
DPOC Test for Risk of Severe Dengue
engue is typically a mild illness, yet approximately 15% of cases can progress to critical dengue, demanding intensive care and monitoring. Current early detection methods for severe dengue, such as PCR and ELISA tests conducted on blood
Novel Screening Test Capable of Identifying 18 Early-Stage Cancers
Cancer, responsible for onesixth of all global deaths, presents a formidable challenge, particularly because early detection is crucial for improving survival rates. However, current screening tests often fall short due to factors like invasiveness, cost,
and limited accuracy in detecting early-stage diseases. In response to this challenge, researchers have now developed an innovative blood test that can identify 18 types of early-stage cancers across various major organs in the human body.
Cont’d on page 13 Cont’d on page 6
Test Screens for Genetic Diseases in Fetuses
CAI Accurately Predicts Cancer Outcomes from Tissue Samples
AI Accurately Predicts Cancer Outcomes from Tissue Samples
AFirst Affordable and Rapid Test for Beta Thalassemia
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world’s population, with 40,000 newborns diagnosed annually. Beta thalassemia patients often require Cont’d on page 8
Handheld Device Detects Fentanyl in Urine in Seconds
Fentanyl, a synthetic opioid recognized by the Centers for Disease Control and Prevention as being 50 times more potent than heroin and 100 times more potent than morphine, is often illicitly combined with other drugs. Just 2 milligrams of fentanyl,
roughly equivalent to 10 to 15 grains of table salt, can be fatal. Research has shown that fentanyl can be detected in urine up to 72 hours after use. Now, researchers have developed a first-of-its-kind, handheld device that is capable of accurately detecting fentanyl
urrently, the first-generation Non-Invasive Prenatal Test (NIPT) is used widely to screen fetuses for common chromosomal disorders, mainly focusing on conditions like Down syndrome and other anomalies resulting from significant chromosomal changes. However, many congenital disorders stem from more subtle alterations in fetal DNA. To detect these, a compre-
Cont’d on page 4
Measuring Chemotherapy Results in Bone Cancer
The calculation of Percent Necrosis (PN) — the proportion of a tumor considered inactive or “dead” following chemotherapy — serves as a vital predictor of survival outcomes in osteosarcoma, a type of bone cancer. For instance, a PN of 99% signifies that 99% that the tumor is dead, indicating the patient’s positive response to chemotherapy and potentially better survival


n innovative AI
is
capable of examining the spatial organization of cells within tissue samples, offering
predictions on cancer patient outcomes
as indications for prognosis and tailored treatment. ® Cont’d on page 14
model
now
precise
as well
If your subscription is not renewed every 12 months your Free Subscription may be automatically discontinued Renew / Start your Free Subscription Access Interactive Digital Magazine Instant Online Product Information: Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 VISIT READER SERVICE PORTAL LINKXPRESS COM ®
Cont’d on page 10
® INTERNATIONAL Vol.41 No.1 • 2-3/2024 VISIT DAILY CLINICAL LAB NEWS ISSN 1068-1760
See article on page 5
GLOBETECH MEDIA >>> <<< PUBLISHED IN COOPERATION WITH International Federation of Clinical Chemistry and Laboratory Medicine INSIDE LabMedica EXPO . 6-16 Clinical News . . . . 3-16 IFCC News .... 17-20 Industry News . .. . 21 Events Calendar . . 22 WORLD’ S CLINICA L LABORATOR Y NEW S LEADER
Handheld White Blood Cell Tracker to Enable Rapid Testing for Infections
White blood cells (WBC), or leukocytes, are key indicators of an individual’s immune system health. High or low WBC counts can signify the severity of an infection, indicate life-threatening conditions like sepsis, or assist in monitoring patient responses to therapies like chemotherapy and psychotropic drugs. Generally, the blood collected for WBC testing is sent to a central lab for analysis that sends results within hours, making it inconvenient and delaying time-sensitive diagnosis or treatment. Now, an innovative device can rapidly count a person’s WBC with a single drop of blood, similar to the way glucometers rapidly scan for blood sugar levels, enabling rapid testing and improved triaging for infections.
Called the CytoTracker Leukometer, the device developed by researchers at Rutgers startup RizLab Health Inc. (Princeton, NJ, USA; www.rizlabhealth.com) can quickly aid the detection of elevated or reduced WBC counts. The device has undergone comprehensive testing, comparing its performance with conventional lab benchtop hematology analyzers used for standard blood tests. These trials confirmed that the CytoTracker Leukometer is at least 97% accurate, thereby meeting established clinical standards. The potential applications of the device are broad and significant. In emergency room settings, it could expedite the detection of sepsis in patients more swiftly than current methods, which

typically involve blood draws and lab testing. Oncologists might use it to quickly assess whether chemotherapy patients require a WBC stimulant. Additionally, it has important implications in psychiatry, particularly for patients on clozapine, commonly prescribed for conditions like schizophrenia. These patients frequently suffer from neutropenia, a deficiency in neutrophils, and need regular testing to renew their prescriptions. The CytoTracker Leukometer’s ability to rapidly measure neutrophil levels could greatly ease the process of maintaining necessary medication regimens.

“Normally, doing a blood count requires a phlebotomist taking a needle stick and collecting significant amounts of venous blood and sending the samples off to labs where they are tested, sometimes taking hours or even days,” said Mehdi Javanmard, a professor in the Department of Electrical and Computer Engineering in the Rutgers School of Engineering, and the co-founder and CEO of RizLab Health Inc. “Our handheld device enables near-patient testing, while only requiring a tiny amount of blood and returning results within minutes, allowing clinicians to make decisions almost immediately.”
“Rapid test results have revolutionized the field of medicine,” added Dr. Tanaya Bhowmick, an infectious disease physician. “The white blood count is a parameter that physicians routinely order to evaluate a patient for possible infection. Having this information rapidly can help triage patients in the outpatient setting.”
Image: The CytoTracker Leukometer is a portable device that quickly delivers readings based on a single drop of blood (Photo courtesy of RizLab Health)
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 2 LabMedica International February-March/2024








103 LMI-3-24 LINKXPRESS COM
Test Screens for Genetic Diseases in Fetuses
Cont’d from cover
hensive examination of all the genes in the fetal genome, known as exome sequencing, is necessary. Typically, this level of screening is reserved for pregnancies where ultrasound scans suggest abnormalities. This is due to the invasive nature of the required tests, such as chorionic villus sampling or amniocentesis, which are accompanied by discomfort and a slight risk of miscarriage. Consequently, many severe genetic conditions remain undetected until after birth. Now, an innovative test uses a blood sample from expectant mothers to analyze all the genes in the fetus, making it possible to screen pregnant women for serious genetic diseases in their unborn children.
The new test, named desNIPT, was developed by a research team from the University of Southern Denmark (Odense, Denmark; www. sdu.dk) and has been proven capable of detecting mutations in fetal genes, which are often the root of serious congenital diseases. This new test builds upon the foundations of the first-generation NIPT, significantly enhancing its capabilities. Unlike traditional methods that require invasive procedures, desNIPT can be conducted with a simple blood draw from the pregnant woman, offering analysis before the child is born. This technique examines fetal DNA circulating in the mother’s bloodstream, a breakthrough that has revolutionized the potential for prenatal disease screening in recent times. Even when the amount of fetal DNA present in the mother’s blood is relatively low, the heightened sensitivity of the desNIPT test allows for the detection of genetic abnormalities in the fetus.
In a research study that was recently published in the The New England Journal of Medicine, 36 pregnant women were mon-
itored, with blood samples collected during their first or second trimester. Each pregnancy had been flagged by ultrasound scans as potentially carrying a serious genetic disease in the fetus. From these 36 pregnancies, de novo disease-causing mutations were identified in 11 cases through the desNIPT analysis. The findings from desNIPT were then cross-referenced with results from traditional exome sequencing obtained via chorionic villus sampling or amniocentesis. The researchers found that the new method successfully detected all the disease-causing gene variants that were identified through the invasive prenatal tests, proving its efficacy. This innovative test paves the way for more comprehensive genetic screenings in the future, including the detection of conditions that might not be visible via ultrasound scans.
“We are highly optimistic as the study indicates that the desNIPT test is remarkably accurate. In the examined pregnant women, we did not observe any false-positive results,” said Martin Larsen, project leader and associate professor at the University of Southern Denmark. “Presently, our focus is on validating the test through a larger study, as well as refining and scaling the methodology.”
Placental Swabs Found Most Effective for Detection of Maternal Sepsis
Over half of maternal deaths occurring in hospitals are attributed to sepsis, a critical condition where an infection spreads beyond local tissue containment, leading to organ failure. Maternal or perinatal sepsis is a significant health concern globally, affecting more than 20 million women and resulting in approximately 17,000 deaths each year. Identifying the infectious agents responsible for these cases is often a complex task. While blood cultures are the preferred diagnostic method, they frequently yield low positive results. Other specimens like vaginal swabs have limited clinical usefulness, and obtaining microbiological cultures from the uterine cavity for antimicrobial guidance is typically challenging. Now, a recent study of microbiology specimens used to investigate maternal sepsis has demonstrated that placen-
tal swabs could play a vital role in informing antimicrobial therapy decisions.
The study performed by researchers from the University of Limerick (Limerick, Ireland; www.ul.ie) involved the analysis of nearly 2,000 specimens collected over five and a half years. The team retrospectively assessed the bacterial culture results from various specimens collected as part of a ‘septic screen’ designed to identify bacteria causing maternal infections leading to sepsis. The specimens included blood, urine, throat swabs, vaginal swabs, and placental swabs. By examining and comparing the results from these specimens for 430 women, the study found that placental swabs were the most effective in detecting the highest number of pathogens.
The study’s findings, published in the jourCont’d on page 5
Graham Beastall United Kingdom
Hernán Fares Taie Argentina
Bernard Gouget France
Maurizio Ferrari Italy
Tahir S. Pillay South Africa
Andreas Rothstein Colombia
Praveen Sharma India
Rosa I. Sierra-Amor Mexico
Peter Wilding United States
Andrew Wootton United Kingdom

A
Published in cooperation with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC).
LabMedica International • LabMedica en Español • LabMedica.com
HospiMedica International • HospiMedica.com • MedImaging.net
Founder & Editorial Director Marc Gueron
Dan Gueron
David Gueron
Sanjit Dutt
Carolyn Moody, RN
Simone Ciolek Parker Xu
Karina Tornatore
Publisher Managing Editor News Editor Regional Director Regional Director Regional Director Reader Service Manager
Subscriptions:
USA Miami, FL 33280, USA
Carolyn.Moody@globetech.net Tel: (1) 954-686-0838
GERMANY, SWITZ., AUSTRIA Bad Neustadt, Germany
Simone.Ciolek@globetech.net Tel: (49) 9771-1779-007
OTHER EUROPE & UK Miami, FL 33280, USA
Carolyn.Moody@globetech.net Tel: (1) 954-686-0838
JAPAN Tokyo, Japan
Katsuhiro.Ishii@globetech.net Tel: (81) 3-5691-3335
CHINA Shenzen, Guangdong, China
Parker.Xu@globetech.net Tel: (86) 755-8375-3877
OTHER COUNTRIES Contact USA Office ads@globetech.net Tel: (1) 954-686-0838
SUBSCRIPTION
LabMedica lnternational is published eight times a year and is circuIated worldwide (outside the USA and Canada) without charge and by written request, to clinical laboratory specialists and administrators, and other qualified professionals allied to the field.
To all others: Paid Subscription is available for a two-year subscription charge of US$120. Single copy price is US$20. Mail your paid subscription order accompanied with payment to Globetech Media, P.O.B. 800222, Miami, FL 33280-0222.
For change of address or questions on your subscription, write to: LabMedica lnternational, Circulation Services at above address; or visit: www.LinkXpress.com
ISSN 1068-1760
Vol.41 No.1. Published, under license, by Globetech Media LLC; Copyright © 2024. All rights reserved. Reproduction in any form is forbidden without express permission. Opinions expressed are solely those of the authors, and do not represent an endorsement, or lack thereof, by the Publisher of any products or services.
4 LabMedica International February-March/2024
INFORMATION
ADVERTISING SALES OFFICES HOW TO CONTACT US labmedica.com
GLOBETECH
PUBLICATION
HospiMedicaExpo.com • LabMedicaExpo.com • LinkXpress.com
Send Press Releases to: Advertising & Ad Material:
Contacts: www.LinkXpress.com LMNews@globetech.net ads@globetech.net info@globetech.net
BOARD
Other
EDITORIAL
INTERNATIONAL Teknopress Yayıncılık ve Ticaret Ltd. Şti. adına İmtiyaz Sahibi: M. Geren • Yazı işleri Müdürü: Ersin Köklü Müşir Derviş İbrahim Sok. 5/4, Esentepe, 34394 Şişli, İstanbul P. K. 1, AVPIM, 34001 İstanbul • E-mail: Teknopress@yahoo.com Baskı: Postkom A.Ş. • İpkas Sanayi Sitesi 3. Etap C Blok • 34490 Başakşehir • İstanbul Yerel süreli yayındır. Yılda sekiz kere yayınlanır, ücretsiz dagıtılır.
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Image: A new blood test can identify genetic diseases in fetuses (Photo courtesy of 123RF)
AI Accurately Predicts Cancer Outcomes from Tissue Samples
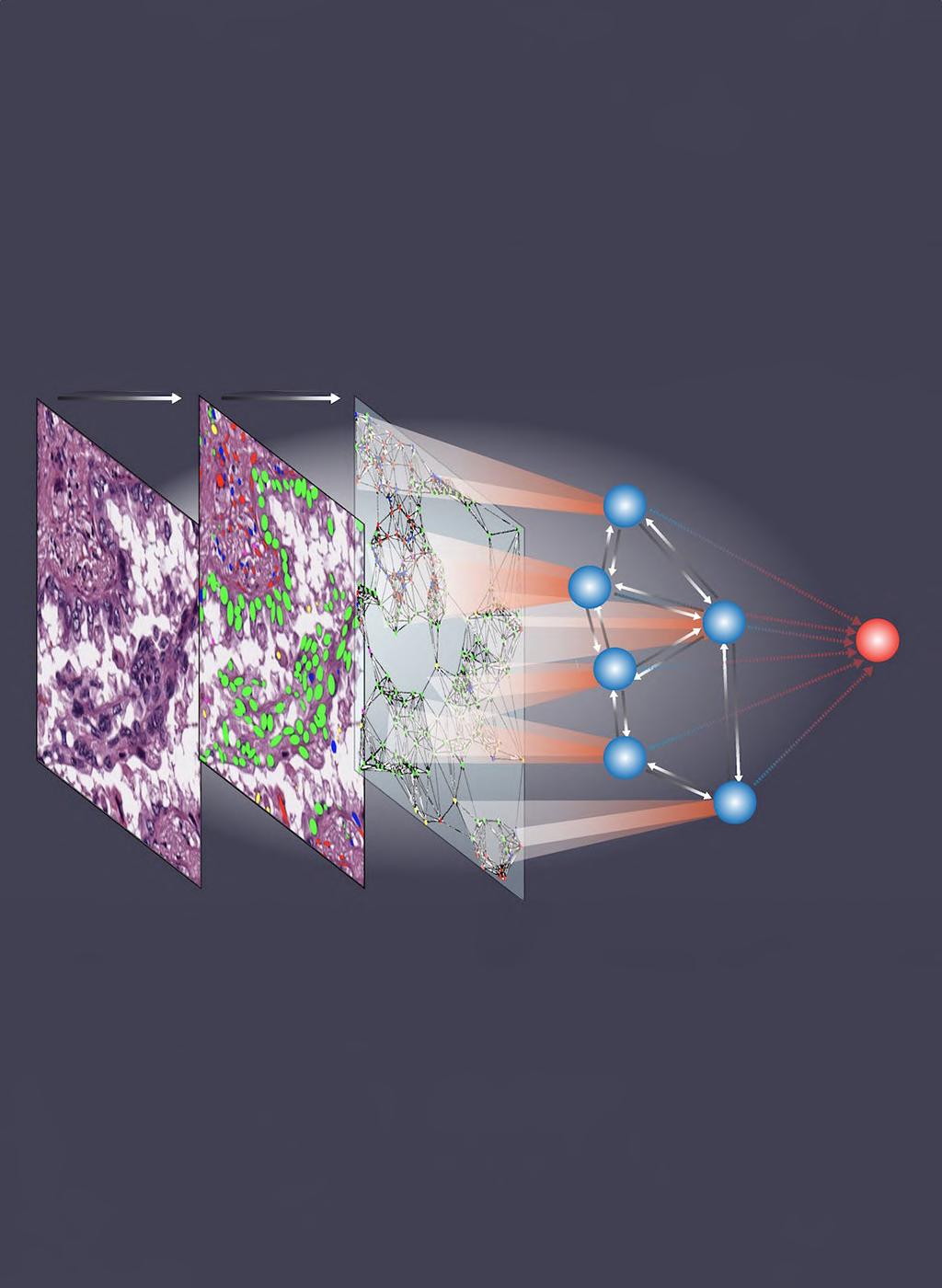
Patient tissue samples are commonly examined on slides by pathologists, a process integral to diagnosis. This traditional method, while effective, is notably time-intensive and subject to variability in interpretations among different pathologists. Moreover, some subtle details in pathology images might escape human observation but could hold critical insights into a patient's health status. Over recent years, several artificial intelligence (AI) models have been developed to undertake certain tasks typically performed by pathologists, such as classifying cell types or gauging cellular interactions based on proximity. Nevertheless, these models have not fully captured the more intricate aspects of tissue image analysis that pathologists conduct, including recognizing complex cell spatial arrangements and filtering out irrelevant image 'noise' that could distort interpretations. Addressing this gap, researchers have now introduced an innovative AI model that is capable of examining the spatial organization of cells within tissue samples, offering precise predictions about cancer patient outcomes and offering new prospects for AI-assisted cancer prognosis and tailored treatment plans.
Dubbed Ceograph, this AI tool, created by researchers at UT Southwestern Medical Center (Dallas, TX, USA; www.utsouthwestern. edu), mimics the approach taken by pathologists for examining tissue slides. It begins by identifying cells and their respective positions within the image. It then classifies cell types and delineates their shapes and spatial distributions, creating a comprehensive map where the arrangement, dispersion, and interactions among cells are detailed for analysis. As detailed in a study published in Nature Communications, the team validated Ceograph in three clinical scenarios using pathology slides. In one instance, Ceograph distinguished between two lung cancer types: adenocarcinoma and squamous cell carcinoma. In another, it gauged the progression risk of potentially cancerous oral conditions to malignancy. Finally, it identified lung cancer patients most likely to benefit from epidermal growth factor receptor inhibitors.
In each scenario, Ceograph's performance in predicting patient outcomes surpassed traditional methods. Notably, the cell spatial organization insights provided by Ceograph are not only interpretable but also shed light on the biological implications of varying individual cell-cell spatial interactions. These developments highlight the increasingly vital role AI can play in healthcare, particularly in enhancing the precision and efficiency of pathology analyses. This technology promises to refine preventive strategies for individuals at high risk and tailor treatment choices to meet each patient's unique needs.
“Cell spatial organization is like a complex jigsaw puzzle where each cell serves as a unique piece, fitting together meticulously to form a cohesive tissue or organ structure,” said study leader Guanghua Xiao, Ph.D. “This re-
Placental Swabs Found Most Effective for Detection of Maternal Sepsis
Cont’d from page 4
nal PLOS ONE on December 27, 2023, indicate that placental swabs are valuable in identifying potential pathogens from the uterine cavity, which is the most common site of perinatal infections. This discovery is crucial as placental swabs are not routinely examined in many hospitals, meaning vital information that could influence the treatment of these infections might be overlooked. Currently, international guidelines for diagnosing maternal infections are inconsistent, and there is scant information in existing scientific literature about the utilization of placental swabs.
“Maternal sepsis may occur during pregnancy or when a C-section incision, tear or other wound from childbirth becomes infected in the days or weeks after giving birth,” explained Professor Colum Dunne who led the unique new study. “This study provides new information on how sepsis can be detected, and the organisms involved identified early, so that the best approach to successful treatment can be selected.”
search showcases the remarkable ability of AI to grasp these intricate spatial

THE ROAD TO ACCURATE SPECIMEN COLLECTION STARTS WITH PURITAN .
Be ready for anything heading your way with Puritan.®
With single-use devices and transport systems for just about every collection site, you’ll be prepared for any type of specimen needed on your path to finding answers Sample with confidence and trust your results.
LEARN MORE ONLINE
info.puritanmedproducts.com/specimen-collection-24
VISIT US IN PERSON
ECCMID Booth #E-61






5 LabMedica International February-March/2024 105 LMI-3-24 LINKXPRESS COM
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Image: The AI model analyzes cell spatial organization to make patient diagnoses and prognoses (Photo courtesy of UT Southwestern)

VITAMIN D TEST LUMIQUICK DIAGNOSTICS
To
The Quick Profile Vitamin D is designed for the quantitative or semi-quantitative determination of total 25-hydroxy Vitamin D (25-OH Vitamin D) in human blood. It is available as one step rapid test and EIA.
202 LMI-3-24 COM
POC Test for Risk of Severe Dengue
Cont’d from cover
samples, often fall short in sensitivity. Additionally, the World Health Organization’s recommended symptom-based guidelines, which include fever, headache, eye pain, rash, and minor bleeding, have limited accuracy (6 to 18%) in detecting severe dengue due to symptom overlap with milder forms and other illnesses.
Scientists and clinicians at NTU Singapore (Singapore; www.ntu. edu.sg) have now identified two biomarkers — sST2 and suPAR — present in the blood of dengue patients that show promise in identifying those at risk of developing severe dengue during the disease’s initial stages. This discovery emerged from a study between 2016 and 2019 involving 129 individuals treated for dengue. Given that lateral flow tests for sST2 and suPAR, proteins associated with heart health and disease, are commercially available for heart failure assessment, the team is exploring the possibility of combining these into a single test kit tailored for severe dengue detection.
Elevated levels of sST2 indicate cardiac stress and fibrosis, useful for gauging the severity of heart failure, while high suPAR levels are indicative of heightened inflammation and an increased risk of cardiac complications. By tracking these markers, researchers gain insights into cardiac conditions and disease progression. The team believes this novel approach could significantly improve accuracy in predicting severe dengue cases, with an estimated 55 to 60% accuracy rate, outper-
RINTEGRATED CHEMISTRY & IMMUNOASSAY ANALYZER MINDRAY IN-VITRO DIAGNOSTICS


The M1000 is an integrated chemistry and immunoassay solution specially designed for large-scale laboratories that supports loading up to 580 samples continuously at one time and decapping samples automatically.
203 LMI-3-24 LINKXPRESS COM


Image: Researchers
forming existing WHO guidelines. The development of these test kits could help healthcare professionals differentiate between mild dengue cases and severe ones requiring hospital admission.
Groundbreaking Rheumatoid Arthritis Blood Test Predicts Treatment Response
heumatoid arthritis (RA), an autoimmune disease affecting joints and other systems in the body, impacts millions globally. Typically, the initial biologic treatment involves anti-inflammatory drugs from the tumor necrosis factor inhibitor (TNFi) family, including FDA-approved Infliximab and Adalimumab. However, only about 30% of RA patients find success with TNFi treatment after six months. This delay in identifying ineffective treatments can be costly, as RA can progress to cause irreversible bone damage. Now, a new predictive blood test promises to revolutionize treatment by guiding doctors to the most effective therapies for individual RA patients.
The PrismRA blood test from Scipher Medicine (Waltham, MA, USA; www. sciphermedicine.com ), a Northeastern University (Boston, MA, USA; www. northeastern.edu) spinoff, is the first clinically validated RA test that predicts treatment response. Marking a significant step forward in RA treatment, the test is capable of determining the suitability of TNFi treatments for RA patients before they begin therapy. This is particularly beneficial as TNFis are also used in treating other immune-mediated conditions like inflammatory bowel disease and psoriasis. Cont’d

6 LabMedica International February-March/2024
receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
have taken a step forward in diagnosing severe cases of dengue (Photo courtesy of NTU Singapore)
on page 8
Image: The PrismRA blood test helps target best treatments for patients with rheumatoid arthritis (Photo courtesy of Scipher Medicine)
Prime Plus provides the most clinical value of any blood gas/critical care analyzer profile by adding essential tests for electrolyte balance (iMg), plasma volume (ePV), kidney function (Urea, Creatinine, eGFR) and differentiating types of anemia (MCHC).
Ionized Magnesium (iMg) Hypomagnesemia is a frequent finding in criticallyill patients.1 Magnesium therapy guided by real time ionized magnesium monitoring has been shown to improve outcome in these patients.2
Estimated Plasma Volume (ePV)
The plasma volume status of a patient is one of the top priorities in evaluating and treating critical illness including CHF, ARDS, AKI,Surgery, and Sepsis.3-5
Urea, Creatinine and eGFR Over 50% of patients admitted to the ICU develop some degree of acute kidney injury.6 Creatinine, eGFR, and Urea monitoring provides early indication of changes in kidney function and helps guide therapy to prevent AKI.
MCHC Mean corpuscular hemoglobin concentration (MCHC) provides insight into chronic low grade blood loss and differentiating types of anemia.
107 LMI-3-24 LINKXPRESS COM Today’s
Care
Include novabiomedical.com References 1. Soliman HM. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit Care Med 2003;31(4):1082-7. 2. Wilkes NJ et al. Correction of ionized plasma magnesium during cardiopulmonary bypass reduces the risk of postoperative cardiac arrhythmia. Anesth and Analg 2002;95(4) 828-834. 3. Kobayashi M et al. Prognostic Value of Estimated Plasma Volume in Heart Failure in Three Cohort Studies; Clin Res Cardiol 2019;108(5): 549-561. 4. Niedermeyer, et al. Calculated Plasma Volume Status Is Associated With Mortality in Acute Respiratory Distress Syndrome. Critical Care Explorations: September 2021, V3(9):1-9. 5. Kim HK et al. Prognostic Value of Estimated Plasma Volume Status in Patients with Sepsis. J Korea Med Sci 2020;9(37):1-10. 6. Mandelbaum T et al. Outcome of critically ill patients with acute kidney injury using the AKIN criteria. Crit Care Med 2011;39(12):2659-2664.
Blood Gas/Critical
Test Menu Must
iMg ePV Urea Creat/eGFR MCHC Test Menu pH P CO2 P O2 SO2 % Hct Hb iMg Na K Cl TCO2 iCa ePV GLU Lac Urea Creat/eGFR CO-Ox MCHC tBil HbF

MULTI-GAS INCUBATOR PHC EUROPE
To

The IncuSafe MCO-170MUVH multi-gas incubator offers the most precise regulation of oxygen and other gases while maintaining superior temperature uniformity, contamination control, and usability.

First Affordable and Rapid Test for Beta Thalassemia
Cont’d from cover
lifelong transfusions for survival. Many individuals with the beta-thal trait are unaware of their condition and the risk of having a child with the disease if their partner also carries the trait. Consequently, early screening and timely diagnosis are critical for managing beta-thalassemia, both for genetic counseling to aid in family planning and for preventing and managing later clinical complications. Now, a beta thalassemia test offers an accurate and convenient solution, aiding couples in making informed reproductive decisions, ensuring proper maternal care, and facilitating early diagnosis in infants.
Hemex Health’s (Portland, OR, USA; hemexhealth.com) Gazelle Hb Variant test is capable of identifying 19 hemoglobinopathy conditions, including both beta-thal disease and trait, and sickle cell disease and trait. The test runs on the Gazelle compact, rugged, battery-powered in vitro diagnostic device that is designed for cost-effective use by entrylevel healthcare workers, even in areas with limited access, resources, or electricity. The device digitally captures patient information and results for storage, printing, or subsequent transmission. Approved for identifying sickle cell disease and beta-thalassemia in
an increasing number of countries, Gazelle integrates miniaturized versions of trusted technologies, innovative optics, and artificial intelligence. Priced similarly to an iPhone, Gazelle offers low-cost individual tests. Its sensitive detection and precise quantification capabilities enable it to identify beta-thal disease or trait in infants as young as six months and sickle cell disease and trait in newborns as early as 37 weeks gestation.
A clinical study validating the Gazelle Hb Variant Test’s diagnostic accuracy for beta thalassemia revealed that it correctly identified subjects with beta-thal major, beta-thal intermedia, and beta-thal trait with over 99% accuracy, compared to the laboratory gold standard of high-performance liquid chromatography (HPLC). The study concluded that Gazelle represents an affordable, rapid solution for beta-thal identification at the point of care. Its demonstrated ability to provide accurate, rapid results (available in 8 minutes) suggests Gazelle’s suitability for widespread beta-thal testing at the point of care. The test’s affordability and convenience could greatly facilitate informed reproductive decisions for millions of couples, ensure appropriate maternal care, and enable prompt diagnosis in infants.
SEPSIS TEST SEEGENE
The Magicplex Sepsis Real-time Test is a comprehensive assay for detection and identification of sepsis-causing pathogens. It can screen for more than 90 pathogens (over 90% of sepsis-causing pathogens) from whole blood samples.

“By combining accuracy and affordability with on-site accessibility, Gazelle can make a significant impact in areas of the world with a high prevalence of beta-thalassemia,” said Patti White, CEO of Hemex Health. “Before Gazelle, lab-based, high-volume batch testing, with its slow turn-around time, was the best option. With Gazelle, young adults can be conveniently tested at primary care centers or even at screening events at schools or other community centers.”
Groundbreaking Rheumatoid Arthritis Blood Test Predicts Treatment Response
Cont’d from page 6
Scipher researchers recognized the urgent need to identify responders and non-responders to TNFis from the outset of treatment. They utilized publicly available datasets and obtained baseline blood data and TNFi response rates from hundreds of RA patients. Through this data, they applied machine-learning algorithms to predict TNFi treatment efficacy using genetic markers in the blood. Further validation was achieved by enrolling around 700 RA patients in a prospective trial
to assess the test’s clinical utility. The trial’s findings were encouraging; patients using the PrismRA biomarker test for guiding therapy were three times more likely to achieve remission compared to those who didn’t.
The PrismRA testing process involves a simple blood draw at a doctor’s office, followed by RNA sequencing analysis. Scipher’s PrismRA test then predicts the likelihood of a patient not responding to TNFis. Based on these results, physicians can decide on an alternative therapy for the patient. With the development
of this biologic test, the focus now shifts to creating similar blood marker tests for other autoimmune diseases. Scipher’s extensive collection of whole blood RNA-seq data from over 20,000 patients offers a robust molecular database for future research and test development.
“This is the first clinically validated rheumatoid arthritis test that predicts treatment response,” said Northeastern graduate Susan Dina Ghiassian, who was the first employee of Scipher Medicine. “Patients will get a chance to be put on the right therapy from day one.”
8 LabMedica International February-March/2024
205 LMI-3-24 COM
206 LMI-3-24 LINKXPRESS COM
receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Image: The Gazelle Hb Variant Test (Photo courtesy of Hemex Health)
AI-Driven Digital Pathology Diagnostic for Colorectal Cancer Biomarker to Optimize Patient Access to Immunotherapy
Colorectal cancer (CRC), with nearly two million new cases and one million deaths globally in 2020, stands as the third most prevalent cancer worldwide and the second leading cause of cancer-related deaths. A critical genomic biomarker for CRC is Microsatellite Instability (MSI), accounting for approximately 15% of all CRC cases. Recent clinical research underscores the prognostic and therapeutic significance of the MSI phenotype, particularly following the approval of immune checkpoint inhibitor (ICI) therapies. Patients with MSI-positive tumors are often good candidates for ICI therapy, while those with microsatellite stable (MSS) tumors typically are not. Consequently, global consensus guidelines now advocate for MSI testing to guide optimal treatment strategies. Pre-screening instruments that can eliminate the necessity for universal patient testing are emerging as a means to streamline this process and alleviate the burden on laboratory personnel and resources.
A team of scientists from from Owkin, Inc. (Paris, France; www.owkin.com), in collaboration with French pathology labs has performed a blind validation of MSIntuit CRC, a groundbreaking AI-powered digital pathology diagnostic created by Owkin. Designed as a pre-screening tool, MSIntuit CRC seeks to refine the precision in diagnosing and treating CRC. The tool, leveraging patient-derived data, is crafted to offer reciprocal benefits to patients. The study's findings indicate that MSIntuit CRC can successfully exclude
Anearly half of the MSS patients while accurately identifying over 96% of MSI patients, in line with the performance of established gold standard methods (92-95%). Such innovations are set to transform the screening process, enabling quicker and more extensive patient screening.
The robustness of the study was evidenced by the blind validation conducted on 600 consecutive CRC cases over two years from nine distinct pathology labs, minimizing the selection bias risk. Additionally, the validation maintained consistent performance across two different pathology slide scanners, with sensitivities of 96% and 98% respectively. The study's focus on sensitivity and specificity as indicators of performance and other methodological strategies underscores the AI model's reliability, ensuring the diagnostic's suitability for clinical use and its applicability across various laboratories and regions. The findings of the study were published in Nature Communications on November 6, 2023.
“With the increasing number of biomarkers to be routinely tested in clinical practice, the need for tools that can both ease bottleneck and resource pressures while ramping up biomarker testing is paramount,” said Meriem Sefta, Chief Diagnostic Officer at Owkin. “Our solution represents the first step towards the development of an AI diagnostic that can identify actionable biomarkers from a single H&E slide used in clinical routine, pushing us closer to realizing a precision medicine future.“
15-Minute Multiplex Molecular Tests to Detect STI at Point-of-Care
nnually, between 35 and 50 million tests for chlamydia and gonorrhea are conducted in the United States, largely driven by guidelines recommending annual STI screenings for many sexually active individuals and even more frequent tests for specific high-risk groups. Despite these recommendations, about half of the women eligible for screening do not undergo proper testing each year, leading to significant undertesting. Additionally, the time delay in lab-based testing can result in patients remaining untreated and potentially spreading infections, or in preemptive treatments based on best guesses that may be inappropriate, causing unnecessary costs, side effects, and potential development of antimicrobial resistance. Now, a new rapid, portable molecular STI test promises to deliver immediate results to patients and providers, enabling prompt treatment and potentially improving health outcomes, especially for patients who might otherwise postpone or forego testing.
Detect (Guilford, CT, USA; www.detect. com) is gearing up to begin clinical validations for its 15-minute multiplex molecular STI test, based on a technology initially developed for tackling the COVID-19 pandemic. This testing relies on loop-mediated isothermal amplification (LAMP) and a simple instrument, aiming for affordability, speed, and ease of use. The instrument performs parallel reactions for quick multiplexing, as opposed to single-tube reactions. While LAMP is a widely used technology, Detect has enhanced it considerably in recent years. Challenges in LAMP for diagnostics developers, such as primer design and reaction speed, have been addressed by Detect’s use of AI-based primer design tools to develop highly sensitive and specific tests. The tests also incorporate proprietary biologics like enzymes, which are self-designed and manufactured by Detect, leading to faster and more accurate results.
Detect has leveraged the same technology





Cont’d on page 10





9 LabMedica International February-March/2024
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Image: AI-generated artistic impression of an H&E-stained whole slide image of cancer tissue (Photo courtesy of Owkin)

DENGUE/ZIKA/CHIKUNGUNYA VIRUS DETECTION KIT SINGUWAY BIOTECH
The Dengue virus/Zika virus/Chikungunya virus (PCR-Fluorescence Probing) detection kit is an IVD test, based on real-time PCR technology, for detection of Dengue virus/Zika virus/Chikungunya virus nucleic acids in serum sample.

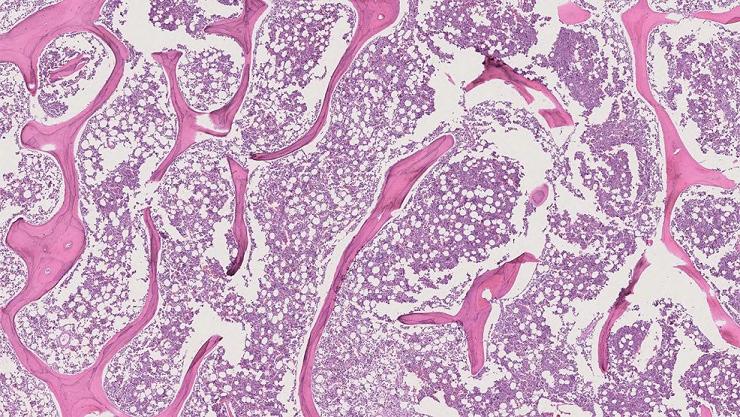
Measuring Chemotherapy Results in Bone Cancer
prospects. Pathologists typically assess PN by meticulously examining, interpreting, and marking up whole-slide images (WSIs), which are detailed cross-sections of specimens (like bone tissue) prepared for microscopic examination. Nevertheless, this traditional method is not only time-consuming and demands specialized expertise but also suffers from significant variability among observers. This means two pathologists might report differing PN estimates from the same WSI. Now, a machine learning model created and trained to calculate PN has shown that its calculation was 85% correct when compared to the results of a musculoskeletal pathologist, with the accuracy improving to 99% upon excluding an outlier.
A research team at Johns Hopkins Medicine (Baltimore, MD, USA; www.hopkinsmedicine.org) is developing a “weakly supervised” machine learning model, one that doesn’t require extensive annotated data for training. By doing so, a pathologist would only need to provide partially annotated WSIs, significantly easing their workload. To develop the machine learning model, the team began by collecting WSIs from patients with intramedullary osteosarcoma (originating within the bone) treated with chemotherapy and surgery between 2011 to 2021. A musculoskeletal pathologist then partially labeled three tissue types on these WSIs: active tumor, dead tumor, and non-tumor tissue and also provided a PN estimate for each case. This data formed the foundation for the model’s training.
The model was trained to recognize and categorize image patterns. The WSIs were segregated into thousands of smaller patches, divided into groups as per the pathologist’s labels, and then fed into the model. This process aimed to provide the model a more robust frame of reference rather than just feeding it one large WSI. Upon completion of the training, the model was tested alongside the musculoskeletal pathologist on six WSIs from two patients. The results demonstrated an 85% correlation in PN calculations and tissue labeling between the model and the pathologist. However, the model struggled to accurately label cartilage, leading to an outlier as a result of an abundance of cartilage on one WSI. When this outlier was removed, the correlation soared to 99%. Future work will focus on incorporating cartilage tissue in the model’s training and broadening the WSIs range to encompass various osteosarcoma types, not just intramedullary.
“If this model were to be validated and produced, it could help expedite the evaluation of chemotherapy’s effectiveness on a patient — and thus, get them a prognosis estimate sooner,” said Christa LiBrizzi, M.D., co-first author of the study and a resident with Johns Hopkins Medicine’s Department of Orthopedic Surgery. “That would reduce health care costs, as well as labor burdens on musculoskeletal pathologists.”
URINALYSIS ANALYZER
URITEST MEDICAL ELECTRONIC

The US-1680 is the world’s first Al-Libre urinalysis analyzer featuring a compact, all-in-one design, throughput of 300 T/H for chemistry analysis, 120 T/H for sediment analysis, and 120 T/H for chemistry and sediment analysis.

15-Minute Multiplex Molecular Tests to Detect STI at Point-of-Care
Cont’d from page 9
used for its COVID-19 test to expand its applications and upgrade the system, making it faster, more user-friendly, and more sensitive. This evolution has resulted in an advanced point-of-care system and new multiplex molecular tests. The company is now advancing its initial isothermal multiplex panel for STIs. Dealing with STI testing presented a unique challenge in sample preparation, as vaginal matrix samples, unlike saliva or nasal secretions, require additional preparation steps to make pathogens accessible. Detect has addressed this major technical hurdle by creating a cost-effective, miniaturized sample preparation component for its system. The test cartridge itself is composed of simple plastic fluidic components, shifting complex electronics or motors from the cartridge to the hub, thus reducing the cost of the cartridge.
Detect’s primary market includes point-of-care testing locations like doctors’ offices, urgent care clinics, nursing homes, and pharmacies, which are typically more concerned with the ongoing cost of consumables rather than the initial cost of the testing device. The company anticipates its platform to be substantially more affordable than other point-of-care systems currently on the market. Detect is focusing on validating its rapid molecular STI assay in the coming months to prepare for submission to the US Food and Drug Administration. In the future, Detect plans to enhance its STI panel to identify specific SNPs in gonorrhea bacteria that render them resistant to ciprofloxacin, a low-cost antibiotic.
10 LabMedica International February-March/2024
208 LMI-3-24 COM
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
209 LMI-3-24 LINKXPRESS COM
Image: A microscopic image of intramedullary osteosarcoma (Photo courtesy of Johns Hopkins Medicine)
Cont’d from cover
New Biomarkers for Diabetic Kidney Disease to Improve Diagnosis and Monitoring
Diabetic kidney disease (DKD) is a major microvascular complication arising from type 2 diabetes mellitus (T2DM), leading to end-stage renal disease (ESRD). Despite advancements in treatment options such as hyperglycemic and blood pressure control and renin-angiotensin system blockades, DKD continues to prevail at alarming rates. Clinically, DKD often progresses from microalbuminuria to macroalbuminuria, marked by increased levels of albumin in the urine and an initial hyperfiltration phase followed by a gradual decline in renal function. However, variations in clinical presentations and progression rates to ESRD have been noted in recent studies. As scientific understanding evolves, the need for specific biomarkers for DKD has become increasingly vital. Now, an analysis of urinary and exosome proteome profiling by a team of scientists has led to the discovery of biomarkers for DKD, offering the potential for early diagnosis and treatment.
The research carried out by scientists at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (Shanghai, China; http:// english.simm.cas.cn) involved extensive urinary proteomics analysis of 144 patients and urinary exosome proteomics of 44 patients with varying degrees of albuminuria related to T2DM. Utilizing exosomes for biomarker discovery offers several benefits, including minimizing the presence of abundant proteins in urine and enriching a subproteome comprising membrane and cytosolic proteins. Urinary exosomes also provide crucial information from a wide range of epithelial origins, reflecting physiological or pathological processes in virtually all epithelial cells exposed to the urinary space. Additionally, proteomic analysis of urinary exosomes can identify proteins specifically linked to certain kidney diseases, as was the case in this study.
The study analyzed urine samples from 144 clinical patients, including 19 healthy controls and 125 DM patients. The diabetic group was further categorized into three stages based on albumin-to-creatinine ratio (ACR) values. The research highlighted the dynamic nature of urinary and exosome proteomes in T2DM patients at different albuminuria stages. Proteins were grouped into six clusters based on their expression patterns throughout DKD progression. The study observed a significant decrease in proteins associated with glycolytic and ubiquitination processes from healthy controls to early DM stages, with a gradual decrease continuing to the late DM stages. Conversely, proteins involved in lipid transport and cholesterol esterification progressively increased, peaking at the most advanced stage. Proteins involved in carbohydrate metabolic processes showed high expression in early DM patients, decreasing as DKD progressed. Additionally, the study identified and validated potential biomarkers for DKD diagnosis or disease monitoring, such as SERPINA1
Image: Novel biomarkers could identify diabetic kidney disease at an earlier stage (Photo courtesy of 123RF)
and TF, in another cohort of diabetic urine samples with varying DKD degrees. Thus, this research has provided a deeper understanding of the biomarkers associated with renal disease, fueling hopes for improved early-stage treatments and management strategies for DKD.
One step HbA1c analyzer

•
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 11 LabMedica International February-March/2024
DiaSys. Total Confidence in Patient Results. www.diasys-diagnostics.com • Precise and reliable HbA1c results traceable to IFCC and NGSP • Fast measurement - results available after 4:30 min
All in one, pre-calibrated cartridge
Storage of tests at 2-30°C for up to 18 months
•
•
Maintenance free analyzer
111 LMI-3-24 LINKXPRESS COM

To

The compact MIRO CANVAS provides true walk-away automation of complex next-generation sequencing (NGS) library preparation and hybridization protocols, requiring only 15 minutes of hands-on

One-Minute COVID-19 Test Adaptable for Detecting Other Viruses with Spike-Like Proteins
Several animals ranging from fireflies to lantern fish have the chemical tools needed to produce light. Such a reaction usually requires the substrate luciferin and the enzyme luciferase. However, a class of less discriminating luciferins, termed as imidazopyrazinone-type (IPT) compounds, has the ability to glow upon coming into contact with other proteins, including ones that are not considered to be enzymes. Previous studies have suggested that IPT luciferins could act as the basis for a new type of medical test that utilizes luminescence to indicate the presence of a target protein in a specimen. Now, a team of researchers has developed a glowing test, based on their belief that an IPT luciferin can react with the SARS-CoV-2 spike protein, which allows the virus particles to invade cells and cause COVID-19.
In their research, the team at the National Institute of Advanced Industrial Science and Technology (AIST, Ibaraki, Japan; www.aist. go.jp) focused on 36 different IPT luciferins, testing their capacity to react with a singular unit of the SARS-CoV-2 spike protein - the critical element that enables the virus to penetrate cells and cause COVID-19. Remarkably, only one of these molecules, derived from small crustaceans in the Cypridina genus, exhibited the ability to emit light. Further investigations were conducted on the luciferin’s interaction with the spike protein in its trimeric, or natural state. The results were promising, as detectable light emissions were observed within a 10-minute span. However, this light emission wasn’t visible to the naked eye and required a commercially available luminescence reader.
POC IMMUNOASSAY SYSTEM ABSOLOGY

The ABSOL CARRY is a point-of-care Immunoassay System with microfluidics technology for providing accurate and stable diagnostics. Designed for POCT, the device provides quantitative results based on immunoassay technology.


Novel Urinary Biomarkers to Enable Early Detection of Lupus Nephritis
Systemic Lupus Erythematosus (SLE), widely known as lupus, is an autoimmune condition where the body's immune system mistakenly attacks its own tissues and organs. This disease can lead to inflammation affecting various body parts, including joints, skin, kidneys, blood cells, brain, and heart. Among its manifestations, lupus nephritis stands out as one of the most severe and common, often resulting in significant morbidity and mortality. Early detection of kidney involvement in lupus patients and prompt intervention are critical in mitigating the associated pain, suffering, and potential fatality. Now, researchers have discovered new biomarkers with improved diagnostic performance for the early detection of lupus nephritis.
In a significant advancement, a research team from the University of Houston (Houston, TX, USA; www.uh.edu) employed Proximity Extension Assay (PEA) proteomics—a method focused on the study of proteins in terms of their interactions, functions, compositions, and structures—on urine samples from lupus patients. This approach led to the discovery of several proteins that are markedly increased in the urine of those with active lupus-related kidney disease. The study reaffirmed the validity of various previously recognized urine biomark-
The team also demonstrated the selectivity of this IPT luciferin as it did not produce light when exposed to six different proteins commonly found in saliva. This unique type of luminescence, termed “biomolecule-catalyzing chemiluminescence (BCL),” is a reaction initiated by biomolecules that aren’t classified as luciferases. Importantly, the researchers found that this luciferin could determine the quantity of the spike protein present in saliva with the same level of accuracy as a method used in vaccine research, but much faster - delivering results in just one minute, a significant improvement over existing rapid point-of-care tests. The researchers envision this BCL-based method could serve as the basis for a straightforward “mix and read” test that would involve adding IPT luciferin to untreated saliva from a person suspected of having COVID-19. This new approach could potentially be adapted to detect other viruses that have spike-like proteins, such as influenza, MERS-CoV, and different coronaviruses. The team’s findings were published in ACS Central Science on January 17, 2024. Cont’d on page 13
12 LabMedica International February-March/2024
NGS PREP SYSTEM INTEGRA BIOSCIENCES
time.
211 LMI-3-24 COM
212 LMI-3-24 LINKXPRESS COM
receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Image: The one-minute COVID-19 test uses a bioluminescent substrate that reacts with SARS-CoV-2 spike protein (Photo courtesy of Ryo Nishihara)
Novel Screening Test Capable of Identifying 18 Early-Stage Cancers
Cont’d from cover
This groundbreaking test from Novelna Inc. (Palo Alto, CA, USA; www.novelna.com) leverages a gender-specific panel of 10 proteins, providing a significant advancement in the ongoing battle against cancer. The foundation for the test was laid by pioneering research that underscores the potential for a new class of cancer screening tests, promising enhanced accuracy, reduced cost, and a move towards personalized healthcare. In their research, the Novelna team analyzed plasma samples from 440 individuals, encompassing both healthy subjects and those diagnosed with 18 different early-stage solid tumors. They employed proximity extension assays technology to assess over 3,000 proteins—both high-abundance and low-abundance—in each sample. Subsequently, they applied a multi-layered statistical process to identify a select group of gender-specific proteins capable of detecting early-stage cancers and identifying their origin tissue with high accuracy.
This test’s focus on gender-specific proteins allows for unparalleled sensitivity and specificity, acknowledging the unique biological differences in cancer development between men and women. Relying on protein markers rather than traditional genomic markers enhances the test’s ability to detect various cancers accurately and promptly. The choice to limit the detection to 10 essential proteins considerably reduces the test’s cost. Compared to Multi-Cancer Early Detection (MCED) tests, which can be priced around USD 1,000, Novelna’s innovative test is expected to cost under USD 100, making early cancer detection more accessible for many.
With a sensitivity exceeding 80% for Stage I and II cancers— stages where patients are often asymptomatic—Novelna’s test surpasses existing methods, which generally show less than 50% sensitivity for detecting cancers at early stages. This breakthrough holds the potential for reshaping public health strategies by integrating this plasma test into regular health check-ups. The high specificity of the test reduces the likelihood of false positives, and its affordability paves the way for broad implementation. The test could prove to be a game-changer in cancer screening by saving a significant number of lives.
“Our study represents a major leap in cancer screening, combining the precision of protein-based biomarkers with the efficiency of sex-specific analysis,” said Dr. Ashkan Afshin, Founder of Novelna Inc. “We’re not only looking at a more effective way of detecting cancer early but also at a cost-effective solution that can be implemented on a large scale.”
Novel Urinary Biomarkers to Enable Early Detection of Lupus Nephritis
Cont’d from page 12
ers for active renal lupus, including ALCAM, CD163, MCP1, SELL, ICAM1, VCAM1, NGAL, and TWEAK. Moreover, the team uncovered additional urine protein biomarkers not previously identified, such as ICAM-2, FABP4, FASLG, IGFBP-2, SELE, and TNFSF13B/BAFF. Analysis of these molecules within the kidneys indicated that they might be released into the urine by both immune and non-immune cells present in the renal tissue.
“We and others have reported several urine proteins that can serve as harbingers of renal involvement in lupus,” said Chandra Mohan, a pioneer in lupus research from the University of Houston. “Here, we report on a novel technique based on the use of antibodies and DNA amplification that can detect even low concentrations of proteins. This technique is called Proximity Extension Assay (PEA).” The study was published in the Journal of Autoimmunity on January 8, 2024.
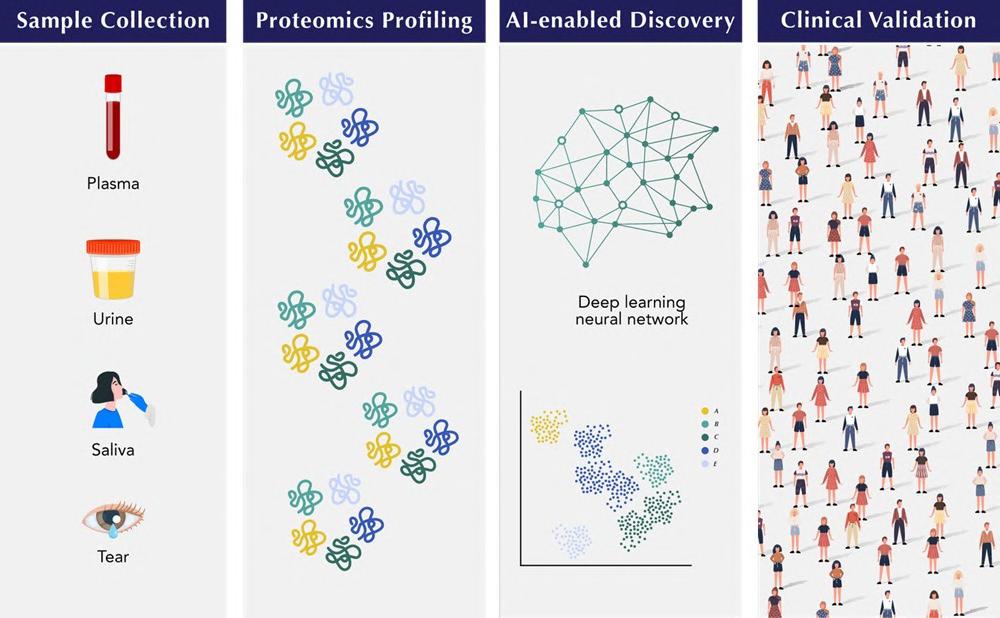
Image: A groundbreaking cancer screening test marks a major step towards early detection (Photo courtesy of Novelna Inc.)
“While further validation in larger population cohorts is necessary, we anticipate that our test will pave the way for more efficient, accurate, and accessible cancer screening,” added Dr. Afshin.






LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 13 LabMedica International February-March/2024
113 LMI-3-24 LINKXPRESS COM
Human - Veterinary - Bio-Threat Diagnostics/ Research/ Forensic QuickProfile TM QuickStatus® Quicknostics® EnviroSafe TM SAFE TM Infectious Diseases Drugs of Abuse Cancer Markers Cardiac Markers Hormones Veterinary Tests Oxidative Stress Bio-Threat Agents ADLM 2024 BOLD MOVE. Visit us at the Clinical Lab Expo Booth 2160 JULY 28-AUGUST 1 CHICAGO, IL www.LumiQuick.com www.QuickProfileDOA.com sales@LumiQuick.com +1(408)855-0061

To
CARDIAC MARKERS QUALITY CONTROL BIO-RAD QUALITY SYSTEMS DIVISION

Liquichek Cardiac Troponins Control is a dedicated three-level Troponins control designed to monitor the precision of high-sensitivity or next-generation Troponin I or Troponin T assays on major analyzer
214 LMI-3-24 COM
Handheld Device Detects Fentanyl in Urine in Seconds
Cont’d from cover
in urine within seconds.
The device developed by researchers at University of Texas at Dallas (Richardson, TX, USA; www.utdallas.edu) contains an electrochemical sensor that operates by generating electrical signals from chemical reactions. Detecting fentanyl was challenging due to its nonvolatile nature, meaning it doesn’t naturally produce an electrochemical signature. To overcome this, the researchers designed a molecular cage-like structure resembling a mousetrap to capture fentanyl. This “trap” incorporates several components, including gold nanoparticles, and utilizes naloxone, a medication that reverses opioid overdoses, to attract and bind to fentanyl. When a urine sample is applied to a test strip, the presence of fentanyl triggers a reaction with the naloxone, resulting in a detectable signal.
This technology can also test other substances for fentanyl by simply diluting a sample in water and applying it to the sensor. The team’s proof-of-concept device can detect trace amounts of fentanyl with 98% accuracy, bypassing the need for expensive and time-consuming lab analyses. The current prototype, designed for urine testing, is a precursor to developing a saliva-based test. Efforts are underway to expand the technology for detecting fentanyl in hair, with the ultimate goal being a saliva test. A saliva-based test would be particularly beneficial for first

AUTOMATIC BIOCHEMISTY ANALYZER ARK DIAGNOSTIC SYSTEM

The Metrolab 2300 GL automatic biochemistry analyzer offers a flexible solution for labs with its optimal throughput and low water consumption. It offers 48 reagent and sample positions and operates with cutting-edge software.
215 LMI-3-24 LINKXPRESS COM


Image: The researchers demonstrate how their sensor can detect fentanyl (Photo courtesy of University of Texas at Dallas)
responders in making timely treatment decisions for overdose cases.
“There is an urgent demand for an easy-to-use, portable, miniaturized device that can detect fentanyl with high specificity and share results immediately to an internet-connected device,” said Dr. Shalini Prasad, professor and department head of bioengineering in the Erik Jonsson School of Engineering and Computer Science. “Our study demonstrates the feasibility of a highly accurate sensor to detect fentanyl within seconds.”
AI Model for Brain Tumor Classification Advances Neuropathology
Diffuse gliomas, which comprise a large portion of malignant brain tumors in adults, include various types such as astrocytoma, oligodendroglioma, and glioblastoma. Diagnosing these types of gliomas traditionally relies on an analysis that integrates histological characteristics with molecular details, a method that presents significant complexities when attempting to develop a comprehensive diagnostic model from whole-slide images (WSIs). The immense gigapixel resolution of WSIs renders the use of standard convolutional neural networks for analysis impractical. To address this challenge, researchers have now introduced a novel integrated diagnostic model that can automatically classify adult-type diffuse gliomas directly from unannotated standard whole-slide pathological images, eliminating the need for additional molecular testing.
Researchers from the Chinese Academy of Sciences (CAS, Beijing, China; www.cas.cn) have devised this deep learning model capable of parsing WSIs and categorizing gliomas without the need for detailed manual annotations. This model adheres to the strict classification guidelines outlined in the 2021 fifth edition of the World Health
Organization Classification of Tumors of the Central Nervous System. The model underwent training and validation across a diverse dataset comprising 2,624 patient cases collected from three different hospitals.
The model's effectiveness was evaluated based on its classification accuracy, sensitivity to various glioma types and grades, and its capability to differentiate between genotypes that exhibit similar histological characteristics. The outcomes of the experiments indicate that the model demonstrates robust performance, with all areas under the receiver operator curve exceeding 0.90. This performance was noted in its ability to classify major tumor types, identify tumor grades within each type, and, notably, distinguish between tumor genotypes that share the same histological features.
"Our integrated diagnosis model has the potential to be used in clinical scenarios for automated and unbiased classification of adulttype diffuse gliomas," said CAS Prof. Li Zhicheng who led the research team. "The future research will focus on improving this model to have multi-center, multi-racial datasets."
14 LabMedica International February-March/2024
receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Electrochemical Sensors with Next-Generation Coating Advance Precision Diagnostics at POC
Current point-of-care (POC) diagnostic technologies are typically limited to measuring a single disease biomarker or several biomarkers from the same class of molecules, such as various RNAs, proteins, or antibodies. However, the ability to measure multiple biomarkers from different molecular classes could provide a more comprehensive understanding of a disease's state, severity, progression, and individual variations in its development. Electrochemical biosensors, which convert the chemical signal of a biomarker found in a small biofluid sample (like blood, saliva, or urine) into an electrical signal proportional to the biomarker's amount, could potentially address many diagnostic challenges at the point of care. These sensors can be assembled into multiplexed arrays to detect different biomarkers, and recent advances have overcome the challenge of “biofouling” – the degradation of electrode surfaces by nonspecific biological molecules in samples – through the development of thin antifouling coatings.
Now, researchers at Wyss Institute at Harvard University (Boston, MA, USA; www.wyss.harvard.edu), in collaboration with several institutes in Korea, have significantly advanced electrochemical diagnostic sensing. They have developed a new nanocomposite porous antifouling coating that is one micrometer thick – about 100 times thicker than previous coatings. This increased thickness, coupled with an engineered porous structure, allows for the integration of a higher number of biomarker-detecting probes into the sensors, achieving up to 17 times greater sensitivity than the


When tested with patient samples, the new sensor demonstrated 3.75 to 17 times higher detection sensitivities compared to a previous sensor fabricated with the same detection systems but using the team’s thinner, non-porous coating. It also accurately distinguished between positive and negative samples with 100% specificity.
“Our novel thick porous emulsion coating directly addresses critical hurdles that currently prevent the widespread use of electrochemical sensors as central components of comprehensive POC diagnostics for many conditions,” said Wyss Founding Director Donald Ingber, M.D., Ph.D. “However, going far beyond that, it could also open up new opportunities
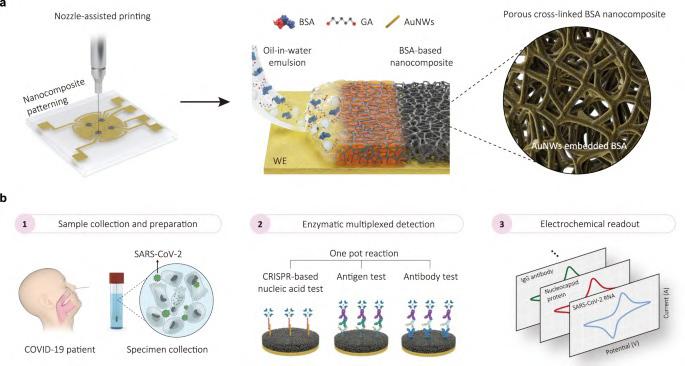
Image: Thick and porous antifouling nanocomposite for electrochemical detection of virus with high accuracy and reliability (Photo courtesy of Wyss Institute at Harvard University)
for developing safer and more functional implantable devices, and other healthcare monitoring systems at multiple disease fronts. Overcoming biofouling and sensitivity problems are challenges that impact many of these efforts.”

For small and medium labs, @SINGUWAY is your answer.






≤30 minutes
≤35 minutes
qPCR Detection Kits (PCR fluorescence method):
Respiratory Diseases: COVID-19, fluA, fluB, AdV, TB and multiplex test
Blood Diseases: HBV, HCV, HIV and multiplex test
Sexually Transmitted Diseases: HPV, CT, NG, UU and multiplex test
Viral Zoonotic Diseases: MPV
Vector-borne Diseases: PF, ZIKV
Genetic Diseases: MTHFR
Animal Diseases: Swine/Avian/Aquatic
Animal/Ruminant/Companion Animal Diseases




15 LabMedica International February-March/2024
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Singu20 Nucleic Acid Extractor AccuRa-32 Real-Time PCR System (32 samples)
Singu20 Nucleic Acid Extractor AccuRa mini Real-Time PCR System (8-16 samples)
5-Part Hematology Analyzer Hematology + CRP +SAA Joint Analyzer (Auto Sampling)
115 LMI-3-24 LINKXPRESS COM

MTo

This assay enables the quantitative determination of the physiologically active forms of vitamin B1, thiamine pyrophosphate, in whole blood samples and of vitamin B6, pyridoxal 5‘-phosphate, in whole blood or plasma samples.

Ultrasensitive Molecular Diagnostic Tools Detect Asymptomatic Malaria
alaria remains a significant cause of illness and death, particularly in low-income countries, striking hardest among young children and pregnant women. In 2022 alone, the World Health Organization reported 608,000 malaria deaths globally, with a staggering 95% of these occurring in the African region. A key challenge in controlling malaria, especially in endemic areas, is identifying and treating asymptomatic carriers of the disease. In response to this challenge, researchers have developed advanced diagnostic tools capable of detecting even minimal traces of malaria in individuals who carry the parasite but do not exhibit symptoms.
A collaborative effort between scientists from the University of Washington (Seattle, WA, USA; www.washington.edu) and Med Biotech Laboratories (Kampala, Uganda; www.mbl.ug) has led to significant advancements in malaria detection. The researchers observed that malaria parasite densities in the blood can suddenly fall below the detec-
tion threshold of traditional tests due to the evolving nature of the malaria pathogens. This issue is exacerbated when using older, less sensitive diagnostic methods and when testing is limited to a single point in time. They found that ultrasensitive molecular diagnostic tools possess greater analytical sensitivity than other testing methods, including blood smears, rapid diagnostic tests, and some other molecular tests. In their study, the team applied these ultrasensitive tools to test non-pregnant adults aged 18 to 59 and children aged eight to 17 in the Katawki district of eastern Uganda, a region with a high incidence of malaria. The participants were not under any malaria medication.
The research involved testing dried blood spots for the presence of Plasmodium ribosomal RNA, essential in the production of parasite proteins, to assess the types and densities of malaria parasites over a month. The goal was to establish a practical sampling schedule that could consistently identify asymptom-

NEAR-PATIENT TESTING PLATFORM BAEBIES

FINDER is a near-patient testing platform powered by digital microfluidics specifically designed to address the need for tests requiring low blood volume. It has a turnaround time of about 15 minutes after sample introduction.

Image: Researchers have made progress in cellfree DNA for early diagnosis of preeclampsia (Photo courtesy of Leandro Lemgruber, University of Glasgow)
atic malaria cases without the need for daily testing. Remarkably, about 60% of the study participants experienced a Plasmodium infection at some point during the research period. However, less than half were detected with an infection at the study’s onset. The average rate of infection stood at 30%. The team has highlighted an increasing trend in asymptomatic malaria among Ugandan children over five years old, indicating the need for further research into the dynamics of asymptomatic malaria. Their findings were published in the journal The Lancet Microbe on January 4, 2024.
“To make anti-infection vaccines, drugs and therapeutics and test them in endemic areas means that you need diagnostic tools that can detect even the lowest density infections,” said Sean Murphy, professor of laboratory medicine and pathology at the University of Washington.
“Our current findings provide critical information on the burden of asymptomatic malaria that we hope one day will be useful to the national malaria control program in Uganda and other malaria-endemic African countries,” added Tonny Owalla, a researcher at Medical Biotech Laboratory in Kampala.
16 LabMedica International February-March/2024
VITAMIN B1/B6 TEST CHROMSYSTEMS
217 LMI-3-24 COM
218 LMI-3-24 LINKXPRESS COM
and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
receive prompt
Edited by Katherina Psarra, MSc, PhD
MESSAGE FROM THE PRESIDENT
By Tomris Ozben • President, IFCC
DearColleagues and Friends,
Stepping into the role as the President of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Executive Board is not only a tremendous honor, but also a great privilege for me. I am excited about the opportunities and challenges that lie ahead as I embark on my journey of serving the IFCC community, commencing my term on January 1st, 2024. I want to express my sincere gratitude to the IFCC member societies within the 6 IFCC Regional Federations for their kind support and trust. I sincerely appreciate their confidence and assure them that I am fully committed to deserving their trust. I am dedicated to contributing my time, expertise, knowledge, and energy to enhance the functions and activities of IFCC.
Taking on a leadership position, with other dedicated members of the IFCC Executive Board, IFCC officers, Regional Federations, National Societies, and Corporate members, our collective mission is to strengthen the robust foundations on which the Federation stands. Our efforts will be centered on identifying new opportunities and formulating innovative strategies that empower Laboratory Medicine to play a crucial role in patient care. Our goal is to ensure that the IFCC continues to stand as the leading global organization representing Laboratory Medicine worldwide.
IFCC demands a dedicated and proficient management team. The President, along with the members of the new IFCC Executive Board and officers of the IFCC Function al Units, will devote both commit ments, time and effort. The day-today operations of IFCC will be man aged efficiently by the exceptional capacity, enthusiasm, and efforts of the IFCC office secretaries working closely with IFCC officers. Our aim is not just to sustain, but to enhance IFCC functions and activities, mak ing significant contributions to the advancement of Laboratory Med icine and the growth of its profes sionals. We are set to carry the IFCC flag even higher, maintaining past initiatives and introducing new projects. The collective expertise, knowledge, and fresh ideas from the Executive Board members and IFCC officers, working with enthusi asm across various functional units, will drive this progress. We emphasize the importance of close collaboration with IFCC Regional
Federations, IFCC Member Societies, and Corporate Members, encouraging their active participation in all IFCC activities.


I express my sincere thanks and congratulations to Prof. Khosrow Adeli for his outstanding leadership, motivating and encouraging IFCC officers and Executive Board members to actively engage in numerous IFCC projects. I extend my heartfelt gratitude to the former IFCC Presidents, outgoing members of the IFCC Executive Boards, and officers of the Functional Units for their exceptional contributions, voluntary services,



www.dubai2024.org

26th International Congress of Clinical Chemistry and Laboratory Medicine


NEWS
IFCC members may send news to Email: enews@ifcc.org 17 LabMedica International February-March/2024
World
Dubai
Trade Centre (DWTC)
The Congress is accredited! IFCC is an approved EFLM CPECS® Provider of Continuing Education Events in Laboratory Medicine. The IFCC Worldlab 2024 has been accredited by the EFLM-CPECS® credit system for a maximum number of 22,5 CPECS® credits.
cooperation with
In
Cont’d on page 18


EDITORIAL
By Katherina Psarra, MSc, PhD
Dear Colleagues,
This is the first IFFC eNews issue for 2024. A new year is in front of us, full of activities and with an IFCC Worldlab in Dubai, full of promises.
This issue begins with our new IFCC President Prof Tomris Ozben’s important first message.
Go through this full of significant content message, dear colleagues, and you will learn a lot about IFCC brilliant future in accordance with its wonderful past.
And what is the most suitable article to follow the second woman IFCC President’s message, but the first woman IFCC President’s, the late Prof. Jocelyn MB Hicks’ own words for inspiration, lessons and counsel?
Dr Sharon M. Geaghan offers us the opportunity to “hear” Jocelyn Hicks’ voice guiding us and especially us, women scientists, in life and work and science. You will be definitely moved by her words and you will feel a kind of empowerment, of support. Do try to mimic her, dear colleagues.
Two ladies, Dr Maria Del Carmen and Dr Maria Schroeder, Chair and member of the Committee on Public Relations together with the Past Chair, Prof. Rajiv Erasmus, who played such an important role in Global Medlab week launching, are inviting us as national societies to participate in the Global Medlab week 2024 with the inspiring title “Labs save lives”.
News from EFLM and the national societies (Saudi, Iran, COLABIOCLI, Japan), from the IFCC PMEP and VLP programs, endocrine cases presented by the TF-YS, a new clinical care group of scientists awarded the UNIVANTS excellence award complete this first 2024 issue of the IFCC eNews.
Happy New Year, dear colleagues and don’t forget to read and to contribute to the eNews.
Message from the IFCC President
Cont’d from page 17
knowledge, and enthusiasm over the years. Their dedication has played a crucial role in building a resilient and continually advancing Federation, establishing it as a leading organization in laboratory medicine worldwide, providing high-quality healthcare services to its members. We will always remember and deeply appreciate their significant contributions. Although they may no longer hold active roles, we are confident that their interests and contributions to the IFCC will endure. We look forward to working closely with them, drawing upon their extensive experience and knowledge for the benefit of IFCC.
In recent years, IFCC’s activities have experienced steady growth. The substantial services and contributions of IFCC to the enhancement of Laboratory Medicine are widely acknowledged. This remarkable success is attributed to the voluntary service, dedication, and time invested by over 300 laboratory scientists from Member Societies and Corporate Members, serving across various IFCC Functional Units. Our heartfelt gratitude extends to the IFCC officers representing Member Societies and Corporate Members for their unwavering support throughout the years. Their ongoing commitment and loyalty have played a pivotal role in the successful execution of numerous IFCC tasks and projects.
I firmly believe that advancing healthcare through Laboratory Medicine relies on the active engagement and dedicated involvement of IFCC Member Societies, Corporate Members, and IFCC officers of the Functional Units. Their collective efforts play a pivotal role in steering our profession toward the objectives and direction which will be outlined in the new IFCC Strategic Action Plans, set to be implemented in the coming years. These plans will be meticulously crafted in collaboration with the IFCC Executive Board, IFCC Functional Units, IFCC National Societies, and Corporate Members, incorporating their valuable suggestions, ideas, and comments in formulating the IFCC Strategic Action Plans. This inclusive approach ensures that the IFCC Strategic Action Plans are a true reflection of the diverse perspectives and expertise within our community.
Success in this evolving landscape requires collaborations among industry, public, private, and academic drivers of in-
novation. The IFCC Executive Board values and welcomes the input of ideas, comments, suggestions, criticisms and the sharing of challenges and concerns from IFCC National Societies and IVD Industry Partners. We particularly encourage young scientists to actively engage and play fundamental roles in IFCC activities. We invite all IFCC community to actively participate in IFCC’s endeavors, fostering collaboration to strengthen IFCC’s leadership role in advancing laboratory medicine, integrated diagnostics and enhancing the quality of healthcare and medical laboratories globally. Your engagement and contributions are crucial as we collectively strive towards these shared goals. Your feedback, participation, and engagement will be integral in steering laboratory medicine forward and shaping the future of our profession.
Achieving our shared objectives requires collaborative action and effort. To facilitate this, we will establish an interactive global platform for IFCC members in laboratory medicine, colleagues from the in vitro diagnostic industry, digital health, and medical devices. This platform will provide opportunities for interaction, sharing, and learning through networking discussions on trends, opportunities, and challenges. The aim is to deliver the latest innovations and cutting-edge technologies in laboratory medicine, establish partnership models for the efficient integration and adoption of emerging technologies, prepare for the new in vitro diagnostic regulations in Europe and beyond, transition to green and sustainable medical laboratories, harmonize and standardize laboratory tests, and enhance collaborations in education and research. This platform will serve as a dynamic space for collective advancement in our field.
I am looking forward to fostering productive cooperation with the IFCC Executive Board members and officers, establishing collaborations and efficient bridges with the IFCC Regional Federations, National Societies and Corporate Members in the ongoing and future activities of IFCC. I wish you all an excellent New Year with new hopes and plans for the year ahead.
With My Best Regards, Prof. Dr
Tomris Ozben IFCC President
News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS 18 LabMedica International February-March/2024

Improving Diagnostic Pathway for Emergency Department Admittees With Suspected Mild Traumatic Brain Injury
Thebrain is still very much an enigma with many things yet to be known, but one thing we are sure of, is that any trauma to the brain can be significant. Thus, in recognition of Brain Injury Awareness month – March 2024 it is important to recognize advancements across the field. Noteworthy is the advent of blood biomarker testing in the emergency department to enable faster triage of patients with suspect mild traumatic brain injury (mTBI).
At the Hospital Universitario Virgen de las Nieves, in Spain, an integrated clinical care team sought to strategically implement blood biomarkers into clinical care. The novel TBI panel is used in conjunction with other clinical information to assist in determining the need for a CT scan of the head for patients 8 years of age or older, who present with suspected mild traumatic brain injury (mTBI, Glasgow Coma Scale score 13-15) within 12 hours of injury. This panel facilitates in vitro diagnostic measurements for the semiquantitative interpretations of GFAP (Glial fibrillary acidic protein) and UCH-L1 (Ubiquitin C-Terminal Hydrolase L1) as found in human plasma or serum.
Implementation of this panel for all patients with suspected mTBI in the emergency department has shifted the way in which suspected TBI patients are assessed and positively impacted care across multiple facets. There has been a 10% reduction in CT scans within the first 3 months of implementation, as well as mitigated overuse of limited resources within the emergency department by 143 total ED hours in a 9-month period. This is coupled with an increase in clinical confidence, with 77.8% of clinicians indicating that the added insights from the panel helped reduce the uncertainty related to the absence of brain lesions, particularly in the non-elderly.
For efforts in improving triage of patients with suspected mTBI, this integrated clinical care team was awarded the UNIVANTS of Healthcare Excellence Recognition of Achievement. Congratulations to Gemma Álverez Corral, Clinical Laboratory Specialist, Maria Isabel Romero Manjon, Radiologist. Department Director, Francisco Ruiz-Cabello Osuna, Clinical Laboratory Specialist, Department Director, Eva Gutierrez Pérez, Emergency Medicine Specialist, Maria Molina Zayas, Clinical Laboratory Specialist.
IFCC OFFICE
Via Carlo Farini 81, 20159 Milan, ITALY
Tel: (39) 02-6680-9912
E-mail: ifcc@ifcc.org • Web: www.ifcc.org
Staff Members: Paola Bramati, Silvia Cardinale, Silvia Colli-Lanzi, Elisa Fossati, Smeralda Skenderaj

MAKE YOUR MOVE.
REGISTER EARLY + SAVE
Ready to discover what’s next for laboratory medicine? Join us for ADLM 2024 in Chicago. Breaking science requires bold thoughts and actions from observation to collaboration to innovation to sharing with lab-minded professionals. Immerse yourself in the experience.
• Five plenaries, 65+ scientific sessions, and 170 roundtables
• 12 ADLM University courses offering practical skills for laboratorians
• 900+ exhibitors, 200+ product categories and live demos
• Networking opportunities to connect with industry peers and leaders
• And much more!
Make your move early for the best rates.
Registration opens early April at meeting.myadlm.org.


19 LabMedica International February-March/2024 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS The views and positions expressed in the IFCC News section are those of the IFCC or the individual authors, and do not necessarily represent the views or positions of LabMedica magazine or its publishers.
JU LY 28–AUGUST 1 • CHICAG O, IL, US A
Patrick Mathias MD, PhD

Activities of the Young Scientists Group on Digital Competence in Laboratory Medicine
Marie Lenski, French YS (Société Française de Biologie Cliniques SFBC), IFCC-TF-YS member
Jakob Adler, German YS (Deutsche Gesellschaft für Klinische Chemie und Laboratoriumsmedizin DGKL), IFCC-TF-YS corresponding member
Thisinitiative was initiated after EuromedLab Munich 2022 by Jakob Adler (Germany) and Marie Lenski (France), and supported by the IFCC Task Force – Young Scientists. A first survey was distributed by IFCC in June 2022 to assess needs of young scientists regarding digital competence on a global scale. The results, published in 2023 in the Journal of Laboratory Medicine, highlighted the necessity of establishing an international working group on digital competence. The aim is to build a learning environment and propose common international resources such as tutorial articles, videos, exercises, technical articles to enhance digital skills in laboratory medicine. Using a dedicated mailing list with nearly 120 subscribers, a second survey was conducted in September 2022. This survey allowed young scientists to express their expectations and needs to improve their digital competence. Results included:
. Programming language and version control (R, Python, Git…)
. Statistics and data science
. Collaborative work
. IT Buzzword Bingo (short explanation of IT buzzwords)
. Concrete application for Laboratory Medicine (reference intervals…)
After an intensive creation of the platform and resources by Jakob Adler, the first online meeting of the young scientists’ group on digital competence in laboratory medicine took place on January 24th, 2024. Twelve young scientists participated in the launch of the activities, engaging in collaborative work and setting future objectives on:


. GitHub: An online platform for code hosting and collaborative work, featuring a dedicated group focused on digital competence in laboratory medicine
. Markdown Language: An article covering the Markdown markup language, accompanied by a toolbox of Markdown tools was presented
. R Programming: An introduction to R programming and resources covering basic operations and functions
. CryptPad: A presentation of this collaborative office suite, which is end-to-end encrypted and open-source
The group of young scientists aims to combine Markdown and R, hosting its work on GitHub, and covering various topics such as programming, data analysis, and practical applications in laboratory medicine.
The next online meeting of this young scientist group on digital competence in laboratory medicine is scheduled for March and will continue monthly thereafter. The mailing list now serves as a newsletter tool. To stay informed, you can visit the Digital Competence in Laboratory Medicine page on pageflow.io and subscribe by sending an email to digcomplabmed@gmx.de.
Information on all mentioned activities, objectives and projects of the TF-YS are available on IFCC website ifcc.org/ task-force-young-scientists-tf-ys, or follow us on instagram (/ ifcc_tfys), facebook (/ifccYOUNG), linkedin (/groups/3049837)

20 LabMedica International February-March/2024 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
Marie Lenski (France)
Jakob Adler (Germany)
Roche (Basel, Switzerland; www.roche.com) entered into a definitive agreement to acquire LumiraDx’s (London, UK; www.lumiradx.com) point-of-care technology. This acquisition is in line with Roche’s strategic goal of fostering patient-centered healthcare through innovative point-ofcare solutions that cover an extensive range of healthcare settings. LumiraDx’s microfluidic technology ensures rapid, precise, and accessible diagnostic services, enabling critical testing in various decentralized healthcare environments. The company boasts an extensive range of tests applicable on a single portable platform, with over 30 assays either available or in development. These assays encompass a variety of medical needs, including infectious diseases, cardiovascular health, diabetes, and coagulation disorders.
Fujirebio (Tokyo, Japan; www.fujirebio.com) and Agappe Diagnostics (Kochi, India; www.agappe.com) have partnered to manufacture cartridge-based CLIA system reagents for the Mispa i60 and Mispa i121 immunology analyzers. These analyzers and reagents will be sold under Agappe’s brand, making it the first Indian local company with a complete chemiluminescence solution made from reagents produced domestically. The collaboration is set to make a substantial impact, with phased launches planned for a complete range of over 30 parameters, spanning various medical segments such as oncology, thyroid, cardiac, fertility, and infectious diseases.
Sysmex Corporation (Kobe, Japan; www.sysmex.co.jp) entered into a strategic alliance agreement with CellaVision (Lund, Sweden; www. cellavision.com) to advance hematology solutions. The partnership between Sysmex and CellaVision has spanned over 20 years, starting in 2001 when Sysmex entered a sales alliance for CellaVision’s cell morphology analyzers. The renewed partnership aims to further improve efficiency and standardization in testing workflows and increase clinical value by providing more precise cell morphology classification to aid in diagnoses. These joint efforts are intended to further improve efficiency and standardization in testing workflows and increase clinical value by providing more precise cell morphology classification to aid in diagnoses.
Sysmex has also entered into a collaboration with Hitachi High-Tech Corporation (Tokyo, Japan; www.hitachi-hightech.com) for the development of genetic testing systems using capillary electrophoresis sequencers (CE sequencers). The goal of the partnership is to produce genetic testing systems that are more efficient and cost-effective, thereby facilitating the widespread clinical application of personalized genetic testing for various diseases. Hitachi will focus on obtaining medical device approvals for their CE sequencers, while Sysmex will develop and seek regulatory approvals for testing reagents compatible with these devices, as well as developing analysis software.
BioMérieux (Marcy-l’Étoile, France; www.biomerieux.com) has acquired LUMED (Sherbrooke, Canada; www.lumed.ca), an innovative software company that has developed a clinical decision support system that helps hospitals optimize their antimicrobial prescriptions. The acquisition is in line with bioMérieux’s efforts to expand its data analytics portfolio, with continued focus and commitment to antimicrobial stewardship and infection prevention and control. bioMérieux plans to leverage its global commercial network to bring LUMED software’s benefits to the largest possible number of patients.
BD (Becton, Dickinson and Company, Franklin Lakes, NJ, USA; www. bd.com) and Techcyte (Orem, UT, USA; www.techcyte.com) are collaborating to offer an AI-based algorithm that will assist cytologists and pathologists in efficiently and effectively identifying signs of cervical cancer and precancer through whole-slide imaging. The agreement will allow BD to provide a comprehensive solution aimed at minimizing human error and increasing throughput. This will help laboratories enhance the standardization, reproducibility, and efficiency of Pap test results.
Seegene, Inc. (Seoul, Korea; www.seegene.com) has selected Microsoft (Redmond, WA, USA; www.microsoft.com) as a technology partner for its SG OneSystem business to realize ‘a world free from all diseases’. With this collaboration, Seegene has adopted a unique strategy of sharing its unique syndromic quantitative PCR technologies with the world’s leading companies to encourage cross-industry innovation in molecular diagnostics. The expansion of participating companies, with whom

Seegene aims to form a global consortium, will empower countries across the world with optimal solutions, enabling them to effectively address future pandemics.


Thermo-Resistant












21 LabMedica International February-March/2024 Industry News To view this issue in interactive digital magazine format visit www.LabMedica.com
121 LMI-3-24 LINKXPRESS COM Size: 2400 x 1200 mm (3 mm thick) 100% Silicone YOUR GLOBAL SOURCE FOR STERILIZATION ACCESSORIES
60 °C to 300 °C)
Washable & Flexible Suitable for central sterilization services Sterilizable STERILIZABLE INSTRUMENT & WORK-SURFACE MATS Front Back WASHING TRAYS MAT Heavy Silicone Cover & Transport Tablet TURBO WASHING MACHINES TRAYS SILICON INSTRUMENT MAT Front Back MICRO INSTRUMENT MAT Exchangable Net Exchangable Nets INVITEDTOAPPLY DISTRIBUTORS Up to 37 cm in length THERMO RESISTANT GLOVES Front Back WASHING TRAYS MAT NEW! VICOTEX Place de la Gare 1 • 1009 Pully • Switzerland Tel: (41) 21-728-4286 • Fax: (41) 21-729-6741 E-Mail: contact@vicotex.com www.vicolab.com S.A. SILICONE TABLET AND STEEL COVER NETS
(-
Fully
Image: The OneSystem strategic collaboration signing ceremony between Seegene and Microsoft (Photo courtesy of Seegene)
2024
MARCH
ICE 2024 – 21st International Congress of Endocrinology. Mar 1-3; Dubai, UAE; isendo.org
China Lab 2024. Mar 5-7; Guangzhou, China; chinalabexpo.com
MASCL 2024 – Congress of the Association for Mass Spectrometry & Advances in Clinical Lab. Mar 17-22; Monterey, CA, USA; msacl.org
USCAP 113th Annual Meeting – United States & Canadian Academy of Pathology. Mar 23-28; Baltimore, MD, USA; uscap.org
APRIL
Analytica 2024. Apr 9-12; Munich, Germany; analytica.de
India Lab Expo & Analytica Anacon India. Apr 15-17; Mumbai, India; analyticaindia.com
Korea Lab 2024. Apr 23-26; Seoul, Korea; korealab.org
ExpoMED Eurasia 2024. Apr 25-27; Istanbul, Turkey; expomedistanbul.com
ECCMID 2024 – 34th European Congress of Clinical Microbiology and Infectious Diseases. Apr 27-30; Barcelona, Spain; eccmid.org
The 15th International & 21st National Congress on Quality Improvement in Clinical Laboratories. Apr 30 - May 3; Tehran, Iran; iqctehran.ir
MAY
24th National Congress of Clinical Chemistry and Laboratory Medicine. May 2-5; Playa del Carmen, Mexico; fenacqc.org.mx
Immunology 2024 – Annual Meeting of the American Association of Immunologists (AAI). May 3-7;
Chicago, IL, USA; immunology2024.aai.org
AACE Annual Meeting 2024 – American Association of Clinical Endocrinology. May 9-11; New Orleans, LA USA; pro.aace.com
ECE 2024 – 26th Annual Congress of the European Society of Endocrinology. May 11-14; Stockholm, Sweden; ese-hormones.org
ASRI 2024 – 43rd Metting of the American Society for Reproductive Immunology. May 18-22; Houston, TX, USA; theasri.org
Hospitalar 2024. May 21-24; Sao Paulo, Brazil; hospitalar.com
107th Annual Meeting of the German Society for Pathology. May 23-25; Munich, Germany; pathologie-dgp.de
IFCC WorldLab 2024 – 26th International Congress of Clinical Chemistry and Laboratory Medicine. May 2630; Dubai, UAE; dubai2024.org
SLAS Europe 2024 Conference and Exhibition - Society of Laboratory Automation and Screening. May 27-29; Barcelona, Spain; slas.org
ISLH 2024 – International Society for Laboratory Hematology. May 30 - Jun 1; Nantes, France; islh.org
EAACI 2024 – Annual Congress of the European Academy of Allergy & Clinical Immunology. May 31Jun 3; Valencia, Spain; eaaci.org
JUNE
IESHG 2024 – European Human Genetics Conference. Jun 1-4; Berlin, Germany; 2024.eshg.org
UKMedLab24 – National Meeting of the Association for Clinical Biochemistry and Laboratory Medicine. Jun 10-12; Brighton, UK; acb.org.uk
9th International Symposium on Critical Care Testing and Blood Gases. Jun 13-14; Saint-Malo, France; criticalcaretesting-saintmalo2024.eu
ASM Microbe 2024 – American Society for Microbiology. Jun 13-17; Atlanta, GA, USA; asm.org
49th CBAC – Congress of the Brazilian Society of
Clinical Analysis. Jun 16-19; Natal, Brazil; sbac.org.br
2024 CSCC Annual Conference – Canadian Society of Clinical Chemists. Jun 17-20; Mont Sainte-Anne, QC, Canada; cscc-sccc.ca
FOCIS 2024 – Annual Meeting of the Federation of Clinical Immunology Societies. Jun 18-21; San Francisco, CA, USA; focisnet.org
FIME 2024 – Florida International Medical Expo. Jun 19-21; Miami, FL, USA; fimeshow.com
ISTH 2024 Congress – International Society on Thrombosis and Haemostasis. Jun 22-26, Bangkok, Thailand; isth2024.org
ECC 2024 – 45th European Congress of Cytology. Jun 23-26; Leipzig, Germany; cytology2024.eu
38th International Congress of the International Society of Blood Transfusion (ISBT). Jun 23-27; Barcelona, Spain; isbtweb.org
AMP Europe 2024. Jun 24-26; Madrid, Spain; amp.org
ASV 2024 – 43rd Annual Meeting of the American Society of Virology. Jun 24-28; Columbus, OH, USA; asv.org
FEBS 2024 – 48th Congress of the Federation of European Biochemical Societies. Jun 29 - Jul 3; Milan, Italy; febs.org
ECB 2024 – European Congress on Biotechnology. Jun 30 - Jul 3; Maastricht, The Netherlands; ecb2024.com
JULY
ESHRE 2024 – 40th Annual Meeting of the European Society of Human Reproduction and Embryology. Jul 7-10; Amsterdam, Netherlands; www.eshre.eu
MedLab Asia 2024. Jul 10-12; Bangkok, Thailand; medlabasia.com
2024 ADLM Annual Scientific Meeting & Clinical Lab Expo. Jul 28 - Aug 1; Chicago, IL, USA; meeting. myadlm.org
64th Annual Academic Assembly of the Japan Soci-
Events Calendar
AUGUST
For a free listing of your event or a paid advertisement contact: LabMedica International Calendar E-mail: info@globetech.net Provided as a service to advertisers. Publisher cannot accept responsibility for any errors or omissions. Advertising Index Inq.No. Advertiser Page Inq.No. Advertiser Page Vol. 41 No. 1 3/2024 LabMedica International ATTENTION: IF YOUR APPLICATION IS NOT RECEIVED AT LEAST ONCE EVERY 12 MONTHS YOUR FREE SUBSCRIPTION MAY BE AUTOMATICALLY DISCONTINUED READER SERVICE PORTAL LINKXPRESS COM ® VISIT Every advertisement or product item contains a LinkXpress number as below: 999 LMI-03-24 LINKXPRESS COM Identify LinkXpress codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress inquiry matrix 1 2 3 Renew/ S tart Your Free Subscription Access Instant Online Product Information – ADLM 19 – APFCB 23 – BCLF 2024 20 – COLABIOCLI 2024 16 111 DiaSys Diagnostic Systems ..................... 11 – EuroMedLab 2025 23 – IFCC WorldLab 2024 .......................... 17 109 Intec Products 9 – LabMedica EXPO ............................. 2 113 Lumiquick 13 107 Nova 7 105 Puritan 5 124 Sekisui 24 115 Singuway 15 103 Snibe 3 121 Vicotex 21
ety of Clinical Chemistry (JSCC). Aug 30 - Sep 1; Utsunomiya, Japan; jscc-jp.gr.jp
SEPTEMBER
ASCP 2024 – Annual Meeting of the American Society for Clinical Pathology. Sep 4-6; Chicago, IL, USA; ascp.org
17th Baltic Congress of Laboratory Medicine. Sep 5-7; Vilnius, Lithuania; balm2024.lt
ECP 2024 – 35th Congress of the European Society of Pathology. Sep 7-11; Florence, Italy; esp-congress.org
EUROTOX 2024 – 58th Congress of the European Societies of Toxicology. Sep 8-11; Copenhagen, Denmark; eurotox2024.com
Thailand LAB International 2024. Sep 11-13; Bangkok, Thailand; thailandlab.com
NFKK 2024 - 39th Nordic Congress of Clinical Chemistry. Sep 17-20; Stockholm, Sweden; nfkk2024.se
ESVC 2024 – Annual Meeting for the European Society for Clinical Virology. Sep 17-21; Frankfurt, Germany; escv.eu
47TH ISOBM CONGRESS – International Society of Oncology and Biomarkers. Sep 19-21; Pilsen, Czech Republic; isobm.org
DKLM 2024 – Annual Congress of the German Society for Clinical Medicine and Laboratory Medicine (DGKL). Sep 25-27; Bremen, Germany; dgkl.de
India Lab Expo & Analytica Anacon India. Sep 26-28; Hyderabad, India; analyticaindia.com
OCTOBER
COLABIOCLI 2024 - 26th Latin American Congress of Clinical Biochemistry. Oct 3-6; Cartagena, Colombia;
colabiocli.com
56th National Congress of the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC). Oct 8-10; Bologna, Italy; sibioc.it
JFBM 2024 - Journées Francophones de Biologie Médicale. Oct 9-11; Troyes, France; jfbm.fr
CAP24 – Annual Meeting of the College of American Pathologists. Oct 19-22; Las Vegas, NV, USA; cap.org
ASHI 2024 – 50th Annual Meeting of the American Society for Histocompatibility and Immunogenetics. Oct 21-24; Anaheim, CA, USA; ashi-hla.org
MedLab Africa 2024. Oct 22-24; Johannesburg, South Africa; africahealthexhibition.com
BCLF 2024 – 31st Meeting of the Balkan Clinical Laboratory Federation & 35th National Congress of the Turkish Biochemical Society. Oct 28 - Nov 1; Antalya, Turkey; turkbiyokimyadernegi.org.tr
APFCB Congress 2024 – Asia Pacific Federation for Clinical Biochemistry and Laboratory Medicine. Oct 31 - Nov 3; Sydney, Australia; apfcbcongress2024.org
NOVEMBER
ALACI 2024 - 14th Latin American and Caribbean Immunology Congress. Nov 4-8; Buenos Aires, Argentina; alaci.org
ASHG 2024 – Annual Meeting of the American Society of Human Genetics. Nov 5-9; Denver, CO, USA; ashg.org
JIB 2024 - Journées de l’innovation en biologie. Nov 7-8; Paris, France; jib-innovation.com
46 Annual ACBI Conference 2024 – Association of

Clinical Biochemists in Ireland. Nov 8-9; Dublin, Ireland; acbi.ie
MEDICA 2024. Nov 11-14; Dusseldorf, Germany; medica-tradefair.com
45th Annual Meeting of the American College of Toxicology (ACT). Nov 17-20; Austin, TX, USA; actox.org Analytica China 2024. Nov 18-20; Shanghai, China; analyticachina.com.cn
AMP 2024 – Annual Meeting & Expo of the Association for Molecular Pathology. Nov 19-23; Vancouver, BC, Canada; amp.org
DECEMBER
ICID 2024 – 20th International Congress on Infectious Diseases. Dec 3-6; Cape Town, South Africa; isidcongress.org
66th ASH Annual Meeting and Exposition – American Society of Hematology. Dec 7-10; San Diego, CA, USA; hematology.org
2025
JANUARY
SLAS 2025 – International Conference & Exhibition of the Society of Laboratory Automation and Screening. Jan 25-29; San Diego, CA, USA; slas.org
FEBRUARY
Medlab Middle East 2025. Feb 3-6; Dubai, UAE; medlabme.com

Events Calendar
23 LabMedica International February-March/2024

132 LMI-4-23 LINKXPRESS COM We make diagnostics that matter At SEKISUI Diagnostics, what matters to you matters to us. Our whole purpose is to partner with you to provide intelligent solutions that enable you to make a timely difference. Because we both understand that there is a patient behind every answer—and that’s what matters most. For more information about our assays and systems, please visit sekisuidiagnostics.com or email us at questions@sekisui-dx.com Clinical Chemistry • Point-of-Care • Enzymes • Pre-Analytic Systems © 2023 SEKISUI Diagnostics, LLC. All rights reserved. Because every result matters is a trademark of SEKISUI Diagnostics, LLC. 124 LMI-3-24 LINKXPRESS COM







































































































































