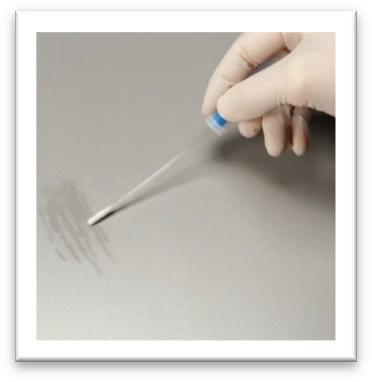
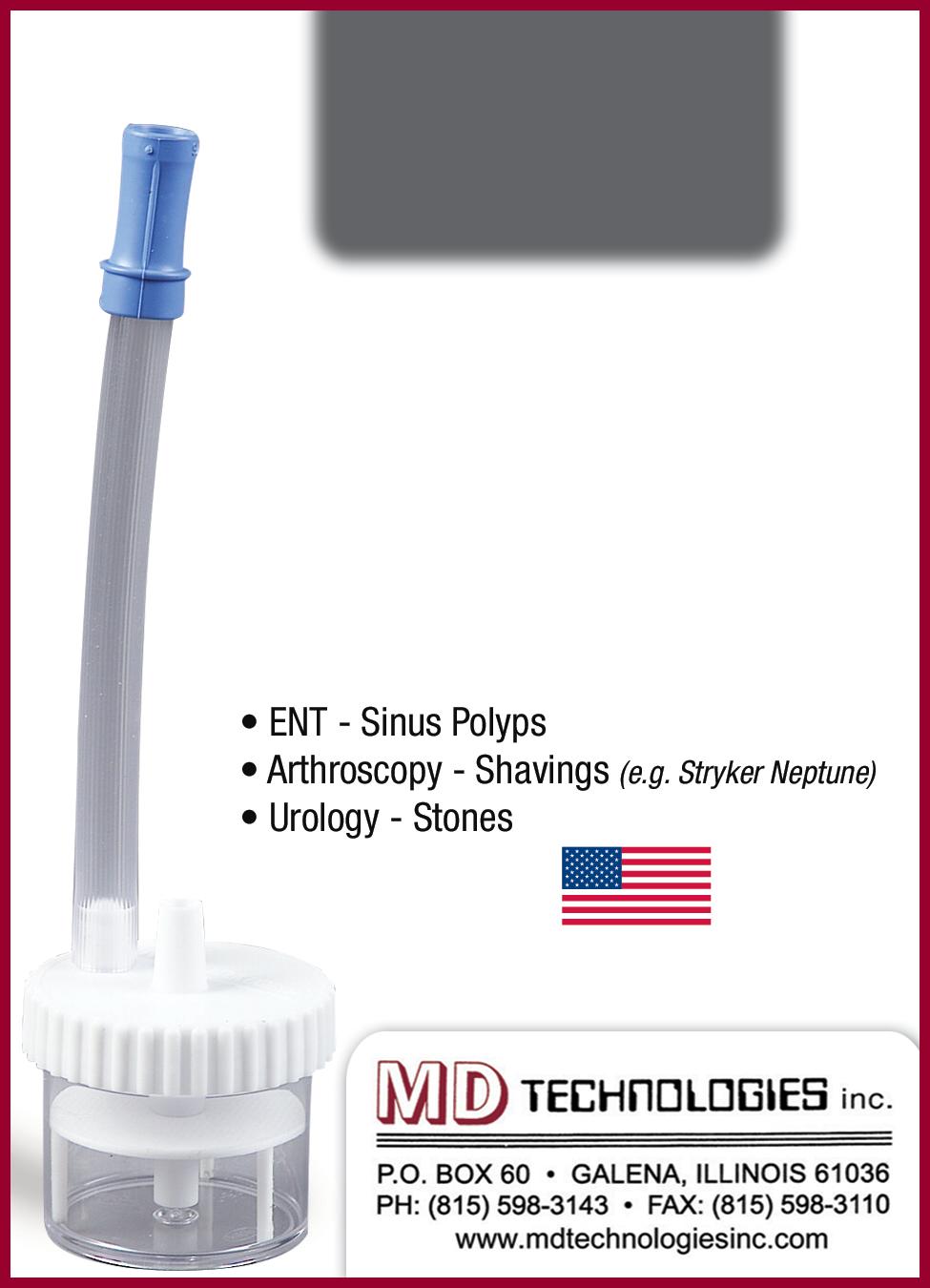
Prepzyme® Forever Wet creates a long lasting moisture barrier. As seen here, instrument remains wet to the touch for days after application.








Prepzyme® Forever Wet creates a long lasting moisture barrier. As seen here, instrument remains wet to the touch for days after application.

The latest breakthrough in enzymatic pre-cleaning sprays, Prepzyme®
Forever Wet’s unique humectant properties form a moist coating over the instruments that lasts for days.
•The humectant formulation creates a moisture retention barrier which keeps soiled instruments and scopes moist for a prolonged period of time – unlike a GEL which HAS NO MOISTURE RETENTION properties
•Operating room safe, non-aerosol, multi-tiered enzymatic spray helps prevent bio-burden from drying on the surface of soiled instruments and scopes
•Ideal for transporting soiled instruments that may sit for an extended period of time
•Reduces tray weight during transport compared to liquid presoaks
•Soiled sharps are visible through humectant
• Decreases spills and potential cross-contamination


HAIs – healthcare-associated infections – continue to ourish, despite dedicated e orts to reduce them, potentially compromising patient and sta safety throughout your facility. Bio lm, bioburden and contaminants can be found everywhere from the CSSD to the OR.
Everything - from a light, in an operating room; a partition curtain or chair in a patient room; a clipboard, exam glove or an endoscope - can be invisibly hazardous and pose a real threat.




An essential step to improving HAI prevention & safety protocols
The SafeStep ATP Monitor veri es the cleanliness of surfaces, endoscopes and cannulated instruments in just 15 seconds. By detecting ATP (adenosine triphosphate) – the energy molecule found in all human, animal, plant, bacterial, yeast, and mold cells – SafeStep gives you the testing power to minimize HAIs and improve patient and sta safety.
Have you heard of UltraSAFE? UltraSAFE addresses the common issues associated with formalin exposure to create a safer, standardized, and cost-effective solution to the challenges Perioperative Nurses face daily. We all know by now that formalin is carcinogenic and bad for our health. We also know that formalin is the accepted standard and is not going anywhere, which is why it is critical that you advocate for your health to ensure you can manage specimens in the safest way possible. That means ZERO EXPOSURE and complete automation. UltraSAFE offers exactly that. Say goodbye to manual filling and costly spills and take the guesswork out of documentation. With UltraSAFE, time to fixation, weight of specimen, and formalin quantity are recorded on a printed label to ensure the integrity of each specimen. The future is safe; UltraSAFE.


Milestone offers a professional development activity for any perioperative team members who participate in specimen management.


By Bill Prentice
T
he Centers for Medicare & Medicaid Services (CMS) issued its 2025 proposed payment rule for ASCs and hospital outpatient departments (HOPD) in July. It invited all interested parties to submit comments by September 9.
ASCA conducted its own analysis and worked with numerous stakeholders, including other national organizations, various professional societies, state leaders, product and service providers, and surgery center physicians and managers, to prepare the association’s comments. ASCA also encouraged others to submit comments of their own.
Highlights of the proposal and ASCA’s responses follow.
Under the proposed rule, on average, ASCs would see an effective inflation update of 2.6 percent. This is the same increase as HOPDs; using the same inflation update factor is something ASCA has been supporting for many years. While ASCA is pleased to see CMS use the hospital market basket update factor for both HOPDs and surgery centers again next year, ASCA is also continuing to encour-
age CMS to make that arrangement permanent.
ASCA also provided CMS with numerous examples of the ways this small update falls short of covering the rising costs associated with the essential services and supplies ASCs need to have in their facilities to be able to continue to provide safe, highquality care.
Back in March, as part of a new process CMS instituted for recommending procedures for addition to Medicare’s ASC Covered Procedures List (ASC-CPL) – a process CMS calls the ASC-CPL Pre-Proposed Rule Recommendation Request – ASCA submitted 18 codes. They included 16 cardiovascular codes and two spine codes. This proposed rule did not mention those codes or provide any insight into any others suggested through that process.
ASCA’s comments conveyed its disappointment that none of those codes were mentioned and expressed concern over the lack of transparency in what CMS originally touted as a new, highly transparent process. Based on the information ASCA shared with CMS about these procedures, we continue to hope
it will decide to add them in its final 2025 payment rule.
CMS did propose to add 20 medical and dental surgical procedures to the ASC-CPL. Whether the addition of these codes, as proposed, would provide greater access to dental procedures in surgery centers remains unclear.
The quality reporting provisions in the proposed rule contained some news we expected and some new requirements we did not anticipate. For example, we were not surprised to find that the proposed rule contained no changes regarding the Outpatient and Ambulatory Surgery Consumer Assessment of Healthcare Providers and Systems (OAS CAHPS) Survey. That survey is still set to become a mandatory component of the ASCQR Program on January 1, 2025. If you are in a surgery center that wants to remain compliant with Medicare’s quality reporting requirements to avoid future cuts in your reimbursement rates but are not already administering this survey, I encourage you to make that a priority now. This survey takes time to set up and, typically, even longer to generate the high-quality results that surgery

centers are used to seeing. ASCA has numerous resources on its website at www.ascassociation.org/prepare-oascahps that can help.
Meanwhile, ASCA will continue to advocate for changes to this survey, such as an electronic-only option, to reduce the cost burden on facilities.
At ASCA, we were also disappointed but unsurprised to see that reporting on the COVID vaccination coverage measure, ASC-20, remains in place. We continue to contest the usefulness of this burdensome measure and request its removal from the ASCQR Program, or at the very least, reporting only once a year instead of selecting one week per month.
The unexpected news related to the ASCQR program is that CMS wants to add three new measures related to health equity.
The first of these would assess a
“facility’s commitment to health equity” across five domains: equity as a strategic priority, data collection, data analysis, quality improvement and leadership engagement. The second would involve screening for Social Drivers of Health (SDOH) in food insecurity, housing instability, transportation needs, utility difficulties and interpersonal safety. The third measure would indicate the “screen positive rate” for patients identified using the second measure.
Both ASCA and the ASC Quality Collaboration oppose these new measures since none of them have been tested in the ASC setting. ASCA also expressed concerns about the benefits of these additional reporting measures and questioned the wisdom of adopting three additional measures in the same year the OAS CAHPS survey becomes mandatory.
CMS is now analyzing all the comments it received on its proposed rule and preparing to release its final rule. That rule is typically released near Halloween and is required by law to be released by November 2, or 60 days prior to its effective date of January 1, 2025.
To help support the critical advocacy work that ASCA is doing on behalf of surgery centers of every specialty across the country, please make sure that if you work in an ASC, your facility is an ASCA member (contact Mykal Cox at mcox@ascassociation. org to learn more).
To learn more about Medicare’s final payment rule, check our website at ascassociation.org for updates.
By Dawn Whiteside
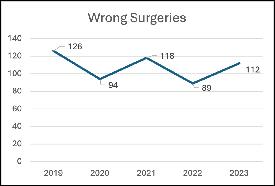
I n May of 2024, the Joint Commission published its annual report regarding sentinel event data for 2023. Reporting sentinel events to The Joint Commission is a voluntary process, which may lead to the conclusion that the actual numbers are significantly higher than reported. (The Joint Commission, 2024) The data reported for 2023 demonstrates a 26% increase in wrong surgeries from 2022 (Diagram 1). Of the 112 wrong surgeries in 2023, the breakdown is as follows: 62% wrong site, 19% wrong procedure, 12% wrong patient and 7% wrong implant. (The Joint Commission, 2024) (The Joint Commission, 2024)
Specific to the perioperative specialty, wrong surgeries encompass surgery or other invasive procedures performed on the wrong person, wrong site or is the wrong procedure. According to the National Quality Forum and the Center’s for Medicare and Medicaid, wrong-site, wrong-procedure and wrong-patient errors are considered never events and sentinel events by The Joint Commission. (Agency for Healthcare Research and Quality, 2006) “The Joint Commission defines a sentinel event as a patient safety event (not primarily related to the natural course of the patient’s illness
or underlying condition) that reaches a patient and results in death, permanent harm (regardless of severity of harm), severe harm (regardless of duration of harm).” (The Joint Commission, 2024, p. 3) After a study in 2004 revealed the rate of wrong surgeries to be estimated at 1:113,000 from 1985-2004, the Joint Commission enacted the Universal Protocol. (Agency for Healthcare Research and Quality, 2006)
The three key components of the Universal Protocol include preprocedure verification, site marking and time-out. The pre-procedure verification is the first gate towards preventing wrong surgeries and includes verifying the correct procedure, for the correct patient, at the correct site with the patient actively involved. This is also the step when all relevant and required documentation is present and accurate. Site marking is the second gate to protect patients and should have patient involvement. Marking the surgical site is required when there is more than one possible location. Site marking should be done by the licensed practitioner, or delegate, be visible after prepping and draping, and include alternative site marking options (armband, etc.) when marking the site is not possible or a patient refuses. The final gate to preventing wrong surgeries is conducting a time-out. The time-out should be a standardized process across the facility.
One individual announces the beginning of the time-out process which is when all conversations and distractions cease. All attention is given to the process and all individuals are actively involved. At a minimum the time-out must include the identification of the correct patient, the correct procedure and the correct procedural site. The current standard in most facilities includes additional information like a fire risk assessment, anesthesia considerations and affirmation, introduction of unknown team members, any expected risks to the patient and patient care interventions already implemented (sequential compression devices, blood screening, etc.). “Root cause analyses of [Wrong-Site, WrongProcedure, and Wrong-Patient Surgery] WSPEs consistently reveal communication issues as a prominent underlying factor.” (Patient Safety Network , 2019) Prevention efforts must start with a just culture and improved communication. “A just culture focuses on identifying and addressing systems issues that lead individuals to engage in unsafe behaviors, while maintaining individual accountability by establishing zero tolerance for reckless behavior.” (Agency for Healthcare Research and Quality, 2019) The evolution of improving efficiencies in the operating room, like turnaround times, may lead some team members to use shortcuts and skip steps that seem unnecessary. This is commonly referred
to as normalized deviance. Effective communication among team members is essential to prevent patient injury. Using a standardized tool like a surgical safety checklist provides a method of communication among all team members to decrease the risk of WSPEs. There are several surgical safety checklists available for use to support the full Universal Protocol procedure. The World Health Organization (WHO) developed a surgical safety checklist in 2009. The Association of perioperative Registered Nurses (AORN) created the Comprehensive Surgical Checklist “using color codes to signify items from the WHO checklist, The Joint Commission Universal Protocol, and areas where the two overlap, the Comprehensive Surgical Checklist offers guidance for preprocedural check in, sign in, time out, and sign out.” (Association of periOperative Registered Nurses, 2024)
As medical professionals, we are very familiar with the phrase “first, do no harm.” If I asked the question where this phrase came from, I am guessing it would be an overwhelming response of “the Hippocratic Oath.” This would have been my response, as well, which is incorrect. Hippocrates, a Greek physician, is credited with this phrase and believed to be by Hippocrates, Of the Epidemics, he wrote “The physician must be able to tell the antecedents, know the present, and foretell the future – must mediate these things, and have two special objects in view with regard to disease, namely, to do good or to do no harm.” (Shmerling, 2020) The nursing equivalent is the Nightingale Oath
taken upon graduation from nursing school at many colleges and universities. Nurses are accountable for their patient’s well-being and are guided by not only their own morale principles but also the American Nurses Association (ANA) Code of Ethics. There are nine provisions within the Code and additional topics within each provision. It is essential that all nurses read and understand the Code. We must live and work by this Code to protect our patients from harm. Within the Code there are specific provision statements related to Respect for Human Dignity, Primacy of the Patient’s Interests, Protection of Patient Health and Safety, and Authority, Accountability, and Responsibility. Is it possible to get it right? The leading causes of sentinel events include ineffective communication, breakdown in teamwork and inconsistently following policies. (The Joint Commission, 2024) Making improvements to each of these causes should certainly decrease the number of possibilities. Using a surgical safety checklist, following and enforcing safety policies, and working to improve teamwork and communication should be at the forefront of organizational goals. Ultimately, we should never underestimate our capacity to do harm and always focus on preventing patient injury.
Agency for Healthcare Research and Quality. (2006, April 26). Universal Protocol for Preventing Wrong Site, Wrong Procedure, Wrong Person Surgery. Retrieved from Patient Safety Network: https://psnet.ahrq.gov/issue/universalprotocol-preventing-wrong-site-wrongprocedure-wrong-person-surgery
Agency for Healthcare Research and Quality. (2019, September 7). Culture of Safety. Retrieved from Patint Safety Network: https://psnet.ahrq.gov/primer/ culture-safety
Association of periOperative Registered Nurses. (2024). AORN Comprehensive Surgical Checklist Toolkit. Retrieved from AORN: https://www.aorn.org/guidelinesresources/tool-kits/comprehensive-surgical-checklist
Patient Safety Network . (2019, September 7). Wrong-Site, Wrong-Procedure, and Wrong-Patient Surgery. Retrieved from Agency for Healthcare Reseach and Quality: https://psnet.ahrq.gov/primer/ wrong-site-wrong-procedure-andwrong-patient-surgery
Shmerling, R. H. (2020, June 22). Harvard Health Blog First, do no harm. Retrieved from Harvard Health Publishing Harvard Medical School: https://www. health.harvard.edu/blog/first-do-noharm-201510138421
The Joint Commission. (2024, May 15). Joint Commission Online-May 15, 2024. Retrieved from The Joint Commission: https://www.jointcommission.org/ resources/news-and-multimedia/newsletters/newsletters/joint-commissiononline/may-15-2024/
The Joint Commission. (2024, May 15). Sentinel Event Data 2023 Annual Review. Retrieved from The Joint Commission: https://www.jointcommission.org/ resources/news-and-multimedia/newsletters/newsletters/joint-commissiononline/may-15-2024/-/media/45c2f4a812 c14f9fbc24cc22dcef8b3f.ashx

– Dawn Whiteside, DNP, MSN-Ed, RN, CNOR, NPD-BC, RNFA, is the director of education and professional development of the Competency & Credentialing Institute. She has over 35 years of experience as a perioperative nurse in many roles including circulator, scrub, first assistant, team leader, charge nurse, manager and educator.




Eliminate
Reduce turnaround time












A report from ResearchAndMarkets.com predicts continued growth for the global market for ultraviolet (UV) disinfection. The report estimated the market at $4.7 billion in the year 2022. It is projected to reach $12.9 billion by 2030.
UV lamps, one of the segments analyzed in the report, is projected to record a 14.6% CAGR and reach $5.4 billion by the end of the analysis period.
Taking into account the ongoing post pandemic recovery, growth in the ballasts/controller units segment is readjusted to a revised 12.9% CAGR for the next 8-year period.
The U.S. market is estimated at $1.2 billion, while China is forecast to grow at 16.2% CAGR.
The UV Disinfection market in the U.S. is estimated at $1.2 billion in the year 2022. China, the world’s second largest economy, is forecast to reach a projected market size of $2.8 billion by the year 2030 trailing a CAGR of 16.2% over the analysis period 2022 to 2030.
Among the other noteworthy geo-
graphic markets are Japan and Canada, each forecast to grow at 10.5% and 11.4% respectively over the 20222030 period. Within Europe, Germany is forecast to grow at approximately 11.2% CAGR. Led by countries such as Australia, India, and South Korea, the market in Asia-Pacific is forecast to reach $2 billion by the year 2030.
The global ultraviolet disinfection equipment market size was estimated at $3.6 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 6.9% from 2023 to 2030, according to Grand View Research.
“The rising demand for ultraviolet (UV) disinfection equipment in health care organizations is likely to boost industry growth,” Grand View Research reports. “The COVID-19 pandemic has impacted the supply of this equipment and its components since manufacturers are unable to satisfy the increasing demand and have reached their maximum production capacity. These firms should expand their manufacturing facilities to address the increasing demand from end-users, which is not possible in a short period.”
UV air purification is an established technique, and several organizations such as WHO and CDC have repeatedly promoted its usage in labs, hospitals, operating rooms, and colleges. Furthermore, UV air purifiers are advised to enhance the air quality in both the residential and commercial sectors by removing particulate matter and dust, which is thought to reduce coronavirus transmission.
With the rise of COVID cases, several hospitals have begun implementing UV systems to disinfect high-risk indoor settings, which is projected to increase product demand in the coming years. UV disinfection systems have a lower environmental effect than sodium hypochlorite and chlorine gas since UV is largely controlled by the composition of the electrical grid. UV disinfection systems have been acknowledged by public health agencies as an effective technique for treating drinking water, reclaimed water, and wastewater. This has prompted nations throughout the world to implement these systems since they are safe and do not require the use of toxic chemicals to conserve water resources and meet sustainability goals.

UVDI’s UV-C whole room surface disinfection technology, the UVDI-360 Room Sanitizer with Smart Connect, has been proven in independent clinical and laboratory studies to achieve 99.99% inactivation of over 35 high-risk microorganisms within an eight-foot radius in five minutes. This powerful disinfecting capability combines with UVDI’s proprietary Smart Connect automated connectivity technology to provide real-time reporting and exceptional durability at significant savings for the hospital. UV Smart Connect technology simplifies device usage, fleet management and device servicing with automatic, real-time communications. Instant UV Smart Connect cloud communications are driven by advanced cellular and wireless technology, connecting the UVDI-360 to UVDI’s easy-to-use, cloudbased operator portal. This next generation room sanitizer, with its user safety features, is now trusted by hospitals worldwide.

The Spectra 1000 is a new advancement in UV disinfection technology. Its patented design (US8791441) ensures maximal UVC output throughout the disinfection cycle, making the Spectra 1000 exceptionally effective against resilient pathogens like C. difficile and Candida auris on hard surfaces in rapid time. It simultaneously disinfects the air, targeting airborne threats that can linger and settle, achieving a >6 log10 reduction against MS2 Bacteriophage, a COVID-19 surrogate. Validated in multiple independent hard surface and aerosol studies. By effectively treating hard surfaces and targeting aerosolized pathogens that ultimately settle, the Spectra 1000 surpasses traditional UV devices to improve the environment of care.
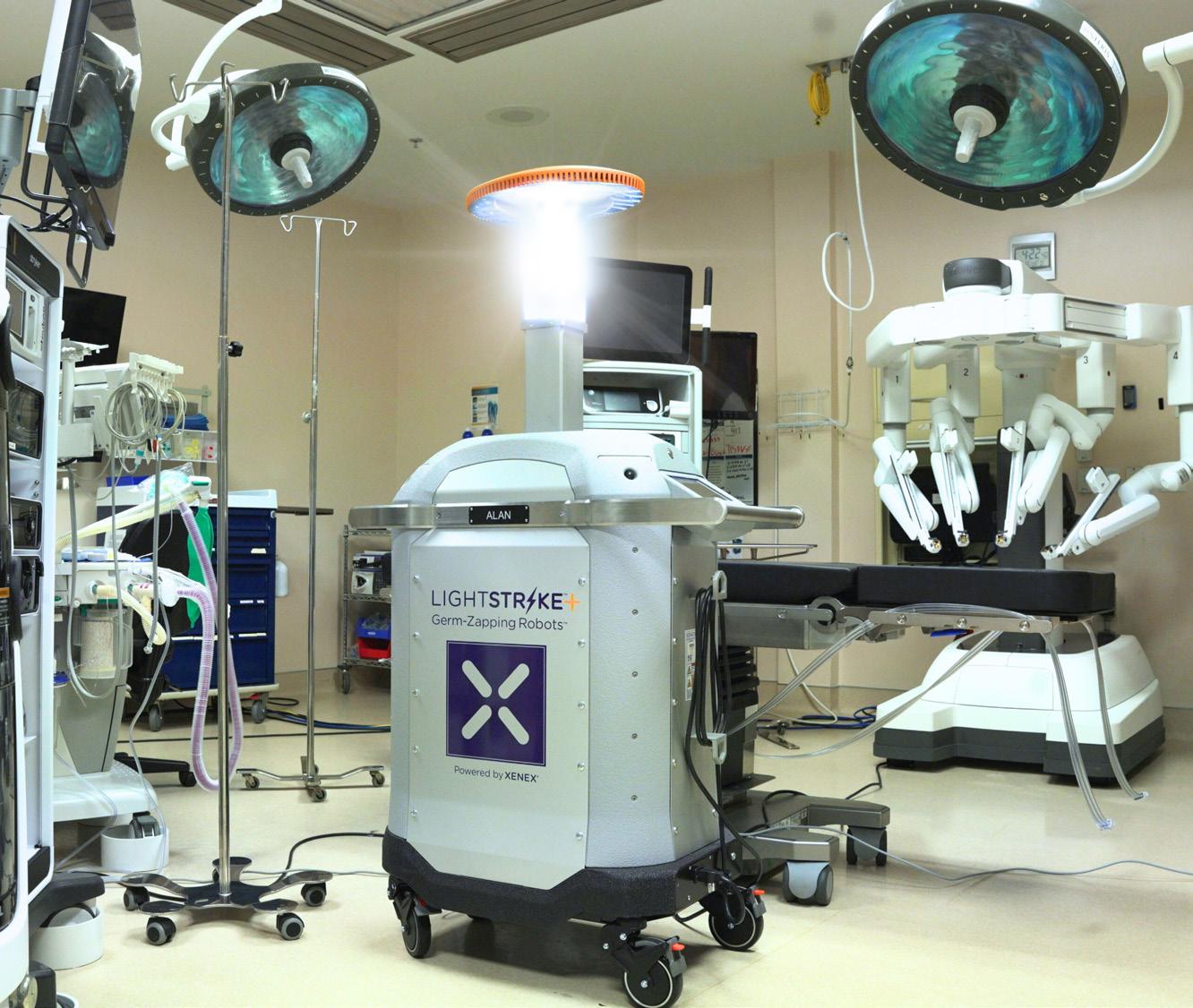
Successful surgical procedures start with a clean operating room. Achieve better microbial reduction in your ORs with LightStrike+’s high-intensity pulsed xenon, broad-spectrum UV light. LightStrike+ is the only FDA authorized UV technology for ORs that is proven to significantly reduce the number of pathogens on high-touch surfaces. Xenex’s LightStrike+
“Whole Room Microbial Reduction Device” is harsh on germs, not on expensive medical equipment. It’s simple to use and provides fast cycle times (as short as 2 minutes for bacteria). Whether it’s the last step of terminal cleaning, after a dirty case, or between every case, LightStrike+ can help you quickly get better pathogen reduction on OR surfaces.

The Surfacide Helios System is the only patented UV-C low-level disinfection solution to use multiple light-emitting robots simultaneously in a single cycle, resulting in greater efficacy in less time. Learn more about why this American-made and manufactured disinfection system offers superior performance advantages in maintaining hygienic environments.
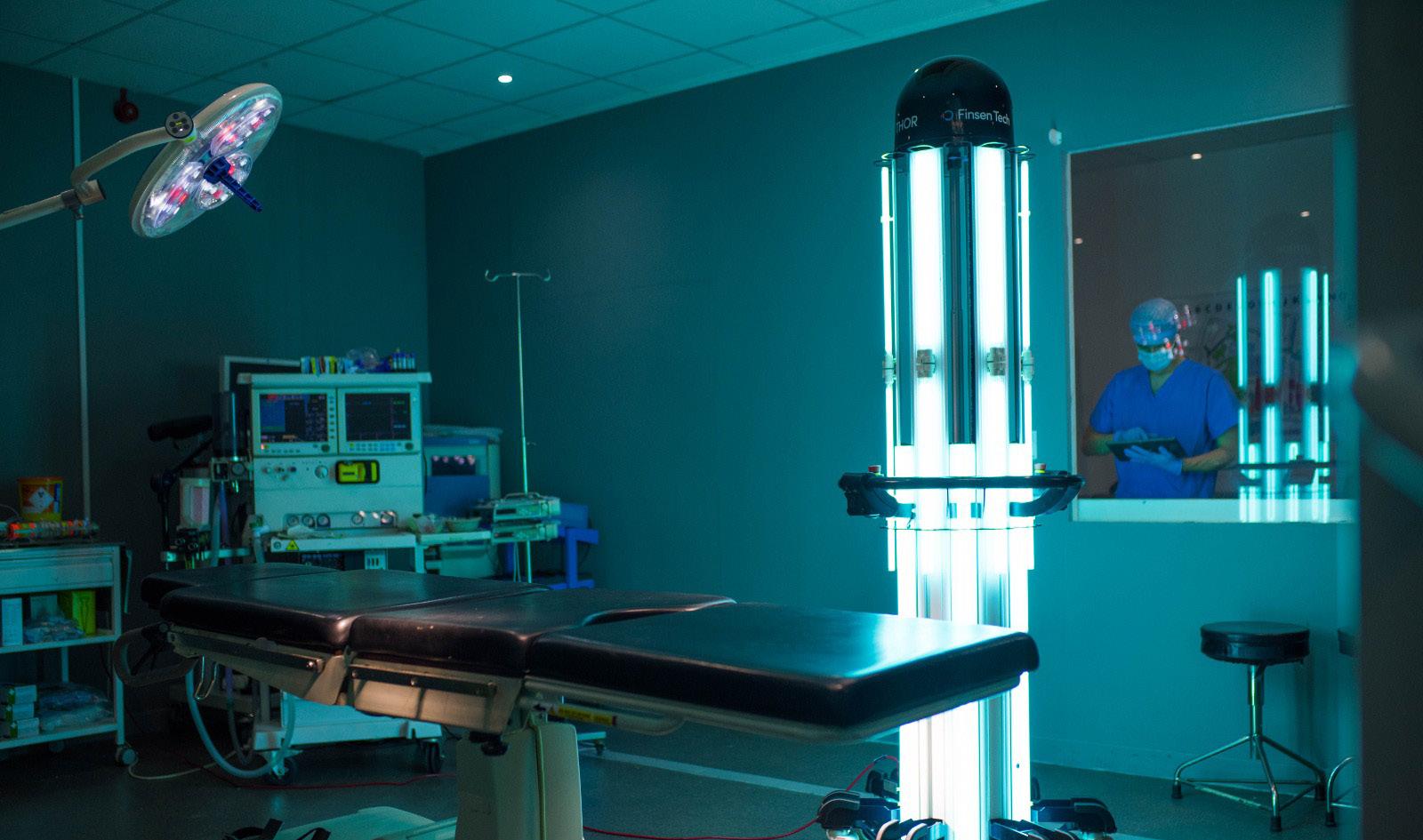
The THOR UVC system is the latest continuous wave UVC disinfection technology suitable for use in any type of hospital, health care or business environment. As an intelligent device, THOR UVC eliminates guesswork and ineffectiveness from regular cleaning. The system is designed with unique patented features to minimize shadowing. Within minutes, log 6 reductions in bacterial kill are achieved (99.9999%). THOR UVC can be moved anywhere. Because it has the same wheelbase as a wheelchair, it can disinfect any space, including ambulances, easily.
Hospitals can terminally disinfect any area regardless of size – from patient rooms to operating theaters, restrooms to cafeterias, and more. With its patented technology, the THOR UVC robot can be adjusted to any height between 1.1m and 2.25m, ensuring complete floor-to-ceiling disinfection and eliminating shadowing. The ergonomic handrail and six-caster wheel design make fast and efficient room turnaround possible. THOR UVC’s unique intelligent placement system puts the unit in the optimal position to eliminate pathogens in dark and shadowed areas. Four motion sensors constantly monitor the process and automatically turn off the tower if any movement is detected.



Are your surgeons feeling the heat in the operating room? The CoolOR® Circulating Water System is a game-changer in surgical comfort. Here’s why it’s a must-have for your team:
• CONTINUOUS COOLING
• WATER-TIGHT RESERVOIR
• QUICK-CONNECT COUPLINGS
• INSULATED WATER LINES
• PORTABILITY
Because



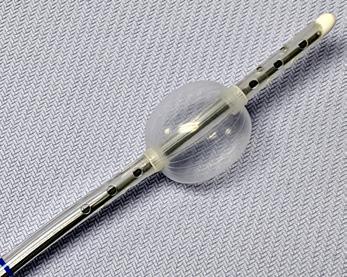

FDA CLEARED
• Vent the left heart during cardiopulmonary bypass surgery
• Securely positioned by inflating a soft positioning balloon in the LV
• Ensure drainage of air, blood and debris
• Disposable for one time use

• Keeps sterile tethered appliances on the surgical field from falling to the floor with 4 hook & loop closures
• Latex free self adhesive back for attachment to the surgical field
• Economical - Saves pre-op time
• Several sizes, configurations, colors
• Sterile shelf life 2 years
• New Users call for FREE SAMPLES


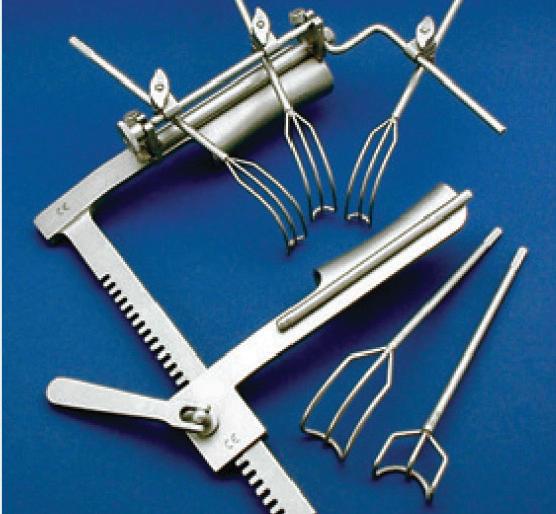
• Universal Self-retaining Heart Retractor
• Mitral Valve Repair or Replacement Surgery
• Exceptional Exposure for Right & Left Atrium



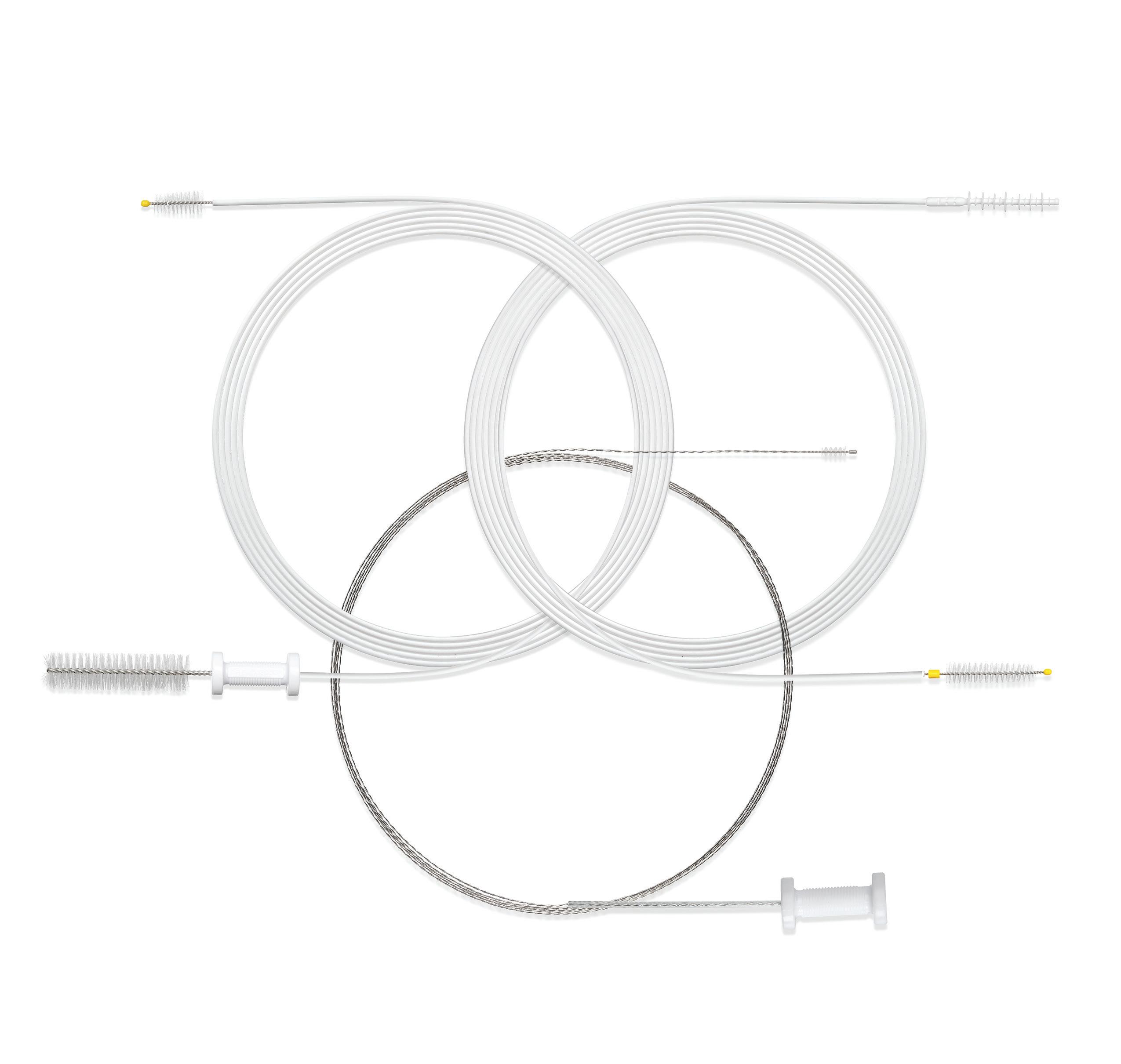
ENDOSCOPE REPROCESSING AND OUR SCOPE BRUSHES... A LOGICAL RELATIONSHIP
No doubt about it... cleaning and reprocessing flexible endoscopes is a detailed process. Manual cleaning, one of the vital steps for effective scope reprocessing, can be challenging if you don’t have the right tools. STERIS brushes are uniquely engineered to help you meet that challenge head on. Let us show you how our cleaning brushes make sense in your processes.

Scan the QR code to learn how our DuoSwift® Cleaning Brush outperforms competitors


Safe patient care is the responsibility of every person and department in the health care facility. Three departments in particular – the operating room, sterile processing department and infection prevention department – have a unique relationship when it comes to ensuring safe processing of surgical instruments.
All three departments are crucial in making sure that surgical processes, products and devices are safe for use with every patient, every time.
“Each department plays a critical role in safe instrument reprocessing,” says Julie Gorog RN, BSN, CNOR, clinical education consultant, Advanced Sterilization Products.
“Therefore, it is essential that staff
members have an understanding of the expectations and barriers faced by their counterparts to assure quality and safe patient care.”
Amanda Heitman, BSN, RN, CNOR, clinical nurse educator and Qlicksmart perioperative educational consultant with Periop Anew, points out that while these three departments are separate and unique, they should work together to promote a culture of patient safety.
“This helps protect both patients and health care workers,” she says.
“Each discipline possesses subject matter expertise that optimizes patient safety for instrument processing,” says Addy Bell, BBA, CSPDT infection preventionist, infection control and prevention at Lehigh Valley Health Network.
“The OR focuses on management
of the instruments in the surgical environment, including inspection, and initiates the cleaning process at the point of use.”
“SP continues the cleaning, disinfecting and sterilization of the instruments according to manufacturer’s instructions for use,” adds Bell. “And IP lends their expertise with knowledge of current guidelines and trends while performing audits to make sure that protocols are being followed to prevent infections.”
IP notification is necessary when breaches in processing are identified, notes Bell.
Karen Reiter, RN, CNOR, RNFA, CASC, vice president of operations for DISC Surgery Centers, likens SP to the heart of the health care organization.
“The sterile processing department must be finely tuned and work the same way every time,” she says. “Strict processes and absolute adherence to policies trickle down to the safety of the patient.”
“It’s essential that staff IN ALL THREE DEPARTMENTS maintain an understanding of the expectations, struggles and barriers faced by their counterparts.”
- Farrah Marsh BSN, RN, CNOR
The OR, meanwhile, is like an orchestra.
“Everything in the OR must be in perfect order, synchronized and working together,” says Reiter. “The patient is the beat of the orchestra. And the infection preventionist is the person who constantly stretches these teams to achieve best practices. Together, these three teams write the music for patient safety.”
Farrah Marsh, BSN, RN, CNOR, director of surgical services for Sanford Health, calls SP “the foundation of the patient’s surgical experience.” Meanwhile, the OR must be well-versed in standards and able to support the work of the surgeon to provide patients with great surgical outcomes.
“IP is seen as the expert voice when decisions regarding patient safety are required,” says Marsh.
According to Rebecca B. Roe, MSN-Ed, RN, NPD-BC, CNOR, the OR is the “start and end” of instrument inspection related to direct patient care.
“I say it is the end of instrument inspection because the scrub personnel must keep the instruments clean and free of debris to the best of their ability,” says Roe. “The decontamination process starts in the OR,” Roe explains. “This means flushing lumens, wiping down drills of blood and other organic material, and keeping instruments wet until official decontamination begins in the SP department. Inspection alerts also require tagging of any broken, dull or non-functioning instrumentation found. This helps prevent use in subsequent trays and allows for proper repair or disposal of the equipment.”
Infection prevention’s influence in the OR has increased with special attention to SP and OR quality inspections and observations.
“IP personnel in surgical services provide an objective view of work activities, along with a deeper understanding of cases involving surgical site infections,” says Roe. “This department has taken on a pivotal role in monitoring surgical cases for readmissions at 30 to 90 days or longer for cases where implants are utilized.”
“Infection control needs to be behind the scenes advocating in their organizations for better safety measures,” says Heitman.
Heitman emphasizes the importance of these three departments working together to improve patient safety by implementing administrative controls. “These measures help support higher levels of controls by ensuring that people are working within safe systems,” she says. Examples include writing policies for sharps safety, ensuring compliance with safe work practices and implementing sharps injury reporting and investigation processes.
Ensuring patient safety requires that the OR, SP and IP departments collaborate and work closely together at all times.
“It can be tempting to assume that because our work is complimentary, we are all experts in each area,” says Marsh. “But this usually isn’t the case.”
For example, an OR nurse may believe the standards that guide the work of the OR team are the same as those that guide SP practices, but they aren’t. “So, it’s important to listen with a willingness to be influenced when working collaboratively,” says Marsh.
It’s vital that staff in each area educate themselves on the standards, workflows and challenges of each specialty.
“Meeting regularly to develop relationships and maintaining open lines of communication is a great way to begin,” says Marsh.
“It’s important for these departments to recognize that their objectives are intertwined. The OR needs to have sterile instruments, SP needs resources to function effectively and IP needs information that enables them to detect trends or outbreaks.”
- Addy Bell, BBA, CSPDT infection preventionist

This includes providing opportunities to shadow staff in each area.
“Shadowing is a great way to debunk myths about processes and influence attitudes,” says Marsh. “In my health system, we encourage IP auditing to bring fresh eyes into the OR. We also welcome SP staff to observe cases so they can learn and develop an understanding of their role in each case. I believe patients benefit from this type of collaboration and transparency.”
Gorog agrees.
“It’s essential that staff in all three departments maintain an understanding of the expectations, struggles and barriers faced by their counterparts,” she says. “Providing them with the ability to shadow roles in real time is one way to accomplish this.”
Roe recommends job sharing as a way for team members in the different departments to better understand each other’s roles and responsibilities. “This helps lessen the silo effect of thinking one department is to blame for things not working well,” she says.
Reiter says that her facility often hires skilled teams trained in hospitals who are used to working in silos.
“So, blurring the lines between departments, cross-training and working together as one team can sometimes be challenging in the beginning and involve a learning curve,” she says.
“Communication between the
departments is key so always keep the lines of communication open,” says Kia Parker, MACPR, CRCST, CER, CHL, a-IPC, CIC, infection preventionist with the department of clinical epidemiology at the James Comprehensive Cancer Center. “For example, OR teams can invite SP personnel to participate in their huddles and SP teams can invite surgical techs, nurses and IP personnel to tour their department during orientation.”
“IP personnel can round in the OR with their SP partners and round in SP with OR partners,” adds Parker. “And SP staff can opt to observe a surgical case. This will facilitate conversations and help break down barriers between departments.”
According to Gorog, collaboration depends on strong communication between each department to build a sense of teamwork.
“Establishing an inter-departmental group with regularly scheduled meetings gives each department the opportunity to provide feedback on process improvements and policy development,” she says.
Bell believes that transparency between the departments, along with communication, is critical to ensuring patient safety. She recommends conducting regular meetings together and creating a feedback system so each department can report any issues or concerns they might have.
“The patient safety trifecta team
should conduct audits and round in the OR and SP monthly,” she says.
At Lehigh Valley Health Network, there are monthly collaborative IP/ OR/SP meetings covering a range of topics, with a focus on IP.
“I think getting this group together once a month has helped increase communication across disciplines and created relationships by connecting people who might not normally have gotten together,” says Bell.
While everyone agrees that open communication and collaboration are the keys to an effective patient safety partnership, there are some common obstacles that often make this difficult. One of the biggest obstacles Bell sees is competing goals between departments.
“For example, if the volume of surgical cases in the OR increases but surgical instrumentation does not increase proportionately, this may put pressure on SP to turn over instruments faster,” says Bell. “If SP is not allocated additional staff, this could have adverse consequences.”
Resources can also be an obstacle.
“If there are many cases of the same type scheduled on the same day, instrument turnover is required to have instruments ready for subsequent cases,” says Roe. “This calls for meticulous coordination and
communication to ensure equipment has been through standard cleaning and sterilizing processes specific for that instrumentation.”
There may be pressure to cut corners and speed up the process if funds are lacking for this level of turnover.
“If facilities are required to limit turnover, budget allocations for more instrumentation may become necessary,” says Roe.
Parker warns against falling into the ‘blame game’ trap.
“Infection prevention is everyone’s responsibility,” she says.
She also warns against what she calls the six most dreaded words in health care: ‘We’ve always done it this way.’ “This helps no one,” she says.
According to Marsh, Sanford Health has implemented SSI drilldowns led by the IP team.
“These meetings have a multidisciplinary approach and include multiple players who impact the patient experience,” she says. “OR team members research the procedure and patient condition while SP reviews any potential opportunities in their processes that could have impacted the outcome.”
Marsh says conversations that arise in these meetings give everyone the opportunity to look through the lens of each other’s expertise and offer different perspectives as they seek to improve.
“I have found this collaboration to be both informative and useful,” she says. “Taking time to debrief together has inspired new ideas and improved patient outcomes.”
“It’s important to approach our work with a curiosity about the contributions of others,” Marsh adds. “We must never stop asking questions of our
“We must never stop asking questions of our partners as we seek to improve. The relationships cultivated by curiosity and respect will improve the working environment for each of us, which will positively impact our patients as well.”
- Farrah Marsh BSN, RN, CNOR
partners as we seek to improve. The relationships cultivated by curiosity and respect will improve the working environment for each of us, which will positively impact our patients as well.”
Many of these obstacles can be
overcome when the OR, SP and IP departments function collaboratively and share resources, which helps create a culture of patient safety. “It’s important for these departments to recognize that their objectives are intertwined,” says Bell. “The OR needs to have sterile instruments, SP needs resources to function effectively and IP needs information that enables them to detect trends or outbreaks.”
To improve collaboration in DISC surgery centers, Reiter says the departments start and end each day with a short huddle.
“Everybody talks about what was good, what was great and what could be improved on that day,” she says.
The departments then review the next few days coming up and how everyone will be educated about any new implants to be used, including how to sterilize them properly.
“The huddles focus not only on what cases we have the next day, but also what trays need to be sterilized and turned over between cases,” says Reiter. “This guarantees that SP remains in the loop, which ensures that work is completed and managed with accountability.”
The need for the OR, SP and IP departments to work closely together cannot be overstated. “Opportunities remain to strengthen these relationships in most organizations,” says Bell. “I believe that more emphasis on this is needed from professional organizations to highlight facilities that have strong relationships between these departments and share best practices.”