free chlorine for several consecutive weeks. Annual free chlorine burns are common in potable water distribution systems using monochloramine as the secondary disinfectant. Based on the historic and current levels of nitrification parameters, such as nitrite, pH, and monochloramine residual in the distribution system, the utility should perform at least a one-time free chlorine burn.
The time frame of the free chlorine burn would be approximately four to six weeks and is dependent on the free chlorine concentrations observed in the distribution system. The goal of the burn is to observe stable free chlorine residuals throughout the system.
The recommended OCCT strategy for the utility is summarized:
S Maintain a buffer intensity of 0.15 mM/pH at the POE by targeting a monthly average finished water alkalinity of 45 to 55 mg/L as CaCO3 to minimize the impacts that nitrification has on pH depression.
• If nitrification is controlled through booster chloramination and free chlorine
Florida Water Resources Journal • November 2022 55
Figure 22. Monthly average of nitrite and free ammonia in the distribution system.
Continued on page 56
burns, then the buffer intensity may be lowered to current levels of 0.12 mM/pH by targeting a monthly average finished water alkalinity of 35 to 45 mg/L as CaCO3 (approximately 10 mg/L reduction in alkalinity).
S Maintain a target pH of 8.9 to 9.1 at the POE and stabilize pH levels in the distribution system to further reduce lead, copper, and manganese corrosion in customers’ plumbing and the distribution mains.
S Reduce iron and manganese concentrations in the system by physical means, such as UDF or swabbing in areas with moderate to significant iron and manganese concentrations, to minimize discolored water complaints. Iron solubility is expected to increase with the recommended water
quality changes, which could potentially cause an increase in discolored water complaints. The UDF will reduce the negative impacts of the higher pH and alkalinity values, as discussed previously.
S Reduce nitrification by installing ammonium sulfate storage and feed systems at the distribution system booster stations to increase monochloramine residual levels in the distribution system.
S Conduct at least a one-time free chlorine burn of the distribution system for an extended time (four to six weeks), with possible repeat chlorine burns in the future.
S Monitor the water quality in the system routinely to proactively identify areas of concern. The utility currently monitors key nitrification water quality parameters. The utility should develop process control charts
that automatically analyze the data and provide routine analysis and visualization of the water quality conditions at sample stations to help utility staff manage distribution water quality and optimize flushing.
The utility’s corrosion control strategy prior to this study was to maintain pH and alkalinity within optimal water quality parameter (OWQP) ranges approved by FDEP. The OWQP ranges were approved by FDEP because the utility has been operating within these conditions and it has been shown to be an effective strategy for control of lead and copper concentrations in the drinking water based on historical LCR sampling. The lead and copper compliance sampling conducted by the utility during the first year of operation of the calcium hydroxide system (while this study was being conducted), were well below the 90th percentile MCL, as shown in Figure 10. Based on the distribution assessment and water quality evaluation performed, the utility can continue to utilize pH and alkalinity adjustment (following the recommendations described herein) to adequately control corrosion in its distribution system without the need for a corrosion inhibitor.
There are no anticipated changes in finished water quality that would require a change in the corrosion control strategy. Table 3 presents the utility’s OWQP prior to this study and the new OWQPs recommended in this study, which were subsequently approved by FDEP.
Implementation of Recommendations
Since completing this study in early 2021, the utility was able to implement some, but not all, of the recommendations identified. Following the completion of the study, the utility was able to increase the finished water alkalinity at the POE to the recommended range of 45 to 55 mg/L as CaCO3 using a combination of its existing soda ash, carbon dioxide, and calcium hydroxide feed systems. The utility also raised the finished water pH consistently, from 8.9 to 9.1 at the POE, as recommended.

Figure 21 presents lead and copper results from LCR monitoring events expressed as a percentile. Sampling events from 2021 and 2022 were conducted after implementation of some of the recommendations. The 90th percentile lead and copper values continue to remain well below the MCL. The maximum lead and copper concentrations observed during sampling events have decreased in 2021 and 2022, compared to 2020 and 2017 and prior to implementation of the recommendations.

56 November 2022 • Florida Water Resources Journal
Figure
23. Monthly average alkalinity at the point of entry and the monthly average pH at the point of entry minus the monthly average pH in the distribution system. Figure 24. Monthly average alkalinity at the point of entry and the monthly average pH in the distribution system. Continued from page 55
The utility has conducted annual fourweek free chlorine burns over the last two years, which have successfully achieved stable free chlorine residuals in the distribution system. The free chlorine burns significantly reduced nitrification activity in the distribution system, but nitrification activity slowly returned to the system over time. Figure 22 presents the monthly average nitrite concentrations and the monthly average free ammonia concentrations measured in the distribution system. The monthly average data are derived from the average of 150 samples collected from 75 locations in the system each month.
As shown, the nitrite levels in the distribution system dropped to near zero during the free chlorine burns, but then slowly returned. There was a relatively high concentration of free ammonia following the free chlorine burns, partly because the chlorine feed pumps at one remote pump station were not operational. This is evidence that nitrification activity was diminished and was not consuming the free ammonia in the system; however, over time the nitrification returned and was able to begin consuming the
free ammonia present in the system, and thus, free ammonia concentrations decreased.
Figure 23 presents the monthly average alkalinity at the POE and the monthly average pH at the POE minus the monthly average pH in the distribution system. The alkalinity at the POE and the pH at the POE are measured three times per day and the monthly average values are derived from the average of the approximately 90 samples collected each month. The monthly average pH in the system is derived from the average of 150 samples collected from 75 locations in the system each month.
As shown, the difference between the POE pH and the system pH was reduced when the alkalinity at the POE increased. In other words, the increased buffer intensity improved the ability of the treated water to resist pH depression caused by nitrification activity, and therefore, the decrease in system pH was mitigated. As previously discussed, decreases in pH increase the solubility of metals like lead, copper, and manganese; therefore, the implemented recommendations improve the ability to control corrosion in the system by reducing the impact of nitrification activity on metal release.
The utility has continued to maintain finished water alkalinity at the POE to the recommended range of 45 to 55 mg/L as CaCO3 and the pH in the distribution system has remained stable, even with the presence of nitrification activity. Figure 24 presents the monthly average alkalinity at the POE and the monthly average pH in the distribution system.
The utility plans to implement UDF or swabbing to reduce iron and manganese concentrations in the distribution system in areas with moderate to significant deposition, but has not implemented these strategies to date. The utility has not received any discolored water complaints, indicating that significant iron release has not been prevalent in the system, even with the slightly increased pH.
The utility also plans to implement ammonium sulfate storage and feed systems at the booster stations to better control nitrification in the further reaches of the distribution system. Even with annual free chlorine burns, it’s apparent that the chloramine booster stations are necessary to control nitrification in the system for the long term. S

Florida Student Receives AWWA Woodard & Curran Scholarship
University of Central Florida (UCF) student Paula Campesino is the 2022 recipient of the Woodard & Curran Scholarship through the AWWA scholarship program.
She received her bachelor of science and master of science in environmental engineering from UCF and plans to receive her Ph.D. from the university in May 2023.
As a graduate research assistant for Dr. Steven Duranceau’s water quality engineering research group, Campesino manages six undergraduate students and is one of the “built-in leaders” for students in the masters and Ph.D. programs. She says she’d like to be an advocate and motivator for women and minority engineers in the water/wastewater industry.
Campesino says she has found few Hispanic female role models in her chosen career of engineering and is grateful for the scholarship from Woodard & Curran. “This support allows me to serve as a role model for future graduate students who may not see themselves represented in typical academic science, technology, engineering, and math groups and who may be stressed with similar cultural dilemmas that I have faced.”
After graduation she plans to find a position at a local engineering firm where she can get more experience working on all kinds of projects, even those not necessarily focused on potable water.
The AWWA Water Equation manages the scholarship program, which includes 28 academic scholarships for students pursuing a career in the water community. Scholarship applications are available from Sept. 1 to Dec. 21, 2022, on the Water Equation website at www.awwa.org.

NEWS BEAT
Hurricane Ian, which made landfall in Florida as a Category 4 storm, left scars not only on the land, but also in the water. The storm’s winds and excessive rain washed leaves, organic matter, and contaminants into streams and bays, signaling the beginning of serious environmental effects that could emerge. Researchers say the degraded water quality could damage aquatic ecosystems for weeks, months, or longer and pose a danger to human health in the short term.
Since Ian’s landfall, authorities have received dozens of emails about overflows from wastewater treatment plants along the western coast of Florida, from Palmetto to Fort Myers. Orlando officials have asked residents to limit how often they flush toilets, take showers, wash dishes, and do laundry because of overflowing sewers.
Satellites show an increase in runoff of some of these materials, soils, and overflowing rivers on land into the ocean before Ian hit. Major discoloration in near-shore waters indicates a change in the clarity, or turbidity, of the water.
The resulting brown water contains a substance called tannins, which is dissolved organic matter that floats near the top of the water, making it look like tea or coffee. Some of the water’s turquoise color is probably from
organic matter and sediment churned to the surface by the hurricane.
The emptying of freshwater streams into the ocean is a natural process and isn’t necessarily a harmful occurrence at a small scale. The organic materials can in fact serve as food for microbial populations, which are consumed by other animals higher in the food chain, but hurricanes can put such systems into overdrive. Too much floating organic matter can block sunlight from reaching plants deeper in the ocean, decreasing their ability to make food through photosynthesis and eventually leading to the death of plant life. Microbes breaking down the organic matter also increase activity, consuming great quantities of oxygen that would otherwise be available for others, and such oxygen-deprived waters make it difficult for plants and fish to survive.
Researchers are particularly concerned for the region’s sea grasses, which require a lot of light and help maintain the local ecosystem. They help prevent erosion, maintain water clarity by trapping sediments and particles with their leaves, and provide food for animals and economically important fish. Poor water quality could wipe out parts of the local sea grass population.
The longer it takes for the water to settle out will define the impact it has on near-shore sea
grass habitats. The storm may have also washed in pesticides and herbicides from farms and yards, as well as wastewater products into bodies of water, posing a risk to people’s health if they are exposed.
Such human-induced pollutants and nutrients coming off the land can also spur harmful algal blooms that are dangerous for animals and people. These harmful blooms, also known as red tides, are especially prevalent off the western coast of Florida and can affect fisheries key to the state’s economy.
Researchers are unsure how long water quality issues will last. Flooding has continued across parts of central Florida, raising river levels and causing more destruction, which complicates cleanup efforts. Property loss is already estimated at more than $60 billion in Florida, according to an industry trade group.
The effects Ian had on water quality are some of the worst in the state’s recent history, topping 2004’s Hurricane Charley, the last Category 4 storm to make landfall along the western coastline of Florida. After Charley, in almost the same location as Ian, it took weeks for improvements in affected areas like the Charlotte Harbor, just north of Fort Myers. The nearby Peace River was in poor condition for up to three months. S
58 November 2022 • Florida Water Resources Journal
Rob Little (left), with Woodard & Curran, presents a check to Paula Campesino.
Choose Experts.

Experts
When it comes to purifying your drinking water and removing contaminants like PFAS compounds to non detectable levels, there is no substitute for over 50 years of experience, superior performance, and optimized equipment design Calgon Carbon offers innovative purification systems for drinking water, including activated carbon, engineered equipment packages, and cost effective carbon recycling programs If safe, clean drinking water is the only option for your community, choose the expertise of Calgon Carbon. AWWA Scholarship Program 31 Blue Planet Environmental Systems 63 Calgon .................................................................. 58 CEU Challenge 13 Cornerstone H2O 49 Data Flow Systems .............................................. 57 FJ Nugent and Associates .................................. 17 Florida Aquastore 35 FSAWWA 2022 Fall Conference 26-30 Florida Water Resources Conference........... 40-41 FWPCOA Training Calendar 45 Gerber Pumps 8 Hudson Pump 53 Heyward .................................................................. 2 Hydro International 5 Lakeside Construction Equipment 7 PolyProcessing 39 US Submergent .................................................... 33 Water Treatment and Controls Technology 30 Xylem 64 Display Advertiser Index Editorial Calendar January Wastewater Treatment February Water Supply; Alternative Sources March Energy Efficiency; Environmental Stewardship April ................... Conservation and Reuse May Operations and Utilities Management June Biosolids Management and Bioenergy Production July Stormwater Management; Emerging Technologies; Florida Water Resources Conference Review August ............... Disinfection; Water Quality September Emerging Issues; Water Resources Management October New Facilities, Expansions, and Upgrades November Water Treatment December .......... Distribution and Collection Januar y 2016Januar y SERVING FLORIDA’S WATER AND WASTEWATER INDUSTRY SINCE 1949 Technical articles are usually scheduled several months in advance and are due 60 days before the issue month (for example, January 1 for the March issue). The closing date for display ad and directory card reservations, notices, announcements, upcoming events, and everything else including classified ads, is 30 days before the issue month (for example, September 1 for the October issue). For further information on submittal requirements, guidelines for writers, advertising rates and conditions, and ad dimensions, as well as the most recent notices, announcements, and classified advertisements, go to www.fwrj.com or call 352-241-6006.
POSITIONS AVAILABLE
City of Titusville - Multiple Positions Available

Water Reclamation Superintendent, Plant Operator Trainee, Utility Asset Program Manager, Maintenance Mechanic, Laboratory Analyst Microbiology, Meter Reader, Equipment Operator, Industrial Electrician. Apply at www.titusville.com
The City of Delray Beach is hiring for Utilities Water Treatment Plant positions, including:

WTP Operations Supervisor * Electrician * Senior Utility Mechanic Licensed Operators * Operator Trainees * Utility Service Workers
Please visit our website: https://www.delraybeachfl.gov/home to learn more about what Delray Beach – “The Village by the Sea” has to offer and submit your on-line application today!
Water Treatment Plant Operators
The Water Treatment Plant at Village of Wellington is currently accepting applications for a full-time Water Operator. Apply online. Job postings and application are available on our website: https://wellingtonfl.munisselfservice. com/employees/EmploymentOpportunities/JobDetail. aspx?req=20&sreq=5&form=WTO3&desc=OPERATOR III, WATER TREATMENT PLANT

We are located in Palm Beach County, Florida. The Village of Wellington offers great benefits. For further information, call Human Resources at (561) 753-2585.
Fern Crest Utilities - Multiple Positions - Davie, Fl.
Fern Crest Utilities is seeking to fill positions for Dual Licensed Operators, Operator Trainee, and Utility Service Tech. Licensed operator must hold at least a C-level water or wastewater license with the ability to obtain the other within 12 months. Operator trainee must have a minimum H.S. diploma and will be required to obtain a water or wastewater license within 18 months of hire. Utility Service tech must hold a minimum H.S. diploma with some mechanical experience preferred. Email resume to styler@thiscd.org
Wastewater Plant Operators
Trainee - $16.75 - $21.78
Class C - $17.60 - $22.88
Class B - $18.49 - $24.04
Class A - $19.43 - $25.26
We offer FREE health, dental, STD/LTD, and a $25k life insurance policy to all employees. Secure retirement solutions through City’s owned pension plan.
To apply, visit www.pcbfl.gov
The Coral Springs Improvement District A GREAT place to further your career and enhance your life!
CSID offers…
Salary levels are at the top of the industry Health Insurance that is unmatched when compared to like sized Districts Promotions from within for qualified employees Continuing education courses to develop your skills and further your growth Retirement plans where an employee can earn 18% of their salary by contributing only 6% toward their future
The Coral Springs Improvement District is seeking qualified employees in the following fields: Water Plant Operator
Applicants must have a valid Class C or higher Drinking water license and experience in Reverse Osmosis/Nano Filtration treatment processes preferred however not required. Position requirements include knowledge of methods, tools and materials used in the controlling, servicing, and minor repairs of all related R.O. water treatment facilities machinery and equipment.
Minimum starting salary - $49,088 ($23.60). Salary to commensurate relative to level of license and experience in this field.
Trainees who have passed the state exam and only need actual hours worked to obtain the license may be considered.
Benefits:
Excellent benefits which include health, life, disability, dental, vison and a retirement plan which includes a 6% non-contributory defined benefit and matching 457b plan with a 100% match up to 6%. EOE. All positions require a valid Florida Drivers license, high school diploma or GED equivalent, be COVID-19 vaccinated, a satisfactory background check, and must pass a pre-employment drug screen test. Please send resume to jzilmer@csidfl.org or fax resume to 954-7536328, attention Jan Zilmer, Director of Human Resources.
60 November 2022 • Florida Water Resources Journal C L A S S I F I E D S CLASSIFIED ADVERTISING RATES Classified ads are $20 per line for a 60 character line (including spaces and punctuation), $60 minimum. The price includes publication in both the magazine and our Web site. Short positions wanted ads are run one time for no charge and are subject to editing. ads@fwrj.com
UTILITY DIRECTOR
Position Open Until Filled
South Martin Regional Utility is a public municipal utility administered by the Town of Jupiter Island. SMRU has a service area of approximately 22,000 residents, and approximately 9,000 customers. The individual assigned to this position coordinates activities of the utility departments for the implementation and completion of capital projects, technical work and utility operations. The Assistant Director is responsible for design and permitting; assists the Director with negotiation of developer agreements, service delivery, quality control and other business relating to the utility’s water, wastewater and irrigation quality water systems. This individual is jointly responsible for the utility administration and assists in overall direction of the utility. Work is performed largely independently with considerable independent judgment and initiative under the direction of the Public Services Director and the Town Manager. Applicants must submit a completed job application which can be obtained at www.townofjupiterisland.com. Applications should be emailed to hr@tji.martin.fl.us or mailed to 2 Bridge Road, Jupiter Island, FL 33455.
City of St Petersburg - Senior Water Resources Manager Wastewater
(IRC55592) This is very responsible professional, supervisory, and administrative work in the City’s Water Resources department, di recting the operations of the wastewater systems divisions. Work involves direct responsibility for planning, organizing, maintaining, operating, and coordinating the activities of the Wastewater division in the collection, treatment and disposal of wastewater including re claimed water programs and provisions; and ensuring compliance with all applicable local, state and federal regulations. Work requires assisting with the development and implementation of long-range plans to meet the City’s future wastewater and reclaimed water ser vice needs; providing direction and leadership for the planning, de velopment, administration, and review of the section’s annual capital improvement and operating budgets. Requirements: Valid Bachelor’s Degree; valid Driver License; Extensive knowledge of the theory, principles, modern methods and practices of the operation of waste water utilities; extensive knowledge of federal, state and municipal environmental laws and standards concerning the treatment and dis posal of wastewater, including provisions for reclaimed water. Close Date: Open Until Filled; $103,334-$163,266; See details at www. stpete.org/jobs EEO-AA-Employer-Vet-Disabled-DFWP-Vets’ Pref
Utility Project Inspector
The City of Eustis is seeking a Utility Project Inspector. Please visit eustis.org for full job description, salary, & online app. Background check/drug screen required. EOE, V/P, DFWP
Utility Lead Worker
The City of Eustis is seeking a Utility Lead Worker. Please visit eustis.org for full job description, salary, & online app. Background check/drug screen required. EOE, V/P, DFWP
Utilities Program Coordinator
The City of Largo (a 2022 Top Workplace winner) is in search of a Utilities Program Coordinator: a hands-on asset management and reliability centered maintenance position that help’s the city achieve operational efficiency.

Apply here! Utilities Program Coordinator (myworkdayjobs.com)
Engineering Support Services Manager/Engineering: $90,529.70 - $144,847.51/annually
Laboratory Technician: $49,094.65 - $74,624.14/annually
Meter Repair Technician I: $40,294.95 - $61,247.81/annually
Project Manager: $78,825.80 - $126,121.29/annually
Public Utilities Asset Manager: $84,607.19 - $135,371.51/annually
Public Utilities Manager (Wastewater Treatment Plant): $84,607.19 - $135,371.51/annually
Senior Utility Field Technician: $46,729.04 - $71,028.55/annually Treatment Plant Mechanic I: $45,588.86 - $69,294.98/annually Utilities Instrumentation and Control Systems Specialist: $58,359.30 - $88,706.40/annually
For More Info and
go to: http://agency.governmentjobs.com/hollywoodfl/default.cfm EOE M/F/D/V

Florida Water Resources Journal • November 2022 61 SOUTH MARTIN REGIONAL UTILITY – ASSISTANT
to Apply
Certified Water Technicians (Florida and all other markets) City of Tampa hiring experienced Water technicians · Up to $35 per hour depending on experience and industry licenses · Bring your years of valve operations and water line repair and maintenance experience to our team · Major medical/dental/comprehensive health coverage · Pension plan · Paid holidays · Employer paid uniforms, safety apparel, continuous training · Overtime opportunities For more information inquire now at: www.tampagov.net
Test Yourself Answer Key
Continued
1. B) maximum-day operating capacity.
Per FAC 62-699.310(2)(e), Classification and Staffing of Domestic Wastewater or Water Treatment Plants and Water Distribution Systems, “…determine the classification and staffing requirements for each water treatment plant using the following two-step procedure: first determine the category of the plant and then, within that category, determine the classification and staffing requirements for the plant. Determine the plant category by identifying the highest category in tables 1 through 5, listing one or more of the plant’s category-determining treatment processes… Determine the plant classification and staffing requirements within the determined plant category by using the permitted maximum-day operating capacity of the plant.”
2. B) automatic control system.
Per FAC 62-699.200(2), Definitions, “‘AUTOMATIC CONTROL SYSTEM’ means an onsite computerized system with sensors and programs that can adjust and control domestic wastewater or water treatment plant equipment and processes over the normal range of expected operating conditions without operator assistance.”
3. D) 24
Per FAC 62-699.310(2)(a)2:
2. Domestic Wastewater Treatment Plant Category II
2. Domestic Wastewater Treatment Plant Category II
Treatment Process Class A Class B Class C Class D
Activated sludge processes, except extended aeration, with or without filtration.
1 mgd up to 5 mgd
5 mgd and above
Staffing by Class C or higher operator: 24 hours/day for seven days/week. The lead/chief operator must be Class A.
Staffing by Class C or higher operator: 16 hours/day for seven days/week. The lead/chief operator must be Class B or higher.
0.25 mgd up to 1 mgd
Staffing by Class C or higher operator: 6 hours/day for fuve days/week and one visit on each weekend day.
0.1 mgd up to 0.25 mgd
Staffing by Class C or higher operator: 3 hours/day for five days/week and one visit on each weekend day.
Less than 0.1 mgd
Staffing by Class C or higher operator: 1/2 hour/day for five days/week and one visit each weekend.
For all of the above plants, the lead/chief operator must be Class C or higher.
Not Applicable
step procedure. . . Determine the distribution system category by using the highest classification of water treatment plant to which the distribution system is connected. Determine the distribution system classification and staffing requirements within the determined distribution system category by using the number of persons served directly by the distribution system.”
6. D) water quality or quantity.
Per FAC 62-699.310(1), “Suppliers of water shall employ only persons appropriately licensed under Chapter 62-602, F.A.C., to be in onsite charge of any water distribution system operation or maintenance activity that may affect water quality or quantity and that is listed in Footnote 1 under the tables in subparagraphs 62-699.310(2)(f)1. and 2., F.A.C.”
7. D) visit.
Per FAC 62-699.200(13) Definitions, “‘Visit’ means an onsite examination of a domestic wastewater or water treatment plant to ensure that equipment is functioning properly, to ensure that chemical supplies are sufficient, and to record the quantity or quality of wastewater or drinking water being treated and other relevant information.”
8. B) if acceptable quality reclaimed water is diverted to the reuse system only when operators are present
Per FAC 62-610.462(3)(a), Reliability and Operator Staffing, “The minimum staffing requirement at the wastewater treatment facility shall be reduced to staffing by a Class C or higher operator 6 hours per day, seven days per week, unless Chapter 62-699, F.A.C., requires additional operator presence or a higher level of operator. The lead/chief operator shall be at minimum Class C, or higher if required by Chapter 62-699, F.A.C. This minimum staffing requirement shall be allowed only in conjunction with at least one of the following: (a) Diversion of acceptable quality reclaimed water to the reuse system only during periods of operator presence.”
9. B) class of biosolids based on pathogen reduction.
FAC 62-640.880(2)(j), Staffing: “(j) Staffing. The level of operator staffing at a biosolids treatment facility shall be as follows:
4. B) year.
Per FAC 62-699.311(5), Additional Classification and Staffing
Requirements, “Upon written request by the permittee or supplier of water, the department shall approve in writing. . . for proposed new domestic wastewater or water treatment plants that are under an electronic surveillance system, automatic control system, or electronic control system, and for existing domestic wastewater or water treatment plants that are under an electronic surveillance system, automatic control system, or electronic control system and that have been in compliance with applicable water quality standards and applicable operation and maintenance requirements for the past year.”
5. A) number of persons served directly by the distribution system.
Per FAC 62-699.310(2)(f), “. determine the classification and staffing requirements for each water distribution system using the following two-
D) during all periods of treatment plant
distribution system
FAC 62-699.311(1), “An operator meeting the lead/chief operator class
plant shall be available during all periods of domestic
water treatment plant operation, and an operator meeting
operator level
class for the water distribution system shall
during all periods of distribution system operation. ‘Available’
to be contacted as needed to initiate the appropriate action in
manner.”
62 November 2022 • Florida Water Resources Journal
from page 23
Per
Class Of Biosolids** Staffing: Type I* Staffing: Type II* Staffing: Type III* A/AA Class A Operator 8 hours/day five days/week Class B OperatoR 4 hours/day five days/week Class B Operator 2 hours/day five days/week B Class A Operator 2 hours/day fuve days/week Class B Operator 1 hour/day five days/week Class C Operator 1 hour/day tthree days/week B*** Class A Operator 1 hour/day five days/week Class B Operator 1 hour/day three days/week 1 hour/week *Classification of type of facility as determined by paragraph 62 640.880(2)(a), F.A.C. **Class of pathogen reduction achieved by the biosolids treatment facility in accordance with subsection 62 640.600(1), F.A.C. ***This category is for Class B liquid alkaline stabilization only." 10.
or
operation. Per
for the treatment
wastewater or
the lead/chief
or
be available
means able
a timely





























































































































































