Diagnosing Allergic Reaction in Kidneys
Acute tubulointerstitial nephritis (AIN) is a medical condition that causes inflammation of the kidneys and can result in acute kidney injury (AKI) — a rapid deterioration of kidney function. Medications used for treating gastroesophageal reflux
Cont’d on page 3
New Platform Brings Power of Multiplex Imaging to Clinical Pathology
For over a hundred years, pathologists have relied on histology, a method that involves examining cells and tissues under a microscope to identify cancerous patterns. Now, a new tool promises to improve this process by providing deep insights into the
cancerous tissue. By combining histological and molecular data, the tool can provide a deeper understanding of the type, behavior, and probable response to the treatment of a tumor.
The tool, named Orion, is the product of extensive research and
Cont’d on page 13
Candida Auris Test Takes Only 3-5 Hours
AACC 2023 Roundup: Highlights of Technical Exhibition
AACC 2023 Roundup: Highlights of Technical Exhibition


See article on page 4
Rapid Molecular Test Detects 8 Common GI Parasites

Gastrointestinal (GI) infections are a significant health concern, causing illness and mortality, especially in developing countries where they claim the lives of approximately two million children under five each year. Gastroenteritis, a common GI illness, can be caused by various Cont’d on page 14
Mindray to Acquire 75% Stake in DiaSys
Candida auris (C. auris) is a fungal pathogen resistant to multiple drugs and is recognized as a significant worldwide health risk due to its high mortality rate of up to 60%. Its identification is challenging with conventional lab techniques, often leading to improper treatment. To reduce the burden of this fungal disease, public health officials are being urged to continue promoting the
Cont’d on page 11
Test Predicts Response to Immunotherapy
In recent years, there has been a significant increase in clinical trials for new cancer drugs. However, only a select group of patients have found success with these newer immunotherapies, which utilize the patient’s immune response to combat cancer. Currently, predicting a patient’s response to therapy depends on isolated, single-analyte biomarkers, which have proven

Cont’d on page 10
M
indray (Shenzhen, China; www.mindray.com) has agreed to acquire a 75% equity interest in DiaSys Diagnostic Systems GmbH (Holzheim, Germany; www.diasys-diagnostics.com ) through its Dutch subsidiary for an all-cash preliminary purchase price of approximately EUR 115



million with the final amount to be determined as of closing. The agreement is slated to be finalized later this year, pending regulatory approvals and the granting of regulatory approvals. Upon finalizing the transaction, Mindray will hold a 75% stake in DiaSys and con-
Cont’d on page 6
he
largest exhibition of in vitro diagnostics was held on July 23-27, 2023, at Anaheim,
a comprehensive review of showcased innovations shaping the future of clinical testing. ®
T
world’s
California. LabMedica’s editors present
If your subscription is not renewed every 12 months your Free Subscription may be automatically discontinued Renew / Start your Free Subscription Access Interactive Digital Magazine Instant Online Product Information: Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 VISIT READER SERVICE PORTAL LINKXPRESS COM ®
® INTERNATIONAL Vol.40 No.5 • 8-9/2023 VISIT DAILY CLINICAL LAB NEWS ISSN 1068-1760 4 OUR YEAR 0 th YEAR OUR WORLD’ S CLINICA L LABORATOR Y NEW S LEADER
GLOBETECH MEDIA >>> <<< PUBLISHED IN COOPERATION WITH International Federation of Clinical Chemistry and Laboratory Medicine INSIDE AACC ’23 Roundup . .. 4 LabMedica EXPO . 6-16 Clinical News . . . 10-20 IFCC News .... 17-19 Industry News . .. . 21 Events Calendar . . 22

102 LMI-09-23 LINKXPRESS COM
Diagnosing Allergic Reaction in Kidneys
Cont’d from cover
disease, bacterial infections, and cancer are believed to trigger AIN in approximately 70% of patients. Diagnosis of AIN is typically challenging due to the absence of disease-specific signs or symptoms, often necessitating a kidney biopsy. To evade the risks associated with kidney biopsies, clinicians may assume the presence of AIN and attempt to halt the progression toward AKI by discontinuing potential instigating drugs and administering corticosteroids. However, such a strategy could lead to excessive treatment if the AIN diagnosis is not accurate. Discontinued medications could encompass crucial therapies like antibiotics and anti-cancer drugs, and corticosteroid treatment presents risks such as high blood sugar, bone loss, gastrointestinal bleeding, and infections.
Now, in a study led by Johns Hopkins Medicine (Baltimore, MD, USA; www.hopkinsmedicine.org), researchers have identified a biomarker detectable via a simple urine test that can aid in diagnosing AIN.
In the study published on July 3, 2023 in the Journal of Clinical Investigation the researchers determined that test ing for the protein CXCL-9 in a per son’s urine could provide a noninvasive method to diagnose AIN, eliminating the need for a kidney biopsy. To identify this top AIN-associated protein, the re searchers tested the urine of more than 200 hospital patients with AKI for 180 potential biomarkers. They discovered that patients with AIN had significantly elevated levels of CXCL-9 in their urine compared to those without AIN, a find ing which was corroborated by exam ination of kidney tissue samples.
The research team suggests that the urine biomarker CXCL-9 could substantially enhance clinical care by helping to confirm or exclude AIN in a large subset of patients, thereby reducing the need for kidney biopsies to cases where biomarker values are in determinate. The team hopes that the insights from this study will also assist in the development of more effective treatments for AIN as a precursor to AKI, and for AKI itself, potentially by targeting inflammatory chemicals in the early stages.

“AIN is an allergic reaction caused by some common medications that are used routinely in a small group of patients,” said Chirag Parikh, M.B.B.S., Ph.D., director of the Division of Nephrology at Johns Hopkins Medicine. With the common assumption of a pa tient having AIN instead of AKI, finding a new biomarker can help eliminate the potential of a misdiagnosis. “Having a method for early diagnosis can help preserve kidney function and long-term chronic kidney disease,” added Parikh.
Image: A new study has identified a biomarker for allergic reaction in kidneys (Photo courtesy of Division of Nephrolo gy, Yale University School of Medicine)

3 LabMedica International August-September/2023 LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
AACC 2023 Roundup: Highlights of Technical Exhibition

INTERNATIONAL
labmedica.com
EDITORIAL BOARD
Graham Beastall United Kingdom
Hernán Fares Taie Argentina
Bernard Gouget France
Maurizio Ferrari Italy
Jocelyn M. Hicks United States
Tahir S. Pillay South Africa
Andreas Rothstein Colombia
Praveen Sharma India
Rosa I. Sierra-Amor Mexico
Peter Wilding United States
Andrew Wootton United Kingdom
A GLOBETECH PUBLICATION
Published in cooperation with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC).
LabMedica International • LabMedica en Español • LabMedica.com HospiMedica International • HospiMedica.com • MedImaging.net HospiMedicaExpo.com • LabMedicaExpo.com • LinkXpress.com
The 2023 AACC Annual Scientific Meeting & Clinical Lab Expo showcased breakthrough innovations shaping the future of clinical testing and patient care. The world’s largest exhibition for in vitro diagnostics is hosted every year by the Association for Diagnostics & Laboratory Medicine (formerly AACC) (ADLM, Washington, DC, USA; myadlm. org). AACC 2023 was held from July 23-27 at the Anaheim Convention Center in California, where laboratory medicine experts presented cutting-edge research and technology.
The AACC Clinical Lab Expo offered attendees the opportunity to browse more than 900 exhibitors across over 200 product categories in person for solutions to their lab-related needs. Attendees benefitted from a stunning lineup of expert speakers and scientific sessions, as well as a vast array of emerging technologies and products at the premier global laboratory medicine exposition. They connected with global leaders in clinical chemistry, molecular diagnostics, mass spectrometry, translational medicine, lab management, and other fields of breaking science in laboratory medicine. With a diverse array of lectures, plenary sessions, scientific sessions, and roundtable sessions, the event provided more than 250 educational opportunities tailored to meet specific needs.
At the AACC 2023 scientific session, “Artificial Intelligence and Machine Learning in the Clinical Laboratories: Fundamental Concepts, Clinical Use Cases, and Future Considerations,” attendees learned about the role that AI and ML can play in the clinical laboratory. In an interesting session, “Infectious Disease Serology: Are You Still Ordering That Test,” different speak-
ers shared case studies, insightful experiences, and guidance on how to effectively manage infectious disease serology testing. Another AACC session titled “Data Analytics Competition: Forecasting Future Preanalytical Errors” focused on the issue of excess of hemolyzed samples faced by several institutions who are keen on understanding how to address this problem while preserving scarce resources. In the “Emerging Trends in Laboratory Medicine: Excellence from the Global Community” session, global lab experts shared their knowledge and insights on several trending topics: pandemic readiness, environmental sustainability, and machine learning.
New research presented at AACC 2023 showed that an AI model can predict the likelihood of individuals developing multiple sclerosis (MS) years before its diagnosis. In other new research presented at AACC 2023, the study results showed that self-collected tests are as effective as provider-collected tests in detecting prevalent sexually transmitted infections (STIs). Another breaking research presented at AACC 2023 threw light on the co-infection rates of SARS-CoV-2, influenza, and respiratory syncytial virus (RSV) in the United States.
Pattern Bioscience (Austin, TX, USA; www.pattern.bio) won the 2023 Disruptive Technology Award for its ground-breaking single-cell microbiology technology. The Disruptive Technology Award competition from ADLM honors innovative testing solutions and disruptive technologies that enhance patient care via diagnostic performance or improved access to high-quality testing. Bypassing the need for the time-consuming steps of current standard culture-based methods, Pattern’s single cell
Cont’d on page 5
Dan Gueron
David Gueron
Sanjit Dutt
Carolyn Moody, RN
Simone Ciolek
Parker Xu
Karina Tornatore
HOW TO CONTACT US
Subscriptions:
Send Press Releases to:
Advertising & Ad Material: Other Contacts:
www.LinkXpress.com LMNews@globetech.net ads@globetech.net info@globetech.net
ADVERTISING SALES OFFICES
SUBSCRIPTION INFORMATION
LabMedica lnternational is published eight times a year and is circuIated worldwide (outside the USA and Canada) without charge and by written request, to clinical laboratory specialists and administrators, and other qualified professionals allied to the field.

To all others: Paid Subscription is available for a two-year subscription charge of US$120. Single copy price is US$20. Mail your paid subscription order accompanied with payment to Globetech Media, P.O.B. 800222, Miami, FL 33280-0222.
For change of address or questions on your subscription, write to: LabMedica lnternational, Circulation Services at above address; or visit: www.LinkXpress.com
ISSN 1068-1760
Vol.40 No.5. Published, under license, by Globetech Media LLC; Copyright © 2023. All rights reserved. Reproduction in any form is forbidden without express permission. Opinions expressed are solely those of the authors, and do not represent an endorsement, or lack thereof, by the Publisher of any products or services.
Teknopress Yayıncılık ve Ticaret Ltd. Şti. adına İmtiyaz Sahibi: M. Geren • Yazı işleri Müdürü: Ersin Köklü Müşir Derviş İbrahim Sok. 5/4, Esentepe, 34394 Şişli, İstanbul P. K. 1, AVPIM, 34001 İstanbul • E-mail: Teknopress@yahoo.com
Baskı: Postkom A.Ş. • İpkas Sanayi Sitesi
3. Etap C Blok • 34490 Başakşehir • İstanbul
Yerel süreli yayındır. Yılda sekiz kere yayınlanır, ücretsiz dagıtılır.
4 LabMedica International August-September/2023
Publisher
Reader
USA Miami, FL 33280, USA Carolyn.Moody@globetech.net Tel: (1) 954-686-0838 GERMANY,
Simone.Ciolek@globetech.net Tel: (49) 9771-1779-007 OTHER EUROPE & UK Miami, FL 33280, USA Carolyn.Moody@globetech.net Tel: (1) 954-686-0838 JAPAN Tokyo, Japan Katsuhiro.Ishii@globetech.net Tel: (81) 3-5691-3335 CHINA Shenzen, Guangdong, China Parker.Xu@globetech.net Tel: (86) 755-8375-3877 OTHER COUNTRIES Contact USA Office ads@globetech.net Tel: (1) 954-686-0838 Founder & Editorial Director Marc Gueron
Managing Editor News Editor Regional Director Regional Director Regional Director
Service Manager
SWITZ., AUSTRIA Bad Neustadt, Germany
AACC 2023 Roundup
microbiology technology directly measures the response of each bacteria cell to antibiotics, thereby determining exactly which agents can be employed for therapy and guiding treatment decisions within hours as opposed days.
Vital Biosciences (Toronto, ON, Canada; www.vitalbio.com ) introduced its game-changing product, the VitalOne which aims to democratize blood diagnostics by making over 50 lab-grade tests — covering 95% of routine lab orders — available at primary care locations, all packaged within a device no bigger than a desktop computer. With the VitalOne, physicians can conduct tests, diagnose, and treat patients all in one appointment, with results obtained in 20 minutes. The underlying technology has been rigorously tested and validated against thousands of samples in comparison to gold-standard instruments, through collaborations with Vital’s lab partners. The VitalOne is currently in its late-stage product development and is yet to be made commercially available. The VitalOne was recognized as the Audience Choice winner at this year’s AACC Disruptive Technology Award.
Bio-Rad Laboratories (Hercules, CA, USA; www.bio-rad.com) showcased its comprehensive range of quality control-related offerings, including the new Cardiac Advance product, Exact Diagnostics branded infectious disease molecular quality controls & panels, the family of InteliQ Load-and-Go controls, and Unity Next Peer QC data management solutions. Bio-Rad also demonstrated the D-100 hemoglobin testing system, a fully automated, best in class HbA1c analyzer for high-volume labs, alongside the BioPlex 2200 System, a fully automated, random-access, track line compatible, multiplex platform.
Thermo Fisher Scientific (Waltham, MA; USA; www.thermofisher. com) presented its dynamic range of products that can help laboratories maximize productivity and innovation. The company also offered live demonstrations of its Indiko and Indiko Plus clinical and specialty chemistry analyzers, and the Applied Biosystems QuantStudio
5 Dx Real-Time PCR System and Applied Biosystems QuantStudio

7 Pro Dx Real-Time PCR System. In addition, Thermo Fisher hosted industry leading workshops where experts discussed the company’s
latest innovations for preeclampsia testing with its new biomarkers PlGF and sFlt-1 that can help manage preeclampsia and neuroendocrine tumors.
Tianlong Science and Technology (Shaanxi, China; www.tlgenetech.cn), an innovative high-tech company specializing in molecular diagnostic products, showcased its extensive range of products as well as solutions for the molecular diagnostic industry. Tianlong demonstrated its game changing GeneRotex 96 nucleic acid extractor which is based on the automatic extraction principle of magnetic bead adsorption separation. The GeneRotex 96 Nucleic Acid Extractor is designed with Tianlong’s innovative Rotational Mixing Technology (RMT), which reduces aerosol
generation during purification, minimizes the risk of false positives due to crosscontamination, and ensures the accuracy of experimental results.

Beckman Coulter, Inc. (Brea, CA, USA; www.beckmancoulter.com) launched its hematology analyzer and slidemaker stainer connected to Scopio Labs’ (Tel Aviv, Israel; www.scopiolabs.com) digital morphology platform for end-to-end digitization with AIassisted peripheral blood smear analysis. The solution features the Beckman Coulter DxH 900 Workcell and DxH Slidemaker Stainer II connected with the Scopio X100HT digital morphology platform capable of continuous loading, scanning and analysis. The company also exhibited its new high-throughput DxI
Cont’d on page 6
5 LabMedica International August-September/2023
AACC 2023 Roundup
Cont’d from page 4
FULLY AUTOMATED ELISA WORKSTATION EUROIMMUN
ENCEPHALITIS VIRUS TEST BIOPERFECTUS TECHNOLOGIES





9000 Access immunoassay analyzer that is designed for superior laboratory performance and demands no daily maintenance. In addition, Beckman Coulter hosted a workshop to discuss new opportunities for laboratories to reduce QC and operational costs, as well as eliminate unnecessary repeats, recalibrations, and troubleshooting episodes, even in the face of CLIA’s tighter standards.

Siemens Healthineers (Erlangen, Germany; www.healthcare. siemens.com) highlighted its newest innovations and trends in clinical diagnostics and point-of-care testing, including its Atellica Integrated Automation which is powered for workflow and workforce efficiency to deliver control and simplicity so labs can drive better outcomes. The company’s experts also demonstrated how the IMMULITE 2000 XPi immunoassay system can address common challenges faced by specialty laboratories. Visitors to the Siemens booth experienced the first view of the company’s newly-launched Atellica CI Analyzer, a compact testing system designed to tackle lab challenges.
Roche Diagnostics (Basel, Switzerland; www.roche.com) showcased its latest advancements in lab automation, core lab systems, and molecular and point-of-care solutions, as well as in breakthrough high-medical-value diagnostic and digital solutions that are transforming patient care. Roche featured its newest innovations including the cobas 5800, a compact, fully-automated molecular PCR-testing solution with a comprehensive menu of assays, and cobas pure integrated solutions, which combine clinical chemistry, immunoassay and Ion-Selective Electrode (ISE) diagnostic testing on a single platform for low- to mid-volume testing needs. The new cobas connection modules (CCM vertical), a flexible modular system that uses lab space effectively and seamlessly integrates into the existing cobas connection modules, was also highlighted, along with Roche’s vision for the transformation of mass spectrometry testing.
Greiner Bio-One (Monroe, NC, USA; www.gbo.com) presented its innovative collection systems and safety products for venous and capillary blood and urine samples. The company also highlighted its research workflow solutions, including specialized products for the cultivation of cell cultures, microplates for high-throughput screening, biobanking solutions, and more. Greiner showcased its new VACUETTE CAT Serum Fast Separator that enables complete clotting of the whole
blood sample in just five minutes, offering a decisive time advantage compared to conventional serum tubes. Greiner also highlighted its Sapphire product family of pipette tips, filter tips and low retention tips alongside its magnetic 3D cell culture solutions based on magnetization of cells with NanoShuttle-PL.
Werfen (Barcelona, Spain; www.werfen.com) offered in-booth demonstrations of its point-of-care and laboratory instruments, reagents, and data management solutions. Werfen provided instrument demonstrations of its wide range of systems, including the ACL TOP Family 50 Series, HemoCell Specialized Lab Automation, HemosIL assays, HemoHub Intelligent Data Manager; GEM Premier 5000, GEM Premier ChemSTAT, GEM Hemochron 100, ROTEM sigma, VerifyNow, GMweb Plus 500, Aptiva, BIO-FLASH, QUANTA-Lyser, QUANTA-Link; Echo Lumena, and NEO Iris. The company also offered a virtual reality experience featuring customized layouts for HemoCell Lab Automation. Additionally, Werfen hosted an educational workshop at the event focusing on the prevalence and impact of pre-analytical errors in point-of-care blood gas testing.
CerTest Biotec (Zaragoza, Spain; www.certest.es), a company specialized in solutions for the diagnosis of infectious diseases, showcased its product range of rapid tests, RT-PCR tests, chemiluminescence (CLIA), and turbidimetry, in addition to raw materials for diagnostic product manufacturers. Certest demonstrated the VIASURE Complete System which is an integrated system offering a comprehensive solution for the diagnosis of infectious diseases, providing fast and reliable results that improve workflow in the diagnostic laboratory. Certest also presented up to 14 publications in the form of posters, reflecting the latest studies conducted by the company, mainly in the field of molecular biology.
CellaVision (Lund, Sweden; www.cellavision.com) showcased some of its latest products, including its new DIFF-Line complete workflow solution for low-volume hematology laboratories. DIFF-Line by CellaVision is a complete workflow for smearing, staining, and analyzing peripheral blood smears in hematology labs that handle a smaller amount of daily blood samples. Advancing into the area of digital bone marrow analysis, CellaVision also shared a preview of its upcoming bone marrow application.
HUMAN Diagnostics (Wiesbaden, Germany; www.human. de), a leading global provider of in-vitro diagnostic solutions, showcased its cutting-edge products and also engaged with industry professionals, researchers, and healthcare experts. Among the company’s products featured at the exhibition was the HumaCount 5D Cont’d on page 7
6 LabMedica International August-September/2023
The EUROLabWorkstation ELISA is a fully automated complete solution for processing ELISA in laboratories with high sample throughput. It has a high capacity of up to 15 microplates and can process more than 200 tests per hour.
The Tick-borne Encephalitis Virus Real Time PCR Kit is an IVD test based on real-time PCR technology for detecting RNA from the tickborne Encephalitis Virus (TBEV). It is compatible with multiple mainstream PCR instruments.
202 LMI-09-23 COM
203 LMI-09-23 LINKXPRESS COM
WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
Cont’d from page 5
AACC 2023 Roundup
CRP, an innovative hematology analyzer that provides rapid and precise measurement of C-reactive protein (CRP), a crucial biomarker for inflammation assessment. The company also highlighted the HumaFIA system, a high-performance fluorescence immunoassay analyzer designed for rapid and sensitive measurement of various analytes.














Puritan Medical Products (Guilford, ME, USA; www.puritanmedproducts. com ), the world’s most trusted manufacturer of swabs, exhibited its patented HydraFlock and PurFlock Ultra flocked swabs and other globallyrenowned products. By combining user feedback and a proprietary research manufacturing process, the company has developed a patented, intricate fiber technology that provides the highest collection and elution capacities available on the market. The company also demonstrated its expertise in topics such as automation, pandemic response, and virus protection, participating in panel discussions, joining in-depth symposiums, and introducing its various experts to their global counterparts.


Jiangsu Bioperfectus Technologies (Shanghai, China; www. bioperfectus.com) showcased its dependable solutions for every laboratory’s PCR needs while also highlighting its automatic nucleic acid extraction systems alongside its range of RUO products and raw materials for life science solutions. Bioperfectus Total PCR Solution offers holistic products of extraction systems, extraction reagents, real-time PCR systems, and RT-PCR reagents. The company’s new SSNP-2000B nucleic acid extraction system exhibited at the show integrates advanced technologies such as machine, electricity and computer software to enable the automatic nucleic acid extraction of a maximum of 32 samples at one time. Bioperfectus also showcased the STC-96A Plus Real-Time PCR System which is a more flexible, efficient and reliable breakthrough in real-time PCR analysis of gene expression and genetic variation.
LGC Clinical Diagnostics (Middlesex, UK; www.lgcgroup.com) showcased is full portfolio of quality measurement tools with complete access to its sub-brands including Maine Standards, SeraCare, Technopath Clinical Diagnostics, and The Native Antigen Company. LGC’s combined capabilities makes it better positioned to support the needs of the clinical diagnostics industry, from early feasibility and research to commercial & laboratory development test (LDT) assay development, installation, validation and ongoing performance moni toring support. LGC presented VALIDATE linearity & calibration ver ification materials, ACCURUN reference materials & QC products, the Multichem range of independent quality controls, and the VirtuE range of antigens & antibodies.
Globe Scientific (Mahwah, NJ, USA; www.globescientific.com) exhibited its newest 2023 laboratory plasticware, glassware and equipment, including its expanded Globe Glass laboratory glassware and PCR product lines. Visitors to the company’s booth were provided an overview of the complete Globe product portfolio with real, live product experts available to walk them through their preferred area of interest. Visitors also discovered Globe’s latest range of high-precision balances, engineered to deliver accurate measurements and enhance workflow efficiency. Among the impressive lineup of lab essentials and equipment showcased by Globe was the Globe | Euromex Microscopes.


Getein Biotech (Nanjing, China; www.getein.com) showcased its latest innovations, including its benchtop immunofluorescence and hematology analyzers as well as its handheld POCT analyzers. Getein demonstrated the Metis 600 integrated system that provides

a suitable solution for immunofluorescence and hematology testing to low volume laboratories. In addition, the company demonstrated the Getein 1160 immunofluorescence quantitative analyzer - a multi-channel POCT analyzer with four incubation channels and one emergency test channel. Alongside the Getein 1160, the company demonstrated the Getein 1100 immunofluorescence quantitative analyzer that is used to measure the concentration of biomarkers in human whole blood, serum, plasma, urine, nasal swab, or saliva samples. The results can be used as an aid in clinical diagnostics of laboratory and point-of-care testing.

Wondfo (Guangzhou, China; www.wondfo.com) provided a glimpse of several newly-launched products alongside its flagship products such as the Finecare FIA Meters (FS-114/FS-205), Accre CLIA Analyzer (A90) and Blood Gas Analyzer (BGA-102). At the

7 LabMedica International August-September/2023
@SINGUWAY is your
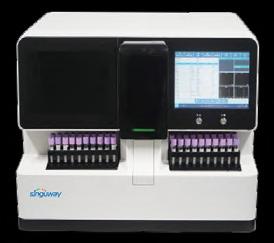
Singu20 Nucleic Acid Extractor AccuRa-32 Real-Time PCR System (32 samples) Singu20 Nucleic Acid Extractor AccuRa mini Real-Time PCR System (8-16 samples) ≤30 minutes qPCR Detection Kits (PCR fluorescence method): Respiratory Diseases: COVID-19 fluA fluB AdV TB and multiplex test Blood Diseases: HBV, HCV, HIV and multiplex test Sexually Transmitted Diseases: HPV, CT, NG, UU and multiplex test Viral Zoonotic Diseases: MPV Vector-borne Diseases: PF ZIKV Genetic Diseases: MTHFR Animal Diseases: Swine/Avian/Aquatic Animal/Ruminant/Companion Animal Diseases 5-Part Hematology Analyzer Hematology + CRP +SAA Joint Analyzer (Auto Sampling) ≤35 minutes 107 LMI-09-23 LINKXPRESS COM Cont’d from page 6 Cont’d on page 8 AACC 2023 Roundup
For small and medium labs,
answer.
5-PART HEMATOLOGY ANALYZER MINDRAY IN-VITRO DIAGNOSTICS



RUBELLA VIRUS TEST



Wondfo booth, Carolina Liquid Chemistries (CLC, Greensboro, NC, USA; www.carolinachemistries.com) showcased the RYAN, the “next generation” of fentanyl testing. RYAN is a portable fluorescence instrument that can be used in a laboratory or pointof-care setting with the Fentanyl Urine Detection Kit which is the first ever FDA cleared and CLIA categorized point-of-care fentanyl test. This fluorescence immunoassay is intended for the qualitative detection of fentanyl in human urine at a cutoff concentration of 1.0 ng/mL.
DiaSys Diagnostic Systems (Holzheim, Germany; www. diasys-diagnostics.com) exhibited its comprehensive portfolio of clinical chemistry analyzers, reagents, calibrators and controls, and also presented a scientific poster on procalcitonin. Among the company’s products highlighted at the show was the DiaSys CRP FS liquid-stable immunoturbidimetric test with dedicated ready-to-use calibrators and controls that allow true differentiation of borderline cases. DiaSys has recently extended its cardiovascular risk assessment portfolio by two innovative new reagents, HDL-c direct FS and LDL-c direct FS. Both are homogenous assays for direct determination. DiaSys has also expanded the application range for its Total bile acids 21 FS reagent and now offers the assay for use in serum and stool samples.
Randox Laboratories (Crumlin, UK; www.randox.com) exhibited its cutting-edge diagnostic products and solutions, while also highlighting its groundbreaking technologies. Randox’s new Acusera Quality Controls for 2022/2023 highlighted at the show included Active B12, Anti Mullerian Hormone (AMH), Brain Natriuretic Peptide (BNP) Control, Bone Markers (Serum) Control, Preeclampsia Control, Serum Indices Control, Ultra-Low PSA Control, Xanthochromia Control. Randox also showcased its recently launched Xylazine ELISA which is the first immunoassay test in the world for this drug of abuse and is available on the Evidence MultiSTAT analyzer. In addition, Randox unveiled Randox Health Labs located in Santa Monica, California. In recent years, Randox has been expanding its Randox Health testing – bringing preventative healthcare directly to the public. Randox’s extensive test menu offers over 150 analytes, allowing individuals to access to some of the most specialized tests available in the market today.
Meridian Bioscience (Cincinnati, OH, USA; www. meridianbioscience.com) unveiled two new animal-free immunoassay interference blockers, K-Block, and Mouse-Free IgG, launched by its Life Science division. These innovative blockers are completely animal-free and provide comparable or superior performance than traditional animal-derived blockers. Meridian’s new K-Block and Mouse-Free IgG overcome the obstacles associated with conventional animal-based blockers. These advanced blockers enhance assay specificity, reduce lot-to-lot variability, comply with sustainability considerations, and minimize contamination risks from animal-derived components.
Fapon Biotech (Guangdong, China; www.faponbiotech.com) introduced its latest chemiluminescence immunoassay (CLIA) system, the Shine i8000/9000. This ultra-high throughput, fully automated analyzer delivers initial results within 10 minutes at a rate of 900 tests per hour. The system’s high throughput per footprint, delivering 377 tests/hour/m2, makes it an ideal choice for labs seeking to boost their testing capacity and efficiency. The launch of the Shine i8000/9000 further expands the company’s extensive portfolio of high, medium, and low-throughput chemiluminescence analyzers. Fapon also delivered an interactive lecture titled “Bioanalytical Performance of Novel Protein-free Blockers & Heterophilic Antibody Interference Blockers for the IVD Industry & Clinical Diagnostics,” offering insights into the latest advancements in IVD blockers.
General Biologicals (GBC, Hsinchu, Taiwan; www.gbc.com. tw) introduced two CellBio circulating tumor cell (CTC) cancer detection systems that are recognized globally as cutting-edge products offering rapid, accessible, and affordable diagnostic solutions for early detection, treatment validation, and therapy monitoring. The CellBio platform employs GBC’s proprietary iFiltration technology to provide positive control and guarantee filtration accuracy. It offers several advantages including high specificity, sensitivity, and cancer capture rate from 7.5-mL blood samples, delivering results within 90 minutes. GBC also launched its automated all-in-one molecular system, GB RealQuant AIO. This integrated instrument combines extraction, amplification, and detection, delivering fast and reliable results within two hours with high flexibility.
Hilab (Manaus, Brazil; www.hilab.com.br), a frontrunner in innovative healthcare solutions, launched a unique point-of-care Hemogram device—the world’s only instrument capable of providing a comprehensive blood count using AI and other cutting-edge
8 LabMedica International August-September/2023
BC-5000 is the lightest and most compact 5-part hematology analyzer so far from Mindray, tailored to assist labs in need of full CBC+5-part results, with relatively low daily sample volume, restricted lab space, and tight budget.
VIRCELL
RUBELLA ELISA is an indirect immunoenzyme assay for antibodies against rubella virus in human serum/plasma, suitable for automated ELISA systems. It includes G1026 reference calibrators for additional semi-quantification protocol.
205 LMI-09-23 COM
WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
206 LMI-09-23 LINKXPRESS COM
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
Cont’d from page 7 Cont’d on page 9
AACC 2023 Roundup
technologies within just 30 minutes. Hilab also introduced two more devices — the Hilab Volt and the Hilab Wave. The Hilab Volt, which works on the principles of electrochemistry, reads an electrode that selectively interacts with the sample, generating an insightful analytical electrical signal. It can help evaluate parameters such as calcium, sodium, potassium, iron, and glucose, among others. The Hilab Wave, functioning via spectrophotometry—an optical analysis methodology used for quantitative measurement of light absorption by solutions—facilitates numerous tests, including those for phosphorus, magnesium, cholesterol, Vitamin D, and even for detecting Malaria.
OnsiteGene (San Diego, CA, USA; www.onsitegene.com) presented the world’s first large sample volume, open-assay, super-fast, ultra-sensitive, and sample-to-answer PCR instrument. The groundbreaking PeakV from OnsiteGene represents a paradigm shift in PCR testing. By utilizing rapid thermal cycling and real-time fluorescent imaging to perform 40 cycles within a span of just five minutes, it delivers an unmatched performance. The device can carry out low copy multiplex qPCR in merely seven minutes and RT-qPCR in nine minutes. Equipped with a substantial sample volume capacity of up to 5mL, PeakV also features automated nested PCR and magnetic beads-based nucleic acid extraction, thereby enhancing its sensitivity and specificity, outperforming conventional qPCR techniques.
Sarstedt (Nümbrecht, Germany; www.sarstedt.com), a leader in pre-analytic innovation, demonstrated its solutions for improving specimen quality, reducing turnaround time, and automating labs of all sizes with customizable modular systems. On display was the SARSTEDT Tempus600®, a dedicated transportation system for sending specimen tubes quickly and reliably to the laboratory without batching or packing into carriers. SARSTEDT demonstrated how the Tempus600® and IVD systems such as the Roche cobas® connection modules work together to shorten the time between tests being ordered and results being ready. Visitors watched how blood collection tubes left the SARSTEDT booth and arrived at the Roche booth in seconds.
Prevencio (Kirkland, WA, USA; www.prevenciomed.com ) showcased its leading-edge, AI-powered HART platform and blood tests. The HART platform is disease agnostic, thereby allowing the development of diagnostics that extend beyond cardiac diseases. Presently, Prevencio’s two primary tests, HART CVE and HART CADhs, are accessible to medical professionals for patient use. HART CVE calcu-
lates a patient’s one-year risk for a heart attack, stroke, or cardiovascular death, whereas HART CADhs evaluates if a patient has a blockage in the heart arteries, indicating an imminent risk of a heart attack.

PBD Biotech (Birmingham, UK; www.pbdbio.com) showcased Actiphage, a phage-based test that detects Mtb in the blood – which is considered an early indicator of ‘active’ TB disease. The test shows potential for screening latent TB and drug management. Actiphage combines phage-based DNA extraction with polymerase chain reaction to identify low levels of viable Mtb in blood. Actiphage was also highlighted in the AACC webinar titled “Phage-Based Laboratory Diagnostics Role in the Detection of Tuberculosis,” sponsored by PBD Biotech. At the webinar, experts discussed the epidemiology of tuberculosis and the limitations of current tests that assess host response. Attendees gained insights into how phage-based diagnostic technology can be employed to detect incipient and active TB.
The ADLM Annual Meeting & Clinical Lab Expo 2024 will take place on 28 July–1 August 2024 in Chicago, IL, USA.

9 LabMedica International August-September/2023 109 LMI-09-23 LINKXPRESS COM
DiaSys. Total Confidence in Patient Results. www.diasys-diagnostics.com · Clinical chemistry and immunoturbidimetric reagents · Automated analyzers for every need · Dedicated calibrators and controls Continuing service and training GERMANY HEADQUARTERS HOLZHEIM DiaSys Diagnostic Systems GmbH Clinical chemistry solutions worldwide SINCE 1991 Your trusted and innovative manufacturer RZ_Anzeige_LabMedic_Clinical_worldwide_141x185_engli_230712.indd 1 12.07.23 08:40 Cont’d from page 8 AACC 2023 Roundup
Predictive Test Improves Immunotherapy Results
inadequate in accurately predicting therapy responses. This inefficiency leads to unnecessary healthcare expenses and, more critically, negative patient outcomes. Now, a new test, that utilizes an advanced multidimensional immune biomarker can predict which cancer patients are likely to respond to monotherapy of immune checkpoint inhibitors like Keytruda.
Cofactor Genomics (San Francisco, CA, USA; www.cofactorgenomics.com) employs Predictive Immune Modeling – a technique that utilizes RNA data and machine learning to combine biological signals, thus creating multidimensional biomarkers that fulfill the goal of precision medicine. Cofactor’s ImmunoPrism platform reveals each patient’s immune profile using RNA from FFPE solid tumor samples.
With its machine learning-based Predictive Immune Modeling approach, the company combines key immune signals into a predictive biomarker. These biomarkers are then utilized in Cofactor’s OncoPrism test, which assists clinicians in determining the probability of a patient’s positive response to immunotherapy.

Cofactor is now leveraging samples and data from one of the largest biobanks in the U.S. to expedite the development of the OncoPrism test for 11 types of cancer. The company’s goal is to establish biomarkers for cancers under study in the national PREDAPT (Predicting Immunotherapy Efficacy From Analysis of Pre-treatment Tumor Biopsies) clinical trial. The initial focus is on examining OncoPrism in head, neck, and lung cancers and will soon broaden to include nine other approved indications such as triple-negative

Image: The first ever diagnostic test accurately predicts patient response to immunotherapy (Photo courtesy of Cofactor)
breast, cervical, colorectal, esophageal, gastric, kidney, liver, and urothelial cancers. Preliminary results for the head and neck cancer biomarker indicate that Cofactor’s method is twice as accurate as the PD-L1 biomarker in identifying the subgroup of patients who respond positively to immune checkpoint inhibitors.
Comprehensive Diabetes Testing Guidelines Released
Diabetes, a condition impacting an estimated 537 million people worldwide, is a disorder where the body either underproduces or underutilizes insulin, causing glucose (or sugar) from food to accumulate in the blood. The condition predisposes people to an elevated risk of heart attacks, stroke, vision loss, kidney disease, and other health issues. Now, the Association for Diagnostics and Laboratory Medicine (ADLM, Washington, DC, USA; myadlm.org), in collaboration with the American Diabetes Association (Arlington, VA, USA; diabetes.org), has released evidence-based guidelines to assist in diagnosing and managing patients with diabetes using the most advanced laboratory analysis tools. These updated guidelines stem from an exhaustive evaluation of cutting-edge diabetes laboratory tests' quality and efficacy.
The recently introduced guidelines replace those published in 2002 and 2011 and offer
comprehensive updates on continuous glucose monitoring and more accurate guidance for glucose and hemoglobin A1c measurements, which serve as an average blood glucose marker. A multidisciplinary team of medical experts has authored the new guidelines, providing specific, actionable suggestions designed to encourage collaboration among healthcare professionals and improve care for millions of individuals.
One of the key inclusions in the 2023 document is its in-depth guidance regarding continuous glucose monitoring (CGM). With this technology, a device gauges a patient's blood glucose every 5-15 minutes, and an automatic pump dispenses insulin when necessary. The expert panel strongly advocates the use of real-time CGM, coupled with insulin, in certain teenagers and adults with type 1 diabetes—a form of disease where the body fails to produce insulin. The guidance applies to type
1 diabetes patients struggling to meet their blood sugar (glycemic) targets, those unaware of their low blood sugar, and/or those prone to hypoglycemia. While CGM isn't often employed for type 2 diabetes—where the body doesn't utilize insulin effectively—the guidelines recommend healthcare providers consider CGM for insulin-dependent type 2 patients who are not meeting glycemic targets.
The new guidelines also suggest that healthcare professionals use blood collection tubes containing a citrate buffer to prevent glucose breakdown after blood sample collection, as this could compromise measurement accuracy. When these tubes aren't available, an alternative technique involving an ice-water bath and other steps is recommended. According to the guidelines, fasting glucose values above 7.0 mmol/L (>126 mg/dL), and hemoglobin A1c measures of at least 6.5% (>48 mmol/mol), should be

Cont’d on page 11



10 LabMedica International August-September/2023
DENGUE IGG/IGM TEST WAMA DIAGNÓSTICA
The Imuno-Rápido Dengue IgG/IgM is a solid-phase immunochromatographic assay for qualitative and differential detection of antibodies against the 4 serotypes of Dengue virus in human serum/plasma (EDTA, Sodium citrate, and Heparin).
DIAGNOSTICS
CLINICAL CHEMISTRY SYSTEM SIEMENS HEALTHINEERS - LABORATORY
208 LMI-09-23 COM 209 LMI-09-23 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
The ADVIA 2400 clinical chemistry system offers a high throughput of up to 2400 tests/hour (1800 photometric, 600 ISE) and high turnaround-time (TAT). It includes testing methods for DOAs, therapeutic drugs, hsCRP and cystatin C.
Cont’d from cover
Candida Auris Test Takes Only 3-5 Hours

Cont’d from cover







crucial benefits of early detection and appropriate treatment. Now, a direct-from-blood molecular diagnostic test can detect C. auris directly from blood in just 3-5 hours, eliminating the need to wait for several days for a positive blood culture.
T2 Biosystems’ (Lexington, MA, USA; www. t2biosystems.com) C. auris direct-from-blood molecular diagnostic test has been granted Breakthrough Device designation by the U.S. Food and Drug Administration (FDA). This is the third product from T2 Biosystems to receive FDA Breakthrough Device designation after the company’s T2Resistance Panel and T2Lyme Panel previously received the same designation. The company intends to expand its FDAapproved T2Dx Instrument’s test options by including the C. auris diagnostic test, designed to detect C. auris directly from the blood within 3-5 hours, thus eliminating the days-long wait for a positive blood culture.
Currently, T2 Biosystems markets and sells the T2Candida Panel, the only FDA-approved diagnostic test capable of detecting sepsis-causing fungal pathogens directly from blood, thereby eliminating the need to wait for days for a positive blood culture. The T2Candida Panel, which runs on the fully automated T2Dx Instrument, can simultaneously detect five Candida species, including Candida albicans, Candida tropicalis, Candida parapsilosis, Candida krusei, and Candida glabrata. Rapid detection


Comprehensive Diabetes Testing Guidelines Released

Cont’d from page 10
deemed indicative of diabetes.
Furthermore, the guidelines offer new guidance for standardizing measurements of insulin and C-peptide—a marker that differentiates between type 1 and type 2 diabetes. The latest updates also cover autoimmune and genetic markers for type 1 diabetes, including a promising gene mutation analysis that can identify rare neonatal diabetes forms. The newly-released guidelines aim to equip the entire healthcare team with the necessary tools to make the most effective clinical decisions by fostering a deeper understanding of lab tests' strengths and limitations.
"It's important to measure accurately—but it's also very important to communicate the relevance to clinicians and to listen to them and share information," said Dr. David Sacks, the guidelines' primary author. "Patient care is a team effort."





of these pathogens, as well as C. auris, is critical to ensuring infected patients receive suitable antifungal therapy, enhancing clinical outcomes.




“We are pleased with the FDA’s decision to grant Breakthrough Device designation for our Candida auris test, which provides greater and more frequent access to the FDA and may accelerate our path to FDA clearance,” said John Sperzel, Chairman and CEO of T2 Biosystems. “We believe adding Candida auris to the test menu on our FDA-cleared T2Dx Instrument will provide clinicians with a valuable tool to rapidly detect a dangerous, multidrug-resistant fungal pathogen much faster than blood culture-based methods, strengthening our value proposition and increasing the attractiveness of our products to U.S. hospitals.”
Digital Microscopy and AI
Clinical and Research Applications

LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 11 LabMedica International August-September/2023
111 LMI-09-23 LINKXPRESS COM
Image: The Candida Auris diagnostic test has received FDA Breakthrough Device Designation (Photo courtesy of T2 Biosystems)
AI Tool Enables In-Surgery



Genomic Profiling of Brain Tumor for Real-Time Guidance
Precise molecular diagnostics, which involve detailing DNA changes within a cell, can significantly influence a neurosurgeon's decision-making during surgery, such as the extent of brain tissue to be excised. Over-removal in the case of less aggressive tumors can negatively impact a patient's neurological and cognitive functioning, while under-removal in the case of highly aggressive ones can leave malignant tissue behind, resulting in rapid growth and spread. Current intraoperative diagnostic methods involve brain tissue freezing and microscopic examination, but these techniques often distort cell appearance and compromise clinical assessment accuracy. Furthermore, the human eye, even with advanced microscopes, can fail to reliably identify subtle genomic variations on a slide. Now, a novel artificial intelligence (AI) approach addresses these issues.


Scientists at Harvard Medical School (Boston, MA, USA; www.hms. harvard.edu) have developed an AI tool capable of swiftly decoding a brain tumor's DNA to determine its molecular identity during surgery. This process can take several days or even weeks using traditional methods. Having immediate access to a tumor's molecular type helps neurosurgeons decide on the extent of brain tissue removal and the application of tumor-killing drugs directly into the brain, all while the patient is still on the operating table. Modern advances in genomics have enabled pathologists to distinguish molecular signatures and associated behaviors among various brain cancer types. Aggressive glioma, for instance, has three main subvariants, each bearing unique molecular markers and growth propensities. Although AI models have been developed to profile other cancer types (e.g., colon, lung, breast), gliomas present unique challenges due to their molecular complexity and vast variation in tumor cell morphology.
The newly developed tool, named CHARM (Cryosection Histopathology Assessment and Review Machine), significantly expedites molecular diagnostics, which can be particularly useful in regions with limited access to technology for quick cancer genetic sequencing. CHARM was developed using 2,334 brain tumor samples from 1,524 individuals with glioma from three distinct patient populations. The tool exhibited a 93% accuracy rate when identifying tumors with specific molecular mutations in an unseen set of brain samples, and it successfully classified three major types of gliomas with distinct
molecular features. Moreover, the tool was adept at visually analyzing tissue surrounding malignant cells, identifying areas of greater cellular density and higher cell death rates, both of which are indicators of more aggressive glioma types.
Additionally, CHARM was able to detect clinically important changes in a subset of low-grade gliomas, a less aggressive glioma subtype that is less likely to invade surrounding tissue. The tool further linked cellular appearance with the molecular profile of the tumor, thereby enabling the algorithm to determine how a cell's appearance relates to the tumor's molecular type. This comprehensive assessment improves the model's accuracy and mirrors how a human pathologist would visually evaluate a tumor sample. While CHARM was initially trained and tested on glioma samples, the researchers believe it can be successfully retrained to identify other brain cancer subtypes. However, the tool would require periodic retraining to reflect new disease classifications as they emerge from new findings. Although CHARM is freely available to other researchers, it needs clinical validation through real-world testing and FDA clearance before it can be used in hospitals.
“Right now, even state-of-the-art clinical practice cannot profile tumors molecularly during surgery. Our tool overcomes this challenge by extracting thus-far untapped biomedical signals from frozen pathology slides,” said study senior author Kun-Hsing Yu, assistant professor of biomedical informatics in the Blavatnik Institute at HMS.
12 LabMedica International August-September/2023
DOA/ALCOHOL PANEL TEST LUMIQUICK DIAGNOSTICS
Quick Profile DOA/Alcohol Panel Test is an immunochromatography-based one-step in vitro test for the qualitative determination of drug substances in human urine specimens. With a reading time of five minutes, it can used at POC.
PIPETTE TIPS ACCUMAX
Accumax’s Pipette Tips are precise and reliable, enabling accurate liquid handling, with an ergonomically optimized cone geometry that provides optimal fit for both Accumax pipettes and pipettes from other manufacturers.
211 LMI-09-23 COM
212 LMI-09-23 LINKXPRESS COM
information on products, log on to www.linkXpress.com
on
mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
To receive prompt and free
or scan the QR code
your
Image: An AI tool can decode brain cancer’s genome during surgery (Photo courtesy of Freepik)
New Platform Brings Power of Multiplex Imaging to Clinical Pathology

development carried out by a team of researchers led by Harvard Medical School (Boston, MA, USA; www.hms.harvard.edu). Orion is a sophisticated digital imaging platform that integrates the insights gathered from traditional histology with the details derived from molecular imaging of a tumor sample. Over the years, the team has been focusing on refining imaging tools for human tissue samples. In a recent study published in the journal Cell, they integrated a technique known as cyclic immunofluorescence, or CyCif, with histology to generate comprehensive maps of colorectal cancer. These maps, accessible to scientists worldwide as part of the Harvard Tissue Atlas (HTA), offer an unprecedented level of detail about the tumors.
However, the researchers wanted to make these imaging tools accessible to clinicians who regularly analyze tumor samples under a microscope to gather essential information for patient diagnosis and treatment. In collaboration with the company RareCyte, they designed a digital imaging platform capable of quickly collecting and analyzing images from both traditional and multiplex immunofluorescence methods on the same tissue sample. The resulting image integrates information from both techniques, providing a comprehensive view of the tumor.
As described in a study published on June 22, 2023 in Nature Cancer, the researchers used Orion to examine tumor samples from over 70 patients with colorectal cancer. The tool provided valuable histological and molecular information for each sample and identified biomarkers that were common in patients with severe disease. These biomarkers, based on specific combinations of tumor features, could predict the progression of colorectal cancer. The researchers are optimistic that, with further refinement, Orion can contribute significantly to the diagnosis and treatment of cancer and other diseases by providing in-depth details about tumors and patient samples. While Orion is in its nascent stages of development, the promising initial results substantiate the potential utility of the platform in a clinical setting.
The researchers plan to refine Orion further by testing it on a larger patient pool and exploring effective combinations of antibodies. The team aims to streamline the platform to make it quicker and more affordable and to extend its application to other cancers like lung cancer and melanoma, and potentially to other conditions such as kidney disease and neurodegenerative diseases. The team envisions Orion as a tool that pathologists can integrate into their existing workflow to supplement their histological expertise with molecular details, providing a holistic understanding of a sample. Significantly, Orion’s digital nature
Machine Learning Solution Helps Detect Precancerous Cervical Lesions
Cervical cancer ranks as the fourth most prevalent cancer in women, with a reported 604,000 new occurrences in 2020, as per the World Health Organization (WHO). Yet, it stands out as one of the most successfully preventable and treatable cancers, provided that it is detected early and managed appropriately. Therefore, early detection of pre-cancerous lesions is critical for disease prevention. Now, researchers have developed an innovative method using large, high-resolution images to detect crucial pre-cancerous lesions.
A team of researchers from INESC TEC (Porto, Portugal; www. inesctec.pt) and IMP Diagnostics (Porto, Portugal; www.impdiagnostics. com) has designed a machine learning solution to aid pathologists in detecting cervical dysplasia, making the diagnosis process of new samples fully automatic. The researchers set out to develop machine learning models to support the subjective classification of lesions in the squamous epithelium - the protective tissue layer against microorganisms
Cont’d on page 14
enables pathologists to examine the images on any computer, thereby eliminating the need for a microscope in a lab or clinic.

“Pathologists already do a huge amount of work with histology to diagnose a patient and understand their disease, but with this tool to augment their knowledge, they will basically have a ‘super view’ of the sample,” said Jia-Ren Lin, study lead author and platform director in the Laboratory of Systems Pharmacology at HMS.
(- 60 °C to 300 °C) Fully Washable & Flexible Suitable for central sterilization services Sterilizable
















LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 13 LabMedica International August-September/2023
Cont’d from cover 113 LMI-09-23 LINKXPRESS COM Size: 2400 x 1200 mm (3 mm thick) 100% Silicone YOUR GLOBAL SOURCE FOR STERILIZATION ACCESSORIES Thermo-Resistant
STERILIZABLE INSTRUMENT & WORK-SURFACE MATS Front Back WASHING TRAYS MAT Heavy Silicone Cover & Transport Tablet TURBO WASHING MACHINES TRAYS SILICON INSTRUMENT MAT Front Back MICRO INSTRUMENT MAT Exchangable Net Exchangable Nets INVITEDTOAPPLY DISTRIBUTORS Up to 37 cm in length THERMO RESISTANT GLOVES Front Back WASHING TRAYS MAT NEW! VICOTEX Place de la Gare 1 • 1009 Pully • Switzerland Tel: (41) 21-728-4286 • Fax: (41) 21-729-6741 E-Mail: contact@vicotex.com www.vicolab.com S.A. SILICONE TABLET AND STEEL COVER NETS
Image: The Orion tool combines structural details with molecular information about a tumor (Photo courtesy of Harvard Medical School)
XYLAZINE IMMUNOASSAY TEST RANDOX LABORATORIES



infectious agents and pathogenic bacteria. In addition to bacteria and viruses, certain protozoa species are major contributors to GI illnesses worldwide. Presently, the diagnosis of GI protozoan infections relies mainly on sample culture, microscopy, antigen detection, and pathogen-specific molecular tests. This traditional approach is well-known for being time-consuming, variable in reliability, labor-intensive, and can take days to yield results. Now, a rapid molecular test can screen for eight of the most common GI parasites, offering significant advantages over currently available diagnostic methods.
The EasyScreen Gastrointestinal Parasite Detection Kit from Genetic Signatures (NSW, Australia; www.geneticsignatures.com) offers a rapid molecular test covering the eight most common and clinically relevant GI parasites. This kit is already available in Australia, Europe, and Canada. Sites that have adopted this kit for syndromic GI screening have experienced faster results, increased reliability, and workflow efficiencies. Genetic Signatures has taken steps to obtain regulatory clearance from the Food and Drug Administration (FDA) through a 510(k) application to market the EasyScreen Gastrointestinal Parasite Detection Kit in the United States (US). The US market represents a substantial opportunity, with an estimated total Addressable market (TAM) of 5.5 million tests annually.


The 510(k) submission includes data from 1,500 clinical samples collected from various sites across the US. Notably, this kit includes GI pathogen targets that are not currently available in other commercial products. The absence of available predicate tests for these specific pathogen
targets required Genetic Signatures to develop new validation methods for the FDA 510(k) submission. The company is actively preparing for the anticipated commercial launch of the EasyScreen Gastrointestinal Parasite Detection Kit upon FDA clearance and is collaborating with pre-qualified customer experience sites in the US to assess the kit’s performance.

“We are very excited to achieve this significant milestone and I am very appreciative of the hard work that has been done by the staff at Genetic Signatures, our advisors and the clinicians,” said John Melki, Managing Director and CEO of Genetic Signatures. “The US is the largest, single market for molecular diagnostic tests and represents significant opportunity for our EasyScreen Gastrointestinal Parasite Detection Kit. With a greater range of GI parasite targets provided in this syndromic solution, and the unique advantages of our 3base technology to detect these parasites, it is the ideal product to launch into the US market. Our plan to achieve additional product registrations in the US continues, with clinical studies to support a FDA 510(k) application of a second 3base product already underway. This is a molecular syndromic test for key viral respiratory infections in a single test.”
Machine Learning Solution Helps Detect Precancerous Cervical Lesions
Cont’d from page 13
- using whole-slide images (WSI) that contain information from the entire tissue.
The team developed a weakly-supervised methodology - a machine learning method that combines annotated and non-annotated data in the model training phase to classify cervical dysplasia. This technique proves particularly beneficial considering the difficulty of obtaining pathology data annotations: the large image sizes make the annotation process extremely time-consuming, tedious, and highly subjective. This methodology enables researchers to establish models with high performance, even when there's some missing information during the training phase. The resulting model can then grade cervical dysplasia,
or abnormal cell growth on the surface, as low (LSIL) or high-grade intraepithelial squamous lesions (HSIL). Given the complexity and subjective nature of the classification process, these machine learning models can provide valuable assistance to pathologists. Furthermore, these systems could act as an early warning mechanism for suspicious cases, alerting pathologists to instances that warrant closer examination.
"In the detection of cervical dysplasia, this was one of the first published works that use the full slides, following an approach that includes the segmentation and subsequent classification of the areas of interest, making the diagnosis of new samples completely automatic," explained Sara Oliveira, a researcher at INESC TEC.
14 LabMedica International August-September/2023
The newly-established Xylazine ELISA is the first immunoassay test in the world for this drug of abuse. It is available on the Evidence MultiSTAT analyzer that screens for up to 29 DOAs from a single sample in less than 31 minutes.
SUITCASE LABORATORY XI’AN TIANLONG SCIENCE & TECHNOLOGY
Tianlong suitcase laboratory iGenecase 1600 with mobile power supply contains all necessary devices (GeneFlex Nucleic Acid Extractor, Gentier mini Portable Real-Time PCR System) and compatible consumables for PCR detection.
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
214 LMI-09-23 COM
215
LMI-09-23 LINKXPRESS COM
Cont’d from cover Rapid Molecular Test Detects 8 Common GI Parasites
Portable Tool Can Diagnose and Monitor Sickle Cell Disease
Sickle cell disease is a lifelong, inherited blood disorder characterized by rigid, sickle-shaped red blood cells due to a severe alteration in their morphology. These misshaped cells can clog blood vessels, impeding blood flow and leading to unpredictable, painful episodes when tissues become oxygen-deprived. The most prevalent and serious complications of sickle cell disease include anemia, pain, and organ failure – with stroke affecting roughly 10 out of every 100 children diagnosed with the condition. The primary objective in managing sickle cell disease is to prevent these painful crises, which require diagnostic and monitoring tools under medical supervision. However, current tools are cumbersome, expensive, and require specialized training. The gold standard methods used to monitor and diagnose the disease, primarily genetic tests and optical microscopy of sickle-shaped red blood cells, are time-consuming, prone to delays, and fail to capture real-time changes.
Currently, there are no commercial tools that allow for the continuous monitoring of sickle cell disease and no portable field sensor that can quantitatively measure and monitor cell sickling events using small blood samples. This leaves patients, whether diagnosed or undiagnosed, vulnerable. As morphological changes from repeated cell sickling events can result in permanent cell damage, rapid diagnosis, and treatment are critical. In response to this challenge, researchers at Florida Atlantic University (Boca Raton, FL, USA; www.fau.edu) have utilized microfluidics, flow cytometry, and electrical impedance to develop an innovative solution that offers patients a better means of managing their disease. Cytometry measures cells and other biological particles, while flow cytometry measures the size, shape, and quantity of cells moving in a fluid stream. Impedance-based flow cytometry provides information on individual particles by measuring changes in impedance values created by particles passing through measurement electrodes. Yet, the current equipment used for these measurements is expensive and cumbersome.
This new invention offers an alternative for patients and healthcare providers. The device consistently and swiftly monitors sickle cell disease using a microfluidics-based electrical
impedance sensor, which can determine the rate of cell sickling and the percentage of sickled cells. It can identify the dynamic processes of cell sickling and unsickling in sickle blood without the need for microscopic imaging or biochemical markers. Assisted by a computer application created for the device, users can conduct impedance scans over specific time lengths, plot the measured impedance magnitude and phase, and directly share the raw data from a smartphone. This portable device, weighing approximately one pound, is handheld and simple to operate.
“There are many advantages to using this
device such as portability and affordability,” said Sarah Du, Ph.D., an associate professor

CALL FOR PROPOSALS
Making your mark in the world of laboratory medicine requires bold thoughts and actions. Every observation, hypothesis and innovation stretches the potential of our profession and improves patient outcomes. This is your opportunity to showcase your hard work to inspire curiosity and discovery in the global community that will gather in Chicago next July.
Ready to show us what you’re made of? Submit your proposal for the 2024 edition of ADLM University, Roundtables and Scientific Sessions.
Visit meeting.myadlm.org for a complete list of submission guidelines and session categories.
Deadline for submissions is November 3.
15 LabMedica International August-September/2023 LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
ZAHRA SHAJANI-YI PHD, DABCC (CC), FAACC
Image: Sickle cell disease is a blood disorder characterized by rigid and sickle-shaped red blood cells (Photo courtesy of Shutterstock) Cont’d on page 16
New Method Can Reveal Bacterial Reaction to Antibiotics in 5 Minutes

Severely sick patients suffering from bacterial infections often require immediate treatment to prevent serious health complications, making it vital for physicians to quickly identify the appropriate antibiotic. However, existing approaches to determining antibiotic resistance can involve extensive periods, sometimes hours or even days. This has led to the frequent prescription of broad-spectrum antibiotics, heightening the risk of antibiotic resistance. Now, a simple method has been developed that can detect bacterial response to antibiotics within just five minutes.
Researchers at Karolinska Institutet (Solna, Sweden; www. karolinskainnovations.ki.se) set out to reduce the unwarranted use of antibiotics by devising a rapid method to assess how bacteria react to different environmental conditions, including antibiotic administration. They developed the 5PSeq method, which relies on sequencing the messenger RNA (mRNA) that is broken down by the bacteria as they synthesize proteins. The researchers employed the 5PSeq method to examine mRNA breakdown intermediates in isolated species and complex microbiomes. They tested the method on 96 bacterial species from diverse phyla in complex clinical samples, such as fecal, gut, and vaginal samples, as well as compost samples. In a matter of minutes, the researchers were able to determine whether the bacteria were reacting to the antibiotic treatment; the effect was most noticeable after about half an hour.

By utilizing metadegradome sequencing - parallel analysis of RNA ends - the team characterized 5’P mRNA decay intermediates in all 96 species, including Bacillus subtilis, Escherichia coli, Synechocystis spp., and Prevotella copri. They discovered co-translational mRNA degradation to be common among bacteria and generated a degradome atlas for the 96 species, facilitating the further study of RNA degradation mechanisms in bacteria. In addition to measuring antibiotic resistance, the method can be employed to help scientists understand how bacteria manage diverse environmental pressures, and how they interact both with each other and with their hosts. The researchers plan to continue investigating complex intestinal samples to gain deeper insights into the interactions of bacterial communities in the gut and their effects on human health. The aim is to refine the method and develop a rapid molecular test for clinical application.

“We demonstrate that metadegradome sequencing provides fast, species-specific posttranscriptional characterization of responses to drug or environmental perturbations,” the researchers wrote. “Our work
paves the way for the application of metadegradome sequencing to investigation of posttranscriptional regulation in unculturable species and complex microbial communities.”

The results of the research were published on May 22, 2023 in Nature Microbiology.


Portable Tool Can Diagnose and Monitor Sickle Cell Disease
Cont’d from page 15 in Florida Atlantic University’s Department of Ocean and Mechanical Engineering within the College of Engineering and Computer Science, who recently received a patent from the United States Patent and Trademark Office for the novel invention. “Importantly, this device will provide users with measurements to diagnose their sickle cell disease severity and compare normal versus diseased sickle cell red blood cell samples. These longitudinal measurements will only require an extremely low sample of blood such as from a finger stick to allow patients to monitor their disease.”
“The combination of electrical impedance measurements and microfluidics provides a promising method to rapidly assess the dynamic processes of cell sickling and unsickling in patients with sickle cell disease,” added Stella Batalama, Ph.D., dean, FAU College of Engineering and Computer Science. “Professor Du’s cutting-edge technology, which has received an important U.S. patent, will provide patients with sickle cell disease opportunities to reliably and conveniently monitor their disease in the same way patients with diabetes can monitor their disease using a glucometer.”
16 LabMedica International August-September/2023
POINT-OF-CARE FENTANYL TEST CAROLINA LIQUID CHEMISTRIES
The Fentanyl Urine Detection Kit is a fluorescence immunoassay for use with RYAN portable immunofluorescence analyzer and is intended for the qualitative detection of fentanyl in human urine at a cutoff concentration of 1.0 ng/mL.
COVID-19 RAPID TEST SEKISUI DIAGNOSTICS
The OSOM COVID-19 Antigen Rapid Test is a lateral flow chromatographic immunoassay intended for the qualitative detection of the SARSCoV-2 nucleocapsid protein antigen in direct mid-turbinate (MT) nasal swab specimens.
217 LMI-09-23 COM
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
218 LMI-09-23 LINKXPRESS COM
Edited by Katherina Psarra, MSc, PhD
MESSAGE FROM THE PRESIDENT
 By Khosrow Adeli • President, IFCC
By Khosrow Adeli • President, IFCC

Greetings to everyone in the IFCC family! I hope you have all had a wonderful summer. The IFCC Executive Board is looking forward to a very productive Fall season in collaboration with all IFCC functional groups and affiliated organizations.
There were several important events held this summer by IFCC national societies. Among these events one holds special significance: the 60th anniversary celebrations held by the Vietnamese Society of Clinical Biochemistry (VACB)—a truly remarkable accomplishment. I had the privilege of representing IFCC during this exceptional event in Hanoi and offering support to VACB as they celebrated this significant milestone. VACB was formed in 1963 even amid the challenging backdrop of the Vietnam War!
Another major event held this summer was the AACC conference in Anaheim during which AACC formally changed its name to ADLM (Association for Diagnostics and Laboratory Medicine). The new name broadens the Association’s scope to encompass several other domains within laboratory medicine and is hoped to expand membership and enhance visibility of the organization in the eye of the public and other stakeholders. During the conference in Anaheim, the IFCC Executive Board met with the AACC/ADLM Executive Board and exchanged ideas on ways to expand collaboration between our two organizations. Several areas were identified where we have common interests and activities and it was agreed to join forces so that we minimize duplication and enhance productivity on various international projects in areas such as newborn screening, global lab quality, as well as educational programs.
The IFCC Executive Board also met separately during the Anaheim conference and discussed many other IFCC priorities including IFCC clinical laboratory practice guidelines, conference organizational matters, and accreditation of future WorldLab Congresses. The Executive Board has formally approved the formation of a new Working Group on Continuing Education/Accreditation to begin planning accreditation of the Dubai WorldLab 2024 scientific program as well as other future IFCC events in collaboration with the CPECS program developed by EFLM.
Progress is also being made on development of the new IFCC Clinical Laboratory Practice Guidelines. The program is aimed at development and dissemination of best practice recommendations/guidelines in all areas of clinical laboratory medicine and facilitating implementation in clinical laboratories worldwide, particularly in developing countries. Under this program, IFCC functional units will be encouraged to employ their expertise to develop one or more of the following documents: best practice recommendations, clinical laboratory consensus statements or position papers based on evidence from wide range
of existing reputable guidelines, peer-reviewed publications, and expert consensus as well as clinical laboratory practice guidelines (CLPG) (evidence-based using a grade system using a standardized methodology for producing guidelines). Stay tuned! a formal announcement will be made over the coming weeks and all IFCC functional units will be invited to participate and submit proposals for development of such best practice recommendations and guideline documents.
Looking forward to a very productive fall season for IFCC member societies, national federations, and colleagues from around the world. As always, feel free to email me at president@ifcc.org with your feedback, questions, or concerns.

17 LabMedica International August-September/2023 NEWS
news
IFCC members may send
to Email: enews@ifcc.org
Cheers, Khosrow
EDITORIAL
By Katherina Psarra, MSc, PhD

Dear Colleagues, I hope you have really enjoyed your holidays, and came back full of energy. This summer was difficult for a lot of people worldwide due to the high temperatures, the fires, the extreme climatic phenomena. Nevertheless, the beautiful holidays memories will keep us company during the months at work lying ahead.
In this relatively small eNews issue you will read about sensational events in the IFCC family, that took place during the summer, as they are described by our President, Prof Khosrow Adeli, who invites all of us to a productive IFCC fall.
Two descriptions of the experiences with the IFCC Scientific Exchange Program make us realize the importance of this program. It permits our colleagues to live in a foreign country, to participate in the life of an important lab, to
acquire and share knowledge with other colleagues, to bring this knowledge to one’s own lab, to make friends and be inspired. Read these reports and get prepared to apply for such a program. You will see that it may change your professional life and it will help your lab as well.
In this issue you can also read about the 8th annual meeting of our colleagues in Sri Lanka, made more interesting and more successful with the participation of Prof Ken Sikaris and Prof Tony Badrick, through the VLP program, another important IFCC program that all IFCC national societies can apply for.
Three scientific groups, winners of the UNIVANTS rewards, are presented in this issue, all of them very interesting. I am sure the NBA COVID-19 bubble will catch your attention. Are we talking about “The NBA”? Well yes go ahead, read and find out why an article about NBA finds its place in the IFCC eNews.
Don’t forget, dear colleagues, that opinions from all over the world are heard in the eNews. We are looking forward to listening to your particular and beautiful voice.
Enjoy the last summer days and the beginning of autumn with the company of IFCC eNews.
The Executive Leadership Exchange: Where Healthcare Excellence Meets Virtual Education
The UNIVANTS of Healthcare Excellence program recognizes, amplifies and inspires replication of best practices involving measurably better healthcare. With hundreds of initiated applications each year, the UNIVANTS award program has become one the highest and most coveted team honors in healthcare today. Cross-disciplinary engagement and collaboration is not only a key requirement for UNIVANTS, but also an important ingredient in healthcare excellence as a whole. With that in mind, and recognizing the importance of crossfunctional education, the UNIVANTS of Healthcare Excellence program is pleased to announce the inaugural Executive Leadership Exchange (ELX) forum.
ELX is an inspiring and engaging educational event for all healthcare professionals, including laboratorians, clinicians, administrators and industry professionals. With a goal to maximize

value, identify solutions that resolve care gaps and promote wellness, this first of its kind program features a diverse agenda, where top leaders across healthcare share best practices, insights and opportunities for measurably better healthcare.
With more than 30 world-renown speakers from around the globe, including Dr. Shannon Haymond, Past AACC President, Quint Studer, Cofounder, Healthcare Plus Solutions Group, David Grenache, Chief Scientific Officer, TriCore Reference laboratories, David Weiss, Senior Vice President of Player Matters, National Basketball Association, Dr. Peter Kavsak, Professor,
Pathology and Molecular Medicine, McMaster University, Michael Dowling, President &CEO, Northwell Health, and more, this brilliant educational forum is a “can’t miss” event of 2023. In addition to the impressive speakers and dynamic presentations, registered attendees can obtain up to 13.0 ACCENT® continuing education credits.
Registration which includes full access to plenary sessions, educational workshops and hot topics for the live Oct 3-4th event is NOW OPEN. Access for those registered will also be available through until October 31st for subsequent viewing. To learn more and register, please visit www. healthcareELX.com
To learn more about the UNIVANTS of Healthcare Excellence program, past winners and to apply for recognition, please www.UnivantsHCE.com. Applications are accepted through to Nov 15.
Toolkit For Emerging Technologies In Laboratory Medicine

CCLM article by the IFCC Emerging Technologies Division (ETD)

An emerging technology (ET) for laboratory medicine can be defined as an analytical method (including biomarkers) or device (software, applications, and algorithms) that by its stage of development, translation into broad routine clinical practice, or geographical adoption and implementation has the potential to add value to clinical diagnostics. Considering the laboratory
medicine-specific definition, this document examines eight key tools, encompassing clinical, analytical, operational, and financial aspects, used throughout the life cycle of ET implementation. Whilst there are differences in clinical priorities between different settings, the use of this set of tools will help support the overall quality and sustainability of the emerging technology implementation.
News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
18 LabMedica International August-September/2023
IFCC-Abbott Visiting Lecturers Enhance Sri Lankan Annual Congress
By Dr Dinesha Maduri Vidanapathirana Joint Secretary, College of Chemical Pathologists of Sri Lanka; Corresponding Member, IFCC Dept of Pathology
This report aims to provide a concise overview of the College of Chemical Pathologists of Sri Lanka’s experience with the IFCC - Abbott Visiting Lecturer Programme (VLP). The report highlights the college’s gratitude for the program support in providing two eminent speakers for their 8th Annual Academic Sessions, held on 14th and 15th July 2023 at Grand Kandyan Hotel, Kandy, Sri Lanka. It acknowledges the successful conference, the positive reception of the lectures delivered by Prof Ken Sikaris and Prof Tony Badrick, the problem-solving achievements, networking opportunities, and the hope for continued support from the IFCC VLP in the future.

The College of Chemical Pathologists of Sri Lanka expresses deep gratitude to the IFCC Abbott Visiting Lecturer Program for their support in providing two eminent speakers for their Annual Academic Sessions. Despite the adverse economic situation Sri Lanka is currently facing, the college is extremely grateful for the opportunity to collaborate with such renowned experts.
The conference organized by the college was a resounding success, attracting a wide range of professionals in the field of Chemical Pathology. There were 75 participants in the main academic program and 233 participants in the Medical Laboratory Science program.
The lectures delivered by Prof Ken Sikaris and Prof Tony Badrick were exceptionally well received by the attendees. Their expertise, knowledge, and engaging presentation styles made a significant impact on the participants, providing them with valuable insights into the latest advancements in the field.
environment that facilitated the sharing of experiences and expertise among professionals.
Also, the VLP provided an excellent platform for networking and establishing connections among the professionals in the field of chemical pathology. Participants had the opportunity to meet and engage with colleagues from different institutions and backgrounds. These networking interactions allowed for the exchange of ideas, best practices, and potential collaborations. The opportunity to build such connections was invaluable for enhancing professional growth and fostering future partnerships.
The College of Chemical Pathologists of Sri Lanka expresses its sincere hope for continued support from the IFCC Abbott Visiting Lecturer Programme in the future. The collaboration with IFCC VLP has been instrumental in the success of the annual sessions, and the college believes that further support would strengthen its ability to provide high-quality education and professional development opportunities in the field of Chemical Pathology. The college is eager to continue working with the IFCC VLP to promote excellence and advancement in the field.

Photo: Speakers and Organizing Committee members of the 8th Annual Academic Sessions which was held on 14th and 15th July 2023 at Grand Kandyan Hotel, Kandy, Sri Lanka with VLP Speakers and IFCC President, Prof Adeli.
Task Force on Outcome Studies in Laboratory Medicine (TF-OSLM) Call For Outcome Study Proposals 2023


IFCC OFFICE
Via Carlo Farini 81, 20159 Milan, ITALY Tel: (39) 02-6680-9912
E-mail: ifcc@ifcc.org • Web: www.ifcc.org
Staff Members: Paola Bramati, Silvia Cardinale, Silvia Colli-Lanzi, Elisa Fossati, Sofia Giardina, Smeralda Skenderaj
The VLP facilitated meaningful discussions and problemsolving sessions, allowing participants to address various challenges faced in the field of chemical pathology. Through interactive exchanges and collaborative efforts, participants were able to find practical solutions to the problems they encountered. The VLP played a vital role in fostering a collaborative
Call For Outcome Study Proposals 2023 Extended Deadline
IFCC's Task Force on Outcome Studies in Laboratory Medicine (TF-OSLM) is seeking research proposals for studies evaluating the impact of laboratory testing on healthcare outcomes. Study proposals should seek to evaluate the clinical effectiveness and impact of new and/or commonly available medical laboratory tests and/or laboratory information on patient care outcomes in clinical practice. It is crucial for the proposed study to link the laboratory testing insights to patient management, and improvements/changes in clinical outcomes. If you, in collaboration with your clinical colleagues, are interested in applying please click here for full eligibility criteria and details on how to apply.
19 LabMedica International August-September/2023 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
The views and positions expressed in the IFCC News section are those
the IFCC or the individual authors, and do not necessarily represent the views or positions of LabMedica magazine or its publishers.
of
ALL APPLICATIONS ARE DUE BY OCT. 1, 2023
Portable Optofluidic Sensing Devices Could Simultaneously Perform Variety of Medical Tests
While a variety of chip-based testing devices have been created, they primarily focus on one particular target or test due to the wide array of forms and quantities that biomolecules take. For instance, proteins that serve as disease biomarkers can have concentrations differing by more than ten orders of magnitude. Now, scientists have utilized novel signal-processing methods on an optofluidic chip-based biosensor, enabling seamless fluorescence detection of a nanobead mixture spanning eight orders of magnitude in concentration, from attomolar to nanomolar. This extends the working concentration range of these sensors by over 10,000 times, paving the way for extremely sensitive portable integrated optofluidic sensors that can conduct various simultaneous medical tests, even if the tests involve different types of bioparticles— like viruses and DNA—in widely varying concentrations.
Researchers at University of California, Santa Cruz (UCSC, Santa Cruz, CA, USA; www. ucsc.edu) are developing a versatile testing platform based on optofluidic chips. These chips merge optics and microfluidic channels on a silicon or plastic chip, and detect particles by illuminating them with a laser beam and subsequently measuring the particle response with a light-sensitive detector. The researchers had previously demonstrated that their platform is capable of conducting various analyses and detecting a multitude of particle types, including
nucleic acids, proteins, viruses, bacteria, and cancer biomarkers. However, until now, they employed separate detectors and signal analysis techniques to measure particles with high and low concentrations. This was necessary as a high concentration of one particle type could generate a large response that drowns out the smaller signals from other particles present in low concentrations.

In their latest study, published in the journal Optica on July 20, 2023, the researchers devised signal-processing techniques that can simultaneously detect particles in both high and low concentrations, regardless of whether the concentrations are known in advance. To achieve this, they mixed different signal modulation frequencies: high-frequency laser modulation to single out particles at low concentrations, and low-frequency laser modulation to detect large signals from numerous particles at high concentrations. They also implemented a highly efficient algorithm they recently developed to identify single particle signals at low concentrations in real-time. Machine learning was beneficial in identifying signal patterns, enabling high-precision differentiation of various particle types. The team demonstrated their new signal analysis method by introducing optofluidic biosensor chips to a solution of nanobeads at varying concentrations and with different fluorescence colors. They were able to accurately determine the concentration of both yel-

low-green and crimson beads, even though their concentrations in the mixture varied by a factor exceeding 10,000.
“This work is our latest step in developing integrated optofluidic sensing devices that are sensitive enough to detect single biomolecules and work over a very wide range of concentrations,” said Holger Schmidt from the W.M. Keck Center for Nanoscale Optofluidics at the USCS who led the research. “We have shown that this can be done with a single method, which allows us to simultaneously measure and distinguish multiple particle types at once even if they have very different concentrations.”
“These signal analysis advances are ideal for enabling device operation at the point of care where signal quality can be poor and where data analysis is required in real time,” added Schmidt.
New Detection Method Diagnoses Ovarian Cancer from Blood, Urine and Saliva
Detecting ovarian cancer in its preliminary stages, when it’s most effectively treatable, poses a significant challenge. One potential method for identifying this cancer is through the analysis of extracellular vesicles (EVs), with a specific focus on small proteins known as exosomes that are discharged from the tumor. These proteins, being extracellular, can be gathered from body fluids such as blood, urine, and saliva. However, the practical application of these biomarkers has been hampered by a scarcity of reliable markers for ovarian cancer detection. Now, researchers have uncovered three previously undiscovered membrane proteins associated with ovarian cancer. Utilizing a novel technology of polyketone-coated nanowires, the research team successfully isolated these proteins, leading to a new detection approach for ovarian cancer detection.
The research, conducted at Nagoya University (Nagoya, Japan; www.nagoya-u.ac.jp) involved extracting both small and medium/large EVs from high-grade serous carcinoma (HGSC), the most prevalent form of ovarian cancer. The researchers then analyzed them using liquid chro-
matography-mass spectrometry. Although the initial phase of the research was demanding and the validation of the identified proteins was particularly challenging, the researchers’ persistent efforts paid off. After examining various antibodies, they finally identified suitable targets. The findings showed that small and medium/ large EVs carry distinctly different molecules, and further study found that the small EVs make better biomarkers than their larger counterparts. The researchers identified FRα, Claudin-3, and
Samples
TACSTD2 as the membrane proteins present in the small EVs connected to HGSC.
Following the successful identification of these proteins, the research team turned their attention to capturing EVs in a manner that would enable cancer detection. To achieve this, they developed polyketone chain-coated nanowires (pNWs), a technology particularly effective for extracting exosomes from blood samples. However, crafting the pNWs was not a straightforward process. The team had to experiment with multiple coatings before settling on polyketones, a completely new material for this application that proved to be a perfect match.
“Our findings showed that each of the three identified proteins is useful as a biomarker for HGSCs,” said Akira Yokoi of the Nagoya University Graduate School of Medicine who led the research group. “The results of this research suggest that these diagnostic biomarkers can be used as predictive markers for specific therapies. Our results allow doctors to optimize their therapeutic strategy for ovarian cancer, therefore, they may be useful for realizing personalized medicine.”
20 LabMedica International August-September/2023 LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Image: Researchers have made major strides toward an all-purpose biosensor chip (Photo courtesy of UCSC)
Cont’d from cover

Mindray to Acquire 75% Stake in DiaSys
solidate DiaSys and its subsidiaries. DiaSys’s wide range of clinical chemistry systems, manufacturing capabilities for controls and calibrators, and a global network of manufacturing and distribution sites synergize fully complement Mindray’s extensive hematology and immunoassays product range, creating a plethora of market opportunities. Recognized globally, DiaSys has over 30 years of experience in the in-vitro diagnostics (IVD) field with production facilities spread across Europe, APAC, and Latin America. Mindray intends to utilize DiaSys’s international team of professionals and global supply platforms to expedite the construction
of overseas supply chains, aiming for further expansion into global medium to large volume customer segments and delivering more timely and comprehensive professional services to clients. Moreover, DiaSys’s vast experience in clinical chemistry is anticipated to bring synergistic value to Mindray’s IVD business. DiaSys’s expertise in developing and producing IVD controls and calibrators will complement Mindray’s IVD product line and enhance its system performance.

“We are very pleased to see this collaboration come to fruition, as Mindray’s and DiaSys’ businesses strongly complement each other,” said Wu Hao, President of Mindray. “Through this acquisition, Mindray will effectively im-
prove its overseas supply chain platform and support the penetration of our IVD business in the medium to large customer segments. We look forward to both parties jointly realizing synergies and creating greater value for our customers.”
“DiaSys with its comprehensive range of products and global manufacturing footprints, and Mindray with its total IVD solutions and worldwide network, are ideal partners for lifting synergies in the IVD field,” added Dr. Günther Gorka, DiaSys’s Founder. “The individual strengths of both partners will support each other and help for a common and sustainable success in all regions of the world.”
City of Florence

LGC Clinical Diagnostics (Middlesex, UK; www.lgcgroup.com), a business unit of LGC and a leading provider of biological materials and viral antigens, has acquired Kova International, Inc. (Garden Grove, CA, USA; www.kovaintl.com), a developer and manufacturer of in vitro urinalysis and toxicology quality control products for clinical laboratories. Kova’s quality control products are employed in hospitals, employee wellness programs, forensic testing and medical device manufacturers production and Quality Assurance (QA) programs. The acquisition expands LGC Clinical Diagnostics’ clinical quality control portfolio, complementing its wide range of offerings across biochemistry, serology, molecular, and clinical genomics quality controls and
the acquisition of Cellix, Randox has extended its technical proficiency into the benchtop flow cytometry area, reaffirming its commitment to personalized medicine and improving preventative healthcare.
is the birthplace of Renaissance and is famous for its Renaissance architecture of noble palaces, squares and churchcountless galleries and museums. Florence is the place and history-lovers. Millions of intellectuals, pioneers, and artisans have been passing through Florence for cenand have shaped the Tuscan capital.
combines modern and efficient congress venues with exhilarating atmosphere. The congress venue is Fortezza da an example of Renaissance architecture, which is located historical city centre. Hotels, restaurants, shops and hisbuildings are in close proximity.


a Contacts /Addresses
Organiser
European Society of Pathology Rue Bara 6 1070 Brussels Belgium
(Waltham, ) has comCorEvitas, LLC www.corevitas.com), a provider of regulatory-grade, real-world evidence for approved medical treatments and therapies, for USD 912.5 million in cash. The rapidly growing area of real-world evidence involves gathering and analyzing data on patient healthcare utilization and outcomes collected from regular clinical practice. CorEvitas has created a multi-therapeutic data intelligence platform that collects structured clinical data from over 400 research sites and tracks more than 100,000 patients over time. Following the completion of the acquisition, CorEvitas will be incorporated into Thermo Fisher’s Laboratory Products and Biopharma Services division.
Roche (Basel, Switzerland; www.roche. com) and Sysmex Corporation (Kobe, Japan; www.sysmex.co.jp ) have expanded their Global Business Partnership Agreement, reinforcing the commitment of both companies to their long-established collaboration of 25 years. Under the revised terms, Roche will continue to offer Sysmex’s hematology products, complementing its comprehensive laboratory solutions portfolio. The revised agreement also ushers in a new dimension of cooperation, incorporating an additional eco-social agreement to jointly investigate more sustainable diagnostic solutions.
Scientific Contact
Raed Al Dieri Rue Bara 6 1070 Brussels Belgium
Email: r.aldieri@esp-pathology.org
Congress and Exhibition Office
CPO HANSER SERVICE GmbH
Paulsborner Str. 44 14193 Berlin
Germany
Phone: +49 – 30 – 300 669-0
Email: ecp-florence@cpo-hanser.de
Randox Laboratories (Crumlin, UK; www. randox.com), a global diagnostics company, has completed its acquisition of Cellix Limited (Dublin, Ireland; www.wearecellix.com), a developer of microfluidic tools and impedance flow cytometers for cell analysis. Randox provides innovative diagnostic solutions for a wide range of sectors including hospitals, clinical and research laboratories, food testing, forensic toxicology, veterinary labs, and life sciences. With
Beckman Coulter, Inc. (Brea, CA, USA; www.beckmancoulter.com) and Fujirebio (Tokyo, Japan; www.fujirebio. com) have combined their respective strengths in immunoassay in a partnership to support the development of therapeutics, clinical trials, and standard clinical adoption in the field of neurodegenerative diseases. The partnership capitalizes on Fujirebio’s skill in developing its pioneering, high-quality neurodegenerative disease biomarker assays and generating clinical evidence to boost clinical adoption. Initial assay development efforts will be focused on Beckman Coulter’s extensive installed base of Access Family Immunoassay Analyzers and the recently introduced DxI 9000 Access Immunoassay Analyzer.
Danaher Corporation (Waltham, MA, USA; www.danaher.com) has entered into a definitive agreement to acquire all of the outstanding shares of Abcam plc (Cambridge, UK; www. abcam.com) for USD 24 per share in cash. Abcam offers a wide range of extensively validated antibodies, reagents,
biomarkers, and assays. These resources serve to target key biological pathways critical for the advancement of drug discovery, life sciences research, and diagnostics. Under the planned arrangement, Abcam is poised to continue operating as an independent entity and brand within Danaher’s Life Sciences sector. The acquisition of Abcam reflects Danaher’s strategy to contribute towards mapping complex diseases and accelerating the process of drug discovery.
36 th European Congress of Pathology
Multidimensional Pathology –Cornerstone of modern diagnostics
7 – 11 September 2024
Fortezza da Basso, Florence, Italy
www.esp-congress.org
21 LabMedica International August-September/2023 Industry News To view this issue in interactive digital magazine format visit www.LabMedica.com
FIRST ANNOUNCEMENT
Ponte Vecchio © adisa - stock.adobe.com
2023
For
a free listing of your event or a paid
SEPTEMBER
Thailand LAB International 2023. Sep 6-8; Bangkok, Thailand; thailandlab.com
ECP 2023 – 35th Congress of the European Society of Pathology. Sep 9-13; Dublin, Ireland; esp-congress.org
EUROTOX 2023 – 57th Congress of the European Societies of Toxicology. Sep 10-13; Ljubljana, Slovenia; eurotox2023.com
45th Mexican National Congress of Clinical Chemists and Expoquím 2023. Sep 11-16; Mazatlán, Mexico; conaquic.com
ACBICON 2023 – 48th Annual Conference of Association of Clinical Biochemists of India. Sep 13-16; Thiruvananthapuram, India; www.acbicon2023kerala.com
India Lab Expo & Analytica Anacon India. Sep 1416; Hyderabad, India; analyticaindia.com
17th National Congress of the Czech Society of Clinical Biochemistry. Sep 17-19; Hradec Králové, Czech Republic; sjezdcskb2023.cz
Arab Lab 2023. Sep 19-21; Dubai, UAE; arablab.com
31st Congress of the Latin American Society of Cytopathology (SLAC). Sep 20-23; San Miguel de Allende, Mexico; congresoslac2023.com
Analitica Latin America 2023. Sep 26-28; Sao Paulo, Brazil; analiticanet.com.br
BCLF 2023 – 30th Meeting of the Balkan Clinical Laboratory Federation. Sep 27-30; Herceg Novi, Montenegro; bclf2023.org
92nd Annual Meeting of the American Thyroid Association (ATA). Sep 27 - Oct 1; Washington, DC, USA; thyroid.org
OCTOBER
ECC 2023 – 44th European Congress of Cytology. Oct 1-4; Budapest, Hungary; cytology2023.eu
EASD 2023 – 59th Annual Meeting of the European
Association for the Study of Diabetes. Oct 3-6; Hamburg, Germany; easd.org
16th Argentinian National Congress of Biochemistry. Oct 5-7; Mendoza, Argentina; cubra.org.ar
AACC Middle East 2023. Oct 7-8; Dubai, UAE; aaccme. com
CAP23 – Annual Meeting of the College of American Pathologists. Oct 7-10; Chicago, IL, USA; cap. org
DKLM 2023 – Annual Congress of the German Society for Clinical Medicine and Laboratory Medicine (DGKL). Oct 10-12; Mannheim, Germany; dgkl.de JFBM 2023 – Journées Francophones de Biologie Médicale. Oct 11-13; Antibes, France; jfbm.fr
CELME 2023 5th Symposium: Cutting Edge of Laboratory Medicine in Europe. Oct 12-13; Prague, Czech Republic; celme2023.cz
21st CNB International Congress 2023 – Colombian National College of Bacteriology. Oct 12-15; Medellín, Colombia; congresocnb.com
60th Annual Scientific Conference of the Australasian Association of Clinical Biochemistry and Laboratory Medicine (AACB). Oct 16-19; Brisbane, Australia; aacb.asn.au
ASHI 2023 – 49th Annual Meeting of the American Society for Histocompatibility and Immunogenetics. Oct 16-20; San Antonio, TX, USA; ashi-hla.org
MedLab Africa 2023. Oct 17-19; Johannesburg, South Africa; africahealthexhibition.com
ASCP 2023 – Annual Meeting of the American Society for Clinical Pathology. Oct 18-20; Long Beach, CA, USA; ascp.org
LABCLIN 2023 – 17th National Congress of the Spanish Societies for Clinical Laboratory (AEBMML, AEFA & SEQCML). Oct 18-20; Zaragoza, Spain; labclin2023.es
EndoBridge 2023. Oct 19-22; Antalya, Turkey; endobridge.org
26th Congress of the Society of Medical Laboratory Technology of South Africa (SMLTSA). Oct 19-22; Johannesburg, South Africa; smltsa.org.za
45th Annual ACBI Conference – Association of Clinical Biochemists in Ireland. Oct 20-21; Dublin,
Ireland; acbi.ie
7th ESPT Congress – European Society for Pharmacogenomics and Personalised Therapy. Oct 25-27; Copenhagen, Denmark; esptcongress.org
5th International Congress & 14th National Congress of the Ecuadorian Society of Clinical Biochemistry. Oct 26-28; Cuenca, Ecuador; congresosebiocliecuador2023.com
63rd Annual Academic Assembly of the Japan Society of Clinical Chemistry (JSCC). Oct 27-29; Toyama, Japan; jscc-jp.gr.jp
34th National Congress of the Turkish Biochemical Society. Oct 29 - Nov 1; Fethiye, Turkey; biyokimyakongresi.org
NOVEMBER
53rd Mexican National Congress of Clinical Pathology. Nov 1-4; Aguascalientes, Mexico; fempac.org. mx
ASHG 2023 – Annual Meeting of the American Society of Human Genetics. Nov 1-5; Washington, DC, USA; ashg.org
44th Annual Meeting of the American College of Toxicology (ACT). Nov 12-15; Orlando, FL, USA; actox.org
MEDICA 2023. Nov 13-16; Dusseldorf, Germany; medica-tradefair.com
AMP 2023 – Annual Meeting & Expo of the Association for Molecular Pathology. Nov 16-18; Salt Lake City, UT, USA; amp.org
71st Annual Scientific Meeting of the American Society of Cytopathology (ASC). Nov 15-19; Austin, TX, USA; cytopathology.org
2023 Annual RBSLM Meeting - Royal Belgian Society of Laboratory Medicine. Nov 17; Brussels, Belgium; rbslm.be
JIB 2023 – Journées de l’innovation en biologie. Nov 17-18; Paris, France; jib-innovation.com
34th Regional Congress of the International Society of Blood Transfusion (ISBT). Nov 18-21; Cape Town, South Africa; isbtweb.org
Events Calendar
IUIS 2023 – International Union of Immunological Societies. Nov 27 - Dec 2; Cape Town, South Africa; iuis2023.org advertisement
contact: LabMedica International Calendar
Provided as a service to advertisers. Publisher cannot accept responsibility for any errors or omissions. Advertising Index Inq.No. Advertiser Page Inq.No. Advertiser Page Vol. 40 No. 5 8-9/2023 LabMedica International – ADLM 15 109 DiaSys Diagnostic Systems 9 – EuroMedLab 2024 23 – European Congress of Pathology 21 105 Feather 5 – IFCC WorldLab 2024 17 – LabMedica EXPO 3 107 Singuway 7 102 Snibe ....................................... 2 113 Vicotex 13 124 Werfen ..................................... 24 111 West Medica 11 ATTENTION: IF YOUR APPLICATION IS NOT RECEIVED AT LEAST ONCE EVERY 12 MONTHS YOUR FREE SUBSCRIPTION MAY BE AUTOMATICALLY DISCONTINUED READER SERVICE PORTAL LINKXPRESS COM ® VISIT Every advertisement or product item contains a LinkXpress number as below: 999 LMI-09-23 LINKXPRESS COM Identify LinkXpress codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress inquiry matrix 1 2 3 Renew/ S tart Your Free Subscription Access Instant Online Product Information
E-mail: info@globetech.net
DECEMBER
ASI 2023 – 51st Annual Scientific Meeting of the Australian and New Zealand Society for Immunology. Dec 4-8; Auckland, New Zealand; asi2023.org
65th Annual Meeting & Exposition of the American Society of Hematology (ASH). Dec 9-12; San Diego, CA, USA; hematology.org
ASLM2023 - 6th Biennial Conference of the African Society for Laboratory Medicine. Dec 12-15; Cape Town, South Africa; aslm2023.org
2024
FEBRUARY
SLAS 2024 – International Conference & Exhibition of the Society of Laboratory Automation and Screening. Feb 3-7; Boston, MA, USA; slas.org
Medlab Middle East 2024. Feb 5-8; Dubai, UAE; medlabme.com
Labquality Days 2024 – International Congress on Quality in Laboratory Medicine. Feb 8-9; Helsinki, Finland; labqualitydays.fi
3rd International Congress of Laboratory Diagnosis 2024. Feb 15-18; Virtual; ldcongress.com Pittcon 2024. Feb 24-28; Philadelphia, PA, USA; pittcon.org
MARCH
ICE 2024 – 21st International Congress of Endocrinology. Mar 1-3; Dubai, UAE; isendo.org China Lab 2024. Mar 5-7; Guangzhou, China; chinalabexpo.com
MASCL 2024 – Congress of the Association for Mass Spectrometry & Advances in Clinical Lab. Mar 17-22; Monterey, CA, USA; msacl.org
USCAP 113th Annual Meeting – United States & Canadian Academy of Pathology. Mar 23-28; Baltimore, MD, USA; uscap.org
APRIL
Analytica 2024. Apr 9-12; Munich, Germany; analytica.de
India Lab Expo & Analytica Anacon India. Apr 1517; Mumbai, India; analyticaindia.com
Korea Lab 2024. Apr 23-26; Seoul, Korea; korealab.org
ExpoMED Eurasia 2024. Apr 25-27; Istanbul, Turkey; expomedistanbul.com

ECCMID 2024 – 34th European Congress of Clinical Microbiology and Infectious Diseases. Apr 2730; Copenhagen, Denmark; eccmid.org
MAY
Immunology 2024 – Annual Meeting of the American Association of Immunologists (AAI). May 3-7; Chicago, IL, USA; immunology2024.aai.org
AACE Annual Meeting 2024 – American Association of Clinical Endocrinology. May 9-11; New Orleans, LA USA; pro.aace.com
ECE 2024 – 26th Annual Congress of the European Society of Endocrinology. May 11-14; Stockholm, Sweden; ese-hormones.org
ASRI 2024 – 43rd Metting of the American Society for Reproductive Immunology. May 18-22; Houston, TX, USA; theasri.org
Hospitalar 2024. May 21-24; Sao Paulo, Brazil; hospitalar.com
107th Annual Meeting of the German Society for Pathology. May 23-25; Munich, Germany; pathologie-dgp.de
IFCC WorldLab 2024 – 26th International Congress of Clinical Chemistry and Laboratory Medicine. May 26-30; Dubai, UAE; dubai2024.org
SLAS Europe 2024 Conference and Exhibition - Society of Laboratory Automation and Screening. May 27-29; Barcelona, Spain; slas.org
ISLH 2024 – International Society for Laboratory Hematology. May 30 - Jun 1; Nantes, France; islh. org
EAACI 2024 – Annual Congress of the European Academy of Allergy & Clinical Immunology. May 31 - Jun 3; Valencia, Spain; eaaci.org
JUNE
ESHG 2024 – European Human Genetics Conference. Jun 1-4; Berlin, Germany; eshg.org
UKMedLab24 – National Meeting of the Association for Clinical Biochemistry and Laboratory Medicine. Jun 10-12; Brighton, UK; acb.org.uk
9th International Symposium on Critical Care Testing and Blood Gases. Jun 13-14; Saint-Malo, France; criticalcaretesting-saintmalo2024.eu
ASM Microbe 2024 – American Society for Microbiology. Jun 13-17; Atlanta, GA, USA; asm.org
49th CBAC – Congress of the Brazilian Society of
Clinical Analysis. Jun 16-19; Natal, Brazil; sbac.org.br
FOCIS 2024 – Annual Meeting of the Federation of Clinical Immunology Societies. Jun 18-21; San Francisco, CA, USA; focisnet.org
FIME 2024 – Florida International Medical Expo. Jun 19-21; Miami, FL, USA; fimeshow.com
ASV 2024 – 43rd Annual Meeting of the American Society of Virology. Jun 24-28; Columbus, OH, USA; asv.org
ECB 2024 – European Congress on Biotechnology. Jun 30 - Jul 3; Maastricht, The Netherlands; ecb2024. com
JULY
ESHRE 2024 – 40th Annual Meeting of the European Society of Human Reproduction and Embryology. Jul 7-10; Amsterdam, Netherlands; eshre.eu MedLab Asia 2024. Jul 10-12; Bangkok, Thailand; medlabasia.com
2024 AACC Annual Scientific Meeting & Clinical Lab Expo. Jul 28 - Aug 1; Chicago, IL, USA; meeting. aacc.org
Events Calendar
23 LabMedica International August-September/2023
The Intelligent Analyzer
GEM Premier 5000 blood gas testing system provides automated quality assurance with every whole-blood* sample. Now with next-generation Intelligent Quality Management (iQM2), featuring IntraSpect™ technology, potential errors are detected not only before and after, but also during sample analysis, along with real-time correction and documentation. Plus, it’s simple—just change the all-in-one GEM PAK once a month. So regardless of testing location or point-of-care operator, quality results and compliance are assured with every sample.

Real-time assurance and advanced simplicity. Now
that’s intelligent.
For more information, contact your local Werfen representative.
werfen.com
*Heparinized.
132 LMI-4-23 LINKXPRESS COM 124 LMI-09-23 LINKXPRESS COM
Acute Care Diagnostics
GEM, Premier and iQM are trademarks of Instrumentation Laboratory Company and/or one of its subsidiaries or parent companies and may be registered in the United States Patent and Trademark Office and in other jurisdictions. The Werfen logo is a trademark of Werfen and may be registered in the Patent and Trademark Offices of jurisdictions throughout the world. ©2021 Instrumentation Laboratory. All rights reserved. 124 LMI-09-23 LINKXPRESS COM











































































































 By Khosrow Adeli • President, IFCC
By Khosrow Adeli • President, IFCC

















