Estadificación y tratamiento del carcinoma mamario según su etapa
La alopecia: causas más comunes y su tratamiento
Reto diagnóstico entre crohn y colitis sistémica
Cuidado preventivo para pacientes con enfermedad inflamatoria de intestino
Especialización deportiva temprana, ¿beneficiosa o perjudicial para el joven atleta?
El cuidado oral va más allá de nuestra boca
Salud en tus manos: exitoso foro entre pacientes, profesionales de la salud y gobierno
ESTADIFICACIÓN Y TRATAMIENTO DEL CARCINOMA MAMARIO SEGÚN SU ETAPA
Por: Bolívar Arboleda-Osorio MD FACS Director
Caribbean Breast Surgery
 Presidente
Presidente
Sociedad Puertorriqueña de Senología
Ponte al día con la agenda médica y las convenciones más relevantes para médicos, pacientes y profesionales de la salud. Pag. 68
@RevistaMSP
12 26 35 42 54 64 70

2 Revista Puertorriqueña de Medicina y Salud Pública



Revista Puertorriqueña de Medicina y Salud Pública 3


4 Revista Puertorriqueña de Medicina y Salud Pública


Revista Puertorriqueña de Medicina y Salud Pública 5








CONTENIDO
ESTADIFICACIÓN Y TRATAMIENTO DEL CARCINOMA MAMARIO SEGÚN SU ETAPA
LA ALOPECIA: CAUSAS MÁS COMUNES Y SU TRATAMIENTO
RETO DIAGNÓSTICO ENTRE CROHN Y COLITIS SISTÉMICA
Metamorfosis, Sandra Reyes
Palabras clave: Mastectomía total con preservación del complejo pezón/areola, cáncer de seno, reconstrucción de seno
Metamorfosis, Sandra Reyes

ESPECIALIZACIÓN DEPORTIVA TEMPRANA, ¿BENEFICIOSA O PERJUDICIAL PARA EL JOVEN ATLETA?
EL CUIDADO ORAL VA MÁS ALLÁ DE NUESTRA BOCA
Key Words: Nipple sparing mastectomy, breast cancer, breast reconstruction


CUIDADO PREVENTIVO PARA PACIENTES CON ENFERMEDAD INFLAMATORIA DE INTESTINO
SALUD EN TUS MANOS: EXITOSO FORO ENTRE PACIENTES, PROFESIONALES DE LA SALUD Y GOBIERNO
EDITOR FUNDADOR Juan Carlos Orengo Valverde, MD, MPH, PhD EDITOR Alberto Santiago Cornier, MD, PhD CONSEJO ASESOR Oscar Soto Raíces, MD, Ahmed Morales, MD, FACP, FACG, FASGE, AGAF, Lcda. Wanda González fisióloga del ejercicio PRINCIPAL OFICIAL EJECUTIVO Pedro Carlos Lugo Hernández III, P.A.C. PRESIDENTA Y FUNDADORA Glorybelle Hernández Figueroa, MBA VICEPRESIDENTA Y FUNDADORA Laila Paloma Lugo, MBA CONTABILIDAD Julio Soto ADMINISTRACIÓN Marta Ivelisse Vélez Ramos, MBA, MARKETING Y SERVICIOS 360 Darlene Rodríguez, Yasmin Morell, Belinda Burgos PERIODISTAS Mayra Acevedo, Luis Penchi, Limarys Suárez DIRECCIÓN GRÁFICA Natalia Zoé Rivera Torres ARTISTA GRÁFICO Jhorman González
DIRECTOR AUDIOVISUAL Christopher Soto REALIZADORA AUDIOVISIAL Salomé Mateus, Duban Valencia FOTOS Revista Medicina y Salud Pública DIRECCIÓN GENERAL / FUNDADOR Carlos Alexis Lugo Marrero DISTRIBUCIÓN OFICINAS Y TORRES MÉDICAS Editorial Mundo
ENVÍO DE REVISTAS Y DISTRIBUCIÓN A GRUPOS MÉDICOS Servicio de correo postal/Comunicación Inteligente
Para ventas y otros servicios pueden comunicarse al 787.848.3333, msp@editorialmundo.com o www.medicinaysaludpublica.com
Revista Puertorriqueña de Medicina y Salud Pública ISSN 1937-8521
COMITÉ EDITORIAL CIENTÍFICO
COMITÉ EDITORIAL Olga Rodríguez, MD - Decana Escuela de Medicina de Ponce (Puerto Rico), Vivian Green, LND, MS, PhD, Sub editora y fundadora (Puerto Rico), José Cordero, MD, MPH - Exdecano Escuela Graduada Salud Pública Recinto de Ciencias Médicas UPR (Puerto Rico), Ángeles Rodríguez, MD, MPH (Puerto Rico), Simón Carlo, MD (Puerto Rico), Bárbara Rosado, MD (Puerto Rico), Idhaliz Flores PhD (Puerto Rico), Jesús Cruz-Correa, MD, FACOG (Puerto Rico), Rafael Bredy, MD, LicMTo, MBE, MS (Puerto Rico), David Caseida, MD, FACOG, (Puerto Rico), José Capriles, MD, MHSA (Puerto Rico) Joaquín Laboy, MD, FACOG (Puerto Rico), Luis Adrian Rivera Pomales, MD, PEMBA, MPH, CMQ (Puerto Rico), Juan Fernández, MS, PhD (Puerto Rico), Nuria Sebate, MD (Puerto Rico), Pedro Amador, MD, MPH (Puerto Rico), Nydia Cappas, PsyD (Puerto Rico), Luis Franco, MD (Puerto Rico), Federico Montealegre, DVM, PhD, Msc (Puerto Rico), Nydia Ortiz, PsyD (Puerto Rico), José Pons, PhD, FPPR (Puerto Rico), Esdrás Vélez, JD, MPH (Puerto Rico), Diego Zavala, MSc, PhD, (Puerto Rico), Ana Torres-Martín, MD (Puerto Rico), Julio Cádiz, MD, MPH (Puerto Rico), Rafael Gómez-Cuevas (Colombia), José Javier Orengo, PhD(c) (España), Cesar A. Del Rey, MD (Panamá), Pedro Serrano, MD, PhD (España), Luis Serra-Majem, MD, PhD (España), José Ramón Calvo, MD, PhD (España).
Síguenos en www.medicinaysaludpublica.com, www.facebook.com/revistamsp, en Twitter @revistamsp, en LinkedIn como Revista Puertorriqueña de Medicina y Salud Pública. Las normas editoriales de la Revista Puertorriqueña de Medicina y Salud Pública para la publicación de artículos originales y cartas al editor pueden ser accesadas en la página web: www.medicinaysaludpublica.com, y solicitadas a través de msp@editorialmundo.com.
Revista Puertorriqueña de Medicina y Salud Pública 7
54 26
12
35 42 64
70




SAN JUAN
En Puerto Rico tenemos un tesoro en nuestra clase médica. Hemos disfrutado del privilegio de tener programas de entrenamiento locales que, bajo mucho esfuerzo y dedicación, gradúan múltiples profesionales de la salud cada año. A pesar de este tesoro, recientemente nos confrontamos con el reto de una clase médica que se sigue encogiendo. Estamos perdiendo nuestros médicos a Estados Unidos, tenemos programas de entrenamiento que han cerrado y otros que están en riesgo de perderse. Como también tenemos médicos que merecidamente, logran el retiro o que por razones de salud se nos van antes de tiempo.
Cada día somos menos y cada día se siente un poco más difícil ejercer una medicina de primera como se merecen nuestros pacientes. Cada día, es más difícil ser un profesional en Puerto Rico, como también cada día, se vuelve más difícil el acceso a nuestros derechos. El derecho a la seguridad, el derecho a la educación y el derecho a la salud. Difícil es ser médico, pero peor aún es ser enfermo o sufrir de una condición donde existe una terapia óptima, pero no hay como recibirla.
Aún con estos retos, no podemos perder nuestro norte. Quiero exhortar a la clase médica a participar más de sus respectivas sociedades. Participar en educar al estudiante de medicina a lograr crear una práctica exitosa cuando se gradúe. Mantenernos todos lo más preparados y educados para luchar en contra de las denegaciones. Sobre todo, y lo más importante, ayudar a educar a los pacientes para saber cómo prevenir las enfermedades, como reconocerlas temprano y de necesitarlo, como respetar la recomendación de su médico y adherirse a su terapia correctamente. Tenemos que ayudarnos a mantener a nuestros médicos primarios con el mejor conocimiento para que cuando le llegue el próximo “formulario preferido” entienda que hay condiciones donde hay medicamentos que tienen una razón por ser superiores independientemente del costo.
Esto lo podemos lograr en foros públicos ya sea en persona o de manera digital. Hay que sobrepasar la realidad de que ya no somos suficientes y no damos abasto para lograr hacer esta intervención en nuestras clínicas individualmente. Hay que buscar como múltiplicar nuestro alcance y así lograr mantener a los nuestros saludables, mientras seguimos la lucha por un mejor Puerto Rico.
Luis A. Renta-Rosa, MD, FACC Presidente Sociedad Puertorriqueña de Cardiología


Revista Puertorriqueña de Medicina y Salud Pública 9
EDITORIAL
EPIDEMIOLOGÍA DE ENFERMEDADES DEL CORAZÓN EN PUERTO RICO
Son la segunda causa de muerte en Puerto Rico y la primera en el resto del mundo, incluyendo los Estados Unidos.
MORTALIDAD POR ENFERMEDADES
CARDIOVASCULARES
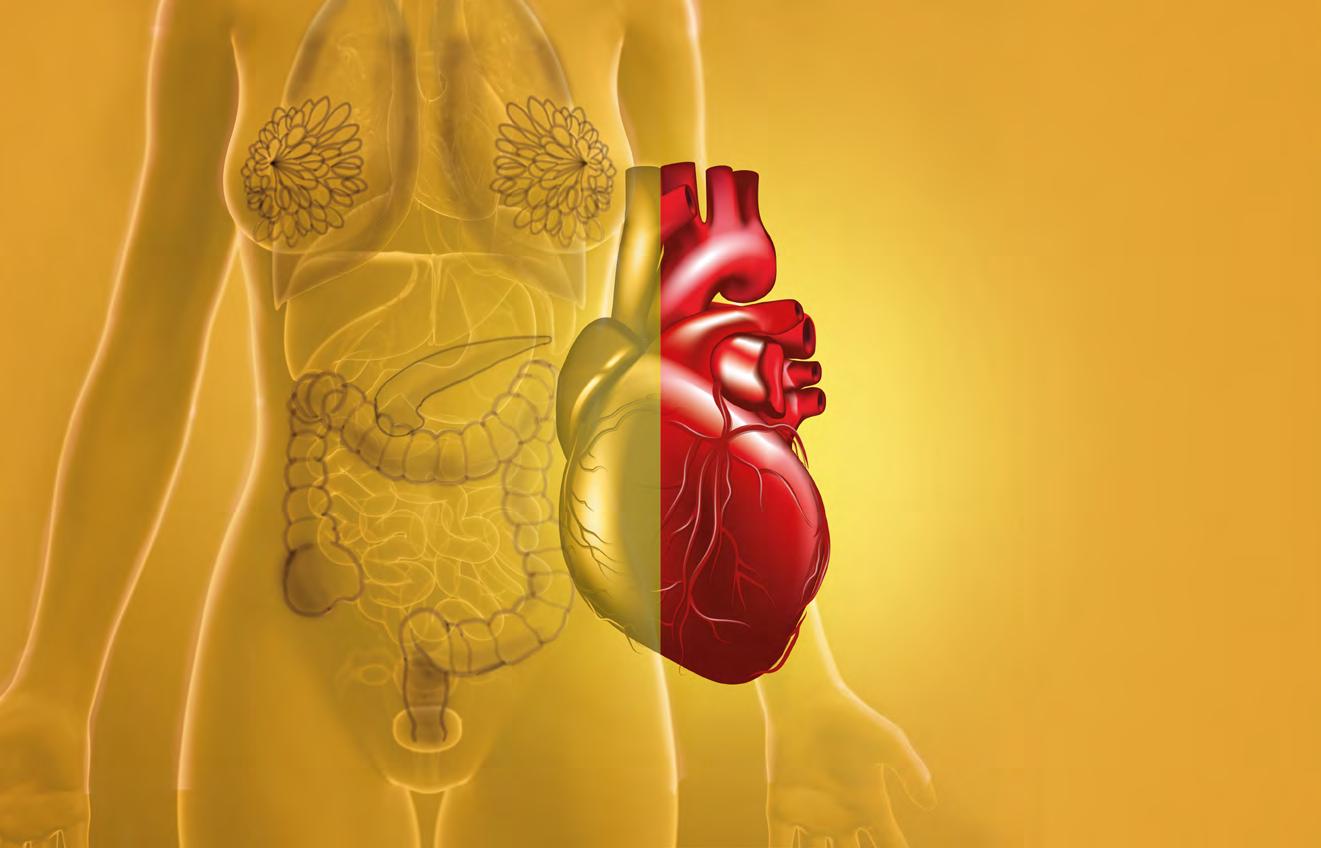







Las personas de 65 años o más reportaron la prevalencia más alta de ataques al miocardio (10.3 %), enfermedad coronaria (13.7 %), hipertensión (69.7 %) y apoplejías (3.8 %).
Las personas con un ingreso anual menor de $ 15,000 reportan la mayor prevalencia de ataques al miocardio (5.6 %), enfermedad coronaria (8.7 %), hipertensión (46.8 %) y apoplejías (stroke) (2.4 %).
324,255 personas utilizan servicios de salud para atender enfermedades cardiovasculares en Puerto Rico. 180,508 son mujeres (56 %) y 143,747 son hombres (44 %). El grupo de edad con mayor diagnóstico fue el de 65 a 69 años.
Las personas que no completaron la escuela superior reportan la prevalencia más alta de ataques al miocardio (7.3 %), enfermedad coronaria (11.1 %), hipertensión (56 %) y apoplejías (2.9 %).(10.3 %), enfermedad coronaria (13.7 %), hipertensión (69.7 %) y apoplejías (3.8 %).
MORTALIDAD POR ENFERMEDADES CARDIOVASCULARES
En los hombres la tasa de mortalidad es de 160.8, por cada 100,000 habitantes
En las mujeres la tasa de mortalidad es de 122.3, por cada 100,000 habitantes
PREVALENCIA DE LAS CONDICIONES CARDIOVASCULARES EN PUERTO RICO
42,2%
de los puertorriqueños padecen hipertensión
La hipertensión es la más común en Puerto Rico y Estados Unidos.
Puerto Rico, tiene una mayor prevalencia de hipertensión y enfermedad coronaria que Estados Unidos.
Aguadilla, Fajardo y Arecibo son las regiones que presentan la mayor prevalencia para las condiciones cardiovasculares.
La región de Mayagüez tiene la menor prevalencia para todas las enfermedades.
Las mujeres tienen mayor prevalencia de hipertensión.
2da fue pagado por los pacientes.
La tasa de mortalidad a causa de las enfermedades cardiovasculares es de 114.1, siendo la segunda causa de muerte.
65%
35% por las aseguradoras de salud.
Los hombres tienen una prevalencia más alta de infarto al miocardio.
Palabras clave: Mastectomía total con preservación del complejo pezón/areola, cáncer de seno, reconstrucción de seno
Key Words:
Palabras Clave: carcinoma mamario, estadificación, tratamiento cáncer de seno, mastectomía parcial, mastectomía total, radioterapia, quimioterapia.
Nipple sparing mastectomy, breast cancer, breast reconstruction

Metamorfosis, Sandra Reyes


El carcinoma mamario es el cáncer invasivo más diagnosticado en el mundo, reemplazando así el carcinoma pulmonar. En el 2020 se diagnosticaron 2.3 millones de casos nuevos, y 685,000 muertes asociadas al cáncer de mama. Para 2023, cerca de 300,000 casos nuevos de
carcinoma mamario invasivo (CMI) serán diagnosticados en los EEUU2. Según datos de la Sociedad Americana Contra el Cáncer, cada año en Puerto Rico aproximadamente 2,205 mujeres son diagnosticadas con cáncer de seno, y unas 444 de estas mueren a causa de esta enfermedad. La tasa de mortalidad
por cáncer de seno en Puerto Rico equivale al 18.9% de la población femenina.
En los pasados años, el Comité Conjunto Americano de Cáncer (AJCC por sus siglas en inglés) actualizó las guías de estadificación para el
12 Revista Puertorriqueña de Medicina y Salud Pública
cáncer de mama Cirugía moderna para el
Resumen:
Bolívar Arboleda-Osorio MD FACS Director Caribbean Breast Surgery Presidente Sociedad Puertorriqueña de Senología


RESUMEN
Por: Bolívar Arboleda Osorio, MD, FACS
Sociedad

La mastectomía total con preservación del complejo pezón/areola ha adquirido más visibilidad entre las opciones quirúrgicas para el tratamiento del cáncer de mama. Las pacientes óptimas para este procedimiento deben ser escogidas con cuidado, es decir pacientes con tumores pequeños limitados a un cuadrante del seno sin envolvimiento de la piel o el pezón y con poca o ninguna ptosis. Las complicaciones de dicho procedimiento pueden ser ligeramente superiores a otras cirugías conservadoras de mama y la tasa de necrosis del pezón ha ido en disminución gracias al uso de los avances tecnológicos disponibles. En los casos apropiados la tasa de recurrencia local debe ser aceptablemente baja.
ESTADIFICACIÓN Y TRATAMIENTO DEL CARCINOMA MAMARIO SEGÚN SU ETAPA
Summary:
Nipple Sparing Mastectomy (NSM) has become more common among the surgical options for the treatment of early stage breast cancer. Patients for this procedure should be selected with care, mostly patients with smaller tumors limited to one quadrant of the breast, without involvement of the breast skin or nipple and with no or very little ptosis. Complications for this procedure could be slightly more than other breast conserving surgeries but the rate of nipple necrosis has been steadily decreasing when the appropriate technological advances are utilized. The rate of nipple necrosis should be acceptably low.
El carcinoma mamario es el carcinoma invasivo más diagnosticado en el mundo. Su manejo depende de la estadificación patológica de la paciente, la cual se define por el tamaño del tumor (T), situación de los ganglios axilares y regionales (N), y la presencia o ausencia de metástasis a distancia (M). También entran en consideración factores adicionales tales como la situación de marcadores hormonales y la firma genómica del tumor.
carcinoma mamario, de manera que además del tradicional método de estadificación utilizando el tamaño del tumor, situación ganglionar, y metástasis a distancia (mejor conocido como sistema TNM) se le incorpora a las guías la situación de los receptores de estrógeno, progesterona, y HER-2,
al igual que el factor de proliferación Ki-67. Esto ha resultado en una clasificación más compleja, pero a la misma vez ha provisto una estadificación más detallada4. De tal suerte, iremos identificando cada uno de los estadios del CMI y, en forma general, las ofertas de tratamiento de las que disponemos
al presente. Dentro de cada estadío hay sub-variantes de manejo, pero en este escrito solo reseñaremos las más comunes.
Revista Puertorriqueña de Medicina y Salud Pública 13
MSP ARTÍCULO DE REVISIÓN
Revista Puertorriqueña de Medicina y Salúd Pública 31
Presidente de la
Puertorriqueña de Senología Pasado Presidente del Colegio Americano de Cirujanos capítulo de PR Director del HIMA San Pablo Breast Institute/ Oncológico, Caguas Profesor Asociado de Cirugía de la Universidad Central del Caribe
Coautora: Diana Avilés Castillo, MD Cirugía plástica reconstructiva Board Certified American Association of Plastic Surgery
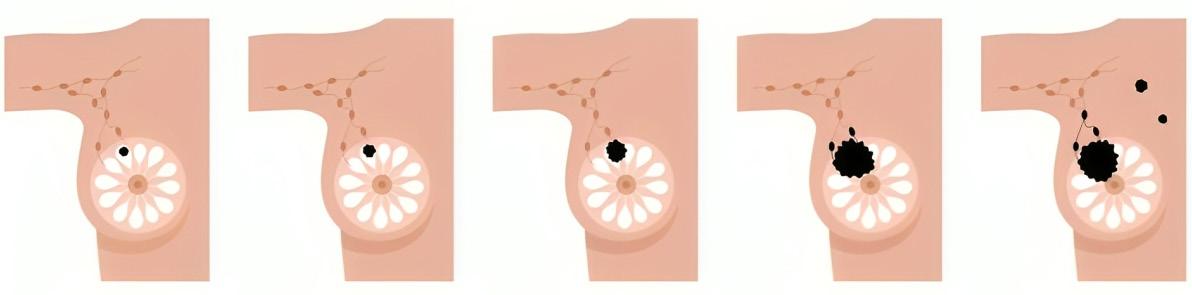
ESTADIOS DEL CÁNCER DE MAMA
ESTADIO 0 ESTADIO 1 ESTADIO 2 ESTADIO 3 ESTADIO 4

ESTADÍO 0
Definición: Llamamos estadio 0 a aquellos tumores que por su naturaleza no representan riesgo conocido de hacer metástasis. En este grupo tenemos primordialmente al carcinoma ductal in situ (DCIS por sus siglas en inglés).
Tratamiento: El manejo de este estadío es muy interesante, ya que puede ir desde una cirugía muy conservadora como lo es una mastectomía parcial (MP), [la cual para propósitos de este escrito se equipara con los términos tumeroctomía, cuadrantectomía, tilectomía y “lumpectomía”], hasta una mastectomía total (MT) con o sin reconstrucción. La extensión de esta cirugía va a depender de la extensión del DCIS. En grupos discretos de micro calcificaciones podemos optar por una MP, pero en DCIS extenso frecuentemente recurrimos a la MT. Hoy en día se recomienda el añadir radioterapia (RXT) a las MP, pero en estudios que están en vía de publicación veremos la omisión de la RXT en casos selectos. Este grupo de pacientes no requiere de quimioterapia, y el uso de hormonoterapia debe considerarse en aquellas que son hormono-positivas.
ESTADÍO 1
Definición: El estadio 1 se define como tumores invasivos que miden 20 mm o menos, y cuyos nódulos axilares están libres de tumor o presentan tumor entre 0.2 y 2 mm. No hay metástasis a distancia (T0 o T1, N1mi, M0)
Tratamiento: Los estadios 1, 2, y 3 son la muestra por excelencia del enlace multidisciplinario en el carcinoma mamario. El ancla de este manejo lo constituye la cirugía, utilizando la radioterapia, quimioterapia y
hormonoterapia en los casos indicados.
a - Cirugía: en estos tumores pequeños, la cirugía conservadora mamaria como lo es la MP con o sin cirugía mamaria oncoplástica (CMO) es el procedimiento de elección. En algunos casos (como por ejemplo pacientes BRCA-mutadas) podemos considerar la MT bilateral como opción de tratamiento. La reconstrucción de la mama siempre debe jugar un papel en nuestra oferta quirúrgica, aunque algunas pacientes opten por no utilizar esta alternativa al momento de su cirugía primaria y relegarla para un segundo tiempo. El manejo de la axila en la mayoría de estos casos se realiza usando la técnica de biopsia de ganglio centinela (BGC). Varios estudios han demostrado la eficacia de dicha técnica.
b - Quimioterapia neo-adyuvante: la neoadjuvancia consiste en el uso de manejo quimioterapéutico antes del proceso quirúrgico. En el estadio 1 consideramos esta opción primordialmente en las pacientes con tumor T1c con alteración HER-2 positivo o que son triple negativos.
c - Radioterapia: al presente, la radioterapia está indicada en toda paciente que recibe cirugía conservadora de la mama. Sin embargo, omitir la radioterapia puede ser una alternativa en pacientes mayores de 70 años de edad.11 Ya en Puerto Rico tenemos disponible la posibilidad de radioterapia intra-operatoria para casos selectos de carcinoma en estadíos tempranos.
d - Hormonoterapia: indicada en las pacientes hormono-sensitivas (ER y/o PR positivas). El largo de tratamiento y la secuencia del mismo puede variar según las características de la paciente.
ESTADÍO 2
Definición: El estadío 2 tiene una combinación potencial de varios tamaños tumorales y condición axilar, pero todos ellos carecen de metástasis a distancia. De tal suerte, el tamaño del tumor puede llegar hasta 50 mm, y el número de ganglios axilares afectados puede ser de 1-3. Las combinaciones posibles son T0N1M0, T1N1M0, T2N0M0, T2N1M0, T3N0M0.
Tratamiento: Al igual que en estadío 1, el manejo principal lo constituye la cirugía, utilizando la radioterapia, quimioterapia y hormonoterapia en los casos indicados.
a - Cirugía: aunque en el estadío 2 los tumores pueden ser más grandes que en estadío 1, la cirugía conservadora mamaria como lo es la MP con o sin cirugía mamaria oncoplástica (CMO) sigue siendo el procedimiento de elección. Aquí la CMO toma más relevancia, ya que la cirugía puede conllevar la reorganización de los tejidos mamarios, solamente posible con las técnicas de CMO nivel 2 y 3. En algunos casos (como por ejemplo pacientes BRCA-mutadas) podemos considerar la MT bilateral como opción de tratamiento. El manejo de la axila en la mayoría de estos casos se realiza usando la técnica de biopsia de ganglio centinela (BGC). Varios estudios han demostrado la eficacia de dicha técnica.
b - Quimioterapia neo-adyuvante: En el estadío 2 esta opción se hace más relevante sobre todo en los tumores T2 y T3. Las pacientes con alteración HER2 positivo o que son triple negativos también pueden beneficiarse de este tratamiento10.
c - Radioterapia: indicaciones similares al estadío 1.
14 Revista Puertorriqueña de Medicina y Salud Pública
d - Hormonoterapia: indicaciones similares al estadío 1.
ESTADÍO 3
Definición: El estadío 3 implica enfermedad loco-regional avanzada, pero sin que exista metástasis a distancia. La invasión de piel o pared torácica es una posibilidad en este estadío. Las combinaciones posibles son (T0, T1, T2, o T3; N2; M0), (T3, N1, M0), (T4; N0, N1, o N2; M0), (cualquier T, N3, M0)
Tratamiento: Al igual que en estadío 1 y 2, el manejo principal lo constituye la cirugía, pero el uso de la neoadjuvancia toma mayor relevancia. Dentro de este estadío se incluye la variante particular de carcinoma inflamatorio de la mama, en donde la combinación de quimioterapia neoadyuvante seguida de cirugía y luego radioterapia y hormonoterapia en los casos indicados ha sido de mucha ayuda.
a - Cirugía: en este estadío la cirugía conservadora mamaria como lo es la MP con o sin cirugía mamaria oncoplástica (CMO) puede todavía ser una opción, pero más frecuentemente se utiliza un MT con BGC o disección ganglionar axilar (DGA). Aquí la CMO toma más relevancia, ya que la cirugía puede conllevar la reorganización de
los tejidos mamarios, solamente posible con las técnicas de CMO nivel 2 y 3. En algunos casos (como por ejemplo pacientes BRCA-mutadas) podemos considerar la MT bilateral como opción de tratamiento. El manejo de la axila en estos casos se realiza usando la técnica de BGC para la situación N1, y DGA para la situación N2 y N3.
b - Quimioterapia neo-adyuvante y adyuvante: En este estadío la enfermedad local avanzada frecuentemente nos obliga a utilizar la neoadjuvancia para disminuir la carga tumoral local y facilitar el proceso quirúrgico14. En algunos casos, el uso de quimioterapia puede también darse luego de la cirugía.
c - Radioterapia: en adición a las indicaciones similares al estadío 1 y 2, la radioterapia a la pared costal suele utilizarse en aquellos tumores con invasión de la misma y en los casos de carcinoma inflamatorio de la mama15 .
d - Hormonoterapia: indicaciones similares al estadío 1.
ESTADÍO 4
Definición: La característica principal de este estadío es que ya existe metástasis a distancia. Muchas veces esto ocurre luego de que ya la paciente ha sido tratada anteriormente
para luego presentar con la situación metastática. Pero en algunos casos puede ser diagnosticada como estadío 4 desde el principio, lo que conocemos como Estadío 4 De Novo7.
Tratamiento: el manejo de este estadío es principalmente médico, con el uso de la radioterapia y la cirugía relegadas a un segundo plano. Para las pacientes con enfermedad hormonopositiva el uso de inhibidores CDK4/6 en combinación con terapia endocrina como primera línea se levanta como una opción factible16, 17. En los casos de pacientes con tumores triple-negativos, tenemos las opciones de quimioterapia en combinación con inhibidores de PARP, carboplatino, pembrolizumab, entre otros. En enfermedad HER-2 positiva, los anticuerpos monoclonales de diferentes tipos siguen siendo importantes en el armamentario contra este tumor.
CONCLUSIÓN
El tratamiento para el carcinoma mamario ha tenido grandes avances en las últimas décadas, logrando reducir la mortalidad de esta terrible enfermedad18. La interacción multidisciplinaria sigue siendo el corazón del manejo de este grupo de pacientes, ejemplificando la multiplicidad de opciones terapéuticas disponibles.
TAMAÑOS DE TUMORES DEL CÁNCER DE MAMA
REFERENCIAS
1. Organización Mundial de la Salud, Cáncer de Mama, datos y cifras, 12 julio 2023, webpage,
2. American Cancer Society, Key Statistics for Breast Cancer, webpage
3. Departamento de Salud, Cáncer, 30 enero 2023
4. Eur J Breast Health. 2021 Jul; 17(3): 234–238.
5. Radiotherapy Omission in Low Risk Ductal in Situ Carcinoma Breast (ROMANCE) website: clinicalTrials.gov ID NCT03878342
6. Pathol Oncol Res. 2020 Jan;26(1):521531
7. ASCO.org Cancer.net Breast Cancer
Stages webpage https://www.cancer. net/cancer-types/breast-cancer/ stages#stage-groups
8. JAMA. 2017;318(10):918-926
9. Lancet Oncol. 2014 Nov; 15(12): 1303–1310
10. Breast Cancer (Dove Med Press). 2021; 13: 199–211

11. J Surg Res. 2022 Nov;279:393-397
12. Asoc hospitales de Puerto Rico, webpage, https://hospitalespr.org/2023/01/18/ tecnica-novel-de-radioterapiaintraoperatoria-hospital-oncologico/
13. Breast Cancer.Org webpage, https:// www.breastcancer.org/treatment/ hormonal-therapy
14. Journal of Clinical Oncology 39, no. 13 (May 01, 2021) 1485-1505.
15. Practical Radiation Oncology, VOLUME 9, ISSUE 6, P402-409, NOVEMBER 2019
16. N Engl J Med. 2016 Nov 17;375(20):1925-1936

17. J Clin Oncol. 2017 Nov 10;35(32):36383646
18. CDC webpage, Disparity in Breast Cancer Deaths, https://www.cdc.gov/cancer/ dcpc/research/articles/disparities-breastcancer-deaths.htm#:~:text=Overall%20 breast%20cancer%20death%20 rates,1.3%25%20per%20year%20 on%20average
Revista Puertorriqueña de Medicina y Salud Pública 15




She needs a treatment shown to reduce risk of recurrence in high-risk early breast cancer (EBC) 1 The first FDA-approved addition to adjuvant ET in nearly 2 decades 1-9 ET=endocrine therapy; HER2−=human epidermal growth factor receptor 2–negative; HR+=hormone receptor–positive.
INDICATION
VERZENIO® (abemaciclib) is indicated in combination with endocrine therapy (tamoxifen or an aromatase inhibitor) for the adjuvant treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive, early breast cancer at high risk of recurrence and a Ki-67 score ≥20% as determined by an FDA-approved test.1





SELECT IMPORTANT SAFETY INFORMATION
Severe diarrhea associated with dehydration and infection occurred in patients treated with Verzenio. Across four clinical trials in 3691 patients, diarrhea occurred in 81 to 90% of patients who received Verzenio. Grade 3 diarrhea occurred in 8 to 20% of patients receiving Verzenio. Most patients experienced diarrhea during the first month of Verzenio treatment. The median time to onset of the first diarrhea event ranged from 6 to 8 days; and the median duration of Grade 2 and Grade 3 diarrhea ranged from 6 to 11 days and 5 to 8 days, respectively. Across trials, 19 to 26% of patients with diarrhea required a Verzenio dose interruption and 13 to 23% required a dose reduction.
Instruct patients to start antidiarrheal therapy, such as loperamide, at the first sign of loose stools, increase oral fluids, and notify their healthcare provider for further instructions and appropriate follow-up. For Grade 3 or 4 diarrhea, or diarrhea that requires hospitalization, discontinue Verzenio until toxicity resolves to ≤Grade 1, and then resume Verzenio at the next lower dose.
Neutropenia, including febrile neutropenia and fatal neutropenic sepsis, occurred in patients treated with Verzenio. Across four clinical trials in 3691 patients, neutropenia occurred in 37 to 46% of patients receiving Verzenio. A Grade ≥3 decrease in neutrophil count (based on laboratory findings) occurred in 19 to 32% of patients receiving Verzenio. Across trials, the median time to first episode of Grade ≥3 neutropenia ranged from 29 to 33 days, and the median duration of Grade ≥3 neutropenia ranged from 11 to 16 days. Febrile neutropenia has been reported in <1% of patients exposed to Verzenio across trials. Two deaths due to neutropenic sepsis were observed in MONARCH 2. Inform patients to promptly report any episodes of fever to their healthcare provider.
Monitor complete blood counts prior to the start of Verzenio therapy, every 2 weeks for the first 2 months, monthly for the next 2 months, and as clinically indicated. Dose interruption, dose reduction, or delay in starting treatment cycles is recommended for patients who develop Grade 3 or 4 neutropenia.
Severe, life-threatening, or fatal interstitial lung disease (ILD) or pneumonitis can occur in patients treated with Verzenio and other CDK4/6 inhibitors. In Verzenio-treated patients in EBC (monarchE), 3% of patients experienced ILD or pneumonitis of any grade: 0.4% were Grade 3 or 4 and there was one fatality (0.1%). In Verzenio-treated patients in MBC (MONARCH 1, MONARCH 2, MONARCH 3), 3.3% of Verzenio-treated patients had ILD or pneumonitis of any grade: 0.6% had Grade 3 or 4, and 0.4% had fatal outcomes. Additional cases of ILD or pneumonitis have been observed in the postmarketing setting, with fatalities reported. Monitor patients for pulmonary symptoms indicative of ILD or pneumonitis. Symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams. Infectious, neoplastic, and other causes for such symptoms should be excluded by means of appropriate investigations. Dose interruption or dose reduction is recommended in patients who develop persistent or recurrent Grade 2 ILD or pneumonitis. Permanently discontinue Verzenio in all patients with Grade 3 or 4 ILD or pneumonitis.
TAKE HOPE FURTHER Please see Select Important Safety Information throughout and Brief Summary of full Prescribing Information for Verzenio on the following pages.
Verzenio : FDA-APPROVED for patients with HR+, HER2–, node-positive EBC at high risk of recurrence and a Ki-67 score ≥20% 1-3
APPROVAL BASED ON RESULTS IN PATIENTS WITH THE INDICATED CLINICAL AND PATHOLOGICAL RISK FACTORS (n=2,003)1
Consider Verzenio for your patients with NODE-POSITIVE DISEASE Ki-67 ≥20%
SELECT IMPORTANT SAFETY INFORMATION (cont’d)



AND
monarchE was a phase III clinical trial that enrolled 5,637 peri- and postmenopausal adult women and men with HR+, HER2−, node-positive EBC at high risk of recurrence. High risk was defined as 4+ positive nodes, or 1-3 positive nodes with Grade 3 disease or tumor size ≥5 cm (central Ki-67 testing was conducted retrospectively for patients with untreated breast tissue samples), or 1-3 positive nodes with Ki-67 ≥20%. All patients completed primary treatment prior to 1:1 randomization to receive either 150-mg, twice-daily Verzenio plus SoC ET or SoC ET alone for 2 years. ET continued through 5-10 years as clinically indicated. The primary endpoint was IDFS.1,2



Grade ≥3 increases in alanine aminotransferase (ALT) (2 to 6%) and aspartate aminotransferase (AST) (2 to 3%) were reported in patients receiving Verzenio. Across three clinical trials in 3559 patients (monarchE, MONARCH 2, MONARCH 3), the median time to onset of Grade ≥3 ALT increases ranged from 57 to 87 days and the median time to resolution to Grade <3 was 13 to 14 days. The median time to onset of Grade ≥3 AST increases ranged from 71 to 185 days and the median time to resolution to Grade <3 ranged from 11 to 15 days.
Monitor liver function tests (LFTs) prior to the start of Verzenio therapy, every 2 weeks for the first 2 months, monthly for the next 2 months, and as clinically indicated. Dose interruption, dose reduction, dose discontinuation, or delay in starting treatment cycles is recommended for patients who develop persistent or recurrent Grade 2, or any Grade 3 or 4 hepatic transaminase elevation.
Please see Select Important Safety Information throughout and Brief Summary of full Prescribing Information for Verzenio on the following pages.
Please see Select Important Safety Information throughout and Brief Summary of full Prescribing Information for Verzenio on the following pages.
4+ nodes OR 1-3 nodes with Grade 3 disease or tumor size ≥5 cm
IDFS=invasive disease–free survival; SoC=standard of care.
In patients with HR+, HER2−, node-positive EBC at high risk of recurrence and a Ki-67 score ≥20% (n=2,003)
In patients with HR+, HER2−, node-positive EBC at high risk of recurrence and a Ki-67 score ≥20% (n=2,003)
Verzenio: The only CDK4 & 6 inhibitor to reduce risk of recurrence in combination with ET 1,7-9
In patients with HR+, HER2−, node-positive EBC at high risk of recurrence and a Ki-67 score ≥20% (n=2,003)
Verzenio: The only CDK4 & 6 inhibitor to reduce risk of recurrence in combination with ET 1,7-9
The only CDK4 & 6 inhibitor to reduce risk of recurrence in combination with ET 1,7-9


Verzenio:
At 3 years, Verzenio reduced the risk of recurrence by more than a third1
At 3 years, Verzenio reduced the risk of recurrence by more than a third1
37 %
See the breakthrough results at VerzenioData.com/EBC
SELECT IMPORTANT SAFETY INFORMATION (cont’d)
SELECT IMPORTANT SAFETY INFORMATION (cont’d)
86.1% of patients remained recurrence-free with Verzenio plus ET vs 79.0% with ET alone.1
At 3 years, Verzenio reduced the risk of recurrence by more than a third1



86.1% of patients remained recurrence-free with Verzenio plus ET vs 79.0% with ET alone.1
86.1% of patients remained recurrence-free with Verzenio plus ET vs 79.0% with ET alone.1










The number of events at the time of analysis was 104 with Verzenio plus ET vs 158 with ET alone.1
The number of events at the time of analysis was 104 with Verzenio plus ET vs 158 with ET alone.1
The number of events at the time of analysis was 104 with Verzenio plus ET vs 158 with ET alone.1

OS was immature. A total of 95 (4.7%) patients had died. Long-term follow-up is planned.1,2
OS was immature. A total of 95 (4.7%) patients had died. Long-term follow-up is planned.1,2
OS was immature. A total of 95 (4.7%) patients had died. Long-term follow-up is planned.1,2
This post hoc efficacy analysis was performed at a median follow-up of 27.1 months. Additional exploratory analyses were performed at this time; efficacy results for the subpopulation with high-risk clinicopathological features and Ki-67 ≥20% are provided.3*
This post hoc efficacy analysis was performed at a median follow-up of 27.1 months. Additional exploratory analyses were performed at this time; efficacy results for the subpopulation with high-risk clinicopathological features and Ki-67 ≥20% are provided.
* Statistical significance was achieved for this subpopulation earlier at the final IDFS analysis. The result in this post hoc analysis cannot be interpreted as statistically significant.1
This post hoc efficacy analysis was performed at a median follow-up of 27.1 months. Additional exploratory analyses were performed at this time; efficacy results for the subpopulation with high-risk clinicopathological features and Ki-67 ≥20% are provided.
Statistical significance was achieved for this subpopulation earlier at the final IDFS analysis. The result in this post hoc analysis cannot be interpreted as statistically significant.
* Statistical significance was achieved for this subpopulation earlier at the final IDFS analysis. The result in this post hoc analysis cannot be interpreted as statistically significant.
Venous thromboembolic events (VTE) were reported in 2 to 5% of patients across three clinical trials in 3559 patients treated with Verzenio (monarchE, MONARCH 2, MONARCH 3). VTE included deep vein thrombosis, pulmonary embolism, pelvic venous thrombosis, cerebral venous sinus thrombosis, subclavian and axillary vein thrombosis, and inferior vena cava thrombosis. In clinical trials, deaths due to VTE have been reported in patients treated with Verzenio.
Venous thromboembolic events (VTE) were reported in 2 to 5% of patients across three clinical trials in 3559 patients treated with Verzenio (monarchE, MONARCH 2, MONARCH 3). VTE included deep vein thrombosis, pulmonary embolism, pelvic venous thrombosis, cerebral venous sinus thrombosis, subclavian and axillary vein thrombosis, and inferior vena cava thrombosis. In clinical trials, deaths due to VTE have been reported in patients treated with Verzenio.
SELECT IMPORTANT SAFETY INFORMATION (cont’d)
Venous thromboembolic events (VTE) were reported in 2 to 5% of patients across three clinical trials in 3559 patients treated with Verzenio (monarchE, MONARCH 2, MONARCH 3). VTE included deep vein thrombosis, pulmonary embolism, pelvic venous thrombosis, cerebral venous sinus thrombosis, subclavian and axillary vein thrombosis, and inferior vena cava thrombosis. In clinical trials, deaths due to VTE have been reported in patients treated with Verzenio.
Verzenio has not been studied in patients with early breast cancer who had a history of VTE. Monitor patients for signs and symptoms of venous thrombosis and pulmonary embolism and treat as medically appropriate. Dose interruption is recommended for EBC patients with any grade VTE and for MBC patients with a Grade 3 or 4 VTE.
Verzenio has not been studied in patients with early breast cancer who had a history of VTE. Monitor patients for signs and symptoms of venous thrombosis and pulmonary embolism and treat as medically appropriate. Dose interruption is recommended for EBC patients with any grade VTE and for MBC patients with a Grade 3 or 4 VTE.
Verzenio has not been studied in patients with early breast cancer who had a history of VTE. Monitor patients for signs and symptoms of venous thrombosis and pulmonary embolism and treat as medically appropriate. Dose interruption is recommended for EBC patients with any grade VTE and for MBC patients with a Grade 3 or 4 VTE.

Verzenio can cause fetal harm when administered to a pregnant woman, based on findings from animal studies and the mechanism of action. In animal reproduction studies, administration of abemaciclib to pregnant rats during the period of organogenesis caused teratogenicity and decreased fetal weight at maternal exposures that were similar to the human clinical exposure based on area under the curve (AUC) at the maximum recommended human dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Verzenio and for 3 weeks after the last dose. Based on findings in animals, Verzenio may impair fertility in males of reproductive potential. There are no data on the presence of Verzenio in human milk or its effects on the breastfed child or on milk production. Advise lactating women not to breastfeed during Verzenio treatment and for at least 3 weeks after the last dose because of the potential for serious adverse reactions in breastfed infants.
Verzenio can cause fetal harm when administered to a pregnant woman, based on findings from animal studies and the mechanism of action. In animal reproduction studies, administration of abemaciclib to pregnant rats during the period of organogenesis caused teratogenicity and decreased fetal weight at maternal exposures that were similar to the human clinical exposure based on area under the curve (AUC) at the maximum recommended human dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Verzenio and for 3 weeks after the last dose. Based on findings in animals, Verzenio may impair fertility in males of reproductive potential. There are no data on the presence of Verzenio in human milk or its effects on the breastfed child or on milk production. Advise lactating women not to breastfeed during Verzenio treatment and for at least 3 weeks after the last dose because of the potential for serious adverse reactions in breastfed infants.
Verzenio can cause fetal harm when administered to a pregnant woman, based on findings from animal studies and the mechanism of action. In animal reproduction studies, administration of abemaciclib to pregnant rats during the period of organogenesis caused teratogenicity and decreased fetal weight at maternal exposures that were similar to the human clinical exposure based on area under the curve (AUC) at the maximum recommended human dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Verzenio and for 3 weeks after the last dose. Based on findings in animals, Verzenio may impair fertility in males of reproductive potential. There are no data on the presence of Verzenio in human milk or its effects on the breastfed child or on milk production. Advise lactating women not to breastfeed during Verzenio treatment and for at least 3 weeks after the last dose because of the potential for serious adverse reactions in breastfed infants.
Revista Puertorriqueña de Medicina y Salud Pública 19 INVASIVE DISEASE-FREE SURVIVAL (%) TIME (MONTHS) 100 90 80 70 60 50 40 30 20 10 0 3 6 9 12 15 18 21 24 27 30 33 36 39 0 Verzenio + ET ET alone NUMBER AT RISK 936 946 963 989 1017 922 908 894 733 484 348 203 109 25 2 0 986 955 938 906 922 883 868 835 687 457 333 197 107 25 3 0 79.0% 86.1%
Verzenio + ET ET
HR=hazard ratio; OS=overall survival.
INVASIVE DISEASE-FREE SURVIVAL (%) TIME (MONTHS) 100 90 80 70 60 50 40 30 20 10 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 0 OF RECURRENCE Verzenio + ET ET alone NUMBER AT RISK 936 946 963 989 1017 922 908 894 733 484 348 203 109 25 2 0 986 955 938 906 922 883 868 835 687 457 333 197 107 25 3 0 79.0% 86.1% Verzenio + ET ET (95% Cl: 0.49-0.80)
37
HR=hazard ratio; OS=overall survival.
VerzenioData.com/EBC
See the breakthrough results at
INVASIVE DISEASE-FREE SURVIVAL (%) TIME (MONTHS) 100 90 80 70 60 50 40 30 20 10 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 0 REDUCTION IN RISK OF RECURRENCE1 Verzenio + ET ET alone NUMBER AT RISK 936 946 963 989 1017 922 908 894 733 484 348 203 109 25 2 0 986 955 938 906 922 883 868 835 687 457 333 197 107 25 3 0 79.0% 86.1% Verzenio + ET ET (95% Cl: 0.49-0.80) HR=0.63
HR=hazard ratio; OS=overall survival.
See the breakthrough results at VerzenioData.com/EBC
therapy (tamoxifen or an aromatase inhibitor) alone. Patients were randomly assigned to receive 150 mg of VERZENIO orally, twice daily, plus tamoxifen or an aromatase inhibitor, or tamoxifen or an aromatase inhibitor, for two years or until discontinuation criteria were met. The median duration of VERZENIO treatment was 24 months.
The most frequently reported (≥5%) Grade 3 or 4 adverse reactions were neutropenia, leukopenia, diarrhea, and lymphopenia.
Fatal adverse reactions occurred in 0.8% of patients who received VERZENIO plus endocrine therapy (tamoxifen or an aromatase inhibitor), including: cardiac failure (0.1%), cardiac arrest, myocardial infarction, ventricular fibrillation, cerebral hemorrhage, cerebrovascular accident, pneumonitis, hypoxia, diarrhea and mesenteric artery thrombosis (0.03% each).
Permanent VERZENIO treatment discontinuation due to an adverse reaction was reported in 19% of patients receiving VERZENIO, plus tamoxifen or an aromatase inhibitor. Of the patients receiving tamoxifen or an aromatase inhibitor, 1% permanently discontinued due to an adverse reaction. The most common adverse reactions leading to VERZENIO discontinuations were diarrhea (5%), fatigue (2%), and neutropenia (0.9%).
Dose interruption of VERZENIO due to an adverse reaction occurred in 62% of patients receiving VERZENIO plus tamoxifen or aromatase inhibitors. Adverse reactions leading to VERZENIO dose interruptions in ≥5% of patients were diarrhea (20%), neutropenia (16%), leukopenia (7%), and fatigue (5%).
Dose reductions of VERZENIO due to an adverse reaction occurred in 44% of patients receiving VERZENIO plus endocrine therapy (tamoxifen or an aromatase inhibitor). Adverse reactions leading to VERZENIO dose reductions in ≥5% were diarrhea (17%), neutropenia (8%), and fatigue (5%).
The most common adverse reactions reported (≥20%) in the VERZENIO, plus tamoxifen or an aromatase inhibitor, arm and ≥2% higher than the tamoxifen or an aromatase inhibitor arm were: diarrhea, infections, neutropenia, fatigue, leukopenia, nausea, anemia, and headache. Adverse reactions are shown in Table 1 and laboratory abnormalities are shown in Table 2.
Table
Plus
or an
e Includes asthenia, fatigue.
f Includes exfoliative rash, mucocutaneous rash, rash, rash erythematous, rash follicular, rash generalized, rash macular, rash maculo-papular, rash maculovesicular, rash morbilliform, rash papular, rash papulosquamous, rash pruritic, rash vesicular, vulvovaginal rash.
Clinically relevant adverse reactions in <10% of patients who received VERZENIO in combination with tamoxifen or an aromatase inhibitor in monarchE include:
• Pruritus-9%
• Dyspepsia-8%
• Nail disorder-6% (includes nail bed disorder, nail bed inflammation, nail discoloration, nail disorder, nail dystrophy, nail pigmentation, nail ridging, nail toxicity, onychalgia, onychoclasis, onycholysis, onychomadesis)
• Lacrimation increased-6%
• Dysgeusia-5%
• Interstitial lung disease (ILD)/pneumonitis-3% (includes pneumonitis, radiation pneumonitis, interstitial lung disease, pulmonary fibrosis, organizing pneumonia, radiation fibrosis – lung, lung opacity, sarcoidosis)
• Venous thromboembolic events (VTEs)-3% (includes catheter site thrombosis, cerebral venous thrombosis, deep vein thrombosis, device related thrombosis, embolism, hepatic vein thrombosis, jugular vein occlusion, jugular vein thrombosis, ovarian vein thrombosis, portal vein thrombosis, pulmonary embolism, subclavian vein thrombosis, venous thrombosis limb)
Table 2: Laboratory Abnormalities (≥10%) in Patients
Receiving VERZENIO Plus Tamoxifen or an Aromatase Inhibitor [with a Difference between Arms of ≥2%] in monarchE VERZENIO Plus Tamoxifen or an

a Includes the following fatal adverse reactions: diarrhea (n=1), and infections (n=4)
b Includes the following fatal adverse reactions: infections (n=5)
c Includes mouth ulceration, mucosal inflammation, oropharyngeal pain, stomatitis.
d Includes all reported preferred terms that are part of the Infections and Infestations system organ class. Most common infections (>5%) include upper respiratory tract infection, urinary tract infection, and nasopharyngitis.
DRUG INTERACTIONS
Effect of
Other Drugs on VERZENIO
CYP3A Inhibitors
Strong and moderate CYP3A4 inhibitors increased the exposure of abemaciclib plus its active metabolites to a clinically meaningful extent and may lead to increased toxicity.
Ketoconazole
Avoid concomitant use of ketoconazole. Ketoconazole is predicted to increase the AUC of abemaciclib by up to 16-fold.
Other Strong CYP3A Inhibitors
In patients with recommended starting doses of 200 mg twice daily or 150 mg twice daily, reduce the VERZENIO dose to 100 mg twice daily with concomitant use of strong CYP3A inhibitors other than ketoconazole. In patients who have had a dose reduction to 100 mg twice daily due to adverse reactions, further reduce the VERZENIO dose to 50 mg twice daily with concomitant use of strong CYP3A inhibitors. If a patient taking VERZENIO
22 Revista Puertorriqueña de Medicina y Salud Pública VERZENIO® (abemaciclib) tablets, for oral use AL HCP BS_MonE 12OCT2021 VERZENIO® (abemaciclib) tablets, for oral use AL HCP BS_MonE 12OCT2021 Verzenio, AL HCP BS_MonE 12OCT2021 - 7 x 10 PRINTER VERSION 2 OF 3
VERZENIO
Tamoxifen
Aromatase Inhibitor N=2791 Tamoxifen or an Aromatase Inhibitor N=2800 All Gradesa % Grade 3 % Grade 4 % All Gradesb % Grade 3 % Grade 4 % Gastrointestinal Disorders Diarrhea 84 8 0 9 0.2 0 Nausea 30 0.5 0 9 <0.1 0 Vomiting 18 0.5 0 4.6 0.1 0 Stomatitisc 14 0.1 0 5 0 0 Infections and Infestations Infectionsd 51 4.9 0.6 39 2.7 0.1 General Disorders and Administration Site Conditions Fatiguee 41 2.9 0 18 0.1 0 Nervous System Disorders Headache 20 0.3 0 15 0.2 0 Dizziness 11 0.1 0 7 <0.1 0 Metabolism and Nutrition Disorders Decreased appetite 12 0.6 0 2.4 <0.1 0 Skin and Subcutaneous Tissue Disorders Rashf 11 0.4 0 4.5 0 0 Alopecia 11 0 0 2.7 0 0
1: Adverse Reactions (≥10%) of Patients Receiving VERZENIO Plus Tamoxifen or an Aromatase Inhibitor [with a Difference between Arms of ≥2%] in monarchE
Aromatase Inhibitor N=2791 Tamoxifen
Aromatase Inhibitor N=2800 All Grades % Grade 3 % Grade 4 % All Grades % Grade 3 % Grade 4 % Creatinine increased 99 0.5 0 91 <0.1 0 White blood cell decreased 89 19 <0.1 28 1.1 0 Neutrophil count decreased 84 18 0.7 23 1.6 0.3 Anemia 68 1.0 0 17 0.1 0 Lymphocyte count decreased 59 13 0.2 24 2.4 0.1 Platelet count decreased 37 0.7 0.2 10 0.1 0.1 Alanine aminotransferase increased 37 2.5 <0.1 24 1.2 0 Aspartate aminotransferase increased 31 1.5 <0.1 18 0.9 0 Hypokalemia 11 1.2 0.1 3.8 0.1 0.1
or an
discontinues a strong CYP3A inhibitor, increase the VERZENIO dose (after 3-5 half-lives of the inhibitor) to the dose that was used before starting the inhibitor. Patients should avoid grapefruit products.
Moderate CYP3A Inhibitors
With concomitant use of moderate CYP3A inhibitors, monitor for adverse reactions and consider reducing the VERZENIO dose in 50 mg decrements, if necessary.
Strong and Moderate CYP3A Inducers

Coadministration of strong or moderate CYP3A inducers decreased the plasma concentrations of abemaciclib plus its active metabolites and may lead to reduced activity. Avoid concomitant use of strong or moderate CYP3A inducers and consider alternative agents.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Based on findings in animals and its mechanism of action, VERZENIO can cause fetal harm when administered to a pregnant woman. There are no available human data informing the drug-associated risk. Advise pregnant women of the potential risk to a fetus. In animal reproduction studies, administration of abemaciclib during organogenesis was teratogenic and caused decreased fetal weight at maternal exposures that were similar to human clinical exposure based on AUC at the maximum recommended human dose (see Data). Advise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies.
Data
Animal Data
In an embryo-fetal development study, pregnant rats received oral doses of abemaciclib up to 15 mg/kg/day during the period of organogenesis. Doses ≥4 mg/kg/day caused decreased fetal body weights and increased incidence of cardiovascular and skeletal malformations and variations. These findings included absent innominate artery and aortic arch, malpositioned subclavian artery, unossified sternebra, bipartite ossification of thoracic centrum, and rudimentary or nodulated ribs. At 4 mg/kg/day in rats, the maternal systemic exposures were approximately equal to the human exposure (AUC) at the recommended dose.
Lactation
Risk Summary
There are no data on the presence of abemaciclib in human milk, or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed infants from VERZENIO, advise lactating women not to breastfeed during VERZENIO treatment and for 3 weeks after the last dose.
Females and Males of Reproductive Potential
Based on animal studies, VERZENIO can cause fetal harm when administered to a pregnant woman.
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating treatment with VERZENIO.
Contraception
Females
Advise females of reproductive potential to use effective contraception during VERZENIO treatment and for 3 weeks after the last dose.
Infertility
Males
Based on findings in animals, VERZENIO may impair fertility in males of reproductive potential.
Pediatric Use
The safety and effectiveness of VERZENIO have not been established in pediatric patients.
Geriatric Use
Of the 2791 VERZENIO-treated patients in monarchE, 15% were 65 years of age or older and 2.7% were 75 years of age or older.
Of the 900 patients who received VERZENIO in MONARCH 1, MONARCH 2, and MONARCH 3, 38% were 65 years of age or older and 10% were 75 years of age or older. The most common adverse reactions (≥5%) Grade 3 or 4 in patients ≥65 years of age across MONARCH 1, 2, and 3 were: neutropenia, diarrhea, fatigue, nausea, dehydration, leukopenia, anemia, infections, and ALT increased.
No overall differences in safety or effectiveness of VERZENIO were observed between these patients and younger patients.
Renal Impairment
No dosage adjustment is required for patients with mild or moderate renal impairment (CLcr ≥30-89 mL/min, estimated by Cockcroft-Gault [C-G]). The pharmacokinetics of abemaciclib in patients with severe renal impairment (CLcr <30 mL/min, C-G), end stage renal disease, or in patients on dialysis is unknown.
Hepatic Impairment
No dosage adjustments are necessary in patients with mild or moderate hepatic impairment (Child-Pugh A or B).
Reduce the dosing frequency when administering VERZENIO to patients with severe hepatic impairment (Child-Pugh C).
Additional information can be found at www.verzenio.com
Revista Puertorriqueña de Medicina y Salud Pública 23 VERZENIO® (abemaciclib) tablets, for oral use AL HCP BS_MonE 12OCT2021 VERZENIO® (abemaciclib) tablets, for oral use AL HCP BS_MonE 12OCT2021 Verzenio, AL HCP BS_MonE 12OCT2021 - 7 x 10 PRINTER VERSION 3 OF 3
AL HCP BS_MonE 12OCT2021
GRUPO DE APOYO

Puerto Rico Dermatitis Atópica
Vivir con Dermatitis Atópica
Vivir con Dermatitis Atópica











¡DESCUBRE TU MEJOR PIEL! FACIAL LÁSER CORPORAL INYECTABLES OTROS Compra Online en: www.novadermpr.com Para más información: Bayamón: 787.798.1993 | Carolina 787.752.3280 Edif. Galería Médica Suit 108 Calle Santa Cruz #64 Bayamón Avenida Comandante Esq. Campo Rico PQ #26 (altos Farmacia Medina) Carolina novadermpr Somos un grupo de especialistas en cabello que ofrecemos las tecnologias más avanzadas en todo tipo de tratamientos para la restauración del cabello del hombre y la mujer. Evaluación Libre de Costo Dirigidos y certificados por Dermatólogos. 787.900.8701 ndhipr | www.ndhipr.com Trasplante Capilar Manual (FUE) Trasplante Capila con tecnología robótica Plasma Rico en Plaquetas Láse Cap Productos
LA ALOPECIA: CAUSAS MÁS COMUNES Y SU TRATAMIENTO
Palabras Clave: Alopecia, cicatricial, no cicatricial, alopecia areata, alopecia androgénica, efluvio telógeno.

RESUMEN
La alopecia es la pérdida parcial o completa de pelo en el cuero cabelludo y/o el cuerpo. Entre las posibles causas de esta condición están los trastornos del ciclo del pelo, reacciones inflamatorias que dañan los folículos pilosos y anomalías hereditarias. La alopecia se puede asociar clínicamente con dolor, sensibilidad, picazón o sensación de ardor en el área afectada. Existen distintos tipos clasificados como cicatriciales y no cicatriciales, entre ellos alopecia areata, alopecia androgénica, efluvio telógeno y
alopecia por tracción. Basado en el historial y examen físico, se determina la causa y los posibles tratamientos. La mayoría de los casos de alopecia requieren asistencia de esteroides tópicos y/o inyectables, dependiendo de la severidad y considerando la edad del paciente. Nuevos estudios presentan recuperación del pelo al utilizar inhibidores de JAK. El tratamiento de la alopecia se debe complementar con consejería psicológica para prevenir y atender preocupaciones relacionadas a la disminución del pelo.

26 Revista Puertorriqueña de Medicina y Salud Pública
1Sección de Dermatología, Escuela de Medicina Ponce Health Sciences University, Ponce, PR

2Escuela de Medicina Ponce Health Sciences University, Ponce, PR
INTRODUCCIÓN
La alopecia es la ausencia o pérdida de pelo en el cuero cabelludo y/o el cuerpo. La misma puede presentarse de manera localizada o generalizada. Debido a las diversas presentaciones clínicas, encuentros patológicos y etiologías, la alopecia se considera como un grupo heterogéneo de condiciones. El impacto psicológico y social puede ser sustancial en los pacientes, propiciando desórdenes como depresión y ansiedad. Asimismo, se ha observado que la alopecia puede ser indicador de afecciones sistémicas que puede aportar significativamente en la identificación de estas.
ALOPECIA NO CICATRICIAL
Se refiere al tipo de alopecia en donde el crecimiento del pelo aún es posible, ya que se mantiene la unidad folicular intacta.
Los tipos más comunes son:
Alopecia androgénica: es provocada por un aumento en la enzima 5-alfa reductasa que se encarga de convertir la testosterona en su forma más potente, dihidrotestosterona (DHT). El aumento de DHT provoca que la fase de crecimiento conocida como la fase anágeno sea acortada, causando la miniaturización del folículo piloso. La presentación clínica de alopecia androgénica es distinta según el género del paciente. En hombres, la alopecia se encuentra presente en la parte frontal y parietal, con poca pérdida en la región occipital. En mujeres, la alopecia se presenta como pérdida de pelo desde la corona, la cual puede extenderse hasta la región frontal.
Alopecia areata: es una condición autoinmune en donde los anticuerpos atacan el folículo piloso provocando
PRESENTACIÓN Y CLASIFICACIÓN
La alopecia se presenta como la pérdida temporera o permanente del pelo. Esta pérdida de pelo afecta el cuero cabelludo u otras áreas del cuerpo de manera focal, en patrón o difusa. Dependiendo del tipo de alopecia, los síntomas pueden presentar con inicio reciente o progresivo y ocurrir una vez o frecuentemente. La alopecia presenta con mayor visibilidad del cuero cabelludo y densidad reducida del pelo. Las características de las áreas

afectadas como eritema, descamación, costras y pústulas y síntomas asociados como picor, dolor y ardor ayudan a distinguir entre los tipos de alopecia, aunque estos pueden no estar presentes. La alopecia se clasifica en dos categorías principales, no cicatricial y cicatricial, la primera de estas siendo la más común. Existen otros desórdenes estructurales del pelo heredados y adquiridos, pero su presentación es poco común.
pérdida de pelo. La alopecia areata puede estar asociada a otras comorbilidades como enfermedades de la tiroides, artritis reumatoide, lupus eritematoso, psoriasis, vitíligo y enfermedad inflamatoria intestinal.
Alopecia por tracción: es el resultado de tensión sostenida al folículo piloso que puede ser propiciada por prácticas de estilización de pelo como trenzas, estilos bollos, moños apretados y permanentes. Aunque la alopecia por tracción se considera como un tipo de alopecia no cicatricial, el trauma repetitivo pudiera causar una alopecia cicatricial.
Efluvio telógeno: se caracteriza por la pérdida de pelos de manera temporera que puede presentarse por acortamiento de la fase telógena o la transición prematura del folículo a la fase telógena debido a un estresor. De conformidad con varias investigaciones realizadas, se ha encontrado una correlación de los pacientes con efluvio telógeno que han sido previamente contagiados con SARS-CoV 2.
Revista Puertorriqueña de Medicina y Salud Pública 27
Reina M. González, MD1; Chavely Calderón, MS32; Myrta Rivera, MS12; María Limardo, MS12; Jashira Babilonia, MS12; Néstor Sánchez, MD FAAD1
ALOPECIA CICATRICIAL DIAGNÓSTICO

Se refiere al tipo de alopecia que resulta en pérdida permanente del pelo a consecuencia de la destrucción del folículo piloso, producido por inflamación, pérdida de glándulas sebáceas y formación de tejido fibroso. Los tipos más comunes son:
Liquen plano pilar (LPP): ocurre con mayor predisposición en mujeres adultas, y aunque se desconoce el mecanismo que lo ocasiona, se sugiere que la pérdida de pelo se debe al daño causado por la inflamación sostenida alrededor del folículo en regiones ricas de células madre pluripotentes. La presentación de pápulas foliculares queratósicas es característico en pacientes con LPP.
Alopecia fibrosa frontal: se considera una variante clínica de LPP que es más común en mujeres postmenopáusicas. La patofisiología de alopecia fibrosa frontal, aunque es similar a LPP, presenta con mayor frecuencia pérdida total de las cejas, ocurriendo esto en un 70% de los pacientes afectados.
Alopecia cicatricial centrífuga central: se pudiera considerar como una condición casi exclusiva para mujeres con descendencia africana, particularmente mujeres con condiciones como diabetes o leiomiomas uterinos, y su patofisiología es desconocida.
DIAGNÓSTICO
Las opciones de tratamiento varían dependiendo de la presentación clínica de la enfermedad. Previo a iniciar un tratamiento, es importante educar al paciente que al momento no existe una cura para la alopecia. Los tratamientos existentes tienen como objetivo suprimir la caída del pelo y promover el crecimiento.
Para las alopecias no cicatriciales se puede utilizar el plasma rico en plaquetas (PRP), la terapia con láser y el trasplante de pelo. Para la alopecia androgénica, el minoxidil tópico y la finasterida oral son los tratamientos de primera línea para los hombres, y el minoxidil tópico para las mujeres. En el caso de la alopecia areata, se recomiendan los corticosteroides tópicos como terapia de primera línea y las inyecciones de corticosteroides intralesionales, solas o combinadas con corticosteroides tópicos. Los corticosteroides orales son
La evaluación de pacientes con pérdida de pelo comienza con un historial médico completo y un examen físico del cuero cabelludo, el cabello y las otras áreas del cuerpo cubiertas de pelo. El médico debe inspeccionar la distribución y densidad del pelo, fragilidad y textura de los tallos de pelo y presencia o ausencia de orificios foliculares. Si es posible, la tricoscopia, la técnica sencilla y no invasiva de diagnóstico por imagen. brinda una examinación más detallada e información adicional sobre la presentación de la condición. El examen físico debe también incluir una prueba de extracción de pelo para determinar pérdida activa de pelo. En la mayoría de los casos, esto es suficiente para diagnosticar tipos de alopecia no cicatricial. En caso del diagnóstico ser incierto luego del examen físico, una biopsia del cuero cabelludo en el área afectada logra distinguir una alopecia cicatricial de una no cicatricial.
En pacientes con caída de pelo no cicatricial de inicio reciente, difuso y sin causa evidente, se deberían completar estudios de laboratorio, incluyendo hierro en suero, ferritina y hormona estimulante de la tiroides. Además, una prueba rápida de reagina plasmática para descartar la alopecia sifilítica es apropiada para pacientes con pérdida de pelo en parches sin inflamación o cicatrización visible.
DIFERENCIALES DIAGNÓSTICOS
Las causas de alopecia antes mencionadas pueden parecerse y confundirse entre sí por lo que se deben considerar en el diagnóstico diferencial de cada una. Otras condiciones médicas que pueden provocar pérdida del pelo incluyen la tiña de la cabeza, tricotilomanía y sífilis secundaria.
Tiña de la cabeza: se manifiesta como perdida de cabello en parchos escamosos con puntos negros visibles en el cuero cabelludo. La prueba de tricoscopia ayuda diagnosticar tiña de la cabeza, evitando un procedimiento largo e invasivo o la necesidad de realizar un examen de KOH. Es más común en niños y menos frecuente en adultos.
Tricotilomanía: presenta como una alopecia irregular, no cicatricial, con pelo corto y prueba de tracción negativa en pacientes, mayormente mujeres, que se halan pelos de la cabeza u otra parte del cuerpo recurrente e involuntariamente. Los pacientes comúnmente deniegan el hábito.
Sífilis secundaria: se presenta con pérdida de pelo irregular en el cuero cabelludo, lo cual ha sido descrito como alopecia apolillada.
más efectivos cuando la enfermedad es de severidad moderada a grave. Se recomiendan la sensibilización tópica con difenilciclopropenona (DPCP), el inhibidor de JAK-baricitinib o antralina y metotrexato, con o sin corticosteroides orales, para adultos con casos graves de alopecia. Los pacientes pediátricos con pocas lesiones pueden tratarse con evaluación continua ya que la mitad de los casos muestra un nuevo crecimiento espontáneo dentro de los seis a doce meses. En el caso de efluvio telógeno, la condición es reversible sin tratamiento, pero el nuevo crecimiento con resultados cosméticamente evidentes puede demorar hasta un año.
Para las alopecias cicatriciales se pueden considerar los corticosteroides tópicos de potencia moderada con o sin inyecciones de acetónido de triamcinolona intralesional. La salud psicológica del paciente debe ser
observada continuamente para prevenir y atender preocupaciones relacionadas a la disminución de pelo. Como medidas alternas se puede orientar a pacientes sobre camuflaje de pelo a través de pelucas y maquillaje, entre otras opciones, para disimular la pérdida de pelo.
CONCLUSIÓN
Es crucial distinguir entre alopecia cicatricial y no cicatricial para orientar a los pacientes sobre los manejos apropiados y resultados factibles. El manejo de la alopecia es principalmente sintomático, complementado por alternativas de camuflaje de alopecia y consejerías de salud mental.
28 Revista Puertorriqueña de Medicina y Salud Pública
REFERENCIAS
1. Phillips TG, Slomiany WP, Allison R. Hair Loss: Common Causes and Treatment. Am Fam Physician. 2017;96(6):371-378.
2. McDonald KA, Shelley AJ, Colantonio S, Beecker J. Hair pull test: Evidence-based update and revision of guidelines. J Am Acad Dermatol. 2017;76(3):472-477. doi:10.1016/j.jaad.2016.10.002
3. Wolff H, Fischer TW, Blume-Peytavi U. The Diagnosis and Treatment of Hair and Scalp Diseases. Dtsch Arztebl Int. 2016;113(21):377-386. doi:10.3238/ arztebl.2016.0377
4. Jackson AJ, Price VH. How to diagnose hair loss. Dermatol Clin. 2013;31(1):2128. doi:10.1016/j.det.2012.08.007

5. Olsen EA, Messenger AG, Shapiro J, et al. Evaluation and treatment of male and female pattern hair loss. J Am Acad Dermatol. 2005;52(2):301-311. doi:10.1016/j.jaad.2004.04.008
6. Blume-Peytavi U, Blumeyer A, Tosti A, et al. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol. 2011;164(1):5-15. doi:10.1111/j.13652133.2010.10011.x
7. Meah N, Wall D, York K, et al. The Alopecia Areata Consensus of Experts (ACE) study part II: Results of an international expert opinion on diagnosis and laboratory evaluation for alopecia areata. J Am Acad Dermatol. 2021;84(6):1594-1601. doi:10.1016/j.jaad.2020.09.028
8. Moghadam-Kia S, Franks AG Jr. Autoimmune disease and hair loss. Dermatol Clin. 2013;31(1):75-91. doi:10.1016/j.det.2012.08.008
9. Aguh C, Jamerson TA. An approach to patients with alopecia. Med Clin N Am. 2021;105:599-610. https://doi. org/10.1016/j.mcna.2021.04.002
10. Asghar F, Shamim N, Farooque U, Sheikh H, Aqeel R. Telogen Effluvium: A Review of the Literature. Cureus. 2020;12(5):e8320. Published 2020 May 27. doi:10.7759/ cureus.8320
11. Melo DF, Lima CDS, Piraccini BM, Tosti A. Trichotillomania: What Do We Know So Far?. Skin Appendage Disord. 2022;8(1):17. doi:10.1159/000518191
12. Dhurat R, Shukla D, Agrawal S, Chitalia J, Ghate S, Jage M. Tinea Capitis Presenting as Diffuse Hair Loss and Significance of Trichoscopy: Four Case Reports. Skin Appendage Disord. 2021;7(4):286-291. doi:10.1159/000513315
13. Chang KM, Nadi L, Wallach F. Secondary syphilis with alopecia and ocular manifestation. J Microbiol Immunol Infect. 2021;54(4):758-759. doi:10.1016/j. jmii.2020.12.015
14. Gjestland T. The Oslo study of untreated syphilis; an epidemiologic investigation of the natural course of the syphilitic infection based upon a re-study of the BoeckBruusgaard material. Acta Derm Venereol Suppl (Stockh). 1955;35(Suppl 34):3-LVI. doi:10.2340/00015555343368
15. Wang EHC, Sallee BN, Tejeda CI, Christiano AM. JAK Inhibitors for Treatment of Alopecia Areata. J Invest Dermatol. 2018;138(9):1911-1916. doi:10.1016/j.jid.2018.05.027
16. Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48(1):103-110. doi:10.1067/ mjd.2003.68
17. Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias [published correction appears in J Am Acad Dermatol. 2005 Sep;53(3):496]. J Am Acad Dermatol. 2005;53(1):1-40. doi:10.1016/j.jaad.2004.06.015
18. Kang H, Alzolibani AA, Otberg N, Shapiro J. Lichen planopilaris. Dermatol Ther. 2008;21(4):249-256. doi:10.1111/ j.1529-8019.2008.00206.x
19. Moghadam-Kia S, Franks AG Jr. Autoimmune disease and hair loss. Dermatol Clin. 2013;31(1):75-91. doi:10.1016/j.det.2012.08.008
20. Tamashunas NL, Bergfeld WF. Male and female pattern hair loss: Treatable and worth treating. Cleve Clin J Med. 2021;88(3):173-182. Published 2021 Mar 1. doi:10.3949/ccjm.88a.20014
21. Wolff H, Fischer TW, Blume-Peytavi U. The Diagnosis and Treatment of Hair and Scalp Diseases. Dtsch Arztebl Int. 2016;113(21):377-386. doi:10.3238/ arztebl.2016.0377
22. Xu L, Liu KX, Senna MM. A Practical Approach to the Diagnosis and Management of Hair Loss in Children and Adolescents. Front Med (Lausanne). 2017;4:112. Published 2017 Jul 24. doi:10.3389/fmed.2017.00112
23. Lintzeri DA, Constantinou A, Hillmann K, Ghoreschi K, Vogt A, Blume-Peytavi U. Alopecia areata - Current understanding
and management. J Dtsch Dermatol Ges. 2022;20(1):59-90. doi:10.1111/ ddg.14689
24. Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97(3):348-357. doi:10.1016/j.abd.2021.08.008
25. Meah N, Wall D, York K, et al. The Alopecia Areata Consensus of Experts (ACE) study: Results of an international expert opinion on treatments for alopecia areata. J Am Acad Dermatol. 2020;83(1):123-130. doi:10.1016/j.jaad.2020.03.004
26. Kanti V, Röwert-Huber J, Vogt A, BlumePeytavi U. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16(4):435-461. doi:10.1111/ddg.13498
27. Cardoso CO, Tolentino S, Gratieri T, Cunha-Filho M, Lopez RFV, Gelfuso GM. Topical Treatment for Scarring and NonScarring Alopecia: An Overview of the Current Evidence. Clin Cosmet Investig Dermatol. 2021;14:485-499. Published 2021 May 12. doi:10.2147/CCID. S284435
Revista Puertorriqueña de Medicina y Salud Pública 29
(ritlecitinib) capsules, for oral use
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
• Increased risk of serious bacterial, fungal, viral, and opportunistic infections leading to hospitalization or death, including tuberculosis (TB). Interrupt treatment if serious infection occurs until the infection is controlled. LITFULO should not be given to patients with active TB. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test
• Higher rate of all-cause mortality, including sudden cardiovascular death with another Janus kinase (JAK) inhibitor vs TNF blockers in rheumatoid arthritis (RA) patients. LITFULO is not approved for use in RA patients
• Malignancies have occurred in patients treated with LITFULO. Higher rate of lymphomas and lung cancers with another JAK inhibitor vs TNF blockers in RA patients
• Higher rate of MACE (defined as cardiovascular death, myocardial infarction, and stroke) with another JAK inhibitor vs TNF blockers in RA patients
• Thrombosis has occurred in patients treated with LITFULO. Increased incidence of pulmonary embolism, venous and arterial thrombosis with another JAK inhibitor vs TNF blockers
INDICATIONS AND USAGE
LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Evaluations and Immunizations Prior to Treatment With LITFULO
• TB infection evaluation: LITFULO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of LITFULO
• Viral hepatitis screening in accordance with clinical guidelines: LITFULO initiation is not recommended in patients with hepatitis B or hepatitis C
• Treatment with LITFULO should not be initiated in patients with absolute lymphocyte count (ALC) <500/mm3 or a platelet count <100,000/mm3
• Update immunizations according to current immunization guidelines
Recommended Dosage
The recommended dosage of LITFULO is 50 mg orally once daily with or without food.
LITFULO capsules should be swallowed whole; not crushed, split, or chewed.
If a dose is missed, the dose should be taken as soon as possible unless it is less than 8 hours before the next dose; in which case, skip the missed dose and resume dosing at the regular scheduled time.
Patients With Severe Hepatic Impairment
LITFULO is not recommended in patients with severe (Child Pugh C) hepatic impairment.
Treatment Interruption or Discontinuation
If treatment interruption is indicated, a temporary treatment interruption for less than 6 weeks is not expected to result in significant loss of regrown scalp hair.
Hematologic Abnormalities
• Treatment with LITFULO should be discontinued if platelet count is <50,000/mm3
• Treatment with LITFULO should be interrupted if ALC is <500/mm3 and may be restarted once ALC returns above this value
ALC and platelet counts are recommended before treatment initiation and at 4 weeks after treatment initiation, and thereafter according to routine patient management.
DOSAGE FORMS AND STRENGTHS
Capsules: 50 mg of ritlecitinib, size 3, opaque capsules with yellow body and blue cap. The body is printed with “RCB 50” and the cap is printed with “Pfizer” in black.
CONTRAINDICATIONS
LITFULO is contraindicated in patients with known hypersensitivity to ritlecitinib or any of its excipients.

WARNINGS AND PRECAUTIONS
Serious infections have been reported in patients receiving LITFULO. The most frequent serious infections have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO.
Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:
• with chronic or recurrent infection
• who have been exposed to TB
• with a history of serious infection or an opportunistic infection
• who have resided or traveled in areas of endemic TB or mycoses, or
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt LITFULO if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Tuberculosis
Screen patients for TB before starting therapy. LITFULO should not be given to patients with active TB. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB. In patients with a negative latent TB test, consider anti-TB therapy before initiating treatment with LITFULO in those at high risk and consider screening patients at high risk for TB during treatment with LITFULO.
Viral Reactivation
Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), was reported in clinical trials. If a patient develops herpes zoster, consider interrupting treatment until the episode resolves.
Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy with LITFULO. Patients with evidence of HIV infection or hepatitis B or C infection were excluded from clinical trials.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO.
Malignancy and lymphoproliferative disorders, including nonmelanoma skin cancer (NMSC), were observed in clinical trials of LITFULO.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding NMSC) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
The risks and benefits of LITFULO treatment should be considered prior to initiating or continuing therapy in patients with a known malignancy other than a successfully treated NMSC or cervical cancer.
Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Major Adverse Cardiovascular Events
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of MACE defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue LITFULO in patients that have experienced an MI or stroke.
Thromboembolic Events
An event of pulmonary embolism (PE) was reported in a patient receiving LITFULO. In a ritlecitinib higher dosing group, 1 patient reported an event of retinal artery occlusion. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, deep vein thrombosis, and PE were observed compared to those treated with TNF blockers. Avoid LITFULO in patients who may be at increased risk of thrombosis. If symptoms of thrombosis or embolism occur, patients should interrupt LITFULO and be evaluated promptly and treated appropriately.
Hypersensitivity
Serious reactions including anaphylactic reactions, urticaria, and rash have been observed in patients receiving LITFULO in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue LITFULO and institute appropriate therapy.
Laboratory Abnormalities
Treatment with LITFULO was associated with decreases in lymphocytes and platelets.
Prior to LITFULO initiation, perform ALC and platelet counts. After initiating treatment with LITFULO, treatment interruption or discontinuation are recommended based on ALC and platelet count abnormalities.
Liver Enzyme Elevations: treatment with LITFULO was associated with increased incidence of liver enzyme elevation compared to placebo. Increases of ALT ≥5 times the upper limit of normal (ULN) and increases of AST ≥5 times the ULN were observed in patients in LITFULO clinical trials. Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in ALT or AST are observed and drug-induced liver injury is suspected, interrupt LITFULO until this diagnosis is excluded. Creatine Phosphokinase (CPK) Elevations: treatment with LITFULO was associated with increased incidence of CPK elevation compared to placebo.
Vaccinations
No data are available on the response to vaccination in patients receiving LITFULO. Use of live attenuated vaccines should be avoided during or shortly prior to initiating treatment. Prior to initiating LITFULO, it is recommended that patients be brought up to date with all immunizations, including prophylactic herpes zoster vaccinations, in agreement with current immunization guidelines.
ADVERSE REACTIONS
Clinical Trials Experience
The safety of LITFULO was evaluated in three randomized, placebo-controlled clinical trials and one long-term trial in patients with alopecia areata, including alopecia totalis and alopecia universalis, who were 12 years of age and older. A total of 1628 patients were treated with LITFULO representing 2085 patient-years of exposure. There were 1011 patients with at least 1 year of exposure to LITFULO. In the placebo-controlled period of clinical trials in alopecia areata, a total of 668 patients were exposed to LITFULO with 130 receiving 50 mg once daily for up to 24 weeks. The median age of patients was 33 years, 105 (11.9%) patients were 12 to <18 years old and 22 (2.5%) patients were 65 years of age or older. The majority of patients were White (70.7%) and female (63.6%).
Adverse reactions occurring at ≥1% in the treated groups and at a higher rate than placebo are presented in the following table. A total of 2 (1.5%) patients treated with LITFULO 50 mg were discontinued from the trials due to adverse reactions.
32 Revista Puertorriqueña de Medicina y Salud Pública
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information
LITFULO™
Specific Adverse Reactions
Exposure adjusted incidence rates were adjusted by clinical trial size for all adverse reactions reported in this section. Overall Infections
In the placebo-controlled trials, for up to 24 weeks, overall infections were reported in 66 patients (80.35 per 100 patient-years) treated with placebo and 43 patients (74.53 per 100 patient-years) treated with LITFULO 50 mg. Across clinical trials, including the long-term trial, overall infections were reported in 645 patients (50.71 per 100 patient-years) treated with LITFULO 50 mg or higher.
Serious Infections
In the placebo-controlled trials, for up to 24 weeks, 3 patients reported serious infections across all ritlecitinib doses studied. Across clinical trials, including the long-term trial, serious infections were reported in 12 patients (0.66 per 100 patient-years) treated with LITFULO 50 mg or higher. The most common serious infections were related to appendicitis, COVID-19 infection (including pneumonia), and sepsis.
Herpes Zoster
In the placebo-controlled trials, for up to 24 weeks, herpes zoster was reported in 4 patients across all ritlecitinib doses studied and 0 patients treated with placebo. Across clinical trials, including the long-term trial, herpes zoster was reported in 21 patients (1.17 per 100 patient-years) treated with LITFULO 50 mg or higher. Opportunistic infections of multi-dermatomal herpes zoster were reported in 1 patient (0.50 per 100 patient-years) treated with the ritlecitinib higher dose in the placebo-controlled trials and 2 patients (0.1 per 100 patient-years) treated with LITFULO 50 mg or higher in all clinical trials.
Malignancy
In the placebo-controlled trials, for up to 24 weeks, 1 malignancy (breast cancer) was reported in 1 patient (1.33 per 100 patient-years) treated with ritlecitinib higher dose and no malignancy was reported in patients treated with placebo. Across clinical trials, including the long-term trial, malignancies excluding NMSC were reported in 7 patients (0.37 per 100 patient-years) treated with LITFULO 50 mg or higher.
Thromboembolic Events
Across clinical trials, including the long-term trial, PE was reported in 1 patient (0.06 per 100 patient-years) treated with LITFULO. There was 1 report of retinal artery occlusion and 1 report of acute MI.
Urticaria
In the placebo-controlled trials, for up to 24 weeks, urticaria was reported in 28 patients treated in all ritlecitinib doses studied and 3 patients treated with placebo. The rate of urticaria was 8.23 per 100 patient-years in patients treated with LITFULO 50 mg and 4.03 per 100 patient-years in patients treated with placebo. Across clinical trials, including the long-term trial, urticaria was reported in 76 patients treated with LITFULO 50 mg or higher. Among all patients treated with LITFULO 50 mg or higher in the integrated safety analysis, the rate of urticaria was 4.10 per 100 patient-years. The median time to onset of an initial event was 8 weeks; median duration of urticaria was 7 days. Most of the cases were mild to moderate in severity.
Decreased Lymphocyte Counts
Across clinical trials, including the long-term trial, confirmed ALC <500/mm3 occurred in 1 patient (<0.1%) treated with LITFULO 50 mg. Age appeared to be a risk factor for lower ALC in patients ≥65 years of age.
Decreased Platelet Count
In the placebo-controlled trials, for up to 24 weeks, treatment with LITFULO was associated with a decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which platelet count remained stable at a lower level with continued therapy. Across clinical trials, including the long-term trial, 1 patient (<0.1%) had a confirmed platelet count <100,000/mm3. No patient had a confirmed platelet count <75,000/mm3
CPK Elevations
In the placebo-controlled trials, for up to 24 weeks, events of blood CPK increased were reported in 2 (1.5%) patients treated with LITFULO 50 mg and 0 patients treated with placebo.
Liver Enzyme Elevations
In the placebo-controlled trials, for up to 24 weeks, events of increases in liver enzymes ≥3 times the ULN were observed in patients treated with LITFULO.
DRUG INTERACTIONS
Effects of LITFULO on Other Drugs
CYP3A Substrates
Ritlecitinib is a CYP3A inhibitor. Concomitant use of ritlecitinib increases area under the curve (AUC) and Cmax of CYP3A substrates, which may increase the risk of adverse reactions of these substrates.
Consider additional monitoring and dosage adjustment in accordance with approved product labeling of CYP3A substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
CYP1A2 Substrates
Ritlecitinib is a CYP1A2 inhibitor. Concomitant use of ritlecitinib increases AUC and Cmax of CYP1A2 substrates, which may increase the risk of adverse reactions of these substrates. Consider additional monitoring and dosage adjustment in accordance with the approved product labeling of CYP1A2 substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
Effects of Other Drugs on LITFULO
CYP3A Inducers
Concomitant use of strong CYP3A inducer (e.g., rifampin) may decrease AUC and Cmax of ritlecitinib, which may result in loss of or reduced clinical response. Coadministration with strong inducers of CYP3A is not recommended.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry
If a patient becomes pregnant while receiving LITFULO, healthcare providers should report LITFULO exposure by calling 1-877-390-2940.
Risk Summary
Available data from clinical trials with LITFULO use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ritlecitinib to pregnant rats and rabbits during organogenesis caused fetotoxicity and fetal malformations at exposures equal to 49 and 55 times the maximum recommended human dose (MRHD) based on an AUC comparison, respectively.
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The estimated background risks in the U.S. general population of major birth defects and miscarriages are 2-4% and 15-20% of clinically recognized pregnancies, respectively.
Data
Animal Data
In an embryo-fetal development study in pregnant rats, oral administration of ritlecitinib from gestation days 6 to 17 decreased fetal body weights and caused fetal skeletal malformations (malformed vertebrae and ribs) and variations (delayed ossification) at doses ≥175 mg/kg/day (49 times the MRHD based on AUC comparison). Maternal toxicity (lower body weights) was noted at 325 mg/kg/day (102 times the MRHD based on AUC comparison). There was no developmental toxicity at 75 mg/kg/day (16 times the MRHD based on AUC comparison).
In an embryo-fetal development study in pregnant rabbits, oral administration of ritlecitinib from gestation days 7 to 19 decreased mean fetal body weights and increased visceral malformations (malpositioned kidneys), skeletal malformations (supernumerary sternebrae,

arch, and/or fused thoracic centra), and skeletal variations (delayed ossification) at 75 mg/kg/day (55 times the MRHD based on AUC comparison). There was no developmental toxicity at doses up to 25 mg/kg/day (12 times the MRHD based on AUC comparison).
In a pre- and postnatal development study in rats, oral administration of ritlecitinib from gestation day 6 through lactation day 20 had no effects on pre- and postnatal development at doses up to 75 mg/kg/day (14 times the MRHD based on AUC comparison). At 175 mg/kg/day (41 times the MRHD based on AUC comparison), ritlecitinib caused adverse lower postnatal survival and lower offspring body weights, which correlated with delayed sexual maturation in both sexes. Bred females in the F1 generation also exhibited lower mean numbers of corpora lutea at 175 mg/kg/day.
Lactation
Risk Summary
There are no data on the presence of ritlecitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Ritlecitinib is present in the milk of lactating rats. When a drug is present in animal milk, it is likely that it will be present in human milk. Because of the serious adverse effects in adults, including risks of serious infection and malignancy, advise women not to breastfeed during treatment with LITFULO and for approximately 14 hours after the last dose (approximately 6 elimination half-lives).
Data
After a single oral 30 mg/kg dose of ritlecitinib to lactating rats, ritlecitinib concentrations in milk over time were higher than those in plasma. The mean milk to plasma AUC ratio was determined to be 2.2.
Pediatric Use
The safety and effectiveness of LITFULO for alopecia areata have been established in pediatric patients ages 12 years and older. A total of 181 pediatric patients ages 12 to <18 years were enrolled in alopecia areata clinical trials, with 105 pediatric patients ages 12 to <18 years with alopecia areata randomized in a pivotal, double-blind, placebo-controlled trial (Trial AA-I). Efficacy was consistent between the pediatric patients and adults. The adverse reaction profile in the pediatric patients was similar to adults.
The safety and efficacy of LITFULO have not been established in pediatric patients under 12 years of age.
Geriatric Use
No dose adjustment is required for patients ≥65 years of age.
A total of 28 patients enrolled in alopecia areata trials were 65 years of age and older, and none were 75 years of age and older. Clinical trials of LITFULO did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients. As there is a higher incidence of infections in the elderly population in general, caution should be used when treating the elderly.
Hepatic Impairment
No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. LITFULO is not recommended in patients with severe (Child Pugh C) hepatic impairment.
OVERDOSAGE
LITFULO was administered in clinical trials up to a single oral dose of 800 mg. Adverse reactions were comparable to those seen at lower doses and no specific toxicities were identified. Pharmacokinetics data up to and including a single oral dose of 800 mg in healthy adult volunteers indicate that more than 90% of the administered dose is expected to be eliminated within 48 hours.
There is no specific antidote for overdose with LITFULO. Treatment should be symptomatic and supportive, and monitor patients for signs and symptoms of adverse reactions.
In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
This brief summary is based on LITFULOTM (ritlecitinib) Prescribing Information LAB-1469-0.5.
Issued: June 2023.
The product’s label may have been updated. For full Prescribing Information, visit LITFULOHCP.com.
Revista Puertorriqueña de Medicina y Salud Pública 33
absent thoracic
Trials of LITFULO for the Treatment of Alopecia Areata LITFULO 50 mg N=130 n (%) Placebo N=213 n (%) Headache 14 (10.8) 18 (8.5) Diarrhea 13 (10.0) 8 (3.8) Acne 8 (6.2) 10 (4.7) Rash 7 (5.4) 2 (0.9) Urticaria 6 (4.6) 3 (1.4) Folliculitis 4 (3.1) 4 (1.9) Pyrexia 4 (3.1) 0 Dermatitis atopic 3 (2.3) 1 (0.5) Dizziness 3 (2.3) 3 (1.4) Blood CPK increased 2 (1.5) 0 Herpes zoster 2 (1.5) 0 Red blood cell count decreased 2 (1.5) 0 Stomatitis 2 (1.5) 0
Adverse Reactions in Clinical
June 2023 LITFULO™
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information
© 2023 Pfizer Inc. All rights reserved. PP-RIL-USA-0653
(ritlecitinib) capsules, for oral use








RETO DIAGNÓSTICO ENTRE CROHN Y COLITIS SISTÉMICA
Por: Dr. Ahmed Morales MD FACP, FACG, FASGE, AGAF Gastroenterólogo
Miembro de la Junta de Editores de la Revista de Medicina y Salud Pública
Palabras clave Crohn, colitis sistémica, evacuación, sangre
Keywords
Crohn’s, systemic colitis, evacuation, blood

Revista Puertorriqueña de Medicina y Salud Pública 35 Revista Puertorriqueña de Medicina y Salud Pública 27
CASO CLÍNICO
RESUMEN
La colitis isquémica ocurre cuando se reduce temporalmente el flujo sanguíneo que va a una parte del intestino grueso (colon) y por lo general, debido a la constricción de los vasos sanguíneos que irrigan el colon o a la reducción del flujo de sangre a través de los vasos debido a presiones bajas.
La colitis isquémica se puede diagnosticar de forma errónea porque se puede confundir fácilmente con otros problemas digestivos.
ABSTRACT
Ischemic colitis occurs when blood flow to part of the large intestine (colon) is temporarily reduced, usually due to constriction of the blood vessels supplying the colon or reduced blood flow through the intestines. vessels due to low pressures.
Ischemic colitis can be misdiagnosed because it can easily be confused with other digestive problems.
betes e hipertensión, que se presentó a las salas de emergencias reportando dolor en el lado izquierdo abdominal dos días previos a su llegada.
Igualmente reportó episodios de diarreas con sangre. Al paciente se le realizaron laboratorios rutinarios y un estudio CT abdominal, que reflejó engrosamiento del lado izquierdo del colon.
Es posible que se necesiten medicamentos para tratar la colitis isquémica o prevenir una infección. Igualmente se pudiera requerir una cirugía si el colon se ha dañado. Sin embargo, la mayoría de las veces la colitis isquémica se cura por sí sola.
Entre sus principales síntomas destacan el dolor abdominal, diarreas, fiebre, pérdida de peso, hemorragia rectal, entre otros síntomas.
Mientras, la enfermedad de Crohn es una afectación inflamatoria de tipo crónico y autoinmune del tubo digestivo que evoluciona de modo recurrente con brotes. Puede afectar desde la boca hasta el ano. La localización más frecuente es el íleon terminal (porción más distal del intestino delgado).
La colitis ulcerativa provoca inflamación y úlceras en el tracto digestivo, que afecta el revestimiento más profundo del intestino grueso (colon) y el recto. Por lo general, los síntomas aparecen con el paso del tiempo. Ambas condiciones guardan similitudes en su presentación, por lo que el cernimiento correcto en estos pacientes determinará el curso clínico idóneo para el paciente. Sin embargo, el no tener ambas enfermedades dentro de los diagnósticos diferenciales puede ser adverso para su salud.
EMedicines may be needed to treat ischemic colitis or prevent infection. Surgery may also be required if the colon has been damaged. However, most of the time ischemic colitis heals on its own. Its main symptoms include abdominal pain, diarrhea, fever, weight loss, rectal bleeding, among other symptoms. Meanwhile, Crohn’s disease is a chronic and autoimmune inflammatory affectation of the digestive tract that evolves recurrently with outbreaks. It can affect from the mouth to the anus. The most common location is the terminal ileum (most distal portion of the small intestine).
Ulcerative colitis causes inflammation and ulcers in the digestive tract, affecting the deeper lining of the large intestine (colon) and the rectum. Symptoms usually appear over time.
Both conditions have similarities in their presentation, so the correct screening in these patients will determine the ideal clinical course for the patient. However, not having both diseases within the differential diagnoses can be detrimental to your health.
RESUMEN
Paciente de 53 años con dolor abdominal y evacuaciones con sangre. Un CT abdominal arrojó engrosamiento del lado izquierdo del colon. Se le hace colonoscopia con biopsias con evidencia de colitis isquémica. Este caso es uno relevante pues el cuadro clínico del paciente puede ser confundido con la enfermedad de Crohn y colitis ulcerativa y recibir un tratamiento incorrecto y adverso a su diagnóstico real.
CASO
Paciente de 53 años con historial clínico de enfermedad coronariana, dia-
Los médicos tomaron la decisión de realizar una colonoscopia que también reflejó la inflamación en el lado izquierdo del colon. No obstante, en las biopsias realizadas al tejido evaluado mediante colonoscopia, arrojó el diagnóstico de colitis isquémica, despejando así la posibilidad de una presentación de la enfermedad de Crohn. Entre los tratamientos a estos pacientes se encuentran la administración de antibióticos e hidratación.
CONCLUSIÓN
El diagnóstico de colitis sistémica provoca un escenario de inflamación en el colon que pudiera ser similar a aquella provocada por la enfermedad de Crohn y/o colitis ulcerativa. Es importante que los médicos estén conscientes de las presentaciones de ambas condiciones para poder administrar el tratamiento correcto, sobre todo en los hallazgos identificados en las colonoscopias.
REFERENCIAS:
Colitis isquémica - Síntomas y causas - Mayo Clinic. (2021, July 29). Mayo Clinic. https://www.mayoclinic.org/ es-es/diseases-conditions/ischemic-colitis/symptoms-causes/syc-20374001 Clínica Universidad de Navarra. (2022). Enfermedad de Crohn: ¿Qué es? Síntomas y tratamiento.
https://www.cun.es/enfermedades-tratamientos/enfermedades/enfermedad-crohn Colitis ulcerosa - Síntomas y causas - Mayo Clinic. (2021, February 23). Mayo Clinic.
https://www.mayoclinic.org/es-es/ diseases-conditions/ulcerative-colitis/ symptoms-causes/syc-20353326#:%7E:text=La%20colitis%20ulcerosa%20 es%20una,tiempo%2C%20no%20se%20 presentan%20s%C3%BAbitamente.

36 Revista Puertorriqueña de Medicina y Salud Pública Revista Puertorriqueña de Medicina y Salud Pública 28
ARRIBA
El Dr. Ahmed Morales verifica sus equipos antes de iniciar la endoscopia.


ABAJO

Plano detalle del equipo para realizar la endoscopia.
Este caso de un paciente de 53 años con dolor abdominal es uno relevante pues el cuadro clínico del paciente puede ser confundido y recibir un tratamiento incorrecto y adverso a su diagnóstico real.
Revista Puertorriqueña de Medicina y Salud Pública 37 Revista Puertorriqueña de Medicina y Salud Pública 29
CREON® (kr
INDICATIONS AND USAGE
CREON®
conditions.
ē ´ŏ
n) (pancrelipase) Delayed-Release Capsules, for oral use
WARNINGS AND PRECAUTIONS
Fibrosing Colonopathy
PROFESSIONAL BRIEF SUMMARY CONSULT PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
DOSAGE AND ADMINISTRATION
CREON is not interchangeable with other pancrelipase products.
CREON is orally administered. Therapy should be initiated at the lowest recommended dose and gradually increased. The dosage of CREON should be individualized based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet as described in the Limitations on Dosing below [see Dosage and Administration and Warnings and Precautions]
Administration
Infants (up to 12 months)
CREON should be administered to infants immediately prior to each feeding, using a dosage of 3,000 lipase units per 120 mL of formula or prior to breastfeeding. Contents of the capsule may be administered directly to the mouth or with a small amount of applesauce. Administration should be followed by breast milk or formula. Contents of the capsule should not be should be taken to ensure that CREON is not crushed or chewed or retained in the mouth, to avoid irritation of the oral mucosa.
Children and Adults
CREON
capsules and capsule contents should not be crushed or chewed. Capsules should be swallowed whole.
For patients who are unable to swallow intact capsules, the capsules may be carefully opened and the contents added to a small amount of acidic soft food with a pH of 4.5 or less, such as applesauce, at room temperature. The CREON-soft food mixture should be swallowed immediately without crushing or chewing, and followed with water or juice to ensure complete ingestion. Care should be taken to ensure that no drug is retained in the mouth.
Dosage
Dosage recommendations for pancreatic enzyme replacement therapy were published following the Cystic Fibrosis Foundation Consensus Conferences. CREON should be administered in a manner consistent with the recommendations of the Cystic Fibrosis Foundation Consensus Conferences (also known as Conferences) provided in the following paragraphs, except for infants. Although the Conferences recommend doses of 2,000 to 4,000 lipase units in infants up to 12 months, CREON is available in a 3,000 lipase unit capsule. Therefore, the recommended dose of CREON in infants up to 12 months is 3,000 lipase units per 120 mL of formula or per breastfeeding. Patients may be dosed on a fat ingestion-based or actual body weight-based dosing scheme.
Additional recommendations for pancreatic enzyme therapy in patients pancreatectomy are based on a clinical trial conducted in these populations.
Infants (up to 12 months)
CREON is available in the strength of 3,000 USP units of lipase thus infants may be given 3,000 lipase units (one capsule) per 120 mL of formula or per breastfeeding. Do not mix CREON capsule contents directly into formula or breast milk prior to administration [see Administration]
Children Older than 12 Months and Younger than 4 Years
Enzyme dosing should begin with 1,000 lipase units/kg of body weight units/kg of body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day.
Children 4 Years and Older and Adults
Enzyme dosing should begin with 500 lipase units/kg of body weight per meal body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day. Usually, half of the prescribed CREON dose for an individualized full meal approximately three meals plus two or three snacks per day. Enzyme doses expressed as lipase units/kg of body weight per meal should be decreased in older patients because they weigh more but tend to ingest less fat per kilogram of body weight.
Pancreatectomy
The initial starting dose and increases in the dose per meal should be individualized based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet.
In one clinical trial, patients received CREON at a dose of 72,000 lipase units per meal while consuming at least 100 g of fat per day]. Lower starting doses recommended in the literature are consistent with the 500 lipase units/kg of body weight per meal lowest starting dose recommended for adults in the Cystic Fibrosis Foundation Consensus Conferences Guidelines. Usually, half of the prescribed CREON dose for an individualized full meal should be given with each snack.
Limitations on Dosing
Dosing should not exceed the recommended maximum dosage set forth by the Cystic Fibrosis Foundation Consensus Conferences Guidelines. If symptoms and signs of steatorrhea persist, the dosage may be increased by the healthcare professional. Patients should be instructed not to increase the dosage on their own. There is great inter-individual variation in response to enzymes; thus, a range of doses is recommended. Changes in dosage may require an adjustment period of several days. If doses are to exceed 2,500 lipase units/kg of body weight per meal, further investigation is warranted. Doses greater than 2,500 lipase units/kg of body weight per meal (or greater than 10,000 lipase units/kg of body weight per day) should be used with caution and only if they are documented to be effective by of fat absorption. Doses greater than 6,000 lipase units/kg of body weight colonopathy, in children less than 12 years of age [see Warnings and Precautions]. Patients currently receiving higher doses than 6,000 lipase units/kg of body weight per meal should be examined and the dosage either immediately decreased or titrated downward to a lower range.
CONTRAINDICATIONS
None.
Fibrosing colonopathy has been reported following treatment with different pancreatic enzyme products. Fibrosing colonopathy is a rare, serious adverse reaction initially described in association with high-dose pancreatic enzyme use, usually over a prolonged period of time and most commonly products exceeding 6,000 lipase units/kg of body weight per meal have been associated with colonic stricture in children less than 12 years of age. some patients may be at risk of progressing to stricture formation. It is recommended, unless clinically indicated, that enzyme doses should be less than 2,500 lipase units/kg of body weight per meal (or less than 10,000 lipase units/kg of body weight per day) or less than 4,000 lipase units/g fat ingested per day [see Dosage and Administration].
Doses greater than 2,500 lipase units/kg of body weight per meal (or greater than 10,000 lipase units/kg of body weight per day) should be used with caution and only if they are documented to be effective by 3-day fecal fat
Patients receiving higher doses than 6,000 lipase units/kg of body weight per meal should be examined and the dosage either immediately decreased or titrated downward to a lower range.
Potential for Irritation to Oral Mucosa
Care should be taken to ensure that no drug is retained in the mouth. CREON should not be crushed or chewed or mixed in foods having a pH greater than 4.5. These actions can disrupt the protective enteric coating resulting in early release of enzymes, irritation of oral mucosa, and/or loss of enzyme activity [see Dosage and Administration and Patient Counseling Information] For patients who are unable to swallow intact capsules, the capsules may be carefully opened and the contents added to a small amount of acidic soft food with a pH of 4.5 or less, such as applesauce, at room temperature. The CREON-soft food mixture should be swallowed immediately and followed with water or juice to ensure complete ingestion.
Potential for Risk of Hyperuricemia
Caution should be exercised when prescribing CREON to patients with gout, renal impairment, or hyperuricemia. Porcine-derived pancreatic enzyme products contain purines that may increase blood uric acid levels.
Potential Viral Exposure from the Product Source CREON is sourced from pancreatic tissue from swine used for food consumption. Although the risk that CREON will transmit an infectious agent to humans has been reduced by testing for certain viruses during manufacturing and by inactivating certain viruses during manufacturing, there is a theoretical risk for transmission of viral disease, including diseases
of transmission of an infectious illness associated with the use of porcine pancreatic extracts have been reported.
Allergic Reactions
Caution should be exercised when administering pancrelipase to a patient with a known allergy to proteins of porcine origin. Rarely, severe allergic reactions including anaphylaxis, asthma, hives, and pruritus, have been reported with other pancreatic enzyme products with different formulations of CREON treatment in patients with severe allergy should be taken into consideration with the overall clinical needs of the patient.
ADVERSE REACTIONS
The most serious adverse reactions reported with different pancreatic enzyme products of the same active ingredient (pancrelipase) that are described allergic reactions [see Warnings and Precautions]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly the rates observed in practice.
The short-term safety of CREON was assessed in clinical trials conducted in or pancreatectomy were treated with CREON.
Cystic Fibrosis
Studies 1 and 2 were randomized, double-blind, placebo-controlled, crossover studies of 49 patients, ages 7 to 43 years, with EPI due to CF. Study 1 included 32 patients ages 12 to 43 years and Study 2 included 17 patients ages 7 to 11 years. In these studies, patients were randomized to receive CREON at a dose of 4,000 lipase units/g fat ingested per day or matching placebo for 5 to 6 days of treatment, followed by crossover to the alternate treatment for an additional 5 to 6 days. The mean exposure to CREON during these studies was 5 days.
In Study 1, one patient experienced duodenitis and gastritis of moderate severity 16 days after completing treatment with CREON. Transient neutropenia without clinical sequelae was observed as an abnormal
In Study 2, adverse reactions that occurred in at least 2 patients (greater than or equal to 12%) treated with CREON were vomiting and headache. Vomiting occurred in 2 patients treated with CREON and did not occur in patients treated with placebo; headache occurred in 2 patients treated with CREON and did not occur in patients treated with placebo.
The most common adverse reactions (greater than or equal to 4%) in Studies 1 and 2 were vomiting, dizziness, and cough. Table 1 enumerates adverse reactions that occurred in at least 2 patients (greater than or equal to 4%) treated with CREON at a higher rate than with placebo in Studies 1 and 2. than or equal to 4%) in Cystic Fibrosis (Studies 1 and 2)
An additional open-label, single-arm study assessed the short-term safety and tolerability of CREON in 18 infants and children, ages 4 months to pancreatic enzyme replacement therapy (mean dose of 7,000 lipase units/kg/day for a mean duration of 18.2 days) followed by CREON (mean dose of 7,500 lipase units/kg/day for a mean duration of 12.6 days).
There were no serious adverse reactions. Adverse reactions that occurred in patients during treatment with CREON were vomiting, irritability, and decreased appetite, each occurring in 6% of patients.
Chronic Pancreatitis or Pancreatectomy
A randomized, double-blind, placebo-controlled, parallel group study was conducted in 54 adult patients, ages 32 to 75 years, with EPI due to chronic pancreatitis or pancreatectomy. Patients received single-blind placebo treatment during a 5-day run-in period followed by an intervening period of up to 16 days of investigator-directed treatment with no restrictions on pancreatic enzyme replacement therapy. Patients were then randomized to receive CREON or matching placebo for 7 days. The CREON dose was 72,000 lipase units per main meal (3 main meals) and 36,000 lipase units per snack (2 snacks). The mean exposure to CREON during this study was 6.8 days in the 25 patients that received CREON.
The most common adverse reactions reported during the study were related to glycemic control and were reported more commonly during CREON treatment than during placebo treatment.
Table 2 enumerates adverse reactions that occurred in at least 1 patient (greater than or equal to 4%) treated with CREON at a higher rate than with placebo.
Table 2: Adverse Reactions in at Least 1 Patient (greater than or equal to 4%) in the Chronic Pancreatitis or Pancreatectomy Trial
Postmarketing Experience
Postmarketing data from this formulation of CREON have been available post approval use of this formulation of CREON. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
constipation and nausea), skin disorders (including pruritus, urticaria and rash), blurred vision, myalgia, muscle spasm, and asymptomatic elevations of liver enzymes have been reported with this formulation of CREON.
Delayed- and immediate-release pancreatic enzyme products with different formulations of the same active ingredient (pancrelipase) have been used for
intestinal obstruction syndrome (DIOS), recurrence of pre-existing carcinoma, and severe allergic reactions including anaphylaxis, asthma, hives, and pruritus.
DRUG INTERACTIONS
been conducted.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Published data from case reports with pancrelipase use in pregnant women or other adverse maternal or fetal outcomes. Pancrelipase is minimally absorbed systematically; therefore, maternal use is not expected to result in fetal exposure to the drug. Animal reproduction studies have not been conducted with pancrelipase.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Lactation
Risk Summary
There are no data on the presence of pancrelipase in either human or animal milk, the effects on the breastfed infant or the effects on milk production. Pancrelipase is minimally absorbed systemically following oral administration; therefore, maternal use is not expected to result in clinically relevant exposure breastfeeding should be considered along with the mother’s clinical need for CREON and any potential adverse effects on the breastfed infant from CREON or from the underlying maternal condition.

Pediatric Use
The short-term safety and effectiveness of CREON were assessed in two randomized, double-blind, placebo-controlled, crossover studies of 49 Study 1 included 8 adolescents between 12 and 17 years of age. Study 2 in pediatric patients in these studies were similar to adult patients [see Adverse Reactions and Clinical Studies]
An open-label, single-arm, short-term study of CREON was conducted in 18 infants and children, ages 4 months to six years of age, with EPI due to therapy (mean dose of 7,000 lipase units/kg/day for a mean duration of 18.2 days) followed by CREON (mean dose of 7,500 lipase units/kg/day for a mean duration of 12.6 days). The mean daily fat intake was 48 grams during treatment with usual pancreatic enzyme replacement therapy and 47 grams
40 Revista Puertorriqueña de Medicina y Salud Pública
Adverse Reaction CREON Capsules n = 49 (%) Placebo n = 47 (%) 3 (6) 1 (2) 2 (4) 1 (2) 2 (4) 0
Adverse Reaction CREON Capsules n = 25 (%) Placebo n = 29 (%) 2 (8) 2 (7) 1 (4) 1 (3) 1 (4) 1 (3) 1 (4) 0 1 (4) 0 1 (4) 0 1 (4) 0
A-F 16-7439 US-CREO-220088 Ad.indd 4 7/14/22 4:25 PM
during treatment with CREON. When patients were switched from their usual pancreatic enzyme replacement therapy to CREON, they demonstrated similar spot fecal fat testing results; the clinical relevance of spot fecal fat testing has not been demonstrated. Adverse reactions that occurred in patients during treatment with CREON were vomiting, irritability, and decreased appetite [see Adverse Reactions]
formulations of pancrelipase consisting of the same active ingredient (lipases, proteases, and amylases) for treatment of children with exocrine pancreatic literature and through clinical experience.
Dosing of pediatric patients should be in accordance with recommended guidance from the Cystic Fibrosis Foundation Consensus Conferences [see Dosage and Administration]. Doses of other pancreatic enzyme products exceeding 6,000 lipase units/kg of body weight per meal have been than 12 years of age [see Warnings and Precautions]
Geriatric Use
65 and over to determine whether they respond differently from younger responses between the elderly and younger patients.
OVERDOSAGE
There have been no reports of overdose in clinical trials or postmarketing surveillance with this formulation of CREON. Chronic high doses of pancreatic colonic strictures [see Dosage and Administration and Warnings and Precautions]. High doses of pancreatic enzyme products have been associated with hyperuricosuria and hyperuricemia, and should be used with caution in patients with a history of hyperuricemia, gout, or renal impairment [see Warnings and Precautions]
NONCLINICAL TOXICOLOGY

Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, genetic toxicology, and animal fertility studies have not been performed with pancrelipase.
PATIENT COUNSELING INFORMATION
Dosing and Administration
• Instruct patients and caregivers that CREON should only be taken as directed by their healthcare professional. Patients should be advised that the total daily dose should not exceed 10,000 lipase units/kg body weight/ day unless clinically indicated. This needs to be especially emphasized for patients eating multiple snacks and meals per day. Patients should be informed that if a dose is missed, the next dose should be taken with the next meal or snack as directed. Doses should not be doubled [see Dosage and Administration (2)]
• Instruct patients and caregivers that CREON should always be taken with food. Patients should be advised that CREON delayed-release capsules and the capsule contents must not be crushed or chewed as doing so could cause early release of enzymes and/or loss of enzymatic activity. Patients should swallow the intact capsules with adequate amounts of liquid at mealtimes. If necessary, the capsule contents can also be sprinkled on soft acidic foods [see Dosage and Administration]
Fibrosing Colonopathy
Advise patients and caregivers to follow dosing instructions carefully, as doses of pancreatic enzyme products exceeding 6,000 lipase units/kg of body weight per meal have been associated with colonic strictures in children below the age of 12 years [see Dosage and Administration]
Allergic Reactions
Advise patients and caregivers to contact their healthcare professional immediately if allergic reactions to CREON develop [see Warnings and Precautions]
Manufactured by: Abbott Laboratories GmbH
Hannover, Germany
Marketed by:
AbbVie Inc.
North Chicago, IL 60064, U.S.A.
© 2009-2020 AbbVie Inc.
Ref: 03-C054-R4 Revised March, 2020
LAB-3604 MASTER US-CREO-220088
Revista Puertorriqueña de Medicina y Salud Pública 41 PM
A-F 16-7439 US-CREO-220088 Ad.indd 5 7/14/22 4:25 PM
CUIDADO PREVENTIVO PARA PACIENTES CON ENFERMEDAD INFLAMATORIA DE INTESTINO

Palabras Clave: Enfermedad inflamatoria de intestino, cuidado preventivo, enfermedad de Crohn, colitis ulcerosa, vacunas, cernimiento de cáncer, osteoporosis, cáncer colorrectal, COVID-19

RESUMEN
El Colegio Americano de Gastroenterología (ACG por sus siglas en inglés) publicó unas guías para el cuidado preventivo requerido en pacientes con enfermedad inflamatoria de intestino (EII), que incluyen vacunas y cernimientos de cáncer, osteoporosis, e infecciones como tuberculosis latente. Debido al impacto que tiene esta condición crónica en la salud emocional de los pacientes con EII, las guías también
hacen un enfoque en el cernimiento para condiciones psiquiátricas como depresión mayor y ansiedad generalizada. Fumar activamente es un factor de riesgo para complicaciones y pobre respuesta a terapia, por lo cual también se recomienda un cernimiento cercano sobre el uso del cigarrillo. Ante la llegada de la pandemia y la severidad del coronavirus, el ACG también recomienda la vacuna contra
el COVID-19 de acuerdo con las recomendaciones del Centro para el Control y la Prevención de Enfermedades (CDC por sus siglas en inglés). Estas guías son una fuente de educación no solo para los gastroenterólogos sino para los médicos primarios con el fin de mejorar el cuidado preventivo y la calidad de vida de estos pacientes.
42 Revista Puertorriqueña de Medicina y Salud Pública
Karelys Burgos Irizarry
 Yue Sai Jao
Yue Sai Jao
La enfermedad inflamatoria de intestino (EII) es un término amplio que se usa para describir desórdenes que causan inflamación crónica del tracto digestivo, como colitis ulcerosa (CU) y enfermedad de Crohn (EC). EII es una de las 5 condiciones crónicas gastrointestinales más prevalentes en los Estados Unidos, con un costo total anual en servicios de salud que sobrepasa los $1.5 billones. Por la severidad de esta condición y la continuidad de cuidado que requiere, estos pacientes tienden a considerar su gastroenterólogo como su médico primario, lo cual lleva a una disminución de visitas al médico primario.
Hay una alta prevalencia de pacientes con EII moderada a severa que requiere tratamiento con terapia inmunosupresora, lo que conlleva un cuidado preventivo específico por las complicaciones asociadas a la inmunosupresión. Sin embargo, los estudios demuestran que estos pacientes no reciben cuidado preventivo con la
INTRODUCCIÓN DISCUSIÓN
Vacunas
Todo paciente con Enfermedad Inflamatoria del Intestino (EII) debe recibir las vacunas inactivadas sin importar su estado de inmunosupresión; estas vacunas incluyen, pero no se limitan a Influenza, Neumococo, Hepatitis A y B, Haemophilus influenza B, Virus del Papilloma Humano, tétano y pertussis. No obstante, la mayoría de las vacunas atenuadas también son parte del mantenimiento de salud y son recomendadas a aquellos pacientes
misma frecuencia que el resto de la población. Una de las razones para esta disparidad es que hay un enfoque primordial en control de síntomas por encima de medidas preventivas. La ausencia de cuidado preventivo en estos pacientes afecta tanto la calidad de cuidado como los resultados de los pacientes. Por lo tanto, no solo los gastroenterólogos, sino los médicos primarios deben crear un enfoque multidisciplinario y tomar un rol proactivo con la meta de empoderar al paciente a responsabilizarse y crear conciencia sobre su enfermedad y el cuidado preventivo que compele.
De acuerdo con las guías del Colegio Americano de Gastroenterología (ACG por sus siglas en inglés) para el cuidado preventivo de pacientes con EII, los asuntos de mantenimiento de salud incluyen: vacunas indicadas para pacientes con EII, cernimiento de tuberculosis latente, osteoporosis, cáncer cervical, melanoma y cáncer de piel no melanoma, cáncer de colon y una
evaluación para depresión, ansiedad y el hábito de fumar. Todos deben ser repasados en cada visita para asegurar la adherencia y el cumplimiento por parte de los pacientes.
Para el año 2013, la prevalencia de EII en Puerto Rico mostró un alza de hasta 4 veces el número de pacientes en comparación con la prevalencia del 2005. Desafortunadamente hay datos limitados sobre el conocimiento y percepción del cuidado preventivo de pacientes con EII en Puerto Rico. Actualmente se está conduciendo un estudio con el fin de evaluar el conocimiento y percepción tanto en los pacientes como en los gastroenterólogos acerca de las guías de cuidado preventivo con el fin de crear conciencia, aumentar el conocimiento y de esa manera mejorar el cuidado multidisciplinario de los pacientes con EII. Mediante este artículo les proveemos un resumen sobre los aspectos más importantes del cuidado preventivo de pacientes con EII.


con EII que no estén inmunosuprimidos (sea por medicamentos, cáncer o malnutrición severa) o que estén a un nivel mínimo de inmunosupresión. Sin embargo, existen excepciones. Hasta el momento, no tenemos evidencia de que las vacunas desencadenen una exacerbación de EII según varios estudios publicados.
La vacuna inactivada contra la influenza se recomienda anualmente.
Las vacunas contra el neumococo PCV13 y PPSV23 se deben administrar en pacientes inmunosuprimidos, pero no simultáneamente. Actualmente, existen dos formulaciones nuevas de la vacuna conjugada contra el neumococo (PCV15 o PCV20). El Centro para Control y Prevención de Enfermedades (CDC) recomienda que se les ofrezca estas alternativas a los adultos de 65 años o más que nunca han recibido la versión de PCV13 o
Revista Puertorriqueña de Medicina y Salud Pública 43
Programa de Adiestramiento en Gastroenterología, Escuela de Medicina, Recinto de Ciencias Médicas UPR
Fellow de Gastroenterología UPR Recinto de Ciencias Médicas
MD, fellow de Gastroenterología, Escuela de Medicina, Recinto de Ciencias Médicas
que su historial de vacunación sea desconocido. A adultos mayores de 50 años, que incluyen los pacientes con EII sin discriminación alguna por su estado de inmunosupresión, se les recomienda que se vacunen contra el Herpes Zoster. El estudio de VERVE, mejor conocido por sus siglas en ingles de “VaricElla ZosteR VaccinE”, demostró seguridad y eficacia de la vacuna en pacientes que fueron administrados la vacuna atenuada de Herpes Zoster mientras estaban recibiendo su tratamiento con biológicos (anti-TNF).
Se recomienda que se administre la vacuna de varicela antes de empezar terapia de inmunosupresión, verificando que el paciente no haya tenido exposición previa al virus o la vacuna. No existe contraindicación para que miembros del hogar que conviven con personas inmunosuprimidas reciban vacunas atenuadas como las vacunas contra paperas, Sarampión o Rubeola, Rotavirus, Varicela y Herpes Zoster, tomando las debidas precauciones para proteger a los pacientes vulnerables. En caso de que una persona saludable se vacune contra la varicela y luego presente una lesión sospechosa en piel, esta persona debe ser aislada para proteger al individuo inmunocomprometido. A los pacientes con EII se les recomienda ser evaluados por un infectólogo por
Prevención de cáncer
El mantenimiento de salud en los pacientes con EII no solo incluye la vacunación sino también los cernimientos adecuados para la detección temprana y correcta de distintos tipos de cáncer. De acuerdo con el Colegio Americano de Obstetricia y Ginecología (ACOG en inglés) y el CDC, se recomienda que se haga un cernimiento anual para la prevención de cáncer cervical con el uso de la prueba de Papanicolaou, ya que los pacientes con EII están predispuestos a tener mayor incidencia de displasia y cáncer cervical en comparación con la población general. A los pacientes con EII se les recomienda que rutinariamente usen bloqueador solar y se hagan cernimientos dermatológicos rutinarios para evaluación de cáncer de la piel incluyendo el melanoma. Se debe enfocar aún más la atención en los pacientes que estén usando medicamentos de inmunosupresión. Se ha identificado una relación entre
lo menos tres meses antes de que se vayan a viajar a países en donde haya diversidad de enfermedades infecciosas para poder administrar las vacunas correspondientes, de ser candidatos. La vacuna contra la fiebre amarilla es atenuada. A aquellos pacientes que por su inmunosupresión no puedan recibir la vacuna contra la fiebre amarilla, se les recomienda que eviten viajar a países endémicos donde puedan contraer esta condición y de ser inevitable el viaje tomar las precauciones necesarias para evitar la infección.
A los pacientes con EII se les recomienda la vacuna contra el meningococo sin importar su estado de inmunosupresión; la vacuna está específicamente destinada para las adolescentes y los adultos jóvenes entre 16 a 23 años que tienen mayor riesgo de infección. A todo paciente de EII se le recomienda recibir las vacunas que le correspondan de acuerdo con su edad, específicamente las vacunas atenuadas e inactivadas al menos 4 y 2 semanas antes de comenzar una terapia de inmunosupresión, respectivamente. Una vez que se le ofrezcan las vacunas inactivadas se recomienda verificar inmunidad en el tiempo de post vacunación para corroborar la necesidad de un refuerzo. La respuesta inmune a la vacuna disminuye a medida que aumente
el estado de inmunosupresión, pero se ha visto que, a pesar de lo antes mencionado, las vacunas siguen siendo beneficiosas y seguras en esta población, según lo descrito en literatura científica. Es importante resaltar que la responsabilidad de las recomendaciones de vacunas descritas en esta publicación debe ser compartida entre el gastroenterólogo y el médico primario para poder fomentar el cuidado preventivo necesario para los pacientes de Crohn y colitis ulcerosa. Por último, la Fundación de Crohn y Colitis, la cual aboga por prácticas seguras en pacientes con EII, apoya la vacunación contra el virus de COVID 19 en pacientes con EII. Ellos entienden que los pacientes con EII no tienen riesgo aumentado de enfermedad severa por coronavirus y no consideran que los pacientes con EII necesiten una dosis adicional de la vacuna de mRNA, ya que muchos no son considerados inmunosuprimidos. Deben seguir las recomendaciones de la población general. Las recomendaciones en cuanto a la vacuna de COVID por parte del ACG se basan en las guías del CDC, por lo cual hay que mantenerse al tanto a los cambios y las actualizaciones más recientes para poder proveerle al paciente la información adecuada.
los biológicos contra factor de necrosis tumoral y el riesgo de melanoma y el uso de tiopurina con el cáncer escamoso de la piel, el último mencionado en adultos mayores de 50 años según el estudio de CESAME.15 A los pacientes con EII se les recomienda cernimiento de cáncer colorrectal después de 8 años de enfermedad mediante evaluación por colonoscopia, dependiendo de la extensión de la enfermedad.
está en remisión.18,19 Hay un estudio en donde se observó una correlación inversa entre la presencia de depresión y la adherencia de medicamentos.20 Lo antes descrito resalta la importancia de identificar las condiciones sicológicas en los pacientes con EII para proveer un manejo completo de la enfermedad y sus complejidades.
Estrés, Depresión y Ansiedad Osteoporosis

Las enfermedades sicológicas son factores que añaden complejidad a los pacientes con Crohn y colitis ulcerosa, ya que afectan como el paciente percibe su condición y el manejo que está recibiendo. Hay diversas publicaciones que han determinado que hay mayor nivel de estrés, depresión y ansiedad en pacientes que tienen la enfermedad activa en comparación con aquellos en quienes su enfermedad
Los pacientes con EII están a mayor riesgo de tener osteoporosis.21 Los factores de riesgo para pérdida de densidad de masa ósea incluyen a mujeres deficientes de estrógeno/ postmenopausia, mujeres de bajo índice de masa corporal, hiperparatiroidismo primario e individuos que estén usando terapia prolongada de corticoesteroides definida como mayor o igual a 5 mg por día de prednisona o una dosis
44 Revista Puertorriqueña de Medicina y Salud Pública
equivalente por al menos 3 meses. A los pacientes que tengan los factores de riesgo antes mencionados se les recomienda el estudio para evaluar la densitometría ósea (DXA), el cual también es útil para mujeres de 65 años o más y hombres de 70 años o más según la Fundación Nacional de Osteoporosis.
Osteoporosis
Se ha identificado una asociación significativa entre el fumador activo y el desarrollo y progresión de la enfermedad de Crohn. Según diversos estudios publicados, los pacientes con
EII que se denominaban fumadores activos estaban relacionados con una alta frecuencia de uso de terapia de mantenimiento específicamente el uso de biológicos, pobre respuesta a terapia y alta frecuencia de intervenciones quirúrgicas en comparación con los no fumadores.
CONCLUSIONES
• Existen guías para el cuidado preventivo requerido para pacientes con EII que incluyen vacunas y cernimientos de cáncer, osteoporosis y tuberculosis latente, indicados para estos pacientes.
• Las guías también hacen enfoque en la evaluación de condiciones psiquiátricas como depresión mayor, ansiedad generalizada y abuso de sustancias, como el cigarrillo, que afectan la percepción y el manejo de la enfermedad.
• No solo los gastroenterólogos deben ser los responsables de inculcar el cuidado preventivo en pacientes con EII y el enfoque debe dirigirse al empoderamiento del paciente con respecto a su cuidado.
• Se debe recomendar a todos los pacientes la vacunación contra el virus de COVID 19 de acuerdo con las recomendaciones del CDC.
CUIDADO DE SALUD PREVENTIVO PARA PACIENTES CON EII: VACUNAS
* Frecuencias y dosis indicadas están disponibles en las recomendaciones del Comité Asesor sobre Prácticas de Inmunización (ACIP).
• Contraindicada en pacientes recibiendo tratamiento inmunosupresor.
† Depende del tipo de medicamento inmunosupresor.
Δ Las recomendaciones resumidas en esta tabla se basan en las guías de cuidado preventivo de EII del ACG y la lista de cotejo de mantenimiento de salud de la Fundación de Crohn y Colitis.
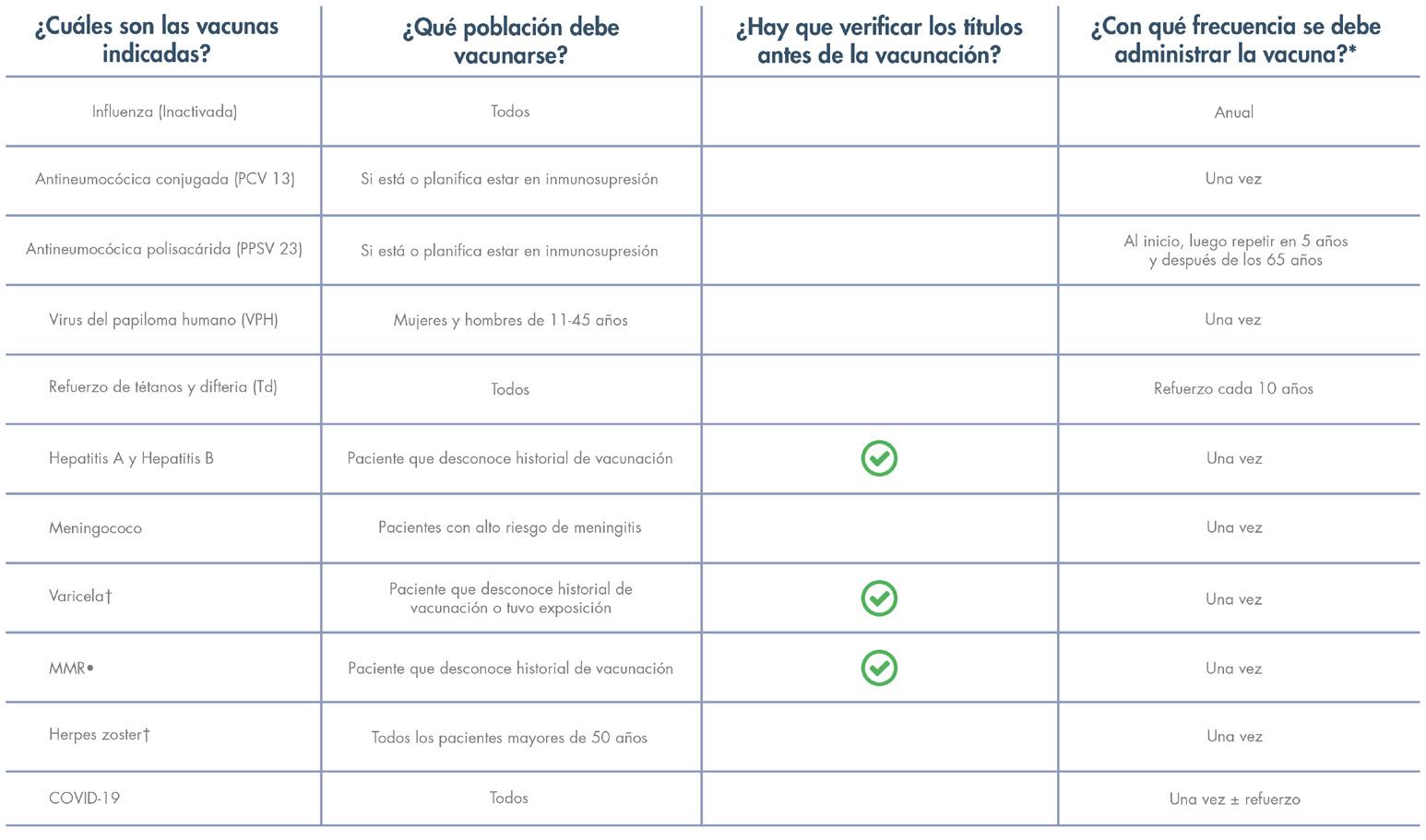
REFERENCIAS
Abegunde AT, Muhammad BH, Ali T. Preventive health measures in inflammatory bowel disease. World J Gastroenterol. 2016 Sep 14;22(34):7625-44. Doi: 10.3748/ wjg.v22.i34.7625.

Farraye F A, Melmed GY, Lichtenstein GR, Kane,SV. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Amer J Gastroenterol: February 2017 – Volume 112 – Issue 2 – p 241258 doi: 10.1038/ajg.2016.537.
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R; National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014 Oct;25(10):2359-81. Doi: 10.1007/s00198-014-2794-2. Epub 2014 Aug 15. Erratum in: Osteoporos Int. 2015 Jul;26(7):2045-7.
Kroger A, Bahta L, Hunter P. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory
Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/aciprecs/general-recs/index.html
Pillai N, Dusheiko M, Burnand B, Pittet V. A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PloS One. 2017 Oct 3;12(10):e0185500. Doi: 10.1371/ journal.pone.0185500.
Revista Puertorriqueña de Medicina y Salud Pública 45
Practice Bulletin No. 157: Cervical Cancer Screening and Prevention. Obstet Gynecol. 2016 Jan;127(1):e1-e20. Doi: 10.1097/ AOG.0000000000001263.
Torres EA, Torres-Cintron M, Velazquez S, Vendrell R, Del Valle A, Pérez CM. Prevalence of Inflammatory Bowel Diseases in Puerto Rico: A Health Care Claims Analysis of an Insured Population. P R Health Sci J. 2021 Sep;40(3):103-109.

Rahier JF, Papay P, Salleron J et al. H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immu- nomodulators and biological therapy. Gut 2011;60:456–62.
Dotan I, Werner L, Vigodman S et al. Normal response to vaccines in inflammatory bowel disease patients treated with thiopurines. Inflamm Bowel Dis 2012;18:261–8.
Agarwal N, Ollington K, Kaneshiro M et al. Are immunosuppressive medications associated with decreased responses to routine immuniza- tions? A systematic review. Vaccine 2012;30:1413–24.
Curtis JR, Cofield SS, Bridges SL, et al; The Safety and Immunologic Effectiveness of the Live Varicella-Zoster Vaccine in Patients Receiving Tumor Necrosis Factor Inhibitor Therapy: A Randomized Controlled Trial. Ann Intern Med.2021;174:1510-1518. [Epub 28 September 2021]. doi:10.7326/
M20-6928
Andrisani G, Frasca D, Romero M et al. Immune response to influenza A/ H1N1 vaccine in inflammatory bowel disease patients treated with anti TNFalpha agents: effects of combined therapy with immunosuppressants. J Crohn’s Colitis 2013;7:301–7.
Hagihara Y, Ohfuji S, Watanabe K et al. Infliximab and/or immunomodu- lators inhibit immune responses to trivalent influenza vaccination in adults with inflammatory bowel disease. J Crohn’s Colitis 2014;8:223–33.
Nguyen DL, Nguyen ET, Bechtold ML. Effect of immunosuppressive therapies for the treatment of inflammatory bowel disease on response to routine vaccinations: a metaanalysis. Dig Dis Sci 2015;60:2446–53.
Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP, Simon T, Maynadié M, Hermine O, Faivre J, Carrat F; CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009 Nov 7;374(9701):1617-25. doi:
10.1016/S0140-6736(09)61302-7. PMID: 19837455.
Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–774, 774.e1-4; quiz e12-13.
Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J Gastroenterol. 2019 Aug 14;25(30):4148-4157. doi: 10.3748/ wjg.v25.i30.4148. PMID: 31435169; PMCID: PMC6700690.
Persoons P, Vermeire S, Demyttenaere K et al. The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Aliment Pharmacol Ther 2005;22: 101–10.
Calvet X, Gallardo O, Coronas R et al. Remission on thiopurinic immunomodulators normalizes quality of life and psychological status in patients with Crohn’s disease. Inflamm Bowel Dis 2006;12:692–6.
Nigro G, Angelini G, Grosso SB et al. Psychiatric predictors of noncompli- ance in inflammatory bowel disease: psychiatry and compliance. J Clin Gastroenterol 2001;32:66–8.
Pollak RD, Karmeli F, Eliakim R et al. Femoral neck osteopenia in patients with inflammatory bowel disease. Am J Gastroenterol 1998;93:1483–90.
Nunes T, Etchevers MJ, Merino O et al. Does smoking influence Crohn’s disease in the biologic era? The TABACROHN study. Inflamm Bowel Dis 2013;19:23–9.
To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the ad- verse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment Pharmacol Ther 2016;43:549–61.
Parsi MA, Achkar JP, Richardson S et al. Predictors of response to infliximab in patients with Crohn’s disease. Gastroenterology 2002;123:707–13.
Moss A, Farraye F, Gordon G, Vrabie R. Health Maintenance Checklist for Adult IBD Patients. Crohn’s & Colitis Foundation’s Professional Education Committee Sub-Group. https://www. crohnscolitisfoundation.org/sites/default/
files/legacy/science-and-professionals/ programs-materials/health-maintenancechecklist.pdf
46 Revista Puertorriqueña de Medicina y Salud Pública
GRUPO DE APOYO




Hidradentitis Suppurativa


Puerto Rico
Golondrinos


¡No estas sol@!
Hidradenitis Suppurativa (HS) Puerto Rico (Golondrinos) hsprgolondrinos
Revista Puertorriqueña de Medicina y Salud Pública 47
Retinal Detachment
Inform patients that retinal detachment has been reported in clinical trials with RINVOQ. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision while receiving RINVOQ [see Adverse Reactions]
Laboratory Abnormalities
Inform patients that RINVOQ may affect certain lab tests, and that blood tests are required before and during RINVOQ treatment [see Warnings and Precautions]
Vaccinations
Advise patients to avoid use of live vaccines with RINVOQ. Instruct patients to inform their healthcare practitioner that they are taking RINVOQ prior to a potential vaccination [see Warnings and Precautions]
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential that exposure to RINVOQ during pregnancy may result in fetal harm. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions and Use in Specific Populations]
Advise females of reproductive potential that effective contraception should be used during treatment and for 4 weeks following the final dose of upadacitinib [see Use in Specific Populations]
Advise females patients who are exposed to RINVOQ during pregnancy to contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Lactation
Advise women not to breastfeed during treatment with RINVOQ and for 6 days after the last dose [see Use in Specific Populations]
Administration
Advise patients not to chew, crush, or split RINVOQ tablets.
Manufactured by: AbbVie Inc., North Chicago, IL 60064, USA

RINVOQ® is a registered trademark of AbbVie Biotechnology Ltd.
©2019-2022 AbbVie Inc.
Ref: 20071756 Revised: October 2022
LAB-8208 MASTER
Revista Puertorriqueña de Medicina y Salud Pública 53 DO NOT RE-SIZE US-RNQG-220641
20071756 Rinvoq PB - 10.5x13 (3).indd 3 25 Oct 2022 3:03 PM US-RNQG-220641



54 Revista Puertorriqueña de Medicina y Salud Pública Revista Puertorriqueña de Medicina y Salud Pública 24 MSP SUPLEMENTO ESPECIAL
ESPECIALIZACIÓN DEPORTIVA TEMPRANA ,
¿BENEFICIOSA O PERJUDICIAL PARA EL JOVEN ATLETA?
PALABRAS CLAVE Especialización temprana, Lesiones , Presión psicológica, Agotamiento
KEYWORDS
Early specialization, injuries, psychological burden, burnout




Catedrático y Director Director Programa Medicina Deportiva Departamento de Medicina Física, Rehabilitación y Salud Deportiva Escuela de Medicina, Universidad de Puerto Rico
RESUMEN
• La especialización deportiva se define como el entrenamiento o régimen competitivo primario en un solo deporte al menos durante 8 meses al año.
• Riesgos de la especialización temprana incluyen: aumento en lesiones de sobreuso, presión psicológica excesiva, agotamiento y abandono del deporte.
• Los beneficios de la especialización temprana incluyen: disciplina, puntualidad, respeto y compromiso a una corta edad.
• Disciplinas como el patinaje artístico sobre hielo, gimnasia y natación pueden requerir especialización temprana y deportes como el tenis, baloncesto, balompié, y béisbol no requieren de la especialización temprana para alcanzar el éxito competitivo.
SUMMARY
• Early sports specialization is defined as intense training or competitive regime in only one sport during at least 8 months per year.
• Risks of early specialization include: increase of overuse injuries, excessive Psychological burden, burnout, and sports withdrawal.
• Early specialization can provide certain benefits such as: discipline, punctuality, respect and Commitment at a young age.
Residente Primer Nivel Medicina Física y Rehabilitación
• Sports such as ice skating, gymnastics, and swimming may require early specialization. In contrast, tennis, basketball, soccer and baseball do not require early specialization in order to reach Competitive success.
Revista Puertorriqueña de Medicina y Salud Pública 55
Daniel Almodóvar Frau, MD
William Micheo, MD
Revista Puertorriqueña de Medicina y Salud Pública 25
MSP SUPLEMENTO ESPECIAL
La implementación del deporte a temprana edad suele ser de gran beneficio para el joven atleta ya que promueve el trabajo en equipo, destrezas de liderazgo, destrezas motoras, destrezas sociales, fomenta la diversión y reduce el ocio, entre otras. El deporte juvenil ha ido cambiando durante los últimos años, donde la norma ha cambiado y establece que los niños y adolescentes deben participar de la actividad deportiva organizada, dirigida por padres y entrenadores, a menudo con régimenes de entrenamiento y aspiraciones para el juego que usualmente difieren al de sus jóvenes participantes. (2) A su vez, esta en la mayoría de las ocasiones es impulsada por la búsqueda de becas universitarias y metas de alcanzar un nivel elite olímpico y/o profesional. (2) A través de los años, el nivel de competitividad en el deporte juvenil ha ido incrementando, promoviendo así una mayor cantidad de eventos competitivos a edades más tempranas y entrenamientos especializados que llevan a instaurar la especialización deportiva en la niñez. La especialización deportiva se define como el entrenamiento o régimen competitivo intenso, durante al menos 8 meses al año, donde el enfoque primario es un solo deporte, excluyendo la participación en otros deportes. (5) El objetivo de la misma es maximizar el potencial del éxito deportivo a través de un régimen de entrenamiento riguroso y la competencia deportiva continua. Esto se basa en la teoría que para alcanzar el mayor nivel en una disciplina, es necesario la práctica temprana enfocada en destrezas y movimientos específicos del deporte seleccionado, existe la controversia sobre si este tipo de práctica es perjudicial para el joven atleta en crecimiento y si contribuye al desarrollo de mejores destrezas y al éxito deportivo alcanzado en un futuro.
Los jóvenes atletas que se especializan en un solo deporte a temprana edad están en mayor riesgo de desarollar problemas físicos, emocionales y sociales. Estos problemas incluyen tasas más altas por lesiones de sobreuso debido a un esqueleto inmaduro, falta de descanso y adaptación neuromuscular, presión psicológica excesiva y agotamiento.
(9) Una lesión de sobreuso se define como un daño micro-traumático a un hueso, músculo, o tendón, que ha

sido sometido a un estrés repetitivo sin proveer el tiempo necesario para que se lleve a cabo el proceso de recuperación adecuada. La literatura establece que hasta un 50% de todas las lesiones en la práctica de la medicina deportiva pediátrica se deben a la sobrecarga. (3) Por otra parte, la presión y/o estrés psicológico son factores elementales que ocasionan un impacto negativo y suelen manifestarse como: aumento en niveles de ansiedad, pobre desempeño académico y autoestima baja que eventualmente podrían llevar al agotamiento. La unión de todos estos factores ocasiona una reacción en cadena que comienza con el agotamiento, que podría causar una disminución en el desempeño atlético de alto nivel y un aumento en la tasa de lesiones, alejando al atleta del escenario deportivo. Esta ausencia puede llevar al atleta a un estado de depresión, como consecuencia, a abandonar el deporte. (9) El abandono del deporte recreacional a temprana edad puede resultar en la inactividad física y abrir paso a una serie de condiciones de salud crónicas en el futuro. (1)
A pesar de los múltiples riesgos que se pueden atribuir a la especialización temprana, esta puede proveer ciertos beneficios como: disciplina, puntualidad, respeto y compromiso a una corta edad. La literatura científica aún no ha podido demostrar de manera consistente que el entrenamiento físico intenso a temprana edad sea esencial y necesario para alcanzar un nivel elite en todas las disciplinas deportivas. Sin embargo, existen estudios que demuestran lo contrario en algunos deportes, por ejemplo, disciplinas como el patinaje artístico sobre hielo, gimnasia y natación, pueden requerir especialización temprana debido a que el rendimiento máximo alcanzado se produce antes que se complete la maduración física del atleta. (2, 6)
Deportes como, el tenis, baloncesto, balompié, y béisbol, en su mayoría no requieren de la especialización temprana para alcanzar el éxito competitivo a un alto nivel; y es en estas disciplinas donde sus participantes se benefician de un método conocido como diversificación deportiva. La diversificación deportiva o la participación en múltiples deportes durante la niñez tiene el potencial de proveer estímulo para






MSP SUPLEMENTO ESPECIAL Revista Puertorriqueña de Medicina y Salud Pública 26 Revista Puertorriqueña de Medicina y Salud Pública 26
MSP SUPLEMENTO ESPECIAL 56 Revista Puertorriqueña de Medicina y Salud Pública
“Los jóvenes atletas que se especializan en un solo deporte a temprana edad están en mayor riesgo de desarollar problemas físicos, emocionales y sociales”
La Asociación Internacional de la Alfabetización Física, define esta como: “la incitación, la confianza, la competencia física y el conocimiento que nos motiva a llevar una vida activa a diario.” (10) Dichos modelos buscan mantener al joven atleta envuelto en el deporte de manera segura con el fin de crear destrezas y relaciones interpersonales saludables entre pares que puede ayudar a minimizar el abandono del deporte y a aumentar las posibilidades de alcanzar un nivel competitivo. Un modelo utilizado por la Asociación de Tenis de los Estados Unidos o USTA (United States Tennis Association) por sus siglas en inglés, divide la participación deportiva en tres etapas: (9)
Edades de 12 años o menos: el atleta descubre, aprende y desarrolla destrezas básicas de un deporte mientras se divierte y se desempeña en otros deportes.
Edades de 12-18 años: el atleta adquiere mejor entendimiento del deporte y la importancia de los entrenamientos que este conlleva para la preparación competitiva.
Edades de 15-18 años: el atleta se dedica al deporte a tiempo completo y trabaja para alcanzar un nivel competitivo de elite.
que el menor adapte y desarrolle destrezas motoras como el equilibrio, la coordinación, la flexibilidad y la fuerza. (9) Este método se enfoca en emplear el juego y la diversión en lugar de la práctica estructurada, en el que el atleta pueda desarrollar pasión y amor propio por el deporte. La diversificación deportiva se basa en la creencia de que las habilidades físicas y cognitivas pueden desarrollarse más rápido y con mejor eficacia al practicar más de un deporte a la vez, donde destrezas aprendidas en un deporte puedan trasladarse y ayudar al desempeño en otro deporte. Por ejemplo, en el béisbol, el lanzar y batear la bola ayuda al desarrollo de la coordinación visual motora, por otro lado, en el balompié, el uso de las piernas para correr y manejar el balón fomenta la coordinación visual motora junto con la agilidad y el juego de pies, un joven que practique ambas disciplinas desarrollará múltiples destrezas que no obtendría si solamente practicara uno de estos deportes. (4) Diversas entidades y organizaciones atléticas han creado modelos de desarrollo para la especialización deportiva con la meta de establecer expectativas apropiadas de acuerdo con la edad del atleta y así reducir los efectos detrimentales de la especialización temprana. Las mismas buscan minimizar estas consecuencias a través de la implementación de programas estructurados para el desarrollo de la alfabetización física y la formación apropiada de atletas de alto rendimiento.
Este modelo toma en cuenta los aspectos físicos, sociales y psicológicos del desarrollo y facilita la transición a un nivel competitivo y a la actividad física o práctica deportiva de por vida. La diversificación deportiva temprana y el retraso en la especialización deportiva hasta después de la pubescencia, en la mayoría de los deportes, puede resultar en una reducción de los riesgos previamente mencionados y en un mayor disfrute y éxito deportivo. (2) La práctica y fomentación de este tipo de modelo de desarrollo es de suma importancia para la salud y bienestar del joven atleta donde el enfoque principal del deporte se debe centrar en la diversión y enseñanza de destrezas que servirán de herramientas para la vida diaria.
Referencias:
1. Biddle, S. J. H., Pearson, N., Ross, G. M., & Braithwaite, R. (2010). Tracking of sedentary behaviours of young people: A systematic review. Preventive Medicine. https://doi.org/10.1016/j.ypmed.2010.07.018
2. Brenner, J. S., LaBella, C. R., Brooks, M. A., Diamond, A., Hennrikus, W., Weiss Kelly, A. K., et. al (2016). Sports specialization and intensive training in young athletes.Pediatrics. https://doi.org/10.1542/peds.2016-2148
3. Brenner, J. S., Small, E. W., Bernhardt, D. T., Congeni, J. A., Gomez, J. E., et. al (2007). Overuse injuries, overtraining, and burnout in child and adolescent athletes. Pediatrics. https://doi.org/10.1542/peds.2007-0887

4. Caruso, T. (2016). Early Sport Specialization Versus Diversification in Youth Athletes. NSCA.
5. Jayanthi, N., Pinkham, C., Dugas, L., Patrick, B., & LaBella, C. (2013). Sports Specialization in Young Athletes: Evidence-Based Recommendations. Sports Health. https://doi.org/10.1177/1941738112464626
6. Law, M. P., Côté, J., & Ericsson, K. A. (2007). Characteristics of expert development in rhythmic gymnastics: A retrospective study. International Journal of Sport and Exercise Psychology. https://doi.org/10.1080/1612197x.2008.9671814
7. Malina, R. M. (2010). Early sport specialization: Roots, effectiveness, risks. Current Sports Medicine Reports. https://doi.org/10.1249/JSR.0b013e3181fe3166
9. Micheo, W. Especialización deportiva temprana en tenis: Riesgos y Beneficios






https://www.usta.com/es/home/stay-current/caribbean/virginislands/early-sports-specialization-in-tennis--risks-and-benefits.html. Accessed December 1, 2019.
10. Sport for Life Society. What is physical literacy? CS4L Physical Literacy. Available at: http://www.physicalliteracy.ca/ what- is- physical- literacy. Accessed December 9, 2019
MSP SUPLEMENTO ESPECIAL Revista Puertorriqueña de Medicina y Salud Pública 27
1.
2.
3.
Revista Puertorriqueña de Medicina y Salud Pública 27 MSP SUPLEMENTO ESPECIAL Revista Puertorriqueña de Medicina y Salud Pública 57
“Diversas entidades y organizaciones atléticas han creado modelos de desarrollo para la especialización deportiva con la meta de establecer expectativas apropiadas de acuerdo con la edad del atleta y así reducir los efectos detrimentales de la especialización temprana”
For moderate to severe rheumatoid arthritis (RA) in adult TNFi-IR patients1

EXPECTATIONS
EXPECTATIONS
CHALLENGE TREATMENT GOALS IN RA WITH A ONCE-DAILY ORAL JAK INHIBITOR
CHALLENGE TREATMENT GOALS IN RA WITH A ONCE-DAILY ORAL JAK INHIBITOR
RINVOQ met primary (ACR20 or ACR50 at Week 12 or 14) and ranked secondary endpoints in clinical trials, with some patients achieving ACR20 as early as Week 1 in SELECT-BEYOND1-3,a,b
RINVOQ met primary (ACR20 or ACR50 at Week 12 or 14) and ranked secondary endpoints in clinical trials, with some patients achieving ACR20 as early as Week 1 in SELECT-BEYOND1-3,a,b
LONG-TERM REMISSION AND LOW DISEASE ACTIVITY DATA observed up to 84 weeks with or without MTX1,3-6
LONG-TERM REMISSION AND LOW DISEASE ACTIVITY DATA observed up to 84 weeks with or without MTX1,3-6
• DAS28-CRP<2.6* and DAS28-CRP≤3.2 evaluated at Week 12 or 14, with response rates from 60 to 84 weeks (in SELECT-BEYOND and SELECT-MONOTHERAPY, respectively)
Discover
• DAS28-CRP<2.6* and DAS28-CRP≤3.2 evaluated at Week 12 or 14, with response rates from 60 to 84 weeks (in SELECT-BEYOND and SELECT-MONOTHERAPY, respectively)
*Clinical remission does not mean drug-free remission or complete absence of disease activity.
Clinical remission does not mean drug-free remission or complete absence of disease activity.
LONG-TERM SAFETY DATA
AEs observed in long-term analysis with ~4.5 years maximum and ~2.6 years median exposure to RINVOQ 15 mg as of 6/30/207,a,b
LONG-TERM SAFETY DATA
AEs observed in long-term analysis with ~4.5 years maximum and ~2.6 years median exposure to RINVOQ 15 mg as of 6/30/207,a,b
• >4400 patients evaluated on upadacitinib,c with >7000 patientyears of long-term exposure to RINVOQ 15 mg as of 6/30/207,a,b
• >4400 patients evaluated on upadacitinib,c with >7000 patientyears of long-term exposure to RINVOQ 15 mg as of 6/30/207,a,b
our commitment to exceptional access and patient support at RinvoqHCPPR.com
Discover our commitment to






exceptional access and patient support at RinvoqHCPPR.com
a SELECT-EARLY (RA-I; MTX-naïve) [primary endpoint at Week 12: ACR50 response vs MTX, select ranked secondary endpoint at Week 24: Δ mTSS vs MTX]; SELECT-MONOTHERAPY (RA-II; MTX-IR) [primary endpoint at Week 14: ACR20 response vs MTX, select ranked secondary endpoints at Week 14: DAS28-CRP<2.6 vs MTX, DAS28-CRP≤3.2 vs MTX]; SELECT-NEXT (RA-III; csDMARD-IR) [RINVOQ + csDMARD; primary endpoint at Week 12: ACR20 response vs placebo + csDMARD]; SELECT-COMPARE (RA-IV; MTX-IR) [RINVOQ + MTX; primary endpoint at Week 12: ACR20 response vs placebo + MTX, select ranked secondary endpoints at Week 26: Δ mTSS vs placebo + MTX]; SELECT-BEYOND (RA-V; bDMARD-IR) [RINVOQ + csDMARD; primary endpoint at Week 12: ACR20 response vs placebo + csDMARD, select ranked secondary endpoints at Week 12: DAS28-CRP≤3.2 vs placebo + csDMARD.] 1,2 bSELECT-CHOICE (bDMARD-IR) [RINVOQ + csDMARDs; primary endpoint at Week 12: Δ DAS28-CRP (noninferiority) vs active comparator + csDMARDs]. 8
c RINVOQ 15 mg; upadacitinib 30 mg; RINVOQ 15 mg is the approved dose.1,7
INDICATION1
a SELECT-EARLY (RA-I; MTX-naïve) [primary endpoint at Week 12: ACR50 response vs MTX, select ranked secondary endpoint at Week 24: Δ mTSS vs MTX]; SELECT-MONOTHERAPY (RA-II; MTX-IR) [primary endpoint at Week 14: ACR20 response vs MTX, select ranked secondary endpoints at Week 14: DAS28-CRP<2.6 vs MTX, DAS28-CRP≤3.2 vs MTX]; SELECT-NEXT (RA-III; csDMARD-IR) [RINVOQ + csDMARD; primary endpoint at Week 12: ACR20 response vs placebo + csDMARD]; SELECT-COMPARE (RA-IV; MTX-IR) [RINVOQ + MTX; primary endpoint at Week 12: ACR20 response vs placebo + MTX, select ranked secondary endpoints at Week 26: Δ mTSS vs placebo + MTX]; SELECT-BEYOND (RA-V; bDMARD-IR) [RINVOQ + csDMARD; primary endpoint at Week 12: ACR20 response vs placebo + csDMARD, select ranked secondary endpoints at Week 12: DAS28-CRP≤3.2 vs placebo + csDMARD.] 1,2 bSELECT-CHOICE (bDMARD-IR) [RINVOQ + csDMARDs; primary endpoint at Week 12: Δ DAS28-CRP (noninferiority) vs active comparator + csDMARDs]. 8 c RINVOQ 15 mg; upadacitinib 30 mg; RINVOQ 15 mg is the approved dose.1,7
RINVOQ is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to one or more TNF blockers.
INDICATION1



RINVOQ is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to one or more TNF blockers.
Limitation of Use: Use of RINVOQ in combination with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants, such as azathioprine and cyclosporine, is not recommended.
Malignancies: Lymphoma and other malignancies have been observed in RINVOQ-treated patients. A higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]), lymphomas, and lung cancer (in current or past smokers) was observed with another JAK inhibitor when compared with TNF blockers in RA patients. Patients who are current or past smokers are at additional increased risk.
Malignancies: Lymphoma and other malignancies have been observed in RINVOQ-treated patients. A higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]), lymphomas, and lung cancer (in current or past smokers) was observed with another JAK inhibitor when compared with TNF blockers in RA patients. Patients who are current or past smokers are at additional increased risk.
SAFETY CONSIDERATIONS1
Limitation of Use: Use of RINVOQ in combination with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants, such as azathioprine and cyclosporine, is not recommended.
Major Adverse Cardiovascular Events: A higher rate of CV death, myocardial infarction, and stroke was observed with a JAK inhibitor in a study comparing another JAK inhibitor with TNF blockers in RA patients ≥50 years of age with at least one CV risk factor. Current or past smokers are at additional increased risk.
SAFETY CONSIDERATIONS1
Serious Infections: Patients treated with RINVOQ are at increased risk for developing serious infections that may lead to hospitalization or death. These infections include tuberculosis (TB), invasive fungal, bacterial, viral, and other infections due to opportunistic pathogens. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids.
Serious Infections: Patients treated with RINVOQ are at increased risk for developing serious infections that may lead to hospitalization or death. These infections include tuberculosis (TB), invasive fungal, bacterial, viral, and other infections due to opportunistic pathogens. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids.
Mortality: A higher rate of all-cause mortality, including sudden cardiovascular (CV) death, was observed with a Janus kinase (JAK) inhibitor in a study comparing another JAK inhibitor with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years of age with at least one CV risk factor.
Mortality: A higher rate of all-cause mortality, including sudden cardiovascular (CV) death, was observed with a Janus kinase (JAK) inhibitor in a study comparing another JAK inhibitor with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years of age with at least one CV risk factor.
Major Adverse Cardiovascular Events: A higher rate of CV death, myocardial infarction, and stroke was observed with a JAK inhibitor in a study comparing another JAK inhibitor with TNF blockers in RA patients ≥50 years of age with at least one CV risk factor. Current or past smokers are at additional increased risk.
Thrombosis: Thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated with JAK inhibitors used to treat inflammatory conditions. A higher rate of thrombosis was observed with another JAK inhibitor when compared with TNF blockers in RA patients.
Hypersensitivity: RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients.
Thrombosis: Thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated with JAK inhibitors used to treat inflammatory conditions. A higher rate of thrombosis was observed with another JAK inhibitor when compared with TNF blockers in RA patients.
Hypersensitivity: RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients.
Other Serious Adverse Reactions: Hypersensitivity Reactions (anaphylaxis and angioedema), Gastrointestinal Perforations, Laboratory Abnormalities (neutropenia, lymphopenia, anemia, lipid elevations, liver enzyme elevations), and Embryo-Fetal Toxicity.
Please see additional Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, on the previous page of this advertisement.
Other Serious Adverse Reactions: Hypersensitivity Reactions (anaphylaxis and angioedema), Gastrointestinal Perforations, Laboratory Abnormalities (neutropenia, lymphopenia, anemia, lipid elevations, liver enzyme elevations), and Embryo-Fetal Toxicity.
Please see Brief Summary of full Prescribing Information on previous pages of this advertisement.
ACR=American College of Rheumatology; AEs=adverse events; bDMARD=biologic DMARD; csDMARD=conventional synthetic DMARD; DAS28-CRP=Disease Activity Score 28 joints, C-reactive protein; DMARD=disease-modifying antirheumatic drug; HAQ-DI=Health Assessment
Please see additional Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, on the previous page of this advertisement. Please see Brief Summary of full Prescribing Information on previous pages of this advertisement.
Questionnaire Disability Index; IR=intolerance or inadequate response; JAK=Janus kinase; mTSS=modified total Sharp score; MTX=methotrexate; TNFi=tumor necrosis factor inhibitor.
ACR=American College of Rheumatology; AEs=adverse events; bDMARD=biologic DMARD; csDMARD=conventional synthetic DMARD; DAS28-CRP=Disease Activity Score 28 joints, C-reactive protein; DMARD=disease-modifying antirheumatic drug; HAQ-DI=Health Assessment Questionnaire Disability Index; IR=intolerance or inadequate response; JAK=Janus kinase; mTSS=modified total Sharp score; MTX=methotrexate; TNFi=tumor necrosis factor inhibitor.
58 Revista Puertorriqueña de Medicina y Salud Pública
RINVOQ® (RIN-VOKE) (upadacitinib) extended-release tablets, for oral use
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, and THROMBOSIS SERIOUS INFECTIONS
Patients treated with RINVOQ are at increased risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions, Adverse Reactions]. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids. If a serious infection develops, interrupt RINVOQ until the infection is controlled.
Reported infections include:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Patients should be tested for latent tuberculosis before RINVOQ use and during therapy. Treatment for latent infection should be considered prior to RINVOQ use.
• Invasive fungal infections, including cryptococcosis and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
The risks and benefits of treatment with RINVOQ should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with RINVOQ, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy [see Warnings and Precautions]
MORTALITY
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another Janus kinase (JAK) inhibitor to tumor necrosis factor (TNF) blockers, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor [see Warnings and Precautions]
MALIGNANCIES
Lymphoma and other malignancies have been observed in patients treated with RINVOQ. In RA patients treated with another JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions].
MAJOR ADVERSE CARDIOVASCULAR EVENTS
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke [see Warnings and Precautions].
THROMBOSIS
Thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated with JAK inhibitors used to treat inflammatory conditions. Many of these adverse events were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid RINVOQ in patients at risk. Patients with symptoms of thrombosis should discontinue RINVOQ and be promptly evaluated [see Warnings and Precautions]
INDICATIONS AND USAGE
Rheumatoid Arthritis
RINVOQ® is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to one or more TNF blockers.
• Limitations of Use: Use of RINVOQ in combination with other JAK inhibitors, biologic disease-modifying antirheumatic drugs (DMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine, is not recommended.
Psoriatic Arthritis
RINVOQ is indicated for the treatment of adults with active psoriatic arthritis who have had an inadequate response or intolerance to one or more TNF blockers.
• Limitations of Use: Use of RINVOQ in combination with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants such as azathioprine and cyclosporine, is not recommended.
Atopic Dermatitis
RINVOQ is indicated for the treatment of adults and pediatric patients
12 years of age and older with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.
• Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
Ulcerative Colitis
RINVOQ is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response or intolerance to one or more TNF blockers.
• Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biological therapies for ulcerative colitis, or with potent immunosuppressants such as azathioprine and cyclosporine.
Ankylosing Spondylitis
RINVOQ is indicated for the treatment of adults with active ankylosing spondylitis who have had an inadequate response or intolerance to one or more TNF blockers.
• Limitations of Use: Use of RINVOQ in combination with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants such as azathioprine and cyclosporine, is not recommended.
CONTRAINDICATIONS
PROFESSIONAL BRIEF SUMMARY
CONSULT PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients [see Warnings and Precautions].
WARNINGS AND PRECAUTIONS
Serious Infections
Serious and sometimes fatal infections have been reported in patients receiving RINVOQ. The most frequent serious infections reported with RINVOQ included pneumonia and cellulitis [see Adverse Reactions]. Among opportunistic infections, tuberculosis, multidermatomal herpes zoster, oral/esophageal candidiasis, and cryptococcosis, were reported with RINVOQ.
Avoid use of RINVOQ in patients with an active, serious infection, including localized infections. Consider the risks and benefits of treatment prior to initiating RINVOQ in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or
• with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with RINVOQ. Interrupt RINVOQ if a patient develops a serious or opportunistic infection.
A patient who develops a new infection during treatment with RINVOQ should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient; appropriate antimicrobial therapy should be initiated, the patient should be closely monitored, and RINVOQ should be interrupted if the patient is not responding to antimicrobial therapy. RINVOQ may be resumed once the infection is controlled.
Tuberculosis
Evaluate and test patients for latent and active tuberculosis (TB) infection prior to administration of RINVOQ. Patients with latent TB should be treated with standard antimycobacterial therapy before initiating RINVOQ. RINVOQ should not be given to patients with active TB. Consider anti-TB therapy prior to initiation of RINVOQ in patients with previously untreated latent TB or active TB in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent TB but who have risk factors for TB infection.
Consultation with a physician with expertise in the treatment of TB is recommended to aid in the decision about whether initiating anti-TB therapy is appropriate for an individual patient.
During RINVOQ use, monitor patients for the development of signs and symptoms of TB, including patients who tested negative for latent TB infection prior to initiating therapy.
Viral Reactivation

Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster) and hepatitis B virus reactivation, were reported in clinical trials with RINVOQ [see Adverse Reactions]. The risk of herpes zoster appears to be higher in patients treated with RINVOQ in Japan. If a patient develops herpes zoster, consider temporarily interrupting RINVOQ until the episode resolves. Screening for viral hepatitis and monitoring for reactivation should be performed in accordance with clinical guidelines before starting and during therapy with RINVOQ. Patients who were positive for hepatitis C antibody and hepatitis C virus RNA, were excluded from clinical trials. Patients who were positive for hepatitis B surface antigen or hepatitis B virus DNA were excluded from clinical trials. However, cases of hepatitis B reactivation were still reported in patients enrolled in the Phase 3 trials of RINVOQ. If hepatitis B virus DNA is detected while receiving RINVOQ, a liver specialist should be consulted.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ.
Malignancy and Lymphoproliferative Disorders
Malignancies, including lymphomas, were observed in clinical trials of RINVOQ [see Adverse Reactions]
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-Melanoma Skin Cancer
NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen.
Major Adverse Cardiovascular Events
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients
should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Thrombosis, including deep venous thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis, have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. Many of these adverse events were serious and some resulted in death.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers.
If symptoms of thrombosis occur, patients should discontinue RINVOQ and be evaluated promptly and treated appropriately. Avoid RINVOQ in patients that may be at increased risk of thrombosis.
Hypersensitivity Reactions
Serious hypersensitivity reactions such as anaphylaxis and angioedema were reported in patients receiving RINVOQ in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue RINVOQ and institute appropriate therapy [see Adverse Reactions].
Gastrointestinal Perforations
Gastrointestinal perforations have been reported in clinical trials with RINVOQ.
Monitor RINVOQ-treated patients who may be at risk for gastrointestinal perforation (e.g., patients with a history of diverticulitis or taking NSAIDs).
Evaluate promptly patients presenting with new onset abdominal pain for early identification of gastrointestinal perforation.
Laboratory Abnormalities
Neutropenia
Treatment with RINVOQ was associated with an increased incidence of neutropenia (ANC less than 1000 cells/mm3).
Evaluate neutrophil counts at baseline and thereafter according to routine patient management. Avoid RINVOQ initiation and interrupt RINVOQ treatment in patients with a low neutrophil count (i.e., ANC less than 1000 cells/mm3).
Lymphopenia
ALC less than 500 cells/mm3 were reported in RINVOQ-treated patients in clinical trials.
Evaluate lymphocyte counts at baseline and thereafter according to routine patient management. Avoid RINVOQ initiation or interrupt RINVOQ treatment in patients with a low lymphocyte count (i.e., less than 500 cells/mm3).
Anemia
Decreases in hemoglobin levels to less than 8 g/dL were reported in RINVOQ-treated patients in clinical trials.
Evaluate hemoglobin at baseline and thereafter according to routine patient management. Avoid RINVOQ initiation or interrupt RINVOQ treatment in patients with a low hemoglobin level (i.e., less than 8 g/dL).
Lipids
Treatment with RINVOQ was associated with increases in lipid parameters, including total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol [see Adverse Reactions]
Elevations in LDL cholesterol decreased to pre-treatment levels in response to statin therapy. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined.
Assess lipid parameters approximately 12 weeks after initiation of treatment, and thereafter according to the clinical guidelines for hyperlipidemia. Manage patients according to clinical guidelines for the management of hyperlipidemia.
Liver Enzyme Elevations
Treatment with RINVOQ was associated with increased incidence of liver enzyme elevations compared to treatment with placebo.
Evaluate liver enzymes at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury.
If increases in ALT or AST are observed during routine patient management and drug-induced liver injury is suspected, RINVOQ should be interrupted until this diagnosis is excluded.
Embryo-Fetal Toxicity
Based on findings in animal studies, RINVOQ may cause fetal harm when administered to a pregnant woman. Administration of upadacitinib to rats and rabbits during organogenesis caused increases in fetal malformations. Verify the pregnancy status of patients of reproductive potential prior to starting treatment. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception during treatment with RINVOQ and for 4 weeks following completion of therapy [see Use in
Specific Populations]
Vaccinations
Avoid use of live vaccines during, or immediately prior to, RINVOQ therapy. Prior to initiating RINVOQ, it is recommended that patients be brought up to date with all immunizations, including varicella zoster or prophylactic herpes zoster vaccinations, in agreement with current immunization guidelines.
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
• Serious Infections [see Warnings and Precautions]
• Mortality [see Warnings and Precautions]
• Malignancy and Lymphoproliferative Disorders [see Warnings and Precautions]
• Major Adverse Cardiovascular Events [see Warnings and Precautions]
• Thrombosis [see Warnings and Precautions]
• Hypersensitivity Reactions [see Warnings and Precautions]
• Gastrointestinal Perforations [see Warnings and Precautions]
• Laboratory Abnormalities [see Warnings and Precautions]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
60 Revista Puertorriqueña de Medicina y Salud Pública
20071734 Rinvoq PB-7.625 x 10.5(3.5).indd 1 04 May 2022 9:00 AM US-RNQR-220149
Adverse Reactions in Patients with Rheumatoid Arthritis
A total of 3833 patients with rheumatoid arthritis were treated with upadacitinib in the Phase 3 clinical trials of whom 2806 were exposed for at least one year.
Patients could advance or switch to RINVOQ 15 mg from placebo, or be rescued to RINVOQ from active comparator or placebo from as early as Week 12 depending on the trial design.
A total of 2630 patients received at least 1 dose of RINVOQ 15 mg, of whom 1860 were exposed for at least one year. In trials RA-I, RA-II, RA-III and RA-V, 1213 patients received at least 1 dose of RINVOQ 15 mg, of which 986 patients were exposed for at least one year, and 1203 patients received at least 1 dose of upadacitinib 30 mg, of which 946 were exposed for at least one year.
Table 1: Adverse Reactions Reported in ≥ 1% of Rheumatoid Arthritis
Patients Treated with RINVOQ 15 mg in Placebo-controlled Trials
treated with RINVOQ 15 mg monotherapy, and 4 patients (3.2 per 100 patient-years) treated with upadacitinib 30 mg monotherapy.
12-Month Exposure Dataset: Opportunistic infections were reported in 7 patients (0.6 per 100 patient-years) treated with RINVOQ 15 mg and 15 patients (1.4 per 100 patient-years) treated with upadacitinib 30 mg.
Malignancies
Placebo-controlled Trials: In RA-III, RA-IV, and RA-V, malignancies excluding NMSC were reported in 1 patient (0.4 per 100 patient-years) treated with placebo, and 1 patient (0.4 per 100 patient-years) treated with RINVOQ 15 mg. In RA-III and RA-V, malignancies excluding NMSC were reported in 0 patients treated with placebo, 1 patient (1.1 per 100 patient-years) treated with RINVOQ 15 mg, and 3 patients (3.5 per 100 patient-years) treated with upadacitinib 30 mg.
MTX-controlled Trials: Malignancies excluding NMSC were reported in 1 patient (0.8 per 100 patient-years) treated with MTX monotherapy, 3 patients (2.4 per 100 patient-years) treated with RINVOQ 15 mg monotherapy, and 0 patients treated with upadacitinib 30 mg monotherapy.
12-Month Exposure Dataset: Malignancies excluding NMSC were reported in 13 patients (1.2 per 100 patient-years) treated with RINVOQ 15 mg and 14 patients (1.3 per 100 patient-years) treated with upadacitinib 30 mg.
Gastrointestinal Perforations
Placebo-controlled Trials: There were no gastrointestinal perforations (based on medical review) reported in patients treated with placebo, RINVOQ 15 mg, and upadacitinib 30 mg.
and 0.7% of patients in the RINVOQ 15 mg and placebo groups, respectively. In RA-III and RA-V, decreases in lymphocyte counts below 500 cells/mm3 in at least one measurement occurred in 0.5% of patients treated with placebo, 0.5% of patients treated with RINVOQ 15 mg, and 2.4% of patients treated with upadacitinib 30 mg.
Anemia
In placebo-controlled trials (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, hemoglobin decreases below 8 g/dL in at least one measurement occurred in <0.1% of patients in both the RINVOQ 15 mg and placebo groups. In RA-III and RA-V, hemoglobin decreases below 8 g/dL in at least one measurement were observed in 0.3% of patients treated with placebo, and none in patients treated with RINVOQ 15 mg and upadacitinib 30 mg.
Adverse Reactions in Patients with Psoriatic Arthritis
A total of 1827 patients with psoriatic arthritis were treated with upadacitinib in clinical trials representing 1639.2 patient-years of exposure, of whom 722 were exposed to upadacitinib for at least one year. In the two Phase 3 trials, 907 patients received at least 1 dose of RINVOQ 15 mg, of whom 359 were exposed for at least one year.
Two placebo-controlled trials were integrated (640 patients on RINVOQ 15 mg once daily and 635 patients on placebo) to evaluate the safety of RINVOQ 15 mg in comparison to placebo for up to 24 weeks after treatment initiation.
*URTI includes: acute sinusitis, laryngitis, nasopharyngitis, oropharyngeal pain, pharyngitis, pharyngotonsillitis, rhinitis, sinusitis, tonsillitis, viral upper respiratory tract infection
Other adverse reactions reported in less than 1% of patients in the RINVOQ 15 mg group and at a higher rate than in the placebo group through Week 12 included pneumonia, herpes zoster, herpes simplex (includes oral herpes), and oral candidiasis.
Four integrated datasets are presented in the Specific Adverse Reaction section:
Placebo-controlled Trials: Trials RA-III, RA-IV, and RA-V were integrated to represent safety through 12/14 weeks for placebo (n=1042) and RINVOQ 15 mg (n=1035). Trials RA-III and RA-V were integrated to represent safety through 12 weeks for placebo (n=390), RINVOQ 15 mg (n=385), and upadacitinib 30 mg (n=384). Trial RA-IV did not include the 30 mg dose and, therefore, safety data for upadacitinib 30 mg can only be compared with placebo and RINVOQ 15 mg rates from pooling trials RA-III and RA-V.
MTX-controlled Trials: Trials RA-I and RA-II were integrated to represent safety through 12/14 weeks for MTX (n=530), RINVOQ 15 mg (n=534), and upadacitinib 30 mg (n=529).
12-Month Exposure Dataset: Trials RA-I, II, III, and V were integrated to represent the long-term safety of RINVOQ 15 mg (n=1213) and upadacitinib 30 mg (n=1203).
Exposure adjusted incidence rates were adjusted by trial for all the adverse events reported in this section.
Specific Adverse Reactions
Infections
Placebo-controlled Trials: In RA-III, RA-IV, and RA-V, infections were reported in 218 patients (95.7 per 100 patient-years) treated with placebo and 284 patients (127.8 per 100 patient-years) treated with RINVOQ 15 mg. In RA-III and RA-V, infections were reported in 99 patients (136.5 per 100 patient-years) treated with placebo, 118 patients (164.5 per 100 patient-years) treated with RINVOQ 15 mg, and 126 patients (180.3 per 100 patient-years) treated with upadacitinib 30 mg.
MTX-controlled Trials: Infections were reported in 127 patients (119.5 per 100 patient-years) treated with MTX monotherapy, 104 patients (91.8 per 100 patient-years) treated with RINVOQ 15 mg monotherapy, and 128 patients (115.1 per 100 patient-years) treated with upadacitinib 30 mg monotherapy.
12-Month Exposure Dataset: Infections were reported in 615 patients (83.8 per 100 patient-years) treated with RINVOQ 15 mg and 674 patients (99.7 per 100 patient-years) treated with upadacitinib 30 mg.
Serious Infections
Placebo-controlled Trials: In RA-III, RA-IV, and RA-V, serious infections were reported in 6 patients (2.3 per 100 patient-years) treated with placebo, and 12 patients (4.6 per 100 patient-years) treated with RINVOQ 15 mg. In RA-III and RA-V, serious infections were reported in 1 patient (1.2 per 100 patientyears) treated with placebo, 2 patients (2.3 per 100 patient-years) treated with RINVOQ 15 mg, and 7 patients (8.2 per 100 patient-years) treated with upadacitinib 30 mg.
MTX-controlled Trials: Serious infections were reported in 2 patients (1.6 per 100 patient-years) treated with MTX monotherapy, 3 patients (2.4 per 100 patient-years) treated with RINVOQ 15 mg monotherapy, and 8 patients (6.4 per 100 patient-years) treated with upadacitinib 30 mg monotherapy.
12-Month Exposure Dataset: Serious infections were reported in 38 patients (3.5 per 100 patient-years) treated with RINVOQ 15 mg and 59 patients (5.6 per 100 patient-years) treated with upadacitinib 30 mg.
The most frequently reported serious infections were pneumonia and cellulitis.
Tuberculosis
Placebo-controlled Trials and MTX-controlled Trials: In the placebo-controlled period, there were no active cases of tuberculosis reported in the placebo, RINVOQ 15 mg, and upadacitinib 30 mg groups. In the MTX-controlled period, there were no active cases of tuberculosis reported in the MTX monotherapy, RINVOQ 15 mg monotherapy, and upadacitinib 30 mg monotherapy groups.

12-Month Exposure Dataset: Active tuberculosis was reported for 2 patients treated with RINVOQ 15 mg and 1 patient treated with upadacitinib 30 mg.
Cases of extra-pulmonary tuberculosis were reported.
Opportunistic Infections (excluding tuberculosis)
Placebo-controlled Trials: In RA-III, RA-IV, and RA-V, opportunistic infections were reported in 3 patients (1.2 per 100 patient-years) treated with placebo, and 5 patients (1.9 per 100 patient-years) treated with RINVOQ 15 mg. In RA-III and RA-V, opportunistic infections were reported in 1 patient (1.2 per 100 patient-years) treated with placebo, 2 patients (2.3 per 100 patient-years) treated with RINVOQ 15 mg, and 6 patients (7.1 per 100 patient-years) treated with upadacitinib 30 mg.
MTX-controlled Trials: Opportunistic infections were reported in 1 patient (0.8 per 100 patient-years) treated with MTX monotherapy, 0 patients
MTX-controlled Trials: There were no cases of gastrointestinal perforations reported in the MTX and RINVOQ 15 mg group through 12/14 weeks. Two cases of gastrointestinal perforations were observed in the upadacitinib 30 mg group.
12-Month Exposure Dataset: Gastrointestinal perforations were reported in 1 patient treated with RINVOQ 15 mg and 4 patients treated with upadacitinib 30 mg.
Thrombosis
Placebo-controlled Trials: In RA-IV, venous thrombosis (pulmonary embolism or deep vein thrombosis) was observed in 1 patient treated with placebo and 1 patient treated with RINVOQ 15 mg. In RA-V, venous thrombosis was observed in 1 patient treated with RINVOQ 15 mg. There were no observed cases of venous thrombosis reported in RA-III. No cases of arterial thrombosis were observed through 12/14 weeks.
MTX-controlled Trials: In RA-II, venous thrombosis was observed in 0 patients treated with MTX monotherapy, 1 patient treated with RINVOQ 15 mg monotherapy and 0 patients treated with upadacitinib 30 mg monotherapy through Week 14. In RA-II, no cases of arterial thrombosis were observed through 12/14 weeks. In RA-I, venous thrombosis was observed in 1 patient treated with MTX, 0 patients treated with RINVOQ 15 mg and 1 patient treated with upadacitinib 30 mg through Week 24. In RA-I, arterial thrombosis was observed in 1 patient treated with upadacitinib 30 mg through Week 24.
12-Month Exposure Dataset: Venous thrombosis events were reported in 5 patients (0.5 per 100 patient-years) treated with RINVOQ 15 mg and 4 patients (0.4 per 100 patient-years) treated with upadacitinib 30 mg.
Arterial thrombosis events were reported in 0 patients treated with RINVOQ 15 mg and 2 patients (0.2 per 100 patient-years) treated with upadacitinib 30 mg.
Laboratory Abnormalities
Hepatic Transaminase Elevations
In placebo-controlled trials (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, alanine transaminase (ALT) and aspartate transaminase (AST) elevations ≥ 3 x upper limit of normal (ULN) in at least one measurement were observed in 2.1% and 1.5% of patients treated with RINVOQ 15 mg, and in 1.5% and 0.7% of patients treated with placebo, respectively. In RA-III and RA-V, ALT and AST elevations ≥ 3 x ULN in at least one measurement were observed in 0.8% and 1.0% of patients treated with RINVOQ 15 mg, 1.0% and 0% of patients treated with upadacitinib 30 mg and in 1.3% and 1.0% of patients treated with placebo, respectively.
In MTX-controlled trials, for up to 12/14 weeks, ALT and AST elevations ≥ 3 x ULN in at least one measurement were observed in 0.8% and 0.4% of patients treated with RINVOQ 15 mg, 1.7% and 1.3% of patients treated with upadacitinib 30 mg and in 1.9% and 0.9% of patients treated with MTX, respectively.
Lipid Elevations
Upadacitinib treatment was associated with dose-related increases in total cholesterol, triglycerides and LDL cholesterol. Upadacitinib was also associated with increases in HDL cholesterol. Elevations in LDL and HDL cholesterol peaked by Week 8 and remained stable thereafter. In controlled trials, for up to 12/14 weeks, changes from baseline in lipid parameters in patients treated with RINVOQ 15 mg and upadacitinib 30 mg, respectively, are summarized below:
• Mean LDL cholesterol increased by 14.81 mg/dL and 17.17 mg/dL.
• Mean HDL cholesterol increased by 8.16 mg/dL and 9.01 mg/dL.
• The mean LDL/HDL ratio remained stable.
• Mean triglycerides increased by 13.55 mg/dL and 14.44 mg/dL.
Creatine Phosphokinase Elevations
In placebo-controlled trials (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, dose-related increases in creatine phosphokinase (CPK) values were observed. CPK elevations > 5 x ULN were reported in 1.0%, and 0.3% of patients over 12/14 weeks in the RINVOQ 15 mg and placebo groups, respectively. Most elevations >5 x ULN were transient and did not require treatment discontinuation. In RA-III and RA-V, CPK elevations > 5 x ULN were observed in 0.3% of patients treated with placebo, 1.6% of patients treated with RINVOQ 15 mg, and none in patients treated with upadacitinib 30 mg.
Neutropenia
In placebo-controlled trials (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, dose-related decreases in neutrophil counts, below 1000 cells/mm3 in at least one measurement occurred in 1.1% and <0.1% of patients in the RINVOQ 15 mg and placebo groups, respectively. In RA-III and RA-V, decreases in neutrophil counts below 1000 cells/mm3 in at least one measurement occurred in 0.3% of patients treated with placebo, 1.3% of patients treated with RINVOQ 15 mg, and 2.4% of patients treated with upadacitinib 30 mg. In clinical trials, treatment was interrupted in response to ANC less than 1000 cells/mm3
Lymphopenia
In placebo-controlled trials (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, dose-related decreases in lymphocyte counts below 500 cells/mm3 in at least one measurement occurred in 0.9%
Overall, the safety profile observed in patients with active psoriatic arthritis treated with RINVOQ 15 mg was consistent with the safety profile observed in patients with rheumatoid arthritis. During the 24-week placebo-controlled period, the frequencies of herpes zoster and herpes simplex were ≥1% (1.1% and 1.4%, respectively) with RINVOQ 15 mg and 0.8% and 1.3%, respectively with placebo. A higher incidence of acne and bronchitis was also observed in patients treated with RINVOQ 15 mg (1.3% and 3.9%, respectively) compared to placebo (0.3% and 2.7%, respectively).
Adverse Reactions in Patients with Atopic Dermatitis
Three Phase 3 (AD-1, AD-2, and AD-3) and one Phase 2b (AD-4) randomized, double-blind, placebo-controlled, multicenter trials evaluated the safety of RINVOQ in patients with moderate-to-severe atopic dermatitis. The majority of patients were White (68%) and male (57%). The mean age was 34 years (ranged from 12 to 75 years) and 13% of the patients were 12 to less than 18 years. In these 4 trials, 2612 patients were treated with RINVOQ 15 mg or 30 mg orally once daily, with or without concomitant topical corticosteroids (TCS).
In the Phase 3 clinical trials (AD-1, AD-2, and AD-3), a total of 1239 patients received RINVOQ 15 mg, of whom 791 were exposed for at least one year and 1246 patients received RINVOQ 30 mg, of whom 826 were exposed for at least one year.
Trials AD-1, AD-2, and AD-4 compared the safety of RINVOQ monotherapy to placebo through Week 16. Trial AD-3 compared the safety of RINVOQ + TCS to placebo + TCS through Week 16.
Weeks 0 to 16 (Trials AD-1 to AD-4)
In RINVOQ trials with and without TCS (Trials AD-1, 2, 3 and 4) through Week 16, the proportion of patients who discontinued treatment because of adverse reactions in the RINVOQ 15 mg, 30 mg and placebo groups were 2.3%, 2.9% and 3.8%, respectively. Table 2 summarizes the adverse reactions that occurred at a rate of at least 1% in the RINVOQ 15 mg or 30 mg groups during the first 16 weeks of treatment.
Table 2: Adverse Reactions Reported in ≥ 1% of Patients with Atopic Dermatitis Treated with RINVOQ 15 mg or 30 mg
* Includes: laryngitis, laryngitis viral, nasopharyngitis, oropharyngeal pain, pharyngeal abscess, pharyngitis, pharyngitis streptococcal, pharyngotonsillitis, respiratory tract infection, respiratory tract infection viral, rhinitis, rhinolaryngitis, sinusitis, tonsillitis, tonsillitis bacterial, upper respiratory tract infection, viral pharyngitis, viral upper respiratory tract infection
** Includes: acne and dermatitis acneiform
*** Includes: genital herpes, genital herpes simplex, herpes dermatitis, herpes ophthalmic, herpes simplex, nasal herpes, ophthalmic herpes simplex, herpes virus infection, oral herpes
**** Includes anaphylactic reaction, anaphylactic shock, angioedema, dermatitis exfoliative generalized, drug hypersensitivity, eyelid oedema, face oedema, hypersensitivity, periorbital swelling, pharyngeal swelling, swelling face, toxic skin eruption, type I hypersensitivity, urticaria
***** Includes abdominal pain and abdominal pain upper
****** Includes herpes zoster and varicella
Revista Puertorriqueña de Medicina y Salud Pública 61
Adverse Reaction Placebo RINVOQ 15 mg n=1042 (%) n=1035 (%) Upper respiratory tract infection (URTI)* 9.5 13.5 Nausea 2.2 3.5 Cough 1.0 2.2 Pyrexia 0 1.2
Adverse Reaction Placebo RINVOQ 15 mg RINVOQ 30 mg n=902 (%) n=899 (%) n=906 (%) Upper respiratory tract infection (URTI)* 17 23 25 Acne** 2 10 16 Herpes simplex*** 2 4 8 Headache 4 6 6 Increased blood creatine phosphokinase 2 5 6 Cough 1 3 3 Hypersensitivity**** 2 2 3 Folliculitis 1 2 3 Nausea 1 3 3 Abdominal pain***** 1 3 2 Pyrexia 1 2 2 Increased Weight 1 2 2 Herpes zoster****** 1 2 2 Influenza <1 2 2 Fatigue 1 1 2 Neutropenia <1 1 2 Myalgia 1 1 2 Influenza like illness 1 1 2
20071734 Rinvoq PB-7.625 x 10.5(3.5).indd 2 04 May 2022 9:00 AM US-RNQR-220149
Other adverse reactions reported in less than 1% of patients in the RINVOQ 15 mg and/or 30 mg group and at a higher rate than in the placebo group through Week 16 included anemia, oral candidiasis, pneumonia, and the adverse event of retinal detachment.
The safety profile of RINVOQ through Week 52 was generally consistent with the safety profile observed at Week 16.
Overall, the safety profile observed in patients with AD treated with RINVOQ was similar to the safety profile in patients with RA. Other specific adverse reactions that were reported in patients with AD included eczema herpeticum/Kaposi’s varicelliform eruption.
Eczema Herpeticum/Kaposi’s Varicelliform Eruption
Placebo-controlled Period (16 weeks): Eczema herpeticum was reported in 4 patients (1.6 per 100 patient-years) treated with placebo, 6 patients (2.2 per 100 patient-years) treated with RINVOQ 15 mg and 7 patients (2.6 per 100 patient-years) treated with RINVOQ 30 mg.
12-Month Exposure (Weeks 0 to 52): Eczema herpeticum was reported in 18 patients (1.6 per 100 patient-years) treated with RINVOQ 15 mg and 17 patients (1.5 per 100 patient-years) treated with RINVOQ 30 mg.
Adverse Reactions in Patients with Ulcerative Colitis
RINVOQ was studied up to 8 weeks in patients with moderately to severely active ulcerative colitis in two randomized, double-blind, placebo-controlled induction studies (UC-1, UC-2) and a randomized, double-blind, placebo controlled, dose-finding study (UC-4; NCT02819635). Long term safety up to 52-weeks was evaluated in patients who responded to induction therapy in a randomized, double-blind, placebo-controlled maintenance study (UC-3) and a long-term extension study.
In the two induction studies (UC-1, UC-2) and a dose finding study (UC-4), 1097 patients were enrolled of whom 719 patients received RINVOQ 45 mg once daily.
In the maintenance study (UC-3), 746 patients were enrolled of whom 250 patients received RINVOQ 15 mg once daily and 251 patients received RINVOQ 30 mg once daily.
Adverse reactions reported in ≥2% of patients in any treatment arm in the induction and maintenance studies are shown in Tables 3 and 4, respectively.
Table 3. Adverse Reactions Reported in ≥2% of Patients with Ulcerative Colitis Treated with RINVOQ 45 mg in Placebo-Controlled Induction Studies (UC-1, UC-2 and UC-4)
Specific Adverse Reactions
Serious Infections
Induction Studies: In UC-1, UC-2, and UC-4, serious infections were reported in 5 patients (8.4 per 100 patient-years) treated with placebo and 9 patients (8.4 per 100 patient-years) treated with RINVOQ 45 mg through 8 weeks. Placebo-controlled Maintenance Study: In UC-3, serious infections were reported in 8 patients (6.3 per 100 patient-years) treated with placebo, 8 patients (4.5 per 100 patient-years) treated with RINVOQ 15 mg, and 6 patients (3.1 per 100 patient-years) treated with RINVOQ 30 mg through 52 weeks.
Laboratory Abnormalities
Hepatic Transaminase Elevations
In studies UC-1, UC-2, and UC-4, elevations of ALT to ≥ 3 x ULN in at least one measurement were observed in 1.5% of patients treated with RINVOQ 45 mg, and 0% of patients treated with placebo for 8 weeks. AST elevations to ≥ 3 x ULN occurred in 1.5% of patients treated with RINVOQ 45 mg, and 0.3% of patients treated with placebo. Elevations of ALT to ≥ 5 x ULN occurred in 0.4% of patients treated with RINVOQ 45 mg and 0% of patients treated with placebo.
In UC-3, elevations of ALT to ≥ 3 x ULN in at least one measurement were observed in 4% of patients treated with RINVOQ 30 mg, 2% of patients treated with RINVOQ 15 mg, and 0.8% of patients treated with placebo for 52 weeks. Elevations of AST to ≥ 3 x ULN in at least one measurement were observed in 2% of patients treated with RINVOQ 30 mg, 1.6% of patients treated with RINVOQ 15 mg and 0.4% of patients treated with placebo. Elevations of ALT to ≥ 5 x ULN were observed in 0.8% of patients treated with 30 mg, 0.4% of patients treated with 15 mg, and 0.4% of patients treated with placebo.
Overall, laboratory abnormalities observed in patients with ulcerative colitis treated with RINVOQ were similar to those described in patients with RA.
Adverse Reactions in Patients with Ankylosing Spondylitis
A total of 596 patients with ankylosing spondylitis were treated with RINVOQ 15 mg in the two clinical trials representing 577.3 patient-years of exposure, of whom 228 were exposed to RINVOQ 15 mg for at least one year.
Overall, the safety profile observed in patients with active ankylosing spondylitis treated with RINVOQ 15 mg was consistent with the safety profile observed in patients with rheumatoid arthritis and psoriatic arthritis.
During the 14-week placebo-controlled period in Trial AS-I, the frequency of headache was 5.4% with RINVOQ 15 mg and 2.1% with placebo. During the 14-week placebo-controlled period in Trial AS-II, the frequency of headache was 3.3% with RINVOQ 15 mg and 1.4% with placebo.
DRUG INTERACTIONS
Strong CYP3A4 Inhibitors
Upadacitinib exposure is increased when RINVOQ is co-administered with a strong CYP3A4 inhibitor (such as ketoconazole and clarithromycin), which may increase the risk of RINVOQ adverse reactions. Monitor patients closely for adverse reactions when co-administering RINVOQ 15 mg once daily with strong CYP3A4 inhibitors.
For patients with atopic dermatitis, coadministration of RINVOQ 30 mg once daily with strong CYP3A4 inhibitors is not recommended. For patients with ulcerative colitis taking strong CYP3A4 inhibitors, reduce the RINVOQ induction dosage to 30 mg once daily. The recommended maintenance dosage is 15 mg once daily.
Strong CYP3A4 Inducers
15 mg dose, 0.9 times the 30 mg dose, and 0.6 times the MRHD (on an AUC basis at maternal oral doses of 5 mg/kg/day and higher). Additional skeletal malformations (bent forelimbs/hindlimbs and rib/vertebral defects) and decreased fetal body weights were observed in the absence of maternal toxicity at an exposure approximately 84 times the 15 mg dose, 43 times the 30 mg dose, and 31 times the MRHD (on an AUC basis at a maternal oral dose of 75 mg/kg/day).
In a second oral embryo-fetal development study, pregnant rats received upadacitinib at doses of 1.5 and 4 mg/kg/day during the period of organogenesis from gestation day 6 to 17. Upadacitinib was teratogenic (skeletal malformations that included bent humerus and scapula) at exposures approximately 1.6 times the 15 mg dose, 0.8 times the 30 mg dose, and 0.6 times the MRHD (on an AUC basis at maternal oral doses of 4 mg/kg/day). No developmental toxicity was observed in rats at an exposure approximately 0.29 times the 15 mg dose, 0.15 times the 30 mg dose, and 0.11 times the MRHD (on an AUC basis at a maternal oral dose of 1.5 mg/kg/day).
In an oral embryo-fetal developmental study, pregnant rabbits received upadacitinib at doses of 2.5, 10, and 25 mg/kg/day during the period of organogenesis from gestation day 7 to 19. Embryolethality, decreased fetal body weights, and cardiovascular malformations were observed in the presence of maternal toxicity at an exposure approximately 15 times the 15 mg dose, 7.6 times the 30 mg dose, and 5.6 times the MRHD (on an AUC basis at a maternal oral dose of 25 mg/kg/day). Embryolethality consisted of increased post-implantation loss that was due to elevated incidences of both total and early resorptions. No developmental toxicity was observed in rabbits at an exposure approximately 2.2 times the 15 mg dose, 1.1 times the 30 mg dose, and 0.82 times the MRHD (on an AUC basis at a maternal oral dose of 10 mg/kg/day).
In an oral pre- and post-natal development study, pregnant female rats received upadacitinib at doses of 2.5, 5, and 10 mg/kg/day from gestation day 6 through lactation day 20. No maternal or developmental toxicity was observed in either mothers or offspring, respectively, at an exposure approximately 3 times the 15 mg dose, 1.4 times the 30 mg dose, and at approximately the same exposure as the MRHD (on an AUC basis at a maternal oral dose of 10 mg/kg/day).
Lactation
Risk Summary
There are no data on the presence of upadacitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Available pharmacodynamic/toxicological data in animals have shown excretion of upadacitinib in milk (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the potential for serious adverse reactions in the breastfed infant, advise patients that breastfeeding is not recommended during treatment with RINVOQ, and for 6 days (approximately 10 half-lives) after the last dose.
Data
A single oral dose of 10 mg/kg radiolabeled upadacitinib was administered to lactating female Sprague-Dawley rats on post-partum days 7-8. Drug exposure was approximately 30-fold greater in milk than in maternal plasma based on AUC0-t values. Approximately 97% of drug-related material in milk was parent drug.
Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to starting treatment with RINVOQ [see Use in Specific Populations]
* Composed of several similar terms
** Elevated liver enzymes composed of elevated ALT, AST, GGT, ALP, liver transaminases, hepatic enzymes, bilirubin, drug-induced liver injury and cholestasis.
Other adverse reactions reported in less than 2% of patients in the RINVOQ 45 mg group and at a higher rate than in the placebo group through Week 8 included herpes zoster and pneumonia.
Table 4. Adverse Reactions Reported in ≥2% of Patients with Ulcerative Colitis Treated with RINVOQ 15 mg or 30 mg in the Placebo-Controlled Maintenance Study (UC-3)1
Upadacitinib exposure is decreased when RINVOQ is co-administered with strong CYP3A4 inducers (such as rifampin), which may lead to reduced therapeutic effect of RINVOQ. Coadministration of RINVOQ with strong CYP3A4 inducers is not recommended.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Available data from the pharmacovigilance safety database and postmarketing case reports on use of RINVOQ in pregnant women are not sufficient to evaluate a drug-associated risk for major birth defects or miscarriage. Based on animal studies, RINVOQ has the potential to adversely affect a developing fetus. Advise patients of reproductive potential and pregnant patients of the potential risk to the fetus.
In animal embryo-fetal development studies, oral upadacitinib administration to pregnant rats and rabbits at exposures equal to or greater than approximately 1.6 and 15 times the 15 mg dose, 0.8 and 7.6 times the 30 mg dose, and 0.6 and 5.6 times the maximum recommended human dose (MRHD) of 45 mg (on an AUC basis) resulted in dose-related increases in skeletal malformations (rats only), an increased incidence of cardiovascular malformations (rabbits only), increased post-implantation loss (rabbits only), and decreased fetal body weights in both rats and rabbits. No developmental toxicity was observed in pregnant rats and rabbits treated with oral upadacitinib during organogenesis at exposures approximately 0.29 and 2.2 times the 15 mg dose, 0.15 times and 1.1 times the 30 mg dose, and at 0.11 and 0.82 times the MHRD (on an AUC basis).
In a pre- and post-natal development study in pregnant female rats, oral upadacitinib administration at exposures approximately 3 times the 15 mg dose, 1.4 times the 30 mg dose, and the same as the MRHD (on an AUC basis) resulted in no maternal or developmental toxicity (see Data)
The background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages are 2-4% and 15-20%, respectively.
Report pregnancies to the AbbVie Inc.’s Adverse Event reporting line at 1-888-633-9110, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
1 Patients who were responders to 8 weeks induction therapy with RINVOQ 45 mg once daily
* Composed of several similar terms
** Elevated liver enzymes composed of elevated ALT, AST, GGT, ALP, liver transaminases, hepatic enzymes, bilirubin, drug-induced liver injury, and cholestasis.
The safety profile of RINVOQ in the long-term extension study was similar to the safety profile observed in the placebo-controlled induction and maintenance periods.
Overall, the safety profile observed in patients with ulcerative colitis treated with RINVOQ was generally similar to the safety profile in patients with RA and AD.
Published data suggest that increased disease activity is associated with the risk of developing adverse pregnancy outcomes in women with rheumatoid arthritis or ulcerative colitis. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.
Data
Animal Data
In an oral embryo-fetal development study, pregnant rats received upadacitinib at doses of 5, 25, and 75 mg/kg/day during the period of organogenesis from gestation day 6 to 17. Upadacitinib was teratogenic (skeletal malformations that consisted of misshapen humerus and bent scapula) at exposures equal to or greater than approximately 1.7 times the
Contraception
Females
Based on animal studies, upadacitinib may cause embryo-fetal harm when administered to pregnant women [see Use in Specific Populations]. Advise female patients of reproductive potential to use effective contraception during treatment with RINVOQ and for 4 weeks after the final dose.
Pediatric Use
Juvenile Idiopathic Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis

The safety and effectiveness of RINVOQ in pediatric patients with juvenile idiopathic arthritis, psoriatic arthritis, and ankylosing spondylitis have not been established.
Atopic Dermatitis
The safety and effectiveness of RINVOQ in pediatric patients 12 years of age and older weighing at least 40 kg with atopic dermatitis have been established. A total of 344 pediatric patients aged 12 to 17 years with moderate to severe atopic dermatitis were randomized across three trials (AD-1, AD-2 and AD-3) to receive either RINVOQ 15 mg (N=114) or 30 mg (N=114) or matching placebo (N=116) in monotherapy or combination with topical corticosteroids. Efficacy was consistent between the pediatric patients and adults. The adverse reaction profile in the pediatric patients was similar to the adults [see Adverse Reactions].
The safety and effectiveness of RINVOQ in pediatric patients less than 12 years of age with atopic dermatitis have not been established.
Ulcerative Colitis
The safety and effectiveness of RINVOQ in pediatric patients with ulcerative colitis have not been established.
Geriatric Use
Rheumatoid Arthritis and Psoriatic Arthritis
Of the 4381 patients treated in the five clinical trials, a total of 906 rheumatoid arthritis patients were 65 years of age or older, including 146 patients 75 years and older. Of the 1827 patients treated in the two psoriatic arthritis Phase 3 clinical trials, a total of 274 patients were 65 years of age or older, including 34 patients 75 years and older. No differences in effectiveness were observed between these patients and younger patients; however, there was a higher rate of overall adverse events, including serious infections, in patients 65 years of age and older.
Atopic Dermatitis
Of the 2583 patients treated in the three Phase 3 clinical trials, a total of 120 patients with atopic dermatitis were 65 years of age or older, including 6 patients 75 years of age. No differences in effectiveness were observed between these patients and younger patients; however, there was a higher rate of serious infections and malignancies in those patients 65 years of age or older in the 30 mg dosing group in the long-term trials.
Ulcerative Colitis
Of the 1097 patients treated in the controlled clinical trials, a total of 95 patients with ulcerative colitis were 65 years and older. Clinical studies of RINVOQ did not include sufficient numbers of patients 65 years of age and older with ulcerative colitis to determine whether they respond differently from younger adult patients.
62 Revista Puertorriqueña de Medicina y Salud Pública
Adverse Reaction Placebo RINVOQ 45 mg Once Daily N= 378 (%) N = 719 (%) Upper respiratory tract infection* 7 9 Acne* 1 6 Increased blood creatine phosphokinase 1 5 Neutropenia* <1 5 Rash* 1 4 Elevated liver enzymes** 2 3 Lymphopenia* 1 3 Folliculitis 1 2 Herpes simplex* <1 2
Adverse Reaction Placebo RINVOQ 15 mg Once Daily RINVOQ 30 mg Once Daily n = 245 (%) n = 250 (%) n = 251 (%) Upper respiratory tract infection* 18 16 20 Increased blood creatine phosphokinase 2 6 8 Neutropenia* 2 3 6 Elevated liver enzymes** 1 6 4 Rash* 4 5 5 Herpes zoster 0 4 4 Folliculitis 2 2 4 Hypercholesterolemia* 1 2 4 Influenza 1 3 3 Herpes simplex* 1 2 3 Lymphopenia* 2 3 2 Hyperlipidemia* 0 2
2
20071734 Rinvoq PB-7.625 x 10.5(3.5).indd 3 04 May 2022 9:00 AM US-RNQR-220149
Renal Impairment
For patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, no dosage adjustment is needed in patients with mild (eGFR 60 to < 90 mL/min/1.73 m2), moderate (eGFR 30 to < 60 mL/min/1.73 m2), or severe renal impairment (eGFR 15 to < 30 mL/min/1.73 m2).
For patients with atopic dermatitis, the maximum recommended dosage is 15 mg once daily for patients with severe renal impairment. No dosage adjustment is needed in patients with mild or moderate renal impairment. For patients with ulcerative colitis, the recommended dosage for severe renal impairment is 30 mg once daily for induction and 15 mg once daily for maintenance. No dosage adjustment is needed in patients with mild or moderate renal impairment.
RINVOQ has not been studied in patients with end stage renal disease (eGFR <15 mL/min/1.73m2). Use in patients with atopic dermatitis or ulcerative colitis with end stage renal disease is not recommended. Hepatic Impairment
The use of RINVOQ has not been studied in patients with severe hepatic impairment (Child Pugh C), and therefore not recommended for use in patients with rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, ulcerative colitis, or ankylosing spondylitis.
For patients with rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, and ankylosing spondylitis, no dosage adjustment is needed in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. For patients with ulcerative colitis, the recommended dosage for mild to moderate hepatic impairment is 30 mg once daily for induction and 15 mg once daily for maintenance
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Serious Infections
Inform patients that they may be more likely to develop infections when taking RINVOQ. Instruct patients to contact their healthcare provider immediately during treatment if they develop any signs or symptoms of an infection [see Warnings and Precautions]
Advise patients that the risk of herpes zoster is increased in patients taking RINVOQ and in some cases can be serious [see Warnings and Precautions]
Malignancies
Inform patients that RINVOQ may increase their risk of certain cancers and that periodic skin examinations should be performed while using RINVOQ. Advise patients that exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions]
Major Adverse Cardiovascular Events
Inform patients that RINVOQ may increase their risk of major adverse cardiovascular events (MACE) including myocardial infarction, stroke,
and cardiovascular death. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions]
Thrombosis
Inform patients that events of deep venous thrombosis and pulmonary embolism have been reported in clinical trials with RINVOQ. Instruct patients to seek immediate medical attention if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions]
Hypersensitivity Reactions
Advise patients to discontinue RINVOQ and seek immediate medical attention if they develop any signs and symptoms of allergic reactions [see Warnings and Precautions].
Gastrointestinal Perforations
Inform patients that gastrointestinal perforations have been reported in clinical trials with RINVOQ and that risk factors include the use of NSAIDS or history of diverticulitis. Instruct patients to seek medical care immediately if they experience new onset of abdominal pain, fever, chills, nausea, or vomiting [see Warnings and Precautions].
Retinal Detachment

Inform patients that retinal detachment has been reported in clinical trials with RINVOQ. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision while receiving RINVOQ [see Adverse Reactions]
Laboratory Abnormalities
Inform patients that RINVOQ may affect certain lab tests, and that blood tests are required before and during RINVOQ treatment [see Warnings and Precautions]
Vaccinations
Advise patients to avoid use of live vaccines with RINVOQ. Instruct patients to inform their healthcare practitioner that they are taking RINVOQ prior to a potential vaccination [see Warnings and Precautions] Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential that exposure to RINVOQ during pregnancy may result in fetal harm. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions and Use in Specific Populations]
Advise females of reproductive potential that effective contraception should be used during treatment and for 4 weeks following the final dose of upadacitinib [see Use in Specific Populations]
Advise females patients who are exposed to RINVOQ during pregnancy to contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Lactation
Advise women not to breastfeed during treatment with RINVOQ and for 6 days after the last dose [see Use in Specific Populations]
Administration Advise patients not to chew, crush, or split RINVOQ tablets. Manufactured by: AbbVie Inc., North Chicago, IL 60064, USA
RINVOQ® is a registered trademark of AbbVie Biotechnology Ltd.
©2019-2022 AbbVie Inc.
Ref: 20071734 Revised: April 2022
LAB-7082 MASTER
US-RNQR-220149
Revista Puertorriqueña de Medicina y Salud Pública 63
20071734 Rinvoq PB-7.625 x 10.5(3.5).indd 4 04 May 2022 9:00 AM US-RNQR-220149
EL CUIDADO ORAL VA MÁS ALLÁ DE NUESTRA BOCA
Al preguntarle a un paciente sobre cómo está su salud o qué hace para mantenerse saludable, no siempre hace referencia a su estado de salud oral. Esto puede entenderse por el pensamiento desligado del paciente de que el cuidado oral no forma parte de la salud general del individuo. La Asociación Dental Americana (ADA) ha establecido una estrecha relación entre la pobre higiene oral y algunas condiciones sistémicas, como enfermedades cardiovasculares, diabetes, accidentes cerebrovasculares y hasta complicaciones en el embarazo. Por esto cobra vital importancia educar a la población para que conozca que cuidar su salud oral para prevenir enfermedades periodontales es esencial para disfrutar de una buena salud.
IMPORTANCIA DE LA HIGIENE ORAL
Una buena salud oral va desde cepillarse los dientes, al menos dos veces al día, utilizar hilo dental y tener una buena alimentación, hasta la modificación de conductas que aumentan los riesgos de desarrollar enfermedades periodontales, y otras enfermedades sistémicas, como lo es el fumar o mascar tabaco. Igualmente, parte del cuidado oral es visitar al dentista regularmente según le sea recomendado, de acuerdo
La Asociación Dental Americana, Sociedad Española de Neurología (SEN), la Sociedad Española de Periodoncia y Osteointegración (SEPA) y la Asociación Americana de Diabetes han publicado estudios e investigaciones sobre los riesgos de la enfermedad periodontal con otras condiciones de salud y las recomendaciones para el manejo en la clínica dental u oficina médica:
• Accidentes cerebrovasculares. Un paciente con periodontitis tiene un riesgo de 2.8 veces las posibilidades de sufrir un accidente cerebrovascular isquémico. Se recomienda a los médicos dialogar con su paciente sobre integrar la higiene oral como parte un estilo de vida saludable para mejorar sus resultados de salud.
• Enfermedad de Alzheimer.
con su plan de tratamiento, y hablar con su médico sobre cualquier cambio o molestia en su boca o encías. Esto ayudará al paciente a recibir tratamientos oportunamente y mantener en buen estado su salud oral y salud general.
CONEXIÓN ENTRE LA HIGIENE ORAL Y LA SALUD GENERAL
De acuerdo con la literatura de la Asociación Dental Americana (ADA) y otras organizaciones, existe una relación estrecha entre enfermedades orales y la salud general, aun cuando es un tema que se está investigando continuamente porque no se conocen las causas específicas. Tan es así que se recomienda que en pacientes que visitan al dentista y son diagnosticados con enfermedad periodontal, su médico debe validar si cuentan con un cernimiento adecuado de riesgos de condiciones cardiovasculares. Así sucede también en pacientes con descontrol en sus niveles glicémicos, donde el médico debe asegurarse de que tengan una evaluación con un dentista para evitar otras complicaciones de salud, incluyendo el deterioro de su salud oral y el desarrollo de enfermedades periodontales.
La enfermedad periodontal se asocia con un riesgo de 1.7 veces mayor a este tipo de demencia y el riesgo pude triplicarse en pacientes con enfermedad periodontal más grave. Los estudios también ponen de manifiesto que las personas con periodontitis obtienen pobres resultados en pruebas neuropsicológicas de función cognitiva.
• Embarazo. Debido a los cambios hormonales en las mujeres embarazadas, estas pueden sufrir de gingivitis (enfermedad en las encías en etapa temprana), lo que puede convertirse, eventualmente, en periodontitis, de acuerdo con la ADA. Algunos síntomas durante el embarazo, como las náuseas y vómitos, pueden provocar la pérdida del esmalte del diente, lo que se conoce como erosión dental, y que pudiera llevar a la extracción
de uno o varios dientes. La mayoría de los tratamientos dentales son seguros para las pacientes embarazadas, por lo que visitar el dentista y realizar un plan de tratamiento, de ser necesario, no debería poner en riesgo el embarazo.
• Infarto agudo al miocardio. Un estudio reciente de la Universidad de Michigan revela que pacientes que habían sufrido un infarto agudo al miocardio y no habían recibido tratamiento dental tuvieron un mayor número de días de hospitalización en comparación con los que habían recibido tratamiento de salud oral. Estos que recibieron tratamiento tuvieron menos días de hospitalización y más visitas de seguimiento.3 El estudio sugiere que pacientes con riesgo de sufrir infartos se beneficiarían de tener su condición periodontal estable.

64 Revista Puertorriqueña de Medicina y Salud Pública
• Diabetes. La relación de la diabetes con la salud oral es una bidireccional. La Asociación Americana de Diabetes establece que el paciente con diabetes tiene un riesgo mayor de desarrollar gingivitis y si esta no se trata adecuadamente, eventualmente, podría convertirse en periodontitis. Las personas con diabetes son más propensas a desarrollar resequedad en la boca y propiciar la acumulación de bacterias, aumentando el riego de enfermedad periodontal. A su vez los pacientes diabéticos con periodontitis tienen mayor riesgo de complicaciones sistémicas debido a la dificultad de mantener los niveles glicémicos controlados. Sin embargo, los expertos aseguran que un cuidado oral adecuado y la evaluación periódica reduce el riesgo de complicaciones.
La ADA continúa apoyando y fomentando la investigación sobre tratamientos adecuados entre dentistas y otros componentes del equipo multidisciplinario para ayudar a identificar otras enfermedades sistémicas que se sospecha tienen una relación con la salud oral.
REFERENCIAS
1. “Medical-Dental Integration Emphasizes Mouth-Body Connection.” American Dental Association, adanews.ada.org/ ada-news/2022/october/medical-dentalintegration-emphasizes-mouth-bodyconnection/. Accessed 13 July 2023.
2. Martínez, Carla Nieto. “Are Periodontitis, Stroke, and Alzheimer’s Disease Linked?” Medscape, 15 June 2023, www.medscape.com/ viewarticle/993282?icd=login_success_ email_match_norm.
3. Romesh P. Nalliah DDS, et al. “Association between Periodontal Care and Hospitalization with Acute Myocardial
Dra. Wanda Nazario

Administradora de Servicios Dentales de MCS
ESENCIAL EL TRABAJO EN EQUIPO Y LA EDUCACIÓN
La integración del equipo multidisciplinario que brinda servicios al paciente, incluyendo los de servicios dentales y servicios médicos, es imprescindible para alcanzar los mejores resultados de salud en un paciente. Igualmente, la educación al paciente sobre los hábitos de higiene oral diarios en el hogar y las visitas periódicas al dentista son primordiales para reducir los factores de riesgo y complicaciones en estos pacientes.
Esto va a la par con avances tecnológicos que permiten que los procedimientos dentales sean más fáciles de tolerar y la experiencia del paciente al visitar el dentista sea una más positiva. Además, estos también consideran una variedad de artículos y recomendaciones para el cuidado oral en casa, ayudando a un cuidado más accesible y continuo.
En la medida en que todos los componentes del equipo de cuidado, incluyendo al paciente, trabajen unidos para promover la higiene oral, lograremos una sociedad más saludable. Es responsabilidad de todos educar a la población sobre lo fácil y beneficioso que es tener una salud oral en óptimas condiciones para disfrutar de Salud Completa.
Infarction.” The Journal of the American Dental Association, 20 Apr. 2022, www. sciencedirect.com/science/article/abs/ pii/S0002817722000873.
4. ADA News, ADA releases new clinical practice guideline on caries restorations. https://adanews.ada.org/ ada-news/2023/june/ada-releasesnew-clinical-practice-guideline-on-cariesrestorations/
5. Pregnancy and Oral Health - the Journal of the American Dental Association, jada. ada.org/article/S0002-8177(21)000179/fulltext. Accessed 13 July 2023.

6. “Oral Health.” Diabetes and Oral Health | ADA, diabetes.org/diabetes/keepingyour-mouth-healthy. Accessed 13 July 2023.
7. Paunica I, Giurgiu M, Dumitriu AS, Paunica S, Pantea Stoian AM, Martu MA, Serafinceanu C. The Bidirectional Relationship between Periodontal Disease and Diabetes Mellitus-A Review. Diagnostics (Basel). 2023 Feb 11;13(4):681. doi: 10.3390/diagnostics13040681. PMID: 36832168; PMCID: PMC9954907.
Revista Puertorriqueña de Medicina y Salud Pública 65





Revista Puertorriqueña de Medicina y Salud Pública 67
AGENDA MÉDICA 2023
EVENTOS Y CONVENCIONES
www.medicinaysaludpublica.com
Puerto Rico
Fecha Actividad Lugar Contacto TIPO DE EVENTO
15 al 18 de marzo de 2023
19 de marzo de 2023
30 de marzo de 2023
1 al 2 de abril de 2023
21 al 22 de abril de 2023
21 al 22 de abril de 2023
28 al 29 de abril de 2023
28 al 30 de abril de 2023
20 de mayo de 2023
26 al 29 de mayo de 2023
26 al 29 de mayo de 202
Julio 2023
11 al 13 de agosto de 2023
11 al 13 de agosto de 2023
12 al 13 de agosto de 2023
Convención anual #49 de la Academia Médica del Sur & Casa del Médico
Nephrology Symposium for Primary Physicians 2023
Convención anual de PIA
Academia de Patología y Medicina de Laboratorio de Puerto Rico
Convención de Médicos de Familia
Convención anual American College of Cardiology- PR Chapter
Convención anual Sociedad Radiológica de PR
Sociedad de Enfermedades Infecciosas de PR (SEINPR)
Caribbean Breast SymposiumSociedad Puertorriqueña de Senología
Ponce Hilton Hotel & Casino Ponce, PR
Antonio’s Restaurant, Condado, PR
Academia Médica del Sur 787-987-8301 / 787-843-0610 academiamedicadelsur@gmail.com
Business Planners - Merna Morales 787-645-9914 bplanner21@gmail.com
Convención
Simposio
Sheraton Puerto Rico Hotel & Casino, San Juan Virginia de los Reyes Convención
Caribe Hilton Hotel San Juan, PR
Serra & Serra 787 406-4571 / 787 640-5776 patologospr@serrayserra.com
Marriot Resort, San Juan, Stelaris Casino, Condado, Puerto Rico Nelly Feliciano
Royal Sonesta San Juan Carolina, PR Aixa Vélez 787-649-7681 / genteinc@gmail.com
Sheraton Convention Center Hotel San Juan, PR
Embassy Isla Verde
Embassy Suites, Isla Verde, Puerto Rico
Convención Sociedad Puertorriqueña de Oftalmología El Conquistador Resort, Fajardo, Puerto Rico
Convención Semi anual Sociedad Puertorriqueña de Endocrinología y Diabetología (SPED)
Convención anual Sociedad Puertorriqueña de Cardiología
Convención Colegio de Optómetras de Puerto Rico
13ma Convención Anual de la Academia de Médicos Generalistas y Primarios de PR
Convención anual Sociedad de Cirujanos Vasculares y Endovasculares de PR
17 al 19 de agosto de 2023 Convención Sociedad de Médicos Podiatras

19 de agosto de 2023
Convención anual de la Asociación de Gastroenterología y Hepatología Pediátrica de PR
19 de agosto de 2023 2023 Excellence in GI Nursing - Sociedad Puertorriqueña de Asistentes de Gastroenterología
23 al 24 de agosto de 2023
Convención Sociedad Puertorriqueña de Nefrología
Ponce Hilton Hotel & Casino Ponce, PR
Hyatt Regency Grand Reserve PR Río Grande, PR
Serra & Serra 787 406-4571 / 787 640-5776 info@socrad.com / info@serrayserra.com
Enid Rivera (804) 774-6326 / enidrm27@gmail.com
Priscilla González 787-615-6796 sociedadsenologapr@gmail.com
Rivs Marketing 787-294-9185 , 787-409-6497 rinavega@rivsmarketing.com
Educational Partners & Coaching 787-646-0780 perez.vilma@gmail.com
Sociedad Puertorriqueña de Cardiología 787-620-2228 socprcardio@gmail.com
Ponce Hilton Hotel & Casino Ponce, PR info@colegiooptometraspr.com
Hotel Sheraton Convention Center, San Juan, PR
La Concha Hotel, San Juan, P.R.
El Convento Hotel, San Juan, PR
Royal Sonesta SJ Hotel, Carolina, PR
Hotel La Concha, San Juan, PR
Royal Sonesta SJ Hotel, Carolina, PR
SR Consultants & Events 939-292-4115 / 787-649-2367 srconsultants&events@gmail.com
AMEC amec@amec-pr.com 787-289-8989

Aixa Vélez genteinc@gmail.com 787-649-7681
IC Planners ivettecolon@icplannerspr.com 787-504-3655
Rivs Marketing 787-548-0047 / info@rivsmarketing.com
Business Planners- Merna Morales 787-645-9914 bplanner21@gmail.com
Convención
Convención
Convención
Convención
Convención
Simposio
Convención
Convención
Convención
Convención
Convención
Convención
Convención
Convención
Convención
68 Revista Puertorriqueña de Medicina y Salud Pública
ACTIVIDADES ESTÁN SUJETAS A CAMBIO
AGENDA MÉDICA 2023
EVENTOS Y CONVENCIONES
www.medicinaysaludpublica.com
24 al 27 de agosto de 2023
25 al 27 de agosto de 2023
26 de agosto de 2023
26 de agosto de 2023
2 al 4 de septiembre de 2023
8 al 9 de septiembre de 2023
22 al 24 de septiembre de 2023
23 de septiembre de 2023
Septiembre 2023
6 al 9 de octubre 2023
Convención anual del Colegio de Farmacéuticos de PR
Convención Sociedad Puertorriqueña de Neurología
Simposio de Alergistas e Immunólogos del Oeste - Asociación Puertorriqueña Médicos Alergistas
Educación Continua - Congreso de Psiquiatras de PR“Mental Health: New Frontiers of Hope”
Convención Anual de la Asociación de Médicos Pediatras de la Región Oeste (AMPRO)
2023 Caribbean Congress on Emergency Medicine - Colegio de Emergenciólogos de Puerto Rico
Congreso Coalición de Asma y otras Condiciones Respiraciones Crónicas de PR
Clinical Gastroenterology and Hepatology Update for Primary Care Physicians - Asociación Puertorriqueña de Gastroenterología

Convención Sociedad Puertorriqueña de Cardiología Intervencional
Convención anual de la Asociación de Hematología y Oncología Médica de PR (AHOMPR)
14 de octubre de 2023 Hot Topics in Internal Medicine - Hospital Metropolitano Dr. Susoni & Pavia Arecibo Medical Faculty Convention
18 al 20 de octubre de 2023
26 al 29 de octubre de 2023
Convención annual de la Asociación de Hospitales de PR
Sheraton Convention Center, San Juan, PR
Fairmont El San Juan Hotel, San Juan, PR
Rincón beach resort, PR
Royal Sonesta SJ Hotel, Carolina, PR
Mayagüez Resort & Casino, Mayagüez, PR
Royal Sonesta Hotel, Isla Verde, PR
Sheraton, San Juan, PR
Sheraton Convention Center, San Juan, PR
Fairmont El San Juan Hotel, 6063 Av. Isla Verde
Ponce Hilton
Embassy Dorado del Mar, Dorado, PR
Sheraton Convention Center Hotel San Juan, PR
Caribe Gyn Ponce Hilton
26 al 28 de octubre de 2023 Convención anual Puerto Rico Urological Association (PRUA)
28 de octubre de 2023 Congreso anual Asociación Puertorriqueña de Médicos Alergistas
28 de octubre de 2023 40ma Conferencia Epilepsia del Caribe - Sociedad Puertorriqueña de Epilepsia
3 al 5 de noviembre de 2023
3 al 5 de noviembre de 2023
10 al 12 noviembre de 2023
15 al 18 de noviembre de 2023
17 al 19 de noviembre de 2023
18 de noviembre de 2023
Diciembre 2023
Diciembre 2023
Puerto Rico HIV Treaters Medical Association Annual Convention
Convención Sociedad de Cirugía Plástica de PR
Convención annual de la Asociación de Médicos Pediatras de la Región Este (AMPRE)
XIII Congreso Asoc Latinoamericana de Nefrología Pediátrica (ALANEPE)
Convención Anual Sociedad Puertorriqueña de Neumología
Improving Elderly Patient Care & 2023 Clinical Vignettes & Research Competition - American College of Physicians

Sheraton Convention Center HotelSan Juan, PR
Sheraton Convention Center Hotel San Juan, PR
Embassy Suites, Isla Verde, PR
Embassy Suites, Dorado, PR
Hyatt Regency Grand Reserve PR Río Grande, PR
Hotel Marriott Condado
Centro de Convenciones Gobernador Pedro Roselló González, San Juan, PR
Royal Sonesta SJ Hotel, Carolina, PR
Sheraton Convention Center, San Juan, PR
Convención Anual SPED Por confirmar
Convención anual Colegio de Médicos Cirujanos de PR Por confirmar
Colegio de Farmacéuticos de PR 787-753-7157
AMEC 787-289-8989 - amec@amec-pr.com
IC Planners ivettecolon@icplannerspr.com 787-504-3655
Japri Planners - 787-612-6775 cmejapri@hotmail.com/japriplanners@hotmail.com
AMPRO ampropediatras@gmail.com
Rivs Marketing 787-548-0047 / info@rivsmarketing.com
IC Planners ivettecolon@icplannerspr.com / 787-504-3655
Rivs Marketing 787-548-0047 / info@rivsmarketing.com
Enid Rivera (804) 774-6326 enidrm27@gmail.com
Germaine Quiñones ahomprgq@gmail.com 787-608-1477
Rivs Marketing - 787-548-0047 info@rivsmarketing.com
Business Planners- Merna Morales 787-645-9914 bplanner21@gmail.com
Germaine Quiñones ahomprgq@gmail.com
Puerto Rico
Convención
Convención
Simposio
Convención
Congreso
Congreso
Convención
Convención
Convención
Convención
787-608-1477 Convención
Aixa Vélez genteinc@gmail.com 787-649-7681
IC Planners ivettecolon@icplannerspr.com 787-504-3655
Sociedad Puertorriqueña de Epilepsia info@sociedadepilepsiapr.org
787-782-6200
HIV Treaters Medical Association
787-646-0780
Educational Partners/Vilma Pérez
Aixa Vélez genteinc@gmail.com787-649-7681
Business Planners- Merna Morales 787-645-9914 bplanner21@gmail.com
www.alanepe.org + 56 2 3251 4970 info@alanepe.org
Business Planners- Merna Morales 787-645-9914 bplanner21@gmail.com
Rivs Marketing - 787-548-0047 info@rivsmarketing.com
Educational Partners & Coaching 787-646-0780 perez.vilma@gmail.com
Colegio de Médicos Cirujanos de PR 787-751-5979 info@colegiomedicopr.org
Convención
Congreso
Conferencia
Convención
Convención
Convención
Congreso
Convención
Convención
Convención
ACTIVIDADES ESTÁN SUJETAS A CAMBIO
Revista Puertorriqueña de Medicina y Salud Pública 69
Fecha Actividad Lugar Contacto TIPO DE EVENTO
Primer encuentro entre pacientes, profesionales de la salud y el Gobierno de Puerto Rico: Foro Salud en Tus Manos


La RevistaMSP estrenó este año el primer encuentro que reunió a pacientes, Gobierno, y profesionales de la salud, donde emplearon un diálogo abierto en torno a los desafíos y necesidades de la población puertorriqueña para el acceso de servicios en salud.

Entre los temas discutidos estuvieron: recertificación de la cubierta médica, autorizaciones de tratamientos, paridad en salud y más.
Conoce más en www.revistamsp.com y reviva parte del encuentro a través de la siguiente fotogalería.
26 de agosto de 2023 en San Juan, PR
El mismo ensambló los esfuerzos para lograr un sistema de salud en Puerto Rico que salvaguarde la inclusión y la accesibilidad a los servicios de salud.
Los participantes del Foro Salud en tus Manos se beneficiaron de conocer de que anteriormente, el programa Medicaid estaba en un 85% del Federal Poverty Level (FPL), sin embargo, recientemente el gobierno federal decidió ampliarlo en su totalidad para que más personas puedan acceder a un plan médico. Foto: Revista Medicina y Salud Pública.
Dentro del conversatorio se dio a conocer de que en el año 2023, Puerto Rico recibió una asignación federal de 19 billones de dólares que ha permitido favorecer la administración del programa de Medicare en la isla. Foto: Revista Medicina y Salud Pública.

70 Revista Puertorriqueña de Medicina y Salud Pública
MUNDO
MSP /
DIGITAL
Pedro Lugo, Vicepresidente de Grupo Editorial Mundo; Lilliam Rodríguez, directora ejecutiva de VOCESPR; Lcdo. Esdras Vélez, PM Unwiding del Programa Medicaid de Puerto Rico; Dinorah Collazo, Directora del programa Medicaid Departamento de Salud de Puerto Rico; Roxanna Rosario, directora interina de la Administración de Seguros de Salud de Puerto Rico (ASES); Jorge Rivera Nieves e Isamari Castrodad, moderadores. Foto: Revista Medicina y Salud Pública.
De izquierda a derecha: Yadira Torres, representante de la Asociación Puertorriqueña de Ayuda al Paciente de Psoriasis; Nelson González, presidente de la Asociación Puertorriqueña de Ayuda al Paciente de Psoriasis; Lilliam Rodríguez, directora ejecutiva de VOCES; Licenciado Esdras Vélez, PM Unwiding del Programa Medicaid de Puerto Rico y la Licenciada Dinorah Collazo, Directora del programa Medicaid Departamento de Salud de Puerto Rico. Foto: Revista MSP






Lilliam Rodríguez, directora ejecutiva de VOCES: Licenciada Dinorah Collazo, Directora del programa Medicaid Departamento de Salud de Puerto Rico; Rodrigo Rueda de Vivero y Dereck Figueroa, brands manager de AbbVie Puerto Rico y Pedro Lugo, videpresidente de Grupo Editorial Mundo. Foto: Revista MSP
Dra. Marisel Bosques, presidenta de los HIV Treaters y Dra. Carmen Zorrilla, ginecóloga obstetra, decana interina de Investigación del Recinto de Ciencias Médicas, durante el turno de preguntas. Fotos: Revista MSP
Más de 40 organizaciones de pacientes y profesionales de la salud se dieron cita a la Fundación Luis Muñoz Marín y el primer Foro: Salud en tus Manos. Fotos: Revista MSP.
La Dra. Alma Cruz, dermatóloga y catedrática del Recinto de Ciencias Médicas, habló en beneficio de los pacientes con hidradenitis supurativa en Puerto Rico y la necesidad de la aprobación de los tratamientos para esta población. Foto: Revista de Medicina y Salud Pública.

Dr. Bolivar Arboleda, Director, Caribbean Breast Surgery Presidente, Sociedad Puertorriqueña de Senología, habló sobre la importancia del apoyo expedito a las cubiertas de las pacientes con cáncer de seno. Foto: Revista MSP.
Pacientes, profesionales de la salud y gobierno se dieron cita en la Fundación Luis Muñoz Marín.

Àngela Díaz, de Nutriendo PR y quien dirigía el Consejo Renal de Puerto Rico, abogó por la necesidad de acceso para pacientes con diálisis en Puerto Rico. Foto: Revista de Medicina y Salud Pública

Revista Puertorriqueña de Medicina y Salud Pública 71 MSP / REVISTAMSP.COM
Visita Nuestro Mundo Digital
Web - Podcast - Video

Visita nuestra plataforma web, redes sociales y podcast ahora también disponibles en iTunes y Spotify, Suscríbete y no te pierdas el mejor contenido de Medicina y Salud Pública
SE CELEBRÓ LA TERCERA EDICIÓN
DE PROTEGE TU PIEL PR
25 DE AGOSTO DE 2023 EN PONCE, PUERTO RICO
Fue un encuentro exitoso en donde el nuevo programa de residencia en dermatología se unió a esta iniciativa para abordar sobre las enfermedades de la piel y la evolución en sus tratamientos.

En vanguardia la disciplina de gastroenterología pediátrica
Sábado, 19 de agosto de 2023 en San Juan
Durante el simposio se discutieron las condiciones gastrointestinales en la población pediátrica más prevalentes y recientes.
Transcurrió la convención anual de la Academia de Médicos Generalistas y Primarios
11 al 13 de agosto de 2023 en San Juan, PR


El evento reunió a un sinnúmero de profesionales especializados en distintas áreas de la medicina.
Exitoso simposio sobre patologías vasculares y endovasculares
Sociedad de Cirujanos Vasculares y Endovasculares de PR 12 al 13 de agosto en San Juan, PR
Se enfatizó la importancia de difundir la educación no tan solo a los cirujanos vasculares sino a los médicos primarios sobre el manejo de estas enfermedades.
Transcurrió la convención anual del colegiado de farmacéuticos
Convención anual Colegio de Farmacéuticos de Puerto Rico 23 al 27 de agosto de 2023
El Colegio de Farmacéuticos de Puerto Rico celebró sus 85 años de fundación durante su convención anual en donde se abordaron temas relacionados a los variados servicios y roles farmacéuticos.


72 Revista Puertorriqueña de Medicina y Salud Pública
www.medicinaysaludpublica.com
@revistaMSP @revistaMSP @revistaMSP
Dr. Andrés J. Fantauzzi; Dra. Reina M. González; Dra. Lillian V. Rivera; y Dr. Lorna M. Torres. Foto: Revista Medicina y Salud Pública.
Dra. Chiara Biaggi, gastroenteróloga pediátrica y Dr. Leonardo Hormaza, presidente de la Asociación de Gastroenterología y Hepatología Pediátrica de Puerto Rico. Foto: Revista Medicina y Salud Pública.
Dra. Suasy Acevedo, farmacéutica y la Lcda. Idalia Bonilla, presidenta del Colegio de Farmacéuticos de Puerto Rico. Foto: Revista Medicina y Salud Pública.
Dr. José Figueroa Figueroa, presidente de la Academia de Médicos Generalistas y Primarios de Puerto Rico. Foto: Revista Medicina y Salud Pública.
Dr. Gabriel Pereira, cirujano vascular y endovascular y el Dr. Rafael Santini, presidente de la Sociedad de Cirugía Vascular y Endovascular de Puerto Rico. Foto: Revista Medicina y Salud Pública.
Fructífera la convención anual de la Sociedad Puertorriqueña de Nefrología


Convención anual Sociedad Puertorriqueña de Nefrología 23 al 26 de agosto de 2023
Se abordaron los avances científicos en la disciplina de nefrología y la salud renal.
Exitoso el segundo simposio de alergistas e inmunólogos del oeste
Asociación Puertorriqueña de Médicos Alergistas

Cumbre de inmunólogos, alergistas, médicos primarios, entre otras especialidades se reunieron para dialogar sobre las actualizaciones en las condiciones vistas en el campo.
Exitoso congreso de educación continua de los psiquiatras
Sociedad Puertorriqueña de Neurología
25 al 27 de agosto de 2023

Especialistas y profesionales de la salud en el área de neurología se reunieron para abordar las más recientes actualizaciones de las condiciones prevalentes en la población.
Academia de Psiquiatría de Puerto Rico 26 de agosto de 2023 en San Juan, PR
En el mismo se hizo hincapié en la importancia del abordaje de la salud mental como un problema de salud pública.

Revista Puertorriqueña de Medicina y Salud Pública 73 MSP / MUNDO DIGITAL
“Jornadas Neurológicas 2023”: Exitosa la convención anual
Dr. Josué Castresana, presidente de la Sociedad de Nefrología de Puerto Rico. Foto: Revista Medicina y Salud Pública.
Dr. Anardi Agosto Mujica, presidente de la Asociación Puertorriqueña de Médicos Alergistas. Foto: Revista Medicina y Salud Pública.
Dr. Edgardo Prieto, presidente de la Academia de Psiquiatría de Puerto Rico Foto: Revista Medicina y Salud Pública.
Dra. Franchesca Fiorito, neuróloga, subespecialista en dolores de cabeza y presidenta de la Academia Puertorriqueña de Neurología. Foto: Revista Medicina y Salud Pública.
UROLOGÍA SIN LÍMITES: LA CARRERA DEL DR. RUIZ DEYÁ
Desde Ponce protagoniza una de las más destacadas prácticas en urología, una de las carreras médicas más necesitadas en el País en cuanto a acceso y el número de pacientes con condiciones que ameritan estos especialistas, según han recalcado durante los últimos cinco años distintos expertos de dicho campo.
El Dr. Gilberto Ruiz Deyá, también un apasionado de la academia desde las aulas de la Ponce Health Science School, ejerce una práctica desde el Centro Médico Episcopal San Lucas de Ponce, que incluye cirugía urológica mínimamente invasiva, oncología quirúrgica, adrenalectomía laparoscópica, nefrectomía laparoscópica, entre otros servicios.

Su pasión en el campo lo ha llevado desde plantear una problemática que da apertura a una solución. El especialista ha sido enfático en la problemática que enfrenta el País, ya que la mayoría de sus colegas son médicos mayores de 65 años, lo que pudiera provocar una disminución de estos profesionales de la salud en la isla durante los próximos años.
Por tal razón, en exclusiva a la Revista MSP, este año anunció que gracias a un convenio entre la academia y la institución hospitalaria desde donde ejerce, se inició el primer programa de residencia en urología, con dos médicos residentes, totalmente acreditados.
DR. RUIZ DEYÁ

Esos no son los únicos desafíos que con total placer el médico puertorriqueño ha reseñado, sino que también ha dado a conocer distintos casos clínicos que han culminado con éxito dentro de su práctica, incluyendo aneurismas renales en jóvenes.
Sobre el impacto de los estilos de vida en el cáncer de próstata, la importancia del examen para esta condición, tratamiento a tiempo de la incontinencia urinaria, la implementación de cernimientos urológicos mediante cirugía y más, el Dr. Ruiz Deyá ha escrito páginas en la historia del rescate y desarrollo de esta práctica desde el sur de la isla.
Por tal razón, la Revista MSP dedica su A Ciencia Cierta a este gran puertorriqueño que ha utilizado su talento para devolverle la salud a cientos de pacientes.
74 Revista Puertorriqueña de Medicina y Salud Pública MSP / A CIENCIA CIERTA
Urólogo especializado en procesos laparoscópicos.



Revista Puertorriqueña de Medicina y Salud Pública 75 Nuestro compromiso con la educación y prevención de enfermedades cardiovasculares continua en beneficio de nuestros socios y pacientes. 787-620-2228 www.cardiologíapr.org @SocPRCardio




~
 Presidente
Presidente

























































 Yue Sai Jao
Yue Sai Jao














































