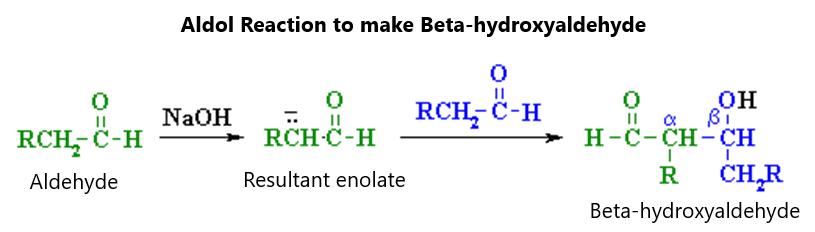
In the halogenation of benzene, it will not work if just the halogen gas or liquid is mixed with the benzene. They need to be acted on by Lewis acid catalysts in order to activate the halogen. These can be iron (III) halides or aluminum (III) halides. Aluminum bromide, for example is a Lewis acid that can mix with bromine gas to make a Br+ ion. This is much more electrophilic than bromine gas alone. The catalyst will polarize the bromine-bromine bond so that there can be a positive bromine end and a negative bromine end. It is the Br+ ion that will ultimately react with benzene. This will leave behind AlBr4- as an end product ion. This ion allows for the hydrogen ion to be “pulled off” the benzene compound, effectively substituting bromine and hydrogen. Figure 58 shows this reaction:
Figure 58.
As you can see, Aluminum bromide isn’t consumed in this reaction but is regenerated as a catalyst that will be useful for other halogenation processes in the reaction. The reaction involving bromine is exothermic but not as exothermic as fluorine (which is explosive in nature). The electrophilicity decreases as one goes down the group of halogens so that this will be endothermic when iodine is the halogen involved.
NITRATION OF BENZENE The reaction yielding nitrobenzene (nitration of benzene) doesn’t happen without the help of sulfuric acid. It takes the sulfuric acid to make the NO2+ ion from nitric acid in order to make it electrophilic enough to bind with benzene. Interestingly, when nitrobenzene is mixed again with iron and reduced, it makes benzene with an amine group attached.
103