NORTH AMERIC AN EDITION
MEDICAL PLASTICS news + SECRETS TO SUCCESSFUL SCALE UP VIRTUAL SUPPLIER AUDITS — THE NEW NORM?
TUBING SOLUTIONS FOR EMERGING APPLICATIONS
MICROMANUFACTURING BY NUMBERS ACCUMOLD’S AARON JOHNSON FOCUSES ON HIGH-VOLUME MANUFACTURING OF MICRO MEDICAL COMPONENTS
ISSUE 17
Apr/ May / June 2021
WWW.MEDICALPLASTICSNEWS.COM
ADVANCING MEDICAL PLASTICS
CONTENTS MPN North America | Issue 17 | Apr/May/June 2021
Regulars 3 Comment Corrine Lawrence muses about remote capabilities. 5 Industry Q&A: The three MASKeteers MPN NA talks to one of the three founding members of CAMMM. 6 Digital spy 10 Cover story Accumold’s Aaron Johnson focuses on volume manufacture of micro medical components. 32 Back to the future
Features 8 Drug delivery: The X factor Peik-Christian Witte, Sanner, discusses developing intelligent products with DfE. 12 Components & assembly: Opening the doors of perception Del R. Lawson, 3M, shares the secrets to successful scale-up.
16 Components & assembly: Step up to scaling up Web Industries’ Kevin Young on how to keep pace with demand for rapid diagnostic tests. 20 Events: MD&M West What to expect from this 2021 in-person event. 22 Supply chain: VSAs not VISAs Lloyd R. DeShane, Yongshen Mould, on virtual supplier audits. 24 Tubing: The eight-point path to design enlightenment Carl Goundry, Tekni-Plex, discusses engineering solutions for emerging applications. 26 Sponsored content: TridAnt infection protection Arjun Luthra, BioInteractions, explains how multi-action technology prevents future pandemics. 28 Regulatory: Prepare to meet thy regulator Sandi Schaible, WuXi, and Michelle Lott, RAQA, on FDA pre-submission meetings.
WWW.MEDICALPLASTICSNEWS.COM
1
Aug 10-12 2021 Anaheim, CA Anaheim Convention Center
JOIN North America’s Largest Annual Medtech Event
MDMWest.com/MPN
17959_MDM_W21
REGISTER NOW at
CREDITS editor | corrine lawrence corrine.lawrence@rapidnews.com advertising | caroline jackson caroline.jackson@rapidnews.com | david geltman david.geltman@rapidnews.com
Editor’s Comment C O R R I N E L AW R E N C E
head of media sales plastics & life sciences | lisa montgomery
REMOTE CONTROL
head of studio & production | sam hamlyn graphic design | matt clarke junior designer | ellie gaskell publisher | duncan wood Medical Plastics News NA Print subscription - qualifying criteria US/Canada – Free UK & Europe – £249 ROW – £249 Medical Plastics News Europe Print subscription - qualifying criteria UK & Europe – Free US/Canada – £249 ROW – £249 FREE on iOS and Android devices Subscription enquiries to subscriptions@rapidnews.com Medical Plastics News is published by: Rapid Life Sciences Ltd, Carlton House, Sandpiper Way, Chester Business Park, Chester, CH4 9QE T: +44(0)1244 680222 F: +44(0)1244 671074 © 2020 Rapid Life Sciences Ltd While every attempt has been made to ensure that the information contained within this publication is accurate the publisher accepts no liability for information published in error, or for views expressed. All rights for Medical Plastics News are reserved. Reproduction in whole or in part without prior written permission from the publisher is strictly prohibited.
ISSN No: 2632 - 3818 (Print) 2632 - 3826 (Digital)
H
ello and welcome to the Q2 issue of MPN NA. To be frank, I’ve hit the ground running. Starting a new job — learning its ways, its people, willing the unfamiliar to become familiar in as short a time as possible — although exciting, isn’t necessarily easy, particularly on a working from home basis. I’m not, however, alone. With global travel restrictions in place, the industry has had its wings clipped in more ways than one. But rather than sit the time out waiting for life to return to normal (whenever — if ever? — that is), the medical plastics industry has pushed ahead, poking at its boundaries to discover and develop new routes to its intended destinations. Although the curtains have been closed on in-person trade fairs, digital exhibitions have become the new normal, and seminars and conferences have been delivered via webinars. These platforms have kept many industry wheels turning. They have also provided savings, be it on dollars that would otherwise have been spent on air fares, accommodation and associated expenses (aka a fortifying cold beer at the end of the day), time, and travelrelated pollution.
component missing in online replacements. This human element provides the way for ‘gut feeling’ or instinct, which may seem odd or at a variance for an industry focused on numbers, measurements, and science. There is something reassuring about being physically present at, for example, a new machine demonstration, or holding a remodelled tool, just as there is in an in-person meeting with a potential business partner. Now, as restrictions gradually lift allowing event organizers to throw open the doors of exhibition halls and manufacturing facilities to welcome on-site visitors, we have a choice: to go, and feel the real deal … or stay and take part online, saving time and money. Weathering the sharp learning curve of doing things remotely, we have come to realize that this is a viable and credible alternative in instances where previously we suspected it would be ineffective. Either way, we now have the option to choose …. and I’m keen to discover which remote activities make it through to the next round of development.
Technology and digital capabilities have also facilitated the continuity of site and facility inspections, as well as machine maintenance. We have had to learn new tricks or overcome an inner reluctance to do things differently, but now that we have, and are getting better at them, why bother returning to how things were? What started off as survival tools, keeping industry rolling, may have longevity, may be our preferred option. Yet, despite the above, people talk of looking forward to returning to in-person events, whatever they are, for the inherent
WWW.MEDICALPLASTICSNEWS.COM
3
INDUSTRY Q&A
THE THREE MASKETEERS COVID-19 HAS BEEN A HARSH TEACHER. AS COUNTRIES SCRAMBLED TO SOURCE ESSENTIAL PPE ITEMS, THE REALITIES OF RELYING ON FOREIGN SUPPLIERS HIT HOME. MPN NA TALKS TO ROBERT BALAZS, CHAIR OF THE CANADIAN ASSOCIATION OF MEDICAL MASK MANUFACTURERS ABOUT ITS MISSION. Please tell us who or what CAMMM is. The newly established Canadian Association of Medical Mask Manufacturers (CAMMM) was created to be the guiding voice for Canadian medical mask manufacturers in establishing standards, communicating with legislators and regulators, and supporting new methodologies and technologies to enhance the economic health of our emerging medical mask manufacturing industry in Canada. As the voice of the industry’s community, CAMMM will promote the industry and engage in advocacy so that Canadian medical mask manufacturers have strong and respected authority in the development of policy and critical debates. Our vision is to ensure Canadian medical mask manufacturers are recognized for leading innovation, global competitiveness, and driving economic growth. Why masks specifically? The three founding members of CAMMM — Breathe Medical Manufacturing (Breathe Medical), The Canadian Shield, and Inno Lifecare — were in discussions with one another and identified similar observations; for example, we noted that many imported masks didn’t meet the quality standards. Our discussions resulted in identifying the need to create CAMMM.
To do this, it is vital for governments to rethink their buying practices if they want a permanent domestic manufacturing base for PPE. Is it sustainable to manufacture in Canada? 100% it is. Is it going to cost a little bit more? Possibly. But why not spend that money at home where it goes right back into the economy? All levels of government need to think about this long-term. When you spend that money offshore, you have not invested a penny into your country. But when you spend it at home, look at how many jobs are created. Look at how many tax dollars are generated back into the economy. You’ve got to do the math on that. So, is it actually costing us more? No, not really.
What has been the response to CAMMM? It’s been tremendous. Canadian companies, organizations, individuals, and suppliers within the industry are very enthusiastic about the association and its goals. Members strongly support the mission of CAMMM in influencing legislation and policy that benefits the Canadian mask industry. It’s an effective way to make contacts, strike up partnerships and potential new sales opportunities. They are excited to have access to developments that affect their businesses and the medical mask sector, and be part of a forum that shares ideas and develops new ways to improve the industry. In addition, members are pleased that they can take advantage of brand elevation, public relations, and networking opportunities. What will CAMMM’s place be post-COVID? CAMMM will continue to focus on two main goals: 1) to help enhance the economic health of the medical mask manufacturing industry in Canada, and 2) to help build an established standards and regulatory system to protect Canadians. Our two main goals will enable our members to be more productive and profitable in an ever-changing and increasingly challenging marketplace. Do you think the ‘Made in Canada’ philosophy has longevity? Yes. Rebuilding Canada’s manufacturing sector is a cornerstone of CAMMM. The COVID-19 outbreak served as a stark reminder that having a robust, sustainable manufacturing industry in Canada is imperative in reducing reliance on global supply chains and keeping Canadians safe. For this reason, CAMMM is strategically assisting mask manufacturers to be an established North American supply chain, bolstering domestic PPE supplies and, at the same time, reinvigorating local economies with thousands of quality jobs for Canadians.
WWW.MEDICALPLASTICSNEWS.COM
5
DIGITAL SPY
DIGITAL
spy
www.qosina.com EXPANDED PRODUCT LINE FOR SINGLE-USE BIOPROCESS COMMUNITY
PARTNERSHIP UPDATE
www.fosterpolymers.com www.drakemedicalplastics.com
Foster and Drake Medical Plastics create one-stop shop Foster Corp., a provider of polymer solutions for medical devices, has signed an exclusive sales agreement with Drake Medical Plastics, a converter of highperformance polymers into stock shapes and precision parts. The agreement streamlines their services to customers in the medical device marketplace globally, and creates a one-stop shop for high performance polymers in the form of machinable shapes. Based in Cypress, Texas, Drake Medical Plastics provides ‘resin-to-shape’ polymer conversion into rod, plate, and tube for machined components for the medical and
PRODUCT UPDATE
life science industries. Stock shapes provide customers with a quick and economical route for prototypes and scaling up to commercial manufacturing. Depending on volume, machined parts can also be a flexible platform for production parts with little to no capital investment. Currently, Foster provides highly engineered materials and compounds to be used in specialty medical and life sciences applications. With this collaboration, medical compounds including highly filled materials will be available in machinable shapes made from Foster’s full range of polymer compounds.
Global supplier of OEM single-use components to the medical and pharmaceutical industries Qosina has extended its product portfolio, developed specifically for the needs of the single-use bioprocess industry. The company now offers a comprehensive selection of components for the design, development and manufacture of single-use systems. Scott Herskovitz, Qosina President and CEO, said: “Our experience serving the needs of regulated customers positions us well to expand our products and services
COMPANY UPDATE
www.siigroup.com
SI GROUP INVESTS TO EXPAND THE US CHEMICAL MARKET Performance additives company SI Group has said it plans to invest more than $50 million across three manufacturing sites in North America to install globally competitive main antioxidant capacity to increase its supply for this important and growing market segment. This investment will expand the US chemical market, with a focus on supplying strategically located, critical raw materials to key partners in the region. With SI Group’s current extensive antioxidant manufacturing footprint in the US and backward integration, this planned expansion will increase its security of supply by offering a fully integrated portfolio of phenolic antioxidants in the US. The new capacity is anticipated to come online in the second half of
6
for the single-use bioprocess community. We have leveraged this experience and our extensive supply chain to provide a muchneeded resource for bioprocess, vaccine production, and cell and gene therapy.”
WWW.MEDICALPLASTICSNEWS.COM
2022. These projects are expected to create short-term construction jobs, followed by permanent manufacturing jobs once construction is complete. With projected growth in the polyolefins market in the US, the expansions will enable SI Group to increase its domestic supply to match growing demand, and further strengthen its position as a key partner to its customers.
DIGITAL SPY
PPE UPDATE
www.canadianshieldppe.ca
Manufacturer launches new line of PPE for Canada’s frontline The Canadian Shield, a Waterloo Region-based medical device manufacturer has added a new proprietary PPE solution to its product line. The new attachable eye shield, called the Vizor, is a patent pending piece of PPE designed to be a simple, affordable, and practical solution for personal protection on Canada’s frontline.
for frontline workers, including reduced availability of traditional combination masks, and limited space for storage and distribution of PPE. Furthermore, the ability to separate the Vizor from the mask body means that the Vizor can be fully recycled, helping to reduce the costs associated with healthcare waste removal.
Made of 100% recyclable plastic, the Vizor aims to improve patient and worker safety in healthcare settings by offering an easily accessible additional barrier of protection.
Andy Jorgensen, CEO of Material & Design Solutions
SUSTAINABILITY UPDATE
www.newageindustries.com
NEWAGE CELEBRATES A DECADE OF SOLAR ENERGY
The power system, consisting of 4,082 rooftop solar panels, has the capacity to produce up to one megawatt continuously and has been doing so for the past 10 years. “It was our most ambitious project to date,” said Ken Baker, the company’s CEO. “As a US manufacturer, we insisted on USmanufactured products — the solar panels, the racking system that
POINT
www.materialanddesign.com
The Vizor, which comes flat-packed for easy storage and distribution, attaches to almost any face mask in seconds. This simple, innovative design alleviates a number of issues
Back in the summer of 2010, NewAge Industries made the decision to embrace solar power and reinforced the roof at its headquarters. The construction culminated on June 8, 2011, when a group of the plastic tubing manufacturer’s employees flipped a switch and began using solar energy.
talking
What was the motivation behind MDS’ recent acquisition of APS Plastics & Manufacturing? Material & Design Solution’s (MDS) acquired the assets of APS Plastics to have better control in our supply chain as it relates to the machining of tight tolerance thermoplastics components. Having control of the planning of work and scheduling allows MDS to shorten lead times and improve on time delivery to our customers. The acquisition gives MDS milling capabilities beyond that which our suppliers could provide and ensure the demanding quality requirements of the customers we serve.
holds all the panels, the inverters that convert the electrical current — and it took a lot of research to source everything.”
Does this acquisition represent MDS’ first interaction with the medical plastics sector? Yes, although John Jorgensen, MDS Chairman, has done business in the field with major players in the US and Europe (while President and CEO of Greene Tweed).
NewAge, which manufactures single-use molded tubing assemblies used in vaccine production, in addition to plastic tubing, made the switch to solar power in conjunction with several other green initiatives including installing energy-efficient lighting.
How will medical plastics customers benefit from the acquisition? All the different industries that MDS serves demand the highest levels of quality. The medical plastics customers’ demands are a good fit with the values and strategic objectives shared by all MDS employees.
The solar energy system originally contributed more than half of the energy needed at NewAge’s headquarters. Expansions at the facility have since increased electricity needs, but the company says it is proud to be harnessing the sun’s energy and putting less pressure on the community’s power grid.
What’s your view on the “Made in the US” ethos? MDS is committed to providing our customers with high-quality manufactured parts, in short lead times with on time delivery. We have a strong preference in sourcing our raw material and other subcomponents with made-in-USA product. Our commitment is to our customers, and if the technology or supply base is unable to support us in meeting those customer requirements, we will source globally.
WWW.MEDICALPLASTICSNEWS.COM
7
DRUG DELIVERY
DEVELOPING A SMART MEDICAL DEVICE IS A TASK THAT LIES SOMEWHERE BETWEEN COMPLICATED AND COMPLEX. STRICT REGULATIONS AND MULTILAYERED REQUIREMENTS NECESSITATE AN ARRAY OF SKILLS FROM PRODUCT DESIGN TO MANUFACTURING AND PROCESSING TO RISK MANAGEMENT. PEIK-CHRISTIAN WITTE, HEAD OF ENGINEERING & INNOVATION, SANNER GMBH, EXPLAINS HOW TO DEVELOP INTELLIGENT PRODUCTS WITH DESIGN FOR EXCELLENCE.
THE X FACTOR mart medical devices with certain functions such as reminders or dosage can increase therapy adherence. Products range from intelligent tablet dispensers to retrofittable add-ons for inhalers or injection systems.
S
DESIGN FOR PRODUCT Requirements management already plays an important role in the initial development phase. Based on numerous customer requirements, product designers should develop concepts that incorporate functionality, manufacturability and costs — also for subsequent series production — and physically convert the results in CAD (computer-aided design) models.
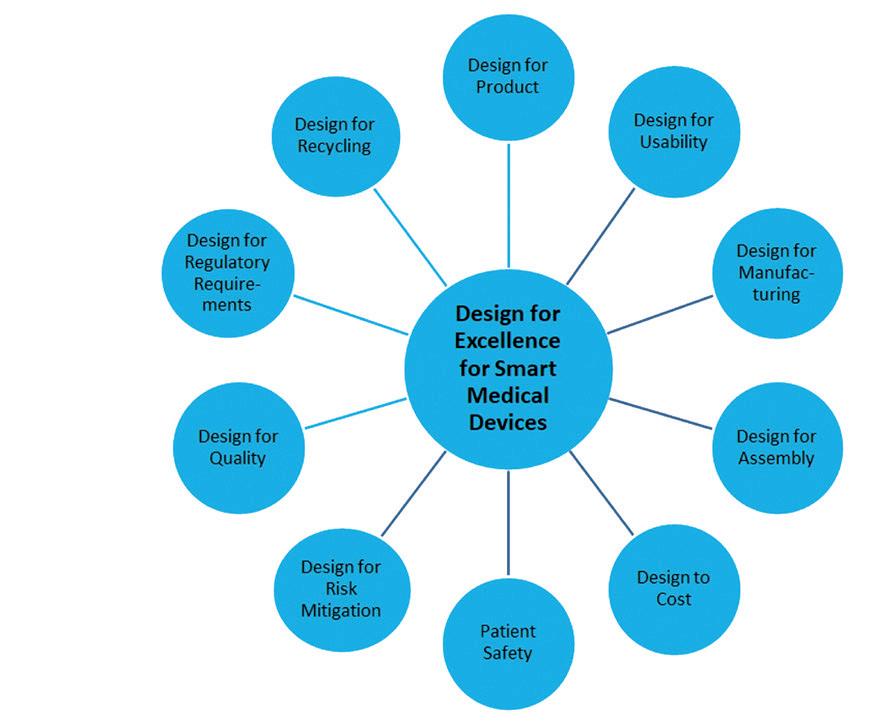
Numerous factors feed into the development and subsequent production of such products. Each factor has a different emphasis depending on the complexity and design of the device. In Design for Excellence (DfX), the X stands for a variable that makes a development successful only in optimal interaction with the others (Figure 1).
DESIGN FOR USABILITY User-friendliness and ergonomics make decisive contributions to the correct use and acceptance of the smart medical device, which ultimately improves the therapeutic success. Particular attention must therefore be paid to usability with the aim of minimizing risks from application errors, ensuring patient safety, and creating the framework conditions for the highest possible level of adherence. DESIGN FOR MANUFACTURING The product must be optimally designed at an early stage regarding the planned manufacturing and assembly conditions, quality, and costs. Particularly in the case of plastic components, product and process development must go hand in hand, hence material selection, tooling and injection molding technology, the lowest possible number of components and functional integration are considered. DESIGN FOR ASSEMBLY The product design, including product structure, is optimized with regard to assembly. The assembly concept (that is, a partially versus a fully automated process) depends on the number of parts to be assembled. DESIGN TO COST An optimum cost–benefit ratio and low follow-up and modification costs are also relevant. Considerations include the optimum use of materials, the lowest possible number of components, and optimizing manufacturing process cycle times. As electronic components for smart medical devices can also boost manufacturing costs, these too warrant detailed analysis.
Figure 1: According to the Design for Excellence (DfX) strategy, only the optimal interaction with numerous other variables will lead to success.
8
DESIGN FOR PATIENT SAFETY Patient protection, patient well-being and patient benefits are important issues in the development, production and application of smart medical WWW.MEDICALPLASTICSNEWS.COM
DRUG DELIVERY
devices. Adverse outcomes or harm from healthcare interventions must be avoided, prevented and/or improved; for example, by ensuring the device is used correctly through sensors that indicate incorrect handling. DESIGN FOR RISK MITIGATION To ensure patient safety, risk management in accordance with ISO 14971 is a key element in the development of smart medical devices. Manufacturers must ensure that their products are safe when used properly and eliminate or minimize the risk of error from handling the product. DESIGN FOR QUALITY Process reliability and speed play major roles in developing and implementing inspection concepts. Precise, often fully automated, error detection is essential. Today, it can be implemented almost in real time via video assistance systems integrated into the production process. These systems compare the actual results with the specifications from the CAQ (computer-aided quality assurance) system.
Figure 2: Complexity in a nutshell: the injection-molded mouthpiece for inhalers is filled with different media and components in several stations.
DESIGN FOR REGULATORY REQUIREMENTS Smart medical devices are usually categorized as ‘active medical devices’ and are subject to the same strict documentation requirements as all other medical devices. These include risk analysis and risk assessment to prove the safety and performance of a clinical evaluation, as well as comprehensive quality management, especially when using electronic assemblies.
BEST PRACTICE 1: MOUTHPIECE FOR INHALER A mouthpiece for a measuring device to determine inflammatory activity in chronic respiratory diseases shows how the aspects of Design for Excellence interact: Product and Usability Design ensure high functionality and ease of use; Design for Manufacturing and Assembly defined the multistage assembly line, including filling, welding, and packaging (Figure 2). The main challenges in assembly were how to feed the various parts and filling media in a way that would be gentle on the product, to meter them accurately to 1/100 of a gram, and to position and weld components and filter materials in the correct position and fit. The result is multistage, precise, and fast assembly with high reproducibility and quality. In addition, a fully automated, software-supported 100% inspection of each work step ensures quality control: 15 camera systems inspect presence, correct execution and completeness, and, at the same time, six weighing stations check the adequate quantity. This combination eliminates subsequent testing on the finished assembled part, which conserves resources and saves time and costs.
BEST PRACTICE 2: ADD-ON DEVICE FOR INHALER Another example is an add-on device for a DPI (dry powder inhaler [Figure 3]). First, the development team had to answer the following: where and how can we position and attach the electronic components to ensure their functionality and to make the assemblies reproducible for large quantities? The position of the sensors is crucial: for example, one of the sensors ensures the vibration characteristics are optimally transmitted during the inhalation process. This avoids overdosage or under-dosage and helps maintain both usability and patient safety. The exact position of the sensor also prevents impairments that could arise from the user’s grip. Special attention must also be paid to a flexible tolerance compensation and to the simplest possible tool geometries for fixing the sensor boards, which minimize subsequent cycle times. A pre-assembled battery with holder, which is placed in the bottom of the add-on, facilitates the assembly steps and cabling. WWW.MEDICALPLASTICSNEWS.COM
Figure 3: Add-on devices for DPIs illustrate the challenges of developing intelligent medical devices.
DESIGN FOR RECYCLING In the case of installed electronic components, environmental aspects such as disposal, recycling, retrofitting and rechargeability play a central role in development. Recycling considerations maximize the proportion of recoverable and recyclable materials; they also help to develop products that facilitate the dismantling and recovery of recyclable materials. MANY YEARS OF EXPERIENCE As the two best practice examples show, smart medical devices must be designed to make them ready for series production. These multilayered requirements can only be met by a team of engineers, project and quality managers, as well as compliance experts that precisely coordinate all process steps in Design for Excellence and works together flexibly.
9
COVER STORY
Micromanufacturing by numbers ENGAGEMENT WITH A MICROMOLDER FOR A MEDICAL DEVICE PRODUCT DEVELOPMENT PROCESS WILL ULTIMATELY RESULT IN THE NEED TO MANUFACTURE PARTS, OFTEN AT VERY HIGH VOLUMES. MICROMOLDERS NEED TO EXCEL AT EVERY STAGE OF PRODUCT DEVELOPMENT, BUT, AS AARON JOHNSON, VP OF MARKETING AND CUSTOMER STRATEGY, ACCUMOLD, HIGHLIGHTS, THERE ARE PARTICULAR CONTINGENCIES THAT SURROUND VOLUME MANUFACTURING THAT MAKES SUPPLIER SELECTION ESPECIALLY IMPORTANT.
M
icromolding typically requires the achievement of truly exacting and sometimes almost impossibly tight tolerances. In many instances the demand is for tiny parts or slightly larger parts with submicron feature sizes. When micron tolerances matter, the customer and micromolding provider must enter into a close partnership in product development, and it becomes hugely important that the micromolder owns, manages, develops, and innovates in every aspect of the supply chain.
The “get it right the first time” headline over every activity plays to the vital importance of vertical integration, which enhances the quality, compliance, and conformance to design intent. Micromolders need to understand the critical to quality (CTQ) characteristics to manufacture parts successfully; these characteristics — including molding, assembly, and packaging — are important to the functionality of the product with regard to end-user experience. In basic terms, the longer the value stream, the more disconnected the value stream, and the more variables can be introduced causing customer issues. Vertical integration supports a shorter value stream reducing silos and suboptimized processes. VOLUME MANUFACTURING Variability is the enemy of high-volume micromanufacturing, and so focus needs to be maintained on implementing process controls that drive repeatability all the way from cutting micro tool steel to measurement and validation methods. Typically, medical device OEMs want the highest quality products at the lowest possible cost. Product quality requirements are driven by the Voice of the Customer (VOC), which enables good micromolders to identify critical medical product characteristics which need to be verified during production. Cost, however, is more than just the price charged to mold a part; it also includes the time it takes to get a product to market, whether defects make it to market, and the levels of supply consistency. Micromolders should strive to supply the highest quality products, through validated processes, with short timelines to market. Realistically, this can only really be accomplished through vertically integrated processes and utilizing process validation. When short-listing potential micromolding suppliers, from a production perspective there are some key questions that medical device OEMs need to ask to ensure the micromolder is equipped to achieve often exacting objectives. First, do you design, build, and maintain your molds? Second, what measurement capabilities do you have on site? Third, what are your methods of validation (DOE, IQ, OQ and PQ)? Finally, what resins do you have experience with in production? The successful manufacture of micromolded medical products is almost entirely down to the VOC. But it is important to realize that the end user isn’t the only customer — there are customers throughout the entire value
10
WWW.MEDICALPLASTICSNEWS.COM
COVER STORY
Variability is the enemy of highvolume micromanufacturing, and so focus needs to be maintained on implementing process controls that drive repeatability … stream. The shorter and more centrally located the value stream the quicker concerns can be raised and resolved. ASSEMBLY When dealing with miniaturized plastic medical parts and components, the assembly stage of the product development process must be discussed and considered early in the design cycle, demanding a collaborative and pragmatic relationship between medical device OEM and micromolder. When dealing with microscale parts and components, the cost of manual assembly is prohibitive and often requires levels of precision when dealing with submicron tolerances that are impossible to achieve. Automated assembly is, therefore, a must in most micromolding scenarios, requiring that medical device OEMs select a micromolding partner that is able to understand the methodology of micro assembly and achieve the extreme positional accuracy required. The demand is for a laser-like focus on design for micromanufacturing (DfMM), which crucially influences the success of every part of the overall product development process, including assembly. The importance of considering
WWW.MEDICALPLASTICSNEWS.COM
assembly at the design stage of a micromolding product development process is huge, and is an important factor for any project. Micromolding just adds more variables that need to be controlled than a standardsized product for which there are many different manufacturing solution partners in the marketplace. Micromolded parts can be difficult to feed, inspect, and manipulate without causing damage. Capturing a micromolded part as it comes out of the mold is often difficult, as is orienting a micro part after molding. Capturing a part at mold ejection and immediately assembling is often the only route to efficient assembly with low waste. All such considerations should be bottomed-out at the design stage to keep costs under control so a medical device OEM can maintain margins and provide a product to the marketplace at an acceptable price. SUMMARY Before embarking on a micromolding project, medical device OEMs should focus on the mold design, mold build, and quality measuring capabilities of the vendor they are engaging to mold their parts. If molders lack the ability to design, build, and measure high-quality molds to extremely tight tolerances they will struggle to produce consistently high quality parts to tight tolerances at high volume. Producing products to specifications that only allow microns of variations to a specification is much more challenging than producing a product with significantly larger variation allowances. A poorly designed and built tool can eat up your entire variation before you even mold a part, essentially killing the project. So, when micron tolerances matter, informed supplier selection is the absolute key to high volume manufacturing success.
11
COMPONENTS & ASSEMBLY
OPENING THE D ORS OF PERCEPTION ALTHOUGH THERE IS NO CRYSTAL BALL TO HELP PREDICT THE COHESIVENESS OF A MEDICAL DEVICE’S DESIGN, MATERIALS AND PRODUCTION PROCESS, THERE ARE TWO DOORS DEVELOPMENT TEAMS CAN UNLOCK TO OPEN UP THEIR PROBABILITY FOR A SUCCESSFUL SCALE-UP. DEL R. LAWSON, PHD, R&D MANAGER IN 3M’S MEDICAL SOLUTIONS DIVISION, PROVIDES THE KEYS TO THOSE DOORS.
T
he medical device design process is bound to have challenges as it progresses into the production stage. Catching all of them can be tricky, but the good news is that many are preventable. Although development teams cannot employ a crystal ball to help predict the cohesiveness of a medical device’s design, materials and production process, there are two doors they can unlock to open up their probability for a successful scale-up. Both rely on proper planning and, when done correctly, can test for potential setbacks before challenges evolve into more significant issues during full-scale production.
friction on certain materials and designs often follow suit. It can be difficult to predict where friction might manifest production issues; one area particularly subject to such stress, however, is the adhesive liner. The adhesive release liner supports a major aspect of a medical device’s designed functionality, and ensuring it is applied properly protects the product’s usability. Adhesive liners must be applied with balanced tension to reduce the impacts of manufacturing. Too tight, and the liner can wrinkle or inhibit the run speed. Yet when liner release values are too loose (low adhesive holding force between the liner and adhesive layer), they could fall off prematurely on the converting equipment. If a team plans to remove and reuse liners, look for any scratches or damage that could affect the final product.
Engineers looking to advance or refresh their design mindset with a more holistic approach should question two factors in the early stages of the development process: the production and material effects on device performance. KEY #1: MANAGING PROCESS EFFECTS FOR SUCCESS Development teams who challenge their assumptions and processes may uncover obstacles they may not have otherwise caught presented by the speed, friction and pressure during scale-up. To improve medical device developers’ overall design, walking through the below questions can be an excellent place to start this effort. How have you factored in friction? As production rates and machinery increase to meet the advancing needs of a project, the impacts of
12
WWW.MEDICALPLASTICSNEWS.COM
MEDICAL PACKAGING SOLUTIONS AS UNIQUELY DESIGNED AS THE DEVICES YOU DEVELOP A proven industry pioneer and innovator, Technipaq has been providing flexible, sterilizable packaging solutions to the world’s leading healthcare, medical device, diagnostic and life sciences companies for more than 35 years. And with the recent installation of a new coating production line, we have continued to expand and enhance the breadth and diversity of our products and capabilities—which now also includes the in-house ability to apply coatings to medical grade DuPont™ Tyvek®. We welcome the opportunity to customize a packaging solution that meets your unique needs. Please also visit www.technipaq.com to see our full range of capabilities, get a product sample kit, and even design your own custom pouch in minutes.
LAMINATING | COATING | PRINTING | SLITTING | CONVERTING
www.technipaq.com 975 Lutter Drive Crystal Lake, IL 60014 Phone: 815.477.1800 Fax: 815.477.0777 info@technipaq.com
DESIGN YOUR OWN POUCH IN MINUTES
SEND ME A NEW PRODUCT SAMPLE KIT
COMPONENTS & ASSEMBLY
Development teams can execute a more seamless scale-up by managing how the selected components and assembly process will affect their device design How might heat or other curing procedures affect adhesives? If the answer to this question is unclear, stability and aging studies can forecast how adhesives may react to heat-based production steps. If the selected bonding agent is heat activated, the production process may need to be adjusted to avoid exposing the device prematurely. The same may be true for exposure to UV wavelengths and other common ‘cure’ conditions. Will cleaning and maintenance influence design? The cleaning and maintenance of manufacturing equipment are a given part of any medical device’s production. Yet what manufacturers use to clean and maintain equipment is not often thought of as an influential factor in design. Certain cleaning products or methods could be incompatible with the product’s materials or construction. Conversely, ineffective cleaning could gum up or contaminate equipment, especially when running different materials on the same line. Thoroughly evaluating cleaning agents, methods and preventative maintenance can reduce unexpected stops to production or impact to the final device performance. Understanding how the production process can influence the final device does not have to take a ‘wait and see’ approach. Rather, teams that use the questions above from the start of a project can stay ahead of potential issues and adjust accordingly. KEY #2: MANAGING MATERIAL EFFECTS FOR SUCCESS Material compatibility is paramount to achieve optimal product performance and functioning components. When every material is thoughtfully selected, manufacturers can reduce the odds of premature failures, and in many cases, they can improve overall device performance. By leveraging the questions below, companies can stay ahead in ensuring material compatibility.
afterthought during medical device development. If teams factored this into the design earlier, picking the right adhesive could drastically contribute to performance and overall efficacy. Companies that leverage the full potential of an adhesive can add to a device’s functionality significantly. Have material and manufacturing compatibility been thoroughly vetted? Testing the final version of a product with the given manufacturing process is fundamental to ensuring material compatibility. In addition, evaluating more than one lot of production equivalent materials can act as an approach to proactively assure critical material compatibility in terms of regulatory, safety, efficacy and appearance performance. ASKING QUESTIONS TO IMPROVE OUTCOMES Development teams can execute a more seamless scale-up by managing how the selected components and assembly process will affect their device design. Although some are already asking several or all of these important questions, they must remember to bring this curiosity into the conversation early in the process. Doing so helps mitigate potential escalating issues.
How will the device materials hold up over time? The test of time can reveal many insights about a device’s materials. Teams must prioritize their device’s essential properties and be mindful of potential contamination points, including naturally occurring sources and those triggered during manufacturing or sterilization. Two ways to do so are through aging and sterilization studies. They help evaluate material compatibility and identify potential stressors by manipulating temperature and time to show where the development process should focus before progressing further. Early sterilization studies can help with selecting the best method for the finish device. These two studies can help companies to identify sources of concern and problem-solve before they scale up. Will the product need to adhere to the skin? Selecting an adhesive can often occur as an afterthought during medical device development. Selecting an adhesive can frequently occur as an
14
WWW.MEDICALPLASTICSNEWS.COM
CYROLITE® has been working in hospitals and
Lipid-resistant, BPA-free, and highly transparent: CYROLITE® acrylics are the reliable invisible helpers in hospitals and labs.
labs for more than 40 years. Thanks to their excellent properties, our high-performance acrylics are perfect for use in a wide range of medical devices. CYROLITE® is highly transparent and easily processed into intricate parts. It can be reliably sterilized using most common methods and is BPA- and DEHP-free. This has impressed both patients and healthcare professionals alike: CYROLITE® meets the requirements of USP Class VI, ISO 10993-1, and REACH. You can find more details at www.cyrolite.com.
COMPONENTS & ASSEMBLY
STEP UP TO SCALING UP ADVANCEMENTS IN DETECTING AND PREVENTING COVID-19 BODE WELL FOR SIMILAR TESTS FOR OTHER MEDICAL CONDITIONS. KEVIN YOUNG, VICE PRESIDENT OF CORPORATE DEVELOPMENT & MEDICAL, WEB INDUSTRIES, INC., EXPLAINS HOW MANUFACTURERS CAN SCALE UP TO KEEP PACE WITH CURRENT AND POST-COVID DEMAND FOR RAPID DIAGNOSTIC TESTS.
I
n response to COVID-19, medical device OEMs and their suppliers are facing demand for vast quantities of reliable diagnostic tests that yield timely results. The demand will likely outlast the current pandemic and extend well beyond the many tests developed to detect the virus. The widespread distribution of COVID-19 tests, such as lateral flow immunoassay (LFI) antigen tests, is already generating increased interest in rapid tests for other medical conditions. Advancements in COVID-19 detection and prevention will assist in their development. Scaling up to produce millions of rapid tests often requires manufacturers to make significant operations changes, especially in three categories: • Technology and automation • Supply and infrastructure • Staffing and workforce development. TECHNOLOGY AND AUTOMATION Before COVID-19, diagnostic tests were usually made manually and in small quantities (Figure 1). Production took place in separate batches as
components moved from one process to the next. This was fine for making thousands or even tens of thousands of test devices per week but inadequate for largescale production. For large quantities, manufacturers and their suppliers need fully automated and integrated production lines, including those that assemble multiple test device components. Each step of the test-making process must be mechanized, from the preparation of test chemistries to the application and lamination of test formulations to materials. Reel-to-reel (Figure 2) and pickand-place systems are examples of equipment that provide accuracy and speed, with little need for human intervention. Beyond this, the sheer pace of production necessitates automated camera and vision systems to monitor the process. Such cameras use sensors and software algorithms to produce images and automate visual inspection tasks. They can quickly identify defects and alert operators when problems arise.
Figure 1: Pre-pandemic, LFI diagnostic test manufacturing was a highly manual batch-based process. (Photo courtesy of Web Industries)
16
SUPPLY AND INFRASTRUCTURE Manufacturers may need to overhaul their plant infrastructure to house new production lines,
WWW.MEDICALPLASTICSNEWS.COM
SUBSCRIBE TODAY
11083 High Flow Check Valve, Adjustable Pressure 23278 FLL to FLL Connector
80199 Check Valve Tubing Port Inlet MLL Outlet Coated Stem
80361 Luer-Activated Valve Female Luer Lock Tubing Port
13619 Pinch Clamp 11499 Elbow Connector FLL, ML with Spin Lock 80368 Endoscopic Valve
61202 Cross Connector Barbed
65515 FLL Connector
99780 1-Way Stopcock FLL, MLS
All trademarks and registered trademarks are the property of their respective owners.
97365 Flow Control Switch FLL Inlet MLL Outlet
57030 Flexible Suction Connector
28305 Hydrophilic Filter FLL Inlet, MLL Outlet
Log on to qosina.com today to see our full product offering. Qosina is a leading global supplier of thousands of OEM single-use components to the medical and pharmaceutical industries. 2002-Q Orville Drive North, Ronkonkoma, NY 11779 USA
qosina.com
info@qosina.com
Qosina Europe Srl:
Via Ticino 6, 20095 - Cusano Milanino (MI) - Italy
info@qosinaeurope.com
COMPONENTS & ASSEMBLY
including expanding existing facilities or building new ones. The additional space might accommodate provisions such as dry rooms, which control temperature and humidity and are essential for diagnostic test production. In addition, new warehousing space is frequently needed to store test components and materials. Scaling up also entails assembling a network of raw material suppliers and production partners to support high volumes. Here, experienced diagnostic test device CMOs can be a central partner. CMOs are often skilled at transferring technology among different production locations and are likely to be expert in flexible materials formatting and converting, and in the precisioncutting needed to manufacture the lateral flow test strips.
Figure 2: Reel-to-reel assembly and lamination of multi-layer test strips support high-speed manufacturing for large-scale medical testing programs. (Photo courtesy of Web Industries.)
Scaling up to produce millions of rapid diagnostic tests will pose technical and organizational challenges like those encountered by producers who have stepped up to make COVID-19 tests. These include technology transfer, automation and human resources — areas where established outside contracting resources are available to smooth the transition.
STAFFING AND WORKFORCE Needs for high-volume production usually mandate a corresponding increase in labor and staffing. Workforces in some processes can grow by a factor of more than five. Although full automation eliminates some manual tasks, it creates greater demand for employees with specialized engineering, bioscience, quality assurance, and inventory management skills. Finding experienced workers to fill skill positions might call for training current employees, and new employees for new equipment and processes.
CASE IN POINT: MAKING RAPID LFI ANTIGEN TESTS In early 2020, the pandemic destabilized the aerospace industry, then Web Industries’ dominant business sector, and prompted the company to turn much of its CMO energies to its budding medical market operations in Holliston, Massachusetts. There, the company addressed sample orders from a potential OEM customer for rapid LFI antigen COVID-19 detection kits. Rapid LFI antigen tests recognize proteins on COVID-19’s surface by sampling a nasal or throat swab. Test results appear within 15–30 minutes.
NEW ERA OF RAPID TESTS Post COVID-19, needs are likely to grow for other diagnostic tests, a coming demand that offers business potential for medical device manufacturers and their suppliers. Many will be available over the counter and prove as easy to use as today’s pregnancy tests. They will be administered outside of clinical settings, be available over the counter at pharmacies, and performed at home. Results will be available in minutes instead of days.
18
Test devices are packaged in kits. Point-of-care test kits consisted of 30 LFI strips, individually housed in a plastic cassette and pouched in foil, along with a corresponding amount of extraction reagent buffer solution and collection swabs, and positive and negative control swabs. Dividers, a reagent holding tray and an IFU (Instructions for Use) card are also included. At-home tests contain most of the same components but are packaged in a smaller subset, for example, one test device, swab, and extraction reagent. With the aid of supply partners, the Web Industries’ Holliston plant was soon producing thousands of antigen tests per week, earning approvals from the US FDA and the company’s now-contracted customer. But tens of millions of tests would be needed to satisfy incoming orders. Accordingly, the company accelerated its efforts by fully automating plant operations with pick-and-place and reel-to-reel machines and flow wrappers for automatic packaging. Management also linked reagent deposition, lamination and test strip cutting. By early 2021, the company was producing and shipping several million LFI antigen test devices per week to its OEM customer.
WWW.MEDICALPLASTICSNEWS.COM
MicroAir
SilcoTek CVD Coatings for Medical Devices and Molding
Ultra Low Air Pressure Regulators and Controllers for plastic tubing extrusion AIR Support to control diameter and ovality during the extrusion of medical, catheter, multi-lumen, taper, and bump profiles.
Tel: 978-562-5353
• • • • •
Increase Surface Release Improve corrosion resistance Prevent contamination Eliminate frequent maintenance Excellent adhesion properties SilcoTek Corporation Game-Changing Coatings www.SilcoTek.com TechService@SilcoTek.com +1(814) 353-1778
Email: olc@onlinecontrols.com
www.onlinecontrols.com
Dynamic Extrusion for Today’s Medical Industry.
Your medical tubing operation deserves precision and profitability. From microbore to multi-lumen, catheter to radioopque, we’re ready to engineer a versatile, tight-tolerance solution for your cleanroom process. Our control and feedscrew technology, compact coextruders, and direct-drive MEDD extruder deliver results that maximize your ROI. What’s more, you can run a range of thermoplastic materials and high-temperature resins with ease.
Call +1 860-934-0575 today to discover the difference!
davis-standard.com
EVENTS — MD&M WEST
MD&M WEST BRINGING TOGETHER ENGINEERS, BUSINESS LEADERS, DISRUPTIVE COMPANIES, AND INNOVATIVE THINKERS TO FIND POWERFUL SOLUTIONS TO CREATE AND BUILD LIFE-CHANGING AND LIFE-SAVING MEDICAL DEVICES.
WHAT, WHERE, WHEN Medical Design & Manufacturing (MD&M) West along with sister shows WestPack, Automation Technology Expo (ATX) West, Design & Manufacturing (D&M) West, Plastec West, and Cannabis Packaging Summit have opened registration for the 2021 in-person event to be held at the Anaheim Convention Center, California, August 10–12, 2021. WESTPACK Unites the entire packaging community from design engineers to distribution leaders to find the most creative packaging solutions and efficient automation systems. ATX WEST The epicenter of robotics technology, thought leaders, and smart manufacturing innovation. This event brings together the industry to continue to push boundaries in automation to create a smarter world. D&M WEST Gathers great thinkers, designers, and engineers from across the design & manufacturing continuum, to take on universal challenges, and find solutions that will inspire and bring forth global change. PLASTEC WEST Goes above and beyond plastics and polymers. Discover the latest biocompatible polymers and
20
cutting-edge large-scale injection molding solutions, and uncover technology solutions in medical design and manufacturing, 3D printing, and robotics. The event will feature a number of complimentary activities: 3DPX ZONE This year, event organizers, Informa Markets Engineers, launch an all-new 3D Printing eXperiential zone featuring live demos, free content, and networking within a dedicated 3D printing hub. CENTER STAGE Attendees can stop by the lobby theater for panel discussions, live demos, thought leadership presentations, and more.
EVENTS — MD&M WEST
... the revamped name reflects the allinlusivity of the program within the ... industry TECH THEATER Technical sessions will also be accessible on the show floor, hosted and presented by suppliers. LOBBY PRODUCT SHOWCASE See the latest innovation within the industry at the Lobby Product Showcase. NEWS DESK Drop by the all-new News Desk for the opportunity to listen in on some of the brightest minds being interviewed by journalists onsite. NETWORKING EVENTS Informa Markets Engineering’s West attendees can take part in formal and informal networking events across the expo floor. MD&M WEST BY NUMBERS • 70 countries • 120 educational sessions • 600 conference delegates • 1,400 exhibitors • 13,000 attendees • 300,000 sq. ft of expo floor DESIGN. ENGINEER. BUILD. CONFERENCE New name, same great content. Formerly known as the Smart Manufacturing Summit and 3D Printing Summit, the revamped name reflects the all-inclusivity of the program within the advanced design & manufacturing industry. With an upgraded pass, visitors can gain exclusive access to eight tracks dedicated to topics, on-demand conference content (post-event), partake in roundtable discussions, and more.
Track: Optimizing your smart manufacturing strategy Title: How to teach your people to stop worrying and love the robot Speaker: Jason Walker, CEO & cofounder at Waypoint Robotics, Inc. Date/ time: Tuesday, August 10 | 14:15–15:00 Location: 210CD Track: Saving the world through medical plastics & sustainable solutions Title: Manufacturing of micro medical parts without cold runners Speaker: Vijay Kudchadkar, director of advanced engineering at WestfallTechnik, Inc. Date/time: Tuesday, August 10 | 14:50–15:20 Location: 208AB Track: New technologies & strategies for better product development Title: New product innovation: integrating the experience economy into your next product Speaker: Avishek Mishra, regulatory affairs specialist at Medtronic Date/time: Wednesday, August 11 | 14:15–15:00 Location: 210AB Track: New materials, methods & technologies in 3D printing Title: Material optimization for scaling up additive manufacturing processes Speaker: Rod Reber, applications development and technical service engineer at Arkema Date/time: Wednesday, August 11 | 15:15–16:00 Location: 210CD
W W W. M E D I C A L P L A S T I C S N E W S . C O M
21
SUPPLY CHAIN
WHEN SUCH EVENTS AS THE CORONAVIRUS PANDEMIC RESULT IN GLOBAL TRAVEL RESTRICTIONS, COMPANIES NEED AN ALTERNATIVE TO IN PERSON SUPPLIER APPROVAL AUDITS. LLOYD R. DESHANE, VICE PRESIDENT, CHINA OPERATIONS, YONGSHEN MOULD, OUTLINES HOW VIRTUAL SUPPLIER AUDITS NOT ONLY RESOLVE THE IMMEDIATE SITUATION, THEY ALSO LOOK TO BECOME THE NEW NORMAL.
Replacing VISAs with VSAs
M
aintaining a strong supply base with the ability to grow the supply chain is an essential requirement for all companies that source products and production in Asia. Pre-COVID supplier approval audits in China/ Asia were fairly standard: supplier quality engineers (SQEs) and program managers would visit plastic tooling and moulding suppliers to audit the quality, capabilities and systems the supplier has in place to understand whether the supplier has the capabilities required to manage programmes for them. They will also check the supplier to confirm they are using certified resins and polymers from approved sources. These detailed audits typically take 6–8 hours of onsite investigation and review with another few hours of follow up if any details require further clarification at a later date. The supplier audit results in a Pass (approved supplier), Fail or Preliminary Approval (based on an agreed timeline in which to correct or improve a specific issue before resubmitting the data to confirm the issue has been addressed and meets the customer’s requirements. The supplier audit includes every aspect of the supplier’s ability to manage not only production but also general business practices (Table I). TRAVEL RESTRICTIONS The global pandemic saw Asia close its doors to visitors in 2020. According to the China Government, travel restrictions are expected to remain in place for all of Asia during 2021, with speculation of opening China in the spring of 2022 once herd immunity is achieved.¹ Even when borders open up, it will be expensive and
22
complicated to travel. Airfares to Asia are at an all-time high and a COVID Vaccine Passport will be required.²,³ China may still require a 2-week quarantine at the traveller’s expense when entering China. Furthermore, China will only accept a vaccine passport that details a China-produced vaccine; no other countries’ vaccine will be accepted. Travel costs and complications aside, visitors still have the health risks. Many companies do not want to subject their employees to these health risks and the liabilities that come with travel due to new strains of the virus and potential infection. These external factors have (and will continue to do so) forced companies to find a way to perform remote Supplier Audits, which are required for three main reasons: 1. Supplier Requalification — a supplier maintenance procedure, typically performed once a year, to ensure a current supplier is maintaining its quality systems and production requirements. 2. Supplier Production Audit — validation of current production; cost reduction ideas including implementation of automation and other manufacturing improvements; delivery schedules; quality assessment of production; and review of increased or reduced production requirements. 3. New Supplier Qualification — introducing a new supplier into the supply base to replace a supplier that cannot meet current standards or demands, or a new supplier that has a new technology or manufacturing capability/ capacity that is desired by the customer. THE VSA For the reasons stated above the Virtual Supplier Audit (VSA) system was born out of necessity. In preparation for the VSA you must communicate with the Asian supplier in advance to ensure they have the tools needed for the VSA and have made the necessary arrangements (Table II). Although customers prefer to see the information in person, using a hand-held phone/camera comes a close second: it can be used during the factory tour, to facilitate questions, and guide the tour if/ when needed. There really is little difference between a VSA and being present at the audit. A followup audit can also be arranged if the customer did not see exactly what
WWW.MEDICALPLASTICSNEWS.COM
SUPPLY CHAIN
what they wanted or needed further validation. On-site supplier validations, however, are not necessarily destined to become things of the past. Many manufacturers still prefer in-person and on-site meetings to gain a personal feel for the teams and management that will produce the product for them. Limited travel has made VSAs essential — a status that looks set to remain certainly for the next 2 years, particularly for those businesses attempting to maintain and grow a strong supply base. Improvements in virtual meeting technologies are making VSAs particularly beneficial as they control the high cost of sending audit staff to Asia, and facilitate real-time reporting of any production issues or the approval process. Once the suppliers have been trained in using VSAs they can apply the same skills and equipment to provide immediate virtual updates on all issues. A significant amount of preparation is required by the customer and the supplier for a smooth VSA. The customer should provide a clear agenda with timings for each topic to be covered and a list of employees required to attend the meeting. The customer should submit the agenda to the supplier at least one week in advance of the VSA affording them enough preparation time, and schedule the time with the staff required for the meeting. A well-planned VSA will ensure that all the information is ready to present via the share screen and the people needed to answer questions are all present in the meeting. References 1. https://mp.weixin.qq.com/s/HKAYkNv4S0TxidzKEcdMpw 2. https://www.trip.com/blog/covid-19vaccine-passports/ 3. https://www.straitstimes.com/asia/ east-asia/covid-19-vaccine-travelrules-widen-the-rift-between-chinaand-the-west
Table I: An example of a VSA agenda outlined by a customer (Philips Medical) and given to the supplier 3 days prior to the audit. The information requested for review is determined by the customer based on what is important to them. Quality certifications (ISO, TS, etc.)
Design review and approval
HR review, staff growth and training, retention rate
Quality review, equipment, calibrations, software, processes, etc.
Employee safety, human rights and working conditions
Production build schedule reporting and updates
Environment regulations compliance
Inventory controls (incoming and outgoing)
Quotation review
Sampling of tooling, TO and T1
Data release controls, ECN (engineering change notice) process
Final dimensional buyoff (first article approval)
Equipment list
Logistics, shipping, packaging review
Design for manufacturability and analysis review
IP (intellectual property) protection and security of data
Engineering review, capabilities, CADD systems, software, etc.
LEAN manufacturing initiatives
Manufacturing review, equipment, calibrations of equipment, etc. Table II: An example of VSA requirements outlined by a customer (Philips Medical) and given to the supplier 7 days prior to the audit. The customer decides who will attend the meeting and the platform through which it will be conducted (for example, Microsoft Teams). Video camera in the conference room working at high efficiency (no delays accepted). Audio in the conference room working at high efficiency with microphones by each presenter. Cell phone with 5G that can be used for factory tour without delay in audio or video; cell phone also connected to audio/video conferencing platform, such as Teams Meetings. Entire management and factory department leaders available for questions. All documentation for review can be done via share screen. Technical managers or support personnel from other countries tied into the virtual meeting for questions. Allow 6–8 hours for the meeting, with a 30-minute break.
WWW.MEDICALPLASTICSNEWS.COM
23
TUBING, CATHETERS & STENTS
The eight-point path to design enlightenment CARL GOUNDRY, PRODUCT MANAGER, TEKNI-PLEX, DISCUSSES EIGHT KEY FACTORS MEDICAL DEVICE INNOVATORS SHOULD CONSIDER WHEN ENGINEERING TUBING SOLUTIONS FOR EMERGING APPLICATIONS.
M
edical device companies are innovating at an exponential rate, but they must consider the following eight factors to ensure a successful product development design process. INTRICACY OF DESIGN Improvements in patient safety and procedural efficiencies are driving innovative design technologies that require more complex components to ensure product performance. Demands on product specifications are tighter and quality standards more stringent. There is increased pressure to ensure fit, form, and function are right first time. Device design engineers are constantly seeking to partner with organizations that can push boundaries and work collaboratively towards meeting new product innovation requirements. Aligning with a well-experienced team — skilled on all aspects of the design journey, from conception to commercialization — is vital to ensure the correct decisions are made at every stage of the development process. Early engagement on design, material selection,
24
conversion, and assembly with the right partner is invaluable towards the success of new product development and launch. INCREASING PRECISION Demands are increasing for even higher levels of surgical procedural precision, putting advances in material and tubing technology at the forefront for device innovation. Comfort, speed and minimally invasive procedures are all attributes that positively affect health and wellbeing. Working with a solution provider that can engineer tubing with these desirable attributes is critical to procedure success. For example, surgeons are relying on robots to perform surgical procedures that would previously have been impossible; pills with cameras are replacing endoscopes; tiny catheters can be used to access every vessel in the body. These technologies have been designed to minimize trauma and improve patient recovery rates. This need for miniaturization is driving more demanding and tighter tolerances. The end result is more pressure on medical device engineers and further pushing of the boundaries of material science. AFFORDABILITY FOCUS People worldwide are living longer, primarily because of access to better healthcare and the advances in medicine during the past 20 years. People are, therefore, more likely to go through an increasing number of medical procedures during their lifetime. This added cost pressure on the healthcare system requires control of cost to allow device manufacturers to keep pace with ever-growing demand volumes which allows equal access for all. Efficiency of design and material selection are critical elements in this regard and as such must be afforded a great deal of consideration to ensure a device remains viable during its expected lifetime.
TUBING, CATHETERS & STENTS
REGULATORY EXPERTISE Being aware of, and compliant with, ever-changing regulatory requirements around the world is critical for any medical device manufacturer. The latest medical device regulations (MDR) in Europe, for example, have different requirements than those found in the US and China. Suppliers to medical device companies must be well versed and up to date with these stipulations, including approved ingredients, toxicity testing, validation steps, labeling requirements, and registration documentation and recording. Your medical tubing partner should exhibit proficiency in best-in-practice manufacturing processes, as well as quality systems optimized for medical device products. Look for companies with decades of experience supporting its customers throughout the regulatory submission phase, regardless of geography. SPEED-TO-MARKET Working with a tubing supplier who knows how to remove weeks and months from the development timeline is an important asset to meeting project milestones. For example, suppliers heavily focused on R&D can help you to reach your end goal quicker, while at the same time meeting critical performance and commercial criteria. Rapid turnaround on material/formulation changes, customized trial runs and prototype sampling are essential for delivering shorter development times. These capabilities, therefore, are an important ask of any supply partner. The greater the depth of expertise in material science and processing technology, the greater the rate of success. PLAN FOR THE FUTURE As conception, design, development, validation, and final commercialization of a medical device can take years to complete, medical device companies do well to work with tubing suppliers who can support their long-term vision and aspirations. Need for change may arise at any stage, and every effort should be taken to limit the impact of such a change. The three most common reasons that we have encountered necessitating a change are transfer of manufacturing site (localization or globalization), obsolescence of a raw material (more and more common nowadays), and engineered change (usually an efficiency improvement or cost-saving initiative). Partnering with companies specializing in the medical sector
Working with a tubing supplier who knows how to remove weeks and months from the development timeline is an important asset to meeting project milestones
will proactively address all three. When evaluating options look for global reach and high levels of raw material/formulation design control and integration. VISION FOR SUSTAINABILITY There is a global spotlight on sustainability, whatever the product or market segment. Medical devices are not exempt, particularly as more disposable devices enter the marketplace. Ensuring the world has access to medical devices that are both safe and sustainable requires a high level of know-how combined with a commitment towards social accountability. Responsible companies should look to associate themselves with like-minded parties that share the same environmental values and are willing to work collaboratively, with passion and urgency, towards a common goal. Innovations in material science are likely to play a big part in this arena in the coming years; it is important to act early, or risk being left behind. MEDICAL SECTOR EXPERIENCE Now, more than ever, it is essential to align with a tubing manufacturer that is fully ensconced in the medical sector. Supply partnerships with skilled companies that are proven experts in the medical arena need to be nurtured to ensure all aspects of new product development are covered. By doing so, you will help to mitigate potential problems/ risks in quality, regulatory failures (or product recalls), bridge technology gaps and prevent shelf-life problems — all of which can stem from a lack of in-depth understanding of the demands placed on the medical device sector.
25
SPONSORED CONTENT
TridAnt
INFECTION PREVENTION INFRASTRUCTURE ARJUN LUTHRA, COMMERCIAL DIRECTOR, BIOINTERACTIONS, EXPLAINS HOW MULTI-ACTION TECHNOLOGY IS A STRAIGHTFORWARD SOLUTION TO FIGHT COVID-19, PREVENT FUTURE PANDEMICS AND ENHANCE GLOBAL HEALTHCARE INFRASTRUCTURE.
T
he case for revolutionary technologies and solutions that greatly enhance our quality of living has never been more important than now. Innovations that protect and enhance our daily routines are in constant demand and are especially critical considering the global Covid-19 pandemic. As the pandemic has persisted, our healthcare services and innovations have battled to keep up with the viral mutations as well as mitigate impact to core areas of healthcare services. This highlights the importance of a preventative measure to be in place so we can return to a better normal. The prevention measure must focus on confronting the current and future variants of Covid-19, preventing other harmful pathogens in our daily lives, as well as enhancing global infrastructure to prevent another pandemic from occurring. THE ROLE OF MATERIALS Materials play a critical role in improving the performance of technology and can improve the applicability of the products. The antimicrobial efficacy of materials involves critical interactions between the pathogen and surface interface, and the successes of applications
depend on a variety of biological events occurring at this interface. These events intensify the complications, which must be addressed during innovation and development. The challenge of infection prevention will help long-term prevention mechanisms which enhance medical devices as well as consumer applications. The biological events which occur at the pathogen-surface interface is critical to improving the prevention technology against pathogens. Biological responses are complex processes which are governed by a variety of factors. These factors range from surface properties, which include the chemistry, topography, wettability, and composition of a surface, to the biological entities present at the interface. Infections on surfaces have long been studied as they pose a significant challenge which has impeded long-term medical device applications. A variety of preventative techniques including silver, copper, and other chemistries used on medical devices have proven not to be significantly effective and highlight the need for an innovative solution. It has been seen in a randomized study of surface treatments to prevent infections that silver surface treatments have failed to reduce infection rates. It has also been seen that the use of silverimpregnated collagen cuffs can impede catheter fixation due to the killing of fibroblasts and cause the catheter to dislodge, thereby highlighting the toxic effects of similar chemistries to silver technology on eukaryotic cells. Furthermore, it has been recommended that any catheter which has caused bacteremia should be immediately removed and only replaced once results of blood cultures normalised. This highlights the difficulty previous technologies experienced when combating infections safely on a surface over long periods of time. Such as approach has had limited success, requiring the use of antibiotics which leads to resistance and further complications. The biological events that occur on a surface are a complex result of interactions between the surface and the proteins and cells, which are present at the pathogen-surface interface. These complex challenges require a multiaction material which considers all these varying factors in one straightforward technology. Multi-action materials provide a high-performance surface which prevents pathogen growth, enhances protection durability, and uses safe components all in one straight-forward solution. THE POWER OF PARTNERSHIPS BioInteractions Ltd and Phoel Ltd have partnered together to provide TridAnt®, the most advanced infection prevention infrastructure that enhances the control of pathogens in high-risk environments such as hospitals and GP practices. TridAnt® technology ensures broad spectrum protection of up to 99.99% against bacteria, viruses, and yeast as well as enhanced durability of up to 365 days — all while using technology comprised of chemistry which has been safe enough to use on medical devices for decades.
26
WWW.MEDICALPLASTICSNEWS.COM
SPONSORED CONTENT
TRIDANT® INFECTION PREVENTION INFRASTRUCTURE CONSISTS OF: • TridAnt® Shield — Protective film immediately protects all hard surfaces and kills 99.99% of bacteria, viruses, and yeast for up to 365 days while reducing cleaning maintenance and routines. • TridAnt® PPE — PPE items enhanced to kill 99.99% of bacteria, viruses, and yeast for up to 365 days without the need to change from patient to patient whilst reducing single-use items. • TridAnt® Skin Sanitiser — Sanitiser and cleansing technology protects skin for up to 48 hours and kills 99.99% of bacteria, viruses, and yeast while preventing transfer of host flora as well as reducing water requirements • TridAnt® Dry-wipe — Antimicrobial dry wipe kills 99.99% of bacteria, viruses, and yeast for up to 365 days without using liquids. TridAnt® Infection Prevention Infrastructure is a straightforward, long-term solution to protecting surfaces, preventing transmission, and controlling pathogens. The infrastructure provides enhanced protection against a broad spectrum of bacteria, viruses and fungi on surfaces ranging from stainless steel to skin. The various products utilise TridAnt® technology to enhance protection and provide further long-term benefits. TridAnt® tools provide a viable method of improving hygiene and healthcare standards while reducing maintenance costs, as well as reliance on single-use items. The various products reduce the level of consumption of harsh chemicals and provide enhanced hygiene infrastructures that prevent future pathogens. Furthermore, TridAnt® items actively clean surfaces on contact, actively cleaning the environment throughout the lifetime of the products. This helps us to maintain hygienic and healthy environments, and greatly reduces the costs of controlling pathogens on surfaces. PHOELING GOOD TridAnt® Infection Prevention Infrastructure is the first line of defence and the missing tool in the toolbox to prevent and control pathogenic transmission. The use of rapid testing as well as vaccines requires additional support from technology to allow these areas to develop fast and effectively enough to keep up with future risks. The use of a prevention tool allows rapid testing and vaccines to use TridAnt® Infection Prevention Infrastructure to gain time and create safe and effective vaccines. The additional TridAnt® tool, works in conjunction with rapid testing and vaccines to provide complete protection structure against Covid-19, its mutations, and the possibility of
future pathogens. This complete infrastructure can be applied safely into schools, restaurants, hotels and hospitals to allow a swift recovery to our normal routines. TridAnt® technology uses the hygiene of the future to bring back the good ‘phoelings’ (pronounced ‘feelings’) of the past. Covid-19 has shown us the significant damage that can be done to our existing healthcare services, our daily routines and our public utilities when we are not proactive against these challenges. TridAnt® Infection Prevention Infrastructure provides us with the tools to combat Covid-19, as well as prevent a similar pandemic from occurring whilst enhancing healthcare infrastructure for the longterm future and returning us to a better normal.
WWW.MEDICALPLASTICSNEWS.COM
27
REGULATORY
Prepare to meet thy regulator MANUFACTURERS STAND TO GAIN TIME AND COST BENEFITS IF THEY CAN FACILITATE A SUCCESSFUL PRE-SUBMISSION MEETING WITH THE FDA PRIOR TO MAKING THEIR FINAL MEDICAL DEVICE APPLICATION SUBMISSION. SANDI SCHAIBLE, SENIOR DIRECTOR OF ANALYTICAL CHEMISTRY AND REGULATORY TOXICOLOGY, WUXI APPTEC, AND MICHELLE LOTT, FOUNDER AND PRINCIPAL OF LEAN RAQA, ADVISE HOW TO SQUEEZE THE MOST OUT OF THESE ONE-TIME-ONLY MEETINGS.
T
he more a manufacturer can collaborate with regulators, the better the manufacturer can identify submission expectations and potential challenges. An effective way to do so is through a successful pre-submission meeting with the US Food and Drug Administration (FDA). These meetings allow manufacturers to work with the FDA to review their medical device testing plans and address concerns before final submission. Facilitating a pre-submission meeting with a regulatory reviewer is a critical resource for a manufacturer trying to get a device cleared with the FDA. Currently, there is no similar pre-submission
pathway available in the European Union (EU) as notified bodies (NBs) are not available for consultive purposes. Competent Authorities are inconsistently interpreting and applying the EU Medical Device Regulation (MDR), creating a wide difference in what each NB will accept. An effective pre-submission meeting can reduce time and money spent preparing the submission and increases the chance of gaining approval. There are three forms of feedback manufacturers can request from the FDA: written responses only; a 60-minute teleconference; or an in-person meeting. In light of the pandemic, meetings are held in a digital format via video or teleconferencing. No matter what they choose, manufacturers must come prepared to make the most of their time. Understanding how to best leverage a pre-submission meeting can set companies and their device up for successful regulatory submission. GETTING STARTED Manufacturers must have a detailed understanding of their device before initiating a pre-submission meeting. This includes knowing the intended use, features, functions, testing strategy, along with how it compares to a predicate. In addition, it is critical to develop clarifying questions on FDA’s initial response to send to the FDA before the meeting. These questions should be sorted by priority and consider regulatory updates and other highrisk topics that need addressing before final submission. Once the FDA responds to the questions, this feedback will guide the specific talking points for the meeting. WHAT TO EXPECT The reviewers will only come prepared to discuss their responses to the questions submitted by the manufacturer ahead of the meeting. Anything outside the preliminary topics should not be addressed during the pre-submission meeting. The pre-submission process allows manufacturers to work with the FDA directly to gain their insight. Based on the responses provided before the meeting, manufacturers can follow up and pinpoint specific areas of their plan where they would like greater detail about the reviewers’ feedback. Prioritize potential talking points to improve the testing plan and minimize additional information requests. Keep in mind that this meeting is not the time to negotiate or barter. Even if the feedback is unexpected or not ideal, essential factors such as patient safety and submission approval may be at stake if manufacturers don’t take
28
WWW.MEDICALPLASTICSNEWS.COM
REGULATORY
attend the meeting. Everyone in attendance should have defined areas of expertise relevant to questions already posed to the FDA. Attendees should be designated to speak one at a time to those topics and should otherwise remain silent.
the feedback seriously. Manufacturers should work with the team offline to provide potential follow-up questions for the final call. MAKING THE MOST OF THE PRE-SUBMISSION MEETING Prior to attending the pre-submission meeting, manufacturers should understand that the FDA will not be leading the conversation. In this meeting, they can discuss their clarifying questions in detail and provide rationale. Reviewers will voice if they agree, disagree or have further questions. With only an hour, manufacturers should not repeat the same content addressed in the pre-submission document that the FDA has already reviewed in detail. Instead, come with a high-level outline of thoughts on the responses, prioritized by most significant concerns. For example, if a manufacturer’s device uses a novel material or manufacturing method, the biocompatibility test strategy may be particularly complex. The manufacturer may want to focus on more complex topics rather than a line-by-line review of the FDA’s feedback. In short, prioritize topics and prepare direct follow-up questions. And finally, practice. With an established team, prepare what each attendee is going to address and when. A point person should lead the call and direct questions to the appropriate team members. As for any important presentation, it’s essential to rehearse and ensure roles are understood. Remember, there’s only one opportunity for this meeting. Those who attend are crucial to the meeting’s success. Considering team expertise can help determine who should
The pre-submission process allows manufacturers to work with the FDA directly to gain their insight
AFTER A PRE-SUBMISSION MEETING If a manufacturer involves a laboratory testing partner or consultant in their submission process, the partner(s) may attend the pre-submission meeting. If they do attend, they cannot be the sole expert guiding the discussion. The manufacturer will need to drive the conversation. The FDA will bring their in-house expert counsel. Manufacturers should consider inviting testing partners and consultants to provide any additional support to the team and interpret any feedback as needed. If the testing partner does not attend, it is critical to relay the exact feedback from the meeting to them. Do not interpret or paraphrase when communicating the results received from the FDA. Any misinformation can affect testing decisions and potentially put the entire submission at risk. Since preparation may change after the pre-submission meeting, manufacturers need to re-evaluate their testing and submission timelines to be realistic. REAP THE BENEFITS Facilitating a pre-submission meeting before moving forward with medical device testing will provide the solid foundation manufacturers need for a successful submission. The more input from regulators a manufacturer can receive early on, the smoother their submission experience will be. Testing and submitting a medical device for regulatory approval is not the time for trial and error. When approaching the pre-submission meeting, manufacturers must focus on detail, be open to feedback and allocate enough time to incorporate any changes to their plan. Doing this work upfront not only saves money and time later on, is also sets manufacturers up to get their submission right the first time.
WWW.MEDICALPLASTICSNEWS.COM
29
3D PRINTING & PROTOTYPING
THE TINY REVOLUTION AS THE CURRENT GLOBAL IMMUNIZATION AGAINST COVID-19 ROLLOUT HAS SHOWN ADMINISTERING CONVENTIONAL NEEDLE VACCINES AT SCALE HAS ITS LIMITATIONS. JOHN KAWOLA, CEO — GLOBAL, BOSTON MICRO FABRICATION, EXPLAINS HOW MICRO 3D PRINTING MICRONEEDLES COULD RADICALLY CHANGE HOW THE WORLD RESPONDS TO VACCINE DISTRIBUTION.
M
ore than 1.5 billion doses of the COVID-19 vaccine have been administered around the world, according to the latest figures from Bloomberg.1 Those numbers are certainly impressive considering the short timeline from vaccine development to deployment, but the last several months have exposed some major logistical challenges of conventional needle vaccines — most notably, their difficulty in administering at scale. Imagine how much easier distribution could be if a vaccine could be mailed directly to your door and administered via a tiny, painless patch that sticks to your arm. What if vaccines in this form could be sent out to impoverished or remote locations that don’t have access to healthcare? Advanced immunization technologies, namely microneedles, are bringing these ideas closer to reality. The concept of microneedles for vaccinations or other drug delivery has been around for a while, but COVID accelerated demand and research to make it happen. But once the delivery method for microneedles is finalized, the industry faces another hurdle:
30
3D PRINTING & PROTOTYPING
figuring out how to manufacture these tiny, functional devices at scale, and as cost-effectively as traditional inoculation methods. The answer lies in micro-precision 3D printing.
3D printing will be used to produce the final microneedle vaccine is up for debate.
BRINGING US CLOSER TO THE FUTURE A team of collaborators, led by Carnegie Mellon University with participation from Boston Micro Fabrication (BMF), recently revealed that they are developing a novel approach to vaccinations with a low dose, inexpensive and hybrid microneedle array technology.² The microneedle arrays consist of hundreds of tiny needles on a small patch that, when applied to the skin, can quickly dissolve and deliver the vaccination.
In a perfect world, PμSL would be used to print the microneedles for end use using a dissolvable, biocompatible material. This would be the most efficient way of going about bringing the arrays to production and distribution, and would cut out other processes such as molding, but this method is reliant on developing the proper dissolvable material. With time of the essence, there are a number of alternatives to consider as well.
This delivery method requires a small dosage amount (as little as 1/100th of the dose of a traditional vaccine) without the same level of cold chain storage requirements, meaning it has the potential to simplify the transport and storage of vaccines, reduce vaccine shortages, and more easily distribute vaccines to people around the world. These are all concerns that have been exposed in recent months with traditional methods of vaccine deployment. The research, so far, has been promising — it found that the smaller the microneedles, the easier it is to puncture the skin and deliver the vaccine effectively. This finding introduces a whole new set of challenges for putting the microneedle arrays into use, and it’s where micro 3D printing comes into play. WHEN SMALLER IS BETTER, BIGGER CHALLENGES ARISE As with any type of manufacturing and product engineering, the smaller the part, the harder it is to design, the more expensive it is to make, and the more complicated it is to put into production at scale. This notion is especially true in traditional manufacturing methods, such as micro injection molding and computer numerical control (CNC) machining, where the smaller and more detailed the part, the higher the cost and the longer the wait to get it (and, if this pandemic taught us anything about manufacturing, it’s that time is of the essence). Additive manufacturing has long been considered a more cost-effective and time-efficient approach, but even 3D printing has traditionally lost its appeal as parts become smaller. Challenges with precision and accuracy have caused innovation roadblocks for years; quite simply, there has been a lack of viable additive manufacturing technologies available that can print with the right resolution and at scale. The high costs associated with traditional manufacturing methods, combined with the lack of capable 3D printing alternatives, would have crushed the promise of microneedle vaccinations before the research was even complete if it weren’t for recent developments in micro 3D printing. Newer technologies, such as stereolithography (SLA), digital light processing (DLP), and a combination of the two called projection micro stereolithography (PµSL), can finally produce ultra high precision, micro sized parts that are suitable both for rapid prototyping and production. MICRO 3D PRINTING FOR MICRONEEDLES Carnegie Mellon invited BMF to participate in the research because of the company’s PμSL technology, which can print small parts down to a 2 µm resolution. The technology makes it possible to print at the right resolution, size, and accuracy with precision and efficiency … but how exactly the micro
One of these options would be using PμSL to print mold patterns that have enough strength to be used to create molds, which would then be used for production. This would allow for many microneedles to be printed off the same 3D printed mold, and would still be more cost effective, accurate and precise compared with traditional manufacturing methods. Although microneedle vaccines are still in the early stages of research and development, there’s no doubt that micro 3D printing will play an integral role in making their mass manufacturing and distribution a reality. These tiny devices have the potential to completely revolutionize the way the world responds to health crises and immunizes millions of people, rapidly, at scale, and although COVID-19 was hopefully a once in a lifetime event, the innovative vaccine manufacturing and delivery techniques being developed today will ensure a smoother fight against any future threats.
References 1. https://www.bloomberg.com/ graphics/covid-vaccine-tracker-globaldistribution/ 2. https://www.meche.engineering. cmu.edu/news/2021/03/scalablemanufacturing-for-painless-vaccines. html
WWW.MEDICALPLASTICSNEWS.COM
31
MTD Micro Molding opens expanded facility 1
On May 27, MTD Micro Molding celebrated the grand opening of its expanded medical micro injection molding facility.
2
The exterior of the expansion — which was completed last fall — effectively doubles the manufacturing space.
3
The new interior space required a few more months to finalize details, more equipment, and move new cuttingedge machinery into place.
4
The new space has been planned to ensure project flow and tracking, improving time and efficiency, which will help customers achieve faster speed-to-market.
5
The company is continuing to respond to the demand for custom packaging and assembly services for micro medical components.
ENvizion Medical scoops innovation award for its feeding tube navigation system
BTG Labs earns ISO 9001:2015 certification
B
B
ased on its recent analysis of the North American enteral feeding tube navigation market, Frost & Sullivan has recognized ENvizion Medical with the 2021 North American Technology Innovation Leadership Award. Addressing the limitations of blind insertions and radiography-based adjuvant method, ENvizion Medical has developed a revolutionary feeding tube navigation system for accurate enteral tube placement in adult patients. The FDA 510(k)-cleared ENvue system consists of an electro-mechanical device with embedded software and enteral feeding tubes. Leveraging the power of electromagnetic technology, the device creates a personalized body map of
32
patients, allowing the medical staff to precisely place the tube in the stomach or small intestine through the oral or nasoenteric route. “Unlike competing products, the ENvue system creatively displays a frontal, lateral, and axial view of the patients’ body contours simultaneously, while also highlighting the enteral feeding tube’s direction in real time,” said Neeraj Nitin Jadhav, Senior Research Analyst. “Such real-time and multifaceted visualization and directional guidance provides increased confidence to healthcare support staff when inserting the feeding tube, eliminating the risk of placement in the patients’ lungs, and, therefore, associated complications.”
TG Labs, a specialist in industrial cleaning, surface treatment, and adhesion process control, has earned ISO 9001:2015 Certification. Internationally recognized as the standard for quality management, ISO 9001:2015 certification signals to customers that BTG Labs’ entire organization is committed to maintaining rigorous quality standards. The company relies on stringent internal quality standards to develop innovative and one-of-akind surface quality and inspection equipment for manufacturers who are bonding, coating, painting, printing and cleaning. “Process control is a critical part of the adhesive bonding and material cleaning success BTG Labs helps customers achieve. It was a natural fit for us to pursue ISO 9001:2015
W W W. M E D I C A L P L A S T I C S N E W S . C O M
Certification so that we would have a common framework and shared vocabulary for pursuing quality objectives,” said Mike Geren, Product Manager at BTG Labs.
COV3