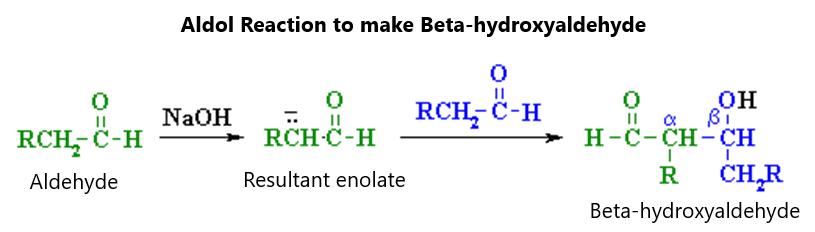
Second, there can be a Strecker synthesis, in which an aldehyde is mixed with ammonium chloride and sodium cyanide to make an intermediate that is then heated and acidified to make a carboxylic acid as is shown in figure 117:
Figure 117.
In the Strecker synthesis, there is nucleophilic addition first and then nucleophilic acyl substitution—essentially two parts to the reaction. It starts with ammonium chloride and sodium cyanide, which add an ammonium group and a cyanide group to an aldehyde. This gives rise to an amino nitrile intermediate. Hydrolysis then converts the nitrile molecule into the carboxylic acid molecule to give rise to the alpha amino acid.
REACTIONS OF AMINO ACIDS These are reactions that take place on the amine side chain and the carboxylic acid side chain. These are not much different from reactions that we have already covered in this course. The most important reactions that take place are those that happen in the course of making peptides and protein molecules. When it comes to amines, there is acylation to form amides that has already been talked about. The R2NH molecule is acted upon by acid chlorides or acid anhydrides under basic circumstances to make an amide compound. This has already been shown in figure 107 in a previous chapter. This is how amides get formed in the making of peptides.
210