PCR Faster than Self-Diagnosis Kits

PCR technology which detects target nucleic acids by amplifying the DNA amount has witnessed significant progress in the life sciences field since it was first developed in 1984. The molecular diagnostics technology achieved public familiarity during
Cont’d on page 4
AI Model Analyzes Cells in Tissue Samples Without Need for Trained Pathologists
Fast and precise information regarding the operated tissue is crucial for guiding a surgeon’s next steps during cancer surgery. In cases where solid tumors are present in a cancer patient, the surgeon typically sends a biopsy sample to a
pathologist for a rapid assessment. The pathologist must determine, among other things, whether the tissue is healthy, the extent of cancer’s spread into the organs, etc. The traditional intraoperative diagnostic process is laborious, time-con-
Cont’d on page 13
ApoB Protein Improves Cardiac Risk Prediction
As part of the annual physical exam, individuals are typically tested for their levels of HDL (the good) and LDL (the bad) cholesterol to assess their risk for heart disease. However, recent research has raised questions about the accuracy of these standard tests in predicting heart disease risk. Instead, emerging data suggest that testing for levels of Apolipoprotein B-100 (ApoB),
Cont’d on page 8
Early Screening Test for Pancreatic Cancer
Pancreatic cancer, a lethal tumor of the pancreas, has one of the highest mortality rates among all major cancers. Every year, this disease claims approximately 466,000 lives worldwide, ranking it as the seventh most common cause of cancer-related fatalities. Its survival rates are among the lowest for any cancer, primarily due to late detection and poor outcomes with conven-
See article on page 11
Smartphone-Powered Lipid Test Enables Early Detection of CVD


Cardiovascular disease (CVD), accountable for 32% of global deaths annually, is the world’s leading cause of death. These largely preventable fatalities highlight the urgent need for improved access to CVD testing and subsequent treatment, a pressing concern worldwide. Now, an innovative diagnostic tool for CVD enables early detection Cont’d on page 15
Pediatric Reference Intervals for Common Cardiovascular Disease Tests

Numerous pediatric hospitals have begun utilizing two cardiac tests, high-sensitivity cardiac troponin (hs-cTn) and N-terminal pro B-type natriuretic peptide (NT-proBNP), which measure levels of proteins cTn I or T and NT-proBNP
respectively. Recent research indicates that these tests may enhance care for children with various conditions, including congenital heart disease, heart failure, and multi-system organ failure due to sepsis. However, a significant limitation is the
Cont’d on page 15
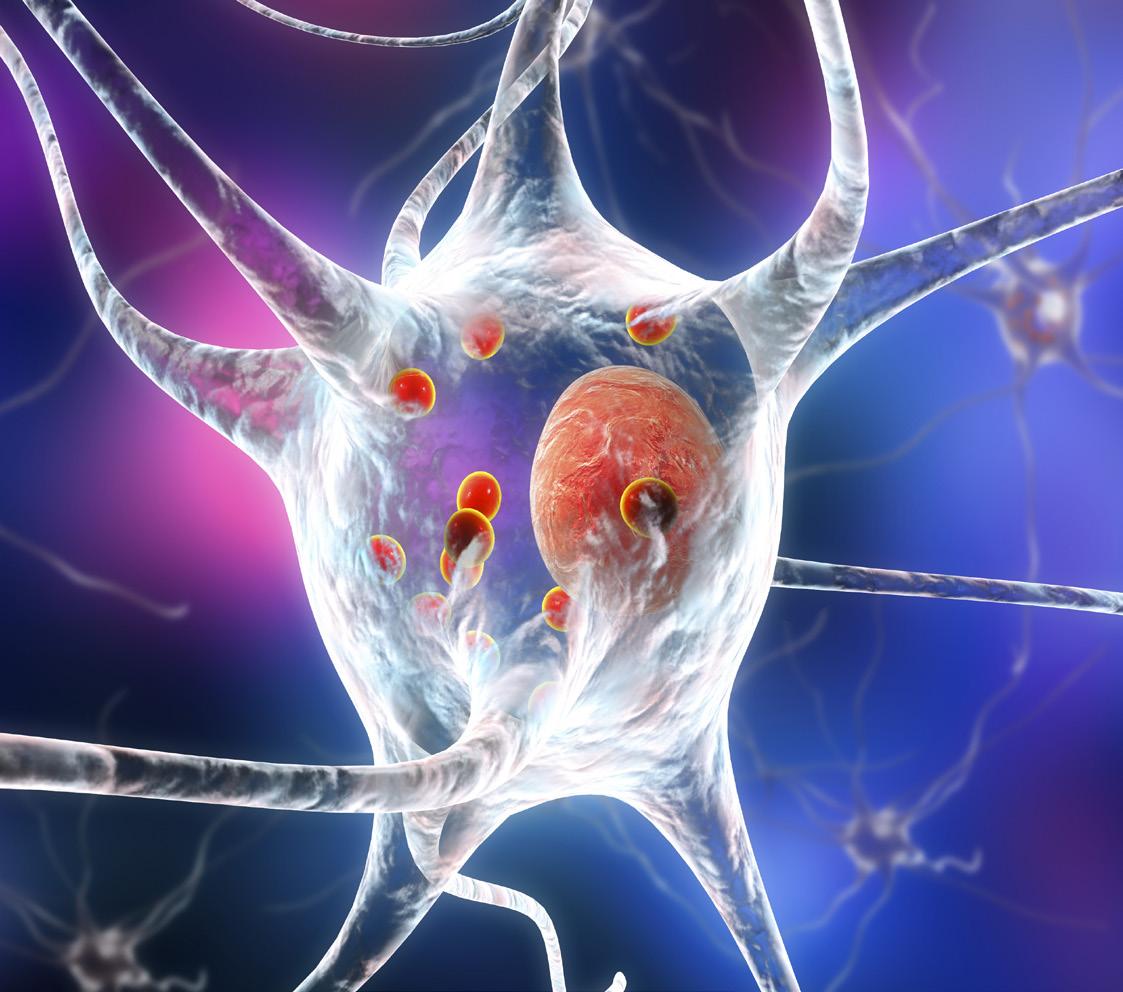
A method capable of detecting the accumulation of abnormal protein deposits associated with Parkinson’s disease in cerebrospinal fluid, has been proven to accurately identify pa-
with the condition. ®
on page 6
tients
Cont’d
If your subscription is not renewed every 12 months your Free Subscription may be automatically discontinued Renew / Start your Free Subscription Access Interactive Digital Magazine Instant Online Product Information: Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 VISIT READER SERVICE PORTAL LINKXPRESS COM ®
Offers Earlier
of Parkinson’s Disease Breakthrough
Offers Earlier Detection of Parkinson’s Disease GLOBETECH MEDIA >>> <<< PUBLISHED IN COOPERATION WITH International Federation of Clinical Chemistry and Laboratory Medicine INSIDE Clinical News ..... 4 LabMedica EXPO . 6-16 IFCC News ....... 17 Industry News . ... 21 Events Calendar . . 22
Breakthrough Test
Detection
Test
® INTERNATIONAL Vol.40 No.3 • 5/2023 VISIT DAILY CLINICAL LAB NEWS ISSN 1068-1760 4 OUR YEAR 0 th YEAR OUR WORLD’ S CLINICA L LABORATOR Y NEW S LEADER
Image: 3D illustration of Parkinson’s Disease showing neurons with small red spheres (Lewy Bodies) that are deposits of proteins accumulated in brain cells, causing their progressive degeneration (Shutterstock).


102 LMI-5-23 LINKXPRESS COM
Biomarkers in Urine and Blood Can Predict Risk of Developing Chronic Kidney Disease
The kidneys play a crucial role in purifying blood and eliminating waste from the body. Acute kidney injury (AKI) occurs when the kidneys abruptly and temporarily lose their functionality, causing a buildup of waste in the blood and hindering the body’s fluid balance. While AKI is treatable, if left unchecked, it can lead to chronic kidney disease (CKD), a more severe and potentially fatal condition, as well as other cardiac issues. AKI is frequently observed in hospitalized patients whose kidneys have been subjected to medical or surgical stresses and complications, potentially prolonging the kidneys’ recovery and causing lasting damage. Now, researchers have identified biomarkers to predict CKD risk in hospitalized patients with AKI.

In a new study that examined the long-term consequences of AKI in hospitalized patients, researchers at Johns Hopkins Medicine (Baltimore, MD, USA; www.hopkinsmedicine.org) have found that elevated levels of specific biomarkers in urine and blood can predict a patient’s likelihood of developing CKD. These results could assist medical professionals in gauging the efficacy of kidney damage recovery and potentially averting the advancement of AKI to CKD.
The researchers conducted a study involving 656 hospitalized patients with AKI, in which they measured several urine and plasma biomarkers related to kidney damage, inflammation, and tubular health at multiple intervals over a year following diagnosis. Their aim was to determine the correlation between changes in these biomarkers over time and the progression of kidney disease after AKI. The researchers discovered that an increase in the biomarkers KIM-1, MCP-1, and TNFRI in urine and plasma, respectively, was associated with a two- to three-fold heightened risk for CKD for each deviation change from baseline to 12 months. These findings indicate that prolonged tissue damage and inflammation, as well as slower restoration of tubular health, increase the risk of kidney disease progression. However, they also noted that an increase in the urine biomarker UMOD was linked to a 40% reduction in CKD risk.
“Longitudinal measurement of some of these proteins have the potential to guide management of patients with AKI after discharge, which includes follow-up with a nephrologist; optimizing diabetes and cardiac medications; and accurate dosing of all medications with reduced kidney function,” said Chirag Parikh, M.D., Ph.D. director of the Division of Nephrology at the Johns Hopkins University School of Medicine and the study’s corresponding author who underscored the need for more research into these ongoing biological processes to help better understand the transition from AKI to CKD.
The study was published on March 23, 2023 in the Journal of Clinical Investigation.

· Optimized UV-test according to IFCC (modified)


· Wide measuring range to limit unnecessary sample dilution

· Minimized interferences by common blood components
· Excellent onboard stability combined with low calibration frequency








3 LabMedica International May/2023 LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Total confidence in patient results. www.diasys-diagnostics.com
DiaSys.
LDH 21 FS
New reagent to determine lactate dehydrogenase
103 LMI-05-23 LINKXPRESS COM
Image: Certain substances in urine, blood can predict kidney disease progression (Photo courtesy of Pexels)
PCR Faster than Self-Diagnosis Kits
Cont’d from cover the COVID-19 pandemic, as PCR is capable of detecting nucleic acids that identify the COVID-19 virus. However, the technical nature of the PCR test makes it impossible for the results to be delivered before one to two hours due to its need for repeated temperature cycles (60~95 oC). Now, a new ultrafast PCR technology uses photothermal nanomaterials to shorten the test time by 10-fold, as compared with the time taken by the existing test. The new method can be completed in five minutes and delivers a diagnostic performance that is similar to that of the existing test method.
Photothermal nanomaterials generate heat immediately upon light irradiation and rapidly increase in temperature, although the performance can be difficult to maintain due to their low stability. A research team at Korea Institute of Science and Technology (KIST; Seoul, Korea; eng.kist. re.kr) has developed a polymer composite that physically holds photothermal nanomaterials and can overcome their instability. By applying it to a PCR system, the team has developed a compact PCR system without a heat plate. Additionally, the researchers have implemented a multiplex
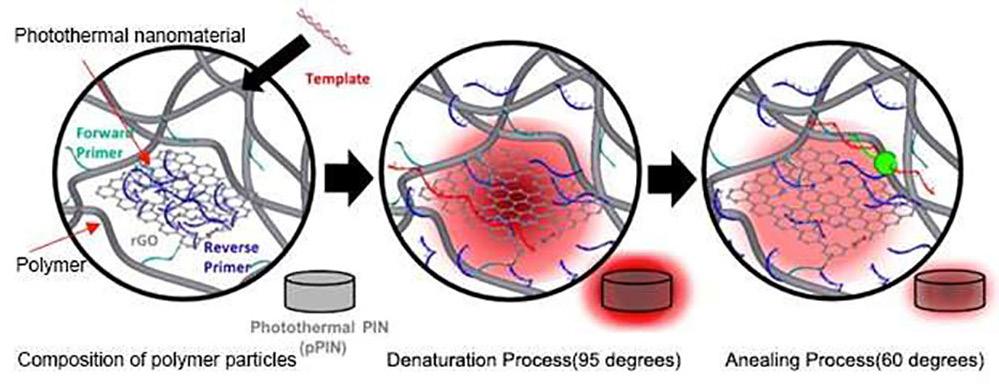
diagnostic technology that detects several genes simultaneously, enabling it to distinguish several types of COVID-19 variants in a single reaction.
“Through additional research, we plan to miniaturize the developed ultrafast PCR technology this year, to develop a device that can be utilized anywhere,” said Dr. Sang Kyung Kim, Director at the Center for Augmented Safety System with Intelligence, Sensing of the KIST. “While maintaining the strength of PCR as an accurate diagnostic method, we will increase its convenience, field applicability, and promptness, by which we expect that it will become a precision diagnostic device that can be used at primary local clinics, pharmacies, and even at home. In addition, PCR technology is a universal molecular diagnostic technology that can be applied to various diseases other than infectious diseases, so it will become more applicable.”
Real-Time Quick-Response PCR Test Marks Innovation in Subtyping Breast Cancer
Breast cancer is the most prevalent cancer among women, with one in seven expected to be diagnosed during their lifetime. The early detection and treatment of the disease have greatly improved survival rates. However, for women with invasive breast cancer, receiving the best possible treatment quickly is crucial for their recovery. Accurately subtyping the tumor is a critical aspect of treatment, but the process has remained largely unchanged for over 50 years and is susceptible to inaccuracies. Now, a new real-time quick-response PCR (RTqPCR) test is set to usher in the first real innovation in subtyping breast cancer in decades.
Cerca Biotech’s (Berlin, Germany; www. cercabiotech.com) MammaTyper RT-qPCR test represents a modern approach to subtyping breast cancer, providing reliable, accurate, and prompt results for every tissue sample. This technology offers a strong basis for treatment planning, providing every woman with the best possible chance of overcoming breast cancer. By employing RT-qPCR technology validated during the COVID-19 pandemic, this multigene test works through an easily replicated and quantitative measurement of marker gene expression, classifying each sample as a St. Gallen subtype. The results are objective and standardized, displaying high consistency with IHC. Furthermore, MammaTyper can precisely evaluate HER2-low tumors and Ki-67 markers, broadening the range of treatment options available to patients.
The MammaTyper kit employs RNA extracted from standard formalin-fixed, paraffin-embedded (FFPE) sample material commonly used in clinical routine, and offers a streamlined molecular pathology workflow to deliver reliable results on the same day, including ERBB2 (HER2) results. This method is highly reproducible and reduces common pre-analytical errors, especially for the proliferation marker Ki-67 which is critical in luminal subtyping and prognosis. Using RT-qPCR technology for breast cancer tissue subtyping provides valuable assistance for pathologists and ensures that accurate subtyping leads to personalized and effective treatment plans for women with breast cancer.
One of the crucial components in selecting the best therapy for breast cancer patients is accurately determining their molecular subtypes. MammaTyper enhances subtyping by providing an easily replicated and quantitative measurement of marker gene expression. This approach overcomes the drawbacks of semi-quantitative staining methods and enables pathologists to improve subtyping precision. The MammaTyper test is suitable for use as a primary diagnostic test (biopsy) for all breast cancer cases. It is also applicable for analyzing resection specimens and metastases, and can serve as a secondary assessment in cases with ambiguous IHC results, or as a rapid test for ERBB2 (HER2) determination. Additionally, it can be utilized as a substitute for IHC and in situ hybridization (FISH/CISH).
INTERNATIONAL
labmedica.com
EDITORIAL BOARD
Graham Beastall United Kingdom
Hernán Fares Taie Argentina
Bernard Gouget France Maurizio Ferrari Italy
Jocelyn M. Hicks United States
Tahir S. Pillay South Africa
Andreas Rothstein Colombia Praveen Sharma India
Rosa I. Sierra-Amor Mexico
Peter Wilding United States
Andrew Wootton United Kingdom
A GLOBETECH PUBLICATION
Published in cooperation with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). LabMedica International • LabMedica en Español • LabMedica.com HospiMedica International • HospiMedica.com • MedImaging.net HospiMedicaExpo.com • LabMedicaExpo.com • LinkXpress.com
Dan Gueron
David Gueron
Sanjit Dutt
Carolyn Moody, RN
Simone Ciolek
Parker Xu
Publisher
Managing Editor News Editor
Regional Director
Regional Director
Regional Director
Karina Tornatore
HOW TO CONTACT US
Subscriptions: Send Press Releases to:
Advertising & Ad Material:
Other Contacts:
www.LinkXpress.com LMNews@globetech.net ads@globetech.net info@globetech.net
ADVERTISING SALES OFFICES
Reader Service Manager USA Miami, FL 33280, USA

Carolyn.Moody@globetech.net Tel: (1) 954-686-0838
GERMANY, SWITZ., AUSTRIA Bad Neustadt, Germany Simone.Ciolek@globetech.net Tel: (49) 9771-1779-007
OTHER EUROPE & UK Miami, FL 33280, USA Carolyn.Moody@globetech.net Tel: (1) 954-686-0838
JAPAN
SUBSCRIPTION INFORMATION
LabMedica lnternational is published eight times a year and is circuIated worldwide (outside the USA and Canada) without charge and by written request, to clinical laboratory specialists and administrators, and other qualified professionals allied to the field.
To all others: Paid Subscription is available for a two-year subscription charge of US$120. Single copy price is US$20. Mail your paid subscription order accompanied with payment to Globetech Media, P.O.B. 800222, Miami, FL 33280-0222.
For change of address or questions on your subscription, write to: LabMedica lnternational, Circulation Services at above address; or visit: www.LinkXpress.com
ISSN 1068-1760
Vol.40 No.3. Published, under license, by Globetech Media LLC; Copyright © 2023. All rights reserved. Reproduction in any form is forbidden without express permission. Opinions expressed are solely those of the authors, and do not represent an endorsement, or lack thereof, by the Publisher of any products or services.
Teknopress Yayıncılık ve Ticaret Ltd. Şti. adına İmtiyaz Sahibi: M. Geren • Yazı işleri Müdürü: Ersin Köklü Müşir Derviş İbrahim Sok. 5/4, Esentepe, 34394 Şişli, İstanbul P. K. 1, AVPIM, 34001 İstanbul • E-mail: Teknopress@yahoo.com
Baskı: Postkom A.Ş. • İpkas Sanayi Sitesi
3. Etap C Blok • 34490 Başakşehir • İstanbul Yerel süreli yayındır. Yılda sekiz kere yayınlanır, ücretsiz dagıtılır.
4 LabMedica International May/2023
Tokyo, Japan Katsuhiro.Ishii@globetech.net Tel: (81) 3-5691-3335 CHINA Shenzen, Guangdong, China Parker.Xu@globetech.net Tel: (86) 755-8375-3877 OTHER COUNTRIES Contact USA Office ads@globetech.net Tel: (1) 954-686-0838 Founder & Editorial Director Marc Gueron LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Image: Schematic diagram of PCR temperature cycle using photothermal effect in polymeric microparticles (Photo courtesy of KSIT)
Electronic Biosensor Detects Biomarkers in Whole Blood Samples Without Addition of Reagents
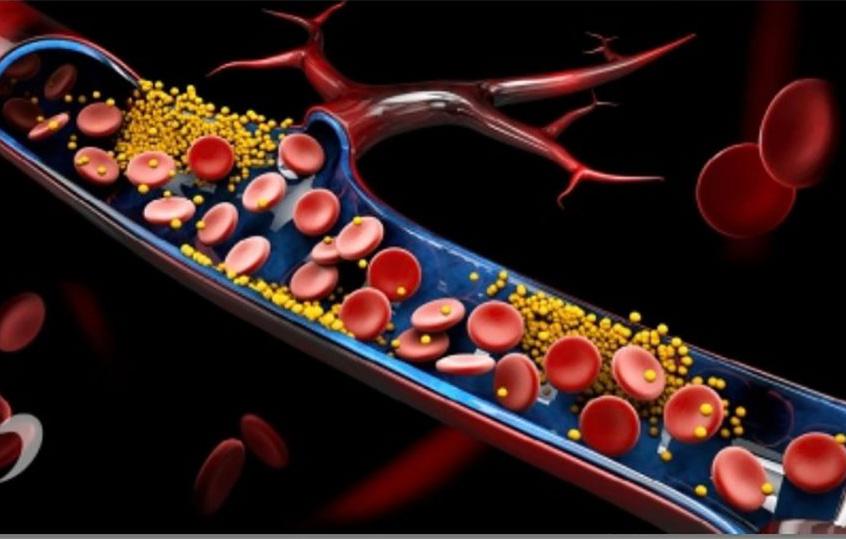
The absence of robust, reliable, and user-friendly bioanalytical tools for early and timely diagnosis of cardiovascular diseases, particularly sudden cardiac arrest, leads to preventable deaths and imposes a significant economic burden on global healthcare systems. Numerous studies have established connections between various cardiovascular diseases and specific protein biomarkers in the blood. However, current methods for analyzing these protein biomarkers, such as ELISA or western blot assays, involve multistep, reagent-intensive processes and require specialized laboratory equipment, limiting their practical applicability and resulting in delayed treatment, reduced compliance, and worse outcomes. Consequently, there is an urgent need for novel methods that enable direct, reagent-free analysis of molecular analytes to identify cardio vascular abnormalities in their early stages and prevent or mitigate their progression.
A team of researchers at University of Toronto (Ontario, Canada; www. utoronto.ca ) and Northwestern University (Evanston, IL, USA; www. northwestern.edu) has designed an electronic biosensor utilizing DNA ap tamers to detect biomarkers in whole blood samples without the need for additional reagents. These DNA ap tamers recognize marker proteins as effectively as antibodies, but they are simpler to produce and more versatile. The biosensor successfully identified clinically relevant levels of a cardiovas cular disease marker protein without further sample preparation.
The researchers’ goal was to create diagnostic tools capable of directly, reliably, and in-field detection of disease biomarkers, eliminating the need to send samples to specialized labs for analysis. The chip-based device developed by the researchers employs chronoamperometric measurements to identify marker proteins in complex samples. Their nanoscale sensor system functions as a molecular “pendulum,” measuring the extra load a protein places on the pendulum, which consists of a DNA strand attached to an electrode, without requiring external reagents.
and inexpensive to produce, and their structures can be customized. Like antibodies, DNA aptamers can bind marker proteins through molecular and structural interactions but are simpler to design.
The researchers developed an aptamer-based sensor by creating a DNA aptamer specific to B-type natriuretic peptide (BNP), a cardiovascular disease biomarker, and connecting it to the DNA pendulum strand tethered to a gold electrode, forming the molecular pendulum sensor. This biosensor effectively detected BNP, even in complex samples such as unprocessed whole blood from cardiac patients. As the sensitivity of the aptamer-based system was found to be comparable to that of antibody-based detection,
the researchers recommend further exploration and adoption of DNA aptamers for laboratory-independent diagnostics.
AMPLIRUN® TOTAL C M Y CM MY CY CMY K
While antibodies are typically used to locate and bind marker proteins in complex mixtures, their complexity makes designing and producing them a challenge. Instead, researchers found that smaller, simpler DNA aptamers can be used as alternatives to antibodies. DNA aptamers are short synthetic fragments with specific shapes and structures, relatively easy
Are
Controls Vircell Anuncio LABMEDICA 140x185 AMPLIRUN TOTAL ZIKV DENV CHIKV_EN.pdf 1 05/04/2023 14:30:05
• Inactivated whole pathogens - Contains the entire genome and is compatible with any analysis.
• Total control in a matrix that mimics a human specimen - For monitoring the entire process.
• Quantified, low positive controls - Prepared to produce results at a significant clinical concentration.
Act confidently with AMPLIRUN® TOTAL to achieve independent assessment of your diagnostic devices and methods, comply with lab accreditation and improve patient care.

• Monodose format and lyophilized presentationIt guarantees stability and avoids handling and additional transport costs.
biosensor uses DNA aptamers for detecting biomarkers in whole blood samples (Photo courtesy of Freepik) info@vircell.com
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 5 LabMedica International May/2023 105 LMI-05-23 LINKXPRESS COM
Electronic
you certain about your molecular assays? AMPLIRUN® TOTAL ZIKV/DENV/CHIKV CONTROL
Independent Molecular
• Non-infectious material for infectious diseases testing
C-PEPTIDE TEST MONOBIND




Pediatric Reference Intervals for Common Cardiovascular Disease Tests
Cont’d from cover
lack of established pediatric reference intervals for hs-cTn and NTproBNP. These intervals, which account for age, developmental stage, ethnicity, and gender, are crucial for accurate test interpretation, as their absence can lead to misdiagnosis and potentially harmful medical care. A groundbreaking study has now defined pediatric reference intervals for these two common cardiovascular disease tests, which is vital for improving heart condition diagnosis and treatment in children.
Researchers at The Hospital for Sick Children (SickKids, Toronto, ON, Canada; www.sickkids.ca) conducted a study to establish pediatric reference intervals for both hs-cTnI and NT-proBNP. They analyzed around 200 blood samples from healthy pediatric patients (ranging from newborns to 18-year-olds) using hs-cTnI and NT-proBNP tests from a major diagnostic manufacturer. Based on the analysis results, the researchers followed the Clinical and Laboratory Standards Institute EP-28A3c guidelines to determine reference limits at the 2.5th, 97.5th, and 99th percentiles.
Importantly, the researchers discovered that hs-cTnI and NTproBNP blood concentrations are significantly higher in newborns, with 99th percentiles at 55.8 ng/L and 1,785 ng/L, respectively. This indicates that test results for hs-cTnI and NT-proBNP not exceeding these levels are normal for newborns, although such levels in adults would suggest cardiovascular disease. This finding could prevent misdiagnosis of heart issues in newborns and highlights the importance of pediatric reference intervals for these tests.

“Lack of evidence-based pediatric reference standards for cardiac biomarker interpretation complicates test interpretation,” said Khosrow Adeli, PhD, and PhD candidate Mary Kathryn Bohn of SickKids, who conducted the study. “The current study establishes comprehensive pediatric reference limits for high sensitivity cardiac troponin I and NT-proBNP in the CALIPER cohort and demonstrates the importance of considering age in interpretation. These data valuably contribute to the limited literature on expected health-associated values for cardiac biomarkers in children and will be helpful to clinical laboratories in interpreting these [increasingly] utilized assays in neonates, children, and adolescents.” The study was published on April 6, 2023 in the AACC’s The Journal of Applied Laboratory Medicine.
High-Sensitivity Troponin I Could Predict Preeclampsia in High-Risk Women
Pre-eclampsia is a dangerous pregnancy complication characterized by high blood pressure that complicates up to 8% of pregnancies, although data on biomarkers that can predict onset and disease severity has so far been limited. Now, a new study has found High Sensitivity Cardiac Troponin I (hs-cTnI) to be significantly higher in pregnant women who go on to develop preeclampsia starting at 14 weeks.
The multi-center observational study by researchers at University Heart Center Freiburg (Freiburg, Germany; www.uniklinik-freiburg.de) involved four international cohorts of pregnant women (SCOPE, MAViS, PRINCE, and OCC) and examined the relation between high-sensitivity troponin and pre-eclampsia onset and severity using the Abbott hs-cTnI assay. The preeclampsia definition varied based on cohort (PRINCE utilizing GSGO guidelines, MAViS and SCOPE utilizing ISSHP, and OCC utilizing DSOG). Severe preeclampsia was defined as preeclampsia requiring delivery <34 weeks gestation; high preeclampsia risk was defined as anyone meeting NICE guidelines for prophylactic aspirin.
A total of 2,293 pregnant women were included in the study, with
Cont’d on page 8
6 LabMedica International May/2023
The C-Peptide AccuBind ELISA Kit is intended for the quantitative determination of circulating C-Peptide concentrations in human serum by the microplate enzyme immunoassay, using the colorimetric
HEMOGLOBIN ONE STEP CARD TEST CERTEST BIOTEC
CerTest FOB one step card test is a colored chromatographic immunoassay for the semi-quantitative determination of human hemoglobin (hHb) in stool samples, offering a simple, highly sensitive and non-invasive screening assay.
202 LMI-05-23 COM
203 LMI-05-23 LINKXPRESS COM
WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
Image: A breaking research could improve cardiac care for children (Photo courtesy of Freepik)
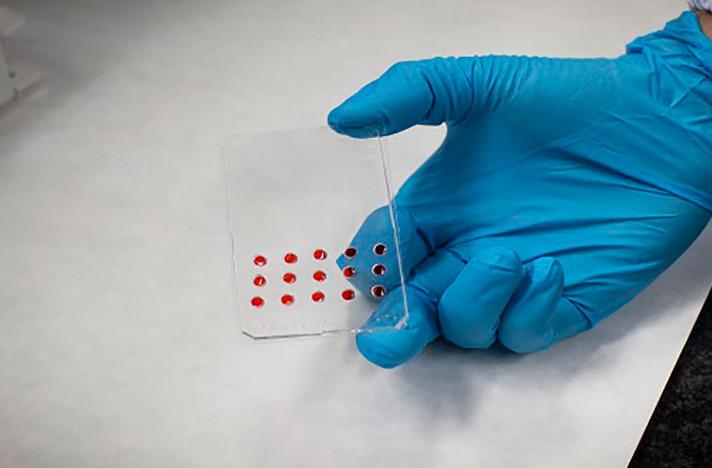
Fully Automated Microfluidic Device Predicts Effectiveness of Cancer Treatment





Designing new technologies for personalized cancer treatment is a significant challenge in the ongoing battle against cancer. Tumors possess unique characteristics and predictive indicators of a patient's response to treatment, based on the tumor's molecular characteristics, such as DNA mutations, is a crucial step forward in oncology. Precision medicine seeks to provide a tailored treatment for cancer patients, both adult and pediatric, that is specific to their pathology. Determining whether a patient can benefit from a particular treatment before initiating therapy could provide a boost to personalized cancer treatment. Now, a microfluidic device can quickly and automatically predict the effectiveness of cancer treatment by using a tiny amount of cells from biopsies and without the need for specialized technical staff.
The microfluidic device called microfluidic dynamic BH3 profiling (μDBP) has been developed by researchers at the University of Barcelona (Barcelona, Spain; https://web.ub.edu) and the Institute for Bioengineering of Catalonia (IBEC, Barcelona, Spain; www. ibecbarcelona.eu). The dynamic BH3 profiling (DBP) was among the first functional assays to be successfully tested for predicting treatment in various types of cancer. This system exposes cancer cells to different therapeutic options to quickly identify the most effective treatment for a patient. This approach is conceptually similar to the use of antibiograms to identify appropriate antibiotics for bacterial infections. However, the μDBP microfluidic device represents a significant improvement over previous DBP systems. This new device requires fewer cancer cells to test potential therapies, automates the process, and can be used without requiring specialized technical staff, making it easier to apply in a clinical setting.


The initial step in testing a biopsy sample involves dissociating it into individual cells using mechanical and enzymatic methods. The processed sample is then filtered to obtain individual cells, which are subjected to the desired treatments before being seeded into the microfluidic device. The use of the μDBP microfluidic platform, which contains small wells for cell seeding, reduces the number of cells required to test a treatment, making it possible to test a larger number of drugs, which is a significant breakthrough. The paper published in the journal npj Precision Oncology is the first to use microfluidics for performing the functional assay of the DBP. Unlike other versions of the assay that require expensive machinery and specialized staff, such as the high-throughput DBP with automated plates and dispensers that test hundreds of treatments, the new μDBP device is designed to test treatments in situ quickly, easily, and automatically, without the need for expensive machinery or specialized staff. The team is currently working on a new prototype of the μDBP device that incorporates technical improvements and aims to gather more experimental evidence with primary samples to demonstrate its clinical usefulness in improving the treatment of various cancers, both pediatric and adult.


“The biggest advantage of the μDBP device is also the automation of the whole process, which would help to implement this functional methodology on a clinical scale. All these advantages would ease the adoption of DBP in hospitals as a routine trial,” according to the researchers.


“DBP has been used to identify the efficacy of treatments on a preclinical and clinical scale in many different cancers, both solid and liquid. These studies have used cell lines, animal models and primary samples with high predictive ability in all cases. However, this assay has not yet been widely applied in hospitals,” said lecturer Joan Montero. “So far, several studies have found a good correlation between DBP results and clinical response in primary leukemia samples. There are currently several clinical trials underway, and we would like this technology to be implemented in hospitals in the coming years to improve cancer therapies.”














LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 7 LabMedica International May/2023
107 LMI-05-23 LINKXPRESS COM
Image: New microfluidics physics-based device to predict cancer therapy response (Photo courtesy of University of Barcelona)
ApoB Protein Improves Cardiac Risk Prediction
Cont’d from cover

a protein responsible for transporting fat molecules including LDL cholesterol - notoriously known as the “bad cholesterol” - throughout the body, may be a more precise indicator of atherosclerotic cardiovascular disease.
In a new study presented at the 2023 American College of Cardiology Annual Scientific Sessions in New Orleans, researchers from Intermountain Health (Salt Lake City, UT, USA; www. intermountainhealthcare.org) found that ApoB testing can help identify patients who may continue to face a higher risk for a cardiovascular event, despite their normal LDL cholesterol levels. ApoB testing remains fairly uncommon, but is on the rise. ApoB levels measure atherogeneic particle numbers, and several studies suggest that particle numbers are superior than cholesterol levels as risk predictors of disease. An improved assessment of particle numbers could make ApoB better at evaluating risk, particularly for patients having normal LDL cholesterol levels, including those with metabolic syndrome, like diabetes or prediabetes or low HDL and high triglyceride levels.
The researchers conducted a retrospective study by analyzing electronic health records of patients from 2010 to February 2022. The study revealed that the number of Apo B tests administered increased from 29 cases in 2010 to 131 in 2021. Additionally, the team observed a positive correlation between ApoB and LDL cholesterol levels, though the ApoB/LDL cholesterol ratio rose as LDL cholesterol decreased. This indicated a high number of atherogenic small, dense LDL particles characterized by smaller amounts of LDL cholesterol per particle. However, the researchers do not expect ApoB to replace standard HDL and LDL testing soon, as it is costlier and not yet firmly established in the healthcare system. Nevertheless, clinicians should increasingly consider ApoB testing a valuable tool for refining cardiovascular risk, particularly in specific patient groups.

“Testing for ApoB doesn’t tell you how much cholesterol a patient has, but instead it measures the number of particles that carry it,” said Jeffrey L. Anderson, Intermountain Health cardiologist and principal investigator of the study. “While it’s still not a commonly ordered test, we found that it’s both being used more often, and it could lead to a more accurate way to test for lipoprotein-related risk than how we do it now.”
Image: ApoB may be a more accurate risk predictor of atherosclerotic cardiovascular disease (Photo courtesy of Intermountain Health)
High-Sensitivity Troponin I Could Predict Preeclampsia in High-Risk Women
Cont’d from page 6


7.8% of them developing preeclampsia and 0.9% developing severe preeclampsia. Of the total, 17.3% were identified as high-risk prior to the start of the study, and the average age of the women was 32 years. Women who developed preeclampsia had a higher prevalence of hypertension, diabetes, African American ancestry, and a history of multiple pregnancies. hs-cTnI levels were persistently high in women who ultimately developed preeclampsia, starting from 14 weeks (and lasting up to 29 weeks) of gestational age (all p<0.001) in the women who ultimately developed preeclampsia (all p<0.001). A hs-cTnI cutoff value of >2.2pg/ml at 14 weeks and >2.6pg/ml at 26 weeks was found to have a combined 100% negative predictive value in predicting severe preeclampsia in women who had a high a priori risk (meeting NICE criteria). Additionally, a hs-cTnI cutoff value of >1.8pg/ml at 14 weeks and >1.9pg/ml at 26 weeks predicted the presence of any preeclampsia.
“Elevated levels of hs-cTnI may precede the development of preeclampsia….and may also have considerable potential in grading preeclampsia risk [and severity] in pregnant women…a randomized, prospective trial needs to assess the potential benefits,” stated Dr. Dirk Westermann from the University Heart Center Freiburg who led the research team.
8 LabMedica International May/2023
MINI CENTRIFUGE GREINER BIO-ONE
The EU PLUG Mini Centrifuge, intended only for indoor use, for separating aqueous solutions and suspensions of various densities. It is designed for quick spin downs of approved microcentrifuge tubes (0.2
PORTABLE TEST READER GRIFOLS DIAGNOSTIC
PQreader is a portable test reader intended for in vitro diagnostic use in the detection and/or quantification of target analytes on lateral flow test strips. It is compatible with Promonitor Quick cassettes.
205 LMI-05-23 COM
206 LMI-05-23 LINKXPRESS COM
to
WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
To receive prompt and free information on products, log on
www.linkXpress.com or scan the QR code on your mobile device
Novel Testing Method Detects HIV and Hepatitis B/C from Single Drop of Blood

very year, over one million people succumb to hepatitis B or hepatitis C, while 650,000 die from HIV-related causes and 1.5 million contract HIV. The World Health Organization aims to eliminate all three viruses by 2030, requiring new testing methods to decrease case numbers. The most common test for hepatitis B, hepatitis C, and HIV involves drawing blood from a vein with a needle. However, this approach is not always suitable in prisons, drug rehabilitation centers, and homeless shelters or in countries where blood sample shipping and refrigerated storage pose challenges. Alternative methods, such as dried blood spot tests, can detect nucleic acid from the three viruses in a single blood spot.
At this year's European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) in Copenhagen, Denmark, (April 15–18), researchers at Copenhagen University Hospital (Copenhagen, Denmark; www.rigshospitalet.dk) presented data on a test capable of detecting HIV, hepatitis B, and hepatitis C using a single drop of blood. The test involves pricking the individual's finger, collecting a few blood spots on filter paper, and allowing them to dry. The Hologic Panther System, commonly found in public health laboratories, then employs transcription mediated amplification to analyze one blood spot for genetic material from the three viruses.
The researchers assessed the test by analyzing 20 samples containing known amounts of HIV, hepatitis B, and hepatitis C using the dried blood spot method (60 samples in total) and successfully detected the viruses in all samples. Plasma dilution determined the lower detection limit, revealing the capability to detect the viruses at levels significantly lower than those typically found in untreated patients.
Image: A study has shown that HIV and hepatitis B & C can be detected from a single drop of blood (Photo courtesy of Freepik)
“We've shown that using existing hospital equipment, it is possible to detect HIV, hepatitis B and hepatitis C from a single drop of blood,” said Stephen Nilsson-Møller at the Department of Clinical Microbiology, Copenhagen University Hospital. “The dried blood spot test is ideal for places where you don't want to use a needle for safety reasons or where it is less practical. It is also suitable for developing countries or places where you run the risk of a blood sample being ruined before it is transferred to a laboratory that can analyze it. Blood samples need to be analyzed within six hours when kept at room temperature, while dried blood spots can last for nine months without refrigeration.”
‘Glow-in-the-Dark’ Proteins Diagnose Viral Diseases with Greater Speed and Ease
Despite undergoing technological advancements, most diagnostic tests for viral diseases that are highly sensitive still involve complex techniques to prepare a sample or interpret the results, making it hard to administer these tests in point-of-care settings or in locations with limited resources. Now, a team of researchers has come up with a sensitive technique that analyzes viral nucleic acids in as little as 20 minutes and can be finished in one step using “glow-inthe-dark” proteins.
Bioluminescence is a scientific phenomenon caused by a chemical reaction involving the luciferase protein that creates the luminescent, glow-in-the-dark effect. The luciferase protein has been utilized in creating sensors that emit observable light when they detect their target, making them perfect for point-of-care testing. Nonetheless, these sensors lack the high levels of sensitivity required of a clinical diagnostic test. A gene-editing method known as CRISPR has shown promise in providing this ability, although it requires multiple steps and additional specialized instruments to de-
tect low signals from a complex and noisy sample. So, researchers at Eindhoven University of Technology (Eindhoven, Netherlands; www. tue.nl) aimed to use CRISPR-related proteins, but combine them with a bioluminescence technique whose signal could be detected with only a digital camera.

In order to ensure that there were sufficient RNA or DNA samples for analysis, the scientists employed recombinase polymerase amplification (RPA), which is a straightforward technique that operates at a constant temperature of around 100 F. The researchers devised a new technique, LUNAS (luminescent nucleic acid sensor), that is comprised of two
Cont’d on page 10
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 9 LabMedica International May/2023
109 LMI-05-23 LINKXPRESS COM
E
Image: Proteins that glow bright blue or green could make disease diagnosis quicker and easier (Photo courtesy of Eindhoven University of Technology)
Novel Biosensor to Detect DNA Damage in Real Time Could Revolutionize Cancer Treatment
Touble-strand breaks (DSBs) are a form of DNA damage in which both strands of DNA break at the same place, adversely affecting cell growth and functioning. Presently, immunostaining techniques are utilized to detect DSBs by identifying the markers accompanying DNA damage, such as the γH2AX protein. Nonetheless, these processes are laborious and incapable of recognizing DSBs in real-time in living specimens.
Scientists from Pusan National University (Busan, South Korea; www.pusan.ac.kr) have invented a new biosensor that utilizes fluorescence resonance energy transfer (FRET) to spot DNA double-strand breaks in real-time in living specimens. The biosensor has the potential to transform cancer treatment by providing doctors with an understanding of how cells respond to therapeutic treatments and also aid in the discovery of new DNA repair drugs. Moreover, the new biosensor can be instrumental in discovering new treatments for DNA damage-related diseases by providing insights into how the human body repairs damaged DNA.
In a study, published on February 17, 2023 in Biomaterials Research, researchers
describe a FRET biosensor that is capable of detecting DSBs in real-time, and providing time- and location-based data on yH2AX. The FRET sensor comprises two fluorescent proteins or dyes - a donor and an acceptor - which examine the interactions between biological molecules. The energy transfer, and consequently, the amount of emitted light (the FRET signal) depends on the distance and orientation between the two dyes. The research team attached the fluorescent dyes with proteins involved in the cellular response to DNA damage, namely the H2AX substrate and BRCT1 domain. The H2AX substrate is a target for the H2AX protein to bind and become phosphorylated (forming γH2AX).
On the other hand, the BRCT1 domain serves as a site for the collection of repair proteins, including γH2AX. Thus, when a DSB occurs, γH2AX is attracted to the BRCT1 domain, resulting in a conformational change in the fluorescent proteins, thus resulting in a change in the FRET signal. The researchers then proceeded to confirm the validity of the sensor by introducing plasmids (DNA that, here, contains instructions to make the FRET sensor inside
the cells) encoding the FRET sensor into human embryonic kidney cells (HEK293T) cells. The biosensor was found to be more sensitive at reacting to the presence of γH2AX than conventional immunostaining techniques, thus making it better at detecting drug- and radiation-induced DSBs.
“The biosensor we have designed could be useful in areas such as cancer treatment and drug discovery,” said Associate Professor Tae-Jin Kim, from Pusan National University, Korea, who led the study. “Moreover, as changes in the FRET signal give useful indications of the extent of the DNA damage, the sensor can also be used to examine DNA damage and repair mechanisms, optimize cancer treatments, discover and assess DNA repair drugs, and identify DNA damaging factors in the environment.”
‘Glow-in-the-Dark’ Proteins Diagnose Viral Diseases with Greater Speed and Ease
Cont’d from page 9



CRISPR/Cas9 proteins specific for different neighboring parts of a viral genome each have a distinct fragment of luciferase attached to them. Upon detecting the presence of a specific viral genome being tested, the two CRISPR/ Cas9 proteins bind to the designated nucleic acid sections and are drawn close to one an-
other, enabling the complete luciferase protein to form and shine blue light in the presence of a chemical substrate.

To take into account the depletion of the substrate, the researchers utilized a control reaction that radiated green light. The presence of a positive result was indicated by a tube that changed from green to blue. The
RPA-LUNAS technique successfully detected SARS-CoV-2 RNA in clinical samples obtained from nasal swabs in just 20 minutes, even at concentrations as low as 200 copies per microliter. The researchers believe that the LUNAS assay holds immense potential for quickly and efficiently detecting various other viruses.
10 LabMedica International May/2023
POINT-OF-CARE READER HEMEX HEALTH
Gazelle is an inexpensive, portable, and easy-to-use diagnostic device that provides accurate testing at sites where it is needed most. The compact, battery-operated device is used inexpensively, with no cold chain requirements.
ONE STEP IVD CARDIAC TEST HUMASIS
208 LMI-05-23 COM 209 LMI-05-23 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
HUBI Cardiac DUO is a one-step IVD test based on immunochromatography, designed for the quantitative determination of CK-MB, Cardiac Troponin I in human whole blood, plasma or serum. It is intended for use with the HUBI QUAN pro.
Image: The biosensor could help optimize cancer treatment, identify DNA damage factors, and elucidate repair mechanisms (Photo courtesy of Pexels)




LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
111 LMI-05-23 LINKXPRESS COM
Breakthrough Test Offers Earlier Detection of Parkinson’s Disease
New Blood Test Identifies Type 2 Diabetes Risk by Analyzing DNA Changes
Type 2 diabetes is a medical condition that arises when the insulin produced by the pancreas fails to function properly or is inadequate. This can lead to increased levels of blood sugar and subsequently, a host of health complications such as heart diseases, stroke, nerve damage, and foot problems. At present, risk assessment tools for type 2 diabetes rely on factors like age, gender, body mass index (BMI), and family history of the disease. Now, a new study indicates that by analyzing changes in the DNA present in blood samples, it is possible to significantly improve the ability to predict the likelihood of an individual developing type 2 diabetes within a decade.

Methylation is a chemical process in the body in which a small molecule called a methyl group is added to DNA. Scientists at the University of Edinburgh (Edinburgh, Scotland, UK; www.ed.ac.uk) examined how these alterations, in combination with other risk factors, could predict the probability of developing type 2 diabetes in almost 15,000 individuals long before any symptoms appear. The researchers found that incorporating DNA methylation data along with conventional risk factors improved the prediction accuracy.
To evaluate the predictive performance, the scientists adopted a hypothetical screening scenario involving 10,000 people where onethird of them developed type 2 diabetes over ten years. The model that incorporated DNA methylation accurately classified an additional 449 individuals compared to using traditional risk factors alone. The addition or removal of these methyl groups can impact the manner in which specific molecules act in the human body. These methylation patterns can be used to monitor the aging process and disease development. These findings could potentially enable the implementation of preventative measures earlier, thus lessening the health and economic burden of type 2 diabetes.
“Similar approaches could be taken for other common diseases to generate broad health predictors from a single blood or saliva sample,” said Professor Riccardo Marioni, Principal Investigator for the study, Centre for Genomic and Experimental Medicine at the University of Edinburgh. “We are incredibly grateful for our study volunteers who make this research possible – the more people that join our study, the more precisely we can identify signals that will help delay or reduce the onset of diseases as we age.”
Image: A new test could help identify type 2 diabetes risk (Photo courtesy of Pexels)
Novel Reference Materials Meet Needs of Labs Testing for BRCA1/2 Gene Variants
Testing of the tumor suppressor genes BRCA1 and BRCA2 for genetic variants can identify DNA variations linked to significantly increased lifetime risks of breast, ovarian, pancreatic, and prostate cancers. Large genomic rearrangements (LGRs), which involve deletions, duplications, and insertions often affecting whole exons, account for up to 27% of BRCA1 and 5% of BRCA2 disease-causing mutations in populations with a strong founder effect. LGRs, typically pathogenic, are difficult to detect and are often overlooked by PCR-based and targeted NGS assays that do not identify partial or complete exon losses or gains. Nevertheless, accurately detecting BRCA1 or BRCA2 pathogenic variants is crucial for clinical disease management, particularly regarding patients’ eligibility for PARP inhibitor treatments.
LGC Clinical Diagnostics (Milford, MA, USA; www. lgcclinicaldiagnostics.com) has released its Seraseq BRCA1/2 Large Genomic Rearrangements Reference Materials to assist clinical laboratories in developing, characterizing, validating, and routinely assessing NGS assays. These innovative reference materials are designed to meet the needs of clinical laboratories testing for BRCA1/2 gene variants at both somatic and germline levels. They contain 20 pathogenic BRCA1 and BRCA2 variants, including 11 exon-level large rearrangements, with 10 variants each in BRCA1 and BRCA2.



These variants range in size from single nucleotide variants (SNVs)
Cont’d on page 13

12 LabMedica International May/2023
LAB REFRIGERATOR HELMER SCIENTIFIC
The iLR120-GX lab refrigerator is a single door, upright model that provides optimal conditions for storing reagents, samples, and controls, with tight temperature uniformity and reliable performance that ensures
TSH (THYROID STIMULATING HORMONE) TEST GOLDSITE DIAGNOSTICS
The Kemilo TSH Kit is used on the Kemilo CLIA platform for the quantitative determination of TSH (Thyroid Stimulating Hormone). It uses an all-in-one cartridge for a single test and is based on the classic CLIA technology.
211 LMI-05-23 COM
To receive
information on products, log on to www.linkXpress.com
device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
212 LMI-05-23 LINKXPRESS COM
prompt and free
or scan the QR code on your mobile
AI Model Analyzes Cells in Tissue Samples Without Need for Trained Pathologists
suming, and resource-intensive. Now, scientists have developed a new technique that can perform a reliable analysis of solid tumors in as little as 30 minutes, without the need for a trained pathologist.
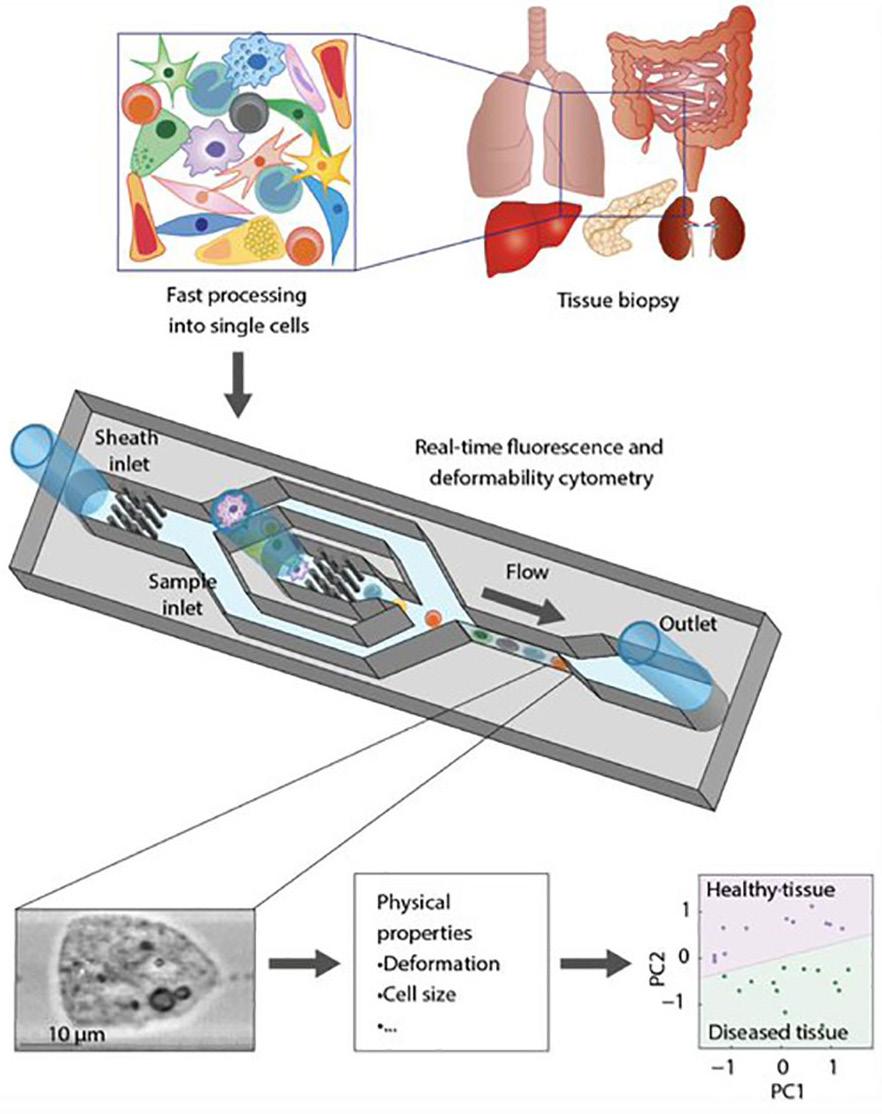
A research team from the Max Planck Institute for the Science of Light (MPL, Erlangen, Germany; www.mpl.mpg.de) has created a novel technique that enables clinicians to analyze cells in tissue samples from cancer patients quickly and precisely, without requiring the expertise of a trained pathologist. The team used artificial intelligence (AI) to evaluate the data generated by their method. For their study, the researchers utilized a tissue grinder to quickly tear apart biopsy samples down to the single-cell level. Subsequently, these single cells were analyzed using real-time deformability cytometry (RT-DC), an approach that is label-free and capable of examining the physical properties of up to 1,000 cells per second. This method is 36,000 times faster than the conventional methods used to evaluate cell deformability.
RT-DC involves pushing single cells at high speed through a microscopic channel, where they undergo deformation due to stress and pressure. Images are captured of each cell, which are then utilized by scientists to ascertain a variety of physical characteristics of the cells, including their size, shape, and deformability. However, solely conducting a physical analysis of cells is insufficient for diagnostic purposes. Physicians must be able to interpret these outcomes independently, without the need for the expertise of a trained pathologist or physicist. Therefore, to accomplish this, the researchers combined the tissue grinder and RT-DC with AI. The AI model evaluates the extensive, complex datasets obtained through RT-DC analysis and rapidly assesses whether a biopsy sample comprises cancerous tissue or not. Furthermore, the use of AI confirmed the significance of cell deformability as a biomarker, as the outcomes were markedly inferior when the AI was not trained with this variable.
Overall, the complete procedure, which includes sample processing and automated data analysis, can be executed in under 30 minutes, making it sufficiently fast to be carried out during surgery. One significant advantage of this method is that it does not require the immediate availability of a pathologist to analyze the sample. This is particularly advantageous since intraoperative consultations may not always be feasible, and in some cases, samples can only be examined after the surgery is completed. Based on the results, patients may need to return to the hospital for further surgery, often days later. Aside from testing for tumor presence, this technique was also utilized to detect tissue inflammation in a model of inflammatory bowel disease (IBD). In the future, this method could assist clinicians in evaluating disease severity or distinguishing between various types of IBD. The team aims to eventually transition their method into a clinical setting to support or perhaps even supplant the traditional
Novel Reference Materials Meet Needs of Labs Testing for BRCA1/2 Gene Variants
Cont’d from page 12
to insertions and/or deletions over 500 bp, encompassing missense, nonsense, frameshift, stop-gain/loss, splice-site, and insertion/deletion of partial or up to two exons, resulting in diverse alterations at the amino acid level. The products combine BRCA variants in the well-characterized GM24385 genomic background at clinically relevant allele frequencies, precisely quantitated by digital PCR and further analyzed by NGS. They are available in either formalin-fixed, paraffin-embedded (FFPE) format (for somatic testing) or purified genomic DNA (gDNA) format (for germline or somatic testing).

pathological analysis.
“This was a proof of concept study - the method could accurately determine the presence of tumor tissue in our samples very quickly,” said Dr. Despina Soteriou, a member of the research team. “The next step will be to continue to work very closely with clinicians to determine how this method can best be translated into the clinic.” The study was published on April 6, 2023 in the journal Nature Biomedical Engineering.
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 13 LabMedica International May/2023
from cover 113 LMI-05-23 LINKXPRESS COM
Cont’d
Image: The tissue biopsy is processed through a tissue grind and then analyzed using real-time deformability cytometry (Photo courtesy of MPL)
AI-Based Method Could Replace Chemical Staining of Histopathological Tissue Samples
For over a century, chemical staining has been a foundational technique in the study of histopathology, particularly in areas like cancer diagnostics. One major drawback of chemical staining, however, is its irreversible nature, which often restricts the sample's use in other tests or experiments. To address this limitation, researchers have developed an artificial intelligence (AI)-based approach to virtually stain histopathological tissue samples, potentially replacing the need for chemical staining.

A study led by researchers from the University of Eastern Finland (Kuopio, Finland; www.uef.fi) has resulted in the development of an AI method that generates computational images closely resembling those obtained through actual chemical staining. These virtually stained images can then be examined for tissue morphology. The virtual staining technique not only reduces the chemical load and manual labor required for sample processing but also allows the tissue to be used for other purposes beyond staining. A key advantage of the proposed method is that it only requires a standard light microscope and an appropriate computer, with no need for specialized hardware or infrastructure.
The rapid advancement of deep neural networks, which learn from vast amounts of data, has revolutionized biomedical image analysis. These methods are not only suitable for traditional image analysis tasks, such as interpretation, but also excel in image-to-image transformations. Virtual staining exemplifies this type of task and was effectively demonstrated by the research team.
“The results are very widely applicable. There are plenty of topics for follow-up research, and the computational methods can still be improved. However, we can already envision several application areas where virtual staining can have a major impact in histopathology,” says Associate Professor Pekka Ruusuvuori from the University of Turku, who led the computational part of the study.
“Deep neural networks are capable of performing at a level we were not able to imagine a while ago. Artificial intelligence-based virtual staining can have a major impact towards more efficient sample processing in histopathology,” said Doctoral Researcher Umair Khan from the University of Turku who was the lead developer.
Simple Blood Test Predicts Neuroendocrine Tumor Response to Radiopharmaceutical Therapy

Biomarkers have been employed to forecast treatment outcomes for breast, prostate, and other types of cancer, although no objective methods currently exist for predicting the success of radiopharmaceutical therapy for neuroendocrine tumors. Now, a new study has revealed that a simple blood test can supply doctors with crucial information to ascertain if peptide receptor radionuclide therapy (PRRT) is likely to be effective in patients with neuroendocrine cancer. The blood-based biomarker PPQ can accurately predict PRRT responsiveness in 96% of patients, and changes in another biomarker, NETest, correlate with PRRT response in 90% of cases.
Previously, a group of nuclear medicine physicians at Memorial Sloan Kettering Cancer Center (MSK, New York, NY, USA; www.mskcc. org) had introduced the blood-based biomarkers PPQ and NETest as indicators for the success of PRRT treatment. In this new study, the team aimed to validate the significance of PPQ and NETest in predicting and monitoring PRRT response. The new study included 67 patients with somatostatin receptor-positive gastroenteropancreatic and lung neuroendocrine tumors, all of whom had metastatic disease and prior treatments. The participants submitted blood samples before each PRRT cycle and during follow-up. PPQ was scored as either positive (likely to respond) or negative (unlikely to respond), while NETest was measured on a scale of zero to 100, with 20 being the upper limit of normality. Of the 67 patients, 40 were classified as PPQ+ and 39 of them (98%) responded to PRRT. Among the 27 PPQ- patients, 25 experienced disease progression despite PRRT. The overall predictive accuracy of PPQ was 96%. Prior to PRRT therapy, all patients exhibited elevated NETest levels. In PRRT responders, baseline NETest levels were 67, decreasing by 37% after treatment. For non-responders, baseline NETest levels were 44, which rose by 76% during follow-up. NETest's accuracy in determining PRRT response was 90%.

“The results of this study demonstrate that not all tumors are created equal; some are more prone to respond to PRRT, and some are less susceptible to radiopharmaceutical therapy,” said Lisa Bodei, MD, PhD, nuclear medicine physician at Memorial Sloan Kettering Cancer Center. “This is the reason why our research is important: to understand from the start which patients will require an intensified treatment plan and which patients, instead, can benefit from a lighter regimen will tremendously improve their management.”

14 LabMedica International May/2023
MALARIA ANTIGEN TEST LUMIQUICK DIAGNOSTICS
The QuickProfile Malaria pf/pv Antigen Test Card is a qualitative immunochromatographic assay for rapid detection and differentiation of P. falciparum-specific HRP-2 and P. vivax-specific LDH in human whole blood specimens.
AUTOMATIC CHEMILUMINESCENCE ANALYZER MACCURA BIOTECHOLOGY
214 LMI-05-23 COM 215 LMI-05-23 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
The i800 automatic chemiluminescence analyzer uses magnetic particle based acridinium ester direct chemiluminescence technology. It has the fastest analysis speed of 200 T/H and offers 20 refrigerated reagent positions.
Smartphone-Powered Lipid Test Enables Early Detection of CVD

Cont’d from cover




by facilitating accurate blood testing via a smartphone or tablet, with results accessible through an app.
PocDoc (Cambridge, UK; www.mypocdoc.co.uk) has pioneered a groundbreaking smartphone-based lipid test capable of delivering a 5 marker lipid panel via the PocDoc app in under six minutes, with results instantaneously shared with the healthcare system. This technology allows lipid testing to extend beyond the confines of general practice surgeries, thereby drastically enhancing accessibility to testing and subsequently preventing more individuals from developing CVD.

Despite its importance, comprehensive 5 marker cholesterol testing is a significant hurdle in diagnosing and treating CVD. The PocDoc Lipid test is the world’s first app-based 5 marker lipid test that checks for five biomarkers, including HDL, Non-HDL, Triglycerides, and Total Cholesterol. By
delivering results almost instantly via a mobile app, the

test significantly reduces the time to treatment. The quantitative lateral flow test is capable of expanding access to CVD testing and follow-up treatment, which poses a significant challenge globally.






Early Screening Test for Pancreatic Cancer
tional treatment options. Globally, the 5-year survival rate is around 9%. Nevertheless, an early diagnosis significantly improves this survival rate. Now, a first-in-class screening test based on novel microbiome markers is under development for this deadly cancer.

Mainz Biomed N.V. (Mainz, Germany; www.mainzbiomed.com) and Microba Life Sciences (Brisbane, Australia; www.microba.com) have entered into a research collaboration for conducting a pilot study that uses Microba’s exclusive metagenomic sequencing technology and bioinformatic tools, with the aim of identifying new microbiome biomarkers for the detection of pancreatic cancer. Mainz Biomed is already progressing with an early-stage pancreatic cancer screening test, known as PancAlert. The test uses multiplex real-time PCR to identify genetic biomarkers in stool samples, and it is expected that this approach will be enhanced by incorporating microbiome biomarkers.
The project, scheduled to continue until the end of 2023, will employ Microba’s Community Profiler (MCP), a unique metagenomic platform technology. MCP has proven to be a top-tier research tool, able to generate detailed and precise species profiles of human gastrointestinal samples. The microbiome offers a plethora of medically relevant biomarkers that can be harnessed for therapeutic development or creating diagnostic tools. As the gut microbiome is adjustable, informed clinicians can enhance the biomarkers linked with treatment response. Microba’s biomarker discovery method employs its robust analysis platform and state-of-the-art informatic strategies to ensure superior resolution, access to new uncultured bacteria, and minimal false positives. Its artificial intelligence capabilities allow it to swiftly pinpoint diagnostic microbiome signatures from vast datasets.
“We are excited by the opportunity to collaborate with Microba as PancAlert is being developed for early-stage disease detection with the goal of being a first-in-class screening test for this deadly form of cancer,” said Guido Baechler, Chief Executive Officer of Mainz Biomed. “Given the growing understanding of the microbiome’s role in pancreatic cancer, we believe it’s of paramount importance to explore integrating diagnostic microbiome biomarkers into the test as it advances to the clinical stage of development and as such, are delighted to align with a global leader in sourcing and analyzing microbiome generated species and datasets.”

15 LabMedica International May/2023 LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Size: 2400 x 1200 mm (3 mm thick) 100% Silicone YOUR GLOBAL SOURCE FOR STERILIZATION ACCESSORIES Thermo-Resistant (- 60 °C to 300 °C) Fully Washable & Flexible Suitable for central sterilization services Sterilizable STERILIZABLE INSTRUMENT & WORK-SURFACE MATS Front Back WASHING TRAYS MAT Heavy Silicone Cover & Transport Tablet TURBO WASHING MACHINES TRAYS SILICON INSTRUMENT MAT Front Back MICRO INSTRUMENT MAT Exchangable Net Exchangable Nets INVITEDTOAPPLY DISTRIBUTORS Up to 37 cm in length THERMO RESISTANT GLOVES Front Back WASHING TRAYS MAT NEW! VICOTEX Place de la Gare 1 • 1009 Pully • Switzerland Tel: (41) 21-728-4286 • Fax: (41) 21-729-6741 E-Mail: contact@vicotex.com www.vicolab.com S.A. SILICONE TABLET
COVER NETS 115 LMI-05-23 LINKXPRESS COM
Cont’d from cover
AND STEEL
Image: PocDoc offers the world’s first app based five marker lipid test (Photo courtesy of PocDoc)
PocDoc Lipid
LeadCare II is the only CLIA waived POC test for blood lead poisoning. It can screen with a simple finger-stick in just three minutes. The system includes the LeadCare II Blood Lead Analyzer and the LeadCare II Test Kit.
Device Converts Smartphone into Fluorescence Microscope for Just USD 50
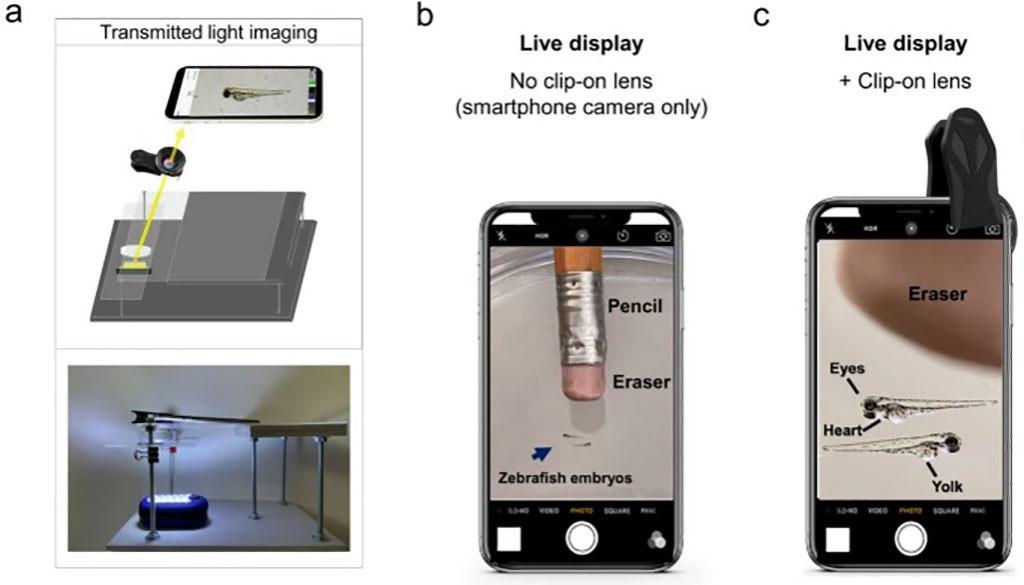
Fluorescence microscopes are utilized to examine specimens labeled with fluorescent stains or expressing fluorescent proteins, like those tagged with green fluorescent protein. However, since these microscopes come with a hefty price tag of several thousand US dollars, their use is typically limited to well-funded research labs. Now, a new device called the “Glowscope” can transform a smartphone or tablet into a fluorescence microscope for less than USD 50. This device allows for imaging of cells, tissues, and organisms under low magnification and can be used in schools, science outreach settings, and even in research labs.
The glowscope, devised by scientists at Winona State University (Winona, MN, USA; www.winona.edu), is built using materials like plexiglass and plywood frame, a clip-on camera lens, an LED torch, and theatre stage lighting filters. Using the frame, a smartphone or tablet is positioned above a specimen while the lens is clipped onto the phone or tablet camera to enable magnification. The specimen is illuminated by the LED torch and a lighting filter is placed over the lens to filter out unwanted wavelengths of light, allowing for visualization of fluorescent light emitted by the specimen.
The scientists used live zebrafish embryos which are between two to three millimeters long and express fluorescent proteins in the spinal cord, cardiac tissue, or hindbrain to demonstrate the efficiency of the glowscope. The clip-on lens provided an approximately fivefold magnification and could image green and red fluorescent tissues
BOND-PRIME is a fast, advanced staining solution, delivering all elements of Universal Access – able to handle any slide, in any combination, at any time, using any reagent. Single or STAT slides can be turned around in one hour.

with a resolution of up to 10 micrometers which is adequate to view individual pigment cells. With the glowscope, the scientists were able to measure the embryos’ heart rates and even observe the movements of individual heart chambers after enhancing the clarity of the videos using free software.
With the materials cost ranging from USD 30 to USD 50, the glowscope presents an affordable alternative to expensive fluorescence microscopes. Scientists have suggested that school students could use glowscopes to study behavior, physiology, development, genetics, and anatomy in small organisms expressing fluorescent proteins that could be obtained from research labs. Additionally, research labs without access to multiple fluorescence microscopes could acquire video data by simultaneously utilizing several glowscopes and smartphones, according to the scientists.
Rapid POC Sensing Kit to Determine Gut Health from Blood Serum and Stool Samples

Digestive disorders represent a significant health burden in our society, with chronic gut inflammation and microbial imbalances playing a critical role in obesity, cardio-metabolic diseases, premature aging, and cancer. Indole-3-propionic acid (IPA), a small metabolite generated by human gut bacteria, has emerged as a vital marker of gut health due to its regulation of the gut’s immune response and suppression of excessive inflammation. Patients experiencing active gut inflammation exhibit reduced IPA levels, while recovery from inflammation leads to a restoration of IPA concentrations. Now, researchers are developing the first-ever sensor for the instant detection of IPA in blood serum and stool samples.
A research team from the Singapore-MIT Alliance for Research and Technology (SMART, Singapore; www.smart.mit.edu) plans to use portable spectrometers previously developed by SMART’s Disruptive &



Sustainable Technologies for Agricultural Precision (DiSTAP) for other biological applications like non-invasive plant health monitoring, to quickly measure IPA levels in blood or stool samples. This advancement will streamline the testing process since traditional IPA analysis involves conventional mass spectrometry-based methods and specialized laboratory equipment.
“Nutrition, microbiome and anti-inflammatory approaches have received great attention in recent years and are hailed as some of the most promising research areas that will address the country’s healthcare challenges,” said Dr. Mervin Chun-Yi Ang, SMART DiSTAP Associate Scientific Director. “Building on SMART DiSTAP’s success in customising portable spectrometers for different agricultural applications, we are excited to further develop these tools and see how they can be also applied to healthcare and medicine.”
16 LabMedica International May/2023
POC BLOOD LEAD POISONING TEST MERIDIAN BIOSCIENCE
ADVANCED STAINING SOLUTION LEICA BIOSYSTEMS
217 LMI-05-23 COM 218 LMI-05-23 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Edited by Katherina Psarra, MSc, PhD
Winners of 2023 IFCC Distinguished Awards Announced
TheIFCC Distinguished Awards are bestowed to laboratory medicine professionals to recognize their outstanding achievements, publicize their exceptional research and contributions to medicine and healthcare, and encourage the overall advancement of clinical chemistry and laboratory medicine.
On occasion of the IFCC WorldLab-EuroMedLab Congress in Rome from 21 to 25 May 2023, four IFCC Distinguished Awards are to be bestowed during the Awards and Speaker Dinner, in magnificent Villa Dino on the ancient roman road “Appia Antica”.
During the Ceremony, the four IFCC Distinguished Awardee and their sponsors are to be congratulated and cherished up by their colleagues. They will receive a Plaque from the hands of the IFCC President, Prof Khosrow Adeli, and by the chair of the IFCC Awards Committee, Prof Maurizio Ferrari.
Dr Robert H. CHRISTENSON (United States) is the winner of the 2023 IFCC Henry Wishinsky Award for Distinguished International Services, sponsored by Siemens.


This award recognizes specifically an individual who has made unique contributions to the promotion and understanding of Clinical Chemistry and Laboratory Medicine throughout the world.
Dr David S. HAGE (United States) is the winner of the 2023 IFCC Award for Distinguished Contributions in Education, sponsored by Abbott Laboratories.
This award recognizes specifically an individual who has made extraordinary contributions in establishing and developing educational materials for the Clinical Chemistry discipline to improve training and educational programs worldwide or in a region.

Dr Anne J. VASSAULT (France) is the winner of the 2023 IFCC-Robert Schaffer Award for Outstanding Achievements in the Development of Standards for Use in Laboratory Medicine, cosponsored by NIST and CLSI.

This award recognizes specifically an individual who has made outstanding and unique contributions to the advancement of reference methods and/ or reference materials for laboratory medicine to facilitate improved quality of clinical diagnostics and therapies, which would in turn lead to reduced costs and improved patient care.
Dr Joe M. EL-KHOURY (United States) is the winner of the 2023 IFCC Young Scientist Award, sponsored by Snibe.

This award recognizes and encourages the academic and professional development of a young investigator (under 40 years of age) who has demonstrated exceptional scientific achievements in Clinical Chemistry and Laboratory Medicine in his/her career.
Prof. Maurizio Ferrari, Chair of the IFCC Awards Committee, said: "We are delighted in electing these colleagues for the 2023 IFCC Awards. The Awardees are a witness of the contribution that IFCC gives to advancement of excellence in laboratory medicine for better healthcare worldwide. I’m happy that so many National Societies submitted excellent candidates: we had a very hard task selecting the Awardees among them. It has been a privilege considering them and we are sure that the Awardees will inspire a new generation of clinician-scientists worldwide".

17 LabMedica International May/2023 NEWS
send news to Email: enews@ifcc.org
IFCC members may
Dr Robert H. CHRISTENSON (United States)
Dr David S. HAGE (United States)
Dr Anne J. VASSAULT (France)
Dr Joe M. EL-KHOURY (United States)
EDITORIAL
By Katherina Psarra, MSc, PhD

Dear Colleagues,
As we brace to enter the month of May, lots of congresses are taking place all over the world.
People can at last meet with their longtime colleagues and friends, and participate with a new intensity, with a new joy.
Soon we’ll all meet in Rome! Lots of people – more than ever – from all over the world will be attending the meeting and strolling in the Eternal City’s streets.
The Exhibition is expected to be extensive and the Scientific Program should meet all expectations.

The music from all over the world can be
heard in this issue too, with “testimonials” –testimonials from all over the world about the use of a new learning software. It seems very successful! Why not listen to all these people and use it too? I think I’ll try it.

AI is the hot topic nowadays. We have just completed the Hellenic Cytometry Congress and AI was at the center of our discussions. The same seems to have happened at the Moroccan Congress of Clinical Chemistry. Dr Bernard Gouget and Dr Alexander Haliassos discussed and explained AI within the Moroccan Health Service.
Go through this issue dear friends. I hope you’ll be discussing with your colleagues from all over the world in Rome, enjoy one another’s company, and also learn a lot!
I am sure we’ll all have a marvelous time in Rome!
Top 5 Reasons to Apply for Recognition Through the UNIVANTS of Healthcare Excellence Program
TheUNIVANTS of Healthcare Excellence award program is global, prestigious award program that, through 8 prestigious program partners, including Abbott, IFCC, AACC, Modern Healthcare, National Association for Healthcare Quality (NAHQ), European Health Management Association (EHMA), Institute of Health Economics (IHE), Healthcare Information and Management Systems Society (HIMSS), seeks to recognize, amplify, and celebrate best practices in healthcare that are facilitated by laboratory medicine.
Laboratory medicine is an essential element of healthcare and a strategic asset for maximizing value and improving healthcare. The opportunity to showcase and celebrate cross-disciplinary achievements associated with measurable differences in healthcare is unique and rewarding, as well as crucial for elevating laboratory medicine.
Thus, as the 2023 UNIVANTS of Healthcare Excellence award program opens its 5th award cycle on August 1st, 2023, here are the top 5 reasons to apply for recognition this year:
1. Celebration of achievements associated with achieving measurably better health outcomes
The measurable difference that strategic use of laboratory medicine and laboratory insights have on patients, payors, clinicians and entire health systems is both unique and impressive. As healthcare professionals, you have dedicated your professional lives to ensuring high-quality, timely and patient-centered care for better health outcomes – this is cause for celebration!
2. Recognition from teammates, colleagues and superiors, both locally and globally
Although many, if not most, recognized teams associated with the UNIVANTS of Healthcare Excellence program do not
set out to receive any awards or recognition, the accolades and exposure associated with UNIVANTS provides a platform for recognition and amplification.
3. Amplification of best practices
Sharing of best practices and outcomes across institutions, care teams and the globe is a key component of UNIVANTS, with all best practices shared globally to enable idea sharing and to serve as inspiration to others.
4. Education across disciplines
When something truly excellent happens, such as the outcomes associated with recognized teams of the UNVIANTS of Healthcare Excellence program, the world wants to know how. Thus, top winning sites associated with UNIVANTS receive opportunities to disseminate and educate on their initiatives, how they achieved their outcomes, and insights associated with their initiative.
5. Replication of proven best practices
While there is no perfect roadmap to healthcare excellence, the recognized care teams and best practices associated with UNIVANTS are outstanding examples of integrated clinical care teams unifying to achieve something greater and can serve as inspiration on what more can be done today.
Applications for the 2023 UNIVANTS of Healthcare Excellence awards open Aug 1, 2023. What can be done? Start thinking about your application, start collating metrics and visit www.UnivantsHCE.com for details on how to apply, as well as tips and tricks for maximizing your application.

News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
18 LabMedica International May/2023
Adaptive Learning in the Training of Future Laboratory Medicine Professionals: Testimonials from Around the World
 Prof Nader Rifai Chair, Education and Management Division of the IFCC
Prof Nader Rifai Chair, Education and Management Division of the IFCC
Adaptive learning, the closest to personalized education, is becoming widely used in high-school and college education as well as in the preparation for professional certification exams and in corporate training programs in certain countries. Studies have shown that adaptive learning enables faster learning, a higher retention rate of learned materials, a higher passing rate, and higher exam scores compared to traditional eLearning methods. Adaptive learning, powered by artificial intelligence, is efficient because it adapts the information to the learner’s needs and considers what the learner already knows.
Learning Lab for Laboratory Medicine (www.area9lyceum. com/laboratorymedicine) was born 8 years ago and was endorsed by the IFCC in 2022. The program can be characterized as follows:
• Consists of >120 advanced and >80 Medical Laboratory Specialists courses that cover all aspects of laboratory medicine

• Courses have been prepared, reviewed, and translated by >430 experts from around the world and are routinely updated.
• The program is free of charge to individual users, to eliminate financial barriers.
• In addition to the English version, the program is available, in part, in Chinese (simplified & traditional), Bahasa Indonesia, and French. In addition, the program is being translated to Arabic, Japanese, Korean, and Portuguese; these websites will be available in 2023.
• All editors, authors, reviewers and translators are volunteers; none received any financial compensation for their efforts.
• Currently, >11,000 individuals from 150 countries are using this program.
Learning Lab is designed for laboratory medicine professionals working in hospital and commercial clinical laboratories as well as those practicing in the in-vitro diagnostics industry. Furthermore, the program is very useful to those undergoing training in one of the disciplines of laboratory medicine. Educators in our field from around the world are starting to incorporate the Learning Lab into the curriculum of their training programs. Some of these educators and their trainees shared their expe-
riences and opinions about the Learning Lab in their own voice.

Dr. Neil Anderson, an Associate Professor of Pathology and Immunology at Washington University School of Medicine and the Director of Molecular Infectious Disease Laboratory and Assistant Medical Director of Microbiology at Barnes Jewish Hospital in St. Louis, MO, USA as well as the Pathology Residency Director, stated “We have used the Learning Lab in our training program and have found it to be a terrific tool for teaching labo-
Staff Members: Paola Bramati, Silvia
19 LabMedica International May/2023 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
JULY 23-27 I ANAHEIM, CA WE ARE DREAMERS+ DISRUPTORS LEADERS + LUMINARIES SEEKERS + SEARCHERS GLOBAL LAB MEDICINE COMMUNITY Rates Go Up Soon. Register Today. In 2023, AACC celebrates 75 years of bringing pioneering product innovations, emerging technologies and revolutionary research to the global laboratory medicine community. Attend the 2023 AACC Annual Scientific Meeting & Clinical Lab Expo to: • Connect with colleagues for five dynamic days of discovery. • Experience 250+ plenary presentations, scientific sessions, roundtables, and more. • Expand our thinking with 300+ expert speakers and 800+ exhibitors showcasing 200+ product categories. Learn about Anaheim All Access full conference registration. meeting.aacc.org Cont’d on page 20
The views and positions expressed in the IFCC News section are those of the IFCC or the individual authors, and do not necessarily represent the views or positions of LabMedica magazine or its publishers.
Via Carlo Farini 81, 20159 Milan, ITALY Tel: (39) 02-6680-9912 E-mail: ifcc@ifcc.org • Web: www.ifcc.org
IFCC OFFICE
Cardinale,
Silvia Colli-Lanzi, Smeralda Skenderaj
Adaptive Learning in the Training of Future Laboratory Medicine Professionals:
Testimonials from Around the World

Cont’d from page 19
ratory medicine. The educational content is well curated by subject matter experts and aligns with the concepts we teach our trainees. The modular nature allows us to provide study resources to trainees with specific knowledge deficits and selectively adopt material into the existing curriculum. While there are many “question set” resources available, the adaptive learning aspect of the Learning Lab is a unique way to promote learner engagement.” His chief resident in clinical pathology, Dr. Patricia Hernandez indicated “I started using the Learning Lab during my first year of residency and it has been an important tool for learning. It is a very dynamic resource: it includes questions with comprehensive explanations making the information easy to assimilate. What I like the most is that the Learning Lab includes diverse fields of Laboratory Medicine, which has been pretty helpful on different rotations. Also, it’s not necessary to complete a topic in order to start another one. There is no established sequence, which makes Learning Lab very flexible, allowing it to fit my needs”.
Dr. Bernard L. Croal, the President of The Association for Clinical Biochemistry and Laboratory Medicine (UK) and the Chair of the Royal College of Pathologists (RCPath)-Scotland and an RCPath Trustee, stated in a recent correspondence “I would also like to fully endorse this free program of online resource. A huge amount of work has gone into it and it is strongly relevant to those working towards FRCPath as well as a great Continuing Professional Development (CPD) resource for all stages of the profession.”
Endang Hoyaranda from Prodia Labs Indonesia and the Indonesian Association for Clinical Chemistry in Jakarta stated, “The Learning Lab as a practical educational program is a great way to fill in the gaps of what students, lab technologists, and lab scientists do not get at their academic trainings, to be directly implemented in their daily work.” Dr. Miswar Fattah, MSI is the Head of Research and Specialty Development also at Prodia Clinical Laboratory Indonesia; his labs include Mass Spectrometry and Separation Science, Molecular Laboratory, Cytogenetic Laboratory, Pathological Anatomy Laboratory, Advanced Microbiology Laboratory, and Advanced Immunology Laboratory. Dr. Fattah stated “The Learning Lab is very beneficial for us because we have so many specialty laboratories. With these comprehensive courses, we have an advantage in training our new staff members
and expanding their expertise. The fact that the Learning Lab contains everything necessary to enhance learning, it is a very welcomed and beneficial development for us. Because of the provided questions, the Learning Lab makes it simple to determine how far the team’s knowledge has progressed, and it also enables us to determine where the trainees’ knowledge is lacking”.
Prof. Pradeep Kumar Dabla, a Professor of Biochemistry at the G.B.Pant Institute of Postgraduate Medical Education & Research (GIPMER), Associated Maulana Azad Medical College in Delhi, India, stated that “In today’s COVID era, the restrictions made us learn several lessons. The digital innovation and competencies tools are highly needed for students and faculty members in educational institutions. The Learning Lab has given us that opportunity to learn together with free access for up to 80 courses for medical lab specialists across the field of lab medicine. These courses, prepared by international experts, provide an opportunity to an excellent adaptive learning experience and enable students and trainees to learn through a thoughtful and interactive process. Our students were helped tremendously to have a first-hand experience in learning and understanding of the various concepts of lab medicine through the Learning Lab and this tool was highly appreciated compared to other teaching methods available”.
Prof. Bobbi S. Pritt, a Professor of Laboratory Medicine and Pathology and Interim Chair of the Department of Laboratory Medicine and Pathology at Mayo Clinic in Rochester, MN, USA has indicated that “The Learning Lab is an innovative and invaluable resource for teaching laboratory medicine. It was put together by subject matter experts around the world, and thoughtfully designed to test key laboratory concepts across the full range of subjects.”
Dr. Lusia Sepiashvili, Assistant Professor at the University of Toronto and a Clinical Biochemist at The Hospital for Sick Children, Toronto, ON, Canada, shared her experience by saying “The Learning Lab adaptive learning program provides a unique format for learning since it is an interactive teaching platform learners can use at their own pace to either expand their knowledge or refresh their understanding of specific topics in laboratory medicine. Therefore, I think it is highly complementary to other types of learning like listening to presentations/webinars,
reading textbooks or journal articles, and practice-based learning.” Dr. Sepiashvili’s Clinical Chemistry Post-Doctoral Fellow at the University of Toronto, Dr. Samantha Logan, indicated that “The Learning lab has been helping me throughout my fellowship to build frameworks for learning new topics. The course on central and peripheral nervous system autoimmunity was particularly helpful because autoimmune testing is an important aspect of our practice as clinical biochemists, but resources to learn the clinical and analytical aspects of this subject are not yet consolidated into textbooks directed at fellows. I also use the Learning Lab to test myself on concepts I have already covered, identify any gaps in my knowledge, and periodically refresh my understanding of core topics.”
Leifur Franzson, MScPharm, Specialist in Clinical Biochemistry, Clinical Associate Professor and Lab Director for Newborn Screening in the Department of Genetics and Molecular Medicine at the University of Iceland in Reykjavik indicated “The Learning Lab is an ingenious, clever and a fun way to learn. I have used it routinely with my trainees in genetic metabolic diseases and clinical chemistry. For example, the statistical courses – and other general courses, are also ideal for students prior to doing their BS, Master or PhD degrees. These courses will be used at the University of Iceland as a teaching aid for medical laboratory scientists and for students in the medical sciences. The courses are prepared by international experts and the program enables learning on the go and at the learner’s own pace. I highly recommend this valuable resource.” His trainee at the Children’s Hospital of Reykjavik, Dr. Jens Georg Waagsbo Stensrud, added “I used the Learning Lab in my training in genetic metabolism and found it to be invaluable. It is a great way to learn and reinforce what you already know. Trainees should take advantage of this free program; it is really great.”
These testimonials from different parts of the world attest to the value of the Learning Lab and its role in educating the future generation of laboratory medicine professionals. Both educators and trainees who have not yet taken advantage of this free resource are encouraged to register and examine the program at their leisure. The registration is very simple and takes 1 min to complete (https://area9lyceum.com/laboratorymedicine/). Give it a try, it may be just what you need!
20 LabMedica International May/2023 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
Global Market for Biomarker-Based Infectious Disease Tests to Approach

USD 21 Billion in 2027



Biomarkers encompass over a thousand substances utilized as reagents, consumables, and/or test components for a variety of diagnostics and medical research procedures. They also include substances that can detect and measure genetic alterations in patient samples. Biomarkers for infectious diseases include proteins (antigens), antibodies generated due to the presence of infectious agents, and genetic markers. Recent advancements like mass spectrometry are being employed for pathogen identification. The global market for infectious disease tests that utilize biomarkers is anticipated to expand at a compound annual growth rate (CAGR) of 5.8% from USD 14.8 billion in 2021 to USD 20.7 billion in 2027, fueled by factors such as an aging population with higher disease vulnerability, an increase in targeted therapies, and a large and expanding clinical diagnostic test market.
These are the latest findings of Kalorama Information (Arlington, VA, USA; www.kaloramainformation.com), a leading publisher of market research in medical markets.
Laboratory immunoassays are expected to account for more than 60% of global infectious disease biomarker sales over the long term. This can be attributed to the technology’s ability in detecting a wide range of common pathogens, including antigens or antibodies associated with pneumonia, influenza, Clostridium difficile (C. diff), hepatitis C, HIV, herpes simplex virus (HSV), and Lyme disease. Enzyme-linked immunoassays are especially suitable for diagnosing these diseases. In the case of more complex infectious disease testing applications, polymerase chain reaction (PCR), microarrays, and other molecular technologies are likely to garner a higher share of the global value and volume. These technol-
Werfen and Thermo Fisher in Worldwide Distribution Alliance for Hemostasis Testing





Werfen (Barcelona, Spain; www.werfen.com) has entered into a new long-term, exclusive partnership agreement with Thermo Fisher Scientific (Waltham, MA, USA; www.thermofisher. com) to globally distribute the TCAutomation laboratory automation system, specifically tailored for hemostasis diagnostics. This agreement, a follow-up to their exclusive contract signed in 2015, allows Werfen to continue offering HemoCell Specialized Lab Automation, which has the largest global installed base of hemostasis automation workcells.

HemoCell streamlines laboratory workflow by integrating all stages of testing. It comprises Werfen’s ACL TOP Family 50 Series Hemostasis Testing Systems and HemoHub Intelligent Data Manager, paired with Thermo Fisher’s TCAutomation system. This combination standardizes sample processing, ensuring consistent turnaround times for routine and urgent samples. This efficiency frees up laboratory staff, improving overall lab efficiency and quality of work. Ultimately, HemoCell contributes to improving the quality of care while minimizing costs.
The TCAutomation laboratory automation system is designed to provide truly efficient workflow for laboratories through its modularity, flexibility, and connectivity. It aids in managing escalating workloads and improving quality. By offering flexible and cost-effective solutions, it automates the most labor-intensive pre- and post-analytical tasks. Its open concept enables direct interfaces with a broad range of analyzers. The TCAutomation system can be easily expanded with additional modules, thereby helping laboratories adapt to future needs and requirements and boost efficiency, productivity, and safety.
“This significant continuation of our long-term distribution agreement is a testament to the strength of our partnership with Thermo Fisher Scientific,” said Remo Tazzi, Vice President, Worldwide Marketing and Service, Hemostasis and Acute Care Diagnostics at Werfen. “It also speaks volumes about the success of HemoCell, and the quality of Werfen’s workflow experts, their innovative tools and processes, and the value they offer hospital laboratories around the world.”

ogies are capable of identifying DNA, RNA, and genes associated with pathogens, enabling a much more definitive diagnosis.
Molecular tests can identify infectious agents such as HPV, Chlamydia trachomatis/Neisseria gonorrhea (CT/NG), and tuberculosis. Moreover, these tests are increasingly being used to detect genes associated with resistance to healthcare-related infections and disease-resistant pathogens. Rapid immunoassays employing lateral flow immunochromatographic techniques are being developed to allow detection of an increasing array of infectious diseases at point-of-care locations, including clinics, doctors’ offices, hospital emergency rooms, and patient homes. Specific tests now available in this area include ones for bacterial vaginosis (BV), chlamydia, dengue, Ebola, HIV, influenza, Legionnaires’ disease, malaria, rotavirus, respiratory syncytial virus (RSV), group A Streptococcus, Treponema pallidum, and Trichomonas vaginalis. The sources for specimens for rapid immunoassays encompass a wide variety of substances, such as whole blood, serum, urine, nasal and throat swabs, stool samples, and vaginal swabs.
21 LabMedica International May/2023 Industry News To view this issue in interactive digital magazine format visit www.LabMedica.com
What awaits you at the EAACI Congress 2023? DISCOVER THE SCIENTIFIC PROGRAMME NOW! It covers a wide range of topics in allergy and clinical immunology. It will provide you with the opportunity to learn about the latest research, treatments, and best practices in the field. www.eaaci.org #EAACI2023 > - HAMBURG HYBRID CONGRESS 2023 EAACI 9-11 JUNE GERMANY SEE YOU SOON IN HAMBURG IN JUNE 2023!
2023
JUNE
106th Annual Meeting of the German Society for Pathology. Jun 1-3; Leipzig, Germany; pathologie-dgp.de
ASCO 2023 – Annual Meeting of the American Society of Clinical Oncology. Jun 2-6; Chicago, IL, USA; asco.org
66th Congress of the German Society for Endocrinology. Jun 5-7; Baden-Baden, Germany; endokrinologie.net
EHA 2023 – Annual Congress of the European Hematology Association. Jun 8-11; Frankfurt, Germany; ehaweb.org
EAACI 2023 – Annual Congress of the European Academy of Allergy & Clinical Immunology. Jun 911; Hamburg, Germany; eaaci.org
ESHG 2023 – European Human Genetics Conference. Jun 10-13; Glasgow, UK; 2023.eshg.org
UKMedLab23 – National Meeting of the Association for Clinical Biochemistry and Laboratory Medicine. Jun 12-14; Leeds, UK; acb.org.uk
14th National Conference of the Association of Laboratory Medicine from Romania. Jun 14-16; Timisoara, Romania; amlr.medical-congresses.ro
ASM Microbe 2023 – American Society for Microbiology. Jun 15-19; Houston, TX, USA; asm.org
33rd Regional Congress of the International Society of Blood Transfusion (ISBT). Jun 17-21; Gothenburg, Sweden; isbtweb.org
48th CBAC – Congress of the Brazilian Society of Clinical Analysis. Jun 18-21; Florianopolis, Brazil; sbac.org.br
FOCIS 2023 – Annual Meeting of the Federation of Clinical Immunology Societies. Jun 20-23; Boston, MA, USA; focisnet.org
FIME 2023 – Florida International Medical Expo. Jun 21-23; Miami, FL, USA; fimeshow.com
83rd Scientific Sessions of the American Diabetes Association. Jun 23-26; San Diego, CA, USA; professional.diabetes.org
ASV 2023 – 42nd Annual Meeting of the American Society of Virology. Jun 24-28; Athens, GA, USA; Web: asv.org
ISTH 2023 Congress – International Society on Thrombosis and c. Jun 24-28, Montreal, Canada; isth2023.org
ESHRE 2023 – 39th Meeting of the European Society of Human Reproduction and Embryology. Jun 25-28; Copenhagen, Denmark; eshre.eu
JULY
Analytica Lab Africa 2023. Jul 5-7; Johannesburg, South Africa; analytica-africa.com
APCCMI 2023 – 19th Asia Pacific Congress of Clinical Microbiology and Infection. Jul 6-8; Seoul, Korea; apccmi2023.com
FEBS 2023 – 47th Congress of the Federation of European Biochemical Societies. Jul 8-12; Tours, France; febs.org
FEMS 2023 – 10th Congress of European Microbiologists. Jul 9-13; Hamburg, Germany; fems2023.org
Analytica China. Jul 11-13; Shanghai, China; analyticachina.com
2023 AACC Annual Scientific Meeting & Clinical Lab Expo. Jul 23-27; Anaheim, CA, USA; meeting.aacc.org
AUGUST
MedLab Asia 2023. Aug 16-18; Bangkok, Thailand; medlabasia.com
12th International Palestinian Conference for Laboratory Medicine (IPCLM). Aug 24-26; Ramallah, Palestine; ipclm.ps
SEPTEMBER
33rd MACB Annual Scientific Conference – Malaysian Association of Clinical Biochemists. Sep 5-6; Kuala Lumpur, Malaysia; macb.org.my
Thailand LAB International 2023. Sep 6-8; Bangkok, Thailand; thailandlab.com
ECP 2023 – 35th Congress of the European Society of Pathology. Sep 9-13; Dublin, Ireland; esp-congress.org
EUROTOX 2023 – 57th Congress of the European Societies of Toxicology. Sep 10-13; Ljubljana, Slovenia; eurotox2023.com
ACBICON 2023 – 48th Annual Conference of Association of Clinical Biochemists of India. Sep 13-16; Thiruvananthapuram, India; acbindia.org.in
India Lab Expo & Analytica Anacon India. Sep 1416; Hyderabad, India; analyticaindia.com
Arab Lab 2023. Sep 19-21; Dubai, UAE; arablab.com
ESPE 2023 – 61st Annual Meeting of the European Society for Paediatric Endocrinology. Sep 21-23; The Hague Netherlands; eurospe.org
Analitica Latin America 2023. Sep 26-28; Sao Paulo, Brazil; analiticanet.com.br
BCLF 2023 – 30th Meeting of the Balkan Clinical Laboratory Federation. Sep 27-30; Herceg Novi, Montenegro; bclf2023.org
92nd Annual Meeting of the American Thyroid Association (ATA). Oct 29 - Sep 1; Washington, DC, USA; thyroid.org
OCTOBER
ECC 2023 – 44th European Congress of Cytology. Oct 1-4; Budapest, Hungary; cytology2023.eu
EASD 2023 – 59th Annual Meeting of the European Association for the Study of Diabetes. Oct 3-6; Hamburg, Germany; easd.org
CAP23 – Annual Meeting of the College of American Pathologists. Oct 7-10; Chicago, IL, USA; cap.org
DKLM 2023 – Annual Congress of the German Society for Clinical Medicine and Laboratory Medicine (DGKL). Oct 10-12; Mannheim, Germany; dgkl.de
CELME 2023 5th Symposium: Cutting Edge of Laboratory Medicine in Europe. Oct 12-13; Prague, Czech Republic; celme2023.cz
Events Calendar
Provided as a service to advertisers. Publisher cannot accept responsibility for any errors or omissions. Advertising Index Inq.No. Advertiser Page Inq.No. Advertiser Page Vol. 40 No. 3 5/2023 LabMedica International – AACC 19 103 DiaSys Diagnostic Systems ...................... 3 113 Dymind 13 – EAACI 2023 ................................. 21 – IFCC WorldLab 2024 17 – LabMedica EXPO 23 124 Nova Biomedical ............................. 24 109 Realmind Biotech 9 107 Singuway .................................... 7 102 Snibe 2 111 Vedalab 11 115 Vicotex 15 105 Vircell 5 ATTENTION: IF YOUR APPLICATION IS NOT RECEIVED AT LEAST ONCE EVERY 12 MONTHS YOUR FREE SUBSCRIPTION MAY BE AUTOMATICALLY DISCONTINUED READER SERVICE PORTAL LINKXPRESS COM ® VISIT Every advertisement or product item contains a LinkXpress number as below: 999 LINKXPRESS COM LMI-05-23 Identify LinkXpress codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress inquiry matrix 1 2 3 Renew/ S tart Your Free Subscription Access Instant Online Product Information
For a free listing of your event or a paid advertisement contact: LabMedica International Calendar E-mail: info@globetech.net
JFBM 2023 – Journées Francophones de Biologie Médicale. Oct 11-13; France; jfbm.fr
85th Annual Meeting of the Japanese Society of Hematology. Oct 13-15; Tokyo, Japan; jshem.org.jp
60th Annual Scientific Conference of the Australasian Association of Clinical Biochemistry and Laboratory Medicine (AACB). Oct 16-19; Brisbane, Australia; aacb.asn.au
ASHI 2023 – 49th Annual Meeting of the American Society for Histocompatibility and Immunogenetics. Oct 16-20; San Antonio, TX, USA; ashi-hla.org
MedLab Africa 2023. Oct 17-19; Johannesburg, South Africa; africahealthexhibition.com
ASCP 2023 – Annual Meeting of the American So ciety for Clinical Pathology. Oct 18-20; Long Beach, CA, USA; ascp.org
LABCLIN 2023 – 17th National Congress of the Spanish Societies for Clinical Laboratory (AEBMML, AEFA & SEQCML). Oct 18-20; Zaragoza, Spain; seqc.es
EndoBridge 2023. Oct 19-22; Antalya, Turkey; endobridge.org
7th ESPT Congress – European Society for Phar macogenomics and Personalised Therapy. Oct 25-27; Copenhagen, Denmark; esptcongress.org
63rd Annual Academic Assembly of the Japan Society of Clinical Chemistry (JSCC). Oct 27-29; Toyama, Japan; jscc-jp.gr.jp
34th National Congress of the Turkish Biochem ical Society. Oct 29 - Nov 1; Fethiye, Turkey; biyokimyakongresi.org
NOVEMBER
ASHG 2023 – Annual Meeting of the American Society of Human Genetics. Nov 1-5; Washing ton, DC, USA; ashg.org
44th Annual Meeting of the American College of Toxicology (ACT). Nov 12-15; Orlando, FL, USA; actox.org
MEDICA 2023. Nov 13-16; Dusseldorf, Germany; medica-tradefair.com
AMP 2023 – Annual Meeting & Expo of the Asso ciation for Molecular Pathology. Nov 16-18; Salt Lake City, UT, USA; amp.org
71st Annual Scientific Meeting of the American Society of Cytopathology (ASC). Nov 16-19; Aus tin, TX, USA; cytopathology.org
JIB 2023 – Journées de l’innovation en biologie. Nov 17-18; Paris, France; jib-innovation.com
34th Regional Congress of the International Soci ety of Blood Transfusion (ISBT). Nov 18-21; Cape Town, South Africa; isbtweb.org
IUIS 2023 – International Union of Immunolog ical Societies. Nov 27 - Dec 2; Cape Town, South Africa; iuis2023.org
DECEMBER
ASI 2023 – 51st Annual Scientific Meeting of the Australian and New Zealand Society for Immu nology. Dec 4-8; Auckland, New Zealand; asi2023. org
65th Annual Meeting & Exposition of the American Society of Hematology (ASH). Dec 9-12; San Diego, CA, USA; hematology.org

2024 FEBRUARY
SLAS 2024 – International Conference & Exhibi-
tion of the Society of Laboratory Automation and Screening. Feb 3-7; Boston, MA, USA; slas.org
Medlab Middle East 2024. Feb 5-8; Dubai, UAE; medlabme.com
Labquality Days 2024 – International Congress on Quality in Laboratory Medicine. Feb 8-9; Helsinki, Finland; labqualitydays.fi
Pittcon 2024. Feb 26-28; Philadelphia, PA, USA; pittcon.org
MARCH
Analytica 2024. Apr 9-12; Munich, Germany; analytica.de
ExpoMED Eurasia 2024. Apr 25-27; Istanbul, Turkey; expomedistanbul.com
ECCMID 2024 – 34th European Congress of Clinical Microbiology and Infectious Diseases. Apr 27-30; Copenhagen, Denmark; eccmid.org

MAY
Immunology 2024 – Annual Meeting of the American Association of Immunologists (AAI). May 3-7;
AACE Annual Meeting 2024 – American Association
LabMedica EXPO provides a sophisticated yet easy-to-use global B2B platform for sourcing clinical laboratory equipment. LabMedica EXPO connects buyers and sellers worldwide through a safe, secure and dynamic network, regardless of size or budget.

Events Calendar
23 LabMedica International May/2023
Send inquiries directly to suppliers... Receive latest product alerts... Chat live with suppliers, and more...
WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
References
Better Tests = Better Patient Outcomes
The Modern Critical Care Profile
Prime Plus provides the most clinical value by completing the modern blood gas/critical care profile by adding essential tests for electrolyte balance (iMg), plasma volume (ePV), kidney function (BUN, Creatinine, eGFR), and mean corpuscular hemoglobin concentration (MCHC).
Ionized Magnesium (iMg) Hypomagnesemia is a frequent finding in critically ill patients.1 Magnesium therapy guided by real time ionized magnesium monitoring has been shown to improve outcome in these patients.2
Estimated Plasma Volume (ePV) The plasma volume status of a patient is one of the top priorities in evaluating and treating critical illness including CHF, ARDS, AKI, Surgery, and Sepsis.3-5
Urea, Creatinine and eGFR Over 50% of patients admitted to the ICU develop some degree of acute kidney injury.6 Creatinine, eGFR, and Urea point-of-care monitoring provides early indication of changes in kidney function and helps guide therapy to prevent AKI.
MCHC Mean corpuscular hemoglobin concentration (MCHC) provides insight into certain causes of anemia, such as iron deficiency or inability of the body to absorb iron, chronic low grade blood loss over time, and autoimmune hemolysis.
novabiomedical.com
1. Soliman HM. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit Care Med 2003;31(4):1082-7.
2. Wilkes NJ et al. Correction of ionized plasma magnesium during cardiopulmonary bypass reduces the risk of postoperative cardiac arrhythmia. Anesth and Analg 2002;95(4) 828-834.
3. Kobayashi M et al. Prognostic Value of Estimated Plasma Volume in Heart Failure in Three Cohort Studies; Clin Res Cardiol 2019;108(5): 549-561.
4. Niedermeyer, et al. Calculated Plasma Volume Status Is Associated With Mortality in Acute Respiratory Distress Syndrome. Critical Care Explorations: September 2021, V3(9):1-9.
5. Kim HK et al. Prognostic Value of Estimated Plasma Volume Status in Patients with Sepsis. J Korea Med Sci 2020;9(37):1-10.
6. Mandelbaum T et al. Outcome of critically ill patients with acute kidney injury using the AKIN criteria. Crit Care Med 2011;39(12):2659-2664.
132 LMI-4-23 LINKXPRESS COM 124 LMI-05-23 LINKXPRESS COM
Critical Care Tests Available: pH P CO2 P O2 SO2 % Hct Hb Na K Cl TCO2 iCa GLU Lac Urea tBil CO-Ox HbF