Robotic-Assisted Bronchoscopy: Game Changer in Earlier Lung Cancer Diagnosis
Robotic-Assisted Bronchoscopy: Game Changer in Earlier Lung Cancer Diagnosis
A new minimally invasive technique allows clinicians to biopsy difficult-to-reach nodules in the peripheral lung, eliminating the need for multiple biopsies prior to lung cancer detection .

AI-Aided Handheld ULS Detects Musculoskeletal Tendons
A n artificial intelligence (AI) application for musculoskeletal (MSK) imaging that works with handheld point-of-care ultrasound devices automatically identifies, highlights, and measures tendon structures in the foot, ankle, and knee, thus accelerating ultrasound mastery Cont’d on page 6

POC Testing Enables Expansion of Near-Patient Care Capabilities



Point of care (POC) diagnostics is medical diagnostic testing that is performed at or near the POC, and at the place and time of patient care. POC diagnostics includes blood glucose testing, rapid coagulation testing, drugs of abuse screen-
ing, blood gas and electrolyte analysis, quick cardiac marker diagnostic, pregnancy testing, infectious disease testing, cholesterol screening, hemoglobin diagnostic, etc. POC diagnostic testing differs from traditional diagnostics that is restricted to

First 4K Single-Use Surgical Arthroscope
Conventional surgical visualization systems are complex, expensive, and difficult to maintain. Dirty or damaged endoscopes negatively affect visualization, disrupt workflow, and present unnecessary safety risks to the operating suite. Hidden costs in the form of service contracts, repairs, and sterilization services negatively impact the bottom line. Now, a first-of-its-kind 4K single-use
Cont’d on page 21 Cont’d on page 18 Cont’d on page 16 INSIDE GLOBETECH MEDIA >>> <<< International Calendar 22 Industry News . . . . . . . . . 21 News Update 3 HospiMedica EXPO . . . . 6-8 News Update . . . . . . . . . 17 HospiMedica EXPO 16-20 News Update 9 HospiMedica EXPO . . 10-14
If your subscription is not renewed every 12 months your Free Subscription may be automatically discontinued Renew / Start your Free Subscription Access Interactive Digital Magazine Instant Online Product Information: Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 VISIT READER SERVICE PORTAL LINKXPRESS COM ® See article on Page 19
INTERNATIONAL ® Vol.41 No.1 • 2-3/2023 ISSN 0898-7270

102 HMI-03-23 LINKXPRESS COM
Minuscule Transducer Generates Ultrasound ‘Storm’ to Break Down Clots in the Brain




formed in an in vitro model of CVST as compared to the existing techniques.

The new tool developed by researchers at North Carolina State University (Raleigh, NC, USA; www.ncsu.edu) consists of a single transducer that has been specifically designed to create the swirling, vortex effect. The transducer is so small that it can be incorporated into a catheter, which is then fed through the circulatory system to the site of the blood clot. The researchers conducted proof-of-concept in vitro testing by using cow blood in a 3D-printed model of the cerebral venous sinus and found that the new tool could dissolve an acute blood clot in less than 30 minutes. Additionally, the researchers performed experiments by applying vortex ultrasound to animal blood vein samples and found there was no damage to the walls of the blood vessels. They also conducted tests to determine whether the vortex ultrasound caused significant damage to red blood cells and found no substantial damage.
“Our previous work looked at various techniques that use ultrasound to eliminate blood clots using what are essentially forward-facing waves,” said Xiaoning Jiang, co-corresponding author of the study. “Our new work uses vortex ultrasound, where the ultrasound waves have a helical wavefront. In other words, the ultrasound is swirling as it moves forward. Based on our in vitro testing, this approach eliminates blood clots more quickly than existing techniques, largely because of the shear stress induced by the vortex wave.”
“The fact that our new technique works quickly is important, because CVST clots increase pressure on blood vessels in the brain. This increases the risk of a hemorrhage in the brain, which can be catastrophic for patients,” added Chengzhi Shi, co-corresponding author of the study. “Existing techniques rely in large part on interventions that dissolve the
AI-Aided MRI Helps Measure Brain Atrophy

Alzheimer’s is the most common form of dementia and accounts for 60% to 80% of cases. One way to measure its progress is via magnetic resonance imaging (MRI) images that show cortical thinning. However, assessing the onset and progression of Alzheimer’s using brain MRI poses a challenge as changes in the thickness of the brain's cortex are extremely small, usually in the sub-millimeter range. Advanced machine learning techniques are generally used for brain research to examine changes in cortical thickness, although the absence of a clinically accurate ‘ground truth’ dataset meant that their sensitivity to the detection of small atrophy levels could not be evaluated. Until now, the only way to obtain a ground truth measure of cortical thickness was by studying the brain post-mortem. However, this again poses a challenge as the brain begins to shrink immediately after death, resulting in inaccurate readings.
Now, scientists from CSIRO (Canberra, Australia; www.csiro.au), in partnership with Queensland University of Technology (Brisbane, Australia; www.qut.edu.au), have used artificial intelligence (AI) to develop a world-first benchmark for measuring brain atrophy – or thinning - in neurodegenerative diseases, including Alzheimer’s disease. Cortical atrophy – thinning of the brain’s cortex – can begin up to 10 years before the appearance of clinical symptoms of Alzheimer’s disease. The new technique allows researchers to set the amount and location of brain degeneration they wish to compare against in order to achieve a clear picture of the best method for cortical thickness quantification. The technique can test the sensitivity of methods to a miniscule level and determine if a method can detect changes in thickness of just 0.01 millimeters.
The scientists believe they have strong evidence that DL+DiReCT – a deep learning-based method for measuring cortical thickness – is robust and sensitive to subtle changes in atrophy. The technique can be applied to research in any brain disease involving neurodegeneration and marks a significant step forward in better understanding dementia and other debili tating brain diseases. The technique could also be used to predict the level

Cont’d on page 4
blood

3 HospiMedica International February-March/2023 HospiMedica International To view this issue in interactive digital magazine format visit www.HospiMedica.com
Cont’d from cover
clot. But this is a time-consuming process. Our approach has the potential to address these clots more quickly, reducing risk for patients.”
Image: The new tool uses “vortex ultrasound” to break down blood clots (Photo courtesy of CDC, Stephanie Rossow)
DAP vc HospiMedica_clr_Eng 3.75 x 7.5 due 2/10/2023 Radcal Touches the World! Features: • Simple to use – Accurate and reliable
Customizable Touch Screen
Wi-Fi and USB Computer Connectivity
Report Generation Visit Us at ECR Booth #D08, 3/1-3/5 Need to check the performance of X-ray machines? Then the Radcal Touch meter is your tool of choice. For further details: contact us at +1 (626) 357-7921 sales@radcal.com • www.radcal.com vc HospiMedica_clr_3.75x7.5_Eng23Feb10_12366.pdf 1 2/10/23 4:33 PM 103 HMI-03-23 LINKXPRESS COM
•
•
•
COVID-19 Update
AI-Aided MRI Helps Measure Brain Atrophy
of cortical degeneration expected in a person over time. The technology was developed on the back of the commonly used and relatively inexpensive MRI images. The researchers have made the synthetic dataset images publicly available for clinicians and scientists who can use the synthetic images to perform their own assessments of cortical thickness quantification methods.
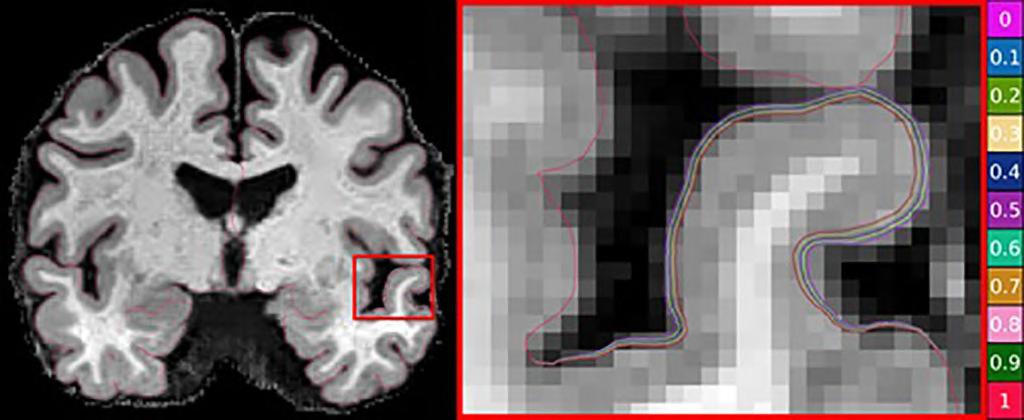
“Using the power of machine learning, we
were able to produce a set of artificial MRI images of brains with predefined signs of neurodegeneration in the cortex region, the outer layer of the brain most affected by Alzheimer’s,” said Filip Rusak, research scientist from CSIRO’s Australian e-Health Research Centre. “Before these findings, there was no way to conclusively determine the sensitivity of the various methods used to measure cortical thickness in Alzheimer’s patients.”
Real-Time 3D Imaging Provides View of X-Rays Hitting Inside Body During Radiation Therapy

Radiation is used in treatment for hundreds of thousands of cancer patients each year, bombarding an area of the body with high energy waves and particles, usually X-rays. The radiation can kill cancer cells outright or damage them so that they can’t spread. These benefits are undermined by a lack of precision, as radiation treatment often kills and damages healthy cells in the areas surrounding a tumor. It can also raise the risk of developing new cancers. Now, radiation, used to treat half of all cancer patients, can be measured during treatment for the first time with precise 3D imaging. By capturing and amplifying tiny sound waves created when X-rays heat tissues in the body, medical professionals can map the radiation dose within the body, giving them new data to guide treatments in real time. It’s a first-of-its-kind view of an interaction doctors have previously been unable to “see.”
With real-time 3D imaging developed at the University of Michigan (Ann Arbor, MI, USA; www.umich.edu), doctors can more accurately direct the radiation toward cancerous cells and limit the exposure of adjacent tissues. To do that, they simply need to “listen.” When Xrays are absorbed by tissues in the body, they are turned into thermal energy. That heating causes the tissue to expand rapidly, and that expansion creates a sound wave. The acoustic wave is weak and usually undetectable by typical ultrasound technology. U-M’s new ionizing radiation acoustic imaging system detects the wave with an array of ultrasonic transducers positioned on the patient’s side. The signal is amplified and then transferred into an ultrasound device for image reconstruction. With the images in-hand, an oncology clinic can alter
the level or trajectory of radiation during the process to ensure safer and more effective treatments. Another benefit of the technology is it can be easily added to current radiation therapy equipment without drastically changing the processes that clinicians are used to.
“In the future, we could use the imaging information to compensate for uncertainties that arise from positioning, organ motion and anatomical variation during radiation therapy,” said Wei Zhang, a research investigator in biomedical engineering and the study’s first author. “That would allow us to deliver the dose to the cancer tumor with pinpoint accuracy.”
“In future applications, this technology can be used to personalize and adapt each radiation treatment to assure normal tissues are kept to a safe dose and that the tumor receives the dose intended,” said Kyle Cuneo, associate professor of radiation oncology at Michigan Medicine. “This technology would be especially beneficial in situations where the target is adjacent to radiation sensitive organs such as the small bowel or stomach.”
INTERNATIONAL
A GLOBETECH PUBLICATION
Publishers of: HospiMedica International • LabMedica International LabMedica en Español • HospiMedicaExpo.com • LabMedicaExpo.com HospiMedica.com • HospiMedica.es • MedImaging.net LabMedica.com • LabMedica.es
HOW TO CONTACT US
Subscriptions:
Send Press Releases to:
Advertising & Ad Material:
Other Contacts:
www LinkXpress com HMNews@globetech net ads@globetech .net info@globetech net
ADVERTISING SALES OFFICES
USA, UK Miami, FL 33280, USA
Carolyn.Moody@globetech.net
Joffre.Lores@globetech.net
Tel: (1) 954-686-0838
Tel: (1) 954-686-0838
GERMANY, SWITZ , AUSTRIA Bad Neustadt, Germany
Simone.Ciolek@globetech.net Tel: (49) 9771-1779-007
BENELUX, FRANCE Hasselt, Belgium
Nadia.Liefsoens@globetech.net Tel: (32) 11-22-4397
JAPAN Tokyo, Japan
Katsuhiro.Ishii@globetech.net Tel: (81) 3-5691-3335
CHINA Shenzhen, Guangdong, China
Parker.Xu@globetech.net Tel: (86) 755-8375-3877
ALL OTHER
SUBSCRIPTION INFORMATION
HospiMedica is published 4 times a year and is circuIated worldwide (outside the USA and Canada), without charge and by written request, to medical department chiefs and senior medical specialists related to critical care, surgical techniques and other hospital-based specialties; hospital directors/administrators; and major distributors/dealers or others allied to the field.
To all others: Paid Subscription is available for a twoyear subscription charge of US$ 100. Single copy price is US$ 20. Mail your paid subscription order accompanied with payment to Globetech Media, LLC, P.O.B. 800222, Miami, FL 33280-0222, USA.
For change of address or questions on your subscription, write to: HospiMedica lnternational, Circulation Services at above address or visit: www LinkXpress com
ISSN 0898-7270
Vol 41 No 1 • Published, under license, by Globetech Media LLC Copyright © 2023. All rights reserved. Reproduction in any form is forbidden without express permission.
Teknopress Yayıncılık ve Ticaret Ltd. Şti adına
İmtiyaz Sahibi: M. Geren • Yazı işleri Müdürü: Ersin Köklü Müşir Derviş İbrahim Sok. 5/4, Esentepe, 34394 Şişli, İstanbul P. K. 1, AVPIM, 34001 İstanbul • E-mail: Teknopress@yahoo.com Baskı: Postkom A.Ş. • İpkas Sanayi Sitesi 3. Etap C Blok • 34490 Başakşehir • İstanbul Yerel süreli yayındır. Yılda dört kere yayınlanır, ücretsiz dagıtılır.
ads@globetech.net Tel: (1) 954-686-0838
COUNTRIES Contact USA Office
www . hospimedica
. com
RN Simone Ciolek Parker Xu Karina Tornatore Publisher News Editor New Products Editor Regional Director Regional Director Regional Director Reader Service Manager 4 HospiMedica International February-March/2023 Marc Gueron Founder & Editorial Director HospiMedica International To view this issue in interactive digital magazine format visit www.HospiMedica.com
Dan Gueron Sanjit Dutt David Gueron Carolyn Moody,
Cont’d from page 3
Image: Tracking radiation treatment in real time promises safer, more effective cancer therapy (Photo courtesy of Pexels)
AI Improves Lung Nodule Detection on Chest X-Rays

Lung nodules are common abnormal growths that typically form on the lungs due to previous lung infections but can rarely be a sign of lung cancer. Chest X-ray is a common screening method used to identifying lung nodules. Artificial intelligence (AI) can serve as a powerful tool to help identify lung nodules, particularly when radiologists have a high volume of cases. Now, a pioneering, randomized controlled study evaluating the effect of AI-based software in real clinical practice has found that AI significantly improved the detection of lung nodules on chest X-rays.
In order to identify the actual effect that AI has in clinical practice, researchers at Seoul National University Hospital (Seoul, Korea; www.snuh. org) conducted a study involving 10,476 patients with an average age of 59 years, who had undergone chest X-rays at a health screening center between June 2020 and December 2021. Patients were also asked to complete a self-reported health questionnaire for identifying baseline characteristics such as age, sex, smoking status and previous history of lung cancer. Within the group of patients, 11% were current or former smokers. The researchers randomly divided the patients evenly into two groups - AI or non-AI. Radiologists aided by AI analyzed the X-rays of the first group while the X-rays of the second group were interpreted without using AI.
Solid nodules with diameters either larger than 8 millimeters or subsolid nodules with a solid portion larger than six millimeters were identified as actionable, meaning that the nodule required follow-up based on lung cancer screening criteria. The researchers identified lung nodules in 2% of the patients. Their analysis showed that the detection rate for actionable lung nodules on chest X-rays was higher when aided by AI (0.59%) as compared to without AI assistance (0.25%). They found no differences in the false-referral rates between the AI and non-AI interpreted groups.
Older age and a history of lung cancer or tuberculosis were associated with positive reports, although these and other health characteristics did not impact the efficacy of the AI system. This indicates that AI can perform consistently across different populations, including those with diseased or postoperative lungs. The researchers now plan to conduct a similar study using chest CT which will also identify clinical outcomes and efficiency of workflow.
Ultrafast MRI Predicts DCIS Upgrade to Invasive Cancer at Breast Surgery
Biopsy-proven ductal carcinoma in situ (DCIS) lesions are often upgraded to invasive cancer at surgery. As a result, accurate prediction of the likelihood of invasion can be helpful for surgical planning, including the need to perform sentinel lymph node biopsy (SLNB). Now, a new study has found that ultrafast (UF) MRI provides beneficial information that can be used in surgical planning, including determining the need for SLNB.
In the study, researchers at NYU Langone Health (New York, NY, USA; www.nyulangone.org) identified consecutive women with biopsy-proven pure DCIS lesions who underwent UF-MRI with DCE-MRI and had subsequent surgery between August 2019 and January 2021. To determine predictors of upgrade to invasive cancer, the researchers assessed patient and lesion characteristics; biopsy method and pathology; as well as lesion features on mammography, ultrasound, DCE-MRI, and UF-MRI.

Ultimately, at surgery, 38% of lesions diagnosed as DCIS at percutaneous biopsy were upgraded to invasive cancer. Time to enhancement on UF-MRI was associated with upgrade from DCIS to invasive cancer (p=.03) with an optimal threshold of 11 seconds (specificity, 50%; sensitivity, 76%). The researchers suggest that short time to enhancement can assist prediction of lesions diagnosed as DCIS at percutaneous biopsy that will be upgraded to invasive cancer at surgery.
“Preoperative UF-MRI, time to enhancement, and lesion size on conventional dynamic contrast-enhanced (DCE) MRI and mammography show potential in predicting upgrade of ductal carcinoma in situ (DCIS) to invasive cancer at surgery,” wrote first author Rachel Miceli, MD, of NYU Langone Health.
"Our study provided strong evidence that AI could really help in interpreting chest radiography. This will contribute to identifying chest diseases, especially lung cancer, more effectively at an earlier stage," said study co-author Jin Mo Goo, M.D., Ph.D., from the Department of Radiology at Seoul National University Hospital.
A Stronger Sun Nuclear
Sun Nuclear and CIRS are now one, as part of Mirion Medical.




With complementary and proven product portfolios, we share a commitment to easing technology adoption, optimizing Quality Management, and ensuring Patient Safety.
Learn more: sunnuclear.com/cirs

5 HospiMedica International February-March/2023
105 HMI-03-23 LINKXPRESS COM
CT Treatment for the Cause of Hypertension
culprit was a gene mutation in the adrenal glands that resulted in the production of excessive levels of the steroid hormone, aldosterone. This steroid hormone leads to salt being retained in the body, thereby driving blood pressure levels higher. Patients having excessive aldosterone levels in their blood exhibit resistance to treatment with commonly used Hypertension drugs, increasing their risk of heart attack and stroke. Now, the same research group has demonstrated that by using a new type of CT scan to light up tiny nodules in a hormone gland and removing them, it is possible to cure high blood pressure. These nodules can be found in one-in-twenty people suffering from high blood pressure.
The research led by doctors at Queen Mary University of London (London, UK; www. qmul.ac.uk) has solved a 60-year problem of how the hormone producing nodules can be detected without a complicated catheter study that is performed in few hospitals and has a high failure rate. The research also found that upon combining it with a urine test, the scan can detect a group of patients who come off all their blood pressure medicines after treatment.
After discovering that their high blood pressure was caused by aldosterone, the doctors conducted a study of the new scan that involved 128 people. The scan found that in two thirds of patients with elevated aldosterone levels, the secretion was from a benign nodule in just one of the adrenal glands, which can be easily removed.
The scan uses a very short-acting dose of metomidate, a radioactive dye that sticks only to the aldosterone-producing nodule. The scan was not only as accurate as the old catheter test, but also quick, painless and technically successful in all the patients. Until now, the catheter test was not capable of predicting which patients could be fully cured of hypertension by surgically removing the gland. In contrast, a ‘hot nodule’ on the scan combined with a urine steroid test detected 18 of the 24 patients who achieved normal blood pressure off all their drugs.


“These aldosterone-producing nodules are very small and easily overlooked on a regular CT scan,” said Professor Morris Brown, co-senior author of the study and Professor of Endocrine Hypertension at Queen Mary University of London. “When they glow for a few minutes after our injection, they are revealed
as the obvious cause of Hypertension, which can often then be cured. Until now, 99% are never diagnosed because of the difficulty and unavailability of tests. Hopefully this is about to change.”
AI-Aided Handheld ULS Detects Musculoskeletal Tendons
for new users and hastening diagnosis and treatment of MSK injuries.




Clarius Mobile Health’s (Vancouver, BC, Canada; www.clarius.com) MSK AI model is designed to streamline workflows, inform clinical management, and provide training assistance during MSK scanning for specific anatomical sites, which include: the plantar fascia (foot), Achilles tendon (ankle), and patellar tendon (knee). The AI analyzes ultrasound imaging in real-time and displays a transparent color overlay to identify the
tendon in view. Upon pausing the image, the AI labels the tendon and determines the greatest thickness, automatically placing measurement calipers that correspond to the top and bottom of the tendon at its thickest region. The user may alter the measurement calipers to make any necessary adjustments to support clinical decision-making. The Clarius MSK AI model is the first FDAcleared AI ultrasound application for MSK imaging and is available with the Clarius L7 HD3 and Clarius L15 HD3 high-frequency wireless handheld ultrasound scanners.
“Ultrasound imaging has long been recognized by the medical community as the best way to see inside the body in real time but learning to detect and recognize anatomy comes with a learning curve,” explained Clarius President and CEO Ohad Arazi. “AI automation is the new frontier and we’re excited to be the world’s first to receive FDA clearance to use AI for musculoskeletal ultrasound. This application will blow the doors open for physiotherapists and orthopedic clinicians to use ultrasound to deliver faster patient care.”

PREMIUM ULTRASOUND SCANNER
The ARIETTA 850 premium ultrasound scanner provides high quality diagnostic imaging to a wide range of clinical areas. Designed with sophisticated ergonomics and multiple new tools it offers greater precision
MOBILE FULL-BODY CT SYSTEM XORAN TECHNOLOGIES
202 203 HMI-03-23 LINKXPRESS COM 6 HospiMedica International February-March/2023
TRON is a truly mobile, full-body CT system that provides high-definition 3D CT images in real-time. It is equipped with easy-to-use software for viewing and reconstructing scan image and an open-bore for easy scanning.
Image: A ten-minute scan enables detection and cure of the commonest cause of high blood pressure (Photo courtesy of Pexels)
WORLD’S MEDICAL DEVICE MARKETPLACE
information
to
Cont’d from cover
To receive prompt and free
on products, log on
www.linkXpress.com or scan the QR code on your mobile device

Identify latest products and technologies . . . Send inquiries directly to suppliers . . . Receive latest product alerts . . . Chat live with suppliers, and more . . . HospiMedica EXPO provides a sophisticated yet easy-to-use global B2B platform for sourcing medical equipment. HospiMedica EXPO connects buyers and sellers worldwide through a safe, secure and dynamic network, regardless of size or budget. WORLD’S MEDICAL DEVICE MARKETPLACE Connecting Buyers with Suppliers Worldwide
Stamp-Size Wearable Ultrasound Patch Provides Cardiac Imaging on the Go


entral blood pressure, the pressure in the central blood vessels, sends blood directly from the heart to other vital organs in the body and is different from peripheral blood pressure that is measured using an inflatable cuff strapped around the upper arm. Medical experts believe that central blood pressure is more accurate than peripheral blood pressure and better at predicting heart disease. However, the measurement of central blood pressure is generally not done during routine exams as it requires a state-of-the-art clinical method that is invasive and involves a catheter inserted into a blood vessel in the patient’s arm, groin or neck and
guided to the heart. While a non-invasive method exists, it is unable to consistently produce accurate readings. The non-invasive method involves holding a pen-like probe, called a tonometer, on the skin directly over a major blood vessel. It is important to hold the tonometer steady and at the exact right angle with the right amount of pressure each time in order to get a good reading. However, this can vary between tests and different technicians. Now, all this could change with a new wearable ultrasound patch that non-invasively monitors blood pressure in arteries deep beneath the skin to detect cardiovascular problems much earlier and with more



A team of researchers, led by the University of California San Diego (La ), has developed a new patch that uses ultrasound waves to continuously record the diameter of a pulsing blood vessel located four centimeters deep below the skin. Customized software then translates this information into a waveform. Each peak, valley and notch in the waveform, as well as its overall shape, indicates a particular activity or event in the heart. The signals offer detailed information to doctors for assessing the cardiovascular health of patients who can use it to predict heart failure or determine if there is no problem with the blood supply. Some of its applications include real-time, continuous monitoring of blood pressure changes in patients diagnosed with heart or lung disease, as well as those who are seri-
The new patch uses ultrasound, which allows it to be used for non-invasively tracking other vital signs and

physiological signals from places deep inside the body. The soft, stretchy ultrasound patch can be worn on the skin for obtaining precise readings of central blood pressure each time, even when the user is on the go, and can also get a good reading through fatty tissue. The researchers performed some tests in which the patch measured blood pressure as well as clinical methods. The researchers tested the patch on a male subject by making him wear it on the forearm, wrist, neck and foot when he was stationary as well as exercising. The recordings collected with the patch were found to be more consistent and precise as compared to the recordings from a commercial tonometer. The researchers also found the patch recordings to be comparable to those collected using a traditional ultrasound probe. The technology can be useful in various inpatient procedures, according to the physicians involved in the study.
“A major advance of this work is it transforms ultrasound technology into a wearable platform,” said co-first author Chonghe Wang, a nanoengineering graduate student at UC San Diego. “This is important because now we can start to do continuous, non-invasive monitoring of major blood vessels deep underneath the skin, not just in shallow tissues.”


640-SLICE CT SCANNER UNITED IMAGING HEALTHCARE
The uCT 960+ is an ultra-premium 640-slice CT scanner, using industry-leading AI-empowered technologies, it features 16 cm z-axis detector coverage, 0.25 s rotation speed, ultra-wide 82 cm bore, and 700 lbs. table weight capacity.
RADIOGRAPHY SYSTEM PRIMAX INTERNATIONAL
205 206 HMI-03-23 LINKXPRESS COM 8 HospiMedica International February-March/2023
The Riviera SPV is designed for high patient throughput and can be configured to the user’s requirements. It grants easier and faster examinations of any anatomical district, with vertical, horizontal and angled
C South-East
Show 1 9 - 2 1 A p r i l 2 0 2 3 - K L C C w w w . a b c e x . c o m WORLD’S MEDICAL DEVICE MARKETPLACE To
Image: Wearable ultrasound patch tracks blood pressure in a deep artery or vein (Photo courtesy of Chonghe Wang/Nature Biomedical Engineering)
Asian Healthcare
receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
Device Monitors Breath Sounds to Predict Respiratory Failure in ICU Patients After Extubation
espiratory failure occurs in 10-20% of post-extubation cases in ICUs, with a mortality rate of 25-50%. Non-invasive ventilation (NIV), such as oxygen delivery via face mask, or high-flow nasal cannula (HFNC) may prevent respiratory failure and the need for reintubation. However, the high cost of these devices makes it difficult to provide them to all patients who are removed from breathing support. Predicting the likelihood of respiratory failure and other breathing difficulties is useful in determining whether a patient will need an unscheduled NIV or HFNC, reintubation, or a more invasive procedure such as cricothyroidotomy, which involves puncturing the throat to create an airway. Now, researchers have developed a novel device that detects abnormal breathing sounds to predict whether an ICU patient is likely to suffer from respiratory complications after removal from a mechanical ventilator, alerting intensive care teams to the need for emergency interventions at an early phase after extubation.

The monitoring device designed by emergency and critical care medicine specialists at Hiroshima University (Hiroshima, Japan; www. hiroshima-u.ac.jp) is powered by an AI they had previously created and trained to analyze and visualize abnormal respiratory sounds. The device’s creation was funded by the Japan Agency for Medical Research and Development (AMED). In their pilot study, the researchers detailed how converting abnormal respiratory sounds into quantitative values as a real-time monitor through their device proved to be useful in predicting respiratory complications after extubation. It could help healthcare professionals in predicting respiratory failure and other life-threatening airway emergencies.
The device provides a continuous monitoring system for respiratory sounds as well as improving prognosis by assisting critical care staff in objectively evaluating respiratory status. Respiratory sound, including stridor, rhonchi, gargling, wheezes, and crackles, are captured at multiple locations by a sensor and visualized in real-time as a spectrogram. Their machine learning algorithm then analyzes and quantifies these frequency signals. The algorithm calculated the quantitative value (QV) of gargling, stridor, and rhonchi in the cervical region or neck and wheezes, rhonchi, coarse crackles, and fine crackles in the thorax area or chest.

The study included 57 patients. Eighteen patients experienced the composite outcome, requiring airway and respiration medical interventions within 48 hours after extubation. The rest belonged to the non-outcome group. According to the researchers, the QVs of stridor and rhonchi in the cervical region were significantly higher in the composite outcome group than in

the non-outcome group. Meanwhile, the QVs of wheezes, rhonchi, and coarse crackles in the anterior thorax region were significantly higher in the outcome group than in the non-outcome group. The QV of fine crackles in the bilateral lateral thorax region was significantly higher in the outcome group than in the non-outcome group. They also stated that inhalation sound volume (average of 5 breaths) in the cervical region immediately after extubation was significantly louder in the outcome group (63.3 dB) than in the non-outcome group (54.3 dB).
Although the device’s predictive score remains to be validated due to the small sample size, the researchers believe that the continuous objective evaluation of respiratory sounds made possible by their apparatus might lead to increased patient safety in ICUs after extubation. Recently, the team of researchers had used their technology to develop a remote respiratory sound monitoring device that could be helpful during a pandemic. The remote medical device combines an electronic stethoscope with a smartphone app that can easily be used by non-doctor medical staff or even patients themselves to auscultate and quickly send information to a specialist for diagnosis. The innovation arose from the experience during the spread of coronavirus where direct auscultation and follow-up check-ups became difficult due
to the risk of infection. The researchers hope that a “respiratory sound monitor” will soon be included in standard cardiorespiratory monitoring system used in hospitals, such as ECGs.
“Respiratory failure in the intensive care unit (ICU) frequently occurs, particularly in patients after extubation, but there has been a lack of sufficient monitors to detect such abnormalities earlier,” said Nobuaki Shime, professor at HU’s Graduate School of Biomedical and Health Sciences, who led the research team. “It will definitely contribute to improving the quality of the cardiorespiratory monitoring system to detect respiratory abnormalities earlier.”
9 HospiMedica International February-March/2023 109 HMI-03-23 LINKXPRESS COM
R
Image: The new remote respiratory sound monitoring device (Photo courtesy of Hiroshima University)
REAL-TIME DIAGNOSTICS ONSCREEN WERFEN




PATIENT MONITOR CONTEC MEDICAL SYSTEMS


Sdevices are assembled with the help of various modules having different material characteristics like softness or rigidity. However, the commercial pastes presently being applied for connecting the modules are usually unable to transmit mechanical and electrical signals with reliability when they become deformed or break easily. For creating a reliably functioning device, module connectors (interfaces) have to be custom-built with sufficient strength to perform the tasks for which they are built. Easily assembling stretchable devices while retaining their strength and reliability under stress still remains a challenge that has limited their development.
Now, an international team led by researchers from Nanyang Technological University, Singapore (NTU Singapore; www.ntu.edu. sg) has developed a universal connector for assembling stretchable devices simply and quickly. Their BIND interface (biphasic, nano-dispersed interface) simplifies the assembly of stretchable devices and also offers an excellent mechanical and electrical performance. Similar to building structures using Lego blocks, it is possible to assemble high-performing stretchable devices by just pressing together any module bearing the BIND interface. This easy and simple method of connecting electronic modules could allow producers to assemble future stretchable devices by using ‘plug-and-play’ components based on their designs.
The researchers developed the BIND interface by thermally evaporating metal (gold or silver) nanoparticles to create a robust interpenetrating nanostructure inside a soft thermoplastic generally used in stretchable electronics (styrene-ethylene-butylene-styrene). This nanostructure offers continuous mechanical as well as electrical pathways, enabling modules with BIND connections to remain robust despite being deformed. The team conducted experiments in which the modules joined by the interface demonstrated an excellent performance. During stretching tests, the modules could be stretched up to seven times their original length before finally breaking. In addition, the electrical transmission of the modules remained robust up to 2.8 times the original length when stretched. The researchers also evaluated the interfacial toughness of the BIND interface by using a standard Peel Adhesion Test, in which the adhesive strength between two modules is put to test by pulling it apart at a constant speed at 180°. In the case of encapsulation modules, the researchers found the BIND interface to be 60 times tougher than conventional connectors. Additionally, the researchers demonstrated the feasibility of its use in real-life applications by building stretchable devices using the BIND

interface, and then testing them on rat models and human skin. The recordings from the stretchable monitoring device attached to rat models displayed reliable signal quality despite interferences with the wirings like touching or tugging. The device stuck on human skin managed to collect high-quality electromyography (EMG) signals which measure the electrical activity generated in muscles during contraction and relaxation, even underwater. The research team has filed an international patent and is now developing a more efficient printing technology to expand the material choice and final application of their innovation. This will accelerate its transition from the laboratory to the designing and manufacturing of products.
“Our breakthrough innovation makes it very easy to form and use a stretchable device since it works like a ‘universal connector’. Any electronic module bearing the BIND interface can be connected simply by pressing them together for less than 10 seconds,” said Chen Xiaodong, lead author of the study. “Moreover, we do away with the cumbersome process of building customized interfaces for specific systems, which we believe will help accelerate the development of stretchable devices.”
“These impressive results prove that our interface can be used to build highly functional and reliable wearable devices or soft robots,” added Dr. Jiang Ying, Research Fellow at the NTU School of Materials Science & Engineering. “For example, it can be used in high-quality wearable fitness trackers where users can stretch, gesture, and move in whichever way they are most comfortable with, without impacting the device’s ability to capture and monitor their physiological signals.”
The CMS8000 patient monitor allows users to select different parameter configurations according to specific requirements and can be used for clinical monitoring in the case of adult, pediatric and neonate patients.
208 209 HMI-03-23 LINKXPRESS COM 10 HospiMedica International February-March/2023
GEMweb Live is a real-time onscreen viewer that consolidates results from four Werfen systems in the cardiovascular operating room. These comprehensive test results, all on one screen, enable faster clin-
WORLD’S MEDICAL DEVICE MARKETPLACE To
Image: In stretching tests, modules were able to withstand stretching of up to seven times their original length before breaking (Photo courtesy of NTU Singapore)
receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
Ingestible Sensor Could Replace Invasive Procedures for Diagnosing GI Motility Disorders
Gastrointestinal (GI) motility disorders such as constipation, gastro esophageal reflux disease, and gastroparesis affect
to a nearby computer or smartphone. The system’s current version can take a measurement any time it receives a wireless trigger from a



within 5 to 10 millimeters. Such level of monitoring can allow doctors to more easily identify the section of the GI tract causing a slowdown in

Critical Care To view this issue in interactive digital magazine format visit www.HospiMedica.com
ANESTHESIA MACHINE MINDRAY PATIENT MONITORING & LIFE SUPPORT





Following a heart attack, there is development of scar tissue, which affects muscle function and can result in congestive heart failure. However, there is still no established treatment available for repairing the damage caused to cardiac tissue after a heart attack. Now, a newly-developed biomaterial that can be injected intravenously reduces inflammation in tissue and encourages cell and tissue repair. Researchers who developed the biomaterial also tested it and proved its effectiveness in treating tissue damage as a result of heart attacks in rodent as well as large animal models. They also provided proof of concept in a rodent model that the biomaterial may benefit patients with traumatic brain injury and pulmonary arterial hypertension.
In previous studies, a team of bioengineers and physicians at the University of California San Diego (La Jolla, CA, USA; www.ucsd.edu) had developed a hydrogel from the natural scaffolding of cardiac muscle tissue, also known as the extracellular matrix (ECM). This gel can be injected into damaged heart muscle tissue using a catheter and forms a scaffold in the damaged areas, promoting new cell growth and repair. The researchers had reported successful results from a phase 1 human clinical trial although the gel can only be used a week or more after a heart attack as it has to be injected directly into the heart muscle – risking damage caused by the needle-based injection procedure. This time, the team set out to develop a treatment that could be administered immediately after a heart attack. For this purpose, the team developed a biomaterial that could be infused into a blood vessel in the heart at the same time when other treatments such as angioplasty or a stent were being administered, or injected intravenously.

The researchers began with the hydrogel they had developed, which had proved to be compatible with blood injections in safety trials. However, the particle size in the hydrogel was too large to target leaky blood vessels. The researchers resolved this issue by putting the liquid precursor of the hydrogel through a centrifuge, enabling them to sift out bigger particles and retain only nano-sized particles. The resultant material was made to go through dialysis and sterile filtering before being freeze dried. After the addition of sterile water to the final powder, a biomaterial is obtained that can be injected intravenously or infused into a coronary artery in the heart. The new biomaterial offers the advantage of even distribution throughout the damaged tissue, as it is infused or injected intravenously. In contrast, hydrogel injected using a catheter stays in specific locations and does not spread out.
The researchers went on to test the biomaterial on a rodent model of heart attacks. The material was expected to pass through the blood
vessels and into the tissue due to the development of gaps between endothelial cells in blood vessels after a heart attack. However, the researchers found that the biomaterial instead bound to those cells, closing the gaps and accelerating healing of the blood vessels, as a result of which inflammation was reduced. Testing the biomaterial in a porcine model of heart attack generated similar results. The team also successfully tested the hypothesis that the biomaterial could help treat other types of inflammation in rat models of traumatic brain injury and pulmonary arterial hypertension. The researchers will now undertake preclinical studies for these conditions with a study on the safety and efficacy of the biomaterial in human subjects expected to begin within one to two years.
“This biomaterial allows for treating damaged tissue from the inside out,” said Karen Christman, a professor of bioengineering at the University of California San Diego, and the lead researcher on the team that developed the material. “It’s a new approach to regenerative engineering.”
“We sought to design a biomaterial therapy that could be delivered to difficult-to-access organs and tissues, and we came up with the method to take advantage of the bloodstream – the vessels that already supply blood to these organs and tissues,” said Martin Spang, the paper’s first author. “While the majority of work in this study involved the heart, the possibilities of treating other difficult-to-access organs and tissues can open up the field of biomaterials/tissue engineering into treating new diseases.”
 The WATO EX-20 high-end anesthesia machine features the latest anesthesia architecture, high quality components and technology for improved performance. Its dynamic gas compensation allows it to deliver
FETAL & MATERNAL MONITOR PHILIPS HEALTHCARE
The WATO EX-20 high-end anesthesia machine features the latest anesthesia architecture, high quality components and technology for improved performance. Its dynamic gas compensation allows it to deliver
FETAL & MATERNAL MONITOR PHILIPS HEALTHCARE
211 212 HMI-03-23 LINKXPRESS COM 12 HospiMedica International February-March/2023
The Avalon FM20 fetal and maternal monitor offers automated coincidence detection (cross-channel verification) using Smart Pulse. It measures the fetal and maternal heart rates separately to enhance diagnostic confidence.
Image: The new biomaterial heals tissues from the inside out (Photo courtesy of UC San Diego)
WORLD’S MEDICAL DEVICE MARKETPLACE
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
All-in-One System Cleans Up Blood Culture Contamination, Cuts Sepsis False Positives
Blood cultures are considered the gold standard diagnostic test for the detection of blood stream infections, such as sepsis. However, positive blood culture results can be frequently wrong, and about 40% of positive results return a false-positive result owing to contamination. Such false-positive results can cause misdiagnosis of sepsis and expose the patient to unnecessary, prolonged, and harmful broad-spectrum antibiotic treatment and extended length of in-patient hospital stay. This may put the patent at a higher risk for acute kidney injury, Clostridioides difficile infection (CDI), Multidrug-Resistant Organism (MDRO) infections, other hospital-acquired infections (HAI), and significantly high hospital costs. Preventing false-positive results and sepsis misdiagnosis has to begin with reducing blood culture contaminations. Now, a new technology can help reduce blood culture contamination and false-positive test results, thereby preventing the patient from being harmed, and reducing unnecessary and prolonged usage of antibiotics, duration of stay, and hospital expenses.
Magnolia Medical Technologies’ (Seattle, WA, USA; www. magnolia-medical.com) Steripath is the only FDA 510(k)-cleared device that is specifically indicated to reduce blood culture contamination with an FDA-cleared labeling claim for an 83% and 88% reduction in contamination rates. Optimally designed for blood culture contamination prevention, Steripath comes pre-assembled and sterile to actively divert and sequester the initial 1.5-2.0mL of blood, the volume that is known to contain contaminants. Blood cultures are collected through an independent, second flow path, creating a closed vein-to-bottle collection system meant to avoid bypassing diversion.
Magnolia is entering into partnerships with hospitals and healthcare providers in the U.S. to prevent sepsis misdiagnosis by improving the accuracy of blood culture results using Steripath. A recent peer-reviewed study published in a leading medical journal constituted the largest controlled clinical dataset ever documented with zero blood culture contamination events. Importantly, the study demonstrated that it was possible to eliminate blood culture contamination and “get to zero” with the use of Steripath. Another large-scale, peer-reviewed study quantified multiple devastating patient harms associated with blood culture contamination and most significantly, a 74% higher risk of in-hospital patient mortality.
“This study includes one of the largest data sets to date examining and directly quantifying the preventable consequences of blood culture contamination on patient safety and clinical outcomes,” said Greg Bullington, CEO and Co-Founder of Magnolia Medical. “This definitive data adds to the substantial body of evidence that already

exists demonstrating that contaminated blood culture results drive avoidable, severely negative outcomes for patients and the healthcare system, including increased costs, inappropriate antibiotic usage that contributes to the rise of multidrug-resistant organisms, increased length of stay, and other preventable adverse events.”

Critical Care To view this issue in interactive digital magazine format visit www.HospiMedica.com 13 HospiMedica International February-March/2023
113 HMI-03-23 LINKXPRESS COM
SUN 5 is a safe, efficient, economical and simple medical heating blanket for the prevention of intraoperative hypothermia. It features a smart, ergonomic design to provide a comfortable experience and prevent cross infection.

Ning, serpentine structures which power wearable electronics.
In a study led by Washington State University (Pullman, WA, USA; www.wsu.edu), researchers demonstrated that it is possible to make electrodes using just screen printing by creating a stretchable, durable circuit pattern which can be transferred to fabric and worn directly on the human skin. These wearable electronics can be used for monitoring the health of patients admitted in hospitals or being treated at home. Currently, commercial manufacturing of wearable electronics involves expensive processes that require clean rooms. Screen printing is used by some for parts of the process, although the new method relies completely on screen printing, making it advantageous for manufacturers and consumers.
In their study, published in the ACS Applied Materials and Interfaces journal, the researchers have detailed the electrode screen-printing process and demonstrated how the produced electrodes can be used for electrocardiogram monitoring, or ECG. Using a multi-step process to layer polymer and metal inks, the researchers created snake-like structures of the electrode. The resulting thin pattern looks delicate, although the electrodes are not fragile. The study demonstrated that the electrodes can be stretched by 30% and bent to 180 degrees.
Multiple electrodes are printed onto a pre-treated glass slide, allowing them to be easily peeled off and transferred onto fabric or other material. After printing the electrodes, they were transferred onto an adhesive fabric which was worn directly on the skin by the subjects. The research-

ers found that the wireless electrodes accurately recorded heart and respiratory rates, and transmitted the data to a mobile phone. The main focus of the study was on ECG monitoring, although the screen-printing process can be utilized to make electrodes for different applications, including those with functions similar to those of smart watches or fitness trackers, according to the researchers. The team is currently working on expanding the technology for printing different electrodes as well as entire electronic chips and even potentially, whole circuit boards.



“We wanted to make flexible, wearable electronics in a way that is much easier, more convenient and lower cost,” said corresponding author Jong-Hoon Kim, associate professor at the WSU Vancouver’s School of Engineering and Computer Science. “That’s why we focused on screen printing: it’s easy to use. It has a simple setup, and it is suitable for mass production.”
Novel Microneedle Patch Immediately Stops Bleeding After Injury
Secondary, uncontrolled bleeding from traumatic injury is a leading cause of death. That could now change with a novel microneedle patch that is capable of immediately stopping bleeding after injury.
The hemostatic microneedle technology developed by researchers at Penn State (University Park, PA, USA; www.psu.edu) can be applied like a typical adhesive bandage to quickly stop bleeding. The biocompatible and biodegradable microneedle arrays (MNAs) on the patch increase its surface contact with blood and accelerate the clotting process. The needles also increase the adhesive properties of the patch through mechanical interlocking to promote wound closure.
The MNA patch is similar to the hydrogel technology currently used to treat bleeding wounds in hospitals, although hydrogel applications need preparation and medical expertise. In contrast, the microneedle patch is pre-engineered for immediate application so that it can be

used by anyone to stop bleeding, similar to an adhesive bandage that is available over-the-counter. Microneedles are already being used to deliver biologics, such as cells or drugs, through the skin or for cosmetic procedures to stimulate collagen production. They are tiny, making their application pain-free. The researchers now plan to bring the patch from the lab to the market by further testing the technology.
“Excessive bleeding is a serious challenge for human health. With hemorrhaging injuries, it is often the loss of blood - not the injury itself - that causes death. There is an unmet medical need for ready-to-use biomaterials that promote rapid blood coagulation,” said Amir Sheikhi, assistant professor of chemical engineering and of biomedical engineering at Penn State. “In vitro, the engineered MNAs reduced clotting time from 11.5 minutes to 1.3 minutes; and in a rat liver bleeding model, they reduced bleeding by more than 90%. Those 10 minutes could be the difference between life and death.”
MEDICAL HEATING BLANKET AEONMED
214 215 HMI-03-23 LINKXPRESS COM 14 HospiMedica International February-March/2023
The ZOLL AED 3 BLS defibrillator provides in-depth rescue support to treat both adult and child victims of sudden cardiac arrest. It is one of the fastest AEDs in the industry at delivering a shock after chest com-
WORLD’S MEDICAL DEVICE MARKETPLACE To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
Hydrogel-Based Spray Kills Antibiotic-Resistant Bacteria in Wounds and Biomedical Implants
Antibiotic resistance has been ranked among the top ten threats to global health by the World Health Organization (WHO). Antibiotic-resistant bacteria is estimated to cause almost 1.3 million deaths annually across the world. Hence, there is a great need for new solutions to tackle antibiotic-resistant bacteria and reduce the use of antibiotics. Infections are a major problem for treatments in which materials like implants and catheters are inserted into the patient’s body. This makes it vital to develop new antibacterial biomaterials that can treat, replace or modify organs, tissue or functions in a biological body. Now, a group of researchers is developing a new spray that can kill even antibiotic-resistant bacteria, and can be used for wound care as well as directly on implants and other medical devices.
In an effort to slow down the spread and development of drug resistance, researchers at Chalmers University of Technology (Gothenburg, Sweden; www.chalmers.se) are developing a new antibacterial material for use in healthcare settings that can become an effective tool against antibiotic resistance. The material consists of small hydrogel particles that are equipped with a type of peptide that effectively kills and binds bacteria. Attaching the peptides to the particles creates a protective environment and increases their stability, allowing them to work with body fluids such as blood, which otherwise inactivates the peptides and make them difficult to use in healthcare settings.
In previous studies, the researchers had shown how the peptides can be used for wound care materials like wound dressings. Now, in two of their latest studies, the team has shown that the bactericidal material can be used both in the form of a wound spray and as a coating on medical devices implanted in the body. For both the spray and the coating, the researchers measured the bactericidal effect of the materials and found that it can last for up to 48 hours in contact with body fluids and for as long as a few years without contact with body fluids. The researchers also found that the material can kill 99.99% of the bacteria, enabling a wide range of clinical applications. Since the usage of urinary catheters is one of the primary causes of hospital-acquired infection, the researchers tested the coating on silicone materials used for catheters, although it can be also used on other biomaterials. Being non-toxic, the material can also be used directly on or in the body for preventing or curing an infection without any adverse impact on the natural healing process.






“Our innovation can have a dual impact in the fight against antibiotic resistance. The material has been shown to be effective against many different types of bacteria, including those that are resistant to antibiotics, such as Methicillin-resistant Staphylococcus aureus (MRSA), while also having the potential to prevent infections and thus reduce the need for antibiotics,” said Martin Andersson, head of research for the study and Professor at the Department of Chemistry and Chemical Engineering at Chalmers.



“The substance in this wound spray is completely non-toxic and does not affect human cells. Unlike existing bactericidal sprays, it does not inhibit the body’s healing process. The materials, which are simply sprayed onto the wound, can also kill the bacteria in a shorter time,” explained Edvin Blomstrand, PhD student at the Department of Chemistry and Chemical Engineering at Chalmers.

“Although the catheters are sterile when unpacked, they can become contaminated with bacteria while they are being introduced into the body, which can lead to infection. One major advantage of this coating is that the bacteria are killed as soon as they come into contact with the surface. Another is that it can be applied to existing products that are already used in healthcare, so it is not necessary to produce new ones,” added Annija Stepulane, PhD student at the Department of Chemistry and Chemical Engineering at Chalmers.
Image: New spray fights infections and antibiotic resistance (Photo courtesy of Chalmers University of Technology)

Critical Care To view this issue in interactive digital magazine format visit www.HospiMedica.com 15 HospiMedica International February-March/2023
MEDICAL COMMUNITY Anytime, Anywhere, On the Go... PRINT MAGAZINE INTERACTIVE DIGITAL EDITION WEB PORTAL ENGLISH • SPANISH MOBILE VERSION PRINT MAGAZINE • INTERACTIVE DIGITAL EDITION WEB PORTAL • MOBILE VERSION • MOBILE APPS E-NEWSLETTER • E-MAIL MARKETING ONLINE SOLUTIONS • HOSPIMEDICA EXPO DAILY CLINICAL NEWS .com
PREMIER MULTIMEDIA PLATFORM SERVING THE WORLD’S HOSPITAL /
upon exertion with lower and lower exertion and arrhythmias, thus gradually affecting their daily routine. Patients with leaky mitral valve have a substantially shorter life expectancy than age and gender-matched people. Currently, complex open-heart surgery to repair or replace the leaky heart valve is the most effective treatment for such patients but is offered to just 2% of patients due to a high surgical risk. Now, a unique platform offers a first-of-its-kind technology for allowing implantation of a biological bioprosthesis to replace the diseased valve through catheterization only.
The unique RoseDoc platform developed by TruLeaf Medical (Or Akiva, Israel; www.truleaf-medical.com) enables a ground-breaking procedure that is minimally invasive, performed on a beating heart via two needle punctures without surgery or the use of a heart-lung machine. The procedure is associated with significantly lower risk than traditional open-heart mitral valve surgery. This means that millions of patients across the world, who until now were considered to be inoperable, can receive a new valve and look forward to a substantial improvement in their functional capacity, quality of life and life expectancy.


TruLeaf has received the Helsinki Ethics Committee’s approval to conduct clinical trial of its innovative platform in humans. As part of the trial, a prosthetic mitral valve will be implanted via two needle sticks in the groins in a two-stage catheterization procedure without the need for open-heart surgery (transcatheter mitral valve replacement, TMVR). The implantation of the RoseDoc, which replaces the patient’s leaky heart valve, will be done in two stages. In the first stage, a docking station will be implanted in the left atrium, followed by implantation of an artificial ‘biological’ mitral valve prosthesis after a few weeks. The approval to carry out implantation of TruLeaf’s TMVR platform in humans


comes a few months after completing the R&D of all the components of the platform, as well as a series of successful short-term and long-term animal experiments.

“The main challenge with existing TMVR technologies is achieving optimal anchoring of the valve prosthesis to the heart, given the complex native mitral valve anatomy and physiology,” explained Benjamin Spencer, TruLeaf Medical CEO. “The RoseDoc TMVR platform is technically simple, safe and has been proven to be effective in rigorous long-term animal testing. Complete elimination of the leak prevents progressive dilation of the heart, which, by itself, in a vicious cycle, worsens the leak, leading to a progressive weakening of the heart muscle and intractable heart failure. At present, patients with severe mitral valve leak refractory to maximal medical treatment remain without an effective treatment. The vast majority of these patients are declined surgery due to prohibitive risk. The RoseDoc unique TMVR platform literally gives these patients new hope.”
Imaging Technology for Brain Tumor Surgery Improves Patient Outcomes
Glioma, a primary brain tumor, that begins in the glial cells surrounding and supporting nerve cells is the most common type of adult brain tumor. It comprises 78% of malignant brain tumors in adults and is also the most common pediatric solid tumor, accounting for about 53% of tumors in children younger than 14 years. Despite modern technology, surgeons find it difficult to determine if they have removed the entire brain tumor. Glioma treatment can be particularly challenging as the tumors usually have “finger-like” projections extending into various parts of the brain. Now, a one-of-
a-kind optical imaging agent illuminates glioma, making it easier for neurosurgeons to see the tumor tissue to be removed. The enhanced visualization enables neurosurgeons to identify and remove as much of the cancer as possible, thus improving patient outcomes.
Gleolan from Medexus Pharmaceuticals (Bolton, ON, Canada; www.medexus.com) is the first and only FDA-approved optical imaging agent for use during fluorescence-guided surgery (FGS) in patients with high grade gliomas, which means the tumor is suspected World Health
Cont’d on page 20


ENDOSCOPY DISPLAY NDS SURGICAL IMAGING
EndoVue Plus 24” is a high-end endoscopy display that accommodates standard analog and digital HD-SDI and DVI-I high-definition signals from a variety of sources, including ultrasound, PACS, and
IRRIGATION PUMP ERBE ELEKTROMEDIZIN
217 218 HMI-03-23 LINKXPRESS COM 16 HospiMedica International February-March/2023
The EIP 2 irrigation pump for endoscopic procedures provides a pulsed jet of sterile water for better procedural site visualization, ideal for rinsing substances such as blood and fecal matter from procedural site and instruments.
Image: The innovative RoseDoc platform replaces the patient\’s leaky heart valve (Photo courtesy of TruLeaf Medical)
WORLD’S MEDICAL DEVICE MARKETPLACE
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
Robotic Assistants in the OR Enable Safer Surgical Procedures










t present, some manual operations are so difficult they can be performed by only a small number of surgeons worldwide, while others are invasive and depend on a surgeon’s specific skill. Now, advanced robotics are providing tools that have the potential to enable more surgeons to carry out such operations and do so with a higher rate of success. With extreme precision needed for certain medical operations, state-of-the-art robots offer a way to make surgery easier, safer and more successful. For instance, the EU-funded Ganymed project is developing a compact robot to make joint-replacement operations more precise, less invasive and – by extension – safer. Similarly, the EU-funded MEETMUSA project has been further developing what it describes as the world’s first surgical robot for microsurgery certified under the EU’s ‘CE’ regulatory regime.
The initial focus of the Ganymed project is on a type of surgery called total knee arthroplasty (TKA), though Ganymed Robotics (Paris, France; www.ganymedrobotics.com) is looking to expand to other joints including the shoulder, ankle and hip. Ageing populations and lifestyle changes are accelerating demand for such surgery. Ganymed’s robot will aim to perform two main functions: contactless localization of bones and collaboration with surgeons to support joint-replacement procedures. It comprises an arm mounted with ‘eyes’, which use advanced computer-vision-driven intelligence to examine the exact position and orientation of a patient’s anatomical structure. This avoids the need to insert invasive rods and optical trackers into the body.
Surgeons can then perform operations using tools such as sagittal saws – used for orthopedic procedures – in collaboration with the robotic arm. The ‘eyes’ aid precision by providing so-called haptic feedback, which prevents the movement of instruments beyond predefined virtual boundaries. The robot also collects data that it can process in real time and use to hone procedures further. Ganymed has already carried out a clinical study on 100 patients of the bone-localization technology that has achieved the desired precision. The company is now performing studies on the TKA procedure, with hopes that the robot will be fully available commercially by the end of 2025 and become a mainstream tool used globally.
Robots are being explored not only for orthopedics but also for highly complex surgery at the microscopic level. Microsure (Eindhoven, the Netherlands; www.microsure.nl) is developing MUSA, the world’s first surgical robot for microsurgery certified under the EU’s ‘CE’ regulatory regime. The small, lightweight robot is attached to a platform equipped with arms able to hold and manipulate microsurgical instruments with a high degree of precision. The platform is suspended above the patient during an operation and is controlled by the surgeon through specially adapted joysticks.
In a 2020 study, surgeons reported using MUSA to treat breast-cancer-related lymphedema – a chronic condition that commonly occurs as a side effect of cancer treatment and is characterized by a swelling of body tissues as a result of a build-up of fluids. To carry out the surgery, the robot successfully sutured – or connected – tiny lymph vessels measuring 0.3 to 0.8 millimeter in diameter to nearby veins in the affected area. When such delicate operations are conducted manually, they are affected by slight shaking in the hands, even with highly skilled surgeons. With the robot, this problem can be avoided. MUSA can also significantly scale down the surgeon’s general hand movements rather than simply repeating them one-to-one, allowing for even greater accuracy than with conventional surgery.






In addition to treating lymphedema, the current version of MUSA – the second, after a previous prototype – has been used for other procedures including nerve repair and soft-tissue reconstruction of the lower leg. Microsure is now developing a third version of the robot, MUSA-3, which is expected to become the first one available on a widespread commercial basis. This new version will have various upgrades, such



17 HospiMedica International February-March/2023
117 HMI-03-23 LINKXPRESS COM Size: 2400 x 1200 mm (3 mm thick) 100% Silicone YOUR GLOBAL SOURCE FOR STERILIZATION ACCESSORIES Thermo-Resistant (- 60 °C to 300 °C) Fully Washable & Flexible Suitable for central sterilization services Sterilizable STERILIZABLE INSTRUMENT & WORK-SURFACE MATS Front Back WASHING TRAYS MAT Heavy Silicone Cover & Transport Tablet TURBO WASHING MACHINES TRAYS SILICON INSTRUMENT MAT Front Back MICRO INSTRUMENT MAT Exchangable Net Exchangable Nets INVITEDTOAPPLY DISTRIBUTORS Up to 37 cm in length THERMO RESISTANT GLOVES Front Back WASHING TRAYS MAT NEW! VICOTEX Place de la Gare 1 • 1009 Pully • Switzerland Tel: (41) 21-728-4286 • Fax: (41) 21-729-6741 E-Mail: contact@vicotex.com www.vicolab.com S.A. SILICONE TABLET AND STEEL COVER NETS
A
Cont’d on page 18
Image: MUSA’s robotic arms (Photo courtesy of Microsure)
surgical arthroscope is designed to improve the efficiency, consistency, and safety of arthroscopic procedures.

Summit, a 4K single-use surgical arthroscope from Pristine Surgical (Manchester, NH, USA; www.pristinesurgical.com), features Pristine Connect cloud-based software to deliver a high-definition image, every time, with a device that is always new and never obsolete. The scope is simple to set up and 100% sterile, ensuring patient and staff safety. Its transparent pricing model and automated inventory management also improve predictability and workflow. The Summit single-use arthroscope has been designed to address challenges inherent to conventional surgical visualization, which are complex and expensive, and feature reusable arthroscopes that are difficult and costly to maintain. Prone to wear, damage, and potential infection, these scopes can bring inefficiency and variability into the operating room.
Pristine has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Summit single-use arthroscope which it expects to launch during the first quarter of 2023 with a predictable and transparent subscription pricing model and a convenient, automated re-ordering system. Through industry leading sustainability partnerships, Pristine will also provide recycling options to its customers.

“The Summit single-use arthroscope represents a new paradigm in arthroscopy, improving a procedure that hasn’t changed much in my 42 years of practice and beyond,” said Dr. Stephen Snyder, chief medical officer of Pristine Surgical and a recognized pioneer in the field of arthroscopy. “We’re removing the well-known barriers of legacy, reusable systems that eventually become obsolete by offering a new 4K single-use scope that improves safety and reliability while decreasing room turnover time and the cost of arthroscopic treatment.”
“Summit simplifies arthroscopic procedures, offering a fully integrated single-use scope with 4K resolution that’s one-of-a-kind,” said Bryan Lord, CEO of Pristine Surgical. “Our FDA clearance is a significant milestone for the company. More importantly, it means that we can now begin offering the benefits of these single-use devices to surgeons and staff, and bring our single-use, cloud-based platform to more than 125 million endoscopy patients treated worldwide each year.”

Image: Summit, the world’s first 4K single-use surgical arthroscope, has received US FDA 510(k) clearance (Photo courtesy of Pristine Surgical)
Robotic Assistants in the OR Enable Safer Surgical Procedures

Cont’d from page 17



as better sensors to enhance precision and improved maneuverability of the robot’s arms. It will also be mounted on a cart with wheels rather than a fixed table to enable easy transport within and between operating theatres.
Furthermore, the robots will be used with exoscopes – a novel high-definition digital camera system. This will allow the surgeon to view a three-dimensional screen through goggles in order to perform ‘heads-up microsurgery’ rather than the less-comfortable process of looking through a microscope. The company is confident that MUSA-3 will be widely used across Europe and the US before a 2029 target date. MEETMUSA is also looking into the potential of artificial intelligence (AI) to further enhance robots. However, the company believes that the aim of AI solutions may be to guide surgeons towards their goals and support them in excelling rather than achieving completely autonomous surgery.
“We’re entering the next revolution in medicine,” said Sophie Cahen, chief executive officer and co-founder of Ganymed Robotics. “Demand is super-high because arthroplasty is driven by the age and weight of patients, which is increasing all over the world.”
“I think the surgeon will always be there in the feedback loop, but these tools will definitely help the surgeon perform at the highest level in the future,” said Tom Konert, who leads MEETMUSA and is a clinical field specialist at Microsure.
PORTABLE SUCTION DEVICE
The 330 Multifunction Aspirator is a portable, self-contained suction device that can customize suction based on the clinical procedure. It provides continuous and intermittent suction capabilities and a fully reflec-
FLEXIBLE VIDEO ENDOCSCOPY SYSTEM XION MEDICAL
220 221 HMI-03-23 LINKXPRESS COM 18 HospiMedica International February-March/2023
The EndoFLEX system configuration comprises the EndoFLEX console, a PanelPC and DiVAS software for acquiring, managing and evaluating examination data and for connecting the system to the HIS or PACS via standard interfaces.
WORLD’S MEDICAL DEVICE MARKETPLACE To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
Robotic-Assisted Bronchoscopy: Game Changer in Earlier Lung Cancer Diagnosis
Cont’d from cover
Lung cancer is the leading cause of cancer death among both men and women. According to the World Health Organization, cancer in its myriad forms accounted for 10 million deaths worldwide in 2020; of that multitude, lung cancer accounted for 1.8 million deaths globally - the single largest share by type of cancer. More than half of people with lung cancer die within one year of being diagnosed. Detecting lung cancer early is a way we can fight back. However, early detection can be challenging in part because around 70% of cancerous lung nodules are found in the outer third of the lung, which may be difficult to reach and adequately biopsy via bronchoscopy. Now, the next innovation in lung nodule biopsy uses robotic-assisted surgery that allows clinicians to reach and biopsy difficult-to-reach nodules in the peripheral lung, eliminating the need for multiple biopsies prior to lung cancer diagnosis.
Intuitive Surgical’s (Silicon Valley, CA, USA; is a robotic-assisted endoluminal platform for minimally invasive periph eral lung nodule biopsy. Using Ion’s fully articulating catheter, physicians can identify a target, plan a path to the pulmonary nodule, and navigate far into the periphery of the lung for biopsy. Ion’s ultrathin, ultramaneuverable catheter allows physicians to reach small lesions in all 18 segments of the lung while its unprecedented stability enables the precision needed for biopsy.
Intuitive Surgical has enhanced Ion’s biopsy workflow by integrating Ion and Siemens Healthineers’ Cios Spin mobile imaging system. With this connection, a 3D scan is performed by Cios Spin and automatically sent to the Ion system. From the Ion console touchscreen, the physicians can now refine navigation and verify tool-in-lesion. The integration offers guidance when managing differences in nodule locations seen on preprocedure CT images and intraprocedural imaging.
Ion brings an immersive bronchoscopy experience that begins with planning, moves through procedures, and powers a virtuous cycle of improvement. Ion’s distinct combination of tools and technologies help visualize each patient’s lung anatomy to support biopsy. With Ion, physicians can reach small lesions in the far reaches of the lung. Distal tip articulation allows clinicians to aim the catheter at small targets, even when located outside the airway. Results from early studies have demonstrated relatively low occurrence of pneumothorax requiring intervention. For physicians, Ion provides more reach, more stability, and more precision. This in turn can help them give patients something to hope for - potentially earlier diagnosis.

36th Annual Congress of the European Association of Nuclear Medicine

SEPTEMBER 9 – 13, 2023
eanm23.eanm.org
To view this issue in interactive digital magazine format visit www.HospiMedica.com Surgical Techniques
19 HospiMedica International February-March/2023
Bladder cancer is the 10th commonest cancer worldwide and the 6th commonest cancer amongst men. It is known to have high recurrence rates and significant risks of disease progression. Early detection of bladder cancers and recognition of disease recurrence can substantially reduce patient morbidity and healthcare costs, reduce the risks of disease progression, and improve overall survival. Now, a new image enhancement and artificial intelligence (AI) diagnostic tool for bladder cancer detection in images (videos and camera stream) seen on white light cystoscopy and narrow band imaging (NBI) cystoscopy could be beneficial in improving bladder cancer diagnostics and patient care.
Claritas HealthTech (London, UK; www.claritashealthtech.com) is commencing clinical validation of CystoSmart, a Software as a Medical Device (SaMD), that has been jointly developed through a research collaboration with Khoo Teck Puat Hospital (KTPH, Singapore; www.ktph.com.sg).


“Our aim has been to develop an AI diagnostic adjunct that enhances the accuracy of detection of bladder cancers. This will be beneficial in allowing: 1) Appropriate treatment for newly diagnosed cancers 2) Accurate recognition of tumor recurrences 3) Complete tumor resection during surgery,” explained Dr. Yeow Siying, a consultant urologist at the Department of Urology, KTPH, who is the medical principal investigator for the project. “The detection of bladder cancer often involves cystoscopy, where a fibre optic camera is inserted into the bladder to visualize its inner lining (mucosa). Most commonly, white light cystoscopy is utilized, whilst other adjuncts such as Narrow Band Imaging (NBI), can help improve the accuracy
Image:
AI diagnostic tool will enhance cancer detection (Photo courtesy of Claritas HealthTech)


of cancer detection to a limited extent. Detection of bladder cancer can be challenging, particularly for flat lesions such as carcinoma-in-situ. Certain benign conditions may appear visually similar to bladder cancers as well.”

“Training AI tools that are reliable in clinical settings have been challenging because it is dependent on the quality of the captured cystoscopic images. Claritas has leveraged its image enhancement platform to create enhanced, clinically validated data sets, thereby allowing more detail to be extracted in training our neural network,” said Dr. Arup Paul, Chief Clinical Strategy Officer at Claritas. “Our pre-clinical studies have allowed the appropriate calibration and development with resulting evidence of high sensitivity and specificity. We are confident of moving into the next stage, with clinical evaluations utilizing CystoSmart to demonstrate benefits for patients, clinicians, and healthcare systems.”
Imaging Technology for Brain Tumor Surgery Improves Patient Outcomes
Cont’d from page 16

Organization Grades III or IV on preoperative imaging, as an adjunct for the visualization of malignant tissue during surgery. Gleolan is the only FDA-approved product that highlights a glioma tumor and surrounding cancer tissue in a bright hue.
Gleolan is an oral solution that contains aminolevulinic acid hydrochloride (ALA HCl) and is given to the patient to drink three hours (between two to four hours) before receiving anesthesia for the surgery. With the proper dosage of Gleolan administered during surgery, the neurosurgeon can view the brain through special blue light filters on a surgical microscope. Under the blue light, Gleolan helps the tumor “fluoresce” or glow a red-violet color. Since non-cancerous brain cells are
unlikely to glow when using the blue light filters, the neurosurgeons can better distinguish the tumor from normal tissue, allowing them to remove more of the tumor tissue.
“When surgically resecting a brain tumor, our aim is always to remove it completely,” said Adam Robin, M.D., neurosurgeon at Henry Ford’s Hermelin Brain Tumor Center (HBTC), who performed the first brain surgery using Gleolan at Henry Ford West Bloomfield Hospital. “When using this imaging agent, we perform the tumor resection procedure under a special blue light, which causes the tumor cells to be bright pink or magenta in color. This visual aid enhances our ability to identify tumor cells that otherwise may have been hidden or more challenging to identify.”

RADIAL ECHOENDOSCOPE
The EG-UR5 is a radial echoendoscope that combines advanced ultrasound and endoscopy technology for the precise diagnosis in the GI tract and its extra-luminal structure. It has a large field of view with a 360°
SURGICAL LIGHT TRUMPF MEDIZIN SYSTEME
223 224 HMI-03-23 LINKXPRESS COM 20 HospiMedica International February-March/2023
TruLight 5000 surgical light is versatile enough for any surgical application, with automatic readjustment of illumination level in typical working distances, adjustable color temperature, and possibility of camera integration.
CystoSmart image enhancement and
WORLD’S MEDICAL DEVICE MARKETPLACE
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
POC Testing Enables Expansion of Near-Patient Care Capabilities

the laboratory. POC diagnostic tests can be performed at the bedside and brings the test immediately to the patient. POC diagnostic testing raises the likelihood of the patient receiving the test results quickly and improves clinical management. Portable and handheld instruments are used in POC diagnostics, enabling the test results to be shared quickly with the patients. It helps in the rapid detection of analytes near the patient, facilitates better disease diagnosis, monitoring, and management, and enables quick medical decisions. POC diagnostics plays a vital role in patient management by reducing the time to treatment.
The COVID-19 outbreak led to increased demand for POC diagnosis. This has been accompanied by the increasing incidence of chronic and infectious diseases globally. As a result, there has been growing demand for portable, transportable, and handheld devices that is fueling the growth of the POC diagnostics market. In addition, the increase in technological advancements has also contributed significantly to the market growth. The global POC diagnostics market is projected to grow at a CAGR of 11.1%, from nearly USD 40 billion in 2022 to over USD 93 billion in 2030, driven by the rising incidence of chronic diseases such as cardiovascular disorders, diabetes, and infectious diseases, as well as an increasing geriatric population. These are the latest findings of Precedence Research (Ottawa, Canada; www.precedenceresearch.com), a market research firm.
Rapid technological advancements such as the emergence of cloudbased deep learning systems and chip technology miniaturization are expected to expand the applications of POC diagnostics. For instance, lab-on-a-chip technology is now used at the POC. The development of nanoparticle-based POC technology has also attracted the attention of the market players and helped doctors to take advantage of nanoparticles for the diagnosis of various chronic conditions. The market players are adopting modern technologies such as artificial intelligence (AI) to produce more efficient products and are focusing on integrating ELISA into products for diagnosing infectious diseases. However, the high cost of product development due to various technology areas depending upon the test type could hamper market growth.
Based on product, the blood glucose monitoring segment dom-
AI Systems Could Induce Great Savings in Healthcare Sector
The wider adoption of artificial intelligence (AI) in healthcare could save the U.S. up to USD 360 billion annually although its uptake in the industry is presently limited owing to the absence of trust among patients and doctors, heterogeneous data and misaligned incentives. Nevertheless, there is likely to be broader adoption of AI in healthcare in the near future, along with various non-financial benefits such as improved healthcare quality, greater care access and better patient and doctor satisfaction levels.

These are the latest findings by researchers at McKinsey & Company (New York City, NY, USA; www.mckinsey.com) and Harvard University (Cambridge, MA, USA; www.harvard.edu).
Presently, the healthcare industry has low adoption of AI-based tools despite its benefits discovered by researchers. In the new paper, researchers estimate that the broader adoption of AI in the healthcare industry could lead to savings in the range of 5% to 10% in healthcare spending, or between approximately USD 200 billion and USD 360 billion per year. Their estimates are based on AI use cases utilizing current technologies that are achievable within the next five years, without compromising quality or access. Hospitals could see cost savings mainly through improved clinical operations, quality and safety – such as optimizing operating rooms, or identifying adverse events. Physician groups can experience similar benefits by leveraging AI for continuity of care, such as referral management.
Health insurers could experience savings from use cases that improve claims management, such as automating prior authoriza-
inates the POC diagnostics market with the highest revenue share due to increasing demand for products for monitoring blood sugar levels and growing preference for home glucose testing. On the other hand, the infectious diseases segment is expected to register a promising CAGR during the forecast period due to the rising prevalence of various contagious diseases. Based on end user, the hospital bedside segment dominates the POC diagnostics market with the highest revenue share due to the rising prevalence of chronic diseases which require long-term care and frequent monitoring. The increasing awareness about the availability of cost-effective and highly innovative POC diagnostics creates a positive outlook for the market. On the other hand, the urgent care & retail clinics segment are expected to register a promising CAGR over the coming years due to the growing number of clinics and urgent care centers.
Based on region, North America dominated the global POC diagnostics market with the largest revenue share due to the high prevalence of lifestyle diseases, increasing awareness of self-testing and home care kits, and rapid technological advancements in the region. On the other hand, the Asia-Pacific market for POC diagnostics is expected to register a promising CAGR during the forecast period due to the increasing number of market players and growing use of POC diagnostics in the developing countries.
tion, along with healthcare and provider relationship management, including preventing readmissions and provider directory management. Based on AI-driven use cases, private payers could save approximately 7% to 9% of their total costs, or between USD 80 billion and USD110 billion annually, within the next five years. Physician groups could save 3% to 8% of their costs, translating into savings of between USD 20 billion and USD 60 billion. Additionally, the report estimates that hospitals could register savings between 4% to 11%, or between USD 60 billion and USD 120 billion per year.
To view this issue in interactive digital magazine format visit www.HospiMedica.com Industry News
21 HospiMedica International February-March/2023
Cont’d from cover
Image: The global point-of-care diagnostics market is expected to surpass USD 93 billion by 2030 (Photo courtesy of Pexels)
Image: Researchers expect broader adoption of AI in healthcare in the near future (Photo courtesy of Pexels)
2023
APRIL
APSCVIR 2023 – 17th Annual Meeting of the Asia Pacific Society of Cardiovascular and Interventional Radiology. Apr 12-15; Seoul, Korea; apscvir.com
ECIO 2023 – European Conference on Interventional Oncology. Apr 16-19; Stockholm, Sweden; ecio.org
ARRS 2023 Annual Meeting – American Roentgen Ray Society. Apr 16-20; Honolulu, HI, USA; arrs.org
HIMSS 2023 – Healthcare Information and Management Systems Society. Apr 17-21; Chicago, IL, USA; himss.org
SEACare 2023 – 23rd Southeast Asian Healthcare & Pharma Show. Apr 19-21; Kuala Lumpur; Malaysia; abcex.com
143rd Annual Meeting of the American Surgical Association (ASA). Apr 20-22; Toronto, Canada; americansurgical.org
AAEM23 – 29th Annual Scientific Assembly of the American Academy of Emergency Medicine. Apr 21-25; New Orleans, LA, USA; aaem.org
AAN 2023 – 75th Annual Meeting of the American Academy of Neurology. Apr 22-27; Boston, MA, USA; aan.com
Charing Cross International Symposium 2023. Apr 25-27; London, UK; cxsymposium.com
DCK 2023 – 139th Congress of the German Society for Surgery (DGCH). Apr 25-28; Munich, Germany; dgch.de
KSCCM-ACCC 2023 – Annual Congress of the Korean Society of Critical Care Medicine. Apr 27-28; Seoul, Korea; accc.or.kr
DAC 2023– Annual Congress of the German Society of Anaesthesiology and Intensive Care Medicine. Apr 27-29; Düsseldorf, Germany; dac-congress.de
JPR 2023 – 53rd Sao, Paulo Conference on Ra-
diology & 25th Meeting of the Latin American Congress of Pediatric Radiology. Apr 27-30; Sao Paulo, Brazil; spr.org.br
Medical Fair India 2023. Apr 27-29; New Delhi, India; medicalfair-india.com
123rd Congress of the Japan Surgical Society (JSS). Apr 27-29; Tokyo, Japan; jp.jssoc.or.jp
CAR 2023 Annual Scientific Meeting – Canadian Association of Radiologists. Apr 27-30; Montreal, Canada; car-asm.ca
AUA 2023 – Annual Meeting of the American Urological Association. Apr 28 - May 1; Chicago, IL, USA; auanet.org
MAY
WCO-IOF-ESCEO 2023 - World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. May 4-7; Barcelona, Spain; wco-iofesceo.org
ECTES 2023 – 22nd Congress of the European Society for Trauma & Emergency Surgery (ESTES). May 7-9; Ljubljana, Slovenia; estes-congress.org
SIOP Europe 2023– 4th Annual Meeting of the European Society for Paediatric Oncology. May 8-12; Valencia, Spain; siopeurope.eu
Vietnam Medi-Pharm Hanoi 2023. May 10-13; Hanoi, Vietnam; vietnammedipharm.vn
ECCC Dubai 2023 – 18th Emirates Critical Care Conference. May 12-14; Dubai, UAE; eccc-dubai. com
ESTRO 2023 – Annual Congress of the European Society of Radiology & Oncology. May 12-16; Vienna, Austria; estro.org
ECE 2023 – 25th European Congress of Endocrinology. May 13-16; Istanbul, Turkey; ese-hormones.org
SPR 2023 – Annual Meeting of the Society for Pediatric Radiology. May 16-20; Austin, TX, USA; pedrad.org
KIHE 2023 – Kazakhstan International Health-
care Exhibition. May 17-19; Almaty, Kazakhstan; kihe.kz
ATS 2023 – International Conference of the American Thoracic Society. May 19-24; Washington, DC USA; thoracic.org
CMEF 2023 – China International Medical Equipment Fair. May 14-17; Shanghai, China; cmef. com.cn
91st EAS Congress 2023 – European Atherosclerosis Society. May 21-24; Mannheim, Germany; eas-society.org
MedtecLIVE 2023. May 23-25; Nuremburg, Germany; medteclive.com
Hospitalar 2023. May 23-26; Sao Paulo, Brazil; hospitalar.com
Nordic Congress of Radiology 2023. May 24-26; Helsinki, Finland; ncr2023.fi
ESOC 2023 – 9th European Stroke Conference. May 24-26; Munich, Germany; eso-conference.org
EFORT Congress 2023 – 24th Annual Congress of European Federation of National Associations of Orthopaedics and Traumatology. May 24-26; Vienna, Austria; congress.efort.org
EUROSON 2023 – 34th Congress of European Federation of Societies for Ultrasound in Medicine and Biology. May 25-27; Riga, Latvia; euroson2023.com

EULAR 2023 – European Congress of Rheumatology. May 31 - Jun 3; Milan, Italy; congress.eular.org
JUNE
EuroAnaesthesia 2023 – European Society of Anaesthesiology. Jun 3-5; Glasgow, UK; esaic.org
2023 ISMRM & ISMRT Annual Meeting & Exhibition – International Society for Magnetic Resonance in Medicine. Jun 3-8; Toronto, Canada; ismrm.org
ESTS 2023 – 31st Meeting of the European Society of Thoracic Surgeons. Jun 4-6; Milan, Italy; ests.org
Medical Taiwan 2023. Jun 8-10; Taipei, Taiwan; medicaltaiwan.com.tw
EHA 2023 - Annual Congress of the European Hematology Association. Jun 8-11; Frankfurt, Germany; ehaweb.org
ESICM EuroAsia 2023. Jun 9-11; Mumbai, India;
isccm.org
ESGAR 2023 – 34th Annual Meeting of the European Society of Gastrointestinal and Abdominal Radiology. Jun 13-16; Valencia, Spain; esgar.org
ICEM 2023 – 22nd International Conference on Emergency Medicine. Jun 13-16; Amsterdam, Netherlands; icem2023.com
SIIM 2023 – Annual Meeting of the Society for Imaging Informatics in Medicine. Jun 14-16; Austin, TX, USA; siim.org
60th ERA Congress – European Renal Association. Jun 15-18; Milan, Italy; era-online.org
CARS 2023 – Computer Assisted Radiology and Surgery. Jun 20-23; Munich, Germany; cars-int.org
FIME 2023 – Florida International Medical Expo. Jun 21-23; Miami, FL, USA; fimeshow.com
23rd MEDEXPO Africa 2023. Jun 21-23; Nairobi, Kenya; expogr.com/kenyamed
ESHRE 2023 – 39th Annual Meeting of the European Society of Human Reproduction and Embryology. Jun 25-28; Copenhagen, Denmark; eshre.eu
JULY
9th Congress of the European Academy of Neurology (EAN). Jul 1-4; Budapest, Hungary; ean.org
SCCT 2023 – 18th Annual Scientific Meeting of the Society of Cardiovascular Computed Tomography. Jul 28-30; Boston, MA, USA; scct.org
33rd Medicall Expo. Jul 28-30; Chennai, India; medicall.in
AUGUST
ASCI 2023 – 16th Congress of the Asian Society of Cardiovascular Imaging. Aug 10-12; Bali, Indonesia; ascibali2023.org
Asia Health 2023. Aug 16-18; Bangkok, Thailand; medlabasia.com
Medical Fair China 2023. Aug 23-25; Suzhou, China; medicalfair.cn
ESC Congress 2023 – European Society of Cardiology. Aug 25-28; Amsterdam, Netherlands; escardio.org
WICC 2023 - 16th World Intensive and Critical Care Congress. Aug 26-30; Istanbul, Turkey; wicc2023.org
Expo Med 2023. Aug 30 – Sep 1; Mexico City,
International Calendar
22 HospiMedica International February-March/2023
For a free listing of your event, or a paid advertisement in this section, contact: International Calendar, HospiMedica International E-mail: info@globetech.net
Mexico; expomed.com.mx
SEPTEMBER
6th World Congress on Regional Anaesthesia & Pain Medicine and 40th Annual Congress of the European Society of Regional Anaesthesia and Pain Therapy (ESRA). Sep 6-9; Paris, France; esraworld2023.com
ERS International Congress 2023 – European Respiratory Society. Sep 9-13; Milan, Italy; erscongress.org
EANM 2023 – 36th Annual Congress of the European Association of Nuclear Medicine. Sep 9-13; Vienna, Austria; eanm.org
CIRSE 2023 – Annual Congress of the Cardiovascular and Interventional Radiological Society of Europe. Sep 9-13 Copenhagen, Denmark; cirse.org
Medical Fair Thailand. Sep 13-15; Bangkok, Thailand; medicalfair-thailand.com
REHACARE 2023 – International Trade Fair for Rehabilitation and Care. Sep 13-16; Dusseldorf, Germany; rehacare.com
ExpoMedical 2023. Sep 20-22; Buenos Aires, Argentina; expomedical.com.ar
19th EuGMS Congress – European Geriatric Medicine Society. Sep 20-22 Helsinki, Finland; eugms.org
IndoHealthCare 2023. Sep 21-23; Jakarta, Indonesia; indohealthcareexpo.com
Medical Fair Brasil. Sep 26-28; Sao Paulo, Brazil; medicalfair-brasil.com.br
Medic West Africa. Sep 26-28; Lagos, Nigeria; medicwestafrica.com
ESVS 2023 – Annual Meeting of the European Society for Vascular Surgery. Sep 26-29; Belfast, UK; esvs.org
EUSOBI 2023 – Annual Scientific Meeting of the European Society of Breast Imaging. Sep 28-30;
Valencia, Spain; eusobi.org
UAA 2023 – 20th Urological Association of Asia Congress. Sep 28 - Oct 1; Dubai, UAE; uaanet.org
ESGO 2023 – 24th European Congress on Gynaecological Oncology. Sep 28 – Oct 1; Istanbul, Turkey; esgo.org
OCTOBER
ASTRO 2023 – 65th Annual Scientific Meeting of the American Society for Radiation Oncology. Oct 1-4; San Diego, CA, USA; astro.org
Global Health Exhibition 2023. Oct 2-4; Riyadh, Saudi Arabia; globalhealthsaudi.com
EASD 2023 – 59th Annual Meeting of the European Association for the Study of Diabetes. Oct 3-6; Hamburg, Germany; easd.org
ISS 50th Annual Meeting – International Skeletal Society. Oct 7-13; London, UK; internationalskeletalsociety.com
34th Medicall Expo. Oct 7-9, New Delhi, India; medicall.in
MICCAI 2023 – 26th International Conference on Medical Image Computing and Computer Assisted Intervention. Oct 8-12; Vancouver, BC, Canada; miccai.org
ACEP23 – Scientific Assembly of the American College of Emergency Physicians. Oct 9-12; Philadelphia, PA, USA; acep.org
15th World Stroke Congress – World Stroke Organization. Oct 10-12; Toronto, Canada; worldstrokecongress.org
Medical Japan 2023 Tokyo– International Medical and Elderly Care Expo. Oct 11-13; Tokyo, Japan; medical-jpn.jp
CBR23 – 52nd Congress of the Brazilian College of Radiology and Diagnostic Imaging. Oct 12-14; Rio de Janeiro, Brazil; cbr.org.br
JFR 2023 - Journées Francophones de Radiologie.
Oct 13-16; Paris, France; jfr.radiologie.fr
ANESTHESIOLOGY 2023 – Annual Meeting of the American Society of Anesthesiologists. Oct 13-17; San Francisco, CA, USA; asahq.org
UEG Week 2023 – United European Gastroenterology. Oct 14-17; Copenhagen, Denmark; ueg. eu/week
ISUOG World Congress 2023 - International Society of Ultrasound in Obstetrics & Gynecology. Oct 16-19; Seoul, Korea; isuog.org
EUSEM 2023 – 17th European Emergency Medicine Congress. Oct 16-20; Barcelona, Spain; eusemcongress.org
Africa Health 2023. Oct 17-19; Johannesburg, South Africa; africahealthexhibition.com
RANZCR 2023 – Annual Scientific Meeting of The Royal Australian and New Zealand College of Radiologists. Oct 19-21; Brisbane, Australia; ranzcr.com
ESMO Congress 2023 - European Society for Medical Oncology. Oct 20-24; Madrid, Spain; esmo.org
ECISM LIVES 2023 – Annual Congress of European Society of Intensive Care Medicine. Oct 21-25; Milan, Italy; esicm.org
ESSO 42 – 42nd Congress of the European Society of Surgical Oncology. Oct 25-27; Florence, Italy; esso42.org
46th World Hospital Congress of the International Hospital Federation (IHF). Oct 25-27; Lisbon, Portugal; worldhospitalcongress.org
ESCR ESTI Joint Meeting 2023 – European Societies of Cardiovascular Radiology and Thoracic Imaging. Oct 26-28; Berlin, Germany; escr.org
NOVEMBER
WUFMB Congress 2023 – World Federation for Ultrasound in Medicine and Biology. Nov 4-7; Muscat, Oman; wfumb.info
BSIR 2023 – Annual Scientific Meeting of the British Society of Interventional Radiology. Nov 8-10; Newport, UK; bsirmeeting.org
MEDICA 2023. Nov 13-16; Dusseldorf, Germany; medica-tradefair.com
APSR 2023 – 27th Congress of the Asian Pacific Society of Respirology. Nov 16-19; Singapore; apsr2023.sg
ASUS 2023 – 6th Congress of the Asian Surgical Ultrasound Society. Nov 18-20; Seoul, Korea; asus2023.org
CBMI 2023 – 28th Brazilian Congress of Intensive Care Medicine. Nov 23-25; Florianopolis, Brazil; amib.org.br
RSNA 2023 – Annual Meeting of the Radiological Society of North America. Nov 26-30; Chicago, IL, USA; rsna.org
DECEMBER
35th Medicall Expo. Dec 8-10, Kolkata, India; medicall.in
2024
JANUARY
Critical Care Congress 2024 – 53rd Annual Meeting of the Society of Critical Care Medicine (SCCM). Jan 21-24; Phoenix, AZ, USA; sccm.org
Arab Health 2024. Jan 29 - Feb 1; Dubai, UAE; arabhealthonline.com
MARCH
ECR 2024 – European Congress of Radiology. Feb 28 - Mar 3; Vienna, Austria; myesr.org
SIR 2024 – Annual Meeting of the Society of Interventional Radiology. Mar 23-28; Salt Lake City, UT, USA; sirmeeting.org
APRIL
Expomed Euroasia 2024. Apr 25-27; Istanbul, Turkey; expomedistanbul.com
International Calendar Provided as a service to advertisers. Publisher cannot accept responsibility for any errors or omissions. Advertising Index Inq No . Advertiser Page 102 COMEN .............................. 2 – EANM 2023 .......................... 19 – HospiMedica 15 – HospiMedica EXPO 7 – IHF 2024 22 113 Mayo Clinic 13 103 Radcal 3 124 Randox 24 105 Sun Nuclear ........................... 5 109 Undis ................................ 9 111 Vedalab ............................. 11 117 Vicolab .............................. 17 Vol. 41 Issue 1 3/2023 HospiMedica International
ATTENTION: IF YOUR APPLICATION IS NOT RECEIVED AT LEAST ONCE EVERY 12 MONTHS YOUR FREE SUBSCRIPTION MAY BE AUTOMATICALLY DISCONTINUED READER SERVICE PORTAL LINKXPRESS COM ® VISIT Every advertisement or product item in this issue contains a LinkXpress number as shown below: 999 HMI-03-23 LINKXPRESS COM Identify LinkXpress codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress inquiry matrix 1 2 3 Renew/ S tart Your Free Subscription Access Instant Online Product Information 23 HospiMedica International February-March/2023
POINT OF CARE TESTING
SEXUALLY TRANSMITTED INFECTION ARRAY
• Sample to answer, cartridge based PCR testing.

• Suitable for in-clinic testing.
• Analytical sensitivity of 95% detection.
• PCR results in 2 hours.
• Detecting 10 bacterial, viral and protozoan infections.
DETECTABLE INFECTIONS
Chlamydia trachomatis (CT)
Herpes simplex virus 1 (HSV-1)
Neisseria gonorrhoeae (NG)
Herpes simplex virus 2 (HSV-2)
Trichomonas vaginalis (TV)
Haemophilus ducreyi (HD)
Mycoplasma genitalium (MG)

Mycoplasma hominis (MH)
Treponema pallidum (Syphilis) (TP)
Ureaplasma urealyticum (UU)
VISIT US AT ECCMIDSTAND
C3-16 15TH –APRIL18TH
1 SAMPLE • 1 TEST • 1 0 INFECTIONS
Visit store.randox.com to buy directly from Randox today randox.com marketing@randox.com
124 HMI-03-23 LINKXPRESS COM























































 The WATO EX-20 high-end anesthesia machine features the latest anesthesia architecture, high quality components and technology for improved performance. Its dynamic gas compensation allows it to deliver
FETAL & MATERNAL MONITOR PHILIPS HEALTHCARE
The WATO EX-20 high-end anesthesia machine features the latest anesthesia architecture, high quality components and technology for improved performance. Its dynamic gas compensation allows it to deliver
FETAL & MATERNAL MONITOR PHILIPS HEALTHCARE


























































