Chemistry Woman in











Say something about yourself and the changes in you, brought by the courseduringthequarter.

More of a math person, I was not excited for Chemistry. Or, generally, this science-filled year as a whole. I knew that this would be harder for me. I’ve been raised in a STEM environment, so I was fully prepared to go and ask for help since Day 1.
General Chemistry 1 impacted this. In the past two months I’ve been exposed to more chemistry than ever, it has been one big experiment.
As my journey has been a whole experiment, there are a few things that I’ve learned and changed in myself. First and foremost, this quarter emphasized that I am truly a challenge seeker. Undoubtedly, chemistry is challenging. Facing this subject head-on, despite all the obstacles it has brought, only solidified that I strive in and for challenge. For instance, when the laboratory report was given, I was intimidated. I felt that it was always lacking. This did not lead me into a breakdown, this only made me work on it non-stop. I made sure, together with my partner, that there weren’t any loopholes Given this, I also learned that I’ve grown to be stronger and I am continuously becoming stronger. If these tasks were handed to me in Grade 11, I would’ve cried about it. Now, I feel that this course changed me in a way that it pushed me to something unfamiliar. It brought me out of my comfort zone to the point that I wanted to do things on my own and not ask help in order for me to fully learn.
Moreover, I also used to be very grade conscious. I was studying for the wrong reasons, ones that harmed my mental state. I was previously a student who cared about nothing else but her grades, setting the highest standard for herself at all times. And, I was accustomed to always being able to reach this. But, this course was a humbling one. It taught me to value learning more than grades. With this, I was able to really set my focus into studying and fully grasping the lessons handed. With making my learning value more, I was able to accept whatever grades or mistakes I would make, while still striving to be the best. I would treat these as lessons for me to learn further; thus, valuing my learnings more. The perfect example was midterms. I knew it was going to be almost impossible to get an E but I still did my best. I studied a lot and in the end I would’ve gotten a P. Upon receiving the results, I




Include a test item from a formative or summative assessment that you are proudof.Explainwhytheitemissomethingworthytoshare.
The first challenge I faced was the Hot Air Balloon. This was a question asked as an extended homework in the lesson about gases. It asked: “In a hot air balloon, how do gas variables such as pressure, volume, temperature, density, and altitude affect each other? Please limit your answer to 3 sentences only.” To that I answered,
“In the whole process, temperature affects most of the variables, especially because the air pumped into the balloon is heated (increased temperature), which also increases the pressure inside the hot air balloon and the volume which causes its inflation. When the temperature is increased, density (because density is also related to volume; density = mass/volume) is decreased so that the air inside the hot air balloon is less than the air outside, which causes the whole hot air balloon to rise. As the hot air balloon rises, it increases its altitude while the pressure decreases inside and outside the hot air balloon; this means that they need to increase temperature to increase pressure inside the balloon, stay inflated, and rise or maintain their altitude.”
With uncertainty and the desire for clarity, I commented: "Hi sir! I was supposed to add this extra information I researched to my answer:
‘Aside from that, since higher altitude means lower pressure, because the air is heated and the relationship of pressure and temperature, it also rises as a result of there being greater pressure (higher temperature, higher pressure) inside the hot air balloon than outside at that certain altitude creating an upward force (then, increasing altitude); if they wish to maintain that altitude, temperature should also be mainted, but if they want to go down, temperature should be decreased which would also decrease the pressure and volume while increasing density causing its deflation.’
However, I was not sure so I didn't include it. I just want to know if i understood it correctly and I hope you can clarify it with me. Thank you, Sir!”



Incorporate a question from lesson content, home tasks, or any assessment that you made a mistake on. Describe why you failed to arrive at the correct answer.Now,youmuststatethecorrectanswertothatquestion.
For many, hearing Mayon Volcano is a trigger for trauma. This was the subject of the question in the midterms that I got completely wrong on. It was a question about total gas and partial pressure. At the moment I was answering, I just put a completely random solution to prevent me from failing. I poured all my knowledge out for that one question in the last few minutes.
Even then, I was incorrect. Looking back, there were major factors which, if they were avoided, would’ve turned the situation completely. Time pressure was a key factor. This to me was the main concern which led to the other aspects. Time pressure made me overlook the question and the simplicity of it. It made me panic so much that I don’t think I was even able to fully read and comprehend the question. This confused me a lot and I got lost which prolonged my process more. I think it was also a factor that we haven’t encountered a problem like this before, nor did I try to even look at similar problems.
Because of this, In asking for the partial pressure, I thought that we would have to go the long way in which it would require us to use the formula. When in fact, all I needed to do was to equate the sum of the partial pressures, including the unknown as a variable, to 1 atm which was given already. Likewise, seeing the atomic weight intimidated me, and led me into a panic. This is because I recall going over this in the reviewer but it was not fully discussed. I think that it was also the lack of perseverance and determination. When I saw the time, I already knew that I wouldn’t get this item correctly so I almost just wrote random things for it. If I were able to budget my time wisely among the different items and made myself familiar with them on my own, this would have been an easy item to handle. A new “Mayon Volcano” problem will arise, but I will now learn from experience to persevere through it.






Focus on the onsite laboratory activity of the course. Elaborate on scientific skills or attitudes you have acquired and practiced along the way. Narrate whyithasmadeyouaproudSTEMpersoninthe
All mixed up. For this laboratory experiment, patience was heavily practiced. For the most part of this experiment, we just have to perform the initial step to get things going. For instance, in filtration we could not do as much to it once we had poured it into the filtration system. We tried tapping it for the process to be quicker but it didn’t help as much and only messed up our set up. Following this, we also had to be patient in the evaporation process. Here, I realized that patience is key in experiments. If in filtration this may have helped the tiniest bit, in evaporation we could not do anything. Hence, we had to be patient and just wait for the solution to evaporate completely and leave the salt crystals.
True Colors. This second laboratory activity helped me acquire the skill of observing. Here we are required to use our sight to observe and record the color shown of each. I believe that, if not acquired, this scientific skill was strengthened because of the difficulty there is in identifying the color of the flame. This stems from the fact that only part of the flame will reveal the color of the chemical At the same time, it will not last long This was a problem mostly with the color yellow and orange; as well as the red at times. Due to this, we had to keep repeating the first few samples. Once we got the hang of it, we could easily observe what colors would. Thus, our observing skills were truly enhanced as we paid close attention to the flame.
Furthermore, for these two experiments, I developed the skill of interpreting and communicating. In the first activity, I really had to deep dive into it in order to aid Hannah in developing the data and results. Here I helped her in adding the necessary tables to support our statements, thus practicing the skill of interpreting. With this, the skill of communicating was also employed as we had to talk to each other regarding the contents. Similarly, this skill was applied as we made the laboratory report. Interpreting and communicating were also skills that we enriched in our second activity, even without the laboratory report. During this, we had to interpret the color we were seeing, and communicate through the worksheet and with each other if we agreed on the color we were seeing. More so, we also had to interpret the mystery chemical by using the flame color to identify what chemical it is.



Look for a short TV or movie clip that details or explains a wrong chemistry concept and idea. Apply what you have learned in the course to explain why the information is erroneous. Further, present a short correct solution oralternatescientific-basednotiontoresolvetheinaccuracy.
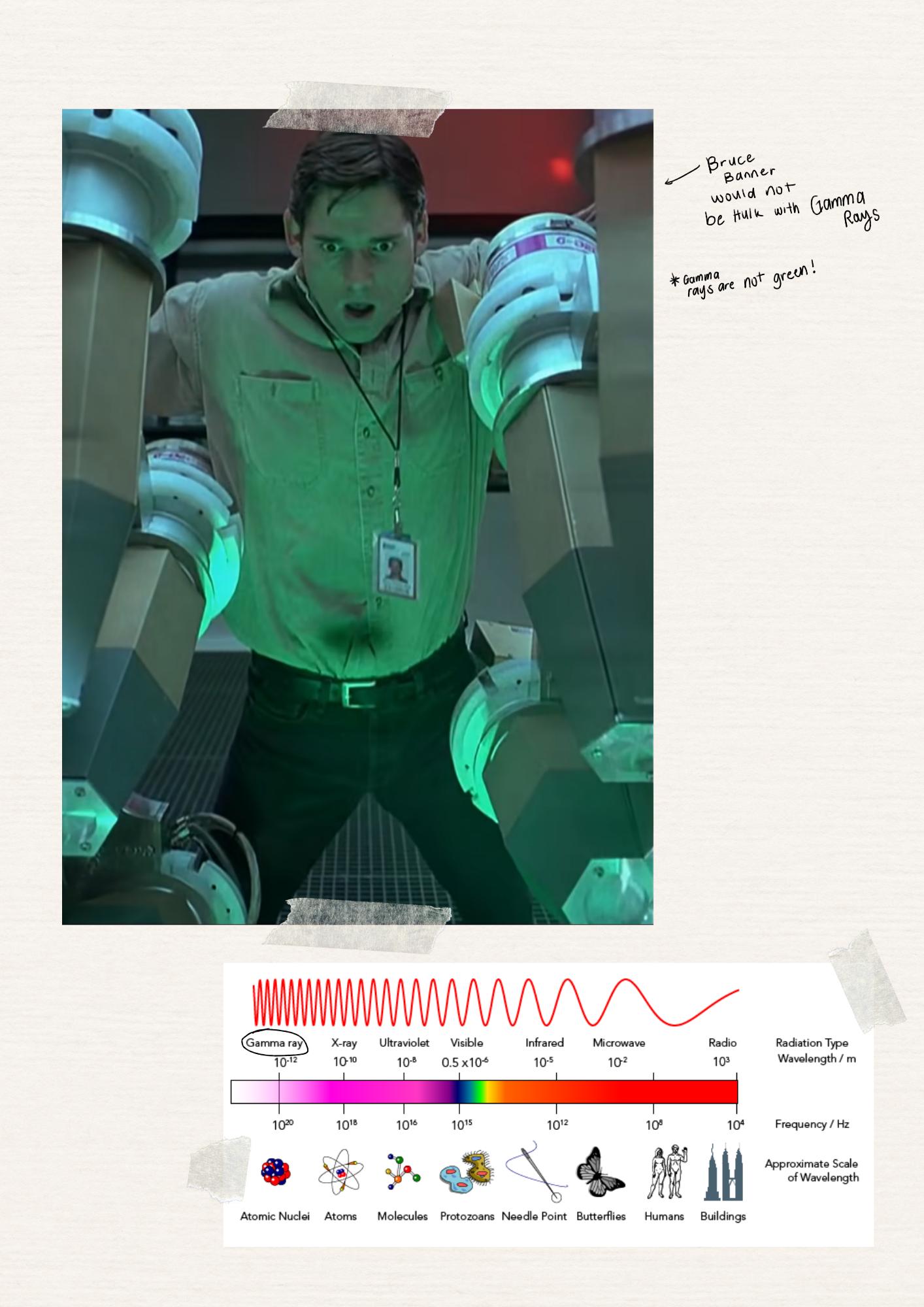
The Hulk. As shown in ”Hulk” (2003), the history behind Bruce Banner turning into the Hulk is the Gamma rays he absorbed. This happened because of an experiment gone wrong and he was trying to save him. Depicted by green light, there were a lot of Gamma rays that were emitted because of this which causes Bruce Banner turning green and into this huge hero when he is angry.
Being a fictional movie, there is a lot of incorrect Chemistry in this scene. The most evident is the fact that Gamma rays turn you into a superhero.
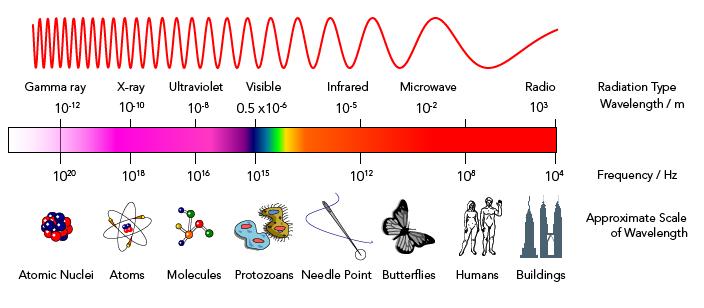
On the electromagnetic spectrum, as discussed in the third module, Gamma rays are the shortest in wavelength and have the highest energy. Even then, it would not be able to give you superpowers. The only effects that this will give, especially with the excessive amount shown in the scene, can mutate genes, destroy living cells, and cause cancer; then, lead to death (“Gamma Rays,” 2019). Overall, it will not turn you into the Hulk (Erdogan, 2019).
On top of that, the portrayal of Gamma rays with the green color is completely wrong. Gamma rays are not part of the visible rays on the electromagnetic spectrum. It goes way beyond violet.
In the real world, where this scene is scientifically correct, Gamma rays would not even be visible. It would be some sort of radiation only. Further, Bruce Banner would have just gotten the actual effects which could lead to his death. Ultimately, there would not be a Hulk if this were to happen in a nonfiction world.
GammaRays (2019) Retrievedfromhttps://flexbooks ck12 org/cbook/ck 12 middle
science
2 0/section/18 9/primary/lesson/gamma rays ms ps/ Erdogan, S. (2019, November 23). Gamma Rays: Helper
6, 2022, from https://letstalkscience.ca/educational resources/stem in context/gamma rays helper or hazard
Gamma Radiation
(Scene)
(2017, October 25) [Video] Retrieved October 6, 2022, from https://www youtube com/watch?v=GTXnxA0qEDY







Summarize all you learned from entries 1 to 5 into a reflection piece. Clarify what transpired during the semester, and why and how these improved you, that might be a takeaway even after the course.
A lab report that encompasses each and every step of the experiment The experiment being: A Woman in Chemistry An experiment that had to do with the new environment and a new set up. It brought an almost never ending challenge, from already challenging formatives, to an extensive laboratory report, further to an almost impossible midterm. These taught to value learning more than the grade itself. Learning in a sense of grasping the lesson completely. More so, learning in a sense that you do not only learn about the lesson, but also the yourself.
With that, the woman in chem that came to be is a challenge seeker. One that uses the attitude of curiosity to learn more, to not back down from an obstacle. She asks questions, observes, interprets, and communicates. She has learned to develop connections among previous, present, and future lessons and discoveries to embody chemistry as a central science. And, when she commits a mistake, she learns even more. But, she also knows her tendency to panic with pressure, to lose hope. She is yet to develop to stay calm and persevere even more in this pressure. More than that, a woman in chem who sees what she has learned in some remarkable movies, such as Hulk (2003), wherein she can evaluate it in a scientific way; and even go further into correcting it
This self awareness as a chemist of her own journey improved her by grounding her to accept who she is as a person She was handed tasks that gave opportunities for her to explore her potential in a field that is not what she is used to not math It opened her eyes to how much she has grown since Because of this, she was able to recenter on things she is good at, things she can handle. She was able to manage all of it. At the same time, it only made her enjoy the whole journey. Enjoy it enough for her to realize that the experiment does not stop there. There is still a lot that is yet to be discovered. Bringing all that she has acquired throughout this will be an aid to her in succeeding. It will allow her to keep moving forward, to keep the experiment going. For, this is a woman in chem