


































































The Insulation tester, Leak Tester Tester, Shaver Leak Tester and Cable Continuity Tester are easy-to-use devices for verifying the functionality of equipment for safety
McGan Insulation Tester
Detect & locate defects such as pinholes, cracks and bare spots in the jacket or coating of laparoscopic and bi-polar electrosurgical instruments

Test the functionality of automated & handheld endoscope leakage testers with healthmark’s new Leak Tester Tester.

Test the quality of monopolar and bipolar cords with this user-friendly, durable device. A green light noti es the user that the cable passed testing.

Shaver Leak Tester

Designed for pressure testing arthroscopic shavers to help identify leaks caused by failing seals that degrade over time from repeated use and processing.
For more of Healthmark’s intelligent solutions for instrument care & infection control, visit hmark.com hmark.com | 800.521.6224 | healthmark@hmark.com












P.12 SPOTLIGHT
p.12 Professional of the Month: WO1 Tyler J Scott, M.S.
p.14 Association of the Month: Oklahoma City Biomedical Association (OKCBA)
p.16 Next Gen: Kaleb Kasitz
p.18 Department of the Month: The Christ Hospital Healthcare Technology Management Department
p.20 Company Showcase: Collaborative Medical Solutions
P.22 INDUSTRY UPDATES
p.22 News & Notes
p.30 Ribbon Cutting: Emeritus Clinical Solutions
p.32 AAMI Update
p.34 ECRI Update
p.36 Tech Choice Awards Nominations

P.40 THE BENCH
p.40 Biomed 101
p.43 Tools of the Trade
p.44 Webinar Wednesday
P.46 FEATURE ARTICLES
p.46 Roundtable: CMMS
p.52 Corporate Profile: Tenacore
p.56 Cover Story: Non-Conformance Challenge: Repair as Needed Benefits Explained
P.63 EXPERT ADVICE
p.63 Career Center
p.64 SPONSORED: Avante Health Solutions

46
p.66 Networking Notes
p.68 SPONSORED: TKA
p.70 The Future
p.72 SPONSORED: Innovatus Imaging
p.74 Cybersecurity
p.78 Health-ISAC
PUBLISHER John M. Krieg
VICE PRESIDENT Kristin Leavoy
VICE PRESIDENT Jayme McKelvey OF SALES
EDITORIAL John Wallace
CONTRIBUTORS Roger Bowles
K. Richard Douglas
Jim Fedele
Joie Marhefka
Manny Roman
Steven J. Yelton
ACCOUNT Megan Cabot
EXECUTIVES Emily Hise
ART DEPARTMENT Karlee Gower
Taylor Hayes Kameryn King
DIGITAL SERVICES Cindy Galindo Kennedy Krieg Haley Harris
EVENTS Kristin Leavoy
WEBINARS Linda Hasluem
HTMJOBS.COM Kristen Register Sydney Krieg
ACCOUNTING Diane Costea
CIRCULATION Joanna Manjarrez
Rob Bundick, Director HTM & Biomedical Engineering, ProHealth Care
Carol Davis-Smith, CCE, FACCE, AAMIF, Owner/ President of Carol Davis-Smith & Associates, LLC
Jim Fedele, CBET, Senior Director of Clinical Engineering, UPMC
Bryant Hawkins Sr., Site Manager, Children’s Hospital of New Orleans
Benjamin Scoggin, MBA, MMCi, Director, Clinical Engineering | Biomedical Operations, Equipment Distribution, Clinical IT, DHTS, Duke Health Technology Solutions
Allison Woollford, Biomedical Equipment Specialist at Duke University Health System
Bryant Hawkins Sr., HTM on the Line, Trimedx Site Manager at Children’s of New Orleans
Dr. Brian Bell, HTM Workshop, Faculty Biomedical Engineering at St. Petersburg College in St. Petersburg, Florida
Carlos Villafane, BMET Latino, Certified Biomedical Engineering Technician III, Baycare Health Systems
Chace Torres, Bearded Biomed, Lead Technician SPBS, Dallas-Fort Worth Metroplex

p.83 Bulletin Board Sponsored by MedWrench
p.84 [Contest] What’s on Your Bench?
p.85 Biomed Brainbuster
p.86 HBA Scrapbook
p.87 CABMET Scrapbook
p.88 NCBA Scrapbook
p.98 #HTMLife
p.92 Preferred Vendors
p.94 Service Index
p.97 Alphabetical Index







At the forefront of Healthcare Technology Management education, the College of Biomedical Equipment Technology (CBET) is redefining what it means to be a leader in this critical field. Our cutting-edge programs in Biomedical Equipment Technology (BMET) and Healthcare Information Systems Management (HISM) are designed to equip you with the advanced skills needed to excel in today’s healthcare landscape.
At the forefront of Healthcare Technology Management education, the College of Biomedical Equipment Technology (CBET) is redefining what it means to be a leader in this critical field. Our cutting-edge programs in Biomedical Equipment Technology (BMET) and Healthcare Information Systems Management (HISM) are designed to equip you with the advanced skills needed to excel in today’s healthcare landscape.

BY K. RICHARD DOUGLAS
Colonel William Lordan Keller was an Army doctor and surgeon who served during World War I and later as the chief of the surgical service at Walter Reed General Hospital. After retiring in 1935, he went on to be appointed by President Franklin D. Roosevelt as a lifetime consultant in surgery at Walter Reed. His contributions to Army medicine left a legacy.
Naming a hospital in his honor was part of that legacy.
Keller Army Community Hospital can be found in West Point, New York. West Point is also home to the nation’s first military academy. West Point prepares young men and women to become officers in the U.S. Army. Cadets attend the academy for four years, graduating with a Bachelor of Science degree and a commission as a second lieutenant.
Keller Army Community Hospital Warrant Officer Tyler J. Scott, M.S., is chief of the equipment management branch at the hospitals.
“I come from a long lineage of military members, but was unsure if the military was for me, so I decided that I would instead go to college. I was unsure what I wanted to do for a career upon my completion of college. My father, Mark Scott, had worked on communication equipment while he served in the U.S. Army, and he suggested that I might like a career in repairing equipment,” Scott says.
He says that his dad was absolutely right.
“He encouraged me to become a 68a biomedical equipment technician and I grew a passion for repairing/
maintaining equipment. I have since spent 10 years in the field and progressed to becoming a 670a health services maintenance technician operating at Keller Army Community Hospital in West Point, New York,” Scott adds.
He says that he attended an 11-month biomedical equipment technician program at the medical education and training campus at Fort Sam Houston, Texas as part of his advanced individual training to become a 68a biomedical equipment specialist.
Scott explains that the biomedical equipment specialist (68a) is someone responsible for servicing and maintaining all medical equipment, calibrations and preventative maintenance, installations and keeping records of services performed.
“I also attended the warrant officer basic course at Fort Sam Houston as part of my 670a health services maintenance technician program. I have also completed the 70K medical logistics officer’s course to gain a wider scope of logistics as a whole,” Scott says.
He started his biomed career at the 14th Combat Support Hospital in Fort Benning, Georgia. He deployed with that unit three times to Kosovo, Iraq and to Puerto Rico during Hurricane Maria.
“I then became the chief of operations of the United States Army Medical Materiel Agency Medical Maintenance Operations Division Utah at Hill Air Force Base, Utah. From there, I became the Chief of the Equipment Management Branch at Keller Army Community Hospital, West Point, New York,” Scott says.
Currently, Scott’s area of specialty is project and life cycle management.
“I ensure that my staff acquire, maintain, sustain and repair our medical equipment within Keller,” he says.
Scott is a demonstrated logistician through the International Society of Logistics.
One of the challenges in the biomed field is staying up to date with the ever-changing medical field.
“The medical device industry is advancing so quickly, by the time you think you have the newest and greatest equipment; something new is on the market. This is great for us biomeds, though as we get to work on the latest and greatest equipment and integrate more with the information technology personnel. This allows us to have a greater presence among the hospital and build strong cohesive teams,” Scott says.
When not working, Scott remains the technician and keeps tools close at hand.
“I enjoy working on and repairing anything I can get my hands on from vehicles to Roomba robot vacuums. I also enjoy the outdoors and going fishing. I am a huge large-mouth bass fisherman,” he says.
Scott was recently married to his fiancée, Andrea Mills, in Central Park.
“We have a 10-year-old son, Braxton, and a 10-year-old daughter, Makenzie,” he says.
Scott still has several goals as he continues through his career.
“I want to continue to make a positive impact on the HTM field. I want to build strong independent teams that have the same passion as I do. I desire to eventually work at a strategic level of planning and development. My overall goal is to integrate emerging technologies to provide quick, accurate and precise diagnosis of patients,” he says.
The U.S. Army is lucky to have an HTM expert of Scott’s caliber.



FAVORITE BOOK:
“The Reaper” by Nicholas Irving
FAVORITE MOVIE:
“Black Hawk Down”
HIDDEN TALENT:

The ability to remember almost everything I have ever heard. I am great with random knowledge.
FAVORITE FOOD:
Texas Roadhouse ribeye rare with mushrooms
FAVORITE PART OF BEING A BIOMED?
Working with a close-knit team that ensures patient safety by placing life-saving equipment into the hands of providers.
WHY DO YOU READ TECHNATION?
To stay up to date with anything and everything HTM-related.
BY K. RICHARD DOUGLAS
Ok lahoma City is the capital and most populace city in the state of Oklahoma. It sits in the central portion of the state. The city offers visitors a cowboy and western museum, a banjo museum, a zoo, a botanical garden, walking trails to a water taxi cruise down the Bricktown Canal and more. The city juxtaposes its western heritage with its modern accouterments.
The biomed community is represented by an association focused on bringing biomeds together. The Oklahoma City Biomedical Association (OKCBA) evolved after a delayed beginning.
“There were several attempts by the original members going back to 1989, but the first official meeting was in 1994 at Southwest Medical Center with Juan Lara, Steve McCartney, Bob Thompson, Ken Reimer, Gene Butler and Larry Henderson. There may have been others, but these were the names that could be remembered,” says OKCBA President Shannon Landsberger.
He says that Juan Lara, Steve McCartney, Bob Thompson and Ken Reimer are credited as the founding members.
“Juan Lara had the initial idea of creating a biomed association and was the one who attempted to get meetings together from 1989 to 1994. He worked at
Midwest Regional Hospital at the time. Steve McCartney was voted in as president of the association early on and kept it going until October 2017 when I was voted in as president. Steve still continues supporting the association as vice president today,” Landsberger says.
He says the original group maintained annual meetings (often at restaurants) from 1994 through 2017, typically around October.
“Steve McCartney was president during most of that timeframe, with me joining the group in 2016, and becoming president in 2017. At that time, Steve stepped down into the vice president position. In 2018, we changed to a bi-annual meeting schedule with April and October being the months we typically planned for. I have worked towards bringing more content to our meetings, with a focus on continued education,” Landsberger says.
OKCBA typically has vendor education as well as discussion topics related to the biomed field at each meeting.
“I also added in a spotlight on retirees, whom we do a more in-depth review of their career with them during the meeting after they retire, often turning into some educational discussions on career plans. Moving forward, I am working with others in the group to move to a quarterly biomed association meeting, which will hopefully entail vendor education at every single meeting, and rotate hospitals in the area so that we are not favoring one facility over another,” Landsberger says.


The OKCBA does not have a formal conference or symposium, although it makes the most of its scheduled meetings. Landsberger tries to get the most productive use out of the regular meetings.
“Our last meeting consisted of a discussion about Ken Reimer, a long-time member and local biomed company owner who recently retired. Ken sponsored nearly all of our meetings until our last meeting before his retirement in October of 2023. I gave a 30-plus minute discussion/code review on AAMI ST108 and its impact on facility design, biomed interaction, and the positive impact of high-water quality in our SPD areas,” Landsberger says.
He says that the group also had plenty of discussion around the general status of the biomed space in Oklahoma including a talk about outsourcing, open positions and future meetings changing to four times per year.
As the HTM profession still seeks methods to recruit new talent to replace the baby-boomer retirees, it has been the biomed associations, in their respective cities and states on the front lines.
“Our biomed association meetings are 100 percent free. We make it a point to let all of our traveling vendors and sister hospitals know about the meetings as well as when to attend meetings, and our influx of new people is fairly steady. I have about 70 members on my email list at this time, and our attendance has grown from an average of five to seven people per meeting in 2016, to around 20 per meeting now in 2024,” Landsberger says.
He says that in Oklahoma, there is no requirement for certification or an education minimum to be a biomed.
“Due to this, biomed is not always recognized as an occupation with a high-level skillset and pay remains relatively low for many. I want to work towards getting our membership AAMI-certified, with continuing education credit mostly provided during our meetings as educational content from vendors. Once this is established, I
will work towards discussions with hospital leaders about the benefits that come with a certified and better educated biomed shop,” Landsberger says.
He says that the intent is for hospital organizations to see the value and offer incentives for those who become certified.
“Once the pay level starts to increase, I think that will help draw in more applicants to the region,” Landsberger adds.
Some biomed associations have a local technical school or dedicated biomed training program that the association can build a relationship with.
“We do not have any biomed schools in Oklahoma, and many of the technical schools have moved away from an in-depth electronics program in favor of short, focused courses that can be completed in a few weeks each. This has limited our ability to recruit biomedical staff locally, and we must often look to biomed programs in other states for added personnel,” Landsberger says.
How did the pandemic impact the way the association operates or affect its members?
“Operationally, it slowed progress down quite a bit, forcing places and personnel to perform better planning around shipment and install timeframes. There was also a need to increase contingency funds on projects a bit more to accommodate the rapid increase in the price of goods and services. Vendor labor prices have risen significantly in the last several years, and I believe it will have a long-term effect on the cost-of-service ratio (COSR) for facilities. Until things stabilize, there is likely to be some added financial tension between biomed groups and administration regarding the added costs for maintenance,” Landsberger says.
Biomeds in the Sooner State have a place to rub elbows, catch up on the community and receive education in centrally located Oklahoma City.
Kaleb Kasitz
Kaleb Kasitz earned a Bachelor of Science in Biological Engineering at the University of Missouri. He currently serves as a Biomedical Engineering Technician II with the Choctaw Nation of Oklahoma Durant Regional Medical Clinic. Kasitz enjoys the problem-solving aspect of his biomed career. TechNation found out more about the Kansas native during a Q&A.
Q: WHERE DID YOU GROW UP?
A: Louisburg, Kansas.
Q: WHERE DID YOU RECEIVE YOUR HTM TRAINING/ EDUCATION?
A: Received a B.S. in Biological Engineering from the University of Missouri and have gained multiple training certificates from different manufacturers on their equipment throughout the years.
Q: HOW DID YOU FIRST DISCOVER HTM?
A: Leaned about the field in college, but more so after when I was applying for jobs and was having a hard time breaking into the industry on the R&D side of biomed.
Q: HOW DID YOU CHOOSE TO GET INTO THIS FIELD?
A: After college I was really trying to get a job in the R&D side of biomed devices but was having a hard time breaking into the industry with no experience aside from my education. So, I took a different approach to the industry and found a clinical biomed job, started there and have progressed in that over the years.
Q: WHAT DO YOU LIKE MOST ABOUT YOUR POSITION?
A: The thing I like most about my position is that it is a good mix of engineering problem solving and that mindset mixed with the


direct impact of helping people in their daily lives by facilitating their medical care.
Q: WHAT INTERESTS YOU THE MOST ABOUT HTM?
A: The most interesting part of HTM is that the field is always changing. Medical equipment and technology are always advancing making it so that there is always something new and challenging to learn and grow with.
Q: WHAT HAS BEEN YOUR GREATEST ACCOMPLISHMENT IN YOUR FIELD THUS FAR?
A: My greatest accomplishment so far has been receiving an IHS award for excellence in customer service. Due to the nature of this award, it was nominated by a peer for my dedication and accomplishments for the company and health care in the region as a whole. The fact that I was nominated and recognized by my peers and chosen from a large field of nominees for this award makes it special and a great accomplishment.
Q: WHAT GOALS DO YOU HAVE FOR YOURSELF IN THE NEXT 5 YEARS?
A: To continue to grow in the field and hone my skills and move up in the HTM industry.
FAVORITE HOBBY: Traveling
FAVORITE SHOW OR MOVIE: “Letterkenny”
FAVORITE MEAL: Love to cook at home and try new things.
WHAT WOULD YOUR SUPERPOWER BE? Green Lantern Ring of Power
1 THING ON YOUR BUCKET LIST: Hot air balloon ride over Cappadocia, Turkey.
SOMETHING YOUR CO-WORKERS DON’T KNOW ABOUT YOU:
I was a competitive water skier in college. I lived in Australia for a year. I love to cook and try new foods.


BOOTH 501 B BOOTH 501


Flat & Fixed Rate Repairs
New AIV-Manufactured Parts & Pump Accessories





New AIV-Manufactured Fetal Monitor Transducers


AIV-Certified Refurbished Pumps Replacement Case Kits for GE Telemetry






BY K. RICHARD DOUGLAS
Th e Christ Hospital in Cincinnati, Ohio, has been caring for patients for more than 130 years. The hospital has developed into a health network that includes several facilities and more than 100 physician locations.
The extensive network requires management of 30,000 devices with an approximate $210 million replacement value.
“HTM is responsible for hospital, AOC, physician offices, nursing school equipment, research center, across Ohio, Kentucky and Indiana,” says Greg Herr, director of healthcare technology management (HTM) department at The Christ Hospital.
The network’s HTM department has 32 full-time employees, which includes two managers, a supervisor of business operations and a biomed equipment lead. The team also has three specialist BMET 2s, a respiratory specialist BMET 2, two specialists for anesthesia, an imaging team with three specialist 3s, two specialist 2s and a specialist 1. The department also has an IT medical security specialist. There are also other biomeds and specialists that round out the diverse team.
“HTM handles systems in cath/hemo, EP, oncology, surgery, infusion pumps (two-way integration), data integration engine (data capsule), several different PACS (cardiology, orthopedics, OB/neonatal, vascular, and just
in process of implementing a new all-in-one 3D portal for cardiology, and next radiology.) Starting work on enterprise imaging architecture,” Herr says.
“The department has one team dedicated to CE/IT integration and works with the biomedical and imaging team to ensure equipment is integrated safely with proper documentation,” Herr says.
Herr says that service contracts are centralized in HTM except for lab, a few in the operating room and central. He says they are managed by the business operations supervisor and director, and coordinated with purchasing and legal.
In terms of data collection, when onboarding new equipment, the process to capture required information and a master class table is used to reduce errors and duplicate descriptions.
“Data is collected from the purchasing process, much of the medical capital – but not all – goes through HTM. The CMMS vendor reviews new system entries, cleans up master equipment index names, and other data quality. Data collection for contracts are collected as contracts are signed and POs created. Date, amount, vendor, term of contract and coverage are identified,” Herr says.
He says that a new process is being developed to streamline workflow and get more accurate data.
The department is involved in several projects with IT, nursing and other clinical services.
Some of those projects include new equipment in the


NICU, cath lab replacements and an imaging wing at Liberty Medical Center.
“During COVID, HTM moved systems from hospital to hospital, setup a temporary ICUs, and converted anesthesia machines to ventilators during the worst of the crisis. Today, mostly recovered, the department is still getting back on track with projects delayed at the time,” Herr says.
He says that the team also worked on an EP project to develop a new five-room suite, with ancillary support areas. It has been a project for 30 months and was recently completed.
“HTM planned and moved much of the existing systems. Included new imaging systems, but also HTM worked out new team sharing in the room, lighting and sound needs. Now, the department is swinging back to the old area and developing it for IR/ vascular,” Herr says.
“During COVID, HTM moved systems from hospital to hospital, setup a temporary ICUs, and converted anesthesia machines to ventilators during the worst of the crisis.”
Herr says that a few years ago, the hospital lost considerable dollars with fridge/freezer failures, either because to the devices failed or loss of power. The health care system was searched and the system began targeting high-risk fridges/freezers with drugs (pharmacy or lab), food and nutrition, off-site physician offices for vaccines/drugs, and med fridges in nursing units, Herr adds.
- Greg Herr
He adds that a current big project is a centralized monitor unit, to collapse all the unit stations into one centrally located system.
The HTM team has also applied its collective expertise to problem-solving.
He says that HTM worked with users on alarm escalation, managed continuity and helped clinical users develop standardized practices using the temp tracking software. This is an ongoing project that is already several years in duration. The health care system lost only small amounts of items in a physician office fridge with loss of power when the owner did not respond in time. Otherwise, it has cut losses substantially.
At The Christ Hospital, the healthcare technology management department keeps the large inventory operating and refrigerators cold.
Collaborative Medical Solutions Inc. is built on a proven foundation with more than 15 years of helping domestic and international clients manage previously owned medical imaging equipment. Collaborative Medical Solutions is successful helping clients capture the best value for their used equipment and creating an easy path to upgrade technology as well as capture cash from their assets.
“We pride ourselves on making the process easy, providing great value and being well funded to create fast and easy transactions,” the company’s website states.
Collaborative Medical Solutions Inc. Principal Trey McIntyre recently shared more information about the company with TechNation
Q: CAN YOU TALK ABOUT THE COMPANY’S HISTORY AND HOW IT ACHIEVES SUCCESS?
MCINTYRE: We formed Collaborative Medical after nearly 20 years in the pre-owned medical imaging equipment space. Then, five years ago, it was an international client from those early days in that business who came to us with an endoscopy opportunity. We had become friends over the years, and it was great to connect again and work together. While endoscopy equipment was somewhat of a new category, the root of what we do remains the same. Relationships matter to us, so we take a collaborative approach with our customers, and work hard to offer flexible solutions for their biggest problems.
We’ve had a lot of success by helping providers rethink scope repair strategies, create simple paths to upgraded technology, and better manage their assets.
Q: WHAT ARE YOUR UNIQUE SELLING POINTS?
MCINTYRE: We think of ourselves as well-rounded, meaning that we have built an in-house repair lab with expert technicians and rigorous quality measures. We’ve maintained the right inventory of patient-ready, cost-effective equipment – and it all comes together to create strong maintenance, loaner and equipment upgrade programs which have proven to be powerful and dynamic for our customers.

Q: WHAT ARE SOME CHALLENGES THAT YOUR COMPANY FACED LAST YEAR?
MCINTYRE: Last year, we focused on investing in a great new facility in the Charlotte, North Carolina area. Space had quickly become a challenge for us as we were growing the business but not our building. It was a massive project, and of course, there were delays along the way, but as of January 1, we’re operating from a purposely designed space where our team can thrive and grow into our next phase.
Q: WHAT PRODUCT OR SERVICE THAT YOUR COMPANY OFFERS ARE YOU MOST EXCITED ABOUT RIGHT NOW?
MCINTYRE: We’ve done away with the traditional maintenance contract style, and instead we put together a program that really aligns everything we do to solve the biggest challenges our customers

face. Our Collaborative Approach is a scope maintenance program that helps plan for the future and reduce downtime while avoiding the unnecessary costs of managing endoscopy assets.
Q: CAN YOU DESCRIBE YOUR COMPANY’S FACILITY FOR TECHNATION READERS?
MCINTYRE: As I mentioned, we’re headquartered just outside of Charlotte, a great location minutes from the airport and the uptown area. While we’re still enjoying the fresh new space, we moved into earlier this year, we’re already committed to our expansion: another new building which will double our current square-footage. From here, our team will grow, our repair capabilities will continue to increase and we’ll launch advanced training programs. We’ve built our current facility with an open concept, and there’s a lot of interaction between our lab and the office teams. And even though we are team-minded, we love visitors and always welcome customers to come see us! We’re proud of the team and the space we’ve put together and certainly want to share it.


year, he brought with him decades of experience and a clear vision of the endoscopy repair lab he wanted to build. From working directly with OEMs, hospital administration, doctors, and technical staff, he knows the pain points that providers face every day. He’s designed our quality and maintenance programs with this in mind.
Our Collaborative Approach is a scope maintenance program that helps plan for the future and reduce downtime while avoiding the unnecessary costs of managing endoscopy assets.
Q: PLEASE TELL ME ABOUT YOUR EMPLOYEES AND HOW THEY CONTRIBUTE?
MCINTYRE: When Alan Koreneff joined the team last
The senior technicians he has hired are among the most talented in the repair industry, and we’re thrilled to have them on board. The techs at our benches have an average of 15 years spent with their hands on flexible scopes from all major brands.
Q: IS THERE ANYTHING ELSE YOU WANT READERS TO KNOW ABOUT YOUR COMPANY?
MCINTYRE: If you want a new approach to scope maintenance, I’d encourage you to talk with us. Discover what our Step-Up program would mean to your facility, or how our transparent scope evaluations ultimately reduce your risk, and much more. We’ll get creative with you, and we’ll keep it simple. Our solution can be comprehensive, it can be flexible – but it will always be collaborative.
For more information, contact CMS at Support@CMSscopes.com.
Bryant Hawkins Sr., a leading voice in the healthcare technology management (HTM) industry and host of the acclaimed podcast “HTM On The Line,” proudly announces the release of his first book, “Dare To Be Great.” Scheduled for publication on October 1, 2024, this inspirational work offers a blueprint for those seeking to unlock their full potential and rise to greatness in their personal and professional lives.
Hawkins is a recognized HTM leader. He won the inaugural Tech Choice Award for Influencer of the Year and serves on the TechNation Digital Advisory Board.
“Dare To Be Great” distills Bryant Hawkins’ years of experience as a motivational speaker, industry leader and mentor into a powerful narrative that challenges readers to embrace the discipline, commitment, and mindset necessary to achieve their dreams. Drawing on real-life examples, including stories of triumph over adversity and insights from some of the HTM industry’s successful figures, Hawkins provides actionable strategies to help readers navigate their paths to success.
“I wrote ‘Dare To Be Great’ to inspire individuals to pursue their highest ambitions, to step out of their comfort zones, and to transform their lives through a firm commitment and relentless work ethic,” said Hawkins. “This book is for anyone who dares to dream big and is ready to take the necessary steps to turn those dreams into reality.”
Key themes in the book include:
• Power of Consistency
• Personal Accountability
• Overcoming Obstacles
• Inspiring Quotes and Insights
“Dare To Be Great” is not just a book; it’s a movement. It’s

an invitation to anyone ready to elevate their life, to break free from mediocrity, and to pursue excellence with passion and purpose, a press release states.
Hawkins is the founder of “Elevating HTM”; a non-profit organization dedicated to inspiring the next generation of HTM professionals. He is also the host of the popular podcast “HTM On The Line,” now in its second year, where he has interviewed over 50 distinguished guests. Known for his motivational speaking, motivational Monday videos and HTM newsletters, Hawkins has a passion for helping others achieve success by sharing the principles that have guided him throughout his journey.


UptimeHealth, in collaboration with the College of Biomedical Equipment Technology (CBET), is excited to announce the Dental Fix Summit 2024, taking place October 3-6, 2024, at the Embassy Suites by Hilton Nashville Airport. This four-day event is designed to equip independent dental equipment repair technicians and biomedical technicians with the latest technical training, business strategies and networking opportunities in the dental industry.
The Dental Fix Summit 2024 is a unique opportunity for technicians to advance their skills and knowledge through hands-on workshops, insightful sessions and interaction with industry leaders. Attendees will have access to OEM technical training and business solutions tailored to the needs of independent service organizations. This event also offers a chance to earn continuing education units (CEUs), enhancing the professional growth of participants.
Key Highlights of Dental Fix Summit 2024:
• OEM Technical Training: Attendees will receive comprehensive training on the latest dental equipment and technology from original equipment manufacturers (OEMs), ensuring they stay ahead in the fast-evolving dental market.
• Business Growth Opportunities: Independent technicians will learn how to expand their businesses, leverage shared resources, and utilize group buying power to access discounts on merchant processing, accounting services, freight rates and other essential business solutions.
• Networking and Community Building: The summit will foster a sense of community among like-minded professionals, offering invaluable networking opportunities to build connections that can support long-term business success.
• Expanded Skill Sets for Biomedical Technicians: Biomedical technicians will have the chance to level up their expertise by adding dental services to their skill sets, opening up new avenues for career growth.
“This collaboration between UptimeHealth and CBET represents a significant step forward in providing top-notch education and resources to the biomedical and dental equipment repair community. We are committed to supporting these technicians as they continue to drive innovation and excellence in their field,” said CBET President Richard “Monty” Gonzales.
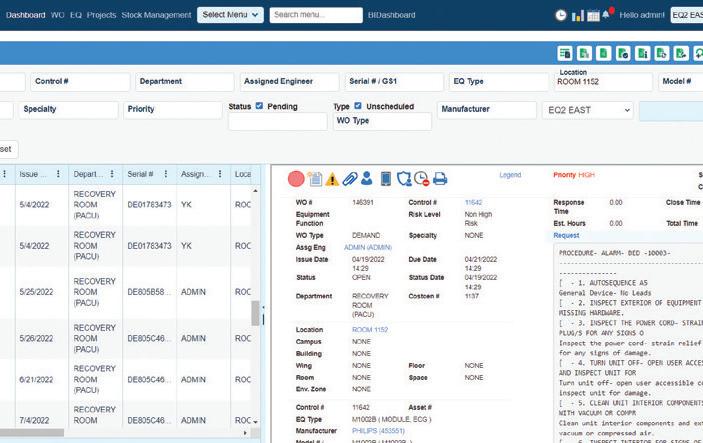
EQ2 HEMS: A "Smart CMMS" that Makes Everything from Managing Work Orders to Maintaining Compliance, Reporting, and Capital Planning Easier
Numerous integrations, to streamline workflows
Superior regulatory compliance reporting included
Mobile app designed for offline and online work means you can always access what you need when you need it
Data and information in the format that fits the needs of all levels of management and the hospital as a whole



St. Philip’s College (SPC) hosted Legacy Day: The Legacy Continues on Friday, Aug. 16, to celebrate the feast day of the college’s founding president, Saint Artemisia Bowden, and unveil three newly renovated campus buildings.
Saint Artemisia Bowden was canonized by the Episcopal Church in 2015. Her feast day is recognized worldwide on Aug. 18 in the Episcopal calendar. The same year she was elevated to sainthood, she was named President Emeritus by the Alamo Colleges District in recognition of her leadership and service to SPC.
The three-hour event showcased the revitalization of the Cybersecurity Innovations Center, the Bowden Legacy Building, and the Applied Science Building, while highlighting the college’s rich history with a ribbon cutting and tour of each building.
The Cybersecurity Innovations Center, previously named the Campus Center, has been transformed into a central hub for government, university, and industry partners in the cybersecurity field. Over the last 70 years, this building underwent four renovations, enlarging its capacity in 1970, 1978, 1987, and 2022.
The Bowden Legacy Building, constructed in 1954 on the original land purchased by Artemisia Bowden in 1922, is now home to the St. Philip’s College Early College High School with
San Antonio ISD. This building honors the college’s founder, continuing her legacy of inspiring future generations.
The Applied Science Building has been a hub of innovation and hands-on learning since its construction in 1992. Renovations have expanded the college’s program offerings in Automotive Technology, Histology, Biomedical Engineering, Vision Care, and Nurse Aide.
Legacy Day activities included check presentations, refreshments, a cornerstone ceremony by area Masonic lodges, gifts to mark the occasion, and remarks by special guests, including Saint Artemisia Bowden’s great-great-niece, Jennifer Walker. To connect the three locations there was a New Orleans-style Second Line Parade, led by the SPC cheer team, Student Government Association student leaders, employees with decorated umbrellas, and a drumline.
“As we celebrate the transformation and expansion of St. Philip’s College, we remember our 126-year heritage and history,” said St. Philip’s College President Dr. Adena Williams Loston. “On this Legacy Day, we honor the unwavering determination of one woman—our founding president, Saint Artemisia Bowden. Her 52 years of devotion to the college leaves us with a sense of pride that challenges us to strive for greatness.”

Biomedical device test and measurement specialist Rigel Medical has agreed to a new partnership with a U.S. government and military contractor as it continues to expand its North America operations.
The collaboration will see Tampa-based Rigel Medical link-up with small business certified service-disabled and veteran-owned (SDVOSB) MEAK Solutions in a move that marks a “significant step forward” in developing and improving the supply and support of biomedical test equipment provided to U.S. government and military sectors.
Working together, both companies will bring their respective technologies and specialist expertise to a broader market, while driving the development of solutions for customers, including additional reach into military veterans’ hospitals and healthcare facilities across the country.
Rigel Medical testers comply with U.S. regulations, so leveraging MEAK’s SDVOSB status will effectively enable the company to meet the requirements of VA hospitals with sector leading biomed equipment solutions, said Lewis Lennard, product manager-North America.
“MEAK Solutions’ SDVOSB status positions us perfectly to serve government agencies, particularly the Department of Veterans Affairs (VA),” he added. “Our new partnership allows us to provide VA hospitals with the high-quality biomedical test equipment, ensuring the best care for the country’s veterans. Indeed, supporting VA hospitals with advanced biomedical solutions directly contributes to the health and well-being of veterans, aligning with our commitment to improve patient care.”
Located in Mentor, Ohio, MEAK Solutions specializes in providing solutions for government, military, and commercial clients. The company has more than 50 years of combined industry experience and a best-inclass quality assurance team to meet government and industry requirements.
Eric Kettani, director at MEAK, said: “We are excited to partner with Rigel Medical, which has a strong track record of delivering innovative biomed testers into the U.S. market. We will be working together to strengthen our offering and in partnership, we will be committed to advancing the development of innovative applications and solutions for the benefit of VA hospitals and their patients.”
Seaward Group USA supplies a wide range of electrical safety instruments to ensure the safety of any product or appliance from the design, compliance and manufacturing stages through to servicing repair and calibration. This includes advanced technology laboratory and production line testers capable of carrying out hi-pot, ground bond and a variety of other tests, as well as EMC test equipment.
The company has an extensive national U.S. sales, service and calibration capability.

The Oregon Biomed Association recently announced the dates for its 2024 Expo & Education Vendor Fair. The event will be held November 7-8 at Holiday Inn Portland-Airport (8439 NE Columbia Ct, Portland, OR 97220).
This is the first OBA Expo since before the COVID pandemic. It will feature educational seminars each day. Breakfast, lunch and

snacks will be provided with registration.
The exhibit hall will host the vendor fair with a happy hour, prizes and awards!
The event is FREE for OBA members! Visit www.orbmet.org/ expo2024 for details.

Spearhead-class expeditionary fast transport ship USNS Burlington (T-EPF 10) arrived in Colón, Panama on Aug. 18, 2024 as part of U.S. Naval Forces Southern Command/U.S. 4th Fleet’s Continuing Promise 2024.
Panama is the final stop on this iteration of the Continuing Promise mission, which has seen previous stops in Jamaica, Costa Rica, Honduras, and Colombia. This visit to Panama is the seventh such visit for Continuing Promise since the exercise first began in 2007, and first visit to Colón since 2019. Burlington served as the primary ship for Continuing Promise 2023, which stopped in Almirante, Panama.
“We are excited for Continuing Promise’s return to Panama,” said Lt. Cmdr. Zachary Smith, Continuing Promise 2024 mission commander. “We have seen so much success on this mission with our various partners and we aim to finish strong with our
partners here.”
Thirty U.S. Navy medical professionals specializing in dentistry, family and geriatric medicine, optometry, nursing, pharmacy, biomedical equipment repair, land radiology will partner with Panamanian Ministry of Health personnel and civilian medical professionals to provide care at Clinica Dr. Hugo Spadafora and Parque de Juventud during the mission’s visit to Colón.
Along with medical personnel, the CP24 team also includes a detachment from the U.S. Fleet Forces Band called “Uncharted Waters.” The band will perform and conduct music clinics at seven separate events throughout the visit.
Panama is the last mission stop of Continuing Promise 2024. After departing Panama, USNS Burlington will sail back to the United States to complete the mission.
— Story from Story by Lt.j.g. Daniel Ehrlich
TRIMEDX – an industry-leading, independent clinical asset management company delivering clinical engineering services, clinical asset informatics, and medical device cybersecurity – has been recertified with the highly regarded International Organization for Standardization’s Medical Device Quality Management System (MD QMS) certification ISO 13485, a widely recognized international standard outlining best practices in developing an effective MD QMS that covers the safety and quality of medical devices throughout their lifecycle.
This recertification, now valid through Aug. 2027, reverifies that TRIMEDX continues its dedication to maintaining a comprehensive quality management system (business policies and procedures), ultimately impacting its service excellence to clients.
“This reaffirms our commitment to excellence for our clients, associates and stakeholders,” says TRIMEDX CEO Henry Hummel. “Our quality of service standardizes work and contributes to continuous improvement as we progress. By verifying these processes, we aim to achieve best-in-class customer satisfaction, quality service and, ultimately, impact clients’ ability to ensure patient safety.”
This standard analyzes and evaluates everything TRIMEDX does from a risk perspective, so risk control is included in all parts of the business. ISO 13485 outlines best practices in

developing an effective MD QMS that covers the safety and quality of medical devices throughout their lifecycle. TRIMEDX was recertified by third-party registrar DEKRA.
The ISO 13485 certification couples with TRIMEDX’s Service Organization Control (SOC) 2 Type 2 and ISO 27001 certification (valid through Dec. 2026) to make up TRIMEDX’s Quality Management System covering MD QMS and Information Security Management System (ISMS) standards.
“Achieving this level of compliance requires a continued commitment and demonstrated ability to monitor system activity and quickly take corrective action with the people, process, and technology in place,” says Denisa Lambert, vice president of quality and regulatory compliance. “Our comprehensive approach to data protection and responsive action sets us apart in delivering service excellence to clients.”



MD Expo is headed to New England with support from two of the top HTM organizations, AAMI and the New England Society of Clinical Engineering (NESCE). HTM professionals from the United States and beyond will gather this October at the Mohegan Sun in Uncasville, Connecticut. The conference is free for all hospital employees, active military and students.
The MD Expo to be held October 8-10, 2024 has been pre-approved for up to 8 CEUs.
One highlight (of which there are many) is the AAMI CBET Study Course led by Dave Scott. This training will cover each area of the CBET exam outline including:
• Anatomy and physiology
• Public safety in the health care facility
• Fundamentals of electricity and electronics
• Healthcare technology and function
• Healthcare technology problem-solving
• Healthcare information technology
Learners who attend this course will have access to course materials which include module slides, practice exams and key test-taking notes. All attendees in this live session can direct questions to the instructor in real-time that will either be answered at the end of the session or addressed via the AAMI discussion group. The AAMI discussion board feature is accessible in the AAMI LMS where they are encouraged to
share information and ask questions throughout the duration of the course and the days that follow. Attendees will also have access to the recording of the October CBET Study Course online which they’ll be able to view for up to 1 year.
There is a $125 fee for the two-day CBET Review Course. However, several free educational sessions are included in the free MD Expo registration including:
• AI Demystified: How to Effectively Use AI to Develop or Improve Your Quality Management System presented by Mark Cooksey
• An Aging Model for Medical Equipment presented by Binseng Wang and Torgeir Rui
• Bridging the Gap Between Nursing and Biomed presented by Leah Goldberg and Cassandra Eilers
For a complete list of educational sessions, visit mdexposhow.com/education/
Another highlight of MD Expo New England is the Reverse Expo – Real Decision Makers. Real Opportunities on October 9.
The Reverse Expo will allow medical equipment sales and service vendors who are registered exhibitors of MD Expo a unique opportunity to meet with more than 20 supply chain, procurement and healthcare technology directors from America’s most prestigious hospitals, imaging centers and health systems.
Find out more about MD Expo at MDExpoShow.com
MD Publishing is planning ahead with events scheduled for 2025. Sign up for the newsletter at MDExpoShow.com to stay abreast of the latest details.
For a sneak peek, pencil in these dates on your calendar:

MD Expo Spring 2025
April 15-17, 2025
Proudly supported by CMIA
The spring 2025 MD Expo will be held at the Pechanga Resort Casino in Temecula, California.
HTM Mixer Denver
May 15-16, 2025
Proudly supported by CABMET
Join us at the Omni Interlocken Hotel in Broomfield, Colorado.
HTM Mixer Milwaukee
July 31- Aug. 1, 2025
Proudly supported by WBA (Wisconsin Biomedical Association)
Join us at the Hyatt Regency Milwaukee in Milwaukee, Wisconsin.
Online registration is open for the HTM Mixer. Find out more via the short video: tinyurl.com/37rymw27
The HTM Mixer Lexington, proudly supported by KAMI, will start on Friday, November 15 and conclude on Saturday, November 16 at the Marriott Resort Griffin Gate (1800 Newtown Pike, Lexington, KY 40511). A kickoff party will be held at 6 p.m. on Saturday night with live music, a bourbon tasting, food and entertainment.
An HTM Mixer has everything an MD Expo offers. Think of it as a hybrid event that combines the best of a state association annual meeting and an MD Expo. The two-day conference includes an exhibit hall, educational opportunities and networking events.
The education line-up features a wide range of topics including:
• Digital Portable and Mobile X-ray Devices
• How to Maximize HTM’s Role in the Capital Process
• Immersive Training for Technicians: Using Extended Realities and AI to Create Better HTMs
• Resilience: A tool for today’s HTM challenges
• Project Management Involving Medical Equipment Installations
• Data-Driven Decision-Making to Increase Asset Availability and Reduce Costs
• HTM Equipment Integration Challenges Working with

• Top-Notch Customer Service within the Clinical Environment, for Non-Clinical Associates
• Care and Handling of Endoscopes and Ultrasound TEE Transducers
Register today at HTMMixer.com


















At Emeritus Clinical Solutions, they believe that hospital beds are the most critical medical devices in a health care setting. While others may overlook them, Emeritus loves and cares for hospital beds, recognizing their paramount importance. They have proven there is a better way to provide hospital bed and stretcher maintenance services, one that prioritizes the needs of hospitals and their patients. The company’s innovative approach revolutionizes these services, making them more patient-centric and efficient.
Emeritus Clinical Solutions Senior Sales Manager Webb Clark recently shared more information about the company.
A: Our staple program is called Bed Maintenance 2.0. This is an outsourced solution with a truly in-house feel. Emeritus will place technicians on-site at your facility full time Monday to Friday to run the patient bed and stretcher program. The Always-On-Site methodology returns hundreds of labor hours back to the HTM department so your biomeds can focus on modalities that they’re a) vested in and b) have actual expertise in. Emeritus will stock OEM parts on-site and your brand neutral, bed expert is working out of your hospital full time. This approach completely erases wait times for parts and service. Travel fees are also non-existent. This is a full-service program, so all costs associated with PMs and repairs are paid for via a firm-fixed monthly rate.

Q: HOW DOES YOUR COMPANY STAND OUT IN THE MEDICAL EQUIPMENT FIELD?
A: 1) We are an HTM company solely focused on patient beds and stretcher maintenance. Furthermore, we are reinventing the way bed maintenance is thought of and performed. 2) Case in point: Always-On-Site. Nobody shows up in a van a week after a service request is placed – Emeritus is already there. Thus, even the back-and-forth correspondence associated with scheduling service is eliminated. Likewise, the need to approve hundreds of POs for parts and services throughout the calendar year is gone. 3) Operations. This is something I can’t overstate enough – from an operations standpoint, Emeritus is exceptional. One of the biggest frustrations HTM managers experience with their vendors is found in communication, or lack thereof. Services can be finicky because of the inherent human element. Managers rely on clear expectations, communication and robust reporting. I would put Emeritus’ operations against any OEM or ISO in the HTM field.
Q: WHAT IS ON THE HORIZON FOR YOUR COMPANY?
A: We are constantly looking to improve our processes. We do this by examining what our competitors offer, then go where they’re not willing to go. Beds exist in a no-man’s land. In roughly half the hospitals in the United States, bed maintenance is assumed by HTM. The other half of the time, beds will fall under the Facilities Department. With that in mind, we have developed a Targeted Transporter Training Program to help hospitals who have transport teams with high turnover. This education program requires quarterly, or even monthly, training. When we talk about cost savings, Emeritus will go well beyond just doing the repairs required. We are looking to cut the head off the snake and reduce the actual number of repairs required. Your Emeritus bed-technician is not only an extension of your HTM team; but will also maintain


rapport with Facilities, Nursing, EVS, and transporters so that the bed and stretcher program is run efficiently. Recently, the Joint Commission has introduced new regulations pertaining to mattress management. Emeritus is already performing mattress audits on behalf of EVS teams, and we are fine-tuning a comprehensive mattress management program to boot.
A: I would challenge your readers to consider how effectively they’re currently tackling bed maintenance. Patient beds do not get the respect they deserve. Forty years ago, a bed was little more than a glorified shopping cart with a mattress on top. Today, beds are outfitted with bed exit alarms, inflatable mattresses, nurse call systems, etc. They’re not the beds of yesteryear and they shouldn’t be treated as such. There is no device at your facility that affects the inpatient experience more. Biomeds are the silent heroes of health care. Your patients don’t understand if the sterilizers in the basement aren’t humming. But they do know if they slept comfortably. They will remember if nursing staff had to move them into another room in the middle of the night because of a broken bed. Furthermore, their families will know and the reputation of your hospital largely hinges on this experience.
We find that too many in-house programs are run inefficiently. Your technicians did not enter this field to work on beds and stretchers. Frankly, most of them loathe working on beds. If you’re not invested in advancing your
technicians’ careers, rest assured – someone else will. Hiring good biomeds is tougher than ever. While beds may feel rudimentary enough to make first call on, you must ask yourself – where does this lead? Often, it leads to a wormhole of trial and error parts ordering. This is how beds rack up in our hallways which invites scrutiny from leadership and regulatory agencies. We find in-house programs lean on OEM support 40-70% of the time and there is a great cost associated with module level repairs.
Traditionally, HTM managers reserve full-service solutions for OR, SPD or imaging equipment. Equipment that, when it breaks, the hospital starts cancelling cases immediately resulting in loss of revenue. By contrast, there are a lot of redundancies when it comes to your bed and stretcher inventory. So why full service? Because an ineffective bed program is a waste of time and money. And the redundancies only compound this error. HTM’s bandwidth is precious. If your technicians are not proficient in this modality, they’re wasting hundreds (in some cases over a thousand) labor hours annually. Couple this with how money is being spent. How often are your technicians leaning on OEM support for troubleshooting or even on-site services? Ask yourself, is this truly an in-house program?
Emeritus is on a mission to reinvent bed maintenance. Bed Maintenance 2.0 is a program that’s meant to optimize your workflow in an often overlooked and misunderstood modality.
For more information, visit emerituscs.com.

On June 28, the Supreme Court issued a decision in Loper Bright Enterprises v. Raimondo, which overturned the legal doctrine known as Chevron Deference. Since 1984, it required Courts to give broad deference to a government agency’s interpretation of ambiguous Federal law. Under Loper Bright, courts must now instead “exercise independent judgment … [and] not defer to an agency interpretation of the law simply because the statue is ambiguous.”
It’s shaken up the industry. In a July 9 AdvaMed webinar, Selina P. Coleman, partner at the Life Sciences Health Industry Group, called it “a landmark supreme court decision, which is ushering in a seismic shift in the balance of power between agencies and courts.”
This decision could have a wide-ranging impact on many aspects of business in America, including medical devices and healthcare technology management. It might also elevate the importance of standards, making them more vital in the lawmaking process, and affecting the role of the work standards developers like AAMI regularly accomplish.
authority granted to them by congress. Once established and made known, agency rules still have the force of law.
Now, though, courts can exercise more discretion in reviewing any challenges to agency interpretations of law, reducing the likelihood that an agency issuing a new rule will go unchallenged.
The role of voluntary consensus standards in supporting medical device regulation may not lessen in this new environment. “When FDA recognizes a standard, they’re saying they will accept conformance with that standard as evidence to support a manufacturer’s claim of compliance with related regulations. But meeting the standard is not required. You don’t have to use the standard,” explained Joe Lewelling, vice president of industry at AAMI. “Right now, it is not clear that Loper Bright would hinder FDA’s current practice.”
In fact, standards might become more important, he said.
She noted that amicus briefs may carry more weight in the future, because these filings may provide more context for judges interpreting statutes.
To start, overturning Chevron deference does not change the function of regulatory agencies, nor does it limit Congress’s power. Agencies like EPA and FDA retain the rulemaking
“They will allow the FDA, in partnership with the medical device stakeholders, to declare what is necessary for safety and effectiveness without putting those specific details in the statutory requirement as well,” he explains.
Diane Wurzburger, vice-chair of industry for AAMI’s Board of Directors, is the executive of regulatory affairs, developed markets, and global strategic policy for GE HealthCare. She agrees that standards will continue to be very important as they bring “an aspect of objectivity.”
“Many standards set criteria that are black and white,” she told AAMI News. “Very simplified, it’s ‘yes’ or ‘no’ – it’s either going to meet the spec or it doesn’t meet the spec” – which is a level of clarity desired by regulators and companies alike.
Ending Chevron deference allows different interpretations of a regulation and “that’s where there may be an opportunity

for a work product of AAMI [or another standard developer] to help clarify what an interpretation means or is intended to be,” she added.
What will actually happen is up in the air, in part because the ruling is so recent, and it did not wipe any current regulations off the books. The decision in Loper Bright Enterprises v. Raimondo only applies to new or pending litigation, and did not retroactively affect rulings on past challenges to regulation. However, a ruling issued by the Court three days after Loper Bright in Corner Post Inc. v. Board of Governors, found that the six-year statute of limitations to challenge agency rules under the APA was triggered not by the issuance of the regulation but from the date a party suffered injury as a result of the rule. This finding greatly expands the universe of regulations that may be challenged. As such, a new business may challenge an old regulation that was formerly upheld under pro-Chevron framework.
“It could work in several directions, so it’s very complicated and may not have the impact everybody predicts the same way,” said Jeff Gibbs, director at Washington, D.C.-based law firm Hyman, Phelps & McNamara. “We live in a very big country with a lot of legal issues and a lot of legal systems. It’s not a simple decision that’s just going to have a clearly visible outcome.”
Patricia Griffin is general counsel for the American National Standards Institute (ANSI), which coordinates the U.S. voluntary consensus standardization system. She told AAMI News that “there are many unknowns regarding Loper, including whether the decision will have any impact on the federal government’s reliance on voluntary consensus standards, what kind of deference will remain now that Chevron is gone, how many new challenges will be permitted under Corner Post (issued after Loper), how congress and the judiciary will respond to the challenges brought about by these landmark decisions, whether voluntary consensus standards will become more important in the regulatory/congressional context, or how these cases impact current debates about standards Incorporated By Reference.”
She noted that amicus briefs may carry more weight in the future, because these filings may provide more context for judges interpreting statutes.
At the July 9 AdvaMed seminar, David A. Bender, senior associate at the Life Sciences Health Industry Group, presented an analysis of past challenges to FDA, CMS and EPA. He found that when circuit courts applied Chevron deference in their ruling, agencies won over 93% of cases. Agencies only won 38.5% of cases when courts reviewed agency action de novo. Since 2000, FDA won in every appellate opinion that applied Chevron deference.
With Chevron deference ended, Coleman posits that there will be “more emphasis on technical legislation drafting to the extent that clarity can be achieved through Congress and stakeholder support.” Industry stakeholders, including standards developers, may also be called on to collaborate with lawmakers to help work clarified, specific language into legislative drafting.
“Industry input will become more valuable on the front end before statutes are enacted to try to get things right the first time, which we know can be a tall order,” she said.
Wurzburger expects it to take six months to year to see some clarity.
“Maybe there’s going to be some cases to help us understand of how those decisions are going to be made,” she said. We can’t say “whether some courts will continue to provide deference to the agencies while others will not.”
But the impacts may not be as widespread as if Chevron hadn’t already been weakened by decades of other court rulings, added Lewelling.
“Chevron had been dying the death of 1,000 cuts for some time now … Loper Bright wasn’t the first case restricting it’s impact, but it does appear to put the nail in the coffin of the Chevron deference.”




BY TOM TOCZYLOWSKI, ECRI’S ASSOCIATE DIRECTOR OF HEALTHCARE PRODUCT ALERTS
Challenges associated with legacy equipment asset information can lead to safety issues. Standardization and normalization of CMMS data can lead to better outcomes and can be a part of a robust Total Systems Safety Approach.
Healthcare technology management (HTM) teams leverage computerized maintenance management systems (CMMS) to enable more efficient work order management. A well-organized CMMS can also help reduce asset downtime and improve maintenance workflows. Often, inconsistent equipment naming conventions and descriptions, and variable device classifications, can negatively impact predictive replacement planning and management of preventive maintenance. This could include variable manufacturer and vendor names (which may change over time), model names and device types.
This inconsistency in CMMS data can also lead to missed cybersecurity threats, recalls and field corrections that could impact the safety of patients and staff. Standardized CMMS data can lead to better operations while mitigating risk and enhancing regulatory compliance. This is evident in the matching of recalls to CMMS inventory for departments utilizing robust recall management solutions (like ECRI’s Alerts Workflow). As work orders are assigned, whether it be for preventive maintenance or corrective maintenance resulting from recalls or incident reports, the process will run smoother with normalized data in the
system. In addition, if there are assets under alternative equipment maintenance (AEM) or advanced analytics and reports being run, the documentation in the CMMS can more easily be organized and tracked with a clean data set.
Each year ECRI produces a “Top 10 Patient Safety Concerns” list. For 2024, “Unintended Consequences of Technology Adoption” was Number 4. As healthcare technologies have been adopted, their widespread implementation without full appreciation of their potential impact is associated with serious risks, including injury or death. Part of that proper adoption of medical equipment includes maintaining performance and maintenance data, and having a system to quickly respond when there are safety issues or recalls. Organizations must have good processes for evaluating, implementing, and maintaining technologies to prevent unintended consequences and to address emerging issues.
At ECRI, we consider recall management and consistent data to be part of Total Systems Safety for a health care organization. We have seen the danger of improperly maintained equipment, missed recalls or corrections, or even improper use of technology. Misapplication or misuse of health technology could lead to negative consequences, including patient harm. ECRI’s Total Systems Approach to Safety is a comprehensive programmatic approach to improving patient safety in health care organizations. It is a transparent learning system that builds reliability and resiliency that supports optimal behaviors for safety.
By redesigning the elements of the safety system,
health care providers are supported to deliver care in a more reliable and resilient way. Our proactive systems approach is anchored in safety science, clinically informed human factors engineering, and health equity to prevent errors, reduce harm, and enhance the overall quality of care by integrating various safety-related processes. This can include preventive maintenance schedules, plans for keeping up-to-date instructions and labeling documentation, and, crucially, maintaining a clean equipment inventory. This approach requires a culture of safety and a commitment to continuous improvement, enterprise-wide communication, and engagement of staff and patients to reduce adverse events, improve patient outcomes, and increase patient and staff satisfaction.
ECRI is an independent, nonprofit organization improving
the safety, quality, and cost-effectiveness of care across all healthcare settings. ECRI is the only organization worldwide to conduct independent medical device evaluations, with labs located in North America and Asia Pacific. ECRI is designated an Evidence-based Practice Center by the U.S. Agency for Healthcare Research and Quality. ECRI and the Institute for Safe Medication Practices PSO is a federally certified Patient Safety Organization as designated by the U.S. Department of Health and Human Services.
For more information, visit www.ecri.org.

Tom Toczylowski, ECRI’s Associate Director of Healthcare Product Alerts

HTM professionals generated over 1,200 nominations for the 2025 Tech Choice Awards with the top 5 nominees for each of the 13 categories named as finalists. Voting begins October 1 and is open until January 15, 2025.
The Tech Choice Awards (also known as the Wrenchies) are the people’s choice award for the HTM community. The winners are based 100 percent on the number of votes each finalist






received during the voting period. A special thank you goes out to the sponsors who help make the Tech Choice Awards possible. The sponsors do not have any input when it comes to selecting winners.
Wrenchie winners will be announced in the March issue of TechNation with an award ceremony planned for the MD Expo set for Southern California in April 2025.

OF THE YEAR, sponsored by USOC
OF THE YEAR, sponsored by MedWrench

• Rana Helou, biomedical engineer II, Crothall
• The ProHealth Care Biomedical Engineering Department
• Memorial Hermann Health System Biomedical Engineering Department
• University Hospitals Health System Healthcare Technology Management Division
• Washington Regional Medical Center Biomedical Department
• UT Southwestern Medical Center’s Healthcare Technology Management Department
OF THE YEAR, sponsored by 626 Holdings
• Earl Morris, BMET, Harrison County Hospital
• David Scott, senior biomedical technician, UC Health
• Charles Woolfolk, HTM manager, Baylor Scott & White Health
• Greg Czajka, support services operations director, Advocate Aurora Health
• Stephen Ellithorpe, executive director, Providence
• Nicole Ruffin, BMET II, Renovo Solutions
• Codi Nelson, program director, Crothall
• Brandi Caton, supervisor of clinical engineering, Trimedx
• Manuel Vanderpool, senior site manager, Trimedx
OF THE YEAR, sponsored by Prescott's

• Webb Clark, senior sales manager, Emeritus
• Samantha Moriarty, manager of clinical engineering operations and inpatient compliance, Brigham and Women's Hospital
• Lewis Lennard, product manager (North America), Rigel Medical
• Michael Toomalatai, chief biomedical engineer, Department of Veteran Affairs
• Millicent Alooh, regional director, BMET implementation at Rice360 Institute for Global Health
MILITARY BMET OF THE YEAR, sponsored by College of Biomedical Equipment Technology

• Tyler Scott, chief, healthcare technology management, Keller Army Community Hospital, U.S. Army
• Bailey Messina, FSE Boston Imaging, U.S. Air Force
• Eric Dalsin, information systems biomedical equipment support specialist, Southeast Louisiana Veterans Health Care System, U.S. Navy
• David Lindsay, CBET, Renew Biomedical, U.S. Army
• Phillip Villegas, hospital corpsman and biomedical equipment technician, U.S. Navy
EDUCATOR OF THE YEAR, sponsored by Maull Biomedical

• Jim Durocher, biomedical professor, St. Clair College
• Dr. Jeffrey Smoot, professor of HTM, MiraCosta Community College
• Phil Pash, director of HETM, IU Indianapolis
• Donald Armstrong, technical training program manager, Renovo Solutions
• Larry Fennigkoh, Ph.D., educator, Milwaukee School of Engineering
DIRECTOR/MANAGER OF THE YEAR, sponsored by MW Imaging

• Mike Busdicker, system director, clinical engineering, Intermountain Healthcare
• Ashley O’Mara, VISN 1 chief clinical engineer, VA New England Healthcare System
• Douglas Redwine, operations manager, healthcare technology management, Texas Health Resources
• Sandy Mason, senior manager, Trimedx
• Rudy Flores, market director, Central United States, UHS
sponsored by Avante Health Solutions
• April Lebo, vice president of demand generation, Probo Medical
• Wanda Legate, senior vice president business development, Tri-Imaging Solutions
• Jenn Nichols, general manager clinical engineering-west operations, Trimedx
• Carol Davis Smith, director, clinical engineering internship program, University of Connecticut
• Izabella Gieras, director clinical engineering, Cedars-Sinai
OF THE YEAR, sponsored by Pronk Technologies

• David Ebo Anderson, biomed & lecturer, Koforidua Technical University
• Edward Reyes, clinical engineering professional, U.S. Department of Veterans Affairs
• Ratish Kumar Mohan, biomedical engineer, Hospital Sisters Mission Outreach
• Clement Appiah Anokye, senior clinical engineering manager, Ghana Health Service & Operation Smiles
• Jeff Ruiz, senior site manager, Trimedx, Miles for Myles
OF THE YEAR, sponsored by Soma Tech, Intl
OF THE YEAR, sponsored by MultiMedical Systems
• North Carolina Biomedical Association (NCBA)
• Clinical Engineering Association of Illinois (CEAI)
• HTMA-NTX
• HTMA-OH
• New England Society of Clinical Engineers (NESCE)
• PM Biomedical, pmbiomedical.com
• Prescott’s, prescottsmed.com
• RSTI, rsti-training.com
• Tri-Imaging Solutions, triimaging.com
• USOC Medical, usocmedical.com
Visit 1technation.com/tech-choice-awards to vote for your favorites. Voting ends January 15, 2025.
• Jennifer Chester, creator of “Bella the BMET”
• Kim Rowland, vice president Women In Leadership
• Jewel Newell, co-founder of I-HTM
• Chyrill Sandrini, host of HTM Insider
• Carlos Villafane, founder of Biomedtechnicians.com

BY CARLOS VILLAFANE
Recently, I read an article from Gallup titled “The New Challenge of Engaging Younger Workers” from February 2024. This article explained that since March 2020, there has been a dramatic decline in engagement at work among younger generations. Per their study, “the younger group of millennial and Gen Z employees (born 1989 or later) have experienced a five-point decline in engagement.”
This trend has been happening for years, as another Gallup survey from 2019 mentioned that “only 29% of millennials are engaged at work, meaning only about three in 10 are emotionally and behaviorally connected to their job and company.”
Why is this happening? The article mentioned something that grabbed my attention: “This generation of workers, especially, is looking for an employer with a purpose they can identify with.”
What can be done to connect and engage the next generation in the HTM/biomed field?
This is something that should concern each one of us. We already know there is a need for new HTM professionals, as so many BMETS and field engineers who started working in our field in the 1970s, ’80s and ’90s are retiring. Also, there are not many schools that offer biomedical engineering courses today when compared to previous decades.
We need to recognize that each one of us can do something to help, as we have a very important purpose in health care and in our communities that we can share with the newer generation. We contribute to the maintenance and improvement of devices that can diagnose, treat, and manage health conditions, directly enhancing patient care and saving lives.
What can you do to share your passion for your career as a BMET?
LOOK FOR OPPORTUNITIES TO SHARE YOUR EXPERIENCE.
• You don’t need to be a teacher to talk to students. Many local middle & high schools enjoy having professionals talk about their careers during career
fairs and similar activities. Can you volunteer to visit a local school, vocational or trade school? AAMI has plenty of printed material and resources that you can use to interest the students.
• Is there a local biomed engineering college near your area? Be a mentor! You can provide guidance, support and inspiration to the students already interested in our field.
• Are you a member of your local biomed/HTM association? If not, get involved! Your support helps the local association to spread the word in the community about our jobs.
• Familiarize with and use social media and online tools to reach the younger generations, as they are more receptive to the use of technology to learn. There are plenty of places online you can use to share success stories, breakthroughs, opportunities, thoughts, and suggestions: Lists, question-and-answer platforms (like Quora), Facebook groups, YouTube (if you want to share videos), etc. Recently there has been an increase of BMET/ HTM-related channels online, podcasts, motivational videos, etc. Follow them, subscribe and volunteer to be interviewed on any of these channels if you can!
• Write articles for your favorite HTM magazine.
• Think about the aspects that make HTM a great career path and share them. For example:
• Everyone involved in the HTM field has the privilege to work in health care, so job security is almost guaranteed. (Even during COVID, we were always busy taking care of the medical devices without interruption).
• The HTM field has tremendous growth potential: Reportlinker.com predicts a “compound annual growth rate of 15.5% through 2026.”
• We work with cutting-edge technology that evolves rapidly. There are constant opportunities to familiarize and learn about new medical devices and new technology.
• We are helping the community by keeping life-saving medical devices in optimal performance to take care of their health needs.
• We never get bored at work!
• Emphasize the real-world Impact that we have.
• Showcase how careers in the HTM field contribute to solving global health challenges, improving patient outcomes and advancing medical research.
By looking for opportunities to talk about our experiences, we can effectively engage and inspire the newer generation to pursue careers in our exciting HTM field!

Carlos Villafañe, CBET, CET, can be found online at biomedtechnicians.com and youtube.com/ @tecnicosbiomedicos



















Register at WebinarWednesday.live Eligible for 1 CE credit from the ACI.
The next generation of infusion device analyzers
The revolutionary IDA-6 Infusion Device Analyzer puts simplified infusion device testing right at your fingertips. With just one touch, effortlessly navigate through the intuitive interface, boost the accuracy of your tests, and easily generate reports —all on a user-friendly 10-inch touchscreen.
Modular design – Simplified calibration with field exchangeable channels to eliminate downtime
4-Channel testing – Test up to four infusion pumps at once
No priming after initial setup – Accelerate test time
IntelliPump Technology – No mess testing with a water recirculation system
OneQA-enabled – Workflow automation simplifies testing, enhancing accuracy and efficiency
Transform your infusion pump testing with the IDA-6 Infusion Device Analyzer today.
For more information, visit flukebiomedical.com


The Webinar Wednesday presentation “Research to Implementation – Understanding Key Components of a Successful RTLS Platform” sponsored by Cognosos is eligible for 1 CE credit from the ACI. Access the on-demand version of the webinar at WebinarWednesday.live.
Cognosos Director of Product Marketing-Healthcare Jeff Stiffler informed participants regarding key components of RTLS platforms, in addition to expected benefits and future trends.
Takeaways from the session included:
• RTLS platform definition
• Key components
• Hardware technology – IR, ultrasound, BLE, UWB
• Software technology – AI/ML, on-premise
• Expected Benefits and ROI
• Current trends and future outlook
Stiffler also answered attendees’ questions during a Q&A session. The Q&A session is included in the on-demand version at WebinarWednesday.live
Todd Peterson, a biomedical technician at Mary Greeley Medical Center in Iowa won an Amazon gift card as part of Webinar Wednesday’s 10th anniversary celebration.
Attendees were asked “Excluding CE credits, why do you attend Webinar Wednesday?”
“To keep up with the fast-moving changes in the health care industry.”
- Brian Caruso, CBET/Clinical Engineering Tech II at Methodist LeBonheur Healthcare
“This Wednesday Webinar series is a pretty good educational source for HTM fields. We, HTM, can apply this knowledge to our daily work and prepare for the TJC survey in a more effective way. It teaches us new technologies such as RTLS, cybersecurity, new standards and regulations. It is the main reason for attending the webinars.”
- Tedd Koh, BMET, Olive View UCLA Medical Center.
“Learn about new information and get refreshers on stuff I’ve learned about.”
- Jason Chaffin, Lead BMET, St. Luke’s Hospital.

The Webinar Wednesday Tools of the Trade Product Demo featured the Claroty xDome. It was a very popular webinar with an international audience. The session was sponsored by Claroty and eligible for 1 CE credit from the ACI.
Claroty Director of Customer Success Justin Brayman presented the August Tools of the Trade Live Demo of the Claroty xDome. He explored the industry’s leading health care cyber-physical systems protection platform. It enables health care organizations to safely deliver connected care while enhancing efficiencies across the clinical environment. Claroty xDome spans the entire health care cybersecurity journey regardless of the scale or maturity of the environment through asset inventory, exposure management, network protection, threat detection and operational efficiency.
As a modular solution, Claroty xDome is suited for organizations at any stage in their health care cybersecurity journey, regardless of their scale, staffing or program maturity. The solution consists of platform essentials, offering foundational capabilities across all core areas mentioned above, as well as advanced modules that provide increased value and enhanced programmatic capabilities.
Brayman also answered questions from attendees after his presentation. The Q&A session proved very informative.
Jody Blunt, a biomedical equipment technician with Memorial Hospital of Sweetwater County, Wyoming, was the lucky winner of a $100 Amazon gift card as part of the Webinar Wednesday 10th anniversary celebration.
Based on survey results, all the attendees were winners. Attendees were asked, “What was your single biggest takeaway from today’s product demo?”
“Enhanced my knowledge about Internet security for medical devices integrations.”
- Yousri M.F. Okasha, a senior clinical engineer with King Faisal Hospital.
“Health care security is something that we need to stay on top of.”
- Jeremiah Yohe, lead biomedical equipment technician, WellSpan Health-York Hospital.
“The importance of protecting our network’s information in an ever-expanding cyber space.”
- Brian Caruso, CBET/Clinical Engineering, Methodist LeBonheur Healthcare.

TechNation’s Webinar Wednesday series presentation of the July Tools of the Trade Live Demo of Kontakt.io’s Asset Tracking Platform was a success. It was sponsored by Kontakt.io and is eligible for 1 CE credit from the ACI. Senior Solutions Architect Tim Young did an amazing job of displaying the many features of the Kontakt.io Asset Tracking Platform.
Never search for equipment or medical devices again. Young showed how to track the exact location of every asset in real time with clear insights into equipment utilization and AI-powered analytics. Beyond asset location visibility, Kontakt.io’s solution automates PAR-level inventory management and increases mobile equipment utilization to over 60%. Stop paying for equipment you don’t use and see day one ROI in three fast, easy steps: reduce your rental fleet, “right-size” your inventory, and defer future CAPEX purchases.
He showed how HTM professionals can help unlock significant savings with an RTLS asset tracking solution that increases productivity, optimizes workflow and eliminates inefficiencies.
With just a glance at the asset tracking platform dashboard – on a smartphone or computer – you’ll see exactly where your equipment is located, how it’s being utilized, where it’s needed most, and how much inventory you really need. Plus, Kontatk.io’s

All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.
solution is future-proof. With open APIs and easy integrations, the platform can scale to include dozens of use cases to help optimize your operations while saving time and money.
Young fielded questions from attendees after his presentation. His answers provided additional knowledge regarding the benefits of the Kontakt.io Asset Tracking Platform.
Attendee Annye Notman in North Carolina was the lucky attendee who won Apple AirPods as part of Webinar Wednesday’s ongoing 10th anniversary celebration.
Attendees answered the question, “What was your single biggest takeaway from today’s product demo?””
“The versatility of the software.”
- Mitch Hoffman, CBET, Parkview Health System. “Benefits of asset tracking.”
- Rafael Chavira, BMET, Loma Linda University. “Ease of deployment.”
- Costas Latinakis, general manager, Theralife.
For more information, including on-demand and upcoming webinars eligible for 1 CE credit from the ACI, visit WebinarWednesday.live.


computerized maintenance management system (CMMS) is a tool that can improve overall medical equipment management at the facility level. The information included in a CMMS varies depending on the individual situation but always includes the medical equipment inventory and typically includes information such as service history, preventive maintenance procedures, equipment and performance indicators, and costing information.
TechNation contacted CMMS leaders in the health care sector to find out more about CMMS options and features for HTM professionals. Participants are:
• Phoenix Data Systems Inc. Vice President of HTM Consulting Al Gresch
• TruAsset Director of Business Development Amanda Moser
• EQ2 HEMS Product Manager Rich Sable
• FSI CEO Zachary Seely
• Accruent Product Line General Manager Hayley Wick
WHAT SHOULD HTM PROFESSIONALS LOOK FOR WHEN PURCHASING A CMMS?
GRESCH: In today’s health care environment, it really comes down to two questions that need to be answered:
• Can my CMMS help me streamline workflows and automate processes to help my team be as efficient and effective as possible, so I can not only easily meet regulatory compliance, but also save significant time and money?
• Does my CMMS partner have the industry expertise to help me optimize my data and operations consistent with industry best practices?
With the immense pressures to reduce costs and meet the needs of the organization with fewer qualified people to fill key roles, these are essential to your success.
MOSER: Anyone who is looking to switch CMMS programs should first evaluate the current process and compile a list of features they are seeking, challenges they are looking to overcome, and requirements that they must meet to obtain the necessary approval to move forward. This allows them to pare down the many options available and maximize their time during the evaluation process. A CMMS solution should provide tools for compliance, efficiency for all user levels, and great customer service and training options.
SABLE: HTM professionals should look for a CMMS that presents data and information (especially in reporting and dashboards) in the format that fits the needs of technicians, management and the hospital as a whole. Also important is the ability to interface with other information systems such as cybersecurity, procurement, real-time location services
(RTLS), test equipment like electrical safety analyzers, and other systems. This includes smaller basic applications, all the way up to larger, more robust systems like ServiceNow. Since the CMMS contains full inventories, the interfaces can leverage this information to automate workflows or enhance technician productivity. For example, ordering parts from within the work order, updating the location information for a device scheduled for PM to reduce time searching for assets, or creating work orders or alerts for assets affected (or potentially to be affected) by a cybersecurity event. Finally, having a mobile application will enhance the benefits of the interface for the technician as real-time alerts or orders can be accomplished in the field; and having one that also works while offline improves productivity since the technician can still access and update work orders.
SEELY: While there are several CMMS providers on the market, one-size does not fit all – true value stems from a healthcare-specific CMMS/EAM that integrates across several HTM systems (materials/parts ordering, device testing, asset location tracking, device calls, and network device security). A seamless solution saves teams resources in areas that are always top-of-mind: constrained staffing and budgets.
WICK: A purpose-built health care CMMS addresses the specific needs of hospitals and other health care organizations. It provides a single source of information easily accessible and customizable, empowering maintenance teams to streamline workflows, automate processes, ensure data integrity, enhance work documentation, and offer key stakeholders data-driven insights into the asset life cycle through easy visualization of critical KPIs. It ensures equipment remains operational and reliable, while improving cybersecurity, maintaining compliance, and helping facilities and biomedical teams to meet daily production expectations, all towards a more efficient operation and better financial performance over time.
HOW CAN A CMMS HELP ADDRESS CYBERSECURITY CONCERNS?
GRESCH: The first way is to make it simple and easy to capture all data relative to a device’s network connectivity and software vulnerabilities. The second is to have comprehensive, bi-directional integrations to cybersecurity and network monitoring platforms. When hit with a cyberattack, time is money, so the sooner you can identify and remediate affected devices, the better. Some are using these integrations to not only ensure device security, but also monitor equipment utilization that can feed into capital replacement decisions.
MOSER: A CMMS solution can provide integrations to security companies to allow the HTM professional to evaluate
potential risks and develop a smooth process to resolution of any concerns with a solid paper trail to document actions that were taken or reasons why actions were unnecessary. The CMMS itself should also have measures in place to protect client data as an industry standard.
SABLE: A CMMS contains many important cybersecurity details that are helpful for prevention and mitigation of an incident. It contains all the hospital assets that are connected to the network and identifies those with ePHI data. With data attributes for network fields stored in the CMMS, it’s easier for the HTM manager or cybersecurity team to query devices with certain operating systems, software and revision level along with MAC address, IP address, and Application Entity (AE) Title information. When the CMMS is interfaced to a cybersecurity application, the cybersecurity application can update the network information in the CMMS or create work orders for recalls or alerts for devices experiencing malicious activity or in need of a patch. It can tell you where the device is located, who supports it, and even whether it’s in use. Our CMMS, HEMS, can also alert the user when new equipment is added to the network; and several IT data points are automatically updated in HEMS as well. Finally, if your CMMS is hosted in the cloud as part of a SaaS arrangement, you will want to make sure that it is with a major provider dedicated to security such as Microsoft Azure, Google Cloud or Amazon AWS.
SEELY: A CMMS should be a centralized hub for integrating across all your HTM systems –today, there are more devices and more connections, leading to increased vulnerabilities and oftentimes fewer resources for managing cybersecurity risk. When correctly implemented, your CMMS is a gateway for cybersecurity management – providing real-time insights into risk through creation of notifications and work orders. FSI recently partnered with Cynerio, a leading platform for health care cybersecurity, building an integration that focuses on the management of connected medical devices, automating the creation of work orders for the identification of vulnerable assets and their proper assignments within our CMMS solution’s normal workflows.
WICK: The health care CMMS should seamlessly integrate with leading cybersecurity solutions to identify issues before attacks occur and track remediation or mitigation efforts. It should facilitate creating or updating standard operating procedures (SOPs) for onboarding, maintaining and retiring equipment. It should also automate the calculation of cybersecurity risk per device based on attributes such as the type of information handled and connectivity. Additionally, it should monitor the network traffic and the information going to and from





devices, send alerts to the appropriate staff for any unusual activity, and offer advanced reports to stay ahead of vulnerabilities, protecting the organization, its assets and its patients.
WHAT ARE SOME MUST-HAVE FEATURES HTM SHOULD REQUIRE OF CMMS?
GRESCH: We’ve touched on some. Automation of processes, integration capabilities to cybersecurity platforms, real time location system technologies, enterprise resource planning software, IoT devices, configuration management database/IT ticketing system like ServiceNow, recall platforms, time/resource platforms and automated test equipment are all important to streamline and optimize workflows. Parts and contract management, mobility, and a comprehensive and easy to use reporting and dashboarding packages are also key to effective management of the HTM function.
MOSER: HTM departments are often running lean and one of the most important features should be a user-friendly platform that offers a quick and easy documentation process for the technicians so that they may maximize their productivity and spend more time with their hands on equipment than on a keyboard. Also, the ability for facility staff to submit work requests in a quick and convenient way, managers to be able to access necessary KPI metrics and reporting, and a way to track capital planning and compliance data without spending weeks preparing for an inspection.
SABLE: One feature HTM should require of CMMS is the ability to use the CMMS in the field with a mobile application because this will enhance technician productivity as mentioned above. Another feature would be the ability to order parts from within the work order, making the workflow more efficient. Dashboards for the technician,
manager, end user (nurse, clinician), and alternate equipment management (AEM) simplify work and increase productivity and feedback on the performance for both the individual and department. With staffing challenges, the CMMS must provide workflow improvements designed specifically for technicians such as handling “unable to locate” devices and the ability to transfer assets to other healthcare delivery organization or lines of service (HTM, Facilities, IT).
SEELY: First and foremost, a CMMS for HTM should assist in regulatory compliance, providing tools and features that help ensure adherence to health care regulations and standards. A CMMS should excel in core features such as asset management, work order management, preventive maintenance scheduling, inventory management, and reporting and analytics. Some innovative capabilities your CMMS should have, integration capabilities, mobile access and tools, user-friendly interface, AI adoption, and security features. All the above features and capabilities provide a comprehensive solution for HTM teams to address compliance, revenue protection and operational efficiencies.
WICK: HTMs should expect their CMMS to have robust features that streamline complex asset and workplace management challenges. A CMMS should offer advanced procedures and sub-procedures that ensure a comprehensive maintenance workflow complemented by dynamic custom forms, process logic, and automation. Cutting-edge dashboards and reports are crucial for real-time insights, alongside superior implementation and support services to facilitate smooth operations. Furthermore, a CMMS should have comprehensive compliance capabilities, providing thorough documentation and visualization for all system components and supporting seamless audits and inspections. Security is also para -
mount, with the need for integrated data security tools to safeguard information. Innovative asset management tools are equally important, including asset scanning and a mobile-first approach to enable end-to-end work order management while ensuring data integrity. A CMMS with these features helps empower HTMs to thrive in an ever-changing health care environment.
HOW CAN AI IMPROVE CMMS?
GRESCH: Greater standardization (nomenclature, CMMS codes, processes and procedures), AEM automation, VR training, predictive maintenance integrations from smarter equipment, and automated staffing analyses are just some ways AI can improve CMMS.
MOSER: Possible AI implementations could include predictive maintenance, automated workflows, duplicate reductions, compliance watchguards, and data driven insights. Something as simple as reducing duplicate vendors and manufacturers can be invaluable in reducing administrative time constraints. More time working on equipment and less time in front of a PC.
SABLE: AI can be used to simplify certain functions of the CMMS to enhance productivity for managers and technicians. One area is an AI-based HELP system where questions can be entered; the HELP system will present the user with the requested information as opposed to manually searching the HELP menus or manuals. Another use is for text-based custom report building using AI where queries can be entered and the system creates the desired reports; this eliminates the need to learn a report design application. The CMMS can leverage AI for monitoring metrics in the system and then alert managers and technicians immediately for parameters that are out of limit or critical trends on an asset’s performance. This way, AI can optimize maintenance planning and spare parts ordering for your inventory. Additionally, hospitals are beginning to look at hospital-wide AI where rather than each application doing its own AI services. This way there is one overall AI analyzing information and data from all sources together. So, it is a good idea to make sure that you have a CMMS that will be able to connect into that AI system.
SEELY: Incorporating AI into CMMS solutions addresses challenges like data access, workflow inefficiencies, regulatory compliance, cost management, and safety risks. AI enables predictive analytics and machine learning to forecast equipment servicing needs, preventing costly breakdowns. Additionally, an AI-driven CMMS
automates routine tasks, streamlines inventory management, and offers insights to enhance operational efficiency, increasing asset lifespan and reducing costs. If used properly, AI improves the experience to have efficient access to the data to make effective informed decisions. Looking ahead, FSI plans to leverage AI to improve the overall user experience and HTM efficiency. We’re committed to using AI responsibly to revolutionize workflows and drive innovation in the healthcare maintenance and HTM markets.
WICK: Customers can leverage AI to identify equipment trends such as failures, downtime, and usage increases. It can also be used to understand equipment life cycles, enhance capital planning, and understand equipment end-of-life expectations. Technicians can leverage AI to identify the correct parts to have on hand when responding to a work order and use it for guided troubleshooting.
HOW CAN BIOMEDS UPGRADE THEIR CMMS TO MEET THE EVER-EVOLVING NEEDS OF HEALTH CARE?
GRESCH: The first is to ensure you have clean, high-quality data (including equipment nomenclature and a consistent, clearly defined set of CMMS codes). Putting bad data into the best CMMS will result in less-than-optimal functionality. The second is to find a partner that not only offers all the functionality listed above, but also has the knowledge and expertise to assist you in optimizing your processes.
MOSER: One great way is to select a CMMS solution that listens to their customers. If you find a company that hears what the HTM community needs and makes continual changes to meet those needs, it won’t be necessary to shop around as often or risk working in an outdated application. Discuss with any potential CMMS solution what some of the changes they made were to their program and always evaluate what upgrade costs will be in the future as many times a program becomes outdated because of upgrade expenses not fitting the budgetary allotments and being cut. Then, over time, HTM departments are backed into a corner where their version is not supported, and the funding isn’t readily available to make the upgrade.
SABLE: First, I recommend making a list of features that you want to add to your CMMS and ask your current vendor if they have solutions that can be added to your current system or if they have a new version of the CMMS. If your current vendor does not have the desired solution, then you can begin searching and scheduling demonstrations with
other CMMS vendors to find products that can meet your current requirements and a new vendor that can meet future requirements. It is important to identify CMMS vendors with extensive experience in health care for biomed, since the requirements are so unique compared to any other industry. Look for a CMMS provider that is exclusive and responsive to hospitals and their needs. You’ll need to present a replacement plan to your executive leadership clearly outlining the costs and benefits to the organization focusing on patient safety, regulatory compliance, and cybersecurity along with increasing biomed efficiency and productivity. Finally, ensure your prospective vendor has a good track record of interfacing with other information systems because the CMMS is the hub and additional features and workflows will come from other systems like cybersecurity, service manuals, recalls, etc.
SEELY: Biomeds should consider a CMMS that can evolve alongside with those needs – what a CMMS offered 5 years ago, or even 6 months ago, may not be as relevant in today’s health care setting. For example, we heard from HTM professionals at AAMI eXchange that integrations related to device testing were a top priority for efficiency and compliance – our product and customer success teams have shared goals of incorporating customer/ industry feedback into our product roadmap, specifically designed for health care service operations and support.
WICK: Significant CMMS enhancements will address top-down digitalization pressure and reduce long-term health care costs. Tools that support lean maintenance practices can bridge gaps caused by worker shortages. Technologies that enhance mobility, data integrity, visualization, and documentation accuracy are crucial for compliance and proactive maintenance. Centralized data with a single source of truth and broader integration capabilities will strengthen cybersecurity. Finally, highly configurable solutions tailored to user experience and specific organizational needs will provide the best fit.
GRESCH: The first is to ensure you have clean, high-quality data (including equipment nomenclature and a consistent, clearly defined set of CMMS codes). Putting bad data into the best CMMS will result in less-than-optimal functionality. The second is to find a partner that not only offers all the functionality listed above, but also has the knowledge and expertise to assist you in optimizing your processes.
MOSER: There are many thriving CMMS options out there.
The process of any software change can be scary and cause hesitation. There is also the expense of import, training, licensing, and continual support. Not every CMMS is the same and some are more affordable than others and, at times, can work with you to help you make the needed changes to continue to move forward with technology.
SABLE: A CMMS can certainly exist as a standalone application to manage your assets, work orders, regulatory compliance, parts inventory, and automated workflows, and some clients still implement their CMMS in this fashion to start, knowing that advanced functionality can be added at a later time. Interfacing with other information systems further increases the workflow efficiency and staff productivity along with better data integrity and addressing cybersecurity concerns of the devices managed in your CMMS. Artificial intelligence is the next evolution to CMMS, and AI can leverage the CMMS data and data from other systems for better asset management, predict failures and parts inventory needs, improve capital planning, and suggest planned maintenance scheduling. EQ2’s HEMS CMMS is ready for this new frontier of asset management and many clients are requesting and adding the mentioned functionality to their existing HEMS/CMMS system.
SEELY: A CMMS is a core operations tool – when correctly implemented and designed to meet the specific needs of HTM departments, a CMMS will alleviate daily challenges around documenting ITM activity (including retests) for compliance, making financial decisions, ensuring devices are safe for patient use and creating time and cost-saving opportunities for BMETs and admins.
WICK: Many hospitals are using legacy CMMS solutions that primarily handle work order management, do not integrate with other systems throughout the organization and lead to a cycle of waste and redundancy. To keep up with the times, health care systems need to modernize their CMMS to improve mobile capabilities, reporting and analytics and automate workflows within and between departments for superior user adoption and experience. It is also critical that the CMMS be able to manage cybersecurity initiatives, AEM programs, equipment capital planning and the complete asset life cycle. A truly modern health care CMMS can connect and transform your HTM, Healthcare Facilities Management, Supply Chain and IT departments to transform the patient experience and ensure ongoing compliance with policies, laws and regulations.


Buzz. Excitement. Energy. Whatever you call it, you can feel it on the Tenacore campus in Southern California. The company has the “it” factor that professional athletes talk about when they are in “the zone.”
One reason is because of a new, first-of-its-kind offering from Tenacore. The introduction of Vending Management Services created a stir and continues the Tenacore track record of meeting the health care industry’s needs.
We are getting ahead of ourselves. I blame it on all the excitement. Let’s back up and set the scene.
Tenacore is a leading provider of comprehensive medical device services, specializing in the repair, recertification, and sale of medical equipment. For over 20 years, Tenacore has been committed to delivering high-quality solutions that ensure health care providers can rely on their equipment for optimal performance and patient care.
“At Tenacore, we’re on a mission to revolutionize the
clinical equipment lifecycle with top-notch service and a relentless drive for improvement. Our vision? To be the premier, most trusted source for exceptional healthcare technology,” CEO Riley Van Hofwegen said.
“Our core values are the heartbeat of Tenacore’s culture, igniting passion and guiding our every move,” he added. “These values keep our team energized and focused on delivering excellence:
• RESPECT: We place our customers at the center of everything we do. By deeply understanding their needs and surpassing their expectations with personalized service, we turn every interaction into a meaningful connection.
• COLLABORATION: Teamwork is our secret sauce. Our diverse group joins forces to tackle challenges and achieve remarkable outcomes, proving that together, we can accomplish anything.
• INTEGRITY: Honesty and transparency are our cornerstones. We conduct our business with unwavering integrity, ensuring ethical practices and clear communication in every interaction.
• ACCOUNTABILITY: We’re committed to excellence. Our products and services adhere to the highest industry standards, backed by transparent communica -
tion, prompt support, and comprehensive warranties that put you first.
• QUALITY: Quality isn’t just a standard; it’s our passion. From our top-tier products to our outstanding services, we infuse excellence into every aspect of our operations.”
OK, OK, OK. I know what you are thinking. All companies have boring mission statements and core values. Those are just words on paper some PR whiz kids dreamed up for the website. While that may be true for some companies it is not the case at Tenacore!
“Our core values are our foundation. If there is ever a question of whether we should take action or not, we refer back to these!” one employee shared.
Tenacore’s core services and offerings are divided into five categories, according to CEO Riley Van Hofwegen.
• CORRECTIVE MAINTENANCE: Our team of technicians offers expert repair services for a wide range of medical devices, ensuring they meet OEM standards.
• PREVENTATIVE MAINTENANCE: Regular maintenance services to prolong the lifespan of your equipment and prevent unexpected downtimes.
“Tenacore is incredible! They’re always responsive and deliver an excellent repair experience. Whenever we need repairs or parts, they’re there for us!” one customer recently exclaimed.
Don’t forget to save some money with proven pre-owned equipment.
• MEDICAL EQUIPMENT: Tenacore’s “T.Certified” preowned medical equipment is the best option available, providing cost-effective, reliable solutions for healthcare facilities.
• RENTAL OPTIONS are also available for all “T.Certified” equipment if the customer is looking for more short-term use options.
All pre-owned equipment undergoes rigorous quality assurance, testing,, certification processes to ensure optimal performance and reliability.
“Always a great experience with Tenacore. These guys either have what I need or can get it,” one client said. “They recently saved us a lot of headache with a large order and got the product to us extremely fast.“
Don’t stop reading now. Tenacore also provides its own signature brand of devices to health care facilities. And, we are almost to the part about the new Vending Management Services!
• O2 BLENDERS: Tenacore’s O2 blenders provide precise and reliable oxygen delivery solutions for healthcare settings. Built to withstand rigorous use, Tenacore O2
“At Tenacore, we’re on a mission to revolutionize the clinical equipment lifecycle with top-notch service and a relentless drive for improvement. Our vision? To be the premier, most trusted source for exceptional healthcare technology.”
– Riley Van Hofwegen, CEO


“As we look to the future, Tenacore remains committed to innovation and excellence.”
– Riley Van Hofwegen, CEO
blenders are designed for durability and ease of maintenance, making them a trusted choice for medical professionals.
• WALL SUCTION REGULATORS: performance, delivering consistent and powerful suction for various medical applications. With intuitive controls and durable construction, Tenacore wall suction are crafted to support patient care with minimal downtime and maximum reliability.
• FETAL TRANSDUCERS:
and dependable monitoring during labor and delivery. They provide exceptional performance and quality, comparable to competing national brands and are available at great cost savings. Lightweight, ergonomic, and easy to clean,
Don’t need a new device? You just want to fix the one you have with a little DIY action? That’s right, Tenacore is your hook up.
• REPAIR PARTS:
Certified, replacement parts for various medical devices.
• PATIENT CABLES:
cables and sensors that meet the highest standards of safety and performance.
“I have been sending infusion pumps, patient monitors and telemetry for service to Tenacore for the past few years. I have always received on time and quality service from the team,” a satisfied customer said.
And now, we learn all about the newest offering from Tenacore!
The Tenacore Vending Management Services delivers outstanding solutions.
It provides Automated Inventory Management.
“Our innovative vending services offer automated inventory manage ment, ensuring that health care providers have immediate access to essential medical supplies and parts,” Riley Van Hofwegen said.
Additional benefits are customized solutions, real-time tracking and cost efficiency.
Here, let me explain with some easy-to-read bullet points.
• CUSTOMIZED SOLUTIONS:
the specific needs of your facility, offering a personalized solution that maximizes convenience and efficiency.
• REAL-TIME TRACKING:
complete visibility and control over your inventory.
• COST EFFICIENCY:
improve operational efficiency, ultimately lowering costs.
“As we look to the future, Tenacore remains committed to innovation and excellence. We will continue to expand our product offerings, enhance our services, and invest in technology to meet the evolving needs of the health care industry. Our focus on quality, customer satisfaction, and sustainability will guide us as we strive to achieve our vision of becoming the leader in medical equipment services and solutions,” Riley Van Hofwegen said.
I know you want to find out more, so I am sharing some contact information below.
WEBSITE: PHONE: EMAIL:















By K. Richard Douglas

Buckminster Fuller, the American architect and designer, once noted; “You never change things by fighting the existing reality. To change something, build a new model that makes the existing model obsolete.”
This may be why certain individuals gain prominence for thinking outside the box. There is often a way of doing something that has historical precedent or time-tested substantiation. In medicine, there is evidence-based medicine that provides clinicians with guidance based on the efficacy of a procedure or therapeutic in the past.
Biomeds have their own evidence-based maintenance that many utilize. The procedures used fall under compliance requirements set forth by regulators. Yet, testing alternatives, when the circumstances demand it, can provide solutions that are workable.
The COVID-19 pandemic challenged biomeds on many levels. It increased the patient load for most hospitals substantially, it restricted access to patients and required a more general use of PPEs and an increased need for respirators and ECMO machines.

At one hospital, one of these pandemic-related challenges was the combination of the numbers of patients being treated with the inability of biomed to access beds for scheduled PMs. How would they inspect, maintain and repair the beds if they had no access? Was there a viable alternative?
“To validate this observation, I conducted a statistical analysis using the Chi-Square test. (A Chi-Square test is a statistical procedure used to determine if there is a significant difference between the observed and expected frequencies of the outcomes. In this case, we looked at the number of bed repairs following the OEM versus the RAN PM schedule.) The results revealed no statistically significant difference in the rate of repairs between the OEM PM schedule and the repair-as-needed (RAN) approach,” Cooksey says.
What happened in confronting this challenge, and the information learned, was put into a 2023 MD Expo presentation by Cooksey, which he delivered at the event in Orlando, Florida.
The challenge of non-conformance, and the search for a solution, leaned on Cooksey’s lengthy Lean Six Sigma training and experience.
Lean Six Sigma is a process improvement methodology that seeks to improve performance by systematically removing operational waste and reducing process variation. It is a combination of Lean Management and Six Sigma.
“Faced with an increased patient census, after COVID restrictions were lifted, we found ourselves unable to access the VersaCare beds and had to postpone our original equipment manufacturer (OEM) preventive maintenance (PM) schedule,” says Mark Cooksey, DME quality engineer at Norton Healthcare in Louisville, Kentucky.
An internal audit resulted in a non-conformance in this area. Faced with having the routine method of inspecting the beds impeded, a quick assessment was in order to devise an alternative approach that would assure that the beds were being maintained and the procedures adapted met scrutiny.
The situation led to some important questions. Did the OEM schedule actually prevent failures? Could beds be put on a repair-as-needed (RAN) schedule without incurring the risk of increasing repair downtime?
Lean Six Sigma is a process improvement methodology that seeks to improve performance by systematically removing operational waste and reducing process variation. It is a combination of Lean Management and Six Sigma.
One of the principles of Lean Six Sigma is to focus on the problem and that is what Cooksey did.
“Not every nonconformance necessitates the full application of a Lean Six Sigma project. We often select tools from our Lean Six Sigma toolkit based on the complexity of the problem at hand. The issues identified with the bed PM nonconformance, tied to a surge in patient census following the easing of COVID restrictions, made it a suitable candidate for a more comprehensive application of the Lean Six Sigma tools,” Cooksey says.
He says that recognizing the surge in patient census as a key factor impeding the department’s preventive maintenance schedule, they decided to leverage their experience with Lean Six Sigma to develop a comprehensive response.
“We assessed the risks using Lean Six Sigma tools, ensuring patient safety and equipment reliability while adapting to the restricted access to preventative maintenance,” Cooksey adds.
The biomed team members at Norton Healthcare hold themselves to high standards. When their audit discovered a problem, meaning non-conformance, that was not an acceptable status. Cooksey suggests changing perspective on this scenario and seeing a non-conformance as an opportunity for improvement.
“Non-conformances can emerge in a multitude of ways: a defective part or vendor service, a customer complaint, an
audit finding, or a deviation from standard work instructions. If a non-conformance requires additional investigation, a formal CAPA (Corrective and Preventive Action) is generated. Our department views any non-conformance as an opportunity for improvement, aligning with our commitment to ISO 13485,” Cooksey explains.
He says that in this case, there was also a cost/benefit analysis of the PM.
“Upon identifying a non-conformance, our immediate step is to evaluate the associated risk. This evaluation helps us understand the severity and potential impact of the non-conformance. We then conduct a root cause analysis to understand the underlying issues. This analysis is crucial in identifying the factors contributing to the non-conformance,” Cooksey says.
At this point, corrective and preventive actions can be formulated and applied.
“Guided by the root cause analysis, we devise immediate corrective actions to address the current non-conformance. Additionally, we establish long-term preventive measures to avoid recurrence of similar non-conformances in the future. In essence, our department’s initial response to any non-conformance is a systematic approach that not only addresses the immediate issue but also aims to prevent future occurrences. This approach underscores our commitment to continuous improvement and quality assurance,” Cooksey says.
In addition to applying Lean Six Sigma principles to the evaluation of the problem, and search for a solution, the team applied ISO 13485 QMS procedures as a guiding standard.
“Relatively few in-house medical device service organizations have achieved ISO 13485 certification, yet it provides a universal structure for maintaining high standards of quality and compliance,” Cooksey says.
He says that the ISO 13485-based QMS helped his team identify the best solution for the VersaCare bed non-conformance. It guided the team in establishing standardized procedures for maintenance and repair, ensuring the safety and performance of the beds. The ISO 13485 QMS provided a framework for identifying and addressing potential issues, minimizing downtime and enhancing patient care. ISO 13485 QMS ensures robust processes for risk assessment, root cause analysis, and implementing corrective and preventive actions.
“In situations like the one mentioned, adhering to ISO 13485 enables us to effectively address non-conformances and
maintain the safety and performance of our medical devices, even under challenging circumstances such as a spike in patient census,” Cooksey says.
He says that seeking ISO 13485 certification can provide numerous benefits to a company that services medical equipment, enhancing its reputation, operational efficiency, and customer satisfaction. It’s a strategic decision that Norton Healthcare chose for the following reasons:
• Quality Assurance: ISO 13485 is an internationally recognized standard that ensures a high level of quality in the design, manufacture, and servicing and is specific to medical devices. By adhering to this standard, the company demonstrates its commitment to providing high-quality services.
• Regulatory Compliance: Many regulatory bodies around the world recognize ISO 13485 certification as a mark of compliance with certain regulatory and legal requirements related to medical devices. This promotes consistency across Norton’s different locations.
• Risk Management: ISO 13485 emphasizes risk management throughout the product life cycle, which is crucial in the medical device industry because of potential impacts on patient safety.
• Customer Confidence: Whether your organization is an in-house servicer like Norton Healthcare or a third-party external servicer of medical equipment, certification can enhance customer confidence in the company’s services, as it serves as an assurance that the company follows internationally recognized best practices in its operations.
• Continuous Improvement: The ISO 13485 standard promotes the implementation of a quality management system (QMS) that focuses on continuous improvement, helping the company to continually refine its processes and services.
• Competitive Advantage: Health care organizations operate in a competitive market. Being ISO 13485 certified can provide a significant advantage, as it demonstrates the company’s commitment to quality and customer satisfaction.
Will the solution stand up to the scrutiny of an audit? That is the last component of the changes instituted to assure continued safety, functionality, cost-savings, resource-utilization and robustness.
The adopted solution must meet muster, reflected in both internal audits and the scrutiny of an accreditation organization’s audit. The use of a tested quality
system by the team means that solutions are carefully crafted and more refined.
“Auditors seek evidence of adherence to procedures and continuous improvement, which is documented through our Corrective and Preventive Actions (CAPA) processes. This ensures that all audits can verify our commitment to maintaining high standards and improving patient care,” Cooksey says.
He says that by integrating ISO 13485 into the department’s processes, they facilitate the evaluation of improvements by auditors, whether they are internal or external DNV auditors.
Cooksey notes that before changing the bed PM to a Repair as Needed (RAN) candidate, they conducted a comprehensive risk assessment, considering factors such as potential patient safety, device reliability and maintenance requirements. They evaluated key PM components and subsystems of the bed, such as: bed frame, hydraulics, mattress, control panel, side rails, casters, brakes, power supply unit and motor assembly.
“The VersaCare bed was selected for this approach due to its high usage rate, limited maintenance access, and the availability of dedicated repair resources. These characteristics made it a prime candidate for a RAN approach, allowing us to optimize our resources and ensure timely maintenance,” Cooksey says.
first place, and we continually review and refine our processes to achieve this. We are committed to continuous improvement and welcome feedback from all stakeholders to help us enhance our PM process,” Cooksey says.
Are there other examples of equipment that might be a good candidate for this approach beyond the VersaCare bed?
“The first step in applying this approach to other equipment is identifying devices with high usage and limited access. Similar to the VersaCare bed, you should evaluate the OEM PM process to understand the preventative measures involved. OEM PMs are designed to prevent failures and potential hazards by ensuring that the equipment is functioning correctly and safely. In theory, regular maintenance can help identify and resolve issues before they become serious problems, enhancing patient safety and device longevity,” Cooksey says.
“Our goal is to prevent these issues from occurring in the first place, and we continually review and refine our processes to achieve this. We are committed to continuous improvement and welcome feedback from all stakeholders to help us enhance our PM process.”
– Mark Cooksey
He says that they found that the OEM PM procedure basically consisted of operating the bed, which is done on a regular basis by end-users.
“The bed has built-in diagnostics and safe stop (fail-safe functionality) to mitigate risks. The observation of the OEM PM procedure, and the low risk, further reinforced our decision to designate the bed PM as a RAN candidate. By leveraging the bed’s built-in features and the regular operation by end users, we can ensure that potential issues are identified and addressed promptly,” Cooksey says.
He added that the CE team frequently brainstorm to identify other devices that might benefit from a similar approach, focusing on those that are often in use and thus hinder the ability to carry out scheduled preventive maintenance (PMs).
“Under our ISO 13485 methodology, we work closely with our customers to address potential issues. This involves regular communication, feedback sessions and collaborative problem-solving to ensure we meet their needs and maintain the highest standards of patient safety,” Cooksey says.
He says that this approach helps mitigate potential patient injuries, inconvenience to clinicians, or other possible electrical or contamination problems that could arise if a problem is discovered when the patient is in a bed.
“Our goal is to prevent these issues from occurring in the
He says that data analysis is used to answer this simple question: “Does the OEM PM schedule reduce failures and improve longevity?”
“Next, conduct a risk analysis to assess the severity of not performing PMs per the OEM schedule. After brainstorming potential hazards associated with the proposed change,” Cooksey says. “The risk analysis must take the following factors into account:
• Risk Severity: The potential impact of the hazard if it were to occur.
• Likelihood of Occurrence: The probability of the hazard occurring.
• Detectability: The ability to detect the hazard “He adds that medical devices with hazards of low severity, relatively rare occurrence, and that have self-diagnostic capabilities or safe stop functionality are ideal candidates for a RAN PM schedule.”
In the end, evaluating a PM cost/benefit analysis from a risk perspective and a calculation of FTE savings are just two of the considerations in moving from an OEM PM schedule to a repair-as-needed approach.
The old adage that states that “when life gives you lemons; make lemonade” has a real application in the biomed department when the right quality system and problem-solving model can be applied to form a solution.
All of these considerations can help an HTM department apply logic from the experience of the CE department at Norton Healthcare.
Scan the QR code to access the MD Expo presentation on this topic.



As an in-house manufacturer, Elite is dedicated to providing high-quality replacement parts and services for medical devices to improve patient care nationwide. We are FDA Registered and dual ISO Certified (ISO 9001:2015 and ISO 13485:2016) resulting in complete traceability from raw material to finished goods.

BY KATHLEEN FURORE
The offer of a new job can be exciting but stressful at the same time – especially if you’ve been offered a position that sounds interesting, but involves a lateral move. How can you decide if it makes sense from a career perspective?
“When it comes to lateral moves, I think it’s time to ditch the traditional view that upward is the only direction worth considering,” says Chris Sorensen, CEO of the sales dialer company PhoneBurner. “Instead, think of your career as a mosaic rather than a ladder. A lateral move could be the piece that adds unexpected value to the bigger picture.”
Focus on growth potential is the advice the business pros I reached out to offered.
That can be more important that the immediate title or salary, according to Lissa Poirot, head of content at Joy Wallet, a publisher network focused on financial education.
“A lateral move might not seem like a step up, but it can open doors to new experiences, networks, and skill sets,” explains Poirot, who suggests considering if the new role offers exposure to different aspects of the business or allows you to develop skills that are in demand in your industry.
“For instance, if the position involves working with new technology or managing a different type of project, it could set you up for future advancement that wouldn’t be possible in your current role,” Poirot says. “Think long-term: If this move can broaden your expertise or position you for a more strategic role down the line, it’s worth considering.”
David Primrose, president of Metal Marker Manufacturing, echoes Poirot’s advice.
“Zoom out and look at the big picture. Think about how this new role fits into your career roadmap,” Primrose suggests. “Will it help plug some holes in your skill set or give you a peek into parts of the business you haven’t explored yet? Sometimes, taking a step to the side can actually fast-track your career by making you a more well-rounded pro.”
There are myriad examples of people who have taken a lateral move that turned into a positive career step. Sorensen shares one with which he is personally familiar.
“One of the most successful professionals I know made a lateral move from engineering to customer support, which at first seemed like a detour,” Sorensen says. “But the deep understanding he gained of customer pain points led him to develop a game-changing product feature, catapulting his career forward in ways he hadn’t anticipated.”
The key, he adds, is to stay open to where these lateral moves might lead.
“They might just unlock the door to the next great chapter of your career that you didn’t even know was there,” he concludes.

Kathleen Furore is a Chicago-based writer and editor who has covered personal finance and other business-related topics for a variety of trade and consumer publications. You can email her your career questions at kfurore@yahoo.com.
BY VINCENT OLIVA, TECHNICIAN III
What is an oxygen blender?
A medical oxygen blender is a specialized device used to precisely mix medical grade oxygen with medical grade air to deliver a specific concentration of oxygen to patients. This device plays a crucial role in various medical settings, including hospitals, clinics, and emergency care environments, where accurate oxygen delivery is essential for patient care. Here’s a breakdown of what a medical oxygen blender does:
• Mixed Gases: The primary function of the oxygen blender is to mix pure medical oxygen with room air (which contains approximately 21% oxygen) to create a gas mixture with a precise oxygen concentration. This allows healthcare providers to tailor oxygen therapy to the specific needs of individual patients.
• Provides Adjustable Oxygen Concentrations: The device can be adjusted to deliver different levels of oxygen concentration, typically ranging from 21% (room air) to 100% oxygen. This flexibility is vital for managing various respiratory conditions, such as
chronic obstructive pulmonary disease (COPD), hypoxemia, and neonatal respiratory distress.
• Delivers Controlled Oxygen Therapy: The oxygen blender ensures that patients receive a steady and accurate concentration of oxygen, which is critical for effective treatment. This controlled delivery helps prevent complications associated with both too much and too little oxygen.
• Real-Time Monitoring: Many modern oxygen blenders come equipped with sensors and monitoring systems that provide real-time feedback on the oxygen concentration being delivered. This helps in maintaining the correct oxygen levels and allows for immediate adjustments if needed.
• Supports Various Delivery Devices: The blended oxygen is typically delivered to patients through various devices such as nasal cannulas, oxygen masks, or ventilators, depending on the patient’s condition and requirements.
• Enhances Safety and Efficiency: By providing precise control over oxygen concentration, the blender helps optimize patient outcomes and minimizes risks associated with improper oxygen levels, such as oxygen toxicity or insufficient oxygenation.
In summary, a medical oxygen blender is an essential tool in healthcare settings that ensures the accurate delivery of oxygen therapy tailored to each patient’s specific needs, playing a critical role in respiratory care and management.
Inaccurate Oxygen Concentration
Possible Causes:
• Calibration Issues: The blender may need recalibration.
• Faulty Sensors: The sensors that measure the oxygen concentration might be malfunctioning.
• Air Leaks: There may be leaks in the connections or fittings.
Steps to Resolve:
• Recalibrate the Device: This often involves setting the device to known concentrations of gases and adjusting as needed.
• Check for Leaks: Inspect all connections and fittings for leaks. Tighten or replace any components as necessary.
• Verify Sensor Functionality: Sensors might need cleaning or replacement if suspected to be faulty.
Erratic or Fluctuating Flow Rates
Possible Causes:
• Obstructions: There may be blockages in the gas lines or filters.
• Faulty Flow Meters: The flow meters or regulators may be malfunctioning.
Steps to Resolve:
• Inspect for Blockages: Check and clear any obstructions in the gas lines or filters. Replace any clogged filters if needed.
• Examine Flow Meters: Verify that the flow meters are functioning correctly and replace them if necessary.
Alarm or Error Messages
Possible Causes:
• Low Gas Supply: The oxygen or air supply might be running low or empty.
mixers or valves may be malfunctioning.
Steps to Resolve:
• Recalibrate the Blender
• Inspect Internal Components: Check for any signs of wear or damage in the internal components. If necessary, consult the manufacturer for repair or replacement parts.
Noise or Vibration
Possible Causes:
• Loose Parts: There might be loose or improperly secured components.
• Mechanical Issues: Internal mechanisms may be malfunctioning.
Steps to Resolve:
• Tighten Components: Ensure that all external and internal components are securely fastened.
• Consult Manufacturer: If the noise or vibration persists, consult the device manual or contact the manufacturer for technical support.
Medical oxygen blenders are essential for delivering precise oxygen levels tailored to each patient’s needs, making their proper function critical in healthcare settings.
• Internal Malfunctions: There might be an internal issue with the device.
Steps to Resolve:
• Check Gas Supplies: Ensure that the oxygen and air supplies are adequate. Replace or refill cylinders as needed.
• Review Error Codes: Consult the device manual for the meaning of any error codes or alarms and follow the recommended troubleshooting steps.
Inconsistent or Poor Mixing
Possible Causes:
• Calibration Problems: The blending mechanism might be out of calibration.
• Component Issues: Internal components such as
Medical oxygen blenders are essential for delivering precise oxygen levels tailored to each patient’s needs, making their proper function critical in healthcare settings. To ensure these devices continue to operate effectively, original equipment manufacturers (OEMs) recommend overhauling oxygen blenders every two years. Regular overhauls help maintain optimal performance, prevent common issues like inaccurate oxygen concentration or erratic flow rates, and ensure patient safety. Avante is here to assist with these overhauls, providing the expertise and support needed to keep your oxygen blenders functioning at their best and enhancing patient care outcomes.
For more information about Avante’s oxygen blender repair capabilities, call one of our experts at 800449-5328 or visit avantehs.com
- Vincent Oliva is a Level II Technician in the patient monitoring division at Avante in San Clemente. With five years of experience at Avante, he specializes in medical oxygen blenders and suction regulators.
BY GARRET SEELEY
In all the complexities of a modern hospital network, employees often take for granted the reliability of a network. Sure, it is obvious when a device will not communicate at all, such as when a cable is disconnected from a network switch. However, some troubleshooting involves looking for intermittent issues. This is especially true with wireless devices. Despite the biomedical field’s effort to reduce these intermittent issues, we still end up troubleshooting wireless systems. This is how.
Let’s first start by discussing an assumption. Namely, if the Wireless Medical Telemetry Service (WMTS) ruling by the Federal Communication Commission (FCC) should prevent a lot of telemetry problems. It does help a great deal. To summarize the history of the WMTS, since the early 2000s, medical devices were granted their own frequency. It is not supposed to conflict with surrounding wireless devices. This is called the WMTS band and is the 608 to 614 MHz range, just outside of the emergency services radios. To capitalize on newer GHz technology, a smaller section was created using the 1395 to 1400 MHz and 1427 to 1431 MHz bands. These frequencies are important as they describe who will compete for the same radio bandwidth, which is the individual channel used to communicate information.
In theory, nobody other than a licensed WMTS system should be in operation around a medical facility. Such devices must be registered with the American Society for Healthcare Engineering (ASHE) to operate, this includes healthcare facilities departments. However, when there is a gap in transmissions, people try to gain access. In Austin, Texas, for example, the 1427 range is limited to a 1429 to 1431.5 MHz cap because of existing transmissions below 1429 MHz. Occasionally, television transmissions and the public use walkie-talkies and sometimes Ham radio will use the same frequencies as well. Although there are restrictions on newer equipment, existing transmissions were grandfathered into the law and may still affect WMTS telemetry systems.

For these reasons, we must use the same techniques to troubleshoot wireless networks in WMTS networks. However, due to the frequencies used, we cannot use standard tools such as off-the-shelf wireless network site scanners. For a biomed to check for interference, we must perform a site evaluation using a spectrum analyzer. There are a wide range of options ranging in price. Just make sure the frequencies the scanner is tuned to will include the entire range of 600 to 1500 MHz This is a typical frequency range for UHF transmissions. Often the scanners for that equipment will also tell if there is an interference in these ranges. When selecting an analyzer, I suggest one with a waterfall option, which records bands over time. In this way, it is easier to see periodic burst transmissions that a display may be too fast to see on the display. Try getting readings or scanning for transmissions in the area around the hospital or when the telemetry system is not in use, such as during upgrades. If need be, the waterfall or logging method can show spikes that interfere with the telemetry transmission. Keep in mind that the telemetry systems should list their frequencies of use, so setting the detection of signals that are being interfered with should be straightforward.
A way to see dead zones (a place without a signal) is with a heat map. In this technique, take measurements of the dB intensity of the telemetry signals and overlay this data on a floor plan. Use topographical map-style circles or groupings to show the intensity of transmissions and their locations. A
heat map is typically shaded in color to describe the intensity of the transmission. This is a very useful tool when looking for losses in WMTS coverage. Such zones occur when a telemetry transmitter is the same distance to multiple access points. Much like a cell phone in a car, the transmitter doesn’t know which “tower” or access point to communicate with. In such areas, dropouts naturally occur. This effect is in every wireless network using multiple transmitters. A heat map is the easiest way to see inadequate coverage or potential dropout zones.
For familiarity, I suggest contacting IT for help troubleshooting wireless systems. Although their tools may differ, the concepts are the same. They have a great deal more experience troubleshooting wireless transmissions. They use heat maps and site scanners (networking-specific spectrum analyzers). Unlike biomeds, their equipment is not licensed, and the tools are much easier to get familiar with. Perhaps ask a friend in the field how they troubleshoot such issues. Remember to ask installers of WMTS systems if they are familiar with any of these techniques. You may be pleasantly surprised.

Garrett Seeley, MS, CBET, IS A Biomedical Equipment Support Specialist-Imaging with VISN 17: VA North Texas Health Care System/ Dallas Veterans Affairs Medical Center.



BY HEATHER MARTIN, RN, BSN
As a nurse who now specializes in healthcare technology management (HTM) solutions, I’ve experienced firsthand the challenges of managing mobile medical equipment in a bustling hospital setting. Let me share my journey and how my transition from bedside care to HTM solutions has shaped my understanding of these critical issues.
It was another hectic shift in the hospital. I was juggling a heavy assignment – caring for post-OR patients, administering medications, providing wound care treatments, collaborating with the health care team, admitting and discharging complex patients, documentation and more. The pace was relentless, and every minute of my day was filled with interruptions.
During this particularly chaotic shift, I got a new admission and urgently needed an infusion pump. I finally found one in the soiled utility room. In the rush, I wiped it down and set it up. My focus was to check another box and move forward. But did I follow the specific cleaning protocols? Likely not, and this lapse could have potentially exposed my patient to infection or even damaged the equipment.
This scenario isn’t unique to me. Many health care workers face similar dilemmas. Struggles with equipment availability, proper cleaning and interdepartmental coordination often lead to inefficiencies and mistrust within the hospital setting. Nurses might stash equipment away “just in case,” and during patient discharges, crucial items can get mixed with linen or trash, resulting in their destruction. Equipment also goes missing – whether through theft or simple misplace-
ment – compounding the problem. These issues create a ripple effect: biomedical technicians face difficulties with preventive maintenance completion due to unavailable equipment, supply chain departments wrestle with the need for additional rentals or purchases, and frustration between departments builds.
So, how do we tackle these challenges effectively? Enter Tech Knowledge Associates (TKA) and their READI+ program. This innovative solution is designed to streamline mobile medical equipment management and enhance hospital operations. READI+ is a key part of TKA’s broader healthcare technology management (HTM) services, aimed at solving these pervasive issues.
READI+ stands for Reliable Equipment, Available, Disinfected and Inspected. It’s a comprehensive program designed to address common equipment management issues. Here’s how READI+ can revolutionize hospital operations:
• Dedicated Equipment Management Team: READI+ assigns a specialized team to manage mobile medical equipment. This team is responsible for equipment movement, processing, and management, ensuring everything is available, properly cleaned, and efficiently tracked. This dedicated approach reduces the burden on clinical staff and prevents issues like improper cleaning and equipment damage.
• Real-Time Location System (RTLS): READI+ integrates RTLS technology to track tagged equipment throughout the hospital. This system minimizes the risk of equipment being misplaced, lost or stolen. Staff can quickly locate and retrieve items, reducing downtime and frustration. RTLS also aids in inventory management and predictive maintenance by tracking equipment usage patterns.
• Enhanced Visibility and Accountability: By centralizing equipment management and tracking, READI+ reduces the “blame game” between departments. Nurses no longer need to hide or hoard equipment, biomedical

technicians can access equipment for maintenance more efficiently, and supply chain departments can make better decisions regarding rentals and purchases. This transparency fosters a more collaborative environment.
• Improved Hygiene and Compliance: READI+ emphasizes rigorous disinfection and inspection protocols. With dedicated staff managing these tasks, equipment is cleaned to manufacturer standards before patient use. This approach reduces cross-contamination risks and ensures compliance with infection control protocols and regulatory standards.
READI+ is a complement to TKA’s broad range of clinical engineering capabilities:
• Comprehensive Equipment Maintenance: TKA provides proactive and reactive maintenance services. Its certified biomedical technicians perform routine checks, calibrations, and repairs to ensure equipment reliability and extend its life cycle.
• Technology Integration and Support: TKA assists hospitals with integrating new technologies into existing systems. They offer configuration, software updates, and troubleshooting to ensure smooth operation and minimal disruptions.
• Regulatory Compliance and Risk Management: TKA helps hospitals navigate complex regulatory requirements and implement risk management strategies. TKA ensures that all equipment meets safety standards and complies with industry regulations.
• Training and Education: TKA offers training programs on equipment usage, maintenance and best practices. This training empowers staff to use equipment effectively and adhere to safety protocols.
• Strategic Planning and Consultation: TKA provides consulting services for long-term technology management, including equipment life cycle planning, budget forecasting and technology assessments.
Implementing READI+ alongside TKA’s comprehensive HTM services can bring significant benefits to hospitals:
• Increased Efficiency: With a dedicated team managing equipment and robust clinical engineering support, staff
can focus on their core responsibilities, leading to a more streamlined workflow and improved patient care.
• Reduced Equipment Loss and Damage: RTLS and organized management minimize the risk of equipment going missing or being damaged. Hospitals can better track usage and ensure equipment longevity.
• Enhanced Interdepartmental Trust: READI+ and TKA’s HTM services promote accountability and transparency. Departments can rely on accurate information and timely access to equipment, reducing conflicts and enhancing collaboration.
• Cost Savings: Improved equipment management and strategic planning reduce the need for rentals and unnecessary purchases. Hospitals can make more informed decisions, leading to cost savings and better resource allocation.
Tech Knowledge Associates’ READI+ program, as part of its comprehensive HTM services, offers a practical solution to the common challenges of mobile equipment asset management. By providing a dedicated team, leveraging RTLS technology, and focusing on reliability, disinfection, and inspection, READI+ tackles the root causes of equipment-related issues. Importantly, READI+ fosters a culture of trust and collaboration between nursing, clinical engineering and supply chain teams. By centralizing equipment management and improving transparency, it eliminates the blame game and promotes a more cohesive working environment. Nurses can rely on well-maintained and readily available equipment, clinical engineers can perform timely maintenance without disruptions, and supply chain staff can make informed decisions based on accurate data. In a high-stakes environment where every moment counts, having a reliable system and robust support isn’t just an option – it’s a necessity.

Heather Martin, RN BSN is the Director of Business Development at TKA.
BY ROGER A. BOWLES, MS, EDD, CBET
When I enrolled in the biomedical equipment technology program at Texas State Technical College in Waco, Texas, over 35 years ago, starting a business was the last thing on my mind. Growing up, I was always taught that I should get a steady job, work 40 hours a week for 40 years with one company and then retire. That was the American dream, or so I thought.
In those days, the main things I thought about were how much I was making an hour and what the health benefits included. The program had classes in electronics, biomedical equipment, the typical academics like English and math, a computer course (Word Perfect, databases, and a spreadsheet program I can’t remember) and a couple of internship courses. There wasn’t a business course. I worked for two great hospitals as a biomedical equipment technician.
When I had the chance to teach at Texas State Technical College over 27 years ago, I jumped at it. It was a steady job, doing something I loved doing and I didn’t care that it didn’t pay as much as I could have made in the field. I figured I would retire here and that will be the case in a few more years. I can’t complain. It has been a good run.
Recently, I began noticing that many former students of mine are now entrepreneurs and run their own businesses in HTM (and some outside of HTM). I have also started a couple of part-time businesses outside of HTM and they may soon become full-time endeavors. Some of the things I’ve learned along the way of starting those businesses could have been easier if I would have had the sense to take an entrepreneurial class or two first. These former students of mine are doing excellent. I thought I would ask them some questions about how they started and why they gave up the regular 40-hour week working for someone else.
Jacob Rowan, CEO of Prime Solutions Biomedical LLC, told me that while he was in school he really didn’t think about starting a business but he was definitely interested in pursuing a leadership position. His drive to start his own business stemmed from his experience as a BMET and his
connections. He said a basic business course about the process of starting a business, taxes, deductions, etc., would have been helpful. He believes, as do I, that financial instability/security is a big reason BMETs don’t start their own business. His business is doing well, growing tremendously in the past 6 months, with 8 total employees and much more growth expected in the coming months.
Bill Picot, CEO of Q1 Medical Equipment LLC, is a familiar name to many BMETs because he has been around a while. He has worked as a BMET for a major hospital in Dallas and as a senior manager for a medical supply company. He has always been an entrepreneur and even thought about it when he was a student at TSTC. He has a couple of businesses, but Q1 Medical Equipment is the most recent and is growing quickly. He plans on a tenfold increase in business in the next 5 years. He agrees that being your own boss can be risky, but it is rewarding at the same time.
Ted Kehtel, CEO of IXS Technologies LLC, is another former student with the entrepreneurial spirit. He believes that some people just have that attribute. I agree with you Ted! I just wish I would have started sooner. IXS Technologies has about 14 employees now and is growing. Ted is also part owner of another biomedical company and has three real estate companies and a gun company. He believes that having several income streams is the key. I’m just starting to realize this myself. It is amazing what teachers can learn from their students!
Omar Ahmed Sandhu, CEO of Mr. Biomed Tech Services, another former student, knew from the beginning that he wanted to have his own business in the biomedical industry. He set out to earn his technical degree and gain experience in the field in order to accomplish this. He credits his upbringing with having the entrepreneurial mindset. He too is doing very well, and I admire his success.
There are more students that are successful business owners, and I wish I could list them all. Looking back, I wish I had started my entrepreneurial journey much earlier. However, it is never too late. I plan on introducing some of these entrepreneurial concepts to students, if even on a conversational basis in class.

Roger A. Bowles, MS, EdD, CBET, is a professor of biomedical equipment technology at Texas State Technical College.
Your Ultrasound Par tner
Diagnostic Imaging Solutions




FREE TECH SUPPORT
FREE TECH SUPPORT
QUALIT Y, TESTED PARTS
QUALIT Y, TESTED PARTS
SERVICE TRAINING
ULTRASOUND SERVICE TRAINING
PROBE REPAIR WITH FREE LOANERS
PROBE REPAIR WITH FREE LOANERS
SERVICE ACROSS THE US
SERVICE ACROSS THE US
Mention this ad for 10% OFF your nex t par t or repair.
Mention this ad for 10% OFF your nex t par t or repair.
HAVING PROBLEMS WITH YOUR ULTRASOUND PROBE?
HAVING PROBLEMS WITH YOUR ULTRASOUND PROBE?
Scan the QR code and get an instant quote by using our free ultrasound probe evaluation tool.
Scan the QR code and get an instant quote by using our free ultrasound probe evaluation tool.

BY TED LUCIDI, CBET
e ’re going to continue focusing on some of the key components within an ultrasound probe. If you missed last month’s content (tinyurl.com/39jdwhak) , we focused on the acoustic lens. Many consider it to be just a rubber membrane that contacts the patient. The truth is that the lens is much more complex than it appears, and it serves multiple purposes.
The most technically complex component within an ultrasound probe is the acoustic array. Ever wonder why ultrasound probes are referred to as transducers? A transducer is just a generic term for any device that converts energy from one form to another. Technically, a speaker is a transducer, as is a microphone. Following that thought stream, a lightbulb is a transducer, as is a thermistor, as is a solar panel. Each of these examples converts energy from one form to another. Look out, here comes the piezoelectric element. It has the ability to convert electrical energy into sound (technically mechanical) energy, and then reverse the process by converting sound (or mechanical) energy into electrical energy. Few, if any, other materials are able to translate energy in this manner. Think about it, a lightbulb cannot create electrical energy as a result of light being applied to it. To the layperson, the array might just seem to be some type of mystical component.
At the heart of an acoustic array are piezoelectric elements.
Some still refer to these as “the crystals.” Why? In the early days of ultrasound, researchers discovered that applying pressure to crystals, such as quartz, tourmaline and Rochelle salt generated electrical energy. The term stuck. The colloquial term “crystals” is quite a departure from the highly advanced acoustic materials used in today’s acoustic arrays. For much of the time that ultrasound technology has existed, ceramics, such as PZT (Lead Zirconate Titanate), have been used in acoustic arrays. Now, single crystal and CMUT technology are in mainstream use. We won’t dive into the technical differences or advantages and disadvantages of each. Keep in mind that, as acoustic arrays have become more advanced, they have also become more fragile.
The acoustic array is much more than just a collection of piezoelectric elements. In design and manufacturing, the array is referred to as the acoustic stack. Today’s acoustic arrays resemble a sandwich that consists of multiple layers. At the core of the array are the piezoelectric elements which are commonly referred to as the “crystals.” Despite the size and weight of the entire array, the elements themselves, are extremely small. The height or thickness of a single element may be less than 0.4mm. Their thickness is typically compared to that of less than a hair.
Beneath the array is a backing material to which the elements are bonded. The backing material serves two purposes, 1) To dampen the high frequency vibrations of the elements and provide a crisp clean signal, and 2) To direct the acoustic energy created in the appropriate direction … out of the probe, and into the patient.
On top of the elements are acoustic impedance matching layers. Matching layers are designed to maximize energy transfer from the array into the patient. The scan or acoustic coupling gel, applied between the probe and the patient is another type of a matching layer. Without scan gel, it’s almost

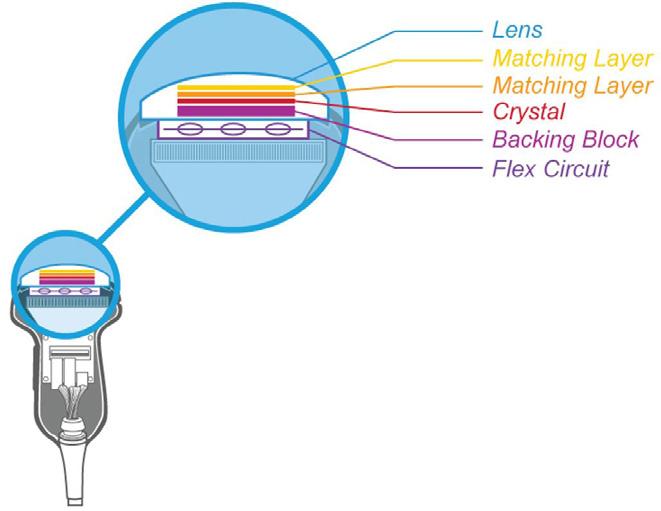
impossible to acquire an image. The latest matrix array probes from Philips utilize 10 matching layers between the acoustic elements and the patient.
We can say, with confidence, that a strong majority of probes sent-in for repair have presented with performance problems because of traumatic array damage. Yet, there is no, or very little, evidence of an impact to the probe itself. Knowing that the ceramic-like acoustic elements are extremely thin and fragile, and knowing that the scan head weighs about a pound … when a probe falls to the floor or is accidentally dropped, the impact shatters a portion of the elements. The damaged elements may no longer resonate at the designed frequency or may not function at all.
Another root cause of array damage is gross fluid invasion, related to TEE probes and other probe types that are high-level disinfected. If there’s a break in physical integrity (missing seal, hole in the lens, or open seam) disinfectant can enter the probe, short-circuit the scanhead electronics, and/ or damage the array.
Typically, traumatic damage presents as a dark vertical line or vertical area of no information, called image dropout. In fact, ANYTHING that causes an acoustic element not to function can result in image dropout. Damage via fluid invasion (or internal contamination) usually presents as

intermittent vertical white lines, streaking through the image. With more advanced probe designs, such as the Philips X5-1c, X8-2t, or GE 6VT-D, error codes may be displayed on the scanner console.
For years, a damaged acoustic array translated to a probe’s replacement. Since 2008, Innovatus Imaging has had the ability to surgically repair or fully replace the acoustic array on over 100 of the most popular probe models. We maintain an FDA-registered manufacturing facility and acoustic lab in Denver, Colorado, which manufactures ultrasound probes for several prominent OEMs. They also develop precise replacement arrays for some of the most popular probe makes and models. By being able to repair or replace the array, fully replace the wiring harness, and perform complex electrical repairs, Innovatus is able to restore your probe’s performance … not just get it working again. And, we do so at a fraction of the cost of a replacement. For more information on how to identify array damage or to request a copy of our how-to guide on performing probe assessments, reach out to training@innovatusimaging.com

Ted Lucidi, CBET, is the director of commercial operations and business analytics at Innovatus Imaging.
BY NADIA ELKAISSI, CHTM
Picture this: Dr. Smith was a well-respected oncologist, known for his forward-thinking approach to patient care. Always eager to embrace new technology, he stumbled upon a sleek, new Bluetooth-enabled chemotherapy infusion pump that promised to streamline his workflow by allowing him to monitor a patient’s treatments directly from his smartphone. It sounded perfect. The only issue was that the device was not Federal Information Processing Standards (FIPS) compliant. Dr. Smith had previously encountered the term “FIPS” in relation to Wi-Fi capabilities and assumed that using the equipment’s Bluetooth functionality would be similarly acceptable. The vendor neglected to fully disclose that while the device’s Bluetooth capability was cutting-edge, it was unencrypted, leaving it vulnerable to cyber threats. Within weeks of integrating the pump, a cybercriminal intercepted the unencrypted Bluetooth signal, and began to manipulate the dosage data. This resulted in a patient receiving a dangerously incorrect amount of medication. The incident, which went unnoticed until after the damage was done, led to a significant patient safety event. This scenario underscores the critical risks associated with deploying non-compliant technology in health care environments. Dr. Smith learned quickly that prioritizing convenience over security can have catastrophic consequences.
In the ever-evolving world of medical technology, it’s easy to get swept up in the excitement of new technology. However, as Dr. Smith learned the hard way, a little due diligence can prevent a lot of damage. With new technology, there is an increasing need to understand and know how to secure the
new wireless technologies, especially Bluetooth. So, what is Bluetooth, and why is it becoming more common in medical devices than Wi-Fi? With the ongoing expansion of telehealth and the need for reliable, secure wireless communication, Bluetooth has become more prevalent in medical devices than ever before. Bluetooth is a radio frequency (RF) communication protocol that enables the short-range data exchanges between devices. It is energy-efficient and reliable, using frequency-hopping to minimize interference with other wireless technologies. For patients managing their health remotely, Bluetooth-enabled devices like blood pressure monitors, glucose meters, and wearable ECGs provide critical connectivity, transmitting vital health information directly to health care providers without the need for extensive network infrastructure.
As Bluetooth continues to play a larger role in the medical field, it is essential to prioritize the most secure Bluetooth configurations and document potential threats associated with the implementation of the medical technology. As wireless communication between medical devices becomes more prevalent, a solid understand of Bluetooth security measures – such as pairing methods, encryption, frequency hopping, authentication, and authorization becomes vital. Following are some key considerations and questions that should guide discussions with vendors when evaluating Bluetooth-enabled medical devices:
When evaluating a Bluetooth configuration, the best way to start is with the device’s pairing method. New technologies often boast quick and easy ways to establish connections, but these can be vulnerable if not properly secured. For instance, the “Just Works” pairing method, while convenient, offers minimal protection and leaves the device susceptible to man-in-the-middle attacks. It’s advisable to inquire about additional security measures, such as a Passkey Entry or Numeric Comparison method, which requires user verification before pairing.
When discussing Bluetooth capabilities, it is important to assess the encryption algorithms in use. Ask for the Bluetooth version and specific features, to provide an accurate representation of the device’s wireless communication technologies. Vendors may claim that their products are Bluetooth-compatible, but they often fail to provide specifics about the Bluetooth security mode, levels, or version. Security modes will define how devices authenticate, encrypt and manage access during communication. There are 4 different levels of security modes and Security Mode 4 is mandatory for all Bluetooth 2.1 and later devices since it enforces Secure Simple Pairing (SSP) and supports robust encryption standards. Security levels, on the other hand, categorize the strength of security within a mode, ranging from unauthenticated, low-security connections to high-security connections that require encryption and mutual authentication. Using higher security levels within the appropriate security modes ensures that data transmitted over Bluetooth is protected against threats like unauthorized access. Some questions you can ask a vendor to understand how the device is configured:
• Which Bluetooth version does the device use? Always look for the latest version possible as it will over more advanced secure capabilities.
• What security mode(s) does the device support? Remember that Security Mode 4 is mandatory for Bluetooth 2.1 and later.
• Is the Bluetooth communication encrypted? If so, what type of encryption is used? Ask if the device uses AES-CCM encryption (with 128-bit keys), which is standard in Bluetooth for protecting data in transit.
Bluetooth’s Frequency Hopping Spread Spectrum (FHSS) technique is a cornerstone of Bluetooth’s security architecture. It minimizes the likelihood of interference by rapidly switching the communication channel between different frequencies. When two Bluetooth devices communicate, they “hop” between different frequencies within a designated range. Bluetooth uses 79 channels in the 2.4 GHz frequency band and can switch between channels up to 1,600 times per second. The changing of frequencies makes it difficult for other devices to interfere with the connection and helps Bluetooth stay within Federal Communication Commission (FCC) guidelines. During the evaluation, confirm that the device employs FHSS and inquire about its compliance with the FCC’s guidelines, which are designed to ensure co-existence among multiple wireless systems operating within the same frequency band.
Authentication and authorization are critical components of Bluetooth security, working in tandem with pair methods to ensure that only trusted devices can connect and access sensitive data. Having a medical device with Bluetooth enabled and no authentication poses significant security risks. Without authentication, the device is vulnerable to unauthorized access, allowing malicious actors to connect and potentially manipulate or steal sensitive data. While authentication verifies the identity of a device before allowing to pair, authorization controls what a device is permitted to do once connected. When discussing Bluetooth security with a vendor, it’s important to confirm what method of secure authentication the device uses. You should also inquire about controls in place to ensure that connected devices have access only to the data and functions necessary for their intended use.
While understanding Bluetooth security for medical devices is crucial, it is equally important to take specific steps to ensure that clinical staff are using these devices correctly, particularly when connecting the device to a phone or tablet. Even the most secure Bluetooth setup can be compromised if staff are not properly trained or if they inadvertently connect to unauthorized devices. Comprehensive training, clear protocols, and regular audits are essential to ensure that staff are pairing devices securely and following best practices. Additionally, limiting device connectivity to approved phones or tablets and using secure applications can further safeguard against unauthorized access. By combining robust security measures with thorough staff education and adherence to protocols, health care facilities can effectively protect patient data and maintain in the integrity of Bluetooth-enabled medical devices.
Bluetooth offers numerous advantages in medical technology such as telehealth and therefore it’s critical to evaluate its security architecture thoroughly. By choosing configurations that maximize security – through strong pairing mechanisms, robust encryption and effective authentication – you can ensure that your healthcare environment remains protected against emerging threats.

REFERENCE:
Nadia ElKaissi, CHTM, is a biomedical engineer with healthcare technology management at VA Central Office (19HTM).
National Institute for Standards and Technology (NIST) Interagency or Internal Reports 8267 DRAFT, Security Review of Consumer Home Internet of Things (IoT) Products, October 2019
Website: www.InternationalXrayBrokers.com Email: admin@intxray.com Call:







•
•
•
•




BY PHIL ENGLERT
The ISO 81001-5-1:2021 standard Health software, and health IT systems safety, effectiveness, and security provides guidelines for the cybersecurity of health software and health IT systems, including medical devices. Part 5-1 focuses on security activities in the product life cycle. This standard is critical for ensuring that medical devices are secure by design, protect patient data and maintain the integrity of health care operations. Japan, Singapore and the European Union have harmonized on the standard for assessing cybersecurity controls during the regulatory review process. The FDA calls out 81001-5-1 three times in the premarket guidance sections on the Secure Product Development Framework (SPDF) to manage cybersecurity risks and implement security controls and testing requirements. When procuring medical device technology, health care providers can leverage the cybersecurity requirements of ISO 810015-1 to reduce risks introduced to their networks and clinical environments. Let’s review the cybersecurity considerations of ISO 81001-5-1 and then discuss how to leverage the standard during the procurement process.
The Secure Product Development Framework (SPDF) provides manufacturers with a set of processes that, when effectively implemented, can help manufacturers demonstrate a reasonable assurance of safety and effectiveness during the regulatory submission process. Manufacturers should integrate security into each phase of the development process, from design to deployment. The standard outlines the need for a secure development life cycle, which includes secure coding practices, regular security testing, and code reviews. The standard emphasizes a robust threat modeling
and risk management process, including identifying, assessing and mitigating cybersecurity risks throughout the device’s life cycle. Medical device manufacturers must perform threat modeling and continuously evaluate the potential cybersecurity risks posed by their devices. Device design should include security features that protect against unauthorized access, tampering and other security threats. Security measures should include implementing strong authentication, encryption and secure communication protocols within the device. Manufacturers must establish a process for monitoring, identifying and addressing vulnerabilities in medical devices, including a commitment to providing timely security patches and updates to address newly discovered vulnerabilities. The 81001-5-1 mandates that medical devices must protect sensitive data, including patient information, from unauthorized access and breaches. The standard recommends data encryption at rest and in transit to ensure data integrity and confidentiality.
Manufacturers should develop an incident response plan to address cybersecurity breaches, including communication, containment and recovery procedures to ensure devices can be restored to a secure state following an incident. ISO 810015-1 emphasizes the importance of robust access controls such as multi-factor authentication (MFA) and role-based access controls (RBAC to limit device access to authorized personnel only). Manufacturers should perform regularly rigorous security testing, including penetration testing, vulnerability assessments and validation of security controls under realistic conditions to identify and address potential security flaws. Finally, the standard mandates comprehensive documentation of the device’s security features, risk management processes and incident response procedures. This documentation should be made available to health care providers to assist them in understanding and managing the cybersecurity risks associated with the device.
In summary, the eight primary product development functions required by ISO 810001-5-1 are:
• Secure Software Development Lifecycle (SDLC)
• Risk Management and Threat Modeling

• Security by Design
• Data Protection and Privacy
• Incident Response and Recovery
• User Access Control
• Security Testing and Validation
• Documentation and Transparency
When procuring medical devices, health care providers can leverage the requirements of the ISO 81001-5-1 standard to ensure that the devices they acquire meet high cybersecurity standards, thereby reducing risks to the organization, protecting patient data, keeping patients safer and building organizational resilience.
Begin by including specific ISO 81001-5-1 cybersecurity requirements in requests for proposals (RFPs) to ensure that only vendors understand and adhere to these standards and can meet stringent cybersecurity criteria are considered. One tool that may assist with this process is PDM24064 Software Acquisition Guide for Government Enterprise Consumers, released by CISA on August 1, 2024, and developed in response to the core challenges of software assurance and cybersecurity transparency in the acquisition process. Evaluate potential vendors based on their adherence to ISO 81001-5-1 and overall cybersecurity approach to incentivize vendors to prioritize cybersecurity, knowing it is a key factor in purchasing decisions. Verify that the device has been developed with security in mind and is regularly assessed for vulnerabilities by requesting detailed documentation from vendors, including evidence of compliance with ISO 81001-51, cybersecurity risk assessments and security testing results. Manufacturers may resist providing documentation submitted for regulatory review, but you want to understand the identified threats and how component selection and controls mitigate them. You have a right to know the residual risks and how they can be managed within your environment. When
practicable, conduct independent security assessments or require the vendor to provide results from third-party security audits to gain additional assurance that the device meets your organization’s security requirements.
Ensure that service level agreements (SLAs) with vendors include provisions for ongoing cybersecurity support, including patch management, vulnerability disclosures, and incident response to help maintain the security of devices throughout their operational life in your clinical environment. Utilize the Healthcare Sector Security Council’s Model Contract language of Medtech Cybersecurity to negotiate more explicit obligations and accountability through the product life cycle. Include terms in the contract that require vendors to provide post-market security updates, including patches and ongoing risk assessments to ensure the device remains secure against emerging threats after deployment. Once devices are deployed, integrate them into your network’s cybersecurity monitoring and incident response systems. For medical devices, this may require passive monitoring technology providing real-time detection and response to any security incidents involving the device.
By leveraging the ISO 81001-5-1 standard in the procurement process, health care providers can significantly reduce the cybersecurity risks associated with medical devices, thereby protecting patient data, ensuring device functionality and maintaining overall clinical safety.

Phil Englert is the vice president of medical device security for Health-ISAC Inc.








Choose the right capital equipment, supplies, and health IT for your facility with guidance from ECRI Instituteʼs team of biomedical and cybersecurity engineers, clinicians, and human factors experts.


ECRI is a trusted authority on healthcare technologies that improve the quality and cost-effectiveness of patient care.
Get test results and ratings, free of vendor bias


Stay on top of risks with hazard and recall notifications
Compare technology with overviews and specifications
Learn more at www.ecri.org/solutions/device-evaluations or contact us today at 610-825-6000, x5891.











Ben Calibrating, or Ben C. for short, is the face of MedWrench! While he may just be a small lego figure, you can find him all over the world.
At MedWrench we have an ongoing contest to see where Ben C. is traveling to and what he’s been up to! Here’s how you can participate:
STEP 1: Like the MedWrench Facebook & LinkedIn pages STEP 2: Post your picture of

#BenC visits Kentucky!
STEP 3: Post a funny caption with your picture telling us what Ben C. is up to

An online resource where medical equipment professionals can find all the information needed to help them be more successful! The easy to navigate Bulletin Board gives you access to informative blogs, expos and events, continuing education opportunities, and a job board. Visit MedWrench.com/BulletinBoard to find out more about this resource.
Follow MedWrench on Facebook & LinkedIn





Next stop - New Orleans!

OCTOBER 23-24
BY KATERINA HARRISON

Radiation therapy is a non-invasive method of cancer treatment that has been successfully used in patients for over 100 years. It uses ionizing radiation which, although highly toxic to living cells, can painlessly kill and control cancerous growthby targeting the growth with a beam of energy. Due to the highly harmful nature ofthis treatment, machines administering this radiation should be designed with robust safety features and thoroughly tested to prevent possibly overdosing the patient, which unfortunately was not the case with the Therac-25. READ
STEP 4: Make sure you tag @MedWrench in your post so our team can see it
Where is #BenC off to next?!
John Samuelson
Biomedical Specialist at AtlantiCare Regional Medical Center
• Power outlets for testing equipment
• JEGS 5-drawer toolbox
• Milwaukee rolling toolbox
• Patient monitor
• Computer
• IV pumps
Submit your bench to be featured in TechNation at 1technation.com/my-bench/. You could win a $25 Amazon gift card via the “What’s On Your Bench” Contest!



Instructions and step-by-step pictures
• Android tablet platform
&
• Preprogrammed auto sequences, including FT-10 AND the new FX-8
• Automate test sequences
• User Programmable
• Eliminate human error
• Easy-to-read graphics & reports



The Heartland Biomedical Association (HBA) celebrated its 30th anniversary during its annual symposium this year, Maggie Berkey shared. It wasn’t all fun and games, but rest assured a good time was had by all! The annual event started off with a golf tournament at the beautiful Players Club at Deer Creek. AAMI Vice President of HTM Danielle McGeary kicked off the education with a keynote address. There were several exceptional presentations throughout Thursday and Friday followed by a lively exhibit hall. HBA is already looking forward to doing it again in September 2025!
For more information, Heartlandbiomed.org
1. These guys got a second chance to golf at The Fat Putter thanks to event sponsors First Call Parts and Avante.
2. The HBA is a strong proponent of education which drives its partnership with John DeLong, program director at Iowa Western Community College, by offering scholarships to students interested in studying to become BMETs.
3. Everyone brought out their smiles for the after party.




4. Golfers enjoyed a delicious meal at The Players Club at Deer Creek after 18 holes of fun!
5. These guys are smiling so big because it was literally the perfect day for some friendly competition at our annual golf tournament.
6. Jesse Crowe and David Ohland from Precision Laser Specialist walked attendees through some key points of laser safety.
7. A huge THANK YOU to our vendor partner(sponsor)ships.

2
1 3 5 6 7 4


The 50th annual CABMET Symposium was a success. The education, vendor hall, networking and great times met the expectations of those who have attended previous symposiums. It was CABMET’s largest symposium ever with 25 different classes for biomeds to choose from. Attendance exceeded 200 people with 40 companies exhibiting. It was a great event this year and CABMET President Leticia Reynolds says the organization is looking forward to the 2025 HTM Mixer in Denver.
1. HTM leaders at the 50th annual CABMET Symposium are (pictured from left to right) Logan Zeien, Stephen Zeien, Danielle McGeary, Matt Oetker, Leticia Reynolds and Robert Smothers.
2. The poker tournament was a hit with attendees.
3. CABMET continues to provide outstanding education at its annual symposiums.


1 3 5 6 4


4. The Thursday Night Happy Hour was a treat for everyone involved.
5. The golf tournament is a staple of the annual symposium and always delivers a fun time.
6. The Zeien family enjoys lunch during the golf tournament.

2


The 2024 NCBA Symposium was held at the Cherokee Casino and Resort in Cherokee, North Carolina on August 19-21. The event offered a fun golf outing, networking, education and an exhibit hall.
For more information, visit ncbiomedassoc.com/
1. Breakfast and registration drew a crowd at the 46th annual North Carolina Biomed Association Symposium in Cherokee, North Carolina.
2. Mark Elliot from Asimily teaches a cybersecurity class.
3. Aaron Watts presents the 7th annual Mike McCoy golf trophy to Shawn O’Donnell from United Infusion.


1 3 5 6 4



4. Kevin Winnie presents Iredell Memorial Health System with the 2024 NCBA Shop of the Year award!
5. Attendees had fun networking at the TechNation Happy Hour held at Wicked Weed.
6. The 2023-2024 NCBA Board of Directors pose for a group photo.





If your ultrasound transducer is deemed beyond repair at any point, you will not be charged for the repair attempt.




All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.
ON-DEMAND:

Tools of the Trade with Tenacore on Tenacore Vending Solution


Unified Efficiency: Building Trust in Mobile Equipment Asset Management Across Departments,
Revolutionizing Support: The Power of REACTS in Field Service
Enhancing Device Management and Extending Cyber Protections with Cynerio + AiRISTA
TUNE IN TO ANY LIVE WEBINAR TO WIN AIRPODS OR $100 AMAZON GIFT CARD
LIVE:



OCT. 2 | Fluke Biomedical Tools of the Trade
OCT. 9 | Kontakt.io
Save the date for this live webinar.
OCT. 16 | Claroty
Save the date for this live webinar.

OCT. 23 | BC Group
Save the date for this live webinar.
PODCASTS:


sponsored by Healthmark AI and Automation Inspection for Sterile Processing & Endoscopy
sponsored by MMS
Zen and The Art of Medical Equipment Maintenance
• $300 a month
• 1/6 page ad
• Up to 5 categories in our Service Index
• Service Index listings includes your company name, website and phone
• Your company name in the alphabetical business index You can be featured in our preferred vendor playlist on TN TV!

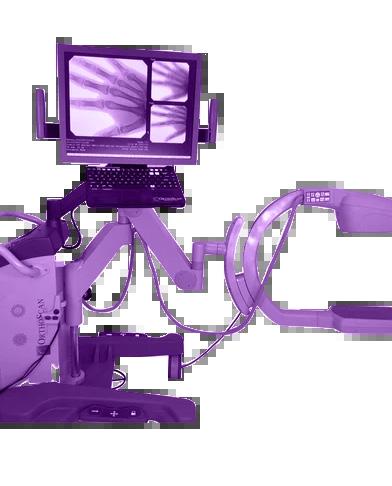





















Consultancy
KEI Medical Imaging Services keimedicalimaging.com
Laboratory College of Biomedical Equipment Technology cbet.edu • 866-866-9027
Labratory
Ozark Biomedical ozarkbiomedical.com
Mammography
Microscopes
Prescott’s, Inc. prescottsmed.com • 833-423-3786
Monitors/CRTs BMES
Innovatus Imaging innovatusimaging.com
Probo
Diagnostic Solutions diagnostic-solutions.com • 330-296-9729
International X-Ray Brokers internationalxraybrokers.com • 508-559-9441
Oxygen
Pronk Technologies, Inc. pronktech.com • 800-609-9802
QRS Solutions
• 877-254-7086
Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010
Ultrasound
Innovatus Imaging innovatusimaging.com • 844-687-5100
Probo Medical probomedical.com • 3174947872
Summit Imaging Inc mysummitimaging.com • 866-586-3744
X-Ray
RSTI rsti-training.com • 800-229-7784
RTI Group
800-222-7537
Tri-Imaging Solutions triimaging.com • 855-401-4890














“I’m grateful for the opportunity to work with Will Andersen! Will is the Vice President of Cardiovascular Ultrasound at GE HealthCare and has been an excellent leader and consistent face of our organization.”
– Jason Velick

“Ending an incredible week in Telford, PA. Thanks to the great leadership at SodexoHTM and Frank Cabrera, training on service and maintenance of the latest model Drager anesthesia machine.”
– Laura Placencia BSBE, CBET Use the hashtag #HTMLife when posting to LinkedIn and a member of the TechNation team will contact you.



Our training program at Tri-Imaging transcends traditional education by instilling skills that will resonate throughout your professional journey for years to come. Unlike conventional training experiences focused solely on facts and figures, we prioritize imparting practical, applicable skills.
Our commitment extends to fostering a deep understanding of the intricate inner workings of various devices and machines that our students will service in their careers. Here at Tri-Imaging we believe in nurturing individual growth, and to that end, we provide one-on-one assistance for each student.
Step into the future of education with Tri-Imaging, where our training program transcends boundaries to equip you with skills that stand the test of time.
























