Testing Breakthrough on Effects of Long COVID
With no current framework or diagnostic tools, pa tients suffering from the effects of COVID-19 and long COVID-19 present general practitioners with a major health challenge. Now, a new discovery will aid the di agnosis of debilitating symptoms
Cont’d on page 20
AI-Enabled Diagnostic Solutions Improve Treatment of Breast and Colorectal Cancers

Two first-in-class rapid, af fordable AI-based diagnos tic solutions that are designed to improve outcomes for pa tients with breast cancer and colorectal cancer have been approved for use in Europe. By using AI to analyze digital

pathology images, they are de signed to help clinicians make precision medicine diagnos tic and treatment based on a patient’s individual characteris tics more accessible to more patients at an earlier stage of their disease.
Cont’d on page 13
New Guidelines for POC Fertility Testing
P oint-of-care tests are clinical tests that are performed near the patient instead of in a central lab. Due to their convenience and rapid turnaround times, these tests can help patients to get treat ment much faster than traditional tests. As a result, the use of pointof-care testing has risen steadily in all areas of healthcare. In the fertility
reproductive
Cord Blood IgE Levels
Tied to Allergy, Asthma
Approximately one in four people worldwide, or approximately two billion people, have non-alco holic fatty liver disease (NAFLD) and therefore are at risk of devel oping nonalcoholic steatohepatitis (NASH). NASH is the most severe form of NAFLD, a silent disease
Microchip Imaging Cytometry

Economic globalization and the aging population of many countries in the world generate an enormous need for rapid and cost-effective point-of-need labora tory tests. Over the past two years, the entire world has been tackling challenges from the COVID-19
Testing
pandemic. The general population in many countries are routinely taking nucleic acid tests and/or rapid antigen tests for screening purposes. Healthcare workers are in need of more economical, and easy-to-use diagnostic testing tools to support their healthcare prac
T
he rising prevalence of al lergic diseases worldwide represents an important health problem. Early identification of children at high risk of allergic diseases could be helpful for phy sicians to recommend preventive measures and apply early inter ventions.
Cord blood immunoglobulin E (IgE) has been considered as a potential marker for years due to

A breakthrough viral testing method known as RTF-EXPAR, matches current gold standard tests in accuracy, while faster, simpler and able to detect lower levels. ® INTERNATIONAL Vol 39 No 6 • 10/ 2022ISSN 1068-1760 WORLD’S CLINICAL LABORATORY NEWS LEADER Cont’d on page 17 Cont’d on page 18 Cont’d on page 4 If your subscription is not renewed every 12 months your Free Subscription may be automatically discontinued Renew / Start your Free Subscription Access Interactive Digital Magazine Instant Online Product Information: Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 VISIT READER SERVICE PORTAL LINKXPRESS COM ® Cont’d on page 14 VISIT DAILY CLINICAL LAB NEWS New Diagnostic Platform, As Sensitive As PCR, LAMP or LFT, But Much Faster New Diagnostic Platform, As Sensitive As PCR, LAMP or LFT, But Much Faster GLOBETECH MEDIA>>> <<< PUBLISHED IN COOPERATION WITH International Federation of Clinical Chemistry and Laboratory Medicine INSIDE Clinical News ..... 4 IFCC News ...... 21 Product News . 6-20 Industry News . .. 25 Events Calendar . 26
and
health fields in particular, it is now used
Improves Laboratory
Blood-Based Liver Test Could Replace Invasive Biopsies
See article on page 7
Two More Studies Show Nova POC Creatinine/eGFR is As Accurate or More Accurate Than Laboratory Methods
Study One: Accuracy Equal to Traceable Gold Standard Creatinine Method
Nova StatSensor capillary and venous whole blood POC specimens were directly compared to the same patient’s venous samples measured by the laboratory gold standard ID-LCMS creatinine method. StatSensor POC concordance with ID-LCMS at a creatinine decision-making level of 1.2 mg/dL performed equally to the laboratory.
“Consequently, the sensitivity and specificity of the capillary blood testing post-calibration alignment was 100% and 98.3% respectively, indicating that the device is suitable to screen for CKD in POC settings and is a reliable method to assess a patient’s renal status in the field.”
Dubois JA et al. Creatinine standardization: a key consideration in evaluating whole blood creatinine monitoring systems for CK D screening. Analytical and Bioanalytical Chemistry (2022) 414:3279–3289.
Study Two:
Accuracy Better Than the Laboratory Jaffe Creatinine/eGFR Method
StatSensor Creatinine/eGFR showed better accuracy than the Jaffe creatinine/eGFR at identifying patients in the decision-making eGFR range of 60-89 when both methods were compared to the gold standard measured GFR.
“The performance of POC devices [StatSensor] to detect eGFR in the range 60–89 mL/min/1.73 m2 is of particular interest. With limited access to renal replacement therapy for severe kidney disease, it is advantageous to detect individuals with early disease who may benefit from renal protective measures. There was improved accuracy in this area compared to laboratory Jaffe measurements.”
Currin S et al. Evaluating chronic kidney disease in rural South Africa: comparing estimated glomerular filtration rate using point-of-care creatinine to iohexol measured GFR. Clin Chem Med Lab 2021

Point-of-Care Whole Blood Creatinine and eGFR Testing
novabiomedical.com
102LMI-10-22LINKXPRESS COM
Consistency in every slide with DIFF-Line
Empowering your laboratory to prepare and analyze peripheral blood smears with consistency and control.






™
103LMI-10-22LINKXPRESS COM
World’s First Preventative DNA Screening for Cancer and Heart Disease Risk
Until now, genetic testing for the DNA changes that increase the risk of can cers and heart disease has only been available on a small scale for those with a known family history or prior disease diagno sis. Population testing, open to everyone, has the potential to drastically improve access and maximize the preventive benefits of DNA test ing. Now, a world-first DNA screening study will provide young Australians with access to a free DNA saliva test in order to learn whether they face increased risk of some cancers and heart disease, which can be prevented or treated early if detected.
The collaborative project, led by scientists at Monash University (Melbourne, Australia; www.monash.edu) and supported by research ers and clinicians across Australia, will screen at least 10,000 people aged 18-40 for genes that increase risk of certain types of cancers and heart disease that often go undetected. DNA Screen is the world’s first preventive DNA screening study designed specifically to assess population DNA screening through a national healthcare system. DNA Screen will identify people with DNA variants in the BRCA1 and BRCA2 genes that lead to an increased risk of hereditary breast and ovarian cancer in women. These genes are also linked to breast and prostate cancer in men, although not as strongly. Men and women who carry DNA variants in the BRCA1 and BRCA2 genes can also pass them onto their children.
The DNA Screen test will also focus on Lynch Syndrome another condition that increases risk for colorectal, endometrial, and other gastrointestinal cancers. Both cancer-re lated conditions have effective, proven inter ventions available to reduce risk if identified early. This includes attending annual check-ups and screens from age 30, and the option of risk-reducing surgery for some people. Early de tection and prevention are often life-saving for cancer. The DNA test also encompasses heart
Image: The test involves placing a saliva sample into a small tube received by mail and sending it back in a postage paid envelope (Photo courtesy of Monash University)
disease risk, focusing on familial hypercholes terolemia (FH) or ‘genetic high cholesterol’, which results in high risk of heart disease from a young age. Despite effective medications such as statins being available to reduce risk, an estimated 95% of FH carriers are currently undiagnosed.
The test is free and involves placing a saliva sample into a small tube received by mail, and sending it back in a postage paid envelope. Those found to be at high risk after DNA test ing about one in 75 or 1.3% – will have their situation explained by experts and be offered genetic counseling and prevention measures, such as regular scans and check-ups. The even tual goal is to develop a new population-based DNA screening program that could be offered through the Australian public healthcare sys tem, available to everyone but targeted on certain medically-actionable conditions where early detection is key.

“Providing genetic testing based on family history alone is not enough. Up to 90 per cent of those at high risk in the general population are not identified by current family histo ry-based testing,” said Monash University’s Assoc. Prof. Paul Lacaze. “Most people don’t find out about their genetic risk until it’s too late, like after an incurable cancer or heart attack is diagnosed. We want to change that.”
Blood-Based Liver Test Could Replace Invasive Biopsies
associated with fatty deposit build-up of the liver. NASH leads to advanced liver diseases such as liver fibrosis (scarring), cir rhosis, and liver cancer, and may ultimately result in death. Currently, the only way to diagnose NASH is through the standard of diagnosis, invasive liver biopsy, which is expensive and is associated with significant complications and discomfort. Until now, no reliable blood test has been developed for either NASH or liver fibrosis. Most im portantly, no available test can define the severity (stage), or monitor the progression, of either NASH or liver fibrosis. Now, a new test uses artificial intelligence (AI) to accurately predict the stages of the whole spectrum of liver diseases, from NAFLD to
NASH and liver fibrosis.
Metadeq Inc. (Boston, MA, USA; www. metadeq.com) has announced a breakthrough non-invasive blood test that utilizes two novel circulating proteins to accurately diagnose NASH and liver fibrosis, and can score the stages of both diseases, without the need for invasive liver biopsy. A study that set out to discover a more accurate liquid biopsy test in support of patient care identified two novel protein biomarkers, PLIN2 and RAB14, to assist in the diagnosis of patients with NASH and/or liver fibrosis. The ability of these proteins to detect both diseases was tested in cohorts with either NASH and/or liver fibrosis that were confirmed with liver biopsy, the
EDITORIAL BOARD
Graham Beastall United Kingdom
Hernán Fares Taie Argentina
Bernard Gouget France
Maurizio Ferrari Italy
Jocelyn M. Hicks United States
Tahir S. Pillay South Africa
Andreas Rothstein Colombia
Praveen Sharma India
Rosa I. Sierra-Amor Mexico
Peter Wilding United States
Andrew Wootton United Kingdom
Dan Gueron
Raymond L Jacobson, PhD
Gerald M Slutzky, PhD
Andreas Rothstein
Sanjit Dutt Carolyn Moody, RN
Simone Ciolek
Parker Xu David Gueron
Publisher
Editor
Editor
Editor
New Products Editor Regional Director Regional Director Regional Director Reader Service Manager
USA Miami, FL 33280, USA
Carolyn.Moody@globetech.net Tel: (1) 954-686-0838
GERMANY, SWITZ., AUSTRIA Bad Neustadt, Germany
Simone.Ciolek@globetech.net Tel: (49) 9771-1779-007
OTHER EUROPE & UK Miami, FL 33280, USA Carolyn.Moody@globetech.net Tel: (1) 954-686-0838
JAPAN Tokyo, Japan
Katsuhiro.Ishii@globetech.net Tel: (81) 3-5691-3335
CHINA Shenzen, Guangdong, China Parker.Xu@globetech.net Tel: (86) 755-8375-3877

OTHER COUNTRIES Contact USA Office ads@globetech.net Tel: (1) 954-686-0838
lnternational is published eight times a year and is circuIated
(outside the USA and
and by written request, to clinical laboratory
without
and administrators, and other qualified professionals allied to the field.
To all others: Paid Subscription
subscription
available for a
of US$120. Single co
paid subscription order
Globetech Media,
FL
4LabMedica International October/2022 LabMedica
worldwide
Canada)
charge
specialists
is
two-year
charge
py price is US$20. Mail your
accompanied with payment to
P.O.B. 800222, Miami,
33280-0222. For change of address or questions on your subscription, write to: LabMedica lnternational, Circulation Services at above address; or visit: www.LinkXpress.com SUBSCRIPTION INFORMATION Vol.39 No.6. Published, under license, by Globetech Media LLC; Copyright © 2022. All rights reserved. Reproduction in any form is forbidden without express permission. Opinions expressed are solely those of the authors, and do not rep resent an endorsement, or lack thereof, by the Publisher of any products or services. ISSN 1068-1760 ADVERTISING SALES OFFICES HOW TO CONTACT US labmedica.com A GLOBETECH PUBLICATION Published in cooperation with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). LabMedica International • LabMedica en Español • LabMedica.com HospiMedica International • HospiMedica.com • MedImaging.net TradeMed.com • LabMedicaExpo.com • LinkXpress.com Subscriptions: Send Press Releases to: Advertising & Ad Material: Other Contacts: www.LinkXpress.com LMNews@globetech.net ads@globetech.net info@globetech.net
INTERNATIONAL Teknopress Yayıncılık ve Ticaret Ltd. Şti. adına İmtiyaz Sahibi: M. Geren • Yazı işleri Müdürü: Ersin Köklü Müşir Derviş İbrahim Sok. 5/4, Esentepe, 34394 Şişli, İstanbul P. K. 1, AVPIM, 34001 İstanbul • E-mail: Teknopress@yahoo.com Baskı: Postkom A.Ş. • İpkas Sanayi Sitesi 3. Etap C Blok • 34490 Başakşehir • İstanbul Yerel süreli yayındır. Yılda sekiz kere yayınlanır, ücretsiz dagıtılır.
News
News
News
Founder & Editorial DirectorMarc Gueron
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Cont’d from cover Cont’d on page 6

105LMI-10-22LINKXPRESS COM
ACE DIAGNOSTIC TEST



CARDIAC MARKERS PLUS CONTROL BIO-RAD QUALITY SYSTEMS DIVISION

ZIKA VIRUS ELISA TEST CTK BIOTECH

ACEcolor is an in-vitro diagnostic test kit for the mea surement of angiotensin converting enzyme (ACE) activity in human serum. The simple test and has a good reproducibility compared with conventional assays for serum ACE.

Liquichek Cardiac Markers Plus Control is a com prehensive cardiac assessment control with high Troponin I & T values for non-high sensitivity assays and assayed val ues for multiple analytes including BNP, NT-pro-BNP and hs-CRP.
The RecombiLISA Zika IgM ELISA is a solid-phase enzyme-linked immunosorbent assay based on the principle of the indirect immu noassay technique and contains a 96-well breakable microplate pre-coated with Zika NS1 antigen.
Wearable Rapid Gas Analyzer Could Detect Illness On the Spot
A
dvancements in micro-gas chroma tography in the past 20 years have demonstrated great potential for the development of powerful portable gas analysis devices. But it remains a challenge to achieve efficient separation and rapid detection for effective gas analysis in a highly integrated and cost-effective platform, as well as in a mobile device. Now, researchers are creating a porta ble, wearable device for rapid gas analysis that could detect illness immediately.
The device being created by electrical en gineering researchers at The University of Texas at Arlington (Arlington, TX, USA; www. uta.edu) will play an important role in health care, among other sectors. The project will create technology to transform a powerful gas analysis instrument traditionally used in research labs into portable and wearable de vices that are easily accepted and accessible by the public. The project has received a USD
550,000 National Science Foundation (NSF) grant from the Partnerships for Innovation–Research Partnerships program, which aims to take devices developed in the laboratory to the marketplace more quickly.

The vast applications could propel the de vice to the marketplace more quickly. In health care, the device could analyze a person’s breath samples to find chemical markers that are specifically linked to infections, cancers and other health conditions. In turn, that could lead to a convenient and rapid health screening and monitoring tool to be used at home.
“The key to the project is system-level inte gration, including creating new micro-gas chro matography architecture and using photonic integrated circuits to achieve rapid and compre hensive volatile organic compounds gas analy sis,” said Yuze “Alice” Sun, an associate profes sor in the Department of Electrical Engineering, who is the principal investigator on the project.
“Not only does the project have the poten tial of improving our lives, but it could take up so much less space than current chromatogra phy analysis machines,” said Diane Huffaker, chair and professor of electrical engineering. “This could save an enormous amount of test ing time, too.”
Blood-Based Liver Test Could Replace Invasive Biopsies
current standard of diagnosis.
The test results show that the proteins can provide rapid and cost-effective testing to combat the growing epidemic of NASH and liver fibrosis. This could be an invaluable tool in diagnosing and monitoring cases of liver diseases, allowing people to receive earlier treatment, from lifestyle adjustments to sur gical and pharmacological interventions. The Metadeq predictive algorithm, which uses A.I., provided unprecedented sensitivity (88-95%), specificity (90-100%), and overall accuracy (92-93%) for NASH, and also has near-perfect sensitivity (99%-100%), specificity (90%-96%), and accuracy (98%-99%) for liver fibrosis.
Currently no NASH drug has been ap proved by either the FDA or EMA, which may
be a direct result of the lack of an accurate, reliable, and non-invasive test. More than 65% of patients who enroll in clinical trials for NASH-related therapies are found to be ineligible for the trial due to screen failure, which causes major monetary losses to com panies developing and testing NASH drugs. The improved accuracy and ability to detect NASH staging will help in identifying and en rolling the appropriate people in clinical trials, speeding up the development of NASH drugs.
“This blood test will allow researchers and clinicians to define the prevalence of NASH across populations, including chil dren and adolescents, avoiding the need for invasive liver biopsy,” said Professor Geltrude Mingrone from the School of Cardiovascular and Metabolic Medicine
& Sciences at King’s College London, who led the study in collaboration with Metadeq. “Critically, it will allow care-giv ers to monitor the efficacy of NASH treat ments over time, reducing screen failures and helping generate better drugs.”
“Since HEPAR-Q is the only diagnostic modality that can diagnose NASH and fibro sis staging we expect it to be instrumental in the development of new therapeutics for the management of patients,” said Frank Jaksch, Chairman of Metadeq. “We believe that HEPAR-Q will advance for the benefit of patients the problem of regular screening, that has resulted in late drug failures during clinical trials, since it is now possible to ac curately measure the severity of disease in a non-invasive manner.”
6LabMedica International October/2022 To receive prompt and free information on products, log on to www. LinkXpress.com or fill out reader service form located on last page, or scan the QR code on your mobile devicePRODUCT NEWS
FUJIREBIO
201LMI-10-22LINKXPRESS COM 202LMI-10-22LINKXPRESS COM 203LMI-10-22LINKXPRESS COM
Image: Assoc. Prof. Yuze “Alice” Sun is creating a wearable device for rapid gas analysis (Photo courtesy of University of Texas at Arlington)
Cont’d from 4
New Diagnostic Platform, As Sensitive As PCR, LAMP or LFT, But Much Faster


unique testing method is just as sen sitive as both PCR and LAMP tests currently used in hospital settings and is also faster and more sensitive than lat eral flow tests, enabling detection at low viral levels. Known as Reverse Transcription-Free EXPAR (RTF-EXPAR) testing, this new technol ogy offers detection in as little as 10 minutes.

The testing method invented at the University of Birmingham (Birmingham, UK; www.birmingham.ac.uk) is set for a global rollout after its commercial rights were exclu sively licensed to the world’s largest COVID19 test provider, Innova Medical Group, Inc. (Pasadena, CA, USA; www.innovamedgroup. com). The speed of the test is based, in part, on its avoidance of slower, reverse transcrip tase-based technologies, and provides the ease of use and speed of lateral flow tests with the sensitivity of PCR testing. Detailed test evaluations reveal the method delivers a fast, accurate, highly sensitive and simple test for COVID-19, meaning the test could be deployed at entertainment venues, airport ar rival terminals, and in remote settings where clinical testing laboratories are not available.

The method can be used with testing tech niques which bypass the need for laboratory equipment, and this is expected to reduce delays in waiting for test results, which currently require samples to be sent to spe cialist laboratories. The assay was invented and tested at the University of Birmingham, which found its sensitivity to be equivalent to quantitative PCR testing. The new RTFEXPAR testing platform is also being adapted for the detection of other viruses, meaning the tests can be quickly adapted to cover both new variants and new viruses. The technol ogy’s new license holder, Innova, is aiming to accelerate RTF-EXPAR’s global rollout for widespread use by 2023.
“The RFT test rapidly amplifies small quantities of viral genetic material, producing a detectable signal within 10 minutes, much faster than PCR or LAMP testing, and even quicker than lateral flow tests,” explained Professor Tim Dafforn from the University of Birmingham. “The reverse transcription and amplification steps slow down existing COVID assays like LAMP and PCR, which are based on nucleic acid detection. An ideal test would have the ‘best of both worlds’both sufficiently sensitive and speedy.”
“The new RTF test achieves that goal in two ways. Firstly, the assay team de signed a new RNA-to-DNA conversion step that avoids reverse transcription and sec ondly, the amplification step to generate the read-out signal uses EXPAR, an alternative DNA amplification process,” added Professor Dafforn.
“EXPAR amplifies DNA at a single tempera ture, thus avoiding lengthy heating and cooling steps found in PCR,” said Professor James Tucker from the University of Birmingham. “However, while LAMP also uses a single tem perature for amplification, EXPAR is a simpler and a more direct process, in which much smaller strands are amplified. This makes EXPAR an even faster DNA amplification tech nique than not only PCR but also LAMP.”
“The RTF technology developed at the University of Birmingham hits a testing sweet spot. It’s just as sensitive as PCR and LAMP tests, but without the time constraints and laboratory equip ment required for these methods,” said Robert Kasprzak, Chief Executive Officer at Innova.

P
Image: The new testing method enables detection at low viral levels (Photo courtesy of Unsplash)
n
7 LabMedica International October/2022 LabMedica InternationalTo view this issue in interactive digital magazine format visit www.LabMedica.com
A
Particle enhanced immunoturbidimetric assay High precision at clinical cut offs Outstanding onboard and calibration stability Dedicated calibrators & controls DiaSys. Total confidence in patient results. www.diasys diagnostics.com
r o c a l c i t o n i
F S T h e r e l i a b l e a l t e r n a t i v e , when time matters in sepsis management Dedicated kits for DiaSys respons® and BioMajesty® JCA-BM6010/C NOW AVAILABLE 107LMI-10-22LINKXPRESS COM
C-REACTIVE PROTEIN
IMMUNOASSAY

is a liquid-stable
dedicated ready-to-use
test results are fully quantitative
allows true differentiation


Gentier96R Real-Time PCR system is designed to meet the needs of high-end laboratories with easyto-use software and user- friendly operational design. With 4 fluores cence channels,

can process 96

Whole Blood Collection Technology Enables
Isolation of cfDNA, CTC, and RNA from Same Sample
RNA is a crucial tool in advancing our understanding of health and disease in humans and animals. However, RNA is particularly fragile and degrades quickly as soon the cells leave the internal environment of the body, such as when blood is drawn. A reliable technology to stabilize the sample right after collection is needed to reduce the degradation rate and maintain RNA integrity for proper anal ysis. Currently, efficient stabilization of RNA in body fluids, cells or tissues requires expensive cold chain involvement or relatively toxic fix ative, which increases the cost per sample and urgency for processing the sample, leaving very little room for error. Whole blood can be a great source of stable RNA. However, commercially available whole blood collection tubes for RNA stabilization have certain limitations. Now, a groundbreaking product for ambient collection, stabilization, and transportation of whole blood samples enables isolation of cfDNA, CTC, and RNA from the same sample. Collected cells are
maintained intact, making them also suitable for FACS analysis.
In order to overcome existing hurdles and enable widespread and efficient collection and analysis of whole blood, Mawi DNA Technologies (Hayward, CA, USA; www. mawidna.com) has developed the HemaSureOMICs Collection Tube products that offer non-toxic stabilization of whole blood, free of formaldehyde or formaldehyde-like ingredients. HemaSure-OMICs Whole Blood Collection Tubes are validated for room temperature stabi lization of viral DNA and RNA for several days in viral load and detection assays applicable to the HIV, MERS-CoV, 2019-nCoV and other DNA and RNA viruses.
cfDNA (up to 14 days), CTC (up to 8 days), and RNA (up to 8 days) are stabilized at room temperature in the same blood tube (tempera ture range of 25° – 45° C). HemaSure-OMICs Whole Blood Collection Tubes are available in 3, 6, and 9 mL plastic vacuum filled blood
Multichem IA Speciality Immunoassay Controls are intended for use as third party, tri- level, liquid stable, multi-analyte quality control materials to monitor the precision of laboratory testing procedures for immunoassays.

Image: HemaSure-OMICs collection tubes (Photo courtesy of Mawi DNA)

collection tubes as well as a 250µl microtainer for blood drops for easy shipping and durability. They are compatible with any commercially available manual or automation-enabled kits for the purification of cfDNA and CTC from whole blood. HemaSure-OMICs Whole Blood Collection Tubes are for research use only and not for use in diagnostic or therapeutic procedures.
Prenatal Test Reduces Time of Detecting Chromosomal Abnormalities
Anewly developed prenatal test can determine if a fetus or embryo has the right number of chromosomes at a fraction of the time and cost of currently available clinical genetic tests.
Currently available prenatal genetic tests cost thousands of dollars and take days to weeks to deliver results, adding to the emotional and financial stress of fertility treatment and preg nancy and impacting treatment options.
Clinical Scientists from the Columbia University Irving Medical Center (New York, NY, USA; www.cuimc.columbia.edu) and their colleagues developed the new test,
called STORK (Short-read Transpore Rapid Karyotyping), that can be used in the doctor's office at the point of care, delivers results in under two hours, and is about 10 times less expensive to process per sample than current tests. The nanopore-based sequencing technol ogy was used to analyze tiny fragments of DNA 15,000 times faster than currently available chromosomal testing methods, significantly reducing the amount of time to get results. The test also uses much smaller equipment, about the size of a harmonica and weighing just 450 grams, making it accessible for use in physician offices.
The chromosomal abnormalities that this test can detect are, by far, the most common causes of miscarriage, structural anomalies, and developmental delays. Prenatal genetic testing is recommended for pregnant women who are age 35 or older, have a family history of genetic disorders, or have had one or more miscarriages. It is also used increasingly during in vitro fertilization (IVF) to test embryos prior to implantation to improve the chances of pregnancy and reduce the risk of miscarriage.
The team tested STORK in 218 blinded samples from miscarriages, pregnancies, via
8LabMedica International October/2022 To receive prompt and free information on products, log on to www. LinkXpress.com or fill out reader service form located on last page, or scan the QR code on your mobile devicePRODUCT NEWS
(CRP) TEST DIASYS DIAGNOSTIC SYSTEMS CRP FS
immunoturbidimetric test with
calibrators and controls. The
which
of borderline cases. REAL TIME PCR SYSTEM XI’AN TIANLONG SCIENCE & TECHNOLOGY The
it
samples in one run.
CONTROLS TECHNOPATH CLINICAL DIAGNOSTICS
204LMI-10-22LINKXPRESS COM 205LMI-10-22LINKXPRESS COM 206LMI-10-22LINKXPRESS COM
Cont’d on page 10
MONOTEST

INVASIVE ASPERGILLOSIS
The reliable result just when you need it
MONOTEST FORMAT
AUTOMATED RESULTS
OPTIMISED TREATMENT
BETTER PATIENT CARE
Fully automated CLIA Random Access system.

Same day results, no batching, no sample cumulation
On-demand testing: One monotest = one reportable result. Nothing else is required.
Objective method, comparable to ELISA technique, with reported results as index values.
Compatible test with the broadest panel of infectious diseases in CLIA monotest format (>90 parametres)
Empowering labs for a healthy future

ASPERGILLUS GALACTOMANNAN Ag VIRCLIA®
•
•
•
•
•
Let’s meet at Booth # 6.F18vircell.com 109LMI-10-22LINKXPRESS COM
HEMOGLOBIN TESTING SYSTEM BIO-RAD LABORATORIES

PORTABLE POC READER
LABORATORIES
The VARIANT II TURBO Hemoglobin Testing Sys tem combines Bio-Rad’s HPLC precision and vari ant detection with fast throughput to provide a comprehensive solution
testing



VeraSTAT is a simple, accurate, portable POC device that delivers fast results via use of pat ented cathodic electrochemilumi nescence technology (C-ECL), designed to offer users the next generation of rapid
Prenatal Test Reduces Time of Detecting Chromosomal Abnormalities
amniotic fluid or chorionic villi, and biopsies from embryos prior to implantation. Results were compared with those obtained using standard clinical testing. STORK results agreed with standard clinical testing in all of the pregnancy-related samples and in 98% of the embryos tested. For miscarriage sam ples, STORK was more accurate than stan dard testing and was determined to have correctly identified chromosome numbers in the 10 cases for which the two tests dis agreed. An additional 60 pregnancy samples were tested with STORK at an independent certified laboratory, and those results were identical to results obtained with standard clinical testing.
Zev Williams, MD, PhD, Assoc. Prof. of Women's Health and a senior author of the study, said, “The affordability of this test also means that individuals who have suffered a miscarriage do not have to wait until a second or third loss before insurance will cover expensive lab tests, leaving many women in the dark and often blaming themselves. Our study also shows that our rapid test was better than the gold standard for testing miscarriage samples, giving women who have suffered a pregnancy loss a sense of closure and the ability to take steps to prevent another loss.” The study was published on August 18, 2022 in the New England Journal of Medicine.
Portable PCR Device Delivers


Results in 30 Minutes at Point-of-Need
When the requirement is for infectious disease testing, every minute counts to inform treatment decisions and slow the risk of transmission. Having rapid multiplex PCR testing at the point of need can make life better for millions of people in hos pitals, clinics, airports, care homes, events, workplaces, and a wider variety of other settings. Now, a portable and simple to use rapid multiplex PCR testing system delivers fast, accurate and actionable results in approximately 30 minutes at the point-of-need.

The Q-POC platform from QuantuMDx Group Ltd. (Tyne and Wear, UK; www.quantumdx.com) compresses an entire molecular diagnosis laboratory into a simple-to-use, accessible and portable single device that produces results in approximately 30 minutes. The many innovative features incorporated into the Q-POC platform make it a safe rapid multiplex PCR testing solution for all clinical en vironments. With the Q-POC supercharged platform, it is no longer necessary to wait hours or days for test results.
The Q-POCT platform has been engineered to limit the need for highly skilled operators and offer a smooth user experience behind each interaction with its intuitive interface. The software is designed
with the simplicity and familiarity of smartphone and tablet inter faces. It requires only three minutes hands-on-time to get each test started, using easy to follow, guided workflows on the touch screen. A ready to use solution, with all its simplicity, accuracy and speed, the Q-POCT requires no calibration or preventive maintenance. Each test cassette contains all the necessary reagents to complete a test and can be stored and shipped at room temperature. Users can just load the cassette and press go.
A ton of tech is behind every result, with intelligent multilingual software algorithms integrated into the Q-POCT platform. Clear, simple test results are automatically generated by sophisticated software algorithms, removing any complicated interpretation of the result. The Q-POCT software has been designed with the user in mind and uses intuitive, user centric software design similar to a smartphone allowing clear and easy reporting. The small, porta ble, battery-operated Q-POCT system marks a huge leap in rapid multiplex PCR testing. Its compact design, as well as its internal lithium-ion battery, supports use in a wide variety of clinical settings, at the point-of-need.
10LabMedica International October/2022 To receive prompt and free information on products, log on to www. LinkXpress.com or fill out reader service form located on last page, or scan the QR code on your mobile devicePRODUCT NEWS CLIA MICROWELL STRIP LUMINOMETER AWARENESS TECHNOLOGY, INC. The LumiStat 4900 is a stand-alone CLIA microwell strip luminometer with the LumiStat Auto-Track fea ture that reads all three strips auto matically. It features touch-screen interface with built-in printer.
for HbA1c
that delivers qual ity results at high test volumes.
RANDOX
The
diagnosis. 207LMI-10-22LINKXPRESS COM 208LMI-10-22LINKXPRESS COM 209LMI-10-22LINKXPRESS COM
Image: Results from STORK prenatal genetic test, shown in foreground (Photo courtesy of Columbia University Irving Medical Center).
Cont’d from page 8
Accurate
Blood Abnormalities Found in People with Long COVID













ARS-CoV-2 infection can result in the development of a con stellation of persistent sequelae following acute disease called post-acute sequelae of COVID-19 (PASC) or Long COVID.



A recent study has shown that Long Covid patients, many of whom struggle with intense fatigue, brain fog, and other symptoms, have low levels of cortisol, a stress hormone that helps the body control inflam mation, glucose, sleep cycles.

A large group of Imunobiologists at the Yale School of Medicine (New Haven, CT, USA; medicine.yale.edu) and their colleagues included 215 individuals in an exploratory, cross-sectional study to perform multi-dimensional immune phenotyping in conjunc tion with machine learning methods to identify key immunolog ical features distinguishing Long COVID. The Healthy Controls (HC), Convalescent Controls (CC) and Long COVID (LC) groups had samples collected within the Mount Sinai Healthcare System (New York, NY, USA). The Healthcare Workers (HCW) group had samples collected within the Yale New Haven Healthcare System.
Whole blood was collected in sodium-heparin-coated vacutainers from participants at Mount Sinai Hospital. The following methods were implemented: Flow cytometry, prepared for analysis on an Attune NXT (Thermo Fisher Scientific, Waltham, MA, USA; www. thermofisher.com) SARS-CoV-2 antibody testing by ELISA plates were read at an excitation/emission wavelength of 450 nm and 570 nm; Multiplex proteomic analysis; Linear Peptide Profiling (Serimmune) and samples were normalized to a final concentration of 4 nM for each pool and run on the NextSeq500 (Illumina, San Diego, CA, USA; www.illumina.com) Protein-based Immunome Wide Association Study (PIWAS) analysis; IMUNE-based motif discovery; and Rapid Extracellular Antigen Profiling (REAP) and analysis.

The scientists reported that marked differences were noted in specific circulating myeloid and lymphocyte populations relative to matched control groups, as well as evidence of elevated humoral responses directed against SARS-CoV-2 among participants with Long COVID. Further, unexpected increases were observed in antibody responses directed against non-SARS-CoV-2 viral patho gens, particularly Epstein-Barr virus. Analysis of circulating im mune mediators and various hormones also revealed pronounced differences, with levels of cortisol being uniformly lower among participants with Long COVID relative to matched control groups. The Long Covid blood samples were also awash with a category of “exhausted” T cells that can be recognized by certain markers they express. Such cells surge in the ongoing presence of patho gens, suggesting the bodies of people with Long Covid are actively fighting something.
The authors concluded that significant biological differences have been identified between participants with Long COVID and demographically and medically matched convalescent and healthy control groups, validating the extensive reports of persistent symp toms by various Long COVID advocacy groups. Unbiased machine learning models further identified both putative biomarkers of Long COVID, as well as potential mediators of Long COVID disease pathogenesis. The study was published on August 10, 2022 in the journal medRxiv
Image: The Attune NxT Flow Cytometer is ideal for immunophe notyping and signaling studies, cell cycle analysis, detection of rare events, stem cell analysis, cancer and apoptosis studies, microbiological assays and more (Photo courtesy of Thermo Fisher Scientific).






LabMedica InternationalTo view this issue in interactive digital magazine format visit www.LabMedica.com 11 LabMedica International October/2022
S 111LMI-10-22LINKXPRESS COM
MONKEYPOX VIRUS CONTROL VIRCELL
AUTOMATIC BLOOD
BCA-1000


automatic blood
a maximum throughput of 1200
independent
AI Detects Parasite from Photos of Blood Samples Taken with Smartphone

Chagas disease caused by the parasite Trypanosoma cruzi is a chronic infectious condition whose prevention requires con trol of its vectors, the triatomines (kissing bugs), and hence a response by public health services. Endemic in 21 countries in the Americas, Chagas disease affects some six million people, with an annual incidence of 30,000 new cases in the region, leading to 14,000 deaths per year on average. Some 70 million people are estimated to risk con tracting the disease because they live in areas exposed to triatomines. One of the techniques used to diagnose Chagas is performed by micros copists trained to detect the parasite in blood samples. This requires a professional microscope, which can be coupled to a high-resolution camera, but the method tends to be too expensive and unaffordable for low-income patients. Now, a new study has shown that artificial intelligence (AI) can be used to detect Trypanosoma cruzi in images of blood samples taken with a smartphone camera and analyzed by optical microscope.
The machine learning approach developed by researchers at the University of São Paulo (São Paulo, Brazil; www.5.usp.br) was based on a random forest algorithm trained to detect and count T. cruzi trypomastigotes in mobile phone images. Trypomastigotes are the extracellular form of the protozoan and the only stage that circulates in the bloodstream of patients with acute Chagas. Images of blood smear samples taken with a camera capable of 12 megapixel resolu tion were analyzed to arrive at a set of features common to 1,314 parasites, including morphometric parameters (shape and size), color and texture.



In the study, parasite specialists trained the algorithm to recognize Trypanosoma cruzi, assisted by machine learning and image processing specialists. The features were divided into training and testing sets and classified using the random forest algorithm. The resulting values for accuracy and sensitivity were considered high (87.6% and 90.5% respectively). The researchers also analyzed the area under the receiver operating characteristic curve (AUC-ROC), a graphical representation widely used to assess diagnostic accuracy and optimal test cut-off. The result was 0.942, considered outstanding (the higher the area under the curve, the more accurate the test). The researchers concluded that auto mating the analysis of images acquired with a mobile device is a viable alternative for reducing costs and gaining efficiency in the use of the
optical microscope. The algorithm is open software so that the scientific community can contribute data and resources.
“We got good results in this machine learning initiative. The algo rithm works well for Chagas and can be adapted for other purposes that depend on images, such as analyzing samples of feces, skin and colposcopies,” said Helder Nakaya, a principal investigator at the Center for Research on Inflammatory Diseases. “The point is to generate images and analyze them under a microscope that can be sent to remote parts of Brazil. The app itself must say whether they are images of the parasite that causes Chagas. It’s therefore important to have a robust and afford able microscope that can collect the images automatically.”
AACC Releases Guidelines on Point-of-Care Testing for Non-Laboratorians
Point-of-care testing (POCT), or near-patient testing, is diagnostic testing conducted close to the patient, often by clinical person nel outside of the laboratory. POCT is regularly performed by personnel without a laboratory science degree or credentialing in labora tory medicine. Now, a new guidance document is available for non-lab oratorians who are involved in setting up a process or physical space designed to provide testing at or near where patient care is provided.
The guide released by the American Association for Clinical Chemistry (AACC, Washington, DC, USA; aacc.org) presents the chal lenges to consider when selecting and implementing diagnostic testing. It provides an overview of POCT, including a discussion of the cost-ben
12LabMedica International October/2022 To receive prompt and free information on products, log on to www. LinkXpress.com or fill out reader service form located on last page, or scan the QR code on your mobile devicePRODUCT NEWS
The Amplirun Monkeypox Virus DNA Control contains the purified DNA of Monkeypox virus to be used as a control in nucleic acid amplifica tion-based research techniques. It provides precise concentration in copies/µl. FULLY AUTOMATED IIFT SYSTEM EUROIMMUN The UNIQO 160 is a fully automated system that provides full IIFT automation – from the primary sam ple to the micros copy result with only one instrument. The all-in-one solution maximizes efficiency of the entire IIFT process.
COAGULATION ANALYZER DIRUI INDUSTRIAL The
is an
coagulation analyzer with
T/H/4 modules, five
STAT positions on each module, 42 re frigerated reagent positions and four room temperature positions. 210LMI-10-22LINKXPRESS COM 211LMI-10-22LINKXPRESS COM 212LMI-10-22LINKXPRESS COM
Image: Algorithm identifies protozoan Trypanosoma cruzi in photo of blood samples taken with smartphone (Photo courtesy of University of São Paulo)
Cont’d on page 13
AI-Enabled Diagnostic Solutions Improve Treatment of Breast and Colorectal Cancers
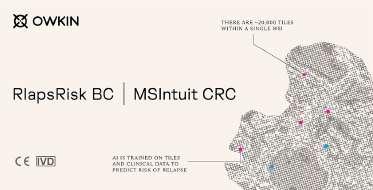
Owkin Dx RlapsRisk BC and Owkin Dx MSIntuit CRC from Owkin (New York, NY, USA; www.owkin.com) have received CE-IVD approval for diagnostic use in the European Union. RlapsRisk BC is the first CE-IVD approved digital pathology-based AI diagnostic that predicts the risk that early breast cancer patients will relapse. MSIntuit CRC is the first CE-IVD approved AI solution that enables the identification of microsatellite stable (MSS) patients from routine histology slides, enabling a significant reduction in time and the number of diagnostic tests for detect ing MSI in clinical practice.
RlapsRisk BC is an AI prognostic solution that predicts whether a breast cancer patient (ER+/HER2-) will go on to relapse after treat ment, informing oncologists which high-risk patients may benefit from targeted therapies and which low-risk patients could potentially avoid chemotherapy. The solution aims to revolutionize breast cancer care management by using AI to assess the risk of relapse after treatment from digital images of tissue sam ples. Currently, breast cancer patients at risk of relapse are identified through expensive, time-consuming molecular or genetic testing. RlapsRisk BC offers doctors a way to identify patients at risk of relapsing by using AI on digital pathology data that will already be held on nearly all breast cancer patients in around 15 minutes.
MSIntuit CRC is an AI digital pathology diagnostic solution that pre-screens for a bio marker in colorectal cancer tumors known as microsatellite instability (MSI) – a defect in a cell’s ability to correct mistakes that occur when DNA is copied. The diagnostic solution rules out MSS phenotypes, allowing pathologists to concentrate their resources on confirming those patients who exhibit MSI. By using an AI model to analyze digital images of tissue samples, doctors are able to more efficiently screen patients when compared to
standard testing techniques, which include time and patient tissue sample-consuming polymerase chain reaction (PCR) testing or immunohistochemistry.
“Owkin’s RlapsRisk BC enables patholo gists to provide oncologists and patients with insights on the risk of relapse, crucial infor mation for the treatment decision-making to avoid unnecessary chemotherapy. Thanks to RlapsRisk, HER2-, ER+ patients will benefit from an automated and accurate prognosis test using just a simple, standard-stained tumor slide,” said Dr. Magali Lacroix-Triki, MD, PhD, an expert breast pathologist at the Department of Medical Biology and Pathology, Institut Gustave Roussy. “AI-based digital pathology diagnostics could help us to provide a comprehensive analysis of each tumor from just one representative stan dard-stained tumor slide, in a complementary process to the pathologist’ diagnosis. This would democratize access to precision med icine, unlocking a new era of treatment for patients across the world.”

“As colorectal cancer is the third most common cancer in the world, it is crucial that we are able to screen patients for biomarkers that make them eligible for po tentially lifesaving treatments. dMMR/MSI screening should be performed on all col orectal cancer patients, but pathologists’ time and resources are often too stretched,” said Professor Magali Svrcek, MD, PhD, Sorbonne Université, AP-HP, Saint-Antoine Hospital, Department of Pathology. “Owkin’s new AI diagnostic solution MSIntuit CRC will roughly half the number of cases requiring screening. It is designed to help more patients receive treatment sooner. AI-based digital pathology algorithms like these, developed in consultation with pathologists, will soon become an integral part of our daily practice. They will help patients to benefit from the best treatment for them sooner – a new era of personalized medicine.”
AACC Releases Guidelines on Point-of-Care Testing for Non-Laboratorians
efit analysis of implementing POCT and the use of POCT in various healthcare settings. The AACC guide presents the challenges to consider when selecting and implementing POCT. It also offers tools to address and manage those challenges and offers solutions to overcome barriers faced in various settings to ensure quality test results that are reliable for patient care.
The AACC guide includes addenda, includ ing glossaries, templates, and forms, to serve as tools in the implementation of POCT. These
are for reference use only; individual POCT laboratories may have unique needs. The guide is not meant to be an all-inclusive ‘how to’ set up a POCT laboratory and is to be used as supportive instruction and education to im plement good laboratory practices, according to the AACC. The guide is most helpful when used in conjunction with all information pro vided by the test manufacturer, including the Manufacturer’s Instructions for Use (MIFU) for testing materials, products, and devices that one has selected to provide laboratory testing services to patients.
LabMedica InternationalTo view this issue in interactive digital magazine format visit www.LabMedica.com 13 LabMedica International October/2022 113LMI-10-22LINKXPRESS COM
Image: Owkin’s AI diagnostic solutions for breast cancer and colorectal cancer have been approved for use in Europe (Photo courtesy of Owkin)
Cont’d from cover
Cont’d from page 12
AUTOMATED ELISA ANALYZER SEBIA

FECAL OCCULT BLOOD DIAGNOSTIC SYSTEM W.H.P.M / HEMOSURE
high speed
Alegria 2 is a fully automated analytical instrument with a walk away design that offers flexibility to run any test from the large ORGENTEC portfolio. Tailor-made biomarker pro files can be analyzed for individual patient

The Hemosure Accu-Reader A100, in conjunction with the Hemosure Accu-Reader A100 FIT test car tridge and sample collection tube, is an automated in-vitro Fecal Occult Blood diagnostic system.

AI Holography System Accurately Checks Quality of Red Blood Cells
R
ed blood cells are a major component of blood that carries oxygen. Red blood cells collected through blood donation are stored for a certain period of time until they are used for transfusion when needed. This process is necessary because unhealthy red blood cells do not function properly and may lead to fatal side effects such as acute lung damage. Conventionally, image-based red blood cell analysis technology is used, which is an invasive method that destroys the three-di mensional structure of red blood cells as red blood cells are observed after staining. In addition, there are tech nical limitations in rapidly analyzing state changes such as three-dimen sional shape, density change, and motility characteristics of red blood cells. To overcome this problem, a research team has developed an AI holography system that automatically extracts important information and inspects the quality of red blood cells. The new system is expected to become a key technology for enabling cleaner and healthier red blood cell injections to patients through accurate quality inspection of red blood cells stored for a certain period of time for blood transfusion.

Scientists at Daegu Gyeongbuk Institute of Science and Technology (DGIST, Daegu, Korea) had earlier developed ‘holography-based red blood cell division and classification technology.’ However, this requires a number of pre-processing algorithms before analysis, which takes a long time, and involves difficulties in performing accurate analysis and classification. In response, the team successfully developed an AI holog
raphy system that automatically inspects the quality of red blood cells stored for a certain time by combining the 3D structure image data of red blood cells obtained with holography technology and generative adversarial neural network technology.
If the developed technology is used, it will be possible to automatically extract important values of judgment for red blood cells by applying the automatic red blood cell 3D structural image analysis algorithm and also check its quality. In particular, it is possible to test the quality of red blood cells precisely and simply as there is no need for invasive methods or pre-treatment procedures required by existing tech nologies. It is expected to be used as a core technology to help minimize the side effects of transfusion by injecting clean and healthy red blood cells to patients needing blood transfusions.
“The technology developed through this research is the source technology that can automatically analyze how red blood cells, stored for transfusion, change their three-dimensional shape depending on the storage period and determine whether stored red blood cells are healthy red blood cells that can be transfused,” said Professor Moon In-kyu of the Department of Robotics and Mechatronics Engineering at DGIST who led the research team. “It is expected that it will help minimize the occurrence of side effects after transfusion in the future as it can check the state of stored red blood cells more minutely and test whether the red blood cells are safe for the patient before transfusion.”
New Guidelines for POC Fertility Testing
for everything from predicting ovulation and diagnosing pregnancy to managing premature rupture of membranes (PROM) - also known as a patient’s water breaking - and high-risk deliveries. However, when pointof-care tests are used inappropriately or performed incorrectly, this can lead to unnecessary follow-up tests and procedures and can even put the patient’s health at risk or lead to death.
Now, the American Association for Clinical Chemistry (Washington, DC, USA; aacc.org) has issued a new guidance document with expert recommendations for performing point-of-care tests for fertility and re productive health. As the use of point-of-care testing rises in these fields, this guidance is intended to ensure that patients and their babies fully benefit from it. AACC has updated guidance that it originally published in 2007 to inform healthcare professionals of the most current best prac
tices for point-of-care testing in reproductive medicine. The highlights of the key recommendations from this document are:
◻ Testing for PROM using commercial kits alone is not recommended without clinical signs that a patient’s water has broken. Additionally, results from these tests must be interpreted in the context of a patient’s clinical presentation to prevent patient harm.
◻
Urine luteinizing hormone tests are accurate and reliable predictors of ovulation. These tests can improve the likelihood of conception among healthy fertile women and can also be used to time certain assisted reproduction procedures. However, further study is still needed to de termine the efficacy of at-home ovulation prediction kits that use saliva or measure basal body temperature.
◻ While blood laboratory pregnancy tests are the gold standard,



14LabMedica International October/2022 To receive prompt and free information on products, log on to www. LinkXpress.com or fill out reader service form located on last page, or scan the QR code on your mobile devicePRODUCT NEWS
needs. REAL-TIME PCR SYSTEM BIOPERFECTUS TECHNOLOGIES The STC-96A Real-Time PCR system features an in novative hardware design to deliver accurate results at
without crosstalk calibration. It maintains temperature uniformity across 96 wells within a remarkable 0.1°C.
213LMI-10-22LINKXPRESS COM 214LMI-10-22LINKXPRESS COM 215LMI-10-22LINKXPRESS COM
Cont’d from cover Cont’d on page 15
Novel Blood Test Can Detect Early-Onset Colorectal Cancer



Colorectal cancer (CRC) represents the third leading cause of cancer-related deaths in the U.S. Patients diagnosed with distant stage of the disease represent 22% of all CRC cases and have 15% 5-years survival rates, in contrast to 91% 5-years sur vival rate of patients with localized disease at diagnosis, indicating how CRC early detection represents an urgent clinical need. Tissue microbiome has been identified as a promising biomarker for the early identification of several cancer types, including CRC. Now, a new study has demonstrated that the analysis of microbiome signatures in plasma can assist in early detection of CRC.
This advancement follows promising early findings by Universal Diagnostics (Cambridge, MA, USA; www.universaldx.com) that revealed early-stage CRC detection through analysis of cell-free circulating tumor DNA (ctDNA) methylation, mutation and frag mentation patterns by using targeted sequencing analysis, advanced computational biology and machine learning algorithms to detect CRC and advanced adenomas. Universal leverages proprietary, state-of-the-art computational biology tools combined with targeted next generation sequencing (NGS) assay platform that allows for simultaneous detection of methylation and microbiome signals for highly-sensitive cancer signal scoring of cell-free DNA regions linked to cancer of interest.
Over the last two decades, microbiome has become a key focus in the nutrition field, giving clinicians and patients a window into how diet and gut bacteria plays into overall health. The proof of principle study by Universal found that changes in gut microbiota have a link into CRC development and progression. Measuring cancer-related microbiome alterations in plasma cell-free DNA (cfDNA) could offer an accurate, non-invasive approach for early cancer detection, leading to decreased cancer mortality. The study found that cfDNA analysis coupled with model building achieved high sensitivity, including at stage I/II and stage III/IV, at equally accurate specificity. The data presented by Universal shows that microbiome research can have a profound impact on other areas of healthcare, such as oncology. This body of research from Universal is being used to build “Signal-X”, a platform to detect multiple types of cancer. Its first product, “Signal-C”, detects early-stage colorectal cancer and adenomatous polyps.
“While methylation, mutation and fragmentation are still the core of early CRC detection, we think microbiome is an interesting addi tion to our proprietary technological platform Signal-X as we build out the platform,” said Christian Hense, COO at Universal DX. “As we learn more about microorganisms and how they interact in commu nities within our bodies to change the way we feel and function, we hope this type of data analysis will help patients get access to earlier and more sensitive screening, individualized guidance for treatment, and advanced monitoring techniques.”



New Guidelines for POC Fertility Testing


healthcare providers should consider using pregnancy point-of-care tests in situations where rapid diagnosis of pregnancy is needed for treatment decisions. One such scenario is if a patient presents to the emergency department with unstable vital signs and symp toms indicative of a ruptured ectopic pregnancy that might require surgery.
“Point-of-care testing is growing in popularity as a means of deliv ering faster turnaround time of test results closer to the patient,” said the guidance document authors, “Guidance is needed for optimizing the implementation of [point-of-care testing] in patient care. This guidance document revises previous recommendations and offers best practices for the use of [point-of-care testing] in fertility and reproductive health.”


15 LabMedica International October/2022 LabMedica InternationalTo view this issue in interactive digital magazine format visit www.LabMedica.com
Image: A new study has shown the link between microbiome bacteria and early colorectal cancer detection (Photo courtesy of Universal Di agnostics)
115LMI-10-22LINKXPRESS COM
Cont’d from page 14
AIR-DISPLACEMENT PIPETTES



MENINGITIS TEST VIRCELL
HEMATOLOGY WORKFLOW SOLUTION CELLAVISION






Select fully-autoclavable, air-displace ment pipettes are ergonomic, safe, and reliable, offering volume setting choice and optional handle personalization for optimal use in various protocols.


The Viral Meningitis Real Time PCR kit is intended for the one-step detection of the main agents causing viral meningitis in human cerebro spinal fluid samples, offering 8 tar gets Multiplex PCR with two reaction tubes

sample.
DIFF-Line by CellaVision is a new workflow solution for low-volume hematology laboratories with three instruments for smearing, staining, and analyzing peripheral blood smears: CellaVision DC-1, RAL SmearBox, and RAL StainBox.

Mitochondrial Biomarker Predicts Type 2 Diabetes Risk
Type 2 diabetes (T2D) is characterized by chronic hyperglycemia primarily caused by both impaired insulin secretion by pancre atic β-cells (insulinopenia) and defective insulin signaling in metabolically active tissues (insulin resistance).
The nuclear-encoded protein ATPase inhibitory factor 1 (IF1) is an en dogenous inhibitor of the mitochondrial ATP synthase. For a long time, IF1 was thought to act only as an inhibitor of the reverse ATPase activity of the ATP synthase. However, recent data indicate that IF1 also partially inhibits the synthetic activity of the ATP synthase in mitochondria, thus limiting oxidative phosphorylation (OXPHOS).
Molecular Biochemists at the Université de Toulouse (Toulouse, France; www.univ-toulouse.fr) and their colleagues conducted a prospective study, where the baseline plasma level of IF1 was measured in 307 participants with prediabetes. The primary outcome was the incidence of new onset diabetes (NOD) within five years of follow-up. Cross-sectional analysis of the IF1 level was also
done in two independent interventional studies. Correlations between plasma IF1 and metabolic parameters at baseline were assessed by Spearman’s correlation coefficients, and the association with the risk of NOD was determined using Cox proportional-hazards models.
Biological analyses including plasma triglycerides (TG), total cholesterol, HDLcholesterol (HDL-C), glucose, glycated hemoglobin (HbA1c), aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (g-GT), insulin and high-molecular-weight adiponectin (HMWadiponectin) were performed. Plasma apoA-I and IF1 were measured by a liquid chromatography – tandem mass spectrometry (LC-MS/MS) method. Analyses were performed on a Xevo TQD mass spectrometer with an electrospray interface and an Acquity H-Class UPLC device (Waters, Milford, MA, USA; www.waters.com).


The scientists reported that the mean IF1 plasma level was lower in participants who developed NOD than in those who did not (537 ± 248 versus 621 ± 313 ng/mL). The plasma IF1 level negatively correlated with clinical variables associated with obesity and insulin resistance, including the BMI and HOMA-IR. Conversely, IF1 was positively associated with plasma markers of cardiometabolic health, such as HDL-C and apoA-I. These correlations were confirmed in cross-sectional analyses. In Therapeutic Innovation in Type 2 DIABetes (IT-DIAB) cohort, the IF1 level was significantly associated with a lower risk of T2D after adjustment for age, sex, and fasting plasma glucose (HR [95% CI] per 1 SD = 0.76 [0.62; 0.94].
The authors concluded that they had identified plasma IF1 as a de terminant of T2D onset in high-risk populations, independently of age, sex, and fasting plasma glucose levels. IF1 measurements are foreseen within the framework of other prospective cohorts of individuals at different risks of T2D to more firmly establish the predictive value of IF1 measurements in the assessment of T2D risk along with established risk factors. The study was published on July 26, 2022 in the journal
To receive prompt and free information on products, log on to www. LinkXpress.com or fill out reader service form located on last page, or scan the QR code on your mobile devicePRODUCT NEWS
GILSON MyPIPETMAN
per
216LMI-10-22LINKXPRESS COM 217LMI-10-22LINKXPRESS COM 218LMI-10-22LINKXPRESS COM 16LabMedica International October/2022 116LMI-10-22LINKXPRESS COM
Research Square
Rapid, simple, cost effective carbapenemase detection Contact us for more information sales@mast-group.com www.mast-group.com MAST CARBA PAcE® Results in <10 minutes
Microchip Imaging Cytometry Improves Laboratory Testing



tice. Public health agencies also need powerful diagnostic tools to help them make critical policy decisions. In a typical clinic appointment, labora tory tests go through procedures such as lab requisition, sample collection, sample processing, and reporting. The average turn-around-time may vary from several hours to several days. For many disease diagnoses and mon itoring requiring instant information and rapid decision-making, the tradi tional technology and workflow cannot effectively meet the clinical needs.
Meanwhile, there is the “rapid test strip” option such as the COVID antigen test strip and the hCG pregnancy test strip that provides instant test results. These rapid test strips are an important diagnostic tool for screening and monitoring, although the application of the test strips is usually restricted to qualitative tests. Additionally, because of their rela tively lower analytical sensitivity, these rapid test strips may not detect biomarkers that have a low quantity in the specimen. Therefore, there is a growing need to develop a quantitative, easy-to-use, and accessible diagnostic instrument and reagents. Given the emerging healthcare needs, scientists and engineers continuously come up with creative diagnostic solutions using a variety of technological approaches. Among these tech nologies, microfluidics has become a highly valuable approach to poten tially address many of the requirements. Microchip imaging cytometry (MIC) based on microfluidic technologies is such an innovative analytical platform that may change the landscape of the clinical lab testing field.

A team of researchers at the University of Toronto (Toronto, ON, Canada; www.utoronto.ca) have published a paper in Opto-Electronic Advances (OEA) that addresses scientific and technical advances in the field of MIC and shows the applications of MIC that may bring more economical, easy-to-use, and accessible healthcare to the public. MIC is a platform technology that can rapidly detect and analyze human biochemical substances such as cells, proteins, and nucleic acids. MIC devices have the attributes of portability, cost-effectiveness, and adapt ability while providing quantitative measurements to meet the needs of laboratory testing in a variety of healthcare settings. Based on the use of microfluidic chips, MIC requires less sample and may complete sample preparation automatically. Therefore, it can provide quantitative testing results simply using a finger prick specimen. The decreased reagent con sumption and reduced form factor also help improve the accessibility and affordability of healthcare services in remote and resource-limited settings.
The article reviews notable clinical applications of MIC technol ogies, such as HIV-patient monitoring, sickle disease screening, in fectious disease diagnosis, etc. Depending on the level of automa tion and image capturing formats, MIC devices were classified into three approaches: Static-chip-static-fluid (SCSF), Static-chip-moving-fluid (SCMF) and Moving-chip-static-fluid (MCSF). Brightfield imaging, fluo rescence imaging, and lens-free imaging techniques have been adopted in MIC systems. Image acquisition techniques such as time delay inte gration and temporally coded excitation were demonstrated to achieve higher sensitivity in detecting fast-moving objects in low light levels.
Compared with traditional flow cytometers, MIC analyzes objects such as cells and particles through a relatively wide and shallow microfluidic chip channel. As a result of the breakthrough development of semiconduc tor sensor devices and information technology in recent years, the light source and imaging detection components of MIC can also achieve higher optoelectronic performance. Thanks to the innovation and development of biotechnology, micro-nano manufacturing, semiconductor materials, information technology, and other fields, MIC will find more important clinical test applications in the future, and promote the development of more economical, easy-to-use, and accessible point-of-need tests. Recent advances in photonics, integrated optics, and imaging technologies prom ise to increase the sensitivity and functionality of MIC systems while decreasing their size and cost. Colors can be differentiated directly on the silicon CMOS image sensors using several techniques. Progress towards
Image: MIC could change the landscape of the clinical lab testing field
OEA)
LabMedica InternationalTo view this issue in interactive digital magazine format visit www.LabMedica.com MICROWELL ELISA & CHEMILUMINESCENCE sales@awaretech.com • PPID via bar code • LIS enabled • Option for disposable tips • Robust and reliable ChemWell®2’s unique and easy to use software allows customization of every operation the analyzer performs, making ChemWell®2 Flexible for many lab applications. AUTOMATED PROCESSOR ChemWell®2 is an automated ELISA/CLIA analyzer: An open, programmable, walk away system. ISO 13485:2016 17 LabMedica International October/2022
Cont’d from cover 117LMI-10-22LINKXPRESS COM Cont’d on page 18
(Photo courtesy of
CREATININE/EGFR




The Nova Max Pro is an easy-to-use meter and creatinine biosensor that measures blood creatinine and calculates estimated glomerular filtration rate (eGFR) from a tiny 1.2 microliter capillary blood sample in just 30 seconds.

AUTOMATIC
ANALYZER
The DIESTRO 103 AP V4 Automatic Basic is an au tomatic electrolyte analyzer designed for medium lab oratories that features auto standby mode for reagents saving, built-in printer and connectivity, and touch screen display.

Cord Blood IgE Levels Tied to Allergy, Asthma
its predictability for allergic diseases, but the results are controversial. Several studies have reported that elevated cord blood IgE may predict allergic diseases and/or allergic sensitization in childhood, whereas other studies failed to find it as a good predictor.
Immunologists at the Chang Gung Memorial Hospital (Taoyuan, Taiwan; www.cgmh.org.tw) included 566 children (321 boys; mean age, 6.5 ± 0.4 years) in a prospective population-based cohort study designed to longitudinally investigate the effects of early-life environmental expo sures and genetic predisposition on childhood allergic outcomes. Blood samples were drawn for subsequent measurement of serum total and al lergen-specific IgE. Measurements of nitric oxide (FeNO) and pulmonary function were performed in standard procedures.
Serum total IgE was determined by ImmunoCAP (Phadia, Uppsala, Sweden; www.phadia.com). Allergic sensitization was defined as a positive Phadia Phadiatop Infant test result (0.35 PAU/L), detecting allergen-specific IgE against a mix of common inhalant and food allergens. Serum levels of allergen-specific IgE were measured using an automated microfluidic-based multiplexed immunoassay system, the BioIC Allergen-specific IgE Detection Kit AD40 Panel (Agnitio Science and Technology, Hsinchu, Taiwan; www.agnitiost.com). FeNO measurement was performed by chemiluminescence analyzer (CLD 88sp NO analyzer, Ecomedics, Dürnten, Switzerland; www. ecomedics.com).
The investigators reported that cord blood IgE levels were significantly associated with FeNO levels and serum total IgE levels. Cord blood IgE levels were positively associated with allergic sensitization (adjusted odds ratio [AOR] = 2.22). Specifically, they found significant associations between elevated levels of IgE in cord blood and higher likelihoods for sensitization to house dust mites (Dermatophagoides pteronyssinus and D. farina), dog dander, egg yolk, garlic and baker’s yeast.
Subjects with cord blood IgE ≥0.24 kU/L (the optimal cutoff) were significantly associated with an increased risk of allergic sensitization (AOR = 2.63,) and asthma (AOR = 2.35) than those with cord blood IgE <0.24 kU/L. Subjects with cord blood IgE ≥0.24 kU/L had significantly higher FeNO levels than those with cord blood IgE <0.24 kU/L. There were no significant associations between cord blood IgE levels and pul monary function parameters.
The authors concluded that that cord blood IgE ≥0.24 kU/L predicts allergic sensitization, FeNO elevation, and asthma among Asian school children. Specifically, cord blood IgE levels ≥0.24 kU/L was associated with a 2.6-fold increased risk of allergic sensitization, particularly sensi
HemaPrep is a small, portable blood smearing instru ment that standardizes the making of smears and does not need any power source. By adjusting the speed of the spreader, the operator can control smear length and thickness.

tization to mites, animals, and foods, at six years of age. This study sug gests that cord blood IgE levels would be useful for early identification of newborns at risk of subsequent allergic sensitization and allergic airway inflammation at school age. The study was published on August 1, 2022 in the journal Pediatric Allergy and Immunology.
Microchip Imaging Cytometry Improves Laboratory Testing

higher sensitivity detectors has also been made by integrating single-pho ton avalanche diodes in standard CMOS with microfluidic systems.
The development of MIC devices should focus on the follow ing aspects: 1) the device should be portable to fit the diagnostic purpose in varying healthcare scenarios; 2) the device should be easy to use and provide sample-to-answer results rapidly (e.g. 15 minutes); 3) the microfluidic assembly should contain pre-loaded reagents and be disposable. Additionally, the analytical perfor mance of MIC devices, such as sensitivity, accuracy, precision, robustness, needs to meet the certain testing requirements. In the process of instrument and reagent design and development, all these aspects need to be considered. Therefore, engineering design and development need to find the sophisticated balance between complexity, performance and cost, to meet the needs in healthcare and to benefit more patients.
18LabMedica International October/2022 To receive prompt and free information on products, log on to www. LinkXpress.com or fill out reader service form located on last page, or scan the QR code on your mobile devicePRODUCT NEWS
METER NOVA BIOMEDICAL
ELECTROLYTE
JS MEDICINA ELECTRONICA
PORTABLE BLOOD SMEARING INSTRUMENT
HORIBA
MEDICAL
219LMI-10-22LINKXPRESS COM 220LMI-10-22LINKXPRESS COM 221LMI-10-22LINKXPRESS COM
Cont’d from cover
Image: The BioIC Allergen Specific-IgE Detection Kit-AD 40 panel (Photo courtesy of Agnitio Science and Technology).
Cont’d from page 17
POC Blood Test Could Diagnose Brain Tumors Earlier


New research could lead to the development of a simple blood test for glioblastomas (GBMs), resulting in earlier diagnosis and more effective and personalized treatment options for the most common type of malignant brain cancer. The research involves the development of mathematical models to assess the current use of biomarkers in the detection of GBMs and how such biomarker-based strategies can be improved.
This research is part of a wider CRUX project led by the University of Bristol (Bristol, UK; www.bristol.ac.uk) to develop an affordable, point of care blood test to diagnose brain tumors. This cross-disciplinary project combines biomarker discovery, development of fluorescent nanoparticle and new testing techniques with computational modeling. In their recent study, mathematical models were developed and paired with experimental data. The researchers found that for the prospective GBM biomarker Glial fibrillary acidic protein (GFAP) lowering the current biomarker threshold could lead to earlier detection of GBMs. The team also used computational modeling to explore the impact of tumor characteristics and patient differences on detection and strategies for improvements.

“Our findings provide the basis for further clinical data on the impact of lowering the current detection threshold for the known biomarker, GFAP, to allow earlier detection of GBMs using blood tests,” said Dr. Johanna Blee, lead author and Research Associate in the University of Bristol’s Department of Engineering Mathematics. “With further ex perimental data, it may also be possible to quantify tumor and patient heterogeneities and incorporate errors into our models and predictions for blood levels for different tumors. We have also demonstrated how our models can be combined with other diagnostics such as scans to enhance clinical insight with a view to developing more personalized and effective treatments.”
“These mathematical models could be used to examine and com pare new biomarkers and tests for brain tumors as they emerge. We are hopeful this research will ultimately aid the development of a simple blood test for brain tumors, enabling earlier and more detailed diagno ses,” added Dr. Blee.
Gene Deletion Underlies









Resistance to Chemotherapy in Triple-Negative Breast Cancer




proteognomics approach was used to identify biomarkers in samples from triple negative breast cancer (TNBC) patients that were associated with resistance to chemotherapy treat ment.

Proteogenomics is a field of biological research that utilizes a com bination of proteomics, genomics, and transcriptomics to aid in the dis covery and identification of peptides. Proteogenomics is used to identify new peptides by comparing MS/MS spectra against a protein database that has been derived from genomic and transcriptomic information.
Triple-negative breast cancer (TNBC) is a type of breast cancer that lacks or shows low levels of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) overexpression and/or gene amplification. TNBC comprises 15–20% of all breast cancer cases and affects more young women or women with a mutation in the BRCA1 gene than other breast cancers. Triplenegative breast cancers, which comprise a very heterogeneous group of cancers, are the most challenging breast cancer type to treat, as hormone therapy that is used for other breast cancers does not work for TNBC.
In its early stages, TNBC is typically treated through surgery, radia tion, and chemotherapy. In later stages where surgery is not possible or

19 LabMedica International October/2022 LabMedica InternationalTo view this issue in interactive digital magazine format visit www.LabMedica.com
Image: A simple blood test for glioblastomas could mean earlier diag nosis (Photo courtesy of University of Bristol)
119LMI-10-22LINKXPRESS COM
A Cont’d on page 20 Size: 2400 x 1200 mm (3 mm thick)100% Silicone YOUR GLOBAL SOURCE FOR STERILIZATION ACCESSORIES Thermo-Resistant ( 60 °C to 300 °C) Fully Washable & Flexible Suitable for central sterilization services Sterilizable STERILIZABLE INSTRUMENT & WORK-SURFACE MATS Front Back WASHING TRAYS MAT Heavy Silicone Cover & Transport Tablet TURBO WASHING MACHINES TRAYS SILICON INSTRUMENT MAT Front Back MICRO INSTRUMENT MAT Exchangable Net Exchangable Nets INVITEDTOAPPLY DISTRIBUTORS Up to 37 cm in length THERMO RESISTANT GLOVES Front Back WASHING TRAYS MAT NEW! VICOTEX Place de la Gare 1 • 1009 Pully • Switzerland Tel: (41) 21-728-4286 • Fax: (41) 21-729-6741 E-Mail: contact@vicotex.com www.vicolab.com S.A. SILICONE TABLET AND STEEL COVER NETS
ESR ANALYZER


that can include severe headaches, extreme exhaustion, heart palpitations and brain fog. The discovery comes in the form of a new diagnostic tool that could be easily deployed in medical practices across the world, at low cost.
Eighteen months ago, researchers at Murdoch University (Perth, Australia) used multi-million-dollar nuclear magnetic res onance (NMR) technology to identify new diagnostic molecular biomarkers that tell if someone has the disease, without the need to detect the disease itself. They then used this work to develop an inexpensive clinical NRM that general practitioners can use to detect vital blood markers to predict the long-term effects of the conditions. The technology uses a specially designed set of radio pulses to extract signals from highly specific biomarker signals (from inflammatory glycoprotein markers and fats bound to lipoproteins) that gives a rapid
a double recog nition immunochromatographic assay for the qualita


COVID 19 SPIKE CROM
total antibodies
to SARS-CoV-2
protein in
Testing Breakthrough on Effects of Long COVID
diagnosis in approximately a minute. The re searchers believe that their findings represent a translational triumph that will ultimately ben efit COVID-19 patients in clinics throughout the world, as well as have the possibility for application across many other diagnostic areas including cardiovascular disease.
“We only discovered these signals about 18 months ago using a more expensive NRM instrument, but with some pulse sequence modifications, we are now able to get identical results on small machines that costs one tenth of the price,” said Professor Jeremy Nicholson, Director of the ANPC and Pro Vice Chancellor for the Health Futures Institute. “We think this technology (low field NMR spectroscopy) will probably have many other clinical applications in the future and may be of particular value in monitoring some of the residual effects of long COVID in individual patients.”
“It ticks all the boxes for a successful
IRIA 5 POS is a compact, easy function, low-cost Erythrocyte Sedimentation Rate (ESR) analyzer with a working load of 18 samples per hour, 5 test tube positions, delivering results in mm Westergren with test time of 17 minutes.
Image: A new diagnostic tool will aid the diag nosis of debilitating symptoms of long COVID19 (Photo courtesy of Pexels)
translational technology: low costs, low main tenance, no specialist required and no need for complex algorithms to understand the data,” said Professor Julien Wist from the ANPC who believes the technological advancement has many benefits, including cutting costs.
Gene Deletion Underlies Resistance to Chemotherapy in Triple-Negative Breast Cancer

the cancer has spread from the initial localized area, treatment is limited to chemotherapy and in some cases further targeted therapy. Triplenegative breast cancers have a relapse pattern that is very different from hormone-positive breast cancers where the risk of relapse is much higher for the first three to five years, but drops sharply and substantially below that of hormone-positive breast cancers afterwards.
In order to predict response to treatment, investigators at Baylor Medical College (Houston, TX, USA; www.bcm.edu) and their colleagues used an innovative analytic ap proach called “microscaled proteogenomics” to analyze tumor biopsies taken from TNBC patients prior to treatment with carboplatin and docetaxel combination chemotherapy.


Data from standard DNA and RNA sequencing approaches were integrated with mass spec trometry-based proteomics and phosphopro teomic analyses to derive more complete mo lecular portraits of treatment-responsive versus treatment-resistant tumors.
Results of proteogenomic analyses of so matic copy number aberrations identified a resistance-associated 19q13.31-33 deletion where the genes LIG1, POLD1, and XRCC1 are located. LIG1 (DNA ligase I) gene deletion and/or low mRNA expression levels were as sociated with lack of pathological complete re sponse, higher chromosomal instability (CIN), and poor prognosis in TNBC, as well as carbo platin-selective resistance in TNBC pre-clinical models. Hemizygous loss of LIG1 was also associated with higher CIN and poor prognosis
in other cancer types, demonstrating broader clinical implications.
“TNBC is the most difficult to treat form of breast cancer, with standard treatment requir ing multiple chemotherapy drugs that unfortu nately often fail to cure the patient,” said first author Dr. Meenakshi Anurag, assistant pro fessor of medicine at Baylor Medical College. “It is imperative that we develop approaches to predict response so that only effective treat ments are given. Furthermore, patients who do not respond to standard drugs need entirely new treatment approaches. The discovery of therapeutic alternatives will depend on new insights into how TNBC arises.”
The study was published in the August 24, 2022, online edition of the journal Cancer Discovery.
20LabMedica International October/2022 To receive prompt and free information on products, log on to www. LinkXpress.com or fill out reader service form located on last page, or scan the QR code on your mobile devicePRODUCT NEWS MONKEYPOX RAPID ANTIGEN TEST LABNOVATION TECHNOLOGIES LABNOVATION’s Monkeypox Antigen Rapid Test Kit is a lateral flow chromatographic immunoassay for the qualitative detection of monkeypox virus antigen in human whole blood, serum, plasma or rash exudate. 222LMI-10-22LINKXPRESS COM 223LMI-10-22LINKXPRESS COM SARS-COV-2 ASSAY EUROFINS TECHNOLOGIES INgezim
is
tive determination of
specific
S
vaccinated individuals, in serum and blood samples. 224LMI-10-22LINKXPRESS COM
LINEAR CHEMICALS
Cont’d from cover
Cont’d from page 19
Twice-Postponed, WorldLab Seoul 2022 Convenes in Conjunction with APFCB And Delivers on All Promises
 By Helen MARTIN, Chemical Pathology, SA Pathology - Adelaide South Australia, APFCB Secretary Congresses and Conferences Committee Corresponding Member
By Helen MARTIN, Chemical Pathology, SA Pathology - Adelaide South Australia, APFCB Secretary Congresses and Conferences Committee Corresponding Member
The 24th International WorldLab Congress of the IFCC and 16th Congress of the Asia-Pacific Federation for Clinical Bio chemistry and Laboratory Medicine (APFCB) was hosted by the Korean Society for Clinical Chemistry (KSCC) in the vibrant and beautiful city of Seoul, at once a bustling mecca of technology and skyscrapers and an oasis of tranquil gardens and Buddhist Temples and Palaces. Postponed due to the COVID-19 pan demic from its original dates in 2020, the 24th IFCC WorldLab was rescheduled to January 2021 and again, finally, to 26-30th June 2022; this later time opened the wonderful opportunity to combine WorldLab for the first time with the APFCB Congress.
Laboratory Medicine is fundamental for optimal healthcare and laboratory professionals worldwide strive to provide high quality and cost-efficient service to their communities. Hence the Congress theme of “Value-Based Laboratory Medicine” was most appropriate and provided delegates with the opportunity to meet and be informed by experts in this area from around the world, enabling sharing of knowledge and facilitating improved health outcomes. Following the opening ceremony with its wel come addresses from IFCC President Khosrow Adeli, APFCB Vice-President Endang Hoyaranda and KSCC Congress President Won-Ki Min, and entertaining and thought-provoking lecture entitled “Almighty Google knows everything about you”, Congress delegates enjoyed a welcome cocktail banquet, for many the first opportunity in over two years to meet with international colleagues and friends, a truly welcome and joyous evening.

The scientific program flowed over the next three and a half days with four plenary lectures addressing the challenges of “Maximizing the value of laboratory medicine in Korea”, “Adding clinical utility to laboratory reports” as well as state of the art developments “Pharmacogenom ics from discovery to clinic” and “Next Genera tion Sequencing analysis of genetic diseases”. Twenty-eight symposia, including five provided by IFCC and three by APFCB, provided expert insights into current challenges and opportuni ties such as utilization management of labora tory tests, using big data to improve healthcare, applications of advanced mobile web technology and laboratory information system technology. Advances in laboratory aspects of major disease burdens worldwide such as kidney disease, dia betes and sepsis were shared as well as educa tional sessions covering essential fundamentals such as standardization, harmonization, tracea bility, method assessment and quality control. In addition, many diagnostic companies provided workshops.
The exhibition space housed thirty-eight di agnostic company booths each eager to share information regarding their latest tests and equip ment. An interesting initiative by the organisers was that, for the first time at an IFCC meeting, posters were presented electronically within the exhibition space.
While the congress offered the opportunity of virtual partic ipation, a positive and valuable development arising from the pandemic and one which I believe is likely to persist into the future, for those who could participate face-to-face the 24th IFCC and 16th APFCB Congress in Seoul was a worthwhile and memorable experience.
21 LabMedica International October/2022 NEWS
Image:
IFCC
President Adeli addressing WorldLab Seoul
2022
plenum at the opening ceremony.
Edited by Katherina Psarra MSc, PhD IFCC members may send news to: Email: enews@ifcc.org www.2023roma.org
EDITORIAL
By Katherina Psarra, MSc, PhD

Dear Colleagues,
With the first raindrops the summer is gone... As the summer is still here in Greece and especially in the islands; I’m glad that I had the great opportunity to attend the IFCCGSCC Joint Symposium on October 2nd in Crete.
In the meantime, full of images of sunsets, seasides or green mountains, we are back to work in our labs. It is a strange time of the year!
IFCC, our international society that makes us, the lab people, visible and shows our importance all over the world, is planning lots
of events for this Fall. The most important event is the IFCC General Conference in Brussels, where we will celebrate the 70 years anniversary of IFCC. Our President, Prof Adeli is inviting us to this wonderful event in his message.
In this issue of IFCC News you can find a very interesting description of the Worldlab in Seoul by Ms Helen Martin, with a lot of information about the city, the congress, the people, the atmosphere.
On the IFCC eNews you will find more articles from the committees, working groups and corporate members.
With my best wishes for a wonderful fall season.
UNIVANTS of Healthcare Excellence Award Program Accepting Applications:

Are you a part of an integrat ed clinical care team? Have you made changes to delivery of care that are associated with measurable improvements to pa tients, payors, clinicians and the health system/ administration? If yes, you may be eligible to ap ply for the prestigious UNIVANTS of Healthcare Excellence award program.
The UNIVANTS of Healthcare Excellence award program is a global award program created to recognize, celebrate, and inspire healthcare excellence. Now in its 5th year, teams from across the globe have been recognized for their innovative and transformative delivery of care that has positively affected key stakeholders, includ ing patients, payors, clinicians, and the health system/ administration. Whether these teams are implementing new test methods, leveraging the power of informat ics or improving processes within their institution, they all have sev eral things in common: (1) team members from across disciplines have united to address some of the most pressing issues in their institution; (2) laboratory insights and laboratory medicine has played an integral part in their suc cess; and (3) they have outcomes that demonstrate a measurable
difference to patients, payors, cli nicians and the health system/ administration.
Don’t miss your chance to be recognized by this prestigious award program and to join in celebrating and sharing your achievements globally. Appli cations will be accepted now through Nov 2022.
Looking to maximize your chances of recognition? Review these tips for applying
1. Download and review the ap plication guidance documents at: https://www.univantshce.com/int/ en/apply#guidance
2. Reach out to the award adminis tration team with any questions at: UNIVANTSofHealthcareExcellence @abbott.com
3. Have ALL members from your integrated clinical care team re view the application before sub mitting.
To learn more about past winners, eligibility criteria and to apply at: www.univantshce.com
22LabMedica International October/2022 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more informationNEWS
Apply Now! The UNIVANTS of Healthcare Excellence Award recognizes teams who collaborate across disciplines and transform healthcare delivery, and ultimately patient lives. Submit your team application to the UNIVANTS of Healthcare Excellence Award program on or before November 15th at UnivantsHCE.com. The time is now to highlight your healthcare excellence! © 2022 Abbott. All rights reserved. ADD-132545-GBL-EN. ACT NOW! DON’T MISS OUT… The views and positions expressed in the IFCC News section are those of the IFCC or the individual authors, and do not necessarily represent the views or positions of LabMedica magazine or its publishers. IFCC OFFICE Via Carlo Farini 81, 20159 Milan, ITALY Tel: (39) 02-6680-9912 E-mail: ifcc@ifcc.org • Web: www.ifcc.org Staff Members: Paola Bramati, Silvia Cardinale, Silvia Colli-Lanzi, Smeralda Skenderaj
MESSAGE FROM THE PRESIDENT
By Khosrow Adeli • President, IFCC

Greetings to everyone in the IFCC family! I hope you have all had a wonderful summer. Here at IFCC, we are looking forward to a very productive Fall season.There are many upcoming events planned this quarter as we come out of the COVID-19 pandemic. These include the AFCB Congress in Broummana, Lebanon (September 8-10), IFCC-GSCC Joint Symposium in Crete, Greece (October 2), as well as several other meetings, workshops, and live webinars. For more informa tion, visit the IFCC website. I look forward to seeing our IFCC community at these important events!
This Fall, IFCC will also be hosting the General Conference for invited IF CC Officers in Brussels, Belgium from October 28-31, 2022. Members of Com mittees and Taskforces as well as Presi dents of National Societies and Regional Federations have been invited and are encouraged to attend. This year’s meet ing will present a special opportunity to celebrate the 70th Anniversary of IFCC, recognizing 70 years of global leader ship in laboratory medicine and cele brating our contributions to advancing excellence in laboratory medicine for better healthcare worldwide. This occa sion will be commemorated throughout the meeting with special events, includ ing a social dinner with live jazz music. Alongside updates from IFCC functional groups including new IFCC Task Forces, several special symposia are planned including: presentations from IFCC Past Presidents, presentations from all six Regional Federation Presidents, a Spe cial Symposium on the Central Role of Lab Medicine in Patient Care & Public Health with expert presentations from AACC, WHO, and others, as well as an Industry Forum by diagnostic industry leaders on the Future of IVD over the Next Decade. Brussels will set the per fect backdrop for these activities as a truly global city, often referred to as the “capital of Europe”.
In addition to upcoming events, the IFCC Task Force on Outcome Studies in Laboratory Medicine (TF-OSLM) is de veloping a funded research program for investigators in clinical labs, hospitals, and other institutions located around the world to conduct new retrospective and prospective research with outcomes that assess the value of laboratory medicine in healthcare overall. A call for proposals has been sent out, seeking research proposals for studies evaluating the im


pact of laboratory testing on health out comes. These activities will fulfill the strategic objectives set out by TF-OSLM, which include promotion of directed re search evaluating the role of laboratory medicine on clinical outcomes as well as building awareness and understanding with regard to the critical role laboratory medicine plays in healthcare outcomes.
Looking forward to a very productive fall season for IFCC member societies, national federations, and colleagues from around the world. As always, feel free to email me at president@ifcc. org with your feedback, questions, or concerns.
CALL FOR PROPOSALS IS OPEN

23 LabMedica International October/2022 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
Cheers, Khosrow
For five incredible days, a global body of thinkers, trailblazers, dreamers and disruptors will converge in Anaheim, CA. This is where genius is shared, theories are proven, correlations are discovered and ideas are challenged. Share your expertise as we come together as one global laboratory medicine community. Submit your proposals for AACC University, Roundtables, and Scientific Sessions. Visit aacc.org/proposals for a list of complete submission guidelines and session categories. Deadline for submissions is Friday, November 4, 2022. 2023 AACC ANNUAL SCIENTIFIC MEETING + CLINICAL LAB EXPO JULY 23-27 I ANAHEIM, CA GLOBAL LAB MEDICINE COMMUNITY
IFCC Welcomes Three New Corporate Members

We are a company committed to the permanent education of clinical laboratory personnel and blood banks in Latin America.

We have a digital platform to offer ondemand academic programs such as diplomas, workshops, courses on topics related to analytical quality control in the

nmatched Multimedia ess to the World’s Clinical aborator y Community
Since its founding in 2010, BioPerfectus (SSE:688399) has been dedicated to providing holistic molecular diag nostic solutions specializing in emerging infectious diseases for generations and generations to come.






We are leading the change by offering qRT PCR Kits, Nucleic Acid Extraction Systems, Automation Solutions, and Rapid Tests to laboratories, hospitals, institutions, and CDCs across the globe. As one of the leading global IVD suppliers, BioPerfectus is committed to delivering excellent services and products that meet the highest international quality and safety standards.
Our global presence enables us to build close relationships with our clients and leverage our expertise to support them worldwide. To "embrace the world with health" has always been our mission. But that's not where our mission ends. We are taking what we have learned, researched, and prepared for the subsequent emergency calls from infectious diseases and saving people's lives.

This is us; this is BioPerfectus. See how we're using diagnostics to save the world: www.bioperfectus.com
Founded in 1960 in Kyoto, Japan, ARKRAY has been the world leader in the field of In Vitro Diagnostics (IVD) testing.

As a pioneer in HbA1c testing and urine testing, we developed and released the first fully automated HbA1c analyzer in 1981 and developed the world's first automated analysis system for use with urine test strips in 1972. We currently provide comprehensive support to the medical field ranging from development, manufacturing, sales and servicing including diagnosis support tools such as software.
Willing to take on new challenges, we solve issues in the medical field and support the healthy livelihood of people around the world.
Website: www.arkray.co.jp/english
24LabMedica International October/2022 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more informationNEWS
GAZINE • INTERACTIVE DIGITAL EDITION AL • MOBILE VERSION • MOBILE APPS TTER • E MAIL MARKETING OLUTIONS • SHOW DISTRIBUTION DAILY CLINICAL LAB NEWS .com
bMedica International • LabMedica en Español P U B L I S H E D I N C O O P E R AT I O N W I T H URCEICSNEWS2021 Media inforMation PUBLISHED IN COOPERATION WITH PRINT MAGAZINE • INTERACTIVE DIGITAL EDITION WEB PORTAL • LABMEDICA EXPO • E-NEWS LETTER MOBILE VERSION • MOBILE APS • E-MAIL MARKETING ONLINE SOLUTIONS • SHOW DISTRIBUTION
BD to Market Accelerate’s Antibiotic Resistance Tests Worldwide
Becton, Dickinson and Company (BD, Franklin Lakes, NJ, USA; www.bd.com) and Accelerate Diagnostics, Inc. (Tucson, AZ, USA; www.axdx.com) have entered into a worldwide com mercial collaboration agreement where BD will offer Accelerate’s rapid testing solution for antibiotic resistance and susceptibility of fering results in hours, versus one to two days with some traditional laboratory methods. Under the agreement, BD will market and sell the Accelerate Pheno system and Accelerate Arc module and associated test kits through its global sales network in territories where products have regulatory approval or registration.
These solutions complement BD’s existing Clinical Microbiology portfolio and advance the shared goal of both companies to address the global threat of antimicrobial resistance. The Accelerate PhenoTest BC kit is the first test cleared by the US-FDA that can deliver both rapid identification and phenotypic antibiotic susceptibility results in hours direct from positive blood cultures.
Recent external studies indicate this solution offers results one to two days faster than traditional laboratory methods, which can include culturing samples for 18 to 24 hours, and then performing a susceptibility test that can take eight to 24 hours to result. This enables clinicians to optimize antibiotic selection and dosage specific to the individual patient, days earlier.

“When a patient is very sick, every minute matters,” said Brooke Story, president of Integrated Diagnostic Solutions for BD. “Rapid
testing can quickly determine if an antibiotic should be used for treat ment, and if so, which one. Through our collaboration with Accelerate Diagnostics, we can help clinicians more quickly, efficiently and effec tively treat patients, which may lead to a reduction in health care costs and help slow the spread of antimicrobial resistance.”
Guangzhou Pluslife Biotechnology (Pluslife, Guangzhou, China; www.pluslife.com) and the Foundation for Innovative New Diagnostics (FIND, Geneva, Switzerland; www.finddx.org) have entered into a strategic collaboration to support low- and mid dle-income countries (LMICs) in the development of diagnostic tools for women’s healthcare by providing access to next generation point of care (POC) molecular testing technology.
The collaboration between Pluslife and FIND aims to strengthen the MDx platforms in LMICs by providing affordable POC MDx platforms to increase accessibility to essential diagnostics and tests for women. Currently, only 1% of primary healthcare facilities have access to essential diagnostics, and some tests that are specifically required for women are not available to all women in LMICs. Applying the Pluslife POC molecular system in such resource-limited settings can increase the testing capability in primary healthcare facilities in an efficient and economic manner and rapidly expand its usage to lower-level medical facilities such as clinics and test centers. Pluslife will remain dedicated to its collaboration with FIND, and both parties will together make diagnosis more accessible to improve the living standards of the people in LMICs.
“The COVID-19 pandemic has accelerated the advancement of tech nologies that have the potential to make a huge impact. Point-of-care molecular testing, with the capacity to test for multiple diseases, can help us change the question from ‘is this COVID?’ to ‘what is this?’, in primary care clinics where most people first seek care,” said Dr. Marta Fernández Suárez, Chief Technology Officer at FIND.
“Pluslife and FIND shared the same goal to spur diagnostic inno vation and make diagnostic more accessible to LMICs, it is truly a sig nificant social responsibility for Pluslife to honor,” commented Pluslife CEO Noah Chen, “Pluslife Next-Generation POC molecular system will be launched as a reform of LMICs health system, to improve the efficiency of testing and the resilience of the different health channels, and finally achieves more accessible and affordable test for all.”
Image: The collaboration will provide affordable POC MDx platforms for LMICs to increase women’s accessibility to diagnostics
Bio-Rad Acquires PCR Developer in Poland for $170 Million

B
io-Rad Laboratories (Hercules, CA, USA; www.biorad.com) has agreed to acquire all of the outstanding shares of Curiosity Diagnostics from Scope Fluidics, S.A. (Warsaw, Poland; www. scopefluidics.com) for a total consideration of up to USD 170 million, consisting of approximately USD 100 million in cash, and up to USD 70 million in future milestone payments. Curiosity Diagnostics, a latestage, pre-commercial platform company, is in the process of developing a sample-to-answer, rapid diagnostics PCR system for the molecular diagnostics market.
“We are excited to have the Curiosity Diagnostics team join BioRad’s Clinical Diagnostics Group and to work closely together to bring a new generation of rapid PCR systems to market,” said Dara Wright, Bio-Rad’s EVP and President, Clinical Diagnostics Group. “Curiosity’s PCR platform, PCR|ONE, offers a streamlined workflow and rapid turn around times, and is expected to extend our reach beyond high-com plexity labs into near-patient molecular diagnostics labs.”
25 LabMedica International October/2022 Industry NewsTo view this issue in interactive digital magazine format visit www.LabMedica.com
Image: The Accelerate Arc Module & BC kit is registered as an IVD (Photo courtesy of Accelerate Diagnostics)
Pluslife to Partner with International Foundation in Providing Affordable POC MDx platforms to Developing Countries
For a free listing of your event or a paid advertisement in this section contact: International Calendar LabMedica International E-mail: info@globetech.net

OCTOBER
54th National Congress of the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC). Oct 5-7; Genoa, Italy; sibioc.it
JFBM 2022 - Journées Francophones de Biologie Médicale. Oct 5-7; Saint-Etienne, France; jfmb.fr
35th IFBLS World Congress 2022 –International Federation of Biomedical Laboratory Science. Oct 5-9; Suwon, Korea; ifbls2022.org
CAP22 – Annual Meeting of the College of American Pathologists. Oct 8-11; New Orleans, LA, USA; capannualmeeting.org
IAP 2022 – 34th Congress of the International Academy of Pathology. Oct 1115; Sydney, Australia; iap2022.com
ESID 2022 – 20th Biennial Meeting of The European Society for Immunodeficiencies. Oct 12-15; Gothenburg, Sweden; esidmeeting.org
DKLM 2022 – Annual Congress of the German Society for Clinical Medicine and Laboratory Medicine (DGKL). Oct 13-14; Mannheim, Germany; dgkl.de
46th ISOBM Congress – International Society of Oncology and Biomarkers. Oct 13-17;
Bled, Slovenia; isobm2022.net
84th Annual Meeting of the Japanese Society of Hematology. Oct 14-16; Fukuoka, Japan; jshem.or.jp
59th Annual Scientific Conference of the Australasian Association of Clinical Biochemistry and Laboratory Medicine (AACB). Oct 18-20; Perth, Australia; aacb. asn.au
20th Congress of the Polish Society for Laboratory Diagnostics. Oct 19-22; Kielce, Poland; ptdl.pl
91st Annual Meeting of the American Thyroid Association (ATA). Oct 19-23; Montreal, Canada; thyroid.org
LABCLIN 2022 – 16th National Congress of the Spanish Societies for Clinical Laboratory (AEBM-ML, AEFA & SEQCML). Oct 19-21; Malaga, Spain; labclin2022.es
MEDLAB Asia 2022. Oct 19-22; Bangkok, Thailand; medlabasia.com
EndoBridge 2022. Oct 20-23; Antalya, Turkey; endobridge.org
ASHI 2022 – 48th Annual Meeting of the American Society for Histocompatibility and Immunogenetics. Oct 24-28; Las Vegas, NV, USA; ashi-hla.org
Arab Lab 2022. Oct 25-27; Dubai, UAE; arablab.com
ASHG 2022 – Annual Meeting of the American Society of Human Genetics. Oct 25-29; Los Angeles, CA, USA; ashg.org
ACPID 2022 – 10th Asian Congress of Pediatric Infectious Diseases. Oct 26-28; Seoul, Korea; acpid2022.org
33rd National Congress of the Turkish Biochemical Society. Oct 26-30; Çeşme,
Turkey; turkbiyokimyadernegi.org.tr
NOVEMBER
AMP 2022 –Annual Meeting & Expo of the Association for Molecular Pathology. Nov 3-5; Phoenix, AZ, USA; amp.org
KAI International Meeting 2022 – Korean Association of Immunologists. Nov 3-5; Incheon, Korea; kai2022.kr
CALILAB 2022 – 6th Argentine Congress of Quality in the Clinical Laboratory. Nov 7-9; Mar Del Plata, Argentina; calilab.fba.org.ar
FIDSSA Congress 2022 – Federation of Infectious Diseases Societies of Southern Africa. Nov 3-5; Durban, South Africa; fidssacongress.co.za
UKMedLab22 - Annual Conference of the Association for Clinical Biochemistry and Laboratory Medicine (UK). Nov 7-9; London, UK; acb.org.uk
43rd Annual Meeting of the American College of Toxicology (ACT). Nov 13-16; Denver, CO, USA; actox.org
Analytica China. Nov 14-16; Shanghai, China; analyticachina.com
MEDICA 2022. Nov 14-17; Dusseldorf, Germany; medica-tradefair.com
ICC 2022 – 21st International Congress of Cytology. Nov 15-20; Baltimore, MD, USA; cytology-iac.org
2022 Annual RBSLM Meeting – The Royal Belgian Society of Laboratory Medicine. Nov 18-20; Brussels, Belgium; rbslm.be
ISID Congress 2022 – International Society for Infectious Diseases. Nov 17-20; Kuala Lumpur, Malaysia; isidcongress.org
ACBICON 2022 – 48th Annual Conference
of the Association of Clinical Biochemists of India. Nov 23-26; New Delhi, India; acbicon2022delhi.com
ChemCon 2022 – 13th Annual Conference of the Pakistan Society of Chemical Pathology (PSCP). Nov 25-26; Karachi, Pakistan; pscp. org.pk
ASI 2022 – 50th Annual Scientific Meeting of the Australian and New Zealand Society for Immunology. Nov 29 – Dec 2; Melbourne, Australia; asi2022.org
DECEMBER
JIB 2022 - Journees de l`Innovation en Biol ogie. Dec 1-2; Paris, France; jib-innovation. com
BSI Congress 2022 – British Society for Immunology. Dec 5-8; Liverpool, UK; bsicon gress.com
8th Annual Conference of the Saudi Society for Clinical Chemistry. Dec 6-8; Riyadh, Saudi Arabia; sscc.med.sa
64th Annual Meeting & Exposition of the American Society of Hematology (ASH). Dec 10-13; New Orleans, LA, USA; hematology.org
2023
JANUARY
Fertility 2023– Joint Conference of the UK Fertility Societies. Jan 10-13; Belfast, UK; fertilityconference.org
FEBRUARY
Medlab Middle East 2023. Feb 6-9; Dubai, UAE; medlabme.com
Labquality Days 2023 – International Congress on Quality in Laboratory Medicine. Feb 9-10; Helsinki, Finland; labqualitydays.fi
26LabMedica International November/2020 Every advertisement or product item in this issue contains a LinkXpress ® number as shown below: Or, Circle LinkXpress Numbers of Interest on Reader Service Card and Scan and Email this form to: subs@globetech.net FREE PRODUCT INFORMATION 999LMI-10-22LINKXPRESS COM Renew / Start your Free Subscription Instant Online Product Information Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 LMI-10-22 LAB MEDICA INTERNATIONAL Reader Service Form PLEASE COMPLETE THE FOLLOWING WRITE CLEARLY IN BLOCK LETTERS or AFFIX YOUR SUBSCRIBER LABEL AP PEARING ON COVER For EXPRESS service: visit www.LinkXpress.com or Scan and Email this form to: subs@globetech.net I. TYPE OF LABORATORY (a) o Hospital Laboratory (i) o Independent/Reference Laboratory (c) o Blood Bank Laboratory (d) o Public Health Laboratory (f) o Industrial/Biomedical Laboratory (g) o Government Authority/Health Agency (l) o Research/Educational Laboratory (h) o Distributor/Dealer/Manufacturer (t) o Other Please Specify . . . . . . . . . . . . . . . . . . . . . . . II. YOUR TITLE OR FUNCTION (1) o Director of Laboratory(ies) (2) o Dept. Chief/Supervisor (3) o Chief Technologist (4) o Technologist (5) o Administrator/Manager (6) o Medical Practitioner (7) o Other Please Specify . . . . . . . . . . . . . . . . . . . . . . . III. Are you a Ph.D. or M.D.? o YES IV. YOUR DEPARTMENT OR SPECIALTY (h) o General Lab Diagnostics (b) o Clinical Chemistry/Biochemistry (c) o Microbiology (d) o Hematology (e) o Blood Bank (p) o Immunology (a) o Anat. Pathology (o) o Serology (q) o Histology (r) o Cytology (g) o Toxicology (k) o Virology (l) o Oncology (m) o Endocrinology (j) o Administration/Purchasing (t) o Other Please Specify . . . . . . . . . . . . . . . . . . . . . . . V. With how many readers do you share this copy of Lab Medica Intl ? . . . . . . . . . The publisher reserves the right to qualify requests Subscriber Code on Your Label (Needed for All Renewals) Name of Individual Position and Department Name of Institution Mailing Address City, ProvincePostal Code Country VI. CIRCLE LINKXPRESS NUMBERS OF INTEREST TO RECEIVE FREE INFORMATION For EXPRESS service: visit www.LinkXpress.com or Scan and Email this form to: subs@globetech.net Yes, I wish to receive free copies of Lab Medica International SIGNATURE (REQUIRED) DATE: DAY ................. MONTH ........................ YEAR .................... 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 Tel: ( )( ) @ E-MAIL (REQUIRED): Events Calendar 2022
October/2022 ATTENTION: Due to the CORONAVIRUS PANDEMIC, many events are being rescheduled for a later date, converted into virtual venues, or altogether cancelled. Please check with the event organizer or website prior to planning for any forthcoming event
MARCH
CHINA LAB 2023. Mar 9-11; Guangzhou, China; chinalabexpo.com
USCAP 112th Annual Meeting - United States & Canadian Academy of Pathology. Mar 11-16; New Orleans, LA, USA; uscap.org
ExpoMED Eurasia 2023. Mar 16-18; Istanbul, Turkey; expomedistanbul.com
Pittcon 2023. Mar 18-22; Philadelphia, PA, USA; pittcon.org
APRIL
MSACL 2023 – Congress of the Association for Mass Spectrometry & Advances in Clinical Lab. Apr 3-6; Monterey, CA, USA; msacl.org
AACR 2023 – Annual Meeting of the Amer ican Association for Cancer Research. Apr 14-19; Orlando, FL, USA; aacr.org
ECCMID 2023 – 33rd European Congress of Clinical Microbiology and Infectious Diseases. Apr 15-18; Copenhagen, Denmark; eccmid.org
Korea Lab 2023. Apr 18-21; Seoul, Korea; korealab.org
Analytica Vietnam 2023. Apr 19-21; Ho Chi Minh City, Vietnam; analyticavietnam.com
India Lab Expo & Analytica Anacon India. Apr 27-28; Mumbai, India; analyticaindia. com
MAY
AACE Annual Meeting 2023 – American Association of Clinical Endocrinology. May
4-6; Seattle, WA, USA; aace.com
ECV 2023 – 8th European Congress of Virology. May 4-7; Gdansk, Poland; eusv.eu
ESPID 2023 – 41st Annual Meeting of the European Society for Paediatric Infectious Disease. May 8-12; Lisbon, Portugal; espidmeeting.org
ISLH 2023 – International Society for Laboratory Hematology. May 11-13; New Orleans, LA, USA; islh.org
Immunology 2023 – Annual Meeting of the American Association of Immunologists (AAI). May 11-15; Washington, DC, USA; aai.org
ECE 2023 – 25th European Congress of Endocrinology. May 13-16; Istanbul, Turkey; ese-hormones.org
IFCC-EFLM WorldLab & EuroMedLab 2023 - 25th International Congress of Clinical Chemistry and Laboratory Medicine. May 21-25; Rome, Italy; 2023roma.org
SLAS Europe 2023 Conference and Exhibition. May 22-26; Brussels, Belgium; slas.org/europe2023
Hospitalar 2023. May 23-26; Sao Paulo, Brazil; hospitalar.com
JUNE
106th Annual Meeting of the German Society for Pathology. Jun 1-3; Leipzig, Germany; pathologie-dgp.de
ASCO 2023 – Annual Meeting of the American Society of Clinical Oncology. Jun 2-6; Chicago, IL, USA; asco.org
66th Congress of the German Society for Endocrinology. Jun 5-7; Baden-Baden, Germany; endokrinologie.net
EHA 2023 - Annual Congress of the European Hematology Association. Jun 8-11; Frankfurt, Germany; ehaweb.org
EAACI 2023 – Annual Congress of the European Academy of Allergy & Clinical Immunology. Jun 9-11; Hamburg, Germany; eaaci.org
ESHG 2023 – European Human Genetics Conference. Jun 10-13; Glasgow, UK; eshg.org
ASM Microbe 2023 – American Society for Microbiology. Jun 15-19; Houston, TX, USA; asm.org
ENDO 2023 – Annual Meeting of the Endocrine Society. Jun 15-18; Chicago, IL, USA; endocrine.org
33rd Regional Congress of the International Society of Blood Transfusion (ISBT). Jun 1721; Gothenburg, Sweden; isbtweb.org
48th CBAC – Congress of the Brazilian Society of Clinical Analysis. Jun 18-21; Florianopolis, Brazil; sbac.org.br
FOCIS 2023 – Annual Meeting of the Federation of Clinical Immunology Societies. Jun 20-23; Boston, MA, USA; focisnet.org
FIME 2023 – Florida International Medical Expo. Jun 21-23; Miami, FL, USA; fimeshow. com
ESHRE 2023 – 39th Meeting of the European Society of Human Reproduction and Embryology. Jun 25-28; Copenhagen, Denmark; eshre.eu
JULY
Analytica Lab Africa 2023. Jul 5-7; Johannesburg, South Africa; analytica-africa. com
FEMS 2023 - 10th Congress of European Microbiologists. Jul 9-13; Hamburg, Germany; fems2023.org
2023 AACC Annual Scientific Meeting & Clinical Lab Expo. Jul 23-27; Anaheim, CA, USA; meeting.aacc.org
SEPTEMBER
Thailand LAB International 2023. Sep 6-8; Bangkok, Thailand; thailandlab.com
India Lab Expo & Analytica Anacon India. Sep 14-16; Hyderabad, India; analyticaindia. com
ESPE 2023 – 61st Annual Meeting of the European Society for Paediatric Endocrinology. Sep 21-23; The Hague Netherlands; eurospe.org
Analitica Latin America 2023. Sep 26-28; Sao Paulo, Brazil; analiticanet.com.br
OCTOBER
ECC 2023 – 44th European Congress of Cytology. Oct 1-4; Budapest, Hungary; cytology2023.eu
EASD 2023 – 59th Annual Meeting of the European Association for the Study of Diabetes. Oct 3-6; Hamburg, Germany; easd.org
CAP23 – Annual Meeting of the College of American Pathologists. Oct 7-10; Chicago, IL, USA; cap.org
ASCP 2023 – Annual Meeting of the American Society for Clinical Pathology. Oct 18-20; Long Beach, CA, USA; ascp.org
NOVEMBER
MEDICA 2023. Nov; Dusseldorf, Germany; medica-tradefair.com
IUIS 2023 – International Union of Immunological Societies. Nov 27 - Dec 2; Cape Town, South Africa; iuis2023.org
BY WEBSITE
BY EMAIL
27 LabMedica International November/2020 2 E asy W ays To Continu e / Start Your FREE Subscription 1 2
• Fill-out all required data on Read er Service Card including signature and date (incomplete or unsigned cards cannot be processed). • Circle inquiry numbers of interest to receive free information • Scan card and Email to: subs@globetech.net
Visit LinkXpress.com to enter your subscription data and reader inquiries. ATTENTION: IF YOUR APPLICATION IS NOT RECEIVED AT LEAST ONCE EVERY 12 MONTHS YOUR FREE SUBSCRIPTION MAY BE AUTOMATICALLY DISCONTINUED READER SERVICE PORTAL LINKXPRESS COM ® VISIT Provided as a service to advertisers. Publisher cannot accept responsibility for any errors or omissions. Advertising Index Inq.No. Advertiser Page Inq.No. Advertiser Page Vol. 39 No. 6 10/2022 LabMedica International – AACC .............................. 23 115 AB Analitica 15 117 Awareness Technology, Inc 17 113 Bioperfectus 13 103 Cellavision ........................... 3 107 DiaSys Diagnostic Systems .............. 7 – EuroMedLab 2023 ................... 21 – LabMedica 24 116 Mast Group 16 102 Nova Biomedical 2 111 Singuway ........................... 11 105 Snibe ............................... 5 119 Vicotex ............................. 19 109 Vircell 9 128 Werfen 28 Events Calendar ATTENTION: Due to the CORONAVIRUS PANDEMIC, many events are being rescheduled for a later date, converted into virtual venues, or altogether cancelled. Please check with the event organizer or website prior to planning for any forthcoming event SLAS 2023 – International Conference & Exhibition of the Society of Laboratory Automation and Screening. Feb 25 - Mar 1; San Diego, CA, USA; slas.org
October/2022
The Intelligent Analyzer
GEM Premier 5000 blood gas testing system provides automated quality assurance with every whole-blood* sample. Now with next-generation Intelligent Quality Management (iQM2), featuring IntraSpect™ technology, potential errors are detected not only before and after, but also during sample analysis, along with real-time correction and documentation. Plus, it’s simple—just change the all-in-one GEM PAK once a month. So regardless of testing location or point-of-care operator, quality results and compliance are assured with every sample.

Real-time assurance and advanced simplicity. Now that’s intelligent.
For more information, contact your local Werfen representative. werfen.com
*Heparinized.
GEM, Premier and iQM are
may be registered
the United States Patent and Trademark
128LMI-09-22LINKXPRESS COM
Acute Care Diagnostics
trademarks of Instrumentation Laboratory Company and/or one of its subsidiaries or parent companies and
in
Office and in other jurisdictions. The Werfen logo is a trademark of Werfen and may be registered in the Patent and Trademark Offices of jurisdictions throughout the world. ©2021 Instrumentation Laboratory. All rights reserved. 128LMI-10-22LINKXPRESS COM




























































































































 By Helen MARTIN, Chemical Pathology, SA Pathology - Adelaide South Australia, APFCB Secretary Congresses and Conferences Committee Corresponding Member
By Helen MARTIN, Chemical Pathology, SA Pathology - Adelaide South Australia, APFCB Secretary Congresses and Conferences Committee Corresponding Member



















